- 1Department of Nutritional Biochemistry, University of Hohenheim, Stuttgart, Germany
- 2Laboratory of Medical Microbiology, Hirszfeld Institute of Immunology and Experimental Therapy, Polish Academy of Sciences, Wroclaw, Poland
- 3Department of Medical Statistics, Biomathematics and Information Processing, University Clinic Mannheim, Mannheim, Germany
- 4Department of Food Microbiology and Hygiene, Institute of Food Science and Biotechnology, University of Hohenheim, Stuttgart, Germany
- 5Department of Vegetative and Clinical Physiology, Institute of Physiology, University Hospital Tübingen, Tübingen, Germany
- 6HoLMiR-Hohenheim Center for Livestock Microbiome Research, University of Hohenheim, Stuttgart, Germany
- 7Institute of Animal Science, University of Hohenheim, Stuttgart, Germany
Non-nutritive sweeteners (NNS) are present in various commercial articles, from foodstuffs to oral hygiene products. Despite their alleged safety, mounting evidence indicates that NNS intake is associated with an alteration of intestinal bacterial populations (dysbiosis) in animals and humans. Since NNS are commercialized based on the assumption that they are not metabolized by human cells and negligible effect on bacterial, the insurgence of dysbiosis associated with NNS intake remains unexplained. The current review aims to assess the effect of selected NNS (acesulfame potassium, advantame, aspartame, neotame, saccharin, stevia, and sucralose) on the human intestinal microbiota. Findings from this review suggests that NNS intake is linked not only to alterations in human physiology but also to modifications of bacterial biochemistry, including the hindrance of quorum sensing pathways, in a species-specific manner. Moreover, there were suggestions that NNS could also affect the biology of phages, namely by binding to the active sites of proteins involved in the infection process and altering the induction rate of prophages. The studies gathered in the present review provide a framework for understanding how NNS might be connected to dysbiosis, both directly through alterations in bacterial biochemistry and indirectly through impaired phage activity.
Introduction
Currently, obesity affects roughly one in eight people worldwide and, since 1990, the percentage of obese adults and adolescents has doubled and quadrupled, respectively, with over 40% of adults being overweight (1). Obesity and high body weight are linked to various medical conditions, including type 2 diabetes, atherosclerosis, depression, and cancer, with a high burden on people’s quality of life and the health systems around the world (2). To address this ongoing outbreak of weight-related problems, the World Health Organization (WHO) has suggested limiting the amount of free sugar in foodstuffs to 10% the daily energy intake (3). Such a goal was followed to the food industry’s massive employment of non-nutritive sweeteners (NNS, also called non-caloric, high-intensity, or artificial sweeteners) (4).
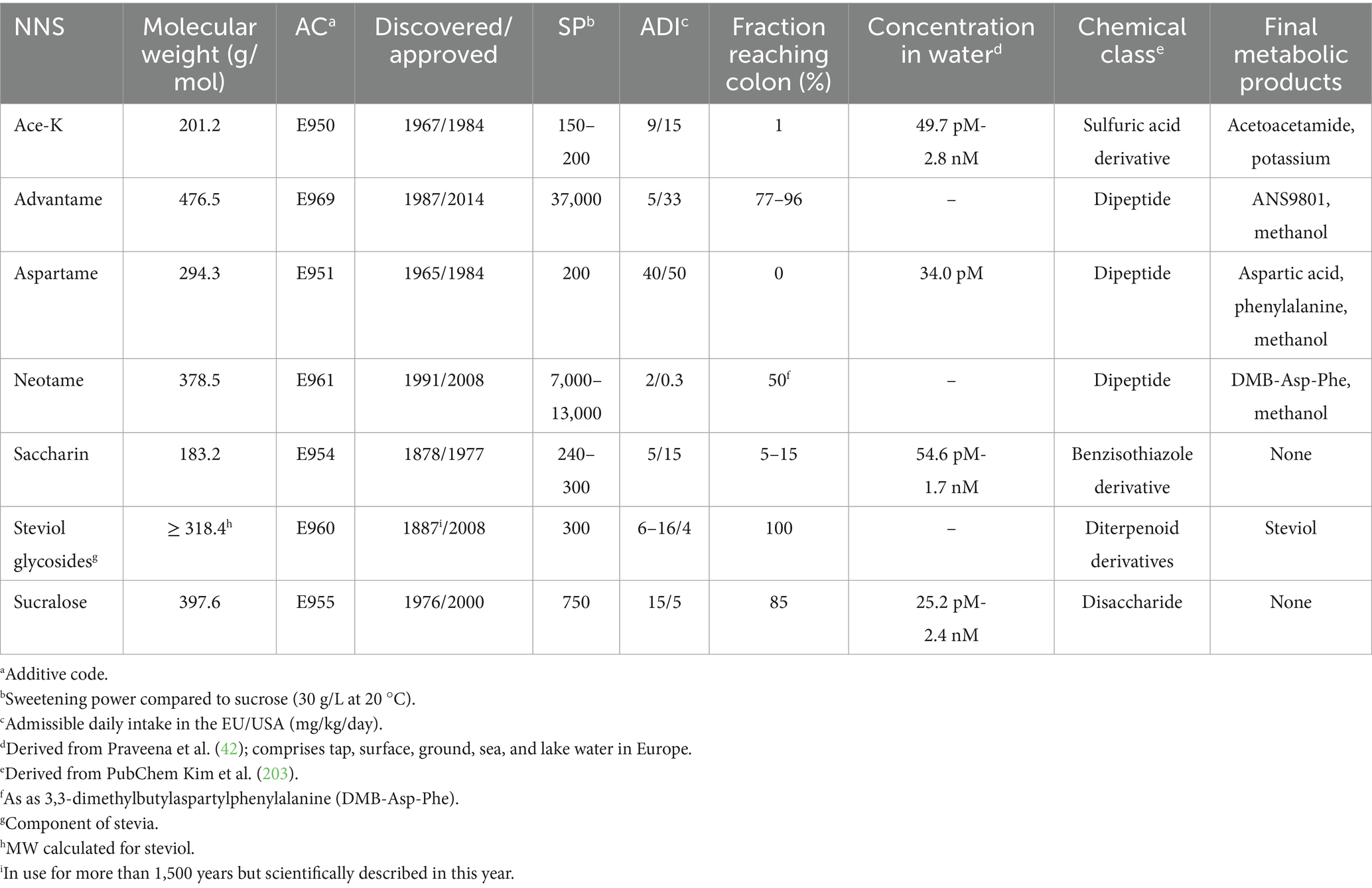
NNS are so defined because, unlike natural sweeteners such as glucose (dextrose), fructose, or maltose, they do not provide energetic input to human cells (5). The WHO, the American Food and Drug Administration (FDA), and the European Food Safety Authority (EFSA) all declared NNS safe for human consumption (6). However, the food authorities also introduced in 1961 a quantitative evaluator of NNS intake, the acceptable daily intake (ADI), which is defined as the daily amount of a sweetener per body-weight ingestible throughout a person’s lifetime without appreciable health risk (7–11).
NNS are now present in a wide variety of goods, sometimes in the absence of consumer awareness, ranging from soft drinks to cereals, from vitamin supplements to oral hygiene products (12–14). The global yearly consumption of NNS reached 117,000 tons in 2021 (15), and it has been observed that the intake of NNS-containing drinks doubled in children between 2000 and 2008 (16).
The NNS ADI varies according to the sweetener and different countries might adopt distinct ADI levels (17, 18). It has been estimated that one person should drink about 20 cans of diet soda containing aspartame, 800 cans of diet beverages containing saccharin, or 14 servings of iced tea containing sucralose to reach the respective ADI (19, 20). Although such amounts appear unattainable (21–23), it has been reported that the ADI is exceeded in several cases, particularity by youngsters (24–26).
In Germany, where about 89% of soft drinks contain NNS (27), a study carried out on a group of 2,291 individuals assessed that 99.8% of the participant did not exceed ADI intake (25). Nonetheless, extrapolating from these data and considering a German population of 83.5 million (28), one might speculate that 0.2% of individuals who consume NNS above the ADI level would correspond to approximately 1.7 million people at risk of excessive NNS intake. Although the exposure to NNS, particularly stevia, is increasing worldwide, especially due to consumption of soft drinks, there is a paucity of recent data monitoring the actual consumption of NNS in the German population (29–31).
Recently, the WHO carried out a systematic re-evaluation of clinical trials on physiological markers, finding that not only does NNS intake not reduce body fat, as previously claimed, but it could also increase the risk of type 2 diabetes, cardiovascular disease, and mortality in consumers (32). Thus, the WHO has revised its stance on NNS safety and now recommends avoiding NNS consumption to control body weight (33, 34). Such re-evaluation highlights the necessity of understanding the effect of NNS intake on human physiology. The data derived from clinical trials regarding the effect of NNS intake on human subjects is controversial, and, in general, the long-term effect of NNS on human health needs to be more adequately investigated (35), especially considering that more people are exposed to NNS than those who knowingly consume them. For instance, an NNS intervention study reported that eight (44.4%) of 18 healthy participants chosen as baseline non-NNS consumers showed sucralose in their urine (average concentration 0.6 mM) even before the actual trial begun (36).
Each consumer can excrete tens to hundreds of NNS milligrams per day in the urine (37–39) and NNS have also been detected in amniotic fluids at concentrations of nanograms per milliliter (40). Due to their chemical stability, NNS are not removed during wastewater treatment; thus, they can reach concentrations of about 2.5 mg/L in effluvial water and have become a widespread environmental contaminant that is employed as trackers of human pollution (41–44). For example, environmental exposure to ace-K has been linked to an increased cellular damage in carp (45). Contaminated water and soil carry the risk of transferring NNS back into the food chain, with the potential of indirect NNS exposure through environmental contamination (46).
Moreover, the scientific assessment of NNS safety is more demanding than it might appear. For instance, several NNS are often mixed in foodstuffs, making it more challenging to assess the individual roles of NNS on human physiology (47). In particular, while dysbiosis is a common outcome associated with NNS intake, its effects are highly variable, depending on individual dietary habits and the genetic backgrounds of both the host and microbes, suggesting a personalized approach for further investigation in this area of nutrition (48, 49). Furthermore, the effects of NNS on human physiology are typical of prolonged contact with low concentrations, a combination that is difficult to replicate experimentally (50). In addition, the type of study can also determine a substantial bias in the results. For instance, it has been suggested that trials funded by sweetener manufacturers tended to report a lower NNS (namely, aspartame) risk association than independent studies (51).
Nonetheless, a growing number of reports suggest that NNS consumption can be associated with adverse physiological effects, particularly due to alterations in the intestinal microbiome compared to normal conditions (dysbiosis). A cross-sectional observational study of human volunteers showed a negative correlation between NNS intake and the abundance of different types of bacteria, including butyric acid-producing ones, in the colon (52). It has been shown that ace-K, aspartame, saccharin, and sucralose increased the horizontal gene transfer among bacteria, both at the intra-species (among Acinetobacter baylyi strains) and the inter-species (between A. baylyi and Bacillus subtilis) levels (53). Remarkably, it has also been shown that these NNS added at a concentration of 1–5 mM for 24 h decreased the growth of the acidogenic Streptococcus mutans but left unaffected that of the alkali-producing Streptococcus sanguis (54). Based on a survey of 28 clinical studies, there was a tendency for an increased abundance of members of the Enterobacteriaceae family and decreased abundance of members of the Clostridium cluster XIVa in NNS consumers compared to healthy controls (55). In addition, bacteria extracted from 13 healthy volunteers and exposed to sucralose showed an increased abundance of Escherichia and Shigella genera, whereas aspartame increased the abundance of bifidobacteria; the production of short-chain fatty acids (SCFA) was also altered by these sweeteners (56). Sucralose has been indicated as a possible cause of the insurgence of inflammatory bowel disease (57). If NNS can affect some bacterial species or even strain differently from others, it can be speculated that NNS intake could raise the risk for an imbalance in the microbial growth that could explain the observed dysbiosis in NNS consumers.
The goal of this review was to determine the direct effects of popular NNS on gut microbes to identify the mechanism underlying the development of dysbiosis associated with the consumption of sweeteners. The most prevalent NNS include acesulfame potassium (ace-K), advantame, aspartame, neotame, saccharin, stevia, and sucralose (4, 58, 59). This review will focus on the direct interaction between NNS and bacteria, based on in vivo and ex vivo trials in animals, as well as in vitro experiments. The review will also examine the potential impact of NNS on phages, considering the crucial role these microbes play in shaping the intestinal microbiome.
The articles presented in the present review were obtained primarily by searching the National Library of Medicine (NLM) with the PubMed tool (60) with the keywords: ((((((Inflammation[MeSH Terms]) OR (Inflammation)) OR ((Dysbiosis[MeSH Terms]) OR (Dysbiosis))) OR (Pathological Conditions, Signs and Symptoms[MeSH Terms])) OR ((obesity) OR (obesity[MeSH Terms]))) OR ((Gastrointestinal Microbiome[MeSH Terms]) OR (Gastrointestinal Microbiome))) AND ((Non-Nutritive Sweeteners[MeSH Terms]) OR (Non-Nutritive Sweeteners)). Furthermore, the NLM’s database of clinical trials (61) was searched using the following keywords: Condition/disease: Non-Nutritive Sweeteners; Other terms: Inflammation OR obesity OR microbiome. Both queries were initiated on November 15, 2022. Relevant papers were included based on their titles and abstracts. Additional literature was obtained by further exploring works related to the selected studies.
NNS structure and metabolism
Ace-K
Ace-K is an oxathiazinone dioxide salt that is efficiently absorbed by the small intestine without metabolization. Thus, ace-K is rapidly transported into the bloodstream and then eliminated through the urine, although 1% of the ingested dose is released into the feces (62). In pregnant women, ace-K has been recovered in amniotic fluid (40) and is transmitted to newborns through breastfeeding (63). However, there are no reports of adverse effects in children. The by-products of ace-K are potassium and acetoacetamide, and dietary NNS generates concentrations of these metabolites that are considered well within the safety levels (64, 65). Ace-K was cleared for human consumption in the 1970s, albeit the clinical testing was later deemed unsatisfactory and additional trials were never performed (66). Moreover, it has been shown that both mouse adipocytes 3 T3-L1 and human primary mesenchymal stem cells exposed to ace-K exhibited enhanced adipogenesis (67).
It has also been reported that several environmental bacteria (belonging to the families Boseaceae, Bradyrhizobiaceae, Chelatococcaceae, Methylophilaceae, Phyllobacteriaceae, and Pseudomonadaceae) can metabolize this NNS using it as an energetic source (68, 69). The biodegradation of ace-K to sulfamic acid has been reported in lakes, rivers, and wastewater treatment plants, but only in aerobic conditions (70, 71). Since the gastrointestinal tract (GIT) is virtually anaerobic (72), the fate of this NNS in the GIT remains largely unknown (see Figure 1).
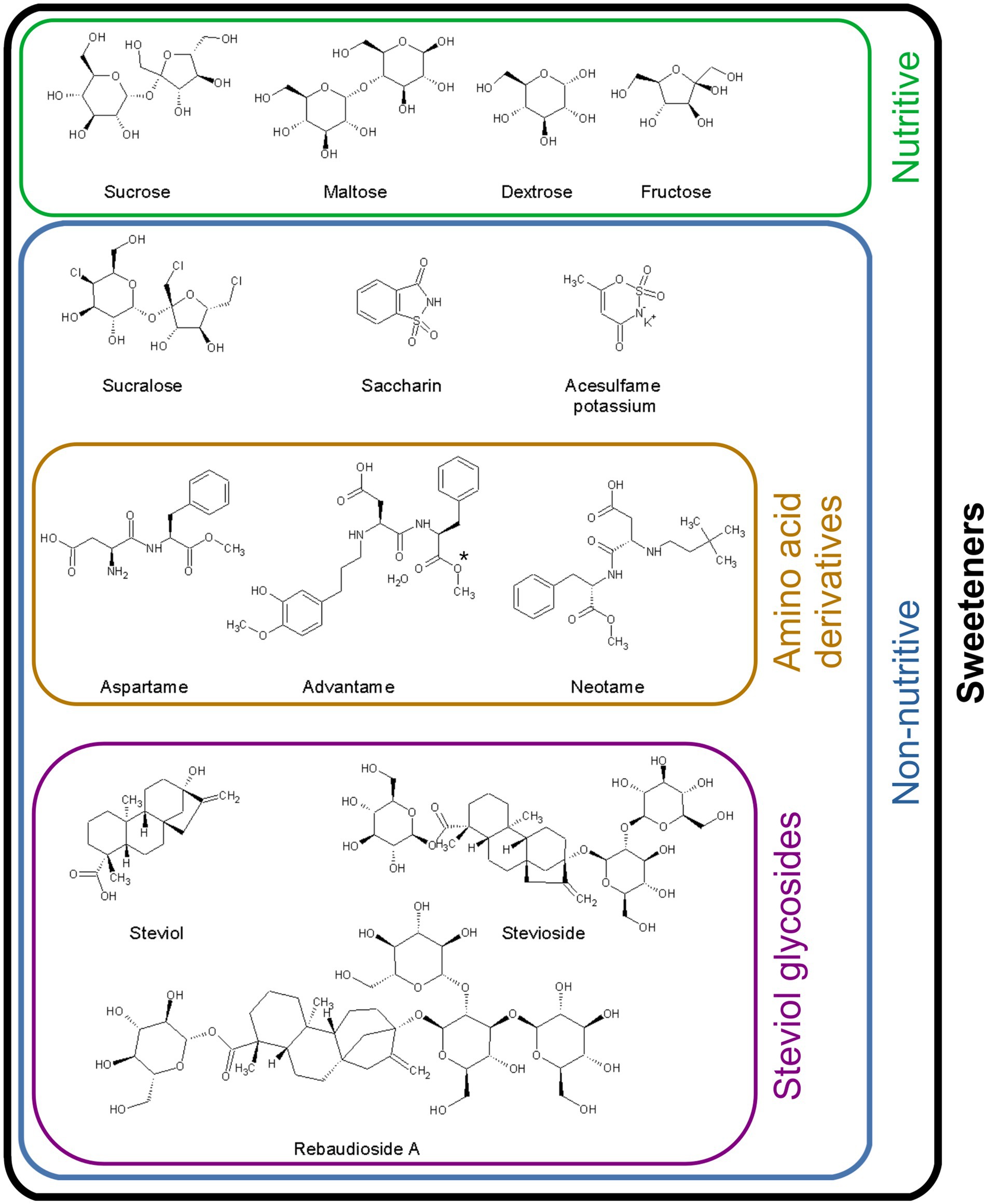
Figure 1. Chemical structure of the sweeteners included in the present review. Comparison of the structures of natural and non-nutritive sweeteners. Dextrose (also known as glucose) and fructose are the basic units of monosaccharides; maltose and sucrose are disaccharides. Sucrose is the reference molecule for the sweetening power of sweeteners. The de-esterification of advantame (*) generates ANS9801-acid.
Aspartame, advantame, and neotame
Aspartame, formally known as aspartyl-phenylalanine methyl ester (or (N-L-α-aspartyl)-L-phenylalanine methyl ester), is a methylated dipeptide of the amino acids L-aspartic acid and L-phenylalanine. Advantame (N-[N-[3-(3-hydroxy-4-methoxyphenyl)propyl]-α-aspartyl]-L-phenylalanine 1-methyl ester) and neotame (N-[N-(3,3-dimethylbutyl)-1-α-aspartyl]-L-phenylalanine 1-methyl ester) are aspartame derivatives (73). Compared to aspartame, the former (developed through computer-based design by the sweetener producer firm Ajinomoto in 1987) has higher thermostability (74); the latter, released in 1991, has higher water solubility. The International Agency for Research on Cancer (IARC) has registered aspartame as a possible carcinogenic agent for humans (75).
Aspartic acid and phenylalanine are released, together with methanol, from the matabolization of aspartame by peptidases and esterases present in the intestinal cells (62). The hydrolysis of advantame in the intestinal tract also produces methanol as well as de-esterified advantame (also known as ANS9801-acid) (76, 77). The latter molecule is further metabolized to N-(3-(3-hydroxy-4-methoxyphenyl))-propyl-L-aspartic acid (HF-1) (78). The hydrolysis of neotame produces dimethylbutylaspartylphenylalanine (DMB-Asp-Phe) and methanol (73).
Inside the enterocytes, aspartic acid is converted to oxalacetate, which is involved in gluconeogenesis; phenylalanine is transformed to tyrosine, whereas methanol is converted into formaldehyde and, in turn, to formic acid and carbon dioxide. The excess of these metabolites, the same as those obtained from the digestion of natural food, is excreted in the urine. Although methanol is toxic, the levels generated by the digestion of aspartame are considered well below the safety threshold (79). No aspartame has been recovered during breastfeeding (80). Aspartame-derived metabolites are readily adsorbed by the small intestine and delivered by the portal vein to the liver; therefore, it does not reach the colon, whereas both advantame and neotame metabolites were recovered from feces and urine (73).
High levels of aspartic acid or phenylalanine may cause health issues, but dietary amounts of aspartame are considered to produce negligible amounts of these metabolites (80). However, people with phenylketonuria should avoid ingesting aspartame because they lack a functional phenylalanine hydroxylase and, therefore, cannot convert phenylalanine to tyrosine (81). High levels of phenylalanine have been linked to an increased risk of seizures (82).
Saccharin
Saccharin is a benzoic acid sulfimide that is moderately absorbed by the small intestine: while about 85–95% of it is delivered to the bloodstream and excreted in the urine, the rest reaches the colon unaltered (62). Saccharin is not metabolized by animal cells (83), indicating physiological inertness in humans, which has led to its widespread use in food products.
Experimental models performed in the 1970s-1980s reported an increased risk of bladder cancer in rodents fed with high-dose saccharin, leading to a legal requirement to display a warning label on saccharin-containing items (51). However, such a requirement was removed in 2000 based on the objection that rodents do not constitute a proper model for human physiology and that the concentrations utilized in early experiments were well above the ADI (84). Nonetheless, it was later demonstrated that human and mouse adipocytes treated with saccharin exhibited an abnormally activated Akt signaling pathway, leading to enhanced adipogenesis and reduced lipolysis (67).
Stevia
Stevia is a generic term for the extract of the shrub Stevia rebaudiana (fam. Asteraceae), also known as candyleaf, which is widespread in south America (85). Stevia has been utilized by indigenous tribes for centuries, but it was not officially recognized by Western scientists until 1887. It was introduced to the Japanese food industry in the 1970s, received approval from the FDA in the 1990s, and was introduced in the European market in 2011 (86, 87).
In 1931, the active factor of candyleaf extracts was described to contain a mixture of glycosides, the most abundant being steviol, stevioside, rebaudioside (reb) A-F, dulcoside, and steviolbioside (86, 88). The steviol glycosides share a diterpene steviol moiety (89). The term ‘stevia’ will be used in this review as a synonym for steviol glycosides to indicate that no active factor has explicitly been disclosed in the cited literature.
Stevia reaches the colon unaltered because animal cells are unable to metabolize its glycosides (62). Intestinal bacteria, specifically members of the genus Bacteroides, have been shown to metabolize steviol glycosides, producing steviol as the final product, which is resistant to further degradation (90, 91). Steviol is then absorbed by the colonic epithelium and released into the bloodstream (89). Interestingly, the microbial enzyme involved in the degradation of steviosides, sennoside hydrolyzing glycosidase, is usually inhibited by glucose (92); thus, it might be expected that its expression would be increased in diets that are poor in this natural saccharine (93).
Sucralose
Sucralose is a chlorinated disaccharide obtained by exchanging three hydroxyl groups of sucrose with chlorine. It is poorly absorbed by the small intestine so that about 85% of it reaches the colon and is excreted in the feces (62, 94). It is commonly assumed that sucralose is metabolized neither by animal nor by bacterial enzymes (95), a feature that advocated for its physiological safety. Nonetheless, it has been reported that environmental bacteria can degrade this NNS, although without a substantial energetic gain, to 1,6-dichloro-1,6-dideoxy-D-fructose and uronic acid (96, 97).
NNS impact on bacteria
Overview
In the following sections, experimental studies investigating the direct effect of NNS on the biology of bacteria will be discussed. The results are summarized in Table 1; selected characteristics of NNS are reported in Table 2.
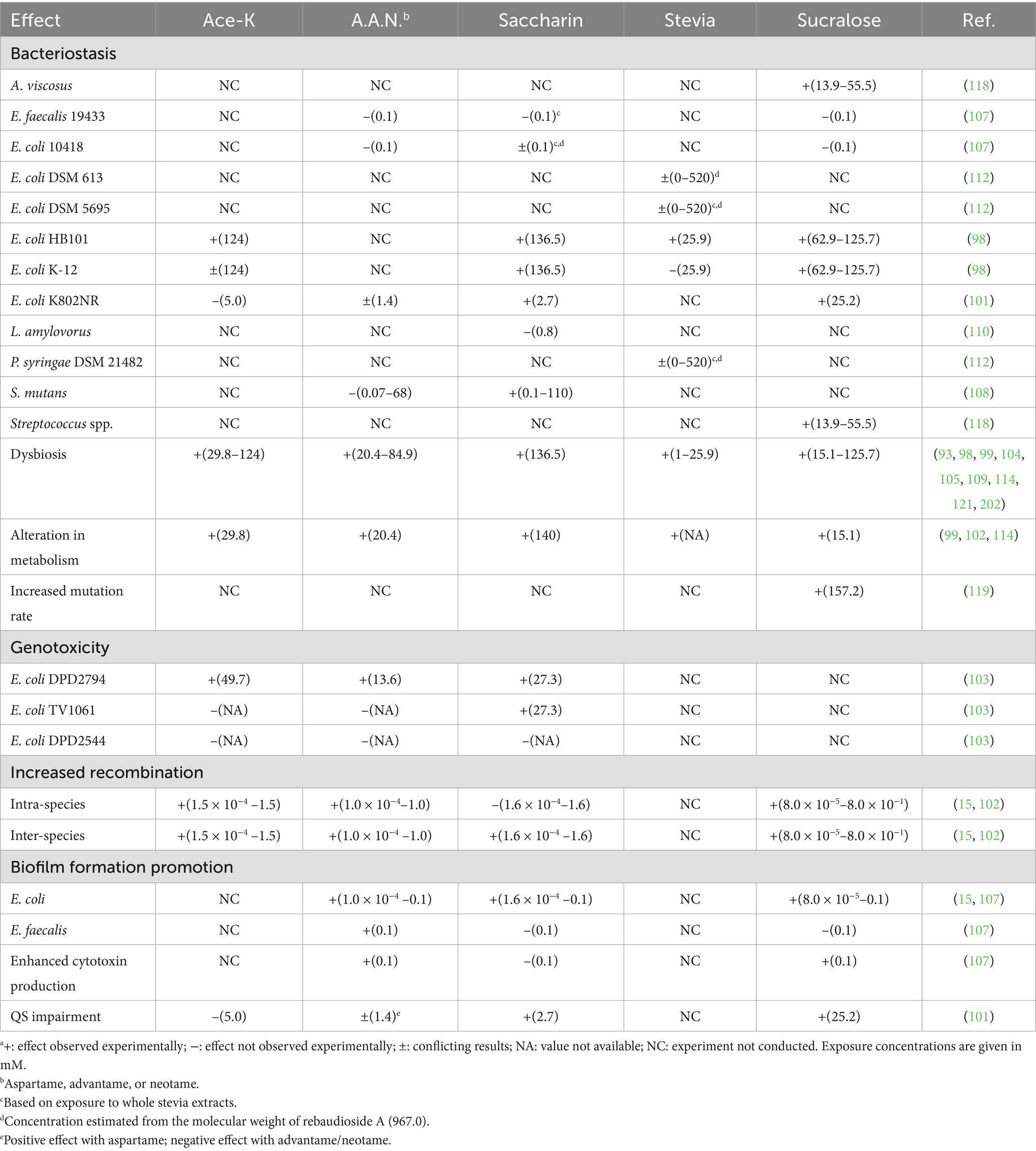
Table 1. Effects of non-nutritive sweeteners on bacteriaa.
Ace-K
Ace-K had a strain-specific bacteriostatic effect on E. coli in vitro: Luria-Bertani (LB) agar supplemented with 2.5% w/v (124 mM) ace-K reduced the number of colony-forming units (CFU) by 90% for E. coli strain HB101, and 98% for K-12 (98). Other studies reported a boost in the growth of E. coli upon exposure to 6 mg/mL of ace-K, an effect associated with alterations in the bacterial metabolism (99). Others, however, did not report substantial differences in bacterial growth: mice fed ace-K supplements within the ADI for 8 weeks did not show a difference in microbial density or cecal butyrate concentration compared to a placebo group (100). The growth of E. coli strain K802NR was not affected by exposure to ace-K at a concentration of 5 mM (101). Bacteria isolated from rat guts and exposed to ace-K displayed inhibited glucose fermentation (102).
Ace-K induced an anti-genotoxic response in E. coli strain DPD2794 but not in strains TV1061 and DPD2544 (103). Ace-K at a concentration of at least 0.03 mg/L increased over three times the recombination frequency between E. coli K-12 strains and four times between E. coli and Pseudomonas alloputida (15). The authors of such a study also reported that the high recombination rate was decreased upon treatment with radical scavengers, suggesting that reactive oxygen species (ROS) were involved in the process. Membrane permeability was also increased upon treatment with ace-K (15). Because the concentration in the experiment was lower than the concentration of NNS in the urine, the authors argued that ace-K could increase the rate of horizontal gene transfer in the human gut, with a higher risk of spreading antibiotic resistance genes (15).
Aspartame, advantame, and neotame
Dysbiosis was observed in newborn mice breastfed by mothers provided with chow supplemented with aspartame, correspondent to the human ADI, in particular, with higher production of propionate and butyrate and decreased lactose fermentation (104). Mice fed with a concentration within the ADI for aspartame over a period of 8 weeks showed impairment of glucose tolerance and higher abundance of members of the family Enterobacteriaceae as well as Clostridium leptum, an SCFA producer compared to controls (105, 106).
Aspartame was not bacteriostatic against either E. coli 10,418 or Streptococcus mutans 19,433, but it increased biofilm formation in E. coli and Enterococcus faecalis as well as enhanced their adhesion to Caco-2 intestinal cells (107, 108). Similarly, advantame (0.4 mM) and neotame (0.5 mM) did not affect the growth of E. coli K802NR whereas aspartame (1.4 mM) showed bacteriostatic activity (101). However, other studies did report a bacteriostatic effect of aspartame (20.4 mM) on E. coli along with altered fatty acid metabolism (99). Moreover, aspartame stimulated the production of cytotoxins in E. faecalis, with subsequent reduced viability of Caco-2 cells (107). Aspartame induced DNA damage in E. coli strain DPD2794 but not in strains TV1061 and DPD2544 (103). Molecular docking studies showed that aspartame could bind the hydrophobic pocket involved in the detection of the quorum sensing (QS) modulator 3-oxo-C12-HSL (LasR) of Pseudonomas aeruginosa, in particular by establishing connections with residue Val76, with an overall affinity of −8.6 kcal/mol, impairing the quorum sensing pathway of this bacterium (101).
Aspartame (0.1 μM) increased four-fold the recombination frequency among E. coli K-12 strains as well as inter-species recombination (E. coli to P. alloputida) (15). The increased recombination rate was associated with a higher ROS concentration in the bacterial cells and higher membrane permeability (15).
Models based on CD-1 mice fed for 4 weeks with neotame at a concentration equivalent to 2.5 times the human ADI showed alterations of the enteric microbiome (109). The alterations in treated mice compared to controls included enrichment of members of the genus Bacteroides (particularly those belonging to the family S24-7) and depletion of members of the families Lachnospiraceae and Ruminococcaceae. Such a modification of the microbiota was also associated with a shift in bacterial biochemistry, characterized by a reduction in the concentrations of malic acid, mannose-6-phosphate, and glyceric acid, among others. On the other hand, there was an increase in lipids such as linoleic and stearic acids.
Saccharin
Saccharin at a concentration of 2.7 mM inhibited the growth of E. coli strain K802NR (101) and exhibited bacteriostatic effect on E. coli strain 10,418 at a concentration of 1 mM but not at 0.1 mM, although it did not affect the growth of E. faecalis (107). Saccharin displayed a species-specific bacteriostatic effect on E. coli: LB agar supplemented with 2.5% w/v (137 mM) saccharin reduced the number of CFU by 90% for strain HB101, and almost 100% for K-12 (98). Studies in rats reported discordant results. It was shown that saccharin did not affect the growth of Lactobacillus amylovorus strain 4,228 (110). In contrast, others have shown that saccharin inhibits the fermentation of glucose in the gut (102). Saccharin at a concentration of 0.1 μM did not affect the recombination frequency between E. coli strains or between E. coli and P. alloputida (15).
Streptococcus mutans was inhibited in a dose-dependent manner by saccharin (108). Saccharin provided at a concentration corresponding to the ADI increased biofilm formation and cellular adhesion to Caco-2 cells in E. coli and Enterococcus faecalis cultures (107). Furthermore, saccharin significantly increased the invasion index of E. faecalis but not that of E. coli; however, E. coli exposed to saccharin increased the production of cytotoxins reducing the viability of Caco-2 cells (107). Saccharin induced chromosomal damage in E. coli strains DPD2794 and TV1061 but not in strain DPD2544 (103).
Like aspartame, saccharin was shown by molecular docking to bind the QS receptor LasR, in particularly by binding to Val76, with an affinity of −7.3 kcal/mol; thus, impairing P. aeruginosa QS pattern and its inhibiting the bacterial growth and motility (101).
Saccharin also increased tissue inflammation. C57BL/6 J mice fed with saccharin at a concentration of 0.3 mg/mL for 6 months displayed a higher expression of pro-inflammatory markers such as inducible nitric-oxide synthase (iNOS) and TNF-α, as well as a higher abundance of some genera such as Corynebacterium and Roseburia, and a lower abundance of Ruminococcus compared to controls (111).
Stevia
Stevia extracts elicited concentration-dependent species-specific responses in selected bacteria (112). For instance, a 1.5% w/v solution of methanol-extracted stevia caused a significant decrease in the growth of E. coli strains 613 and 5,695 but not in Pseudomonas syringae strain DSM 21482. However, at a concentration of 3.1%, stevia extracts exhibited a significant bacteriostatic effect on P. syringae 21,482, but did not alter the growth of the E. coli strains. Instead, at a stevia concentration of 6.2%, E. coli 5,695 showed a significant growth increase over the control, whereas E. coli 613 and P. syringae 21,482 remained unaffected.
It has been reported that rebA could be metabolized by selected members of the genera Bifidumbacterium and Lactobacillus in a strain-specific fashion: B. breve CCDM 562, B. bifidum CCDM 559, B. adolescentis AVNB3-P1, and L. mucosae SP1TA2-P1 showed faster growth than a panel of eleven other strains (113). While the increase in growth rate was deemed too small to provide a significant advantage to these strains, newborn mice fed with stevia showed an increased abundance of propionate- and butyrate-producing bacteria and a decreased abundance of lactose fermenters compared to controls, leading to increased body weight and fat accumulation (104).
RebA displayed a strain-specific bacteriostatic effect in vitro on E. coli HB101 but not on K-12 (98). Exposure to steviol, a compound produced by bacteria harvested from the colon of volunteers, resulted in a tenfold reduction in propionate production and a change in pH associated with a higher density of bifidobacteria (114). It has been shown that, compared to glucose, stevioside is an inhibitor of anaerobic bacteria, whereas RebA is an inhibitor of aerobic bacteria (93).
Not all effects associated with stevia are adverse. It has been demonstrated that stevia stimulates the expression of sodium/glucose cotransporter 1 (SGLT1) on the surface of rabbit intestinal cells, alleviating the pathogenic symptoms of experimental E. coli infection (115). SGLT1, which is activated by glucose, facilitates the absorption of water and other electrolytes into the cell, thereby counteracting the effects of colitis (116). SGLT1 expression is induced by the hormone glucagon-like peptide 2 (GLP-2), released by enteroendocrine cells of the intestine in response to glucose intake (117). Stevia can also activate the excretion of GLP-2 from the enteroendocrine cells by binding to the taste family 1 receptor (T1R) present on the surface of the intestinal epithelial cells (115). The physiological consequences of such an alteration are unknown.
Sucralose
Sucralose showed a bacteriostatic effect on E. coli HB101 and K-12 in vitro: LB agar supplemented with 2.5% w/v (63 mM) sucralose decreased the bacterial density by 74%; moreover, the size of the colonies also showed a dose-dependent reduction related to the content of sucralose in the culture medium (98). Similarly, 25 mM sucralose significantly reduced the growth of E. coli strain K802NR (101). A slight bacteriostatic effect upon E. coli was confirmed at a concentration of 15.1 mM (99). However, E. coli strain 10,418 was not affected by sucralose (107). Sucralose at a concentration of 126 mM inhibited the growth of Streptococcus sobrinus, S. sanguis, S. challis, S. salivarius, and Actinomyces viscosus, all of which are commonly found in the oral microbiome, without entering the bacterial cells (118). Sucralose at a concentration of at least 27.8 mM inhibited the growth of a panel of environmental bacteria (50). Even in this case, the inhibitory effect was obtained without the transportation of sucralose into the bacterial cell. Sub-inhibitory concentrations of sucralose, however, increased the survival rate of E. coli BW25113 when exposed to the antibiotic moxifloxacin and enhanced the mutation rate of this strain (119).
Mice fed a supplement of sucralose showed increased body weight and a higher abundance of Bacillota compared to controls (98). Sucralose within the ADI increased biofilm formation and cellular adhesion to Caco-2 cells in E. coli and E. faecalis cultures (107). Additionally, sucralose enhanced the expression of cytotoxins in both E. coli and E. faecalis, thereby reducing the viability of Caco-2 cells (107). Maternal sucralose in mice also down-regulated the expression of mucin type 2 and tight junction protein ZO-1, while boosting pro-inflammatory cytokines such as IL-1β and IFN-γ, suggesting a morphological alteration of the intestinal epithelium associated with local inflammation (120).
Remarkably, different concentrations of sucralose were linked to dinstinct dysbiotic profiles. Sucralose administered to rats at 0.54 mM increased Bacillota abundance while decreasing the abundance of Bacteroidetes, whereas sucralose at 0.78 mM had the opposite effect (121). Nonetheless, both concentrations reduced the abundance of members of the commensal families Lactobacillaceae and Akkermansiaceae.
Sucralose was also involved in generating an inflammatory micro-environment. C57BL/6 mice fed sucralose at a concentration of 0.3 mM for 6 months exhibited a higher expression of pro-inflammatory markers, such as matrix metalloproteinase 2 and iNOS, along with altered expression of amino acid metabolism and modifications in the microbiome relative abundances (122).
It has been reported that sucralose administered within the ADI to mice for 8 weeks resulted in a dose-dependent reduction in the abundance of bacteria of the Clostridium cluster XIVa group and a decrease in the amount of cecal butyrate (100). Sucralose binds to the P. aeruginosa QS receptor LasR, particularly by forming a connection with residue Val76, with an affinity of −6.1 kcal/mol, thereby inhibiting the growth and motility of this bacterium through impairment of the quorum sensing pathway (101).
Sucralose at a concentration of 0.1 μM was sufficient to promote intra-species recombination in E. coli K-12, and inter-species recombination between E. coli and P. alloputida, with rate increases of 1.5 and 2.6 times over controls, respectively (15). Sucralose treatment increased the production of ROS in the bacterial cells and the permeability of the cells (15).
NNS impact on bacteriophages
Overview
Bacteriophages (phages for short) represent a major modulator of bacterial communities (123, 124). Lytic phages provide one level of regulation (‘Kill-the-Winner’ model) by lysing the more abundant species in the community and allowing the proliferation of less competitive bacteria (125). Recent data have shed light on the crucial role of phages in modulating the development and response of the immune system (126–129). To infect their hosts, phages require not only receptors to recognize bacterial surface receptors, such as proteins and carbohydrates present in capsule and cell wall compounds, but also enzymes that can digest the polysaccharides present not only in these structures but also in biofilms’ extracellular matrices (130). For example, the tail tubular proteins TTPAgp31 (gp31 for short) of Klebsiella pneumoniae phage KP32 possess glycolytic activity, which enables the virus to diffuse within biofilms (131–133). Nonetheless, it is assumed that most phages in the human GIT are temperate (134). The theoretical frameworks (such as the ‘Piggyback-the-Winner’ and ‘community shuffling’ models) predict that prophage induction at high host densities is a key aspect to stabilize dominant bacterial species and promote diversity through genetic transfer (135–139).
Despite the momentous role that phages play in shaping the intestinal microbiome, the effect of NNS on phage biology has mostly gone overlooked. Due to the lack of experimental data on the impact of non-nutritive sweeteners (NNS) on phage biology, the following sections will investigate how substances like sucrose and polyethylene glycol affect phage particles and their infectivity. These molecules were chosen because they share similar chemical properties with NNS and carbohydrates.
Phage stabilization
While the literature on how NNS affect the morphology of phages is sparse, it is crucial to recognize that carbohydrates and other compounds significantly influence virion structure. These studies primarily focus on the need to enhance virion stability during industrial storage. In particular, lyophilization is a necessary step in the long-term preservation of viruses and the delivery of phage preparations through spraying; however, it can cause disruptions to virions, resulting in the loss of infectivity (140). Carbohydrates such as lactose, mannitol, polyethylene glycol (PEG), and trehalose are known to prevent virion disruption during lyophilization by forming a protective matrix around the virus shell (141–143). Several sweeteners (dextran, glucose, sucrose, trehalose, mannitol, and xylitol) have been investigated for their properties in protecting phage particles during phage preparation, with 10% w/v sucrose (292 mM) being the most effective (144). Other studies confirmed the protective power of sucralose, applied at a concentration of 2% (58 mM) (145).
By way of example, sucrose is routinely used to stabilize phage particles in lyophilized phage preparations (146). In a process known as “preferential exclusion,” disaccharides can surround a capsid, trapping a layer of liquid water around the virion and protecting it from structural deformation caused by freezing (147). Dextran can protect the capsid from osmotic and heat shocks (148). Steviol glycosides are known, apart from their sweetness, for their emulsification power and are employed to improve food texture (149–151). It has been shown that rebA can form apolar bonds with proteins, such as those found in soy extracts, thereby improving the emulsification of the matrix (152). Remarkably, it has been reported that emulsifiers can alter the intestinal microbiome (153). Nonetheless, the role of rebA in particular and steviol glycosides in general in modulating the homeostasis of the GIT remains poorly characterized.
The concentration of these protective molecules is an important factor to consider. Sucrose at a low concentration (100 mM) showed protective activity against E. coli phage CA933P during the lyophilization process (which includes both freezing and drying steps), whereas higher concentrations increased virion disruption (154).
Alteration of infectivity
There is very limited information regarding the role of NNS on phage infectivity. One study demonstrated that stevia extracts can either enhance or reduce the infectivity of selected phages (112). In particular, methanol-derived stevia extracts exhibited not only different activities against various viruses but also a concentration-dependent behavior. For instance, a concentration of 50% w/v of stevia significantly increased phage MS2 (host: E. coli DSM 5695) and T4 (host: E. coli DSM 613) densities in comparison with unexposed controls but not that of phage Φ6 (host: Pseudomonas syringae DSM 21482). Conversely, 1.5% stevia significantly decreased MS2 and T4 densities compared to unexposed controls, but exposure to 3% solution increased the amount of phages; Φ6 had the opposite trend (112).
Phage infection is affected by its environment. For instance, the infectivity of phage lambda towards E. coli was decreased in the presence of lactose, possibly due to this carbohydrate hindering the adsorption step (155).
Only one study analyzed the direct interaction between sweeteners, albeit natural, and phage proteins involved in the infection process (132). Docking analysis demonstrated that maltose could fit into a pocket within gp31, establishing a hydrogen bond with residues Asp131, Asp133, and Glu134, with Asp133 being part of the catalytic site (132). Consequently, it was hypothesized that the binding side of gp31 would accommodate disaccharides because larger molecules would cause the protein to unfold. The authors of that study also noted that the binding to maltose was not very specific, implying that gp31 might bind to various saccharides. Such data can lead to speculation that NNS might have the potential to bind gp31 or other phage proteins.
In our laboratory, we sought to assess whether this hypothesis had a foundation by investigating the binding of selected NNS on two phage proteins. We used gp31 in conjunction with the fiber protein gp17 of phage ɸYeO3-12, which has Yersinia enterocolitica as its host (156). We first assess the potential for NNS to bind to these proteins using biodocking. Our results indicated that several NNS could not only bind gp31 and gp17 but also overlap with the pocket binding maltose, a natural carbohydrate that represents a natural ligand for these proteins. In particular, we observed that rebA could overlap with maltose on gp31 (Figure 2A) and gp17 (Figure 2B). We confirmed the binding of rebA to recombinant gp31 by microscale thermophoresis (157) and that of gp17 by ELISA (158). Since these proteins are involved in the infection process, we sought to assess whether the binding of rebA could hamper the activity of these proteins. We observed that exposure to rebA decreased the processivity rate of gp31 compared to unexposed controls. Similarly, the addition of rebA to recombinant gp17 decreased the adsorption rate of this protein compared to controls. Unexpectedly, however, when we exposed whole phages derived from bacterial lysates, we observed that the infection process occurred about 30 min faster than in unexposed controls.
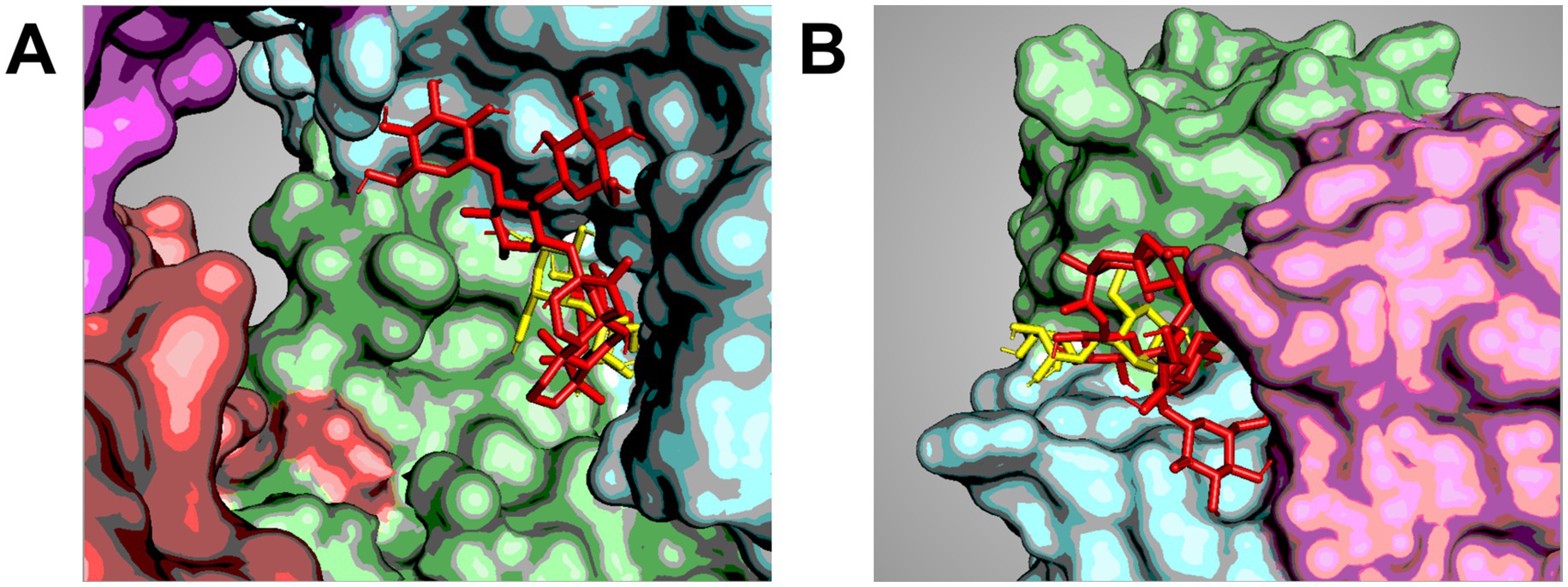
Figure 2. Co-localization of sweeteners on phage proteins. Cartoon showing selected poses of the docking between gp31 (a tubular protein with enzymatic activity) of Klebsiella phage 32 (A) and gp17 (a fiber protein involved in the recognition of host’s surface moieties) of Yersinia phage ɸYeO3-12 (B). The images show the overlapping between maltose (yellow) and rebA (red) in the same pocket, suggesting a direct competition between these molecules. The images were obtained by the docking analysis carried out by Marongiu et al. (156) generated using PyMol ver. 2.5.0 and Schrödinger, LLC (201).
To the best of our knowledge, these experiments were the first to specifically investigate the effect of NNS on phage infectivity. These results confirmed the hypothesis that NNS (namely, rebA) could not only bind to phages but also alter the biology of these viruses. Additional experimental evidence is needed to expand these observations and understand their impact on the microbiome.
Prophage induction
There is only one paper regarding the NNS-driven alteration of prophage induction rates (159). According to this study, stevia increased the prophage induction rate of Bacteroides thetaiotaomicron by 410% but decreased that of Enterococcus fecalis, which was instead highly induced by aspartame (+579%).
Nonetheless, carbohydrates have long been known to induce prophages with species-specific efficacy (160). For instance, glucose boosted the induction rate of Salmonella enterica ser. Typhimurium (161) and fructose can induce prophages Φ1 and Φ2 carried by Limosilactobacillus reuteri, an important commensal species of the human gut (162). Newly formed Φ1 and Φ2 virions were generated upon cultivating L. reuteri with either galactose, xylose, or fructose but not glucose (163). The induction mechanism was based on the reduction of fructose to mannitol, and through the action of acetate kinase A (AckA), it led to the production of the SCFA acetic acid (164). Subsequently, acetate activates the recA (163), the key regulator of the SOS response, which in turn cleaves the prophage suppressor, triggering the activation of the lytic genes (165). Interestingly, other SCFAs, such as propionate and butyrate, could induce prophage in L. reuteri (166). Since recA is present in virtually all bacteria and is one of the most conserved bacterial proteins (167), it is plausible that a similar induction mechanism might occur in bacteria other than L. reuteri (168).
Discussion
Summary of the data
Despite the widespread use of NNS in foodstuffs and other oral products, a consensus on the safety of these sweeteners for human consumption remains necessary. The alleged NNS food hygiene relies on the triple assumption that (i) human cells cannot metabolize these substances, (ii) they do not impact bacteria, and (iii) they reach the colon in negligible (169). Nonetheless, recent evidence suggests that NNS can cause dysbiosis in both humans and rodents, a disorder that has been linked to increased risk of conditions such as type 2 diabetes (170, 171). The purpose of this review was to provide a summary of the relationship between NNS intake and microbial activity, focusing on the experimental evidence investigating the direct effect of NNS on bacterial growth. The present work also focused on the NNS’ potential role in phage biology, a feature that is frequently overlooked in the literature.
The NNS discussed in the present review (ace-K, advantame, aspartame, neotame, saccharin, stevia, and sucralose) were consistently reported to cause dysbiosis, alter bacterial metabolism, and impair QS pathways in a species-specific fashion. These differences suggest a diverse response from selected bacterial species or even strains of the same species, which can explain the onset of dysbiosis. The data gathered in this review suggested that the primary impact of non-nutritive sweeteners (NNS) on bacterial growth is related to the induction of oxidative stress, changes in membrane permeability, and QS response. Nonetheless, it is not clear thus far whether NNS can affect the bacterial biochemistry from within after internalization or could act from outside the cell by activating signal pathways that can alter bacterial growth and environmental adaptation.
Remarkably, in vitro treatment of eukaryotic cells (human glioblastoma-derived SH-SY5Y and mouse cell lines TM3 and TM4) with aspartame (270 μM) or sucralose (≥1 μM) resulted in increased cellular oxidative stress (172, 173), while mouse models reported discordant results on the antioxidant effects of aspartame in vivo (174, 175). These results suggest that not only do NNS have the potential to affect cellular biochemistry in both prokaryotic and eukaryotic cells, but that some additional factors might superimpose on the NNS activity in vivo, leading to more inconsistent results.
Therefore, NNS-induced oxidative damage and cellular damage in general could be considered as the main candidates to explain the observed impairment of bacterial growth, although the details of the molecular mechanisms underlying this process are still poorly understood. Furthermore, the activation of QS pathways does not necessarily require the metabolization of NNS, which aligns with the observation that these molecules can be retrieved unaltered in biological samples (51, 176).
The possible molecular mechanisms linking NNS exposure to oxidative stress or QS alteration remain unclear. However, it is well established that oxidative stress can lead to modifications in bacterial biochemistry, including DNA damage and lipid peroxidation (177, 178). Among the bacterial responses to oxidative stress, there is the alteration of membrane fluidity (homeoviscous adaptation) and alteration of permeability through the activation of porins such as OmpC (179–181). Furthermore, oxidative stress is understood to activate the nucleotide excision repair, specifically the transcription-coupled repair pathway, which is recombinogenic (182). These responses are consistent with the results of the studies presented in the present review (53, 102).
In this review, we propose an additional putative scenario to explain NNS-linked dysbiosis: phage hindrance. The impairment of phages would not involve NNS metabolism and would most likely occur at minute levels of sweeteners due to the delicate position that phages hold in the balance of bacterial homeostasis. Such a scenario remains speculative, but so does the model of the NNS-induced QS alteration (101). The concept conveyed here is based on the observations that sucrose and other carbohydrates, most with sweetening capability, could protect the virion structure through preferential exclusion (146, 154) as well as by reducing the aggregation of viral particles (140).
In addition, recent evidence reported on NNS influence on phage infectivity (112). Because phage structural enzymes may bind saccharides such as maltose (132), it is possible that NNS could overlap with the carbohydrate-binding pocket of these proteins. In our laboratory, we have substantiated this hypothesis by showing through preliminary experiments that rebA bound proteins of phages KS32 (host: Klebsiella pneumoniae) and ɸYeO3-12 (host: Yersinia enterocolitica) and how rebA could interfere with phage infection by speeding up the lytic cycle of ɸYeO3-12 (156). The mechanisms of this enhancement, though, remain elusive.
Prophage induction might also be affected by NNS. It has been reported that stevia altered the activation of prophage in a species-specific manner (159). Since induction is linked to damage responses like the SOS pathway and QS systems (163, 183–185), the cellular stress observed in bacteria upon exposure to NNS might lead to the speculation that induction could be another by-product of NNS intake. Moreover, given the close relationship between QS and prophage induction, which is linked to horizontal gene transfer, metabolic alteration in bacteria, and predator/prey interactions (186–188), understanding the possible influence of NNS on the QS is important both for medical and microbial ecology purposes.
Any imbalance in bacterial abundance resulting from NNS exposure may be amplified within the microbiome, as phages influence the immune system, for example, by controlling SCFA levels (126). As a result, alterations to the immune system may produce local inflammation, which can cause cellular damage to intestinal cells and promote the spread of additional pathogens. Figure 3 illustrates this speculative framework linking the consumption of NNS to the onset of dysbiosis.
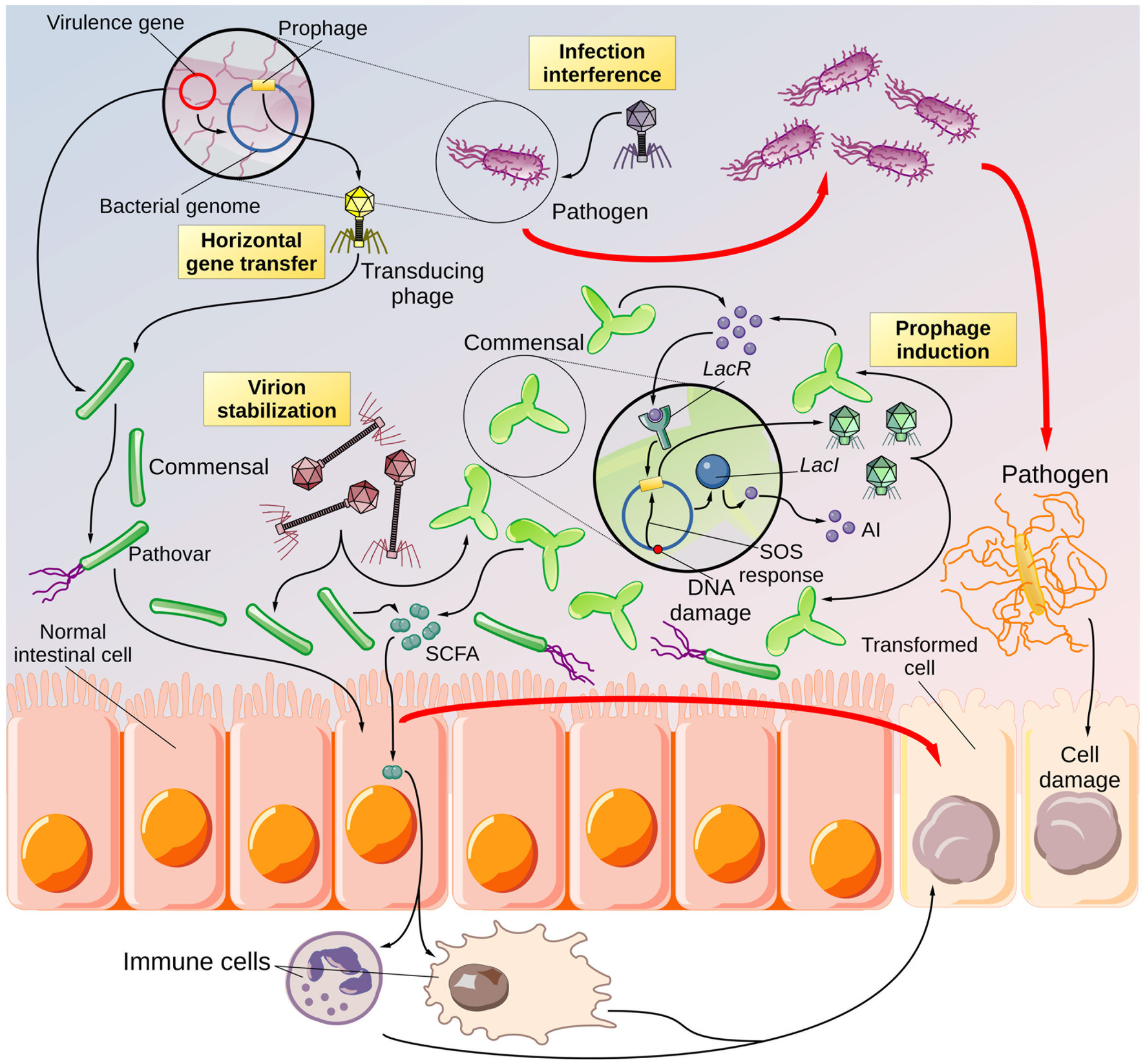
Figure 3. Overview of the speculative NNS-driven phage hindrance. Cartoon depicting the potential effects of NNS on phage biology and the consequences for the gut microbiota. According to the literature, NNS can increase the rate of horizontal gene transfer. It is reasonable to believe that a pathobiont (a bacterium with virulence capabilities present in the digestive system in low abundance) could exchange its virulence traits (encoded in plasmids or prophages) with a more abundant and avirulent commensal species. Thus, a commensal symbiont would be transformed into a pathovar. The virulence factors may cause harm to intestinal epithelial cells, promoting the formation of altered cells that can degenerate into cancer precursors. Inhibiting lytic phages may increase the population of pathogenic bacteria that can cause local inflammation and cellular damage if phages target pathogenic species. These conditions may, in turn, lead to the proliferation of more pathogenic species and the development of altered cells. In addition, NNS have been shown to stabilize phage virions. If the stabilized phages use a commensal species as a host, the infection rate of symbionts may increase, leading to a decrease in the abundance of beneficial species, which may promote the spread of pathogenic species. The imbalance in bacterial abundance due to phages may cause alterations in immune cells, for instance, through the action of short-chain fatty acids (SCFAs) that can lead to chronic inflammation and, consequently, cellular damage and a higher abundance of opportunistic bacteria. Finally, there are reports from the literature. NNS can influence the rate of prophage induction. Prophages can be activated in response to DNA damage, as well as to bacterial densities, due to their sensitivity to the bacterial autoinducers (AIs). Induced prophages generate a wave of infectious phages that can target other bacteria, specifically variants of the same species that do not carry the prophage, thus causing an imbalance in the bacterial community—a feature described by the community shuffling model. Even in this case, the result would be a higher risk of promoting the growth and spread of opportunistic bacteria. The end product of phage hindrance, combined with other factors such as diet and concurrent illnesses, would be the establishment of dysbiosis.
Limitations and perspectives
Assessing NNS food hygiene is a challenging task. First, safety testing is typically performed in animal models; however, it has been noted that animal microbiomes have not proven to be a viable substitute for human intestinal microbiota (189). Second, NNS are expected to exert their activity in minute amounts over prolonged periods, making it challenging to evaluate their impact on human or bacterial cells through experimental models (50). Since most bacteria do not metabolize NNS, the mechanism underlying NNS-associated dysbiosis remains largely unknown (190). Third, studies that expose bacteria to NNS have used varying concentrations of sweeteners, complicating comparisons between results. Four, individual differences in dietary habits, genetic backgrounds, and microbiome compositions further contribute to the variability of findings across studies. Finally, it should be considered that clinical studies assessing the impact of NNS on the human microbiome are largely based on questionnaires, sometimes in the quantification of NNS levels in urine, and more rarely determine the direct impact of NNS on bacteria.
The NNS concentrations employed in the studies reported herein showed a wide variation (10−5-102 mM) but it is not known thus far what are the physiological levels within the human GIT. To the best of our knowledge, the NNS concentration in feces has yet to be established, while only few studies reported the concentration of NNS in urine, which was in the range of 0.1–0.3% of the ADI (191). Based on an average volume of the colon of about 700 mL (192, 193), we estimated the colonic concentration of rebA at about 5 μM (Marongiu, manuscript submitted) while the environmental NNS concentration is even lower than that. Therefore, only a subset if the experiments described in the present review were performed with concentrations similar to the physiological NNS concentration.
Since alterations in bacterial growth have been observed both in the human GIT and in the environment, it can be speculated that either NNS are active at very low concentrations or some other, yet unknown, mechanisms are responsible for the reported changes. Experimental investigation, even using high NNS doses, is therefore fundamental for discriminating between these two scenarios, along with an empirical quantification of the NNS physiological level at which the bacteria are exposed within the GIT. Similarly, the data regarding the NNS effect on phages is virtually absent altogether. Additional studies addressing whether NNS could stabilize phage particles or reduce virion aggregation, for instance, would shed light on the observed alteration of phage infectivity (156).
The ramifications of NNS affecting commensal species in the intestinal tract could be far-reaching, given that these bacteria not only compete with each other for nutrient intake but also oppose the colonization of the intestinal mucosa by foreign pathogens as well as the proliferation of resident pathobionts, a process known as colonization resistance (194). For example, commensal bacteria can reduce the spread of intestinal pathogens, such as Vibrio cholerae, whose cholera toxin is encoded by prophage CTXΦ (195, 196), through direct competition. Bacteroides thetaiotaomicron, on the other hand, produces compounds that limit the growth of the pathogen E. coli O157: H7 as well as expression of its Shiga toxin, which is produced through the induction of Stx prophages present in this strain (197). Moreover, commensal bacteria can also counteract the invasion of pathogens by stimulating the immune system through the release of SCFA (198, 199). Thus, alterations in the growth of commensal bacteria (symbionts) might increase the susceptibility to intestinal pathogens.
Understanding the interaction between NNS and phage biology is important not only for food safety and microbial ecology but may also have direct medical applications. For instance, the addition of the sweetener xylitol to phage preparations increased the reduction in Pseudomonas aeruginosa and Klebsiella pneumoniae loads compared to phages alone (200). Nonetheless, little experimental evidence is at the moment available to fully understand how NNS might modulate phage structure and infection.
Conclusion
The analysis of the current literature revealed a limited but growing body of evidence suggesting a connection between the consumption of NNS and dysbiosis. Despite the paucity of clinical trials, preliminary laboratory results suggest that such dysbiosis may be mediated primarily by alterations in biochemical pathways and interference with quorum sensing signaling within bacterial communities. Furthermore, NNS might influence phage biology, potentially having far-reaching consequences for the gut microbiome.
Author contributions
LM: Conceptualization, Data curation, Formal analysis, Validation, Visualization, Writing – original draft, Writing – review & editing. EB: Data curation, Formal analysis, Validation, Writing – review & editing. SH: Data curation, Formal analysis, Writing – review & editing. LEH: Supervision, Validation, Writing – review & editing. SV: Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. LM was supported by a grant of the Ministry of Rural Affairs and Consumer Protection Baden-Württemberg (Az. 16 (34) 8402.43). SV was supported by a grant from the Dr. Hans Fritz Stiftung (funding 3140080501).
Acknowledgments
We would like to thank Dr. Markus Burkard, University of Hohenheim, Germany, for his constructive comments on the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. WHO Obesity and overweight. World health organ newsroom (2024) Available online at: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (Accessed January 23, 2025).
2. Bray, GA, Kim, KK, and Wilding, JPH. Obesity: a chronic relapsing progressive disease process. A position statement of the world obesity federation. Obes Rev. (2017) 18:715–23. doi: 10.1111/obr.12551
3. Russell, C, Grimes, C, Baker, P, Sievert, K, and Lawrence, MA. The drivers, trends and dietary impacts of non-nutritive sweeteners in the food supply: a narrative review. Nutr Res Rev. (2021) 34:185–208. doi: 10.1017/S0954422420000268
4. Cavagnari, BM. Non-caloric sweeteners: specific characteristics and safety assessment. Arch Argent Pediatr. (2019) 117:e1–e7. doi: 10.5546/aap.2019.eng.e1
5. Carocho, M, Morales, P, and Ferreira, ICFR. Sweeteners as food additives in the XXI century: a review of what is known, and what is to come. Food Chem Toxicol. (2017) 107:302–17. doi: 10.1016/j.fct.2017.06.046
6. European Food Safety Authority. Minutes of the 33rd meeting of the working group on the reevaluation of sweeteners (2021) Available online at: https://www.efsa.europa.eu/sites/default/files/wgs/food-ingredients-and-packaging/sweeteners-m.pdf (Accessed November 25, 2021).
7. Fitch, SE, Payne, LE, van de Ligt, JLG, Doepker, C, Handu, D, Cohen, SM, et al. Use of acceptable daily intake (ADI) as a health-based benchmark in nutrition research studies that consider the safety of low-calorie sweeteners (LCS): a systematic map. BMC Public Health. (2021) 21:956. doi: 10.1186/s12889-021-10934-2
8. Butchko, HH, and Kotsonis, FN. Acceptable daily intake vs actual intake: the aspartame example. J Am Coll Nutr. (1991) 10:258–66. doi: 10.1080/07315724.1991.10718153
9. Mortensen, A. Sweeteners permitted in the European Union: safety aspects. Scand J Food Nutr. (2006) 50:104–16. doi: 10.1080/17482970600982719
10. Shwide-Slavin, C, Swift, C, and Ross, T. Nonnutritive sweeteners: where are we today? Diabetes Spectr. (2012) 25:104–10. doi: 10.2337/diaspect.25.2.104
11. Lohner, S, Toews, I, and Meerpohl, JJ. Health outcomes of non-nutritive sweeteners: analysis of the research landscape. Nutr J. (2017) 16:55. doi: 10.1186/s12937-017-0278-x
12. Dunford, EK, Taillie, LS, Miles, DR, Eyles, H, Tolentino-Mayo, L, and Ng, SW. Non-nutritive sweeteners in the packaged food supply-an assessment across 4 countries. Nutrients. (2018) 10:257. doi: 10.3390/nu10020257
13. Lange, FT, Scheurer, M, and Brauch, H-J. Artificial sweeteners--a recently recognized class of emerging environmental contaminants: a review. Anal Bioanal Chem. (2012) 403:2503–18. doi: 10.1007/s00216-012-5892-z
14. Sambra, V, López-Arana, S, Cáceres, P, Abrigo, K, Collinao, J, Espinoza, A, et al. Overuse of non-caloric sweeteners in foods and beverages in Chile: a threat to consumers’ free choice? Front Nutr. (2020) 7:68. doi: 10.3389/fnut.2020.00068
15. Yu, Z, Wang, Y, Lu, J, Bond, PL, and Guo, J. Nonnutritive sweeteners can promote the dissemination of antibiotic resistance through conjugative gene transfer. ISME J. (2021) 15:2117–30. doi: 10.1038/s41396-021-00909-x
16. Sylvetsky, AC, Welsh, JA, Brown, RJ, and Vos, MB. Low-calorie sweetener consumption is increasing in the United States. Am J Clin Nutr. (2012) 96:640–6. doi: 10.3945/ajcn.112.034751
17. Schiano, C, Grimaldi, V, Scognamiglio, M, Costa, D, Soricelli, A, Nicoletti, GF, et al. Soft drinks and sweeteners intake: possible contribution to the development of metabolic syndrome and cardiovascular diseases. Beneficial or detrimental action of alternative sweeteners? Food Res Int. (2021) 142:110220. doi: 10.1016/j.foodres.2021.110220
18. European Commission. Acceptable daily intake of sweeteners in the EU. (2021) Available online at: https://knowledge4policy.ec.europa.eu/health-promotion-knowledge-gateway/sugars-sweeteners-7_en (Accessed October 23, 2023).
19. Touyz, LZG. Saccharin deemed “not hazardous” in United States and abroad. Curr Oncol. (2011) 18:213–4. doi: 10.3747/co.v18i5.836
20. Ahmad, SY, Friel, J, and Mackay, D. The effects of non-nutritive artificial sweeteners, aspartame and sucralose, on the gut microbiome in healthy adults: secondary outcomes of a randomized double-blinded crossover clinical trial. Nutrients. (2020) 12:408. doi: 10.3390/nu12113408
21. Martínez, X, Zapata, Y, Pinto, V, Cornejo, C, Elbers, M, Graaf, M, et al. Intake of non-nutritive sweeteners in Chilean children after enforcement of a new food labeling law that regulates added sugar content in processed foods. Nutrients. (2020) 12:1594. doi: 10.3390/nu12061594
22. Barraj, L, Bi, X, and Tran, N. Screening level intake estimates of low and no-calorie sweeteners in Argentina, Chile, and Peru. Food Addit Contam Part Chem Anal Control Expo Risk Assess. (2021) 38:1995–2011. doi: 10.1080/19440049.2021.1956692
23. Basílio, M, Silva, LJG, Pereira, AMPT, Pena, A, and Lino, CM. Artificial sweeteners in non-alcoholic beverages: occurrence and exposure estimation of the Portuguese population. Food Addit Contam Part Chem Anal Control Expo Risk Assess. (2020) 37:2040–50. doi: 10.1080/19440049.2020.1812734
24. Garavaglia, MB, Rodríguez García, V, Zapata, ME, Rovirosa, A, González, V, Flax Marcó, F, et al. Non-nutritive sweeteners: children and adolescent consumption and food sources. Arch Argent Pediatr. (2018) 116:186–91. doi: 10.5546/aap.2018.eng.186
25. Bär, A, and Biermann, C. Intake of intense sweeteners in Germany. Z Ernahrungswiss. (1992) 31:25–39. doi: 10.1007/BF01612550
26. Takehara, CT, Nicoluci, ÍG, Andrade, TFS, and Arisseto-Bragotto, AP. A comprehensive database of declared high-intensity sweeteners in Brazilian commercial products and updated exposure assessment. Food Res Int. (2022) 161:111899. doi: 10.1016/j.foodres.2022.111899
27. Huizinga, O, and Hubert, M. The content of caloric and non-caloric sweeteners in soft drinks in Germany. Obes Med. (2017) 6:11–4. doi: 10.1016/j.obmed.2017.03.001
28. Fed Stat Off (2023) Current population. Available online at: https://www.destatis.de/EN/Themes/Society-Environment/Population/Current-Population/_node.html (Accessed January 23, 2025).
29. Russell, C, Baker, P, Grimes, C, Lindberg, R, and Lawrence, MA. Global trends in added sugars and non-nutritive sweetener use in the packaged food supply: drivers and implications for public health. Public Health Nutr. (2023) 26:952–64. doi: 10.1017/S1368980022001598
30. Schorb, S, Gleiss, K, Wedekind, R, Suonio, E, Kull, A-K, Kuntz, M, et al. Assessment of aspartame (E951) occurrence in selected foods and beverages on the German market 2000-2022. Foods. (2023) 12:2156. doi: 10.3390/foods12112156
31. Krüger, R, Watzl, B, and Merz, B. Urinary excretion of low- and no-calorie sweeteners (LNCS) and associated food sources, as observed in the German cross-sectional KarMeN-study. Eur J Nutr. (2025) 64:136. doi: 10.1007/s00394-025-03644-7
32. WHO Team GRC. Use of non-sugar sweeteners: WHO guideline. World Health Organization. (2023). Available online at: https://www.who.int/publications/i/item/9789240073616 (Accessed June 20, 2023).
33. WHO CO. WHO advises not to use non-sugar sweeteners for weight control in newly released guideline. WHO news (2023) Available online at: https://www.who.int/news/item/15-05-2023-who-advises-not-to-use-non-sugar-sweeteners-for-weight-control-in-newly-released-guideline (Accessed June 15, 2023).
34. Harris, E. WHO warns against artificial sugars for weight loss. JAMA. (2023) 329:2011. doi: 10.1001/jama.2023.9600
35. Del Pozo, S, Gómez-Martínez, S, Díaz, LE, Nova, E, Urrialde, R, and Marcos, A. Potential effects of sucralose and saccharin on gut microbiota: a review. Nutrients. (2022) 14:682. doi: 10.3390/nu14081682
36. Sylvetsky, AC, Walter, PJ, Garraffo, HM, Robien, K, and Rother, KI. Widespread sucralose exposure in a randomized clinical trial in healthy young adults. Am J Clin Nutr. (2017) 105:820–3. doi: 10.3945/ajcn.116.144402
37. Diepeveen-de Bruin, M, Maho, W, Buso, MEC, Naomi, ND, Brouwer-Brolsma, EM, Feskens, EJM, et al. Development and validation of a UPLC-MS/MS method for the quantification of sugars and non-nutritive sweeteners in human urine. J Chromatogr B Analyt Technol Biomed Life Sci. (2023) 1225:123741. doi: 10.1016/j.jchromb.2023.123741
38. Tasevska, N, Runswick, SA, McTaggart, A, and Bingham, SA. Urinary sucrose and fructose as biomarkers for sugar consumption. Cancer Epidemiol Biomarkers Prev. (2005) 14:1287–94. doi: 10.1158/1055-9965.EPI-04-0827
39. Buso, ME, Boshuizen, HC, Naomi, ND, Maho, W, Diepeveen-de Bruin, M, Balvers, MG, et al. Relative validity of habitual sugar and low/no-calorie sweetener consumption assessed by food frequency questionnaire, multiple 24-h dietary recalls and urinary biomarkers: an observational study within the SWEET project. Am J Clin Nutr. (2023) 119:546–59. doi: 10.1016/j.ajcnut.2023.11.019
40. Halasa, BC, Sylvetsky, AC, Conway, EM, Shouppe, EL, Walter, MF, Walter, PJ, et al. Non-nutritive sweeteners in human amniotic fluid and cord blood: evidence of transplacental fetal exposure. Am J Perinatol. (2021) 40:1286–91. doi: 10.1055/s-0041-1735555
41. Loos, R, Carvalho, R, António, DC, Comero, S, Locoro, G, Tavazzi, S, et al. EU-wide monitoring survey on emerging polar organic contaminants in wastewater treatment plant effluents. Water Res. (2013) 47:6475–87. doi: 10.1016/j.watres.2013.08.024
42. Praveena, SM, Cheema, MS, and Guo, H-R. Non-nutritive artificial sweeteners as an emerging contaminant in environment: a global review and risks perspectives. Ecotoxicol Environ Saf. (2019) 170:699–707. doi: 10.1016/j.ecoenv.2018.12.048
43. Anim, AK, Thompson, K, Duodu, GO, Tscharke, B, Birch, G, Goonetilleke, A, et al. Pharmaceuticals, personal care products, food additive and pesticides in surface waters from three Australian east coast estuaries (Sydney, Yarra and Brisbane). Mar Pollut Bull. (2020) 153:111014. doi: 10.1016/j.marpolbul.2020.111014
44. Ma, L, Liu, Y, Zhang, J, Yang, Q, Li, G, and Zhang, D. Impacts of irrigation water sources and geochemical conditions on vertical distribution of pharmaceutical and personal care products (PPCPs) in the vadose zone soils. Sci Total Environ. (2018) 626:1148–56. doi: 10.1016/j.scitotenv.2018.01.168
45. Cruz-Rojas, C, SanJuan-Reyes, N, Fuentes-Benites, MPAG, Dublan-García, O, Galar-Martínez, M, Islas-Flores, H, et al. Acesulfame potassium: its ecotoxicity measured through oxidative stress biomarkers in common carp (Cyprinus carpio). Sci Total Environ. (2019) 647:772–84. doi: 10.1016/j.scitotenv.2018.08.034
46. Li, Z, Yu, X, Yu, F, and Huang, X. Occurrence, sources and fate of pharmaceuticals and personal care products and artificial sweeteners in groundwater. Environ Sci Pollut Res Int. (2021) 28:20903–20. doi: 10.1007/s11356-021-12721-3
47. Carniel Beltrami, M, Doring, T, and De Dea Linder, J. Sweeteners and sweet taste enhancers in the food industry. Food Sci Technol. (2018) 38:181–7. doi: 10.1590/fst.31117
48. Suez, J, Cohen, Y, Valdés-Mas, R, Mor, U, Dori-Bachash, M, Federici, S, et al. Personalized microbiome-driven effects of non-nutritive sweeteners on human glucose tolerance. Cell. (2022) 185:3307–3328.e19. doi: 10.1016/j.cell.2022.07.016
49. Crakes, KR, Questell, L, Soni, S, and Suez, J. Impacts of non-nutritive sweeteners on the human microbiome. Immunometab Cobham Surrey. (2025) 7:e00060. doi: 10.1097/IN9.0000000000000060
50. Omran, A, Ahearn, G, Bowers, D, Swenson, J, and Coughlin, C. Metabolic effects of sucralose on environmental bacteria. J Toxicol. (2013) 2013:372986. doi: 10.1155/2013/372986
51. Shankar, P, Ahuja, S, and Sriram, K. Non-nutritive sweeteners: review and update. Nutrition. (2013) 29:1293–9. doi: 10.1016/j.nut.2013.03.024
52. Farup, PG, Lydersen, S, and Valeur, J. Are nonnutritive sweeteners obesogenic? Associations between diet, Faecal microbiota, and short-chain fatty acids in morbidly obese subjects. J Obes. (2019) 2019:1–8. doi: 10.1155/2019/4608315
53. Yu, Z, Wang, Y, Henderson, IR, and Guo, J. Artificial sweeteners stimulate horizontal transfer of extracellular antibiotic resistance genes through natural transformation. ISME J. (2021) 16:543–54. doi: 10.1038/s41396-021-01095-6
54. Zhu, J, Liu, J, Li, Z, Xi, R, Li, Y, Peng, X, et al. The effects of nonnutritive sweeteners on the cariogenic potential of Oral microbiome. Biomed Res Int. (2021) 2021:9967035. doi: 10.1155/2021/9967035
55. Harrington, V, Lau, L, Crits-Christoph, A, and Suez, J. Interactions of non-nutritive artificial sweeteners with the microbiome in metabolic syndrome. Immunometabolism. (2022) 4:e220012. doi: 10.20900/immunometab20220012
56. Gerasimidis, K, Bryden, K, Chen, X, Papachristou, E, Verney, A, Roig, M, et al. The impact of food additives, artificial sweeteners and domestic hygiene products on the human gut microbiome and its fibre fermentation capacity. Eur J Nutr. (2020) 59:3213–30. doi: 10.1007/s00394-019-02161-8
57. Qin, X. What made Canada become a country with the highest incidence of inflammatory bowel disease: could sucralose be the culprit? Can J Gastroenterol J Can Gastroenterol. (2011) 25:511. doi: 10.1155/2011/451036
58. Harvard, T.H. Chan School of public health. Low-Calorie Sweeteners. (2023) Available online at: https://www.hsph.harvard.edu/nutritionsource/healthy-drinks/artificial-sweeteners/ [Accessed January 10, 2023]
59. National Library of Medicine. Nutritive and non-nutritive low-calorie sweeteners approved by FDA or recognized as generally recognized as safe (GRAS) (242-291). Diet Treat Obes (2023) Available online at: https://www.ncbi.nlm.nih.gov/books/NBK278991/table/diet-treatment-obes.table20nut/ (Accessed January 10, 2023).
60. National Center for Biotechnology Information. PubMed (National Library of medicine). PubMed (2025) Available online at: https://pubmed.ncbi.nlm.nih.gov/about/ (Accessed September 26, 2025).
61. ClinicalTrials.gov National Center for Biotechnology Information. (2024) Available online at: https://clinicaltrials.gov/ (Accessed September 26, 2025).
62. Magnuson, BA, Carakostas, MC, Moore, NH, Poulos, SP, and Renwick, AG. Biological fate of low-calorie sweeteners. Nutr Rev. (2016) 74:670–89. doi: 10.1093/nutrit/nuw032
63. Stampe, S, Leth-Møller, M, Greibe, E, Hoffmann-Lücke, E, Pedersen, M, and Ovesen, P. Artificial sweeteners in breast milk: a clinical investigation with a kinetic perspective. Nutrients. (2022) 14:635. doi: 10.3390/nu14132635
64. Franz, MJ, Powers, MA, Leontos, C, Holzmeister, LA, Kulkarni, K, Monk, A, et al. The evidence for medical nutrition therapy for type 1 and type 2 diabetes in adults. J Am Diet Assoc. (2010) 110:1852–89. doi: 10.1016/j.jada.2010.09.014
65. Kroger, M, Meister, K, and Kava, R. Low-calorie sweeteners and other sugar substitutes: a review of the safety issues. Compr Rev Food Sci Food Saf. (2006) 5:35–47. doi: 10.1111/j.1541-4337.2006.tb00081.x
66. Woods, D. US scientists challenge approval of sweetener. BMJ. (1996) 313:386. doi: 10.1136/bmj.313.7054.386
67. Simon, BR, Parlee, SD, Learman, BS, Mori, H, Scheller, EL, Cawthorn, WP, et al. Artificial sweeteners stimulate adipogenesis and suppress lipolysis independently of sweet taste receptors. J Biol Chem. (2013) 288:32475–89. doi: 10.1074/jbc.M113.514034
68. Kahle, M, Buerge, IJ, Müller, MD, and Poiger, T. Hydrophilic anthropogenic markers for quantification of wastewater contamination in ground- and surface waters. Environ Toxicol Chem. (2009) 28:2528–36. doi: 10.1897/08-606.1
69. Kleinsteuber, S, Rohwerder, T, Lohse, U, Seiwert, B, and Reemtsma, T. Sated by a zero-calorie sweetener: wastewater Bacteria can feed on Acesulfame. Front Microbiol. (2019) 10:2606. doi: 10.3389/fmicb.2019.02606
70. Belton, K, Schaefer, E, and Guiney, PD. A review of the environmental fate and effects of Acesulfame-potassium. Integr Environ Assess Manag. (2020) 16:421–37. doi: 10.1002/ieam.4248
71. Kahl, S, Kleinsteuber, S, Nivala, J, van Afferden, M, and Reemtsma, T. Emerging biodegradation of the previously persistent artificial sweetener Acesulfame in biological wastewater treatment. Environ Sci Technol. (2018) 52:2717–25. doi: 10.1021/acs.est.7b05619
72. Hentges, DJ. The anaerobic microflora of the human body. Clin Infect Dis Off Publ Infect Dis Soc Am. (1993) 16:S175–80. doi: 10.1093/clinids/16.supplement_4.s175
73. Farag, MA, Rezk, MM, Hamdi Elashal, M, El-Araby, M, Khalifa, SAM, and El-Seedi, HR. An updated multifaceted overview of sweet proteins and dipeptides as sugar substitutes; the chemistry, health benefits, gut interactions, and safety. Food Res Int Ott Ont. (2022) 162:111853. doi: 10.1016/j.foodres.2022.111853
74. Birch, GG. Sweetness and sweeteners. Endeavour. (1987) 11:21–4. doi: 10.1016/0160-9327(87)90165-7
75. Meenakshi, S, and Mohan, V. Decoding the mystery of non-nutritive sweeteners. Int J Diabetes Dev Ctries. (2024) 44:3–9. doi: 10.1007/s13410-024-01323-7
76. Chattopadhyay, S, Raychaudhuri, U, and Chakraborty, R. Artificial sweeteners - a review. J Food Sci Technol. (2014) 51:611–21. doi: 10.1007/s13197-011-0571-1
77. Otabe, A, Fujieda, T, Masuyama, T, Ubukata, K, and Lee, C. Advantame--an overview of the toxicity data. Food Chem Toxicol. (2011) 49:S2–7. doi: 10.1016/j.fct.2011.06.046
78. Ubukata, K, Nakayama, A, and Mihara, R. Pharmacokinetics and metabolism of N-[N-[3-(3-hydroxy-4-methoxyphenyl) propyl]-α-aspartyl]-L-phenylalanine 1-methyl ester, monohydrate (advantame) in the rat, dog, and man. Food Chem Toxicol. (2011) 49:S8–S29. doi: 10.1016/j.fct.2011.06.042
79. Ranney, RE, Oppermann, JA, Muldoon, E, and McMahon, FG. Comparative metabolism of aspartame in experimental animals and humans. J Toxicol Environ Health. (1976) 2:441–51. doi: 10.1080/15287397609529445
80. Magnuson, BA, Burdock, GA, Doull, J, Kroes, RM, Marsh, GM, Pariza, MW, et al. Aspartame: a safety evaluation based on current use levels, regulations, and toxicological and epidemiological studies. Crit Rev Toxicol. (2007) 37:629–727. doi: 10.1080/10408440701516184
81. Elhawary, NA, AlJahdali, IA, Abumansour, IS, Elhawary, EN, Gaboon, N, Dandini, M, et al. Genetic etiology and clinical challenges of phenylketonuria. Hum Genomics. (2022) 16:22. doi: 10.1186/s40246-022-00398-9
82. Maher, TJ, and Wurtman, RJ. Possible neurologic effects of aspartame, a widely used food additive. Environ Health Perspect. (1987) 75:53–7. doi: 10.1289/ehp.877553
83. Renwick, AG. The disposition of saccharin in animals and man--a review. Food Chem Toxicol. (1985) 23:429–35. doi: 10.1016/0278-6915(85)90136-x
84. OEHHA Saccharin Delisted Effective April 6, 2001 as Known to the State to Cause Cancer. Calif off environ health Hazard Assess (2001) Available online at: https://oehha.ca.gov/proposition-65/crnr/saccharin-delisted-effective-april-6-2001-known-state-cause-cancer (Accessed August 25, 2025).
85. Borgo, J, Laurella, LC, Martini, F, Catalán, CAN, and Sülsen, VP. Stevia genus: phytochemistry and biological activities update. Molecules. (2021) 26:733. doi: 10.3390/molecules26092733
86. Samuel, P, Ayoob, KT, Magnuson, BA, Wölwer-Rieck, U, Jeppesen, PB, Rogers, PJ, et al. Stevia leaf to Stevia sweetener: exploring its science, benefits, and future potential. J Nutr. (2018) 148:1186S–205S. doi: 10.1093/jn/nxy102
87. Brempt, KV. Parliamentary question | approval of Stevia on the European market | E-011267/2011 | European Parliament. (2012) Available online at: https://www.europarl.europa.eu/doceo/document/E-7-2011-011267_EN.html (Accessed October 27, 2023).
88. Ceunen, S, and Geuns, JMC. Steviol glycosides: chemical diversity, metabolism, and function. J Nat Prod. (2013) 76:1201–28. doi: 10.1021/np400203b
89. Wheeler, A, Boileau, AC, Winkler, PC, Compton, JC, Prakash, I, Jiang, X, et al. Pharmacokinetics of rebaudioside a and stevioside after single oral doses in healthy men. Food Chem Toxicol Int J Publ Br Ind Biol Res Assoc. (2008) 46:S54–60. doi: 10.1016/j.fct.2008.04.041
90. Gardana, C, Simonetti, P, Canzi, E, Zanchi, R, and Pietta, P. Metabolism of stevioside and rebaudioside a from Stevia rebaudiana extracts by human microflora. J Agric Food Chem. (2003) 51:6618–22. doi: 10.1021/jf0303619
91. Koyama, E, Kitazawa, K, Ohori, Y, Izawa, O, Kakegawa, K, Fujino, A, et al. In vitro metabolism of the glycosidic sweeteners, stevia mixture and enzymatically modified stevia in human intestinal microflora. Food Chem Toxicol. (2003) 41:359–74. doi: 10.1016/s0278-6915(02)00235-1
92. Yang, L, Akao, T, Kobashi, K, and Hattori, M. A sennoside-hydrolyzing beta-glucosidase from Bifidobacterium sp. strain SEN is inducible. Biol Pharm Bull. (1996) 19:701–4. doi: 10.1248/bpb.19.701
93. Renwick, AG, and Tarka, SM. Microbial hydrolysis of steviol glycosides. Food Chem Toxicol Int J Publ Br Ind Biol Res Assoc. (2008) 46:S70–4. doi: 10.1016/j.fct.2008.05.008
94. Roberts, A, Renwick, AG, Sims, J, and Snodin, DJ. Sucralose metabolism and pharmacokinetics in man. Food Chem Toxicol. (2000) 38:S31–41. doi: 10.1016/s0278-6915(00)00026-0
95. John, BA, Wood, SG, and Hawkins, DR. The pharmacokinetics and metabolism of sucralose in the mouse. Food Chem Toxicol. (2000) 38:S107–10. doi: 10.1016/s0278-6915(00)00032-6
96. Labare, MP, and Alexander, M. Biodegradation of sucralose, a chlorinated carbohydrate, in samples of natural environments. Environ Toxicol Chem. (1993) 12:797–804. doi: 10.1002/etc.5620120502
97. Labare, MP, and Alexander, M. Microbial cometabolism of sucralose, a chlorinated disaccharide, in environmental samples. Appl Microbiol Biotechnol. (1994) 42:173–8. doi: 10.1007/BF00170242
98. Wang, Q-P, Browman, D, Herzog, H, and Neely, GG. Non-nutritive sweeteners possess a bacteriostatic effect and alter gut microbiota in mice. PLoS One. (2018) 13:e0199080. doi: 10.1371/journal.pone.0199080
99. Shahriar, S, Ahsan, T, Khan, A, Akhteruzzaman, S, Shehreen, S, and Sajib, AA. Aspartame, acesulfame K and sucralose- influence on the metabolism of Escherichia coli. Metab Open. (2020) 8:100072. doi: 10.1016/j.metop.2020.100072
100. Uebanso, T, Ohnishi, A, Kitayama, R, Yoshimoto, A, Nakahashi, M, Shimohata, T, et al. Effects of low-dose non-caloric sweetener consumption on gut microbiota in mice. Nutrients. (2017) 9:560. doi: 10.3390/nu9060560
101. Markus, V, Share, O, Shagan, M, Halpern, B, Bar, T, Kramarsky-Winter, E, et al. Inhibitory effects of artificial sweeteners on bacterial quorum sensing. Int J Mol Sci. (2021) 22:863. doi: 10.3390/ijms22189863
102. Pfeffer, M, Ziesenitz, SC, and Siebert, G. Acesulfame K, cyclamate and saccharin inhibit the anaerobic fermentation of glucose by intestinal bacteria. Z Ernahrungswiss. (1985) 24:231–5. doi: 10.1007/BF02023668
103. Harpaz, D, Yeo, LP, Cecchini, F, Koon, THP, Kushmaro, A, Tok, AIY, et al. Measuring artificial sweeteners toxicity using a bioluminescent bacterial panel. Molecules. (2018) 23:454. doi: 10.3390/molecules23102454
104. Wang, W, Nettleton, JE, Gänzle, MG, and Reimer, RA. A metagenomics investigation of intergenerational effects of non-nutritive sweeteners on gut microbiome. Front Nutr. (2021) 8:795848. doi: 10.3389/fnut.2021.795848
105. Palmnäs, MSA, Cowan, TE, Bomhof, MR, Su, J, Reimer, RA, Vogel, HJ, et al. Low-dose aspartame consumption differentially affects gut microbiota-host metabolic interactions in the diet-induced obese rat. PLoS One. (2014) 9:e109841. doi: 10.1371/journal.pone.0109841
106. Barber, TM, Kabisch, S, Pfeiffer, AFH, and Weickert, MO. The effects of the Mediterranean diet on health and gut microbiota. Nutrients. (2023) 15:2150. doi: 10.3390/nu15092150
107. Shil, A, and Chichger, H. Artificial sweeteners negatively regulate pathogenic characteristics of two model gut Bacteria, E. coli and E. faecalis. Int J Mol Sci. (2021) 22:228. doi: 10.3390/ijms22105228
108. Linke, HA, and Chang, CA. Physiological effects of sucrose substitutes and artificial sweeteners on growth pattern and acid production of glucose-grown Streptococcus mutans strains in vitro. Z Naturforsch C. (1976) 31:245–51. doi: 10.1515/znc-1976-5-605
109. Chi, L, Bian, X, Gao, B, Tu, P, Lai, Y, Ru, H, et al. Effects of the artificial sweetener Neotame on the gut microbiome and fecal metabolites in mice. Molecules. (2018) 23:367. doi: 10.3390/molecules23020367
110. Daly, K, Darby, AC, and Shirazi-Beechey, SP. Low calorie sweeteners and gut microbiota. Physiol Behav. (2016) 164:494–500. doi: 10.1016/j.physbeh.2016.03.014
111. Bian, X, Tu, P, Chi, L, Gao, B, Ru, H, and Lu, K. Saccharin induced liver inflammation in mice by altering the gut microbiota and its metabolic functions. Food Chem Toxicol. (2017) 107:530–9. doi: 10.1016/j.fct.2017.04.045
112. Stachurska, X, Mizielińska, M, Ordon, M, and Nawrotek, P. The use of plant extracts and bacteriophages as an alternative therapy approach in combatting bacterial infections: the study of lytic phages and Stevia rebaudiana. J Vet Res. (2023) 67:545–57. doi: 10.2478/jvetres-2023-0059
113. Kunová, G, Rada, V, Vidaillac, A, and Lisova, I. Utilisation of steviol glycosides from Stevia rebaudiana (Bertoni) by lactobacilli and bifidobacteria in in vitro conditions. Folia Microbiol (Praha). (2014) 59:251–5. doi: 10.1007/s12223-013-0291-1
114. Vamanu, E, Pelinescu, D, Gatea, F, and Sârbu, I. Altered in vitro Metabolomic response of the human microbiota to sweeteners. Genes. (2019) 10:535. doi: 10.3390/genes10070535
115. Moran, AW, Al-Rammahi, MA, Daly, K, Grand, E, Ionescu, C, Bravo, DM, et al. Consumption of a natural high-intensity sweetener enhances activity and expression of rabbit intestinal Na(+)/glucose cotransporter 1 (SGLT1) and improves Colibacillosis-induced enteric disorders. J Agric Food Chem. (2020) 68:441–50. doi: 10.1021/acs.jafc.9b04995
116. Hamilton, KL. Robert K. Crane-Na(+)-glucose cotransporter to cure? Front Physiol. (2013) 4:53. doi: 10.3389/fphys.2013.00053
117. Moran, AW, Al-Rammahi, MA, Batchelor, DJ, Bravo, DM, and Shirazi-Beechey, SP. Glucagon-like Peptide-2 and the enteric nervous system are components of cell-cell communication pathway regulating intestinal Na(+)/glucose co-transport. Front Nutr. (2018) 5:101. doi: 10.3389/fnut.2018.00101
118. Young, DA, and Bowen, WH. The influence of sucralose on bacterial metabolism. J Dent Res. (1990) 69:1480–4. doi: 10.1177/00220345900690080601
119. Qu, Y, Li, R, Jiang, M, and Wang, X. Sucralose increases antimicrobial resistance and stimulates recovery of Escherichia coli mutants. Curr Microbiol. (2017) 74:885–8. doi: 10.1007/s00284-017-1255-5
120. Dai, X, Guo, Z, Chen, D, Li, L, Song, X, Liu, T, et al. Maternal sucralose intake alters gut microbiota of offspring and exacerbates hepatic steatosis in adulthood. Gut Microbes. (2020) 11:1043–63. doi: 10.1080/19490976.2020.1738187
121. Zhang, M, Chen, J, Yang, M, Qian, C, Liu, Y, Qi, Y, et al. Low doses of sucralose Alter fecal microbiota in high-fat diet-induced obese rats. Front Nutr. (2021) 8:787055. doi: 10.3389/fnut.2021.787055
122. Bian, X, Chi, L, Gao, B, Tu, P, Ru, H, and Lu, K. Gut microbiome response to sucralose and its potential role in inducing liver inflammation in mice. Front Physiol. (2017) 8:487. doi: 10.3389/fphys.2017.00487
123. Sutcliffe, SG, Reyes, A, and Maurice, CF. Bacteriophages playing nice: lysogenic bacteriophage replication stable in the human gut microbiota. iScience. (2023) 26:106007. doi: 10.1016/j.isci.2023.106007
124. Townsend, E, Kelly, L, Muscatt, B, Box, J, Hargraves, N, Lilley, D, et al. The human gut phageome: origins and roles in the human gut microbiome. Front Cell Infect Microbiol. (2021) 11:643214. doi: 10.3389/fcimb.2021.643214
125. Maslov, S, and Sneppen, K. Population cycles and species diversity in dynamic kill-the-winner model of microbial ecosystems. Sci Rep. (2017) 7:39642. doi: 10.1038/srep39642
126. Van Belleghem, JD, Dąbrowska, K, Vaneechoutte, M, Barr, JJ, and Bollyky, PL. Interactions between bacteriophage, bacteria, and the mammalian immune system. Viruses. (2018) 11:10. doi: 10.3390/v11010010
127. Górski, A, Międzybrodzki, R, Borysowski, J, Dąbrowska, K, Wierzbicki, P, Ohams, M, et al. Phage as a modulator of immune responses: practical implications for phage therapy In: M Łobocka and W Szybalski, editors. Advances in virus research. Bacteriophages, part B. Cambridge, Massachusetts: Academic Press, (2012). 41–71.
128. Barr, JJ, Auro, R, Furlan, M, Whiteson, KL, Erb, ML, Pogliano, J, et al. Bacteriophage adhering to mucus provide a non-host-derived immunity. Proc Natl Acad Sci USA. (2013) 110:10771–6. doi: 10.1073/pnas.1305923110
129. Sausset, R, Petit, MA, Gaboriau-Routhiau, V, and De Paepe, M. New insights into intestinal phages. Mucosal Immunol. (2020) 13:205–15. doi: 10.1038/s41385-019-0250-5
130. Gerstmans, H, Rodríguez-Rubio, L, Lavigne, R, and Briers, Y. From endolysins to Artilysin®s: novel enzyme-based approaches to kill drug-resistant bacteria. Biochem Soc Trans. (2016) 44:123–8. doi: 10.1042/BST20150192
131. Pyra, A, Brzozowska, E, Pawlik, K, Gamian, A, Dauter, M, and Dauter, Z. Tail tubular protein a: a dual-function tail protein of Klebsiella pneumoniae bacteriophage KP32. Sci Rep. (2017) 7:2223. doi: 10.1038/s41598-017-02451-3
132. Swietnicki, W, and Brzozowska, E. In silico analysis of bacteriophage tail tubular proteins suggests a putative sugar binding site and a catalytic mechanism. J Mol Graph Model. (2019) 92:8–16. doi: 10.1016/j.jmgm.2019.07.002
133. Brzozowska, E, Pyra, A, Pawlik, K, Janik, M, Górska, S, Urbańska, N, et al. Hydrolytic activity determination of tail tubular protein a of Klebsiella pneumoniae bacteriophages towards saccharide substrates. Sci Rep. (2017) 7:18048. doi: 10.1038/s41598-017-18096-1
134. Clooney, AG, Sutton, TDS, Shkoporov, AN, Holohan, RK, Daly, KM, O’Regan, O, et al. Whole-Virome analysis sheds light on viral dark matter in inflammatory bowel disease. Cell Host Microbe. (2019) 26:764–778.e5. doi: 10.1016/j.chom.2019.10.009
135. Silveira, CB, Luque, A, and Rohwer, F. The landscape of lysogeny across microbial community density, diversity and energetics. Environ Microbiol. (2021) 23:4098–111. doi: 10.1111/1462-2920.15640
136. Mills, S, Shanahan, F, Stanton, C, Hill, C, Coffey, A, and Ross, RP. Movers and shakers: influence of bacteriophages in shaping the mammalian gut microbiota. Gut Microbes. (2013) 4:4–16. doi: 10.4161/gmic.22371
137. Touchon, M, de Moura Sousa, JA, and Rocha, EP. Embracing the enemy: the diversification of microbial gene repertoires by phage-mediated horizontal gene transfer. Curr Opin Microbiol. (2017) 38:66–73. doi: 10.1016/j.mib.2017.04.010
138. Rodriguez-Valera, F, Martin-Cuadrado, A-B, Rodriguez-Brito, B, Pasić, L, Thingstad, TF, Rohwer, F, et al. Explaining microbial population genomics through phage predation. Nat Rev Microbiol. (2009) 7:828–36. doi: 10.1038/nrmicro2235
139. Golomidova, A, Kulikov, E, Isaeva, A, Manykin, A, and Letarov, A. The diversity of coliphages and coliforms in horse feces reveals a complex pattern of ecological interactions. Appl Environ Microbiol. (2007) 73:5975–81. doi: 10.1128/AEM.01145-07
140. Wang, W. Lyophilization and development of solid protein pharmaceuticals. Int J Pharm. (2000) 203:1–60. doi: 10.1016/s0378-5173(00)00423-3
141. Merabishvili, M, Vervaet, C, Pirnay, J-P, De Vos, D, Verbeken, G, Mast, J, et al. Stability of Staphylococcus aureus phage ISP after freeze-drying (lyophilization). PLoS One. (2013) 8:e68797. doi: 10.1371/journal.pone.0068797
142. Chang, RYK, Wallin, M, Kutter, E, Morales, S, Britton, W, Li, J, et al. Storage stability of inhalable phage powders containing lactose at ambient conditions. Int J Pharm. (2019) 560:11–8. doi: 10.1016/j.ijpharm.2019.01.050
143. Bolsan, AC, Sampaio, GV, Rodrigues, HC, Silva De Souza, S, Edwiges, T, Celant De Prá, M, et al. Phage formulations and delivery strategies: unleashing the potential against antibiotic-resistant bacteria. Microbiol Res. (2024) 282:127662. doi: 10.1016/j.micres.2024.127662
144. Zheng, H. Devitrification of lyoprotectants: a critical determinant for bacteriophages inactivation in freeze-drying and storage. Food Res Int Ott Ont. (2023) 173:113307. doi: 10.1016/j.foodres.2023.113307
145. Zhang, Y, Peng, X, Zhang, H, Watts, AB, and Ghosh, D. Manufacturing and ambient stability of shelf freeze dried bacteriophage powder formulations. Int J Pharm. (2018) 542:1–7. doi: 10.1016/j.ijpharm.2018.02.023
146. Puapermpoonsiri, U, Ford, SJ, and van der Walle, CF. Stabilization of bacteriophage during freeze drying. Int J Pharm. (2010) 389:168–75. doi: 10.1016/j.ijpharm.2010.01.034
147. McLellan, MR, and Day, JG. Cryopreservation and freeze-drying protocols. Mol Biol. (1995) 38:1–5. doi: 10.1385/0-89603-296-5:1
148. Serwer, P, Masker, WE, and Allen, JL. Stability and in vitro DNA packaging of bacteriophages: effects of dextrans, sugars, and polyols. J Virol. (1983) 45:665–71. doi: 10.1128/JVI.45.2.665-671.1983
149. Carbonell-Capella, JM, Buniowska, M, Cortes, C, Zulueta, A, Frigola, A, and Esteve, MJ. Influence of pulsed electric field processing on the quality of fruit juice beverages sweetened with Stevia rebaudiana. Food Bioprod Process. (2017) 101:214–22. doi: 10.1016/j.fbp.2016.11.012
150. Vatankhah, M, Garavand, F, Mohammadi, B, and Elhamirad, A. Quality attributes of reduced-sugar Iranian traditional sweet bread containing stevioside. J Food Meas Charact. (2017) 11:1233–9. doi: 10.1007/s11694-017-9500-y
151. Gliemmo, MF, Montagnani, MA, Schelegueda, LI, González, MM, and Campos, CA. Effect of xantham gum, steviosides, clove, and cinnamon essential oils on the sensory and microbiological quality of a low sugar tomato jam. Food Sci Technol Int. (2016) 22:122–31. doi: 10.1177/1082013215574400
152. Zhang, T, Peng, Q, Xia, Y, Zhang, Y, Myint, K, and Wu, J. Steviol glycosides, an edible sweet surfactant that can modulate the interfacial and emulsifying properties of soy protein isolate solution. J Food Eng. (2021) 289:110264. doi: 10.1016/j.jfoodeng.2020.110264
153. Van Hul, M, Cani, PD, Petitfils, C, De Vos, WM, Tilg, H, and El-Omar, EM. What defines a healthy gut microbiome? Gut. (2024) 73:1893–908. doi: 10.1136/gutjnl-2024-333378
154. Dini, C, and de Urraza, PJ. Effect of buffer systems and disaccharides concentration on Podoviridae coliphage stability during freeze drying and storage. Cryobiology. (2013) 66:339–42. doi: 10.1016/j.cryobiol.2013.03.007
155. Gabig, M, Herman-Antosiewicz, A, Kwiatkowska, M, Los, M, Thomas, MS, and Wegrzyn, G. The cell surface protein Ag43 facilitates phage infection of Escherichia coli in the presence of bile salts and carbohydrates. Microbiology. (2002) 148:1533–42. doi: 10.1099/00221287-148-5-1533
156. Marongiu, L, Brzozowska, E, Brykała, J, Burkard, M, Schmidt, H, Szermer-Olearnik, B, et al. The non-nutritive sweetener rebaudioside a enhances phage infectivity. Sci Rep. (2025) 15:1–12. doi: 10.1038/s41598-025-85186-w
157. Jerabek-Willemsen, M, Wienken, CJ, Braun, D, Baaske, P, and Duhr, S. Molecular interaction studies using microscale thermophoresis. Assay Drug Dev Technol. (2011) 9:342–53. doi: 10.1089/adt.2011.0380
158. Filik, K, Szermer-Olearnik, B, Niedziółka-Jönson, J, Roźniecka, E, Ciekot, J, Pyra, A, et al. φYeO3-12 phage tail fiber Gp17 as a promising high specific tool for recognition of Yersinia enterocolitica pathogenic serotype O:3. AMB Express. (2022) 12:1. doi: 10.1186/s13568-021-01341-2
159. Boling, L, Cuevas, DA, Grasis, JA, Kang, HS, Knowles, B, Levi, K, et al. Dietary prophage inducers and antimicrobials: toward landscaping the human gut microbiome. Gut Microbes. (2020) 11:721–34. doi: 10.1080/19490976.2019.1701353
160. Kato, A, Ando, K, and Arima, K. Effect of carbohydrates on induction of bacteriophage lambda. Biochem Biophys Res Commun. (1970) 41:837–40. doi: 10.1016/0006-291x(70)90158-0
161. Entner, N, and Engelsberg, E. An effect of glucose on bacteriophage synthesis in Salmonella typhimurium. Nature. (1958) 182:1808–9. doi: 10.1038/1821808a0
162. Duar, RM, Lin, XB, Zheng, J, Martino, ME, Grenier, T, Pérez-Muñoz, ME, et al. Lifestyles in transition: evolution and natural history of the genus Lactobacillus. FEMS Microbiol Rev. (2017) 41:S27–48. doi: 10.1093/femsre/fux030
163. Oh, J-H, Alexander, LM, Pan, M, Schueler, KL, Keller, MP, Attie, AD, et al. Dietary fructose and microbiota-derived short-chain fatty acids promote bacteriophage production in the gut symbiont Lactobacillus reuteri. Cell Host Microbe. (2019) 25:273–284.e6. doi: 10.1016/j.chom.2018.11.016
164. O’Riordan, KJ, Collins, MK, Moloney, GM, Knox, EG, Aburto, MR, Fülling, C, et al. Short chain fatty acids: microbial metabolites for gut-brain axis signalling. Mol Cell Endocrinol. (2022) 546:111572. doi: 10.1016/j.mce.2022.111572
166. Chatterjee, A, and Duerkop, BA. Sugar and fatty acids Ack-celerate prophage induction. Cell Host Microbe. (2019) 25:175–6. doi: 10.1016/j.chom.2019.01.012
167. Bell, JC, and Kowalczykowski, SC. RecA: regulation and mechanism of a molecular search engine. Trends Biochem Sci. (2016) 41:491–507. doi: 10.1016/j.tibs.2016.04.002
168. Chintapalli, SV, Bhardwaj, G, Babu, J, Hadjiyianni, L, Hong, Y, Todd, GK, et al. Reevaluation of the evolutionary events within recA/RAD51 phylogeny. BMC Genomics. (2013) 14:240. doi: 10.1186/1471-2164-14-240
169. Plaza-Diaz, J, Pastor-Villaescusa, B, Rueda-Robles, A, Abadia-Molina, F, and Ruiz-Ojeda, FJ. Plausible biological interactions of low- and non-calorie sweeteners with the intestinal microbiota: an update of recent studies. Nutrients. (2020) 12:153. doi: 10.3390/nu12041153
170. Fowler, SPG. Low-calorie sweetener use and energy balance: results from experimental studies in animals, and large-scale prospective studies in humans. Physiol Behav. (2016) 164:517–23. doi: 10.1016/j.physbeh.2016.04.047
171. Katan, MB, de Ruyter, JC, Kuijper, LDJ, Chow, CC, Hall, KD, and Olthof, MR. Impact of masked replacement of sugar-sweetened with sugar-free beverages on body weight increases with initial BMI: secondary analysis of data from an 18 month double-blind trial in children. PLoS One. (2016) 11:e0159771. doi: 10.1371/journal.pone.0159771
172. Griebsch, LV, Theiss, EL, Janitschke, D, Erhardt, VKJ, Erhardt, T, Haas, EC, et al. Aspartame and its metabolites cause oxidative stress and mitochondrial and lipid alterations in SH-SY5Y cells. Nutrients. (2023) 15:1467. doi: 10.3390/nu15061467
173. Chiang, Y-F, Chen, Y-C, Huang, K-C, Ali, M, and Hsia, S-M. Exposure to sucralose and its effects on testicular damage and male infertility: insights into oxidative stress and autophagy. Environ Health Perspect. (2025). doi: 10.1289/EHP15919
174. Mchunu, N, Chukwuma, CI, Ibrahim, MA, Oyebode, OA, Dlamini, SN, and Islam, MS. Commercially available non-nutritive sweeteners modulate the antioxidant status of type 2 diabetic rats. J Food Biochem. (2019) 43:e12775. doi: 10.1111/jfbc.12775
175. Anbara, H, Kian, M, Darya, G-H, and Sheibani, MT. Long-term intake of aspartame-induced cardiovascular toxicity is reflected in altered histochemical parameters, evokes oxidative stress, and trigger P53-dependent apoptosis in a mouse model. Int J Exp Pathol. (2022) 103:252–62. doi: 10.1111/iep.12458
176. Higgins, KA, and Mattes, RD. A randomized controlled trial contrasting the effects of 4 low-calorie sweeteners and sucrose on body weight in adults with overweight or obesity. Am J Clin Nutr. (2019) 109:1288–301. doi: 10.1093/ajcn/nqy381
177. Lushchak, VI. Oxidative stress and mechanisms of protection against it in bacteria. Biochem Mosc. (2001) 66:476–89. doi: 10.1023/a:1010294415625
178. Seixas, AF, Quendera, AP, Sousa, JP, Silva, AFQ, Arraiano, CM, and Andrade, JM. Bacterial response to oxidative stress and RNA oxidation. Front Genet. (2021) 12:821535. doi: 10.3389/fgene.2021.821535
179. Maslovska, O, Komplikevych, S, and Hnatush, S. Oxidative stress and protection against it in bacteria. Stud Biol. (2023) 17:153–72. doi: 10.30970/sbi.1702.716
180. Dam, S, Pagès, J-M, and Masi, M. Stress responses, outer membrane permeability control and antimicrobial resistance in Enterobacteriaceae. Microbiology. (2018) 164:260–7. doi: 10.1099/mic.0.000613
181. van der Heijden, J, Reynolds, LA, Deng, W, Mills, A, Scholz, R, Imami, K, et al. Salmonella rapidly regulates membrane permeability to survive oxidative stress. mBio. (2016) 7:e01238–16. doi: 10.1128/mBio.01238-16
182. Carvajal-Garcia, J, Samadpour, AN, Hernandez Viera, AJ, and Merrikh, H. Oxidative stress drives mutagenesis through transcription-coupled repair in bacteria. Proc Natl Acad Sci USA. (2023) 120:e2300761120. doi: 10.1073/pnas.2300761120
183. Rossmann, FS, Racek, T, Wobser, D, Puchalka, J, Rabener, EM, Reiger, M, et al. Phage-mediated dispersal of biofilm and distribution of bacterial virulence genes is induced by quorum sensing. PLoS Pathog. (2015) 11:e1004653. doi: 10.1371/journal.ppat.1004653
184. Erez, Z, Steinberger-Levy, I, Shamir, M, Doron, S, Stokar-Avihail, A, Peleg, Y, et al. Communication between viruses guides lysis-lysogeny decisions. Nature. (2017) 541:488–93. doi: 10.1038/nature21049
185. Laganenka, L, Sander, T, Lagonenko, A, Chen, Y, Link, H, and Sourjik, V. Quorum sensing and metabolic state of the host control lysogeny-lysis switch of bacteriophage T1. mBio. (2019) 10:e01884–19. doi: 10.1128/mBio.01884-19
186. de Maganha Almeida Kumlien, AC, Borrego, CM, and Balcázar, JL. Antimicrobial resistance and bacteriophages: an overlooked intersection in water disinfection. Trends Microbiol. (2021) 29:517–27. doi: 10.1016/j.tim.2020.12.011
187. Zhang, Y, Guo, Y, Qiu, T, Gao, M, and Wang, X. Bacteriophages: underestimated vehicles of antibiotic resistance genes in the soil. Front Microbiol. (2022) 13:936267. doi: 10.3389/fmicb.2022.936267
188. León-Félix, J, and Villicaña, C. The impact of quorum sensing on the modulation of phage-host interactions. J Bacteriol. (2021) 203:e00687-20. doi: 10.1128/JB.00687-20
189. Rowland, IR, Mallett, AK, Bearne, CA, and Farthing, MJ. Enzyme activities of the hindgut microflora of laboratory animals and man. Xenobiotica. (1986) 16:519–23. doi: 10.3109/00498258609043540
190. Lobach, AR, Roberts, A, and Rowland, IR. Assessing the in vivo data on low/no-calorie sweeteners and the gut microbiota. Food Chem Toxicol. (2019) 124:385–99. doi: 10.1016/j.fct.2018.12.005
191. Chu, Y-Y, Chen, Y-H, Hsieh, R-H, Hsia, S-M, Wu, H-T, and Chen, Y-C. Development and validation of the Chinese version non-nutritive sweetener FFQ with urinary biomarker in children and adolescents. Public Health Nutr. (2022) 25:2056–63. doi: 10.1017/S136898002200088X
192. Pritchard, SE, Marciani, L, Garsed, KC, Hoad, CL, Thongborisute, W, Roberts, E, et al. Fasting and postprandial volumes of the undisturbed colon: normal values and changes in diarrhea-predominant irritable bowel syndrome measured using serial MRI. Neurogastroenterol Motil. (2014) 26:124–30. doi: 10.1111/nmo.12243
193. Nilsson, M, Sandberg, TH, Poulsen, JL, Gram, M, Frøkjaer, JB, Østergaard, LR, et al. Quantification and variability in colonic volume with a novel magnetic resonance imaging method. Neurogastroenterol Motil. (2015) 27:1755–63. doi: 10.1111/nmo.12673
194. Horrocks, V, King, OG, Yip, AYG, Marques, IM, and McDonald, JAK. Role of the gut microbiota in nutrient competition and protection against intestinal pathogen colonization. Microbiology. (2023) 169:001377. doi: 10.1099/mic.0.001377
195. Waldor, M, and Mekalanos, J. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science. (1996) 272:1910–4. doi: 10.1126/science.272.5270.1910
196. Cho, JY, Liu, R, Macbeth, JC, and Hsiao, A. The interface of vibrio cholerae and the gut microbiome. Gut Microbes. (2021) 13:1937015. doi: 10.1080/19490976.2021.1937015
197. de Sablet Thibaut, C, Christophe, B-D, Annick, V, Marjolaine, G, Alain, M, and Christine, P. Human microbiota-secreted factors inhibit Shiga toxin synthesis by enterohemorrhagic Escherichia coli O157:H7. Infect Immun. (2009) 77:783–90. doi: 10.1128/IAI.01048-08
198. Buffie, CG, and Pamer, EG. Microbiota-mediated colonization resistance against intestinal pathogens. Nat Rev Immunol. (2013) 13:790–801. doi: 10.1038/nri3535
199. Martin-Gallausiaux, C, Marinelli, L, Blottière, HM, Larraufie, P, and Lapaque, N. SCFA: mechanisms and functional importance in the gut. Proc Nutr Soc. (2021) 80:37–49. doi: 10.1017/S0029665120006916
200. Chhibber, S, Bansal, S, and Kaur, K. Disrupting the mixed-species biofilm of Klebsiella pneumoniae B5055 and Pseudomonas aeruginosa PAO using bacteriophages alone or in combination with xylitol. Microbiol Read Engl. (2015) 161:1369–77. doi: 10.1099/mic.0.000104
201. Schrödinger, LLC. The PyMOL molecular graphics system, version 1.8. PyMOL Schrödinger (2015) Available online at: https://pymol.org/2/support.html?#citing (Accessed June 11, 2023).
202. Nettleton, JA, Lutsey, PL, Wang, Y, Lima, JA, Michos, ED, and Jacobs, DRJ. Diet soda intake and risk of incident metabolic syndrome and type 2 diabetes in the multi-ethnic study of atherosclerosis (MESA). Diabetes Care. (2009) 32:688–94. doi: 10.2337/dc08-1799
Keywords: non-nutritive sweeteners, dysbiosis, ace-K, advantame, aspartame, neotame, saccharin, stevia
Citation: Marongiu L, Brzozowska E, Hetjens S, Hoelzle LE and Venturelli S (2025) Intestinal dysbiosis associated with non-nutritive sweeteners intake: an effect without a cause? Front. Nutr. 12:1694264. doi: 10.3389/fnut.2025.1694264
Edited by:
Michael Gänzle, University of Alberta, CanadaReviewed by:
Yuan Gao, Zhejiang University of Science and Technology, ChinaArkadiusz Jaworski, Medical University of Warsaw, Poland
Copyright © 2025 Marongiu, Brzozowska, Hetjens, Hoelzle and Venturelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luigi Marongiu, bHVpZ2kubWFyb25naXVAdW5pLWhvaGVuaGVpbS5kZQ==
 Luigi Marongiu
Luigi Marongiu Ewa Brzozowska
Ewa Brzozowska Svetlana Hetjens3
Svetlana Hetjens3 Ludwig E. Hoelzle
Ludwig E. Hoelzle