- 1School of Public Health, Bengbu Medical University, Bengbu, China
- 2School of Nursing, Bengbu Medical University, Bengbu, China
- 3Department of Nephrology, The Second Affiliated Hospital of Bengbu Medical University, Bengbu, China
Background: Although determinants of glycaemic control in type 2 diabetes mellitus (T2DM) have been extensively investigated, the joint influence of sleep quality and physical activity (PA) remains insufficiently studied. We aimed to examine the independent and combined associations of sleep quality and PA with glycaemic control in patients with T2DM.
Methods: We conducted a cross-sectional study of 329 patients with T2DM. Sleep quality was evaluated using the Pittsburgh Sleep Quality Index (PSQI). PA was self-reported through questionnaires and expressed as metabolic equivalents (METs). Associations between sleep quality, PA, and glycaemic control were assessed using multivariable logistic regression.
Results: After adjusting for confounding factors, we observed that declining habitual sleep efficiency was associated with an increased risk of suboptimal glycaemic control (OR 1.64, 95% CI 1.14–2.43). Participants with poorer sleep quality had a higher risk of suboptimal glycaemic control compared with those with better sleep quality (OR 2.09, 95% CI 1.09–4.09). High PA was associated with a significantly lower risk of poor glycaemic control compared with low PA (OR 0.20, 95% CI 0.05–0.68). In combined analyses, the greatest reduction in the risk of poor glycaemic control was observed in participants with good sleep quality and moderate PA, compared to those with poor sleep quality and low PA (OR 0.38, 95% CI 0.14–0.98).
Conclusion: In patients with T2DM, achieving optimal glycaemic control requires not only maintaining PA but also improving sleep quality.
1 Introduction
Given the increasing prevalence of type 2 diabetes mellitus (T2DM) globally and the significant burden it imposes, identifying modifiable risk factors for poor glycaemic control is crucial to prevent complications (1). Adequate glycaemic control plays a pivotal role in slowing the progression of T2DM and reducing its associated complications (2, 3). Poor glycaemic control can lead to hyperglycaemic symptoms and immediate complications (4). However, a recent study indicated that only half of patients with diabetes achieve adequate glycaemic control (5), suggesting that inadequate control is becoming more prevalent.
Among the various factors influencing glycaemic control, sleep has emerged as an important candidate (6, 7). Sleep disturbances are common both in the general population and among patients with T2DM, and their prevalence is rising due to the demands of modern society (1, 8, 9). Previous observational studies have shown that both sleep duration and quality are associated with hemoglobin A1c (HbA1c) levels in adults. For instance, the Fukuoka Diabetes Registry's cross-sectional study found a significant link between short or prolonged sleep and elevated HbA1c levels (10).
Physical activity (PA) is another key factor that has demonstrated effectiveness in improving clinical outcomes, including mortality, comorbidities, and HbA1c levels in T2DM patients (11–13). Regular PA is considered a core component of glycaemic management in diabetes, with moderate to vigorous physical activity (MVPA) shown to reduce HbA1c in a dose-dependent manner (14). Furthermore, MVPA enhances muscle glucose uptake and long-term glycaemic control by increasing skeletal muscle oxidative capacity and improving insulin signaling (15).
Sleep and PA are interrelated health behaviors (16, 17). Sleep and PA are closely related in terms of energy expenditure and time utilization (18, 19). Both acute and regular PA have been shown to improve sleep quality, while the quality and duration of sleep also affect PA performance (20–22). Previous studies have examined the separate effects of sleep quality and PA on glycaemic control in T2DM patients, yet inconsistencies in the measures used and the findings reported remain. Therefore, this study aims to explore both the independent and joint associations of sleep quality and PA with glycaemic control in community-based patients with T2DM, providing evidence-based guidance for improving glycaemic control through sleep and PA interventions.
2 Methods
2.1 Data source and study sample
Tis cross-sectional study used data from the community disease profiles of the Provincial Chronic Disease Prevention and Control Demonstration Area programme, conducted between May and July, 2019, in Bengbu City, China. All patients with T2DM—diagnosed in hospital before the survey—within the jurisdiction of a community health service station were recruited using whole-population sampling. The study protocol was approved by the Ethics Committee of Bengbu Medical College [Approval No. (2016) 15], and written informed consent was obtained from all participants.
Eligible participants were adults (≥18 years) with T2DM who had resided in the study area for more than 6 months during the preceding 12 months. Exclusion criteria were: (1) history of psychiatric or cognitive disorders; (2) severe organic disease or mobility impairment; (3) pregnancy or lactation; and (4) missing HbA1c data. A total of 329 patients with T2DM were included in the final analysis.
2.2 Evaluation of glycaemic control
Glycaemic control was defined according to the comprehensive control targets for type 2 diabetes mellitus outlined in the Chinese Guidelines for the Prevention and Control of Type 2 Diabetes Mellitus (2020 Edition), developed by the Diabetes Branch of the Chinese Medical Association. Achievement of glycaemic control was defined as a HbA1c level of <7.0% (23).
2.3 Sleep quality assessment
Sleep quality over the past month was assessed using the Pittsburgh Sleep Quality Index (PSQI). The PSQI consists of 19 items grouped into seven components: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction. Each component is scored from 0 to 3, yielding a total score of 0–21, with higher scores indicating poorer sleep quality. A total PSQI score ≥5 was considered indicative of poor sleep quality (24–26).
2.4 PA levels assessment
PA levels were assessed using the Chinese version of the International Physical Activity Questionnaire (IPAQ) (26), and were expressed in terms of Metabolic Equivalents (METs). Participants were categorized into three groups based on the World Health Organization (WHO) guidelines on PA for adults, which have been operationalized in previous studies using IPAQ data as follows: low PA (<600 MET-min/week), moderate PA (600–1,200 MET-min/week), and high PA (≥1,200 MET-min/week) (17, 27).
2.5 Covariates
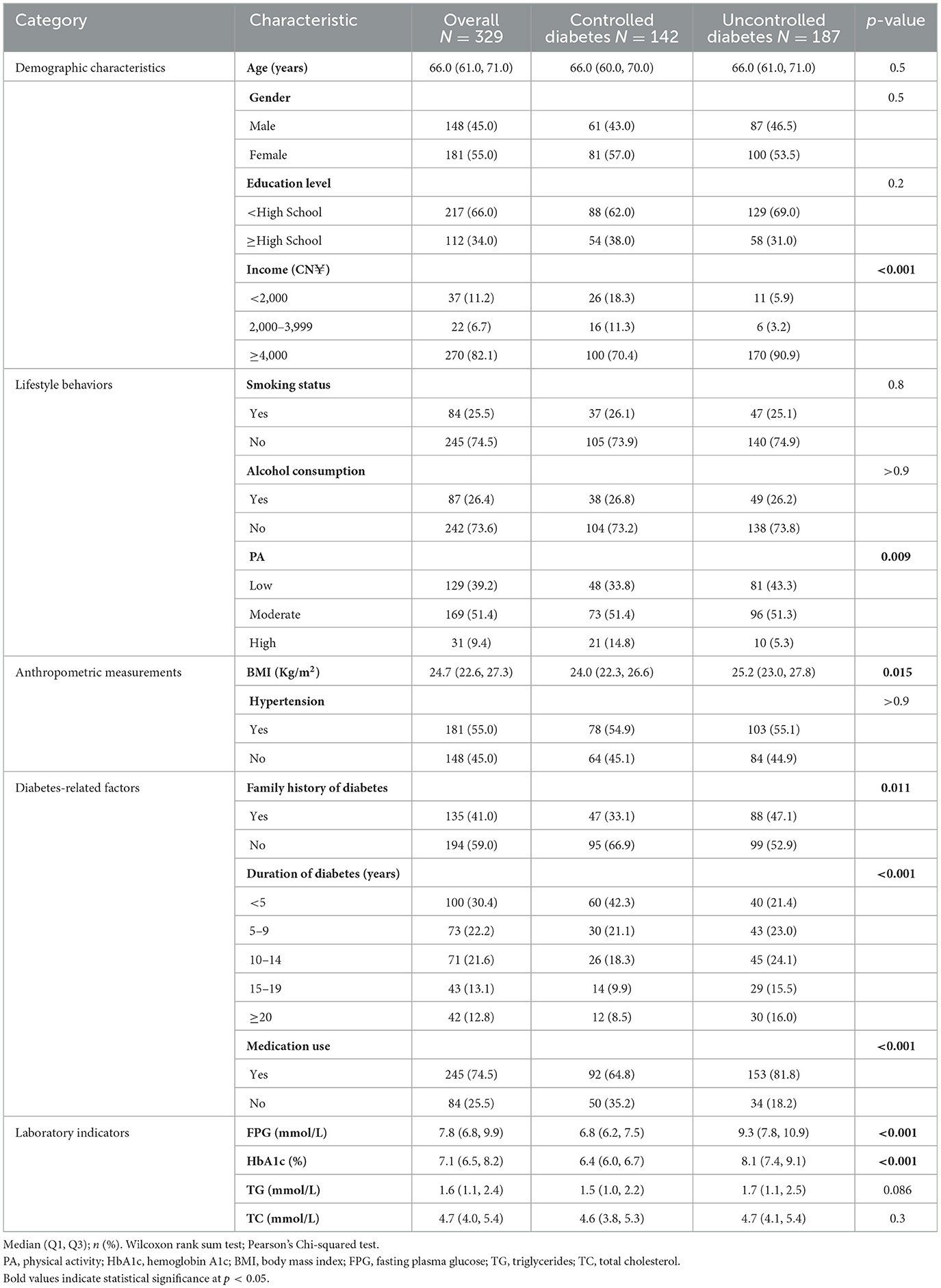
Based on prior research, potential baseline covariates included age, sex, income, smoking status, alcohol consumption, body-mass index (BMI), family history of diabetes, diabetes duration, medication use, fasting plasma glucose (FPG), and triglycerides (TG). Sociodemographic information (age, sex, monthly income, education level, and family history of diabetes), lifestyle behaviors (smoking and alcohol consumption), and diabetes-related factors (duration of diabetes and medication use) were obtained through structured questionnaires administered via centralized and household surveys. Anthropometric measurements—including systolic blood pressure (SBP), diastolic blood pressure (DBP), weight, height, and BMI—were collected by trained researchers using standardized procedures and calibrated instruments. BMI was calculated as weight (kg)/height (m2). For laboratory testing, participants fasted from 8:00 pm the night before the examination, resulting in a total fasting duration of at least 10 h prior to venous blood sample collection the following morning. Venous blood samples were collected the following morning to assess HbA1c, FPG, TG, and total cholesterol (TC), all expressed in mmol/L. Community physicians provided reminders about fasting requirements and verified the timing of the last meal before blood collection. Samples were transported to the affiliated hospital laboratory for analysis.
2.6 Statistical analysis
Normality tests were performed on continuous variables, revealing that all exhibited non-normal distributions. Non-normally distributed continuous variables were reported as medians with interquartile ranges, and intergroup differences were assessed using the Wilcoxon signed-rank test. Categorical variables were presented as frequencies with proportions, and intergroup differences were evaluated using the chi-square test. Multivariate logistic regression analyses were conducted to explore the associations between sleep quality, PA, and glycaemic control in patients with T2DM. Adjustments were as follows: Model 1 was unadjusted; Model 2 was adjusted for age, sex, income, smoking, and alcohol consumption; and Model 3 was further adjusted for additional potential confounders, including BMI, family history of diabetes, duration of diabetes, medication use, FPG, and TG. Joint analyses were performed based on combined strata of sleep quality (good/poor) and PA (low/moderate/high), with the poor sleep quality and low PA group serving as the reference. In addition, a multiplicative interaction between sleep quality and PA was formally tested by including their product term in the logistic regression model. Complementing these primary analyses, we also examined the associations by treating PSQI scores and log-MET as continuous exposures, assessing potential non-linear relationships.
Finally, two sensitivity analyses were conducted to assess the robustness of the findings. First, categorical variables for age and BMI were used for adjustment. Second, additional adjustments were made for carbohydrate, fat, protein, and total energy intake to determine whether diet influenced the relationship between sleep quality/PA and glycaemic control. Multiple imputation was applied to minimize the reduction in sample size due to missing covariates. All statistical tests were conducted at a significance level of 0.05. Data analyses were performed using R version 4.40 and MSTATA software (https://www.mstata.com/).
3 Results
3.1 Participant characteristics
A total of 329 participants (45.0% male, 55.0% female) were included in the study, comprising 142 individuals with well-controlled blood glucose and 187 with poorly controlled blood glucose. Baseline characteristics of the study population are presented in Table 1. Significant differences were observed between the groups in terms of income, BMI, family history of diabetes, duration of diabetes, medication use, FPG, and HbA1c levels (p < 0.05). Compared to the poorly controlled group, the well-controlled group had lower BMI, FPG, and HbA1c levels. Within the well-controlled group, a smaller proportion of participants had an income ≥4,000 CN¥ (70.4%), engaged in low PA (33.8%), had a family history of diabetes (33.1%), had a diabetes duration of ≥20 years (9.9%), or were using diabetes medication (64.8%). No significant differences were found between the groups in terms of age, gender, education level, smoking status, alcohol consumption, hypertension, TG, or TC levels (p > 0.05).
3.2 Association between sleep quality and glycaemic control
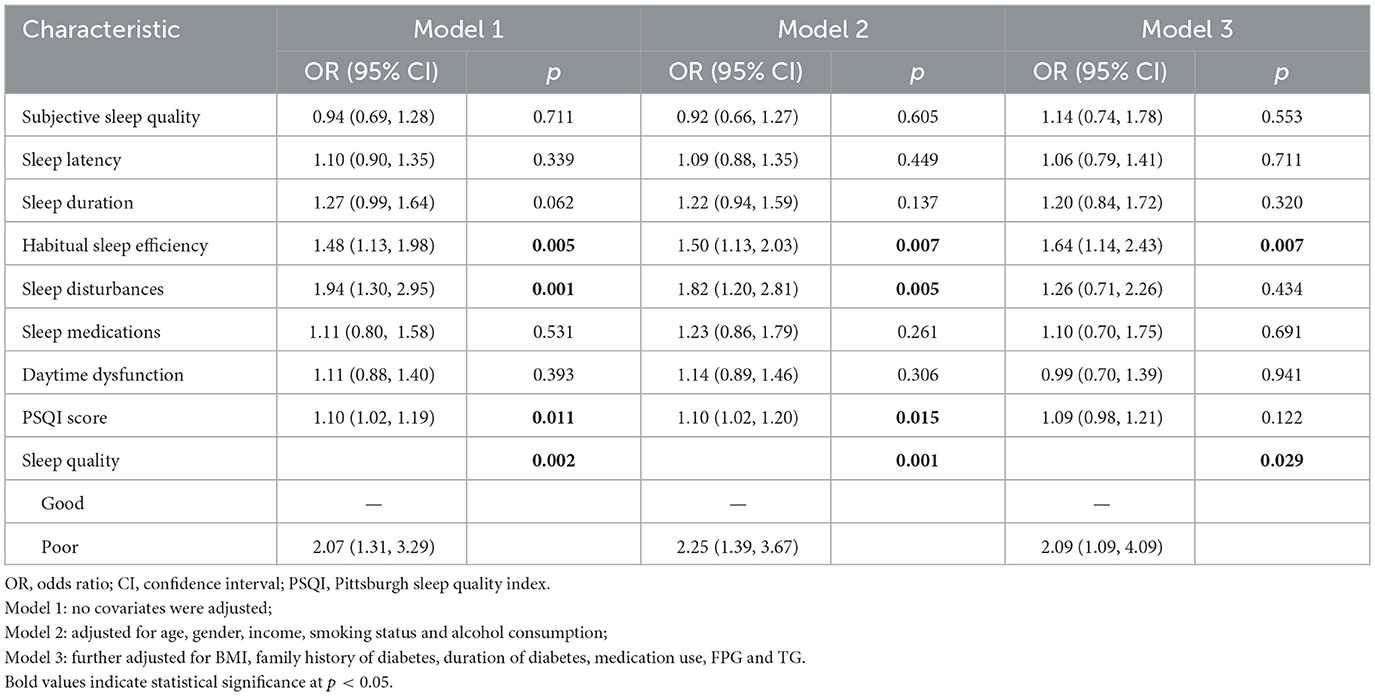
Table 2 presents the association between sleep quality and glycaemic control in patients with T2DM. In the multivariate logistic regression model (Model 3), habitual sleep efficiency was significantly associated with glycaemic control (OR = 1.64, 95% CI: 1.14–2.43), indicating that a decline in habitual sleep efficiency was associated with an increased risk of suboptimal glycaemic control. In Models 1 and 2, sleep disturbance emerged as a risk factor for poor glycaemic control (OR = 1.94, 95% CI: 1.30–2.95; OR = 1.82, 95% CI: 1.20–2.81), with higher PSQI scores reflecting a higher risk of suboptimal glycaemic control (OR = 1.10, 95% CI: 1.02–1.19; OR = 1.10, 95% CI: 1.02–1.20). However, this association was not statistically significant in Model 3. Additionally, participants with poor sleep quality had a higher risk of suboptimal glycaemic control compared to those with good sleep quality (OR = 2.09, 95% CI: 1.09–4.09). When treated as a continuous variable, the PSQI score was not significantly associated with glycaemic control, although a non-linear relationship was suggested (p for non-linearity = 0.038; see Supplementary Table 1).
3.3 Association between PA and glycaemic control
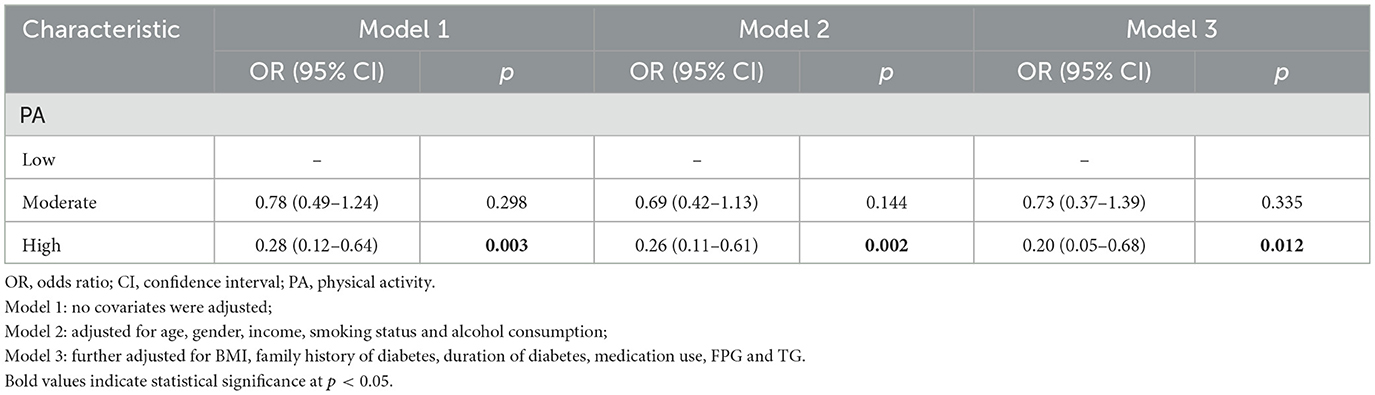
Multivariate logistic regression analysis was conducted to examine the association between PA and glycaemic control. In the unadjusted model, the risk of suboptimal glycaemic control was significantly lower in the high PA group compared to the low PA group (OR = 0.28, 95% CI: 0.12–0.64). After adjusting for all covariates, the risk of suboptimal glycaemic control remained significantly lower in the high PA group (OR = 0.20, 95% CI: 0.05–0.68). The moderate PA group did not show a statistically significant association in any of the models. The results are presented in Table 3. The median weekly PA volume for each category is provided in Supplementary Table 2. The analysis of log-transformed MET as a continuous variable confirmed a significant protective effect against poor glycaemic control (OR: 0.77, 95% CI: 0.59–0.97), with evidence of a non-linear dose-response relationship (p for non-linearity = 0.021; Supplementary Table 1).
3.4 Joint effect of sleep quality and PA on glycaemic control
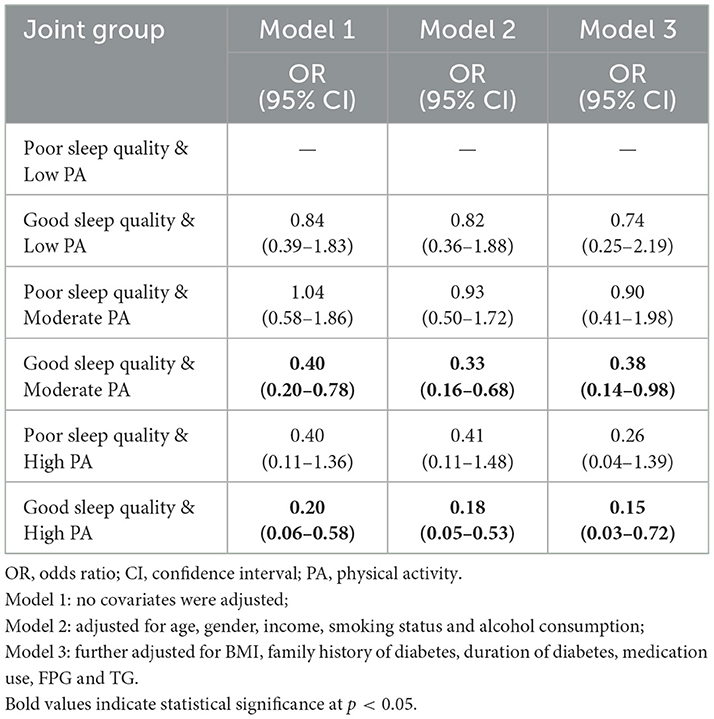
The results of the joint effects analysis are summarized in Table 4. In Model 3, the risk of suboptimal glycaemic control was most significantly reduced in the group with good sleep quality and moderate PA compared to the group with poor sleep quality and low PA (OR = 0.38, 95% CI: 0.14–0.98). The risk of suboptimal glycaemic control was also significantly lower in the good sleep quality and high PA group compared to the poor sleep quality and low PA group (OR = 0.15, 95% CI: 0.03–0.72). No statistically significant differences were observed in the remaining groups. Additionally, the interaction between sleep quality and PA on glycaemic control was not significant (see Supplementary Table 3). Finally, Supplementary Table 4 displays the unadjusted HbA1c levels for all combinations of sleep quality and PA.
3.5 Sensitivity analysis
In sensitivity analyses, we observed consistent results when adjusting for age (<60 and ≥60 years) and BMI (<28 and ≥28 kg/m2) by categorizing continuous variables (Model 4, Supplementary Table 5). Furthermore, in Model 5, based on Model 4, the associations between subjective sleep efficiency, sleep quality, PA, and glycaemic control remained largely unchanged after additional adjustment for protein, fat, carbohydrate, and total energy intake (Supplementary Table 6). Similar results were observed for interaction effects (Supplementary Tables 7, 8).
4 Discussion
Our findings suggest that in community-based patients with T2DM, decreased habitual sleep efficiency and poorer sleep quality are associated with an increased risk of suboptimal glycaemic control. Compared with individuals with low PA, those engaging in high PA exhibited a significantly lower risk of poor glycaemic control. Additionally, the combined analysis revealed that individuals with good sleep quality and moderate PA experienced the greatest reduction in the risk of suboptimal glycaemic control.
Several prior studies have explored the relationship between sleep and glycaemic control in T2DM patients. For instance, poorer subjective sleep quality (10) and reduced sleep efficiency (28–30) have been linked to higher HbA1c levels in individuals with T2DM. Our results are consistent with a recent study in Mexico, which found that poor sleep quality doubled the risk of poor glycaemic control, even after accounting for other risk factors (31). Additionally, a cross-sectional study from Iran also highlighted a significant association between habitual sleep efficiency and poor glycaemic control, aligning with our findings (32).
The American Diabetes Association's Standards of Care have recently incorporated sleep health, reflecting its growing relevance to glycemic management (33, 34). Our study strengthens this emerging evidence by demonstrating a clear link between poor sleep quality and suboptimal glycemic control in a community-based T2DM population. From a pathophysiological perspective, our observations are consistent with mechanisms highlighted in experimental research, including disruptions in sleep architecture (e.g., suppressed slow-wave sleep) and consequent sympathetic nervous system activation, which are known to impair insulin secretion and glucose metabolism (35–37). While causal inferences cannot be drawn from our cross-sectional data, these findings collectively underscore the value of considering sleep quality in clinical practice. Further studies are needed to clarify these underlying mechanisms.
In addition to sleep, PA plays a critical role in improving glycaemic control in T2DM patients. Numerous meta-analyses have demonstrated the effectiveness of various PA regimens in managing glycaemic levels (38–41). However, no studies have definitively identified the optimal type or dose of PA needed to validate these recommendations. A recent study identified a non-linear dose-response relationship between PA and glycaemic control, showing that the optimal dose for T2DM patients was 1,100 MET-min per week, irrespective of baseline HbA1c levels (42).
The association between PA and improved HbA1c observed in our study is consistent with several established physiological mechanisms, such as increased glucose uptake in skeletal muscle (43), improved adipocyte function, and reduced cytokine production (44). Furthermore, evidence suggests that moderate-intensity endurance training can enhance glycaemic control and late-phase β-cell function (45), which may partly explain the beneficial association observed in the group with both good sleep quality and moderate PA. However, individual responses to PA are highly variable (46), and excessive PA can lead to mitochondrial damage and impaired glucose tolerance (47), which may influence the relationship between PA and HbA1c changes.
Our study also found that good sleep quality combined with moderate PA significantly reduced the risk of suboptimal glycaemic control. However, we did not observe a significant interaction between sleep quality and PA in relation to glycaemic control. Existing literature suggests a degree of interdependence between PA and sleep, with both influencing health through distinct mechanisms (16, 48–50). PA potentially influencing sleep through mechanisms such as fluctuations in body temperature, glucose metabolism, autonomic nervous system activity, and changes in body composition, mood, and cardiorespiratory health (21, 22, 51).
Moreover, the observed benefit of combining good sleep with moderate PA supports the notion that good sleep quality may help mitigate the risks associated with lower activity levels. This could be facilitated by mechanisms that promote metabolic homeostasis, such as those improving glucose regulation (35, 43). This observation helps explain our finding that while only high levels of PA reduced the risk of suboptimal glycaemic control, the combination of good sleep quality and moderate PA significantly lowered the risk. Future research should further explore the underlying mechanisms between sleep and PA through longitudinal studies, and investigate personalized exercise intervention strategies for individuals with sleep disorders.
5 Strengths and limitations
Our study has several strengths. First, it is the first to examine the joint association between sleep quality, PA, and glycaemic control, while also exploring potential interactions between these factors. Second, we carefully adjusted for a range of potential confounders, including sociodemographic factors, lifestyle behaviors, and diabetes-related variables such as duration and medication status. Lastly, we conducted sensitivity analyses, particularly excluding dietary factors, to assess the robustness of our findings.
However, this study has several limitations. First, it involves a relatively small sample, limiting the generalizability of the results. Moreover, the sample size may have provided insufficient statistical power to detect significant interaction effects between sleep quality and PA, even if such interactions truly exist. Second, the cross-sectional design precludes causal inference regarding the relationships between sleep quality, PA, and glycemic control. The observed associations may be subject to reverse causality, whereby poor glycemic control could lead to sleep disturbances and reduced PA, as well as residual confounding. Furthermore, several specific limitations in confounding control must be noted. Although we adjusted for the use of hypoglycemic medications, we could not account for specific drug classes (e.g., insulin, sulfonylureas, GLP-1 receptor agonists, SGLT2 inhibitors), which have distinct effects on glycemia and sleep. We also did not systematically assess or adjust for obstructive sleep apnea (OSA) or depressive symptoms, both of which are strongly associated with sleep, PA, and glycemic control (52). Finally, the reliance on self-reported questionnaires for both sleep quality and PA introduces the potential for recall and response biases. Additionally, the multiple statistical comparisons conducted for the various components of sleep quality should be interpreted with caution. Self-reported PA may not adequately capture absolute activity levels compared to device-based measures (53–55), and similarly, objective sleep metrics are often more accurate than self-reports (56–58). The use of objective or combined assessment tools in future studies is therefore strongly recommended.
6 Conclusion
In conclusion, this study found that in patients with T2DM, reduced habitual sleep efficiency and poorer sleep quality are associated with an increased risk of suboptimal glycaemic control. Additionally, individuals with high PA levels were at a significantly lower risk of suboptimal glycaemic control compared to those with low PA. The combination of good sleep quality and moderate PA was linked to the greatest reduction in the risk of poor glycaemic control. These findings illustrate the potential of a comprehensive approach to T2DM management that addresses both sleep quality and PA to achieve optimal glycemic control. Future prospective and intervention studies are warranted to confirm these observed associations and establish their temporal sequence.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. Requests to access these datasets should be directed to Hong Xie, eGhAYmJtdS5lZHUuY24=.
Ethics statement
The study protocol was approved by the Ethics Committee of Bengbu Medical College (Approval No. [2016] 15), and written informed consent was obtained from all participants. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
LZ: Formal analysis, Methodology, Writing – original draft. YL: Investigation, Supervision, Writing – review & editing. SZ: Investigation, Visualization, Writing – review & editing. HZ: Data curation, Methodology, Writing – original draft. XJ: Data curation, Project administration, Supervision, Writing – original draft. YY: Project administration, Visualization, Writing – original draft. HX: Conceptualization, Funding acquisition, Methodology, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Humanities and Social Sciences Planning Fund Project of the Ministry of Education of China (No. 15YJAZH085).
Acknowledgments
The authors thank Yaqin Yang for her valuable contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1694982/full#supplementary-material
References
1. Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. (2018) 14:88–98. doi: 10.1038/nrendo.2017.151
2. Hu C, Jia W. Diabetes in China: epidemiology and genetic risk factors and their clinical utility in personalized medication. Diabetes. (2018) 67:3–11. doi: 10.2337/dbi17-0013
3. Nathan DM The The DCCT/EDIC Research Group. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. (2014) 37:9–16. doi: 10.2337/dc13-2112
4. Rodríguez-Gutiérrez R, Millan-Alanis JM, Barrera FJ, McCoy RG. Value of patient-centered glycemic control in patients with type 2 diabetes. Curr Diab Rep. (2021) 21:63. doi: 10.1007/s11892-021-01433-0
5. Wang L, Peng W, Zhao Z, Zhang M, Shi Z, Song Z, et al. Prevalence and treatment of diabetes in China, 2013–2018. JAMA. (2021) 326:2498. doi: 10.1001/jama.2021.22208
6. Anothaisintawee T, Reutrakul S, Van Cauter E, Thakkinstian A. Sleep disturbances compared to traditional risk factors for diabetes development: systematic review and meta-analysis. Sleep Med Rev. (2016) 30:11–24. doi: 10.1016/j.smrv.2015.10.002
7. Schmid SM, Hallschmid M, Schultes B. The metabolic burden of sleep loss. Lancet Diabetes Endocrinol. (2015) 3:52–62. doi: 10.1016/S2213-8587(14)70012-9
8. Plantinga L, Rao M, Schillinger D. Prevalence of self-reported sleep problems among people with diabetes in the United States, 2005-2008. Prev Chronic Dis. (2012) 9:110244. doi: 10.5888/pcd9.110244
9. Kerkhof GA. Epidemiology of sleep and sleep disorders in The Netherlands. Sleep Med. (2017) 30:229–39. doi: 10.1016/j.sleep.2016.09.015
10. Lee SWH, Ng KY, Chin WK. The impact of sleep amount and sleep quality on glycemic control in type 2 diabetes: a systematic review and meta-analysis. Sleep Med Rev. (2017) 31:91–101. doi: 10.1016/j.smrv.2016.02.001
11. Hou L, Wang Q, Pan B, Li R, Li Y, He J, et al. Exercise modalities for type 2 diabetes: a systematic review and network meta-analysis of randomized trials. Diabetes Metabolism Res. (2023) 39:e3591. doi: 10.1002/dmrr.3591
12. Hemmingsen B, Gimenez-Perez G, Mauricio D, Roqué I, Figuls M, Metzendorf M-I, Richter B. Diet, physical activity or both for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus Cochrane. Database Syst Rev. (2017) 12:CD003054. doi: 10.1002/14651858.CD003054.pub4
13. Chang C-H, Kuo C-P, Huang C-N, Hwang S-L, Liao W-C, Lee M-C. Habitual physical activity and diabetes control in young and older adults with type II diabetes: a longitudinal correlational study. IJERPH. (2021) 18:1330. doi: 10.3390/ijerph18031330
14. Boniol M, Dragomir M, Autier P, Boyle P. Physical activity and change in fasting glucose and HbA1c: a quantitative meta-analysis of randomized trials. Acta Diabetol. (2017) 54:983–91. doi: 10.1007/s00592-017-1037-3
15. Stanford KI, Goodyear LJ. Exercise and type 2 diabetes: molecular mechanisms regulating glucose uptake in skeletal muscle. Adv Physiol Educ. (2014) 38:308–14. doi: 10.1152/advan.00080.2014
16. Huang B-H, Hamer M, Duncan MJ, Cistulli PA, Stamatakis E. The bidirectional association between sleep and physical activity: A 69 years longitudinal analysis of 38,601 UK Biobank participants Prev Med. (2021) 143:106315. doi: 10.1016/j.ypmed.2020.106315
17. Liu H, Shi Y, Yu M, Guo X, Ruan Y, Qin F, et al. Individual and joint associations between sleep duration and physical activity with cognitive function: a longitudinal analysis among middle-aged and older adults in China. Alzheimer's Dement. (2025) 21:e14212. doi: 10.1002/alz.14212
18. Tremblay MS, Colley RC, Saunders TJ, Healy GN, Owen N. Physiological and health implications of a sedentary lifestyle. Appl Physiol Nutr Metab. (2010) 35:725–40. doi: 10.1139/H10-079
19. Tudor-Locke C, Leonardi C, Johnson WD, Katzmarzyk PT. Time spent in physical activity and sedentary behaviors on the working day: the American time use survey. J Occup Environ Med. (2011) 53:1382–7. doi: 10.1097/JOM.0b013e31823c1402
20. Kredlow MA, Capozzoli MC, Hearon BA, Calkins AW, Otto MW. The effects of physical activity on sleep: a meta-analytic review. J Behav Med. (2015) 38:427–49. doi: 10.1007/s10865-015-9617-6
21. Driver HS, Taylor SR. Exercise and sleep. Sleep Med Rev. (2000) 4:387–402. doi: 10.1053/smrv.2000.0110
22. Chennaoui M, Arnal PJ, Sauvet F, Léger D. Sleep and exercise: a reciprocal issue? Sleep Med Rev. (2015) 20:59–72. doi: 10.1016/j.smrv.2014.06.008
23. Chinese Society of Diabetes. Guideline for the prevention and treatment of type 2 diabetes mellitus in China (2020 edition) (Part 1). Chin J Pract Intern Med. (2021) 41:668–95. doi: 10.3760/cma.j.cn311282-20210304-00142
24. Abdu Y, Naja S, Mohamed Ibrahim MI, Abdou M, Ahmed R, Elhag S, et al. Sleep quality among people with type 2 diabetes mellitus during COVID-19 pandemic: evidence from Qatar's national diabetes center. DMSO. (2023) 16:2803–12. doi: 10.2147/DMSO.S421878
25. Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28:193–213. doi: 10.1016/0165-1781(89)90047-4
26. Bergmann M, Tschiderer L, Stefani A, Heidbreder A, Willeit P, Högl B. Sleep quality and daytime sleepiness in epilepsy: systematic review and meta-analysis of 25 studies including 8,196 individuals. Sleep Med Rev. (2021) 57:101466. doi: 10.1016/j.smrv.2021.101466
27. Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. (2020) 54:1451–62. doi: 10.1136/bjsports-2020-102955
28. Curtis DS, Fuller-Rowell TE, El-Sheikh M, Carnethon MR, Ryff CD. Habitual sleep as a contributor to racial differences in cardiometabolic risk. Proc Natl Acad Sci USA. (2017) 114:8889–94. doi: 10.1073/pnas.1618167114
29. Whitaker KM, Lutsey PL, Ogilvie RP, Pankow JS, Bertoni A, Michos ED, et al. Associations between polysomnography and actigraphy-based sleep indices and glycemic control among those with and without type 2 diabetes: the Multi-Ethnic Study of Atherosclerosis. Sleep. (2018) 41: doi: 10.1093/sleep/zsy172
30. Trento M, Broglio F, Riganti F, Basile M, Borgo E, Kucich C, et al. Sleep abnormalities in type 2 diabetes may be associated with glycemic control. Acta Diabetol. (2008) 45:225–9. doi: 10.1007/s00592-008-0047-6
31. Suárez-Torres I, García-García F, Morales-Romero J, Melgarejo-Gutiérrez M, Demeneghi-Marini VP, Luna-Ceballos RI, et al. Poor quality of sleep in Mexican patients with type 2 diabetes and its association with lack of glycemic control. Prim Care Diabetes. (2023) 17:155–60. doi: 10.1016/j.pcd.2023.01.011
32. Borzouei S, Ahmadi A, Pirdehghan A. Sleep quality and glycemic control in adults with type 2 diabetes mellitus. J Family Med Prim Care. (2024) 13:3398–402. doi: 10.4103/jfmpc.jfmpc_118_24
33. Henson J, Covenant A, Hall AP, Herring L, Rowlands AV, Yates T, et al. Waking up to the importance of sleep in type 2 diabetes management: a narrative review. Diabetes Care. (2024) 47:331–43. doi: 10.2337/dci23-0037
34. Davies MJ, Aroda VR, Collins BS, Gabbay RA, Green J, Maruthur NM, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. (2022) 45:2753–86. doi: 10.2337/dci22-0034
35. Herzog N, Jauch-Chara K, Hyzy F, Richter A, Friedrich A, Benedict C, et al. Selective slow wave sleep but not rapid eye movement sleep suppression impairs morning glucose tolerance in healthy men. Psychoneuroendocrinology. (2013) 38:2075–82. doi: 10.1016/j.psyneuen.2013.03.018
36. Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. (1999) 354:1435–9. doi: 10.1016/S0140-6736(99)01376-8
37. Buxton OM, Cain SW, O'Connor SP, Porter JH, Duffy JF, Wang W, et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med. (2012) 4:129ra43. doi: 10.1126/scitranslmed.3003200
38. Liu Y, Ye W, Chen Q, Zhang Y, Kuo C-H, Korivi M. Resistance exercise intensity is correlated with attenuation of HbA1c and insulin in patients with type 2 diabetes: a systematic review and meta-analysis. IJERPH. (2019) 16:140. doi: 10.3390/ijerph16010140
39. Grace A, Chan E, Giallauria F, Graham PL, Smart NA. Clinical outcomes and glycaemic responses to different aerobic exercise training intensities in type II diabetes: a systematic review and meta-analysis. Cardiovasc Diabetol. (2017) 16:37. doi: 10.1186/s12933-017-0518-6
40. Mannucci E, Bonifazi A, Monami M. Comparison between different types of exercise training in patients with type 2 diabetes mellitus: a systematic review and network metanalysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. (2021) 31:1985–92. doi: 10.1016/j.numecd.2021.02.030
41. Pan B, Ge L, Xun Y, Chen Y, Gao C, Han X, et al. Exercise training modalities in patients with type 2 diabetes mellitus: a systematic review and network meta-analysis. Int J Behav Nutr Phys Act. (2018) 15:72. doi: 10.1186/s12966-018-0703-3
42. Gallardo-Gómez D, Salazar-Martínez E, Alfonso-Rosa RM, Ramos-Munell J, Del Pozo-Cruz J, Del Pozo Cruz B, et al. Optimal dose and type of physical activity to improve glycemic control in people diagnosed with type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. (2024) 47:295–303. doi: 10.2337/dc23-0800
43. Holloszy JO. Exercise-induced increase in muscle insulin sensitivity. J Appl Physiol. (2005) 99:338–43. doi: 10.1152/japplphysiol.00123.2005
44. Balducci S, Zanuso S, Nicolucci A, Fernando F, Cavallo S, Cardelli P, et al. Anti-inflammatory effect of exercise training in subjects with type 2 diabetes and the metabolic syndrome is dependent on exercise modalities and independent of weight loss. Nutr Metab Cardiovasc Dis. (2010) 20:608–17. doi: 10.1016/j.numecd.2009.04.015
45. Zhang H, Simpson LK, Carbone NP, Hirshman MF, Nigro P, Vamvini M, et al. Moderate-intensity endurance training improves late phase β-cell function in adults with type 2 diabetes. iScience. (2023) 26:107226. doi: 10.1016/j.isci.2023.107226
46. Solomon TPJ. Sources of inter-individual variability in the therapeutic response of blood glucose control to exercise in type 2 diabetes: going beyond exercise dose. Front Physiol. (2018) 9:896. doi: 10.3389/fphys.2018.00896
47. Flockhart M, Nilsson LC, Tais S, Ekblom B, Apró W, Larsen FJ. Excessive exercise training causes mitochondrial functional impairment and decreases glucose tolerance in healthy volunteers. Cell Metab. (2021) 33:957–70.e6. doi: 10.1016/j.cmet.2021.02.017
48. Wennman H, Kronholm E, Heinonen OJ, Kujala UM, Kaprio J, Partonen T, et al. Leisure time physical activity and sleep predict mortality in men irrespective of background in competitive sports. Progress Prev Med. (2017) 2:e0009. doi: 10.1097/pp9.0000000000000009
49. Chen L-J, Hamer M, Lai Y-J, Huang B-H, Ku P-W, Stamatakis E. Can physical activity eliminate the mortality risk associated with poor sleep? A 15-year follow-up of 341,248 MJ Cohort participants. J Sport Health Sci. (2022) 11:596–604. doi: 10.1016/j.jshs.2021.03.001
50. Kline CE. The bidirectional relationship between exercise and sleep: implications for exercise adherence and sleep improvement. Am J Lifestyle Med. (2014) 8:375–9. doi: 10.1177/1559827614544437
51. Uchida S, Shioda K, Morita Y, Kubota C, Ganeko M, Takeda N. Exercise effects on sleep physiology. Front Neurol. (2012) 3:48. doi: 10.3389/fneur.2012.00048
52. Yin X, Bao W, Ley SH, Yang J, Cuffe SB, Yu G, et al. Sleep characteristics and long-term risk of type 2 diabetes among women with gestational diabetes. JAMA Netw Open. (2025) 8:e250142. doi: 10.1001/jamanetworkopen.2025.0142
53. Hammer TM, Johansson J, Emaus N, Furberg A-S, Gracia-Marco L, Morseth B, et al. Changes in accelerometer-measured physical activity and self-reported leisure time physical activity from adolescence to young adulthood: a longitudinal cohort study from the Fit Futures Study. Int J Behav Nutr Phys Act. (2025) 22:99. doi: 10.1186/s12966-025-01799-4
54. Shephard RJ. Limits to the measurement of habitual physical activity by questionnaires. Br J Sports Med. (2003) 37:197–206. doi: 10.1136/bjsm.37.3.197
55. Prince SA, Adamo KB, Hamel M, Hardt J, Connor Gorber S, Tremblay M, et al. Comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. (2008) 5:56. doi: 10.1186/1479-5868-5-56
56. Nauha L, Farrahi V, Jurvelin H, Jämsä T, Niemelä M, Kangas M, et al. Comparison and agreement between device-estimated and self-reported sleep periods in adults. Ann Med. (2023) 55:2191001. doi: 10.1080/07853890.2023.2191001
57. Cespedes EM, Hu FB, Redline S, Rosner B, Alcantara C, Cai J, et al. Comparison of self-reported sleep duration with actigraphy: results from the hispanic community health study/study of Latinos Sueño Ancillary Study. Am J Epidemiol. (2016) 183:561–73. doi: 10.1093/aje/kwv251
Keywords: T2DM, sleep quality, physical activity, glycaemic control, joint effect
Citation: Zhou L, Li Y, Zhang S, Zhang H, Ji X, Yu Y and Xie H (2025) Independent and joint associations of sleep quality and physical activity with glycaemic control in patients with type 2 diabetes mellitus. Front. Nutr. 12:1694982. doi: 10.3389/fnut.2025.1694982
Received: 29 August 2025; Accepted: 14 October 2025;
Published: 03 November 2025.
Edited by:
David Christopher Nieman, Appalachian State University, United StatesReviewed by:
Laurel M. Wentz, Appalachian State University, United StatesCamila Akemi Sakaguchi, Appalachian State University, United States
Copyright © 2025 Zhou, Li, Zhang, Zhang, Ji, Yu and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Xie, eGhAYmJtdS5lZHUuY24=
 Lei Zhou
Lei Zhou Yize Li1
Yize Li1