- 1Physical Education College, Shandong University of Finance and Economics, Jinan, Shandong, China
- 2Department of Physical Education, Shandong University of Traditional Chinese Medicine, Jinan, Shandong, China
Age-related diseases, including cardiovascular disorders, type 2 diabetes, neurodegenerative conditions such as Alzheimer's and Parkinson's disease, and age-related eye diseases, represent leading causes of disability and mortality worldwide. Growing evidence highlights the therapeutic promise of non-pharmacological interventions, notably saffron (Crocus sativus L.) and structured exercise, both of which exert pleiotropic effects through antioxidant, anti-inflammatory, and neuroprotective pathways. In this review, we summarize current experimental and clinical data on saffron's bioactive compounds, crocin, crocetin, and safranal, and their capacity to modulate lipid metabolism, insulin sensitivity, mitochondrial function, and protein aggregation. Parallel findings from exercise research demonstrate improvements in cardiovascular function, glycemic control, neuroplasticity, and ocular health. Importantly, emerging studies reveal synergistic benefits when saffron supplementation is combined with physical activity, resulting in amplified improvements in vascular remodeling, glycemic regulation, neurotrophic signaling, and behavioral outcomes. These complementary interventions target shared molecular pathways, including PI3K/Akt/mTOR signaling, SIRT1–PGC-1α activation, Nrf2-mediated antioxidant defense, and modulation of inflammatory cytokines. Taken together, saffron and exercise represent safe, accessible, and multi-target strategies that may delay or attenuate the progression of aging-related diseases. Future large-scale, long-term clinical trials are warranted to establish optimal protocols and to integrate these interventions into preventive and therapeutic frameworks for healthy aging.
1 Introduction
The global demographic shift toward an increasingly older population has become one of the most pressing public health concerns of the current era. Advances in medicine, technology, and socioeconomic development have extended life expectancy, but these gains are accompanied by a steep rise in age-associated diseases (1). By 2030, one in six individuals worldwide will be aged 60 years or older, and by 2050, the number of people in this age group is expected to reach 2.1 billion, with the majority living in low- and middle-income countries. The extension of human life span is not necessarily paralleled by a similar expansion in health span. Many individuals spend their final decades coping with chronic, debilitating disorders that severely impact independence and quality of life (2). Neurological decline, cardiometabolic dysfunction, and psychiatric disturbances rank among the leading contributors to morbidity in older adults, creating both medical and socioeconomic burdens for families and healthcare systems (3).
Age-related diseases encompass a broad spectrum of conditions, including neurodegenerative disorders such as Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease (HD), multiple sclerosis (MS), and amyotrophic lateral sclerosis (ALS), as well as cardiovascular disease (CVD), type 2 diabetes (T2D), metabolic syndrome, and certain cancers (4, 5). These conditions are driven by a complex interplay of genetic, molecular, and environmental factors, with aging itself serving as the strongest risk factor (6). Biological aging is marked by cumulative damage at the cellular and molecular levels, involving oxidative stress, mitochondrial dysfunction, chronic inflammation, impaired proteostasis, and dysregulated signaling networks (7). These alterations ultimately compromise physiological resilience, leading to cognitive decline, emotional instability, impaired mobility, and increased vulnerability to chronic diseases.
Neurodegenerative and cardiometabolic disorders are leading contributors to age-related morbidity, characterized by progressive neuronal loss, impaired cognition and movement, metabolic dysregulation, and heightened vulnerability to conditions such as dementia, Parkinson's disease, type 2 diabetes, and cardiovascular disease (8). These conditions often coexist with sarcopenia, depression, and anxiety, creating a cycle of functional decline and diminished quality of life (9). Current pharmacological options provide only temporary symptomatic relief and are limited by side effects, underscoring the urgent need for safe, affordable, and multi-targeted strategies (10). Increasingly, nutraceuticals like saffron and lifestyle approaches, such as structured physical activity, are being explored as promising non-pharmacological interventions to address these interconnected challenges of aging.
Among natural products, Crocus sativus L., commonly known as saffron, stands out as a promising candidate. Saffron is a highly valued spice with a long history of use in traditional medicine across Persian, Mediterranean, and South Asian cultures (11). Modern scientific research has revealed that saffron and its active constituents, including crocin, crocetin, safranal, and picrocrocin, possess a wide range of pharmacological activities (12, 13). These compounds exhibit strong antioxidant capacity, modulate inflammatory signaling, regulate apoptosis and autophagy, and influence neurotransmitter systems, such as serotonin, dopamine, and glutamate (14). Through these mechanisms, saffron has been shown to protect neuronal cells, enhance cognitive performance, and alleviate mood disorders (15). Clinical trials suggest that saffron supplementation can improve symptoms of mild-to-moderate depression with fewer side effects compared to conventional antidepressants (16). In models of AD, saffron reduces amyloid-beta accumulation and improves memory performance, while in PD, it preserves dopaminergic neurons and mitigates motor dysfunction. Importantly, saffron's safety profile and minimal toxicity make it an attractive long-term intervention for older adults (17, 18).
Parallel to nutraceutical research, physical activity has long been recognized as a cornerstone of preventive medicine in aging. Regular exercise confers multidimensional benefits across neurological, psychological, and metabolic domains (19). Aerobic exercise enhances cardiovascular health, increases insulin sensitivity, and supports neurogenesis, while resistance training is particularly effective in combating sarcopenia and mobility disability (20–23). Exercise also stimulates the release of neurotrophic factors, such as brain-derived neurotrophic factor (BDNF), which promote synaptic plasticity, learning, and memory (24). Moreover, physical activity modulates the hypothalamic–pituitary–adrenal (HPA) axis, thereby reducing stress reactivity and improving mood stability (25). Evidence from large epidemiological studies shows that physically active individuals have a significantly lower risk of dementia, depression, cardiovascular events, and premature mortality (26).
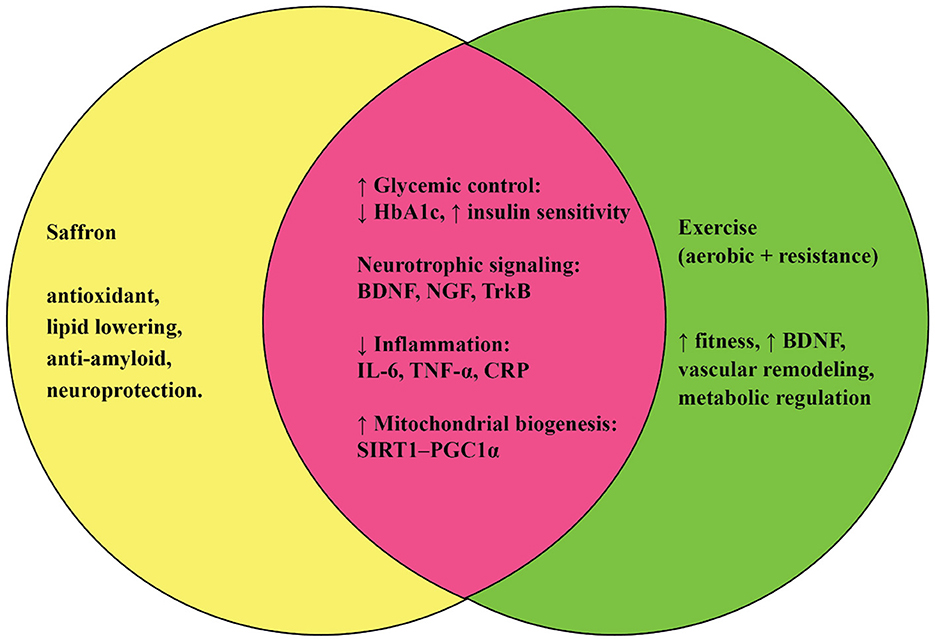
Interestingly, both saffron and physical activity appear to converge on similar molecular pathways, though they act through distinct upstream triggers. For instance, both interventions enhance mitochondrial function, reduce oxidative stress, and attenuate chronic low-grade inflammation, all of which are central to the biology of aging (27, 28). Both also regulate neurotransmitter systems and promote neurotrophic signaling, supporting cognitive resilience (29, 30). Moreover, they complement each other: while exercise directly improves metabolic health and muscular function, saffron provides bioactive compounds that can cross the blood–brain barrier and modulate neurochemical balance (15, 31). Together, these interventions may reinforce each other, creating synergistic effects that are greater than either strategy alone.
Another important dimension is their combined potential to improve the quality of life. Older adults often struggle with fatigue, sleep disturbances, reduced motivation, and emotional instability, which can undermine adherence to exercise regimens (32). Saffron supplementation, by improving mood, sleep quality, and energy (33), may enhance the capacity and willingness of individuals to engage in physical activity. Conversely, exercise may optimize the body's utilization and metabolism of saffron's bioactives, potentially amplifying their efficacy. Such bidirectional reinforcement highlights the value of integrative approaches that bridge nutraceutical and lifestyle domains.
The urgency of developing such integrative strategies is underscored by global policy frameworks. The World Health Organization has declared 2021–2030 the “Decade of Healthy Ageing,” emphasizing the need for holistic, person-centered interventions that enable older adults to maintain functional ability and wellbeing. This initiative calls for the development of evidence-based, low-cost strategies that can be implemented across diverse cultural and socioeconomic contexts (34, 35). Saffron, with its long-standing cultural acceptance and safety, combined with universally applicable exercise programs, aligns well with these global goals.
The present review aims to synthesize current evidence on the individual and combined effects of saffron supplementation and physical activity in aging. We specifically highlight their roles in modulating cognitive, emotional, and metabolic outcomes, and delineate shared and distinct molecular pathways that underlie these effects. By mapping areas of convergence and synergy, this review proposes an integrative framework for non-pharmacological strategies to enhance quality of life and resilience in older adults. Ultimately, combining saffron with structured exercise may represent a safe, accessible, and culturally adaptable approach to promote healthy longevity and mitigate the burden of age-related diseases.
2 Bioactive compounds of saffron
Saffron (Crocus sativus L.) is considered one of the most chemically diverse medicinal plants, with over 160 bioactive molecules identified in its floral stigmas, tepals, and corms (36). Among these, four compounds, crocin, crocetin, picrocrocin, and safranal (Figure 1), are regarded as the main active constituents that account for its unique sensory properties and its wide spectrum of biological activities (37). Crocin represents the most abundant apocarotenoid in saffron, responsible for its characteristic deep red coloration and high solubility in water (38). Beyond its role as a natural dye, crocin acts as a potent antioxidant, efficiently scavenging reactive oxygen species (ROS) and protecting neuronal and cardiovascular tissues from oxidative injury (39, 40). Experimental studies further highlight its neuroprotective actions, including enhancement of memory performance, attenuation of β-amyloid–induced toxicity in Alzheimer's disease models, and improvement of dopaminergic survival in Parkinson's models (41, 42). Crocin has also been linked to antidepressant-like effects, partly through modulation of serotonin and dopamine signaling (43, 44).
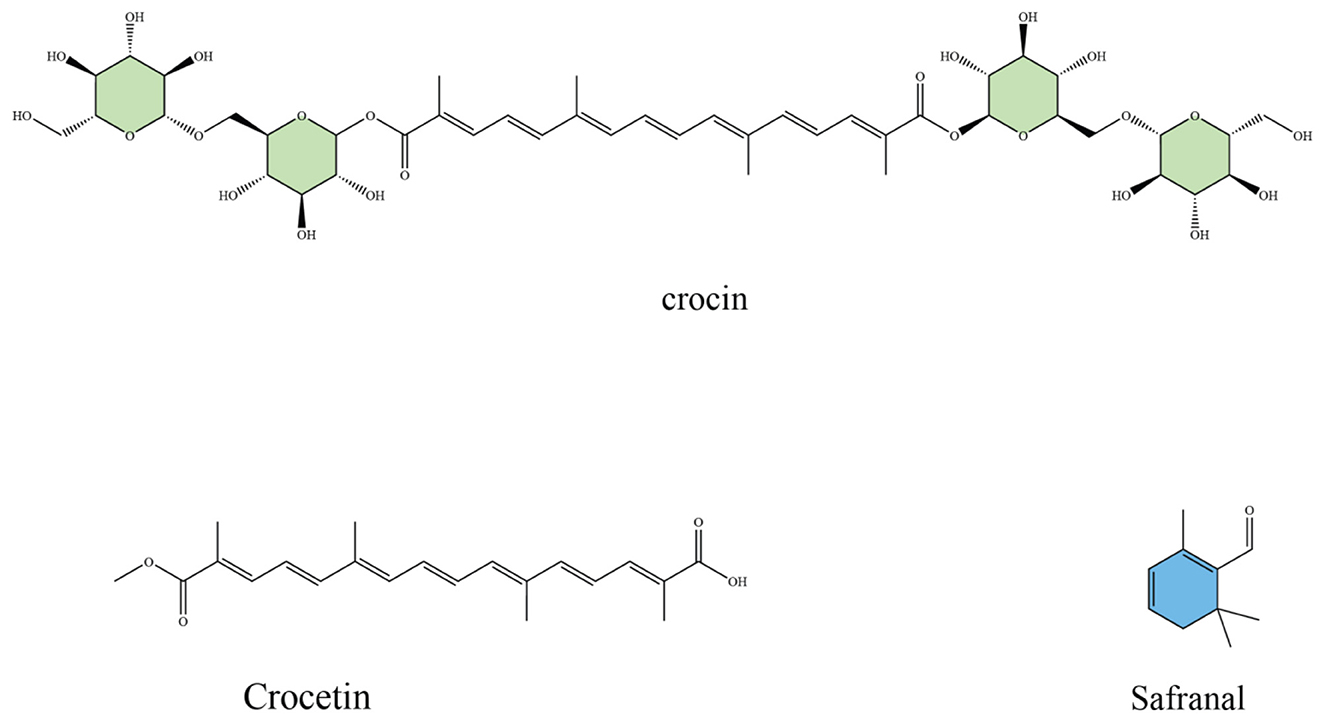
Figure 1. Chemical structures of major saffron bioactive constituents: crocin (a glycosylated carotenoid responsible for saffron's color and antioxidant activity), crocetin (an aglycone carotenoid with vascular and metabolic effects), and safranal (a monoterpene aldehyde contributing to aroma and neuroprotective properties).
Crocetin, the aglycone form of crocin, is a lipophilic carotenoid that contributes to saffron's orange–red hue. Although less abundant, crocetin exhibits important pharmacological effects such as suppression of lipid peroxidation, regulation of mitochondrial function, and anti-inflammatory activity via downregulation of pro-inflammatory cytokines (45, 46). Animal and human studies indicate that crocetin improves insulin sensitivity, lowers serum lipids, and protects vascular endothelium, suggesting a role in metabolic health and cardiovascular disease prevention (47–49).
Picrocrocin, a monoterpene glycoside, is the precursor of saffron's distinctive bitter flavor (50). While traditionally valued as a flavoring compound, picrocrocin has demonstrated bioactivity in experimental systems, including anti-proliferative effects on certain cancer cell lines and modulation of apoptotic pathways (51). Its breakdown during dehydration or storage leads to the formation of safranal, another major bioactive (52). Safranal, the main volatile aldehyde generated from picrocrocin, is primarily responsible for saffron's aroma (53). Despite being present in relatively low concentrations, safranal displays significant pharmacological effects. Studies have reported its strong antioxidant capacity, cytotoxicity against tumor cells, as well as antidepressant, anxiolytic, and anticonvulsant activities. Its role in neuronal protection has been attributed to both free radical scavenging and regulation of neurotransmitter systems (54).
Together, these compounds act through overlapping mechanisms, particularly antioxidant, anti-inflammatory, and anti-apoptotic pathways, that are central to aging-related disorders. In addition, they modulate neurotransmitter balance, support mitochondrial function, and regulate glucose and lipid metabolism, linking saffron not only to neuroprotection but also to cardiometabolic health. The unique combination of sensory attributes and pharmacological properties makes saffron an exceptional source of natural bioactives with wide therapeutic potential. Collectively, these molecular and physiological properties highlight saffron as a scientifically substantiated nutraceutical rather than merely a traditional remedy. Its key constituents, crocin, crocetin, safranal, and picrocrocin, act on convergent cellular pathways that regulate mitochondrial bioenergetics, neurotransmitter balance, and protein aggregation, providing a mechanistic justification for its antioxidant, anti-inflammatory, and neuroprotective efficacy. By bridging traditional ethnopharmacological knowledge with modern biochemical validation, saffron exemplifies how plant-derived bioactives can contribute to evidence-based preventive and therapeutic strategies in aging and neurodegenerative disease.
3 Saffron and age-related health
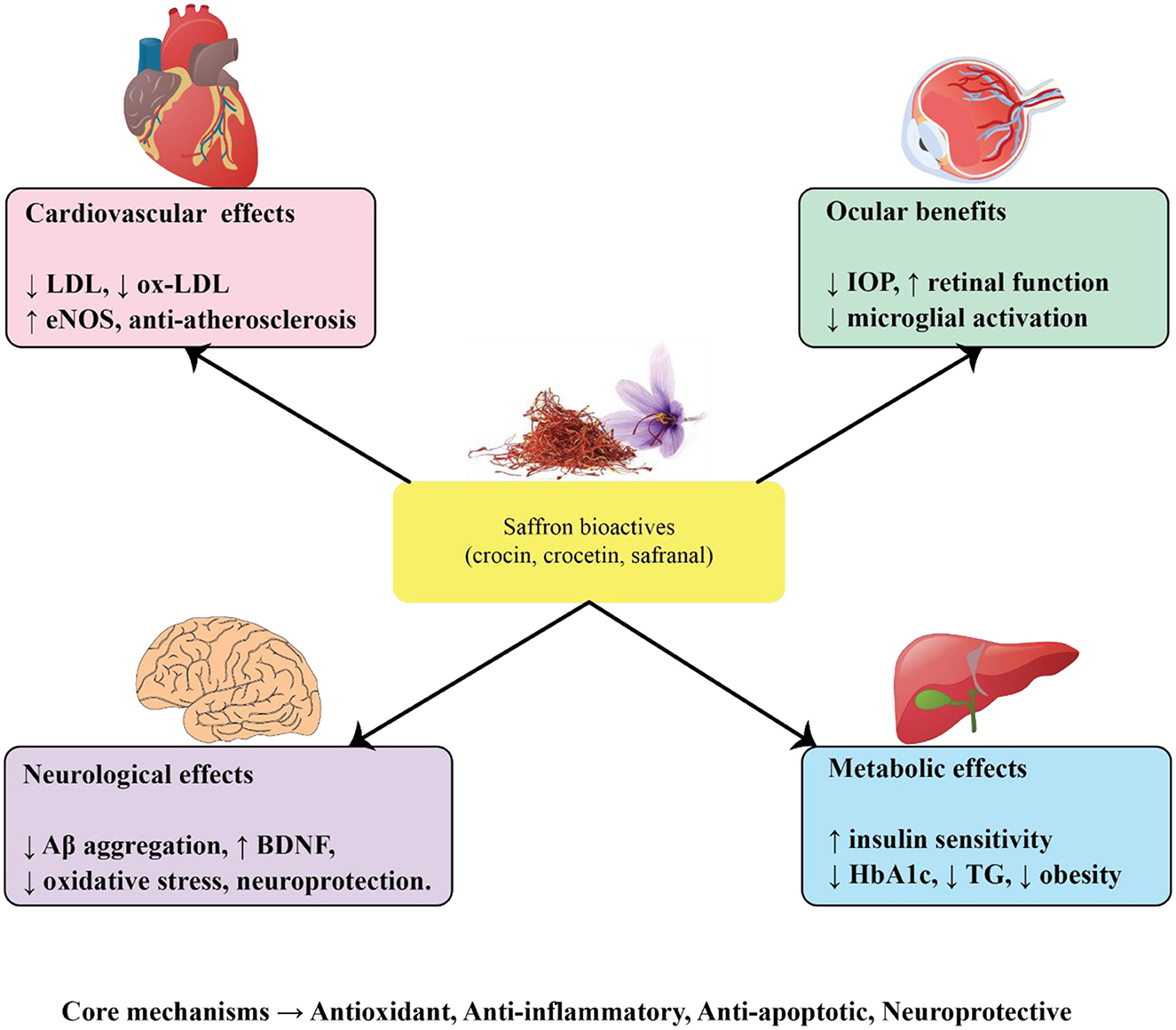
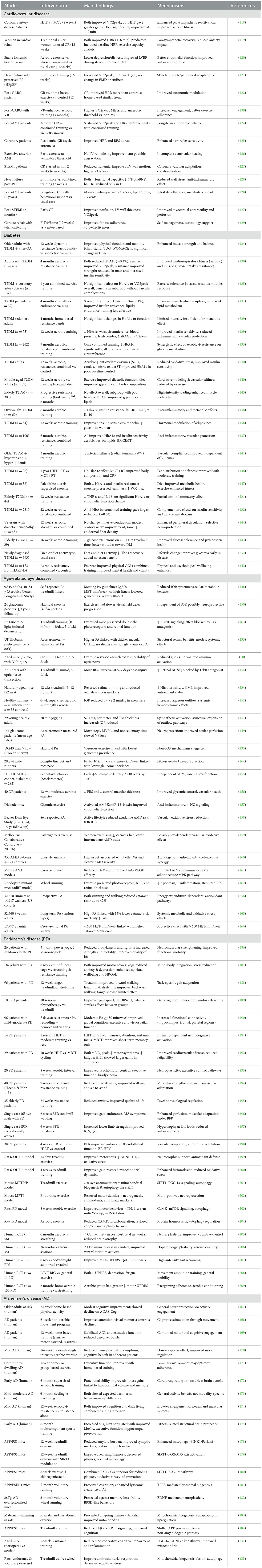
The progressive rise in age-related disorders, including cardiovascular disease, diabetes, ocular pathologies, and neurodegenerative conditions such as Alzheimer's and Parkinson's disease, represents a major global health challenge. A central theme across these disorders is the interplay of oxidative stress, chronic inflammation, mitochondrial dysfunction, and apoptotic cell loss. Saffron (Crocus sativus L.) and its major bioactive constituents, crocin, crocetin, and safranal, have drawn increasing scientific attention due to their antioxidant, anti-inflammatory, neuroprotective, and metabolic regulatory properties. Evidence from preclinical studies and clinical trials indicates that saffron may exert multi-target benefits that extend across vascular, metabolic, visual, and neurological domains of aging (Figure 2). To provide an integrative overview, the following subsections summarize saffron's effects on cardiovascular diseases, metabolic disorders, age-related eye diseases, and neurodegenerative conditions. A consolidated summary of experimental and clinical findings across these domains is presented in Table 1.
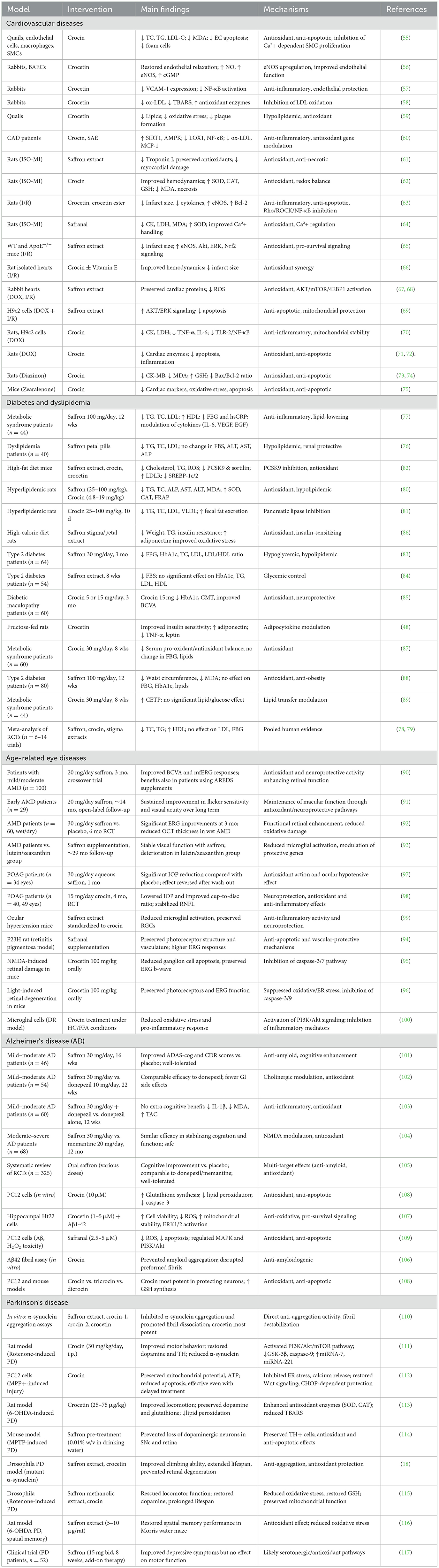
Table 1. Summary of preclinical and clinical evidence on saffron and its bioactive compounds in age-related cardiometabolic, neurodegenerative, and ocular diseases.
3.1 Saffron and cardiovascular diseases
Cardiovascular diseases (CVDs) remain the leading cause of death worldwide, with atherosclerosis, myocardial infarction, and heart failure forming the major pathological outcomes. Increasing evidence indicates that saffron and its active constituents, particularly crocin, crocetin, and safranal, exert protective effects on cardiovascular health through their antioxidant, anti-inflammatory, and anti-apoptotic properties. Experimental studies in animals, cellular models, and recent clinical trials provide a solid basis for their role as complementary therapeutic agents in cardiovascular disorders. Crocin has been shown to reduce the development of atherosclerosis in quails fed with a hyperlipidemic diet. Its administration significantly lowered serum cholesterol, triglycerides, and LDL-C, decreased malondialdehyde (MDA), and prevented nitric oxide (NO) depletion. Mechanistically, crocin improved endothelial cell survival by preventing oxidized LDL (ox-LDL)–induced apoptosis, reduced cholesterol ester accumulation in macrophages, and inhibited smooth muscle cell proliferation by modulating intracellular Ca2+ signaling. Collectively, these actions limited foam cell formation and plaque development, key processes in atherosclerosis initiation and progression (55).
Crocetin, another carotenoid derivative, has also demonstrated vascular protection. In hypercholesterolemic rabbits, crocetin restored acetylcholine-mediated endothelium-dependent relaxation by upregulating endothelial nitric oxide synthase (eNOS) activity and increasing cGMP levels, while leaving endothelium-independent vasodilation intact (56). Additionally, crocetin supplementation suppressed vascular cell adhesion molecule-1 (VCAM-1) expression, a pivotal mediator of leukocyte recruitment during atherogenesis, through inhibition of NF-κB activation (57). These results highlight its dual role in improving endothelial function and attenuating vascular inflammation.
Another study confirmed that crocetin supplementation reduced oxidative modification of LDL, thereby lowering ox-LDL and thiobarbituric acid reactive substances (TBARS) while enhancing antioxidant enzyme activity in rabbits with diet-induced hyperlipidemia (58). Similarly, quail models confirmed crocetin's anti-atherogenic effects, showing decreased serum lipid levels, reduced oxidative stress, and suppressed plaque formation (59). Together, these findings indicate that saffron bioactives act at multiple stages of atherosclerosis by regulating lipid metabolism, protecting endothelial cells, and modulating vascular inflammation.
Beyond animal studies, clinical investigations have begun to explore saffron's cardiovascular benefits. A randomized controlled trial in coronary artery disease (CAD) patients demonstrated that 8 weeks of crocin supplementation (30 mg/day) significantly reduced serum ox-LDL and MCP-1 while upregulating SIRT1 and AMPK gene expression and downregulating LOX1 and NF-κB in peripheral blood mononuclear cells (60). These molecular changes indicate crocin's capacity to improve endothelial health and reduce inflammatory signaling in humans, supporting its translational potential for atherosclerosis management.
Saffron extracts and isolated constituents also display cardioprotective effects in myocardial injury models. In isoproterenol-induced myocardial infarction (MI) in rats, saffron administration decreased serum troponin I, preserved glutathione peroxidase activity, and reduced histopathological damage (61). Crocin supplementation in a similar model improved blood pressure, ventricular function, and antioxidant enzyme activity, while lowering MDA and myocardial necrosis, suggesting potent protective effects against oxidative stress–mediated cardiac damage (62). Crocetin has also been reported to ameliorate ischemia-reperfusion (I/R) injury in rats by reducing infarct size, attenuating inflammatory cytokines (TNF-α, IL-1β, and IL-6), and enhancing antioxidant defenses. Its cardioprotective effect was linked to inhibition of the Rho/ROCK/NF-κB pathway and upregulation of anti-apoptotic proteins such as Bcl-2 (63). Similarly, safranal showed protective effects in isoproterenol-induced MI by reducing oxidative stress markers, modulating intracellular Ca2+ homeostasis, and improving myocardial structure (64).
Several studies also highlight saffron's role in ischemia-reperfusion injury and drug-induced cardiotoxicity. In mouse models, saffron aqueous extract significantly reduced infarct size and oxidative damage via activation of Akt/eNOS/ERK and Nrf2 pathways (65). Crocin was shown to reduce infarct size and improve hemodynamic parameters in isolated rat hearts subjected to ischemia-reperfusion, with effects comparable to vitamin E, and an even stronger protection when combined (66). Cardioprotection has also been observed against anthracycline-induced cardiotoxicity. Saffron extracts reduced oxidative stress and preserved cardiac architecture in isolated rabbit hearts exposed to doxorubicin (67, 68). In vitro, saffron extract limited apoptosis and mitochondrial damage in cardiomyocytes subjected to combined ischemia-reperfusion and doxorubicin exposure, mainly by activating AKT/ERK pathways (69). Crocin specifically protected against doxorubicin-induced myocardial toxicity by downregulating TLR-2/NF-κB signaling and restoring mitochondrial potential in both in vivo and in vitro models (70). Other studies confirmed crocin's role in reducing doxorubicin-induced cardiac apoptosis and inflammatory infiltration by normalizing Bax/Bcl-2 ratios, caspase-3 activation, and cytokine imbalance (71, 72).
Saffron constituents have also been evaluated against toxic cardiovascular insults. Crocin mitigated diazinon-induced cardiotoxicity in rats by reducing oxidative stress, restoring glutathione levels, and preventing mitochondrial-mediated apoptosis (73, 74). Likewise, crocin protected cardiac tissue from zearalenone-induced oxidative stress and apoptosis in mice, reducing serum cardiac markers and improving antioxidant status (75).
Overall, saffron and its bioactive molecules protect against cardiovascular injury through multiple complementary mechanisms: lowering serum lipids, limiting LDL oxidation, improving endothelial function, modulating inflammatory signaling, and preserving antioxidant defenses. Preclinical models consistently demonstrate benefits in atherosclerosis, ischemia, and cardiotoxicity, while early clinical data confirm anti-inflammatory and endothelial-protective effects in CAD patients. These findings suggest that saffron could serve as a low-risk nutraceutical adjunct for cardiovascular prevention and therapy, though larger human trials are warranted.
3.2 Saffron and its constituents in dyslipidemia and diabetes
Saffron and its active carotenoids have been increasingly investigated as potential adjuncts for managing dyslipidemia and diabetes, two interrelated metabolic disorders that strongly predispose individuals to cardiovascular complications. Clinical and preclinical studies consistently show that saffron exerts hypolipidemic, antioxidant, and insulin-sensitizing effects, although outcomes vary depending on preparation, dosage, and study population.
Randomized clinical trials in humans highlight saffron's lipid-lowering potential. In patients with dyslipidemia, supplementation with saffron petal extract significantly reduced triglycerides, total cholesterol, and LDL cholesterol while simultaneously improving renal function indices, with no evidence of hepatic toxicity (76). Another clinical study in individuals with metabolic syndrome reported similar benefits, demonstrating decreased triglycerides and LDL cholesterol alongside increased HDL levels and reductions in inflammatory cytokines, including IL-6 and VEGF (77). Such findings suggest that saffron addresses not only lipid imbalance but also the underlying inflammatory milieu characteristic of metabolic disorders. Supporting these outcomes, a systematic review and meta-analysis of randomized controlled trials confirmed that saffron supplementation reduces total cholesterol and triglycerides while modestly elevating HDL, although effects on LDL remain inconsistent across studies (78, 79).
Animal research provides mechanistic explanations for these clinical effects. Oral administration of saffron and its main carotenoid, crocin, in hyperlipidemic rats decreased triglycerides, cholesterol, and liver enzyme markers of injury while enhancing antioxidant defenses, such as superoxide dismutase and catalase, thereby attenuating lipid peroxidation (80). Crocin specifically reduced serum triglycerides and cholesterol in a dose-dependent manner through selective inhibition of pancreatic lipase, limiting fat absorption and promoting fecal excretion of cholesterol (81). More recently, saffron, crocin, and crocetin were identified as natural inhibitors of proprotein convertase subtilisin/kexin type 9 (PCSK9), a central regulator of LDL receptor degradation. In high-fat diet–fed mice, these compounds reduced cholesterol and triglycerides, alleviated hepatic steatosis, and downregulated PCSK9 and sortilin expression while upregulating LDL receptors and suppressing transcription factors SREBP-1c and SREBP-2, collectively improving lipid clearance (82). This newly identified pathway positions saffron as a nutraceutical with effects comparable to pharmacological PCSK9 inhibitors.
In addition to lipid regulation, saffron influences glucose metabolism and insulin sensitivity. Clinical trials in patients with type 2 diabetes have shown promising outcomes. In a 3-month randomized trial, saffron supplementation significantly lowered fasting plasma glucose, HbA1c, total cholesterol, and LDL cholesterol compared with placebo, without adverse effects on hepatic or renal function (83). Another trial confirmed that saffron hydroalcoholic extract reduced fasting blood glucose over 8 weeks, though no significant improvements were detected in HbA1c or lipid levels (84). Crocin supplementation has demonstrated benefits in diabetic complications; in patients with diabetic maculopathy, crocin at 15 mg/day not only decreased HbA1c and fasting glucose but also improved ocular outcomes such as central macular thickness and visual acuity (85).
Preclinical findings provide biological support for these outcomes. In fructose-fed rats, crocetin improved insulin sensitivity by enhancing adiponectin expression and suppressing tumor necrosis factor-α and leptin, thereby restoring the balance of adipocytokines that regulate glucose homeostasis (48). In obese rats fed a high-calorie diet, saffron stigma and petal extracts reduced insulin resistance, lowered body weight, and improved oxidative stress and lipid profiles, with stigma showing a greater effect, likely due to its higher content of phenolic compounds (86). Moreover, crocin supplementation in metabolic syndrome patients reduced oxidative stress markers by significantly lowering serum pro-oxidant/antioxidant balance, even though no major changes in fasting glucose or lipid profile were observed (87).
Nevertheless, some human trials report modest or no significant improvements in certain markers. For example, saffron supplementation in type 2 diabetic patients reduced waist circumference and oxidative stress marker malondialdehyde but did not significantly influence fasting glucose, insulin sensitivity, or lipid profile compared with placebo (88). Similarly, crocin supplementation increased cholesteryl ester transfer protein levels but produced no meaningful improvements in lipids or fasting glucose in metabolic syndrome subjects (89). These mixed results highlight the variability in responses and underscore the importance of factors such as dosage, intervention duration, and bioavailability of saffron formulations.
Taken together, the evidence indicates that saffron and its bioactive compounds exert a broad spectrum of actions on metabolic health. By lowering triglycerides and cholesterol, inhibiting PCSK9-mediated LDL receptor degradation, reducing oxidative stress, and improving adipocytokine signaling, saffron offers a multifaceted approach to ameliorating dyslipidemia and enhancing insulin sensitivity. Although several clinical trials report promising results, heterogeneity across studies underscores the need for well-designed, longer-term investigations using standardized saffron preparations to clarify its role as an adjunct therapy in dyslipidemia and diabetes management.
3.3 Saffron and age-related eye diseases
Age-related eye disorders such as age-related macular degeneration (AMD), glaucoma, diabetic retinopathy, and inherited retinal degenerations remain among the most common causes of irreversible vision loss worldwide. A shared hallmark in the pathogenesis of these conditions is the contribution of oxidative stress, mitochondrial dysfunction, inflammation, and progressive neuronal cell death. Since saffron and its bioactive constituents (crocin, crocetin, and safranal) exhibit strong antioxidant, anti-inflammatory, and neuroprotective properties, they have been investigated as adjunctive therapies to slow disease progression and preserve visual function. Evidence from clinical and experimental studies provides a growing rationale for saffron supplementation in several age-related ocular diseases.
AMD is one of the leading causes of blindness in the elderly. Several randomized controlled trials have shown that saffron can improve retinal function and visual outcomes in patients with both dry and wet forms of AMD. In a crossover clinical trial with over 100 participants, oral saffron supplementation (20 mg/day) led to measurable improvements in best-corrected visual acuity (BCVA) and multifocal electroretinogram (mfERG) responses when compared with placebo. Importantly, these benefits were observed even in patients already using standard Age-Related Eye Disease Study (AREDS) supplements, suggesting that saffron provides additional advantages beyond conventional antioxidant therapy (90). Longer-term studies have confirmed that saffron's benefits are not transient. In a follow-up trial spanning more than a year, patients with early AMD receiving daily saffron supplementation maintained improved flicker sensitivity and visual acuity compared with baseline, and these changes remained stable throughout the observation period (91). Other randomized trials with 6-month treatment durations also demonstrated functional gains, particularly in electroretinographic outcomes and macular sensitivity. In patients with wet AMD, saffron use was associated with reduced retinal thickness and improved electrophysiological parameters, although some of these effects diminished at longer follow-up intervals (92).
Animal and cellular studies provide mechanistic evidence explaining saffron's protective effects. In light-induced retinal degeneration models, saffron treatment preserved photoreceptor integrity, reduced microglial activation, and maintained visual function. These effects were linked to the modulation of matrix metalloproteinase activity and stabilization of retinal architecture (93). In inherited retinal degeneration models, safranal delayed photoreceptor loss and preserved retinal vasculature, while electroretinogram recordings demonstrated preserved retinal responses in treated animals compared with untreated controls (94). Crocetin, another active component, has been shown to protect against excitotoxic damage in NMDA-induced retinal injury in mice. Oral administration reduced ganglion cell apoptosis, maintained b-wave amplitudes in ERG recordings, and inhibited caspase activation, supporting its anti-apoptotic and neuroprotective role (95). Similar effects were observed in models of oxidative and endoplasmic reticulum stress, where crocetin reduced cell death and preserved mitochondrial membrane potential, again highlighting its ability to counter stress-induced degeneration (96).
Glaucoma is a chronic optic neuropathy characterized by retinal ganglion cell (RGC) degeneration, often associated with elevated intraocular pressure (IOP). In a pilot trial involving patients with primary open-angle glaucoma (POAG), oral saffron supplementation (30 mg/day) for 1 month produced a significant reduction in IOP when used in combination with conventional topical medications. After discontinuation, IOP values gradually returned to baseline, indicating that the effect is treatment-dependent (97).
A more recent randomized, triple-blind trial with crocin supplementation in POAG patients confirmed these findings. Crocin (15 mg/day for 4 months) significantly lowered IOP and improved cup-to-disc ratio compared to placebo. While best-corrected visual acuity and retinal nerve fiber layer thickness were not significantly altered, disease progression was stabilized, suggesting a potential neuroprotective role (98). Complementary preclinical studies further support these clinical outcomes. In mouse models of ocular hypertension, saffron extracts reduced microglial activation, attenuated neuroinflammation, and prevented RGC loss, indicating that saffron's benefits extend beyond pressure control and include direct neuronal protection (99).
Diabetic retinopathy (DR) represents another major cause of visual impairment in aging populations. Crocin has been shown to counteract oxidative stress and inflammation in microglial cells exposed to high glucose and free fatty acid conditions, mimicking the diabetic environment. Through activation of the PI3K/Akt pathway, crocin suppressed pro-inflammatory mediators, reduced oxidative markers, and promoted neuronal survival (100). These findings indicate that saffron may provide protection against early inflammatory and neurodegenerative processes in DR, although more clinical validation is needed.
Taken together, both clinical and experimental data support saffron as a promising adjunct therapy for several age-related eye diseases. In AMD, consistent improvements in retinal function, visual acuity, and contrast sensitivity have been reported across trials. In glaucoma, saffron and crocin supplementation lower intraocular pressure and provide neuroprotection. Preclinical studies extend these observations to inherited retinal degenerations and diabetic retinopathy, showing broad neuroprotective and anti-inflammatory effects. Although results are encouraging, most clinical studies have relatively short follow-up periods and modest sample sizes. Long-term, large-scale trials are necessary to establish the durability of benefits and to optimize dosage regimens. Nevertheless, the current body of evidence positions saffron as a safe, low-risk, and biologically active nutraceutical that holds therapeutic potential in the management of degenerative eye diseases in aging populations.
3.4 Saffron and its potential in Alzheimer's disease
Alzheimer's disease (AD) is the most prevalent form of dementia, characterized by progressive memory loss, impaired cognition, and functional decline. Its neuropathology involves amyloid-β (Aβ) plaques, neurofibrillary tangles of hyperphosphorylated tau, oxidative stress, and chronic neuroinflammation, all of which contribute to synaptic dysfunction and neuronal death. Available pharmacological treatments, such as cholinesterase inhibitors (e.g., donepezil) and N-methyl-D-aspartate (NMDA) receptor antagonists (e.g., memantine), provide only symptomatic relief and do not halt disease progression. This has led researchers to explore natural compounds with multi-target neuroprotective properties. Among these, saffron and its constituents, crocin, crocetin, and safranal, have attracted considerable interest for their antioxidant, anti-amyloid, and anti-inflammatory activities.
Several randomized clinical trials have investigated saffron supplementation in patients with mild to moderate AD. One of the earliest studies demonstrated that 30 mg/day of saffron extract over 16 weeks significantly improved cognitive scores, measured by the Alzheimer's Disease Assessment Scale–Cognitive Subscale (ADAS-cog) and the Clinical Dementia Rating scale, compared with placebo. Importantly, saffron was well-tolerated, and adverse events did not differ from those in the placebo group, suggesting a favorable safety profile (101). A subsequent 22-week multicenter study compared saffron with donepezil, a standard therapy for AD. Results showed that saffron had comparable efficacy to donepezil in improving cognition, with similar adverse event rates, except for vomiting, which was more frequent in the donepezil group (102). This finding raised the possibility that saffron may offer equivalent therapeutic benefits with fewer gastrointestinal side effects.
More recent work has examined saffron in combination with existing medications. A randomized double-blind trial in patients receiving donepezil found that adding saffron did not significantly enhance cognitive scores beyond donepezil alone, as measured by the Mini-Mental State Examination (MMSE). However, saffron supplementation significantly reduced inflammatory markers such as IL-1β and oxidative stress marker malondialdehyde (MDA), while improving total antioxidant capacity (103). This suggests that saffron may provide adjunctive systemic benefits, even if the additive effect on cognition is modest in patients already on pharmacological therapy.
In patients with more advanced AD, saffron was also compared with memantine. A 12-month randomized trial found no significant differences between the two treatments in cognitive decline, as measured by the Severe Cognitive Impairment Rating Scale (SCIRS) and Functional Assessment Staging (FAST). Both groups experienced stabilization of symptoms, and adverse event rates were similar (104). These findings further strengthen the argument that saffron may be considered as a therapeutic alternative or complementary approach in AD management. A systematic review of randomized controlled trials, including more than 300 participants, concluded that saffron supplementation consistently improved cognitive outcomes compared with placebo and produced results comparable to donepezil and memantine. Importantly, saffron was well-tolerated, and no serious safety concerns were reported (105).
While clinical findings support saffron's symptomatic benefits, preclinical studies have provided valuable insight into the underlying mechanisms. Crocin, the most abundant water-soluble carotenoid in saffron, has been shown to inhibit the aggregation of Aβ42 fibrils and even disrupt preformed fibrils. In vitro assays using thioflavin T fluorescence and electron microscopy demonstrated that crocin reduced amyloid load and altered peptide conformation, favoring less toxic structures (106). Crocetin, another carotenoid from saffron, demonstrated protective effects against Aβ1-42-induced toxicity in hippocampal-derived cells. Treatment with crocetin improved mitochondrial membrane potential, reduced reactive oxygen species, and increased cell viability. Moreover, crocetin promoted extracellular signal-regulated kinase (ERK1/2) activation, a pathway involved in neuronal survival (107). Animal studies have also confirmed the neuroprotective potential of saffron constituents. In rodent models, crocin prevented cell death under oxidative and hypoxic stress by enhancing glutathione synthesis and inhibiting caspase-3 activation, thereby reducing apoptosis (108). Similarly, crocetin administration in mice exposed to neurotoxic stimuli prevented retinal and neuronal cell damage by modulating caspase pathways and reducing oxidative burden (96). Safranal, a volatile component of saffron, has also demonstrated protective activity against Aβ-induced toxicity. In PC12 cell models, safranal reduced ROS production, attenuated apoptosis, and modulated MAPK and PI3K/Akt signaling pathways, both of which play central roles in neuronal survival and synaptic plasticity (109).
Neuroinflammation and oxidative stress are recognized as central drivers of AD progression. Several studies have demonstrated that saffron reduces pro-inflammatory cytokines such as TNF-α and IL-6 while enhancing antioxidant defenses. Clinical data confirm these effects, with saffron supplementation lowering circulating markers of lipid peroxidation and boosting enzymatic antioxidants like superoxide dismutase (SOD) and glutathione peroxidase (GPx). This systemic improvement in redox balance may help slow neurodegeneration indirectly by reducing vascular and metabolic stressors that exacerbate AD pathology (103).
Saffron represents a safe and potentially effective complementary therapy for Alzheimer's disease. Clinical trials have shown that saffron supplementation improves cognitive outcomes in mild to moderate AD, with efficacy comparable to established drugs such as donepezil and memantine. Preclinical evidence highlights crocin, crocetin, and safranal as the key compounds mediating neuroprotection through anti-amyloid, antioxidant, and anti-inflammatory effects. Although larger and longer trials are necessary, current evidence positions saffron as a promising natural candidate for integrative management of Alzheimer's disease.
3.5 Saffron and Parkinson's disease
Parkinson's disease (PD) is a progressive neurodegenerative disorder marked primarily by the selective loss of dopaminergic neurons in the substantia nigra and the pathological accumulation of misfolded α-synuclein. These events lead to characteristic motor impairments, such as tremor, rigidity, and bradykinesia, as well as non-motor symptoms including depression and cognitive decline. Current pharmacological approaches, most notably levodopa and dopamine agonists, alleviate symptoms but do not halt the underlying neurodegeneration. This therapeutic gap has prompted growing interest in natural compounds with antioxidant, anti-inflammatory, and anti-apoptotic potential. Among these, saffron and its main bioactive components have been increasingly investigated for their neuroprotective properties in PD.
One of the hallmarks of PD pathology is the misfolding and aggregation of α-synuclein into toxic fibrils that form Lewy bodies. Inoue et al. (110) showed that saffron extracts and specific constituents, particularly crocin-1, crocin-2, and crocetin, were capable of both preventing α-synuclein aggregation and breaking down preformed fibrils. Using thioflavin T assays and electron microscopy, they observed that these molecules shortened and reduced fibrillar structures, with crocetin being the most potent. Such anti-aggregation effects suggest that saffron may intervene at an upstream stage of PD pathology by directly targeting misfolded proteins responsible for neuronal toxicity (110). Oxidative stress and apoptosis contribute heavily to dopaminergic cell death. Salama et al. (111) explored crocin's role in rotenone-induced PD in rats, a model that closely mimics the mitochondrial dysfunction observed in patients. Administration of crocin not only improved behavioral deficits but also activated the PI3K/Akt/mTOR signaling pathway, a critical regulator of neuronal survival. This modulation resulted in reduced activity of glycogen synthase kinase-3β (GSK-3β) and caspase-9, alongside decreased α-synuclein accumulation. Crocin also upregulated microRNA-7 and microRNA-221, which reinforced Akt/mTOR signaling. These findings indicate that crocin protects dopaminergic neurons through a multi-layered mechanism that integrates survival signaling, mitochondrial stabilization, and reduced apoptosis (111).
Mitochondrial dysfunction and endoplasmic reticulum (ER) stress are both implicated in PD progression. Experiments in MPP+-treated PC12 cells revealed that crocin preserved mitochondrial membrane potential, maintained ATP production, and reduced apoptotic cell death even when treatment was delayed after injury (112). These benefits were associated with suppression of ER stress markers, regulation of calcium release, and restoration of Wnt signaling pathways. Such findings underscore crocin's ability to interfere with multiple damaging cascades triggered by mitochondrial toxins, highlighting its therapeutic versatility. Crocetin, another major saffron carotenoid, has also shown efficacy in PD models. In a 6-hydroxydopamine (6-OHDA) rat model, crocetin treatment improved locomotor function, preserved dopamine levels in the striatum, and restored antioxidant enzyme activity in both the striatum and substantia nigra (113). Markers of lipid peroxidation were significantly reduced, and histopathological analysis confirmed preservation of nigral neurons. These effects support the notion that crocetin counteracts oxidative stress that drives dopaminergic cell death, thereby offering neuroprotection.
The neuroprotective effects of saffron have also been demonstrated in whole-animal models. In a mouse model of PD induced by MPTP, saffron pre-treatment prevented the typical loss of tyrosine hydroxylase-positive neurons in both the substantia nigra and the retina (114). Similarly, experiments in Drosophila overexpressing mutant forms of α-synuclein showed that saffron and crocetin preserved climbing ability, extended lifespan, and protected against retinal degeneration (18). Rao et al. (115) further confirmed these findings in a rotenone-induced fly model, showing that saffron extract and crocin reduced oxidative stress, restored glutathione, preserved dopamine levels, and delayed locomotor decline. While PD is primarily recognized as a motor disorder, patients often suffer from cognitive deficits and mood disturbances. In rat models, saffron extract improved spatial memory performance impaired by 6-OHDA injection (116). Clinically, adjunct saffron supplementation has been shown to significantly improve depressive symptoms in PD patients, though without significant benefit on motor outcomes (117). These results indicate that saffron may play a supportive role in addressing non-motor complications of PD, which substantially affect patients' quality of life. Evidence from in vitro experiments, animal models, and early-phase clinical trials strongly supports saffron's neuroprotective role in Parkinson's disease. Its bioactive constituents, crocin, crocetin, and safranal, target diverse pathological mechanisms, including α-synuclein aggregation, oxidative stress, mitochondrial dysfunction, ER stress, and inflammation. Additionally, saffron shows promise in alleviating depressive symptoms associated with PD. While larger clinical trials are required to determine its definitive therapeutic value, saffron appears to be a safe and promising adjunctive strategy for improving both neurological and psychological outcomes in PD.
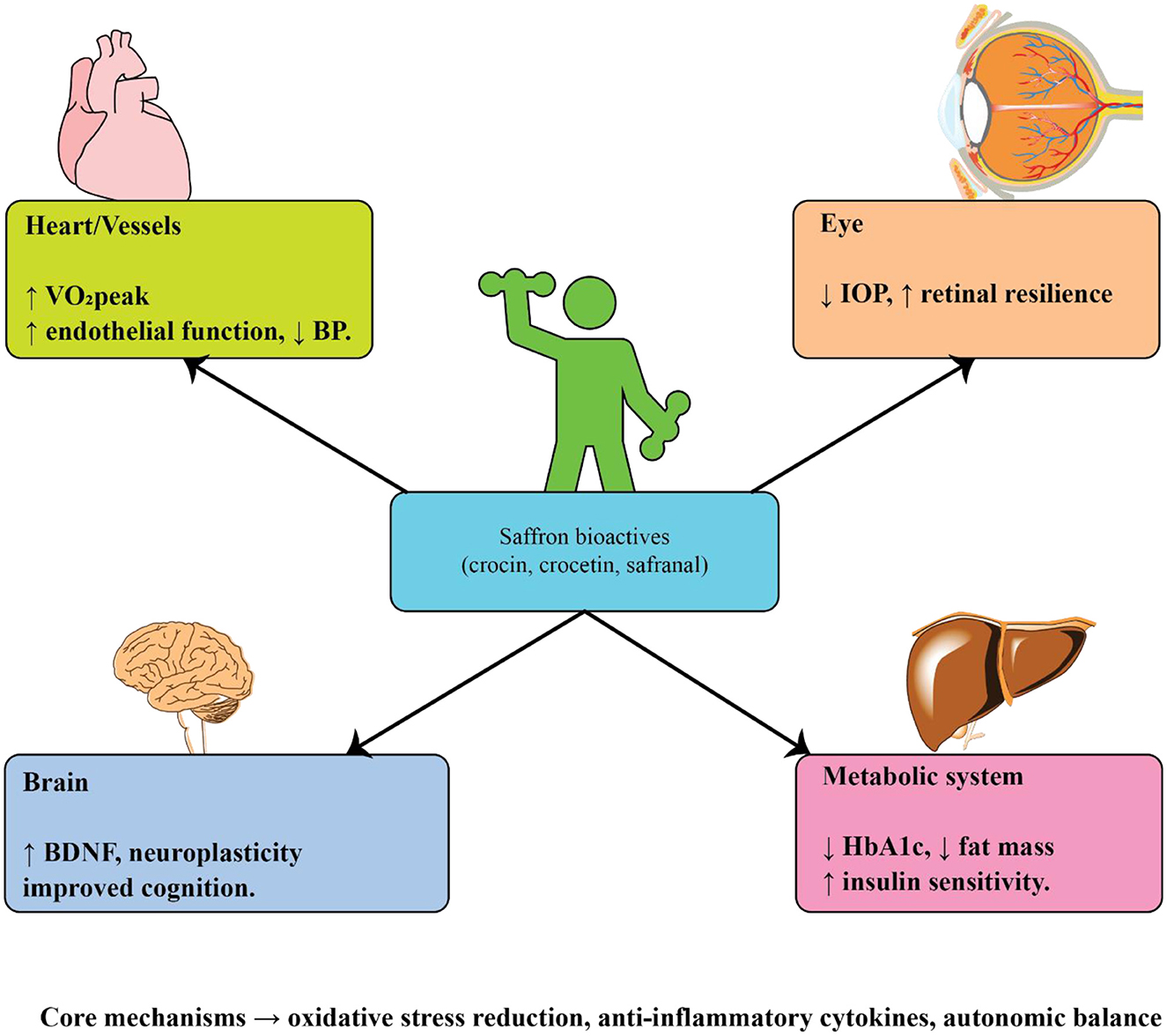
4 Exercise and age-related health
Regular physical activity is one of the most effective lifestyle interventions for preventing and managing chronic age-related diseases. Beyond improving general fitness, structured exercise exerts wide-ranging benefits on cardiovascular function, metabolic regulation, cognitive performance, and psychological wellbeing. Evidence from epidemiological studies, randomized clinical trials, and experimental animal models consistently shows that exercise reduces morbidity and enhances quality of life in older populations. Mechanistically, these effects are mediated through improvements in autonomic regulation, mitochondrial function, neurotrophic support, vascular integrity, and inflammatory balance. Given the multidimensional role of exercise, its impact has been extensively studied in cardiovascular diseases, type 2 diabetes, age-related eye disorders, Alzheimer's disease, and Parkinson's disease (Figure 3). A comprehensive summary of key experimental and clinical findings across these conditions is provided in Table 2, which highlights the diversity of exercise modalities, patient populations, and mechanistic pathways investigated to date.
4.1 Exercise and cardiovascular diseases
Cardiovascular diseases (CVD) remain the leading cause of morbidity and mortality worldwide, and their management increasingly incorporates lifestyle-based interventions alongside pharmacological care. Exercise training has been identified as a cornerstone in both primary and secondary prevention of coronary artery disease, myocardial infarction, and heart failure. The beneficial effects of exercise are mediated through improvements in autonomic function, myocardial perfusion, vascular function, metabolic capacity, and psychosocial wellbeing. Evidence from randomized clinical trials and rehabilitation studies strongly supports its role in enhancing prognosis and quality of life across a broad spectrum of cardiac patients. One of the most widely studied markers of autonomic balance and prognosis in cardiac patients is heart rate recovery (HRR) after exercise. HRR reflects parasympathetic reactivation following exertion and has been shown to predict long-term survival. Villelabeitia-Jaureguizar et al. (118) compared high-intensity interval training (HIIT) with moderate continuous training (MCT) in patients with stable coronary artery disease. Both programs significantly improved peak oxygen uptake (VO2peak), but HIIT induced larger gains in both VO2peak and HRR at 1 and 2 min post-exercise, suggesting a superior effect on autonomic recovery and aerobic fitness. These findings highlight HIIT as a safe and more effective option than traditional continuous exercise in low-risk coronary patients (118). Similarly, Beckie et al.(119) examined women undergoing cardiac rehabilitation (CR) and found that both traditional and women-tailored CR programs improved HRR between 1 and 6 min after exercise cessation. Although no difference was observed between program types, both significantly enhanced autonomic recovery. Predictors of better HRR improvement included baseline HRR, higher exercise capacity measured in metabolic equivalents (METs), and reduced anxiety levels, whereas older age and insulin use were associated with poorer responses. These results emphasize the role of both physical capacity and psychosocial health in mediating the cardiovascular benefits of exercise (119).
Beyond HRR, exercise training also influences psychological wellbeing and vascular health in ischemic heart disease. A randomized trial by Blumenthal et al. (120) evaluated aerobic exercise and stress management in patients with stable ischemic heart disease. Compared with usual medical care, both exercise and stress management groups showed significant reductions in depression and general distress, smaller decreases in left ventricular ejection fraction during stress testing, and improved flow-mediated dilation. In addition, stress management enhanced baroreflex sensitivity and heart rate variability, further supporting the integrative benefits of behavioral interventions alongside exercise (120). In the setting of heart failure with preserved ejection fraction (HFpEF), exercise training has also demonstrated functional benefits. Kitzman et al. (121) randomized older patients with HFpEF to 16 weeks of endurance training or attention control. The exercise group showed significant improvements in VO2peak and quality of life, although measures of endothelial function and arterial stiffness remained unchanged. These results indicate that gains in exercise capacity may be driven more by skeletal muscle adaptations and peripheral oxygen utilization than by central vascular changes in this population (121).
Post-surgical cardiac patients also benefit from structured exercise programs. Wu et al. (122) compared cardiac rehabilitation with home-based exercise after coronary artery bypass grafting (CABG). Both approaches improved HRR compared with baseline, but only the supervised CR group showed statistically greater improvements over the control group. This suggests that while home-based exercise is helpful, structured and supervised programs may yield superior autonomic recovery (122). Extending this concept, Chuang et al. (123) introduced virtual reality (VR) to enhance patient engagement in post-CABG exercise. Their study revealed that patients in the VR-enhanced group achieved higher VO2peak, METs, and anaerobic threshold values than those in conventional programs, supporting the role of novel technologies in optimizing rehabilitation outcomes (123).
The long-term impact of exercise training on autonomic recovery has also been explored. Giallauria et al. (124) studied patients after acute myocardial infarction (AMI) who completed a 3-month hospital-based exercise program. Those who continued structured home-based training maintained improvements in HRR and VO2peak at 6 months, while those with only general advice experienced a decline. These findings underscore the need for ongoing, structured activity to preserve cardiovascular benefits beyond the initial rehabilitation period (124). Additional insights come from Legramante et al. (125), who found that residential cardiac rehabilitation improved HRR and baroreflex sensitivity in coronary artery patients. The parallel improvement in HRR and baroreflex measures further validates HRR as a simple, clinically meaningful marker of exercise-induced autonomic adaptation (125).
Exercise is not without caution. Kubo et al. (126) investigated patients with extensive anterior AMI and reduced ejection fraction, randomized to early supervised exercise at ventilatory threshold vs. standard care. While control patients showed reductions in left ventricular volumes, those in the exercise group exhibited no such improvement, and some measures suggested worsening remodeling. These results highlight the need for careful patient selection and timing, as exercise during early ventricular healing may aggravate adverse remodeling in severe infarcts (126).
Nonetheless, most studies support exercise as safe and beneficial when introduced appropriately. For example, Giallauria et al. (127) showed that beginning exercise rehabilitation within 2 weeks after STEMI reduced stress-induced myocardial hypoperfusion, improved wall motion and ejection fraction, and prevented unfavorable remodeling, leading to better functional recovery. Long-term programs combining educational and behavioral interventions, as evaluated by the same group, further demonstrated sustained improvements in VO2peak, lipid profile, and reduced clinical events over 2 years in post-AMI patients (127). Exercise also is proven to be effective in patients undergoing percutaneous coronary intervention (PCI). Abolahrari-Shirazi et al. (128) compared endurance training (ET) with combined endurance-resistance training (CT) in heart failure patients after PCI. Both exercise regimens significantly improved functional capacity and reduced NT-proBNP levels, while CRP decreased only in the endurance group. These findings indicate that both modalities are safe and effective, with subtle differences in inflammatory and biomarker responses (128). Recent strategies also seek to expand rehabilitation access through home-based and telemonitored approaches. The FIT@Home study compared 12 weeks of telemonitored home-based training with traditional center-based rehabilitation in low-to-moderate risk patients. Both approaches improved physical fitness and activity levels, but telemonitoring offered additional benefits in adherence, self-efficacy, and cost-effectiveness. This reflects a broader trend toward integrating technology to extend long-term rehabilitation support outside the hospital (129).
Taken together, these studies consistently demonstrate that exercise exerts beneficial effects on multiple domains of cardiovascular health. Improvements include enhanced autonomic recovery (HRR, baroreflex sensitivity), increased VO2peak and functional capacity, better myocardial perfusion, reduced ischemia, improved left ventricular function, and favorable effects on psychological wellbeing. However, benefits depend on exercise modality, intensity, patient-risk profile, and timing of initiation. High-intensity interval training, when appropriately prescribed, appears particularly effective in improving aerobic fitness and autonomic recovery, whereas structured long-term programs are crucial for maintaining sustained cardiovascular protection. Thus, exercise training represents an essential non-pharmacological intervention in the prevention and management of cardiovascular diseases. Across diverse patient populations, from those recovering from myocardial infarction or bypass surgery to individuals with stable coronary artery disease or heart failure, structured physical activity improves autonomic balance, myocardial function, vascular health, and overall quality of life. While caution is warranted in high-risk or recently infarcted patients, the overall evidence strongly supports exercise as a safe, adaptable, and cost-effective component of cardiovascular care.
4.2 Exercise and type 2 diabetes
Type 2 diabetes mellitus (T2DM) is a chronic disease characterized by hyperglycemia, insulin resistance, and progressive β-cell dysfunction. Alongside pharmacological therapy, lifestyle interventions, particularly exercise, are considered essential for glycemic control and reduction of long-term complications. Different forms of physical activity, including aerobic, resistance, and combined modalities, have been studied for their effects on glycemic control, cardiovascular fitness, inflammation, and quality of life in patients with T2DM. The evidence shows that while results vary depending on exercise type, intensity, and patient comorbidities, structured training consistently improves metabolic health and functional outcomes. Both aerobic and resistance exercise offer benefits for patients with T2DM, but their effects on metabolic markers and functional capacity differ. A randomized trial compared aerobic and resistance training over 4 months and found that both modalities significantly reduced HbA1c by approximately 0.35–0.40%, with concurrent improvements in insulin sensitivity, lean body mass, and reductions in visceral and subcutaneous adiposity. Aerobic training more effectively enhanced cardiorespiratory fitness, while resistance training was superior for muscle strength. This suggests that each modality targets different components of physical health but provides similar metabolic improvements (130). A study by Cauza et al. (131) highlighted that resistance training might offer particular advantages. In their trial, patients who engaged in strength training demonstrated a significant reduction in HbA1c (from 8.3% to 7.1%), improved insulin sensitivity, and favorable changes in lipid profiles, including increased HDL and reduced triglycerides. By contrast, aerobic training did not produce significant changes in glycemic markers or lipids in that study, suggesting that resistance training may play a central role in metabolic management for patients with poor baseline control (131).
The integration of aerobic and resistance training may yield the most pronounced benefits. Sigal et al. (132) reported that combined training reduced HbA1c by 0.5% compared to sedentary controls, a greater reduction than achieved by aerobic or resistance training alone (132). Similarly, Church et al. (133) demonstrated that only the combined program significantly improved HbA1c, while single-modality training alone was insufficient. These results emphasize that combination training enhances glycemic control through complementary mechanisms, such as improving both cardiovascular efficiency and muscle glucose uptake (133).
The presence of comorbidities may influence exercise responses. Chen et al. (134) studied older patients with T2DM and knee osteoarthritis and compared dynamic vs. isometric resistance training. Both improved functional performance, but dynamic resistance training yielded superior outcomes in strength and mobility. However, neither intervention significantly altered HbA1c. This highlights that exercise improves quality of life and mobility even when direct glycemic effects are modest, particularly in populations with multiple chronic conditions (134). Similarly, Byrkjeland et al. (135) examined patients with T2DM and coronary artery disease and reported no overall significant improvement in HbA1c or VO2peak after 1 year of combined training. Yet, patients without prior myocardial infarction or microvascular complications did show improvements, suggesting that the degree of vascular disease modifies exercise effectiveness. Importantly, even when glycemic benefits were limited, exercise improved ventilatory threshold and time to exhaustion, reflecting enhanced functional performance (135).
Chronic low-grade inflammation is closely linked with insulin resistance and cardiovascular risk in T2DM. Aerobic exercise appears particularly effective in reducing inflammatory markers. Kadoglou et al. (136) showed that 6 months of aerobic training reduced hsCRP and IL-18 while increasing IL-10, thereby shifting the balance toward an anti-inflammatory state. Another study by Kadoglou et al. (137) found that aerobic training reduced carotid intima-media thickness progression, which was independently associated with changes in inflammatory markers and improvements in VO2peak. These findings suggest that the vascular protective effects of exercise extend beyond glycemic control (137). Exercise also influences novel adipokines. Kadoglou et al. (138) demonstrated that aerobic exercise increased circulating apelin and improved insulin sensitivity, while ghrelin responses were modest and gender-dependent. These results highlight exercise-induced hormonal modulation as an additional mechanism supporting vascular and metabolic health (138).
Exercise interventions frequently improve body composition and cardiovascular function. Choi et al. (139) reported that aerobic exercise increased soluble receptor for advanced glycation end-products (sRAGE) and reduced body weight, waist circumference, and blood pressure, in parallel with decreased hsCRP. Improvements in sRAGE may provide vascular protection by neutralizing harmful AGE interactions (139). Gulsin et al. (140) further showed that supervised aerobic training improved diastolic function in middle-aged adults with T2DM, even without substantial weight loss, underscoring the independent cardiovascular benefits of exercise (140). A trial by Madden et al. (141) demonstrated that 3 months of aerobic training reduced arterial stiffness in older adults with T2DM and comorbid hypertension and hyperlipidemia. Notably, these benefits occurred without significant improvement in VO2max, suggesting that vascular adaptations may occur independently of cardiorespiratory capacity (141).
Exercise also enhances daily functioning and overall quality of life (QOL). Myers et al. (142), in the HART-D study, showed that aerobic, resistance, and combined training improved physical health scores compared to controls, with combined training producing additional benefits in vitality and mental health domains. Improvements in physical function are especially important in older diabetic adults, who face an increased risk of disability (142). Tessier et al. (143) also demonstrated that aerobic exercise reduced glucose excursions during oral glucose tolerance testing and improved attitudes toward diabetes in older participants, suggesting psychosocial as well as metabolic benefits (143). Exercise may help preserve nerve function in diabetic neuropathy. Stubbs et al. (144) studied veterans with length-dependent polyneuropathy and found that while nerve conduction parameters were largely unchanged, exercise modestly improved sensory nerve function and, in some cases, epidermal nerve fiber density. This suggests potential neuroprotective effects that require confirmation in larger studies (144).
Novel training protocols are being explored to enhance outcomes. Hangping et al. (145) tested a high-intensity progressive resistance training method (bioDensity™) in older Chinese patients. While overall effects on HbA1c were not significant, patients with poor baseline control (HbA1c >7.5%) showed meaningful improvements, as well as favorable lipid changes (145). Similarly, Magalhães et al. (146) compared high-intensity interval training (HIIT) plus resistance training vs. moderate-intensity continuous training plus resistance training. Neither improved HbA1c, but moderate continuous training improved body composition and fitness, reinforcing that not all exercise intensities translate into glycemic benefits (146).
Otten et al. (147) combined a Paleolithic diet with or without supervised exercise and found that both groups improved insulin sensitivity and HbA1c, but exercise preserved lean mass and increased cardiovascular fitness, indicating an additive role of physical activity in structured lifestyle programs. Collectively, evidence supports the role of exercise as a cornerstone of type 2 diabetes management. Aerobic and resistance training independently improve glycemic control, insulin sensitivity, and body composition, while combined training offers the most consistent improvements in HbA1c. Beyond metabolic effects, exercise reduces inflammation, improves vascular health, enhances functional performance, and contributes to a better quality of life. While outcomes vary depending on comorbidities and baseline metabolic health, structured and sustained physical activity remains one of the most effective non-pharmacological strategies for improving overall outcomes in individuals with type 2 diabetes.
4.3 Exercise and age-related eye diseases
Age-related eye disorders such as glaucoma, diabetic retinopathy (DR), age-related macular degeneration (AMD), and cataracts are among the leading causes of visual impairment and blindness in older adults. Increasing evidence highlights physical activity as a low-cost, accessible intervention with the potential to preserve visual health by modulating intraocular pressure (IOP), retinal metabolism, oxidative stress, and neurotrophic signaling. Findings from epidemiological studies, clinical trials, and experimental animal models collectively suggest that exercise exerts protective effects across multiple ocular pathologies, though the mechanisms and magnitude of benefits vary between diseases.
Glaucoma is strongly linked to age and characterized by progressive optic neuropathy and visual field (VF) loss. Physical activity appears to influence both disease onset and progression. In a large prospective cohort (n ≈ 9,500 adults, mean age 50), individuals meeting weekly activity recommendations (>500 MET-min/week) had nearly half the risk of developing glaucoma compared with inactive peers. High cardiorespiratory fitness, measured by treadmill testing, further reduced risk, and the combination of both behaviors conferred the lowest hazard ratio (148). Other longitudinal studies reinforce these associations. Among older adults with glaucoma, accelerometer-measured steps and time in moderate-to-vigorous activity correlated with slower VF deterioration. Specifically, an additional 5,000 steps per day or >2.5 h of non-sedentary activity reduced VF loss by approximately 10% (149). Retrospective work further showed that patients who reported regular exercise experienced slower progression of glaucomatous visual field defects, even when mean IOP values were similar between exercisers and non-exercisers, suggesting benefits beyond pressure reduction (150).
Mechanistic investigations help explain these findings. Jogging and other aerobic activities acutely reduce IOP, likely through increased aqueous outflow mediated by sympathetic stimulation and expansion of the trabecular meshwork and Schlemm's canal (151). Controlled interventions in healthy volunteers show that 6 weeks of supervised aerobic and strength training lowered mean IOP by more than 2 mmHg, while no changes were seen in controls (152). Animal studies provide additional mechanistic depth: treadmill and swimming exercise in aged rodents enhanced resilience of the optic nerve to pressure injury, reduced retinal gliosis, and normalized macrophage activation (153, 154). Collectively, these data indicate that exercise protects retinal ganglion cells (RGCs) through both pressure-dependent and neurotrophic mechanisms, the latter largely mediated by exercise-induced increases in BDNF.
DR is a microvascular complication of diabetes that accelerates with age and poor metabolic control. Physical activity may play dual roles in improving systemic glucose regulation and protecting retinal tissue directly. Cross-sectional U.S. data revealed that sedentary behavior independently predicted higher odds of DR in adults with diabetes, even after adjusting for total activity and glycemic parameters (155). This suggests that prolonged inactivity may contribute to microvascular damage irrespective of exercise levels. Clinical trials confirm the benefits of structured aerobic exercise. In a 12-week intervention with moderate-intensity training, patients with non-proliferative DR experienced significant reductions in fasting blood glucose and central macular thickness, both markers of disease progression (156). Exercise-induced improvements in systemic metabolism, such as enhanced insulin sensitivity and reduced vascular inflammation, are thought to underlie these retinal outcomes. Preclinical studies support these mechanisms: in diabetic mice, endurance training activated the AMPK/miR-181b signaling axis, which alleviated endothelial dysfunction and reduced inflammatory markers relevant to DR progression (157). Together, these findings highlight exercise as a modifiable factor capable of reducing both systemic and ocular risks in diabetic patients.
AMD, particularly its neovascular form, is a major cause of irreversible blindness in older populations. Early population-based data from the Beaver Dam Eye Study showed that individuals reporting regular physical activity at baseline were less likely to develop exudative AMD 15 years later (158). Similarly, the Melbourne Collaborative Cohort Study linked frequent vigorous activity, particularly in women, with reduced odds of intermediate AMD (159). Lifestyle analyses in AMD patients further demonstrate that higher physical activity scores are associated with better visual acuity and slower disease progression, likely due to enhanced antioxidant defenses and reduced oxidative stress (160). Mechanistic work in mouse models indicates that exercise reduces choroidal neovascularization by inhibiting AIM2 inflammasome activation in myeloid cells. Serum from exercised animals transferred similar benefits to sedentary counterparts, suggesting that systemic mediators, including adiponectin, may play a role (161). These findings collectively point to exercise as a protective factor in AMD, possibly delaying onset and enhancing responses to anti-VEGF therapies.
Beyond AMD and DR, exercise has shown neuroprotective effects in inherited and light-induced retinal degeneration models. Wheel running in mice carrying rhodopsin mutations (I307N) prevented photoreceptor apoptosis, preserved retinal thickness, and reduced inflammatory responses (162). Earlier studies demonstrated similar protection in light-induced degeneration, with exercised animals retaining twice as many photoreceptors compared to sedentary controls, an effect abolished by blocking BDNF signaling (163). These data strongly suggest that systemic exercise elevates neurotrophic support within the retina, contributing to resilience against degenerative stress.
Cataracts are the leading cause of blindness worldwide and are closely tied to aging. Epidemiological studies show that habitual walking and running are associated with reduced cataract risk. In large cohorts of U.S. runners and walkers, cataract incidence declined linearly with increasing exercise energy expenditure, with up to a 42% lower risk among the most active individuals (164). Similarly, Swedish population-based data demonstrated that long-term high physical activity, particularly occupational activity and regular walking or cycling, was linked with a 13–24% decreased risk of cataract (165). Spanish survey data confirmed that meeting WHO activity guidelines (>600 MET-min/week) was associated with lower cataract prevalence, especially in older age groups (166). Although mechanisms are not fully defined, exercise may prevent cataracts by improving systemic antioxidant capacity, reducing oxidative stress in the lens, and lowering chronic inflammation, factors known to contribute to lens opacification.
Across ocular conditions, several common mechanisms appear to underlie exercise benefits. These include acute and chronic reductions in IOP, improved ocular blood flow, upregulation of BDNF and other neurotrophic factors, attenuation of oxidative stress, and suppression of inflammatory signaling. Exercise also indirectly benefits the eye by improving systemic metabolic control, vascular health, and reducing sedentary behavior, all of which influence ocular aging. Evidence from both human and animal studies strongly supports exercise as a protective factor against multiple age-related eye diseases. Regular physical activity reduces glaucoma risk and slows its progression, attenuates DR severity through improved glycemic and vascular function, decreases AMD incidence and progression by modulating oxidative and inflammatory pathways, and lowers cataract risk through systemic antioxidant mechanisms. Experimental models consistently show neuroprotective and anti-inflammatory effects mediated by factors such as BDNF and adiponectin. Although not a substitute for medical treatment, exercise represents a powerful and accessible tool to preserve visual function and delay the onset of vision-threatening diseases in aging populations.
4.4 Exercise and Alzheimer's disease
Alzheimer's disease (AD) is the most common cause of dementia, characterized by progressive decline in memory, cognition, and functional abilities. While pharmacological approaches provide modest symptomatic relief, they do not halt disease progression. In recent decades, physical exercise has emerged as a promising non-pharmacological intervention to slow cognitive decline and improve quality of life. Findings from randomized clinical trials, pilot studies, and animal experiments offer growing evidence that different forms of physical activity, ranging from aerobic training to multimodal and resistance-based programs, can provide both functional and neurobiological benefits in AD. Early clinical evidence came from the randomized controlled trial by Lautenschlager et al. (167), who investigated a 6-month home-based physical activity program in older adults with subjective memory complaints. Compared with controls, participants in the intervention group experienced modest improvements on the Alzheimer's disease Assessment Scale–Cognitive Subscale (ADAS-Cog) that persisted at 18 months. Although the effect size was small, it suggested that regular exercise can slow cognitive deterioration even before dementia is diagnosed (167). Other trials in patients with diagnosed AD have supported these findings. Yágüez et al. (168) tested a 6-week non-aerobic movement program in individuals with Alzheimer-type dementia and found improvements in attention and visual memory. Importantly, patients in the control group declined over the same period, while those exercising maintained or enhanced cognitive performance, underlining the protective effect of even short interventions (168).
A pilot randomized controlled trial by Holthoff et al. (169) extended these results by showing that home-based physical activity not only improved executive function and daily living skills but also stabilized caregiver burden. Patients engaged in structured leg and movement training demonstrated better outcomes than those receiving standard care, indicating potential transfer benefits from motor activity to daily functioning and caregiver quality of life (169). The role of exercise intensity has also been examined. In the largest trial to date, Hoffmann et al. (170) randomized 200 patients with mild AD to supervised moderate-to-high intensity aerobic exercise or usual care. While intention-to-treat analysis did not show global cognitive benefits, patients who adhered closely to the exercise program improved on the Symbol Digit Modalities Test, suggesting a dose–response effect. Notably, exercise also reduced neuropsychiatric symptoms such as agitation and mood disturbances, which are highly burdensome in AD care (170). Long-term interventions highlight that sustained engagement matters. The Finnish Alzheimer's Disease Exercise Trial by Öhman et al. (171) evaluated 1 year of either home-based or group-based training. Although global cognition did not significantly differ across groups, home-based exercise improved executive function compared with controls, suggesting that familiar environments and individualized routines may maximize adherence and benefit (171).
Aerobic-focused studies offer additional insights. Morris and Vidoni (172) showed that 6 months of supervised aerobic training improved functional ability, and changes in cardiorespiratory fitness correlated with memory performance and hippocampal volume preservation. This link between fitness gains and brain outcomes suggests that physiological adaptation, rather than exercise alone, drives cognitive resilience (172). Similarly, Yu et al. (173) reported that 6 months of cycling attenuated expected declines in ADAS-Cog scores, although differences with stretching controls were not statistically significant. Nevertheless, both groups performed better than the natural progression of AD, pointing to a general benefit of structured activity (173). More recent work has emphasized multimodal programs. Papatsimpas et al. (174) demonstrated that combining aerobic and resistance training for 12 weeks significantly enhanced global cognition and daily function compared with controls, while resistance training alone provided more modest improvements. This highlights the value of diverse training modes targeting both strength and endurance (174). Likewise, David et al. (175) showed that increases in cardiorespiratory fitness during a 6-month multicomponent sports intervention correlated with better executive function, higher MoCA scores, and preservation of hippocampal volume, reinforcing the neuroprotective role of physical fitness (175). Other controlled studies have found less consistent cognitive outcomes but still report functional or psychosocial benefits. Toots et al. (176) observed no significant cognitive improvement from high-intensity exercise in nursing home residents with dementia, while Henskens et al. (177) noted gains in mobility, endurance, and mood, particularly when training incorporated daily life activities. Together, these findings suggest that population characteristics, stage of disease, and type of training may influence outcomes (176, 177).
Preclinical models provide strong support for the neurobiological mechanisms underlying exercise benefits in AD. Transgenic mouse and rat models consistently demonstrate that aerobic or resistance training reduces amyloid-β burden, improves synaptic function, and enhances mitochondrial quality. For example, treadmill running in APP/PS1 mice reduced amyloid plaque deposition, restored mitochondrial structure, and increased synaptic proteins, such as synaptophysin and GAP43 (178). Follow-up work showed that these effects were mediated by activation of the PINK1/Parkin mitophagy pathway via the SIRT1–FOXO axis, highlighting a link between exercise, mitochondrial clearance, and neuronal survival (179). Complementary evidence comes from combined lifestyle interventions. Shi et al. (180) demonstrated that exercise plus chlorogenic acid supplementation in AD mice synergistically reduced oxidative stress, neuroinflammation, and amyloid accumulation through activation of the SIRT1/PGC-1α pathway, producing greater improvements than either intervention alone (180). Similarly, Wang et al. (181) reported that long-term voluntary running enhanced lysosomal function and amyloid clearance via TFEB-mediated gene transcription, leading to significant cognitive improvements in APP/PSEN1 mice.
Sex- and hormone-specific models have also been examined. In ovariectomized 3xTg-AD mice, exercise protected against memory loss and behavioral deficits by upregulating BDNF signaling, suggesting particular benefits for postmenopausal women at risk for AD (182). Maternal exercise during pregnancy has even been shown to protect offspring from amyloid-induced neurotoxicity in adulthood, through long-lasting enhancements in mitochondrial function and synaptic plasticity (183). Mechanistically, across studies, exercise consistently upregulates neurotrophic factors (BDNF, PGC-1α), enhances mitochondrial biogenesis and turnover, decreases oxidative stress, and promotes non-amyloidogenic APP processing through SIRT1-mediated signaling (184, 185). These converging pathways demonstrate how physical activity directly counteracts hallmark pathologies of AD. Overall, the evidence from human and animal studies demonstrates that physical exercise offers meaningful benefits for individuals with Alzheimer's disease. While not all trials show consistent effects on global cognition, improvements are frequently seen in executive function, daily living skills, neuropsychiatric symptoms, and caregiver burden. Mechanistic studies strongly support that exercise promotes brain resilience by reducing amyloid accumulation, enhancing mitochondrial health, and boosting neurotrophic signaling. Collectively, these findings underscore that structured physical activity, whether aerobic, resistance-based, or multimodal, represents a practical and powerful adjunct therapy to slow functional decline and improve quality of life in people living with AD.
4.5 Exercise and Parkinson's disease
Parkinson's disease (PD) is a progressive neurodegenerative disorder characterized by tremor, rigidity, bradykinesia, postural instability, and a broad range of non-motor symptoms, including depression, fatigue, and cognitive impairment. While pharmacological treatments remain the mainstay of care, exercise has emerged as a valuable adjunct that can alleviate symptoms, enhance function, and possibly influence disease progression. Evidence from clinical and preclinical studies highlights how structured exercise, from yoga and resistance training to treadmill walking and high-intensity aerobic interventions, improves motor outcomes, quality of life, and even neurobiological markers of PD.
Mind–body exercises, such as yoga, provide both motor and psychological benefits. A controlled trial showed that a 3-month power yoga program significantly reduced bradykinesia and rigidity while improving muscle strength, leg power, and daily mobility scores in older adults with PD (186). Beyond motor function, mindfulness-based yoga yielded additional benefits. In a large randomized trial, yoga was more effective than stretching and resistance training in reducing anxiety and depression, while also improving spiritual wellbeing and health-related quality of life (187). These results suggest yoga is a holistic intervention capable of addressing both physical and emotional aspects of PD. Comparisons among different exercise modalities have produced valuable insights. In one head-to-head trial, treadmill training led to sustained improvements in forward gait, while backward walking improved with treadmill and stretching but not with tango dance, contrary to expectations (188). Other investigations confirmed that treadmill training and individualized physiotherapy both enhance gait speed and stability during dual-task conditions, which are critical for fall prevention (189). Collectively, these findings reinforce the role of walking-based interventions in improving functional mobility in PD patients.
Cognitive impairment is a common complication of PD. Observational work found that patients who engaged in moderate-intensity physical activity, meeting at least 150 min per week as recommended by WHO, performed better in executive function, visuospatial ability, and memory. These benefits correlated with stronger functional connectivity between the hippocampus, brainstem, and frontal–parietal regions (190). Short-term studies also suggest intensity matters: acute moderate training enhanced short-term recall, while high-intensity interval training (HIIT) produced broader effects on attention and sustained focus (191). Thus, aerobic activity can mitigate cognitive decline in PD, likely through neuroplastic adaptations in corticostriatal and cortical networks. The optimal intensity of aerobic training remains debated. In a recent randomized trial, both HIIT and moderate continuous training improved cardiorespiratory fitness and motor symptoms, but HIIT achieved a larger, clinically meaningful increase in peak oxygen uptake and reduced knee extensor fatigability (192). Another study found that aerobic interval training improved psychomotor function, executive ability, and manual dexterity after 8 weeks (193). These data indicate that both modes are beneficial, with HIIT potentially offering additional advantages in endurance and strength.
Resistance training has shown strong effects on bradykinesia and muscle weakness, two disabling features of PD. Nine weeks of progressive resistance training improved motor scores, walking performance, and sit-to-stand tests (194). A 24-week program further reduced anxiety symptoms and enhanced quality of life in elderly PD patients (195). Novel approaches such as blood flow restriction (BFR) training are also promising. Case-based and controlled studies reported that low-load resistance exercise with BFR improved lower limb strength, walking endurance, and autonomic function, while reducing symptoms of restless leg syndrome (196–198). Compared to high-intensity resistance training, low-intensity BFR was equally or more effective in improving vascular and autonomic markers, offering a safer option for frailer patients.
Preclinical studies shed light on the mechanisms through which exercise exerts neuroprotection. In rodent PD models, treadmill and endurance training improved motor performance, preserved dopaminergic neurons, reduced α-synuclein accumulation, and enhanced mitochondrial function (199–202). These effects were associated with antioxidant upregulation, autophagy activation, and mitochondrial quality control, mediated in part by SIRT1-PGC-1α signaling and lysosomal pathways. Regular aerobic activity also altered microRNA expression (upregulating miR-3557, downregulating miR-324), which modulated CaMK and mTOR signaling cascades to promote neuroprotection (203, 204). Human neuroimaging adds convergent evidence. Aerobic exercise increased functional connectivity between the putamen and motor cortex, reduced brain atrophy, and improved executive control tasks (205). PET imaging demonstrated enhanced dopamine release in the caudate nucleus and increased striatal responsiveness during reward tasks after aerobic training (206). These findings suggest that exercise not only improves symptoms but also promotes dopaminergic neurotransmission and corticostriatal plasticity.
Innovative protocols further expand exercise's role in PD. Body-weight–supported treadmill training allowed participants to achieve high-intensity walking safely and resulted in improved motor scores, quality of life, and walking distance (207). Similarly, LSVT BIG therapy, designed to encourage exaggerated movement patterns, produced improvements in motor and non-motor symptoms comparable to general exercise (208). Importantly, home-based and remotely supervised aerobic programs, such as the Park-in-Shape trial, have shown efficacy in reducing motor symptoms, highlighting their practicality and long-term feasibility (209). Overall, a wide range of exercise modalities, from yoga and resistance training to treadmill and high-intensity aerobic interventions, have demonstrated significant benefits for people with Parkinson's disease. These include improved gait, muscle strength, motor performance, cognitive function, and mood, along with enhanced quality of life. Mechanistic studies indicate that these outcomes are mediated through mitochondrial support, antioxidant defense, neuroplasticity, and dopaminergic regulation. Exercise is therefore a powerful, non-pharmacological adjunct in PD management, adaptable to different stages and patient needs.
5 Synergistic benefits of saffron and exercise in age-related diseases
The growing evidence on the convergence of nutraceutical and lifestyle interventions highlights the unique potential of saffron and physical activity as complementary strategies in aging-related disorders. While each intervention independently exerts significant effects on metabolism, vascular health, and neurodegeneration, their combined use produces amplified outcomes across multiple biological systems. Recent human and animal studies consistently indicate that saffron potentiates the effects of exercise on glycemic regulation, vascular remodeling, neurotrophic signaling, and inflammatory suppression, offering a multi-layered defense against the hallmarks of age-related diseases (Figure 4). A summary of the current experimental and clinical evidence on combined saffron–exercise interventions is presented in Table 3, highlighting their synergistic effects across metabolic, cardiovascular, and neurodegenerative outcomes.
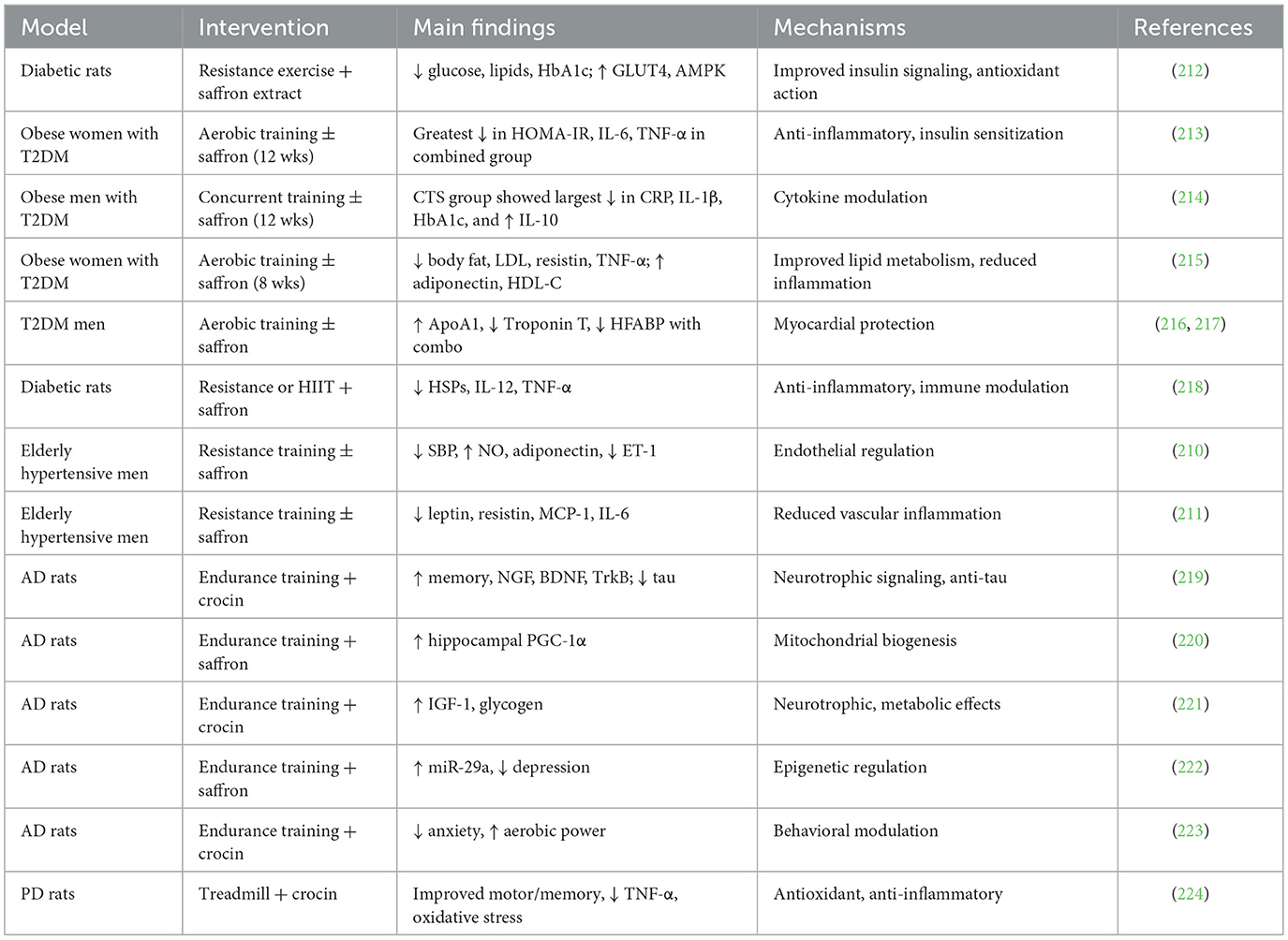
Table 3. Combined effects of exercise and saffron supplementation on metabolic and neurodegenerative disorders.
The growing body of mechanistic research reveals that saffron and exercise converge on interconnected signaling cascades, particularly the PI3K/Akt/mTOR, AMPK/SIRT1-PGC-1α, and Nrf2-mediated pathways, that coordinate oxidative balance, mitochondrial biogenesis, and neurotrophic signaling. Activation of these shared molecular targets promotes autophagic clearance of damaged proteins, improves cellular energy homeostasis, and enhances the expression of BDNF. Through these overlapping mechanisms, saffron and exercise reinforce each other's antioxidant and anti-inflammatory actions, providing a biological rationale for their observed synergy across metabolic and neurodegenerative disorders. Conceptually, this framework underscores the novelty of integrating nutraceutical and lifestyle strategies as complementary, interdependent components of a unified adaptive system for promoting healthy aging.
5.1 Cardiovascular benefits in hypertensive aging
Hypertension and vascular dysfunction are critical contributors to frailty and mortality in older adults. Studies indicate that saffron and exercise complement one another in improving endothelial health. In a randomized controlled trial with hypertensive older men, resistance training combined with saffron supplementation reduced systolic blood pressure more effectively than either alone, with significant increases in nitric oxide and adiponectin and decreases in endothelin-1 (210). These vascular adaptations suggest improved endothelial relaxation and reduced vasoconstrictor tone. Mojtahedi et al. (211) extended these findings by showing that combined saffron and resistance training produced stronger reductions in leptin, resistin, MCP-1, and IL-6 than either treatment individually. While saffron and exercise each improved lipid profiles by raising HDL and lowering total cholesterol, their combination did not further augment these effects, suggesting that lipid changes may plateau. However, the additive impact on inflammatory cytokines underscores their shared but distinct pathways, where saffron primarily suppresses NF-κB–mediated inflammation and exercise enhances vascular shear stress–driven nitric oxide bioavailability (211).
5.2 Metabolic interactions in type 2 diabetes
Diabetes mellitus represents a paradigm where saffron and exercise act synergistically. At the cellular level, saffron enhances insulin secretion and glucose uptake in β-cells and myotubes, mediated by upregulation of GLUT4 and AMPK pathways. Resistance exercise alone activates these same pathways through mechanical and metabolic stress, but their co-activation markedly improves systemic glycemic control. In rodent models, combined saffron and exercise interventions lowered fasting glucose, HbA1c, and serum lipids while increasing antioxidant defenses, demonstrating improvements that exceeded single treatments (212).
Human trials mirror these preclinical findings. In obese women with T2DM, 12 weeks of aerobic training plus saffron supplementation produced the strongest reductions in HOMA-IR, fibrinogen, homocysteine, and pro-inflammatory cytokines compared with exercise or saffron alone (213). Likewise, in obese men, concurrent training with saffron supplementation resulted in significantly greater declines in TNF-α, IL-6, IL-1β, and hs-CRP, with a concomitant rise in IL-10, pointing to an enhanced anti-inflammatory phenotype (214). Importantly, markers of lipid metabolism, including triglycerides and LDL-C, improved most robustly in the combination groups. These findings suggest that saffron not only mirrors the effects of exercise but also magnifies them through redox-mediated and cytokine-modulating pathways. Notably, the metabolic improvements appear sustainable beyond active training. Rajabi et al. (215) observed that women who received saffron plus aerobic training maintained improvements in insulin sensitivity, adiponectin, and lipid profile for up to 2 weeks after detraining, whereas benefits faded more quickly in groups without saffron. This indicates that saffron may stabilize or prolong the adaptive metabolic response induced by exercise, perhaps through its capacity to buffer oxidative stress and regulate adipocytokines (215).
Beyond glucose control, combined saffron and exercise improves cardiac risk markers. Barari et al. (216, 217) reported that men with diabetes who underwent aerobic training while receiving saffron extract showed significant reductions in ApoA1, Troponin T, and HFABP, suggesting myocardial protection. Given that Troponin T and HFABP are sensitive markers of subclinical cardiac injury, these results emphasize the cardioprotective value of integrating saffron into exercise programs for high-risk patients (216, 217). Animal data further reinforce this conclusion. In diabetic rats, resistance training or HIIT combined with saffron reduced expression of inflammatory mediators such as HSPs, IL-12, and TNF-α (218). Together, these outcomes highlight a coordinated effect on glucose homeostasis, lipid metabolism, and systemic inflammation.
5.3 Neuroprotective interactions in Alzheimer's disease
The role of saffron and exercise in neurodegeneration is particularly evident in Alzheimer's disease (AD). In rodent models, both interventions have been shown to influence neurotrophic pathways, tau phosphorylation, and mitochondrial quality control. Moghadasi et al. (219) demonstrated that crocin supplementation combined with endurance training significantly improved memory and learning in TMT-induced AD rats. These effects were associated with the upregulation of BDNF, NGF, and TrkB expression and reduced tau, highlighting enhanced synaptic plasticity and attenuated pathological burden (219). Mitochondrial biogenesis is another area of convergence. Azarian et al. (220) found that endurance training with saffron increased hippocampal expression of PGC-1α, a master regulator of mitochondrial metabolism, beyond the effects of either alone (220). Similarly, Negarandeh et al. (221) showed that endurance training combined with crocin increased IGF-1 and glycogen expression in hippocampal tissue of AD rats, suggesting improved energy supply for neuronal survival. These findings underscore how saffron stabilizes neuronal energy metabolism while exercise drives structural and synaptic adaptations (221).
Psychological dimensions of AD also appear responsive. Negarandeh et al. (222) reported that the combination of endurance training and saffron increased miR-29a expression and produced greater reductions in depression-like behaviors compared with either treatment alone. Another study found that while crocin and exercise independently reduced anxiety and improved aerobic power, their combination did not produce additive effects in these domains (223). Nevertheless, the overall evidence indicates a clear synergy in cognitive and mood-related outcomes.
5.4 Interactions in Parkinson's disease
Parkinson's disease (PD) is characterized by dopaminergic degeneration and oxidative injury, both of which are targeted by saffron and exercise. Shahidani et al. (224) investigated treadmill training with crocin in 6-OHDA-lesioned rats, a standard PD model. The combined intervention reduced motor asymmetry, improved spatial and aversive memory, and significantly decreased TNF-α and lipid peroxidation. Biochemical assays showed that crocin enhanced antioxidant capacity, while exercise increased thiol concentration, together producing robust neuroprotection. These findings illustrate that saffron amplifies the neurobiological gains of exercise by directly scavenging free radicals and modulating inflammatory cascades, whereas exercise reinforces synaptic and motor plasticity.
Together, these studies point toward a consistent theme: saffron reinforces the biochemical and cellular adaptations induced by exercise, while exercise facilitates the functional expression of saffron's molecular effects. Their convergence on mitochondrial function, antioxidant defense, and inflammatory regulation explains their broad utility across metabolic, cardiovascular, and neurodegenerative diseases of aging. Thus, accumulating preclinical and clinical data demonstrate that saffron and exercise are not merely additive but often synergistic in combating age-related disease. They converge on AMPK/GLUT4 signaling, nitric oxide production, neurotrophic factor expression, and suppression of inflammatory cytokines, while addressing psychological and functional domains such as depression, fatigue, and mobility. These integrative effects provide compelling evidence that combining saffron supplementation with structured exercise represents a safe, culturally adaptable, and mechanistically grounded strategy to promote health span in older adults.
6 Limitations
Although the evidence supporting saffron and exercise as complementary interventions in age-related diseases is steadily growing, several limitations constrain the strength of current conclusions. First, most clinical studies remain small in sample size, short in duration, and often single-centered, which reduces their generalizability to broader populations. Differences in trial design, participant characteristics (age, comorbidities, and baseline disease severity), and outcome measures further complicate cross-study comparisons. Second, saffron preparations are heterogeneous, with variable dosages, extraction methods, and purity across studies. This lack of standardization makes it difficult to establish optimal dosing regimens or to compare results across trials. Moreover, many studies do not perform chemical characterization of saffron supplements, leaving uncertainty about the precise concentrations of crocin, crocetin, and safranal being administered.
Third, while animal models provide mechanistic insight, they cannot fully replicate the multifactorial complexity of human aging. Rodent models of Alzheimer's, Parkinson's, or diabetes capture only selected aspects of human disease, which limits the translation of preclinical findings to clinical practice. Fourth, most human trials assess short-term biochemical or functional endpoints (e.g., insulin resistance, inflammatory markers, cognition over 12–24 weeks), but long-term data on clinical outcomes such as cardiovascular events, disease progression, or mortality are lacking. Additionally, adherence to both exercise and supplementation protocols is rarely monitored beyond the intervention period, leaving the sustainability of benefits unclear. Finally, the interaction between saffron and exercise at the molecular level remains incompletely understood. While both converge on pathways such as AMPK, NF-κB, and BDNF signaling, the precise temporal sequence and dose–response relationships are not well-defined. This gap makes it difficult to determine whether saffron enhances exercise adaptations directly or whether its benefits arise mainly from independent parallel mechanisms.
7 Future perspectives
Future work should prioritize large-scale, multicenter randomized controlled trials that investigate the combined effects of saffron supplementation and structured exercise programs in diverse populations. Standardization of saffron formulations is essential, with precise quantification of active compounds such as crocin and crocetin to ensure reproducibility. Dose–response studies are particularly needed to determine the minimal effective and maximal safe doses when combined with exercise. Longitudinal research should assess not only surrogate markers (e.g., HbA1c, inflammatory cytokines, and neurotrophins) but also hard clinical outcomes, including incidence of dementia, cardiovascular events, functional disability, and quality-adjusted life years. Extending follow-up beyond the typical 8–24 weeks will help establish the durability of benefits and adherence patterns.
Mechanistic studies should focus on dissecting how saffron influences exercise-induced adaptations in mitochondria, synaptic plasticity, endothelial function, and immune regulation. Integrative omics approaches (transcriptomics, metabolomics, and proteomics) may uncover novel pathways and biomarkers that reflect the synergy between nutraceuticals and lifestyle interventions. Another priority is personalization. Not all individuals respond equally to exercise or saffron, and genetic, metabolic, and microbiome profiles may influence outcomes. Precision-nutrition and precision-exercise frameworks could help tailor interventions to maximize benefit for specific subgroups, such as postmenopausal women, patients with metabolic syndrome, or individuals at high risk for neurodegeneration. In alignment with the latest advances, recent comprehensive reviews have broadened the therapeutic scope of saffron beyond metabolic and neurodegenerative contexts. Razavi et al. (225) demonstrated that saffron and its key constituents, crocin, crocetin, safranal, and kaempferol, ameliorate oxidative stress, inflammation, and apoptosis in models of traumatic brain injury via modulation of NF-κB, NLRP3, Nrf2/HO-1, Bcl-2/Bax, and SIRT1 signaling. These mechanistic pathways parallel those activated by exercise-induced neuroplastic and mitochondrial adaptations, supporting the broader neuroprotective relevance of saffron. Likewise, Goyal et al. (226) summarized saffron's benefits in reproductive and sexual health, including improved erectile and menstrual function through serotonergic, nitric-oxide–dependent, and anti-inflammatory mechanisms. Together, these findings highlight saffron's multifaceted physiological influence across neuroendocrine and systemic domains, reinforcing its potential as a holistic adjunct to lifestyle-based interventions such as structured physical activity. Building upon these recent insights, future research should explore how saffron's multi-systemic effects can be harnessed synergistically with exercise training, for example, by integrating neuroprotective, endocrine, and vascular endpoints into combined intervention trials. Such cross-domain designs could clarify whether saffron enhances exercise-mediated adaptations in cognitive, reproductive, and metabolic health, thereby providing a mechanistic foundation for personalized lifestyle–nutraceutical strategies across the lifespan.
In addition to scientific and mechanistic advances, the integration of saffron supplementation and structured exercise also carries important global-health and policy implications. Within the framework of the WHO Decade of Healthy Ageing (2021–2030), such strategies align with international priorities to promote functional ability, equity, and active participation of older adults. Both saffron and exercise represent safe, low-cost, and culturally adaptable interventions that can be implemented across diverse socioeconomic settings. Their accessibility is particularly relevant for low- and middle-income countries, where pharmaceutical options for chronic disease prevention are limited but community-based health programs are expanding. Future interdisciplinary collaborations between clinicians, public-health professionals, and policymakers will be crucial to translate this nutraceutical-lifestyle synergy into scalable, sustainable models for healthy aging worldwide. Finally, pragmatic trials and implementation studies are needed to evaluate the feasibility, cultural adaptability, and cost-effectiveness of combined saffron–exercise programs in real-world settings. This is particularly relevant in low- and middle-income countries, where saffron is culturally accepted and exercise interventions are cost-efficient but underutilized.
8 Conclusion
The current body of evidence underscores saffron and exercise as two promising non-pharmacological interventions with complementary effects on age-related diseases. Saffron and its bioactive components modulate oxidative stress, inflammation, mitochondrial dysfunction, and aberrant protein aggregation, while exercise improves cardiovascular fitness, metabolic health, neuronal resilience, and psychological wellbeing. When combined, these interventions appear to exert synergistic benefits, enhancing glycemic control, vascular integrity, neurotrophic signaling, and functional outcomes across experimental and clinical models. Although encouraging results support their potential as low-risk adjunct therapies, existing clinical studies remain limited in size, duration, and methodological consistency. Future well-designed trials are essential to confirm the long-term efficacy, clarify optimal dosing and exercise regimens, and identify patient populations most likely to benefit. Collectively, saffron and exercise represent complementary and biologically active strategies with significant potential to improve healthspan and mitigate the burden of age-related diseases.
Author contributions
LL: Writing – review & editing, Writing – original draft, Conceptualization, Methodology, Visualization, Validation, Investigation. KW: Writing – review & editing, Visualization, Writing – original draft, Methodology, Supervision, Data curation, Conceptualization, Validation, Investigation.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gianfredi V, Nucci D, Pennisi F, Maggi S, Veronese N, Soysal P. Aging, longevity, and healthy aging: the public health approach. Aging Clin Exp Res. (2025) 37:125. doi: 10.1007/s40520-025-03021-8
2. Drake JC, Yan Z. Targeting healthspan to optimally combat non-communicable disease in an aging world. Sports Med Health Sci. (2019) 1:59–60. doi: 10.1016/j.smhs.2019.08.005
3. Prince MJ, Wu F, Guo Y, Gutierrez Robledo LM, O'Donnell M, Sullivan R, et al. The burden of disease in older people and implications for health policy and practice. Lancet. (2015) 385:549–62. doi: 10.1016/S0140-6736(14)61347-7
4. Pathak N, Vimal SK, Tandon I, Agrawal L, Hongyi C, Bhattacharyya S. Neurodegenerative disorders of Alzheimer, Parkinsonism, amyotrophic lateral sclerosis and multiple sclerosis: an early diagnostic approach for precision treatment. Metab Brain Dis. (2022) 37:67–104. doi: 10.1007/s11011-021-00800-w
5. Nurkolis F, Utami TW, Alatas AI, Wicaksono D, Kurniawan R, Ratmandhika SR, et al. Can salivary and skin microbiome become a biodetector for aging-associated diseases? Current insights and future perspectives. Front Aging. (2024) 5:2024. doi: 10.3389/fragi.2024.1462569
6. Franceschi C, Garagnani P. Suggestions from geroscience for the genetics of age-related diseases. PLoS Genet. (2016) 12:e1006399. doi: 10.1371/journal.pgen.1006399
7. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: an expanding universe. Cell. (2023) 186:243–78. doi: 10.1016/j.cell.2022.11.001
8. Duarte AI. Recovery of insulin/IGF-1/GLP-1 signaling protects the brain against cardiometabolic and neurodegenerative diseases. Alzheimer's Dement. (2024) 20:e089823. doi: 10.1002/alz.89823
9. Pasco JA, Williams LJ, Jacka FN, Stupka N, Brennan-Olsen SL, Holloway KL, et al. Sarcopenia and the common mental disorders: a potential regulatory role of skeletal muscle on brain function? Curr Osteoporos Rep. (2015) 13:351–7. doi: 10.1007/s11914-015-0279-7
10. Van der Schyf CJ. The use of multi-target drugs in the treatment of neurodegenerative diseases. Expert Rev Clin Pharmacol. (2011) 4:293–8. doi: 10.1586/ecp.11.13
11. Marrone G, Urciuoli S, Di Lauro M, Cornali K, Montalto G, Masci C, et al. Saffron (Crocus sativus L.) and its by-products: healthy effects in internal medicine. Nutrients. (2024) 16:2319. doi: 10.3390/nu16142319
12. Hosseinzadeh H, Nassiri-Asl M. Avicenna's (Ibn Sina) the canon of medicine and saffron (Crocus sativus): a review. Phytother Res. (2013) 27:475–83. doi: 10.1002/ptr.4784
13. Hosseini A, Razavi BM, Hosseinzadeh H. Pharmacokinetic properties of saffron and its active components. Eur J Drug Metab Pharmacokinet. (2018) 43:383–90. doi: 10.1007/s13318-017-0449-3
14. Yang W, Qiu X, Wu Q, Chang F, Zhou T, Zhou M. Pei J. Active constituents of saffron (Crocus sativus L.) and their prospects in treating neurodegenerative diseases (Review). Exp Ther Med. (2023) 25:235. doi: 10.3892/etm.2023.11934
15. Bej E, Volpe AR, Cesare P, Cimini A. d'Angelo M, Castelli V. Therapeutic potential of saffron in brain disorders: from bench to bedside. Phytother Res. (2024) 38:2482–95. doi: 10.1002/ptr.8169
16. Hausenblas HA, Saha D, Dubyak PJ. Anton SD. Saffron (Crocus sativus L) and major depressive disorder: a meta-analysis of randomized clinical trials. J Integr Med. (2013) 11:377–83. doi: 10.3736/jintegrmed2013056
17. Batarseh YS, Bharate SS, Kumar V, Kumar A, Vishwakarma RA, Bharate SB, et al. Crocus sativus extract tightens the blood-brain barrier, reduces amyloid β load and related toxicity in 5XFAD mice. ACS Chem Neurosci. (2017) 8:1756–66. doi: 10.1021/acschemneuro.7b00101
18. Inoue E, Suzuki T, Shimizu Y, Sudo K, Kawasaki H, Ishida N. Saffron ameliorated motor symptoms, short life span and retinal degeneration in Parkinson's disease fly models. Gene. (2021) 799:145811. doi: 10.1016/j.gene.2021.145811
19. Clemente-Suárez VJ, Rubio-Zarapuz A, Belinchón-deMiguel P, Beltrán-Velasco AI, Martín-Rodríguez A, Tornero-Aguilera JF. Impact of physical activity on cellular metabolism across both neurodegenerative and general neurological conditions: a narrative review. Cells. (2024) 13:1940. doi: 10.3390/cells13231940
20. Stimpson NJ, Davison G, Javadi A-H. Joggin' the noggin: towards a physiological understanding of exercise-induced cognitive benefits. Neurosci Biobehav Rev. (2018) 88:177–86. doi: 10.1016/j.neubiorev.2018.03.018
21. Yaribeygi H, Atkin SL, Simental-Mendía LE, Sahebkar A. Molecular mechanisms by which aerobic exercise induces insulin sensitivity. J Cell Physiol. (2019) 234:12385–92. doi: 10.1002/jcp.28066
22. Tharmaratnam T, Civitarese RA, Tabobondung T, Tabobondung TA. Exercise becomes brain: sustained aerobic exercise enhances hippocampal neurogenesis. J Physiol. (2017) 595:7–8. doi: 10.1113/JP272761
23. Yasuda T. Selected methods of resistance training for prevention and treatment of sarcopenia. Cells. (2022) 11:1389. doi: 10.3390/cells11091389
24. Loprinzi PD, Frith E, A. brief primer on the mediational role of BDNF in the exercise-memory link. Clin Physiol Funct Imaging. (2019) 39:9–14. doi: 10.1111/cpf.12522
25. Hu S, Li X, Yang L. Effects of physical activity in child and adolescent depression and anxiety: role of inflammatory cytokines and stress-related peptide hormones. Front Neurosci. (2023) 17:1234409. doi: 10.3389/fnins.2023.1234409
26. Hamer M, Chida Y. Physical activity and risk of neurodegenerative disease: a systematic review of prospective evidence. Psychol Med. (2009) 39:3–11. doi: 10.1017/S0033291708003681
27. Scuto M, Modafferi S, Rampulla F, Zimbone V, Tomasello M. Spano' S, et al. Redox modulation of stress resilience by Crocus sativus L for potential neuroprotective and anti-neuroinflammatory applications in brain disorders: from molecular basis to therapy. Mech Ageing Dev. (2022) 205:111686. doi: 10.1016/j.mad.2022.111686
28. El Assar M, Álvarez-Bustos A, Sosa P, Angulo J, Rodríguez-Mañas L. Effect of physical activity/exercise on oxidative stress and inflammation in muscle and vascular aging. Int J Mol Sci. (2022) 23:8713. doi: 10.3390/ijms23158713
29. Lippi G, Mattiuzzi C, Sanchis-Gomar F. Updated overview on interplay between physical exercise, neurotrophins, and cognitive function in humans. J Sport Health Sci. (2020) 9:74–81. doi: 10.1016/j.jshs.2019.07.012
30. Abdian S, Fakhri S, Moradi SZ, Khirehgesh MR, Echeverría J. Saffron and its major constituents against neurodegenerative diseases: a mechanistic review. Phytomedicine. (2024) 135:156097. doi: 10.1016/j.phymed.2024.156097
31. Aoi W, Naito Y, Yoshikawa T. Dietary exercise as a novel strategy for the prevention and treatment of metabolic syndrome: effects on skeletal muscle function. J Nutr Metab. (2011) 2011:676208. doi: 10.1155/2011/676208
32. Rivera-Torres S, Fahey TD, Rivera MA. Adherence to exercise programs in older adults: informative report. Gerontol Geriatr Med. (2019) 5:2333721418823604. doi: 10.1177/2333721418823604
33. Lopresti AL, Smith SJ, Drummond PD. An investigation into an evening intake of a saffron extract (affron®) on sleep quality, cortisol, and melatonin concentrations in adults with poor sleep: a randomised, double-blind, placebo-controlled, multi-dose study. Sleep Med. (2021) 86:7–18. doi: 10.1016/j.sleep.2021.08.001
34. The Lancet Healthy L. The Decade of Healthy Ageing: progress and challenges ahead. Lancet Healthy Longev. (2024) 5:e1. doi: 10.1016/S2666-7568(23)00271-4
35. Rudnicka E, Napierała P, Podfigurna A, Meczekalski B, Smolarczyk R, Grymowicz M. The World Health Organization (WHO) approach to healthy ageing. Maturitas. (2020) 139:6–11. doi: 10.1016/j.maturitas.2020.05.018
36. Cusano E, Consonni R, Petrakis EA, Astraka K, Cagliani LR, Polissiou MG. Integrated analytical methodology to investigate bioactive compounds in Crocus sativus L. flowers Phytochem Anal. (2018) 29:476–86. doi: 10.1002/pca.2753
37. Cerdá-Bernad D, Valero-Cases E, Pastor J-J, Frutos MJ. Saffron bioactives crocin, crocetin and safranal: effect on oxidative stress and mechanisms of action. Crit Rev Food Sci Nutr. (2022) 62:3232–49. doi: 10.1080/10408398.2020.1864279
38. Finley JW, Gao S, A. Perspective on Crocus sativus L. (Saffron) constituent crocin: a potent water-soluble antioxidant and potential therapy for Alzheimer's disease. J Agric Food Chem. (2017) 65:1005–20. doi: 10.1021/acs.jafc.6b04398
39. Bastani S, Vahedian V, Rashidi M, Mir A, Mirzaei S, Alipourfard I, et al. An evaluation on potential anti-oxidant and anti-inflammatory effects of Crocin. Biomed Pharmacother. (2022) 153:113297. doi: 10.1016/j.biopha.2022.113297
40. Zununi Vahed S, Zuluaga Tamayo M, Rodriguez-Ruiz V, Thibaudeau O, Aboulhassanzadeh S, Abdolalizadeh J, et al. Functional mechanisms of dietary crocin protection in cardiovascular models under oxidative stress. Pharmaceutics. (2024) 16:840. doi: 10.3390/pharmaceutics16070840
41. Asadi F, Jamshidi AH, Khodagholi F, Yans A, Azimi L, Faizi M, et al. Reversal effects of crocin on amyloid β-induced memory deficit: modification of autophagy or apoptosis markers. Pharmacol Biochem Behav. (2015) 139:47–58. doi: 10.1016/j.pbb.2015.10.011
42. Haeri P, Mohammadipour A, Heidari Z. Ebrahimzadeh-bideskan A. Neuroprotective effect of crocin on substantia nigra in MPTP-induced Parkinson's disease model of mice. Anat Sci Int. (2019) 94:119–27. doi: 10.1007/s12565-018-0457-7
43. Salek R, Dehghani M, Mohajeri SA, Talaei A, Fanipakdel A, Javadinia SA. Amelioration of anxiety, depression, and chemotherapy related toxicity after crocin administration during chemotherapy of breast cancer: a double blind, randomized clinical trial. Phytother Res. (2021) 35:5143–53. doi: 10.1002/ptr.7180
44. Wang P, Lin S, Chen Z, Jiang Y, Jiang J, Liu L. Antidepressant effects of crocin-1 from saffron in mice with chronic unpredictable mild stress-induced depression. FASEB J. (2019) 33:lb75. doi: 10.1096/fasebj.2019.33.1_supplement.lb75
45. Guo Z-L, Li M-X, Li X-L, Wang P, Wang W-G, Du W-Z, et al. Crocetin: a systematic review. Front Pharmacol. (2022) 12:745683. doi: 10.3389/fphar.2021.745683
46. Hashemi M, Hosseinzadeh H. A. comprehensive review on biological activities and toxicology of crocetin. Food Chem Toxicol. (2019) 130:44–60. doi: 10.1016/j.fct.2019.05.017
47. Abedimanesh S, Bathaie SZ, Ostadrahimi A, Asghari Jafarabadi M, Taban Sadeghi M. The effect of crocetin supplementation on markers of atherogenic risk in patients with coronary artery disease: a pilot, randomized, double-blind, placebo-controlled clinical trial. Food Funct. (2019) 10:7461–75. doi: 10.1039/C9FO01166H
48. Xi L, Qian Z, Xu G, Zheng S, Sun S, Wen N, et al. Beneficial impact of crocetin, a carotenoid from saffron, on insulin sensitivity in fructose-fed rats. J Nutr Biochem. (2007) 18:64–72. doi: 10.1016/j.jnutbio.2006.03.010
49. Song L, Kang C, Sun Y, Huang W, Liu W, Qian Z. Crocetin inhibits lipopolysaccharide-induced inflammatory response in human umbilical vein endothelial cells. Cell Physiol Biochem. (2016) 40:443–52. doi: 10.1159/000452559
50. Chrysanthou A, Pouliou E, Kyriakoudi A, Tsimidou MZ. Sensory threshold studies of picrocrocin, the major bitter compound of saffron. J Food Sci. (2016) 81:S189–S98. doi: 10.1111/1750-3841.13152
51. Yu L, Li J, Xiao M. Picrocrocin exhibits growth inhibitory effects against SKMEL- 2 human malignant melanoma cells by targeting JAK/STAT5 signaling pathway, cell cycle arrest and mitochondrial mediated apoptosis. J BUON. (2018) 23:1163–8.
52. Diretto G, Ahrazem O, Rubio-Moraga Á, Fiore A, Sevi F, Argandoña J, et al. UGT709G1: a novel uridine diphosphate glycosyltransferase involved in the biosynthesis of picrocrocin, the precursor of safranal in saffron (Crocus sativus). New Phytol. (2019) 224:725–40. doi: 10.1111/nph.16079
53. Farag MA, Hegazi N, Dokhalahy E, Khattab AR. Chemometrics based GC-MS aroma profiling for revealing freshness, origin and roasting indices in saffron spice and its adulteration. Food Chem. (2020) 331:127358. doi: 10.1016/j.foodchem.2020.127358
54. Nanda S, Madan K. The role of Safranal and saffron stigma extracts in oxidative stress, diseases and photoaging: a systematic review. Heliyon. (2021) 7:e06117. doi: 10.1016/j.heliyon.2021.e06117
55. He S-Y, Qian Z-Y, Tang F-T, Wen N, Xu G-L, Sheng L. Effect of crocin on experimental atherosclerosis in quails and its mechanisms. Life Sci. (2005) 77:907–21. doi: 10.1016/j.lfs.2005.02.006
56. Tang FT, Qian ZY, Liu PQ, Zheng SG, He SY, Bao LP, et al. Crocetin improves endothelium-dependent relaxation of thoracic aorta in hypercholesterolemic rabbit by increasing eNOS activity. Biochem Pharmacol. (2006) 72:558–65. doi: 10.1016/j.bcp.2006.05.018
57. Zheng S, Qian Z, Tang F, Sheng L. Suppression of vascular cell adhesion molecule-1 expression by crocetin contributes to attenuation of atherosclerosis in hypercholesterolemic rabbits. Biochem Pharmacol. (2005) 70:1192–9. doi: 10.1016/j.bcp.2005.07.034
58. Zheng S, Qian Z, Sheng L, Wen N. Crocetin attenuates atherosclerosis in hyperlipidemic rabbits through inhibition of LDL oxidation. J Cardiovasc Pharmacol. (2006) 47:70–6. doi: 10.1097/01.fjc.0000194686.11712.02
59. He S-Y, Qian Z-Y, Wen N, Tang F-T, Xu G-L, Zhou C-H. Influence of Crocetin on experimental atherosclerosis in hyperlipidamic-diet quails. Eur J Pharmacol. (2007) 554:191–5. doi: 10.1016/j.ejphar.2006.09.071
60. Abedimanesh N, Motlagh B, Abedimanesh S, Bathaie SZ, Separham A, Ostadrahimi A. Effects of crocin and saffron aqueous extract on gene expression of SIRT1, AMPK, LOX1, NF-κB, and MCP-1 in patients with coronary artery disease: a randomized placebo-controlled clinical trial. Phytother Res. (2020) 34:1114–22. doi: 10.1002/ptr.6580
61. Joukar S, Najafipour H, Khaksari M, Sepehri G, Shahrokhi N, Dabiri S, et al. The effect of saffron consumption on biochemical and histopathological heart indices of rats with myocardial infarction. Cardiovasc Toxicol. (2010) 10:66–71. doi: 10.1007/s12012-010-9063-1
62. Goyal SN, Arora S, Sharma AK, Joshi S, Ray R, Bhatia J, et al. Preventive effect of crocin of Crocus sativus on hemodynamic, biochemical, histopathological and ultrastuctural alterations in isoproterenol-induced cardiotoxicity in rats. Phytomedicine. (2010) 17:227–32. doi: 10.1016/j.phymed.2009.08.009
63. Huang Z, Nan C, Wang H, Su Q, Xue W, Chen Y, et al. Crocetin ester improves myocardial ischemia via Rho/ROCK/NF-κB pathway. Int Immunopharmacol. (2016) 38:186–93. doi: 10.1016/j.intimp.2016.05.025
64. Xue Y, Jin W, Xue Y, Zhang Y, Wang H, Zhang Y, et al. Safranal, an active constituent of saffron, ameliorates myocardial ischemia via reduction of oxidative stress and regulation of Ca2+ homeostasis. J Pharmacol Sci. (2020) 143:156–64. doi: 10.1016/j.jphs.2020.03.005
65. Efentakis P, Rizakou A, Christodoulou E, Chatzianastasiou A, López MG, León R, et al. Saffron (Crocus sativus) intake provides nutritional preconditioning against myocardial ischemia–reperfusion injury in Wild Type and ApoE(–/–) mice: involvement of Nrf2 activation. Nutr Metab Cardiovasc Dis. (2017) 27:919–29. doi: 10.1016/j.numecd.2017.08.005
66. Dianat M, Esmaeilizadeh M, Badavi M, Samarbafzadeh A, Naghizadeh B. Protective effects of crocin on hemodynamic parameters and infarct size in comparison with vitamin e after ischemia reperfusion in isolated rat hearts. Planta Med. (2014) 80:393–8. doi: 10.1055/s-0033-1360383
67. Chahine N, Hanna J, Makhlouf H, Duca L, Martiny L, Chahine R. Protective effect of saffron extract against doxorubicin cardiotoxicity in isolated rabbit heart. Pharm Biol. (2013) 51:1564–71. doi: 10.3109/13880209.2013.802812
68. Chahine N, Makhlouf H, Duca L, Martiny L. Chahine R. Cardioprotective effect of saffron extracts against acute doxorubicin toxicity in isolated rabbit hearts submitted to ischemia-reperfusion. Injury. (2014) 69:459–70. doi: 10.5560/znc.2014-0124
69. Chahine N, Nader M, Duca L, Martiny L, Chahine R. Saffron extracts alleviate cardiomyocytes injury induced by doxorubicin and ischemia-reperfusion in vitro. Drug Chem Toxicol. (2016) 39:87–96. doi: 10.3109/01480545.2015.1036281
70. Chu X, Zhang Y, Xue Y, Li Z, Shi J, Wang H, et al. Crocin protects against cardiotoxicity induced by doxorubicin through TLR-2/NF-κB signal pathway in vivo and vitro. Int Immunopharmacol. (2020) 84:106548. doi: 10.1016/j.intimp.2020.106548
71. Elsherbiny NM, Salama MF, Said E, El-Sherbiny M, Al-Gayyar MMH. Crocin protects against doxorubicin-induced myocardial toxicity in rats through down-regulation of inflammatory and apoptic pathways. Chem Biol Interact. (2016) 247:39–48. doi: 10.1016/j.cbi.2016.01.014
72. Razmaraii N, Babaei H, Mohajjel Nayebi A, Assadnassab G, Ashrafi Helan J, Azarmi Y. Crocin treatment prevents doxorubicin-induced cardiotoxicity in rats. Life Sci. (2016) 157:145–51. doi: 10.1016/j.lfs.2016.06.012
73. Razavi BM, Hosseinzadeh H, Movassaghi AR, Imenshahidi M, Abnous K. Protective effect of crocin on diazinon induced cardiotoxicity in rats in subchronic exposure. Chem Biol Interact. (2013) 203:547–55. doi: 10.1016/j.cbi.2013.03.010
74. Razavi BM, Hosseinzadeh H, Imenshahidi M, Malekian M, Ramezani M, Abnous K. Evaluation of protein ubiquitylation in heart tissue of rats exposed to diazinon (an organophosphate insecticide) and crocin (an active saffron ingredient): role of HIF-1α. Drug Res (Stuttg). (2015) 65:561–6. doi: 10.1055/s-0034-1384533
75. Salem IB, Boussabbeh M, Neffati F, Najjar M, Abid-Essefi S, Bacha H. Zearalenone-induced changes in biochemical parameters, oxidative stress and apoptosis in cardiac tissue: protective role of crocin. Hum Exp Toxicol. (2015) 35:623–34. doi: 10.1177/0960327115597467
76. Kazemi T, Mollaei H, Takhviji V, Bijari B, Zarban A, Rostami Z, et al. The anti-dyslipidemia property of saffron petal hydroalcoholicextract in cardiovascular patients: a double-blinded randomized clinical trial. Clin Nutr ESPEN. (2023) 55:314–9. doi: 10.1016/j.clnesp.2023.04.002
77. Kermani T, Zebarjadi M, Mehrad-Majd H, Mirhafez S-R, Shemshian M, Ghasemi F, et al. Anti-inflammatory effect of Crocus sativus on serum cytokine levels in subjects with metabolic syndrome: a randomized, double-blind, placebo- controlled trial. Curr Clin Pharmacol. (2017) 12:122–6. doi: 10.2174/1574884712666170622082737
78. Asbaghi O, Soltani S, Norouzi N, Milajerdi A, Choobkar S, Asemi Z. The effect of saffron supplementation on blood glucose and lipid profile: a systematic review and meta-analysis of randomized controlled trials. Complement Ther Med. (2019) 47:102158. doi: 10.1016/j.ctim.2019.07.017
79. Rahmani J, Manzari N, Thompson J, Clark CCT, Villanueva G, Varkaneh HK, et al. The effect of saffron on weight and lipid profile: a systematic review, meta-analysis, and dose–response of randomized clinical trials. Phytother Res. (2019) 33:2244–55. doi: 10.1002/ptr.6420
80. Asdaq SMB, Inamdar MN. Potential of Crocus sativus (saffron) and its constituent, crocin, as hypolipidemic and antioxidant in rats. Appl Biochem Biotechnol. (2010) 162:358–72. doi: 10.1007/s12010-009-8740-7
81. Sheng L, Qian Z, Zheng S, Xi L. Mechanism of hypolipidemic effect of crocin in rats: crocin inhibits pancreatic lipase. Eur J Pharmacol. (2006) 543:116–22. doi: 10.1016/j.ejphar.2006.05.038
82. Siddiq A A, Dileep SA, Sj AR, Singam SSR, Martin A. Saffron and its active constituents ameliorate hypercholesterolemia by inhibiting PCSK9 and modulating Sortilin, LDLR, and SREBP-2 signaling in high fat diet induced hypercholesterolemic C57BL/6 mice. J Ethnopharmacol. (2025) 346:119697. doi: 10.1016/j.jep.2025.119697
83. Moravej Aleali A, Amani R, Shahbazian H, Namjooyan F, Latifi SM. Cheraghian B. The effect of hydroalcoholic Saffron (Crocus sativus L) extract on fasting plasma glucose, HbA1c, lipid profile, liver, and renal function tests in patients with type 2 diabetes mellitus: a randomized double-blind clinical trial. Phytother Res. (2019) 33:1648–57. doi: 10.1002/ptr.6351
84. Milajerdi A, Jazayeri S, Hashemzadeh N, Shirzadi E, Derakhshan Z, Djazayeri A, et al. The effect of saffron (Crocus sativus L.) hydroalcoholic extract on metabolic control in type 2 diabetes mellitus: a triple-blinded randomized clinical trial. J Res Med Sci. (2018). 23(1). doi: 10.4103/jrms.JRMS_286_17
85. Sepahi S, Mohajeri SA, Hosseini SM, Khodaverdi E, Shoeibi N, Namdari M, et al. Effects of crocin on diabetic maculopathy: a placebo-controlled randomized clinical trial. Am J Ophthalmol. (2018) 190:89–98. doi: 10.1016/j.ajo.2018.03.007
86. Mohaqiq Z, Moossavi M, Hemmati M, Kazemi T, Mehrpour O. Antioxidant properties of saffron stigma and petals: a potential therapeutic approach for insulin resistance through an insulin-sensitizing adipocytokine in high-calorie diet rats. Int J Prev Med. (2020) 11:184. doi: 10.4103/ijpvm.IJPVM_275_19
87. Nikbakht-Jam I, Khademi M, Nosrati M, Eslami S, Foroutan-Tanha M, Sahebkar A, et al. Effect of crocin extracted from saffron on pro-oxidant–anti-oxidant balance in subjects with metabolic syndrome: a randomized, placebo-controlled clinical trial. Eur J Integr Med. (2016) 8:307–12. doi: 10.1016/j.eujim.2015.12.008
88. Ebrahimi F, Sahebkar A, Aryaeian N, Pahlavani N, Fallah S, Moradi N, et al. Effects of saffron supplementation on inflammation and metabolic responses in type 2 diabetic patients: a randomized, double-blind, placebo-controlled trial. Diab Metab Syndrome Obesity. (2019) 12:2107–15. doi: 10.2147/DMSO.S216666
89. Javandoost A, Afshari A, Nikbakht-Jam I, Khademi M, Eslami S, Nosrati M, et al. Effect of crocin, a carotenoid from saffron, on plasma cholesteryl ester transfer protein and lipid profile in subjects with metabolic syndrome: a double blind randomized clinical trial. ARYA Atheroscler. (2017) 13:245–52.
90. Broadhead GK, Grigg JR, McCluskey P, Hong T, Schlub TE, Chang AA. Saffron therapy for the treatment of mild/moderate age-related macular degeneration: a randomised clinical trial. Graefe's Arch Clin Exp Ophthalmol. (2019) 257:31–40. doi: 10.1007/s00417-018-4163-x
91. Piccardi M, Marangoni D, Minnella AM, Savastano MC, Valentini P, Ambrosio L, et al. A longitudinal follow-up study of saffron supplementation in early age-related macular degeneration: sustained benefits to central retinal function. Evid.-Based Complement Altern Med. (2012) 2012:429124. doi: 10.1155/2012/429124
92. Lashay A, Sadough G, Ashrafi E, Lashay M, Movassat M, Akhondzadeh S. Short-term outcomes of saffron supplementation in patients with age-related macular degeneration: a double-blind, placebo-controlled, randomized trial. Med Hypothesis Discov Innov Ophthalmol. (2016) 5:32–8.
93. Di Marco S, Carnicelli V, Franceschini N, Di Paolo M, Piccardi M, Bisti S, et al. Saffron: a multitask neuroprotective agent for retinal degenerative diseases. Antioxidants. (2019) 8:224. doi: 10.3390/antiox8070224
94. Fernández-Sánchez L, Lax P, Esquiva G, Martín-Nieto J, Pinilla I, Cuenca N. Safranal, a saffron constituent, attenuates retinal degeneration in P23H rats. PLoS ONE. (2012) 7:e43074. doi: 10.1371/journal.pone.0043074
95. Ohno Y, Nakanishi T, Umigai N, Tsuruma K, Shimazawa M, Hara H. Oral administration of crocetin prevents inner retinal damage induced by N-methyl-d-aspartate in mice. Eur J Pharmacol. (2012) 690:84–9. doi: 10.1016/j.ejphar.2012.06.035
96. Yamauchi M, Tsuruma K, Imai S, Nakanishi T, Umigai N, Shimazawa M, et al. Crocetin prevents retinal degeneration induced by oxidative and endoplasmic reticulum stresses via inhibition of caspase activity. Eur J Pharmacol. (2011) 650:110–9. doi: 10.1016/j.ejphar.2010.09.081
97. Jabbarpoor Bonyadi MH, Yazdani S, Saadat S. The ocular hypotensive effect of saffron extract in primary open angle glaucoma: a pilot study. BMC Complement Altern Med. (2014) 14:399. doi: 10.1186/1472-6882-14-399
98. Mahdiani S, Shokoohi-Rad S, Sepahi S, Motamedshariaty VS, Mohajeri SA, Sahebkar A. Crocin supplementation in primary open angle glaucoma: a randomized, triple-blind, placebo-controlled clinical trial. Med Drug Discov. (2024) 21:100169. doi: 10.1016/j.medidd.2023.100169
99. Fernández-Albarral JA, Ramírez AI, de Hoz R, López-Villarín N, Salobrar-García E, López-Cuenca I, et al. Neuroprotective and anti-inflammatory effects of a hydrophilic saffron extract in a model of glaucoma. Int J Mol Sci. (2019) 204110. doi: 10.3390/ijms20174110
100. Yang X, Huo F, Liu B, Liu J, Chen T, Li J, et al. Crocin inhibits oxidative stress and pro-inflammatory response of microglial cells associated with diabetic retinopathy through the activation of PI3K/Akt signaling pathway. J Mol Neurosci. (2017) 61:581–9. doi: 10.1007/s12031-017-0899-8
101. Akhondzadeh S, Sabet MS, Harirchian MH, Togha M, Cheraghmakani H, Razeghi S, et al. Saffron in the treatment of patients with mild to moderate Alzheimer's disease: a 16-week, randomized and placebo-controlled trial. J Clin Pharm Ther. (2010) 35:581–8. doi: 10.1111/j.1365-2710.2009.01133.x
102. Akhondzadeh S, Shafiee Sabet M, Harirchian MH, Togha M, Cheraghmakani H, Razeghi S, et al. A 22-week, multicenter, randomized, double-blind controlled trial of Crocus sativus in the treatment of mild-to-moderate Alzheimer's disease. Psychopharmacology. (2010) 207:637–43. doi: 10.1007/s00213-009-1706-1
103. Rasi Marzabadi L, Fazljou SMB, Araj-Khodaei M, Sadigh-Eteghad S, Naseri A, Talebi M. Saffron reduces some inflammation and oxidative stress markers in donepezil-treated mild-to-moderate Alzheimer's disease patients: a randomized double-blind placebo-control trial. J Herbal Med. (2022) 34:100574. doi: 10.1016/j.hermed.2022.100574
104. Farokhnia M, Shafiee Sabet M, Iranpour N, Gougol A, Yekehtaz H, Alimardani R, et al. Comparing the efficacy and safety of Crocus sativus L. with memantine in patients with moderate to severe Alzheimer's disease: a double-blind randomized clinical trial. Hum Psychopharmacol: Clin Exp. (2014) 29:351–9. doi: 10.1002/hup.2412
105. Avgerinos KI, Vrysis C, Chaitidis N, Kolotsiou K, Myserlis PG. Kapogiannis D. Effects of saffron (Crocus sativus L.) on cognitive function: a systematic review of RCTs. Neurol Sci. (2020) 41:2747–54. doi: 10.1007/s10072-020-04427-0
106. Ghahghaei A, Bathaie SZ, Kheirkhah H, Bahraminejad E. The protective effect of crocin on the amyloid fibril formation of aβ42 peptide in vitro. Cell Mol Biol Lett. (2013) 18:328–39. doi: 10.2478/s11658-013-0092-1
107. Kong Y, Kong L-P, Luo T, Li G-W, Jiang W, Li S, et al. The protective effects of crocetin on Aβ1−−42-induced toxicity in Ht22 cells. CNS Neurol Disord- Drug Targets. (2014) 13:1627–32. doi: 10.2174/1871527313666140806125410
108. Ochiai T, Shimeno H, Mishima K-i, Iwasaki K, Fujiwara M, Tanaka H, et al. Protective effects of carotenoids from saffron on neuronal injury in vitro and in vivo. Biochim Biophys Acta Gen Sub. (2007) 1770:578–84. doi: 10.1016/j.bbagen.2006.11.012
109. Rafieipour F, Hadipour E, Emami SA, Asili J, Tayarani-Najaran Z. Safranal protects against beta-amyloid peptide-induced cell toxicity in PC12 cells via MAPK and PI3 K pathways. Metab Brain Dis. (2019) 34:165–72. doi: 10.1007/s11011-018-0329-9
110. Inoue E, Shimizu Y, Masui R, Hayakawa T, Tsubonoya T, Hori S, et al. Effects of saffron and its constituents, crocin-1, crocin-2, and crocetin on α-synuclein fibrils. J Nat Med. (2018) 72:274–9. doi: 10.1007/s11418-017-1150-1
111. Salama RM, Abdel-Latif GA, Abbas SS, El Magdoub HM, Schaalan MF. Neuroprotective effect of crocin against rotenone-induced Parkinson's disease in rats: interplay between PI3K/Akt/mTOR signaling pathway and enhanced expression of miRNA-7 and miRNA-221. Neuropharmacology. (2020) 164:107900. doi: 10.1016/j.neuropharm.2019.107900
112. Zhang G-F, Zhang Y, Zhao G. Crocin protects PC12 cells against MPP+-induced injury through inhibition of mitochondrial dysfunction and ER stress. Neurochem Int. (2015) 89:101–10. doi: 10.1016/j.neuint.2015.07.011
113. Ahmad AS, Ansari MA, Ahmad M, Saleem S, Yousuf S, Hoda MN, et al. Neuroprotection by crocetin in a hemi-parkinsonian rat model. Pharmacol Biochem Behav. (2005) 81:805–13. doi: 10.1016/j.pbb.2005.06.007
114. Purushothuman S, Nandasena C, Peoples CL, El Massri N, Johnstone DM, Mitrofanis J, et al. Saffron pre-treatment offers neuroprotection to nigral and retinal dopaminergic cells of MPTP-treated mice. J Parkinson's Dis. (2013) 3:77–83. doi: 10.3233/JPD-130173
115. Rao SV. Muralidhara, Yenisetti SC, Rajini PS. Evidence of neuroprotective effects of saffron and crocin in a Drosophila model of parkinsonism. Neuro Toxicol. (2016) 52:230–42. doi: 10.1016/j.neuro.2015.12.010
116. Hatami H. Dehghan G. The effect of ethanolic extract of saffron (Crocus sativus L.) on improving the spatial memory parameters in the experimental models of Parkinson's disease in male rats. JABS. (2015) 5:534.
117. Lalezari V, Aghamollaii V, Moslehi A, Najafi A, Parsaei M, Beikmarzehei A, et al. Evaluating efficacy and safety of Saffron add-on treatment in improvement of motor and depressive symptoms of patients with Parkinson's disease: a randomized, double-blind, placebo-controlled clinical trial. J Herbal Med. (2024) 48:100968. doi: 10.1016/j.hermed.2024.100968
118. Villelabeitia-Jaureguizar K, Vicente-Campos D, Senen AB, Jiménez VH, Garrido-Lestache MEB, Chicharro JL. Effects of high-intensity interval versus continuous exercise training on post-exercise heart rate recovery in coronary heart-disease patients. Int J Cardiol. (2017) 244:17–23. doi: 10.1016/j.ijcard.2017.06.067
119. Beckie TM, Beckstead JW, Kip KE, Fletcher G. Improvements in heart rate recovery among women after cardiac rehabilitation completion. J Cardiovasc Nurs. (2014) 29:38–47. doi: 10.1097/JCN.0b013e31827324e2
120. Blumenthal JA, Sherwood A, Babyak MA, Watkins LL, Waugh R, Georgiades A, et al. Effects of exercise and stress management training on markers of cardiovascular risk in patients with ischemic heart disease: a randomized controlled trial. JAMA. (2005) 293:1626–34. doi: 10.1001/jama.293.13.1626
121. Kitzman Dalane W, Brubaker Peter H, Herrington David M, Morgan Timothy M, Stewart Kathryn P, Hundley WG, et al. Effect of endurance exercise training on endothelial function and arterial stiffness in older patients with heart failure and preserved ejection fraction. JACC. (2013) 62:584–92. doi: 10.1016/j.jacc.2013.04.033
122. Wu S-K, Lin Y-W, Chen C-L, Tsai S-W. Cardiac rehabilitation vs. home exercise after coronary artery bypass graft surgery: a comparison of heart rate recovery. American J Phys Med Rehabil. (2006) 85:66. doi: 10.1097/01.phm.0000228597.64057.66
123. Chuang T-Y, Sung W-H, Lin C-Y. Application of a virtual reality–enhanced exercise protocol in patients after coronary bypass. Arch Phys Med Rehabil. (2005) 86:1929–32. doi: 10.1016/j.apmr.2005.05.003
124. Giallauria F, Lorenzo AD, Pilerci F, Manakos A, Lucci R, Psaroudaki M, et al. Long-term effects of cardiac rehabilitation on end-exercise heart rate recovery after myocardial infarction. Eur J Cardiovasc Prev Rehabil. (2006) 13:544–50. doi: 10.1097/01.hjr.0000216547.07432.fb
125. Legramante JM, Iellamo F, Massaro M, Sacco S, Galante A. Effects of residential exercise training on heart rate recovery in coronary artery patients. Am J Physiol-Heart Circul Physiol. (2007) 292:H510–H5. doi: 10.1152/ajpheart.00748.2006
126. Kubo N, Ohmura N, Nakada I, Yasu T. Katsuki Ta, Fujii M, Saito M. Exercise at ventilatory threshold aggravates left ventricular remodeling in patients with extensive anterior acute myocardial infarction. Am Heart J. (2004) 147:113–20. doi: 10.1016/S0002-8703(03)00521-0
127. Giallauria F, Acampa W, Ricci F, Vitelli A, Torella G, Lucci R, et al. Exercise training early after acute myocardial infarction reduces stress-induced hypoperfusion and improves left ventricular function. Eur J Nucl Med Mol Imaging. (2013) 40:315–24. doi: 10.1007/s00259-012-2302-x
128. Abolahrari-Shirazi S, Kojuri J, Bagheri Z, Rojhani-Shirazi Z. Efficacy of combined endurance-resistance training versus endurance training in patients with heart failure after percutaneous coronary intervention: a randomized controlled trial. J Res Med Sci. (2018) 23:12. doi: 10.4103/jrms.JRMS_743_17
129. Kraal JJ, Peek N, van den Akker-Van Marle ME, Kemps HMC. Effects and costs of home-based training with telemonitoring guidance in low to moderate risk patients entering cardiac rehabilitation: the FIT@Home study. BMC Cardiovasc Disord. (2013) 13:82. doi: 10.1186/1471-2261-13-82
130. Bacchi E, Negri C, Zanolin ME, Milanese C, Faccioli N, Trombetta M, et al. Metabolic effects of aerobic training and resistance training in type 2 diabetic subjects: a randomized controlled trial (the RAED2 study). Diabetes Care. (2012) 35:676–82. doi: 10.2337/dc11-1655
131. Cauza E, Hanusch-Enserer U, Strasser B, Ludvik B, Metz-Schimmerl S, Pacini G, et al. The relative benefits of endurance and strength training on the metabolic factors and muscle function of people with type 2 diabetes mellitus. Arch Phys Med Rehabil. (2005) 86:1527–33. doi: 10.1016/j.apmr.2005.01.007
132. Sigal RJ, Kenny GP, Boulé NG, Wells GA. Prud'homme D, Fortier M, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes. Ann Intern Med. (2007) 147:357–69. doi: 10.7326/0003-4819-147-6-200709180-00005
133. Church TS, Blair SN, Cocreham S, Johannsen N, Johnson W, Kramer K, et al. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial. JAMA. (2010) 304:2253–62. doi: 10.1001/jama.2010.1710
134. Chen S-M, Shen F-C, Chen J-F, Chang W-D, Chang N-J. Effects of resistance exercise on glycated hemoglobin and functional performance in older patients with comorbid diabetes mellitus and knee osteoarthritis: a randomized trial. Int J Environ Res Public Health. (2020) 17:224. doi: 10.3390/ijerph17010224
135. Byrkjeland R, Njerve IU, Anderssen S, Arnesen H, Seljeflot I, Solheim S. Effects of exercise training on HbA1c and VO2peak in patients with type 2 diabetes and coronary artery disease: a randomised clinical trial. Diab Vasc Dis Res. (2015) 12:325–33. doi: 10.1177/1479164115590552
136. Kadoglou NPE, Iliadis F, Angelopoulou N, Perrea D, Ampatzidis G, Liapis CD, et al. The anti-inflammatory effects of exercise training in patients with type 2 diabetes mellitus. Eur J Cardiovasc Prev Rehabil. (2007) 14:837–43. doi: 10.1097/HJR.0b013e3282efaf50
137. Kadoglou NPE, Fotiadis G, Kapelouzou A, Kostakis A, Liapis CD, Vrabas IS. The differential anti-inflammatory effects of exercise modalities and their association with early carotid atherosclerosis progression in patients with Type 2 diabetes. Diabetic Med. (2013) 30:e41–50. doi: 10.1111/dme.12055
138. Kadoglou NP, Vrabas IS, Kapelouzou A, Lampropoulos S, Sailer N, Kostakis A, et al. The impact of aerobic exercise training on novel adipokines, apelin and ghrelin, in patients with type 2 diabetes. Med Sci Monit. (2012) 18:Cr290–5. doi: 10.12659/MSM.882734
139. Choi KM, Han KA, Ahn HJ, Hwang SY, Hong HC, Choi HY, et al. Effects of exercise on sRAGE levels and cardiometabolic risk factors in patients with type 2 diabetes: a randomized controlled trial. J Clin Endocrinol Metab. (2012) 97:3751–8. doi: 10.1210/jc.2012-1951
140. Gulsin GS, Swarbrick DJ, Athithan L, Brady EM, Henson J, Baldry E, et al. Effects of low-energy diet or exercise on cardiovascular function in working-age adults with type 2 diabetes: a prospective, randomized, open-label, blinded end point trial. Diabetes Care. (2020) 43:1300–10. doi: 10.2337/dc20-0129
141. Madden KM, Lockhart C, Cuff D, Potter TF, Meneilly GS. Short-term aerobic exercise reduces arterial stiffness in older adults with type 2 diabetes, hypertension, and hypercholesterolemia. Diabetes Care. (2009) 32:1531–5. doi: 10.2337/dc09-0149
142. Myers VH, McVay MA, Brashear MM, Johannsen NM, Swift DL, Kramer K, et al. Exercise training and quality of life in individuals with type 2 diabetes: a randomized controlled trial. Diabetes Care. (2013) 36:1884–90. doi: 10.2337/dc12-1153
143. Tessier D, Ménard J, Fülöp T, Ardilouze J-L, Roy M-A, Dubuc N, et al. Effects of aerobic physical exercise in the elderly with type 2 diabetes mellitus. Arch Gerontol Geriatr. (2000) 31:121–32. doi: 10.1016/S0167-4943(00)00076-5
144. Stubbs EB, Fisher MA, Miller CM, Jelinek C, Butler J, McBurney C, et al. Randomized controlled trial of physical exercise in diabetic veterans with length-dependent distal symmetric polyneuropathy. Front Neurosci. (2019) 13:51. doi: 10.3389/fnins.2019.00051
145. Hangping Z, Xiaona Q, Qi Z, Qingchun L, Na Y, Lijin J, et al. The impact on glycemic control through progressive resistance training with bioDensityTM in Chinese elderly patients with type 2 diabetes: the PReTTy2 (progressive resistance training in type 2 diabetes) trial. Diabetes Res Clin Pract. (2019) 150:64–71. doi: 10.1016/j.diabres.2019.02.011
146. Magalhães JP, Júdice PB, Ribeiro R, Andrade R, Raposo J, Dores H, et al. Effectiveness of high-intensity interval training combined with resistance training versus continuous moderate-intensity training combined with resistance training in patients with type 2 diabetes: a one-year randomized controlled trial. Diab Obes Metab. (2019) 21:550–9. doi: 10.1111/dom.13551
147. Otten J, Stomby A, Waling M, Isaksson A, Tellström A, Lundin-Olsson L, et al. Benefits of a Paleolithic diet with and without supervised exercise on fat mass, insulin sensitivity, and glycemic control: a randomized controlled trial in individuals with type 2 diabetes. Diabetes Metab Res Rev. (2017) 33:e2828. doi: 10.1002/dmrr.2828
148. Meier NF, Lee DC, Sui X, Blair SN. Physical activity, cardiorespiratory fitness, and incident glaucoma. Med Sci Sports Exerc. (2018) 50:2253–8. doi: 10.1249/MSS.0000000000001692
149. Lee MJ, Wang J, Friedman DS, Boland MV, De Moraes CG, Ramulu PY. Greater physical activity is associated with slower visual field loss in glaucoma. Ophthalmology. (2019) 126:958–64. doi: 10.1016/j.ophtha.2018.10.012
150. Yokota S, Takihara Y, Kimura K, Takamura Y, Inatani M. The relationship between self-reported habitual exercise and visual field defect progression: a retrospective cohort study. BMC Ophthalmol. (2016) 16:147. doi: 10.1186/s12886-016-0326-x
151. Yan X, Li M, Song Y, Guo J, Zhao Y, Chen W, et al. Influence of exercise on intraocular pressure, schlemm's canal, and the trabecular meshwork. Invest Ophthalmol Vis Sci. (2016) 57:4733–9. doi: 10.1167/iovs.16-19475
152. Yeak Dieu Siang J, Mohamed MNAB, Mohd Ramli NB, Zahari MB. Effects of regular exercise on intraocular pressure. Eur J Ophthalmol. (2021) 32:2265–73. doi: 10.1177/11206721211051236
153. Chrysostomou V, Kezic JM, Trounce IA, Crowston JG. Forced exercise protects the aged optic nerve against intraocular pressure injury. Neurobiol Aging. (2014) 35:1722–5. doi: 10.1016/j.neurobiolaging.2014.01.019
154. He Y-Y, Wang L, Zhang T, Weng S-J, Lu J, Zhong Y-M. Aerobic exercise delays retinal ganglion cell death after optic nerve injury. Exp Eye Res. (2020) 200:108240. doi: 10.1016/j.exer.2020.108240
155. Loprinzi PD. Association of accelerometer-assessed sedentary behavior with diabetic retinopathy in the United States. JAMA Ophthalmol. (2016) 134:1197–8. doi: 10.1001/jamaophthalmol.2016.2400
156. Soleimani A, Soltani P, Karimi H, Mirzaei M, Esfahanian F, Yavari M, et al. The effect of moderate-intensity aerobic exercise on non-proliferative diabetic retinopathy in type II diabetes mellitus patients: a clinical trial. Microvasc Res. (2023) 149:104556. doi: 10.1016/j.mvr.2023.104556
157. Cheng C-K, Shang W, Liu J, Cheang W-S, Wang Y, Xiang L, et al. Activation of AMPK/miR-181b axis alleviates endothelial dysfunction and vascular inflammation in diabetic mice. Antioxidants. (2022) 11:1137. doi: 10.3390/antiox11061137
158. Knudtson MD, Klein R, Klein BEK. Physical activity and the 15-year cumulative incidence of age-related macular degeneration: the Beaver Dam Eye Study. Br J Ophthalmol. (2006) 90:1461. doi: 10.1136/bjo.2006.103796
159. McGuinness MB, Karahalios A, Simpson JA, Guymer RH, Robman LD, Hodge AM, et al. Past physical activity and age-related macular degeneration: the Melbourne Collaborative Cohort Study. Br J Ophthalmol. (2016) 100:1353. doi: 10.1136/bjophthalmol-2015-307663
160. Ulańczyk Z, Grabowicz A, Cecerska-Heryć E, Sleboda-Taront D, Krytkowska E, Mozolewska-Piotrowska K, et al. Dietary and lifestyle factors modulate the activity of the endogenous antioxidant system in patients with age-related macular degeneration: correlations with disease severity. Antioxidants. (2020) 9:954. doi: 10.3390/antiox9100954
161. Cui B, Guo X, Zhou W, Zhang X, He K, Bai T, et al. Exercise alleviates neovascular age-related macular degeneration by inhibiting AIM2 inflammasome in myeloid cells. Metabolism. (2023) 144:155584. doi: 10.1016/j.metabol.2023.155584
162. Zhang X, Girardot PE, Sellers JT Li Y, Wang J, Chrenek MA, et al. Wheel running exercise protects against retinal degeneration in the I307N rhodopsin mouse model of inducible autosomal dominant retinitis pigmentosa. Mol Vis. (2019) 25:462–76.
163. Lawson EC, Han MK, Sellers JT, Chrenek MA, Hanif A, Gogniat MA, et al. Aerobic exercise protects retinal function and structure from light-induced retinal degeneration. J Neurosci. (2014) 34:2406. doi: 10.1523/JNEUROSCI.2062-13.2014
164. Williams PT. Relationship of incident glaucoma versus physical activity and fitness in male runners. Med Sci Sports Exerc. (2009) 41:1566–72. doi: 10.1249/MSS.0b013e31819e420f
165. Zheng Selin J, Orsini N, Ejdervik Lindblad B, Wolk A. Long-term physical activity and risk of age-related cataract: a population-based prospective study of male and female cohorts. Ophthalmology. (2015) 122:274–80. doi: 10.1016/j.ophtha.2014.08.023
166. López-Sánchez GF, Pardhan S, Trott M, Sánchez-Castillo S, Jackson SE, Tully M, et al. The association between physical activity and cataracts among 17,777 people aged 15–69 years residing in Spain. Ophthalmic Epidemiol. (2020) 27:272–7. doi: 10.1080/09286586.2020.1730911
167. Lautenschlager NT, Cox KL, Flicker L, Foster JK, van Bockxmeer FM, Xiao J, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer's disease: a randomized trial. JAMA. (2008) 300:1027–37. doi: 10.1001/jama.300.9.1027
168. Yágüez L, Shaw KN, Morris R, Matthews D. The effects on cognitive functions of a movement-based intervention in patients with Alzheimer's type dementia: a pilot study. Int J Geriatr Psychiatry. (2011) 26:173–81. doi: 10.1002/gps.2510
169. Holthoff VA, Marschner K, Scharf M, Steding J, Meyer S, Koch R, et al. Effects of physical activity training in patients with Alzheimer's dementia: results of a pilot RCT study. PLoS ONE. (2015) 10:e0121478. doi: 10.1371/journal.pone.0121478
170. Hoffmann K, Sobol NA, Frederiksen KS, Beyer N, Vogel A, Vestergaard K, et al. Moderate-to-high intensity physical exercise in patients with Alzheimer's disease: a randomized controlled trial. J Alzheimers Dis. (2015) 50:443–53. doi: 10.3233/JAD-150817
171. Öhman H, Savikko N, Strandberg TE, Kautiainen H, Raivio MM, Laakkonen M-L, et al. Effects of exercise on cognition: the finnish Alzheimer's disease exercise trial: a randomized, controlled trial. J Am Geriatr Soc. (2016) 64:731–8. doi: 10.1111/jgs.14059
172. Morris JK, Vidoni ED, Johnson DK, Van Sciver A, Mahnken JD, Honea RA, et al. Aerobic exercise for Alzheimer's disease: a randomized controlled pilot trial. PLoS ONE. (2017) 12:e0170547. doi: 10.1371/journal.pone.0170547
173. Yu F, Vock DM, Zhang L, Salisbury D, Nelson NW, Chow LS, et al. Cognitive effects of aerobic exercise in Alzheimer's disease: a pilot randomized controlled trial. J Alzheimers Dis. (2021) 80:233–44. doi: 10.3233/JAD-201100
174. Papatsimpas V, Vrouva S, Papathanasiou G, Papadopoulou M, Bouzineki C, Kanellopoulou S, et al. Does therapeutic exercise support improvement in cognitive function and instrumental activities of daily living in patients with mild Alzheimer's disease? A randomized controlled trial. Brain Sci. (2023) 13:1112. doi: 10.3390/brainsci13071112
175. David S, Costa AS, Hohenfeld C, Romanzetti S, Mirzazade S, Pahl J, et al. Modulating effects of fitness and physical activity on Alzheimer's disease: implications from a six-month randomized controlled sports intervention. J Alzheimers Dis. (2025) 103:552–69. doi: 10.1177/13872877241303764
176. Toots A, Littbrand H, Boström G, Hörnsten C, Holmberg H, Lundin-Olsson L, et al. Effects of exercise on cognitive function in older people with dementia: a randomized controlled trial. J Alzheimers Dis. (2017) 60:323–32. doi: 10.3233/JAD-170014
177. Henskens M, Nauta Ilse M, van Eekeren Marieke CA, Scherder Erik JA. Effects of physical activity in nursing home residents with dementia: a randomized controlled trial. Dement Geriatr Cogn Disord. (2018) 46:60–80. doi: 10.1159/000491818
178. Zhao N, Yan Q-W, Xia J, Zhang X-L, Li B-X, Yin L-Y, et al. Treadmill exercise attenuates Aβ-induced mitochondrial dysfunction and enhances mitophagy activity in APP/PS1 transgenic mice. Neurochem Res. (2020) 45:1202–14. doi: 10.1007/s11064-020-03003-4
179. Zhao N, Zhang X, Li B, Wang J, Zhang C, Xu B. Treadmill exercise improves PINK1/parkin-mediated mitophagy activity against Alzheimer's disease pathologies by upregulated SIRT1-FOXO1/3 axis in APP/PS1 Mice. Mol Neurobiol. (2023) 60:277–91. doi: 10.1007/s12035-022-03035-7
180. Shi D, Hao Z, Qi W, Jiang F, Liu K, Shi X. Aerobic exercise combined with chlorogenic acid exerts neuroprotective effects and reverses cognitive decline in Alzheimer's disease model mice (APP/PS1) via the SIRT1//PGC-1α/PPARγ signaling pathway. Front Aging Neurosci. (2023) 15:1269952. doi: 10.3389/fnagi.2023.1269952
181. Wang X. Zhu Y-t, Zhu Y, Sun Y-l, Huang J, Li Z, et al. Long-term running exercise alleviates cognitive dysfunction in APP/PSEN1 transgenic mice via enhancing brain lysosomal function. Acta Pharmacol Sin. (2022) 43:850–61. doi: 10.1038/s41401-021-00720-6
182. García-Mesa Y, Pareja-Galeano H, Bonet-Costa V, Revilla S, Gómez-Cabrera MC, Gambini J, et al. Physical exercise neuroprotects ovariectomized 3xTg-AD mice through BDNF mechanisms. Psychoneuroendocrinology. (2014) 45:154–66. doi: 10.1016/j.psyneuen.2014.03.021
183. Klein CP, Hoppe JB, Saccomori AB, dos Santos BG, Sagini JP, Crestani MS, et al. Physical exercise during pregnancy prevents cognitive impairment induced by amyloid-β in adult offspring rats. Mol Neurobiol. (2019) 56:2022–38. doi: 10.1007/s12035-018-1210-x
184. Koo J-H, Kang E-B, Oh Y-S, Yang D-S, Cho J-Y. Treadmill exercise decreases amyloid-β burden possibly via activation of SIRT-1 signaling in a mouse model of Alzheimer's disease. Exp Neurol. (2017) 288:142–52. doi: 10.1016/j.expneurol.2016.11.014
185. Marques-Aleixo I, Santos-Alves E, Balça MM, Rizo-Roca D, Moreira PI, Oliveira PJ, et al. Physical exercise improves brain cortex and cerebellum mitochondrial bioenergetics and alters apoptotic, dynamic and auto(mito)phagy markers. Neuroscience. (2015) 301:480–95. doi: 10.1016/j.neuroscience.2015.06.027
186. Ni M, Mooney K, Signorile JF. Controlled pilot study of the effects of power yoga in Parkinson's disease. Complement Ther Med. (2016) 25:126–31. doi: 10.1016/j.ctim.2016.01.007
187. Kwok JYY, Kwan JCY, Auyeung M, Mok VCT, Lau CKY, Choi KC, et al. Effects of mindfulness yoga vs. stretching and resistance training exercises on anxiety and depression for people with Parkinson disease: a randomized clinical trial. JAMA Neurol. (2019) 76:755–63. doi: 10.1001/jamaneurol.2019.0534
188. Rawson KS, McNeely ME, Duncan RP, Pickett KA, Perlmutter JS, Earhart GM. Exercise and Parkinson's disease: comparing tango, treadmill, and stretching. J Neurol Phys Ther. (2019) 43:245. doi: 10.1097/NPT.0000000000000245
189. Gaßner H, Trutt E, Seifferth S, Friedrich J, Zucker D, Salhani Z, et al. Treadmill training and physiotherapy similarly improve dual task gait performance: a randomized-controlled trial in Parkinson's disease. J Neural Transm. (2022) 129:1189–200. doi: 10.1007/s00702-022-02514-4
190. Donahue EK, Venkadesh S, Bui V, Tuazon AC, Wang RK, Haase D, et al. Physical activity intensity is associated with cognition and functional connectivity in Parkinson's disease. Parkinsonism Relat Disord. (2022) 104:7–14. doi: 10.1016/j.parkreldis.2022.09.005
191. Fiorelli CM, Ciolac EG, Simieli L, Silva FA, Fernandes B, Christofoletti G, et al. Differential acute effect of high-intensity interval or continuous moderate exercise on cognition in individuals with Parkinson's disease. J Phys Activity Health. (2019) 16:157–64. doi: 10.1123/jpah.2018-0189
192. Kathia MM, Duplea S-G, Bommarito JC, Hinks A, Leake E, Shannon J, et al. High-intensity interval versus moderate-intensity continuous cycling training in Parkinson's disease: a randomized trial. J Appl Physiol. (2024) 137:603–15. doi: 10.1152/japplphysiol.00219.2024
193. Marusiak J, Fisher BE, Jaskólska A, Słotwiński K, Budrewicz S, Koszewicz M, et al. Eight Weeks of aerobic interval training improves psychomotor function in patients with Parkinson's disease—randomized controlled trial. Int J Environ Res Public Health. (2019) 16:880. doi: 10.3390/ijerph16050880
194. Vieira de. Moraes Filho A, Chaves SN, Martins WR, Tolentino GP, de Cássia Pereira Pinto Homem R, Landim de Farias G, et al. Progressive resistance training improves bradykinesia, motor symptoms and functional performance in patients with Parkinson's disease. Cli Interv Aging. (2020) 15:87–95. doi: 10.2147/CIA.S231359
195. Ferreira RM. Alves WMGdC, Lima TA, Alves TGG, Alves PAMF, Pimentel CP, et al. The effect of resistance training on the anxiety symptoms and quality of life in elderly people with Parkinson's disease: a randomized controlled trial. Arq Neuropsiquiatr. (2018) 76:499–506. doi: 10.1590/0004-282x20180071
196. Douris PC, Cogen ZS, Fields HT, Greco LC, Hasley MR, Machado CM, et al. The effects of blood flow restriction training on functional improvements in an active single subject with Parkinson's disease. Int J Sports Phys Ther. (2018) 13:247–54. doi: 10.26603/ijspt20180247
197. Douris PC, D'Agostino N, Werner WG, Petrizzo J, DiFrancisco-Donoghue J. Blood flow restriction resistance training in a recreationally active person with Parkinson's disease. Physiother Theory Pract. (2022) 38:422–30. doi: 10.1080/09593985.2020.1762812
198. Bane A, Wilson L, Jumper J, Spindler L, Wyatt P, Willoughby D. Effects of Blood flow restriction resistance training on autonomic and endothelial function in persons with Parkinson's disease. J Parkinson's Dis. (2024) 14:761–75. doi: 10.3233/JPD-230259
199. Costa ROd, Gadelha-Filho CVJ, Costa AEMd, Feitosa ML, Araújo DPd, Lucena JDd, et al. The treadmill exercise protects against dopaminergic neuron loss and brain oxidative stress in Parkinsonian rats. Oxid Med Cell Longe. (2017) 2017:2138169. doi: 10.1155/2017/2138169
200. Chuang C-S, Chang J-C, Cheng F-C, Liu K-H, Su H-L, Liu C-S. Modulation of mitochondrial dynamics by treadmill training to improve gait and mitochondrial deficiency in a rat model of Parkinson's disease. Life Sci. (2017) 191:236–44. doi: 10.1016/j.lfs.2017.10.003
201. Koo J-H, Cho J-Y. Treadmill exercise attenuates α-synuclein levels by promoting mitochondrial function and autophagy possibly via SIRT1 in the chronic MPTP/P-induced mouse model of Parkinson's disease. Neurotox Res. (2017) 32:473–86. doi: 10.1007/s12640-017-9770-5
202. Jang Y, Kwon I, Song W, Cosio-Lima LM, Lee Y. Endurance exercise mediates neuroprotection against MPTP-mediated Parkinson's disease via enhanced neurogenesis, antioxidant capacity, and autophagy. Neuroscience. (2018) 379:292–301. doi: 10.1016/j.neuroscience.2018.03.015
203. Liu W, Li L, Liu S, Wang Z, Kuang H, Xia Y, et al. MicroRNA expression profiling screen miR-3557/324-targeted CaMK/mTOR in the rat striatum of Parkinson's disease in regular aerobic exercise. Biomed Res Int. (2019) 2019:7654798. doi: 10.1155/2019/7654798
204. Liu W, Fu R, Wang Z, Liu S, Tang C, Li L, et al. Regular aerobic exercise-alleviated dysregulation of CAMKIIα carbonylation to mitigate Parkinsonism via homeostasis of apoptosis with autophagy. J Neuropathol Exp Neurol. (2020) 79:46–61. doi: 10.1093/jnen/nlz106
205. Johansson ME, Cameron IGM, Van der Kolk NM, de Vries NM, Klimars E, Toni I, et al. Aerobic exercise alters brain function and structure in Parkinson's disease: a randomized controlled trial. Ann Neurol. (2022) 91:203–16. doi: 10.1002/ana.26291
206. Sacheli MA, Neva JL, Lakhani B, Murray DK, Vafai N, Shahinfard E, et al. Exercise increases caudate dopamine release and ventral striatal activation in Parkinson's disease. Mov Disord. (2019) 34:1891–900. doi: 10.1002/mds.27865
207. Rose MH, Løkkegaard A, Sonne-Holm S, Jensen BR. Improved clinical status, quality of life, and walking capacity in Parkinson's disease after body weight-supported high-intensity locomotor training. Arch Phys Med Rehabil. (2013) 94:687–92. doi: 10.1016/j.apmr.2012.11.025
208. Dashtipour K, Johnson E, Kani C, Kani K, Hadi E, Ghamsary M, et al. Effect of exercise on motor and nonmotor symptoms of Parkinson's disease. Parkinsons Dis. (2015) 2015:586378. doi: 10.1155/2015/586378
209. van der Kolk NM, de Vries NM, Kessels RPC, Joosten H, Zwinderman AH, Post B, et al. Effectiveness of home-based and remotely supervised aerobic exercise in Parkinson's disease: a double-blind, randomised controlled trial. Lancet Neurol. (2019) 18:998–1008. doi: 10.1016/S1474-4422(19)30285-6
210. Hooshmand-Moghadam B, Eskandari M, Shabkhiz F, Mojtahedi S. Mahmoudi N. Saffron (Crocus sativus L.) in combination with resistance training reduced blood pressure in the elderly hypertensive men: a randomized controlled trial. Br J Clin Pharmacol. (2021) 87:3255–67. doi: 10.1111/bcp.14746
211. Mojtahedi S, Hooshmand-Moghadam B, Rosenkranz S, Shourideh Z, Amirshaghaghi F. Shabkhiz F. Improvement of inflammatory status following saffron (Crocus sativus L.) and resistance training in elderly hypertensive men: a randomized controlled trial. Exp Gerontol. (2022) 162:111756. doi: 10.1016/j.exger.2022.111756
212. Dehghan F, Hajiaghaalipour F, Yusof A, Muniandy S, Hosseini SA, Heydari S, et al. Saffron with resistance exercise improves diabetic parameters through the GLUT4/AMPK pathway in-vitro and in-vivo. Sci Rep. (2016) 6:25139. doi: 10.1038/srep25139
213. Rajabi A, Akbar Nezhad Gharehlo A, Madadizadeh E, Basereh A, Khoramipoor K, Pirani H, et al. The effect of 12 weeks of aerobic exercise training with or without saffron supplementation on diabetes-specific markers and inflammation in women with type 2 diabetes: a randomized double-blind placebo-controlled trial. Eur J Sport Sci. (2024) 24:899–906. doi: 10.1002/ejsc.12125
214. Hooshmand Moghadam B, Rashidlamir A, Attarzadeh Hosseini SR, Gaeini AA. Kaviani M. The effects of saffron (Crocus sativus L.) in conjunction with concurrent training on body composition, glycaemic status, and inflammatory markers in obese men with type 2 diabetes mellitus: a randomized double-blind clinical trial. Br J Clin Pharmacol. (2022) 88:3256–71. doi: 10.1111/bcp.15222
215. Rajabi A, Khajehlandi M, Siahkuhian M, Akbarnejad A, Khoramipour K, Suzuki K. Effect of 8 weeks aerobic training and saffron supplementation on inflammation and metabolism in middle-aged obese women with type 2 diabetes mellitus. Sports. (2022) 10:167. doi: 10.3390/sports10110167
216. Barari A, Amini S, Mansouri B. The effect of saffron extract and aerobic training on serum HbA1C and Apo A1 in men with Diabetes Mellitus, Type II type 2 diabetes. Jundishapur J Physiol. (2018) 1:1–8.
217. Barari A, Shirali S, Amini S, Abbassi Daloii A, Golizade Gangraj P. Effect of Saffron extract and aerobic exercises on Troponin T and heart-type fatty acid binding protein in type 2 diabetes patients. IJDO. (2017) 9:45.
218. Rajaei Z, Ghaedi H, Ghahramani M, Ghahramani M. Comparison of the effects of eight weeks of resistance training and high-intensity interval training combined with saffron extract on inflammatory markers in male wistar rats with diabetes. J Health Rep Technol. (2025) 11:159238. doi: 10.5812/jhrt-159238
219. Moghadasi M, Akbari F, Najafi P. Interaction of aerobic exercise and crocin improves memory, learning and hypocampic tau and neurotrophins gene expression in rats treated with trimethytin as a model of Alzheimer's disease. Mol Biol Rep. (2024) 51:111. doi: 10.1007/s11033-023-09197-4
220. Azarian F, Farsi S, Hosseini SA, Azarbayjani MA. Effect of endurance training with saffron consumption on PGC1-α gene expression in hippocampus tissue of rats with Alzheimer's disease. Ann Military Health Sci Res. (2020) 18:9131. doi: 10.5812/amh.99131
221. Negarandeh Z, Salamat KM, Hosseini SA, Etemad Z. The effect of endurance training with crocin consumption on IGF-1 and glycogen expression in rat hippocampus tissue of trimethyltin-treated model of Alzheimer's disease. Asian J Sports Med. (2019) 10:2246. doi: 10.5812/asjsm.92246
222. Negarandeh Z, Salamat KM, Hosseini SA. The effect of eight weeks of endurance training with saffron on miR133bFC, miR29aFC in the hippocampus tissue and depression in rats with alzheimer's disease. Jundishapur J Nat Pharm Prod. (2021) 16:3333. doi: 10.5812/jjnpp.103333
223. Azarian F, Farsi S, Hosseini SA, Azarbayjani MA. The effect of endurance training and crocin consumption on anxiety-like behaviors and aerobic power in rats with Alzheimer's. Iran J Psychiatry Behav Sci. (2019) 13:9011. doi: 10.5812/ijpbs.89011
224. Shahidani S, Rajaei Z, Alaei H. Pretreatment with crocin along with treadmill exercise ameliorates motor and memory deficits in hemiparkinsonian rats by anti-inflammatory and antioxidant mechanisms. Metab Brain Dis. (2019) 34:459–68. doi: 10.1007/s11011-018-0379-z
225. Razavi SM, Hosseini Y, Niknejad A, Esmaealzadeh N, Najafi Arab Z, Mavaddat H, et al. A comprehensive literature review on the effects of saffron and its bioactive components on traumatic brain injury (TBI). Naunyn-Schmiedeberg's Arch Pharmacol. (2025) 398:7785–800. doi: 10.1007/s00210-025-03868-8
226. Goyal A, Raza FA, Sulaiman SA, Shahzad A, Aaqil SI, Iqbal M, et al. Saffron extract as an emerging novel therapeutic option in reproduction and sexual health: recent advances and future prospectives. Ann Med Surg (Lond). (2024) 86:2856–65. doi: 10.1097/MS9.0000000000002013
227. Giallauria F, Acampa W, Ricci F, Vitelli A, Maresca L, Mancini M, et al. Effects of exercise training started within 2 weeks after acute myocardial infarction on myocardial perfusion and left ventricular function: a gated SPECT imaging study. Eur J Prev Cardiol. (2012) 19:1410–9. doi: 10.1177/1741826711425427
228. Giallauria F, Lucci R, D'Agostino M, Vitelli A, Maresca L, Mancini M, et al. Two-year multicomprehensive secondary prevention program: favorable effects on cardiovascular functional capacity and coronary risk profile after acute myocardial infarction. J Cardiovasc Med. (2009) 10:772–80. doi: 10.2459/JCM.0b013e32832d55fe
229. Cheung NW, Cinnadaio N, Russo M, Marek S, A. pilot randomised controlled trial of resistance exercise bands in the management of sedentary subjects with type 2 diabetes. Diabetes Res Clin Pract. (2009) 83:e68–71. doi: 10.1016/j.diabres.2008.12.009
230. Oliveira VNd, Bessa A, Jorge MLMP, Oliveira RJdS, de Mello MT, De Agostini GG, et al. The effect of different training programs on antioxidant status, oxidative stress, and metabolic control in type 2 diabetes. Appl Physiol Nutr Metab. (2012) 37:334–44. doi: 10.1139/h2012-004
231. Rech A, Botton CE, Lopez P, Quincozes-Santos A, Umpierre D, Pinto RS. Effects of short-term resistance training on endothelial function and inflammation markers in elderly patients with type 2 diabetes: a randomized controlled trial. Exp Gerontol. (2019) 118:19–25. doi: 10.1016/j.exger.2019.01.003
232. Andrews RC, Cooper AR, Montgomery AA, Norcross AJ, Peters TJ, Sharp DJ, et al. Diet or diet plus physical activity versus usual care in patients with newly diagnosed type 2 diabetes: the early ACTID randomised controlled trial. Lancet. (2011) 378:129–39. doi: 10.1016/S0140-6736(11)60442-X
233. Madjedi KM, Stuart KV, Chua SYL, Ramulu PY, Warwick A, Luben RN, et al. The association of physical activity with glaucoma and related traits in the UK biobank. Ophthalmology. (2023) 130:1024–36. doi: 10.1016/j.ophtha.2023.06.009
234. Kim C-S, Park S, Chun Y, Song W, Kim H-J, Kim J. Treadmill exercise attenuates retinal oxidative stress in naturally-aged mice: an immunohistochemical study. Int J Mol Sci. (2015) 16:21008. doi: 10.3390/ijms160921008
235. Seo J-H, Lee Y. Association of exercise intensity with the prevalence of glaucoma and intraocular pressure in men: a study based on the Korea National Health and Nutrition Examination survey. J Clin Med. (2022) 11:4725. doi: 10.3390/jcm11164725
236. Williams PT. Walking and running are associated with similar reductions in cataract risk. Med Sci Sports Exerc. (2013) 45:1089–96. doi: 10.1249/MSS.0b013e31828121d0
Keywords: saffron, exercise, aging, Alzheimer's disease, Parkinson's disease
Citation: Li L and Wang K (2025) Integrative effects of saffron and physical activity on endurance performance, quality of life, cognitive, emotional, and metabolic outcomes in age-related and neurodegenerative diseases. Front. Nutr. 12:1698135. doi: 10.3389/fnut.2025.1698135
Received: 04 September 2025; Accepted: 27 October 2025;
Published: 27 November 2025.
Edited by:
Gianpiero Greco, University of Bari Aldo Moro, ItalyReviewed by:
Debajit Karmakar, Lakshmibai National Institute of Physical Education, IndiaGawel Solowski, Bingöl University, Türkiye
Copyright © 2025 Li and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kehai Wang, d2toNzcxMkBzZHV0Y20uZWR1LmNu
 Lingyun Li1
Lingyun Li1 Kehai Wang
Kehai Wang