- 1School of Physical Education, Nanjing Xiaozhuang University, Nanjing, China
- 2School of Sports and Health, Nanjing Sport Institute, Nanjing, China
- 3Laboratory of Kinesiology, Nanjing Sport Institute, Nanjing, China
Background: Metabolic syndrome (MetS) is characterized by a cluster of metabolic risk factors, including abdominal obesity, raised triglycerides (TGs), lowered high-density lipoprotein cholesterol (HDL-c), hypertension, and impaired glucose tolerance. Multifaceted interventions, including dietary intervention (DI) and exercise training (Ex), are recommended for the treatment of MetS.
Objective: Our study is a systematic review and meta-analysis comparing the efficacy of DI alone vs. DI with Ex (DI + Ex) on body composition, blood pressure (BP), and other cardiometabolic risk factors in patients with obesity and MetS.
Method: We searched PubMed, Web of Science, SPORTDiscus, and the Cochrane Library from their inception to 10 August 2025, for randomized controlled trials. We calculated mean differences (MD) or standardized mean differences (SMD) and 95% confidence intervals (CIs) using random- or fixed-effect models (depending on heterogeneity). The major outcome was improvement in MetS risk factors, including changes in waist circumference (WC), TGs, HDL-c, BP, and fasting plasma glucose (FPG). The secondary outcomes were body weight, body mass index (BMI), body fat (BF), fasting insulin, total cholesterol (TC), low-density lipoprotein cholesterol (LDL-c), and glycated hemoglobin (HbA1c).
Results: In this meta-analysis of 16 studies involving 902 individuals with obesity and MetS, the DI + Ex group demonstrated a significant advantage over DI for improving specific MetS risk factors. Specifically, the DI + Ex group showed superior efficacy in reducing WC [MD = 2.11 cm, 95% CI: (0.99, 3.23)] and FPG [SMD = 0.22, 95% CI: (0.03, 0.40)]. However, the addition of exercise did not confer a significant benefit for HDL-c, BP, or TG. Beyond the primary MetS factors, the combined intervention was also more effective for a range of secondary outcomes, including body weight, BMI, BF, TC, and LDL-c.
Conclusion: Our findings demonstrate that DI + Ex interventions yield significant improvements in multiple MetS risk factors among individuals with obesity. We recommend that healthcare providers and public health initiatives prioritize these integrated programs to optimize cardiometabolic health outcomes in this population.
Introduction
Metabolic syndrome (MetS) is a major risk factor for cardiovascular diseases, type 2 diabetes, and other negative health outcomes, posing a major challenge to clinical practice and public health (1). Against the backdrop of current lifestyles and dietary habits, the incidence of MetS is increasing annually, a trend that is particularly pronounced among obese populations (2, 3). Although the current understanding of the pathogenesis of MetS remains incomplete, research suggests that it results from the interaction of factors, such as obesity, high-calorie diets, and lack of physical exercise (4, 5). The main characteristics of MetS include obesity, insulin resistance, hypertension, hyperglycemia, and dyslipidemia; these characteristics also form the defining criteria for this condition (5, 6).
The core of MetS is obesity-related glucose and lipid metabolic disorders. Therefore, current non-surgical intervention strategies primarily focus on achieving a negative energy balance, mainly through exercise training (Ex) and dietary intervention (DI). Achieving a negative energy balance through Ex or increased physical activity levels is an important clinical approach for obesity management or health maintenance (7). In recent years, DI has gained increasing attention. Caloric restriction through DI can effectively improve MetS by enhancing insulin sensitivity and regulating lipid metabolism, among other benefits (8–10). Studies indicate that dietary caloric restriction appears to be more effective for weight management than Ex (7) but may lead to a reduction in lean body mass and basal metabolic rate (11). By contrast, Ex is effective in preserving lean body mass during weight loss (12). For obese individuals—particularly those who belong to exercise-based weight-loss plans—adhering to the weight-loss programs is often difficult (13). Consequently, an increasing number of studies are exploring the combination of DI and Ex (DI + Ex) to mitigate the drawbacks of single interventions and achieve synergistic benefits. Although several meta-analyses have investigated the effects of DI alone vs. those of DI + Ex on blood lipids (14), inflammatory markers (15), blood glucose (16), and body composition (17) in populations with obesity and MetS, no studies have compared DI alone with DI + Ex specifically in individuals with obesity and MetS.
In this study, we explored the intervention effects of DI alone vs. those of DI + Ex in individuals with obesity and MetS.
Methods
This review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) (18).
Types of studies
Randomized controlled trials (RCTs) were eligible.
Control
All studies compared DI with Ex+DI, and their outcomes were measured at pre- and post-intervention.
Literature search
Several databases, including PubMed, Web of Science, SPORTDiscus, and the Cochrane Library, were searched to identify relevant RCTs published from database inception to 10 August 2025, by two investigators (Cao and Xu). The search strategy involved a Boolean search using a combination of subject-related and free words to identify pertinent data in titles, abstracts, and keywords. The following search terms were used: (“exercise” OR “physical activity” OR “aerobic training” OR “AT” OR “combine training” OR “CT” OR “resistance training” OR “RT” OR “high-intensity interval training” OR “HIIT”) AND (“dietary intervention” OR “caloric restriction” OR “lifestyle intervention) AND (“obese” OR “obesity”) (Supplementary File).
Study selection
The selection criteria for this meta-analysis were as follows: (a) The participants were adults (age > 18 years) with MetS (incorporating the definitions of the International Diabetes Federation and the American Heart Association/National Heart, Lung, and Blood Institute) (19). (b) The intervention program was conducted between DI and DI + Ex and lasted for at least 2 weeks. (c) The studies were RCTs or had parallel study designs. (d) The reported outcomes included waist circumference (WC), triglycerides (TGs), high-density lipoprotein cholesterol (HDL-c), blood pressure (BP), fasting plasma glucose (FPG), and fasting insulin (each study included at least three of these outcomes), with measurements taken at pre- and post-intervention. (e) Articles were in the English language. Exclusion criteria were as follows: (a) Non-original research. (b) Studies with unreliable designs or substantial statistical errors. (c) Interventions with Ex, DI, or Ex+DI only or other interventions. (d) Participants with cancer, HIV, chronic heart and/or liver failure, or other conditions limiting their ability to perform Ex or DI. (e) Articles in non-English languages. In the case of DI, studies included used any type of DI. In the case of Ex, studies involving any type of Ex or physical activity were included.
Measured outcomes
The major outcomes were the improvement in MetS, including changes in WC, TG, HDL-c, BP, and FPG levels. Secondary outcomes were body weight (BW), body mass index (BMI), body fat (BF), fasting insulin (FINs), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-c), and glycated hemoglobin (HbA1c).
Data extraction
Two researchers (Cao and Xu) extracted data and research characteristics from the qualifying literature, and disagreements were resolved by discussion with another reviewer. Duplicate studies were excluded from different databases. The extracted content from the literature included the following: first author, publication year, country, participant characteristics (sample size, age, and sex), intervention characteristics, and outcomes. Raw pre- and post-intervention values or differences (post-intervention values − pre-intervention values) were extracted for main variable measurements. Values presented as quartiles, confidence intervals, median, or standard errors were all converted into means and standard deviations (20–22).
Assessment of study quality
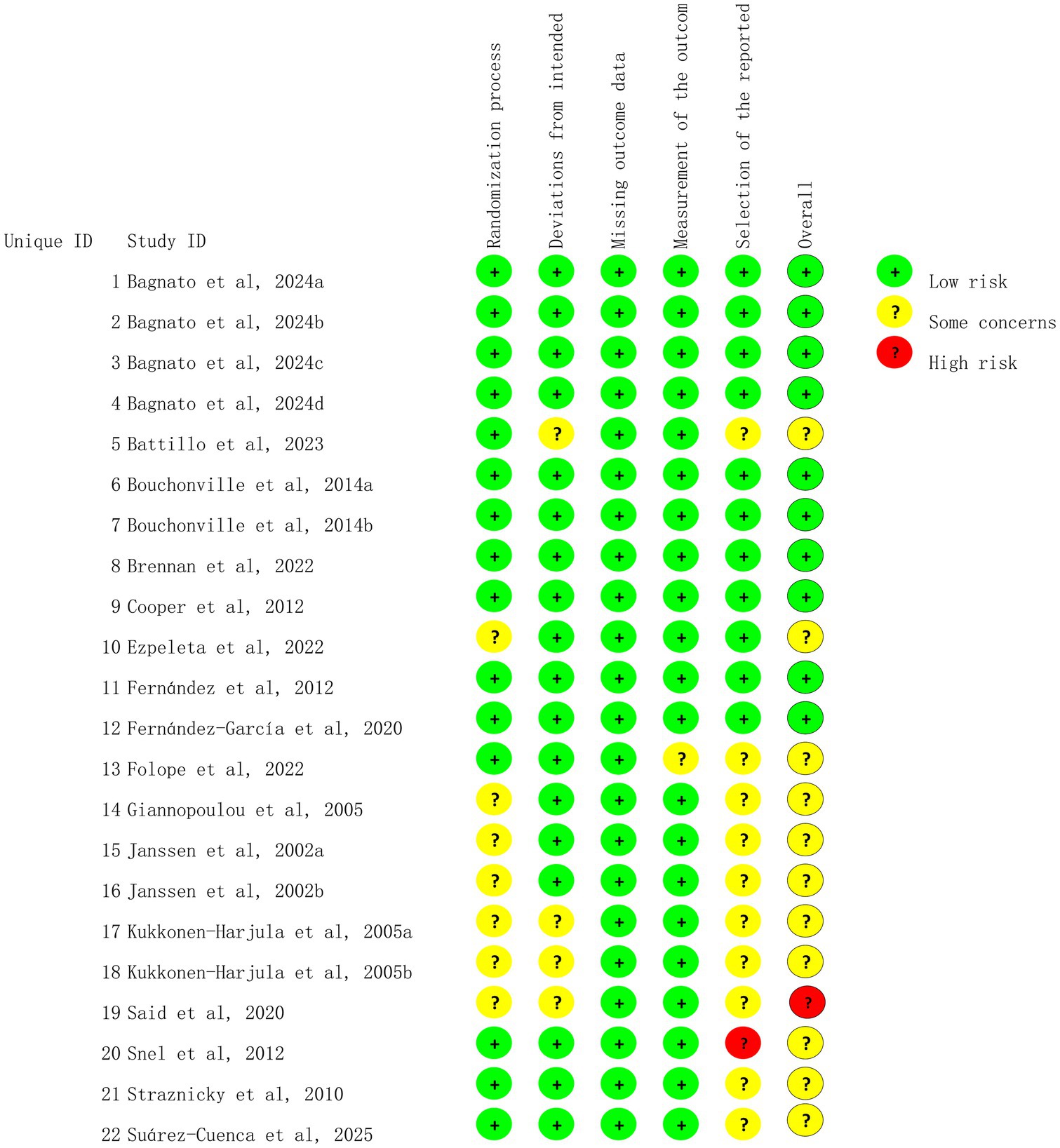
Study quality was independently assessed by two investigators (Cao and Zhang) using the revised Cochrane risk-of-bias tool for randomized trials (RoB 2) (23). The RoB 2 tool was employed to assess the following variables: (I) bias arising from randomization, (II) bias because of deviations from intended interventions, (III) bias because of missing data, (IV) bias in outcome measurement, and (V) bias in the selection of the reported outcomes. Any discrepancy was resolved by consensus. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system was applied to assess the quality of the evidence (24).
Heterogeneity
Heterogeneity between trials was analyzed through the χ2 test, and the test level of p<0.1 was regarded as indicating significant heterogeneity. Cochran Q statistics were calculated, and the following formula was utilized to calculate I2, which was used to qualify the level of heterogeneity (25). The magnitude of heterogeneity (I2) was categorized as low (0–24%), moderate (25–49%), substantial (50–74%), and considerable (75–100%) (25).
Data analysis
Meta-analyses were conducted by employing Review Manager software (version 5.3; The Nordic Cochrane Center, London, United Kingdom) or Statistics and Data Science software (version 17.0; Stata Corporation). Measurement data were calculated as mean differences (MD) or standardized mean differences (SMD) by using random or fixed-effects models (fixed models were used when I2 was less than 25%, whereas random models were applied when I2 was higher than 25%). In addition, for SMDs, effect size analysis statistics were considered as small (0.2), medium (0.5), and large (0.8) (26). The precision of the pooled effect size was reported as 95% confidence intervals (CI). Sources of heterogeneity were identified through subgroup analyses based on exercise type, duration, and year. If statistical heterogeneity existed among the results, the source of heterogeneity was further analyzed by using sensitivity analyses, which were conducted by removing each study individually. All analyses were conducted separately for each outcome variable.
Results
Literature search
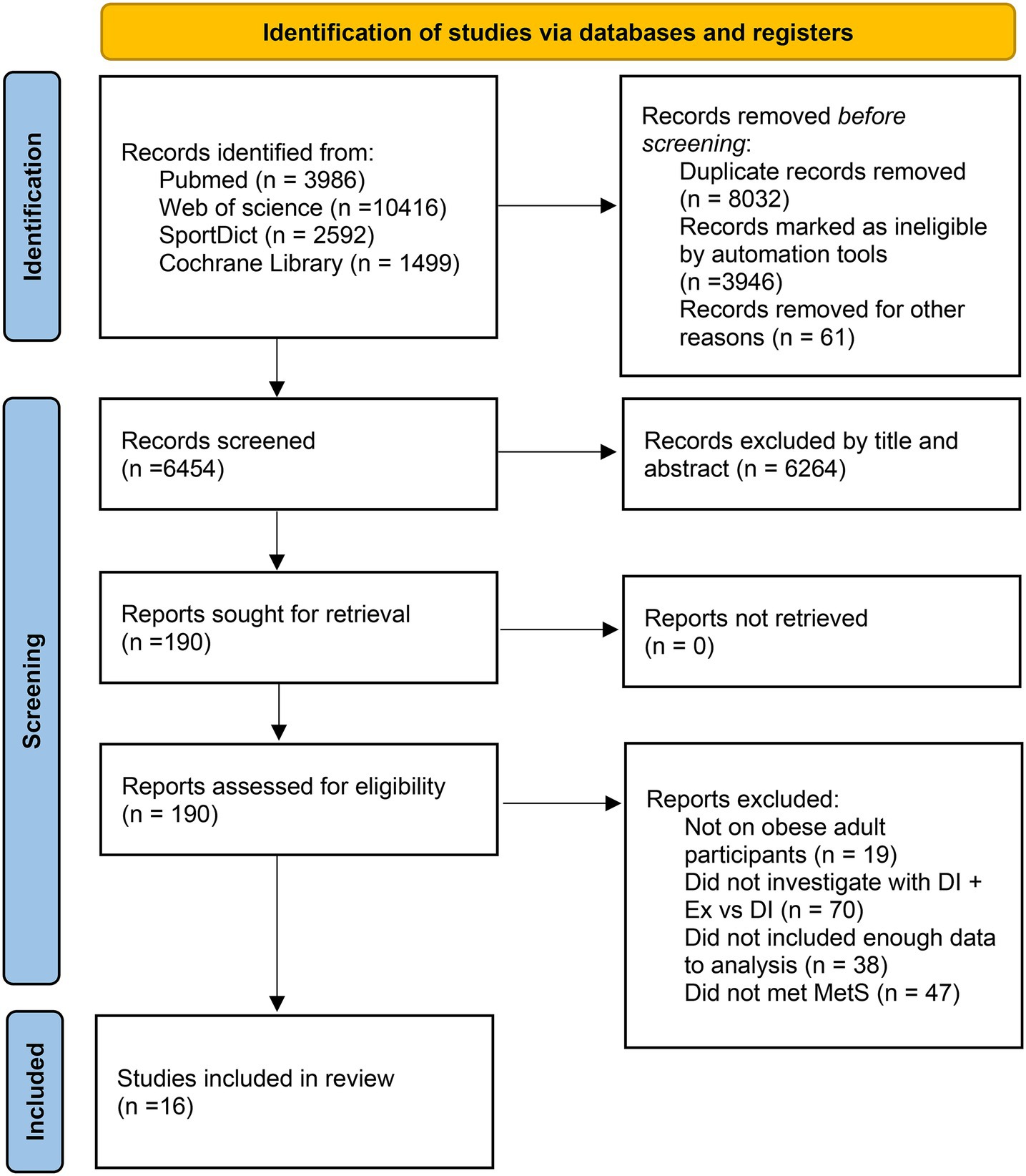
We identified a total of 18,493 potential studies across PubMed, Cochrane Library, Web of Science, and SPORTDict. After removing duplicates and eliminating papers on the basis of the eligibility criteria, 190 articles for full-text checking remained. After reviewing the full texts, we excluded 174 studies that did not meet the requirements. Finally, 16 studies met the selection criteria (27–42). The details of the search are shown in Figure 1.
Included trials
We included 16 studies (22 trials) with 840 participants in our meta-analysis. All of these participants were diagnosed as obese with MetS. The largest number of participants in single studies was 90 (31), and the smallest was 22 (28). Three studies included only male participants (29, 36), and three studies included only female participants (27, 28). Two studies were conducted with resistance training (RT) + DI (27, 29). Four studies involved high-intensity interval training (HIIT) + DI (39, 41, 42); four studies involved combined training (CT) + DI (34, 36, 38), and 14 studies included aerobics training (AT) + DI (27–33, 35, 37, 40, 41). The mean age of the participants ranged from 19 to 70 years, and the publication dates of the articles ranged primarily from 2002 to 2025. The detailed characteristics of participants are summarized in Table 1.
Risk of bias among the included trials
We assessed 22 trials for risk of bias, and the results are shown in Figures 2, 3. All studies were deemed to be at low risk of bias for the missing outcome data domain, and the selection of the reported results domain was rated as low risk or of some concern. The majority of the studies were considered to be of some concern regarding the risk of bias for the rest of the items.
Meta-analysis of overall effects
Effect of DI vs. DI + ex on risk factors for mets
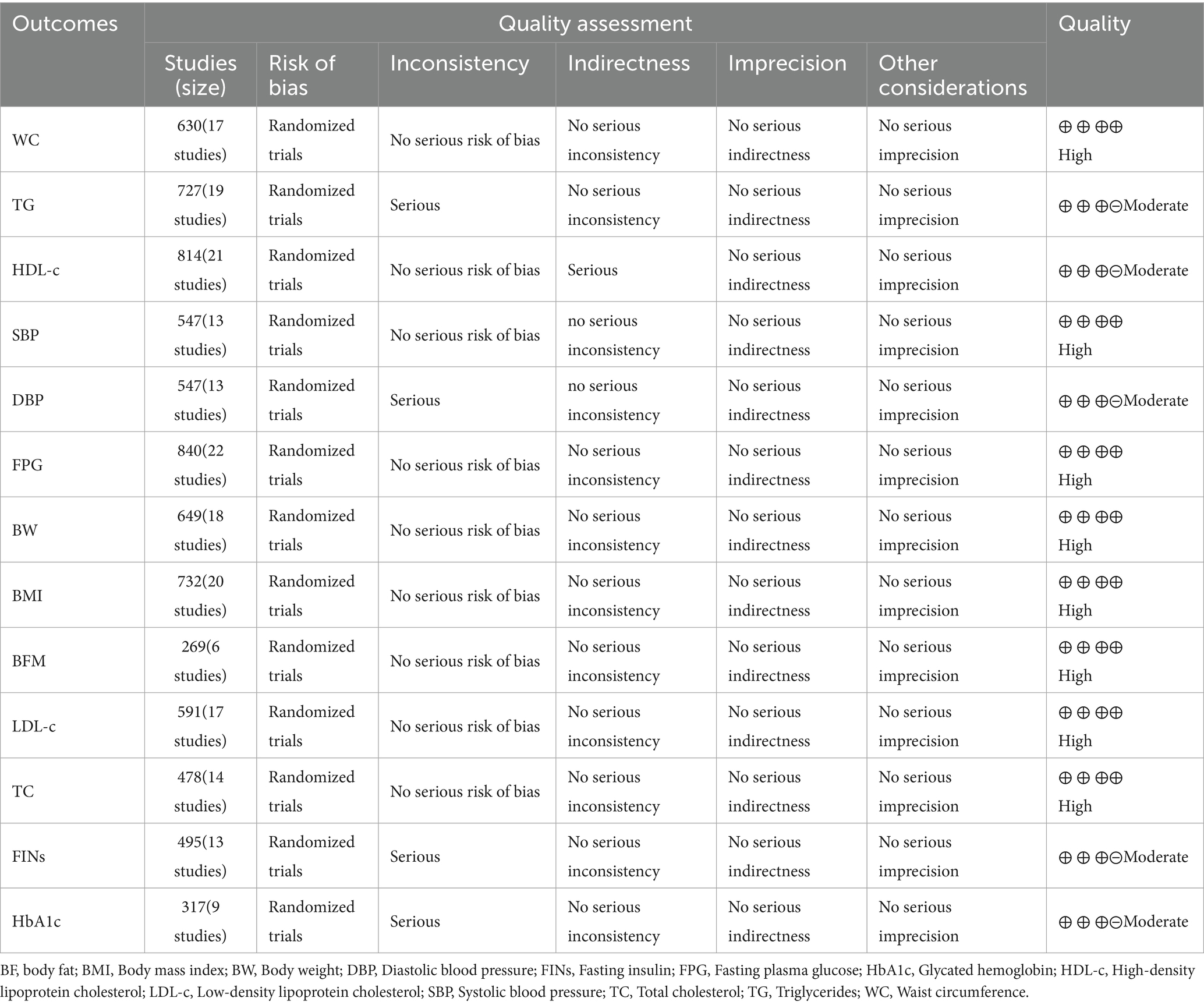
The within-group analysis found that DI [MD = −5.69 cm, 95% CI: (−8.63, −2.75), p < 0.001, I2 = 89%] and DI + Ex [MD = −8.29 cm, 95% CI: (−12.06, −4.51), p < 0.001, I2 = 94%] resulted in significant improvements in WC (Table 2). Compared with DI, DI + Ex showed greater benefits for WC [MD = 2.11 cm, 95% CI: (0.99, 3.23), p = 0.03, I2 = 44%] (Figure 4).
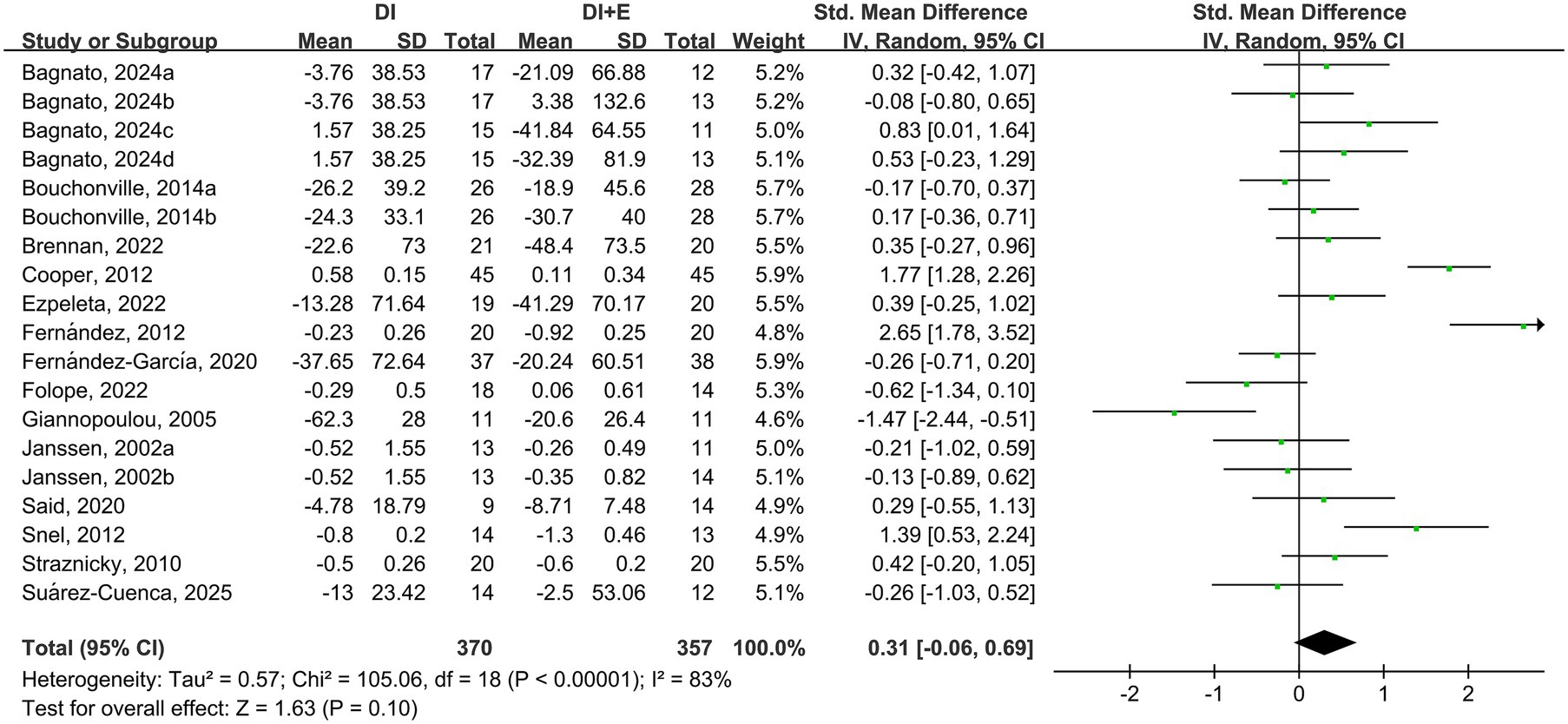
The within-group analysis found that DI [SMD = −0.46, 95% CI: (−0.85, −0.07), p < 0.001, I2 = 85%] and DI + Ex [SMD = −0.77, 95% CI: (−1.15, −0.39), p < 0.001, I2 = 82%] resulted in significant improvements in TG (Table 2). However, directly comparing DI and DI + Ex revealed no difference between groups [SMD = 0.31, 95% CI: (−0.06, 0.69), p = 0.10, I2 = 83%] (Figure 5).
The within-group analysis showed that DI [SMD = −0.55, 95% CI: (−0.91, −0.19), p < 0.001, I2 = 84%] and DI + Ex [SMD = −0.78, 95% CI: (−1.13, −0.43), p < 0.001, I2 = 82%] resulted in significant improvements in FPG (Table 2). Compared with DI, DI + Ex had greater benefits for FPG [SMD = 0.22, 95% CI: (0.03, 0.40), p = 0.03, I2 = 41%] (Figure 6).
For BP, the within-group analysis found that DI [MD = −7.92 mm Hg, 95% CI: (−12.49, −3.35), p < 0.001, I2 = 91%] and DI + Ex [MD = −6.81 mm Hg, 95% CI: (−11.40, −2.23), p < 0.001, I2 = 91%] resulted in significant improvements in SBP, whereas no difference was found between DI and DI + Ex [MD = −0.68 mm Hg, 95% CI: (−3.27, 1.91), p = 0.61, I2 = 66%]. The within-group analysis also showed that DI [MD = −4.50 mm Hg, 95% CI: (−6.16, −2.84), p < 0.001, I2 = 70%] and DI + Ex [MD = −4.84 mm Hg, 95% CI: (−7.41, −2.28), p < 0.001, I2 = 89%] resulted in significant improvements in DBP, and no difference was found between groups [MD = 0.33 mm Hg, 95% CI: (−2.72, 3.38), p = 0.83, I2 = 92%; Figure 7].
The within-group analysis revealed that DI or DI + Ex did not result in significant improvements in HDL-c and no difference between groups (Figure 8).
Effect of DI vs. DI + ex intervention on anthropometric indices
Eighteen trials discovered significant decreases in terms of BW, 20 trials found significant reductions in BMI, and five reported changes in FFM after DI or DI + E intervention. Compared with DI, DI + Ex had greater benefits for BW [MD = 1.12 kg, 95% CI: (0.27, 1.96), p = 0.02, I2 = 44%], BMI [MD = 0.58 kg/m2, 95% CI: (0.37, 0.78), p < 0.001, I2 = 0%], and BF [MD = 2.27 kg, 95% CI: (0.79, 3.74), p < 0.001, I2 = 79%] (Table 3).
Effect of DI vs. that of DI + E on blood indices
Fourteen trials discovered significant decreases in TC, 17 trials found significant decreases in LDL-c, and 13 trials reported FINs after DI or DI + Ex. The results showed that HbA1c significantly improved after DI + Ex [MD = −0.35, 95% CI: (−0.63, −0.07), p < 0.001, I2 = 90%] but not after DI. Compared with DI, DI + Ex had greater benefits for TC [SMD = 0.18, 95% CI: (0.00, 0.36), p = 0.05, I2 = 23%] and LDL-c [SMD = 0.17, 95% CI: (0.00, 0.33), p = 0.05] but not for FINs or HbA1c (Table 4).
Subgroup analysis
Intervention duration (≤16 and >16 weeks), exercise type (AT, RT, HIIT, and CT), and participants’ age (≤ 50 and > 50 years) were subjected to subgroup analysis because of high heterogeneity. For MetS risk factors, the results showed that compared with DI, DI + Ex resulted in significant improvements in WC over long (p = 0.023) or short (p = 0.003) durations, under AT (p = 0.002), and in older (p = 0.003) or younger (p = 0.033) people. Subgroup analysis also revealed that compared with DI, DI + Ex resulted in significant improvements in FPG over short durations (p = 0.011), under AT (p = 0.038), under HIIT (p = 0.024), and in older people (p = 0.020). However, subgroup analysis did not reveal significant effects on TG, HDL-c, SBP, and DBP.
The results for anthropometric indices showed that compared with DI, DI + Ex resulted in significant improvements in BW over a short duration (p = 0.013), under AT (p = 0.001), and in older people (p = 0.023). Subgroup analysis demonstrated that compared with DI, DI + Ex resulted in a significant improvement over long (p = 0.005) or short (p < 0.001) durations, under AT (p < 0.001), and in older people (p < 0.001). Subgroup analysis showed that compared with DI, DI + Ex resulted in a significant improvement in BF over a long duration (p < 0.001), under AT (p = 0.010), under CT (p = 0.049), and in older people (p = 0.002).
For blood indices, subgroup analyses revealed a significant decrease in TC in older people (p = 0.018). It also revealed a significant decrease in LDL-c over a long duration (p = 0.018), under AT (p = 0.028), and in older people (p = 0.029). However, subgroup analysis did not reveal a significant effect on FINs and HbA1c (Supplementary File).
Sensitivity analysis
We used sensitivity analysis to exclude the included studies individually from the overall study to assess the effect of each study on the outcome effect size and to explore the stability of results. Sensitivity analysis demonstrated that by omitting the study by Giannopoulou et al. (28), DI + Ex can significantly improve TG compared with DI. Excluding a particular study on the did not significantly affect the effect sizes of other outcomes, and the results remained stable (Supplementary File).
Quality of studies
We examined the quality of the included studies from several aspects (Table 5).
Discussion
Meta-analysis results indicated that compared with DI alone, DI + Ex more effectively improved key MetS risk factors, WC, and FPG in individuals with obesity and MetS [SMD = 0.22, 95% CI: (0.03, 0.40), p = 0.03]. However, no significant effects were observed for TG, HDL-c, SBP, or DBP (p > 0.05). Regarding other health parameters, DI + Ex significantly improved BW [MD = 1.12 kg, 95% CI: (0.27, 1.96), p = 0.02], BMI [MD = 0.58 kg/m2, 95% CI: (0.37, 0.78), p < 0.001], BF [MD = 2.27 kg, 95% CI: (0.79, 3.74), p < 0.001], TC [SMD = 0.18, 95% CI: (0.00, 0.36), p = 0.05], and LDL-c [SMD = 0.17, 95% CI: (0.00, 0.33), p = 0.05]. By contrast, no significant improvements were found for FINs or glycated hemoglobin (HbA1c). These findings suggest that DI + Ex may offer potential advantages for improving health outcomes in individuals with obesity and MetS.
Previous reviews or meta-analyses have confirmed that Ex or DI can effectively improve health outcomes in obese individuals with insulin resistance (43–46). This finding is consistent with the statistical results of the single intervention modalities included in this study. Between-group comparative results demonstrated that compared with DI alone, DI + Ex significantly improved BW [MD = 1.12 kg, 95% CI: (0.27, 1.96), p = 0.02], BMI [MD = 0.58 kg/m2, 95% CI: (0.37, 0.78), p < 0.001], BFM [MD = 2.27 kg, 95% CI: (0.79, 3.74), p < 0.001], and WC [MD = 2.11 cm, 95% CI: (0.99, 3.23), p = 0.03]. These findings align with the results of previous research (47). A primary reason for this consistency is that adding Ex to DI further increases energy expenditure. Additionally, DI alone may lead to a reduction in skeletal muscle mass, which can further decrease basal metabolic rate (48). By contrast, the combination of DI + Ex effectively prevents the loss of skeletal muscle (12, 49). Furthermore, subgroup analysis revealed that short-term (≤16 weeks) interventions produced more significant effects compared with long-term (>16 weeks) interventions. These effects may be related to physiological adaptations that occur with prolonged exercise. Chen et al. found that a 2-week combined DI + Ex intervention effectively improved health parameters in individuals with MetS (50). Roberts et al. reported that a 3-week combined intervention reduced the prevalence of MetS by 60% (51). Additionally, subgroup analysis indicated that AT+DI significantly improved body composition indicators, whereas other forms of exercise did not yield significant effects. This finding is consistent with the results of Wewege et al. (52).
In terms of cardiac health markers, such as BP and blood lipids, DI and combined DI + Ex effectively improved SBP, DBP, TG, TC, and LDL-c; this finding is consistent with the results of previous meta-analyses (14, 53, 54). However, further between-group comparisons revealed that, apart from TC and LDL-c, other indicators showed no significant differences, a finding also reported by Khalafi et al. (55). A possible explanation for this discrepancy is that any intervention modality may have a ceiling effect on improvement. Not all indicators in each included study were abnormal because some fell within the normal range. Therefore, interventions produced more significant improvements in abnormal indicators than in normal ones, suggesting that the baseline values of the indices are closely related to intervention outcomes (55, 56). The analysis of anthropometric indices further supports the hypothesis that when all relevant indicators were within abnormal ranges, the DI + Ex intervention showed more significant effects than diet alone, as evidenced by metrics such as BW and BMI. The specific mechanisms behind this effect remain to be further investigated. Additionally, owing to the high heterogeneity in certain outcome indicators, we conducted subgroup and sensitivity analyses to identify potential sources. Although the exact origins of heterogeneity remain unclear, the results appear to depend partly on participant characteristics, such as age. This finding suggests that age plays a vital role in lipid profile changes. In addition to common exercise interventions, lifestyle interventions may modulate lipid metabolism, which is tightly linked to the cardiometabolic continuum. Researchers found that intensive lipid-lowering with small interfering RNA (siRNA) therapy leads not only to substantial LDL-c reduction but also to improved vascular function and arterial stiffness (57).
In terms of glycemic metabolism indicators, DI and DI + Ex effectively improved FPG and FINs. Between-group comparisons showed that compared with DI alone, DI + Ex led to greater improvement in FPG but not to a statistically significant improvement in FINs, a finding that is consistent with previous results (16, 58). The favorable effect of DI + Ex on glucose metabolism may be attributed to increased energy expenditure and reduced BF resulting from DI + Ex (59). We also found that DI + Ex was more effective than DI alone in improving BF (MD = 2.27 kg, p < 0.001). Abnormal lipid metabolism often leads to lipid accumulation in skeletal muscle, which can further impair insulin signaling and adversely affect glucose metabolism (60). While DI alone effectively reduces BW, it does not significantly enhance mitochondrial oxidative capacity in skeletal muscle (61). By contrast, Ex has been shown to improve mitochondrial oxidative function (62). Subgroup analysis further revealed that DI + AT and DI + HIIT were more effective than DI alone in improving FPG. A possible explanation is that AT and HIIT are more effective than other exercise modalities in reducing visceral adipose tissue (62, 63), whereas RT does not significantly improve visceral fat levels (64). In addition, studies also found that alterations in TG metabolism and HDL-c function represent early determinants of vascular injury in individuals with metabolic disturbances (65), DI + Ex can effectively improve TG, which suggests that DI + Ex can also effectively improve vascular health.
Limitations
Our study has several limitations. First, its results showed heterogeneity, which may be introduced by differences in exercise program (type, duration, intensity, and frequency), DI characteristics, and population cohorts. Second, aerobics was the most prevalent exercise; other exercise types are needed in future studies. Third, DI protocols exhibited heterogeneity in terms of energy restriction intensity and dietary composition. Fourth, the geographic scope of the included studies is narrow, as the population is largely restricted to developed countries and lacks representation from key regions such as East Asia and Africa. First, the age range of participants (mean age from 44 to over 70 years) is high, which may limit the applicability of the results to younger adult populations. Finally, SMDs were calculated for inconsistent data units in some outcomes. However, their actual clinical importance is unknown.
Conclusion
Our findings demonstrate that DI + Ex interventions yield significant improvements in multiple MetS risk factors among individuals with obesity. We recommend that healthcare providers and public health initiatives prioritize these integrated programs to optimize cardiometabolic health outcomes in this population.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YC: Data curation, Formal analysis, Funding acquisition, Methodology, Software, Writing – original draft, Writing – review & editing. RX: Formal analysis, Funding acquisition, Methodology, Project administration, Writing – review & editing. JZ: Data curation, Methodology, Software, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by a research grant of Key Laboratory of Nanjing Sport Institute (Grant no. SYS202102) and Talent Introduction Research Start-Up Funds (4172521).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1703600/full#supplementary-material
References
1. Neeland, IJ, Lim, S, Tchernof, A, Gastaldelli, A, Rangaswami, J, Ndumele, CE, et al. Metabolic syndrome. Nat Rev Dis Primers. (2024) 10:77. doi: 10.1038/s41572-024-00563-5,
2. Saklayen, MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. (2018) 20:12. doi: 10.1007/s11906-018-0812-z,
3. Obeidat, AA, Ahmad, MN, Ghabashi, MA, Alazzeh, AY, Habib, SM, Abu Al-Haijaa, D, et al. Developmental trends of metabolic syndrome in the past two decades: a narrative review. J Clin Med. (2025) 14:402. doi: 10.3390/jcm14072402,
4. Lemieux, I, and Després, JP. Metabolic syndrome: past, present and future. Nutrients. (2020) 12:501. doi: 10.3390/nu12113501,
5. Reaven, GM. The metabolic syndrome: time to get off the merry-go-round? J Intern Med. (2011) 269:127–36. doi: 10.1111/j.1365-2796.2010.02325.x,
6. Alberti, KG, Zimmet, P, and Shaw, J. The metabolic syndrome--a new worldwide definition. Lancet. (2005) 366:1059–62. doi: 10.1016/S0140-6736(05)67402-8,
7. Verheggen, RJ, Maessen, MF, Green, DJ, Hermus, AR, Hopman, MT, and Thijssen, DH. A systematic review and meta-analysis on the effects of exercise training versus hypocaloric diet: distinct effects on body weight and visceral adipose tissue. Obes Rev. (2016) 17:664–90. doi: 10.1111/obr.12406,
8. Fontana, L, Partridge, L, and Longo, VD. Extending healthy life span--from yeast to humans. Science. (2010) 328:321–6. doi: 10.1126/science.1172539,
9. Giacco, A, Cioffi, F, and Silvestri, E. Mediterranean diet and metabolic syndrome. Nutrients. (2025) 17:14. doi: 10.3390/nu17142364,
10. García-García, FJ, Monistrol-Mula, A, Cardellach, F, and Garrabou, G. Nutrition, bioenergetics, and metabolic syndrome. Nutrients. (2020) 12:2785. doi: 10.3390/nu12092785,
11. Sundfør, TM, Svendsen, M, and Tonstad, S. Effect of intermittent versus continuous energy restriction on weight loss, maintenance and cardiometabolic risk: a randomized 1-year trial. Nutr Metab Cardiovasc Dis. (2018) 28:698–706. doi: 10.1016/j.numecd.2018.03.009,
12. Brubaker, PH, Nicklas, BJ, Houston, DK, Hundley, WG, Chen, H, Molina, AJA, et al. A randomized, controlled trial of resistance training added to caloric restriction plus aerobic exercise training in obese heart failure with preserved ejection fraction. Circ Heart Fail. (2023) 16:e010161. doi: 10.1161/CIRCHEARTFAILURE.122.010161,
13. Lemstra, M, Bird, Y, Nwankwo, C, Rogers, M, and Moraros, J. Weight loss intervention adherence and factors promoting adherence: a meta-analysis. Patient Prefer Adherence. (2016) 10:1547–59. doi: 10.2147/PPA.S103649,
14. Khalafi, M, Sakhaei, MH, Kazeminasab, F, Rosenkranz, SK, and Symonds, ME. Exercise training, dietary intervention, or combined interventions and their effects on lipid profiles in adults with overweight and obesity: a systematic review and meta-analysis of randomized clinical trials. Nutr Metab Cardiovasc Dis. (2023) 33:1662–83. doi: 10.1016/j.numecd.2023.05.024,
15. Khalafi, M, Symonds, ME, and Akbari, A. The impact of exercise training versus caloric restriction on inflammation markers: a systemic review and meta-analysis. Crit Rev Food Sci Nutr. (2022) 62:4226–41. doi: 10.1080/10408398.2021.1873732,
16. Khalafi, M, Azali Alamdari, K, Symonds, ME, Rohani, H, and Sakhaei, MH. A comparison of the impact of exercise training with dietary intervention versus dietary intervention alone on insulin resistance and glucose regulation in individual with overweight or obesity: a systemic review and meta-analysis. Crit Rev Food Sci Nutr. (2023) 63:9349–63. doi: 10.1080/10408398.2022.2064424,
17. Cheng, CC, Hsu, CY, and Liu, JF. Effects of dietary and exercise intervention on weight loss and body composition in obese postmenopausal women: a systematic review and meta-analysis. Menopause. (2018) 25:772–82. doi: 10.1097/GME.0000000000001085,
18. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71,
19. Alberti, KG, Eckel, RH, Grundy, SM, Zimmet, PZ, Cleeman, JI, Donato, KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; National Heart, Lung, and Blood Institute; American Heart Association; world heart federation; international atherosclerosis society; and International Association for the Study of obesity. Circulation. (2009) 120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644,
20. Higgins, JPT, Thomas, J, Chandler, J, Cumpston, M, and Welch, VA. Cochrane handbook for systematic reviews of interventions: Cochrane handbook for systematic reviews of interventions. London, England: Cochrane Collaboration (2019).
21. Wan, X, Wang, W, Liu, J, and Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135,
22. Hozo, SP, Djulbegovic, B, and Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. (2005) 5:13. doi: 10.1186/1471-2288-5-13,
23. Sterne, JAC, Savović, J, Page, MJ, Elbers, RG, Blencowe, NS, Boutron, I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898,
24. Guyatt, GH, Oxman, AD, Vist, GE, Kunz, R, Falck-Ytter, Y, Alonso-Coello, P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD,
25. Higgins, JP, Thompson, SG, Deeks, JJ, and Altman, DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557,
26. Cohen, J. Statistical power analysis for the behavioral sciences. Cambridge, MA: Academic Press (1988).
27. Janssen, I, Fortier, A, Hudson, R, and Ross, R. Effects of an energy-restrictive diet with or without exercise on abdominal fat, intermuscular fat, and metabolic risk factors in obese women. Diabetes Care. (2002) 25:431–8. doi: 10.2337/diacare.25.3.431,
28. Giannopoulou, I, Fernhall, B, Carhart, R, Weinstock, RS, Baynard, T, Figueroa, A, et al. Effects of diet and/or exercise on the adipocytokine and inflammatory cytokine levels of postmenopausal women with type 2 diabetes. Metabolism. (2005) 54:866–75. doi: 10.1016/j.metabol.2005.01.033,
29. Kukkonen-Harjula, KT, Borg, PT, Nenonen, AM, and Fogelholm, MG. Effects of a weight maintenance program with or without exercise on the metabolic syndrome: a randomized trial in obese men. Prev Med. (2005) 41:784–90. doi: 10.1016/j.ypmed.2005.07.008,
30. Straznicky, NE, Lambert, EA, Nestel, PJ, McGrane, MT, Dawood, T, Schlaich, MP, et al. Sympathetic neural adaptation to hypocaloric diet with or without exercise training in obese metabolic syndrome subjects. Diabetes. (2010) 59:71–9. doi: 10.2337/db09-0934,
31. Cooper, JN, Columbus, ML, Shields, KJ, Asubonteng, J, Meyer, ML, Sutton-Tyrrell, K, et al. Effects of an intensive behavioral weight loss intervention consisting of caloric restriction with or without physical activity on common carotid artery remodeling in severely obese adults. Metabolism. (2012) 61:1589–97. doi: 10.1016/j.metabol.2012.04.012,
32. Fernández, JM, Rosado-Álvarez, D, Da Silva Grigoletto, ME, Rangel-Zúñiga, OA, Landaeta-Díaz, LL, Caballero-Villarraso, J, et al. Moderate-to-high-intensity training and a hypocaloric Mediterranean diet enhance endothelial progenitor cells and fitness in subjects with the metabolic syndrome. Clin Sci (Lond). (2012) 123:361–73. doi: 10.1042/CS20110477,
33. Snel, M, Gastaldelli, A, Ouwens, DM, Hesselink, MK, Schaart, G, Buzzigoli, E, et al. Effects of adding exercise to a 16-week very low-calorie diet in obese, insulin-dependent type 2 diabetes mellitus patients. J Clin Endocrinol Metab. (2012) 97:2512–20. doi: 10.1210/jc.2011-3178,
34. Bouchonville, M, Armamento-Villareal, R, Shah, K, Napoli, N, Sinacore, DR, Qualls, C, et al. Weight loss, exercise or both and cardiometabolic risk factors in obese older adults: results of a randomized controlled trial. Int J Obes. (2014) 38:423–31. doi: 10.1038/ijo.2013.122,
35. Fernández-García, JC, Martínez-Sánchez, MA, Bernal-López, MR, Muñoz-Garach, A, Martínez-González, MA, Fitó, M, et al. Effect of a lifestyle intervention program with energy-restricted Mediterranean diet and exercise on the serum polyamine metabolome in individuals at high cardiovascular disease risk: a randomized clinical trial. Am J Clin Nutr. (2020) 111:975–82. doi: 10.1093/ajcn/nqaa064,
36. Said, MA, Abdelmoneem, M, Alibrahim, MC, Elsebee, MA, and Kotb, AAH. Effects of diet versus diet plus aerobic and resistance exercise on metabolic syndrome in obese young men. J Exerc Sci Fit. (2020) 18:101–8. doi: 10.1016/j.jesf.2020.03.002,
37. Brennan, AM, Standley, RA, Anthony, SJ, Grench, KE, Helbling, NL, DeLany, JP, et al. Weight loss and exercise differentially affect insulin sensitivity, body composition, cardiorespiratory fitness, and muscle strength in older adults with obesity: a randomized controlled trial. J Gerontol A Biol Sci Med Sci. (2022) 77:1088–97. doi: 10.1093/gerona/glab240,
38. Folope, V, Meret, C, Castres, I, Tourny, C, Houivet, E, Grigioni, S, et al. Evaluation of a supervised adapted physical activity program associated or not with Oral supplementation with arginine and leucine in subjects with obesity and metabolic syndrome: a randomized controlled trial. Nutrients. (2022) 14:708. doi: 10.3390/nu14183708,
39. Battillo, DJ, and Malin, SK. Impact of caloric restriction and exercise on trimethylamine N-oxide metabolism in women with obesity. Nutrients. (2023) 15:1455. doi: 10.3390/nu15061455,
40. Ezpeleta, M, Gabel, K, Cienfuegos, S, Kalam, F, Lin, S, Pavlou, V, et al. Effect of alternate day fasting combined with aerobic exercise on non-alcoholic fatty liver disease: a randomized controlled trial. Cell Metab. (2023) 35:56–70.e3. doi: 10.1016/j.cmet.2022.12.001,
41. Bagnato, CB, Bianco, A, Bonfiglio, C, Franco, I, Verrelli, N, Carella, N, et al. Healthy lifestyle changes improve cortisol levels and liver steatosis in MASLD patients: results from a randomized clinical trial. Nutrients. (2024) 16:4225. doi: 10.3390/nu16234225,
42. Suárez-Cuenca, JA, Díaz-Jiménez, DE, Pineda-Juárez, JA, Mendoza-Mota, AG, Valencia-Aldana, OD, Núñez-Angeles, S, et al. Effect of Mediterranean diet in combination with isokinetic exercise therapy on body composition and cytokine profile in patients with metabolic syndrome. Nutrients. (2025) 17:256. doi: 10.3390/nu17020256,
43. Manoogian, ENC, Wilkinson, MJ, O’Neal, M, Laing, K, Nguyen, J, Van, D, et al. Time-restricted eating in adults with metabolic syndrome: a randomized controlled trial. Ann Intern Med. (2024) 177:1462–70. doi: 10.7326/M24-0859,
44. Maruthur, NM, Pilla, SJ, White, K, Wu, B, Maw, MTT, Duan, D, et al. Effect of Isocaloric, time-restricted eating on body weight in adults with obesity: a randomized controlled trial. Ann Intern Med. (2024) 177:549–58. doi: 10.7326/M23-3132,
45. Świątkiewicz, I, Nuszkiewicz, J, Wróblewska, J, Nartowicz, M, Sokołowski, K, Sutkowy, P, et al. Feasibility and Cardiometabolic effects of time-restricted eating in patients with metabolic syndrome. Nutrients. (2024) 16:802. doi: 10.3390/nu16121802,
46. Liang, M, Pan, Y, Zhong, T, Zeng, Y, and Cheng, ASK. Effects of aerobic, resistance, and combined exercise on metabolic syndrome parameters and cardiovascular risk factors: a systematic review and network meta-analysis. Rev Cardiovasc Med. (2021) 22:1523–33. doi: 10.31083/j.rcm2204156,
47. Xie, Y, Gu, Y, Li, Z, He, B, and Zhang, L. Effects of different exercises combined with different dietary interventions on body composition: a systematic review and network meta-analysis. Nutrients. (2024) 16:3007. doi: 10.3390/nu16173007,
48. Trepanowski, JF, Kroeger, CM, Barnosky, A, Klempel, MC, Bhutani, S, Hoddy, KK, et al. Effect of alternate-day fasting on weight loss, weight maintenance, and Cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Intern Med. (2017) 177:930–8. doi: 10.1001/jamainternmed.2017.0936,
49. Selvaraj, S, Kim, J, Ansari, BA, Zhao, L, Cvijic, ME, Fronheiser, M, et al. Body composition, natriuretic peptides, and adverse outcomes in heart failure with preserved and reduced ejection fraction. JACC Cardiovasc Imaging. (2021) 14:203–15. doi: 10.1016/j.jcmg.2020.07.022,
50. Chen, AK, Roberts, CK, and Barnard, RJ. Effect of a short-term diet and exercise intervention on metabolic syndrome in overweight children. Metabolism. (2006) 55:871–8. doi: 10.1016/j.metabol.2006.03.001,
51. Roberts, CK, Won, D, Pruthi, S, Kurtovic, S, Sindhu, RK, Vaziri, ND, et al. Effect of a short-term diet and exercise intervention on oxidative stress, inflammation, MMP-9, and monocyte chemotactic activity in men with metabolic syndrome factors. J Appl Physiol. (1985) 100:1657–65. doi: 10.1152/japplphysiol.01292.2005
52. Wewege, MA, Thom, JM, Rye, KA, and Parmenter, BJ. Aerobic, resistance or combined training: a systematic review and meta-analysis of exercise to reduce cardiovascular risk in adults with metabolic syndrome. Atherosclerosis. (2018) 274:162–71. doi: 10.1016/j.atherosclerosis.2018.05.002,
53. Valenzuela-Fuenzalida, JJ, Bravo, VS, Valarezo, LM, Delgado Retamal, MF, Leiva, JM, Bruna-Mejías, A, et al. Effectiveness of DASH diet versus other diet modalities in patients with metabolic syndrome: a systematic review and meta-analysis. Nutrients. (2024) 16:3054. doi: 10.3390/nu16183054,
54. Mohammadzadeh, M, Amirpour, M, Ahmadirad, H, Abdi, F, Khalesi, S, Naghshi, N, et al. Impact of fasting mimicking diet (FMD) on cardiovascular risk factors: a systematic review and meta-analysis of randomized control trials. Diabetol Metab Syndr. (2025) 17:137. doi: 10.1186/s13098-025-01709-5,
55. Khalafi, M, Symonds, ME, Maleki, AH, Sakhaei, MH, Ehsanifar, M, and Rosenkranz, SK. Combined versus independent effects of exercise training and intermittent fasting on body composition and cardiometabolic health in adults: a systematic review and meta-analysis. Nutr J. (2024) 23:7. doi: 10.1186/s12937-023-00909-x,
56. Mølmen, KS, Almquist, NW, and Skattebo, Ø. Effects of exercise training on mitochondrial and capillary growth in human skeletal muscle: a systematic review and meta-regression. Sports Med. (2025) 55:115–44. doi: 10.1007/s40279-024-02120-2,
57. Bosco, G, Di Giacomo Barbagallo, F, Di Marco, M, Scilletta, S, Miano, N, Esposto, S, et al. Effect of inclisiran on lipid and mechanical vascular profiles in familial hypercholesterolemia subjects: results from a single lipid center real-world experience. Prog Cardiovasc Dis. (2025) 92:108–17. doi: 10.1016/j.pcad.2025.05.008,
58. Snowling, NJ, and Hopkins, WG. Effects of different modes of exercise training on glucose control and risk factors for complications in type 2 diabetic patients: a meta-analysis. Diabetes Care. (2006) 29:2518–27. doi: 10.2337/dc06-1317,
59. Redman, LM, Huffman, KM, Landerman, LR, Pieper, CF, Bain, JR, Muehlbauer, MJ, et al. Effect of caloric restriction with and without exercise on metabolic intermediates in nonobese men and women. J Clin Endocrinol Metab. (2011) 96:E312–21. doi: 10.1210/jc.2010-1971,
60. Luo, Y, Luo, D, Li, M, and Tang, B. Insulin resistance in Pediatric obesity: from mechanisms to treatment strategies. Pediatr Diabetes. (2024) 2024:2298306. doi: 10.1155/2024/2298306,
61. Ben Ounis, O, Elloumi, M, Lac, G, Makni, E, Van Praagh, E, Zouhal, H, et al. Two-month effects of individualized exercise training with or without caloric restriction on plasma adipocytokine levels in obese female adolescents. Ann Endocrinol (Paris). (2009) 70:235–41. doi: 10.1016/j.ando.2009.03.003,
62. Abrego-Guandique, DM, Aguilera Rojas, NM, Chiari, A, Luciani, F, Cione, E, and Cannataro, R. The impact of exercise on mitochondrial biogenesis in skeletal muscle: a systematic review and meta-analysis of randomized trials. Biomol Concepts. (2025) 16:55. doi: 10.1515/bmc-2025-0055,
63. Maillard, F, Pereira, B, and Boisseau, N. Effect of high-intensity interval training on Total, abdominal and visceral fat mass: a meta-analysis. Sports Med. (2018) 48:269–88. doi: 10.1007/s40279-017-0807-y,
64. Khalafi, M, Malandish, A, Rosenkranz, SK, and Ravasi, AA. Effect of resistance training with and without caloric restriction on visceral fat: a systemic review and meta-analysis. Obes Rev. (2021) 22:e13275. doi: 10.1111/obr.13275,
65. Di Marco, M, Scilletta, S, Miano, N, Capuccio, S, Musmeci, M, Di Mauro, S, et al. Triglycerides to high density lipoprotein cholesterol ratio (TG/HDL), but not triglycerides and glucose product (TyG) index, is associated with arterial stiffness in prediabetes. Diabetes Res Clin Pract. (2025) 224:112189. doi: 10.1016/j.diabres.2025.112189,
Keywords: diet intervention, exercise training, metabolic syndrome, obesity, weight loss
Citation: Cao Y, Xu R and Zhang J (2025) Comparison of the impact of exercise training combined with dietary intervention vs. dietary intervention alone in patients with obesity and metabolic syndrome—a systematic review. Front. Nutr. 12:1703600. doi: 10.3389/fnut.2025.1703600
Edited by:
Daichi Sumi, ASICS Institute of Sport Science, JapanReviewed by:
Yujia Zhang, Centers for Disease Control and Prevention (CDC), United StatesFrancesco Di Giacomo Barbagallo, University of Catania, Italy
Copyright © 2025 Cao, Xu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Zhang, emhhbmdqaWVnenR5QDEyNi5jb20=
†These authors have contributed equally to this work
 Youxiang Cao
Youxiang Cao Rui Xu
Rui Xu Jie Zhang1*
Jie Zhang1*



![Forest plot showing the standardized mean difference and 95% confidence intervals for multiple studies. The plot displays individual study results with green dots and horizontal lines, along with a diamond representing the overall effect size. The studies listed include Bagnato, Battilo, Bouchonville, and others. Heterogeneity is indicated by Tau-squared equals 0.08, Chi-squared equals 35.87, degrees of freedom equals 21, I-squared equals 41%. The test for overall effect shows Z equals 2.31, P equals 0.02. The overall effect size is 0.22 [0.03, 0.40].](https://www.frontiersin.org/files/Articles/1703600/fnut-12-1703600-HTML/image_m/fnut-12-1703600-g006.jpg)