- 1Clinical Anatomy & Reproductive Medicine Application Institute, Hengyang Medical School, University of South China, Hengyang, Hunan, China
- 2Hengyang Hospital of Hunan Normal University & Hengyang Central Hospital, Hengyang, Hunan, China
Introduction: Polycystic ovary syndrome (PCOS) affects 5–15% of reproductive-aged women and involves significant metabolic dysregulation, for which nutritional interventions show therapeutic potential. Methods: This umbrella meta-analysis synthesizes evidence from 46 randomized trials (n = 30,133) to evaluate dietary supplements targeting core PCOS pathways.
Methods: This umbrella meta-analysis synthesizes evidence from 46 randomized trials (n = 30,133) to evaluate dietary supplements targeting core PCOS pathways.
Results: Key nutraceuticals demonstrate clinically relevant benefits: myo-inositol significantly improves insulin sensitivity (HOMA-IR SMD = −0.81) and SHBG levels (SMD = 9.65) by enhancing glucose transporter activity; probiotics reduce systemic inflammation (CRP SMD = −0.82) via gut-microbiota modulation; omega-3 fatty acids ameliorate dyslipidemia (LDL-C SMD = −9.57; HDL-C SMD = 2.31) through anti-inflammatory mechanisms. Plant-derived compounds like curcumin lower fasting glucose (SMD = −3.43) via NF-ĸB pathway inhibition, while green tea catechins reduce adiposity. Significant heterogeneity arises from variations in supplement bioavailability, dosing protocols, and patient metabolic phenotypes. Nevertheless, consistent evidence confirms that targeted nutrient supplementation modulates insulin signaling, lipid metabolism, and hormonal balance in PCOS. Emerging research priorities include personalized nutrition protocols leveraging nutrigenomic interactions and antioxidant-rich formulations (e.g., vitamin E, selenium).
Discussion: This work establishes a mechanistic foundation for integrating evidence-based nutraceuticals—particularly myo-inositol, probiotics, and omega-3s—into PCOS management, offering clinically actionable strategies while highlighting needs for standardized dosing and bioavailability studies.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD42024602681.
1 Introduction
Over the past half-century, global birth rates have plummeted significantly (1). Polycystic ovary syndrome (PCOS) is a leading cause of female infertility, affecting 5–15% of women of reproductive age (2, 3). The syndrome is characterized by pathological features such as abnormal secretion of gonadotropins, ovarian folliculogenesis, steroidogenesis, insulin secretion, and adipose tissue function (4). Studies have indicated that PCOS is often associated with symptoms like central obesity and insulin resistance (5). Weight gain and obesity exacerbate insulin resistance and metabolic syndrome features (6). In individuals with PCOS, excess weight contributes to reproductive and metabolic risks (7–9). Weight gain and obesity in women with PCOS can also lead to worsened insulin resistance and metabolic dysfunction (mediated by further impairment of the PI3 kinase downstream insulin receptor pathway), as well as the typical reproductive disorders and hyperandrogenism associated with the condition (10). An umbrella meta-analysis suggests that insulin resistance is considered a primary factor in the pathophysiology of PCOS and is involved in the development of hyperandrogenemia and reproductive dysfunction through various mechanisms (11). Therefore, nutrition and dietary interventions play a crucial role in the progression or management of the disease (12).
Previous research has shown metabolic disturbances in many nutrients in PCOS patients, such as vitamin D, minerals, and the ratio of omega-6 to omega-3 fatty acids (13). Supplementation with natural molecules like inositol, vitamin E, vitamin D, and omega-3 may help alleviate pathological symptoms of PCOS, such as oocyte immaturity, insulin resistance, hyperandrogenism, oxidative stress, and inflammation (14). Alesi et al. (15) highlighted the potential benefits of specific nutrients—including vitamins (e.g., B-12, inositol, vitamin D, E, K), minerals (e.g., calcium, selenium), and other compounds (e.g., omega-3 fatty acids, probiotics)—for PCOS management. A network umbrella meta-analysis by Hu et al. (16) shows that carnitine, inositol, and probiotics are effective in reducing weight and body mass index (BMI). Omega-3 is effective in lowering fasting blood glucose (FBG), chromium is effective in lowering fasting insulin (FINS), and coenzyme Q10 is the most effective in improving lipid metabolism (16). However, a systematic review by Menichini et al. (17) notes that while some nutritional supplements (such as inositol, probiotics, vitamin D, and E) show potential benefits in certain aspects, these results are not consistent and further research is needed to confirm their efficacy. A review article published in J Turk Ger Gynecol Assoc points out that although some studies suggest that nutritional supplements may be beneficial for the metabolic and endocrine functions of PCOS (18). Several systematic reviews and umbrella meta-analyses have indicated that while individual studies show potential benefits of certain nutritional supplements, the overall evidence is insufficient and more high-quality studies are needed to validate these findings (19).
The current umbrella meta-analysis aims to critically evaluate the efficacy of nutritional supplements in the treatment of PCOS, intending to provide a more nuanced understanding of their potential benefits and to address the existing discrepancies in the literature.
2 Materials and methods
This study adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (20). The protocol for this systematic review and umbrella meta-analysis has been prospectively registered in the PROSPERO database (registration number: CRD42024600102).
2.1 Eligibility criteria for this review
This review includes umbrella meta-analyses evaluating the efficacy of nutritional supplements—such as n-3 polyunsaturated fatty acids, alpha-lipoic acid, polyphenols, minerals, melatonin, vitamins, inositol, probiotics, and L-carnitine—compared to placebo or alternative treatments in women diagnosed with PCOS using the Rotterdam criteria (21).
2.2 Search strategy
We conducted a comprehensive literature search across PubMed, Web of Science, Embase, and Scopus up to September 13, 2024, identifying umbrella meta-analyses on nutritional supplement interventions for PCOS, and included a thorough review of relevant publications, review articles, and the reference lists of included studies. To further expand our literature resources, We manually reviewed reference lists of included studies and contacted field experts to identify additional or unpublished sources. Our search strategy skillfully combined MeSH terms and keywords, and our search was limited to human studies without language restrictions. The detailed search strategy, keywords, and specific search syntax for each database are listed in Appendix A. We applied specific exclusion criteria to ensure the quality and relevance of the included studies.
Exclusion Criteria:
Studies were excluded if they:
• Were non-randomized controlled trials (non-RCTs)
• Included fewer than 20 participants
• Were not published in peer-reviewed journals
• Did not focus on nutritional supplement treatment for PCOS
• Lacked sufficient data for analysis
By applying these exclusion criteria, we aimed to include only high-quality studies that directly addressed the research question.
2.3 Data synthesis and quality assessment
Two reviewers (RTW and MYW) independently screened titles and abstracts using predefined criteria. Full texts of potentially eligible studies were assessed, with disagreements resolved by consensus or a third reviewer (XCL). Data extracted from the included studies encompassed publication year, sample size, geographical location of the study, duration of nutritional supplementation, standardized mean difference (SMD), mean difference (MD), odds ratio (OR), relative risk (RR), and their corresponding 95% confidence intervals (CI). To standardize effect sizes, odds ratios (ORs) were converted to relative risks (RRs), and mean differences (MDs) to standardized mean differences (SMDs).
2.4 Statistical analysis
Statistical analyses were conducted using STATA version 16.0 (StataCorp, College Station, TX, United States). A random-effects model was applied when I2 exceeded 50%, accounting for heterogeneity across studies. Sensitivity analyses assessed the robustness of findings, with all estimates reported as 95% confidence intervals (CI).
3 Results
3.1 Study selection and characteristics
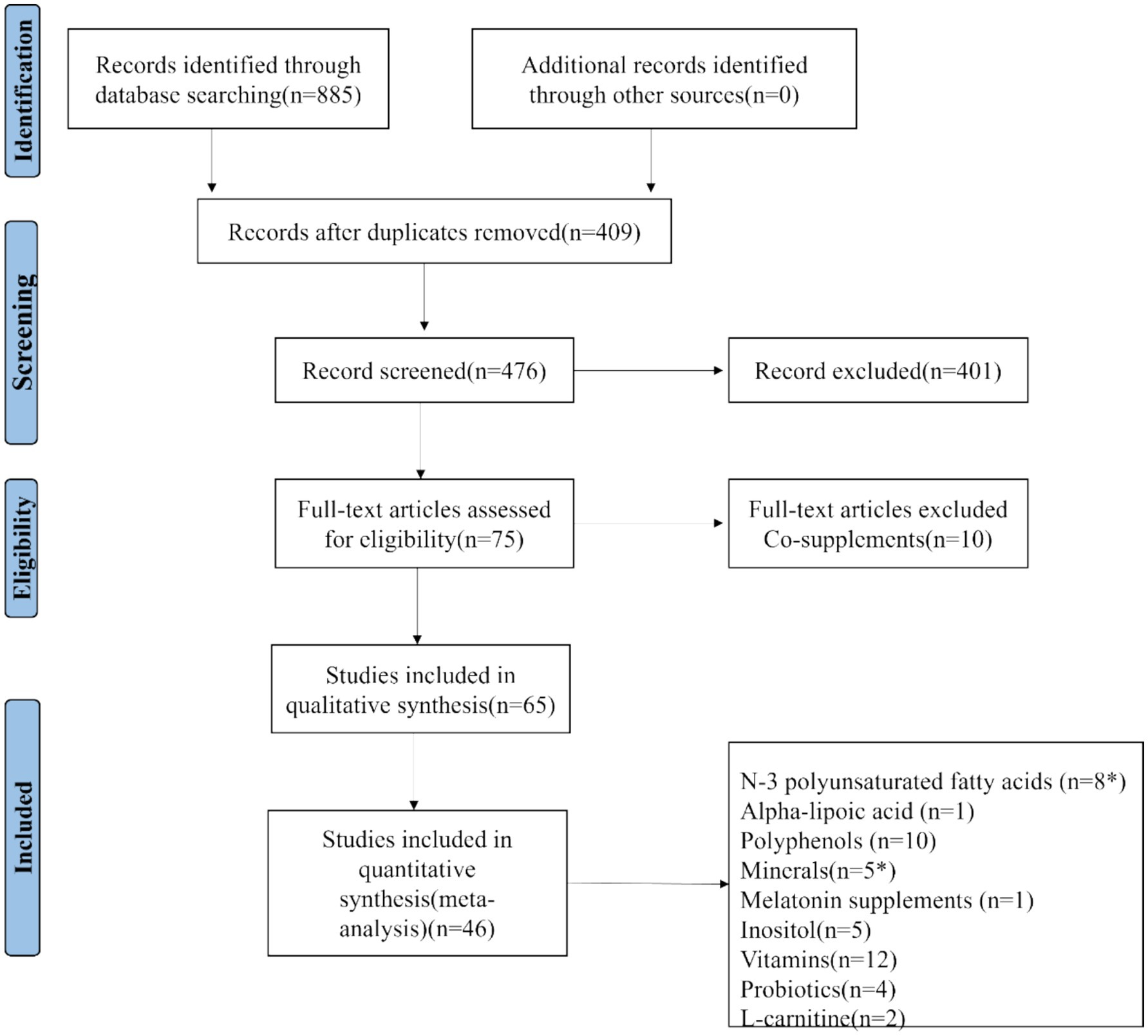
The initial search yielded 885 umbrella meta-analysis articles on the treatment of PCOS with nutritional supplements: 130 from PubMed, 227 from Embase, 248 from Scopus, and 280 from Web of Science. No unpublished studies were identified. Among these, 409 studies were determined to be duplicates. After rigorous screening of the titles and abstracts of the remaining 476 articles, 75 articles were assessed in full text, resulting in 65 articles that met the qualitative synthesis criteria. For quantitative synthesis, a total of 46 studies were ultimately included (22–62). The process is depicted in Figure 1. The selected 46 studies comprised 30,133 female participants. The studies that met the inclusion criteria spanned from 2016 to 2024. These investigations were geographically distributed across 12 countries: Australia (1 study), Iran (7 studies), Brazil (2 studies), China (11 studies), Saudi Arabia (3 studies), United States (1 study), Taiwan (1 study), Mexico (1 study), Hungary (1 study), United Kingdom (1 study), Greece (1 study), and Malaysia (1 study). Each randomized controlled trial included in the umbrella meta-analysis utilized nutritional supplements as a therapy for PCOS. The detailed characteristics of the included umbrella meta-analyses are summarized in Appendix C: Characteristics of umbrella meta-analyses examining the effects of nutritional supplements on PCOS.
3.2 Umbrella meta-analysis results
3.2.1 N-3 unsaturated fatty acids
The umbrella meta-analysis encompassed 8 studies reporting on n-3 unsaturated fatty acids (22–29). A total of 29 relevant outcomes were recorded, with the experimental group receiving oral n-3 unsaturated fatty acids and the control group receiving a placebo. Notably, adiponectin levels (SMD: 1.43; 95% CI: 1.20, 1.66; I2 = 0.0%, p = 0.962), BMI (SMD: −0.56; 95% CI: −1.00, −0.11; I2 = 0.0%, p = 0.983), fasting blood glucose (SMD: −3.62; 95% CI: −5.25, −1.98; I2 = 0.0%, p = 0.414), FSH (SMD: −0.01; 95% CI: −0.66, 0.65; I2 = 22.9%, p = 0.255), GSH (SMD: 0.24; 95% CI: −0.42, 0.90; I2 = 0.0%, p = 0.504), HDL-c (SMD: 2.31; 95% CI: 0.81, 3.81; I2 = 0.0%, p = 0.732), HOMA-IR (SMD: −0.73; 95% CI: −0.96, −0.50; I2 = 75.0%, p = 0.003), LDL-C (SMD: −9.57; 95% CI: −10.24, −8.90; I2 = 0.0%, p = 0.492), LH (SMD: −0.84; 95% CI: −1.82, 0.15; I2 = 23.7%, p = 0.252), MDA (SMD: −0.39; 95% CI: −0.54, −0.24; I2 = 0.0%, p = 0.596), SHBG (SMD: 0.75; 95% CI: 0.15, 1.36; I2 = 0.0%, p = 0.669), TG (SMD: −6.70; 95% CI: −11.89, −1.50; I2 = 96.5%, p < 0.001), total cholesterol (SMD: −8.84; 95% CI: −10.77, −6.92; I2 = 20.1%, p = 0.286), and total testosterone (SMD: −0.14; 95% CI: −0.28, 0.00; I2 = 0.0%, p = 0.452) were among the outcomes assessed (Figure 2).
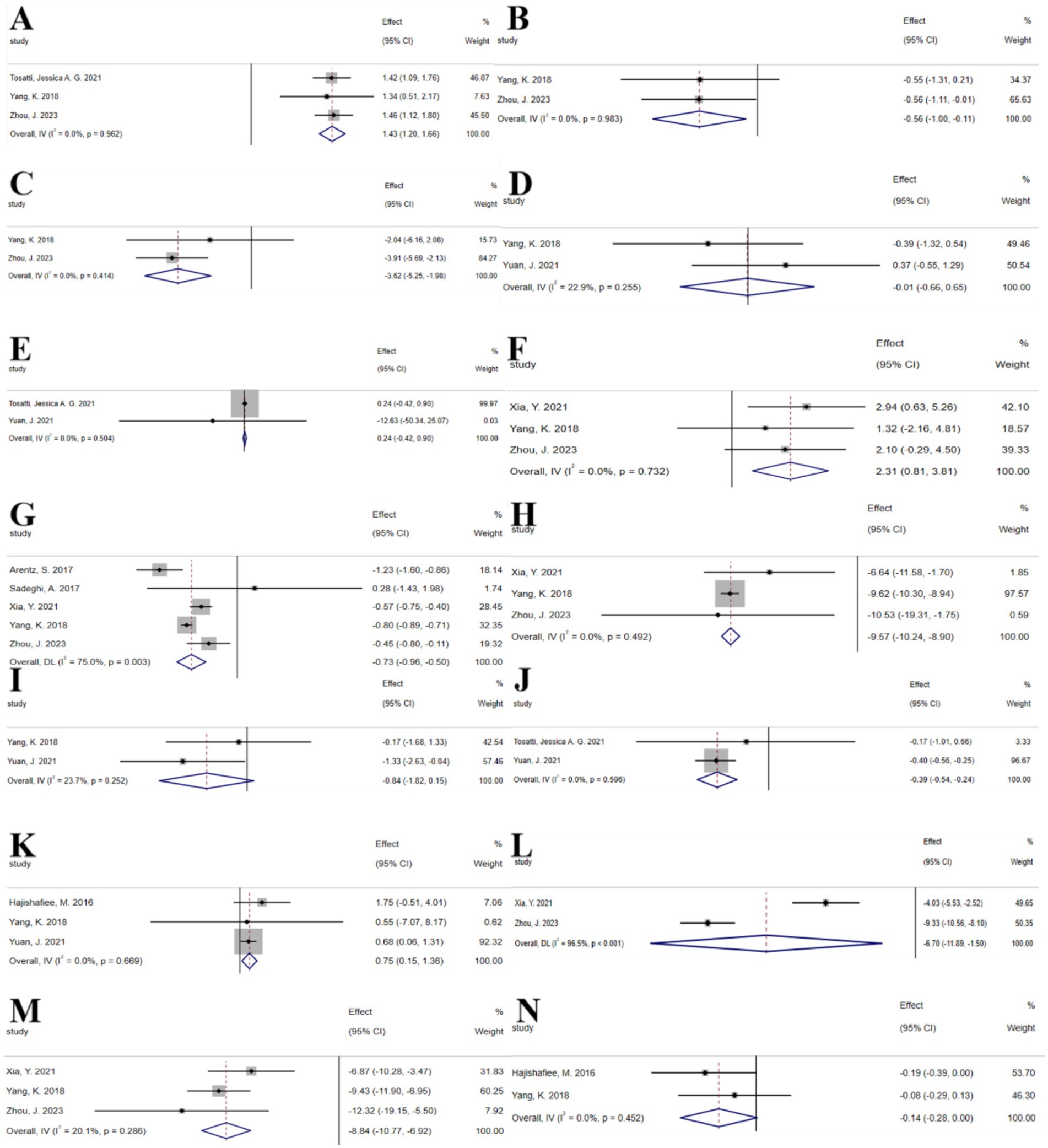
Figure 2. N-3 unsaturated fatty acids forest plot. (A) Adiponectin concentration, (B) BMI, (C) Fasting glucose, (D) FSH, (E) GSH, (F) HDL-c, (G) HOMA-IR, (H) LDL-c, (I) LH, (J) MDA, (K) SHBG, (L) TG, (M) Total cholesterol, (N) Total testosterone.
3.2.2 Inositol
The umbrella meta-analysis included 5 studies reporting on inositol (25, 30–33). A total of 24 relevant outcomes were recorded, with the experimental group receiving oral inositol and the control group receiving a placebo, except for the study by Zeng et al. (30), which used metformin as the control. Notably, androstenedione (SMD: −1.07; 95% CI: −2.03, −0.12; I2 = 92.2%, p < 0.001), free testosterone (SMD: −0.24; 95% CI: −0.55, 0.06; I2 = 70.3%, p = 0.009), HOMA-IR (SMD: −0.81; 95% CI: −1.21, −0.41; I2 = 82.6%, p < 0.001), LH (SMD: −2.42; 95% CI: −4.59, −0.25; I2 = 92.5%, p < 0.001), SHBG (SMD: 9.65; 95% CI: 3.17, 16.13; I2 = 88.1%, p < 0.001), total testosterone (SMD: −1.48; 95% CI: −3.21, 0.24; I2 = 72.1%, p = 0.006), BMI (SMD: −0.17; 95% CI: −0.30, −0.04; I2 = 43.7%, p = 0.169), fasting blood glucose (SMD: −1.09; 95% CI: −1.49, −0.68; I2 = 15.3%, p = 0.317), fasting insulin (SMD: −1.44; 95% CI: −1.95, −0.93; I2 = 21.2%, p = 0.281), FSH (SMD: −1.34; 95% CI: −1.56, −1.13; I2 = 51.8%, p = 0.150), and pregnancy rate (RR: 1.38; 95% CI: 1.09, 1.74; I2 = 6.1%, p = 0.372) were among the outcomes assessed (Figure 3). Given the significant high heterogeneity observed in the related outcomes, sensitivity analyses were conducted (Supplementary Figure S1). After excluding studies with considerable heterogeneity, androstenedione (SMD: −1.47; 95% CI: −2.83, −0.12; I2 = 93.4%, p < 0.001), free testosterone (SMD: −0.39; 95% CI: −0.61, −0.17; I2 = 18.3%, p = 0.299), HOMA-IR (SMD: −0.90; 95% CI: −1.51, −0.30; I2 = 74.5%, p = 0.020), LH (SMD: −3.43; 95% CI: −4.29, −2.57; I2 = 0.0%, p = 0.895), and SHBG (SMD: 19.17; 95% CI: 2.79, 35.54; I2 = 87.1%, p < 0.001) were re-evaluated (Supplementary Figure S2).

Figure 3. Inositol forest plot. (A) Androstenedione, (B) Free testosterone, (C) HOMA-IR, (D) LH, (E) SHBG, (F) Total testosterone, (G) BMI, (H) Fasting glucose, (I) Fasting insulin, (J) FSH, (K) Pregnancy.
3.2.3 Plant polyphenols
3.2.3.1 Curcumin
The umbrella meta-analysis included 7 studies reporting on curcumin (34–38). A total of 38 relevant outcomes were recorded, with the experimental group receiving oral curcumin and the control group receiving a placebo. Notably, BMI (SMD: −0.25; 95% CI: −0.41, −0.09; I2 = 0.0%, p = 0.492), C-reactive protein (SMD: −0.58; 95% CI: −1.25, 0.08; I2 = 2.6%, p = 0.311), FAI (SMD: −0.05; 95% CI: −1.87, 0.17; I2 = 77.1%, p = 0.036), fasting blood glucose (SMD: −3.43; 95% CI: −4.13, −2.73; I2 = 0.0%, p = 0.884), fasting insulin (SMD: −1.20; 95% CI: −1.88, −0.52; I2 = 0.0%, p = 0.601), FSH (SMD: 0.00; 95% CI: −0.02, 0.03; I2 = 0.0%, p = 0.587), gastrointestinal adverse events (RR: 1.16; 95% CI: 0.95, 1.42; I2 = 42.7%, p = 0.186), HDL-C (SMD: 3.83; 95% CI: 0.36, 7.30; I2 = 0.0%, p = 0.934), HOMA-IR (SMD: −0.69; 95% CI: −1.04, −0.34; I2 = 89.7%, p < 0.001), insulin (SMD: −2.26; 95% CI: −3.19, −1.33; I2 = 70.4%, p = 0.009), LDL (SMD: −0.11; 95% CI: −0.38, 0.16; I2 = 0.0%, p = 0.803), LDL-C (SMD: −7.06; 95% CI: −15.82, 1.70; I2 = 0.0%, p = 0.849), LH (SMD: −0.41; 95% CI: −1.06, 0.24; I2 = 72.8%, p = 0.025), postprandial blood glucose (SMD: −0.37; 95% CI: −0.83, 0.10; I2 = 66.0%, p = 0.087), pregnancy rate (RR: 0.95; 95% CI: 0.52, 1.73; I2 = 84.6%, p = 0.001), QUICK (SMD: 0.01; 95% CI: 0.01, 0.01; I2 = 0.0%, p = 0.996), SHBG (SMD: 9.87; 95% CI: 2.28, 17.45; I2 = 79.5%, p = 0.027), TC (SMD: −0.30; 95% CI: −0.54, −0.05; I2 = 35.1%, p = 0.214), TG (SMD: −6.67; 95% CI: −19.84, 6.49; I2 = 0.0%, p = 0.653), total cholesterol (SMD: −14.06; 95% CI: −20.19, −7.93; I2 = 0.0%, p = 0.976), total testosterone (SMD: −0.19; 95% CI: −0.37, −0.02; I2 = 79.2%, p = 0.008), waist circumference (SMD: −2.73; 95% CI: −4.01, −1.46; I2 = 0.0%, p = 0.607), and WHR (SMD: −0.04; 95% CI: −0.05, −0.03; I2 = 31.3%, p = 0.228) were among the outcomes assessed (Figure 4).

Figure 4. Curcumin forest plot. (A) BMI, (B) C-reactive protein, (C) FAI, (D) Fasting blood glucose, (E) Fasting insulin, (F) FSH, (G) Gastrointestinal adverse events, (H) HDL-c, (I) HOMA-IR, (J) Insulin, (K) LDL, (L) LDL-c, (M) LH, (N) Postprandial plasma glucose, (O) Pregnancy rates, (P) QUICKI, (Q) SHBG, (R) TC, (S) TG, (T) Total cholesterol, (U) Total testosterone, (V) Waist circumference, (W) WHR.
3.2.3.2 Green tea
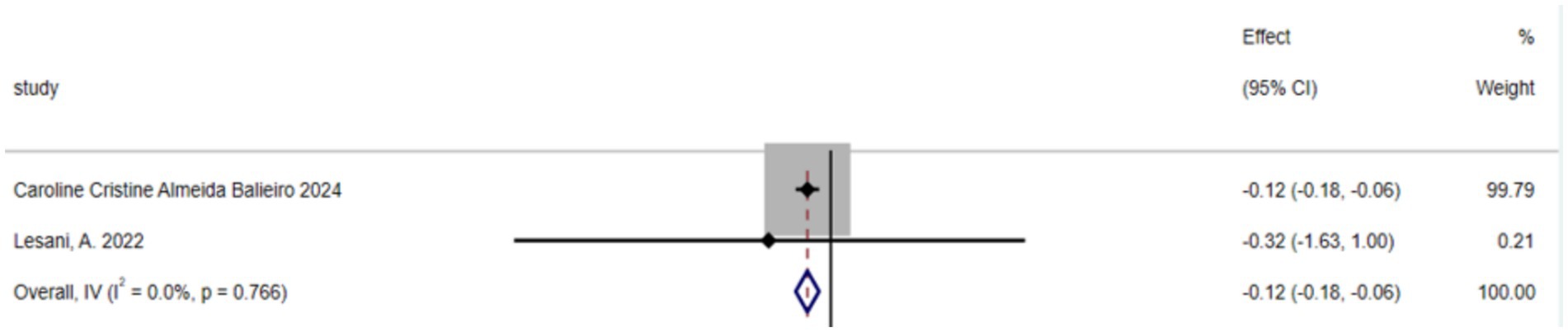
The umbrella meta-analysis included 2 studies reporting on green tea (39). A total of 8 relevant outcomes were recorded, with the experimental group receiving oral green tea and the control group receiving a placebo. Notably, BMI (SMD: −0.12; 95% CI: −0.18, −0.06; I2 = 0.0%, p = 0.766) was among the outcomes assessed (Figure 5).
3.2.4 Mineral substances
The umbrella meta-analysis encompassed 5 studies reporting on mineral substances (25, 40–42), including three studies on selenium supplements (40, 41) and two studies on chromium supplements (42, 43). A total of 24 relevant outcomes were recorded, with the experimental group receiving oral chromium and selenium supplements and the control group receiving a placebo. Notably, FBS (SMD: −0.26; 95% CI: −0.65, 0.13; I2 = 0.0%, p = 0.681), FG (SMD: −0.23; 95% CI: −0.52, 0.06; I2 = 11.1%, p = 0.289), FSI (SMD: 0.14; 95% CI: −1.19, 1.48; I2 = 0.0%, p = 0.983), HOMA-IR (SMD: −0.54; 95% CI: −1.49, 0.40; I2 = 89.0%, p < 0.001), and SHBG (SMD: −0.07; 95% CI: −0.34, 0.20; I2 = 32.2%, p = 0.224), as well as total testosterone (SMD: −0.35; 95% CI: −0.54, −0.15; I2 = 0.0%, p = 0.804) were among the outcomes assessed (Figure 6).
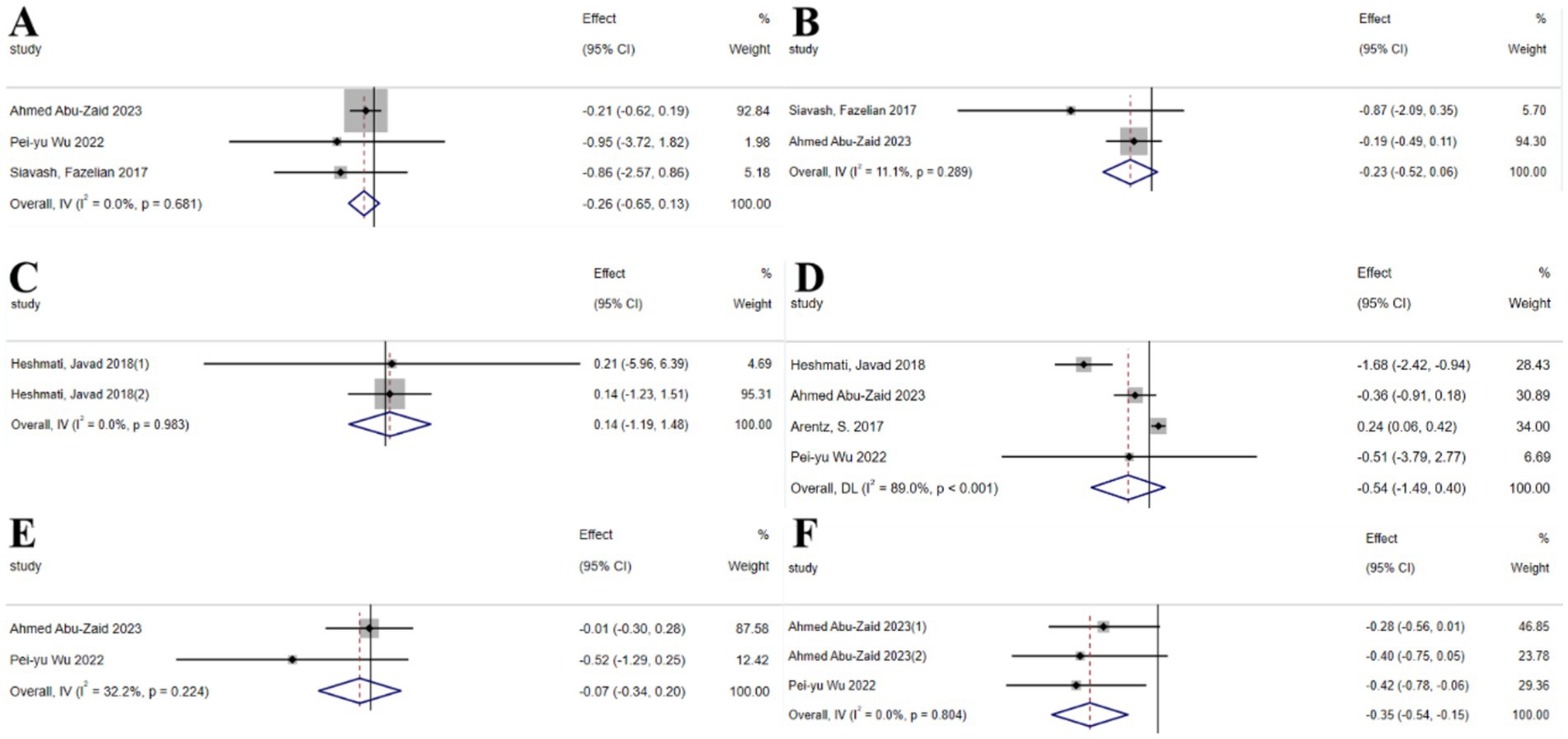
Figure 6. Mineral substance forest plot. (A) FBS, (B) FG, (C) FSI, (D) HOMA-IR, (E) SHBG, (F) Total testosterone.
3.2.5 Vitamins
3.2.5.1 Vitamin D
The umbrella meta-analysis included 10 studies reporting on Vitamin D (43–52). A total of 29 relevant outcomes were recorded, with the experimental group receiving oral Vitamin D and the control group receiving a placebo. Notably, HOMA-IR (SMD: 0.09; 95% CI: −0.38, 0.56; I2 = 92.4%, p < 0.001), TC (SMD: −0.55; 95% CI: −0.82, −0.27; I2 = 96.2%, p < 0.001), TG (SMD: −1.02; 95% CI: −2.14, 0.10; I2 = 93.0%, p < 0.001), LDL-c (SMD: −2.62; 95% CI: −4.27, −0.97; I2 = 95.1%, p < 0.001), hs-CRP (SMD: -0.74; 95% CI: −1.10, −0.39; I2 = 44.9%, p = 0.178), LH (SMD: −0.41; 95% CI: −0.54, −0.28; I2 = 0.0%, p = 0.927), regular menstrual cycles (SMD: 1.38; 95% CI: 1.21, 1.56; I2 = 0.0%, p = 0.583), and total testosterone (SMD: −0.04; 95% CI: −0.18, 0.10; I2 = 69.1%, p = 0.072) were among the outcomes assessed (Figure 7). Given the significant high heterogeneity observed in the related outcomes, sensitivity analyses were conducted (Supplementary Figure S4). After excluding studies with considerable heterogeneity, HOMA-IR (SMD: −0.14; 95% CI: −0.66, 0.37; I2 = 80.0%, p = 0.026), TC (SMD: −11.28; 95% CI: −13.36, −9.21; I2 = 0.0%, p = 0.873), and TG (SMD: −8.99; 95% CI: −12.10, −5.89; I2 = 14.1%, p = 0.312) were re-evaluated (Supplementary Figure S4).
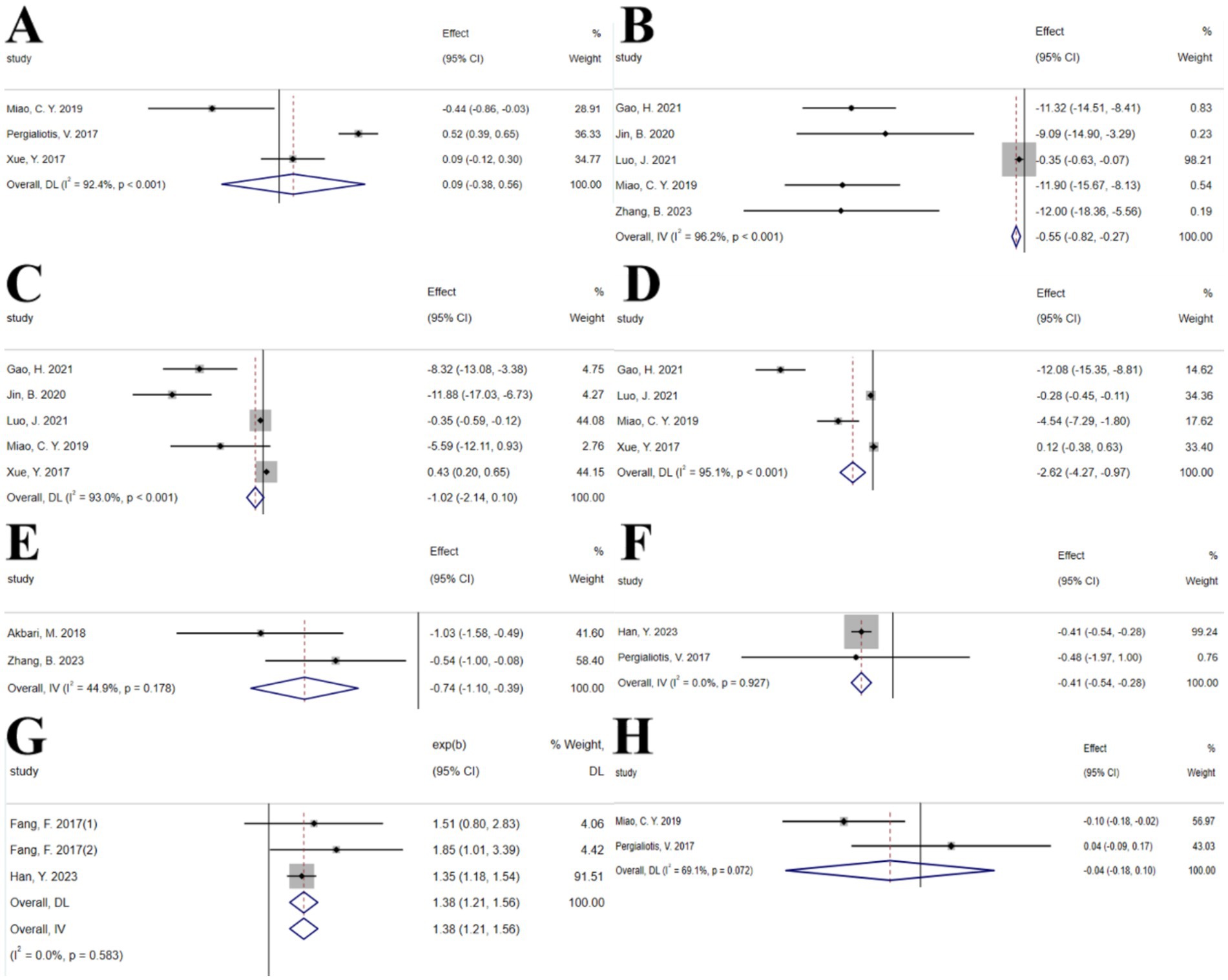
Figure 7. Vitamin D forest plot. (A) HOMA-IR, (B) TC, (C) TG, (D) LDL-c, (E) hs-CRP, (F) LH, (G) Regular menstrual cycles, (H) Total testosterone.
3.2.5.2 Vitamin E
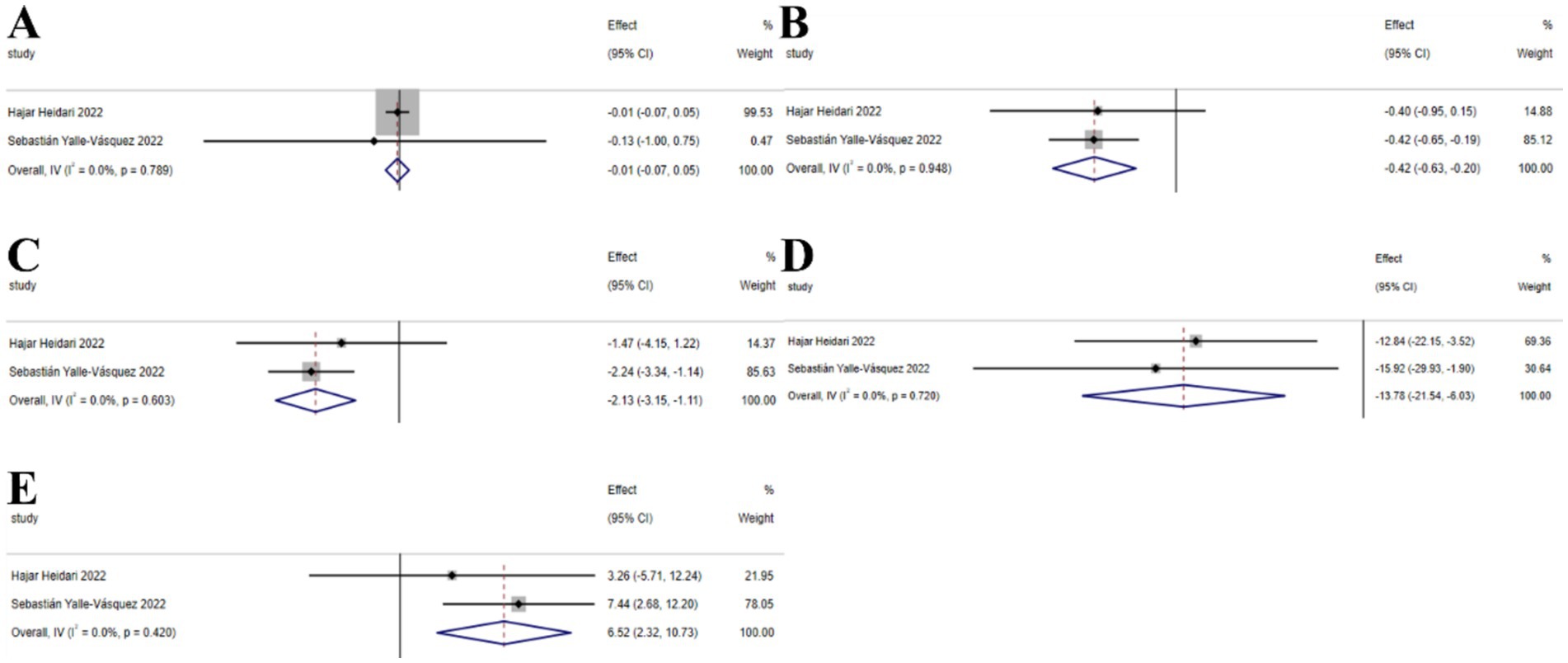
The umbrella meta-analysis included 2 studies reporting on Vitamin E (53, 54). A total of 26 relevant outcomes were recorded, with the experimental group receiving oral Vitamin E and the control group receiving a placebo. Notably, BMI (SMD: −0.01; 95% CI: −0.07, 0.05; I2 = 0.0%, p = 0.789), HOMA-IR (SMD: −0.42; 95% CI: −0.63, −0.20; I2 = 0.0%, p = 0.948), insulin (SMD: −2.13; 95% CI: −3.15, −1.11; I2 = 0.0%, p = 0.603), LDL-c (SMD: −13.78; 95% CI: −21.54, −6.03; I2 = 0.0%, p = 0.720), and SHBG (SMD: 6.52; 95% CI: 2.32, 10.73; I2 = 0.0%, p = 0.420) were among the outcomes assessed (Figure 8).
3.2.6 Probiotics
The umbrella meta-analysis included 4 studies reporting on probiotics (55–58). A total of 24 relevant outcomes were recorded, with the experimental group receiving oral probiotics and the control group receiving a placebo. Notably, BMI (SMD: −0.19; 95% CI: −0.34, −0.04; I2 = 34.1%, p = 0.219), CRP (SMD: −0.82; 95% CI: −1.31, −0.32; I2 = 0.0%, p = 0.495), FPG (SMD: −0.72; 95% CI: −1.78, 0.34; I2 = 82.8%, p = 0.016), GSH (SMD: 0.31; 95% CI: 0.08, 0.54; I2 = 0.0%, p = 0.368), hirsutism (SMD: −0.32; 95% CI: −0.76, 0.13; I2 = 69.8%, p = 0.069), HOMA-IR (SMD: −0.51; 95% CI: −0.72, −0.30; I2 = 0.0%, p = 0.407), insulin (SMD: −0.55; 95% CI: −0.80, −0.30; I2 = 0.0%, p = 0.651), MDA (SMD: −0.88; 95% CI: −1.13, −0.63; I2 = 0.0%, p = 0.716), NO (SMD: 0.35; 95% CI: 0.16, 0.54; I2 = 0.0%, p = 0.802), SHBG (SMD: 0.52; 95% CI: 0.29, 0.76; I2 = 0.0%, p = 0.688), TAC (SMD: 0.53; 95% CI: 0.33, 0.72; I2 = 40.5%, p = 0.195), total testosterone (SMD: −0.57; 95% CI: −0.80, −0.34; I2 = 0.0%, p = 0.842), and vLDL-C (SMD: −0.55; 95% CI: −0.74, −0.35; I2 = 34.4%, p = 0.217) were among the outcomes assessed (Figure 9).

Figure 9. Probiotics forest plot. (A) BMI, (B) CRP, (C) FPG, (D) GSH, (E) Hirsutism, (F) HOMA-IR, (G) Insulin, (H) Malondialdehyde, (I) NO, (J) SHBG, (K) TAC, (L) Total testosterone levels, (M) vLDL-C.
3.2.7 Other indicators
In this umbrella review, indicators reported by only a single umbrella meta-analysis could not be subjected to traditional umbrella meta-analysis to assess their effect sizes and consistency (59–62). However, the reporting of these indicators provides insights into potentially overlooked aspects of PCOS treatment with nutritional supplements. Appendix B records the relevant indicators.
3.2.8 Bibliometric analysis
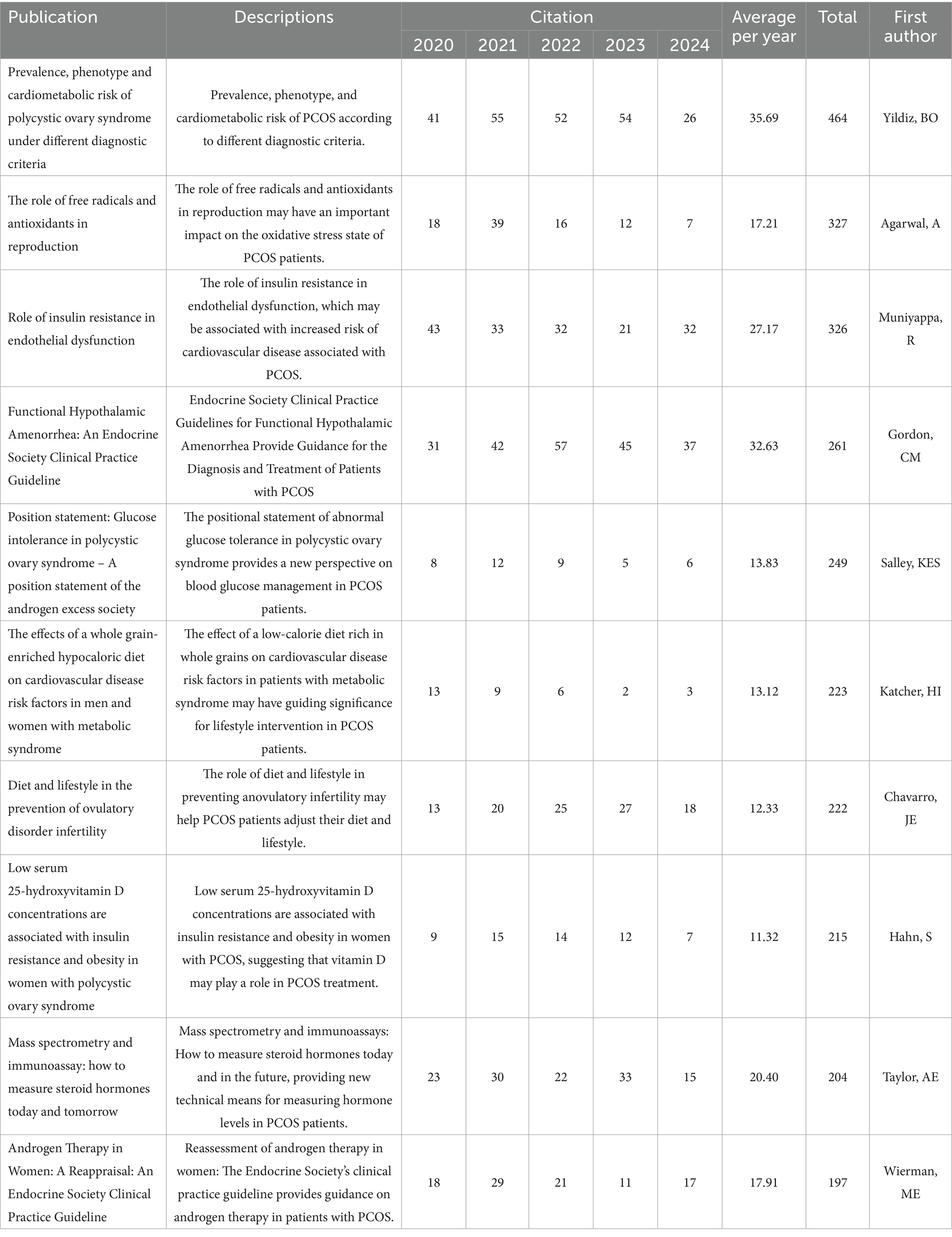
The therapeutic potential of nutritional supplements for PCOS is gaining increasing recognition, with a growing body of literature emphasizing their clinical application. To assess the scientific trajectory of this field, a comprehensive bibliometric analysis was conducted using the WoS database from January 1, 2001, to September 13, 2024. The search strategy is recorded in Appendix A. The search yielded 2,345 articles, with only 1,468 experimental papers included. These articles were cited a total of 35,989 times, with an average of 24.52 citations per paper. Quantitative and visual analysis was performed using VOSviewer. Among 464 journals, 58 published 5 or more articles; among 7,352 authors, 82 published 5 or more articles; among 1,941 institutions, 135 published 5 or more articles; among 78 countries, 48 published 5 or more articles; among 5,016 keywords, 538 appeared 5 or more times (Figure 10). Table 1 lists the top 10 most-cited articles. The surge in citations for different keywords from 2006 to 2024 reflects the evolution of research hotspots in PCOS. It is evident that the focus on nutritional supplements has gradually shifted from early cell models to current clinical trials. However, to date, individualized dosing regimens for nutritional supplements in the treatment of PCOS have not been clearly defined. In the future, nutritional supplements will likely place greater emphasis on individualized plans to achieve optimal outcomes (63).
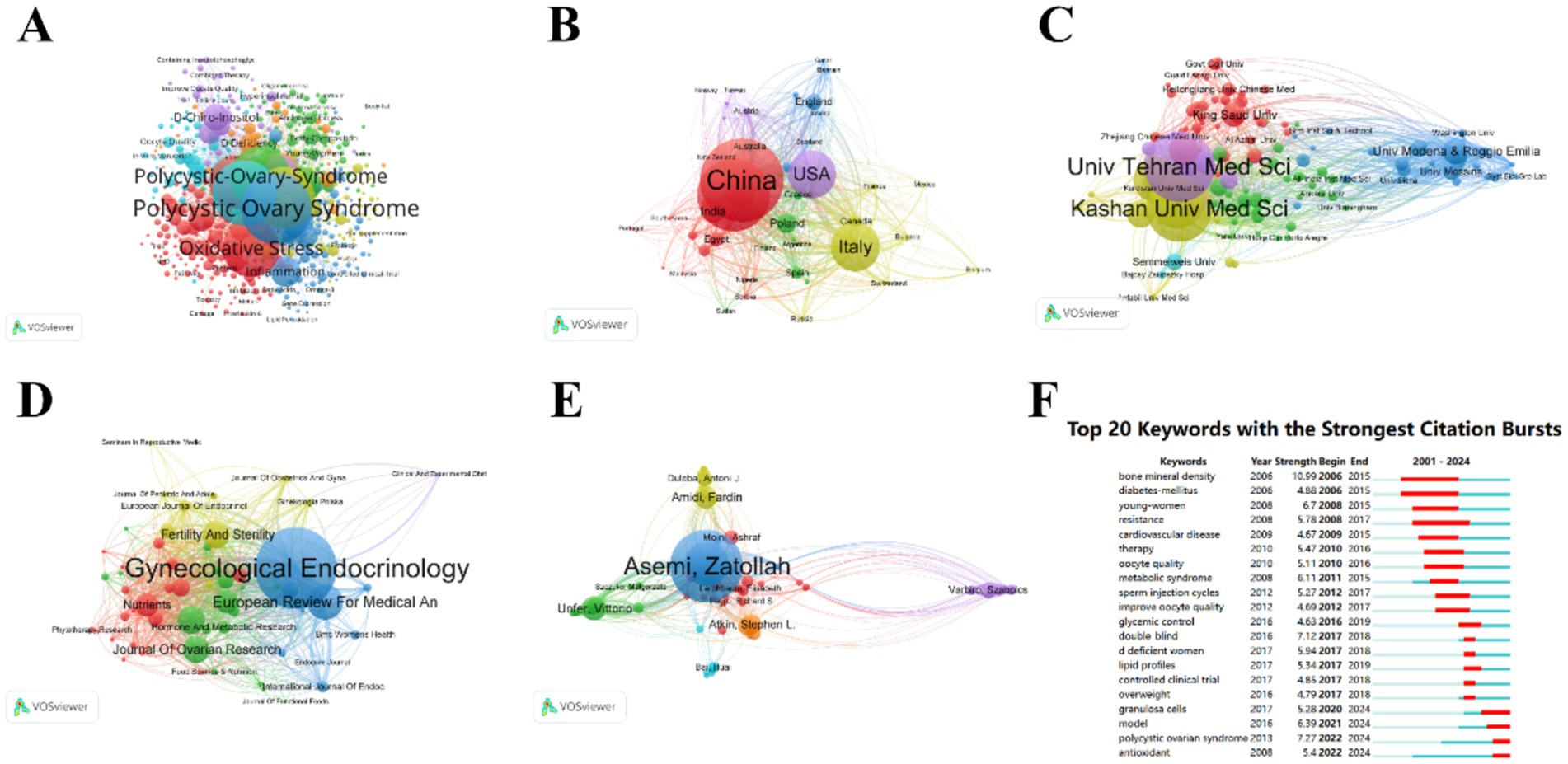
Figure 10. Visualization. (A) Key words, (B) Country, (C) Institution, (D) Periodical, (E) Author, (F) Top 20 Keyword Bursts. In the map, larger nodes represent a higher number of publications. The thickness of the connecting lines indicates the intensity of collaboration, with thicker lines signifying more frequent joint publications. Nodes of different colors represent different clusters.
4 Discussion
Previous research has suggested that nutritional supplement therapy may be a promising treatment strategy (64). We conducted a comprehensive search on nutritional supplements, including a total of 45 studies encompassing umbrella meta-analyses on the treatment of PCOS with n-3 polyunsaturated fatty acids, α-lipoic acid, plant polyphenols (curcumin, green tea, resveratrol), minerals (chromium, selenium), melatonin supplements, vitamins, inositol, probiotics, and L-carnitine. Our results indicate that n-3 polyunsaturated fatty acids, inositol, plant polyphenols (curcumin and green tea), minerals (chromium and selenium), vitamins D and E, and probiotics all show potential benefits in improving the metabolic and reproductive health of PCOS patients. The keyword burst plot in the bibliometric analysis (Figure 10E) indicates that the early research focus, as shown by the burst of keywords such as “bone mineral density,” “diabetes mellitus,” and “young women,” has shifted toward the antioxidant and personalized aspects of nutritional supplements in recent years, as indicated by the burst of keywords like “polycystic ovarian syndrome” and “antioxidant” (65–67).
Our results align with existing evidence that nutritional supplements ameliorate insulin resistance, lipid profiles, and hormonal imbalances in PCOS (68, 69). n-3 Polyunsaturated fatty acids significantly improved the adiponectin levels, BMI, fasting blood glucose, HDL-c, HOMA-IR, LDL-C, MDA, SHBG, TG, and total cholesterol in PCOS patients, but not FSH and total testosterone levels. Studies by Yuan et al. (27) and Zhou et al. (29) both indicated that supplementation with n-3 polyunsaturated fatty acids had no significant effect on FSH levels, which is consistent with the previous report by Salek et al. (70), indicating a lack of comprehensive evidence specifically targeting FSH improvement. The studies by Hajishafiee et al. (23) and Zeng and Yang et al. (30) both indicated that supplementation with n-3 polyunsaturated fatty acids had no significant effect on total testosterone levels, which is not consistent with the results of the study by Albardan et al. (71). In the study by Hajishafiee, et al. (23), most clinical trials used fish oil or flaxseed oil; in Hajishafiee 2016’s study, different types of n-3 polyunsaturated fatty acids were used, including EPA and DHA from fish oil and plant-derived ALA (such as ALA from flaxseed oil and walnuts). Different doses and types of n-3 PUFA may have inconsistent effects on testosterone metabolic parameters (72). Inositol improved androstenedione, HOMA-IR, LH, SHBG, BMI, fasting blood glucose, fasting insulin, FSH, and pregnancy rates, which is consistent with the study by Gudović et al. (73). This is because inositol improves insulin resistance (IR) by promoting the translocation of glucose transporter (GLUT4), enhancing cellular glucose uptake, thereby lowering fasting insulin levels (74) and regulating the signaling of FSH and LH, improving the LH/FSH ratio, reducing androgen levels, and thus alleviating hyperandrogenemia (75). Curcumin significantly improved BMI, fasting blood glucose, fasting insulin, HDL-C, HOMA-IR, insulin, QUICK, SHBG, TC, total cholesterol, total testosterone, waist circumference, and WHR in PCOS patients, but had no significant effect on gastrointestinal adverse events, postprandial blood glucose, or pregnancy rates. Curcumin has significant anti-inflammatory effects, mainly by downregulating the NLRP3 inflammasome, and regulating NF-κB signaling and interleukin secretion are the most prominent functional mechanisms of curcumin in modulating the inflammasome (76), thus potentially focusing more on improving the inflammatory levels in PCOS patients. The study by Yongwatana et al. (77) suggests that curcumin may have a positive effect on gastrointestinal symptoms, but whether it significantly affects gastrointestinal adverse events in PCOS patients requires further research to confirm. Green tea can improve BMI in PCOS patients, and the health benefits of green tea are attributed to its natural antioxidants, namely catechins, especially epigallocatechin-3-gallate (EGCG), which has beneficial effects on health, including high antioxidant, bone protection, neuroprotection, anticancer, antihyperlipidemic, and antidiabetic effects (78). Regarding minerals, our results show no significant improvement in FBS, FG, FSI, HOMA-IR, or SHBG in PCOS patients. This is consistent with the conclusion by Pokorska-Niewiada et al. (79), suggesting that a more comprehensive treatment approach, including lifestyle changes, pharmacological treatment, and possibly nutritional supplements, rather than relying solely on mineral supplements, may be needed for PCOS patients. Our results indicate that vitamin D does not significantly affect HOMA-IR, TG, or total testosterone, which is contrary to the studies by Miao et al. (51) and Kiani et al. (68), and may be related to the dose and treatment duration of vitamin D (80). Vitamin E does not significantly affect BMI, with one study reporting a small but significant decrease in waist circumference (d = 0.3), but no significant change in weight (81). Probiotics significantly improved BMI, CRP, GSH, HOMA-IR, insulin, MDA, NO, SHBG, TAC, total testosterone, and vLDL-C in PCOS patients, which is consistent with the study by Angoorani et al. (82). We found that probiotics did not significantly improve hirsutism in PCOS patients, which is consistent with the study by Wu et al. (83).
We assessed the methodological quality of the included meta-analyses using the AMSTAR 2 checklist. Among the 46 studies, 21 (45.7%) were rated as high quality, 13 (28.3%) as medium quality, and 12 (26.1%) as low quality. The overall fair-to-good quality of the included studies reinforces the reliability of our umbrella review findings. The detailed item-by-item quality assessment results for each study are presented in Supplementary Table S1.
In this study, there was heterogeneity among different umbrella meta-analyses. The persistent heterogeneity, even after sensitivity analyses, underscores the influence of factors like dosing regimens and supplement formulations. Despite significant overall effects observed in previous umbrella meta-analyses, there are still some controversies. The differences in results can be attributed to different treatment doses and durations, different types of analyses, different umbrella meta-analysis qualities, and different sample sizes. For example, in the case of probiotics, except for the synbiotic pomegranate juice used in the study by (88), other studies used probiotic capsules, and the strains of probiotics in each study were not consistent. For instance, the probiotic capsule used in the study by (84, 89) included Lactobacillus casei, Lactobacillus acidophilus, Streptococcus thermophilus, Lactobacillus bulgaricus, Bifidobacterium longum, Bifidobacterium breve, and Streptococcus thermophilus (each strain at a concentration of 7 × 10^9 CFU/g), while the probiotic capsule used in the study by (85, 90) included Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactobacillus fermentum, and Bifidobacterium (each strain at a concentration of 2 × 10^9 CFU/g), plus 200 micrograms of selenium.
This study is the first comprehensive analysis of the impact of nutritional supplements on the treatment of PCOS. We adhered to the Preferred Reporting Items for Systematic Reviews and Umbrella meta-analyses (PRISMA) guidelines, enhancing the transparency and reproducibility of our research. We have clarified that inositol and probiotics have significant outcomes in the treatment of PCOS. Concurrently, bibliometric analysis indicates a shift toward the study of the antioxidant aspects of nutritional supplements, aligning with significant advancements in research on inositol and probiotics (84, 85). A study discusses the importance of personalized diets in the treatment of PCOS, emphasizing the relationship between healthy eating, physiological homeostasis regulation, and disease recovery (86). Relevant research suggests that individual genetic variations affect metabolic responses to diet, highlighting the importance of integrating nutrigenomics with nutritional supplementation in formulating personalized dietary guidelines (86). Szczuko et al. (63) emphasize that the clinical phenotypes of PCOS can change over the life cycle and can coexist within the same patient. While personalized treatment remains the primary approach, phenotypic grouping and adherence to treatment recommendations may also be clinically applicable. Precise recommendations should be implemented well before the onset of metabolic complications, which is particularly crucial for women with PCOS (63). Cowan et al. (87) suggest that self-management strategies support lifestyle changes in PCOS patients and increase interaction with qualified health professionals, thereby enhancing treatment outcomes. As demonstrated by our bibliometric research, the treatment of PCOS with nutritional supplements is increasingly trending toward personalization in the future (Figure 11).
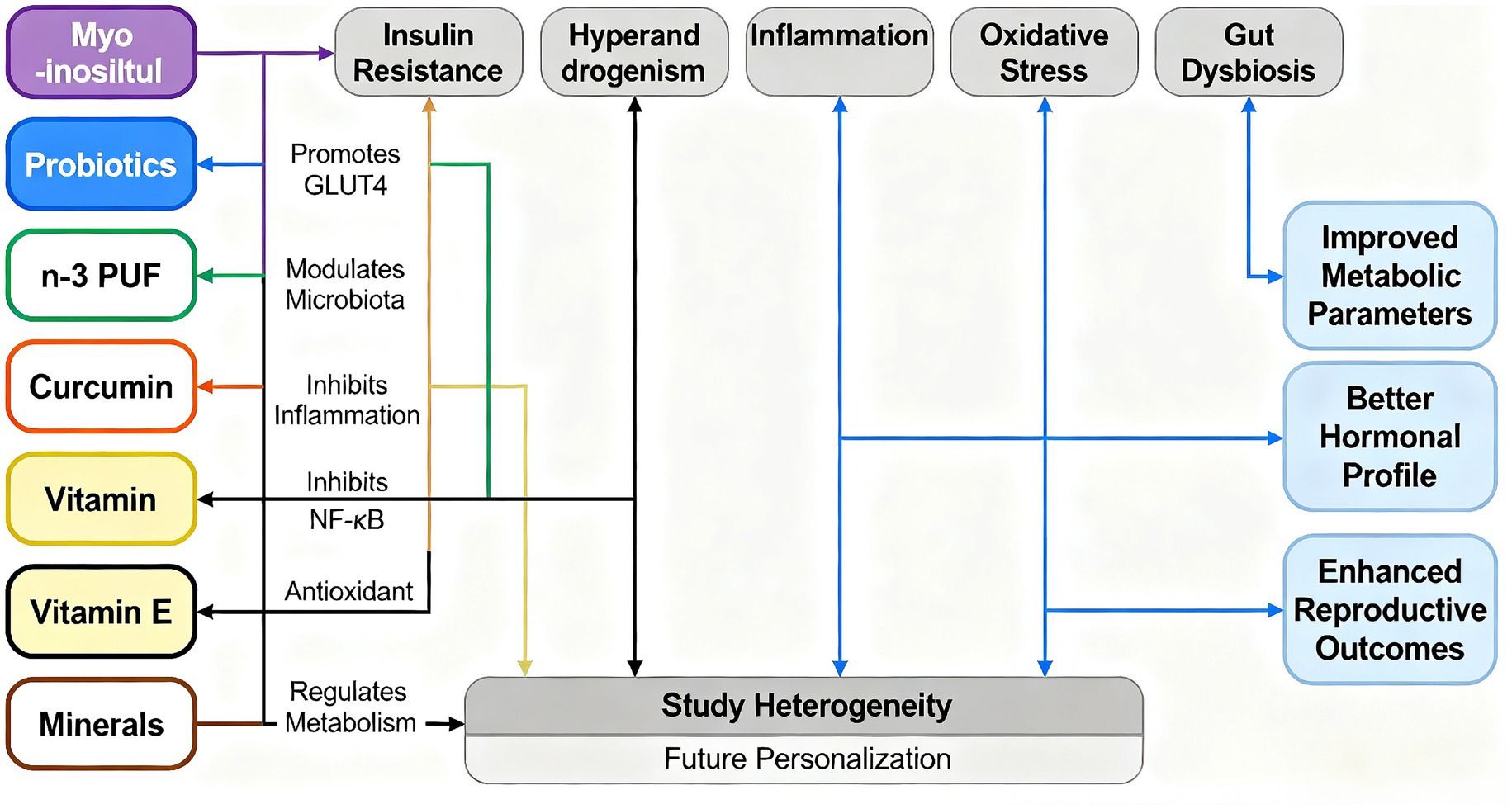
Figure 11. Schematic diagram illustrating the mechanism by which nutritional supplements improve metabolic and reproductive dysfunction. This figure summarizes the potential mechanisms by which various nutritional supplements (such as inositol, probiotics, n-3 polyunsaturated fatty acids, curcumin, vitamins and minerals) exert beneficial effects. These supplements target core pathological links such as insulin resistance, hyperandrogenemia, inflammation, oxidative stress and dysbiosis of the intestinal flora (e.g., polycystic ovary syndrome, PCOS). By regulating these key pathways, these interventions can work synergistically to regulate metabolism and ultimately achieve the effects of improving metabolic parameters, optimizing hormone levels and enhancing reproductive health outcomes. This figure also highlights the heterogeneity in current research and points to the direction of future personalized nutritional therapy.
5 Conclusion
Nutritional supplements show promise for improving metabolic and reproductive outcomes in PCOS, but heterogeneity highlights the need for personalized approaches and further validation. Future research should focus on personalized treatment regimens and the antioxidant properties of nutritional supplements to achieve optimal therapeutic outcomes for PCOS.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
RW: Conceptualization, Methodology, Project administration, Validation, Visualization, Writing – original draft. KH: Conceptualization, Formal analysis, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MW: Conceptualization, Data curation, Formal analysis, Software, Validation, Visualization, Writing – original draft. WZ: Conceptualization, Data curation, Funding acquisition, Project administration, Software, Validation, Visualization, Writing – original draft. YH: Conceptualization, Formal analysis, Methodology, Resources, Software, Validation, Visualization, Writing – original draft. WJ: Conceptualization, Data curation, Formal analysis, Methodology, Validation, Visualization, Writing – original draft. YF: Data curation, Formal analysis, Investigation, Software, Validation, Visualization, Writing – original draft. HS: Formal analysis, Funding acquisition, Project administration, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. XL: Conceptualization, Data curation, Investigation, Project administration, Software, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Hunan Province clinical innovation guidance project (No. 2021SK50402) and Hunan Province Innovation and Entrepreneurship Training Program for College Students (No. S202410555233).
Acknowledgments
Thanks are extended to Clinical Anatomy & Reproductive Medicine Application Institute, Hengyang Medical School, University of South China, for their assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1705284/full#supplementary-material
References
1. Skakkebæk, NE, Lindahl-Jacobsen, R, Levine, H, Andersson, AM, Jørgensen, N, Main, KM, et al. Environmental factors in declining human fertility. Nat Rev Endocrinol. (2022) 18:139–57. doi: 10.1038/s41574-021-00598-8
2. Khan, MJ, Ullah, A, and Basit, S. Genetic basis of polycystic ovary syndrome (Pcos): current perspectives. Appl Clin Genet. (2019) 12:249–60. doi: 10.2147/TACG.S200341
3. Deshpande, PS, and Gupta, AS. Causes and prevalence of factors causing infertility in a public health facility. J Hum Reprod Sci. (2019) 12:287–93. doi: 10.4103/jhrs.JHRS_140_18
4. Azziz, R, Carmina, E, Chen, Z, Dunaif, A, Laven, JSE, Legro, RS, et al. Polycystic ovary syndrome. Nat Rev Dis Primers. (2016) 2:16057. doi: 10.1038/nrdp.2016.57
5. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (Ncep) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA. (2001) 285:2486–97. doi: 10.1001/jama.285.19.2486
6. Reaven, GM. The metabolic syndrome: requiescat in pace. Clin Chem. (2005) 51:931–8. doi: 10.1373/clinchem.2005.048611
7. Lim, SS, Davies, MJ, Norman, RJ, and Moran, LJ. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and umbrella meta-analysis. Hum Reprod Update. (2012) 18:618–37. doi: 10.1093/humupd/dms030
8. Dobbie, LJ, Pittam, B, Zhao, SS, Alam, U, Hydes, TJ, Barber, TM, et al. Childhood, adolescent, and adulthood adiposity are associated with risk of Pcos: a mendelian randomization study with umbrella meta-analysis. Hum Reprod. (2023) 38:1168–82. doi: 10.1093/humrep/dead053
9. Koivuaho, E, Laru, J, Ojaniemi, M, Puukka, K, Kettunen, J, Tapanainen, JS, et al. Age at adiposity rebound in childhood is associated with Pcos diagnosis and obesity in adulthood-longitudinal analysis of Bmi data from birth to age 46 in cases of Pcos. Int J Obes. (2019) 43:1370–9. doi: 10.1038/s41366-019-0318-z
10. Barber, TM, Hanson, P, Weickert, MO, and Franks, S. Obesity and polycystic ovary syndrome: implications for pathogenesis and novel management strategies. Clin Med Insights Reprod Health. (2019) 13:1179558119874042. doi: 10.1177/1179558119874042
11. Cassar, S, Misso, ML, Hopkins, WG, Shaw, CS, Teede, HJ, and Stepto, NK. Insulin resistance in polycystic ovary syndrome: a systematic review and umbrella meta-analysis of euglycaemic-hyperinsulinaemic clamp studies. Hum Reprod. (2016) 31:2619–31. doi: 10.1093/humrep/dew243
12. Cowan, S, Lim, S, Alycia, C, Pirotta, S, Thomson, R, Gibson-Helm, M, et al. Lifestyle management in polycystic ovary syndrome – beyond diet and physical activity. BMC Endocr Disord. (2023) 23:14. doi: 10.1186/s12902-022-01208-y
13. Rondanelli, M, Perna, S, Faliva, M, Monteferrario, F, Repaci, E, and Allieri, F. Focus on metabolic and nutritional correlates of polycystic ovary syndrome and update on nutritional management of these critical phenomena. Arch Gynecol Obstet. (2014) 290:1079–92. doi: 10.1007/s00404-014-3433-z
14. Iervolino, M, Lepore, E, Forte, G, Laganà, AS, Buzzaccarini, G, and Unfer, V. Natural molecules in the Management of Polycystic Ovary Syndrome (Pcos): an analytical review. Nutrients. (2021) 13:1677. doi: 10.3390/nu13051677
15. Alesi, S, Ee, C, Moran, LJ, Rao, V, and Mousa, A. Nutritional supplements and complementary therapies in polycystic ovary syndrome. Adv Nutr. (2022) 13:1243–66. doi: 10.1093/advances/nmab141
16. Hu, X, Wang, W, Su, X, Peng, H, Tan, Z, Li, Y, et al. Comparison of nutritional supplements in improving glycolipid metabolism and endocrine function in polycystic ovary syndrome: a systematic review and network umbrella meta-analysis. PeerJ. (2023) 11:e16410. doi: 10.7717/peerj.16410
17. Menichini, D, Ughetti, C, Monari, F, di Vinci, PL, Neri, I, and Facchinetti, F. Nutraceuticals and polycystic ovary syndrome: a systematic review of the literature. Gynecol Endocrinol. (2022) 38:623–31. doi: 10.1080/09513590.2022.2089106
18. Günalan, E, Yaba, A, and Yılmaz, B. The effect of nutrient supplementation in the management of polycystic ovary syndrome-associated metabolic dysfunctions: a critical review. J Turk Ger Gynecol Assoc. (2018) 19:220–32. doi: 10.4274/jtgga.2018.0077
19. Escobar-Morreale, HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. (2018) 14:270–84. doi: 10.1038/nrendo.2018.24
20. Shamseer, L, Moher, D, Clarke, M, Ghersi, D, Liberati, A, Petticrew, M, et al. Preferred reporting items for systematic review and umbrella meta-analysis protocols (Prisma-P) 2015: elaboration and explanation. BMJ. (2015) 350:g7647. doi: 10.1136/bmj.g7647
21. Teede, HJ, Tay, CT, Laven, JJE, Dokras, A, Moran, LJ, Piltonen, TT, et al. Recommendations from the 2023 international evidence-based guideline for the assessment and Management of Polycystic Ovary Syndrome. J Clin Endocrinol Metab. (2023) 108:2447–69. doi: 10.1210/clinem/dgad463
22. Tosatti, JAG, Alves, MT, Cândido, AL, Reis, FM, Araújo, VE, and Gomes, KB. Influence of n-3 fatty acid supplementation on inflammatory and oxidative stress markers in patients with polycystic ovary syndrome: a systematic review and umbrella meta-analysis. Br J Nutr. (2021) 125:657–68. doi: 10.1017/S0007114520003207
23. Hajishafiee, M, Askari, G, Iranj, B, Ghiasvand, R, Bellissimo, N, de Zepetnek, JT, et al. The effect of n-3 polyunsaturated fatty acid supplementation on androgen status in patients with polycystic ovary syndrome: a systematic review and umbrella meta-analysis of clinical trials. Horm Metab Res. (2016) 48:281–9. doi: 10.1055/s-0042-105288
24. Xia, Y, Wang, Y, Cui, M, and Su, D. Efficacy of omega-3 fatty acid supplementation on cardiovascular risk factors in patients with polycystic ovary syndrome: a systematic review and umbrella meta-analysis. Ann Palliat Med. (2021) 10:6425–37. doi: 10.21037/apm-21-1050
25. Arentz, S, Smith, CA, Abbott, J, and Bensoussan, A. Nutritional supplements and herbal medicines for women with polycystic ovary syndrome; a systematic review and umbrella meta-analysis. BMC Complement Altern Med. (2017) 17:500. doi: 10.1186/s12906-017-2011-x
26. Sadeghi, A, Djafarian, K, Mohammadi, H, and Shab-Bidar, S. Effect of omega-3 fatty acids supplementation on insulin resistance in women with polycystic ovary syndrome: umbrella meta-analysis of randomized controlled trials. Diabetes Metab Syndr. (2017) 11:157–62. doi: 10.1016/j.dsx.2016.06.025
27. Yuan, J, Wen, X, and Jia, M. Efficacy of omega-3 polyunsaturated fatty acids on hormones, oxidative stress, and inflammatory parameters among polycystic ovary syndrome: a systematic review and umbrella meta-analysis. Ann Palliat Med. (2021) 10:8991–9001. doi: 10.21037/apm-21-2018
28. Yang, K, Zeng, L, Bao, T, and Ge, J. Effectiveness of omega-3 fatty acid for polycystic ovary syndrome: a systematic review and umbrella meta-analysis. Reprod Biol Endocrinol. (2018) 16:27. doi: 10.1186/s12958-018-0346-x
29. Zhou, J, Zuo, W, Tan, Y, Wang, X, Zhu, M, and Zhang, H. Effects of n-3 polyunsaturated fatty acid on metabolic status in women with polycystic ovary syndrome: an umbrella meta-analysis of randomized controlled trials. J Ovarian Res. (2023) 16:54. doi: 10.1186/s13048-023-01130-4
30. Zeng, L, and Yang, K. Effectiveness of myoinositol for polycystic ovary syndrome: a systematic review and umbrella meta-analysis. Endocrine. (2018) 59:30–8. doi: 10.1007/s12020-017-1442-y
31. Pundir, J, Psaroudakis, D, Savnur, P, Bhide, P, Sabatini, L, Teede, H, et al. Inositol treatment of anovulation in women with polycystic ovary syndrome: an umbrella meta-analysis of randomised trials. BJOG. (2018) 125:299–308. doi: 10.1111/1471-0528.14754
32. Unfer, V, Facchinetti, F, Orrù, B, Giordani, B, and Nestler, J. Myo-inositol effects in women with Pcos: an umbrella meta-analysis of randomized controlled trials. Endocr Connect. (2017) 6:647–58. doi: 10.1530/EC-17-0243
33. Greff, D, Juhász, AE, Váncsa, S, Váradi, A, Sipos, Z, Szinte, J, et al. Inositol is an effective and safe treatment in polycystic ovary syndrome: a systematic review and umbrella meta-analysis of randomized controlled trials. Reprod Biol Endocrinol. (2023) 21:10. doi: 10.1186/s12958-023-01055-z
34. Shen, W, Qu, Y, Jiang, H, Wang, H, Pan, Y, Zhang, Y, et al. Therapeutic effect and safety of curcumin in women with Pcos: a systematic review and umbrella meta-analysis. Front Endocrinol. (2022) 13:1051111. doi: 10.3389/fendo.2022.1051111
35. Simental-Mendía, LE, Shah, N, Sathyapalan, T, Majeed, M, Orekhov, AN, Jamialahmadi, T, et al. Effect of curcumin on glycaemic and lipid parameters in polycystic ovary syndrome: a systematic review and umbrella meta-analysis of randomized controlled trials. Reprod Sci. (2022) 29:3124–33. doi: 10.1007/s43032-021-00761-6
36. Abdelazeem, B, Abbas, KS, Shehata, J, Baral, N, Banour, S, and Hassan, M. The effects of curcumin as dietary supplement for patients with polycystic ovary syndrome: an updated systematic review and umbrella meta-analysis of randomized clinical trials. Phytother Res. (2022) 36:22–32. doi: 10.1002/ptr.7274
37. Chien, YJ, Chang, CY, Wu, MY, Chen, C-H, Horng, Y-S, and Wu, H-C. Effects of curcumin on glycemic control and lipid profile in polycystic ovary syndrome: systematic review with umbrella meta-analysis and trial sequential analysis. Nutrients. (2021) 13:684. doi: 10.3390/nu13020684
38. Xie, L, Zhang, D, Ma, H, He, H, Xia, Q, Shen, W, et al. The effect of berberine on reproduction and metabolism in women with polycystic ovary syndrome: a systematic review and umbrella meta-analysis of randomized control trials. Evid Based Complement Alternat Med. (2019) 2019:7918631. doi: 10.1155/2019/7918631
39. Almeida Balieiro, CC, Hespanhol, LC, Mendes Fonseca, L, Wantowski, S, Freitas, MAA, Dias, YJM, et al. Effects of polyphenol in women with polycystic ovary syndrome: a systematic review and umbrella meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. (2024) 294:84–91. doi: 10.1016/j.ejogrb.2023.12.038
40. Wu, PY, Tan, X, Wang, M, Zheng, X, and Lou, J-H. Selenium supplementation for polycystic ovary syndrome: an umbrella meta-analysis of randomized controlled trials. Gynecol Endocrinol. (2022) 38:928–34. doi: 10.1080/09513590.2022.2118709
41. Heshmati, J, Omani-Samani, R, Vesali, S, Maroufizadeh, S, Rezaeinejad, M, Razavi, M, et al. The effects of supplementation with chromium on insulin resistance indices in women with polycystic ovarian syndrome: a systematic review and umbrella meta-analysis of randomized clinical trials. Horm Metab Res. (2018) 50:193–200. doi: 10.1055/s-0044-101835
42. Fazelian, S, Rouhani, MH, Bank, SS, and Amani, R. Chromium supplementation and polycystic ovary syndrome: a systematic review and umbrella meta-analysis. J Trace Elem Med Biol. (2017) 42:92–6. doi: 10.1016/j.jtemb.2017.04.008
43. Gao, H, Li, Y, Yan, W, and Gao, F. The effect of vitamin D supplementation on blood lipids in patients with polycystic ovary syndrome: an umbrella meta-analysis of randomized controlled trials. Int J Endocrinol. (2021) 2021:8849688. doi: 10.1155/2021/8849688
44. Jin, B, Qian, L, Fu, X, Zhu, J, and Shu, J. Influence of vitamin D supplementation on lipid levels in polycystic ovary syndrome patients: an umbrella meta-analysis of randomized controlled trials. J Int Med Res. (2020) 48:300060520935313. doi: 10.1177/0300060520935313
45. Han, Y, Cao, Q, Qiao, X, and Huang, W. Effect of vitamin D supplementation on hormones and menstrual cycle regularization in polycystic ovary syndrome women: a systemic review and umbrella meta-analysis. J Obstet Gynaecol Res. (2023) 49:2232–44. doi: 10.1111/jog.15727
46. Akbari, M, Ostadmohammadi, V, Lankarani, KB, Tabrizi, R, Kolahdooz, F, Heydari, ST, et al. The effects of vitamin D supplementation on biomarkers of inflammation and oxidative stress among women with polycystic ovary syndrome: a systematic review and umbrella meta-analysis of randomized controlled trials. Horm Metab Res. (2018) 50:271–9. doi: 10.1055/s-0044-101355
47. Fang, F, Ni, K, Cai, Y, Shang, J, Zhang, X, and Xiong, C. Effect of vitamin D supplementation on polycystic ovary syndrome: a systematic review and umbrella meta-analysis of randomized controlled trials. Complement Ther Clin Pract. (2017) 26:53–60. doi: 10.1016/j.ctcp.2016.11.008
48. Zhang, B, Yao, X, Zhong, X, Hu, Y, and Xu, J. Vitamin D supplementation in the treatment of polycystic ovary syndrome: an umbrella meta-analysis of randomized controlled trials. Heliyon. (2023) 9:e14291. doi: 10.1016/j.heliyon.2023.e14291
49. Pergialiotis, V, Karampetsou, N, Panagopoulos, P, Trakakis, E, and Papantoniou, N. The effect of vitamin D supplementation on hormonal and glycaemic profile of patients with Pcos: an umbrella meta-analysis of randomised trials. Int J Clin Pract. (2017) 71. doi: 10.1111/ijcp.12957
50. Luo, J, Li, T, and Yuan, J. Effectiveness of vitamin D supplementation on lipid profile in polycystic ovary syndrome women: an umbrella meta-analysis of randomized controlled trials. Ann Palliat Med. (2021) 10:114–29. doi: 10.21037/apm-20-2492
51. Miao, CY, Fang, XJ, Chen, Y, and Zhang, Q. Effect of vitamin D supplementation on polycystic ovary syndrome: an umbrella meta-analysis. Exp Ther Med. (2020) 19:2641–9. doi: 10.3892/etm.2020.8525
52. Xue, Y, Xu, P, Xue, K, Duan, X, Cao, J, Luan, T, et al. Effect of vitamin D on biochemical parameters in polycystic ovary syndrome women: an umbrella meta-analysis. Arch Gynecol Obstet. (2017) 295:487–96. doi: 10.1007/s00404-016-4247-y
53. Heidari, H, Hajhashemy, Z, and Saneei, P. An umbrella meta-analysis of effects of vitamin E supplementation alone and in combination with omega-3 or magnesium on polycystic ovary syndrome. Sci Rep. (2022) 12:19927. doi: 10.1038/s41598-022-24467-0
54. Yalle-Vásquez, S, Osco-Rosales, K, Nieto-Gutierrez, W, Benites-Zapata, V, Pérez-López, FR, and Alarcon-Ruiz, CA. Vitamin E supplementation improves testosterone, glucose- and lipid-related metabolism in women with polycystic ovary syndrome: an umbrella meta-analysis of randomized clinical trials. Gynecol Endocrinol. (2022) 38:548–57. doi: 10.1080/09513590.2022.2079629
55. Tabrizi, R, Ostadmohammadi, V, Akbari, M, Lankarani, KB, Vakili, S, Peymani, P, et al. The effects of probiotic supplementation on clinical symptom, weight loss, glycemic control, lipid and hormonal profiles, biomarkers of inflammation, and oxidative stress in women with polycystic ovary syndrome: a systematic review and umbrella meta-analysis of randomized controlled trials. Probiotics Antimicrob Proteins. (2022) 14:1–14. doi: 10.1007/s12602-019-09559-0
56. Li, Y, Tan, Y, Xia, G, and Shuai, J. Effects of probiotics, prebiotics, and synbiotics on polycystic ovary syndrome: a systematic review and umbrella meta-analysis. Crit Rev Food Sci Nutr. (2023) 63:522–38. doi: 10.1080/10408398.2021.1951155
57. Shamasbi, SG, Ghanbari-Homayi, S, and Mirghafourvand, M. The effect of probiotics, prebiotics, and synbiotics on hormonal and inflammatory indices in women with polycystic ovary syndrome: a systematic review and umbrella meta-analysis. Eur J Nutr. (2020) 59:433–50. doi: 10.1007/s00394-019-02033-1
58. Miao, C, Guo, Q, Fang, X, Chen, Y, Zhao, Y, and Zhang, Q. Effects of probiotic and synbiotic supplementation on insulin resistance in women with polycystic ovary syndrome: an umbrella meta-analysis. J Int Med Res. (2021) 49:3000605211031758. doi: 10.1177/03000605211031758
59. Ali Fadlalmola, H, Elhusein, AM, Al-Sayaghi, KM, Albadrani, MS, Swamy, DV, Mamanao, DM, et al. Efficacy of resveratrol in women with polycystic ovary syndrome: a systematic review and umbrella meta-analysis of randomized clinical trials. Pan Afr Med J. (2023) 44:134. doi: 10.11604/pamj.2023.44.134.32404
60. Ziaei, S, Hasani, M, Malekahmadi, M, Daneshzad, E, Kadkhodazadeh, K, and Heshmati, J. Effect of melatonin supplementation on cardiometabolic risk factors, oxidative stress and hormonal profile in Pcos patients: a systematic review and umbrella meta-analysis of randomized clinical trials. J Ovarian Res. (2024) 17:138. doi: 10.1186/s13048-024-01450-z
61. Gong, Y, Jiang, T, He, H, Wang, Y, Wu, G-L, Shi, Y, et al. Effects of carnitine on glucose and lipid metabolic profiles and fertility outcomes in women with polycystic ovary syndrome: a systematic review and umbrella meta-analysis. Clin Endocrinol. (2023) 98:682–91. doi: 10.1111/cen.14885
62. Mohd Shukri, MF, Norhayati, MN, Badrin, S, and Abdul Kadir, A. Effects of L-carnitine supplementation for women with polycystic ovary syndrome: a systematic review and umbrella meta-analysis. PeerJ. (2022) 10:e13992. doi: 10.7717/peerj.13992
63. Szczuko, M, Kikut, J, Szczuko, U, Szydłowska, I, Nawrocka-Rutkowska, J, Ziętek, M, et al. Nutrition strategy and life style in polycystic ovary syndrome-narrative review. Nutrients. (2021) 13:2452. doi: 10.3390/nu13072452
64. Di Lorenzo, M, Cacciapuoti, N, Lonardo, MS, Nasti, G, Gautiero, C, Belfiore, A, et al. Pathophysiology and nutritional approaches in polycystic ovary syndrome (Pcos): a comprehensive review. Curr Nutr Rep. (2023) 12:527–44. doi: 10.1007/s13668-023-00479-8
65. He, H, Chen, X, Ding, Y, Chen, X, and He, X. Composite dietary antioxidant index associated with delayed biological aging: a population-based study. Aging. (2024) 16:15–27. doi: 10.18632/aging.205232
66. Lourenço, SC, Moldão-Martins, M, and Alves, VD. Antioxidants of natural plant origins: from sources to food industry applications. Molecules. (2019) 24:4132. doi: 10.3390/molecules24224132
67. Baroni, L, Sarni, AR, and Zuliani, C. Plant foods rich in antioxidants and human cognition: a systematic review. Antioxidants. (2021) 10:714. doi: 10.3390/antiox10050714
68. Kiani, AK, Donato, K, Dhuli, K, Stuppia, L, and Bertelli, M. Dietary supplements for polycystic ovary syndrome. J Prev Med Hyg. (2022) 63:E206–13. doi: 10.15167/2421-4248/jpmh2022.63.2S3.2762
69. Faghfoori, Z, Fazelian, S, Shadnoush, M, and Goodarzi, R. Nutritional management in women with polycystic ovary syndrome: a review study. Diabetes Metab Syndr. (2017) 11:S429–32. doi: 10.1016/j.dsx.2017.03.030
70. Salek, M, Clark, CCT, Taghizadeh, M, and Jafarnejad, S. N-3 fatty acids as preventive and therapeutic agents in attenuating Pcos complications. EXCLI J. (2019) 18:558–75. doi: 10.17179/excli2019-1534
71. Albardan, L, Platat, C, and Kalupahana, NS. Role of omega-3 fatty acids in improving metabolic dysfunctions in polycystic ovary syndrome. Nutrients. (2024) 16:2961. doi: 10.3390/nu16172961
72. Wynne-Ellis, MM, Mursu, JJ, Tuomainen, TP, Bertone-Johnson, E, Salonen, JT, Virtanen, JK, et al. Dietary fat quality nd serum androgen concentrations in middle-aged men. Eur J Clin Nutr. (2024) 78:99–106. doi: 10.1038/s41430-023-01358-9
73. Gudović, A, Bukumirić, Z, Milincic, M, Pupovac, M, Andjić, M, Ivanovic, K, et al. The comparative effects of Myo-inositol and metformin therapy on the clinical and biochemical parameters of women of Normal weight suffering from polycystic ovary syndrome. Biomedicine. (2024) 12:349. doi: 10.3390/biomedicines12020349
74. Wang, T, Wang, J, Hu, X, Huang, X, and Chen, GX. Current understanding of glucose transporter 4 expression and functional mechanisms. World J Biol Chem. (2020) 11:76–98. doi: 10.4331/wjbc.v11.i3.76
75. Gambioli, R, Forte, G, Buzzaccarini, G, Unfer, V, and Laganà, AS. Myo-inositol as a key supporter of fertility and physiological gestation. Pharmaceuticals. (2021) 14:504. doi: 10.3390/ph14060504
76. Hasanzadeh, S, Read, MI, Bland, AR, Majeed, M, Jamialahmadi, T, and Sahebkar, A. Curcumin: an inflammasome silencer. Pharmacol Res. (2020) 159:104921. doi: 10.1016/j.phrs.2020.104921
77. Yongwatana, K, Harinwan, K, Chirapongsathorn, S, Opuchar, K, Sanpajit, T, Piyanirun, W, et al. Curcuma longa Linn versus omeprazole in treatment of functional dyspepsia: a randomized, double-blind, placebo-controlled trial. J Gastroenterol Hepatol. (2022) 37:335–41. doi: 10.1111/jgh.15705
78. Kamal, DAM, Salamt, N, Zaid, SSM, and Mokhtar, MH. Beneficial effects of green tea catechins on female reproductive disorders: a review. Molecules. (2021) 26:2675. doi: 10.3390/molecules26092675
79. Pokorska-Niewiada, K, Brodowska, A, and Szczuko, M. The content of minerals in the PCOS group and the correlation with the parameters of metabolism. Nutrients. (2021) 13:2214. doi: 10.3390/nu13072214
80. Bouillon, R, Manousaki, D, Rosen, C, Trajanoska, K, Rivadeneira, F, and Richards, JB. The health effects of vitamin D supplementation: evidence from human studies. Nat Rev Endocrinol. (2022) 18:96–110. doi: 10.1038/s41574-021-00593-z
81. Tefagh, G, Payab, M, Qorbani, M, Sharifi, F, Sharifi, Y, Shirvani, MSE, et al. Effect of vitamin E supplementation on cardiometabolic risk factors, inflammatory and oxidative markers and hormonal functions in Pcos (polycystic ovary syndrome): a systematic review and umbrella meta-analysis. Sci Rep. (2022) 12:5770. doi: 10.1038/s41598-022-09082-3
82. Angoorani, P, Ejtahed, HS, Ettehad Marvasti, F, Taghavi, MS, Mohammadpour Ahranjani, B, Hasani-Ranjbar, S, et al. The effects of probiotics, prebiotics, and synbiotics on polycystic ovarian syndrome: an overview of systematic reviews. Front Med. (2023) 10:1141355. doi: 10.3389/fmed.2023.1141355
83. Wu, LY, Yang, TH, Ou, YC, and Lin, H. The role of probiotics in women’s health: an update narrative review. Taiwan J Obstet Gynecol. (2024) 63:29–36. doi: 10.1016/j.tjog.2023.09.018
84. López-Gambero, AJ, Sanjuan, C, Serrano-Castro, PJ, Suárez, J, and de Fonseca, FR. The biomedical uses of Inositols: a nutraceutical approach to metabolic dysfunction in aging and neurodegenerative diseases. Biomedicine. (2020) 8:295. doi: 10.3390/biomedicines8090295
85. Wang, Y, Wu, Y, Wang, Y, Xu, H, Mei, X, Yu, D, et al. Antioxidant properties of probiotic Bacteria. Nutrients. (2017) 9:5. doi: 10.3390/nu9050521
86. Singh, S, Pal, N, Shubham, S, Sarma, DK, Verma, V, Marotta, F, et al. Polycystic ovary syndrome: etiology, current management, and future therapeutics. J Clin Med. (2023) 12:1454. doi: 10.3390/jcm12041454
87. Cowan, S, Grassi, A, Monahan Couch, L, Jeanes, Y, Lim, S, Pirotta, S, et al. Evidence-based lifestyle guidelines and self-management strategies utilized by women with polycystic ovary syndrome. Nutrients. (2023) 15:589. doi: 10.3390/nu15030589
88. Esmaeilinezhad, Z, Babajafari, S, Sohrabi, Z, Eskandari, M. H, Amooee, S, and Barati-Boldaji, R. Effect of synbiotic pomegranate juice on glycemic, sex hormone profile and anthropometric indices in PCOS: A randomized, triple blind, controlled trial. Nutrition, metabolism, and cardiovascular diseases: NMCD. (2019) 29:201–208. doi: 10.1016/j.numecd.2018.07.002
89. Shoaei, T, Heidari-Beni, M, Tehrani, HG, Feizi, A, Esmaillzadeh, A, and Askari, G. Effects of probiotic supplementation on pancreatic β-cell function and c-reactive protein in women with polycystic ovary syndrome: a randomized double-blind placebo-controlled clinical trial. Int J Prev Med. (2015) 6:27. doi: 10.4103/2008-7802.15386
90. Jamilian, M, Mansury, S, Bahmani, F, Heidar, Z, Amirani, E, and Asemi, Z. The effects of probiotic and selenium co-supplementation on parameters of mental health, hormonal profiles, and biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome. J Ovarian Res. (2018) 11:80. doi: 10.1186/s13048-018-0457-1
Glossary
AMSTAR - A MeaSurement Tool to Assess systematic Review
BMI - Body Mass Index
CI - Confidence Interval
CRP - C-reactive Protein
DHA - Docosahexaenoic Acid
EPA - Eicosapentaenoic Acid
FBG - Fasting Blood Glucose
FG - Fasting Glucose
FI - Fasting Insulin
FSH - Follicle-Stimulating Hormone
GSH - Glutathione
HDL-C - High-Density Lipoprotein Cholesterol
HOMA-IR - Homeostatic Model Assessment of Insulin Resistance
hs-CRP - high-sensitivity C-reactive Protein
LDL-C - Low-Density Lipoprotein Cholesterol
LH - Luteinizing Hormone
MDA - Malondialdehyde
MD - Mean Difference
NO - Nitric Oxide
OR - Odds Ratio
PCOS - Polycystic Ovary Syndrome
PRISMA - Preferred Reporting Items for Systematic Reviews and Meta-Analyses
QUICKI - Quantitative Insulin Sensitivity Check Index
RCT - Randomized Controlled Trial
RR - Risk Ratio
SHBG - Sex Hormone-Binding Globulin
SMD - Standardized Mean Difference
TAC - Total Antioxidant Capacity
TC - Total Cholesterol
TG - Triglycerides
vLDL-C - very Low-Density Lipoprotein Cholesterol
WHR - Waist-to-Hip Ratio
Keywords: dietary supplements, polycystic ovary syndrome, umbrella meta-analysis, bibliometrics, future trend
Citation: Wang R, Huang K, Wang M, Zou W, Huang Y, Jiang W, Feng Y, Shen H and Lei X (2025) Efficacy of dietary supplements as an adjunctive therapy for polycystic ovary syndrome: an umbrella meta-analysis. Front. Nutr. 12:1705284. doi: 10.3389/fnut.2025.1705284
Edited by:
Ivana Šarac, University of Belgrade, SerbiaReviewed by:
Akingbolabo Daniel Ogunlakin, Bowen University, NigeriaSukrasno Sukrasno, Bandung Institute of Technology, Indonesia
Copyright © 2025 Wang, Huang, Wang, Zou, Huang, Jiang, Feng, Shen and Lei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaocan Lei, MjAxOTAwMDAxM0B1c2MuZWR1LmNu; Huilong Shen, MTM1NzUxMjg5MjNAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Rutong Wang
Rutong Wang Kongwei Huang
Kongwei Huang Mengyao Wang1†
Mengyao Wang1† Xiaocan Lei
Xiaocan Lei