- 1Department of Pharmacy, The Second Affiliated Hospital of Dalian Medical University, Dalian, China
- 2Office of the Ethics Committee, The Second Affiliated Hospital of Dalian Medical University, Dalian, China
Background: Malnutrition is a prevalent complication in older adults with gastric cancer and significantly impacts postoperative outcomes following curative gastrectomy. This study aimed to investigate the clinical value of nutrition support team (NST) in the perioperative management of gastric cancer older adults with concomitant malnutrition.
Methods: This retrospective cohort study included patients aged ≥65 years who underwent curative gastrectomy and met the Global Leadership Initiative on Malnutrition (GLIM) criteria for malnutrition between 2021 and 2024. Outcomes were compared between the NST group and conventional nutritional management group to analyze differences in nutritional support efficacy and clinical outcomes.
Results: NST group showed lower mortality at day 1 and 30 (0.0 vs. 0.4%, 0.8 vs. 4.6%, P < 0.05) and higher compliance for energy (71.4 vs. 10.0%, P < 0.001) and protein intake (56.0 vs. 10.8%, P < 0.001) compared to traditional nutrition (TN) group. Prognostic nutritional index (PNI) [46.60 (43.45, 50.05), P < 0.0001; 47.30 (43.45, 50.05), P < 0.0001] and prealbumin [136.8 (123.2, 172.0), P < 0.0001; 157.0 (128.2, 183.4), P < 0.0001] were significantly higher in NST group at day 7 and discharge. NST reduced the incidence of anastomotic leakage (1.7 vs. 5.0%, P < 0.05) and infection rates (4.5 vs. 10.4%, P < 0.05), weight loss at day 7 and before discharge [2.12 ± 0.10% (95% CI: 1.95, 2.28) vs. 6.63 ± 0.20% (95% CI: 6.23, 7.03), P < 0.001; 1.92 ± 0.07% (1.78, 2.07) vs. 6.53 ± 0.20% (6.13, 6.93), P < 0.001]. NST group had a shorter length of stay [15.00 (14.00, 17.00), P < 0.05], postoperative stay [12.00 (9.00, 14.00), P < 0.05], and lower readmission rates (10.8 vs. 17.8%, P < 0.05). NST significantly reduced the time to drain removal after surgery [9.00 (8.00, 11.00), P < 0.001], time to first flatus [3.00 (3.00, 3.00), P < 0.001] and bowel movement [4.00 (4.00, 4.00), P < 0.001] were shorter in NST group.
Conclusion: Our results demonstrated that NST intervention was associated with superior postoperative survival outcomes in malnourished older adults with gastric cancer. These findings supported that NST may serve as a valuable component of routine perioperative care for this vulnerable population.
1 Introduction
Gastric cancer represents one of the most prevalent malignancies globally, with GLOBOCAN 2024 data ranking it fifth in both incidence and mortality (1). The disease demonstrates marked age-dependent distribution, with epidemiological projections indicating that patients aged ≥65 years will comprise 68.6% of all gastric cancer cases by 2040, representing a substantial increase from 58.9% in 2020 (2). Tumor-associated catabolism, reduced oral intake, and gastrointestinal dysmotility significantly increase malnutrition risk in gastric cancer patients compared to other solid malignancies, particularly among the older adults (3). Malnutrition manifests as involuntary weight loss, decreased serum prealbumin levels, and lymphocytopenia, among other features, which collectively compromise immune function and reduce tolerance to surgical intervention (4).
Despite significant technical advances in radical gastrectomy (5, 6), this procedure remains highly invasive and induces a profound hypercatabolic state accompanied by immunosuppression, thereby predisposing patients to malnutrition of varying severity. Based on the Global Leadership Initiative on Malnutrition (GLIM) criteria, the perioperative prevalence of malnutrition among older adults with gastric cancer approaches 70% (7). The pathophysiology of perioperative malnutrition follows a distinct temporal pattern: pre-existing nutritional deficits are exacerbated by intraoperative metabolic derangements and further compounded by postoperative hypercatabolism, a cascade that is particularly severe in geriatric populations (8). Substantial evidence demonstrates that malnutrition is associated with significantly increased rates of postoperative infectious complications, prolonged hospital length of stay, and reduced long-term survival in this vulnerable cohort (9–11). Therefore, implementing comprehensive perioperative nutritional interventions to optimize recovery outcomes in older adults with gastric cancer represents a critical and unmet clinical need.
In response to this challenge, Nutrition Support Team (NST), multidisciplinary collaborative units based on evidence-based medicine, had emerged as critical components of modern healthcare systems for optimizing patient nutritional status (12, 13). By integrating specialized expertise from physicians, dietitians, pharmacists, and nurses, NSTs deliver individualized, precision-based nutritional interventions for malnourished patients. Strong evidence demonstrates that NST intervention significantly improves nutritional outcomes in hospitalized patients and reduces nutrition-related complications (14, 15). Following NST implementation, Kennedy et al. observed a substantial reduction in catheter-associated infection rates from 71 to 29%, along with a decrease in hospital length of stay by 2–4 days (16). A systematic review incorporating an economic model of 27 randomized controlled trials demonstrated that nutritional support yields average cost savings of $2,818 per patient (17).
However, evidence regarding perioperative NST implementation in malnourished older adults with gastric cancer remains limited, with both mechanistic insights and clinical benefits requiring further investigation. This study assessed the impact of NST-guided intervention on perioperative nutritional status, complications, and clinical outcomes in this population. By establishing optimal perioperative nutrition support strategies and promoting multidisciplinary collaboration in oncologic nutrition, this investigation aimed to provide robust evidence for improving prognosis and quality of life in this vulnerable patient cohort.
2 Method
2.1 Study design and participants
This retrospective cohort study was approved by the Ethics Committee of the Second Hospital of Dalian Medical University (approval number: KY2025-697-01, approved on September 1, 2025). We screened consecutive patients who underwent primary radical gastrectomy for gastric cancer at the Department of Gastrointestinal Surgery, Second Affiliated Hospital of Dalian Medical University, between January 2021 and September 2024.
2.1.1 Participant selection and nutritional assessment
Selection of 1,086 patients with gastric cancer followed a 2-step approach based on established nutritional assessment frameworks (18). First, nutritional risk screening was conducted using the Mini Nutritional Assessment-Short Form (MNA-SF), a validated tool comprising six items [food intake decline, weight loss, mobility, psychological stress/acute disease, neuropsychological problems, and body mass index (BMI)] with a total score ranging from 0 to 14 points (19). Patients with MNA-SF scores ≤ 11 were identified as being at nutritional risk and proceeded to comprehensive malnutrition diagnosis (20).
Second, patients identified with nutritional risk underwent comprehensive malnutrition diagnosis using the GLIM criteria (21). The GLIM framework requires the presence of at least one phenotypic criterion and one etiologic criterion for malnutrition diagnosis. The phenotypic criteria assessed were: (1) non-volitional weight loss (>5% within the past 6 months or >10% beyond 6 months); (2) low BMI (< 20 kg/m2 for patients < 70 years or < 22 kg/m2 for patients ≥70 years); and (3) reduced muscle mass. The etiologic criteria included: (1) reduced food intake or assimilation ( ≤ 50% of energy requirements for >1 week, or any reduction for >2 weeks, or chronic gastrointestinal conditions affecting nutrient absorption); and (2) inflammation/disease burden (presence of acute disease/injury or chronic disease-related inflammation).
Enteral nutrition (EN) via feeding tube was prioritized when gastrointestinal function was adequate (presence of bowel sounds, absence of abdominal distention, and tolerance to trial feeding). Transition from EN to supplemental parenteral nutrition (SPN) occurred when: (1) EN alone could not meet 50% of calculated energy requirements for 7 days, or (2) gastrointestinal intolerance developed (severe nausea, vomiting, diarrhea, or abdominal distention). Transition from SPN to total parenteral nutrition (TPN) was indicated when: (1) EN was contraindicated (intestinal obstruction, severe ileus, or anastomotic leakage), or (2) combined EN and PN could not achieve nutritional targets. Transition from parenteral nutrition (PN) to EN was attempted when gastrointestinal function recovered, with progressive advancement of enteral feeding as tolerated.
2.1.2 Inclusion and exclusion criteria
Subsequently, 512 patients with nutritional risk and malnutrition were enrolled, after applying inclusion and exclusion criteria. Inclusion criteria were: (1) age ≥65 years; (2) MNA-SF score ≤ 11; (3) confirmed malnutrition diagnosis based on GLIM criteria. Exclusion criteria were: (1) BMI ≥28 kg/m2 (to exclude patients with obesity, where nutritional management strategies differ substantially); (2) concurrent malignancies; (3) incomplete medical records. Traditional nutrition (TN) group (n = 241) and NST (n = 241) were followed by the propensity score matching (PSM). PSM was employed to mitigate potential biases when comparing the TN and NST groups.
2.1.3 Grouping and interventions
The NST was established in January 2021, comprising a multidisciplinary team of healthcare professionals: a gastrointestinal surgeon, a registered dietitian, a clinical pharmacist, and a specialized nurse (Figure 1). Based on the nutritional intervention strategy employed, eligible patients were stratified into two groups. The TN group received conventional nutritional support, which consisted of routine dietary counseling and empirical nutritional supplementation prescribed by the attending physician in accordance with standard institutional protocols. In contrast, the NST group received comprehensive multidisciplinary nutritional care, including individualized nutritional assessment and intervention plans, along with regular follow-up throughout the perioperative period. Assignment to NST services was determined through a shared decision-making process involving the attending surgeon and the patient or their family members, with consideration given to the patient's willingness to participate in structured nutritional management and the availability of NST resources at the time of hospitalization.

Figure 1. NST teamwork mode. NST teamwork mode for hospitalized older adults with gastric cancer: Nutritional risk is screened via Mini Nutritional Assessment-Short Form (MNA-SF)/Global Leadership Initiative on Malnutrition (GLIM) to confirm support needs. NST members collaborate on nutrition plans, monitor tolerance, electrolytes, and complications. Guided by evidence-based practices, the team analyzes key outcomes (30-day mortality, intake achievement, nutritional/immune improvement) to enable accelerated perioperative recovery. NST, nutrition support team; EN, enteral nutrition via feeding tube; PN, parenteral nutrition; PSM, propensity score matching; MR, mortality rate; LOS, length of stay.
2.2 Screening and baseline measurements
Preoperative baseline demographic and clinical characteristics of the patients were systematically documented, encompassing gender, age, body weight, height, BMI, surgical procedure (total gastrectomy or partial gastrectomy), surgical approach (laparoscopic or robot-assisted), duration of surgery, Charlson comorbidity Index (CCI), prognostic nutritional index (PNI), and MNA-SF score. Additionally, preoperative baseline biochemical parameters were measured, including levels of prealbumin, hemoglobin, and total bilirubin and creatinine, all of which were measured under fasting conditions. The preoperative body weight was defined as the body weight measured on the day of surgery. The body weight was then measured again at 1 month after surgery (postoperative weight) to calculate the percentage of postoperative weight loss (= [postoperative weight – preoperative weight]/preoperative weight × 100).
2.3 Process of NST activity
Clinical physicians initiated a 2-step GLIM protocol within 24 h of admission, consisting of nutritional risk screening and malnutrition diagnosis. For patients with confirmed malnutrition, a multidisciplinary team comprising physicians, dietitians, and clinical pharmacists developed individualized nutritional intervention plans. These evidence-based strategies were tailored to each patient's nutritional status, biochemical parameters, and gastrointestinal function, with specifications for caloric requirements and delivery routes. Registered nurses provided nutritional education, implemented standardized support protocols, and monitored and documented any adverse events.
For patients with confirmed malnutrition undergoing elective surgery, nutritional support was initiated preoperatively within 24–48 h of admission. For patients unable to resume oral intake within 48 h postoperatively, nutritional support therapy was promptly commenced. The NST conducted multidisciplinary rounds at least twice weekly to ensure appropriate implementation and safety of nutritional interventions.
Due to the impracticality of measuring individual energy expenditure, target energy intake was established at 25–30 kcal/kg/day and protein intake at 1.5 g/kg/day, following perioperative nutrition guidelines (22). The NST employed a progressive 5-tier nutritional support strategy: (1) oral intake (PO); (2) oral nutritional supplementation (ONS); (3) EN; (4) EN + SPN; and (5) TPN. When oral intake or ONS provided less than 50% of target energy requirements for 7 days, EN was initiated. When EN failed to meet more than 50% of patients' energy requirements within 7 days, SPN was commenced. For patients with contraindications to enteral nutrition, such as intestinal obstruction, severe paralytic ileus, or high-output enterocutaneous fistula, TPN was implemented immediately (23). PN formulations consisted of dextrose (50%−60% of non-protein calories), lipid emulsions (30%−40% of non-protein calories), and amino acids, with a non-protein calorie-to-nitrogen ratio of 100–150:1. All patients received vitamins and trace elements according to ASPEN guidelines (24), with daily electrolyte adjustments based on laboratory monitoring. Parenteral nutrition was administered via peripherally inserted central catheter lines.
Patient adherence to nutritional prescriptions was monitored by registered nurses through daily documentation of oral intake, enteral feeding volumes, and parenteral nutrition records. Adherence data were systematically reviewed and verified during multidisciplinary NST rounds. To ensure inter-rater reliability within the NST, standardized training was conducted prior to study initiation, unified assessment protocols were implemented, and consensus-based discussions were held during team meetings to minimize subjective variability in nutritional assessment and intervention decisions.
2.4 Outcomes
The primary outcome was the 30-day mortality rate following surgery. The diagnostic criterion for 30-day mortality was defined as any death from any cause occurring within 30 days of the day on which the patient underwent radical or palliative gastrectomy for gastric cancer. Secondary outcomes were assessed across three categories: (1) nutritional parameters: compliance rates for energy and protein intake targets were monitored throughout the perioperative period; postoperative nutritional status was evaluated using the PNI, along with serum levels of prealbumin, albumin, and hemoglobin measured on postoperative days 3 and 7, and at discharge; weight loss from baseline was documented for all patients; (2) surgical complications: anastomotic leakage rates were compared between groups; postoperative infections were defined according to the Centers for Disease Control and Prevention (CDC) criteria for healthcare-associated infections (25), with specific infectious complications assessed including pneumonia, intra-abdominal infection, urinary tract infection, and catheter-related bloodstream infection; and (3) recovery metrics: total hospital length of stay, postoperative length of stay, unplanned readmission rate, time to drain removal, time to first flatus, and time to first bowel movement were recorded.
2.5 Statistical analysis
Statistical analyses were conducted using SPSS version 27.0 for Windows (SPSS Inc., Chicago, IL, USA). Survival outcomes were estimated using the Kaplan–Meier method. PSM was employed to mitigate potential biases when comparing the TN and NST groups. A logistic regression model was fitted to estimate the propensity score, controlling for these covariates: gender, age, BMI, surgical type, operation, duration of surgery, prealbumin, hemoglobin, total bilirubin, creatinine, PNI, MNA-SF and Charlson comorbidity index (CCI). PSM was conducted using a 1:1 nearest-neighbor algorithm without replacement and a caliper width of 0.1. Successful balance between the groups was confirmed, with all absolute standardized mean differences (ASD) falling below the threshold of 0.2 post-matching. Continuous variables were assessed using the Kolmogorov-Smirnov test and compared using t-tests (normal distribution) or Mann–Whitney U test (abnormal distribution), with results expressed as mean ± standard error (SE) or median (P25, P75). Two-sided P-values < 0.05 were considered statistically significant. Categorical variables were compared using the χ2 test.
3 Results
3.1 Patient baseline characteristics
A total of 513 patients consented to participate in this study between January 1, 2021, and December 30, 2024. After propensity score matching, 71 patients were excluded, leaving 482 patients (TN: 241, NST: 241) for analysis (Figure 2). No significant differences in baseline characteristics were observed between the two groups. The baseline characteristics of the included patients are presented in Table 1. Among the NST participants, the number of male participants is nearly twice that of female participants. Participants were on average about 70 years old. BMI was under 24 kg/m2. Regarding surgical procedures, threefold cases underwent partial gastrectomy rather than total gastrectomy, with twofold performed using robotic surgery. The mean duration of surgery was half-years. The values of nutritional indicators, renal function, bilirubin levels, and nutrition prognostic-related scores were comparable between the NST intervention group and the TN group.
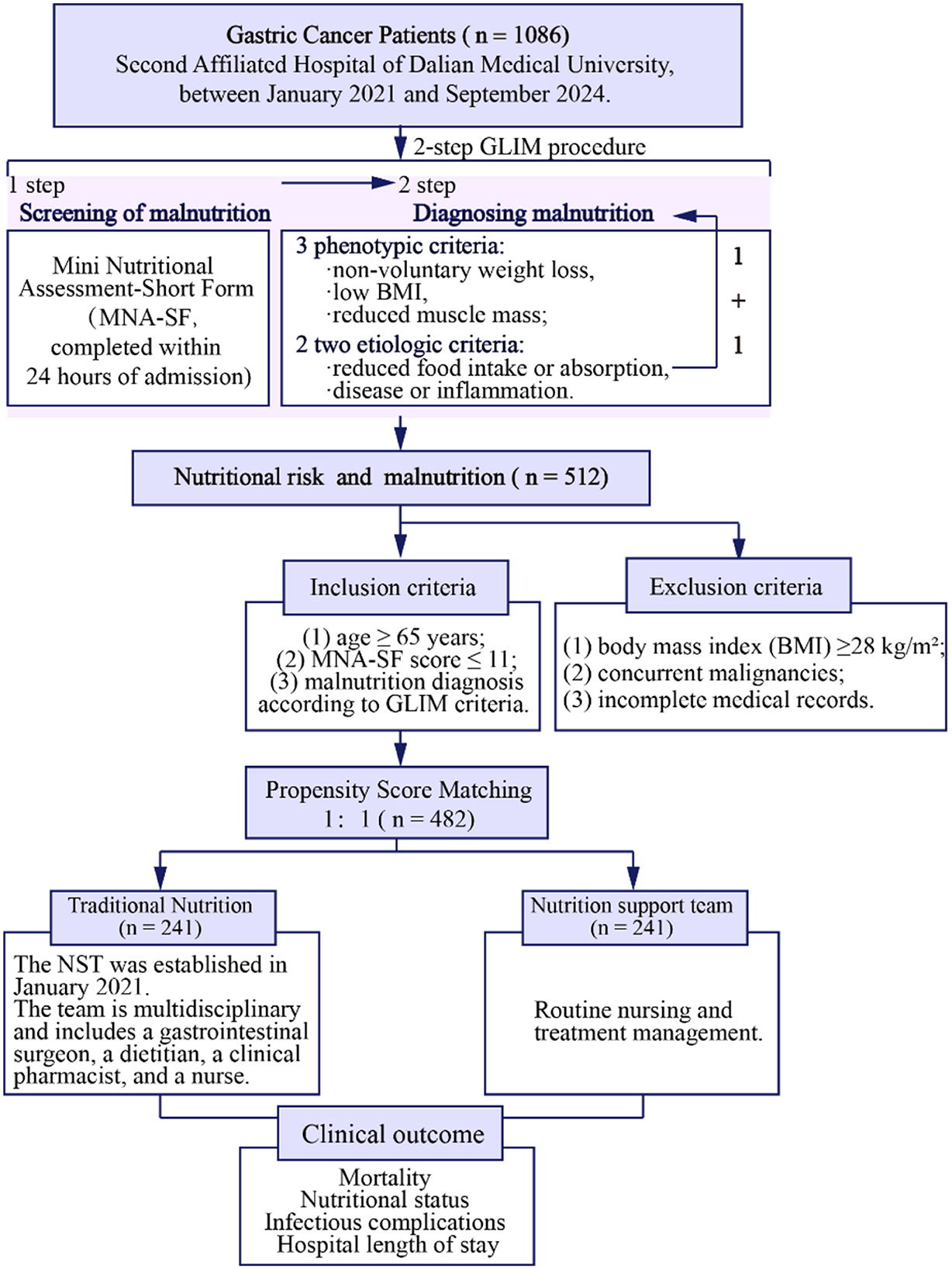
Figure 2. Flowchart. This retrospective cohort study enrolled gastric cancer patients ≥65 years undergoing radical gastrectomy (Jan 2021–Sep 2024) with Mini Nutritional Assessment-Short Form (MNA-SF) ≤ 11 and Global Leadership Initiative on Malnutrition (GLIM)-defined malnutrition. Exclusions: body mass index (BMI) ≥28 kg/m2, other malignancies, incomplete data. Two hundred and forty-one patients each in the nutrition support team (NST) group and traditional nutrition (TN) group.
3.2 Prognosis
Figure 3 showed 30-day mortality rates curves. The mortality rates at day 1 and 30 were 0.4%−4.1% (95% CI: 1.9%−7.2%) in the TN group, compared to 0.0%−0.8% (95% CI: 0.0%, 2.0%) in the NST group, respectively (P < 0.05). Our data indicated that NST prominently declined the mortality rate of older adults with gastric carcinoma within 30 days. Among the 10 deaths in the TN group within 30 days postoperatively, the causes were septic shock (n = 6), respiratory failure (n = 1), heart failure (n = 1), pulmonary embolism (n = 1), and multiple organ dysfunction syndrome (MODS, n = 1). In the NST group, two deaths occurred, attributed to septic shock (n = 1) and MODS (n = 1).
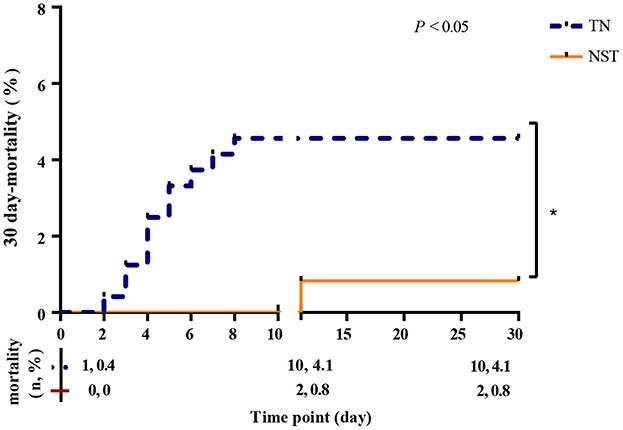
Figure 3. NST group declined the 30 day-mortality rates in malnourished older adults with gastric cancer. Thirty day-mortality of the TN group (n = 241; blue dash line) and NST group (n = 241; orange line). TN, traditional nutrition; NST, nutrition support team.
3.3 Nutritional support outcomes
3.3.1 Compliance rates for energy and protein intake
A total of 236 patients in the TN group and 221 patients in the intervention group received PN therapy. The compliance rates for energy and protein intake were compared between the two groups before discharge after gastrectomy, for supply a more standardized the nutritional support according to the ESPEN guidelines which calls for 25–30 kcal/kg/d energy supply and 1.2–1.5 g/kg/d protein intake. The NST group, compared with the TN group, showed a higher compliance rates for energy (71.4 vs. 10.0%, respectively, P < 0.001), and higher compliance rates for protein intake (56.0 vs. 10.8%, respectively, P < 0.001, Figure 4).
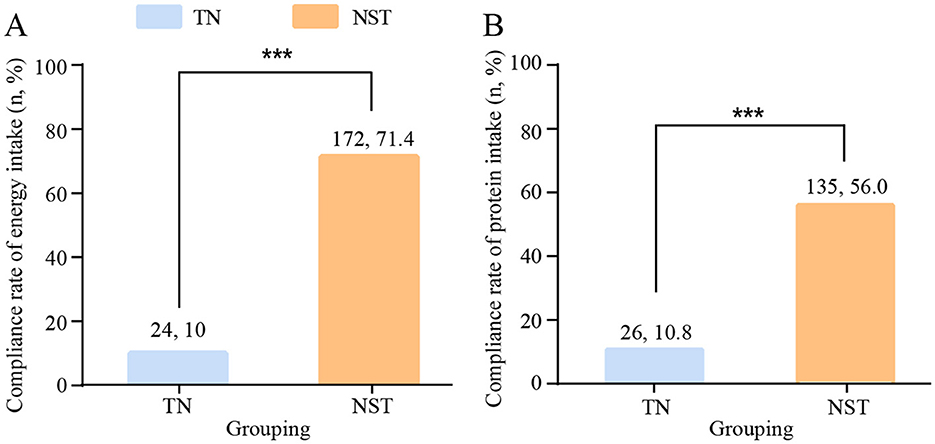
Figure 4. NST-led care boosted the compliance rates for (A) energy and (B) protein intake in malnourished older adults with gastric cancer. Compared to the TN group, ***P < 0.001. TN, traditional nutrition; NST, nutrition support team.
3.3.2 Postoperative nutritional status
The postoperative nutritional indicators PNI, prealbumin, albumin and hemoglobin were compared between the two groups at day 3, day 7 and before discharge, separately (Supplementary Table S1). The PNI were increased by NST group in comparison of the TN groups at day 7 and before discharge (P < 0.0001, Figure 5). These differences were clinically meaningful, as the mean improvements of 2.42 points at day 7 and 1.56 points before discharge elevated PNI above the critical threshold of 46, which was associated with reduced postoperative complication risk in gastric cancer patients (26). The prealbumin of NST group were observably elevated at day 7 and before discharge (P < 0.0001). The NST group reached prealbumin concentrations of approximately 150 mg/L before discharge, a threshold associated with adequate protein synthesis and reduced postoperative complications (27). The serum albumin level and hemoglobin were not significantly different between the TN and NST groups at day 3, day 7 nor before discharge.
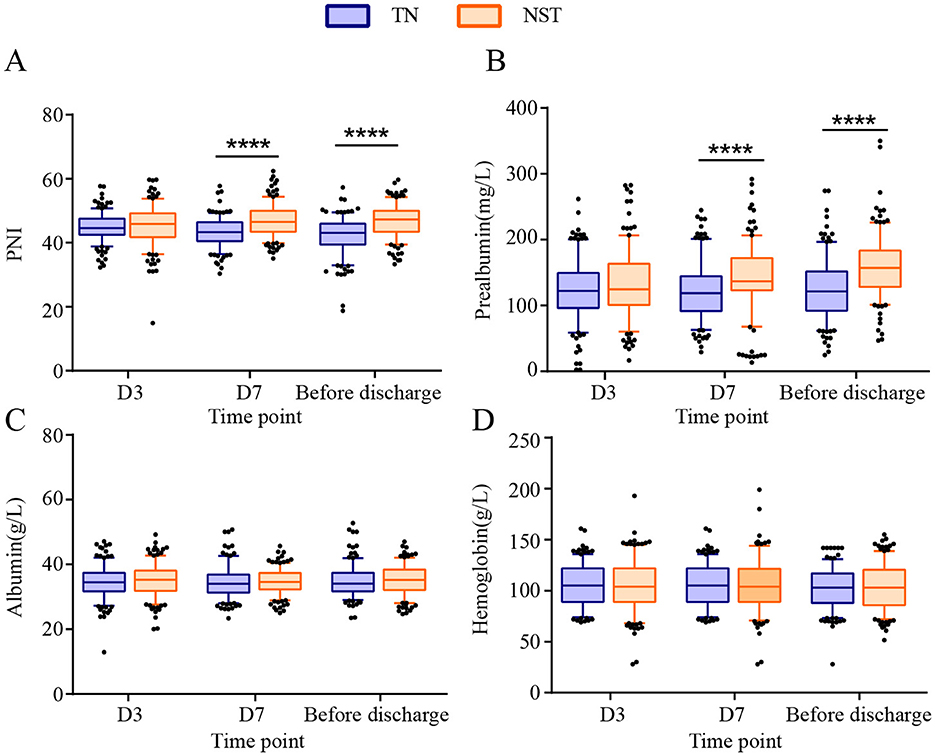
Figure 5. NST-led enhanced the postoperative nutritional status at day 3, 7 and before discharge in malnourished older adults with gastric cancer. Statistics analysis of (A) prognostic nutritional index (PNI), (B) prealbumin, (C) albumin and (D) hemoglobin. Compared to the TN group, ****P < 0.0001. PNI, prognostic nutritional index; TN, traditional nutrition; NST, nutrition support team.
3.3.3 Postoperative complications
Postoperative complications were shown in Figure 6. After NST intervention, the incidence of anastomotic leakage in older adults with gastric carcinoma was significantly decreased compared to the TN group (1.7 vs. 5.0%, P < 0.05). Similarly, infectious complications occurred less frequently in the NST group than in the TN group (4.5 vs. 10.4%, P < 0.05). A total of 23 infection episodes occurred in the TN group, comprising pneumonia (n = 13), catheter-related bloodstream infection (n = 1), abdominal infections (n = 7), and urinary tract infections (n = 2). In the NST group, 10 infection episodes were documented, including pneumonia (n = 5), abdominal infections (n = 4), and urinary tract infection (n = 1). In addition to anastomotic leakage and infectious complications, other major postoperative complications were also recorded. Intra-abdominal bleeding occurred in three patients (1.2%) in the TN group and two patients (0.8%) in the NST group. Chylothorax was observed in one patient (0.4%) in each group. Pulmonary embolism occurred in one patient (0.4%) in the TN group, with no cases documented in the NST group.
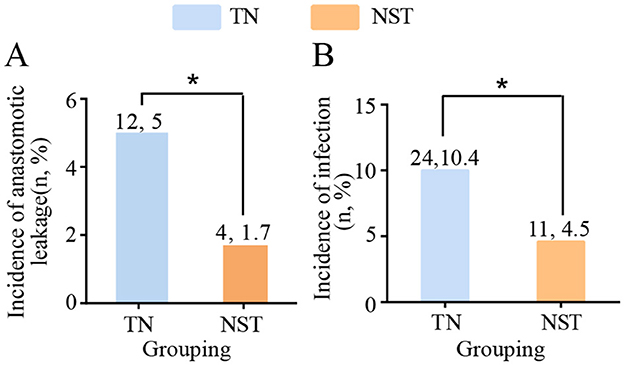
Figure 6. NST-led care impeded the postoperative complications before discharge in malnourished older adults with gastric cancer. Statistics of the number of patients with (A) anastomotic leakage and (B) incidence of infection. Compared to the TN group, *P < 0.05. TN, traditional nutrition; NST, nutrition support team.
3.3.4 Weight loss
A significant reduction of weight loss (D3: 2.11 ± 0.08 vs. 6.63 ± 0.20, P < 0.001; before discharge: 1.92 ± 0.07 vs. 6.53 ± 0.20, P < 0.001) in the NST-group was noted compared to the TN group at day 7 and before discharge (Figure 7).
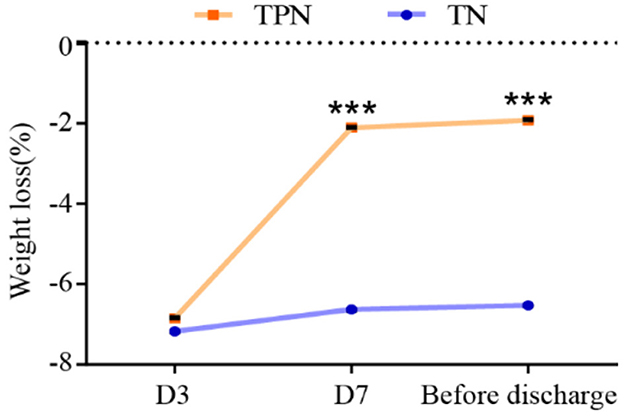
Figure 7. NST-led care rescued the perioperative weight loss in malnourished older adults with gastric cancer. Compared to the TN group, ***P < 0.001. TN, traditional nutrition; NST, nutrition support team.
3.3.5 Recovery metrics
NST group significantly decreased the length of stay [15.00 (14.00, 17.00) vs. 16.00 (14.00, 19.00), P < 0.05] and postoperative length of stay [12.00 (9.00, 14.00) vs. 12.00 (9.00, 16.00), P < 0.05] and unplanned admission rate (10.8 vs. 17.8%, P < 0.05) compared to the TN group (Figure 8). Furthermore, NST significantly reduced the time to drain removal after surgery [9.00 (8.00, 11.00) vs. 10.00 (8.00, 12.00), P < 0.001], the first time to first flatus [3.00 (3.00, 3.00) vs. 4.00 (4.00, 4.00), P < 0.001] and first bowel movement [4.00 (4.00, 4.00) vs. 5.00 (5.00, 5.00), P < 0.001] compared with the TN group.
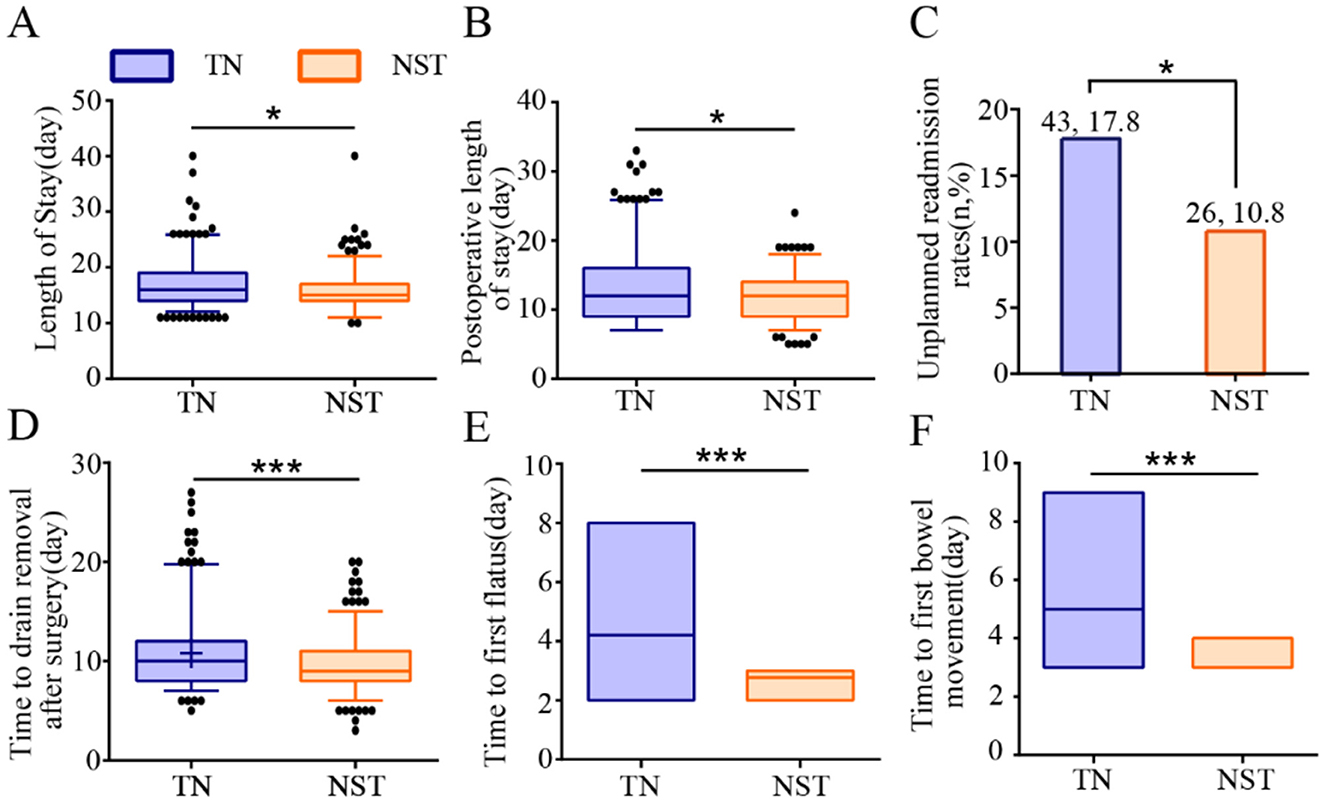
Figure 8. NST-led care improved recovery metrics in malnourished older adults with gastric cancer. Analysis of (A) length of stay, (B) postoperative length of stay, (C) unplanned readmission rates, (D) time to drain removal after surgery, (E) time to first flatus and (F) time to first bowel movement. Compared to the TN group, ***P < 0.001 or *P < 0.05. TN, traditional nutrition; NST, nutrition support team.
4 Discussion
This study evaluated the clinical value of NST in the perioperative management of malnourished older adults with gastric cancer. Our findings demonstrated that multidisciplinary NST involvement was associated with improved clinical outcomes through individualized nutrition regimens with continuous monitoring and adjustment. While the observational design of this study precludes definitive causal inference, the observed benefits followed a biologically plausible stepwise progression: adequate nutrient intake was associated with enhanced nutritional status, which correlated with reduced infection rates and shortened hospital stays. These improvements ultimately coincided with lower mortality, accelerated recovery, and improved quality of life. Our study was well-referenced with up-to-date literature and strong alignment with ESPEN and ASPEN guidelines.
These findings align with recent evidence demonstrating the beneficial impact of nutritional interventions in surgical populations. Schuetz et al. reported that individualized nutritional support to reach protein and caloric goals reduced the risk of adverse clinical outcomes in medical inpatients at nutritional risk (28), while Gomes et al. demonstrated that nutritional support interventions was associated with clinically significant improvements of important clinical outcomes in the medical inpatient population, in whom malnutrition is highly prevalent (29).
Causes of mortality in older adults with gastric cancer include postoperative malnutrition, infectious complications, age-related immunosuppression, and disease progression (11, 30, 31). The high prevalence of malnutrition in this population significantly increases the risk of postoperative complications and early mortality (32). Adequate caloric and protein supplementation represents a critical modifiable factor for improving prognosis (28, 33). In this study, the NST established evidence-based nutritional targets, which improving nutrition forms part of the ESPEN guidelines, recommending an energy supply of 25–30 kcal/kg/d and a protein intake of 1.2–1.5 g/kg/d (22). Through individualized nutritional prescriptions and a standardized titration protocol, the NST intervention group achieved significantly higher compliance rates for both energy and protein intake. Notably, conventional nutritional management alone proved insufficient to meet these targets (34). By comparison, the NST's systematic intervention ensures the effective delivery of nutritional therapy and underscores nutrition improvement as a global priority, a core element of United Nations Sustainable Development Goal 2 (35).
Malnutrition amelioration through enhanced nutrient intake correlates directly with improvements in biochemical parameters (36). Prealbumin, as a negative acute phase response protein with a short half-life, responds rapidly to nutritional interventions (37). Our results showed that the NST group exhibited a significant increase in prealbumin levels by postoperative day 7. Recent evidence has indicated that changes in prealbumin during the first week post-surgery can serve as an accurate predictor of patient mortality and are a reliable indicator for assessing the effectiveness of nutritional support (38, 39). Our findings suggest that NST may improve the nutritional status of patients, potentially contributing to a reduction in mortality rates. Additionally, the PNI first proposed by Onodera et al. (40), integrates serum albumin levels and peripheral blood lymphocyte counts and has been validated as an independent prognostic factor for gastrointestinal malignancies (41, 42). A significant increase in PNI was demonstrated in the NST group. PNI reflects both nutritional status and immune function, with its improvement being negatively correlated with the incidence of postoperative complications (43–45). Our findings indicated that NST facilitated the effective recovery of nutritional status and immune function.
Infectious complications constitute a major source of perioperative morbidity and mortality in older adults with gastric cancer (46), with their incidence being closely associated with patients' nutritional status (47, 48). The findings of this study demonstrated that the incidence of infectious complications was significantly lower in the NST intervention group compared to the TN group, consistent with results reported in previous studies (49, 50). Notably, NST intervention significantly decreased all-cause mortality in the subset of patients with infectious complications. Malnourished patients are more susceptible to progression from infection to sepsis (51), while NST enhanced patients' resistance to infectious insults by improving nutritional status. Furthermore, optimal nutritional support preserved the integrity of the intestinal barrier, reduced bacterial translocation, and mitigated the risk of sepsis-related mortality (52).
The superior infection control in the NST group reflects multiple interconnected immunological mechanisms. Adequate protein supports immunoglobulin synthesis and lymphocyte proliferation (49), with specific amino acids (glutamine, arginine) enhancing immune cell function and maintaining intestinal barrier integrity (53, 54). Sufficient energy intake maintains neutrophil and macrophage phagocytic capacity (52), while omega-3 fatty acids modulate inflammatory responses toward anti-inflammatory pathways (55). Critical micronutrients serve as immune cofactors: zinc enables T-cell maturation (56), selenium provides antioxidant protection (57), vitamin D induces antimicrobial peptides (58), and vitamin C supports phagocyte function (59).
NST protocols emphasizing early enteral nutrition preserve gut-associated lymphoid tissue (60), maintain microbiota diversity, and prevent bacterial translocation (61, 62), Comprehensive nutritional support restores antioxidant defenses, particularly important in older adults with immunosenescence (63). The multidisciplinary NST approach provides distinct advantages: individualized assessment prevents both underfeeding and overfeeding complications (64), early intervention optimizes the post-operative immune window (47). These synergistic mechanisms, including macronutrient-driven energy restoration, micronutrient-enhanced immune signaling, and gut health maintenance, ultimately reduce infection complications and improve clinical outcomes (65).
Maintaining body weight independently and significantly predicts the prognosis of surgical patients (66). Numerous previous studies have consistently demonstrated a strong correlation between perioperative unintentional weight loss and increased rates of postoperative complications, prolonged hospital stays, and higher mortality (67–69). In the present study, our results indicated that unintentional weight loss in patients receiving NST interventions was significantly lower than that in the TN group, which has considerable clinical significance. It has been reported that nutritional support establishes a necessary metabolic foundation for the body to cope with surgical trauma stress and promotes postoperative recovery by preserving lean body mass and maintaining the reserve function of visceral protein pools (70). This finding further underscores the critical value of individualized perioperative nutritional support strategies in improving the prognosis of surgical patients.
The accelerated recovery of gastrointestinal function and the reduced length of hospital stay are comprehensive indicators reflecting all of the aforementioned improvements (71). In this study, we observed that the time to first postoperative flatus and defecation was significantly shortened in the intervention group. This can be attributed not only to the direct facilitative effect of adequate energy and protein supplementation on intestinal mucosal repair (47), but also to the attenuation of systemic inflammatory responses and reduction in infectious complications as a result of improved nutritional status (72). The synergistic effects of these factors contribute to an accelerated postoperative recovery process. As an integrated outcome measure, the reduction in length of hospital stay reflects not only improvements in the quality of medical care but also demonstrates the comprehensive cascade of NST intervention effects (28, 29), spanning from initial nutritional optimization to ultimate clinical outcome improvements.
4.1 Limitation
Despite the positive results observed in this study, several limitations should be acknowledged. First, as a single-center study, the sample size was relatively limited, the study population consisted primarily of Asian patients from a single Chinese medical center, which may limit the generalizability of our findings to non-Asian populations, and acknowledge possible selection bias might exist and the generalizability of these findings requires validation in larger, multicenter studies. Second, owing to a pragmatic approach, the follow-up period in this study was relatively short, and the long-term effects of NST intervention on patient survival and quality of life remain to be further investigated. This study was conducted at a tertiary hospital with well-established NST infrastructure, and the feasibility and effectiveness of implementation in centers without specialized nutritional support teams require further validation. In the future, multicenter studies in different ethnic populations and healthcare resource settings are needed to further validate the broader applicability of our findings and to explore NST implementation models suitable for various healthcare institutions. Finally, the absence of a cost-effectiveness analysis precludes a comprehensive evaluation of the health economic value of the NST model.
5 Conclusion
This study demonstrates that systematic perioperative management by an NST in older adults malnourished patients with gastric cancer was associated with higher attainment of energy and protein targets, improvements in nutritional and immune function, lower postoperative 30-day mortality, and faster clinical recovery. In light of current guidelines and emerging evidence, NST-led nutritional intervention should be considered an essential component of perioperative care for older adults with malnutrition.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Second Hospital of Dalian Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LQ: Resources, Writing – original draft. ZL: Data curation, Writing – original draft. LY: Conceptualization, Data curation, Validation, Writing – original draft. ML: Supervision, Writing – review & editing. LC: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by grants from Lichun Cheng, grant number No. LJ212510161026 and the APC was funded by the Liaoning Provincial Department of Education General Project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1707892/full#supplementary-material
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 3:229–63. doi: 10.3322/caac.21834
2. Yan C, Shan F, Ying X, Li Z. Global burden prediction of gastric cancer during demographic transition from 2020 to 2040. Chin Med J. (2023) 4:397–406. doi: 10.1097/CM9.0000000000002626
3. Seid A, Debebe Z, Ayelign A, Abeje M, Endris BS, Assefa M, et al. Malnutrition diagnosed by patient-generated subjective global assessment and the risk of all-cause mortality in adults with gastrointestinal cancer: a systematic review and meta-analysis. J Hum Nutr Diet. (2025) 1:e70012. doi: 10.1111/jhn.70012
4. Wang HM, Wang TJ, Huang CS, Liang SY, Yu CH, Lin TR, et al. Nutritional status and related factors in patients with gastric cancer after gastrectomy: a cross-sectional study. Nutrients. (2022) 13:2634. doi: 10.3390/nu14132634
5. Huang C, Liu H, Hu Y, Sun Y, Su X, Cao H, et al. Laparoscopic vs open distal gastrectomy for locally advanced gastric cancer: five-year outcomes from the CLASS-01 randomized clinical trial. JAMA Surg. (2022) 1:9–17. doi: 10.1001/jamasurg.2021.5104
6. Hyung WJ, Yang HK, Park YK, Lee HJ, An JY, Kim W, et al. Long-term outcomes of laparoscopic distal gastrectomy for locally advanced gastric cancer: the KLASS-02-RCT Randomized Clinical Trial. J Clin Oncol. (2020) 28:3304–13. doi: 10.1200/JCO.20.01210
7. Zhang X, Tang M, Zhang Q, Zhang KP, Guo ZQ. Xu, et al. The GLIM criteria as an effective tool for nutrition assessment and survival prediction in older adult cancer patients. Clin Nutr. (2021) 3:1224–32. doi: 10.1016/j.clnu.2020.08.004
8. Gillis C, Carli F. Promoting perioperative metabolic and nutritional care. Anesthesiology. (2015) 6:1455–72. doi: 10.1097/ALN.0000000000000795
9. Pacelli F, Bossola M, Rosa F, Tortorelli AP, Papa V, Doglietto GB. Is malnutrition still a risk factor of postoperative complications in gastric cancer surgery? Clin Nutr. (2008) 3:398–407. doi: 10.1016/j.clnu.2008.03.002
10. Kang MK, Lee HJ. Impact of malnutrition and nutritional support after gastrectomy in patients with gastric cancer. Ann Gastroenterol Surg. (2024) 4:534–52. doi: 10.1002/ags3.12788
11. GlobalSurg Collaborative and NIHR Global Health Unit on Global Surgery. Impact of malnutrition on early outcomes after cancer surgery: an international, multicentre, prospective cohort study. Lancet Glob Health. (2023) 3:e341–9. doi: 10.1016/S2214-109X(22)00550-2
12. Mcclave SA, Dibaise JK, Mullin GE, Martindale RG. ACG clinical guideline: nutrition therapy in the adult hospitalized patient. Am J Gastroenterol. (2016). 3: 315–34; quiz 335. doi: 10.1038/ajg.2016.28
13. Hua Y, Mingwei Z, Wei C, Xinying W. Guideline for clinical application of parenteral and enteral nutrition in adults patients in China (2023 edition). Chin Society of Parenteral and Enteral Nutrition. (2023) 13:946–74. doi: 10.3760/cma.j.cn112137-20221116-02407
14. Eriksen MK, Crooks B, Baunwall SMD, Rud CL, Lal S, Hvas CL. Systematic review with meta-analysis: effects of implementing a nutrition support team for in-hospital parenteral nutrition. Aliment Pharmacol Ther. (2021) 5:560–70. doi: 10.1111/apt.16530
15. Gonzalez-Granda A, Schollenberger A, Thorsteinsson R, Haap M, Bischoff SC. Impact of an interdisciplinary nutrition support team (NST) on the clinical outcome of critically ill patients. A pre/post NST intervention study. Clin Nutr ESPEN. (2021). 486–91. doi: 10.1016/j.clnesp.2021.06.018
16. Kennedy JF, Nightingale JM. Cost savings of an adult hospital nutrition support team. Nutrition. (2005). 11-12:1127–33. doi: 10.1016/j.nut.2005.08.002
17. Roberts MF, Levine GM. Nutrition support team recommendations can reduce hospital costs. Nutr Clin Pract. (1992) 5:227–30. doi: 10.1177/0115426592007005227
18. Cederholm T, Jensen GL, Correia M, Gonzalez MC, Fukushima R, Higashiguchi T, et al. GLIM criteria for the diagnosis of malnutrition - A consensus report from the global clinical nutrition community. Clin Nutr. (2019) 38:1–9. doi: 10.1016/j.clnu.2018.08.002
19. Koren-Hakim T, Weiss A, Hershkovitz A, Otzrateni I, Anbar R, Gross Nevo RF, et al. Comparing the adequacy of the MNA-SF, NRS-2002 and MUST nutritional tools in assessing malnutrition in hip fracture operated elderly patients. Clin Nutr. (2016) 5:1053–8. doi: 10.1016/j.clnu.2015.07.014
20. Guigoz Y, Vellas B, Garry PJ. Assessing the nutritional status of the elderly: the Mini Nutritional Assessment as part of the geriatric evaluation. Nutr Rev. (1996). 1(Pt 2):S59–65. doi: 10.1111/j.1753-4887.1996.tb03793.x
21. Cederholm T, Jensen GL, Correia M, Gonzalez MC, Fukushima R, Pisprasert V, et al. The GLIM consensus approach to diagnosis of malnutrition: a 5-year update. Clin Nutr. (2025). 49:11–20. doi: 10.1016/j.clnu.2025.03.018
22. Weimann A, Braga M, Carli F, Higashiguchi T, Hubner M, Klek S, et al. ESPEN guideline: clinical nutrition in surgery. Clin Nutr. (2017) 3:623–50. doi: 10.1016/j.clnu.2017.02.013
23. Lobo DN, Gianotti L, Adiamah A, Barazzoni R, Deutz NEP, Dhatariya K, et al. A Perioperative nutrition: recommendations from the ESPEN expert group. Clin Nutr. (2020) 11:3211–27. doi: 10.1016/j.clnu.2020.03.038
24. Blaauw R, Osland E, Sriram K, Ali A, Allard JP, Ball P, et al. Parenteral provision of micronutrients to adult patients: an expert consensus paper. JPEN J Parenter Enteral Nutr. (2019) 1:S5–S23. doi: 10.1002/jpen.1525
25. Horan T, Crus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. (2008) 5:309–32. doi: 10.1016/j.ajic.2008.03.002
26. Jiang N, Deng JY, Ding XW, Ke B, Liu N, Zhang RP, et al. Prognostic nutritional index predicts postoperative complications and long-term outcomes of gastric cancer. World J Gastroenterol. (2014) 30:10537–44. doi: 10.3748/wjg.v20.i30.10537
27. Zhou J, Hiki N, Mine S, Kumagai K, Ida S, Jiang X, et al. Role of prealbumin as a powerful and simple index for predicting postoperative complications after gastric cancer surgery. Ann Surg Oncol. (2017) 2:510–7. doi: 10.1245/s10434-016-5548-x
28. Schuetz P, Fehr R, Baechli V, Geiser M, Deiss M, Gomes F, et al. Individualised nutritional support in medical inpatients at nutritional risk: a randomised clinical trial. Lancet. (2019) 10188:2312–21. doi: 10.1016/S0140-6736(18)32776-4
29. Gomes F, Baumgartner A, Bounoure L, Bally M, Deutz NE, Greenwald JL, et al. Association of nutritional support with clinical outcomes among medical inpatients who are malnourished or at nutritional risk: an updated systematic review and meta-analysis. JAMA Netw Open. (2019) 11:e1915138. doi: 10.1001/jamanetworkopen.2019.15138
30. Fukuda Y, Yamamoto K, Hirao M, Nishikawa K, Nagatsuma Y, Nakayama T, et al. Sarcopenia is associated with severe postoperative complications in elderly gastric cancer patients undergoing gastrectomy. Gastric Cancer. (2016) 3:986–93. doi: 10.1007/s10120-015-0546-4
31. Hirahara N, Tajima Y, Fujii Y, Kaji S, Kawabata Y, Hyakudomi R, et al. Prediction of postoperative complications and survival after laparoscopic gastrectomy using preoperative Geriatric Nutritional Risk Index in elderly gastric cancer patients. Surg Endosc. (2021) 3:1202–9. doi: 10.1007/s00464-020-07487-7
32. Zhao XN, Lu J, He HY, Ge SJ. Clinical significance of preoperative nutritional status in elderly gastric cancer patients undergoing radical gastrectomy: a single-center retrospective study. World J Gastrointest Surg. (2024) 7:2211–20. doi: 10.4240/wjgs.v16.i7.2211
33. Tamiya H, Yasunaga H, Hosoi T, Yamana H, Matsui H, Fushimi K, et al. Association between protein intake and mortality in older patients receiving parenteral nutrition: a retrospective observational study. Am J Clin Nutr. (2021) 6:1907–16. doi: 10.1093/ajcn/nqab292
34. Chen J, Zou L, Sun W, Zhou J, He Q. The effects of nutritional support team intervention on postoperative immune function, nutritional statuses, inflammatory responses, clinical outcomes of elderly patients with gastric cancer. BMC Surg. (2022) 1:353. doi: 10.1186/s12893-022-01784-9
35. Meara JG, Hagander L, Leather AJM. Surgery and global health: a Lancet Commission. Lancet. (2014) 9911:12–3. doi: 10.1016/S0140-6736(13)62345-4
36. Gao X, Liu Y, Zhang L, Zhou D, Tian F, Gao T, et al. Effect of early vs late supplemental parenteral nutrition in patients undergoing abdominal surgery: a randomized clinical trial. JAMA Surg. (2022) 5:384–93. doi: 10.1001/jamasurg.2022.0269
37. Pardo E, Jabaudon M, Godet T, Pereira B, Morand D, Futier E, et al. Dynamic assessment of prealbumin for nutrition support effectiveness in critically ill patients. Clin Nutr. (2024) 6:1343–52. doi: 10.1016/j.clnu.2024.04.015
38. Zhang Y, Gu F, Wang F, Zhang Y. Effects of early enteral nutrition on the gastrointestinal motility and intestinal mucosal barrier of patients with burn-induced invasive fungal infection. Pak J Med Sci. (2016) 3:599–603. doi: 10.12669/pjms.323.9717
39. Yu PJ, Cassiere HA, Dellis SL, Manetta F, Kohn N, Hartman AR. Impact of preoperative prealbumin on outcomes after cardiac surgery. JPEN J Parenter Enteral Nutr. (2015) 7:870–4. doi: 10.1177/0148607114536735
40. Onodera T, Goseki N, Kosaki G. [Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients]. Nihon Geka Gakkai Zasshi. (1984) 9:1001–5.
41. Migita K, Takayama T, Saeki K, Matsumoto S, Wakatsuki K, Enomoto K, et al. The prognostic nutritional index predicts long-term outcomes of gastric cancer patients independent of tumor stage. Ann Surg Oncol. (2013) 8:2647–54. doi: 10.1245/s10434-013-2926-5
42. Okugawa Y, Toiyama Y, Yamamoto A, Shigemori T, Ichikawa T, Yin C, et al. Lymphocyte-to-C-reactive protein ratio and score are clinically feasible nutrition-inflammation markers of outcome in patients with gastric cancer. Clin Nutr. (2020) 4:1209–17. doi: 10.1016/j.clnu.2019.05.009
43. Yang Y, Gao P, Song Y, Sun J, Chen X, Zhao J, et al. The prognostic nutritional index is a predictive indicator of prognosis and postoperative complications in gastric cancer: a meta-analysis. Eur J Surg Oncol. (2016) 8:1176–82. doi: 10.1016/j.ejso.2016.05.029
44. Zhang X, Wang Y, Xu M, Zhang Y, Lyu Q. The malnutrition in AECOPD and its association with unfavorable outcomes by comparing PNI, GNRI with the GLIM criteria: a retrospective cohort study. Front Nutr. (2024) 11:1365462. doi: 10.21203/rs.3.rs-3280965/v1
45. Chan AW, Chan SL, Wong GL, Wong VW, Chong CC, Lai PB, et al. Prognostic Nutritional Index (PNI) predicts tumor recurrence of very early/early stage hepatocellular carcinoma after surgical resection. Ann Surg Oncol. (2015) 13:4138–48. doi: 10.1245/s10434-015-4516-1
46. Liu X, Xue Z, Yu J, Li Z, Ma Z, Kang W, et al. Risk factors for postoperative infectious complications in elderly patients with gastric cancer. Cancer Manag Res. (2020) 12:4391–8. doi: 10.2147/CMAR.S253649
47. Weimann A, Braga M, Carli F, Higashiguchi T, Hubner M, Klek S, et al. ESPEN practical guideline: clinical nutrition in surgery. Clin Nutr. (2021) 7:4745–61. doi: 10.1016/j.clnu.2021.03.031
48. Yang ZY, Yang F. Nutritional status of patients with gastrointestinal cancers and analysis of factors for postoperative infections. BMC Cancer. (2024) 24:1389. doi: 10.1186/s12885-024-13093-w
49. Vlug LE, Nagelkerke SCJ, Jonkers-Schuitema CF, Rings E, Tabbers MM. The role of a nutrition support team in the management of intestinal failure patients. Nutrients. (2020) 12:172. doi: 10.3390/nu12010172
50. Cong MH Li SL, Cheng GW, Liu JY, Song CX, Deng YB, et al. An interdisciplinary nutrition support team improves clinical and hospitalized outcomes of esophageal cancer patients with concurrent chemoradiotherapy. Chin Med J. (2015) 22:3003–7. doi: 10.4103/0366-6999.168963
51. Adejumo AC, Akanbi O, Pani L. Protein energy malnutrition is associated with worse outcomes in sepsis-A nationwide analysis. J Acad Nutr Diet. (2019) 12:2069–84. doi: 10.1016/j.jand.2019.04.019
52. Moore FA, Feliciano D, Vrassy RJ, Mcardle AH, Booth FV. Morgenstein-Wagner, et al. Early enteral feeding, compared with parenteral, reduces postoperative septic complications: the results of a meta-analysis. Ann Surg. (1992) 2:172–83. doi: 10.1097/00000658-199208000-00008
53. Cruzat V, Macedo Rogero M, Noel Keane K, Curi R, Newsholme P. Glutamine: metabolism and immune function, supplementation and clinical translation. Nutrients. (2018) 10:1564. doi: 10.20944/preprints201809.0459.v1
54. Dimeji IY, Abass KS, Audu NM, Ayodeji AS. L-Arginine and immune modulation: a pharmacological perspective on inflammation and autoimmune disorders. Eur J Pharmacol. (2025) 997:177615. doi: 10.1016/j.ejphar.2025.177615
55. Hong K, Hun M, Wu F, Mao J, Wang Y, Zhu J, et al. Association between Omega-3 fatty acids and autoimmune disease: evidence from the umbrella review and Mendelian randomization analysis. Autoimmun Rev. (2024) 11:103651. doi: 10.1016/j.autrev.2024.103651
56. Wessels I, Maywald M, Rink L. Zinc as a gatekeeper of immune function. Nutrients. (2017) 9:1286. doi: 10.3390/nu9121286
57. Landucci F, Mancinelli P, De Gaudio AR, Virgili G. Selenium supplementation in critically ill patients: a systematic review and meta-analysis. J Crit Care. (2014) 1:150–6. doi: 10.1016/j.jcrc.2013.08.017
58. Wimalawansa SJ. Infections and autoimmunity-the immune system and vitamin D: a systematic review. Nutrients. (2023) 17:3842. doi: 10.3390/nu15173842
59. Carr AC, Maggini S. Vitamin C and immune function. Nutrients. (2017) 11:1211. doi: 10.3390/nu9111211
60. Ayers P, Adams S, Boullata J, Gervasio J, Holcombe B, Kraft MD, et al. A.S.P.E.N. parenteral nutrition safety consensus recommendations. JPEN J Parenter Enteral Nutr. (2014) 3:296–333. doi: 10.1177/0148607113511992
61. Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. (2014) 1:121–41. doi: 10.1016/j.cell.2014.03.011
62. Manzanares W, Lemieux M, Langlois PL, Wischmeyer PE. Probiotic and synbiotic therapy in critical illness: a systematic review and meta-analysis. Crit Care. (2016) 19:262. doi: 10.1186/s13054-016-1434-y
63. Fulop T, Larbi A, Dupuis G, Le Page A, Frost EH, Cohen AA, et al. Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front Immunol. (2018) 8:1960. doi: 10.3389/fimmu.2017.01960
64. Singer P, Blaser AR, Berger MM, Alhazzani W, Calder PC, Casaer MP, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. (2019) 1:48–79. doi: 10.1016/j.clnu.2018.08.037
65. Adiamah A, Skorepa P, Weimann A, Lobo DN. The impact of preoperative immune modulating nutrition on outcomes in patients undergoing surgery for gastrointestinal cancer: a systematic review and meta-analysis. Ann Surg. (2019) 2:247–56. doi: 10.1097/SLA.0000000000003256
66. Pache B, Grass F, Hubner M, Kefleyesus A, Mathevet P, Achtari C. Prevalence and consequences of preoperative weight loss in gynecologic surgery. Nutrients. (2019) 11:1094. doi: 10.3390/nu11051094
67. Chacon E, Vilchez V, Eman P, Marti F, Morris-Stiff G, Dugan A, et al. Effect of critical care complications on perioperative mortality and hospital length of stay after hepatectomy: a multicenter analysis of 21,443 patients. Am J Surg. (2019) 1:151–6. doi: 10.1016/j.amjsurg.2018.11.016
68. Ho JW, Wu AH, Lee MW, Lau SY, Lam PS, Lau WS, et al. Malnutrition risk predicts surgical outcomes in patients undergoing gastrointestinal operations: results of a prospective study. Clin Nutr. (2015) 4:679–84. doi: 10.1016/j.clnu.2014.07.012
69. Andreyev HJ, Norman AR, Oates J, Cunningham D. Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur J Cancer. (1998) 4:503–9. doi: 10.1016/S0959-8049(97)10090-9
70. Wischmeyer PE, Carli F, Evans DC, Guilbert S, Kozar R, Pryor A, et al. American Society for Enhanced Recovery and Perioperative Quality Initiative Joint Consensus Statement on Nutrition Screening and Therapy Within a Surgical Enhanced Recovery Pathway. Anesth Analg. (2018) 6:1883–95. doi: 10.1213/ANE.0000000000002743
71. Rinninella E, Borriello R, D'angelo M, Galasso T, Cintoni M, Raoul P, et al. COntrolling NUTritional Status (CONUT) as Predictive Score of Hospital Length of Stay (LOS) and Mortality: a prospective cohort study in an internal medicine and gastroenterology unit in Italy. Nutrients. (2023) 15:1472. doi: 10.3390/nu15061472
Keywords: nutrition support team, malnutrition, gastric cancer, older adults, recovery
Citation: Qiu L, Lin Z, Yang L, Li M and Cheng L (2025) Effectiveness of nutrition support team-led care on perioperative outcomes in malnourished older adults with gastric cancer. Front. Nutr. 12:1707892. doi: 10.3389/fnut.2025.1707892
Received: 18 September 2025; Revised: 21 November 2025;
Accepted: 24 November 2025; Published: 10 December 2025.
Edited by:
Katarzyna Milana Broczek, Maria Sklodowska-Curie National Research Institute of Oncology, PolandReviewed by:
Bing Feng, Pennington Biomedical Research Center, United StatesVasilios Lygizos, Alexandra General Hospital, Greece
Copyright © 2025 Qiu, Lin, Yang, Li and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Miao Li, bGltaWFvZHlleS0xN0BkbXUuZWR1LmNu; Lichun Cheng, Y2hlbmdkYW9qaW5nLTg4QDE2My5jb20=
†These authors share first authorship
 Lulu Qiu1†
Lulu Qiu1† Le Yang
Le Yang Miao Li
Miao Li