- 1Renal Division, Department of Medicine, Heilongjiang Academy of Chinese Medicine Sciences, Harbin, China
- 2Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 3Jiangxi University of Chinese Medicine, Nanchang, Jiangxi, China
Diabetic kidney disease (DKD), a prevalent microvascular complication of diabetes, is driven by a complex pathogenesis. A key pathological hallmark of early DKD is intrarenal lipid deposition, a process pivotally driven by impaired cholesterol efflux. This efflux is critically mediated by ATP-binding cassette (ABC) transporters, primarily ABCA1 and ABCG1. Dysfunction of these transporters precipitates cholesterol accumulation in renal cells, subsequently inducing oxidative stress, inflammation, and fibrosis. Recently, a diverse range of herbal monomers has emerged as a promising class of therapeutic agents for DKD. Compounds—including Anthocyanins, Morroniside, Resveratrol, Tanshinone, Puerarin, Baicalin, Curcumin, Protocatechuic acid, and Kaempferol—have been shown to activate the PPARγ and LXRα signaling pathways. This activation upregulates the expression of ABCA1 and ABCG1, thereby enhancing cholesterol efflux, mitigating renal lipid deposition, and ultimately slowing DKD progression. However, this body of research is largely limited to preclinical studies in vitro and in animal models. Consequently, the complex, multi-target mechanisms of these compounds in vivo remain poorly understood. Future investigations should therefore leverage multi-omics technologies to comprehensively delineate these mechanisms. Furthermore, large-scale clinical trials are imperative to validate the therapeutic efficacy and safety of these agents, potentially establishing novel strategies for the prevention and treatment of DKD.
1 Introduction
Diabetic kidney disease (DKD), a major microvascular complication of diabetes, is a leading cause of end-stage renal disease (ESRD). Affecting approximately one-third of individuals with diabetes worldwide, the incidence of DKD is escalating in parallel with the global diabetes pandemic (1–3). The disease is clinically characterized by an initial presentation of proteinuria that progresses to nephrotic syndrome and, ultimately, renal failure (4). While its progression is driven by multiple factors, including persistent hyperglycemia and hypertension, dyslipidemia has emerged as a particularly critical and modifiable driver, making its management a key therapeutic strategy (5). However, current therapeutic regimens, which primarily target glycemic and blood pressure control, have proven insufficient to halt disease progression in many patients (6, 7).
Growing evidence implicates intrarenal lipid accumulation as a central pathogenic mechanism in DKD (8–10). Specifically, this accumulation is driven by impaired cholesterol efflux—the process of transporting intracellular cholesterol to extracellular acceptors like apolipoprotein A-I (apoA-I) via ATP-binding cassette (ABC) transporters (11, 12). The transporter ABCA1 is a master regulator of this process; its dysfunction leads to cholesterol overload in podocytes and tubular epithelial cells. This lipid toxicity triggers a cascade of deleterious events, including oxidative and endoplasmic reticulum stress (ERS), inflammation, and ultimately, renal fibrosis (13, 14). This pathogenic cascade is further substantiated by evidence linking ABCA1 dysfunction to cardiolipin-driven mitochondrial damage and by clinical observations where reduced cholesterol efflux in DKD patients accelerates disease progression (15, 16). Preclinical validation comes from animal models, where podocyte-specific Abca1 knockout exacerbates glomerulosclerosis, whereas treatment with ABCA1 agonists like cyclodextrin ameliorates DKD pathology (14). Collectively, these findings establish the impairment of ABCA1-mediated cholesterol efflux as a critical, early event in DKD pathogenesis.
Given this complex pathophysiology, therapeutic strategies capable of addressing multiple targets are highly sought after. Traditional Chinese medicine (TCM) offers a promising source of such agents, valued for its multi-component, multi-target nature and favorable safety profiles (17–19). Indeed, isolated monomers from TCM have shown efficacy in DKD by modulating diverse pathological processes, including inflammation, hyperglycemia, and insulin resistance (20–23). Critically, a key emerging mechanism is their ability to regulate cholesterol efflux by modulating ABC transporter expression. Despite growing interest, a systematic synthesis of this specific mechanism is currently lacking. This review, therefore, consolidates and analyzes the existing evidence on how TCM-derived monomers modulate ABC transporters to treat DKD, providing a mechanistic framework summarized in Table 1 and Figure 1.
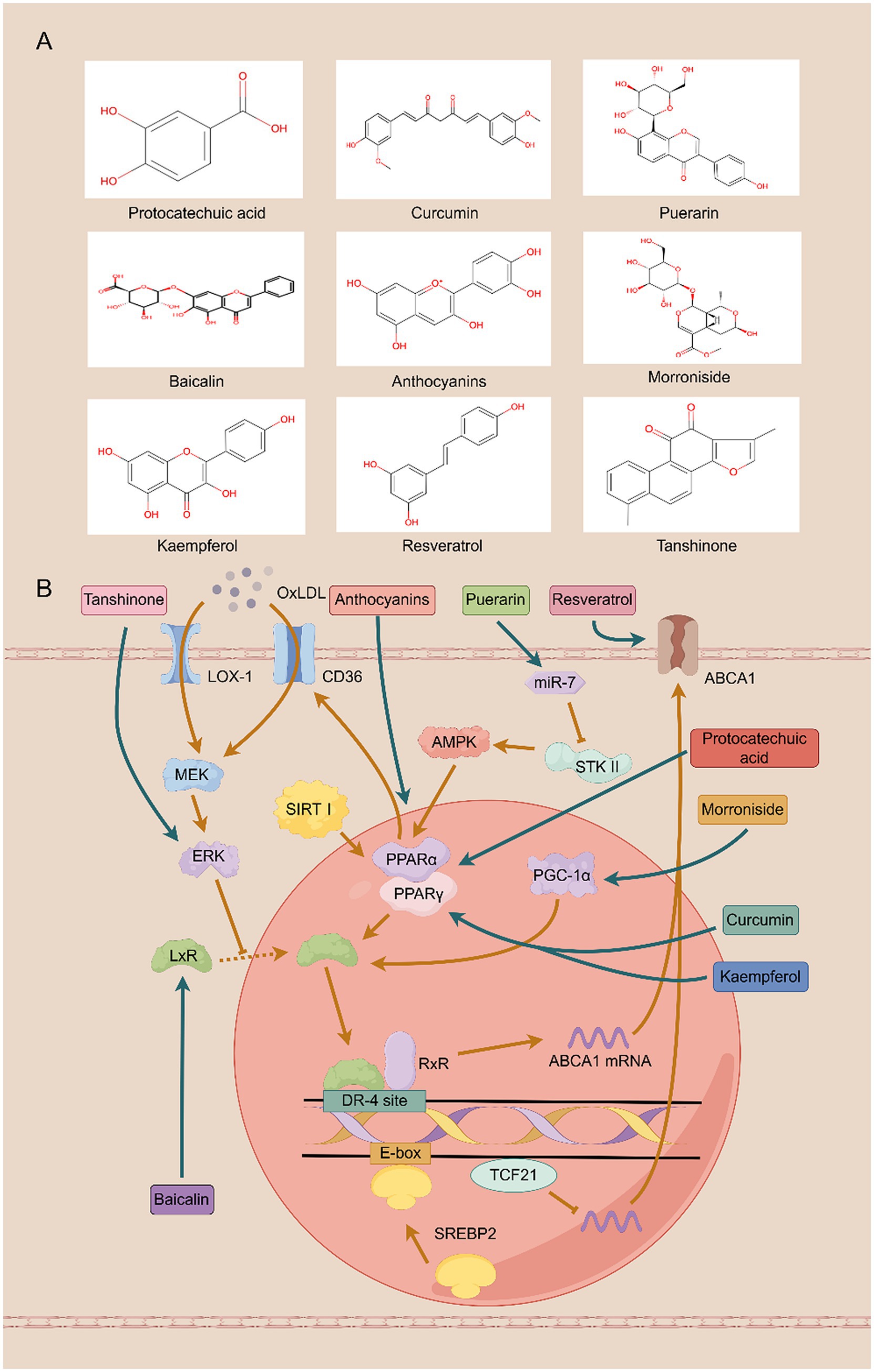
Figure 1. Traditional Chinese medicine monomers protect against diabetic kidney disease by improving cholesterol efflux. (A) The structure of the compound. (B) The mechanism involved in cholesterol efflux.
2 Traditional Chinese medicine monomers regulate cholesterol efflux
2.1 Treatment of DKD by direct action
Anthocyanins, a class of pleiotropic flavonoids widely found in plants, manifest potent renal protective effects by modulating inflammation, oxidative stress, and metabolic homeostasis (24). Mechanistically, they suppress the nuclear factor kappa B (NF-κB) signaling pathway, which in turn attenuates the expression of pro-inflammatory mediators, including tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and monocyte chemotactic protein-1 (MCP-1) in renal tissues. This anti-inflammatory action has been validated in vitro, where anthocyanins dose-dependently inhibit palmitic acid-induced cytokine expression and NF-κB activity in human renal tubular epithelial cells (25). Furthermore, anthocyanins counteract oxidative stress and fibrosis by upregulating core components of the glutathione (GSH) antioxidant system (GCLC, GCLM) while concurrently inhibiting key fibrotic signaling molecules such as transforming growth factor-β1 (TGF-β1), α-smooth muscle actin (α-SMA), and Collagen IV (26). In the context of glucose homeostasis, they modulate intestinal absorption by inhibiting sodium-glucose cotransporter 1 (SGLT1) and glucose transporter 2 (GLUT2) and enhance systemic glucose disposal by promoting AMP-activated protein kinase (AMPK)-mediated glucose transporter 4 translocation, thereby improving insulin sensitivity (27). Building on these metabolic benefits, recent work has identified a critical role for anthocyanins in regulating lipid metabolism. These compounds activate the peroxisome proliferator-activated receptor alpha (PPARα) / liver X receptor alpha (LXRα) signaling axis, leading to the upregulation of ABCA1. This, in turn, promotes cholesterol efflux, reduces intrarenal lipid deposition, and slows the progression of renal fibrosis. Crucially, the functional necessity of this pathway was confirmed in experiments where pharmacological or genetic inhibition of either PPARα or LXRα completely abrogated the protective effects of anthocyanins (28).
Morroniside, the principal bioactive iridoid glycoside derived from the medicinal herb Cornus officinalis, exhibits a spectrum of organ-protective functions, including potent nephroprotection (29). Its renoprotective effects are multifaceted. Within podocytes, it restores cellular homeostasis by modulating autophagy via mechanistic target of rapamycin (mTOR) inhibition and by suppressing NOX4-mediated reactive oxygen species (ROS) accumulation (30). Concurrently, morroniside counteracts glomerulosclerosis by blocking the interaction between advanced glycation end products (AGEs) and their receptor (RAGE). This action disrupts downstream pro-fibrotic signaling cascades (p38/MAPK/NF-κB/TGF-β), thereby curtailing mesangial cell proliferation and extracellular matrix deposition (31). Beyond these established mechanisms, morroniside fundamentally reshapes the renal lipid landscape in DKD by downregulating the master lipid regulators sterol regulatory element-binding protein 1/2 (SREBP-1/2) (32). It orchestrates a dual strategy to combat lipid accumulation. First, it promotes cholesterol efflux by activating the peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α)/LXRα signaling axis, leading to the transcriptional upregulation of the transporters ABCA1 and ABCG1 (11). Second, it simultaneously curtails cholesterol uptake by engaging the PGC-1α/PPARγ pathway to suppress the expression of the fatty acid translocase CD36 (33). This synergistic regulation—enhancing efflux while inhibiting influx—corrects intracellular lipid imbalance, preserves podocyte integrity, and robustly delays the progression of DKD.
2.2 Traditional Chinese medicine monomers with potential therapeutic value
Resveratrol, an extensively studied natural polyphenol found in medicinal herbs such as Polygonum cuspidatum and in dietary sources like grapes, exerts pleiotropic renoprotective effects (34). A primary mechanism involves its engagement of the pivotal metabolic regulators AMPK and sirtuin 1 (SIRT1), which in turn activate PGC-1α. This signaling cascade orchestrates a multi-pronged defense against lipotoxicity by upregulating adiponectin receptors 1 and 2 (AdipoR1/2) to reduce intracellular fatty acids and triglycerides, while simultaneously suppressing pro-apoptotic pathways (PI3K/Akt/FOXO3a) and bolstering antioxidant defenses (SOD1/2, BCL-2) (35, 36). Critically, beyond managing lipotoxicity, Resveratrol directly targets the machinery of cholesterol efflux. Resveratrol dose-dependently upregulates the expression of the key transporters ABCA1 and ABCG1, as well as the cholesterol-metabolizing enzyme 27-hydroxylase. This effect is mediated through the PPARγ signaling axis, a conclusion supported by experiments where the upregulation was abrogated by PPARγ antagonists (37). The convergence of these pathways—mitigating lipid-induced damage while actively promoting cholesterol removal—translates into significant attenuation of key DKD pathologies, including proteinuria, glomerular matrix expansion, and inflammation.
Salvia miltiorrhiza is a traditional Chinese medicinal herb with a long history of use. Tanshinones represent the most pharmacologically active class of compounds in Salvia miltiorrhiza (38), encompassing multiple related compounds such as Tanshinone I, Tanshinone IIA, and Tanshinone IIB (39). Tan IIA is known to exert broad renoprotective effects by concurrently mitigating inflammation, oxidative stress, endoplasmic reticulum (ER) stress, and pyroptosis. These actions collectively reduce renal fibrosis and preserve kidney function. Tanshinone IIA (Tan IIA) reduces inflammatory responses in the kidneys by inhibiting the expression of TGF-β1, MCP-1, and P-selectin, thereby decreasing the production of inflammatory mediators. It also alleviates oxidative stress induced by diabetes through lowering malondialdehyde (MDA) levels and increasing superoxide dismutase (SOD) activity, thus protecting the kidneys from oxidative damage (40). Animal studies demonstrate that Tan IIA also alleviates endoplasmic reticulum (ER) stress by inhibiting activation of the protein kinase RNA-like ER kinase (PERK) signaling pathway. This prevents phosphorylation of eukaryotic translation initiation factor 2 alpha, reduces levels of the transcription factor activating transcription factor 4 (ATF4), thereby downregulating TGF-β1 expression and decreasing extracellular matrix (ECM) accumulation, ultimately mitigating renal fibrosis in diabetic nephropathy (41). Furthermore, Tan IIA inhibits NOD-like receptor family pyridine domain-containing protein 3 (NLRP3), reduces cleavage of caspase-1 and interleukin-1β (IL-1β), indicating Tan IIA mitigates pyroptosis in human renal glomerular endothelial cells (HRGECs) by suppressing NLRP3 inflammasome activation (42). Recent studies reveal that Tan IIA inhibits scavenger receptor A (SR-A)-mediated uptake of oxidized low-density lipoprotein (oxLDL) and enhances ABCA1/G1-mediated cholesterol efflux by activating the ERK/Nrf2/HO-1 pathway and suppressing mRNA rapid decay, thereby mitigating foam cell formation damage to normal cells (43). This dual action—blocking lipid influx while promoting efflux—prevents the formation of lipid-laden foam cells within the renal microenvironment. This regulation of cholesterol trafficking is now understood as a critical mechanism through which Tan IIA preserves renal cell integrity and slows the progression of DKD.
Puerarin, the primary active component extracted from Pueraria root, belongs to the isoflavone class. As a natural antioxidant, Puerarin possesses significant health benefits, exhibiting a range of biological activities including antioxidant, anti-inflammatory, antitumor effects, immune enhancement, and protection of cardiovascular and neural cells (44, 45). In vitro experiments demonstrated that Puerarin significantly reduced the expression of pro-inflammatory cytokines IL-1β, IL-6, IL-18, and TNFα, with the most pronounced inhibition observed for IL-1β. In vivo studies revealed that Puerarin suppresses vascular inflammation, mitigates vascular calcification, and delays chronic kidney disease progression by inhibiting NLRP3 inflammasome activation, thereby reducing Caspase1 activation and IL-1β production (46). Furthermore, Puerarin enhances NF-κB inhibition by activating SIRT1 to reduce p65 acetylation, thereby suppressing NF-κB-mediated expression of other inflammatory factors and protecting glomerular function. Combining Puerarin with arctigenin potentiates their renal protective effects (47). Building on this established anti-inflammatory profile, recent studies have elucidated a sophisticated molecular mechanism by which Puerarin directly regulates lipid homeostasis. Puerarin downregulates microRNA-7 (miR-7), a microRNA that post-transcriptionally silences serine/threonine kinase 11 (STK11) by targeting its 3′ untranslated region (3′ UTR). This de-repression of STK11 unleashes the downstream AMPK/PPARγ/LXRα signaling cascade, culminating in the transcriptional upregulation of ABCA1. The resulting enhancement of cholesterol efflux from macrophages effectively reduces intracellular lipid accumulation and lipotoxicity (48). This microRNA-mediated regulation of cholesterol trafficking represents a key mechanism by which Puerarin may mitigate the lipotoxic component of DKD.
Baicalin (BAI), a principal flavonoid isolated from Scutellaria baicalensis, is recognized for its potent renoprotective properties, which stem from its ability to concurrently disarm pro-inflammatory signaling and neutralize oxidative stress (49). BAI inhibits activation of the MAPK signaling pathway by reducing the phosphorylation levels of extracellular signal-regulated kinase 1/2 (ERK1/2), c-Jun N-terminal kinase (JNK), and p38 mitogen-activated protein kinase (p38) phosphorylation levels. This downregulates mRNA levels of inflammatory cytokines (IL-1β, IL-6, MCP-1, and TNFα), thereby alleviating renal fibrosis (50). Concurrently, it bolsters cellular antioxidant defenses by upregulating key enzymes such as SOD and GSH-px, thereby mitigating oxidative damage (51). Crucially, the therapeutic efficacy of BAI in DKD is now understood to be critically dependent on its direct regulation of cholesterol homeostasis. In vitro studies have demonstrated that BAI drives the expression of the master lipid regulators PPARγ and LXRα at both the mRNA and protein levels. This, in turn, transcriptionally upregulates the cholesterol transporters ABCA1 and ABCG1 to facilitate robust cholesterol efflux and ameliorate cellular lipotoxicity. The indispensability of this axis is confirmed by gene-silencing experiments, where siRNA-mediated knockdown of either PPARγ or LXRα completely abrogated the BAI-induced upregulation of ABCA1 and ABCG1 (52). Therefore, Baicalin’s capacity to simultaneously resolve inflammation and correct dysregulated lipid metabolism positions it as a compelling therapeutic candidate for DKD.
Turmeric, a spice native to India, has a long history of use in China. Curcumin, an active polyphenolic compound extracted from turmeric, exhibits anti-inflammatory, anticancer, anti-aging, and blood glucose-regulating effects (53, 54). It effectively neutralizes multiple reactive oxygen species and suppresses the master inflammatory transcription factor NF-κB, providing a broad cellular defense against metabolic stressors (55). In the specific context of DKD, a key mechanism underlying its renoprotective action involves its ability to thwart the pathogenic actions of the chemokine CXCL16 in podocytes. Under diabetic conditions, CXCL16 drives lipotoxicity by concurrently suppressing the lipid sensor PPARγ and upregulating the pro-inflammatory enzyme cyclooxygenase-2 (COX2). Curcumin intervenes directly by inhibiting CXCL16 expression, an action that prevents podocyte lipid accumulation and apoptosis (56). The centrality of this target is underscored by gene-silencing studies, where CXCL16 knockdown not only phenocopied Curcumin’s protective effects but also completely abrogated its regulatory influence on PPARγ and COX2. This CXCL16-mediated rescue of PPARγ subsequently unleashes the canonical LXRα-ABCA1 signaling axis, culminating in the enhanced expression of the ABCA1 transporter and its partner, apoA-I, to drive cholesterol efflux (55, 57). Therefore, Curcumin’s capacity to dismantle this specific, lipotoxic CXCL16-PPARγ signaling cascade illuminates a critical pathway through which it restores podocyte lipid homeostasis and slows DKD progression.
Protocatechuic acid (PCA) is a naturally occurring phenolic acid widely present in our daily diet and herbal medicines. It is also one of the primary metabolites of complex polyphenols such as Anthocyanins and proanthocyanidins (58). Protocatechuic acid exerts functions including antioxidant, anti-inflammatory, and protective effects on the cardiovascular system, liver, and kidneys (59). Recent mechanistic studies have pinpointed a sophisticated regulatory role for PCA in cholesterol homeostasis. In pathological contexts, microRNA-10b (miRNA-10b) acts as a post-transcriptional repressor of the cholesterol transporters ABCA1 and ABCG1, driving lipid accumulation and cellular lipotoxicity. PCA directly counteracts this process by suppressing the expression of miRNA-10b. This de-repression restores the functional expression of ABCA1 and ABCG1, thereby re-establishing efficient cholesterol efflux from macrophages (60). This targeted regulation of lipid metabolism complements PCA’s systemic benefits observed in diabetic animal models. There, PCA administration not only improves key metabolic parameters—reducing blood glucose and BUN while enhancing insulin levels and creatinine clearance—but also directly modulates renal gene expression. Specifically, it restores the expression of the crucial lipid sensors PPARα and PPARγ and suppresses the expression of the pro-inflammatory receptor for advanced glycation end-products (RAGE) (61). Therefore, PCA’s therapeutic potential in DKD stems from a dual-front attack: its ability to precisely target the miRNA-10b/ABCA1 axis to resolve cellular lipotoxicity and its capacity to concurrently improve systemic glycemic control and suppress renal inflammation.
Kaempferol (KPF), a prominent flavonoid abundant in various medicinal herbs, is renowned for a spectrum of therapeutic properties, including potent anti-diabetic and renoprotective activities (62, 63). At the level of renal histopathology, KPF directly counters the structural degradation characteristic of DKD. In vivo studies demonstrate that KPF reverses key pathological hallmarks, including mesangial matrix expansion, podocyte foot process effacement, and glomerular basement membrane thickening. This structural preservation is driven by its ability to restore cellular homeostasis. KPF recalibrates the central energy sensor AMP-activated protein kinase (AMPK), which in turn restrains the anabolic mTOR pathway to suppress aberrant cell growth and unleashes protective autophagy, a critical cellular quality control mechanism (64). A significant portion of KPF’s efficacy is mediated by its active metabolite, kaempferol-3-O-gallate, which orchestrates a dual correction of the dysregulated lipid and glucose metabolism central to DKD. It systematically improves the lipid profile and enhances insulin sensitivity in diabetic models. Mechanistically, this metabolite stimulates cholesterol efflux by activating the canonical PPARγ/LXRα/ABCA1 signaling cascade. Concurrently, it enhances insulin signaling by engaging a distinct PPARγ-PI3K/AKT pathway to improve cellular glucose uptake (65). Therefore, the therapeutic potential of kaempferol in DKD arises from a sophisticated, dual-component mechanism: the parent flavonoid preserves renal architecture and cellular integrity via the AMPK-autophagy axis, while its key metabolite systemically corrects the underlying metabolic pathologies of lipid and glucose dysregulation.
3 Summary and outlook
The chronic lipotoxic and glucotoxic milieu of DKD progressively suppresses the expression of pivotal cholesterol transporters, thereby trapping lipids within renal cells and driving a relentless cycle of cellular injury and disease progression. The evidence presented herein delineates a compelling therapeutic strategy: the targeted reactivation of this cholesterol efflux machinery by a diverse pharmacopeia of herbal monomers. By engaging key regulatory nodes such as the PPARγ/LXRα axis, these natural compounds restore the function of ABCA1/G1, offering a powerful mechanism to mitigate lipotoxicity and preserve renal function.
However, translating this preclinical promise into clinical reality requires surmounting critical knowledge gaps. Current research, while mechanistically insightful, has been largely constrained to a “single-target, single-pathway” paradigm, focusing predominantly on the ABCA1 axis. This approach fails to capture the holistic, multi-target synergy that likely underpins the efficacy of these pleiotropic compounds. Current studies have also revealed several limitations of natural products, particularly their poor oral bioavailability and rapid metabolic clearance. Future research may address these issues through the use of advanced delivery systems such as nanocarriers (e.g., solid lipid nanoparticles and SEDDS), phospholipid complexes (phytosomes), or co-administration with metabolic inhibitors like piperine to markedly enhance bioavailability (66–68). Furthermore, the field has certain limitations in terms of preclinical research models. In in vitro experiments, immortalized cell lines (e.g., HK-2 cells) and primary cells derived from non-diabetic individuals fail to replicate the chronic stress state associated with human DKD. In animal experiments, streptozotocin-induced diabetic models and db/db mouse models exhibit notable differences from humans in both pathological characteristics and physiological mechanisms. More critically, the severity and progression rate of human DKD are significantly influenced by age, comorbidities, and genetic background, and existing models fail to recapitulate the heterogeneity of human DKD. In the future, priority should be given to the adoption of humanized models, combined with multi-omics technologies, to enhance the reliability of research findings.
The path forward must therefore be twofold. First, a paradigm shift toward systems-level inquiry is essential. The application of multi-omics technologies (e.g., transcriptomics, proteomics, metabolomics) is imperative to comprehensively map the network-wide effects of these compounds in vivo, deciphering their true multi-target mechanisms beyond the established ABC transporter axis. Secondly, it is necessary to conduct large-scale screening of traditional Chinese medicine monomers that have been proven to have cholesterol efflux regulatory activity, in order to identify candidate compounds with significant therapeutic effects, good pharmacokinetic characteristics, and low toxicity. And validate its efficacy and safety in the humanized DKD model to better simulate human pathological states. Upon establishing robust preclinical evidence of these network-level effects, the ultimate validation must come from rigorously designed, large-scale, multicenter, randomized controlled trials. These trials are indispensable for definitively assessing the clinical efficacy and safety of the most promising candidates. Such a strategy will not only illuminate novel biology but also pave the way for a new class of evidence-based, mechanism-driven therapeutics for the prevention and treatment of DKD.
Author contributions
CW: Writing – original draft. JSu: Writing – original draft. JSh: Writing – original draft. ZL: Supervision, Writing – original draft. YA: Supervision, Writing – original draft. HZ: Writing – review & editing. LW: Writing – review & editing. PL: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the National Natural Science Foundation of China (Nos. 82274489, 82575000).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gupta, S, Dominguez, M, and Golestaneh, L. Diabetic kidney disease: an update. Med Clin North Am. (2023) 107:689–705. doi: 10.1016/j.mcna.2023.03.004
2. Liu, D, Chen, X, He, W, Lu, M, Li, Q, Zhang, S, et al. Update on the pathogenesis, diagnosis, and treatment of diabetic tubulopathy. Integr Med Nephrol Androl. (2024) 11:e23. doi: 10.1097/IMNA-D-23-00029
3. Zhao, H, Li, Z, Yan, M, Ma, L, Dong, X, Li, X, et al. Irbesartan ameliorates diabetic kidney injury in db/db mice by restoring circadian rhythm and cell cycle. J Transl Intern Med. (2024) 12:157–69. doi: 10.2478/jtim-2022-0049
4. Wal, P, Tyagi, S, Pal, RS, Yadav, A, and Jaiswal, R. A strategic investigation on diabetic nephropathy; its conceptual model and clinical manifestations: a review. Curr Diabetes Rev. (2023) 19:e260422204036. doi: 10.2174/1573399818666220426091238
5. Deng, Y, Zhu, H, Xing, J, Gao, J, Duan, J, Liu, P, et al. The role of natural products in improving lipid metabolism disorder-induced mitochondrial dysfunction of diabetic kidney disease. Front Physiol. (2025) 16:1624077. doi: 10.3389/fphys.2025.1624077
6. Samsu, N. Diabetic nephropathy: challenges in pathogenesis, diagnosis, and treatment. Biomed Res Int. (2021) 2021:1497449. doi: 10.1155/2021/1497449
7. Chung, JY-F, Lan, H-Y, and Tang, PM-K. New insights into traditional Chinese medicine in treatment of diabetic nephropathy. Integr Med Nephrol Androl. (2023) 10:e00026. doi: 10.1097/IMNA-D-22-00026
8. Zhao, H, Zhao, T, and Li, P. Gut microbiota-derived metabolites: a new perspective of traditional Chinese medicine against diabetic kidney disease. Integr Med Nephrol Androl. (2024) 11:e23. doi: 10.1097/IMNA-D-23-00024
9. Liu, P, Zhu, W, Wang, Y, Ma, G, Zhao, H, and Li, P. Chinese herbal medicine and its active compounds in attenuating renal injury via regulating autophagy in diabetic kidney disease. Front Endocrinol. (2023) 14:1142805. doi: 10.3389/fendo.2023.1142805
10. Ma, L, Feng, Z, and Li, P. New insights into the use of traditional Chinese medicine for treating diabetic kidney disease by regulating DNA methylation. Integr Med Nephrol Androl. (2024) 11:e24. doi: 10.1097/IMNA-D-24-00018
11. Gao, J, Liu, P, Shen, Z, Xu, K, Wu, C, Tian, F, et al. Morroniside promotes PGC-1α-mediated cholesterol efflux in sodium palmitate or high glucose-induced mouse renal tubular epithelial cells. Biomed Res Int. (2021) 2021:9942152. doi: 10.1155/2021/9942152
12. Liu, P, Peng, L, Zhang, H, Tang, PM-K, Zhao, T, Yan, M, et al. Tangshen formula attenuates diabetic nephropathy by promoting ABCA1-mediated renal cholesterol efflux in db/db mice. Front Physiol. (2018) 9:343. doi: 10.3389/fphys.2018.00343
13. Schelling, JR. The contribution of lipotoxicity to diabetic kidney disease. Cells. (2022) 11:3236. doi: 10.3390/cells11203236
14. Zhang, J, Wu, Y, Zhang, J, Zhang, R, Wang, Y, and Liu, F. ABCA1 deficiency-mediated glomerular cholesterol accumulation exacerbates glomerular endothelial injury and dysfunction in diabetic kidney disease. Metab Clin Exp. (2023) 139:155377. doi: 10.1016/j.metabol.2022.155377
15. Ducasa, GM, Mitrofanova, A, Mallela, SK, Liu, X, Molina, J, Sloan, A, et al. ATP-binding cassette A1 deficiency causes cardiolipin-driven mitochondrial dysfunction in podocytes. J Clin Invest. (2019) 129:3387–400. doi: 10.1172/JCI125316
16. Liu, P, Ma, L, Zhao, H, Shen, Z, Zhou, X, Yan, M, et al. Association between LXR-α and ABCA1 gene polymorphisms and the risk of diabetic kidney disease in patients with type 2 diabetes mellitus in a Chinese han population. J Diabetes Res. (2020) 2020:8721536. doi: 10.1155/2020/8721536
17. Zhong, X, Jia, J, Tan, R, and Wang, L. Hederagenin improves adriamycin-induced nephropathy by inhibiting the JAK/STAT signaling pathway. Integr Med Nephrol Androl. (2024) 11:e22. doi: 10.1097/IMNA-D-22-00016
18. Wang, Y, Liu, P, Ma, G, Wu, C, Zhu, W, Sun, P, et al. Mechanism of dioscin ameliorating renal fibrosis through NF-κB signaling pathway-mediated inflammatory response. Mol Med Rep. (2023) 27:93. doi: 10.3892/mmr.2023.12980
19. Jian, J, Yu-Qing, L, Rang-Yue, H, Xia, Z, Ke-Huan, X, Ying, Y, et al. Isorhamnetin ameliorates cisplatin-induced acute kidney injury in mice by activating SLPI-mediated anti-inflammatory effect in macrophage. Immunopharmacol Immunotoxicol. (2024) 46:319–29. doi: 10.1080/08923973.2024.2329621
20. Yan, Y, Tan, R, Liu, P, Li, J, Zhong, X, Liao, Y, et al. Oridonin alleviates IRI-induced kidney injury by inhibiting inflammatory response of macrophages via AKT-related pathways. Med Sci Monit. (2020) 26:e921114. doi: 10.12659/MSM.921114
21. Jia, J, Tan, R, Xu, L, Wang, H, Li, J, Su, H, et al. Hederagenin improves renal fibrosis in diabetic nephropathy by regulating Smad3/NOX4/SLC7A11 signaling-mediated tubular cell ferroptosis. Int Immunopharmacol. (2024) 135:112303. doi: 10.1016/j.intimp.2024.112303
22. Wang, J, Zhang, R, Wu, C, Wang, L, Liu, P, and Li, P. Exploring potential targets for natural product therapy of DN: the role of SUMOylation. Front Pharmacol. (2024) 15:1432724. doi: 10.3389/fphar.2024.1432724
23. Tong, G, Wang, S, Gao, G, He, Y, Li, Q, Yang, X, et al. The efficacy of sulodexide combined with jinshuibao for treating early diabetic nephropathy patients. Int J Clin Exp Med. (2020) 13:8308–831.
24. Tiscornia, C, Tapia, V, Águila, D, Lorca-Ponce, E, Aicardi, V, and Vásquez, F. Maqui and chronic kidney disease: a narrative review on the potential nephroprotective role of anthocyanins. Nutrients. (2025) 17:1058. doi: 10.3390/nu17061058
25. Madduma Hewage, S, Prashar, S, Debnath, SC, K, O, and Siow, YL. Inhibition of inflammatory cytokine expression prevents high-fat diet-induced kidney injury: role of lingonberry supplementation. Front Med. (2020) 7:80. doi: 10.3389/fmed.2020.00080
26. Qin, Y, Zhai, Q, Li, Y, Cao, M, Xu, Y, Zhao, K, et al. Cyanidin-3-O-glucoside ameliorates diabetic nephropathy through regulation of glutathione pool. Biomed Pharmacother. (2018) 103:1223–30. doi: 10.1016/j.biopha.2018.04.137
27. Solverson, P. Anthocyanin bioactivity in obesity and diabetes: the essential role of glucose transporters in the gut and periphery. Cells. (2020) 9:2515. doi: 10.3390/cells9112515
28. Du, C, Shi, Y, Ren, Y, Wu, H, Yao, F, Wei, J, et al. Anthocyanins inhibit high-glucose-induced cholesterol accumulation and inflammation by activating LXRα pathway in HK-2 cells. Drug Des Dev Ther. (2015) 9:5099–113. doi: 10.2147/DDDT.S90201
29. Wu, C, Wang, J, Zhang, R, Zhao, H, Li, X, Wang, L, et al. Research progress on cornus officinalis and its active compounds in the treatment of diabetic nephropathy. Front Pharmacol. (2023) 14:1207777. doi: 10.3389/fphar.2023.1207777
30. Gao, X, Liu, Y, Wang, L, Sai, N, Liu, Y, and Ni, J. Morroniside inhibits H2O2-induced podocyte apoptosis by down-regulating NOX4 expression controlled by autophagy in vitro. Front Pharmacol. (2020) 11:533809. doi: 10.3389/fphar.2020.533809
31. Lv, G, Lv, X, Tao, Y, and Xu, H. Effect of morroniside on glomerular mesangial cells through AGE-RAGE pathway. Hum Cell. (2016) 29:148–54. doi: 10.1007/s13577-015-0128-0
32. Zhu, W, Chen, M, Wang, Y, Chen, Y, Zhang, Y, Wang, Y, et al. Regulation of renal lipid deposition in diabetic nephropathy on morroniside via inhibition of NF-KB/TNF-a/SREBP1c signaling pathway. Chem Biol Interact. (2023) 385:110711. doi: 10.1016/j.cbi.2023.110711
33. Chen, Y, Chen, M, Zhu, W, Zhang, Y, Liu, P, and Li, P. Morroniside attenuates podocytes lipid deposition in diabetic nephropathy: a network pharmacology, molecular docking and experimental validation study. Int Immunopharmacol. (2024) 138:112560. doi: 10.1016/j.intimp.2024.112560
34. Tian, B, and Liu, J. Resveratrol: a review of plant sources, synthesis, stability, modification and food application. J Sci Food Agric. (2020) 100:1392–404. doi: 10.1002/jsfa.10152
35. Kim, MY, Lim, JH, Youn, HH, Hong, YA, Yang, KS, Park, HS, et al. Resveratrol prevents renal lipotoxicity and inhibits mesangial cell glucotoxicity in a manner dependent on the AMPK-SIRT1-PGC1α axis in db/db mice. Diabetologia. (2013) 56:204–17. doi: 10.1007/s00125-012-2747-2
36. Park, HS, Lim, JH, Kim, MY, Kim, Y, Hong, YA, Choi, SR, et al. Resveratrol increases Adipo R1 and AdipoR2 expression in type 2 diabetic nephropathy. J Transl Med. (2016) 14:176. doi: 10.1186/s12967-016-0922-9
37. Voloshyna, I, Hai, O, Littlefield, MJ, Carsons, S, and Reiss, AB. Resveratrol mediates anti-atherogenic effects on cholesterol flux in human macrophages and endothelium via PPARγ and adenosine. Eur J Pharmacol. (2013) 698:299–309. doi: 10.1016/j.ejphar.2012.08.024
38. Li, Z, Xu, S, and Liu, P. Salvia miltiorrhiza Burge (danshen): a golden herbal medicine in cardiovascular therapeutics. Acta Pharmacol Sin. (2018) 39:802–24. doi: 10.1038/aps.2017.193
39. Zhou, L, Zuo, Z, and Chow, MSS. Danshen: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharmacol. (2005) 45:1345–59. doi: 10.1177/0091270005282630
40. Chen, X, Wu, R, Kong, Y, Yang, Y, Gao, Y, Sun, D, et al. Tanshinone II aattenuates renal damage in STZ-induced diabetic rats via inhibiting oxidative stress and inflammation. Oncotarget. (2017) 8:31915–22. doi: 10.18632/oncotarget.16651
41. Xu, S, He, L, Ding, K, Zhang, L, Xu, X, Wang, S, et al. Tanshinone IIA ameliorates streptozotocin-induced diabetic nephropathy, partly by attenuating PERK pathway-induced fibrosis. Drug Des Dev Ther. (2020) 14:5773–82. doi: 10.2147/DDDT.S257734
42. Wu, Q, Guan, Y-B, Zhang, K-J, Li, L, and Zhou, Y. Tanshinone IIA mediates protection from diabetes kidney disease by inhibiting oxidative stress induced pyroptosis. J Ethnopharmacol. (2023) 316:116667. doi: 10.1016/j.jep.2023.116667
43. Liu, Z, Wang, J, Huang, E, Gao, S, Li, H, Lu, J, et al. Tanshinone IIA suppresses cholesterol accumulation in human macrophages: role of heme oxygenase-1. J Lipid Res. (2014) 55:201–13. doi: 10.1194/jlr.M040394
44. Wang, D, Bu, T, Li, Y, He, Y, Yang, F, and Zou, L. Pharmacological activity, pharmacokinetics, and clinical research progress of puerarin. Antioxidants. (2022) 11:2121. doi: 10.3390/antiox11112121
45. Lai, W, Wang, B, Huang, R, Zhang, C, Fu, P, and Ma, L. Ferroptosis in organ fibrosis: from mechanisms to therapeutic medicines. J Transl Intern Med. (2024) 12:22–34. doi: 10.2478/jtim-2023-0137
46. Liu, H, Zhang, X, Zhong, X, Li, Z, Cai, S, Yang, P, et al. Puerarin inhibits vascular calcification of uremic rats. Eur J Pharmacol. (2019) 855:235–43. doi: 10.1016/j.ejphar.2019.05.023
47. Li, X, Wang, J, Yan, J, He, JC, Li, Y, and Zhong, Y. Additive renal protective effects between arctigenin and puerarin in diabetic kidney disease. Biomed Pharmacother. (2024) 171:116107. doi: 10.1016/j.biopha.2023.116107
48. Li, C-H, Gong, D, Chen, L-Y, Zhang, M, Xia, X-D, Cheng, H-P, et al. Puerarin promotes ABCA1-mediated cholesterol efflux and decreases cellular lipid accumulation in THP-1 macrophages. Eur J Pharmacol. (2017) 811:74–86. doi: 10.1016/j.ejphar.2017.05.055
49. Wen, Y, Wang, Y, Zhao, C, Zhao, B, and Wang, J. The pharmacological efficacy of baicalin in inflammatory diseases. Int J Mol Sci. (2023) 24:9317. doi: 10.3390/ijms24119317
50. Ma, L, Wu, F, Shao, Q, Chen, G, Xu, L, and Lu, F. Baicalin alleviates oxidative stress and inflammation in diabetic nephropathy via Nrf2 and MAPK signaling pathway. Drug Des Dev Ther. (2021) 15:3207–21. doi: 10.2147/DDDT.S319260
51. Li, X, Xu, R, Zhang, D, Cai, J, Zhou, H, Song, T, et al. Baicalin: a potential therapeutic agent for acute kidney injury and renal fibrosis. Front Pharmacol. (2025) 16:1511083. doi: 10.3389/fphar.2025.1511083
52. He, X-W, Yu, D, Li, W-L, Zheng, Z, Lv, C-L, Li, C, et al. Anti-atherosclerotic potential of baicalin mediated by promoting cholesterol efflux from macrophages via the PPARγ-LXRα-ABCA1/ABCG1 pathway. Biomed Pharmacother. (2016) 83:257–64. doi: 10.1016/j.biopha.2016.06.046
53. Marton, LT, Pescinini-E-Salzedas, LM, Camargo, MEC, Barbalho, SM, Haber, JFDS, Sinatora, RV, et al. The effects of curcumin on diabetes mellitus: a systematic review. Front Endocrinol. (2021) 12:669448. doi: 10.3389/fendo.2021.669448
54. Tan, R-Z, Liu, J, Zhang, Y-Y, Wang, H-L, Li, J-C, Liu, Y-H, et al. Curcumin relieved cisplatin-induced kidney inflammation through inhibiting mincle-maintained M1 macrophage phenotype. Phytomed. (2019) 52:284–94. doi: 10.1016/j.phymed.2018.09.210
55. Panahi, Y, Ahmadi, Y, Teymouri, M, Johnston, TP, and Sahebkar, A. Curcumin as a potential candidate for treating hyperlipidemia: a review of cellular and metabolic mechanisms. J Cell Physiol. (2018) 233:141–52. doi: 10.1002/jcp.25756
56. Chen, Y, Tao, J, He, Y, Hou, X, Fang, J, Huang, J, et al. Curcumin targets CXCL16-mediated podocyte injury and lipid accumulation in diabetic kidney disease treatment. Arch Pharm Res. (2024) 47:924–39. doi: 10.1007/s12272-024-01521-1
57. Dong, S, Zhao, S, Wu, Z, Yang, J, Xie, X, Yu, B, et al. Curcumin promotes cholesterol efflux from adipocytes related to PPARgamma-LXRalpha-ABCA1 passway. Mol Cell Biochem. (2011) 358:281–5. doi: 10.1007/s11010-011-0978-z
58. Song, J, He, Y, Luo, C, Feng, B, Ran, F, Xu, H, et al. New progress in the pharmacology of protocatechuic acid: a compound ingested in daily foods and herbs frequently and heavily. Pharmacol Res. (2020) 161:105109. doi: 10.1016/j.phrs.2020.105109
59. Zhang, S, Gai, Z, Gui, T, Chen, J, Chen, Q, and Li, Y. Antioxidant effects of protocatechuic acid and protocatechuic aldehyde: old wine in a new bottle. Evid Based Complement Altern Med. (2021) 2021:6139308. doi: 10.1155/2021/6139308
60. Wang, D, Xia, M, Yan, X, Li, D, Wang, L, Xu, Y, et al. Gut microbiota metabolism of anthocyanin promotes reverse cholesterol transport in mice via repressing mi RNA-10b. Circ Res. (2012) 111:967–81. doi: 10.1161/CIRCRESAHA.112.266502
61. Lin, C-Y, Tsai, S-J, Huang, C-S, and Yin, M-C. Antiglycative effects of protocatechuic acid in the kidneys of diabetic mice. J Agric Food Chem. (2011) 59:5117–24. doi: 10.1021/jf200103f
62. Calderón-Montaño, JM, Burgos-Morón, E, Pérez-Guerrero, C, and López-Lázaro, M. A review on the dietary flavonoid kaempferol. Mini-Rev Med Chem. (2011) 11:298–344. doi: 10.2174/138955711795305335
63. Periferakis, A, Periferakis, K, Badarau, IA, Petran, EM, Popa, DC, Caruntu, A, et al. Kaempferol: antimicrobial properties, sources, clinical, and traditional applications. Int J Mol Sci. (2022) 23:15054. doi: 10.3390/ijms232315054
64. Sheng, H, Zhang, D, Zhang, J, Zhang, Y, Lu, Z, Mao, W, et al. Kaempferol attenuated diabetic nephropathy by reducing apoptosis and promoting autophagy through AMPK/mTOR pathways. Front Med. (2022) 9:986825. doi: 10.3389/fmed.2022.986825
65. Tang, H, Zeng, Q, Tang, T, Wei, Y, and Pu, P. Kaempferide improves glycolipid metabolism disorder by activating PPARγ in high-fat-diet-fed mice. Life Sci. (2021) 270:119133. doi: 10.1016/j.lfs.2021.119133
66. Wang, T, Li, L, Liu, L, Tan, R, Wu, Q, Zhu, X, et al. Overview of pharmacodynamical research of traditional Chinese medicine on hyperuricemic nephropathy: from the perspective of dual-regulatory effect on the intestines and kidneys. Front Pharmacol. (2025) 16:1517047. doi: 10.3389/fphar.2025.1517047
67. Cao, Z, Liu, J, and Yang, X. Deformable nanocarriers for enhanced drug delivery and cancer therapy. Exploration. (2024) 4:20230037. doi: 10.1002/EXP.20230037
Keywords: diabetic kidney disease, cholesterol efflux, traditional Chinese medicine, ABCA1, PPARs
Citation: Wu C, Sun J, Shi J, Li Z, An Y, Zhu H, Wang L and Liu P (2025) Traditional Chinese medicine monomers: a new strategy against diabetic kidney disease by regulating cholesterol efflux. Front. Nutr. 12:1708809. doi: 10.3389/fnut.2025.1708809
Edited by:
Imran Khan, Abdul Wali Khan University Mardan, PakistanReviewed by:
Tongtong Liu, Guang’anmen Hospital, China Academy of Chinese Medical Sciences, ChinaShahid Hussain, Kohsar University, Pakistan
Copyright © 2025 Wu, Sun, Shi, Li, An, Zhu, Wang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Han Zhu, emh0Y20wMjAzQDE2My5jb20=; Lifan Wang, d2xmNDY0ODM3NEAxNjMuY29t; Peng Liu, ZHJsaXVwZW5nQHNpbmEuY24=
†These authors have contributed equally to this work
 Chenguang Wu
Chenguang Wu Jianing Sun
Jianing Sun Junwei Shi1†
Junwei Shi1† Han Zhu
Han Zhu Lifan Wang
Lifan Wang Peng Liu
Peng Liu