- 1Pediatrics and Neonatology Unit, Guglielmo da Saliceto Hospital, Piacenza, Italy
- 2Pediatric Clinic, Department of Medicine and Surgery, University of Parma, Parma, Italy
- 3Department of Medicine and Surgery, University of Parma, Parma, Italy
Growing awareness of gluten-related disorders has led to a rising number of diagnoses of celiac disease (CD) and increasing adoption of the gluten-free diet (GFD), often without medical necessity. This narrative review summarizes current evidence on the main gluten-related conditions—CD, wheat allergy (WA), and non-celiac gluten sensitivity (NCGS)—and their nutritional implications, with particular focus on pediatric populations. Although these disorders share overlapping clinical features, they differ in pathogenesis, diagnostic criteria, and management. In CD, strict lifelong gluten exclusion remains essential for intestinal healing and symptom resolution, whereas in WA, wheat avoidance is the cornerstone of therapy. NCGS is characterized by gluten-related gastrointestinal and extra-intestinal symptoms in the absence of CD or WA, with notable clinical overlap with irritable bowel syndrome. Across all conditions, adherence to a GFD can lead to nutritional imbalances, including deficiencies in iron, folate, vitamin B12, vitamin D, calcium, zinc, and magnesium, as well as reduced fiber intake and unfavorable changes in gut microbiota. Overreliance on processed gluten-free foods may further increase cardiometabolic risks. In children, unmonitored GFDs may impair growth and neurodevelopment. Clinicians should ensure accurate differential diagnosis, provide nutritional counseling, and monitor long-term outcomes to balance the therapeutic benefits of GFD with potential risks.
1 Background
In recent years, interest in gluten-related disorders has expanded considerably, not only within the medical community but also among the wider public. This heightened awareness has resulted in increased recognition of celiac disease (CD) and, at the same time, a substantial proportion of people choosing to follow a self-initiated gluten-free diet (GFD). Such developments have contributed to widespread uncertainty about the full spectrum of gluten-associated conditions, which encompasses celiac disease, wheat allergy (WA), and non-celiac gluten sensitivity (NCGS). Although these entities share some overlapping clinical features, they are fundamentally distinct with respect to pathophysiology, diagnostic approaches, and therapeutic strategies (1–4).
CD is a chronic, immune-mediated disorder triggered by gluten ingestion in genetically predisposed individuals, leading to characteristic small-bowel mucosal damage and extra-intestinal manifestations. By contrast, WA is an IgE-driven immune reaction that may provoke acute hypersensitivity symptoms following wheat exposure. NCGS refers to a condition in which gluten consumption provokes both gastrointestinal and extra-intestinal complaints, in the absence of autoimmune mechanisms or IgE-mediated allergy (5–8).
Distinguishing among these conditions remains a clinical challenge, particularly in terms of long-term management and therapeutic decision-making. Furthermore, the widespread adoption of GFD has raised concern regarding its nutritional adequacy and possible health risks for individuals without a clear medical indication (9, 10).
The present narrative review seeks to provide a broad overview of gluten-related disorders, emphasizing the key distinctions among CD, WA, and NCGS. In addition, it discusses potential adverse effects associated with GFD and examines emerging evidence on nutritional issues linked to gluten exclusion.
2 Methods
This narrative review was conducted in three main stages: an initial literature search, a screening of abstracts and complete manuscripts, and a final evaluation of the selected studies. Publications from 1990 to 2025 were retrieved from major scientific databases, including PubMed, EMBASE, Scopus, ScienceDirect, Web of Science, and Google Scholar, to ensure a comprehensive overview of the available evidence. The most recent search was performed in June 2025.
Eligible study designs comprised randomized placebo-controlled trials, controlled clinical investigations, double-blind randomized studies, and systematic reviews. The search strategy combined the following keywords: “celiac disease” OR “gluten-free diet” OR “wheat allergy” OR “non-celiac gluten sensitivity” AND “diagnosis” OR “treatment” OR “weight changes” OR “cardiovascular disease” OR “dyslipidemia” OR “vitamin” OR “nutritional challenges” OR “eating behavior.” In addition, reference lists of the included publications were manually reviewed to identify further relevant contributions.
Only complete articles published in English were considered. After duplicates were removed, abstracts were screened for relevance, and the remaining manuscripts were assessed against the inclusion criteria. The evidence collected was subsequently synthesized into an integrated narrative review, with a specific focus on nutritional issues related to gluten exposure and restriction.
3 Results
3.1 Celiac disease
CD is a chronic, immune-mediated disorder of the small intestine, triggered by the ingestion of gluten-containing cereals such as wheat, barley, and rye in genetically predisposed individuals. The condition arises from an abnormal immune reaction to gluten-derived peptides, which induces intestinal inflammation and villous atrophy (11, 12). Both innate and adaptive immune mechanisms are implicated, with HLA-DQ2 and HLA-DQ8 molecules playing a central role by presenting deamidated gluten peptides to CD4+ T cells, thereby initiating the inflammatory cascade (12, 13).
Globally, CD affects roughly 1% of the population, though prevalence rates differ according to geographic, genetic, and environmental factors (14, 15). Recent evidence indicates a rising incidence, likely reflecting increased disease awareness, improved diagnostic tools, and shifts in dietary habits (13, 14, 16). Despite this, many individuals remain undiagnosed, highlighting the importance of enhanced screening strategies (12, 14).
3.1.1 Clinical presentation and diagnosis
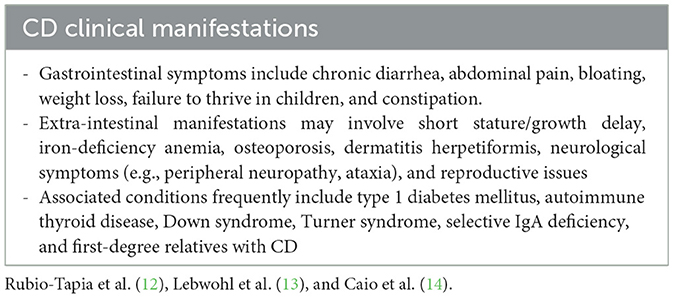
The clinical spectrum of CD is highly variable, ranging from classical gastrointestinal symptoms to extra-intestinal features and even silent or asymptomatic forms (Table 1) (12–14). Such heterogeneity frequently results in diagnostic delays, reinforcing the need to consider CD in diverse clinical scenarios (12, 15).
Diagnosis typically requires a combination of serological and, in some cases, histological investigations (11):
- Serological tests: First-line assessments include serum total IgA and IgA anti–tissue transglutaminase antibodies (TGA-IgA). In IgA-deficient individuals, IgG-based assays, such as anti-deamidated gliadin peptide IgG, are recommended (12).
- No-biopsy approach: The 2020 ESPGHAN guidelines allow a diagnosis without biopsy in symptomatic pediatric patients with TGA-IgA ≥10 times the upper limit of normal and positive endomysial antibodies (EMA-IgA) on a second sample.
- Histological evaluation: When serological findings are discordant or below the threshold, duodenal biopsies remain necessary. Current recommendations call for at least four samples from the distal duodenum and one from the bulb (11).
- HLA typing: Although not routinely required, genotyping for HLA-DQ2 and DQ8 can help exclude CD, as the absence of these alleles makes the diagnosis highly improbable (13).
All testing should be performed while the patient consumes a gluten-containing diet, to minimize false negatives and maintain diagnostic reliability (11).
3.1.2 Treatment and follow-up
The only effective therapy for CD is lifelong adherence to a strict gluten-free diet, which generally results in clinical remission and restoration of mucosal integrity (11, 12). Compliance must be monitored through regular clinical evaluations and repeated measurement of TGA-IgA (11). Follow-up visits are typically advised at 3–6 months post-diagnosis, and subsequently every 6–12 months depending on clinical status and serological trends (13).
If symptoms persist or antibody levels remain elevated, dietary adherence should be reassessed, and alternative explanations such as refractory CD or coexisting conditions should be investigated (14, 16).
3.2 Wheat allergy
WA is an immune-mediated hypersensitivity reaction that may involve IgE- or non-IgE–dependent mechanisms against wheat proteins such as albumins, globulins, gliadins, and glutenins. Unlike celiac disease, which represents an autoimmune condition, WA is a typical food allergy characterized by an inappropriate immune response directed at wheat-derived antigens (17). The IgE-mediated pathway commonly involves mast cell and basophil activation with subsequent histamine and mediator release (18).
3.2.1 Clinical manifestations and diagnosis
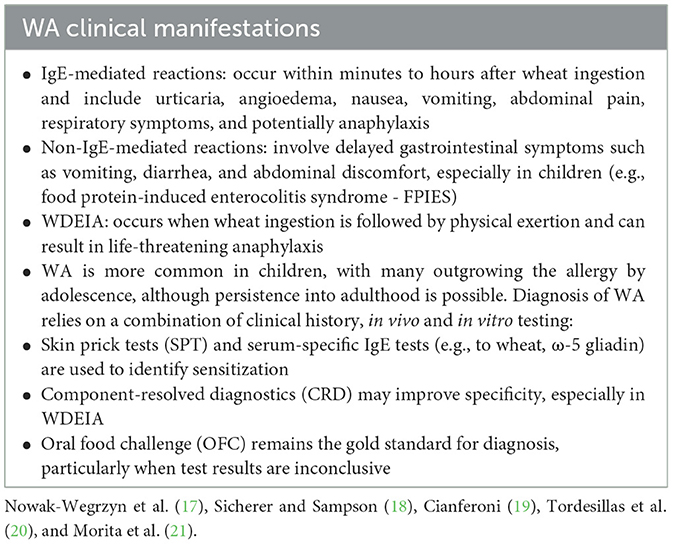
WA can present in several distinct clinical forms, including immediate-type food allergy, wheat-dependent exercise-induced anaphylaxis (WDEIA), occupational asthma or rhinitis (commonly referred to as baker's asthma), and contact urticaria (19).
The symptoms vary depending on the underlying immune mechanism and the route of exposure (Table 2) (17–21). Accurate differentiation between WA, CD, and NCGS is essential, as their pathophysiology, clinical profile, and therapeutic approaches differ significantly (22).
3.2.2 Treatment and follow-up
The primary treatment for WA is strict elimination of wheat and wheat-derived products from the diet. In contrast to celiac disease, exclusion of other gluten-containing grains such as barley or rye is generally unnecessary unless cross-reactivity has been demonstrated (18).
Patient education plays a central role and should focus on label reading, early recognition of allergic symptoms, and, when indicated, the availability of rescue medication such as epinephrine auto-injectors. In pediatric patients, regular follow-up visits and laboratory testing are advised to monitor for potential resolution of the allergy over time (19).
Although allergen-specific immunotherapy is being explored as a therapeutic option, it is not yet established as standard practice (21).
3.3 Non-celiac gluten sensitivity
NCGS is defined by the onset of gastrointestinal and extra-intestinal symptoms following gluten ingestion in individuals without evidence of CD or WA. Symptoms usually improve upon gluten withdrawal and recur after reintroduction. Unlike CD and WA, no validated biomarkers exist for NCGS, making diagnosis difficult and largely dependent on clinical assessment and exclusion of alternative conditions (23, 24).
Typical gastrointestinal complaints include abdominal pain, bloating, diarrhea, and constipation, whereas extra-intestinal manifestations may involve fatigue, headache, musculoskeletal pain, and cognitive disturbances such as “brain fog” (23, 25).
The underlying mechanisms of NCGS are not fully understood. Current evidence points toward a predominant role of innate immunity, rather than adaptive responses (23, 26). Amylase–trypsin inhibitors (ATIs), naturally occurring wheat proteins, have been implicated in activating toll-like receptor 4 (TLR4), thereby promoting intestinal inflammation (23). Additionally, fermentable oligo-, di-, monosaccharides, and polyols (FODMAPs)—particularly fructans found in wheat—may contribute to gastrointestinal symptoms, although they are unlikely to explain the extra-intestinal features commonly reported in NCGS (27, 28).
3.3.1 Clinical manifestations and diagnosis
The diagnostic process for NCGS is based on systematically excluding CD and WA. This typically involves serological testing for CD-specific antibodies and skin prick tests (SPT) or serum-specific IgE to rule out WA. Once these conditions are excluded, improvement of symptoms on a gluten-free diet and their recurrence following gluten reintroduction may support the diagnosis.
According to the Salerno Experts' Criteria, a double-blind, placebo-controlled crossover challenge represents the gold standard for diagnosis. However, given its complexity, this approach is rarely feasible in everyday clinical practice (23). In routine settings, clinicians often rely on the pattern of symptom remission after gluten withdrawal and recurrence after re-exposure (24).
An important aspect to consider in the clinical evaluation of NCGS is its substantial overlap with irritable bowel syndrome (IBS) (9). Both conditions share common gastrointestinal manifestations, including abdominal pain, bloating, altered bowel habits, and variable symptom fluctuation, which can complicate differential diagnosis. Recent studies have suggested that a subset of patients with IBS, particularly those with diarrhea-predominant or mixed subtypes, may experience symptom improvement on gluten-free or low-FODMAP diets, highlighting potential shared pathophysiological mechanisms (29–31). These mechanisms may include visceral hypersensitivity, altered intestinal permeability, low-grade mucosal inflammation, and activation of the innate immune system. Moreover, wheat components other than gluten—such as amylase–trypsin inhibitors and fructans—can act as triggers in both NCGS and IBS, further blurring the clinical boundaries between the two entities (9, 29). Careful diagnostic assessment is therefore required to distinguish NCGS from IBS, ensuring that dietary interventions are tailored appropriately and unnecessary dietary restrictions are avoided.
3.3.2 Treatment and follow-up
A GFD remains the primary therapeutic approach for NCGS. In contrast to CD, the required level of dietary strictness and the long-term duration of gluten avoidance are not well-defined, and some individuals appear to tolerate small amounts of gluten without symptoms (25).
Regular follow-up is recommended to monitor adherence, evaluate nutritional status, and track symptom progression (5, 23). Importantly, unnecessary adoption of a GFD in individuals without medical indications carries the risk of nutritional deficiencies and should therefore be pursued with caution (5).
3.4 Gluten-free diet: beyond treatment
3.4.1 Nutritional risks of GFD
Although a GFD is indispensable for patients with CD and frequently adopted by those with NCGS, it may present nutritional challenges, particularly when followed without medical necessity. The increasing popularity of GFD among the general population has intensified concerns about its long-term health implications (32, 33).
Several studies indicate that processed gluten-free foods often contain lower amounts of protein and fiber while providing higher levels of saturated fat and sugar compared to gluten-containing alternatives (34, 35). Moreover, the lack of systematic micronutrient fortification in gluten-free products can predispose individuals to deficiencies (36, 37).
3.4.1.1 Micronutrient deficiencies
Nutritional inadequacies on a GFD are common when the diet is poorly balanced. Key deficiencies include:
- Iron: Frequently deficient in CD both at diagnosis and follow-up. The lack of iron fortification in gluten-free foods and the limited bioavailability of non-heme iron contribute to persistent anemia (38, 39).
- Folate and B vitamins: Wheat-based foods are major contributors of folate, thiamine, niacin, and riboflavin, particularly in regions with mandatory fortification. Gluten-free products often fail to compensate, increasing cardiovascular risk through elevated homocysteine (40).
- Vitamin B12: Deficiency may occur due to malabsorption or inadequate intake, especially in elderly individuals or those with neurological symptoms (39).
- Vitamin D and calcium: Essential for bone health but often insufficient in GFDs, increasing risk for osteopenia and osteoporosis (38, 41, 42).
- Magnesium and zinc: Reduced intake of whole grains, legumes, and nuts leads to deficiencies that impair enzymatic activity, immunity, and metabolism (39, 43).
- Fiber: GFDs are consistently low in fiber, contributing to constipation, reduced satiety, and unfavorable microbiota changes (34, 44).
- Selenium and Copper: Less frequently studied, but deficiencies can compromise antioxidant defense, thyroid function, and cardiovascular health (39).
- Vitamin K: In cases of fat malabsorption, deficiency may affect coagulation and bone metabolism (38).
To counteract these risks, patients should prioritize naturally gluten-free, nutrient-rich foods such as pseudo-cereals (e.g., quinoa, buckwheat, amaranth), legumes, fruits, and vegetables, and incorporate fortified gluten-free products when available. Ongoing dietary supervision by trained dietitians is strongly recommended (36).
3.4.1.2 Macronutrient imbalances and food quality
Processed gluten-free foods are often nutritionally inferior. They may contain:
- Excess saturated fats and refined sugars added to improve palatability.
- Lower-quality protein, as common gluten-free flours (rice, corn) lack essential amino acids.
- Reduced fiber, negatively impacting satiety, gut function, and metabolic health.
- A higher glycemic index, which can impair glycemic control in individuals with metabolic disorders (34, 44).
3.4.1.3 Gut microbiota and functional implications
Evidence suggests that long-term GFD may induce dysbiosis, characterized by decreased levels of beneficial bacteria such as Bifidobacteria and Lactobacilli and increased prevalence of potentially harmful taxa like Enterobacteriaceae (45). These shifts, largely attributable to reduced intake of whole-grain fibers, may compromise immune regulation, barrier integrity, and nutrient metabolism, particularly in those without a medical indication for GFD.
3.4.1.4 Special considerations for non-celiac individuals
Among individuals without CD or NCGS, adherence to a GFD for perceived health benefits may reduce overall dietary quality. Studies show reduced intake of essential nutrients alongside increased consumption of processed gluten-free snacks, often rich in sugars and fats (33, 36). Without professional guidance, this population may inadvertently heighten their risk of chronic disease.
3.4.2 GFD and weight change
Weight outcomes on a GFD can be bidirectional. In newly diagnosed CD patients, improved nutrient absorption after mucosal healing may result in weight gain, which can reduce dietary adherence in some cases (46). Conversely, an increasing number of patients are overweight or obese at diagnosis, raising questions about GFD's metabolic impact (47, 48).
A Finnish nationwide study (n = 698) reported BMI improvements across diagnostic categories after 1 year of GFD, with underweight patients gaining weight and overweight/obese patients showing partial normalization (47). Brambilla et al. observed BMI stabilization in children after diagnosis, with fewer underweight cases and only a slight increase in overweight prevalence (49). A 2022 prospective study (n = 215) found significant weight gain in patients following GFD for more than 2 years (50). A 2023 systematic review (n = 45 studies, ~28,000 participants) showed BMI redistribution, with some individuals moving into higher or lower BMI categories, but no increased risk of pathological weight gain (51). Another meta-analysis confirmed increased weight and fat mass in CD patients but no significant changes in healthy controls (52).
Overall, these findings suggest that GFD may normalize weight in CD patients, but long-term risks related to processed gluten-free foods (high in fat and salt) remain a concern (33).
3.4.3 GFD and eating disorders
Strict dietary control may predispose certain individuals to maladaptive eating behaviors. Shared features between CD and eating disorders (EDs)—including weight loss, fatigue, and nonspecific gastrointestinal complaints—can complicate diagnosis (53, 54).
The constant vigilance required to maintain a GFD may mimic or exacerbate disordered eating, particularly in adolescents and women (55, 56). A 2021 meta-analysis reported a pooled prevalence of EDs of 8.88% among CD patients, markedly higher than the general population (57).
Strong associations have been reported between CD and anorexia nervosa (AN). A Swedish cohort study (n = 107,000) found increased hazard ratios for AN following CD diagnosis and vice versa (58, 59). Smaller surveys confirm changes in eating attitudes post-diagnosis, with heightened awareness and negative emotions toward food (60).
Clinicians should remain vigilant for ED symptoms in CD patients, as they can compromise adherence to GFD and adversely affect psychological and physical health (61).
3.4.4 GFD and cardiovascular risk
While GFD is the only effective treatment for CD (62), it is not inherently cardioprotective. Gluten-free starches and flours typically have higher glycemic indices and lower fiber and nutrient density (63). Adequate fiber intake is essential for cardiovascular health due to its lipid-lowering and anti-inflammatory properties (64, 65).
Evidence regarding the cardiometabolic effects of GFD is mixed. Some studies and reviews report improvements in HDL cholesterol, systolic blood pressure, and inflammatory markers, but increases in total and LDL cholesterol (66–72). Pediatric cohorts have shown increases in both total and HDL cholesterol after adopting GFD (71). Differences in lipid responses between genders have also been observed (73). Lipoprotein(a), an independent cardiovascular risk factor, appears unaffected by GFD (74–76).
Overall, GFD may contribute to metabolic alterations, including weight gain, dyslipidemia, and glucose imbalance, particularly when based on processed gluten-free foods (77, 78). Patients should be counseled on balanced dietary choices to mitigate these risks.
3.4.5 Environmental factors in CD
Although genetic predisposition (HLA-DQ2/DQ8) is central to CD development, environmental influences also play a role. Gluten exposure is the most critical factor, but breastfeeding, early-life infections, microbiome alterations, and antibiotic use may modulate risk (79–81).
Early observational studies suggested protective effects of breastfeeding and timing of gluten introduction, but large prospective trials such as PreventCD and TEDDY found no significant influence on CD risk (82, 83). As a result, ESPGHAN guidelines do not recommend breastfeeding as a preventive measure against CD, although it is still encouraged for its many other health benefits (84).
Additional concerns have been raised regarding industrial food processing. Use of microbial transglutaminase as an additive may negatively affect gluten-sensitive individuals, potentially influencing disease risk (85).
4 Discussion
Gluten-related disorders comprise a heterogeneous spectrum of conditions—CD, WA, and NCGS—that present with overlapping symptoms but differ substantially in immunological mechanisms, diagnostic criteria, and management. Despite notable progress in diagnostic strategies, differentiating between these conditions remains clinically challenging, particularly in the absence of reliable biomarkers for NCGS and the wide variability of gastrointestinal and extra-intestinal manifestations. These diagnostic uncertainties can contribute both to delayed treatment initiation and to unnecessary adoption of dietary restrictions.
The GFD represents the cornerstone of therapy for CD and a valuable approach for many cases of WA and NCGS. Nevertheless, its increasing popularity among individuals without confirmed diagnoses has raised legitimate concerns. Evidence consistently shows that GFD, especially when based on industrially processed gluten-free products, is associated with micronutrient deficiencies (iron, folate, B vitamins, vitamin D, calcium, zinc, magnesium), reduced dietary fiber, and potential alterations in gut microbiota. Moreover, imbalances in macronutrient composition, higher glycemic index, and increased levels of fats and sugars in commercial gluten-free products may predispose individuals to metabolic complications, including overweight, obesity, and cardiovascular risk.
These issues are particularly relevant in pediatric populations. Unlike adults, children require carefully tailored diets to support optimal growth, bone mineralization, and neurodevelopment. Applying restrictive dietary interventions without medical justification may compromise nutritional adequacy and expose younger patients to psychosocial burdens, including heightened risk of maladaptive eating behaviors. Such considerations highlight the duality of GFD: indispensable for those with CD and often beneficial for WA and NCGS, yet potentially harmful when adopted indiscriminately.
A key strength of this narrative review lies in its comprehensive scope, integrating recent findings across multiple domains—including pathogenesis, diagnosis, management, and nutritional outcomes—while drawing on a wide range of clinical studies and systematic reviews. This broad perspective allows for an up-to-date synthesis of the clinical, nutritional, and public health implications of gluten-related disorders. However, certain limitations must be acknowledged. As a narrative review, this work is not based on systematic evidence appraisal, and the selection of studies may have introduced bias. Furthermore, the heterogeneity of available studies, varying diagnostic definitions of NCGS, and differences in study populations and dietary assessments complicate direct comparison of findings. Finally, the rapidly evolving nature of research in this field means that new data may soon refine or challenge current conclusions.
Taken together, these strengths and limitations underscore the importance of interpreting the present findings as a broad overview rather than definitive guidance, while pointing to the need for well-designed prospective studies to clarify unresolved questions.
While the present review supports the therapeutic value of the GFD for patients with confirmed CD, WA, and NCGS, it is important to acknowledge the ongoing debate regarding the extent of gluten's role in symptom generation, particularly in NCGS and IBS. Some studies suggest that gluten itself is not the primary trigger in many self-reported NCGS cases, pointing instead to other wheat components such as fructans or amylase–trypsin inhibitors as symptom-inducing agents (27, 28). Conversely, other investigations have demonstrated reproducible symptom recurrence after blinded gluten challenges, supporting gluten as a distinct trigger in a subset of sensitive individuals (23, 25). Similarly, while numerous reports highlight nutritional deficiencies and cardiometabolic concerns associated with GFD, some evidence indicates improved metabolic profiles and quality of life when the diet is appropriately balanced and supervised (70). These contrasting perspectives emphasize that the impact of gluten and GFD is heterogeneous and context-dependent. Future studies should therefore aim to stratify patients according to immunological and metabolic phenotypes, enabling more precise dietary recommendations and minimizing unnecessary restrictions.
5 Conclusions
The growing recognition of gluten-related disorders has improved the diagnosis of CD and expanded clinical awareness of WA and NCGS. At the same time, the widespread self-prescribed adoption of the GFD in individuals without medical necessity highlights the need for caution. While GFD is indispensable for CD and often beneficial in WA and NCGS, its indiscriminate use can result in micronutrient deficiencies, altered gut microbiota, and potential cardiometabolic risks, particularly when the diet relies on processed gluten-free products.
This narrative review emphasizes the importance of accurate differential diagnosis, individualized dietary counseling, and continuous follow-up in both adult and pediatric populations. Particular attention must be paid to children, where nutritional adequacy and healthy growth should remain the primary objectives. Future research should prioritize high-quality prospective studies to better define the long-term nutritional and metabolic consequences of GFD, with special focus on pediatric populations and individuals without confirmed diagnoses. Such evidence is essential to balance the therapeutic benefits of GFD with its potential risks, ensuring safe, effective, and patient-centered care.
Author contributions
MC: Writing – original draft, Investigation, Conceptualization, Methodology, Writing – review & editing. TS: Writing – original draft, Writing – review & editing, Methodology, Investigation, Data curation. VA: Investigation, Writing – review & editing, Writing – original draft, Methodology, Data curation. GP: Methodology, Investigation, Writing – review & editing, Data curation, Writing – original draft. AB: Methodology, Data curation, Writing – review & editing, Writing – original draft, Investigation. MB: Investigation, Data curation, Writing – review & editing, Writing – original draft, Methodology. AM: Investigation, Data curation, Writing – review & editing, Writing – original draft, Methodology. SE: Writing – original draft, Supervision, Resources, Conceptualization, Validation, Writing – review & editing. GB: Validation, Supervision, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
SE declared that she was an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Elli L, Branchi F, Tomba C, Villalta D, Norsa L, Ferretti F, et al. Diagnosis of gluten related disorders: celiac disease, wheat allergy and non-celiac gluten sensitivity. World J Gastroenterol. (2015) 21:7110–9. doi: 10.3748/wjg.v21.i23.7110
2. Catassi C, Bai JC, Bonaz B, Bouma G, Calabrò A, Carroccio A, et al. Non-celiac gluten sensitivity: the new frontier of gluten related disorders. Nutrients. (2013) 5:3839–53. doi: 10.3390/nu5103839
3. Valentina P, Victoria K, Monica M, Pasqua P, Ruggiero F, Lorenzo N, et al. Elevation of tTG-IgA antibodies in A prospective cohort of children at risk for celiac disease: insights from the CDGEMM cohort. Am J Gastroenterol. (2025). doi: 10.14309/ajg.0000000000003772
4. Mpakosi A, Kaliouli-Antonopoulou C, Cholevas V, Cholevas S, Tzouvelekis I, Mironidou-Tzouveleki M, et al. Challenges in the pediatric celiac disease diagnosis: an up-to-date review. Diagnostics. (2025) 15:2392. doi: 10.3390/diagnostics15182392
5. Volta U, Caio G, De Giorgio R, Henriksen C, Skodje G, Lundin KE. Non-celiac gluten sensitivity: a work-in-progress entity in the spectrum of wheat-related disorders. Best Pract Res Clin Gastroenterol. (2015) 29:477–91. doi: 10.1016/j.bpg.2015.04.006
6. Tanveer M, Ahmed A. Non-celiac gluten sensitivity: a systematic review. J Coll Physicians Surg Pak. (2019) 29:51–7. doi: 10.29271/jcpsp.2019.01.51
7. Al-Toma A, Zingone F, Branchi F, Schiepatti A, Malamut G, Canova C, et al. European Society Coeliac Disease in Adults. part 1: diagnostic approach. United Eur Gastroenterol J. (2025). doi: 10.1002/ueg2.70119
8. Stordal K, Kurppa K. Celiac disease, non-celiac wheat sensitivity, wheat allergy - clinical and diagnostic aspects. Semin Immunol. (2025) 77:101930. doi: 10.1016/j.smim.2025.101930
9. Makharia A, Catassi C, Makharia GK. The Overlap between Irritable Bowel Syndrome and Non-Celiac Gluten Sensitivity: A Clinical Dilemma. Nutrients. (2015) 7:10417–26. doi: 10.3390/nu7125541
10. Fingerle M, Salaorni S, Pietrobelli A, Piacentini G, Banzato C, Pecoraro L. Wheat-related disorders in children: a 360-degree view. Children (Basel). (2024) 11:707. doi: 10.3390/children11060707
11. Husby S, Koletzko S, Korponay-Szabó I, Kurppa K, Mearin ML, Ribes-Koninckx C, et al. European Society Paediatric Gastroenterology, hepatology and nutrition guidelines for diagnosing coeliac disease 2020. J Pediatr Gastroenterol Nutr. (2020) 70:141–56. doi: 10.1097/MPG.0000000000002497
12. Rubio-Tapia A, Hill ID, Semrad C, Kelly CP, Greer KB, Limketkai BN, et al. American College of Gastroenterology Guidelines Update: diagnosis and management of celiac disease. Am J Gastroenterol. (2023) 118:59–76. doi: 10.14309/ajg.0000000000002075
13. Lebwohl B, Sanders DS, Green PHR. Coeliac disease. Lancet. (2018) 391:70–81. doi: 10.1016/S0140-6736(17)31796-8
14. Caio G, Volta U, Sapone A, Leffler DA, De Giorgio R, Catassi C, et al. Celiac disease: a comprehensive current review. BMC Med. (2019) 17:142. doi: 10.1186/s12916-019-1380-z
15. Li T, Feng Y, Wang M, Wang C, Gao F. Factors influencing diagnostic delays in celiac disease. World J Gastroenterol. (2025) 31:109585. doi: 10.3748/wjg.v31.i30.109585
16. Cenni S, Sesenna V, Boiardi G, Casertano M, Russo G, Reginelli A, et al. The role of gluten in gastrointestinal disorders: a review. Nutrients. (2023) 15:1615. doi: 10.3390/nu15071615
17. Nowak-Wegrzyn A, Katz Y, Mehr SS, Koletzko S. Non-IgE-mediated gastrointestinal food allergy. J Allergy Clin Immunol. (2015) 135:1114–24. doi: 10.1016/j.jaci.2015.03.025
18. Sicherer SH, Sampson HA. Food allergy: a review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J Allergy Clin Immunol. (2018) 141:41–58. doi: 10.1016/j.jaci.2017.11.003
19. Cianferoni A. Wheat allergy: diagnosis and management. J Asthma Allergy. (2016) 9:13–25. doi: 10.2147/JAA.S81550
20. Tordesillas L, Berin MC, Sampson HA. Immunology of food allergy. Immunity. (2017) 47:32–50. doi: 10.1016/j.immuni.2017.07.004
21. Morita E, Matsuo H, Chinuki Y, Takahashi H, Dahlström J, Tanaka A. Food-dependent exercise-induced anaphylaxis -importance of omega-5 gliadin and HMW-glutenin as causative antigens for wheat-dependent exercise-induced anaphylaxis. Allergol Int. (2009) 58:493–8. doi: 10.2332/allergolint.09-RAI-0125
22. Kajita N, Kusakawa G, Hirao K, Yokoyama S, Morikawa E, Morita K, et al. Lymphocyte-stimulation test for diagnosis of wheat- induced enterocolitis syndrome in children. J Investig Allergol Clin Immunol. (2025). doi: 10.18176/jiaci.1085
23. Manza F, Lungaro L, Costanzini A, Caputo F, Carroccio A, Mansueto P, et al. Non-celiac gluten/wheat sensitivity-state of the art: a five-year narrative review. Nutrients. (2025) 17:220. doi: 10.3390/nu17020220
24. Siddiqui UN, Pervaiz A, Khan ZB, Sultana T. Diagnostic dilemma, possible non-celiac gluten sensitivity: consideration in approach and management. Cureus. (2022) 14:e25302. doi: 10.7759/cureus.25302
25. Molina-Infante J, Carroccio A. Suspected nonceliac gluten sensitivity confirmed in few patients after gluten challenge in double-blind, placebo-controlled trials. Clin Gastroenterol Hepatol. (2017) 15:339–48. doi: 10.1016/j.cgh.2016.08.007
26. Volta U, Caio G, Tovoli F, De Giorgio R. Non-celiac gluten sensitivity: questions still to be answered despite increasing awareness. Cell Mol Immunol. (2013) 10:383–92. doi: 10.1038/cmi.2013.28
27. Biesiekierski JR, Peters SL, Newnham ED, Rosella O, Muir JG, Gibson PR. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology. (2013) 145:320–8.e1–3. doi: 10.1053/j.gastro.2013.04.051
28. Skodje GI, Sarna VK, Minelle IH, Rolfsen KL, Muir JG, Gibson PR, et al. Fructan, rather than gluten, induces symptoms in patients with self-reported non-celiac gluten sensitivity. Gastroenterology. (2018) 154: 529–539.e2. doi: 10.1053/j.gastro.2017.10.040
29. Siragusa N, Baldassari G, Ferrario L, Passera L, Rota B, Pavan F, et al. The ten dietary commandments for patients with irritable bowel syndrome: a narrative review with pragmatic indications. Nutrients. (2025) 17:2496. doi: 10.3390/nu17152496
30. Catassi G, Catassi C. An overview of progress in establishing a diagnostic tool for non-celiac gluten sensitivity. Expert Rev Mol Diagn. (2025) 25:59–66. doi: 10.1080/14737159.2025.2458469
31. Borghini R, Spagnuolo A, Donato G, Borghini G. Gluten-Free Diet for Fashion or Necessity? Review with new speculations on irritable bowel syndrome-like disorders. Nutrients. (2024) 16:4236. doi: 10.3390/nu16234236
32. Reilly NR. The gluten-free diet: recognizing fact, fiction, and fad. J Pediatr. (2016) 175:206–10. doi: 10.1016/j.jpeds.2016.04.014
33. Melini V, Melini F. Gluten-free diet: gaps and needs for a healthier diet. Nutrients. (2019) 11:170. doi: 10.3390/nu11010170
34. Missbach B, Schwingshackl L, Billmann A, Mystek A, Hickelsberger M, Bauer G, et al. Gluten-free food database: the nutritional quality and cost of packaged gluten-free foods. PeerJ. (2015) 3:e1337. doi: 10.7717/peerj.1337
35. Saturni L, Ferretti G, Bacchetti T. The gluten-free diet: safety and nutritional quality. Nutrients. (2010) 2:16–34. doi: 10.3390/nu20100016
36. Martin J, Geisel T, Maresch C, Krieger K, Stein J. Inadequate nutrient intake in patients with celiac disease: results from a German dietary survey. Digestion. (2013) 87:240–6. doi: 10.1159/000348850
37. Russell LA, Alliston P, Armstrong D, Verdu EF, Moayyedi P, Pinto-Sanchez MI. Micronutrient deficiencies associated with a gluten-free diet in patients with celiac disease and non-celiac gluten or wheat sensitivity: a systematic review and meta-analysis. J Clin Med. (2025) 14:4848. doi: 10.3390/jcm14144848
38. Vici G, Belli L, Biondi M, Polzonetti V. Gluten free diet and nutrient deficiencies: a review. Clin Nutr. (2016) 35:1236–41. doi: 10.1016/j.clnu.2016.05.002
39. Thompson T, Dennis M, Higgins LA, Lee AR, Sharrett MK. Gluten-free diet survey: are Americans with coeliac disease consuming recommended amounts of fibre, iron, calcium and grain foods? J Hum Nutr Diet. (2005) 18:163–9. doi: 10.1111/j.1365-277X.2005.00607.x
40. Hallert C, Grant C, Grehn S, Grännö C, Hultén S, Midhagen G, et al. Evidence of poor vitamin status in coeliac patients on a gluten-free diet for 10 years. Aliment Pharmacol Ther. (2002) 16:1333–9. doi: 10.1046/j.1365-2036.2002.01283.x
41. Fasano A, Catassi C. Clinical practice. Celiac disease N Engl J Med. (2012) 367:2419–26. doi: 10.1056/NEJMcp1113994
42. Infantino C, Francavilla R, Vella A, Cenni S, Principi N, Strisciuglio C, et al. Role of vitamin D in celiac disease and inflammatory bowel diseases. Nutrients. (2022) 14:5154. doi: 10.3390/nu14235154
43. Miranda J, Lasa A, Bustamante MA, Churruca I, Simon E. Nutritional differences between a gluten-free diet and a diet containing equivalent products with gluten. Plant Foods Hum Nutr. (2014) 69:182–7. doi: 10.1007/s11130-014-0410-4
44. Pellegrini N, Agostoni C. Nutritional aspects of gluten-free products. J Sci Food Agric. (2015) 95:2380–5. doi: 10.1002/jsfa.7101
45. Caminero A, Meisel M, Jabri B, Verdu EF. Mechanisms by which gut microorganisms influence food sensitivities. Nat Rev Gastroenterol Hepatol. (2019) 16:7–18. doi: 10.1038/s41575-018-0064-z
46. Rodrigues M, Yonamine GH, Fernandes Satiro CA. Rate and determinants of non-adherence to a gluten-free diet and nutritional status assessment in children and adolescents with celiac disease in a tertiary Brazilian referral center: a cross-sectional and retrospective study. BMC Gastroenterol. (2018) 18:15. doi: 10.1186/s12876-018-0740-z
47. Ukkola A, Mäki M, Kurppa K, Collin P, Huhtala H, Kekkonen L, et al. Changes in body mass index on a gluten-free diet in coeliac disease: a nationwide study. Eur J Intern Med. (2012) 23:384–8. doi: 10.1016/j.ejim.2011.12.012
48. Maleki F, Hosseinpour M, Delpisheh A, Bahardoust M, Hajizadeh-Sharafabad F, Pashaei MR. The prevalence of obesity and underweight in celiac patients at the time of diagnosis: a systematic review and meta-analysis. BMC Gastroenterol. (2024) 24:357. doi: 10.1186/s12876-024-03446-x
49. Brambilla P, Picca M, Dilillo D, Meneghin F, Cravidi C, Tischer MC, et al. Changes of body mass index in celiac children on a gluten-free diet. Nutr Metab Cardiovasc Dis. (2013) 23:177–82. doi: 10.1016/j.numecd.2011.10.002
50. Asri N, Taraghikhah N, Baniasadi R, Ishaq S, Rezaei-Tavirani M, Sadeghi A, et al. The effect of gluten-free diet duration on body mass index of iranian patients with celiac disease. Middle East J Dig Dis. (2022) 14:323–9. doi: 10.34172/mejdd.2022.290
51. Barone M, Iannone A, Cristofori F, Dargenio VN, Indrio F, Verduci E, et al. Risk of obesity during a gluten-free diet in pediatric and adult patients with celiac disease: a systematic review with meta-analysis. Nutr Rev. (2023) 81:252–66. doi: 10.1093/nutrit/nuac052
52. Xin C, Imanifard R, Jarahzadeh M, Rohani P, Velu P, Sohouli MH. Impact of Gluten-free Diet on Anthropometric indicators in individuals with and without celiac disease: a systematic review and meta-analysis. Clin Ther. (2023) 45:e243–51. doi: 10.1016/j.clinthera.2023.09.018
53. Cadenhead JW, Wolf RL, Lebwohl B, Lee AR, Zybert P, Reilly NR, et al. Diminished quality of life among adolescents with coeliac disease using maladaptive eating behaviours to manage a gluten-free diet: a cross-sectional, mixed-methods study. J Hum Nutr Dietet. (2019) 32:12638. doi: 10.1111/jhn.12638
54. Wei Y, Wang Y, Yuan Y, Chen J. Celiac disease, gluten-free diet, and eating disorders: from bench to bedside. Foods. (2024) 14:74. doi: 10.3390/foods14010074
55. Roos S, Kärner A, Hallert C. Psychological well-being of adult coeliac patients treated for 10 years. Dig Liver Dis. (2006) 38:177–80. doi: 10.1016/j.dld.2006.01.004
56. Hallert C, Sandlund O, Broqvist M. Perceptions of health-related quality of life of men and women living with coeliac disease. Scand J Caring Sci. (2003) 17:301–7. doi: 10.1046/j.1471-6712.2003.00228.x
57. Galmiche M, Déchelotte P, Lambert G, Tavolacci MP. Prevalence of eating disorders over the 2000-2018 period: a systematic literature review. Am J Clin Nutr. (2019) 109:1402–13. doi: 10.1093/ajcn/nqy342
58. Abber SR, Burton Murray H. Does gluten avoidance in patients with celiac disease increase the risk of developing eating disorders? Dig Dis Sci. (2023) 68:2790–2. doi: 10.1007/s10620-023-07915-3
59. Mårild K, Størdal K, Bulik CM, Rewers M, Ekbom A, Liu E, et al. Celiac disease and anorexia nervosa: a nationwide study. Pediatrics. (2017) 139:4367. doi: 10.1542/peds.2016-4367
60. Satherley R-M, Higgs S, Howard R. Disordered eating patterns in coeliac disease: a framework analysis. J Hum Nutr Diet. (2017) 30:724–36. doi: 10.1111/jhn.12475
61. Rabiee R, Mahdavi R, Shirmohammadi M, Nikniaz Z. Eating disorders, body image dissatisfaction and their association with gluten-free diet adherence among patients with celiac disease. BMC Nutr. (2024) 10:100. doi: 10.1186/s40795-024-00910-5
62. Marciniak M, Szymczak-Tomczak A, Mahadea D, Eder P, Dobrowolska A, Krela-Kazmierczak I. Multidimensional disadvantages of a gluten-free diet in celiac disease: a narrative review. Nutrients. (2021) 13:643. doi: 10.3390/nu13020643
63. Mehtab W, Agarwal S, Agarwal H, Ahmed A, Agarwal A, Prasad S, et al. Gluten-free foods are expensive and nutritionally imbalanced than their gluten-containing counterparts. Indian J Gastroenterol. (2024) 43:668–78. doi: 10.1007/s12664-024-01519-z
64. Banderali G, Capra ME, Viggiano C, Biasucci G, Pederiva C. Nutraceuticals in paediatric patients with dyslipidaemia. Nutrients. (2022) 14:569. doi: 10.3390/nu14030569
65. Capra ME, Biasucci G, Travaglia E, Sodero R, Banderali G, Pederiva C. Fiber in the treatment of dyslipidemia in pediatric patients. Children. (2025) 12:427. doi: 10.3390/children12040427
66. Romão B, Falcomer AL, Palos G, Cavalcante S, Botelho RBA, Nakano EY, et al. Glycemic index of gluten-free bread and their main ingredients: a systematic review and meta-analysis. Foods. (2021) 10:506. doi: 10.3390/foods10030506
67. Capra ME, Monopoli D, Decarolis NM, Giudice A, Stanyevic B, Esposito S, et al. Dietary models and cardiovascular risk prevention in pediatric patients. Nutrients. (2023) 15:3664. doi: 10.3390/nu15163664
68. Defeudis G, Massari MC, Terrana G, Coppola L, Napoli N, Migliaccio S. Gluten-free diet and metabolic syndrome: could be a not benevolent encounter? Nutrients. (2023) 15:627. doi: 10.3390/nu15030627
69. Burayzat S, Elsahoryi N, Freitekh A, Alzoubi O, Al-Najjar R, Tayyem R. Does a gluten-free diet affect BMI and glycosylated hemoglobin in children and adolescents with type 1 diabetes and asymptomatic celiac disease? A meta-analysis and systematic review. Children. (2022) 9:1247. doi: 10.3390/children9081247
70. Rohani P, Izze da Silva Magalhães E, Imanifard R, Jarahzadeh M, Ziamanesh F, et al. Impact of gluten-free diet (GFD) on some of cardiovascular risk factors: a systematic review and meta-analysis. J Nutr Sci. (2024) 13:e37. doi: 10.1017/jns.2024.39
71. Norsa L, Shamir R, Zevit N, Verduci E, Hartman C, Ghisleni D, et al. Cardiovascular disease risk factor profiles in children with celiac disease on gluten-free diets. World J Gastroenterol. (2013) 19:5658–64. doi: 10.3748/wjg.v19.i34.5658
72. Potter MDE, Brienesse SC, Walker MM, Boyle A, Talley NJ. Effect of the gluten-free diet on cardiovascular risk factors in patients with coeliac disease: a systematic review. J Gastroenterol Hepatol. (2018) 33:781–91. doi: 10.1111/jgh.14039
73. Forchielli ML, Fernicola P, Diani L, Scrivo B, Salfi NC, Pessina AC, et al. Gluten-free diet and lipid profile in children with celiac disease: comparison with general population standards. J Pediatr Gastroenterol Nutr. (2015) 61:224–9. doi: 10.1097/MPG.0000000000000785
74. Pederiva C, Capra ME, Biasucci G, Banderali G, Fabrizi E, Gazzotti M, et al. ipoprotein(a) and family history for cardiovascular disease in paediatric patients: a new frontier in cardiovascular risk stratification Data from the LIPIGEN paediatric group. Atherosclerosis. (2022) 349:233–9. doi: 10.1016/j.atherosclerosis.2022.04.021
75. Pillan MN, Spandrio S, Sleiman I, Meini A, Scalvini T, Balestrieri GP. Effects of a gluten-free diet on serum lipids and lipoprotein (a) levels in a group of patients with celiac disease. J Pediatr Gastroenterol Nutr. (1994) 18:183–5. doi: 10.1097/00005176-199402000-00010
76. Ti Y, Xu D, Qin X, Hu Y, Xu Y, Zhao Q, et al. Mendelian randomization analysis does not support a causal influence between lipoprotein(A) and immune-mediated inflammatory diseases. Sci Rep. (2025) 15:3834. doi: 10.1038/s41598-025-88375-9
77. Valvano M, Longo S, Stefanelli G, Frieri G, Viscido A, Latella G. Celiac disease, gluten-free diet, and metabolic and liver disorders. Nutrients. (2020) 12:940. doi: 10.3390/nu12040940
78. Ciccone A, Gabrieli D, Cardinale R, Di Ruscio M, Vernia F, Stefanelli G, et al. Metabolic alterations in celiac disease occurring after following a gluten-free diet. Digestion. (2019) 100:262–8. doi: 10.1159/000495749
79. Ivarsson A, Myléus A, Norström F, van der Pals M, Rosén A, Högberg L, et al. Prevalence of childhood celiac disease and changes in infant feeding. Pediatrics. (2013) 131:e687–94. doi: 10.1542/peds.2012-1015
80. Bielik M, Selvek M, Suchánková M, Shawkatová I. A case-control epidemiological survey on potential risk factors for celiac disease. Cent Eur J Public Health. (2024) 32:119–24. doi: 10.21101/cejph.a8010
81. Capra ME, Aliverti V, Bellani AM, Berzieri M, Montani AG, Pisseri G, et al. Breastfeeding and non-communicable diseases: a narrative review. Nutrients. (2025) 17:511. doi: 10.3390/nu17030511
82. Vriezinga SL, Auricchio R, Bravi E, Castillejo G, Chmielewska A, Crespo Escobar P, et al. Randomized feeding intervention in infants at high risk for celiac disease. N Engl J Med. (2014) 371:1304–15. doi: 10.1056/NEJMoa1404172
83. Andrén Aronsson C, Lee HS, Liu E, Uusitalo U, Hummel S, Yang J, et al. Age at gluten introduction and risk of celiac disease. Pediatrics. (2015) 135:239–45. doi: 10.1542/peds.2014-1787
84. Szajewska H, Shamir R, Mearin L, Ribes-Koninckx C, Catassi C, Domellöf M, et al. Gluten introduction and the risk of coeliac disease. J Pediatr Gastroenterol Nutr. (2016) 62:507–13. doi: 10.1097/MPG.0000000000001105
Keywords: gluten, celiac disease, wheat allergy, non-celiac gluten sensitivity, nutrition, vitamin, challenges
Citation: Capra ME, Sguerso T, Aliverti V, Pisseri G, Bellani AM, Berzieri M, Montani AG, Esposito S and Biasucci G (2025) Gluten-related nutritional challenges in pediatric subjects: treatment and beyond. Front. Nutr. 12:1709121. doi: 10.3389/fnut.2025.1709121
Received: 19 September 2025; Accepted: 28 October 2025;
Published: 25 November 2025.
Edited by:
Malika Bouchenak, Oran University 1 Ahmed Ben Bella, AlgeriaReviewed by:
Tatiana Palotta Minari, Federal University of São Paulo, BrazilAbdelghani Yagoubi, Independent Researcher, Algiers, Algeria
Copyright © 2025 Capra, Sguerso, Aliverti, Pisseri, Bellani, Berzieri, Montani, Esposito and Biasucci. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Susanna Esposito, c3VzYW5uYW1hcmlhcm9iZXJ0YS5lc3Bvc2l0b0B1bmlwci5pdA==
 Maria Elena Capra1
Maria Elena Capra1 Anna Giuseppina Montani
Anna Giuseppina Montani Susanna Esposito
Susanna Esposito Giacomo Biasucci
Giacomo Biasucci