- 1Department of Thoracic Surgery, The First Affiliated Hospital, Fujian Medical University, Fuzhou, China
- 2Department of Thoracic Surgery, National Regional Medical Center, Binhai Campus of the First Affiliated Hospital, Fujian Medical University, Fuzhou, China
Objective: The global immune-nutrition-inflammation index (GINI) is a new composite indicator that assesses nutrition and inflammation and has been linked to prognosis in various cancers. Neoadjuvant immunochemotherapy (nICT) is becoming more common for treating locally advanced esophageal squamous cell carcinoma (ESCC). However, the potential of GINI to predict outcomes for ESCC patients undergoing nICT has not yet been explored. This study aims to examine the predictive value of the pretreatment GINI in relation to pathological response and prognosis for ESCC patients receiving nICT.
Methods: A total of 138 patients with locally advanced ESCC who underwent nICT followed by radical resection at our institution between 2022 and 2024 were retrospectively included in this study. The GINI index was calculated from pretreatment blood parameters. The receiver operating characteristic (ROC) curve analysis was conducted to determine the optimal cutoff value of the GINI index for predicting pathological response, which was defined using the Becker tumor regression grade (TRG). Logistic regression and Cox proportional hazards models were used to analyze the associations between the GINI index and both pathological response and survival outcomes.
Results: The optimal cutoff value of GINI for predicting pathological response was 73.47 (AUC = 0.912). Multivariate analysis identified high-GINI as an independent risk factor for both poor pathological response (OR = 1.05, p < 0.001) and shorter overall survival (OS) (HR = 1.01, p = 0.012). Compared to the low-GINI group, patients in the high-GINI group had significantly poorer tumor differentiation, more advanced pathological stage, and a higher incidence of complications (all p < 0.05). Survival analysis demonstrated that the low-GINI group had significantly better 3-year OS (87.8% vs. 68.7%, p = 0.014) and disease-free survival (DFS) (82.7% vs. 63.3%, p = 0.011) than the high-GINI group.
Conclusion: Pretreatment GINI is a promising biomarker for predicting pathological response and survival outcomes in locally advanced ESCC patients treated with nICT. A high GINI level is significantly associated with treatment resistance and poorer prognosis, suggesting its potential utility in risk stratification and guiding individualized treatment strategies.
1 Introduction
Esophageal cancer (EC) is a significant global public health challenge. In 2022, there were approximately 511,000 new cases and 445,000 deaths worldwide, placing its incidence and mortality as the 11th and 7th highest, respectively, among all malignancies globally (1). While the incidence of esophageal cancer in China has been declining due to early screening and better control of risk factors, the country still bears one of the highest burdens of Esophageal Squamous Cell Carcinoma (ESCC) worldwide. The anatomical structure of the esophagus, combined with the non-specific early symptoms—such as difficulty swallowing (dysphagia) and chest pain (retrosternal pain)—often leads to most patients being diagnosed at an advanced or metastatic stage (2). This late diagnosis significantly affects treatment options and patient prognosis. In recent years, neoadjuvant immunochemotherapy (nICT) has emerged as a crucial treatment strategy for locally advanced ESCC. This approach can downstage tumors, eliminate micrometastases, and reduce the risks of intraoperative dissemination and postoperative recurrence, potentially improving patient outcomes. However, due to the heterogeneity of tumors, not all patients benefit from nICT. Current data suggest that only about 20 to 40% of patients achieve an optimal pathological response, with an even smaller percentage attaining long-term disease control (3). Therefore, it is essential to identify effective biomarkers that can predict both the pathological response and prognosis of nICT.
In clinical management, patients with EC face significant nutritional management challenges, as dysphagia often leads to a high incidence of malnutrition and cachexia. Concurrently, side effects from essential treatments such as chemotherapy, radiotherapy, and surgery—including anorexia and metabolic consumption—synergize with disease-related factors, further exacerbating the decline in nutritional status. As a result, the relationship between nutritional status and cancer prognosis has garnered increasing attention from researchers (4). To assess and predict prognosis in esophageal cancer, several nutritional assessment tools have been utilized, including the Prognostic Nutritional Index (PNI) (5), the global immune-nutrition-inflammation index (GINI) (6), Geriatric Nutritional Risk Index (GNRI) (7), Controlling Nutritional Status (CONUT) score (8), and Nutritional Risk Screening 2002 (NRS2002) score (9).
GINI is a composite indicator that combines six hematological parameters: C-reactive protein, platelets, monocytes, neutrophils, albumin, and lymphocytes. This index allows for a comprehensive assessment of the body’s nutritional and inflammatory status. It was initially developed and validated by Topkan et al. (10) as a prognostic factor for stage IIIC non-small cell lung cancer (NSCLC) patients undergoing chemoradiotherapy. Subsequently, its predictive value has been further confirmed in various gastrointestinal malignancies, including gastric cancer (11), esophageal cancer (6), and colorectal cancer (12), demonstrating a close association with tumor progression and outcomes.
Currently, no studies have examined the clinical value of GINI in patients with ESCC who are receiving nICT. To fill this research gap, this study is the first to systematically investigate the predictive role of GINI concerning treatment effectiveness and prognosis in ESCC patients undergoing nICT. The aim is to clarify its potential as a novel biomarker.
2 Materials and methods
2.1 Participants
We conducted a retrospective analysis of patients with locally advanced ESCC who received nICT at our institution from January 2022 to April 2024. The inclusion criteria were as follows: (1) age 18–75 years; (2) pathologically confirmed ESCC; (3) an Eastern Cooperative Oncology Group performance status (ECOG-PS) score of 0–1; (4) clinical TNM stage II-IVA; (5) successful R0 radical resection following nICT; and (6) availability of complete clinical data with a follow-up duration of ≥6 months. The exclusion criteria were: (1) pathological type other than ESCC; (2) failure to achieve R0 resection after nICT (i.e., R1 or R2 resection); (3) concomitant active infection, hematological disease, or autoimmune disease; (4) history of or concurrent other primary malignant tumors; or (5) receipt of other antitumor therapies during the study period. The study protocol was approved by the Medical Ethics Committee of the First Affiliated Hospital of Fujian Medical University (Approval No.: [2015]084–2) and strictly adhered to the ethical principles of the Declaration of Helsinki (2013 revision). Since this was a retrospective analysis, all patient data were anonymized, and retrospective informed consent for data usage was obtained, ensuring compliance with patient privacy and ethical standards.
A total of 161 patients with ESCC underwent nICT. Among them, 10 patients refused further treatment, and 13 patients had incomplete clinical data. Therefore, this study retrospectively included 138 patients (Figure 1).
2.2 Treatment protocols
All patients received nICT according to the Chinese Society of Clinical Oncology (CSCO) guidelines for esophageal cancer. The regimen consisted of albumin-bound paclitaxel (135 mg/m2, day 1), cisplatin (75 mg/m2, day 1), and tislelizumab/sintilimab/camrelizumab (200 mg, day 2), administered every 3 weeks for two cycles. Surgical resection was performed 4–6 weeks after the last nICT cycle. The surgical approach was determined by a multidisciplinary team (MDT) discussion and included minimally invasive esophagectomy (MIE, incorporating thoracoscopic/laparoscopic approaches), open surgery, or robot-assisted procedures, selected based on individual patient characteristics. All surgeries were performed by an experienced thoracic surgery team (averaging over 100 esophagectomies annually) at our institution.
2.3 Follow-up
To monitor disease progression, all patients were enrolled in a regular follow-up program after completion of treatment: follow-ups were conducted every 3 months for the first 2 years postoperatively, every 6 months during years 3–5, and annually thereafter. This study utilized the 8th edition AJCC/UICC staging system, and data collection continued until June 2025.
2.4 Calculation of the GINI
GINI is a composite parameter calculated based on six hematological indicators: neutrophil count (NEU#), monocyte count (MON#), platelet count (Plt), lymphocyte count (LYM#), albumin level, and C-reactive protein (CRP) level. In accordance with established research protocols, peripheral blood was collected from patients within 1 week prior to initiating nICT, and data for the aforementioned parameters were extracted. The GINI is calculated using the following formula:
2.5 Assessment of treatment response
Postoperative tumor regression was graded using the Becker Tumor Regression Grading (13), with the following criteria: TRG Grade 1a (Complete Regression): No residual tumor cells; TRG Grade 1b (Near-Complete Regression): Residual tumor cells occupy <10% of the tumor bed area; TRG Grade 2 (partial regression): residual tumor cells occupy 10–50% of the tumor bed area; TRG Grade 3 (poor regression): residual tumor cells occupy >50% of the tumor bed area. Pathological response to therapy was stratified as favorable (TRG 1a–1b, representing significant tumor remission) or suboptimal (TRG 2–3). For statistical analysis, patients were stratified by tumor regression grade (TRG) into two groups: TRG 1a–1b and TRG 2–3.
2.6 Statistical analysis
All cases included in this study underwent rigorous screening to ensure the completeness of key variables, including hematologic parameters and clinical pathological variables used for GINI calculation. Consequently, no missing values existed in the final analysis dataset, eliminating the need for imputation or exclusion.
Statistical analyses were performed using IBM SPSS Statistics software (Version 26.0) and R (version 4.x). Continuous data conforming to a normal distribution are presented as mean ± standard deviation and were compared using the Student’s t-test. Categorical data are presented as numbers (percentages) and were compared using the Chi-square test or Fisher’s exact test (when expected frequencies were <5). To evaluate the predictive ability of GINI for treatment response, a receiver operating characteristic (ROC) curve was plotted, and the area under the curve (AUC) was calculated in R. To assess further the model’s internal validation performance and optimism bias, 1,000 bootstrap resamples were performed, and corrected performance metrics including accuracy, Kappa value, sensitivity, specificity, and AUC were computed. The optimal cutoff value for GINI was determined using the Youden’s index. Variables showing statistical significance in univariate analysis were subsequently included in a multivariate logistic regression model to identify independent predictors of pathological response. The Cox proportional hazards regression model was used to analyze independent prognostic factors for disease-free survival (DFS) and overall survival (OS), with results expressed as hazard ratios (HRs) and 95% confidence intervals (CIs). Survival curves were plotted using the Kaplan–Meier method, the median follow-up time was calculated using the reverse Kaplan–Meier method, and intergroup comparisons were performed using the log-rank test. To enhance the clinical interpretability of the GINI index’s effect size, we standardized it using different units to recalculate the effect sizes: per standard deviation change (61.30 units), per 25-unit change, and per 50-unit change. Additionally, we assessed the linear relationship between the GINI index and outcome measures using the chi-square linear-by-linear association test and the Log-rank test for trend. All tests were two-sided, and a p-value < 0.05 was considered statistically significant.
3 Results
3.1 Patient characteristics
This study ultimately enrolled 138 patients with locally advanced ESCC who received nICT. The mean age of the entire cohort was 60.5 years (range: 47–75 years), comprising 121 males (87.7%) and 17 females (12.3%). The median follow-up time was 24.5 months (range: 6–41 months). Tumor locations were predominantly in the middle esophagus (50.7%) and lower esophagus (36.2%). The majority of tumors (58.0%) were moderately differentiated, while 16.7% were poorly differentiated. Postoperative pathological (ypT) staging was as follows: ypT0 in 26 cases (18.8%), ypT1-2 in 56 cases (40.6%), and ypT3-4a in 56 cases (40.6%). Postoperative complications occurred in 55 patients (39.9%). During the follow-up period, 27 (19.6%) patients experienced recurrence. Among them, there were 13 (48.1%) patients with distant recurrence after treatment, including peritoneal metastasis and non-regional lymph node metastasis (LNM), while there were 14 (51.9%) cases with local recurrence, including locoregional LNM and anastomotic site recurrence (Figure 2). Patient clinicopathological characteristics are shown in Table 1.

Figure 2. Detailed recurrence patterns after nICT. There were 13 (48.1%) cases with distant recurrence and 14 (51.9%) cases with local recurrence.
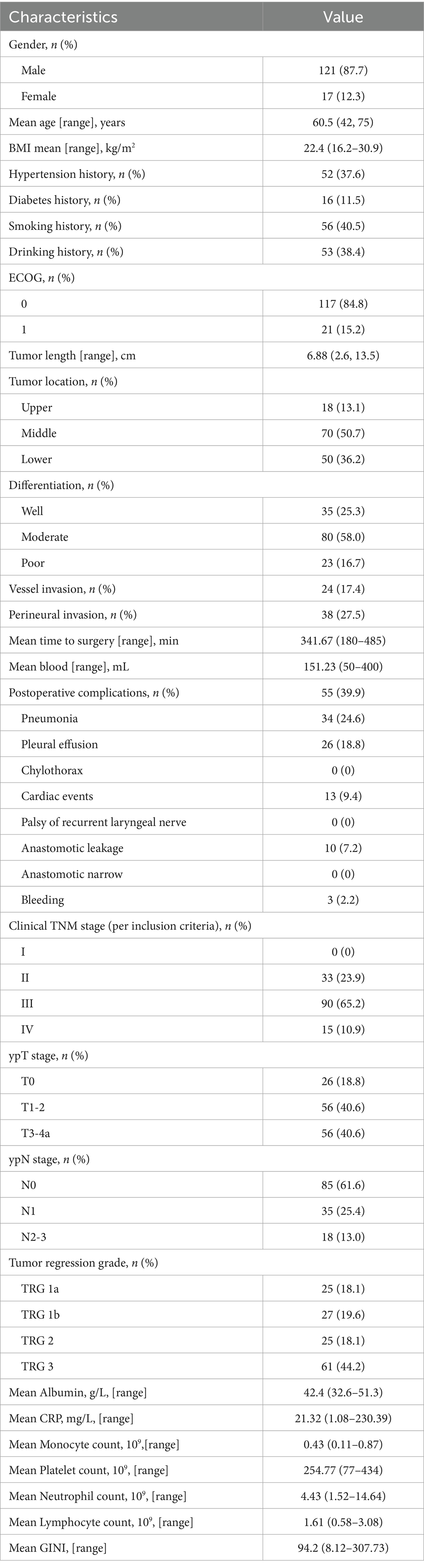
Table 1. Clinicopathological characteristics of 138 patients with esophageal squamous cell carcinoma.
3.2 Univariate and multivariate analyses of pathological tumor regression response
To identify predictors of pathological tumor regression response, univariate and multivariate logistic regression analyses were performed (results shown in Table 2). Univariate analysis demonstrated that age (p = 0.025), lymphocyte count (LYM#, p = 0.021), and GINI score (p < 0.001) were significantly associated with pathological response. Multivariate analysis further confirmed that both LYM# (OR = 0.29, 95% CI: 0.09–0.90, p = 0.033) and GINI score (OR = 1.05 95% CI: 1.03–1.06, p < 0.001) were independent predictive factors for pathological tumor regression following nICT.
3.3 Optimal cut-off values of GINI before nICT
To evaluate the predictive efficacy of GINI for tumor regression response, we constructed a ROC curve (Figure 3). The results demonstrated significant predictive value for GINI, with an area under the curve (AUC) of 91.2%. The optimal cutoff value was determined to be 73.47, corresponding to a sensitivity of 86.5%, specificity of 84.9%, and a Youden’s index of 0.714. And bootstrap internal validation (1,000 repetitions) demonstrated robust discriminative ability of the model, with a corrected mean AUC of 0.909, which is closely aligned with the original AUC of 0.912. Furthermore, the model exhibited strong predictive accuracy (accuracy = 0.840, Kappa = 0.658) and stable classification performance (sensitivity = 86.4%, specificity = 80.3%), indicating excellent generalizability and a low risk of overfitting. To assess the robustness of the identified optimal cutoff value (73.47), we performed a sensitivity analysis by evaluating the performance of adjacent cutoff values. As summarized in Supplementary Table 2, the predictive performance remained highly stable across a range of cutoff values from 72.72 to 75.42. Specifically, sensitivity ranged from 84.6 to 88.5%, specificity from 82.6 to 84.9%, and the negative predictive value consistently exceeded 90% at all tested thresholds. This stability confirms that the discriminative ability of the GINI index is not critically dependent on a single cutoff value and reinforces its reliability for clinical risk stratification.

Figure 3. The left figure displays a single ROC curve based on the entire sample, while the right figure shows the results of 1,000 Bootstrap validations. The gray lines represent the ROC curve for each resampling, and the red line indicates the averaged ROC curve along with its corresponding AUC value. GINI, the global immune-nutrition-inflammation index; ROC, receiver operating characteristic.
Patients were dichotomized based on this cutoff into low-GINI (<73.47; n = 58) and high-GINI (≥73.47; n = 80) groups. Comparison of therapeutic efficacy between groups revealed that 77.6% of patients in the low-GINI group achieved a favorable pathological response, compared to only 8.6% in the high-GINI group. This difference was statistically significant (p < 0.001), indicating that a higher baseline GINI level is significantly associated with poorer nICT efficacy.
To enhance the clinical interpretability of the GINI index, we further analyzed its effect on outcomes using a continuous scale with standardized and clinically relevant units (Supplementary Table 1). In multivariate analyses, each one-standard-deviation increase in GINI (61.30 units) was associated with a significantly increased risk of poor pathological response (OR = 15.252, 95% CI: 6.216–37.422, p < 0.001) and worse overall survival (HR = 1.909, 95% CI: 1.155–3.156, p = 0.012). When scaled to smaller, clinically practical increments, each 25-unit and 50-unit increase in GINI remained significantly associated with adverse outcomes for both pathological response and survival (all p < 0.05).
Furthermore, tests for linear trend confirmed a significant dose–response relationship between the continuous GINI index and both pathological response (linear-by-linear association χ2 = 64.122, p < 0.001) and overall survival (log-rank test for trend χ2 = 7.147, p = 0.008), supporting its use as a robust and monotonically increasing risk factor.
3.4 Patient characteristics stratified by GINI
Based on the optimal cutoff value (73.47) determined by the ROC curve, the 138 patients were divided into a low-GINI group (58 patients, 42.0%) and a high-GINI group (80 patients, 58.0%). A comparison of the baseline clinical characteristics between the two groups is shown in Table 3. The high-GINI group showed significantly higher proportions of patients with poor tumor differentiation (p < 0.001), vascular invasion (p < 0.001), perineural invasion (p < 0.001), postoperative pneumonia (p = 0.016), pleural effusion (p = 0.014), as well as more advanced ypT stage (p < 0.001) and ypTNM stage (p < 0.001) compared to the low-GINI group.
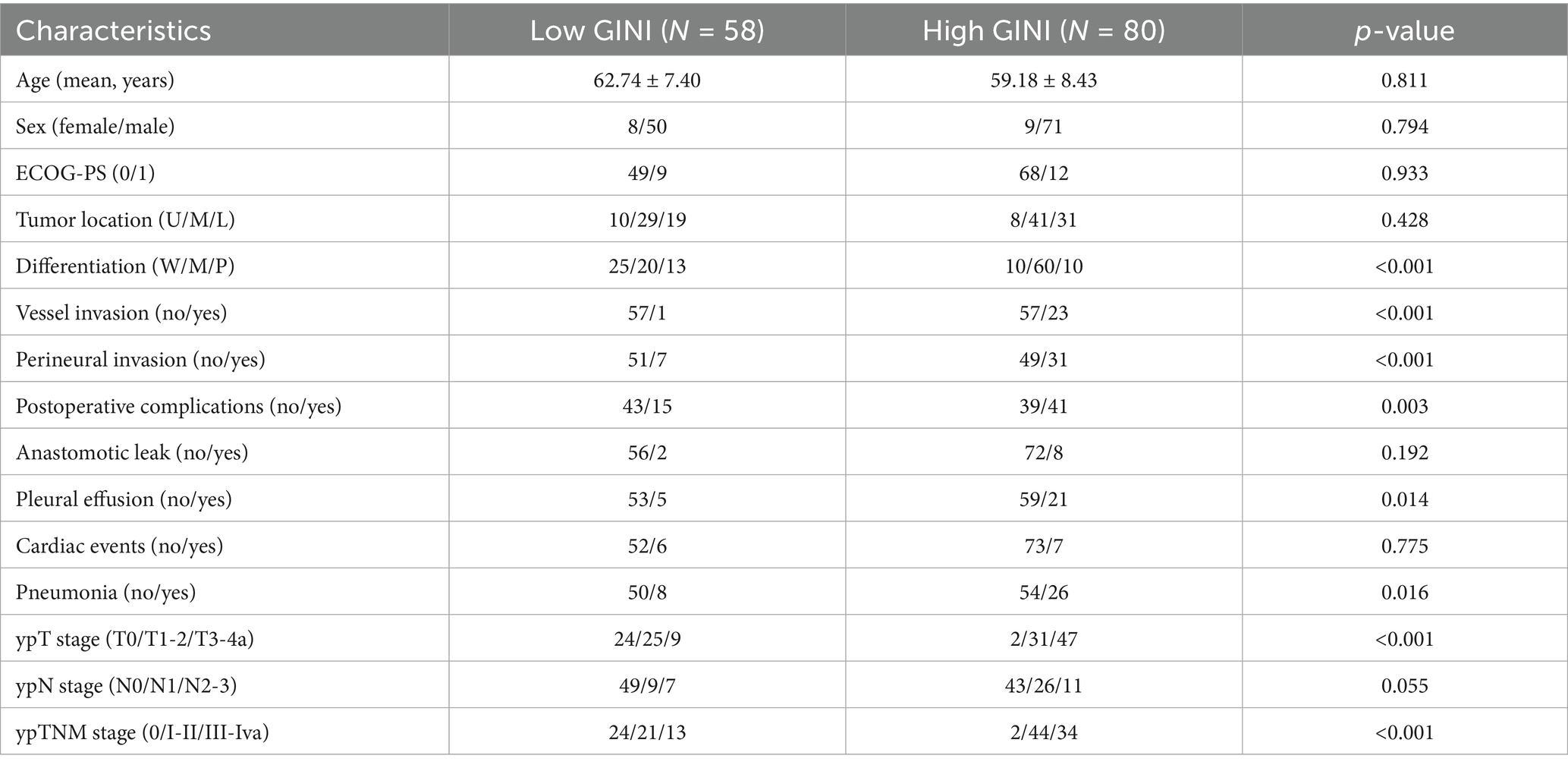
Table 3. Comparison of clinical variables in esophageal squamous cell carcinoma patients stratified by GINI.
3.5 Survival analysis
At the time of data analysis, the median survival time of this study had not been reached. For the entire cohort, the 1-, 2-, and 3-year survival rates were as follows: overall survival (OS), 96.4, 90.1, and 77.5%; disease-free survival (DFS), 86.2, 78.8, and 71.8%. Kaplan–Meier survival analysis (Figure 4) demonstrated that the low-GINI group had significantly better 3-year OS (87.8% vs. 68.7%, p = 0.014) and DFS (82.7% vs. 63.3%, p = 0.011) compared to the high-GINI group, indicating that a higher pretreatment GINI is a predictor of poor survival outcomes.
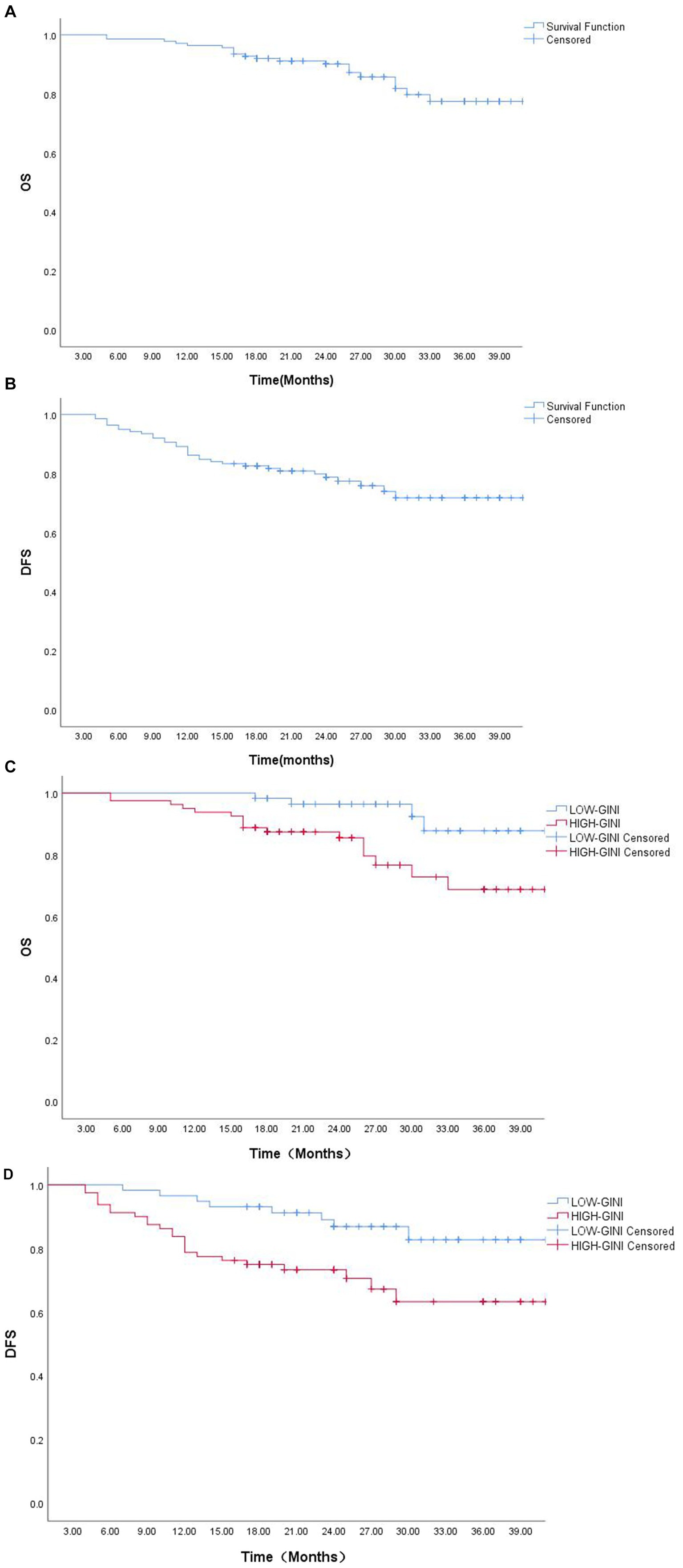
Figure 4. Survival analysis curves: (A) Overall survival (OS) curve for the entire cohort; (B) Disease-free survival (DFS) curve for the entire cohort; (C) OS curve stratified by GINI; (D) DFS curve stratified by GINI.
To further investigate the predictive value of GINI for survival outcomes following nICT, we conducted separate Cox regression analyses for DFS and OS. Univariate analysis for DFS initially identified six potential prognostic factors (Table 4). After adjusting for potential confounders, multivariate analysis identified only lymph node metastasis status, ypTNM stage, and postoperative complications as independent predictors of DFS. In contrast, for OS, multivariate analysis demonstrated that the GINI score (HR = 1.01, p = 0.012) exhibited independent prognostic value, highlighting its significant role in predicting long-term patient survival.
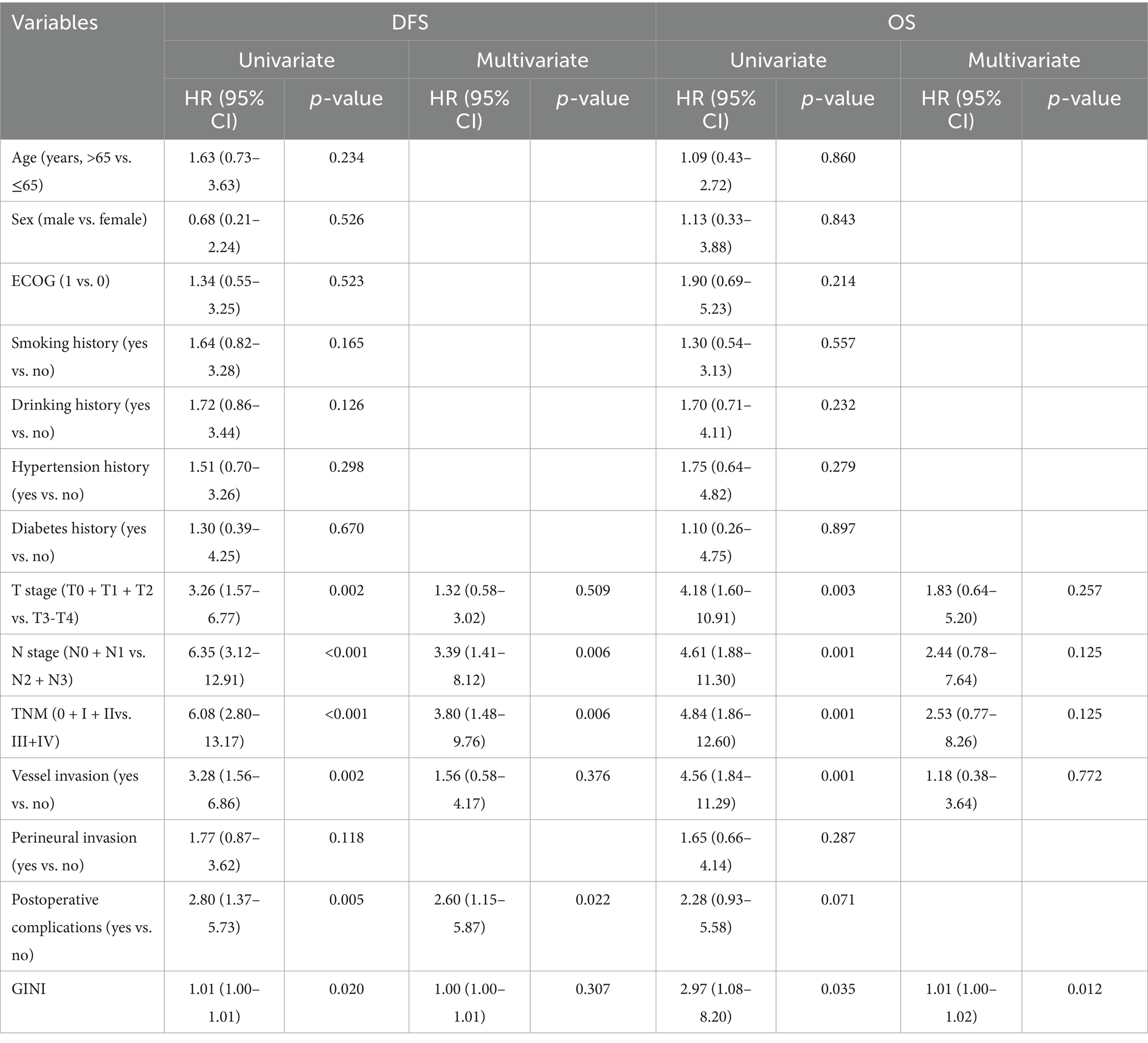
Table 4. Univariate and multivariate cox proportional hazards analyses of clinicopathological factors for DFS and OS.
4 Discussion
Malnutrition is a prevalent complication during the treatment of malignant tumors, with approximately 80% of cancer patients experiencing varying degrees of malnutrition at different stages of therapy (14). In patients with esophageal cancer, the occurrence of cachexia is particularly prominent. The underlying mechanisms of this condition include reduced food intake and metabolic disorders, which encompass increased energy expenditure, excessive catabolism, and systemic inflammatory responses (15). Moreover, chronic inflammation is recognized as a key characteristic of cancer, influencing the entire process of tumor development and progression (16). In light of recent findings, the importance of nutrition and inflammation-related indicators in assessing prognosis has gained significant attention. Serum albumin levels are not only indicative of the body’s nutritional reserves, but low levels (hypoalbuminemia) are also closely linked to poor postoperative recovery and the onset of cachexia (17). Additionally, C-reactive protein (CRP), a vital marker of systemic inflammation, usually suggests a worse prognosis when present at elevated levels (18); neutrophils contribute to disease progression by promoting tumor cell proliferation, suppressing immune function, and stimulating angiogenesis (19); Conversely, lymphocytes play a crucial role in antitumor immunity through their cytotoxic effects (20). Moreover, platelets enhance tumor angiogenesis and invasive behavior by releasing various bioactive factors (21).
GINI combines key indicators to create a comprehensive biomarker that reflects an individual’s nutritional, inflammatory, and immune status. This index is calculated by multiplying the C-reactive protein/albumin ratio (CAR) by the pan-immunological inflammation value (PIV) (10). Previous studies have shown that CAR is an effective tool for assessing both systemic inflammation and nutritional status (22, 23). As highlighted by Topkan et al. (10), GINI’s primary advantage is its ability to simultaneously incorporate both albumin and CRP—two critical parameters essential for accurate diagnosis. In cancer-associated inflammatory states, inflammatory cytokines such as IL-6 and TNF-α not only stimulate the synthesis of CRP (24, 25) but also inhibit the synthesis of albumin, accelerate its degradation, and promote vascular extravasation (26). GINI effectively captures the fundamental aspects of this pathophysiological process, indicating its potential for predicting treatment efficacy and prognosis. Existing studies confirm that GINI is strongly associated with prognosis across various malignancies. For instance, Bozkurt et al. (12) found that GINI correlated with poor prognosis in metastatic colorectal cancer; Yamamoto et al. (6) identified GINI as an independent predictor of overall survival following radical treatment for esophageal cancer; and Topkan et al. (27) reported that baseline GINI is an effective predictor of progression-free survival and overall survival in patients with glioblastoma.
In recent years, nICT has emerged as a crucial treatment strategy for locally advanced ESCC; however, reliable nutrition-related biomarkers for early prediction of treatment response remain lacking. This study is the first to systematically evaluate the impact of GINI on pathological tumor regression response and prognosis in ESCC patients receiving nICT. The results showed that GINI and lymphocyte count served as independent predictive factors for pathological tumor regression response. The optimal cutoff value of GINI determined by the Youden index effectively stratified patients into subgroups with significant clinical differences. Our analysis demonstrated that patients in the low-GINI group exhibited favorable pathological characteristics, including better tumor differentiation and earlier disease stage, and achieved superior survival outcomes. These findings suggest that GINI serves as a robust biomarker for pretreatment risk stratification. This indicator helps identify high-risk patients who may respond poorly to nICT, providing a basis for developing intensified adjuvant treatment strategies for the high-GINI population, thereby advancing the progress of individualized precision therapy for esophageal squamous cell carcinoma.
Furthermore, in this study, we systematically investigated the association between GINI and clinical outcomes in ESCC patients following nICT using multiple analytical methods. Kaplan–Meier survival analysis demonstrated that patients in the low-GINI group had significantly better survival outcomes. Multivariate Cox regression analysis further confirmed that GINI was an independent prognostic factor for overall survival, reinforcing its biological value in comprehensively reflecting the body’s inflammatory, nutritional, and immune imbalance status. However, it is noteworthy that GINI did not demonstrate independent predictive value for disease-free survival in multivariate analysis. We speculate that this may stem from GINI’s influence on recurrence risk being largely indirect, potentially mediated through intermediate factors such as tumor burden and postoperative complications. In contrast, GINI’s independent predictive power for overall survival suggests that nutritional-inflammatory status may more directly determine long-term prognosis through non-recurrence pathways, including effects on treatment tolerance and non-cancer-related survival. This finding highlights the need in clinical practice to consider not only tumor characteristics but also the host’s systemic response status.
Limitations of this study include: (1) The single-center retrospective design may introduce selection bias; (2) The optimal cutoff value for GINI should be validated in prospective, multicenter observational studies rather than additional retrospective analyses to confirm its generalizability and clinical utility; (3) This study focused on validating the prognostic value of GINI itself. We did not compare its predictive performance with other established or emerging biomarkers in ESCC, such as PD-L1 combined positive score (CPS) or tumor mutational burden (TMB), as their assessment was beyond the scope of this retrospective analysis; (4) Only baseline GINI values were used without considering dynamic changes during treatment; (5) Although GINI demonstrates good comprehensive predictive capability, its specific predictive mechanisms in the context of nICT require further in-depth investigation; (6)The nICT regimen in this study included one of three different PD-1 inhibitors. Due to the limited sample size, we were unable to conduct a thorough subgroup analysis and therefore could not evaluate the effect of each PD-1 inhibitor on treatment efficacy. As a result, we cannot rule out the possibility of unmeasured bias in our analysis arising from the combination of these inhibitors. Future research should focus on establishing standardized cutoff values, implementing dynamic monitoring, elucidating the underlying predictive mechanisms, and exploring whether GINI has a complementary or substitute relationship with other biomarkers.
5 Conclusion
This study demonstrates that the pretreatment GINI serves as an effective biomarker for predicting treatment response and prognosis in patients with locally advanced ESCC undergoing nICT. A higher pretreatment GINI level was independently associated with unfavorable pathological characteristics, lower tumor regression rates, and significantly shorter survival. By identifying high-risk patients before nICT, GINI paves the way for more individualized therapy. For instance, patients identified as high-risk by an elevated GINI score could be candidates for more intensive neoadjuvant regimens, closer monitoring during treatment, or be prioritized for subsequent adjuvant therapies. Conversely, low-GINI patients, who are likely to respond well to standard nICT, could be spared from unnecessary treatment intensification and its associated toxicities. The integration of GINI into clinical decision-making, however, requires further validation through multicenter prospective trials.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Ethics statement
Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article because the study was approved by the Ethics Committee of Fujian Medical University (No.: [2015]084–2) and individual consent for this retrospective analysis was waived.
Author contributions
ZF: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Validation, Writing – original draft, Writing – review & editing, Visualization. HY: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Validation, Writing – original draft, Writing – review & editing. JX: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing, Funding acquisition, Validation. ZH: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. YT: Conceptualization, Data curation, Formal analysis, Methodology, Resources, Supervision, Writing – review & editing, Investigation, Project administration. BL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the Fujian Provincial Health Commission’s Young and Middle-aged Backbone Talent Project (No. 2024GGB08), the Fujian Provincial Finance Department’s Special Fund (No. BPB2022LB), and the University-Industry Research Cooperation Project of Science and Technology, Fujian Province (No. 2020Y4008).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer ZY declared a shared parent affiliation with the authors to the handling editor at the time of review.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1717477/full#supplementary-material
References
1. Bray, F, Laversanne, M, Sung, H, Ferlay, J, Siegel, RL, Soerjomataram, I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Li, J, Xu, J, Zheng, Y, Gao, Y, He, S, Li, H, et al. Esophageal cancer: epidemiology, risk factors and screening. Chin J Cancer Res. (2021) 33:535–47. doi: 10.21147/j.issn.1000-9604.2021.05.01
3. Doroshow, DB, Bhalla, S, Beasley, MB, Sholl, LM, Kerr, KM, Gnjatic, S, et al. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat Rev Clin Oncol. (2021) 18:345–62. doi: 10.1038/s41571-021-00473-5
4. Ruan, GT, Yang, M, Zhang, XW, Zhang, HL, Li, RQ, Liu, LM, et al. Association of systemic inflammation and overall survival in elderly patients with cancer cachexia - results from a multicenter study. J Inflamm Res. (2021) 14:5527–40. doi: 10.2147/JIR.S332408
5. Zhang, L, Ma, W, Qiu, Z, Li, J, Wang, Y, He, Y, et al. Prognostic nutritional index as a prognostic biomarker for gastrointestinal cancer patients treated with immune checkpoint inhibitors. Front Immunol. (2023) 14:1219929. doi: 10.3389/fimmu.2023.1219929
6. Yamamoto, S, Aoyama, T, Maezawa, Y, Hara, K, Esashi, M, Tamagawa, A, et al. The global immune-nutrition-information index (GINI) is an independent prognostic factor for esophageal cancer patients who receive curative treatment. Cancer Diagn Progn. (2025) 5:115–21. doi: 10.21873/cdp.10419
7. Fang, P, Yang, Q, Zhou, J, Wang, X, Liu, Y, Song, C, et al. The impact of geriatric nutritional risk index on esophageal squamous cell carcinoma patients with neoadjuvant therapy followed by esophagectomy. Front Nutr. (2022) 9:983038. doi: 10.3389/fnut.2022.983038
8. Yoshida, N, Baba, Y, Shigaki, H, Harada, K, Kosumi, K, Kurashige, J, et al. Preoperative nutritional assessment by controlling nutritional status (CONUT) is useful to estimate postoperative morbidity after esophagectomy for esophageal cancer. World J Surg. (2016) 40:1910–7. doi: 10.1007/s00268-016-3549-3
9. Hersberger, L, Bargetzi, L, Bargetzi, A, Tribolet, P, Baechli, V, Geiser, M, et al. Nutritional risk screening (NRS 2002) is a strong and modifiable predictor risk score for short-term and long-term clinical outcomes: secondary analysis of a prospective randomised trial. Clin Nutr. (2020) 39:2720–9. doi: 10.1016/j.clnu.2019.11.041
10. Topkan, E, Selek, U, Pehlivan, B, Kucuk, A, Ozturk, D, Ozdemir, BS, et al. The prognostic value of the novel global immune-nutrition-inflammation index (GINI) in stage IIIC non-small cell lung Cancer patients treated with concurrent Chemoradiotherapy. Cancers (Basel). (2023) 15:4512. doi: 10.3390/cancers15184512
11. Aoyama, T, Hashimoto, I, Maezawa, Y, Hara, K, Yamamoto, S, Esashi, R, et al. Global immune-nutrition-information index is independent prognostic factor for gastric Cancer patients who received curative treatment. Cancer Diagn Progn. (2024) 4:489–95. doi: 10.21873/cdp.10353
12. Bozkurt, O, Gönül, R, Kaya, BU, Zararsiz, GE, İnanc, M, and Özkan, M. The prognostic importance of the global immune-nutrition-information index (GINI) in patients with Ras wild type metastatic colorectal cancer. Sci Rep. (2025) 15:25525. doi: 10.1038/s41598-025-11148-x
13. Becker, K, Mueller, JD, Schulmacher, C, Ott, K, Fink, U, Busch, R, et al. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer. (2003) 98:1521–30. doi: 10.1002/cncr.11660
14. Trujillo, EB, Dixon, SW, Claghorn, K, Levin, RM, Mills, JB, and Spees, CK. Closing the gap in nutrition care at outpatient cancer centers: ongoing initiatives of the oncology nutrition dietetic practice group. J Acad Nutr Diet. (2018) 118:749–60. doi: 10.1016/j.jand.2018.02.010
15. Baracos, VE, Martin, L, Korc, M, Guttridge, DC, and Fearon, KCH. Cancer-associated cachexia. Nat Rev Dis Primers. (2018) 4:17105. doi: 10.1038/nrdp.2017.105
16. Sethi, G, Shanmugam, MK, Ramachandran, L, Kumar, AP, and Tergaonkar, V. Multifaceted link between cancer and inflammation. Biosci Rep. (2012) 32:1–15. doi: 10.1042/BSR20100136
17. Haskins, IN, Baginsky, M, Amdur, RL, and Agarwal, S. Preoperative hypoalbuminemia is associated with worse outcomes in colon cancer patients. Clin Nutr. (2017) 36:1333–8. doi: 10.1016/j.clnu.2016.08.023
18. Groblewska, M, Mroczko, B, Sosnowska, D, and Szmitkowski, M. Interleukin 6 and C-reactive protein in esophageal cancer. Clin Chim Acta. (2012) 413:1583–90. doi: 10.1016/j.cca.2012.05.009
19. Xiong, S, Dong, L, and Cheng, L. Neutrophils in cancer carcinogenesis and metastasis. J Hematol Oncol. (2021) 14:173. doi: 10.1186/s13045-021-01187-y
20. Mantovani, A, Allavena, P, Sica, A, and Balkwill, F. Cancer-related inflammation. Nature. (2008) 454:436–44. doi: 10.1038/nature07205
21. Bambace, NM, and Holmes, CE. The platelet contribution to cancer progression. J Thromb Haemost. (2011) 9:237–49. doi: 10.1111/j.1538-7836.2010.04131.x
22. Frey, A, Martin, D, D'Cruz, L, Fokas, E, Rödel, C, Fleischmann, M, et al. C-reactive protein to albumin ratio as prognostic marker in locally advanced non-small cell lung cancer treated with chemoradiotherapy. Biomedicine. (2022) 10:598. doi: 10.3390/biomedicines10030598
23. He, D, Yang, Y, Yang, Y, Tang, X, and Huang, K. Prognostic significance of preoperative C-reactive protein to albumin ratio in non-small cell lung cancer patients: a meta-analysis. Front Surg. (2023) 9:1056795. doi: 10.3389/fsurg.2022.1056795
24. Greten, FR, and Grivennikov, SI. Inflammation and Cancer: triggers, mechanisms, and consequences. Immunity. (2019) 51:27–41. doi: 10.1016/j.immuni.2019.06.025
25. Lippitz, BE, and Harris, RA. Cytokine patterns in cancer patients: a review of the correlation between interleukin 6 and prognosis. Onco Targets Ther. (2016) 5:e1093722. doi: 10.1080/2162402X.2015.1093722
26. Keller, U. Nutritional laboratory markers in malnutrition. J Clin Med. (2019) 8:775. doi: 10.3390/jcm8060775
27. Topkan, E, Kilic Durankus, N, Senyurek, S, Öztürk, D, Besen, AA, Mertsoylu, H, et al. The global immune-nutrition-inflammation index (GINI) as a robust prognostic factor in glioblastoma patients treated with the standard stupp protocol. Int J Immunopathol Pharmacol. (2024) 38:3946320241284089. doi: 10.1177/03946320241284089
Keywords: global immune-nutrition-inflammation index (GINI), esophageal squamous cell carcinoma, neoadjuvant immunochemotherapy, prognosis, biomarker
Citation: Feng Z, Yi H, Xiao J, Huang Z, Tu Y and Liu B (2025) The global immune-nutrition-inflammation index predicts pathological response and survival in esophageal squamous cell carcinoma treated with neoadjuvant immunochemotherapy. Front. Nutr. 12:1717477. doi: 10.3389/fnut.2025.1717477
Edited by:
Guangming Zhang, University of Texas Health Science Center at Houston, United StatesReviewed by:
Jing Xue, Wuxi People’s Hospital Affiliated to Nanjing Medical University, ChinaZhang Yang, Fujian Medical University Union Hospital, China
Copyright © 2025 Feng, Yi, Xiao, Huang, Tu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Liu, MzI4MjIxNzIwQHFxLmNvbQ==
 Zhouxv Feng
Zhouxv Feng Huihan Yi
Huihan Yi Jiazhou Xiao1,2
Jiazhou Xiao1,2
