Abstract
Owing to the complex nature of vector-borne diseases (VBDs), whereby monitoring of human case patients does not suffice, public health authorities experience challenges in surveillance and control of VBDs. Knowledge on the presence and distribution of vectors and the pathogens that they transmit is vital to the risk assessment process to permit effective early warning, surveillance, and control of VBDs. Upon accepting this reality, public health authorities face an ever-increasing range of possible surveillance targets and an associated prioritization process. Here, we propose a comprehensive approach that integrates three surveillance strategies: population-based surveillance, disease-based surveillance, and context-based surveillance for EU member states to tailor the best surveillance strategy for control of VBDs in their geographic region. By classifying the surveillance structure into five different contexts, we hope to provide guidance in optimizing surveillance efforts. Contextual surveillance strategies for VBDs entail combining organization and data collection approaches that result in disease intelligence rather than a preset static structure.
Introduction
Globalization and human population growth continue to put increasing pressures on human health and well-being. Emerging diseases have become a growing threat leading to the development of epidemic intelligence systems to aid public health authorities. The main aim of this epidemic intelligence is to encompass all activities that permit the early identification of potential health hazards, their verification, risk assessment, and investigation in order to inform and improve public health control measures in a timely manner (1). Epidemic intelligence integrates both an indicator-based and an event-based component; the former referring to structured collection of data through routine surveillance systems and the latter referring to data gathered from sources of intelligence of any nature (2). Finally, good epidemiologic judgment, the reasoning process that indicates when there is sufficient data on which to make public health decisions (1), is essential. This has been a challenging component in the past but even more so today with mandated transparency of public decisions, and a continuously changing interconnected world. Further, it has become apparent that an interdisciplinary approach to the prevention and control of zoonoses is invaluable. Cross-sector working ensures better preparedness and contingency planning, more efficient and effective surveillance systems, cost-sharing between sectors according to their benefits of control, increased health equity, and improved sharing of logistics and costs for service provision (3). In recent years, efforts to improve the collaboration between the public and veterinary health, has paid off (4). Since the transmission cycles of several zoonotic pathogens occur in nature, involvement of stakeholders of forest and nature management is a logical next step. This means combining organization and data collection approaches that result in disease intelligence (2) rather than a preset static structure.
In recent decades, vector-borne diseases (VBDs) have emerged as a significant threat to human health in temperate areas (5). In Europe, the incidence and geographical distribution of endemic VBDs, specifically Lyme borreliosis, are increasing in several areas (6). Also, West Nile fever is emerging in Europe (7). Other VBDs have appeared outside the regions where they originally circulated such as dengue in Madeira (8). And last but not least, novel VBDs have emerged or have recently been recognized, such as infections with the tick-borne Borrelia miyamotoi (9). Considering the already occurring disease burden and various emerging threats for the future, cost-effective VBD surveillance is challenging. Investment in networks keeping a close watch on the matters within their expertise, public health, veterinary, and ecohealth (Figure 1) is needed. Here, ecohealth is defined as the combined health effects of (changes to) the ecologic network in which we function. This also includes the implications of nature management decisions and land use on factors influencing VBD epidemiology. Each and every pathogen has its own disease ecology; therefore, the combined effect of changes affecting factors like, for instance, reservoir species distribution, vector densities, and human exposure, is inheritably complex.
Figure 1
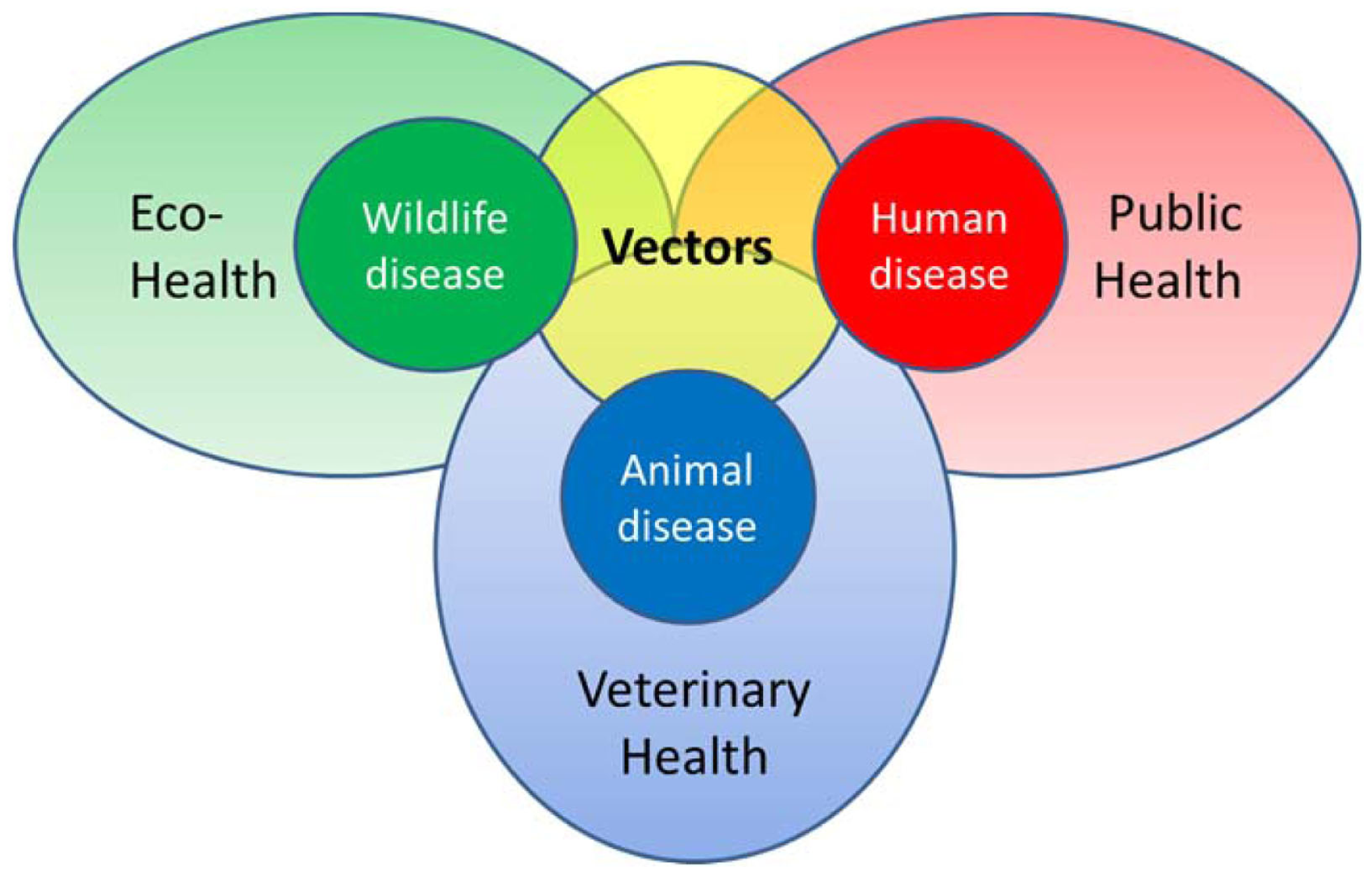
Schematic representation of the various health disciplines involved in vector-borne diseases.
We aim to develop a strategy for the surveillance of vector-borne pathogens, on a national as well as a Pan-European context. When developing and structuring VBD surveillance strategies, consideration of potential prospectives for action is important. Here, we present the building blocks of such contextual surveillance strategy. We expand the surveillance pyramids conventionally used as the basis of public health surveillance to pyramid assemblies, encompassing data from livestock (and pets), wildlife, and vector population. Next, we customize the pyramid assemblies to specific diseases, here focusing on those transmitted by mosquitoes and ticks. The contextual surveillance strategy of VBDs is further illustrated using examples from the Netherlands. Finally, we link the national with Pan-European VBD surveillance strategies.
Building Blocks
Population-based surveillance
To adequately monitor the disease burden of VBDs related to their occurrence, information is needed from different subgroups of the human population, represented by a disease burden pyramid. However, the forecasting, early detection, prevention and control of VBDs requires knowledge of other parameters. Acquiring data on the presence and density of (infected) vectors complements the disease burden pyramid for VBDs. In the case of a zoonotic VBD, an additional level is required to encompass the density of (infected) reservoir hosts (10). Actually, for each of these layers (10), a surveillance pyramid of their own can be drawn, which can be used when developing a contextual surveillance strategy. Separate surveillance pyramids can also be drawn for each of the additional population groups (e.g., sentinels or affected animal populations), highlighting all possible targets and levels of surveillance, forming a surveillance pyramid assembly (Figure 2). The distinction between wild and domestic animals is necessary to match the different health disciplines involved in the data collection (see also Figure 1). For all pyramids, the level of “infectious population” is added to better assess transmission risks. While all surveillance pyramids have the same organization, not all levels occur in every pyramid. For example, the top four surveillance levels of the pyramid do not exist for vectors. An additional point is that some pyramids might be absent in the surveillance pyramid assembly (see below).
Figure 2
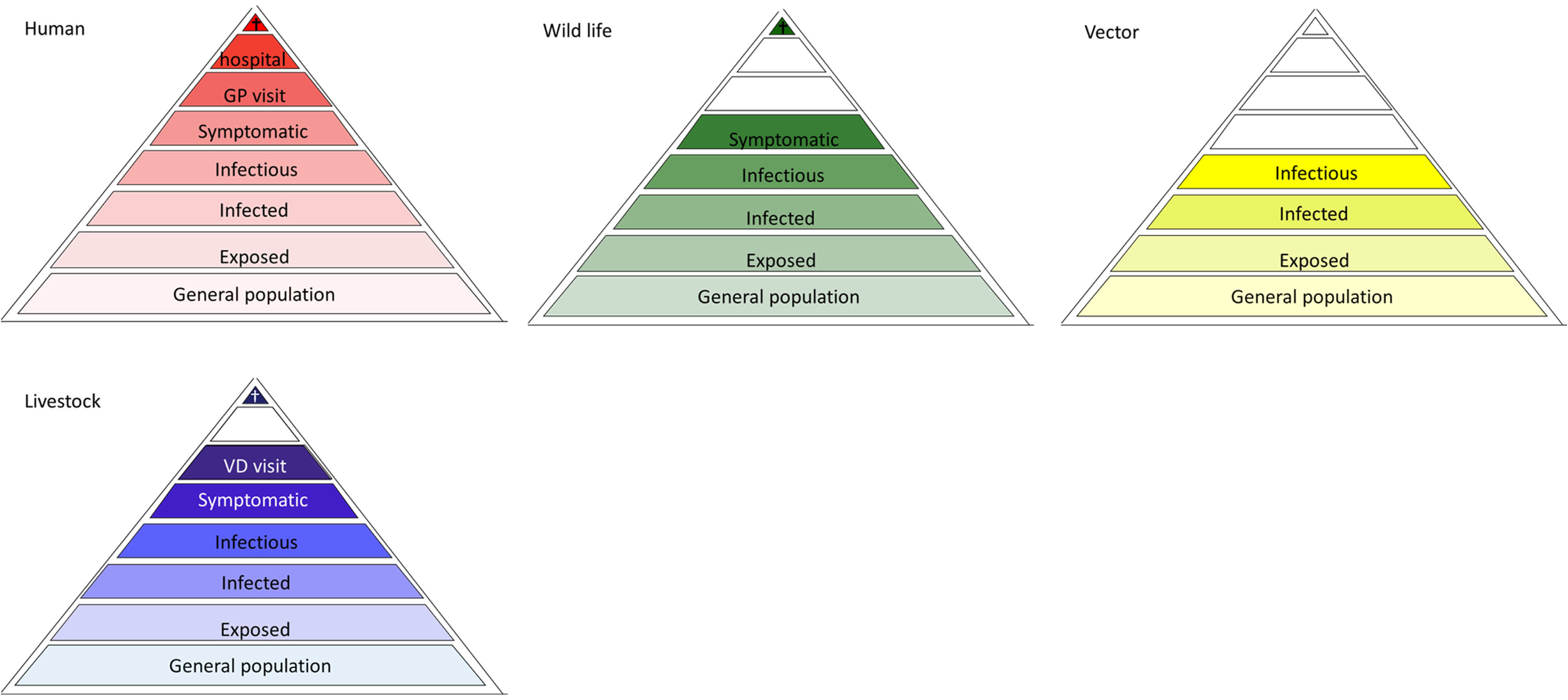
Surveillance pyramid assembly of a hypothetical vector-borne zoonosis. Not all levels exist in surveillance pyramid of all populations. The color scheme used refers to Figure 1.
Disease-based surveillance
To accurately describe all possible targets of surveillance for a specific VBD, tailoring the surveillance pyramid assembly is important. Some vector-borne pathogens, such as malaria, dengue, and chikungunya, can be maintained in a human-vector-human cycle, with no involvement of animals in the transmission cycle. Most vector-borne pathogens, however, circulate among vectors and (wild) reservoir animals. Here, humans frequently act as dead-end hosts, from which pathogens are not transmitted to other susceptible hosts, in nature; non-natural routes of transmission (e.g., through blood transfusion) or “rare” routes (e.g., mother-to-child transmission) are not considered here. In these cases, the infectious level is omitted from the human surveillance pyramid (Figures 3A–C and 4B). The same holds for animal species that are dead-end hosts (Figures 3A and 4B). In some cases, the addition of a pyramid for animals, that are neither a reservoir nor a host, but may serve as a sentinel for exposure, can be useful. Spill-over of tick-borne encephalitis virus, for example, has been revealed in dogs and deer in Belgium (11, 12). Similarly, chickens may serve as sensitive sentinels for West Nile virus (WNV) without contributing to the virus transmission cycle. Vector surveillance usually entails data on the population sizes and the infection rates.
Figure 3
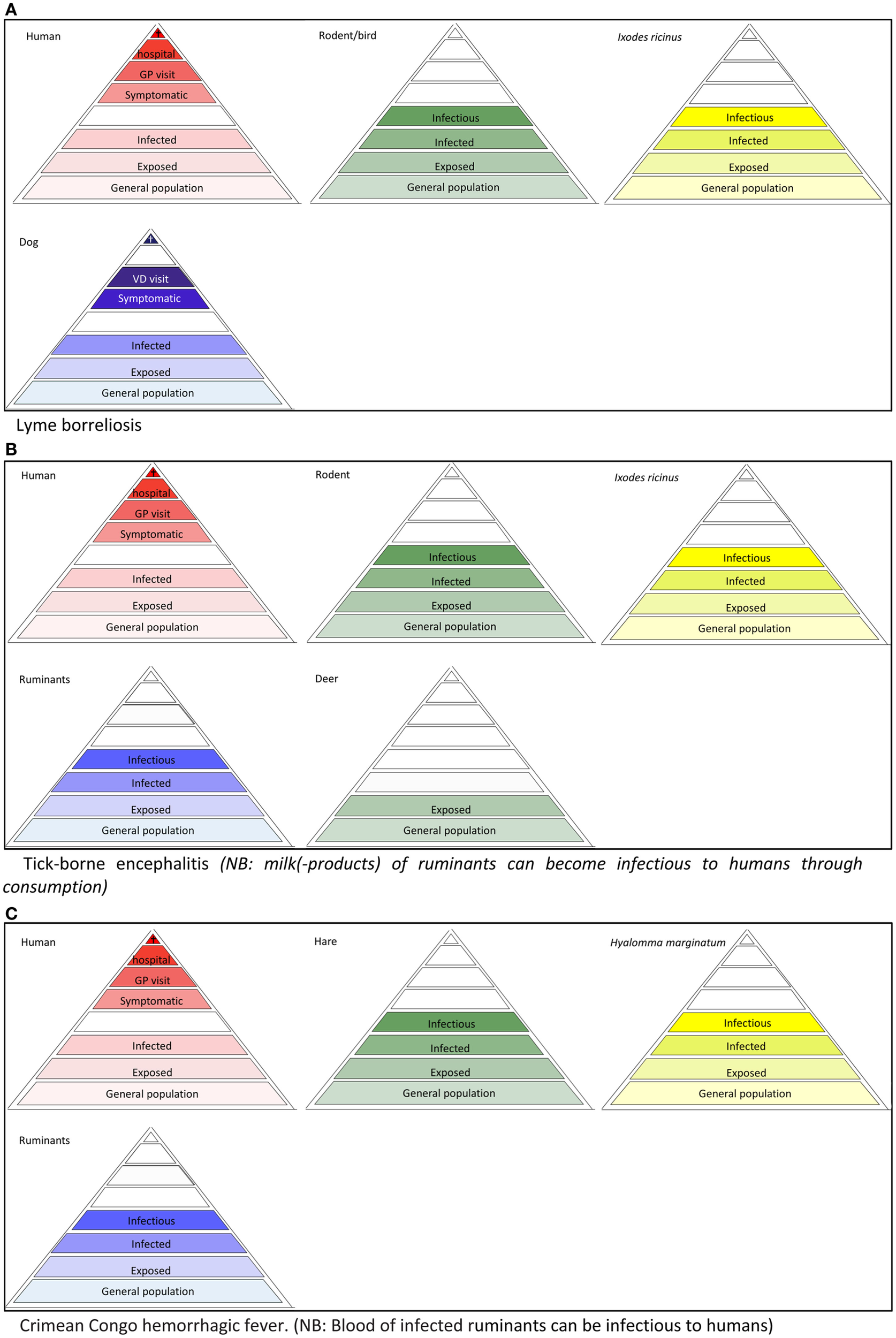
Disease depended surveillance pyramids of tick-borne diseases, (A) Lyme borreliosis (B) tick-borne encephalitis, (C) Crimean Congo hemorrhagic fever.
Figure 4
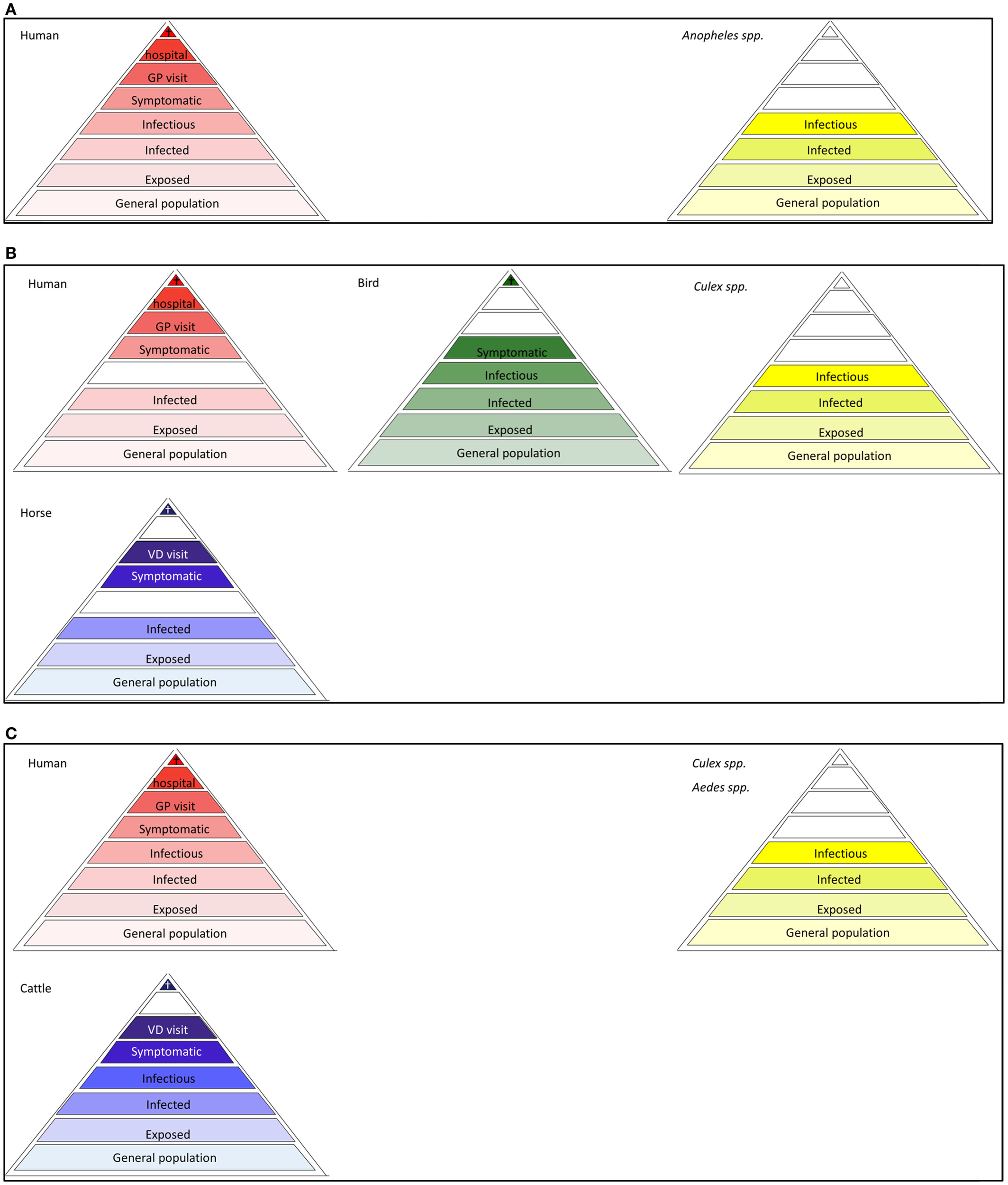
Disease dependent surveillance pyramids of mosquito-borne diseases (A) Malaria, (B) West Nile fever, (C) Rift Valley fever. The pyramid assemblies for dengue and chikungunya resemble that of malaria, provided that Anopheles spp is replaced by Ae. albopictus and Ae. aegypti as vector.
Context-based surveillance
The concept of surveillance pyramid assemblies offers a systematic approach for identifying all possible targets and levels of surveillance of any VBD. The next step is to develop a cost-effective strategy for surveillance for VBD, which largely depends on the purpose of the surveillance. Selecting the particular layers of each population to include as sensible targets for surveillance depend on the contexts of VBDs. A surveillance strategy should be based on the identification of the involved levels for a certain disease and the technical/laboratory methods (defining the feasibility) that can support the surveillance of that level (10).
The presence of a vector is a prerequisite for the transmission of a VBD. While the absence of a vector prevents transmission, the presence of a vector does not imply that actual transmission of a pathogen is occurring or will occur in the future. Based on this premise, the status of a VBD can be described in five different contexts based on the presence or absence of the three facets of VBDs important for public health: human cases, pathogens, and vectors (Table 1) (10). In short, VBDs with disease burden in a country fall under context 1 and the remainder fall under one of the other four contexts (Contexts 2–5).
Table 1
| Context | Disease burden1 | Pathogen2 | Vector | Tick-borne pathogens | Mosquito-borne pathogen |
|---|---|---|---|---|---|
| 1a | √ (every year) | √ | √ | Borrelia burgdorferi spp. | No examples |
| 1b | √ (not every year) | √ | √ | Borrelia miyamotoi | No examples |
| 2 | – | √ | √ | Rickettsia helvetica, Neoehrlichia mikurensis, Anaplasma phagocytophilum, Ehrlichia & Babesia, Bartonella | Plasmodium spp |
| 3 | – | – | √ | Tick-borne encephalitis virus | Dirofilaria spp., Mosquito-borne viruses (Batai, Inkoo, Rift Valley, Sindbis Snowshoe hare, Tahyna, Usutu, West Nile) |
| 4 | – | √ | – | Coxiella burnetti, Francisella tularensis | Chikungunya virus; Dengue virus |
| 5 | – | – | – | Crimean Congo Hemorrhagic Fever virus | Japanese encephalitis virus3 |
Current status of vector-borne diseases in the Netherlands.
1Locally acquired human case.
2Imported human case, infected animal reservoir, or vector.
3Potentially European mosquitoes are competent to transmit JEV (13), but this has not been validated.
To identify, assess the risk, communicate, and ultimately control VBDs, monitoring and surveillance tools, appropriate to the context, are needed (10). Generally, the current impact of a health problem is assessed through burden of disease calculations, while the impact of future outbreaks in an area is determined through quantitative risk assessments. Although applicable for endemic VBDs (context 1, Table 1), such quantitative assessments would not be sensible for the remaining VBD contexts without actual disease burden. Nevertheless, information on these so-called threats to human health is desirable (10). Consequently, surveillance efforts for VBDs, that are endemic and cause disease burden, need to have a different focus and structure than those for VBDs that are a threat. As a consequence, any surveillance strategy for a VBD needs to be developed considering its context.
National Context
When comparing tick-borne with mosquito-borne diseases in Netherlands (Table 1), for example, the most striking difference arises in context 1, i.e., endemic diseases. While the disease burden of mosquito-borne diseases is currently zero, the disease burden of tick-borne diseases, such as Lyme disease, is significant and growing (14). In the Netherlands, an increase in reported cases of tick bites and erythema migrans, the first clinical symptom of Lyme disease, has been reported over the last 15 years (14, 15).
For mosquito-borne diseases in the Netherlands, risk estimates, preparedness, and early warning are the more important components of risk analyses (10). Risk estimates are very useful in providing insight into the complexity of VBD emergence, provided that their assumptions, uncertainties, and ambiguities are taken into account (16, 17). Between 30 and 40 species of mosquito are indigenous to north-west Europe, many of which act as potential vectors of pathogens. However, since the eradication of malaria in the 1960s, no mosquito-borne diseases have been reported. Still, several endemic mosquito species are proven vectors of zoonoses or malaria in the laboratory or in the field elsewhere in Europe. With this in mind, various mosquito-borne diseases are categorized as context 3. Whether actual transmission of mosquito-borne pathogens occurs after introduction depends upon the vector capacity. The consequences of pathogen introduction in a region with a competent mosquito vector population can be very quick and significant, as illustrated with WNV introduction in the United States in 1999 (18) or chikungunya virus introduction in the Caribbean in 2013 (19). Development of contingency plans (including outbreak exercises) for potential emergence and databases with background information are tools to improve preparedness. For early warning purposes, focus is placed on improving and updating detection tools for the laboratory (20) and field (21) to enable rapid pathogen detection in a vector, reservoir, or host, when introduced. To this end, knowledge of the contexts of VBDs in the rest of Europe and more globally through international collaboration and networks is essential to enhance preparedness. This also applies to non-endemic tick-borne pathogens (22).
Here, we further describe surveillance strategies when placed in context, in reverse order (Contexts 5–1). Overviews of surveillance strategies for a mosquito- and tick-borne example (when available) in the Netherlands are shown in a box.
Context 5 deals with VBDs that currently do not pose any risk to the country owing to the current absence of both the pathogen and vector. The main concern centers therefore on the future establishment of the vector upon its introduction. Surveillance of pathogens and/or human cases is not a priority at this stage. If vector establishment on the basis of climatic or environmental constraints is impossible or highly improbable, no surveillance activities are recommended. However, this assessment needs to be iterative accounting for changes in the geographic distribution of the vectors in Europe.
|
Japanese encephalitis risk to the Netherlands (Context 5)
Japanese encephalitis is endemic to South East Asia. The principal vectors are Culex mosquitoes, and to a lesser degree, Aedes. The main vector in Asia, Cx. tritaeniorhynchus, is not present in Europe. Recently, an ambiguous report on the detection of fractions of this virus in Cx. pipiens in Italy appeared (13). Until there is conclusive evidence of Japanese encephalitis virus in Europe, this virus and its main vector are considered absent in Europe (23). Therefore, no surveillance activities are recommended. |
|
Crimean Congo Hemorrhagic fever risk to the Netherlands (Context 5)
The main vector of Crimean Congo hemorrhagic fever virus is the hard tick Hyalomma marginatum and its current known European distribution is shown in Figure 5. Transmission only occurs in regions within the vector’s geographic distribution. These ticks have been occasionally imported to Northwestern Europe on migratory birds (24, 25). Modeling suggests that the current climate of the Netherlands is unsuitable to sustain a population of these ticks, with little prospect of their establishment in the foreseeable future (26, 27), making any surveillance activity in the surveillance pyramid assembly obsolete. Nevertheless, source-finding activities in response to incidental findings of specimen to confirm absence of a local population should be considered. |
Context 4 deals with VBDs where although the vector is absent, the pathogen is regularly introduced. In comparison to context 5, owing to the introduction of the pathogen, assessments whether or not the vector can establish, upon introduction, has priority. If local climatic and environmental conditions permit establishment, surveillance focusing on detecting the introduction of the vector at potential ports of entry/risk locations is required. Only the vector pyramid within the surveillance pyramid assembly needs to be considered, preferentially also in an international context.
|
Dengue virus risk in the Netherlands (Context 4) – excluding current risks in the Dutch Overseas Territories
Dengue virus is regularly introduced with infected humans returning from their travel to dengue endemic countries. However, there are no established populations of the principal vector, Ae. aegypti nor of Aedes albopictus, in the Netherlands, although Ae. albopictus has been regularly introduced, but intercepted (28). The current European geographic distributions of these mosquito species are shown in Figure 5. The climate is suitable for establishment of Ae. albopictus in Northwestern Europe, but is unsuitable for Ae. aegypti. Expansions of the latter in Europe is expected, but even with climate change it is not anticipated to establish in Northwestern Europe, where the temperature will remain unsuitable (29). Upon establishment of Ae. albopictus, the risk status will change from context 4 to 2. The current Dutch policy is therefore to prevent establishment of vectors by active surveillance and control using biocides at high-risk areas, since exotic mosquitoes are frequently imported. |
Context 3 deals with VBDs that already have established putative vector populations, but so far there is no evidence of either pathogen circulation nor pathogen introductions. Surveillance is therefore focused on detecting the introduction or circulation of the disease causing pathogen as early as possible to enable adequate preventive and control measures. This may necessitate surveillance of the reservoir or sentinels (which may be humans or animals depending on the disease) and vector pyramids. In instances, where human cases of vector-borne zoonoses have been detected earlier than pathogen circulation in an enzootic cycle, human surveillance needs to be added to the surveillance strategy. In other cases, the availability or costs of samples determines the focus of surveillance. For all diseases belonging to this context, monitoring on the geographic distribution of the diseases in Europe is advisable.
|
West Nile fever risk in the Netherlands (Context 3)
Since human cases (humans as well as horses are dead-end hosts Figure 4B) can only occur following virus amplification cycles between bird-biting mosquitoes and birds. Given that endemic mosquitoes are putative WNV vectors, potentially infected populations of both birds (the host) and mosquitoes (the vector) are targeted for surveillance in high-risk areas suitable for transmission from an environmental/climatic point of view. Upon detection of an enzootic cycle of WNV, adequate preventive measures including public awareness campaigns, veterinary horse vaccination campaigns, and mosquito control activities may be implemented. In the Netherlands, a combined mosquito and WNV surveillance in the Oostvaardersplassen was started in 2009 and continued in 2010 with a wider screening for arboviruses, namely WNV, Usutu virus, Sindbis virus, Tahyna virus, and Batai virus (20, 30) and potential vector composition (30). In 2012, dozens of wild songbirds that were found dead in the Netherlands were tested for Usutu virus, but found negative (31). Up to now, no evidence of mosquito-borne virus circulation has been found in the Netherlands. Cerebrospinal liquors of humans with encephalitis of unknown cause were tested for the presence of flavivirus (32, 33). In collaboration with the Dutch Animal Health Service, a similar survey was performed on horses. In the UK, routine mosquito surveillance detected the presence of an additional WNV mosquito vector, Culex modestus in north Kent. To ensure an updated risk assessment on WNV for the UK, enhanced surveillance for the mosquito, and virus testing in mosquitoes and birds was implemented. The mosquito has shown evidence of expansion, but there is so far no evidence of the virus (34). |
|
Tick-borne encephalitis risk in the Netherlands (Context 3)
While its main vector, the sheep tick I. ricinus, occurs commonly, no autochthonous cases of tick-borne encephalitis (TBE) has been reported, nor any evidence of this virus circulating in an enzootic cycle in Netherlands. A closely related tick-borne flavivirus, named louping ill virus (LIV), however, is causing disease, predominantly in animals in upland areas of the UK and Ireland (35). In comparison with mosquito-borne disease outbreaks, the consequences of pathogen introduction in a region with a competent tick vector population are generally much more transient. In endemic areas, the permissive locations for transmission of tick-borne encephalitis virus are very local and patchy. In addition, the transmission of the virus between vectors occasionally occurs through co-feeding of immature stages of the tick on a non-viremic rodent host. Consequently, the detection of the pathogen soon after introduction is difficult and requires a very costly and intensive surveillance system. Recent publications suggest that animals that are neither a reservoir nor a sensitive host can act as sentinels for the circulation of tick-borne encephalitis virus, as shown with dogs and deer in Belgium (11, 12). However, the fact that a safe human vaccine against tick-borne encephalitis exists enables public health authorities to take effective preventive measures upon detection the first human case to protect the population for additional human cases, but not to prevent circulation. Such surveillance strategy aiming at an early detection of the first human case may be the most cost-effective one. |
Context 2 includes VBDs with enzootic circulation of the pathogen, which so far has not resulted in reported human cases in the country and VBDs with frequent introduction of infectious reservoirs into the country, which so far has not resulted in autochthonous human cases. The presence of an established vector population for both categories is a requirement within this context.
|
Malaria risk in the Netherlands (Context 2)
The indigenous mosquito species, Anopheles atroparvus, is capable of transmitting malaria parasites Plasmodium vivax, but transmission has ceased in the second half of the last century through reduction of the vector population, changes in farm management, improved health, and eradicating the pathogen (36). In recent years, intense mosquito nuisance caused by An. plumbeus, a putative vector for Plasmodium falciparum, has been reported regularly in local agricultural area in the Netherlands and Belgium. Until recently, this species had been breeding only in tree holes, but has adapted to new breeding ground, namely manure gutters under abandoned pig stables in Netherlands and Belgium (37). Although human cases are imported, there is no autochthonous transmission to humans. The chance of malaria transmission is much more unlikely than arboviral transmission given the non-zoonotic nature of malaria. While dengue cases are infectious before or even without developing symptoms, patients infected with falciparum-malaria become infectious only after developing symptoms, which permits an opportunity to prevent subsequent transmission. |
|
Non-Borrelia tick-borne disease risk in the Netherlands (Context 2)
The sheep tick I. ricinus is responsible for the vast majority of human tick bites. In ticks in the Netherlands, a total of six (groups of) pathogens can be distinguished. For four groups, no association with human cases has been found (yet) (Table 2) (38). The latter may be due to one of the following causes. First, humans do not develop symptoms when infected with these pathogens (in this case, microorganisms would be the correct term). Second, humans do develop symptoms when infected with these pathogens, but these symptoms are mild and go unnoticed. Third, humans do develop symptoms when infected with these pathogens, but these symptoms are atypical and ambiguous and diagnosed under another cause. Fourth, humans do develop symptoms when infected with these pathogens, but these infections are travel-related. Rickettsia helvetica, can infect humans, but whether it causes symptoms and therefore disease burden is unclear. |
Table 2
| Pathogen | I. ricinus (n) | Tick infection rate (%) | Dutch human cases reported |
|---|---|---|---|
| Borrelia burgdorferi s.l. | 628 (5308) | 11.8 | Yes |
| Rickettsia helvetica | 1265 (4061) | 31.1 | No |
| Anaplasma phagocytophilum | 44 (5343) | 0.8 | No |
| Babesia spp. | 112 (4238) | 2.0b | No |
| Neoehrlichia mikurensisa | 300 (5343) | 5.6 | No |
| Borrelia miyamotoia | 6 (300)b | 2.7 | Yes |
Pathogens found in Dutch I. ricinus ticks.
aNew to the Netherlands.
btested in pools.
Context 1 deals with VBDs that result in human cases or disease burden every year (Context 1a) or infrequently (Context 1b) (Table 1). In the Netherlands, as mentioned earlier, there are two tick- and no mosquito-borne diseases belonging to these contexts. To halt the increase in disease incidences, management (control and intervention) strategies for one or more of the three components (disease burden, pathogen, and vector) and the environment are important.
|
Borrelia miyamotoi risk in the Netherlands (Context 1b)
In 2012 in the Netherlands, a tick-borne disease switched from context 2 to context 1b, as a result of a case of meningoencephalitis caused by relapsing fever spirochete Borrelia miyamotoi (9). Since there are no diagnostics available yet, the possibilities for surveillance are limited. Development of diagnostics has been prioritized to help to investigate the form of the human disease surveillance pyramid. Possibly the disease belongs to context 1a, but until evidence is provided it will be assigned to context 1b. |
|
Lyme disease risk in the Netherlands (Context 1a)
For the majority of Europe, including the Netherlands, Lyme disease belongs to context 1a. Because of the large and growing number of human infections, the epidemiology, ecology, and prevention of Lyme borreliosis is receiving vast public, political, and scientific interest in the Netherlands (14, 39–43). The strong increase in incidence has several biological, environmental, and societal reasons. The two main reasons for the increase are increases in the level of exposure of humans to ticks and increases in the abundance of infected ticks (44). Environmental change factors are also exacerbating the risk (45). |
Pan-European Context
Public health authorities are required to prepare for future threats and call for predictions of the likely changes in public health risks. Usually, they focus their preparedness on their own geographical region. Whereas, the context of a disease is essential to design a surveillance strategy, assessing the context of VBDs is actually an issue for many public health authorities as the necessary information is not always readily available. In other words, in some cases surveillance data are required to assess the context, in which the subsequent surveillance strategy is prioritized. In addition, for all diseases belonging to contexts 3–5, national health experts are required to monitor the geographic distribution of the diseases, pathogens, and vectors in Europe.
To assist EU member states in this task, the European Centre for Disease Prevention and Control (ECDC) runs various programs to aggregate data to develop Pan-European maps on vector distribution and VBD incidence, to identify drivers of change and to provide guidance (10, 45–48) (Figures 5 and 6). Based on such data, assessing the context of a particular VBD in each European country seems to be a rather straightforward task. Central databases, however, on infections of wildlife with vector-borne pathogens are lacking or incomplete, making the assessment of the pathogens category difficult. When ignoring this category, context 2 and 4 are effectively omitted from the Pan-European maps on VBD contexts, hindering appropriate risk assessments on national and international level. Through the Eden FP6 and Edenext FP7 programs, huge steps have been made with collecting data and identifying drivers for pathogen circulation (49). Following on from data collected on human cases and vectors, the aggregation of data on pathogen circulation in reservoir species is the final step in developing Pan-European maps of VBDs, based on their national context.
Figure 5
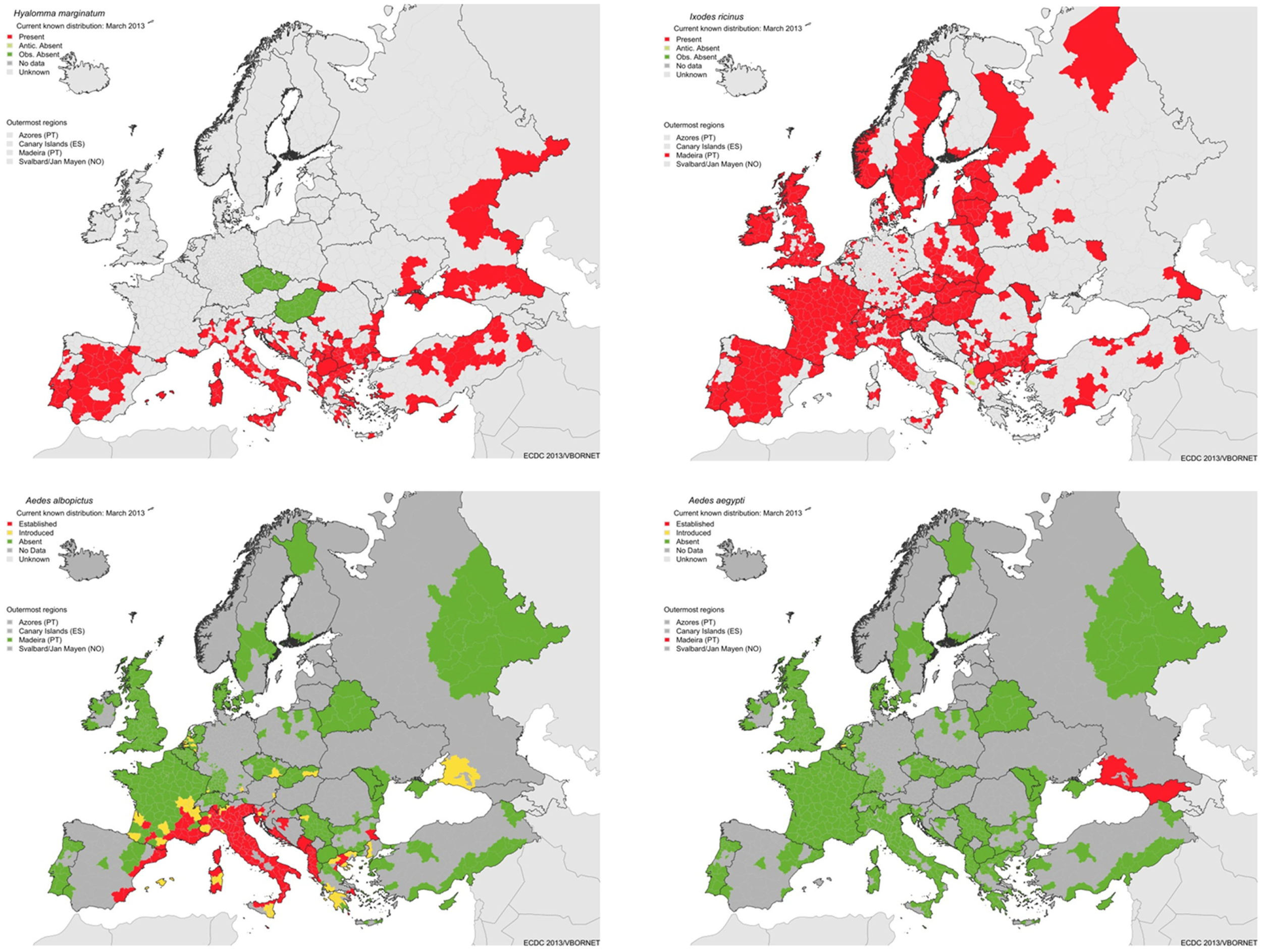
Geographic distribution of major vectors in Europe: ticks: Hyalomma marginatum, vector of Crimean Congo hemorrhagic fever (top left), Ixodes ricinus, vector of Lyme borreliosis and tick-borne encephalitis (top right), and Ae. albopictus (bottom left) and Ae. aegypti (bottom right), vectors of dengue (source: ECDC).
Figure 6
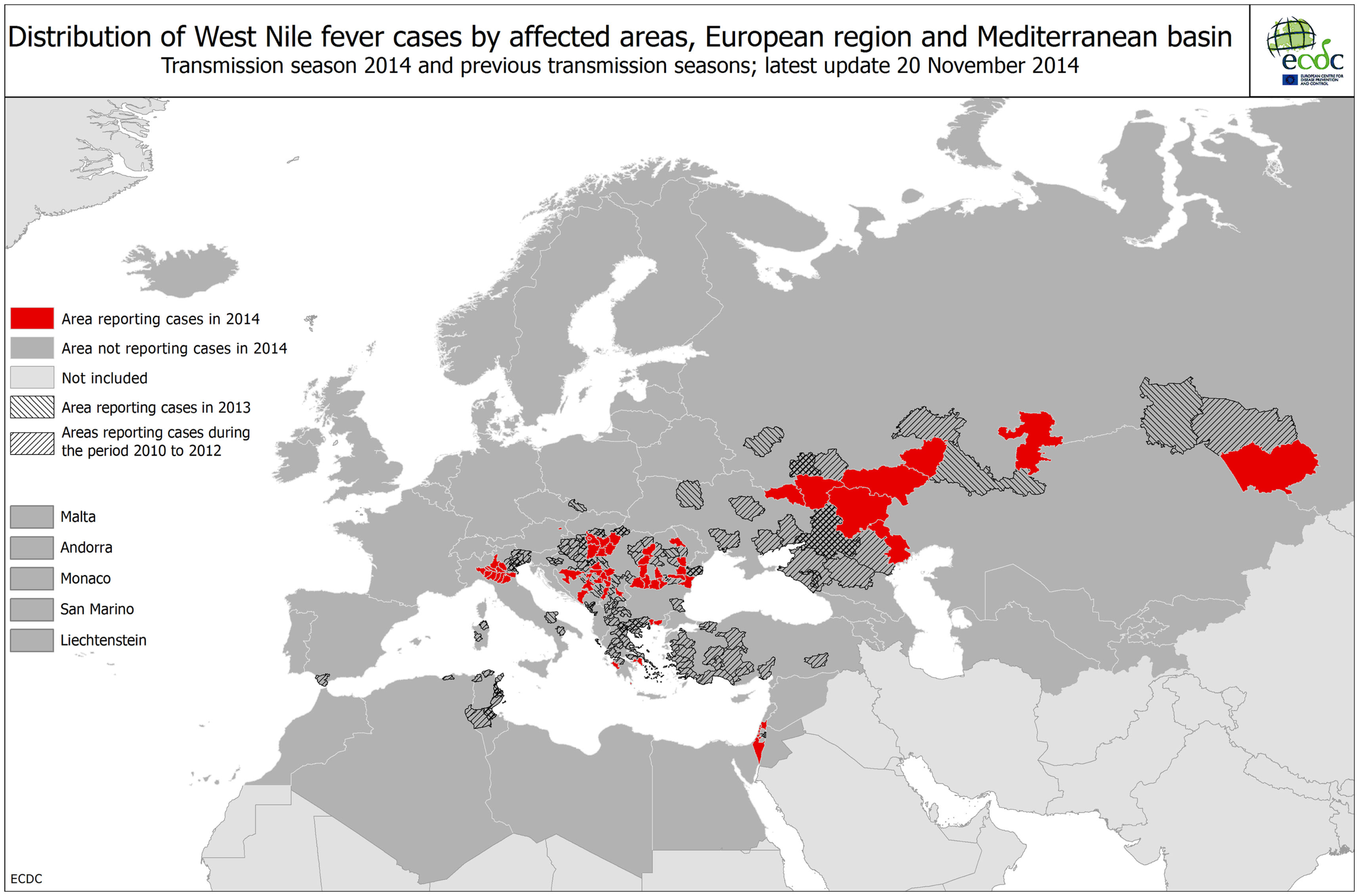
West Nile fever map of Europe (Source: ECDC).
Conclusion
Cataloging all possible surveillance activities on VBDs without putting these into context does not assist public health authorities with assessing comparative risk. Here, we propose a way forward for public health authorities to assess potential surveillance approaches for VBD based on its context and on a country-by-country basis. Surveillance efforts for VBDs that are endemic have a different focus and structure than those for VBDs that do not pose any immediate risk as neither the pathogen nor vector is present. Within the context of a VBD, the best surveillance strategy depends on the potential prospects for action and the costs/benefit analysis. This is particularly important given economic constraints, and therefore, a focus on interventions that achieve the largest health gain per euro spent seems eminently appropriate. For some VBDs, taking action prior to a VBD becoming an issue is preferable. Once a decision for intervention to decrease the disease burden (or group/category of diseases) or to mitigate a threat has been made, surveillance should be implemented in order to measure the effectiveness of this intervention (10).
It has become apparent that an interdisciplinary approach to the prevention and control of zoonoses is invaluable. Cross-sector working also ensures better preparedness and contingency planning, more efficient and effective surveillance systems, cost-sharing between sectors according to the benefits of control, increased health equity and improved sharing of logistics and costs for service provision (3). The field of integration of animal and human disease surveillance is new, but growing (4). Since the transmission cycles of several zoonotic pathogens occur in nature, involvement of stakeholders involved in environmental management and biodiversity-enhancing strategies is the next logical step. This requires an extension of the data collection approaches to further enhance disease intelligence (2) rather than a preset static structure. The described contextual surveillance for VBD extended with veterinary and wildlife health along with public health is highly applicable in a One Health approach.
Statements
Acknowledgments
The authors would like to thank Wim van Bortel, Hervé Zeller, Chantal Reusken, and Wim Ooms for their comments on earlier version of the document. The authors wish to thank the Vbornet consortium for helpful discussions and two anonymous reviewers for their valuable input. In addition, they highly appreciated the support of the Dutch Food and Consumer Product Safety Authority and by the Dutch Ministry of Health, Welfare and Sports (VWS).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1
Thacker SB Dannenberg AL Hamilton DH . Epidemic intelligence service of the centers for disease control and prevention: 50 years of training and service in applied epidemiology. Am J Epidemiol (2001) 154(11):985–92.10.1093/aje/154.11.985
2
Paquet C Coulombier D Kaiser R Ciotti M . Epidemic intelligence: a new framework for strengthening disease surveillance in Europe. Euro Surveill (2006) 11(12):212–4.
3
Leach M Scoones I . The social and political lives of zoonotic disease models: narratives, science and policy. Soc Sci Med (2013) 88:10–7.10.1016/j.socscimed.2013.03.017
4
Wendt A Kreienbrock L Campe A . Zoonotic disease surveillance – inventory of systems integrating human and animal disease information. Zoonoses Public Health (2014).10.1111/zph.12120
5
World health Organization. The Vector-Borne Human Infections of Europe: Their Distribution and Burden on Public Health. Copenhagen: WHO Regional Office for Europe (2004).
6
Rizzoli A Hauffe HC Carpi G Vourc’h GI Neteler M Rosà R . Lyme borreliosis in Europe. Euro Surveill (2011) 16(27):pii:19906.
7
Hubalek Z Halouzka J . West Nile fever – a reemerging mosquito-borne viral disease in Europe. Emerg Infect Dis (1999) 5(5):643–50.10.3201/eid0505.990506
8
Sousa CA Clairouin M Seixas G Viveiros B Novo MT Silva AC et al Ongoing outbreak of dengue type 1 in the autonomous region of Madeira, Portugal: preliminary report. Euro Surveill (2012) 17(49):pii:20333.
9
Hovius JW de Wever B Sohne M Brouwer MC Coumou J Wagemakers A et al A case of meningoencephalitis by the relapsing fever spirochaete Borrelia miyamotoi in Europe. Lancet (2013) 382(9892):658.10.1016/S0140-6736(13)61644-X
10
Braks M van der Giessen J Kretzschmar M van Pelt W Scholte EJ Reusken C et al Towards an integrated approach in surveillance of vector-borne diseases in Europe. Parasit Vectors (2011) 4:192.10.1186/1756-3305-4-192
11
Roelandt S Heyman P De Filette M Vene S Van derStede Y Caij AB et al Tick-borne encephalitis virus seropositive dog detected in Belgium: screening of the canine population as sentinels for public health. Vector Borne Zoonotic Dis (2011) 11(10):1371–6.10.1089/vbz.2011.0647
12
Linden A Wirtgen M Nahayo A Heyman P Niedrig M Schulze Y . Tickborne encephalitis virus antibodies in wild cervids in Belgium. Vet Rec (2012) 170(4):108.10.1136/vr.e646
13
Ravanini P Huhtamo E Ilaria V Crobu MG Nicosia AM Servino L et al Japanese encephalitis virus RNA detected in Culex pipiens mosquitoes in Italy. Euro Surveill (2012) 17(28):pii:20221.
14
Hofhuis A Herremans T Notermans DW Sprong H Fonville M van der Giessen JW et al A prospective study among patients presenting at the general practitioner with a tick bite or erythema migrans in The Netherlands. PLoS One (2013) 8(5):e64361.10.1371/journal.pone.0064361
15
Hofhuis A van der Giessen JW Borgsteede FH Wielinga PR Notermans DW van Pelt W . Lyme borreliosis in the Netherlands: strong increase in GP consultations and hospital admissions in past 10 years. Euro Surveill (2006) 11(6):E0606222.
16
Sedda L Morley DW Braks MA De Simone L Benz D Rogers DJ . Risk assessment of vector-borne diseases for public health governance. Public Health (2014) 128(12):1049–58.10.1016/j.puhe.2014.08.018
17
Fischer EA Boender GJ Nodelijk G de Koeijer AA van Roermund HJ . The transmission potential of Rift Valley fever virus among livestock in the Netherlands: a modelling study. Vet Res (2013) 44(1):58.10.1186/1297-9716-44-58
18
Kilpatrick AM . Globalization, land use, and the invasion of West Nile virus. Science (2011) 334(6054):323–7.10.1126/science.1201010
19
Van Bortel W Dorleans F Rosine J Blateau A Rousset D Matheus S et al Chikungunya outbreak in the Caribbean region, December 2013 to March 2014, and the significance for Europe. Euro Surveill (2014) 19(13):pii:20759.
20
Reusken C de Vries A Ceelen E Beeuwkes J Scholte EJ . A study of the circulation of West Nile virus, Sindbis virus, Batai virus and Usutu virus in mosquitoes in a potential high risk area for arbovirus circulation in the Netherlands, “De Oostvaardersplassen”. Eur Mosq Bull (2011) 29:66–81.
21
Ibáñez-Justicia A Scholte EJ Reusken C de Vries A Dik M Braks M . An additional tool for arbovirus surveillance in the Netherlands: the use of honey-baited cards to detect circulating mosquito-borne viruses. Proc Dutch Entomol Soc (2012) 23:63–71.
22
Jahfari S Fonville M Hengeveld P Reusken C Scholte EJ Takken W et al Prevalence of Neoehrlichia mikurensis in ticks and rodents from North-west Europe. Parasit Vectors (2012) 5(1):74.10.1186/1756-3305-5-74
23
Cleton N Koopmans M Braks M Reusken C . Japanse encefalitis in Zuid-Europa?Ned Tijdschr Med Microbiol (2013) 21(1):7–11.
24
Nijhof AM Bodaan C Postigo M Nieuwenhuijs H Opsteegh M Franssen L et al Ticks and associated pathogens collected from domestic animals in the Netherlands. Vector Borne Zoonotic Dis (2007) 7(4):585–95.10.1089/vbz.2007.0130
25
Jameson LJ Morgan PJ Medlock JM Watola G Vaux AG . Importation of Hyalomma marginatum, vector of Crimean-Congo haemorrhagic fever virus, into the United Kingdom by migratory birds. Ticks Tick Borne Dis (2012) 3(2):95–9.10.1016/j.ttbdis.2011.12.002
26
Gale P Stephenson B Brouwer A Martinez M de laTorre A Bosch J et al Impact of climate change on risk of incursion of Crimean-Congo haemorrhagic fever virus in livestock in Europe through migratory birds. J Appl Microbiol (2012) 112(2):246–57.10.1111/j.1365-2672.2011.05203.x
27
Estrada-Pena A Sanchez N Estrada-Sanchez A . An assessment of the distribution and spread of the tick Hyalomma marginatum in the western Palearctic under different climate scenarios. Vector Borne Zoonotic Dis (2012) 12(9):758–68.10.1089/vbz.2011.0771
28
Scholte E Den HartogW Dik M Schoelitsz B Brooks M Schaffner F et al Introduction and control of three invasive mosquito species in the Netherlands, July-October 2010. Euro Surveill (2010) 15(45):pii:19710.
29
Rogers DJ Suk JE Semenza JC . Using global maps to predict the risk of dengue in Europe. Acta Trop (2014) 129:1–14.10.1016/j.actatropica.2013.08.008
30
Reusken CB de Vries A Buijs J Braks MA den Hartog W Scholte EJ . First evidence for presence of Culex pipiens biotype molestus in the Netherlands, and of hybrid biotype pipiens and molestus in northern Europe. J Vector Ecol (2010) 35(1):210–2.10.1111/j.1948-7134.2010.00080.x
31
Reusken C Zutta DE Kik M Cleton A Rijks J Schmidt-Chantasit J et al Geen bewijs voor Usutuvirus als oorzaak van sterfte bij zang vogels in Nederland (herfst 2012). Tijdschr Diergeneeskd (2014) 139(3):28–30.
32
Rockx B van Asten L van den Wijngaard C Godeke GJ Goehring L Vennema H et al Syndromic surveillance in the Netherlands for the early detection of West Nile virus epidemics. Vector Borne Zoonotic Dis (2006) 6(2):161–9.10.1089/vbz.2006.6.161
33
Koppelman MH Sjerps MS de Waal M Reesink HW Cuypers HT . No evidence of West Nile virus infection in Dutch blood donors. Vox Sang (2006) 90(3):166–9.10.1111/j.1423-0410.2006.00754.x
34
Medlock JM Vaux AG . Distribution of West Nile virus vector, Culex modestus, in England. Vet Rec (2012) 171(11):278.10.1136/vr.e6123
35
Jeffries CL Mansfield KL Phipps LP Wakeley PR Mearns R Schock A et al Louping ill virus: an endemic tick-borne disease of Great Britain. J Gen Virol (2014) 95(Pt 5):1005–14.10.1099/vir.0.062356-0
36
Verhave JP . Experimental, therapeutic and natural transmission of Plasmodium vivax tertian malaria: scientific and anecdotal data on the history of Dutch malaria studies. Parasit Vectors (2013) 6(1):19.10.1186/1756-3305-6-19
37
Dekoninck W Hendrickx F Vasn Bortel W Versteirt V Coosemans M Damiens D et al Human-induced expanded distribution of Anopheles plumbeus, experimental vector of West Nile virus and a potential vector of human malaria in Belgium. J Med Entomol (2011) 48(4):924–8.10.1603/ME10235
38
Coipan EC Jahfari S Fonville M Maassen CB van der Giessen J Takken W et al Spatiotemporal dynamics of emerging pathogens in questing Ixodes ricinus. Front Cell Infect Microbiol (2013) 3:36.10.3389/fcimb.2013.00036
39
Coumou J Hovius JW van Dam AP . Borrelia burgdorferi sensu lato serology in the Netherlands: guidelines versus daily practice. Eur J Clin Microbiol Infect Dis (2014).10.1007/s10096-014-2129-4
40
Tijsse-Klasen E Jacobs JJ Swart A Fonville M Reimerink JH Brandenburg AH et al Small risk of developing symptomatic tick-borne diseases following a tick bite in The Netherlands. Parasit Vectors (2011) 4:17.10.1186/1756-3305-4-17
41
Gassner F Verbaarschot P Smallegange RC Spitzen J Van Wieren SE Takken W . Variations in Ixodes ricinus density and Borrelia infections associated with cattle introduced into a woodland in The Netherlands. Appl Environ Microbiol (2008) 74(23):7138–44.10.1128/AEM.00310-08
42
Mulder S van Vliet AJ Bron WA Gassner F Takken W . High risk of tick bites in Dutch gardens. Vector Borne Zoonotic Dis (2013) 13(12):865–71.10.1089/vbz.2012.1194
43
Beaujean DJ Gassner F Wong A van Steenbergen JE Crutzen R Ruwaard D . Determinants and protective behaviours regarding tick bites among school children in the Netherlands: a cross-sectional study. BMC Public Health (2013) 13:1148.10.1186/1471-2458-13-1148
44
Sprong H Hofhuis A Gassner F Takken W Jacobs F van Vliet AJ et al Circumstantial evidence for an increase in the total number and activity of Borrelia-infected Ixodes ricinus in the Netherlands. Parasit Vectors (2012) 5:294.10.1186/1756-3305-5-294
45
Medlock JM Hansford KM Bormane A Derdakova M Estrada-Peña A George JC et al Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit Vectors (2013) 6:1.10.1186/1756-3305-6-1
46
Medlock JM Hansford KM Schaffner F Versteirt V Hendrickx G Zeller H et al A review of the invasive mosquitoes in Europe: ecology, public health risks, and control options. Vector Borne Zoonotic Dis (2012) 12(6):435–47.10.1089/vbz.2011.0814
47
Zeller H Marrama L Sudre B Van Bortel W Warns-Petit E . Mosquito-borne disease surveillance by the European centre for disease prevention and control. Clin Microbiol Infect (2013) 19(8):693–8.10.1111/1469-0691.12230
48
Guidelines for the Control of Mosquitoes of Public Health Importance in Europe. WHO Regional Office for Europe & EMCA (2013). 42 p. Available from: http://www.emca-online.eu/documents/visitors/EMCA_guidelines_Speyer_2011.pdf
49
Available from: http://www.edenext.eu/publications
Summary
Keywords
vector-borne diseases, surveillance, one health, disease burden, threat, emerging diseases
Citation
Braks M, Medlock JM, Hubalek Z, Hjertqvist M, Perrin Y, Lancelot R, Duchyene E, Hendrickx G, Stroo A, Heyman P and Sprong H (2014) Vector-Borne Disease Intelligence: Strategies to Deal with Disease Burden and Threats. Front. Public Health 2:280. doi: 10.3389/fpubh.2014.00280
Received
15 July 2014
Accepted
01 December 2014
Published
22 December 2014
Volume
2 - 2014
Edited by
A. Paulo Gouveia Almeida, Universidade nova de Lisboa, Portugal
Reviewed by
Yingchen Wang, University of North Carolina, USA; Rubén Bueno-Marí, University of Valencia, Spain
Copyright
© 2014 Braks, Medlock, Hubalek, Hjertqvist, Perrin, Lancelot, Duchyene, Hendrickx, Stroo, Heyman and Sprong.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marieta Braks, National Institute for Public Health and the Environment, Antonie van Leeuwenhoeklaan 9, 3721 MA Bilthoven, P.O. Box 1, 3720 BA Bilthoven, Netherlands e-mail: marieta.braks@rivm.nl
This article was submitted to Epidemiology, a section of the journal Frontiers in Public Health.
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.