Abstract
The genomics revolution over the past three decades has led to great strides in rare disease (RD) research, which presents a major shift in global policy landscape. While RDs are individually rare, there are common challenges and unmet medical and social needs experienced by the RD population globally. The various disabilities arising from RDs as well as diagnostic and treatment uncertainty were demonstrated to have detrimental influence on the health, psychosocial, and economic aspects of RD families. Despite the collective large number of patients and families affected by RDs internationally, the general lack of public awareness and expertise constraints have neglected and marginalized the RD population in health systems and in health- and social-care policies. The current Coronavirus Disease of 2019 (COVID-19) pandemic has exposed the long-standing and fundamental challenges of the RD population, and has reminded us of the critical need of addressing the systemic inequalities and widespread disparities across populations and jurisdictions. Owing to the commonality in goals between RD movements and universal health coverage targets, the United Nations (UN) has highlighted the importance of recognizing RDs in policies, and has recently adopted the UN Resolution to promote greater integration of RDs in the UN agenda, advancing UN's commitment in achieving the 2030 Sustainable Development Goals of “leav[ing] no one behind.” Governments have also started to launch Genome Projects in their respective jurisdictions, aiming to integrate genomic medicine into mainstream healthcare. In this paper, we review the challenges experienced by the RD population, the establishment and adoption of RD policies, and the state of evidence in addressing these challenges from a global perspective. The Hong Kong Genome Project was illustrated as a case study to highlight the role of Genome Projects in enhancing clinical application of genomic medicine for personalized medicine and in improving equity of access and return in global genomics. Through reviewing what has been achieved to date, this paper will provide future directions as RD emerges as a global public health priority, in hopes of moving a step toward a more equitable and inclusive community for the RD population in times of pandemics and beyond.
Introduction
Rare diseases (RDs) are an emerging public health priority. RD refers to a disease that affects a small number of people in a population (1). There are 6,000–8,000 unique RDs identified, with approximately 80% being genetic in origin, and 50–75% being pediatric onset (1–3). They are often chronic, progressive, and debilitating, and can lead to significant morbidity and mortality (4). With RDs' nature being heterogeneous, complex, and individually rare, they are difficult to be diagnosed, and are challenging to be assessed in aggregate. Currently there is no universal definition for RDs, with differing prevalence among different parts of the world. The European Union Regulation on orphan medicinal products defined RDs as conditions affecting < 50 per 100,000 individuals in the European population (5), whereas the American Orphan Drug Act defined RDs as conditions affecting < 200,000 individuals in the United States (6, 7). Other definitions have been proposed by different jurisdictions, ranging from five per 100,000 to 76 per 100,000 individuals, with the global average being 40 per 100,000 individuals (Figure 1) (8–10).
Figure 1
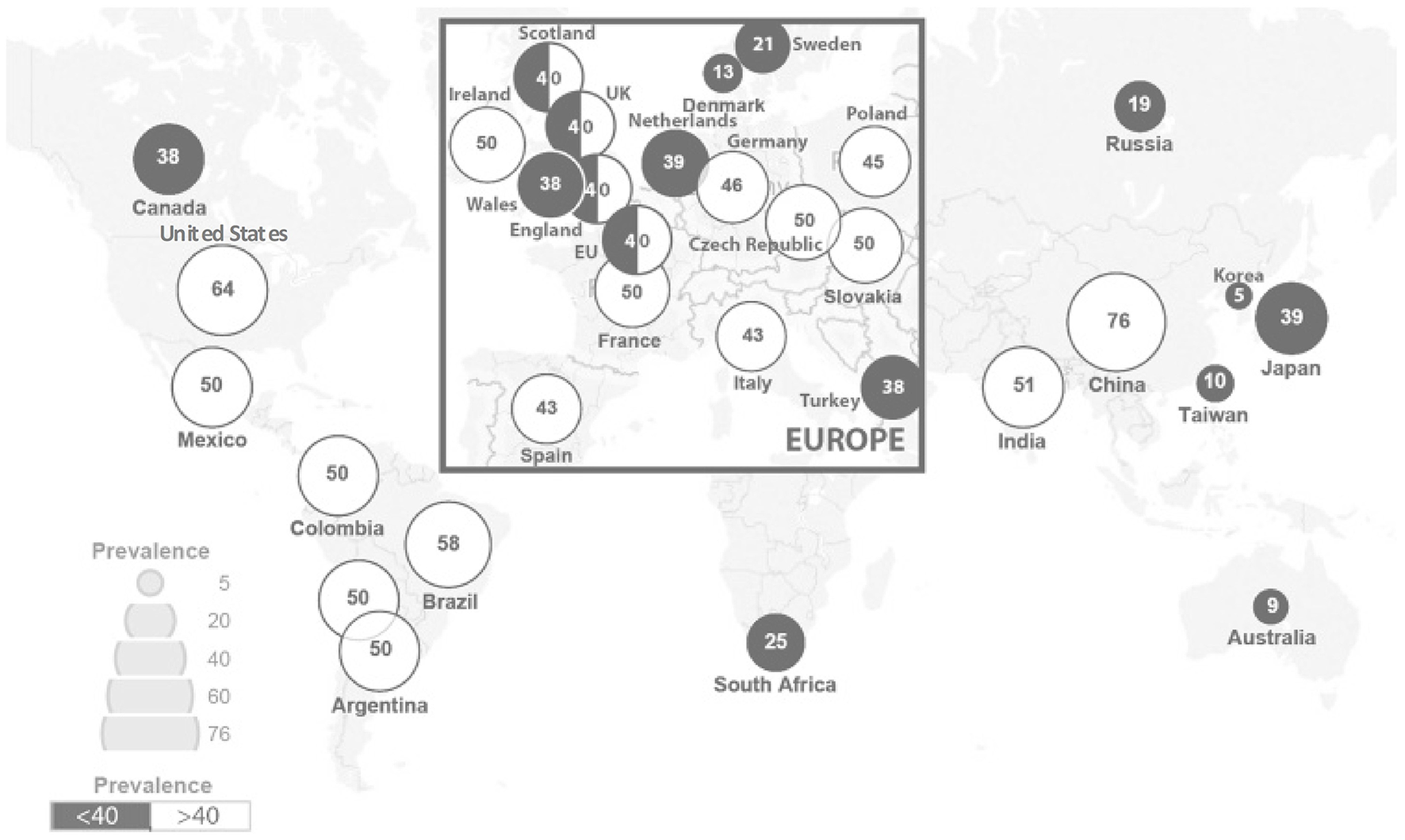
RD prevalence per 100,000 across jurisdictions [adapted from Richter et al. (9)].
Although individually rare, the collective number of people affected by RDs was equivalent to the population of the world's third largest country (11). A recent global RD prevalence based on 3,585 RDs was estimated to be 3.5–5.9% of the world's population, which corresponds to 263 to 446 million people worldwide (10). When the impact of RDs extends to family members and carers of the RD patient, it was expected that RDs affect approximately 1.05–1.4 billion people globally (12).
Long diagnostic odyssey, lifelong disabilities, lack of compensatory support, and few but costly effective treatments are some of the unmet needs that plagues the lives of RD patients (13, 14). The various disabilities arising from the disease as well as diagnostic and treatment uncertainty have been demonstrated to have detrimental influence on the health, psychosocial, and economic aspects of the lives of the RD families (15, 16). In 2019, Rare Diseases International released a position paper emphasizing the need for universal health coverage (UHC) policies to account for RDs, owing to the commonality in goals between RD movements and the UHC targets (17). The United Nations (UN) political declaration on UHC has recognized the RD population as a marginalized group that should be considered during healthcare planning, claiming that UHC “shall never be fully attained nor realized if persons living with RDs are left behind and their needs left unmet” (17). Despite the significant challenges faced, under allocation of resources and inadequate healthcare planning for the RD population remains prevalent (13).
Challenges experienced by the RD population
While RDs are individually rare, there are common features across the range of RDs and common challenges experienced by the RD population. Unmet medical and social needs of RD patients, families, and carers exist globally. Approximately half of the individuals with suspected RDs are undiagnosed, while RD patients who have received a diagnosis encounter fundamental myriad challenges due to delays or incorrect diagnoses, treatment, care, and social acceptance (18).
From the individual's perspective, long diagnostic odyssey often plagues the lives of RD patients. In Europe, 25% of the RD patients had to wait between 5 and 30 years from disease onset to receiving a genetic diagnosis for their condition, and 40% had initially received multiple misdiagnoses, leading to ineffective and unnecessary medical management (19). In another survey of RD families from the United Kingdom and United States, patients typically visit eight physicians and receive two to three misdiagnoses prior to receiving a correct genetic diagnosis, which spanned over a period of 5.6–7.6 years (2, 20). Not only do the individuals endure years of diagnostic odyssey, but it is also expensive for the health systems to undergo a succession of unnecessary medical follow-ups and conventional diagnostic approaches.
Undeniably, a genetic diagnosis offers the potential for personalized medicine, yet opens another door of challenges in treatment availability, accessibility, and affordability. With RDs being heterogeneous and individually rare, interventions and therapies, including orphan drugs, are seldom available due to the lack of market incentives and small market opportunity for the biopharmaceutical industry (21, 22). Currently, < 3% of diagnosed RDs have a suitable drug treatment (21, 23); it was estimated that fewer than one-tenth of RD patients have received disease-specific treatment globally (24). Where a treatment has been approved for a RD, cost of the drug is generally extremely costly, with RD drugs reported to be as high as 13.8 times more than conventional drugs (21, 23). This can be financially overwhelming for many, especially when RD drugs usually require out-of-pocket (OOP) cost-sharing by the patient. Consequently, RD patients may need to bear the catastrophically high OOP expenditure on health services and resources, posing a higher risk of financial hardship. For patients who are not able to afford the extremely costly therapies, they will continue to be managed with conventional approaches, adding to the never-ending socio-economic costs of RDs. Accessibility also remains to be a problem, with access and reimbursement recommendations on the same intervention varying vastly across jurisdictions.
From a wider socio-economic perspective, both patients and carers have highlighted the challenges in maintaining employment and education due to frequent medical follow-ups and the unprecedented and uncertainty nature of their condition (25–28). In the United Kingdom, 66% of the RD patients and carers indicated that their ability to hold paid employment was affected, with many of them being forced to retire early or reduce working hours due to the condition or the related caring responsibilities (25). Importantly, a significant number of RD patients and carers were forced to reduce working days or quit their job completely by their employer because they were considered as “unreliable.” These ultimately manifest as significant opportunities and productivity loss, and can be a burden for the RD patients, families, and the society as a whole. Due to social discrimination and stigmatization, low social awareness, and lack of knowledge and understanding from the general public, both RD patients and carers often feel isolated and excluded from the society (29). As such, the RD population experience extraordinary healthcare, psychosocial, and economic burden, contributing to the decreased wellbeing and quality of life.
Despite the collective large number of patients and families affected by RDs internationally, the general lack of public awareness and expertise constraints have neglected and marginalized the RD population in healthcare systems and in health- and social-care policies.
Challenges in funding treatments and therapies for RDs
In the era of resource and budget constraints, health economic evidence plays a critical role in guiding decision makers to prioritize and allocate resources efficiently and effectively. Although cost-effectiveness and cost-utility analyses are often considered to be more useful in informing health and social care decisions, as they take account into both costs and outcomes simultaneously as compared to other option alternatives, such types of analyses are relatively difficult to be conducted within the RD population. This is due to the limited intervention alternatives that are available in the market, the small number of patients that can be recruited into clinical studies, and the conflicting ethical considerations for funding RD treatment (21, 22, 30, 31). Orphan drugs and RD interventions are often considered to be cost-ineffective against standard cost-effectiveness thresholds, such as the £20k – £30k (US$26k–$39k) per quality-adjusted life year (QALY) threshold proposed by National Institute for Health and Care Excellence (NICE), due to treatment's epidemiological and economic specifics (31, 32). Future health technology assessments concerning epidemiological, clinical, and economic evidence are warranted for assessing and appraising RD treatment and medications at a territory-wide or national level (31).
Genomic medicine challenges conventional health economic evaluation paradigms, which fails to capture the multi-dimensional outcomes that genomic medicine generates. Some health economists and ethicists have argued for an adjusted threshold for the RD population, such as the £78.3k (US$102.4k) per QALY threshold at the RD mid-point population and £937.1k (US$1,225.9k) per QALY for ultra-rare orphan drugs, based on the principles of equity and “veil of ignorance” (22, 33–35). Nevertheless, the adjusted threshold does not fully encompass the challenges associated with rarity. While the QALY can be a useful measure to evaluate health-related quality of life and survival, its simplicity in methodological calculation does not capture multi-dimensional patient benefits. The QALY is only one of the many elements of value in the “value flower” proposed by Lakdawalla et al., which could all contribute to how a healthcare intervention is valued (36). Elements such as the severity of disease, insurance value, real option value, and equity, are particularly relevant and important for RD therapies and should also be considered (37). Others have proposed that efficiency assessments such as cost per QALY should not be employed when the alternative choice is between an only treatment and no treatment (35). On the other hand, the multi-criteria decision analysis approach is proposed to provide more transparent and inclusive evidence in identifying and combining the relative importance of different criteria and stakeholder perspectives in a single health technology assessment for RD therapies, with the aim of balancing evidence among different stakeholders. More recently, the new NICE methods and processes for technology appraisals have been adopted in February 2022, with some of the changes made of particular relevance to determining the value of RD therapies. These include consideration of disease severity, different types of evidence including qualitative and expert elicitation, flexibility to accept uncertainty in specific situations, and commercial and managed access. It is recommended that NICE appraisals should consider the degree of need and desirability to promote innovation in addition to the clinical effectiveness and value for money. Health system's obligations for equality and human rights must also be considered. Flexibilities should be adopted rather than strictly following the cost-effectiveness threshold. These provide an innovative and sustainable framework to assess and appraise RD interventions. In the future, decision makers and health authorities should take account into the spill over effect, the broader social value of RD treatment and intervention, and their potential and innovativeness for other non-rare cases (31).
RDs under the COVID-19 pandemic
The current Coronavirus Disease of 2019 (COVID-19) pandemic remains to be an unprecedented global health challenge due to its persistent spread and unpredictable clinical course. As of August 23, 2022, over 595.1 million cases were confirmed and over 6.4 million deaths were reported across 222 jurisdictions since the outbreak of COVID-19 in December 2019 in Wuhan China (38). The pandemic has reminded us of the critical need of addressing the systemic inequalities in the determinants of health and illnesses, including genomic, social, and environmental factors, which has resulted in widespread disparities across populations and jurisdictions. This highlights the paramount importance of engaging a more diverse and inclusive research workforce, including the RD population.
The COVID-19 pandemic has further perpetuated and exacerbated the unmet needs and challenges experienced by the RD community, regardless of whether they were infected with COVID-19.
First, RD has been identified as a risk factor for COVID-19 related mortality. While RD patients had a similar rate of COVID-19 infection as the general population, Chung et al. reported that RD patients were associated with an adjusted 3.4 times odds of COVID-19 related hospital mortality compared to the general population in Hong Kong (95% CI 1.24–9.41; p = 0.017) (39). Similar findings were observed in a retrospective cohort study in Genomics England 100k Genomes participants, in which RD patients were found to have a 3.5 times odds of COVID-19-related deaths compared to the unaffected relatives (95% CI 1.21–12.2), although the effect was insignificant after adjusting for age and number of comorbidities (OR 1.94; 95% CI 0.65–5.80) (40). COVID-19-related mortality was not confined to one specific group of RD patients, as suggested by both studies. Results from these studies suggested that RD as a group is a pre-existing comorbidity that is associated with COVID-19-related mortality, and should be considered in healthcare prioritization (39, 40).
In addition to RD patients who were infected with COVID-19, patients without infection had also experienced enormous and multifaceted challenges during the pandemic. Interruptions of care, particularly delays and cessation of diagnostic workups, therapies, rehabilitation, surgeries, and medications, pose substantial impact on the health and social wellbeing of the RD patients. Genetic laboratories and hospitals were required to provide urgent services only, to focus manpower and resources on combating COVID-19. In the United Kingdom, referrals to Clinical Genetics Service fell over 50% during April to June 2020 as compared to the same period in 2019 (41). Request for genetic testing such as microarrays, which is often the first line genetic diagnostic test for patients with suspected undiagnosed genetic disease, was markedly reduced (41). There was also substantial decrease in the number of other diagnostic tests performed, including echocardiograms, radiological investigation, and gastroscopies (41). The pandemic has disproportionately exacerbated the problem of diagnostic delay for RDs, affecting all points on the path to diagnosis, from initial engagement with health services, referral for investigation or specialist assessment, to the availability of definitive testing and registering with patient advocacy groups for support. In addition to the significant drop in RD diagnosis, health service utilization was also substantially affected. In Hong Kong, over 70% of the RD patients had reduce health service utilization during the pandemic (42). Importantly, health status was affected in 46% of the patients due to reduced service provision. Psychological health and rehabilitation were affected in 79% and 78% of the patients respectively, especially among patients who are severely or totally dependent according to the Barthel Index for Activities of Daily Living (42). Moreover, patients' social life, daily living, and financial status were also severely impacted by the COVID-19 pandemic, affecting 92%, 89%, and 81%, respectively (42). Almost 60% of the patients reported increased expenditure during the pandemic, while 56% of the patients experienced reduction in household income, indicating the magnified financial burden on the RD population (42). Similar patterns were also identified in other cohorts in the West, all highlighting the significant repercussion of the pandemic on regular healthcare service, physical and psychological health, and financial status of the RD population (43–47). The COVID-19 pandemic has inspired and accelerated the adoption of telemedicine and telehealth in some parts of the world (43, 48). Future implementation of telemedicine into the healthcare systems may serve as a sustainable healthcare delivery model beyond the COVID-19 pandemic.
For carers of the RD patients, lifelong caring has posed substantial psychological and financial burden in the best of times, and these challenges have been further exacerbated during the COVID-19 pandemic. In a study by Fuerboeter et al. to assess the mental health and overall quality of life in parents of children with rare congenital surgical diseases in Germany, the parents, especially mothers, reported severe psychosocial impairment during the pandemic (49). Parents of the RD patients had a significantly lower quality of life than parents in the control group, potentially due to the lockdown measures imposed, daily care for the patient, work-from-home measures, and the concerns of their children being at a higher risk of infection after surgeries (49). This study highlighted the need to provide support and raise awareness for parents in addition to the RD patient via a family-centered approach, especially during difficult periods such as the era of COVID-19 pandemic.
Besides RD patients and carers, the pandemic has also brought unprecedented challenges to RD patient organizations internationally. A multinational cross-sectional study was conducted to evaluate the impact of the COVID-19 pandemic on 80 RD organizations across 10 jurisdictions in the Asia Pacific region, namely Australia, Hong Kong, India, Japan, mainland China, Malaysia, New Zealand, the Philippines, Singapore and Taiwan (48). The study found that almost 90% of the patient organization representatives were concerned about the pandemic's impact on their organizations. In particular, over 60% and over 40% of the participants have highlighted reduction in organization capacity and funding as their biggest challenges during the pandemic respectively (48). They have also experienced difficulties in supporting their members as physical interactions were restricted. Importantly, patient group representatives underpinned the need to move toward a digitalised era, both in organization operation and healthcare, especially amidst confinement measures. In particular, operation of RD patient organizations in Australia and New Zealand were not impacted or were less affected by the pandemic as they had greater digital capacities and have digitalised their operations prior to the pandemic (48). The pandemic has brought myriad challenges to the RD patients and organizations, yet has also created opportunities by accelerating the adaption of tele-operation and telehealth, complementing face-to-face visits and consultations.
The current COVID-19 pandemic has highlighted and exposed the long-standing and fundamental challenges and healthcare needs of the RD population. The healthcare, social care, economic, and organizational challenges experienced by the RD community indicate the importance of ensuring adequate and continuity of diagnostic and priority management strategies for RDs during pandemics and beyond.
RDs: A global public health priority
The challenges arising from the nature of RDs have led RDs to emerge as a global public health priority. Unprecedented global integration of RD research is crucial to raise awareness, enhance understanding, accelerate diagnosis, and improve treatment for RDs. Recognizing its importance, the International Rare Diseases Research Consortium (IRDiRC) was established in 2011 to facilitate international collaboration between public and private sectors, and among stakeholders active in RDs research across government research funding bodies, companies, academia, and patient advocacy organizations around the world (50, 51). The IRDiRC have set out three 10-year goals for 2017 to 2027, with the vision to enable RD patients to achieve an accurate diagnosis, and to receive appropriate care and available therapy within 1 year of seeking medical attention (50). The three IRDiRC goals are:
-
To provide all individuals with suspected RDs who have seek medical attention with a diagnosis within 1 year if the RD is reported in medical literature; and to put those who remain undiagnosed in an international coordinated diagnostic and research pipeline;
-
To approve a thousand new therapies for RDs, with the majority focusing on RDs without approved options; and
-
To develop new methodologies for assessing the impact of RD diagnoses and therapies.
The three IRDiRC goals mainly target the healthcare challenges of RDs, with the overarching aim being to galvanize the broad RD community to enable universal diagnosis and treatment, to ensure that the programmes and interventions can reach RD patients and families, and to pose the intended positive impact on the health and wellbeing of the RD population (50, 51).
Recognizing the importance of promoting inclusion and protecting the human rights of the RD population, EURORDIS, Rare Disease International, and the Committee on Non-Governmental Organizations (NGOs) for RDs, together called for a UN Resolution for RDs in 2019, urging the 193 UN Member States of the General Assembly to adopt the Resolution by the end of 2021. This campaign targets the RD patients and families by recognizing and addressing their needs and challenges, which aims to promote greater integration of RDs in the agenda of the UN, and advances UN's commitment in achieving the Sustainable Development Goals (SDGs) of the 2030 Agenda, with the endeavor to “leave no one behind.” The UN resolution has five key asks:
-
Social inclusion and participation of RD patients and families;
-
Universal and equitable access of quality healthcare without having to experience financial hardship;
-
Promotion of RD strategies and actions at a national level;
-
Integration of RDs into UN programmes, agencies, and priorities; and
-
Routine publication of UN reports for resolution progress monitoring.
The Call for UN Resolution has promoted research and global coalition to tackle the socio-economic challenges of the RD population. The UN resolution was subsequently adopted on December 16, 2021 (52); this is an important milestone toward greater awareness and recognition for the RD community, allowing implementation of international policies to address the needs and challenges of the RD population.
The state of evidence in addressing the challenges of the RD population
National and international stakeholders across academia, health systems, governments, funding bodies, NGOs, and patient advocacy organizations have set out research projects and programmes to tackle the challenges experienced by the RD population, contributing to achieving the three IRDiRC goals and the five UN Resolution key asks. There has been tremendous progress in RD research over the past decade, especially in RD diagnoses and gene discoveries, achieved by the advancement in genomic technologies. Positive trend in RD-related therapeutic development was also observed, with the IRDiRC's 2020 goal for 200 new therapies being achieved in early 2017, three years ahead of the agenda (50). The socio-economic burden of RDs is harder to gauge and is rather limited in literature, given the collective number of unique RDs identified and the lack of standardized methodologies to collect related data.
Improving RD diagnoses and its implications
Traditionally, making a diagnosis is particularly challenging due to the heterogeneity and the rarity of each of the 6,000–8,000 RDs, multisystemic involvement, and pleiotropic manifestations (53–55). In the era of genomic medicine, our understanding on RDs has been transformed by the rapid expansion and translational application of next-generation sequencing (NGS) technologies in the past decade. NGS technology utilizes massively parallel sequencing methods to simultaneously and comprehensively sequence multiple genes, the entire protein-coding region of the genome (the “exome”), or the entire human genome (56). The diagnostic capacity of whole-exome sequencing (WES) and whole-genome sequencing (WGS), both NGS approaches, were shown to be effective over conventional diagnostic approaches across multiple studies in different populations. In a meta-analysis including 37 studies and comprising 20,068 children, the pooled diagnostic rates among WES and WGS were found to be 0.36 (95% CI 0.33–0.40, I2 = 83%) and 0.41 (95% CI 0.34–0.48, I2 = 44%), respectively, higher than the conventional diagnostic method of chromosomal microarray (0.10, 95% CI 0.08–0.12, I2 = 81%) (57). In critically ill patients with urgent needs, previous clinical studies have illustrated the vast amount of potential of rapid WES (rWES) and rapid WGS (rWGS) in diagnostic capacity, speed, and clinical utility in acute care (53, 58–60). The diagnostic capacity of rWES and rWGS was corroborated by findings from 18 studies comprising 1,049 patients from different countries, combined as part of a meta-analysis, with the pooled diagnostic yield being 0.43 (95% CI 0.36–0.50, I2 = 80.7%) (61). The successful application of WES and WGS in diagnosing patients with RDs in different settings has also allowed new gene discoveries over the years since its introduction in 2010 (Figure 2) (62, 63). The speed of new gene discoveries has been increasing substantially, with discoveries made by WES and WGS almost tripled the discoveries made by conventional methods since 2013 (62).
Figure 2
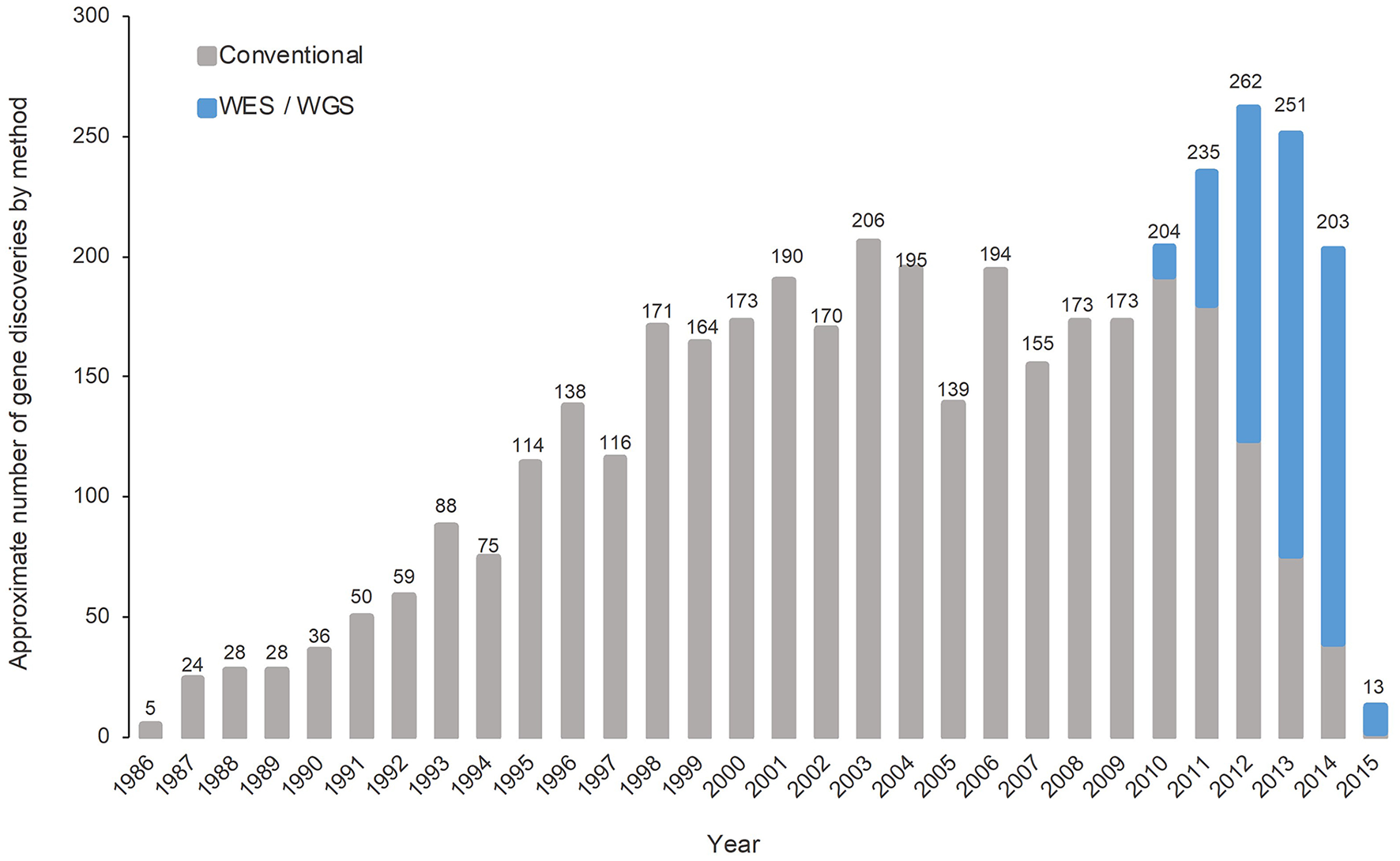
Number of gene discoveries made by WES/WGS compared with conventional diagnostic methods [adapted from Chong et al. (62)].
More importantly, WES and WGS offer the potential for the development of pragmatic, phenotype-driven management with genotype-differentiated personalized treatment (64). Personalized medicine, according to the National Human Genome Research Institute (NHGRI), was defined as an emerging practice of medicine that utilizes an individual's genetic profile to guide clinical decision-making in disease prevention, diagnosis, and treatment (65). WES and WGS have the potential to impact diagnosis-predicated clinical management, often referred as clinical utility, which includes but not limited to referral to specialists, surveillance for potential future complications, lifestyle changes, and indication or contraindication of investigations, procedures, surgeries, and medications (61, 66). In the meta-analysis by Clark et al. that included four WGS studies and 12 WES studies with data on clinical utility, 27% (95% CI 17–40%, I2 = 54%) and 17% (95% CI 12–24%, I2 = 76%) of children with genetic diagnoses had subsequent changes in their clinical management respectively (57). Early and rapid adoption of rWES or rWGS within a median of two to three weeks of results turnaround time could potentially impact clinical management promptly and profoundly, thus improving patient's clinical outcome and quality of life, and reducing morbidity and mortality (58–60, 67). In the intensive care setting of the National Health Service (NHS) of the United Kingdom, the use of rWGS led to changes in clinical management in 65% of the diagnosed patients (60). Chung et al. investigated the diagnostic utility of rWES and rWGS as a meta-analysis, and illustrated that genetic diagnoses could impact clinical management in up to 100% of the diagnosed patients in some cohorts (61). A rapid and timely genetic diagnosis is particularly important among critically ill patients with urgent needs, as it is potentially lifesaving.
The implication of RD diagnoses is beyond that on patients. In the era of resource and budget constraints, the evaluation of economic implications of providing WES and WGS within clinical settings has a principal role in informing efficient and effective healthcare resource allocation. Despite the high unit costs of WES and WGS, studies have demonstrated the cost-effectiveness of WES and WGS across clinical settings (68–72). On the other hand, health-economic evidence of rWES and rWGS is rather limited, with the fact that parallel comparison of rWES/rWGS and conventional diagnostic methods is more challenging due to the critical and urgent clinical setting that requires immediate clinical management decisions. Studies however illustrated the potential of rWES and rWGS to reduce healthcare costs, with costs being saved in the avoidance of unnecessary investigations, procedures, hospitalisations, and medications (58, 59, 61). In particular, Stark et al. reported a cost-saving of AU$543,178 (US$408,090) from avoidance of planned procedures and hospital days using rWES in Australia (59). In Hong Kong, Chung et al. demonstrated a reduction of 566 hospital days and a cost-saving of HK$8 million (US$1.03 million) from clinical management changes using rWES (61). In the United States, Farnaes et al. illustrated a net cost-saving of US$128,555 from reduced inpatient days using rWGS (58). Available evidence shed light on the consideration of integrating WES/WGS into clinical workflows to enable precision medicine and reduce healthcare costs.
The importance of an early genetic diagnosis for RD patients was demonstrated and highlighted in many of the previous studies, contributing to and reinforcing the 10-year goal of IRDiRC to provide an early definitive molecular diagnosis within 1 year of medical attention (50, 63). An accurate genetic diagnosis is the first step in managing the RD properly, allowing the identification of useful resources and treatment for the best possible clinical outcome for patients. The diagnosis-predicated changes in management not only improved clinical outcomes for patients, but could also lead to net cost-savings, stressing its multi-level significance. In addition to the immediate clinical changes and associated cost-savings from a rapid genetic diagnosis, an early genetic diagnosis has the power to aid better understanding in RD epidemiology and target health disparities, which all act as strong advocacies for the RD population (Figure 3) (73). It also contributes to existing literature and provides empirical evidence for better health- and social-care planning, such as implementation of population-wide sequencing and prevention strategies (74).
Figure 3
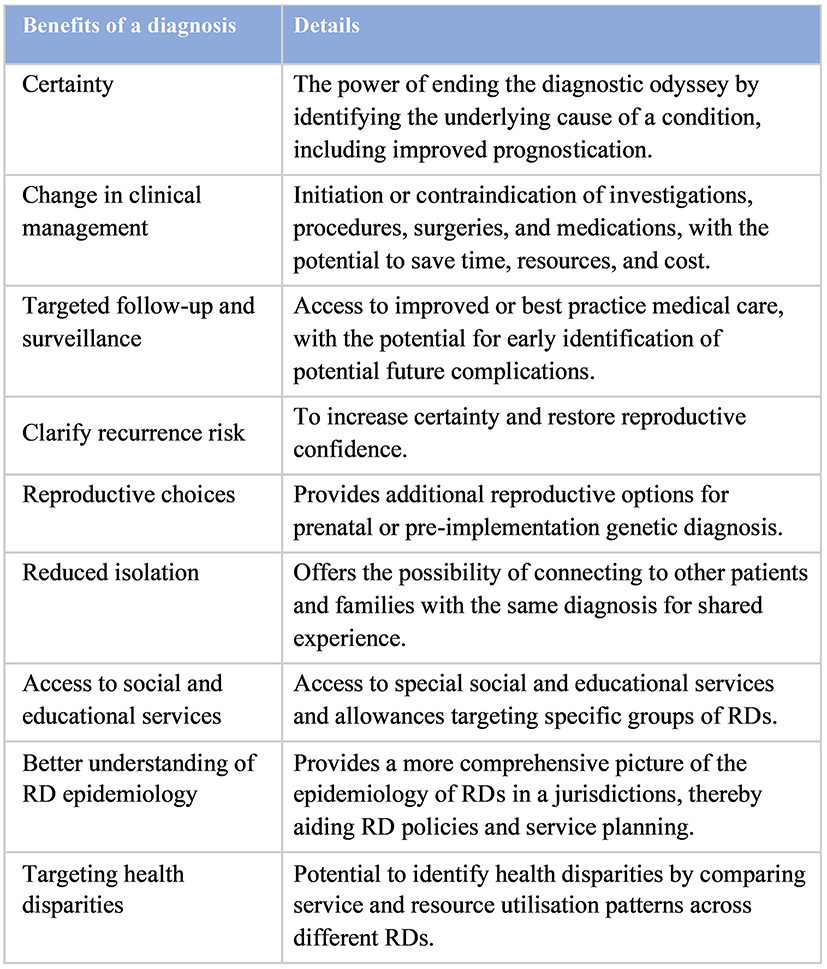
Benefits of an early genetic diagnosis [adapted from Tan et al. (73)].
Socio-economic costs of RDs
The societal impact of RDs has an economic dimension. In literature, majority of the economic evidence was based on individual RDs that are relatively “common,” while recent studies accounting for a wider range of RDs often quantify direct healthcare costs from a health system perspective due to the lack of standardized methodologies to collect cost-related data beyond health administrative dataset for the RD population (15, 75).
The largest study to date, estimated healthcare utilization and related costs across 1,600 RDs from a health system perspective in the United States. The study highlighted the disproportionately higher number of inpatient stays, readmissions, emergency visits, and the related costs of the RD population as compared to other common conditions (76). Similarly, the direct immediate healthcare burden of RDs was also estimated in studies conducted in Australia, Hong Kong, Shanghai, and Taiwan (77–81), suggesting the high direct healthcare costs in the RD population.
Although often neglected and rather challenging to estimate, it is also extremely important to evaluate and estimate the direct non-healthcare and indirect economic consequences for healthcare and related planning, especially in a chronic disease population. With RDs often being medically devastating and life-threatening, unpaid informal carers, usually a family member or a friend of the patient, play an extremely important role in supporting and assisting the patient's daily healthcare and social needs. This harbors a unique set of challenges and burden in RD carers, which encompasses coordination of care as well as helping with daily activities, both of which have spill over effects onto the carers' own personal lives, especially work responsibilities. The nature of RDs thus potentially inhibits patient's and carer's participation and integration into society, resulting in significant productivity loss, posing financial constraints for the RD family in addition to the substantial medical costs that often requires cost-sharing by the patient (82). On the other hand, many of these carers, usually both parents of the patients with RDs, have to sustain family's financial income by staying in the workforce. Therefore, paid carers, such as live-in domestic helpers, are commonly hired as an alternative to provide formal care support. This is particularly prevalent in Asia, such as the case in Hong Kong. In fact, previous evidence has demonstrated that direct non-healthcare and indirect costs of RDs (including paid and unpaid carers) are higher than direct healthcare costs of RDs, reflecting the importance to consider the broader socio-economic consequences of RDs in health- and social-care policies (16).
As highlighted by a meta-analysis published in 2021 that identified 19 studies in literature, economic evidence from a wider societal perspective has been very limited, with majority of the evidence focusing on individual RDs (75). Almost all of the identified studies were conducted in European populations, with many of them collected as part of the “Social Economic Burden and Health-Related Quality of Life in Patients with Rare Diseases in Europe” (BURQOL-RD) project series, which estimated the costs of 10 relatively “common” RDs across eight jurisdictions in Europe (15, 83–92). The results undoubtedly aided understanding on the patterns of resource use and areas that require prioritization, supporting appropriate healthcare planning for these 10 RDs. Nevertheless, the 10 selected RDs may be insufficient to encompass the heterogeneity and differential impacts of the 6,000–8,000 known RDs. It is important to note that the economic impact of RDs that are relatively “rare” was never reported in literature, due to the challenges in patient recruitment. Recently, the EverydayLife Foundation has published a report that estimated the costs of 379 RDs in the United States from a societal perspective, which was found to be US$62,141 per patient per year (16). In 2019, the national cost of RDs in the United States totalled US$966 billion (non-healthcare and indirect costs accounting for 56.7%), significantly higher than the costs estimated for some of the most expensive chronic illnesses, including cancer, diabetes, and heart disease as indicated by the Centers for Disease Control and Prevention (CDC) (16). Although only 379 of the 6,000–8,000 RDs were included for estimation, to the best of knowledge, this represents the only and the most comprehensive study to evaluate the socio-economic burden of RDs as a collective group.
In addition to the high societal costs of RDs, it was anticipated that the disproportionately high service and resource needs, and the RD-related productivity loss might pose significant financial burden on the RD families, putting them at a higher risk of experiencing financial hardship. Only two studies have attempted to evaluate the proportion of financial hardship brought about by extremely high OOP health expenditure in the RD population to date, one being in China where the authors have estimated the rate of catastrophic health expenditure (CHE) across seven RD groups (93), and another study being in Turkey where the authors estimated the CHE incidence mainly in patients with metabolic and neuromuscular diseases (94). These two studies have reported very different rates of CHE at different thresholds (0.0015–0.1670% vs. 47.35%), reflecting the differences in healthcare and social care contexts, and the availability and accessibility of resources across jurisdictions.
Health-related quality of life of the RD population
The impact of RDs can also be determined by quantifying patient's health-related quality of life (HRQoL). HRQoL is defined as “an individual's perception of his/her living quality, encompassing physical, mental, and social wellbeing” (95). Most RDs are typically chronic, progressive, degenerative, and life-threatening, with effective drugs being costly and scarce. Social exclusion and discrimination based on RD health conditions further depletes available resources for coping with RDs (27). It is therefore crucial to identify and understand the impact of disease and social related difficulties on the quality of life of RD patients. Previous studies have attempted to investigate the HRQoL of the RD population in more than one RD group, and have highlighted the significantly lowered HRQoL as compared to the general population (15, 28, 95, 96). In particular, the meta-analysis by Ng et al. included four studies comprising 2,079 RD patients and demonstrated a pooled utility score of 0.57 (95% CI 0.48–0.66), consistently lower than that of the general public (95). Importantly, Ng et al. has also demonstrated the “spill over effects” on carers' HRQoL in Hong Kong. Lifelong caring, high dependency of patient, and economic strain are all factors that contribute to the decreased wellbeing of patient family members and carers. In Hong Kong, both RD patients (mean utility score of 0.53) and their carers (mean utility score of 0.78) reported lower utility scores than the general population (mean utility score of 0.92) (95). More strikingly, they reported utility scores even lower than that of patients with other chronic illnesses, including patients with heart disease (0.88), hypertension (0.88), diabetes (0.87), and cancer (0.87), reflecting the disproportionate impact of RDs on healthcare and social wellbeing (95, 97, 98).
The role of Genome Projects in advancing genomic medicine
The importance of generating greater representation and diversity across genomic datasets is becoming more widely recognized. Initially, genetic research and genomic databases were biased toward data from Caucasians, particularly of European ancestry. In 2009, 96% of genome-wide association studies were of European descent (99). Groups of other ancestries were very poorly represented. The lack of ethnic diversity in genomics was limiting the usefulness of genomic technologies and widening inequalities across different populations. To address this, contribution of genomic data of other ethnicities has increased over the past few years, increasing from 4% in 2009 to 19% in 2016 (99).
Besides research, governments have also started to launch Genome Projects in their respective jurisdictions to apply WGS to the study of RDs, and to a lesser extent, cancers and common disorders, at a much bigger population size, or even at a nationwide level, to integrate genomic medicine into mainstream healthcare and to improve global genomic diversity and equity (100).
The government of the United Kingdom has launched the 100,000 Genomes Project in 2013, and it has been a huge success in providing grounds for the NHS Genomic Medicine Service to be the first national health care system to offer WGS as part of routine clinical care for patients with undiagnosed RDs and cancers (101, 102). This has inspired governments worldwide, even in middle-income countries, to launch Genome Projects in their respective jurisdictions, aiming to enhance clinical application of genomic medicine for personalized medicine (Figure 4) (100, 103). In the upcoming years, results from Genome Projects worldwide would potentially enhance our capability to better diagnose and manage RDs, and would provide empirical evidence for implementation of WES/WGS in health systems. More importantly, genomic data across populations, especially those beyond Europe and North America, will together contribute to improving equity of access and return in global genomics.
Figure 4
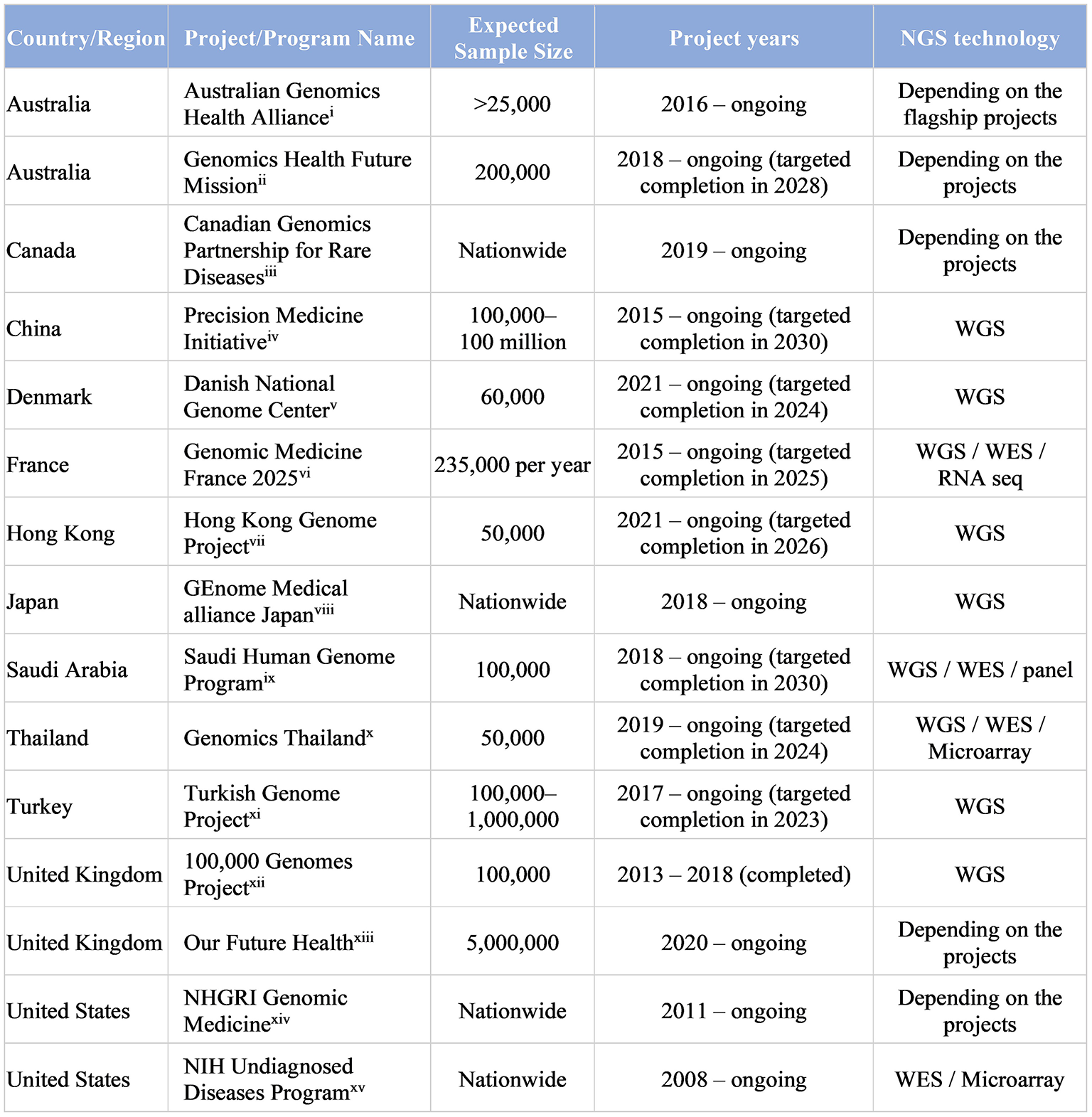
Large-scale Genome Projects targeting RDs and undiagnosed diseases (>20,000 subject genomes) (i) (104, 105); (ii) (106); (iii) (107); (iv) (108, 109); (v) (110); (vi) (111); (vii) (112); (viii) (113, 114); (ix) (115); (x) (116, 117); (xi) (118, 119); (xii)(101, 120); (xiii) (121, 122); (xiv) (123); (xv) (124). [adapted from Chung et al. (100) and Chu et al. (103)].
Case study: The Hong Kong Genome Project
As discussed above, genomic data of non-European ancestries has been increasing over the years. Genome Projects in Asia for example, are playing a major role in contributing genomic data of Asian ancestry to improve global genomic diversity. In Asia, Hong Kong has a relatively homogeneous Chinese population. The case study of the Hong Kong Genome Project (HKGP) was selected to illustrate the contribution of Chinese genomic data.
In the 7.5 million population in Hong Kong with 94% of the population being Chinese (ethnically speaking, Han Chinese), one in 67 individuals is living with one or more RDs, with 35% being pediatric patients (78, 125). As of 2018, over 470 RDs have been identified in Hong Kong, affecting approximately 1.5% of the population (78). In order to enhance clinical application of WGS to benefit patients and families, particularly the RD population, and to strive for excellence and adherence to international standards, the Hong Kong Genome Institute (HKGI) was established in May 2020 by the former Food and Health Bureau (currently the Health Bureau), Hong Kong Special Administrative Region, to implement the HKGP, with the vision being “to avail genomic medicine to all for better health and wellbeing” (126).
The HKGP, which is implemented in two phases, the pilot phase and the main phase, is the first large-scale genome sequencing project in Hong Kong. It is set to conduct WGS for 20,000 cases with the aim to enhance clinical application of genomic medicine to benefit patients and their families with more precise diagnoses and personalized treatment (126). The pilot phase of the HKGP was launched in July 2021, focusing on undiagnosed diseases and hereditary cancers. Lessons learnt during the pilot phase of HKGP would guide the directions of the Project's main phase, which is set out to be rolled out in July 2022, expanding eligibility to cover other hereditary diseases and research cohorts related to “genomics and precision health.”
With WGS being offered as part of the HKGP, it could potentially lead to diagnosis-predicated precision medicine for patients and families, thereby improving patient outcomes whilst minimizing healthcare expenditure and related financial hardships, achieving diagnostic, clinical, and economic utility. In addition to the clinical benefits, the HKGP also aims to advance research, establish infrastructure and protocols, nurture talents, enhance public genomic literacy and engagement, and drive health- and social- care policy measures to pioneer the development of genomic medicine in Hong Kong (126). The potentials and prospects that have emerged in the launching of the HKGP pilot phase were highlighted by Chu et al., providing insights to prepare for the launching of the main phase (103). With HKGP being the first and the largest local clinical genomic database, it creates novel research opportunities for studying various diseases, including RDs, contributing to improving genomic equity in healthcare. With a relatively homogenous population, genomic data generated from the HKGP would contribute to global genomic diversity in the foreseeable future.
Future directions of RDs
With the three 10-year goals laid out by IRDiRC and the adoption of the UN Resolution in December 2021 to stress the importance of including RD population in the UN 2030 Agenda, great strides have been made in RD research over the past decade, presenting a major shift in the global policy landscape. In particular, previous studies have highlighted the importance of an early diagnosis and the significant consequences of RDs on the living quality and socio-economic burden of patients. It should be recognized that the health, social, and economic implications of RDs are inherently the results of insufficient social support, limited medical expertise, and the lack of public awareness on RDs. As RD emerges as a global public health priority, RD policies and strategies in the sectors of healthcare, social care, insurance, education, and many more, are required to foster a more equitable and inclusive community for the RD population. Progress in RD research and analysis will likely improve all disease understanding in the future. Here, we recommend future action plans for RDs through a patient-centered and multidisciplinary approach, focusing on the implementation of education and training programmes, elimination of discrimination and stigmatization, and global coalition among multi-disciplinary stakeholders.
Firstly, providing education and training to clinicians at the primary, secondary, and tertiary care level is the first step to an accurate genetic diagnosis. Despite the technological advancement and increased data sharing, many RD patients still experience extensive diagnostic odysseys, and some remain undiagnosed. One of the major barriers to obtaining a diagnosis is the lack of knowledge and insufficient training on RDs. According to the National Organization for Rare Disorders (NORD) survey 2019, almost half of the patients and carers identified limited medical specialization to be a major barrier to delays in RD diagnosis (127). Previous studies have shown that many primary care physicians profess low confidence in their skillsets in managing patients with genetic-related issues and in using genetic information to make clinical decisions (128–131). Primary care physicians have identified lack of knowledge and training opportunities to be the major barriers to genomic medicine in primary care (128, 131, 132). Emphasis on further education and training in genomic medicine among medical specialists should be prioritized in order to improve RD diagnosis and management. Continuous technological and technical advancement in genomics is also required to diagnose patients and to transform sequencing information into diagnostic knowledge, such as the application of bioinformatics, analytic algorithm, functional analysis, health informatics, data linkage capability, data sharing, etc. (74, 133). Global network involving full participation by clinicians, researchers, and patients and carers should be formed to tackle the undiagnosed cases (74). International efforts have been made over the years to investigate and diagnose patients who had long sought one without success, such as the initiation of the Undiagnosed Disease Program by the National Institutes of Health (NIH) and the Undiagnosed Diseases Network International (134, 135). The establishment of these programmes and networks have supported global improvements in diagnosis of RDs via core principles and implementation methods (74).
Secondly, in addressing the significant socio-economic burden and the lowered HRQoL of the RD population, the government and healthcare system should work together to provide affordable and accessible resources, thereby improving the HRQoL of patients and carers. Previous cost-of-illness studies highlighted the unique and complex challenges the RD population face, providing strong evidence that management of these challenges should be treated differently to other common disease (15, 16, 77–79, 83–92). With RD patients requiring services and care across multi-disciplines, patients often experience frustration in service fragmentation. The implementation of “one-stop” clinics may improve coordination of care through providing various services at a single location, tackling multiple and complex problems simultaneously (29). In France and the United Kingdom, the integration of “one-stop” clinics was shown to improve coordination between services, providing timely and informed care to the RD population (136). Overall, the utilization of such “one-stop” clinics have yielded better patient outcomes and are more cost-effective and thus, are a possible solution to the high socio-economic burden and the lowered HRQoL of RDs (137). In addition, implementation of reimbursement regulations would improve affordability and accessibility of treatments, potentially reducing the risk of financial hardship of the RD population (138). Other action plans in Australia and New Zealand have also been put forward to support the RD population (139). These aim to reduce the healthcare, social care, and economic burden through empowering, improving diagnosis and intervention, coordinating care and increasing research for RDs.
Thirdly, the government plays a pivotal role in raising awareness and in mitigating discrimination and stigmatization of the RD population. Efforts have been made in different parts of the world to implement Genome Projects to integrate genomic medicine in mainstream healthcare. In order to enhance understanding and mitigate genetic discrimination, unprecedented global coalition is of paramount importance to improve inclusivity of the RD population. Previous studies have demonstrated participants' concerns on genetic discrimination in the context of employment and insurance (140–142). In particular, undergraduates in Hong Kong were found to be pessimistic toward unfavorable genetic testing results, with almost 60% of the respondents claiming that they would feel “inadequate or different,” 56% would feel helpless, and nearly 60% perceived that they would be disadvantaged in job seeking in case of unfavorable genetic testing results (140). It is of utmost importance to eliminate the root causes of stigmatization and discrimination of the RD population in order to improve social inclusion and reduce opportunities and productivity loss. This can be done through the implementation of anti-discrimination policies such as Genetic Information Nondiscrimination Act (GINA) in the United States to aid assimilation of the RD population into society (143, 144). Legislations in Japan and Taiwan have also incorporated social care services into their RD framework, thereby facilitating the inclusion and integration of the RD population into the society in addition to providing quality healthcare (145). Additional education and awareness for the public on the RDs should also be implemented to increase acceptance and reduce stigma. Both these strategies in conjunction will work toward improving social integration of the RD population, thereby improving their HRQoL and reducing socio-economic burden.
Fourthly, a more widespread utilization of telehealth or telemedicine constitutes a sustainable and alternate model amidst the COVID-19 pandemic and beyond. Telemedicine has the potential to revolutionize patient access to clinical specialists around the world without geographical boundaries. The COVID-19 pandemic has accelerated the digitalisation of healthcare across the world and have inspired healthcare professional licensing agencies to address this in various nations and states. This improves access for all patients in both urban and rural areas, and in both developed and developing countries regardless of their economic status. Adoption of telemedicine into routine clinical care would require innovative approaches to increase capacity and the strengthening of health systems. In the long run, greater reliance on telemedicine is undeniably the way forward, which constitutes a sustainable healthcare delivery model in times of and beyond pandemics.
Finally, RD patient organizations have the power to drive forward the adoption of necessary policies and help to coordinate care (146). Governments should strive to strengthen the public's awareness on the needs of RD populations through in-depth conversations and focus group meetings with patient representatives. On one hand, RD patient groups have important roles in advocating for patients' rights and research opportunity. On the other hand, patient groups are the pillar of psychologic support for patients and their families. The recently adopted ground-breaking UN Resolution led by Rare Disease International, EURORDIS, and the Committee on NGOs for RDs serves as a strong example. It represents a major shift in the global policy landscape, by promoting greater integration and prioritization of the RD population in the UN agenda. Through this global campaign, the needs of the RD community are brought to light, allowing for the development of necessary strategies and plans to provide affordable and accessible care. Acting as the voice of the RD population, RD patient organizations can empower patients and carers alike while raising awareness to educate the community.
Taken collectively, there is a scientific, social, ethical, and political imperative to promote greater integration and inclusiveness of RDs in research and policies, contributing to the goal of the UN Resolution, to “leave no one behind.”
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Author contributions
CC and BC contributed to the conception of the review. CC performed the literature review and drafted the manuscript. Hong Kong Genome Project, AC, and BC critically reviewed and revised the manuscript for important intellectual content. AC and BC oversaw and supervised the review. All authors contributed to the overall interpretation, reviewed, and approved the final draft for submission.
Acknowledgments
We would like to thank all members of the Hong Kong Genome Institute in preparing the launch of the Hong Kong Genome Project. This work would not have been possible without the instrumental leadership and guidance from Dr. Su-vui Lo, Chief Executive Officer. We extend our gratitude to the Board of Directors and Advisory Committees for their continuous support and advice. We also wish to acknowledge the support of the Hong Kong Genome Project stakeholders: the Health Bureau; Hospital Authority; and the Department of Health.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
- NHGRI
National Human Genome Research Institute
- NIH
National Institutes of Health
- RD
rare disease
- RNA Seq
RNA sequencing
- WES
whole-exome sequencing
- WGS
whole-genome sequencing.
Abbreviations
References
1.
Orphanet . The Portal for Rare Diseases and Orphan Drugs 2012. Available online at: https://www.orpha.net/consor/cgi-bin/Education_AboutRareDiseases.php?lng=EN (accessed May 20, 2022).
2.
The Lancet Diabetes E . Spotlight on rare diseases. Lancet Diabetes Endocrinol. (2019) 7:75. 10.1016/S2213-8587(19)30006-3
3.
Plaiasu V Nanu M Matei D . Rare disease day - at a glance. Maedica (Bucur). (2010) 5:65–6.
4.
Gopal-Srivastava R Kaufmann P . Facilitating clinical studies in rare diseases. In:Posada de la PazMTaruscioDGroftSC, editors. Rare Diseases Epidemiology: Update and Overview. Cham: Springer International Publishing. (2017). p. 125–40.
5.
European Union . Regulation (EC) N°141/2000 of the European Parliament of the Council of 16 December 1999 on Orphan Medicinal Products. (2000). Available online at: https://eur-lex.europa.eu/EN/legal-content/summary/medicines-for-rare-diseases-orphan-drugs.html (accessed May 20, 2022).
6.
The The Food Drug Administration United, States . Rare Diseases at FDA. (2022). Available online at: https://www.fda.gov/patients/rare-diseases-fda (accessed May 20, 2022).
7.
The The Food Drug Administration United, States . Orphan Drug Act - Relevant Excerpts. (2018). Available online at: https://www.fda.gov/industry/designating-orphan-product-drugs-and-biological-products/orphan-drug-act-relevant-excerpts (accessed May 20, 2022).
8.
Song P Gao J Inagaki Y Kokudo N Tang W . Rare diseases, orphan drugs, and their regulation in Asia: current status and future perspectives. Intractable Rare Dis Res. (2012) 1:3–9. 10.5582/irdr.2012.v1.1.3
9.
Richter T Nestler-Parr S Babela R Khan ZM Tesoro T Molsen E et al . Rare disease terminology and definitions-a systematic global review: report of the ISPOR rare disease special interest group. Value Health. (2015) 18:906–14. 10.1016/j.jval.2015.05.008
10.
Nguengang Wakap S Lambert DM Olry A Rodwell C Gueydan C Lanneau V et al . Estimating cumulative point prevalence of rare diseases: analysis of the Orphanet database. Eur J Hum Genet. (2020) 28:165–73. 10.1038/s41431-019-0508-0
11.
EURORDIS. #Resolution4Rare: Global campaign for the first UN Resolution on Persons Living with a Rare Disease. (2021). Available online at: https://www.eurordis.org/news/resolution4rare-global-campaign-first-un-resolution-persons-living-rare-disease (accessed May 20, 2022).
12.
Groft SC Posada de la Paz M . Rare diseases: joining mainstream research and treatment based on reliable epidemiological data. In:Posada de la PazMTaruscioDGroftSC, editors. Rare Diseases Epidemiology: Update and Overview. Cham: Springer International Publishing. (2017). p. 3–21.
13.
Valdez R Ouyang L Bolen J . Public health and rare diseases: oxymoron no more. Prev Chronic Dis. (2016) 13:150491. 10.5888/pcd13.150491
14.
NGO Committee for Rare Diseases . The “Right to Health” in Rare Diseases. (2018). Available online at: https://www.ngocommitteerarediseases.org/wp-content/uploads/2018/05/NGO-CfRDs-Submission-The-Right-to-Health-in-Rare-Diseases_Feb-15-2018.pdf (accessed May 20, 2022).
15.
Lopez-Bastida J Oliva-Moreno J Linertova R Serrano-Aguilar P . Social/economic costs and health-related quality of life in patients with rare diseases in Europe. Eur J Health Econ. (2016) 17:1–5. 10.1007/s10198-016-0780-7
16.
Lewin Group . The National Economic Burden of Rare Disease Study. (2021). Available online at: https://everylifefoundation.org/wp-content/uploads/2021/02/The_National_Economic_Burden_of_Rare_Disease_Study_Summary_Report_February_2021.pdf (accessed June 23, 2022).
17.
Rare Diseases International . Rare Diseases: Leaving no one Behind in Universal Health Coverage. (2019). Available online at: https://d254mlohi4u805.cloudfront.net/rdi/2019/RDI%20UHC%20Paper%20Final%20October%202019.pdf (accessed May 20, 2022).
18.
Groft SC Posada M Taruscio D . Progress, challenges and global approaches to rare diseases. Acta Paediatr. (2021) 110:2711–6. 10.1111/apa.15974
19.
EURORDIS. Survey of the Delay in Diagnosis for 8 Rare Diseases in Europe ('Eurordiscare 2'). (2007). Available online at: https://www.eurordis.org/IMG/pdf/Fact_Sheet_Eurordiscare2.pdf
20.
Shire . Rare Disease Impact Report: Insights from Patients and the Medical Community. (2013). Available online at: https://globalgenes.org/wp-content/uploads/2013/04/ShireReport-1.pdf (accessed May 20, 2022).
21.
Makarova EV Krysanov IS Valilyeva TP Vasiliev MD Zinchenko RA . Evaluation of orphan diseases global burden. Eur J Transl Myol. (2021) 31:9610. 10.4081/ejtm.2021.9610
22.
Ollendorf DA Chapman RH Pearson SD . Evaluating and valuing drugs for rare conditions: no easy answers. Value Health. (2018) 21:547–52. 10.1016/j.jval.2018.01.008
23.
Czech M Baran-Kooiker A Atikeler K Demirtshyan M Gaitova K Holownia-Voloskova M et al . A review of rare disease policies and orphan drug reimbursement systems in 12 Eurasian countries. Front Public Health. (2019) 7:416. 10.3389/fpubh.2019.00416
24.
Chan AYL Chan VKY Olsson S Fan M Jit M Gong M et al . Access and unmet needs of orphan drugs in 194 countries and 6 areas: a global policy review with content analysis. Value Health. (2020) 23:1580–91. 10.1016/j.jval.2020.06.020
25.
Emily Muir . Rare Disease UK: The Rare Reality - an Insight to the Patient Family Experience of Rare Disease. (2016). Available online at: https://www.raredisease.org.uk/media/1588/the-rare-reality-an-insight-into-the-patient-and-family-experience-of-rare-disease.pdf (accessed May 20, 2022).
26.
Chen S Wang J Zhu J Chung RY Dong D . Quality of life and its contributors among adults with late-onset Pompe disease in China. Orphanet J Rare Dis. (2021) 16:199. 10.1186/s13023-021-01836-y
27.
Zhu X Smith RA Parrott RL . Living with a rare health condition: the influence of a support community and public stigma on communication, stress, and available support. J Appl Commun Res. (2017) 45:179–98. 10.1080/00909882.2017.1288292
28.
Efthymiadou O Mossman J Kanavos P . Differentiation of health-related quality of life outcomes between five disease areas: results from an international survey of patients. Int J Technol Assess Health Care. (2018) 34:498–506. 10.1017/S0266462318000557
29.
Castro R Senecat J de Chalendar M Vajda I Dan D Boncz B . Bridging the Gap between Health and Social Care for Rare Diseases: Key Issues and Innovative Solutions. In:Posada de la PazMTaruscioDGroftSC, editors. Rare Diseases Epidemiology: Update and Overview. Cham: Springer International Publishing. (2017). p. 605–27.
30.
Griggs RC Batshaw M Dunkle M Gopal-Srivastava R Kaye E Krischer J et al . Clinical research for rare disease: opportunities, challenges, and solutions. Mol Genet Metab. (2009) 96:20–6. 10.1016/j.ymgme.2008.10.003
31.
Iskrov G Miteva-Katrandzhieva T Stefanov R . Health Technology Assessment and Appraisal of Therapies for Rare Diseases. In:Posada de la PazMTaruscioDGroftSC, editors. Rare Diseases Epidemiology: Update and Overview. Cham: Springer International Publishing. (2017). p. 221–31.
32.
McCabe C Claxton K Culyer AJ . The NICE cost-effectiveness threshold: what it is and what that means. Pharmacoeconomics. (2008) 26:733–44. 10.2165/00019053-200826090-00004
33.
Berdud M Drummond M Towse A . Establishing a reasonable price for an orphan drug. Cost Eff Resour Alloc. (2020) 18:31. 10.1186/s12962-020-00223-x
34.
Hughes DA Tunnage B Yeo ST . Drugs for exceptionally rare diseases: do they deserve special status for funding?Qjm. (2005) 98:829–36. 10.1093/qjmed/hci128
35.
Hyry HI Roos JC Cox TM . Orphan drugs: expensive yet necessary. Qjm. (2015) 108:269–72. 10.1093/qjmed/hcu240
36.
Lakdawalla DN Doshi JA Garrison LP Jr Phelps CE Basu A Danzon PM . Defining elements of value in health care-A health economics approach: an ISPOR special task force report [3]. Value Health. (2018) 21:131–9. 10.1016/j.jval.2017.12.007
37.
Granados. A Beresniak A Drummond M Upadhyaya S Wong-Rieger D . Are We capturing the Multidimensional Value of Rare Disease Therapies Through the QALY? Report of ISPOR 2022 Issue Panel. (2022). Available online at: http://www.datamining-international.com/new/wp-content/uploads/2022/07/220715-ISPOR-2022-Issue-Panel-Report-QALYs-and-RDs.pdf (accessed September 22, 2022).
38.
Centre for Health Protection Department Department of Health The The Government of the Hong Kong Special Administrative Region. Countries/Areas with Reported Cases of Coronavirus Disease-2019 (COVID-19). (2022). Available online at: https://www.chp.gov.hk/files/pdf/statistics_of_the_cases_novel_coronavirus_infection_en.pdf
39.
Chung CCY Wong WHS Chung BHY . Hospital mortality in patients with rare diseases during pandemics: lessons learnt from the COVID-19 and SARS pandemics. Orphanet J Rare Dis. (2021) 16:361. 10.1186/s13023-021-01994-z
40.
Zhang H Thygesen JH Shi T Gkoutos GV Hemingway H Guthrie B et al . Increased COVID-19 mortality rate in rare disease patients: a retrospective cohort study in participants of the Genomics England 100,000 Genomes project. Orphanet J Rare Dis. (2022) 17:166. 10.1186/s13023-022-02312-x
41.
Hampson C Evans W Menzies L McKay L . Measuring the impact of the COVID-19 pandemic on diagnostic delay in rare disease. Eur Med J. (2022). 10.33590/emj/21-00181 (accessed September 22, 2022).
42.
Chung CC Wong WH Fung JL Hong Kong RD Chung BH . Impact of COVID-19 pandemic on patients with rare disease in Hong Kong. Eur J Med Genet. (2020) 63:104062. 10.1016/j.ejmg.2020.104062
43.
Chowdhury SF Sium SMA Anwar S . Research and management of rare diseases in the COVID-19 pandemic era: challenges and countermeasures. Front Public Health. (2021) 9:640282. 10.3389/fpubh.2021.640282
44.
National Organization for Rare Disorders (NORD) . COVID-19 Community Follow-up Survey Report. (2020). Available online at: https://rarediseases.org/wp-content/uploads/2020/11/NRD-2061-RareInsights-CV19-Report-2_FNL.pdf (accessed May 20, 2022).
45.
Rare Disease Ireland . Living With a Rare Disease in Ireland During the COVID-19 Pandemic. (2020). Available online at: https://rdi.ie/wp-content/uploads/2020/05/Research-Report-Living-with-a-rare-disease-in-Ireland-during-the-COVID-19-pandemic.pdf (accessed May 20, 2022).
46.
Canadian Organization for Rare Disorders . Applying Lessons From COVID-19 to Better Healthcare for Rare Diseases. (2020). Available online at: http://www.raredisorders.ca/content/uploads/Applying-Lessons-from-COVID-19-3.pdf (accessed May 20, 2022).
47.
Byun M Feller H Ferrie M Best S . Living with a genetic, undiagnosed or rare disease: a longitudinal journalling study through the COVID-19 pandemic. Health Expect. (2022). 10.1111/hex.13405
48.
Chung CCY Ng YNC Jain R Chung BHY . A thematic study: impact of COVID-19 pandemic on rare disease organisations and patients across ten jurisdictions in the Asia Pacific region. Orphanet J Rare Dis. (2021) 16:119. 10.1186/s13023-021-01766-9
49.
Fuerboeter M Boettcher J Barkmann C Zapf H Nazarian R Wiegand-Grefe S et al . Quality of life and mental health of children with rare congenital surgical diseases and their parents during the COVID-19 pandemic. Orphanet J Rare Dis. (2021) 16:498. 10.1186/s13023-021-02129-0
50.
Austin CP Cutillo CM Lau LPL Jonker AH Rath A Julkowska D et al . Future of rare diseases research 2017-2027: an IRDiRC perspective. Clin Transl Sci. (2018) 11:21–7. 10.1111/cts.12500
51.
Cutillo CM Austin CP Groft SC . A global approach to rare diseases research and orphan products development: the International Rare Diseases Research Consortium (IRDiRC). In:Posada de la PazMTaruscioDGroftSC, editors. Rare Diseases Epidemiology: Update and Overview. Cham: Springer International Publishing. (2017). p. 349–69.
52.
Rare Disease International . Un Resolution On Persons Living With A Rare Disease. (2021). Available online at: https://www.rarediseasesinternational.org/un-resolution/ (accessed May 20, 2022).
53.
Saunders CJ Miller NA Soden SE Dinwiddie DL Noll A Alnadi NA et al . Rapid whole-genome sequencing for genetic disease diagnosis in neonatal intensive care units. Sci Transl Med. (2012) 4:154ra35. 10.1126/scitranslmed.3004041
54.
Council Recommendation of 8 June 2009 on European action in the field of rare diseases . Off J Eur Union. (2009) C151:7–10. Available online at: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:C:2009:151:0007:0010:EN:PDF (accessed May 20, 2022).
55.
Genetic Disease Foundation. (2010). Available online at: http://www.geneticdiseasefoundation.org/ (accessed May 20, 2022).
56.
Behjati S Tarpey PS . What is next generation sequencing?Arch Dis Child Educ Pract Ed. (2013) 98:236–8. 10.1136/archdischild-2013-304340
57.
Clark MM Stark Z Farnaes L Tan TY White SM Dimmock D et al . Meta-analysis of the diagnostic and clinical utility of genome and exome sequencing and chromosomal microarray in children with suspected genetic diseases. NPJ Genom Med. (2018) 3:16. 10.1038/s41525-018-0053-8
58.
Farnaes L Hildreth A Sweeney NM Clark MM Chowdhury S Nahas S et al . Rapid whole-genome sequencing decreases infant morbidity and cost of hospitalization. NPJ Genom Med. (2018) 3:10. 10.1038/s41525-018-0049-4
59.
Stark Z Lunke S Brett GR Tan NB Stapleton R Kumble S et al . Meeting the challenges of implementing rapid genomic testing in acute pediatric care. Genet Med. (2018) 20:1554–63. 10.1038/gim.2018.37
60.
French CE Delon I Dolling H Sanchis-Juan A Shamardina O Megy K et al . Whole genome sequencing reveals that genetic conditions are frequent in intensively ill children. Intensive Care Med. (2019) 45:627–36. 10.1007/s00134-019-05552-x
61.
Chung CCY Leung GKC Mak CCY Fung JLF Lee M Pei SLC et al . Rapid whole-exome sequencing facilitates precision medicine in paediatric rare disease patients and reduces healthcare costs. Lancet Reg Health West Pac. (2020) 1:100001. 10.1016/j.lanwpc.2020.100001
62.
Chong JX Buckingham KJ Jhangiani SN Boehm C Sobreira N Smith JD et al . The genetic basis of mendelian phenotypes: discoveries, challenges, and opportunities. Am J Hum Genet. (2015) 97:199–215. 10.1016/j.ajhg.2015.06.009
63.
Boycott KM Rath A Chong JX Hartley T Alkuraya FS Baynam G et al . International cooperation to enable the diagnosis of all rare genetic diseases. Am J Hum Genet. (2017) 100:695–705. 10.1016/j.ajhg.2017.04.003
64.
Willig LK Petrikin JE Smith LD Saunders CJ Thiffault I Miller NA et al . Whole-genome sequencing for identification of Mendelian disorders in critically ill infants: a retrospective analysis of diagnostic and clinical findings. Lancet Respir Med. (2015) 3:377–87. 10.1016/S2213-2600(15)00139-3
65.
National Human Genome Research Institute . Personalized Medicine. (2022). Available online at: https://www.genome.gov/genetics-glossary/Personalized-Medicine (accessed July 29, 2022).
66.
Riggs ER Wain KE Riethmaier D Smith-Packard B Faucett WA Hoppman N et al . Chromosomal microarray impacts clinical management. Clin Genet. (2014) 85:147–53. 10.1111/cge.12107
67.
Smith LD Willig LK Kingsmore SF . Whole-exome sequencing and whole-genome sequencing in critically Ill neonates suspected to have single-gene disorders. Cold Spring Harb Perspect Med. (2015) 6:a023168. 10.1101/cshperspect.a023168
68.
Schwarze K Buchanan J Taylor JC Wordsworth S . Are whole-exome and whole-genome sequencing approaches cost-effective? A systematic review of the literature. Genet Med. (2018) 20:1122–30. 10.1038/gim.2017.247
69.
Tan TY Dillon OJ Stark Z Schofield D Alam K Shrestha R et al . Diagnostic Impact and cost-effectiveness of whole-exome sequencing for ambulant children with suspected monogenic conditions. JAMA Pediatr. (2017) 171:855–62. 10.1001/jamapediatrics.2017.1755
70.
Soden SE Saunders CJ Willig LK Farrow EG Smith LD Petrikin JE et al . Effectiveness of exome and genome sequencing guided by acuity of illness for diagnosis of neurodevelopmental disorders. Sci Transl Med. (2014) 6:265ra168. 10.1126/scitranslmed.3010076
71.
Schofield D Alam K Douglas L Shrestha R MacArthur DG Davis M et al . Cost-effectiveness of massively parallel sequencing for diagnosis of paediatric muscle diseases. npj Genom Med. (2017) 2:4. 10.1038/s41525-017-0006-7
72.
Stark Z Schofield D Alam K Wilson W Mupfeki N Macciocca I et al . Prospective comparison of the cost-effectiveness of clinical whole-exome sequencing with that of usual care overwhelmingly supports early use and reimbursement. Genet Med. (2017) 19:867–74. 10.1038/gim.2016.221
73.
Baynam G Pachter N McKenzie F Townshend S Slee J Kiraly-Borri C et al . The rare and undiagnosed diseases diagnostic service - application of massively parallel sequencing in a state-wide clinical service. Orphanet J Rare Dis. (2016) 11:77.
74.
Baynam G Bowman F Lister K Walker CE Pachter N Goldblatt J et al . Improved Diagnosis and care for rare diseases through implementation of precision public health framework. In:Posada de la PazMTaruscioDGroftSC, editors. Rare Diseases Epidemiology: Update and Overview. Cham: Springer International Publishing. (2017). p. 55–94.
75.
Sequeira AR Mentzakis E Archangelidi O Paolucci F . The economic and health impact of rare diseases: a meta-analysis. Health Policy Technol. (2021) 10:32–44. 10.1016/j.hlpt.2021.02.002
76.
Navarrete-Opazo AA Singh M Tisdale A Cutillo CM Garrison SR . Can you hear us now? The impact of health-care utilization by rare disease patients in the United States. Genet Med. (2021) 23:2194–2201. 10.1038/s41436-021-01241-7
77.
Walker CE Mahede T Davis G Miller LJ Girschik J Brameld K et al . The collective impact of rare diseases in Western Australia: an estimate using a population-based cohort. Genet Med. (2017) 19:546–52. 10.1038/gim.2016.143
78.
Chiu ATG Chung CCY Wong WHS Lee SL Chung BHY . Healthcare burden of rare diseases in Hong Kong - adopting ORPHAcodes in ICD-10 based healthcare administrative datasets. Orphanet J Rare Dis. (2018) 13:147. 10.1186/s13023-018-0892-5
79.
Tisdale A Cutillo CM Nathan R Russo P Laraway B Haendel M et al . The IDeaS initiative: pilot study to assess the impact of rare diseases on patients and healthcare systems. Orphanet J Rare Dis. (2021) 16:429. 10.1186/s13023-021-02061-3
80.
Cai X Yang H Genchev GZ Lu H Yu G . Analysis of economic burden and its associated factors of twenty-three rare diseases in Shanghai. Orphanet J Rare Dis. (2019) 14:233. 10.1186/s13023-019-1168-4
81.
Hsu JC Wu HC Feng WC Chou CH Lai EC Lu CY . Disease and economic burden for rare diseases in Taiwan: A longitudinal study using Taiwan's National Health Insurance Research Database. PLoS ONE. (2018) 13:e0204206. 10.1371/journal.pone.0204206
82.
von der Lippe C Diesen PS Feragen KB . Living with a rare disorder: a systematic review of the qualitative literature. Mol Genet Genomic Med. (2017) 5:758–73. 10.1002/mgg3.315
83.
Angelis A Kanavos P Lopez-Bastida J Linertova R Oliva-Moreno J Serrano-Aguilar P et al . Social/economic costs and health-related quality of life in patients with epidermolysis bullosa in Europe. Eur J Health Econ. (2016) 17 (Suppl 1):31–42. 10.1007/s10198-016-0783-4
84.
Cavazza M Kodra Y Armeni P De Santis M Lopez-Bastida J Linertova R et al . Social/economic costs and quality of life in patients with haemophilia in Europe. Eur J Health Econ. (2016) 17:53–65. 10.1007/s10198-016-0785-2
85.
Cavazza M Kodra Y Armeni P De Santis M Lopez-Bastida J Linertova R et al . Social/economic costs and health-related quality of life in patients with Duchenne muscular dystrophy in Europe. Eur J Health Econ. (2016) 17:19–29. 10.1007/s10198-016-0782-5
86.
Chevreul K Gandre C Brigham KB Lopez-Bastida J Linertova R Oliva-Moreno J et al . Social/economic costs and health-related quality of life in patients with fragile X syndrome in Europe. Eur J Health Econ. (2016) 17:43–52. 10.1007/s10198-016-0784-3
87.
Chevreul K Michel M Brigham KB Lopez-Bastida J Linertova R Oliva-Moreno J et al . Social/economic costs and health-related quality of life in patients with cystic fibrosis in Europe. Eur J Health Econ. (2016) 17:7–18. 10.1007/s10198-016-0781-6
88.
Iskrov G Astigarraga I Stefanov R Lopez-Bastida J Linertova R Oliva-Moreno J et al . Social/economic costs and health-related quality of life in patients with histiocytosis in Europe. Eur J Health Econ. (2016) 17:67–78. 10.1007/s10198-016-0790-5
89.
Kuhlmann A Schmidt T Treskova M Lopez-Bastida J Linertova R Oliva-Moreno J et al . Social/economic costs and health-related quality of life in patients with juvenile idiopathic arthritis in Europe. Eur J Health Econ. (2016) 17:79–87. 10.1007/s10198-016-0786-1
90.
Lopez-Bastida J Linertova R Oliva-Moreno J. Posada-de-la-Paz M Serrano-Aguilar P Kanavos P et al . Social/economic costs and health-related quality of life in patients with Prader-Willi syndrome in Europe. Eur J Health Econ. (2016) 17:99–108. 10.1007/s10198-016-0788-z
91.
Lopez-Bastida J Linertova R Oliva-Moreno J Serrano-Aguilar P. Posada-de-la-Paz M Kanavos P et al . Social/economic costs and health-related quality of life in patients with scleroderma in Europe. Eur J Health Econ. (2016) 17:109–17. 10.1007/s10198-016-0789-y
92.
Pentek M Gulacsi L Brodszky V Baji P Boncz I Pogany G et al . Social/economic costs and health-related quality of life of mucopolysaccharidosis patients and their caregivers in Europe. Eur J Health Econ. (2016) 17:89–98. 10.1007/s10198-016-0787-0
93.
Xin XX Guan XD Shi LW . Catastrophic expenditure and impoverishment of patients affected by 7 rare diseases in China. Orphanet J Rare Dis. (2016) 11:74. 10.1186/s13023-016-0454-7
94.
Oguzhan G Ökçün S Kurnaz M Çalişkan Z Koçkaya G Karahan EB et al . Out-of-Pocket Healthcare Expenditures of Households Living with Rare Diseases. Res Squ. [Preprint]. 10.21203/rs.3.rs-540029/v1
95.
Ng YNC Ng NYT Fung JLF Lui ACY Cheung NYC Wong WHS et al . Evaluating the health-related quality of life of the rare disease population in Hong Kong using EQ-5D 3-level. Value Health. (2022). 10.1016/j.jval.2022.04.1725
96.
Forestier-Zhang L Watts L Turner A Teare H Kaye J Barrett J et al . Health-related quality of life and a cost-utility simulation of adults in the UK with osteogenesis imperfecta, X-linked hypophosphatemia and fibrous dysplasia. Orphanet J Rare Dis. (2016) 11:160. 10.1186/s13023-016-0538-4
97.
Wong EL Cheung AW Wong AY Xu RH Ramos-Goni JM Rivero-Arias O . Normative Profile of Health-Related Quality of Life for Hong Kong General Population Using Preference-Based Instrument EQ-5D-5L. Value Health. (2019) 22:916–24. 10.1016/j.jval.2019.02.014
98.
Xu RH Cheung AWL Wong EL . Examining the health-related quality of life using EQ-5D-5L in patients with four kinds of chronic diseases from specialist outpatient clinics in Hong Kong SAR, China. Patient Prefer Adherence. (2017) 11:1565–72. 10.2147/PPA.S143944
99.
Popejoy AB Fullerton SM . Genomics is failing on diversity. Nature. (2016) 538:161–4. 10.1038/538161a
100.
Chung BHY Chau JFT Wong GK . Rare versus common diseases: a false dichotomy in precision medicine. NPJ Genom Med. (2021) 6:19. 10.1038/s41525-021-00176-x
101.
Investigators GPP Smedley D Smith KR Martin A Thomas EA McDonagh EM et al . 100,000 genomes pilot on rare-disease diagnosis in health care - preliminary report. N Engl J Med. (2021) 385:1868–80. 10.1056/NEJMoa2035790
102.
National Health Service United Kingdom . NHS Genomic Medicine Service. (2020). Available online at: https://www.england.nhs.uk/genomics/nhs-genomic-med-service/ (accessed May 20, 2022).
103.
Chu ATW Fung JLF Tong AHY Chow SM Chan KYK Yeung KS et al . Potentials and challenges of launching the pilot phase of Hong Kong Genome Project. J Transl Genet Genom. (2022) 6:290–303. 10.20517/jtgg.2022.02
104.
Australian Genomics . Available online at: https://www.australiangenomics.org.au/our-history/
105.
Stark Z Boughtwood T Phillips P Christodoulou J Hansen DP Braithwaite J et al . Australian genomics: A federated model for integrating genomics into healthcare. Am J Hum Genet. (2019) 105:7–14. 10.1016/j.ajhg.2019.06.003
106.
Department Department of Health and Aged Care Australian Government . Genomics Health Futures Mission. Available online at: https://www.health.gov.au/initiatives-and-programs/genomics-health-futures-mission
107.
Genome Canada . Available online at: https://genomecanada.ca/challenge-areas/all-for-one/
108.
Stark Z Boughtwood T Phillips P Christodoulou J Hansen DP Braithwaite J et al . Australian genomics: A federated model for integrating genomics into healthcare. Am J Hum Genet. (2019) 105:7–14.
109.
Liu J Hui RT Song L . Precision cardiovascular medicine in China. J Geriatr Cardiol. (2020) 17:638–41. 10.11909/j.issn.1671-5411.2020.10.005
110.
Danish Ministry of Health . Personalised Medicine for the Benefit of the Patients. (2022). Available online at: https://eng.ngc.dk/Media/637614364621421665/Danish%20Strategy%20for%20personalised%20medicine%202021%202022.pdf
111.
Aviesan . Genomic Medicine France 2025. (2022). Available online at: https://solidarites-sante.gouv.fr/IMG/pdf/genomic_medicine_france_2025.pdf
112.
Steering Committee on Genomic Medicine . Strategic Development of Genomic Medicine in Hong Kong. (2022). Available online at: https://www.healthbureau.gov.hk/download/press_and_publications/otherinfo/200300_genomic/SCGM_report_en.pdf
113.
Japan Agency for Medical Research and Development . GA4HG. GEM Japn (GEnome Medical alliance Japan) (2022). Available online at: https://www.amed.go.jp/en/aboutus/collaboration/ga4gh_gem_japan.html
114.
Global Alliance for Genomics & Health . GEM Japan Releases Largest-Ever Open-Access Japanese Variant Frequency Panel. (2022). Available online at: https://www.ga4gh.org/news/gem-japan-releases-largest-ever-open-access-japanese-variant-frequency-panel/
115.
Saudi Human Genome Program . Available online at: https://shgp.kacst.edu.sa/index.en.html
116.
Nature Research Thailand Thailand Center of Excellence for Life Sciences . A Twisted Ladder to Prosperity. (2022). Available online at: https://www.nature.com/articles/d42473-020-00211-y
117.
Genomics Thailand . Available online at: https://genomicsthailand.com/Genomic/home
118.
BBMRI-ERIC . Turkish Genome Project Launched. (2022). Available online from: https://www.bbmri-eric.eu/news-events/turkish-genome-project-launched/
119.
Özçelik T . Medical genetics and genomic medicine in Turkey: a bright future at a new era in life sciences. Mol Genet Genomic Med. (2017) 5:466–72.
120.
Genomics England . The 100,000 Genomes Project by Numbers. Available online at: https://www.genomicsengland.co.uk/news/the-100000-genomes-project-by-numbers
121.
Our Future Health . Available online at: https://ourfuturehealth.org.uk/research-programme/
122.
GOV.UK. Genome UK: 2021 to 2022 Implementation Plan. (2021). Available online at: https://www.gov.uk/government/publications/genome-uk-2021-to-2022-implementation-plan/genome-uk-2021-to-2022-implementation-plan
123.
National Institutes of Health . NIH Genome Sequencing Program Targets the Genomic Bases of Common, Rare Disease. (2022). Available online at: https://www.nih.gov/news-events/news-releases/nih-genome-sequencing-program-targets-genomic-bases-common-rare-disease
124.
National Human Genome Research Institute . The Undiagnosed Diseases Program. (2022). Available online at: https://www.genome.gov/Current-NHGRI-Clinical-Studies/Undiagnosed-Diseases-Program-UDN
125.
Census Statistics Department Hong Kong Special Administrative Region . Thematic Report: Ethnic Minorities. (2017). Available online at: https://www.statistics.gov.hk/pub/B11201002016XXXXB0100.pdf (accessed August 20, 2022).
126.
Hong Kong Genome Institute . Strategic Plan 2022-25. (2022). Available online at: https://hkgp.org/wp-content/uploads/2022/07/HKGI-Strategic-Plan-2022-25.pdf (accessed September 22, 2022).
127.
National Organization for Rare Disorders (NORD) . Barriers to Rare Disease Diagnosis, Care and Treatment in the US: A 30-Year Comparative Analysis. (2020). Available online at: https://rarediseases.org/wp-content/uploads/2020/11/NRD-2088-Barriers-30-Yr-Survey-Report_FNL-2.pdf (accessed May 20, 2022).
128.
Yu MWC Fung JLF Ng APP Li Z Lan W Chung CCY et al . Preparing genomic revolution: Attitudes, clinical practice, and training needs in delivering genetic counseling in primary care in Hong Kong and Shenzhen, China. Mol Genet Genomic Med. (2021) 9:e1702. 10.1002/mgg3.1702
129.
Nippert I Harris HJ Julian-Reynier C Kristoffersson U Ten Kate LP Anionwu E et al . Confidence of primary care physicians in their ability to carry out basic medical genetic tasks-a European survey in five countries-Part 1. J Community Genet. (2011) 2:1–11. 10.1007/s12687-010-0030-0
130.
Vassy JL Green RC Lehmann LS . Genomic medicine in primary care: barriers and assets. Postgrad Med J. (2013) 89:615–6. 10.1136/postgradmedj-2013-132093
131.
Carroll JC Makuwaza T Manca DP Sopcak N Permaul JA O'Brien MA et al . Primary care providers' experiences with and perceptions of personalized genomic medicine. Can Fam Phys. (2016) 62:e626–e35.
132.
Houwink EJ van Luijk SJ Henneman L van der Vleuten C Jan Dinant G Cornel MC . Genetic educational needs and the role of genetics in primary care: a focus group study with multiple perspectives. BMC Fam Pract. (2011) 12:5. 10.1186/1471-2296-12-5
133.
Khoury MJ . Planning for the future of epidemiology in the era of big data and precision medicine. Am J Epidemiol. (2015) 182:977–9. 10.1093/aje/kwv228
134.
Gahl WA Tifft CJ . The NIH undiagnosed diseases program: lessons learned. JAMA. (2011) 305:1904–5. 10.1001/jama.2011.613
135.
Undiagnosed Disease Network International (UDNI). (2022). Available online at: https://www.udninternational.org/ (accessed May 20, 2022).
136.
Van Groenendael S Giacovazzi L Davison F Holtkemper O Huang Z Wang Q et al . High quality, patient centred and coordinated care for Alstrom syndrome: a model of care for an ultra-rare disease. Orphanet J Rare Dis. (2015) 10:149. 10.1186/s13023-015-0366-y
137.
Harari S . Why we should care about ultra-rare disease. Eur Respir Soc. (2016) 25:101–3. 10.1183/16000617.0017-2016
138.
Nicod E Whittal A Drummond M Facey K . Are supplemental appraisal/reimbursement processes needed for rare disease treatments? An international comparison of country approaches. Orphanet J Rare Dis. (2020) 15:189. 10.1186/s13023-020-01462-0
139.
HealthiNZ . Impact of Living with a Rare Disorder in NZ: Why a Different Approach is Needed to Improve Outcomes for People Living With Rare Disorders, Their Family and Whānau. Available online at: https://www.raredisorders.org.nz/assets/VOICE-OF-RARE-DISORDERS_WhitePaperV5.pdf (accessed May 20, 2022).
140.
Cheung NYC Fung JLF Ng YNC Wong WHS Chung CCY Mak CCY et al . Perception of personalized medicine, pharmacogenomics, and genetic testing among undergraduates in Hong Kong. Hum Genom. (2021) 15:54. 10.1186/s40246-021-00353-0
141.
Hui E Chow K Leung D Chan H Wu D . Attitudes of university students in Hong Kong about the use of genomic science and technology. New Genet Soc. (2012) 31:323–41. 10.1080/14636778.2012.662040
142.
Wauters A Van Hoyweghen I . Global trends on fears and concerns of genetic discrimination: a systematic literature review. J Hum Genet. (2016) 61:275–82. 10.1038/jhg.2015.151
143.
Kim H Ho CWL Ho CH Athira PS Kato K De Castro L et al . Genetic discrimination: introducing the Asian perspective to the debate. NPJ Genom Med. (2021) 6:54. 10.1038/s41525-021-00218-4
144.
Yaneva-Deliverska . Rare diseases and genetic discrimination. J IMAB. (2011) 17:116–9. 10.5272/jimab.2011171.116
145.
Research Office Legislative Council Secretariat . Supplementary information on rare disease policies in selected palces. In: Legislative Council. Hong Kong. (2017).
146.
Dharssi S Wong-Rieger D Harold M Terry S . Review of 11 national policies for rare diseases in the context of key patient needs. Orphanet J Rare Dis. (2017) 12:63. 10.1186/s13023-017-0618-0
Summary
Keywords
rare disease, genomic equity, diversity, public health priority, inclusiveness, Hong Kong Genome Project
Citation
Chung CCY, Hong Kong Genome Project, Chu ATW and Chung BHY (2022) Rare disease emerging as a global public health priority. Front. Public Health 10:1028545. doi: 10.3389/fpubh.2022.1028545
Received
30 August 2022
Accepted
30 September 2022
Published
18 October 2022
Volume
10 - 2022
Edited by
Chiuhui Mary Wang, Rare Diseases International, France
Reviewed by
Daniel Wainstock, Pontifical Catholic University of Rio de Janeiro, Brazil; Thaisa Gois Farias de Moura Santos Lima, Ministry of Health, Brazil; Hugh Dawkins, University of Notre Dame Australia, Australia
Updates
Copyright
© 2022 Chung, Hong Kong Genome Project, Chu and Chung.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Brian Hon Yin Chung bhychung@genomics.org.hkAnnie Tsz Wai Chu atwchu@genomics.org.hk
This article was submitted to Public Health Policy, a section of the journal Frontiers in Public Health
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.