- Department of Public Health, Vrije Universiteit Brussel, Brussels, Belgium
Background: Patient-level simulation (PLS) models overcome some major limitations of conventional cohort models and have broad applicability in healthcare, yet limited knowledge exists about their potential in cancer care.
Objectives: This systematic review aims to: (1) describe the application areas of PLS models in cancer care, (2) identify commonly used model structures, (3) evaluate the quality of reporting based on established guidelines, and (4) critically discuss the potential and limitations of PLS models in this context.
Methods: A systematic literature search was completed in Web of Science, PubMed, EMBASE and EconLit. Reasons underlying the use of PLS models were identified with a conventional inductive content analysis and reporting quality was assessed with an 18-item checklist based on the ISPOR-SMDM guidelines.
Results: The number of publications increased over time and most studies used state-transition microsimulation (49.25%) or discrete event simulation (48.51%). Two main application areas could be discerned, namely disease progression modelling (DPM) (78.36%) and health and care systems operation (HCSO) (21.64%). In the DPM domain, the use of PLS models was mainly motivated by the need to represent patient heterogeneity and history. In the HCSO domain, PLS models were used to better understand and improve cancer care delivery. Average reporting quality was 65.2% and did not improve over time.
Conclusion: PLS models can be used to simulate the progression of cancer and to model cancer care delivery. In the DPM domain more direct comparisons with cohort models are required to establish the relative advantages of PLS models and in the HCSO domain the impact on clinical practice needs to be systematically assessed. Furthermore, adherence to the ISPOR-SMDM guidelines should be improved.
Introduction
Cancer is a critical global health challenge, with close to 20 million new cases and 9.7 million cancer-related deaths reported globally in 2022 alone (1). According to updated estimates from the International Agency for Research on Cancer, approximately one in five individuals will develop cancer in their lifetime, and around one in nine men and one in 12 women will die from it. With predictions that new cancer cases may rise to 35 million annually by 2050, the importance of preventive measures, early detection, and equitable access to care is more urgent than ever.
Cancer incidence tends to increase with a country’s Human Development Index (1), and in Europe and North America, the disease has become the leading cause of death among middle-aged adults (2). However, in low- and middle-income countries (LMICs), the cancer burden is also rapidly increasing, with cancer rates rising as these nations undergo demographic and epidemiological transitions. Survival rates are lower in LMICs due to limited access to screening, early diagnosis, and effective treatment, and global efforts to address these disparities are therefore critical for improving cancer outcomes across all regions.
To encourage progress against cancer the pace of innovation in oncology has accelerated at an unprecedented speed in recent years, yet novel treatments come with an increasing price tag (3, 4). In a world where resources are finite and healthcare systems are under increasing strain, the question of how to improve value in cancer care has never been more pertinent. In this context, “value” is defined as the ratio of patient outcomes to the cost of care (5–7). The goal is to achieve the best possible outcomes for patients while ensuring that the cost of treatments, remains sustainable for healthcare systems and affordable for patients. The rising costs associated with cutting-edge cancer treatments, coupled with the need to ensure access to quality care for all patients, have made value-based healthcare models a focal point of policy discussions and health economic research.
Achieving high-value cancer care requires an evidence-based approach to assess the effectiveness and cost-effectiveness of treatments. Decision analytic models address this need and provide a framework to integrate the best available evidence on the decision problems encountered within healthcare (8, 9). Models must, however, adequately represent the complexities of clinical reality to be a valid foundation on which to base decisions (10, 11). With respect to cancer care, this is a particularly challenging endeavor (12). Treatment for cancer generally involves a combination of therapies (13), delivered simultaneously or sequentially, and requires coordination between multiple disciplines (14). Moreover, in the fast-paced field of oncology, more sophisticated therapies emerge continuously, and treatment paradigms are rapidly evolving. In this dynamic new era, cancer is gradually changing from a death sentence to a chronic disease that requires long-term management (15) and treatments are becoming increasingly personalized (16, 17) in recognition of the substantial heterogeneity that underlies the disease (18).
The growing complexity of cancer care may pose challenges for Markov and other cohort models, the dominant modelling approach today (19, 20). In a cohort model outcomes are calculated for a cohort of supposedly average patients, without considering potential differences between patients. This traditional modelling technique is subject to a number of restrictive assumptions, which may limit its capacity to accurately represent the nature of cancer and the realities of cancer care delivery (12). Markov models presume that the population of interest is homogenous, and may therefore struggle to adequately reflect the heterogeneity in cancer patients (21). Furthermore, the Markovian memoryless property and the frequent use of discrete time cycles could hamper a proper consideration of the dynamics of cancer (22–24).
For these reasons, it has been suggested that researchers should turn to alternative methods such as patient-level simulation (PLS) models (25, 26). As defined by Drummond et al. (9), these models calculate the outcomes of a sufficiently large sample of simulated patients and subsequently average across patients. Since PLS models provide estimates for the outcomes of interest for each individual patient they allow modelers to examine variability in outcomes and to track individual patient histories (21). Moreover, PLS models are not constrained by the Markov property and may therefore be better able to accurately reflect the dynamics of cancer (21, 27). Errors arising from the use of discrete time cycles could be averted as well, either by opting for continuous time state-transition microsimulation (22) or for discrete event simulation (DES) (25).
Importantly, the application of PLS models is not limited to the area of health economics and health technology. In the field of health policy, the approach is used to simulate health trajectories and to predict the impact of alternative policy interventions (28). Notable examples include the Future Elderly Model (FEM) (29) and the Population Health Model (POHEM) (30), two continuous time state-transition microsimulation models. Discrete event simulation, on the other hand, is increasingly applied in healthcare management to analyze how resources can be optimally employed (31). The apparent versatility of PLS models thus suggests they may be of use to researchers in a variety of healthcare fields (25).
While PLS models have demonstrated their utility in various healthcare domains, their application to cancer care remains underexplored. To our knowledge, no systematic review has examined the application of PLS models in cancer care (19). This knowledge gap may reflect both the technical challenges of implementing PLS models and the historical dominance of cohort-based approaches. Nevertheless, as cancer care continues to evolve, the time is ripe to investigate the potential of PLS models to enhance decision-making in oncology.
In this systematic review, we aim to address this gap by laying the groundwork for future research and providing a roadmap for integrating PLS models into oncology. We document the utilization of PLS models across time, examine which specific techniques are favored and which questions they are applied to. Moreover, we systematically assess the reporting quality of papers and conduct a content analysis to uncover the main reasons for choosing a PLS model. Finally, we critically discuss the potential contribution of PLS models in cancer care.
Methods
Search strategy
A systematic search of the peer-reviewed literature was conducted in four major databases, namely, Web of Science, PubMed, EMBASE and EconLit. The search strategy was first developed for Web of Science, and subsequently adapted for the remaining databases. Search terms were identified through consultation of guidelines (32), textbooks (9) and earlier reviews (19, 26, 33), as well as through exploration of databases. The full search strategy for each database can be consulted in Supplementary Appendix 1. Since PLS models were relatively rare before 2010 (26, 33), January 2010 was chosen as the start of the time frame.
Inclusion and exclusion criteria
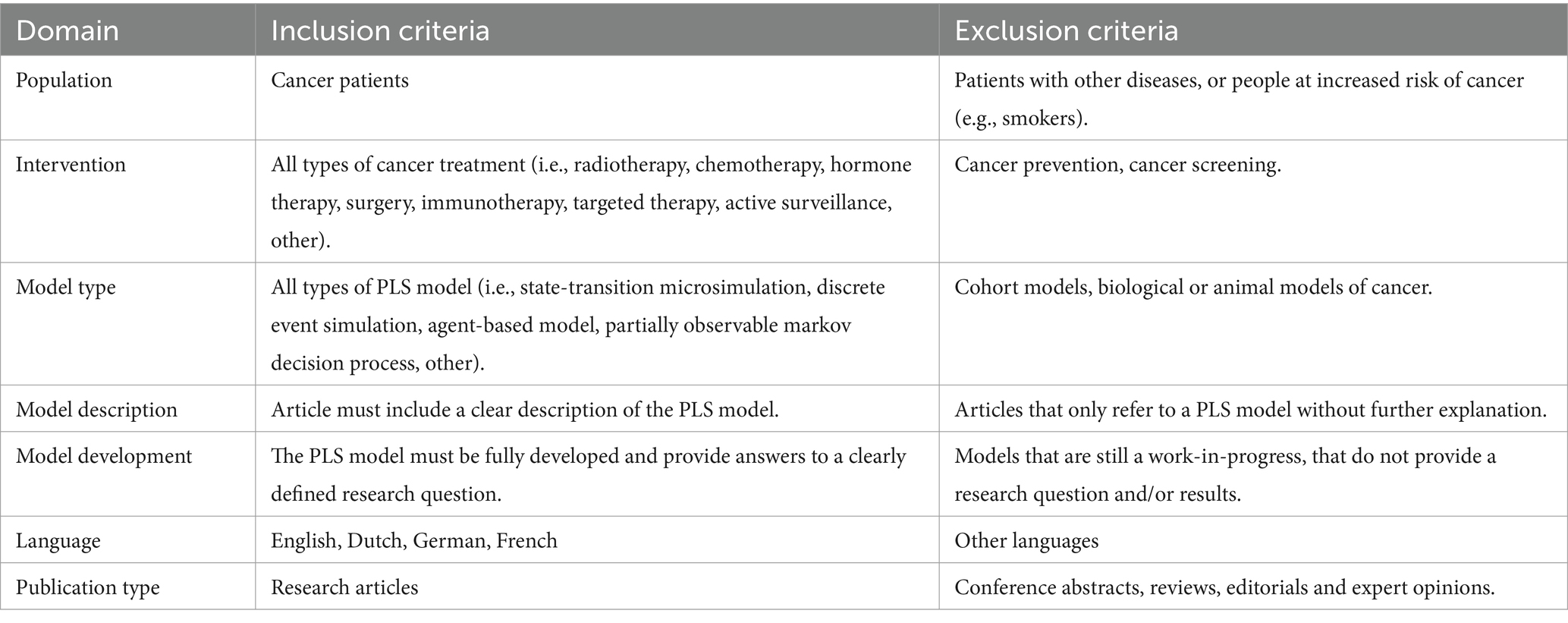
To be eligible for inclusion publications needed to include an application of the PLS approach to cancer treatment. In addition, models needed to be clearly described to permit a classification of modelling techniques according to the International Society for Pharmacoeconomics and Outcomes Research–Society for Medical Decision Making (ISPOR-SMDM) Modelling Good Research Practices Task Force guidelines (11, 32). Models also needed to be fully developed and applied to a certain research question to enable us to explore the application areas of the PLS approach. Gray literature was excluded to maintain a focus on peer-reviewed publications. The inclusion and exclusion criteria are summarized in Table 1.
Data extraction
Titles and abstracts of initially retrieved studies were screened independently by two reviewers using the web application Rayyan. Selection was based on the predefined eligibility criteria and the two reviewers (SLB and HVD) were blinded to each other’s decision. In cases of disagreement, consensus was reached through discussion, and if necessary, a third reviewer (KP) was consulted. Subsequently, the full texts of eligible articles were obtained and screened according to the same criteria. One reviewer (SLB) assessed all full-text articles, and a second reviewer independently checked a random sample of 20%, which is judged to be representative (19). Agreement rate was 100% and inter-rater reliability was therefore deemed sufficiently high. Key data, including study objectives, modelling techniques, application areas, and results, were extracted using a standardized data extraction form developed based on PRISMA guidelines.
To gain insight into the primary reasons underlying the use of PLS models a conventional inductive content analysis was conducted according to Elo and Kyngäs (34). This rigorous and thorough method for analysing text-based data is well-suited for identifying major themes and objectives and is recommended when there is little existing research on a topic (35, 36). The approach involved open coding of the text by two reviewers (SLB and KP), who iteratively developed and refined categories to capture emerging themes. The analysis was performed using Nvivo, which is well-suited for qualitative analysis of text-based data.
Reporting quality was assessed with an 18-item checklist for PLS models based on the ISPOR-SMDM guidelines (37). The checklist was designed to be applicable to model-based analyses in a variety of healthcare fields, including but not limited to health economics and healthcare management. Scoring was conducted independently by two reviewers (SLB and KP), and discrepancies were resolved through consensus. A two-way ANOVA was performed that examined the effect of application area and modelling technique on reporting quality. Prior to analysis, the assumptions of ANOVA were tested, including normality of residuals (using the Shapiro–Wilk test) and homogeneity of variances (using Levene’s test). Results were analyzed with IBM SPSS Statistics 28.0.
Results
Search results
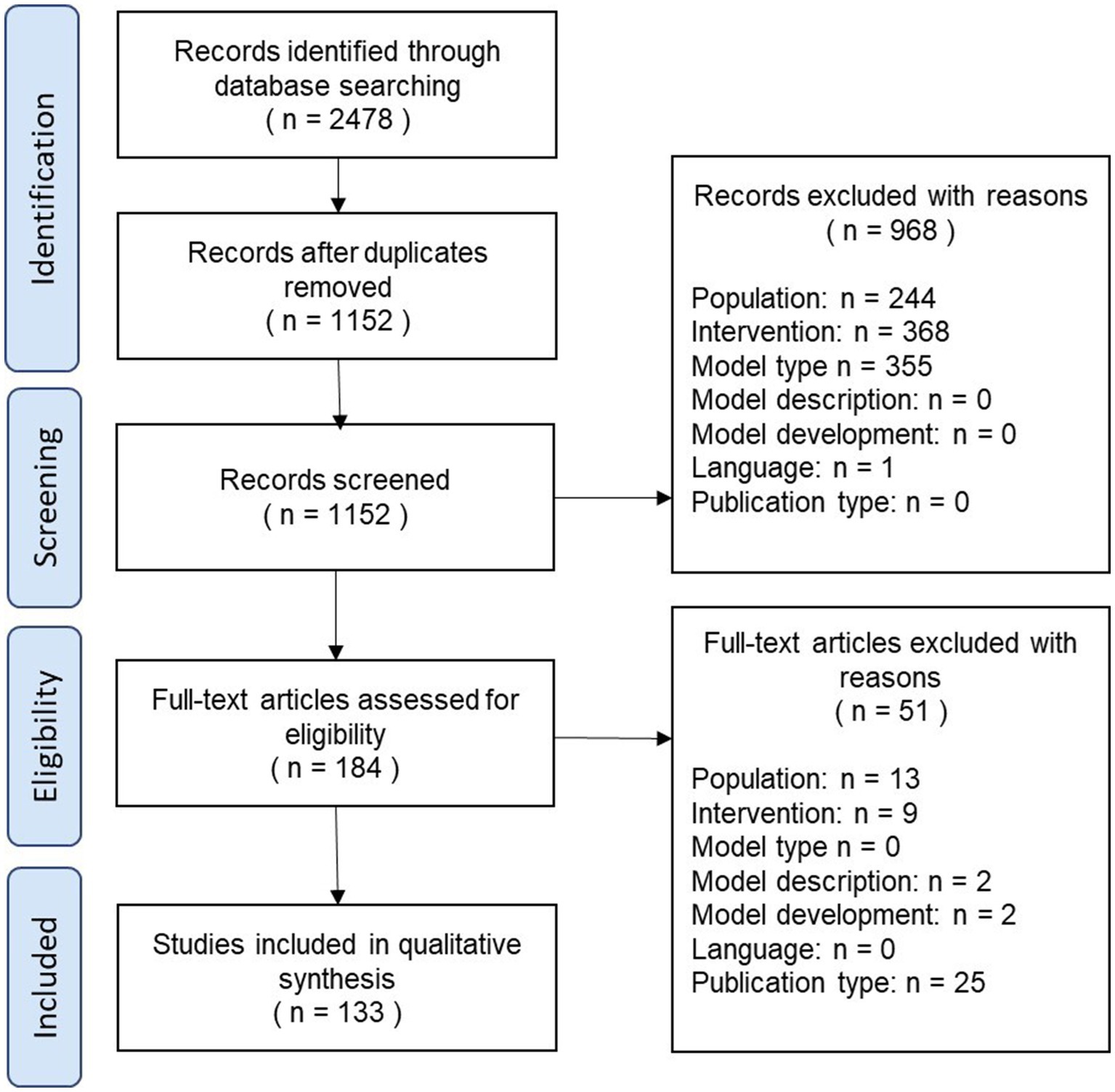
The results of the review process are illustrated as a PRISMA diagram in Figure 1.
Publication trends over time
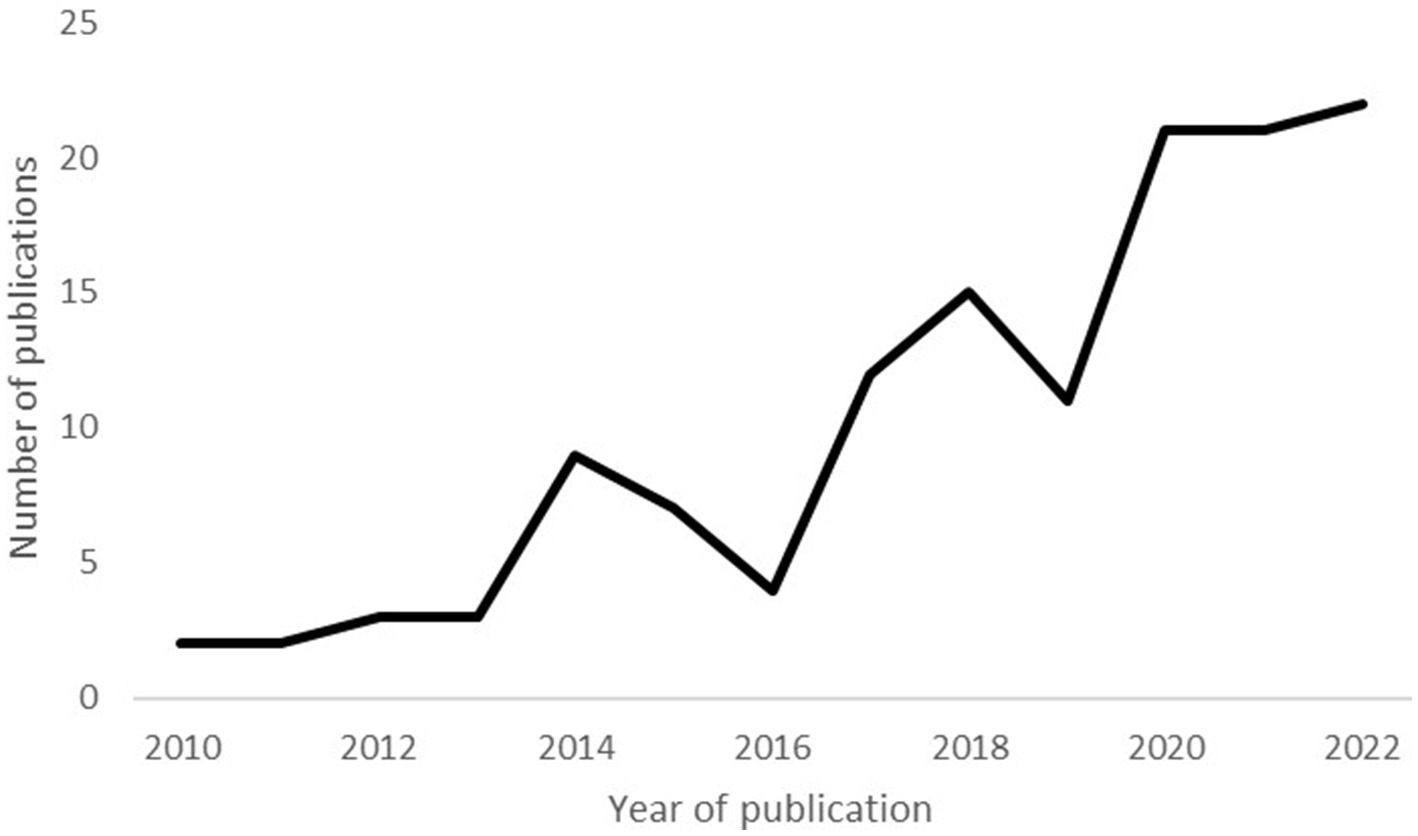
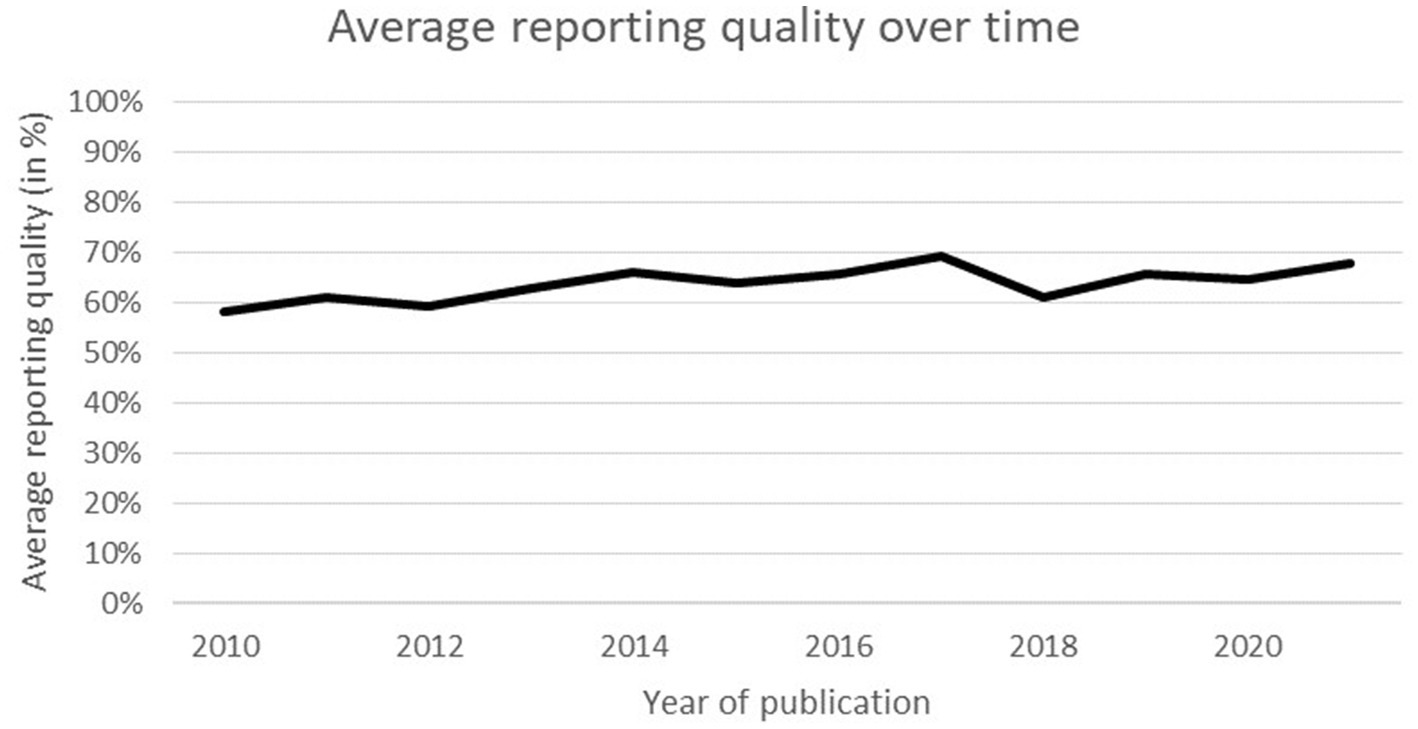
Figure 2 illustrates a clear increase in the number of published PLS models in recent years. Only eight papers were published between 2010 and 2012, but this number increased eightfold between 2019 and 2021. These results are consistent with earlier reviews which documented an increasing utilization of PLS models (26, 33).
Model structures
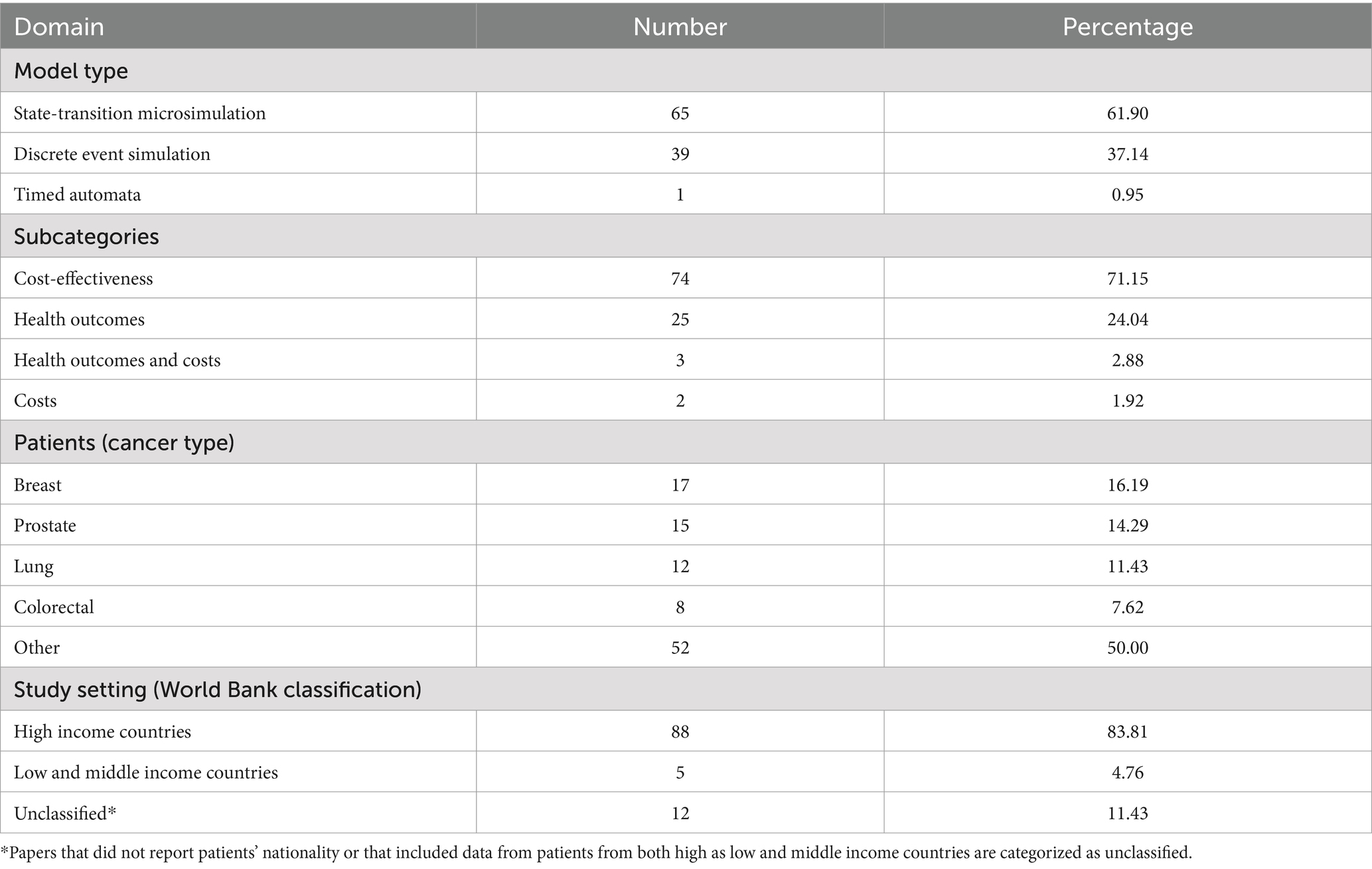
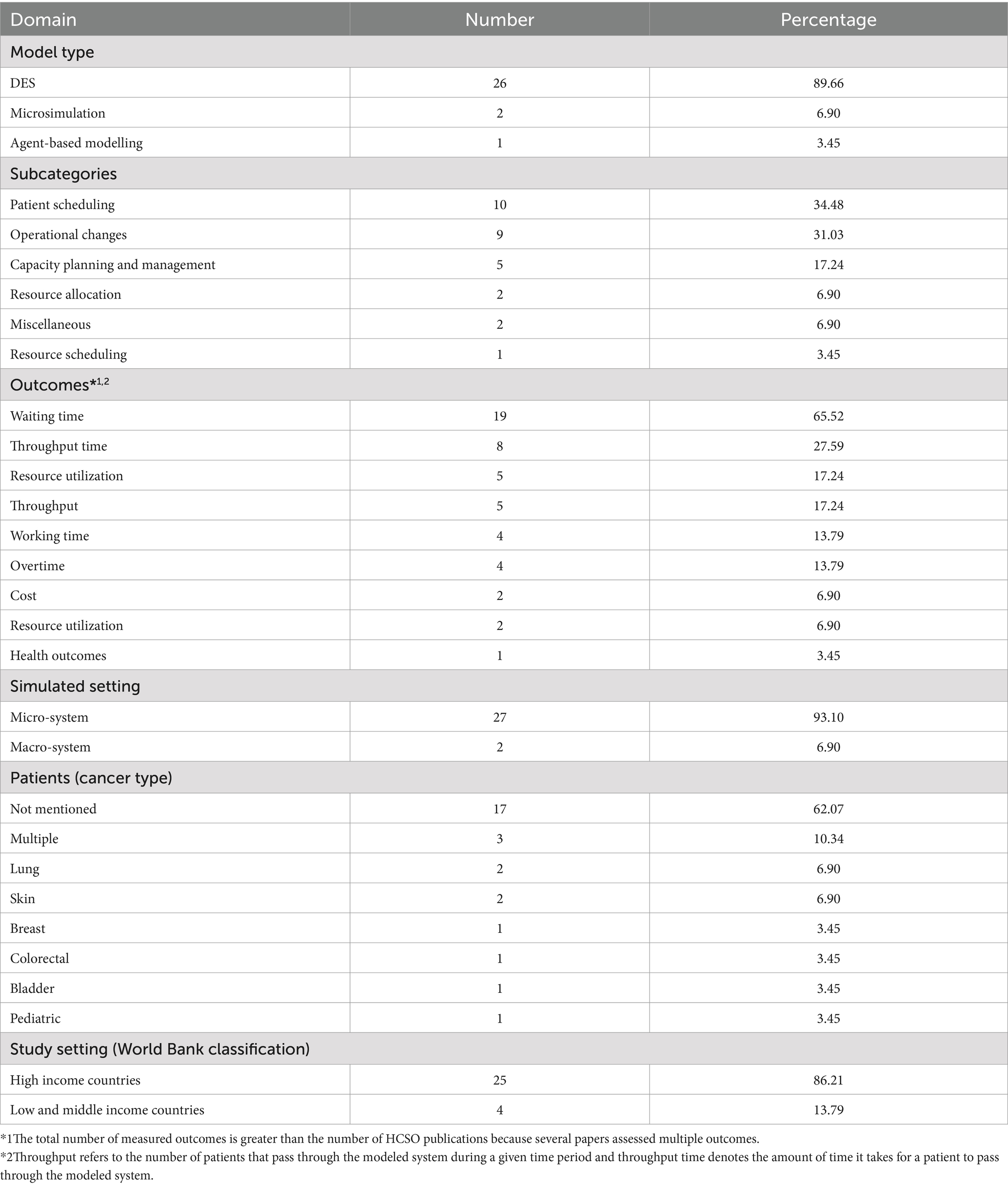
The majority of studies used either state-transition microsimulation (STMS) (49.25%) or discrete event simulation (DES) (48.51%). Other model structures (2.24%) were relatively rare and included agent-based modelling (n = 1) and timed automata (n = 1).
Application areas
To investigate the areas in which the PLS approach has been applied, the models were categorized according to a classification scheme inspired by an earlier review (19). Broadly, the models in the present review can be grouped into two major categories, namely disease progression modelling (DPM) and health and care systems operation (HCSO). In what follows, we give an overview of the general features of these models. A summary of the results for DPM and HCSO models can be found in Tables 2, 3, respectively. More detailed information is provided. More detailed information is provided in Supplementary Appendices A1, A2.
Disease progression modelling
Disease progression models, which comprise the largest category (78.36%), describe the time course of diseases and simulate the influence of treatment options on disease status (38–40). Accordingly, these models provide guidance in medical decision-making by indicating the best course of action based on clinical benefits and harms, consumed resources or both. Within this area of application, STMS was the most commonly employed technique (61.90%). DPM models can be organized into subcategories according to the outcomes that are used to compare alternative treatment strategies. The majority of DPM models evaluated therapeutic interventions for cancer based on their cost-effectiveness (71.15%), expressed as an incremental cost-effectiveness ratio (ICER). The remainder considered only health outcomes (e.g., QALYs), only costs (e.g., healthcare costs) or both costs as health outcomes without combining them in a cost-effectiveness analysis. With regard to the patient populations, the four cancer types with the highest incidence worldwide (i.e., breast, prostate, lung, and colorectal cancer) were predominantly studied (41). Furthermore, patient populations generally came from high-income countries, which constitute the regions with the highest cancer incidence (41).
Notably, certain research questions were frequently addressed. To begin, several authors investigated the value of applications in the domain of personalized oncology (42–64), for example by assessing the cost-effectiveness of molecular diagnostics (47, 50, 60, 61), or by demonstrating that PLS models can be used to guide radiotherapy decisions (56, 59). The PLS approach was likewise often applied to model sequences of treatments and/or tests (42, 44, 51–55, 61, 64–78). Blommestein et al. (67), for instance, determined the cost-effectiveness of thirty treatment sequences including up to three lines of therapy for patients with multiple myeloma. A number of authors also used PLS models to represent the full clinical trajectory of a disease (79–81). An example is the model by Wang et al. (81), which simulates costs, survival and QALYs for patients with follicular lymphoma across the treatment pathway. Other models included not only treatments, but also elements such as screening and prevention (69, 82–89). Tappenden et al. (84), for instance, constructed a whole disease model of colorectal cancer, which proved capable of evaluating the majority of topics within the UK’s NICE colorectal cancer guideline within one consistent framework.
Health and care systems operation
HCSO models simulate healthcare systems and can be harnessed by healthcare managers to better understand and improve the delivery of care (90). The domain of HCSO was nearly monopolized by DES models (89.65%), and most researchers turned their attention to issues touching on patient scheduling and operational changes. To evaluate the effects of proposed alterations modelers typically focused on operational outcomes, particularly waiting time. The ABM model, as well as most DES models, were unit-specific, that is, they represented specific hospital departments dedicated to the delivery of cancer drugs, radiotherapy or surgery (i.e., micro-systems). The two papers on STMS, on the contrary, modeled the healthcare system at a national level (91, 92) (i.e., macro-systems).
We also examined the extent to which proposed quality improvements were implemented in clinical practice, given that it is assumed that the impact of HCSO models in healthcare is quite limited (93, 94). In this review, 27.59% of the authors did not explore any scenarios to improve the modeled system or explicitly stated that implementation was not their intention (91, 92, 95–99). In the remaining publications an implementation was mentioned in 38.10% of the cases (100–107) and 19.05% (100, 101, 105, 106) also reported an evaluation of the intervention.
Reasons for using PLS models
The reasons for choosing a PLS model were broadly in line with the presumed advantages reported in the literature. Within the DPM category, model choice was frequently motivated by the presence of heterogeneity in patient characteristics and pathways (47, 50, 51, 53, 55, 61, 63, 65, 67, 74, 78–81, 108–118) or the importance of taking into account patient history (47, 50, 52, 68, 72, 79, 80, 108–110, 113, 115, 116, 119–122). Notably, a number of authors within the DPM domain also drew attention to specific advantages of the DES framework, such as the avoidance of errors due to the use of discrete time cycles (42, 51, 115, 119, 123) and a more efficient handling of competing risks (124). One paper also cited the ability to represent resources and to simulate the effects of wait times (125). All other DES models in the DPM category were, however, non-constrained resource models. Of note, a small number of DPM papers directly contrasted the PLS with the cohort approach. Jahn et al. (54) performed a cross-model validation of two models for personalized breast cancer treatment, a DES model and a cohort state-transition model, and observed that model choice can affect cost-effectiveness results. Gibson et al. (110) came to the same conclusion based on a comparison of the PLS approach with a survival partition and cohort model. Degeling et al. (115), in contrast, found comparable cost-effectiveness outcomes for a DES and a Markov model for metastatic colorectal cancer. Yet, the authors remarked that the DES model provided a more accurate representation of the clinical trial data in terms of mean health-state durations. In a similar vein, Pan et al. (116) reported that a DES model for prostate cancer predicted clinical outcomes from trial data more accurately than a survival partition model, especially over a longer time horizon.
HCSO modelers mainly used the PLS approach as a tool to gain knowledge about health care systems (91, 97–99, 101, 126–129) and to improve care delivery processes (101, 126, 130). In particular, PLS models can be used to explore a variety of “what if?” scenarios (100–103, 105, 106, 126, 127, 129–133) while avoiding the risks and costs that real-life experimentation would entail (101, 106, 130, 131, 133). Improvements that are cost-free or even cost-saving can be uncovered (101, 106, 130, 131, 133), and users can learn how to more efficiently employ scarce resources (134). Furthermore, the model can not only support informed decisions (103, 130), but can also serve as a communication tool to convince other stakeholder s (101, 133, 134).
Reporting quality
The average reporting quality was 65.2% (SD = 11.4), and no significant time trend was observed, R2 = 0.011, F(1, 132) = 1.427, p = 0.234 (see Figure 3). There was no significant interaction between the independent variables F(1,127) = 0.001, p = 0.981 and no significant difference between modelling techniques (p = 0.648). Nonetheless, simple main effects analysis did demonstrate that DPM models (x̄ = 68.59, SD = 8.07) obtained significantly higher scores than HCSO models (x̄ = 53.06, SD = 10.77) (p < 0.001).
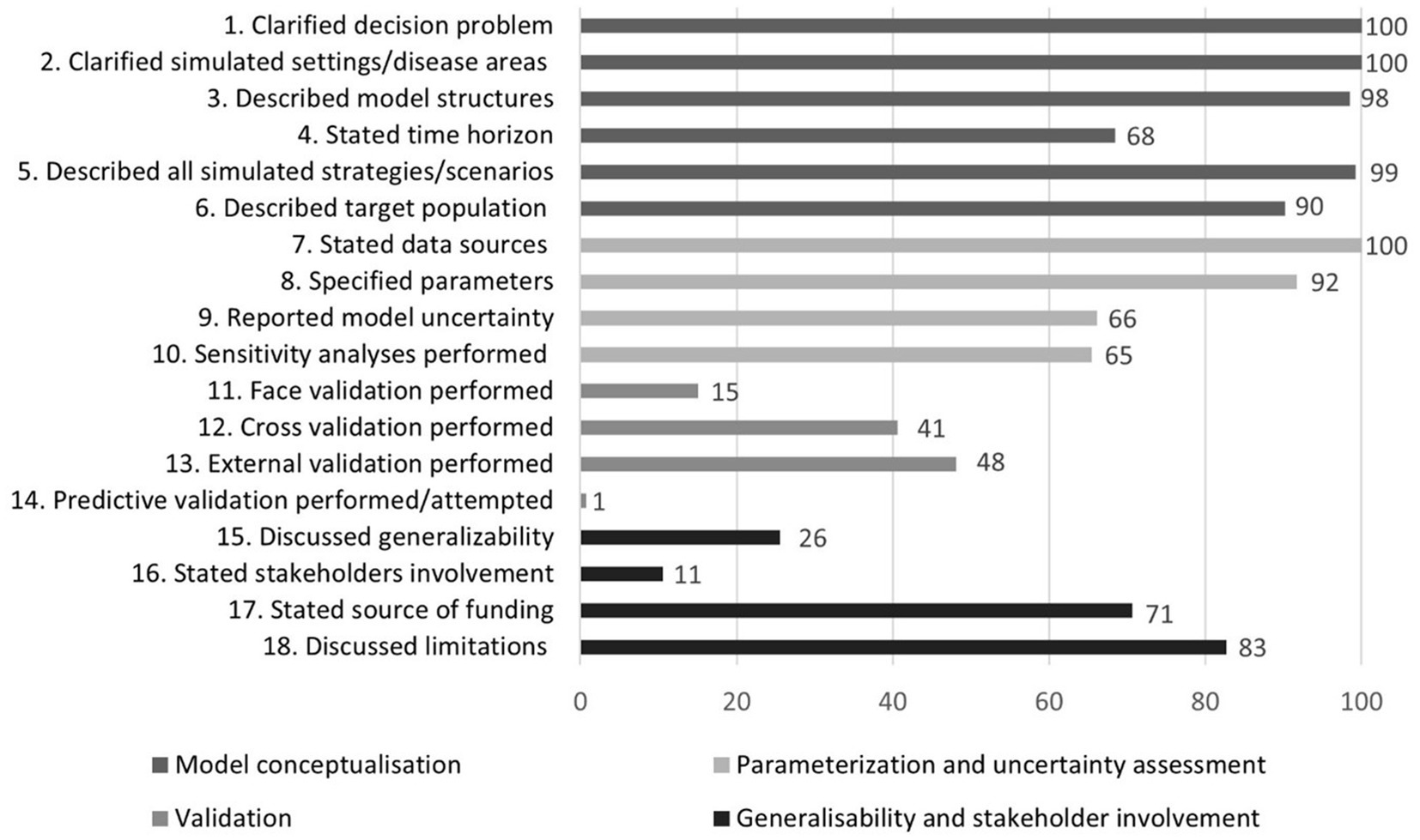
The fulfilment rate of individual checklist items is shown in Figure 4. Most criteria concerning model conceptualization (1–6) as well as those related to parameterization and uncertainty assessment (7–10) were fulfilled at high rates. Fulfilment rates for criteria regarding generalizability and stakeholder involvement (15–18), and especially those pertinent to validation (11–14), were, however, substantially lower.
Discussion
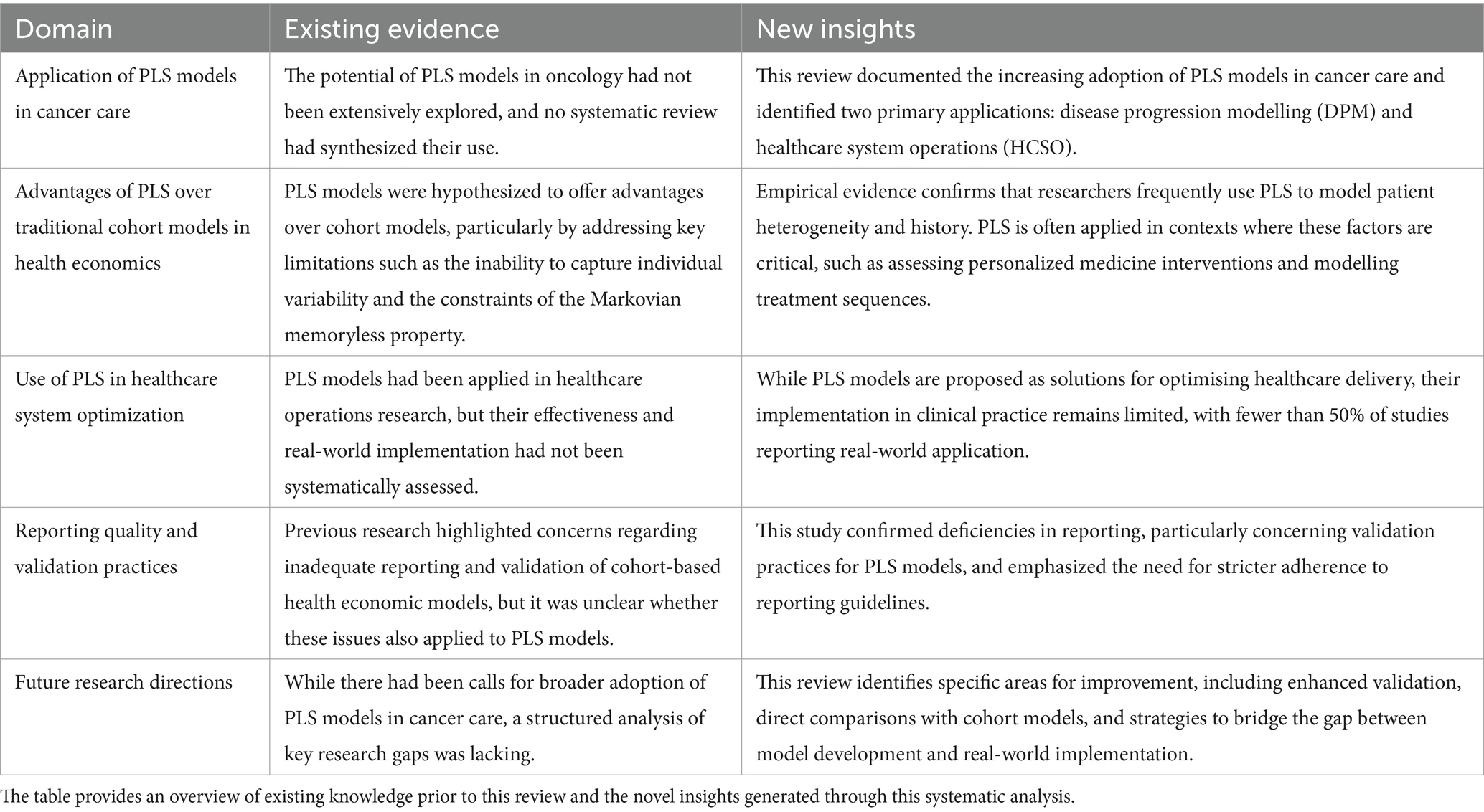
We have documented an increasing use of PLS models in cancer care and identified two distinct application areas of PLS models, namely the simulation of the progression of cancer and the modelling of the healthcare systems in which patients are treated. Although DPM and HCSO models have different functions and intended users, they could both contribute to the achievement of high-value cancer care. To fully tap the potential of these models, however, some points warrant closer scrutiny (Table 4).
Choosing valuable treatments
DPM models, the most frequently used category in this review, primarily evaluated treatment cost-effectiveness. The decision to adopt the PLS approach was frequently motivated by the need to represent heterogeneity in patient characteristics and pathways or to model patient history. Accordingly, PLS models were often applied in areas where these aspects are likely to be of particular importance. A prime example can be found in personalized medicine, a burgeoning field of research in oncology (135). As it is challenging to accurately capture dynamic and diverse patient pathways in conventional cohort models, it has been argued that PLS models may be relatively superior in this area (26).
The modelling of sequences of treatments and/or tests is another area that may be well-suited to the PLS approach. Treatment sequences are a common feature of the contemporary management of metastatic cancer and identifying an optimal sequence for (subpopulations of) patients is the ambition of many researchers (136, 137). Answering such questions using randomized controlled trials is, however, unfeasible (65, 67) and the difficulty of efficiently representing downstream consequences of earlier treatments in cohort models could hinder an accurate assessment of the (cost) effectiveness of treatments across the entire sequence (138). The PLS approach likewise appears suitable for the construction of generic models of the full disease course of a certain type of cancer. Such comprehensive and adaptive models allow the comparison of multiple interventions across the whole cycle of care and permit nuanced judgments on which treatments to use for which patient subgroups (84, 139). These models go beyond standard technology adoption questions and could support the move toward a health technology assessment paradigm that supports health technology management, aims for efficiency and considers whole system value from a long-term perspective (140–142). This paradigm change is becoming more and more necessary due to the increasing strain on healthcare expenditures (143).
Although these results suggest that the PLS approach may have merit in a few noteworthy fields of research, the true litmus test for PLS models in the DPM domain is a head-to-head comparison with cohort models. In this review, four model comparisons were identified. This review’s observation that model choice can impact outcomes is particularly significant. In some cases, such as for the analysis of patient-level time-to-event data, a PLS model may be more appropriate than a cohort model (115, 116). Nevertheless, the scarcity of identified model comparisons precludes drawing conclusions about the relative merits of PLS models in cancer care and continuing work in this area therefore represents an important avenue for further research. Researchers should ideally also take into account potential drawbacks of the PLS approach, such as increased complexity, computational intensity and data requirements (8, 27). Although validity should always take precedence over simplicity, the ISPOR-SMDM Modeling Good Research Practices Task Force recommends that models should be kept as simple as possible (11). PLS models should therefore not replace cohort models as the default option in cancer care, but rather be employed when required by the demands of the decision problem (32).
Improving the value of cancer care delivery
The HCSO domain formed the second important application area of PLS models in this review. Consistent with previous reviews (33, 93), models were generally unit-specific and simulated a certain clinic or a specific hospital department. In the HCSO domain, authors employed the PLS approach to acquire knowledge of health care systems and to improve care delivery processes. Nevertheless, although many papers in this review described solutions to enhance the quality of cancer care (95, 100, 101, 103, 107, 128, 130, 131, 144), it can currently not be established whether HCSO models lead to genuine benefits for patients or the organizations that care for them. Previous reviews have concluded that the application of modelling results in healthcare is remarkably low and it is estimated that 90% of these models have no influence on clinical practice (93, 145). In the present review, less than half of the papers that described experiments to improve modeled system mentioned an implementation in clinical practice and only about one in five also reported an evaluation of the intervention. It remains unclear whether these results stem from an implementation gap or inconsistent reporting. Promoting higher quality reporting, for example by encouraging the adherence to reporting guidelines such as those published by the World Health Organisation (146), could enable us to assess the scope of the implementation gap and may also reveal some of its causes. Bridging this gap is crucial, as research confined to academia does not directly benefit patients. Modelers may be advised to work out a systematic implementation strategy with key stakeholders to bridge the know-do gap. The Consolidated Framework for Implementation Research (CFIR) (147, 148), one of the most widely used implementation science frameworks, can assist researchers in planning for a successful implementation.
Setting higher standards: enhancing reporting quality
The assessment of reporting quality in PLS models revealed notable deficiencies, particularly in the area of validation, underscoring the need for greater transparency and rigor in future research. The lack of detailed reporting on model validation—defined as the evaluation of whether a model is a proper and sufficient representation of the system for a particular application (149)—aligns with findings from previous reviews (37, 150). The importance of validation is paramount, as decision-analytic models are intended to guide decision-making. For these models to effectively serve that purpose, decision-makers must have confidence in the accuracy and reliability of the results.
Different types of validation play a crucial role in strengthening the credibility of decision-analytic models (151). Although 72% of the papers reviewed reported conducting one or more types of validation, significant gaps remain. Face validation, reported in only 15% of studies, ensures that the model aligns with current medical science and the best available evidence. Cross-validation, noted in 41% of studies, compares models using different methods to ensure similar outcomes. External validation, present in 48% of papers, confirms that model predictions align with real-world results, such as clinical trial outcomes. Predictive validation, regarded as the most esteemed form of validation due to its alignment with the core purpose of modelling—forecasting future outcomes—was reported in only 1% of studies.
Growing awareness of the significance of validation has prompted calls for adherence to standardized validation guidelines (152). Researchers employing PLS models should particularly heed this call to action, as these models tend to be more complex and less transparent, potentially undermining trust in their findings (27). Actively engaging with stakeholders can enhance the development of valid models, making them more relevant and practical (153). This collaborative approach could bridge the gap between academia and real-world healthcare, transforming theoretical exercises into actionable tools that provide tangible benefits.
Limitations
This review is subject to some limitations that need to be mentioned. Firstly, the search was limited to peer-reviewed research articles, which may have led to the exclusion of some relevant papers. Nevertheless, our comprehensive search of four major databases likely provides a representative snapshot of the literature. Secondly, although agreement rate was 100%, the second reviewer only screened a representative sample of full-text papers.
Conclusion
The cancer care landscape is rapidly evolving and new additions to the expanding therapeutic armamentarium arrive continuously, often accompanied with stratospheric costs and unclear benefits (154). Policymakers, healthcare managers and clinicians face difficult decisions under high uncertainty and require models that accurately reflect the complexities of cancer care. PLS models could assist the cancer community in evaluating the (cost) effectiveness of cancer therapies, and due to their ability to take into account patient heterogeneity and history, these models may in some cases be more appropriate than conventional cohort models. Nevertheless, comprehensive comparisons with cohort models will be necessary to definitely ascertain the relative advantages and disadvantages of PLS models in the DPM domain. Additionally, PLS models may be used to better understand cancer care systems and to improve the delivery of care. The actual contribution of HCSO models to cancer care remains, however, unestablished and future studies will need to systematically assess and report the impact these models have on clinical practice. Finally, in both the DPM and HCSO domain more attention should be paid to the reporting quality of PLS models. In agreement with previous research (37), the assessment of papers in the present review revealed room for improvement in several areas, particularly with respect to model validation. Moreover, average reporting quality did not improve over time.
Author contributions
S-LB: Conceptualization, Formal analysis, Writing – original draft. HV: Conceptualization, Writing – review & editing. MD: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing. KP: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Strategic Research Programme (zwaartepunt, SRP 53, 2019–2024) ‘Societal Benefit of Markerless Stereotactic Body Radiotherapy: a Statistical Support based on Quantitative Imaging’ (SMARTQI of the Vrije Universiteit Brussel) and the ‘Value-Based Breast Radiotherapy’ research grant from Kom op tegen Kanker (KOTK_VUB/2024/13906).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1335300/full#supplementary-material
References
1. Bray, F, Laversanne, M, Sung, H, Ferlay, J, Siegel, RL, Soerjomataram, I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Dagenais, GR, Leong, DP, Rangarajan, S, Lanas, F, Lopez-Jaramillo, P, Gupta, R, et al. Variations in common diseases, hospital admissions, and deaths in middle-aged adults in 21 countries from five continents (PURE): a prospective cohort study. Lancet. (2020) 395:785–94. doi: 10.1016/S0140-6736(19)32007-0
3. Mailankody, S, and Prasad, V. Five years of Cancer drug approvals: innovation, efficacy, and costs. JAMA Oncol. (2015) 1:539–40. doi: 10.1001/jamaoncol.2015.0373
4. Hofmarcher, T, Lindgren, P, Wilking, N, and Jönsson, B. The cost of cancer in Europe 2018. Eur J Cancer. (2020) 129:41–9. doi: 10.1016/j.ejca.2020.01.011
5. Porter, ME. Value-based health care delivery. Ann Surg. (2008) 248:503–9. doi: 10.1097/SLA.0b013e31818a43af
6. Porter, ME. A strategy for health care reform--toward a value-based system. N Engl J Med. (2009) 361:109–12. doi: 10.1056/NEJMp0904131
7. Porter, ME. What is value in health care? N Engl J Med. (2010) 363:2477–81. doi: 10.1056/NEJMp1011024
8. Briggs, A, Sculpher, M, and Claxton, K. Decision modelling for health economic evaluation. (Oxford, United Kingdom: Oxford University Press) (2006).
9. Drummond, MF, Sculpher, MJ, Claxton, K, Stoddart, GL, and Torrance, GW. Methods for the economic evaluation of health care programmes. (Oxford, United Kingdom: Oxford University Press) (2015).
10. Weinstein, MC, O'Brien, B, Hornberger, J, Jackson, J, Johannesson, M, McCabe, C, et al. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR task force on good research practices—modeling studies. Value Health. (2003) 6:9–17. doi: 10.1046/j.1524-4733.2003.00234.x
11. Caro, JJ, Briggs, AH, Siebert, U, and Kuntz, KMISPOR-SMDM Modeling Good Research Practices Task Force. Modeling good research practices—overview: a report of the ISPOR-SMDM modeling good research practices task force–1. Med Decis Mak. (2012) 32:667–77. doi: 10.1177/0272989X12454577
12. Miller, JD, Foley, KA, and Russell, MW. Current challenges in health economic modeling of cancer therapies: a research inquiry. American Health Drug Benefits. (2014) 7:153–62.
13. Mokhtari, RB, Homayouni, TS, Baluch, N, Morgatskaya, E, Kumar, S, and Das, B. Combination therapy in combating cancer. Oncotarget. (2017) 8:38022. doi: 10.18632/oncotarget.16723
14. Levit, LA, Balogh, E, Nass, SJ, and Ganz, P. (editors). Institute of Medicine. Delivering high-quality cancer care: Charting a new course for a system in crisis (2013). doi: 10.17226/18359
15. Phillips, JL, and Currow, DC. Cancer as a chronic disease. Collegian. (2010) 17:47–50. doi: 10.1016/j.colegn.2010.04.007
16. Krzyszczyk, P, Acevedo, A, Davidoff, EJ, Timmins, LM, Marrero-Berrios, I, Patel, M, et al. The growing role of precision and personalized medicine for cancer treatment. Technology. (2018) 6:79–100. doi: 10.1142/S2339547818300020
17. Shin, SH, Bode, AM, and Dong, Z. Precision medicine: the foundation of future cancer therapeutics. NPJ Precision Oncol. (2017) 1:1–3. doi: 10.1038/s41698-017-0016-z
18. Alizadeh, AA, Aranda, V, Bardelli, A, Blanpain, C, Bock, C, Borowski, C, et al. Toward understanding and exploiting tumor heterogeneity. Nat Med. (2015) 21:846–53. doi: 10.1038/nm.3915
19. Ball, G, Levine, M, Thabane, L, and Tarride, JE. Onwards and upwards: a systematic survey of economic evaluation methods in oncology. Pharmacoecon Open. (2021) 5:397–410. doi: 10.1007/s41669-021-00263-w
20. Bullement, A, Cranmer, HL, and Shields, GE. A review of recent decision-analytic models used to evaluate the economic value of cancer treatments. Appl Health Econ Health Policy. (2019) 17:771–80. doi: 10.1007/s40258-019-00513-3
21. Davis, S, Stevenson, M, Tappenden, P, and Wailoo, A. NICE DSU technical support document 15: Cost-effectiveness modelling using patient-level simulation. Rep BY Decis Support UNIT, (2014).
22. Stahl, JE. Modelling methods for pharmacoeconomics and health technology assessment. PharmacoEconomics. (2008) 26:131–48. doi: 10.2165/00019053-200826020-00004
23. Caro, JJ, and Möller, J. Advantages and disadvantages of discrete-event simulation for health economic analyses. Expert Rev Pharmacoecon Outcomes Res. (2016) 16:327–9. doi: 10.1586/14737167.2016.1165608
24. Caro, JJ, and Möller, J. Decision-analytic models: current methodological challenges. PharmacoEconomics. (2014) 32:943–50. doi: 10.1007/s40273-014-0183-5
25. Caro, JJ, Möller, J, Karnon, J, Stahl, J, and Ishak, J. Discrete event simulation for health technology assessment. Boca Raton, Florida: CRC Press (2015).
26. Degeling, K, Koffijberg, H, and MJ, IJ. A systematic review and checklist presenting the main challenges for health economic modeling in personalized medicine: towards implementing patient-level models. Expert Rev Pharmacoecon Outcomes Res. (2017) 17:17–25. doi: 10.1080/14737167.2017.1273110
27. Karnon, J, Haji, H, and Afzali, A. When to use discrete event simulation (DES) for the economic evaluation of health technologies? A review and critique of the costs and benefits of DES. PharmacoEconomics. (2014) 32:547–58. doi: 10.1007/s40273-014-0147-9
28. Zucchelli, E, Jones, A, and Rice, N. The evaluation of health policies through microsimulation methods. York, United Kingdom HEDG, c/o Department of Economics, University of York (2010).
29. Goldman, DP, Leaf, DE, and Tysinger, B. The future adult model: technical documentation. Technical Report. (2018)
30. Hennessy, DA, Flanagan, WM, Tanuseputro, P, Bennett, C, Tuna, M, and Kopec, J. The population health model (POHEM): an overview of rationale, methods and applications. Popul Health Metrics. (2015) 13:1–12. doi: 10.1186/s12963-015-0057-x
31. Liu, S, Li, Y, Triantis, KP, Xue, H, and Wang, Y. The diffusion of discrete event simulation approaches in health Care Management in the Past Four Decades: a comprehensive review. MDM Policy Pract. (2020) 5:2381468320915242. doi: 10.1177/2381468320915242
32. Roberts, M, Russell, LB, Paltiel, AD, Chambers, M, McEwan, P, Krahn, M, et al. Conceptualizing a model: a report of the ISPOR-SMDM modeling good research practices task force–2. Med Decis Mak. (2012) 32:678–89. doi: 10.1177/0272989X12454941
33. Zhang, X. Application of discrete event simulation in health care: a systematic review. BMC Health Serv Res. (2018) 18:687. doi: 10.1186/s12913-018-3456-4
34. Elo, S, and Kyngäs, H. The qualitative content analysis process. J Adv Nurs. (2008) 62:107–15. doi: 10.1111/j.1365-2648.2007.04569.x
35. Hsieh, H-F, and Shannon, SE. Three approaches to qualitative content analysis. Qual Health Res. (2005) 15:1277–88. doi: 10.1177/1049732305276687
36. Finfgeld-Connett, D. Use of content analysis to conduct knowledge-building and theory-generating qualitative systematic reviews. Qual Res. (2014) 14:341–52. doi: 10.1177/1468794113481790
37. Zhang, X, Lhachimi, SK, and Rogowski, WH. Reporting quality of discrete event simulations in healthcare—results from a generic reporting checklist. Value Health. (2020) 23:506–14. doi: 10.1016/j.jval.2020.01.005
38. Cook, SF, and Bies, RR. Disease progression modeling: key concepts and recent developments. Curr Pharmacol Rep. (2016) 2:221–30. doi: 10.1007/s40495-016-0066-x
39. Mould, DR. Models for disease progression: new approaches and uses. Clin Pharmacol Ther. (2012) 92:125–31. doi: 10.1038/clpt.2012.53
40. Dobler, CC, Guyatt, GH, Wang, Z, and Murad, MH. Users’ guide to medical decision analysis. Mayo Clin Proc. (2021) 96:2205–17. doi: 10.1016/j.mayocp.2021.02.003
41. Sung, H, Ferlay, J, Siegel, RL, Laversanne, M, Soerjomataram, I, Jemal, A, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
42. Arrieta, O, Anaya, P, Morales-Oyarvide, V, Ramírez-Tirado, LA, and Polanco, AC. Cost-effectiveness analysis of EGFR mutation testing in patients with non-small cell lung cancer (NSCLC) with gefitinib or carboplatin–paclitaxel. Eur J Health Econ. (2016) 17:855–63. doi: 10.1007/s10198-015-0726-5
43. Birnbaum, JK, Duggan, C, Anderson, BO, and Etzioni, R. Early detection and treatment strategies for breast cancer in low-income and upper middle-income countries: a modelling study. Lancet Glob Health. (2018) 6:e885–93. doi: 10.1016/S2214-109X(18)30257-2
44. Chen, Q, Staton, AD, Ayer, T, Goldstein, DA, Koff, JL, and Flowers, CR. Exploring the potential cost-effectiveness of precision medicine treatment strategies for diffuse large B-cell lymphoma. Leuk Lymphoma. (2018) 59:1700–9. doi: 10.1080/10428194.2017.1390230
45. Criss, SD, Palazzo, L, Watson, TR, Paquette, AM, Sigel, K, Wisnivesky, J, et al. Cost-effectiveness of pembrolizumab for advanced non-small cell lung cancer patients with varying comorbidity burden. PLoS One. (2020) 15:e0228288. doi: 10.1371/journal.pone.0228288
46. Criss, SD, Weaver, DT, Sheehan, DF, Lee, RJ, Pandharipande, PV, and Kong, CY. Effect of PD-L1 testing on the cost-effectiveness and budget impact of pembrolizumab for advanced urothelial carcinoma of the bladder in the United States. Urol Oncol. (2019) 37:180.e11–8. doi: 10.1016/j.urolonc.2018.11.016
47. Degeling, K, Schivo, S, Mehra, N, Koffijberg, H, Langerak, R, de Bono, JS, et al. Comparison of timed automata with discrete event simulation for modeling of biomarker-based treatment decisions: an illustration for metastatic castration-resistant prostate Cancer. Value Health. (2017) 20:1411–9. doi: 10.1016/j.jval.2017.05.024
48. Harrison, RF, Cantor, SB, Sun, CC, Villanueva, M, Westin, SN, Fleming, ND, et al. Cost-effectiveness of laparoscopic disease assessment in patients with newly diagnosed advanced ovarian cancer*. Gynecol Oncol. (2021) 161:56–62. doi: 10.1016/j.ygyno.2021.01.024
49. Health Quality Ontario. Minimal residual disease evaluation in childhood acute lymphoblastic leukemia: an economic analysis. Ont Health Technol Assess Ser. (2016) 16:1–83.
50. Hörster, L, Schlenk, RF, Stadler, M, Gabriel, M, Thol, F, Schildmann, J, et al. Cost-effectiveness of methods in personalized medicine. Results of a decision-analytic model in patients with acute myeloid leukemia with normal karyotype. Leuk Res. (2017) 62:84–90. doi: 10.1016/j.leukres.2017.09.009
51. Hoyer, T, Bekkers, R, Gooszen, H, Massuger, L, Rovers, M, and Grutters, JPC. Cost-effectiveness of early-initiated treatment for advanced-stage epithelial ovarian Cancer patients a modeling study. Int J Gynecol Cancer. (2014) 24:75–84. doi: 10.1097/IGC.0000000000000025
52. Ibarrondo, O, Álvarez-López, I, Freundlich, F, Arrospide, A, Galve-Calvo, E, Gutiérrez-Toribio, M, et al. Probabilistic cost-utility analysis and expected value of perfect information for the Oncotype multigenic test: a discrete event simulation model. Gac Sanit. (2020) 34:61–8. doi: 10.1016/j.gaceta.2018.07.012
53. Jahn, B, et al. Cost effectiveness of personalized treatment in women with early breast cancer: the application of OncotypeDX and adjuvant! Online to guide adjuvant chemotherapy in Austria. Springerplus. (2015):4.
54. Jahn, B, Rochau, U, Kurzthaler, C, Paulden, M, Kluibenschädl, M, Arvandi, M, et al. Lessons learned from a cross-model validation between a discrete event simulation model and a cohort state-transition model for personalized breast Cancer treatment. Med Decis Mak. (2016) 36:375–90. doi: 10.1177/0272989X15604158
55. Jahn, B, Rochau, U, Kurzthaler, C, Hubalek, M, Miksad, R, Sroczynski, G, et al. Personalized treatment of women with early breast cancer: a risk-group specific cost-effectiveness analysis of adjuvant chemotherapy accounting for companion prognostic tests OncotypeDX and adjuvant!Online. BMC Cancer. (2017) 17:685. doi: 10.1186/s12885-017-3603-z
56. Jones, DA, et al. Informing radiotherapy decisions in stage I/IIa Hodgkin lymphoma: modelling life expectancy using radiation dosimetry. Blood Adv. (2021)
57. Lange, JM, Laviana, AA, Penson, DF, Lin, DW, Bill-Axelson, A, Carlsson, SV, et al. Prostate cancer mortality and metastasis under different biopsy frequencies in north American active surveillance cohorts. Cancer. (2020) 126:583–92. doi: 10.1002/cncr.32557
58. Penn, CA, Wong, MS, and Walsh, CS. Cost-effectiveness of maintenance therapy based on molecular classification following treatment of primary epithelial ovarian Cancer in the United States. JAMA Netw Open. (2020) 3:e2028620. doi: 10.1001/jamanetworkopen.2020.28620
59. Quik, EH, Feenstra, TL, Postmus, D, Slotman, BJ, Leemans, CR, Krabbe, PFM, et al. Individual patient information to select patients for different radiation techniques. Eur J Cancer. (2016) 62:18–27. doi: 10.1016/j.ejca.2016.04.008
60. Rios, JD, Velummailum, R, Bennett, J, Nobre, L, Tsang, DS, Bouffet, E, et al. Clinical and economic impact of molecular testing for BRAF fusion in pediatric low-grade glioma. BMC Pediatr. (2022) 22:13. doi: 10.1186/s12887-021-03069-1
61. Schluckebier, L, Caetano, R, Garay, OU, Montenegro, GT, Custodio, M, Aran, V, et al. Cost-effectiveness analysis comparing companion diagnostic tests for EGFR, ALK, and ROS1 versus next-generation sequencing (NGS) in advanced adenocarcinoma lung cancer patients. BMC Cancer. (2020) 20:875. doi: 10.1186/s12885-020-07240-2
62. Stenehjem, DD, Bellows, BK, Yager, KM, Jones, J, Kaldate, R, Siebert, U, et al. Cost-utility of a prognostic test guiding adjuvant chemotherapy decisions in early-stage non-small cell lung Cancer. Oncologist. (2016) 21:196–204. doi: 10.1634/theoncologist.2015-0162
63. Wang, HI, Smith, A, Aas, E, Roman, E, Crouch, S, Burton, C, et al. Treatment cost and life expectancy of diffuse large B-cell lymphoma (DLBCL): a discrete event simulation model on a UK population-based observational cohort. Eur J Health Econ. (2017) 18:255–67. doi: 10.1007/s10198-016-0775-4
64. Weaver, DT, Raphel, TJ, Melamed, A, Rauh-Hain, JA, Schorge, JO, Knudsen, AB, et al. Modeling treatment outcomes for patients with advanced ovarian cancer: projected benefits of a test to optimize treatment selection. Gynecol Oncol. (2018) 149:256–62. doi: 10.1016/j.ygyno.2018.02.007
65. Ambavane, A, Yang, S, Atkins, MB, Rao, S, Shah, A, Regan, MM, et al. Clinical and economic outcomes of treatment sequences for intermediate- to poor-risk advanced renal cell carcinoma. Immunotherapy. (2020) 12:37–51. doi: 10.2217/imt-2019-0199
66. Bieri, U, Hübel, K, Seeger, H, Kulkarni, GS, Sulser, T, Hermanns, T, et al. Management of Active Surveillance-Eligible Prostate Cancer during Pretransplantation workup of patients with kidney failure: a simulation study. Clin J Am Soc Nephrol. (2020) 15:822–9. doi: 10.2215/CJN.14041119
67. Blommestein, HM, Franken, MG, van Beurden-Tan, CHY, Blijlevens, NMA, Huijgens, PC, Sonneveld, P, et al. Cost-effectiveness of novel treatment sequences for transplant-ineligible patients with multiple myeloma. JAMA Netw Open. (2021) 4:e213497. doi: 10.1001/jamanetworkopen.2021.3497
68. Blommestein, HM, Verelst, SGR, de Groot, S, Huijgens, PC, Sonneveld, P, and Uyl-de Groot, CA. A cost-effectiveness analysis of real-world treatment for elderly patients with multiple myeloma using a full disease model. Eur J Haematol. (2016) 96:198–208. doi: 10.1111/ejh.12571
69. de Carvalho, TM, Heijnsdijk, EAM, and de Koning, HJ. Estimating the risks and benefits of active surveillance protocols for prostate cancer: a microsimulation study. BJU Int. (2017) 119:560–6. doi: 10.1111/bju.13542
70. De Groot, S, et al. Potential health gains for patients with metastatic renal cell carcinoma in daily clinical practice: a real-world cost-effectiveness analysis of sequential first- and second-line treatments. PLoS One. (2017) 12:e0177364. doi: 10.1371/journal.pone.0177364
71. Degeling, K, Wong, HL, Koffijberg, H, Jalali, A, Shapiro, J, Kosmider, S, et al. Simulating progression-free and overall survival for first-line doublet chemotherapy with or without bevacizumab in metastatic colorectal Cancer patients based on real-world registry data. PharmacoEconomics. (2020) 38:1263–75. doi: 10.1007/s40273-020-00951-1
72. Dragomir, A, et al. Drug costs in the management of metastatic castration-resistant prostate cancer in Canada. BMC Health Serv Res. (2014):14.
73. Hird, AE, Magee, DE, Cheung, DC, Sander, B, Sridhar, S, Nam, RK, et al. Neoadjuvant versus adjuvant chemotherapy for upper tract urothelial carcinoma: a microsimulation model. Clin Genitourin Cancer. (2021) 19:e135–47. doi: 10.1016/j.clgc.2020.10.001
74. Kang, SK, Mali, RD, Prabhu, V, Ferket, BS, and Loeb, S. Active surveillance strategies for low-grade prostate Cancer: comparative benefits and cost-effectiveness. Radiology. (2021) 300:594–604. doi: 10.1148/radiol.2021204321
75. Parikh, RC, du, XL, Robert, MO, and Lairson, DR. Cost-effectiveness of treatment sequences of chemotherapies and targeted biologics for elderly metastatic colorectal Cancer patients. J Manag Care Spec Pharm. (2017) 23:64–73. doi: 10.18553/jmcp.2017.23.1.64
76. Savitz, ST, Chen, RC, and Sher, DJ. Cost-effectiveness analysis of neurocognitive-sparing treatments for brain metastases. Cancer. (2015) 121:4231–9. doi: 10.1002/cncr.29642
77. Tarhini, A, Benedict, A, McDermott, D, Rao, S, Ambavane, A, Gupte-Singh, K, et al. Sequential treatment approaches in the management of BRAF wild-type advanced melanoma: a cost-effectiveness analysis. Immunotherapy. (2018) 10:1241–52. doi: 10.2217/imt-2018-0085
78. Tarhini, A, McDermott, D, Ambavane, A, Gupte-Singh, K, Aponte-Ribero, V, Ritchings, C, et al. Clinical and economic outcomes associated with treatment sequences in patients with BRAF-mutant advanced melanoma. Immunotherapy. (2019) 11:283–95. doi: 10.2217/imt-2018-0168
79. Leunis, A, Redekop, WK, van Montfort, KAGM, Löwenberg, B, and Uyl-de Groot, CA. The development and validation of a decision-analytic model representing the full disease course of acute myeloid leukemia. PharmacoEconomics. (2013) 31:605–21. doi: 10.1007/s40273-013-0058-1
80. Moldaver, D, Hurry, M, Evans, WK, Cheema, PK, Sangha, R, Burkes, R, et al. Development, validation and results from the impact of treatment evolution in non-small cell lung cancer (iTEN) model. Lung Cancer. (2020) 139:185–94. doi: 10.1016/j.lungcan.2019.10.019
81. Wang, HI, Roman, E, Crouch, S, Aas, E, Burton, C, Patmore, R, et al. A generic model for follicular lymphoma: predicting cost, life expectancy, and quality-adjusted-life-year using UK population–based observational data. Value Health. (2018) 21:1176–85. doi: 10.1016/j.jval.2018.03.007
82. Evans, WK, Wolfson, MC, Flanagan, WM, Shin, J, Goffin, J, Miller, AB, et al. Canadian CANCER risk management model: evaluation of CANCER control. Int J Technol Assess Health Care. (2013) 29:131–9. doi: 10.1017/S0266462313000044
83. Louie, AV, Rodrigues, GB, Palma, DA, and Senan, S. Measuring the population impact of introducing stereotactic ablative radiotherapy for stage I non-small cell lung cancer in Canada. Oncologist. (2014) 19:880–5. doi: 10.1634/theoncologist.2013-0469
84. Tappenden, P, Chilcott, J, Brennan, A, Squires, H, Glynne-Jones, R, and Tappenden, J. Using whole disease modeling to inform resource allocation decisions: economic evaluation of a clinical guideline for colorectal cancer using a single model. Value Health. (2013) 16:542–53. doi: 10.1016/j.jval.2013.02.012
85. Tramontano, AC, Cipriano, LE, Kong, CY, Shepard, JAO, Lanuti, M, Gazelle, GS, et al. Microsimulation model predicts survival benefit of radiofrequency ablation and stereotactic body radiotherapy versus radiotherapy for treating inoperable stage I non-small cell lung Cancer. Am J Roentgenol. (2013) 200:1020–7. doi: 10.2214/AJR.12.8968
86. Ward, ZJ, Yeh, JM, Bhakta, N, Frazier, AL, Girardi, F, and Atun, R. Global childhood cancer survival estimates and priority-setting: a simulation-based analysis. Lancet Oncol. (2019) 20:972–83. doi: 10.1016/S1470-2045(19)30273-6
87. Ward, ZJ, Scott, AM, Hricak, H, Abdel-Wahab, M, Paez, D, Lette, MM, et al. Estimating the impact of treatment and imaging modalities on 5-year net survival of 11 cancers in 200 countries: a simulation-based analysis. Lancet Oncol. (2020) 21:1077–88. doi: 10.1016/S1470-2045(20)30317-X
88. Ward, ZJ, Grover, S, Scott, AM, Woo, S, Salama, DH, Jones, EC, et al. The role and contribution of treatment and imaging modalities in global cervical cancer management: survival estimates from a simulation-based analysis. Lancet Oncol. (2020) 21:1089–98. doi: 10.1016/S1470-2045(20)30316-8
89. Ward, ZJ, Atun, R, Hricak, H, Asante, K, McGinty, G, Sutton, EJ, et al. The impact of scaling up access to treatment and imaging modalities on global disparities in breast cancer survival: a simulation-based analysis. Lancet Oncol. (2021) 22:1301–11. doi: 10.1016/S1470-2045(21)00403-4
90. Lagergren, M. What is the role and contribution of models to management and research in the health services? A view from Europe. Eur J Oper Res. (1998) 105:257–66. doi: 10.1016/S0377-2217(97)00233-6
91. Edwards, JP, Datta, I, Hunt, JD, Stefan, K, Ball, CG, Dixon, E, et al. A novel approach for the accurate prediction of thoracic surgery workforce requirements in Canada. J Thorac Cardiovasc Surg. (2014) 148:7–12. doi: 10.1016/j.jtcvs.2014.03.031
92. Edwards, JP, Datta, I, Hunt, JD, Stefan, K, Ball, CG, Dixon, E, et al. Forecasting the impact of stereotactic ablative radiotherapy for early-stage lung cancer on the thoracic surgery workforce(aEuro). Eur J Cardiothorac Surg. (2016) 49:1599–606. doi: 10.1093/ejcts/ezv421
93. Günal, MM, and Pidd, M. Discrete event simulation for performance modelling in health care: a review of the literature. J Simul. (2010) 4:42–51. doi: 10.1057/jos.2009.25
94. Vieira, B, Hans, EW, van Vliet-Vroegindeweij, C, van de Kamer, J, and van Harten, W. Operations research for resource planning and -use in radiotherapy: a literature review. BMC Med Inform Decis Mak. (2016) 16:149.
95. Alvarado, MM, Cotton, TG, Ntaimo, L, Pérez, E, and Carpentier, WR. Modeling and simulation of oncology clinic operations in discrete event system specification. Simul Transact Society Model Simul Int. (2018) 94:105–21. doi: 10.1177/0037549717708246
96. Bardet, A, Fraslin, AM, Marghadi, J, Borget, I, Faron, M, Honoré, C, et al. Impact of COVID-19 on healthcare organisation and cancer outcomes. Eur J Cancer. (2021) 153:123–32. doi: 10.1016/j.ejca.2021.05.012
97. Baril, C, Gascon, V, Miller, J, and Bounhol, C. The importance of considering resource's tasks when modeling healthcare services with discrete-event simulation: an approach using work sampling method. J Simul. (2017) 11:103–14. doi: 10.1057/jos.2016.6
98. Baril, C, Gascon, V, and Miller, J. Design of experiments and discrete-event simulation to study oncology nurse workload. IISE Trans Healthc Syst Eng. (2020) 10:74–86. doi: 10.1080/24725579.2019.1680581
99. Corazza, U, Filippini, R, and Setola, R. Discrete event simulation of a proton therapy facility: a case study. Comput Methods Prog Biomed. (2011) 102:305–16. doi: 10.1016/j.cmpb.2010.05.011
100. Arafeh, M, et al. Using six sigma DMAIC methodology and discrete event simulation to reduce patient discharge time in king Hussein Cancer center. J Healthc Eng. (2018) 2018:3832151.
101. Chalk, D, Trent, N, Vennam, S, McGrane, J, and Mantle, M. Reducing delays in the diagnosis and treatment of muscle-invasive bladder cancer using simulation modelling. J Clin Urol. (2019) 12:129–33. doi: 10.1177/2051415818794089
102. Corsini, RR, et al. A configurable computer simulation model for reducing patient waiting time in oncology departments. Health Systems.
103. Kang, H, and Haswell, E. Patient flow analysis using real-time locating system data: a case study in an outpatient oncology center. JCO Oncol Pract. (2022) 16:e1471–80. doi: 10.1200/OP.20.00119
104. Liang, BH, et al. Improvement of chemotherapy patient flow and scheduling in an outpatient oncology clinic. Int J Prod Res. (2015) 53:7177–90. doi: 10.1080/00207543.2014.988891
105. McKinley, KW, et al. Discrete event simulation modelling to evaluate the impact of a quality improvement initiative on patient flow in a paediatric emergency department. Emerg Med J. (2020) 37, 193–199.
106. Romero, HL, Dellaert, NP, van der Geer, S, Frunt, M, Jansen-Vullers, MH, and Krekels, GAM. Admission and capacity planning for the implementation of one-stop-shop in skin cancer treatment using simulation-based optimization. Health Care Manag Sci. (2013) 16:75–86. doi: 10.1007/s10729-012-9213-z
107. Woodall, JC, Gosselin, T, Boswell, A, Murr, M, and Denton, BT. Improving patient access to chemotherapy treatment at Duke Cancer institute. Interfaces. (2013) 43:449–61. doi: 10.1287/inte.2013.0695
108. Doan, TN, and Barendregt, J. Adjuvant trastuzumab chemotherapy in early breast cancer: meta-analysis of randomised trials and cost-effectiveness analysis. Swiss Med Wkly. (1920) 2019:w20082–2.
109. Dewilde, S, Woods, B, Castaigne, JG, Parker, C, and Dunlop, W. Bendamustine-rituximab: a cost-utility analysis in first-line treatment of indolent non-Hodgkin's lymphoma in England and Wales. J Med Econ. (2014) 17:111–24. doi: 10.3111/13696998.2013.873044
110. Gibson, EJ, Begum, N, Koblbauer, I, Dranitsaris, G, Liew, D, McEwan, P, et al. Cohort versus patient level simulation for the economic evaluation of single versus combination immuno-oncology therapies in metastatic melanoma. J Med Econ. (2019) 22:531–44. doi: 10.1080/13696998.2019.1569446
111. Li, S, et al. Cost-effectiveness of Lorlatinib as a first-line therapy for untreated advanced anaplastic lymphoma kinase-positive non-small cell lung Cancer. Front Oncol. (2021):11.
112. Möller, J, Nicklasson, L, and Murthy, A. Cost-effectiveness of novel relapsed-refractory multiple myeloma therapies in Norway: lenalidomide plus dexamethasone vs bortezomib. J Med Econ. (2011) 14:690–7. doi: 10.3111/13696998.2011.611841
113. Mamiya, H, Tahara, RK, Tolaney, SM, Choudhry, NK, and Najafzadeh, M. Cost-effectiveness of palbociclib in hormone receptor-positive advanced breast cancer. Ann Oncol. (2017) 28:1825–31. doi: 10.1093/annonc/mdx201
114. Degeling, K, Corcoran, NM, Pereira-Salgado, A, Hamid, AA, Siva, S, and IJzerman, MJ. Lifetime health and economic outcomes of active surveillance, radical prostatectomy, and radiotherapy for favorable-risk localized prostate Cancer. Value Health. (2021) 24:1737–45. doi: 10.1016/j.jval.2021.06.004
115. Degeling, K, Franken, MD, May, AM, van Oijen, MGH, Koopman, M, Punt, CJA, et al. Matching the model with the evidence: comparing discrete event simulation and state-transition modeling for time-to-event predictions in a cost-effectiveness analysis of treatment in metastatic colorectal cancer patients. Cancer Epidemiol. (2018) 57:60–7. doi: 10.1016/j.canep.2018.09.008
116. Pan, F, Reifsnider, O, Zheng, Y, Proskorovsky, I, Li, T, He, J, et al. Modeling clinical outcomes in prostate Cancer: application and validation of the discrete event simulation approach. Value Health. (2018) 21:416–22. doi: 10.1016/j.jval.2017.09.022
117. Soeteman, DI, Stout, NK, Ozanne, EM, Greenberg, C, Hassett, MJ, Schrag, D, et al. Modeling the effectiveness of initial management strategies for ductal carcinoma in situ. JNCI J Natl Cancer Inst. (2013) 105:774–81. doi: 10.1093/jnci/djt096
118. Davies, KR, Brewster, AM, Bedrosian, I, Parker, PA, Crosby, MA, Peterson, SK, et al. Outcomes of contralateral prophylactic mastectomy in relation to familial history: a decision analysis (BRCR-D-16-00033). Breast Cancer Res. (2016) 18:93. doi: 10.1186/s13058-016-0752-y
119. Hummel, SR, Stevenson, MD, Simpson, EL, and Staffurth, J. A model of the cost-effectiveness of intensity-modulated radiotherapy in comparison with three-dimensional conformal radiotherapy for the treatment of localised prostate cancer. Clin Oncol (R Coll Radiol). (2012) 24:e159–67. doi: 10.1016/j.clon.2012.09.003
120. Mojtahed, SA, Boyer, NR, Rao, SA, Gajewski, TF, Tseng, J, and Turaga, KK. Cost-effectiveness analysis of adjuvant therapy for BRAF-mutant resected stage III melanoma in Medicare patients. Ann Surg Oncol. (2021) 28:9039–47. doi: 10.1245/s10434-021-10288-4
121. Sarkar, RR, Gloude, NJ, Schiff, D, and Murphy, JD. Cost-effectiveness of chimeric antigen receptor T-cell therapy in pediatric relapsed/refractory B-cell acute lymphoblastic leukemia. J Natl Cancer Inst. (2019) 111:719–26. doi: 10.1093/jnci/djy193
122. Wan, X, Zhang, YC, Ma, JA, Tan, C, Zeng, XH, and Peng, LB. Ribociclib in hormone-receptor-positive advanced breast cancer: establishing a value-based cost in China. Breast. (2019) 43:1–6. doi: 10.1016/j.breast.2018.10.004
123. Raphael, J, Helou, J, Pritchard, KI, and Naimark, DM. Palbociclib in hormone receptor positive advanced breast cancer: a cost-utility analysis. Eur J Cancer. (2017) 85:146–54. doi: 10.1016/j.ejca.2017.08.018
124. Blakely, T, Collinson, L, Kvizhinadze, G, Nair, N, Foster, R, Dennett, E, et al. Cancer care coordinators in stage III colon cancer: a cost-utility analysis. BMC Health Serv Res. (2015) 15:306. doi: 10.1186/s12913-015-0970-5
125. Tully, S, Feng, Z, Grindrod, K, McFarlane, T, Chan, KKW, and Wong, WWL. Impact of increasing wait times on overall mortality of chimeric antigen receptor T-cell therapy in large B-cell lymphoma: a discrete event simulation model. JCO Clin Cancer Inform. (2019) 3:1–9. doi: 10.1200/CCI.19.00086
126. Vieira, B, Demirtas, D, van de Kamer, J, Hans, EW, and van Harten, W. Improving workflow control in radiotherapy using discrete-event simulation. BMC Med Inform Decis Mak. (2019) 19:199. doi: 10.1186/s12911-019-0910-0
127. Rejeb, O, Pilet, C, Hamana, S, Xie, X, Durand, T, Aloui, S, et al. Performance and cost evaluation of health information systems using micro-costing and discrete-event simulation. Health Care Manag Sci. (2018) 21:204–23. doi: 10.1007/s10729-017-9402-x
128. Joustra, P, van der Sluis, E, and van Dijk, NM. To pool or not to pool in hospitals: a theoretical and practical comparison for a radiotherapy outpatient department. Ann Oper Res. (2010) 178:77–89. doi: 10.1007/s10479-009-0559-7
129. Joustra, PE, Kolfin, R, van Dijk, NM, Koning, CCE, and Bakker, PJM. Reduce fluctuations in capacity to improve the accessibility of radiotherapy treatment cost-effectively. Flex Serv Manuf J. (2012) 24:448–64. doi: 10.1007/s10696-011-9119-y
130. Baril, C, Gascon, V, Miller, J, and Bounhol, C. Studying nurse workload and patient waiting time in a hematology-oncology clinic with discrete event simulation. IIE Transact Healthcare Syst Eng. (2016) 6:223–34. doi: 10.1080/19488300.2016.1226212
131. Babashov, V, Aivas, I, Begen, MA, Cao, JQ, Rodrigues, G, D’Souza, D, et al. Reducing patient waiting times for radiation therapy and improving the treatment planning process: a discrete-event simulation model (radiation treatment planning). Clin Oncol (R Coll Radiol). (2017) 29:385–91. doi: 10.1016/j.clon.2017.01.039
132. Kazemian, P, Sir, MY, van Oyen, MP, Lovely, JK, Larson, DW, and Pasupathy, KS. Coordinating clinic and surgery appointments to meet access service levels for elective surgery. J Biomed Inform. (2017) 66:105–15. doi: 10.1016/j.jbi.2016.11.007
133. Lame, G, Jouini, O, and Stal-Le Cardinal, J. Combining soft systems methodology, ethnographic observation, and discrete-event simulation: a case study in cancer care. J Oper Res Soc. (2020) 71:1545–62. doi: 10.1080/01605682.2019.1610339
134. Mohamed, I, El-Henawy, I, and El-Din, RZ. An early discharge approach for managing hospital capacity. Int J Model Simul Scient Comput. (2017) 8
135. Jackson, SE, and Chester, JD. Personalised cancer medicine. Int J Cancer. (2015) 137:262–6. doi: 10.1002/ijc.28940
136. Lorente, D, Fizazi, K, Sweeney, C, and de Bono, JS. Optimal treatment sequence for metastatic castration-resistant prostate Cancer. Eur Urol Focus. (2016) 2:488–98. doi: 10.1016/j.euf.2016.10.008
137. McEachron, J, Zhou, N, Spencer, C, Shanahan, L, Chatterton, C, Singhal, P, et al. Evaluation of the optimal sequence of adjuvant chemotherapy and radiation therapy in the treatment of advanced endometrial cancer. J Gynecol Oncol. (2020) 31:e90. doi: 10.3802/jgo.2020.31.e90
138. Degeling, K, Vu, M, Koffijberg, H, Wong, HL, Koopman, M, Gibbs, P, et al. Health economic models for metastatic colorectal Cancer: a methodological review. PharmacoEconomics. (2020) 38:683–713. doi: 10.1007/s40273-020-00908-4
139. Tappenden, P, Chilcott, J, Brennan, A, Squires, H, and Stevenson, M. Whole disease modeling to inform resource allocation decisions in Cancer: a methodological framework. Value Health. (2012) 15:1127–36. doi: 10.1016/j.jval.2012.07.008
140. Bryan, S, Mitton, C, and Donaldson, C. BREAKING THE ADDICTION TO TECHNOLOGY ADOPTION. Health Econ. (2014) 23:379–83. doi: 10.1002/hec.3034
141. Husereau, D, Henshall, C, Sampietro-Colom, L, and Thomas, S. Changing health technology assessment paradigms? Int J Technol Assess Health Care. (2016) 32:191–9. doi: 10.1017/S0266462316000386
142. Marshall, DA, Grazziotin, LR, Regier, DA, Wordsworth, S, Buchanan, J, Phillips, K, et al. Addressing challenges of economic evaluation in precision medicine using dynamic simulation modeling. Value Health. (2020) 23:566–73. doi: 10.1016/j.jval.2020.01.016
143. Scotland, G, and Bryan, S. Why do health economists promote technology adoption rather than the search for efficiency? A proposal for a change in our approach to economic evaluation in health care. Med Decis Mak. (2016) 37:139–47. doi: 10.1177/0272989X16653397
144. Heshmat, M, and Eltawil, A. Solving operational problems in outpatient chemotherapy clinics using mathematical programming and simulation. Ann Oper Res. (2021) 298:289–306. doi: 10.1007/s10479-019-03500-y
145. Vázquez-Serrano, JI, Peimbert-García, RE, and Cárdenas-Barrón, LE. Discrete-event simulation modeling in healthcare: a comprehensive review. Int J Environ Res Public Health. (2021) 18:58–64. doi: 10.3390/ijerph182212262
146. Hales, S, Lesher-Trevino, A, Ford, N, Maher, D, Ramsay, A, and Tran, N. Reporting guidelines for implementation and operational research. Bull World Health Organ. (2016) 94:58–64. doi: 10.2471/BLT.15.167585
147. Damschroder, LJ, Aron, DC, Keith, RE, Kirsh, SR, Alexander, JA, and Lowery, JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. (2009) 4:1–15. doi: 10.1186/1748-5908-4-50
148. Kirk, MA, Kelley, C, Yankey, N, Birken, SA, Abadie, B, and Damschroder, L. A systematic review of the use of the consolidated framework for implementation research. Implement Sci. (2015) 11:1–13. doi: 10.1186/s13012-016-0437-z
149. Vemer, P, Krabbe, PFM, Feenstra, TL, van Voorn, GAK, Ramos, C, and al, MJ. Improving model validation in health technology assessment: comments on guidelines of the ISPOR-SMDM modeling good research practices task force. Value Health. (2013) 16:1106–7. doi: 10.1016/j.jval.2013.06.015
150. de Boer, PT, Frederix, GWJ, Feenstra, TL, and Vemer, P. Unremarked or unperformed? Systematic review on reporting of validation efforts of health economic decision models in seasonal influenza and early breast cancer. PharmacoEconomics. (2016) 34:833–45. doi: 10.1007/s40273-016-0410-3
151. Eddy, DM, Hollingworth, W, Caro, JJ, Tsevat, J, McDonald, KM, and Wong, JB. Model transparency and validation: a report of the ISPOR-SMDM modeling good research practices task force–7. Med Decis Mak. (2012) 32:733–43. doi: 10.1177/0272989X12454579
152. Corro Ramos, I, et al. Evaluating the validation process: embracing complexity and transparency in health economic modelling. PharmacoEconomics. (2024) 40:1–5.
153. Feenstra, T, Corro-Ramos, I, Hamerlijnck, D, van Voorn, G, and Ghabri, S. Four aspects affecting health economic decision models and their validation. PharmacoEconomics. (2022) 40:241–8. doi: 10.1007/s40273-021-01110-w
Keywords: discrete event simulation, microsimulation, cancer, health economics, health policy and management
Citation: Busschaert S-L, Van Deynse H, De Ridder M and Putman K (2025) Patient-level simulation models in cancer care: a systematic review. Front. Public Health. 13:1335300. doi: 10.3389/fpubh.2025.1335300
Edited by:
Xiaozhen Lai, Peking University, ChinaReviewed by:
Alexandre Morais Nunes, University of Lisbon, PortugalMatthew Koehler, The MITRE Corporation, United States
Copyright © 2025 Busschaert, Van Deynse, De Ridder and Putman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sara-Lise Busschaert, c2FyYS1saXNlLmJ1c3NjaGFlcnRAdnViLmJl
†ORCID: Sara-Lise Busschaert, orcid.org/0000-0003-0248-9164
Helena Van Deynse, orcid.org/0000-0001-7744-1478
Mark De Ridder, orcid.org/0000-0003-4433-8807
Koen Putman, orcid.org/0000-0002-7235-3759
 Sara-Lise Busschaert
Sara-Lise Busschaert Helena Van Deynse
Helena Van Deynse Mark De Ridder
Mark De Ridder Koen Putman
Koen Putman