- 1Vaccines R&D/Infectious Disease, GSK, Wavre, Belgium
- 2Vaccines Institute of Global Health, GSK, Siena, Italy
Introduction: Widespread implementation of pneumococcal conjugate vaccines (PCVs)—namely the 7-valent PCV (PCV7), 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV), and 13-valent PCV (PCV13)—in infant national immunization programs has reduced pneumococcal diseases in children, including invasive pneumococcal disease (IPD), acute otitis media (AOM), and community-acquired pneumonia (CAP). However, as the use of PCV impacts pneumococcal epidemiology, identifying the serotypes associated with remaining disease is crucial to guide future vaccination strategies for this population.
Methods: We systematically searched the literature for observational studies (2006–2020) on pneumococcal serotype distribution in IPD, AOM, and CAP among ≤5-year-old children post-PCV introduction. Serotype-specific pooled percentage averages were calculated by post-PCV period (post-PCV7 or pooled post-PHiD-CV/PCV13), or by PCV product (PHiD-CV or PCV13) to determine the contribution of each serotype to a certain clinical manifestation.
Results: Our analysis of 86 studies (47 on IPD, 30 on AOM, and 9 on CAP) shows continued reporting of several vaccine serotypes in all clinical manifestations post-PHiD-CV/PCV13, particularly serotypes 19A, 3, and 1. In PCV13 settings, serotype 19A reporting was reduced but still prevalent compared to PHiD-CV settings. Predominant non-PCV13 serotypes varied by clinical manifestation.
Conclusion: Post-PCV implementation, pneumococcal epidemiology in children is intricate. The persistence of some vaccine serotypes, variations across clinical manifestations, rising antimicrobial resistance, and other factors highlight the need for new vaccine technologies providing enhanced and broader protection to children.
1 Introduction
Streptococcus pneumoniae (Spn) is a major bacterial cause of a wide range of infections, which can be broadly grouped into invasive pneumococcal diseases (IPD), including meningitis and septicemia, and non-invasive diseases, such as acute otitis media (AOM) and community-acquired pneumonia (CAP) (1–5). Non-invasive forms of these infections may become invasive (e.g., when CAP is accompanied by bacteremia) (6, 7). Young children (≤5 years of age), older adults (≥65 years of age), and those with underlying medical conditions are at increased risk of pneumococcal infections (1, 6, 8, 9).
Although all of the at least 100 identified Spn serotypes are theoretically capable of causing disease (10), only a subset is responsible for most pneumococcal infections. The prevalence and distribution of (disease-causing) serotypes vary by age, geographical location, clinical manifestation, and antibiotic use (8, 11–17). Nonetheless, the major driver of changes in pneumococcal epidemiology over time has been the global implementation of pneumococcal conjugate vaccines (PCVs) which has led to serotype replacement (18). This occurs as serotypes included in the PCV decline following vaccination, allowing non-vaccine serotypes to expand—a process that typically becomes evident around 4 years after vaccine introduction (19). The 7-valent PCV (PCV7; Prevenar/Prevnar, Pfizer Inc.) (which contains serotypes 4, 6B, 9 V, 14, 18C, 19F, and 23F) was the first approved PCV and was included in many infant national immunization programs (NIPs) between 2006 and 2008 (20). The pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV, Synflorix, GSK) and 13-valent PCV (PCV13, Prevenar 13/Prevnar 13, Pfizer Inc.) replaced PCV7 in NIPs since 2009 and provided coverage for additional serotypes (PHiD-CV contains all PCV7 serotypes + 1, 5, and 7F; PCV13 contains all PHiD-CV serotypes + 3, 6A, and 19A) (11). Starting from 2015, the World Health Organization (WHO) estimated PCV coverage in 1-year-old children in high-income countries to be ≥80% (21). In low-and middle-income countries, PCV coverage has been lower, with estimates of 28 to 60% in 2015, slowly increasing in subsequent years (21).
Despite the considerable reduction in disease burden by infant vaccination with PHiD-CV and PCV13 (22–27), Spn remains a major cause of morbidity and mortality in children (28). Monitoring the evolution of pneumococcal epidemiology to evaluate the (long-term) effectiveness of vaccines and vaccination strategies is critical. Spn serotype distribution is best characterized for IPD, as it is a reportable disease, and serotyping is routinely conducted as part of many IPD surveillance programs (11). However, IPD-focused surveillance strategies may not reflect the true prevalence of pneumococcal serotypes. CAP (mainly in adults) and AOM (in children) represent the highest proportion of the overall pneumococcal disease burden (8, 29), but data on serotypes causing these manifestations are scarcer. This is because their diagnosis is often based on clinical presentation without routine collection of biological specimens, as obtaining samples for etiological diagnosis can be challenging and the conventional diagnostic tools for CAP exhibit limited sensitivity (11, 30–33).
The objective of this systematic literature review (SLR) was to summarize the global evidence from published observational studies on the serotype distribution in both invasive and non-invasive pneumococcal disease among children ≤5 years of age after the implementation of PHiD-CV and PCV13, compared to the post-PCV7 era. With the recent introduction of the 15-valent (PCV15, Vaxneuvance, Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc. [MSD]) and 20-valent (PCV20, Prevenar 20/Prevnar 20, Pfizer Inc.) PCVs in infant NIPs, whose epidemiological impact is still to be determined, along with ongoing pneumococcal vaccine development, we aimed to better understand the impact of PHiD-CV and PCV13—both widely implemented for many years—on the pneumococcal epidemiology. Specifically, we focused on the contribution of individual serotypes to remaining IPD, CAP, and AOM in children.
2 Methods
This analysis is part of a larger SLR that aimed to assess the effect of widespread PHiD-CV/PCV13 usage in infants on the serotype distribution in remaining invasive and non-invasive pneumococcal disease in children aged ≤5 years and adults aged ≥65 years. This manuscript presents the results in children. Results in older adults are summarized separately (34). The SLR was conducted in accordance with its protocol and with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines (35).
2.1 Systematic search strategy
PubMed and EMBASE were searched for articles published from 1 January 2006 to 31 December 2020 on pneumococcal serotype distribution in IPD, CAP, or AOM after infant uptake of PCV7, PHiD-CV, or PCV13. The 2006 cutoff was chosen because several countries (including the United States, Canada, Australia, the United Kingdom, France, Belgium, Germany, and Italy) had already universally introduced PCV7 by 2006. We used a 2020 cutoff to avoid the immediate and rebound effects of the coronavirus disease 2019 (COVID-19) pandemic, an exceptional event that significantly disrupted surveillance systems, vaccination programs, medical care access, and disease trends for several years (36–39). Post-PHiD-CV/PCV13 serotyping data were of primary interest; data collected after PCV7 implementation were included to assess changes in serotype distribution before and after PHiD-CV/PCV13 uptake. A broad search strategy was applied using combinations of search strings, consisting of terms for Spn serotypes, PCVs, and pneumococcal diseases (Supplementary methods).
2.2 Eligibility criteria and study selection
All eligibility criteria were determined upfront in the protocol and were applied at screening and the data extraction phase.
As many studies on IPD were expected, eligible IPD studies were first limited to SLRs/meta-analyses. Since only 2 SLRs were retrieved, both with several overlapping studies among their respective datasets (40, 41), eligibility was expanded, as predefined, to include the most recent observational studies published between 2018 and 2020. Only studies reporting serotyping data of at least 30 isolates obtained from sterile sites were included (42).
For AOM and CAP, only observational studies were included. AOM was to be defined by clinical diagnosis (the presence of inflammation of the middle ear, associated with effusion, accompanied by a rapid onset of symptoms, and signs of an ear infection) (43, 44). Only studies that reported serotyping data of at least 30 isolates from middle ear fluid samples were included.
CAP was to be defined as pneumonia acquired outside of the hospital (45), and studies reporting serotyping data on samples obtained from either sterile sites (aligned with definition of invasive CAP) or non-sterile sites (non-invasive CAP) were included. Given the overall limited number of studies on this outcome in children aged ≤5 years, no distinction was made between non-invasive and invasive CAP, and the minimal number of reported serotyped isolates was set at 20 instead of 30.
Further details on inclusion/exclusion criteria and study selection workflow are provided in Supplementary methods, Supplementary Tables S1–S3.
2.3 Data extraction and analysis
The contribution of each serotype to a certain clinical manifestation was determined by calculating pooled percentage averages for each serotype using the following formula:
whereby the “sum” corresponds to the total number of samples across studies included in the corresponding analysis.
For each clinical manifestation, studies were categorized into 2 vaccine periods—post-PCV7 or post-PHiD-CV/PCV13 (pooled)—based on information provided in the publications. To increase robustness and address limitations in interpreting data from settings with low PCV uptake, the primary analysis for each clinical manifestation was restricted to data from countries where the PCV was implemented through infant NIPs; studies from countries with PCV introduction limited to private markets were excluded. A sensitivity analysis was conducted for each clinical manifestation on all identified studies, including those in countries that implemented PCVs in the private market only. Furthermore, subgroup analyses on all eligible studies were conducted based on the PCV product (PHiD-CV or PCV13) for each clinical manifestation.
Serotype-specific pooled percentage averages were reported only if data from at least 5 studies were available for that specific serotype, to ensure that serotype distribution was based on a sufficiently robust number of studies and to reduce the influence of isolated or potentially biased findings. This criterion was not applied for CAP-related analyses given the limited number of studies. Similarly, it was not applied to the subgroup analyses per PCV product.
All analyses were performed with R studio and were descriptive. No formal statistical testing was performed because of the methodological heterogeneity in design, sampling methods, and population selection of the included studies. The data extraction procedure and study categorization are detailed in Supplementary methods.
3 Results
3.1 Study selection and characteristics
After screening 3,822 publications, 126 studies were selected for data extraction, of which 109 were included in the final analysis. The main reason for study exclusion was the lack of sufficient details for further analysis. Among the selected studies, 86 covered serotype distribution data in children ≤5 years. Of these, 47 reported data on IPD (46–92), 30 on AOM (93–122), and 9 on CAP (123–131) (Figure 1). Of the 86 studies included, most were conducted in Europe (n = 42), followed by Asia (n = 21), South America (n = 7), North America (n = 6), Africa (n = 5), the Middle East (n = 4), and Australia (n = 1).
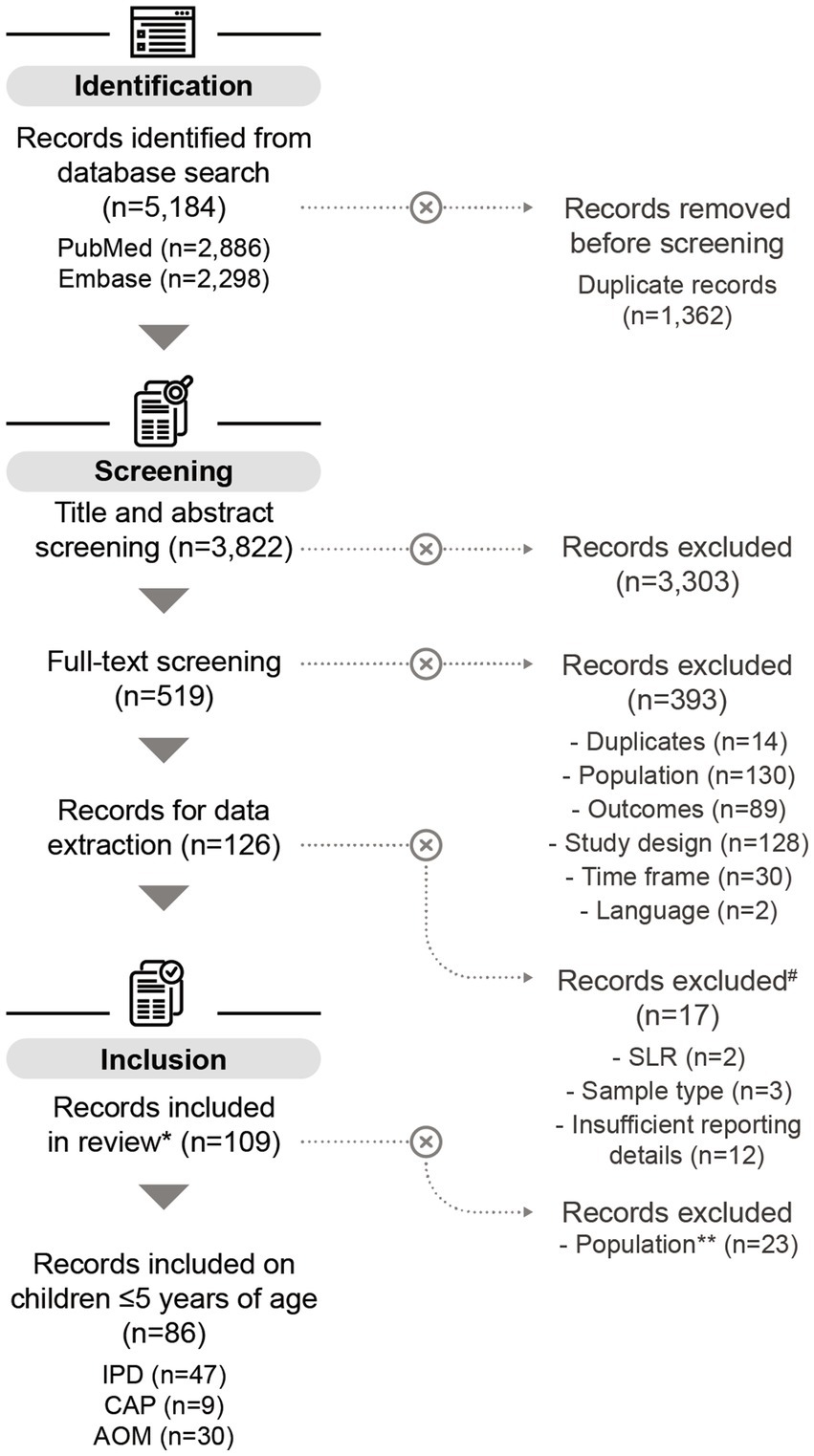
Figure 1. PRISMA flow chart of the systematic literature search. #SLRs on IPD, records on AOM that exclusively reported serotypes from nasopharyngeal samples, and records with insufficient reporting details, were excluded from the data analysis. More information can be found in the methods section. *Some of the records included in this review also contain data on adults ≥65 years (n = 11), but these datapoints were not included in the analysis on children ≤5 years. **Records containing only data on adults ≥65 years were excluded for the purpose of this publication. n, number of records; SLR, systematic literature review; IPD, invasive pneumococcal disease; CAP, community-acquired pneumonia; AOM, acute otitis media.
3.2 IPD
3.2.1 IPD study characteristics
Among the 47 IPD-related publications, 13 reported data following PCV7 implementation and 38 following PHiD-CV or PCV13 (Supplementary Table S4); 4 reported data following both PCV7 and PHiD-CV/PCV13. Overall, serotyping data from 15,511 isolates from IPD cases were extracted from the 38 post-PHiD-CV/PCV13 studies. Most of these (32/38 studies, 84%) were conducted in a setting where the PCV was implemented through an infant NIP, and where PCV7 had been used previously. Most of the included post-PHiD-CV/PCV13 studies (32/38 studies, 84%) contained post-PCV13 data (1–9 years post-introduction), and 8 (21%) contained post-PHiD-CV data (2–6 years). Two studies were conducted in a setting where both PHiD-CV and PCV13 were implemented, each providing separable data per PCV product. In one study, data were indistinguishable due to the concurrent use of both PCV products and could thus not be used for the stratified analysis per PCV product. One post-PCV13 study presented inconsistent data that rendered it unsuitable for the stratified analysis per PCV product.
3.2.2 Serotype distribution in children with IPD post-PHiD-CV/PCV13 uptake
Post-PHiD-CV/PCV13 implementation through infant NIPs (primary analysis, 32 studies), the top 10 serotypes responsible for causing IPD were 12F (pooled percentage average of 8.9%), 24F (8.6%), 19A (6.9%), 6A (6.0%), 33F (5.5%), 1 (5.4%), 10A (5.0%), 3 (4.9%), 15A (4.6%), and 22F (4.4%) (Figure 2A, Supplementary Table S5). The sensitivity analysis, including 6 additional studies where the PCV was introduced to the private market only (38 studies), showed a shift in the rankings of these serotypes. In this analysis, serotype 19A (9.9%) was the leading serotype, followed by 24F (8.4%), 12F (8.3%), 1 (6.5%), 33F (5.2%), 3 (5.1%), 6A (4.9%), 10A (4.7%), 15A (4.5%), and 22F (4.2%) (Supplementary Figure S1). Overall, this suggests that vaccine implementation through NIPs, and consequently increasing vaccine uptake, provided additional impact on vaccine-type disease, particularly on serotype 19A. Nevertheless, post-PHiD-CV/PCV13, serotypes 19A, 6A, and 3, included in PCV13, and serotype 1, included in both PHiD-CV and PCV13, still contributed frequently to remaining IPD (Figure 2A, Supplementary Figure S1).
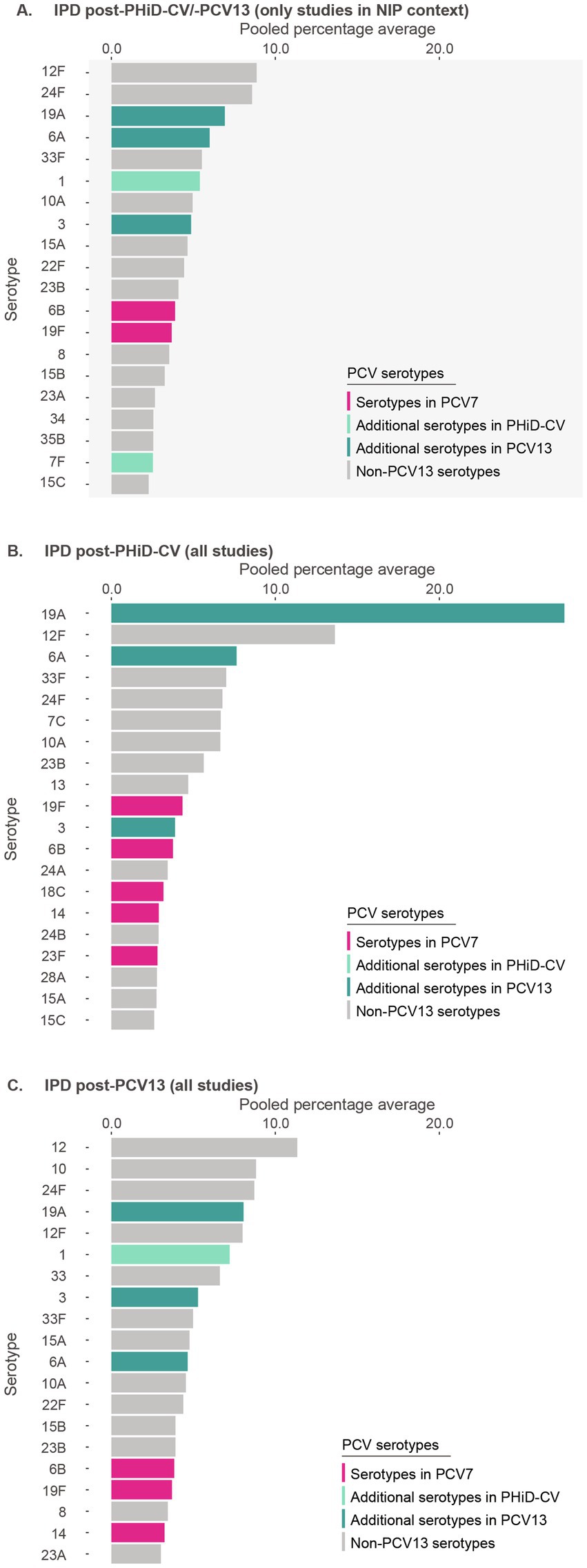
Figure 2. Serotype distribution in invasive pneumococcal disease among children ≤5 years of age (A) post-PHiD-CV/PCV13 implementation through infant national immunization programs (n = 32), (B) post-PHiD-CV (n = 7), and (C) post-PCV13 (n = 31) uptake in infants (either through infant national immunization programs or private markets). The top 20 serotypes are shown. Serotypes are represented by colors corresponding to the lowest valency PCV product in which they are included. In the PCV legend, the additional serotypes included in the product are relative to the next lower valency product. Pooled percentage averages were calculated for each serotype individually, thus the sum of all serotypes may exceed 100%. For panel A, serotype-specific pooled percentage averages were calculated only if 5 or more studies reported on the respective serotype. For panel B and C, the pooled percentage averages were calculated irrespective of the number of studies reporting on it. For panel B, although the legend includes all PCVs, no PHiD-CV-specific serotypes were identified in the top-20 serotypes. IPD, invasive pneumococcal disease; n, number of studies that were included in the analysis; NIP, national immunization program; PCV, pneumococcal conjugate vaccine; PCV7, 7-valent PCV; PCV13, 13-valent PCV; PHiD-CV, pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine.
In PHiD-CV settings, serotype 19A was prominently reported (27.6%), followed by serotypes 6A (7.6%), 3 (3.9%), and 1 (1.0%) (Figure 2B). In PCV13 settings, serotype 19A still contributed to disease, but at much lower frequency (8.1%) (Figure 2C), with differing contributions for serotypes 6A (4.7%), 3 (5.3%), and 1 (7.3%).
Post-PCV7 in NIP settings, 19A was identified as the leading serotype (24.7%), followed by serotype 24F (15.0%) (Supplementary Figure S2).
3.3 AOM
3.3.1 AOM study characteristics
Among the 30 included AOM-related publications, 22 reported serotype distribution data following the adoption of PCV7 and 8 following PHiD-CV or PCV13 (Supplementary Table S4). Two studies reported data from both PCV periods. Overall, data from 731 serotyped isolates from AOM cases were extracted from the 8 post-PHiD-CV/PCV13 studies, which were all conducted in NIP setting. Of these, 5 studies (63%) were in a setting of PCV13 use (4–8 years post-introduction), 2 (25%) in a setting of PHiD-CV use (6–7 years), and 1 in context of mixed PHiD-CV/PCV13 use (4 years). The post-PHiD-CV studies were conducted in locations without previous PCV7 use, while the PCV13 studies were in a location with previous PCV7 use.
3.3.2 Serotype distribution in children with AOM post-PHiD-CV/PCV13 uptake
Post-PHiD-CV/PCV13 implementation, 8 serotypes were reported: 19F (11.9%), 3 (8.5%), 19A (6.9%), 23A (5.0%), 11A (4.8%), 35B (4.4%), 23B (4.0%), and 21 (2.8%) (Figure 3A, Supplementary Table S6). This suggests that serotype 19F, included in both PHiD-CV and PCV13, as well as serotypes 3 and 19A, included in PCV13, contributed frequently to remaining AOM.
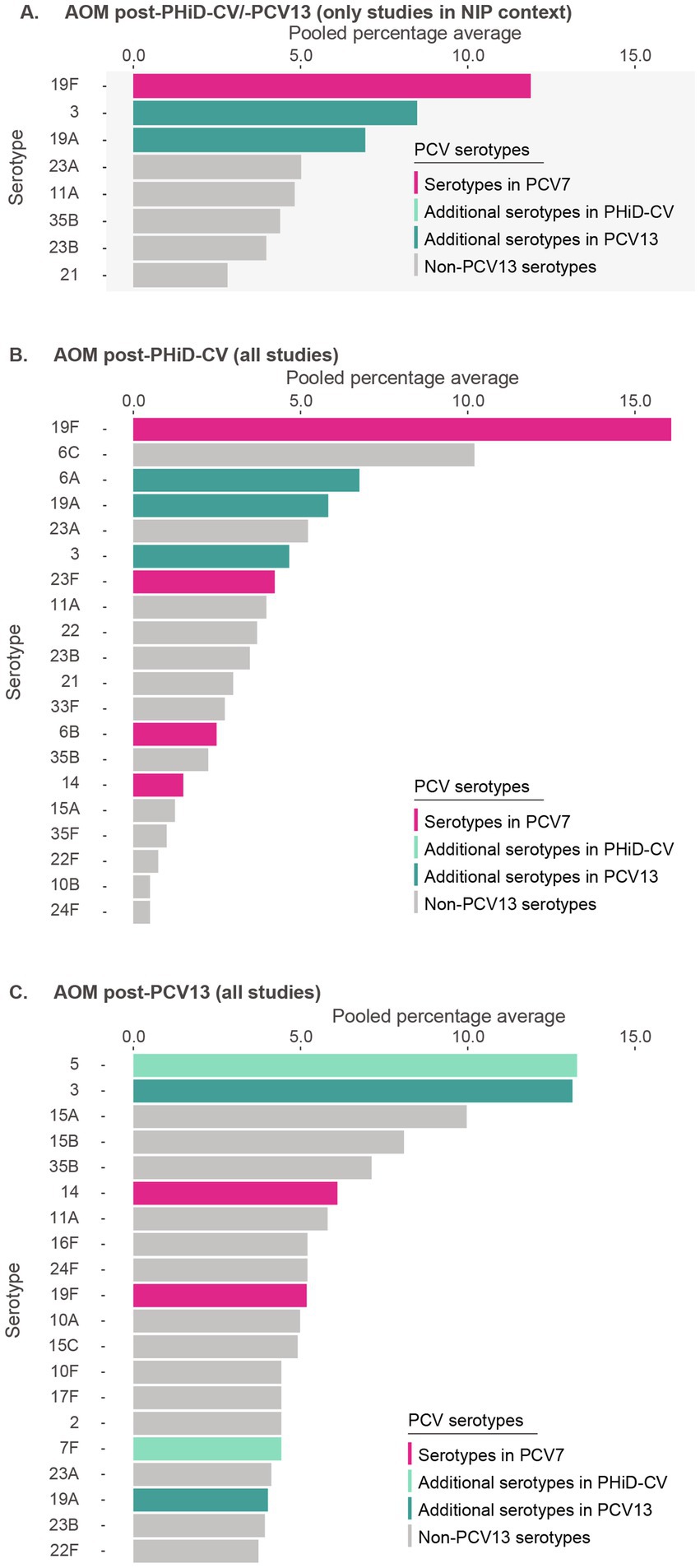
Figure 3. Serotype distribution in acute otitis media among children ≤5 years of age (A) post-PHiD-CV/PCV13 implementation through infant national immunization programs (n = 8), (B) post-PHiD-CV (n = 2), and (C) post-PCV13 (n = 5) uptake in infants (either through infant national immunization programs or private markets). The top 20 serotypes are shown. Serotypes are represented by colors corresponding to the lowest valency PCV product in which they are included. In the PCV legend, the additional serotypes included in the product are relative to the next lower valency product. Pooled percentage averages were calculated for each serotype individually, thus the sum of all serotypes may exceed 100%. For panel A, serotype-specific pooled percentage averages were calculated only if 5 or more studies reported on the respective serotype. For panel B and C, the pooled percentage averages were calculated irrespective of the number of studies reporting on it. AOM, acute otitis media; n, number of studies that were included in the analysis; NIP, national immunization program; PCV, pneumococcal conjugate vaccine; PCV7, 7-valent PCV; PCV13, 13-valent PCV; PHiD-CV, pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine.
In PHiD-CV settings, the contribution of serotypes 19F, 3, and 19A was 16.1, 4.7, and 5.8%, respectively (Figure 3B). In PCV13 settings, their contribution was 5.9, 14.8, and 4.5%, respectively (Figure 3C).
Post-PCV7, serotype 19A clearly dominated rankings (30.5%), followed by serotypes 3 (9.2%) and 19F (7.7%) (Supplementary Figure S3).
3.4 CAP
3.4.1 CAP study characteristics
Nine CAP-related studies were identified, of which 6 were performed following the adoption of PCV7 and 3 following PHiD-CV or PCV13 (Supplementary Table S4). Most CAP studies identified Spn from blood cultures, confirming bacteremic CAP cases. Overall, data from 235 serotyped isolates from CAP cases were extracted post-PHiD-CV/PCV13, all from studies in NIP context. Of the 3 post-PHiD-CV/PCV13 studies, 1 (33%) contained data in PHiD-CV setting (5 years of use), and 2 (67%) in PCV13 setting (6–7 years). The post-PHiD-CV study was conducted in a location without previous PCV7 use; the PCV13 studies were conducted in a location with previous PCV7 use.
3.4.2 Serotype distribution in children with CAP post-PHiD-CV/PCV13 uptake
Post-PHiD-CV/PCV13 adoption, serotypes 19A (18.2%) and 1 (16.6%) were the most frequently reported serotypes in CAP cases. These were followed by serotypes 7F (6.2%), 6A (5.1%), 35B (5.1%), 16F (4.7%), 22F (4.6%), 23B (4.6%), 8 (4.3%), and 11A (4.3%) (Figure 4, Supplementary Table S7).
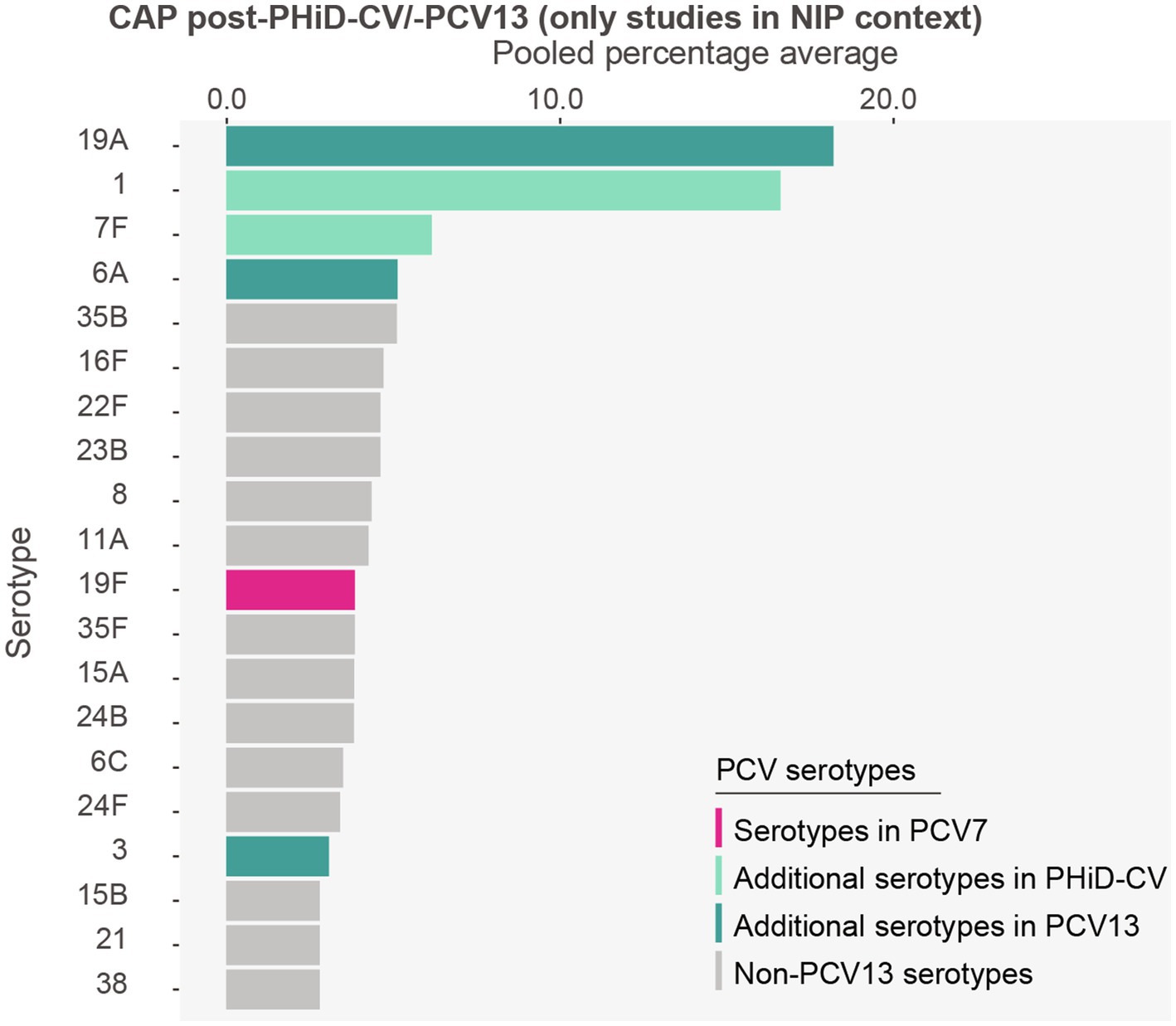
Figure 4. Serotype distribution in community-acquired pneumonia among children ≤5 years of age post-PHiD-CV/-PCV13 implementation through infant national immunization programs (n = 3). The top 20 serotypes are shown. Serotypes are represented by colors corresponding to the lowest valency PCV product in which they are included. In the PCV legend, the additional serotypes included in the product are relative to the next lower valency product. Pooled percentage averages were calculated for each serotype individually, thus the sum of all serotypes may exceed 100%. Serotype-specific pooled percentage averages were calculated irrespective of the number of studies reporting on it. Given the limited number of studies on CAP, results from the subgroup analyses per PCV product need to be interpreted with caution and are included in the Supplementary results. CAP, community-acquired pneumonia; n, number of studies that were included in the analysis; PCV, pneumococcal conjugate vaccine; PCV7, 7-valent PCV; PHiD-CV, pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine; PCV13, 13-valent PCV.
Serotype 19A was the main serotype in the PHiD-CV setting (28.2%) (Supplementary Figure S4), while serotype 1 was predominant in PCV13 settings (24.2%) (Supplementary Figure S5).
Post-PCV7, serotypes 1 (21.0%) and 19A (19.7%) were predominantly identified (Supplementary Figure S6).
4 Discussion
Our SLR indicates that despite widespread PCV implementation, some vaccine serotypes were still frequently reported. Particularly serotype 19A, which dominated rankings across all clinical manifestations post-PCV7, was still commonly identified, although its pooled percentage average appeared reduced in PCV13 settings. Serotype 3 continued to be regularly detected in IPD and AOM cases post-PHiD-CV/PCV13, across both PHiD-CV and PCV13 settings. Serotype 1 was another highly common vaccine serotype detected in IPD and CAP cases in the post-PCV7 era, that persisted post-PHiD-CV/PCV13 adoption (mostly in PCV13 settings); in contrast, this serotype contributed minimally to AOM in either of the 2 PCV periods. In AOM, serotype 19F, which was already included in PCV7, remained prevalent post-PHiD-CV/PCV13 implementation, though mostly in PHiD-CV settings without previous PCV7 use.
Our study also indicated an increase in several non-PCV13 serotypes post-PHiD-CV/PCV13 introduction compared to the post-PCV7 era. In IPD, serotypes 12F and 24F were frequently reported. Common non-PCV13 serotypes detected in AOM and CAP were 11A, 35B, and 23B.
This SLR presents a comprehensive summary that may extend beyond the pivotal PCV Review of Impact Evidence (PRIME) SLR on IPD conducted by the WHO in 2017 (132). Our review covers a broader scope of serotype-specific data across the 3 major clinical manifestations of pneumococcal disease—namely IPD, AOM, and CAP. While the PRIME report, based on published impact and effectiveness data up to 2017, underscored the impact of PCV13 on serotype 19-mediated IPD, it noted insufficient evidence to evaluate the impact on serotypes 3 and 6A. By extending our analyses up to 2020, we potentially captured more recent insights on these serotypes, and on those not covered in the earlier PRIME analysis.
Parallels of our IPD data were also noted with the ongoing, global Pneumococcal Serotype Replacement and Distribution Estimation (PSERENADE) project, (22, 133) a significant IPD study that used “raw” surveillance data potentially less influenced by serotype reporting bias (22, 133). Similar to our observations, these recent data collected between 2015 and 2018 show that serotypes 19A and 3 were the leading serotypes, with serotype 19A mainly detected in PHiD-CV settings and serotype 3 in both PHiD-CV and PCV13 settings. Serotype 6C was among the leading serotypes at PHiD-CV sites in the PSERENADE study, but scarcely reported in both PHiD-CV and PCV13 settings in our IPD analysis. In contrast to our analysis, serotypes 6A and 1 were contributing minimally to IPD in the PSERENADE study, which may be due to differences in geographical diversity or maturity of the PCV programs. Our IPD findings also align with other SLRs/meta-analyses that showed that serotypes 19A and 3 were the main IPD serotypes post-PHiD-CV/PCV13 (41, 134–137). Furthermore, several of the most prominent non-PCV13 serotypes reported by the PSERENADE project (22, 133) and another SLR on individual serotypes (41)—in particular 10A, 12F, 22F, 24F, and 33F—were also highly ranked in our IPD analysis.
AOM and CAP represent the largest burden of pneumococcal disease, but serotype distribution data in these manifestations are scarce. Our AOM analysis confirmed the observation of a recent AOM-focused SLR that 19F, 3, and 19A were the predominant PCV13 serotypes associated with AOM post-PHiD-CV/PCV13 implementation (138).
While our CAP analysis should be interpreted with caution due to the low number of included studies, PCV13 serotypes 1 and 19A still seemed to dominate rankings in the post-PHiD-CV/PCV13 period, similar to the post-PCV7 period. Serotype 3, commonly reported post-PCV7 in CAP cases, was nearly not reported post-PHiD-CV/PCV13. Given that our search strategy for CAP studies mostly retrieved publications on invasive cases, our CAP data might be largely representative of this smaller CAP population (139). Importantly, the CAP and IPD results might be partially overlapping, since 12–16% of IPD patients aged <2 years are estimated to have invasive CAP (140).
The persistence of disease caused by particular vaccine serotypes is probably multifactorial. One factor may be the variation in PCV effectiveness for different serotypes, e.g., PCV13 effectiveness against serotype 3 has not been consistently demonstrated (134, 141) and PHiD-CV has not been effective at controlling 19A-mediated disease (11, 142). Although our analysis excluded studies involving immunocompromised children and those with other comorbidities, which are well-known risk factors for vaccine failure (143–145), the remaining vaccine-type disease may be partially attributed to unreported underlying comorbidities or other individual risk factors (146–149). Lastly, studies have suggested that vaccine and antimicrobial pressure can both induce clonal changes and capsular switching, leading to the genetic transformation of virulent vaccine serotypes into variants that escape vaccine-mediated immunity, thereby being able to occupy the ecological niche (150–156).
PCV15 and PCV20 were recently approved for use in children and adults in different countries. Also the first pneumococcal vaccine specifically designed for adults, a 21-valent PCV (PCV21, Capvaxive, Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc., [MSD]), has been recently introduced (157). In addition to these, some regionally used PCVs are contributing to the expanding pneumococcal vaccine landscape, though their broader impact remains more limited (158, 159). Considering that the full impact of a PCV is only evident after about 4 years, provided high immunization rates are achieved, it is too soon to evaluate changes in pneumococcal epidemiology following the introduction of these new PCVs (19). In addition, since PCV15 and PCV20 were licensed based on immunological non-inferiority compared to PCV13, their potential benefit on disease remains to be determined (157, 160–164). Current pneumococcal vaccine technologies have also been shown to exert carrier-induced immune suppression—a phenomenon in which the antibody response to the carrier protein compromises the response to the serotype polysaccharide—which increases as the number of glycoconjugates (valencies) included in PCVs increases (165). This may explain why phase 3 trials evaluating 3- and 4-dose infant vaccination series of PCV20 showed that immune responses to some serotypes did not meet some pre-specified statistical non-inferiority criteria compared to PCV13 (162, 163). Our results also highlight differences in pneumococcal epidemiology across the different clinical manifestations. Current PCVs do not cover the full spectrum of pneumococcal diseases, potentially leaving gaps in protection. Additional factors amplifying serotype diversity, such as geographical location and the age of the at-risk population (41, 135), make it challenging for current vaccines to provide complete protection. Therefore, novel pneumococcal vaccination strategies and technologies are needed that can provide enhanced and broader protection.
Our review has several limitations. As the search on studies was limited to those published up to 2020, the collected data might not reflect the most up-to-date pneumococcal epidemiology. Nevertheless, this time restriction was chosen to avoid introducing effects of the COVID-19 pandemic, as the social preventive measures that were applied led to an intermittent global decrease in Spn transmission and subsequent rebound effect (16). Several studies evaluating serotype distribution in IPD cases after relaxation of the preventive measures indicated a generally similar serotype distribution as before the pandemic. IPD was mainly caused by non-PCV13 serotypes, but several vaccine serotypes (including 3 and 19A) were still prominent (166–170).
It was not feasible to accurately account for the heterogeneity inherent to observational studies. Overall, the study heterogeneity did not allow us to perform a meta-analysis. Instead, serotype-specific pooled percentage averages were calculated for this descriptive analysis, which considered for each serotype the sizes of the studies reporting on the respective serotype. This approach accounts for the varying availability of data for each serotype across studies, which allows for an estimation that reflects the prevalence of each serotype within the subset of studies reporting on it. However, it is a less robust approach compared to meta-analysis, and it does not allow for confirmatory statistical analyses or conclusive outcomes. Additionally, it may disproportionally bias the serotype distribution to certain serotypes with higher reporting rates. For our main post-PHiD-CV/PCV13 analyses, we focused only on serotypes that were reported by at least 5 studies, to reduce the impact of isolated or potentially biased findings. Nevertheless, this may have resulted in the omission of an important emerging serotype reported in a small number of studies. In addition, our analyses are largely driven by studies in high-income countries, which mostly have high-quality surveillance systems in place. As serotype circulation, vaccination programs and uptake, and pneumococcal disease burden differ in developing countries, this may limit the generalizability of our findings (21, 171). Lastly, the availability of studies conducted in PHiD-CV settings was limited and unbalanced in comparison to those conducted in PCV13 settings. Therefore, our analyses remained descriptive, and the outcomes and comparisons should be interpreted with caution.
In conclusion, while the overall incidence of pneumococcal disease has consistently declined with the introduction of PHiD-CV and PCV13, the serotype distribution responsible for remaining disease has changed, with non-PCV13 serotypes becoming predominant. Several vaccine serotypes—in particular serotypes 19A, 1, and 3—are still responsible for a substantial proportion of remaining invasive and non-invasive pneumococcal disease. Continued monitoring of serotype evolution therefore remains critical to appraise optimal vaccination strategies for the prevention of pneumococcal disease, including new vaccine technologies that could provide broader and improved protection in children.
Trademark statement
Synflorix is a trademark licensed to or owned by GSK. Prevenar/Prevnar, Prevenar 13/Prevnar 13 and Prevenar 20/Prevnar 20 are trademarks of Pfizer Inc. Vaxneuvance and Capvaxive are trademarks of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc. (MSD).
Author contributions
PI: Conceptualization, Data curation, Formal analysis, Writing – review & editing. MA: Conceptualization, Data curation, Formal analysis, Writing – review & editing. DB: Conceptualization, Data curation, Formal analysis, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by GSK, which was involved in all stages of the conduct of this review and covered the costs associated with the development and publication of this manuscript.
Acknowledgments
The authors would like to thank P95 for performing the systematic literature review search and analyses, and Naveen Karkada (GSK) for providing statistical support. The authors would also like to thank the Akkodis Belgium platform for writing and editorial assistance (by Elisabeth Rossaert), manuscript coordination, and design support, on behalf of GSK.
Conflict of interest
PI, MA, and DB are employed by GSK and hold financial equities in GSK.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1544359/full#supplementary-material
References
1. Lynch, JP, and Zhanel, GG. Streptococcus pneumoniae: epidemiology, risk factors, and strategies for prevention. Semin Respir Crit Care Med. (2009) 30:189–209. doi: 10.1055/s-0029-1202938
2. Walker, CLF, Rudan, I, Liu, L, Nair, H, Theodoratou, E, Bhutta, ZA, et al. Global burden of childhood pneumonia and diarrhoea. Lancet. (2013) 381:1405–16. doi: 10.1016/s0140-6736(13)60222-6
3. File, TM Jr. Streptococcus pneumoniae and community-acquired pneumonia: a cause for concern. Am J Med. (2004) 117 Suppl 3A:39S–50S. doi: 10.1016/j.amjmed.2004.07.007
4. Bergenfelz, C, and Hakansson, AP. Streptococcus pneumoniae otitis media pathogenesis and how it informs our understanding of vaccine strategies. Curr Otorhinolaryngol Rep. (2017) 5:115–24. doi: 10.1007/s40136-017-0152-6
5. Ngo, CC, Massa, HM, Thornton, RB, and Cripps, AW. Predominant bacteria detected from the middle ear fluid of children experiencing otitis media: a systematic review. PLoS One. (2016) 11:e0150949. doi: 10.1371/journal.pone.0150949
6. Drijkoningen, JJC, and Rohde, GGU. Pneumococcal infection in adults: burden of disease. Clin Microbiol Infect. (2014) 20:45–51. doi: 10.1111/1469-0691.12461
7. van Mens, SP, van Deursen, AMM, de Greeff, SC, de Melker, HE, Schouls, LM, van der Ende, A, et al. Bacteraemic and non-bacteraemic/urinary antigen-positive pneumococcal community-acquired pneumonia compared. Eur J Clin Microbiol Infect Dis. (2015) 34:115–22. doi: 10.1007/s10096-014-2209-5
8. O’Brien, KL, Wolfson, LJ, Watt, JP, Henkle, E, Deloria-Knoll, M, McCall, N, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. (2009) 374:893–902. doi: 10.1016/S0140-6736(09)61204-6
9. Russell, F, Sanderson, C, Temple, B, and Mulholland, K. (2011). Global review of the distribution of pneumococcal disease by age and region. Available online at: https://blogs.lshtm.ac.uk/vaccineschedules/files/6.-Russel-review-age-specific-epidemiology-PCV-schedules-session-nov11.pdf. [Accessed April 14, 2025].
10. Ganaie, F, Saad, JS, McGee, L, van Tonder, AJ, Bentley, SD, Lo, SW, et al. A new pneumococcal capsule type, 10D, is the 100th serotype and has a large cps fragment from an oral Streptococcus. MBio. (2020) 11:e00937-20. doi: 10.1128/mBio.00937-20
11. World Health Organization. Pneumococcal conjugate vaccines in infants and children under 5 years of age: WHO position paper. Wkly Epidemiol Rec. (2019) 94:85–103.
12. Hausdorff, WP, Bryant, J, Paradiso, PR, and Siber, GR. Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use, part I. Clin Infect Dis. (2000) 30:100–21. doi: 10.1086/313608
13. Johnson, HL, Deloria-Knoll, M, Levine, OS, Stoszek, SK, Freimanis Hance, L, Reithinger, R, et al. Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: the pneumococcal global serotype project. PLoS Med. (2010) 7:e1000348. doi: 10.1371/journal.pmed.1000348
14. Hausdorff, WP, Bryant, J, Kloek, C, Paradiso, PR, and Siber, GR. The contribution of specific pneumococcal serogroups to different disease manifestations: implications for conjugate vaccine formulation and use, part II. Clin Infect Dis. (2000) 30:122–40. doi: 10.1086/313609
15. Hausdorff, WP, Feikin, DR, and Klugman, KP. Epidemiological differences among pneumococcal serotypes. Lancet Infect Dis. (2005) 5:83–93. doi: 10.1016/s1473-3099(05)01280-6
16. Brueggemann, AB, Jansen van Rensburg, MJ, Shaw, D, McCarthy, ND, Jolley, KA, Maiden, MCJ, et al. Changes in the incidence of invasive disease due to Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis during the COVID-19 pandemic in 26 countries and territories in the invasive respiratory infection surveillance initiative: a prospective analysis of surveillance data. Lancet Digit Health. (2021) 3:e360–70. doi: 10.1016/s2589-7500(21)00077-7
17. Liñares, J, Ardanuy, C, Pallares, R, and Fenoll, A. Changes in antimicrobial resistance, serotypes and genotypes in Streptococcus pneumoniae over a 30-year period. Clin Microbiol Infect. (2010) 16:402–10. doi: 10.1111/j.1469-0691.2010.03182.x
18. Weinberger, DM, Malley, R, and Lipsitch, M. Serotype replacement in disease after pneumococcal vaccination. Lancet. (2011) 378:1962–73. doi: 10.1016/S0140-6736(10)62225-8
19. Hanquet, G, Krizova, P, Dalby, T, Ladhani, SN, Nuorti, JP, Danis, K, et al. Serotype replacement after introduction of 10-valent and 13-valent pneumococcal conjugate vaccines in 10 countries, Europe. Emerg Infect Dis. (2022) 28:137–8. doi: 10.3201/eid2801.210734
20. World Health Organization. (2007). Pneumococcal conjugate vaccine for childhood immunization - WHO position paper. Available online at: https://apps.who.int/iris/handle/10665/240895. [Accessed April 14, 2025].
21. World Health Organization. (2022). Pneumococcal conjugate immunization (PCV3) coverage estimates by World Bank income group. Available online at: https://apps.who.int/gho/data/view.main.PCV3vREGWB?lang=en. [Accessed April 14, 2025].
22. Knoll, MD, Bennett, JC, Garcia Quesada, M, Kagucia, EW, Peterson, ME, Feikin, DR, et al. Global landscape review of serotype-specific invasive pneumococcal disease surveillance among countries using PCV10/13: the pneumococcal serotype replacement and distribution estimation (PSERENADE) project. Microorganisms. (2021) 9:742. doi: 10.3390/microorganisms9040742
23. de Oliveira, LH, Camacho, LAB, Coutinho, ESF, Martinez-Silveira, MS, Carvalho, AF, Ruiz-Matus, C, et al. Impact and effectiveness of 10 and 13-valent pneumococcal conjugate vaccines on hospitalization and mortality in children aged less than 5 years in Latin American countries: a systematic review. PLoS One. (2016) 11:e0166736. doi: 10.1371/journal.pone.0166736
24. Mrkvan, T, Pelton, SI, Ruiz-Guiñazú, J, Palmu, AA, and Borys, D. Effectiveness and impact of the 10-valent pneumococcal conjugate vaccine, PHiD-CV: review of clinical trials and post-marketing experience. Expert Rev Vaccines. (2018) 17:797–818. doi: 10.1080/14760584.2018.1516551
25. Ngocho, JS, Magoma, B, Olomi, GA, Mahande, MJ, Msuya, SE, de Jonge, MI, et al. Effectiveness of pneumococcal conjugate vaccines against invasive pneumococcal disease among children under five years of age in Africa: a systematic review. PLoS One. (2019) 14:e0212295. doi: 10.1371/journal.pone.0212295
26. Izurieta, P, Scherbakov, M, Nieto Guevara, J, Vetter, V, and Soumahoro, L. Systematic review of the efficacy, effectiveness and impact of high-valency pneumococcal conjugate vaccines on otitis media. Hum Vaccin Immunother. (2022) 18:2013693. doi: 10.1080/21645515.2021.2013693
27. Izurieta, P, Bahety, P, Adegbola, R, Clarke, C, and Hoet, B. Public health impact of pneumococcal conjugate vaccine infant immunization programs: assessment of invasive pneumococcal disease burden and serotype distribution. Expert Rev Vaccines. (2018) 17:479–93. doi: 10.1080/14760584.2018.1413354
28. Wahl, B, O’Brien, KL, Greenbaum, A, Majumder, A, Liu, L, Chu, Y, et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000-15. Lancet Glob Health. (2018) 6:e744–57. doi: 10.1016/S2214-109X(18)30247-X
29. Liese, JG, Silfverdal, SA, Giaquinto, C, Carmona, A, Larcombe, JH, Garcia-Sicilia, J, et al. Incidence and clinical presentation of acute otitis media in children aged <6 years in European medical practices. Epidemiol Infect. (2014) 142:1778–88. doi: 10.1017/s0950268813002744
30. Rodrigues, CMC, and Groves, H. Community-acquired pneumonia in children: the challenges of microbiological diagnosis. J Clin Microbiol. (2018) 56:e01318–7. doi: 10.1128/jcm.01318-17
31. Feikin, DR, Hammitt, LL, Murdoch, DR, O’Brien, KL, and Scott, JAG. The enduring challenge of determining pneumonia etiology in children: considerations for future research priorities. Clin Infect Dis. (2017) 64:S188–96. doi: 10.1093/cid/cix143
32. Blomgren, K, and Pitkäranta, A. Current challenges in diagnosis of acute otitis media. Int J Pediatr Otorhinolaryngol. (2005) 69:295–9. doi: 10.1016/j.ijporl.2004.09.012
33. Elberse, K, van Mens, S, Cremers, AJ, Meijvis, SCA, Vlaminckx, B, de Jonge, MI, et al. Detection and serotyping of pneumococci in community acquired pneumonia patients without culture using blood and urine samples. BMC Infect Dis. (2015) 15:56. doi: 10.1186/s12879-015-0788-0
34. Izurieta, P, and Borys, D. Serotype distribution of invasive and non-invasive pneumococcal disease in adults ≥65 years of age following the introduction of 10-and 13-valent pneumococcal conjugate vaccines in infant national immunization programs: a systematic literature review. Front. Public Health. (2025) 13. doi: 10.3389/fpubh.2025.154433
35. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
36. Shaw, D, Abad, R, Amin-Chowdhury, Z, Bautista, A, Bennett, D, Broughton, K, et al. Trends in invasive bacterial diseases during the first 2 years of the COVID-19 pandemic: analyses of prospective surveillance data from 30 countries and territories in the IRIS consortium. Lancet Digit Health. (2023) 5:e582–93. doi: 10.1016/s2589-7500(23)00108-5
37. Ota, MOC, Badur, S, Romano-Mazzotti, L, and Friedland, LR. Impact of COVID-19 pandemic on routine immunization. Ann Med. (2021) 53:2286–97. doi: 10.1080/07853890.2021.2009128
38. Bigouette, JP, Callaghan, AW, Donadel, M, Porter, AM, Rosencrans, L, Lickness, JS, et al. Effects of COVID-19 on vaccine-preventable disease surveillance Systems in the World Health Organization African Region, 2020. Emerg Infect Dis. (2022) 28:S203–7. doi: 10.3201/eid2813.220088
39. Principi, N, Autore, G, Ramundo, G, and Esposito, S. Epidemiology of respiratory infections during the COVID-19 pandemic. Viruses. (2023) 15:15. doi: 10.3390/v15051160
40. Balsells, E, Dagan, R, Yildirim, I, Gounder, PP, Steens, A, Munoz-Almagro, C, et al. The relative invasive disease potential of Streptococcus pneumoniae among children after PCV introduction: a systematic review and meta-analysis. J Infect. (2018) 77:368–78. doi: 10.1016/j.jinf.2018.06.004
41. Balsells, E, Guillot, L, Nair, H, and Kyaw, MH. Serotype distribution of Streptococcus pneumoniae causing invasive disease in children in the post-PCV era: a systematic review and meta-analysis. PLoS One. (2017) 12:e0177113. doi: 10.1371/journal.pone.0177113
42. Randle, E, Ninis, N, and Inwald, D. Invasive pneumococcal disease. Arch Dis Child Educ Pract Ed. (2011) 96:183–90. doi: 10.1136/adc.2010.191718
43. National Institute for Health and Care Excellence. (2021). Clinical knowledge summaries (CKS) acute otitis media. Available online at: https://cks.nice.org.uk/topics/otitis-media-acute/. [Accessed April 14, 2025].
44. Lieberthal, AS, Carroll, AE, Chonmaitree, T, Ganiats, TG, Hoberman, A, Jackson, MA, et al. The diagnosis and management of acute otitis media. Pediatrics. (2013) 131:e964–99. doi: 10.1542/peds.2012-3488
45. National Institute for Health and Care Excellence. (2012). Clinical guideline: pneumonia scope. Available online at: https://www.nice.org.uk/guidance/cg191/documents/pneumonia-final-scope2. [Accessed April 14, 2025].
46. Amin-Chowdhury, Z, Collins, S, Sheppard, C, Litt, D, Fry, NK, Andrews, N, et al. Characteristics of invasive pneumococcal disease caused by emerging serotypes after the introduction of the 13-valent pneumococcal conjugate vaccine in England: a prospective observational cohort study, 2014-2018. Clin Infect Dis. (2020) 71:e235–43. doi: 10.1093/cid/ciaa043
47. Bao, Y, Wang, Q, Yao, K, Xie, G, Gao, W, Huang, L, et al. The changing phenotypes and genotypes of invasive pneumococcal isolates from children in Shenzhen during 2013-2017. Vaccine. (2019) 37:7248–55. doi: 10.1016/j.vaccine.2019.09.069
48. Baxter, R, Aukes, L, Pelton, SI, Yee, A, Klein, NP, Gruber, WC, et al. Impact of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease after introduction into routine pediatric use. J Pediatric Infect Dis Soc. (2021) 10:141–50. doi: 10.1093/jpids/piaa035
49. Berezin, EN, Jarovsky, D, Cardoso, MRA, and Mantese, OC. Invasive pneumococcal disease among hospitalized children in Brazil before and after the introduction of a pneumococcal conjugate vaccine. Vaccine. (2020) 38:1740–5. doi: 10.1016/j.vaccine.2019.12.038
50. Ceyhan, M, Aykac, K, Gurler, N, Ozsurekci, Y, Öksüz, L, Altay Akısoglu, Ö, et al. Serotype distribution of Streptococcus pneumonia in children with invasive disease in Turkey: 2015-2018. Hum Vaccin Immunother. (2020) 16:2773–8. doi: 10.1080/21645515.2020.1747931
51. Cohen, R, Levy, C, Ouldali, N, Goldrey, M, Béchet, S, Bonacorsi, S, et al. Invasive disease potential of pneumococcal serotypes in children after PCV13 implementation. Clin Infect Dis. (2021) 72:1453–6. doi: 10.1093/cid/ciaa917
52. Corcoran, M, Mereckiene, J, Cotter, S, Murchan, S, Cunney, R, and Humphreys, H. Invasive Streptococcus pneumoniae infections and vaccine failures in children in Ireland from the postvaccine era from 2007 to 2018. Pediatr Infect Dis J. (2020) 39:339–44. doi: 10.1097/inf.0000000000002549
53. de Miguel, S, Domenech, M, González-Camacho, F, Sempere, J, Vicioso, D, Sanz, JC, et al. Nationwide trends of invasive pneumococcal disease in Spain (2009-2019) in children and adults during the pneumococcal conjugate vaccine era. Clin Infect Dis. (2021) 73:e3778–87. doi: 10.1093/cid/ciaa1483
54. Desmet, S, Lagrou, K, Wyndham-Thomas, C, Braeye, T, Verhaegen, J, Maes, P, et al. Dynamic changes in paediatric invasive pneumococcal disease after sequential switches of conjugate vaccine in Belgium: a national retrospective observational study. Lancet Infect Dis. (2021) 21:127–36. doi: 10.1016/s1473-3099(20)30173-0
55. Desmet, S, Wouters, I, Van Heirstraeten, L, Beutels, P, Van Damme, P, Malhotra-Kumar, S, et al. In-depth analysis of pneumococcal serotypes in Belgian children (2015–2018): diversity, invasive disease potential, and antimicrobial susceptibility in carriage and disease. Vaccine. (2021) 39:372–9. doi: 10.1016/j.vaccine.2020.11.044
56. Hernández, S, Muñoz-Almagro, C, Ciruela, P, Soldevila, N, Izquierdo, C, Codina, MG, et al. Invasive pneumococcal disease and influenza activity in a pediatric population: impact of PCV13 vaccination in pandemic and nonpandemic influenza periods. J Clin Microbiol. (2019) 57:e00363–19. doi: 10.1128/JCM.00363-19
57. Hernstadt, H, Cheung, A, Hurem, D, Vasilunas, N, Phuong, LK, Quinn, P, et al. Changing epidemiology and predisposing factors for invasive pneumococcal disease at two Australian tertiary hospitals. Pediatr Infect Dis J. (2020) 39:1–6. doi: 10.1097/inf.0000000000002489
58. Iwata, S, Takata, M, Morozumi, M, Miyairi, I, Matsubara, K, and Ubukata, K. Drastic reduction in pneumococcal meningitis in children owing to the introduction of pneumococcal conjugate vaccines: longitudinal analysis from 2002 to 2016 in Japan. J Infect Chemother. (2021) 27:604–12. doi: 10.1016/j.jiac.2020.11.019
59. Izquierdo, C, Ciruela, P, Hernández, S, García-García, JJ, Esteva, C, Moraga-Llop, F, et al. Pneumococcal serotypes in children, clinical presentation and antimicrobial susceptibility in the PCV13 era. Epidemiol Infect. (2020) 148:e279:1–37. doi: 10.1017/s0950268820002708
60. Kambire, D, Soeters, HM, Ouedraogo-Traore, R, Medah, I, Sangare, L, Yameogo, I, et al. Early impact of 13-valent pneumococcal conjugate vaccine on pneumococcal meningitis-Burkina Faso, 2014-2015. J Infect. (2018) 76:270–9. doi: 10.1016/j.jinf.2017.12.002
61. Kaplan, SL, Barson, WJ, Lin, PL, Romero, JR, Bradley, JS, Tan, TQ, et al. Invasive pneumococcal disease in children’s hospitals: 2014-2017. Pediatrics. (2019) 144:e20190567. doi: 10.1542/peds.2019-0567
62. Ladhani, SN, Collins, S, Djennad, A, Sheppard, CL, Borrow, R, Fry, NK, et al. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000–17: a prospective national observational cohort study. Lancet Infect Dis. (2018) 18:441–51. doi: 10.1016/S1473-3099(18)30052-5
63. Levy, C, Varon, E, Ouldali, N, Béchet, S, Bonacorsi, S, and Cohen, R. Changes in invasive pneumococcal disease spectrum after 13-valent pneumococcal conjugate vaccine implementation. Clin Infect Dis. (2020) 70:446–54. doi: 10.1093/cid/ciz221
64. Li, M-C, Wang, Y, Zhang, H, Liu, Y, Chen, XJ, Yang, HW, et al. Serotype distribution and clinical characteristics associated with streptococcus pneumoniae among Chinese children and adults with invasive pneumococcal disease: a multicenter observational study. Hum Vaccin Immunother. (2021) 17:146–56. doi: 10.1080/21645515.2020.1757996
65. Massora, S, Lessa, FC, Moiane, B, Pimenta, FC, Mucavele, H, Chaúque, A, et al. Invasive disease potential of Streptococcus pneumoniae serotypes before and after 10-valent pneumococcal conjugate vaccine introduction in a rural area, southern Mozambique. Vaccine. (2019) 37:7470–7. doi: 10.1016/j.vaccine.2019.09.079
66. Metcalf, BJ, Chochua, S, Walker, H, Tran, T, Li, Z, Varghese, J, et al. Invasive pneumococcal strain distributions and isolate clusters associated with persons experiencing homelessness during 2018. Clin Infect Dis. (2020) 72:e948–56. doi: 10.1093/cid/ciaa1680
67. Nakano, S, Fujisawa, T, Ito, Y, Chang, B, Matsumura, Y, Yamamoto, M, et al. Nationwide surveillance of paediatric invasive and non-invasive pneumococcal disease in Japan after the introduction of the 13-valent conjugated vaccine, 2015-2017. Vaccine. (2020) 38:1818–24. doi: 10.1016/j.vaccine.2019.12.022
68. Nicolosi, L, Bozzola, E, Krzysztofiak, A, Pantosti, A, Lancella, L, Bernaschi, P, et al. Serotype distribution of Streptococcus pneumoniae causing invasive pneumococcal disease at bambino Gesù Children’s Hospital in Rome: is it time for a new vaccine? J Pediatr Infect Dis. (2019) 14:013–5. doi: 10.1055/s-0037-1615784
69. Ouldali, N, Varon, E, Levy, C, Angoulvant, F, Georges, S, Ploy, MC, et al. Invasive pneumococcal disease incidence in children and adults in France during the pneumococcal conjugate vaccine era: an interrupted time-series analysis of data from a 17-year national prospective surveillance study. Lancet Infect Dis. (2021) 21:137–47. doi: 10.1016/s1473-3099(20)30165-1
70. Park, DC, Kim, SH, Yong, D, Suh, IB, Kim, YR, Yi, J, et al. Serotype distribution and antimicrobial resistance of invasive and noninvasive Streptococcus pneumoniae isolates in Korea between 2014 and 2016. Ann Lab Med. (2019) 39:537–44. doi: 10.3343/alm.2019.39.6.537
71. Picazo, JJ, Ruiz-Contreras, J, Casado-Flores, J, Negreira, S, Baquero-Artigao, F, Hernández-Sampelayo, T, et al. Impact of 13-valent pneumococcal conjugate vaccination on invasive pneumococcal disease in children under 15 years old in Madrid, Spain, 2007 to 2016: the HERACLES clinical surveillance study. Vaccine. (2019) 37:2200–7. doi: 10.1016/j.vaccine.2019.03.015
72. Soeters, HM, Kambiré, D, Sawadogo, G, Ouédraogo-Traoré, R, Bicaba, B, Medah, I, et al. Impact of 13-valent pneumococcal conjugate vaccine on pneumococcal meningitis, Burkina Faso, 2016-2017. J Infect Dis. (2019) 220:S253–62. doi: 10.1093/infdis/jiz301
73. Ubukata, K, Takata, M, Morozumi, M, Chiba, N, Wajima, T, Hanada, S, et al. Effects of pneumococcal conjugate vaccine on genotypic penicillin resistance and serotype changes, Japan, 2010-2017. Emerg Infect Dis. (2018) 24:2010–20. doi: 10.3201/eid2411.180326
74. Varghese, J, Chochua, S, Tran, T, Walker, H, Li, Z, Snippes Vagnone, PM, et al. Multistate population and whole genome sequence-based strain surveillance of invasive pneumococci recovered in the USA during 2017. Clin Microbiol Infect. (2020) 26:512.e1–512.e10. doi: 10.1016/j.cmi.2019.09.008
75. Wang, Q, Shi, W, Li, Y, Gao, W, Yuan, L, Dong, F, et al. Serotype distribution of Streptococcus pneumoniae isolated from children hospitalized in Beijing children’s hospital (2013–2019). Vaccine. (2020) 38:7858–64. doi: 10.1016/j.vaccine.2020.10.005
76. Hernández-Bou, S, Gomez, B, Mintegi, S, and Garcia-Garcia, JJ Bacteraemia Study Working Group of the Infectious Diseases Working Group of the Spanish Society of Paediatric Emergencies (SEUP). Occult bacteremia etiology following the introduction of 13-valent pneumococcal conjugate vaccine: a multicenter study in Spain. Eur J Clin Microbiol Infect Dis. (2018) 37:1449–55. doi: 10.1007/s10096-018-3270-2
77. Hammitt, LL, Etyang, AO, Morpeth, SC, Ojal, J, Mutuku, A, Mturi, N, et al. Effect of ten-valent pneumococcal conjugate vaccine on invasive pneumococcal disease and nasopharyngeal carriage in Kenya: a longitudinal surveillance study. Lancet. (2019) 393:2146–54. doi: 10.1016/s0140-6736(18)33005-8
78. Luna-Muschi, A, Castillo-Tokumori, F, Deza, MP, Mercado, EH, Egoavil, M, Sedano, K, et al. Invasive pneumococcal disease in hospitalised children from Lima, Peru before and after introduction of the 7-valent conjugated vaccine. Epidemiol Infect. (2019) 147:e91. doi: 10.1017/S0950268819000037
79. Lu, C-Y, Chiang, C-S, Chiu, C-H, Wang, ET, Chen, YY, Yao, SM, et al. Successful control of Streptococcus pneumoniae 19A replacement with a catch-up primary vaccination program in Taiwan. Clin Infect Dis. (2019) 69:1581–7. doi: 10.1093/cid/ciy1127
80. Lee, M-C, and Kuo, K-C. The clinical implication of serotype distribution and drug resistance of invasive pneumococcal disease in children: a single center study in southern Taiwan during 2010-2016. J Microbiol Immunol Infect. (2019) 52:937–46. doi: 10.1016/j.jmii.2019.04.006
81. Polkowska, A, Skoczyńska, A, Paradowska-Stankiewicz, I, Stefanoff, P, Hryniewicz, W, Kuch, A, et al. Pneumococcal meningitis before the introduction of 10-valent pneumococcal conjugate vaccine into the National Childhood Immunization Program in Poland. Vaccine. (2019) 37:1365–73. doi: 10.1016/j.vaccine.2018.12.028
82. Lee, H-Y, Wu, T-L, Su, L-H, Li, HC, Janapatla, RP, Chen, CL, et al. Invasive pneumococcal disease caused by ceftriaxone-resistant Streptococcus pneumoniae in Taiwan. J Microbiol Immunol Infect. (2018) 51:500–9. doi: 10.1016/j.jmii.2016.12.004
83. Ciruela, P, Izquierdo, C, Broner, S, Munoz-Almagro, C, Hernandez, S, Ardanuy, C, et al. The changing epidemiology of invasive pneumococcal disease after PCV13 vaccination in a country with intermediate vaccination coverage. Vaccine. (2018) 36:7744–52. doi: 10.1016/j.vaccine.2018.05.026
84. Kent, A, Makwana, A, Sheppard, CL, Collins, S, Fry, NK, Heath, PT, et al. Invasive pneumococcal disease in UK children <1 year of age in the post-13-valent pneumococcal conjugate vaccine era: what are the risks now? Clin Infect Dis. (2019) 69:84–90. doi: 10.1093/cid/ciy842
85. Makwana, A, Sheppard, C, Borrow, R, Fry, N, Andrews, NJ, and Ladhani, SN. Characteristics of children with invasive pneumococcal disease after the introduction of the 13-valent pneumococcal conjugate vaccine in England and Wales, 2010-2016. Pediatr Infect Dis J. (2018) 37:697–703. doi: 10.1097/INF.0000000000001845
86. Turner, P, Leab, P, Ly, S, Sao, S, Miliya, T, Heffelfinger, JD, et al. Impact of 13-valent pneumococcal conjugate vaccine on colonization and invasive disease in Cambodian children. Clin Infect Dis. (2020) 70:1580–8. doi: 10.1093/cid/ciz481
87. Richter, L, Schmid, D, Kanitz, EE, Zwazl, I, Pollabauer, E, Jasinska, J, et al. Invasive pneumococcal diseases in children and adults before and after introduction of the 10-valent pneumococcal conjugate vaccine into the Austrian national immunization program. PLoS One. (2019) 14:e0210081. doi: 10.1371/journal.pone.0210081
88. Silva-Costa, C, Brito, MJ, Aguiar, SI, Lopes, JP, Ramirez, M, Melo-Cristino, J, et al. Dominance of vaccine serotypes in pediatric invasive pneumococcal infections in Portugal (2012-2015). Sci Rep. (2019) 9:6. doi: 10.1038/s41598-018-36799-x
89. Berger, Y, Adler, A, Ariel, T, Rokney, A, Averbuch, D, and Grisaru-Soen, G. Paediatric community-acquired bacteraemia, pneumococcal invasive disease and antibiotic resistance fell after the pneumococcal conjugate vaccine was introduced. Acta Paediatr. (2019) 108:1321–8. doi: 10.1111/apa.14670
90. Al-Jardani, A, Al Rashdi, A, Al Jaaidi, A, Al Bulushi, M, Al Mahrouqi, S, Al-Abri, S, et al. Serotype distribution and antibiotic resistance among invasive Streptococcus pneumoniae from Oman post 13-valent vaccine introduction. Int J Infect Dis. (2019) 85:135–40. doi: 10.1016/j.ijid.2019.05.027
91. Shi, W, Li, J, Dong, F, Qian, S, Liu, G, Xu, B, et al. Serotype distribution, antibiotic resistance pattern, and multilocus sequence types of invasive Streptococcus pneumoniae isolates in two tertiary pediatric hospitals in Beijing prior to PCV13 availability. Expert Rev Vaccines. (2019) 18:89–94. doi: 10.1080/14760584.2019.1557523
92. Díaz-Conradi, A, Hernández, S, García-García, JJ, Munoz-Almagro, C, Moraga-Llop, F, Ciruela, P, et al. Complicated pneumococcal pneumonia with pleural effusion or empyema in the 13-valent pneumococcal conjugate vaccine era. Pediatr Pulmonol. (2019) 54:517–24. doi: 10.1002/ppul.24279
93. Casey, JR, Adlowitz, DG, and Pichichero, ME. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. (2010) 29:304–9. doi: 10.1097/INF.0b013e3181c1bc48
94. Casey, JR, Kaur, R, Friedel, VC, and Pichichero, ME. Acute otitis media otopathogens during 2008 to 2010 in Rochester, New York. Pediatr Infect Dis J. (2013) 32:805–9. doi: 10.1097/INF.0b013e31828d9acc
95. Marchisio, P, Esposito, S, Picca, M, Baggi, E, Terranova, L, Orenti, A, et al. Serotypes not included in 13-valent pneumococcal vaccine as causes of acute otitis media with spontaneous tympanic membrane perforation in a geographic area with high vaccination coverage. Pediatr Infect Dis J. (2017) 36:521–3. doi: 10.1097/INF.0000000000001485
96. Setchanova, L, Stancheva, I, Popova, D, Alexandrova, A, and Mitov, I. Bacterial spectrum of acute otitis media in Bulgarian children during the 10-valent pneumococcal conjugate vaccine era. J Pediatr Infect Dis. (2020) 15:135–43. doi: 10.1055/s-0040-1701647
97. van der Linden, M, Imöhl, M, Busse, A, Rose, M, and Adam, D. Bacterial spectrum of spontaneously ruptured otitis media in the era of pneumococcal conjugate vaccination in Germany. Eur J Pediatr. (2015) 174:355–64. doi: 10.1007/s00431-014-2409-0
98. Hays, C, Vermee, Q, Agathine, A, Dupuis, A, Varon, E, Poyart, C, et al. Demonstration of the herd effect in adults after the implementation of pneumococcal vaccination with PCV13 in children. Eur J Clin Microbiol Infect Dis. (2017) 36:831–8. doi: 10.1007/s10096-016-2868-5
99. Sierra, A, Lopez, P, Zapata, MA, Vanegas, B, Castrejon, MM, Deantonio, R, et al. Non-typeable Haemophilus influenzae and Streptococcus pneumoniae as primary causes of acute otitis media in colombian children: a prospective study. BMC Infect Dis. (2011) 11:4. doi: 10.1186/1471-2334-11-4
100. Fu, J, Li, L, Liang, Z, Xu, S, Lin, N, Qin, P, et al. Etiology of acute otitis media and phenotypic-molecular characterization of Streptococcus pneumoniae isolated from children in Liuzhou, China. BMC Infect Dis. (2019) 19:168. doi: 10.1186/s12879-019-3795-8
101. Guevara, S, Abdelnour, A, Soley, C, Porat, N, Dagan, R, and Arguedas, A. Streptococcus pneumoniae serotypes isolated from the middle ear fluid of Costa Rican children following introduction of the heptavalent pneumococcal conjugate vaccine into a limited population. Vaccine. (2012) 30:3857–61. doi: 10.1016/j.vaccine.2012.04.010
102. Kempf, M, Varon, E, Lepoutre, A, Gravet, A, Baraduc, R, Brun, M, et al. Decline in antibiotic resistance and changes in the serotype distribution of Streptococcus pneumoniae isolates from children with acute otitis media; a 2001-2011 survey by the French pneumococcal network. Clin Microbiol Infect. (2015) 21:35–42. doi: 10.1016/j.cmi.2014.08.009
103. Chi, H, Chiu, N-C, Huang, F-Y, Hsu, CH, Lee, KS, Huang, LM, et al. Acute otitis media caused by Streptococcus pneumoniae serotype 19A ST320 clone: epidemiological and clinical characteristics. J Microbiol Immunol Infect. (2018) 51:337–43. doi: 10.1016/j.jmii.2016.08.002
104. Quirk, SJ, Haraldsson, G, Erlendsdottir, H, Hjalmarsdottir, MA, van Tonder, AJ, Hrafnkelsson, B, et al. Effect of vaccination on pneumococci isolated from the nasopharynx of healthy children and the middle ear of children with otitis media in Iceland. J Clin Microbiol. (2018) 56:e01046–18. doi: 10.1128/jcm.01046-18
105. Dupont, D, Mahjoub-Messai, F, Francois, M, Doit, C, Mariani-Kurkdjian, P, Bidet, P, et al. Evolving microbiology of complicated acute otitis media before and after introduction of the pneumococcal conjugate vaccine in France. Diagn Microbiol Infect Dis. (2010) 68:89–92. doi: 10.1016/j.diagmicrobio.2010.04.012
106. Chen, Y-J, Hsieh, Y-C, Huang, Y-C, and Chiu, CH. Clinical manifestations and microbiology of acute otitis media with spontaneous otorrhea in children. J Microbiol Immunol Infect. (2013) 46:382–8. doi: 10.1016/j.jmii.2013.04.001
107. Alonso, M, Marimon, JM, Ercibengoa, M, Perez-Yarza, EG, and Perez-Trallero, E. Dynamics of Streptococcus pneumoniae serotypes causing acute otitis media isolated from children with spontaneous middle-ear drainage over a 12-year period (1999-2010) in a region of northern Spain. PLoS One. (2013) 8:e54333. doi: 10.1371/journal.pone.0054333
108. Abdelnour, A, Arguedas, A, Dagan, R, Soley, C, Porat, N, Castrejon, MM, et al. Etiology and antimicrobial susceptibility of middle ear fluid pathogens in Costa Rican children with otitis media before and after the introduction of the 7-valent pneumococcal conjugate vaccine in the National Immunization Program: acute otitis media microbiology in Costa Rican children. Medicine. (2015) 94:e320. doi: 10.1097/md.0000000000000320
109. Levy, C, Varon, E, Ouldali, N, Wollner, A, Thollot, F, Corrard, F, et al. Bacterial causes of otitis media with spontaneous perforation of the tympanic membrane in the era of 13 valent pneumococcal conjugate vaccine. PLoS One. (2019) 14:e0211712. doi: 10.1371/journal.pone.0211712
110. Ziv, O, Kraus, M, Holcberg, R, Dinur, AB, Kordeluk, S, Kaplan, D, et al. Acute otitis media in infants younger than two months of age: epidemiologic and microbiologic characteristics in the era of pneumococcal conjugate vaccines. Int J Pediatr Otorhinolaryngol. (2019) 119:123–30. doi: 10.1016/j.ijporl.2019.01.031
111. Dortet, L, Ploy, M-C, Poyart, C, and Raymond, J. Emergence of Streptococcus pneumoniae of serotype 19A in France: molecular capsular serotyping, antimicrobial susceptibilities, and epidemiology. Diagn Microbiol Infect Dis. (2009) 65:49–57. doi: 10.1016/j.diagmicrobio.2009.05.009
112. Parra, MM, Aguilar, GM, Echaniz-Aviles, G, Rionda, RG, Estrada Mde, L, Cervantes, Y, et al. Bacterial etiology and serotypes of acute otitis media in Mexican children. Vaccine. (2011) 29:5544–9. doi: 10.1016/j.vaccine.2011.04.128
113. Hoshino, T, Takeuchi, N, Fukasawa, C, Hirose, S, Okui, H, Sato, H, et al. Analysis of Streptococcus pneumoniae and Haemophilus influenzae isolated from middle ear fluid before and after the introduction of government subsidies for pneumococcal and H. influenzae type b vaccines in Japan. J Infect Chemother. (2017) 23:85–9. doi: 10.1016/j.jiac.2016.10.008
114. Kung, Y-H, Chiu, N-C, Lee, K-S, Chang, L, Huang, DT, Huang, FY, et al. Bacterial etiology of acute otitis media in the era prior to universal pneumococcal vaccination in Taiwanese children. J Microbiol Immunol Infect. (2014) 47:239–44. doi: 10.1016/j.jmii.2013.08.016
115. Fenoll, A, Aguilar, L, Vicioso, M-D, Gimenez, MJ, Robledo, O, and Granizo, JJ. Increase in serotype 19A prevalence and amoxicillin non-susceptibility among paediatric Streptococcus pneumoniae isolates from middle ear fluid in a passive laboratory-based surveillance in Spain, 1997-2009. BMC Infect Dis. (2011) 11:239. doi: 10.1186/1471-2334-11-239
116. Ochoa-Gondar, O, Figuerola-Massana, E, Vila-Corcoles, A, Aguirre, CA, de Diego, C, Satue, E, et al. Epidemiology of Streptococcus pneumoniae causing acute otitis media among children in southern Catalonia throughout 2007-2013: incidence, serotype distribution and vaccine’s effectiveness. Int J Pediatr Otorhinolaryngol. (2015) 79:2104–8. doi: 10.1016/j.ijporl.2015.09.022
117. Ding, Y, Geng, Q, Tao, Y, Lin, Y, Wang, Y, Black, S, et al. Etiology and epidemiology of children with acute otitis media and spontaneous otorrhea in Suzhou, China. Pediatr Infect Dis J. (2015) 34:e102–6. doi: 10.1097/inf.0000000000000617
118. Gene, A, del Amo, E, Iñigo, M, Monsonis, M, Pallares, R, and Munoz-Almagro, C. Pneumococcal serotypes causing acute otitis media among children in Barcelona (1992-2011): emergence of the multiresistant clone ST320 of serotype 19A. Pediatr Infect Dis J. (2013) 32:e128–33. doi: 10.1097/INF.0b013e31827c54dc
119. Koutouzis, EI, Michos, A, Koutouzi, FI, Chatzichristou, P, Parpounas, K, Georgaki, A, et al. Pneumococcal mastoiditis in children before and after the introduction of conjugate pneumococcal vaccines. Pediatr Infect Dis J. (2016) 35:292–6. doi: 10.1097/inf.0000000000000995
120. Ozawa, D, Yano, H, Endo, S, Hidaka, H, Kakuta, R, Okitsu, N, et al. Impact of the seven-valent pneumococcal conjugate vaccine on acute otitis media in Japanese children: emergence of serotype 15A multidrug-resistant Streptococcus pneumoniae in middle ear fluid isolates. Pediatr Infect Dis J. (2015) 34:e217–21. doi: 10.1097/inf.0000000000000776
121. Stamboulidis, K, Chatzaki, D, Poulakou, G, Ioannidou, S, Lebessi, E, Katsarolis, I, et al. The impact of the heptavalent pneumococcal conjugate vaccine on the epidemiology of acute otitis media complicated by otorrhea. Pediatr Infect Dis J. (2011) 30:551–5. doi: 10.1097/INF.0b013e31821038d9
122. Ubukata, K, Morozumi, M, Sakuma, M, Takata, M, Mokuno, E, Tajima, T, et al. Etiology of acute otitis media and characterization of pneumococcal isolates after introduction of 13-valent pneumococcal conjugate vaccine in Japanese children. Pediatr Infect Dis J. (2018) 37:598–604. doi: 10.1097/inf.0000000000001956
123. Elemraid, MA, Sails, AD, Thomas, MF, Rushton, SP, Perry, JD, Eltringham, GJA, et al. Pneumococcal diagnosis and serotypes in childhood community-acquired pneumonia. Diagn Microbiol Infect Dis. (2013) 76:129–32. doi: 10.1016/j.diagmicrobio.2013.02.012
124. Negash, AA, Asrat, D, Abebe, W, Hailemariam, T, Gebre, M, Verhaegen, J, et al. Pneumococcal carriage, serotype distribution, and risk factors in children with community-acquired pneumonia, 5 years after introduction of the 10-valent pneumococcal conjugate vaccine in Ethiopia. Open Forum Infect Dis. (2019) 6:6. doi: 10.1093/ofid/ofz259
125. Takeuchi, N, Naito, S, Ohkusu, M, Abe, K, Shizuno, K, Takahashi, Y, et al. Epidemiology of hospitalised paediatric community-acquired pneumonia and bacterial pneumonia following the introduction of 13-valent pneumococcal conjugate vaccine in the national immunisation programme in Japan. Epidemiol Infect. (2020) 148:e91. doi: 10.1017/s0950268820000813
126. Esposito, S, Marchese, A, Tozzi, AE, Rossi, GA, Da Dalt, L, Bona, G, et al. Bacteremic pneumococcal community-acquired pneumonia in children less than 5 years of age in Italy. Pediatr Infect Dis J. (2012) 31:705–10. doi: 10.1097/INF.0b013e31825384ae
127. Marchese, A, Esposito, S, Coppo, E, Rossi, GA, Tozzi, A, Romano, M, et al. Detection of Streptococcus pneumoniae and identification of pneumococcal serotypes by real-time polymerase chain reaction using blood samples from Italian children ≤ 5 years of age with community-acquired pneumonia. Microb Drug Resist. (2011) 17:419–24. doi: 10.1089/mdr.2011.0031
128. Pírez, MC, Algorta, G, Cedrés, A, Sobrero, H, Varela, A, Giachetto, G, et al. Impact of universal pneumococcal vaccination on hospitalizations for pneumonia and meningitis in children in Montevideo, Uruguay. Pediatr Infect Dis J. (2011) 30:669–74. doi: 10.1097/INF.0b013e3182152bf1
129. Moisi, JC, Makawa, MS, Tall, H, Agbenoko, K, Njanpop-Lafourcade, BM, Tamekloe, S, et al. Burden of pneumococcal disease in northern Togo before the introduction of pneumococcal conjugate vaccine. PLoS One. (2017) 12:e0170412. doi: 10.1371/journal.pone.0170412
130. De Schutter, I, Vergison, A, Tuerlinckx, D, Raes, M, Smet, J, Smeesters, PR, et al. Pneumococcal aetiology and serotype distribution in paediatric community-acquired pneumonia. PLoS One. (2014) 9:e89013. doi: 10.1371/journal.pone.0089013
131. Ouldali, N, Levy, C, Minodier, P, Morin, L, Biscardi, S, Aurel, M, et al. Long-term association of 13-valent pneumococcal conjugate vaccine implementation with rates of community-acquired pneumonia in children. JAMA Pediatr. (2019) 173:362–70. doi: 10.1001/jamapediatrics.2018.5273
132. Cohen, O, Knoll, MD, O’Brien, KL, Ramakrishnan, M, Farrar, J, Pilishvili, T, et al. (2017). Pneumococcal conjugate vaccine (PCV) review of impact evidence (PRIME): summary of findings from systematic review. Available online at: https://terrance.who.int/mediacentre/data/sage/SAGE_Docs_Ppt_Oct2017/9_session_PCV/Oct2019_session9_PCV_PRIMEsummary.pdf. [Accessed April 14, 2025].
133. Garcia Quesada, M, Peterson, ME, Bennett, JC, Hayford, K, Zeger, SL, Yang, Y, et al. Serotype distribution of remaining invasive pneumococcal disease after extensive use of ten-valent and 13-valent pneumococcal conjugate vaccines (the PSERENADE project): a global surveillance analysis. Lancet Infect Dis. (2024) 25:445–56. doi: 10.1016/s1473-3099(24)00588-7
134. Tin Tin Htar, M, Christopoulou, D, and Schmitt, HJ. Pneumococcal serotype evolution in Western Europe. BMC Infect Dis. (2015) 15:419. doi: 10.1186/s12879-015-1147-x
135. Løchen, A, Croucher, NJ, and Anderson, RM. Divergent serotype replacement trends and increasing diversity in pneumococcal disease in high income settings reduce the benefit of expanding vaccine valency. Sci Rep. (2020) 10:18977. doi: 10.1038/s41598-020-75691-5
136. Mungall, BA, Hoet, B, Nieto Guevara, J, and Soumahoro, L. A systematic review of invasive pneumococcal disease vaccine failures and breakthrough with higher-valency pneumococcal conjugate vaccines in children. Expert Rev Vaccines. (2022) 21:201–14. doi: 10.1080/14760584.2022.2012455
137. Grant, LR, Slack, MPE, Theilacker, C, Vojicic, J, Dion, S, Reinert, RR, et al. Distribution of serotypes causing invasive pneumococcal disease in children from high-income countries and the impact of pediatric pneumococcal vaccination. Clin Infect Dis. (2023) 76:e1062–70. doi: 10.1093/cid/ciac475
138. Pichichero, M, Malley, R, Kaur, R, Zagursky, R, and Anderson, P. Acute otitis media pneumococcal disease burden and nasopharyngeal colonization in children due to serotypes included and not included in current and new pneumococcal conjugate vaccines. Expert Rev Vaccines. (2023) 22:118–38. doi: 10.1080/14760584.2023.2162506
139. Iroh Tam, P-Y, Bernstein, E, Ma, X, and Ferrieri, P. Blood culture in evaluation of pediatric community-acquired pneumonia: a systematic review and meta-analysis. Hosp Pediatr. (2015) 5:324–36. doi: 10.1542/hpeds.2014-0138
140. ACIP. Preventing pneumococcal disease among infants and young children. Recommendations of the advisory committee on immunization practices (ACIP). MMWR Recomm Rep. (2000) 49:1–35.
141. Slotved, H-C, Dalby, T, Harboe, ZB, Valentiner-Branth, P, Casadevante, VF, Espenhain, L, et al. The incidence of invasive pneumococcal serotype 3 disease in the Danish population is not reduced by PCV-13 vaccination. Heliyon. (2016) 2:e00198. doi: 10.1016/j.heliyon.2016.e00198
142. De Wals, P, Lefebvre, B, Deceuninck, G, and Longtin, J. Incidence of invasive pneumococcal disease before and during an era of use of three different pneumococcal conjugate vaccines in Quebec. Vaccine. (2018) 36:421–6. doi: 10.1016/j.vaccine.2017.11.054
143. Oligbu, G, Hsia, Y, Folgori, L, Collins, S, and Ladhani, S. Pneumococcal conjugate vaccine failure in children: a systematic review of the literature. Vaccine. (2016) 34:6126–32. doi: 10.1016/j.vaccine.2016.10.050
144. Bliss, SJ, O’Brien, KL, Janoff, EN, Cotton, MF, Musoke, P, Coovadia, H, et al. The evidence for using conjugate vaccines to protect HIV-infected children against pneumococcal disease. Lancet Infect Dis. (2008) 8:67–80. doi: 10.1016/S1473-3099(07)70242-6
145. Pelton, SI, Weycker, D, Farkouh, RA, Strutton, DR, Shea, KM, and Edelsberg, J. Risk of pneumococcal disease in children with chronic medical conditions in the era of pneumococcal conjugate vaccine. Clin Infect Dis. (2014) 59:615–23. doi: 10.1093/cid/ciu348
146. Feemster, K, Weaver, J, Buchwald, U, Banniettis, N, Cox, KS, McIntosh, ED, et al. Pneumococcal vaccine breakthrough and failure in infants and children: a narrative review. Vaccines. (2023) 11:1750. doi: 10.3390/vaccines11121750
147. Lim, FJ, Lehmann, D, McLoughlin, A, Harrison, C, Willis, J, Giele, C, et al. Risk factors and comorbidities for invasive pneumococcal disease in Western Australian aboriginal and non-aboriginal people. Pneumonia. (2014) 4:24–34. doi: 10.15172/pneu.2014.4/463
148. Flory, JH, Joffe, M, Fishman, NO, Edelstein, PH, and Metlay, JP. Socioeconomic risk factors for bacteraemic pneumococcal pneumonia in adults. Epidemiol Infect. (2009) 137:717–26. doi: 10.1017/s0950268808001489
149. Chapman, KE, Wilson, D, and Gorton, R. Invasive pneumococcal disease and socioeconomic deprivation: a population study from the north east of England. J Public Health (Oxf). (2013) 35:558–69. doi: 10.1093/pubmed/fdt011
150. Moore, MR, Gertz, RE Jr, Woodbury, RL, Barkocy-Gallagher, GA, Schaffner, W, Lexau, C, et al. Population snapshot of emergent Streptococcus pneumoniae serotype 19A in the United States, 2005. J Infect Dis. (2008) 197:1016–27. doi: 10.1086/528996
151. Wyres, KL, Lambertsen, LM, Croucher, NJ, McGee, L, von Gottberg, A, Linares, J, et al. Pneumococcal capsular switching: a historical perspective. J Infect Dis. (2013) 207:439–49. doi: 10.1093/infdis/jis703
152. Jefferies, JMC, Smith, A, Clarke, SC, Dowson, C, and Mitchell, TJ. Genetic analysis of diverse disease-causing pneumococci indicates high levels of diversity within serotypes and capsule switching. J Clin Microbiol. (2004) 42:5681–8. doi: 10.1128/JCM.42.12.5681-5688.2004
153. Tyrrell, GJ. The changing epidemiology of Streptococcus pneumoniae serotype 19A clonal complexes. J Infect Dis. (2011) 203:1345–7. doi: 10.1093/infdis/jir056
154. Temime, L, Boelle, P-Y, Opatowski, L, and Guillemot, D. Impact of capsular switch on invasive pneumococcal disease incidence in a vaccinated population. PLoS One. (2008) 3:e3244. doi: 10.1371/journal.pone.0003244
155. Ruiz García, Y, Nieto Guevara, J, Izurieta, P, Vojtek, I, Ortega-Barría, E, and Guzman-Holst, A. Circulating clonal complexes and sequence types of Streptococcus pneumoniae serotype 19A worldwide: the importance of multidrug resistance: a systematic literature review. Expert Rev Vaccines. (2021) 20:45–57. doi: 10.1080/14760584.2021.1873136
156. Johnson, CN, Wilde, S, Tuomanen, E, and Rosch, JW. Convergent impact of vaccination and antibiotic pressures on pneumococcal populations. Cell Chem Biol. (2024) 31:195–206. doi: 10.1016/j.chembiol.2023.11.003
157. Platt, HL, Bruno, C, Buntinx, E, Pelayo, E, Garcia-Huidobro, D, Barranco-Santana, EA, et al. Safety, tolerability, and immunogenicity of an adult pneumococcal conjugate vaccine, V116 (STRIDE-3): a randomised, double-blind, active comparator controlled, international phase 3 trial. Lancet Infect Dis. (2024) 24:1141–50. doi: 10.1016/s1473-3099(24)00344-x
158. Alderson, MR, Sethna, V, Newhouse, LC, Lamola, S, and Dhere, R. Development strategy and lessons learned for a 10-valent pneumococcal conjugate vaccine (PNEUMOSIL®). Hum Vaccin Immunother. (2021) 17:2670–7. doi: 10.1080/21645515.2021.1874219
159. Wang, C, Su, L, Mu, Q, Gu, X, Guo, X, and Wang, X. Cost-effectiveness analysis of domestic 13-valent pneumococcal conjugate vaccine for children under 5 years of age in mainland China. Hum Vaccin Immunother. (2021) 17:2241–8. doi: 10.1080/21645515.2020.1870396
160. Lupinacci, R, Rupp, R, Wittawatmongkol, O, Jones, J, Quinones, J, Ulukol, B, et al. A phase 3, multicenter, randomized, double-blind, active-comparator-controlled study to evaluate the safety, tolerability, and immunogenicity of a 4-dose regimen of V114, a 15-valent pneumococcal conjugate vaccine, in healthy infants (PNEU-PED). Vaccine. (2023) 41:1142–52. doi: 10.1016/j.vaccine.2022.12.054
161. Ishihara, Y, Fukazawa, M, Enomoto, S, de Solom, R, Yamaji, M, Kline, M, et al. A phase 3 randomized study to evaluate safety and immunogenicity of 20-valent pneumococcal conjugate vaccine in healthy Japanese infants. J Infect Dis. (2024) 141:106942. doi: 10.1016/j.ijid.2024.01.009
162. Korbal, P, Wysocki, J, Jackowska, T, Kline, M, Tamimi, N, Drozd, J, et al. Phase 3 safety and immunogenicity study of a three-dose series of twenty-valent pneumococcal conjugate vaccine in healthy infants and toddlers. Pediatr Infect Dis J. (2024) 43:587–95. doi: 10.1097/inf.0000000000004300
163. Senders, S, Klein, NP, Tamimi, N, Thompson, A, Baugher, G, Trammel, J, et al. A phase three study of the safety and immunogenicity of a four-dose series of 20-Valent pneumococcal conjugate vaccine in healthy infants. Pediatr Infect Dis J. (2024) 43:596–603. doi: 10.1097/inf.0000000000004334
164. Shelly, S, Klein, N, Tamimi, N, Thompson, A, Baugher, G, Trammel, J, et al. Phase 3 safety and immunogenicity study of 20-valent pneumococcal conjugate vaccine (PCV20) administered in a 4-dose infant immunization series. Lisbon, Portugal: European Society for Paediatric Infectious Diseases (2023).
165. Dagan, R, Poolman, J, and Siegrist, CA. Glycoconjugate vaccines and immune interference: a review. Vaccine. (2010) 28:5513–23. doi: 10.1016/j.vaccine.2010.06.026
166. Pérez-García, C, Sempere, J, de Miguel, S, Hita, S, Úbeda, A, Vidal, EJ, et al. Surveillance of invasive pneumococcal disease in Spain exploring the impact of the COVID-19 pandemic (2019-2023). J Infect. (2024) 89:106204. doi: 10.1016/j.jinf.2024.106204
167. Griffith, A, Golden, AR, Lefebvre, B, McGeer, A, Tyrrell, GJ, Zhanel, GG, et al. Invasive pneumococcal disease surveillance in Canada, 2021-2022. Can Commun Dis Rep. (2024) 50:121–34. doi: 10.14745/ccdr.v50i05a02
168. Silva-Costa, C, Gomes-Silva, J, Pinho, M, Friães, A, Subtil-Limpo, F, Ramirez, M, et al. Rebound of pediatric invasive pneumococcal disease in Portugal after the COVID-19 pandemic was not associated with significant serotype changes. J Infect. (2024) 89:106242. doi: 10.1016/j.jinf.2024.106242
169. Perniciaro, S, van der Linden, M, and Weinberger, DM. Reemergence of invasive pneumococcal disease in Germany during the spring and summer of 2021. Clin Infect Dis. (2022) 75:1149–53. doi: 10.1093/cid/ciac100
170. Almeida, SCG, Lemos, APS, Bierrenbach, AL, Moraes, JC, and Brandileone, MCC. Serotype distribution and antimicrobial susceptibility pattern of Streptococcus pneumoniae in COVID-19 pandemic era in Brazil. Microorganisms. (2024) 12:12. doi: 10.3390/microorganisms12020401
Keywords: acute otitis media, children, community-acquired pneumonia, invasive pneumococcal disease, pneumococcal conjugate vaccine, serotype distribution, Streptococcus pneumoniae
Citation: Izurieta P, AbdelGhany M and Borys D (2025) Serotype distribution of invasive and non-invasive pneumococcal disease in children ≤5 years of age following the introduction of 10- and 13-valent pneumococcal conjugate vaccines in infant national immunization programs: a systematic literature review. Front. Public Health. 13:1544359. doi: 10.3389/fpubh.2025.1544359
Edited by:
Eitan Naaman Berezin, Santa Casa of São Paulo, BrazilReviewed by:
Kanny Diallo, Centre Suisse de Recherches Scientifiques en Côte d’Ivoire, Côte d’IvoireEustachio Cuscianna, University of Bari Aldo Moro, Italy
Copyright © 2025 Izurieta, AbdelGhany and Borys. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Patricia Izurieta, cGF0cmljaWEucy5penVyaWV0YUBnc2suY29t
 Patricia Izurieta
Patricia Izurieta Mohammad AbdelGhany2
Mohammad AbdelGhany2