- 1Shandong Academy of Occupational Health and Occupational Medicine, Shandong First Medical University & Shandong Academy of Medical Sciences, Jinan, China
- 2Shandong Center for Disease Control and Prevention, Jinan, China
- 3School of Public Health Jilin University, Changchun, China
- 4College of Traditional Chinese Medicine, Shandong University of Traditional Chinese Medicine, Jinan, China
Background: Chronic diseases have emerged as a significant public health challenge, impacting the well-being of the Chinese populace, despite scant research exploring the influence of dietary factors on these conditions. This article aimed to investigate the dietary patterns of adult residents in Shandong Province, China, and explore the relationship between these dietary patterns and common chronic diseases.
Methods: We used data from the Total Diet Study of the Population of Shandong Province in China between 2015 and 2016. After further screening, a total of 2,828 adult residents with complete dietary and chronic disease prevalence information were included in this study. Food frequency questionnaires were used to ascertain dietary consumption. Dietary patterns were derived through factor analysis. Multivariate logistic regression models were employed to assess the associations between dietary patterns and the risk of common chronic diseases, while adjusting for potential confounders.
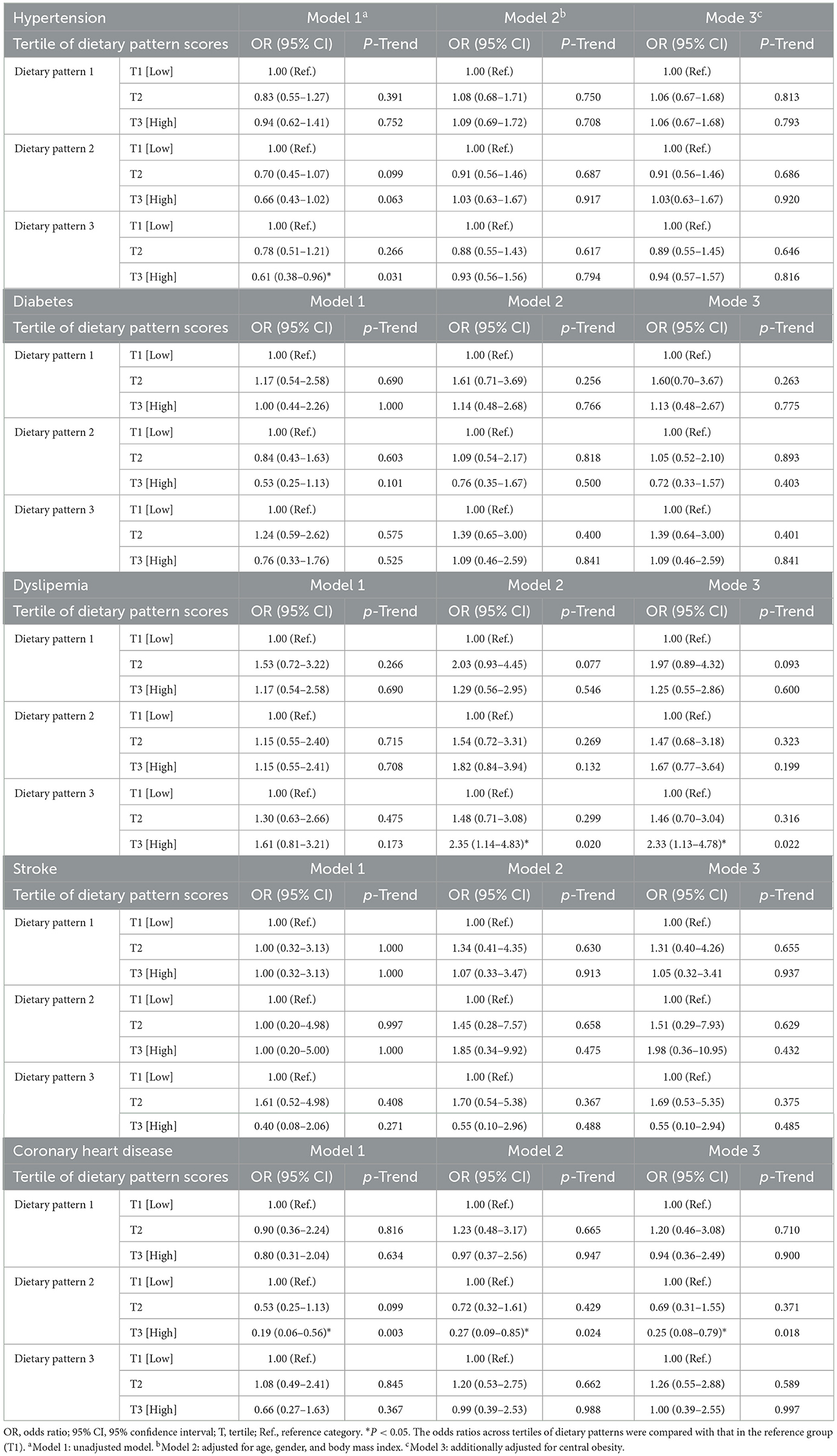
Results: Three dietary patterns were identified: dietary pattern 1 (characterized by high intake of grains and tubers, vegetables, fruits, eggs, meat, nuts, and legumes); dietary pattern 2 (with high consumption of edible fungi and algae, legumes, snacks, aquatic products, and vegetables, but low in eggs); and dietary pattern 3 (high in dairy, beverages, and snacks). Notably, dietary pattern 2 was associated with a decreased risk of coronary heart disease, even after adjusting for potential confounders [odds ratio (OR) = 0.25, 95% confidence interval (CI) = 0.08-0.79, P < 0.05]. A higher incidence of dyslipidemia was significantly correlated with dietary pattern 3 (OR = 2.33, 95% CI = 1.13–4.78, P < 0.05).
Conclusions: Our findings demonstrated that adherence to specific dietary patterns can influence the risk of dyslipidemia and coronary heart disease. Higher adherence to dietary pattern 3 was linked to a higher risk of dyslipidemia, while dietary pattern 2 helped reduce the risk of coronary heart disease.
1 Introduction
The rapid economic growth and aging population in China have led to a significant rise in chronic non-communicable diseases, which have become a major public health concern. Data indicates that conditions such as cardiovascular diseases, diabetes, and cancers are prevalent, with notable urban-rural disparities and a trend toward younger onset ages. From 12.33% in 2003 to 34.29% in 2018, the prevalence of chronic diseases among the Chinese population has risen annually due to a variety of variables, including lifestyle, environment, and population age distribution (1). According to reports, 88.5% of all deaths among Chinese citizens in 2019 were caused by chronic illnesses, with diabetes, cancer, cardiovascular disease, and chronic respiratory conditions accounting for 47.1%, 24.1%, 8.8%, and 2.5% of deaths, respectively (2). The rapid increase in morbidity and mortality from chronic diseases poses a heavy burden on society, with up to 100.2 million disability-adjusted life years due to cardiovascular disease in 2021 alone (3).
Numerous studies show that dietary interventions are crucial in preventing chronic disease (4–7). However, single nutrient or food studies do not accurately reflect the overall effects of dietary combinations because of the interactions and synergy between nutrients (8). Hence, as an alternative way of explaining dietary complexity, dietary patterns better reflect reality. Factor analysis is one of the most commonly used methods in the study of dietary patterns and is widely used to capture different dietary characteristics in a population, to assess the overall quality of a diet and to construct statistical models (9, 10). The high-protein pattern extracted by factor analysis was found to be in negative association with the risk of dyslipidemia by Guo et al. (11). A previous cross-sectional study linked the cautious pattern with a higher prevalence of hypertension and coronary heart disease (CHD) (12). A 10-year cohort study showed that participants with higher adherence to traditional Nordic and modern dietary patterns had a lower risk of haemorrhagic stroke, ischaemic stroke and diabetes (13).
Due to a combination of factors such as geographical location, socio-economic situation, cultural practices and eating habits, there are great differences in the dietary structure of different groups of people (14, 15). The rapid development of Shandong Province in recent decades has dramatically changed the lifestyles and dietary habits of the local population (16). In 2018, Shandong had the highest age-standardized mortality rate and diet-related mortality rate of ischemic heart disease in China, with 92.8 deaths per 100,000 people (16). A large cross-sectional study showed that the prevalence rates of hypertension, diabetes and hyperlipidemia in rural areas of Shandong Province were 29.36%, 15.03% and 5.68% respectively (17). However, despite the prevalence of various chronic diseases in Shandong Province, there is a relative paucity of in-depth research on the potential links between specific dietary patterns and common chronic diseases in the region Consequently, this study aimed to identify the primary dietary patterns among adult residents in Shandong Province through factor analysis, and subsequently investigate the potential associations between these patterns and prevalent chronic diseases.
2 Materials and methods
2.1 Study participants
The data for this study was obtained from the Total Diet Study of the Population of Shandong Province (18). The project used a multi-stage, stratified, whole-cluster random sampling approach to recruit participants. Six municipal survey sites in Yantai, Weifang, Jinan, Liaocheng, Tai'an, and Linyi were chosen as they reflect the typical dietary habits and consumption levels of Shandong Province's residents, as evidenced by the province's rich food culture and specific dietary patterns. Subsequently, one district and two counties, each with a medium economic status, were selected from within each city. Two street offices per urban district and four neighborhoods per street office were selected; two townships per county and three villages per township were selected. The number of households taken from each community/village is not < 30. Survey respondents were household members or residents ≥2 years of age who had lived in the survey site for more than 6 months.
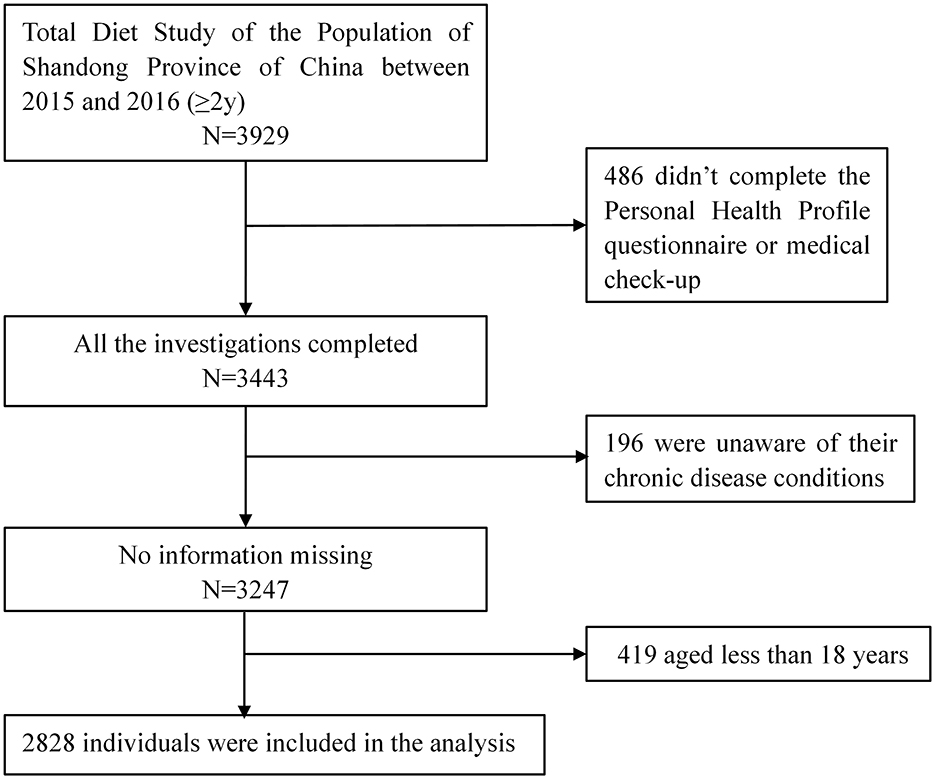
In the project, a total of 11,821 individuals from 3,667 households across Shandong Province were sampled, among whom 3,929 were investigated using the Food Frequency Questionnaire (FFQ). The project commenced on September 1, 2015, and concluded with a site survey at the end of March 31, 2016. The following were the inclusion criteria for this study: (1) participants who were 18 years of age or older; (2) completion of the Personal Health Profile questionnaire, FFQ, and a medical examination with no missing information. Ultimately, 2,828 participants were included in the final dataset. An informed consent was signed by each participant before the survey. The study was approved by the Shandong Academy of Occupational Health and Occupational Medicine in China, with the ethics number SDZFY-EC-H-2024-10. The screening flowchart was shown in Figure 1.
2.2 Dietary assessment
A standardized FFQ was used to measure dietary intake and gather data on participants' eating patterns throughout the previous 12 months. The 106 food items in the FFQ were categorized into 12 major groups, including grains and tubers, legumes, edible fungi and algae, fruits, vegetables, nuts, meat, eggs, aquatic products, dairy, snacks and beverages. Fractions from nutrient supplements, cooking oils and spices were not included in this study. The detailed food component categories were shown in Supplementary Table S1.
Participants were asked to fill in the number of intakes (daily, weekly, monthly, yearly, or never) and the weight of a single intake. The sum of the average daily intakes of all foods in each food group is the intake of that food group. Factor analysis was used to derive dietary patterns. The dietary sample data was first subjected to the Bartlett's test of sphericity and the Kaiser-Meyer-Olkin appropriateness test before factor analyses. The data was deemed appropriate for exploratory factor analysis if the Kaiser-Meyer-Olkin value was ≥0.5 and the P value for the Bartlett's test statistic was less than 0.05. Eigenvalues > 1 were used as criteria for extracting the common factors, which were then combined with the scree plot, cumulative contribution, and interpretability of the factors to determine the number of factors to retain, i.e., the main dietary patterns. Maximum variance rotation was used to obtain a more condensed factor structure. The intake of each food group and the associated factor loadings were used to compute factor scores for this dietary pattern. Each dietary pattern's factor scores were separated into tertiles, which rose from T1 to T3. The study participants' preference for the related food pattern increased with a higher score.
2.3 Measurement of anthropometry and other variables
Trained investigators used conventional procedures to perform medical examinations on the participants. A metal stadiometer with an accuracy of 0.1 cm was used to measure height. Weight was measured using electronic scales with an accuracy of 0.1 kg. Body mass index (BMI) was calculated by dividing weight in kilograms by height in meters squared (BMI = weight (kg)/(height (meters))2). Underweight (< 18.5 kg/m2), normal weight (18.5– < 25 kg/m2), overweight (25.0– < 30.0 kg/m2), and obese (≥30.0 kg/m2) were the BMI classifications (19). To test blood pressure, an electronic sphygmomanometer was used. A soft ruler was used to measure the participants' waist and hip circumferences to the closest 0.1 cm after they were instructed to change into light clothing. The measurement of waist circumference divided by hip circumference was used to compute the waist-to-hip ratio. For men and women, a healthy waist-to-hip ratio was < 0.9 and < 0.8, respectively; if not, central obesity was diagnosed (20).
Face-to-face enquiry surveys were conducted in households by trained and qualified enumerators, who collected comprehensive health-related information. Participants filled out a basic family profile registration form and a personal health profile questionnaire, which encompassed essential details about family members, demographic data (including gender, age, ethnicity, etc.), the current status of prevalent chronic diseases such as hypertension, diabetes, dyslipidaemia, stroke, and CHD, and family medical history.
2.4 Assessment of common chronic diseases
Adults with systolic blood pressure of 140 mmHg or higher and diastolic blood pressure of 90 mmHg or higher, tested three times on different days without the use of antihypertensive medications, were diagnosed with hypertension (21). Other diseases (diabetes mellitus, dyslipidaemia, stroke and coronary heart disease) were based on previous diagnosis in community/township hospitals and higher. The diagnosis of diabetes required a fasting blood glucose level of ≥7.00 mmol/L or self-reported diabetes (22). The diagnosis of dyslipidemia required meeting any of the following criteria: total cholesterol ≥6.22 mmol/L, or low-density lipoprotein cholesterol (LDL-C) ≥4.14 mmol/L, or high-density lipoprotein cholesterol < 1.04 mmol/L, or triglycerides ≥2.26 mmol/L, or a self-reported history of hyperlipidemia (23). In clinical practice, the diagnosis of stroke was mainly based on the symptoms of acute onset neurological deficits, imaging examinations, and biomarker measurements (24, 25). The diagnosis of coronary heart disease required a combination of typical angina pectoris symptoms, ischemic changes in electrocardiogram, elevated myocardial enzymes and coronary angiography (26).
2.5 Statistical analysis
Among this study, the basic features of patients in the various disease populations were reported after a descriptive analysis of each disease population. Means and standard deviations were used to characterize continuous variables, while rates and composition ratios were used to convey categorical variables. For measurement data, the t-test was used to compare differences, and for count data, the chi-square test was used for between-group comparisons.
The relationship between each dietary pattern and the risk of common chronic diseases was evaluated using logistic regression. In several models, the results were displayed as odds ratios (ORs) and 95% confidence intervals (CIs) in relation to the reference tertile, which was the lowest one. For this investigation, three models were created. Model 1 was a rudimentary model that did not account for variables. Age, gender, and BMI adjustments were made to Model 2. Based on Model 2, Model 3 was also modified to account for central obesity. SPSS 27.0 was used for all statistical analyses (IBM SPSS Inc., USA). In the study, a difference was deemed statistically significant if the bilateral P was < 0.05.
3 Results
3.1 Basic information of population
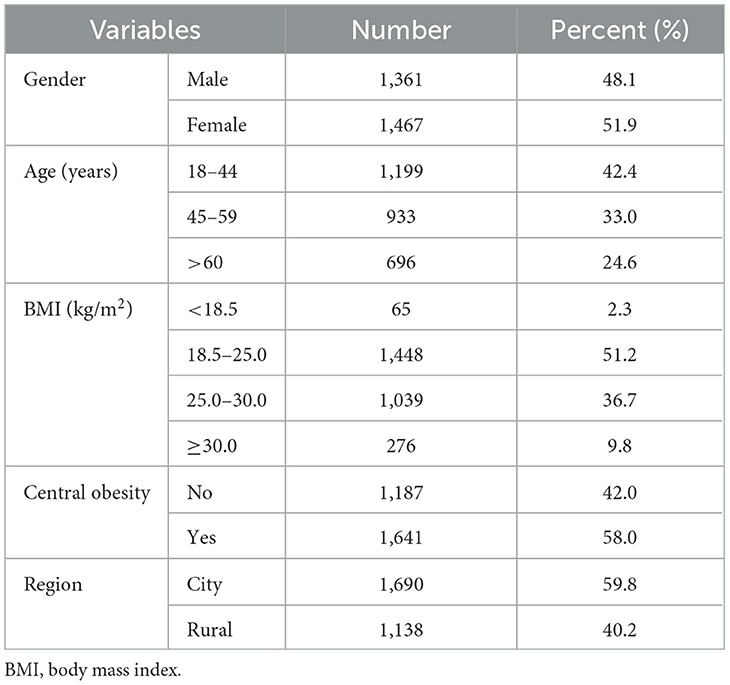
This study comprised 2,828 participants, ages 18 to 95, of which 1,361 (48.1%) were male and 1,467 (51.9%) were female. The characteristics of each participant are displayed in Table 1. Most participants (42.4%) fell within the age range of 18– 44. Regarding BMI, 46.5% of participants were overweight or obese, whereas 51.2% had a normal classification. Central obesity was present in 58% of the participants. 59.8% of the participants lived in cities, while the rest lived in rural areas.
3.2 Characteristics of different affected populations
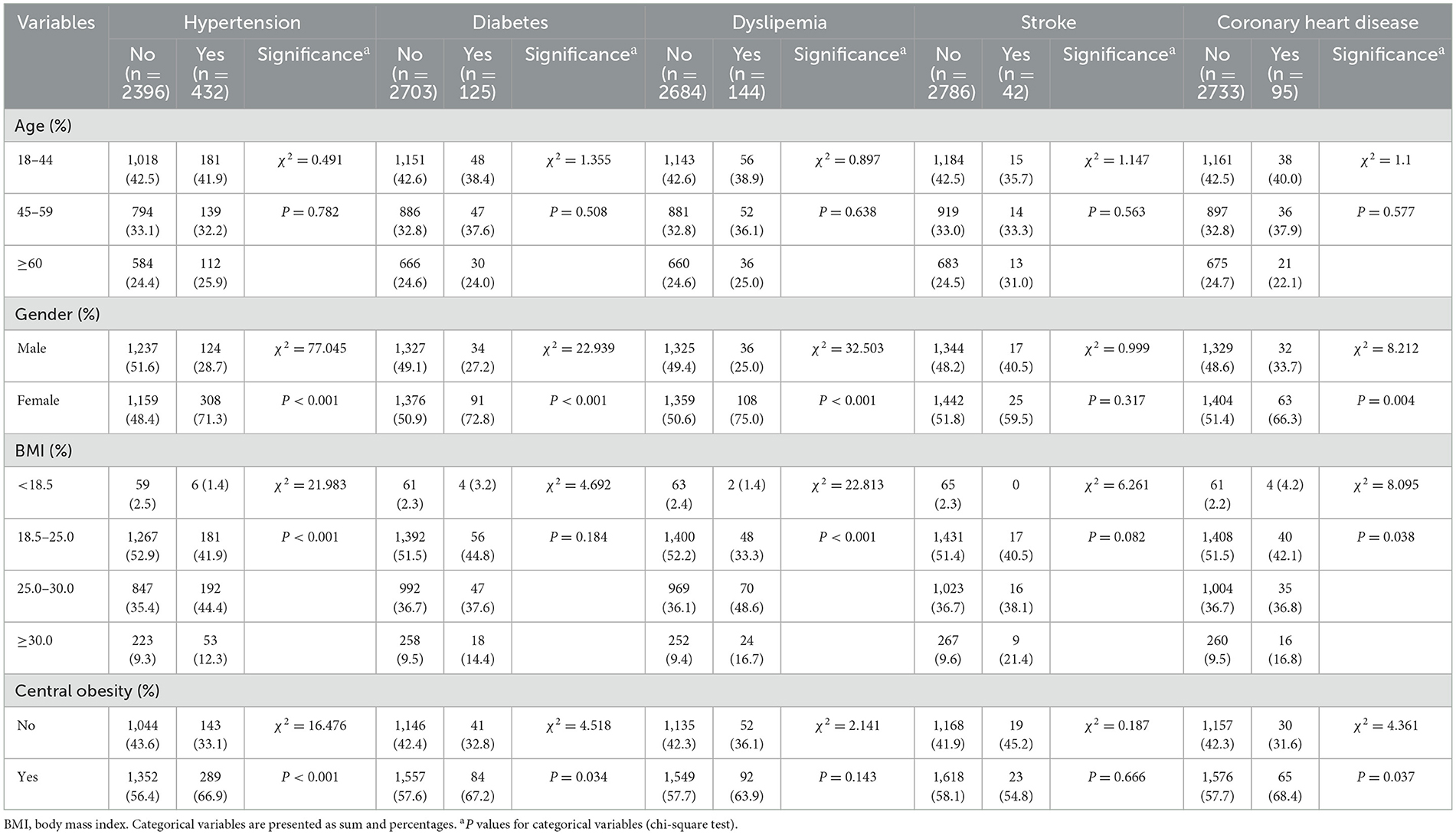
Table 2 displays the participants' characteristics based on their prevalence of common chronic conditions. The study subjects were grouped according to whether they were diseased or not. The prevalence of hypertension was 432, with a prevalence rate of 15.28%, and the difference between affected and unaffected residents was statistically significant for gender, BMI and central obesity (P < 0.05). The prevalence of diabetes mellitus was 125 with a prevalence rate of 4.42%, with differences in the distribution of gender and central obesity in both groups (P < 0.05). The prevalence of dyslipidaemia was 144 with a prevalence rate of 5.09%, it was significantly different in both groups in terms of gender and BMI (P < 0.05). The number of patients with CHD was 95, with a prevalence rate of 3.36%, and significant differences were observed with regard to gender, BMI and central obesity (P < 0.05).
3.3 Dietary patterns
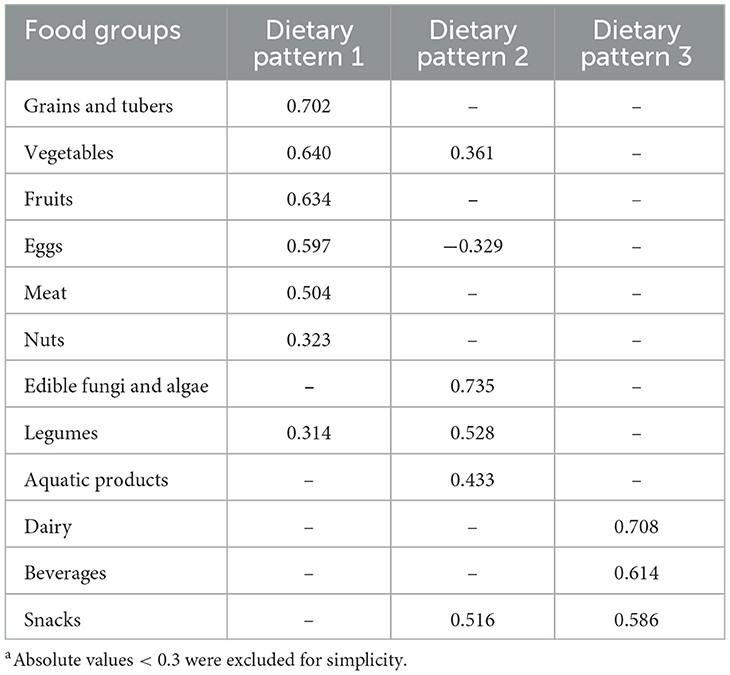
We verified that the Kaiser–Meyer–Olkin index (0.756) and Bartlett's test (P < 0.001) supported the applicability of the factor analysis of the dietary data. After extracting the factors with feature values greater than 1, combined with the lithotripsy plot and the interpretability of the factors, three common factors were finally determined. The lithotripsy pattern is shown in Supplementary Figure S1. The eigenvalues of these three common factors were 2.76, 1.41 and 1.13 respectively, and the explanatory variances were 23.0, 11.7 and 9.4% respectively. These factors combined accounted for more than 44% of the variance in food intake observed. Table 3 displays the factor loadings for each of the three primary dietary patterns that this study's factor analysis revealed. Grains and tubers, vegetables, fruits, eggs, meat, nuts, and legumes were the staples of dietary pattern 1. Dietary pattern 2 was defined by a low intake of eggs and a high intake of edible fungi and algae, legumes, snacks, aquatic products, and vegetables. High consumption of dairy products, beverages, and snacks characterized dietary pattern 3.
3.4 Associations between dietary patterns and common chronic diseases
The relationships between the three dietary patterns and CHD, stroke, diabetes, dyslipidemia, and hypertension are shown in Table 4. The chances of CHD were considerably lower for individuals in the highest tertile of dietary pattern 2 intake than for those in the lowest tertile (R = 0.19, 95%CI = 0.06–0.56, P < 0.05). When age, gender, BMI, and central obesity were taken into account, this connection persisted (OR = 0.25, 95%CI = 0.08–0.79, P < 0.05). After controlling for model 3 covariates, the incidence of dyslipidemia was higher for those in the highest tertile of dietary pattern 3 adherence than for those in the lowest adherence (OR = 2.33, 95% CI = 1.13–4.78, P < 0.05). Additionally, compared to the lowest tertile of dietary pattern 3, the highest tertile was adversely linked to hypertension. After controlling for other variables, however, this association between T3 and T1 vanished (model 1: OR = 0.61, 95% CI = 0.38–0.96, P < 0.05; model 2: OR = 0.93, 95% CI = 0.56–1.56, P = 0.79; model 3: OR = 0.94, 95% CI = 0.57–1.57, P = 0.82).
4 Discussion
Three dietary patterns among adult residents of Shandong Province were extracted for this study using factor analysis. Dietary pattern 1 was characterized by high factor loadings for staple foods such as grains and tubers, as well as vegetables, fruits, eggs, meat, nuts, and legumes. Dietary pattern 2 was characterized by low egg consumption and high consumption of edible fungi and algae, legumes, snacks, aquatic products, and vegetables. Dietary pattern 3 tended to be highly loaded with dairy, beverage and snacks. Among these, a decreased risk of CHD was linked to greater adherence to dietary pattern 2. After accounting for a number of factors, a higher risk of dyslipidemia was linked to high adherence to dietary pattern 3. Conversely, there was no significant correlation found between dietary pattern 1 and CHD, stroke, diabetes, dyslipidemia, or hypertension.
In this study, we observed that participants who adhered to dietary pattern 2 experienced a significantly reduced risk of CHD, with a 75% lower incidence compared to those who did not follow this pattern. Numerous investigations into the connection between dietary patterns and CHD have been carried out (27–29). However, due to the frequent variation in the quantity and composition of food groups used in previous studies, as well as the significant differences in dietary patterns compared to dietary pattern 2, direct comparison of their results with ours is challenging. Dietary pattern 2's negative correlation with CHD risk may be due to a combination of the dietary components and the characteristics of the nutrients it contains. Edible fungi and algae in the FFQ included mushrooms, edible non-mushroom fungi (such as agaric and tremella), nori, and kelp. A recent systematic review found that eating mushrooms decreased blood triglyceride levels but did not correlate with the incidence of CHD (30). Meanwhile, agaricus bisporus, auricularia auricular, lentinula edodes and pleurotus ostreatus that we often consume are rich in glucan, which has cholesterol-lowering properties (31, 32). In addition, nori and kelp are also known to have lipid-lowering effects, as they contain bioactive compounds such as porphyran and fucoidan, respectively (33–35). Lower blood cholesterol and triglyceride levels mean a lower risk of atherosclerosis. According to an early study, higher consumption of legumes was substantially linked to a reduced risk of CHD (36). Beans are an excellent source of providing fiber, protein and phytosterols, all of which have been shown to lower LDL-C (37–39). In addition, a number of bioactive peptides derived from soy protein have been validated in various models for their lipid-lowering, angiotensin-converting enzyme-inhibiting, and antioxidant effects (40). Consuming fish was linked to lower rates of CHD morbidity and death, according to a study by Zhang et al. (41). Omega-3 fatty acids, which have been shown to be highly effective in reducing blood triglyceride levels, preventing thrombosis, functioning as an anti-inflammatory, and preventing cardiac arrhythmias, are primarily responsible for fish's capacity to prevent CHD (42–44). Li et al. (45) found a link between consuming 100 g/d of green leafy vegetables and a lower risk of CHD after combining 24 meta-analyses. On the one hand, the soluble dietary fiber in vegetables binds with bile acids, thereby reducing the reabsorption of cholesterol, thus lowering cholesterol levels (46). On the other hand, the antioxidant substances in vegetables can scavenge free radicals and protect vascular endothelial cells (47–49). Although belonging to the same snack category, relevant studies have shown that intake of whole grain bread and chocolate was significantly linked to a lower risk of CHD (50, 51), whereas high intake of white bread, biscuits, cream cakes and fried foods significantly increased the risk of CHD (52, 53). It is reasonable to speculate that some of the adverse cardiovascular effects of snacking are canceled out by other beneficial ingredients. There is ongoing debate on the relationship between eating eggs and the risk of CHD. Even increasing the frequency of egg consumption from less than one egg per month to more than seven eggs per week did not raise the risk of CHD overall, according to a study that combined seven US prospective cohorts (54). Another meta-analysis of prospective dose-response trials came to the same conclusion (55). However, more frequent egg consumption was linked to a lower incidence of ischemic heart disease, according to a cohort research that included over 500,000 Chinese individuals (56). In conclusion, there was no correlation found between decreased egg intake and an increased risk of CHD.
Our findings indicated an increased risk of dyslipidaemia among individuals who followed dietary pattern 3. Similarly, a Brazilian cross-sectional study made clear that girls' high triglyceride levels were linked to dietary patterns high in dairy products, sugary sodas, and sweets (57). A prospective cohort study of adults in Harbin, China, suggested that adherence to a snacking dietary pattern dominated by biscuits, fried crisps, liquid beverages, candies, and ice cream raised the risk of hypercholesterolemia and hypertriglyceridemia (58). Moreover, the results of a study conducted by the National Health and Nutrition Examination Survey in Japan revealed a positive correlation between a dietary pattern characterized by the consumption of bread and dairy products and elevated LDL-C levels, as well as increased total cholesterol levels, in female subjects (59). A cross-sectional study encompassing dietary data from 11,404 Korean women revealed that dietary patterns characterized by elevated consumption of red meat, milk and dairy products, and bread and snacks exhibited a positive correlation with increased total cholesterol levels (60). There are several possible explanations for this association. Firstly, beverages usually contain high levels of fructose, which can induce dyslipidaemia by providing substrates for fatty acid and triglyceride synthesis as well as activating key transcription factors to increase lipogenesis (61, 62). The intake of sugary drinks is positively correlated with triglycerides and LDL-C and negatively correlated with high density lipoprotein cholesterol, which has been confirmed by a large number of scholars (63–66). Second, a lot of processed meals, such baked products and fried foods, are frequently heavy in trans fatty acids and saturated fats, which raise LDL-C levels (67–70). At the same time, snacks are generally high in calories, and excessive intake of snacks can easily lead to obesity, which is one of the important triggers of high blood cholesterol (71). However, the effect of dairy products on blood lipids remains controversial. Low-fat dairy products, which are high in saturated fatty acids, are advised to be substituted for full-fat dairy products according to traditional dietary standards. However, recent studies have demonstrated that full-fat dairy products do not have a negative effect on the lipid profile (72–74). The specific mechanism of dairy products' role in this needs to be explained by further research.
In this study, dietary pattern 1 was not associated with the risk of cardiovascular disease (CVD). This might be due to the fact that the protective components counteracted the effects of potential risk factors. For instance, grains were classified into whole grains and refined grains, and meats included poultry and red meat, but they had different effects on CVD. A meta-analysis that included 43 observational studies indicated that red meat consumption was positively correlated with CVD (75). Another systematic review of prospective cohort studies pointed out that replacing red meat or processed meat with poultry was negatively correlated with the risk of CVD, CHD and stroke (76). The 2020 Dietary Guidelines Advisory Committee concluded that one of the characteristics of dietary patterns associated with reducing the risk of CVD is a higher intake of whole grains and a lower intake of red meat and processed meat, as well as refined grains (77). In addition, the lack of association might be due to the fact that our analysis did not take into account some potential confounding factors, such as alcohol consumption and smoking status.
This study boasts several advantages. Firstly, dietary patterns derived through factor analysis offer a more realistic portrayal of actual eating habits compared to priori patterns. Secondly, data collection was handled by highly skilled professionals who ensured quality control throughout the process, guaranteeing information accuracy. Lastly, to address key potential confounders, three statistical models were employed for this investigation. However, this study has certain limitations. Initially, the causal relationship between common chronic diseases and dietary habits was difficult to as-certain due to the inherent limitations of cross-sectional studies. Further validation of our results is required through either prospective cohort studies or randomized con-trolled trials to ensure robustness. Secondly, the use of the FFQ to assess dietary intake over the year may be impacted by recall bias. Meanwhile, the categorization of food groups, the retention of factor numbers and the definition of dietary patterns were subjectively determined by the researchers. Therefore, the current dietary patterns may not reflect the full reality of the study population. Third, due to differences in ethnicity, culture, and dietary habits, different populations have different dietary pat-terns, and all the data in this study came from Shandong, China, which would limit the generalisability of the results. Fourth, many potential residual confounding factors were not included in our model, such as educational level, income level, smoking, drinking status, etc., which would affect our results. Fifth, the age range of the population in this study was relatively wide. Although the age factor had been adjusted, it may mask the heterogeneous influence of dietary patterns among different age groups.
5 Conclusions
In summary, the current study found that adult residents of Shandong Province, China, had different dietary patterns and that these patterns were related to cardiovascular health. In particular, it was discovered that dietary pattern 2, which was defined by reduced consumption of eggs and higher consumption of edible fungi and algae, legumes, snacks, aquatic products, and vegetables, had a preventive impact against CHD. On the other hand, a higher risk of dyslipidemia was associated with dietary pattern 3, which was characterized by a high intake of dairy products, beverages, and snacks. These findings highlight how important dietary practices are in preventing chronic illnesses and offer insightful information for the creation of nutrition-related policy. Further prospective studies in diverse populations are warranted to validate these findings and enhance our understanding of the broader implications of dietary patterns on health outcomes.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: The data are not publicly available due to privacy and legal reason. Requests to access these datasets should be directed to Guangjian Wu, bml1bml1MTAyODg4ODhAMTYzLmNvbQ==.
Ethics statement
The studies involving humans were approved by Shandong Academy of Occupational Health and Occupational Medicine in China (Approval Number: SDZFY-EC-H-2024-10). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XW: Conceptualization, Data curation, Formal analysis, Methodology, Software, Validation, Writing – original draft, Writing – review & editing. JL: Conceptualization, Data curation, Software, Writing – original draft. XL: Conceptualization, Data curation, Software, Writing – original draft. WZ: Conceptualization, Data curation, Software, Writing – original draft. YY: Conceptualization, Methodology, Writing – original draft. JM: Conceptualization, Methodology, Writing – original draft. MCa: Conceptualization, Methodology, Writing – original draft. MCh: Conceptualization, Methodology, Writing – original draft. GW: Conceptualization, Methodology, Project administration, Software, Supervision, Writing – review & editing. HX: Conceptualization, Methodology, Project administration, Software, Supervision, Writing – review & editing. ZD: Conceptualization, Methodology, Project administration, Software, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Key Research and Development Program of China [grant number 2022YFC2503202], National Natural Science Foundation of China (NSFC) [grant numbers 81602893, 81872575], Natural Science Foundation of Shandong Province [grant numbers ZR2015YL049, ZR2021MH218 and ZR2022MH184]; Shandong Province Medical and Health Technology Development Plan [grant numbers 202104020224, 202212040403, 202312010854]; Shandong Province Traditional Chinese Medicine Science and Technology Plan [grant numbers 2021M151, Z-2023114], and Jinan Science and Technology Plan [grant number 202328074] and the Innovation Project of Shandong Academy of Medical Science.
Acknowledgments
We are really appreciative of the help provided by all team members and study participants.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1629284/full#supplementary-material
References
1. Zheng W, Han X, Lv Y. The general situation and group differences of chronic diseases in Chinese population. Soc Sci J. (2022) 11:139–49.
2. Peng W, Chen S, Chen X, Ma Y, Wang T, Sun X, et al. Trends in major non-communicable diseases and related risk factors in China 2002–2019: an analysis of nationally representative survey data. Lancet Reg Health West Pac. (2024) 43:100809. doi: 10.1016/j.lanwpc.2023.100809
3. Liu H, Yin P, Qi J, Zhou M. Burden of non-communicable diseases in China and its provinces, 1990-2021: results from the global burden of disease study 2021. Chin Med J. (2024) 137:2325–33. doi: 10.1097/cm9.0000000000003270
4. Micha R, Peñalvo JL, Cudhea F, Imamura F, Rehm CD, Mozaffarian D. Association between dietary factors and mortality from heart disease, stroke, and type 2 diabetes in the United States. JAMA. (2017) 317:912–24. doi: 10.1001/jama.2017.0947
5. Aune D, Keum N, Giovannucci E, Fadnes LT, Boffetta P, Greenwood DC, et al. Nut consumption and risk of cardiovascular disease, total cancer, all-cause and cause-specific mortality: a systematic review and dose-response meta-analysis of prospective studies. BMC Med. (2016) 14:207. doi: 10.1186/s12916-016-0730-3
6. Tammi R, Männistö S, Maukonen M, Kaartinen NE. Whole grain intake, diet quality and risk factors of chronic diseases: results from a population-based study in Finnish adults. Eur J Nutr. (2024) 63:397–408. doi: 10.1007/s00394-023-03272-z
7. Zekrumah M, Begua P, Razak A, Wahab J, Moffo N, Ivane A, et al. Role of dietary polyphenols in non-communicable chronic disease prevention, and interactions in food systems: an overview. Nutrition. (2023) 112:112034. doi: 10.1016/j.nut.2023.112034
8. Tapsell LC, Neale EP, Satija A, Hu FB. Foods, nutrients, and dietary patterns: interconnections and implications for dietary guidelines. Adv Nutr. (2016) 7:445–54. doi: 10.3945/an.115.011718
9. Hong X, Ye Q, Wang Z, Yang H, Chen X, Zhou H, et al. Reproducibility and validity of dietary patterns identified using factor analysis among Chinese populations. Br J Nutr. (2016) 116:842–52. doi: 10.1017/S000711451600249X
10. Zhao J, Li Z, Gao Q, Zhao H, Chen S, Huang L, et al. A review of statistical methods for dietary pattern analysis. Nutr J. (2021) 20:37. doi: 10.1186/s12937-021-00692-7
11. Guo Q, Ma Z, Zhu C, Zeng Q. Association of dietary pattern and physical activity with lipid-related indices among Chinese population: a cross-sectional study. Lipids Health Dis. (2020) 19:244. doi: 10.1186/s12944-020-01420-6
12. Wang H, Qu M, Yang P, Yang B, Deng F. Dietary patterns and cardio-cerebrovascular disease in a Chinese population. Nutr Res Pract. (2015) 9:313–8. doi: 10.4162/nrp.2015.9.3.313
13. Qin C, Lv J, Yu C, Guo Y, Bian Z, Gao M. et al. Dietary patterns and cardiometabolic diseases in 05 million Chinese adults: a 10-year cohort study. Nutr J. (2021) 20:74. doi: 10.1186/s12937-021-00730-4
14. Sobal J, Bisogni CA. Constructing food choice decisions. Ann Behav Med. (2009) 38(Suppl 1):S37–46. doi: 10.1007/s12160-009-9124-5
15. Martinho Vjpd, Bartkiene E, Djekic I, Tarcea M, Barić IC, Cernelič-Bizjak M, et al. Determinants of economic motivations for food choice: insights for the understanding of consumer behaviour. Int J Food Sci Nutr. (2022) 73:127–39. doi: 10.1080/09637486.2021.1939659
16. Fang Y, Xia J, Lian Y, Zhang M, Kang Y, Zhao Z, et al. The burden of cardiovascular disease attributable to dietary risk factors in the provinces of China, 2002–2018: a nationwide population-based study. Lancet Reg Health West Pac. (2023) 37:100784. doi: 10.1016/j.lanwpc.2023.100784
17. Lyu J, Zhang W, Li W, Wang S, Zhang J. Epidemic of chronic diseases and the related healthy lifestyle interventions in rural areas of Shandong Province, China. BMC Public Health. (2020) 20:606. doi: 10.1186/s12889-020-08729-y
18. Wu G. Dietary survey of residents in Shandong province and the relationship between dietary pattern and common chronic diseases (doctoral thesis). Jilin University, Changchun, China (2024).
19. World Health Organization. A Healthy Lifestyle - Who Recommendations. (2010). Available online at: https://www.who.int/europe/news-room/fact-sheets/item/a-healthy-lifestyle-who-recommendations (Acccessed January 10, 2025).
20. World Health Organization. Waist Circumference and Waist-Hip Ratio: Report of a Who Expert Consultation. (2008). Available online at: https://www.who.int/publications/i/item/9789241501491 (Accessed January 8, 2025).
21. World Health Organization. Hypertension. (2023). Available from: https://www.who.int/news-room/fact-sheets/detail/hypertension (Accessed January 20, 2025).
22. Chen Z, Zou Y, Yang F, Ding XH, Cao C, Hu H, et al. Relationship between creatinine to body weight ratios and diabetes mellitus: a Chinese cohort study. J Diabetes. (2022) 14:167–78. doi: 10.1111/1753-0407.13248
23. Zhu J, Zhang Y, Wu Y, Xiang Y, Tong X, Yu Y, et al. Obesity and dyslipidemia in Chinese adults: a cross-sectional study in Shanghai, China. Nutrients. (2022) 14:2321. doi: 10.3390/nu14112321
24. Kamtchum-Tatuene J, Jickling GC. Blood biomarkers for stroke diagnosis and management. Neuromolecular Med. (2019) 21:344–68. doi: 10.1007/s12017-019-08530-0
25. Liu L, Chen W, Zhou H, Duan W, Li S, Huo X, et al. Chinese stroke association guidelines for clinical management of cerebrovascular disorders: executive summary and 2019 update of clinical management of ischaemic cerebrovascular diseases. Stroke Vasc Neurol. (2020) 5:159–76. doi: 10.1136/svn-2020-000378
26. Wang H, Yuan Y, Song L, Qiu G, Lai X, Yang L, et al. Association between education and the risk of incident coronary heart disease among middle-aged and older Chinese: the Dongfeng-Tongji cohort. Sci Rep. (2017) 7:776. doi: 10.1038/s41598-017-00880-8
27. Tayyem RF, Al-Shudifat AE, Johannessen A, Bawadi HA, AbuMweis SS, Agraib LM, et al. Dietary patterns and the risk of coronary heart disease among Jordanians: a case-control study. Nutr Metab Cardiovasc Dis. (2018) 28:262–9. doi: 10.1016/j.numecd.2017.10.026
28. Chiavaroli L, Viguiliouk E, Nishi SK, Blanco Mejia S, Rahelić D, Kahleová H, et al. Dash dietary pattern and cardiometabolic outcomes: an umbrella review of systematic reviews and meta-analyses. Nutrients. (2019) 11:338. doi: 10.3390/nu11020338
29. Ghasempour Dabaghi G, Zarepur E, Rabiee Rad M, Mohammadifard N, Haghighatdoost F, Khosravi A, et al. Dietary patterns and premature coronary artery disease: result from the Iran premature coronary artery disease (Ipad) study. BMC Cardiovasc Disord. (2024) 24:683. doi: 10.1186/s12872-024-04333-9
30. Uffelman CN, Chan NI, Davis EM, Wang Y, McGowan BS, Campbell WW. An assessment of mushroom consumption on cardiometabolic disease risk factors and morbidities in humans: a systematic review. Nutrients. (2023) 15:1079. doi: 10.3390/nu15051079
31. Kumar K, Mehra R, Guiné RPF, Lima MJ, Kumar N, Kaushik R, et al. Edible mushrooms: a comprehensive review on bioactive compounds with health benefits and processing aspects. Foods. (2021) 10:2996. doi: 10.3390/foods10122996
32. Kalita P, Ahmed AB, Sen S, Chakraborty R. A comprehensive review on polysaccharides with hypolipidemic activity: occurrence, chemistry and molecular mechanism. Int J Biol Macromol. (2022) 206:681–98. doi: 10.1016/j.ijbiomac.2022.02.189
33. Cho TJ, Rhee MS. Health functionality and quality control of laver (porphyra, pyropia): current issues and future perspectives as an edible seaweed. Mar Drugs. (2019) 18:14. doi: 10.3390/md18010014
34. Inoue N, Yamano N, Sakata K, Nagao K, Hama Y, Yanagita T. The sulfated polysaccharide porphyran reduces apolipoprotein B100 secretion and lipid synthesis in Hepg2 cells. Biosci Biotechnol Biochem. (2009) 73:447–9. doi: 10.1271/bbb.80688
35. Zhang Y, Liu T, Qu ZJ, Wang X, Song WG, Guo SD. Laminaria Japonica Aresch-derived fucoidan ameliorates hyperlipidemia by upregulating LXRs and suppressing SREBPs. Cardiovasc Ther. (2024) 2024:8649365. doi: 10.1155/2024/8649365
36. Marventano S, Izquierdo Pulido M, Sánchez-González C, Godos J, Speciani A, Galvano F, et al. Legume consumption and CVD risk: a systematic review and meta-analysis. Public Health Nutr. (2017) 20:245–54. doi: 10.1017/S1368980016002299
37. Bazzano LA. Effects of soluble dietary fiber on low-density lipoprotein cholesterol and coronary heart disease risk. Curr Atheroscler Rep. (2008) 10:473–7. doi: 10.1007/s11883-008-0074-3
38. Schoeneck M, Iggman D. The effects of foods on LDL cholesterol levels: a systematic review of the accumulated evidence from systematic reviews and meta-analyses of randomized controlled trials. Nutr Metab Cardiovasc Dis. (2021) 31:1325–38. doi: 10.1016/j.numecd.2020.12.032
39. Gylling H, Plat J, Turley S, Ginsberg HN, Ellegård L, Jessup W, et al. Plant sterols and plant stanols in the management of dyslipidaemia and prevention of cardiovascular disease. Atherosclerosis. (2014) 232:346–60. doi: 10.1016/j.atherosclerosis.2013.11.043
40. Chatterjee C, Gleddie S, Xiao CW. Soybean bioactive peptides and their functional properties. Nutrients. (2018) 10:1211. doi: 10.3390/nu10091211
41. Zhang B, Xiong K, Cai J, Ma A. Fish consumption and coronary heart disease: a meta-analysis. Nutrients. (2020) 12:2278. doi: 10.3390/nu12082278
42. Khan SU, Lone AN, Khan MS, Virani SS, Blumenthal RS, Nasir K, et al. Effect of omega-3 fatty acids on cardiovascular outcomes: a systematic review and meta-analysis. EClinicalMedicine. (2021) 38:100997. doi: 10.1016/j.eclinm.2021.100997
43. Mason RP, Libby P, Bhatt DL. Emerging mechanisms of cardiovascular protection for the omega-3 fatty acid eicosapentaenoic acid. Arterioscler Thromb Vasc Biol. (2020) 40:1135–47. doi: 10.1161/ATVBAHA.119.313286
44. Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. (2011) 58:2047–67. doi: 10.1016/j.jacc.2011.06.063
45. Li N, Wu X, Zhuang W, Xia L, Chen Y, Wang Y, et al. Green leafy vegetable and lutein intake and multiple health outcomes. Food Chem. (2021) 360:130145. doi: 10.1016/j.foodchem.2021.130145
46. Trautwein EA, McKay S. The role of specific components of a plant-based diet in management of dyslipidemia and the impact on cardiovascular risk. Nutrients. (2020) 12:2671. doi: 10.3390/nu12092671
47. Sarker U, Hossain MM, Oba S. Nutritional and antioxidant components and antioxidant capacity in green morph amaranthus leafy vegetable. Sci Rep. (2020) 10:1336. doi: 10.1038/s41598-020-57687-3
48. Santhakumar AB, Battino M, Alvarez-Suarez JM. Dietary polyphenols: structures, bioavailability and protective effects against atherosclerosis. Food Chem Toxicol. (2018) 113:49–65. doi: 10.1016/j.fct.2018.01.022
49. Song W, Derito CM, Liu MK, He X, Dong M, Liu RH. Cellular antioxidant activity of common vegetables. J Agric Food Chem. (2010) 58:6621–9. doi: 10.1021/jf9035832
50. Hu Y, Willett WC, Manson JAE, Rosner B, Hu FB, Sun Q. Intake of whole grain foods and risk of coronary heart disease in US men and women. BMC Med. (2022) 20:192. doi: 10.1186/s12916-022-02396-z
51. Ren Y, Liu Y, Sun XZ, Wang BY, Zhao Y, Liu DC, et al. Chocolate consumption and risk of cardiovascular diseases: a meta-analysis of prospective studies. Heart. (2019) 105:49–55. doi: 10.1136/heartjnl-2018-313131
52. Willett WC, Stampfer MJ, Manson JE, Colditz GA, Speizer FE, Rosner BA, et al. Intake of trans fatty acids and risk of coronary heart disease among women. Lancet. (1993) 341:581–5. doi: 10.1016/0140-6736(93)90350-P
53. Qin P, Zhang M, Han M, Liu D, Luo X, Xu L, et al. Fried-food consumption and risk of cardiovascular disease and all-cause mortality: a meta-analysis of observational studies. Heart. (2021) 107:1567–75. doi: 10.1136/heartjnl-2020-317883
54. Djoussé L, Zhou G, McClelland RL, Ma N, Zhou X, Kabagambe EK, et al. Egg consumption, overall diet quality, and risk of type 2 diabetes and coronary heart disease: a pooling project of US prospective cohorts. Clin Nutr. (2021) 40:2475–82. doi: 10.1016/j.clnu.2021.03.003
55. Mousavi SM, Zargarzadeh N, Rigi S, Persad E, Pizarro AB, Hasani-Ranjbar S, et al. Egg consumption and risk of all-cause and cause-specific mortality: a systematic review and dose-response meta-analysis of prospective studies. Adv Nutr. (2022) 13:1762–73. doi: 10.1093/advances/nmac040
56. Qin C, Lv J, Guo Y, Bian Z, Si J, Yang L, et al. Associations of egg consumption with cardiovascular disease in a cohort study of 05 million Chinese adults. Heart. (2018) 104:1756–63. doi: 10.1136/heartjnl-2017-312651
57. Vaz JDS, Buffarini R, Kac G, Bielemann RM, Oliveira I, Menezes AB, et al. Dietary patterns are associated with blood lipids at 18-year-olds: a cross-sectional analysis nested in the 1993 Pelotas (Brazil) birth cohort. Nutr J. (2018) 17:77. doi: 10.1186/s12937-018-0389-z
58. Na L, Han T, Zhang W, Wu X, Na G, Du S, et al. A snack dietary pattern increases the risk of hypercholesterolemia in Northern Chinese adults: a prospective cohort study. PLoS ONE. (2015) 10:e0134294. doi: 10.1371/journal.pone.0134294
59. Htun NC, Suga H, Imai S, Shimizu W, Ishikawa-Takata K, Takimoto H. Dietary pattern and its association with blood pressure and blood lipid profiles among Japanese adults in the 2012 Japan national health and nutrition survey. Asia Pac J Clin Nutr. (2018) 27:1048–61. doi: 10.6133/apjcn.072018.04
60. Kim HA, Shin HR, Song S. Dietary patterns derived by reduced rank regression are associated with lipid disorders among Korean adults: a cross-sectional analysis. Lipids Health Dis. (2024) 23:25. doi: 10.1186/s12944-024-02007-1
61. Hannou SA, Haslam DE, McKeown NM, Herman MA. Fructose metabolism and metabolic disease. J Clin Invest. (2018) 128:545–55. doi: 10.1172/JCI96702
62. Herman MA, Samuel VT. The sweet path to metabolic demise: fructose and lipid synthesis. Trends Endocrinol Metab. (2016) 27:719–30. doi: 10.1016/j.tem.2016.06.005
63. Haslam DE, Chasman DI, Peloso GM, Herman MA, Dupuis J, Lichtenstein AH, et al. Sugar-sweetened beverage consumption and plasma lipoprotein cholesterol, apolipoprotein, and lipoprotein particle size concentrations in US adults. J Nutr. (2022) 152:2534–45. doi: 10.1093/jn/nxac166
64. Zhu Z, He Y, Wang Z, He X, Zang J, Guo C, et al. The associations between sugar-sweetened beverage intake and cardiometabolic risks in Chinese children and adolescents. Pediatr Obes. (2020) 15:e12634. doi: 10.1111/ijpo.12634
65. Ejtahed HS, Bahadoran Z, Mirmiran P, Azizi F. Sugar-sweetened beverage consumption is associated with metabolic syndrome in Iranian adults: Tehran lipid and glucose study. Endocrinol Metab. (2015) 30:334–42. doi: 10.3803/EnM.2015.30.3.334
66. Haslam DE, Peloso GM, Herman MA, Dupuis J, Lichtenstein AH, Smith CE, et al. Beverage consumption and longitudinal changes in lipoprotein concentrations and incident dyslipidemia in US adults: the Framingham heart study. J Am Heart Assoc. (2020) 9:e014083. doi: 10.1161/JAHA.119.014083
67. Wali JA, Jarzebska N, Raubenheimer D, Simpson SJ, Rodionov RN, O'Sullivan JF. Cardio-metabolic effects of high-fat diets and their underlying mechanisms-a narrative review. Nutrients. (2020) 12:1505. doi: 10.3390/nu12051505
68. Chiu S, Williams PT, Krauss RM. Effects of a very high saturated fat diet on LDL particles in adults with atherogenic dyslipidemia: a randomized controlled trial. PLoS ONE. (2017) 12:e0170664. doi: 10.1371/journal.pone.0170664
69. Oteng AB, Kersten S. Mechanisms of action of trans fatty acids. Adv Nutr. (2020) 11:697–708. doi: 10.1093/advances/nmz125
70. Mavlanov U, Czaja TP, Nuriddinov S, Dalimova D, Dragsted LO, Engelsen SB, et al. The effects of industrial processing and home cooking practices on trans-fatty acid profiles of vegetable oils. Food Chem. (2025) 469:142571. doi: 10.1016/j.foodchem.2024.142571
71. Klop B, Elte JW, Cabezas MC. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients. (2013) 5:1218–40. doi: 10.3390/nu5041218
72. Astrup A, Geiker NRW, Magkos F. Effects of full-fat and fermented dairy products on cardiometabolic disease: food is more than the sum of its parts. Adv Nutr. (2019) 10:924s−30s. doi: 10.1093/advances/nmz069
73. Poppitt SD. Cow's milk and dairy consumption: is there now consensus for cardiometabolic health? Front Nutr. (2020) 7:574725. doi: 10.3389/fnut.2020.574725
74. Schmidt KA, Cromer G, Burhans MS, Kuzma JN, Hagman DK, Fernando I, et al. Impact of low-fat and full-fat dairy foods on fasting lipid profile and blood pressure: exploratory endpoints of a randomized controlled trial. Am J Clin Nutr. (2021) 114:882–92. doi: 10.1093/ajcn/nqab131
75. Shi W, Huang X, Schooling CM, Zhao JV. Red meat consumption, cardiovascular diseases, and diabetes: a systematic review and meta-analysis. Eur Heart J. (2023) 44:2626–35. doi: 10.1093/eurheartj/ehad336
76. Papp RE, Hasenegger V, Ekmekcioglu C, Schwingshackl L. Association of poultry consumption with cardiovascular diseases and all-cause mortality: a systematic review and dose response meta-analysis of prospective cohort studies. Crit Rev Food Sci Nutr. (2023) 63:2366–87. doi: 10.1080/10408398.2021.1975092
Keywords: dietary pattern, common chronic diseases, factor analysis, Chinese adults, cross-sectional study
Citation: Wu X, Liu J, Li X, Zhang W, Yang Y, Ma J, Cao M, Cheng M, Wu G, Xiu H and Du Z (2025) The association of dietary pattern with the risk of common chronic diseases in Shandong Province, China: a cross-sectional study. Front. Public Health 13:1629284. doi: 10.3389/fpubh.2025.1629284
Received: 15 May 2025; Accepted: 30 July 2025;
Published: 26 August 2025.
Edited by:
Sisi Cao, San Diego State University, United StatesReviewed by:
Jinyu Wang, Qingdao University, ChinaHesti Permata Sari, Jenderal Soedirman University, Indonesia
Copyright © 2025 Wu, Liu, Li, Zhang, Yang, Ma, Cao, Cheng, Wu, Xiu and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guangjian Wu, bml1bml1MTAyODg4ODhAMTYzLmNvbQ==; Haidi Xiu, eGhkMTk4MjQxM0AxNjMuY29t; Zhongjun Du, ZHV6ajE5ODFAMTYzLmNvbQ==; ZHV6aG9uZ2p1bkBzZGZtdS5lZHUuY24=
 Xi Wu1
Xi Wu1 Zhongjun Du
Zhongjun Du