Abstract
The global concern about plastics has been amplified due to their widespread contamination in the environment and their ability to cross biological barriers in living organisms. However, our understanding of their bioaccumulation, toxicity, and interaction with other environmental pollutants remains limited. Plastics are classified into three categories: macro-(MAP > 5 mm), micro-(MIP, <5 mm), and nanoplastics (NAP≤ 100 nm). Among these, NAPs have superior sorption capacity, a large surface area, and a greater ability to release co-contaminants into tissues, resulting in more complex and harmful effects compared to MAPs and MIPs. To assess the toxic effects of NAPs, particularly their genotoxicity in fish, we carried out a bibliographic search in PubMed using the search terms “nanoplastics” and “fish,” which yielded 233 articles. These studies focused on various polymers including polyamide (PA), polycarbonate (PC), polyethylene (PE), polyethylene terephthalate (PET), polymethylmethacrylate (PMMA), polypropylene (PPP), polystyrene (PS), and polyvinyl chloride (PVC). We further refined our search by including fish species such as common carp, fathead minnows, medaka, tilapia, trout, and zebrafish and selected 114 articles for review. This article provides a comprehensive overview of the current state of knowledge on the effects of NAPs on fishes, emphasizing their interaction with co-contaminants including metals, polycyclic aromatic hydrocarbons, pharmaceuticals, pesticides, antibiotics, plastic additives, and endocrine disruptors found in the aquatic environments. Our findings indicate that among fish species, zebrafish (∼68%) is the most frequently studied, while PS (∼89%) is the most commonly encountered NAP in the aquatic ecosystems. Despite substantial experimental variability, our systematic review highlights that NAPs accumulate in various tissues of fish including the skin, muscle, gill, gut, liver, heart, gonads, and brain across all developmental stages, from embryos to adults. NAP exposure leads to significant adverse effects including increased oxidative stress, decreased locomotor and foraging activities, altered growth, immunity, lipid metabolism, and induced neurotoxicity. Furthermore, NAP exposure modulates estrogen–androgen–thyroid–steroidogenesis (EATS) pathways and shows potential intergenerational effects. Although the USEPA and EU are aware of the global impacts of plastic pollution, the prolonged persistence of plastics continues to pose a significant risk to both aquatic life and human health.
1 Introduction
Plastic particles are introduced into the environment through industrial activities, human practices, and inadequate waste management systems (Chen et al., 2017a; Gigault et al., 2018; Cox et al., 2019; Ebere et al., 2019; Strungaru et al., 2019; Kokalj et al., 2021). In recent decades, plastic pollution has emerged as the second largest environmental challenge, ranking among global threats such as ocean acidification, climate change, and ozone depletion (Amaral-Zettler et al., 2015; Ma et al., 2016; Vethaak and Leslie, 2016; Schymanski et al., 2018; Alimba and Faggio, 2019). The predominant source of plastic pollution stems from poor waste management practices including garbage dumping, improper disposal of waste, and runoff from industrial or agricultural activities (Leslie et al., 2017; Mahon et al., 2017; Triebskorn et al., 2019). The onset of the COVID-19 pandemic further exacerbated plastic contamination with the widespread use of personal protective equipment (e.g., face masks) and single-use packaging materials, contributing to a significant rise in plastic waste (Aragaw, 2020; Fadare and Okoffo, 2020; Yudell et al., 2020; Patricio Silva et al., 2021; Vanapalli et al., 2021; Afrin et al., 2022; Cho et al., 2022). Plastic waste once released into the environment does not decompose rapidly. Instead, it undergoes gradual decomposition, involving photolysis, oxidation, abrasion, hydrolysis, and biodegradation over an extended period of time (Sudhakar et al., 2007; Watters et al., 2010; Andrady, 2011; Maity and Pramanick, 2020). Larger plastic particles eventually break down into microplastics (MIPs; diameter ranging between 100 and 50,00,000 nm) and nanoplastics (NAPs, diameter ≤100 nm) through mechanisms such as wave action, mechanical wear and tear, photooxidation, and microbial degradation (O’Brine and Thompson, 2010; Lambert et al., 2013; Cozar et al., 2014; Gigault et al., 2016; Lambert and Wagner, 2016). NAPs are potentially more hazardous than MIPs (Rochman et al., 2013; Almeida et al., 2019; Domenech et al., 2020; Liang et al., 2021; Yang and Wang, 2022; Yang and Wang, 2023; Huang et al., 2023; Huang et al., 2023). The European Food Safety Authority (EFSA) has indicated that particles less than 150 µm (150,000 nm) in diameter may cross the intestinal mucosal barrier, while particles less than 1.5 µm (1,500 nm) in diameter can be transported into deeper tissues, including vital organs. Several types of MIPs (<50,00,000 nm), including polystyrene (PS), polyvinyl chloride (PVC), polyethylene (PE), polyethylene terephthalate (PET), polymethyl methacrylate (PMMA), polyoxymethylene, and polypropylene (PPP), have been found in various environmental compartments (de Sa et al., 2018) and have also been detected in the liver tissue of individuals with liver cirrhosis (Horvatits et al., 2022).
NAPs, often used as raw materials in products such as facial cleaners, scrubs, toothpaste, and other personal care items, are unintentional byproducts of plastic degradation and manufacturing processes (Enfrin et al., 2020; Kim, 2021; Kim et al., 2021). These particles, typically less than 1,000 nm in size, exhibit colloidal behavior and possess distinct chemical and physical characteristics compared to bulk plastics (Sharifi et al., 2012; Chen et al., 2017b; Pitt et al., 2018a; Lee et al., 2019). Due to their small size and high surface area, NAPs are highly efficient at both physical and chemical absorption of other environmental contaminants (Hartmann et al., 2017; Lee et al., 2019; Trevisan et al., 2019; Bhagat et al., 2020; Bhagat et al., 2021). Moreover, they are easily transferred through the food chain (Chae et al., 2018). Once absorbed into the body, NAPs can spread into the organs, including the brain and gonads, by overcoming the biological barriers (Lehner et al., 2019). Therefore, understanding their environmental fate, bioavailability, intake, and the potential effects on different organisms, is critical (Parenti et al., 2019; Lins et al., 2022) for humans. The persistence and degradation of macro- and MIPs contribute to the increase in NAPs in aquatic environments, including seas (Thompson et al., 2004; Cole et al., 2011; Harshvardhan and Jha, 2013; Earni-Cassola et al., 2019; Gigault et al., 2016), shorelines (Browne, 2011), estuaries (Saedi and Thompson, 2014), beach sediments (Imhof et al., 2013), lakes (Eriksen et al., 2013; Free et al., 2014), and freshwater ecosystems (Wagner et al., 2014; Vendel et al., 2017; Brandts et al., 2018; Pitt et al., 2018a; b; Parenti et al., 2019; Barria et al., 2020). These particles not only pose a direct toxicological threat but can also adsorb harmful chemicals, further enhancing their potential for inflicting biological harm (Jinhui et al., 2019; Campanale et al., 2020; Gonzalez-Fernandez et al., 2021). In aquatic organisms, such as zebrafish, NPs can be ingested and bio-fragmented within the body, potentially leading to toxicity and other physiological disruptions (Jovanovic, 2017; Khan and Ali, 2023; Barria et al., 2020; Duan et al, 2020).
Although PS is often used in risk assessments due to its commercial availability and varied sizes and surface charges, other plastics such as PE and PPP are also prevalent in environmental debris but have been less studied (Koelmans et al., 2019; de Ruijter et al., 2020). The current research gap necessitates a more comprehensive investigation of NAPs from various plastic types to assess their toxicity and ecological impacts. The aim of this systematic review is to evaluate the toxicological potential of NAPs in relation to plastic type, particle size, and their ability to adsorb hydrophobic pollutants, with a particular focus on the genotoxic effects in aquatic organisms such as fish. We hypothesize that NAPs upon crossing biological barriers and entering cells may trigger oxidative stress, induce DNA damage, and enhance the bioactivity of adsorbed contaminants. These processes may disrupt critical biological functions, including digestion, metabolism, neural activity and behavior, reproduction, and development, and potentially lead to intergenerational/transgenerational effects that could have significant implications on human health.
2 Materials and methods
2.1 Literature search strategy
We conducted a comprehensive literature search to find journal articles that examine the toxic effects of NAPs on fish, with a special focus on the impacts at the molecular level. The electronic search was performed in PubMed (http://www.ncbi.nlm.nih.gov/pubmed) until 29 February 2024, using the following search terms: “nanoplastics,” “fish,” and the different polymers of NAPs found in the aquatic environment (e.g., PA, PC, PE, PET, PMMA, PPP, PS, and PVC) (Table 1). The search also included the common names of the six fish species: common carp, fathead minnows, medaka, tilapia, trout, and zebrafish, previously followed in the studies by Dasmahapatra et al. (2023), Dasmahapatra et al. (2024). PubMed was selected as the primary database due to its reputation as a reliable and authoritative source for peer-reviewed scientific literature.
TABLE 1
| Serial number | Common name and molecular formula | IUPAC name | Chemical structure | Molecular weight (Da)/molar mass (g/mol) |
|---|---|---|---|---|
| 1 | Polyamide | Poly [imino (alkanedioyl)] |

|
10,000–50,000 Da |
| 2 | Polycarbonate (C16H18O5) | Acrylonitrile–butadiene–styrene |

|
290.32 |
| 3 | Polyethylene (C2H4) | Poly (methylene) |

|
28.05 |
| 4 | Polyethylene terephthalate (C10H12O6) | Ploy (ethyl benzene-1,4-dicarboxylate) |

|
228.19 |
| 5 | Polypropylene (C22H42O3) | Poly (1-methylethylene) |

|
354.56 |
| 6 | Polyethylene methacrylate (C5H10O2) | Poly (methyl 2-methylpropenoate) |

|
102.13 |
| 7 | Polystyrene (CH2CH(C6H5) | Poly (1-phenylethylene) |

|
2.01 |
| 8 | Polyvinyl chloride (C2H3Cl) | Poly (1-chloroethylene) |

|
62.49 |
Chemical structures of plastic polymers followed in this review.
LDPE = Low-density polyethylene; PA = polyamide; PC = polycarbonate; PE = polyethylene, PET = polyethylene terephthalate; PMMA = polyethylene methacrylate; PPP = polypropylene, PS = polystyrene; PVC = polyvinyl chloride. In two articles, part of the studies used plastic sizes ≤ 100 nm, and part of the studies used plastic sizes ≥ 100 nm. For this reason, these articles are mentioned in the exclusion as well as in the inclusion boxes.
For this review, we focused primarily on bony fish, with the selected species serving as representative examples of the class Osteichthyes (Figure 1). The term carp was used to refer collectively to several species, including common carp (Cyprinus carpio), grass carp (Ctenopharyngodon idella), silver carp (Hypophthalmichthys molitrix) and tooth carp (Aphaniops hormuzensis) (Estrela et al., 2021; Guimaraes et al., 2021; Hamed et al., 2022; Liu S. et al., 2022; Wu et al., 2022; Zhang X. et al., 2022; Saemi-Komsari et al., 2023; Li Z. et al., 2024; Zhang et al., 2024a). Similarly, the term medaka encompassed Chinese rice fish (Oryzias sinensis), Hainan medaka (Oryzias curvinotus), Japanese medaka (Oryzias latipes), and marine medaka (Oryzias melastigma) (Chae et al., 2018; Kang et al., 2021; Zhang et al., 2021; Zhang et al., 2024 YT.; He et al., 2022; Chen Y. et al., 2023; Gao D. et al., 2023; Li X. et al., 2023; Wang F. et al., 2023; Yu et al., 2023; Zhou et al., 2023a; Zhou et al., 2023b; Li X. et al., 2024). The term tilapia was used to refer to various species such as red tilapia (Oreochromis niloticus), Nile tilapia (Oreochromis niloticus), and Mozambique tilapia (O. mossambicus) (Ding et al., 2018; Pang et al., 2021; Hao et al., 2023; Wang W. et al., 2023; Zheng and Wang, 2024; Zheng et al., 2024).
FIGURE 1
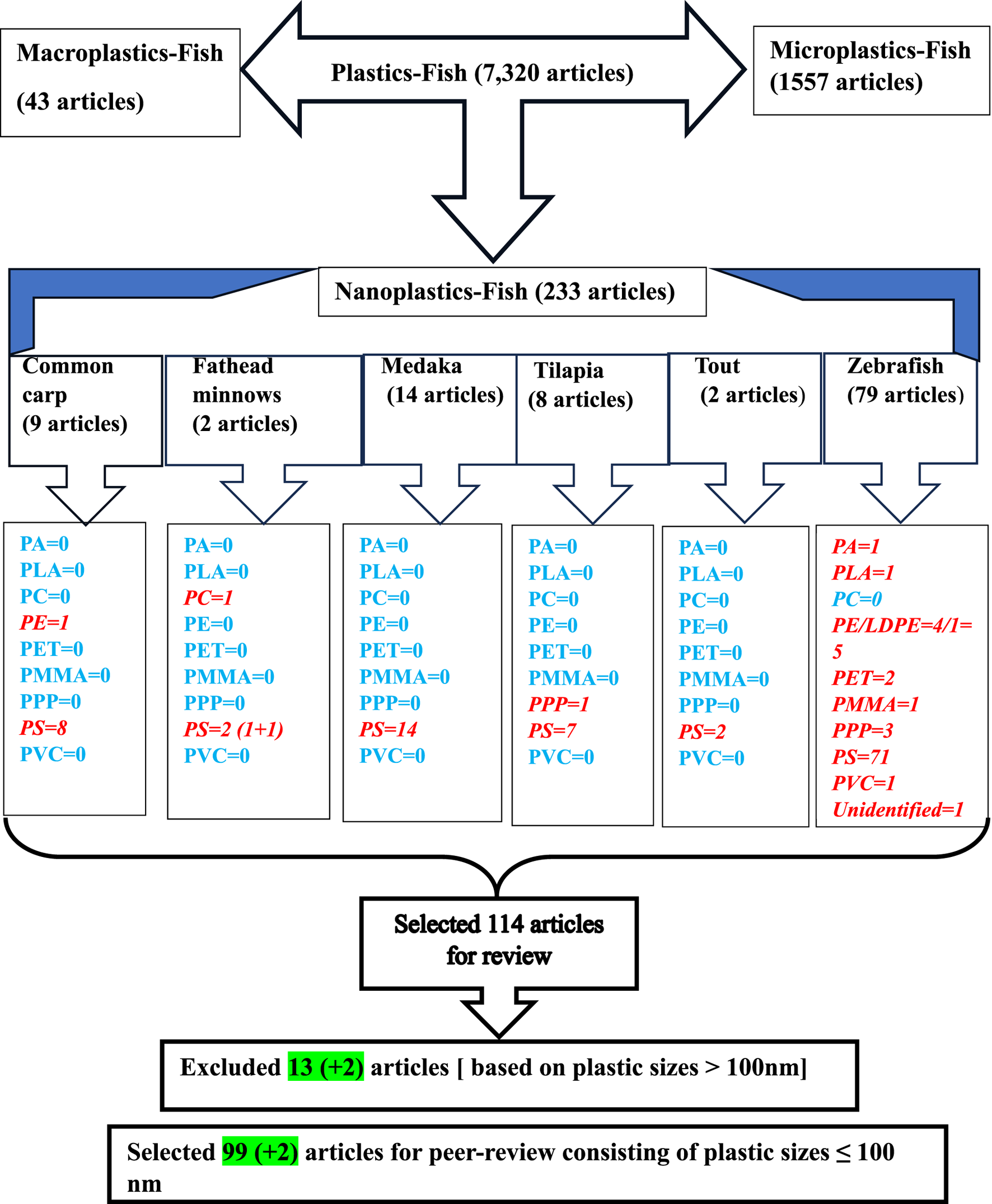
Flow chart of the literature search in PubMed (http://www.ncbi.nlm.nih.gov/pubmed).
The search yielded 114 peer-reviewed articles that highlight potential developmental, reproductive, neurological, immunological, and behavioral disorders in fish exposed to NAPs (Figure 1; Tables 2–9). A comprehensive summary of the findings has been compiled in Supplementary Table S1, which has been deposited in a public repository [Figshare (https://figshare.com) for reference and future update, if necessary.
TABLE 2
| Serial number | Authors | Polymer | Fish/stage of Development | Sizes | Concentration /dose |
Duration | Mode of exposure /additives |
|---|---|---|---|---|---|---|---|
| 1 | Aliakbarzadeh et al., (2023) | PS | Zebrafish (Danio rerio)/adults | 20-80 nm (average 57.5 nm) | 0.1, 1, 10 and 100 µg/L | 45 days | Waterborne/4- nonylphenol (1µg/L) |
| 2 | Barreto et al., (2021) | PS | Zebrafish (Danio rerio)/embryos | 60 nm | 0.015, 1.5, and 150 mg/L | 96 h | Waterborne/SIM (0.015-150 µg/L) |
| 3 | Barreto et al., (2023) | PS | Zebrafish (Danio rerio)/embryos | 44 nm | 0.015, 1.5 mg/L | 96-120 hpf | Waterborne/DPH (0.01 and 10 mg/L) |
| 4 | Bashirova et al., (2023) | PET | Zebrafish (Danio rerio)/embryos (6 and 72 hpf) | Hydrodynamic diameter 70±5 nm | to 5, 10, 50, 100, 200 mg/L | Until 96- 120 hpf | Waterborne |
| 5 | Bhagat et al., (2022) | PS | Zebrafish (Danio rerio)/embryos | 50 nm | 1 mg/L | 96 h | Waterborne/ nAL2O3(1 mg/L) and nCeO3 (1 mg/L) |
| 6 | Brun et al., (2019) | PS | Zebrafish (Danio rerio) /embryos (72 hpf) | 25 nm | 20 mg/L | Until 120 hpf | Waterborne |
| 7 | Chackal et al., (2022) | PS | Zebrafish (Danio rerio)/embryos | 100 nm | 2.5 and 25 µg/L | Until 7 dpf | Waterborne/BDE-47 (10 ng/L) |
| 8 | Chae et al., (2018) | PS | Chinese rice fish (Oryzias sinensis)/ adults (F0) and larvae (F1) | 60.39, 57.45, 57.29 nm | 5 mg/L | Adults (F0) exposed for 7 days; larvae (F1) exposed for 24 h | Waterborne |
| 9 | Chen et al., (2017a) | PS | Zebrafish (Danio rerio)/embryos | 47 and 41000 nm | 1 mg/L | 120 h | Waterborne /EE2 (2 and 20 µg/L) |
| 10 | Chen et al., (2017b) | PS | Zebrafish (Danio rerio)/ adults (6 months old) | 47 nm | 1 mg/L | 3 days | Waterborne/BPA (0.78 µg/L) |
| 11 | Chen et al., (2022) | PS | Marine medaka (Oryzias melastigma) /embryos | 50, 500, and 6000 nm | 106 particles/L | 19 days | Waterborne |
| 12 | Chen et al., (2023a) | PS-NH2 and PS-COOH | Marine medaka (Oryzias melastigma) /embryos | 80 nm | 10 µg/L | 10 days with additional 10 days depuration | Waterborne (regular or acidified sea water) |
| 13 | Chen et al., (2023b) | PS, UV-PS, O3-PS | Zebrafish (Danio rerio)/embryos (8 hpf) | 80 nm | 0.5 and 5 mg/L | Until 120 hpf | Waterborne/ penicillin (1 and 10 µg/L) |
| 14 | Chen et al., (2023c) | PS | Zebrafish (Danio rerio)/embryos | 50 nm | 0.1, 1, 5, 10, 20, 30, and 50 mg/L | Until 120 hpf; evaluated on 5th, 7th, and 12th day | Waterborne/ Sodium nitroprusside (0.1,1, 10, 20, 30 and 40 µM) |
| 15 | Chen et al., (2024) | PS | Zebrafish (Danio rerio)/embryos (8hpf) | 80, 200, 500 nm | 0.1, 0.5, 1, 5, 10, 25, and 50 mg/L | 120 hpf, depurate 10 days | Waterborne |
| 16 | Cheng et al., (2022) | PS | Zebrafish (Danio rerio)/embryos | 50, 100 nm and micro-PS | 0.1, 0.5, 2 and 10 mg/L | 120 hpf | Waterborne |
| 17 | Clark et al., (2023a) | PS-Pd | Rainbow trout (Oncorhynchus mykiss)/juvenile | 200 nm | 10 mg/kg food | 3 and 7 days; depurated 7 days | Dietary |
| 18 | Clark et al., (2023b) | PS | Rainbow trout (Oncorhynchus mykiss)/juvenile | 35±8 nm | 5.9 µg/g food | 3,7,14 days | Dietary |
| 19 | Dai et al., (2023) | PS | Zebrafish (Danio rerio)/embryos | 20 nm | 2, 5, and 8 mg/L | 22, 46, and 72 hpf | Waterborne |
| 20 | Deng et al., (2023) | PS | Zebrafish (Danio rerio)/adults | 100 nm | 500 ng/mL | 28 days | Waterborne |
| 21 | De Souza Teodoro et al., (2024) | PET | Zebrafish (Danio rerio)/embryos | 68.06-955 nm and 1305000-2032000 nm | 0.5, 1, 5, 10, and 20 mg/L | 6 days | Waterborne |
| 22 | Ding et al., (2018) | PS | Red Tilapia (Oreochromis niloticus)/juveniles | 100 nm | 1, 10, 100 µg/L | 14 days | Waterborne |
| 23 | Ding et al., (2020) | PS | Red Tilapia (Oreochromis niloticus)/juveniles | 300, 5000, 7000-9000 nm | 100 µg/L | 6 and 14 days | Waterborne |
| 24 | Du et al., (2024) | PS | Zebrafish (Danio rerio)/adults | 50-100 nm | 1000 µg/L | 21 days | Waterborne/dietary exposure to high fat diet (24% crude fat) |
| 25 | Duan et al., (2023) | PS | Zebrafish (Danio rerio)/embryos (4 hpf) | 50 nm | 0.1, 0.5, and 1 mg/L | 72 h | Waterborne |
| 26a | Elizalde-Velazquez et al., (2020) | PS | Fathead minnows (Pimephales promelas)/ adult (males) | 50 nm | 5 µg/L (0.1 ml injected volume) | 48 h | IP |
| 26b | Elizalde-Velazquez et al., (2020) | PS | Fathead minnows (Pimephales promelas)/ adult (males) | 50 nm | 5 µg/L | 48 h | Trophic transfer (fed with daphnia which were consumed PS-exposed green algae) |
| 27 | Estrela et al., (2021) | PS | Grass carp (Ctenopharyngodon idella)/juveniles | 23.03±0.266 nm | 760 µg/L | 72 h | Waterborne/ZnO2 (760 µg/L) |
| 28 | Feng et al., (2022) | PS | Zebrafish (Danio rerio)/embryos | 100 nm | 100, 200, and 400 mg/L | 96 h | Waterborne |
| 29 | Gao et al., (2023a) | PS | Hainan medaka (Oryzias curvinotus) | 80 nm | 200 µg/L | 7 days | Waterborne/F53B (500 µg/L) |
| 30 | Gao et al., (2023b) | PS | Zebrafish (Danio rerio) /embryos (3 hpf) | 80 nm | 5, 10, 25, 50, 100 µg/L | 96 hpf | Waterborne/APAP (2-8 mM) |
| 31 | Geum and Yeo, (2022) | PS | Zebrafish (Danio rerio)/embryos | 50 nm | 5 mg/L | 4,8,12,24,32, 48, 72 hpf | Waterborne/PHE (0.5 and 1 mg/L and mucin from jelly fish (50 µg/L) |
| 32a | Greven et al., (2016) | PC | Fathead minnows (Pimephales promelas)/ neutrophils of adults | 158.7 nm | 0.025, 0.05, 0.1, 0.2, and 100 µg/mL | 2h | In vitro |
| 32b | Greven et al., (2016) | PS | Fathead minnows (Pimephales promelas)/ neutrophils of adults | 41 nm | 0.025, 0.05, 0.1, 0.2, and 100 µg/mL | 1-2 h | In vitro |
| 33 | Guimaraes et al., (2021) | PS | Grass carp (Ctenopharyngodon Idella)/juveniles | 23.03±0.266 nm (20-26 nm) | 0.04 ng/L, 34 ng/L, and 34 µg/L | 20 days | Waterborne |
| 34 | Habumugisha et al., (2023) | PS | Zebrafish (Danio rerio)/adults (males) | 50 nm | 5, 10, 15 mg/L | 30 days; depurated 16 days; evaluated; evaluated on 3, 6, 12, 18, 24, 30, 34, 38, 42, and 46 days. | Waterborne |
| 35 | Hamed et al., (2022) | PE | Common carp (Cyprinus carpio)/juvenile | 100 nm and > 100 nm | 100 mg/L | 15 days | Waterborne |
| 36 | Hao et al., (2023) | PS | Tilapia (Oreochromis niloticus)/ juveniles | 86 and 185 nm | 1 mg/L | 21 days, depurated 7 days | Waterborne |
| 37 | He et al., (2021). | PS | Zebrafish (Danio rerio)/adults (males and females) | 46 and 5800 nm | 2 mg/L | 21 days | Waterborne /TPhP (0.08, 0.5, 0.7, 1, 1.2, 1.5 mg/L) |
| 38 | He et al., (2022) | PS | Marine medaka (Oryzias melastigma)/adults | 100 nm | 3.45 mg/g | 30 days [F0]. [F1 offspring were evaluated 60 dph without any exposure) |
Dietary [F0]/ /SMG (94.62 mg/g) |
| 39 | Kang et al., (2021) | PS | Marine medaka (Oryzias melastigma)/larvae (7 dph) | 50 nm and 45 µm (45,000 nm) | 10 µg/mL and 2.5 µg/mL | 24 h (10µg/L). 1, 7, 14, and 120 days (2.5 µg/mL) |
Waterborne |
| 40 | Kantha et al., (2022) | PS | Zebrafish (Danio rerio)/ embryos | 25 nm | 10, 25, and 50 mg/L | 96 h | Waterborne |
| 41 | Khan and Ali (2023) | PE | Zebrafish (Danio rerio)/ adults | 10-100 µm (10,000-100,000 nm) | Unknown | 24h | Waterborne |
| 42 | Lee et al., (2019) | PS | Zebrafish (Danio rerio)/ embryos | 50, 200, 500 nm | 0.1 mg/L | 6, 24, 96 h | Waterborne |
| 43 | Lee et al., (2022) | PPP | Zebrafish (Danio rerio)/ embryos (24 hpf and 72 hpf) | 562.15±118.47 nm | 50 mg/L | 24 h | Waterborne |
| 44 | Li et al., (2023a) | PS | Zebrafish (Danio rerio)/ adults | 80 nm | 15 and 150 mg/L | 28 days | Waterborne/vitamin D (280 and 2800 IU/kg) |
| 45 | Li et al., (2023b) | PS | Marine medaka (Oryzias melastigma) /juveniles (2 months old) | 100 nm | 1 mg/L | 30 days | Waterborne/SMX (100µg/L) |
| 46 | Li et al., (2023c) | PE | Zebrafish (Danio rerio)/adults | 70 and 13500 nm | 20 mg/L | 21 days | Waterborne/PEMIP (20 mg/L) |
| 47 | Li et al., (2024a) | PS | Grass carp (Ctenopharyngodon idella)/juveniles | 80 nm | 10, 100, 1000 µg/L | 8 days; coexposure 3 days with 5 days preexposure with PS | Waterborne/Aeromonas hydrophilia (2X107CFU/mL) |
| 48 | Li et al., (2024b) | PS | Marine medaka (Oryzias melastigma) / larvae (3 dph) | 70, 500 and 2000 nm | 20, 200, and 2000 /L | 90 days | Trophic transfer (fed to rotifers and the rotifers were fed by the fish) |
| 49 | Lin et al., (2023) | PS | Zebrafish (Danio rerio)/adults (males and females) | 70 nm | 2 mg/L | 21 days | Waterborne/DES (1,10, 100 ng/L) |
| 50 | Ling et al., (2022) | PS | Zebrafish (Danio rerio)/adults (males and females) | 70 nm | 100µg/L | 90 days | Waterborne /MCLR (0.9, 4.5, and 22.5 µg/L) |
| 51 | Liu et al., (2021) | PS | Zebrafish (Danio rerio)/embryos | 100 nm | 10 µg/L | Until 120 hpf | Waterborne/BMDBM (1,10, and 100 µg/L) |
| 52 | Liu et al., (2022a) | PS | Grass carp (Ctenopharyngodon idella)/juveniles | 80 nm | 20, 200, 2000 µg/L | 7 days | Waterborne/TC (5000 µg/L) |
| 53 | Liu et al., (2022b) | PS | Zebrafish (Danio rerio)/embryos | 100 nm | 10 µg/L | 144h and depurated 72 h | Waterborne/AV0 (10 µg/L) |
| 54a | Manuel et al., (2022) | PMMA | Zebrafish (Danio rerio)/embryos | 32 nm | 0.001, 0.01,0.1, 1, 10, 100 mg/L | 96 h | Waterborne |
| 54b | Manuel et al., (2022) | PS | Zebrafish (Danio rerio)/embryos | 22 nm | 0.001, 0.01, 0.1, 1, 10, 100 mg/L | 96 h | Waterborne |
| 55 | Martin et al., (2023) | PS | Zebrafish (Danio rerio)/embryos | 30 and 100 nm | 0.1, 1, and 10 mg/L | 96 h | Waterborne |
| 56 | Martinez-Alvarez et al., (2022) | PS | Zebrafish (Danio rerio)/embryos | 50, 500, and 4500 nm | 0.069 µg/L- 50.1 mg/L | 120 h | Waterborne /B(a)P (0.1-10 mg/L) |
| 57 | Martin-Folgar et al., (2023) | PS | Zebrafish (Danio rerio)/embryos | 30 nm | 0.1, 0.5 and 3 mg/L | 120 hpf | Waterborne |
| 58a | Monikh et al., (2022) | PE | Zebrafish (Danio rerio) /embryos (6 hpf) |
50 nm | 3X1010 particles/L (0.000 25 mg/L) | 24 h | Waterborne |
| 58b | Monikh et al., (2022) | PPP | Zebrafish (Danio rerio) /embryos (6 hpf) |
50 nm | 3X1010 particles/L (.00022 mg/L) | 24h | Waterborne |
| 58c | Monikh et al., (2022) | PS | Zebrafish (Danio rerio) /embryos (6 hpf) |
200 and 600 nm | 3X1010 particles/L (PS 200 nm =0.13 mg/L; PS 600=3.5 mg/L) | 24 h | Waterborne/B(a)P (10 µg/L) |
| 58d | Monikh et al., (2022) | PVC | Zebrafish (Danio rerio) /embryos (6 hpf) |
200 nm | 3X1010 particle/L (0.17 mg/L) | 24 h | Waterborne/B(a)P (10 µg/L) |
| 59 | Pang et al., (2021) | PS | Tilapia (Oreochromis mossambicus)/larvae | 100 nm | 20 mg/L | 7 days and depurated 7 days | Waterborne |
| 60 | Parenti et al., (2019) | PS | Zebrafish (Danio rerio) /embryos (72 hpf) | 500 nm | 1 mg/L | 2 days (until 120 hpf) | Waterborne |
| 61 | Park and Kim (2022) | PS | Zebrafish (Danio rerio) /embryos (1 dpf) | 400 and 1000 nm | 7.5-60 mg/L | 3 days | Waterborne |
| 62 | Pedersen et al., (2020) | PS | Zebrafish (Danio rerio) /embryos (6 hpf) | 50, 200 nm | 10, 100, 1000, 10,000 µg/L | Until 120 hpf | Waterborne |
| 63 | Pitt et al., (2018a) | PS | Zebrafish (Danio rerio) /embryos (6 hpf) | 51 nm | 0.1, 1, and 10 mg/L | 120 h | Waterborne |
| 64 | Pitt et al., (2018b) | PS | Zebrafish (Danio rerio)/adults | 42 nm | 1 mg/g | 7 days | Dietary |
| 65 | Saemi-Komsari et al., (2023) | PS | Tooth Carp (Aphaniops hormuzensis)/ adults | 100-300 nm (average 185 nm) | 1, 5,10,25, 100, 200 mg/L and 1.1. 0.1, 1, 5 mg/L | 96h (waterborne)/3, 14, 28 days (dietary exposure) | Waterborne and dietary/ TCS (0.5 mg/kg) |
| 66 | Santos et al., (2022) | PS | Zebrafish (Danio rerio)/embryos | 44 nm | 0.015, 1.5,15, and 150 mg/L | 96-120 hpf | Waterborne/PHN (0.2, 2, and 20 mg/L) |
| 67 | Santos et al., (2024) | PS | Zebrafish (Danio rerio)/embryos | 23.03 ±0.266 nm | 0.04 ng/l, 34 ng/L and 34 µg/L | 144 hpf | Waterborne |
| 68 | Sarasamma et al., (2020) | PS | Zebrafish (Danio rerio)/adults | 70 nm | 0.5, 1.5 and 5 mg/L | 7 days, 30 days, 7 weeks |
Waterborne |
| 69 | Sendra et al., (2021) | PS | Zebrafish (Danio rerio) /larvae (120 hpf) | 50, 1000, 50,000 nm | 10 mg/L | 7 days | Waterborne |
| 70 | Senol et al., (2023) | PS | Zebrafish (Danio rerio)/adults | 134±2.9 nm | 25 mg/L | 96 h | Waterborne at 28°, 29°, and 30° C |
| 71 | Sokmen et al., (2020) | PS | Zebrafish (Danio rerio)/embryos | 20 nm | 3 nL of 270 mg/L | 120 h | Injected to fertilized eggs |
| 72 | Sulukan et al., (2022a) | PS | Zebrafish (Danio rerio) /embryos (4 hpf) | 20 nm | 3 nL of 270 mg/L | Grown 6 months and evaluated F1 offspring | Injected to fertilized eggs |
| 73 | Sulukan et al., (2022b) | PS | Zebrafish (Danio rerio)/adults | 100 nm | 25 mg/L | 96 h | Waterborne at 28°, 29°, and 30° C |
| 74 | Suman et al., (2023) | PS | Zebrafish (Danio rerio)/embryos | 500 nm | 0.1, 1, and 10 mg/L | 6 days | Waterborne |
| 75 | Sun et al., (2021) | PE | Zebrafish (Danio rerio) /embryos (6 hpf) | Hydrodynamic size 191.10 ±3.13 nm | 25, 50, 100, 200, 400, 600, 800, 1000 µg/mL | 48-96 h | Waterborne |
| 76a | Tamayo-Belda et al. (2023)) | LDPE | Zebrafish (Danio rerio) /embryos (4 hpf) | 164-91 nm | 0.001, 0.01,0.1,1, and 10 mg/L | 4h-96 h | Waterborne |
| 76b | Tamayo-Belda et al. (2023)) | PLA | Zebrafish (Danio rerio) /embryos (4 hpf) | 122-712 nm | 0.001, 0.01,0.1,1, and 10 mg/L | 4h-96 h | Waterborne |
| 76c | Tamayo-Belda et al. (2023)) | PPP | Zebrafish (Danio rerio) /embryos (4 hpf) | 164-220 nm | 0.001, 0.01,0.1,1, and 10 mg/L | 4h-96 h | Waterborne |
| 76d | Tamayo-Belda et al. (2023)) | PS | Zebrafish (Danio rerio) /embryos (4 hpf) | 91-825 nm | 0.001, 0.01,0.1,1, and 10 mg/L | 4h-96 h | Waterborne |
| 77 | Teng et al., (2022a) | PS-NH2 PS-COOH |
Zebrafish (Danio rerio)/embryos | 30-51 nm | 30 and 50 mg/L | 120 h | Waterborne |
| 78 | Teng et al., (2022b) | PS | Zebrafish (Danio rerio)/juveniles and adults | 44 nm | 1, 10, and 100 µg/L | 30 and 60 days | Waterborne |
| 79 | Teng et al., (2023) | PS | Zebrafish (Danio rerio)/ adults | 80 nm | 15 and 150 µg/L | 21 days | Waterborne/ vit D (280-2800 IU/kg, via food) |
| 80 | Trevisan et al., (2019) | PS | Zebrafish (Danio rerio) /embryos (6 hpf) | 44 nm | 1.1. 1, 10 mg/L | 96 h | Waterborne/PAH (5.07-25.36 µg/L) |
| 81 | Trevisan et al., (2020) | PS | Zebrafish (Danio rerio)/embryos | 44 nm | 1 mg/L | 7 days | Waterborne/PAH (5.073 ng/mL) |
| 82 | Van Pomeren et al., (2017) | PS | Zebrafish (Danio rerio)/embryos | 25, 50, 250,700 nm | 5-50 mg/L | 48 h | Waterborne |
| 83 | Varshney et al., (2023) | PS | Zebrafish (Danio rerio)/embryos | 15 nm | 50 mg/L | 96 h | Waterborne/ p, p’-DDE (100 µg/L) |
| 84 | Wang et al, (2022) | PS | Zebrafish (Danio rerio)/embryos | 80 nm | 0.05, 0.1, 1, and 10 mg/L | 120 hpf | Waterborne/BDE-47 (0.1 mg/L) |
| 85 | Wang et al., (2023a) | PS | Marine medaka (Oryzias melastigma) / adults | 100 nm | 5 mg/ g food | 30 days | Feeding/ SMG (0.5 and 5 mg/g food) |
| 86 | Wang et al., (2023b) | PS | Tilapia/juveniles | 100, 500, and 5,000 nm | 1, 10, 100 µg/L | 7 days | Waterborne |
| 87 | Wang et al, (2023c) | PS | Zebrafish (Danio rerio)/embryos | 80 nm | 0.05, 0.1, 1, 5, and 10 mg/L | 12-120hpf | Waterborne/BDE-47 (0.1 and 10 mg/L) |
| 88 | Wang et al., (2023d) | PS-COOH | Zebrafish (Danio rerio)/embryos | 50 nm | 1, 5, and 10 mg/L | 144 h | Waterborne |
| 89 | Wang et al., (2023e) | Nanoplastics (NAPs) | Zebrafish (Danio rerio)/adults (120 dpf) | 100 nm | 1 mg/L | 45 days | Waterborne/BPAF (200 µg/L) |
| 90 | Wu et al., (2021) | PS | Zebrafish (Danio rerio)/adults | 70 nm | 100 µg/L | 45 days; F1 embryos were evaluated without any further exposure | Waterborne/MCLR (0.9, 4.5, and 22.5 µg/L) |
| 91 | Wu et al., (2022) | PS | Carp /adult | 50, 100, and 400 nm | 1000 µg/L | 28 days | Waterborne |
| 92 | Wu et al., (2023) | PPP | Tilapia (Oreochromis niloticus)/juveniles | 100 nm and 100 µm (100,000 nm) | 1, 10, and 100 mg/L | 21 days | Waterborne |
| 93 | Xie et al., (2021) | PS | Zebrafish (Danio rerio)/adults | 80 and 8000 nm | 1 mg/L (80 nm); 10 µg/L (8000 nm) | 21 days | Waterborne |
| 94 | Yang et al., (2023) | PS | Zebrafish (Danio rerio)/adults | 100 and 20,000 nm | 100 and 1000 µg/L | 4 days, depurate 3 days | Waterborne |
| 95 | Ye et al., (2024) | PS | Zebrafish (Danio rerio)/adults | 50 nm | 1 mg/L | 21 days | Waterborne/ homosolate (0.0262-262 µg/L) |
| 96 | Yu et al., (2022a) | PS | Zebrafish (Danio rerio)/adults | 100 nm | 20 and 200 µg/L | 3 weeks | Waterbone/lead (50 µg/L) |
| 97 | Yu et al., (2022b) | PS | Zebrafish (Danio rerio)/adults | 40-54 nm; 394-407 nm; 4,000-8,000 nm; 45,000-85,000 nm; 158,000-234,000 nm | 60-338 µg/L | 30 days | Waterborne/tetracycline (100 µg/L) |
| 98 | Yu et al., (2023) | PS | Marine medaka (Oryzias melastigma) / embryos (6hpf) | 50 nm | 55 µg/L | 21 days | Waterborne /BPA (100 µg/L) |
| 99a | Zhang et al., (2020) | PS | Zebrafish (Danio rerio)/embryos | 70 ± 9.21 nm | Injected 0.52 nL of 1000, 3000, and 5000 mg/L | Hatched larvae depurate 4 weeks | Injected to eggs |
| 99b | Zhang et al., (2020) | PS | Zebrafish (Danio rerio)/embryos | 70 ± 9.21 nm | 0.5 and 5 mg/L | Until the hatching, depurate 4 weeks | Waterborne |
| 100 | Zhang et al., (2021) | PS | Marine medaka (Oryzias melastigma) | 100 nm | 5 mg/g food | 30 days | Feeding/SMG 0.5, and 5 mg/g |
| 101a | Zhang et al., (2022b) | PS | Grass carp (Ctenopharyngodon idella)/ embryos (12hpf) | 80 and 8000 nm | 5, 15, and 45 µg/L | 2-8 h | Waterborne |
| 101b | Zhang et al., (2022b) | PS | Grass carp (Ctenopharyngodon idella)/ larvae (24 hph) | 50 and 5000 nm (green fluorescence). 1000 and 5000 (red fluorescence |
10 µg/L | 12-96 h | Waterborne |
| 102 | Zhang et al., (2022c) | Polyamide (PA) | Zebrafish (Danio rerio)/embryos | 5-50 µm (5,000-50,000 nm) | 1, 10, and 20 mg/L | 2hpf-10dpf | Waterborne |
| 103 | Zhang et al., (2023) | PS | Zebrafish (Danio rerio)/adults | 100 nm | 1 mg/L | 30 days | Waterborne/arsenic (200 µg/L) |
| 104 | Zhang et al., (2024a) | PS | Silver carp (Hypophthalmichthys molitrix)/ adults | 80 nm | 10 and 1000 µg/L | 96 h | Waterborne/Microcystin-LR (1µg/L) |
| 105 | Zhang et al., (2024b) | PS-plain, PS-COOH, PS-NH2 | Marine medaka (Oryzias melastigma) /adults (10-12 months old) | Z-average of plain PS =244.0±11.6 nm, PS-COOH =294.7±8.6 nm, and PS-NH2 = 277.0±15.9 nm | 3.62 mg/g of food | 30 days, depurated for 21 days | Feeding/SMZ (4.62 mg/g food) |
| 106 | Zhang et al., (2024c) | PS | Zebrafish (Danio rerio)/adults | 100 nm | 1 ng/L | 30 days | Waterborne/arsenic (1 mg/L) |
| 107 | Zhao et al., (2021) | PS | Zebrafish (Danio rerio)/adults (males and females) | 54.5 ±2.8 nm | 10 mg/L | 120 days; evaluated F0 and F1 larvae without further exposure | Waterborne/TDCIPP (0.47, 2.64, or 12.78 µg/L) |
| 108 | Zheng and Wang (2024) | PS | Tilapia (Oreochromis niloticus)/larvae | 80 nm and 20 µm (20,000 nm) | 100 µg/L | 28 days | Waterborne |
| 109 | Zheng et al., (2024) | PS | Tilapia (Oreochromis niloticus)/larvae | 80, 2000, 20,000 nm | 100 µg/L | 28 days | Waterborne |
| 110 | Zhou et al., (2023a) | PS | Japanese medaka (Oryzias latipes)/ adults | 100 nm | 10, 104, 106 particles/ L (1.79589 X1013 particles/10 mg concentration) | 3 months | Waterborne |
| 111 | Zhou et al., (2023b) | PS | Japanese medaka (Oryzias latipes)/ larvae (9 dph) and adults (60 dph) | 100 nm | Larvae= (1014 items/L or 55 mg/L). Adults= (10 items/L or 5.5X10-12 mg/L; 104/L or 5.5X10-9 mg/L; 106 items/L or 5.5X10-7 mg/L) |
Larvae 48 h. Adults 3 months. |
Waterborne |
| 112 | Zhou et al., (2023c) | PS | Zebrafish (Danio rerio)/embryos | 100, 500, 1000 nm | 10 mg/L or 2.2 X1012 particles/L for 100 nm;1.76X1010 particles/L for 500 nm; 2.2X109 particle/L for 1000 nm. | 5 days | Waterborne |
| 113 | Zhou et al., (2023d) | PS | Zebrafish (Danio rerio)/adults | 50 ±3 nm | 1 mg/L | 4 weeks | Waterborne |
| 114. | Zuo et al., (2021) | PS | Zebrafish (Danio rerio)/adults | 70 nm | 100µg/L | 21 days; F1 (120 hpf) were evaluated without further exposure | Waterborne/MCLR (0.9, 4.5, and 22.5 µg/L) |
List of authors who studied the effects of NAPs on fish.
Blocks highlighted in yellow are coexposure studies. Elizalde-Velazquez et al. (2020) used two different methods ofexposure (injection and trophic transfer) of PS and mentioned inone article. Greven et al. (2016) studied the effects of PC and PS in one article. Manuel et al. (2022) reported the effects of PMMA and PS inzebrafish in one article. Monikh et al. (2022) reported the effects of PE, PPP, PS, and PVC in one article. Tamayo-Belda et al. (2023) reported the effects of PLA, PP, PS, and LDPE in one article. Zhang et al. (2020) used two different methods of exposure (injection and waterborne) of PS and mentioned in one article. Zhang C. et al. (2022) used two different life stages of zebrafish(embryo larvae) for PS exposure and described in one article. Wang L. et al. (2023) did not mention the type of NAPs used inthe experiment.AVO = avobenzone; BDE-47 = Polybrominated diphenyl ether: BMDBM = methoxydibenzoylmethane; BPA = bisphenol A; EE2 =17 α-ethynyl estradiol; IP = intraperitoneal injection; LDPE = lowdensitypolyethylene; MCLR = microcystin-LR; PA = polyamide; PC = polycarbonate; PE = polyethylene; PET = polyethyleneterephthalate; PHN = phenmedipham; PLA = polylactic acid; PMMA = polymethylmethacrylate; PPP = polypropylene; PS =polystyrene; SIM = simvastatin; SMZ = sulfamethazine; TDCIPP = tris (1,3-dichloro-2-propyl) phosphate; TPhP =triphenyl phosphate; TC = tetracycline; TCS = triclosan.
TABLE 3
| Fish | Polymer (name) | Sizes (mode of exposure) | Developmental stages | References | |
|---|---|---|---|---|---|
| 1 | Zebrafish | PA | ∼32, 500 nm | Embryos (2 hpf) | Zhang et al. (2022c) |
| 2a | Fathead minnows | PC | 158.7 nm (in vitro) | Adults (neutrophils) | Greven et al. (2016) |
| 2b | Fathead minnows | PC | 41 nm (in vitro) | Adults (neutrophils) | Greven et al. (2016) |
| 3 | Zebrafish | PE | 191.10 ± 3.13 nm | Embryos (6 hpf) | Sun et al. (2021) |
| 4 | Zebrafish | PE | 10,000–100,000 nm | Adults (8–10 months old) | Khan and Ali (2023) |
| 5 | Zebrafish | PPP | 562.15 ± 118.47 nm | Embryos (24 hpf and 72 hpf) | Lee et al. (2022) |
| 6a | Zebrafish | PPP | 164–220 nm | Embryos (4 hpf | Tamayo-Belda et al. (2023) |
| 6b | Zebrafish | PLA | 122–712 nm | Embryos (4 hpf | Tamayo-Belda et al. (2023) |
| 7 | Marine medaka (Oryzias melastigma) | PS | 244–277 nm | Adult | Zhang et al. (2024b) |
| 8 | Rainbow trout (Oncorhynchus mykiss) | PS | ∼200 nm | Juveniles | Clark et al. (2023a) |
| 9 | Red tilapia (Oreochromis niloticus) | PS | 300, 500, and 7,000–9,000 nm | Juveniles | Ding et al. (2020) |
| 10 | Zebrafish | PS | 500 nm | Embryos (72 hpf) | Parenti et al. (2019) |
| 11a | Zebrafish | PS | 200 and 600 nm | Embryos (6 hpf) | Monikh et al. (2022) |
| 11b | Zebrafish | PVC | 200 nm | Embryos | Monikh et al. (2022) |
| 12 | Zebrafish | PS | 400–1,000 nm | Embryos (1 dpf) | Park and Kim (2022) |
| 13 | Zebrafish | PS | 500 nm | Embryos | Suman et al. (2023) |
| 14 | Zebrafish | PS | 134 ± 2.9 nm | Adult | Senol et al. (2023) |
| 15 | Zebrafish | Nanoplastics | 100 nm | Adults (120 dpf) | Wang et al. (2023e) |
Articles excluded from reviews (based on the size and the mode of exposure).
Greven et al. (2016) studied the effects of PC and PS on RBCs of adult fathead minnows in vitro. Monikh et al. (2022) studied the effects of PS and PVC on zebrafish and included in one article. Tamayo-Belda et al. (2023) described the effects of PPP and PLA on zebrafish embryos in one article; Wang L. et al. (2023) did not mention the types of NAPs used in this study.
TABLE 4
| Fish | Polymer | MIP (size) | NAP (size) | Developmental stage | References | |
|---|---|---|---|---|---|---|
| 1 | Common carp (Cyprinus carpio) | PE | >5 mm->100 nm | <100 nm | Juvenile | Hamed et al. (2022) |
| 2 | Zebrafish | PE | 13.5 µm (13,500 nm) | 70 nm | Adult | Li et al. (2023c) |
| 3 | Zebrafish | PET | >100 nm −2032 µm (20,32,000 nm) | 68.06–100 nm | Embryos | de Souza Toedoro et al. (2024) |
| 4 | Carp | PS | 400 nm | 50 and 100 nm | Adult | Wu et al. (2022) |
| 5a | Grass carp (Ctenopharyngodon idella) | PS | 8 µm (8,000 nm) | 80 nm | Embryos | Zhang et al. (2022b) |
| 5b | Grass carp (Ctenopharyngodon idella) | PS | 5 µm (8,000 nm) | 50 nm | Larvae | Zhang et al. (2022b) |
| 6 | Tooth carp (Aphaniops hormuzensis) | PS | 300 nm | 100 nm | Adult | Saemi-Komsari et al. (2023) |
| 7 | Marine medaka (Oryzias melastigma) | PS | 500 and 6,000 nm | 50 nm | Embryos | Chen et al. (2022) |
| 8 | Marine medaka (Oryzias melastigma) | PS | 45 µm (45,000 nm) | 50 nm | Larvae (7 dph) | Kang et al. (2021) |
| 9 | Marine medaka (Oryzias melastigma) | PS | 500 nm and 2 µm (2,000 nm) | 70 nm | Larvae (3 dph) | Li et al. (2024b) |
| 10 | Tilapia (Oreochromis niloticus) | PPP | 100 µm (100,000 nm) | 100 nm | Juveniles | Wu et al. (2023) |
| 11 | Tilapia (Oreochromis niloticus) | PS | 2 and 20 µm (2,000 and 20,000 nm) | 80 nm | Larvae | Zheng et al. (2024) |
| 12 | Nile tilapia (Oreochromis niloticus) | PS | 185 nm | 100 nm | Juveniles | Hao et al. (2023) |
| 13 | Nile tilapia (Oreochromis niloticus) | PS | 500 and 5,000 nm | 100 nm | Juveniles | Wang et al. (2023b) |
| 14 | Zebrafish | PS | 41 µm (41,000 nm) | 47 nm | Embryos | Chen et al. (2017a) |
| 15 | Zebrafish | PS | 250 and 700 nm | 25 and 50 nm | Embryos | Van Pomeren et al. (2017) |
| 16 | Zebrafish | PS | 200 and 500 nm | 50 nm | Embryos | Lee et al. (2019) |
| 17 | Zebrafish | PS | 200 nm | 50 nm | Embryos | Pedersen et al. (2020) |
| 18 | Zebrafish | PS | 500 and 4,500 nm | 50 nm | Embryos | Martinez-Alvarez et al. (2022) |
| 19 | Zebrafish | PS | 500 and 1,000 nm | 100 nm | Embryos | Zhou et al. (2023c) |
| 20 | Zebrafish | PS | 200 and 500 nm | 80 nm | Embryos and larvae | Chen et al. (2024) |
| 21 | Zebrafish | PS | 1,000 nm and 50 µm | 50 nm | Larvae | Sendra et al. (2021) |
| 22 | Zebrafish | PS | 5,800 nm | 46 nm | Adults (male and female) | He et al. (2021) |
| 23 | Zebrafish | PS | 8,000 nm | 80 nm | Adults | Xie et al. (2021) |
| 24 | Zebrafish | PS | 394–407 nm, 4–8 μm, (4,000–8,000 nm), 45–85 µm (45,000–85,000 nm), and 158–234 µm (158,000–234,000 nm) | 40–54 nm | Adults | Yu et al. (2022b) |
| 25 | Zebrafish | PS | 20 µm (20,000 nm) | 100 nm | Adults | Yang et al. (2023) |
| 26a | Zebrafish | PS | 122, 220, 712, and 825 nm | 91 nm | Embryos (4 hpf–96 hpf) | Tamayo-Belda et al. (2023) |
| 26b | Zebrafish | LDPE | 164,106, 342, and 122 nm | 91 nm | Embryos (4 hpf–96 hpf) | Tamayo-Belda et al. (2023) |
Articles included both MIPs and NAPs during investigations.
MIP , microplastics (diameter of the polymer is > 100 nm); NAPs , nanoplastics (diameter of the polymer is ≤100 nm); Tamayo-Belda et al. (2023) measured the diameter of the plastic every day during the exposure period (day 0, day 1, day 2, day 3, and day 4).
TABLE 5
| Name of the plastics | Fish | Developmental stages | Nanoplastic size/diameter | Mode of exposure/duration | Accumulated (tissues/organs) or studied organs | References |
|---|---|---|---|---|---|---|
| PE | Common carp (Cyprinus carpio) | Juveniles | 100 nm | Waterborne-(15 mg/L)-15 days) | Brain and eye | Hamed et al. (2022) |
| LDPE | Zebrafish (Danio rerio) | Embryos (4 hpf) | 91 nm | Waterborne (0.001, 0.01, 0.1, 1, 10, and 10 mg/L), 96 hpf | Vitelline membrane | Tamayo-Belda et al. (2023) |
| PE | Zebrafish (Danio rerio) | Embryos (6 hpf) | 50 nm | Waterborne (3 × 1010 particles/L or 0.00025 mg/L), 24 h | Whole embryo | Monikh et al. (2022) |
| PE | Zebrafish (Danio rerio) | Adults (3 months) | 70 nm | Waterborne (20 mg/L), 21 days | Gill/gut/intestine /liver | Li et al. (2023c) |
| PET | Zebrafish (Danio rerio) | Embryos (6 and 72 hpf) | 70 ± 5 nm | Waterborne (5, 10, 50, 100, and 200 mg/L), until 96–120 hpf | Liver, intestine, and kidney | Bashirova et al. (2023) |
| PET | Zebrafish (Danio rerio) | Embryos | 68.06 nm and above | Waterborne (0.5, 1, 5, 10, and 20 mg/L), 6 days | Chorion surface | de Souza Toedoro et al. (2024) |
| PMMA | Zebrafish (Danio rerio) | Embryos | 32 nm | Waterborne (0.001, 0.01, 0.1, 1, 10, and 100 mg/L), 96 h | Whole embryo | Manuel et al. (2022) |
| PPP | Tilapia (Oreochromis niloticus) | Juveniles (10 ± 1 g; length 13 ± 1 cm) | 100 nm | Waterborne (0.001, 0.01, and 0.1 mg/L), 21 days | Liver | Wu et al. (2023) |
| PPP | Zebrafish (Danio rerio) | Embryos (6 hpf) | 50 nm | Waterborne (3 × 1010 particles/L or 0.000022 mg/L), 24 h | Whole embryos | Monikh et al. (2022) |
| PS | Carp | Adults | 50 and 100 nm | Waterborne (0.1 mg/L), 28 days | Heart | Wu et al. (2022) |
| PS | Grass carp (Ctenopharyngodon idella) | Embryos (12 hpf) | 50–80 nm | Waterborne (0.005–0.045 mg/L); 2,4, and 8 h | On the chorion | Zhang et al. (2022b) |
| PS | Grass carp (Ctenopharyngodon idella) | Juveniles | 23.03 ± 0.266 nm | Waterborne (0.76 mg/L), 72 h | Blood/liver/brain | Estrela et al. (2021) |
| PS | Grass carp (Ctenopharyngodon idella) | Juveniles | 20–26 nm | Waterborne (0.00000004–0.034 mg/L), 20 days | Liver/brain | Guimaraes et al. (2021) |
| PS | Grass carp (Ctenopharyngodon idella) | Juveniles | 80 nm | Waterborne, 0.02, 0.2, and 2 mg/L (7 days) | Liver and intestine | Liu et al. (2022a) |
| PS | Grass carp (Ctenopharyngodon idella) | Juveniles | 80 nm | Waterborne (0.01, 0.1, and 1 mg/L), 8 days | Gut/intestine | Li et al. (2024a) |
| PS | Silver carp (Hypophthalmichthys molitrix) | Adults | 80 nm | Waterborne (0.01 and 1 mg/L), 96 h | Gut/intestine/liver | Zhang et al. (2024a) |
| PS | Tooth carp (Aphaniops hormuzensis) | Adult | 100 nm | Waterborne (1, 5, 10, 25, 50, 100, and 200 mg/L), 96 h Diet (0.01, 0.1, 1, and 5 mg/kg), 3, 14, and 28 days |
Gut, gill, liver, muscle, and skin | Saemi-Komsari et al. (2023) |
| PS | Fathead minnows (Pimephales promelas) | Adult males | 50 nm | IP-injected (0.1 mL of 0.005 mg/L), 48 h | Liver and head kidney | Elizalde-Velazquez et al. (2020) |
| PS | Fathead minnows (Pimephales promelas) | Adult males | 50 nm | Trophic transfer (0.005 mg/L), 48 h | Liver and head kidney | Elizalde-Velazquez et al. (2020) |
| PS | Chinese rice fish (Oryzias sinensis) | Adults and F1 larvae | 57.29–60.39 nm | Waterborne (5 mg/L); (adults 7 days; F1 larvae 24 h) | Yolk sac | Chae et al. (2018) |
| PS | Hainan medaka (Oryzias curvinotus) | Adults | 80 nm | Waterborne (0.2 mg/L), 7 days | Gills and intestine | Gao et al. (2023a) |
| PS | Japanese medaka (Oryzias latipes) | Adults | 100 nm | 10, 104, and 106 particles/L (1.79589 × 1013 particles/10 mg concentration) | Gut | Zhou et al. (2023b) |
| PS | Japanese medaka (Oryzias latipes) | Adults | 100 nm | Waterborne (10, 104, and 106 particles/L) or (5.5 × 10−12, 5.5 × 10−9, and 5.5 × 10−7 mg/L), 3 months | Gonads (ovary/testis) | Zhou et al. (2023a) |
| PS | Japanese medaka (Oryzias latipes) | Larvae (9 dph) | 100 nm | Waterborne (1014 items/L or 55 mg/L), 48 h | Gut | Zhou et al. (2023b) |
| PS | Japanese medaka (Oryzias latipes) | Adults (60 dph) | 100 nm | Waterborne (5.5 × 10−12 mg/L, 5.5 × 10−9 mg/L, and 5.5 × 10−7 mg/L), 90 days | Gut | Zhou et al. (2023b) |
| PS | Marine medaka (Oryzias melastigma) | Embryos | PS (50 nm) | Waterborne (106 particles/L), 19 days | Whole embryo | Chen et al. (2022) |
| PS | Marine medaka (Oryzias melastigma) | Embryos | PS-NH2 (80 nm); PS-COOH (80 nm) | Waterborne (0.01 mg/L), 10 days (depurated for 10 days) | Gastrointestinal tract and intestinal villi | Chen et al. (2023a) |
| PS | Marine medaka (Oryzias melastigma) | Embryos (6 hpf) | 50 nm | Waterborne (0.055 mg/L), 21 days | Abdominal area/liver/heart | Yu et al. (2023) |
| PS | Marine medaka (Oryzias melastigma) | Larvae (7 dph) | 50 nm | Waterborne (0.0025–0.01 mg/L); 1, 7, 14, and 120 dph | Gut | Kang et al. (2021) |
| PS | Marine medaka (Oryzias melastigma) | Larvae (3 dph) | 70 nm | Trophic transfer (0.02, 0.2, and 2 mg/L), 90 days | Intestine/liver /muscle/gonad | Li et al. (2024b) |
| PS | Marine medaka (Oryzias melastigma) | Juveniles (2 months) | 100 nm | Waterborne (1 mg/L), 30 days | Intestine | Li et al. (2023b) |
| PS | Marine medaka (Oryzias melastigma) | Adults | 100 nm | Waterborne (5 mg/g), 30 days | Gut/intestine | Zhang et al. (2021) |
| PS | Marine medaka (Oryzias melastigma) | Adults | 100 nm | Dietary (3.45 mg/g), 30 days | Gut/liver of 60 dph F1 larvae | He et al. (2022) |
| PS | Marine medaka (Oryzias melastigma) | Adults (4 months) | 100 nm | Dietary (5 mg/g), 30 days (depurated for 21 days) | Gut | Wang et al. (2023a) |
| PS | Rainbow trout (Oncorhynchus mykiss) | Juvenile | 35 ± 8 nm | Dietary (0.0059 mg/g food); 3, 7, and 14 days | Hind intestine and liver | Clark et al. (2023b) |
| PS | Red tilapia (Oreochromis niloticus) | Juveniles | 100 nm | Waterborne (0.001, 0.01, and 0.1 mg/L), 14 days | Gut, gills, liver, and brain | Ding et al. (2018) |
| PS | Tilapia (Oreochromis niloticus) | Larvae | 80 nm | Waterborne (0.1 mg/L), 28 days | Gills | Zheng and Wang (2024) |
| PS | Tilapia (Oreochromis niloticus) | Larvae | 80 nm | Waterborne (0.1 mg/L), 28 days | Gills | Zheng et al. (2024) |
| PS | Tilapia (Oreochromis niloticus) | Larvae (4 weeks old) | 100 nm | Waterborne (20 mg/L), 7 days (depurated for 7 days) | Whole fish | Pang et al. (2021) |
| PS | Tilapia (Oreochromis niloticus) | Juveniles | 86 nm | Waterborne (1 mg/L), 21 days (depurated 7 days) | Gill, stomach, intestine, liver, and muscle | Hao et al. (2023) |
| PS | Tilapia (Oreochromis niloticus) | Juveniles | 100 nm | Waterborne (1, 10, and 100 mg/L), 7 days | Gill, liver, intestine, and muscle | Wang et al. (2023b) |
| PS | Zebrafish (Danio rerio) | Embryos (3 hpf) | 47 nm | Waterborne (1 mg/L), 120 h | Whole embryo | Chen et al. (2017a) |
| PS | Zebrafish (Danio rerio) | Embryos | 25 and 50 nm | Waterborne (25 mg/L; 25 nm) (50 mg/L; 50 nm); 0–48 hpf, 24–72 hpf, and 72–120 hpf | Chorion (0 hpf); eye (72 hpf) | Van Pomeren et al. (2017) |
| PS | Zebrafish (Danio rerio) | Embryos (6 hpf) | 51 nm | Waterborne (0.1, 1, and 10 mg/L), 120 hpf | Yolk sac, GI tract, gall bladder, liver, pancreas, heart, and brain | Pitt et al. (2018a) |
| PS | Zebrafish (Danio rerio) | Embryos (72 hpf) | 25 nm | Waterborne (20 mg/L), 72–120 hpf, 48 h | Intestine, pancreas, and gall bladder | Brun et al. (2019) |
| PS | Zebrafish (Danio rerio) | Embryos | 50 nm | Waterborne (0.1 mg/L); 6, 24, and 96 hpf | Whole body | Lee et al. (2019) |
| PS | Zebrafish (Danio rerio) | Embryos (6 hpf) | 44 nm | Waterborne (0.1, 1, and 10 mg/L), 96 hpf | Whole body | Trevisan et al. (2019) |
| PS | Zebrafish (Danio rerio) | Embryos (6 hpf) | 50 nm | Waterborne (0.01, 0.1, 1, and 10 mg/L), 120 hpf | GI tract, eye, liver, and cranial region | Pedersen et al. (2020) |
| PS | Zebrafish (Danio rerio) | Embryos | 20 nm | Microinjected to eggs (3 µL of 270 mg/L), 120 hpf | Brain | Sokmen et al. (2020) |
| PS | Zebrafish (Danio rerio) | Embryos | 44 nm | Waterborne (1 mg/L), 7 days | Yolk sac and brain | Trevisan et al. (2020) |
| PS | Zebrafish (Danio rerio) | Embryos | 70 ± 9.21 nm | Microinjected to eggs (0.52 nL of 1,000, 3,000, and 5,000 mg/L), 4 weeks | Maximum in the yolk sac and followed by brain > eyes > gut > swim bladder (maximum accumulation in the trunk region | Zhang et al. (2020) |
| PS | Zebrafish (Danio rerio) | Embryos | 70 ± 9.21 nm | Waterborne (0.5 and 5 mg/L), exposed until hatching and depurated for 4 weeks | Maximum accumulation in the brain and eyes | Zhang et al. (2020) |
| PS | Zebrafish (Danio rerio) | Embryos | 60 nm | Waterborne (0.015, 1.5, and 150 mg/L, 96 h | Whole embryos | Barreto et al. (2021) |
| PS | Zebrafish (Danio rerio) | Embryos (2 hpf) | 100 nm | Waterborne (0.01 mg/L); 12 h (depurated 120 hpf) | Whole embryos | Liu et al. (2021) |
| PS | Zebrafish (Danio rerio) | Embryos | 50 nm | Waterborne 1 mg/L (96 h) | Whole embryo | Bhagat et al. (2022) |
| PS | Zebrafish (Danio rerio) | Embryos | 100 nm | Waterborne (0.0025 and 0.025 mg/L) 7 days | Anterior part containing the yolk sac and digestive tract | Chackal et al. (2022) |
| PS | Zebrafish (Danio rerio) | Embryos | 50 and 100 nm | Waterborne ((0.1, 0.5, 2 and 10 mg/L), 120 hpf | Intestine and areas of excretion | Cheng et al. (2022) |
| PS | Zebrafish (Danio rerio) | Embryos | 100 nm | Waterborne (100, 200, and 400 mg/L), 96 h | Whole embryo | Feng et al. (2022) |
| PS | Zebrafish (Danio rerio) | Embryos | 50 nm | Waterborne (5 mg/L), 4–96 hpf | Surface of the chorion and the embryos | Geum and Yeo, (2022) |
| PS | Zebrafish (Danio rerio) | Embryos | 25 nm | Waterborne (10, 25, and 50 mg/L), 96 hpf | Whole embryo | Kantha et al. (2022) |
| PS | Zebrafish (Danio rerio) | Embryos (2 hpf) | 100 nm | Waterborne (0.01 mg/L) (144 hpf, depurated for 3 days) | Whole embryo | Liu et al. (2022b) |
| PS | Zebrafish (Danio rerio) | Embryos | 22 nm | Waterborne (0.001, 0.01, 0.1, 1, 10, and 100 mg/L), 96 hpf | Whole embryo | Manuel et al. (2022) |
| PS | Zebrafish (Danio rerio) | Embryos | 50 nm | Waterborne (0.000069, 0.00069, 0.069, 0.687, and 6.87 mg/L), 120 hpf | Chorion, eye, tail, and yolk sac | Martinez-Alvarez et al. (2022) |
| PS | Zebrafish (Danio rerio) | Embryos | 44 nm | Waterborne (0.015, 0.15, 1.5, 15, and 150 mg/L), 96–120 hpf | Whole embryo | Santos et al. (2022) |
| PS | Zebrafish (Danio rerio) | Embryos (4 hpf) | 20 nm | Injected (3 nL of 270 mg/L); grown for 6 months; F1 embryos were evaluated | Whole embryo | Sulukan et al. (2022a) |
| PS | Zebrafish (Danio rerio) | Embryos | PS-NH2 (50 nm fluorescent) PS-COOH (30 nm fluorescent) PS-NH2 (51 nm, unlabeled) (+ve charge) PS-COOH (50 nm unlabeled) (-ve charge) |
Waterborne (30 and 50 mg/L to labeled or unlabeled PS-NH2 or PS-COOH), 120 hpf | GI tract, pericardium, and brain | Teng et al. (2022a) |
| PS | Zebrafish (Danio rerio) | Embryos | 80 nm | Waterborne (0.05 mg/L, 0.1 mg/L, 1 mg/L, 5 mg/L, and 10 mg/L) (120 hpf) | Surface of the chorion, brain, gills, mouth, trunk, heart, liver, and digestive tract | Wang et al. (2022) |
| PS | Zebrafish (Danio rerio) | Embryos | 44 nm | Waterborne (0.015 and 1.5 mg/L), 96–120 h | Whole embryo | Barreto et al. (2023) |
| PS | Zebrafish (Danio rerio) | Embryos (8 hpf) | 80 nm | Waterborne (0.5 and 5 mg/L), 96 hpf | Yolk sac, eye, head, and nerve tubes | Chen et al. (2023b) |
| PS | Zebrafish (Danio rerio) | Embryos | 50 nm | Waterborne (0.1, 1, 5, 10, 20, 30, and 50 mg/L), 5 days | Whole embryo | Chen et al. (2023c) |
| PS | Zebrafish (Danio rerio) | Embryos | 20 nm | Waterborne (2, 5, and 8 mg/L); 22, 46, and 70 h | Whole embryo | Dai et al. (2023) |
| PS | Zebrafish (Danio rerio) | Embryos | 50 nm | Waterborne (0.1, 0.5, and 1 mg/L); 4–72 h at 24°C, 27°C, and 30°C | Chorion, abdomen, circulatory system, intestinal tract, and excretory regions | Duan et al. (2023) |
| PS | Zebrafish (Danio rerio) | Embryos (3 hpf) | 80 nm | Waterborne (0.005, 0.01, 0.025, 0.05, and 0.1 mg/L), 96 h | Whole embryo | Gao et al. (2023b) |
| PS | Zebrafish (Danio rerio) | Embryos | 30 and 100 nm | Waterborne (0.1, 1, and 10 mg/L), 96 h | Chorion, head, trunk, and in the yolk | Martin et al. (2023) |
| PS | Zebrafish (Danio rerio) | Embryos | 30 nm | Waterborne (0.1, 0.5, and 3 mg/L), 120 hpf | Whole embryo | Martin-Folgar et al. (2023) |
| PS | Zebrafish (Danio rerio) | Embryos (4 hpf) | PS (91, nm) | Waterborne (0.001, 0.01, 0.1, 1, 10, and 10 mg/L), 96 hpf | Vitelline membrane | Tamayo-Belda et al. (2023) |
| PS | Zebrafish (Danio rerio) | Embryos (2 hpf) | 15 nm | Waterborne (50 mg/L), 96 h | GI tract, pericardium, eye, and cranial regions | Varshney et al. (2023) |
| PS | Zebrafish (Danio rerio) | Embryos | 80 nm | Waterborne (0.05, 0.1, 1, 5, and 10 mg/L), 120 hpf | Gills, GI, liver, and heart | Wang et al. (2023c) |
| PS | Zebrafish (Danio rerio) | Embryos (4 hpf) | 50 nm | Waterborne (1, 5, and 10 mg/L), 144 hpf | Whole embryo | Wang et al. (2023d) |
| PS | Zebrafish (Danio rerio) | Embryos (4 hpf) | 100 nm | Waterborne (10 mg/L), 5 days | Chorion, brain, yolk sac, muscle, GI tract, pancreas, gall bladder, liver, and swim bladder | Zhou et al. (2023c) |
| PS | Zebrafish (Danio rerio) | Embryos (8 hpf) | 80 nm | Waterborne (0.1, 0.5, 1, 5, 10, 25, and 50 mg/L); 120 hpf; some were depurated for 10 days | Chorion, eye, brain, and dorsal trunk | Chen et al. (2024) |
| PS | Zebrafish (Danio rerio) | Embryos | PS (23.03 ± 0.266 nm) | Waterborne (0.00000004 mg/L, 0.000034 mg/L, and 0.034 mg/L), 144 hpf | In embryos, accumulation occurred in the chorion, muscle, gills, and head of the fish; in larvae, accumulation occurred in the digestive system, gills, and somite | Santos et al. (2024) |
| PS | Zebrafish (Danio rerio) | Larvae (120 hpf) | 50 nm | Waterborne (10 mg/L), 24 h–7 days | Gut, skin, caudal fin, and eyes | Sendra et al. (2021) |
| PS | Zebrafish (Danio rerio) | Adults (6 months old) | 47 nm | Waterborne 1 mg/L (3 days) | Viscera, gills, head, and muscle | Chen et al. (2017b) |
| PS | Zebrafish (Danio rerio) | Adults | 42 nm | Dietary (1 mg/L); 7 days; F1 larvae were evaluated | Yolk sac, GI tract, liver, pancreas, and gall bladder | Pitt et al. (2018b) |
| PS | Zebrafish (Danio rerio) | Adults (6 months old) | 70 nm | Waterborne (0.5, 1.5, and 5 mg/L); 7 days, 30 days, and 7 weeks | Gonads, intestine, liver, and brain tissues (observed after 30 days of exposure) | Sarasamma et al. (2020) |
| PS | Zebrafish (Danio rerio) | Adults (male and female) | 46 nm | Waterborne (2 mg/L), 21 days | Gonads | He et al. (2021) |
| PS | Zebrafish (Danio rerio) | Adults (male and female) | 70 nm | Waterborne (0.1 mg/L), 45 days; F1 embryos were evaluated | Whole embryos (F1) | Wu et al. (2021) |
| PS | Zebrafish (Danio rerio) | Adults | 80 nm | Waterborne (1 mg/L), 21 days | Gut | Xie et al. (2021) |
| PS | Zebrafish (Danio rerio) | Adults (90 days old) | 54.5 ± 2.8 nm | Waterborne (10 mg/L), 120 days; both F0 parents and F1 embryos were evaluated | F0 = gut > gills > gonad > liver F1 = whole embryo/larvae |
Zhao et al. (2021) |
| PS | Zebrafish (Danio rerio) | Adults (90 days old) | 70 nm | Waterborne (0.1 mg/L), 21 days; F1 larvae were evaluated at 120 hpf | Testis and ovary (F1 larvae) | Zuo et al. (2021) |
| PS | Zebrafish (Danio rerio) | Adults (male and female) | 70 nm | Waterborne (0.1 mg/L), 3 months | Liver | Ling et al. (2022) |
| PS | Zebrafish (Danio rerio) | Adults (3 months old) | 100 nm | Waterborne (25 mg/L); 96 h at 28°C, 29°C, and 30°C | Brain | Sulukan et al. (2022b) |
| PS | Zebrafish (Danio rerio) | Juveniles and adults | 44 nm | Waterborne (0.001, 0.01, and 0.1 mg/L); 30 and 60 days | Gut–brain axis | Teng et al. (2022b) |
| PS | Zebrafish (Danio rerio) | Adults | 100 nm | Waterborne (0.02 and 0.2 mg/L), 3 weeks | Intestine | Yu et al. (2022a) |
| PS | Zebrafish (Danio rerio) | Adults | 40–54 nm | Waterborne (0.06–0.186 mg/L), 30 days | Intestine | Yu et al. (2022b) |
| PS | Zebrafish (Danio rerio) | Adults | 20–80 nm | Waterborne (0.0001, 0.001, 0.01, and 0.1 mg/L), 45 days | Brain | Aliakbarzadeh et al. (2023) |
| PS | Zebrafish (Danio rerio) | Adults | 100 nm | Waterborne (0.5 mg/L0), 28 days | Liver | Deng et al. (2023) |
| PS | Zebrafish (Danio rerio) | Adults (male, 4 months old) | 50 nm | Waterborne (5, 10, and 15 mg/L), exposed for 30 days and depurated for 16 days | Intestine > liver > gill> muscle > brain | Habumugisha et al. (2023) |
| PS | Zebrafish (Danio rerio) | Adults | 80 nm | Waterborne (15 and 150 mg/L) (21 days) | Liver | Li et al. (2023a) |
| PS | Zebrafish (Danio rerio) | Adults (5 months old; male and female) | 70 nm | Waterborne (2 mg/L), 21 days | Gonads (testis and ovary) | Lin et al. (2023) |
| PS | Zebrafish (Danio rerio) | Adults | 100 nm | Waterborne (0.1 and 1 mg/L); 4 days (depurated for 3 days) | Gut | Yang et al. (2023) |
| PS | Zebrafish (Danio rerio) | Adults | 100 nm | Waterborne (1 mg/L), 30 days | Brain | Zhang et al. (2023) |
| PS | Zebrafish (Danio rerio) | Adults | (50 ± 3 nm) | Waterborne (1 mg/L), 4 weeks | Brain | Zhou et al. (2023d) |
| PS | Zebrafish (Danio rerio) | Adults | 50–100 nm | Waterborne/dietary exposure to a high-fat diet (21 days) | Gut | Du et al. (2024) |
| PS | Zebrafish (Danio rerio) | Adults | 50 nm | Waterborne (1 mg/L), 21 days | Liver, brain, and gonads (testis and ovary) | Ye et al. (2024) |
| PS | Zebrafish (Danio rerio) | Adults | 100 nm | Waterborne (1 mg/L), 30 days | Blood, intestine, and brain | Zhang et al. (2024c) |
Accumulation of nanoplastics in the specific organs of fish at various stages of development.
Elizalde-Velazquez et al. (2020) used two different methods of exposure (injection and trophic transfer) of PS in fathead minnows and mentioned it in one article. Manuel et al. (2022) reported the effects of PMMA, and PS in zebrafish in one article. Monikh et al. (2022) reported the effects of PE, and PPP in one article in zebrafish. Tamayo-Belda et al. (2023) reported the effects of PS, and LDPE in one article in zebrafish. Zhang et al. (2020) used two different methods of exposure (injection and waterborne) of PS in zebrafish and mentioned in one article.
TABLE 6
| Fish | Plastic polymers | Developmental stage | Observed effects | References |
|---|---|---|---|---|
| Common carp (Cyprinus carpio) | PE | Juvenile |
|
Hamed et al. (2022) |
| Carp | PS | Adults |
|
Wu et al. (2022) |
| Grass carp (Ctenopharyngodon idella) | PS | Embryos |
|
Zhang et al. (2022b) |
| Grass carp (Ctenopharyngodon idella) | PS | Juveniles |
|
Estrela et al. (2021), Guimaraes et al. (2021), Liu et al. (2022a), Li et al. (2024a) |
| Silver carp (Hypophthalmichthys molitrix) | PS | Adults |
|
Zhang et al. (2024a) |
| Tooth carp (Aphaniops hormuzensis) | PS | Adults |
|
Saemi-Komsari et al. (2023) |
| Fathead minnows (Pimephales promelas) | PS | Adult (male) |
|
Elizalde-Velazquez et al. (2020) |
| Chinese rice fish (Oryzias sinensis) | PS | Adults and F1 embryos |
|
Chae et al. (2018) |
| Hainan medaka (Oryzias curvinotus) | PS | Adults |
|
Gao et al. (2023a) |
| Japanese medaka (Oryzias latipes) | PS | Adult |
|
Zhou et al. (2023a), Zhou et al. (2023b) |
| Marine medaka (Oryzias melastigma) | PS, PS-NH2, and PS-COOH | Embryos |
|
Chen et al. (2023a), Yu et al. (2023) |
| Marine medaka (Oryzias melastigma) | PS | Larvae |
|
Kang et al. (2021), Li et al. (2024b) |
| Marine medaka (Oryzias melastigma) | PS | Juveniles |
|
Li et al. (2023b) |
| Marine medaka (Oryzias melastigma) | PS | Adult |
|
Zhang et al. (2021); He et al. (2022), Wang et al. (2023a); Zhang et al. (2024b) |
| Rainbow trout (Oncorhynchus mykiss) | PS | Juveniles |
|
Clark et al. (2023b) |
| Tilapia (Oreochromis niloticus) | PPP | Juveniles |
|
Wu et al. (2023) |
| Tilapia (Oreochromis niloticus) | PS | Larvae |
|
Zheng and Wang (2024), Zheng et al. (2024) |
| Tilapia (Oreochromis niloticus) | PS | Juveniles |
|
Ding et al. (2018), Ding et al. (2020); Hao et al. (2023); Wang et al. (2023b) |
| Zebrafish (Danio rerio) | LDPE | Embryos |
|
Tamayo-Belda et al. (2023) |
| Zebrafish (Danio rerio) | PE | Embryos |
|
Monikh et al. (2022) |
| Zebrafish (Danio rerio) | PE | Adults |
|
Khan and Ali (2023); Li et al. (2023c) |
| Zebrafish (Danio rerio) | PET | Embryos |
|
Bashirova et al. (2023); De Souza Toedoro et al. (2024) |
| Zebrafish (Danio rerio) | PMMA | Embryos |
|
Manuel et al. (2022) |
| Zebrafish (Danio rerio) | PPP | Embryos |
|
Monikh et al. (2022) |
| Zebrafish (Danio rerio) | PS, PS-NH2, and PS-COOH | Embryos |
|
Chen et al. (2017a); Van Pomeren et al. (2017); Pitt et al. (2018a); Brun et al. (2019); Lee et al. (2019); Trevisan et al. (2019), Trevisan et al. (2020); Pedersen et al. (2020); Sokmen et al. (2020), Zhang et al. (2020), Barreto et al. (2021), Liu et al. (2021); Bhagat et al. (2022); Chackal et al. (2022); Cheng et al. (2022); Feng et al. (2022); Geum and Yeo, (2022); Kantha et al. (2022); Liu et al. (2022b); Manuel et al. (2022); Martinez-Alvarez et al. (2022); Santos et al. (2022), Teng et al. (2022a); Wang et al. (2022); Barreto et al. (2023); Chen et al. (2023b); Chen et al. (2023c); Dai et al. (2023); Duan et al. (2023); Gao et al. (2023b); Martin et al. (2023); Martin-Folgar et al. (2023); Tamayo-Belda et al. (2023); Varshney et al. (2023); Wang et al. (2023d), Wang et al. (2023e); Zhou et al. (2023c); Chen et al. (2024); Santos et al. (2024) |
| Zebrafish (Danio rerio) | PS | Larvae |
|
Sendra et al. (2021) |
| Zebrafish (Danio rerio) | PS | Adult |
|
Chen et al. (2017b); Pitt et al. (2018b); Sarasamma et al. (2020); He et al. (2021); Wu et al. (2021); Xie et al. (2021); Zhao et al. (2021); Zuo et al. (2021); Ling et al. (2022); Sulukan et al. (2022a); Teng et al. (2022b); Yu et al. (2022a); Yu et al. (2022b); Aliakbarzadeh et al. (2023); Deng et al. (2023); Habumugisha et al. (2023); Li et al. (2023a); Lin et al. (2023); Yang et al. (2023); Zhang et al. (2023); Zhang et al. (2024c); Zhou et al. (2023d); Du et al. (2024); Ye et al. (2024) |
Effects of NAPs on fish targeting toxicological endpoints.
TABLE 7
| Polymer (name, method of application, and duration) | Fish (name and developmental stage) | Organ and gene types | Upregulated | Downregulated | Unchanged | References |
|---|---|---|---|---|---|---|
| PS (80 nm) (10, 100, and 1,000 μg/L; waterborne, 8 days) | Grass carp (juveniles) | Gut/intestine | In intestine IL-6, IL-8, IL-10, IL-1β, TNF-α, and INF-γ2 | Li et al. (2024a) | ||
| PS (50 nm) (IP injected) (0.1 mL of 5 μg/L injected volume) exposed for 48 h | Fathead minnows (adult male) | Liver and head kidney |
|
|
Elizalde-Velazquez et al. (2020) | |
| PS (50 nm) (trophic transfer) (48 h) | Fathead minnows (adult male) | Liver and kidney |
|
|
|
Elizalde-Velazquez et al. (2020) |
| PS (100 nm) [(5 mg/g); dietary, 30 days] | Marine medaka (adults) | Gut |
|
Zhang et al. (2021) | ||
| PS (100 nm) (3.5 mg/g; dietary for 30 days) Parental exposure (F0); F1 was not exposed (observed after 60 dpf) | Marine medaka (adults) | Liver |
|
|
|
He et al. (2022) |
| PS (70 nm) (20, 200, and 2000 μg/L trophic transfer, 90 days) | Marine medaka (adults) | Intestine, liver, muscle, and F1 offspring |
|
|
Li et al. (2024b) | |
| PS (100 nm) (20 mg/L, waterborne); exposed for 7 days and depurated for 7 days | Mozambique tilapia (larvae) (4 weeks old) (0.57 ± 0.13 g body weight) | Whole fish |
|
|
Pang et al. (2021) | |
| PS (86 nm) (1 mg/L, (waterborne, exposed for 21 days and depurated for 7 days) | Nile tilapia (juveniles) (10.9 ± 3.9 g body weight) | Gut/intestine |
|
|
Hao et al. (2023) | |
| PS (100 nm) (waterborne, 1, 10, and 100 μg/L for 7 days) | Nile tilapia (juvenile body weight 15 ± 5 g) | Liver |
|
|
Wang et al. (2023b) | |
| PS (47 nm) (1 mg/L, 120 h waterborne) | Zebrafish (embryos) | Whole larvae |
|
|
Chen et al. (2017a) | |
| PS (70 ± 9.21 nm) (injected 0.52 nL volume of 1,000, 3,000, and 5,000 mg/L and also exposed to 0.5 and 5 mg/L PSNAP waterborne until hatching), depurated until 4 weeks) | Zebrafish (embryos) | Whole larvae |
|
|
Zhang et al. (2020) | |
| PS (100 nm) (exposed to 10 μg/L; waterborne until 12 hpf) and depurated until 120 hpf) | Zebrafish (embryos 2 hpf) | Whole larvae |
|
Liu et al. (2021) | ||
| PS (50 nm) (exposed to 1 mg/L; waterborne until 96 hpf) | Zebrafish (embryos) | Whole larvae |
|
|
|
Bhagat et al. (2022) |
| PS (50 and 100 nm) (0.1, 0.5, 2, and 10 mg/L; waterborne exposure 120 hpf | Zebrafish (embryos) | Liver |
|
Cheng et al. (2022) | ||
| PS (100 nm) (100, 200, and 400 mg/L; 24 h waterborne) | Zebrafish (embryos) | Whole embryo |
|
|
Feng et al. (2022) | |
| PS (100 nm) (10 μg/L, waterborne) exposed for 144 hpf and depurated for 3 days | Zebrafish (embryos, 2 hpf) | Whole embryo |
|
|
Liu et al. (2022b) | |
| PS (80 nm) (50 μg/L, 100 μg/L, 1 mg/L, 5 mg/L, and 10 mg/L; waterborne, 120 hpf) | Zebrafish (embryos) | Whole larvae |
|
|
|
Wang et al. (2022) |
| PS (50 nm) (0.1, 1, 5, 10, 20, 30, and 50 mg/L) (waterborne exposure for 5 days and depurated until 12 days) | Zebrafish (embryos) | Whole larvae |
|
|
Chen et al. (2023c) | |
| PS (20 nm) (2, 5, and 8 mg/L) (waterborne, exposed for 22, 46, and 70 h) | Zebrafish (embryos, 2 hpf) | Whole embryo |
|
|
Dai et al. (2023) | |
| PS (80 nm) (5, 10, 25, 50, and 100 μg/L) (waterborne, exposed until 96 hpf) | Zebrafish (embryos 2 hpf) | Whole larvae |
|
Gao et al. (2023b) | ||
| PS (30 nm and 100 nm) (0.1, 1, and 10 mg/L, exposed for 96 h) | Zebrafish (embryos 5 hpf) | Whole larvae |
|
Martin et al. (2023) | ||
| PS (30 nm) (0.1, 0.5, and 3 mg/L, waterborne, exposed for 120 hpf) | Zebrafish (embryos, 1 hpf) | Whole larvae |
|
|
|
Martin-Folgar et al. (2023) |
| PS (80 nm) (0.05, 0.1, 1, 5, and 10 mg/L, waterborne, exposed for 120 hpf) | Zebrafish (fertilized eggs) | Whole larvae |
|
|
Wang et al. (2023c) | |
| PS (100 nm) (10 mg/L, waterborne, exposed for 5 days) | Zebrafish embryos (2 hpf) | Whole larvae |
|
|
|
Zhou et al. (2023c) |
| PS (80 nm) (0.1, 0.5, 1, 5, 10, 25, and 50 mg/L) (waterborne, exposed for 120 hpf) | Zebrafish embryos (8 hpf)) | Whole larvae |
|
Chen et al. (2024) | ||
| PS (80 nm) (1 mg/L) waterborne, exposed for 21 days | Zebrafish (adults) | Gut |
|
|
Xie et al. (2021) | |
| PS (54.5 ± 2.8 nm) (10 mg/L), waterborne, exposed for 120 days. Both P1 and F1 | Zebrafish (adults) | Brain and liver |
|
Zhao et al. (2021) | ||
| PS (70 nm) (100 μg/L, waterborne, exposed for 3 months | Zebrafish (adult male and female fish) | Liver |
|
Ling et al. (2022) | ||
| PS (100 nm) (25 mg/L; exposed at 28-, 29-, and 30°C for 96 h) | Zebrafish (adults, 3 months old) | Brain |
|
Sulukan et al. (2022a) | ||
| PS (44 nm) (1, 10, and 100 μg/L, waterborne, exposed for 30 and 60 days) | Zebrafish (juveniles and adults) | Intestine |
|
|
Teng et al. (2022b) | |
| PS (100 nm) (500 ng/mL) waterborne, exposed for 28 days | Zebrafish (adults) | Liver (hepatocytes) |
|
|
Deng et al. (2023) | |
| PS (80 nm) (15 and 150 mg/L, waterborne, exposed for 21 days | Zebrafish (adults) | Liver |
|
|
Li et al. (2023a) | |
| PS (100 nm) (1 mg/L, waterborne, exposed for 30 days) | Zebrafish (adults) | Brain |
|
|
|
Zhang et al. (2023) |
| PS (50 nm) (1.0 mg/L, waterborne, exposed for 21 days) | Zebrafish (adults) | Gonad (ovary) and liver |
|
Ye et al. (2024) |
Genotoxic effects of NAPs on fish.
TABLE 8
| Additives (name/concentration) | Type/nature | Fish | Developmental stages | Nanoplastics (name/size/concentrations) | Mode of exposure and duration | Results | References |
|---|---|---|---|---|---|---|---|
| Acetaminophen (APAP) (2 and 8 mM) | Drug | Zebrafish (Danio rerio) | Embryos (3 hpf) | PS (80 nm) (100 μg/L) | Waterborne (96 hpf) |
|
Gao et al. (2023b) |
| Aeromonas hydrophilia (2 × 107 CFU/mL) | Bacteria | Grass carp (Ctenopharyngodon idella) | Juveniles | PS (80 nm) (10, 100, and 1,000 μg/L) | PS = waterborne (5 days) bacteria = injection; depurated for 3 days |
|
Li et al. (2024a) |
| nAL2O3 (1 mg/L) | Metal | Zebrafish (Danio rerio) | Embryos | PS (50 nm) (1 mg/L) | Waterborne (96 hpf) |
|
Bhagat et al. (2022) |
| Arsenic (As; 200 μg/L) | Metalloid | Zebrafish (Danio rerio) | Adults | PS (100 nm) (1 mg/L) | Waterborne (30 days) |
|
Zhang et al. (2023) |
| As (1 mg/L) | Metalloid | Zebrafish (Danio rerio) | Adult | PS (100 nm) (1 mg/L) | Waterborne (30 days) |
|
Zhang et al. (2024c) |
| Avobenzone (AVO) or butyl methoxydibenzoylmethane (1, 10, and 100 μg/L) | PCP/sunscreen | Zebrafish (Danio rerio) | Embryos | PS (100 nm) (10 μg/L) | Waterborne (2–12 hpf). Depurated until 120 hpf |
|
Liu et al. (2021) |
| Avobenzone (AVO; 10 μg/L) | PCP/sunscreen | Zebrafish (Danio rerio) | Embryos (2 hpf) | PS (100 nm) (10 μg/L) | Waterborne (144 hpf). Depurated for 3 days |
|
Liu et al. (2022b) |
| Benzo [a] pyrene (BAP) (0.1, 0.5, 1, 5, and 10 mg/L) | PAH | Zebrafish (Danio rerio) | Embryos | PS (50 nm) (0.069, 0.69, 69, 687, and 6,870 μg/L) (120 hpf) | Waterborne (120 hpf) |
|
Martinez-Alvarez et al. (2022) |
| BDE-47 (10 ng/L) | Flame retardant | Zebrafish (Danio rerio) | Embryos | PS (100 nm) (2.5 and 25 μg/L) | Waterborne (7 days) |
|
Chackal et al. (2022) |
| BDE-47 (0.1 mg/L) | Flame retardant | Zebrafish (Danio rerio) | Embryos | PS (80 nm) 0.05, 0.1, 1, 5, and 10 mg/L | Waterborne (120 hpf) |
|
Wang et al. (2022) |
| BDE-47 (0.1 and 10 μg/L) | Flame retardant | Zebrafish (Danio rerio) | Embryos | PS (80 nm) (0.05, 0.1, 1, 5, and 10 mg/L) | Waterborne (120 hpf) |
|
Wang et al. (2023c) |
| Butylmethoxydibenzoylmethane (BMDBM) or avobenzone (1,10, and 100 μg/L) | PCP/sunscreen | Zebrafish | Embryos (2 hpf) | PS (100 nm) (10 μg/L) | Waterborne (2–12 hpf) |
|
Liu et al. (2021) |
| Bisphenol A (BPA) (100 μg/L) | Plastic additive | Marine medaka (Oryzias melastigma) | Embryos (6 hpf) | PS (50 nm) (55 μg/L) | Waterborne (21 days) |
|
Yu et al. (2023) |
| BPA (0.78 μg/L) | Plastic additive | Zebrafish (Danio rerio) | Adults (6 months old) | PS (47 nm) (1 mg/L) | Waterborne (3 days) |
|
Chen et al. (2017b) |
| nCeOs (1 mg/L) | Metal | Zebrafish (Danio rerio) | Embryos | PS (50 nm) (1 mg/L) | Waterborne (96 hpf) |
|
Bhagat et al. (2022) |
| Chloroauric acid (1 μg/mL) | Inorganic compound | Zebrafish (Danio rerio) | Embryos | PS (50 nm) (0.1 mg/L) | Waterborne (6, 24, and 96 hpf) |
|
Lee et al. (2019) |
| p, p’-DDE (100 μg/L) | Insecticide | Zebrafish (Danio rerio) | Embryos | PS (15 nm) (50 mg/L) | Waterborne (96 hpf) |
|
Varshney et al. (2023) |
| Diethylstilbesterol (DES) (1,10, and 100 ng/L) | Synthetic hormone (estradiol) | Zebrafish (Danio rerio) | Adults (male and female) 5 months old | PS (70 nm) (2 mg/L) | Waterborne (21 days) |
|
Lin et al. (2023) |
| Diphenhydramine (DPH) (0.01 and 10 mg/L) | Antihistamine | Zebrafish (Danio rerio) | Embryos | PS (44 nm) (0.015 and 1.5 mg/L) | Waterborne (96–120 f) |
|
Barreto et al. (2023) |
| 17α-ethinylestradiol (EE2) (2 and 20 μg/L) | Hormone (synthetic estrogen) | Zebrafish (Danio rerio) | Embryos | PS (47 nm) (1 mg/L | Waterborne (120 h) |
|
Chen et al. (2017a) |
| F-53B (500 μg/L) | Polyfluoroalkyl substance | Hainan medaka (Oryzias curvinotus) | Adults (length 2.85 ± 0.17 cm; weight 440 ± 90 mg) | PS (80 nm) (200 μg/L) | Waterborne (7 days) |
|
Gao et al. (2023a) |
| Glucose (40 mM) | Carbohydrate | Zebrafish (Danio rerio) | Larvae (72 hpf) | PS (25 nm) (20 mg/L) | Waterborne (exposed 72–120 hpf) |
|
Brun et al. (2019) |
| Homosolate (0.0262–262 μg/L) | Organic compound/UV filter | Zebrafish (Danio rerio) | Adults | PS (50 nm) (1 mg/L) | Waterborne days) |
|
Ye et al. (2024) |
| Lead (50 μg/L) | Metal | Zebrafish (Danio rerio) | Adults | PS (100 nm) (20 and 200 μg/L) | Waterborne (exposed for 3 weeks) |
|
Yu et al. (2022a) |
| Microcystin LR (MCLR) (0.9, 4.5, and 22.5 μg/L) | Antibiotics | Zebrafish (Danio rerio) | Adults | PS (70 nm) (100 μg/L) | Waterborne (96 h) 21 days parental exposure (F0) and F1 larvae (120 hpf) were evaluated without exposure |
|
Zuo et al. (2021) |
| Microcystin LR (MCL) (0.9, 4.5, and 22.5 μg/L) | Antibiotics | Zebrafish (Danio rerio) | Adults (male and female) | PS (70 nm) (100 μg/L) | Waterborne (96 h) 3 months |
|
Ling et al. (2022) |
| Microcystin-LR (MSL) (1 μg/L) | Antibiotics | Silver carp (Hypophthalmichthys molitrix) | Adults (9.33 ± 1.01 cm length, 10.43 ± 3.41 g weight) | PS (80 nm) (10 and 1,000 μg/L) | Waterborne (96 h) |
|
Zhang et al. (2024a) |
| 4-Nonylphenol (1 μg/L) | Nonionic surfactant | Zebrafish (Danio rerio) | Adults | PS (20–80 nm) average size 57.5 nm (0.1, 1, 10, and 100 μg/L) | Waterborne (45 days) |
|
Aliakbarzadeh et al. (2023) |
| Oxytetracycline (100 μg/L) | Antibiotics | Zebrafish (Danio rerio) | Adults (6 months old) | PS (40–54 nm) (60–338 μg/L) | Waterborne (30 days) |
|
Yu et al. (2022b) |
| Penicillin (1 and 10 μg/L) | Antibiotics | Zebrafish (Danio rerio) | Embryos (8 hpf) | PS (80 nm) (0.5 and 5 mg/L) | Waterborne (120 hpf) |
|
Chen et al. (2023b) |
| Phenanthrene (PHE) (0.1, 0.5, and 1.0 mg/L) and jellyfish mucin (50 μg/mL) | PHE (polycyclic aromatic hydrocarbon); mucin (biological substance) | Zebrafish (Danio rerio) | Embryos | PS (50 nm) (5 mg/L) | Waterborne (4, 8, 12, 24, 32, 48, and 72 hpf) |
|
Geum and Yeo, (2022) |
| Phenmedipham (PHN) (0.02, 0.2, and 20 mg/L) | Herbicide | Zebrafish (Danio rerio) | Embryos | PS (44 nm) (0.015 and 1.5 mg/L) | Waterborne (96–120 hpf) |
|
Santos et al. (2022) |
| Polycyclic aromatic hydrocarbons (PAH) (5.07–25.36 μg/L) | Organic substance | Zebrafish (Danio rerio) | Embryos | PS (44 nm) (0.1, 1, and 10 mg/L) | Waterborne (96 hpf) |
|
Trevisan et al. (2019) |
| Polycyclic aromatic hydrocarbons (PAH) (1 mg/L) | Organic substance | Zebrafish (Danio rerio) | Embryos | PS (44 nm) (1 mg/L) | Waterborne (96 hpf) (7 days) |
|
Trevisan et al. (2020) |
| Simvastatin (SIM) (0.015–150 μg/L) | Statin | Zebrafish (Danio rerio) | Embryos | PS (60 nm) (0.05 or 1.5 mg/L) | Waterborne (96 h) |
|
Barreto et al. (2021) |
| Sodium nitroprusside (8 µM) | Inorganic compound/ | Zebrafish (Danio rerio) | Embryos | PS (50 nm) (20 mg/L) | Waterborne (12 days) |
|
Chen et al. (2023c) |
| Sulfamethazine (SMZ) (0.5 and 5 mg/g) | Antimicrobial agent | Marine medaka (Oryzias melastigma) | Adults | PS (100 nm) (5 mg/g) | Dietary (30 days) |
|
Zhang et al. (2021) |
| Sulfamethazine (SMZ) (4.62 mg/g) | Antimicrobial agent | Marine medaka (Oryzias melastigma) | Adults (580.2 ± 189.5 mg body weight) | PS (100 nm) (3.45 mg/g) | Dietary (30 days) parental (F0) exposure; F1 evaluated after 60 days |
|
He et al. (2022) |
| Sulfamethazine (SMZ) (0.5 and 5 mg/g) | Antimicrobial agent | Marine medaka (Oryzias melastigma) | Adults (4 months old) | PS (100 nm) (5 mg/g) | Dietary (30 days) depurated 21 days |
|
Wang et al. (2023a) |
| Sulfamethoxazole (SMX) (100 μg/L | Antibiotics | Marine medaka (Oryzias melastigma) | Juveniles (2 months old) | PS (100 nm) (1 mg/L) | Waterborne (30 days) |
|
Li et al. (2023b) |
| Tetracycline (TC) (5,000 μg/L) | Antibiotics | Grass carp (Ctenopharyngodon idella) | Juveniles | PS (80 nm) (20, 200, and 2000 μg/L) - | Waterborne (7 days) |
|
Liu et al. (2022a) |
| Triclosan (TCS) (0.01, 0.1, and 1 mg/kg) | Biocide | Tooth carp (Aphaniops hormuzensis) | Adults | PS (100 nm) (0.5 mg/L) | Dietary (3, 14, and 28 days) |
|
Saemi-Komsari et al. (2023) |
| Triphenyl phosphate (TPhP) (0.08, 0.5, 0.7, 1, 1.2, and 1.5 mg/L) | Flame-retardant and plasticizer | Zebrafish (Danio rerio) | Adults (male and female) | PSNAP (46 nm) (2 mg/L) | Waterborne (21 days) |
|
He et al. (2021) |
| Tris (1,3-dichloro-2-propyl) phosphate (TDCIPP) (0.47, 2.64, or 12.78 μg/L) | Flame-retardant | Zebrafish (Danio rerio) | Adults | PS (54.5 ± 2.8 nm) (10 mg/L) | Waterborne (120 days) evaluated F0 and F1 larvae (without exposure) |
|
Zhao et al. (2021) |
| Vitamin D (280 and 2,800 IU/kg body weight) | Vitamin | Zebrafish (Danio rerio) | Adults | PS (80 nm) (15 and 150 mg/L) | Dietary (for 21 days) |
|
Li et al. (2023a) |
| ZnO (760 μg/L) | Metal oxide | Grass carp (Ctenopharyngodon idella) | Juveniles | PS (23.03 ± 0.266 nm) (760 μg/L) | Waterborne (72 h) |
|
Estrela et al. (2021) |
Effects of NAPs and various environmental contaminants used in coexposure studies on the toxicological endpoints of fish.
TABLE 9
| Additives (name/concentration) | Type/nature | Fish | Developmental stages | Nanoplastics (name/size/concentrations) | Mode of exposure and duration | Gene expressions | References |
|---|---|---|---|---|---|---|---|
| Acetaminophen (APAP) (2 and 8 mM) | Drug | Zebrafish (Danio rerio) | Embryos (3 hpf) | PS (80 nm) (100 μg/L) | Waterborne (96 hpf) |
|
Gao et al. (2023b) |
| Aeromonas hydrophilia (2 × 107 CFU/mL) | Bacteria | Grass carp (Ctenopharyngodon idella) | Juveniles | PS (80 nm) (10, 100, and 1,000 μg/L) | PS = waterborne (5 days); bacteria = injection; depurated for 3 days |
|
Li et al. (2024a) |
| nAL2O3 (1 mg/L) | Metal | Zebrafish (Danio rerio) | Embryos | PS (50 nm) (1 mg/L) | Waterborne (96 hpf) |
|
Bhagat et al. (2022) |
| Arsenic (As; 200 μg/L) | Metalloid | Zebrafish (Danio rerio) | Adults | PS (100 nm) (1 mg/L) | Waterborne (30 days) |
|
Zhang et al. (2023) |
| As (1 mg/L) | Metalloid | Zebrafish (Danio rerio) | Adult | PS (100 nm) (1 mg/L) | Waterborne (30 days) |
|
Zhang et al. (2024c) |
| Avobenzone (AVO) or butyl methoxydibenzoylmethane (BMDZM) (1, 10, and 100 μg/L) | POP | Zebrafish (Danio rerio) | Embryos | PS (100 nm) (10 μg/L) | Waterborne (2–12 hpf) Depurated until 120 hpf |
|
Liu et al. (2021) |
| BDE-47 (0.1 mg/L) | Flame-retardant | Zebrafish (Danio rerio) | Embryos | PS (80 nm) 0.05, 0.1, 1, 5, and 10 mg/L | Waterborne (120 hpf) |
|
Wang et al. (2022) |
| BDE-47 (0.1 and 10 μg/L) | Flame retardant | Zebrafish (Danio rerio) | Embryos | PS (80 nm) (0.05, 0.1, 1, 5, and 10 mg/L) | Waterborne (120 hpf) |
|
Wang et al. (2023e) |
| BMDBM or avobenzone (1, 10, and 100 μg/L) | PCP/sunscreen | Zebrafish (Danio rerio) | Embryos | PSNAP (100 nm) (10 μg/L) | Waterborne (120 hpf) |
|
Liu et al. (2021) |
| nCeO2 (1 mg/L) | Metal | Zebrafish (Danio rerio) | Embryos | PS (50 nm) (1 mg/L) | Waterborne (9 hpf) |
|
Bhagat et al. (2022) |
| 17α-ethinylestradiol (EE2) (2 and 20 μg/L) | Hormone | Zebrafish (Danio rerio) | Embryos | PS (47 nm) (1 mg/L | Waterborne (120 |
|
Chen et al. (2017a) |
| Homosolate (0.0262–262 μg/L) | Organic compound/UV filter | Zebrafish (Danio rerio) | Adults | PS (50 nm) (1 mg/L) | Waterborne (21 days) |
|
Ye et al. (2024) |
| Lead (50 μg/L) | Metal | Zebrafish (Danio rerio) | Adults | PS (100 nm) (20 and 200 μg/L) | Waterborne (exposed for 3 weeks) |
|
Yu et al. (2022a) |
| Microcystin LR (MCLR) (0.9, 4.5, and 22.5 μg/L) | Antibiotics | Zebrafish (Danio rerio) | Adults | PS (70 nm) (100 μg/L) | Waterborne (96 h) 21 days parental exposure (F0) and F1 larvae (120 hpf) were evaluated without exposure |
|
Zuo et al. (2021) |
| Microcystin LR (MCL) (0.9, 4.5, and 22.5 μg/L) | Antibiotics | Zebrafish (Danio rerio) | Adults (male and female) | PS (70 nm) (100 μg/L) | Waterborne (96 h) 3 months |
|
Ling et al. (2022) |
| Sodium nitroprusside (8 µM) | Inorganic compound/ | Zebrafish (Danio rerio) | Embryos | PS (50 nm) (20 mg/L) | Waterborne (12 days) |
|
Chen et al. (2023c) |
| Sulfamethazine (SMZ) (0.5 and 5 mg/g) | Antimicrobial agent | Marine medaka (Oryzias melastigma) | Adults | PS (100 nm) (5 mg/g) | Dietary (30 days) |
|
Zhang et al. (2021) |
| Sulfamethazine (SMZ) (4.62 mg/g) | Antimicrobial agent | Marine medaka (Oryzias melastigma) | Adults (580.2 ± 189.5 mg body weight) | PS (100 nm) (3.45 mg/g) | Dietary (30 days) parental (F0) exposure; F1 evaluated after 60 days |
|
He et al. (2022) |
| Tetracycline (TC) (5,000 μg/L) | Antibiotics | Grass carp (Ctenopharyngodon idella) | Juveniles | PS (80 nm) (20, 200, and 2000 μg/L) | Waterborne (7 days) |
|
Liu et al. (2022a) |
| Tris (1,3-dichloro-2-propyl) phosphate (TDCIPP) (0.47, 2.64, or 12.78 μg/L) | Flame-retardant | Zebrafish (Danio rerio) | Adults | PS (54.5 ± 2.8 nm) (10 mg/L) | Waterborne (120 days); evaluated F0 and F1 larvae (without exposure) |
|
Zhao et al. (2021) |
| Vitamin D (280 and 2,800 IU/kg body weight) | Vitamin | Zebrafish (Denio rerio) | Adults | PS (80 nm) (15 and 150 mg/L) | Dietary (for 21 days) |
|
Li et al. (2023a) |
| Vitamin D (280 and 2,800 IU/kg body weight) | Vitamin | Zebrafish (Denio rerio) | Adults | PS (80 nm) (15 and 150 mg/L) | Waterborne (21 days) |
|
Teng et al. (2023) |
| ZnO (760 μg/L) | Metal oxide | Grass carp (Ctenopharyngodon idella) | Juveniles | PS (23.03 ± 0.266 nm) (760 μg/L) | Waterborne (72 h) |
|
Estrela et al. (2021) |
Genotoxic effects of NAPs with various environmental contaminants used in coexposure studies.
Among the 114 selected articles, we further screened by focusing only on studies on NAPs that are ≤100 nm in diameter/size; therefore, studies made focusing on plastic sizes >100 nm (15 articles) were excluded during evaluation (Table 3). Among these 15 articles, two articles, Monikh et al., 2022 (PE, PPP, PS, and PVC), and Tamayo-Belda et al., 2023 (LDPE, PLA, PPP, and PS), focused on more than one plastic type and included together in one article. Moreover, their studies examined various sizes of plastics, belonging to both NAPs and MIPs. Therefore, these two articles were included in both inclusion (Tables 2, 4) exclusion (Table 3) tables. Wang L. et al. (2023) did not mention the plastic types used for zebrafish embryos, although the size of the NAP was 100 nm. Therefore, we did not consider Wang L. et al. (2023) for review (Table 3). In addition, 26 articles included both MIP (>100 nm) and NAP (≤100 nm) in their investigations (Table 4). During the review process, we considered these 26 articles and focused only on the studies carried out on NAPs and excluded the studies carried out on MIPs (Table 4). Moreover, Tamayo-Belda et al. (2023) measured the diameter of the plastics (LDPP, PLA, PPP, and PS) every day during embryo development (4–96 hpf), and the diameter of the plastic particle was widely variable (>100 nm) within the days of exposure. However, in case of PS, the diameter of the plastic particle during the exposure (4 hpf) was 91 nm, which was below the exclusion limit of the MIPs (≤100 nm) followed in this study. In addition, for LDPE, the diameter of the plastic particle is 91 nm only on 4 dpf (96 hpf) of development (Table 4). We, therefore, consider PS and LDPE as NAPs during evaluation. Furthermore, three articles, namely, Manuel et al. (2022) (studies on PMMA and PS on zebrafish embryos); Monikh et al. (2022) (studies on PPP, PE, PS, and PVC on zebrafish embryos); and Tamayo-Belda et al. (2023) (studies on LDPE, PLA, PPP, and PS on zebrafish embryos), studied multiple plastic particles and described the results together in one article. Elizalde-Velazquez et al. (2020) studied the effects of PS on fathead minnows using two methods of exposure (IP and trophic transfer) and described the results together in one article. Moreover, we confined our search to in vivo studies and excluded in vitro studies (Greven et al., 2016). However, Greven et al. (2016), used two different sizes of PS (158.7 nm and 41 nm sizes) on fathead minnows and described the results together in one article. Therefore, 15 (13 + 2) articles, including studies by Monikh et al. (2022) and Tamayo-Belda et al. (2023), were excluded (Table 3), 26 articles were partly excluded from the review, and finally, 101 (99 + 2) articles were selected for NAP evaluation (Tables 5–9).
3 Results
In laboratory studies, fish at different developmental stages (embryos, larvae, juveniles, and adults) were used for the assessment of NAP toxicity (Table 2). In embryos, NAPs were accumulated/agglomerated on the chorion after exposure (waterborne) and depending on the size of the NAPs and the pore diameter of the chorion (in zebrafish, the size of the chorion was 200–700 nm in diameter, Chen et al., 2020), NAP particles crossed the barrier and entered into the body of the developing embryos and gradually accumulated on different organs over time. In some experiments, NAPs were directly injected inside the eggs (Sokmen et al., 2020; Zhang et al., 2020). However, in larvae, juveniles, and adults, the fish when exposed to NAPs through waterborne mode, trophic transfer, or through diet entered inside the body through the mouth, gills, and skin. In a few cases, NAPs were directly administered through injections (Elizalde-Velazquez et al., 2020).
3.1 Effects of NAPs on fish
3.1.1 Polyethylene
Polyethylene (PE) is also known as polythene, is a synthetic resin and the most commonly used plastic in the world. It can only generate nonspecific van der Walls interactions (Geum and Yeo, 2022). Our literature search found only two fish species; common carp (one article) and zebrafish (four articles on PE and one article on LDPE; three on embryos and two on adults) were used to evaluate the toxic potential of PE/LDPE as NAPs. Moreover, two more studies were conducted on PE where the particle size was >100 nm (Sun et al., 2021; Khan and Ali, 2023), and were therefore excluded from evaluation. The 96 hpf no observed adverse effect level (NOAEL) found on the toxicity of PE in zebrafish embryos was 0.05 mg/L (hydrodynamic size 191.10 ± 3.13 nm) (Sun et al., 2021). Zebrafish adults exposed to pristine polyethylene (76,740 ± 14,070 nm) were able to excrete small PE (5,920 ± 4,960 nm) within 24 h of exposure (Supplementary Table S1), which indicates that PEMIP enters the gut, metabolizes to smaller fragments, and is excreted in the fecal material (Khan and Ali, 2023).
3.1.1.1 Common carp
In juvenile common carp (Cyprinus carpio), PE significantly decreased the enzyme activities (AChE and MAO) and NO content in the brain (Hamed et al., 2022) and caused histological damages, indicating varying degrees of necrosis, fibrosis, changes in blood capillaries, tissue detachment, edema, degenerated connective tissues, and necrosis of large cerebellar neurons and ganglion cells (Tables 2, 5, 6, Supplementary Table S1). In eyes, necrosis, degeneration, vacuolation, and curvature in the inner layer were observed after PE exposure.
3.1.1.2 Zebrafish
Both embryos and adults of zebrafish were used for the evaluation of PE toxicity (Tables 2, 5, 6; Supplementary Table S1). Zebrafish embryos within 6 hpf were exposed to PE (50 nm; 3 × 10−10/L) for 24 h or to LDPE (91–342 nm) for 96 h, and mortality and development were evaluated until 4–5 dpf (Tables 2, 5, 6; Supplementary Table S1). It was observed that PE did not induce mortality; however, delayed hatching was observed, and the hatched embryos were normal, although the larval body length was reduced when compared with that of controls (Monikh et al., 2022). The zebrafish larvae (120 hpf) exposed to LDPE during development showed slight locomotor activity during the light phase (Tamayo-Belda et al., 2023). Zebrafish adults were exposed to PE (70 nm) at a concentration of 20 μg/mL for 21 days (Tables 2, 5, 6; Supplementary Table S1), and the oxidative stress and AChE enzyme activity in the gill, gut, and liver of fish on 7, 14, and 21 days of exposure (Li R. et al., 2023) were investigated. Moreover, gut dysbiosis was also analyzed. Organ-dependent oxidative damage induced by PE was observed after chronic exposure. Insignificant differences in the neurotoxicity (inhibition of AChE activity) and dysbiosis of gut microbiota were also observed in fish exposed to PE (Li R. et al., 2023). The effects on GST, GSH, CAT, LPO, and SOD showed that PE induced organ-specific oxidative damage in the gill, gut, and liver (Li R. et al., 2023).
Taken together, it was observed that PE (50 nm) was able to reduce the length of zebrafish larvae when the embryos were exposed only for 24 h (Monikh et al., 2022).Juvenile common carp exposed to PE (<100 nm; 15 mg/L) for 15 days had disrupted brain structure (histology) and function (AChE and MAO activities and NO contents), while in adult zebrafish, PE (70 nm; 20 μg/L for 21 days) induced organ-specific oxidative stress (gill/gut/liver), inhibited AChE activity, and induced dysbiosis in gut bacterial communities (Li R. et al., 2023). Therefore, although the study is limited only to two fish models and studies on gene expression are lacking, PE was found to induce toxicity in fish, depending on the developmental stages, concentration, sizes, and the duration of exposure, as well as in different organs of the fish (Table 6).
3.2 Polyethylene terephthalate
Polyethylene terephthalate (PET) is one of the most used plastic polymers, particularly for containers (container for food, drinks, and plastic bags), owing to its transparency, flexibility, and innocuity (Dhaka et al., 2022). It is also used in textiles and as parts of automotives and electronics (Gwada et al., 2019; Dhaka et al., 2022). PET particles have been found in ground water, drinking water, soils, and sediments in the air (Dhaka et al., 2022; Jiang et al., 2022; Lin et al., 2022; Zhang H. et al., 2022). The hazardous effects of PET in the form of nanoparticles (PETNAPs) in marine organisms such as amphipods, copepods, and fish have been studied (Heinder et al., 2017; Ji et al., 2020). PETNAPs have raised severe concerns regarding potential danger and risks for nature and human wellbeing (Dhaka et al., 2022; Zhang H. et al., 2022). Studies on human cell culture showed that PETNAPs at a higher concentration have inhibitory effects on the cell viability (Margi et al., 2021; Zhang H. et al., 2022; Villacorta et al., 2022), and the interaction of PETNAPs with different contaminants (Hg2+, glyphosate, and levofloxacin) can significantly change the cell physiology (Margi et al. (2021)). Using human lung carcinoma cell culture, Zhang H. et al (2022) have shown that PETNAP increased levels of reactive oxygen species (ROS), which may affect mitochondrial potential. A comprehensive system-level tracking of the toxicity pathways affected by PETNAPs is necessary to understand the toxicity mechanisms of PETNAPs. Our literature search found that only zebrafish embryos were used (two articles) to evaluate the toxic potential of PETNAPs in fish (Bashirova et al., 2023; de Souza Toedoro et al., 2024).
3.2.1 Zebrafish
Zebrafish embryos (6 hpf and 72 hpf) were exposed to PET (70 ± 5 nm and 68.06+ nm) until 96–120 hpf (Bashirova et al. (2023) or 6 days (de Souza Toedoro et al. (2024) at concentrations ranging from 0.5 to 200 mg/L (Tables 2, 5, 6; Supplementary Table S1). PET was accumulated in liver, kidney, and intestine of the larvae (Table 5), and its exposure reduced the survivability and hatching of the embryos in a concentration-dependent manner. The heart rates remained unaltered. The locomotor activity of the larvae in the dark phase was reduced in a concentration-dependent manner. Quantitative analysis of the metabolites indicated a significant decrease in acetate, glucose, alanine, leucine, isoleucine, valine, glutamate, cystine, glycine, and GSH levels; however, a significant increase was noticed in lactate, choline, glycerophosphorylcholine and ethanolamine, tryptophan, phenylalanine, tyrosine, free fatty acids, and cholesterol levels (Bashirova et al., 2023). Higher levels of ROS were generated in the intestine, liver, and kidney region of the larvae (Bashirova et al., 2023). In contrast to the study, de Souza Toedoro et al. (2024) observed that PET accumulated on the surface of the chorion in a concentration-dependent manner, and no effect on the mortality and hatching of the embryos was observed. The heart rates of the treated embryos at 48 hpf increased significantly in a concentration-dependent manner, and the length of the hatched larvae did not change significantly; also, no effect on locomotor activity was observed. The interocular distance reduced significantly in embryos exposed to PET. Moreover, spontaneous tail coiling was diminished by PET exposure. No significant effect was observed in lipid peroxidation or total antioxidant capacity during embryo–larval development (de Souza Toedoro et al., 2024). Therefore, despite the differences between the two studies, PET was able to modulate the embryonic development as well as the behavior of the zebrafish larvae; however, there are few studies on the genotoxicity.
3.3 Polymethylmethacrylate
Polymethylmethacrylate (PMMA), is often used in electronic equipment and prosthetics, and 0.26 million tons were used in Europe in 2019 (Plastic Europe, 2022). However, the effect of PMMA on aquatic animals is poorly understood (Manuel et al., 2022). A recent study showed that 40-nm PMMA nanoparticles, at higher concentrations, impaired survival and growth in tadpoles and induced deformities (Venancio et al., 2022). In the marine fish, Sparus aurata, 40-nm PMMA nanoparticles demonstrated the ability to alter the antioxidant status and lipid metabolism pathways and induced genotoxic effects on red blood cells (Brandts et al., 2021). In our literature search, only zebrafish embryos (one article) were used to evaluate the toxic potential of PMMA in fish (Manuel et al., 2022).
Zebrafish embryos (2 hpf) were exposed to PMMA (32 nm; 0.001–100 mg/L) until 96 hpf (Tables 2, 5, 6; Supplementary Table S1), and the larvae (96 hpf) were used for evaluation of mortality, hatching, and pericardial edema (Manuel et al., 2022). The swimming behavior of the larvae was assessed after 120 hpf. It was observed that PMMA at the highest concentration induced mortality and delayed hatching of the embryos. No significant effect on the swimming behavior of the larvae was observed. AChE activity did not show any significant alterations, except for the larvae exposed to a concentration of 0.01 mg/L, in whom the activity significantly decreased when compared with controls. Among the antioxidant enzymes, GST did not show any significant alterations; however, GPX activity was enhanced only in larvae exposed to 10 mg/L PMMA. CAT activity, though nonlinear, was found to be enhanced in larvae exposed to concentrations of 0.001, 0.1, and 10 mg/L. Concerning energy reserves, no significant effect in terms of glycogen was observed (Manuel et al., 2022). Although the concentrations limited the toxic potential of PMMA in zebrafish, the effects were mediated through ROS and oxidative stress.
3.4 Polypropylene
Polypropylene (PPP) is one of the most widely used plastics, with the application ranging from food packaging to use as automotive parts, and it is also one among the most significant components of personal protective equipment such as masks, the use of which has increased since the COVID-19 pandemic (Aragaw, 2020; Patricio Silva et al., 2021; Vanapalli et al., 2021). A considerable amount of PPP waste has accumulated in the environment and is continuously converted to PPPMIPs by action of external factors such as UV radiation, oxidation, and biofilms (Min et al., 2020). PPPMIPs have been detected in the gastrointestinal tracts of sea turtles of the Atlantic Coastlines of Florida (White et al., 2018). In zebrafish embryos, PPP are internalized by ingestion and distributed in the intestine and eventually excreted (Lee et al., 2022). Adult zebrafish were exposed to the micro/nanoplastics extracted from food-grade PPP nonwoven bags for 2 and 14 days, and the activities/contents of several oxidative-stress related biomarkers (ROS, GSH, SOD, CAT, and MDA) were modulated in the gill and liver of the exposed fish (Li J. et al., 2023). Additionally, a recent study reported that PPPMIPs were released from infant feeding bottles during formula preparations (Li et al., 2020). Moreover, in a study on human-derived cell and animal models (zebrafish and nematodes), PPPMIPs induced cytotoxicity, proinflammatory cytokine activity, oxidative stress, and intestinal damage (Lei et al., 2018b; Hwang et al., 2019). Therefore, it was suggested that the preparation and labeling techniques for PPPNAPs as model plastic nanomaterials are important for enhancing toxicological and biodistribution studies (Cassano et al., 2021). Our literature search found that two fish species tilapia juveniles (one article) and zebrafish embryos (three articles) were used to study the toxic potential of PPPNAP; however, two of the articles (Lee et al., 2022 and Tomayo-Belda et al., 2023) used PPPMIPs.
3.4.1 Tilapia
Tilapia (body weight 10 ± 1 g; length 13 ± 1 cm) were exposed to PPPNAP (100 nm) in water for 21 days at three different concentrations (1, 10, and 100 mg/L), and the liver was used for metabolomics analysis (Tables 2, 5, 6; Supplementary Table S1). It was observed that the body weight and the hepatosomatic index (HSI) of the fish did not change after 21 days of exposure to PPP (Wu et al., 2023). However, the plastics induced significant effects on glycerophospholipid, arginine, and proline metabolism and on aminoacyl-tRNA biosynthesis (Wu et al., 2023).
3.4.2 Zebrafish
Embryos of zebrafish within 6 hpf were exposed to 3 × 1010 particles/L of PPP (50 nm) for 24 h (Tables 2, 5, 6; Supplementary Table S1). It was observed that although there was no induction in the mortality among the embryos, the hatching was delayed, and the larval length was reduced significantly. Moreover, 18% of the larvae exposed to PPP showed a curved spine (Monikh et al., 2022).
3.5 Polystyrene
Polystyrene (PS) plastic used in producing Styrofoam, which is used in food containers and packaging products (Kik et al., 2020). It is one of the most produced plastic polymers in the world; in 2019, there was a demand of 1.58 million tons alone in Europe (Manuel et al., 2022). Due to its significant use, often in single-use products associated with food packing, PS is the most detected plastic in the environment (Fahrenfeld et al., 2019) and the most studied plastic on aquatic organisms (Lu et al., 2016; de Sa et al., 2018; Peng et al., 2024). In addition, it is one of the most abundantly found plastics in the marine environment (Pitt et al., 2018b). Among the plastic polymers, PS has an intermediate density (1.05 g/cm3), with a value close to density of water (1–1.03 g/cm3); this makes PS plastics behave differently in waters of different salinity and thus become bioavailable for aquatic organisms, from surface waters to bottom waters or in sediments (Earni-Cassola et al., 2019). PS has a relatively higher adsorption capacity than PE (Geum and Yeo, 2022). The 96-h LC50 as determined in tooth carp (Aphaniops hormuzenis) was 19.3 mg/L (Saemi-Komsari et al., 2023). PSNAP produces ROS, which results in oxidative stress-mediated toxicity (Schirinzi et al., 2017; Lei et al., 2018a; Eom et al., 2020; Kim and Rhee, 2021). Our literature search showed that PS is the only plastic for which almost all the selected fish species were studied and the highest number (104) of articles (∼89%) were considered for review (Figure 1).
3.5.1 Carp
The search terms nanoplastics, PS, and carp identified articles on carp (one article), grass carp (five articles), silver carp (one article), and tooth carp (one article). Our search indicated that among all these carps, the toxic effects of PS were evaluated on embryos, larvae, and juveniles of grass carp and on adults of carp, silver carp, and tooth carp. Moreover, the size (20–8,000 nm), concentrations (5 μg–200 mg/L), duration (2 hpf–20 days), and the modes of exposure (waterborne and dietary) were widely variable (Tables 2, 5, 6). It was observed that in embryos (grass carp), the accumulation of NAPs was mostly on the chorion; in larvae (grass carp) in the intestine and nose area; in juveniles (grass carp) in the gut, intestine, blood, liver, and brain; while in adults (carp, silver carp, and tooth carp), PS was accumulated in the gill, gut, intestine, liver, heart, muscle, and skin (Table 5). The studied effects were mostly focused on toxicological endpoints (Table 6), while genotoxic effects were also investigated (Table 7).
PS (80 nm) was unable to induce any disorder in heart rates or mortality in grass carp embryos (Zhang C. et al., 2022), while in juveniles, PS increased liver weight (HSI), induced DNA damage in erythrocytes, lesion in the gills and intestine, and histological damages in the gut and brain (Table 5). Moreover, the overall antioxidant activities and LPO contents in the brain (CAT, GST, GPx, and SOD activities and GSH and MDA contents) increased, while NO contents remained unaltered. The enhancement of AChE activity in the brain did not affect locomotory movements (Table 6). Moreover, in the intestine of juveniles (grass carp), the expressions of several immunomodulatory genes (IL-6, IL-8, IL-10, IL-1β, TNF-α, and INF-γ2) were upregulated (Li Z. et al., 2024). In adults, PS induced apoptosis, inhibited antioxidant capacity, and increased the protein contents of TL4 and NOX2, which resulted in induction of apoptosis and myocardial injury (Wu et al., 2022). Moreover, the diversity and richness of gut microbiota increased after PS exposure (Zhang et al., 2024a). Taken together, despite the variations in the dose, duration, mode of exposure, and developmental stages, PS was found to be toxic to carps, and PS accumulation in the brain and induction of oxidative stress resulted in immunomodulatory effects in the intestine that disrupted the gut microbial communities.
3.5.2 Fathead minnows
The effects of PS were studied in fathead minnows both in vitro and in vivo. For in vitro effects, neutrophils were collected from adult fish and exposed to PS (41.0 nm diameter) either for 1 h (100 μg/L) or for 2 h at four different concentrations (0.025, 0.05, 0.1, and 0.2 μg/L) (Supplementary Table S1). PS induced degranulation of primary granules, and neutrophil extracellular traps were released in a concentration-dependent manner (Greven et al., 2016), even though nonlinear. However, oxidative burst was less affected.
Adult male fish were exposed to PS (50 nm) either by IP injection (0.1 mL of 5 μg/L) or by trophic transfer [fed PS-exposed (5 mg/L) daphnia to the experimental fish] and sacrificed after 48 h (Elizalde-Velazquez et al., 2020). PS was accumulated in the liver and head kidney of the exposed fish and regulated the expressions of four immune-related genes (ncf2, nox2, mst1, and c3) (Table 7; Supplementary Table S1). The expressions of mst1 and c3 were upregulated in fed animals and downregulated in injected fish (Elizalde-Velazquez et al., 2020). Moreover, the expression of ncf2 was downregulated and that of nox2 remained unaltered in both the liver and head kidney of fish exposed to PS either by injection or by feeding (Elizalde-Velazquez et al., 2020). In the head kidney, significant downregulation was observed in ncf2 expression in both methods of exposure, while mst1 expression was downregulated in injected fish and remained unaltered in fed ones. C3 in the head kidney was downregulated in fed fish and remained unaltered in PSNAP-injected fish (Tables 5, 6; Supplementary Table S1). Therefore, modes of exposure of PS to the fish played a significant role in the expression of immunomodulatory genes in fathead minnows.
3.5.3 Medaka
The search terms, nanoplastics, PS, and medaka identified 14 articles belonging to Chinese rice fish (one article), Hainan medaka (one article), Japanese medaka (two articles), and marine medaka (10 articles). Among these fish (medaka), embryos of marine medaka (Chen et al., 2022; Chen et al., 2023 Y.; Yu et al., 2023), larvae (9 dph) of Japanese medaka (Zhou et al., 2023b) and marine medaka (Kang et al., 2021; Li X. et al., 2024), juveniles of marine medaka (Li Y. et al., 2023; Li X. et al., 2023) and adults of Hainan medaka (Gao D. et al., 2023), Japanese medaka (Zhou et al., 2023a; Zhou et al., 2023b), and marine medaka (Zhang et al., 2021; He et al., 2022; Wang F. et al., 2023) were used for evaluation of PS toxicity. Accordingly, in these studies, the sizes (50 nm–45 µm or 50–45,000 nm), concentrations/doses (5.5 × 10−12 mg/L −5 mg/L), modes of exposure (waterborne, trophic transfer, and dietary), and duration (24 h–120 dph) of exposure with PSNAP were widely variable (Tables 2, 5). Moreover, the accumulation and the effects of PSNAPs in embryos (yolk sac, GI tract, intestinal villi, liver, and heart), larvae (gut, intestine, liver, muscle, and gonads), juveniles (intestine), and adults (gills, intestine, gut, liver, ovary, and testis) were dependent on the developmental stages of the fish (Tables 2, 5). Although the studies were focused on toxicological endpoints (Table 6), investigations on genotoxic effects (Table 7) as well as intergenerational effects have also been done. Moreover, because the diameter of the exposed PS particle was >100 nm, we have excluded the studies carried out by Zhang YT. et al. (2024) on adults of marine medaka in this review (Table 3).
The embryos of marine medaka with PSNAP (50 nm; 55 μg/L) exposure exhibited reduced heart rates (6 dpf), induced mortality, and reduced larval body length (21 dpf); also, deformities in craniofacial structures and abnormalities were also observed in the histology of the liver and heart of the larvae (21 dpf) (Table 6). Moreover, embryos of marine medaka were exposed to PS-NH2 (80 nm) and PS-COOH (80 nm) at 10 μg/L concentration in regular sea water (pH 8.2) or in acidified sea water (pH 7.4) for 10 days and allowed hatching under a PS-free environment in sea water (Chen Y. et al., 2023). It was observed that both PS-NH2 and PS-COOH accumulated in the gut and intestinal villi of the larvae and induced toxic effects (mortality, hatching, heart rates, morphological abnormalities, malformations, and swimming speed and distance) during embryo–larval development (Chen Q. et al., 2023). PS-NH2 showed greater toxicity than PS-COOH; however, in acidified conditions (pH 7.4), the toxicity of PS-COOH was greater than that of PS-NH2 (Chen J. et al., 2023).
PSNAP has no effect on the length, weight, and eye diameter of the fish larvae exposed to PSNAP. Moreover, the oxidative stress (ROS content and the activities of CAT, SOD, and GST) induced by PSNAP exposure exhibited stronger effects and disruption of gut microbiota (Kang et al., 2021). In juveniles (2-month-old marine medaka), PSNAP (100 nm; 5 mg/L, 30 days) was unable to induce histopathological changes in the intestine; however, the mucus content was slightly increased, and the number of intestinal goblet cells significantly decreased with alteration in the gut microbial community (Li X. et al., 2023).
Exposure to PSNAP (80 nm; 250 μg/L) for 7 days in fasting conditions in Hainan medaka adults damaged the gills (fusion of the gill lamellae), liver (appearance of eosinophilic vesicles and vacuolization), and intestine (erosion of intestinal villi) (Table 6). Moreover, the oxidative enzymes (CAT and SOD) and the LPO content (MDA) were altered in these organs (Gao X. et al., 2023). The gut microbiota was also affected by PSNAP exposure. In adults of Japanese medaka, PSNAP induced concentration-dependent mortality and intestinal damage by enhancing the activities of trypsin and chymotrypsin and reducing the amylase activity (Table 6). Moreover, intestinal lipase contents tended to increase, and alkaline phosphatase contents decreased in a concentration-dependent manner (Zhou et al., 2023a; Zhou et al., 2023b). The gut permeability was also disrupted by PSNAP exposure, with enhancement in the diamine oxidase activity and decrease in the d-lactate contents. The oxidative stress-related enzyme (CAT) and MDA contents in the intestine were enhanced, and that of SOD was suppressed after PSNAP exposure. In contrast, the antioxidant enzymatic activities (SOD, CAT, GPx, and LZM) and the MDA content in the gonads were altered in a nonlinear fashion (Zhao et al., 2021). Gut microbial community diversity exhibited a decrease, and changes were observed in the composition (Zhou et al., 2023b). In gonads, a concentration-dependent inhibition in spermatogenesis and oogenesis was observed in Japanese medaka exposed to PSNAPs for 3 months (Zhou et al., 2023a).
Adults of marine medaka were exposed to PSNAPs (70 nm) by trophic transfer (fed with rotifers exposed to PSNAPs), which indicated accumulation in the gut of the fish. Moreover, long-term exposure (90 days) through trophic transfer not only damaged the tissues, including the intestine, liver (induced inflammation), muscle (decreased nutrient contents), and gonads (disrupted spermatogenesis and oogenesis) but also disrupted the gut microbial community. Moreover, reduction in fertility, inhibition of hatching, and disruption in the growth of the offspring were also observed (Li X. et al., 2024). Gene expression analysis indicated that the expressions of il6, il8, il1b, il10, and tnf, in the liver and intestine of the PSNAP (70 nm)-fed fish were upregulated, and in the liver, the expressions of lipid synthesis-related genes (fasn, srebf1, and pparg) and lipid transport-related genes (cetp, and ldlr) were upregulated and those of the lipid degradation-related genes (atg1, ppara, and aco) were downregulated (Li X. et al., 2024). The gene expressions of the Toll-like receptor 4 (TLR4) pathway (irf3, irak4, traf6, and tbk1) in the liver showed a trend of upregulation, while those in muscle development-related genes (myog, myod, mstn, myf5, and fgf6b) were downregulated after PSNAP exposure by trophic transfer (Table 6).
Marine medaka adults fed 5 mg/g (actual concentration was 3.45 mg/g) PSNAPs (100 nm) for 30 days and depurated for 21 days showed sex-specific dysbiosis in the gut microbial community (male fish were more effective than female fish), and during depuration, male fish recovered quickly than female fish (He et al., 2022). Moreover, the eggs produced at the 30th day of exposure by the parents (F0) were reared for 60 days without any additional treatments (F1), and the intergenerational effects on growth, gut microbial content, and the hepatic gene expressions related to oxidative stress (cat, sod, and gpx) and igf1 were evaluated (He et al., 2022). It was observed that parental exposure to PSNAP significantly reduced the body weight of F1 male fish and decreased the hepatic igf1 and decreased sod mRNA content than controls (F1); in female fish, no alteration in the hepatic igf1 mRNA level was observed (Tables 7). The composition of the gut microbiota of the F1 fish was altered when the parents (F0) were fed with PSNAP. The mRNA expression pattern of sod, cat, and gpx remained unaltered in female F1 fish (He et al., 2022). Adults of Chinese rice fish were exposed to PSNAPs (57.29–60.39 nm) either directly (5 mg/L) for 7 days or through trophic transfer by feeding daphnia (Daphnia magna), which consumed algae (Chlamydomonas reinhardtii) exposed to PSNAPs (Chae et al., 2018). Moreover, fertilized eggs laid by the parents during direct exposure periods were further exposed to PSNAPs (5 mg/L) for 24 h, and the unhatched embryos (144 hpf) and larvae (0 dph) were evaluated for accumulation of the PSNAPs (Supplementary Table S1). It was observed that both in trophic transfer and direct exposure, accumulation of PSNAPs was observed in the gut of the parents; in larvae (0 dph) and embryos (144 hpf), the PSNAPs were accumulated on the yolk sac. The locomotor activity of the larvae was also affected by PSNAP exposure. It was observed that the total distance covered during swimming tended to increase; however, the area traveled tended to decrease by the larvae (Chae et al., 2018).
3.5.4 Rainbow trout
The search terms nanoplastics, PS, and rainbow trout identified two articles focused only on juvenile fish (Supplementary Table S1). Moreover, among these studies, in one study (Clark et al., 2023a), the diameter of the exposed PS particle was >100 nm, which was excluded from this review (Table 3). Juvenile rainbow trout (5–10 g bodyweight) were exposed to PSNAP (35 ± 8 nm) through diet (5.9 μg/kg food; fed 2% of body weight) for 3, 7, and 14 days (Table 5), and it was observed that PSNAPs were accumulated in the hind intestine after 3 days and transferred to the liver after 7 days of exposure (Clark et al., 2023b).
3.5.5 Tilapia
The search terms nanoplastics, PS, and tilapia identified seven articles focusing on of two species, Oreochromis mossambicus (Mozambique tilapia, one article) and Oreochromis niloticus (Nile tilapia, six articles). Moreover, our literature search did not find any study on embryos or on adult tilapia; only larvae (Pang et al., 2021Zheng and Wang, 2024; Zheng et al., 2024) and juveniles (Ding et al., 2018; 2020; Hao et al., 2023; Wang W. et al., 2023) were used in the studies. Although the mode of exposure of PSNAP was waterborne, the sizes (80 nm–90 µm or 80–90,000 nm), concentrations/doses (1 µg–100 mg/L), and duration (7–28 days) of exposure were highly variable (Tables 2, 5). Moreover, the whole larvae of Mozambique tilapia and gill, stomach, liver, intestine, muscle, and brain of Nile tilapia were considered targets of PSNAP toxicity. Although the studies were focused on toxicological endpoints (Table 6), investigations on genotoxic effects (Table 7) have also been done. Because the diameter of the exposed PS particle was >100 nm, we have excluded the studies conducted by Ding et al. (2020) on juvenile tilapia in this review (Table 3).
The gill of Nile Tilapia larvae consisted of twelve types of cells (Zheng and Wang, 2024; Zheng et al., 2024). After PSNAP exposure (80 nm, 100 μg/L, 28 days) differential damage in the gill tissue was induced, with a 22% decrease in cell types including endothelial cells, fibroblasts, macrophages, natural killer cells, and B-cells; only H+-ATPase-rich cells exhibited significantly higher cell counts (Zheng and Wang, 2024). The oxygen consumption, gill histopathology, and transcriptomic and metabolomics analyses of the genes in gills indicate that PSNAP exposure induced severe respiratory distress in tilapia (Table 6).
The larvae of Mozambique tilapia were exposed to PSNAP (100 nm, 20 mg/L) for 7 days and depurated for a week (Table 5). Transcriptomic and metabolomic analyses identified a total of 203 significantly changed metabolites and 2,152 differentially expressed unigenes after PSNAP treatment and recovery (Pang et al., 2021). Moreover, the study indicated that short-term exposure to PSNAPs induced abnormal metabolism of glycolipids, energy, and amino acids (Pang et al., 2021). Transcriptomic results suggested that PSNAP exposure caused signaling disorders, particularly the pathways associated with cell adhesion molecules (CAMs), neuroactive ligand–receptor interaction, and extracellular matrix (ECM)–receptor interactions. A series of differentially expressed genes related to CAMs revealed that PSNAP exposure might have caused early inflammatory responses (Pang et al., 2021). Moreover, the biological processes of “detection of chemical stimulus involved in sensory perception of smell” are affected by PSNAP exposure (Pang et al., 2021).
Juvenile Nile tilapia were exposed to PSNAPs (86–100 nm, 1–1,000 μg/L for 7–21 days), and some of them were under depuration for a week (Hao et al., 2023, Wang et al., 2023b). It was observed that PSNAP exposure did not induce any mortality or mechanical injury in the body and produced insignificant effects on feeding or swimming behavior. Moreover, PSNAP was internalized and accumulated in the gill, gut, intestine, liver, brain, and muscle tissues of the fish (Ding et al., 2018; Hao et al., 2023; Wang W. et al., 2023). The intestine exhibits severe damage in the mucosal layers, which leads to an impact on the microbial community. The intestinal injury was related to the induction of inflammation (upregulation of tnfα, il1β, and il8 and downregulation of il10) and oxidative stress (enhanced activities of SOD and GPx and MDA content) (Hao et al., 2023). In the liver, PSNAP induced hepatic steatosis, modulated the inflammatory response, and disrupted liver functions (Wang W. et al., 2023). The oxidative stress induced in the liver showed enhanced SOD activity with no alterations in the MDA content (Ding et al., 2018). The CYP enzymes, EROD (cyp1a) and BFCOD (cyp3a), showed inconsistent effects. Mechanistically, PSNAP perturbed protein homeostasis in the endoplasmic reticulum by inhibiting the expression of chaperon proteins and genes involved in endoplasmic reticulum-related degradation (Wang W. et al., 2023). The dysfunction of lipid metabolism in the liver was due to the activation of PERK-eIF2α and Nrf2/Keap1 pathways by PSNAP. Moreover, induction of oxidative stress (inhibition of SOD activity and enhanced level of MDA) is also involved in hepatic lipid accumulation (Wang W. et al., 2023). However, in the brain, the AChE enzymatic activity was significantly reduced by PSNAP exposure (Ding et al., 2018).
3.5.6 Zebrafish
The search terms nanoplastics, PS, and zebrafish identified 69 articles belonging to embryo larval development (45 articles) and adults (26 articles). Five articles (4 on embryos and 1 on adults) were excluded from the review because the diameter of the studied PSNAP was >100 nm (Table 3). In these studies, the structure of PS (pristine/acidic/alkaline/aged/non-aged), sizes (15 nm–234 µm or 15–234,000 nm), concentrations/doses (0.04 ng–400 mg/L), modes of exposure (waterborne, injection, trophic transfer, and dietary) exposure conditions (temperature, pH, and depuration), and duration of exposure (4 h–120 dph; with or without depuration) were highly variable (Tables 2, 5). The accumulation and the effects of PSNAPs in embryos (chorion, yolk sac, mouth, trunk, eye, tail, caudal fin, muscle, somite, gill, GI tract, gall bladder, liver, intestine, pancreas, pericardium, heart, brain, nerve tubes, neuromast, and swim bladder) and in adults (gills, blood, GI tract, intestine, liver, gall bladder, pancreas, testis, ovary, brain, muscle) were dependent on the developmental stages (embryos) and the age (larvae, juveniles, and adults) of the fish (Tables 2, 5). Moreover, the studies indicated that PSNAP accumulated in different tissues of zebrafish larvae and adults have altered transcriptomes affecting the physiology and behavior of the fish (Pedersen et al., 2020).
3.5.6.1 Embryo–larval development
Zebrafish embryos at different stages of development and transgenic zebrafish embryos were exposed to PSNAPs, and their effects on development (mortality, hatching rates, and morphology), cardiovasculature (heart rates, circulation, vessel formation, and endothelial cells), neurobehavior (spontaneous contraction in the early period of development, neurotransmitters, brain, eye, and movements), inflammation, oxidative stress, apoptosis, and gene expression were evaluated (Tables 6, 7). Although the 96-h LC50 of PSNAP (100 nm) on the 24-hpf zebrafish embryos was 431.1 mg/L (Feng et al., 2022), depending on the exposure routes and the concentration and duration of PSNAP exposure, inconsistent effects on survivability, malformation rates (pericardial edema, yolk sac edema, short tail, malformed head, jaw abnormalities, spontaneous movements of the embryos, sprouting of the transverse blood vessels, inhibition of myocardial diastolic functions, curved spine, scoliosis, and uninflated swim bladder), and hatching rates were observed; however, heart beats (bradycardia) and larval body length tended to reduce (Table 6). Moreover, concentration-dependent decline in ion contents (Na+, K+, and Ca 2+) and acid/ammonia excretion by skin cells of the embryos was observed after PSNAP exposure (Kantha et al., 2022). The number of active mitochondria in the ionocytes of the skin cells was also decreased by PSNAP accumulation in embryos in a concentration-dependent manner. Vascular malformations, including the ectopic sprouting of intersegmental vessels (ISVs), malformations of superficial ocular vessels (SOVs), and overgrowth of common cardinal veins (CCVs), as well as disorganized vasculature of the sub-intestinal venous plexus (SIVPs), were also observed in zebrafish embryos after PSNAP exposure (Dai et al., 2023). The gene expression analysis of the VEGFA/VEGFR pathways including vegfa, nrp1, klf6a, flt1, fih1, flk1, cldn5a, and rspa3 were altered in a time- and concentration-dependent manner (Dai et al., 2023). These studies indicated that PSNAP interferes with the VEGFA/VEGFR pathways during embryogenesis and induced malformed vasculature in zebrafish.
The metabolic levels of the liver were significantly increased in larvae owing to PSNAP exposure (Chen J. et al., 2023). Particles with smaller sizes and longer duration of exposure (PSNAP; 50 and 100 nm diameter, for 24–120 hpf.) induced higher aggregations of neutrophils and apoptosis of macrophages in the abdominal region of the larvae (Cheng et al., 2022). The glycogen concentrations showed a concentration-dependent increase and isocitrate dehydrogenase concentrations inconsistently decreased during larval development when exposed to PSNAPs (Manuel et al., 2022). Cortisol concentration in the whole larvae (72–120 hpf exposure) was increased significantly by PSNAP exposure in a concentration-dependent manner (Brun et al., 2019). The glucocorticoid receptor mutant zebrafish larvae (gr−/−) have high cortisol levels, and no significant difference was observed in these larvae (gr−/−) exposed to PSNAPs (Brun et al., 2019). The expression of fabp10a (liver-specific fatty acid binding protein) was enhanced in the larval liver by PSNAP exposure (Cheng et al., 2022) and upregulation of the expression of tg, trβ, and esr2 genes and enhanced expression of tshβ, thyroglobulin (tg), nis, dio2, and trβ and no effect on cyp1a1 expression by PSNAP were also observed (Wang et al., 2022).
Decrease in the frequency of the spontaneous contraction of the embryos during development (Santos et al., 2024) indicated that PSNAP modulated nervous system development in zebrafish embryos. Administration by microinjection also showed bioaccumulation of PSNAP in the brain, which induced DNA damage and resulted in excessive ROS and apoptosis (Sokmen et al., 2020). PSNAP exposure decreased the number of larval neurons, axonal abnormalities in motor neurons, and induced neuronal apoptosis (Zhou W. et al., 2023). Compared with controls, there was a decrease in the GAD1 activity and GABA and 5-HT contents of larvae and no effect on the activities of AChE, tyrosine hydroxylase (THY), TPH and acetylcholine (ACh), and dopamine (DA) contents in larvae exposed to PSNAP (Zhou W. et al., 2023). Cholinesterase activity remained unaltered in larvae exposed to PSNAP. However, the activity of AChE significantly decreased in lower concentrations (0.01 and 0.1 mg/L) of PSNAP and increased in the higher concentration (1 mg/L; 22 nm) group (Manuel et al., 2022). The AChE activity was significantly enhanced after 144 hpf, while during recovery (maintained in PSNAP-free media), there was no significant difference between control and the exposure groups (Liu Y. et al., 2022). Compared with the controls, PSNAP (50 nm) with concentrations 1, 5, and 10 mg/L for 144 hpf (6 days) enhanced AChE activity and dopamine content of the larvae (Wang Y. et al., 2023). Moreover, exposure to PSNAP (80 nm) increased neural and optical-specific mRNAs (Chen et al., 2024). Transcriptomic analysis indicated that neurodegeneration and motor dysfunction were induced during larval development when exposed to PSNAPs. Expressions of mbp (responsible for myelination of axons) and syn2α (a neuronal phosphoprotein which induced synaptogenesis) were downregulated only in injected groups, and that of gfap (an intermediate filament protein, expressed in astrocytes) was downregulated only in waterborne exposure groups (Zhang et al., 2020). In transgenic zebrafish larvae [Tg (atoh1a: dTomato)], PSNAP (50 nm; 1, 5, and 10 mg/L for 144 hpf) inhibited the expression of atoha1 mRNA in the cerebellum, thereby indicating damage to the central nervous system (Wang Y. et al., 2023). Single-cell RNA sequencing indicated PSNAP (12 h with 100 nm size PSNAP, 10 μg/L) regulated the expressions of olig2, foxg1a, fzd8b, sis3a, rx1, lhx2b, nkx2.1a, and sfrp5 to alter nervous system development, retinal development, and stem cell differentiation (Liu et al., 2021). Upregulation of gfap and α1-tubulin mRNAs (related to nervous system) by PSNAP was also observed (Chen et al., 2017a).
PSNAP induced morphological changes in the eyes (decreased eye area with reduced interocular distance) and head (increased head area and reduction in head width and depth) (Santos et al., 2024). Expressions of visual system cone genes (opn1sw2, opn1lw2 and opn1mw1) were downregulated by injection of PSNAP to the embryos; however waterborne exposure downregulated the expressions of opn1w2 and opn1mw1 only (Zhang et al., 2020). The gene expression analysis indicated PSNAP dominated the regulation of retinal system development genes (pax1, pax2, six3, lax9, and six6). However, increased cell density and disintegration of the retinal pigment epithelium occurred (Wang et al., 2022; Wang et al., 2023 L.). Genes related to visual system (rhodopsin, zfrho; blue opsin, zfblue) did not undergo significant alterations with PSNAP exposure (Chen et al., 2017a).
Metabolomic analysis revealed that the metabolic pathways of catabolic processes, amino acids, and purines were highly promoted by PSNAP exposure (Supplementary Table S1). Moreover, PSNAP induced the upregulation of several stress and immune-responsive genes (il6 and il1b), cytochrome P450s (cyp1a and cyp51), and initiation of ROS removal protein-encoding genes (sod and cat). Moreover, PSNAP was also accumulated in macrophages during early development of zebrafish (Martin et al., 2023). ROS generation was induced by PSNAPs during embryo–larval development (Cheng et al., 2022). The activities of GST, GPx, and CAT decreased, although inconsistent (Santos et al., 2022). Moreover, the LPO levels showed inconsistent effects (Manuel et al., 2022). No effect was observed on CAT and GPx activity on larvae (96 hpf) with PSNAP exposure; however, GSH content decreased significantly (Chen et al., 2017a). The integrated biomarker response/index based on the seven oxidative stress-related biomarkers (SOD, CAT, GPx, GSH, GR, MDA, and ROS) showed an increase after PSNAP exposure (Bhagat et al., 2022; Chen J. et al., 2023). Enhanced ROS content induced apoptosis and ferroptosis (cell death due to iron accumulation). Significantly increase in NO content and decrease in the activities of soluble guanylate cyclase (sGC) and protein kinase G (PKG) enzymes were observed. Gene expression analysis indicated that PSNAP exposure significantly upregulated gene expressions related to antioxidant enzymes (SOD, GPx, and GST) and downregulated the expression of aromatase (cyp19a1a and cyp19a1b) and DNA methyl transferases (dnmt3bb1) (Liu et al., 2021). The expression of GPX4, the key protein for ferroptosis, and of the genes Slc7a11, Acs14a, Keap1b, and Ncoa4 were higher in larvae exposed to PSNAP (Chen J. et al., 2023). The mRNA expressions of cat, gpx1a, sod1, and sod2 were downregulated in embryos exposed to PSNAP, however, the expression of casp3a (apoptotic marker) mRNA was upregulated and that of bcl2 mRNA (non-apoptotic marker) was downregulated in embryos exposed to PSNAP (Kantha et al., 2022). The activity of the caspase-3 and the expressions of bik, bad, bax, bim, bid, and bok were significantly increased by PSNAP exposure (Chen J. et al., 2023). Moreover, the expressions of several base excision pathway genes (lig1, lig3, polb, parp1, pold, fen1, nthl1, apex, xrcc1, and ogg1) were altered by PSNAP exposure (Feng et al., 2022).
The locomotor activity of the PSNAP-exposed larvae showed increased activity in the dark phase (Brun et al., 2019); however, the swimming behavior of the larvae exposed to PSNAPs (50 nm) did not show any significant change (Pedersen et al., 2020) but reduced counterclockwise and anticlockwise rotations (Zhang et al., 2020). Other behaviors (meander, angular velocity, and moving distance) remained unaltered (Zhang et al., 2020). In contrast, swimming behavior significantly decreased in the larvae (120 hpf) when the embryos were exposed to PSNAP (Barreto et al., 2023), or the effects observed in swimming behavior were found to be very insignificant (Parenti et al., 2019; Manuel et al., 2022; Tamayo-Belda et al., 2023). PSNAP exposure increased (50 nm; 1, 5, and 10 mg/L for 144 hpf) the swimming distance significantly by decreasing the swimming speed (Santos et al., 2024). However, PSNAP exposure suppressed the locomotor activity (total distance traveled) during the dark phase (Chen et al., 2017a). PSNAP exposure elicited complex effects on locomotor behavior with increased long distance and decreased short distance movements (Supplementary Table S1). When fish were allowed to recover (72 h), the locomotor behavior (swimming speed), compared with that in controls, significantly reduced during 144 hpf of development (Liu Y. et al., 2022). Behavioral analysis indicated that PSNAP exposure induced hyperactivity compared to control larvae (Santos et al., 2022; Gao X. et al., 2023). All these data suggested that PSNAPs have the potential to induce movement disorders in zebrafish.
Positively charged PSNAPs (PS-NH2) induced stronger developmental toxicity (decreased spontaneous movements of the embryos, heart beats, hatching rates, and larval length) and cellular apoptosis in the brain and greater impairment of neurobehavioral disorders (locomotor activity and behavior) than negatively charged PSNAPs (PS-COOH) (Teng et al., 2022a). A study compared the effects of pristine PS (80 nm, 0.5 and 5 mg/L), aged UV-PS (0.5 and 5 mg/L), and non-aged O3-PS (0.5 and 5 mg/L) on zebrafish embryos exposed for 8-120 hpf, indicated that these PSNAPs did not induce developmental toxicity (hatching, malformation, and mortality) (Chen J. et al., 2023). Cellular apoptosis was induced in 24 hpf embryos and 120 hpf larvae in all experimental groups (apoptosis mostly seen in embryonic tail and larval head region), except those exposed to O3-PS (Chen J. et al., 2023). Moreover, PS-NH2 interacted with neurotransmitter receptor N-methyl-D-aspartate receptor 2b (NMDA2B), whereas PS-COOH impacted on the G-protein coupled receptor (GPR1). The differences in the binding ability and affinity between neurotransmitter receptors (NMDA2B, and GPR1) as a function of positive or negative charge revealed the mechanism of different toxicity (Teng et al., 2022a).
The influence of temperature on the toxic effects of PSNAP on zebrafish embryos were studied after exposing the 4 hpf embryos to PSNAP (0.1, 0.5, and 1.0 mg/L) and then maintained at three different temperatures (24°C, 27°C, and 30°C) (Supplementary Table S1). The evaluation was made from 24 to 72 hpf (Duan et al., 2023). The elevated temperature promoted the accumulation of PSNAP during zebrafish development and resulted in an increase in the mortality of zebrafish larvae (Duan et al., 2023).
3.5.6.2 Juveniles and adult zebrafish
Juveniles and adults of zebrafish were exposed to PSNAPs, and the effects on mortality, morphology, cardiovasculature (heart rates, circulation, vessel formation, and endothelial cells), neurobehavior (swimming activity, aggressiveness, predator avoidance, and shoal formation), inflammation, oxidative stress and apoptosis, gut microbiota, and gene expressions (Tables 6, 7) were evaluated. Depending on the exposure routes and the concentration and duration of PSNAP exposure, inconsistent effects on survivability and malformation rates were observed; however, heart beats (bradycardia) and body length tended to reduce (Table 6).
In zebrafish larvae (72 hpf), PSNAPs (20 mg/L) were accumulated in the intestine, exocrine pancreas, and gall bladder (Table 5; Supplementary Table S1), while the swim bladder failed to inflate (Brun et al., 2019). No effect was observed on growth, although the length of the larvae tended to reduce after PSNAP exposure. Cortisol concentration in the whole larvae (72–120 hpf exposure) was increased significantly by PSNAP exposure in a concentration-dependent manner (Supplementary Table S1).
Zebrafish juveniles were exposed to 1,000 μg/L PSNAP (50 nm diameter) through diet (Tables 2, 5, 6; Supplementary Table S1). The feeding with regular diet was done for 3 weeks, while for PSNAP exposure, it was only for 1 week. It was observed that PSNAPs perturb lipid metabolism and gut microbiota stability in zebrafish (Du et al., 2024) despite no effects on the body weight. The CAT activity increased, and MDA content decreased, while SOD activities remained unaltered in the liver. The mRNA expression of cpt1ab was upregulated, that of fasn was downregulated, and that of hmgcra remained unaltered after PSNAP exposure (Du et al., 2024).
Juvenile/adult zebrafish were exposed to PSNAPs (44 nm) for 30 days (1, 10, and 100 μg/L), and growth and the brain–intestine–microbe axis were evaluated. It was observed that the growth of the fish (body length) was significantly inhibited in a concentration-dependent manner (Table 2; Supplementary Table S1). Moreover, metabolomic analysis revealed alterations in 42 metabolites involved in neurotransmission (Teng et al., 2022b). Moreover, changes in fourteen metabolites correlated to changes in three microbial groups, including Proteobacteria, Firmicutes, and Bacteroidetes, in fish exposed to PSNAPs. These findings suggest that PSNAPs cause intestinal inflammation, growth inhibition, and restricted development of zebrafish, which are strongly linked to the disrupted regulation within the brain–intestine–microbiota axis (Teng et al., 2022b).
In zebrafish adults, PSNAP exposure (either fluorescently labeled or regular) did not significantly affect the survivability, body length, BMI, or the observable health of the fish. The bioaccumulation of the PSNAP was dependent on the concentrations, duration of exposure, and tissue types (intestine, liver, gill, muscle, brain, and gonads) (Chen et al., 2017b; Sarasamma et al., 2020; He et al., 2021; Habumugisha et al., 2023; Lin et al., 2023; Yang et al., 2023; Ye et al., 2024; Zhang et al., 2024c). During depuration, PSNAP was eliminated from the gut within 2–3 days in a concentration-dependent manner (Yang et al., 2023).
In the intestine, the damage of the epithelium including a cilia defect and enhanced mucus secretion induced by PSNAP exposure depended on the size of the plastic; as the size decreased, the damage of the intestinal epithelium increased (Yu J. et al., 2022; Yu et al., 2022 Z.). The histophysiology indicated vacuolization of the intestinal goblet cells and mitochondria (Teng et al., 2023), and the intestinal villi were swollen and disorganized in the fish exposed to PSNAP, even though the height of the villi significantly decreased. Moreover, the ratio of the villus height/crypt depth or the ratio of the villus height/villus width was also significantly decreased by PSNAP exposure when compared with controls (Teng et al., 2023; Zhang et al., 2024c). The level of ROS in the intestine markedly increased and GSH content significantly decreased; however, SOD activity and MDA content remained unaltered (Zhang et al., 2024c). In contrast to these studies, Teng et al. (2023) observed a significant concentration-dependent increase of SOD activity and an inconsistent increase in MDA content in the intestine of zebrafish adults exposed to PSNAP (80 nm, 15–150 μg/L, 21 days). The mitochondrial DNA content was significantly reduced and that of TNF-α and immunoglobulin IgM was increased by PSNAP exposure in the intestine in a concentration-dependent manner. Moreover, in the intestine, 5-HT level tended to decrease in fish exposed to PSNAP (Zhang et al., 2024c). Compared with controls, the activity of MAO (the catalytic enzyme of 5-HT) and the mRNA level of mao in the intestine tended to decrease in fish exposed to PSNAP. The mRNAs (tph1a, tph1b, and tph2) of tryptophan hydroxylase (TPH), the rate-limiting enzyme for 5-HT synthesis, showed a tendency to downregulate in fish exposed to PSNAP (Zhang et al., 2024c). Concentration-dependent dysregulation of the gene expression of several genes in the intestine was observed in adult zebrafish exposed to PSNAP (downregulation of tnfα, il1β, il10, and chemokine 8a in fish exposed to 1 and 10 μg/L; upregulation of tnf, il1b, il6, il10, cxcl8a, inflammatory caspase B, and tight junction protein 2a in fish exposed to 100 μg/L), while the expression of ahr was downregulated by all concentrations of PSNAP used in the experiments (Teng et al., 2022b). PSNAP exposure decreased the expression of IL-6 and increased the expression of nuclear factor kappa-B (nf-κb) in the intestine. The expression of IL-1β in the intestine was upregulated by PSNAP exposure (15 μg/L) while downregulated by a higher concentration (150 μg/L). The expressions of tight junction proteins 2a (tjp2a) and tjp2b, cyp1a1, and cyp1b1 increased significantly in the intestine of fish when exposed to a lower concentration of PSNAP (15 μg/L) (Teng et al., 2023).
There are seven types of cells identified in zebrafish intestine: enterocytes, macrophages, neutrophils, B cells, T cells, enteroendocrine cells, and goblet cells (Yu J. et al., 2022), and the effects of PSNAP were found to be cell-specific. In macrophages, immune system-related DEGs (ctsba, nfkbiab, and pycard) were significantly altered by PSNAP exposure, and the genes related to MAPK signaling pathways (hsp70.1, hsp70.2, and hsp70l) remained unaltered. In enterocytes, genes related to GSH metabolism (gsta2, gsto1, gsto2, gpx1a, and mgst1.2) and cytochrome P450 remained unaltered. In B and T cells, upregulation of hsp70.1, hsp70.2, and hsp70.3 occurred in fish exposed to PSNAP. Gene ontology (GO) analysis found several other DEGs such as gadd45ba, jun, ccl35.2, and ccl35.2 remained altered in macrophages after PSNAP exposure. In enterocytes, GO analysis showed alterations in the expression of apoa4a, apoa1a, and apoea in fish exposed to PSNAP. Moreover, PSNAP (1 mg/L) induced dysbiosis in gut microbiota and significantly increased the abundance of Proteobacteria and decreased that of Fusobacteria, Firmicutes, and Verrucomicrobiota at the phylum level; at the genus level, Aeromonas abundance was increased by PSNAP exposure ((Xie et al., 2021; Yu Z. et al., 2022; Yang et al., 2023; Zhang et al., 2024c). Therefore, the diversity and abundance of the gut virome were also disrupted by PSNAP exposure (Teng et al., 2023).
In adult fish, PSNAP exposure increased HSI and also vacuoles and lipid droplets in the liver cell matrices (Li Y. et al., 2023). Moreover, the triglycerides and total cholesterol content also increased in the liver (Tables 5; Supplementary Table S1). A significant increase in MDA content and decrease in CAT activities and GSH levels suggests significant oxidative damage induced by PSNAP in zebrafish liver (Deng et al., 2023). Like the intestine, zebrafish liver also consists of nine different types of cells, of which 85% cells were hepatocytes belonged to male (52.39%) and female (33.63%) fish (Deng et al., 2023). The single-cell transcriptomic analysis (scRNA-seq) observed the heterogeneous response patterns of hepatocytes belonging to male and female fish (Supplementary Table S1; Deng et al., 2023). The peroxisome proliferator receptor activator (PPAR) signaling pathway was upregulated in hepatocytes of both male and female zebrafish (Deng et al., 2023). Lipid-metabolism-related functions were altered more notably in male-derived hepatocytes, while female-derived hepatocytes were more sensitive to estrogen stimulus. In macrophages, oxidation–reduction process and immune responses were significantly altered, while in lymphocytes, oxidation–reduction process, ATP synthesis, and DNA binding were mostly altered (Deng et al., 2023). Moreover, a nonlinear increase in the gene hydroxy-3-methylglutaryl-coenzyme A (hmgcra), sterol regulatory element-binding protein (srebp1), diacylglycerol aceyltransferase 1b (dgat1b), acetyl coenzyme A carboxylase (acc), and carbohydrate response element-binding protein (cvhrebp) by PSNAP exposure in the liver was observed; however, the expression of carnitine palmitoyl transferase 1 (cpt1) was decreased significantly by PSNAP exposure (Sarasamma et al., 2020). In the liver, biochemical biomarkers (tnfα, cortisol, vitellogenin, cyp1a1, cyp11a1, and cyp19a1) were altered after 30 days of exposure to PSNAPs; however, no alteration was observed in MDA content and EROD activities (Sarasamma et al., 2020). In addition, PSNAP exposure did not show any induction of esr2b, vtg1, or vtg2 mRNAs in the liver of both males and female fish (Ye et al., 2024). In contrast to the studies mentioned above, the studies carried out by Ling et al. (2022) indicated that the histology of the liver remained unaltered in the fish exposed to PSNAP (70 nm, 100 μg/L for 3 months) (Ling et al., 2022). HSI either remained unchanged (He et al., 2021) or a significant decrease was observed in both male and female fish (70 nm, 2 mg/L, 3 weeks) with exposure to PSNAP (Lin et al., 2023). The biochemical analysis of the oxidative stress-related mechanisms also showed that PSNAP was unable to induce any significant effects on the ROS, GSH, and MDA contents and the CAT activity (Ling et al., 2022). Consequently, gene expression analysis related to antioxidant mechanisms (p38a, p38b, ERK2, ERK3, Nrf2, H O -1, cat1, sod1, gax, JINK1, and gstr1), remained unaffected after PSNAP exposure (Ling et al., 2022).
In the muscle, PSNAP exposure enhanced ROS content and reduced GR activity in female fish, while ATP content was decreased, and no alteration was observed in creatine kinase and hif1α contents (Pitt et al., 2018b; Sarasamma et al., 2020).
PSNAP, when accumulated in the brain of adult zebrafish, slightly increased (not significant) the craniosomatic index (CSI), resulted in damage to the brain histology, and reduced the number of neurons in a concentration-dependent manner (Aliakbarzadeh et al., 2023; Teng et al., 2023). Moreover, the basement membrane of the blood–brain barrier (BBB) was damaged, and a small amount of microthrombosis consisting of aggregated and dissolved red blood cells was observed; also, the mitochondria with a damaged membrane and loss of cristae were observed. Consequently, mitochondrial DNA copy number was significantly reduced, and the genes related to mitochondrial synthesis (pgc1-a and pgc1-b) in the zebrafish brain did not show any significant effects. However, the mitochondrial fusion-related gene (mfn1a, mf1b, and opa1) expressions were downregulated and those of mitochondrial division-related genes (drp1, mff, fis 1, mid49, and mid51) showed a tendency to upregulate (Zhang et al., 2023). The expression of genes related to mitophagy (ulk1a, and parl) were also upregulated by PSNAP exposure. The enzymatic activities of CAT, SOD, AChE, GR (females), glutamine synthase, and GSH contents in the brain were reduced by PSNAP exposure (Pitt et al., 2018b); moreover, GPx (only females) and glutamate dehydrogenase activity in the brain was increased in fish exposed to PSNAP, and upregulation of myelin/basic protein gene expressions occurred in the central nervous system of adult zebrafish (Chen et al., 2017b; Pitt et al., 2018b). Several neurotransmitter biomarkers (AChE, dopamine, melatonin, GABA, serotonin, vasopressin, kisspeptin, and oxytocin) were significantly altered in a concentration-dependent manner in fish exposed to PSNAPs, even though the acetylcholine, prolactin, and vasotocin levels remained unaltered (Chen et al., 2017b; Sarasamma et al., 2020).
The 5-HT level in the brain was significantly reduced in fish exposed to PSNAP, while the serum 5-HT levels remained unaltered. Among the 5-HT receptor mRNAs, expressions of htr1aa, htr1ab, and htr2c were significantly upregulated, while the expressions of htr1b and htr4 showed downregulation in the brain of fish. In addition to 5-HT, PSNAP exposure decreased GABA, dopamine, and oxytocin levels and enhanced cortisol content in the brain (Teng et al., 2023). The activity of MAO tended to decrease, while AChE activity remained unaltered (Zhang et al., 2023). The neurotransmitter catabolic gene mao was significantly downregulated, while the expression of ache tended to increase in the brain of fish exposed to PSNAP (Zhang et al., 2023). Compared with controls, the γ-H2AX levels (marker for DNA damage), 8-hydroxydeoxyguanosine (8-OHdG), and MDA contents were significantly higher in the brain of male and female fish exposed to PSNAP (Zhang et al., 2023). Moreover, the ATP and cyclin-dependent kinase levels were significantly lower and p53 levels were significantly higher in the brains of male and female zebrafish exposed to PSNAP, and the β-galactosidase and lipofuscin levels (aging markers) are significantly higher in the brain of zebrafish (both males and females) exposed to PSNAP, with higher levels of H2O2 and O2− in the brain (Zhou W. et al., 2023).
The impacts of PSNAP exposure (50 nm; 1.0 mg/L, 21 days) on the adult zebrafish were also focused on reproductive endpoints (Tables 6,7). It was observed that PSNAP was unable to alter the GSI in both males and female fish, cause histological alterations in the ovary and testis, egg production (fecundity) and hatching of the embryos, and the expressions of sgk1 (glucocorticoid-regulated kinase 1) and stc mRNAs in the ovary; moreover, the E2 level of the ovary and serum, T, GnRH, FSH, and LH contents in the ovary also remained unaltered after PSNAP exposure (Ye et al., 2024). In male fish, E2 levels in the serum and testis and the GnRH, FSH, and LH levels in the testis remained unaltered (Ye et al., 2024). The expressions of cyp17a2 and hsdβ1 mRNAs in the ovary and testis remained unaffected after PSNAP exposure.
Adult male and female zebrafish exposed to 2 mg/L PSNAP (46 nm) for 21 days (Table 2; Supplementary Table S1) showed no significant effects on HSI, GSI, histological alterations in the testis and ovary, spermatogenesis and oogenesis, VTG content, and E2 and T levels in male and female fish (He et al., 2021). However, the amount of mature sperm in the testis and the fecundity (total eggs produced during the experimental period) of the fish decreased in fish exposed to PSNAP (He et al., 2021). The spawning events, fertilization, and hatching rates of the eggs remained unaltered in fish exposed to PSNAP (He et al., 2021).
The studies conducted by Lin et al. (2023) indicated that PSNAP (70 nm, 2 mg/L, 21 days) exposure can decrease HSI and GSI in both male and female fish. Moreover, in male fish, the seminiferous tubules were deformed, and lacunae appeared in the testis; the spermatogonium and spermatocytes were increased (Lin et al., 2023). In female fish, PSNAP exposures showed more preovulatory oocytes and smaller mature oocytes than controls. The levels of E2 and T in PSNAP-exposed fish decreased in both male and female zebrafish (Lin et al., 2023). However, no effect of PSNAP on the E2/T ratio of male and female fish was observed. The VTG content of male fish remained unaltered, while in female fish, VTG content was induced by PSNAP exposure in a concentration-dependent manner. Moreover, no significant effects on the T3 and T4 levels of both male and female fish were observed after PSNAP exposure (Lin et al., 2023). Compared to controls, PSNAP exposure reduced fecundity, spawning events, fertilization, and hatchability of the embryos. In addition, PSNAP exposure induced abnormal development (teratogenic effects) of the larvae observed at 96 hpf (spinal curvature, pericardial cyst, and growth retardation) (Lin et al., 2023).
Behavioral alterations in locomotor activities (aggressiveness, shoal formation, and predator avoidance behavior) in adult zebrafish were affected by PSNAP exposure in a concentration-dependent manner, while the circadian rhythm of locomotor activity was dysregulated (Sarasamma et al., 2020). PSNAP exposure induced anxiety-like behavior; however, the average velocity and acceleration were unaffected by the treatment (Teng et al., 2023). Adult male and female zebrafish were exposed to 1 mg/L PSNAP (50 ± 3 nm) for 28 days, and the learning and memory (the primary cognitive functions of the brain) were assessed with classic T-maze exploration tasks. It was observed that PSNAP-exposed zebrafish (both males and female) took significantly longer time for their first entry and spent significantly less time in the reward zone in the T-maze task, indicating deficit in the learning and memory (Zhou W. et al., 2023). Adult male and female zebrafish were exposed to PSNAP (100 nm sizes) at a concentration of 1 mg/L for 30 days (Table 2; Supplementary Table S1). The anxiety-like behavior (evaluated by the open field test) showed those exposed to PSNAP alone spent more time in the lower layer than the upper layer, while controls spent uniform time in both upper and lower layers. Furthermore, in the T-maze test, control and PSNAP groups swam quickly in the feeding zone (F zone) and stayed there for long time (Zhang et al., 2024c), indicating effective learning and memory ability of the fish.
Zebrafish adults (3 months old, AB strain) were exposed to 25 mg/L PSNAP (134 ± 2.9 nm) at 28°C, 29°C, and 30°C for 96 h (Table 2; Supplementary Table S1). It was observed that PSNAP exposure with increased temperature induced DNA damage, degeneration, necrosis, and hyperemia in the liver, while in gills, adhesion of lamellae, desquamation, and inflammation in the lamellar epithelium and in muscle alteration in oxidative stress were observed (Senol et al., 2023). Moreover, the locomotor activity (total distance traveled, average speed, and average angular velocity) was decreased in PSNAP-exposed fish, and these effects were modulated by temperature (Sulukan et al., 2022b). The PSNAP was accumulated in the brain and induced degenerative necrosis changes in the medulla oblongata, medial longitudinal fascicle, lateral valvula nucleus, and thalamus, and the effect was increased with the increased in temperature (Sulukan et al., 2022b). Moreover, two proteins, Gfap (indicator of brain injuries) and 8-OHdG (indicator of oxidative DNA damage), were found to be increased in the damaged region of the brain, which is also temperature-sensitive (Sulukan et al., 2022b). Moreover, the temperature and PSNAP exposure caused a synergistic effect on the brain metabolomic alteration (Sulukan et al., 2022b)
3.5.6.3 Intergenerational effects
The intergenerational effects were evaluated in F1 embryos or adults exposing zebrafish embryos (1 article) or adults (3 articles) to PSNAPs in the F0/P1 generation for a reasonable period of time, and the effects on offspring (F1) without exposing them to the plastics were evaluated. In a study on zebrafish, fertilized eggs (4 hpf) were injected with PSNAPs (20 nm, ∼270 mg/L; 3 nL injected volume/egg) and grown in plastic-free media for 6 months (P1) and were allowed to breed, and the offspring (F1) were evaluated for morphological, molecular, and metabolomic disorders (Table 5; Supplementary Table S1). It was observed that compared with controls, parental PSNAP exposure (P1) induced significant malformations, decreased survival rates, increased heart rates, as well as decreased eye size and locomotor activity in the F1 offspring (Sulukan et al., 2022a). In addition, cell death and ROS were increased significantly; however, lipid accumulation was decreased in the F1 generation (Sulukan et al., 2022a).
AB strain zebrafish adults (90 dpf) were exposed to PSNAP (54.5 ± 2.8 nm; 10 mg/L, 90 days), waterborne and F1 larvae (without exposure to PSNAP) were evaluated for disruptions induced in the HPT axis (Table 2; Supplementary Table S1). Parental exposure (F0) to PSNAP reduced survival rates, hatching rates, and body length (7 dpf) and significantly enhanced the malformation rates during the embryo–larval development of F1 larvae (Zhao et al., 2021). Compared with controls, total T3 and T4 levels in F1 larvae remained unaltered; in F1 eggs, T4 level reduced significantly, while T3 level remained unaltered (Zhao et al., 2021). However, in F1 larvae, no significant changes in T3 and T4 contents were observed. In another experiment, adult zebrafish were exposed to 100 μg/L PSNAP (70 nm) for 21 days (P1), and the F1 larvae (120 hpf) were evaluated for intergenerational effects (Table 6; Supplementary Table S1). It was observed that due to parental exposure (F0), accumulation of PSNAP was detected in the testis and ovary of the F1 larvae (Zuo et al., 2021). PSNAP exposure to parents had no effect on the induction of developmental disorders and no alterations in the T4 and T3 levels. Gene expressions in the HPT axis and GH/IGF axis remained unaltered. In a study by Wu et al. (2021) in which parents (P1) were exposed to PSNAP (70 nm, 100 μg/L) for 45 days (Table 2; Supplementary Table S1), the F1 embryos/larvae were evaluated for intergenerational effects. It was observed that PSNAP was accumulated in the F1 embryos (Wu et al., 2021); however, compared with controls, no significant effect was observed on hatching rates (72 hpf), hatching enzymatic activities, and spontaneous tail movements (wagging). Moreover, no significant effect was observed on the AChE activity of the F1 embryos exposed to PSNAP, parentally; gene expression analysis related to hatching enzymes (tox 16, foxp1, ctslb, xpb1, klf4, cap1, bmp4, cd63, He1.2, zhe1, and prl), cholinergic system (ache and chrnα7), and muscle development (Wnt, MyoD, Myf5, Myogenin, and MRF4) indicated alterations in the F1 larvae exposed parentally to PSNAP (Wu et al., 2021). In another study, juvenile/adult zebrafish were exposed to PSNAPs (44 nm) for 60 days (1, 10, and 100 μg/L), and the intergenerational effects during embryo–larval development (F1) were evaluated (Teng et al., 2022b). Accumulation of PSNAPs in the GI tract after 60 days of exposure to the fish impaired the development of the F1 embryos, including reduced spontaneous movement, hatching rates, and larval length (Teng et al., 2022b). Moreover, accumulation of PSNAPs was observed in the intestine, liver, and pancreas of the F1 fish (Teng et al., 2022b).
Taken together, it was observed that PSNAP as a chemical is transferred to the next generation and is accumulated in the whole embryos, intestine, liver, pancreas, and gonads (testis and ovary) of the F1 offspring. Moreover, several of the toxic potentials observed in the P1 fish were also observed in F1 fish, which indicate that intergenerational effects of PSNAP were independent of the dose, duration, mode of exposure, and developmental stage of zebrafish.
3.6 Coexposure
NAPs with small particle sizes and high surface area/volume ratios easily absorb environmental pollutants and affect their bioavailability (Liu et al., 2021). Due to high adsorption activity, the toxic effects of NAPs could be modified by exposure to other toxic chemicals found in the environment. Moreover, NAPs can absorb contaminants and potentially decrease their uptake due to particle agglomeration or function as a vector to accumulate the hazardous chemicals inside the cell, which were unable to enter by themselves. Our literature search found several chemicals including hormones, pesticides, antibiotics, metals, organic chemicals, biological materials, and bacteria disposed/found in the environments used as additional contaminants along with NAPs during experiments (Tables 8, 9). In coexposure studies, the diameter of the PVC particles is 200 nm (Monikh et al., 2022). We therefore excluded this article from the review. Among thirteen fish species, only six species, grass carps (juveniles), silver carp (adults), tooth carp (adults), marine medaka (embryos, juveniles, and adults), Hainan medaka (adults), and zebrafish (embryo–larvae–juveniles–adults), were used in coexposure experiments (Tables 8, 9).
3.6.1 Carps
Juveniles of grass carp were coexposed with tetracycline (TC), ZnO, and also infected with pathogenic bacteria (Aeromonas hydrophilia) during PSNAP exposure (Table 8). TC coexposure showed pathogenic lesions in the gills and intestine and enhanced the oxidative stress-related changes (total antioxidant capacity and the activities of CAT and SOD) in the liver and intestine (Liu S. et al., 2022). The expressions of MMP2, MMP9, and IL-8 in the liver and intestine of the coexposed fish were also upregulated (Table 9; Supplementary Table S1; Liu S. et al., 2022). Coexposure with ZnO (750 μg/L) did not induce alterations in the locomotor activity, biochemical concentrations of the liver and brain (carbohydrates, proteins, and triglycerides in the liver and carbohydrate and protein contents in the brain), while it increased the oxidative stress-related activities and AChE activity in the brain (Estrela et al., 2021). Moreover, DNA damage in the erythrocytes was also observed. Injection of the pathogenic bacteria to grass carp, pre-exposed to PSNAP (80 nm diameter, 10–1,000 μg/L), showed enhancement in the enzymatic activities of CAT, SOD, and GST, and MPO and MDA contents were enhanced in the oxidative stress-related mechanisms in the grass carp gut after bacterial infection (Li Z. et al., 2024). Moreover, the microbial communities in the gut were also modified after injection of A. hydrophilia (Li Z. et al., 2024). In silver carp adults (Hypopthalmichthys molitrics), MCLR (1 μg/L) coexposure caused pathological damages in the gill, liver, and intestine of the fish (Zhang et al., 2024a) and aggravated the changes in the microbial community in the intestine and the metabolic patterns in the liver (Table 7). In tooth carp, coexposure with triclosan (TCS) did not significantly affect the uptake of PSNAPs in the organs of tooth carp and reduced the toxic effects induced by PSNAP in this fish (Saemi-Komsari et al., 2023).
3.6.2 Medaka
Embryos, juveniles, and adults of marine medaka were used in coexposure studies. Embryos were coexposed with BPA, juveniles with SMX, and adults with SMZ (Table 8). BPA reduced the accumulation of PSNAP in the embryos and thus mitigated the toxic effects of PSNAP on embryo mortality, heart rates, and larval body length during embryo larval development (Yu et al., 2023). In juveniles, SMX coexposure was unable to modulate the toxic effects (mucus content in the intestine, goblet cell number, and gut microbial community) induced by PSNAP exposure alone (Li X. et al., 2023). Coexposure of SMZ in adults (through diet) modulated the gut microbial community (Wang F. et al., 2023) and the intergenerational effects of PSNAP on growth, gut microbial content, and the hepatic gene expressions (cat, sod, gpx, and igf1) in F1 generation (He et al., 2022). Hainan medaka adults were coexposed with F-53B, which can interact with the effects induced by PSNAPs and modulated the effects on the accumulation, histology, antioxidant activity, and gut microbiota induced in fish after PSNAP exposure (Gao X. et al., 2023).
3.6.3 Zebrafish
In zebrafish, embryos along with PSNAP were coexposed with varieties of chemicals including acetaminophen (APAPM), Al2O3, Au, avobenzone (AVO), B(a)P, BDE-47, CeO2, diphenhydramine (DPH), DDE, EE2, glucose, PAHs, penicillin, mucin (jelly fish), phenmedipham, simvastatin (SIM), and sodium nitroprusside (SNP), and the toxic effects of PSNAP with interaction of these compounds were evaluated (Tables 8, 9).
It was observed that APAPM, a non-opioid and antipyretic agent used for treating pain and fever, potentiated the toxic effects of PSNAP in inducing edema, spinal curvature, pigment deficiency, melanocyte abnormalities, and reducing larval body length, and in the swimming behavior of zebrafish (Gao X. et al., 2023). Moreover, the downregulation of genes related to osteogenesis (runx2a, runx2b, sp7, bmp2b, and shh) by PSNAP was also observed with APAMP coexposure (Gao X. et al., 2023). AVO is an organic molecule used in sunscreens (cosmetics), and exposure to PSNAP alone enhanced the accumulation of AVO in zebrafish embryos in a time-dependent manner and did not produce any lethal effects and morphological disorders (Table 8); however, the heart rates increased and the locomotor behavior (swimming speed) significantly reduced (Liu et al., 2021; Liu Y. et al., 2022). In addition, oxidative stress, which was enhanced by exposure with PSNAP and AVO alone, was reduced in coexposed embryos (Liu et al., 2021). The AChE activity significantly enhanced during coexposure, while during recovery (maintained in treatment-free medium), there was no significant difference with the controls (Liu Y. et al., 2022). Gene expression analysis indicates that exposure to AVO and PSNAP alone significantly upregulated gene expressions related to antioxidant enzymes (CAT, SOD, GPx, and GST by AVO and SOD, GPx, and GST by PSNAP) and downregulated the expressions of aromatase (cyp19a1a and cyp19a1b) and DNA methyl transferases (dnmt1 and dnmt3aa by AVO and dnmt3bb1 by PSNAP); however, the coexposure reduced the adverse effects induced by PSNAP and AVO alone during the expression of all these genes (Liu et al., 2021). Moreover, genes in stem cells (foxg1, her5, her6, shha, and sox2) were responsive to exposure of both AVO and PSNAP (Liu Y. et al., 2022). During the early life stages of zebrafish, AVO dominated the regulation of nervous system-related genes (α1-tubulin, elav13, gap43, gfap, mbp, syn2a, lfing, her5, her6, her11, lfng, pax2a, and fgfr4), while PSNAP alters gene expression related to nervous system development, retinal development, and stem cell differentiation (pax1, pax2, six3, lax9, six6, olig2, foxg1a, fzd8b, sis3a, rx1, lhx2b, nkx2.1a, and sfrp5) (Liu et al., 2021; Liu Y. et al., 2022).
Zebrafish embryos were coexposed with BDE-47 (2,2′,4′-tetrabromodiphenyl ether; 10 ng/L), a flame-retardant, and the effects on accumulation, morphological deformities (pericardial edema, yolk sac edema, tail curvature, jaw malformation, and fin and heart malformation), spontaneous movement during embryonic development, survival and hatching, growth, feeding, oxygen consumption, larval movement, histopathology of the eye, muscle, and cartilage, and gene expressions in the HPT-, HPI-, and HPG-axis,VTG, and other genes (apoa1a, apoba, insa, insb, pck, pomca, and pomcb) were evaluated. It was observed that PSNAPs alone were quickly aggregated on the surface of the embryonic chorions and accumulated in the brain, mouth, trunk, gills, heart, liver, and GI tract of the larvae (Chackal et al., 2022; Wang et al., 2022; Wang Q. et al., 2023) and served as a vector for accumulation of B(a)P in the embryos (Martinez-Alvarez et al., 2022). Moreover, coexposure with BDE-47 exacerbates the morphological deformities induced by PSNAP with regard to hemorrhage, small head and eyes, yolk edema, pericardial edema, spine curvature, swim bladder deficiency, and curved tail (Wang et al., 2022; Wang et al., 2023 L.). In addition, coexposure caused lower survival rates and shorter body lengths and accelerated spontaneous movements of the embryos. Histopathological observations revealed that coexposure caused damage to retinal structures, muscle fiber, liver morphology (color), and cartilage tissues. Gene expression analysis further indicated that exposure to PSNAP alone upregulated the expressions of tshβ, tg, nis, dio2, and trβ and had no effect on cyp1a1 (Wang et al., 2022; Wang et al., 2023 L.); however, coexposure with BDE-47 upregulated the expressions of cyp1a1 and tg, while downregulating the expressions of tshβ, nis, ttr, doi2, trβ, and gpx1a in larvae (Wang et al., 2022; Wang et al., 2023 L.), which indicates the negative interaction with the gene expression made by BDE-47 was abolished by PSNAP (Chackal et al., 2022).
Zebrafish embryos (6hpf) were exposed to PSNAP either alone or with a mixture of river sediment extracts that contain PAHs for 96 hpf (Tables 8; Supplementary Table S1). It was observed that in coexposure, the incidence of disorders induced by PAH alone was reduced (Trevisan et al., 2019). Moreover, PSNAP, either alone or in coexposure increased NADH production. PSNAP alone accumulated in the yolk sac and brain; however, accumulation of PAH was observed only in the yolk sac when exposed to PAH alone; during coexposure, PAH accumulation was observed in the brain (Trevisan et al., 2020). This study indicates that PSNAPs can absorb contaminants and potentially decrease their uptake due to particle agglomeration or function as a vector to accumulate the hazardous chemicals inside the cell, which were unable to enter by themselves. Zebrafish embryos coexposed with PHE (an aromatic hydrocarbon; PSNAP + PHE) and jellyfish mucin (PSNAP + PHE + mucin) (Table 8) showed that PSNAP and PHE alone induced pericardial edema, yolk sac edema, and decreased hatching rates (Geum and Yeo, 2022), and PSNAP was agglomerated on the surface of the chorion of the embryos in PSNAP + PHE groups, while in coexposure with mucin (jellyfish), a clean chorion was observed (Table 8).
PSNAP enhanced the accumulation of aluminum and cerium in zebrafish embryos by inhibiting the ATP-binding cassette (ABC) transporter inhibitor activity, while no effect was observed on embryo mortality or malformation rates (pericardial edema, yolk sac edema, curved tail, and spinal curvature). The hatching rate declined in embryos co-exposed with CeO2. Coexposure with chloroauric acid (Au) synergistically exacerbated the marginal effects induced by PSNAP on the survival, hatching rate, developmental abnormalities, and cell death of zebrafish embryos, which was dependent on the production of ROS and the proinflammatory responses synergized by the combined toxicity of PSNAP and metal ions (Lee et al., 2019; Bhagat et al., 2022). Enhanced ROS production and oxidative stress lead to the activation of genes (gadd45a, p53, xrcc2, rad51, and trl3) associated with DNA damage and repair. Al2O3 alone upregulated the expression of gadd45a and xrcc2, and coexposure with PSNAP enhanced the expression of rad51 and p53; moreover, coexposure with CeO2 downregulated tlr3 and mt2 gene expressions (Bhagat et al., 2022). There was no change in metallothionine (mt2) expression by PSNAP alone, while both Al2O3 and CeO2 alone enhanced mt2 expression; surprisingly, coexposure with PSNAP significantly decreased the expression of mt2 compared to the expression induced by AL2O3 and CeO2 alone (Table 9). The expressions of abcc2 and P-gp mRNAs were upregulated, and those of abcc1, abcc4, and abcb4 mRNAs were downregulated (efflux transporter genes) by PSNAP exposure. Al2O3 alone, except abbcc2, downregulated the expression of the efflux transporter genes studied, while CeO2 alone downregulated the expressions of abcc1, abcc4, abcb4, and p-gp. Coexposure with Al2O3 (increased abcc4) and CeO2 (reduced abcc1 and p-gp) modulated the expression patterns of efflux transporter genes regulated by PSNAP (Table 9). The synergistic effects of PS on toxicity appeared to relate to the mitochondrial damage. Taken together, the effects of PSNAPs were marginal but could be a trigger for exacerbating the toxicity induced by metal ions (Lee et al., 2019; Bhagat et al., 2022).
Coexposure with antihistamine diphenhydramine (DPH) for 96 h induced embryo mortality, malformations, and decreased heart beats and hatching rates; moreover, the activities of GST and AChE increased, while that of CAT remained unaltered (Barreto et al., 2023). The movement disorders were also induced in larvae with PSNAP and DPH coexposure (Barreto et al., 2023). Moreover, coexposure of zebrafish embryos with phenmedipham (PHN), an herbicide, did not induce any significant change in embryo mortality or deformities; however, at 96 hpf, the PSNAP increased CAT activity, while coexposure increased both CAT and GST enzymatic activities (Santos et al., 2022). Behavioral analysis indicates that during 120 hpf (larvae), PS alone or coexposed with PHN induced hyperactivity (Santos et al., 2022). Moreover, cholinesterase activity was found to be decreased only in coexposed larvae and not in larvae exposed to PSNAP or PHN alone. In coexposure with DDE, due to its large surface area, PSNAP served as a carrier of the pesticide and enhanced toxicity (morphological, cardiac, and respiratory) in zebrafish embryos (Varshney et al., 2023). DDE alone or in combination with PSNAP induced pericardial edema, lordosis, and uninflated swim bladder (Table 8). No significant difference was observed in the oxygen consumption rate of the larvae exposed to PSNAP only; however, in DDE and PSNAP + DDE, oxygen consumption rates increased significantly. The locomotor behavior of the larvae (movement, distance moved, velocity, angular velocity, and rotations) did not change after PSNAP exposure, while significant alterations (reductions) were noticed in larvae exposed to DDE alone or DDE + PSNAP (Varshney et al., 2023). The uptake of EE2, a synthetic estrogen, by zebrafish embryos was reduced by PSNAP in coexposure; however, the body length of the larvae was reduced and locomotor activity (total distance travelled) during the dark phase was suppressed (Table 8). Upregulation of gfap and α1-tubulin mRNAs (related to nervous system) by PSNAP alone or coexposed with EE2 occurred in zebrafish larvae (Chen et al., 2017a).
Zebrafish embryos were exposed to pristine PS, aged UV-PS, non-aged O3-PS, and penicillin either alone or coexposed with antibiotics (Table 8). Penicillin alone did not induce developmental toxicity (hatching, malformation, and mortality); however, accumulation of PSNAP in the yolk sac, eye, head, and nerve tubes was interrupted by penicillin coexposure (Chen J. et al., 2023). It was observed that pristine PS and penicillin coexposure synergistically suppressed heart rates and spontaneous movements of the embryos and swimming behavior and touch responses of the larvae (Chen J. et al., 2023). Except those exposed to O3-PS, ROS levels were significantly increased in PS + penicillin and UV-PS + penicillin groups resulted in induction of cellular apoptosis (apoptosis mostly seen in the embryonic tail and larval head region) (Chen J. et al., 2023). Coexposure with penicillin affected the motor behaviors (spontaneous movements, touch response, and swimming) and heart beats of the embryos during development. Upon exposure with PS, aged PS, or penicillin co-exposed with PS, neurotransmitter metabolite expressions in zebrafish larvae were significantly dysregulated (Chen J. et al., 2023).
Coexposure with simvastatin (SIM) (an anticholesterolemic drug) increased hatching rates and heart beats, while SIM alone can delay hatching, reduce heart beats, induce edema, and cause mortality after 96 h of exposure (Barreto et al., 2021). Coexposure of zebrafish embryos with sodium nitroprusside (SNP) significantly reduced the accumulation of PSNAP in the larvae and antagonized the effects induced by PSNAP (20 mg/L) during embryo–larval development (spinal curvature, organ edema, and survival rates) (Table 8; Chen Q. et al., 2023). Moreover, the activities of several enzymes including soluble guanylate cyclase (sGC), protein kinase G (PKG), caspase 3, which were regulated by PSNAP exposure, were also antagonized by SNP coexposure. The oxidative stress and ROS levels, apoptosis and ferroptosis, GPX4 (the key protein for ferroptosis) content, and the expression of several PSNAP-responsive genes including Adma, Nos, Pde6d, prkg, bik, bad, bax, bim, bid, bok, Slc7a11, Acs14a, Keap1b, and Ncoa4 were also modulated by SNP exposure during embryo–larval development of zebrafish (Table 9; Chen Q. et al., 2023). Moreover, the increased proliferation of macrophages and neutrophils and the upregulation of tnfα, tgfβ, il-4, and il-6 mRNAs by PSNAP were alleviated by SNP exposure in coexposed embryos (Tables 8, 9; Chen Q. et al., 2023).
In larval zebrafish, PSNAP accumulated in the intestine, pancreas, and gall bladder and disrupted glucose homeostasis with increased cortisol secretion (Table 8). Moreover, coexposure with glucose did not show any significant response (Brun et al., 2019). The locomotor activity of the PSNAP-exposed larvae showed increased activity in the dark phase; coexposure with glucose diminished the hyperactivity. It was suggested that the adverse effects of PSNAPs are at least in part are mediated by glucocorticoid receptor activation, leading to aberrant locomotor activity (Brun et al., 2019).
Zebrafish juveniles were fed with regular diet, high-fat diet, and exposed to 1,000 μg/L PSNAP (50–1,000 nm diameter) either to fish fed with normal diet or fed with high-fat diet (Supplementary Table S1). The feeding with regular diet and high-fat diet has been done for 3 weeks, while for PSNAP exposure, it was only for 1 week. Despite no effects on the body weight, it was observed that PSNAP exposure perturbs lipid metabolism and gut microbiota stability in zebrafish (Du et al., 2024). Combined exposure of PSNAP with high-fat diet resulted in gastrointestinal injury and reduced the number of goblet cells in the intestinal layer (Du et al., 2024). The CAT activity increased, and MDA content decreased, while SOD activities remained unaltered in the liver of zebrafish after PSNAP exposure (Du et al., 2024). Moreover, the mRNA expression of cpt1ab was upregulated, that of fasn was downregulated, and that of hmgcra remained unaltered after PSNAP exposure (Du et al., 2024).
In adult zebrafish, the toxic potentials of PSNAP were also evaluated in the presence of other environmental pollutants, including arsenic, BPA, diethylstilbestrol (DES), homosolate, lead, MCLR, 4-nonylphenol (4-NP); oxytetracycline, triphenyl phosphate (TPhP), tris (1,3-dichloro-2-propyl) phosphate (TDCIPP), and vit D (Tables 8). Moreover, the expressions of several genes related to metabolism, immunity, oxidative stress, apoptosis, neurobehavior, reproduction, and growth were also evaluated (Table 9). Furthermore, the intergenerational effects of PSNAP exposure were also evaluated in some of these experiments in coexposure (Wu et al., 2021; Zhu et al., 2021).
During coexposure, the accumulation of PSNAP in different organs of adult zebrafish was interrupted by the presence of coexposed chemicals. For example, PSNAP nonlinearly enhanced the accumulation of TDCIPP in the whole fish (body burden) as well as in the eggs (ovary), and the order of accumulation was gut > gills > gonad > liver. The accumulation of TDCIPP in female fish tended to be higher than that in male fish (sex-specific) (Zhao et al., 2021). Moreover, the accumulation of PSNAP in the liver of zebrafish was independent of MCLR, while accumulation of MCLR in the liver of zebrafish was enhanced by PSNAP exposure (Ling et al., 2022). In addition, PSNAP exposure enhanced the accumulation of BPA in viscera, gills, head, and muscle of zebrafish (Chen et al., 2017b) and As in the intestine and brain. Accumulation of homosolate in the testis, ovary, liver, and brain of male and female fish was enhanced by PSNAP exposure (not significant). Coexposure with As or OTC has no effect on mortality (Zhang et al., 2023); however, exposure to TPhP alone was highly toxic to zebrafish (LC50 was 976 μg/L). It was also observed that Pb enhanced the accumulation of PSNAP in the intestine, while excessive Pb reduced the accumulation (Yu J. et al., 2022).
The effect of PSNAP in coexposure with Pb, As, and OTC was evaluated in intestines of adult zebrafish (Yu J. et al., 2022; Zhang et al., 2024c). The intestinal villi were swollen, and the ratio of the villus height/crypt depth or the ratio of the villus height/villus width were decreased in fish exposed to As either alone or in combinations (Zhang et al., 2024c). Moreover, exposure of the fish to OTC alone caused damage of the lining epithelium of intestinal villi and vacuolation of intestinal epithelial cells, while coexposure with PSNAP alleviated the processes (Ye et al., 2024). There are seven types of cells found in the intestine (enterocytes, macrophages, neutrophils, B cells, T cells, enteroendocrine cells, and goblet cells) of adult zebrafish, and PSNAP and Pb exposure influenced enterocytes, macrophages, B cells, T cells, and goblet cells during coexposure (Yu J. et al., 2022). The PSNAP exposure induced the effects on macrophages by affecting the expressions of genes related to immunologic (ctsba, nfkbiab, and pycard) and apoptotic processes, while Pb exposure influenced the enterocytes by altering genes related to oxidative stress (gsta2, gsto 1, gsto2, gpx1a, and mgst1.2) and lipid metabolism. Consequently, in coexposure, the effects induced by PSNAP on macrophages were decreased by Pb, while in enterocytes, the Pb-induced effects were decreased by PSNAP exposure (Yu J. et al., 2022). In B and T cells, upregulation of hsp70.1, hsp70.2, and hsp70.3 occurred in fish exposed to PSNAP and Pb alone, and also in coexposure (Table 9; Yu et al.). The 8-hydroxy-2′-deoxygluconate (8-OHdG) and TNF-α levels were enhanced in the intestine by Pb exposure, and PSNAP synergized the effects. As, either alone or in combinations, markedly increased ROS and decreased GSH content in the intestine, while SOD activity and MDA content remained unaltered. The mitochondrial DNA copy number significantly reduced in fish exposed to PSNAP or As, either alone or in combinations. Moreover, 5-HT level in the intestine was decreased by As in coexposure, while in serum, it (5-HT) remained unaltered (Zhang et al., 2024c). The mRNA (tph1a, tph1b, and tph2) expressions of tryptophan hydroxylase (TPH), the rate-limiting enzyme for 5-HT synthesis, tended to downregulate in fish exposed to PSNAP and As either alone or in combinations (Zhang et al., 2024c). The intestinal microbiota was also altered by Pb, As, and OTC, either alone or in coexposed conditions (Yu Z. et al., 2022; Zhang et al., 2024c).
The effect of PSNAP in coexposure with TDCIPP, BPA, MCLR, and vit-D (dietary) was evaluated in the liver of adult zebrafish (Zhao et al., 2021; Ling et al., 2022; Li Y. et al., 2023). The HSI was increased by PSNAP and remained unaltered when fed with vit D (Li Y. et al., 2023), while MCLR induced cellular swelling, fat vacuolization, and cytoarchitecture of the organ, and coexposure with PSNAP exacerbated the effects (Ling et al., 2022). The biochemical analysis showed that MCLR alone enhanced ROS and MDA contents and reduced GSH and CAT activities in a concentration-dependent manner, while coexposure with PSNAP aggravated the effects (Ling et al., 2022). Consequently, gene expressions related to antioxidant mechanisms (p38a, p38b, ERK2, ERK3, Nrf2, HO-1, cat1, sod1, gax, JINK1, and gstr1) remained unaffected after PSNAP exposure, while MCLR enhanced the expression of several genes (ERK2, ERK3, p38a, Nrf2, gpx1a, gstr1, at1, and sod1) in a concentration-dependent manner, and coexposure with PSNAP exacerbated the expression of Nfr2 (Ling et al., 2022). TDCIPP alone or in combination with PSNAP upregulated the expressions of thyroglobulin (tg) and uridine diphosphate glucuronosyltransferase (ugt1ab) genes in the liver of female zebrafish. Moreover, the expressions of deiodinase 1 (dio1) and transthyretin (ttr) were downregulated, and the expression of deiodinase 2 (dio2) gene was upregulated in female fish exposed to TDCIPP either alone or in combination with PSNAP (Zhao et al., 2021). In the liver of male fish, the transcription of tg and ugt1ab genes was upregulated in fish exposed with TDCIPP alone or in combinations. Moreover, the expression of trβ remained unaltered in all the experimental groups, while trα expression in the liver of male fish was upregulated when exposed to TDCIPP alone or in combinations with PSNAP. In addition, a significant downregulation of ttr expression was observed in the liver of male fish exposed to TDCIPP either alone or in combinations (Zhao et al., 2021). Vit D altered the number of lipid droplets as well as the triglyceride and total cholesterol contents in the liver (Li Y. et al., 2023). Moreover, inconsistent effects were observed in CAT and SOD enzymatic levels and MDA contents in the liver. A nonlinear increase in the gene hydroxy-3-methylglutaryl-coenzyme A (hmgcra), sterol regulatory element binding protein (srebp1), diacylglycerol acetyltransferase 1b (dgat1b), acetyl coenzyme A carboxylase (acc), and carbohydrate response element binding protein (cvhrebp) by PSNAPs in the liver was ameliorated by high vit D diet (2800 IU/kg); in contrast, the expression of carnitine palmitoyl transferase 1 (cpt1) was decreased significantly by PSNAPs and was increased by vit D.
The effects of PSNAP in coexposure with BPA, TDCIPP, NP-4, and As were evaluated in the brain of adult zebrafish (Chen et al., 2017b; Zhao et al., 2021; Aliakbarzadeh et al., 2023; Zhang et al., 2023). It was observed that in the brain, similar to PSNAP, BPA alone can inhibit AChE activity and upregulate myelin basic protein (MBP) gene expression, while coexposure upregulated the expressions of myelin and tubulin protein/gene, dopamine content, and the mRNA expression of mesencephalic astrocyte-derived neurotrophic factor (MANF). However, AChE activity in the brain remained unaltered by coexposure (Chen et al., 2017b). Therefore, PSNAP by increasing the BPA concentration in the brain induced neurotoxic effects through a mechanism other than AChE inhibition (Chen et al., 2017b). TDCIPP alone can interrupt the thyroid hormone-dependent mechanisms in the brain of adult zebrafish. In female fish, the transcription of corticotropin-releasing hormone (crh) was upregulated in a nonlinear fashion in fish exposed to TDCIPP either alone or in combinations. However, the transcription of tshβ remained unaltered in fish exposed to PSNAP and TDCIPP either alone or in combinations. In the brain of male fish, transcription of crh and tshβ increased only in coexposed fish (TDCPP + PSNAP). The enzymatic activities of CAT, AChE, glutamine synthase, and GSH contents in the brain were reduced by 4-nolnynphenol (4-NP), either alone or in coexposure. However, the glutamate dehydrogenase activity in the brain was found to increase in fish exposed to PSNAP either alone or in combination with 4-NP (Aliakbarzadeh et al., 2023). The metalloid As was able to cross the blood–brain barrier and accumulated in the brain and enhanced ROS production by increasing the SOD activity and MDA content and decreasing the GSH levels. As a result, microthrombi were observed in the brain, and the mitochondrial DNA significantly reduced; the expressions of genes related to mitochondrial synthesis (pgc1-a and pgc1-b) and fusion (mfn1a, mf1b, and opa1) were downregulated, while those of the genes related to mitochondrial division (drp1, mff, fis 1, mid49, and mid51) were upregulated (Zhang et al., 2023). Moreover, the expressions of genes related to mitophagy (ulk1a, parl, parkin, pink 1 and fundc1) were upregulated. The neurotransmitter dopamine (DA) activity significantly decreased, and ACh activity increased. The activity of neurotransmitter catabolic gene mao was significantly downregulated, and the activity of MAO was significantly decreased, and the activity of AChE significantly increased in the brain of fish exposed to As. The expression of ache mRNA in the brain was upregulated, while 5-HT level in the brain was significantly reduced. PSNAP was able to promote the accumulation of As in the brain of adult zebrafish and potentiated most of the effects induced by As alone (Zhang et al., 2023). Moreover, PSNAP when coexposed with As decreased the swimming speed and induced anxiety-like behavior and affected learning and memory of the adult zebrafish (Zhang et al., 2024c).
The effect of PSNAP in coexposure with TPhP, TDCIPP, DES, and homosolate was evaluated in the gonads and hormone levels of adult zebrafish (He et al., 2021; Zhao et al., 2021; Lin et al., 2023; Ye et al., 2024). TPhP alone enhanced liver weight (HSI) and ovarian weight and disrupted spermatogenesis and oogenesis as well as the histological structure of the testis and ovary (He et al., 2021). Moreover, TPhP alone did not significantly disrupt the sex steroid levels (E2 and T), and thus the VTG content in male fish, even though VTG decreased in female fish (He et al., 2021). The fecundity (total eggs produced during the experimental period) of the fish decreased in fish exposed to TPhP alone (He et al., 2021). Coexposure of PSNAP along with TPhP (PSNAP + TPhP) increased HSI and GSI and reduced VTG content in both male and female fish. Moreover, coexposure also inhibited spermatogenesis with structural derangements (formation of lacunae and interstitial tissue) in the testis and induced follicular atresia (atretic follicles) in the ovary (He et al., 2021). The E2 level in male fish enhanced, while T level remained unaltered in both male and female fish in coexposure (He et al., 2021). The fecundity significantly reduced, and the number of spawning events, fertilization, and hatching rates of the embryos were also reduced (He et al., 2021). The synthetic estrogen, DES, like TPhP, decreased HSI and GSI in both male and female fish. Moreover, in the testis, DES alone or in coexposure induced lacunae and increased the number of spermatogonium and spermatocytes and induced the deformation of seminiferous tubules (Lin et al., 2023). In female fish, PSNAP and DES exposure showed more preovulatory oocytes and smaller mature oocytes. The levels of E2 and T in PSNAP- and DES-exposed fish either alone or in coexposure decreased in both male and female zebrafish (Lin et al., 2023). However, DES alone or in combination with PSNAP increased the E2/T ratio in a concentration-dependent manner in male fish. In female fish, a concentration-dependent reduction in the E2/T ratio was observed in fish coexposed with PSNAP and DES (Lin et al., 2023). DES alone or coexposed with PSNAP enhanced the VTG content in a concentration-dependent manner in both males and female fish. PSNAP exposure has no significant effects on the T3 and T4 levels of both male and female fish; however, DES alone or in combination with PSNAP decreased both T3 and T4 contents in male and female fish in a concentration-dependent manner (Lin et al., 2023). Moreover, PSNAP and DES alone or in combination reduced fecundity, spawning events, fertilization, and hatchability of the embryos. In addition, PSNAP and DES either alone or in combination induced abnormal development (teratogenic effects) of the larvae (spinal curvature, pericardial cyst, and growth retardation) (Lin et al., 2023). Adult zebrafish exposed to TDCIPP alone or in combinations with PSNAP decreased T3 and T4 levels in female and T4 level in male fish (Zhao et al., 2021). In eggs, only T4 level (no T3) was reduced significantly when the fish were exposed to PSNAP alone and in combination with TDCIPP (concentration-dependent). A concentration-dependent reduction in the T3 level was observed when the fish was exposed in a combination of TDCIPP and PSNAP. Coexposure with homosolate, an emerging POP, did not induce any alteration in the GSI of both male and female fish; however, it resulted in higher expression of sgk1 and promoted ovary development, while inhibiting spermatogenesis (Ye et al., 2024). Coexposure also modulated steroid hormone synthesis genes (cyp17a2 and hsd 17β1) and esr2b, vtg1, and vtg2 and resulted in higher E2 release in female fish. Conversely, male fish showed lower T and E2 levels and altered the expressions of cyp11a1, cyp11a2, cyp17a1, cyp17a2, and hsdβ1 (Ye et al., 2024).
The intergenerational effect of PSNAP in coexposure with MCLR was evaluated in F1 embryos/larvae, which were obtained from the parents exposed to PSNAP and MCLR either alone or in combinations for 45 days (Wu et al., 2021; Zhu et al., 2021). It was observed that PSNAP was accumulated also in the F1 embryos and influenced the accumulation of MCLR (Wu et al., 2021). A concentration-dependent reduction in hatching rates, hatching enzymatic activities, and tail wagging of the F1 embryos exposed to MCLR alone or in combination with PSNAP was observed (Wu et al., 2021). Pathological alterations in somite muscles (irregular somite boundaries) were observed in F1 larvae exposed parentally to MCLR alone or coexposed with PSNAP, while no significant effect was observed on the AChE activity; however, a concentration-dependent increase in the AChE activity was observed in F1 larvae coexposed to MCLR and PSNAP. Gene expression analysis related to hatching enzymes (tox 16, foxp1, ctslb, xpb1, klf4, cap1, bmp4, cd63, He1.2, zhe1, and prl), cholinergic system (ache and chrnα7), and muscle development (Wnt, MyoD, Myf5, myogenin, and MRF4) indicated alterations in the F1 larvae exposed parentally to PSNAP and MCLR either alone or in combinations (Wu et al., 2021). It was also observed that, due to parental exposure (F0) to PSNAP and PSNAP + MCLR, accumulation of PSNAP was detected in the testis and ovary of the F1 larvae, and the presence of PSNAP in the environment increased the accumulation of MCLR in F1 larvae (Zuo et al., 2021). Moreover, parental exposure of MCLR and PSNAP + MCLR affects the hatchability (decreased), malformation (decreased), mortality (increased), body length (decreased), and heart rates (decreased) of the F1 larvae; even though parents with PSNAP exposure alone had no effects on the induction of developmental defects in F1 larvae. Parental exposure to PSNAP alone did not alter the T4 and T3 levels in the F1 larvae. However, MCLR either alone or in coexposure reduced T4 and T3 levels of the F1 larvae. Gene expression in the F1 larvae of the HPT axis and GH/IGF axis remained unaltered when the parents were exposed to PSNAP alone; however, the expressions of HPT axis genes (trα, trβ, dio2, dio1, ttr, tg, tshr, nis, crh, pax8, and nkx2.1), except ugt1ab and tpo, were altered in F1 larvae after parental exposure either to MCLR alone or coexposed with PSNAP. Among GH/IGF axis genes (igf2α, igf1, gh, ghrh, ghrα, igf1ra, igf1rβ, igf2β, and igf2r), only igf1, igf2α, and ghrβ altered in F1 larvae when the parents were exposed to MCLR + PSNAP (Zuo et al., 2021).
4 Discussion
In the systematic review, our search strategy collected literature on eight plastic polymers (PA, PC, PE, PET, PMMA, PPP, PS, and PVC) (Table 1) studied on 13 fish species, consisting of 114 articles (Figure 1; Table 2). The effects of the plastics on fish were evaluated either alone or when coexposed with other environmental pollutants, including heavy metals, POP, drugs, and bacteria. The accumulation of NAPs by fish was also influenced by the surface charge of the plastics and environmental conditions (temperature, pH, and diet). The information collected on plastic toxicity summarized from all these literatures was assembled in Supplementary Table S1 and deposited at Figshare (www.figshare.com) for reference and future upgradation, if needed.
Our strategies found a wide variation in the diameters of the plastic polymers used in these studies. Although the size of the MIPs is usually considered to be < 5,000,000 nm (5,000 µm), the size of NAPs has not yet achieved a consensus, with some considering it to be < 1,000 nm and others <100 nm (Torres-Ruiz et al., 2021). During the review, we considered the size/diameter of the NAPs as ≤100 nm and excluded 15 (13 + 2) articles, where the sizes of the studied plastic particles were >100 nm (Table 3). In addition, the diameter of the studied plastics (PE, PPP, PET, and PS) in 26 articles was ≤100 nm as well as > 100 nm (Table 4). In these studies, we have considered the effects observed on the plastic sizes ≤100, and the effects found on diameters >100 nm were excluded (Table 4). Moreover, our review focused mostly on whole/intact animals and embryos; therefore, the studies performed in vitro were also excluded from this review (Greven et al., 2016). In addition, in 48 articles, NAPs were coexposed with various environmental pollutants (Table 8). Moreover, in some studies, modifications in diet (high-fat diet) and environmental conditions (temperature and pH) were made. Considering all these variations, we have finally selected 101 (99 + 2) articles for review (Figure 1; Table 5).
Our findings revealed that among the five plastic polymers (PE, PET, PMMA, PPP, and PS), the studies were limited either to plastic types or the developmental stages (embryos, larvae, juveniles, and adults) of the fish (Table 5). For example, effects of PE/LDPE were studied on embryos and adults of zebrafish and juveniles of common carp; PET and PMMA were found on embryos of zebrafish, PPP in juveniles of tilapia and zebrafish, and PS on grass carp (embryos, larvae, and juveniles), silver carp (adults), tooth carp (adult), fathead minnows (adult male), Chinese rice fish (adults), Japanese medaka (larvae and adults), marine medaka (embryos, larvae, juveniles, and adults), rainbow trout (juveniles), Nile/red tilapia (larvae and juveniles), Mozambique tilapia (larvae), and zebrafish (embryos, larvae, and adults). Moreover, most of the studies on fish were focused on the effects of PS (∼89%), probably because of their wide availability and a well-characterized research material that can be manufactured with a large range of particle sizes, fluorescence labeling, as well as various surface modifications (Torres-Ruiz et al., 2021; Xu et al., 2022). In addition, among thirteen fish species, our search strategies found that zebrafish was the most studied fish (78 articles out of 114; ∼69%) than any other fish species included in this review. However, despite wide arrays of variability in the mode of exposures (waterborne, trophic transfer, dietary, injections, or coexposure with other environmental pollutants) and durations and concentrations, the study showed bioaccumulation of NAPs on chorion and embryos during embryo–larval development as well as in the gill, gut/intestine, liver, kidney, gonads (testis and ovary), muscle, and brain of larvae, juveniles, and adult fish. Moreover, accumulation of NAPs in the tissues/organs of fish induced multiple biological effects including body and bone morphology, teratogenic, cardiac, oxidative stress, inflammatory, genotoxic, hepatotoxic, neurotoxic, behavioral, reproductive, endocrine disruptions, and an intergenerational impact (Tables 5–9). In coexposure experiments, the combined effects of NAP and other environmental pollutants on fish can be observed as synergistic or antagonistic, while no influence of some of the chemicals was also noticed (Table 8). Our studies agree with the concept that in fish, NAPs due to their small size are able to penetrate tissues by crossing the biological barriers (chorions in the embryos and gill, skin, and gut in larvae, juveniles, and adults), as observed in humans (lung, skin, and gastrointestinal barriers in humans) and can induce toxicogenomic effects at the cellular level (Lehner et al., 2019; Mantovani et al., 2019). Although the bioaccumulation of NAPs in fish was evident from our literature survey, the data on LC50, NOEC, or LOEC are very limited. The 96 hpf NOAEL as determined on PE (hydrodynamic size 191.10 ± 3.13 nm; Sun et al., 2021) in zebrafish embryos was 50 μg/L, the 96 h LC50 for PS (diameter 100 nm) on zebrafish embryos (24 hpf) was 431.1 mg/L (Feng et al., 2022), while in tooth carp adults (PS, average diameter was 185 nm), it was 19.3 mg/L (Saemi-Komsari et al., 2023), which are significantly higher than the plastic concentrations found in the aquatic environments (Mojiri et al., 2024).
Oxidative stress and inflammation are the two major pathways commonly affected by exposure to NAPs in fish (Brun et al., 2019). Engineered nanoparticles are known as potent inducers of immune and inflammatory responses as well as for the generation of reactive oxygen species (Khanna et al., 2015). Although we have limited the diameter of NAPs to ≤ 100 nm (minimum is 15 nm), our literature survey showed that small NAPs can reach internal organs (brain, eyes, liver, pancreas, and heart), and comparatively larger particles accumulated in the gut, gill, and skin of fish (Table 5). In embryos, NAPs after crossing the chorion (probably through chorionic pores) were initially accumulated in the yolk sac and later transported to various organs, including the GI tract, liver, pancreas, gall bladder, kidney, heart, and brain (Table 6; Supplementary Table S1); while, in larvae, juveniles, and adults, the accumulation was initially observed on the gill, skin, and gut and then gradually transferred to the liver, pancreas, kidney, gonads, and brain. Consequently, as a part of the detoxification process (mediated by cytochrome P450-dependent mechanisms), the Oxidative stress induced, resulted in cellular apoptosis, histological damage in the accumulated organs, and activated immunomodulatory mechanisms. Accordingly, the genes belonging to these pathways were functional and controlled the processes as well (Aschner et al., 2025).
Oxidative stress is a key putative mechanism of NAPs causing imbalance of ROS (Sharpton, 2018), which is an intracellular chemical species that contain oxygen (O2) and are reactive toward lipids, proteins, and DNA (Glasauer and Chandel, 2013). Excessive ROS is a major cause of oxidative damage and weakens the immunity of fish (Ding et al., 2018; Sun et al., 2019). Enzymatic antioxidants such as SOD and CAT participate in protecting organisms from excesses of ROS, which was induced by exposure to xenobiotics (Mates, 2000). SOD encompasses mitochondrial Mn-SOD and cytosolic SOD (Cu and Zn-SOD) enzymes that convert the superoxide anion into H2O2, which was then converted by CAT into water and oxygen (Abele et al., 2011). The impairment of these oxidative enzymes damaged the cell membrane and DNA, resulting in a loss of defense capability (Matos et al., 2019). Both in embryos and adult fish, the major oxidative enzymes are CAT, SOD, GPx, GST, and the GSH and MDA, which were used as important biomarkers for NAP toxicity. The oxidative stress index (based on CAT, peroxidase, and SOD activities and GSH and MDA contents) was found to be increased in fish after NAP exposure (Bhagat et al., 2022; Chen J. et al., 2023). Our review indicated that the plastic particles we surveyed (PE, PET, PMMA, PPP, and PS) have the potential to regulate oxidative stress and ROS in the fish. Therefore, oxidative stress, calculated as the oxidative stress index, should be considered a potential indicator of NAP toxicity.
Our literature search also indicated that the effects of NAPs on gene expression analysis were observed in 33 articles (∼29%) and restricted only to PS (Tables 7, 9). No other plastic types were used for gene expression analysis. Moreover, in larvae (Mozambique tilapia and zebrafish), juveniles (grass carp and Nile tilapia), and adults (FHM, marine medaka, and zebrafish), the gene analyses were also restricted to PS, and the studied organs were gut/intestine (grass carp, marine medaka, Nile tilapia, and zebrafish), liver (FHM, marine medaka, Nile Tilapia, and zebrafish), kidney (FHM), ovary (zebrafish), brain (zebrafish), and muscle (marine medaka) of the fish (Table 7).
Our studies indicate that in zebrafish embryos, PSNAP either alone or in coexposure upregulated several genes which belonged to membrane transport, detoxification, oxidative stress, apoptosis and ferroptosis, inflammation, base excision pathways, VEGFA/VEGFR pathways, and also related to the liver, vasculature, nervous system, visual system, and HPT and HPG axis (Tables 7, 9), while downregulation of several genes was related to membrane transport, apoptosis, steroidogenesis, neurodegeneration and motor dysfunction, visual system, epigenome, VEGFA/VEGFR pathways, osteogenesis, thyroxin transport, and synthesis. Moreover, several of the studied genes belonged to detoxification, visual system, oxidative stress, metallothionein, DNA damage, and mitochondrial metabolism, and the central nervous system development remained unaltered (Tables 7, 9). In larvae, juveniles and adults, gene regulations were organ-specific and mostly related to the functions of the organs. Moreover, as in embryo–larval development, in coexposure with environmental pollutants, synergistic/antagonistic or no significant effects in gene expressions were observed (Table 9). In the gut/intestine, the gut microbiota played a significant role in gene regulations, which could be synergistic/antagonistic to the effects induced by PSNAP in other organs. The expressions of several genes related to oxidative stress and immunomodulation (IL-6, IL-8, IL-10, IL-1β, TNF-α, and INF-γ2) were upregulated by PSNAP (Li Z. et al., 2024). Moreover, in macrophages of the intestine, immune system-related DEGs (ctsba, nfkbiab, and pycard) were significantly altered by PSNAP exposure, and the genes related to MAPK signaling pathways (hsp70.1, hsp70.2, and hsp70l) remained unaltered. In intestinal enterocytes, genes related to GSH metabolism (gsta2, gsto1, gsto2, gpx1a, and mgst1.2) and cytochrome P450 remained unaltered. In intestinal B and T cells, upregulation of hsp70.1, hsp70.2, and hsp70.3 was observed in fish exposed to PSNAP.
In the liver, in addition to immunomodulation, lipid synthesis-related genes (fasn, srebf1, and pparg), and lipid transport-related genes (cetp and ldlr) were upregulated, and the lipid degradation-related genes (atg1, ppara, and aco) were downregulated (Li X. et al., 2024). The genes of the Toll-like receptor 4 (TLR4) pathway (irf3, irak4, traf6, and tbk1) in the liver showed a trend of upregulation, while muscle development-related gene (myog, myod, mstn, myf5, and fgf6b) expressions were downregulated, and no alteration was observed in creatine kinase and hif1α contents after PSNAP exposure (Pitt et al., 2018b; Sarasamma et al., 2020).
In the brain, the development of microthrombi in the basement membrane of the blood–brain barrier, a well-known toxicogenomic index, was associated with the downregulation of mitochondrial fusion-related genes (mfn1a, mf1b, and opa1), while the mitochondrial division-related genes (drp1, mff, fis 1, mid49, and mid51) showed a tendency of upregulation (Zhang et al., 2023). The expressions of genes related to mitophagy (ulk1a, and parl) were also upregulated by PSNAP exposure. Moreover, among the 5-HT receptor mRNAs, htr1aa, htr1ab, and htr2c were significantly upregulated, while the expressions of htr1b and htr4 showed downregulation in the brain of fish.
In zebrafish, PSNAP have the potential to accumulate in the gonads (testis and ovary), disrupted endocrine functions, impaired gametogenesis, interfere with intergenerational inheritance and thus embryonic development, and modulated the gene expressions related to hatching enzymes (tox 16, foxp1, ctslb, xpb1, klf4, cap1, bmp4, cd63, He1.2,zhe1,and prl), cholinergic system (ache and chrnα7), and muscle development in F1 offspring (Wnt, MyoD, Myf5, myogenin, and MRF4) (Wu et al., 2021). The molecular mechanisms underlying these effects, including oxidative stress, inflammation, and epigenetic modifications, highlighted the complex and multifaceted nature of NAP toxicity.
Taken together, even though much work remains to be done, our systematic review analysis on the effects of NAP on fish embryos and adults together with genetic analysis in vivo revealed a toxicity pathway starting with the particles entering the cell and inducing oxidative stress and immune responses that generated inflammation. Further intrusion of NAPs on the organelles such as mitochondria induced alterations in energy (carbohydrate) metabolism. The accumulation of NAPs in different organs was dependent on size, concentrations, and durations, influenced on specific neurobehavioral, cardiac, lipid metabolism, reproduction, and intergenerational inheritance.
Plastic pollution is a global problem and poses a significant threat to ecosystems, wildlife, and human health, with plastics taking hundreds of years to decompose in the environment. Several countries have recently introduced regulations and legislations focused on plastic. These are primarily aimed to reduce the consumption and improve waste management; however, attention should be given to plastic production. More than 60 countries have implemented bans and levies on plastic packaging and single-use waste. In 2018, the European Commission published its strategy to reduce usage of single-use plastics, followed by legislation in the form of the Single-Use Plastics Directive. In 2021, the EU has levied a “plastic tax” on all unrecycled plastic waste generated within the region. The EPA’s “National Strategy to Prevent Plastic Pollution” aims to eliminate the release of plastic waste into the environment by 2040. However, despite all these regulations and rules, we may all be aware of the problem and cooperate to implement the government policies to reduce plastic pollution in the environment.
5 Conclusion
Our systematic review has synthesized current knowledge on the toxicogenomic effects of NAPs in fish, using them as a model to assess the potential health risk to humans. Although methodological challenges and the limited scope of studies in plastics beyond PS remain, our findings indicate that the toxicity of NAPs can be influenced by several factors, including particle size, exposure duration, exposure route, tissue accumulation, and the chemical composition of plastics. Furthermore, NAPs pose risks to various organs through mechanisms such as oxidative stress, immune system modulation, and specific organ effects, including neurotoxicity, cardiotoxicity, genotoxicity, teratogenesis, endocrine disruption, energy metabolism alterations, and intergenerational inheritance. Despite the variability in fish species, sizes and types of the plastics, surface charge, environmental conditions, exposure routes, duration of exposure, and developmental stages of the experimental fish, our review highlights that NAPs can cross the biological barriers and gradually accumulate in the various parts/organs of the body in a non-specific manner. This accumulation occurs over time, further emphasizing the complex and potentially widespread impact of NAP exposure on aquatic organisms, with implications for human health. In summary, NAPs possess significant adsorptive properties and serve as vectors for other environmental contaminants, potentially exerting synergistic, antagonistic, or neutral effects on the tissues and organs of fish. The biotransformation process activates oxidative stress-dependent mechanisms, which in turn induce specific gene regulatory responses. In the gut/intestine, the toxicogenomic responses to NAPs exhibited either synergistic or antagonistic interactions with the gut microbiota. Intergenerational transfer of NAPs has been shown to disrupt embryo–larval development in the F1 generation. Although significant knowledge gaps remain, our systematic review addresses several critical scientific questions regarding the toxicological effects of NAPs, paving the way for future research into their environmental and health impacts.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author contributions
AD: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, and Writing – review and editing. JC: Data curation, Formal analysis, Methodology, Resources, Software, and Writing – original draft. PT: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, and Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The research was supported by NIH/NIMHD grant #G12MD07581 (RCMI Center for Environmental Health), NIH/NIMHD grant #U54MD015929 (RCMI Center for Health Disparities Research) at Jackson State University, Jackson, Mississippi, United States, and NIH/NIMHD grant #U54MD013376 (RCMI Center for Urban Health Disparities Research and Innovation) at Morgan State University, Baltimore, Maryland, United States.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ftox.2025.1530209/full#supplementary-material
Glossary
- Ach
Acetylcholine
- AChE
Acetyl cholinesterase
- αKGPD
alpha keto glutarate dehydrogenase
- APAP
Acetaminophen
- AVO
Avobenzone
- FHM
Fathead minnows
- BAP
Benzo [a] pyrene
- BDE-47
Polybrominated diphenyl ethers
- BFCOD
7-benzyloxy-4-trifluoromethyl-coumarin O-dibenzyloxylase
- BMDMB
butyl methoxydibenzoylmethane
- BPA
Bisphenol A
- CAT
Catalase
- CCV
Common cardinal vein
- CO
Cardiac output
- c3
Complement component 3
- DES
Diethylstilbestrol
- DA
Dopamine
- DPH
Diphenhydramine
- dph
Day post hatch
- EROD
7-Etoxyresorufin O-deethylase
- EE2
17 α-Ethynyl estradiol
- FHM
Fathead minnows
- GABA
Gamma-aminobutyric acid
- GAD
Glutamic acid decarboxylase
- GDH
Glutamate dehydrogenase
- GI-tract
Gastrointestinal tract
- GR
Glutatione reductase
- GS
Glutammine synthetase
- GSH
Glutathione
- GSSG
Oxidized glutathione
- GST
Glutathione-S-transferase
- HSI
Hepatosomatic index
- HPT
Hypothalamus–pituitary–thyroid
- 5-HT
Serotonin
- ISV
Intersegmental vessel
- LDPE
Low-density polyethylene
- LOEL
Lowest observed effect level
- LZM
Lysozyme
- mst1
Macrophage-stimulating factor 1
- MAO
Monoamine oxidase
- MAPs
Macroplastics
- MCL
Microcystin-LR
- MIPs
Microplastics
- NAPs
Nanoplastics
- ncf2
Neutrophil cytosolic factor 2
- No
Nitric oxide
- NOAEL
No observed adverse effect level
- Noel
No observed effect level
- nox2
NADPH oxidase 2
- PC
Polycarbonate
- PCP
Personal care products
- PE
Polyethylene
- PLA
Polylactic acid
- PMME
Polymethylmethacrylate
- PEMIP
Polyethylene microplastics
- PENAPs
Polyethylene nanoplastics
- PET
Polyethylene terephthalate
- PETNAPs
Polyethylene terephthalate nanoplastics
- PHE
Phenanthrene
- PHN
Phenmediphamµ
- POP
Persistent organic pollutants
- PP
Polypropylene
- PPAR
Peroxisome proliferator activator receptor
- PPPMIP
Polypropylene microplastics
- PS
Polystyrene
- PSMIPs
Polystyrene microplastics
- PSNAPs
Polystyrene nanoplastics.
- PU
Polyurethane
- PVC
Polyvinyl chloride
- SIM
Simvastatin
- SIVP
Sub-intestinal venous plexus
- SMX
Sulfamethoxazole
- SMZ
Sulfamethazine
- SNP
Sodium nitroprusside
- SOV
Superficial ocular vessels
- T-AOC
Total antioxidant content
- TC
Tetracycline
- TCS
Triclosan
- TDCIPP
Tris 1,3-dichloro-2-propyl phosphate
- TG
Thyroglobulin
- TGL
Triglyceride
- TLR
Toll-like-receptor
- TPH
Tryptophan hydroxylase
- TPhP
Triphenyl phosphate
- VEGFA
Vascular endothelial growth factor
- VEGFR
Vascular endothelial growth factor receptor
- vit D
Vitamin D
- VTG
Vitellogenin
- wph
Weeks post hatch
References
1
Abele D. Vazquez-Medina J. P. Zenteno-Savin T. (2011). Oxidative stress in aquatic ecosystems. Oxidative stress Aquat. Ecosyst. 1-524. 10.1002/9781444345988
2
Afrin S. Rahman M. Hossain N. Uddin K. Malafaia G. (2022). Are there plastic particles in my sugar? A pioneering study in the characterization of microplastics in the commercial sugars and risk assessment. Sci. Total Environ.837, 155849. 10.1016/j.scitotenv.2022.155849
3
Aliakbarzadeh F. Rafiee M. Khodagholi F. Khorramizadeh M. R. Manouchehhri H. Eslami A. et al (2023). Advanced effects of polystyrene nanoplastics and its binary mixtures with nonylphenol on zebrafish nervous system: from oxidative stress to impaired neurotransmitter system. Environ. Pollut.312, 120597. 10.1016//j.envipol.2022.120587
4
Alimba C. G. Faggio C. (2019). Microplastics in the marine environment: current trends in environmental pollution and mechanisms of toxicological profile. Environ. Toxicol. Pharmacol.68, 61–74. 10.1016/j.etap.2019.03.001
5
Almeida M. Martins M. A. Soares A. M. V. Cuesta A. Oliviera M. (2019). Polystyrene nanoplastics alter the cytotoxicity of human pharmaceuticals on marine fish cell lines. Environ. Toxicol. Pharmacol.69, 57–65. 10.1016/j.etap.2019.03.019
6
Amaral-Zettler L. A. Zettler E. R. Slikas B. Boyd G. D. Melvin D. W. Morral C. E. et al (2015). The biogeography of the plastisphere: implications for policy. Front. Ecol. Environ.13, 541–546. 10.1890/150017
7
Andrady A. L. (2011). Microplastics in the marine environment. Mar. Pollut. Bull.62, 1596–1605. 10.1016/j.marpolbul.2011.05.030
8
Aragaw T. A. (2020). Surgical face masks as a potential source for microplastic pollution in the COVID-19 scenario. Mar. Pollut. Bull.159, 111517. 10.1016/j.marpolbul.2020.111517
9
Aschner M. Dasmahapatra A. K. Muriana A. (2025). Editorial: model organisms in toxicology. Front. Toxicol.7, 1560492. 10.3389/ftox.2025.1560492
10
Barreto A. Santos J. Amorim M. J. B. Maria V. L. (2021). Polystyrene nanoplastics can alter the toxicological effects of simvastatin on Danio rerio. Toxics9, 44. 10.3390/toxics9030044
11
Barreto A. Santos J. Calisto V. Rocha L. S. Amorim M. J. B. Maria V. L. (2023). Cocktail effects of emerging contaminants on zebrafish: nanoplastics and the pharmaceutical diphenhydramine. Nanoimpact30, 100456. 10.1016/j.impact.2023.100456
12
Barria C. Brandts I. Tort L. Oliveira M. Teles M. (2020). Effect of nanoplastics on fish health and performance: a review. Mar. Pollut. Bull.151, 110791. 10.1016/j.marpolbul.2019.110791
13
Bashirova N. Poppitz D. Kluver N. Scholz S. Matysik J. Alia A. (2023). A mechanistic understanding of the effects of polyethylene terephthalate nanoplastics in the zebrafish (Danio rerio) embryo. Sci. Rep.13, 1891. 10.1038/s41598-023-28712-y
14
Bhagat J. Nishimura N. Shimada Y. (2021). Toxicological interactions of microplastics/nanoplastics and environmental contaminants: current knowledge and future perspectives. J. Hazard Mat.405, 123913. 10.1016/j.jhazmat.2020.123913
15
Bhagat J. Zang L. Kaneko S. Nishimura N. Shimada Y. (2022). Combined exposure in nanoplastics and metal oxide nanoparticles inhibits efflux pumps and causes oxidative stress in zebrafish embryos. Sci. Total Environ.835, 155436. 10.1010/j.scitotevn.2022.155436
16
Bhagat J. Zang L. Nishimura N. Shimada Y. (2020). Zebrafish: an emerging model to study microplastic and nanoplastic toxicity. Sci. Total Environ.728, 138707. 10.1016/j.scitotenv.2020.138707
17
Brandts I. Barria C. Martins M. A. Franco-Martinez L. Barreto A. Tvarijonaviciute A. et al (2021). Water borne exposure of gilthead seabream (Sparus aurata) to polymethylmethacrylate nanoplastics causes effects at cellular and molecular levels. J. hazard Mater403, 123590. 10.1016/j.jhazmat.2020.123590
18
Brandts L. Teles M. Goncalves A. P. Barreto A. Franco-Martinez L. Tavarijonaviciute A. et al (2018). Effects of nanoplastics on Mytilus galloprovincialis after individual and combined exposure with carbamazepine. Sci. Total Environ.643, 775–784. 10.1016/j.scitotenv.2018.06.257
19
Browne M. A. Crump P. Niven S. J. Teuten E. Tonkin A. Galloway T. et al (2011). Accumulation of microplastic on shorelines woldwide: sources and sinks. Environ. Sci. Tech.45, 9175–9179. 10.1021/es201811s
20
Brun N. R. van Hage P. Hunting E. R. Haramis A. P. G. Vink S. C. Vijver M. J. et al (2019). Polystyrene nanoplastics disrupt glucose metabolism and cortisol levels with a possible link to behavioural changes in larval zebrafish. Commun. Biol.2, 382. 10.1038/s42003-019-0629-6
21
Campanale C. Massarelli C. Savino I. Locaputo V. Uricchio V. F. (2020). A detailed review study on potential effects of microplastics and additives of concern on human health. Int. J. Environ. Res. Publ. Health17, 1212. 10.3390/ijerph17041212
22
Cassano D. La Spina R. Ponti J. Bianchi I. Gilliland D. (2021). Inorganic species-doped polypropylene nanoparticles for multifunctional detection. ACS Appl. Nano Mater4, 1551–1557. 10.1021/acsanm.0c03039
23
Chackal R. Eng T. Rodrigues E. M. Matthews S. Page-Lariviere F. Avery-Gomm S. et al (2022). Metabolic consequences of developmental exposure to polystyrene nanoplastics, the flame retardant BDE-47 and their combination in zebrafish. Front. Pharmacol.13, 822111. 10.3389/fphar.2022.822111
24
Chae Y. Kim D. Kim S. W. An Y.-Z. (2018). Trophic transfer and individual impact of nano-sized polystyrene in a four-species freshwater food chain. Sci. Rep.8, 284. 10.1038/s41598-017-18849-y
25
Chen J. Fang C. Zheng R. Bo J. (2022). Embryo toxicity of polystyrene microspheres of different sizes to the marine medaka (Oryzias melastigma) (McClelland, 1839). Water Res.14 (12), 1831. 10.3390/w14121831
26
Chen J. Lei Y. Wen J. Zheng Y. Gan X. Liang Q. et al (2023b). The neurodevelopmental toxicity induced by combined exposure of nanoplastics and penicillin in embryonic zebrafish: the role of aging processes. Environ. Pollut.335, 122281. 10.1016/j.envpol.2023.122281
27
Chen J. Liang Q. Zheng Y. Lei Y. Gan X. Mei H. et al (2024). Polystyrene nanoplastics induced size-dependent developmental and neurobehavioral toxicities in embryonic and juvenile zebrafish. Aqua Tox267, 106842. 10.1016./jaquatox.2024.106842
28
Chen Q. Cao Y. Li H. Liu H. Liu Y. Bi L. et al (2023c). Sodium nitroprusside alleviates nanoplastics-induced developmental toxicity by suppressing apoptosis, ferroptosis and inflammation. J. Env. Manag.345, 118702. 10.1016/j.jenvman.2023.118702
29
Chen Q. Gundlach M. Yang S. Jiang J. Velki M. Yin D. et al (2017a). Quantitative investigation of the mechanisms of microplastics and nanoplastics toward zebrafish larvae locomotor activity. Sci. Total Environ.584-585, 1022–1031. 10.1016/j.scitotenv.2017.01.156
30
Chen Q. Yin D. Jia Y. Schiwy S. Legradi J. Yang S. et al (2017b). Enhanced uptake of BPA in the presence of nanoplastics can lead to neurotoxic effects in adult zebrafish. Sci. Total Environ.609, 1312–1321. 10.1016/j.scitotenv.2017.07.144
31
Chen Y. Wang X. Sui Q. Chang G. Sun X. Zhu L. et al (2023a). Charge-dependent negative effects of polystyrene nanoplastics on Oryzias melastigma under ocean acidification conditions. Sci. Total Environ.865, 161248. 10.1016/j.scitotenv.2022.161248
32
Chen Z.-Y. Li N.-J. Cheng F.-Y. Hsueh J.-F. Huang C.-C. Lu F.-I. et al (2020). The effect of the chorion on size-dependent acute toxicity and underlying mechanisms of amine-modified silver nanoparticles in zebrafish embryos. Int. J. Mol. Sci.21, 2864. 10.3390/ijms21082864
33
Cheng H. Duan Z. Wu Y. Wang Y. Zhang H. Shi Y. et al (2022). Immunotoxicity responses to polystyrene nanoplastics and their related mechanisms in the liver of zebrafish (Danio rerio) larvae. Environ. Int.161, 107128. 10.1016/j.envint.2022.107128
34
Cho H.-J. Lee W. S. Jeong J. Lee J.-S. (2022). A review on the impacts of nanomaterials on neuromodulation and neurological dysfunction using a zebrafish animal model. Comp. Biochem. Physiol. Part C261, 262109428. 10.1016/jcbpc.2022.109428
35
Clark N. J. Fischer A. C. Durndell L. Galloway T. S. Thompson R. C. (2023b). Translocation of 14C-polystyrene nanoplastics into fish during a very-low concentration dietary exposure. Chemosphere341, 140058. 10.1016/j.chemosphere.2023.140058
36
Clark N. J. Khan F. R. Crowther C. Mitrano D. M. Thompson R. C. (2023a). Uptake, distribution and elimination of palladium-doped polystyrene nanoplastics in rainbow trout (Oncorhynchus mykiss) following dietary exposure (Oncorhynchus mykiss) following dietary exposure. Sci. Total Environ.854, 158765. 10.1016/j.scitotenv.2022.158765
37
Cole M. Lindeque P. Halsband C. Galloway T. S. (2011). Microplastics as contaminants in the marine environment: a review. Mar. Poll. Bull.62, 2588–2597. 10.1016/j.marpolbul.2011.09.025
38
Cox K. D. Covernton G. A. Davies H. L. Dower J. F. Juanes F. Dudas S. E. (2019). Human consumption of microplastics. Environ. Sci. Technol.53, 7068–7074. 10.1021/acs.est.9b01517
39
Cozar A. Echevarria F. Gonzalez-Gordillo J. I. Irijogen X. Ubeda B. Harnendez-Leon S. et al (2014). Plastic debris in the open ocean. Proc. Nat. Acad. Sci. U. S. A.111, 10239–10244. 10.1073/pnas.1314705111
40
Dai L. Luo J. Feng M. Wang M. Zhang J. Cao X. et al (2023). Nanoplastics exposure induces vascular malformation by interfering with the VEGFA/VEGFR pathway in zebrafish (Danio rerio). Chemosphere313, 137360. 10.1016/j.chemosphere.2022.137360
41
Dasmahapatra A. K. Chatterjee J. Tchounwou P. B. (2024). A systematic review of the toxic potential of parabens in fish. Front. Toxicol.6, 1399467. 10.3389/ftox20241399467
42
Dasmahapatra A. K. Williams C. B. Myla A. Tiwary S. K. Tchounwou P. B. (2023). A systematic review of the evaluation of endocrine-disrupting chemicals in the Japanese medaka (Oryzias latipes) fish. Front. Toxicol.5, 1272368. 10.3389/ftox.2023.1272368
43
Deng J. Zeng X. Li J. Luo L. Yang Y. Luan T. (2023). Single-cell transcriptomic analysis reveals heterogeneity of the patterns of responsive genes and cell communications in liver cell populations of zebrafish exposed to polystyrene nanoplastics. Sci. Total Environ.889, 164082. 10.1016/j.scitotenv.2023.164082
44
de Ruijter V. N. Redondo-Hasselerharm P. E. Gouin T. Koelmans A. A. (2020). Quality criteria for microplastic effect studies in the context of risk assessment: a critical review. Environ. Sci. Technol.54, 11692–11705. 10.1021/acs.est0c03057
45
de Sa L. C. Oliveira M. Ribeiro F. Rocha T. L. Futter M. L. (2018). Studies of the effects of microplastics on aquatic organisms: what do we know and where should we focus our efforts in the future?Sci. Total Environ.645, 1029–1039. 10.1016/j.scitotenv.2018.07.207
46
de Souza Toedoro L. Jablonski C. A. Pelegrini K. Carneiro T. Pereira B. Maraschin T. G. et al (2024). Toxic effects of environmental-relevant exposure to polyethylene terephthalate (PET) micro and nanoparticles in zebrafish early development. NanoImpact33, 100497. 10.1016/j.impact.2024.100497
47
Dhaka V. Singh S. Anil A. G. Naik S. K. Garg S. Samuel J. et al (2022). Occurrence, toxicity, and remediation of polyethylene terephthalate plastics. A review. Environ. Chem. Lett.20, 1777–1800. 10.1007/s10311-021-01384-8
48
Ding J. Huang Y. Liu S. Zhang S. Zou H. Wang Z. et al (2020). Toxicological effects of nano- and micro-polystyrene plastics on red tilapia: are larger plastic particles more harmless?J. Hazard Mat.396, 122693. 10.1016/j.jhazmat.2020.122693
49
Ding J. Zhang S. Razanajatovo R. M. Zou H. Zhu W. (2018). Accumulation, tissue distribution, and biochemical effects of polystyrene microplastics in the freshwater fish red tilapia (Oreochromis niloticus). Environ. Pollut.238, 1–9. 10.1016/j.envpol.2018.03.001
50
Domenech J. Hernandez A. Rubio L. Marcos R. Cortes C. (2020). Interactions of polystyrene nanoplastics with in vitro models of the human intestinal barrier. Arch. Toxicol.94, 2997–3012. 10.1007/s00204-020-02805-3
51
Du J. Hu Y. Li Z. Zhou J. Xiang F. Zheng H. et al (2024). Toxicity of polystyrene Nanoplastics in the liver and intestine of normal and high-fat-diet juvenile zebrafish. Environ. Toxicol. Chem.43, 147–158. 10.1002/etc.5767
52
Duan Z. Duan X. Zhao X. Wang X. Wang J. Liu Y. et al (2020). Barrier function of zebrafish embryonic chorions against microplastics and nanoplastics and its impact on embryo development. J. Hazard Mater.395, 122621. 10.1016/j.jhazmat.2020.122621
53
Duan Z. Wang J. Zhang H. Wang H. Wang Y. Chen Y. et al (2023). Elevated temperature decreases cardiovascular toxicity of nanoplastics but adds to their lethality: a case study during zebrafish (danio rerio) development. J. Hazard Mat.458, 131679. 10.1016/j.jhazmat.2023.131679
54
Earni-Cassola G. Zadjelovic V. Gibson M. I. Christie-Oleza J. A. (2019). Distribution of plastic polymer types in the marine environment: a meta-analysis. J. Hazard Mat.369, 691–698. 10.1016/j.jhazmat.2019.02.067
55
Ebere E. C. Wirnkor V. A. Ngozi V. E. (2019). Uptake of microplastics by plant; a reason to worry or to be happy?World Sci. News131, 256–267. Available online at: www.worldscientific news.com
56
Elizalde-Velazquez A. Cargo J. Zhao X. Green M. J. Canas-Carrell J. E. (2020). In vivo effects of the immune function of fathead minnow (Pimephales promelas) following injection and intraperitoneal injection of polystyrene nanoplastics. Sci. Total Environ.735, 139461. 10.1016/jscitotenv.2020.139461
57
Enfrin M. Lee J. Gibert Y. Basheer F. Kong L. Dumee L. F. (2020). Release of hazardous nanoplastic contaminants due to microplastics fragmentation under shear stress forces. J. hazard Mat.384, 121393. 10.1016/j.jhazmat.2019.121393
58
Eom H. J. Nam S. E. Rhee J. S. (2020). Polystyrene microplastics induce mortality through acute cell stress and inhibition of cholinergic activity in a brine shrimp. Mol. Cell Toxicol.16, 233–243. 10.1007/s13273-020-00088-4
59
Eriksen M. Mason S. Wilson S. Box C. Zellers A. Edwards W. et al (2013). Microplastic pollution in the surface waters of the laurentian great lakes. Mar. Pollut. Bull.77, 177–182. 10.1016/j.marpolbul.2013.10.007
60
Estrela F. N. Guimaraes A. T. B. Silva F. G. da Luz T. M. Silva A. M. Pereira P. S. et al (2021). Effects of polystyrene nanoplastics on Ctenopharyngodon idella (grass carp) after individual and combined exposure with zinc oxide nanoparticles. J. Hazard. Mat.403, 123879. 10.1016/j.jhazmat.2020.123879
61
Fadare O. O. Okoffo E. D. (2020). Covid-19 face masks: a potential source of microplastic fibers in the environment. Sci. Total Environ.737, 140279. 10.1016/j.scitotenv.2020.140279
62
Fahrenfeld N. L. Arbuckle-Keil G. Beni N. N. Barlett-Hunt S. L. (2019). Source tracking microplastics in the aquatic environment. Trends Anal. Chem.112, 248–254. 10.1016/j.trac2018.11.030
63
Feng M. Luo J. Wan Y. Zhang J. Lu C. Wang M. et al (2022). Polystyrene nanoplastic exposure induces developmental toxicity by activating the oxidative stress response and base excision repair pathway in zebrafish (Danio rerio). ACS Omega7, 32153–32163. 10.1021/acsomega.2c03378
64
Free C. M. Jensen O. P. Mason S. A. Eriksen M. Willimson N. J. Boldgiv B. (2014). High levels of microplastic pollution in a large remote mountain lake. Mar. Pollut. Bull.85, 156–163. 10.1016/j.marpolbul.2014.06.001
65
Gao D. Kong C. Liao H. Junaid M. Pan T. Chen X. et al (2023a). Interactive effects of polystyrene nanoplastics and 6:2 chlorinated polyfluorinated ether sulfonates on the histomorphology, oxidative stress and gut microbiota in Hainan Medaka (Oryzias curvinotus). Sci. Total Environ.880, 163307. 10.1016/j.scitotenv.2023.163307
66
Gao X. Zhang Y. Hou L. Zhao Y. Zhang H. Jia Z. et al (2023b). Co-exposure to nanoplastics and acetaminophen causes skeletal dysplasia and behavioral abnormalities in zebrafish. Ecotoxicol. Environ. Saf.253, 114640. 10.1016/j.ecoenv.2023.114640
67
Geum S. W. Yeo M.-K. (2022). Reduction in toxicity of polystyrene nanoplastics combined with phenanthrene through binding of jellyfish mucin with nanoplastics. Nanomaterials12, 1427. 10.3390/nano12091427
68
Gigault J. Pedrono B. Maxit B. Ter Halle A. (2016). Marine plastic litter: the unanalyzed nano-fraction. Environ. Sci. Nano3, 346–350. 10.1039/C6EN00008H
69
Gigault J. Ter Halle A. Baudrimont M. Pascal P.-Y. Gauffre F. Phi T.-L. et al (2018). Current opinion: what is nanoplastics?Environ. Pollut.235, 1030–1034. 10.1016/j.envpol.2018.01.024
70
Glasauer A. Chandel N. S. (2013). ROS. Curr. Biol.23, R100–R102. 10.1016/j.cub.2012.12.011
71
Gonzalez-Fernandez C. Diaz Banos F. G. Esteban M. A. Cuesta (2021). Functionalized nanoplastics (NPs) increase the toxicity of metals in fish cell lines. Int. J. Mol. Sci.22, 7141. 10.3390/ijms.22137141
72
Greven A.-C. Merk T. Karagoz F. Mohr K. Klapper M. Jovanovic B. et al (2016). Polycarbonate and polystyrene nanoplastic particles act as stressors to the innate immune system of fathead minnow (Pimephales promelas). Environ. Toxicol. Chem.35, 3093–3100. 10.1002/etc.3501
73
Guimaraes A. T. B. Estrela F. N. de Lima Rodrigues A. S. Chagas T. Q. Pereira P. S. Silva F. G. et al (2021). Nanopolystyrene particles at environmentally relevant concentrations causes behavioral and biochemical changes in juvenile grass carp (Ctenopharyngodon idella). J. Haz Mat.403, 123864. 10.1016/j.jhazmat.2020.123864
74
Gwada B. Ogendi G. Makindi S. Trott S. (2019). Composition of plastic waste discarded by household s and its management approaches. Glob. J. Environ. Sci. Manag.5, 83–84. 10.22034/gjesm.2019.01.07
75
Habumugisha T. Zhang Z. Fang C. Yan C. Zhang X. (2023). Uptake, bioaccumulation, biodistribution and depuration of polystyrene nanoplastics in zebrafish (danio rerio). Sci. Total Environ.893, 164840. 10.1016/j.scitotenv.2023.164840
76
Hamed M. Martyniuk C. J. Naguib M. Lee J. S. Sayed A. E.-D. H. (2022). Neurotoxic effects of different sizes of plastics (nano, micro, and macro) on juvenile common carp (Cyprinus carpio). Front. Mol. Neurosci.15, 1028364. 10.3389/fnmol.2022.1028364
77
Hao T. Gao Y. Li Z.-C. Zhou X.-X. Yan B. (2023). Size-dependent uptake and depuration of nanoplastics in tilapia (Oreochromis niloticus) and distinct intestinal impacts. Environ. Sci. Technol.57, 2804–2812. 10.1021/acs.est.2c08059
78
Harshvardhan K. Jha B. (2013). Biodegradation of low-density polyethylene by marine bacteria from pelagic waters. Arabian Sea. India. Mar. Pollut. Bull.77, 100–106. 10.1016/j.marpolbul.2013.10.025
79
Hartmann N. B. Rist S. Bodin J. Jensen L. H. S. Schmidt S. N. Mayer P. et al (2017). Microplastics as vectors for environmental contaminants; exploring sorption desorption, and transfer to biota. Integr. Environ. Assess. Manag.13, 488–493. 10.1002/ieam.1904
80
He J. Yang X. Liu H. (2021). Enhanced toxicity of triphenyl phosphate to zebrafish in the presence of micro- and nano-plastics. Sci. Total Environ.756, 143986. 10.1016/j.scitotenv.2020.143986
81
He S. Li D. Wang F. Zhang C. Yue C. Huang Y. et al (2022). Parental exposure to sulfamethazine and nanoplastics alters the gut microbial communities in the offspring of marine madaka (Oryzias melastigma). J. Haz Mat.423, 127003. 10.1016/j.jhazmat.2021.127003
82
Heinder F. M. Alajmi F. Huerlimann R. Zeng C. Newmann S. J. Vamvounis G. et al (2017). Toxic effects of polyethylene terephthalate microparticles and Di(2-ethylhexyl) phthalate on the calanoid copepod Parvocalanus crassirostris. Ecotoxicol. Environ. Saf.141, 298–305. 10.1016/j.ecoenv.2017.03.029
83
Horvatits T. Tamminga M. Liu B. Sebode M. Carambia A. Fischer L. et al (2022). Microplastics detected in cirrhotic liver tissue. EBIO Med.82, 104147. 10.1016/j.ebiom.2022.104147
84
Huang W. Wu T. Wu R. Peng J. Zhang Q. Shi X. et al (2023). Fish to learn: insights into the effects of environmental chemicals on eye development and visual function in zebrafish. Environ. Sci. Poll. Res.30, 73018–73030. 10.1007/s11356-023-27629-3
85
Hwang J. Choi D. Han H. Choi J. Hong J. (2019). An assessment of the toxicity of polypropylene microplastics in human derived cells. Sci. Total Environ.684, 657–669. 10.1016/j.scitotenv.2019.05.071
86
Imhof H. K. Ivleva N. P. Schmid J. Niessner R. Laforsch C. (2013). Contamination of beach sediments of a subalpine lake with microplastic particles. Curr. Biol.23, R867–R868. 10.1016/j.cub.2013.09.001
87
Ji Y. Wang C. Wang Y. Fu L. Man M. Chen L. (2020). Realistic polyethylene terephthalate nanoplastics and the size- and surface coating-dependent toxicological impacts on zebrafish embryos. Environ. Sci. Nano7, 2313–2324. 10.1039/d0en00464b
88
Jiang Q. Chen X. Jiang H. Wang M. Zhang T. Zhang W. (2022). Effects of acute exposure to polystyrene nanoplastics on the channel catfish larvae: insights from energy metabolism and transcriptomic analysis. Front. Physiol.13, 923278. 10.3389/fphys.2022.923278
89
Jinhui J. Sudong S. Yan Y. Xia X. Jiahao J. Yongjian Y. et al (2019). Effects of microplastics and attached heavy metals on growth, immunity, and heavy metal accumulation in the yellow seahorse, Hippocampus kuda Bleeker. Mar. Pollute Bull.149, 110510. 10.1016/j.marpolbul.2019.110510
90
Jovanovic B. (2017). Ingestion of microplastics by fish and its potential consequences from a physical perspective. Integr. Environ. Assess. Manag.13, 510–515. 10.1002/ieam.1913
91
Kang H.-M. Byeon E. Jeong H. Kim M.-S. Chen Q. Lee J. S. (2021). Different effects of nano- and microplastics on oxidative status and gut microbiota in the marine medaka Oryzias melastigma. J. Haz. Mat.405, 124207. 10.1016/j.jhazmat.2020.124207
92
Kantha P. Liu S.-T. Horng J.-L. Lin L.-Y. (2022). Acute exposure to polystyrene nanoplastics impairs skin cells and ion regulation in zebrafish embryos. Aquat. Toxicol.248, 106203. 10.1016/jaquatox.2022.106203
93
Khan D. Ali S. A. (2023). On the novel process of pristine microplastic bio-fragmentation by zebrafish (Danio rerio). Arch. Environ. Contam. Toxicol.84, 299–306. 10.1007/s00244-023-00987-2
94
Khanna P. Ong C. Bay B. H. Baeg G. H. (2015). Nanotoxicity: an interplay of oxidative stress, inflammation, and cell death. J. Nanomater5, 1163–1180. 10.3390/nano5031163
95
Kik K. Bukowska B. Sicinska P. (2020). Polystyrene nanoparticles: sources, occurrence in the environment, distribution in tissues, Accumulation and Toxicity to various organisms. Environ. Pollut.262, 114297. 10.1016./j.envpol.2020.114297
96
Kim J. Rhee J. S. (2021). Biochemical and physiological responses of the water flea Moina macrocopa to microplastics: a multigenerational study. Mol. Cell Toxicol.17, 523–532. 10.1007/s13273-021-00162-5
97
Kim M. S. (2021). Modeling study on fate of micro/nano-plastics in micro/nano-hydrodynamic flow of freshwater. J. Hazard Mater419, 126397. 10.1016/j.jhazmat.2021.126397
98
Kim S. E. Robles-Lopez K. Cao X. Liu K. Chotani P. J. Bhabani N. et al (2021). Wnt1 lineage-specific deletion of gpr161results in embryonic midbrain malformation and failure of craniofacial skeletal development. Front. Genet.12, 761418. 10.3389/fgene.2021.761418
99
Koelmans A. A. Mohamed N. N. H. Hermsen E. Kooi M. Mintenig S. M. DE France J. (2019). Microplastics in freshwater and drinking water. Critical reviews and assessment of data quality. Water Res.155, 410–422. 10.1016/j.watres.2019.02.054
100
Kokalj A. J. Hartmann N. B. Drobne D. Potthoff A. Kuhnel D. (2021). Quality of nanoplastics and microplastics ecotoxicity studies: refining quality criteria for nanomaterial studies. J. Hazard Mater.415, 125751. 10.1016/j.j.hazmat2021.125751
101
Lambert S. Sinclair C. J. Bradly E. L. Boxall A. B. (2013). Effect of environmental conditions and latex degradation in aquatic systems. Sci. Total Environ.447, 225–234. 10.1016/scitotenv.2012.12.067
102
Lambert S. Wagner M. (2016). Characterisation of nanoplastics during the degradation of polystyrene. Chemosphere145, 265–268. 10.1016/j.chemosphere.2015.11.078
103
Lee W. S. Cho H.-J. Kim E. Huh Y. H. Kim H.-J. Kim B. et al (2019). Bioaccumulation of polystyrene nanoplastics and their effect on the toxicity of Au ions in zebrafish embryos. Nanoscale11, 3173–3185. 10.1039/c8nr90280a
104
Lee W. S. Kim H. Sim Y. Kang T. Jeong J. (2022). Fluorescent polypropylene nanoplastics for studying uptake, biodistribution, and excretion in zebrafish embryos. ACS Omega7, 2467–2473. 10.1021/acsomega1c06779
105
Lehner R. Weder C. Petri-Fink A. Rothen-Rutishauser B. (2019). Emergence of nanoplastic in the environment and possible impact on human health. Environ. Sci. Technol.53, 1748–1765. 10.1021/acs.est8b05512
106
Lei L. Liu M. Song Y. Lu S. Hu J. Cao C. et al (2018a). Polystyrene (nano)microplastics cause size-dependent neurotoxicity, oxidative damage and other adverse effects inCaenorhabditis elegans. Environ. Sci. Nano5, 2009–2020. 10.1039./C8EN00412A
107
Lei L. Wu S. Lu S. Liu M. Song Y. Fu Z. et al (2018b). Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Sci. Total Environ.619-620, 1–8. 10.1016/j.scitotenv.2017.11.103
108
Leslie H. A. Brandsma S. H. van Velzen M. J. M. Vethaak A. D. (2017). Microplastics en route: Field measurements in the Dutch river delta and Amsterdam canals, wastewater treatment plants, North Sea sediments and biota. Environ. Int.101, 133–142. 10.1016/j.envint.2017.01.018
109
Li D. Shi Y. Yang L. Xiao L. Kehoe D. K. GunKo Y. K. et al (2020). Microplastic release from the degradation of polypropylene feeding bottles during infant formula preparation. Nat. Food1, 746–754. 10.1038/s43016-020-00171-y
110
Li J. Wang Q. Cui M. Yu S. Chen X. Wang J. (2023d). Release characteristics and toxicity assessment of micro/nanoplastics from food-grade nonwoven bags. Sci. Total Environ.883, 163642. 10.1016/j.scitotenv.2023.163642
111
Li R. Nie J. Qiu D. Li S. Sun Y. Wang C. (2023c). Toxic effects of chronic exposure to polyethylene nano/microplastics on oxidative stress, neurotoxicity, and gut microbiota of adult zebrafish (Danio rerio). Chemosphere339, 139774. 10.1016/j.chemosphere.2023.139774
112
Li X. Luo J. Ham C. Lu X. (2023b). Nanoplastics enhance the intestinal damage and genotoxicity of sulfamethoxazole to medaka juveniles (Oryzias melastigma) in costal environment. Sci. Total Environ.894, 164943. 10.1016/j.scitotenv.2023.164943
113
Li X. Zheng Y. Lu L. Eom J. Ru S. Li Y. et al (2024b). Trophic transfer of micro- and nanoplastics and toxicity induced by long-term exposure of nanoplastics along the rotifer (Brachionus plicatilis)-marine medaka (Oryzias melastigma) food chain. Environ. Pollut.346, 123599. 10.1016/j.envpol.2024.123599
114
Li Y. Teng M. Zhao L. Sun J. Yan J. Zhu W. et al (2023a). Vitamin D modulates disordered lipid metabolism in zebrafish (Danio rerio) liver caused by exposure to polystyrene nanoplastics. Environ. Int.182, 108328. 10.1016/jenvint.2023.108328
115
Li Z. Chen F. Wei M. Zhi L. Su Z. Chong Y. et al (2024a). Concurrent impacts of polystyrene nanoplastic exposure and Aeromonas hydrophila infection on oxidative stress, immune response, and intestinal microbiota of grass carp (Ctenopharyngodon idella). Sci. Total Environ.912, 169225. 10.1016/j.scitotenv.2023.169225
116
Liang B. X. Zhong Y. Z. Huang Y. J. Lin X. Liu J. Lin L. et al (2021). Underestimated health risks: polystyrene micro- and nanoplastics jointly induce intestinal barrier dysfunction by ROS-mediated epithelial cell apoptosis. Part Fibre Toxicol.18, 20. 10.1186/s12989-021-00414-1
117
Lin S. Zhang H. Wang C. Su X.-L. Song Y. Wu P. et al (2022). Metabolomics reveal nanoplastic-induced mitochondrial damage in human liver and lung cells. Environ. Sci. Technol.56, 12483–12493. 10.1021/acsest2c03980
118
Lin X. Wang Y. Yang X. Watson P. Yang F. Liu H. (2023). Endocrine disrupting effect and reproductive toxicity of the separate exposure and co-exposure of nano-polystyrene and diethylstilbestrol to zebrafish. Sci. Total Env.865, 161100. 10.1016/j.scitotenv.2022.161100
119
Ling X. Zuo J. Pan M. Nie H. Shen J. Yang Q. et al (2022). The presence of polystyrene nanoplastics enhances the MCLR uptake in zebrafish leading to the exacerbation of oxidative liver damage. Sci. Total Env.818, 151749. 10.1016/jscitotenv.2021.151749
120
Lins T. F. O’Brien A. M. Zargartalebi M. Sinton D. (2022). Nanoplastic state and fate in aquatic environments: multiscale modeling. Environ. Sci. Technol.56, 4017–4028. 10.1021/acs.est.1c03922
121
Liu S. Yan L. Zhang Y. Junaid M. Wang J. (2022a). Polystyrene nanoplastics exacerbated the ecotoxicological and potential carcinogenic effects of tetracycline in juvenile grass carp (Ctenopharyngodon idella). Sci. Total Environ.803, 150027. 10.1016/j.scitotenv.2021.150027
122
Liu Y. Wang Y. Li N. Jiang S. (2022b). Avobenzone and nanoplastics affect the development of zebrafish nervous system and retinal system and inhibit their locomotor behavior. Sci. Total Environ.806, 150681. 10.1016/j.scitotenv.2021.150681
123
Liu Y. Wang Y. Ling X. Yan Z. Wu D. Liu J. et al (2021). Effects of nanoplastics and butyl methoxydibenzoylmethane on early zebrafish embryos identified by single-cell RNA sequencing. Environ. Sci. Technol.55, 1885–1896. 10.1021/acs.est.0c06479
124
Lu Y. Zhang Y. Deng Y. Jiang W. Zhao Y. Geng J. et al (2016). Uptake and accumulation of polystyrene microplastics in zebrafish (Danio rerio) and toxic effects in liver. Environ. Sci. Technol.50, 4054–4060. 10.1021/acs.est.6b00183
125
Ma Y. Huanga A. cao S. Sun F. Wang L. Guo H. et al (2016). Effects of nanoplastics and microplastics on toxicity, bioaccumulation, and environmental fate of phenanthrene in fresh water. Environ. Pollut.219, 166–173. 10.1016/j.envpol.2016.10.061
126
Mahon A. M. O’Connel B. Healy M. G. O’Conner L. Officer R. Nash R. et al (2017). Microplastics in sewage sludge: effects of treatments. Environ. Sci. Technol.51, 810–818.
127
Maity S. Pramanick K. (2020). Perspectives and challenges of micro/nanoplastics-induced toxicity with special reference to phytotoxicity. Biol26, 3241–3250. 10.1111/gcb15074
128
Mantovani A. Dinarello C. A. Molgora M. Garlanda C. (2019). Interleukin-1 and related cytokines in the regulation of inflammation and immunity. Immunity50, 778–795. 10.1016/j.immuni.2019.03.012
129
Manuel P. Almeida M. MartinsM O. M. (2022). Effects of nanoplastics on zebrafish embryo-larval stages: a case study with polystyrene (PS) and polymethylmethacrylate (PMMA) particles. Environ. Res.213, 113584. 10.1016/j.envres.2022.113584
130
Margi D. Veronesi M. Sanchez-Moreno P. Tolardo V. Bandaera T. Pompa P. P. et al (2021). PET nanoplastics interactions with water contaminants and their impact on human cells. Environ. Pollut.271, 116262. 10.1016/j.envpol.2020.116262
131
Martin L. Marbach S. Zimba P. Liu Q. Xu W. (2023). Uptake of nanoplastic particles by zebrafish embryos triggers the macrophage response at early developmental stage. Chemosphere341, 140069. 10.1016/j.chemosphere.2023.140069
132
Martinez-Alvarez I. Le Manach K. Devier M.-H. Cajaraville M. P. Budzinski H. Orbea A. (2022). Screening of the toxicity of polystyrene nano- and microplastics alone and in combination with benzo(a)pyrene in brine shrimp larvae and zebrafish embryos. Nanomaterials12, 941. 10.3390/nano12060941
133
Martin-Folgar R. Torres-Ruiz M. de Alba M. Canax-Portilla A. I. Gonzalez M. C. Morales M. (2023). Molecular effects of polystyrene nanoplastics toxicity in zebrafish embryos (Danio rerio). Chemosphere312, 137077. 10.1016/j.chemosphere.2022.137077
134
Mates J. M. (2000). Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology153, 83–104. 10.1016/s0300-483x(00)00306-1
135
Matos B. Martins M. Samamed A. C. Sousa D. Ferreira I. Diniz M. S. (2019). Toxicity evaluation of quantum dots (ZnS and CdS) singly and combined in zebrafish (Danio rerio). Int. J. Environ. Res. Public Health17, 232. 10.3390/ijerph17010232
136
Min K. Cuiffi J. D. Mathers R. T. (2020). Ranking environmental degradation trends of plastic marine debris based on physical properties and molecular structure. Nat. Commun.11, 727. 10.1038/s41467-020-14538-z
137
Mojiri A. Zhou J. L. Lou Z. (2024). Micro(nano) plastics in aquatic environments: state of the art and beyond. Water16, 902. 10.3390/w16060902
138
Monikh F. Durao M. Kipriianov P. V. Huuskonen H. Kekalainen J. Uusi-Heikkila S. et al (2022). Chemical composition and particle size influence the toxicity of nanoscale plastic debris and their co-occurring benzo(α) pyrene in the model aquatic organisms Daphnia magna and Danio rerio. NanoImpact25, 100382. 10.1016/j.impact.2022.100382
139
O’Brine T. Thompson R. C. (2010). Degradation of plastic carrier bags in the marine environment. Mar. Pollut. Bull.60, 2279–2283. 10.1016/jmarpolbul.2010.08.005
140
Pang M. Wang Y. Tang Y. Dai J. Tong J. Jin G. (2021). Transcriptome sequencing and metabolite analysis reveal the toxic effects of nanoplastics on tilapia after exposure to polystyrene. Environ. Pollut.277, 116860. 10.1016/j.envpol.2021.116860
141
Parenti C. C. Ghilardi A. Della Torre C. Magni S. Del Giacco L. Binelli A. (2019). Evaluation of the infiltration of polystyrene nanobeads in zebrafish embryo tissues after short-term exposure and the related biochemical and behavioural effects. Environ. Pollut.254, 112947. 10.1016/j.envpol.2019.07.115
142
Park S. H. Kim K. (2022). Microplastics induced developmental toxicity with microcirculation dysfunction in zebrafish embryos. Chemosphere286, 131868. 10.1016/j.chemosphere.2021.131868
143
Patricio Silva A. L. Prata J. C. Walker T. R. Duarte A. C. Oyuang W. Barcelo D. et al (2021). Increased plastic pollution due to COVID-19 pandemic challenges and recommendations. Chem. E J.405, 126683. 10.1016/j.cej.2020.126683
144
Pedersen A. F. Meyer D. N. Petriv A.-M. V. Soto A. L. Shields J. N. Akermann C. et al (2020). Nanoplastics impact the zebrafish (Danio rerio) transcriptome: associated developmental and neurobehavioral consequences. Environ. Pollut.266 (pt2), 115090. 10.1016/j.envpol.2020.115090
145
Peng M. Felix R. C. Canario A. V. M. Power D. M. (2024). The physiological effect of polystyrene nanoplastic particles on fish and human fibroblasts. Sci. Total Environ.914, 169979. 10.1016/j.scitotenv.2024.169979
146
Pitt J. A. Kozal J. S. Jayasundara N. Massarsky A. Trevisan R. Geitner N. et al (2018a). Uptake tissue distribution and toxicity of polystyrene nanoparticles in developing zebrafish (Danio rerio). Aquat. Toxicol.194, 185–194. 10.1016/jaquatox.2017.11017
147
Pitt J. A. Trevisan R. Massarsky A. Kozal J. S. Levin E. D. Di Giulio R. T. (2018b). Maternal transfer of nanoplastics to offspring in zebrafish (Danio rerio). A case study with nanopolystyrene. Sci. Total Environ.643, 324–334. 10.1016/j.scitotenv.2018.06.186
148
Plastic Europe (2022). Plastics the facts 2021. An analysis of European plastic production.
149
Rochman C. M. Hoh E. H. Kurobo T. The S. J. (2013). Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Sci. Rep.3, 3263. 10.1038/srep03263
150
Saedi S. S. Thompson R. C. (2014). On the quantity and composition of floating plastic entering and leaving the Tamar estuary, southwest England Mar. Pollut. Bull.81, 55–60. 10.1016/j.marpolbul.2014.02.020
151
Saemi-Komsari M. Pashaei R. Abbasi S. Esmaeili H. R. Dzingeleviciene R. Hadavand B. S. et al (2023). Accumulation of polystyrene nanoplastics and triclosan by a model tooth-carp fish, Aphaniops hormuzensis (Teleostei: aphaniidae). Environ. Pollut.333, 121997. 10.1016/jenvpol.2023.121997
152
Santos A. L. Rodrigues L. C. Rodrigues C. C. Cirqueria F. Malafaia G. Rocha T. L. (2024). Polystyrene nanoplastics induced developmental impairments and vasotoxicity in zebrafish (Danio rerio). J. Hazard Mat.464, 880. 10.1016/j.hazrdmat.2023.132880
153
Santos J. Barreto A. Sousa E. M. L. Calisto V. Amorim M. J. B. Maria V. L. (2022). The role of nanoplastics on the toxicity of the herbicide phenmedipham, using Danio rerio embryos as model organisms. Environ. Pollut.303, 119166. 10.1016/j.envpol.2022.119166
154
Sarasamma S. Audira G. Siregar P. Malhotra N. lai Y.-H. Liang Z.-T. et al (2020). Nanoplastics cause neurobehavioral impairments, reproductive and oxidative damages, and biomarker responses in zebrafish: throwing up alarms of wide spread health risk of exposure. Int. J. Mol. Sci.21, 1410. 10.3390/ijms21041410
155
Schirinzi G. F. Perez-Pomeda I. Sanchis J. Rossini C. Farre M. Barcelo D. (2017). Cytotoxic effects of commonly used nanomaterials and microplastics on cerebral and epithelial human cells. Environ. Res.159, 579–587. 10.1016/j.envres.2017.08.043
156
Schymanski D. Goldbeck C. Humpf H. U. Furst P. (2018). Analysis of microplastics in water by micro-Raman spectroscopy: release of plastic particles from different packaging into mineral water. Water Res.129, 154–162. 10.1016/j.watres.2017.11.011
157
Sendra M. Pereiro P. Yeste M. P. Mercado L. Figueras A. Novoa B. (2021). Size matters: zebrafish (Danio rerio) as a model to study toxicity of nanoplastics from cells to whole organisms. Environ. Pollut.268, 115759. 10.1016/j.envpol.2020.115769
158
Senol O. Sulukan E. Baran A. Bolat I. Toraman E. Alak G. et al (2023). Global warming and nanoplastic toxicity; small temperature increases can make gill and liver toxicity more dramatic, which affects fillet quality caused by polystyrene nanoplastics in the adult zebrafish model. Sci. Total Environ.892, 164682. 10.1016/j.scitotenv.2023.164682
159
Sharifi S. Behzadi S. Laurent S. Laird Forrest M. L. Stroeve P. Mahmoudi M. M. (2012). Toxicity of nanomaterials. Chem. Soc. Rev.41, 2323–2343. 10.1039/c1cs15188f
160
Sharpton T. J. (2018). Role of the gut microbiome in vertebrate evolution. mSystems3, e00174–e00175. 10.1128/msystems.00174-17
161
Sokmen T. Q. Sulukan E. Turkoglu M. Baran A. Ozkaraca M. Ceyhum B. (2020). Polystyrene nanoplastics (20 nm) are able to bioaccumulate and cause oxidative DNA damages in the brain tissue of zebrafish embryo (Danio rerio). Neurotoxicology77, 51–59. 10.1016/j.neuro.2019.12.010
162
Strungaru S.-A. Jijie R. Nicoara M. Plavan G. Faggio C. (2019). Micro(nano) plastics in freshwater ecosystems. Abundance, toxicological impact, and quantification methodology. Trac. Trends Anal. Chem.110, 116–128. 10.1016/j.trac.2018.10.025
163
Sudhakar M. Trishul A. Doble M. Suresh Kumar K. SyedJahan S. Inbakandan D. et al (2007). Biofouling and Biodegradation of polyolefins in ocean waters. Polymdegr. Stab.92, 1743–1752. 10.1016/j.polymdegradstab.2007.03.029
164
Sulukan E. Baran A. Senol O. Yildirim S. Mavi A. Ceyhun H. A. et al (2022b). The synergistic toxicity of temperature increases and nanopolystyrene on zebrafish brain implies that global warming may worsen the current risk based on plastic debris. Sci. Tot Environ.808, 152092. 10.1016/j.scitotenv.2021.152092
165
Sulukan E. Senol O. Baran A. Kankaynar M. Yildirim S. Kiziltan T. et al (2022a). Nano-sized polystyrene plastic particles affect many cancer-related biological processes even in the next generations, zebrafish modeling. Sci. Total Env.838, 156391. 10.1016/j.scitotenv.2022.156391
166
Suman A. Mahapatra A. Gupta P. Ray S. S. Singh R. K. (2023). Polystyrene microplastics modulated bdnf expression triggering neurotoxicity via apoptotic pathway in zebrafish embryos. Comp. Biochem. Physiol. partC271, 109699. 10.1016/jcbpc.2023.109699
167
Sun J. Xia S. Ning Y. Pan X. Qu J. Xu Y. (2019). Effects of microplastics and attached heavy metals on growth, immunity, and heavy metal accumulation in the yellow seahorse, Hippocampus kuda Bleeker. Mar. Pollut. Bull.149, 110510. 10.1016/j.marpolbul.2019.110510
168
Sun M. Ding R. Ma Y. Sun Q. Ren X. Sun Z. et al (2021). Cardiovascular toxicity assessment of polyethylene nanoplastics on developing zebrafish embryos. Chemosphere282, 131124. 10.1016/j.chemosphere.2021.131124
169
Tamayo-Belda M. Venancio C. Fernandez-Pinas F. Rosal R. Lopes I. Oliveira M. (2023). Effects of petroleum-based and biopolymer-based nanoplastics on aquatic organisms: a case study with mechanically degraded pristine polymers. Sci. Total Environ.883, 163447. 10.1016/j.scitotenv.2023.163447
170
Teng M. Li Y. Zhao X. White J. C. Zhao L. Sun J. et al (2023). Vitamin D modulation of brain-gut-virome disorder caused by polystyrene nanoplastics exposure in zebrafish (Danio rerio). Microbiome11, 266. 10.1186/s40168-023-01680-1
171
Teng M. Zhao X. Wang C. Wang C. White J. C. Zhao W. et al (2022b). Polystyrene nanoplastics toxicity to zebrafish: dysregulation of the brain-intestine-microbiota axis. ACS Nano< 16, 8190–8204. 10.1021/acsnano.2c01872
172
Teng M. Zhao X. Wu F. Wang C. Wang C. White J. C. et al (2022a). Charge-specific adverse effects of polystyrene nanoplastics on zebrafish (Danio rerio) development and behavior. Environ. Int.163, 107154. 10.1016/j.envint.2022.107154
173
Thompson R. C. Olsen Y. Mitchell R. P. Davis A. Rowland S. J. John A. W. et al (2004). Lost at sea: where is all the plastic. Science304, 838. 10.1126/science.1094559
174
Torres-Ruiz M. de la Vieja A. de alba Gonzalez M. LopezME C. A. C. Portilla A. S. C. Cañas Portilla A. I. (2021). Toxicity of nanoplastics for zebrafish embryos, what we know and where to go next. Sci. Total Environ.797, 149125. 10.1016/jscitotenv.2021.149125
175
Trevisan R. Uzochukwu D. Di Giulio R. T. (2020). PAH soeption to nanoplastics and the Trojan horse effect as drivers of mitochondrial toxicity and PAH localization in zebrafish.
176
Trevisan R. Voy C. Chen S. Di Giulio R. T. (2019). Nanoplastics decrease the toxicity of a complex PAH mixture but impair mitochondrial energy production in developing zebrafish. Environ. Sci. Technol.53, 8405–8415. 10.1021/acs.est.9b02003
177
Triebskorn R. Braunbeck T. Grummt T. Hanslik I. Huppertsberg S. Jekel M. et al (2019). Relevance of nano- and microplastics for freshwater ecosystems: a critical review. Trends Anal. Chem.110, 375–392. 10.1016/j.trac.2018.11.023
178
Vanapalli K. R. Sharma H. B. Ranjan V. P. Samal B. Bhattacharya J. Dubey B. K. et al (2021). Challenges for strategies for effective plastic waste management during and post COVID-19 pandemic. Sci. Total Environ.250, 141514. 10.1016/j.scitotenv.2020.141514
179
Van Pomeren M. Brun N. R. Peijnenburg WJGM Vijver M. G. (2017). Exploring uptake and biodistribution of polystyrene (nano)particles in zebrafish embryos at different developmental stages. Aquat. Toxicol.190, 40–45. 10.1016/j.aquatox.2017.06.017
180
Varshney S. Gora A. H. Kiron V. Siriyappagouder P. Dahle D. Kogel T. et al (2023). Polystyrene nanoplastics enhance the toxicological effects of DDE in zebrafish (Danio rerio) larvae. Sci. Total Environ.859, 160457. 10.1016/j.scitotenv.2022.160457
181
Venancio C. Melnic L. Tamayo-Belda M. Oliveira M. Martins M. A. Lopes I. (2022). Avian scavengers' contributions to people: the cultural dimension of wildlife-based tourism. Sci. Total Environ.806, 150419. 10.1016/j.scitotenv.2021.150419
182
Vendel A. J. Bessa F. Alves V. E. N. Amorim A. L. A. Patrico J. Palma A. R. T. (2017). Widespread microplastic ingestion by fish assemblages in tropical estuaries subjected to anthropogenic pressures. Mar. Pollut. Bull117117, 448–455. 10.1016/j.marpolbul.2017.01.081
183
Vethaak A. D. Leslie H. A. (2016). Plastic debris is a human health issue. Environ. Sci. Technol50, 6825–6826. 10.1021/acs.est.6b02569
184
Villacorta A. Rubio L. Alaraby M. Lopez-Mesas M. Fuentes-Cebrian V. Moriones O. H. et al (2022). A new source of representative secondary PET nanoplastics. Obtention, characterization, and hazard evaluation. J. Hazard Mater.439, 129593. 10.1016/j.jhazmat.2022.129593
185
Wagner M. Engwall M. Hollet H. (2014). (Micro) plastics in the environment. Environ. Sci. Eur.26, 16. 10.1186/s12302-014-0016-3
186
Wang F. Zhang C. Cai S. Yang Z. Li F. Liu X. et al (2023a). Incomplete recovery of gut microbiota in marine medaka (Oryzias melastigma) during the depuration phase, after exposure to sulfamethazine/nanoplastics. Sci. Total Environ.893, 164841. 10.1016/J.scitotenv.2023.164841
187
Wang L. Zhu Y. Gu J. Yin X. Guo L. Qian L. et al (2023e). The toxic effect of bisphenol AF and nanoplastic coexposure in parental and offspring generation zebrafish. Ecotoxicol. Environ. Saf.251, 114565. 10.1016/j.ecoenv.2023.114565
188
Wang Q. Chen G. Tian L. Kong C. Gao D. Chen Y. et al (2023c). Neuro- and hepato-toxicity of polystyrene nanoplastics and polybrominated diphenyl ethers on early life stages of zebrafish. Sci. Total Environ.857, 159567. 10.1016/j.scitotenv,2022.159567
189
Wang Q. Li Y. Chen Y. Tian L. Gao D. Liao H. et al (2022). Toxic effects of polystyrene nanoplastics and polybrominated diphenyl ethers to zebrafish (Danio rerio). Fish. Shellfish Immunol.126, 21–33. 10.1016/j.fsi.2022.05.025
190
Wang W. Mao X. Zhang R. Zhou X.-X. Lio Y. Zhou H. et al (2023b). Nanoplastic exposure at environmental concentrations disrupts hepatic lipid metabolism through oxidative stress induction and endoplasmic reticulum homeostasis perturbation. Environ. Sci. Technol.57, 14127–14137. 10.1021/acs.est.3c02769
191
Wang Y. Wang J. Cong J. Zhang H. Gong Z. Sun H. et al (2023d). Nanoplastics induce neuroexcitatory symptoms in zebrafish (Danio rerio) larvae through a manner contrary to Parkinsonian’s way in proteomics. Sci. Total Environ.905, 166898. 10.1016/j.scitotenv.2023.166898
192
Watters D. L. Yoklavich M. M. Love M. S. Schroeder D. M. (2010). Assessing marine debris in deep seafloor habitats off California. Mar. Pollut. Bull.60, 131–138. 10.1016/j.marpolbul.2009.08.019
193
White E. M. Clark S. Manire C. A. Crawford B. Wang S. Locklin J. et al (2018). Ingested micronizing plastic particle compositions and size distributions within stranded post-hatchling sea turtles. Environ. Sci. Technol.52, 10307–10316. 10.1021/acs.est.8b02776
194
Wu H. Guo J. Yao Y. Xu S. (2022). Polystyrene nanoplastics induced cardiomyocyte apoptosis and myocardial inflammation in carp by promoting ROS production. Fish. Shellfish Immunol.125, 1–8. 10.1016/j.fsi.2022.04.048
195
Wu Q. Li G. Huo T. Du X. Yang Q. Hung T.-C. et al (2021). Mechanisms of parental co-exposure to polystyrene nanoplastics and microcystin-LR aggravated hatching inhibition of zebrafish offspring. Sci. Total Envirn774, 145766. 10.1016/j.scitotenv.2021.145766
196
Wu X. Li M. Yang S. Dong J. Pan W. Ning Y. et al (2023). Liver metabolic dysregulation induced by polypropylene nano- and microplastics in Nile Tilapia using internal extractive electrospray ionization mass spectrometry. Anal. Chem.95, 7863–7871. 10.1021/acs.analchem.2c05672
197
Xie S. Zhou A. Wei T. Li S. Yang B. Xi G. et al (2021). Nanoplastics induce more serious microbiota dysbiosis and inflammation in the gut of adult zebrafish than microplastics. Bull. Env. Contamin Toxicol.107, 640–650. 10.1007/s00128-021-03348-8
198
Xu J.-L. Lin X. Wang J. J. Gowen A. A. (2022). A review of potential human health impacts of micro-and nanoplastics exposure. Sci. Total Environ.10 (pt 1), 158111. 10.1016/j.scitotenv.2022.158111
199
Yang L. Tang B. Z. Wang W.-X. (2023). Near-infrared-II in vivo visualization and quantitative tracking of micro/nanoplastics in fish. ACS Nano17, 19410–19420. 10.1021/acsnano.3c07571
200
Yang M. Wang W.-X. (2022). Differential cascading cellular and subcellular toxicity induced by two sizes of nanoplastics. S. C. Total Environ.829, 154593. 10.1016/j.scitotenv.2022.154593
201
Yang M. Wang W.-X. (2023). Recognition and movement of polystyrene nanoplastics in fish cells. Environ. Pollut.316, 120627. 10.1016/j.envpol.2022.120627
202
Ye R. Li Z. Xian H. Zhong Y. Liang B. Huang Y. et al (2024). Combined effects of polystyrene nanosphere and homosolate exposures on estrogenic end points in MCF-7 cells and zebrafish. Environ. Health Persp132, 27011. 10.1289/EHP13696
203
Yu F. Jin F. Cong Y. Lou Y. Li Z. Li R. et al (2023). Bisphenol A decreases the developmental toxicity and histopathological alterations caused by polystyrene nanoplastics in developing marine medaka Oryzias melastigma. Chemosphere336, 139174. 10.1016/j.chemosphere.2023.139174
204
Yu J. Chen L. Gu W. Liu S. Wu B. (2022a). Heterogeneity effects of nanoplastics and lead on zebrafish intestinal cells identified by single cell sequencing. Chemosphere289, 133133. 10.1016/j.chemosphere.2021.133133
205
Yu Z. Zhang L. Huang Q. Dong S. Wang X. Yan C. (2022b). Combined effects of micro-/nano-plastics and oxytetracycline on the intestinal histopathology and microbiome in zebrafish (Danio rerio). Sci. Total Environ.843, 156917. 10.1016/j.scitotenv.2022.156917
206
Yudell M. Roberts D. DeSalle R. Tishkoff S. (2020). Accumulation of plastic waste during COVID-19. Science369 (80-) 369), 1314–1315. 10.1126/SCIENCE.ABD9925
207
Zhang C. Bao F. Wang F. Xue Z. Lin D. (2024a). Toxic effects of nanoplastics and microcystin-LR coexposure on the liver-gut axis of Hypophthalmichthys molitrix. Sci. Tot Environ.916, 170011. 10.1016/j.scitotenv.2024.170011
208
Zhang C. Li Y. Yu H. Li T. Ye L. Zhang X. et al (2024c). Co-exposure of microplastics and arsenic causes neurotoxicity in zebrafish (Danio rerio) through disrupting homeostasis of microbiota-intestine-brain axis. Sci. Total Env.912, 169430. 10.1016/j.scitotenv.2023.169430
209
Zhang C. Li Y. Yu H. Ye L. Li T. Zhang X. et al (2023). Nanoplastics promotes arsenic-induced ROS accumulation, mitochondrial damage, and disturbances in neurotransmitter metabolism of zebrafish (Danio rerio). Sci. Total Environ.863, 161005. 10.1016/j.scitotenv.2022.161005
210
Zhang C. Zuo Z. Wang Q. Wang S. Lv L. Zou J. (2022b). Size effects of microplastics on embryos and observation of toxicity kinetics in larvae of grass carp (Ctenopharyngodon idella). Toxics10, 76. 10.3390/toxics10020076
211
Zhang H. Zhang S. Duan G. Wang L. (2022a). Pulmonary toxicology assessment of polyethylene terephthalate nanoplastic particles in vitro. Environ. Int.162, 107177. 10.1016/j.envint.2022.107177
212
Zhang R. Silic M. R. Schaber A. Wasel O. Freeman J. L. Sepulveda M. S. (2020). Exposure route affects the distribution and toxicity of polystyrene nanoplastics in zebrafish. Sci. Total Environ.724, 138065. 10.1016/j.scitotenv.2020.138065
213
Zhang X. Xia M. Zhao J. Cao Z. Zou W. Zhou Q. (2022c). Photoaging enhanced the adverse effects of polyamide microplastics on the growth, intestinal health, and lipid absorption in developing zebrafish. Environ. Int.158, 106922. 10.1016/j.envint.2021.106922
214
Zhang Y. T. Chen H. He S. Wang F. Liu Y. Chen M. et al (2021). Subchronic toxicity of dietary sulfamethazine and nanoplastics in marine medaka (Oryzias melastigma): insights from the gut microbiota and intestinal oxidative status. Ecotoxicol. Env. Saf.226, 112820. 10.1016/j.ecoenv.2021.112820
215
Zhang Y. T. Zhang Z. Zhang M. Zhang C. Chen H. Wang F. et al (2024b). Surface functional groups on nanoplastics delay the recovery of gut microbiota after combined exposure to sulfamethazine in marine medaka (Oryzias melastigma). Aqua. Toxicol.267, 106813. 10.1016/j.aquatox.2023.106813
216
Zhao X. Liu Z. Ren X. Duan X. (2021). Parental transfer of nanopolystyrene-enhanced tris (1,3-dichloro-2-propyl) phosphate induces transgenerational thyroid disruption in zebrafish. Aqu. Tox236, 105871. 10.1016/j.aquatox.2021.105871
217
Zheng S. Tang B. Z. Wang W.-X. (2024). Microplastics and nanoplastics induced differential respiratory damage in tilapia fish Oreochromis niloticus. J. Hazrd Mat.465, 133181. 10.1016/j.jhazmat.2023.133181
218
Zheng S. Wang W.-X. (2024). Single cell RNA sequencing profiling cellular heterogeneity and specific responses of fish gills to microplastics and nanoplastics. Environ. Sci. Technol.58, 5974–5986. 10.1021/acs.est.3c10338
219
Zhou R. Zhou D. Yang S. Shi Z. Pan H. Jin Q. et al (2023c). Neurotoxicity of polystyrene nanoplastics with different particle sizes at environment-related concentrations on early zebrafish embryos. Sci. Total Environ.872, 162096. 10.1016/j.scitotenv.2023.162096
220
Zhou W. Tong D. Tian D. Yu Y. Huang L. Zhang W. et al (2023d). Exposure to polystyrene nanoplastics led to learning and memory deficits in zebrafish by inducing oxidative damage and aggravating brain aging. Adv. Healthc. Mater17, 2301799. 10.1002/adhm.202301799
221
Zhou Y. Gui L. Wei W. Xu E. G. Zhou W. Sokolova I. M. et al (2023b). Low particle concentrations of nanoplastics impair the gut health of medaka. Aquat. Toxicol.256, 106422. 10.1016/j.aquatox.2023.106422
222
Zhou Y. Jin Q. Xu H. Wang Y. Li M. (2023a). Chronic nanoplastic exposure induced oxidative and immune stress in medaka gonad. Sci. Total Environ.869, 161838. 10.1016/j.scitotenv.2023.161838
223
Zuo J. Huo T. Du X. Yang Q. Wu Q. Shen J. et al (2021). The joint effect of parental exposure to microcystin-LR and polystyrene nanoplastics on the growth of zebrafish offspring. J. Hazard Mat.410, 124677. 10.1016/j.jhazmat.2020.124677
Summary
Keywords
nanoplastics, fish, oxidative stress, genotoxic effects, development, intergenerational effects
Citation
Dasmahapatra AK, Chatterjee J and Tchounwou PB (2025) A systematic review of the effects of nanoplastics on fish. Front. Toxicol. 7:1530209. doi: 10.3389/ftox.2025.1530209
Received
19 November 2024
Accepted
24 March 2025
Published
30 May 2025
Volume
7 - 2025
Edited by
Marisa Passos, RWTH Aachen University, Germany
Reviewed by
Yue Ge, United States Environmental Protection Agency (EPA), United States
Arianna Giorgetti, University of Bologna, Italy
Updates
Copyright
© 2025 Dasmahapatra, Chatterjee and Tchounwou.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paul B. Tchounwou, paul.tchounwou@morgan.edu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.