- 1Health and Environmental Sciences Institute, Washington, DC, United States
- 2L’Oréal Research and Innovation, Clark, NJ, United States
- 3Frankel Cardiovascular Center Cell Regeneration Core/Internal Medicine-Cardiology, University of Michigan, Ann Arbor, MI, United States
- 4innoVitro GmbH, Juelich, Germany
- 5Consultant, Beaufort, SC, United States
- 6Stanford Cardiovascular Institute, Stanford University, Stanford, CA, United States
- 7FUJIFILM Cellular Dynamics, Inc., Madison, WI, United States
- 8Food and Drug Administration, Human Foods Program, Office of Food Chemical Safety Dietary Supplements and Innovation, College Park, MD, United States
- 9Food and Drug Administration, Human Foods Program, Office of Chemistry and Toxicology, Laurel, MD, United States
- 10Neurotoxicology Research Group, Division of Toxicology, Institute for Risk Assessment Sciences (IRAS), Faculty of Veterinary Medicine, Utrecht University, Utrecht, Netherlands
Botanicals (e.g., extracts derived from plants, algae, or fungi) are increasingly utilized by consumers with the hope of enhancing their health, managing symptoms, or preventing ailments; however, these products have often had limited pre-market toxicity testing. Traditional toxicity testing (e.g., rodent testing) is complicated by the nature of botanicals as complex mixtures and the potential for lot-to-lot variability in chemical constituents. Cardiotoxicity is a key area of concern, as adverse effects on the cardiovascular system can have severe consequences, and although not commonly reported, there have been reports of adverse cardiac events. New approach methodologies (NAMs) offer human-relevant, efficient, innovative, and cost-effective solutions for evaluating the cardiotoxicity of botanicals. The Botanical Safety Consortium (BSC) was established to focus on identifying suitable NAMs to screen for potential toxicities associated with these widely used products. This manuscript outlines the BSC Cardiotoxicity Working Group’s approach for evaluating NAMs for assessing the potential cardiotoxicity of botanicals. These NAMs leverage in vitro models, such as human-induced pluripotent stem cell-derived cardiomyocytes, and techniques like microelectrode arrays, voltage and calcium optical mapping, contractile force measurement, and mitochondrial function assays to evaluate botanical-induced effects on the cardiovascular system. Using well-characterized botanical extracts as case studies, the BSC aims to refine a toolkit for high-throughput and human-relevant cardiotoxicity screening. This foundational work supports the broader goal of improving botanical safety assessment practices and advancing the application of NAMs in regulatory toxicology.
1 Introduction
The use of botanical supplements, herbal remedies, and various botanical-based products has become widespread across the globe. Many consumers use these products with the hope of enhancing their health, managing symptoms, or preventing ailments. In the United States alone, the market for botanical dietary supplements reached an estimated $12.5 billion in 2023 (Smith et al., 2024). While not common, some botanical products have caused unintended adverse effects (Di Lorenzo et al., 2015). In severe cases, these effects led to permanent organ damage or even fatalities, underscoring the importance of screening botanical products to ensure their safety. Unlike other industries, where pre-market testing and assessments are standard, regulatory actions related to botanicals often occur reactively after reports of adverse events (EFSA Scientific Committee, 2009; Institute of Medicine and National Research Council of the National Academies, 2005). As such, most botanicals do not undergo specific toxicity assessments (Bent, 2008), including cardiotoxicity, leaving potential hazards unknown (Geller et al., 2015).
Assessing the safety of botanicals, particularly botanical extracts, can be difficult due to their complex and highly variable chemistry. Botanical extracts derived from plants, algae, or fungi may contain hundreds of different chemical constituents (Mitchell et al., 2022; Pyne et al., 2019). In addition, the chemical composition of a botanical extract can vary even within products sourced from the same species based on things like growing conditions, harvesting practices, manufacturing processes, and potential contamination or adulteration (Bruni and Sacchetti, 2009; Kulić et al., 2022). The inherent variation in botanical extracts makes it difficult to identify just one sample for testing in traditional animal tests with rodents or other models, as one sample may not represent the variety found in products that are available for purchase (Rombolà et al., 2020). Testing multiple samples would require considerable resources in terms of cost, time, and animal use. In addition, for cardiotoxicity, traditional methods using experimental rodent models do not always offer sufficient relevance to human biology (Beeton, 2018; Glukhov et al., 2010; Kaese and Verheule, 2012; US FDA, 2005). There is a growing demand for predictive techniques capable of efficiently evaluating cardiotoxicity in complex mixtures using rapid and resource-conscious methods. Furthermore, regulatory agencies in the United States and other jurisdictions are prioritizing the adoption of alternatives to animal testing in toxicological assessments (Reddy et al., 2023).
In response to this need, the Botanical Safety Consortium (BSC) was formed to explore and evaluate new approach methodologies (NAMs) that could help identify adverse effects induced by botanicals, including, but not limited to, potential cardiotoxicity (Mitchell et al., 2022). The BSC comprises experts from diverse fields, such as toxicology, in vitro methods, analytical chemistry, and pharmacognosy, across academia, industry, and government. Within the BSC, the Cardiotoxicity Working Group is focused on established in vitro models that may be used to screen for cardiotoxic effects. Other groups in the BSC are focused on genotoxicity, hepatotoxicity, developmental and reproductive toxicity, neurotoxicity, and dermal toxicity (Mitchell et al., 2022).
The Cardiotoxicity Working Group selected specific in vitro assays and botanical case studies to evaluate whether these methods are appropriate for assessing the cardiotoxic potential of botanicals as complex mixtures. This manuscript describes the approach taken by the group and includes an overview of the selected botanicals known to have cardiotoxicity potential (or lack thereof) and a discussion of relevant assays used already in single-chemical toxicity testing. These assays are being adapted to fit the complex nature of botanicals as mixtures, with the goal of advancing the understanding and safety assessment of botanicals in this crucial area of cardiotoxicity. The selected NAMs are intended to be incorporated into a larger battery of assays that could be used to comprehensively evaluate botanical safety. While currently limited to the selected screening assays, the working group recognizes the need for a broader range of tools, which can evolve over time.
Cardiovascular toxicity encompasses adverse effects on the heart and blood vessels, which may arise through direct exposure to toxicants or indirectly via toxic metabolites or inflammatory agents. (Berridge et al., 2024). The heart’s structure and function are complex, with numerous possible targets for adverse effects from botanicals (Figure 1). Currently, the primary focus of the BSC Cardiotoxicity Working Group is on adverse effects directly impacting the heart muscle cells (cardiomyocytes). However, botanicals may also affect other types of cells in the heart (e.g., endothelial cells, fibroblasts, nodal cells, leukocytes), the cardiac vasculature, and the pulmonary and systemic vascular systems. Key cardiac functions of cardiomyocytes that can be compromised include cardiac rhythm, left-ventricular contraction, and myocardial injury. These adverse effects can have serious consequences, impacting quality of life or even posing life-threatening risks, underscoring the importance of understanding and mitigating potential cardiotoxic effects associated with botanicals.
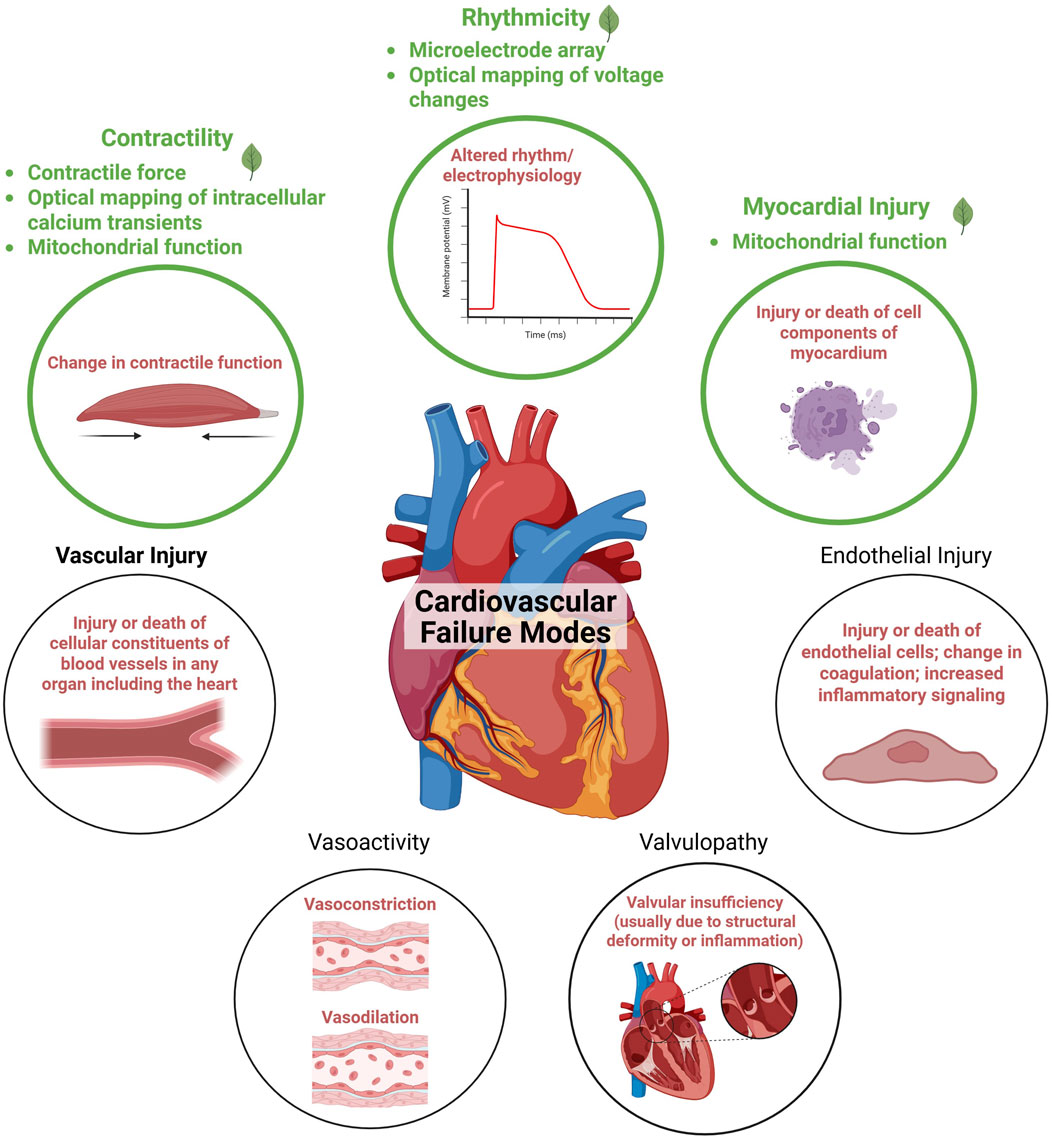
Figure 1. Adapted from the cardiovascular failure modes described in Berridge et al. (2024). Endpoints currently prioritized by the Botanical Safety Consortium’s cardiotoxicity working group are highlighted in green. Figure created in BioRender.
Given the prevalence of cardiovascular disease and its significant contribution to morbidity and mortality, safeguarding cardiac health is a priority in both clinical and public health contexts. Cardiovascular disease remains the leading cause of death for men, women, and most racial and ethnic groups in the United States, accounting for approximately 20% of all deaths in the United States in 2022, with over 700,000 deaths (Martin et al., 2024). Although reports of botanical-induced cardiotoxicity are uncommon, this does not imply safety, as many botanicals on the market have not undergone cardiotoxicity evaluations. Examples below describe instances where botanicals have led to cardiotoxicity. Without routine testing, there remains a risk of potential cardiac effects. Indeed, studies have linked some botanicals to cardiotoxicity (Bhalla et al., 2015; Puschner, 2012).
The Cardiotoxicity Working Group of the BSC focuses on developing efficient screening strategies to aid in identifying botanicals with potential cardiotoxicity. Their work involves selecting and evaluating NAMs to investigate if they can accommodate botanicals as complex chemical mixtures. Using candidate botanicals chosen based on known or suspected cardiotoxicity, the working group is establishing a series of case studies to evaluate these new methodologies. This manuscript describes the approach taken by the Cardiotoxicity Working Group and includes an overview of botanicals known to have cardiotoxicity potential or no known cardiotoxicity and a discussion of relevant tools traditionally used for single-chemical toxicity testing. These tools are being adapted to fit the nature of botanicals as complex mixtures to advance the understanding and safety assessment of botanicals in this crucial area of cardiotoxicity.
2 Selected assays for cardiotoxicity screening
To effectively screen for botanical-induced cardiotoxicity, there is a need for a battery of assays that capture a range of mechanisms relevant to the heart. The proposal outlined here will evaluate multiple tools for their potential to screen for the cardiotoxicity of botanicals and investigate if there are considerations unique to botanicals as complex mixtures (Figure 2). The assays included in the current battery were suggested and selected by experts in cardiac in vitro tools, most with experience from initiatives like the Comprehensive In vitro Proarrhythmia Assay (CiPA), which seeks to redefine preclinical cardiac safety assessment for new drugs. When selecting assays, experts identified tools that were established and reproducible, relevant for key mechanisms associated with botanical-induced cardiotoxicity, and accessible.
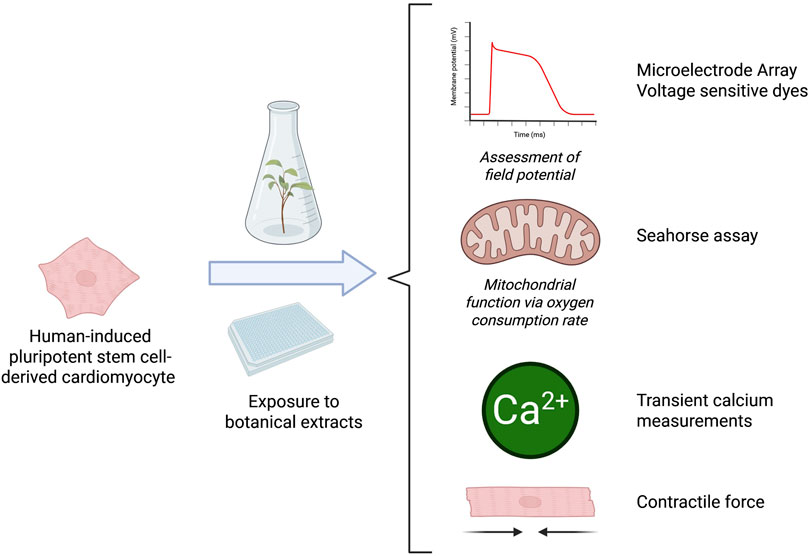
Figure 2. Overview of the model system and experimental setups used to determine the suitability of these assays for botanicals. Microelectrode Array (MEA) recordings can be used to assess cardiac-modulatory potential of botanical extracts including changes in several functional parameters, like beat rate, field potential duration, and spike amplitude. Mitochondrial function assessment is used to assess mitochondrial dysfunction in cardiomyocytes that could contribute to impaired ATP production, increased oxidative stress, and cell death. The optical mapping of transient calcium measurements can provide insights into calcium-handling abnormalities associated with arrhythmogenesis, contractile dysfunction, and other cardiac pathologies. The contractile force assay provides key insights into potential effects on the contractile properties of heart cells. Figure created in BioRender.
For assay qualification in cardiotoxicity studies, sematilide, quinidine, and procainamide are appropriate positive controls because they produce reproducible, mechanistically informative perturbations of cardiac electrophysiology: sematilide is a class III IKr blocker that prolongs action potential duration, while quinidine and procainamide are class Ia agents that primarily inhibit fast Na+ current with additional repolarization effects that lengthen QT (the time between the start of ventricular depolarization and the end of repolarization; prolongation of QT interval can indicate an increased risk for arrhythmia) and can unmask proarrhythmic liability; inclusion of such mechanistically diverse reference drugs is consistent with best-practice recommendations for repolarization studies in human stem cell-derived cardiomyocyte assays (Gintant et al., 2020).
2.1 In vitro cell lines
Most of the assays selected for this effort utilized human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CM). Some safety pharmacology screen assays use other cell lines, including human embryonic kidney (HEK)293. Although hiPSC-CMs often display fetal-like phenotypes [e.g., low expression of inward rectifying potassium channels or a more depolarized resting membrane potential (Sala et al., 2017a; Garg et al., 2018; Savoji et al., 2019)], they express all major cardiac ion channels found in adult cardiomyocytes (Garg et al., 2018; Karakikes et al., 2015). Furthermore, hiPSC-CMs are widely being evaluated as an alternative model for cardiac safety assessment, such as in the Comprehensive In vitro Proarrhythmia Assay (CiPA) initiative (Blinova et al., 2018).
Although hiPSC-CM assays have been extensively used to test drugs (Ma et al., 2024; Malan et al., 2016; Yanagida et al., 2024; Yanagida and Kanda, 2024; Zeng et al., 2019; Zhao et al., 2018) and to inform authorities of cardiac safety in investigational new drug applications (Yang et al., 2022), the use of these assays to detect cardiotoxicity induced by complex mixtures such as environmental samples or plant extracts has not been extensively reported and still requires testing and evaluation (Burnett et al., 2021).
2.2 Microelectrode array
Microelectrode array (MEA) recordings provide a non-invasive, kinetic method to study cardiomyocyte function by recording extracellular field potentials in hiPSC-CM monolayers (Figure 3) (Sala et al., 2017b). This approach assesses the impact on multiple cardiac targets, including ion channels, using spontaneously active hiPSC-CMs cultured on MEAs as a physiologically relevant model for cardiotoxicity assessment (Sala et al., 2017a). The field potentials recorded with the MEA correlate to intracellular membrane action potentials typically measured using patch-clamp recording. Moreover, drug-induced effects on field potentials correlate well with standard functional cardiac electrophysiology methods (Harris et al., 2013).
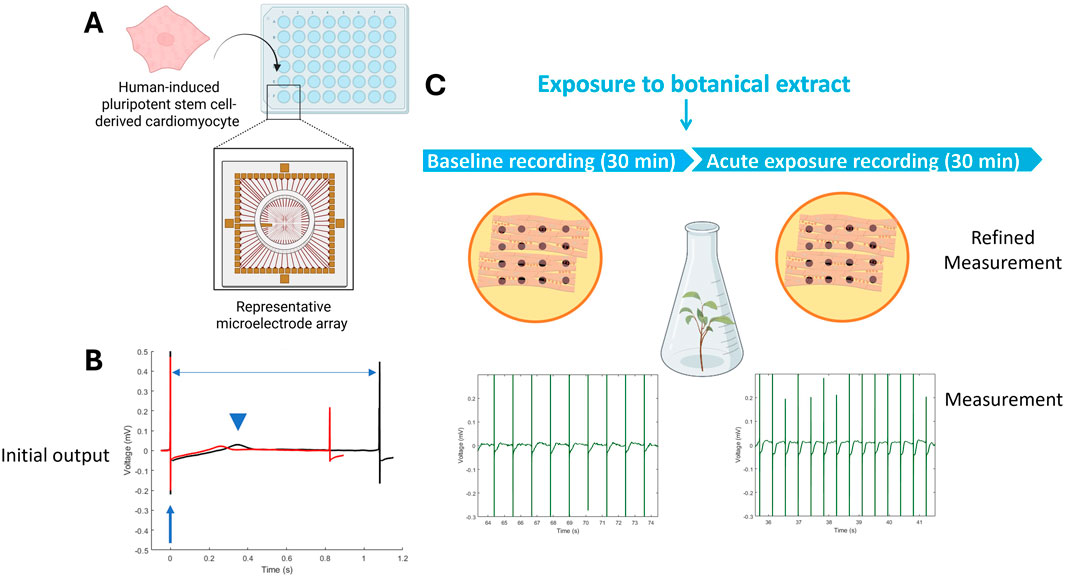
Figure 3. Microelectrode Array (MEA) recordings rely on 48-well or 96-well plates that have 8–16 electrodes on the bottom of each well, on top of which cardiomyocytes can be cultured (A). Cardiac function is recorded as an electrical signal with distinct features, such as the depolarization peak (arrow), repolarization (arrowhead), and beat period (horizontal arrow) that can change between baseline (black) and exposure (red) (B). The cardiac-modulatory potential of botanical extracts can be assessed by comparing activity before (i.e., baseline recording) and after acute exposure (C). Figure created in BioRender.
While MEAs provide useful functional readouts, they cannot identify precise molecular targets, so complementary assays are often needed for mechanistic clarity. They are also only moderate in throughput compared to simpler screening methods, which limits their use for very large libraries. Finally, although MEA parameters map to electrocardiogram (ECG)-like features, translating these signals into clinical outcomes remains uncertain without considering human absorption, metabolism, and exposure. The extracellular field potential recordings allow for the investigation of several functional parameters, including beat rate, field potential duration, and spike amplitude. These parameters closely resemble the heart rate, QT interval, and QRS amplitude (the height of a wave or deflection on an ECG graph that represents ventricular depolarization; important in the diagnosis of ventricular hypertrophy) in an ECG, thereby enabling translation of in vitro findings to in vivo effects. Consequently, hiPSC-CMs cultured on MEA recordings hold great potential to study drug-induced electrophysiological alterations, torsadogenic potential, and arrhythmias (Andrysiak et al., 2021; Asakura et al., 2015; Blinova et al., 2018; Kanda et al., 2018; Satsuka and Kanda, 2020; Takasuna et al., 2020; Zhao et al., 2022).
Recently, hiPSC-CMs monolayers cultured on MEAs have been used to detect proarrhythmic effects of plant extracts (Evodia rutaecarpa preparations containing different amounts of the human ether-a-go-go related gene (hERG) inhibitors dehydroevodiamine and hortiamine). Using this approach, it was shown that the extracts dose-dependently prolonged the field potential duration (Baltov et al., 2023). This recent study highlights the applicability of hiPSC-CMs cultured on MEAs for predicting human cardiotoxicity by plant extracts.
2.3 Optical mapping of voltage changes
Voltage-sensitive dyes allow for the assessment of membrane potential changes through optical imaging. These dyes (such as electrochromism-based styryl dyes and dyes that rely on photo-induced electron transfer) bind to the external surface of cell membranes without disrupting function, and their fluorescence intensity varies with changes in membrane potential (Chemla and Chavane, 2010; O’Shea et al., 2020). This method enables the evaluation of action potential waveform and duration in hiPSC-CMs, providing insights relevant to QT interval assessment (Figure 4).
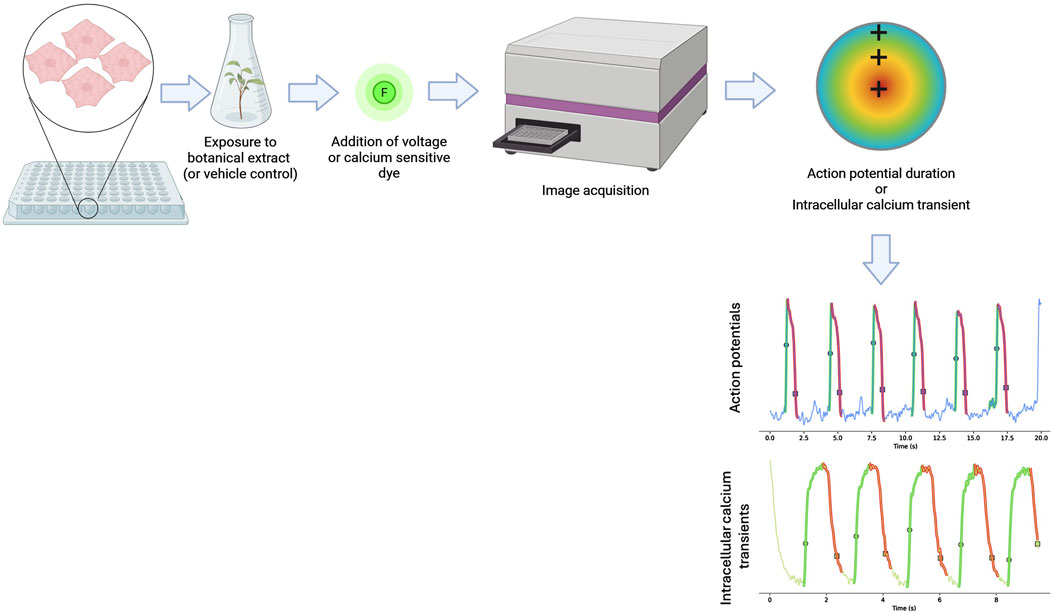
Figure 4. Schematic diagram showing how optical mapping is conducted. Human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) are plated onto well plates and exposed to a botanical extract or a solvent control for a period of time before a voltage or calcium-sensitive dye is added. The activation of the dye by the depolarizing hiPSC-CMs is then measured and used to measure action potentials and intracellular calcium transients to capture key electrophysiological parameters. Figure created in BioRender.
Optical mapping for investigating drug-induced changes in action potentials has been validated in multicenter and international consortium studies (Blinova et al., 2018; 2017; Kanda et al., 2018). While MEA have been widely used for arrhythmogenic assessment, studies indicate that optical mapping can provide higher spatiotemporal resolution (Gintant et al., 2020). Optical mapping can capture key electrophysiological parameters such as action potential duration, heterogeneity, and conduction velocity using high-resolution charged-coupled device cameras. Incorporating conduction velocity into arrhythmogenesis risk assessment models has improved predictive accuracy (da Rocha et al., 2020). Given its ability to detect subtle electrophysiological alterations, optical mapping has been widely applied in pharmaceutical screening (Blinova et al., 2018; Gunawan et al., 2021; Strauss et al., 2019).
Optical mapping can be affected by dye-related artifacts such as photobleaching or cytotoxicity (O’Shea et al., 2020). While it provides detailed information on action potentials, it does not identify specific molecular targets, and the use of simplified monolayers limits its physiological relevance compared to intact cardiac tissue. While extensively used for single chemicals, the applicability of optical mapping for botanicals and complex chemical mixtures remains largely unexplored. Determining whether this method can reliably assess their electrophysiological effects is an area of ongoing investigation.
2.4 Optical mapping of intracellular calcium transients
Changes in intracellular calcium transients offer critical insights into cardiomyocyte function, as calcium-handling abnormalities are associated with arrhythmogenesis, contractile dysfunction, and other cardiac pathologies. Optical mapping of calcium transients is conducted in a similar manner as the optical mapping of voltage sensitive dyes, with the primary difference being the type of dye used (Figure 4). Fluorescent dyes used for the mapping of intracellular calcium transients react to the presence of calcium ions released from the plated cells. Numerous studies have used different optical imaging modalities to investigate calcium transient alterations caused by mutations, acquired diseases, and drug exposure (Allan et al., 2023; Davis et al., 2021; Di Pasquale et al., 2013; Kim J. J. et al., 2015; Lee et al., 2012; Monteiro Da Rocha et al., 2023; Shafaattalab et al., 2019).
In hiPSC-CMs, optical mapping of calcium transients has been used to evaluate responses to β-adrenergic stimulation and calcium channel blockers. For instance, the chronotropic, inotropic, and lusitropic effects of isoproterenol treatment are easily observed using this approach (Allan et al., 2023; Davis et al., 2021; Monteiro Da Rocha et al., 2023; Pioner et al., 2019). Additionally, hiPSC-CMs exhibit expected responses to calcium channel blockers, demonstrating the physiological relevance of this system (Bedut et al., 2016; da Rocha et al., 2020). Beyond pharmacological applications, calcium transient mapping has provided insights into conduction abnormalities, including unidirectional conduction block, gap junction uncoupling, ischemia, alternans, and anisotropy in cardiac monolayers (Himel et al., 2012).
Calcium-sensitive indicators can buffer calcium or introduce toxicity, which may alter cell physiology. Because calcium transients are downstream of voltage changes, they provide an indirect view of excitability, and their slower kinetics can obscure rapid electrical events. In addition, simplified monolayer cultures lack the structural complexity of intact myocardium, which limits translation to in vivo outcomes.
Given the potential for botanicals to influence calcium handling, optical mapping could be a valuable tool for assessing their cardiotropic effects. However, the inherent complexity of botanical extracts may introduce challenges in identifying specific bioactive components and interpreting concentration-response relationships. Further studies are needed to determine whether this method can effectively capture the cardiophysiological impact of botanical mixtures.
2.5 Mitochondrial function
Mitochondrial dysfunction is a critical factor in cardiotoxicity, contributing to impaired ATP production, increased oxidative stress, and cell death, all of which can compromise cardiac function (Brown et al., 2017). The Seahorse Cell Mito Stress Test (Agilent Technologies) is a widely used assay that assesses mitochondrial function by measuring the oxygen consumption rate (OCR) of live cells in real time (Leung and Chu, 2018). This method utilizes a multi-well plate with built-in injection ports that introduce modulators of cellular respiration, allowing for the dynamic assessment of mitochondrial activity. The assay measures key parameters, including basal respiration, ATP-linked respiration, maximal and spare respiratory capacities, and non-mitochondrial respiration (Figure 5). Specific modulators include oligomycin, which inhibits ATP synthase and reduces OCR; carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP), an uncoupler that disrupts the mitochondrial membrane potential and increases OCR; and rotenone/antimycin A, which inhibits the electron transport chain and decreases OCR.
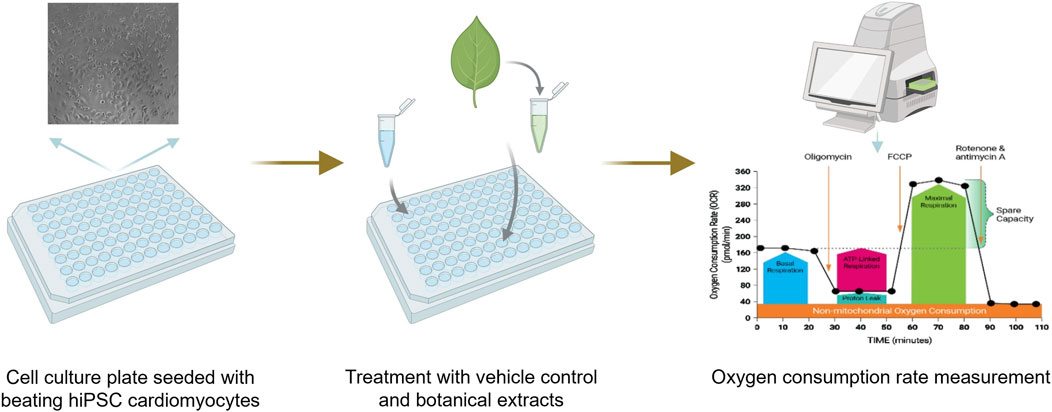
Figure 5. Schematic diagram showing how mitochondrial dysfunction is measured in the seahorse assay. Beating human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CM) are plated and then exposed to a botanical extract or vehicle control. After exposure, oxygen consumption rates are measured to assess potential mitochondrial dysfunction.
Mitochondrial function is particularly important in cardiomyocytes, where ATP production directly supports cardiac contraction and vascular function. Impaired mitochondrial activity can lead to reduced cardiac output and systemic effects on cardiovascular health. The Seahorse assay has been widely applied to evaluate mitochondrial toxicity induced by clinically relevant drugs such as aspirin, doxorubicin, and simvastatin (Holt et al., 2023; Kretzschmar et al., 2021; Kuzyk et al., 2020; Liu et al., 2024). While its use with botanical extracts is less explored, this approach could provide insights into plant-derived compounds that influence mitochondrial function. A key limitation of the assay is that it measures OCR only in cultured cells, not in intact tissues, which may limit physiological relevance (Divakaruni et al., 2014). Additionally, variability in cell adhesion, plating density, and cytoplasmic metabolism can impact reproducibility, necessitating careful optimization of experimental conditions (Brand and Nicholls, 2011; Lange et al., 2012; Luz et al., 2015).
2.6 Contractile force
The in vitro assessment of cardiotoxicity often involves measuring contractile force, which can be performed either directly or through impedance-based techniques. Direct measurement of contractile force typically utilizes isolated cardiomyocytes or tissue constructs, where mechanical sensors detect the force generated during cell contraction (Figure 6). This method provides precise, real-time data on the contractile properties of heart cells, crucial for evaluating the impact of drugs or compounds on cardiac function.
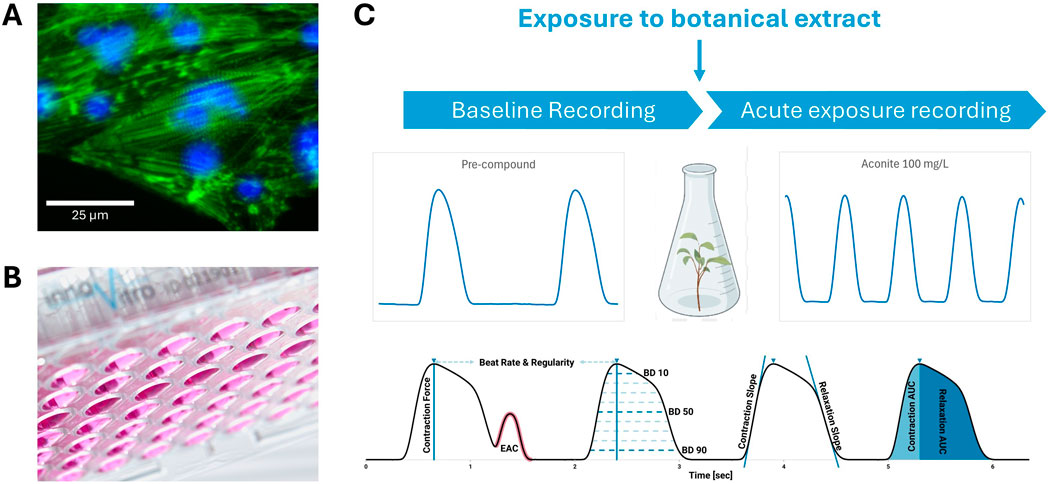
Figure 6. A schematic illustrating the direct measurement of contractile force. Human-induced pluripotent stem cell-derived cardiomyocytes (A) cardiomyocytes stained with a combined phalloidin/DAPI (4′,6-diamidino-2-phenylindole) for visualizing F-actin and nuclei, respectively are plated on freely swinging polydimethylsiloxane membranes (B), which move when the plated cardiomyocytes contract. A baseline recording is taken (C) before the same cells are exposed to a botanical extract or a vehicle control. Then another recording is taken and compared to the baseline to evaluate changes in the contractile force of the cardiomyocytes (C). EAC, early after contraction.
Alternatively, impedance measurements offer a non-invasive approach to assess contractility indirectly by detecting changes in electrical resistance as cardiomyocytes contract and relax. Electrodes embedded in the culture substrate continuously monitor these impedance fluctuations, which correspond to cell movement and morphology changes. This technique enables high-throughput screening of cardiotoxic effects and provides dynamic information on beat rate, amplitude, and rhythmicity.
The FLEXcyte 96 technology (Nanion Technologies GmbH, Munich, Germany) integrates both contractile force measurement and impedance-based assessment in a 96-well format, offering a physiologically relevant mechanical environment (Gossmann et al., 2020). In this system, hiPSC-CMs are seeded on freely swinging polydimethylsiloxane (PDMS) membranes (innoVitro GmbH, Juelich, Germany), which bend under the weight of the culture medium (Figure 6) (Gossmann et al., 2016). As the cardiomyocytes contract, the membranes lift, and this movement is recorded. Contractile parameters—including force of contraction, beat duration, beat frequency, contraction and relaxation velocity, and area under the curve—are derived using Laplace’s Law (Gossmann et al., 2016). Additionally, impedance monitoring in this system detects changes in cellular adhesion, morphology, and conductivity, offering an integrated assessment of cardiomyocyte functionality. This approach has been used to evaluate the potential cardiotoxicity of single chemicals including G protein-coupled receptor (GPCR) agonists, calcium channel agonist, an hyperpolarization-activated cyclic nucleotide-gated (HCN) channel antagonist and potassium channel antagonists (Lickiss et al., 2024); however, there is a need for additional work to evaluate the suitability of the method for use with complex mixtures such as botanical extracts.
Contractility assays may miss subtle electrophysiological changes that do not translate into altered contraction, and they provide only an indirect view of ion channel activity. Immature sarcomere organization in iPSC-derived cardiomyocytes can further limit fidelity to adult myocardium. In addition, both monolayer and engineered tissue models lack systemic inputs such as neurohormonal regulation and hemodynamic load, which are key determinants of contractile performance in vivo.
2.7 Safety pharmacology screen
Safety pharmacology screens are widely used to identify potential adverse effects on key organ systems, with the heart being a particular area of concern. These screens bring together complementary assays that capture different aspects of cardiomyocyte function, such as excitability, calcium handling, and contractility. For the purposes of this strategy paper, we reference one version of a safety pharmacology screen that illustrates how such assays can be combined to provide an integrated view of cardiac activity (Table 1).
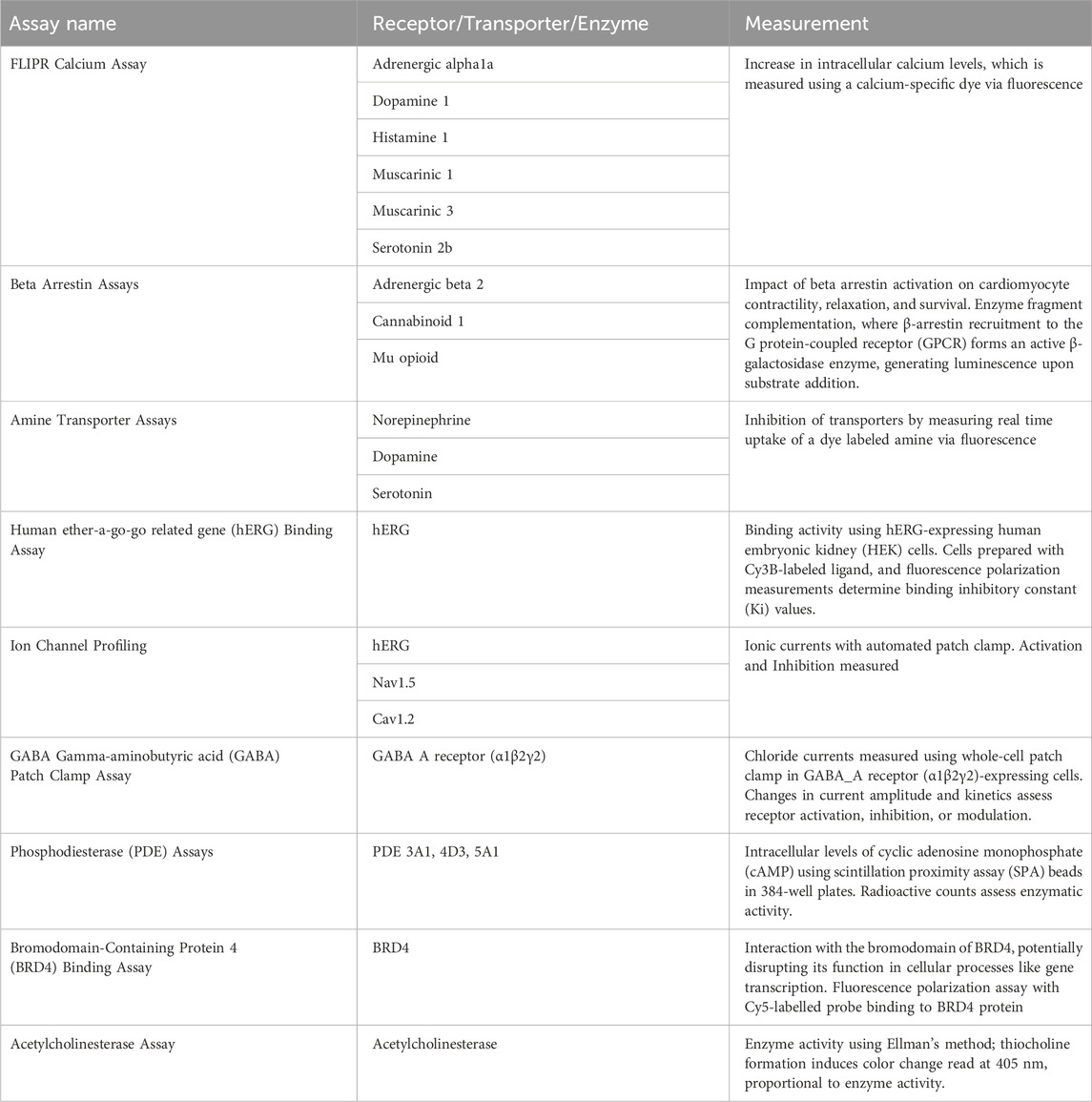
Table 1. A list of the assays evaluated in the selected safety pharmacology screen as well as the specific mechanisms the assay assessed and the information that could be derived from the measurements.
While widely used in the pharmaceutical industry, safety pharmacology assays often focus on single targets, such as hERG, which can miss broader pharmacological effects. In addition, these assays do not capture absorption, metabolism, or systemic distribution, which are critical for translating findings to human exposure.
3 Botanical case studies
To evaluate the above-described screening assays, the Cardiotoxicity Working Group nominated botanicals to be used as demonstrative case studies. Of those nominated (Table 2), several are not typically taken as dietary supplements (e.g., aconite, oleander, and comfrey), but their known toxicity makes them useful case studies for the assays. In addition to the botanicals specifically selected by the Cardiotoxicity Working Group, other botanicals were selected as case studies by other working groups and may have limited or no data with respect to cardiotoxicity. The botanicals selected by the BSC were based on the existing literature, including data in humans (e.g., adverse event reporting or clinical trials), animal data (e.g., rodent studies), or mechanistic studies (e.g., cell-based studies), and often a combination of these data types. Prioritization for inclusion in the study was based on availability of chemical analyses data (e.g., constituent identification and qualification). These data are described below in a nonsystematic review. It should be noted that botanical safety information is varied with respect to information reported and quality (Patel et al., 2023). The selected botanicals have been sourced and chemically analyzed to verify the authentication of the test materials (National Toxicology Program, 2023; Waidyanatha et al., 2024). Table 3 provides an overview of available cardiotoxicity data for each botanical case study.
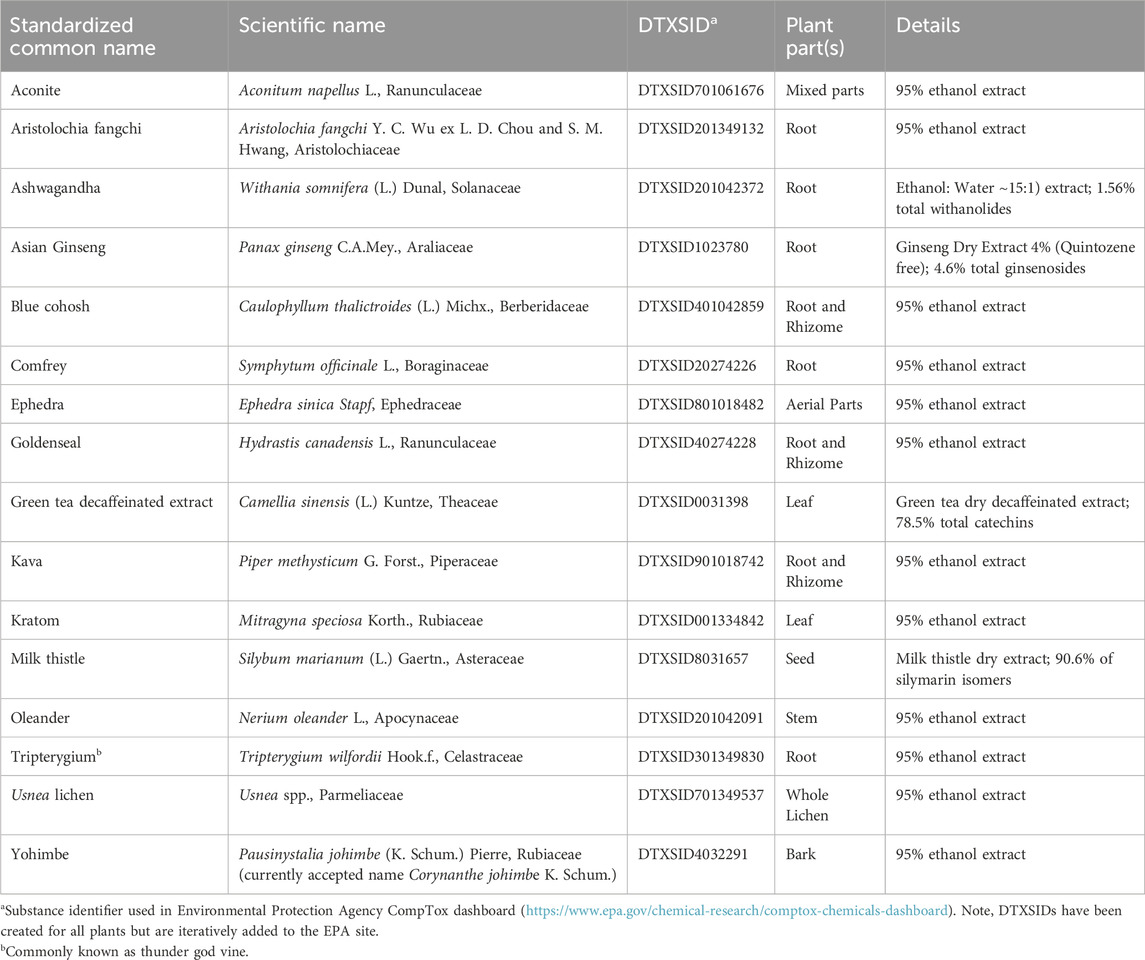
Table 2. List of botanical extracts used in the BSC, including their standardized common and scientific names, Distributed Structure-Searchable Toxicity substance identifier (DTXSID), and part(s) of the plant used to derive the extract. Botanicals with suspected cardiotoxicity are bold. Table is modified from Waidyanatha et al. (2024).
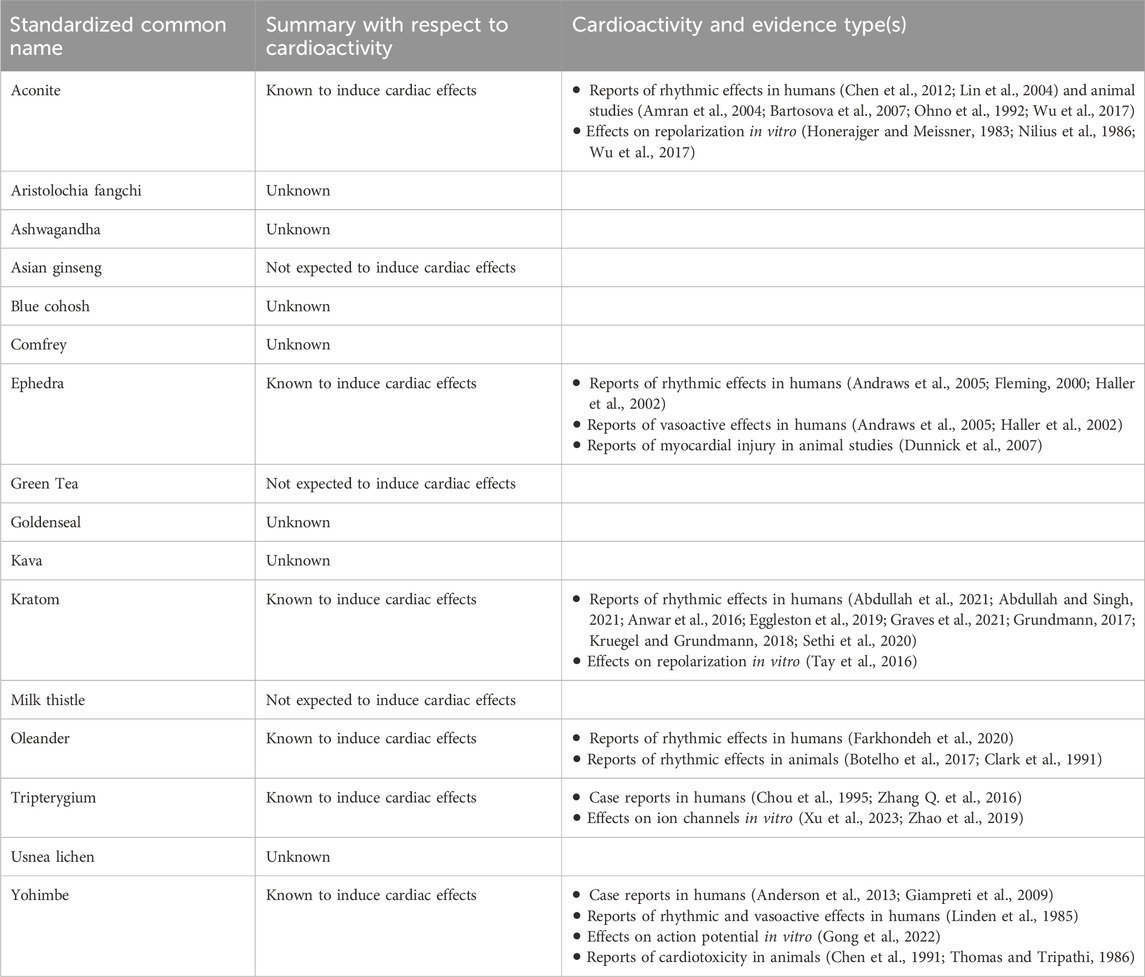
Table 3. Summary of available data types for selected botanicals. This was not a systematic literature search, and the quality of the studies was not evaluated and may vary. Given the complex nature of botanical extracts, the chemical composition of the tested materials included in the review and the extracts prepared by the Consortia may be different due to differences in the source and preparation of the materials.
3.1 Aconite
Aconite (Aconitum napellus L.), also known as monkshood, is a flowering plant indigenous to West and Central Europe. Traditional medicines prepared from Aconitum spp. (including A. napellus) are generally prepared from the whole plant or root and purported to have some effects, including anesthetic, analgesic, anti-inflammatory, and antidiarrheal properties (Povšnar et al., 2017). However, there are contemporary case studies documenting fatal or near-fatal poisonings associated with aconite (Pullela et al., 2008; Strzelecki et al., 2010). In cases of overdose, aconite is known to elicit cardiotoxic and neurotoxic effects (Gao et al., 2020; Li et al., 2022). Aconitine [DTXSID4046319] is a toxic alkaloid found in the leaves and roots of the plant.
The total content of aconitine and other toxic alkaloids can be reduced by soaking aconite root in an alkaline solution and boiling; however, even when it is processed using this technique, a poor-quality aconite preparation, when ingested, can cause toxicity, including muscle spasms, paralysis of the respiratory system, and heart rhythm disorders (Chen et al., 2012), and several cases of life-threatening ventricular tachycardia have also been reported (Lin et al., 2004).
Aconitine has been shown to induce ventricular tachycardia and ventricular fibrillation in various animal studies (Amran et al., 2004; Bartosova et al., 2007; Ohno et al., 1992; Wu et al., 2017) and has been demonstrated to act as a late Na+ current agonist, leading to delayed repolarization, in isolated cardiomyocytes (Honerajger and Meissner, 1983; Nilius et al., 1986). Additionally, data from hiPSC-CMs indicated that inhibition of the L-type calcium channel currents, as well as increased beating frequency and decreased action potential duration, although no change in late sodium currents, by aconitine could play a key role in the proarrhythmic effects of aconitine in humans (Wu et al., 2017).
Overall, aconite is expected to induce cardiac effects in humans.
3.2 Aristolochia fangchi
Aristolochia fangchi is a perennial climbing vine native to southeast Asia. It is also known as fang ji or guang fang ji, and other names that have led to mistaken identity with other plants. In the 1990s in Belgium, over 100 people mistakenly took A. fangchi instead of Stephania tetrandra for weight loss, leading to kidney damage, severe kidney failure requiring transplants or dialysis in 70 cases, and subsequent cancers or urinary tract diseases. (Debelle et al., 2008; Grady, 2000). A. fangchi and other related Aristolochia species contain aristolochic acids (such as aristolochic acid I [DTXSID0040969] and II [DTXSID00197166]), which are acknowledged for their genotoxic and renal toxic properties. This species was singled out for investigation by the BSC due to its genotoxic effects (Debelle et al., 2008; Grady, 2000).
Based on searches in Google Scholar and PubMed as of the publication of this article, there are no studies in humans or rodent models for Aristolochia with respect to cardiotoxicity. One study in zebrafish embryos exposed to aristolochic acid I increased the pericardial area, widened sinus venosus and the bulbus arteriosus (a measurement used to assess cardiac development), and reduced heart size and linearization of both ventricles. However, this could be due to the compound causing genotoxicity or developmental toxicity in the developing embryo (Hu et al., 2024).
While there were effects in the zebrafish embryo study, it is unclear what cardiac effects to anticipate for A. fangchi in the select assays.
3.3 Ashwagandha
Ashwagandha (Withania somnifera), also known as Indian ginseng, is an evergreen shrub indigenous to arid regions of tropical and subtropical zones across Asia, Africa, and the Middle East. It has been used in Ayurveda and other traditional medicinal practices and is often purported to enhance resilience against various stressors (Speers et al., 2021). The selection of ashwagandha root extract by the BSC stemmed from the existing developmental toxicity studies with negative results, and there have been documented instances of hepatotoxicity (Lubarska et al., 2023; Siddiqui et al., 2021). Biologically significant constituents, including ashwagandhanolide [DTXSID301353590], withaferin A [DTXSID10965459], and withanoside I [DTXSID801355610] have been identified in its root (Pal et al., 2012).
Short-term administration of ashwagandha extract (e.g., up to 3 months) may be safe, but there is not enough information on its long-term safety (NCCIH, 2023). A review paper from Brown (2018), reports in a table that there is shock and ventricular tachycardia in case reports related to ashwagandha. This comes from another review which reports a case in a 60-year-old male taking W. somnifera with “VT, ventricular bigemini, LBBB” and a case of a 55-year old that presented with ventricular tachycardia (Dwivedi et al., 2011). The review is on 12 patients treated at the University of Delhi, but no details are given on the proper identification and characterization of the material.
Standardized ashwagandha root extracts (1.5% withanolides) given to rats at a dose of 300 mg/kg were reported to reduce cardiotoxicity induced by doxorubicin and restore several biochemical changes, such as reduction of elevated malondialdehyde levels, catalase, superoxide dismutase activity, calcium content, Bcl-2 protein levels (Hamza et al., 2008).
A review by Wiciński et al. (2024) summarized data for rodents, cell lines, clinical studies, and other models on ashwagandha’s cardiovascular effects, with results from many of the assays indicating that ashwagandha results in lowering inflammation and anti-angiogenic effects.
While cardiotoxic effects from ashwagandha seem unlikely, it is unclear whether ashwagandha will elicit cardiac effects in the selected assays.
3.4 Asian ginseng
Asian ginseng (Panax ginseng) is a perennial herb characterized by its branched root shape. Asian ginseng is typically cultivated for a span of 6–10 years before being harvested. Originating from regions across Asia, including China and Korea, it has been integral to health-related practices for hundreds of years. According to the National Center for Complementary and Integrative Health (NCCIH), short-term oral usage (up to 6 months) is generally considered safe for most individuals (NCCIH, 2020). The selection of the root extract of this plant by the BSC was based on the absence of adverse effects for various endpoints supported in the literature. Asian ginseng contains ginsenoside saponins. These chemicals have been shown to have some therapeutic effects (Jia et al., 2009; Vijayakumar and Kim, 2023; Wang et al., 2009).
Clinical trials have been conducted using different doses in either healthy subjects or patients with various conditions, including cardiovascular and metabolic diseases (Kim Y.-S. et al., 2015). Single doses up to 800 mg of P. ginseng in caffeinated beverages, such as energy drinks, have not been associated with changes in electrocardiogram parameters or increases in heart rate or blood pressure (Shah et al., 2016). Studies in in vitro cell cultures and in vivo animal models have shown ginseng’s potential cardiovascular benefits through various mechanisms, including antioxidation, modifying vasomotor function, reducing platelet adhesion, influencing ion channels, altering autonomic neurotransmitter release, and improving lipid profiles (Kim, 2012), as well as enhancing mitochondrial respiration capacity and ATP production in cardiomyocytes (Huang et al., 2022).
Overall, Asian ginseng is not expected to cause cardiotoxicity but may have effects on cardiological activity in the select assays.
3.5 Blue cohosh
Blue cohosh (Caulophyllum thalictroides), not to be confused with black cohosh (Actaea racemose), is a flowering plant in the barberry family of plants native to eastern North America. Blue cohosh has been used by midwives to help induce labor; however, it has been associated with perinatal stroke, congestive heart failure, and neonatal shock (Datta et al., 2014). It has been used in combination with other botanicals for abortive or contraceptive purposes.
Blue cohosh root contains N-methylcytosine [DTXSID60149949], which has nicotine-like effects (Scott and Chen, 1943), and caulosaponin (no DTXSID or CASRN available), a glycoside that constricts coronary vessels (NICHD, 2006). While there were no extensive studies conducted investigating the effects of blue cohosh on the cardiovascular system, acute exposure to nicotinic and nicotinic-like alkaloids can have cardiovascular effects such as tachycardia and bradycardia (Schep et al., 2009).
Blue cohosh root extract disrupted cardiovascular and craniofacial cartilage development in medaka embryos in a dose and developmental stage-specific manner (Wu et al., 2010).
More research is needed to see if blue cohosh will induce any effects on the heart, especially in adults.
3.6 Comfrey
Comfrey (Symphytum officinale) is a perennial flowering plant indigenous to Asia and Europe. It has historically been used in wound healing applications. However, it contains known genotoxic compounds, specifically pyrrolizidine alkaloids (PAs) (Schramm et al., 2019). Among the identified PAs in comfrey are lycopsamine [DTXSID60145542] and 7-acetyllycopsamine [DTXSID50223742].
One clinical study monitored heart rate and blood pressure when patients were using a daily topical application that contained comfrey extract and reported no differences between comfrey and placebo (Grube et al., 2007). While there were several cardiovascular-related adverse event reports in the literature where individuals mistakenly consumed foxglove (Digitalis purpurea) instead of comfrey (Lin et al., 2010; Vithayathil and Edwards, 2016) there have been no extensive studies conducted on comfrey’s (or its constituents’) effects on the heart.
It is unknown whether comfrey will induce cardiotoxic effects in the selected in vitro assays.
3.7 Ephedra
Ephedra (Ephedra sinica), also known as Ma Huang, is an herbaceous perennial plant native to China (NIDDK, 2012). Constituents of biological interest include alkaloids like ephedrine [DTXSID0022985], pseudoephedrine [DTXSID0023537], and N-methylephedrine [DTXSID401021166] (NIDDK, 2012). In China and India, it has been used to treat various conditions, such as colds, fever, and headache, and has been purported to promote weight loss. Ephedra was used in weight loss supplements; however, adverse effects including cardiovascular effects and neurological effects (e.g., heart attack, seizure, stroke, and death) led to the US Food and Drug Administration (US FDA) banning all dietary supplements containing ephedrine alkaloids from being sold in the US in 2004 (US FDA, 2004a; 2004b).
If abused, taken in high dosages, or taken by consumers who have pre-existing cardiovascular diseases, ephedra may contribute to adverse events. There are several deaths reported from an overdose of ephedra. (Gurley et al., 1998; Theoharides, 1997). Symptoms of overdose include heart palpitations, extreme nervousness, sweating, enlarged pupils, severe headache, dizziness, dyspnea, elevated body temperature, and, ultimately, death (Fleming, 2000).
Reports have suggested that ephedra alkaloids taken at well-controlled doses showed cardiovascular side effects such as increased blood pressure, heart rate, and mild palpitations (Andraws et al., 2005). In a single-dose study in humans, the biological effects of an oral dose of an ephedrine alkaloids supplement included central nervous system stimulation, peripheral vasoconstriction, elevation of blood pressure, bronchodilation, and cardiac stimulation (Haller et al., 2002). A study investigating powdered ephedra in humans reported increased pulse rate, decreased intestinal tone and motility, mydriasis, and tachycardia (White et al., 1997). There is evidence that potential interactions of orally ingested ephedrine alkaloids (mainly ephedrine and/or pseudoephedrine, not necessarily the herb ephedra itself) with cardiac glycosides or halothane can cause arrhythmia (Brinker, 2001).
Weight loss supplements containing ephedra and caffeine have been purported to increase weight loss in individuals when compared to a placebo (Boozer et al., 2002); however, the combination of ephedra or ephedrine with caffeine enhanced the cardiotoxicity over that with the herbal medicine or the active ingredient alone (Dunnick et al., 2007). In a rodent model, cardiotoxicity included hemorrhage, necrosis, and degeneration in the ventricles or interventricular septum within 2–4 h after treatment with ephedra extract/caffeine or ephedrine/caffeine (Dunnick et al., 2007).
Based on the adverse event reports associated with ephedra consumption, ephedra is anticipated to induce an effect in the selected assays for assessing cardiotoxicity.
3.8 Goldenseal
Goldenseal (Hydrastis canadensis) is a flowering, herbaceous, perennial native to the eastern United States and southeastern Canada. Traditionally, goldenseal has been purported to treat skin disorders, digestive issues, urinary tract infections, and more. However, the NCCIH says “the scientific evidence does not support the use of goldenseal for any health-related purpose.” (NCCIH, 2021). Additionally, goldenseal is known to induce botanical-drug interactions, which could affect people taking medicines for heart conditions. Some of its known constituents include berberine [DTXSID9043857] and hydrastine [DTXSID9025409]. Per NCCIH, “Berberine … has been studied for heart failure, diarrhea, infections, and other health conditions” (NCCIH, 2021).
The National Toxicology Program (NTP) completed feed studies in rats and mice for goldenseal root powder for 2 weeks, 3 months, and 2 years. In the 2-year study, there was increased liver cancer in the high-dose male and female rats and the high-dose male mice. The 2-year NTP study found that goldenseal extract may reduce the background level of cardiomyopathy in F344/N rats (National Toxicology Program, 2010). Berberine has been reported to have both cardiotoxic and cardioprotective effects (Wang et al., 2023) and some studies point to anti-inflammatory properties and potential cardioprotective effects (for review, see Mandal et al., 2020).
Whether goldenseal will induce cardiac effects in the selected in vitro assays is unclear.
3.9 Green tea dry decaffeinated extract
Green tea (Camellia sinensis) is one of the most common beverages in the world and has numerous studies purporting various benefits (NICCIH, 2020). As a steeped beverage with natural levels of caffeine, it is believed to be safe to drink up to eight cups per day. The polyphenols in green tea may account for up to 30% of its dry weight. Most of these polyphenols are catechins, with one of the major polyphenol monomers being epigallocatechin gallate (EGCG [DTXSID1029889]) (Lin et al., 2003).
Several completed clinical phase I-III trials on green tea extracts and/or catechins demonstrated the bioavailability, safety, and effectiveness in modulating clinical and biological markers associated with cancer and non-cancer endpoints. While the tea is considered safe, the BSC focused on a decaffeinated concentrated green tea extract containing high amounts of catechins, similar to extracts commonly used as dietary supplements (National Toxicology Program, 2016). In this project, the Hepatotoxicity Working Group selected decaffeinated concentrated green tea extract due to adverse event reports and by the Genotoxicity Working Group due to its previous evaluation by NTP (National Toxicology Program, 2016). Some data have indicated that higher doses of green tea extracts and/or catechins (e.g., higher than 800 mg EGCG/day) may be associated with moderate to severe abnormalities in liver function (Wu et al., 2011; Yu et al., 2017).
Some studies assessed the cardiotoxic or cardioprotective potential of green tea extracts. One study in healthy human volunteers with different genotypes involved in the inactivation of various catechol-containing chemicals (low vs high activity groups) suggested that green tea extract may have a beneficial effect on small vessel tone in the low activity group (Miller et al., 2012). In animals, many studies report green tea to have cardioprotective effects (Cheng et al., 2016; Ibrahim et al., 2019; Khan et al., 2014; Wang et al., 2016).
Overall, it is unlikely green tea will produce cardiotoxic effects, but it may induce cardiological activity in the select assays.
3.10 Kava
Kava (Piper methysticum) is a perennial shrub of the pepper family native to the Pacific Islands. The aqueous extracts of kava roots and rhizome have been historically used as a ceremonial beverage but are modernly used as an herbal product purported to have anti-anxiety and pain relief effects (Norton and Ruze, 1994). The BSC Hepatotoxicity working group selected Kava due to adverse event reports associated with kava consumption starting in the 1990s (Soares et al., 2022).
Many lactones have been identified in kava (Soares et al., 2022) including kavain [DTXSID5033595] and 7,8-dihydrokavain [DTXSID101018162]. On average, the kavalactones account for 3%–20% of the dry weight of the kava root (Chua et al., 2016; Sarris et al., 2011).
The NTP conducted 2-week, 3-month, and 2-year studies of kava extract in rats and mice, finding increased liver cancer rates in both male and female mice, higher incidences of liver lesions in both male and female rats, and a slight increase in testicular tumors in male rats. (National Toxicology Program, 2012). There were no reported effects on the cardiovascular system in any of the groups (National Toxicology Program, 2012).
While hepatotoxicity can be considered the main health concern, other reversible (upon interruption of the ingestion or treatment) reported side effects are kava dermopathy, depressant effect along with its anxiolytic properties, drug interaction, gastrointestinal discomfort, nausea, headaches, memory problems, and tremor (Soares et al., 2022). Tachycardia and electrocardiogram abnormalities (tall P waves) have also been reported in heavy kava users (Mathews et al., 1988). One study suggested kava was able to improve reflex vagal control of heart rate in humans with anxiety disorder (Watkins et al., 2001).
Kavain was shown to have an antithrombotic effect in vitro on human platelets (Gleitz et al., 1997).
It is unclear whether kava will have an expected cardiac effect in the select in vitro assays due to the mix of available scientific literature.
3.11 Kratom
Kratom (Mitragyna speciosa) is a tropical evergreen tree native to Southeast Asia. More than 40 alkaloids have been identified in kratom extracts, with three of them, mitragynine [DTXSID701032140], corynantheidine (CASRN 23407-35-4), and 7-hydroxymitragynine [DTXSID20903988] known to have pharmacological effects (Meireles et al., 2019). Mitragynine and 7-hydroxymitragynine act on the µ-opioid receptor (Alford et al., 2025; Babu et al., 2008), which has increasingly led to kratom being taken to treat pain and opioid withdrawal without consultation from healthcare providers (Garcia-Romeu et al., 2020). The US FDA has warned that kratom users could experience serious adverse events, including liver toxicity, seizures, and substance use disorder, and ultimately concluded that there is inadequate information regarding kratom to provide reasonable assurance that it does not present a significant or unreasonable risk of illness or injury and, therefore, cannot be legally marketed as a dietary supplement and cannot be lawfully added to conventional foods (Office of the Commissioner, 2023).
There are adverse effect reports resulting from kratom use, including one of a young man who consumed both kratom and Adderall and was found to have a small area of hemorrhage in his brain (Castillo et al., 2017), and others have reported addiction (Vento et al., 2021) and death (McIntyre et al., 2015). Kratom can also cause tachycardia and changes in blood pressure, (Eggleston et al., 2019; Grundmann, 2017; Kruegel and Grundmann, 2018; Sethi et al., 2020). A Poison Control Center report on 3,484 kratom exposures in the US from 2014 to 2019, primarily involving single-substance oral exposures in older adults, found that 45% of cases included cardiovascular effects like hypertension and tachycardia. (Graves et al., 2021). According to an earlier Poison Control Center’s report for kratom exposures between 2010 and 2014, the reported signs and symptoms included tachycardia (25%) and hypertension (12%) (Anwar et al., 2016).
One observational study reported that regular kratom use was associated with at least an 8-fold increase in the odds of individuals presenting with sinus tachycardia when compared with non-kratom users. However, there was no difference in the odds of having other ECG abnormalities (Abdullah et al., 2021). In this study, kratom use was associated with an increased probability of borderline QT interval (QTc) but not prolonged QTc.
In regular kratom users, a study found that higher serum mitragynine levels (at least 9.6 mg/L) were associated with prolongation of the QT interval, and such effects were found to be dose-dependent (Abdullah and Singh, 2021). A mechanistic study showed that mitragynine did not affect the hERG expression at the transcriptional level but inhibited the protein expression (Tay et al., 2016). The hERG tail currents following depolarization pulses were also inhibited by mitragynine (IC50 value of 1.62 μM in the hERG-transfected HEK293 cells). In addition, mitragynine inhibited the acetylcholine-activated potassium current through G protein-coupled inwardly rectifying potassium (GIRK) channels (IC50 value of 3.32 μM). The study authors concluded that blocking both hERG and GIRK channels may cause additive cardiotoxicity risks (Tay et al., 2016).
Overall, we expect kratom to induce cardiac effects in the select in vitro assays.
3.12 Milk thistle
Milk thistle (Silybum marianum) is an annual plant belonging to the Asteraceae family. It originates from Southern Europe, Russia, Asia Minor, and Northern Africa and has been naturalized in North and South America and Australia (Bijak, 2017). Western herbalists and naturopathic physicians utilize milk thistle fruit for its purported aid for digestive issues. Modern formulations claim to help with liver health (Blumenthal and Busse, 1998).
Generally perceived as safe, milk thistle was chosen by the BSC due to its documented lack of toxicity, notably following an NTP 2-year study conducted on rats and mice (National Toxicology Program, 2011) that found no differences in cardiac effects compared to controls in mice or rats. In addition, a few papers in the literature report that milk thistle can counter chemical-induced cardiotoxicity (Ali et al., 2018; Karimian and Mahmoudi, 2024).
Overall, milk thistle is not expected to induce cardiotoxicity.
3.13 Oleander
Oleander (Nerium oleander) is a flowering subtropical shrub native to the Mediterranean region but cultivated worldwide as an ornamental plant; it is now found in parts of Asia, Australia, and the Southern United States (INCHEM, 1997). The primary constituent of pharmacological interest is oleandrin [DTXSID40861950], a glycoside. Oleandrin has been used in ethno-phytomedicine as a treatment for a broad spectrum of diseases, including asthma, eczema, and ringworm (Zhai et al., 2022). However, it needs to be noted that oleander is a poison when ingested in sufficient amounts. This has occurred in livestock (Galey et al., 1996) pets, and humans (both intentionally and unintentionally) (Langford and Boor, 1996).
Oleander extracts contain oleandrin and other cardiac glycosides, which can inhibit the activity of Na+/K + ATPase, leading to hyperkalemia. Cardiac glycosides were initially used in the treatment of congestive heart failure and other conditions (Digitalis Investigation Group, 1997). The cardiotoxic effects include hemorrhage, necrosis, arrhythmia, sinus bradycardia, and a prolonged P-R interval for ECG recordings (Farkhondeh et al., 2020).
Oleander has been tested using animal models. In mice and rats, there were elevated troponin levels and indications of hyperemia and hemorrhage (Khordadmehr and Nazifi, 2018). In guinea pigs, there were signs of arrhythmias due to Na+/K+ pump inhibition (Botelho et al., 2017) and in dogs, there were induced dysrhythmias (Clark et al., 1991).
Overall, oleander is known to have cardiac effects and is expected to elicit effects in the selected assays.
3.14 Tripterygium
Tripterygium (Tripterygium wilfordii), also known as Thunder God Vine, has historically been utilized in traditional Chinese medicine to purportedly treat inflammatory and autoimmune disorders (Dinesh and Rasool, 2019). The root extracts of the plant contain triptolide [DTXSID5041144] and celastrol [DTXSID2040993] as major constituents (Liu et al., 2020).
There are reported overdose cases for tripterygium and other adverse event reports in humans (Chou et al., 1995; Zhang Q. et al., 2016). One review highlighted various toxicities associated with tripterygium, including intestinal, liver, kidney, and other endpoints (Ru et al., 2019). Another review reported that 13% of adverse events in the literature of tripterygium were cardiac-related (Zhang C. et al., 2016). There is a documented case of a young man who suffered cardiac damage and intense vomiting, diarrhea, and other severe symptoms after ingesting tripterygium extract: he tragically passed away within 3 days (Chou et al., 1995).
A study using HEK293 cells found that an aqueous crude extract of tripterygium inhibited the amplitude of the hERG current (Zhao et al., 2019). Another in vitro study predicted that tripterygium can target voltage-gated sodium channels (Xu et al., 2023).
Other studies have reported that tripterygium can ameliorate damage from other chemicals. This could be due to its pharmacological activities, such as anticancer, anti-inflammation, antifibrosis, and antiatherosclerosis, at low doses below toxic levels, similar to glycosides having therapeutic or toxic effects depending on the dose (Huang et al., 2021; Song et al., 2023).
Overall, we expect tripterygium to induce cardiac effects in the selected assays.
3.15 Usnea
Usnea lichen, also known as “beard lichen” due to its filamentous strands that grow from tree branches, is found around the world, with over 350 species (National Toxicology Program, 2022). In Chinese herbal medicine, Usnea spp. has been used as a purported treatment for many ailments, including headaches, ocular irritation, malaria, and snake bites (Crawford, 2015). The primary constituent of biological interest is usnic acid [DTXSID0040123], which is found in Asian, European, and North American Usnea species (National Toxicology Program, 2022). The sample used by the BSC is wild-sourced in North America.
A 3-month NTP study demonstrated that exposure to an ethanolic extract of U. barbata and U. hirta containing 60 mg/kg/day (+/−)-usnic acid can be toxic to male and female F344/N NCTR rats, as evidenced by significant weight loss, morbidity, or death after 3 months of exposure (National Toxicology Program, 2022). No evidence of cardiotoxicity was noted in the NTP study. There is one 14-day study that reported the thinning of the cell content in the myocardium and gene expression changes related to oxidative stress in usnic acid-exposed rats, though this could be due to a high dose (100 mg/kg; Yokouchi et al., 2015). Mendonça et al., 2017, the authors report that usnic acid isolated from Cladonia substellata impaired myocardial contractility and reduced atrial contraction in guinea pigs. The authors found this effect was related to reduced calcium entry into myocardial cells. Additionally, in vitro in isolated cardiomyocytes, exposure to usnic acid caused irreversible cardiac contracture—again related to calcium homeostasis (Mendonça et al., 2017).
Overall, it is unknown if usnea will induce cardiac effects in the selected in vitro assays, given the mix of literature.
3.16 Yohimbe
Yohimbe (Corynanthe johimbe) is an evergreen of the Rubiaceae family native to tropical regions of the African west coast. The bark has traditionally been taken as a purported treatment for fever, leprosy, and cough, as well as for erectile dysfunction, and as an aphrodisiac in West Africa (EFSA Panel on Food Additives, 2013; Lo Faro et al., 2020). More recently, yohimbe has been sold as an aphrodisiac and an athletic performance enhancer (Clark et al., 1984; Grunewald and Bailey, 1993). The indole alkaloid yohimbine [DTXSID9040130] is one of the primary constituents of biological relevance (EFSA Panel on Food Additives, 2013).
There are several adverse effect reports associated with the yohimbe constituent yohimbine, including a male bodybuilder who experienced severe, but reversible, acute effects, including vomiting, loss of consciousness, and seizures after ingesting 5 g of yohimbine (Giampreti et al., 2009). There also have been reports of yohimbine being found at very high blood levels in two individuals who died unexpectedly (Anderson et al., 2013).
Overdose of yohimbine has been linked to causing transient hypertension, accelerated heart rate, and atrial fibrillation (Linden et al., 1985). In a hiPSC-CM model, yohimbine inhibited the frequency and prolonged the duration of spontaneous action potentials by inhibiting sodium and calcium currents (Gong et al., 2022). Yohimbine was identified as an α2-adrenoceptor antagonist (Goldberg and Robertson, 1983) and has been used as a model α2-adrenoceptor antagonist in animal studies. In cats, yohimbine antagonizes the antiarrhythmic effect of clonidine (α2-adrenoceptor agonist) on intravenous acetylstrophanthidin ventricular tachycardia (Chen et al., 1991). In guinea pigs, yohimbine exacerbated the cardiotoxicity of ouabain (a plant-derived toxic substance), which is linked to arrhythmias and cardiac arrest (Thomas and Tripathi, 1986).
Overall, yohimbe is expected to have cardiac effects.
4 Conclusions and next steps
Botanicals are used worldwide, and it is critical to ensure their safety, including the potential for them to elicit cardiotoxic effects. To address this need, the cross-sector team of experts from the BSC has identified several NAMs to test their applicability for evaluating botanicals as complex mixtures, using well-characterized, data-rich botanicals as case studies. Assays were selected based on their reproducibility, relevance to key mechanisms, and accessibility. This work aims to equip researchers with tools to better understand cardiotoxicity, prioritize and guide further testing, and deepen our knowledge of botanical products under evaluation by expanding the botanical safety toolkit. It exemplifies a more mechanism-informed approach to botanical safety assessment, shifting away from reliance on traditional animal testing methods towards more predictive, human-relevant models. The next steps of the Consortium involve testing the selected botanical case studies in these assays, analyzing the data, comparing the findings to existing literature, and developing a toolkit of in vitro assays for cardiotoxicity assessment.
While this initial work will focus on hazard identification and screening, future efforts could encompass a more exposure-based approach and aid in investigating the predictivity of NAMs for human responses. Critical considerations in the future will include attempts to evaluate the relevance of in vitro findings, considering absorption, distribution, and metabolism in humans. In vitro to in vivo extrapolation using physiologically based pharmacokinetic modeling and simulation could also aid in defining human-relevant concentrations. Advanced complex cellular co-culture models could also provide additional insights into the clinical relevance of toxicity findings and help identify specific effects and mechanisms of action beyond the capabilities of screening-level assays. In addition, exploring how these assays perform with a wider array of botanical extract matrices will help evaluate the domain of applicability for these methods.
This cardiotoxicity-focused effort represents one component of a broader effort by the Botanical Safety Consortium, which also includes ongoing initiatives in genotoxicity, hepatotoxicity, neurotoxicity, reproductive, and dermal toxicity. These novel studies plan to expand the botanical safety toolkit and give researchers tools to better understand cardiotoxicity, prioritize and plan future testing as needed, and better understand the botanical being tested.
Author contributions
JK: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Visualization, Writing – original draft, Writing – review and editing. HC: Funding acquisition, Resources, Writing – original draft, Writing – review and editing. AM: Visualization, Writing – original draft, Writing – review and editing. MG: Visualization, Writing – original draft, Writing – review and editing. PH: Writing – original draft, Writing – review and editing. YK: Writing – original draft, Writing – review and editing. NM: Writing – original draft, Writing – review and editing. J-YP: Writing – original draft, Writing – review and editing. RS: Writing – original draft, Writing – review and editing. RV: Writing – original draft, Writing – review and editing. RW: Writing – original draft, Writing – review and editing, Visualization. JW: Writing – original draft, Writing – review and editing. JY: Writing – original draft, Writing – review and editing. SZ: Visualization, Writing – original draft, Writing – review and editing. CM: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Visualization, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported in part by the Health and Environmental Sciences Institute’s (HESI) Botanical Safety Consortium. It is recognized via a Memorandum of Understanding between the U.S. Food and Drug Administration (FDA), Office of Dietary Supplement Programs and Department of Health and Human Services (HHS); National Institute of Environmental Health Sciences (NIEHS); Division of the National Toxicology Program, Office of Liaison, Policy, and Review; and Health and Environmental Sciences Institute (HESI) via Department of the Interior (DOI) Federal Consulting Group (FCG) under Blanket Purchase Agreement Order 140D0421F0068. This work is also supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences, Intramural Research project ZIA ES103316-04.
Acknowledgments
We acknowledge the committee members for their support and helpful feedback during the development of this document.
Conflict of interest
Author HSC was employed by L’Oréal Research and Innovation.
Author MG was employed by innoVitro GmbH.
Authors NM and RV were employed by FUJIFILM Cellular Dynamics, Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. Generative AI was used to edit/refine text after it was written by several authors, for readability and length. All text was reviewed and edited by authors.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdullah, M. F. I. L. B., and Singh, D. (2021). Assessment of cardiovascular functioning among regular kratom (Mitragyna speciosa korth) users: a case series. Front. Pharmacol. 12, 723567. doi:10.3389/fphar.2021.723567
Abdullah, M. F. I. L., Tan, K. L., Narayanan, S., Yuvashnee, N., Chear, N. J. Y., Singh, D., et al. (2021). Is kratom (Mitragyna speciosa korth.) use associated with ECG abnormalities? Electrocardiogram comparisons between regular kratom users and controls. Clin. Toxicol. Phila. PA 59, 400–408. doi:10.1080/15563650.2020.1812627
Alford, A. S., Moreno, H. L., Benjamin, M. M., Dickinson, C. F., and Hamann, M. T. (2025). Exploring the therapeutic potential of mitragynine and corynoxeine: Kratom-derived indole and oxindole alkaloids for pain management. Pharmaceuticals 18, 222. doi:10.3390/ph18020222
Ali, E. H., Sharifpanah, F., Taha, A., Tsang, S. Y., Wartenberg, M., and Sauer, H. (2018). The milk thistle (Silybum marianum) compound silibinin inhibits cardiomyogenesis of embryonic stem cells by interfering with angiotensin ii signaling. Stem Cells Int. 2018, 9215792. doi:10.1155/2018/9215792
Allan, A., Creech, J., Hausner, C., Krajcarski, P., Gunawan, B., Poulin, N., et al. (2023). High-throughput longitudinal electrophysiology screening of mature chamber-specific hiPSC-CMs using optical mapping. iScience 26, 107142. doi:10.1016/j.isci.2023.107142
Amran, Md.S., Hashimoto, K., and Homma, N. (2004). Effects of sodium-calcium exchange inhibitors, KB-R7943 and SEA0400, on aconitine-induced arrhythmias in Guinea pigs in vivo, in vitro, and in computer simulation studies. J. Pharmacol. Exp. Ther. 310, 83–89. doi:10.1124/jpet.104.066951
Anderson, C., Anderson, D., Harre, N., and Wade, N. (2013). Case study: two fatal case reports of acute yohimbine intoxication. J. Anal. Toxicol. 37, 611–614. doi:10.1093/jat/bkt057
Andraws, R., Chawla, P., and Brown, D. L. (2005). Cardiovascular effects of ephedra alkaloids: a comprehensive review. Prog. Cardiovasc. Dis. 47, 217–225. doi:10.1016/j.pcad.2004.07.006
Andrysiak, K., Stępniewski, J., and Dulak, J. (2021). Human-induced pluripotent stem cell-derived cardiomyocytes, 3D cardiac structures, and heart-on-a-chip as tools for drug research. Pflüg. Arch. - Eur. J. Physiol. 473, 1061–1085. doi:10.1007/s00424-021-02536-z
Anwar, M., Law, R., and Schier, J. (2016). Notes from the field: kratom (Mitragyna speciosa) exposures reported to poison centers - united States, 2010–2015. MMWR Morb. Mortal. Wkly. Rep. 65, 748–749. doi:10.15585/mmwr.mm6529a4
Asakura, K., Hayashi, S., Ojima, A., Taniguchi, T., Miyamoto, N., Nakamori, C., et al. (2015). Improvement of acquisition and analysis methods in multi-electrode array experiments with iPS cell-derived cardiomyocytes. J. Pharmacol. Toxicol. Methods 75, 17–26. doi:10.1016/j.vascn.2015.04.002
Babu, K. M., McCurdy, C. R., and Boyer, E. W. (2008). Opioid receptors and legal highs: salvia divinorum and Kratom. Clin. Toxicol. Phila. Pa 46, 146–152. doi:10.1080/15563650701241795
Baltov, B., Beyl, S., Baburin, I., Reinhardt, J., Szkokan, P., Garifulina, A., et al. (2023). Assay for evaluation of proarrhythmic effects of herbal products: case study with 12 Evodia preparations. Toxicol. Rep. 10, 589–599. doi:10.1016/j.toxrep.2023.04.014
Bartosova, L., Novak, F., Bebarova, M., Frydrych, M., Brunclik, V., Opatrilova, R., et al. (2007). Antiarrhythmic effect of newly synthesized compound 44Bu on model of aconitine-induced arrhythmia — compared to lidocaine. Eur. J. Pharmacol. 575, 127–133. doi:10.1016/j.ejphar.2007.07.044
Bedut, S., Seminatore-Nole, C., Lamamy, V., Caignard, S., Boutin, J. A., Nosjean, O., et al. (2016). High-throughput drug profiling with voltage- and calcium-sensitive fluorescent probes in human iPSC-derived cardiomyocytes. Am. J. Physiol.-Heart Circ. Physiol. 311, H44–H53. doi:10.1152/ajpheart.00793.2015
Beeton, C., and Beeton, C. (2018). Differences in ion channel phenotype and function between humans and animal models. Front. Biosci. 23, 43–64. doi:10.2741/4581
Bent, S. (2008). Herbal medicine in the United States: review of efficacy, safety, and regulation: grand rounds at University of California, San Francisco Medical Center. J. Gen. Intern. Med. 23, 854–859. doi:10.1007/s11606-008-0632-y
Berridge, B., Pierson, J., Pettit, S., and Stockbridge, N. (2024). Challenging the status quo: a framework for mechanistic and human-relevant cardiovascular safety screening. Front. Toxicol. 6, 1352783. doi:10.3389/ftox.2024.1352783
Bhalla, A., Thirumalaikolundusubramanian, P., Fung, J., Cordero-Schmidt, G., Soghoian, S., Sikka, V. K., et al. (2015). “Native medicines and cardiovascular toxicity,” in Heart and toxins (London, United Kingdom: Academic Press), 175–202. doi:10.1016/B978-0-12-416595-3.00006-2
Bijak, M. (2017). Silybin, a major bioactive component of milk thistle (Silybum marianum L. Gaernt.)—Chemistry, bioavailability, and metabolism. Molecules 22, 1942. doi:10.3390/molecules22111942
Blinova, K., Stohlman, J., Vicente, J., Chan, D., Johannesen, L., Hortigon-Vinagre, M. P., et al. (2017). Comprehensive translational assessment of human-induced pluripotent stem cell derived cardiomyocytes for evaluating drug-induced arrhythmias. Toxicol. Sci. 155, 234–247. doi:10.1093/toxsci/kfw200
Blinova, K., Dang, Q., Millard, D., Smith, G., Pierson, J., Guo, L., et al. (2018). International multisite study of human-induced pluripotent stem cell-derived cardiomyocytes for drug proarrhythmic potential assessment. Cell Rep. 24, 3582–3592. doi:10.1016/j.celrep.2018.08.079
Blumenthal, M., and Busse, W. R. (1998). “Bundesinstitut für Arzneimittel und Medizinprodukte (Germany),” in The complete German commission E monographs. Therapeutic guide to herbal medicines. Austin, Texas: Boston: American Botanical Council; Integrative Medicine Communications.
Boozer, C., Daly, P., Homel, P., Solomon, J., Blanchard, D., Nasser, J., et al. (2002). Herbal ephedra/caffeine for weight loss: a 6-month randomized safety and efficacy trial. Int. J. Obes. 26, 593–604. doi:10.1038/sj.ijo.0802023
Botelho, A. F. M., Santos-Miranda, A., Joca, H. C., Mattoso, C. R. S., De Oliveira, M. S., Pierezan, F., et al. (2017). Hydroalcoholic extract from Nerium oleander L. (Apocynaceae) elicits arrhythmogenic activity. J. Ethnopharmacol. 206, 170–177. doi:10.1016/j.jep.2017.05.031
Brand, M. D., and Nicholls, D. G. (2011). Assessing mitochondrial dysfunction in cells. Biochem. J. 435, 297–312. doi:10.1042/BJ20110162
Brinker, F. J. (2001). Herb contraindications and drug interactions. 3rd ed. Sandy, OR: Eclectic Medical Publications.
Brown, A. C. (2018). Heart toxicity related to herbs and dietary supplements: online table of case reports. Part 4 of 5. J. Diet. Suppl. 15, 516–555. doi:10.1080/19390211.2017.1356418
Brown, D. A., Perry, J. B., Allen, M. E., Sabbah, H. N., Stauffer, B. L., Shaikh, S. R., et al. (2017). Expert consensus document: mitochondrial function as a therapeutic target in heart failure. Nat. Rev. Cardiol. 14, 238–250. doi:10.1038/nrcardio.2016.203
Bruni, R., and Sacchetti, G. (2009). Factors affecting polyphenol biosynthesis in wild and field grown St. John’s Wort (Hypericum perforatum L. Hypericaceae/Guttiferae). Molecules 14, 682–725. doi:10.3390/molecules14020682
Burnett, S. D., Blanchette, A. D., Chiu, W. A., and Rusyn, I. (2021). Cardiotoxicity hazard and risk characterization of ToxCast chemicals using human induced pluripotent stem cell-derived cardiomyocytes from multiple donors. Chem. Res. Toxicol. 34, 2110–2124. doi:10.1021/acs.chemrestox.1c00203
Castillo, A., Payne, J. D., and Nugent, K. (2017). Posterior reversible leukoencephalopathy syndrome after kratom ingestion. Bayl. Univ. Med. Cent. Proc. 30, 355–357. doi:10.1080/08998280.2017.11929647
Chemla, S., and Chavane, F. (2010). Voltage-sensitive dye imaging: technique review and models. J. Physiol.-Paris 104, 40–50. doi:10.1016/j.jphysparis.2009.11.009
Chen, S. A., Liu, R. H., Ting, T. H., Chang, M. S., Chiang, B. N., and Kuo, J. S. (1991). Termination of digitalis-induced ventricular tachycardias by clonidine involves central alpha 2-adrenoceptors in cats. Br. J. Pharmacol. 103, 1114–1118. doi:10.1111/j.1476-5381.1991.tb12309.x
Chen, S. P. L., Ng, S. W., Poon, W. T., Lai, C. K., Ngan, T. M. S., Tse, M. L., et al. (2012). Aconite poisoning over 5 years: a case series in Hong Kong and lessons towards herbal safety. Drug Saf. 35, 575–587. doi:10.2165/11597470-000000000-00000
Cheng, T., Liu, J., Ren, J., Huang, F., Ou, H., Ding, Y., et al. (2016). Green tea catechin-based complex micelles combined with doxorubicin to overcome cardiotoxicity and multidrug resistance. Theranostics 6, 1277–1292. doi:10.7150/thno.15133
Chou, W.-C., Wu, C.-C., Yang, P.-C., and Lee, Y.-T. (1995). Hypovolemic shock and mortality after ingestion of Tripterygium wilfordii Hook F.: a case report. Int. J. Cardiol. 49, 173–177. doi:10.1016/0167-5273(95)02282-2
Chua, H. C., Christensen, E. T. H., Hoestgaard-Jensen, K., Hartiadi, L. Y., Ramzan, I., Jensen, A. A., et al. (2016). Kavain, the major constituent of the anxiolytic Kava extract, potentiates GABAA receptors: functional characteristics and molecular mechanism. PLoS One 11, e0157700. doi:10.1371/journal.pone.0157700
Clark, J. T., Smith, E. R., and Davidson, J. M. (1984). Enhancement of sexual motivation in male rats by yohimbine. Science 225, 847–849. doi:10.1126/science.6474156
Clark, R. F., Selden, B. S., and Curry, S. C. (1991). Digoxin-specific fab fragments in the treatment of oleander toxicity in a canine model. Ann. Emerg. Med. 20, 1073–1077. doi:10.1016/S0196-0644(05)81355-1
Crawford, S. D. (2015). “Lichens used in traditional medicine,” in Lichen secondary metabolites. Editor B. Ranković (Cham: Springer International Publishing), 27–80. doi:10.1007/978-3-319-13374-4_2
da Rocha, A. M., Creech, J., Thonn, E., Mironov, S., and Herron, T. J. (2020). Detection of drug-induced torsades de pointes arrhythmia mechanisms using hiPSC-CM syncytial monolayers in a high-throughput screening voltage sensitive dye assay. Toxicol. Sci. Off. J. Soc. Toxicol. 173, 402–415. doi:10.1093/toxsci/kfz235
Datta, S., Mahdi, F., Ali, Z., Jekabsons, M. B., Khan, I. A., Nagle, D. G., et al. (2014). Toxins in botanical dietary supplements: blue cohosh components disrupt cellular respiration and mitochondrial membrane potential. J. Nat. Prod. 77, 111–117. doi:10.1021/np400758t
Davis, J., Chouman, A., Creech, J., Monteiro Da Rocha, A., Ponce-Balbuena, D., Jimenez Vazquez, E. N., et al. (2021). In vitro model of ischemic heart failure using human induced pluripotent stem cell–derived cardiomyocytes. JCI Insight 6, e134368. doi:10.1172/jci.insight.134368
Debelle, F. D., Vanherweghem, J.-L., and Nortier, J. L. (2008). Aristolochic acid nephropathy: a worldwide problem. Kidney Int. 74, 158–169. doi:10.1038/ki.2008.129
Di Lorenzo, C., Ceschi, A., Kupferschmidt, H., Lüde, S., De Souza Nascimento, E., Dos Santos, A., et al. (2015). Adverse effects of plant food supplements and botanical preparations: a systematic review with critical evaluation of causality. Br. J. Clin. Pharmacol. 79, 578–592. doi:10.1111/bcp.12519
Di Pasquale, E., Lodola, F., Miragoli, M., Denegri, M., Avelino-Cruz, J. E., Buonocore, M., et al. (2013). CaMKII inhibition rectifies arrhythmic phenotype in a patient-specific model of catecholaminergic polymorphic ventricular tachycardia. Cell Death Dis. 4, e843. doi:10.1038/cddis.2013.369
Digitalis Investigation Group (1997). The effect of digoxin on mortality and morbidity in patients with heart failure. N. Engl. J. Med. 336, 525–533. doi:10.1056/NEJM199702203360801
Dinesh, P., and Rasool, M. (2019). “Herbal formulations and their bioactive components as dietary supplements for treating rheumatoid arthritis,” in Bioactive food as dietary interventions for arthritis and related inflammatory diseases (London, United Kingdom: Academic Press), 385–399. doi:10.1016/B978-0-12-813820-5.00022-2
Divakaruni, A. S., Rogers, G. W., and Murphy, A. N. (2014). Measuring mitochondrial function in permeabilized cells using the seahorse XF analyzer or a clark-type oxygen electrode. Curr. Protoc. Toxicol. 60, 25.2.1–25.2.16. doi:10.1002/0471140856.tx2502s60
Dunnick, J. K., Kissling, G., Gerken, D. K., Vallant, M. A., and Nyska, A. (2007). Cardiotoxicity of Ma Huang/caffeine or Ephedrine/caffeine in a rodent model system. Toxicol. Pathol. 35, 657–664. doi:10.1080/01926230701459978
Dwivedi, S., Aggarwal, A., and Sharma, V. (2011). Cardiotoxicity from ‘safe’ herbomineral formulations. Trop. Doct. 41, 113–115. doi:10.1258/td.2010.100304
EFSA Panel on Food Additives (2013). Scientific opinion on the evaluation of the safety in use of Yohimbe (Pausinystalia yohimbe (K. Schum.) Pierre ex Beille). EFSA J. 11. doi:10.2903/j.efsa.2013.3302
EFSA Scientific Committee (2009). Guidance on safety assessment of botanicals and botanical preparations intended for use as ingredients in food supplements. EFSA J. 7. doi:10.2903/j.efsa.2009.1249
Eggleston, W., Stoppacher, R., Suen, K., Marraffa, J. M., and Nelson, L. S. (2019). Kratom use and toxicities in the United States. Pharmacotherapy 39, 775–777. doi:10.1002/phar.2280
Farkhondeh, T., Kianmehr, M., Kazemi, T., Samarghandian, S., and Khazdair, M. (2020). Toxicity effects of Nerium oleander, basic and clinical evidence: a comprehensive review. Hum. Exp. Toxicol. 39, 773–784. doi:10.1177/0960327120901571
Galey, F. D., Holstege, D. M., Plumlee, K. H., Tor, E., Johnson, B., Anderson, M. L., et al. (1996). Diagnosis of oleander poisoning in livestock. J. Vet. Diagn. Invest. 8, 358–364. doi:10.1177/104063879600800314
Gao, X., Hu, J., Zhang, X., Zuo, Y., Wang, Y., and Zhu, S. (2020). Research progress of aconitine toxicity and forensic analysis of aconitine poisoning. Forensic Sci. Res. 5, 25–31. doi:10.1080/20961790.2018.1452346
Garcia-Romeu, A., Cox, D. J., Smith, K. E., Dunn, K. E., and Griffiths, R. R. (2020). Kratom (Mitragyna speciosa): user demographics, use patterns, and implications for the opioid epidemic. Drug Alcohol Depend. 208, 107849. doi:10.1016/j.drugalcdep.2020.107849
Garg, P., Garg, V., Shrestha, R., Sanguinetti, M. C., Kamp, T. J., and Wu, J. C. (2018). Human induced pluripotent stem cell–derived cardiomyocytes as models for cardiac channelopathies: a primer for non-electrophysiologists. Circ. Res. 123, 224–243. doi:10.1161/CIRCRESAHA.118.311209
Geller, A. I., Shehab, N., Weidle, N. J., Lovegrove, M. C., Wolpert, B. J., Timbo, B. B., et al. (2015). Emergency department visits for adverse events related to dietary supplements. N. Engl. J. Med. 373, 1531–1540. doi:10.1056/NEJMsa1504267
Giampreti, A., Lonati, D., Locatelli, C., Rocchi, L., and Campailla, M. T. (2009). Acute neurotoxicity after yohimbine ingestion by a body builder. Clin. Toxicol. 47, 827–829. doi:10.1080/15563650903081601
Gintant, G., Kaushik, E. P., Feaster, T., Stoelzle-Feix, S., Kanda, Y., Osada, T., et al. (2020). Repolarization studies using human stem cell-derived cardiomyocytes: validation studies and best practice recommendations. Regul. Toxicol. Pharmacol. 117, 104756. doi:10.1016/j.yrtph.2020.104756
Gleitz, J., Beile, A., Wilkens, P., Ameri, A., and Peters, T. (1997). Antithrombotic action of the kava pyrone (+)-kavain prepared from Piper methysticum on human platelets. Planta Med. 63, 27–30. doi:10.1055/s-2006-957597
Glukhov, A. V., Flagg, T. P., Fedorov, V. V., Efimov, I. R., and Nichols, C. G. (2010). Differential KATP channel pharmacology in intact mouse heart. J. Mol. Cell. Cardiol. 48, 152–160. doi:10.1016/j.yjmcc.2009.08.026
Goldberg, M. R., and Robertson, D. (1983). Yohimbine: a pharmacological probe for study of the alpha 2-adrenoreceptor. Pharmacol. Rev. 35, 143–180.
Gong, Y., Yang, L., Tang, J., Zheng, J., Witman, N., Jakob, P., et al. (2022). Yohimbine directly induces cardiotoxicity on human-induced pluripotent stem cell-derived cardiomyocytes. Cardiovasc. Toxicol. 22, 141–151. doi:10.1007/s12012-021-09709-3
Gossmann, M., Frotscher, R., Linder, P., Neumann, S., Bayer, R., Epple, M., et al. (2016). Mechano-Pharmacological characterization of cardiomyocytes derived from human induced pluripotent stem cells. Cell. Physiol. Biochem. 38, 1182–1198. doi:10.1159/000443124
Gossmann, M., Linder, P., Thomas, U., Juhasz, K., Lemme, M., George, M., et al. (2020). Integration of mechanical conditioning into a high throughput contractility assay for cardiac safety assessment. J. Pharmacol. Toxicol. Methods 105, 106892. doi:10.1016/j.vascn.2020.106892
Graves, J. M., Dilley, J. A., Terpak, L., Brooks-Russell, A., Whitehill, J. M., Klein, T. A., et al. (2021). Kratom exposures among older adults reported to U.S. poison centers, 2014–2019. J. Am. Geriatr. Soc. 69, 2176–2184. doi:10.1111/jgs.17326
Grube, B., Grünwald, J., Krug, L., and Staiger, C. (2007). Efficacy of a comfrey root (Symphyti offic. radix) extract ointment in the treatment of patients with painful osteoarthritis of the knee: results of a double-blind, randomised, bicenter, placebo-controlled trial. Phytomedicine 14, 2–10. doi:10.1016/j.phymed.2006.11.006
Grundmann, O. (2017). Patterns of Kratom use and health impact in the US-results from an online survey. Drug Alcohol Depend. 176, 63–70. doi:10.1016/j.drugalcdep.2017.03.007
Grunewald, K. K., and Bailey, R. S. (1993). Commercially marketed supplements for bodybuilding athletes. Sports Med. 15, 90–103. doi:10.2165/00007256-199315020-00003
Gunawan, M. G., Sangha, S. S., Shafaattalab, S., Lin, E., Heims-Waldron, D. A., Bezzerides, V. J., et al. (2021). Drug screening platform using human induced pluripotent stem cell-derived atrial cardiomyocytes and optical mapping. Stem Cells Transl. Med. 10, 68–82. doi:10.1002/sctm.19-0440
Gurley, B. J., Gardner, S. F., White, L. M., and Wang, P.-L. (1998). Ephedrine pharmacokinetics after the ingestion of nutritional supplements containing Ephedra sinica (Ma Huang). Ther. Drug Monit. 20, 439–445. doi:10.1097/00007691-199808000-00015
Haller, C. A., Jacob, P., and Benowitz, N. L. (2002). Pharmacology of ephedra alkaloids and caffeine after single-dose dietary supplement use. Clin. Pharmacol. Ther. 71, 421–432. doi:10.1067/mcp.2002.124523
Hamza, A., Amin, A., and Daoud, S. (2008). The protective effect of a purified extract of Withania somnifera against doxorubicin-induced cardiac toxicity in rats. Cell Biol. Toxicol. 24, 63–73. doi:10.1007/s10565-007-9016-z
Harris, K., Aylott, M., Cui, Y., Louttit, J. B., McMahon, N. C., and Sridhar, A. (2013). Comparison of electrophysiological data from human-induced pluripotent stem cell–derived cardiomyocytes to functional preclinical safety assays. Toxicol. Sci. 134, 412–426. doi:10.1093/toxsci/kft113
Himel, H. D., Bub, G., Lakireddy, P., and El-Sherif, N. (2012). Optical imaging of arrhythmias in the cardiomyocyte monolayer. Heart Rhythm. 9, 2077–2082. doi:10.1016/j.hrthm.2012.08.035
Holt, A. K., Najumudeen, A. K., Collard, T. J., Li, H., Millett, L. M., Hoskin, A. J., et al. (2023). Aspirin reprogrammes colorectal cancer cell metabolism and sensitises to glutaminase inhibition. Cancer Metab. 11, 18. doi:10.1186/s40170-023-00318-y
Honerajger, P., and Meissner, A. (1983). The positive inotropic effect of aconitine. Naunyn. Schmiedeb. Arch. Pharmacol. 322, 49–58. doi:10.1007/BF00649352
Hu, Y., Chen, L., Wu, Y., Zhang, J., Sheng, Z., Zhou, Z., et al. (2024). Palmatine reverse aristolochic acid-induced heart failure through activating EGFR pathway via upregulating IKBKB. Ecotoxicol. Environ. Saf. 285, 117100. doi:10.1016/j.ecoenv.2024.117100
Huang, B., Huang, C., Zhu, L., Xie, L., Wang, Y., and Zhu, N. (2021). Exploring the pharmacological mechanisms of Tripterygium wilfordii Hook F. against cardiovascular disease using network pharmacology and molecular docking. Biomed. Res. Int. 2021, 5575621–11. doi:10.1155/2021/5575621
Huang, Q., Lou, T., Lu, J., Wang, M., Chen, X., Xue, L., et al. (2022). Major ginsenosides from Panax ginseng promote aerobic cellular respiration and SIRT1-mediated mitochondrial biosynthesis in cardiomyocytes and neurons. J. Ginseng Res. 46, 759–770. doi:10.1016/j.jgr.2022.02.002
Ibrahim, M. A., Bakhaat, G. A., Tammam, H. G., Mohamed, R. M., and El-Naggar, S. A. (2019). Cardioprotective effect of green tea extract and vitamin E on Cisplatin-induced cardiotoxicity in mice: toxicological, histological and immunohistochemical studies. Biomed. Pharmacother. 113, 108731. doi:10.1016/j.biopha.2019.108731
INCHEM (1997). Nerium oleander L. [WWW Document]. Available online at: https://inchem.org/documents/pims/plant/pim366.htm#SectionTitle:1.3%20%20Common%20name(s)%20and%20synonyms (Accessed 20 October, 2023).
Institute of Medicine and National Research Council of the National Academies (2005). Dietary supplements: a framework for evaluating safety. Washington, DC: National Academies Press. doi:10.17226/10882
Jia, L., Zhao, Y., and Liang, X.-J. (2009). Current evaluation of the millennium phytomedicine- Ginseng (II): collected chemical entities, modern pharmacology, and clinical applications emanated from traditional Chinese medicine. Curr. Med. Chem. 16, 2924–2942. doi:10.2174/092986709788803204
Kaese, S., and Verheule, S. (2012). Cardiac electrophysiology in mice: a matter of size. Front. Physiol. 3, 345. doi:10.3389/fphys.2012.00345
Kanda, Y., Yamazaki, D., Osada, T., Yoshinaga, T., and Sawada, K. (2018). Development of torsadogenic risk assessment using human induced pluripotent stem cell-derived cardiomyocytes: Japan iPS Cardiac Safety Assessment (JiCSA) update. J. Pharmacol. Sci. 138, 233–239. doi:10.1016/j.jphs.2018.10.010
Karakikes, I., Ameen, M., Termglinchan, V., and Wu, J. C. (2015). Human induced pluripotent stem cell-derived cardiomyocytes: insights into molecular, cellular, and functional phenotypes. Circ. Res. 117, 80–88. doi:10.1161/CIRCRESAHA.117.305365
Karimian, A., and Mahmoudi, L. (2024). Silymarin’s potential in countering drug-induced cardiotoxicity, nephrotoxicity, and hepatotoxicity: a narrative review. Trends Pharm. Sci. 10. doi:10.30476/tips.2024.100638.1220
Khan, G., Haque, S. E., Anwer, T., Ahsan, M. N., Safhi, M. M., and Alam, M. F. (2014). Cardioprotective effect of green tea extract on doxorubicin-induced cardiotoxicity in rats. Acta Pol. Pharm. 71, 861–868.
Khordadmehr, M., and Nazifi, S. (2018). Study of troponin, creatine kinase biomarkers, and histopathological lesions in experimental Nerium oleander toxicity in rats and mice. J. Vet. Res. 62, 97–102. doi:10.1515/jvetres-2018-0013
Kim, J.-H. (2012). Cardiovascular diseases and Panax ginseng: a review on molecular mechanisms and medical applications. J. Ginseng Res. 36, 16–26. doi:10.5142/jgr.2012.36.1.16
Kim, J. J., Yang, L., Lin, B., Zhu, X., Sun, B., Kaplan, A. D., et al. (2015). Mechanism of automaticity in cardiomyocytes derived from human induced pluripotent stem cells. J. Mol. Cell. Cardiol. 81, 81–93. doi:10.1016/j.yjmcc.2015.01.013
Kim, Y.-S., Woo, J.-Y., Han, C.-K., and Chang, I.-M. (2015). Safety analysis of Panax ginseng in randomized clinical trials: a systematic review. Medicines 2, 106–126. doi:10.3390/medicines2020106
Kretzschmar, T., Bekhite, M. M., Wu, J. M. F., Haase, D., Förster, M., Müller, T., et al. (2021). Long-chain and very long-chain ceramides mediate doxorubicin-induced toxicity and fibrosis. Int. J. Mol. Sci. 22, 11852. doi:10.3390/ijms222111852
Kruegel, A. C., and Grundmann, O. (2018). The medicinal chemistry and neuropharmacology of kratom: a preliminary discussion of a promising medicinal plant and analysis of its potential for abuse. Neuropharmacology 134, 108–120. doi:10.1016/j.neuropharm.2017.08.026
Kulić, Ž., Lehner, M. D., and Dietz, G. P. H. (2022). Ginkgo biloba leaf extract EGb 761® as a paragon of the product by process concept. Front. Pharmacol. 13, 1007746. doi:10.3389/fphar.2022.1007746
Kuzyk, C. L., Anderson, C. C., and Roede, J. R. (2020). Simvastatin induces delayed apoptosis through disruption of glycolysis and mitochondrial impairment in neuroblastoma cells. Clin. Transl. Sci. 13, 563–572. doi:10.1111/cts.12740
Lange, M., Zeng, Y., Knight, A., Windebank, A., and Trushina, E. (2012). Comprehensive method for culturing embryonic dorsal root ganglion neurons for seahorse extracellular flux XF24 analysis. Front. Neurol. 3, 175. doi:10.3389/fneur.2012.00175
Langford, S. D., and Boor, P. J. (1996). Oleander toxicity: an examination of human and animal toxic exposures. Toxicology 109, 1–13. doi:10.1016/0300-483X(95)03296-R
Lee, P., Klos, M., Bollensdorff, C., Hou, L., Ewart, P., Kamp, T. J., et al. (2012). Simultaneous voltage and calcium mapping of genetically purified human induced pluripotent stem cell-derived cardiac myocyte monolayers. Circ. Res. 110, 1556–1563. doi:10.1161/CIRCRESAHA.111.262535
Leung, D. T. H., and Chu, S. (2018). “Measurement of oxidative stress: mitochondrial function using the seahorse System,” in Preeclampsia, methods in molecular biology. Editors P. Murthi, and C. Vaillancourt (New York, NY: Springer), 285–293. doi:10.1007/978-1-4939-7498-6_22
Li, S., Yu, L., Shi, Q., Liu, Y., Zhang, Y., Wang, S., et al. (2022). An insight into current advances on pharmacology, pharmacokinetics, toxicity and detoxification of aconitine. Biomed. Pharmacother. 151, 113115. doi:10.1016/j.biopha.2022.113115
Lickiss, B., Hunker, J., Bhagwan, J., Linder, P., Thomas, U., Lotay, H., et al. (2024). Chamber-specific contractile responses of atrial and ventricular hiPSC-cardiomyocytes to GPCR and ion channel targeting compounds: a microphysiological system for cardiac drug development. J. Pharmacol. Toxicol. Methods 128, 107529. doi:10.1016/j.vascn.2024.107529
Lin, Y.-S., Tsai, Y.-J., Tsay, J.-S., and Lin, J.-K. (2003). Factors affecting the levels of tea polyphenols and caffeine in tea leaves. J. Agric. Food Chem. 51, 1864–1873. doi:10.1021/jf021066b
Lin, C.-C., Chan, T. Y. K., and Deng, J.-F. (2004). Clinical features and management of herb-induced aconitine poisoning. Ann. Emerg. Med. 43, 574–579. doi:10.1016/j.annemergmed.2003.10.046
Lin, C.-C., Yang, C.-C., Phua, D.-H., Deng, J.-F., and Lu, L.-H. (2010). An outbreak of foxglove leaf poisoning. J. Chin. Med. Assoc. 73, 97–100. doi:10.1016/S1726-4901(10)70009-5
Linden, C. H., Vellman, W. P., and Rumack, B. (1985). Yohimbine: a new street drug. Ann. Emerg. Med. 14, 1002–1004. doi:10.1016/S0196-0644(85)80249-3
Liu, R., Li, X., Huang, N., Fan, M., and Sun, R. (2020). “Toxicity of traditional Chinese medicine herbal and mineral products,” in Advances in pharmacology (Cambridge MA: Academic Press), 301–346. doi:10.1016/bs.apha.2019.08.001
Liu, C., Shen, M., Liu, Y., Manhas, A., Zhao, S. R., Zhang, M., et al. (2024). CRISPRi/a screens in human iPSC-cardiomyocytes identify glycolytic activation as a druggable target for doxorubicin-induced cardiotoxicity. Cell Stem Cell 31, 1760–1776.e9. doi:10.1016/j.stem.2024.10.007
Lo Faro, A. F., Di Trana, A., La Maida, N., Tagliabracci, A., Giorgetti, R., and Busardò, F. P. (2020). Biomedical analysis of New Psychoactive Substances (NPS) of natural origin. J. Pharm. Biomed. Anal. 179, 112945. doi:10.1016/j.jpba.2019.112945
Lubarska, M., Hałasiński, P., Hryhorowicz, S., Mahadea, D. S., Łykowska-Szuber, L., Eder, P., et al. (2023). Liver dangers of herbal products: a case report of Ashwagandha-induced liver injury. Int. J. Environ. Res. Public. Health 20, 3921. doi:10.3390/ijerph20053921
Luz, A. L., Smith, L. L., Rooney, J. P., and Meyer, J. N. (2015). Seahorse Xfe24 extracellular flux analyzer-based analysis of cellular respiration in Caenorhabditis elegans. Curr. Protoc. Toxicol. 66, 25.7.1–25.7.15. doi:10.1002/0471140856.tx2507s66
Ma, J., Ross, L., Grube, C., and Wang, H.-S. (2024). Toxicity of low dose bisphenols in human iPSC-derived cardiomyocytes and human cardiac organoids – impact on contractile function and hypertrophy. Chemosphere 353, 141567. doi:10.1016/j.chemosphere.2024.141567
Malan, D., Zhang, M., Stallmeyer, B., Müller, J., Fleischmann, B. K., Schulze-Bahr, E., et al. (2016). Human iPS cell model of type 3 long QT syndrome recapitulates drug-based phenotype correction. Basic Res. Cardiol. 111, 14. doi:10.1007/s00395-016-0530-0
Mandal, S. K., Maji, A. K., Mishra, S. K., Ishfaq, P. M., Devkota, H. P., Silva, A. S., et al. (2020). Goldenseal (Hydrastis canadensis L.) and its active constituents: a critical review of their efficacy and toxicological issues. Pharmacol. Res. 160, 105085. doi:10.1016/j.phrs.2020.105085
Martin, S. S., Aday, A. W., Almarzooq, Z. I., Anderson, C. A. M., Arora, P., Avery, C. L., et al. (2024). Centennial collection: health applications of digital technologies. Circulation 149, 1701–1703. doi:10.1161/CIRCULATIONAHA.124.068243
Mathews, J. D., Riley, M. D., Fejo, L., Munoz, E., Milns, N. R., Gardner, I. D., et al. (1988). Effects of the heavy usage of Kava on physical health: summary of a pilot survey in an aboriginal community. Med. J. Aust. 148, 548–555. doi:10.5694/j.1326-5377.1988.tb93809.x
McIntyre, I. M., Trochta, A., Stolberg, S., and Campman, S. C. (2015). Mitragynine ‘Kratom’ related fatality: a case report with postmortem concentrations. J. Anal. Toxicol. 39, 152–155. doi:10.1093/jat/bku137
Meireles, V., Rosado, T., Barroso, M., Soares, S., Gonçalves, J., Luís, Â., et al. (2019). Mitragyna speciosa: clinical, toxicological aspects and analysis in biological and non-biological samples. Med. Basel Switz. 6, 35. doi:10.3390/medicines6010035
Mendonça, S., De Vasconcelos, C., Cruz, J., Roman-Campos, D., Menezes-Filho, J., Anjos-Neto, R., et al. (2017). (+)-Usnic acid isolated from the lichen Cladonia substellata impairs myocardial contractility. Planta Medica Int. Open 4, e59–e65. doi:10.1055/s-0043-114423
Miller, R. J., Jackson, K. G., Dadd, T., Mayes, A. E., Brown, A. L., Lovegrove, J. A., et al. (2012). The impact of the catechol-O-methyltransferase genotype on vascular function and blood pressure after acute green tea ingestion. Mol. Nutr. Food Res. 56, 966–975. doi:10.1002/mnfr.201100726
Mitchell, C. A., Dever, J. T., Gafner, S., Griffiths, J. C., Marsman, D. S., Rider, C., et al. (2022). The Botanical Safety Consortium: a public-private partnership to enhance the botanical safety toolkit. Regul. Toxicol. Pharmacol. 128, 105090. doi:10.1016/j.yrtph.2021.105090
Monteiro Da Rocha, A., Allan, A., Block, T., Creech, J., and Herron, T. J. (2023). High-throughput cardiotoxicity screening using mature human induced pluripotent stem cell-derived cardiomyocyte monolayers. J. Vis. Exp. 64364. doi:10.3791/64364
National Toxicology Program (2010). Toxicology and carcinogenesis studies of goldenseal root powder (Hydrastis canadensis) in F344/N rats and B6C3F1 mice (feed studies). Natl. Toxicol. Program Tech. Rep. Ser., 1–188.
National Toxicology Program (2011). Toxicology and carcinogenesis studies of milk thistle extract (CAS No. 84604-20-6) in F344/N rats and B6C3F1 mice (feed studies). Natl. Toxicol. Program Tech. Rep. Ser., 1–177.
National Toxicology Program (2012). Toxicology and carcinogenesis studies of kava kava extract (CAS No. 9000-38-8) in F344/N rats and B6C3F1 mice (Gavage Studies). Natl. Toxicol. Program Tech. Rep. Ser. 1–186.
National Toxicology Program (2016). Toxicology studies of green tea extract in F344/NTac rats and B6C3F1/N mice and toxicology and carcinogenesis studies of green tea extract in Wistar Han [Crl:WI(Han)] rats and B6C3F1/N mice (gavage studies). Natl. Toxicol. Program Tech. Rep. Ser. 1–238. doi:10.22427/NTP-TR-585
National Toxicology Program (2022). Toxicity studies of Usnea lichens containing (+/−) usnic acid (CASRN 125-46-2) administered in feed to F344/N NCTR Rats and B6C3F1/NCTR mice. Natl. Toxicol. Program Tech. Rep. Ser., 1–143. doi:10.22427/NTP-TOX-105
National Toxicology Program (2023). Botanical safety consortium – Chemical analysis. doi:10.22427/NTP-DATA-500-007-001-000-3
NCCIH (2020). Asian Ginseng [WWW Document]. US Natl. Cent. Complement. Integr. Health. Available online at: https://www.nccih.nih.gov/health/asian-ginseng (Accessed October 19, 2023).
NCCIH (2021). Goldenseal. [WWW Document]. NCCIH. Available online at: https://www.nccih.nih.gov/health/goldenseal (Accessed December 26, 2024).
NCCIH (2023). Ashwagandha [WWW Document]. US Natl. Cent. Complement. Integr. Health. Available online at: https://www.nccih.nih.gov/health/ashwagandha (Accessed January 4, 2024).
NICCIH (2020). Green Tea [WWW Document]. US Natl. Cent. Complement. Integr. Health. Available online at: https://www.nccih.nih.gov/health/green-tea (Accessed October 20, 2023).
NICHD (2006). “Blue cohosh,” in Drugs and lactation database (LactMed®) (Bethesda, MD: National Institute of Child Health and Human Development).
NIDDK (2012). LiverTox: clinical and research Information on drug-induced liver injury. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases.
Nilius, B., Boldt, W., and Benndorf, K. (1986). Properties of aconitine-modified sodium channels in single cells of mouse ventricular myocardium. Gen. Physiol. Biophys. 5, 473–484.
Norton, S. A., and Ruze, P. (1994). Kava dermopathy. J. Am. Acad. Dermatol. 31, 89–97. doi:10.1016/S0190-9622(94)70142-3
Office of the Commissioner (2023). FDA and kratom. [WWW Document]. FDA. Available online at: https://www.fda.gov/news-events/public-health-focus/fda-and-kratom (Accessed November 29, 2023).
Ohno, Y., Chiba, S., Uchigasaki, S., Uchima, E., Nagamori, H., Mizugaki, M., et al. (1992). The influence of tetrodotoxin on the toxic effects of aconitine in vivo. Tohoku J. Exp. Med. 167, 155–158. doi:10.1620/tjem.167.155
O’Shea, C., Kabir, S. N., Holmes, A. P., Lei, M., Fabritz, L., Rajpoot, K., et al. (2020). Cardiac optical mapping – State-of-the-art and future challenges. Int. J. Biochem. Cell Biol. 126, 105804. doi:10.1016/j.biocel.2020.105804
Pal, A., Bhushan, B., and Khanum, F. (2012). “Therapeutic uses of Withania somnifera (Ashwagandha),” in Recent progress in medicinal plants (RPMP), 34, 97–118.
Patel, D., Sorkin, B. C., Mitchell, C. A., Embry, M. R., Rina-Kong, S., Adams, R. E., et al. (2023). Improving the rigor and utility of botanical toxicity studies: recommended resources. Regul. Toxicol. Pharmacol. 144, 105471. doi:10.1016/j.yrtph.2023.105471
Pioner, J. M., Santini, L., Palandri, C., Martella, D., Lupi, F., Langione, M., et al. (2019). Optical investigation of action potential and calcium handling maturation of hiPSC-cardiomyocytes on biomimetic substrates. Int. J. Mol. Sci. 20, 3799. doi:10.3390/ijms20153799
Povšnar, M., Koželj, G., Kreft, S., and Lumpert, M. (2017). Rare tradition of the folk medicinal use of Aconitum spp. is kept alive in Solčavsko, Slovenia. J. Ethnobiol. Ethnomedicine 13, 45. doi:10.1186/s13002-017-0171-x
Pullela, R., Young, L., Gallagher, B., Avis, S. P., and Randell, E. W. (2008). A case of fatal aconitine poisoning by monkshood ingestion. J. Forensic Sci. 53, 491–494. doi:10.1111/j.1556-4029.2007.00647.x
Puschner, B. (2012). “Cardiotoxic plants,” in Clinical veterinary advisor (St. Louis, MI: Saunders), 89–91. doi:10.1016/B978-1-4160-9979-6.00057-X
Pyne, M. E., Narcross, L., and Martin, V. J. J. (2019). Engineering plant secondary metabolism in microbial systems. Plant Physiol. 179, 844–861. doi:10.1104/pp.18.01291
Reddy, N., Lynch, B., Gujral, J., and Karnik, K. (2023). Regulatory landscape of alternatives to animal testing in food safety evaluations with a focus on the western world. Regul. Toxicol. Pharmacol. 143, 105470. doi:10.1016/j.yrtph.2023.105470
Rombolà, L., Scuteri, D., Marilisa, S., Watanabe, C., Morrone, L. A., Bagetta, G., et al. (2020). Pharmacokinetic interactions between herbal medicines and drugs: their mechanisms and clinical relevance. Life 10, 106. doi:10.3390/life10070106
Ru, Y., Luo, Y., Zhou, Y., Kuai, L., Sun, X., Xing, M., et al. (2019). Adverse events associated with treatment of Tripterygium wilfordii Hook F: a quantitative evidence synthesis. Front. Pharmacol. 10, 1250. doi:10.3389/fphar.2019.01250
Sala, L., Bellin, M., and Mummery, C. L. (2017a). Integrating cardiomyocytes from human pluripotent stem cells in safety pharmacology: has the time come? Br. J. Pharmacol. 174, 3749–3765. doi:10.1111/bph.13577
Sala, L., Ward-van Oostwaard, D., Tertoolen, L. G. J., Mummery, C. L., and Bellin, M. (2017b). Electrophysiological analysis of human pluripotent stem cell-derived cardiomyocytes (hPSC-CMs) using multi-electrode arrays (MEAs). J. Vis. Exp. 55587, 55587. doi:10.3791/55587
Sarris, J., LaPorte, E., and Schweitzer, I. (2011). Kava: a comprehensive review of efficacy, safety, and psychopharmacology. Aust. N. Z. J. Psychiatry 45, 27–35. doi:10.3109/00048674.2010.522554
Satsuka, A., and Kanda, Y. (2020). Cardiotoxicity assessment of drugs using human iPS cell-derived cardiomyocytes: toward proarrhythmic risk and cardio-oncology. Curr. Pharm. Biotechnol. 21, 765–772. doi:10.2174/1389201020666190628143345
Savoji, H., Mohammadi, M. H., Rafatian, N., Toroghi, M. K., Wang, E. Y., Zhao, Y., et al. (2019). Cardiovascular disease models: a game changing paradigm in drug discovery and screening. Biomaterials 198, 3–26. doi:10.1016/j.biomaterials.2018.09.036
Schep, L. J., Slaughter, R. J., and Beasley, D. M. G. (2009). Nicotinic plant poisoning. Clin. Toxicol. 47, 771–781. doi:10.1080/15563650903252186
Schramm, S., Köhler, N., and Rozhon, W. (2019). Pyrrolizidine alkaloids: biosynthesis, biological activities and occurrence in crop plants. Mol. Basel Switz. 24, 498. doi:10.3390/molecules24030498
Scott, C. C., and Chen, K. K. (1943). The pharmacological action of n-methylcytisine. J. Pharmacol. Exp. Ther. 79, 334–339. doi:10.1016/s0022-3565(25)09437-6
Sethi, R., Hoang, N., Ravishankar, D. A., McCracken, M., and Manzardo, A. M. (2020). Kratom (Mitragyna speciosa): friend or foe? Prim. Care Companion CNS Disord. 22, 19nr02507. doi:10.4088/PCC.19nr02507
Shafaattalab, S., Li, A. Y., Lin, E., Stevens, C. M., Dewar, L. J., Lynn, F. C., et al. (2019). In vitro analyses of suspected arrhythmogenic thin filament variants as a cause of sudden cardiac death in infants. Proc. Natl. Acad. Sci. U. S. A. 116, 6969–6974. doi:10.1073/pnas.1819023116
Shah, S. A., Occiano, A., Nguyen, T. A., Chan, A., Sky, J. C., Bhattacharyya, M., et al. (2016). Electrocardiographic and blood pressure effects of energy drinks and Panax ginseng in healthy volunteers: a randomized clinical trial. Int. J. Cardiol. 218, 318–323. doi:10.1016/j.ijcard.2016.05.007
Siddiqui, S., Ahmed, N., Goswami, M., Chakrabarty, A., and Chowdhury, G. (2021). DNA damage by Withanone as a potential cause of liver toxicity observed for herbal products of Withania somnifera (Ashwagandha). Curr. Res. Toxicol. 2, 72–81. doi:10.1016/j.crtox.2021.02.002
Smith, T., Bauman, H., and Resetar, H. (2024). US sales of herbal supplements decline slightly in 2022. HerbalGram, 52–69.
Soares, R. B., Dinis-Oliveira, R. J., and Oliveira, N. G. (2022). An updated review on the psychoactive, toxic and anticancer properties of Kava. J. Clin. Med. 11, 4039. doi:10.3390/jcm11144039
Song, C.-Y., Feng, M.-X., Li, L., Wang, P., Lu, X., and Lu, Y.-Q. (2023). Tripterygium wilfordii Hook F. ameliorates paraquat-induced lung injury by reducing oxidative stress and ferroptosis via Nrf2/HO-1 pathway. Ecotoxicol. Environ. Saf. 252, 114575. doi:10.1016/j.ecoenv.2023.114575
Speers, A. B., Cabey, K. A., Soumyanath, A., and Wright, K. M. (2021). Effects of Withania somnifera (Ashwagandha) on stress and the stress-related neuropsychiatric disorders anxiety, depression, and insomnia. Curr. Neuropharmacol. 19, 1468–1495. doi:10.2174/1570159X19666210712151556
Strauss, D. G., Gintant, G., Li, Z., Wu, W., Blinova, K., Vicente, J., et al. (2019). Comprehensive in vitro proarrhythmia Assay (CiPA) update from a cardiac safety research Consortium/Health and Environmental Sciences Institute/FDA meeting. Ther. Innov. Regul. Sci. 53, 519–525. doi:10.1177/2168479018795117
Strzelecki, A., Pichon, N., Gaulier, J. M., Amiel, J. B., Champy, P., and Clavel, M. (2010). Acute toxic herbal intake in a suicide attempt and fatal refractory ventricular arrhythmia. Basic Clin. Pharmacol. Toxicol. 107, 698–699. doi:10.1111/j.1742-7843.2010.00566.x
Takasuna, K., Kazusa, K., and Hayakawa, T. (2020). Comprehensive Cardiac Safety Assessment using hiPS-cardiomyocytes (Consortium for Safety Assessment using Human iPS Cells: CSAHi). Curr. Pharm. Biotechnol. 21, 829–841. doi:10.2174/1389201020666191024172425
Tay, Y. L., Teah, Y. F., Chong, Y. M., Jamil, M. F. A., Kollert, S., Adenan, M. I., et al. (2016). Mitragynine and its potential blocking effects on specific cardiac potassium channels. Toxicol. Appl. Pharmacol. 305, 22–39. doi:10.1016/j.taap.2016.05.022
Theoharides, T. C. (1997). Sudden death of a healthy college student related to ephedrine toxicity from a Ma Huang-containing drink. J. Clin. Psychopharmacol. 17, 437–439. doi:10.1097/00004714-199710000-00025
Thomas, G. P., and Tripathi, R. M. (1986). Effects of alpha-adrenoceptor agonists and antagonists on ouabain-induced arrhythmias and cardiac arrest in Guinea-pig. Br. J. Pharmacol. 89, 385–388. doi:10.1111/j.1476-5381.1986.tb10271.x
US FDA (2004a). Ephedra and ephedrine alkaloids for weight loss and athletic performance. [WWW Document]. Available online at: https://ods.od.nih.gov/factsheets/EphedraandEphedrine-HealthProfessional/(Accessed November 30, 2023).
US FDA (2004b). Final rule declaring dietary supplements containing ephedrine alkaloids adulterated because they present an unreasonable risk. final rule. Fed. Regist. 69, 6787–6854.
US FDA (2005). S7B nonclinical evaluation of the potential for delayed ventricular repolarization (QT interval prolongation) by human pharmaceuticals (No. FDA-2004-D-0366). Rockville, MD: US Food and Drug Administration. Center for Biologics Evaluation and Research Center for Drug Evaluation and Research.
Vento, A. E., De Persis, S., De Filippis, S., Schifano, F., Napoletano, F., Corkery, J. M., et al. (2021). Case report: treatment of kratom use disorder with a classical tricyclic antidepressant. Front. Psychiatry 12, 640218. doi:10.3389/fpsyt.2021.640218
Vijayakumar, A., and Kim, J.-H. (2023). Ginseng and ginsenosides on cardiovascular and pulmonary diseases; Pharmacological potentials for the coronavirus (COVID-19). J. Ginseng Res. 48, 113–121. doi:10.1016/j.jgr.2023.10.002
Vithayathil, M. K., and Edwards, M. (2016). Comfrey herbal remedy causing second-degree heart block: do not be outfoxed by digitalis. BMJ Case Rep. 2016, bcr2016216995. doi:10.1136/bcr-2016-216995
Waidyanatha, S., Collins, B. J., Cristy, T., Embry, M., Gafner, S., Johnson, H., et al. (2024). Advancing botanical safety: a strategy for selecting, sourcing, and characterizing botanicals for developing toxicological tools. Food Chem. Toxicol. 186, 114537. doi:10.1016/j.fct.2024.114537
Wang, H., Peng, D., and Xie, J. (2009). Ginseng leaf-stem: bioactive constituents and pharmacological functions. Chin. Med. 4, 20. doi:10.1186/1749-8546-4-20
Wang, X., Zhang, Z., Wu, G., Nan, C., Shen, W., Hua, Y., et al. (2016). Green tea extract catechin improves internal cardiac muscle relaxation in RCM mice. J. Biomed. Sci. 23, 51. doi:10.1186/s12929-016-0264-1
Wang, Y., Liao, J., Luo, Y., Li, M., Su, X., Yu, B., et al. (2023). Berberine alleviates doxorubicin-induced myocardial injury and fibrosis by eliminating oxidative stress and mitochondrial damage via promoting nrf-2 pathway activation. Int. J. Mol. Sci. 24, 3257. doi:10.3390/ijms24043257
Watkins, L. L., Connor, K. M., and Davidson, J. R. (2001). Effect of kava extract on vagal cardiac control in generalized anxiety disorder: preliminary findings. J. Psychopharmacol. Oxf. Engl. 15, 283–286. doi:10.1177/026988110101500407
White, L. M., Gardner, S. F., Gurley, B. J., Marx, M. A., Wang, P., and Estes, M. (1997). Pharmacokinetics and cardiovascular effects of Ma-Huang (Ephedra sinica) in normotensive adults. J. Clin. Pharmacol. 37, 116–122. doi:10.1002/j.1552-4604.1997.tb04769.x
Wiciński, M., Fajkiel-Madajczyk, A., Kurant, Z., Liss, S., Szyperski, P., Szambelan, M., et al. (2024). Ashwagandha’s multifaceted effects on human health: impact on vascular endothelium, inflammation, lipid metabolism, and cardiovascular outcomes-a review. Nutrients 16, 2481. doi:10.3390/nu16152481
Wu, M., Hu, Y., Ali, Z., Khan, I. A., Verlangeiri, A. J., and Dasmahapatra, A. K. (2010). Teratogenic Effects of Blue Cohosh (Caulophyllum thalictroides) in Japanese Medaka (Oryzias latipes) are probably mediated through GATA2/EDN1 signaling pathway. Chem. Res. Toxicol. 23, 1405–1416. doi:10.1021/tx100205a
Wu, K.-M., Yao, J., and Boring, D. (2011). Green tea extract-induced lethal toxicity in fasted but not in nonfasted dogs. Int. J. Toxicol. 30, 19–20. doi:10.1177/1091581810387445
Wu, J., Wang, X., Chung, Y. Y., Koh, C. H., Liu, Z., Guo, H., et al. (2017). L-type calcium channel inhibition contributes to the proarrhythmic effects of aconitine in human cardiomyocytes. PLoS One 12, e0168435. doi:10.1371/journal.pone.0168435
Xu, Y., Li, W., Wen, R., Sun, J., Liu, X., Zhao, S., et al. (2023). Voltage-gated sodium channels, potential targets of Tripterygium wilfordii Hook F. to exert activity and produce toxicity. J. Ethnopharmacol. 311, 116448. doi:10.1016/j.jep.2023.116448
Yanagida, S., and Kanda, Y. (2024). Prediction of cardiac toxicity by anti-cancer drugs using iPSC cardiomyocytes. Yakugaku Zasshi 144, 265–271. doi:10.1248/yakushi.23-00164-3
Yanagida, S., Kawagishi, H., and Kanda, Y. (2024). Cardiotoxicity risk assessment of anti-cancer drugs and future perspectives. Folia Pharmacol. Jpn. 159, 83–89. doi:10.1254/fpj.23094
Yang, X., Ribeiro, A. J. S., Pang, L., and Strauss, D. G. (2022). Use of human iPSC-CMs in nonclinical regulatory studies for cardiac safety assessment. Toxicol. Sci. 190, 117–126. doi:10.1093/toxsci/kfac095
Yokouchi, Y., Imaoka, M., Niino, N., Kiyosawa, N., Sayama, A., and Jindo, T. (2015). (+)-Usnic acid-induced myocardial toxicity in rats. Toxicol. Pathol. 43, 424–434. doi:10.1177/0192623313504308
Yu, Z., Samavat, H., Dostal, A. M., Wang, R., Torkelson, C. J., Yang, C. S., et al. (2017). Effect of green tea supplements on liver enzyme elevation: results from a randomized intervention study in the United States. Cancer Prev. Res. (Phila. PA.) 10, 571–579. doi:10.1158/1940-6207.CAPR-17-0160
Zeng, H., Wang, J., Clouse, H., Lagrutta, A., and Sannajust, F. (2019). HiPSC-CMs from different sex and ethnic origin donors exhibit qualitatively different responses to several classes of pharmacological challenges. J. Pharmacol. Toxicol. Methods 99, 106598. doi:10.1016/j.vascn.2019.106598
Zhai, J., Dong, X., Yan, F., Guo, H., and Yang, J. (2022). Oleandrin: a systematic review of its natural sources, structural properties, detection methods, pharmacokinetics and toxicology. Front. Pharmacol. 13, 822726. doi:10.3389/fphar.2022.822726
Zhang, C., Sun, P.-P., Guo, H.-T., Liu, Y., Li, J., He, X.-J., et al. (2016). Safety profiles of Tripterygium wilfordii Hook F: a systematic review and meta-analysis. Front. Pharmacol. 7, 402. doi:10.3389/fphar.2016.00402
Zhang, Q., Chen, X., Chen, S., Liu, Z., Wan, R., and Li, J. (2016). Fatal honey poisoning caused by Tripterygium wilfordii Hook F in southwest China: a case series. Wilderness Environ. Med. 27, 271–273. doi:10.1016/j.wem.2016.01.002
Zhao, Z., Lan, H., Li, X., El-Battrawy, I., Xu, Q., Huang, M., et al. (2018). Isolated intracranial arterial hypertension. Eur. Heart J. 39, 3674. doi:10.1093/eurheartj/ehy550
Zhao, W., Xiao, L., Pan, L., Ke, X., Zhang, Y., Zhong, D., et al. (2019). Cardiac toxicity of Triptergium wilfordii Hook F. may correlate with its inhibition to hERG channel. Heliyon 5, e02527. doi:10.1016/j.heliyon.2019.e02527
Keywords: botanical safety consortium, botanicals, cardiotoxicity, in vitro, new approach methods, complex mixtures
Citation: Krzykwa J, Chaudhari HS, Monteiro Da Rocha A, Gossmann M, Hoffmann P, Khokhar Y, Meyer N, Park J-YK, Sprando R, Vaidyanathan R, Westerink RHS, Wu JC, Yourick J, Zhao SR and Mitchell CA (2025) Developing an approach for evaluating the cardiotoxic potential of botanicals. Front. Toxicol. 7:1646044. doi: 10.3389/ftox.2025.1646044
Received: 12 June 2025; Accepted: 10 October 2025;
Published: 30 October 2025.
Edited by:
Matthew Stevenson, Imperial Brands PLC, United KingdomReviewed by:
Giuseppina Iachetta, Italian Institute of Technology (IIT), ItalyJessica Palmer, Stemina Biomarker Discovery, United States
Copyright © 2025 Krzykwa, Chaudhari, Monteiro Da Rocha, Gossmann, Hoffmann, Khokhar, Meyer, Park, Sprando, Vaidyanathan, Westerink, Wu, Yourick, Zhao and Mitchell. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Julie Krzykwa, amtyenlrd2FAaGVzaWdsb2JhbC5vcmc=
 Julie Krzykwa
Julie Krzykwa Hemantkumar S. Chaudhari
Hemantkumar S. Chaudhari Andre Monteiro Da Rocha3
Andre Monteiro Da Rocha3 Ravi Vaidyanathan
Ravi Vaidyanathan Remco H. S. Westerink
Remco H. S. Westerink Joseph C. Wu
Joseph C. Wu Jeffrey Yourick
Jeffrey Yourick Shane R. Zhao
Shane R. Zhao Constance A. Mitchell
Constance A. Mitchell