- 1Department of Biochemistry, Dong-A University, Busan, South Korea
- 2Department of Physiology, School of Medicine, Dong-A University, Busan, South Korea
- 3Immune-Network Pioneer Research Center, Dong-A University, Busan, South Korea
- 4The Laboratory of Chemistry of Marine Natural Products, G. B. Elyakov Pacific Institute of Bioorganic Chemistry, Far-Eastern Branch of the Russian Academy of Science, Vladivostok, Russia
Many marine triterpene glycosides have in vitro and in vivo activities with very low toxicity, suggesting that they are suitable agents for the prevention and treatment of different diseases, particularly cancer. However, the molecular mechanisms of action of natural marine compounds in cancer, immune, and other various cells are not fully known. This review focuses on the structural characteristics of marine triterpene glycosides and how these affect their biological activities and molecular mechanisms. In particular, the membranotropic and membranolytic activities of frondoside A and cucumariosides from sea cucumbers and their ability to induce cytotoxicity and apoptosis have been discussed, with a focus on structure-activity relationships. In addition, the structural characteristics and antitumor effects of stichoposide C and stichoposide D have been reviewed along with underlying their molecular mechanisms.
Introduction
Many marine natural products have biological activities and low toxicity suitable for administration and exhibit wide diversity in their mechanisms of action. Glycosides, substances consisting of a sugar moiety attached to a triterpene or steroid aglycone, are widely distributed in plants. Triterpene glycosides are also found in marine invertebrates and are characteristic secondary metabolites of echinoderms, octocorals, and sponges (Stonik et al., 1999; Kalinin et al., 2008; Bordbar et al., 2011).
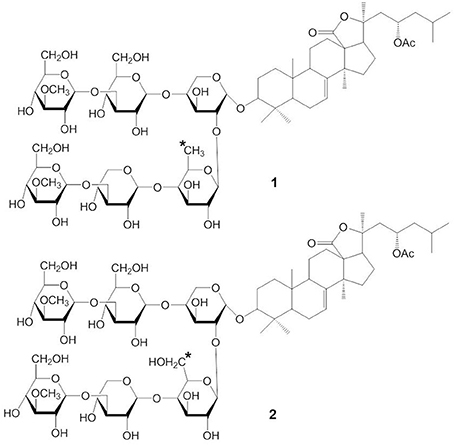
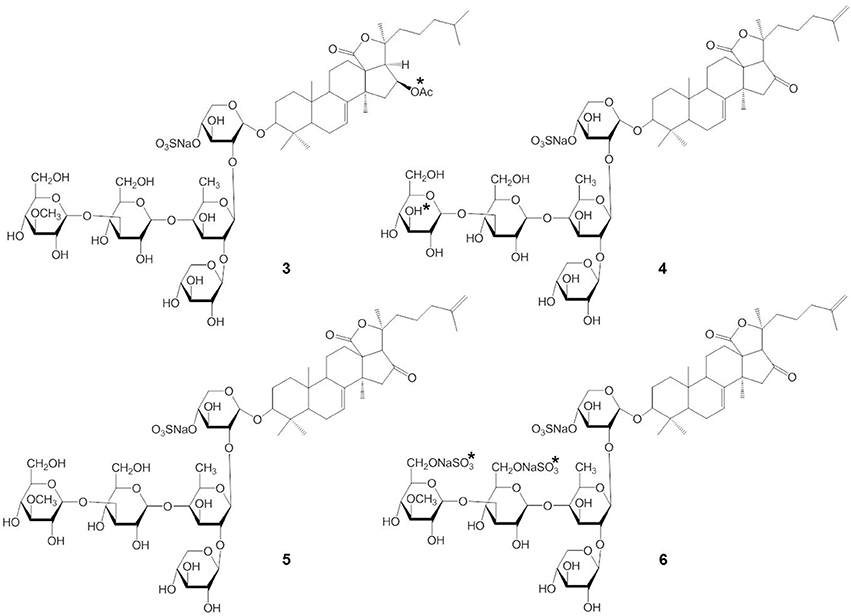
Stichoposide C (STC) (compound 1) and stichoposide D (STD) (compound 2) are hexaosides isolated from the holothurian Stichopus chloronotus (Figure 1) (Kitagawa et al., 1981; Stonik et al., 1982a). These compounds are also found in other representatives of the family Stichopodidae such as Thelenota ananas (Stonik et al., 1982b). The structural differences between STC and STD are a sugar residue; STC has quinovose, while STD has glucose as the second monosaccharide unit (indicated as the asterisk in compound 1 and 2). Frondoside A (compound 3) and cucumariosides are derived from the edible sea cucumbers Cucumaria frondosa and C. japonica, respectively (Girard et al., 1990; Stonik et al., 1999) (Figure 2). C. japonica is a source of several different cucumariosides such as cucumarioside A2-2 (compound 4), A4-2 (compound 5), and A7-1 (compound 6) (Figure 2) (Avilov et al., 1990; Drozdova et al., 1993, 1997; Stonik et al., 1999). Frondoside A and cucumariosides are pentaosides, with the main structural difference between the two compounds being the functional group at C-16 of the aglycone (acetoxy or keto group) and the third carbohydrate unit in the carbohydrate chain (indicated by an asterisk in compound 3 and 4). Interestingly, despite such similar structures, the biological activity and mechanism of frondoside A and cucumariosides appear to differ.
This review highlights the structural characteristics and mechanisms of action of marine triterpene glycosides, such as stichoposides, frondoside A, and cucumariosides. The biological activities and molecular mechanisms of several additional marine triterpene glycosides that have been studied are summarized.
The Structural Characteristics of Marine Triterpene Glycosides
Triterpene glycosides are the most abundant secondary metabolites in terrestrial plants and sea cucumbers. Marine triterpene glycosides are the predominant secondary metabolites of holothurians and are suggested to be responsible for their general cytotoxicity (Zhang et al., 2006a,b,c,d; Kim and Himaya, 2012; Colorado-Ríos et al., 2013). Last 20 years more than 100 new triterpene glycosides were isolated. Really, only by Russian group from Pacific Institute of Bioorganic Chemistry (PIBOC) at Vladivostok, about 30 new glycosides were isolated from Eupentacta (Cucumaria) fraudatrix (Silchenko et al., 2011, 2012a,b,c,d, 2013b,c), 14 new glycosides from Cucumaria frondosa (Girard et al., 1990; Silchenko et al., 2005a,b, 2007b), 7 from Stuarocucumis liouvillei (Antonov et al., 2008, 2011), 6 from Cladolabes schmeltzi (Silchenko et al., 2013d), 6 from Cucumaria okhotensis (Silchenko et al., 2007a, 2008), 5 from Synallactes nozawai (Silchenko et al., 2002), 5 from Actinocucumis typica (Silchenko et al., 2013a), 4 from Cucumaria conicospermium (Avilov et al., 2003), 3 from Mediterranean species (Silchenko et al., 2005c), 3 from Pentamera calcigera (Avilov et al., 2000a,b), 3 from Australostichopus (Stichopus) mollis (Moraes et al., 2005), 3 from Achlionice violaecuspidata (Antonov et al., 2009), 2 from Synapta maculata (Avilov et al., 2008), 1 from Cucumaria koriaiensis (Avilov et al., 1997), 1 from Psolus eximus (Kalinin et al., 1997), and so on. Several series of structures were also reviewed (Kalinin et al., 2012; Kim and Himaya, 2012). Reviews, completely described all the last glycosides were not yet published. Several early reviews are mentioned in our papers (Stonik et al., 1999; Kalinin et al., 2005). These are grouped into four main structural categories, based on their aglycone structures: 3β-hydroxyholost-9(11)-ene aglycone skeleton (structure 7), 9β H-3β-hydroxyholost-7-ene skeleton (structure 8), other holostane type glycosides and nonholostane glycosides (Figure 3) (Kim and Himaya, 2012).
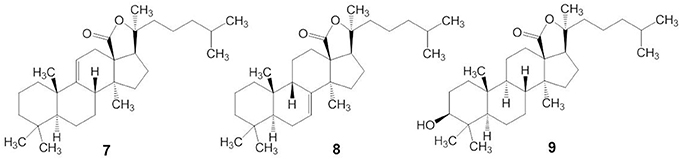
Figure 3. Structures of aglycone skeleton systems with 9(11) double bond (7), 9β-H-7(8)-unsaturation (8) and 3β, 20S-Dihydroxy-5α-lanostano-18(20)-lactone (9).
Triterpene glycosides of holothurians typically have carbohydrates and triterpenoid moieties (Kerr and Chen, 1995; Bordbar et al., 2011). The triterpenoid moieties consist of lanostane derivatives (Zhou et al., 2005) where the majority belongs to the holostane type (Dang et al., 2007). Holostane type triterpene glycosides include a 3β, 20S-dihydroxy-5α-lanostano-18(20)-lactone structural feature (structure 9). The glycone part of natural products isolated from the sea cucumbers consists of two to six sugar units and is linked to the C-3 position of the aglycone unit (Chiludil et al., 2003; Kalinin et al., 2005). Quinovose, glucose, 3-O-methylglucose, xylose, and 3-O-methylxylose are the main sugars present in the carbohydrate moieties of these glycosides (Iñiguez-Martinez et al., 2005). In the structure of the oligosaccharide chain, the first monosaccharide unit is always a xylose, whereas 3-O-methylglucose or 3-O-methylxylose is always at the terminus. In some glycosides, sulfate groups are attached to the oligosaccharide chain. Most of these are mono-sulfated glycosides with a few occurrences of di- and tri-sulfated glycosides (Chiludil et al., 2003).
Membranotropic and Membranolytic Effects of Triterpene Glycosides
Membranolytic effects such as increased membrane permeability, loss of barrier function, and the rupture of cell membrane are considered to be the basic mechanisms underlying a variety of biological activities exerted by triterpene glycosides from both sea cucumbers and higher plants. However, the molecular mechanisms of action of these compounds in biomembranes are not fully understood. The triterpene glycosides attach to cell membranes, interact with membrane lipids, and form glycoside-sterol complexes in biomembranes, modulating the membrane microviscosity and the activities of embedded membrane proteins (Stonik et al., 1999; Pislyagin et al., 2012). The formation of multimeric channels in sterol-containing lipid bilayers by triterpene glycosides may also be a basic mechanisms involved in increasing the permeability of membranes to ions and peptides (Li et al., 2005).
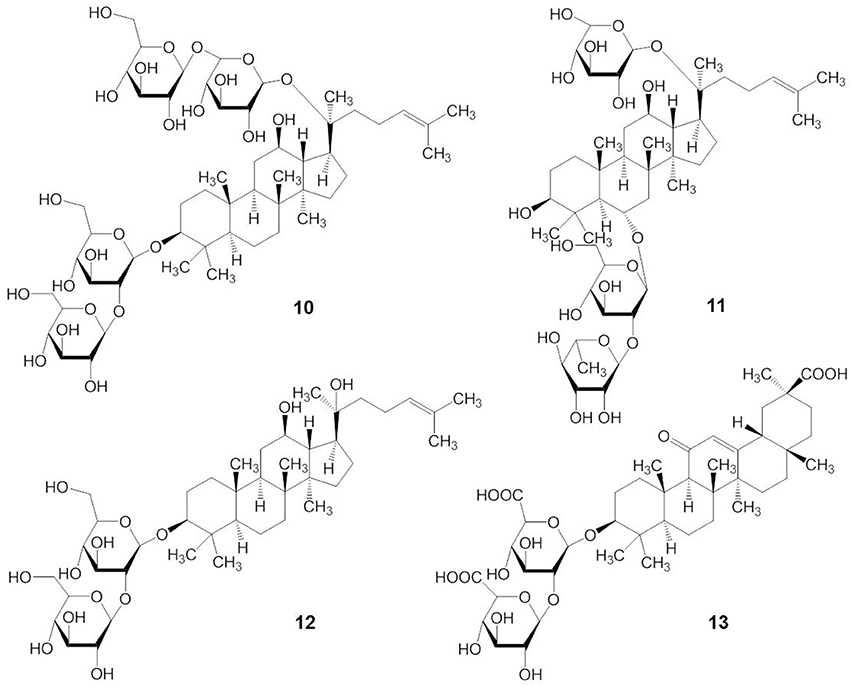
Although there are many subtle structural and functional differences between marine and plant triterpene glycosides, knowledge from earlier research with plant triterpene glycosides suggests that marine triterpene glycosides may have similar effects on membranes. For example, extensive studies on the membranotropic effects of plant triterpene glycosides have been performed for decades, especially with natural compounds from Panax ginseng C.A. Meyer (Im and Nah, 2013). The structures of some pharmacologically important plant triterpene glycosides are shown in Figure 4. Ginsenosides or ginseng saponins are major pharmacologically active ingredients of ginseng, which are composed with an aglycone of a dammarane skeleton and one or more covalently linked sugar moieties (Nah, 2014). Ginsenoside Rb1 has two glucopyranosyl sugar chains at C-3 and C-20 positions, respectively (compound 10) (Figure 4). Ginsenoside Re has one glucose-rhamnose disaccharide moiety at C-6 position and one glucopyranosyl moiety at C-20 position (compound 11). Ginsenoside Rg3 has two glucopyranosyl sugar chains only at C-3 position (compound 12). Glycyrrhizin, the main sweet-tasting constituent of licorice root, consists of a disaccharide of two glucuronic acids linked at C-3 position of the pentacyclic triterpene aglycone, glycyrrhetinic acid (compound 13). Recently, a detailed mechanism for the membrane permeabilization induced by the triterpenoid monodesmosidic saponin, α- and δ-hederin (triterpene saponins) has been proposed (Lorent et al., 2013). This mechanism includes three steps of cholesterol-independent binding to the membrane, interaction with cholesterol and asymmetric lateral distribution of saponin, and pore formation and budding of the lipid bilayer due to the increased curvature stress (Lorent et al., 2013).
More recently, at lower concentrations (in the nanomolar or low micromolar ranges) than those causing hemolytic and cytotoxic effects, the triterpene glycosides from marine sponges and sea cucumbers were found to act on specific membrane transport proteins and change their activities. For example, frondoside A and cucumarioside A2-2 inhibited the ATP-binding cassette (ABC) transporter, multidrug-resistance protein-1 (MDR1) (Wink et al., 2012; Menchinskaya et al., 2013). Membrane transporters which are modulated by triterpene glycosides and thus can be proposed as potential therapeutic targets are summarized in Table 1. Although the majority of researches on the membranotropic effects were done with plant triterpene glycosides, understanding the reported targets of membrane transport proteins might provide a basis for the exploration of target molecules for marine triterpene glycosides and their development as drugs.
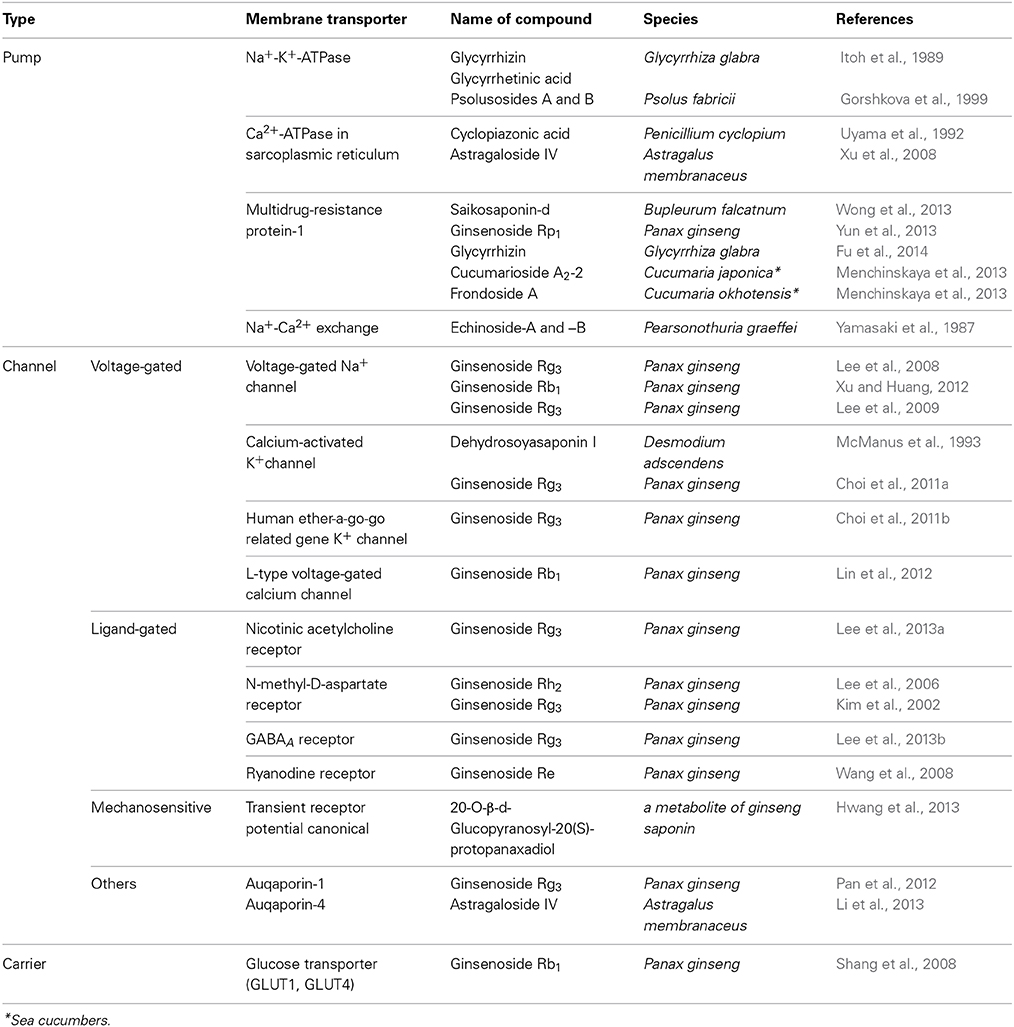
Table 1. Membrane transporters as potential targets of triterpene glycosides from sea cucumbers and plants.
Selective inhibition of Na+-K+-ATPase and Ca2+-ATPase in sarcoplasmic/endoplasmic reticulum (SERCA), in combination with increased Ca2+ influx through L-type voltage-gated calcium channels, transient receptor potential canonical (TRPC) channels, and the ryanodine receptor led to an increase of cytosolic Ca2+ levels. This might explain the positive inotropic effect of triterpene glycosides (Gorshkova et al., 1999; Wang et al., 2008; Lin et al., 2012; Hwang et al., 2013; Wong et al., 2013). Furthermore, triterpene glycosides inhibited voltage-gated Na+ channels (NaV1.2 and NaV1.4) (Lee et al., 2008). In addition, triterpene glycosides could induce K+ currents through voltage-gated K+ channel (KV1.4), calcium-activated K+ channel (BKCa), and human Ether-à-go-go Related Gene (hERG) K+ channels (Kv11.1), which might be responsible for their vasodilatory and antiarrhythmic effects (Lee et al., 2009; Choi et al., 2011a,b; Xu and Huang, 2012). The antiepileptic and neuroprotective effects of triterpene glycosides might be due to the inhibition of excitatory N-methyl-D-aspartate (NMDA) receptors and nicotinic acetylcholine receptors, as well as the activation of inhibitory γ-amino butyric acid (GABA) receptors (Lee et al., 2006, 2013a,b).
Anticancer Activities of Marine Triterpene Glycosides
The antitumor action of the triterpene glycosides from sea cucumbers was discovered by Nigrelli (1952), and most of the marine triterpene glycosides that have been studied since that time are cytotoxic toward cancer cells. Nigrelli (1952) showed that injection of a “holothurin” solution inhibited the growth of Sarcoma-180 tumor cells and induced regression of the tumor. Injection of Krebs-2 ascitic tumor cells treated with holothurin into healthy mice failed to induce marked tumor growth up to 80 days (Sullivan et al., 1955). In addition, holothurin, which is a substance containing as a main constituent holothurin A, inhibited the growth of epidermal carcinoma (KB) tumor cells (Nigrelli et al., 1967).
Many triterpene glycosides from various species of sea cucumbers have diverse biological activities, including anticancer activity. For example, glycosides from 19 species of the families Holothuriidae and Stichopodidae (the glycosides in majority belong to holothurin A and B series) inhibited the growth of Sarcoma-37 cells at in vitro concentrations ranging from 6.2 to 100 μg/ml (Kuznetsova et al., 1982). Although the anticancer mechanisms of the triterpene glycosides have not been investigated in detail, the biologic actions, structure-activity relationships, and molecular mechanisms of stichoposide C, frondoside A, and cucumariosides have been most intensively studied (Aminin et al., 2001; Jin et al., 2009; Yun et al., 2012; Yun, 2014) and are discussed in the following sections. In addition, the potential molecular mechanisms of other triterpene glycosides have been described.
Structure-activity Relationships of Marine Triterpene Glycosides
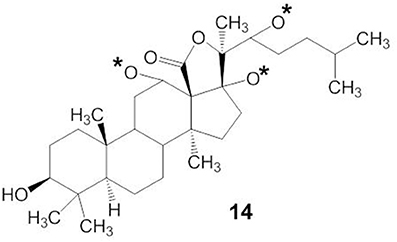
The molecular mechanisms of action of marine triterpene glycosides can be understood by uncovering the relationships between their structure and activities. However, the structure-activity relationships of marine triterpene glycosides have not been intensively studied. As shown in structure 14, the presence of an 18(20)-lactone in the aglycone, with at least one oxygen group nearby (indicated by an asterisk in structure 14), is significant for the biological activity of triterpene glycosides bearing a 9(11)-double bond (Kitagawa, 1988) (Figure 5). Glycosides that have a 7(8)-double bond in their aglycone without a 16-keto group are more active in hemolytic test than those with a 16-keto group (Kalinin et al., 1996). In general, the characteristics of the attached glycone structure may be related to the biological activities of the marine triterpene glycosides.
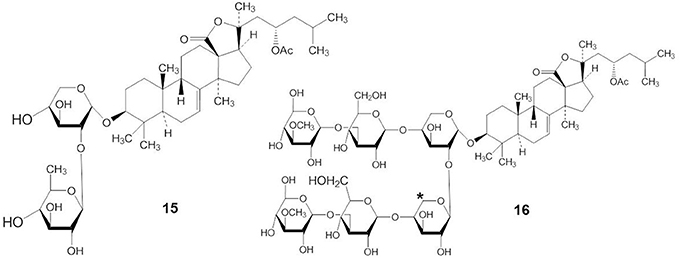
Many investigators have suggested that the bioactivity of the triterpene glycosides results from their strong membranolytic activity. It was reported that the membranolytic activity of triterpene glycosides was due to the formation of complexes between the glycosides and the 5(6)-unsaturated sterols within target cellular membranes (Kalinin, 2000). A linear tetrasaccharide chain of triterpene glycosides is necessary for the effects leading to modification of the cellular membrane (Kitagawa, 1988; Kalinin et al., 1992). Stichoposide A (STA) (compound 15), which had two monosaccharide units, and stichoposide E (STE) (compound 16), which has a xylose residue as the second monosaccharide unit (indicated by an asterisk in compound 16), had lesser membranotropic activity than other stichoposides (Kalinin et al., 2008) (Figure 6). Maltsev et al. (1985) reported that glycosides with quinovose as the second monosaccharide unit were more active hemolytics than other triterpene glycosides.
The presence or absence of a sulfate group in the sugar chain of triterpene glycosides influences their bioactivity (Kalinin, 2000; Kim and Himaya, 2012). A sulfate group at C-4 of the first xylose of non-branched glycosides with a linear tetrasaccharide unit (compound 17) does not significantly affect the activity of triterpene glycosides, but the absence of a sulfate group at C-4 of the xylose residue (compound 18) decreases their activity (indicated by an asterisk in compound 17 and 18) (Figure 7) (Kitagawa, 1988; Kalinin et al., 1992). In contrast, the presence of a sulfate at C-4 of the first xylose in branched pentaosides with 3-O-methyl group on the terminal monosaccharide increases their activities, while the same sulfate decreases the activity of branched pentaosides that have glucose as the terminal residue. Sulfate groups attached to the C-6 position of terminal glucose or 3-O-methylglucose residues in triterpene glycosides greatly reduce their activity (Kalinin, 2000; Kim and Himaya, 2012).
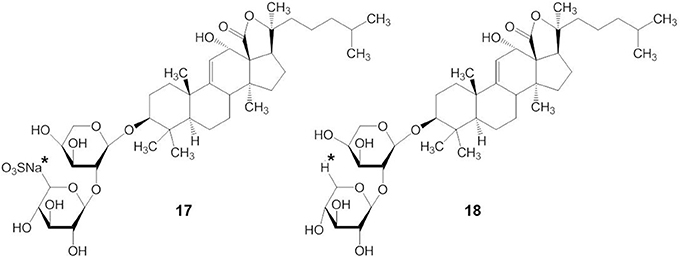
Figure 7. Structure of compound with (17) or without (18) a sulfate group at C-4 of the xylose residue.
Stichoposides
STC
STC (also called stichloroside C1) is a quinovose-containing hexaoside, originally isolated from the holothurian S. chloronotus (Kitagawa et al., 1981; Stonik et al., 1982a) but is also found in other representatives of the family Stichopodidae such as T. ananas (Stonik et al., 1982b). STC has quinovose as the second monosaccharide unit. The antitumor activity of STC appears to be related to its membranotropic effects (Kalinin et al., 2008). We previously reported that STC induced apoptosis of human leukemia and colorectal cancer cells through the activation of both intrinsic and extrinsic pathways (Yun et al., 2012). Anticancer agents increase ceramide levels, to variable extents, in all types of cancer cells (Taha et al., 2006). Ceramide is generated either by de novo synthesis or by sphingomyelin hydrolysis through the action of several types of sphingomyelinase (SMase) such as acid, neutral, or alkaline SMase (Strum et al., 1997; Brown and London, 1998; Kolesnick et al., 2000; Hannun and Obeid, 2008). Both acid and neutral SMase are involved in ceramide generation in response to apoptotic stimuli (Levade and Jaffrezou, 1999; Goni and Alonso, 2002; Gulbins and Kolesnick, 2002). Moreover, under conditions where the classical apoptotic pathway fails, intracellular generation of ceramide may function as part of a backup system that enables caspase-independent programmed cell death (Taha et al., 2006). We demonstrated that STC induced apoptosis through the generation of ceramide by the activation of acid and neutral SMases (Yun et al., 2012). Therefore, the target of STC seems to be SMase leading to increases in ceramide and apoptosis.
STD
STD is a hexaoside containing glucose at the second monosaccharide unit. We have shown that STD can induce apoptosis of human leukemia cells via the extrinsic and intrinsic pathways (Park et al., 2012a). We previously compared the potency of STC and STD in the induction of apoptosis using human leukemia K562 and HL-60 cells. STC was two to five times more potent than STD in inducing cell death [IC50 = 0.5 (K562 cells) and 0.3 (HL-60 cells) μM for STC; 1.0 (K562 cells), and 1.5 (HL-60 cells) μM for STD] (Park et al., 2012a). These results are consistent with the relative membranotropic activities of STC and STD, suggesting that their anticancer activities may be related to their membranotropic activities. More importantly, STC and STD did not have any toxicity in normal hematopoietic progenitor cells or in a mouse tumor model (Yun et al., 2012; Yun, 2014).
It was shown that STD induced apoptosis by activating ceramide synthase 6 (CerS6) and increasing cellular levels of ceramide (Yun, 2014). The activation of CerS6 appears to be subsequent to the activation of the death receptor Fas (CD95) by STD (Yun, 2014). These results suggest that the difference in only one sugar between STC and STD may influence both the potency and the molecular mechanisms for their activities. However, further studies on the relationship between the structure and the activity of these molecules are needed to improve the efficacy and safety of these compounds in treating cancer patients.
Frondoside A and Cucumariosides
Biological Actions of Frondoside A and the Cucumariosides
Frondoside A has a sulfate, an acetoxy group at C-16 of the aglycone, penta-saccharide chain with xylose as the third monosaccharide residue, and 3-O-methylglucose as the terminal monosaccharide residue (Girard et al., 1990). Cucumarioside A4-2 has a 16-keto group in the aglycone and a glucose residue as the third monosaccharide unit in the carbohydrate chain (Kalinin et al., 1992, 1996). Cucumarioside A2-2 has 3-O-methylglucose instead of glucose as the terminal monosaccharide unit. Cucumarioside A2-2 is probably biogenetically connected with A4-2 (Kalinin et al., 1992). Therefore, the main structural differences between frondoside A and the cucumariosides, as shown in Figure 2, are in a functional group at C-16 of the aglycone and the third carbohydrate unit in the carbohydrate chain.
Frondoside A and cucumariosides show anticancer activities in vitro and suppress tumor growth in vivo (Tian et al., 2005; Tong et al., 2005; Li et al., 2008). The antitumor activity of frondoside A and cucumariosides is a result of their activity to induce apoptosis of cancer cells (Li et al., 2008; Jin et al., 2009; Roginsky et al., 2010), including HL-60, NB-4, and THP-1 leukemic cells (Jin et al., 2009).
The cancer inhibitory effect of frondoside A in tumor-bearing mice might partly result from other biological activities, including its antiangiogenic and antimetastatic effects (Li et al., 2008; Al Marzouqi et al., 2011; Ma et al., 2012; Attoub et al., 2013). In addition, frondoside A inhibited the invasion of breast cancer cells via its ability to decrease matrix metalloproteinase (MMP)-9 expression through inhibition of nuclear translocation and transactivation of NF-κB and AP-1 (Park et al., 2012b). Park et al. (2012b) also showed that frondoside A significantly inhibited PI3K/Akt, ERK-1/2, and p38 MAPK activation in 12-O-tetradecanoyl-phorbol-13-acetate (TPA)-stimulated breast cancer cells, indicating that frondoside A inhibited TPA-induced NF-κB and AP-1 activation via inactivation of the PI3K/Akt, ERK1/2 and p38 MAPK pathways. Frondoside A also decreased AP-1-dependent transcriptional activities in JB6-LucAP-1 cells (Silchenko et al., 2008).
It is well established that prostaglandin E receptor, EP4 that is expressed in a number of different malignancies, can promote the migration of tumor cells in vitro (Timoshenko et al., 2003; Wang and Dubois, 2010). EP4 also promotes the invasive behavior of inflammatory breast cancers, one of the more aggressive forms of breast cancers (Robertson et al., 2010). Frondoside A inhibited metastasis of breast cancer cells by antagonizing EP4 and EP2 (Ma et al., 2012).
Cucumariosides increased the lysosomal activity and intracellular Ca++ concentrations of macrophages. These effects are related to the chemical structures of the molecules. For example, although there was no direct correlation, Silchenko et al. (2013c) suggested that the lysosomal activity and cytotoxicity of cucumarioside depended on features of both the aglycone and the carbohydrate chain. Holt et al. (2012) have investigated the effect of frondoside A on NK cells and demonstrated that prostaglandin E2 (PGE2) significantly suppressed the secretion of interferon-γ (IFNγ) from NK cells while frondoside A restored the capacity of NK cells to secrete IFNγ in the presence of PGE2. Other studies reported that in vitro treatment of peritoneal macrophages with cucumarioside A2-2 stimulated cell adhesion as well as their spreading reaction and motility (Aminin et al., 2011), whereas frondoside A suppressed MMP-9 enzymatic activity, secretion, and expression in MBA-MB-231 human breast cancer cells, leading to inhibition of invasion and migration of these cells (Park et al., 2012b). Therefore, it is important to compare the effects of frondoside A and cucumariosides on the migration and spreading of various kinds of cells, including cancer and immune cells.
Effects of Sulfate Groups on the Hemolytic Activity of Cucumariosides
The structures of the aglycone and carbohydrates in cucumariosides may confer membranolytic activity (Stonik et al., 1999). Kalinin et al. (1996) demonstrated that the membranolytic properties of cucumariosides correlated with their cytotoxicity to tumor cells. Cucumarioside A2-2 had in vitro cytotoxic and hemolytic effects on sea urchin embryos with EC50s of 0.45 and 5 μg/mL, respectively (Aminin et al., 2006, 2010). The LD50 of cucumarioside A2-2 for mice was 10 mg/kg after intraperitoneal injection (Polikarpova et al., 1990). The membranolytic action of cucumariosides may be mediated through formation of molecular complexes with sterols in membranes and subsequent generation of solitary ion channels and large aqueous pores (Anisimov, 1987; Verbist, 1993; Kalinin et al., 2008). In addition, the glycosides effectively increased the microviscosity of the lipid bilayer of cell membranes (Pislyagin et al., 2012).
Marine triterpene glycosides contain different numbers of sulfate groups bound with sugars. Cucumarioside A2-2 has a sulfate group at C-4 of the first xylose residue and cucumarioside A6-2 has an additional sulfate group at C-6 of the terminal 3-O-methylglucose residue. The hemolytic activity of cucumarioside A2-2 was significantly greater than its desulfated derivative and was higher than that of cucumarioside A6-2 (Kalinin et al., 1996). Moreover, cucumarioside A2-2 had more active hemolytic activity than cucumarioside A3, which has an additional sulfate group at C-6 of the third monosaccharide unit (Kalinin et al., 1996). The increase in intracellular Ca2+ concentrations was also influenced by the number and positions of sulfate groups in the carbohydrate moiety of the molecules. Cucumarioside A2-2 was more active in inducing a rapid increase in cytosolic Ca2+ content, when compared to the poly-sulfated derivative of A2-2, cucumarioside A7-1 (indicated by an asterisk in compound 5) (Agafonova et al., 2003). In addition, the mono-sulfated cucumariosides A2-2 and A4-2 stimulated peritoneal macrophage lysosomal activity, while desulfation of their carbohydrate moiety completely abolished this activity (Aminin et al., 2001). Therefore, the hemolytic and cytotoxic activities of triterpene glycosides may be dependent on the positions of sulfate groups attached to the carbohydrates.
Cytotoxic Effects of Frondoside A and Cucumariosides on Cancer Cells
Frondoside A showed potent cytotoxicity against various cancer cells, including HeLa, HL-60, and lung cancer cells such as LNM35, A549, and NCI-H460-Luc2 (Silchenko et al., 2008; Jin et al., 2009; Attoub et al., 2013). Moreover, frondoside A enhanced the inhibition of lung tumor growth induced by the anticancer agent, cisplatin (Attoub et al., 2013). The IC50 of frondoside A in HL-60 cells was approximately 5- to 10- fold lower than that of cucumarioside A2-2 (Jin et al., 2009), although the in vivo toxicity of these two compounds for mice was similar (Polikarpova et al., 1990; Aminin et al., 2001). Overall, the structures of both the aglycone and the carbohydrate chain seem to be very important for the cytotoxic activity of frondoside A and the cucumariosides against cancer cells. However, some changes in the carbohydrate residues may not play a significant role in the cytotoxicity of triterpene glycosides because the cucumarioside A2-2 and cucumarioside A4-2 differ only in the structure of their terminal monosaccharide residue having glucose and methylglucose, respectively.
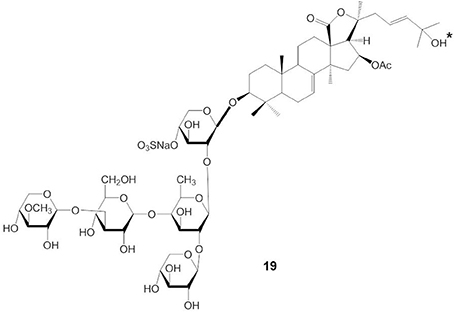
Silchenko et al. (2012d) suggested that amphiphilicity might affect the cytotoxic potency of cucumarioside by demonstrating that the presence of a 25-OH group in the aglycone moiety of triterpene glycosides (cucumarioside H2) (indicated by an asterisk in compound 19) significantly decreased their cytotoxicity, but the cucumarioside having 25-ethoxy group (cucumarioside H4) had potent cytotoxic activity against lymphocytes and very high hemolytic activity (Figure 8). Our study suggested that the acetyl group at C-16 of the aglycone moiety might play a significant role in the cytotoxicity of triterpene glycosides because frondoside A had more potent effects than cucumarioside A2-2 (Jin et al., 2009). In contrast, the presence of acetyl groups in steroids increased their cytotoxic potency (Mimaki et al., 2001).
Apoptotic Effects of Frondoside A and Cucumariosides on Cancer Cells
Frondoside A caused a concentration-dependent reduction in the viability of lung cancer cells (LNM35, A549, and NCI-H460-Luc2), melanoma cells (MDA-MB-435), breast cancer cells (MCF-7), and hepatoma cells (HepG2) over 24 h, and increased the activities of caspases-3 and -7 in LNM35 lung cancer cells (Attoub et al., 2013). It was also shown that treatment of human pancreatic cancer cells with a low concentration of frondoside A induced apoptosis through increased activities of caspases-9, -3, and -7, increased bax, and decreased bcl-2 and mcl-1 (Li et al., 2008). Our results demonstrated that mitochondrial membrane permeability was not changed, and the accumulation of cytochrome c in the cytosolic fraction was not observed in HL-60 cells treated with frondoside A (Jin et al., 2009). Similarly, frondoside A had more potent effects than cucumarioside A4-2 on apoptosis in leukemic cells but did not induce caspase activation before early apoptosis, whereas cucumariosides A2-2 and A4-2 showed similar effects on pro-caspase cleavage and mitochondrial permeability (Jin et al., 2009). Moreover, the annexin-V positivity induced by frondoside A was not inhibited by the pancaspase inhibitor, zVAD-fmk, whereas both the annexin-V positivity and cleavage of caspases induced by cucumarioside A2-2 were efficiently blocked by zVAD-fmk. These results suggest that frondoside A initiates apoptosis in a caspase-independent manner in some cancer cells. Determination of the structural characteristics responsible for the differential effects of frondoside A and cucumariosides on inducing apoptosis in cancer cells will be essential to reveal their mechanisms of action.
Other Marine Triterpene Glycosides
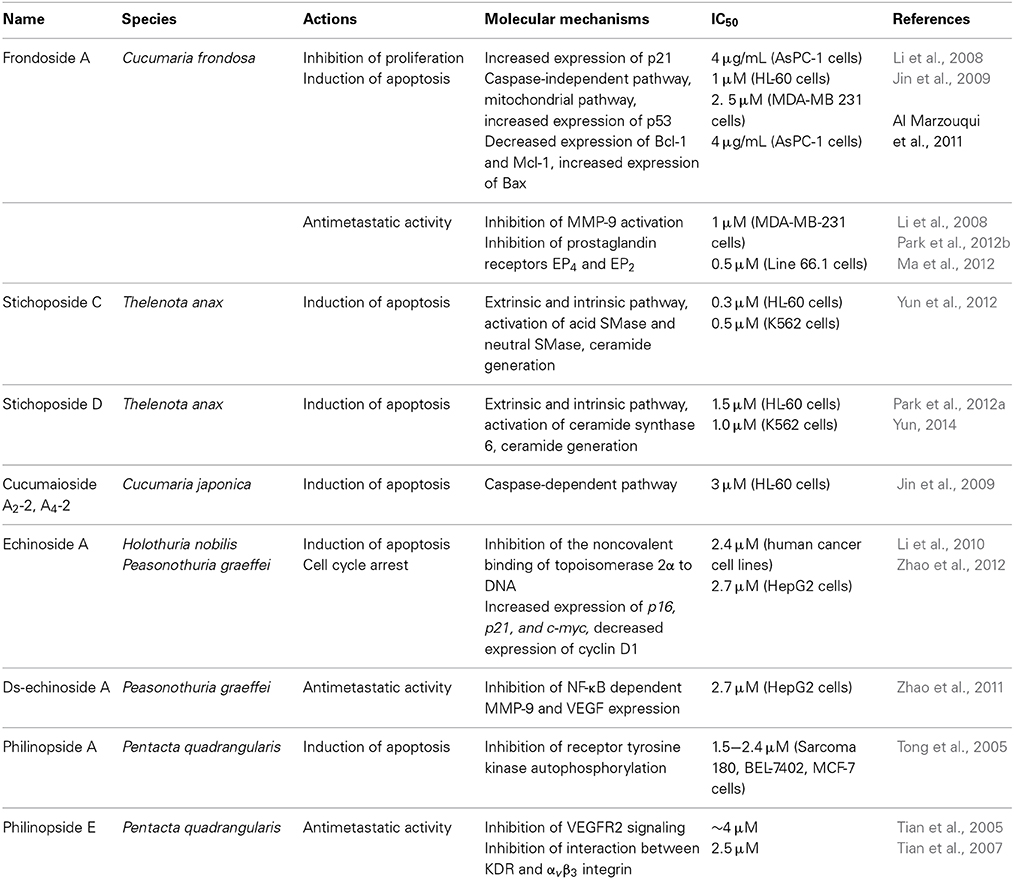
Previous studies demonstrated that marine triterpene glycosides from sea cucumbers had anticancer activities (Stonik, 1986; Stonik et al., 1999). However, the molecular mechanisms for their anticancer activities were only partly defined. Here, we briefly review potential molecular mechanisms for the anticancer activity of several marine triterpene glycosides. A summary of this information is shown in Table 2.
Echinoside A and DS-Echinoside A
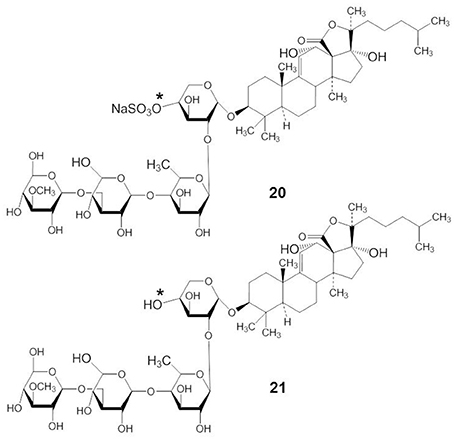
Echinoside A (EA) and DS-echinoside A (DSEA) belong to the holostane glycoside type with an 18(20)-lactone. Both have identical aglycone structures and there are only small structural differences in their carbohydrate chains. EA has a sulfate group at C-4 of the first xylose residue (compound 20) but DSEA is a non-sulfated triterpene glycoside (compound 21) (indicated by an asterisk in compound 20 and 21) (Figure 9). Li et al. (2010) have shown that EA, which was isolated from the sea cucumber Holothuria nobilis, displayed potent anticancer activities through inhibition of the noncovalent binding of topoisomerase 2α to DNA, resulting in double strand breaks and subsequent cell apoptosis. EA is the first marine-derived topoisomerase inhibitor identified with a saponin skeleton. Zhao et al. (2012) demonstrated that EA, isolated from Pearsonothuria graeffei, inhibited cell proliferation by arresting the cell cycle in the G0/G1 phase and inducing apoptosis, with DSEA exerting the strongest effect. DSEA exhibited more potent anticancer activity than EA. This suggests that the sulfate group at C-4 of the first xylose residue may reduce the anticancer activity of EA. Zhao et al. (2011) demonstrated that DSEA, isolated from the sea cucumber Pearsonothuria graeffei, inhibited the main steps involved in metastasis of HepG2 cells, including suppression of cell migration, adhesion, and invasion. DSEA suppressed MMP-9 and VEGF expression and enhanced the expression of TIMP by blocking the NF-κB signaling pathway in a dose-dependent manner. This indicated that a desulfation reaction at xylose C-2 might be related to NF-κB targeting in tumor metastasis.
Philinopside A and E
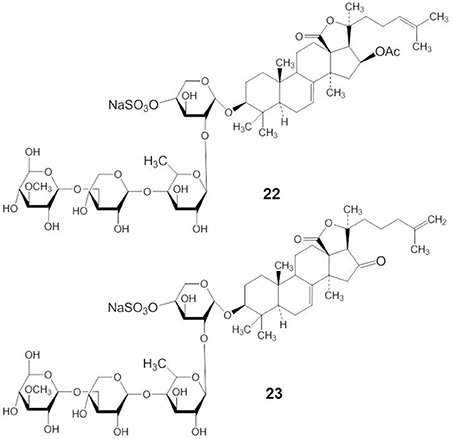
Philinopside A (PA) (compound 22) is a compound isolated from the sea cucumber Pentacta quadrangularis (Figure 10). PA exhibited antitumor effects both in vitro and in vivo through the inhibition of autophosphorylation of receptor tyrosine kinases, including growth factor receptor, platelet derived growth factor receptor-β, and fibroblast growth factor receptor (Tong et al., 2005).
Philinopside E (PE) (compound 23) is a new sulfated saponin from sea cucumbers. PE inhibits cell adhesion, migration, and invasion through the inhibition of vascular endothelial growth factor receptor 2 (VEGFR2) signaling leading to the suppression of Akt, ERK, focal adhesion kinase, and paxillin (Tian et al., 2005). In addition, Tian et al. (2007) have shown that PE specifically interacted with the extracellular domain of the kinase insert domain-containing receptor (KDR) to block its interaction with VEGF and inhibited downstream signaling. More specifically, PE markedly suppressed αvβ 3 integrin-driven downstream signaling as a result of disturbing the physical interaction between KDR and αvβ3 integrin in human microvascular endothelial cells, followed by disruption of the actin cytoskeleton organization and decreased cell adhesion to vitronectin (Tian et al., 2007).
Conclusions
Sea cucumbers contain physiologically active triterpene glycosides. Biological effects, including anticancer activities of several marine triterpene glycosides are observed in vitro and in vivo. Research regarding the mechanisms of action of marine triterpene glycosides on membrane transporters is very limited despite extensive studies on similar compounds in plants. Taking into account the structural and functional differences between marine and plant triterpene glycosides, more intensive studies are required with natural marine triterpene glycosides to assess their potential as novel drugs for the treatment of diseases, including cancer. The STC has anticancer activity through the generation of ceramide by a different mechanism from STD because of the sugar moiety. The anticancer effects of frondoside A and cucumariosides might be through inhibition of tumorigenesis and metastasis and modulation of antitumor immune responses. However, both frondoside A and cucumariosides also possess membranolytic, cytotoxic, and apoptotic properties with different potencies and mechanisms. Structural differences between frondoside A and cucumarioside seem to be responsible for their different biological activities. Thus, identification of the structural characteristics controlling the biological activities of marine triterpene glycosides is essential for developing marine drugs.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the Pioneer Research Center Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning (2012-0009583 and 2012-0009664).
References
Agafonova, I. G., Aminin, D. L., Avilov, S. A., and Stonik, V. A. (2003). Influence of cucumariosides upon intracellular [Ca+2]i and lysosomal activity of macrophages. J. Agric. Food Chem. 51, 6982–6986. doi: 10.1021/jf034439x
Al Marzouqi, N., Iratni, R., Nemmar, A., Arafat, K., Ahmed Al Sultan, M., Yasin, J., et al. (2011). Frondoside A inhibits human breast cancer cell survival, migration, invasion and the growth of breast tumor xenografts. Eur. J. Pharmacol. 668, 25–34. doi: 10.1016/j.ejphar.2011.06.023
Aminin, D. L., Agafonova, I. G., Berdyshev, E. V., Isachenko, E. G., Avilov, S. A., and Stonik, V. A. (2001). Immunomodulatory properties of cucumariosides from the edible Far-Eastern holothurian Cucumaria japonica. J. Med. Food 4, 127–135. doi: 10.1089/109662001753165701
Aminin, D. L., Chaykina, E. L., Agafonova, I. G., Avilov, S. A., Kalinin, V. I., and Stonik, V. A. (2010). Antitumor activity of the immunomodulatory lead Cumaside. Int. Immunopharmacol. 10, 648–654. doi: 10.1016/j.intimp.2010.03.003
Aminin, D. L., Gorpenchenko, T. Y., Bulgakov, V. P., Andryjashchenko, P. V., Avilov, S. A., and Kalinin, V. I. (2011). Triterpene glycoside cucumarioside A2-2 from sea cucumber stimulates mouse immune cell adhesion, spreading, and motility. J. Med. Food 14, 594–600. doi: 10.1089/jmf.2010.1274
Aminin, D. L., Pinegin, B. V., Pichugina, L. V., Zaporozhets, T. S., Agafonova, I. G., Boguslavski, V. M., et al. (2006). Immunomodulatory properties of Cumaside. Int. Immunopharmacol. 6, 1070–1082. doi: 10.1016/j.intimp.2006.01.017
Anisimov, M. M. (1987). Triterpene glycosides and structure-functional properties of biomembranes. Nauchnye Doki. Vyss. Shkoly Biol. Nauki 10, 49–63.
Antonov, A. S., Avilov, S. A., Kalinovsky, A. I., Anastyuk, S. D., Dmitrenok, P. S., Evtushenko, E. V., et al. (2008). Triterpene glycosides from Antartic sea cucumbers. 1. Structure of liouvillosides A1, A2, A3, B1, and B2 from the sea cucumber Staurocucumis liouvillei: new procedure for separation of highly polar glycoside fractions and taxonomic revision. J. Nat. Prod. 71, 1677–1685. doi: 10.1021/np800173c
Antonov, A. S., Avilov, S. A., Kalinovsky, A. I., Anastyuk, S. D., Dmitrenok, P. S., Kalinin, V. I., et al. (2009). Triterpene glycosides from Antarctic sea cucumbers. 2. Structure of Achlioniceosides A1, A2, and A3 from the sea cucumber Achlionice violaecuspidata (=Rhipidothuria racowitzai). J. Nat. Prod. 72, 33–38. doi: 10.1021/np800469v
Antonov, A. S., Avilov, S. A., Kalinovsky, A. I., Dmitrenok, P. S., Kalinin, V. I., Taboada, S., et al. (2011). Triterpene glycosides from Antartic sea cucumbers. III. Structures of liouvillosides A4 and A5, two minor disulphated tetraosides containing 3-O-methylquinovose as terminal monosaccharide units from the sea cucumber Staurocucumis liouvillei (Vaney). Nat. Prod. Res. 25, 1324–1333. doi: 10.1080/14786419.2010.531017
Attoub, S., Arafat, K., Gélaude, A., Al Sultan, M. A., Bracke, M., Collin, P., et al. (2013). Frondoside A suppressive effects on lung cancer survival, tumor growth, angiogenesis, invasion, and metastasis. PLoS ONE 8:e53087. doi: 10.1371/journal.pone.0053087
Avilov, S. A., Antonov, A. S., Drozdova, O. A., Kalinin, V. I., Kalinovsky, A. I., Riguera, R., et al. (2000a). Triterpene glycosides from the far eastern sea cucumber Pentamera calcigera II: disulfated glycosides. J. Nat. Prod. 63, 65–71. doi: 10.1021/np9903447
Avilov, S. A., Antonov, A. S., Drozdova, O. A., Kalinin, V. I., Kalinovsky, A. I., Stonik, V. A., et al. (2000b). Triterpene glycosides from the Far Eastern sea cucumber Pentamera calcigera. 1. Monosulfated glycosides and cytotoxicity of their unsulfated derivatives. J. Nat. Prod. 63, 65–71. doi: 10.1021/np9903447
Avilov, S. A., Antonov, A. S., Silchenko, A. S., Kalinin, V. I., Kalinovsky, A. I., Dmitrenok, P. S., et al. (2003). Triterpene glycosides from the Far Eastern sea cucumber Cucumaria conicospermium. J. Nat. Prod. 66, 910–916. doi: 10.1021/np030005k
Avilov, S. A., Kalinovsky, A. I., Kalinin, V. I., Stonik, V. A., Riguera, R., and Jimenez, C. (1997). Koreoside A, a new nonholostane triterpene glycoside from the sea cucumber Cucumaria koraiensis. J. Nat. Prod. 60, 808–810. doi: 10.1021/np970152g
Avilov, S. A., Silchenko, A. S., Antonov, A. S., Kalinin, V. I., Kalinovsky, A. I., Smirnov, A. V., et al. (2008). Synaptosides A and A1, two triterpene glycosides from the sea cucumber Synapta maculata containing 3-O-methylglucuronic acid and their cytotoxic activity against tumor cells. J. Nat. Prod. 71, 525–531 doi: 10.1021/np070283+
Avilov, S. A., Stonik, V. A., and Kalinovsky, A. I. (1990). Structures of four new triterpene glycosides from the sea cucumber Cucumaria japonica. Khim. Prirodn. Soedin. 6, 787–792.
Bordbar, S., Anwar, F., and Saari, N. (2011). High-value components and bioactives from sea cucumbers for functional foods- a review. Mar. Drugs 9, 1761–1805. doi: 10.3390/md9101761
Brown, D. A., and London, E. (1998). Functions of lipid rafts in biological membranes. Annu. Rev. Cell. Dev. Biol. 14, 111–136. doi: 10.1146/annurev.cellbio.14.1.111
Chiludil, H. D., Murray, A. P., Seldes, A. M., and Maier, M. (2003). Biologically active triterpene glycosides from the sea cucumbers (Holothuroidea, Echinodermata). Stud. Nat. Prod. Chem. 28, 587–591. doi: 10.1016/S1572-5995(03)80150-3
Choi, S. H., Shin, T. J., Hwang, S. H., Lee, B. H., Kang, J., Kim, H. J., et al. (2011b). Ginsenoside Rg3 decelerates hERG K+ channel deactivation through Ser631 residue interaction. Eur. J. Pharmacol. 663, 59–67. doi: 10.1016/j.ejphar.2011.05.006
Choi, S. H., Shin, T. J., Lee, B. H., Hwang, S. H., Lee, S. M., Lee, B. C., et al. (2011a). Ginsenoside Rg3 enhances large conductance Ca2+-activated potassium channel currents: a role of Tyr360 residue. Mol. Cells 31, 133–140. doi: 10.1007/s10059-011-0017-7
Colorado-Ríos, J., Muñoz, D., Montoya, G., Márquez, D., Márquez, M. E., López, J., et al. (2013). HPLC-ESI-IT-MS/MS analysis and biological activity of triterpene glycosides from the Colombian marine sponge Ectyoplasia ferox. Mar. Drugs 11, 4815–4833. doi: 10.3390/md11124815
Dang, N. H., Thanh, N. V., Kiem, P. V., Huang, L. M., Minh, C. V., and Kim, Y. H. (2007). Two new triterpene glycosides from the Vietnamese sea cucumber Holothuria scarbra. Arch. Pharm. Res. 30, 1387–1391. doi: 10.1007/BF02977361
Drozdova, O. A., Avilov, S. A., Kalinin, V. I., Kalinovsky, A. I., Stonik, V. A., Rugiera, R., et al. (1997). Cytotoxic triterpene glycosides from Far-Eastern sea cucumber belonging to the genus Cucumaria. Liebigs. Ann. Chem. 11, 2351–2356. doi: 10.1002/jlac.199719971125
Drozdova, O. A., Avilov, S. A., Kalinovsky, A. I., Stonik, V. A., Milgrom, Y. M., and Rashkes, Y. W. (1993). Trisulfated glycosides from the sea cucumber Cucumaria japonica. Khim. Prirodn. Soedin. 3, 369–374.
Fu, Y., Zhou, E., Wei, Z., Liang, D., Wang, W., Wang, T., et al. (2014). Glycyrrhizin inhibits the inflammatory response in mouse mammary epithelial cells and a mouse mastitis model. FEBS J. 281, 2543–2557. doi: 10.1111/febs.12801
Girard, M., Belanger, J., ApSimon, J. W., Garneau, F. X., Harvey, C., and Brisson, J. R. (1990). Frondoside A, novel triterpene glycoside from the holothurian Cucumaria frondosa. Can. J. Chem. 68, 11–18. doi: 10.1139/v90-003
Goni, F. M., and Alonso, A. (2002). Sphingomyelinases: enzymology and membrane activity. FEBS. Letter. 531, 38–46. doi: 10.1016/S0014-5793(02)03482-8
Gorshkova, I. A., Kalinin, V. I., Gorshkov, B. A., and Stonik, V. A. (1999). Two different modes of inhibition of the rat brain Na+, K+-ATPase by triterpene glycosides, psolusosides A and B from the holothurian Psolus fabricii. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 122, 101–108. doi: 10.1016/S0742-8413(98)10085-3
Gulbins, E., and Kolesnick, R. (2002). Acid sphingomyelinase-derived ceramide signaling in apoptosis. Subcell. Biochem. 36, 229–244. doi: 10.1007/0-306-47931-1_12
Hannun, Y. A., and Obeid, L. M. (2008). Principles of bioactive lipid signaling: lessons from sphingolipids. Nat. Rev. Mol. Cell. Biol. 9, 139–150. doi: 10.1038/nrm2329
Holt, D. M., Ma, X., Kundu, N., Collin, P. D., and Fulton, A. M. (2012). Modulation of host natural killer cell functions in breast cancer via prostaglandin E2 receptors EP2 and EP4. J. Immunother. 35, 179–188. doi: 10.1097/CJI.0b013e318247a5e9
Hwang, J. A., Hwang, M. K., Jang, Y., Lee, E. J., Kim, J. E., Oh, M. H., et al. (2013). 20-O-β-d-glucopyranosyl-20(S)-protopanaxadiol, a metabolite of ginseng, inhibits colon cancer growth by targeting TRPC channel-mediated calcium influx. J. Nutr. Biochem. 24, 1096–1104. doi: 10.1016/j.jnutbio.2012.08.008
Im, D. S., and Nah, S. Y. (2013). Yin and Yang of ginseng pharmacology: ginsenosides vs gintonin. Acta Pharmacol. Sin. 34, 1367–1373. doi: 10.1038/aps.2013.100
Iñiguez-Martinez, A. M., Guerra-Rivas, G., Rios, T., and Quijano, L. (2005). Triterpenoid oligoglycosides from the sea cucumber Stichopus parvimensis. J. Nat. Prod. 68, 1669–1673. doi: 10.1021/np050196m
Itoh, K., Hara, T., Shiraishi, T., Taniguchi, K., Morimoto, S., and Onishi, T. (1989). Effects of glycyrrhizin and glycyrrhetinic acid on Na+-K+-ATPase of renal basolateral membranes in vitro. Biochem. Int. 18, 81–89.
Jin, J. O., Shastina, V. V., Shin, S. W., Xu, Q., Park, J. I., Rasskazov, V. A., et al. (2009). Differential effects of triterpene glycosides, frondoside A and cucumarioside A2-2 isolated from sea cucumbers on caspase activation and apoptosis of human leukemia cells. FEBS. Lett. 583, 697–702. doi: 10.1016/j.febslet.2009.01.010
Kalinin, V. I. (2000). System-theoretical (holistic) approach to the modeling of structural functional relationships of biomolecules and their evolution: an example of triterpene glycosides from sea cucumbers (Echinodermata, Holothurioidea). J. Theor. Biol. 206, 151–168. doi: 10.1006/jtbi.2000.2110
Kalinin, V. I., Aminin, D. L., Avilov, S. A., Silchenko, A. S., and Stonik, V. A. (2008). “Triterpene glycosides from sea cucucmbers (Holothurioidae, Echinodermata), biological activities and functions,” in Studies in Natural Product Chemistry (Bioactive Natural Products), ed Atta-ur-Rahman (Amsterdam: Elsevier Science Publisher), 135–196.
Kalinin, V. I., Avilov, S. A., Kalinina, E. Y., Korolkova, O. G., Kalinovsky, A. I., Stonik, V. A., et al. (1997). Structure of eximisoside A, a novel triterpene glycoside from the Far-Eastern sea cucumber Psolus eximius. J. Nat. Prod. 60, 817–819. doi: 10.1021/np9701541
Kalinin, V. I., Ivanchina, N. V., Krasokhin, V. B., Makarieva, T. N., and Stonik, V. A. (2012). Glycosides from marine sponges (Porifera, Demospongiae): structures, taxonomical distribution, biological activities and biological roles. Mar. Drugs 10, 1671–1710. doi: 10.3390/md10081671
Kalinin, V. I., Prokofieva, N. G., Likhatskaya, G. N., Schentsova, E. B., Agafonova, I. G., Avilov, S. A., et al. (1996). Hemolytic activities of triterpene glycosides from holothurian order Dendrochirotida: some trends in the evolution of this group of toxins. Toxicon 34, 475–483. doi: 10.1016/0041-0101(95)00142-5
Kalinin, V. I., Silchenko, A. S., Avilov, S. A., Stonik, V. A., and Smirnov, A. V. (2005). Sea cucumbers triterpene glycosides, the recent progress in structural elucidation and chemotaxonomy. Phytochem. Rev. 4, 221–236. doi: 10.1007/s11101-005-1354-y
Kalinin, V. I., Volkova, O. V., Likhatskaya, G. N., Prokofieva, N. G., Agafonova, I. G., Anisimov, M. M., et al. (1992). Hemolytic activity of triterpene glycosides from Cucumariidae family holothurians and evolution of this group of toxins. J. Nat. Toxins 1, 17–30.
Kerr, R. G., and Chen, Z. (1995). In vivo and in vitro biosynthesis of saponins in sea cucumbers. J. Nat. Prod. 58, 172–176. doi: 10.1021/np50116a002
Kim, S., Ahn, K., Oh, T. H., Nah, S. Y., and Rhim, H. (2002). Inhibitory effect of ginsenosides on NMDA receptor-mediated signals in rat hippocampal neurons. Biochem. Biophys. Res. Commun. 296, 247–254. doi: 10.1016/S0006-291X(02)00870-7
Kim, S. K., and Himaya, S. W. (2012). Triterpene glycosides from sea cucumbers and their biological activities. Adv. Food Nutr. Res. 65, 297–319. doi: 10.1016/B978-0-12-416003-3.00020-2
Kitagawa, I. (1988). Research of biologically active marine natural products. Yakugaku. Zasshi. 108, 398–416.
Kitagawa, I., Kobayashi, M., Inamoto, T., Yosuzawa, T., and Kyogoku, Y. (1981). The structures of six antifungal oligoglycosides, Stichlorosides A1, A2, B1, B2, C1 and C2 from the sea cucumber Stichopus chloronotus (Brandt). Chem. Phar. Bull. 29, 2387–2391 doi: 10.1248/cpb.29.2387
Kolesnick, R. N., Goni, F. M., and Alonso, A. (2000). Compartmentalization of ceramide signaling: physical foundations and biological effects. J. Cell. Physiol. 184, 285–300. doi: 10.1002/1097-4652(200009)184:3<285::AID-JCP2>3.0.CO;2-3
Kuznetsova, T. A., Anisimov, M. M., Popov, A. M., Baranova, S. I., Afiyatullov, S. H., Kapustina, I. I., et al. (1982). A comparative study in vitro of physiological activity of triterpene glycosides of marine invertebrates of echinoderm type. Comp. Biochem. Physiol. C. 73, 41–43. doi: 10.1016/0306-4492(82)90165-4
Lee, B. H., Choi, S. H., Hwang, S. H., Kim, H. J., Lee, S. M., Kim, H. C., et al. (2013a). Effects of ginsenoside Rg3 on α9α10 nicotinic acetylcholine receptor-mediated ion currents. Biol. Pharm. Bull. 36, 812–818. doi: 10.1248/bpb.b12-01009
Lee, B. H., Kim, H. J., Chung, L., and Nah, S. Y. (2013b). Ginsenoside Rg3 regulates GABA receptor channel activity: involvement of interaction with the γ2 subunit. Eur. J. Pharmacol. 705, 119–125. doi: 10.1016/j.ejphar.2013.02.040
Lee, E., Kim, S., Chung, K. C., Choo, M. K., Kim, D. H., Nam, G., et al. (2006). 20(S)-ginsenoside Rh2, a newly identified active ingredient of ginseng, inhibits NMDA receptors in cultured rat hippocampal neurons. Eur. J. Pharmacol. 536, 69–77. doi: 10.1016/j.ejphar.2006.02.038
Lee, J. H., Choi, S. H., Lee, B. H., Shin, T. J., Pyo, M. K., Hwang, S. H., et al. (2009). The effects of ginsenoside Rg3 on human Kv1.4 channel currents without the N-terminal rapid inactivation domain. Biol. Pharm. Bull. 32, 614–618. doi: 10.1248/bpb.32.614
Lee, J. H., Lee, B. H., Choi, S. H., Yoon, I. S., Shin, T. J., Pyo, M. K., et al. (2008). Involvement of batrachotoxin binding sites in ginsenoside-mediated voltage-gated Na+ channel regulation. Brain Res. 1203, 61–67. doi: 10.1016/j.brainres.2008.01.078
Levade, T., and Jaffrezou, J. P. (1999). Signalling sphingomyelinases: which, where, how and why? Biochim. Biophys. Acta 1438, 1–17. doi: 10.1016/S1388-1981(99)00038-4
Li, M., Ma, R. N., Li, L. H., Qu, Y. Z., and Gao, G. D. (2013). Astragaloside IV reduces cerebral edema post-ischemia/reperfusion correlating the suppression of MMP-9 and AQP4. Eur. J. Pharmacol. 715, 189–195. doi: 10.1016/j.ejphar.2013.05.022
Li, M., Miao, Z. H., Chen, Z., Chen, Q., Gui, M., Lin, L. P., et al. (2010). Echinoside A, a new marine-derived anticancer saponin, targets topoisomerase 2α by unique interference with its DNA binding and catalytic cycle. Ann. Oncol. 21, 597–607. doi: 10.1093/annonc/mdp335
Li, X., Roginsky, A. B., Ding, X. Z., Woodward, C., Collin, P., and Newman, R. A. (2008). Review of the apoptosis pathways in pancreatic cancer and the anti-apoptotic effects of the novel sea cucumber compound, Frondoside A. Ann. N.Y. Acad. Sci. 1138, 181–198. doi: 10.1196/annals.1414.025
Li, X. X., Davis, B., Haridas, V., Gutterman, J. U., and Colombini, M. (2005). Proapoptotic triterpene electrophiles (avicins) form channels in membranes: cholesterol dependence. Biophys. J. 88, 2577–2584. doi: 10.1529/biophysj.104.049403
Lin, Z. Y., Chen, L. M., Zhang, J., Pan, X. D., Zhu, Y. G., Ye, Q. Y., et al. (2012). Ginsenoside Rb1 selectively inhibits the activity of L-type voltage-gated calcium channels in cultured rat hippocampal neurons. Acta Pharmacol. Sin. 33, 438–444. doi: 10.1038/aps.2011.181
Lorent, J., Le Duff, C. S., Quetin-Leclercq, J., and Mingeot-Leclercq, M. P. (2013). Induction of highly curved structures in relation to membrane permeabilization and budding by the triterpenoid saponins, α- and δ-Hederin. J. Biol. Chem. 288, 14000–14017. doi: 10.1074/jbc.M112.407635
Ma, X., Kundu, N., Collin, P. D., Goloubeva, O., and Fulton, A. M. (2012). Frondoside A inhibits breast cancer metastasis and antagonizes prostaglandin E receptors EP4 and EP2. Breast Cancer Res. Treat. 132, 1001–1008. doi: 10.1007/s10549-011-1675-z
Maltsev, I. I., Stekhova, S. I., Schentsova, E. B., Anisimov, M. M., and Stonik, V. A. (1985). Antimicrobial activities of glycosides from the sea cucumbers of family Stichopodidae. Khim. Pharm. Zhurn. 19, 54–56.
McManus, O. B., Harris, G. H., Giangiacomo, K. M., Feigenbaum, P., Reuben, J. P., Addy, M. E., et al. (1993). An activator of calcium-dependent potassium channels isolated from a medicinal herb. Biochemistry 32, 6128–6133. doi: 10.1021/bi00075a002
Menchinskaya, E. S., Pislyagin, E. A., Kovalchuk, S. N., Davydova, V. N., Silchenko, A. S., Avilov, S. A., et al. (2013). Antitumor activity of cucumarioside A2-2. Chemotherapy 59, 181–191. doi: 10.1159/000354156
Mimaki, Y., Yokosuka, A., Kuroda, M., and Sashida, Y. (2001). Cytotoxic activities and structure–cytotoxic relationships of steroidal saponins. Biol. Pharm. Bull. 24, 1286–1289. doi: 10.1248/bpb.24.1286
Moraes, G., Northcote, P. T., Silchenko, A. S., Antonov, A. S., Kalinovsky, A. I., Dmitrenok, P. S., et al. (2005). Mollisosides A, B1, and B2: minor triterpene glycosides from the New Zealand and South Australian sea cucumber Australostichopus mollis. J. Nat. Prod. 68, 842–847. doi: 10.1021/np050049o
Nah, S. Y. (2014). Ginseng ginsenoside pharmacology in the nervous system: involvement in the regulation of ion channels and receptors. Front. Physiol. 5:98. doi: 10.3389/fphys.2014.00098
Nigrelli, R. F. (1952). The effect of holothurin on fish and mice with Sarcoma-180. Zoologica 37, 89–90.
Nigrelli, R. F., Stempien, M. F. Jr., Ruggieri, G. D., Liguori, V. R., and Cecil, J. T. (1967). Substances of potential biomedical importance from marine organisms. Fed. Proc. 26, 1197–1205.
Pan, X. Y., Guo, H., Han, J., Hao, F., An, Y., Xu, Y., et al. (2012). Ginsenoside Rg3 attenuates cell migration via inhibition of aquaporin 1 expression in PC-3M prostate cancer cells. Eur. J. Pharmacol. 683, 27–34. doi: 10.1016/j.ejphar.2012.02.040
Park, E. S., Yun, S. H., Shin, S. W., Kwak, J. Y., and Park, J. I. (2012a). Induction of apoptosis and antitumor activity by stichoposide D through the generation of ceramide in human leukemia cells. J. Life. Sci. 22, 760–771. doi: 10.5352/JLS.2012.22.6.760
Park, S. Y., Kim, Y. H., Kim, Y., and Lee, S. J. (2012b). Frondoside A has an anti-invasive effect by inhibiting TPA-induced MMP-9 activation via NF-κB and AP-1 signaling in human breast cancer cells. Int. J. Oncol. 41, 933–940. doi: 10.3892/ijo.2012.1518
Pislyagin, E. A., Gladkikh, R. V., Kapustina, I. I., Kim, N. Y., Shevchenko, V. P., Nagaev, I. Y., et al. (2012). Interaction of holothurian triterpene glycoside with biomembranes of mouse immune cells. Int. Immunopharmacol. 14, 1–8. doi: 10.1016/j.intimp.2012.05.020
Polikarpova, S. I., Volkova, O. N., Sedov, A. M., Stonik, V. A., and Likhoded, V. G. (1990). Cytogenetic study of the mutagenicity of cucumarioside. Genetika 26, 1682–1685.
Robertson, F. M., Simeone, A. M., Lucci, A., McMurray, J. S., Ghosh, S., and Cristofanilli, M. (2010). Differential regulation of the aggressive phenotype of inflammatory breast cancer cells by prostanoid receptors EP3 and EP4. Cancer 116, 2806–2814. doi: 10.1002/cncr.25167
Roginsky, A. B., Ding, X.-Z., Woodward, C., Ujiki, M. B., Singh, B., Bell, R. H. Jr., et al. (2010). Anti-pancreatic cancer effects of a polar extract from the edible sea cucumber, Cucumaria frondosa. Pancreas 39, 646–652. doi: 10.1097/MPA.0b013e3181c72baf
Shang, W., Yang, Y., Zhou, L., Jiang, B., Jin, H., and Chen, M. (2008). Ginsenoside Rb1 stimulates glucose uptake through insulin-like signaling pathway in 3T3-L1 adipocytes. J. Endocrinol. 198, 561–569. doi: 10.1677/JOE-08-0104
Silchenko, A. S., Avilov, S. A., Antonov, A. A., Kalinin, V. I., Kalinovsky, A. I., Smimov, A. V., et al. (2002). Triterpene glycosides from the deep-water North-Pacific sea cucumber Synallactes nozawai Mitsukuri. J. Nat. Prod. 65, 1802–1808. doi: 10.1021/np0202881
Silchenko, A. S., Avilov, S. A., Antonov, A. S., Kalinovsky, A. I., Dmitrenok, P. S., Kalinin, V. I., et al. (2005a). Glycosides from the sea cucumber Cucumaria frondosa. III. Structure of frondosides A2-1, A2-2, A2-3, and A2-6, four new minor monosulfated triterpene glycosides. Can. J. Chem. 83, 21–27. doi: 10.1139/v04-163
Silchenko, A. S., Avilov, S. A., Antonov, A. S., Kalinovsky, A. I., Dmitrenok, P. S., Kalinin, V. I., et al. (2005b). Glycosides from the sea cucumber Cucumaria frondosa. IV. Structure of frondosides A2-4, A2-7, and A2-8, three new minor monosulfated triterpene glycosides. Can. J. Chem. 83, 2120–2126. doi: 10.1139/v05-243
Silchenko, A. S., Avilov, S. A., Kalinin, V. I., Kalinovsky, A. I., Dmitrenok, P. S., Fedorov, S. N., et al. (2008). Constituents of the sea cucumber Cucumaria okhotensis. Structures of okhotosides B1-B3 and cytotoxic activities of some glycosides from this species. J. Nat. Prod. 71, 351–356. doi: 10.1021/np0705413
Silchenko, A. S., Avilov, S. A., Kalinin, V. I., Stonik, V. A., Kalinovsky, A. I., Dmitrenok, P. S., et al. (2007a). Monosulfated triterpene glycosides from Cucumaria okhotensis Levin et Stepanov, a new species of sea cucumbers from sea of Okhotsk. Russ. J. Bioorg. Chem. 33, 73–82, doi: 10.1134/S1068162007010098
Silchenko, A. S., Avilov, S. A., Kalinovsky, A. I., Dmitrenok, P. S., Kalinin, V. I., Morre, J., et al. (2007b). Glycosides from the North Atlantic sea cucumber Cucumaria frondosa V- Structures of five new minor trisulfated triterpene oligoglycosides, frondosides A7-1, A7-2, A7-3, A7-4, and isofrondoside C. Can. J. Chem. 85, 626–636. doi: 10.1139/v07-087
Silchenko, A. S., Kalinovsky, A. I., Avilov, S. A., Andryjaschenko, P. V., Dmitrenok, P. S., Martyyas, E. A., et al. (2012a). Triterpene glycosides from the sea cucumber Eupentacta fraudatrix. Structure and biological action of cucumariosides A1, A3, A4, A5, A6, A12 and A15, seven new minor non-sulfated tetraosides and unprecedented 25-keto, 27-norholostane aglycone. Nat. Prod. Commun. 7, 517–525.
Silchenko, A. S., Kalinovsky, A. I., Avilov, S. A., Andryjaschenko, P. V., Dmitrenok, P. S., Martyyas, E. A., et al. (2012b). Triterpene glycosides from the sea cucumber Eupentacta fraudatrix. Structure and cytotoxic action of cucumariosides A2, A7, A9, A10, A11, A13 and A14, seven new minor non-sulfated tetraosides and an aglycone with an uncommon 18-hydroxy group. Nat. Prod. Commun. 7, 845–852.
Silchenko, A. S., Kalinovsky, A. I., Avilov, S. A., Andryjaschenko, P. V., Dmitrenok, P. S., Martyyas, E. A., et al. (2012c). Triterpene glycosides from the sea cucumber Eupentacta fraudatrix. Structure and biological activity of cucumariosides B1 and B2, two new minor non-sulfated unprecedented triosides. Nat. Prod. Commun. 7, 1157–1162.
Silchenko, A. S., Kalinovsky, A. I., Avilov, S. A., Andryjaschenko, P. V., Dmitrenok, P. S., Martyyas, E. A., et al. (2013a). Structures and biological activities of typicosides A1, A2, B1, C1 and C2, triterpene glycosides from the sea cucumber Actinocucumis typica. Nat. Prod. Commun. 8, 301–310.
Silchenko, A. S., Kalinovsky, A. I., Avilov, S. A., Andryjaschenko, P. V., Dmitrenok, P. S., Martyyas, E. A., et al. (2013b). Triterpene glycosides from the sea cucumber Eupentacta fraudatrix. Structure and biological action of cucumariosides I1, I3, I4, three new minor disulfated pentaosides. Nat. Prod. Commun. 8, 1053–1058.
Silchenko, A. S., Kalinovsky, A. I., Avilov, S. A., Andryjaschenko, P. V., Dmitrenok, P. S., Menchinskaya, E. S., et al. (2013c). Structure of cucumarioside I2 from the sea cucumber Eupentacta fraudatrix (Djakonov et Baranova) and cytotoxic and immunostimulatory activities of this saponin and relative compounds. Nat. Prod. Res. 27, 1776–1783. doi: 10.1080/14786419.2013.778851
Silchenko, A. S., Kalinovsky, A. I., Avilov, S. A., Andryjaschenko, P. V., Dmitrenok, P. S., Yurchenko, E. A., et al. (2011). Structure of cucumariosides H5, H6, H7 and H8, triterpene glycosides from the sea cucumber Eupentacta fraudatrix and unprecedented aglycone with 16,22-epoxy-group. Nat. Prod. Commun. 6, 1075–1082.
Silchenko, A. S., Kalinovsky, A. I., Avilov, S. A., Andryjaschenko, P. V., Dmitrenok, P. S., Yurchenko, E. A., et al. (2012d). Structures and cytotoxic properties of cucumariosides H2, H3 and H4 from the sea cucumber Eupentacta fraudatrix. Nat. Prod. Res. 26, 1765–1774. doi: 10.1080/14786419.2011.602637
Silchenko, A. S., Kalinovsky, A. I., Avilov, S. A., Andryjaschenko, P. V., Dmitrenok, P. S., Yurchenko, E. A., et al. (2013d). Structure and biological action of cladolosides B1, B2, C1, C2 and D, six new triterpene glycosides from the sea cucumber Cladolabes schmeltzii. Nat. Prod. Commun. 8, 1527–1534.
Silchenko, A. S., Stonik, V. A., Avilov, S. A., Kalinin, V. I., Kalinovsky, A. I., Zaharenko, A. M., et al. (2005c). Holothurins B2, B3, and B4, new triterpene glycosides from Mediterranean sea cucumbers of the genus holothuria. J. Nat. Prod. 68, 564–567. doi: 10.1021/np049631n
Stonik, V. A. (1986). Some terpenoid and steroid derivatives from echinoderms and sponges. Pure Appl. Chem. 58, 423–436.
Stonik, V. A., Kalinin, V. I., and Avilov, S. A. (1999). Toxins from sea cucumbers (holothuroids): chemical structures, properties, taxonomic distribution, biosynthesis and evolution. J. Nat. Toxins 8, 235–248.
Stonik, V. A., Maltsev, I. I., and Elyakov, G. B. (1982b). Structures of thelenotoside-A and thelenoside-B from the sea cucumber Thelenota ananas. Chem. Nat. Prod. 624–627.
Stonik, V. A., Maltsev, I. I., Kalinovsky, A. I., Conde, K., and Elyakov, G. B. (1982a). Glycosides of marine- invertebrates. XI. The two novel triterpene glycosides from holothurian of Stichopodidae family. Chem. Nat. Prod. 194–199.
Strum, J. C., Ghosh, S., and Bell, R. M. (1997). Lipid second messengers. A role in cell growth regulation and cell cycle regulation. Adv. Exp. Mol. Biol. 407, 421–431. doi: 10.1007/978-1-4899-1813-0_63
Sullivan, T. D., Ladue, K. T., and Nigrelli, R. F. (1955). The effects of holothurin, a steroid saponin of animal origin, on Krebs-2 ascites tumors in Swiss mice. Zoologica 40, 49–52.
Taha, T. A., Mullen, T. D., and Obeid, L. M. (2006). A house divided: ceramide, sphingosine, and sphingosine-1-phosphate in programmed cell death. Biochim. Biophys. Acta 1758, 2027–2036. doi: 10.1016/j.bbamem.2006.10.018
Tian, F., Zhang, X., Tong, Y., Yi, Y., Zhang, S., Li, L., et al. (2005). PE, a new sulfated saponin from sea cucumber, exhibits anti-angiogenetic and antitumor activities in vitro and in vivo. Cancer Biol. Ther. 4, 874–882. doi: 10.4161/cbt.4.8.1917
Tian, F., Zhu, C. H., Zhang, X. W., Xie, X., Xin, X. L., Yi, Y. H., et al. (2007). Phillinopside E, a new sulfated saponin from sea cucumber, blocks the interaction between kinase insert domain-containing receptor (KDR) and αvβ3 integrin via binding to the extracellular domain of KDR. Mol. Pharmacol. 72, 545–552. doi: 10.1124/mol.107.036350
Timoshenko, A. V., Xu, G., Chakrabarti, S., Lala, P. K., and Chakraborty, C. (2003). Role of prostaglandin E2 receptors in migration of murine and human breast cancer cells. Exp. Cell Res. 289, 265–274. doi: 10.1016/S0014-4827(03)00269-6
Tong, Y., Zhang, X., Tian, F., Yi, Y., Xu, Q., Li, L., et al. (2005). Philinopside A, a novel marine derived compound possessing dual anti-angiogenetic and anti-tumor effects. Int. J. Cancer 114, 843–853. doi: 10.1002/ijc.20804
Uyama, Y., Imaizumi, Y., and Watanabe, M. (1992). Effects of cyclopiazonic acid, a novel Ca2+-ATPase inhibitor, on contractile responses in skinned ileal smooth muscle. Br. J. Pharmacol. 106, 208–214. doi: 10.1111/j.1476-5381.1992.tb14316.x
Verbist, J. E. (1993). “Pharmacological effects of compounds from Echinoderms,” in Echinoderm Studies, Vol. 4, eds M. Jangoux and J. M. Lawrence (Rotterdam; Brookfild: A. A. Balkema), 111–186.
Wang, D., and Dubois, R. N. (2010). Eicosanoids and cancer. Nat. Rev. Cancer 10, 182–192. doi: 10.1038/nrc2809
Wang, Y. G., Zima, A. V., Ji, X., Pabbidi, R., Blatter, L. A., and Lipsius, S. L. (2008). Ginsenoside Re suppresses electromechanical alternans in cat and human cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 295, H851–H859. doi: 10.1152/ajpheart.01242.2007
Wink, M., Ashour, M. L., and El-Readi, M. Z. (2012). Secondary metabolites from plants inhibiting ABC transporters and reversing resistance of cancer cells and microbes to cytotoxic and antimicrobial agents. Front. Microbiol. 3:130. doi: 10.3389/fmicb.2012.00130
Wong, V. K., Li, T., Law, B. Y., Ma, E. D., Yip, N. C., Michelangeli, F., et al. (2013). Saikosaponin-d, a novel SERCA inhibitor, induces autophagic cell death in apoptosis-defective cells. Cell Death Dis. 11, e720. doi: 10.1038/cddis.2013.217
Xu, L., and Huang, S. P. (2012). Effect of the ginsenoside Rb1 on the spontaneous contraction of intestinal smooth muscle in mice. World J. Gastroenterol. 18, 5462–5469. doi: 10.3748/wjg.v18.i38.5462
Xu, X. L., Chen, X. J., Ji, H., Li, P., Bian, Y. Y., Yang, D., et al. (2008). Astragaloside IV improved intracellular calcium handling in hypoxia-reoxygenated cardiomyocytes via the sarcoplasmic reticulum Ca-ATPase. Pharmacology 81, 325–332. doi: 10.1159/000121335
Yamasaki, Y., Ito, K., Enomoto, Y., and Sutko, J. L. (1987). Alterations by saponins of passive Ca2+ permeability and Na+-Ca2+ exchange activity of canine cardiac sarcolemmal vesicles. Biochim. Biophys. Acta 897, 481–487. doi: 10.1016/0005-2736(87)90445-7
Yun, S. H. (2014). Study on the Molecular Mechanisms for Antitumor Activity of Stichoposide D, an Extract from Sea Cucumbers. Ph.D. Thesis, Dong-A University, Busan, Korea.
Yun, S. H., Park, E. S., Shin, S. W., Na, W. Y., Han, J. Y., Jeong, J. S., et al. (2012). Stichoposide C induces apoptosis through the generation of ceramide in leukemia and colorectal cancer cells and shows in vivo antitumor activity. Clin. Cancer Res. 18, 5934–5948. doi: 10.1158/1078-0432.CCR-12-0655
Yun, U. J., Lee, J. H., Koo, K. H., Ye, S. K., Kim, S. Y., Lee, C. H., et al. (2013). Lipid raft modulation by Rp1 reverses multidrug resistance via inactivating MDR-1 and Src inhibition. Biochem. Pharmacol. 85, 1441–1453. doi: 10.1016/j.bcp.2013.02.025
Zhang, S. H., Li, L., Yi, Y. H., and Sun, P. (2006a). Philipnosides E and F, two new sulfated triterpene glycosides from the sea cucumber Pentacta quadrangularis. Nat. Prod. Res. 20, 399–407. doi: 10.1080/14786410500185584
Zhang, S. Y., Yi, Y. H., and Tang, H. F. (2006b). Bioactive triterpene glycosides from the sea cucumber Holothuria fuscocinerea. J. Nat. Prod. 69, 1492–1495. doi: 10.1021/np060106t
Zhang, S. Y., Yi, Y. H., and Tang, H. F. (2006c). Cytotoxic sulfated triterpene glycosides from the sea cucumber Pseudocolochirus violaceus. Chem. Biodivers. 3, 807–817. doi: 10.1002/cbdv.200690083
Zhang, S. Y., Yi, Y., Tang, H. F., Li, L., Sun, P., and Wu, J. (2006d). Two new bioactive triterpene glycosides from the sea cucumber Pseudocolochirus violaceus. J. Asian Nat. Prod. Res. 8, 1–8. doi: 10.1080/10286020500034972
Zhao, Q., Liu, Z., Xue, Y., Wang, J., Li, H., Tang, Q., et al. (2011). Ds-echinoside A, a new triterpene glycoside derived from sea cucumber, exhibits antimetastatic activity via the inhibition of NF-κB-dependent MMP-9 and VEGF expressions. J. Zhejang Univ. Sci. B 12, 534–544. doi: 10.1631/jzus.B1000217
Zhao, Q., Xue, Y., Wang, J., Li, H., Long, T., Li, Z., et al. (2012). In vitro and in vivo anti-tumour activities of echinoside A and ds-echinoside A from Pearnonothuria graeffei. J. Sci. Food. Agric. 92, 965–974. doi: 10.1002/jsfa.4678
Keywords: triterpene glycosides, frondoside A, cucumarioside, stichoposides, anticancer activity, membrane transporters
Citation: Park J-I, Bae H-R, Kim CG, Stonik VA and Kwak J-Y (2014) Relationships between chemical structures and functions of triterpene glycosides isolated from sea cucumbers. Front. Chem. 2:77. doi: 10.3389/fchem.2014.00077
Received: 28 June 2014; Accepted: 21 August 2014;
Published online: 09 September 2014.
Edited by:
Antonio Trincone, Consiglio Nazionale delle Ricerche, ItalyReviewed by:
Robert Vianello, Rudjer Boskovic Institute, CroatiaDaniel Anthony Dias, The University of Melbourne, Australia
Copyright © 2014 Park, Bae, Kim, Stonik and Kwak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joo-In Park, Department of Biochemistry, Dong-A University College of Medicine, 32, Daesin-gongwon-ro, Seo-Gu, Busan 602-714, South Korea e-mail:amlwYXJrQGRhdS5hYy5rcg==;
Hae-Rahn Bae, Department of Physiology, Dong-A University School of Medicine, 32, Daesin-gongwon-ro, Seo-Gu, Busan 602-714, South Korea e-mail:aHJiYWVAZGF1LmFjLmty;
Jong-Young Kwak, Department of Biochemistry, Immune-network Pioneer Research Center, Dong-A University School of Medicine, Dong-A University, 32, Daesin-gongwon-ro, Seo-Gu, Busan 602-714, South Korea e-mail:anlrd2FrQGRhdS5hYy5rcg==
 Joo-In Park
Joo-In Park Hae-Rahn Bae
Hae-Rahn Bae Chang Gun Kim
Chang Gun Kim Valentin A. Stonik4
Valentin A. Stonik4 Jong-Young Kwak
Jong-Young Kwak