- 1Department of Chemistry, Federal University of Lavras, Lavras, Brazil
- 2Department of Chemistry, Faculty of Science, University of Hradec Kralove, Hradec Kralove, Czechia
Essential to understanding life, the biomolecular phenomena have been an important subject in science, therefore a necessary path to be covered to make progress in human knowledge. To fully comprehend these processes, the non-covalent interactions are the key. In this review, we discuss how specific protein-ligand interactions can be efficiently described by low computational cost methods, such as Molecular Mechanics (MM). We have taken as example the case of the halogen bonds (XB). Albeit generally weaker than the hydrogen bonds (HB), the XBs play a key role to drug design, enhancing the affinity and selectivity toward the biological target. Along with the attraction between two electronegative atoms in XBs explained by the σ-hole model, important orbital interactions, as well as relief of Pauli repulsion take place. Nonetheless, such electronic effects can be only well-described by accurate quantum chemical methods that have strong limitations dealing with supramolecular systems due to their high computational cost. To go beyond the poor description of XBs by MM methods, reparametrizing the force-fields equations can be a way to keep the balance between accuracy and computational cost. Thus, we have shown the steps to be considered when parametrizing force-fields to achieve reliable results of complex non-covalent interactions at MM level for In Silico drug design methods.
Introduction
Biological systems are huge, they change in time and they are very sensitive to in vivo conditions like temperature and environment (Ramalho et al., 2009; Freitas et al., 2014; Nair and Miners, 2014; Jurinovich et al., 2015). These facts are remembered every day by drug designers, structural biologists, biophysicists and many other professionals that need to study these systems (Nair and Miners, 2014). In order to overcome these barriers, many scientists opt to model their systems using the classical atomistic Molecular Dynamics (MD) simulation method.
The classical MD is a computational method based on Molecular Mechanics (MM) physics and its first simulation was performed by Alder and Wainright (Alder and Wainwright, 1959) in the late ‘50s. In this pioneering work, the authors discussed the difficulties to treat the many-body problem and proposed a numerical scheme to deal with multiple interactions of particles by solving Newton's motion equations. Although Alder and Wainright gave the first spark for the beginning of classical MD, the first realistic MD simulation was performed just in 1969 (Allen et al., 1969). In this work, by the implementation of Lennard-Jones potentials (essential to describe van der Waals interactions), Dr. Rahman and co-authors successfully modeled 864 atoms of liquid argon. Over the last decades, MD modeling was refined, and many different codes have been launched. Nowadays, drug design is one of the areas that most benefits from the enormous development that the atomistic MD has acquired over the years. However, the mindset that it is difficult, unnecessary or too time-consuming to parameterize new molecules may turn to final works that mislead the real interactions. Unphysical models or catastrophic geometries with very inaccurate interaction energies can be found along with an MD simulation, especially if the modeled molecule has too many chemical functions or π-conjugations (Davis and Patel, 2010; Prandi et al., 2016; Aytenfisu et al., 2017).
Despite the great effort being made by scientific programmers to enhance the quality of classical molecular simulation techniques, much more can be done by the user to improve inter- and intramolecular interactions outcomes. It should be kept in mind that intramolecular interactions are the driving force of most biomolecular phenomena (Martins et al., 2003; Adesokan et al., 2004; Ramalho et al., 2009; Poater et al., 2011; Ben-Naim, 2012; Hongo et al., 2013; Poznanski and Shugar, 2013). They are known to be quantum chemical phenomena that go beyond the classical description of matter and, in particular cases, they cannot be understood by simple electrostatic or dispersion schemes (Ramalho and da Cunha, 2011; Esrafili and Ahmadi, 2012; Wolters et al., 2014). This is one of the greatest challenges to force-field modeling since there is no classical analog to the quantum behavior of electrons.
That is the case of halogen bonds (XB), a real and relevant tool for rational drug design (Auffinger et al., 2004; Lu et al., 2009, 2010, 2012; Wilcken et al., 2013; Mendez et al., 2017). The XBs are non-covalent interactions between an acceptor (A), often Lewis base, and a halogenated molecule acting as a donor (D) (Figure 1). On one hand, some researchers address the origin of the XBs to the existence of a positive electrostatic potential region on the halogen atom (X) bond called σ-hole (Clark et al., 2007; Politzer et al., 2007, 2013; Kolár et al., 2014). On the other hand, the literature also highlights the importance of the orbital interactions, revealing the covalency part of XB (Wolters and Bickelhaupt, 2012; Wang et al., 2014; Novák et al., 2015; Wolters et al., 2015; Dominikowska et al., 2016; Bora et al., 2017; Santos et al., 2017). In contrast to molecular mechanics approaches, the XB are purely quantum chemical phenomena, whose strength grows with the size of the halogens, making chlorine, bromine, and iodine promising alternatives to promote secondary side-chain interactions inside protein cavities (Lu et al., 2010; Cavallo et al., 2016; Santos et al., 2017).
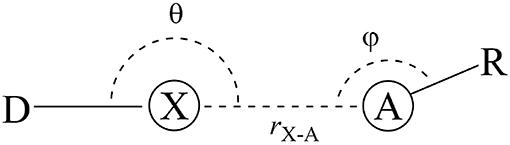
Figure 1. General halogen-bond scheme. The donor (D) is bonded to a halogen atom (X) that interacts with the acceptor (A) in a distance r.
Once many compounds with biological activity have halogen atoms in their composition, the accurate description of XBs by molecular mechanics is crucial. Now, the main questions we pursue to answer are: what can we do to solve this problem?; what are the alternatives we have?; what is the best approach to build up accurate techniques to describe these interactions?
Force-Fields: an Overview
Quantum Mechanics (QM) considers the electronic effects in molecules. On the other hand, Molecular Mechanics (MM) is based just on the interaction among classical charged particles, neglecting direct electronic effects.
Since the electronic environment around an atom changes accordingly to its neighborhood, we need an artifice to describe atoms with the same atomic numbers, but chemically different. For example, we need to distinguish the sp3 from sp2 carbons. To recover most of the electronic effects in MM based simulations different atom types should be employed. The atom types are atomic labels used to indicate chemically different atoms. In the example cited, different atom types should describe the carbons in ethanol.
After the atom types are set, the classical MD software associates each bonded or non-bonded molecular interaction to a set of parameters. In more detail, the MD software calculates the total potential (VTOT) that acts in each particle.
The Equation (1) shows a generic form of a total potential: VS, VA, and VD are the bonded terms, the stretching, angular, and dihedral potentials, respectively; and the last two terms are the non-bonded terms, in which accounts for the van der Waals interactions described by the Lennard-Jones potential (Vvdw) and Coulomb potential (VC) that simulates the electrostatic interactions. It is important noticing that the terms in the total potential equation may vary depending on its implementation in the MD software. For instance, in Equation (1.1) we see the parameters , rμ, and , that are the stretching force constant, the length of the bond and equilibrium distance, respectively; In Equation (1.2), , θμ, and are the angular force constant, the angle, and equilibrium angle, respectively; The term in Equation (1.3) is the dihedral torsional barrier, is the periodicity or the number of minima in the cosine function, δμ is the dihedral angle and is a phase angle that represents the displacement of the dihedral angle (or torsional displacement); in Equation (1.4), εij, σij, and rij are the depth of the potential well, the distance at which Vvdw is a minimum and the distance between two particles, respectively; and finally q and ϵ0 in Equation (1.5) are the charge and the electrostatic constant, respectively. The description of each term in Equation (1) depends on these parameters and a complete set of equations together is named force-field (FF).
In the last decades, the use of MD has been expanded to different areas, being necessary the creation of parameters to describe a huge set of molecular interactions at the same time or, at least, those more relevant to a certain purpose. Thus, large groups of transferable parameters have been created aiming to describe chemically similar molecules. Nowadays, there are many sets of specialized parameters for the description of many different molecular groups like polymers, proteins, solvents, small organic molecules, etc. (Jorgensen et al., 1996; Schuler et al., 2001; Wang et al., 2004; Vanommeslaeghe et al., 2010; Dickson et al., 2014).
Due to the wide use of classical MD for protein modeling, here we may highlight two of the most used sets of parameters for biomodelling: AMBER (Assisted Model Building with Energy Refinement) (Case et al., 2014), created by Peter Kollman and his group at the University of California, and CHARMM (Chemistry at Harvard using Molecular Mechanics) (Vanommeslaeghe et al., 2010), initially developed by Martin Karplus and co-workers at Harvard University. Over the years, CHARMM has expanded and gained new specific parameters for the modeling of smaller molecules.
Other diffused family of parameters for biomolecular systems are OPLS and GROMOS. The OPLS (Optimized Potentials for Liquid Simulations) (Jorgensen et al., 1996) force-field was developed by Jorgensen's group to simulate proteins in solution. In 1976, GROMOS (GROningen MOlecular Simulation) (Schuler et al., 2001) started to be developed at the University of Groningen. Originally created for biomolecules modeling, until today it is constantly updated for many different classes of molecules.
Another example of a set of parameters specially designed for small and medium-sized organic molecules is the MMn (n = 1, 2, 3, 4) family of parameters developed by Allinger and coauthors (Allinger et al., 1971, 1989, 1996; Allinger, 1977; Lii and Allinger, 1989a,b; Nevins et al., 1996; Langley et al., 2001; Langley and Allinger, 2002).
With the expansion of the use of MD simulations in the pharmaceutical field, the development of a set of parameters for drug design research was urgent. Thus, in 2004, the GAFF (General AMBER Force Field) (Wang et al., 2004) family of parameters was specifically created and tested for pharmaceutical purposes. In order to guarantee a great transferability, many GAFF equilibrium parameters were extracted from the average of X-ray and ab initio calculations of different molecules. Besides, pure GAFF is not yet able to model the major part of metallic interactions in complexes and can poorly describe halogen bonds (Rendine et al., 2011; Li and Merz, 2017).
Parameterization: the Key to Realistic Results
In the last decade, the US Food and Drug Administration (FDA)1 approved more than 230 New Molecular Entity (NME) drugs. Almost 42% of the new non-biological approved drugs contain halogen atoms, and more than 3% are metallic complexes (see data in Figure 2). These data show the importance of a specific parameterization for new drugs since most general FFs are not able to describe with high accuracy those bonds for molecular dynamics simulations (Santos et al., 2014, 2017).
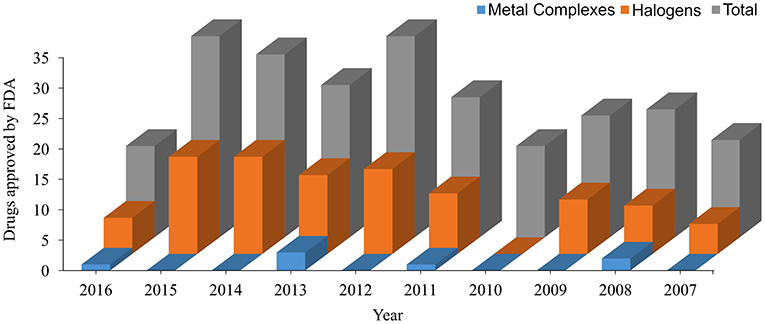
Figure 2. New Molecular Entity (NME) approved by the US Food and Drug Administration (FDA) in the last 10 years. All data are taken from https://www.fda.gov.
Unfortunately, specificity and transferability usually have an inversely proportional relationship. Due to their good transferability, GAFF and other general force-fields are ideal to describe molecules that are indirectly involved in the studies that we would like to do. However, for very specific cases, sets of general parameters are not enough to model physical structures or interactions and we need to remodel them.
Then, theoretical scientists have realized that molecular models need to be accurate to perform a realistic simulation. For this reason, many tools were developed aiming more straightforward paths to parameterization. The greatest part of methodologies is based on the extraction of equilibrium distances, angles and dihedrals from a QM optimized structure and the force constants are derived from the diagonalization of the Hessian matrix (extracted from a QM calculation). Some examples of tools that help computational scientists to parameterize their molecules are the following: Automated Topology Builder (ATB) (Malde et al., 2011), Paratool (Mayne et al., 2013), and Joyce (Barone et al., 2013).
More specifically, ATB is much more than an on-line tool to build biomolecular force-fields for MD or Monte Carlo simulations, it can also calculate free energies and predict hydration free enthalpies. This website is very user-friendly, does not require any installation procedure and sends an e-mail to the user when the parameters are ready.
Paratool is a plugin of the software Visual Molecular Dynamics (VMD) (Humphrey et al., 1996). It was specifically developed to build parameters in CHARMM or AMBER format. It is not as automated as ATB, but it is very user-friendly since it is linked to the VMD graphical interface.
Joyce is a software specially developed to assist the derivation of parameters in GROMACS (GROningen MAchine for Chemical Simulations) (van der Spoel et al., 2005) or Moscito (Paschek and Geiger, 2002) format for MD atomistic simulations. It is also a very versatile and flexible program, in which the user can symmetrize molecular groups, set dependencies between parameters and even impose specific values to the parameters.
The three aforementioned tools are just some examples of how a specific set of parameters can be derived. They were cited in ascendant order of time-consuming and effort to create a new specific FF. The choice of modeling a molecule with an FF created in a very automated way or a much more fitted one depends on the molecule, the required accuracy and how dependent the studied property is from the molecular geometry. However, another issue that cannot be neglected is the more complex intermolecular interactions, such as the halogen bonds. The difficulties of modeling intramolecular parameters are beyond the simple extraction and fitting of the parameters: they are also led by the MD software limitations.
Some MD software like AMBER do not distinguish intra from intermolecular parameters for van der Waals and Coulombic charges. Although MD simulations may give good results for many physical and macro properties of a large number of different systems, many times specific micro-interactions are not modeled in a refined way. This is the case of some vibrational modes: even if a very precise parameterization is done, coupled vibrational modes in very conjugated molecules are extremely hard to describe (Prandi et al., 2016; Andreussi et al., 2017). The difficulty of an accurate description is mirrored in the fact that most MD simulation programs do not couple molecular motions like a stretching mode with an angular bending or the stretching modes of two adjacent atom pairs (Andreussi et al., 2017; Cerezo et al., 2018). More precisely, all mentioned terms in Equation (1) are expressed as sums of contributions, each one depending on a single internal coordinate. In this way, the off-diagonal terms (or hybrid terms) of the Hessian are not explicitly taken into account. Here, it is important to emphasize that it is mathematically and physically possible to derive parameters considering the cited couplings (Cerezo et al., 2018), but the implementation of a force-field functional form that describes the coupled terms can still not be done in many MD software.
It is evident that neither the best set of parameters can completely recover the electronic effects of a given molecule along an MD trajectory. Although some MD simulation programs are starting to be more flexible in terms of a force-field functional implementation, like GROMACS and Moscito (Cerezo et al., 2018). There is a still long path to achieve the full force-field functional form flexibility. Indeed, the maximum refinement that a normal user of mostly MD programs can do is to construct his own set of parameters. However, diving into very specific cases simple parameterizations can still be not enough.
Beyond the Limits
As defined in the introduction, the halogen bonds (XB) are non-covalent interactions between the halogen bond donor (D) and the halogen-bond acceptor (A) (Figure 1). Thus, the force-fields will describe these phenomena through the van der Waals term (Equation 1.4) and by the Coulombic term (Equation 1.5).
Many researchers address the origin of the XBs to the σ-hole, classifying them as σ-hole interactions (Clark et al., 2007; Politzer et al., 2008). The σ-hole is a positive region on the electrostatic potential surface (ESP), that arises from a charge anisotropy effect along with the D–X bond (Clark et al., 2007; Politzer et al., 2013). In other words, the electron density polarizes toward the D–X, generating an electron depletion in the back of the halogen (X) toward the D–X bond axis (see the blue regions over the halogens in Figure 3, in which D = CH3) (Politzer et al., 2012). For the σ-hole model, the strength of the XBs, which increases along X = F < Cl < Br < I, is directly correlated to the increase of the positive electrostatic potential on the halogen. In this sense, to perform a classical FF description of the XBs, the attraction between A and X should be rigorously described by the Coulomb potential. However, here we have at least two barriers to overcome: firstly, the XB cannot be seen as the attraction of two points charges as described by Equation (1.5), but the interaction of two densities; secondly, even using the point charge scheme, halogen atoms often have negative charges that would cause an electrostatic repulsion between X and A, not allowing the XB to happen. The fact is, something totally different from the usual parameterization must be done.
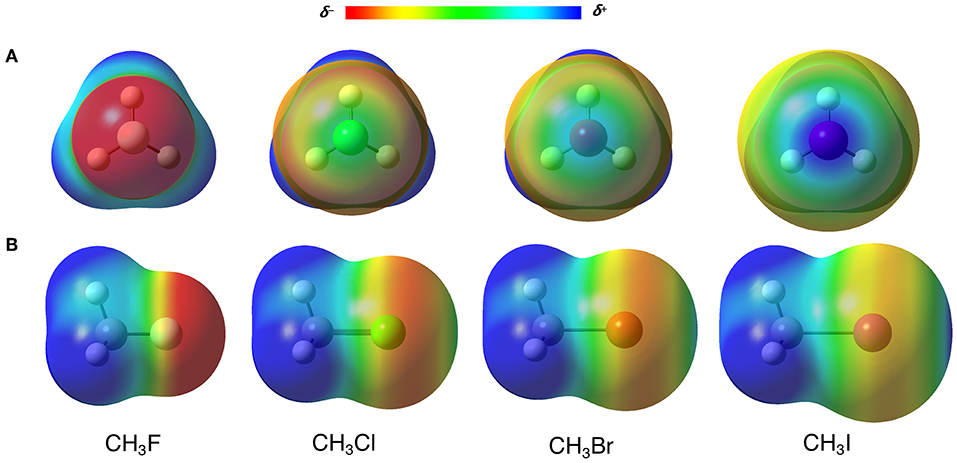
Figure 3. (A) Front and (B) side view of the electrostatic potential surfaces (at 0.02 a.u.) from −0.3 (red) to 0.3 (blue) a.u. of CH3X (X = F, Cl, Br, I) molecules. Computed at B3LYP-D3(BJ)/def2-TZVP, using Gaussian 09 (Frisch et al., 2009).
The first attempt to describe the XB through molecular mechanics was suggested by Ibrahim who introduced the Explicit σ-hole (ESH) theory (Ibrahim, 2011, 2012; Kolár et al., 2013). The ESH is a way to model the σ-hole as a massless positive point charge bonded to the halogen atom at a certain distance (rESH) (Figure 4). In general, there are two parameters to be fitted: the charge of the massless point and its distance to X (rESH).
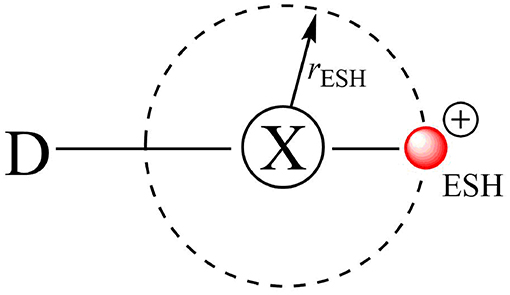
Figure 4. The explicit σ-hole (ESH) scheme to model halogen bonds via the molecular mechanics approach.
The ESH strategy has promoted huge advances for the modeling of XB in a biological environment predicting energy minima points in halogen-bonded systems along the potential energy surfaces. However, it is totally based on the classical electrostatic point of view of chemical interactions, that is, the electrostatic attraction between two point charges.
In fact, the XBs are a mix of attractive dispersion, electrostatics and orbital interactions in balance with repulsive orbital interactions (Pauli repulsion) and should not be described neglecting either one of them (Huber et al., 2013; Wolters et al., 2014; Santos et al., 2017). Figure 5 shows a simplified scheme of the halogen-bonding mechanism by Wolters and Bickelhaupt in the sight of Kohn-Sham density functional molecular orbital theory (Wolters and Bickelhaupt, 2012). An occupied molecular orbital of the acceptor, described by np orbitals of the halides, interacts with an unoccupied molecular orbital of the halogenated molecule (DX) to promote an attractive orbital interaction. Here, the doubly occupied orbital can be further extrapolated to any doubly occupied MO. See that the unoccupied molecular orbital of the DX molecule will have a strong sigma anti-bonding orbital () character. The Pauli repulsion originates from the interaction between the doubly occupied orbitals.
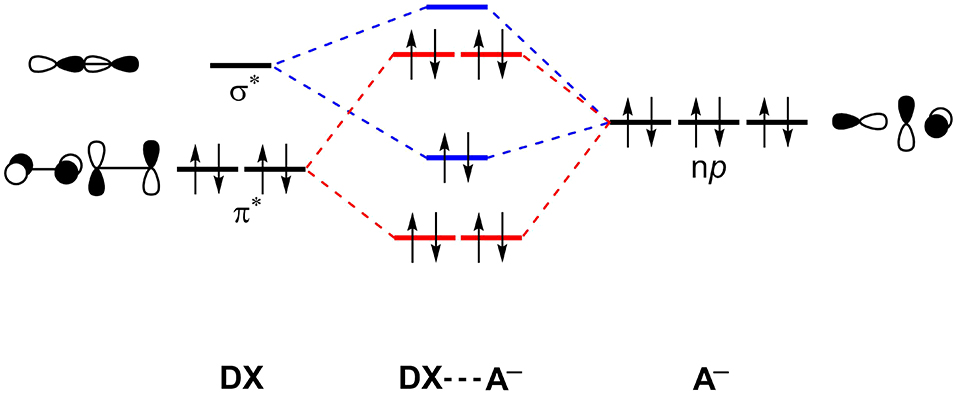
Figure 5. Simplified molecular orbital perspective of halogen-halide bonds. Main attractive interactions (blue) and repulsive interactions (red) are highlighted. X, D, A = F, Cl, Br, I.
In practice, the σ-hole model often seems to work, but just by coincidence. In previous work, we have shown that the maximum ESP values on σ-hole (Vmax) and the unoccupied orbital which contains the contribution of may have a similar origin (Santos et al., 2017). In other words, by increasing the value of Vmax, the will be stabilized and the interaction energy will become more stable.
Once the XBs have an important contribution of non-classical interactions, they cannot be described only by the Coulomb potential to get the ideal parameterization. In the traditional FF equation (Equation 1), the other alternative is to look at the van der Waals term. The Equation (2) is the Lennard-Jones 12-6 potential (Lennard-Jones, 1931) written in a different way than in Equation (1.4). Here, the positive part is the repulsion term and the negative part is the attractive term. The parameters are reduced to ε, the potential energy depth, and re, the equilibrium distance.
In theory, the repulsive part of Equation (2) would account for the Pauli repulsion, which is decently described by the traditional FF being the result of the steric hindrance between two atoms. The attractive term would account for the dispersive and orbital interactions. The first is also quite well-parametrized but the same cannot be said for the orbital interactions (Wu et al., 2012; Santos et al., 2014). The main problem of neglecting the orbital interactions in molecular mechanics is to get overestimated destabilizing energies at low range distances (Santos et al., 2014, 2017). At this interaction bond length, the Pauli repulsion and charge transfer are exponentially intensified.
One way to minimize this problem is to use the LJ 10-6 potential (Equation 3). With a lower exponential factor in the repulsion term, the interaction energies at low range distances are less destabilized (Du et al., 2013; Santos et al., 2014, 2017). However, the LJ 10-6 does not bring geometric improvements in comparison with the LJ 12-6, mainly in the cases that the non-covalent interactions are extremely directional, as the halogen bonds. The Lennard-Jones potential can model if a Lewis base will approximate toward the σD−X bond axis or not and how it would affect the interaction energy (Soteras Gutiérrez et al., 2016; Bernardes and Canongia Lopes, 2017).
Changing the Potential Equations
If the actual model does not work for a specific system, we must reformulate it. So, why not do the same for FF equations? Nevertheless, it is not necessary to build an equation from scratch. Wiser is to modify a well-known model. In the case of reformulating new non-bonded force-field terms, subtly modifications into the VC or VLJ have been done to get more accurate functions.
The directional dependence of halogen bonds can be understood by looking at the σ-hole and MO theories together. The interaction angle θ must be close to 180° to lead the interaction toward the D–X bond axis (Figure 1). This is the geometry configuration that would maximize the electrostatic and orbital interactions for both σ-hole and MO theories (Riley et al., 2009, 2013; Esrafili and Ahmadi, 2012; Santos et al., 2017). The angle φ depends on the electronic structure of the acceptor (A) in order to maximize the attractive donor-acceptor orbital overlaps (see Figure 5). For instance, having an sp2 oxygen as acceptor, φ would be around 120° to provide the frontal overlap between the lone pair of the oxygen (LPO) and the orbital. By the same perspective, for an sp nitrogen as acceptor, φ would be around 180° and, for π acceptors, φ would be around 90° (Cavallo et al., 2016; Nziko and Scheiner, 2016; Santos et al., 2017).
In a very clever way, Carter and co-workers (Carter et al., 2012; Scholfield et al., 2015; Koebel et al., 2016) have introduced the angular dependence into the LJ 12-6 and Coulombic potentials to describe bromine bonds, which was later extended to chlorine and iodine. The Equations (4) and (5) are the ff BXB functions to describe the non-bonding terms of XBs. The effective halogen charge (Zx) is defined by the amplitude (A) and the baseline (B) of the cosine function, which has the period ν and α = 180 − θ. In VLJ, 〈rvdw(X)〉 is now the average radius of the bromine at the energy minimum.
Parameterized to predict the halogen bonds in DNA junctions, the variation in the interaction energies were ~0.06 to ~0.7 kcal.mol−1 in comparison to the experimental data. The ff BXB functions also give good values of θ, from ~146 to ~122°.
Du et al. have introduced new polarizable non-bonded functions to the force-field equations in order to reproduce the XBs, the PEff model (Du et al., 2013). The electrostatic potential is defined by (6), in which Q is a constant, α, β, and ζ are parameters from ab initio electrostatic potential, r1 is a coordinate in the equatorial area, R is the distance from the halogen atom toward the D–X axis and r is the halogen-bond length.
The Lennard-Jones potential was used to simulate the repulsion and dispersion interactions (7), in which re is a function of θ, re, T is the transverse distance, re,L is the longitudinal distance and λ is a steepness parameter manually set to 1.26.
The third and last term is the polarization energy (8), in which E is the electronic field, Etot incorporates the induce dipole effects and α is the isotropic polarizability of the halogen.
The PEff functions have demonstrated a good performance in comparison with MP2 methods to predict the binding energies for chlorine, bromine, and iodine. Applied to well-known crystal structures, the deviation of the halogen-bond lengths was <0.1 Å and giving bond angles close to 180°.
Both ff BXB and PEff are complete force-field and already functional, albeit some tests with molecular dynamics must be carried out. Moreover, they only consider Cl, Br, and I as donors and Lewis basis with available electron lone pairs (i.e., A = S, O, N) as acceptors. Nonetheless, molecules with π orbitals can also act as halogen bond acceptors and must be considered since there are several aromatic structures in the biological environment. In this sight, a new LJ 10-6 function has been proposed that takes into account the halogen-bond acceptor nature and also includes the fluorine in the XB donor, the Emod (Equation 10) (Santos et al., 2017). Indeed, at certain conditions regarding the electronic structure of the whole donor molecule, the fluorine atom can form strong halogen-bonded complexes, sometimes as strong as the chlorine bonds (Wolters and Bickelhaupt, 2012; Santos et al., 2017).
The Emod empirical potential (10) has two new parameters: δ and γ (11). The parameter δ accounts for the attractive orbital interactions regarding the angular dependence to minimize the repulsion term, based on the synergy between Vmax and the energies, as aforementioned. The Vmax is calculated by QM methods, α is the van der Waals radius of the halogen atom and β is a constant, in which β = 2.5 for lone pair acceptors and β = 0.432 for aromatic acceptors. The parameter γ is a function of δ to rebalance the potential.
The Emod was designed to use the re already parameterized by any force-field without halogen-bond corrections. In practice, Vmax should be obtained by a QM calculation and used to fully define the parameters in Equation (11). Also, the Emod could be used the general VLJ function of the FF, since when Vmax is not given (i.e., equal to zero), the parameters δ and γ will tend to zero, and this function will behave like a traditional LJ 10-6 potential. However, Emod has not been tested with a complete force-field equation and there are no parameters for iodine.
Performing subtle modifications in the traditional empirical potentials is a good strategy to improve force-field equations. There are many other examples of modified potentials fitted to obtain reliable results of complex properties at the molecular mechanics level (Bernardes and Canongia Lopes, 2017; Franchini et al., 2018; Lin and MacKerell, 2018; Nunes et al., 2018). This approach eases the implementation of these functions by not requiring a huge effort to build a code from the beginning but using an already existing open-access and well-working code.
The use parameters obtained from previous quantum mechanical calculations can surely improve the results of a molecular dynamics simulation, but the next step is to rework the potentials in Equation (1) to further decrease the level of empiricism. That is where we find the ab initio force-fields (McDaniel et al., 2016; Xu et al., 2018; Pérez-Conesa et al., 2019). In principle, it would be possible to properly describe any non-covalent interactions with ab initio derived potentials, considering their specific properties, with a manageable computational cost.
Summary and Outlook
Through the last decades, computational methods have been employed in the investigations around chemical properties of the matter. The evolution of technology has allowed us to go deeper into the atomic level to retrieve information about chemical bonds and non-covalent interactions. However, the computational cost has been the border of how further our knowledge could go. To overcome these borders, cheap computational approaches based on classical mechanics have emerged.
In this review, we have discussed how cheap approaches, like molecular dynamics (MD) and molecular mechanics (MM) calculations, can be improved. Toward this goal, the parameterization of their force field (FF) equations is the key. Most of the parameters can be obtained by quantum mechanical (QM) calculations together with specific tools to modify and generate more accurate FF. Nevertheless, we have further explored one case that only setting up better parameters is not enough to retrieve the real information from a non-covalent interaction.
Being purely quantum chemical phenomena, the halogen bonds (XBs) have required the replacement of some FF potentials, since simple classical equations could not describe the properties of systems they are involved in. This replacement has been wisely done by modifying and introducing new parameters to well-known potentials. The new potentials to describe XBs were fit to high-level QM calculations, showing good agreement with crystal structure data. Thus, we strongly believe that the classical mechanical approaches will evolve by introducing new potentials based on ab initio calculations. The scope of this review is to highlight the relevance of ab initio parameterizations if the recovering of quantum chemical effects, lost by MM simulations, is wanted.
Author Contributions
LS and IP held the literature research and prepared the initial draft of this review. LS, IP, and TR were responsible for proofing and preparing the final copy of this review.
Funding
We thank the Brazilian agencies FAPEMIG, CNPq, and CAPES for the financial support. This work was also supported by University of Hradec Kralove (Faculty of Science, VT2019-2021).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
1. ^US Food and Drug Administration (FDA). Available online at: https://www.fda.gov/ (accessed November 4, 2017).
References
Adesokan, A. A., Roberts, V. A., Lee, K. W., Lins, R. D., and Briggs, J. M. (2004). Prediction of HIV-1 integrase/viral DNA interactions in the catalytic domain by fast molecular docking. J. Med. Chem. 47, 821–828. doi: 10.1021/jm0301890
Alder, B. J., and Wainwright, T. E. (1959). Studies in molecular dynamics. General method. J. Chem. Phys. 31:459. doi: 10.1063/1.1730376
Allen, R. E., de Wette, F. W., and Rahman, A. (1969). Calculation of dynamical surface properties of noble-gas crystals. II. Phys. Rev. 179:887. doi: 10.1103/PhysRev.179.887
Allinger, N. L. (1977). Conformational analysis. 130. MM2. a hydrocarbon force field utilizing V1 and V2 torsional terms. J. Am. Chem. Soc. 99, 8127–8134. doi: 10.1021/ja00467a001
Allinger, N. L., Chen, K., and Lii, J. H. (1996). An improved force field (MM4) for saturated hydrocarbons. J. Comput. Chem. 17, 642–668. doi: 10.1002/(SICI)1096-987X(199604)17:5/6<;642::AID-JCC6>;3.0.CO;2-U
Allinger, N. L., Thomas Tribble, M., Miller, M. A., and Wertz, D. H. (1971). Conformational analysis. LXIX. an improved force field for the calculation of the structures and energies of hydrocarbons. J. Am. Chem. Soc. 93, 1637–1648. doi: 10.1021/ja00736a012
Allinger, N. L., Yuh, Y. H., and Lii, J. H. (1989). Molecular mechanics. the MM3 force field for hydrocarbons. 1. J. Am. Chem. Soc. 111, 8551–8566. doi: 10.1021/ja00205a001
Andreussi, O., Prandi, I. G., Campetella, M., Prampolini, G., and Mennucci, B. (2017). Classical force fields tailored for QM applications: is it really a feasible strategy? J. Chem. Theory Comput. 13, 4636–4648. doi: 10.1021/acs.jctc.7b00777
Auffinger, P., Hays, F. A., Westhof, E., and Ho, P. S. (2004). Halogen bonds in biological molecules. Proc. Natl. Acad. Sci. U.S.A. 100, 8742–8747. doi: 10.1073/pnas.0407607101
Aytenfisu, A. H., Spasic, A., Grossfield, A., Stern, H. A., and Mathews, D. H. (2017). Revised RNA dihedral parameters for the Amber force field improve RNA molecular dynamics. J. Chem. Theory Comput. 13, 900–915. doi: 10.1021/acs.jctc.6b00870
Barone, V., Cacelli, I., De Mitri, N., Licari, D., Monti, S., and Prampolini, G. (2013). Joyce and Ulysses: integrated and user-friendly tools for the parameterization of intramolecular force fields from quantum mechanical data. Phys. Chem. Chem. Phys. 15, 3736–3751. doi: 10.1039/c3cp44179b
Ben-Naim, A. (2012). Levinthal's question revisited, and answered. J. Biomol. Struct. Dyn. 30, 113–124. doi: 10.1080/07391102.2012.674286
Bernardes, C. E. S., and Canongia Lopes, J. N. (2017). Modeling halogen bonds in ionic liquids: a force field for imidazolium and halo-imidazolium derivatives. J. Chem. Theory Comput. 13, 6167–6176. doi: 10.1021/acs.jctc.7b00645
Bora, P. L., Novák, M., Novotný, J., Foroutan-Nejad, C., and Marek, R. (2017). Supramolecular covalence in bifurcated chalcogen bonding. Chem. Eur. J. 23, 7315–7323. doi: 10.1002/chem.201700179
Carter, M., Rappé, A. K., and Ho, P. S. (2012). Scalable anisotropic shape and electrostatic models for biological bromine halogen bonds. J. Chem. Theory Comput. 8, 2461–2473. doi: 10.1021/ct3001969
Case, D. A., Babin, V., Berryman, J. T., Betz, R. M., Cai, Q., and Cerutti, D. S. (2014), AMBER 14. San Francisco, CA: University of California.
Cavallo, G., Metrangolo, P., Milani, R., Pilati, T., Priimagi, A., Resnati, G., et al. (2016). The halogen bond. Chem. Rev. 116, 2478–2601. doi: 10.1021/acs.chemrev.5b00484
Cerezo, J., Prampolini, G., and Cacelli, I. (2018). Developing accurate intramolecular force fields for conjugated systems through explicit coupling terms. Theor. Chem. Acc. 137:80. doi: 10.1007/s00214-018-2254-8
Clark, T., Hennemann, M., Murray, J. S., and Politzer, P. (2007). Halogen bonding: the σ-Hole. J. Mol. Model. 13, 291–296. doi: 10.1007/s00894-006-0130-2
Davis, J. E., and Patel, S. (2010). Revised charge equilibration parameters for more accurate hydration free energies of alkanes. Chem. Phys. Lett. 484, 173–176. doi: 10.1016/j.cplett.2009.09.061
Dickson, C. J., Madej, B. D., Skjevik, A. A., Betz, R. M., Teigen, K., Gould, I. R., et al. (2014). Lipid14: the amber lipid force field. J. Chem. Theory Comput. 10, 865–879. doi: 10.1021/ct4010307
Dominikowska, J., Bickelhaupt, F. M., Palusiak, M., and Fonseca Guerra, C. (2016). Source of cooperativity in halogen-bonded haloamine tetramers. Chem. Phys. Chem. 17, 474–480. doi: 10.1002/cphc.201501130
Du, L., Gao, J., Bi, F., Wang, L., and Liu, C. (2013). A polarizable ellipsoidal force field for halogen bonds. J. Comput. Chem. 34, 2032–2040. doi: 10.1002/jcc.23362
Esrafili, M. D., and Ahmadi, B. (2012). A theoretical investigation on the nature of Cl…N and Br…N halogen bonds in FArX…NCY complexes (X = Cl, Br and Y = H, F, Cl, Br, OH, NH2, CH3 and CN). Comput. Theor. Chem. 997, 77–82. doi: 10.1016/j.comptc.2012.07.038
Franchini, D., Dapiaggi, F., Pieraccini, S., Forni, A., and Sironi, M. (2018). Halogen bonding in the framework of classical force fields: the case of chlorine. Chem. Phys. Lett. 712, 89–94. doi: 10.1016/j.cplett.2018.09.052
Freitas, L. B., Borgati, T. F., de Freitas, R. P., Ruiz, A. L., Marchetti, G. M., de Carvalho, J. E., et al. (2014). Synthesis and antiproliferative activity of 8-hydroxyquinoline derivatives containing a 1,2,3-triazole moiety. Eur. J. Med. Chem. 84, 595–604. doi: 10.1016/j.ejmech.2014.07.061
Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., et al. (2009). Gaussian 09, Revision D.01. Wallingford, CT: Gaussian Inc.
Hongo, K., Cuong, N. T., and Maezono, R. (2013). The importance of electron correlation on stacking interaction of adenine-thymine base-pair step in B-DNA: a quantum Monte Carlo study. J. Chem. Theory Comput. 9, 1081–1086. doi: 10.1021/ct301065f
Huber, S. M., Scanlon, J. D., Jimenez-Izal, E., Ugalde, J. M., and Infante, I. (2013). On the directionality of halogen bonding. Phys. Chem. Chem. Phys. 15:10350. doi: 10.1039/c3cp50892g
Humphrey, W., Dalke, A., and Schulten, K. (1996). VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38. doi: 10.1016/0263-7855(96)00018-5
Ibrahim, M. A. (2011). Molecular mechanical study of halogen bonding in drug discovery. J. Comput. Chem. 32, 2564–2574. doi: 10.1002/jcc.21836
Ibrahim, M. A. (2012). Molecular mechanical perspective on halogen bonding. J. Mol. Model. 18, 4625–4638. doi: 10.1007/s00894-012-1454-8
Jorgensen, W. L., Maxwell, D. S., and Tirado-Rives, J. (1996). Development and testing of the OLPS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 118, 11225–11236. doi: 10.1021/ja9621760
Jurinovich, S., Viani, L., Prandi, I. G., and Mennucci, B. (2015). Towards an ab initio description of the optical spectra of light-harvesting antennae: application to the CP29 complex of photosystem II. Phys. Chem. Chem. Phys. 17, 14405–14416. doi: 10.1039/C4CP05647G
Koebel, M. R., Schmadeke, G., Posner, R. G., and Sirimulla, S. (2016). AutoDock VinaXB: implementation of XBSF, new empirical halogen bond scoring function, into AutoDock Vina. J. Chem. Inform. 8:27. doi: 10.1186/s13321-016-0139-1
Kolár, M., Hobza, P., and Bronowska, A. K. (2013). Plugging the explicit σ-holes in molecular docking. Chem. Commun. 49, 981–983. doi: 10.1039/C2CC37584B
Kolár, M., Hostaš, J., and Hobza, P. (2014). The strength and directionality of a halogen bond are co-determined by the magnitude and size of the σ-Hole. Phys. Chem. Chem. Phys. 16, 9987–9996. doi: 10.1039/C3CP55188A
Langley, C. H., and Allinger, N. L. (2002). Molecular mechanics (MM4) calculations on amides. J. Phys. Chem. A 106, 5638–5652. doi: 10.1021/jp014426r
Langley, C. H., Lii, J. H., and Allinger, N. L. (2001). Molecular mechanics (MM4) calculations on carbonyl compounds part I: aldehydes. J. Comput. Chem. 24, 1283–1286. doi: 10.1002/jcc.1177
Lennard-Jones, J. E. (1931). Cohesion. Proc. Phys. Soc. 43, 461–482. doi: 10.1088/0959-5309/43/5/301
Li, P., and Merz, K. M. Jr. (2017). Metal ion modeling using classical mechanics. Chem. Rev. 117, 1564–1686. doi: 10.1021/acs.chemrev.6b00440
Lii, J. H., and Allinger, N. L. (1989a). Molecular mechanics. the MM3 force field for hydrocarbons. 2. Vibrational frequencies and thermodynamics. J. Am. Chem. Soc. 111, 8566–8575. doi: 10.1021/ja00205a002
Lii, J. H., and Allinger, N. L. (1989b). Molecular mechanics. the MM3 force field for hydrocarbons. 3. The van Der Waals' potentials and crystal data for aliphatic and aromatic hydrocarbons. J. Am. Chem. Soc. 111, 8576–8582. doi: 10.1021/ja00205a003
Lin, F. Y., and MacKerell, A. D. (2018). Polarizable empirical force field for halogen-containing compounds based on the classical drude oscillator. J. Chem. Theory Comput. 14, 1083–1098. doi: 10.1021/acs.jctc.7b01086
Lu, Y., Liu, Y., Xu, Z., Li, H., Liu, H., and Zhu, W. (2012). Halogen bonding for rational drug design and new drug discovery. Expert Opin. Drug Discov. 7, 375–383. doi: 10.1517/17460441.2012.678829
Lu, Y., Shi, T., Wang, Y., Yang, H., Yan, X., Luo, X., et al. (2009). Halogen bonding-a novel interaction for rational drug design? J. Med. Chem. 52, 2854–2862. doi: 10.1021/jm9000133
Lu, Y., Wang, Y., and Zhu, W. (2010). Nonbonding interactions of organic halogens in biological systems: implications for drug discovery and biomolecular design. Phys. Chem. Chem. Phys. 12, 4543–4551. doi: 10.1039/b926326h
Malde, A. K., Zuo, L., Breeze, M., Stroet, M., Poger, D., Nair, P. C., et al. (2011). An automated force field topology builder (ATB) and repository: version 1.0. J. Chem. Theory Comput. 7, 4026–4037. doi: 10.1021/ct200196m
Martins, T. L. C., Ramalho, T. C., Figueroa-Villar, J. D., Flores, A. F. C., and Pereira, C. M. P. (2003). Theoretical and experimental 13C and 15N NMR investigation of guanylhydrazones in solution. Magn. Reson. Chem. 41, 983–988. doi: 10.1002/mrc.1299
Mayne, C. G., Saam, J., Schulten, K., Tajkhorshid, E., and Gumbart, J. C. (2013). Rapid parameterization of small molecules using the force field toolkit. J. Comput. Chem. 34, 2757–2770. doi: 10.1002/jcc.23422
McDaniel, J. G., Choi, E., Son, C. Y., Schmidt, J. R., and Yethiraj, A. (2016). Ab Initio force fields for imidazolium-based ionic liquids. J. Phys. Chem. B 120, 7024–7036. doi: 10.1021/acs.jpcb.6b05328
Mendez, L., Henriquez, G., Sirimulla, S., and Narayan, M. (2017). Looking back, looking forward at halogen bonding in drug discovery. Molecules 22, 22–25. doi: 10.3390/molecules22091397
Nair, P. C., and Miners, J. O. (2014). Molecular dynamics simulations: from structure function relationships to drug discovery. In Silico Pharmacol. 2:4. doi: 10.1186/s40203-014-0004-8
Nevins, N., Chen, K., and Allinger, N. L. (1996). Molecular mechanics (MM4) calculations on alkenes. J. Comput. Chem. 17, 669–694. doi: 10.1002/(SICI)1096-987X(199604)17:5/6<;669::AID-JCC7>;3.0.CO;2-S
Novák, M., Foroutan-Nejad, C., and Marek, R. (2015). Asymmetric bifurcated halogen bonds. Phys. Chem. Chem. Phys. 17, 6440–6450. doi: 10.1039/C4CP05532B
Nunes, R., Vila-Viçosa, D., Machuqueiro, M., and Costa, P. J. (2018). Biomolecular simulations of halogen bonds with a GROMOS force field. J. Chem. Theory Comput. 14, 5383–5392. doi: 10.1021/acs.jctc.8b00278
Nziko, V. P. N., and Scheiner, N. (2016). Comparison of π-hole tetrel bonding with σ-hole halogen bonds in complexes of XCN (X = F, Cl, Br, I) and NH3. Phys. Chem. Chem. Phys. 18, 3581–3590. doi: 10.1039/C5CP07545A
Paschek, D., and Geiger, A. (2002). MOSCITO 4. Dortmund: Department of Physical Chemistry; University of Dortmund.
Pérez-Conesa, S., Torrico, F., Martínez, J. M., Pappalardo, R. R., and Marcos, E. S. (2019). A general study of actinyl hydration by molecular dynamics simulations using ab initio force fields. J. Chem. Phys. 150:104504. doi: 10.1063/1.5083216
Poater, J., Swart, M., Fonseca Guerra, C., and Bickelhaupt, F. M. (2011). Selectivity in DNA replication. interplay of steric shape, hydrogen bonds, π-stacking and solvent effects. Chem. Commun. 47, 7326–7328. doi: 10.1039/c0cc04707d
Politzer, P., Lane, P., Concha, M. C., Ma, Y., and Murray, J. S. (2007). An overview of halogen bonding. J. Mol. Model. 13, 305–311. doi: 10.1007/s00894-006-0154-7
Politzer, P., Murray, J. S., and Clark, T. (2013). Halogen bonding and other σ-hole interactions: a perspective. Phys. Chem. Chem. Phys. 15, 11178–11189. doi: 10.1039/c3cp00054k
Politzer, P., Murray, J. S., and Concha, M. C. (2008). σ-hole bonding between like atoms; a fallacy of atomic charges. J. Mol. Model. 14, 659–665. doi: 10.1007/s00894-008-0280-5
Politzer, P., Riley, K. E., Bulat, F. A., and Murray, J. S. (2012). Perspectives on halogen bonding and other σ-hole interactions: lex parsimoniae (Occam's Razor). Comput. Theor. Chem. 998, 2–8. doi: 10.1016/j.comptc.2012.06.007
Poznanski, J., and Shugar, D. (2013). Halogen bonding at the ATP binding site of protein kinases: preferred geometry and topology of ligand binding. Biochim. Biophys. Acta 1834, 1381–1396. doi: 10.1016/j.bbapap.2013.01.026
Prandi, I. G., Viani, L., Andreussi, O., and Mennucci, B. (2016). Combining classical molecular dynamics and quantum mechanical methods for the description of electronic excitations: the case of carotenoids. J. Comput. Chem. 37, 981–991. doi: 10.1002/jcc.24286
Ramalho, T. C., and da Cunha, E. F. F. (2011). Thermodynamic framework of the interaction between protein and solvent drives protein folding. J. Biomol. Struct. Dyn. 28, 645–646. doi: 10.1080/073911011010524975
Ramalho, T. C., Rocha, M. V. J., da Cunha, E. F. F., and Freitas, M. P. (2009). The search for new COX-2 inhibitors: a review of 2002-2008 patents. Expert Opin. Ther. Pat. 19, 1193–1228. doi: 10.1517/13543770903059125
Rendine, S., Pieraccini, S., Forni, A., and Sironi, M. (2011). Halogen bonding in ligand–receptor systems in the framework of classical force fields. Phys. Chem. Chem. Phys. 13, 19508–19516. doi: 10.1039/c1cp22436k
Riley, K. E., Murray, J. S., Politzer, P., Concha, M. C., and Hobza, P. (2009). Br…O complexes as probes of factors affecting halogen bonding: interactions of bromobenzenes and bromopyrimidines with acetone. J. Chem. Theory Comput. 5, 155–163. doi: 10.1021/ct8004134
Riley, K. E., Rezáč, J., and Hobza, P. (2013). Competition between halogen dihalogen and hydrogen bonds in bromo- and iodomethanol dimers. J. Mol. Model. 19, 2879–2883. doi: 10.1007/s00894-012-1727-2
Santos, L. A., da Cunha, E. F. F., Freitas, M. P., and Ramalho, T. C. (2014). Hydrophobic noncovalent interactions of inosine-phenylalanine: a theoretical model for investigating the molecular recognition of nucleobases. J. Phys. Chem. A 118, 5808–5817. doi: 10.1021/jp411230w
Santos, L. A., da Cunha, E. F. F., and Ramalho, T. C. (2017). Toward the classical description of halogen bonds: a quantum based generalized empirical potential for fluorine, chlorine, and bromine. J. Phys. Chem. A 121, 2442–2451. doi: 10.1021/acs.jpca.6b13112
Scholfield, M. R., Ford, M. C., Vander Zanden, C. M., Billman, M. M., Ho, P. S., and Rappé, A. K. (2015). Force field model of periodic trends in biomolecular halogen bonds. J. Phys. Chem. B 119, 9140–9149. doi: 10.1021/jp509003r
Schuler, L. D., Daura, X., and van Gunsteren, W. F. (2001). An improved GROMOS96 force field for aliphatic hydrocarbons in the condensed phase. J. Comput. Chem. 22, 1205–1218. doi: 10.1002/jcc.1078
Soteras Gutiérrez, I., Lin, F. Y., Vanommeslaeghe, K., Lemkul, J. A., Armacost, K. A., Brooks, C. L., et al. (2016). Parametrization of halogen bonds in the CHARMM general force field: improved treatment of ligand–protein interactions. Bioorg. Med. Chem. 24, 4812–4825. doi: 10.1016/j.bmc.2016.06.034
van der Spoel, D., Lindahl, E., Hess, B., Groenhof, G., Mark, A. E., and Berendsen, H. J. C. (2005). GROMACS: fast, flexible, and free. J. Comput. Chem. 26, 1701–1718. doi: 10.1002/jcc.20291
Vanommeslaeghe, K., Hatcher, E., Acharya, C., Kundu, S., Zhong, S., Shim, J., et al. (2010). CHARMM general force field: a force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 31, 671–690. doi: 10.1002/jcc.21367
Wang, C., Danovich, D., Mo, Y., and Shaik, S. (2014). On the nature of the halogen bond. J. Chem. Theory Comput. 10, 3726–3737. doi: 10.1021/ct500422t
Wang, J., Wolf, R. M., Caldwell, J. W., Kollman, P. A., and Case, D. A. (2004). Development and testing of a general amber force field. J. Comput. Chem. 25, 1157–1174. doi: 10.1002/jcc.20035
Wilcken, R., Zimmermann, M. O., Lange, A., Joerger, A. C., and Boeckler, F. M. (2013). Principles and applicationsof halogen bonding in medicinal chemistry and chemical biology. J. Med. Chem. 56, 1363–1388. doi: 10.1021/jm3012068
Wolters, L. P., and Bickelhaupt, F. M. (2012). Halogen bonding versus hydrogen bonding: a molecular orbital perspective. Chem. Open 1, 96–105. doi: 10.1002/open.201100015
Wolters, L. P., Schyman, P., Pavan, M. J., Jorgensen, W. L., Bickelhaupt, F. M., and Kozuch, S. (2014). The many faces of halogen bonding: a review of theoretical models and methods. Wiley Interdiscip. Rev. Comput. Mol. Sci. 4, 523–540. doi: 10.1002/wcms.1189
Wolters, L. P., Smits, N. W. G., and Fonseca Guerra, C. (2015). Covalency in resonance-assisted halogen bonds demonstrated with cooperativity in n-halo-guanine quartets. Phys. Chem. Chem. Phys. 17, 1585–1592. doi: 10.1039/C4CP03740E
Wu, X., Sun, Y., Li, C., and Yang, W. (2012). Parametric effects of the potential energy function on the geometrical features of ternary Lennard-Jones clusters. J. Phys. Chem. A 116, 8218–8225. doi: 10.1021/jp3037395
Keywords: halogen bonds, force-fields, molecular dynamics, non-covalent interactions, drug design
Citation: Santos LdA, Prandi IG and Ramalho TC (2019) Could Quantum Mechanical Properties Be Reflected on Classical Molecular Dynamics? The Case of Halogenated Organic Compounds of Biological Interest. Front. Chem. 7:848. doi: 10.3389/fchem.2019.00848
Received: 21 September 2019; Accepted: 21 November 2019;
Published: 13 December 2019.
Edited by:
Jamie Platts, Cardiff University, United KingdomReviewed by:
Pui Shing Ho, Colorado State University, United StatesCina Foroutan-Nejad, Masaryk University, Czechia
Copyright © 2019 Santos, Prandi and Ramalho. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Teodorico C. Ramalho, dGVvQHVmbGEuYnI=
 Lucas de Azevedo Santos1
Lucas de Azevedo Santos1 Ingrid G. Prandi
Ingrid G. Prandi Teodorico C. Ramalho
Teodorico C. Ramalho