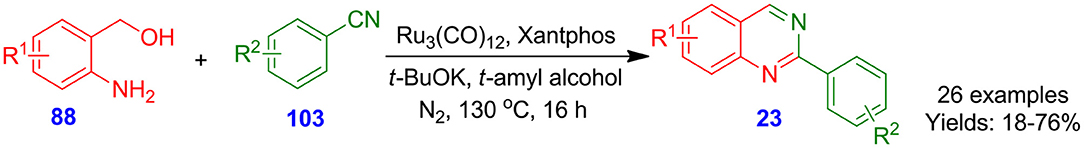- Department of Chemistry, Quaid-i-Azam University, Islamabad, Pakistan
In medicinal chemistry, one of the most significant heterocyclic compounds are quinazolines, possessing broad range of biological properties such as anti-bacterial, anti-fungal, anti-HIV, anti-cancer, anti-inflammatory, and analgesic potencies. Owing to its numerous potential applications, in the past two decades, there is an increase in the importance of designing novel quinazolines, exploring promising routes to synthesize quinazolines, investigating different properties of quinazolines, and seeking for potential applications of quinazolines. The present review article describes synthesis of quinazolines via eco-friendly, mild, atom-efficient, multi-component synthetic strategies reported in the literature. The discussion is divided into different parts as per the key methods involved in the formation of quinazoline skeletons, aiming to provide readers an effective methodology to a better understanding. Consideration has been taken to cover the most recent references. Expectedly, the review will be advantageous in future research for synthesizing quinazolines and developing more promising synthetic approaches.
Introduction
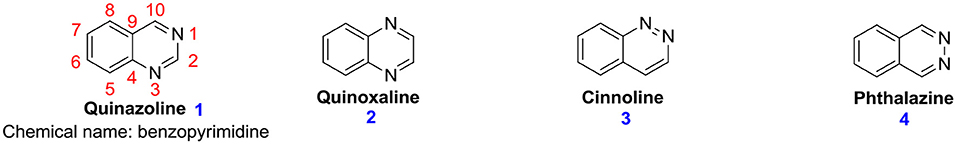
Quinazoline derivatives are among the most significant families of heterocyclic. Quinazoline (1,3-diazanaphthalene; 1) is a moiety made up of two condensed six-membered aromatic rings, a pyrimidine ring, and a benzene ring (Wang and Gao, 2013). It is yellow and amorphous, and its molar mass is 130.15 g.mol−1, and the chemical formula is C8H6N2. On the basis of various substitution patterns of nitrogen atoms, it is isomeric with quinoxaline 2, cinnoline 3, and pthalazine 4 (Figure 1). These isomeric forms are also called diazanaphthalenes. Analogs of this family, which contain a pyrazine ring and a benzene ring, are called Quinoxaline 2. These are also known as benzopyrazine. Cinnoline 3 also comprises a pyrazine ring and a benzene ring (Mishra, 2020). Phthalazine 4 is also called benzopyridiazine or benzo-orthodiazine, which contains a benzene ring and a pyridiazine ring. Gabriel (Ranawat et al., 2011) was the first scientist to prepare a quinazoline nucleus in the laboratory in 1903. Widdege (Asif, 2014) was the first scientist to propose the name quinazoline for this nucleus on the basis of its appearance as an isomer with the quinoxaline ring (Mahato et al., 2011). The synthesis of various compounds containing quinazoline as the main nucleus is largely mediated on the patterns of substitution on the 1,3-diazine entity of the system (Kamel et al., 2016).
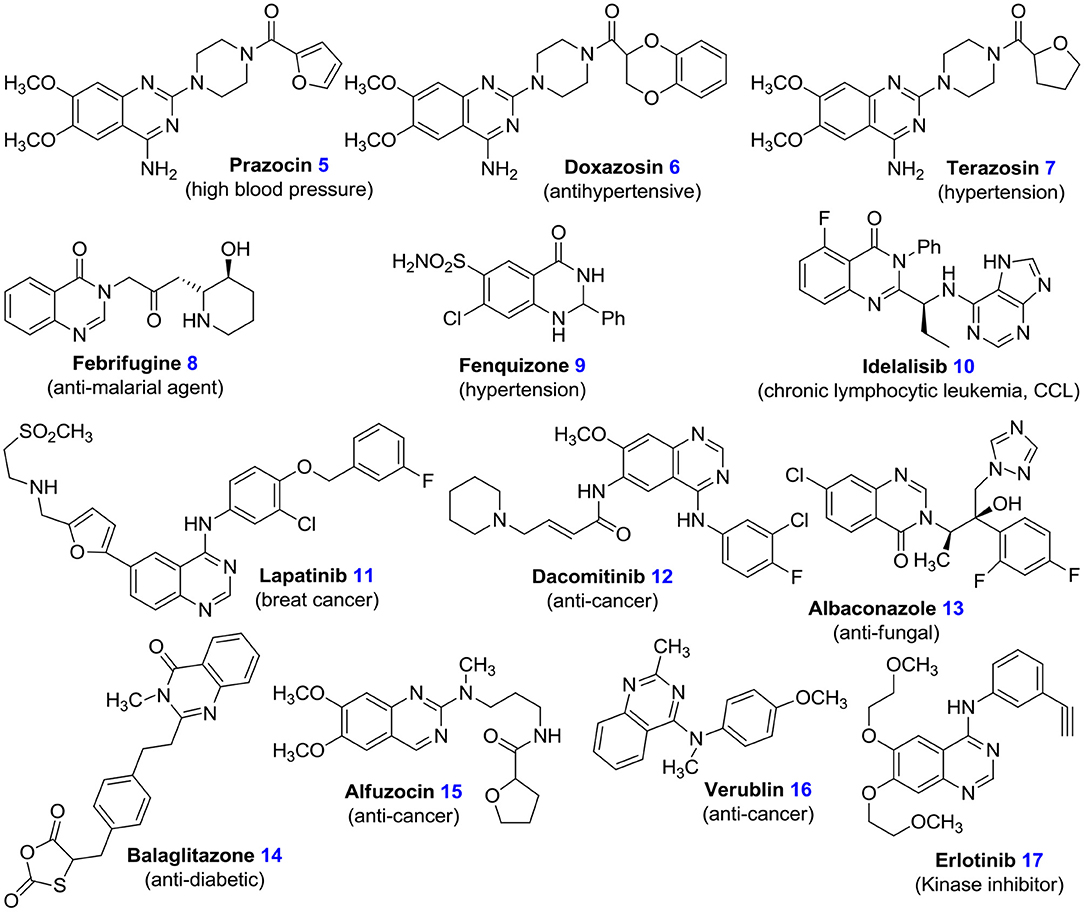
Quinazolines are noteworthy in medicinal chemistry, on account of a wide range of their anti-viral (Alagarsamy et al., 2018), anti-HIV (Vijaychand et al., 2011), anti-malaria (Patel et al., 2017), anti-inflammatory (Karaman et al., 2008), anti-fungal (Alagarsamy et al., 2018), anti-bacterial (Bedi et al., 2004), anti-spasm (Wang and Gao, 2013), anti-cytotoxin (Mishra, 2020), anti-virus (Witt and Bergman, 2000), anti-analgesic (Selvam and Kumar, 2011; Kshirsagar, 2015), anti-cancer (Karaman et al., 2008), anti-oxidation (Iino et al., 2009), anti-hypertensive (Honkanen et al., 1983), anti-depressant (El-Sayed et al., 2019), anti-psychotic (Mizuno et al., 2010), anti-diabetes (Uckun et al., 2002), anti-tuberculosis activities (Kunes et al., 2000), and also their inhibitory effects on thyrosine kinase, poly-(ADP-ribose) polymerase (PARP), and thymidylate synthase (Eswaran et al., 2010). There are several approved drugs with quinazoline structure in the market such as, prazosin hydrochloride 5, doxazosin mesylate 6, and terazosin hydrochloride 7 (Figure 2) (Jafari et al., 2016; Devi et al., 2017). Also, many quinazoline derivatives act as DNA-binding agents or as effective adrenergic blockers (Kamel et al., 2016). Many quinazoline derivatives also constitute the building blocks for about 150 natural alkaloids isolated from numerous families of the plant kingdom, from animals, and from microorganisms. Earlier studies conducted in the 1950s and 1960s led to the discovery of febrifugine 8, a quinazolinone-based alkaloid, which possesses anti-malarial potential, from the Chinese plant aseru (Wattanapiromsakul et al., 2003). Various quinazolinone-mediated drugs, which include fenquizone 9 and idelalisib 10, have been observed to display a wide range of anti-fungal, anti-tumor, anti-microbial, and cytotoxic potencies (Witt and Bergman, 2000). In combination therapy, lapatinib 11 has been shown to be active for breast cancer (McKee et al., 1947). Dacomitinib 12 is used to treat NSCLC (non-small-cell lung carcinoma) (Kumar et al., 2009). Albaconazole 13 has potent and broad-spectrum anti-fungal activity. Balaglitazone 14 has been used in trials studying the treatment of diabetes mellitus. Alfuzocin 15, verublin 16, and erlotinib 17 are anti-cancer agents (Figure 2) (Selvam and Kumar, 2011; Kshirsagar, 2015).
Synthetic chemists prepared a library of quinazolines with different bioactivities by linking several active groups to the quinazoline entity using developing synthetic approaches, and the potential uses of the quinazolines in area of medicine, pesticides, and biology have also been disclosed (Khan et al., 2014, 2015). To be more precise, the position two, six, and eight of quinazoline nucleus is very significant for structural-activity investigations, and 2,3-difunctionalized quinazoline derivatives are observed to possess anti-viral, anti-hypertensive, and anti-bacterial functions (Bouley et al., 2016; Hrast et al., 2017). Incorporation of various heterocyclics, such as phenothiazine, triazole, and pyridine, at second position of quinazoline entity results in the development of insecticidal, anti-bacterial, and anti-fungal properties (McKee et al., 1947). The substitution of heteroaryl and aryl moieties at N-3 and C-2, respectively, has shown improved analagesic and anti-inflammatory activities (Iino et al., 2009). Substitution at second and third position, like bridge phenyl ring, phenyl ring, and heterocyclic rings, are shown to contain anti-microbial potency. Development of the lipophilic character at the C-4 position of the quinazoline ring would be desired for novel inhibitory affinity. Deactivating functional groups in the third position provides enhanced hypotensive efficacy. A phenyl ring at the eighth position and a nitro group at the sixth position of quinazoline entity possess improved anti-cancer potency. Quinazoline derivatives when bonded to thiadiazole ring bear anti-HIV, anti-fungal, and antibacterial activities. Incorporation of a stryl group at the second position in quinazoline (=O) leads to development of enhanced chemotherapeutic actions. Electron-rich substituents or halogens on the sixth position and substituted amine or simple amine on the fourth position are known to assist the potency against bacteria. The third position should be bonded to diverse heterocyclic rings for enhanced chemotherapeutic action (Kung et al., 1999; Bhattacharjee et al., 2004).
In the recent years, numerous synthetic approaches for the formation of quinazoline scaffolds have been disclosed (Rajput and Mishra, 2012; Srivastava and Srivastava, 2015; Hameed et al., 2018). This review delivers a broad picture of progress for the development of quinazolines over the last decade. To be more precise, the review consists of two parts. The first part provides transition metal-free approaches to afford quinazoline derivatives, including heterogeneous catalytic systems, microwave-assisted reactions, ionic liquid-based reactions, and visible light-mediated synthetic systems. The second part focuses on transition metal-catalyzed approaches to afford quinazoline derivatives, including ruthenium-, zinc-, rhodium-, cobalt-, nickel-, gold-, iron-, palladium-, and copper-catalyzed reactions to synthesize quinazolines. Hopefully, the literature review would be valuable for scientists working in the field of medicinal and synthetic chemistry.
Transition Metal-Free Approach for the Synthesis of Quinazolines
In heterocyclic chemistry, transition metal-catalyzed coupling reactions have played a significant part for the formation of medicinally vital compounds. However, these reactions have some confronted challenges and limitation to some extent owing to the catalytic system. That is to say, most of the transition metals are toxic in nature, very expensive, and sensitive to moisture, especially oxygen. Also, the huge transition metal consumption does not meet the prerequisite for sustainable development. Alternative methodologies, therefore, for the development of C–N and C–C bonds under transition metal-free conditions are highly required and advantageous. In recent years, transition metal-free coupling reactions have become one of the attractive systems in synthesis to accomplish reactions with high productivity and to study how the reactions operate in the absence of transition metals.
Microwave-Promoted Synthesis
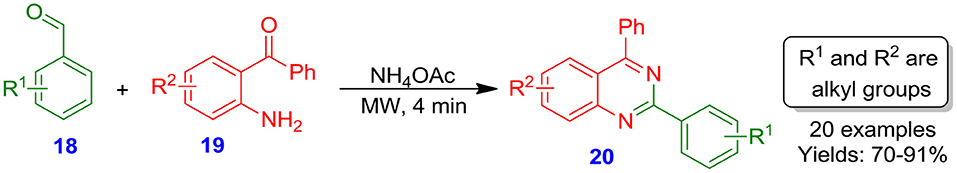
Sarma and Prajapati reported a catalyst- and solvent-free synthesis of quinazoline derivatives 20 from aldehydes 18, 2-aminobenzophenones 19, and ammonium acetate under microwave heating conditions (Scheme 1). The presented protocol was equally operative with a diverse range of electron-deficient and electron-rich benzaldehydes 18, and afforded target quinazolines 20 in good to excellent isolated yields (70–91%) within minutes (Sarma and Prajapati, 2011). The reaction is clean and simple, and provides an eco-friendly alternative toward removing organic solvents from organic synthesis.
4-Dimethylaminopyridine-Catalyzed Three-Component Approach
Boulcina et al. documented a general, efficient, one-pot process for the formation of quinazoline frameworks 20 in good to excellent isolated yields (67–98%) through the DMAP (4-[N,N-dimethylamino] pyridine)-catalyzed reaction of aromatic or hetero-aromatic aldehydes 18 with 2-aminobenzophenone 19 in the presence of NH4OAc under mild conditions (Scheme 2) (Derabli et al., 2014). This technique provides numerous benefits, for example, easy accessibility of starting materials, and high selectivity.
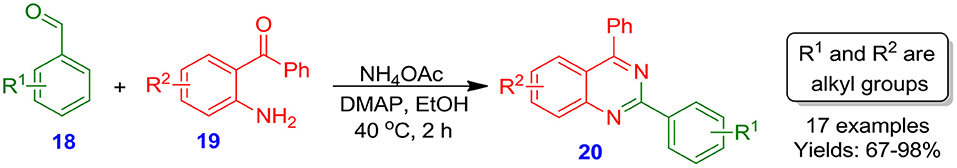
Scheme 2. Reaction of aldehydes and 2-aminobenzophenones catalyzed by 4-dimethylaminopyridine (DMAP).
Iodine/Ammonium Acetate-Assisted Three-Component Methodology
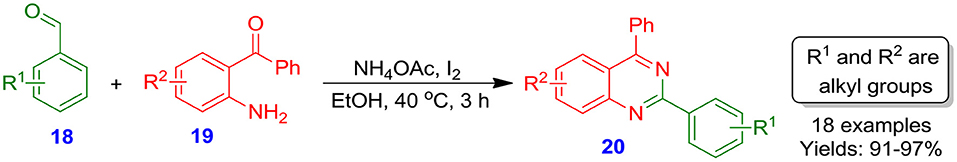
Panja et al. described a three-component one-pot methodology for the synthesis of highly substituted quinazoline derivatives 20 (34) via I2-catalyzed reaction of substituted benzaldehydes 18 with substituted o-aminoarylketones 19 in the presence of NH4OAc (Scheme 3). When performed in neat or with EtOH even at moderate temperature, the reaction results in an excellent yield (91–97%) in lesser time. It was observed that iodine is the appropriate catalyst counterpart in this synthetic approach attributed to its oxidizing properties and Lewis acidity. Moreover, this technique is superior in terms of simplicity and non-involvement of chromatographic purification technique (Panja et al., 2012).
Magnetic Ionic Liquid-Catalyzed Synthesis
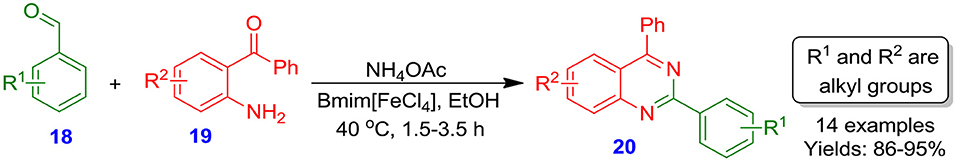
In another report, Panja et al. documented an ionic liquid (IL) Bmim[FeCl4] (butylmethylimidazolium tetrachloroferrate)-catalyzed one-pot, solvent-free, high yielding, multi-component green methodology for the synthesis of quinazolines 20 by the reaction of substituted aldehydes 18 with 2-aminobenzophenones 19 in the presence of NH4OAc at moderate temperature (Scheme 4). This technique was observed to be more valuable compared to other approaches in terms of its high catalyst stability, easier recyclability, high yield simplicity, and absence of any chromatographic purification technique (Panja and Saha, 2013).
Base-Driven Synthesis in Water
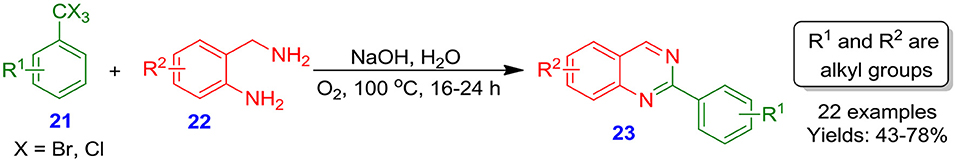
Cho et al. reported sustainable transition-metal-free synthesis of quinazoline derivatives 23 with moderate to good isolated yields (43–78%) from reaction of easily available α,α,α-trihalotoluenes 21 with o-aminobenzylamines 22 in the presence of molecular oxygen and sodium hydroxide in H2O (Scheme 5) (Chatterjee et al., 2018). The recrystallization process of the crude reaction mixture for the purification of the solid quinazolines eliminates the application of chromatographic purification and huge solvent-consuming workup, which make the overall process more economical and sustainable.
Tetrabutylammonium Iodide-Catalyzed Amination
Li et al. disclosed tetrabutylammonium iodide (n-Bu4NI)-catalyzed tandem reaction for the formation of imidazo[1,5-c]-quinazolines 26 (Scheme 6). This technique was investigated by reacting various 4-methyl-2-phenylquinazolines 24 with benzylamines 25, which afforded corresponding imidazo[1,5-c]quinazoline derivatives 26 with appropriate yields (35–98%) (Zhao et al., 2014). Selective dual amination of sp3 C-H bond under mild condition is involved in this reaction. Additionally, the reaction exhibited a wide range of substrates, including the common readily available α-amino acids and benzylamines. The novel procedure serves not only as a technique to develop a new series of imidazo-N-heterocycle derivatives 26 but also as a rare example of oxidative amination of benzylic primary C–H bonds with primary amines. This is a very valuable approach to transform simple quinazolines into highly functionalized quinazolines.
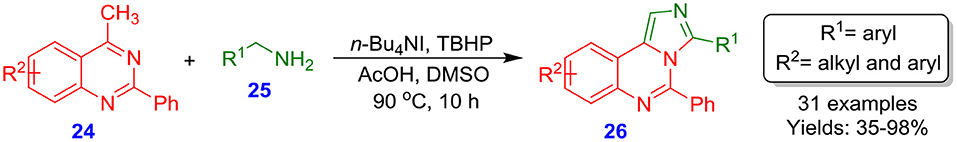
Scheme 6. Reaction of benzylamines with 4-methyl-2-phenylquinazolines catalyzed by tetrabutylammonium iodide.
Iodine/Tert Butylhydroperoxide-Driven C–H Functionalization
Zhang et al. documented an iodine/TBHP-assisted effective and facile one-pot tandem process for the development of 2-phenylquinazolines 29 with good to excellent isolated yields from benzylamines 27 and 2-aminobenzophenones 28 (Zhang et al., 2010). The method avoids the application of any kind of metal or hazard reagents (Scheme 7).
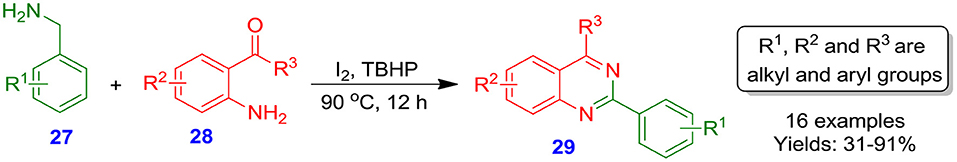
Scheme 7. Reaction of benzylamines with 2-aminobenzophenones assisted by iodine/tert butylhydroperoxide (TBHP).
Ceric Ammonium Nitrate-Catalyzed Synthesis
Nageswar et al. demonstrated the construction of quinazoline scaffolds 29 with good to excellent isolated yields (75–93%) from reaction of benzylamines 27 and 2-aminobenzophenones 28 catalyzed by CAN/TBHP (ceric ammonium nitrate/tert-butylhydroperoxide) in CH3CN (Scheme 8). The CAN/TBHP system was observed to be efficient, mild, and novel reagent for the facile synthesis of quinazolines 29. The yield of reaction was slightly increased when the electron-withdrawing group was present at the para-position of the benzylamine 27, whereas an electron-donating group decreased the yield of product (Karnakar et al., 2011).
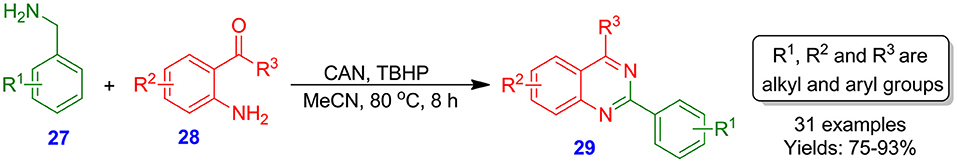
Scheme 8. Reaction of benzylamines with 2-aminobenzophenones catalyzed by ceric ammonium nitrate (CAN).
4-Hydroxy-TEMPO-Catalyzed C-H Bond Amination
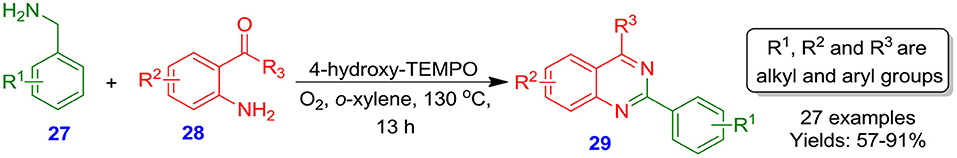
Han et al. described an effective and novel aerobic approach for the oxidative synthesis of 2-aryl quinazoline derivatives 29 via amination of benzyl C-H bonds using a one-pot 4-hydroxy-TEMPO radical-catalyzed reaction of 2-aminobenzaldehydes and 2-aminobenzoketones 28 with arylmethanamines 27, without the use of any additives or metals (Scheme 9) (Han et al., 2011).
Tert Butylhydroperoxide/DDQ-Induced Oxidative Cyclization Approach
Rachakonda et al. explored the synthesis of 2-arylquinazolines 29 under transition-metal-free and mild conditions from commercially available benzylamines 27 and 2-aminobenzophenones or 2-aminoacetophenones 28 via oxidative and condensation cyclization using DDQ as a versatile reagent (Scheme 10). The mechanism of the reaction involved condensation reaction followed by cyclization, giving the desired 2-arylquinazoline in good to excellent isolated yields (71–92%) (Rachakonda et al., 2012).
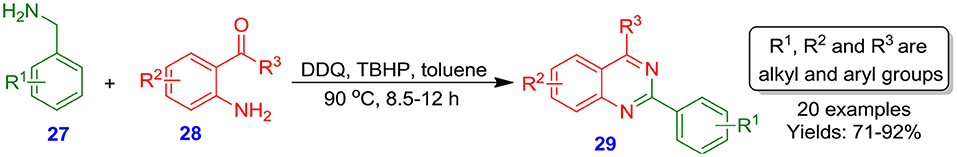
Scheme 10. Reaction of benzylamines with 2-aminobenzophenones induced by tert butylhydroperoxide (TBHP)/DDQ.
Cobalt Zeolite Imidazolate Framework-Catalyzed Synthesis
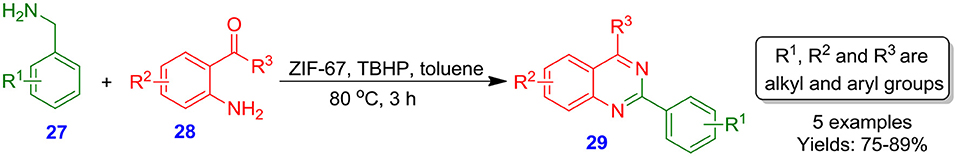
Truong et al. employed a heterogeneous catalytic system (viz. ZIF-67) for the cyclization reaction of benzylamines 27 with 2-aminobenzoketones 28 to afford quinazoline products 29 in excellent isolated yields (Scheme 11). Application of TBHP as an oxidant in toluene solvent at 80°C was observed to be at optimal conditions of reaction. ZIF-67 catalytic system could be regenerated and recycled without important degradation in catalytic potency (Truong et al., 2015).
Molecular Iodine-Catalyzed C-H Bond Amination Using Oxygen as an Oxidant
Very recently, Bhanage et al. reported the preparation of I2-catalyzed quinazoline derivatives 29 from reaction of 2-aminobenzaldehydes 28 or 2-aminobenzophenones with benzyl-amines 27 (Scheme 12). Numerous functionalized hetero-aryl or aryl amines were investigated with an ample range of functionalized 2-aminobenzaldehydes or 2-aminobenzophenones 28 to give the quinazolines 29 in moderate to excellent yields (49–92%) (Deshmukh and Bhanage, 2018). The application of O2 as an eco-friendly oxidant coupled with the solvent-, additive- and transition-metal-free conditions makes the approach greener and economical. The lack of aqueous workup also improves the productivity of this procedure. Moreover, the procedure uses I2 in catalytic amount and provides benzylic sp3 C–H bond functionalization/amination (Eswaran et al., 2010).
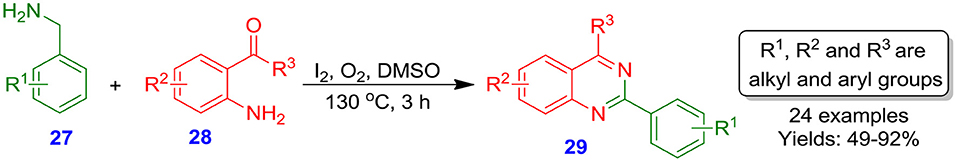
Scheme 12. Reaction of benzyl-amines with 2-aminobenzophenones or 2-aminobenzaldehydes catalyzed by molecular iodine.
Lewis Acid-Catalyzed Synthesis
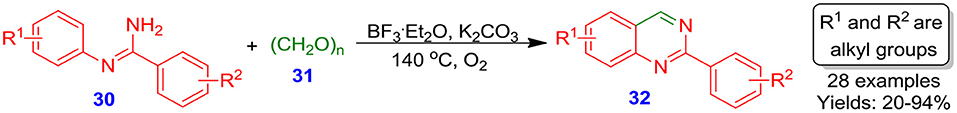
In the recent years, Deng et al. reported an efficient method for the synthesis of quinazoline scaffolds 32 under transition-metal-free conditions from reaction of N-phenyl-benzimidamides 30 and polyoxymethylene 31 as one carbon source (Cheng et al., 2016). The optimized condition of reaction was well-tolerated with electron-deficient and electron-rich substituent on benzene ring with low to excellent yield of respective quinazoline derivatives 32 (20–94%) (Scheme 13). The transition-metal-free reaction and mild conditions are one of most attractive features of this technique. This novel process offers an easily handle-able, eco-friendly, and complementary approach to 2-arylquinazoline scaffolds.
Iodine (III)-Driven Oxidative C–N and C–C Bond Construction
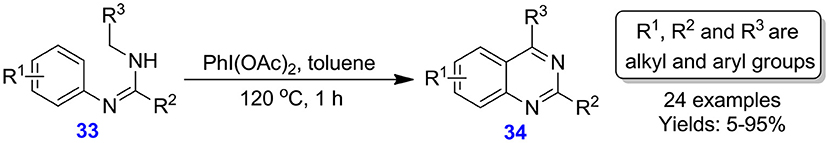
Lin et al. documented the preparation of multi-substituted quinazolines 34 from N-alkyl-N′-arylamidines 33 through the formation of iodine (III)-driven oxidative C(sp2)-N and C(sp3)-C(sp2) bonds under base- and metal-free conditions (Lin et al., 2014). Substrates having electron-deficient and electron-rich substituents on the aromatic ring afforded the respective quinazoline in low to excellent yields (5–95%); however, the reaction was incompatible with an aliphatic substituent (Scheme 14).
Visible Light-Assisted Photo-Redox Oxidative Annulation
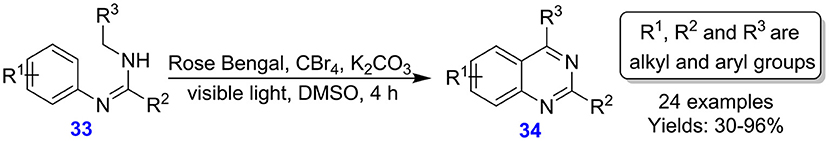
Tang et al. reported the formation of quinazolines 34 from amidine derivatives 33 via formation of visible light-based oxidative C(sp2)–C(sp3) bond (Scheme 15). This synthesis is a metal-free oxidative coupling assisted by photo-redox catalytic system. The procedure features low loading of catalyst (1 mol %) (Shen et al., 2016). The approach was observed to tolerate a broad spectrum of functional groups.
I2/KI-Based Oxidative C–C Bond Construction
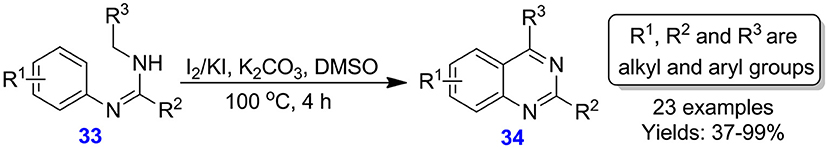
Lv et al. described the I2/KI-based oxidative C–C bond construction for the construction of quinazoline derivatives 34 from N,N′-difunctionalized amidines 33. Under the standard condition, all N,N′-disubstituted amidines 33 converted into the corresponding quinazolines 34 in moderate to excellent yields (37–99%) (Scheme 16) (Lv et al., 2016). This environmentally benign and practical technique can also be performed on a gram scale and operates well with crude amidine precursors.
Metal-Free Oxidative Annulation Using Cyanamide or Carbonitrile
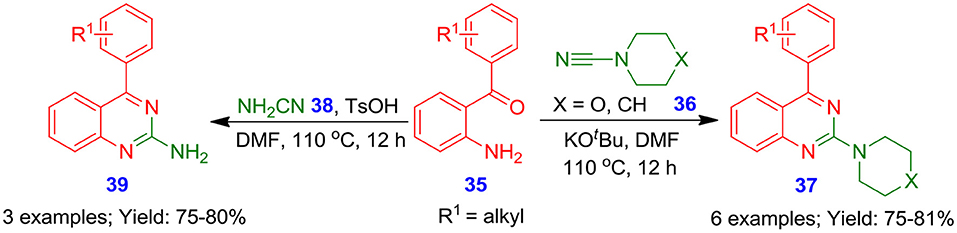
North et al. demonstrated a promising methodology for the formation of 2-aminoquinazolines (37, 39) in moderate to good isolated yields from the reaction of 2-aminobenzophenones 35 and 4-morpholinecarbonitrile 36 or cyanamide 38 (Pandya et al., 2017). The benefit of this synthetic approach is its transition-metal-free and mild conditions (Scheme 17). Of note, this process permits the synthesis of bioactive 2-aminoquinazoline analogous (37, 39) using a cyclic amine or free amine, allowing good atom economy and structural diversity.
Orthoester-Mediated Solvent- and Catalyst-Free Method
Bhat et al. disclosed a catalyst- and solvent-free environmental-friendly procedure for the formation of quinazoline frameworks 39 with good to excellent isolated yields (79–94%) from a one-pot three-component reaction of 2-aminoarylketones 28 and trialkyl orthoesters 38 in the presence of ammonium acetate (Scheme 18) (Bhat et al., 2015). The procedure bypasses some of the limitations and problems associated with the earlier techniques and is beneficial in terms of high product yield, simple work up procedure, moderate reaction time, readily availability of starting materials, clean reaction, and operational simplicity. Moreover, the protocol provides an environmental friendly and facile methodology toward the construction of bioactive novel quinazolines.
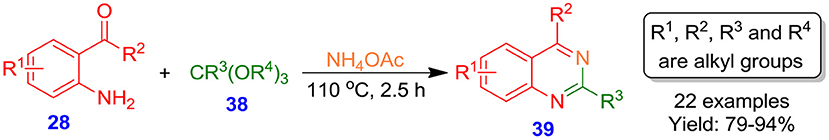
Scheme 18. Reactions of 2-aminoarylketones with orthoesters under catalyst- and solvent-free conditions.
Potassium Iodide-Promoted Three-Component Synthesis
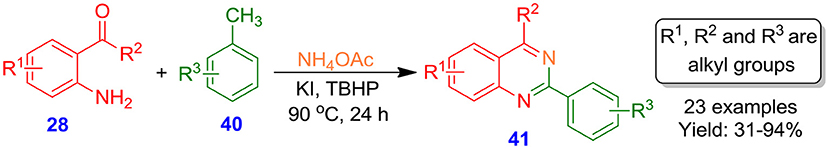
Li et al. synthesized 2-arylquinazoline derivatives 41 through a three-component one-pot mild KI-promoted methodology under transition-metal-free conditions by the reaction of 2-amino-benzophenones 28 with methylarenes 40 as one carbon source in the presence of NH4OAc (Zhao et al., 2015). The reaction demonstrated a wide spectrum of functional group tolerance including electron-deficient and electron-rich 2-aminoarylketones 28 and methylarenes 40 (Scheme 19).
Transition Metal-Catalyzed Methodologies
Since the past century, transition metal-catalyzed C–H activation reactions have been investigated, and these reactions represent a promising achievement and development of organometallic chemistry. These reactions were started in 1960 as a key subject in organometallic chemistry and became one of the most effective catalytic systems for the formation of C–N and C–C bonds (Meijere and Diederich, 2004; Diederich and Stang, 2008). Moreover, transition metal-catalyzed C–H activation and functionalization have some advantages over classical technique such as straightforward method for the development of fused heterocyclic compounds, no need of pre-functionalization of starting material, which offers a more efficient reduction in the generation of waste. In the past decade, remarkable efforts have been made for the construction of heterocycles through the formation of C–N and C–C bonds. A brief summary of the recent literature of metal-catalyzed formation of quinazolines via C–H activation and C–N coupling reactions is described below.
Palladium-Based Catalytic Systems
Vlaar et al. reported palladium(II) acetate-catalyzed aerobic oxidative coupling of (2-aminophenyl)azole derivatives 42 with isocyanide derivatives 43 for the formation of medicinally significant azole fused quinazolines 44 by using air as oxidant at 75°C in 2-methyltetrahydrofuran (Vlaar et al., 2014). An ample spectrum of triazole starting materials was reacted well and furnished annulated products in moderate to excellent isolated yields (11–83%) (Scheme 20A). In related development, Wang et al. described the palladium-catalyzed construction of 2-arylquinazoline scaffolds 47 by reacting E-1-(2′-nitrophenyl)ethanoneo-methyloximes 45 and benzyl alcohols 46 in the presence of dppf as ligand under argon at 160°C through hydrogen transfer approach (Wang et al., 2014). It is supposed that the synthesis of quinazolines proceeds through the dehydrogenation of benzyl alcohols to benzaldehydes, followed by the formation of imine and subsequent intramolecular cyclization resulting in the development of quinazolines (Scheme 20B). Likewise, the reaction was performed out with benzyl alcohols 46, urea 48, and 1-(2-nitrophenyl)ethanone 49 under optimized condition. The efficient synthesis delivers quinazolines 50 in lower to excellent isolated yields (21–90%) (Scheme 20C). Similarly, Xu et al. described the annulation process for the formation of multi-substituted quinazolines 52 from N-allylamidines 51 under microwave heating conditions by employing palladium as an active catalyst in xylene at 170°C (Xu et al., 2015). The scope of this methodology was disclosed by using a spectrum of aryl amidines containing electron-deficient and electron-rich substituents, which provided the corresponding product in good to excellent isolated yields (73–94%) (Scheme 20D). In an interesting study, Chen et al. illustrated a novel and practical technique for the preparation of quinazoline scaffolds 56 from 2-aminobenzylamine 53 with aryl bromides 54 and carbon monoxide 55, involving palladium-catalyzed aminocarbonylation–condensation–oxidation sequence and facilitating the desired quinazolines in poor to excellent isolated yields (5–93%) (Chen et al., 2014). In this procedure, DMSO serves both as oxidant and solvent (Scheme 20E). The generality of the approach was investigated by varying different withdrawing groups (cyano, trifluoromethyl) and electron releasing (methoxy, dimethylamino, or tert-butyl) containing aryl bromide.
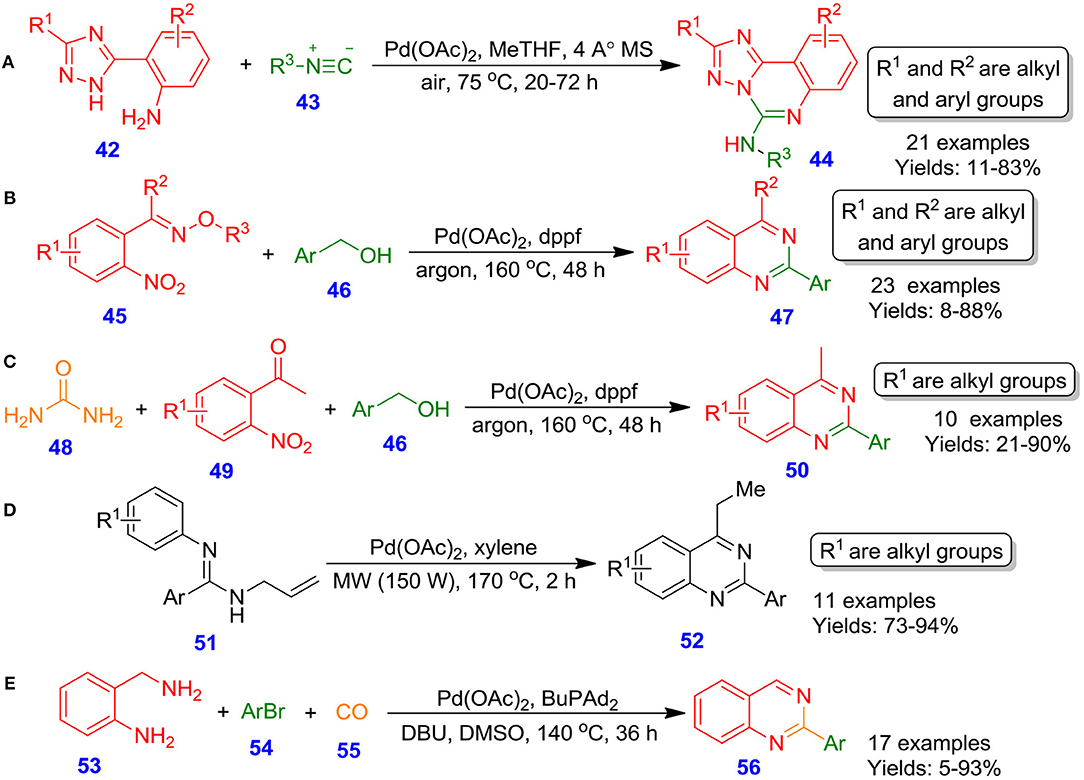
Scheme 20. Pd-catalyzed preparation of diverse azolo-[c] quinazoline, 2-arylquinazoline and substituted quinazoline derivatives.
Watanabe et al. described MoCl5 and Pd(PPh3)Cl2 catalyzing intermolecular reductive N-hetero-cyclization reaction of 2-nitrophenyl ketones or 2-nitrobenzaldehyde 57 with methanamide 58 for the formation of quinazoline analogous 59 (Akazome et al., 1995). The reaction proceeds through the formation of an active imene intermediate by selective carbon monoxide-based deoxygenation of nitro species (Scheme 21A). On the other hand, Chen et al. reported Pd-catalyzed three-component, one-pot tandem assembly for quinazolines 63 by using readily available 2-aminobenzonitriles 60, aryl boronic acids 61, and aldehydes 62. The method displays broad substrate scope and amazing chemoselectivity (Scheme 21B). A notable feature of this technique is the tolerance of iodo and bromo moieties, affording flexibility for further synthetic manipulations (Hu et al., 2018). Later, the same research group disclosed another methodology for quinazoline scaffolds 65 from reaction of aryl boronic acids 61 with 2-(quinazolinone-3(4H)-yl)benzonitriles 64. This tandem synthesis involved nucleophilic addition, followed by intramolecular cyclization and subsequent ring-opening, delivering the corresponding product in moderate to excellent isolated yields (31–93%) (Scheme 21C) (Zhang et al., 2018). In another interesting report by Chen et al., the synthesis of 2,4-disubstituted quinazoline derivatives 66 through Pd–catalyzed reaction of aryl boronic acids 61 with N-(2-cyanoaryl)benzamides 60 by employing 1,10-phen, trifluoroacetic acid in THF at 80°C has been described (Scheme 21D). The reaction revealed a wide spectrum of functional group tolerance, including electron-deficient and electron-rich aryl boronic acids 61 with N-(2-cyanoaryl)benzamides 60 (Zhu et al., 2018).
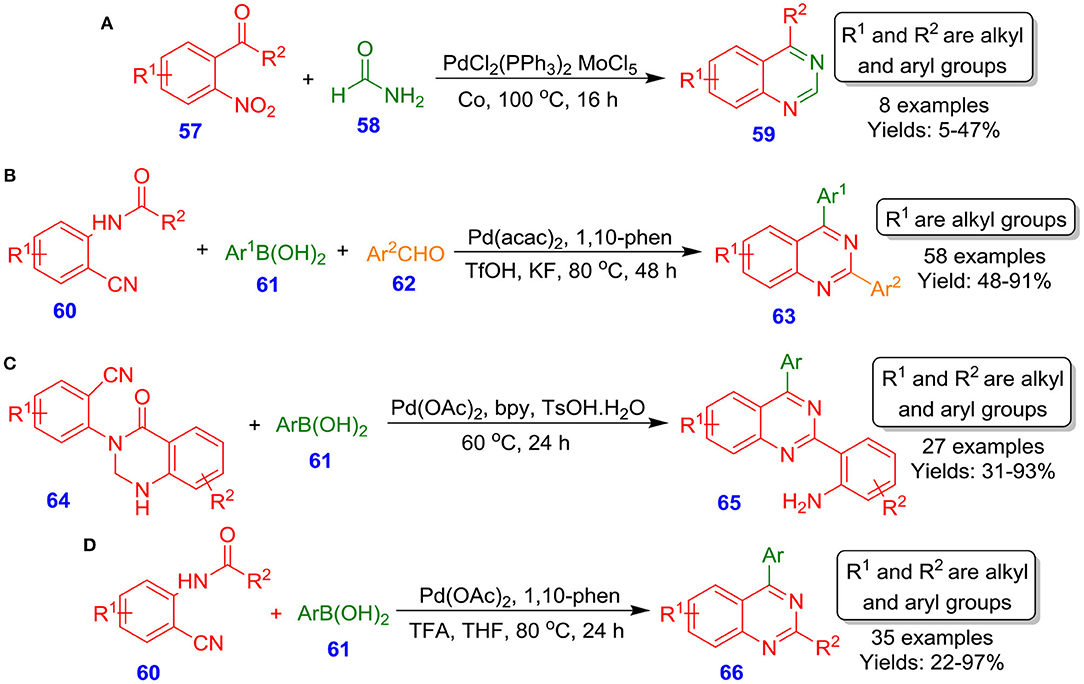
Scheme 21. Palladium-catalyzed synthesis of quinazolinone-based nitriles and multi-substituted quinazolines.
Copper-Mediated Catalytic Systems
In literature, the efficiency of copper-based catalytic systems is well-documented since last century where these salts have demonstrated to be an effective catalytic system in cross-coupling reactions for the preparation of bioactive molecules and natural products, on account of their good functional group tolerance, low toxicity, and economic attractiveness (Deutsch et al., 2008; Allen et al., 2013; Guo X.-X. et al., 2015). Copper salts have become a promising alternative to their costly counterparts, for instance ruthenium-, rhodium-, and palladium-based catalytic systems for the cross-coupling reactions. Cu-catalyzed Ullmann reaction is the pioneering work in the area of synthetic organic chemistry (Sambiagio et al., 2014). Cu-catalyzed cross-coupling reaction has been broadly explored for the newer synthetic approach for fused heterocyclic entities.
Fan et al. documented an effective one-pot method for the formation of diversely functionalized quinazolines 70 via copper-catalyzed tandem reaction among 2-bromobenzyl bromide derivatives 67, aldehyde derivatives 68, and ammonium hydroxide 69 (Fan et al., 2014). The reaction proceeds through cupric acetate-catalyzed amination of 2-bromobenzyl bromides 67 to 2-aminobenzyl amines, followed by condensation with aldehydes and subsequent intramolecular nucleophilic cyclization and aromatization, furnishing quinazoline derivatives (Scheme 22A). By employing simple aliphatic amines and ammonia as the source of nitrogen, the technique offers a practical and versatile approach. Also, this technique has several benefits, such as structural diversity of products and readily available starting materials. Alternatively, Liu et al. reported the direct approach to substituted quinazolines 20 from reaction among 2-aminobenzoketone derivatives 19, toluene 41, and ammonium acetate in the presence of copper(II) chloride at 80°C for 12 h (Liu et al., 2015). The reaction proceeds through oxidative amination of benzylic carbon–hydrogen bonds of methylarenes with 2-aminobenzoketones and ammonia, followed by intramolecular cyclization, affording quinazoline derivatives. Furthermore, the kinetic isotope effect (KIE) suggested that the carbon–hydrogen bond cleavage was the rate-limiting step in this methodology (Scheme 22B). On account of this method, a library of 2-arylquinazoline derivatives can be easily prepared in good isolated yields. Recently, in the same line, Kamal et al. employed 2-aminobenzophenones 28 and phenacyl azides 71 for the construction of quinazolines 72 by using cupric acetate, triethylamines in acetonitrile at ambient temperature (Visweswara Sastry et al., 2017). This procedure proceeded well and constructed two C–N bonds in a single operation (Scheme 22C). Additional, no oxidant or external source of nitrogen is demanded to accomplish the formation of quinazolines 72. The process is practical for production of numerous functionalized quinazolines 72 with high functional group tolerance as well as a broad spectrum of substrates. On the same note, Vishwakarma et al. demonstrated Cu-catalyzed effective approach for forming o-protected-4-hydroxyquinazolines 75 from 2-aminobenzonitriles 73, substituted aldehydes 68, and substituted alcohols 74 through the development of an N-functionalized bicyclic precursor, followed by nucleophilic attack of the alkoxy moiety (Battula et al., 2014). The synthesis was sufficiently explored with a wide spectrum of substituted 2-aminobenzonitriles and aldehydes led to respective quinazolines 75 in moderate to excellent isolated yields (41–88%) (Scheme 22D). Subsequently, Gao et al. disclosed a one-pot tandem method for the efficient and straightforward preparation of pyrazolo[1,5-a]quinazolines 78 by treating 2-bromobenzaldehydes 76 with 5-aminopyrazoles 77 in the presence of potassium carbonate at 110°C through Cu-catalyzed imine creation followed by Ullmann type coupling resulting in fused quinazolines 78 (Scheme 22E). Diverse functionalized 5-aminopyrazoles and 2-bromobenzaldehydes tolerated well and provided respective quinazoline in moderate to good isolated yields (41–79%) (Gao et al., 2014). With benefits such as mild reaction conditions, simple synthetic procedures, and readily available starting materials, the technique developed could be considered as a promising technique. Further, very recently, Wang et al. published Cu-catalyzed one-pot process for the preparation of substituted quinazolines 81 by reaction of 2-ethynylanilines 79 with benzonitriles 80 using O2 as the sole oxidant (Wang et al., 2018). The reaction proceeded via effective cleavage of the carbon–carbon triple bond and formation of new carbon–nitrogen, and carbon–carbon bonds in a one-pot manner (Scheme 22F). Furthermore, the reaction showcased a broad spectrum of substituent tolerance with several 2-ethynylanilines and benzonitriles, offering an array of quinazoline derivatives in moderate to excellent isolated yields (41–88%). These quinazolines also exhibited good fluorescence quantum yield, aggregation-induced emission effect, and lifetime decay, enhancing the importance of quinazolines in material chemistry for future aspect.
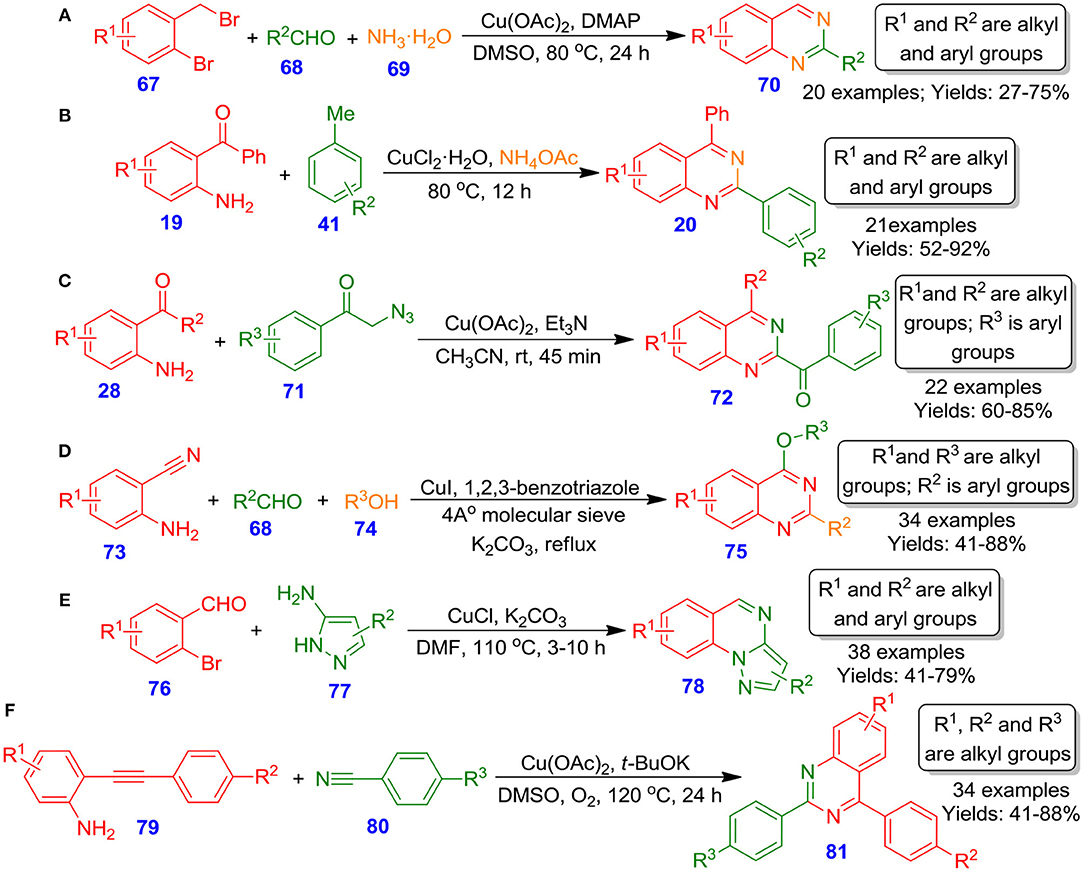
Scheme 22. Copper–catalyzed synthesis of o-protected-4-hydroxyquinazolines, pyrazolo[1,5-a]quinazolines, tetrahydroquinazolines, 2-arylquinazolines, and multi-substituted quinazolines.
Fu et al. described quinazoline derivatives 20 via Cu-catalyzed tandem couplings of functionalized 2-halophenylketones or 2-halobenzaldehydes 82 with amidine hydrochlorides 83 under mild conditions (Huang et al., 2008). The approach was equally operative with aromatic as well as with aliphatic and delivered target quinazolines in good to excellent isolated yields (61–95%) (Scheme 23A). Moreover, the method showed simple, practical, and economical advantages. Similarly, Truong et al. reported an effective one-pot approach for highly functionalized quinazolines 20 in good to excellent isolated yields via ligand-free Cu-catalyzed Ullmann condensation of o-iodobenzaldehyde derivatives 82 with substituted amidine hydrochlorides 83 in the presence of Cs2CO3 in methanol at 60°C (Truong and Morrow, 2010). Mild reaction conditions as well as one-pot conditions make this procedure a striking alternative for the preparation of this family of compounds (Scheme 23B). In the recent years, Raut et al. achieved a facile and green ultrasonic-assisted formation of cuprous oxide nano-cubes as a heterogeneous nanocatalytic system at ambient temperature, and cuprous oxide nano-cubes were utilized for the construction of quinazoline frameworks 20 using one-pot tandem cyclization of 2-bromobenzaldehyde derivatives 82 with amidine hydrochlorides 83 without using any ligands (Scheme 23C). Numerous quinazoline derivatives 20 could be synthesized in excellent isolated yields within a few minutes. Additionally, the cuprous oxide nanocatalytic system could be regenerated and recycled up to four times without any important loss of catalytic potency (Raut et al., 2017). Along the same line, Wang et al. developed an effective one-pot procedure for the region-selective formation of functionalized quinazoline analogous 86 by using diaryl-λ3-iodanes 84 and nitriles 85 in the presence of cupric acetate and potassium tert-butoxide in dimethyl sulfoxide at 120°C (Wang et al., 2013). This approach of electrophilic annulations permits the use of commercially available materials and facilitates great flexibility of the substitution patterns on unsymmetrical or symmetrical diaryl-λ3-iodanes 84 and nitriles 85, gave respective quinazolines 86 in good to excellent isolated yields (52–94%) (Scheme 23D). In another approach, Hua et al. disclosed cuprous chloride-catalyzed multicomponent one-pot formation of quinazoline analogous 50 by the reaction of o-bromo aromatic ketones 87 with aromatic aldehydes 62 or aromatic alcohols 46, and ammonia in H2O (Ju et al., 2012). The most important features of this synthetic tool include good isolated yields and air or DTBP (when primary alcohols are used) as oxidants (Scheme 23E). In a related development, Farhang and Baghbanian documented an effective and eco-friendly one-pot formation of quinazolines 56 via magnetically isolable and recyclable CuFe2O4 nanoparticle catalyzed tandem cyclization reaction among aryl aldehydes 62, 2-amino benzophenones 19, and ammonium acetate (Baghbanian and Farhang, 2014). Nanoparticles of CuFe2O4 was easily synthesized by the thermal decomposition of Fe(NO3)3 and Cu(NO3)2 in H2O in the presence of NaOH (Scheme 23F). The catalytic potency of CuFe2O4 nanoparticles was investigated in aqueous media, revealing that this system is applicable as a promising, reusable, and green catalyst in organic synthesis. Moreover, the main benefits of the technique are (i) chemoselectivity, (ii) an insignificant loss of activity by using recycled catalyst, and (iii) simplicity in the extraction of the substrate/product from the catalysts. In the same connection, Han et al. explored a facile and effective one-pot reaction of 2-aminobenzylamine derivatives 22 with arylaldehyde derivatives 62 for the synthesis of quinazoline skeletons 56 by using DABCO/CuCl/4-HO-TEMPO as the catalytic system and oxygen as the terminal oxidizing agent (Han et al., 2012). Various substituted heteroaryl or aryl aldehydes 62 were treated with a variety of functionalized 2-aminobenzylamines 22 and furnished the functionalized quinazolines 56 in moderate to excellent isolated yields (40–96%) (Scheme 23G).
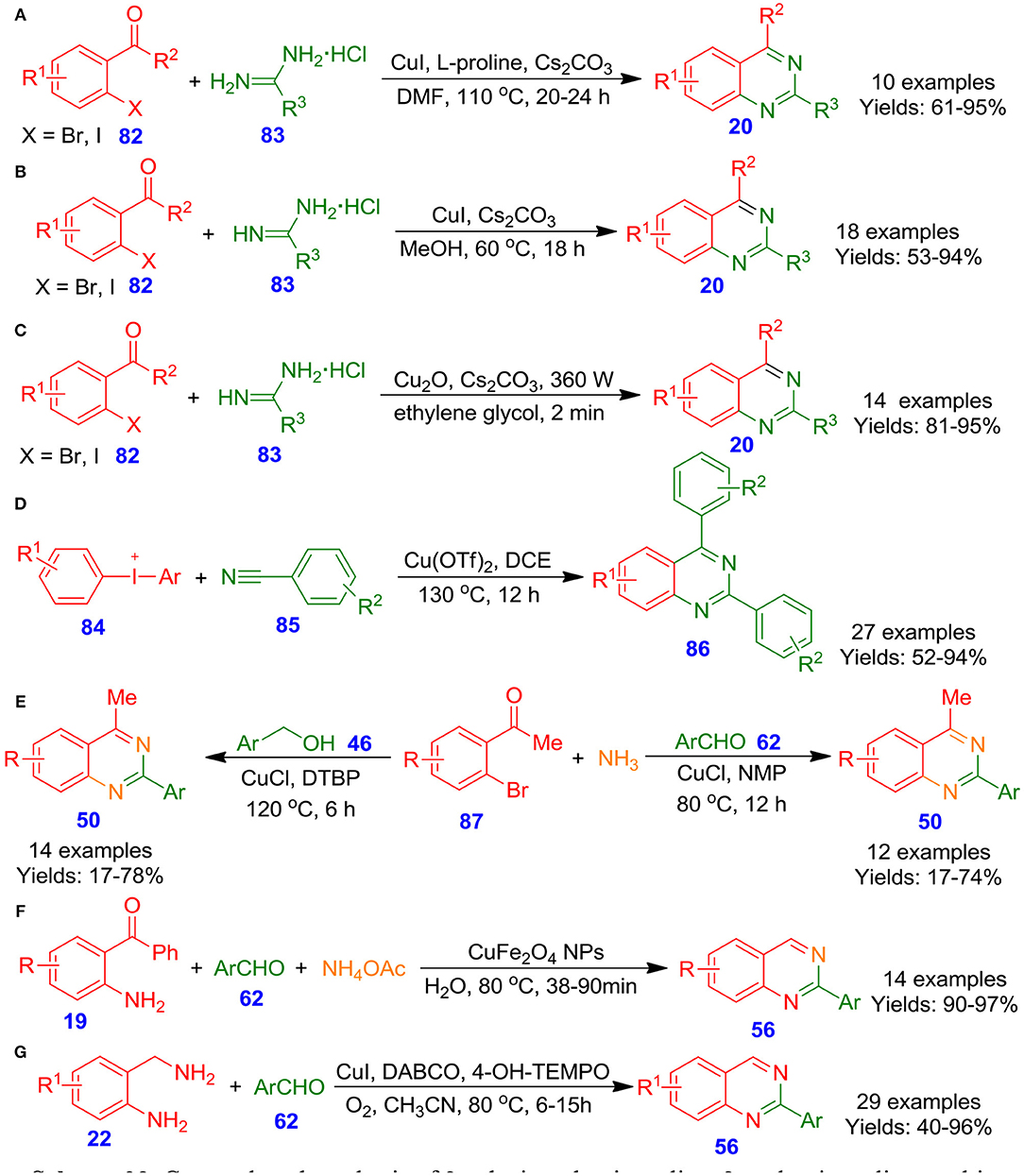
Scheme 23. Cu–catalyzed synthesis of 2-substituted quinazoline, 2-aryl quinazoline, multi-substituted quinoline, and quinazolinone derivatives.
Wu et al. reported the Cu-catalyzed one-pot tandem reaction among aldehydes 46, (2-aminophenyl)methanols 88 and NH4Cl in the presence of TEMPO and cerium trinitrate at 80°C for 24 h. The technique represents a practical and convenient strategy for the formation of 2-functionalized quinazolines 56 (Scheme 24A). The reaction mechanism involved functionalization of 2-aminobenzylalcohols to 2-aminobenzaldehydes by using CuCl/TEMPO/2,2′-bipyridine(bpy) catalytic system. Following, the reaction of 2-aminobenzaldehydes with aldehydes and ammonium chloride afforded cyclized entity dihydroquinazolines, which on aromatization result in the formation of quinazoline frameworks in moderate to excellent isolated yields (55–97%) (Chen et al., 2013). Likewise, Yang et al. documented an efficient and novel process for the production of pyrazolo[1,5-c]quinazoline derivatives 90 via two-step one-pot reactions of commercially available functionalized 1-(2-halophenyl)-3-akylprop-2-yn-1-one derivatives 89, amidine hydrochlorides 83, and hydrazine hydrochloride under mild conditions, and the respective pyrazolo[1,5-c]quinazoline derivatives 90 were achieved in good to excellent isolated yields (Scheme 24B). The unique process can offer useful and diverse N-fused heterocycles for medicinal chemistry and combinatorial chemistry (Yang et al., 2012). In an interesting study, Kiruthika and Perumal disclosed a copper-catalyzed one-pot, intermolecular procedure for the rapid construction of indolo[1,2-a]quinazoline derivatives 93 from the commercially available gem-dibromovinylanilide derivatives 91 and N-tosyl-o-bromobenzamide derivatives 92 by employing Cs2CO3 and 1,10-phen in refluxing THF, followed by refluxing under basic conditions (Kiruthika and Perumal, 2014). The protocol operated well with a diverse range of N-tosyl-obromobenzamide derivatives 92 and converted into respective quinazolines with good isolated yields (70–81%) (Scheme 24C). Moreover, this technique is practical, economical, and more reliable in terms of scalability, yield, and time. Keeping this in view, Gou et al. illustrated an effective technique for the development of pyrazolo[1,5-c]quinazolines 95 and 5,6-dihydropyrazolo[1,5-c]quinazoline derivatives 97 via one-pot Cu–catalyzed tandem reaction of 5-(2-bromoaryl)-1H-pyrazole derivatives 94 with ketones 96 or aldehydes 68 in ammonium hydroxide under aerobic conditions (Guo et al., 2013). A diverse spectrum of ketones and aldehydes, including hetero-aryl, alkenyl, alkyl, and aryl underwent efficiently in this reaction conditions and furnished corresponding functionalized quinazolines in moderate to good isolated yields (32–79%) (Scheme 24D). This synthetic process has the benefits of inexpensive starting materials and reagents, simple operation process, and broad scope of substrates. In another report by Fan et al., copper-catalyzed two-step one-pot sequential reactions of 2-(2-bromoaryl)-1H indole derivatives 98 with substituted aldehydes 68, and ammonium hydroxide for the selective preparation of indolo[1,2-c]quinazolines 99 and 11H-indolo[3,2-c]quinolones 100 has been described (Guo S. et al., 2015). The regioselectivity of synthesis was maintained by regulating the reaction conditions. When the reaction was performed under acidic conditions, carbon–carbon coupling was observed, leading to the formation of 11H-indolo[3,2-c]quinolones in good isolated yields (32–81%). Having said that, in the absence of acid, formation of indolo[1,2-c]quinazolines was observed in moderate to excellent isolated yields (30–89%) (Scheme 24E). The current procedure features simple operation procedures and easily controlled selectivity. Very recently, Fan et al. described Cu-catalyzed aerobic oxygenation of 2-(2-amidoaryl)-1Hindoles 101, followed by intramolecular cyclization reaction under acidic conditions, resulting in the construction of quinazolines 102 in moderate to good isolated yields (40–72%) (Scheme 24F) (Guo et al., 2018).
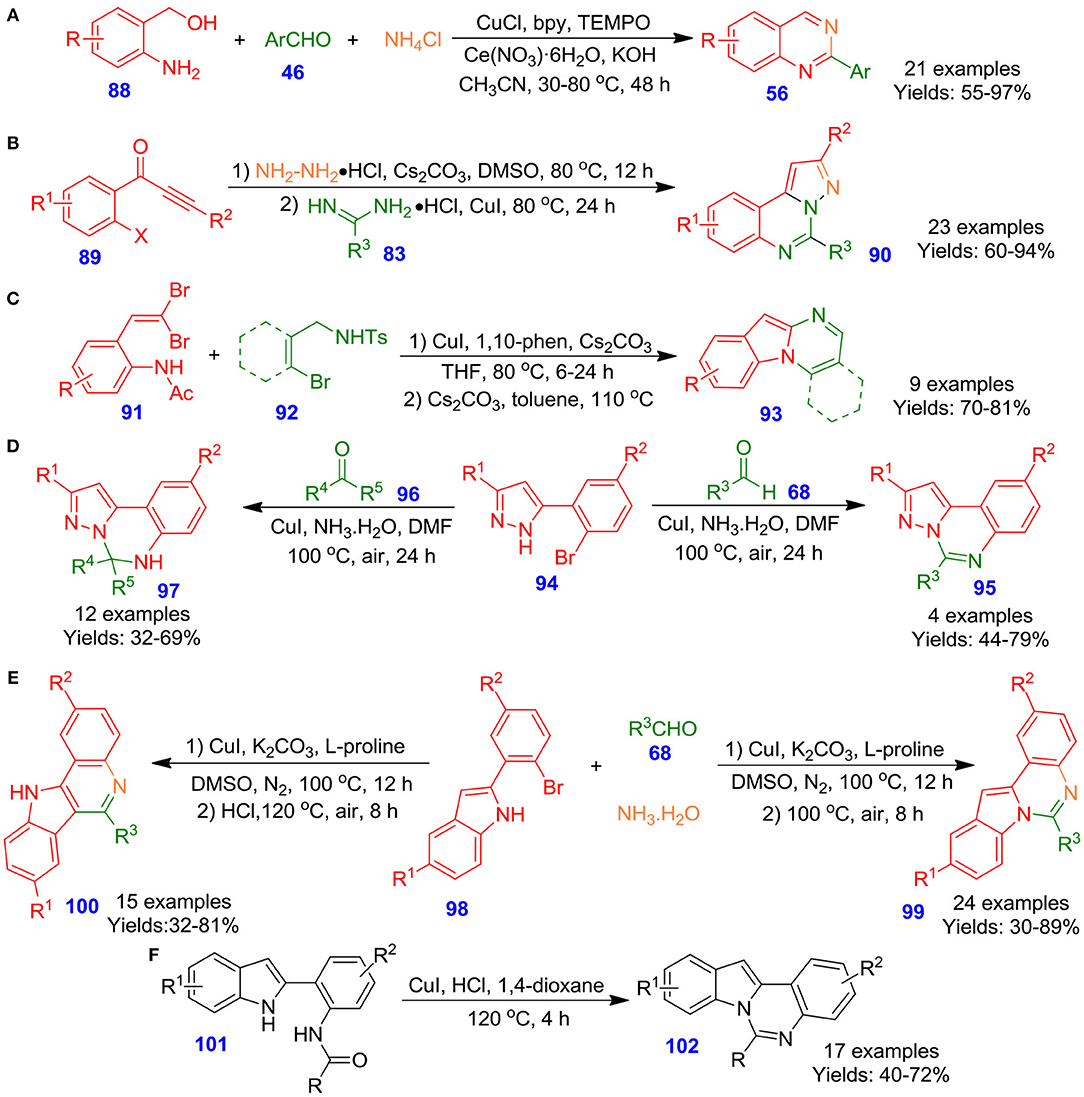
Scheme 24. Cu–catalyzed synthesis of 2-aryl quinazoline, pyrazolo[1,5-c]quinazoline, and indolo[1,2-a]quinazolines, 2-(2-bromoaryl)-1H-indole derivatives.
Ruthenium-Mediated Catalytic System
Chen et al. explored straightforward Ru-catalyzed dehydrogenative synthetic protocol to afford 2-arylquinazoline derivatives 23 from the reaction of 2-aminoaryl methanol derivatives 88 with benzonitrile derivatives 103 in the presence of triruthenium dodecacarbonyl, potassium tert-butoxide, and Xantphos (Chen et al., 2015). A library of 2-aminoaryl methano derivatives 88 was successfully transformed in combination with various kinds of benzonitrile derivatives 103 into numerous desired quinazolines 23 in moderate to good isolated yields (18–76%) (Scheme 25). In this process, there is no need for the utilization of less eco-friendly halogenated substrates, providing a significant basis for constructing 2-arylquinazolines.
Zinc-Based Catalytic System
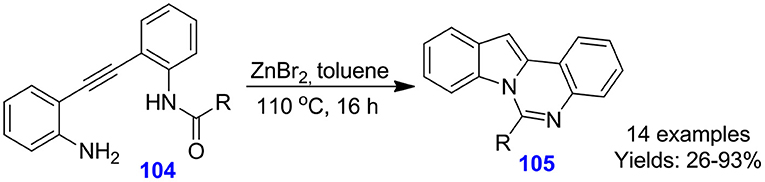
Wang et al. reported the zinc bromide (ZnBr2)-catalyzed domino hydro-amination cyclization approach for the development of indolo[1,2-c]quinazoline frameworks 105 from acyclic alkyne reactants 104 (Xu et al., 2013). The synthesis proceeds through ZnBr2-assisted tandem sequence, involving 5-endo-dig hydro-amination and intramolecular cyclization between an amide group with the indole nitrogen and gave indolo[1,2-c]quinazolines 105 in moderate to excellent isolated yields (26–93%) (Scheme 26). The method features mild condition as well as non-indole substrates, which are suitable for the forming of a panel of indolo[1,2-c]quinazolines.
Rhodium-Mediated Catalytic Systems
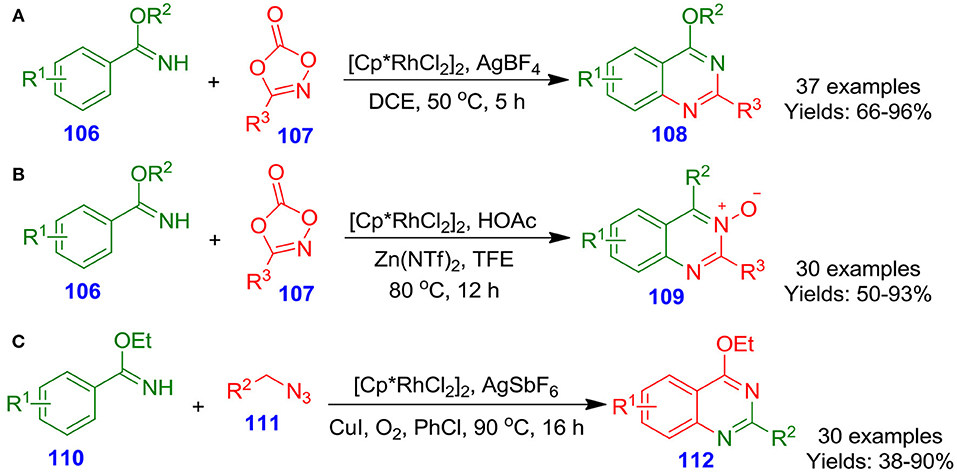
Zhu et al. described the [Cp*RhCl2]2/AgBF4-catalyzed double carbon–nitrogen bond formation sequence for the construction of highly functionalized quinazolines 108 from reaction of benzimidate derivatives 106 with dioxazolone derivatives 107 (Wang J. et al., 2016). In this reaction, dioxazolone derivatives 107 also functioned as an internal oxidizing agent to regulate the catalytic cycle. A library of benzimidate derivatives 106 were transformed into corresponding quinazolines 108 with good to excellent isolated yields (66–96%) (Scheme 27A). The synthetic process proceeded with the benefits of operational simplicity, and high atom efficiency, and offered a significant basis for access to quinazoline derivatives. In the same year, Li et al. reported an effective synthetic procedure to access quinazoline N-oxides oxides 109 from ketoxime derivatives 106 and dioxazolon derivatives 107 through Zn(II)/Rh(III)-catalyzed carbon–hydrogen activation–amidation of the ketoxime derivatives (Wang Q. et al., 2016). This annulation tool proceeded effectively in the absence of any oxidant and delivered target quinazoline N-oxides oxides with good to excellent isolated yields (50–93%) (Scheme 27B). Furthermore, the reaction proceeded in high efficiency under mild conditions with water and carbon dioxide as the byproducts. Interestingly, Wang and Jiao documented an efficient and novel copper- and rhodium-co-catalyzed [4 + 2] carbon–hydrogen bond activation and annulation for the formation of biologically active quinazolines 112 from reaction of imidate derivatives 110 with alkyl azide derivatives 111 (Wang and Jiao, 2016). This aerobic oxidative procedure offers a valuable utilization of simple alkyl azide derivatives 111 in N-heterocycle synthesis with nitrogen and water as co-products (Scheme 27C). High atom efficiency and good functional group tolerance make this procedure suitable in accessing numerous functionalized quinazolines (Patel and Patel, 2019).
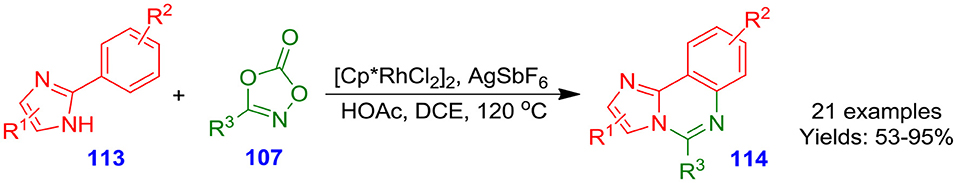
In the recent years, Wu et al. described a rhodium-catalyzed direct and unique methodology for forming a library of 5-arylimidazo[1,2-c]quinazoline derivatives 114 in moderate to excellent isolated yields from annulation of ketones 107 and 2-arylimidazoles 113 (Scheme 28). This process is characterized by (i) free of halo functionalization handles; and (ii) commercially available starting material (Wu et al., 2018).
Cobalt-Based Catalytic Systems
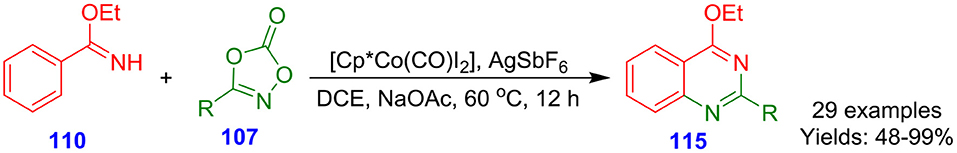
Wang et al. developed cyclopentadienylcobalt dicarbonyl-catalyzed [4 + 2] cycloaddition of rarely explored dioxazolones 107 with imines 110 for the formation of multi-functionlized quinazolines 115 (Wang X. et al., 2016). The reaction involved cobalt-mediated tandem direct carbon–hydrogen amidation followed by intramolecular cyclization to deliver quinazolines with moderate to excellent isolated yields (48–99%) (Scheme 29). Cobalt-based catalytic system is exclusively suited to this conversion owing to its high sensitivity to steric hindrance and strong Lewis acidity.
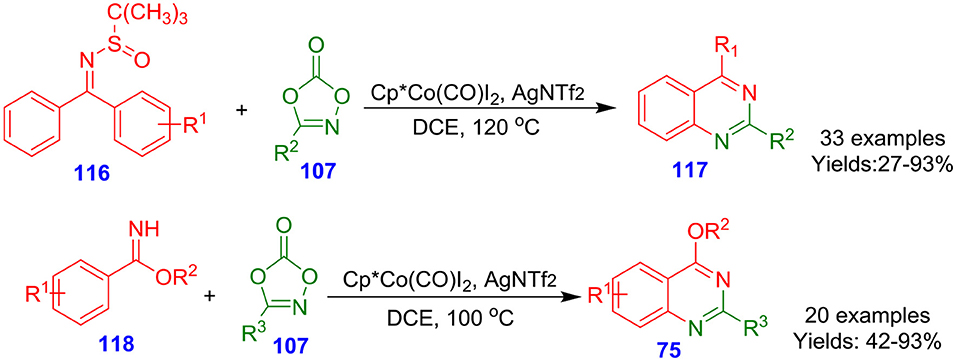
Wang et al. documented cobalt-catalyzed direct functionalization of N-sulfinylimines 116 and benzimidates 118 with dioxazolones 107 for the rapid formation of quinazolines (117 and 75) (Scheme 30). Numerous dioxazolones 107, benzimidates 118, and N-sulfinylimines 116 actively contributed under the optimized reaction condition and afforded quinazolines in lower to excellent isolated yields (27–97%) (Wang F. et al., 2016). The synthesis of quinazolines proceeded with high mono-/di- and regioselectivity. In these synthetic tool, the dioxazolone coupling partners serve as a synthon of (the oxidized form of) nitriles.
In an interesting report, Ahmadi and Bazgir described a cobalt-assisted isonitrile insertion cyclization reaction for the formation of fused quinazoline frameworks 120. To be more precise, treatment of isocyanides 43 with benzo[d]imidazol-anilines 119 in the presence of cobalt catalyst, sodium acetate, and potassium persulfate afforded quinazolines 120 (Ahmadi and Bazgir, 2016). The simple technique is highly useful, and it offers a straightforward methodology to a library of benzoimidazo-quinazoline amines (Scheme 31).
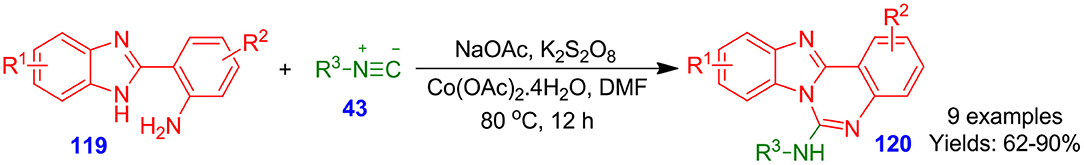
Scheme 31. Co-catalyzed isocyanide insertion-cyclization for constructing benzoimidazoquinazoline frameworks.
Nickel-Mediated Catalytic Systems
Sharada et al. described a ligand-base-free nickel-catalyzed one-pot sequential tandem approach for oxidative insertion of isonitrile under aerobic condition with intramolecular bisamine nucleophiles (Scheme 32). The tandem method involved an ring opening of isatoic anhydrides 121 followed by annulation to benzimidazoles 123 and subsequent nickel(II) bis(acetylacetonate)-catalyzed intramolecular insertion of isocyanide 43 result in fused quinazoline derivatives 124 with moderate to excellent isolated yields (30–75%) (Shinde et al., 2017). The base-/ligand-free features and application of dioxygen as the sole oxidant make this approach novel. The salient characteristics of this technique are the employment of inexpensive and commercially available starting materials, high bond-forming index (BFI), short reaction time, and the construction of four new carbon–nitrogen bonds in one pot fashion. Fluorescence investigation suggested that the synthesized quinazolines exhibit potent fluorescence properties with high quantum yield. These quinazolines have been proposed to be employed as a high fluorescent probe (Patel and Patel, 2019).
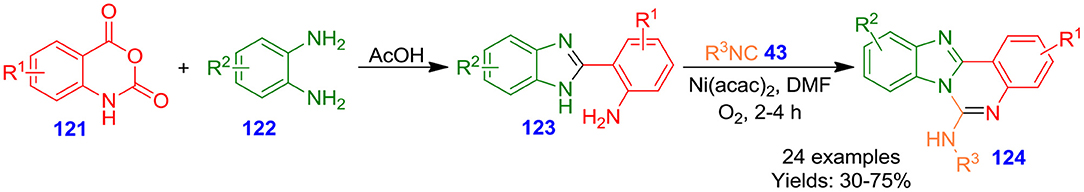
Scheme 32. Ni-catalyzed sequential double annulation cascade (SDAC) approach to access fused quinazolines.
Parua et al. developed nickel [Ni(MeTAA)]-catalyzed approach for the formation of quinazoline derivatives 126 from acceptor-less dehydrogenative coupling of 2-aminobenzylamines 22 with benzyl alcohols 125 and 2-aminobenzylalcohols 88 with benzonitriles 127 in the presence of potassium tert-butoxide in xylene at 100°C for 24 h (Parua et al., 2018). The environmentally benign methodology, easy to prepare nickel catalyst, and broad substrate scope made this methodology beneficial (Scheme 33).
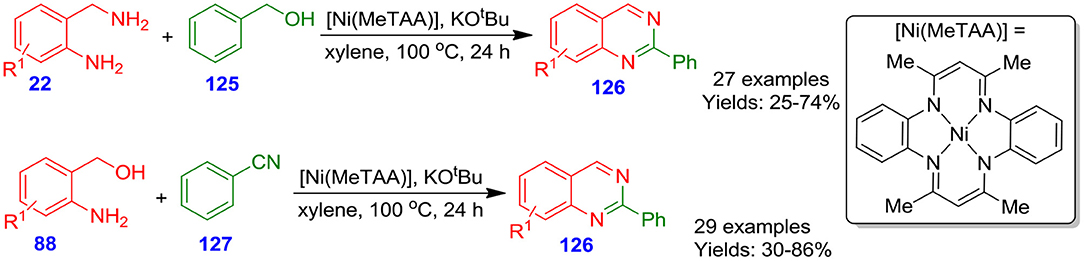
Scheme 33. Ni-catalyzed acceptorless dehydrogenative coupling for accessing poly-substituted quinazolines.
Gold-Based Catalytic Systems
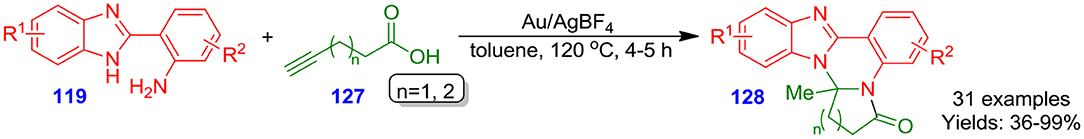
Liu et al. described facile and efficient Ag(I)/Au(I)-catalyzed cascade technique for one-pot formation of benzo[4,5]imidazo[1,2]pyrrolo[1,2]quinazolinones 128 through the reaction of the functionalized 2-(1H-benzo[d]imidazol-2-yl)aniline derivatives 119 with 5-hexynoic acid or 4-pentynoic acid 127 (Ji et al., 2013). Furthermore, in the described procedure, substituent functionality in aniline derivatives 119 was generally well tolerated and gave respective quinazolinone derivatives 128 in moderate to excellent isolated yields (36–99%) (Scheme 34). The approach involved three new carbon–nitrogen bond formation in one-pot fashion.
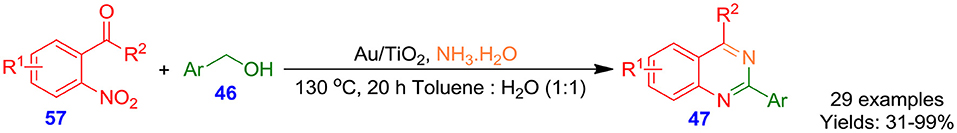
Alternatively, Wang et al. demonstrated hydrogen-transfer strategy for the preparation of 2,4-difunctionalized quinazolines 47 through a highly effective and selective nitrogen source-assisted reaction of aromatic alcohols 46 with o-nitroacetophenones 57 in the presence of Au/TiO2 as a catalytic system (Tang L. et al., 2015). The synthetic protocol is a wide substrate scope, has good tolerance to water and air, and signifies a novel avenue for economical and practical multiple carbon–nitrogen bond formation (Scheme 35). More significantly, no additional reductant, oxidant, and additive are demanded in the synthesis, and the catalytic system can be regenerated and recycled readily.
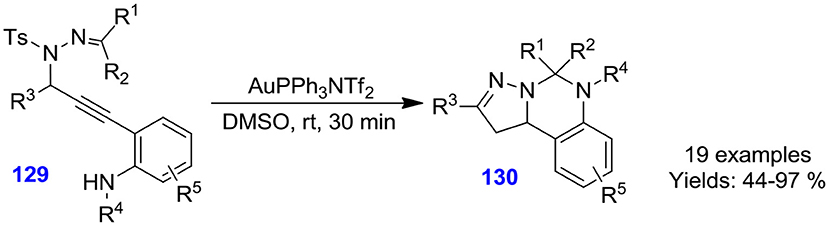
Tang et al. described gold-catalyzed chemo-selective cyclization of N-propargylic sulfonyl hydrazones 129 in dimethyl sulfoxide at ambient temperature for the development of 5,6-dihydropyrazolo[1,5-c]quinazoline derivatives 130 (Scheme 36). Numerous N-propargylic sulfonyl hydrazones actively converted under the optimized reaction condition to furnish quinazolines in good to excellent isolated yields (44–97%) (Tang H.-T. et al., 2015).
Iron-Based Catalytic Systems
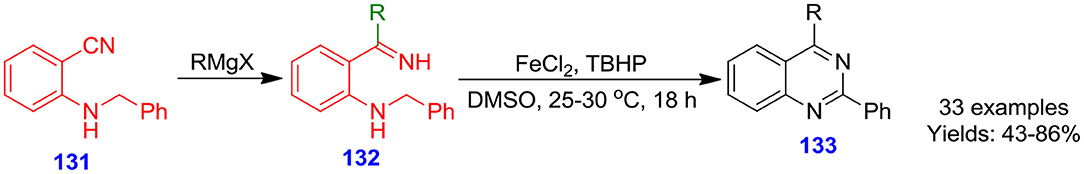
Chen et al. reported ferrous chloride-catalyzed carbon–hydrogen oxidation and intramolecular carbon–nitrogen bond formation for the construction of quinazolines 133 using tert-butyl hydroperoxide as terminal oxidant. 2-Alkylamino N-H ketamines 132 were prepared via reaction of commercially available 2-alkylaminobenzonitrile 131 with Grignard reagent (Chen et al., 2018). The process delivered a broad variety of 2,4-difunctionalized quinazoline derivatives in good to excellent isolated yields (43–86%) (Scheme 37). The oxidation of the N-alkyl moiety in this procedure employs cheap and non-toxic iron salts (FeCl2) in the absence of any privileged ligands.
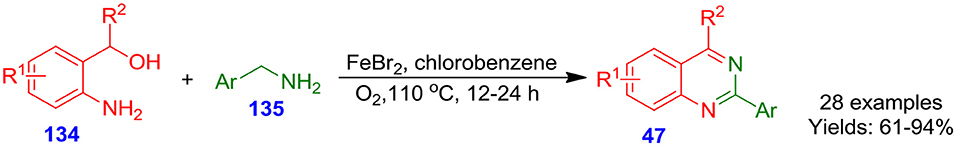
Ferrous bromide-catalyzed one-pot cascade approach for the preparation of quinazoline analogs 47 have been disclosed by Gopalaiah et al. from 2-hydroxymethylanilines 134 with aromatic amines 135 under an aerobic oxidative condition in benzene chloride at 110°C for 12–24 h (Gopalaiah et al., 2017). In a one-pot manner, the reaction proceeds through the construction of N-benzylidenebenzylamine intermediate and subsequent oxidative trapping of ammonia/intramolecular cyclization (Scheme 38). Both heteroaromatic/aromatic amines 135 treated smoothly in this approach and delivered corresponding quinazoline analogs 47 in good to excellent isolated yields (61–94%). This technique shows a wide substrate scope and is applicable to gram-scale synthesis. Moreover, the employment of molecular oxygen as an oxidant and an inexpensive and abundant iron salt as a catalyst makes this conversion very sustainable and practical (Patel and Patel, 2019).
Jeong and Shinde described green and effective method for the construction of functionalized quinazoline derivatives (138 and 140) from indazol-3-amine derivatives 136, aldehyde derivatives 68 and 137 or 139 catalyzed by ferric fluoride (FeF3) under ultrasonication in the absence of any solvent (Shinde and Jeong, 2016). The application of ultrasonication permits to accelerate the formation of a product from hours to only a few minutes, and ferric fluoride demonstrates excellent catalytic potency (Scheme 39). This effective green procedure offers amazing benefits such as easy work-up process, low cost, good to excellent yields, and eliminates the application of chromatographic purification. Also, the catalytic system can be easily regenerated and recycled for at least four runs without any important influence on the productivity of the quinazolines (Patel and Patel, 2019).
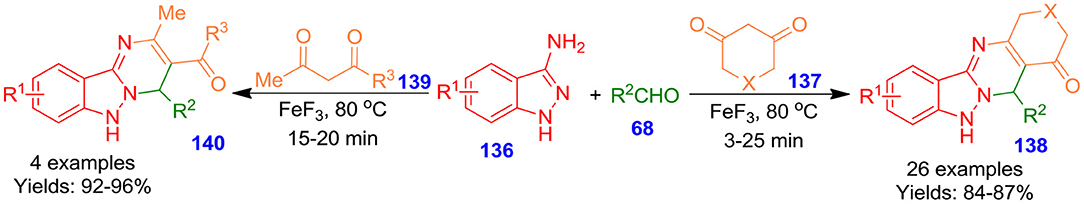
Scheme 39. Sonochemical ferric fluoride-based synthesis of highly functionalized quinazolines under solvent-less conditions.
Conclusion and Perspectives
In the review article, a broad spectrum of simple, mild, effective, novel synthetic routes to afford various functionalized quinazolines through cheap and commercially available starting materials have been reviewed. Clearly, a lot of work has been done for the construction of quinazoline frameworks in the recent past. Magnetic ionic liquid synthesis, nickel- and palladium-catalyzed synthesis, base-driven synthesis in water and microwave-promoted synthesis are highly advanced, novel approaches, with numerous positive aspects like mild reaction conditions, time efficient, recyclable catalysts, and use harmless solvents. Most importantly, several strategies including Lewis acid-catalyzed synthesis, cobalt zeolite imidazolate framework-catalyzed synthesis, CAN-catalyzed synthesis, base-driven synthesis in water, iron-catalyzed synthesis, and zinc-catalyzed synthesis have been successfully utilized to attain diversely decorated frameworks of quinazoline, which are important in agrochemical and pharmaceutical industries. The regularly improved synthetic approaches better the synthetic research on quinazolines with a tendency of faster, more convenient, and more diverse. Furthermore, because of the simplicity of synthetic methods enabling the construction of core scaffolds of many marketed drugs, we hope to see further research in the design of novel functionalized quinazoline derivatives, with exploitation of their biological activities in diverse ways.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Ahmadi, F., and Bazgir, A. (2016). ChemInform abstract: synthesis of benzoimidazoquinazolines by cobalt-catalyzed isocyanide insertion-cyclization. ChemInform. 47. doi: 10.1002/chin.201646182
Akazome, M., Yamamoto, J., Kondo, T., and Watanabe, Y. (1995). Palladium complex-catalyzed intermolecular reductive N-heterocyclization: novel synthesis of quinazoline derivatives from 2-nitrobenzaldehyde or 2-nitrophenyl ketones with formamide. J. Organomet. Chem. 494, 229–233. doi: 10.1016/0022-328X(95)05387-5
Alagarsamy, V., Chitra, K., Saravanan, G., Solomon, V. R., Sulthana, M. T., and Narendhar, B. (2018). An overview of quinazolines: Pharmacological significance and recent developments. Eur. J. Med. Chem. 151, 628–685. doi: 10.1016/j.ejmech.2018.03.076
Allen, S. E., Walvoord, R. R., Padilla-Salinas, R., and Kozlowski, M. C. (2013). Aerobic copper-catalyzed organic reactions. Chem. Rev. 113, 6234–6458. doi: 10.1021/cr300527g
Asif, M. (2014). Chemical characteristics, synthetic methods, and biological potential of quinazoline and quinazolinone derivatives. Int. J. Med. Chem. 12:111. doi: 10.1155/2014/395637
Baghbanian, S. M., and Farhang, M. (2014). CuFe2O4 nanoparticles: a magnetically recoverable and reusable catalyst for the synthesis of quinoline and quinazoline derivatives in aqueous media. RSC Adv. 4, 11624–11633. doi: 10.1039/c3ra46119j
Battula, S., Vishwakarma, R. A., and Ahmed, Q. N. (2014). Cu–benzotriazole-catalyzed electrophilic cyclization of N-arylimines: a methodical tandem approach to O-protected-4hydroxyquinazolines. RSC Adv. 4, 38375–38378. doi: 10.1039/C4RA07377K
Bedi, P. M. S., Kumar, V., and Mahajan, M. P. (2004). Synthesis and biological activity of novel antibacterial quinazolines. Bioorganic Med. Chem. Lett. 14:5211–5213. doi: 10.1016/j.bmcl.2004.07.065
Bhat, S. I., Das, U. K., and Trivedi, D. R. (2015). An efficient three-component, one-pot synthesis of quinazolines under solvent-free and catalyst-free condition. J. Heterocycl. Chem. 52, 1253–1259. doi: 10.1002/jhet.2220
Bhattacharjee, A. K., Hartell, M. G., Nichols, D. A., Hicks, R. P., Stanton, B., Van Hamont, J. E., et al. (2004). Structure-activity relationship study of antimalarial indolo [2, 1-b] quinazoline-6, 12-diones (tryptanthrins). Three dimensional pharmacophore modeling and identification of new antimalarial candidates. Eur. J. Med. Chem. 39, 59–67. doi: 10.1016/j.ejmech.2003.10.004
Bouley, R., Ding, D., Peng, Z., Bastian, M., Lastochkin, E., Song, W., et al. (2016). Structure–activity relationship for the 4 (3 H)-Quinazolinone antibacterials. J. Med. Chem. 59, 5011–5021. doi: 10.1021/acs.jmedchem.6b00372
Chatterjee, T., Kim, D. I., and Cho, E. J. (2018). Base-promoted synthesis of 2-aryl quinazolines from 2-aminobenzylamines in water. J. Org. Chem. 83, 7423–7430. doi: 10.1021/acs.joc.8b00327
Chen, C., He, F., Tang, G., Yuan, H., Li, N., Wang, J., et al. (2018). Synthesis of quinazolines via an iron-catalyzed oxidative amination of N–H ketimines. J. Org. Chem. 83, 2395–2401. doi: 10.1021/acs.joc.7b02943
Chen, J., Natte, K., Neumann, H., and Wu, X. F. (2014). A convenient palladium-catalyzed carbonylative synthesis of quinazolines from 2-aminobenzylamine and aryl bromides†. RSC Adv. 4, 56502–56505. doi: 10.1039/C4RA11303A
Chen, M., Zhang, M., Xiong, B., Tan, Z., Lv, W., and Jiang, H. (2015). ChemInform abstract: a novel ruthenium-catalyzed dehydrogenative synthesis of 2-arylquinazolines from 2-aminoaryl methanols and benzonitriles. ChemInform. 46. doi: 10.1002/chin.201519224
Chen, Z., Chen, J., Liu, M., Ding, J., Gao, W., Huang, X., et al. (2013). Unexpected copper-catalyzed cascade synthesis of quinazoline derivatives. J. Org. Chem. 78, 11342–11348. doi: 10.1021/jo401908g
Cheng, X., Wang, H., Xiao, F., and Deng, G. J. (2016). Lewis acid-catalyzed 2-arylquinazoline formation from: N′-arylbenzimidamides and paraformaldehyde. Green Chem. 18, 5773–5776. doi: 10.1039/C6GC02319C
Derabli, C., Boulcina, R., Kirsch, G., Carboni, B., and Debache, A. (2014). A DMAP-catalyzed mild and efficient synthesis of 1,2-dihydroquinazolines via a one-pot three-component protocol. Tetrahedron Lett. 58, 200–204. doi: 10.1016/j.tetlet.2013.10.157
Deshmukh, D. S., and Bhanage, B. M. (2018). Molecular iodine catalysed benzylic sp 3 C-H bond amination for the synthesis of 2-arylquinazolines from 2-aminobenzaldehydes, 2-aminobenzophenones and 2-aminobenzyl alcohols. Synlett 29, 979–985. doi: 10.1055/s-0037-1609200
Deutsch, C., Krause, N., and Lipshutz, B. H. (2008). CuH-catalyzed reactions. Chem. Rev. 108, 2916–2927. doi: 10.1021/cr0684321
Devi, P., Srivastava, A., Srivastava, K., and Bishnoi, A. (2017). Green approaches towards the synthesis of substituted quinazolines. Curr. Green Chem. 4, 25–37. doi: 10.2174/2213346104666170704153434
Diederich, F., and Stang, P. J. (2008). Metal-Catalyzed Cross-Coupling Reactions, 2nd Edn. John Wiley and Sons.
El-Sayed, N. N. E., Almaneai, N. M., Soliman, S. M., Bacha, A., and Ben Ghabbour, H. A. (2019). Synthesis, Structural Investigation, Antiphospholipases, Antiproteases and Antimicrobial Activities of 2-(4-hydroxy-3-methoxy-benzylidene)-6-methoxy-3,4-dihydro-2H-naphthalen-1-one and 4-(4-hydroxy-3-methoxy-phenyl)-8-methoxy-3,4,5,6-tetrahydro-1H-benzo[h. J. Chem. Soc. Pak. 41, 1074–1089.
Eswaran, S., Adhikari, A. V., Chowdhury, I. H., Pal, N. K., and Thomas, K. D. (2010). New quinoline derivatives: synthesis and investigation of antibacterial and antituberculosis properties. Eur. J. Med. Chem. 45, 3374–3383. doi: 10.1016/j.ejmech.2010.04.022
Fan, X., Li, B., Guo, S., Wang, Y., and Zhang, X. (2014). Synthesis of quinazolines and tetrahydroquinazolines: copper-catalyzed tandem reactions of 2-bromobenzyl bromides with aldehydes and aqueous ammonia or amines. Chem. Asian J. 9, 739–743. doi: 10.1002/asia.201301296
Gao, L., Song, Y., Zhang, X., Guo, S., and Fan, X. (2014). Copper-catalyzed tandem reactions of 2-bromobenzaldehydes/ketones with aminopyrazoles toward the synthesis of pyrazolo[1,5-a]quinazolines. Tetrahedron Lett. 55, 4997–5002. doi: 10.1016/j.tetlet.2014.07.028
Gopalaiah, K., Saini, A., and Devi, A. (2017). Iron-catalyzed cascade reaction of 2-aminobenzyl alcohols with benzylamines: synthesis of quinazolines by trapping of ammonia. Org. Biomol. Chem. 15, 5781–5789. doi: 10.1039/C7OB01159H
Guo, S., Tao, L., Zhang, W., Zhang, X., and Fan, X. (2015). Regioselective synthesis of indolo [1, 2-c] quinazolines and 11 H-indolo [3, 2-c] quinolines via copper-catalyzed cascade reactions of 2-(2-bromoaryl)-1 H-indoles with aldehydes and aqueous ammonia. J. Org. Chem. 80, 10955–10964. doi: 10.1021/acs.joc.5b02076
Guo, S., Wang, F., Tao, L., Zhang, X., and Fan, X. (2018). Solvent-dependent copper-catalyzed indolyl C3-oxygenation and N1-cyclization reactions: selective synthesis of 3 H-indol-3-ones and indolo [1, 2-c] quinazolines. J. Org. Chem. 83, 3889–3896. doi: 10.1021/acs.joc.8b00231
Guo, S., Wang, J., Fan, X., Zhang, X., and Guo, D. (2013). Synthesis of pyrazolo [1, 5-c] quinazoline derivatives through copper-catalyzed tandem reaction of 5-(2-bromoaryl)-1 H-pyrazoles with carbonyl compounds and aqueous ammonia. J. Org. Chem. 78, 3262–3270. doi: 10.1021/jo4001756
Guo, X.-X., Gu, D.-W., Wu, Z., and Zhang, W. (2015). Copper-catalyzed C–H functionalization reactions: efficient synthesis of heterocycles. Chem. Rev. 115, 1622–1651. doi: 10.1021/cr500410y
Hameed, A., Al-Rashida, M., Uroos, M., Ali, S. A., Arshia Ishtiaq, M., and Mohammed, K. K. (2018). Quinazoline and quinazolinone as important medicinal scaffolds: a comparative patent review (2011–2016). Expert Opin. Ther. Pat. 28, 281–297. doi: 10.1080/13543776.2018.1432596
Han, B., Wang, C., Han, R. F., Yu, W., Duan, X. Y., Fang, R., et al. (2011). Efficient aerobic oxidative synthesis of 2-aryl quinazolines via benzyl C-H bond amination catalyzed by 4-hydroxy-TEMPO. Chem. Commun. 47, 7818–7820. doi: 10.1039/c1cc12308d
Han, B., Yang, X. L., Wang, C., Bai, Y. W., Pan, T. C., Chen, X., et al. (2012). CuCl/DABCO/4-HO-TEMPO-catalyzed aerobic oxidative synthesis of 2-substituted quinazolines and 4 H−3,1-benzoxazines. J. Org. Chem. 77, 1136–1142. doi: 10.1021/jo2020399
Honkanen, E., Pippuri, A., Kairisalo, P., Nore, P., Karppanen, H., and Paakkari, I. (1983). Synthesis and antihypertensive activity of some new quinazoline derivatives. J. Med. Chem. 26, 1433–1438. doi: 10.1021/jm00364a014
Hrast, M., RoŽman, K., Jukič, M., Patin, D., Gobec, S., and Sova, M. (2017). Synthesis and structure–activity relationship study of novel quinazolinone-based inhibitors of MurA. Bioorg. Med. Chem. Lett. 27, 3529–3533. doi: 10.1016/j.bmcl.2017.05.064
Hu, K., Zhen, Q., Gong, J., Cheng, T., Qi, L., Shao, Y., et al. (2018). Palladium-catalyzed three-component tandem process: one-pot assembly of quinazolines. Org. Lett. 20, 3083–3087. doi: 10.1021/acs.orglett.8b01070
Huang, C., Fu, Y., Fu, H., Jiang, Y., and Zhao, Y. (2008). Highly efficient copper-catalyzed cascade synthesis of quinazoline and quinazolinone derivatives. Chem. Commun. 2008, 6333–6335. doi: 10.1039/b814011a
Iino, T., Sasaki, Y., Bamba, M., Mitsuya, M., Ohno, A., Kamata, K., et al. (2009). Discovery and structure–activity relationships of a novel class of quinazoline glucokinase activators. Bioorg. Med. Chem. Lett. 19, 5531–5538. doi: 10.1016/j.bmcl.2009.08.064
Jafari, E., Khajouei, M. R., Hassanzadeh, F., Hakimelahi, G. H., and Khodarahmi, G. A. (2016). Quinazolinone and quinazoline derivatives: recent structures with potent antimicrobial and cytotoxic activities. Res. Pharm. Sci. 11, 1–14.
Ji, X., Zhou, Y., Wang, J., Zhao, L., Jiang, H., and Liu, H. (2013). Au (I)/Ag (I)-Catalyzed cascade approach for the synthesis of benzo [4, 5] imidazo [1, 2-c] pyrrolo [1, 2-a] quinazolinones. J. Org. Chem. 78, 4312–4318. doi: 10.1021/jo400228g
Ju, J., Hua, R., and Su, J. (2012). Copper-catalyzed three-component one-pot synthesis of quinazolines. Tetrahedron 68, 9364–9370. doi: 10.1016/j.tet.2012.09.035
Kamel, M., Zaghary, W., Al-Wabli, R., and Anwar, M. (2016). Synthetic approaches and potential bioactivity of different functionalized quinazoline and quinazolinone scaffolds. Egypt. Pharm. J. 15, 98–131. doi: 10.4103/1687-4315.197580
Karaman, M. W., Herrgard, S., Treiber, D. K., Gallant, P., Atteridge, C. E., Campbell, B. T., et al. (2008). A quantitative analysis of kinase inhibitor selectivity. Nat. Biotechnol. 26, 127–132. doi: 10.1038/nbt1358
Karnakar, K., Shankar, J., Murthy, S. N., Ramesh, K., and Nageswar, Y. V. D. (2011). An efficient protocol for the synthesis of 2-phenylquinazolines catalyzed by ceric ammonium nitrate (CAN). Synlett 2011, 1089–1096. doi: 10.1055/s-0030-1259960
Khan, I., Ibrar, A., Abbas, N., and Saeed, A. (2014). Recent advances in the structural library of functionalized quinazoline and quinazolinone scaffolds: synthetic approaches and multifarious applications. Eur. J. Med. Chem. 76, 193–244. doi: 10.1016/j.ejmech.2014.02.005
Khan, I., Ibrar, A., Ahmed, W., and Saeed, A. (2015). Synthetic approaches, functionalization and therapeutic potential of quinazoline and quinazolinone skeletons: the advances continue. Eur. J. Med. Chem. 90, 124–169. doi: 10.1016/j.ejmech.2014.10.084
Kiruthika, S. E., and Perumal, P. T. (2014). CuI-catalyzed coupling of gem-dibromovinylanilides and sulfonamides: an efficient method for the synthesis of 2-Amidoindoles and indolo [1, 2-a] quinazolines. Org. Lett. 16, 484–487. doi: 10.1021/ol403365t
Kshirsagar, U. A. (2015). Recent developments in the chemistry of quinazolinone alkaloids. Org. Biomol. Chem. 13, 9336–9352. doi: 10.1039/C5OB01379H
Kumar, S., Bawa, S., and Gupta, H. (2009). Biological activities of quinoline derivatives. Mini Rev. Med. Chem. 9, 1648–1654. doi: 10.2174/138955709791012247
Kunes, J., Bazant, J., Pour, M., Waisser, K., Slosárek, M., and Janota, J. (2000). Quinazoline derivatives with antitubercular activity. Farmaco 55, 725–729. doi: 10.1016/S0014-827X(00)00100-2
Kung, P.-P., Casper, M. D., Cook, K. L., Wilson-Lingardo, L., Risen, L. M., Vickers, T. A., et al. (1999). Structure– activity relationships of novel 2-substituted quinazoline antibacterial agents. J. Med. Chem. 42, 4705–4713. doi: 10.1021/jm9903500
Lin, J. P., Zhang, F. H., and Long, Y. Q. (2014). Solvent/oxidant-switchable synthesis of multisubstituted quinazolines and benzimidazoles via metal-free selective oxidative annulation of arylamidines. Org. Lett. 16, 2822–2825. doi: 10.1021/ol500864r
Liu, L. Y., Yan, Y. Z., Bao, Y. J., and Wang, Z. Y. (2015). Efficient synthesis of 2-arylquinazolines via copper-catalyzed dual oxidative benzylic C-H aminations of methylarenes. Chinese Chem. Lett. 26, 1216–1220. doi: 10.1016/j.cclet.2015.07.008
Lv, Z., Wang, B., Hu, Z., Zhou, Y., Yu, W., and Chang, J. (2016). Synthesis of Quinazolines from N,N′-Disubstituted Amidines via I2/KI-Mediated Oxidative C-C Bond Formation. J. Org. Chem. 81, 9924–9930. doi: 10.1021/acs.joc.6b02100
Mahato, A. K., Srivastava, B., and Nithya, S. (2011). Chemistry, structure activity relationship and biological activity of quinazoline-4 (3H)-one derivatives. Inven. Rapid Med. Chem. 2, 400–402.
McKee, M., McKee, R. L., and Bost, R. W. (1947). 7-Chloro-4-(1-diethylamino-4-pentylamino)-quinazoline. J. Am. Chem. Soc. 69, 184. doi: 10.1021/ja01193a502
Meijere, A., and Diederich, F. (2004). Metal-Catalyzed Cross-Coupling Reactions. Wiley-VCH. doi: 10.1002/9783527619535
Mishra, S. (2020). “Quinazolinone and quinazoline derivatives: synthesis and biological application,” in Quinazolinone and Quinazoline Derivatives. doi: 10.5772/intechopen.89203
Mizuno, M., Iwakura, Y., Shibuya, M., Zheng, Y., Eda, T., Kato, T., et al. (2010). Antipsychotic potential of quinazoline ErbB1 inhibitors in a schizophrenia model established with neonatal hippocampal lesioning. J. Pharmacol. Sci. 114, 320–331. doi: 10.1254/jphs.10099FP
Pandya, A. N., Villa, E. M., and North, E. J. (2017). A simple and efficient approach for the synthesis of 2-aminated quinazoline derivatives via metal free oxidative annulation. Tetrahedron Lett. 58, 1276–1279. doi: 10.1016/j.tetlet.2017.02.033
Panja, S. K., Dwivedi, N., and Saha, S. (2012). I2-Catalyzed three-component protocol for the synthesis of quinazolines. Tetrahedron Lett. 53, 6167–6172. doi: 10.1016/j.tetlet.2012.08.016
Panja, S. K., and Saha, S. (2013). Recyclable, magnetic ionic liquid bmim[FeCl4]-catalyzed, multicomponent, solvent-free, green synthesis of quinazolines. RSC Adv. 3, 14495–14500. doi: 10.1039/c3ra42039f
Parua, S., Sikari, R., Sinha, S., Chakraborty, G., Mondal, R., and Paul, N. D. (2018). Accessing polysubstituted quinazolines via nickel catalyzed acceptorless dehydrogenative coupling. J. Org. Chem. 83, 11154–11166. doi: 10.1021/acs.joc.8b01479
Patel, D. M., and Patel, H. M. (2019). Trimethylglycine-betaine-based-catalyst-promoted novel and ecocompatible pseudo-four-component reaction for regioselective synthesis of functionalized 6, 8-dihydro-1′ H, 5 H-spiro [[1, 3] dioxolo [4, 5-g] quinoline-7, 5′-pyrimidine]-2′, 4′, 6′(3′ H)-trione derivatives. ACS Sustain. Chem. Eng. 7, 18667–18676. doi: 10.1021/acssuschemeng.9b05184
Patel, T. S., Vanparia, S. F., Patel, U. H., Dixit, R. B., Chudasama, C. J., Patel, B. D., et al. (2017). Novel 2,3-disubstituted quinazoline-4(3H)-one molecules derived from amino acid linked sulphonamide as a potent malarial antifolates for DHFR inhibition. Eur. J. Med. Chem. 129, 251–265. doi: 10.1016/j.ejmech.2017.02.012
Rachakonda, S., Pratap, P. S., and Rao, M. V. B. (2012). DDQ/TBHP-induced oxidative cyclization process: a metal-free approach for the synthesis of 2-phenylquinazolines. Synthesis 44, 2065–2069. doi: 10.1055/s-0031-1289768
Rajput, R., and Mishra, A. P. (2012). A review on biological activity of quinazolinones. Int. J. Pharm. Pharm. Sci. 4, 66–70.
Ranawat, M. S., Amrutkar, S. V., and Phargharmol, P. (2011). Synthesis and pharmacological evaluation of 3-alkyl/aryl−2-methylquinazolin-4-one derivatives. Int. J. Drug Des. Discov. 2, 453–457.
Raut, A. B., Tiwari, A. R., and Bhanage, B. M. (2017). Ultrasound-assisted preparation of copper(I) oxide nanocubes: high catalytic activity in the synthesis of quinazolines. ChemCatChem. 9, 1292–1297. doi: 10.1002/cctc.201601330
Sambiagio, C., Marsden, S. P., Blacker, A. J., and McGowan, P. C. (2014). Copper catalysed Ullmann type chemistry: from mechanistic aspects to modern development. Chem. Soc. Rev. 43, 3525–3550. doi: 10.1039/C3CS60289C
Sarma, R., and Prajapati, D. (2011). Microwave-promoted efficient synthesis of dihydroquinazolines. Green Chem. 13, 718–722. doi: 10.1039/c0gc00838a
Shen, Z. C., Yang, P., and Tang, Y. (2016). Transition metal-free visible light-driven photoredox oxidative annulation of arylamidines. J. Org. Chem. 81, 309–317 doi: 10.1021/acs.joc.5b02366
Shinde, A. H., Arepally, S., Baravkar, M. D., and Sharada, D. S. (2017). Nickel-catalyzed aerobic oxidative isocyanide insertion: access to benzimidazoquinazoline derivatives via a sequential double annulation cascade (SDAC) strategy. J. Org. Chem. 82, 331–342. doi: 10.1021/acs.joc.6b02423
Shinde, V. V., and Jeong, Y. T. (2016). Sonochemical FeF3 catalyzed three-component synthesis of densely functionalized tetrahydroindazolo [3, 2-b] quinazoline under solvent-free conditions. Tetrahedron Lett. 57, 3795–3799. doi: 10.1016/j.tetlet.2016.07.031
Srivastava, S., and Srivastava, S. (2015). Biological activity of Quinazoline: a review. Int. J. Pharma Sci. Res. 6, 1206–1213.
Tang, H.-T., Xiong, K., Li, R.-H., Ding, Z.-C., and Zhan, Z.-P. (2015). Synthesis of 5, 6-dihydropyrazolo [1, 5-c] quinazolines through gold-catalyzed chemoselective bicyclization of N-propargylic sulfonylhydrazones. Org. Lett. 17, 326–329. doi: 10.1021/ol503437n
Tang, L., Yang, Y., Wen, L., Zhang, S., Zha, Z., and Wang, Z. (2015). Supported gold-catalyzed and ammonia-promoted selective synthesis of quinazolines in aqueous media. Org. Chem. Front. 2, 114–118. doi: 10.1039/C4QO00278D
Truong, T., Hoang, T. M., Nguyen, C. K., Huynh, Q. T. N., and Phan, N. T. S. (2015). Expanding applications of zeolite imidazolate frameworks in catalysis: synthesis of quinazolines using ZIF-67 as an efficient heterogeneous catalyst. RSC Adv. 5, 24769–24776. doi: 10.1039/C4RA16168H
Truong, V. L., and Morrow, M. (2010). Mild and efficient ligand-free copper-catalyzed condensation for the synthesis of quinazolines. Tetrahedron Lett. 51, 758–760. doi: 10.1016/j.tetlet.2009.11.133
Uckun, F. M., Sudbeck, E. A., Cetkovic, M., Malaviya, R., and Liu, X.-P. (2002). Dimethoxy Quinazolines for Treating Diabetes. Available online at: https://www.freepatentsonline.com/6495556.html.
Vijaychand, A., Manjula, S. N., Bharath, E. N., and Divya, B. (2011). Medicinal and biological significance of quinazoline: a highly important scaffold for drug discovery: a review. Int. J. Pharma Bio Sci. 2, 780–809.
Visweswara Sastry, K. N., Prasad, B., Nagaraju, B., Ganga Reddy, V., Alarifi, A., Babu, B. N., et al. (2017). Copper-catalysed tandem synthesis of substituted quinazolines from phenacyl azides and O-carbonyl anilines. ChemistrySelect 2, 5378–5383. doi: 10.1002/slct.201700889
Vlaar, T., Bensch, L., Kraakman, J., Vande Velde, C. M. L., Maes, B. U. W., Orru, R. V. A., et al. (2014). Synthesis of diverse azolo [c] quinazolines by palladium (II)-catalyzed aerobic oxidative insertion of isocyanides. Adv. Synth. Catal. 356, 1205–1209. doi: 10.1002/adsc.201301129
Wang, D., and Gao, F. (2013). Quinazoline derivatives: Synthesis and bioactivities. Chem. Cent. J. 7:95. doi: 10.1186/1752-153X-7-95
Wang, F., Wang, H., Wang, Q., Yu, S., and Li, X. (2016). Co (III)-catalyzed synthesis of quinazolines via C–H activation of N-sulfinylimines and benzimidates. Org. Lett. 18, 1306–1309. doi: 10.1021/acs.orglett.6b00227
Wang, H., Chen, H., Chen, Y., and Deng, G.-J. (2014). Palladium-catalyzed one pot 2-arylquinazoline formation via hydrogen-transfer strategy. Org. Biomol. Chem. 12, 7792–7799. doi: 10.1039/C4OB01296H
Wang, J., Zha, S., Chen, K., Zhang, F., Song, C., and Zhu, J. (2016). Quinazoline synthesis via Rh(III)-catalyzed intermolecular C-H functionalization of benzimidates with dioxazolones. Org. Lett. 18, 2062–2065. doi: 10.1021/acs.orglett.6b00691
Wang, Q., Wang, F., Yang, X., Zhou, X., and Li, X. (2016). Rh (III)-and Zn (II)-catalyzed synthesis of quinazoline N-oxides via C–H amidation–cyclization of oximes. Org. Lett. 18, 6144–6147. doi: 10.1021/acs.orglett.6b03155
Wang, X., He, D., Huang, Y., Fan, Q., Wu, W., and Jiang, H. (2018). copper-catalyzed synthesis of substituted quinazolines from benzonitriles and 2-ethynylanilines via carbon-carbon bond cleavage using molecular oxygen. J. Org. Chem. 83, 5458–5466. doi: 10.1021/acs.joc.8b00378
Wang, X., and Jiao, N. (2016). Rh-and Cu-cocatalyzed aerobic oxidative approach to quinazolines via [4+ 2] C–H annulation with alkyl azides. Org. Lett. 18, 2150–2153. doi: 10.1021/acs.orglett.6b00774
Wang, X., Lerchen, A., and Glorius, F. (2016). A comparative investigation: group 9 Cp* M (III)-catalyzed formal [4+ 2] cycloaddition as an atom-economic approach to quinazolines. Org. Lett. 18, 2090–2093. doi: 10.1021/acs.orglett.6b00716
Wang, Y., Su, X., and Chen, C. (2013). One-pot synthesis of multiply substituted quinoline and quinazoline derivatives via [2+2+2] cascade annulation with diaryliodonium salts. Synlett 49, 6752–6754. doi: 10.1055/s-0033-1339878
Wattanapiromsakul, C., Forster, P. I., and Waterman, P. G. (2003). Alkaloids and limonoids from Bouchardatia neurococca: systematic significance. Phytochemistry 64, 609–615. doi: 10.1016/S0031-9422(03)00205-X
Witt, A., and Bergman, J. (2000). Synthesis and reactions of some 2-Vinyl-3H-quinazolin-4-ones. Tetrahedron 56, 7245–7253. doi: 10.1016/S0040-4020(00)00595-0
Wu, X., Sun, S., Xu, S., and Cheng, J. (2018). Rh-catalyzed annulation of ortho-C– H bonds of 2-arylimidazoles with 1, 4, 2-dioxazol-5-ones toward 5-arylimidazo [1, 2-c] quinazolines. Adv. Synth. Catal. 360, 1111–1115. doi: 10.1002/adsc.201701331
Xu, L., Li, H., Liao, Z., Lou, K., Xie, H., Li, H., et al. (2015). ChemInform abstract: divergent synthesis of imidazoles and quinazolines via Pd(OAc) 2-catalyzed annulation of N-allylamidines. ChemInform. 46. doi: 10.1002/chin.201550165
Xu, M., Xu, K., Wang, S., and Yao, Z.-J. (2013). Assembly of indolo [1, 2-c] quinazolines using ZnBr2-promoted domino hydroamination–cyclization. Tetrahedron Lett. 54, 4675–4678. doi: 10.1016/j.tetlet.2013.06.079
Yang, X., Jin, Y., Liu, H., Jiang, Y., and Fu, H. (2012). Easy and efficient one-pot synthesis of pyrazolo [1, 5-c] quinazolines under mild copper-catalyzed conditions. RSC Adv. 2, 11061–11066. doi: 10.1039/c2ra21929h
Zhang, J., Zhu, D., Yu, C., Wan, C., and Wang, Z. (2010). A simple and efficient approach to the synthesis of 2-phenylquinazolines via sp3 C-H functionalization. Org. Lett. 12, 2841–2843. doi: 10.1021/ol100954x
Zhang, Y., Shao, Y., Gong, J., Hu, K., Cheng, T., and Chen, J. (2018). Palladium-catalyzed tandem reaction of quinazolinone-based nitriles with arylboronic acids: synthesis of 2-(4-arylquinazolin-2-yl) anilines. Adv. Synth. Catal. 360, 3260–3265. doi: 10.1002/adsc.201800615
Zhao, D., Shen, Q., and Li, J. X. (2015). Potassium iodide-catalyzed three-component synthesis of 2-arylquinazolines via amination of benzylic C-H bonds of methylarenes. Adv. Synth. Catal. 357, 339–344. doi: 10.1002/adsc.201400827
Zhao, D., Wang, T., Shen, Q., and Li, J. X. (2014). n-Bu4NI-catalyzed selective dual amination of sp3 C-H bonds: oxidative domino synthesis of imidazo[1,5-c]quinazolines on a gram-scale. Chem. Commun. 50, 4302–4304. doi: 10.1039/C4CC01444H
Keywords: pyrimidine, bicyclic compounds, synthesis, green chemistry, quinazolines, quinazolinones
Citation: Faisal M and Saeed A (2021) Chemical Insights Into the Synthetic Chemistry of Quinazolines: Recent Advances. Front. Chem. 8:594717. doi: 10.3389/fchem.2020.594717
Received: 14 August 2020; Accepted: 26 November 2020;
Published: 14 January 2021.
Edited by:
Naohiko Yoshikai, Nanyang Technological University, SingaporeReviewed by:
Zhiyuan Chen, Jiangxi Normal University, ChinaHitendra M. Patel, Sardar Patel University, India
Copyright © 2021 Faisal and Saeed. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aamer Saeed, YWFtZXJzYWVlZEB5YWhvby5jb20=
 Muhammad Faisal
Muhammad Faisal Aamer Saeed
Aamer Saeed