- State Key Laboratory Breeding Base of Green Pesticide and Agricultural Bioengineering, Key Laboratory of Green Pesticide and Agricultural Bioengineering, Ministry of Education, Research and Development Center for Fine Chemicals, Guizhou University, Guiyang, China
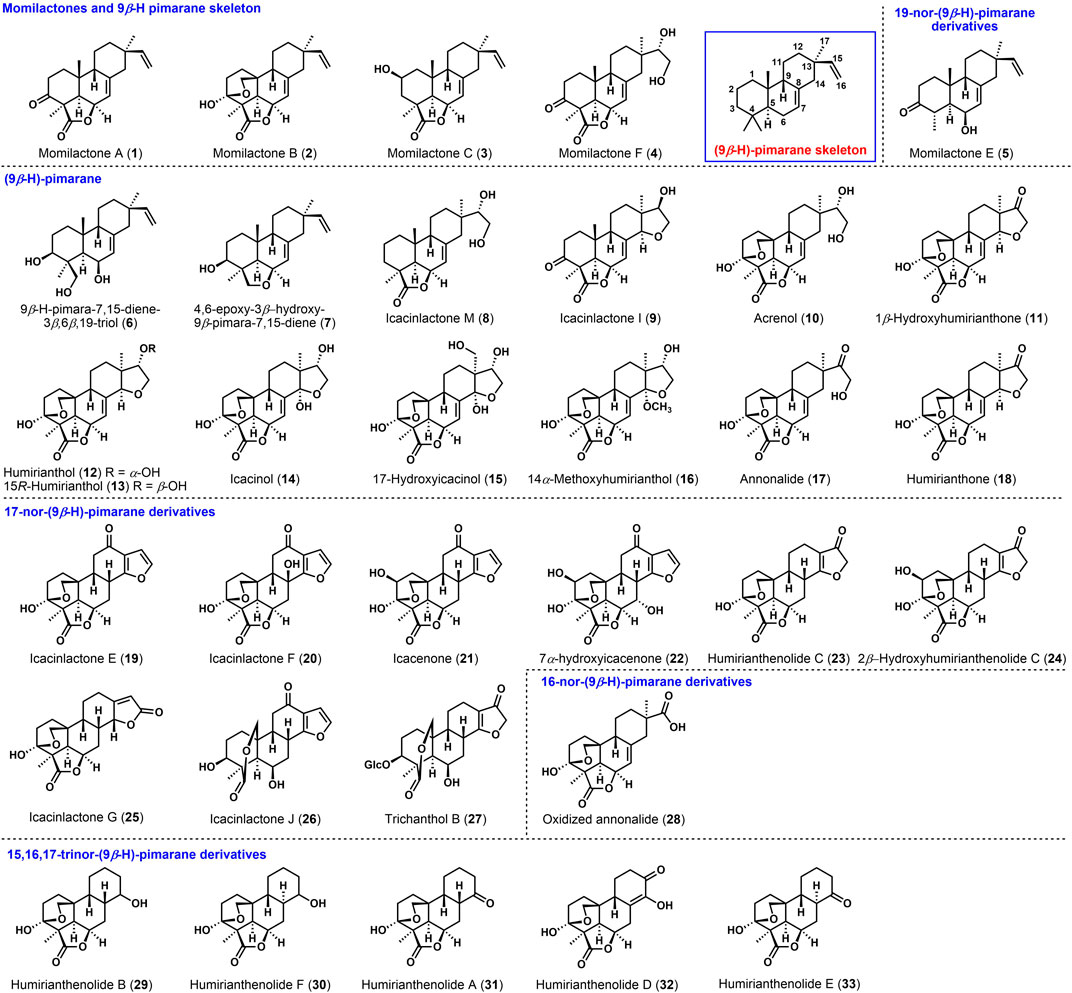
Allelochemicals are secondary metabolites produced from plants and used to prevent and control the invasion of other plants and microorganisms, with broad application prospects in crop protection. Structurally, momilactones belong to 9β-H pimarane diterpenoids, one of rice’s significant allelochemicals with anti-weeds and antibacterial activity. Rare studies have been reported with the synthesis challenges of the unique 9β-H pimarane skeleton. Hence, synthetic strategies of momilactones and related 9β-H pimarane skeleton are reviewed from 1984 to 2021.
Introduction
Modern genetic evidence and recent studies have shown that momilactones are among the most active allelochemicals (Lin et al., 2019) and play a key role in allelopathy and resistance induction in rice (Okada et al., 2016). In 1973, momilactone A (1) and momilactone B (2) were isolated from Oryza sativa L. by Kato (Kato et al., 1973), firstly identified as new growth inhibitors. They have significant bioactivities, including weeds elimination in paddy fields and antimicrobial activity, especially toward Pyricularia oryzae Cav. (Jiang et al., 2016). However, the natural content of momilactones could not meet further research needs. Synthetic approaches to yield these natural products seem to attract synthetic chemists (Mohan et al., 1996). Kato (Kato et al., 1977) determined the stereochemical configuration of momilactone A by X-ray single-crystal diffraction as 9β-H. Momilactone A has continuous chiral centers with a trans-syn-cis tricyclic skeleton named 9β-H pimaranes, as shown in Figure 1, characterized in the family compounds (Zhao et al., 2018). Moreover, the trans-syn-cis tricyclic ring and the stereochemistry at C-9 led to significant challenges in synthesizing these molecules. In the early stage (Deslongchamps and Germain, 1999), the construction of the 9β-H-pimarane skeleton commonly had drawn the attention of scientists devoted to the synthesis of momilactones and related diterpenoids.
9β-H-pimarane diterpenoids are featured with the trans-syn-cis tricyclic skeleton and β-configuration of the proton at C-9. Such studies have been reported to investigate their abundant biological activities (Xu et al., 2021). Figure 1 shows that the known (9β-H)-pimarane related diterpenoids can be classified into (9β-H)-pimarane (1–4, 6–18), 16-nor-(9β-H)-pimarane (28), 17-nor-(9β-H)-pimarane (19–27), 19-nor-(9β-H)-pimarane (5), and 15,16,17-trinor-(9β-H)-pimarane derivatives (29–33). Among the momilactone family, momilactones A and B were obtained from moss Hypnum plumaeforme by Nozaki (Nozaki et al., 2007). Momilactones C (3), F (4), and E (5) were found from the hulls (Liu et al., 2012), leaves, and roots of rice (Cho et al., 2015). Strictly speaking, momilactone E belongs to 19-nor-(9β-H)-pimarane, and momilactone D possesses the 9β-OH, which could not be classified as (9β-H)-pimarane. These natural products exhibited inhibition of weeds and antibacterial activities (Tsunakawa et al., 1976). Momilactone B had the most efficient, currently known bioactivity (Dayan et al., 2009).
For example, (9β-H)-pimaranes, 4,6-epoxy-3β-hydroxy-9β-pimara-7,15-diene (7), and 9β-H-pimara-7,15-diene-3β,6β,19-triol (6) (Horie et al., 2015) were isolated from the rice husks of Oryza sativa L. The anti-fungal activities on Magnaporthe grisea (Li et al., 2014) have been investigated. Icacinlactone M (8), 14α-methoxyhumirianthol (16), and annonalide (17) were found from Icacina oliviformis (Zhao et al., 2015a) for the first time (Sun et al., 2021). Besides (Graebner et al., 2000), humirianthol (12) (Li et al., 2020), icacinol (14), 17-hydroxyicacinol (15), 14α-methoxyhumirianthol (16), and annonalide (17) showed cytotoxic activities (Onakpa et al., 2014) toward human cancer cell lines. These compounds were also obtained from the tuber of Icacina oliviformis (Zhou et al., 2020). Cytotoxic humirianthone (18) and 15R-humirianthol (13) were found from the lianas in the Suriname rainforest (Adou et al., 2005). The 17-nor-(9β-H)-pimarane derivates (Zhao et al., 2015b), humirianthenolide C (23), 2β-hydroxyhumirianthenolide C (24), icacenone (21), 7α-hydroxyicacenone (22), and icacinlactone E-J (19, 20, 25, 26) with cytotoxic activities (Guo et al., 2016) were isolated from the tubers of Icacina trichantha (Zhao et al., 2015a). 7α-Hydroxyicacenone (22), icacenone (21), and trichanthol B (27) (Xu et al., 2021) might also be considered for antimicrobial activities (On'Okoko et al., 1985). Humirianthenolides A, B, D, E, and F (29–33) were separated from the tuber of Humirianthera rupestris, known as the 15,16,17-trinor-(9β-H)-pimarane derivates (Zoghbi et al., 1981). Oxidized annonalide (28) was identified as 16-nor-(9β-H)-pimarane derivates. Most of the above compounds exhibited biological activities such as plant growth inhibition, anti-fungal activity (Shen et al., 2020), and cytotoxicity (Zhou et al., 2020). Given the broad biological activities, the chemical syntheses of 9β-H-pimarane diterpenoids are significant, although there was only one total synthesis of (±)-momilactone A reported by Germain and Deslongchamps Germain and Deslongchamps (2002). This review covers the recent synthetic approaches to momilactones and related 9β-H-pimarane skeleton.
Synthetic Studies Toward 9β-H Pimarane Skeleton Diterpenoids
A few synthetic strategies about 9β-H-pimarane skeleton molecules had been described for the challenging framework, especially the continuous chiral centers. It would be difficult to accomplish the trans-syn-cis tricyclic with stereoselectivity.
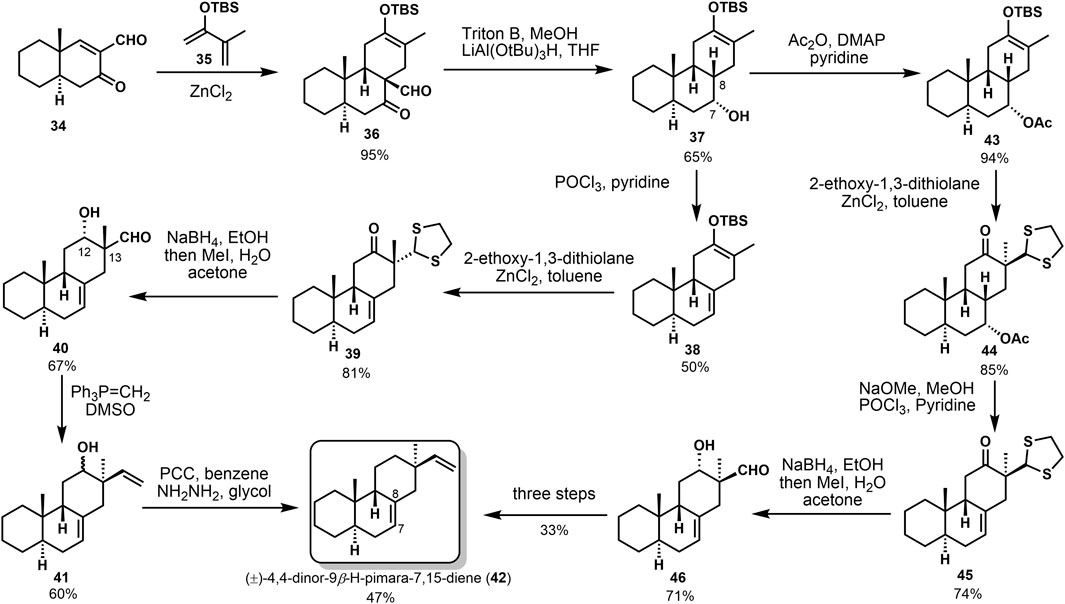
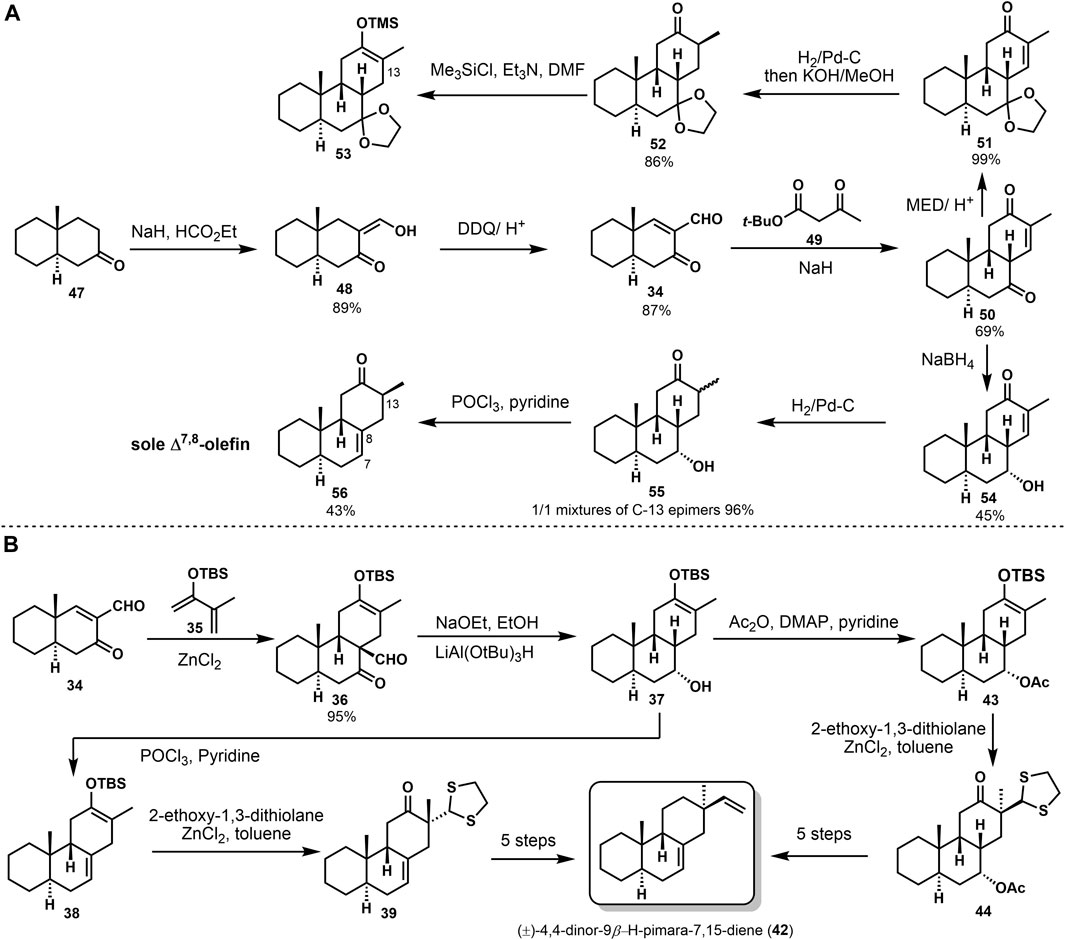
In 1984, Sicherer-Roetman (Sicherer-Roetman et al., 1984) described the synthesis of model compound (±)-4,4-dinor-(9β-H)-pimara-7,15-diene 42, possessing the trans-syn-cis skeleton and α-methyl and β-vinyl groups at C-13. The transannular Diels–Alder strategy had been used to construct the core tricyclic system, as shown in Scheme 1. Product 36 was obtained by the Diels–Alder reaction of ketone formaldehyde 34 and o-diolefin 35 under the catalysis of ZnCl2; the step provided that cis-adduct 36 was deformylated in the presence of triton B and then hydrogenated with LiAl(OtBu)3H to obtain sole reduction product 37. From this point on, compound 42 could be provided by two different strategies. First, compound 37 was dehydrated in POCl3 and pyridine to yield dienolsilane 38. Then, dithioacetal 39 was obtained with 2-ethoxy-1,3-dithiolan, and cis-β-hydroxyaldehyde 40 was afforded by reduction and hydrolysis. They got β-vinyl product 41 through a Wittig reaction of compound 40. Considerable epimerization occurred at C-12 and C-13, a handful of the α-vinyl product was detected. Finally, oxidation of 41 and Wolff–Kishner reduction of the carbonyl gave compound 42 at 47% yield. The second approach protected the hydroxyl group to afford acetyl ester 43. Alkene intermediate 45 was afforded through the alkylation, hydrolyzation, and dehydration, followed by reduction and hydrolyzation. With compound 46 in hand, epimerization also occurred, resulting in a single β-vinyl product. The target compound 42 is finally transformed under the same conditions as the first route.
The stereoselective synthesis of (±)-4,4-dinor-9β-H-pimara-7,15-diene (42) was accomplished by Sicherer-Roetman Sicherer-Roetman et al. (1985). Initially Meyer (Meyer et al. 1975) formed the trans-syn-cis tricyclic product 50 with formyl enone 34 and tert-butyl 3-oxopentanoate 49 (Scheme 2A). Formylation and dehydrogenation of decalone 47 provided the starting compound 34. To investigate the alkylation of 50 and get β-vinyl group at C-13, they prepared trimethylsilyl enol ether 53 after reduction, but product 53 would hydrolyze rapidly. Then they obtained 54 from 50 with the presence of NaBH4. Product 54 could be treated through hydrogenation and elimination to get 56. Elimination of 55 only provided the Δ7,8-olefin in 43% yield. Another approach was based on the Diels–Alder reaction. They obtained regiospecific silyl enol ether 36 and provided the desired stereochemistry at C-9. Deformylation of product 36 and reduction with lithium tri-tert-butoxy aluminum hydride gave alcohol 37. Then, product 37 was converted into model compound (±)-4,4-dinor-(9β-H)-pimara-7,15-diene (42) via several transformations (Scheme 2B). These conversions were reported in 1984 by Sicherer-Roetman (Sicherer-Roetman et al., 1984).
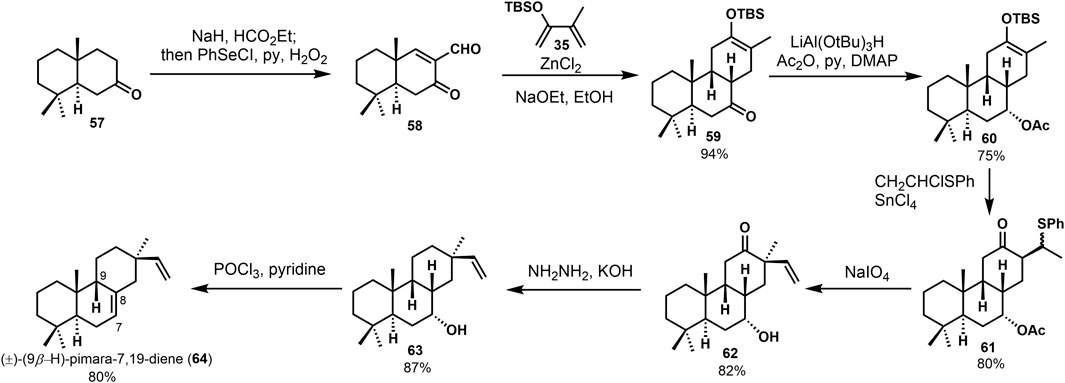
(±)-9β-H-pimara-7,19-diene (64) was seen as one of the intermediates in the biosynthesis of photoalexines in rice. It possessed the A, B, C ring system of momilactones. In 1989, Jansen (Jansen et al., 1989) reported the synthesis of (±)-9β-H-pimara-7,19-diene (64). They followed their previous syntheses to carry out a Diels–Alder reaction between enone aldehyde 57 and 2-(tert-butyldimethylsilyloxy)-3-methyl-1,3-butadiene 35. Through deformylation and hydrogenation, with the hydroxyl group being protected, 7α-acetoxy compound 60 was provided. Stereoselective alkylation of the silyl enol ether 60 with CH2CHClSPh, followed by oxidation and elimination of the sulfoxide group, gained the desired vinyl product 62. The carbonyl was removed during the Wolff–Kishner reduction of 62. Finally, (±)-9β-H-pimara-7,19-diene (64) gave a 28% overall yield (Scheme 3).
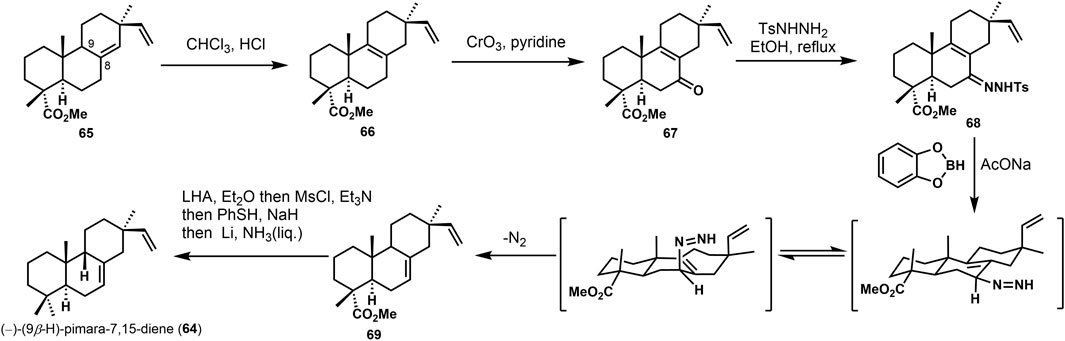
The synthetic challenge of 9β-H pimarane skeleton could be to create the 9,10-syn configuration (Feilner et al., 2021). Several synthetic approaches have been accomplished (Feilner et al., 2020) to construct the stereochemistry at C-9,10 by Michael addition, lithium-ammonia reduction (Yu and Yu, 2015), and Diels–Alder reaction (Deslongchamps et al., 2014). Some of these strategies would gain the 9,10-trans products, inconsistent with the desired goal. In 1992, the 9,10-syn stereochemistry was accomplished via catechol borane reduction by Coates (Chu and Coates, 1992). As shown in Scheme 4, the unsaturated compound 66 was obtained from 65 by isomerization to its Δ8 isomer with HC1/CHC13. Regioselective allylic oxidation of 66 provided ketene 67. It was refluxed with p-toluene sulfonyl hydrazine in ethanol to obtain tosylhydrazone 68 and treated with catechol borane and sodium acetate. Double bond isomerization rearrangement was used, and Δ7,8-olefin 69 was obtained. Subsequently, the 4α-ester group of compound 69 was reduced by lithium aluminum-hydrogen to yield primary alcohol, and hydroxyl was protected after removing the methyl sulfonyl and fulguration. Finally, the target product (−)-(9β-H)-pimara-7,15-diene (64) was obtained by desulphurization with liquid lithium ammonia.
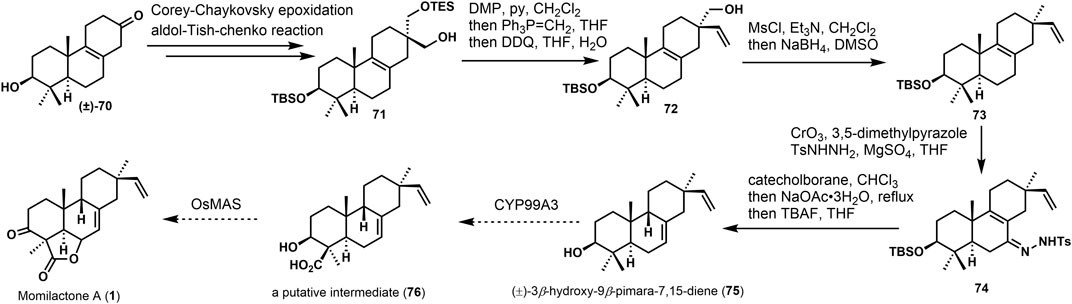
Yajiama (Yajiama et al., 2011) investigated the synthesis of (±)-3β-hydroxy-9β-pimara-7,15-diene (75). The core skeleton was constructed via Hutchins allyldiazene rearrangement (Chu and Coates, 1992). In Scheme 5, the approach started from the known ketone (±)−70, and 71 was gained via several transformations in good yield. Then, the hydroxyl group was oxidized. After the Witting olefination and deprotection, vinyl product 72 was obtained. The hydroxyl group of 72 was removed to get the desired derivative 73. It possesses β-vinyl groups at C-13. After reducing 74 by catechol borane, under the presence of sodium acetate, the desired 9,10-syn tricyclic compound (±)−3α-hydroxy-9β-pimara-7,15-diene (75) was provided, which was considered a putative intermediate of momilactones and other diterpene phytoalexins in rice. It can be converted into 76 and momillactone A (1). In these syntheses, it furnished the configuration of the C-13 quaternary center using a stereoselective approach, and 9,10-syn tricyclic skeleton was constructed via rearrangement. This methodology would also apply to the synthesis of 9β-H pimaranes.
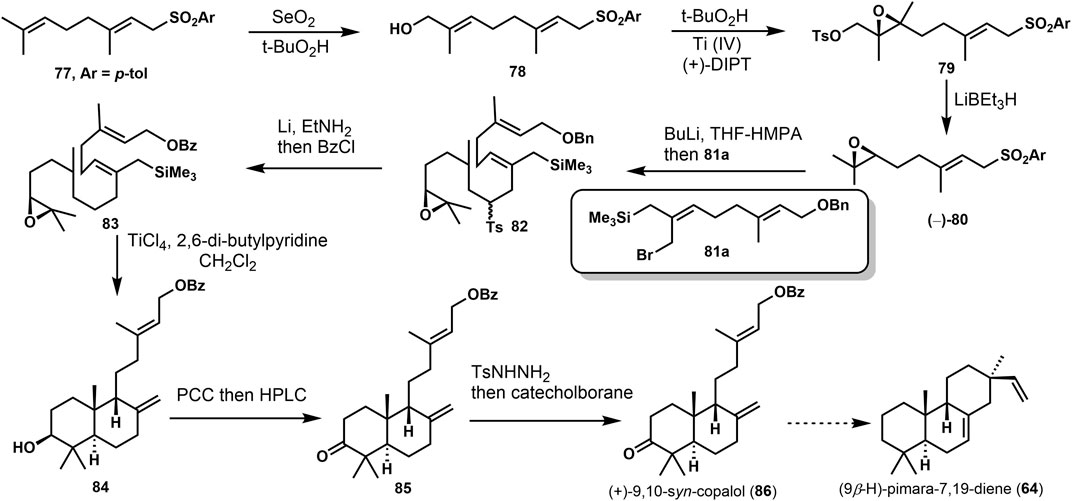
Yee and Coates (Yee and Coates, 1992) accomplished the synthesis of 9,10-syn-Copalol (86). In Scheme 6, the approach was started from 77 via Riley oxidation and Sharpless epoxidation under the presence of TiCl4. A conversion was performed to remove the hydroxyl group with LiBEt3H. Then, 82 was provided via lithiation and alkylation with (E, Z)-8-bromo-9-(trimethylsilyl) geranyl benzyl ethers (81a). Selective reductive cleavage of the toluenesulfonyl and protected benzyl group produced the tandem cyclization precursor 83. Lewis acid treatment (TiCl4) of 83 afforded the stereorandom bicyclizations 84 and its diastereoisomers. Then, mixtures were oxidized and separated to get 85. (+)-9,10-syn-copalol (86) was offered through the reduction with catecholborane. It could be converted to (9β-H)-pimaran-7,19-diene 64) via another tandem cyclization.
Fusidane triterpenes are a relatively small family of natural steroidal antibiotics, including fusidine, helvolic acid, and fusidic acid. These compounds have a unique chair-boat-chair ABC tricyclic ring system seen as a sort of 9β-pimara skeleton (Caron and Deslongchamps, 2010). In 2014, the intermolecular/transannular Michael reaction was first applied to the synthesis of ABC-ring in fusidane triterpenes by Fujii and Nakada (Fujii and Nakada, 2014). In Scheme 7, they developed the stereoselective intramolecular Michael reaction of compound 87 with L-Selectride to provide compound 88 (Scheme 7). Compound 88 was performed with benzyl thiol and potassium carbonate affording the benzyl thioester 89. It was then converted to aldehyde 90 by Fukuyama reduction. Enone 91 was prepared via HWE reaction of aldehyde 90 and keto phosphonate 92. The dimethyl acetal 93 was afforded from 91, followed by reduction, and Dess–Martin oxidation gave aldehyde 94. The intramolecular Cr-mediated reaction of compound 94 was optimized when the reaction was performed in THF/DMF mixture, offering sole product 95 (70%). After that, oxidation of compound 95 provided the bis-enone 96, the substrate for intermolecular/transannular Michael reaction cascade. Then, they carried out the reaction of compound 96 under several conditions. Annulation product 97 was formed when thiophenol and DBU were used in methanol at 0 °C in a 73% yield.
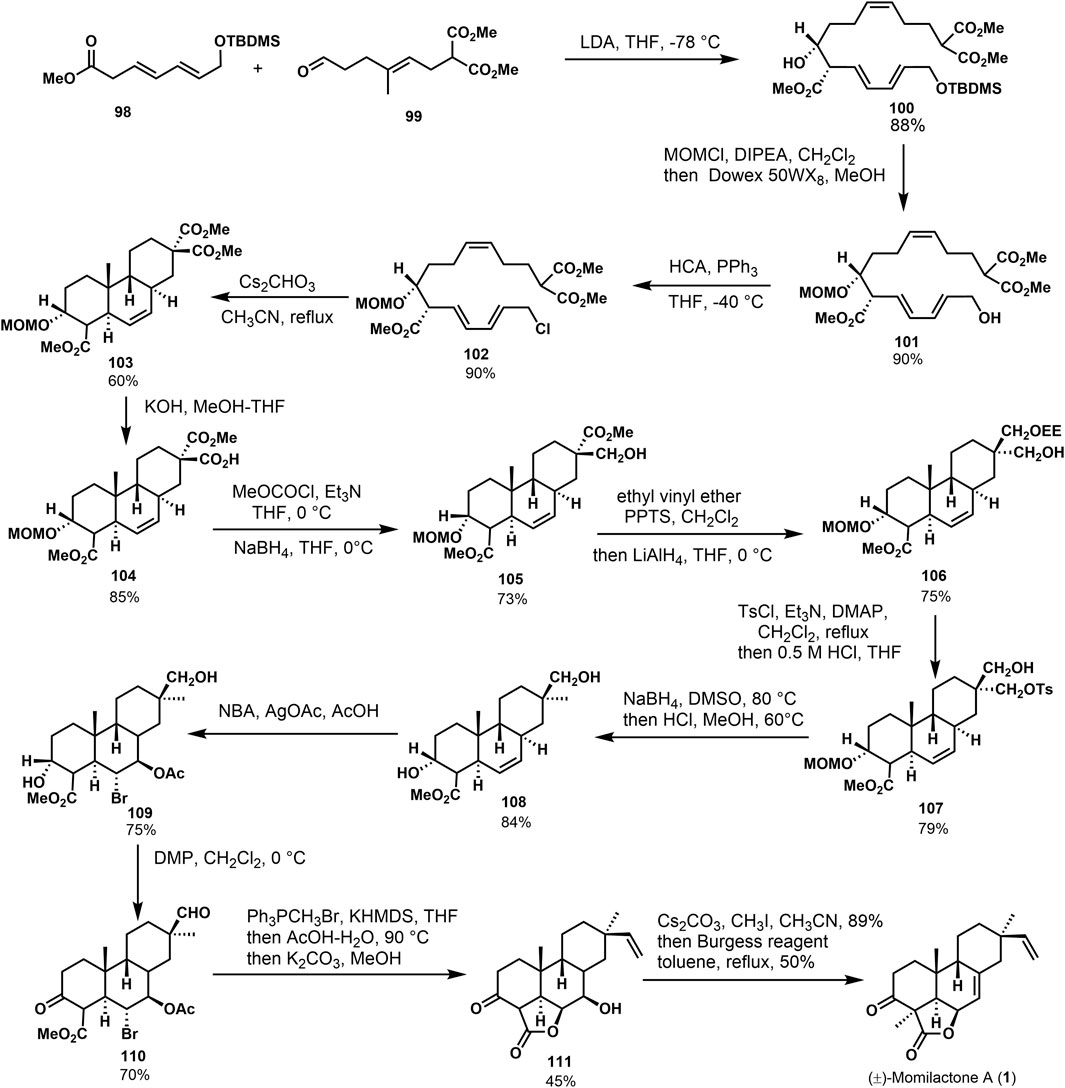
Germain and Deslongchamps (Germain and Deslongchamps, 2002) accomplished the first total synthesis of (±)-momilactone A (1) via a Diels–Alder reaction (Germain and Deslongchamps, 1999). Scheme 8 shows that the condensation was accomplished from conjugated olefins 98 and vinylaldehydes 99 with 88% yield to give diethylisomers 100. Subsequently, MOM ether was obtained from 100 via the protection, followed by selective desilication of primary hydroxyl ether to obtain compound 102. Trans-syn-trans tricyclic compound 103 was offered by Diels–Alder reaction with stereoselectivity under reflux in cesium carbonate acetonitrile solution. In a word, a series of conversions of 100 provided the diastereoisomer 103 in the chair-boat-chair configuration, which is consistent with (±)-momilactone A (1). The target product was obtained through linear strategy transformation starting from intermediate 103. Malonate compound 103 underwent partial hydrolysis and several functional group transformations to afford intermediate 104. Then, the double bond addition was performed under the action of NBS and silver acetate to obtain bromoacetate 109 with high stereoselectivity, followed by the Dess–Martin oxidation and Wittig reaction to obtain the alkenone. Under the condition of acetic acid-water, intramolecular esterification was performed. Moreover, the hydrolysis of acetyl ester was carried out to obtain hydroxylolactone 111. Then, the target product (±)-momilactone A (1) was obtained by the carbonyl α-methylation and dehydration of lactone.
Summary and Further Prospects
Some synthetic strategies have been reported about the construction of the 9β-H piamarane skeleton, such as Diels–Alder reaction, Michael addition, and catechol borane reduction. They carried out the syntheses of the skeleton and the intermediates in natural products using simple procedures. The asymmetric totals synthesis of 9β-H piamaranes has not been reported so far. A new approach must be applied to the natural products in 9β-H pimaranes.
Author Contributions
YZ collected and organized all literature about 9β-H pimarane diterpenoids and reviewed for abstract, introduction, some 9β-H pimarane skeleton, and momilactones syntheses. ML prepared all the scheme and references, summary, and further prospects. QL reviewed Coates’s synthesis of (9β-H)-pimara-7,15-diene and De Groot’s first synthesis of 4,4-dinor-(9β-H)-pimara-7,15-diene. JH reviewed all literature and gave significant discussion. YC reviewed the synthetic efforts towards 9β-H pimarane diterpenoids in the past three decades. He summed up very beautiful reaction schemes.
Funding
We acknowledge financial support from the Science and Technology Foundation of Guizhou Province (No. Qian Ke He platform talents (2018)5781-30), the Science and Technology Foundation of Guizhou Province (2020)1Y108, Department of Education of Guizhou Province (Qian Jiao He KY Zi (2017)375), the PhD Foundation of Guizhou University (Gui Da Ren Ji He (2017)32), and the Plant Protection and Inspection Station of Guizhou Province Project (K19-0201-007) for their financial support.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adou, E., Williams, R. B., Schilling, J. K., Malone, S., Meyer, J., Wisse, J. H., et al. (2005). Cytotoxic Diterpenoids from Two Lianas from the Suriname Rainforest. Bioorg. Med. Chem. 13, 6009–6014. doi:10.1016/j.bmc.2005.07.026
Caron, P.-Y., and Deslongchamps, P. (2010). Versatile Strategy to Access Tricycles Related to Quassinoids and Triterpenes. Org. Lett. 12, 508–511. doi:10.1021/ol902711b
Cho, J.-G., Cha, B.-J., Min Lee, S., Shrestha, S., Jeong, R.-H., Sung Lee, D., et al. (2015). Diterpenes from the Roots ofOryza sativaL. And Their Inhibition Activity on NO Production in LPS-Stimulated RAW264.7 Macrophages. Chem. Biodiversity 12, 1356–1364. doi:10.1002/cbdv.201400239
Chu, M., and Coates, R. M. (1992). Partial Synthesis of 9,10-Syn-Diterpenes via Tosylhydrazone Reduction: (-)-(9β)-Pimara-7,15-Diene and (-)-(9β)-isopimaradiene. J. Org. Chem. 57, 4590–4597. doi:10.1021/jo00043a013
Das G.B. Zoghbi, M., F. Roque, N., and Gottlieb, H. E. (1981). Humirianthenolides, New Degraded Diterpenoids from Humirianthera Rupestris. Phytochemistry 20, 1669–1673. doi:10.1016/S0031-9422(00)98552-2
Dayan, F. E., Cantrell, C. L., and Duke, S. O. (2009). Natural Products in Crop protection. Bioorg. Med. Chem. 17, 4022–4034. doi:10.1016/j.bmc.2009.01.046
Deslongchamps, P., and Germain, J. (1999). Transannular Diels-Alder Approach to the Synthesis of Momilactone A. Tetrahedron Lett. 40, 4051–4054. doi:10.1016/S0040-4039(99)00669-3
Feilner, J. M., Plangger, I., Wurst, K., and Magauer, T. (2021). Bifunctional Polyene Cyclizations: Synthetic Studies on Pimarane Natural Products. Chem. Eur. J. 27, 12410–12421. doi:10.1002/chem.202101926
Feilner, J. M., Wurst, K., and Magauer, T. (2020). A Transannular Polyene Tetracyclization for Rapid Construction of the Pimarane Framework. Angew. Chem. Int. Ed. 59, 12436–12439. doi:10.1002/anie.202003127
Fujii, T., and Nakada, M. (2014). Stereoselective Construction of the ABC-Ring System of Fusidane Triterpenes via Intermolecular/transannular Michael Reaction cascade. Tetrahedron Lett. 55, 1597–1601. doi:10.1016/j.tetlet.2014.01.071
Germain, J., and Deslongchamps, P. (2002). Total Synthesis of (±)-Momilactone A. J. Org. Chem. 67, 5269–5278. doi:10.1021/jo025873l
Germain, J., and Deslongchamps, P. (1999). Transannular Diels-Alder Approach to the Synthesis of Momilactone A. Tetrahedron Lett. 40, 4051–4054. doi:10.1016/S0040-4039(99)00669-3
Graebner, I. B., Mostardeiro, M. A., Ethur, E. M., Burrow, R. A., Dessoy, E. C. S., and Morel, A. F. (2000). Diterpenoids from Humirianthera Ampla. Phytochemistry 53, 955–959. doi:10.1016/S0031-9422(99)00585-3
Guo, B., Onakpa, M. M., Huang, X.-J., Santarsiero, B. D., Chen, W.-L., Zhao, M., et al. (2016). Di-nor- and 17-Nor-Pimaranes from Icacina Trichantha. J. Nat. Prod. 79, 1815–1821. doi:10.1021/acs.jnatprod.6b00289
Horie, K., Inoue, Y., Sakai, M., Yao, Q., Tanimoto, Y., Koga, J., et al. (2015). Identification of UV-Induced Diterpenes Including a New Diterpene Phytoalexin, Phytocassane F, from Rice Leaves by Complementary GC/MS and LC/MS Approaches. J. Agric. Food Chem. 63, 4050–4059. doi:10.1021/acs.jafc.5b00785
Jansen, B. J. M., Schepers, G. C., and De Groot, A. (1989). The Stereoselective Synthesis of (±)-9βh-Pimara-7,19-Diene. Tetrahedron 45, 2773–2776. doi:10.1016/S0040-4020(01)80107-1
Jiang, Z.-Y., Yang, C.-T., Hou, S.-Q., Tian, K., Wang, W., Hu, Q.-F., et al. (2016). Cytotoxic Diterpenoids from the Roots of Aralia Melanocarpa. Planta Med. 82, 742–746. doi:10.1055/s-0042-104349
Kato, T., Aizawa, H., Tsunakawa, M., Sasaki, N., Kitahara, Y., and Takahashi, N. (1977). Chemical Transformations of the Diterpene Lactones Momilactones A and B. J. Chem. Soc. Perkin Trans. 1, 250–254. doi:10.1039/P19770000250
Kato, T., Kabuto, C., Sasaki, N., Tsunagawa, M., Aizawa, H., Fujita, K., et al. (1973). Momilactones, Growth Inhibitors from rice, Oryza Sativa L. Tetrahedron Lett. 14, 3861–3864. doi:10.1016/S0040-4039(01)87058-1
Li, G., Xu, Q.-L., He, C.-M., Zeng, L., and Wang, H.-F. (2014). Two New Anti-fungal Diterpenoids from the Husks of Oryza Sativa. Phytochemistry Lett. 10, 309–312. doi:10.1016/j.phytol.2014.10.023
Li, J. L., Wie, L. L., Chen, C., Liu, D., Gu, Y. Q., Duan‐Mu, J. X., et al. (2020). Bioactive Constituents from the Bryophyta Hypnum Plumaeforme. Chem. Biodiversity 17, 1612–1630. doi:10.1002/cbdv.202000552
Lin, X., Pang, Y., Lu, F., Ding, C., Zeng, R., and Song, Y. (2019). Review on Momilactones of Key Allelochemicals in rice Allelopathy. Guihaia 39, 548–556. doi:10.11931/guihaia.gxzw201801016
Liu, N., Wang, S., and Lou, H. (2012). A New Pimarane-type Diterpenoid from moss Pseudoleskeella Papillosa (Lindb.) Kindb. Acta Pharmaceutica Sinica B 2, 256–259. doi:10.1016/j.apsb.2012.03.003
Meyer, W. L., Clemans, G. B., and Manning, R. A. (1975). Diterpenoid Total Synthesis, an A .Far. B .Far. C Approach. VII. Total Synthesis of DL-Sugiol, DL-Ferruginol, and DL-Nimbiol. J. Org. Chem. 40, 3686–3694. doi:10.1021/jo00913a015
Mohan, R. S., Yee, N. K. N., Coates, R. M., Ren, Y.-Y., Stamenkovic, P., Mendez, I., et al. (1996). Biosynthesis of Cyclic Diterpene Hydrocarbons in Rice Cell Suspensions: Conversion of 9,10-Syn-Labda-8(17),13-Dienyl Diphosphate to 9β-Pimara-7,15-Diene and Stemar-13-Ene. Arch. Biochem. Biophys. 330, 33–47. doi:10.1006/abbi.1996.0223
Monday Onakpa, M., Zhao, M., Gödecke, T., Chen, W.-L., Che, C.-T., Santarsiero, B. D., et al. (2014). Cytotoxic (9βH)-Pimarane and (9βH)-17-Norpimarane Diterpenes from the Tuber ofIcacina Trichantha. Chem. Biodiversity 11, 1914–1922. doi:10.1002/cbdv.201400151
Nozaki, H., Hayashi, K.-i., Nishimura, N., Kawaide, H., Matsuo, A., and Takaoka, D. (2007). Momilactone A and B as Allelochemicals from MossHypnum Plumaeforme: First Occurrence in Bryophytes. Biosci. Biotechnol. Biochem. 71, 3127–3130. doi:10.1271/bbb.70625
Okada, K., Kawaide, H., Miyamoto, K., Miyazaki, S., Kainuma, R., Kimura, H., et al. (2016). HpDTC1, a Stress-Inducible Bifunctional Diterpene Cyclase Involved in Momilactone Biosynthesis, Functions in Chemical Defence in the Moss Hypnum Plumaeforme. Sci. Rep. 6, 25316–25329. doi:10.1038/srep25316
On'Okoko, P., Vanhaelen, M., Vanhaelen-Fastré, R., Declercq, J. P., and Van Meerssche, M. (1985). Icacenone, a Furanoditerpene with a Pimarane Skeleton from Icacina Mannii. Phytochemistry 24, 2452–2453. doi:10.1016/S0031-9422(00)83067-8
Ravindar, K., Caron, P.-Y., and Deslongchamps, P. (2014). Anionic Polycyclization Entry to Tricycles Related to Quassinoids and Terpenoids: A Stereocontrolled Total Synthesis of (+)-Cassaine. J. Org. Chem. 79, 7979–7999. doi:10.1021/jo501122k
Shen, L., Liu, M., He, Y., Al Anbari, W. H., Li, H., Lin, S., et al. (2020). Novel Antimicrobial Compounds as Ophiobolin-type Sesterterpenes and Pimarane-type Diterpene from Bipolaris Species TJ403-B1. Front. Microbiol. 11, 856–868. doi:10.3389/fmicb.2020.00856
Sicherer-Roetman, A., Jansen, B. J. M., and De Groot, A. (1985). ChemInform Abstract: Investigations into the Total Synthesis of Momilactones. Stereoselective Preparation of (±)-4,4-Dinor-9βh-Pimara-7,19-Diene. Chemischer Informationsdienst 16, 193–202. doi:10.1002/chin.198551295
Sicherer-Roetman, A., Jansen, B. J. M., and De Groot, A. (1984). Stereospecific Preparation of (±)-4,4-Dinor-9βh-Pimara-7,15-Diene, a Model for the Total Synthesis of Momilactone Type Diterpenes. Tetrahedron Lett. 25, 2593–2596. doi:10.1016/S0040-4039(01)81239-9
Sun, M., Guo, B., Xu, M., Zhao, M., Onakpa, M. M., Wu, Z., et al. (2021). (9βH)- and 17-Nor-Pimaranes from Icacina Oliviformis. J. Nat. Prod. 84, 949–955. doi:10.1021/acs.jnatprod.9b01131
Tsunakawa, M., Ohba, A., Sasaki, N., Kabuto, C., Kato, T., Kitahara, Y., et al. (1976). Momilactone-c, a Minor Constituent of Growth Inhibitors in Rice Husk. Chem. Lett. 5, 1157–1158. doi:10.1246/cl.1976.1157
Xu, M.-M., Zhou, J., Zeng, L., Xu, J., Onakpa, M. M., Duan, J.-A., et al. (2021). Pimarane-derived Diterpenoids with Anti-Helicobacter pylori Activity from the Tuber of Icacina Trichantha. Org. Chem. Front. 8, 3014–3022. doi:10.1039/d1qo00374g
Yajima, A., Toda, K., Okada, K., Yamane, H., Yamamoto, M., Hasegawa, M., et al. (2011). Stereocontrolled Total Synthesis of (±)-3β-Hydroxy-9β-Pimara-7,15-Diene, a Putative Biosynthetic Intermediate of Momilactones. Tetrahedron Lett. 52, 3212–3215. doi:10.1016/j.tetlet.2011.04.044
Yee, N. K. N., and Coates, R. M. (1992). Total Synthesis of (+)-9,10-syn- and (+)-9,10-Anti-Copalol via Epoxy Trienylsilane Cyclizations. J. Org. Chem. 57, 4598–4608. doi:10.1021/jo00043a014
Yu, J., and Yu, B. (2015). Synthesis of the ABC Skeleton of the Aglycon of Echinoside A. Chin. Chem. Lett. 26, 1331–1335. doi:10.1016/j.cclet.2015.08.010
Zhao, M., Cheng, J., Guo, B., Duan, J., and Che, C.-T. (2018). Momilactone and Related Diterpenoids as Potential Agricultural Chemicals. J. Agric. Food Chem. 66, 7859–7872. doi:10.1021/acs.jafc.8b02602
Zhao, M., Onakpa, M. M., Chen, W.-L., Santarsiero, B. D., Swanson, S. M., Burdette, J. E., et al. (2015b). 17-Norpimaranes and (9βH)-17-Norpimaranes from the Tuber of Icacina Trichantha. J. Nat. Prod. 78, 789–796. doi:10.1021/np5010328
Zhao, M., Onakpa, M. M., Santarsiero, B. D., Chen, W.-L., Szymulanska-Ramamurthy, K. M., Swanson, S. M., et al. (2015a). (9βH)-Pimaranes and Derivatives from the Tuber of Icacina Trichantha. J. Nat. Prod. 78, 2731–2737. doi:10.1021/acs.jnatprod.5b00688
Keywords: 9β-H pimarane, skeleton, momilactones, allelochemical, diterpenoids
Citation: Zhang Y, Li M, Liu Q, Huang J and Chen Y (2022) A Synthetic View on Momilactones and Related 9β-H Pimarane Skeleton Diterpenoids. Front. Chem. 10:882404. doi: 10.3389/fchem.2022.882404
Received: 23 February 2022; Accepted: 28 February 2022;
Published: 21 March 2022.
Edited by:
Yaqiong Su, Xi’an Jiaotong University, ChinaCopyright © 2022 Zhang, Li, Liu, Huang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Huang, amh1YW5nNjZAMTYzLmNvbQ==; Yang Chen, eWNoZW4xQGd6dS5lZHUuY24=
 Yue Zhang
Yue Zhang Mengran Li
Mengran Li Qichang Liu
Qichang Liu Jian Huang
Jian Huang Yang Chen
Yang Chen