- 1Department of Pharmacognosy and Medicinal Plants, Faculty of Pharmacy, Future University in Egypt, Cairo, Egypt
- 2Medicinal Chemistry Department, Faculty of Pharmacy, King Salman International University, Ras-Sedr, South Sinai, Egypt
- 3Department of Pharmaceutical Sciences, College of Pharmacy, Princess Nourah Bint Abdulrahman University, Riyadh, Saudi Arabia
- 4Department of Pharmaceutical Sciences, College of Pharmacy and Thumbay Research Institute for Precision Medicine, Gulf Medical University, Ajman, United Arab Emirates
- 5Department of Pharmaceutical Organic Chemistry, Faculty of Pharmacy, Cairo University, Cairo, Egypt
- 6Department of Pharmacognosy, College of Pharmacy, King Khalid University, Asir, Saudi Arabia
- 7Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Kafrelsheikh University, Kafrelsheikh, Egypt
- 8Department of Pharmacognosy, Faculty of Pharmacy, Ain Shams University, Cairo, Egypt
Introduction: Natural skincare products and cosmetic preparations have gained popularity among consumers in recent years, prompting cosmetic companies to develop more natural offerings. These products often incorporate plant extracts known for their anti-aging, anti-wrinkle, and depigmentation properties.
Methods: Using gas chromatography-mass spectrometry, this study examined the volatile compounds in both fresh and dry Citrus aurantifolia (key lime) fruit essential oils. The oils’ anti-aging and antioxidant activities were assessed through in vitro anti-collagenase and anti-elastase assays.
Results: In vitro analysis revealed good inhibition of elastase and collagenase enzymes by fresh key lime essential oil, with IC50 values of 145.02 and 63.97 μg/mL, respectively, compared to positive controls (daidzein for collagenase, piroxicam for elastase), in comparison to dry key lime oil (IC50 = 223.14 and 109.57 μg/mL, respectively). The antioxidant activity of the oils was evaluated using the ABTS (2,2’-azino-bis(3-ethylbenzothiazoline6-sulfonic acid)) radical scavenging assay. The fresh key lime oil demonstrated stronger antioxidant activity (37.76 ± 0.80 μM Trolox equivalent (TE)/g) compared to the dry key lime oil (27.76 ± 1.11 μM TE/g), suggesting that it retains more bioactive compounds essential for radical scavenging activity. Additionally, molecular docking was performed to analyze interactions between the main metabolites and the targeted enzymes active sites. Molecular docking analysis showed excellent binding scores for the three main metabolites.
Conclusion: The anti-aging potential of fresh key lime essential oil may be attributed to its major compounds. These findings suggest that key lime essential oil could be a promising natural ingredient for anti-aging skincare formulations.
1 Introduction
Citrus species are among the most frequently eaten and extensively distributed fruits (Lin et al., 2019). The genus originated in Southeast Asia and is now found in various tropical and subtropical areas. Citrus fruits are categorized into several categories, including tangerines, mandarins, oranges, limes, lemons … etc. Because of their nutritional benefits and pleasant aroma and taste, these fruits are typically consumed whole fruits or as juices (Prommaban and Chaiyana, 2022). Citrus fruit (pulp, peel, and seed) provide potent bioactive metabolites as essential oils, phenolics, and flavonoids (Tavallali et al., 2021). These active metabolites displayed numerous biological properties as anti-inflammatory, antioxidant, anti-tumor, cardiovascular protective, and neuroprotective activities (Mahato et al., 2018). Essential oils are key secondary bioactive compounds (Rabie et al., 2023) that provide fragrances and are broadly employed in food additives, pharmaceuticals, and medicinal treatments (Prommaban and Chaiyana, 2022; El-Nashar et al., 2022). Citrus essential oils mostly consist of terpene hydrocarbons including monoterpenes, sesquiterpenes, and oxygenated derivatives (González-Mas et al., 2019). The most prevalent monoterpene hydrocarbon identified in various citrus essential oils was limonene (Mohammed et al., 2022).
Citrus aurantifolia belongs to the citrus genus, which is planted mainly in warm regions such as Egypt, Mexico, the Far east, and India (Spadaro et al., 2012). C. aurantifolia is a green colour fruit with smooth and thin skin and acidic juicy flesh with a defined odour and taste (Lin et al., 2019). The essential oil and juice are the major economic products of C. aurantifolia (Spadaro et al., 2012). Its essential oil constitutes mainly limonene, terpinen-4-ol, α-terpineol, β-pinene, eucalyptus, 1,4-cineole, p-cymene, geranial, β- bisabolene, and neral, which show various pharmacological activities as antioxidant, hypolipidemic, antidiabetic, anti-inflammatory, anticarcinogenic, antifungal, and antidepressant properties (Lin et al., 2019). Aside from these reported biological activities, anti-aging effects on the skin were rarely investigated.
Most of the research done on the C. aurantifolia was concerned with the analysis of the essential oils derived from the fruits and leaves (Spadaro et al., 2012; Jain et al., 2020) with significant attention on their antioxidant, antimicrobial, and anticancer activities (Naeini et al., 2018; Boshtam et al., 2011; Patil et al., 2009) that are mostly related to their high monoterpenes and oxygenated monoterpenes (Prommaban and Chaiyana, 2022). We were interested in studying the anti-aging activity of C. aurantifolia essential oils due to its verified antioxidant properties. Particularly, we planned to explore from a comparative point of view which oils, fresh or dried key lime oil, have the ability to inhibit collagenase and elastase enzymes to possibly use them as natural ingredients in managing skin age-related issues.
Skin ageing and wrinkles are significant issues of human skin that can be influenced by intrinsic factors (for example, genotype, endocrine metabolism, or level of hormones) and/or external factors (for example, ultraviolet radiation, nutrition, stress, and pollution) (Eun et al., 2020). Exposure of the skin to these external factors causes an increase in the enzymes (for example, collagenase and elastase) that participate in the ageing process. These enzymes initiate collagen and elastin breakdown, causing accelerated skin ageing which appears as wrinkles (Oulebsir et al., 2022).
Skin aging results from elastin and collagen diminution in the epidermal connective tissue, resulting in skin thinning and an increase in the wrinkles formation (Kim and Bae, 2023). The aging progression is related to the initiation of inflammatory pathways in the epidermis, usually indicated as “inflamm-aging” (Neves and Sousa-Victor, 2020). Ultraviolet radiation from the sun also plays an essential part in skin ageing and damage by promoting cytokines and intensifying the formation of free radicals (Lago and Puzzi, 2019). The cosmetics business is constantly researching active compounds to stop and minimize skin ageing, with cosmeceuticals being a critical part of anti-aging skin treatments (Shanbhag et al., 2019).
Essential oils are commonly used in skin care products and cosmetics because of their active metabolite with scented odor (Sharmeen et al., 2021). Many researchers have stated the usage of Citrus essential oils in cosmetics and pharmaceuticals (Prommaban and Chaiyana, 2022). Several research studies have revealed bioactive compounds and pharmacological properties of essential oils obtained from citrus flowers, leaves, and peels. However, research has been limited to the anti-ageing properties of key lime essential oil. Therefore, our study highlighted the chemical composition of both fresh and dried key lime essential oils, as well as their antioxidant activity and inhibitory effect on elastase and collagenase enzymes in vitro.
2 Results and discussion
2.1 Drying effect on the constituents and the yield of key lime essential oil
Both dried and fresh oil yields were 0.67% and 0.5% v/w, respectively. The essential oils have a distinguishing, faint yellow colour and a strong, lemon-scented odour. The constituents of both key lime essential oils are revealed in Table 1. Thirty-two and twenty-two compounds were separated and identified in fresh and dry key lime. In fresh key lime essential oil, 12 metabolites were monoterpenes, 10 oxygenated monoterpenes, and 10 sesquiterpene hydrocarbons, while in dried key lime essential oil, 11 metabolites were monoterpenes, 9 oxygenated monoterpenes, and 2 sesquiterpene hydrocarbons.
The major metabolite of the fresh key lime essential oil was D-limonene (42.02%), followed by β-pinene (10.88%), γ-terpinene (10.16%), and α-terpineol (7.84%). However, the main dry key lime essential oil compounds were D-limonene (72.64%), followed by γ-terpinene (15.67%). The shade drying method produced an alteration in the constituents, in which the percentage of monoterpenes hydrocarbons revealed a substantial increase, 76.42% in fresh oil to 96.24% in dry oil, whereas the oxygenated monoterpenes and sesquiterpene hydrocarbons decreased significantly (3.2%) in dry oil, in contrast to (13.51%) in fresh oil. This may be attributed to the isomerization of metabolites and short-chain alkenes (Elkhawas et al., 2022).
Furthermore, both α-terpineol, and 4-terpinenol, which have antioxidant, anti-inflammatory, and skin-regenerating characteristics (Chen et al., 2023; Be Tu and Tawata, 2015; Delgado-Adámez et al., 2017), dropped significantly from 7.84% to 1.71%, respectively, in fresh oil to 1.61% and 077%, respectively, in dry oil. Other oxygenated compounds, such as endo-borneol, fenchol, and γ-terpineol, were only present in the fresh sample. Sesquiterpene hydrocarbons, which made up 6.82% of fresh oil, were eradicated in dry oil (0.30%). Compounds including β-bisabolene, α-farnesene, and caryophyllene showed significant decreases.
D- Limonene is the prevalent chemotype found in essential oils produced from Citrus spp. various parts such as peels, fruits, flowers, and leaves (Lota et al., 2002). Lemes et al. reported that terpenes prevail in the essential oil collected from other C. aurantifolia specimens and that the compounds of the essential oil change greatly depending on the plant’s geographical locations and environmental factors (Lemes et al., 2018). Spadaro et al. explored the essential oil of key lime obtained from Italy and reported that D-limonene, γ-terpinene, and β-pinene presented the major compounds (Spadaro et al., 2012). Furthermore, Hong et al. stated that the major compounds identified in C. aurantifolia from South Korea were β-pinene and limonene (Hong et al., 2017).
Limonene, a lemon-fragrant monoterpene found in essential oils of citrus plants, is used in cosmetics, food, and drugs (Kim Y. W. et al., 2013). It was reported that it has various pharmacological properties such as anti-inflammatory, anticancer, antidiabetic, and antioxidant activities (Anandakumar et al., 2021; Secerli et al., 2023). Although previously reported data on C. aurantifolia fruits afforded about 46% of D-limonene (Liu et al., 2022). Yet, the percentage of limonene and γ-terpinene in our study was to some extent within range as previously reported data by Tavallali et al. (2021) and Sreepian et al. (2022). These variations were most likely due to different types of C. aurantifolia (variants, cultivars (Maria and Riccardo, 2020), and regions), and storage duration, harvest year (Gioffrè et al., 2020), season (Vekiari et al., 2002), climate change, and the extraction method (Sulzbach et al., 2021), all of which might change the chemical composition of essential oil (Elkhawas et al., 2023).
The essential oils of fresh and dry key lime fruits were predominantly D-limonene and γ-terpinene, which constitute 52.18% and 88.28%, respectively, of all components (Table 1). These compounds were stated to display antioxidant, anti-inflammatory, and anti-aging activities (Chen et al., 2023; Kumar et al., 2022). Moreover, these compounds can show a synergistic activity together (Boussaada et al., 2007). Kumar et al. have proved that limonene can shield keratinocytes from UVB-induced photoaging by preventing intracellular free radical formation, loss of skin barrier function, and cell death. Thus, it enables the skin to preserve its integrity and function (Kumar et al., 2022).
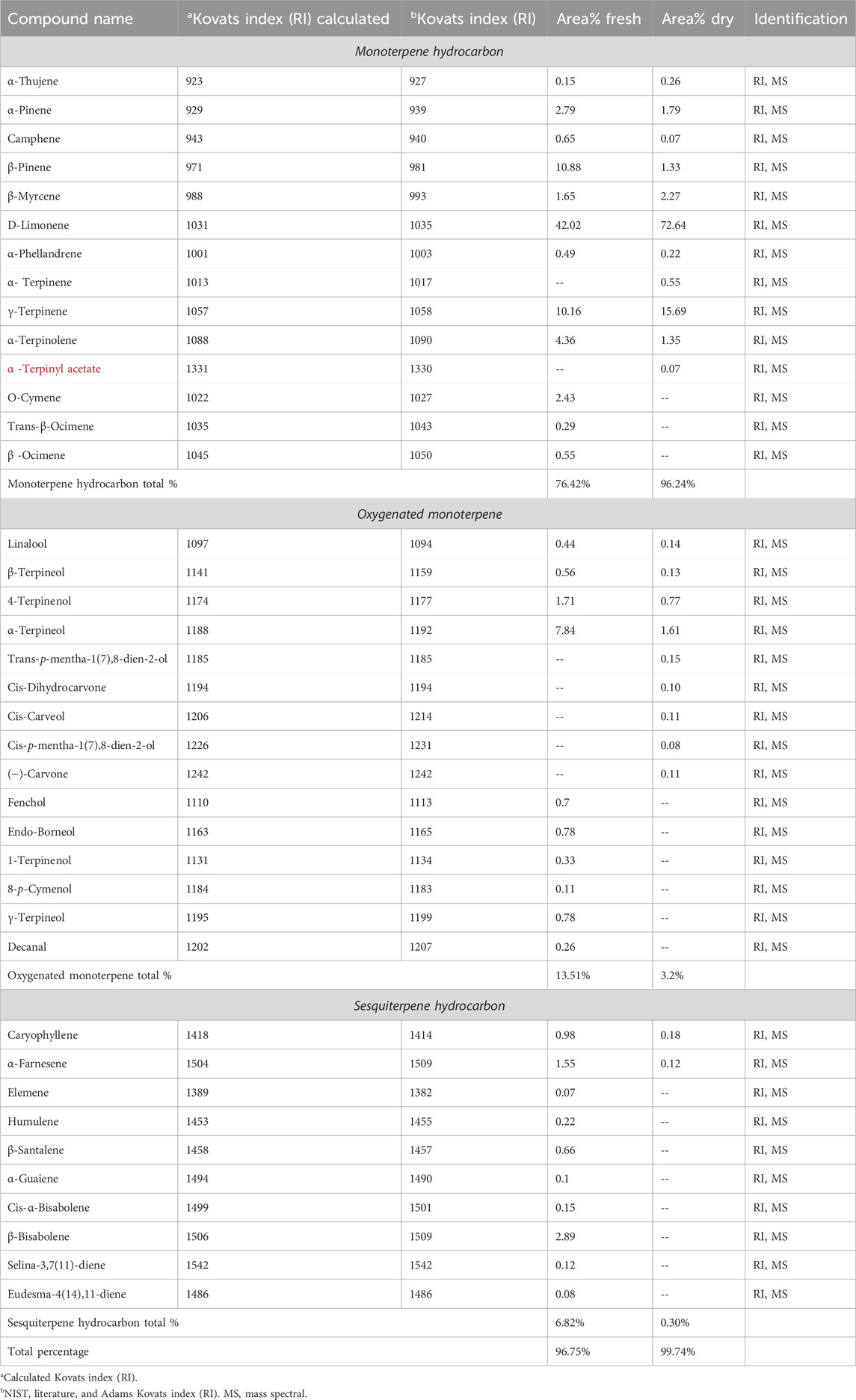
Table 1. Secondary metabolites of fresh and dry key lime’s essential oils are identified by GC-MS analysis.
Several studies reported that various naturally occurring compounds, such as terpenes in essential oils, have anti-aging activity because of their antioxidant potentials (Cavinato et al., 2017), demonstrating that terpenes are capable of capturing free radicals and inhibiting UVB-induced photoaging (González-Burgos et al., 2012). Mohamed et al. stated that limonene exhibited antioxidant potential because of its ability to capture free radicals and has the ability to improve the effects of oxidative stress in vitro and in vivo (Mohammed et al., 2022). Furthermore, it was reported by Garg et al. that limonene exhibited high anti-inflammatory activity (Garg, 2017).
2.2 In vitro antiaging activity of fresh and dry Citrus aurantifolia essential oil
The antiaging capability of both C. aurantifolia essential oils was investigated in vitro. The findings showed promising activity of both essential oils towards collagenase and elastase enzymes and good antioxidant activity. Various quantities of the essential oils were tested and demonstrated dose-dependent inhibitory action against two enzymes (Table 2).
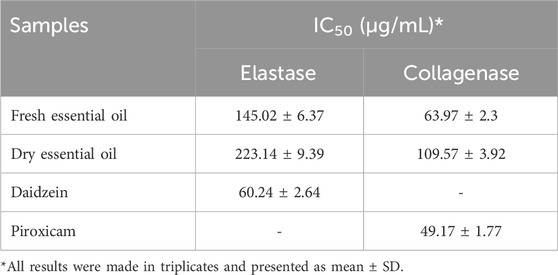
Table 2. IC50 values (µg/mL) for the anti-elastase and anti-collagenase activities for fresh and dry key lime essential oils.
Fresh key lime essential oil showed substantial anti-elastase and anti-collagenase potentials, IC50 = 145.02 ± 6.37 and 63.97 ± 2.3 µg/mL, respectively, close to that of the standard anti-aging drug with IC50 = 60.24 ± 2.64 and 49.17 ± 1.77 µg/mL, respectively as well as good antioxidant activity 37.76 ± 0.80 µM TE/g. Fresh key lime essential oil exhibits superior anti-elastase and anti-collagenase activities due to its higher content of bioactive oxygenated monoterpenes and sesquiterpenes. Key compounds like α-terpineol and 4-terpinenol enhance its antioxidant and anti-inflammatory effects, helping to protect collagen and elastin from degradation (Kim S. H. et al., 2013).
In this study, the compositional variations between fresh and dry key lime essential oils have important implications for their recognized anti-aging activity, especially since fresh oil contains more oxygenated monoterpenes and sesquiterpene hydrocarbons. According to Elgamal et al. sesquiterpenes have an important function as anti-oxidants, anti-inflammatory agents, and hence anti-aging properties (Elgamal et al., 2021).
While drying boosted D-limonene, the loss of oxygenated and sesquiterpene compounds likely lowers the oil’s efficiency in countering oxidative stress, inflammation, and collagen degradation, all of which contribute to skin ageing. As a result, the fresh key lime essential oil, with its more diversified bioactive composition, is predictable to have more anti-aging potential than the dry oil, making it a better choice for cosmetic and dermatological applications.
2.3 Antioxidant 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid (ABTS) assay
The scavenging capacity of both key lime essential oils was also evaluated using ABTS radical cation. The data suggests that the ABTS scavenging activity varied significantly between the essential oils. The ABTS radical scavenging activity of fresh key lime essential oil (37.76 ± 0.80 µM TE/g) was greater than that of dried key lime essential oil (27.76 ± 1.11 µM TE/g). However, when compared to quercetin (48.61 ± 2.00 μM TE/g), a well-known flavonoid with potent antioxidant properties, both fresh and dry key lime oils exhibit moderate antioxidant potential but remain lower in efficacy, which aligns with the expected antioxidant contributions of essential oils versus polyphenolic compounds (Table 3).
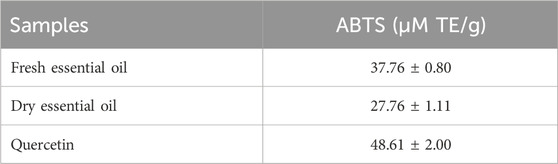
Table 3. ABTS radical scavenging potential of fresh and dry key lime essential oils expressed as Trolox Equivalent Antioxidant Capacity (µM Trolox equivalent (TE)/g).
According to Gargouri et al., Citrus paradisi essential oil has high radical scavenging activity against ABTS free radicals. Our findings (37.76 μM TE/g for fresh key lime oil) are consistent with earlier studies indicating the antioxidant activity of citrus essential oils (Gargouri et al., 2021). Furthermore, Tundris et al. investigated the antioxidant activity of three citrus oils and found that they demonstrated a strong radical scavenging capacity. These findings highlight the potential of citrus essential oils as natural antioxidants, with C. aurantifolia essential oil exhibiting the highest activity among the studied varieties (Tundis et al., 2012).
The detected metabolites of key lime essential oils were stated to exhibit considerable pharmacological activity that is valuable in the skin care industry. Their antiaging and antioxidant properties were observed. These observed positive activities prompted us to investigate the anti-aging potential of both key limes’ essential oils using several in vitro and molecular docking studies. This activity may be ascribed to the percentage of α-Pinene and β-Pinene 2.79% and 10.88% in fresh key lime compared to dry key lime. Fraternale et al. reported that limonene and α-pinene, tested alone and in mixture, exhibited repressive activity against elastase and collagenase enzymes (Fraternale et al., 2019). Elgamal et al. reported that anti-aging effectiveness was directly related to antioxidant potential. The high percentage of sesquiterpene and oxygenated monoterpenes in fresh key lime essential oil, representing 6.82% and 13.51%, respectively, played an important role as an antioxidant agent and, therefore anti-aging (Elgamal et al., 2021). These reports determined that an increase in the free radical scavenging compounds in fresh key lime essential oil caused a rise in the anti-aging potential. All results concluded with the synergetic effect of the compounds of the essential oil. This fact about the role of synergetic action was extremely obvious in our data, where the fresh key lime essential oil demonstrated improved activity compared to dry key lime essential oil.
2.4 In silico studies
2.4.1 Molecular docking
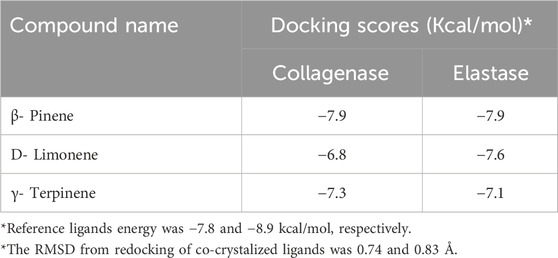
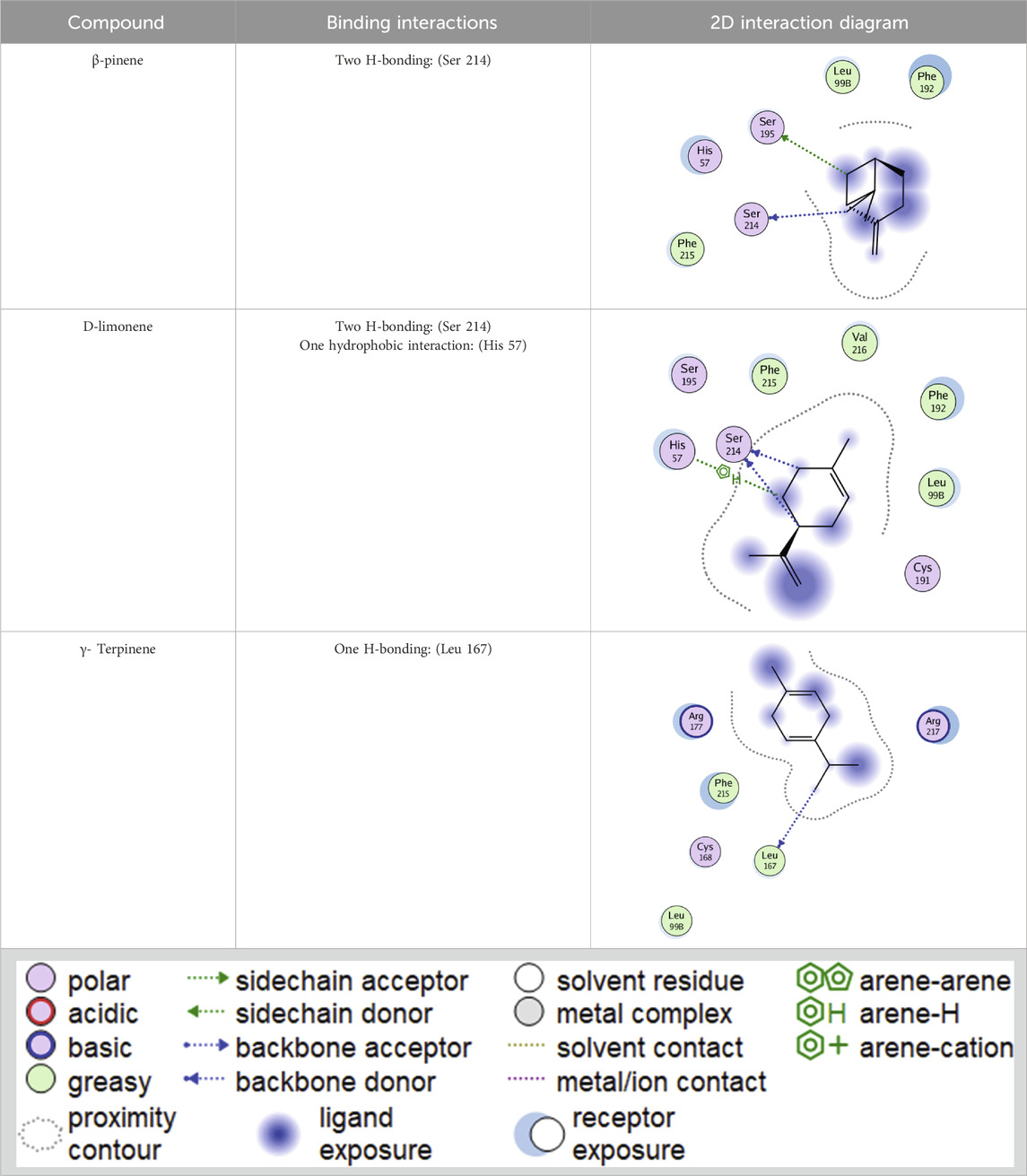
The essential oil’s promising inhibitory effect against collagenase and elastase prompted molecular docking research. Docking studies are carried out to determine the likely binding mechanism and pattern of drugs with potential targets. As previously stated, the major chemicals we found have been shown to have antiaging effects in various trials. Computational drug design research has shed light on the relevance of employing molecular docking in drug development to acquire important insights into molecules’ interactions and possible binding processes in protein binding sites (Singab et al., 2023). The key lime main metabolites were docked to the active sites of collagenase and elastase, and their binding modes, interaction energies, and 2D interaction diagrams are shown in Tables 4–6. The compounds’ interaction energies were in the range of −(6.8–7.9 and 7.1–7.9) Kcal/mol. The β- pinene exhibited the best docking scores with collagenase and elastase enzymes.
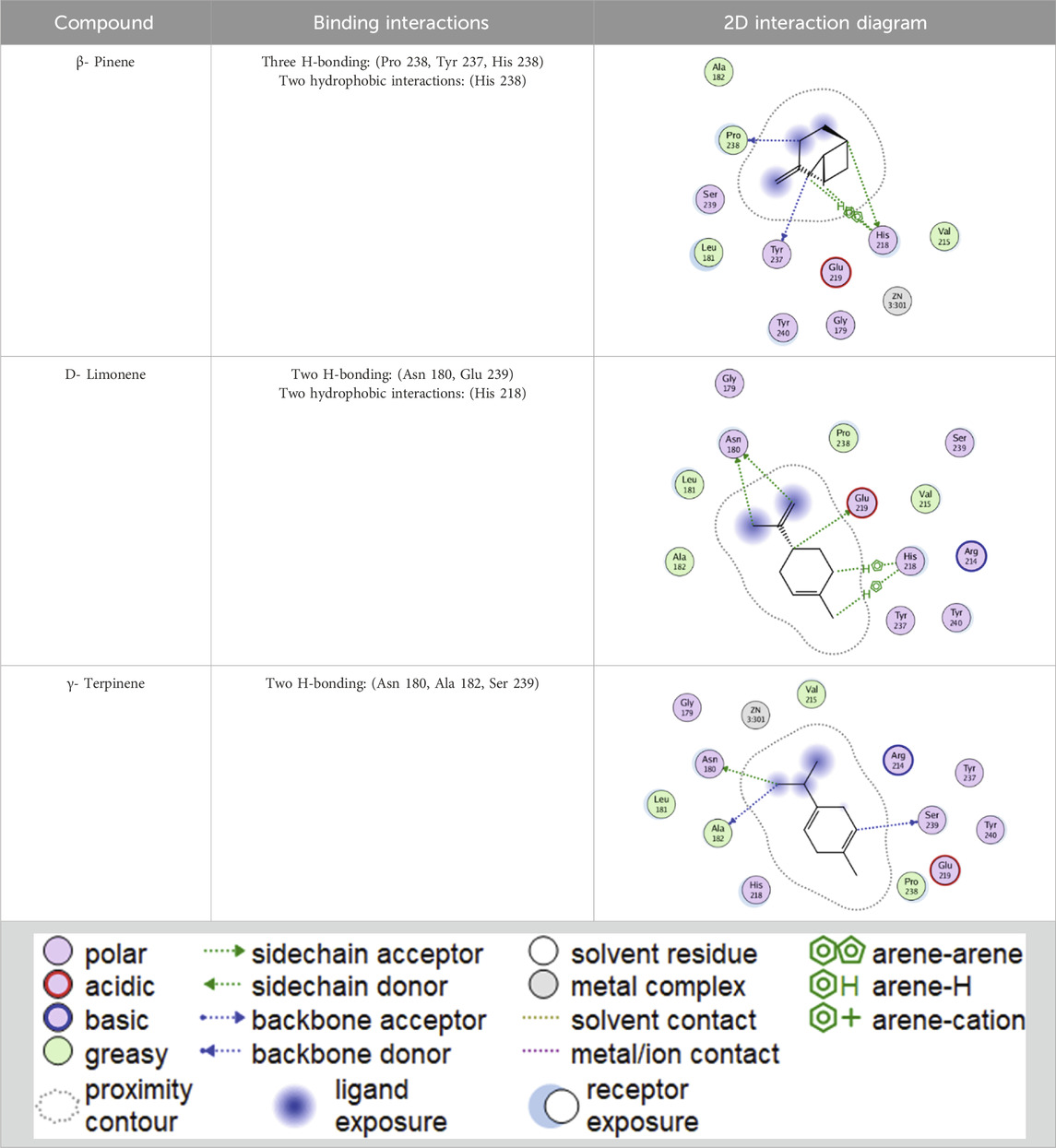
Table 6. 2D interaction diagrams and binding modes of the key lime major metabolite towards collagenase.
2.4.2 Molecular dynamics simulation (MDS)
Molecular dynamics (MD) simulations have offered significant insights into how drugs interact with their targets. These include precise assessments of ligand-target binding affinities, exploration of macromolecular behavior, and evaluation of how specific mutations influence drug resistance profiles. Within this framework, the complexes formed between B-Pinene and the enzymes collagenase and elastase were selected for MD simulation studies.
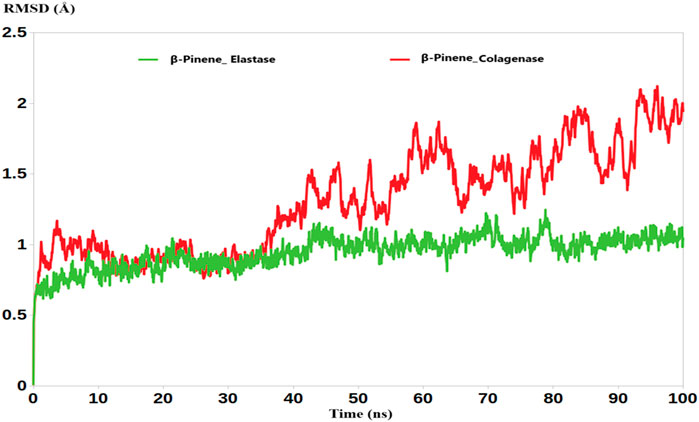
Interestingly, the molecular dynamics (MD) simulations revealed that the average root mean square deviation (RMSD) values for the complexes of B-Pinene with collagenase and elastase were 1.91 Å and 1.34 Å, respectively (Figure 1). These relatively low RMSD values indicate minimal structural fluctuations of the protein-ligand complexes over the simulation time, suggesting that B-Pinene maintains a stable binding conformation within the active sites of both enzymes.
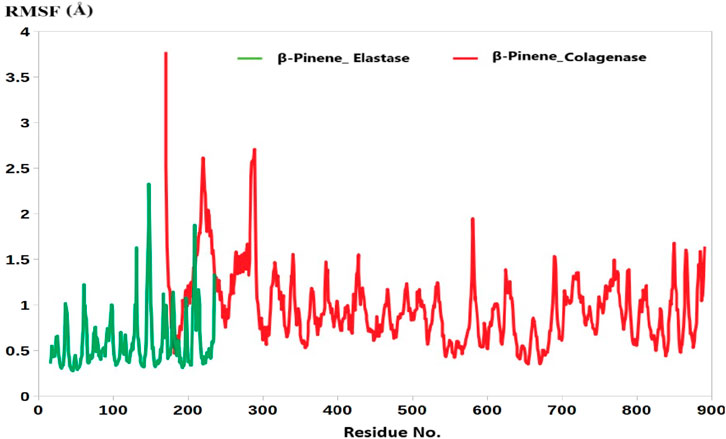
In addition, the root mean square fluctuation (RMSF) analysis further supports this observation. The average RMSF value for the collagenase-B-Pinene complex was calculated at 1.77 Å, while that of the elastase-B-Pinene complex was slightly lower at 1.17 Å (Figure 2). RMSF measures the flexibility of individual amino acid residues throughout the simulation, and the low values observed here indicate that the residues in the binding regions of both enzymes remain relatively stable and are not highly flexible upon ligand binding.
Collectively, these results highlight the potential of B-Pinene to form stable and consistent interactions with both collagenase and elastase. The stable RMSD and RMSF profiles reinforce the reliability of our initial molecular docking results, confirming that the predicted binding poses are not only energetically favorable but also dynamically stable over time. These findings provide strong evidence supporting the use of B-Pinene as a potential inhibitor of these enzymes and validate the robustness of our computational docking and simulation workflow.
3 Conclusion
Finally, this study emphasizes the differences in chemical compositions between fresh and dry key lime (Citrus aurantifolia) essential oils, emphasizing the greater chemical complexity of fresh oil. Fresh oil contains more metabolites, notably oxygenated monoterpenes like α-terpineol and 4-terpinenol, indicating better bioactivity, especially for anti-aging uses. The found anti-elastase and anti-collagenase actions, as validated by molecular docking and in vitro experiments, suggest that fresh key lime oil has potential as a natural cosmetic product. However, because these findings are based on in vitro and in silico techniques, additional in vivo research and clinical validation are required to validate efficacy and safety. Future research should investigate the stability, formulation potential, and long-term production of fresh vs. dry essential oils for industrial uses, ensuring their viability in the skincare and pharmaceutical industries.
4 Methods
4.1 Plant material
From the Egyptian market in Cairo, fifth settlement, fresh Citrus aurantifolia were collected (July, 2024) and authenticated by Dr. Therese Labib Yousef, Director of Herbarium, Consultant of botanical gardens and National Gene Bank. Voucher number (L20-12) was preserved at Pharmacognosy department, Future University in Egypt, Faculty of pharmacy.
4.2 Essential oil isolation
Three kilograms of fruit were washed using flowing water and then toweled to eliminate any surface dust. The clean fruits were divided into two-halves. The first half of key lime was subjected to shade drying for 3 weeks till complete drying (same weight for 3 consecutive days) (temperature range 30°C–36°C with 25%–40% humidity, good airflow and frequent rotation to avoid mold growth). Just before hydro-distillation, fresh and dry fruits were grounded for 4 h (Ngo et al., 2020; Veloso et al., 2020) in a Clevenger apparatus to separate the essential oils. Over anhydrous Na2SO4, the oils were dried and kept in a shaded brown glass container in the refrigerator (4°C) till analysis. Based on the mentioned equation, the yield was calculated:
4.3 Gas chromatography–mass spectrometer analysis
Shimadzu Gas chromatography–Mass spectrometer -QP2010 (Tokyo, Japan), Rtx-5MS column (30 m × 0.25 mm i.d. × 0.25 µm film thickness) and a split–splitless injector as per previous procedures (Elkhawas et al., 2023). Isothermally, the starting temperature of the column was 45°C for 2 min, then programmed to 300°C with a rate of 5°C/min. Helium was used as a carrier gas with a flow rate of 1.41 mL/min and an injection volume 1 μL was employed in split mode at a split ratio of 1:15. The ionization voltage was 60 mA of 70 eV, and the ion source was 200°C. Compounds were identified based on their Kovats index (KI) and cross-referenced with NIST, Adams, and relevant literature data.
4.4 In Vitro anti-ageing evaluation
4.4.1 Anti-collagenase analyse
The anti-collagenase test was achieved colorimetric (ab 196999) as per the procedure stated by (Thring et al., 2009; Imran et al., 2023) with minimal changes. Collagenase from Clostridium histolyticum (ChC—EC.3.4.23.3) was dissolved in the 50 mM Tricine buffer to 0.8 unit/mL initial concentration. N-[3-(2-furyl) acryloyl]-Leu–Gly–Pro–Ala (FALGPA) was used as a substrate and dissolved in Tricine buffer to a final concentration of 2 mM. The absorbance values were measured at 490 nm. A positive control (piroxicam) was used.
4.4.2 Anti-elastase assay
The anti-elastase test was achieved spectrophotometrically as previously described by Kim et al. (Imran et al., 2023; Kim et al., 2004) with minimal changes. Porcine pancreatic elastase was dissolved in sterile water to prepare a stock solution with a concentration of 3.33 mg/mL. N-Succinyl-Ala–Ala–Ala–p-nitroanilide (AAAPVN) was used as a substrate and dissolved in Tris-HCl buffer at a concentration of 1.6 mM. Daidzein was used as positive control. The absorbance was measured at 400 nm.
4.4.3 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) assay
The assay was performed as previously reported by Floegel et al. (2011). The ABTS capacity of both oils was calculated as Trolox equivalent antioxidant capacity (TEAC) and expressed as µM TE/g.
4.5 Statistical analysis
The assays were performed in triplicates, and the values are presented as mean ± SD. For in vitro anti-collagenase, and anti-elastase activities, the (IC50) was predicted from the graph plots of the dose–response curves at each sample concentration by Graph Pad Prism software (San Diego, CA, United States) and statistical analysis was carried out using one way ANOVA.
4.6 In silico studies
4.6.1 Molecular docking
Molecular docking of the major metabolites of key lime was accomplished on the crystal structure of collagenase and elastase enzymes, recovered as co-crystallized with a ligand from the protein data bank (PDB ID: 1W3Y, and 1b0f). Docking exploration was conducted using Accelrys Inc.’s Discovery Studio 4.5 software in San Diego, California, United States. Using the default program protocol, ligands and enzymes were initially created. The active binding site of collagenase and elastase enzymes was then determined by analyzing the binding mode of the co-crystallized reported ligand active conformer. The structures of the compounds under consideration were docked into the enzyme’s active binding site pocket using the C-Docker technique. The program provided 2D binding diagrams and C-Docker binding energies for evaluation (Elkhawas et al., 2023; El-Nashar et al., 2023; Ramlal et al., 2024).
4.6.2 Molecular dynamics simulation (MDS)
The two molecular dynamics of B-Pinene in complex with collagenase and elastase were performed using Gromacs and Acypype. The typical workflow of the reported methodology was applied for 100 ns of molecular simulation (Elgohary et al., 2025; El Hassab et al., 2025; Majrashi et al., 2024).
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
YE: Conceptualization, Methodology, Data curation, Investigation, Validation, Writing – original draft. ME: Methodology, Resources, Software, Visualization, Writing – original draft. OA: Investigation, Methodology, Writing – original draft. AN: Data curation, Investigation, Writing – original draft. TM: Methodology, Visualization, Software, Writing – original draft. WE: Project administration, Validation, Writing – review and editing. NM: Conceptualization, Validation, Data curation, Methodology, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The authors extend their appreciation to the Deanship of Research and Graduate Studies at King Khalid University for funding this work through Large Research Project under grant number (RGP2/500/46).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The reviewer ME declared a shared affiliation with the author WE at the time of review.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Anandakumar, P., Kamaraj, S., and Vanitha, M. K. (2021). D-limonene: a multifunctional compound with potent therapeutic effects. J. Food Biochem. 45 (1), e13566. doi:10.1111/jfbc.13566
Be Tu, P. T., and Tawata, S. (2015). Anti-oxidant, anti-aging, and anti-melanogenic properties of the essential oils from two varieties of Alpinia zerumbet. Molecules 20 (9), 16723–16740. doi:10.3390/molecules200916723
Boshtam, M., Moshtaghian, J., Naderi, G., Asgary, S., and Nayeri, H. (2011). Antioxidant effects of Citrus aurantifolia (Christm) juice and peel extract on LDL oxidation. J. Res. Med. Sci. official J. Isfahan Univ. Med. Sci. 16 (7), 951–955.
Boussaada, O., Skoula, M., Kokkalou, E., and Chemli, R. (2007). Chemical variability of flowers, leaves, and peels oils of four sour orange provenances. J. Essent. Oil-Bear. Plants 10 (6), 453–464. doi:10.1080/0972060x.2007.10643579
Cavinato, M., Waltenberger, B., Baraldo, G., Grade, C. V. C., Stuppner, H., and Jansen-Dürr, P. (2017). Plant extracts and natural compounds used against UVB-induced photoaging. Biogerontology 18 (4), 499–516. doi:10.1007/s10522-017-9715-7
Chen, Y., Zhang, L.-L., Wang, W., and Wang, G. (2023). Recent updates on bioactive properties of α-terpineol. J. Essent. Oil Res. 35 (3), 274–288. doi:10.1080/10412905.2023.2196515
Delgado-Adámez, J., Garrido, M., Bote, M. E., Fuentes-Pérez, M. C., Espino, J., and Martín-Vertedor, D. (2017). Chemical composition and bioactivity of essential oils from flower and fruit of Thymbra capitata and Thymus species. J. Food Sci. Technol. 54 (7), 1857–1865. doi:10.1007/s13197-017-2617-5
Elgamal, A. M., Ahmed, R. F., Abd-ElGawad, A. M., El Gendy, A. E.-N. G., Elshamy, A. I., and Nassar, M. I. (2021). Chemical profiles, anticancer, and anti-aging activities of essential oils of pluchea dioscoridis (L.) DC. And Erigeron bonariensis L plants.
Elgohary, M. K., Elkotamy, M. S., Alkabbani, M. A., El Hassab, M. A., Al-Rashood, S. T., Binjubair, F. A., et al. (2025). Sulfonamide-Pyrazole derivatives as next-generation Cyclooxygenase-2 enzyme inhibitors: from molecular design to in vivo efficacy. Int. J. Biol. Macromol. 293, 139170. doi:10.1016/j.ijbiomac.2024.139170
El Hassab, M. A., Eldehna, W. M., Hassan, G. S., and Abou-Seri, S. M. (2025). Multi-stage structure-based virtual screening approach combining 3D pharmacophore, docking and molecular dynamic simulation towards the identification of potential selective PARP-1 inhibitors. BMC Chem. 19 (1), 30. doi:10.1186/s13065-025-01389-2
Elkhawas, Y. A., Gad, H. A., Lashkar, M. O., Khinkar, R. M., Wani, M. Y., and Khalil, N. (2022). Effect of sun drying on phytoconstituents and antiviral activity of ginger against low-pathogenic human coronavirus. Agronomy 12 (11), 2763. doi:10.3390/agronomy12112763
Elkhawas, Y. A., Seleem, M., Shabayek, M. I., Majrashi, T. A., Al-Warhi, T., Eldehna, W. M., et al. (2023). Phytochemical profiling and mechanistic evaluation of black garlic extract on multiple sclerosis rat model. J. Funct. Foods 111, 105900. doi:10.1016/j.jff.2023.105900
El-Nashar, H. A. S., Mostafa, N. M., Abd El-Ghffar, E. A., Eldahshan, O. A., and Singab, A. N. B. (2022). The genus Schinus (Anacardiaceae): a review on phytochemicals and biological aspects. Nat. Prod. Res. 36 (18), 4839–4857. doi:10.1080/14786419.2021.2012772
El-Nashar, H. A. S., Sayed, A. M., El-Sherief, H. A. M., Rateb, M. E., Akil, L., Khadra, I., et al. (2023). Metabolomic profile, anti-trypanosomal potential and molecular docking studies of Thunbergia grandifolia. J. Enzyme Inhib. Med. Chem. 38 (1), 2199950. doi:10.1080/14756366.2023.2199950
Eun, C.-H., Kang, M.-S., and Kim, I.-J. (2020). Elastase/collagenase inhibition compositions of Citrus unshiu and its association with phenolic content and anti-oxidant activity. Appl. Sci. 10 (14), 4838. doi:10.3390/app10144838
Floegel, A., Kim, D.-O., Chung, S.-J., Koo, S. I., and Chun, O. K. (2011). Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 24 (7), 1043–1048. doi:10.1016/j.jfca.2011.01.008
Fraternale, D., Flamini, G., and Ascrizzi, R. (2019). In vitro anticollagenase and antielastase activities of essential oil of Helichrysum italicum subsp. italicum (roth) G. Don. J. Med. Food 22 (10), 1041–1046. doi:10.1089/jmf.2019.0054
Garg, C. (2017). Molecular mechanisms of skin photoaging and plant inhibitors. IJGP 11 (02). doi:10.22377/ijgp.v11i02.1031
Gargouri, B., Amor, I. B., Messaoud, E. B., Elaguel, A., Bayoudh, A., Kalle, I., et al. (2021). Antioxidant capacity and antitumoral activity of Citrus paradisi essential oil. BJSTR 40 (2), 32121–32141. doi:10.26717/bjstr.2021.40.006434
Gioffrè, G., Ursino, D., Labate, M. L. C., and Giuffrè, A. M. (2020). The peel essential oil composition of bergamot fruit (Citrus bergamia, Risso) of Reggio Calabria (Italy): a review. Emir. J. Food Agric. 32 (11), 835–845. doi:10.9755/ejfa.2020.v32.i11.2197
González-Burgos, E., and Gómez-Serranillos, M. P. (2012). Terpene compounds in nature: a review of their potential antioxidant activity. Curr. Med. Chem. 19 (31), 5319–5341. doi:10.2174/092986712803833335
González-Mas, M. C., Rambla, J. L., López-Gresa, M. P., Blázquez, M. A., and Granell, A. (2019). Volatile compounds in citrus essential oils: a comprehensive review. Front. Plant Sci. 10, 12. doi:10.3389/fpls.2019.00012
Hong, J. H., Khan, N., Jamila, N., Hong, Y. S., Nho, E. Y., Choi, J. Y., et al. (2017). Determination of volatile flavour profiles of citrus spp. fruits by SDE-GC-MS and enantiomeric composition of chiral compounds by MDGC-MS. Phytochem. Anal. PCA 28 (5), 392–403. doi:10.1002/pca.2686
Imran, M., Iqbal, A., Badshah, S. L., Sher, A. A., Ullah, H., Ayaz, M., et al. (2023). Chemical and nutritional profiling of the seaweed dictyota dichotoma and evaluation of its antioxidant, antimicrobial and hypoglycemic potentials. Mar. Drugs 21 (5), 273. doi:10.3390/md21050273
Jain, S., Arora, P., and Popli, H. (2020). A comprehensive review on essential oil: its phyto Citrus aurantifoliachemistry and pharmacological aspects. Braz. J. Nat. Sci. 3 (2), 354. doi:10.31415/bjns.v3i2.101
Kim, B., and Bae, S. (2023). Evaluation of citrus junos essential oil as a promising anti-aging ingredient: in vitro and clinical studies on skin health improvement. Asian J. Beauty Cosmetol. 21, 465–479. doi:10.20402/ajbc.2023.0070
Kim, S. H., Lee, S. Y., Hong, C. Y., Gwak, K. S., Park, M. J., Smith, D., et al. (2013b). Whitening and antioxidant activities of bornyl acetate and nezukol fractionated from Ryptomeria japonica essential oil. Int. J. Cosmet. Sci. 35 (5), 484–490. doi:10.1111/ics.12069
Kim, Y.-J., Uyama, H., and Kobayashi, S. (2004). Inhibition effects of (+)-catechin–aldehyde polycondensates on proteinases causing proteolytic degradation of extracellular matrix. Biochem. Biophys. Res. Commun. 320 (1), 256–261. doi:10.1016/j.bbrc.2004.05.163
Kim, Y. W., Kim, M. J., Chung, B. Y., Bang, D. Y., Lim, S. K., Choi, S. M., et al. (2013a). Safety evaluation and risk assessment of d-limonene. J. Toxicol. Environ. Health B Crit. Rev. 16 (1), 17–38. doi:10.1080/10937404.2013.769418
Kumar, K. J. S., Vani, M. G., and Wang, S.-Y. (2022). Limonene protects human skin keratinocytes against UVB-induced photodamage and photoaging by activating the Nrf2-dependent antioxidant defense system. Environ. Toxicol. 37 (12), 2897–2909. doi:10.1002/tox.23646
Lago, J. C., and Puzzi, M. B. (2019). The effect of aging in primary human dermal fibroblasts. PloS one 14 (7), e0219165. doi:10.1371/journal.pone.0219165
Lemes, R. S., Alves, C. C. F., Estevam, E. B. B., Santiago, M. B., Martins, C. H. G., Santos, T. C. L. D. O. S., et al. (2018). Chemical composition and antibacterial activity of essential oils from Citrus aurantifolia leaves and fruit peel against oral pathogenic bacteria. An. Acad. Bras. Ciênc. 90, 1285–1292. doi:10.1590/0001-3765201820170847
Lin, L.-Y., Chuang, C.-H., Chen, H.-C., and Yang, K.-M. (2019). Lime (Citrus aurantifolia (christm.) swingle) essential oils: volatile compounds, antioxidant capacity, and hypolipidemic effect. Foods 8 (9), 398. doi:10.3390/foods8090398
Liu, S., Li, S., and Ho, C.-T. (2022). Dietary bioactives and essential oils of lemon and lime fruits. Food Sci. Hum. Well 11 (4), 753–764. doi:10.1016/j.fshw.2022.03.001
Lota, M.-L., de Rocca Serra, D., Tomi, F., Jacquemond, C., and Casanova, J. (2002). Volatile components of peel and leaf oils of lemon and lime species. J. Agric. Food Chem. 50 (4), 796–805. doi:10.1021/jf010924l
Mahato, N., Sharma, K., Sinha, M., and Cho, M. H. (2018). Citrus waste derived nutra-/pharmaceuticals for health benefits: current trends and future perspectives. J. Funct. Foods 40, 307–316. doi:10.1016/j.jff.2017.11.015
Majrashi, T. A., El Hassab, M. A., Amin, M. K. A. H., Elkaeed, E. B., Shaldam, M. A., Al-Karmalawy, A. A., et al. (2024). Multistep structure-based virtual screening approach toward the identification of potential potent SARS-CoV-2 Mpro inhibitors. J. Biomol. Struct. and Dyn., 1–10. doi:10.1080/07391102.2024.2427375
Maria, G. A., and Riccardo, N. (2020). Citrus bergamia, Risso: the peel, the juice and the seed oil of the bergamot fruit of Reggio Calabria (South Italy). Emir. J. Food Agric. 32 (7), 522–532. doi:10.9755/ejfa.2020.v32.i7.2128
Mohammed, M. S. O., Babeanu, N., Cornea, C. P., and Radu, N. (2022). Limonene-a biomolecule with potential applications in regenerative medicine. JMPB 26 (2).
Naeini, A. R., Nazeri, M., and Shokri, H. (2018). Inhibitory effect of plant essential oils on Malassezia strains from Iranian dermatitis patients. J. Herbmed Pharmacol. 7 (1), 18–21. doi:10.15171/jhp.2018.04
Neves, J., and Sousa-Victor, P. (2020). Regulation of inflammation as an anti-aging intervention. FEBS J. 287 (1), 43–52. doi:10.1111/febs.15061
Ngo, T. C. Q., Tran, T. K. N., Dao, T. P., Tran, T. H., Ngo, H. D., Huynh, X. P., et al. (2020). Yield and composition analysis of Vietnamese lemon (citrus aurantifolia) essential oils obtained from hydrodistillation and microwave-assisted hydrodistillation. IOP Conf. Ser. Mater. Sci. Eng. 991 (1), 012123. doi:10.1088/1757-899x/991/1/012123
Oulebsir, C., Mefti-Korteby, H., Djazouli, Z.-E., Zebib, B., and Merah, O. (2022). Essential oil of Citrus aurantium L. Leaves: composition, antioxidant activity, elastase and collagenase inhibition. Agronomy 12 (6), 1466. doi:10.3390/agronomy12061466
Patil, J. R., Chidambara Murthy, K. N., Jayaprakasha, G. K., Chetti, M. B., and Patil, B. S. (2009). Bioactive compounds from Mexican lime (Citrus aurantifolia) juice induce apoptosis in human pancreatic cells. J. Agric. Food Chem. 57 (22), 10933–10942. doi:10.1021/jf901718u
Prommaban, A., and Chaiyana, W. (2022). Microemulsion of essential oils from citrus peels and leaves with anti-aging, whitening, and irritation reducing capacity. J. Drug Deliv. Sci. Tech. 69, 103188. doi:10.1016/j.jddst.2022.103188
Rabie, O., El-Nashar, H. A. S., Majrashi, T. A., Al-Warhi, T., El Hassab, M. A., Eldehna, W. M., et al. (2023). Chemical composition, seasonal variation and antiaging activities of essential oil from Callistemon subulatus leaves growing in Egypt. J. Enzyme Inhib. Med. Chem. 38 (1), 2224944. doi:10.1080/14756366.2023.2224944
Ramlal, A., Khari, M., Jakhar, P., Fawzy, I. M., Sogan, N., Liu, X., et al. (2024). In silico analysis of soybean phytocompounds against Plasmodium falciparum. J. Herb. Med. 46, 100888. doi:10.1016/j.hermed.2024.100888
Secerli, J., Erdem, O., and Bacanlı, M. (2023). Antiaging effects of limonene in ageing-induced HaCaT cells. Genet. Appl. 7 (1). doi:10.31383/ga.vol7iss1ga04
Shanbhag, S., Nayak, A., Narayan, R., and Nayak, U. Y. (2019). Anti-aging and sunscreens: paradigm shift in cosmetics. Adv. Pharm. Bull. 9 (3), 348–359. doi:10.15171/apb.2019.042
Sharmeen, J. B., Mahomoodally, F. M., Zengin, G., and Maggi, F. (2021). Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules 26 (3), 666. doi:10.3390/molecules26030666
Singab, A. N. B., Elkhawas, Y. A., Al-Sayed, E., Elissawy, A. M., Fawzy, I. M., and Mostafa, N. M. (2023). Antimicrobial activities of metabolites isolated from endophytic Aspergillus flavus of Sarcophyton ehrenbergi supported by in-silico study and NMR spectroscopy. Fungal Biol. Biotechnol. 10 (1), 16. doi:10.1186/s40694-023-00161-2
Spadaro, F., Costa, R., Circosta, C., and Occhiuto, F. (2012). Volatile composition and biological activity of key lime Citrus aurantifolia essential oil. Nat. Prod. Commun. 7 (11), 1523–1526. doi:10.1177/1934578x1200701128
Sreepian, A., Popruk, S., Nutalai, D., Phutthanu, C., and Sreepian, P. M. (2022). Antibacterial activities and synergistic interaction of citrus essential oils and limonene with gentamicin against clinically isolated methicillin-resistant Staphylococcus aureus. Sci. World J. 2022, 1–12. doi:10.1155/2022/8418287
Sulzbach, M., Silva, M. A. S. D., Gonzatto, M. P., Marques, M. M. O., Böettcher, G. N., Silvestre, W. P., et al. (2021). Effect of distillation methods on the leaf essential oil of some Citrus cultivars. J. Essent. Oil Res. 33 (5), 452–463. doi:10.1080/10412905.2021.1936666
Tavallali, H., Bahmanzadegan, A., Rowshan, V., and Tavallali, V. (2021). Essential oil composition, antioxidant activity, phenolic compounds, total phenolic and flavonoid contents from pomace of Citrus aurantifolia. JMPB 10 (Special), 103–116. doi:10.22092/jmpb.2020.341476.1175
Thring, T. S., Hili, P., and Naughton, D. P. (2009). Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 9, 27–11. doi:10.1186/1472-6882-9-27
Tundis, R., Loizzo, M. R., Bonesi, M., Menichini, F., Mastellone, V., Colica, C., et al. (2012). Comparative study on the antioxidant capacity and cholinesterase inhibitory activity of Citrus aurantifolia swingle, C. aurantium L., and C. bergamia risso and poit. Peel essential oils. J. Food Sci. 77 (1), H40–H46. doi:10.1111/j.1750-3841.2011.02511.x
Vekiari, S. A., Protopapadakis, E. E., Papadopoulou, P., Papanicolaou, D., Panou, C., and Vamvakias, M. (2002). Composition and seasonal variation of the essential oil from leaves and peel of a Cretan lemon variety. J. Agric. Food Chem. 50 (1), 147–153. doi:10.1021/jf001369a
Keywords: 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), antiaging, anticollagenase, anti-elastase, Citrus aurantifolia, GC/MS, key lime
Citation: Elkhawas YA, El Hassab MA, Al kamaly O, Negmeldin AT, Majrashi TA, Eldehna WM and Mostafa NM (2025) Citrus aurantifolia essential oil: antiaging potential through integrated in vitro and in silico studies. Front. Chem. 13:1577871. doi: 10.3389/fchem.2025.1577871
Received: 16 February 2025; Accepted: 21 July 2025;
Published: 01 August 2025.
Edited by:
Rongrui Wei, Wuhan Polytechnic University, ChinaReviewed by:
Mohammed Abu El-Magd, Kafrelsheikh University, EgyptFevzi Topal, Gumushane University, Türkiye
Ahmed A. El-Husseiny, Al Azhar University, Egypt
Copyright © 2025 Elkhawas, El Hassab, Al kamaly, Negmeldin, Majrashi, Eldehna and Mostafa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wagdy M. Eldehna, d2FnZHkyMDAwQGdtYWlsLmNvbQ==; Ahmed T. Negmeldin, ZHIuYWhtZWR0aGFiZXRAZ211LmFjLmFl; Yasmin A. Elkhawas, eWFzbWllbi5hbGFhQGZ1ZS5lZHUuZWc=; Nada M. Mostafa, bmFkYW1vc3RhZmFAcGhhcm1hLmFzdS5lZHUuZWc=
 Yasmin A. Elkhawas
Yasmin A. Elkhawas Mahmoud A. El Hassab
Mahmoud A. El Hassab Omkulthom Al kamaly
Omkulthom Al kamaly Ahmed T. Negmeldin
Ahmed T. Negmeldin Taghreed A. Majrashi6
Taghreed A. Majrashi6 Wagdy M. Eldehna
Wagdy M. Eldehna Nada M. Mostafa
Nada M. Mostafa