Abstract
Supercapacitors (SCs) are high-performance electrochemical energy storage devices, and their performance hinges on the electrode materials. 2D MXene nanomaterials, with their excellent conductivity, tunable interlayer spacing, and rich surface chemistry, have emerged as highly promising electrode materials for SCs. However, the capacitive performance of intrinsic MXene fails to meet application requirements. This review first introduces the composition and principles of SCs in detail, then summarizes the pure MXene nanomaterials in SCs, and systematically explores the regulatory mechanisms of vacancy doping strategies on MXene material structure and capacitive performance. The study reveals the structure-property relationships, providing theoretical basis and direction for designing high-performance MXene-based SCs electrode materials.
1 Introduction
With their outstanding power density and fast charging and discharging capabilities, supercapacitors (SCs) have become important energy storage components in key areas such as electric vehicles (regenerative braking systems), renewable energy grid connection (power fluctuation smoothing), smart grids (fast frequency response), and wearable/flexible electronic devices. As a new type of energy storage device that bridges the gap between traditional capacitors and batteries (Janardhanan et al., 2025), SCs are considered promising electrochemical energy storage devices due to their unique performance and advantages, including ultra-high power density (Yadav and Srivastava, 2025), extremely long cycle life (Zhao et al., 2025), rapid charging and discharging capabilities ranging from seconds to minutes (Lee et al., 2025), high safety (Wang et al., 2018), and a wide temperature range (Xu et al., 2025). Generally speaking, based on two energy storage mechanisms: electric double-layer capacitance achieved through ion adsorption and pseudocapacitance achieved through rapid surface redox reactions between the electrolyte and electrode surface. SCs comprise three types based on charge storage: (i) Electric double-layer capacitors (EDLCs) store charge electrostatically at the electrode-electrolyte interface, offering high power density and long cycle life (Shen et al., 2024); (ii) Pseudocapacitors (PCs) utilize fast, reversible surface redox reactions for higher energy density (Cui et al., 2024); (iii) Hybrid supercapacitors (HSCs) combine EDLC and PC mechanisms/electrodes to balance performance (Gharanli et al., 2025; Xuan et al., 2025).
For EDLCs, when electrode materials (such as activated carbon, carbon fiber, etc.) are immersed in an electrolyte containing ions, the charges on the electrode surface attract ions of opposite charge in the electrolyte, thereby forming a double-layer structure with charge separation between the electrode and the electrolyte (Atlas and Ramon, 2018). Due to its rapid and reversible charge adsorption process, which does not involve redox reactions, it has excellent stability and high-power characteristics. However, its precisely this essentially purely electrostatic charge storage mechanism limits the energy density (Shen et al., 2024). In contrast, pseudocapacitive energy storage of PCs relies on rapid redox reactions, which primarily occur at the electrode/electrolyte interface and its near-surface regions. These reactions typically depend on conductive materials with high specific surface areas and abundant electrochemical active sites, such as transition metal oxides or conductive polymers. These active sites facilitate the rapid reversible accumulation and release of charge (ions/electrons) on the electrode surface, significantly enhancing the device’s charge storage capacity and thereby exhibiting higher energy density and specific capacitance (Xu et al., 2022). However, due to the Faraday effect, PCs typically have lower power than EDLCs (Chuang et al., 2010). HSCs combine double-layer and pseudocapacitive mechanisms, increasing energy density by introducing pseudocapacitive materials while maintaining high power output and fast charging/discharging characteristics of carbon materials (Cheng et al., 2021). Therefore, it is important to develop advanced SCs by constructing electrode materials with double-layer and pseudocapacitive behaviors.
Researchers are continuously exploring new electrode materials, such as metal oxides (Orera et al., 2022), metal sulphides (Oh et al., 2017), metal carbides (Sheikh et al., 2024), metal nitrides (Adalati et al., 2022), metal hydroxides (Gonçalves et al., 2020), metal-organic frameworks (MOFs) (Sarac et al., 2025), and MXene (Jiang et al., 2020), to further enhance the overall performance of SCs, particularly in terms of increasing energy density. Among them, MXenes, two-dimensional transition metal carbides, nitrides, and carbonitrides, are a novel nanomaterial with a structure similar to graphene, prepared by selectively etching its precursor MAX phase (i.e., Mn+1AXn, where M is an early transition metal, A is a Group III or IV element, X is C or N, and n = 1, 2, 3) (Wang et al., 2023; He et al., 2024). Since Michael Naguib et al. first discovered Ti3C2Tx in 2011 (Lukatskaya et al., 2013), exceed 30 MXene variants with different compositions have been successfully synthesized, and over 100 MXene configurations have been theoretically predicted (Zhang et al., 2023). MXene, with its diverse inherent properties, has demonstrated broad application potential in various fields such as optoelectronics (Tan et al., 2020), sensors (Bhardwaj and Hazra, 2021), electromagnetic shielding (Yun et al., 2020), electromagnetic wave absorption (Ma et al., 2023), and energy storage (Liu L. et al., 2022). Especially in the field of energy storage, MXene has become a highly promising electrode candidate material due to its unique two-dimensional layered structure, tunable interlayer spacing, excellent conductivity, high specific surface area, and adjustable surface functional groups (GaneshKumar et al., 2025). Furthermore, among the numerous electrode materials developed for SCs, MXene stands out for its ability to simultaneously provide double-layer capacitance and pseudocapacitance. By integrating MXene with promising materials such as metal oxides/sulfides and conductive polymers to form hybrid structures, both the energy density and power density of SCs can be enhanced (Boota and Gogotsi, 2018; Meng et al., 2021).
The electrochemical behavior of MXene is not only determined by its intrinsic structure but also influenced by the electrolyte. More importantly, during the preparation and post-processing stages, its key structural features (including interlayer spacing, pore structure, surface end groups, heteroatom doping, defects, and vacancies) can be precisely controlled. Therefore, by understanding the composition of SCs and their different energy storage mechanisms, this review systematically summarizes the application progress of pure MXene nanomaterials in SCs, explores the influence patterns of vacancy doping modification strategies on MXene structure and capacitive performance, and reveals their energy storage mechanisms. This review will be crucial for the rational design of high-performance MXene-based SC devices.
2 Research on pure MXene nanomaterials for SCs
It is worth noting that different types of MXene exhibit significant differences in their electrochemical performance. According to reports, Tsyganov et al. (2024) prepared Ti3AlC2 precursors using the molten salt method and obtained Ti3C2Tx under hydrothermal conditions using an HCl+KF mixed etchant. Adhesive-free Ti3AlC2 thin film electrodes prepared via blade coating exhibited an extremely high mass-specific capacitance of 480 F g−1 when tested in 1 M H2SO4 electrolyte at 25°C under ambient pressure, measured at a scan rate of 1 mV s−1 with an electrode mass loading of ∼2 mg cm−2. Due to its unique energy storage mechanism, 2D V4C3Tx demonstrated stable long-term cycling performance (97.23% capacitance retention after 10,000 cycles in H2SO4 solution), which has been highlighted as a high-performance material for SCs. The pseudocapacitance of V4C3Tx accounts for 37% of the total capacitance (268.5 F g−1) in H2SO4, which is attributed to the stability of vanadium’s oxidation states (+2, +3, +4) (Wang et al., 2019a). Additionally, Ghazaly et al. (2021) investigated the electrochemical behavior of Mo1.33C MXene in LiCl electrolyte. The results showed that at a scan rate of 2 mV s−1, the volumetric capacity of Mo1.33C was 815 F cm−3, with a wide operating potential window from −1.2 V to 0.3 V (relative to Ag/AgCl). Further, asymmetric SCs Mo1.33C//MnxOn were constructed, and the device exhibited excellent volumetric performance in 5 M LiCl electrolyte: energy density reached 58 mWh cm−3, and maximum power density reached 31 W cm−3. After 10,000 cycles at 10 A g−1, its capacitance retention rate remained as high as 92%. 2D Mo-based MXenes demonstrate significant potential in energy storage applications, with current research primarily focused on H2SO4 electrolytes. However, H2SO4 electrolytes limit the voltage window of symmetric SCs to within 0.9 V and asymmetric devices to within 1.3 V (Ghazaly et al., 2021).
MXene electrodes exhibit both capacitive (double-layer capacitance) and pseudocapacitive contributions in electrochemical capacitors. Their primary energy storage mechanism depends on the type of electrolyte. In aqueous SCs, the capacitance of 2D titanium carbides in acidic electrolytes primarily stems from the protonation of H+ with MXene oxygen-containing groups (Dall'Agnese et al., 2014). As shown in Figure 1a, hydrated ions are embedded between MXene layers in an aqueous electrolyte system and adsorbed onto the surface via electrostatic forces, thereby primarily forming an EDLC mechanism. Additionally, due to the smaller bare radius of alkali metal cations (Li+), which possess higher hydration energy, changes in cations also significantly affect capacitance (Figures 1b–d). The special closed water molecules surrounding the cations shield the external electric field, reducing the potential difference between the ions and the MXene surface, thereby greatly enhancing capacitance (Sugahara et al., 2019). As shown in Figure 1e, in aqueous electrolytes, cation intercalation behavior is prominent, resulting in near-rectangular cyclic voltammetry (CV) curves (Sugahara et al., 2019); in non-aqueous electrolytes, highly reversible redox reactions occur on the surface (Figure 1f), leading to significantly distorted CV curves (Wang et al., 2019b; Ando et al., 2020; Yadav and Kurra, 2025). The use of non-aqueous electrolytes (i.e., organic electrolytes) promotes cation dehydration (Figure 1g), leading to charge transfer between ions and surface groups on two-dimensional titanium carbide (Zhao et al., 2019). Additionally, expanding the operating voltage is another direction for enhancing the energy density of capacitors. As is well known, most organic electrolytes can maintain a wide electrochemical potential window, meaning that the large overpotential between adsorbed ions and MXene surface groups can be overcome (Wang et al., 2019b). Hydrated-melt electrolytes have been applied in aqueous electrolytes to achieve higher operating voltages and energy densities (Kim et al., 2019). In the study by Kim et al. (2019), hydrated Li+ ions tightly aggregate on the MXene surface within a wide voltage window (Figure 1h), while the accumulation of hydrated ions induces pseudocapacitance in MXene at low voltages (Figures 1i,j). In addition to the aforementioned electrolytes, Lin et al. (2016a) also constructed SCs using ionic liquid electrolytes and MXene electrodes, achieving a large potential range of 3 V (Figure 1k). Then, the charge storage mechanism was revealed using in situ X-ray diffraction. As shown in Figure 1l, unlike the pseudocapacitance generated by the redox reactions of surface functional groups on MXene in aqueous electrolytes, non-boiling ionic liquids cannot induce any redox reactions. The pseudocapacitance of ionic liquid electrolytes, however, originates from the electrostatic attraction between intercalated anions and positively charged MXene sheets during the intercalation process, as well as the steric effects (pillar effects) during the deintercalation process (Lin et al., 2016b).
FIGURE 1

(a) Schematic diagram of the continuous model of a micro-gap capacitor and schematic diagram of the double-layer capacitor model (Sugahara et al., 2019). (b) Experimental specific capacitance of MXene in aqueous solutions with Li+, Na+, K+, Rb+, TMA+, TEA+, and TBA+ electrolytes (Sugahara et al., 2019). (c) Order-of-magnitude relationship between bare ion size, hydrated ion size, and observed capacitance values (Sugahara et al., 2019). (d) Effect of ion-MXene distance (b−a0) on experimental specific capacitance (Sugahara et al., 2019). (e) CV curves for MXene with various aqueous electrolytes at 0.5 mV s−1. Near-rectangular shape indicates cation intercalation-dominated EDLC behavior (Sugahara et al., 2019). (f) CV curves of MXene in Ca(TFSI)2 electrolytes at a scan rate of 2 mV s−1. Peak distortion confirms pseudocapacitance from surface redox reactions (Yadav and Kurra, 2025). (g) Schematic diagram of the charging storage mechanism hypothesis of MXene in non-aqueous electrolytes. Hydrated ion accumulation induces pseudocapacitance at low voltages (Zhao et al., 2019). (h) Schematic illustration of Li+ intercalation to form an electric double-layer in MXene (Kim et al., 2019). (i) Cathodic linear sweep voltammetry of a 1 M Li2SO4 aqueous electrolyte and a hydrate-melt electrolyte with a Ti electrode at a sweep rate of 0.1 mV s−1. Chronoamperometry at various applied potentials vs. Ag/AgCl in a 1 M Li2SO4 aqueous electrolyte and a hydrate-melt electrolyte with MXene (Kim et al., 2019). (j) Cyclic voltammetry curves of MXene with a 1 M Li2SO4 aqueous electrolyte (black line) and a hydrate-melt electrolyte (red line) at a scan rate of 0.5 mV s−1 (Kim et al., 2019). (k) CV curves of a 2-electrode Swagelok cell at scan rate from 20 to 500 mV s−1 within a voltage window of 3 V (Lin et al., 2016a). (l) Electrochemical in situ X-ray diffraction study of IL-Ti3C2Tx film at various constant potentials (0.5 V steps) in EMI-TFSI electrolyte (Lin et al., 2016b).
3 Vacancy-doped MXene nanomaterials and their capacitive performances
While pure MXenes exhibit promising charge storage dynamics, their intrinsic capacitance remains constrained by limited active sites and sluggish ion kinetics. To transcend these limitations, vacancy engineering emerges as a paradigm—strategically tailoring atomic-scale defects to reconfigure electronic and ionic landscapes, as systematically deciphered below. Vacancy engineering strategies are classified by elemental identity and synthetic origin: 1) In-situ homogeneous metal vacancies (e.g., Ti in Ti3C2Tz) arise from controlled etching or thermal oxidation, with concentration tunable via etchant severity (Sang et al., 2016); 2) In-situ heterogeneous metal vacancies inherit ordered structures from i-MAX precursors through selective A-layer etching, enabling zigzag vacancy patterns (Zhou et al., 2025); 3) In-situ carbon vacancies form during MXene synthesis via fluorine-free routes or decarbonization, exhibiting high thermodynamic stability (Hu et al., 2017).
3.1 In-situ homogeneous metal vacancies
The direct and in situ metal vacancies in MXenes originate from the liquid etching process or the thermal energy from post-heat treatment. As shown in Figures 2a–d, during the liquid etching process, MXene atoms, especially the outer metal (e.g., Ti) atoms, are inevitably removed. Since Ti-deficient MXenes originate from direct contact between the etching solution and the MXene, the concentration of Ti vacancies can be regulated by controlling the concentration of the etching solution (Sang et al., 2016). During heat treatment, C-Ti elongates and fractures, with Ti atoms reacting with -O end groups and separating from TiO2 particles (Naguib et al., 2014). Additionally, as shown in Figure 2e, these metal vacancies tend to aggregate and migrate to the edges of MXene layers, forming triangular nanodomains (Karlsson et al., 2015). The introduction of metal vacancies reconfigures the charge distribution around defects, increasing the density of active sites available for ion intercalation (Wang et al., 2022). However, metal vacancies also enhance the material’s catalytic activity (Figure 2f), potentially accelerating electrolyte degradation and significantly limiting its operating voltage window (Shi et al., 2022).
FIGURE 2
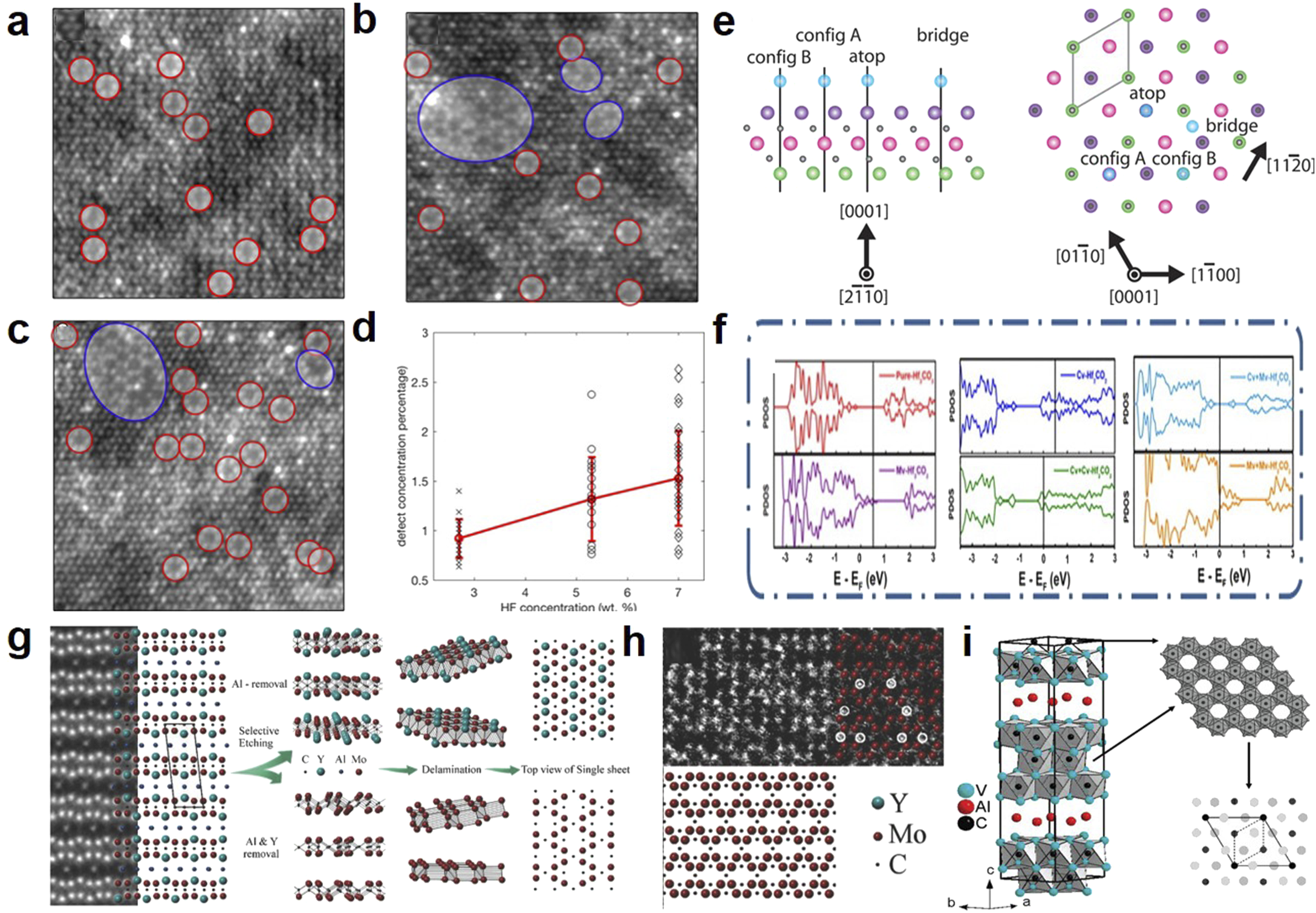
HAADF-STEM images from single-layer Ti3C2Tx MXene flakes prepared using etchants with different HF concentrations: (a) 2.7 wt.% HF, (b) 5.3 wt.% HF, and (c) 7 wt.% HF. Single VTi vacancies are indicated by red circles, while vacancy clusters V are shown by blue circles (Sang et al., 2016). (d) Scatter plot of defect concentration from images acquired from samples produced using different HF concentrations (Sang et al., 2016). (e) Side view and top view of the Ti3C2Tx monolayer. The side view shows three titanium layers (green, pink, purple) and two intermediate carbon layers (black), as well as the Tx positions (blue) distributed along the [2110] zone axis. The top view shows the atomic layers along the [0001] crystal plane axis. The unit cell (approximately 3 Å10) is outlined with solid lines (Karlsson et al., 2015). (f) The projected density of states (PDOS) diagram of the surface O atoms for Hf2CO2 with various types of vacancy (Shi et al., 2022). (g) (Left) STEM image of (Mo2/3Y1/3)2AlC with corresponding structure model, prior to etching. (Middle) Depending on etching protocol two different structures are obtained; one in which only Al is removed (top) or one in which both Al and Y are removed (bottom). (Right) Top view of corresponding structures obtained (Persson et al., 2018). (h) Magnified view of the atomic structure exhibiting the signature zig-zag pattern of vacancy ordered MXene (Persson et al., 2018). (i) Schematic diagram of V2AlC single crystal and new carbides V4AlC3-x and V12Al3C8 (Etzkorn et al., 2007).
3.2 In-situ heterogeneous metal vacancies
The remarkable energy storage capabilities of MXene with ordered/disordered vacancies (Mo1.33C, W1.33C, Nb1.33C) have been a focal point of attention. These MXene with ordered vacancies (i-MXene) can prepared from the etching of in-plane ordered MAX (i-MAX). The structure of i-MXene is closely related to the etching process. As shown in Figures 2g,h, Ingemar Persson et al. applied different etching schemes to the parent i-MAX phase, namely, varying concentrations of hydrofluoric acid and different etching times. Under harsh etching conditions, compounds with partial removal of Y (Mo2/3Y(1-x)/3)2C were formed, and under extended etching times, fully etched Mo1.33C was obtained (Persson et al., 2018). When H2SO4 is chosen as the electrolyte, most MXenes, including Mo1.33C, exhibit higher volumetric capacitance because highly reversible redox reactions occur between the -OH groups and H+ ions. However, (Mo2/3Y(1-x)/3)2C exhibits a completely different trend, showing higher capacitance in KOH electrolyte compared to H2SO4 electrolyte. The specific composition and arrangement of functional groups on (Mo2/3Y(1-x)/3)2C may be the cause of this phenomenon. Through theoretical calculations and experimental verification, it was found that the Mo1.33C surface is more prone to forming -F functional groups compared to the original Mo2C (Halim et al., 2016; Lind et al., 2017). Additionally, Halim et al. (2018) successfully synthesized and characterized Nb1.33C with disordered vacancies. The results indicated that Nb1.33C is similar to Nb2C, with -O end groups dominating.
To further enhance the electrochemical performance of Mo1.33C electrodes, researchers employed two effective strategies: post-etching annealing treatment (Rakhi et al., 2015) and MXene-based hydrogel construction (Jia et al., 2024). The former has been proven to significantly enhance the electrode’s high-rate discharge performance, while the latter is an effective approach to improving its specific capacitance (Ahmed et al., 2020). Additionally, combining Mo1.33C with conductive polymers can further improve its capacitance and stability (Qin et al., 2017; El Ghazaly et al., 2021; Ghazaly et al., 2021). For example, to address the brittleness issue of the original Mo1.33C film, Leiqiang Qin et al. reported a ‘printed electrode’ (an electrode with high flexibility, light weight, and portability) by mixing Mo1.33C with poly(3,4-ethylenedioxythiophene): poly(styrene sulfonate) (PEDOT:PSS) (Qin et al., 2017). SCs constructed using this flexible electrode achieved an energy density of up to 33.2 mWh cm−3, a power density of 19,470 mW m−3, and a maximum capacitance of 568 F cm−3. Considering the synergistic effect between organic and metallic materials, Qin et al. (2019) employed an electrochemical polymerization method to construct interconnected three-dimensional porous polymer-xylene (Mo1.33C) composite nanorings. This newly designed electrode can increase the energy density to 20.05 mWh cm−3. Additionally, when constructing asymmetric micro-SC with MnO2, the operating voltage can be increased to 1.6 V, the areal capacitance can be increased to 69.5 mF cm−2, and the energy density can be increased to 250.1 mWh cm−3 (Qin et al., 2019).
3.3 In-situ carbon vacancies
The in situ carbon vacancies in MXenes originate from their corresponding MAX phases. As shown in Figure 2i, the V4AlC3-x compound can be obtained from a Co-containing melt (Etzkorn et al., 2007). Adjacent vacancies in zirconium carbide can enhance its toughness and flexibility (Xie et al., 2016). In addition to conducting experiments, Hu et al. (2017) also used first-principles calculation methods to verify that the conductivity and flexibility of carbon-vacancy Ti2CT2 are higher than those of perfect Ti2CT2. Furthermore, unlike metal vacancies in MXene, carbon migration is unrestricted under ambient conditions due to the high migration barrier energy.
4 Conclusion and outlook
4.1 Conclusion
This review systematically deciphers how atomic-scale vacancy engineering in MXenes—spanning in situ defects: homogeneous and heterogeneous metal vacancies, and carbon vacancies—reconfigures electronic, ionic, and interfacial properties to transcend intrinsic capacitive limitations. By establishing clear links between vacancy design (type, density, distribution), electrolyte selection, and device architecture, it provides a foundational roadmap for developing next-generation MXene-based SCs. The work underscores vacancy doping not merely as a materials modification tactic, but as a paradigm-shifting strategy to unlock the theoretical limits of 2D energy storage materials. The specific research content is as follows.
4.1.1 Pure MXene nanomaterial system comparison
Titanium-based MXene (Ti3C2Tx) performs best in acidic electrolytes (480 F g−1); vanadium-based MXene (V4C3Tx) exhibits a high pseudocapacitance ratio (37%) due to its multi-valent nature (V2+/3+/4+); Mo-based MXene (Mo1.33C) achieves a wide voltage window (−1.2 to 0.3 V) and high volumetric capacitance (815 F cm−3) in LiCl electrolyte.
4.1.2 Fundamental material design principles
The review establishes that intentional vacancy creation—whether through in situ etching of homogeneous metal (e.g., HF concentration-dependent Ti vacancies in Ti3C2Tx), in situ thermal treatment (e.g., C-Ti bond fracture forming TiO2), or in situ etching of heterogeneous metal (e.g., Mo1.33C)—directly enhances MXene’s electrochemical activity. These vacancies can expand active sites for ion adsorption/intercalation, and modulate electronic structure, reducing ion diffusion barriers, and optimize surface chemistry by promoting -O termination over -F.
4.1.3 Structure-property relationship
Vacancy type and distribution: Ordered heterogeneous metal vacancies (e.g., zigzag-patterned Mo1.33C) outperform disordered ones in ion-accessible surface area and conductivity due to reduced charge recombination. Electrolyte–electrode synergy: Hydrated ion confinement in MXene interlayers (e.g., Li+ in hydrate-melt electrolytes) boosts capacitance by 300% via dielectric constant enhancement. Interlayer spacing control: Vacancies facilitate intercalation of larger ions/organic molecules, widening interlayer gaps and improving EDLC formation kinetics.
4.2 Outlook
4.2.1 Machine learning-assisted material screening
Utilizing computational simulation and AI prediction to accelerate the development of novel MXenes (such as double transition metal carbonitrides), optimizing doping sites and vacancy concentrations to achieve synergistic improvements in conductivity and capacitance performance.
4.2.2 Multi-level structural design
Design MXene nanomaterials composed of different elements, such as M-site bimetallic solid solution MXene, X-site N, B solid solution MXene, and medium-high entropy multi-metal solid solution MXene, further achieve vacancy doping and other element doping, optimise the MXene structure, and enhance surface activity.
Combining 3D printing technology to construct gradient-porous MXene electrodes addresses the ion transport bottleneck caused by nanoplate stacking, while a flexible, ultra-thin, all-MXene device design approach achieves high areal capacitance. For example, 3D-printed gradient-porous Ti3C2Tx electrodes (Liu G. et al., 2022) mitigate ion transport bottlenecks caused by nanosheet stacking, achieving capacitance retention of 95% at 100 mV s−1. In addition, ultra-thin all-MXene micro-supercapacitors (Zhao et al., 2025) demonstrate areal capacitance of 250 mF cm−2 via van der Waals self-assembly, highlighting scalable fabrication potential.
4.2.3 Electrolyte engineering innovation
Develop high-voltage/wide-temperature-range electrolytes (such as deep eutectic electrolytes and ionic liquid composite systems) suitable for vacancy-type MXene electrodes to overcome the voltage window limitations of aqueous electrolytes.
4.2.4 Multifunctional integrated device
Develop vacancy-type MXene-based micro-supercapacitor-sensor integrated devices that combine MXene’s electromagnetic shielding and sensing properties to expand its self-powered applications in wearable energy systems.
4.2.5 Sustainable preparation and recycling
Exploring fluorine-free etching processes and closed-loop recycling technologies for vacancy-type MXene waste materials to meet environmental requirements for large-scale production and reduce energy consumption during preparation.
4.2.6 Unresolved challenges and research imperatives
Vacancy stability: Long-term evolution of vacancies during cycling (e.g., aggregation at edges) remains poorly understood. Multi-ion compatibility: Most vacancy studies focus on Li+/Na+; behavior with multivalent ions (Mg2+, Al3+) is underexplored. Industrial processing: Vacancy consistency across large-scale MXene production (e.g., roll-to-roll) requires standardized protocols.
Statements
Author contributions
YT: Funding acquisition, Writing – original draft, Resources. ZB: Writing – original draft. YX: Software, Writing – review and editing, Formal Analysis, Resources, Methodology. XX: Software, Writing – review and editing, Data curation, Resources. CY: Funding acquisition, Writing – review and editing, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (52302366, 52002329), and the Natural Science Foundation of Chongqing (CSTB2022NSCQ-MSX0759), and the Natural Science Research Project of Education Department of Shaanxi Provincial (23JK0539), and the China Postdoctoral Science Foundation (2024MD753972), and Shaanxi Postdoctoral Science Foundation (2024BSHSDZZ072), and Outstanding Youth Science Fund of Xi’an University of Science and Technology (2024YQ3-05), and Start-up Fund for High-level Researchers of Xi’an University of Science and Technology (2050122071).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
AdalatiR.SharmaM.SharmaS.KumarA.MalikG.BoukherroubR.et al (2022). Metal nitrides as efficient electrode material for supercapacitors: a review. J. Energy Storage56, 105912. 10.1016/j.est.2022.105912
2
AhmedB.GhazalyA. E.RosenJ. (2020). i-MXenes for energy storage and catalysis. Adv. Funct. Mater.30 (47), 2000894. 10.1002/adfm.202000894
3
AndoY.OkuboM.YamadaA.OtaniM. (2020). Capacitive versus pseudocapacitive storage in MXene. Adv. Funct. Mater.30 (47), 2000820. 10.1002/adfm.202000820
4
AtlasI.RamonG. Z. (2018). Periodic energy conversion in an electric-double-layer capacitor. J. Colloid Interface Sci.530, 675–685. 10.1016/j.jcis.2018.06.034
5
BhardwajR.HazraA. (2021). MXene-based gas sensors. J. Mater. Chem. C9 (44), 15735–15754. 10.1039/d1tc04085e
6
BootaM.GogotsiY. (2018). MXene—Conducting polymer asymmetric pseudocapacitors. Adv. Energy Mater.9 (7), 1802917. 10.1002/aenm.201802917
7
ChengW.FuJ.HuH.HoD. (2021). Interlayer structure engineering of MXene-Based capacitor-type electrode for hybrid micro-supercapacitor toward battery-level energy density. Adv. Sci.8 (16), 2100775. 10.1002/advs.202100775
8
ChuangC.-M.HuangC.-W.TengH.TingJ.-M. (2010). Effects of carbon nanotube grafting on the performance of electric double layer capacitors. Energy and Fuels24 (12), 6476–6482. 10.1021/ef101208x
9
CuiZ.WangD.XuT.YaoT.ShenL. (2024). Enabling extreme low-temperature proton pseudocapacitor with tailored pseudocapacitive electrodes and antifreezing electrolytes engineering. Chem. Eng. J.495, 153347. 10.1016/j.cej.2024.153347
10
Dall'AgneseY.LukatskayaM. R.CookK. M.TabernaP.-L.GogotsiY.SimonP. (2014). High capacitance of surface-modified 2D titanium carbide in acidic electrolyte. Electrochem. Commun.48, 118–122. 10.1016/j.elecom.2014.09.002
11
El GhazalyA.Méndez-RomeroU. A.HalimJ.Nestor TsengE. O. Å.PersonP.AhmedB.et al (2021). Improved charge storage performance of a layered Mo1.33C MXene/MoS2/graphene nanocomposite. Nanoscale Adv.3 (23), 6689–6695. 10.1039/d1na00642h
12
EtzkornJ.AdeM.HillebrechtH. (2007). V2AlC, V4AlC3-x (x ≈ 0.31), and V12Al3C8: synthesis, crystal growth, structure, and superstructure. Inorg. Chem.46 (18), 7646–7653. 10.1021/ic700382y
13
GaneshKumarP.PanchabikesanK.DivyaS.OhT. H. (2025). Revolutionizing MXene nanomaterials for hydrogen production and storage: enhancing catalysis, storage, mechanical integrity, and ecosystem compatibility. Adv. Colloid Interface Sci.342, 103528. 10.1016/j.cis.2025.103528
14
GharanliS.GharibA.MoharramnejadM.KarimA.MalekshahR. E.KarimiF.et al (2025). Environmentally friendly nickel-based nanocomposites for energy storage: a review of supercapacitor and battery-type mechanisms. J. Energy Storage122, 116509. 10.1016/j.est.2025.116509
15
GhazalyA. E.ZhengW.HalimJ.TsengE. N.PerssonP. O.AhmedB.et al (2021). Enhanced supercapacitive performance of Mo1.33C MXene based asymmetric supercapacitors in lithium chloride electrolyte. Energy Storage Mater.41, 203–208. 10.1016/j.ensm.2021.05.006
16
GonçalvesJ. M.da SilvaM. I.TomaH. E.AngnesL.MartinsP. R.ArakiK. (2020). Trimetallic oxides/hydroxides as hybrid supercapacitor electrode materials: a review. J. Mater. Chem. A8 (21), 10534–10570. 10.1039/d0ta02939d
17
HalimJ.KotaS.LukatskayaM. R.NaguibM.ZhaoM. Q.MoonE. J.et al (2016). Synthesis and Characterization of 2D Molybdenum Carbide (MXene). Adv. Funct. Mater.26 (18), 3118–3127. 10.1002/adfm.201505328
18
HalimJ.PalisaitisJ.LuJ.ThörnbergJ.MoonE. J.PrecnerM.et al (2018). Synthesis of two-dimensional Nb1.33C (MXene) with randomly distributed vacancies by etching of the Quaternary solid solution (Nb2/3Sc1/3)2AlC MAX phase. ACS Appl. Nano Mater.1 (6), 2455–2460. 10.1021/acsanm.8b00332
19
HeY.SuQ.LiuD.XiaL.HuangX.LanD.et al (2024). Surface engineering strategy for MXene to tailor electromagnetic wave absorption performance. Chem. Eng. J.491, 152041. 10.1016/j.cej.2024.152041
20
HuT.YangJ.WangX. (2017). Carbon vacancies in Ti2CT2MXenes: defects or a new opportunity?Phys. Chem. Chem. Phys.19 (47), 31773–31780. 10.1039/c7cp06593k
21
JanardhananJ. C.BijiC. A.JohnH. (2025). Research trends in ammonium-ion supercapacitors. Chem. Eng. J.507, 160212. 10.1016/j.cej.2025.160212
22
JiaM.ChenQ.ChenK.ZhangX.FengH.FengC.et al (2024). Muscle-inspired MXene-based conductive hydrogel by magnetic induced for flexible multifunctional sensors. Eur. Polym. J.214, 113149. 10.1016/j.eurpolymj.2024.113149
23
JiangQ.LeiY.LiangH.XiK.XiaC.AlshareefH. N. (2020). Review of MXene electrochemical microsupercapacitors. Energy Storage Mater.27, 78–95. 10.1016/j.ensm.2020.01.018
24
KarlssonL. H.BirchJ.HalimJ.BarsoumM. W.PerssonP. O. A. (2015). Atomically resolved structural and chemical investigation of single MXene sheets. Nano Lett.15 (8), 4955–4960. 10.1021/acs.nanolett.5b00737
25
KimK.AndoY.SugaharaA.KoS.YamadaY.OtaniM.et al (2019). Dense charge accumulation in MXene with a hydrate-melt electrolyte. Chem. Mater.31 (14), 5190–5196. 10.1021/acs.chemmater.9b01334
26
LeeD.KimJ.KimC. W.KimJ.-G.JungS. E.HeoS. J.et al (2025). Nanocell-structured carbon nanotube composite fibers for ultrahigh energy and power density supercapacitors. Compos. Part B Eng.295, 112179. 10.1016/j.compositesb.2025.112179
27
LinZ.BarbaraD.TabernaP.-L.Van AkenK. L.AnasoriB.GogotsiY.et al (2016a). Capacitance of Ti3C2Tx MXene in ionic liquid electrolyte. J. Power Sources326, 575–579. 10.1016/j.jpowsour.2016.04.035
28
LinZ.RozierP.DuployerB.TabernaP.-L.AnasoriB.GogotsiY.et al (2016b). Electrochemical and in-situ X-ray diffraction studies of Ti3C2Tx MXene in ionic liquid electrolyte. Electrochem. Commun.72, 50–53. 10.1016/j.elecom.2016.08.023
29
LindH.HalimJ.SimakS. I.RosenJ. (2017). Investigation of vacancy-ordered Mo1.33C MXene from first principles and x-ray photoelectron spectroscopy. Phys. Rev. Mater.1 (4), 044002. 10.1103/PhysRevMaterials.1.044002
30
LiuG.YuR.LiuD.XiaY.PeiX.WangW.et al (2022a). 3D-printed TiO2-Ti3C2Tx heterojunction/rGO/PDMS composites with gradient pore size for electromagnetic interference shielding and thermal management. Compos. Part A Appl. Sci. Manuf.160, 107058. 10.1016/j.compositesa.2022.107058
31
LiuL.ZschiescheH.AntoniettiM.DaffosB.TarakinaN. V.GibilaroM.et al (2022b). Tuning the surface chemistry of MXene to improve energy storage: example of nitrification by salt melt. Adv. Energy Mater.13 (2), 2202709. 10.1002/aenm.202202709
32
LukatskayaM. R.MashtalirO.RenC. E.Dall’AgneseY.RozierP.TabernaP. L.et al (2013). Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide. Science341 (6165), 1502–1505. 10.1126/science.1241488
33
MaC.MaW.WangT.MaF.XuX.FengC.et al (2023). An MXene coating with electromagnetic wave absorbing performance. Inorg. Chem. Commun.151, 110565. 10.1016/j.inoche.2023.110565
34
MengW.LiuX.SongH.XieY.ShiX.DarguschM.et al (2021). Advances and challenges in 2D MXenes: from structures to energy storage and conversions. Nano Today40, 101273. 10.1016/j.nantod.2021.101273
35
NaguibM.MashtalirO.LukatskayaM. R.DyatkinB.ZhangC. F.PresserV.et al (2014). One-step synthesis of nanocrystalline transition metal oxides on thin sheets of disordered graphitic carbon by oxidation of MXenes. Chem. Commun.50 (56), 7420–7423. 10.1039/c4cc01646g
36
OhS. M.KimI. Y.PatilS. B.ParkB.LeeJ. M.AdpakpangK.et al (2017). Improvement of Na ion electrode activity of metal oxide via composite formation with metal sulfide. ACS Appl. Mater Interfaces9 (3), 2249–2260. 10.1021/acsami.6b11220
37
OreraA.BetatoA.Silva-TreviñoJ.LarreaÁ.Laguna-BerceroM. Á. (2022). Advanced metal oxide infiltrated electrodes for boosting the performance of solid oxide cells. J. Mater. Chem. A10 (5), 2541–2549. 10.1039/d1ta07902f
38
PerssonI.el GhazalyA.TaoQ.HalimJ.KotaS.DarakchievaV.et al (2018). Tailoring structure, composition, and energy storage properties of MXenes from selective etching of in‐plane, chemically ordered MAX phases. Small14 (17), e1703676. 10.1002/smll.201703676
39
QinL.TaoQ.El GhazalyA.Fernandez‐RodriguezJ.PerssonP. O. Å.RosenJ.et al (2017). High-performance ultrathin flexible solid-state supercapacitors based on solution processable Mo1.33C MXene and PEDOT:PSS. Adv. Funct. Mater.28 (2), 1703808. 10.1002/adfm.201703808
40
QinL. Q.TaoQ. Z.LiuX. J.FahlmanM.HalimJ.PerssonaP. O. A.et al (2019). Polymer-MXene composite films formed by MXene-facilitated electrochemical polymerization for flexible solid-state microsupercapacitors. Nano Energy60, 734–742. 10.1016/j.nanoen.2019.04.002
41
RakhiR. B.AhmedB.HedhiliM. N.AnjumD. H.AlshareefH. N. (2015). Effect of postetch annealing gas composition on the structural and electrochemical properties of Ti2CTx MXene electrodes for supercapacitor applications. Chem. Mater.27 (15), 5314–5323. 10.1021/acs.chemmater.5b01623
42
SangX.XieY.LinM.-W.AlhabebM.Van AkenK. L.GogotsiY.et al (2016). Atomic defects in monolayer titanium carbide (Ti3C2Tx) MXene. ACS Nano10 (10), 9193–9200. 10.1021/acsnano.6b05240
43
SaracB.YucerS.CiftciF. (2025). MOF-based bioelectronic supercapacitors. Small21 (15), 2412846. 10.1002/smll.202412846
44
SheikhZ. A.KimH.VikramanD.AftabS.BhatA. A.HussainI.et al (2024). Improved charge storage kinetics using metal carbide integrated NiO hybrid composite electrodes for supercapacitors. J. Energy Storage100, 113605. 10.1016/j.est.2024.113605
45
ShenH.ZhuY.ZadaI.LiH.BokhariS. W.ZhuS.et al (2024). Investigation of strategies for improving the energy density of symmetric electrical double-layer capacitors. J. Energy Storage79, 110127. 10.1016/j.est.2023.110127
46
ShiL.-N.CuiL.-T.JiY.-R.XieY.ZhuY.-R.YiT.-F. (2022). Towards high-performance electrocatalysts: activity optimization strategy of 2D MXenes-based nanomaterials for water-splitting. Coord. Chem. Rev.469, 214668. 10.1016/j.ccr.2022.214668
47
SugaharaA.AndoY.KajiyamS.YazawaK.GotohK.OtaniM.et al (2019). Negative dielectric constant of water confined in nanosheets. Nat. Commun.10, 850. 10.1038/s41467-019-08789-8
48
TanT.JiangX.WangC.YaoB.ZhangH. (2020). 2D material optoelectronics for information functional device applications: status and challenges. Adv. Sci.7 (11), 2000058. 10.1002/advs.202000058
49
TsyganovA.VikulovaM.ShindrovA.ZheleznovD.GorokhovskyA.GorshkovN. (2024). Molten salt-shielded synthesis of Ti3AlC2 as a precursor for large-scale preparation of Ti3C2Tx MXene binder-free film electrode supercapacitors. Dalton Trans.53 (13), 5922–5931. 10.1039/d3dt04327d
50
WangH.ZhangF.XiaJ.LuF.ZhouB.YiD.et al (2022). Engineering electronic structures of titanium vacancies in Ti1-xO2 nanosheets enables enhanced Li-ion and Na-ion storage. Green Energy and Environ.7 (4), 734–741. 10.1016/j.gee.2020.11.006
51
WangL.WangJ.OuyangB. (2023). Computational investigation of MAX as intercalation host for rechargeable aluminum-ion battery. Adv. Energy Mater.13 (46). 10.1002/aenm.202302584
52
WangX.LinS.TongH.HuangY.TongP.ZhaoB.et al (2019a). Two-dimensional V4C3 MXene as high performance electrode materials for supercapacitors. Electrochimica Acta307, 414–421. 10.1016/j.electacta.2019.03.205
53
WangX.MathisT. S.LiK.LinZ.VlcekL.ToritaT.et al (2019b). Influences from solvents on charge storage in titanium carbide MXenes. Nat. Energy4 (3), 241–248. 10.1038/s41560-019-0339-9
54
WangZ.ChengJ.ZhouJ.ZhangJ.HuangH.YangJ.et al (2018). All-climate aqueous fiber-shaped supercapacitors with record areal energy density and high safety. Nano Energy50, 106–117. 10.1016/j.nanoen.2018.05.029
55
XieC. W.OganovA. R.LiD.DebelaT. T.LiuN.DongD.et al (2016). Effects of carbon vacancies on the structures, mechanical properties, and chemical bonding of zirconium carbides: a first-principles study. Phys. Chem. Chem. Phys.18 (17), 12299–12306. 10.1039/c5cp07724a
56
XuC.MuJ.ZhouT.TianS.GaoP.YinG.et al (2022). Surface redox pseudocapacitance boosting vanadium nitride for high-power and ultra-stable potassium-ion capacitors. Adv. Funct. Mater.32 (38), 2206501. 10.1002/adfm.202206501
57
XuL.SunD.LvS.TianG.WangG.WangB.et al (2025). Novel semiconductor materials for advanced wide temperature range supercapacitors. J. Mater. Chem. A13 (10), 6954–6992. 10.1039/d4ta07378a
58
XuanX.XieY.TangY.ZhouJ.BiZ.ZouJ.et al (2025). Revealing the ionic storage mechanisms of Mo2VC2Tz (MXene) in multiple aqueous electrolytes for high-performance supercapacitors. Chem. Eng. J.519, 165537. 10.1016/j.cej.2025.165537
59
YadavD.SrivastavaN. (2025). High power density supercapacitor, using a fast ion conducting, flexible, economical, and environment benign polymer-in-salt-electrolytes (PISEs). J. Power Sources647, 237353. 10.1016/j.jpowsour.2025.237353
60
YadavS.KurraN. (2025). Pseudocapacitive charge storage dynamics of vanadium carbide MXene in water-in-salt calcium-ion electrolyte. Small, 2503657. 10.1002/smll.202503657
61
YunT.KimH.IqbalA.ChoY. S.LeeG. S.KimM. K.et al (2020). Electromagnetic shielding of monolayer MXene assemblies. Adv. Mater.32 (9), e1906769. 10.1002/adma.201906769
62
ZhangT.ShuckC. E.ShevchukK.AnayeeM.GogotsiY. (2023). Synthesis of three families of titanium carbonitride MXenes. J. Am. Chem. Soc.145 (41), 22374–22383. 10.1021/jacs.3c04712
63
ZhaoX.Dall'AgneseC.ChuX. F.ZhaoS.ChenG.GogotsiY.et al (2019). Electrochemical behavior of Ti3C2Tx MXene in environmentally friendly methanesulfonic acid electrolyte. ChemSusChem12 (19), 4480–4486. 10.1002/cssc.201901746
64
ZhaoZ.XuZ.WangY.HuangW.ChengY.WongW.-Y. (2025). Scalable assembly of flexible ultrathin all-in-one MXene-based supercapacitors. J. Mater. Chem. A13 (18), 13175–13185. 10.1039/d5ta00327j
65
ZhouJ.ChangN.TangY.XieY.XuanX.BiZ.et al (2025). Uncovering the strengthening mechanisms of metal vacancies in the structure and capacitance performance of defect-controlled Mo2−□CTz MXene. Chem. Eng. J.519, 165391. 10.1016/j.cej.2025.165391
Summary
Keywords
2D materials, MXene, vacancy doping, supercapacitors, energy storage mechanisms
Citation
Tang Y, Bi Z, Xie Y, Xuan X and Yang C (2025) Two-dimensional vacancy-doped MXene nanomaterials for supercapacitors. Front. Chem. 13:1656521. doi: 10.3389/fchem.2025.1656521
Received
30 June 2025
Accepted
14 July 2025
Published
23 July 2025
Volume
13 - 2025
Edited by
Juan Yang, Xi’an Jiaotong University, China
Reviewed by
Shude Liu, National Institute for Materials Science, Japan
Zhuosen Wang, Zhengzhou University, China
Updates
Copyright
© 2025 Tang, Bi, Xie, Xuan and Yang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chenhui Yang, yangch@nwpu.edu.cn
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.