- 1Institute of Soil and Environmental Sciences, University of Agriculture Faisalabad, Faisalabad, Pakistan
- 2Department of Environmental Sciences, COMSATS University Islamabad, Vehari-Campus, Vehari, Pakistan
- 3Department of Agronomy, Faculty of Agriculture, University of Agriculture Faisalabad, Faisalabad, Pakistan
- 4Department of Soil Science and Plant Nutrition Selçuk University, Konya, Türkiye
- 5Department of Soil and Water Science, Indian River Research and Education Center, Institute of Food and Agricultural Sciences, University of Florida, Fort Pierce, FL, United States
Chromium (Cr) contamination of soil threatens environmental and human health, necessitating effective and sustainable remediation solutions. Here we explored the impact of rice husk biochar (RH-BC), nano-RH-BC, vermicompost, nano-zero valent zinc (nZVZn), and manganese-modified RH-BC (MnO/RH-BC) on phytoaccumulation of Cr by industrial hemp (Cannabis sativa L.) and ryegrass (Lolium perenne L.) grown in tannery wastewater-impacted Cr-contaminated soil in a pot trial. Results revealed that maximum shoot Cr concentration was observed in ryegrass with nZVZn mainly in first cutting (45 mg kg-1 DW) followed by vermicompost (42 mg kg-1 DW) > RH-BC (40 mg kg-1 DW) > nano-RH-BC (35 mg kg-1 DW) ∼ MnO/H-BC (34 mg kg-1 DW). In the case of hemp, nZVZn led to maximum shoot Cr concentration (42 mg kg-1 DW) and minimum with RH-BC (4 mg kg-1 DW); notably, hemp survived only for the first harvest. The SPAD value (chlorophyll content) was the highest with MnO/RH-BC for ryegrass and RH-BC for hemp (40 and 51, respectively) and minimum with nZVZn. Results showed that the RH-BC enhanced shoot dry weight of hemp by 664% compared to control, and MnO/RH-BC increased for ryegrass by 400%, 93%, 55%, and 21% at first, second, third, and fourth harvests, respectively. Overall, RH-BC and MnO/RH-BC-amended soils improved plant tolerance against Cr, highlighting their potential for phytoremediation of Cr-contaminated soil. Based on the findings, it is evident that Cr phytoaccumulation is a complex process and varies with plant species and amendment type, which are key factors to consider in future field trials on tannery wastewater-impacted Cr-contaminated soils.
1 Introduction
Soil and water contamination with heavy metals and organic pollutants poses a serious threat to global public health and the environment (Das et al., 2023; Xu et al., 2024). Various anthropogenic activities, such as tanning industry wastewater, mining and smelting operations, use of fertilizers and pesticides in agriculture, contribute to the contamination of soil, ultimately affecting the food chain (Shah and Daverey, 2020). Chromium (Cr), a toxic heavy metal, is carcinogenic (Ding et al., 2021; Deb et al., 2022). It exists mainly in two forms, hexavalent Cr (Cr(VI)) and trivalent Cr (Cr(III)). Hexavalent Cr is a highly mobile and toxic species of Cr and prevails under oxidized environments in soil (Bibi et al., 2018). Given the high toxicity and mobility of Cr(VI) in soil, there is a need to devise suitable, sustainable, and eco-friendly techniques to remediate Cr-contaminated soil, such as those impacted with Cr-containing tannery wastewater (Younas et al., 2023; Liang et al., 2021a).
Compared to conventional soil remediation techniques, such as excavation, capping, and phytoremediation, this could be a sustainable, cost-effective, and environmentally-friendly solution to remediate Cr-contaminated soil (Gebretekle et al., 2024; López Arias et al., 2024). However, the success of phytoremediation depends on the toxic metal extraction (phytoextraction) or retention in soil with roots in the presence of amendments (phytostabilization) (Khan et al., 2022). For example, phytoremediation of Cr-contaminated soil with Arundo donax L. accumulated Cr up to 2.7 mg kg-1 DW in roots than in shoots to 1.7 mg kg-1 DW (Gebretekle et al., 2024). Likewise, plant species such as Dendrocalamus asper, Bambusa vulgaris, Dendrocalamus membranaceus, and Bambusa blumeana have been reported to accumulate Cr from soil (62, 21, 56, and 148 mg kg-1 DW in shoot, respectively) (Were et al., 2017). Previous research has shown that Cannabis sativa L. (hemp) could be used for phytoremediation of heavy-metal contaminated soils. For instance, the strong root and high biomass may make it easier for heavy metals to be absorbed and stored in the root and shoot (Golia et al., 2023). Lolium perenne L. (ryegrass) has been studied for its phytoremediation potential in Cr-contaminated soils (Masotla et al., 2023). It absorbs metals within its root system and transfers to above-ground biomass (shoot) and acting as a protective strategy against metal toxicity (Masotla et al., 2023; Saldarriaga et al., 2023).
In phytoremediation, soil amendments such as biochar and nano-zero metals, and compost may decrease or limit heavy metal transfer to plants in the shoot and root. Yang et al. (2022) used biochar-supported zero-valent iron nanoparticle (BC/nZVI) to remediate Cr-contaminated soil. After 15 days of treatment, the BC/nZVI reduced soil Cr concentration by 86%. Serrano et al. (2024) tested phosphorus-loaded biochar to help ryegrass remediate cadmium, Cr, and lead-contaminated soils. The combination improved soil physicochemical parameters, plant development, and heavy metal absorption, showing biochar’s synergistic potential in phytoremediation. In a recent study, Aransiola et al., 2024 reported that application of vermicompost on potentially toxic elements (lead (Pb), cadmium (Cd), arsenic (As)) contaminated soil slightly improved their concentration in Sida acuta L. up to 1.68–10.7, 0.002–0.43, 0.27–3.79 mg kg-1 DW, respectively. Nanoscale zero-valent metals (nZVMs), such as nZV iron (Fe), zinc (Zn), nickel (Ni), and copper (Cu), may reduce metal mobility in soil and help enhance plant growth and uptake depending on different soil and plant factors, and metal type (Ranaweera et al., 2024). Such as, nanoparticles in soil improved Pb, Ni, and Cd remediation while amended with Catharanthus roseus removing 97%, 76%, and 71%; Tradescantia spathacea 93%, 73%, and 73%; and Alternanthera dentate 92%, 80%, and 76% (Jesitha et al., 2021).
To the best of our knowledge, previous research is limited to enhancing our understanding to compare the Cr accumulation potential between hemp and ryegrass plants, especially under the impact of different carbon-based amendments and nanoparticles. Here we explored the (i) efficiency of rice husk biochar (RH-BC), nano-RH-BC, vermicompost, nano-zero valent zinc (nZVZn), and manganese-modified RH-BC (MnO/RH-BC) on phytoaccumulation of Cr by hemp (C. sativa L.) and ryegrass (L. perenne L.) in tannery wastewater impacted Cr-contaminated soil; and (ii) effect of various amendments on Cr uptake and morphological and physiological attributes of both plant species. The current study is key to understand phytoremediation (phytostabilization) efficiency of hump and ryegrass, notably when Cr-contaminated soil is treated with different soil amendments.
2 Materials and methods
2.1 Preparation of rice husk biochar (RH-BC), manganese modified RH-BC, nano-zero valent zinc, and vermicompost
For the preparation of RH-BC and manganese-modified RH-BC (MnO/RH-BC), rice husk feedstock was collected from a farmer’s field in Faisalabad, Pakistan. The rice husk biomass was sun-dried before pyrolysis under oxygen-free conditions in a muffle furnace at 500°C for a 2-h residence time (Niazi et al., 2018).
To prepare MnO modified RH-BC, 2 g of RH-BC was mixed with 100 mL solution of potassium permanganate (KMnO4) (0.632 g/100 mL) in a conical flask and shaken for 8 h at 30°C constantly in an incubating shaker, and then it was allowed to settle down overnight. This biochar is referred to as manganese oxide modified RH-BC (MnO/RH-BC). The residue was rinsed with deionized water and then dried in an oven at 40°C for 24 h (Tan et al., 2018; Shaheen et al., 2022).
The synthesis of nano-zero valent zinc (nZVZn) was based on the reduction of zinc chloride (ZnCl2) with sodium borohydride (NaBH4) (Masood ul Hasan et al., 2023) (Details are given in the Supplementary Material). Vermicompost was sourced from the Department of Agronomy’s vermicompost preparation unit in the University of Agriculture Faisalabad, Pakistan, as described by Aslam et al. (2019).
All the biochars and nZVZn were characterized after preparation using attenuated total reflectance–Fourier transform infrared spectroscopy (ATR-FTIR; Alpha-II, Bruker, Germany), Brunauer–Emmett–Teller (BET) surface area, and field-emission scanning electron microscopy (FE-SEM, JEOL JSM-6500F, United States) (details are given in Supplementary Material).
2.2 Soil collection and analysis for physicochemical properties
Soil was collected from Kasur in Punjab (31°03′37.7″N; 74°16′44.4″E), Pakistan, at a depth of 0–15 cm. Soil samples were air-dried, ground, and sieved (<2 mm) before analyzing for various relevant physical and chemical properties, including electrical conductivity (EC), pH, total soil Cr, soil texture, and total organic carbon (TOC) content. Soil extract was used to determine calcium + magnesium (Ca2++Mg2+), carbonate (CO32-), bicarbonate (HCO3−), chloride (Cl−), total dissolved solids (TDS) and total suspended solids (TSS). Soil pH and EC were measured in a 1:5 suspension of soil to deionized water. The Bouycous hydrometer method was used to determine soil texture (Rayment and Lyons, 2011). Soil organic carbon (SOC) was measured using the Walkley-Black (Walkley and Black, 1934). Carbonate, HCO3−, Cl−, and Ca2+ + Mg2+ concentrations were determined in soil extract through titration (Subedi and Kumari, 2020).
2.3 Pot experiment
The pot experiment was carried out in a glasshouse at the University of Agriculture Faisalabad (UAF) using a completely randomized design (CRD) with five different treatments (RH-BC, nano-RH-BC, nZVZn, MnO/RH-BC, vermicompost) and a control (without amendment). All the treatments were replicated three times using plastic pots with 3 L capacity (11 cm diameter and 18 cm height). Soil treatments were applied at different levels because of their nature and based on some earlier knowledge (2%, wt/wt, RH-BC, nano-RH-BC, and vermicompost; 0.05% wt/wt nZVZn; 1% MnO/RH-BC). The amendment doses were selected based on prior literature, preliminary experiments, and the need to explore a range of effects from sub-optimal to potentially optimal application rates. The aim was to identify the most effective treatment to reduce Cr bioavailability while promoting plant health without inducing toxicity. Lower doses represent environmentally safe application levels, whereas higher doses test the material’s upper efficacy limits. Similar dosage ranges have been applied in previous studies examining soil remediation using biochar and nanoparticles (Qiao et al., 2019).
Pots were filled with 2 kg of Cr-contaminated soil were internally lined with a plastic sheet to limit leaching of Cr. Ten seeds of hemp and 20 seeds of ryegrass per pot were sown in three replications, and after 15 days of germination, three healthy and uniform plants of each species were maintained during the pot experiment (2 months for hemp until first harvest and 5 months for ryegrass up to four harvests).
To ensure adequate nutrient supply, recommended doses of nitrogen (N), phosphorus (P), and potassium (K) fertilizers were applied at rates of 75–25–55 mg kg-1 for hemp and 50–25–30 mg kg-1 for ryegrass. Nutrient sources included urea (for N), diammonium phosphate (DAP, for N and P), and murate of potash (MOP, for K). Ryegrass was harvested four times, allowing multiple harvests, and each harvest was done after 35 days, while hemp did not yield multiple harvests and was only harvested once after 2 months. This is the reason that ryegrass plants were grown for 5 months to have four successive harvests of plants.
After harvesting, plant samples were dried and subjected to acid digestion in the laboratory using a 1:1 ratio of nitric acid to hydrogen peroxide and analyzed for Cr using a flame atomic absorption spectrometer (F-AAS; Thermo-AA®, Solar Series, Waltham, MA, United States). The limit of detection (LOD) for total Cr was 0.01 mg L-1 and the limit of quantification (LOQ) was 0.03 mg L-1 based on the calibration curve and instrument sensitivity.
Plants were carefully uprooted from the soil to minimize root damage. Roots and shoots (aboveground biomass) were separated using sharp and clean scissors. Roots were gently washed with distilled water to remove soil particles. Excess water was blotted with a paper towel to remove surface moisture. The shoot and root fresh weight (FW) was recorded immediately after harvesting the plants. Plant samples were placed in labelled paper bags and dried in an oven at 65°C for 48 h until a constant weight was achieved. Once oven dried, the dry weight (DW) of root and shoot was recorded.
Roots were scanned using a root scanner to assess root morphology parameters like root area, root average diameter, and root number of tips. Root images were taken immediately after washing and drying to avoid shrinkage and strength.
2.4 Morphological and physiological parameters of hemp and ryegrass plants
The germination percentage (GP) was calculated based on the number of germinated seeds vs. sown seeds to assess the effect of Cr toxicity on the seed germination percentage of hemp and ryegrass. The number of leaves of hemp plants was counted from each of three plants in each of three replicates, and the recorded values were averaged to obtain a representative mean for each treatment. The height of hemp and ryegrass plants was measured for each plant in each pot in three replicates using a tape measure, and the average value is presented for each treatment. Shoot length and root length were measured using a scale meter, and plant biomass (fresh and dry weight) was recorded using a weighing balance.
The SPAD value (chlorophyll content) was measured at the vegetative stage of plants after using a chlorophyll meter. A chlorophyll meter was positioned on a lush green leaf in the morning time between 10.00 a.m. and 11.00 a.m. Three readings were taken from different leaf sites and a mean was calculated from those readings.
2.5 Chromium uptake
The root and shoot uptake of Cr was calculated as follows (Meeinkuirt et al., 2016) Equation 1:
where, shoot or root Cr concentration is given in mg Cr per 1,000 g DW, and the shoot or root dry weight is presented in g.
2.6 Translocation factor, bioaccumulation factor, and remediation factor
The translocation factor for Cr accumulation from root to shoot by both plant species was calculated as (Ignatius et al., 2014) Equation 2–4:
Bioconcentration factor for hemp and ryegrass showing their Cr accumulation from soil was calculated as:
and the remediation factor was calculated using the following formula (Sun et al., 2009):
2.7 Statistical analysis
Statistical analysis was conducted using a two-way analysis of variance (ANOVA) to assess treatment differences, followed by Tukey’s HSD test for treatment comparison. All the analyses were performed using Statistix 8.1 and Origin (version 9.65) at P ≤ 0.05. Data are presented as mean ± standard deviation (SD) from three replicates. Principal component analysis (PCA) was conducted using R software (R Team, 2010).
3 Results and discussion
3.1 Characterization of RH-BC, MnO/RH-BC and nZVZn
The pH and surface area for RH-BC, nano-RH-BC, nZVZn, and MnO/RH-BC are presented in Supplementary Table S2. Surface and morphological characterization of RH-BC, MnO/RH-BC, and nZVZn was done using SEM-EDX, X-ray diffraction, and FTIR spectroscopy (see Supplementary Figures S2, S3).
3.2 Growth of hemp and ryegrass plants
Results showed that RH-BC significantly (p ≤ 0.05) increased the GP of hemp increased the plant height, shoot length, shoot fresh and dry weight, root fresh weight, and root dry weight of hemp over the control. While nZVZn significantly (p ≤ 0.05) decreased shoot and root length, shoot fresh and dry weight, and root fresh and dry weight of hemp than the control (Table 1). Adding MnO/RH-BC to ryegrass led to a significant (p ≤ 0.05) increase in GP, length of shoots, shoot fresh and dry weight, and root fresh and dry weight. (Table 2).
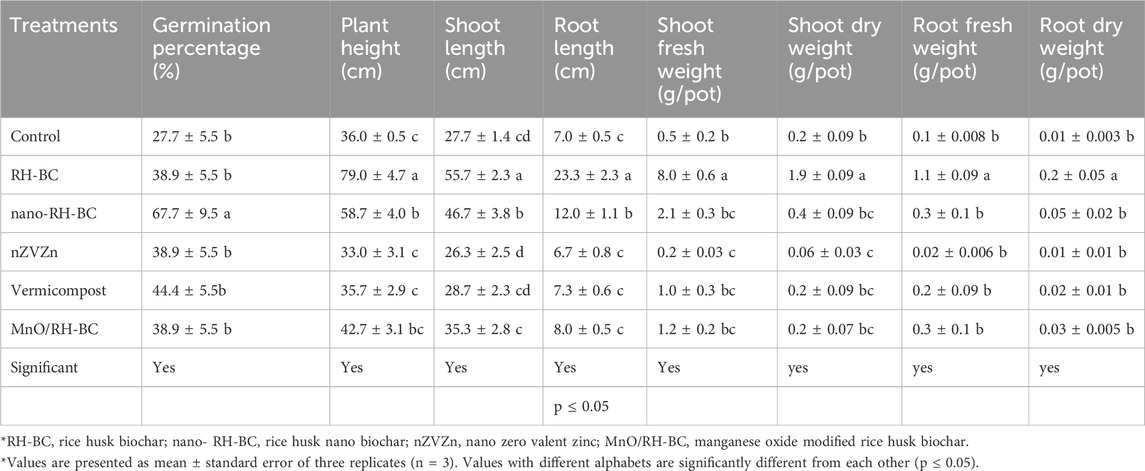
Table 1. Impact of various amendments on morphological parameters of hemp grown in chromium-contaminated soil from Kasur in Punjab, Pakistan.

Table 2. Impact of various amendments on morphological parameters of ryegrass grown in chromium-contaminated soil from Kasur in Punjab, Pakistan.
With RH-BC and nano-RH-BC, the dry weight of hemp shoots increased by 664% and 96%, respectively, over the control. However, the addition of nZVZn resulted in a 70% decrease in shoot dry weight compared to the control. Equation 1 was used to calculate root and shoot uptake of chromium. Compared to control, the shoot dry weight of ryegrass in the first cutting was increased by 150%, 250%, 200%, 250%, and 475% with RH-BC, nano-RH-BC, nZVZn, vermicompost, and MnO/RH-BC, respectively. In the second cutting, the shoot dry weight was enhanced by 104%, 80%, 53%, and 40% with MnO/RH-BC, vermicompost, nano-RH-BC, and nZVZn, respectively. The shoot dry weight of ryegrass in the third cutting was increased by 60%, 13%, and 13% in MnO/RH-BC, vermicompost, and RH-BC, and decreased by 13% and 26% in nano-RH-BC and nZVZn compared to the control. I the fourth cutting, hoot dry weight of ryegrass was increased by 27% with RH-BC and MnO/RH-BC and decreased by 33%, 16%, and 11% with nZVZn, nano-RH-BC, and vermicompost, respectively, compared to the control (Tables 1, 2).
The Equations 2-4 were used to calculate the translocation factor, bioconcentration factor, and remediation factor, respectively, for hemp and ryegrass plants. Results revealed that RH-BC significantly (p ≤ 0.05) increased the chlorophyll content of hemp over the control. While in the case of ryegrass, MnO/RH-BC, vermicompost, nano-RH-BC, and nZVZn significantly (p ≤ 0.05) increased chlorophyll content for all four cuttings compared to the control (Figures 1a,b). In the hemp root area, root average diameter and root number of tips increased with RH-BC and decreased with nZVZn compared to the control. While in the ryegrass root area, root average diameter and root number of tips increased with MnO/RH-BC compared to the control (Figure 2).
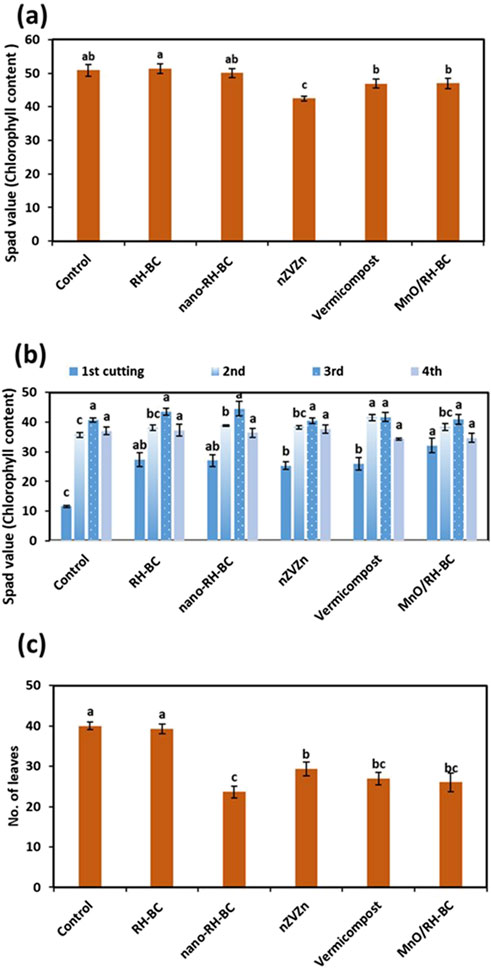
Figure 1. Effect of rice husk-biochar (RH-BC), nano-RH-BC, vermicompost, manganese modified RH-BC (MnO/RH-BC), nano-zero valent zinc (nZVZn) on SPAD value (chlorophyll content) of (a) hemp, (b) ryegrass, (c) number of leaves of hemp grown in Cr-contaminated soil.
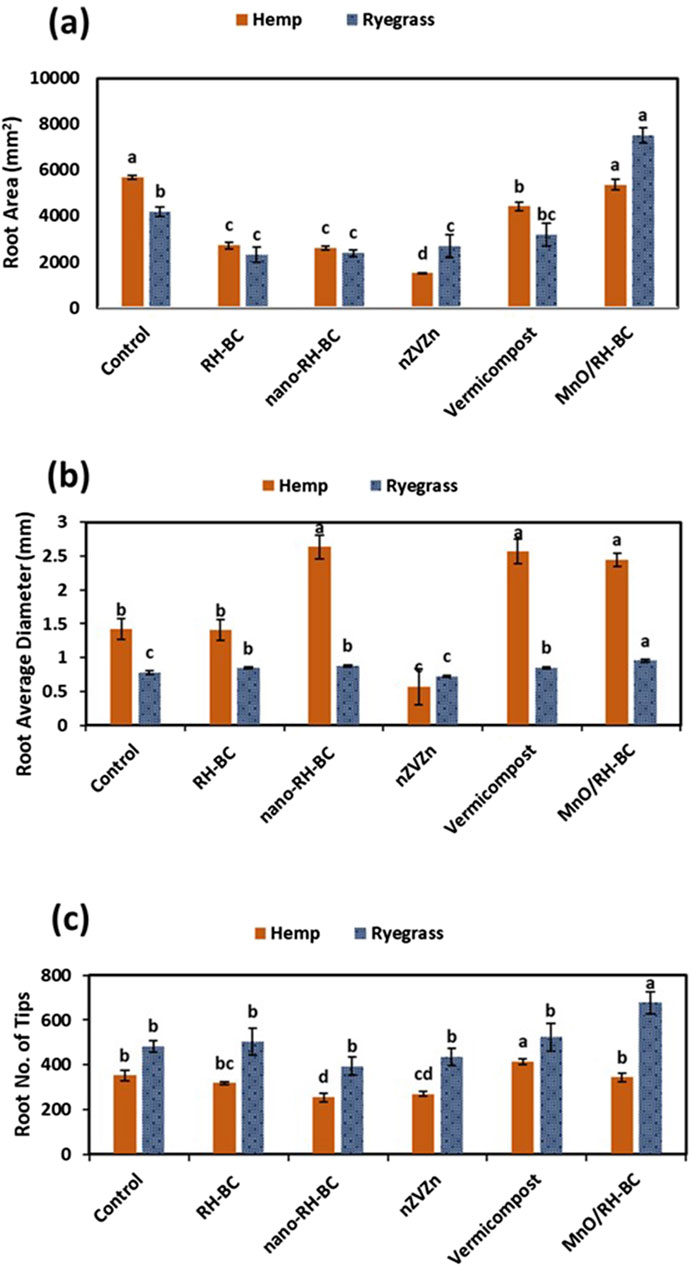
Figure 2. Effect of rice husk-biochar (RH-BC), nano-RH-BC, vermicompost, manganese modified RH-BC (MnO/RH-BC), nano-zero valent zinc (nZVZn) on (a) root areas (mm2) of hemp and ryegrass, (b) root average diameter (mm) of hemp and ryegrass, and (c) root number of tips of hemp and ryegrass grown in Cr-contaminated soil.
3.3 Chromium concentration in different plant parts of hemp and ryegrass
Compared to the control, all the treatments significantly reduced Cr concentration in the root and shoot of both hemp and ryegrass plants (p ≤ 0.05) (Figure 3). In control, maximum shoot Cr concentration in hemp was 49 ± 0.06 mg kg-1 DW and in root it was 16 ± 0.2 mg kg-1 DW. With RH-BC, hemp had lower shoot and root Cr concentration (4.6 ± 0.8 and 4.8 ± 1.0 mg kg-1 DW) than other treatments. Data showed that application of different treatments decreased or slightly increased (like nano-RH-BC, vermicompost) shoot and root Cr concentration in ryegrass and hemp plants compared to the control (Figure 3a).
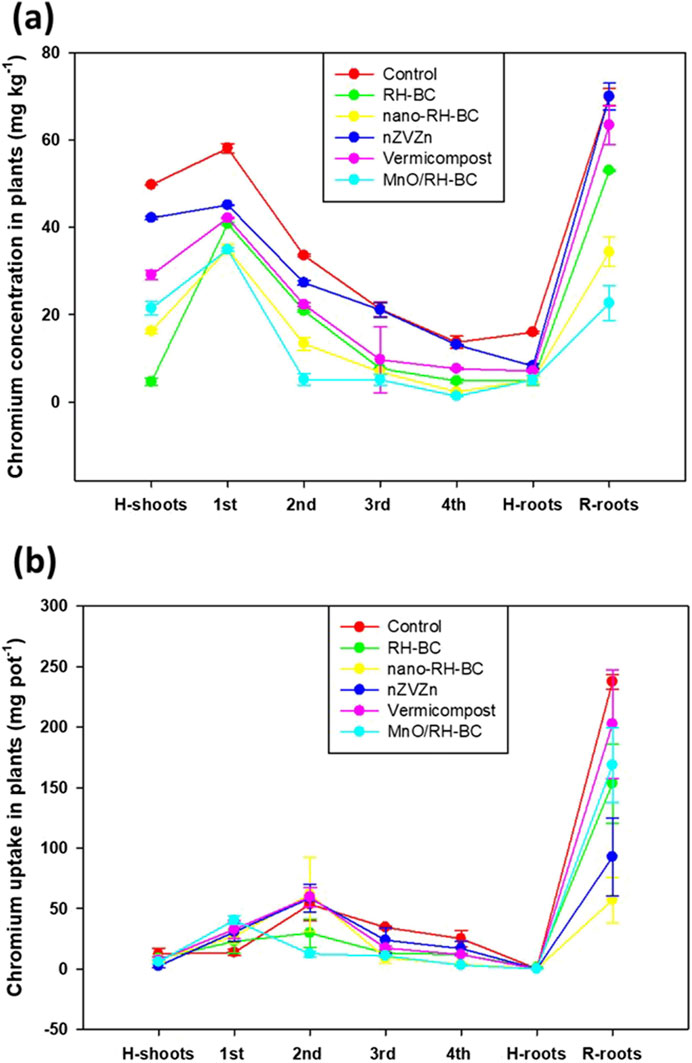
Figure 3. (a) Chromium concentration in plant parts and (b) Cr uptake in plant parts under different soil amendment treatments: rice husk-biochar (RH-BC), nano-RH-BC, vermicompost, manganese modified RH-BC (MnO/RH-BC), nano-zero valent zinc (nZVZn), grown in Cr-contaminated soils.
The addition of RH-BC and MnO/RH-BC significantly decreased plant uptake of Cr (p ≤ 0.05) in hemp and ryegrass, respectively. Data indicate that application of treatments may not be a useful approach to increase phytoremediation and metal removal by both plant species in Cr-contaminated soil in this study. However, these treatments may seem effective to support plant growth by immobilizing Cr in the soil-plant system. Rather, the shoot Cr concentration in controls was higher than in most of the applied treatments, which allows high Cr accumulation. Interestingly, with the presence of RH-BC and MnO/RH-BC, no shoot biomass loss occurred, even at a high Cr concentration of 379 ± 0.06 mg kg-1 DW in soil. For example, the maximum increase in Cr uptake in the root was 95% and 81% in the control in hemp and ryegrass, respectively.
This study aimed to evaluate the combined effects of soil amendments and plant-soil interactions on Cr accumulation by both plant species (Siebielec, and Siebielec, 2025; Kumar et al., 2020). The majority of data indicate that both plant species, especially ryegrass, alone were effective in removing Cr from soil, and the addition of soil amendments may not enhance the soil Cr remediation potential. There was a slight Cr increase with vermicompost or RH-BC, but it was not significantly different from the control, where no amendment was applied to the soil. Also, application of MnO/RH-BC may be a promising strategy for Cr phytostabilization if applied with plants or alone for soil Cr immobilization. This could be due to the presence of carboxyl (-COOH), hydroxyl (-OH), and carbonyl (-C=O) functional groups, and the presence of MnO that enhances Cr bonding to biochar (Shaheen et al., 2019).
3.4 Uptake of chromium by hemp and ryegrass
Shoot and root Cr uptake were non-significantly (p ≤ 0.05) different in both hemp and ryegrass compared to respective controls (Figure 3b). Shoot Cr uptake was maximum (12 mg pot−1) in hemp with the control treatment. The number of leaves of hemp was observed to be higher in the control and RH-BC treatments and decreased in the nano-RH-BC treatment (Figure 1c). In the case of RH-BC, nano-RH-BC, nZVZn, vermicompost, and MnO/RH-BC, there were non-significant (p ≤ 0.05) effects on shoot and root Cr uptake (Figure 3b). Hemp showed minimum shoot Cr uptake (3 mg pot−1) and root Cr uptake (0.1 mg pot−1) with nZVZn treatment compared to other treatments. The maximum uptake of Cr in ryegrass shoot was 40 mg pot-1 with MnO/RH-BC treatment in first cutting, (59 mg pot-1) with vermicompost treatment in second cutting, (34 mg pot-1) with control treatment in third (25 mg pot-1) and fourth cutting. Maximum Cr uptake in the root was (237 mg pot-1) with the control treatment in ryegrass.
In ryegrass, the minimum Cr uptake in shoot (13 mg pot-1) was recorded with control in first cutting (12 mg pot-1), MnO/RH-BC in second cutting (9 mg pot-1), nano-RH-BC in third cutting (3 mg pot-1), and MnO/RH-BC in fourth cutting. In ryegrass, minimum Cr uptake by roots (57 mg pot-1) was recorded with nano-RH-BC, for ryegrass (Figure 3b).
3.5 Pre- and post-experiment properties of soils
The physicochemical properties of the Cr-contaminated soil are presented in Table 3. Soil was classified as sandy loam, with sand (60%), silt (26%), and clay (14%). Total soil Cr was 379 mg kg-1. Soil organic matter content was 2.1%, and the soil pH was 7.1. Electrical conductivity was 0.3 dS m-1.
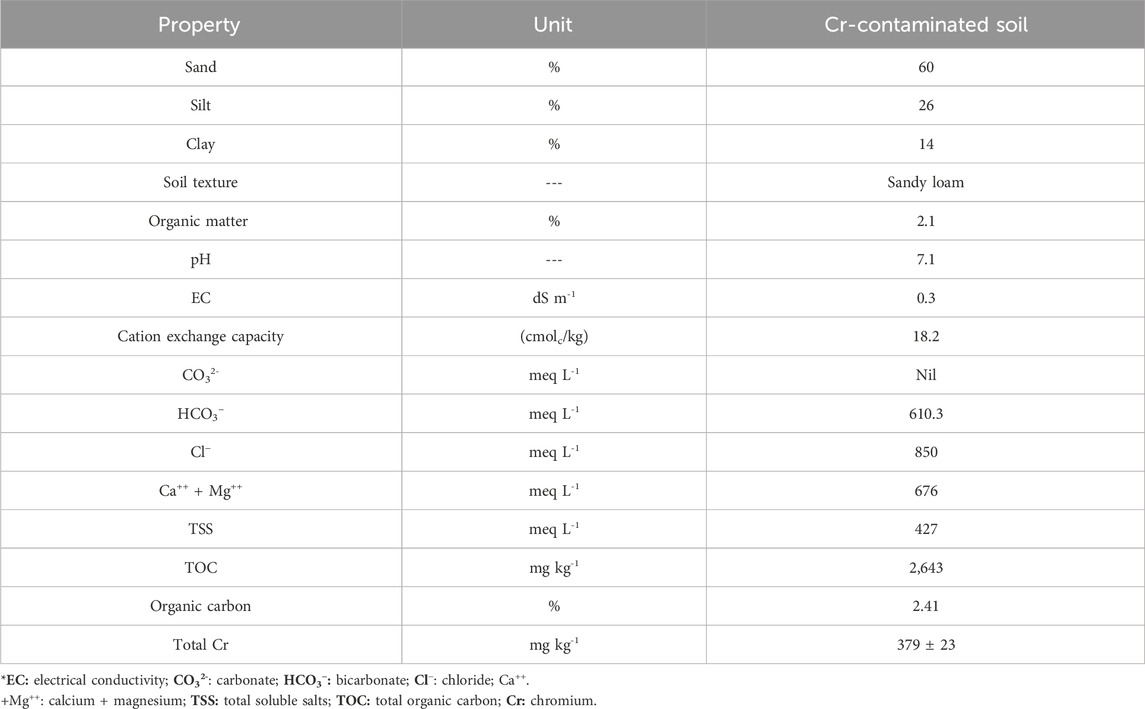
Table 3. Physico-chemical properties of tannery wastewater impacted by Cr-contaminated water from Kasur in Punjab, Pakistan.
The post-harvest chemical properties of soil after the cultivation of ryegrass and hemp under different treatments are summarized in Table 4. The comparison of post-harvest soil properties indicates that MnO/RH-BC and RH-BC treatments showed the most significant effects on improving soil properties in both plant species, with higher organic carbon and nutrient retention observed in hemp soils. These findings suggest that different plant species influence soil chemistry differently, which is critical for selecting the appropriate soil amendment strategies for phytoremediation and nutrient management.
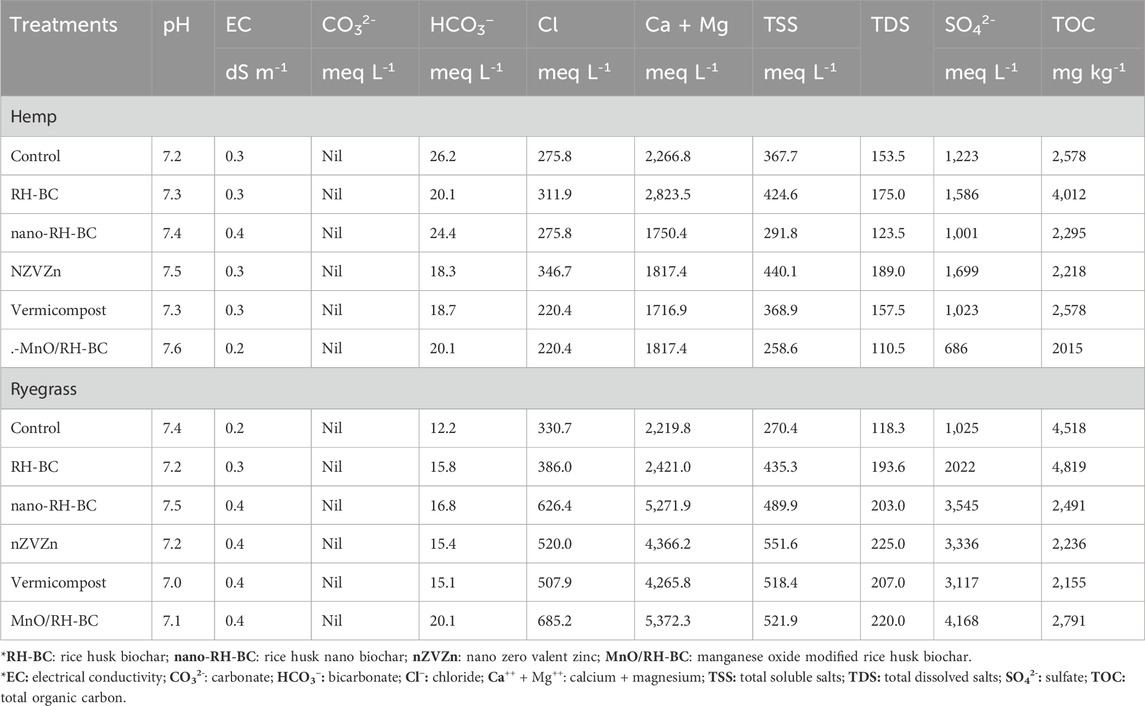
Table 4. Post-harvest soil properties after cultivation of hemp and ryegrass under different treatments.
3.6 Relationship between variables
Supplementary Figure S4 presents the correlation matrix between ryegrass and hemp parameters. The correlation matrix of hemp is presented in Panel A, revealing several pairs that were statistically significantly positively correlated. There are strong links between the Cr concentration of roots and shoots fresh weight (RCr/SFW) (r = 0.92, p ≤ 0.001), shoot Cr concentration and root Cr concentration (SCr/RCr) (r = 0.94, p ≤ 0.001), shoot dry weight and shoot Cr concentration (SDW/SCr) (r = 0.77, p ≤ 0.01), and shoot length and root length (SL/RL) (r = 0.77, p ≤ 0.01). This shows that these variables are connected. There were other correlations higher than r = 0.60, indicative of moderate to strong relationships.
Also, Panel B displays the ryegrass correlation matrix with RyCr concentration in roots/shoot length (RCr/SL) (r = 0.84, p ≤ 0.001), shoot Cr concentration/shoot length (SCr/SL) (r = 0.71, p ≤ 0.01), chlorophyll content/root Cr concentration (CC/RCr) (r = 0.74, p ≤ 0.01), and shoot dry weight/shoot Cr concentration (SDW/SCr) (r = 0.67, p ≤ 0.05). All of these relationships are positive and significant. Other correlations are greater than r = 0.50, showing moderate positive associations. For further clarity, significance levels (p-values) are now reported explicitly in the updated correlation matrix (Supplementary Figure S4).
3.7 Principal component analysis (PCA)
The intended use of PCA is to extract significant trends from the dataset, to better data presentation, and minimize dimensionality (Figure 4). It was possible to use the data for PCA since the Kaiser-Meyer-Olkin (KMO) test scores were above 0.7 and the Bartlett’s test of sphericity scores were significant (p ≤ 0.001). Before analysis, data were standardized (mean = 0, variance = 1), and outliers were removed based on standardized residuals exceeding ±3.0. PC1 and PC2 together account for 55% and 22% of the total variation in hemp, respectively, for a total of 78%. PC1 mostly separates growth-related characteristics (shoot fresh weight (SFW), shoot dry weight (SDW), and shoot length (SL)), while PC2 differentiates root-related characteristics (root dry weight (RDW), root area (RA), and root length (RL). Germination percentage is showing a negative correlation with shoot biomass variables, i.e., an inverse relation. The PCA biplot’s cos2 values reveal a heavy loading of SFW, SDW, SL, and RDW on PC1. Figure 5 shows the translocation factor, bioconcentration factor, and remediation factor of hemp and ryegrass plants in Cr contaminated soil under different treatments. This means that biomass production was the main cause of variation in hemp. Variables that include GP and chlorophyll content (CC) contribute less, suggesting that their impact during the experiment was minimal.
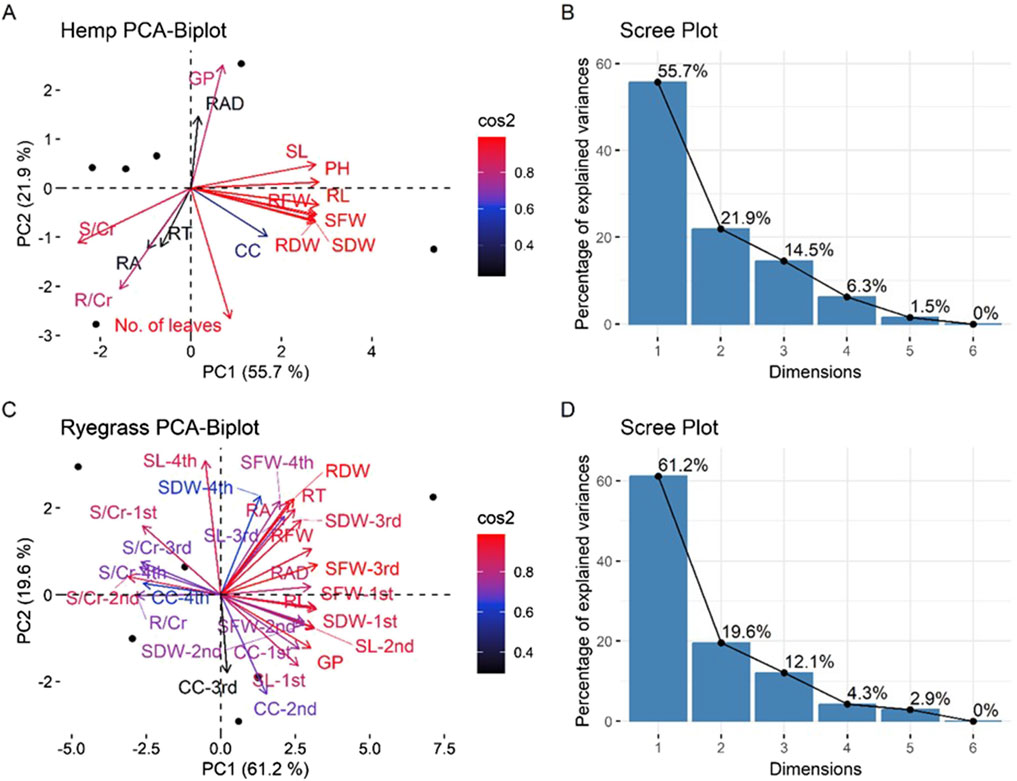
Figure 4. Principal component analysis of hemp and regress plant parameters (A) hemp, (B) scree plot for hemp, (C) ryegrass, (D) scree plot for ryegrass. The following parameters are represented as: GP, Germination percentage; SL, shoot length; SFW, shoot fresh weight; RFW, root fresh weight; SDW, shoot dry weight; RDW, root dry weight; CF, chlorophyll content; RA, root area; RAD, root average diameter; SCr, chromium concentration in shoots; RCr, chromium concentration in roots; 1, 2, 3, and 4 represent the first, second, third, and fourth cuttings of ryegrass.
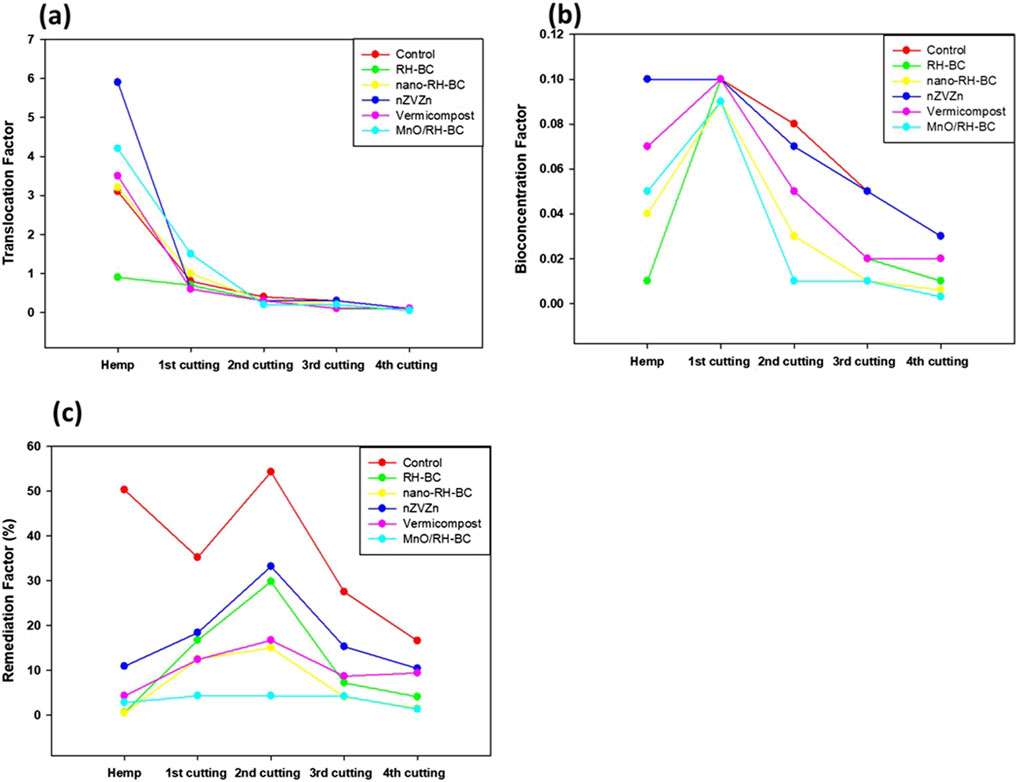
Figure 5. (a) Translocation factor, (b) bioconcentration factor, and (c) remediation factor of hemp and ryegrass plants under the effect of various treatments (rice husk-biochar (RH-BC), nano-RH-BC, vermicompost, Manganese modified RH-BC (MnO/RH-BC), nano-zero valent zinc (nZVZn) grown in Cr-contaminated soil.
For ryegrass, PC1 explains 61%, and PC2, 20%, total variance explained 81%. Once again, the shoot biomass characters (SFW, SDW, SL) dominate the hemp PC1, while the root characters dominate PC2. The cos2 scale shows that SFW, SDW, SL, and root characters load heavily on PC1, affirming their interdependence. The Cr levels in shoots (SCr) make big differences along PC1 and PC2, which means they play a part in differentiating growth. GP and CC, on the other hand, do not make much of a difference. The fact that PC1 can explain 56% of the variance in hemp and 61% of the variance in ryegrass shows that the dataset is very different. A PC1 value of more than 50% is thought to be significant for telling the difference between patterns in plant research. Shoot biomass traits are clearly separated along PC1, and root biomass traits are separated along PC2. This shows that growth allocation is a big part of how things change in different environments, like when biochar and nanoparticles are used.
Results showed that the plants in amended soils, especially RH-BC and MnO/RH-BC, exhibited enhanced growth and tolerance, including increased shoot and root dry biomass, which could be attributed to reduced metal mobility to plants. Hemp (C. sativa L.) did not survive beyond the first harvest, likely due to its comparatively lower tolerance to heavy metal stress, particularly under elevated Cr concentrations. While hemp is known for its rapid biomass production and some phytoextraction capacity, it is sensitive to high levels of Cr, which can impair root elongation, reduce photosynthetic efficiency, and induce oxidative stress. In contrast, ryegrass (L. perenne L.) is more resilient and has been widely reported to tolerate and adapt to metal-contaminated soils due to its fibrous root system and robust physiological mechanisms (Saud et al., 2022; Barbosa et al., 2015). The observed failure of hemp to establish after the first harvest could also be linked to amendment-induced changes in soil, such as micronutrient imbalances, and potential phytotoxicity at higher amendment doses. This species-specific difference underscores the importance of selecting appropriate plant species for phytoremediation based on tolerance thresholds and environmental adaptability (Testa et al., 2023; Ali et al., 2013; Citterio et al., 2003).
With RH-BC and nano-RH-BC treatments, the shoot dry weight of hemp was increased by 664% and 96%, respectively. However, the addition of nZVZn resulted in a 70% decrease compared to the control. Compared to the control, the shoot dry weight of ryegrass was enhanced by 475%, 104%, 60%, and 27% in MnO/RH-BC in first to fourth cuttings. The positive effect of BC was previously reported by Jabborova et al. (2021a) that the addition of 2% and 3% biochar to soil significantly increased shoot dry weight by 24% and 38% over the control. The application of modified biochar made from rice husk led to an increase in the shoot dry weight of the maize plants. (Maharlouei et al., 2021).
In hemp root area, root diameter and root number of tips increased with RH-BC and decreased with nZVZn compared to the control. In ryegrass, the root area, root diameter, and root number of tips increased with MnO/RH-BC compared to the control (Figure 2). These results are in agreement with Jabborova et al. (2021a), who reported an increase in root diameter of lettuce by 22% after being treated with 1% biochar. In another study, compared to control, biochar treatments at 2% and 3% showed the greatest improvement in root morphological characteristics of lettuce (Jabborova et al., 2021b). The authors observed that basil plants treated with 3% biochar had 47% more root surface area, 37% more root diameter, and 45% more root volume than controls.
Different mechanisms are involved to reduce Cr accumulation in plants by biochar and nano zero-valent metals (Li et al., 2017). Figure 4b shows a scree plot for hemp and Figure 4d shows a scree plot for ryegrass plants. While nZVZn increased Cr uptake in ryegrass and hemp, biochar alone or in combination reduced Cr bioavailability in soil through adsorption and complexation mechanisms (Ahmad et al., 2014; Kumpiene et al., 2008). The observed decrease in Cr uptake in hemp and ryegrass under biochar treatments reflects this immobilization effect, supporting the stabilization pathway rather than phytoextraction. These contrasting trends highlight the importance of selecting amendment strategies tailored to the phytoremediation mechanism (extraction vs. stabilization) and plant species used (Ali et al., 2013; Beesley et al., 2011).
Vermicompost can chelate and minimize Cr toxicity, in addition to providing nutrients (Pande et al., 2007). For example, in an earlier study, magnetic Enteromorpha prolifera biomass-derived biochar resulted in immobilization of Cr in soil and decreased its availability (Liang et al., 2021b). Likewise, the application of combined amendments (apatite, biochar, and organic fertilizer) reduced bioavailable Cd and Cr concentrations in soil in comparison to the control (Hong et al., 2022). The observed reduction in bioavailable Cr may be attributed to both physical and chemical mechanisms. Rice husk biochar contains abundant oxygenated functional groups (e.g., –COOH, –OH), which can complex with Cr(III) or promote the reduction of Cr(VI) to the less toxic and less mobile Cr(III) form (Ahmad et al., 2014; Xu et al., 2022). Rice husk biochar is rich in oxygen-containing functional groups such as hydroxyl (–OH), carboxyl (–COOH), and carbonyl (C=O) groups. Functional groups on the biochar surface can form complexes with Cr(III), effectively immobilizing it (Chatzimichailidou et al., 2023).
In MnO-amended treatments, manganese oxides serve as redox-active materials that facilitate the immobilization of Cr(VI) by promoting its reduction to the less toxic Cr(III) form through electron transfer processes and co-precipitation mechanisms (Fan et al., 2022). The MnO/rice husk biochar composite acts synergistically—biochar enhances surface area and provides electron-donating functional groups, while MnO contributes redox buffering capacity and catalyzes Cr transformation (Tariq et al., 2025). These mechanisms collectively contribute to reduced Cr mobility and uptake, especially in hemp, which is more sensitive to Cr-induced oxidative stress.
Guo et al. (2020) found that both the root and shoot Cr concentration of Ipomoea aquatica decreased with biochar and foliar application of selenium compared to the control. For phytoremediation purposes in Cr-contaminated soils, particularly when combined with soil amendments like RH-BC, nZVZn, vermicompost, and MnO/RH-BC ryegrass may be more suitable than hemp because it yielded more biomass under Cr contamination. It is worth mentioning that future studies should be directed to evaluate speciation of Cr in different pools in soil amended with these treatments, which may provide key information on Cr immobilization or release after amendment application by the two different plant species investigated here.
In hemp, the trend of uptake of Cr was control > RH-BC > nano-RH-BC > vermicompost > MnO/RH-BC > nZVZn. While in the roots of hemp, the trend of uptake of Cr was RH-BC > control > nano-RH-BC > MnO/RH-BC > vermicompost > nZVZn. In ryegrass, the maximum Cr uptake was with MnO/RH-BC at the first cutting, vermicompost at the second cutting, and control at the third and fourth cuttings. In the roots of ryegrass, the maximum uptake of Cr was noted in the control. Chromium in soil and plants comes from industrial effluents, however, contamination in plant vegetative sections varies by accumulation capability. The correlation data indicate that Cr in soil and plant tissues has a harmful negative growth of ryegrass plants.
4 Conclusion
This study shows that RH-BC, MnO/RH-BC, and vermicompost slightly increased hemp and ryegrass growth in Cr-contaminated soil. Hemp did not survive well, while ryegrass gave four successful cuttings, with a decreasing trend in shoot and root Cr concentration in ryegrass from the first to the fourth cutting. Data indicated that RH-BC improved hemp growth in Cr stress and performed better than nZVZn, vermicompost, and MnO/RH-BC. In ryegrass, MnO/RH-BC led to better growth of plants than the RH-BC, nZVZn, and vermicompost. Overall, this study shows that application nZVZn accumulates more Cr in hemp and ryegrass than in the other treatments. The observed decrease in Cr uptake in hemp and ryegrass under biochar treatments reflects this immobilization effect, supporting the stabilization pathway. It is evident from the findings that the accumulation of Cr in the two plant species was dependent on amendment type and plant species, which is key to understanding before applying remediation strategies on a large scale in field trials. Further research is warranted to evaluate long-term stability and impact on plant growth and Cr (im)mobilization in the soil, especially in different soil textures and properties.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
AZ: Conceptualization, Formal Analysis, Methodology, Visualization, Writing – original draft. NN: Conceptualization, Supervision, Validation, Project administration, Writing – review and editing. ZS: Conceptualization, Supervision, Writing – review and editing. MS: Writing – review and editing. KH: Writing – original draft. ZH: Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors are thankful to Dr Saddam Hussain for providing industrial hemp seeds and Dr Zubair Aslam for helping prepare vermicompost at the Department of Agronomy, University of Agriculture, Faisalabad. Thanks are also extended to four reviewers for constructive feedback to improve the quality of the paper. The author would like to express her heartfelt gratitude to the Higher Education Commission (HEC) of Pakistan for granting the invaluable opportunity to conduct collaborative research at the University of Florida, United States, through its International Research Supporting Initiative Program (IRSIP) scholarship (No. I-8/HEC/HRD//2023/12834).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2025.1558255/full#supplementary-material
References
Ahmad, M., Rajapaksha, A. U., Lim, J. E., Zhang, M., Bolan, N., Mohan, D., et al. (2014). Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere 99, 19–33. doi:10.1016/j.chemosphere.2013.10.071
Ali, H., Khan, E., and Sajad, M. A. (2013). Phytoremediation of heavy metals—concepts and applications. Chemosphere 91, 869–881. doi:10.1016/j.chemosphere.2013.01.075
Aransiola, S. A., Josiah, I. U. J., Abioye, O. P., Bala, J. D., Rivadeneira-Mendoza, B. F., Prasad, R., et al. (2024). Micro and vermicompost assisted remediation of heavy metal contaminated soils using phytoextractors. Case Stud. Chem. Environ. Eng. 9, 100755. doi:10.1016/j.cscee.2024.100755
Aslam, Z., Bashir, S., Hassan, W., Bellitürk, K., Ahmad, N., Niazi, N. K., et al. (2019). Unveiling the efficiency of vermicompost derived from different biowastes on wheat (Triticum aestivum L.) plant growth and soil health. Agron 9 (12), 791. doi:10.3390/agronomy9120791
Barbosa, B., Boléo, S., Sidella, S., Costa, J., Duarte, M. P., Mendes, B., et al. (2015). Phytoremediation of heavy metal-contaminated soils using the perennial energy crops Miscanthus spp. and Arundo donax L. BioEner. Res. 8, 1500–1511.
Beesley, L., Moreno-Jiménez, E., Gomez-Eyles, J. L., Harris, E., Robinson, B., and Sizmur, T. (2011). A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environ. Poll. 159, 3269–3282. doi:10.1007/s12155-015-9688-9
Bibi, I., Niazi, N. K., Choppala, G., and Burton, E. D. (2018). Chromium (VI) removal by siderite (FeCO3) in anoxic aqueous solutions: an X-ray absorption spectroscopy investigation. Sci. Total Environ. 640, 1424–1431. doi:10.1016/j.envpol.2011.07.023
Chatzimichailidou, S., Xanthopoulou, M., Tolkou, A. K., and Katsoyiannis, I. A. (2023). Biochar derived from rice by-products for arsenic and chromium removal by adsorption: a review. J. Comp. Sci. 7, 59. doi:10.3390/jcs7020059
Citterio, S., Santagostino, A., Fumagalli, P., Prato, N., Ranalli, P., and Sgorbati, S. (2003). Heavy metal tolerance and accumulation of Cd, Cr and Ni by Cannabis sativa L. Plant soil 256, 243–252. doi:10.1023/a:1026113905129
Das, S., Sultana, K. W., Ndhlala, A. R., Mondal, M., and Chandra, I. (2023). Heavy metal pollution in the environment and its impact on health: exploring green technology for remediation. Environ. Health Insig. 17, 11786302231201259. doi:10.1177/11786302231201259
Deb, A. K., Biswas, B., Naidu, R., and Rahman, M. M. (2022). Mechanistic insights of hexavalent chromium remediation by halloysite-supported copper nanoclusters. J. Hazard. Mater. 421, 126812. doi:10.1016/j.jhazmat.2021.126812
Ding, K., Zhou, X., Hadiatullah, H., Lu, Y., Zhao, G., Jia, S., et al. (2021). Removal performance and mechanisms of toxic hexavalent chromium (Cr (VI)) with ZnCl2 enhanced acidic vinegar residue biochar. J. Hazard. Mater. 420, 126551. doi:10.1016/j.jhazmat.2021.126551
Fan, J., Qin, L., Duan, T., Qi, Z., and Zou, L. (2022). Preparation of MnOx-modified biochar and its removal mechanism for Cr (VI) in aqueous solution. Water 14, 2507. doi:10.3390/w14162507
Gebretekle, B. G., Teklu Gebretsadik, T., Mekonnen, K. N., and Asgedom, A. G. (2024). Insights on phytoremediation of chromium from tannery wastewater contaminated soil. Int. J. Phytorem. 1-9.
Golia, E. E., Bethanis, J., Ntinopoulos, N., Kaffe, G. G., Komnou, A. A., and Vasilou, C. (2023). Investigating the potential of heavy metal accumulation from hemp. The use of industrial hemp (Cannabis Sativa L.) for phytoremediation of heavily and moderated polluted soils. Sustain. Chem. Phar. 31, 100961. doi:10.1080/15226514.2024.2366252
Guo, X., Ji, Q., Rizwan, M., Li, H., Li, D., and Chen, G. (2020). Effects of biochar and foliar application of selenium on the uptake and subcellular distribution of chromium in Ipomoea aquatica in chromium-polluted soils. Ecotoxicol. Environ. Saf. 206, 111184. doi:10.1016/j.ecoenv.2020.111184
Hong, Y., Li, D., Xie, C., Zheng, X., Yin, J., Li, Z., et al. (2022). Combined apatite, biochar, and organic fertilizer application for heavy metal co-contaminated soil remediation reduces heavy metal transport and alters soil microbial community structure. Sci. Total Environ. 851, 158033. doi:10.1016/j.scitotenv.2022.158033
Ignatius, A., Arunbabu, V., Neethu, J., and Ramasamy, E. V. (2014). Rhizofiltration of lead using an aromatic medicinal plant Plectranthus amboinicus cultured in a hydroponic nutrient film technique (NFT) system. Environ. Sci. Poll. Res. 21, 13007–13016. doi:10.1007/s11356-014-3204-1
Jabborova, D., Kadirova, D., Narimanov, A., and Wirth, S. (2021a). Beneficial effects of biochar application on lettuce (Lactuca sativa L.) growth, root morphological traits and physiological properties. Ann. Phytomed 10, 93–100. doi:10.21276/ap.2021.10.2.13
Jabborova, D., Ma, H., Bellingrath-Kimura, S. D., and Wirth, S. (2021b). Impacts of biochar on basil (Ocimum basilicum) growth, root morphological traits, plant biochemical and physiological properties and soil enzymatic activities. Scien. Horticul. 290, 110518. doi:10.1016/j.scienta.2021.110518
Jesitha, K., Jaseela, C., and Harikumar, P. S. (2021). “Nanotechnology enhanced phytoremediation and photocatalytic degradation techniques for remediation of soil pollutants,” in Nanomater. Soil remed. (Elsevier), 463–499.
Khan, A. U., Khan, A. N., Waris, A., Ilyas, M., and Zamel, D. (2022). Phytoremediation of pollutants from wastewater: a concise review. Open Life Sci. 17 (1), 488–496. doi:10.1515/biol-2022-0056
Kumar, V., Sharma, P. K., Jatav, H. S., Singh, S. K., Rai, A., Kant, S., et al. (2020). Organic amendments application increases yield and nutrient uptake of mustard (Brassica juncea) grown in chromium-contaminated soils. Commun. Soil Sci. Plant Anal. 51 (1), 149–159. doi:10.1080/00103624.2019.1695831
Kumpiene, J., Lagerkvist, A., and Maurice, C. (2008). Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments–a review. Waste Manag. 28, 215–225. doi:10.1016/j.wasman.2006.12.012
Li, H., Dong, X., da Silva, E. B., de Oliveira, L. M., Chen, Y., and Ma, L. Q. (2017). Mechanisms of metal sorption by biochars: biochar characteristics and modifications. Chemosphere 178, 466–478. doi:10.1016/j.chemosphere.2017.03.072
Liang, J., Huang, X., Yan, J., Li, Y., Zhao, Z., Liu, Y., and Wei, Y. (2021a). A review of the formation of Cr (VI) via Cr (III) oxidation in soils and groundwater. Sci. Total Environ. 774–145762.
Liang, L., Xi, F., Tan, W., Meng, X., Hu, B., and Wang, X. (2021b). Review of organic and inorganic pollutants removal by biochar and biochar-based composites. Biochar 3, 255–281. doi:10.1007/s42773-021-00101-6
López Arias, T. R., Franco, D., Medina, L., Benítez, C., Villagra, V., McGahan, S., et al. (2024). Removal of Chromium (III) and reduction in toxicity in a primary tannery effluent using two floating macrophytes. Toxics 12, 152. doi:10.3390/toxics12020152
Maharlouei, Z. D., Fekri, M., Saljooqi, A., Mahmoodabadi, M., and Hejazi, M. (2021). Effect of modified biochar on the availability of some heavy metals speciation and investigation of contaminated calcareous soil. Environ. Earth Sci. 80, 119–120. doi:10.1007/s12665-021-09418-8
Masood ul Hasan, I., Javed, H., Hussain, M. M., Shakoor, M. B., Bibi, I., Shahid, M., et al. (2023). Biochar/nano-zerovalent zinc-based materials for arsenic removal from contaminated water. Int. J. Phytorem. 25, 1155–1164. doi:10.1080/15226514.2022.2140778
Masotla, M. K. L., Melato, F. A., and Mokgalaka-Fleischmann, N. S. (2023). Extraction potential of Lolium perenne L.(Perennial rye grass) for metals in landfill soil: its tolerance and defense strategies. Minerals 13, 873. doi:10.3390/min13070873
Meeinkuirt, W., Kruatrachue, M., Pichtel, J., Phusantisampan, T., and Saengwilai, P. (2016). Influence of organic amendments on phytostabilization of Cd-contaminated soil by Eucalyptus camaldulensis. Sci. Asia. 42, 83–91. doi:10.2306/scienceasia1513-1874.2016.42.083
Niazi, N. K., Bibi, I., Shahid, M., Ok, Y. S., Shaheen, S. M., Rinklebe, J., et al. (2018). Arsenic removal by Japanese oak wood biochar in aqueous solutions and well water: investigating arsenic fate using integrated spectroscopic and microscopic techniques. Sci. Total Environ. 621, 1642–1651. doi:10.1016/j.scitotenv.2017.10.063
Pande, P., Chand, S., Yadav, V. K., Anwar, M., and Patra, D. D. (2007). Influence of Chromium with vermicompost on growth and accumulation by Brahmi. Commun. Soil Sci. Plant Anal. 38, 2815–2829. doi:10.1080/00103620701663057
Qiao, J., Yu, H., Wang, X., Li, F., Wang, Q., Yuan, Y., et al. (2019). The applicability of biochar and zero-valent iron for the mitigation of arsenic and cadmium contamination in an alkaline paddy soil. Biochar 1, 203–212. doi:10.1007/s42773-019-00015-4
Ranaweera, R. W., Jayaweera, V., De Silva, N., Wijesekara, H., Gunatilake, S., Mubarak, A., et al. (2024). Synthesis of nano zero valent zinc-reduced graphene oxide nanocomposite using a novel electrochemical technique for the adsorptive degradation of methyl orange. Colloids Surfaces A Physicochem. Eng. Asp. 703, 135250. doi:10.1016/j.colsurfa.2024.135250
Rayment, G. E., and Lyons, D. J. (2011). Soil chemical methods: Australasia 3. Clayton, Victoria, Australia: CSIRO Publishing.
Saldarriaga, J. F., López, J. E., Díaz-García, L., and Montoya-Ruiz, C. (2023). Changes in Lolium perenne L. rhizosphere microbiome during phytoremediation of Cd-and Hg-contaminated soils. Environ. Sci. Poll. Res. 30, 49498–49511.
Saud, S., Wang, D., Fahad, S., Javed, T., Jaremko, M., Abdelsalam, N. R., et al. (2022). The impact of chromium ion stress on plant growth, developmental physiology, and molecular regulation. Front. Plant Sci. 13, 994785. doi:10.1007/s11356-023-25501-y
Serrano, M. F., López, J. E., Henao, N., and Saldarriaga, J. F. (2024). Phosphorus-loaded biochar-assisted phytoremediation to immobilize cadmium, chromium, and lead in soils. ACS omega 9, 3574–3587. doi:10.1021/acsomega.3c07433
Shah, V., and Daverey, A. (2020). Phytoremediation: a multidisciplinary approach to clean up heavy metal contaminated soil. Environ. Technol. Innov. 18, 100774. doi:10.1016/j.eti.2020.100774
Shaheen, S. M., Mosa, A., El-Naggar, A., Hossain, M. F., Abdelrahman, H., Niazi, N. K., et al. (2022). Manganese oxide-modified biochar: production, characterization and applications for the removal of pollutants from aqueous environments-a review. Bioresour. Technol. 346, 126581. doi:10.1016/j.biortech.2021.126581
Shaheen, S. M., Niazi, N. K., Hassan, N. E., Bibi, I., Wang, H., Tsang, D. C., et al. (2019). Wood-based biochar for the removal of potentially toxic elements in water and wastewater: a critical review. Int. Mater. Rev. 64, 216–247. doi:10.1080/09506608.2018.1473096
Siebielec, S., and Siebielec, G. (2025). Comparing effects of soil amendments on plant growth and microbial activity in metal-contaminated soils. Sustain 17 (5), 2135. doi:10.3390/su17052135
Subedi, T., and Kumari, M. (2020). An investigation on physico-chemical properties of soil in pokhara. IJRAR-Int. J. Res. Anal. Rev. (IJRAR) 7, 707–710. Available online at: https://ijrar.org/papers/IJRAR2001959.pdf.
Sun, Y.-bing, Qi-xing, Z., Jing, An, Liu, W.-tao, and Liu, R. (2009). Chelator-enhanced phytoextraction of heavy metals from contaminated soil irrigated by industrial wastewater with the hyperaccumulator plant (Sedum alfredii Hance). Geoderma 150 (1-2), 106–112. doi:10.1016/j.geoderma.2009.01.016
Tan, G., Wu, Y., Liu, Y., and Xiao, D. (2018). Removal of Pb (II) ions from aqueous solution by manganese oxide coated rice straw biochar A low-cost and highly effective sorbent. J. Taiwan Inst. Chem. Eng. 84, 85–92. doi:10.1016/j.jtice.2017.12.031
Tariq, R., Imran, M., Nadeem, M., Murtaza, B., Iqbal, J., Shah, N. S., et al. (2025). Exploring Cu-oxide and Mn-oxide impregnated biochar nanocomposites for sustainable chromium removal from water: reusability and kinetic studies. Chem. Eng. Sci. 308, 121410. doi:10.1016/j.ces.2025.121410
Testa, G., Corinzia, S. A., Cosentino, S. L., and Ciaramella, B. R. (2023). Phytoremediation of cadmium-lead-and nickel-polluted soils by industrial hemp. Agron 13, 995. doi:10.3390/agronomy13040995
Walkley, A., and Black, I. A. (1934). An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 37, 29–38. doi:10.1097/00010694-193401000-00003
Were, F. H., Wafula, G. A., and Wairungu, S. (2017). Phytoremediation using bamboo to reduce the risk of chromium exposure from a contaminated tannery site in Kenya. J. Health Poll. 7, 12–25. doi:10.5696/2156-9614-7.16.12
Xu, W., Jin, Y., and Zeng, G. (2024). Introduction of heavy metals contamination in the water and soil: a review on source, toxicity and remediation methods. Green Chem. Lett. Rev. 17, 2404235. doi:10.1080/17518253.2024.2404235
Xu, Z., Wan, Z., Sun, Y., Gao, B., Hou, D., Cao, X., et al. (2022). Electroactive Fe-biochar for redox-related remediation of arsenic and chromium: distinct redox nature with varying iron/carbon speciation. J. Hazard. Mater. 430, 128479. doi:10.1016/j.jhazmat.2022.128479
Yang, J., Tan, X., Shaaban, M., Cai, Y., Wang, B., and Peng, Q. A. (2022). Remediation of Cr (VI)-Contaminated soil by biochar-supported nanoscale zero-valent iron and the consequences for indigenous microbial communities. Nanomater 12, 3541. doi:10.3390/nano12193541
Keywords: contamination, phytostabilization, carbon-based amendments, nanoparticles, chromium
Citation: Zulfqar A, Niazi NK, Saqib ZA, Shahid M, Hussain K and He Z (2025) Phytoaccumulation of chromium by hemp and ryegrass in rice husk biochars, nano-zero valent zinc and vermicompost amended tannery wastewater soil. Front. Environ. Sci. 13:1558255. doi: 10.3389/fenvs.2025.1558255
Received: 09 January 2025; Accepted: 14 May 2025;
Published: 17 June 2025.
Edited by:
Mohammad Faizan, Maulana Azad National Urdu University, IndiaReviewed by:
Paula S. Tourinho, Masaryk University, CzechiaNadeesha Ukwattage, University of Colombo, Sri Lanka
V. N. Meena Devi, Noorul Islam University, India
Haider Sultan, Hainan University, China
Copyright © 2025 Zulfqar, Niazi, Saqib, Shahid, Hussain and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nabeel Khan Niazi, bmFiZWVsa25pYXppQGdtYWlsLmNvbQ==; Zhenli He, emhlQHVmbC5lZHU=
 Amna Zulfqar
Amna Zulfqar Nabeel Khan Niazi
Nabeel Khan Niazi Zulfiqar Ahmad Saqib
Zulfiqar Ahmad Saqib Muhammad Shahid2
Muhammad Shahid2 Zhenli He
Zhenli He