- 1Department of Plankton and Microbial Ecology, Leibniz-Institute of Freshwater Ecology and Inland Fisheries (IGB), Stechlin, Germany
- 2Department of Chemical Sciences, University of Padua, Padova, Italy
- 3Institute for Nanomaterials, Advanced Technologies, and Innovation, Technical University of Liberec, Liberec, Czechia
- 4Key Laboratory of Aquatic Botany and Watershed Ecology, Wuhan Botanical Garden, Chinese Academy of Sciences, Wuhan, China
- 5College of Life Sciences, University of Chinese Academy of Sciences, Beijing, China
- 6Danjiangkou Wetland Ecosystem Field Scientific Observation and Research Station, Chinese Academy of Sciences and Hubei Province, Wuhan, China
- 7Institute of Biochemistry and Biology, University of Potsdam, Potsdam, Germany
Introduction: Plastic pollution poses a significant and increasing threat to aquatic ecosystems, i.e. contaminating water resources and posing health risks for humans and the environment. Yet, plastic leachates can also stimulate microbial growth and activities, impacting biochemical cycles in aquatic ecosystems. Synthetic polymers and their leachates vary in their chemical composition and thus differently impact micro- and higher organisms. This study aims to assess: i) how different synthetic polymer leachates affect free-living aquatic bacteria, and ii) how these effects vary at high vs. low nutrient conditions.
Methods: Leachates were extracted from five synthetic polymers, i.e. low-density polyethylene (LDPE), polypropylene (PP), polyvinyl chloride (PVC), starch-polylactic acid (starch-PLA), and tire rubber via incubation in ultrapure water under UV radiation. Free-living (<5.0 μm) microbial communities of Lake Stechlin, Germany, were exposed to these leachates, and changes in total microbial growth and community composition were analysed using dose response models.
Results: Nutrient availability resulted in different effects on total microbial growth and community composition of the tested synthetic polymers. For instance, PP leachates caused significant community shifts with increased total microbial growth rates at low nutrient conditions, but not at high nutrient conditions, whilst starch-PLA leachates led to community shifts at both nutrient conditions, but didn’t impact total microbial growth.
Discussion: These results highlight the importance of leachate quality and nutrient availability for understanding the effects of leachates on microbial growth and community dynamics. Our findings reveal that synthetic polymer pollution has the potential to alter microbial loop functioning and hence biochemical cycles of aquatic ecosystems.
GRAPHICAL ABSTRACT | Graphical Abstract created in BioRender. Schubert, M. (2025) https://BioRender.com/w84p641.
Highlights
• Leachates of different materials showed varying effects on microbial growth rates and community composition
• Differences in effects of various leachates at low nutrient conditions were dampened at high nutrient conditions
• Inhibitory effects of leachates were maintained at both nutrient conditions, causing significant community shifts
1 Introduction
Plastic production has steadily increased worldwide, reaching >450 million tons in 2019 (OECD, 2022). Plastics have become increasingly diverse in chemical compositions (Chalmin, 2018), and, presently, numerous often unknown additives are added to plastics to modify their mechanical and chemical properties such as plasticizers, flame retardants, impact modifiers, antioxidants, UV-stabilizers and antimicrobials (Hahladakis et al., 2018). These additives are distributed unequally between plastic types and usages. For instance, in 2014, 90% of plasticizers produced were reportedly used for polyvinyl chloride (PVC) plastic, which is the fourth most common plastic-type to date (Czogała et al., 2021).
These chemicals have been reported to leach from plastic into the environment due to ageing processes (Bandow et al., 2017). Plastic aging is the process by which plastics undergo physical and chemical changes over time due to environmental factors such as UV radiation, temperature, mechanical stress, and microbial activity. Microbial activity may also alter the structure of plastics by breaking the bonds and using the polymers as an energy source (Salinas et al., 2023). For example, low density polyethylene (LDPE) is one of the most produced plastic types and releases mainly monoterpenes and fatty acids among the “high priority chemicals in plastics” according to ToxCast data (Zimmermann et al., 2019). Starch-polylactic acid (starch-PLA), a supposedly biodegradable alternative to petrol-based plastics requires a mixture of plasticizers, softeners, and elastomer tougheners to be used for plastic bags (Koh et al., 2018). In particular tire rubber granulates release various chemical cocktails composed of PAHs, bisphenols, phenols, and heavy metals into the environment (Halsband et al., 2020).
Leachates pose variable risks to organisms and the environment (Gunaalan et al., 2020). For instance, plastics leachates, to a large extent, are responsible for the observed adverse effects of plastics on aquatic (micro)organisms such as growth inhibition of the microalgae Dunaliella teriolecta when exposed to polyethene (PE), polystyrene (PS) or polypropylene (PP) (Schiavo et al., 2021). Additionally, plastic leachates of LDPE, PS, and PLA have been shown to cause shifts in the community composition of exposed marine bacteria (Birnstiel et al., 2022). As plastic leachate compositions and quantities can greatly vary between plastic types, we hypothesized that leachates of various synthetic polymers result in variable effects on the growth and community composition of free-living bacteria in freshwater.
Plastics, primarily composed of organic carbon, can release leachates that serve as a carbon source for various aquatic bacteria. For instance, in lake ecosystems, leachates from low-density polyethylene (LDPE) have been shown to increase bacterial biomass by 2.29 times when added at environmentally relevant concentrations (Sheridan et al., 2022). The study also found that bacterial growth efficiency improved by an average of 1.72 times, as the organic matter released from plastic leachates was more accessible than the natural organic matter in boreal lakes of Northern Europe. This has significant ecological implications. According to the microbial loop concept (Azam et al., 1983), bacterial uptake of dissolved organic carbon (DOC) is critical for channelling otherwise inaccessible carbon into aquatic food webs. When leachate DOC, derived from primary and secondary plastic constituents, contributes to bacterial biomass production, it enters nutrient cycles primarily through the consumption of bacteria by protists, potentially altering food web dynamics. Consequently, plastic pollution may coincide with carbon and nutrient enrichment in aquatic environments (Abreo et al., 2015). The ecological impact of plastic leachates likely varies depending on the type of plastic and its influence on microbial growth, potentially reshaping aquatic food web structures and ecosystem functions.
The objectives of this study were twofold: 1) to compare the effects of LDPE, PP, PVC, starch-PLA, and tire rubber leachates on growth rates and community composition of pelagic bacteria of a lake ecosystem, and 2) to investigate how the observed adverse effects change with nutrient availability result from the leaches from plastic. First, plastic leachates were extracted from five commercial synthetic polymer products (LDPE, PP, PVC, compostable starch-PLA, tire crumb rubber). LDPE, PP, PVC, and tire crumb rubber were selected for their high relative occurrences in aquatic environments (Uurasjärvi et al., 2020; Yuan et al., 2019; Capolupo et al., 2020) as well as for their documented effects on aquatic microorganisms (Sheridan et al., 2022; Schiavo et al., 2021; Sarker et al., 2020; Halsband et al., 2020). The starch-PLA bag was selected for comparison with a form of “biodegradable” plastics. Then, complex, free-living bacterial communities from Lake Stechlin were exposed to the extracted leachates at different nutrient conditions.
2 Materials and methods
2.1 Materials
Commercially available polymers were used to generate leachates differing in chemical quality and quantity. LDPE plastic bags (“Beste Wahl Gefrier-Beutel”, Rewe, Cologne, Germany), PP rope (“PP-seil”, Toom, Cologne, Germany), PVC floor (“Grauer PVC-Boden”, Toom, Cologne, Germany), compostable plastic bags, made of starch-PLA (Gut und Günstig Kompostierbare Bio-Beutel), and weathered tire (Bridgestone Europe, Saventem, Belgium) were used for leachate production as described in the section below.
2.2 Preparation of plastic leachates
The extraction of the synthetic polymer leachate was performed following the method of Sheridan et al. (2022). Briefly, before the incubation, all plastic items were treated with 70% ethanol to avoid interferences (through carryover of microorganisms or organics) in leachate release during incubations. Plastic items were cut into approximately 1 cm2 pieces before weighing. For tire rubber, tire crumbs of variable sizes were produced using a hardened steel file on the outer-most layer of a weathered tire (Bridgestone). The tire crumbs were cleaned and disinfected in 70% ethanol, which evaporated entirely before incubation. Plastics were added to 500 mL Milli Q Water in glass beakers at concentrations of 8.88 g/L. The Milli-Q water used in the study was taken from a Millipore® filtration system (RiOs™ Essential 16 Water Purification System). All samples were incubated in triplicates for 7 days at 20°C–25°C on shakers (100 rpm) using a 12 h:12 h cycle of UV-light illumination to increase the weathering of all plastic pieces (Klein, et al., 2021). UVC irradiation was performed by a TNN 15/35 lamp that emits a single narrow line at 254 nm. Here, we applied the spectral irradiance of about 2.6 W cm−2 for an irradiating distance of 50 cm from the lamp to the surface of the water. All samples were kept under cling film to avoid organic carbon contamination from air. In addition, Milli Q water without any addition of plastics (control H2O) was incubated alongside all plastic-containing samples. After incubation, plastic leachate samples were filtered using 3x pre-rinsed, sterile 0.22 µm polycarbonate filters (Nucleopore) to remove any plastic particles as well as any microbial contamination. Our initial results showed that 1x filtration through polycarbonate filters resulted in a number of contaminated samples, in our final protocol, all leachates were filtered twice. Leachate samples were analysed using the Total Organic Carbon analyser TOC-L (Shimazdu CPH-CSN, Japan) for DOC concentrations using the EN 1484 method guidelines with oxidation. This metric was selected as a good proxy for amount of leachates produced since organic carbon constitutes the largest component of all plastic leachates.
2.2.1 Statistical analysis
Standard t-tests (Student) were used for statistical analysis. The experiment was repeated five times. For each repetition, an average leaching rate was determined based on the average of triplicates, subtracting the DOC concentration in the respective controls. Samples were statistically compared to the LDPE leaching rates.
2.3 Complex community exposure
2.3.1 Bacterial community incubations
Complex communities of lake microorganisms were sampled from the well-mixed epilimnion of Lake Stechlin in Germany (53:10N, 13:02E) at a depth of 2 m. Lake water was filtered through pre-rinsed, sterile 5.0 µm polycarbonate filters (Nucleopore, Whatman, Cytiva, Kent, United Kingdom) to retain the free-living fraction of the microbial communities and avoid contamination by particulate organic matter. Filtered lake water was transferred into sterilized Erlenmeyer flasks and the microbial communities thereof were exposed to a concentration of 0.167 mL Leachate.mL−1.
As such, the final concentrations of DOC from plastic leachates were: 46.9 mg DOC L−1 for starch-PLA, 0.4 mg DOC L−1 for LDPE, 9.7 mg DOC L−1 for PP, 4.2 mg DOC L−1 for PVC, and 3.5 mg DOC L−1 for tire rubber exposure groups. To control groups were also incubated, one exposed to the control water of the plastic leachate preparation (control H2O), the other exposed to sterile Milli-Q water (0-control). For LB medium incubations, LB medium was added to a final concentration of 1:200 (ca. 60 mg DOC L−1). The DOC concentration of the sampled lake water was at approximately 6 mg L−1. A large headspace in the Erlenmeyer flasks ensured oxic conditions during the entire incubation. Triplicates were used for each experimental condition. All flasks were incubated on a shaker at 50 rpm in continuous light for 72 h (OECD, 2011). The ambient temperature was 17°C to avoid any influence of a sudden temperature change. Samples, taken from each flask before and after incubation, were fixed with formaldehyde (3% fin. conc.). The rest of the samples was filtered through 0.22 µm Durapore filters and kept frozen at −70°C for later DNA extraction.
2.3.2 Bacterial growth rate determination
Microbial growth rate was calculated using change in bacterial cell counts over time. Cell numbers were counted before and after incubation using a flow cytometer (BD FACS Aria). The samples were stained by DAPI (Sigma-Aldrich, Burlington, United States) (fin. conc. 5 μg mL−1) according to the protocol of Porter and Feig (1980). To determine the correct threshold, a sample was filtered sterile to exclusively measure the background noise to be subtracted as a blank. Additionally, certain samples were randomly selected to be counted manually by fluorescence microscopy to ensure that flow cytometer readings were correct. As the filtration method in the leachate isolation experiment revealed contaminated samples in this experiment, certain expositions and their respective controls were repeated to avoid any bacterial contamination.
2.3.3 Community composition analysis
DNA was extracted from frozen filters of the complex community exposition experiment using the phenol-chloroform method (Montero-Pau et al., 2008). Extracted DNA was quantified through Qubit and the relevant marker genes were PCR-amplified before sending the DNA for 16S rRNA gene amplicon sequencing. The V4 region (515F–806R primers) of the 16S rRNA gene (2 x ∼250 bp) was sequenced on a MiSeq platform (Illumina, San Diego, United States). The sequence data were processed using the DADA2 pipeline (Callahan et al., 2016). Adapters were removed before further analysis. Quality profile analysis revealed a significant decline in quality beyond 270 bases for forward reads and 220 bases for reverse reads. Consequently, the reads were trimmed at these points. Ambiguous bases were removed, bases with a quality score below 2 were truncated, and reads with an expected error rate greater than 2 were discarded.
The DADA2 machine learning algorithm was employed to train error models based on the filtered sequences. The sequences were then dereplicated using the dereplication function. Forward and reverse reads were merged, and chimeric sequences were removed. Finally, merged reads were assigned to taxonomy using the IDTAXA algorithm (Murali et al., 2018) with the Silva database v138.1. Taxonomic classification was performed using the RDP Bayesian classifier algorithm within DADA2.
2.3.4 Statistical analysis
Total abundances between samples groups were compared using t-tests (Student) or Mann-Whitney when the group did not follow a normal distribution.
Total reads per sample were deemed sufficient, ranging between approximately 41,000 and 75,000 reads. Alpha diversity was estimated using the Shannon index. Either regular t-tests (Student) or the Mann-Whitney U test were used to calculate significant differences in comparison to control samples. Bray-Curtis dissimilarity matrices were established for β-diversity. The statistical significance of differences was checked using PERMANOVA. The “vegan” package (Oksanen et al., 2022) was used for visualization of β-diversity using an NMDS analysis. Relative read abundances were used as a proxy for relative abundances of genera present in the sample to compare proportional changes of different genera between leachate exposure groups. Percentages of reads of a genus were compared between exposure groups using standard t-tests (Student).
2.4 Exposure to different concentrations
Leachates of PP, PVC and tire rubber were selected for exposure experiment as they have yielded significant differences in total abundance and growth rates of bacteria compared to the control groups in our previous experiment (2.2.1).
2.4.1 Communities exposure
Complex communities of free-living bacteria from Lake Stechlin were used, and leachates were diluted with sterile Milli-Q water to create four exposure concentrations in the treatments: 0.00167, 0.0167, 0.083, and 0.167 mL leachate per mL liquid. This resulted in final DOC concentrations of 0.09, 0.85, 4.26, and 8.52 mg DOC per L for PP; 0.03, 0.31, 1.57, and 3.14 mg DOC per L for PVC; and 0.04, 0.43, 2.16, and 4.32 mg DOC per L for tire rubber. Control samples were exposed to the same concentration series using control water (control H2O) from the preparation of plastic leachates (2.2), with two additional controls added to account for potential dilution effects: one using double-filtered (0.22 µm) and autoclaved Lake Stechlin water and the other using sterile Milli-Q water (0-control). To increase nutrient and organic matter concentrations, exposure groups were also repeated with the addition of LB medium at a final concentration of 1:200 (ca. 60 mg DOC L−1). The DOC concentration of the sampled lake water was at approximately 6 mg L−1. Triplicates were prepared for all experimental conditions to ensure reproducibility.
2.4.2 Growth rate estimation
Samples of each culture were taken before and after 72 h of incubation and fixed with formaldehyde (3% fin. conc.). Cell abundance was estimated as described above for the complex community experiment.
2.4.3 Statistical analysis
Dose-response models were applied using the R-packages drc (Ritz et al., 2015) and minpack.lm (Elzhov et al., 2023) for regression analysis and determining the EC50 values.
3 Results
3.1 Leachate concentrations based on DOC
The compostable, biodegradable plastic bag (starch-PLA) yielded the highest leaching rates (17.8 mg DOC week−1 g polymer−1, p = 2.128e−07), followed by the PP rope (4.3 mg DOC week−1 g polymer−1, p = 6.118e−09) (Figure 1). PVC floor parts and the tire crumb rubber also leached significant amounts of DOC (1.5 mg DOC week−1 g polymer−1 and 1.1 mg DOC week−1 g polymer−1, respectively, p = 8.467e−05 and p = 0.003643), though these leaching rates were consistently lower than for the PP rope (4.3 mg DOC week−1 g polymer−1, p = 6.118e−09). LDPE leached the lowest amount of DOC as compared to all other synthetic polymer products, and the amount of DOC in the LPDE leachates were close or occasionally even lower than in the control sample (Figure 1). Though leaching rates of LDPE were on average 0.15 mg DOC week−1 g polymer−1 (whilst subtracting the controls), the leaching rates were not significantly different from the controls.
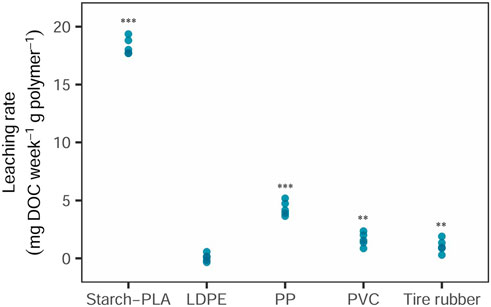
Figure 1. Leaching rates of synthetic polymer products. Leaching rate is calculated as DOC amount released by a given amount of synthetic polymer (mg DOC week−1 g polymer−1). Synthetic polymers included: Starch-PLA: Starch Polylactic Acid, LDPE: Low Density PolyEthylene, PP: PolyPropylene, PVC: PolyVinylChloride, and Tire rubber: Tire crumb rubber. Statistical analysis consisted of t-tests (Student) ** represents significance of p < 0.01; *** represents significance of p < 0.001.
3.2 Microbial growth rate changes
No significant differences in microbial growth rates occurred between the 0-control and control H2O samples at low nutrient conditions (p = 0.11 and p = 0.48 at low nutrient conditions; p = 0.07 and p = 0.34 at high nutrient conditions). As there was an insignificant difference, control H2O samples were used for comparison with exposure samples. At low nutrient conditions (Figure 2), strong growth stimulation was noticed for PP (p = 0.00742) and PVC leachate samples (p = 0.0039). A statistically significant inhibition was noticed for the tire rubber leachate when compared with either the 0-control or control H2O samples (p = 0.006217). However, neither LDPE nor compostable bag leachates showed any significant difference in bacterial growth to control H2O samples. At high nutrient conditions (Figure 2), however, only PVC leachates revealed a significant increase in specific growth rate when compared to control H2O (p = 0.003904). When comparing the normalized growth rates, there was a significant difference for PP (p = 0.006) and tire rubber leachates (p = 0.002) between the two nutrient conditions. For both leachate types, however, the addition of nutrients caused insignificant differences to the control H2O samples in total abundance and growth rates.
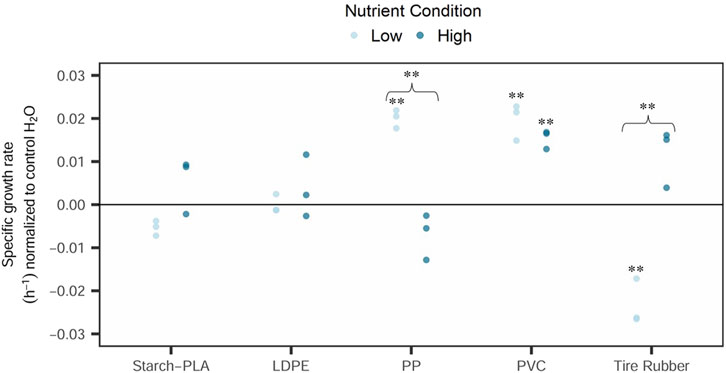
Figure 2. Microbial growth of natural free-living bacteria communities (<5.0 µm) in a 72-h in vitro exposition to synthetic polymer leachates at high vs. low nutrient conditions. Synthetic polymers included: Starch-PLA: Starch Polylactic Acid, LDPE: Low Density PolyEthylene, PP: PolyPropylene, PVC: PolyVinylChloride, and Tire rubber: Tire crumb rubber. Control H2O samples were exposed to the control sample of the leachate extraction experiment. Leachate exposure samples were normalized to the control H2O group by subtracting the median of this group to all values. All samples were run in triplicate. Statistical analysis consisted of t-tests (Student) or Mann-Whitney U test when the respective group does not follow a normal distribution. ** represents a statistical significance of p < 0.01. The curly brace indicates a significant difference between the same leachate types at different nutrient conditions. Both were compared by using the normalized values for each condition.
3.3 Compositional changes
Overall, there were stronger differences in α-diversity between plastic leachate types at low nutrient conditions than at high nutrient conditions (Figure 3). Starch-PLA, PP, and PVC exposure groups showed a significantly stronger decrease in α-diversity than the LDPE exposure group (p = 0.002, p = 0.03, p = 0.001 respectively). This decrease in α-diversity was reflected by the average Shannon indices, which ranged between 0.3 and 0.5 units lower than control samples. Yet, the LDPE exposure group also showed a significant decrease in α-diversity when compared to control H2O samples. The tire rubber exposure also tended toward a decrease in α-diversity though a high variability rendered it insignificant.
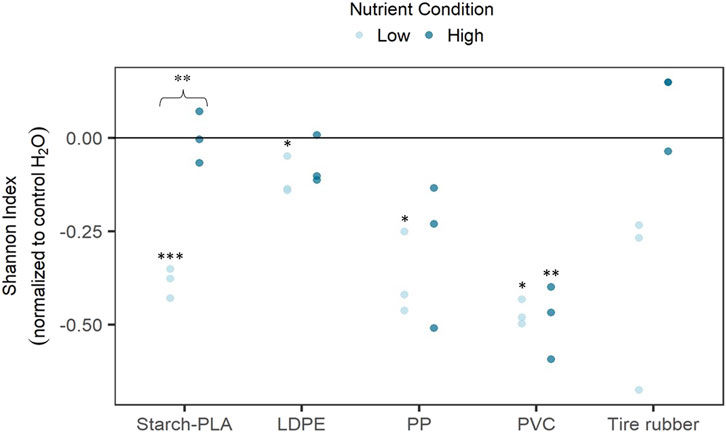
Figure 3. Alpha diversity (Shannon index normalized to the control H2O sample) of natural free-living bacteria (<5.0 µm) after a 72-h in vitro exposition to synthetic polymer leachates at high and low nutrient conditions. Synthetic polymers included: Starch-PLA: Starch Polylactic Acid, LDPE: Low Density PolyEthylene, PP: PolyPropylene, PVC: PolyVinylChloride, and Tire rubber: Tire crumb rubber. Control H2O samples were exposed to the control sample of the leachate extraction experiment. Leachate exposure samples were normalized to the control H2O group by subtracting the median of this group to all values. All samples were run in triplicate. * represents a statistical significance of p < 0.05. ** represents a statistical significance of p < 0.01. Statistical analysis consisted of t-tests (Student) or Mann-Whitney U test when the respective group does not follow a normal distribution. *** represents a statistical significance of p < 0.001. The curly brace indicates a significant difference between the same leachate types at different nutrient conditions. Both were compared by using the normalized values for each condition.
At high nutrient conditions, the only significant decrease in α-diversity occurred in the PVC exposure (p = 0.006). Though the Shannon index for the PP exposure tended to decrease as well, it was insignificant due to the high variance between samples. The starch-PLA exposure was the only exposure group which had significantly different α-diversities between both nutrient conditions (p = 0.003). For tire rubber exposure, this difference was insignificant due to high variability between samples of the same exposure groups.
At high nutrient conditions, the only significant decrease in α-diversity occurred in the PVC exposure group (p = 0.006). Though the Shannon index for the PP exposure group tended to decrease as well, it was insignificant due to the high variance between samples. The starch-PLA exposure was the only exposure group which had significantly different α-diversities between both nutrient conditions (p = 0.003). For tire rubber exposure, this difference was insignificant due to the high variability between samples of the same exposure groups.
NMDS analysis revealed that LDPE exposure was most similar to control H2O samples (Figure 4). Additionally, there were clear differences between all synthetic polymer exposure groups. PERMANOVA using Bray-Curtis distances showed significant differences in community composition between each leachate exposure group and the respective control H2O groups under low nutrient conditions (starch-PLA: p = 0.005, LDPE: p = 0.004, PP: p = 0.005, PVC: p = 0.013, tire rubber: p = 0.004). However, it is evident on the NMDS (Figure 4), that the community composition of the LDPE exposure group was the most similar to the control groups compared to all other exposure groups.
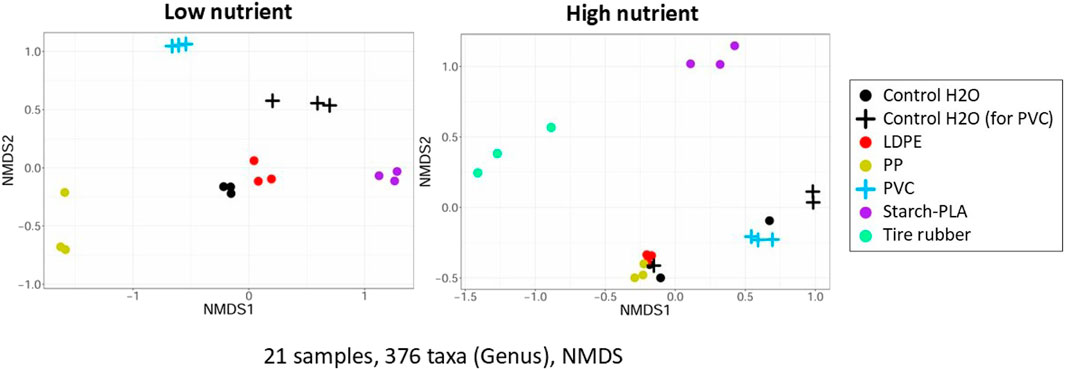
Figure 4. NMDS of Bray Curtis distances of natural free-living bacteria (<5.0 µm) after a 72 h in vitro exposition to synthetic polymer leachates at high and low nutrient conditions. Synthetic polymers included: Starch-PLA: Starch Polylactic Acid, LDPE: Low Density PolyEthylene, PP: PolyPropylene, PVC: PolyVinylChloride, and Tire rubber: Tire crumb rubber. Control H2O samples were exposed to the control sample of the leachate extraction experiment. Leachate exposure samples were exposed to the leachates of the plastic type listed. As the experiment was repeated for the PVC exposure group, this exposure group and its respective control are represented with a plus sign rather than dots.
At high nutrient conditions, only bacterial communities of the tire rubber and starch-PLA exposures were significantly different from the respective control H2O (starch-PLA: p = 0.036, tire rubber: p = 0.037). In contrast, control groups, PP, and LDPE exposures were overlapping. The PVC exposure was also not significantly different according to PERMANOVA. Note that PP exposure at low nutrient conditions was significantly different from the controls, yet at high nutrient conditions, they did not differ from the control H2O exposure (p = 0.435). Similarly, the PVC exposure at high nutrient conditions was also not significantly different from the control H2O exposure at high nutrient conditions (p = 0.318), despite clear differences at low nutrient conditions.
At low nutrient conditions, significant differences occurred between communities exposed to different synthetic polymer types (Figure 5). After exposure to starch-PLA, the hgcI-clade of Actinomycetota and Pseudorhizobium dominated these samples (on average 10.03% ± 2.19% and 5.4% ± 0.79), but Vogesella and Acinetobacter were present in only low proportions (0.09% ± 0.03% and 0.14% ± 0.12, respectively). Additionally, Rhodoferax was entirely absent from these samples. For the tire rubber exposure, the hglc-clade (19.3% ± 0.28) and Pseudomonas (4.8% ± 0.02) formed the dominant groups in these samples. Similar to the starch-PLA exposure, the proportion of Vogesella was also reduced (0.09% ± 0.03). On the other hand, Vogesella (26.2% ± 1.48) and Acinetobacter (25.3% ± 4.35) were by far the two most dominant genera in the PP exposures.
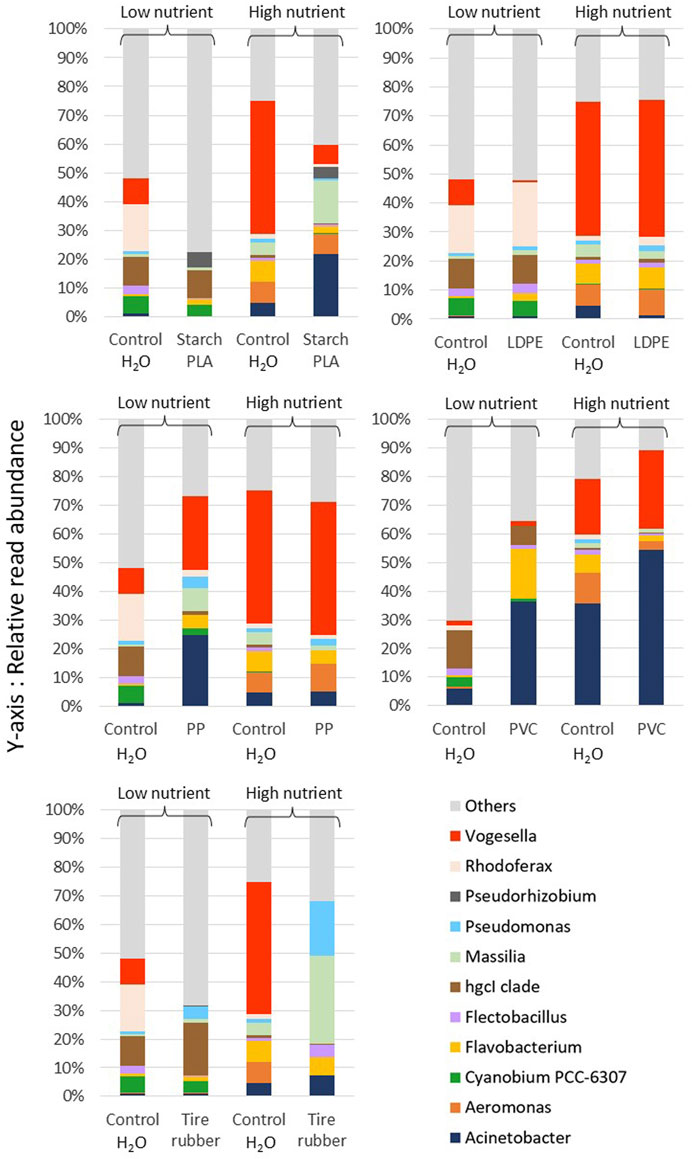
Figure 5. Average relative abundances of reads assigned to the most common genera of free-living freshwater bacteria (<5 µm) in each sample exposed to synthetic polymer leachates at high and low nutrient conditions. Synthetic polymers included: Starch-PLA: Starch Polylactic Acid, LDPE: Low Density PolyEthylene, PP: PolyPropylene, PVC: PolyVinylChloride, and Tire rubber: Tire crumb rubber. Control H2O samples were exposed to the control sample of the leachate extraction experiment. Leachate exposure samples were exposed to the leachates of the plastic type listed. N = 3 for all sample groups except “Control H2O” at high nutrient conditions and tire rubber leachates at low nutrient conditions for which N = 2, due to significant variation in relative abundance of Acinetobacter which skewed the average.
In the PVC exposure, Acinetobacter (36.73% ± 1.15) and Flavobacterium (17.7% ± 0.22) were the most dominant genera. The LDPE exposure group was most similar to control H2O samples, with Rhodoferax (23.5% ± 3.44) and hgcI-clade of Actinomycetota (5.37% ± 2.16) forming, on average, the most dominant groups. Though some bacterial genera seem to be common in several exposures, individual leachate exposures differed in their overall composition (see Figure 4), especially the most dominant bacterial genera.
At high nutrient conditions, also at genus level resolution, there were smaller differences between leachate exposures and controls. The only pronounced compositional changes compared to the control H2O samples occurred in starch-PLA and tire rubber exposures. For starch-PLA exposure, the dominant genera were Acinetobacter and Massilia (21.8% ± 4.32% and 15.0% ± 7.10, respectively), though there was some variability between replicates. On the other hand, Pseudomonas and Massilia were dominant after tire rubber leachate exposure (19.2% ± 1.32% and 30.8% ± 5.48, respectively). For all other exposures, i.e., LDPE, PP and PVC, differences in relative read abundance were insignificant between exposure groups and the respective controls.
Concerning effects on community composition, PVC was the only leachate type for which significant effects on total abundance and growth rates were observed at high nutrient conditions (Figure 2). Our bacterial community composition analysis indicated that this may be the result of a strong proliferation of Acinetobacter and Vogesella, the two dominant genera in the control H2O samples at high nutrient conditions (Figure 5). This may also explain why there is a significant decrease in α-diversity at high nutrient conditions (Figure 3), whilst β-diversity was insignificantly different to control H2O samples. Starch-PLA, despite it had no effect on total bacterial abundance, it resulted in significant community shifts at both nutrient conditions (Figure 4).
For the PP leachate exposure, however, addition of nutrients seemingly dampened the stimulatory effects on total bacterial abundance and growth rate as well as community shifts (Figures 2–5). For the tire rubber exposure groups, however, slight inhibitions across microbial communities occurred at low nutrient conditions. At high nutrient conditions, there was an apparent stimulation of growth rates for certain bacterial genera, which was seemingly equalled by stronger inhibitions, for instance of Vogesella (Figure 5). This may explain the lack of differences in overall growth rates compared to control H2O samples (Figure 2), whilst there were significant differences in β-diversity when compared to the control H2O at both nutrient conditions (Figure 4).
3.4 Concentration series exposure
At low nutrient conditions, there was a significant concentration effect (p = 5.0e−04) for PP leachates exposures (Supplementary Figure 1a). The sigmoidal dose-response model was fitted to the PP leachate concentration series with an R2 value of 0.868. Growth rates yielded an EC50 value of 0.43 mg DOC L−1 (Figure 6a). For PVC leachates (Supplementary Figure 1a), there was also a significant concentration effect (p = 3.0e−05), but only at low nutrient concentrations. The sigmoidal dose-response model was fitted to the PVC leachate concentration series with an R2 value of 0.761. These revealed a higher EC50 4.74 mg DOC L−1 (Figure 6b). Though there is a disparity in the EC50 values for PP and PVC, it is important to note that the specific growth rate effects at these concentrations for PP and PVC exposures at the highest tested concentrations was on average 0.01 h−1 for both exposure groups.
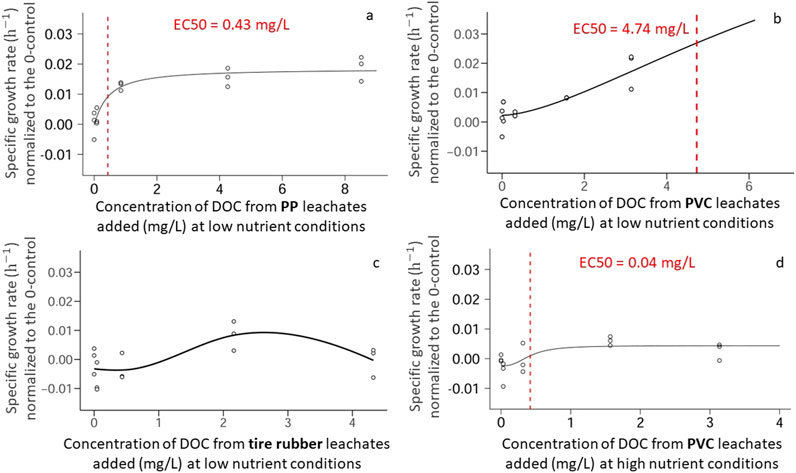
Figure 6. Dose-response models for different synthetic polymer leachates regarding microbial growth rates of natural free-living bacteria (<5.0 µm) from Lake Stechlin over a 72-h exposure period at either high or low nutrient conditions. (a) Exposures to PolyPropylene (PP) at low nutrient conditions. (b) Exposures to PolyVinyl Chloride (PVC) at low nutrient conditions. (c) Exposures to tire crumb rubber at low nutrient conditions. (d) Exposures to PolyVinyl Chloride (PVC) at high nutrient conditions. Concentration is expressed in volume per volume as this better represents the differences in leaching rates between plastic types.
The tire rubber leachates (Figure 6c), on the other hand, did not show data befitting a sigmoidal function thus no EC50 could be calculated. The specific growth rate for the second highest concentration of tire leachate (2.16 mg DOC L−1) was significantly (p = 0.04459, 0.02138) higher than in the samples of 0.00167- and 0.0167 mL Leachate mL−1. However, a linearized model was not statistically significant (p = 0.22). Since the R2 value of this model was only 0.11, a linearized model is likely not ideal to represent this data. Therefore, a LOESS function was used to describe this dataset, which yielded an R2 value of 0.812.
At high nutrient conditions, there was a significant increase in growth rate (Figure 6d) for samples which were exposed to PVC leachates when compared to control samples (p = 0.04). A dose response model was created for PVC at high nutrient conditions as the linearized model was statistically significant. The sigmoidal function yielded an R2 value of 0.465. The EC50 value for the PVC exposure groups at high nutrient conditions was estimated at 0.04 mg DOC L−1. Despite this lowered EC50 value at high compared to low nutrient conditions, the estimated change in specific growth rate at the EC50 concentration was much lower at 0.0001 h−1 (compared to 0.027 h−1 at low nutrient conditions). The linearized models did not show any other significant changes in growth for any other sample (Supplementary Figure 1b).
Overall, changes in total specific growth rates at low nutrient conditions were weaker or disappeared at high nutrient conditions. This was most obvious for PP, though inhibitory effects of PVC were also weaker at high nutrient conditions.
4 Discussion
4.1 Effects of synthetic polymer leachates
Our experiments showed that plastic leachates of different synthetic polymer types have varying effects on microbial communities. A part of these variations can be explained by the differences in leaching rates among the various plastic products used in our study. Another factor is DOC quality of the different leachates and hence bacterial substrate availability (Romera-Castillo et al., 2022). The starch-PLA bag showed leaching rates which were 4–5 times higher than those of the PP rope. However, when natural free-living bacterial communities from Lake Stechlin were exposed to starch-PLA leachates, no significant differences in total growth rates were observed (Figure 2). In contrast, significant community shifts occurred at both high and low nutrient conditions in exposures to starch-PLA leachates, whilst no significant changes in growth rates were recorded (Figure 4). This indicates that starch-PLA leachates are interacting with the microbial communities, causing stimulation as well as inhibition, depending on the respective bacterial genera. This is reinforced by results of our exposure experiment of selected bacterial isolates from Lake Stechlin (Supplementary Figure 2). Thereafter, at high nutrient conditions, increased growth rates were observed for Flavobacterium, whereas a trend towards growth inhibition was observed for Sphingomonas. As starch-PLA leached the highest amounts of organic carbon, the lack of a strong stimulation of any genus implies that the main component of the organic carbon leached is little available for the free-living bacterial community of the lake. Starch-PLA bags, though sold as compostable, have been found to be biodegradable by maximal 90% in industrial compost systems, even at 60°C as per the European norm laboratory test methods (Ciriminna and Pagliaro, 2020, EN 14046). In our study, the biodegradability of this leachate type in natural aquatic settings seems to be very low, as has been found earlier for certain PLA types in the ocean (Egea et al., 2024).
The relative composition of leachates is important in determining effects on microbial communities (Gunaalan et al., 2020). Tire rubber, which is known to harbour a large variety of additives, showed inhibitory effects on growth rates of natural free-living bacterial communities at low nutrient conditions (Figure 2) - though released DOC amounts were similar to those of PVC. Moreover, our concentrations exposure experiment revealed that effects of PP, PVC and tire rubber leachates on microbial dynamics largely depend on exposure concentration, as has been previously reported in the literature (Li et al., 2016). At low nutrient conditions (Figure 6), PP and PVC leachates seem to well fit a typical sigmoidal model. The accuracy of the model can be put into question for PVC, as it seems questionable (from the four applied leachate concentrations) whether near-maximum effects have been reached. The tire rubber leachates do not fit a sigmoidal model at all, but due to statistically significant changes in growth rates for an intermediate concentration and the growth rate inhibition observed of natural free-living bacteria (Figure 2), the data can be represented by a biphasic dose-response model (Figure 6c). It is possible that at intermediate concentrations, the inhibitory chemicals are not concentrated enough to reveal a strong inhibitory effect on lake bacteria, and thus there seems to be additional available organic carbon for the bacterial community.
Though no biphasic effects of tire rubber leachates on microbes have been reported in the literature, there is evidence that tire rubber granules can both stimulate growth of certain microorganisms and inhibit growth of others. Among the organisms growing on tire leachates were members of the Pseudomonas genus (Leff et al., 2007), which were also dominant in our experiment at high nutrient conditions (Figure 5), and isolates had increased growth rates at high nutrient concentrations in tire rubber exposures (Supplementary Figure 2b). On the other hand, the apparent inhibition of Vogesella by tire rubber leachates at high nutrient conditions, one of the dominant genera in the control H2O samples, also illustrates the inhibitory potential of tire rubber leachates. In sediment microbial communities, zinc and benzothiazole were identified as the main potential factors of tire rubber additives leading to pronounced community shifts and these chemicals are proven to be toxic for several microorganisms (Ding et al., 2022). As a consequence, tire particles were also shown to inhibit nutrient cycles in coastal sediments (Ding et al., 2022). The combination of these effects could explain the observed dose-responses in our concentrations experiment.
4.2 Environmental relevance
Exposures to different leachate concentrations also allowed us to compare the measured effects to environmentally realistic concentrations, using the same method as Sheridan et al. (2022) to calculate realistic LDPE leachate concentrations in freshwaters. This was assisted by bibliographical data on the relative occurrence of various plastic types (Uurasjärvi et al., 2020; Yuan et al., 2019; Capolupo et al., 2020).
According to our calculations, PP leachates can result in environmental concentrations of 4.88 mg DOC L−1, taking the high leaching rates of this study into account and the fact that PP microplastics often dominates the entire plastics pollution in aquatic environments. Considering that the EC50 for PP leachates reached 0.43 mg DOC L−1, significant environmental effects can be expected. For PVC, the realistic environmental concentration was found to be much lower, i.e., only 0.39 mg L−1, due to its less frequent occurrence in freshwater and much lower leaching rates (according to the measured leaching rates in our experimental model system). According to our dose-response model, no significant effects on the specific growth rate of the free-living bacterial community can be expected at this concentration. For tire rubber, it is more difficult to estimate its relative contribution, particularly as these tend to be much denser and can rapidly sink onto the sediment. For this reason, the tire rubber particle abundance in the sediment was used to estimate its environmental concentrations. A realistic environmental concentration seems to be 0.32 mg DOC L−1. For this concentration, according to the dose-response model fitted to the data, no significant effect on the bacterial community might be expected.
It must be mentioned that these are rough estimates to provide an idea on possible effect size of the environmentally relevant concentrations of each tested synthetic polymer leachate. Yet, in certain instances of heavily polluted water bodies, these might be much higher or else be significantly lower in more preserved water bodies. Additionally, the use of strong UV-lamps likely disproportionately affects leaching rates of certain products over others (Romera-Castillo et al., 2022), and, as mentioned previously, leaching rates and chemical make-ups are not equally distributed between products of the same synthetic polymer type. Furthermore, as seen in the mixed leachate sample of the bacterial isolates exposure (Supplementary Figure 2), the generally mixed and heterogenous plastic pollution of aquatic systems is likely to have effects which differ from those of leachates of a single plastic type or product. Nevertheless, these values can offer an important orientation for the extent of aquatic pollution by plastic leachates and their potential effect size on aquatic (micro)organisms.
It is also noteworthy to mention that these experiments may be influenced by the “bottle effect” caused by shifting the natural bacterial communities in small and closed containers (Hammes et al., 2010). It is known that this bottle effect can potentially alter the measured effects. Therefore, this incubation may be not entirely representative for natural ecosystems. Yet, microcosms with their highly controlled environmental factors can give important insights into the response of microbial communities to synthetic polymer leachates and thus provide valuable information on the effects of synthetic polymer leachate effects on complex microbial communities (Russo et al., 2016).
4.3 Effects of nutrient availability
Leachate exposures of natural bacterial communities (Figures 2, 6) indicate that effects of nutrient availability are not negligible, as has been found for pesticides (DeLorenzo et al., 2001). Furthermore, the effects of nutrient availability varied depending on the plastic type and for specific bacterial genera of natural free-living lake communities.
For the most part, stimulatory effects on bacterial communities via PP and PVC leachates are greatly dampened at high nutrient conditions compared to their respective controls as can be seen for the entire lake community (Figures 2, 6) and also for Pseudomonas when exposed to PVC leachates (Supplementary Figure 2). This notion can be explained by a more efficient uptake of specific forms of dissolved organic carbon over others. DOC from the LB medium is much more bioavailable than leachates from these synthetic polymers, e.g., represented by differences in bacterial carbon uptake genes (Poretsky et al., 2010). Acinetobacter and Vogesella represent an exception to this notion. Studies have shown that members of both Acinetobacter and Vogesella genera have PAH degrading capabilities (Czarny et al., 2020; Li et al., 2016), a common additive of PP and PVC plastic products. PAH degradation has also been shown to be enhanced when nutrient availability is high (Premnath et al., 2021). Further, bacterial co-metabolism can explain the simultaneous growth stimulation of Acinetobacter and Vogesella via PP and PVC even at low nutrient conditions. Nutrient enrichment has been shown to functionally influence microbial communities, especially via the expression of ABC transporters, which is downregulated when nutrients are enriched (Russo et al., 2016). Additionally, ABC-transporters have also been identified as important for microbial uptake of aromatic compounds, e.g., for an Acinetobacter strain (Mutanda et al., 2022). As aromatic compounds likely constitute an important component of both PP and PVC leachates, it is plausible that bacteria of the complex lake microbial community expressed less ABC transporters at high nutrient conditions, and thus were less impacted by aromatic (or other) leachate compounds.
Conversely, leachates which show inhibitory or no stimulatory effects, e.g., starch-PLA and tire rubber leachates, can cause important bacterial community shifts at high nutrient conditions. ABC-transporters have also been shown to be important in resistance to heavy metals, which are abundant in tire rubber leachates (Nies, 2003; Halsband et al., 2020). Thereby, downregulation of ABC transporters expression at high nutrient conditions can also impede the microbe’s ability to resist to heavy metals. Nevertheless, the addition of nutrients nullified the effects on total bacterial abundance and growth rates. A potential explanation could be that, for instance, Pseudomonas is able to use tire rubber leachates as a growth substrate (Leff et al., 2007), as is suggested by increased growth rates at high nutrient conditions compared to control H2O samples. This effect was absent for the tested Pseudomonas isolate at low nutrient conditions (Supplementary Figure 2b). As for PAHs (Premnath et al., 2021), tire rubber might be more bioavailable at nutrient enriched conditions, which also promote co-metabolism of highly polymeric carbon compounds. Differences in growth rates between exposures, for instance of Rhodoferax and Pseudomonas exposed to tire rubber leachates (Figure 5), indicate that the nature of these shifts varies with bacterial nutrient availability.
Our findings illustrate that plastic pollution in freshwaters leads to leachate effects on bacterial dynamics, which differ in dependence of the present environmental features. Unproductive, nutrient-poor freshwater bodies tend to have DOC concentrations ranging from 1 to 50 mg DOC L−1 (Menzel et al., 2005), though, for instance, concentrations as high as 300 mg DOC L−1 have been found in Canadian wetlands (Blodau et al., 2004). In our exposure experiments, organic carbon concentrations were approximately 6 mg and 60 mg DOC L−1, representing both ends of the trophic spectrum, i.e., unproductive vs. productive freshwater bodies, respectively. Yet, our study does not provide the entire picture of what could happen in other more eutrophic aquatic systems. The addition of nutrients together with the exposure to synthetic polymer leachates is also likely more akin to anthropogenic nutrient enrichment than the leachate pollution of naturally nutrient-poor lake ecosystems. Our results illustrate a variety of effects of leachate pollution on bacterial dynamics depending on the specific environmental settings of complex and chemically diverse freshwater ecosystems.
5 Conclusion
Our results shed a new light on the complexity of effects exerted by synthetic polymer leachates on aquatic microbial communities, which extend beyond previous findings, i.e., the stimulation of microbial growth through LDPE leachates (Sheridan et al., 2022). The extent of these effects ranged from bacterial community shifts to changes in total bacterial growth rates. Our results revealed variable effects between synthetic polymer leachate types and nutrient conditions. This highlights the importance of taking the trophic states and presumably other specific environmental features of aquatic ecosystems into account when evaluating the effects of plastic leachate pollution under real world conditions. In a regulatory context, our findings emphasize the need for prioritizing use-reduction of plastics of higher leaching rates and toxic effects, such as PP, PVC and tire rubber and highlight the risks associated with replacing currently used plastics with starch-PLA based products.
To further proceed, we suggest to not solely analyse community shifts at the 16S rRNA gene level, but also use transcriptomics analyses of bacterial communities exposed to various types of plastic leachates, to highlight functional changes via up- or downregulation of specific functional genes. Transcriptomics and other OMICS approaches will increase our understanding of the mechanisms underlying our findings. Future experiments should use more diverse freshwater bacterial communities and include the particle-attached fractions to elucidate the full extent of effects, exerted by various types of plastic leachate pollution, on microbial diversity and metabolism and thus overall ecosystem functioning.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
MS: Data curation, Formal Analysis, Investigation, Software, Validation, Visualization, Writing – original draft, Writing – review and editing. FM: Methodology, Software, Supervision, Validation, Writing – original draft, Writing – review and editing. YY: Writing – original draft, Writing – review and editing. H-PG: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work received funding from the European Union’s Horizon 2020 Research and Innovation programme, under the Grant Agreement number 965367 (PlasticFate).
Acknowledgments
We would like to thank Lars Ganzert and Solvig Pinnow for methodological discussions and support for incubation experiments. Thanks to all doctoral students and researchers of the MIBI group, who contributed with their input and assistance in conducting and analysing these experiments.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2025.1589648/full#supplementary-material
References
Abreo, N. A. S., Macusi, E. D., Cuenca, G. C., Ranara, C. T. B., Andam, M. B., Cardona, L. C., et al. (2015). Nutrient enrichment, sedimentation, heavy metals and plastic pollution in the marine environment and its implications on philippine marine biodiversity: a review. A Rev. ijec 15. doi:10.7718/ijec.v15i1.999
Azam, F., Fenchel, T., Field, J. G., Gray, J. S., Meyer-Reil, L. A., and Thingstad, F. (1983). “The ecological role of water-column microbes in the sea foundations of ecology II,” in Classic papers with commentaries, 2022. Editors Miller, E. Thomas, and J. Travis (University of Chicago Press), 384–390. doi:10.7208/chicago/9780226125534-024
Bandow, N., Will, V., Wachtendorf, V., and Simon, F.-G. (2017). Contaminant release from aged microplastic. Environ. Chem. 14, 394. doi:10.1071/EN17064
Birnstiel, S., Sebastián, M., and Romera-Castillo, C. (2022). Structure and activity of marine bacterial communities responding to plastic leachates. Sci. Total Environ. 834, 155264. doi:10.1016/j.scitotenv.2022.155264
Blodau, C., Basiliko, N., and Moore, T. R. (2004). Carbon turnover in peatland mesocosms exposed to different water table levels. Biogeochemistry 67, 331–351. doi:10.1023/B:BIOG.0000015788.30164.e2
Callahan, B. J., McMurdie, P. J., Rosen, M. J., Han, A. W., Johnson, A. J. A., and Holmes, S. P. (2016). DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583. doi:10.1038/nmeth.3869
Capolupo, M., Sørensen, L., Jayasena, K. D. R., Booth, A. M., and Fabbri, E. (2020). Chemical composition and ecotoxicity of plastic and car tire rubber leachates to aquatic organisms. Water Res. 169, 115270. doi:10.1016/j.watres.2019.115270
Chalmin, P. (2018). The history of plastics: from the capitol to the tarpeian rock. Field Actions Sci. Rep. Special Issue 19, 6–11. Available online at: https://journals.openedition.org/factsreports/5071.
Ciriminna, R., and Pagliaro, M. (2020). Biodegradable and compostable plastics: a critical perspective on the dawn of their global adoption. ChemistryOpen 9, 8–13. doi:10.1002/open.201900272
Czarny, J., Staninska-Pięta, J., Piotrowska-Cyplik, A., Juzwa, W., Wolniewicz, A., Marecik, R., Ławniczak, Ł., and Chrzanowski, Ł. (2020). Acinetobacter sp. as the key player in diesel oil degrading community exposed to PAHs and heavy metals. J. Hazard. Mater. 383, 121168. doi:10.1016/j.jhazmat.2019.121168
Czogała, J., Pankalla, E., and Turczyn, R. (2021). Recent attempts in the design of efficient PVC plasticizers with reduced migration. Materials 14, 844. doi:10.3390/ma14040844
DeLorenzo, M. E., Scott, G. I., and Ross, P. E. (2001). Toxicity of pesticides to aquatic microorganisms: a review. Environ. Toxicol. Chem. 20, 84–98. doi:10.1002/etc.5620200108
Ding, J., Meng, F., Chen, H., Chen, Q., Hu, A., Yu, C.-P., et al. (2022). Leachable additives of tire particles explain the shift in microbial community composition and function in coastal sediments. Environ. Sci. Technol. 56, 12257–12266. doi:10.1021/acs.est.2c02757
Egea, L. G., Brun, F. G., and Jiménez-Ramos, R. (2024). Dissolved organic carbon leaching from microplastics and bioavailability in coastal ecosystems. Sci. Total Environ. 909, 168673. doi:10.1016/j.scitotenv.2023.168673
Elzhov, T. V., Mullen, K. M., Spiess, A.-N., and Bolker, B. (2023). minpack.lm: R interface to the levenberg-marquardt nonlinear least-squares algorithm found in MINPACK, plus support for bounds. Available online at: https://cran.r-project.org/web/packages/minpack.lm/index.html.
Gunaalan, K., Fabbri, E., and Capolupo, M. (2020). The hidden threat of plastic leachates: a critical review on their impacts on aquatic organisms. Water Res. 184, 116170. doi:10.1016/j.watres.2020.116170
Hahladakis, J. N., Velis, C. A., Weber, R., Iacovidou, E., and Purnell, P. (2018). An overview of chemical additives present in plastics: migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 344, 179–199. doi:10.1016/j.jhazmat.2017.10.014
Halsband, C., Sørensen, L., Booth, A. M., and Herzke, D. (2020). Car tire crumb rubber: does leaching produce a toxic chemical cocktail in coastal marine systems? Front. Environ. Sci. 8, 125. doi:10.3389/fenvs.2020.00125
Hammes, F., Vital, M., and Egli, T. (2010). Critical evaluation of the volumetric “bottle effect” on microbial batch growth. Appl. Environ. Microbiol. 76, 1278–1281. doi:10.1128/AEM.01914-09
Klein, K., Hof, D., Dombrowski, A., Schweyen, P., Dierkes, G., Ternes, T., et al. (2021). Enhanced in vitro toxicity of plastic leachates after UV irradiation. Water Res. 199, 117203. doi:10.1016/j.watres.2021.117203
Koh, J. J., Zhang, X., and He, C. (2018). Fully biodegradable Poly(lactic acid)/Starch blends: a review of toughening strategies. Int. J. Biol. Macromol. 109, 99–113. doi:10.1016/j.ijbiomac.2017.12.048
Leff, A. A., McNamara, C. J., and Leff, L. G. (2007). Bacterial communities of leachate from tire monofill disposal sites. Sci. Total Environ. 387, 310–319. doi:10.1016/j.scitotenv.2007.06.042
Li, H.-X., Getzinger, G. J., Ferguson, P. L., Orihuela, B., Zhu, M., and Rittschof, D. (2016). Effects of toxic leachate from commercial plastics on larval survival and settlement of the barnacle Amphibalanus amphitrite. Environ. Sci. Technol. 50, 924–931. doi:10.1021/acs.est.5b02781
Menzel, R., Stürzenbaum, S., Bärenwaldt, A., Kulas, J., and Steinberg, C. E. W. (2005). Humic material induces behavioral and global transcriptional responses in the nematode Caenorhabditis elegans. Environ. Sci. Technol. 39, 8324–8332. doi:10.1021/es050884s
Montero-Pau, J., Gómez, A., and Muñoz, J. (2008). Application of an inexpensive and high-throughput genomic DNA extraction method for the molecular ecology of zooplanktonic diapausing eggs. Limnol. and Ocean Methods 6, 218–222. doi:10.4319/lom.2008.6.218
Murali, A., Bhargava, A., and Wright, E. S. (2018). IDTAXA: a novel approach for accurate taxonomic classification of microbiome sequences. Microbiome 6, 140. doi:10.1186/s40168-018-0521-5
Mutanda, I., Sun, J., Jiang, J., and Zhu, D. (2022). Bacterial membrane transporter systems for aromatic compounds: regulation, engineering, and biotechnological applications. Biotechnol. Adv. 59, 107952. doi:10.1016/j.biotechadv.2022.107952
Muthukrishnan, T., Govender, A., Dobretsov, S., and Abed, R. (2017). Evaluating the reliability of counting bacteria using epifluorescence microscopy. JMSE 5, 4. doi:10.3390/jmse5010004
Nies, D. H. (2003). Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol. Rev. 27, 313–339. doi:10.1016/S0168-6445(03)00048-2
OECD (2011). Guidelines for the testing of chemicals. Freshwater alga and cyanobacteria, growth inhibition test. Paris: OECD Publishing. Available online at: https://www.oecd.org/content/dam/oecd/en/publications/reports/2011/07/test-no-201-freshwater-alga-and-cyanobacteria-growth-inhibition-test_g1gh28f1/9789264069923-en.pdf.
OECD (2022). Global plastics outlook: economic drivers, environmental impacts and policy options. Paris: OECD Publishing. doi:10.1787/de747aef-en
Oksanen, J., Simpson, G., Blanchet, F., Kindt, R., Legendre, P., Minchin, P., et al. (2022). _vegan: community ecology package_. R. Package version 2.6-4. Available online at: https://CRAN.R-project.org/package=vegan.
Poretsky, R. S., Sun, S., Mou, X., and Moran, M. A. (2010). Transporter genes expressed by coastal bacterioplankton in response to dissolved organic carbon. Environ. Microbiol. 12, 616–627. doi:10.1111/j.1462-2920.2009.02102.x
Porter, K. G., and Feig, Y. S. (1980). The use of DAPI for identifying and counting aquatic microflora1. Limnol. and Oceanogr. 25, 943–948. doi:10.4319/lo.1980.25.5.0943
Premnath, N., Mohanrasu, K., Guru Raj Rao, R., Dinesh, G. H., Prakash, G. S., Ananthi, V., et al. (2021). A crucial review on polycyclic aromatic Hydrocarbons - environmental occurrence and strategies for microbial degradation. Chemosphere 280, 130608. doi:10.1016/j.chemosphere.2021.130608
Ritz, C., Baty, F., Streibig, J. C., and Gerhard, D. (2015). Dose-response analysis using R. PLoS ONE 10, e0146021. doi:10.1371/journal.pone.0146021
Romera-Castillo, C., Mallenco-Fornies, R., Saá-Yánez, M., and Álvarez-Salgado, X. A. (2022). Leaching and bioavailability of dissolved organic matter from petrol-based and biodegradable plastics. Mar. Environ. Res. 176, 105607. doi:10.1016/j.marenvres.2022.105607
Russo, D. A., Couto, N., Beckerman, A. P., and Pandhal, J. (2016). A metaproteomic analysis of the response of a freshwater microbial community under nutrient enrichment. Front. Microbiol. 7, 1172. doi:10.3389/fmicb.2016.01172
Salinas, J., Carpena, V., Martínez-Gallardo, M. R., Segado, M., Estrella-González, M. J., Toribio, A. J., et al. (2023). Development of plastic-degrading microbial consortia by induced selection in microcosms. Front. Microbiol. 14, 1143769. doi:10.3389/fmicb.2023.1143769
Sarker, I., Moore, L. R., Paulsen, I. T., and Tetu, S. G. (2020). Assessing the toxicity of leachates from weathered plastics on photosynthetic marine bacteria prochlorococcus. Front. Mar. Sci. 7, 571929. doi:10.3389/fmars.2020.571929
Schiavo, S., Oliviero, M., Chiavarini, S., Dumontet, S., and Manzo, S. (2021). Polyethylene, Polystyrene, and Polypropylene leachate impact upon marine microalgae Dunaliella tertiolecta. J. Toxicol. Environ. Health, Part A 84, 249–260. doi:10.1080/15287394.2020.1860173
Sheridan, E. A., Fonvielle, J. A., Cottingham, S., Zhang, Y., Dittmar, T., Aldridge, D. C., et al. (2022). Plastic pollution fosters more microbial growth in lakes than natural organic matter. Nat. Commun. 13, 4175. doi:10.1038/s41467-022-31691-9
Uurasjärvi, E., Hartikainen, S., Setälä, O., Lehtiniemi, M., and Koistinen, A. (2020). Microplastic concentrations, size distribution, and polymer types in the surface waters of a northern European lake. Water Environ. Res. 92, 149–156. doi:10.1002/wer.1229
Yuan, W., Liu, X., Wang, W., Di, M., and Wang, J. (2019). Microplastic abundance, distribution and composition in water, sediments, and wild fish from Poyang Lake, China. Ecotoxicol. Environ. Saf. 170, 180–187. doi:10.1016/j.ecoenv.2018.11.126
Keywords: lake bacterial communities, synthetic polymers, biodegradable plastics, nutrient enrichment, bacterial growth and community composition
Citation: Schubert M, Monikh FA, Yang Y and Grossart H-P (2025) Nutrient availability modulates the effects of plastic leachates on the growth and community dynamics of free-living freshwater bacteria. Front. Environ. Sci. 13:1589648. doi: 10.3389/fenvs.2025.1589648
Received: 07 March 2025; Accepted: 23 April 2025;
Published: 23 May 2025.
Edited by:
Pengfei Wu, Nanjing Forestry University, ChinaCopyright © 2025 Schubert, Monikh, Yang and Grossart. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fazel Abdolahpur Monikh, ZmF6ZWwubW9uaWtoQHVuaXBkLml0; Hans-Peter Grossart, aGFuc3BldGVyLmdyb3NzYXJ0QGlnYi1iZXJsaW4uZGU=
†ORCID: Fazel Abdolahpur Monikh, orcid.org/0000-0001-9500-5303
 Marius Schubert
Marius Schubert Fazel Abdolahpur Monikh1,2,3*†
Fazel Abdolahpur Monikh1,2,3*† Yuyi Yang
Yuyi Yang Hans-Peter Grossart
Hans-Peter Grossart