- 1Department of Agricultural, Food and Forest Sciences, University of Palermo, Palermo, Italy
- 2NBFC - National Biodiversity Future Center, Palermo, Italy
Introduction: The Mediterranean is the European region with the lowest woody cover and the highest level of habitat degradation, being highly susceptible to climate change effects and desertification risk. In such worrying conditions, increasing woody cover and restoring forests is a major goal established in several international commitments. However, recruitment limitation of woody species is rather frequent both within natural regeneration processes and active restoration programs, particularly due to drought, overgrazing, and a lack of post-planting tending operations. Therefore, finding suitable tools to improve the recruitment success of native woody species is of crucial importance.
Methods: We assessed woody natural regeneration under abandoned prickly pear orchards, olive trees, and nearby open areas in three sites under high desertification risk in central Sicily (Italy). Then, we tested for differences in density, richness, diversity, height, and basal diameter of the woody recruiting species between these three habitats.
Results and discussion: Natural regeneration was widespread under prickly pear, with 94.6% of the sampled plots showing at least one recruit, in comparison to 61.6% of plots under olive and 22.3% in open areas. Natural regeneration density under prickly pears (114 ± 99 individuals m−2) was significantly higher (p < 0.001) than under olive trees (60.4 ± 76.4) and open areas (4.6 ± 9.3). Recruits’ diversity, basal diameter, and height were also significantly higher under prickly pear, concentrating 94.4% of the individuals higher than 100 cm and all late successional species. Our results indicate a great potential for prickly pears to accelerate the natural regeneration of Mediterranean woody species in areas under desertification. However, a site-specific evaluation must be made taking into account prickly pear’s historical presence, temporary income as a crop, management capacity and, especially, its invasive potential.
Introduction
Large areas in the Mediterranean have been historically deprived of the original woody cover, reducing biodiversity and exposing bare lands to increasing soil erosion and desertification risk (Prăvălie et al., 2017; Pausas and Millán, 2019). On the one hand, such degradation processes are bound to increase in the next decades as a consequence of human population growth as well as land use and climate change (Reynolds et al., 2007; Mulligan et al., 2016). On the other hand, the progressive abandonment of agricultural lands and the reduced pressure on woodlands in the Mediterranean could progressively provide the opportunity to restore native vegetation and correlated ecosystem services in increasingly larger areas (Plieninger et al., 2014; Novara et al., 2017; Bueno et al., 2020b). However, recruitment limitation is often a huge barrier to forest restoration, particularly in areas under desertification threat, and is caused by several factors (Acácio et al., 2007; Granda et al., 2014). Seed limitation is a first bottleneck that can depend on the lack of mother plants (source limitation), seed dispersers, and/or their interactions (Valiente-Banuet et al., 2015; La Mantia et al., 2019). Even when seed limitation is overcome, the high mortality of planted or naturally regenerating seedlings and saplings seems to be more the rule than the exception in the Mediterranean (Duponnois et al., 2009; Mendoza et al., 2009; Andivia et al., 2017). Drought and herbivory have been found to be the main factors causing such limitations; consequently, plant–plant facilitation (e.g., nurse plants, biogroups) may become essential for ecological restoration of degraded and/or harsh environments (Castro et al., 2002; Gómez-Aparicio et al., 2004; Padilla and Pugnaire, 2006; Brooker et al., 2008). This occurs, for instance, in xeric mountain areas, where shrub and tree species were found growing clustered together and around a main and larger species in biogroups (Pedrotti, 2019). Hence, these plant ensembles may allow the progressive spread of woody vegetation into open areas where single woody species are unable to establish and persist. Many studies have investigated plant–plant facilitation in the Mediterranean, although the balance between positive and negative effects is still not straightforward to predict due to largely species-specific and context-dependent outcomes, particularly in drought-prone ecosystems (Gómez-Aparicio, 2009; Filazzola and Lortie, 2014; Gonzalez and Ghermandi, 2019). For example, the facilitative effect of native shrubs can be reduced as aridity increases due to competition (Andivia et al., 2017), or nurse plant functional traits or growth form (e.g., shrub or tree) may generate contrasting effects, either positive or negative, along plant ontogeny (Gómez-Aparicio, 2009; Rolo et al., 2013). However, a critical issue is that if several degraded areas are totally deprived of any woody cover, then the ad-hoc implementation of nurse plants becomes the only chance. Such strategy, in turn, implies careful planning and crucial post-planting tending operations, which are not always carried out due to economic reasons, often undermining the efficacy of restoration interventions (Le Houerou, 2000; Gómez-Aparicio, 2009; Meli et al., 2017). In several cases, non-native plant species are faster-growing or more stress-tolerant than natives, making them more appealing to be used for ecological restoration (Krumm and Vítková, 2016; Badalamenti et al., 2020c; Suzuki et al., 2021). On the one hand, such exotic species may become invasive and seriously threaten native species and habitats, thus leading to expensive control and eradication programs (Simberloff et al., 2013). On the other hand, even invasive species may be useful for restoration or conservation purposes under some circumstances, therefore generating a conservation trade-off and calling for a better understanding of their functional role in ecosystem dynamics and plant community assembly (Schlaepfer et al., 2011; Vimercati et al., 2020; Badalamenti et al., 2020a). Although the risk of a generalized and uncritical approach to non-native species still exists, there is increasing consensus in the scientific community on the need for evidence-based assessment of alien species’ invasiveness and related harmful impacts (e.g., Kumschick et al., 2023). In this research, we assessed the possible role of prickly pear [Opuntia ficus-indica (L.) Mill.] as a tool for forest restoration in Mediterranean areas. The prickly pear is a cactus species native to Mexico but cultivated over 1 million hectares in the Mediterranean basin, mainly due to its edible fruits but also for livestock fodder and fencing (Le Houérou, 1996). In Europe, Italy plays a leading role in prickly pear cultivation, hosting 8,614 hectares of plantations, mostly of spineless varieties located in Sicily, producing up to 87,000 tons a year, more than 12% of the world production (Erre et al., 2009; ISTAT, 2022). Due to their extreme capacity to thrive in harsh and dry conditions and ease of reproduction, opuntias, particularly the spinier species Opuntia maxima Mill. and O. stricta (Haw.) Haw., have also become strongly invasive in some Mediterranean regions, particularly in small islands, rocky habitats, and cliffs (Vilà et al., 2003; Padrón et al., 2011; Guarino et al., 2021; Tesfay and Kreyling, 2021). In turn, positive effects of prickly pear on different ecosystem services such as soil protection, nutrient cycling, carbon sequestration, and refuge to native grasses, forbs, and argan trees have also been reported (Génin et al., 2017; Oduor et al., 2018; Novoa et al., 2021; Stavi, 2022; Jorge et al., 2023). Additionally, prickly pear is being used as a restoration tool in large-scale projects in Africa (Neffar et al., 2018), and its cultivation contributes to sustaining the agricultural socio-economical tissues in many arid areas, therefore representing a typical conservation trade-off (Shackleton et al., 2011; Stavi, 2022). Despite this growing wealth of knowledge, the potential facilitative effects of prickly pear on the natural regeneration of Mediterranean woody species are still unknown. To fill this gap, we aimed to quantify the density, richness, and size of the woody species recruiting underneath prickly pear individuals in comparison with olive trees and nearby abandoned open areas. We hypothesized that, due to its functional and structural characteristics (CAM metabolism, low water requirements, moderate shading effect, and protection against herbivores), prickly pear will significantly facilitate the recruitment of a wide range of native woody species and allow their full establishment beyond the sapling stage.
Materials and methods
Study sites
Field surveys were carried out in three study sites in Sicily (Italy), all classified at a critical risk of desertification (Calvi et al., 2016; Figure 1; Supplementary Table S1). The first prickly pear orchard (PPR) is localized in Roccapalumba (Palermo province), whose landscape is dominated by clay soils and cultivated lands, especially annual crops, olive, and prickly pear orchards. The study site falls within the upper meso-Mediterranean upper dry bioclimatic belt (Bazan et al., 2015), at an altitude of 500 m a.s.l., on slopes less than 20%. Mean annual precipitation is 561 mm, mean annual temperature is 15.7°C, and soils are classified as Typic pelloxererts (Fierotti, 1988). The second prickly pear orchard (PPSP) is localized in Santo Pietro (Catania province), in hilly areas of south-eastern Sicily, dominated by sandy soils and covered by cultivated areas, including annual crops, vineyards, pasturelands, and hardwood forests and shrublands. The study site falls within the upper thermo-Mediterranean with a lower sub-humid bioclimate (Bazan et al., 2015), at an altitude of 262 m a.s.l., on slopes less than 10%. Mean annual precipitation is 690 mm, mean annual temperature is 17.0°C, and the soils are Typic xerochrepts (Fierotti, 1988). The third prickly pear orchard (PPC) is localized in Caltagirone (Catania province), in inner hilly areas dominated by clay soils and cultivated lands, subject to an upper thermo-Mediterranean lower sub-humid bioclimate (Bazan et al., 2015), at an altitude of 427 m a.s.l., on slopes less than 10%. Mean annual precipitation in this area is 690 mm, mean annual temperature is 17.0°C and the soils are Typic xerochrepts (Fierotti, 1988). All the surveyed prickly pear orchards were planted approximately 30 years ago. Most of the surface of the PPR and PPC orchards is currently managed, with the natural regeneration controlled through mowing; however, from 2005 onwards, local farmers have been leaving some rows unmanaged that were selected for sampling. PPSP orchard was totally abandoned after 2005, although it is currently accessed by horses, sheep, and cattle. The PPSP falls within the Site of Community Importance “Bosco di Santo Pietro” (ITA 070005), which hosts some of the most significant cork oak stands in Sicily.
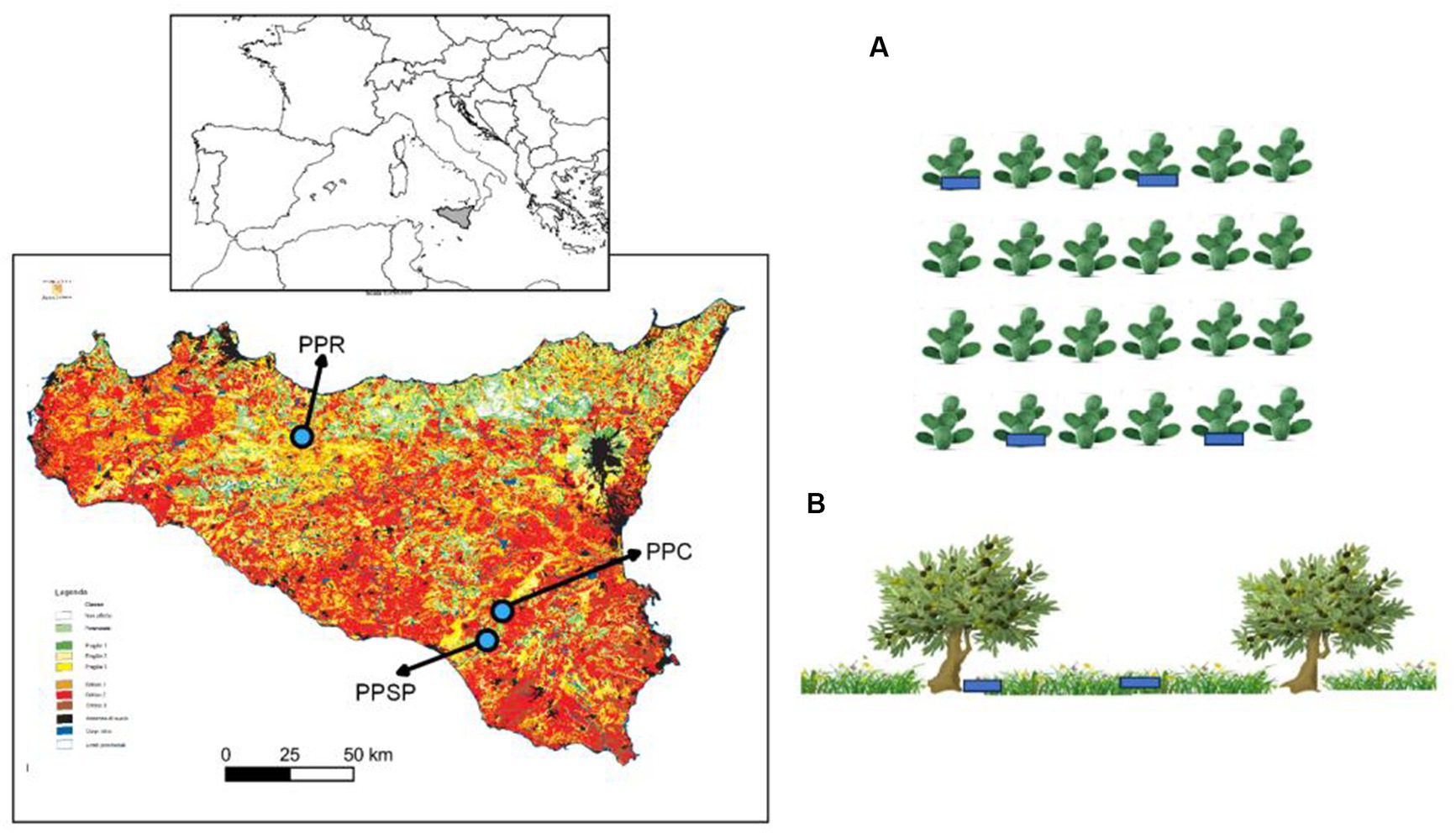
Figure 1. Location of the three prickly pear orchards overlaid onto the desertification risk map of Sicily (Calvi et al., 2016), with study sites located in areas classified as critical 1 and 2 (red/brown color). From left to right: PPR, Roccapalumba; PPSP, Santo Pietro; PPC, Caltagirone. (A) Example of the random distribution of the sampling plots (blue) under the prickly pear individuals, avoiding consecutive individuals and rows and (B) sampling plots under olives and open areas, avoiding nearby individuals. Images: Freepik.com.
Sampling design
Regeneration assessment
In spring 2021, within each of the three prickly pear orchards, we established 50 rectangular plots (3 × 2 m) around the trunk of prickly pear plants, for a total of 150 plots (Figure 1). Prickly pear trunks represented the center of the plots, with the longer axis established along the row and the smaller axis directed to the inter-row. To reduce spatial autocorrelation, a plot was separated by two individuals (i.e., considering only the individuals n. 1, n. 4, n. 7, etc.), and 10 individuals in each row were surveyed, with a minimum distance of three rows (i.e., considering only the rows n. 1, n. 4, n. 7, etc.; Figure 1).
Natural regeneration under olives and in open areas
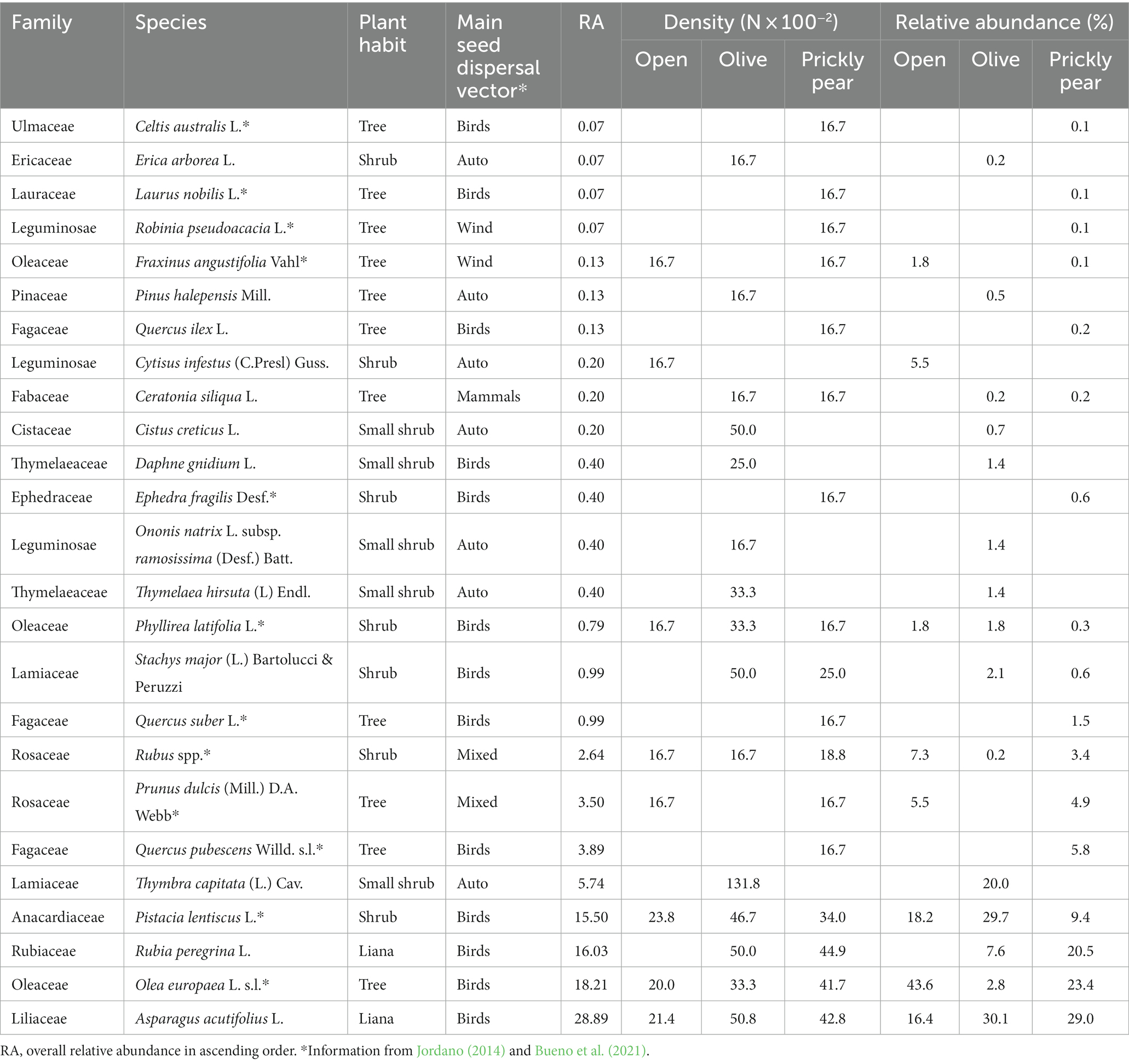
To allow for comparisons with prickly pears, we established the same rectangular plots (3 × 2 m) around the trunk of 120 randomly selected olive individuals (Olea europaea) distributed in one abandoned orchard nearby PPC (N = 40) and two abandoned orchards nearby PPSP (N = 40 each), with all olives located from 130 to 550 m from the respective prickly pear orchards. The higher number of plots in PPSP was to compensate for the sampling effort once we did not find abandoned olive orchards in the surroundings of PPR. As a control, we also randomly established the same rectangular plots at open areas nearby (±3 m) for each olive tree in each abandoned olive orchard (N = 50 in PPC and N = 100 in PPSP) and in an abandoned field on the side of the PPR orchard (N = 50), with a minimum distance of 5 m among plots (Figure 1). Once PPSP was totally abandoned (i.e., no inter-row management as in PPC and PPR), we also established 50 plots in the prickly pear inter-rows, complementing the sampling in open areas. We checked that these open areas were neither managed (i.e., mowing and tilling) nor burned at least in the last 15 years, based on interviews with the landowners, field observations, and the analysis of satellite images (Google Earth Pro®). At each plot, we counted and identified all woody individuals higher than 10 cm, shrub and tree individuals while height and basal diameter were measured only for shrub and tree individuals exceeding 1 m in height. The second edition of Flora d’Italia (Pignatti et al., 2017–2019) was used as a reference for plant identification and nomenclature, while the classification of the main seed dispersal vector of the species was from Jordano (2014) and Bueno et al. (2021).
Characteristics of the nearest forests
Since we were mostly interested in evaluating the facilitation effect of prickly pear to promote forest restoration, we recorded the presence of the nearest patches of native forests as a reliable proxy of the potential seed source for colonization through seed dispersal. Natural forests were searched within a 2-km radius area around each study site, considering a minimum forest area of 5,000 m2. We obtained the forest cover and type from the most recent regional forestry inventory (Camerano et al., 2011). Furthermore, we also assessed the recent wildfire occurrence (from 2007 to 2020) in the same natural forests because a high fire frequency may seriously compromise seed production and subsequent seed dispersal chances (data from the headquarters of the Forest Service of Sicily).1
Statistical analysis
In order to check whether our sampling was representative of the overall natural regeneration richness, we first performed rarefaction tests for each habitat in each study site with the specaccum function and 100 permutations within the vegan package (Supplementary Figure S1). To test for differences in richness, density (number of plants per 100 m2), height, and basal diameter of natural regeneration underneath prickly pear, olive trees, and in open areas, we used generalized linear models (GLM). First, we ran two global models pooling the data from the three sites to account for the potential natural variability across sites. In the first model, we included all species found in the survey (lianas, small shrubs, shrubs, and trees), whereas in the second, we included only the shrub and tree species. Then, to check for local scale differences, we ran separate models for each site, again one model with all species and a second with shrubs and trees. For richness analysis, we used a Poisson distribution with log link, and for density, height, and basal diameter, we used a negative binomial distribution once these data showed a non-normal distribution (Shapiro–Wilk p < 0.05), resulting in high overdispersion in the Poisson and quasi-Poisson models. All analyses were performed with R v4.2.1 (R Core Team, 2021) with the MASS package for the GLM.
Results
Density and species richness of woody natural regeneration
Overall, 1,516 individuals from 25 different woody species were recorded in the sampling plots, with an average density of 53.7 individuals per 100 m−2 (Table 1). The rarefaction analysis indicated that our sampling effort can be considered satisfactory to represent the plant community of recruits at our sites (Supplementary Figure S1). Colonization under prickly pear was widespread, with 94.6% of the plots containing at least one recruiting individual, while this percentage dropped to 61.6% under olives and to 22.3% in open areas. Average natural regeneration density under prickly pear was almost double that under olive trees (114 ± 99 × 100 m−2 vs. 60.4 ± 76.4, respectively) and almost 30 times higher than in open areas (4.6 ± 9.3), with GLM indicating significant differences both considering all plant species and only shrubs and trees (Tables 2, 3; Figure 2). In turn, density and richness were higher under olives than in open areas. Prickly pear’s highest woody species density and richness were confirmed by the separated GLM models, indicating significantly higher values in all intra-site comparisons (Tables 2, 3; Figure 2).
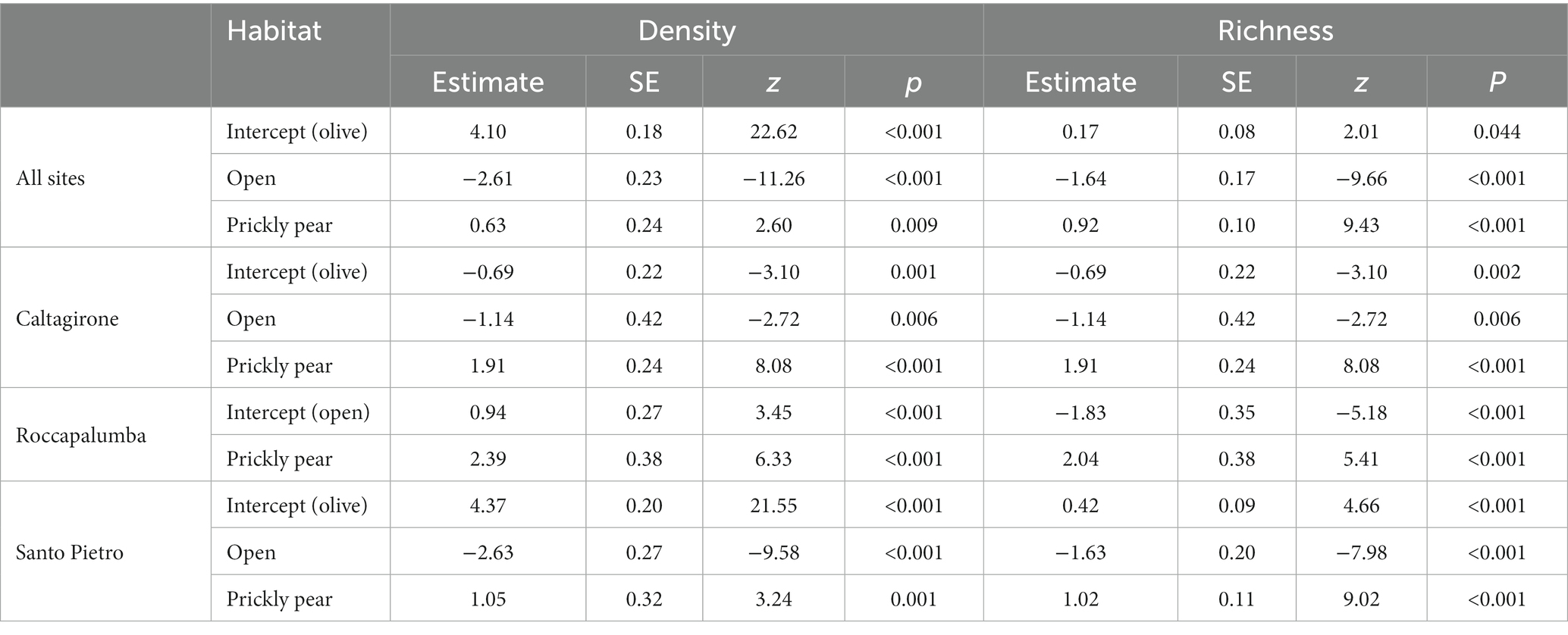
Table 2. Results of the generalized linear models comparing density (n × 100 m−2) and richness considering all plant species with all sites pooled and within each study site.
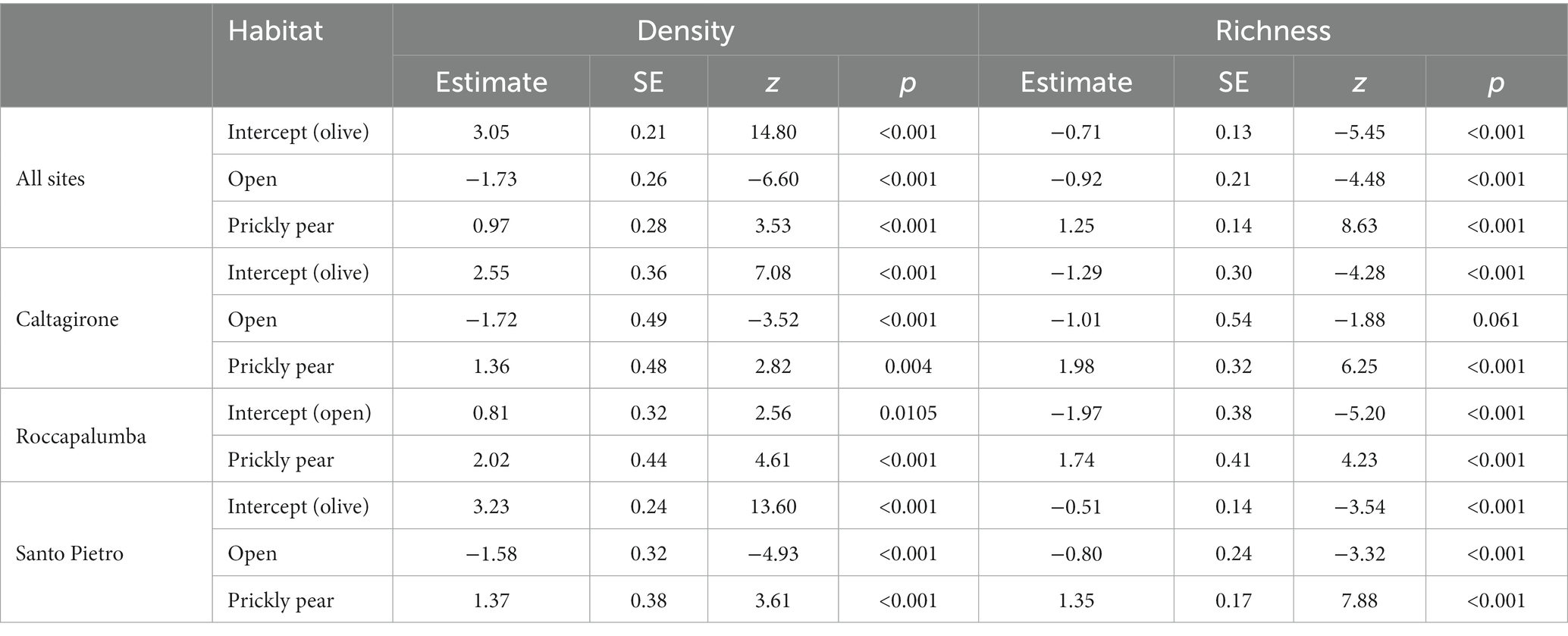
Table 3. Results of the generalized linear models comparing density (n × 100 m−2) and richness of shrub and tree species with all sites pooled and for each study site.
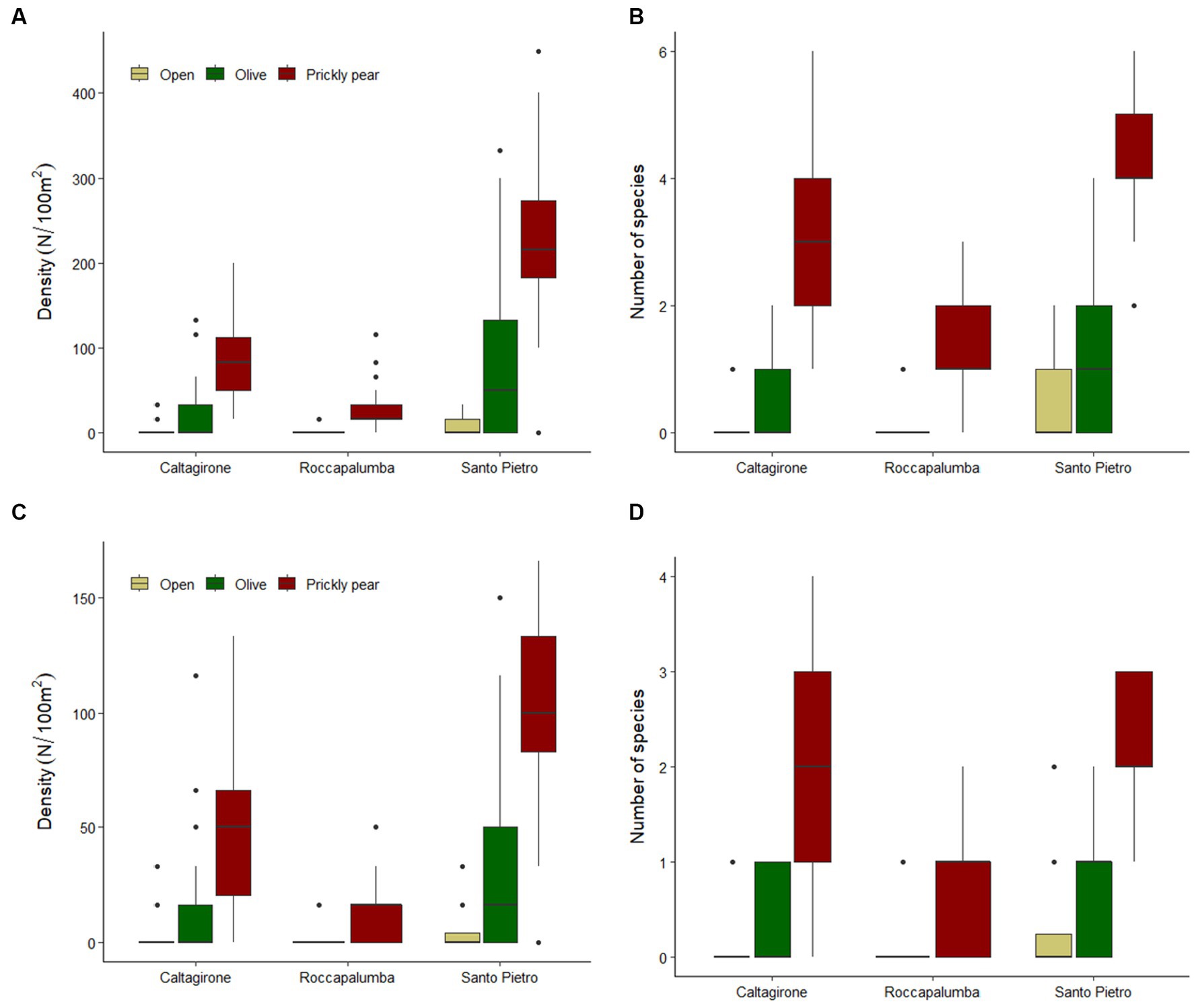
Figure 2. Boxplots showing the natural regeneration density and richness of all woody species (A,B) and of shrubs and trees (C,D) across open areas, under abandoned olive trees, and in prickly pear orchards in the three study sites.
Recruitment of established woody species
Out of the 1,516 woody individuals, 202 (12.9%) were shrubs and trees higher than 100 cm, belonging to 15 species, and 94.4% of them occurred under the prickly pears. Indeed, only six plots in open sites and five plots under olives hosted such recruiting individuals, which were, conversely, widely spread over 117 prickly pear plots, accounting for 1.4, 10.9, and 19.7% of all woody individuals in the three habitats, respectively. Recruits were also significantly higher and larger under prickly pear than under olives and open areas (Table 4), including 53 individuals higher than 300 cm and with basal diameters up to 39 cm (Figure 3). Such differences were also confirmed by the separated GLM models (Table 4; Figure 3).
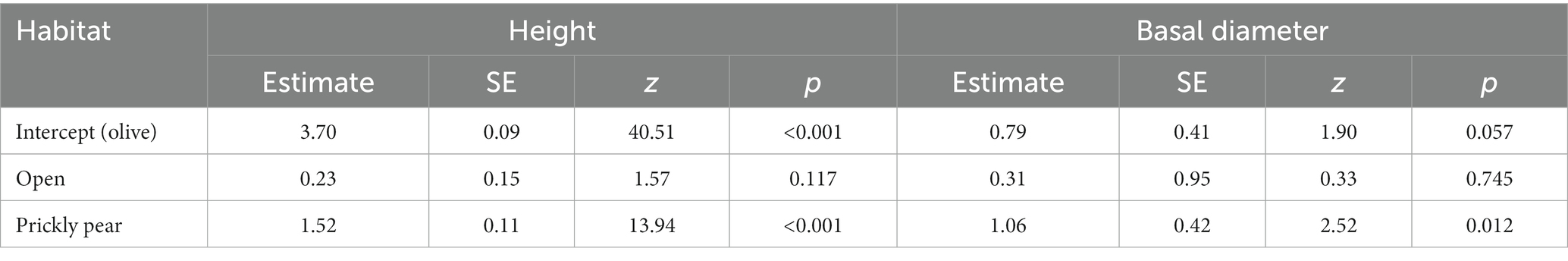
Table 4. Results of the generalized linear models comparing the height and basal diameter of shrub and tree species across habitats.
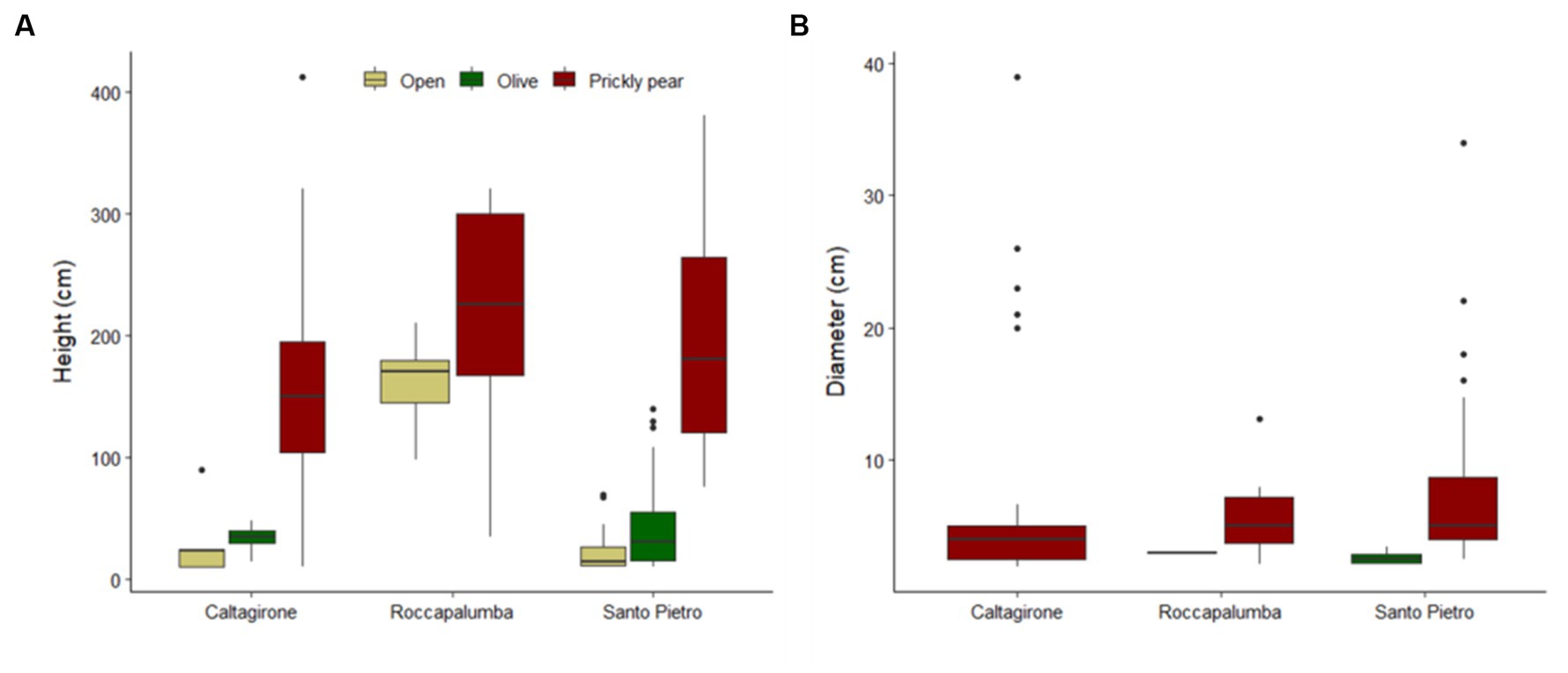
Figure 3. Boxplots showing the height of all shrub and tree individuals (A) and the basal diameter of individuals with a height of ≥100 cm (B) in the open areas, under abandoned olive trees, and in prickly pear orchards in the three study sites.
Natural regeneration species composition
Wild asparagus (Asparagus acutifolius L.) was the most abundant species (28.9% of all the individuals) and, together with olive (Olea europaea L. s.l., 18.2%), mastic (Pistacia lentiscus L., 15.5%), and brambles (Rubus spp., 2.6%), occurred in all three habitats (Table 1). Seventy-six individuals of three oak species, i.e., downy oak (Quercus pubescens Willd. s.l.), cork oak (Q. suber L.), and holm oak (Q. ilex L.), were observed, but only under prickly pears. Downy oak was the most abundant oak species (80.3%), occurring at relatively high densities (19 × 100 m−2) and even reaching heights up to 7.5 m and diameters of 21 cm in PPC. Olives, almonds (Prunus dulcis (Mill.) D.A. Webb), mastic and downy oaks, (Quercus pubescens) accounted for 91% of the established individuals, although only the first two occurred in all prickly pear orchards. The large majority of the recruits were from fleshy-fruited species (92.6%), indicating an active role of animal seed dispersal networks.
Characteristics of the nearest forests
Since we could not perform a statistical analysis of the influence of the nearest forest stands due to low sampling sites, we report here, and in Supplementary Table S2, a descriptive assessment. In PPC, the area with almost all oaks recruited, there were four downy oak stands with average size of 3.7 hectares, occurring 500 m away, and no wildfires were registered in these areas in the last 14 years. In PPR, there were three downy further than oak stands with average size of 8 hectares, but all of them were further than 1.5 km and had burned at least twice in the last 14 years. In PPSP, we found two Q. suber forests (average size of 10 hectares) and one large Q. ilex forest (>10 hectares), although 1.8 km distant.
Discussion
In the last century, massive reforestation projects have significantly increased woody cover in the Mediterranean, while millions of hectares of agricultural fields have been abandoned, although many of them did not evolve toward late successional stages composed of native tree species. In our study, we found that prickly pear strongly facilitated the recruitment and fostered the growth of a wide variety of Mediterranean woody species, including late successional tree species, in comparison with abandoned olive trees and open areas.
Woody species density and richness patterns
Recruits were widely distributed under prickly pear in all three study sites, with 94.6% of the 150 sampled individuals hosting at least one woody species. Olive trees also showed a high frequency of woody recruits (62%), in comparison to open areas (22%). However, virtually all fully established (≥1.0 m high) shrubs and trees were found under prickly pear. In a study comparing the natural regeneration under a native shrub [Retama sphaerocarpa (L.) Boiss.] and paired open sites along an environmental gradient in Spain, just about 10% of the 1,263 Retama shrubs had one recruit, for a total of 211 woody individuals belonging to four species (Andivia et al., 2017). The authors also observed that Retama’s facilitative effect was negatively correlated with aridity and herbivory pressure. Due to the CAM metabolism, the high water-use efficiency, the shallow root system, and the high level of herbivory protection, prickly pear is expected to cause a positive correlation between increasing aridity and herbivory and its facilitative effect. Olive trees showed significantly fewer woody recruits than prickly pears, particularly those lacking established shrub and tree species (Figure 3; Table 4), possibly resulting from a higher competition for light and space because abandoned olive trees often have a high density of lower branches and root shoots. Conversely, prickly pear has a more open architecture, giving support to lianas and allowing the full growth of trees and high shrubs. A facilitative effect was also observed in the dynamics of biogroups in Italian mountain ecosystems, where junipers (such as Juniperus deltoides R.P. Adams and Juniperus hemisphaerica Presl) have been found to act as the development center for several woody species (Lapenna and Fascetti, 2010; Pedrotti, 2019). Indeed, a global meta-analysis indicated that shrubs tend to provide higher facilitative effects than trees, especially when considering later developmental stages (Gómez-Aparicio, 2009), although such ontogenetic trade-off was also observed within the same shrub species (Rolo et al., 2013). Indeed, recruitment was significantly higher under olives than in open areas, and the establishment of lianas such as Asparagus acutifolius and wild madder (Rubia peregrina L.) was widespread there. Interestingly, Andivia et al. (2017) also found that Asparagus acutifolius was the most abundant species occurring under Retama shrubs. The second most abundant recruiting species was olive, occurring in all prickly pear orchards, with some individuals higher than 6 m. A high olive seedling density has also been verified under Mediterranean pine plantations, although saplings and adults were very rare (Badalamenti et al., 2018). In turn, olive recruits were absent or rare under other olives and open areas, indicating both high intra-specific competition and susceptibility to drought and herbivory.
Prickly pear contribution against recruitment limitation
The positive role of prickly pear in the ecological restoration of semi-arid ecosystems has been previously acknowledged (Le Houérou, 1996; Neffar et al., 2013; Génin et al., 2017; Neffar et al., 2018) and the utility of prickly pear to improve soil conditions in order to favor the cultivation of other species has been highlighted in the past, but with exclusive reference to agricultural contexts (Nocito, 1844). Due to its peculiar plant structure, the prickly pear could also be a useful plant for combating erosion in riparian contexts, where, moreover, its invasive potential is effectively zero (Stavi, 2022). However, its possible facilitative role in the natural regeneration of Mediterranean woody species has been barely documented. In one of the few study cases, prickly pear has been reported to aid the development of the argan tree (Sideroxylon spinosum L.) in pre-Saharan Morocco, with an increasing effect of old prickly pear individuals resulting in soil organic matter accumulation (Génin et al., 2017; Hassan et al., 2019). Prickly pear has also been facilitating the natural regeneration of common walnut (Juglans regia L.) and almond (Prunus dulcis) in Mediterranean agroecosystems (Bueno et al., 2020a; Badalamenti et al., 2022). Recently, a study in the savanna ecosystem showed that the invasive Opuntia stricta can create fertility islands that facilitate the establishment of native plants, improving soil abiotic and biotic conditions (Novoa et al., 2021). In another study, Oduor et al. (2018) found that the abundance and diversity of native species were not affected by prickly pear invasion, regardless of the cover percentage, showing that the negative effects exerted by alien plants are strongly context-dependent.
The rarefaction analysis indicated that our results are representative of the plant community of recruits present at our study sites (Supplementary Figure S1). The vast majority of the woody species found in our surveys (Table 3) have fleshy fruits and rely on vertebrates, particularly mammals and especially birds, for seed dispersal (Bueno et al., 2021). This evidence implies a high incidence and effectiveness of animal-mediated seed dispersal networks. This is particularly the case for birds, as they tend to avoid open areas or to stay on the ground, relying both on natural and artificial perches (Pons and Pausas, 2006; La Mantia et al., 2019). Although we did not assess the seed rain, our personal observations clearly indicate that birds frequently used both olive trees and prickly pear individuals. Indeed, during our surveys, we observed many seed dispersers, such as warblers (Sylvia spp.), thrushes (Turdus spp.), robins (Erithacus rubecula), corvids (Corvus cornix, Pica pica, and Garrulus glandarius), pigeons (Columba palumbus), and starlings (Sturnus spp.) perching or foraging inside the prickly pear orchards. Due to its quick development, prickly pear can soon offer suitable perches for birds, consequently fostering secondary succession underneath. Since herbivory and trampling are key biotic factors affecting the recruitment of woody species, the physical protection offered by a nurse plant is of paramount importance (Padilla and Pugnaire, 2006; Andivia et al., 2017). The PPC and PPR orchards are protected to prevent the access of large animals, whereas the PPSP and respective olives and open areas are frequently accessed by livestock (cattle, horses, and sheep), while wild herbivores such as deer are not present. Since spineless prickly pears are the most used varieties in our study sites, the main protective mechanism is the physical barrier created by the cladodes, particularly when they become hard with aging. Despite such protection was also provided by olives and can be offered by other native shrubs (Smit et al., 2008), the multi-stemmed architecture of prickly pear, similar to a candle holder, seemed to represent a great trade-off between protection and space and light competition (Rolo et al., 2013). Effectively, we observed several recruits showing a typical tree habit with a streamlined trunk, in comparison with the shrubby aspect of recruits in open areas that is typical of sites under high herbivory pressure.
Oak regeneration and the role of the remnant forest patches
Oak forests (dominated by Quercus spp.) represent the late successional stages in some areas of the Mediterranean basin, including the surroundings of our study sites. Since oaks are highly susceptible to recruitment limitations and are often lacking in reforestation interventions, they are frequently used as indicators in facilitation studies (Acácio et al., 2007; Gómez-Aparicio, 2009; Bobiec et al., 2018). The main abiotic constraints hampering the natural regeneration of Mediterranean oaks are poor soil conditions and summer drought. These two conditions may not only kill the seedlings but also affect the viability of acorns when moisture content drops below 26% (Acácio et al., 2007; Ganatsas and Tsakaldimi, 2013; Matías et al., 2019). Light limitation is another relevant factor, with reduced development in high-tree-density plantations or dense shrublands (Pausas et al., 2006). To overcome these limiting factors, prickly pear was found to be of crucial importance. Indeed, not only did we find a high oak seedling density under prickly pears, but they also hosted all the established oak individuals that reached up to 7 m high, indicating a good balance between soil organic matter content, water availability, space, and light competition (Bautista-Cruz et al., 2018; Hassan et al., 2019). However, our results also suggest a central role of oak seed limitation conditioned by the characteristics of the nearest forest fragments (especially size and distance) because the vast majority of high oak individuals were found in PPC. Jays and the wood mouse, both observed in our study sites (Cairone et al., 2020; Bueno et al., unpublished data), are the main seed dispersers of Mediterranean oaks. They act at very different spatial scales, with wood mice operating at short distances (10–50 m) and jays at medium distances (approximately 300 m), with rare long-distance dispersal events (Gómez, 2003; La Mantia and Bueno, 2016). Despite patches of oak forests being present in the surroundings of all the study areas and relatively large oak forest patches (up to 18 ha) being present inside the 12.5 km2 surveyed area, only at PPC were they within the mean seed dispersal range of the Eurasian jay (Garrulus glandarius) (as far as 500 m), which seemed to be one major factor driving the highest oak density there. Interestingly, some oak individuals in PPC were already reproductive (Figure 4), indicating a further successional step where seed dispersal from outside may become increasingly less important. Conversely, the very high fire frequency affecting oak forests in the surroundings of PPR is a major detrimental factor, as recurrent wildfires generally curb seed production for several years (Trabaud and Galtié, 1996; Badalamenti et al., 2020b), therefore reducing the chances of long-term seed dispersal. The suitability of prickly pear for oak seedling establishment, as widely observed in PPC, would suggest, hence, that the direct seeding of acorns could be successfully implemented in PPR and PPSP sites in order to overcome seed limitation.
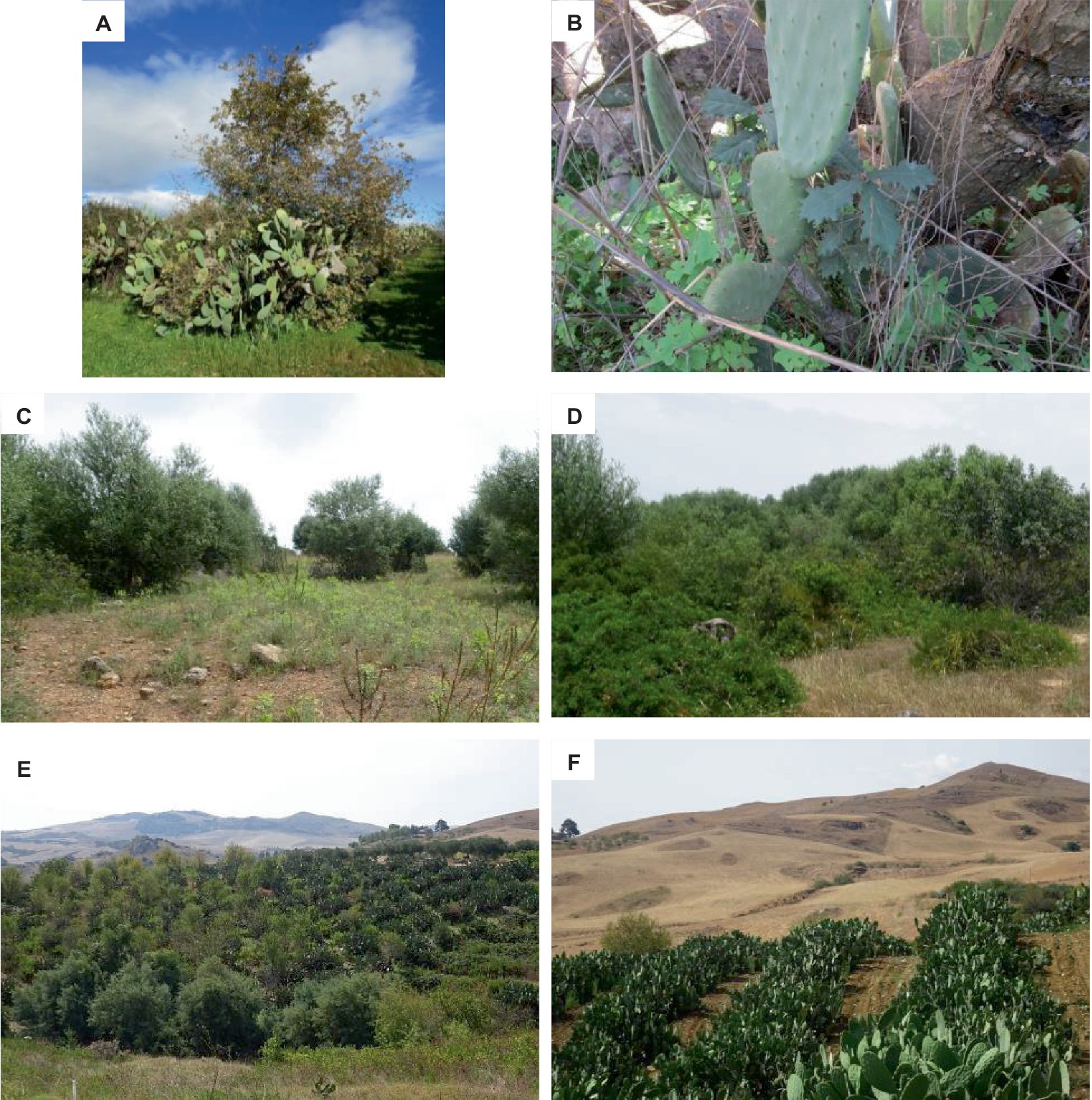
Figure 4. One mature Quercus pubescens individual (A) and one Quercus pubescens sapling growing at the PPC site (B). Abandoned olive trees and respective open areas at PPSP (C), current appearance of the abandoned prickly pear orchard at PPSP, dominated by native woody species (D), aspect of the PPR site with woody species covering the prickly pears in the unmanaged part on the left (E), one managed prickly pear orchard and nearby arable land intermingled with degraded patches that could be suitable to use prickly pear to foster native woody species colonization (F).
Conservation trade-offs for the use of prickly pear
The limited number of studies evaluating prickly pear as a restoration tool largely depends on its prevalent use as a productive crop, so unwanted plant species in prickly pear orchards are usually removed. For this reason, we did not find other suitable areas for our research, which limited the number of sampling sites. However, we clearly recognize that the major constraint that may limit the use of prickly pear as a restoration tool is its invasive potential in the Mediterranean, where it represents a serious ecological threat, particularly for chasmophytic vegetation in rocky habitats (Vilà et al., 2003; Guarino et al., 2021). Even if in our study areas and in nearby abandoned fields and forest patches (e.g., pine and eucalyptus reforestation, oak forests, and maquis), we found no evidence of invasive behavior, we do not recommend its use in areas where it is not already present or if careful management is not feasible. From this side, it should be kept in mind that prickly pear is an agricultural crop already covering millions of hectares and sustaining the economy of entire regions, so that in many areas, including Sicily, there are no restrictions on its cultivation. Most importantly, its management is really feasible, as knocking down all the first fruits when they are unripe is a common agricultural practice made by local farmers, preventing their diffusion through seed dispersal. Prickly pear is also highly sensitive to shading, so that the cover by adult tree individuals, once living underneath its canopy, naturally prevents its future development. In turn, when native woody plants are fully established, prickly pear can be cut, and the residuals can be used to feed livestock (another common practice), mulch, increase soil organic matter, or be used as a natural biogel for reforestation purposes (Le Houérou, 1996; Vimercati et al., 2020; Stavi, 2022). Considering the several biotic and abiotic constraints hampering the restoration success of Mediterranean degraded areas, particularly in arid and semi-arid climates, our results indicate that prickly pear may represent a valid tool to facilitate the restoration of Mediterranean woodlands, provided that its invasive potential is carefully considered.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
RB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. EB: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. GS: Investigation, Methodology, Writing – review & editing. TM: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – review & editing.
Funding
This work has received funding from the European Union-NextGenerationEU through the Italian Ministry of University and Research under PNRR–PNRR MUR Missione 4 Componente 2 Investimento 1.4 “Potenziamento strutture di ricerca e creazione di “Campioni Nazionali di R&S” Centro Nazionale della Biodiversità (National Biodiversity Future Center, NBFC – 2022-2026), Spoke 7. The views and opinions expressed are those of the authors only and do not necessarily reflect those of the European Union or the European Commission. Neither the European Union nor the European Commission can be held responsible for them. The research was also partially funded by the Life Project “Desert-Adapt” LIFE16 CCA/IT/000011.
Acknowledgments
We thank the prickly pear farmers, Michele Russo and Andrea Cairone, for their contribution to the conceptual framework, fieldwork support, and precious information on prickly pear cultivation and natural history. We also thank Salvatore Pasta for the comments on the article and the two reviewers for the constructive suggestions that improved the article.
In memoriam
One of the authors (Tommaso La Mantia) dedicates this article to his friend Enza Chessa, who studied prickly pear throughout her life.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2024.1343069/full#supplementary-material
Footnotes
References
Acácio, V., Holmgren, M., Jansen, P. A., and Schrotter, O. (2007). Multiple recruitment limitation causes arrested succession in Mediterranean Cork oak systems. Ecosystems 10, 1220–1230. doi: 10.1007/s10021-007-9089-9
Andivia, E., Villar-Salvador, P., Tovar, L., Rabasa, S., and Rey Benayas, J. M. (2017). Multiscale assessment of woody species recruitment in Mediterranean shrublands: facilitation and beyond. J. Veg. Sci. 28, 639–648. doi: 10.1111/jvs.12520
Badalamenti, E., Bueno, R. S., Campo, O., Gallo, M., La Mela Veca, D. S., Pasta, S., et al. (2018). Pine stand density influences the regeneration of Acacia saligna Labill. H.L.Wendl. and native Woody species in a Mediterranean coastal pine plantation. Forests 9:359. doi: 10.3390/f9060359
Badalamenti, E., Bueno, R. S., Sala, G., Cusimano, D., La Mantia, T., and Ilardi, V. (2022). The naturalization of the almond Prunus dulcis in different ecological contexts in the Mediterranean: an underestimated process? Flora 294:152117. doi: 10.1016/j.flora.2022.152117
Badalamenti, E., Pasta, S., Sala, G., Catania, V., Quatrini, P., and La Mantia, T. (2020a). The paradox of the alien plant Leucaena leucocephala subsp. glabrata (rose) S. Zárate in Sicily: another threat for the native Flora or a valuable resource? Int. J. Plant Biol. 11:8637. doi: 10.4081/pb.2020.8637
Badalamenti, E., Scalenghe, R., La Mantia, T., Bueno, R., Sala, G., Pizzurro, G., et al. (2020b). The cork oak in the mountains of Palermo (Italy): ecological insights from the south-eastern edge of its distribution range. iForest 13, 336–344. doi: 10.3832/ifor3360-013
Badalamenti, E., Sferlazza, S., La Mela Veca, D. S., Maetzke, F., Sala, G., and La Mantia, T. (2020c). Which are southern Italy’s fastest growing tree species? Lessons from the past for future perspectives, with a special focus on Sicily. Ann. Silvicult. Res. 45, 31–43. doi: 10.12899/asr-1845
Bautista-Cruz, A., Leyva-Pablo, T., de León-González, F., Zornoza, R., Martínez-Gallegos, V., Fuentes-Ponce, M., et al. (2018). Cultivation of Opuntia ficus-indica under different soil management practices: a possible sustainable agricultural system to promote soil carbon sequestration and increase soil microbial biomass and activity. Land Degrad. Dev. 29, 38–46. doi: 10.1002/ldr.2834
Bazan, G., Marino, P., Guarino, R., Domina, G., and Schicchi, R. (2015). Bioclimatology and vegetation series in Sicily: a geostatistical approach. Ann. Bot. Fenn. 52, 1–18. doi: 10.5735/085.052.0202
Bobiec, A., Reif, A., and Öllerer, K. (2018). Seeing the oakscape beyond the forest: a landscape approach to the oak regeneration in Europe. Landsc. Ecol. 33, 513–528. doi: 10.1007/s10980-018-0619-y
Brooker, R. W., Maestre, F. T., Callaway, R. M., Lortie, C. L., Cavieres, L. A., Kunstler, G., et al. (2008). Facilitation in plant communities: the past, the present, and the future. J. Ecol. 96, 18–34. doi: 10.1111/j.1365-2745.2007.01295.x
Bueno, R. S., Badalamenti, E., Barone, E., Cairone, A., Mantia, A. L., Sala, G., et al. (2020a). First assessment of natural regeneration and seed dispersal of Persian walnut (Juglans regia L.) in Mediterranean agroecosystems. Arboriculture & Urban. Forestry 46, 174–184. doi: 10.48044/jauf.2020.013
Bueno, R. S., García, D., Galetti, M., and La Mantia, T. (2020b). Past cover modulates the intense and spatially structured natural regeneration of woody vegetation in a pastureland. Plant Ecol. 221, 205–218. doi: 10.1007/s11258-020-01006-3
Bueno, R. S., García, D., Galetti, M., and La Mantia, T. (2021). Trophic and spatial complementarity on seed dispersal services by birds, wild mammals, and cattle in a Mediterranean woodland pasture. Glob. Ecol. Conserv. 31:e01880. doi: 10.1016/j.gecco.2021.e01880
Cairone, A., Di Leo, C., and La Mantia, T. (2020). “How avifauna changes: forty years of observations on the status of birds in a representative area of Sicily (Roccapalumba, pa)” in Life on islands: biodiversity in Sicily and surrounding islands: studies dedicated to Bruno Massa. eds. T. La Mantia, E. Badalamenti, A. Carapezza, P. Lo Cascio, and A. Troia (Palermo: Edizioni Danaus), 307–325.
Calvi, F., Catena, P., Cibella, R., Cirasa, A., Dolce, F., Drago, A., et al. (2016). Carta della sensibilità alla desertificazione in Sicilia, Scala 1:250000. Palermo: Assessorato Regionale del Territorio e dell’Ambiente.
Camerano, P., Cullotta, S., and Varese, P. (2011). Strumenti Conoscitivi per la Gestione delle Risorse Forestali della Sicilia. Tipi Forestali. Perugia.
Castro, J., Zamora, R., Hódar, J. A., and Gómez, J. M. (2002). Use of shrubs as nurse plants: a new technique for reforestation in Mediterranean Mountains. Restor. Ecol. 10, 297–305. doi: 10.1046/j.1526-100X.2002.01022.x
Duponnois, R., Hafidi, M., Thioulouse, J., Galiana, A., Ouahmane, L., Dreyfus, B., et al. (2009). “Monitoring the development of nurse plant species to improve the performances of reforestation programs in Mediterranean areas” in Microbial strategies for crop improvement. eds. M. S. Khan, A. Zaidi, and J. Musarrat (Berlin, Heidelberg: Springer Berlin Heidelberg), 255–265.
Erre, P., Chessa, I., Nieddu, G., and Jones, P. G. (2009). Diversity and spatial distribution of Opuntia spp. in the Mediterranean Basin. J. Arid Environ. 73, 1058–1066. doi: 10.1016/j.jaridenv.2009.05.010
Fierotti, G. (1988). Carta dei suoli della Sicilia (scala 1:250.000). Palermo: Regione Siciliana, Assessorato Territorio e Ambiente.
Filazzola, A., and Lortie, C. J. (2014). A systematic review and conceptual framework for the mechanistic pathways of nurse plants. Glob. Ecol. Biogeogr. 23, 1335–1345. doi: 10.1111/geb.12202
Ganatsas, P., and Tsakaldimi, M. (2013). A comparative study of desiccation responses of seeds of three drought-resistant Mediterranean oaks. For. Ecol. Manag. 305, 189–194. doi: 10.1016/j.foreco.2013.05.042
Génin, M., Alifriqui, M., Fakhech, A., Hafidi, M., Ouahmane, L., and Génin, D. (2017). Back to forests in pre-Saharan Morocco? When prickly pear cultivation and traditional agropastoralism reduction promote argan tree regeneration. Silva Fennica 51:1618. doi: 10.14214/sf.1618
Gómez, J. M. (2003). Spatial patterns in long-distance dispersal of Quercus ilex acorns by jays in a heterogeneous landscape. Ecography 26, 573–584. doi: 10.1034/j.1600-0587.2003.03586.x
Gómez-Aparicio, L. (2009). The role of plant interactions in the restoration of degraded ecosystems: a meta-analysis across life-forms and ecosystems. J. Ecol. 97, 1202–1214. doi: 10.1111/j.1365-2745.2009.01573.x
Gómez-Aparicio, L., Zamora, R., Gómez, J. M., Hódar, J. A., Castro, J., and Baraza, E. (2004). Applying plant facilitation to forest restoration: a meta-analysis of the use of shrubs as nurse plants. Ecol. Appl. 14, 1128–1138. doi: 10.1890/03-5084
Gonzalez, S. L., and Ghermandi, L. (2019). Dwarf shrub facilitates seedling recruitment and plant diversity in semiarid grasslands. PLoS One 14:e0212058. doi: 10.1371/journal.pone.0212058
Granda, E., Escudero, A., and Valladares, F. (2014). More than just drought: complexity of recruitment patterns in Mediterranean forests. Oecologia 176, 997–1007. doi: 10.1007/s00442-014-3064-x
Guarino, R., Chytrý, M., Attorre, F., Landucci, F., and Marcenò, C. (2021). Alien plant invasions in Mediterranean habitats: an assessment for Sicily. Biol. Invasions 23, 3091–3107. doi: 10.1007/s10530-021-02561-0
Hassan, S., Inglese, P., Gristina, L., Liguori, G., Novara, A., Louhaichi, M., et al. (2019). Root growth and soil carbon turnover in Opuntia ficus-indica as affected by soil volume availability. Eur. J. Agron. 105, 104–110. doi: 10.1016/j.eja.2019.02.012
ISTAT (2022). Coltivazioni legnose fruttifere. Istituto Nazionale di Statistica, Rome. Available at: http://dati.istat.it/Index.aspx?QueryId=33705 (Accessed August 28, 2023).
Jordano, P. (2014). “Fruits and frugivory” in Seeds: the ecology of regeneration in plant communities. ed. R. S. Gallagher. 3rd Edn (Wallingford: CABI), 18–61.
Jorge, A. O. S., Costa, A. S. G., and Oliveira, M. B. P. P. (2023). Adapting to climate change with Opuntia. Plan. Theory 12:2907. doi: 10.3390/plants12162907
Krumm, F., and Vítková, L. (2016). Introduced tree species in European forests: opportunities and challenges. Freiburg: European Forest Institute.
Kumschick, S., Bertolino, S., Blackburn, T. M., Brundu, G., Costello, K. E., De Groot, M., et al. (2023). Using the IUCN environmental impact classification for alien taxa to inform decision-making. Conserv. Biol. :e14214. doi: 10.1111/cobi.14214
La Mantia, T., and Bueno, R. S. (2016). Colonization of eurasian jay Garrulus glandarius and holm oaks Quercus ilex: the establishment of ecological interactions in urban areas. Avocetta 40, 85–87.
La Mantia, T., Rühl, J., Massa, B., Pipitone, S., Lo Verde, G., and Bueno, R. S. (2019). Vertebrate-mediated seed rain and artificial perches contribute to overcome seed dispersal limitation in a Mediterranean old field. Restor. Ecol. 27, 1393–1400. doi: 10.1111/rec.13009
Lapenna, M. R., and Fascetti, S. (2010). Ex situ conservation of dwarf junipers (Juniperus hemisphaerica Presl, Juniperus nana Willd.) in Pollino National Park, southern Italy. Scand. J. For. Res. 25, 109–114. doi: 10.1080/02827581.2010.485782
Le Houérou, H. N. (1996). The role of cacti (Opuntia spp.) in erosion control, land reclamation, rehabilitation and agricultural development in the Mediterranean Basin. J. Arid Environ. 33, 135–159. doi: 10.1006/jare.1996.0053
Le Houerou, H. N. (2000). Restoration and rehabilitation of arid and semiarid Mediterranean ecosystems in North Africa and West Asia: a review. Arid Soil Res. Rehabil. 14, 3–14. doi: 10.1080/089030600263139
Matías, L., Abdelaziz, M., Godoy, O., and Gómez-Aparicio, L. (2019). Disentangling the climatic and biotic factors driving changes in the dynamics of Quercus suber populations across the species latitudinal range. Divers. Distrib. 25, 524–535. doi: 10.1111/ddi.12873
Meli, P., Holl, K. D., Rey Benayas, J. M., Jones, H. P., Jones, P. C., Montoya, D., et al. (2017). A global review of past land use, climate, and active vs. passive restoration effects on forest recovery. PLoS One 12:e0171368. doi: 10.1371/journal.pone.0171368
Mendoza, I., Gómez-Aparicio, L., Zamora, R., and Matías, L. (2009). Recruitment limitation of forest communities in a degraded Mediterranean landscape. J. Veg. Sci. 20, 367–376. doi: 10.1111/j.1654-1103.2009.05705.x
Mulligan, M., Burke, S., and Ogilvie, A. (2016). “Much more than simply “desertification”: understanding agricultural sustainability and change in the Mediterranean” in The end of desertification? Disputing environmental change in the drylands. eds. R. Behnke and M. Mortimore (Berlin, Heidelberg: Springer Berlin Heidelberg), 427–450.
Neffar, S., Chenchouni, H., Beddiar, A., and Redjel, N. (2013). Rehabilitation of degraded rangeland in drylands by prickly pear (Opuntia ficus-indica L.) plantations: effect on soil and spontaneous vegetation. Ecol. Balkan. 5, 63–76.
Neffar, S., Menasria, T., and Chenchouni, H. (2018). Diversity and functional traits of spontaneous plant species in Algerian rangelands rehabilitated with prickly pear (Opuntia ficus-indica L.) plantations. Turk. J. Bot. 42, 448–461. doi: 10.3906/bot-1801-39
Novara, A., Gristina, L., Sala, G., Galati, A., Crescimanno, M., Cerdà, A., et al. (2017). Agricultural land abandonment in Mediterranean environment provides ecosystem services via soil carbon sequestration. Sci. Total Environ. 576, 420–429. doi: 10.1016/j.scitotenv.2016.10.123
Novoa, A., Foxcroft, L. C., Keet, J.-H., Pyšek, P., and Le Roux, J. J. (2021). The invasive cactus Opuntia stricta creates fertility islands in African savannas and benefits from those created by native trees. Sci. Rep. 11:20748. doi: 10.1038/s41598-021-99857-x
Oduor, A. M. O., Long, H., Fandohan, A. B., Liu, J., and Yu, X. (2018). An invasive plant provides refuge to native plant species in an intensely grazed ecosystem. Biol. Invasions 20, 2745–2751. doi: 10.1007/s10530-018-1757-5
Padilla, F. M., and Pugnaire, F. I. (2006). The role of nurse plants in the restoration of degraded environments. Front. Ecol. Environ. 4, 196–202. doi: 10.1890/1540-9295(2006)004[0196:TRONPI]2.0.CO;2
Padrón, B., Nogales, M., Traveset, A., Vilà, M., Martínez-Abraín, A., Padilla, D. P., et al. (2011). Integration of invasive Opuntia spp. by native and alien seed dispersers in the Mediterranean area and the Canary Islands. Biol. Invasions 13, 831–844. doi: 10.1007/s10530-010-9872-y
Pausas, J. G., Bonet, A., Maestre, F. T., and Climent, A. (2006). The role of the perch effect on the nucleation process in Mediterranean semi-arid oldfields. Acta Oecol. 29, 346–352. doi: 10.1016/j.actao.2005.12.004
Pausas, J. G., and Millán, M. M. (2019). Greening and Browning in a climate change hotspot: the Mediterranean Basin. Bioscience 69, 143–151. doi: 10.1093/biosci/biy157
Pedrotti, F. (2019). The vegetation around dry-wall stone huts on the Macereto plateau (Sibillini Mountains, central Apennines). Bocconea 28, 293–306. doi: 10.7320/Bocc28.293
Pignatti, S., Guarino, R., and La Rosa, M. (2017–2019). Flora d’Italia, 2nd ed. Bologna: Edagricole.
Plieninger, T., Hui, C., Gaertner, M., and Huntsinger, L. (2014). The impact of land abandonment on species richness and abundance in the Mediterranean Basin: a meta-analysis. PLoS One 9:e98355. doi: 10.1371/journal.pone.0098355
Pons, J., and Pausas, J. G. (2006). Oak regeneration in heterogeneous landscapes: the case of fragmented Quercus suber forests in the eastern Iberian Peninsula. For. Ecol. Manag. 231, 196–204. doi: 10.1016/j.foreco.2006.05.049
Prăvălie, R., Patriche, C., and Bandoc, G. (2017). Quantification of land degradation sensitivity areas in southern and central southeastern Europe. New results based on improving DISMED methodology with new climate data. Catena 158, 309–320. doi: 10.1016/j.catena.2017.07.006
R Core Team (2021). R: a language and environment for statistical computing. Version 4.1.2. R Foundation for Statistical Computing, Vienna.
Reynolds, J. F., Smith, D. M. S., Lambin, E. F., Turner, B. L., Mortimore, M., Batterbury, S. P. J., et al. (2007). Global desertification: building a science for dryland development. Science 316, 847–851. doi: 10.1126/science.1131634
Rolo, V., Plieninger, T., and Moreno, G. (2013). Facilitation of holm oak recruitment through two contrasted shrubs species in Mediterranean grazed woodlands. J. Veg. Sci. 24, 344–355. doi: 10.1111/j.1654-1103.2012.01458.x
Schlaepfer, M. A., Sax, D. F., and Olden, J. D. (2011). The potential conservation value of non-native species. Conserv. Biol. 25, 428–437. doi: 10.1111/j.1523-1739.2010.01646.x
Shackleton, S., Kirby, D., and Gambiza, J. (2011). Invasive plants – friends or foes? Contribution of prickly pear (Opuntia ficus-indica) to livelihoods in Makana municipality, eastern cape, South Africa. Dev. South. Afr. 28, 177–193. doi: 10.1080/0376835X.2011.570065
Simberloff, D., Martin, J.-L., Genovesi, P., Maris, V., Wardle, D. A., Aronson, J., et al. (2013). Impacts of biological invasions: what’s what and the way forward. Trends Ecol. Evol. 28, 58–66. doi: 10.1016/j.tree.2012.07.013
Smit, C., den Ouden, J., and Díaz, M. (2008). Facilitation of Quercus ilex recruitment by shrubs in Mediterranean open woodlands. J. Veg. Sci. 19, 193–200. doi: 10.3170/2007-8-18352
Stavi, I. (2022). Ecosystem services related with Opuntia ficus-indica (prickly pear cactus): a review of challenges and opportunities. Agroecol. Sustain. Food Syst. 46, 815–841. doi: 10.1080/21683565.2022.2076185
Suzuki, K. F., Kobayashi, Y., Seidl, R., Senf, C., Tatsumi, S., Koide, D., et al. (2021). The potential role of an alien tree species in supporting forest restoration: lessons from Shiretoko National Park, Japan. For. Ecol. Manag. 493:119253. doi: 10.1016/j.foreco.2021.119253
Tesfay, Y. B., and Kreyling, J. (2021). The invasive Opuntia ficus-indica homogenizes native plant species compositions in the highlands of Eritrea. Biol. Invasions 23, 433–442. doi: 10.1007/s10530-020-02373-8
Trabaud, L., and Galtié, J.-F. (1996). Effects of fire frequency on plant communities and landscape pattern in the massif des Aspres (southern France). Landsc. Ecol. 11, 215–224. doi: 10.1007/BF02071812
Valiente-Banuet, A., Aizen, M. A., Alcántara, J. M., Arroyo, J., Cocucci, A., Galetti, M., et al. (2015). Beyond species loss: the extinction of ecological interactions in a changing world. Funct. Ecol. 29, 299–307. doi: 10.1111/1365-2435.12356
Vilà, M., Burriel, J. A., Pino, J., Chamizo, J., Llach, E., Porterias, M., et al. (2003). Association between Opuntia species invasion and changes in land-cover in the Mediterranean region. Glob. Chang. Biol. 9, 1234–1239. doi: 10.1046/j.1365-2486.2003.00652.x
Keywords: desertification, ecological restoration, Mediterranean forests, oak, plant–plant facilitation, recruitment limitation, seed dispersal, Quercus
Citation: Bueno RS, Badalamenti E, Sala G and La Mantia T (2024) A crop for a forest: Opuntia ficus-indica as a tool for the restoration of Mediterranean forests in areas at desertification risk. Front. For. Glob. Change. 7:1343069. doi: 10.3389/ffgc.2024.1343069
Edited by:
Arun Jyoti Nath, Assam University, IndiaReviewed by:
Kevin Cianfaglione, Lille Catholic University, FranceSergio Espinoza, Catholic University of the Maule, Chile
Copyright © 2024 Bueno, Badalamenti, Sala and La Mantia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Emilio Badalamenti, ZW1pbGlvLmJhZGFsYW1lbnRpQHVuaXBhLml0
 Rafael Silveira Bueno
Rafael Silveira Bueno Emilio Badalamenti
Emilio Badalamenti Giovanna Sala
Giovanna Sala Tommaso La Mantia1,2
Tommaso La Mantia1,2