- 1Department of Animal Production and Management, Faculty of Agriculture and Animal Sciences, Busitema University Arapai Campus, Soroti, Uganda
- 2School of Medicine, Kabale University, Kabale, Uganda
- 3Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, Taif University, Taif, Saudi Arabia
- 4National Institute of Laser Enhanced Sciences, Cairo University, Giza, Egypt
- 5Department of Wildlife and Aquatic Animal Resources, School of Veterinary Medicine and Animal Resources, College of Veterinary Medicine, Animal Resources and Biosecurity, Makerere University, Kampala, Uganda
- 6School of Pharmacy, Kampala International University Western Campus, Bushenyi, Uganda
- 7Faculty of Biomedical Sciences, Kampala International University Western Campus, Bushenyi, Uganda
- 8Department of Medical Laboratory Sciences, School of Allied Health Sciences, Kampala International University Western Campus, Bushenyi, Uganda
- 9Faculty of Agriculture and Environmental Science, Muni University, Arua, Uganda
- 10School of Medicine and Health Sciences, Soroti University, Soroti, Uganda
- 11Department of Pharmacology and Therapeutics, Faculty of Veterinary Medicine, Damanhour University, Damanhour, Egypt
- 12Department of Chemistry, Faculty of Science, Gulu University, Gulu, Uganda
- 13Department of Humanities and Sciences, University of California, Los Angeles, Los Angeles, CA, United States
In this study, we initiated an effort to generate information about beef safety in Uganda. Our entry point was to assess by atomic absorption spectrophotometry the levels of essential elements copper (Cu), cobalt (Co), iron (Fe) and zinc (Zn), and non-essential elements lead (Pb), chromium (Cr), nickel (Ni), and cadmium (Cd) in 40 beef samples collected from within and around Soroti (Uganda). The information was used to evaluate the safety of consuming such beef against the World Health Organization (WHO) limits. The latter was accomplished by (i) estimating the daily intake (EDI) of each metal in the study area, (ii) modeling the non-cancer health risk using the target hazard quotient (THQ) and (iii) modeling the cancer risk using the incremental lifetime cancer risk (ILCR). The study finds that the mean concentrations (±95% CI) and EDI were in the order of Fe > Zn > Cr > Ni > Pb > Co > Cu > Cd. Cancer risk was found to be due to Ni > Cr > Cd > Pb and significantly higher in children than adults. The latter particularly demonstrates the importance of Ni poisoning in the study area. Overall, while essential elements in our beef samples were below WHO limits (hence no health risks), non-essential elements had high health and cancer risks due to higher levels of Cr and Ni.
Introduction
Contamination of food by heavy metals such as lead (Pb), iron (Fe), chromium (Cr), cadmium (Cd), copper (Cu), cobalt (Co), Nickel (Ni) and zinc (Zn) in developing countries is a major public health problem (1, 2). This has been demonstrated in Uganda, for example, in beef (3, 4), in drinking water (5, 6), street food (7), alcoholic beverages (8, 9), fish (10), food crops grown at dumpsites (11), and in food consumed around Lake Victoria and Lake George (12–14). Compounding this is human activities such as mining, irrational usage of chemicals and poor policies on industrial waste management which only exacerbate the problem (15, 16). In Africa, there is a general absence of legislations clearly designed to mitigate environmental degradation. This only suggests one natural course for the continent: that environmental contamination with heavy metals will worsen.
In the body, essential elements in moderate amounts play a crucial role in cellular functions. Their bioavailability is required for certain enzymes to aid metabolic reactions in cellular systems. Iron for example, once complexed with Cu, helps in electron transport system during ATP metabolism [see Supplementary Information in (17)]. The World Health Organization (WHO) recommends a daily intake of 6 ppm/day for Fe and 1 ppm for Cu in children. For a 70 kg adult, the recommendation is 392 ppm/week for Fe and 245 ppm/week for Cu (18). Excessive human consumption of Cu can disrupt physiological processes in the digestive and excretory systems leading to intestinal ulcerations and tubular necrosis (19, 20). Concentrations of Fe and Cu in beef samples are 184 and 160 ppm, respectively (21). Furthermore, contamination of red meat with heavy metals has been identified as a major public health risk requiring novel strategies to decontaminate meat (22).
Zn, an importance micronutrient, is an essential co-factor in living cells. It is required in maintaining the integrity of cellular structure, and as a regulator of cellular activities associated with strong antioxidant capacity (23, 24). The recommended maximum consumption of Zn is 7 ppm/week for a child (18) and 490 ppm/week for a 70 kg adult (25). The concentration of Zn in beef has been shown to be 127 ppm (21). This level of Zn is excessive when absorbed into the body since Zn suppresses Cu and Fe absorption leading to deficiencies of these elements in cells. As a consequence, ingestion of high Zn concentrations in a diet leads to gastrointestinal disorders and dysregulation of lipid metabolism in the body (26). Co in the form of vitamin B12 (26), is usually present at 0.25 ppm on in beef average (21) and acts as an essential nutrient for managing anemia associated with malabsorption of vitamin B12 in humans during ulcers (27). Co toxicity mainly leads to cardiovascular, hematological, neurological, and endocrine deficits. (28, 29).
Non-essential elements such as Pb, Cd, Ni and Cr are toxic to the body. Pb exposure for example is associated with gastrointestinal irritation and neurotoxicity in children and adults while chronic exposure may lead to cancer in humans (30, 31). These metals, Cr and Ni for example, arise from tanneries and from black smith industry, respectively, in Uganda. WHO recommends a tolerable intake of 0.025 ppm/week for Pb (32), a change from a previous permissible limit of 1.75 ppm/week for a 70 kg adult (18). Cd is a carcinogenic agent known to induce some form of cancer (33). This includes those of the lung and the prostate. WHO has set a permissible limit of 0.49 ppm/body weight/week for Cd (21, 34). In 2003, the Food and Agricultural Organization (FAO) set a weekly tolerable intake of 7 ppm/body weight. The European Food Safety Agency (EFSA) modified this to 2.5 ppm/body weight/week (21), and then to 0.49 ppm/bodyweight/week day (34) as a more practical limit. Ni is important in the body at low levels as an enzyme activator, but a carcinogen at high concentration (35, 36). WHO has set a Ni tolerable daily intake of 11 ppm/day/body weight for humans (18). Cr has been associated with urticaria, anemia and generalized visceral disorders (37, 38). In a recent study, half of a local population which consumed meat of wild boar was shown to be exposed to levels of Cr >12.5l ppm/week/ person (CI for the median = 0.5l ppm/Cr week/person) (39), much lower than the intake of 31.67 and 28.47 ppm/Cr week/person in both men and women in Nigeria (40). The latter demonstrates the importance of Cr in food safety.
Heavy metals enter the meat though feedlots and water used in raising the donor animals (3, 41). Furthermore, environmental pollution with sewage and urban agriculture which thrives on it helps propagate heavy metal bio-accumulation in foods (11, 42).
In developing countries, information concerning the extent of heavy metal contamination is scarce. Policy makers in the food industry are therefore not well informed to take the necessary steps to protect consumers. There is therefore need to start providing this critical of information for safeguarding public safety. In this study, we begin the process filling this information gap by establishing the heavy metal concentrations in beef consumed in a typical developing country community. We further show how to assess public health risk posed by consuming contaminants at the levels detected in beef.
Methods
Study Design
This study was an observational one conducted in the Soroti district of eastern Uganda during the months of December 2019 to March 2020. The area was chosen because of its cattle keeping culture, along with the capacity to study agriculturally-related problems in a purposely located university in Soroti. Furthermore, Soroti district has numerous butcheries (points of sale) to acquire samples. The belief in the area, anecdotally at best, is that beef sold there are contaminated with heavy metals (among others), the sources of which are anthropogenic in nature.
A total of 40 beef samples were collected from butcher points of sale in local villages. The georeferenced coordinates for each village with an accuracy <3 m were also taken (data files available). These were used for mapping (quantum geographical systems, qGIS®, version 3.03 Cirona) onto images acquired using the Sentinel-2A satellite from the United States Geographical Surveys (Sentinel-2 image ID: L1C_T36NWG_A023913_20200120T080825 and image ID: L1C_T36NWH_AO23484_20191221T080920). The following modifications were made in the process: Band 1 was set to red, interpolation was set to linear, color ramp was set to viridis. These settings were divided to show 6 categories: black (26 units) for deep water = Lake Kyoga, purple (54 units) for shallow water =swamps, light blue (82 units) for moderate vegetation, green (110 units) for sparse vegetation and yellow (138 units) for bare/dry soil in qGIS®.
Preparation of Heavy Metal Standards
Solutions of heavy metals working standards were prepared from stock solutions as previously described (3). For instrument calibration, four (4) calibration standard solutions were prepared at the 0.0, 0.5, 2.0, and 5.0 ppm levels to cover the linear absorbance range of the atomic absorption spectrophotometer used in this study (AAS, PerkinElmer 2380; see Table 1 for outcome).
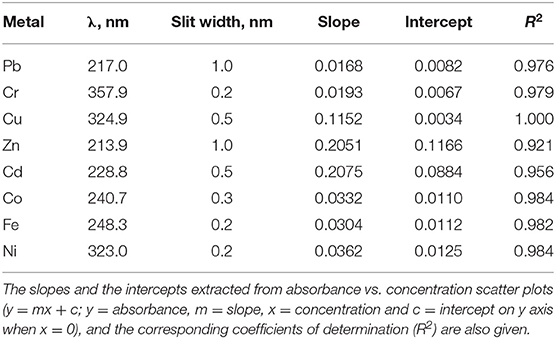
Table 1. The wavelengths and corresponding slit widths used to obtain instrumental linear calibration range for each metal.
Sampling, Preparation, and Analysis of Beef
About 200 g of raw beef samples were collected from the butchers during morning slaughter hours and placed into sterile plastic bottles. These were transported at 4°C to our laboratory for processing. Analysis of the processed samples was based on standard methods for heavy metal determination (3, 43). The concentration of the heavy metals in each beef extract was determined using AAS at wavelength corresponding to, and instrumental linear calibration range for, each metal listed in Table 1. Blank (solvent) analyses were done as well to assure laboratory control over the analytical process. This was inline with standard practice in this field (44).
Modeling of Estimated Daily Intake of Heavy Metals in Beef
To estimate the daily intake of heavy metals from consuming beef from Soroti on the basis of samples collected, the following equation was used;
where, EDI = estimated daily intake, C = heavy metal concentration and BW = body weight (60.7 and 20.5 kg for adults and children, respectively). The beef ingestion rate (IR) for Ugandans is 120 and 56.7 g for both adults and children, respectively (3).
Modeling Non-cancer Risk Associated With Beef Consumption
The target hazard quotient (THQ) was used to generate the hazard index (HI) to determine presence of non-carcinogenic health effects following oral ingestion of beef using the following equation:
where CDI is the chronic daily intake obtained using the equation below, and RfD is the oral reference dose of the contaminant, an estimation of the maximum permissible risk on human population through daily exposure.
where EDI is the estimated daily intake of a metal via ingestion of specific route. EFr is the exposure frequency (365 days/year); EDtot is the exposure duration (i.e., 6 years for children and 30 years for adults); and AT is the period of exposure for noncarcinogenic effects. For non-cancer risk modeling, AT = EFr × EDtot (2,190 days in children and 10,950 days in adults) and the reference dose (RfD) for each hazard was described previously (5). i.e., 0.04, 0.3, 0.0035, 0.0005, 0.03, 0.7, 0.02, and 0.0003 ppm/day for Cu, Zn, Pb, Cd, Co, Fe, Ni, and Cr, respectively. Exposure to multiple contaminants results in additive and interactive effects; thus, the hazard index (HI = ∑THQ) was used as an indication of risk (5).
Modeling Cancer Risk Associated With Beef Consumption
The incremental lifetime cancer risk (ILCR) using standard methods (3) was used to model the cancer risk in the Ugandan population using the following equation:
where CDI is the chronic daily intake of a particular metal and this was estimated over the 70-year lifespan for Ugandans (i.e., AT =70 years × 365 days = 25,550 days). In addition, the cancer slope factor (CSF) of 0.0085 (ppm/day)−1, 0.38 (ppm/day)−1, 0.84 (ppm/day)−1, and 0.5 (ppm/day)−1, for Pb, Cd, Ni, and Cr respectively, were used (45, 46).
Statistical Analysis
Descriptive statistical analyses were conducted in MS Excel version 2010. A one sample t-test was conducted by making comparisons using the WHO/FAO reference values and significance was reported when P < 0.05. A summary was acquired by getting the discrepancy between the actual and theoretical mean (WHO limits) values to define “High” and “Low.” On the modeled estimates, a two sample t-test was conducted between children and adults on the estimated daily intake and cancer risk. Furthermore, presence of cancer risk was identified when thee ILCR was >1 × 10−4. Superscript “a” was used to indicate absence of risk while superscript “b” was used to indicate the presence of risk in the sampled population. Hierarchical clustering on principal components (HCPC) (47) was used to assess natural clustering of data. HCPC combines the benefits of both principal components analysis (PCA) with those of cluster analysis (CA) to group individuals or variables. Factoshiny package running within RStudio platform was used for HCPC (48, 49).
Results
Description of The Study Area
The study was conducted in an agricultural area with a high distribution of sparse green vegetation and water tributaries from Lake Kyoga. These conditions are conducive to livestock productivity in areas shown in Figure 1.
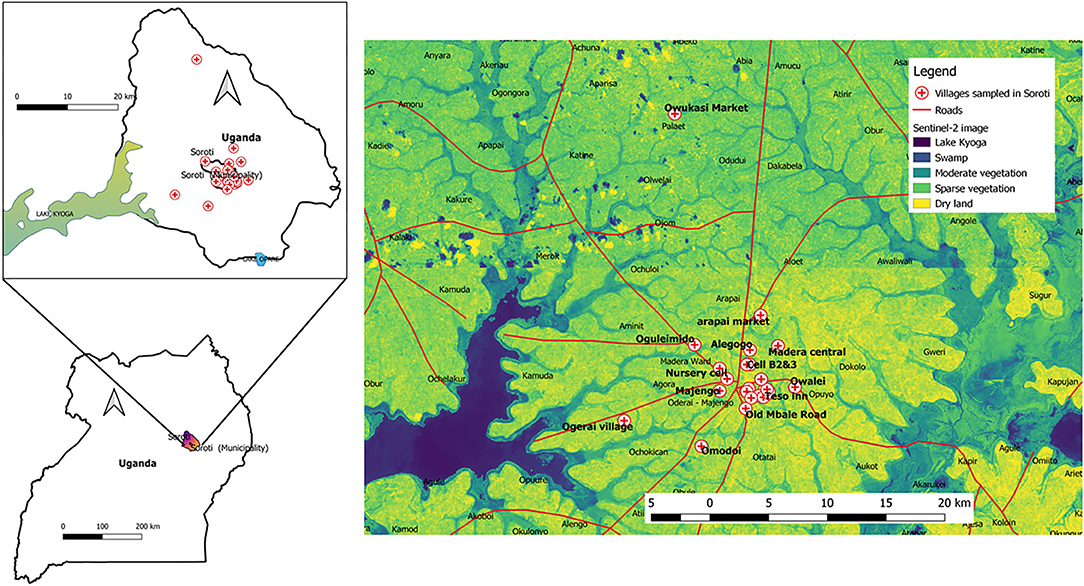
Figure 1. Map of Uganda showing vegetation distribution in the study area. Soroti is the largest district in the Teso sub-region and it is ~116km from Mbale, the largest city in Eastern Uganda. Soroti has a population of about 114,000 and this agricultural area is known for its high productivity of millet, cassava, peas, potatoes, beans, onions, tomatoes, cabbages and simsim as a result of its close proximity to the Lake Kyoga basin. Soroti also lies within the cattle corridor of Uganda where indigenous zebu cattle constitute the major animals kept in rural homesteads.
Description of Heavy Metal Concentrations in Beef From Eastern Uganda
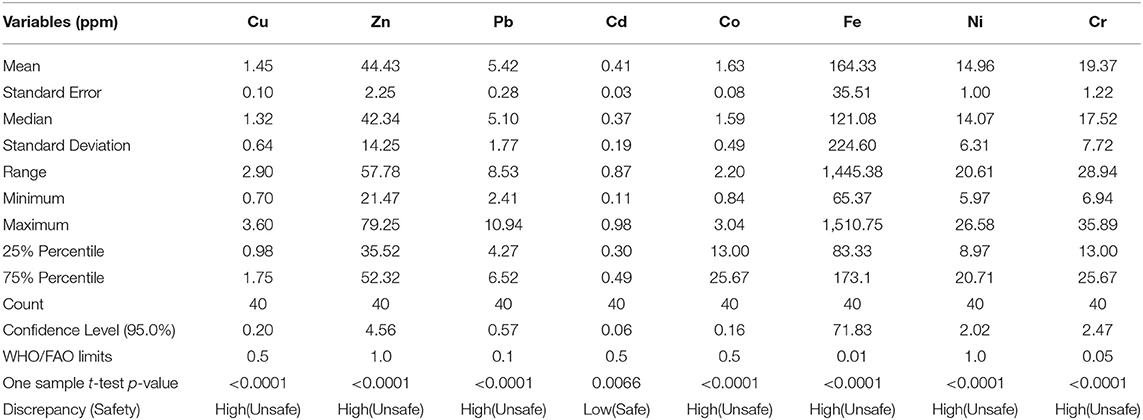
The statistics associated with metals concentrations in this study are displayed in Table 2. It can be seen from the table that only Cd level overall was below the WHO guideline maximum value and, as such, safe in a beef serving in the study area.
Description on Estimated Daily Intake of Heavy Metals From Beef of Eastern Uganda
Oral ingestion of Cu, Zn, Pb, Cd, Co, Ni, and Cr were significantly higher in children (P < 0.05) than adults. The EDIs are shown in Table 3.
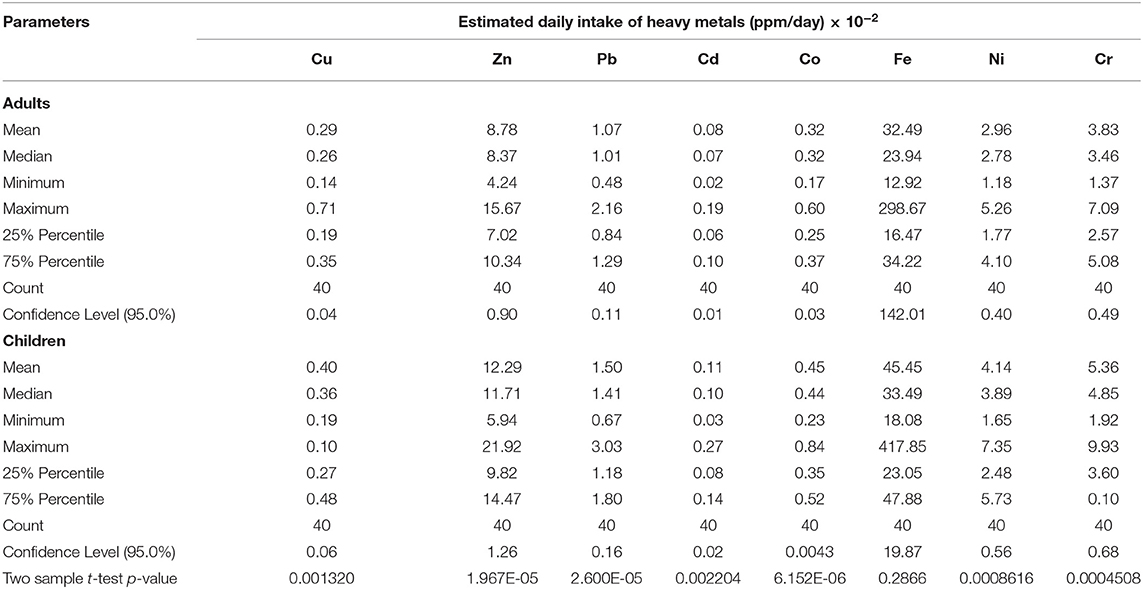
Table 3. Modeled estimated daily intake of heavy metals in beef amongst adults and children of Uganda.
The estimated daily intake of heavy metals by both children and adults were found to fall into three groups by HCPC (Figure 2). In one EDI group, labeled cluster 1, samples had low levels of Ni, Cr, Cu, and Co. In another (cluster 2), samples had high levels of Cr, Ni, and Cu, but low level of Pb, and in the last group (cluster 3), samples had high levels of Cd, Co, Pb, Ni, Fe, and Cu.
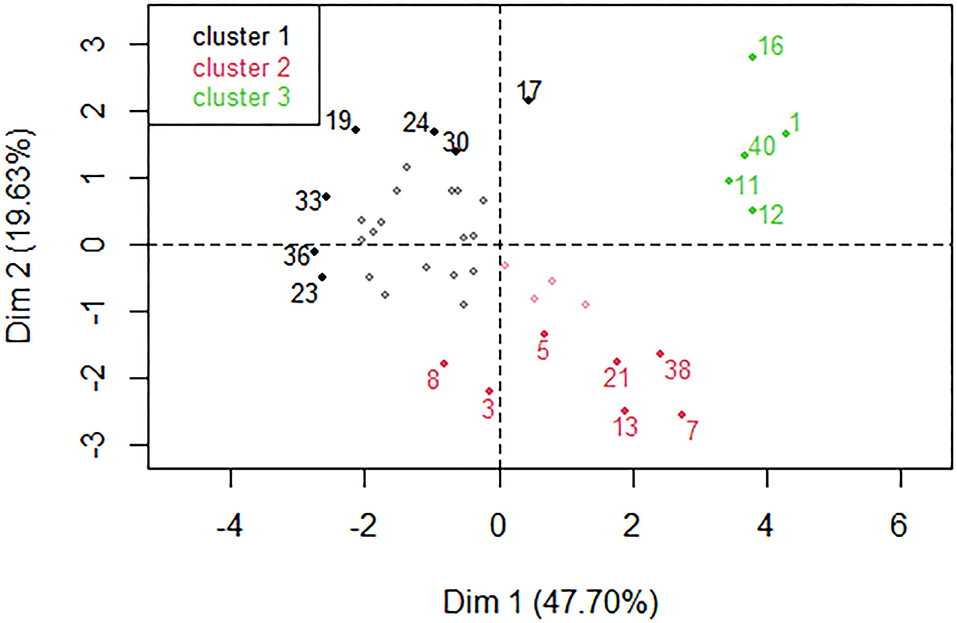
Figure 2. Hierarchical classification of the individual samples by HCPC revealed three clusters color-coded black (cluster 1), red (cluster 2) and green (cluster 3). A few individuals with the strongest influence in each cluster are shown for illustrative purposes.
Modeled Non-cancer Risk Amongst Ugandans
The target hazard quotient (THQ) for the metals studied were above 1 for both children and adults indicating a non-carcinogenic risk. There were no significant differences in THQ values in both adults and children as shown in Table 4.
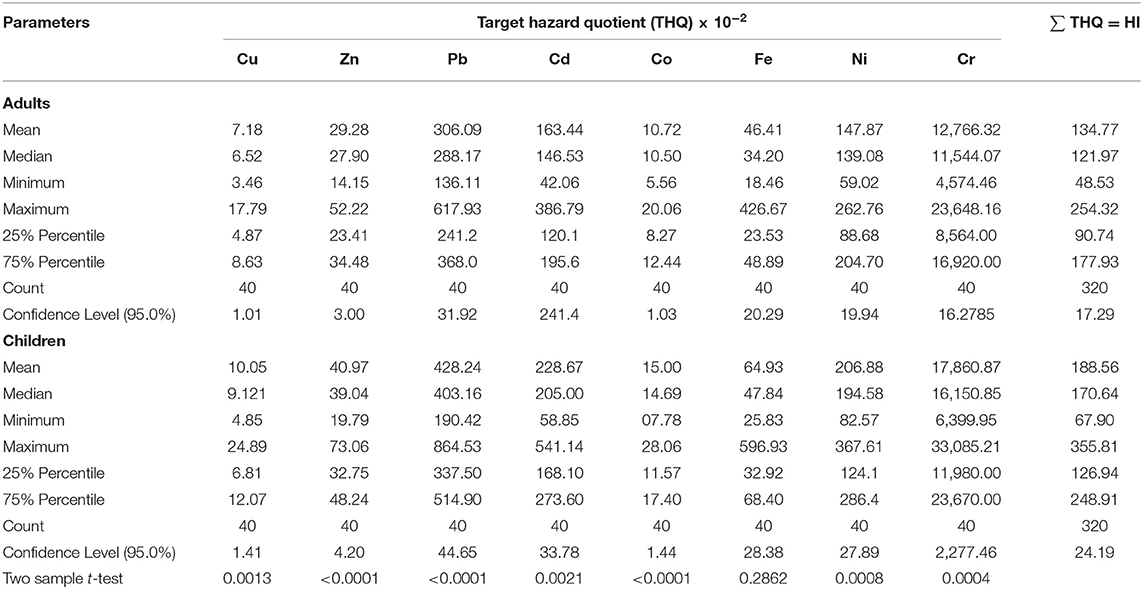
Table 4. Estimation of non-cancer health risks amongst Ugandans associated with oral consumption of beef from Soroti.
Modeled Cancer Risk Amongst Ugandans
The cancer risk was significantly lowest in the order of Pb < Cd < Cr < Ni in adults than children. Cancer risk following consumption of chromium and Nickel as shown in Table 5.
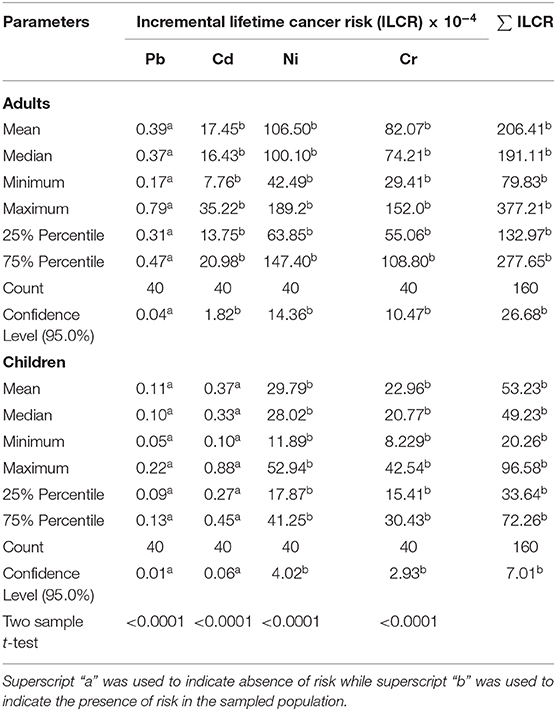
Table 5. Cancer risk associated with oral consumption of beef from Soroti amongst adults and children of Uganda.
Discussion
Eastern Uganda is an agricultural region of Uganda, predominantly dealing with cattle keeping. Soroti district in particular is one of the largest district in the area with numerous butcheries (points of sale) in the communities. Unfortunately, the beef sold in the district have contaminations including heavy metals, sources of which are anthropogenic in nature. The mean concentrations of heavy metals in the samples, from the highest to the lowest, revealed that Fe, Zn, Cr, Ni, Pb, Co, Cu, and Cd should be of concern in that order. Comparatively, this order is contrary to our earlier finding in the southwestern region of Uganda in which the order was Zn > Pb > Fe > Cu, and no Cd was detected (3). The discrepancy is explained by the fact that these two regions have different anthropogenic activities with potential health risks.
Earlier studies by Kasozi et al. (3) showed that accumulation of heavy metals in beef in both eastern and southwestern regions of Uganda (and by extrapolation, the whole country) is a national crisis requiring close attention by policy making bodies. The National Environmental Authority of Uganda (NEMA), could devise routine monitoring strategies to ensure that the situation doesn't depreciate further. On a broader scale, this falls squarely into the ecological cycle of pollution in Uganda which has been shown to involve everything from waste management (11, 15, 16), sources of natural water (12–14), effect on aquatic wildlife (10), state of drinking water (5, 6) to production of alcoholic beverages (8, 9) as well as food sold to the general public in the streets of Uganda (7). Similar findings of heavy metals have been reported in Kenya fishes and beef (2, 47).
It is well-established that absorption of heavy metals subsequently leads to their bioaccumulation into animal tissues such as beef (50, 51)and into human tissues following consumption of contaminated beef (52–54). This study supports this notion by showing that indeed there were high levels of heavy metals in all samples collected, well above the WHO limits with the exception of Cd. These exceedances of WHO limits point toward carcinogenic and non-carcinogenic risks in the study area (and perhaps a glimpse into what is happening in the entire country, Uganda). In Nigeria similarly, high levels of heavy metals were also found in beef demonstrating the global threat (40). Furthermore, depending on the age group and category of person studied, children, expectant mothers and pastoralists who depend on beef as staple diets in these communities are affected by these contaminants.
The importance of the current study might as well be that of revealing an indicator for heavy metals contamination in the environmental in eastern Uganda [similar to the concept of a bio-indicator species reported in Egypt (55)]. In terms of health risks assessment on the other hand, we show that the estimated daily intake (EDI) for the heavy metals was significantly higher in children than adults. High level of non-essential metals in children is destructive to organs such as kidney and liver during development. Additionally, damage to organs like intestines, reproductive system and skin is not uncommon.
Essential elements were in the order of Fe > Zn > Co > Cu. The order of heavy metals reveals pattern of less amounts of essential nutrients from the beef of eastern Uganda as compared to the recommended daily intake. Daily amounts of 1 ppm/day for Zn, 6 ppm/day for Fe; 56 ppm/day for Co and of 35 ppm/day for Cu required for normal body functioning (18). Since Fe, Cu and Co play a crucial role in erythrocyte physiology and metabolism, there is need for nutritional supplementation in order to curb deficiencies (17, 26).
The estimated daily ingestion of non-essential elements was in the order of Cr > Ni > Pb > Cd and these were generally higher than the tolerable daily intake recommended by WHO except for Cd, hence demonstrating the importance of Pb, Cr and Ni poisoning in eastern Uganda (18, 21, 32, 34). Indeed Pb poisoning was in agreement with our previous study in southwestern Uganda executed by (3).
Overall, our data suggest that there is no health risk associated with consumption of the essential elements Cu, Zn, Fe and Co (THQ < 1). On the other hand, a significantly higher risk of consuming the non-essential elements Cr, Pb, Ni, and Cd in the beef samples studied. The risk was more pertinent in adults than in children, the order of impact of which was Cr > Pb > Ni > Cd. Particularly, Cr, which is associated with urticaria, anemia and generalized visceral disorders (37, 38), appears to be a threat to public health in Soroti. This agrees with epidemiological reports in eastern Uganda where anemia rate was found to be 58.8% and stunted growth to be at 27.7% amongst children (56). Cancer risk was found to be highest in the order of Ni > Cr > Cd > Pb, and significantly higher in children than in adults. Most critical though is that the elements Ni, Cr, Cd, and Pb are all established carcinogens (9, 35, 36) and finding them in beef here at any concentration raises serious concerns because there are no “safe” levels of carcinogens. Furthermore, chronic consumption of Cd in beef would predispose children to cancer in their elderly ages (33).
Conclusion
In this study, we have shown that toxic heavy metals are present in beef from Soroti (Uganda) at concentrations far higher than those recommended by WHO. We have also shown that essential elements are present at concentrations much lower than those recommended by WHO, hence increasing the risk of deficiency in these necessary enzyme co-factors. The risk of cancer from the consuming Soroti beef was found to be mainly propagated by chronic ingestion of Ni.
Based on the above, we recommend practical livestock production strategies that minimize livestock exposure to heavy metals in eastern Uganda. To effectively do so, we suggest at the minimum a collaborative effort led by the Uganda Ministries of Agriculture Animal Industry & Fisheries, and of Health with the sole purpose of (i) devising practical strategies to improve beef quality and promote healthy beef consumption in the country, and (ii) designing nutritional guidelines to help communities struggling with malnutrition challenge.
Data Availability Statement
Data files used in the study can be found at https://figshare.com/s/231bb252ceae87d86d12.
Author Contributions
KK conceptualized the study and drafted the initial manuscript. KK, GZ, and JE designed the study. YH acquired the data. KK, KM, FS, KA, and FA conducted data analysis. AT, FS, RM, FK, TP, HK, RM, PB, HM, PE, LO, JO, LOO, JM, GB, and OO conducted data interpretation. All authors revised it for intellectual content, approved final version to be published and remain in agreement on all aspects of the work.
Funding
The authors extend their appreciation to the administration of Busitema University Grant ID: BU/CR/156/1/Kasozi.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by Taif University Researchers Supporting Program (project number: TURSP-2020/153), Taif University, Saudi Arabia.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2021.592340/full#supplementary-material
Abbreviations
Cd, Cadmium; Co, Cobalt; Cr, Chromium; Cu, Copper; EFSA, European Food Safety Agency; FAO, Food and Agricultural Organization; Fe, Iron; HI, Hazard index; ILCR, Incremental lifetime cancer risk; MAAIF, Ministry of Agriculture Animal Industry and Fisheries; MoH, Ministry of Health; NEMA, National Environmental Management Authority; Ni, Nickel; Pb, Lead; ppm, Parts per million; THQ, Target hazard quotient; US EPA, United States Environment Protection Agency; WHO, World Health Organization; Zn, Zinc.
References
1. Orisakwe OE, Amadi CN, Frazzoli C, Dokubo A. Nigerian foods of probiotics relevance and chronic metal exposure: a systematic review. Environ Sci Pollut Res. (2020) 27:19285–97. doi: 10.1007/s11356-020-08537-2
2. Outa JO, Kowenje CO, Avenant-Oldewage A, Jirsa F. Trace elements in crustaceans, mollusks and fish in the kenyan part of Lake Victoria: bioaccumulation, bioindication and health risk analysis. Arch Environ Contam Toxicol. (2020) 78:589–603. doi: 10.1007/s00244-020-00715-0
3. Kasozi KI, Natabo PC, Namubiru S, Tayebwa DS, Tamale A, Bamaiyi PH. Food safety analysis of milk and beef in Southwestern Uganda. J Environ Public Heal. (2018) 2018:1–7. doi: 10.1155/2018/1627180
4. Orisakwe OE, Oladipo OO, Ajaezi GC, Udowelle NA. Horizontal and vertical distribution of heavy metals in farm produce and livestock around lead-contaminated goldmine in Dareta and Abare, Zamfara State, Northern Nigeria. J Environ Public Health. (2017) 2017:3506949. doi: 10.1155/2017/3506949
5. Kasozi KI, Namubiru S, Kamugisha R, Eze ED, Tayebwa DS, Ssempijja F, et al. Safety of drinking water from primary water sources and implications for the general public in Uganda. J Environ Public Health. (2019) 2019:7813962. doi: 10.1155/2019/7813962
6. Bamuwamye M, Ogwok P, Tumuhairwe V, Eragu R, Nakisozi H, Ogwang PE. Human health risk assessment of heavy metals in Kampala (Uganda) Drinking Water. J Food Res. (2017) 6:6. doi: 10.5539/jfr.v6n4p6
7. Bamuwamye M, Ogwok P, Tumuhairwe V. Cancer and non-cancer risks associated with heavy metal exposures from street foods : evaluation of roasted meats in an urban setting. J Environ Pollut Hum Heal. (2015) 3:24–30. doi: 10.12691/jephh-3-2-1
8. Otim EO, Chen IR, Otim O. Applying multivariate analysis to characterize waragi spirits from Acoli, Uganda, by their metal contents. Heliyon. (2019) 5:e01417. doi: 10.1016/j.heliyon.2019.e01417
9. Otim O, Juma T, Otunnu O. Assessing the health risks of consuming ‘sachet' alcohol in Acoli, Uganda. PLoS ONE. (2019) 14:e0212938. doi: 10.1371/journal.pone.0212938
10. Andrew T, Francis E, Charles M, Naigaga I, Jessica N, Micheal O, et al. Mercury concentration in muscle, bellyfat and liver from Oreochromis niloticus and Lates niloticus consumed in Lake Albert fishing communities in Uganda. Cogent Food Agric. (2016) 2:1214996. doi: 10.1080/23311932.2016.1214996
11. Awino FB, Maher WA, Krikowa F, Lynch AJJ. Occurrence of trace metals in food crops grown on the Mbale Dumpsite, Uganda, and Human Health Risks. Integr Environ Assess Manag. (2020) 16:362–77. doi: 10.1002/ieam.4237
12. Bakyayita GK, Norrström AC, Kulabako RN. Assessment of levels, speciation, and toxicity of trace metal contaminants in selected shallow groundwater sources, surface runoff, wastewater, and surface water from designated streams in Lake Victoria Basin, Uganda. J Environ Public Health. (2019) 2019:1–18. doi: 10.1155/2019/6734017
13. Fuhrimann S, Stalder M, Winkler MS, Niwagaba CB, Babu M, Masaba G, et al. Microbial and chemical contamination of water, sediment and soil in the Nakivubo wetland area in Kampala, Uganda. Environ Monit Assess. (2015) 187:475. doi: 10.1007/s10661-015-4689-x
14. Owor M, Hartwig T, Muwanga A, Zachmann D, Pohl W. Impact of tailings from the Kilembe copper mining district on Lake George, Uganda. Environ Geol. (2007) 51:1065–75. doi: 10.1007/s00254-006-0398-7
15. Fashola M, Ngole-Jeme V, Babalola O. Heavy metal pollution from gold mines: environmental effects and bacterial strategies for resistance. Int J Environ Res Public Health. (2016) 13:1047. doi: 10.3390/ijerph13111047
16. Ikenaka Y, Nakayama SMM, Muzandu K, Choongo K, Teraoka H, Mizuno N, et al. Heavy metal contamination of soil and sediment in Zambia. In: Heavy Metal Contamination of Water and Soil. 1st ed. Apple Academic Press (2014). 20 p.
17. Bhagi-Damodaran A, Michael MA, Zhu Q, Reed J, Sandoval BA, Mirts EN, et al. Why copper is preferred over iron for oxygen activation and reduction in haem-copper oxidases. Nat Chem. (2017) 9:257–263. doi: 10.1038/nchem.2643
18. Pandelova M, Lopez WL, Michalke B, Schramm KW. Ca, Cd, Cu, Fe, Hg, Mn, Ni, Pb, Se, and Zn contents in baby foods from the EU market: comparison of assessed infant intakes with the present safety limits for minerals and trace elements. J Food Compos Anal. (2012) 27:120–7. doi: 10.1016/j.jfca.2012.04.011
19. Chuttani HK, Gupta PS, Gulati S, Gupta DN. Acute copper sulfate poisoning. Am J Med. (1965) 39:849–54. doi: 10.1016/0002-9343(65)90105-1
20. Crisponi G, Nurchi VM, Fanni D, Gerosa C, Nemolato S, Faa G. Copper-related diseases: from chemistry to molecular pathology. Coord Chem Rev. (2010) 254(7–8):876–89. doi: 10.1016/j.ccr.2009.12.018
21. Blanco-Penedo I, López-Alonso M, Miranda M, Hernández J, Prieto F, Shore RF. Non-essential and essential trace element concentrations in meat from cattle reared under organic, intensive or conventional production systems. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. (2010) 27:36–42. doi: 10.1080/02652030903161598
22. Atangana E. Production, disposal, and efficient technique used in the separation of heavy metals from red meat abattoir wastewater. Environ Sci Pollut Res. (2020) 27:9424–34. doi: 10.1007/s11356-019-06850-z
23. Jarosz M, Olbert M, Wyszogrodzka G, Młyniec K, Librowski T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology. (2017) 25:11–24. doi: 10.1007/s10787-017-0309-4
24. Chasapis CT, Spiliopoulou CA, Loutsidou AC, Stefanidou ME. Zinc and human health: an update. Arch Toxicol. (2012) 86:521–34. doi: 10.1007/s00204-011-0775-1
25. Shahbazi Y, Ahmadi F, Fakhari F. Voltammetric determination of Pb, Cd, Zn, Cu and Se in milk and dairy products collected from Iran: an emphasis on permissible limits and risk assessment of exposure to heavy metals. Food Chem. (2016) 192:1060–7. doi: 10.1016/j.foodchem.2015.07.123
26. Leyssens L, Vinck B, Van Der Straeten C, Wuyts F, Maes L. Cobalt toxicity in humans—A review of the potential sources and systemic health effects. Toxicology. (2017) 387:43–56. doi: 10.1016/j.tox.2017.05.015
27. Malek E, Sacher RA. Megaloblastic anemia. In: Linda MM, Richard NM, editors. Pathobiology of Human Disease. Academic Press (2014). p. 1499–505
28. Catalani S, Rizzetti M, Padovani A, Apostoli P. Neurotoxicity of cobalt. Hum Exp Toxicol. (2012) 31:421–37. doi: 10.1177/0960327111414280
29. Apostoli P, Catalani S, Zaghini A, Mariotti A, Poliani PL, Vielmi V, et al. High doses of cobalt induce optic and auditory neuropathy. Exp Toxicol Pathol. (2013) 65:719–27. doi: 10.1016/j.etp.2012.09.006
30. Karri V, Schuhmacher M, Kumar V. Heavy metals (Pb, Cd, As and MeHg) as risk factors for cognitive dysfunction: A general review of metal mixture mechanism in brain. Environ Toxicol Pharmacol. (2016) 48:203–13. doi: 10.1016/j.etap.2016.09.016
31. Abbas Q, Yousaf B, Liu G, Zia-ur-Rehman M, Ali MU, Munir MAM, et al. Evaluating the health risks of potentially toxic elements through wheat consumption in multi-industrial metropolis of Faisalabad, Pakistan. Environ Sci Pollut Res. (2017) 24:26646–57. doi: 10.1007/s11356-017-0311-9
32. WHO. Exposure of children to chemical hazards in food. In: European Environment and Health Information Systems. Hoboken, NJ: John Wiley & Sons, Inc. (2007). p. RPG4_Food_Ex1. Available from: http://www.euro.who.int/__data/assets/pdf_file/0003/97446/4.4.pdf (accessed August 12, 2020).
33. United States Environmental Protection Agency. Cadmium (CASRN 7440-43-9). IRIS, US EPA. Integrated Risk Information System (IRIS) (2014). Available from: http://www.epa.gov/iris/subst/0141.htm (accessed August 12, 2020).
34. FAO/WHO. Working document for Information use in discussion related to contaminants toxins in the GSCTFF. In: Joint FAO/WHO Food Standards Programme Codex Committee on Contaminants in Foods. (2011). p. 1–90. Available from: ftp://ftp.fao.org/codex/meetings/CCCF/cccf5/cf05_INF.pdf (accessed August 12, 2020).
35. Sreekanth TVM, Nagajyothi PC, Lee KD, Prasad TNVKV. Occurrence, physiological responses and toxicity of nickel in plants. Int J Environ Sci Technol. (2013) 10:1129–40. doi: 10.1007/s13762-013-0245-9
37. Guertin J. Toxicity and health effects of chromium (all oxidation states). In: Guertin J, Jacobs JA, Avakian CP, editors. Chromium(VI) Handbook. Boca Raton, FL: CRC Press (2004). p. 215–34.
38. Saha R, Nandi R, Saha B. Sources and toxicity of hexavalent chromium. J Coord Chem. (2011) 64:1782–806. doi: 10.1080/00958972.2011.583646
39. Danieli PP, Serrani F, Primi R, Ponzetta MP, Ronchi B, Amici A. Cadmium, lead, and chromium in large game: a local-scale exposure assessment for hunters consuming meat and liver of wild boar. Arch Environ Cont Toxicol. (2012) 63:612–27. doi: 10.1007/s00244-012-9791-2
40. Ihedioha JN, Okoye COB, Onyechi UA. Health risk assessment of zinc, chromium, and nickel from cow meat consumption in an urban Nigerian population. Int J Occup Environ Health. (2014) 20:281–8. doi: 10.1179/2049396714Y.0000000075
41. Broomhead NK, Moodley R, Jonnalagadda SB. Elemental analysis of the edible fruit of Carpobrotus dimidiatus (from Kwazulu-Natal, South Africa) and the influence of soil quality on its elemental uptake. J Environ Sci Health Part B. (2020) 55:406–15. doi: 10.1080/03601234.2019.1707016
42. Iloms E, Ololade OO, Ogola HJO, Selvarajan R. Investigating industrial effluent impact on municipal wastewater treatment plant in vaal, South Africa. Int J Environ Res Public Health. (2020) 17:1–18. doi: 10.3390/ijerph17031096
43. Alam M, Khan M, Khan A, Zeb S, Khan MA, Amin Nul Sajid M, et al. Concentrations, dietary exposure, and human health risk assessment of heavy metals in market vegetables of Peshawar, Pakistan. Environ Monit Assess. (2018) 190:505. doi: 10.1007/s10661-018-6881-2
44. Ishak I, Rosli FD, Mohamed J, Mohd Ismail MF. Comparison of digestion methods for the determination of trace elements and heavy metals in human hair and nails. Malaysian J Med Sci. (2015) 22:11–20.
45. Kamunda C, Mathuthu M, Madhuku M. Health risk assessment of heavy metals in soils from witwatersrand gold mining basin, South Africa. Int J Environ Res Public Health. (2016) 13:663. doi: 10.3390/ijerph13070663
46. Ngole-Jeme VM, Fantke P. Ecological and human health risks associated with abandoned gold mine tailings contaminated soil. PLoS ONE. (2017) 12:1–24. doi: 10.1371/journal.pone.0172517
47. Husson F, Josse J, Pagès J. Principal Component Methods - Hierarchical Clustering - Partitional Clustering: Why Would We Need to Choose for Visualizing Data? Technical Report – Agrocampus (2010). Available from: http://www.sthda.com/english/upload/hcpc_husson_josse.pdf (accessed August 12, 2020).
48. R Core Team. R: A Language and Environment for Statistical Computing. v3.6.3. Vienna: R Foundation for Statistical Computing (2019). Available from: https://www.R-project.org/ (accessed December 22, 2020).
49. RStudio Team. RStudio: Integrated Development for R. RStudio. Boston, MA: PBC (2020). Available from: https://www.rstudio.com/ (accessed August 12, 2020).
50. Salazar MJ, Rodriguez JH, Leonardo Nieto G, Pignata ML. Effects of heavy metal concentrations (Cd, Zn and Pb) in agricultural soils near different emission sources on quality, accumulation and food safety in soybean [Glycine max (L.) Merrill]. J Hazard Mater. (2012) 233–4:244–53. doi: 10.1016/j.jhazmat.2012.07.026
51. Amadi CN, Frazzoli C, Orisakwe OE. Sentinel species for biomonitoring and biosurveillance of environmental heavy metals in Nigeria. J Environ Sci Health C Toxicol Carcinog. (2020) 38:21–60. doi: 10.1080/26896583.2020.1714370
52. Jin L, Liu J, Ye B, Ren A. Concentrations of selected heavy metals in maternal blood and associated factors in rural areas in Shanxi Province, China. Environ Int. (2014) 66:157–64. doi: 10.1016/j.envint.2014.01.016
53. Singh R, Gautam N, Mishra A, Gupta R. Heavy metals and living systems: an overview. Indian J Pharmacol. (2011) 43:246–53. doi: 10.4103/0253-7613.81505
54. Järup L. Hazards of heavy metal contamination. Br Med Bull. (2003) 68:167–82. doi: 10.1093/bmb/ldg032
55. Eid EM, Galal TM, Shaltout KH, El-Sheikh MA, Asaeda T, Alatar AA, et al. Biomonitoring potential of the native aquatic plant Typha domingensis by predicting trace metals accumulation in the Egyptian Lake Burullus. Sci Total Environ. (2020) 714:136603. doi: 10.1016/j.scitotenv.2020.136603
Keywords: heavy metals, food hygiene, food safety, Uganda, beef industry
Citation: Kasozi KI, Hamira Y, Zirintunda G, Alsharif KF, Altalbawy FMA, Ekou J, Tamale A, Matama K, Ssempijja F, Muyinda R, Kawooya F, Pius T, Kisakye H, Bogere P, Matovu H, Omadang L, Etiang P, Mbogua J, Ochieng JJ, Osuwat LO, Mujinya R, Batiha GE-S and Otim O (2021) Descriptive Analysis of Heavy Metals Content of Beef From Eastern Uganda and Their Safety for Public Consumption. Front. Nutr. 8:592340. doi: 10.3389/fnut.2021.592340
Received: 14 August 2020; Accepted: 06 January 2021;
Published: 11 February 2021.
Edited by:
Rakesh Bhardwaj, National Bureau of Plant Genetic Resources (ICAR), IndiaReviewed by:
Orish Ebere Orisakwe, University of Port Harcourt, NigeriaSomnath Mandal, Uttar Banga Krishi Viswavidyalaya, India
Copyright © 2021 Kasozi, Hamira, Zirintunda, Alsharif, Altalbawy, Ekou, Tamale, Matama, Ssempijja, Muyinda, Kawooya, Pius, Kisakye, Bogere, Matovu, Omadang, Etiang, Mbogua, Ochieng, Osuwat, Mujinya, Batiha and Otim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Keneth Iceland Kasozi, a2ljZWxhbmR5QGdtYWlsLmNvbQ==; a2ljZWxhbmR5QGthYi5hYy51Zw==
 Keneth Iceland Kasozi
Keneth Iceland Kasozi Yunusu Hamira
Yunusu Hamira Gerald Zirintunda
Gerald Zirintunda Khalaf F. Alsharif3
Khalaf F. Alsharif3 Justine Ekou
Justine Ekou Kevin Matama
Kevin Matama Fred Ssempijja
Fred Ssempijja Francis Kawooya
Francis Kawooya Theophilus Pius
Theophilus Pius Hellen Kisakye
Hellen Kisakye Paul Bogere
Paul Bogere Henry Matovu
Henry Matovu Leonard Omadang
Leonard Omadang Juma John Ochieng
Juma John Ochieng Regan Mujinya
Regan Mujinya Gaber El-Saber Batiha
Gaber El-Saber Batiha Ochan Otim
Ochan Otim