- 1Department of Anesthesia, McGill University, Montreal, QC, Canada
- 2Department of Dietetics/Speech and Language Therapy, University Hospital Southampton NHS Foundation Trust, Southampton, United Kingdom
- 3Duke Clinical Research Institute, Duke University School of Medicine, Durham, NC, United States
- 4Faculty of Medicine, School of Human Development and Health, University of Southampton, Southampton, United Kingdom
- 5National Institute of Health Research Cancer and Nutrition Collaboration, Southampton, United Kingdom
- 6National Institute for Health Research Biomedical Research Centre, University Hospital Southampton National Health Service Foundation Trust, Southampton, United Kingdom
- 7Department of Anaesthesia, Perioperative and Pain Medicine, Peter MacCallum Cancer Centre, Melbourne, VIC, Australia
- 8Anaethesia, Pain and Perioperative Medicine Unit, The University of Melbourne, Melbourne, VIC, Australia
- 9Centre for Integrated Critical Care Medicine and The Sir Peter MacCallum Department of Oncology, The University of Melbourne, Melbourne, VIC, Australia
- 10Nutrition and Dietetics, Faculty of Health and Well Being, University of Winchester, Winchester, United Kingdom
- 11Anaesthesia, Perioperative and Critical Care Research Group, National Institute for Health Research Biomedical Research Centre, University Hospital Southampton National Health Service Foundation Trust, University of Southampton, Southampton, United Kingdom
- 12Faculty of Medicine, School of Cancer Sciences, University of Southampton, Southampton, United Kingdom
Background: Prehabilitation aims to improve functional capacity prior to cancer treatment to achieve better psychosocial and clinical outcomes. Prehabilitation interventions vary considerably in design and delivery. In order to identify gaps in knowledge and facilitate the design of future studies, we undertook a scoping review of prehabilitation studies to map the range of work on prehabilitation being carried out in any cancer type and with a particular focus on diet or nutrition interventions.
Objectives: Firstly, to describe the type of prehabilitation programs currently being conducted. Secondly, to describe the extent to which prehabilitation studies involved aspects of nutrition, including assessment, interventions, implementation, and outcomes.
Eligibility Criteria: Any study of quantitative or qualitative design that employed a formal prehabilitation program before cancer treatment (“prehabilitation” listed in keywords, title, or abstract).
Sources of Evidence: Search was conducted in July 2020 using MEDLINE, PubMed, EMBASE, EMCARE, CINAHL, and AMED.
Charting Methods: Quantitative data were reported as frequencies. Qualitative nutrition data were charted using a framework analysis that reflects the Nutrition Care Process Model: assessment, intervention, and monitoring/evaluation of the nutrition intervention.
Results: Five hundred fifty unique articles were identified: 110 studies met inclusion criteria of a formal prehabilitation study in oncology. prehabilitation studies were mostly cohort studies (41%) or randomized-controlled trials (38%) of multimodal (49%), or exercise-only (44%) interventions that were applied before surgery (94%). Nutrition assessment was inconsistently applied across these studies, and often conducted without validated tools (46%). Of the 110 studies, 37 (34%) included a nutrition treatment component. Half of these studies provided the goal for the nutrition component of their prehabilitation program; of these goals, less than half referenced accepted nutrition guidelines in surgery or oncology. Nutrition interventions largely consisted of counseling with dietary supplementation. The nutrition intervention was indiscernible in 24% of studies. Two-thirds of studies did not monitor the nutrition intervention nor evaluate nutrition outcomes.
Conclusion: Prehabilitation literature lacks standardized and validated nutritional assessment, is frequently conducted without evidence-based nutrition interventions, and is typically implemented without monitoring the nutrition intervention or evaluating the intervention's contribution to outcomes. We suggest that the development of a core outcome set could improve the quality of the studies, enable pooling of evidence, and address some of the research gaps identified.
Background
Prehabilitation interventions can be applied prior to oncological treatments, including surgery, to fortify functional reserve and enhance functional capacity to prepare patients to weather the imminent physiological and psychological stresses of treatment (1). Preoperative functional capacity is predictive of postsurgical outcomes, such as morbidity in colorectal surgery (2, 3). As an example, frail patients who cannot attain a 400-m 6-min walking distance before surgery suffer three times as many postsurgical complications as those who can walk this distance (2). In the same way, there is an extensive body of evidence that those who are undernourished, as marked by a history of weight loss and symptoms indicative of poor nutritional state, have greater surgical morbidity and mortality (4). Several prospective studies have identified that unimodal (e.g., exercise-only interventions) and multimodal (e.g., exercise interventions with nutrition optimization and/or psychological intervention) prehabilitation programs can be carried out successfully in the period before surgery to improve preoperative functional capacity (5–8).
The findings of available systematic reviews of prehabilitation, however, are somewhat inconsistent regarding effectiveness of the intervention on clinical outcomes such as postoperative complications (9, 10). These seeming contradictions are in part related to the heterogeneity of study populations, study designs, and study interventions that often cannot be melded together into one message for prehabilitation (11). Undernutrition, for instance, leads to adaptive mechanisms that tend to reduce energy expenditure in part by reducing physical activity and basal metabolism with conservation of reserves (12). As a result, malnourished patients participating in exercise-only prehabilitation might not be able to engage with or adapt to exercise and improve their functional capacity prior to surgery as well as those who are better nourished (2). The inconsistent findings of these reviews may also be attributed to the scarcity of process measures/implementation outcomes reported in the prehabilitation literature. Synthesizing and reporting data on the effectiveness of an intervention only limits conclusions: success or failure of any intervention is a combination of treatment effectiveness (in terms of both improved functional endpoints, and the impact on clinical outcomes, e.g., reduced postoperative complications) together with its implementation factors (13). Few, if any, reviews of prehabilitation have reported implementation factors that might influence the effectiveness of the program.
While systematic reviews summarize and assess the quality of the collective evidence of a given topic, scoping reviews determine the coverage of a body of literature on a specific topic to identify the available evidence, to examine how research in the field was conducted, and to identify and assess knowledge gaps (14). We conducted a scoping review to determine what and how interventions have been incorporated as part of prehabilitation in the oncology setting. That is, we sought to identify the type of interventions currently being conducted within prehabilitation programs, the patient populations being studied, and the study designs that have been used in research specifically labeled as “prehabilitation” (i.e., “what”). Additionally, given the relationship between nutrition and functional capacity, we sought to determine the extent to which prehabilitation studies involved nutrition, including assessment, interventions, implementation, and outcomes (i.e., “how”). We aimed to identify any research limitations or omissions that could usefully inform future research design, conduct and interpretation, or that could help improve the coherence and delivery of the nutritional aspects of prehabilitation in clinical practice.
Methods
We performed a scoping review of the literature based on the framework outlined by Arksey and O'Malley (15), recommendations of Levac et al. (16), and in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analysis extension for Scoping Reviews (PRISMA-ScR). The review included the following five key phases: (1) identifying the research question, (2) identifying relevant studies, (3) study selection, (4) charting the data, and (5) collating, summarizing, and reporting the results. A project team consisting of health researchers, physicians, dietitians, an epidemiologist, and perioperative clinic managers was established to develop the research question and oversee the study.
Identifying the Research Question
The overarching goal of this scoping review was to provide an overview of current prehabilitation practices in oncology, to identify the extent to which prehabilitation programs included nutrition, and to generate recommendations for future studies based on identified gaps. Our research questions were as follows:
1. What are the study, patient, and intervention characteristics of published prehabilitation studies?
2. How many prehabilitation studies were conducted with a nutrition treatment component?
3. What are the specific (i) nutrition assessments, (ii) interventions, (iii) process measures (monitoring and evaluation), and (iv) nutrition outcomes associated with the prehabilitation studies that included a nutrition treatment component?
Identifying Relevant Studies
Given that our goal was to map current research practices in oncology-related prehabilitation, we focused our scoping review to studies of interventions applied prior to oncology treatment that were identified as either unimodal or multimodal prehabilitation; that is, published work, including protocols, that contained the term “prehabilitation” in the title, abstract, or keywords. We did not set a time limit to the search to ensure as much evidence as possible was captured.
We used broad search terms that encompassed prehab* or pre-hab* or pre-rehab* AND cancer* or oncolog* or malignan*. The final search was conducted in July 2020 using MEDLINE, PubMed, EMBASE, EMCARE, CINAHL, and AMED. Hand searching the reference lists of key papers, including all identified systematic reviews and meta-analyses of prehabilitation, were also conducted.
Study Selection
Two reviewers (CG and SD) independently reviewed titles and abstracts for inclusion. Articles were considered for full-text review if inclusion criteria were met: (1) a quantitative or qualitative study of a “prehabilitation” program; and (2) included adult patients (age >18 years) with cancer (or where the majority of participants reported in the study had cancer), treated with surgery or other oncological therapies. Studies were excluded if they were narrative reviews, editorials, commentaries, conference abstracts, or were published in a language other than English or French. Selected articles for full-text review were then independently reviewed by the two reviewers. Disagreements were addressed by discussion and consensus.
Charting the Data
The data extraction template (Microsoft 2010, Redmond, WA) was developed in consultation with the project team and included study design, cancer type, specification of the prehabilitation program, primary outcome measure, and whether nutrition was part of the formal prehabilitation program by including the use of nutritional screening/assessment or nutrition treatment. Of the studies identified as having a nutrition intervention component, quantitative and qualitative data were collected on: (1) method of nutritional assessment, (2) validated nutrition screening or assessment tool, (3) goal of the nutrition intervention including the reference standard or accepted nutritional guideline, (4) characteristics of the nutrition intervention, (5) evaluation and monitoring of the intervention, and (6) nutrition outcomes. Two researchers (CG and SD) independently extracted data for the first 10 studies to refine the data form and ensure consistent data extraction that adequately reflected the research question.
Collating and Summarizing Results
Quantitative data were analyzed using descriptive statistics (frequencies). Qualitative data were charted using a framework analysis that reflects the Nutrition Care Process Model: assessment, intervention, and monitoring/evaluation of the nutrition intervention (17). The study team were consulted in the interpretation of the findings, identifying research gaps and creating suggestions for future research.
Results
Search Results
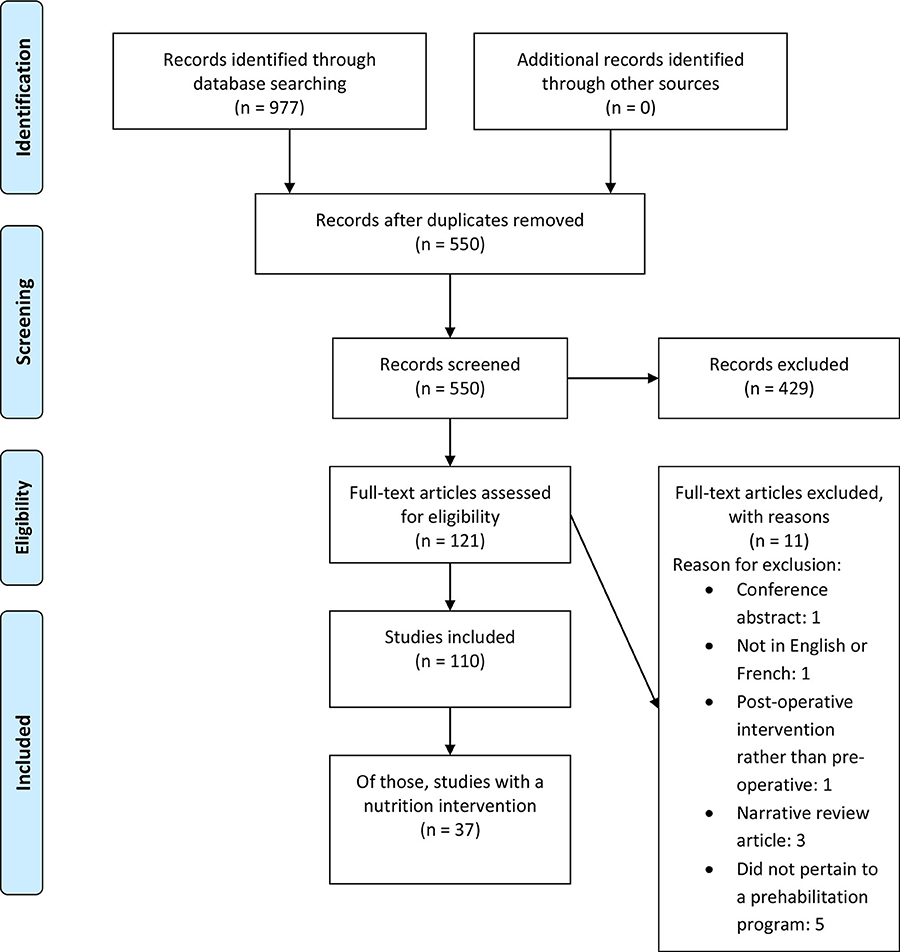
Our search identified 550 unique articles (Figure 1). After abstract screening, 121 articles were suitable for full-text review. Hand searching did not produce any further unique articles. Eleven articles were subsequently excluded because of language (n = 1), a narrative review (n = 3), a conference abstract (n = 1), no preoperative intervention (n = 1), or did not pertain to a prehabilitation program (n = 5). One-hundred and ten studies were included in the final review, of these, 34% (n = 37) included a nutrition intervention component.
All Prehabilitation Studies
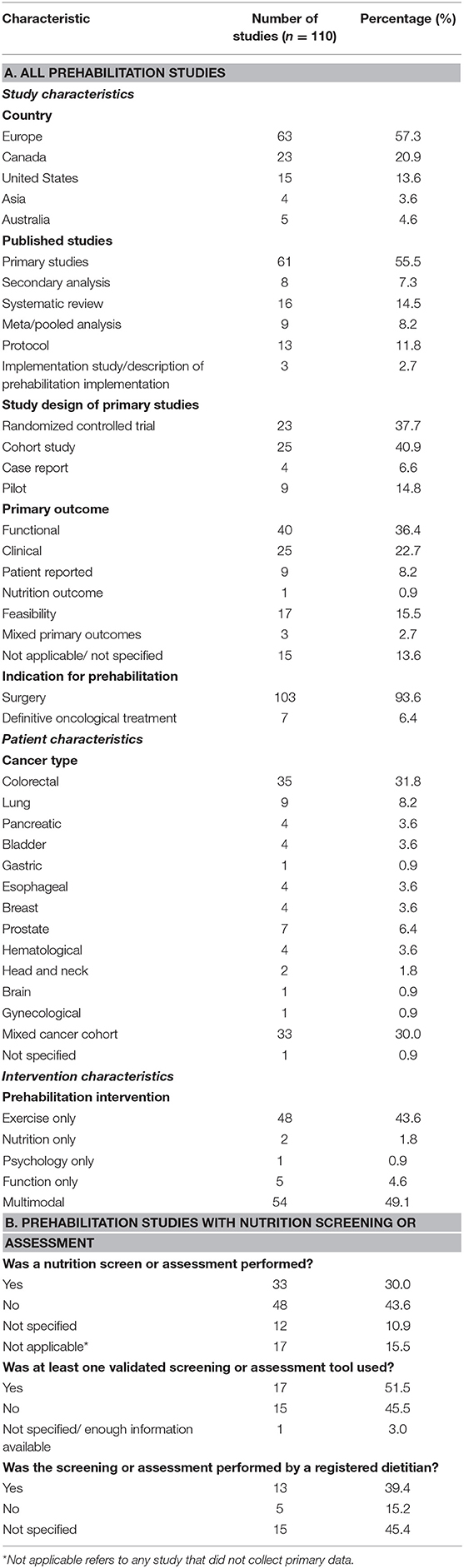
Table 1 describes the findings for all of the prehabilitation studies. These studies were published between 2012 and 2020. Of these 110 studies, 56% (n = 61) were identified as primary research studies; 57% of the prehabilitation studies arose from Europe (n = 63) and 21% from Canada (n = 23). The primary studies were largely conducted as cohort designs (n = 25; 41%) and randomized controlled trials (RCTs) (n = 23; 38%). Systematic reviews, meta-analyses, and pooled analyses comprised 23% (n = 25) of the prehabilitation literature. Functional (n = 40; 36%) and clinical (n = 25; 23%) measures were the most frequently reported primary outcomes.
Most of the prehabilitation literature described multimodal (n = 54, 49%) or exercise-only prehabilitation (n = 48, 44%); two studies reported interventions that were exclusively nutrition related (2%) while one study reported an intervention that was exclusively psychological (1%). We identified that surgical prehabilitation made up 94% of the literature, with the rest related to definitive non-surgical oncological treatments. The patient populations studied most were colorectal cancer (n = 35; 32%) and mixed cancer types (n = 33; 30%).
Screening or assessment for malnutrition was conducted in one-third of prehabilitation studies (n = 33); approximately half of these studies used a validated tool (n = 17) and 39% of these studies (n = 13) employed a registered dietitian to conduct the screening or assessment. The person who conducted the screening/assessment was not specified in 45% of these studies.
Prehabilitation Studies With a Nutrition Treatment Component
Table 2 and Supplementary Table 1 describe the quantitative and qualitative findings of the prehabilitation studies with a nutrition treatment component. Only 37 of the 110 studies of prehabilitation had a nutrition treatment component. The study designs were as follows: 27% (n = 10) were protocols (18–27), 14% (n = 5) were pilot studies (8, 28–31), 5% (n = 2) were descriptions of prehabilitation programs (32, 33), 3% (n = 1) were case reports (34), 3% (n = 1) were feasibility studies (35), and 3% were qualitative studies (36). Of these 37 studies, 30% (n = 11) were cohort studies (37–47) and 16% (n = 6) were RCTs (48–53).
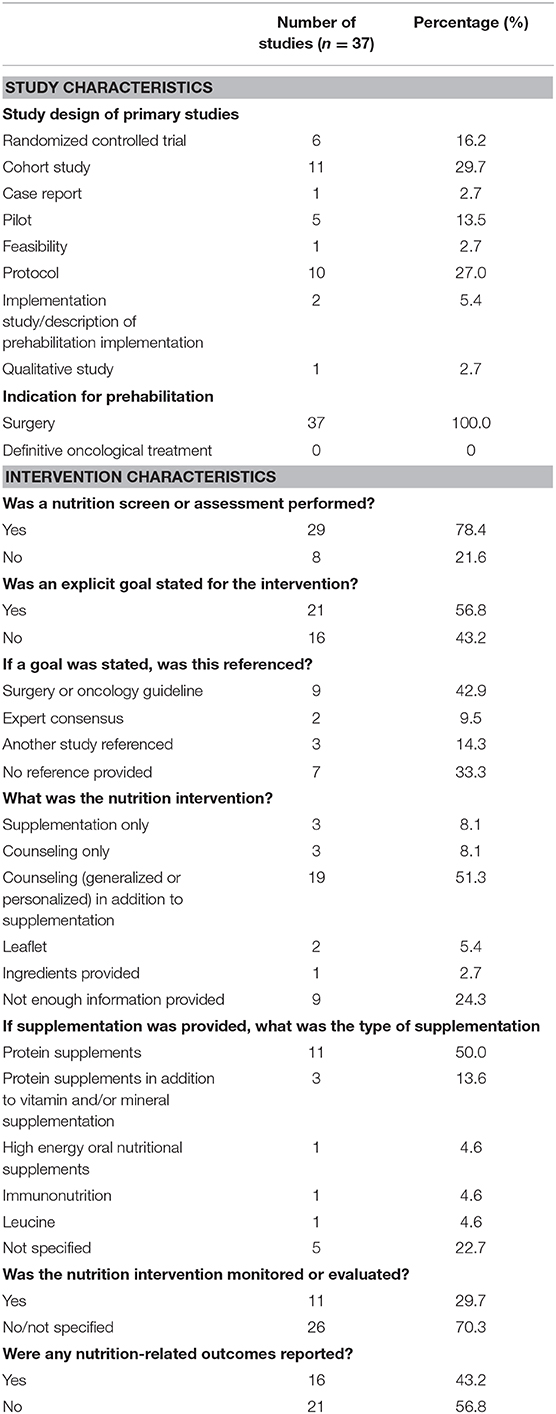
Table 2. Study and intervention characteristics of prehabilitation studies with a nutrition component.
Nutritional Assessment Within Prehabilitation
Seventy-eight percent (n = 29) of the 37 identified studies included a statement regarding the conduct of nutritional assessment [n = 8 studies did not include a nutritional assessment statement (20, 26, 32, 36, 39, 43, 45, 47)]; however, the application of assessment was inconsistent across studies. Each study used a different method for nutritional assessment, with most studies using a combination of various nutritional assessment tools, parameters, and indicators. The most commonly used tools to screen or assess for malnutrition were Subjective Global Assessment/Patient-Generated-Subjective Global Assessment (8, 27, 31, 35, 51), Nutrition Risk Screening-2002 (8, 19, 51, 52), Mini Nutritional Assessment (23, 28, 40, 41), Simplified Nutritional Appetite Questionnaire (23, 37, 41), and Malnutrition Universal Screening Tool (22, 46). The most common nutritional parameters were pre-albumin or albumin (18, 19, 23, 34, 38, 41, 46), which were reported by 19% (n = 7) of studies as a nutritional parameter [although, it is not considered to robustly reflect nutritional status in patients with cancer (54)], and 27% (n = 10) reported use of food records or recalls (8, 18, 27, 34, 35, 48–51, 53). Forty-three percent (n = 16) of studies included nutritional indicators, such as weight, body mass index (BMI), or body composition as an element of the assessment (18, 19, 23, 27–30, 33, 35, 38, 40, 41, 44, 46, 50, 53). Body composition analysis included computed tomography (CT) (18), bioimpedance (19), and skinfold assessments (24, 27, 35).
Eight percent (n = 3) of studies stated that an assessment was conducted without providing details of the method or tool used (21, 25, 42). As examples, “Complete nutritional assessment undertaken by a registered dietitian” (42) and “A nutritionist performed a medical examination running appropriate biological tests to evaluate the nutritional status” (25). Another study provided only vague details of the nutritional parameters used—“the dietitian assessed nutritional status using … and blood vitamin B [the B-vitamin assessed was not specified]” (41). In most cases, the cut-points or criteria for nutritional risk or diagnosis of a nutrition problem requiring treatment (e.g., malnutrition) were not specified. Only 16% (n = 6) of studies specified their diagnostic criteria rather than cut-points (22, 23, 28, 40, 44, 46).
Nutrition Interventions Within Prehabilitation
Eleven percent (n = 4) of studies specified that a nutrition intervention was provided to patients “in need” without defining the mechanism for identifying these patients (18, 20, 32, 47). As an example, “Usual care for all participants included review by specialist dietitians if they were struggling nutritionally (20).” Little more than half (n = 21) of the prehabilitation studies with a nutrition treatment component specified a goal for the nutrition intervention; of these, 67% (n = 14) referenced the stated goals and only 43% (n = 9) used a reference standard or accepted guideline, including European Society for Clinical Nutrition and Metabolism (ESPEN) guidelines (8, 21, 25, 35, 48–51, 53). Most goals were related to meeting estimated protein needs (8, 22, 25, 27, 28, 31, 35, 37, 48, 51, 53) or meeting estimated energy and protein needs (19, 21, 23, 39, 41, 49, 50). Protein needs were estimated at 1.2–2.0 g/kg/day and energy needs were estimated using 25–30 kcal/kg/day, indirect calorimetry, Harris Benedict equation, or WHO formula. Other stated nutrition goals included optimizing nutritional status (30), protein supplementation (32), and caloric and protein supplementation (18). Fifty-one percent (n = 19) of the interventions applied to meet these goals included a combination of both nutrition counseling (personalized or generalized) and supplementation (8, 18, 19, 22, 23, 25, 27, 31, 34, 35, 39, 41, 42, 48–53). Eight percent (n = 3) of studies used counseling alone (30, 44, 45), 5% (n = 2) used a leaflet (26, 36), and 8% (n = 3) used supplementation alone (32, 38, 46). Of the studies that used a nutrition supplement, “protein supplements” or a combination of vitamin/mineral supplements with protein supplements (8, 22, 25, 27, 31, 32, 34, 35, 38, 41, 48–51, 53) were used most often. Other supplements included high-energy oral nutrition supplements (19) and immunonutrition (46). Whey protein supplements (8, 22, 27, 31, 48–51, 53) were among the most prevalent of the protein-only supplements used in prehabilitation studies. Twenty-three percent (n = 5) of studies reported use of a supplement but did not provide any detail on the type of supplement used (18, 23, 39, 42, 52).
Many interventions appeared to be “personalized” to meet individual patient needs (8, 18, 19, 22, 24, 25, 32, 34, 39, 53). For some of the studies, it was clear that the nutrition assessment directed the nutrition care plan, including the need for specialized nutrition support (20, 40, 46), provision of a supplement or the supplemental dose (19, 23, 41, 49–51, 53), need for weight loss/gain (8, 27, 42, 53), or provided dietary advice based on food recalls, dietary patterns, and nutrition-impact symptoms (8, 22, 30, 31, 39, 51, 53). It was unclear how the nutritional assessment influenced the treatment plan in the remaining studies. Standardized instructions revolved around consuming protein supplements or snacks post-exercise (25, 27, 31, 35, 39, 45, 48–51, 53), increasing dietary protein intake (22, 27, 28, 34, 36, 50–52) and tips on consuming balanced meals (22, 44, 48, 53). Twenty-four percent (n = 9) of studies did not provide enough information for us to discern the specific nutrition intervention (20, 21, 24, 29, 33, 37, 40, 43, 47). Examples include, “aimed to incorporate nutrition support (33),” “appropriate supplementation (18),” or leaflets or seminars that “included nutrition (29, 43).”
Monitoring and Evaluation of Nutrition Impact Within Prehabilitation
Finally, a third (n = 11) of studies monitored adherence to the nutrition intervention (8, 19, 22, 25, 28, 30, 35, 45, 49, 52, 53). Self-reported adherence using logbooks/dairies (8, 19, 50, 52, 53) and a mobile app (22) were reported. Twenty-four percent (n = 9) of studies monitored adherence and provided ongoing support through telephone calls (8, 19, 24, 28, 35, 45, 49, 50, 53). However, tailoring of the nutrition intervention based on a follow-up appointment or telephone call was reported in only 8% (n = 3) of studies (24, 25, 50). An objective evaluation of whether the nutrition prescription was meeting patient needs preoperatively was reported in only one study where weight was measured (30). Yet, 43% (n = 16) of the studies reported some form of nutrition outcome, such as weight (18, 24, 29, 30, 33, 35, 38, 44, 51), food records or questionnaire (18, 21, 27, 44), nutrition screening or assessment tools (19, 27, 35), body composition (8, 18–22, 24, 29, 51), and handgrip strength (8, 20, 24, 33, 35). Although food recalls/records were stated to be used in several studies, only one study reported intake data (fiber and fat) (44). Of note, only 5% (n = 2) of studies examined outcomes by sex (38, 51).
Discussion
We conducted a scoping review to map the formal prehabilitation literature and identify opportunities to improve future research with particular emphasis on nutritional support. Currently, much of the available prehabilitation evidence, which could be used to inform practice and policy, is in the form of cohort studies. The majority of prehabilitation studies were conducted as multimodal or exercise-only studies and were applied before surgery. Only one-third of these studies included a dietary/nutrition treatment component. Nutrition assessment was inconsistently applied across these studies. In many studies, it was unclear how the nutrition assessment was used to identify nutrition problems or influence the treatment plan. Nearly one-quarter of these studies stated a nutrition intervention was applied without describing the intervention. Approximately half of the studies reported a nutrition treatment goal; yet, of those studies that reported a goal, one-third were not referenced at all and less than half referenced accepted nutrition guidelines in surgery or oncology. Finally, approximately two-thirds of studies did not monitor the nutrition intervention or evaluate nutrition outcomes.
This review identified several important research gaps. Firstly, two-thirds of the published literature on prehabilitation did not include nutrition risk screening or malnutrition assessment. Given that nutritional status can exert a modifying effect on nutritional (55), clinical (56, 57), and functional (58) outcomes, a failure to examine treatment effects at different levels of nutritional status limits research conclusions and clinical decision making (59–61). Effect modification is considered a natural phenomenon that should be reported and described; therefore, pooling of data should only be considered when the effect of treatment is identified to be homogenous across the strata of a potential modifying variable (e.g., nutritional status) (62). Considering a single treatment effect for prehabilitation on the impact of outcomes, independent of nutritional status, could result in a finding of a null effect (if subgroups respond to treatment in opposing ways), an overestimated, or an underestimated effect of prehabilitation treatment depending on the prevalence of malnutrition in the sample. Similarly, many studies were conducted in mixed cancer types, yet the treatment effect for prehabilitation might differ based on cancer status. While small sample sizes often preclude modification analysis, a failure to investigate heterogeneous effects could be a contributing factor to the conflicting, contradictory reports of the effect of prehabilitation on outcomes.
Overall, nutritional screening and assessment across published prehabilitation studies was heterogeneous and often completed without validated tools. Informal assessments, including clinical parameters and subjective measures result in under recognition of malnutrition (63). Valid nutritional assessment is required to identify malnutrition and any other nutrition-related problems that contribute to adverse outcomes. This finding has three important implications for prehabilitation research: (1) using non-validated tools to identify malnutrition produces findings that are subject to misclassification bias; (2) using a variety of tools to identify malnourished patients limits cross-study comparisons and synthesis of findings for meta-analysis; and (3) even validated tools cannot diagnose malnutrition with 100% sensitivity and specificity, so it is unlikely that the studies employing non-validated tools identified all the nutritionally compromised patients. The latter point is particularly problematic given that the primary outcome for most prehabilitation trials was identified to be functional and/or clinical. Malnourished patients have lower functional capacity (58, 64) and a reduced capacity to gain function through exercise alone (without first correcting malnutrition, which, for malnourished patients, could be the underlying etiology for the compromised function (58, 65, 66). A failure to correctly identify malnutrition for treatment has the potential to produce misleading findings for the effect of prehabilitation.
Of the published prehabilitation studies with a nutrition treatment component, approximately two-thirds of these studies did not monitor or evaluate the nutrition intervention. According to Proctor et al. (13), when an intervention fails to deliver, it is critical that we are able to attribute failure to either the intervention itself, the factors associated with its implementation, or a combination of the two. Inferring success or failure of the prehabilitation program using only functional and clinical endpoints is problematic as it is impossible to discern where the success or failure lies (13). As an example, we identified that 41% of nutrition prehabilitation interventions supplemented protein. Yet, it is difficult to discern whether positive or negative findings can be attributed to this intervention, or to another component of the multimodal prehabilitation, given implementation was poorly documented. If we have failed to monitor whether the nutrition prescription met patient needs (e.g., the intervention was acceptable to the patient, it was feasible to meet estimated therapeutic targets with the given intervention), assess implementation outcomes (e.g., fidelity of the intervention against protocol or patient adherence to the prescribed intervention), or evaluate nutrition outcomes (e.g., weight stabilization for malnourished patients), we cannot conclude with confidence that the intervention itself was (un)successful. Studies that do not monitor the nutrition prescription and evaluate the outcomes, do not contribute to our collective understanding of which interventions work best, how do they work, and for whom do they work best.
Finally, almost half of the published prehabilitation studies with a nutrition treatment component did not report the goal of the nutrition intervention. Several accepted standards exist to form the basis of nutrition goals in surgery (4) or oncology (67, 68) care. This finding has two major implications for prehabilitation research. First, when the goal of an intervention is unknown, critical appraisal of the study design and study's finding is difficult. Second, it is expected that evidence-based interventions that represent accepted standards are most likely to meet patient needs consistently. Treating patients without taking cognizance of and seeking to achieve these standards increases the risk of inadequate nutritional care with the associated inferior outcomes, again, potentially contributing to conflicting findings for multimodal or nutrition prehabilitation.
In order to effectively address the research gaps identified, we recommend that a core outcome set (COS) be developed and adopted for prehabilitation studies. A COS is a standardized set of outcomes to be reported by all trials within a research field (69). Additional outcomes may be reported at the discretion of the researcher, but a minimum standardized set of outcomes would be reported, permitting cross-study comparisons and enabling data synthesis for systematic reviews or meta-analyses that inform clinical practice (70). This need is illustrated by our identification that 23% of the formal prehabilitation literature constitutes systematic reviews and meta-analyses, and many of these reviews were found to be inconclusive, citing heterogeneity as the rationale. Clearly, addressing the extent of heterogeneity would enhance data synthesis and should be seen as a priority for prehabilitation research. For nutrition, the development of a COS that includes standards for nutritional assessment, a requirement to state the goal of the intervention in relation to an appropriate reference standard, along with a standard set of measurements to monitor and evaluate the intervention, could greatly advance the literature.
We would like to acknowledge a few limitations. First, we did not register this trial; although, this is not a prerequisite for scoping reviews. Second, this review was limited to prehabilitation interventions for patients with cancer. As a result, our findings should not be generalized to all prehabilitation research. Third, our search was limited to six databases and languages of English and French; these criteria may have biased our findings. Finally, we limited our review to formal prehabilitation studies (articles with the term prehabilitation in the title, abstract or keywords); this strategy may have introduced misclassification bias. That said, there is no accepted definition of prehabilitation, and our goal was to map the range of studies currently being conducted as a form of “prehabilitation.” We also acknowledge the large body of evidence of nutritional-only interventions such as preoperative nutritional support that have been reported previously that would not be included using our search strategy focusing on prehabilitation.
Conclusion
The prehabilitation literature is lacking standardized and validated nutritional assessment, is frequently conducted without employing evidence-based nutrition interventions, and is typically conducted without monitoring the nutrition intervention or evaluating the intervention's contribution to outcomes. In order to advance our understanding of prehabilitation, the nutrition component of prehabilitation interventions should be based on validated tools of assessment, accepted standards, monitored, and evaluated. We suggest that the development, adoption, and application of a core outcome set would be a first step in addressing the research gaps identified and result in studies that are more likely to inform clinical practice and improve patient outcomes.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
CG, SD, and MW designed the research. CG and SD carried out the data collection. All authors edited, read, and approved the final manuscript.
Disclaimer
The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2021.644723/full#supplementary-material
References
1. Carli F, Silver JK, Feldman LS, McKee A, Gilman S, Gillis C, et al. Surgical prehabilitation in patients with cancer: state-of-the-science and recommendations for future research from a panel of subject matter experts. Phys Med Rehabil Clin North Am. (2017) 28:49–64. doi: 10.1016/j.pmr.2016.09.002
2. Gillis C, Fenton TR, Gramlich L, Sajobi TT, Culos-Reed SN, Bousquet-Dion G, et al. Older frail prehabilitated patients who cannot attain a 400 m 6-min walking distance before colorectal surgery suffer more postoperative complications. Eur J Surg Oncol. (2020) 47:871–81. doi: 10.1016/j.ejso.2020.09.041
3. Minnella EM, Liberman AS, Charlebois P, Stein B, Scheede-Bergdahl C, Awasthi R, et al. The impact of improved functional capacity before surgery on postoperative complications: a study in colorectal cancer. Acta Oncol. (2019) 58:573–8. doi: 10.1080/0284186X.2018.1557343
4. Weimann A, Braga M, Carli F, Higashiguchi T, Hübner M, Klek S, et al. ESPEN guideline: clinical nutrition in surgery. Clin Nutr. (2017) 36:623–50. doi: 10.1016/j.clnu.2017.02.013
5. Chen BP, Awasthi R, Sweet SN, Minnella EM, Bergdahl A, Santa Mina D, et al. Four-week prehabilitation program is sufficient to modify exercise behaviors and improve preoperative functional walking capacity in patients with colorectal cancer. Support Care Cancer. (2017) 25:33–40. doi: 10.1007/s00520-016-3379-8
6. West MA, Loughney L, Lythgoe D, Barben CP, Sripadam R, Kemp GJ, et al. Effect of prehabilitation on objectively measured physical fitness after neoadjuvant treatment in preoperative rectal cancer patients: a blinded interventional pilot study. Br J Anaesth. (2015) 114:244–51. doi: 10.1093/bja/aeu318
7. Barberan-Garcia A, Ubre M, Roca J, Lacy AM, Burgos F, Risco R, et al. Personalised prehabilitation in high-risk patients undergoing elective major abdominal surgery: a randomized blinded controlled trial. Ann Surg. (2018) 267:50–6. doi: 10.1097/SLA.0000000000002293
8. Gillis C, Loiselle SE, Fiore JF Jr, Awasthi R, Wykes L, Liberman AS, et al. Prehabilitation with whey protein supplementation on perioperative functional exercise capacity in patients undergoing colorectal resection for cancer: a pilot double-blinded randomized placebo-controlled trial. J Acad Nutr Diet. (2016) 116:802–12. doi: 10.1016/j.jand.2015.06.007
9. Gillis C, Buhler K, Bresee L, Carli F, Gramlich L, Culos-Reed N, et al. Effects of nutritional prehabilitation, with and without exercise, on outcomes of patients who undergo colorectal surgery: a systematic review and meta-analysis. Gastroenterology. (2018) 155:391–410.e4. doi: 10.1053/j.gastro.2018.05.012
10. Heger P, Probst P, Wiskemann J, Steindorf K, Diener MK, Mihaljevic AL. A systematic review and meta-analysis of physical exercise prehabilitation in major abdominal surgery (PROSPERO 2017 CRD42017080366). J Gastrointest Surg. (2020) 24:1375–85. doi: 10.1007/s11605-019-04287-w
11. Thomas G, Tahir MR, Bongers BC, Kallen VL, Slooter GD, van Meeteren NL. Prehabilitation before major intra-abdominal cancer surgery: a systematic review of randomised controlled trials. Eur J Anaesthesiol. (2019) 36:933–45. doi: 10.1097/EJA.0000000000001030
12. Calloway DH. Functional consequences of malnutrition. Rev Infect Dis. (1982) 4:736. doi: 10.1093/4.4.736
13. Proctor E, Silmere H, Raghavan R, Hovmand P, Aarons G, Bunger A, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. (2011) 38:65–76. doi: 10.1007/s10488-010-0319-7
14. Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. (2018) 18:143. doi: 10.1186/s12874-018-0611-x
15. Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. (2005) 8:19–32. doi: 10.1080/1364557032000119616
16. Levac D, Colquhoun H, O'Brien KK. Scoping studies: advancing the methodology. Implement Sci. (2010) 5:69. doi: 10.1186/1748-5908-5-69
17. Swan WI, Vivanti A, Hakel-Smith NA, Hotson B, Orrevall Y, Trostler N, et al. Nutrition care process and model update: toward realizing people-centered care and outcomes management. J Acad Nutr Diet. (2017) 117:2003–14. doi: 10.1016/j.jand.2017.07.015
18. Tully R, Loughney L, Bolger J, Sorensen J, McAnena O, Collins CG, et al. The effect of a pre- and post-operative exercise programme versus standard care on physical fitness of patients with oesophageal and gastric cancer undergoing neoadjuvant treatment prior to surgery (The PERIOP-OG Trial): study protocol for a randomised controlled trial. Trials. (2020) 21:638. doi: 10.1186/s13063-020-04311-4
19. Bausys A, Luksta M, Kuliavas J, Anglickiene G, Maneikiene V, Gedvilaite L, et al. Personalized trimodal prehabilitation for gastrectomy. Medicine. (2020) 99:e20687–e. doi: 10.1097/MD.0000000000020687
20. Chmelo J, Phillips AW, Greystoke A, Charman SJ, Avery L, Hallsworth K, et al. A feasibility study to investigate the utility of a home-based exercise intervention during and after neo-adjuvant chemotherapy for oesophago-gastric cancer-the ChemoFit study protocol. Pilot Feasibil Stud. (2020) 6:50. doi: 10.1186/s40814-020-00597-y
21. Sheill G, Guinan E, O'Neill L, Normand C, Doyle SL, Moore S, et al. Preoperative exercise to improve fitness in patients undergoing complex surgery for cancer of the lung or oesophagus (PRE-HIIT): protocol for a randomized controlled trial. BMC Cancer. (2020) 20:321. doi: 10.1186/s12885-020-06795-4
22. Barberan-Garcia A, Navarro-Ripoll R, Sánchez-Lorente D, Moisés-Lafuente J, Boada M, Messaggi-Sartor M, et al. Cost-effectiveness of a technology-supported multimodal prehabilitation program in moderate-to-high risk patients undergoing lung cancer resection: randomized controlled trial protocol. BMC Health Serv Res. (2020) 20:207. doi: 10.1186/s12913-020-05078-9
23. Janssen TL, Mosk CA, van Hoof-de Lepper CCHA, Wielders D, Seerden TCJ, Steyerberg EW, et al. A multicomponent prehabilitation pathway to reduce the incidence of delirium in elderly patients in need of major abdominal surgery: study protocol for a before-and-after study. BMC Geriatr. (2019) 19:87. doi: 10.1186/s12877-019-1101-7
24. Allen S, Brown V, Prabhu P, Scott M, Rockall T, Preston S, et al. A randomised controlled trial to assess whether prehabilitation improves fitness in patients undergoing neoadjuvant treatment prior to oesophagogastric cancer surgery: study protocol. BMJ Open. (2018) 8:e023190. doi: 10.1136/bmjopen-2018-023190
25. Le Roy B, Pereira B, Bouteloup C, Costes F, Richard R, Selvy M, et al. Effect of prehabilitation in gastro-oesophageal adenocarcinoma: study protocol of a multicentric, randomised, control trial-the PREHAB study. BMJ Open. (2016) 6:e012876. doi: 10.1136/bmjopen-2016-012876
26. McIsaac DI, Saunders C, Hladkowicz E, Bryson GL, Forster AJ, Gagne S, et al. PREHAB study: a protocol for a prospective randomised clinical trial of exercise therapy for people living with frailty having cancer surgery. BMJ Open. (2018) 8:e022057. doi: 10.1136/bmjopen-2018-022057
27. van Rooijen S, Carli F, Dalton S, Thomas G, Bojesen R, Le Guen M, et al. Multimodal prehabilitation in colorectal cancer patients to improve functional capacity and reduce postoperative complications: the first international randomized controlled trial for multimodal prehabilitation. BMC Cancer. (2019) 19:98. doi: 10.1186/s12885-018-5232-6
28. Bruns ERJ, Argillander TE, Schuijt HJ, van Duijvendijk P, van der Zaag ES, Wassenaar EB, et al. Fit4SurgeryTV at-home prehabilitation for frail older patients planned for colorectal cancer surgery: a pilot study. Am J Phys Med Rehabil. (2019) 98:399–406. doi: 10.1097/PHM.0000000000001108
29. Santa Mina D, Matthew AG, Hilton WJ, Au D, Awasthi R, Alibhai SM, et al. Prehabilitation for men undergoing radical prostatectomy: a multi-centre, pilot randomized controlled trial. BMC Surgery. (2014) 14:89. doi: 10.1186/1471-2482-14-89
30. Dewberry LC, Wingrove LJ, Marsh MD, Glode AE, Schefter TE, Leong S, et al. Pilot prehabilitation program for patients with esophageal cancer during neoadjuvant therapy and surgery. J Surg Res. (2019) 235:66–72. doi: 10.1016/j.jss.2018.09.060
31. Li C, Carli F, Lee L, Charlebois P, Stein B, Liberman AS, et al. Impact of a trimodal prehabilitation program on functional recovery after colorectal cancer surgery: a pilot study. Surg Endosc. (2013) 27:1072–82. doi: 10.1007/s00464-012-2560-5
32. Sell NM, Silver JK, Rando S, Draviam AC, Mina DS, Qadan M. Prehabilitation telemedicine in neoadjuvant surgical oncology patients during the novel COVID-19 coronavirus pandemic. Ann Surg. (2020) 272:e81–3. doi: 10.1097/SLA.0000000000004002
33. Moore J, Merchant Z, Rowlinson K, McEwan K, Evison M, Faulkner G, et al. Implementing a system-wide cancer prehabilitation programme: the journey of greater manchester's ‘Prehab4cancer'. Eur J Surg Oncol. (2020) 47:524–32. doi: 10.1016/j.ejso.2020.04.042
34. Carli F, Brown R, Kennepohl S. Prehabilitation to enhance postoperative recovery for an octogenarian following robotic-assisted hysterectomy with endometrial cancer. Can J Anaesthes. (2012) 59:779–84. doi: 10.1007/s12630-012-9734-4
35. van Rooijen SJ, Molenaar CJL, Schep G, van Lieshout RHMA, Beijer S, Dubbers R, et al. Making patients fit for surgery: introducing a four pillar multimodal prehabilitation program in colorectal cancer. Am J Phys Med Rehabil. (2019) 98:888–96. doi: 10.1097/PHM.0000000000001221
36. Beck A, Thaysen HV, Soegaard CH, Blaakaer J, Seibaek L. Investigating the experiences, thoughts, and feelings underlying and influencing prehabilitation among cancer patients: a qualitative perspective on the what, when, where, who, and why. Disabil Rehabil. (2020) 13:1–8. doi: 10.1080/09638288.2020.1762770
37. Souwer ETD, Bastiaannet E, de Bruijn S, Breugom AJ, van den Bos F, Portielje JEA, et al. Comprehensive multidisciplinary care program for elderly colorectal cancer patients: “From prehabilitation to independence”. Eur J Surg Oncol. (2018) 44:1894–900. doi: 10.1016/j.ejso.2018.08.028
38. Nakajima H, Yokoyama Y, Inoue T, Nagaya M, Mizuno Y, Kadono I, et al. Clinical benefit of preoperative exercise and nutritional therapy for patients undergoing hepato-pancreato-biliary surgeries for malignancy. Ann Surg Oncol. (2019) 26:264–72. doi: 10.1245/s10434-018-6943-2
39. Ngo-Huang A, Parker NH, Bruera E, Lee RE, Simpson R, O'Connor DP, et al. Home-Based exercise prehabilitation during preoperative treatment for pancreatic cancer is associated with improvement in physical function and quality of life. Integr Cancer Ther. (2019) 18:1534735419894061. doi: 10.1177/1534735419894061
40. van der Vlies E, Smits AB, Los M, van Hengel M, Bos WJW, Dijksman LM, et al. Implementation of a preoperative multidisciplinary team approach for frail colorectal cancer patients: influence on patient selection, prehabilitation and outcome. J Geriatr Oncol. (2020) 11:1237–43. doi: 10.1016/j.jgo.2020.04.011
41. Janssen TL, Steyerberg EW, van Hoof-de Lepper CCHA, Seerden TCJ, de Lange DC, Wijsman JH, et al. Long-term outcomes of major abdominal surgery and postoperative delirium after multimodal prehabilitation of older patients. Surg Today. (2020) 50:1461–70. doi: 10.1007/s00595-020-02044-0
42. Ploussard G, Almeras C, Beauval JB, Gautier JR, Garnault V, Frémont N, et al. A combination of enhanced recovery after surgery and prehabilitation pathways improves perioperative outcomes and costs for robotic radical prostatectomy. Cancer. (2020) 126:4148–55. doi: 10.1002/cncr.33061
43. Paterson C, Primeau C, Pullar I, Nabi G. Development of a prehabilitation multimodal supportive care interventions for men and their partners before radical prostatectomy for localized prostate cancer. Cancer Nurs. (2019) 42:E47–53. doi: 10.1097/NCC.0000000000000618
44. Macleod M, Steele RJC, O'Carroll RE, Wells M, Campbell A, Sugden JA, et al. Feasibility study to assess the delivery of a lifestyle intervention (TreatWELL) for patients with colorectal cancer undergoing potentially curative treatment. BMJ Open. (2018) 8:e021117. doi: 10.1136/bmjopen-2017-021117
45. Ngo-Huang A, Parker NH, Wang X, Petzel MQB, Fogelman D, Schadler KL, et al. Home-based exercise during preoperative therapy for pancreatic cancer. Langenbecks Arch Surg. (2017) 402:1175–85. doi: 10.1007/s00423-017-1599-0
46. Mazzola M, Bertoglio C, Boniardi M, Magistro C, De Martini P, Carnevali P, et al. Frailty in major oncologic surgery of upper gastrointestinal tract: how to improve postoperative outcomes. Eur J Surg Oncol. (2017) 43:1566–71. doi: 10.1016/j.ejso.2017.06.006
47. Huang GH, Ismail H, Murnane A, Kim P, Riedel B. Structured exercise program prior to major cancer surgery improves cardiopulmonary fitness: a retrospective cohort study. Support Care Cancer. (2016) 24:2277–85. doi: 10.1007/s00520-015-3028-7
48. Liu Z, Qiu T, Pei L, Zhang Y, Xu L, Cui Y, et al. Two-Week multimodal prehabilitation program improves perioperative functional capability in patients undergoing thoracoscopic lobectomy for lung cancer: a randomized controlled trial. Anesthes Analg. (2020) 131:840–9. doi: 10.1213/ANE.0000000000004342
49. Minnella EM, Awasthi R, Bousquet-Dion G, Ferreira V, Austin B, Audi C, et al. Multimodal prehabilitation to enhance functional capacity following radical cystectomy: a randomized controlled trial. Eur Urol Focus. (2019) 7:132–8. doi: 10.1016/j.euf.2019.05.016
50. Minnella EM, Awasthi R, Loiselle SE, Agnihotram RV, Ferri LE, Carli F. Effect of exercise and nutrition prehabilitation on functional capacity in esophagogastric cancer surgery: a randomized clinical trial. JAMA Surg. (2018) 153:1081–9. doi: 10.1001/jamasurg.2018.1645
51. Bousquet-Dion G, Awasthi R, Loiselle S, Minnella EM, Agnihotram RV, Bergdahl A, et al. Evaluation of supervised multimodal prehabilitation programme in cancer patients undergoing colorectal resection: a randomized control trial. Acta Oncol. (2018) 57:849–59. doi: 10.1080/0284186X.2017.1423180
52. Jensen BT, Laustsen S, Jensen JB, Borre M, Petersen AK. Exercise-based pre-habilitation is feasible and effective in radical cystectomy pathways-secondary results from a randomized controlled trial. Support Care Cancer. (2016) 24:3325–31. doi: 10.1007/s00520-016-3140-3
53. Gillis C, Li C, Lee L, Awasthi R, Augustin B, Gamsa A, et al. Prehabilitation versus rehabilitation: a randomized control trial in patients undergoing colorectal resection for cancer. Anesthesiology. (2014) 121:937–47. doi: 10.1097/ALN.0000000000000393
54. Bharadwaj S, Ginoya S, Tandon P, Gohel TD, Guirguis J, Vallabh H, et al. Malnutrition: laboratory markers vs nutritional assessment. Gastroenterol Rep. (2016) 4:272–80. doi: 10.1093/gastro/gow013
55. Schricker T, Wykes L, Meterissian S, Hatzakorzian R, Eberhart L, Carvalho G, et al. The anabolic effect of perioperative nutrition depends on the patient's catabolic state before surgery. Ann Surg. (2013) 257:155–9. doi: 10.1097/SLA.0b013e31825ffc1f
56. Klute K, Brouwer J, Jhawer M, Sacks H, Gangadin A, Ocean AJ, et al. Chemotherapy toxicity predicted by baseline nutrition assessment in gastrointestinal (GI) malignancies: a multicenter analysis. J Clin Oncol. (2015) 33:410. doi: 10.1200/jco.2015.33.3_suppl.410
57. Curtis LJ, Bernier P, Jeejeebhoy K, Allard J, Duerksen D, Gramlich L, et al. Costs of hospital malnutrition. Clin Nutr. (2017) 36:1391–6. doi: 10.1016/j.clnu.2016.09.009
58. Lopes J, Russell DM, Whitwell J, Jeejeebhoy KN. Skeletal muscle function in malnutrition. Am J Clin Nutr. (1982) 36:602–10. doi: 10.1093/ajcn/36.4.602
59. Chan LN, Compher C, DiBaise JK, Dimaria-Ghalili RA, Guenter P, Resnick HE, et al. American society for parenteral and enteral nutrition research agenda. JPEN J Parent Enter Nutr. (2014) 38:13–8. doi: 10.1177/0148607113508783
60. Compher C, Jain AK, Nichol PF, Blackmer A, Earthman C, Evans DC, et al. Research agenda 2018: the american society for parenteral and enteral nutrition. JPEN J Parent Enter Nutr. (2018) 42:838–44. doi: 10.1002/jpen.1312
61. Peltz G. Nutrition support in cancer patients: a brief review and suggestion for standard indications criteria. Nutr J. (2002) 1:1. doi: 10.1186/1475-2891-1-1
62. Gillis C, Gramlich L, Culos-Reed SN, Sajobi TT, Fiest KM, Carli F, et al. Third-Variable effects: tools to understand who, when, why, and how patients benefit from surgical prehabilitation. J Surg Res. (2020) 258:443–52. doi: 10.1016/j.jss.2020.09.026
63. Aktas A, Walsh D, Galang M, O'Donoghue N, Rybicki L, Hullihen B, et al. Underrecognition of malnutrition in advanced cancer: the role of the dietitian and clinical practice variations. Am J Hosp Palliat Care. (2017) 34:547–55. doi: 10.1177/1049909116639969
64. Wojzischke J, van Wijngaarden J, van den Berg C, Cetinyurek-Yavuz A, Diekmann R, Luiking Y, et al. Nutritional status and functionality in geriatric rehabilitation patients: a systematic review and meta-analysis. Eur Geriatr Med. (2020) 11:195–207. doi: 10.1007/s41999-020-00294-2
65. Kamo T, Ishii H, Suzuki K, Nishida Y. The impact of malnutrition on efficacy of resistance training in community-dwelling older adults. Physiother Res Int. (2019) 24:e1755. doi: 10.1002/pri.1755
66. Gillis C, Fenton TR, Gramlich L, Carli F. Malnutrition and functional capacity: what is the effect of multimodal prehabilitation?
67. Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. (2017) 36:11–48. doi: 10.1016/j.clnu.2016.07.015
68. Zhao X-H, Yang T, Ma X-D, Qi Y-X, Lin Y-Y, Chen X-Z, et al. Heterogeneity of nutrition care procedures in nutrition guidelines for cancer patients. Clin Nutr. (2020) 39:1692–704. doi: 10.1016/j.clnu.2019.08.022
69. Williamson PR, Altman DG, Blazeby JM, Clarke M, Devane D, Gargon E, et al. Developing core outcome sets for clinical trials: issues to consider. Trials. (2012) 13:132. doi: 10.1186/1745-6215-13-132
Keywords: surgical nutrition, oncological nutrition, pre-operative, pre-surgery, prehabilitation
Citation: Gillis C, Davies SJ, Carli F, Wischmeyer PE, Wootton SA, Jackson AA, Riedel B, Marino LV, Levett DZH and West MA (2021) Current Landscape of Nutrition Within Prehabilitation Oncology Research: A Scoping Review. Front. Nutr. 8:644723. doi: 10.3389/fnut.2021.644723
Received: 21 December 2020; Accepted: 08 February 2021;
Published: 09 April 2021.
Edited by:
Donato Angelino, University of Teramo, ItalyReviewed by:
Daniele Nucci, Veneto Institute of Oncology (IRCCS), ItalyRoberta Masella, National Institute of Health (ISS), Italy
Copyright © 2021 Gillis, Davies, Carli, Wischmeyer, Wootton, Jackson, Riedel, Marino, Levett and West. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chelsia Gillis, Y2hlbHNpYS5naWxsaXNAbWNnaWxsLmNh
†These authors have contributed equally to this work and share first and last authorship
 Chelsia Gillis
Chelsia Gillis Sarah J. Davies
Sarah J. Davies Francesco Carli
Francesco Carli Paul E. Wischmeyer3
Paul E. Wischmeyer3 Stephen A. Wootton
Stephen A. Wootton Bernhard Riedel
Bernhard Riedel Luise V. Marino
Luise V. Marino Malcolm A. West
Malcolm A. West