- 1MetroHealth Medical Center, Case Western Reserve University, Cleveland, OH, United States
- 2School of Medicine, Case Western Reserve University, Cleveland, OH, United States
Introduction: Human milk (HM) is the ideal enteral feeding for nearly all infants and offers unique benefits to the very low birthweight (VLBW) infant population. It is a challenge to meet the high nutrient requirements of VLBW infants due to the known variability of HM composition. Human milk analysis (HMA) assesses the composition of HM and allows for individualized fortification. Due to recent U.S. Food and Drug Administration (FDA) approval, it has relatively recent availability for clinical use in the US.
Aim: To identify current practices of HMA and individualized fortification in neonatal intensive care units (NICUs) across the United States (US) and to inform future translational research efforts implementing this nutrition management method.
Methods: An institutional review board (IRB) approved survey was created and collected data on the following subjects such as NICU demographics, feeding practices, HM usage, HM fortification practices, and HMA practices. It was distributed from 10/30–12/21/2020 via online pediatric nutrition groups and listservs selected to reach the intended audience of NICU dietitians and other clinical staff. Each response was assessed prior to inclusion, and descriptive analysis was performed.
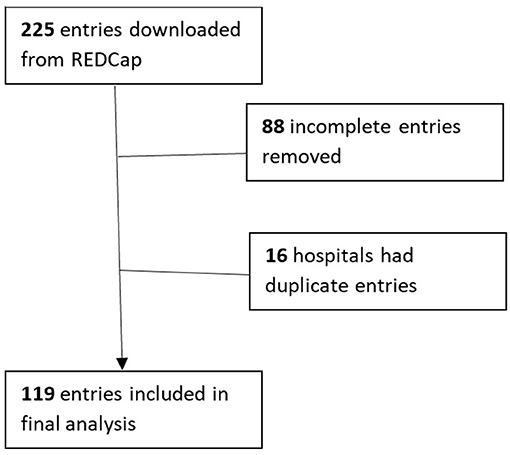
Results: About 225 survey responses were recorded during the survey period with 119 entries included in the analysis. This represented 36 states and Washington D.C., primarily from level III and IV NICUs. HMA was reported in 11.8% of responding NICUs. The most commonly owned technology for HMA is the Creamatocrit Plus TM (EKF Diagnostics), followed by the HM Analyzer by Miris (Uppsala, Sweden). In NICUs practicing HMA, 84.6% are doing so clinically.
Discussion: Feeding guidelines and fortification of HM remain standard of care, and interest in HMA was common in this survey. Despite the interest, very few NICUs are performing HMA and individualized fortification. Barriers identified include determining who should receive individualized fortification and how often, collecting a representative sample, and the cost and personnel required.
Conclusions: Human milk analysis and individualized fortification are emerging practices within NICUs in the US. Few are using it in the clinical setting with large variation in execution among respondents and many logistical concerns regarding implementation. Future research may be beneficial to evaluate how practices change as HMA and individualized fortification gain popularity and become more commonly used in the clinical setting.
Introduction
Human milk (HM) is the ideal enteral feeding for nearly all infants and offers unique benefits to the very low birthweight (VLBW) infant population. HM is associated with a decreased incidence of several life, threatening complications of prematurity, including late-onset sepsis (1), necrotizing enterocolitis (2), bronchopulmonary dysplasia (3, 4), and retinopathy of prematurity (5). HM consumption has also been associated with increased height z-scores and decreases in suprailiac skinfold thickness at 5.5 years of age (6), which may be suggestive of improved long-term growth outcomes for VLBW infants who receive HM rather than donor human milk (DHM) or preterm infant formula.
While HM is the preferred type of feeding for VLBW infants, it requires fortification to meet the extraordinarily high nutrient requirements of preterm infants (7, 8). HM fortification is a standard of care in the neonatal intensive care units (NICUs) but is typically done by means of standard fortification, with the false assumption of known HM nutrient density. HM is a dynamic substance with different macronutrient compositions between individuals throughout the lactation period of an individual and even within a single session of expression (9–12). This variability, combined with the high nutrient requirements creates a challenge in the clinical setting to appropriately meet the nutritional needs of preterm infants.
Human milk analysis (HMA) allows for individualized fortification of HM as a strategy to better ensure adequate macronutrient and total energy administration to premature infants. However, individualized fortification is not standard of practice in the United States (US) due to the relatively recent availability of HMA technology for clinical use. At present, only one device for macronutrient analysis has U.S. Food and Drug Administration (FDA) approval for use in the clinical setting; previous use of HM analyzers in the NICU has only been as a part of an institutional review board (IRB) approved research protocol.
Aim
The aim was to identify current practices of HM analysis and individualized fortification in NICUs across the US and to inform future translational research efforts of implementation of this nutrition management method.
Materials and Methods
A survey was created and administered using Research Electronic Data Capture (REDCap) tools hosted at Case Western Reserve University. REDCap is a secure, web-based software platform designed to support data capture for research studies. The MetroHealth Medical Center IRB gave ethical approval for this study (IRB 20-00413). A waiver of consent was obtained for all survey participants via the REDCap survey.
The survey was piloted within the Ohio Neonatal Nutritionists group (as shown in Table 1) to ensure user-friendly survey design and minimize measurement error. The survey collected data on the following subjects such as NICU demographics, typical feeding practices, HM usage, HM fortification practices, and HM analysis practices. The complete survey can be viewed in Appendix X.
The survey was distributed from 10/30–12/21/2020 via online pediatric nutrition groups and listservs selected to reach the intended audience of NICU dietitians and other clinical staff. Groups and listservs were selected to minimize sampling error and reach a large, diverse group of NICU dietitians and clinicians within the US. As shown in Table 1 for a full description of groups.
Study data were recorded in REDCap and downloaded for descriptive analysis, and each response was assessed prior to inclusion in the analysis. Surveys were included if responses had complete demographic information and partial responses for the remainder of the survey. The recorded city, state, and name of the hospital were used to identify duplicate responses from institutions. In instances of duplicate responses, the more complete entries were retained. If multiple complete entries were present, the entries were compared, and agreeing information was retained. If there was conflicting information in the comparison then: reported estimated daily NICU censuses were averaged; if one person entered a value of “didn't know” and one person entered a known value, the known value was used; If one individual indicated that a product was being used while the other did not, it was assumed that the product is being used; a value of “NA” was entered for all other conflicting answers.
Statistics
Survey data were stored in a secure study database and imported to R Statistical Software (version 4.0.2) (13) for analysis. Descriptive summaries are presented using median [interquartile range (IQR)] or mean (SD) as appropriate for continuous variables and number (%) for categorical variables.
Results
A total of 225 survey responses were recorded during the survey period with 119 entries included in the final analysis (as shown in Figure 1). Survey responses were recorded from 36 separate states and Washington D.C (as shown in Figure 2) and represented mostly level III (62.2%) and level IV (35.3%) NICUs. Additional NICU demographic information is displayed in Table 2.
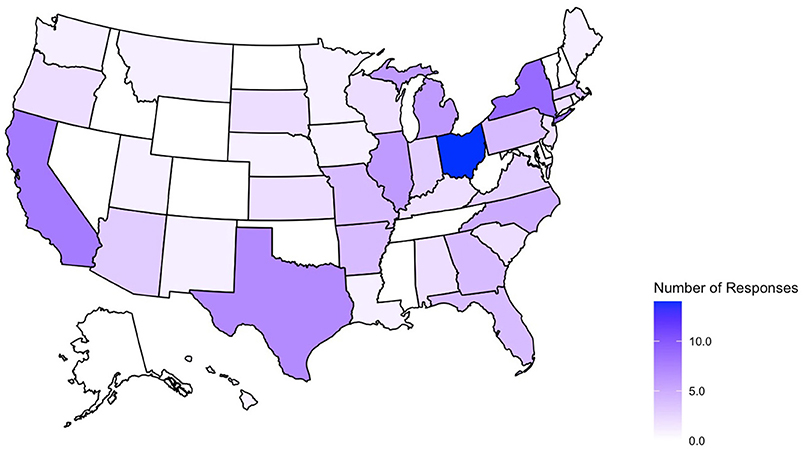
Figure 2. States represented in collected survey responses. No submissions from AK, CO, DE, ID, MS, NV, NH, ND, OK, RI, TN, VT, WV, WY.
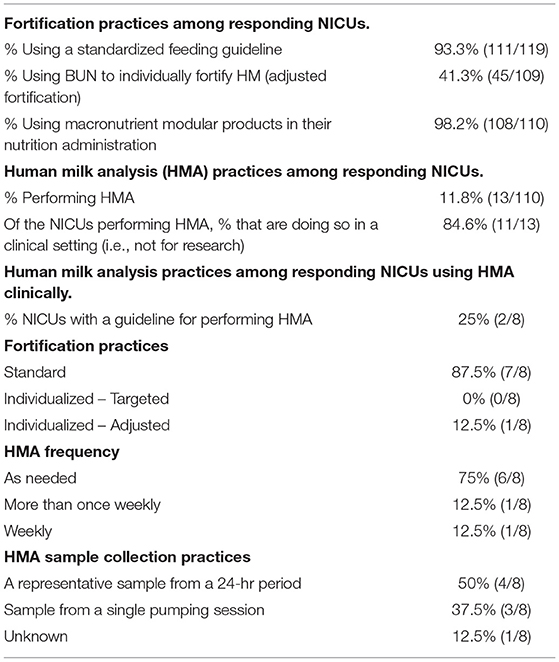
As described in Table 3, 93.3% of responding NICUs utilize a standardized feeding guideline. A total of 98.2% of responding NICUs utilize some macronutrient modular product in addition to HM fortifiers in their practice. The types of macronutrient modular products used in responding NICUs are described in Figure 3. Modular products are defined as products that provide additional nutrient components to HM, by increasing the amount of protein, carbohydrate, fat, or a combination of macronutrients. Adjustable fortification, the practice of using blood urea nitrogen (BUN) levels to adjust added protein to enteral feeds, was reported in 41.3% of responding NICUs.
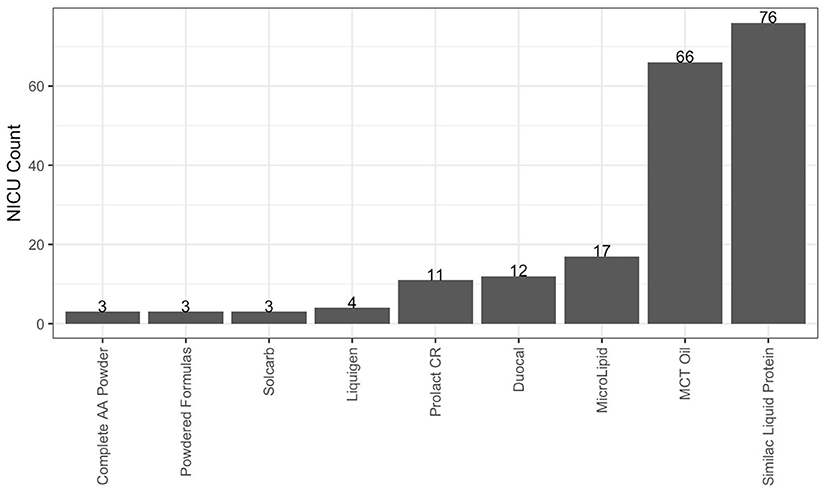
Figure 3. Macronutrient modulars used in responding NICUs. Other products used in fewer than 3 NICUs: canola, oil, fish, oil, olive oil, DHA+ARA, Beneprotein and concentrated liquid formulas.
Human milk analysis was reported in 11.8% of responding NICUs. The most commonly owned technology for HMA is the Creamatocrit PlusTM (EKF Diagnostics, Boerne, TX, USA), followed by the HM Analyzer by Miris (Uppsala, Sweden) (Figure 4). The most commonly reported HM analyzer that NICUs reported considering purchasing was the HM Analyzer by Miris (Uppsala, Sweden) (Figure 4). The roles of individuals who typically performed HMA and maintained HMA quality in the reporting NICUs are described in Figure 5, while a wide variety of medical professionals perform milk analysis, the responsibility of maintaining the quality of HMA measurements fell largely to registered dietitian nutritionists, lactation consultants, or physicians.
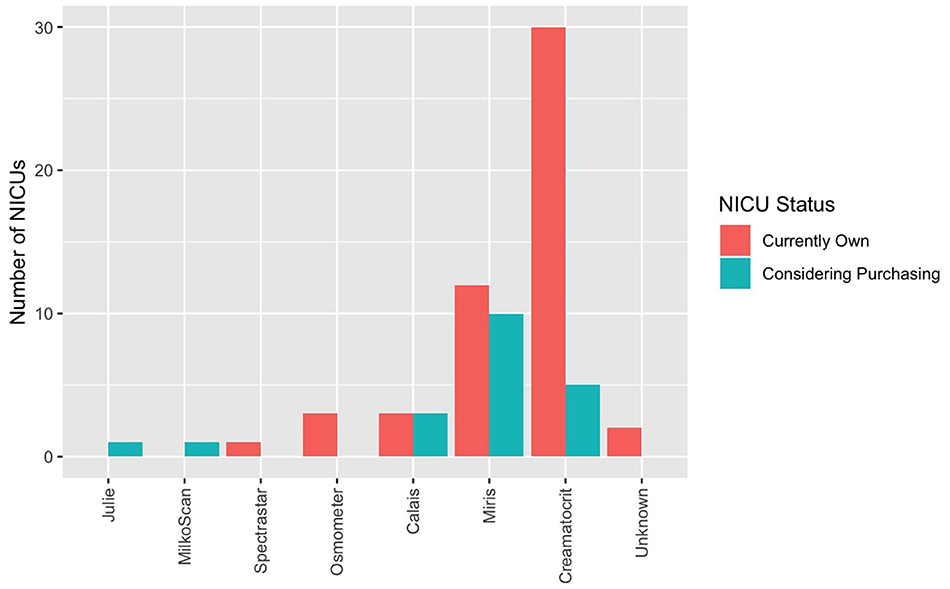
Figure 4. Human milk analyzers in NICUs. What unit currently own (for research or clinical use) and what they are considering purchasing.
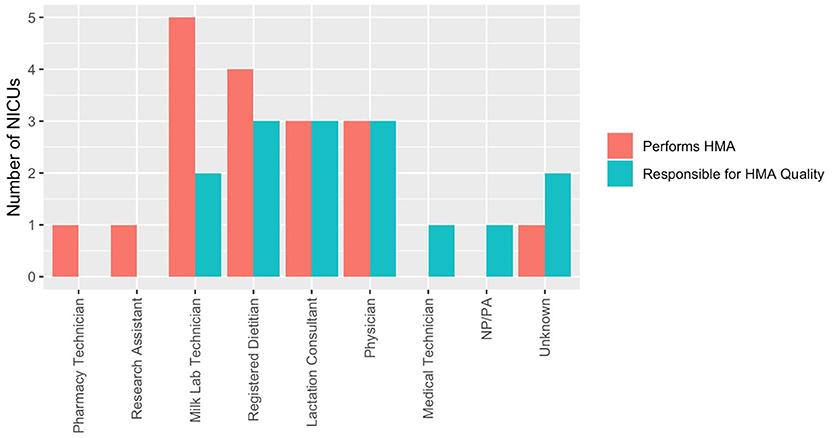
Figure 5. Human milk analysis responsibilities in NICUs. Who performs HMA and who establishes quality of measurements.
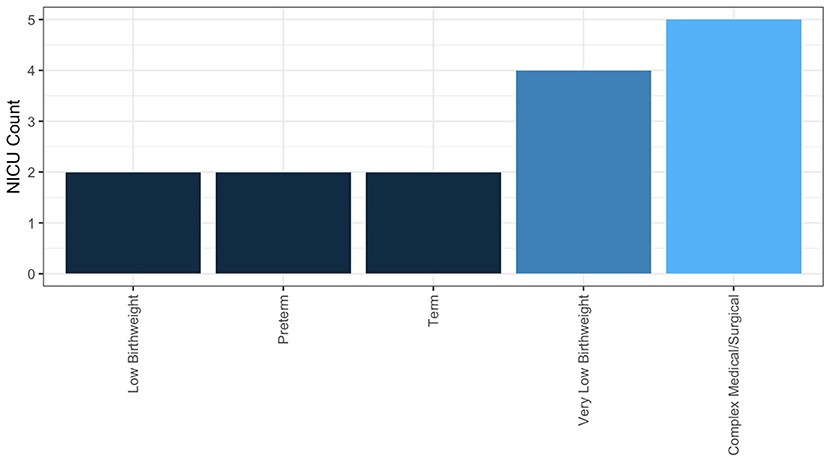
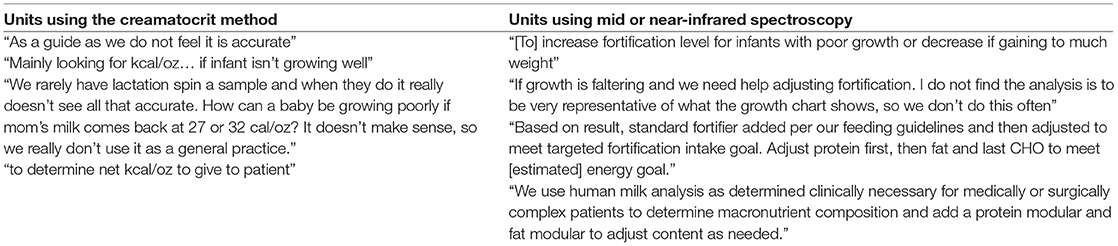
In NICUs practicing HMA, 84.6% are doing so clinically, meaning they are using HMA in the NICU, not under an IRB-approved research protocol. The remainder practice HMA for research alone; 25% of these NICUs having a guideline for performing HMA. Descriptive statistics for frequency of HMA, method of HM sample collection, and fortification practices for NICUs performing HMA clinically are reported in Table 3. Populations, where HMA is being used clinically, are described in Figure 6. How HMA is being used clinically was captured via open-text responses and is displayed in Box 1.
Box 1. Summary of text responses describing how human milk analysis (HMA) is being used in the clinical setting of respondents.
Discussion
Utilization of feeding guidelines and HM fortification remains a standard of care in US NICUs, as evident in the survey responses. There is an increase in interest in individualized fortification methods, and the use of macronutrient modular products in enteral feed administration was common in this survey. HM analysis and individualized fortification are emerging practices within NICUs in the US, with large variation in execution among respondents to this survey. While these practices have been widely discussed in a clinical research setting, translating HMA and individualized fortification into the clinical setting will require implementation research to better evaluate barriers to practice utilization.
The Rationale for Individualized Fortification/HMA
The known variability of HM is a challenge in creating nutrition plans in the NICU environment, where preterm infants have exceptionally high nutrient requirements that cannot be met by HM alone. Even with standard fortification practices, estimated nutrient requirements may not be met due to the variability of HM (14). HM composition varies both within and between lactating individuals and is influenced by postnatal time and potentially degree of prematurity. Zachariassen et al. (15) found that HM samples were higher in fat and energy content for infants <28 weeks gestational age (GA) compared to those of 28–32 weeks GA (15). Decline in protein content in HM in the first weeks of life has been repeatedly reported (9, 12, 15), although the influence of prematurity on protein content is debated in the published literature (12). Trends of lactose and carbohydrate concentrations within HM have been described in early milk expression (9). However, carbohydrates remain the least accurate and arguably an invalid macronutrient measured using infrared (IR) HM analyzers available for clinical use (16, 17). In a longitudinal study of HM-fed very preterm infants, Belfort et al. (18) found substantial variation in intakes of protein and energy. This variation predicted slower weight gain and linear growth, despite standard fortification in HM-fed very preterm infants. This finding supports the hypothesis that providing individually targeted HM fortification may reduce macronutrient deficits and improve physical growth (18).
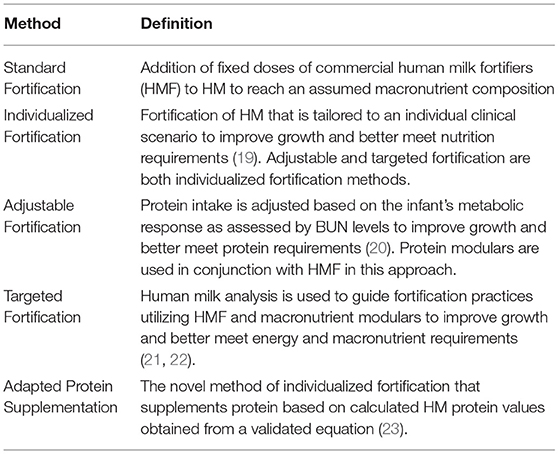
Incorporation of the known variabilities in HM into standard NICU nutritional care is a necessary evolution of practice to meet the nutrition requirements of preterm infants. Multiple individualized fortification strategies have been used to address this variability and nutrient requirements, described in Table 4.
A recent double-blind randomized control trial by Rochow et al. (21) with very low GA infants fed target fortified HM showed that target fortification of HM with low macronutrient content enhances the quality of nutrition and growth of preterm infants. Not only did they show that target fortification improved weight gain but also had a positive impact on increased body composition, length, and head circumference (21).
Responses to the survey indicated that most NICUs utilize standard fortification practices, using assumed energy and macronutrient density values when adding HM fortifier to HM. However, nearly all (98.2%) responding NICUs reported using at least one macronutrient modular in their clinical practice, with the most commonly reported products such as Liquid Protein Fortifier (n = 76, Abbott Nutrition), MCT Oil (n = 66, various), MicroLipidTM (n = 17, Nestlé Health Science), Duocal (n = 12, Neocate), and Prolact CR® (n = 11, Prolacta Bioscience). The common utilization of additional macronutrient modulars shows that individualized fortification is already a common, although not standard, practice in US NICUs and one that is driven by clinical assessment, rather than HMA.
The quality of early life nutrition administration may be predictive of body composition development in the preterm infant period (24). Individualized fortification may be a strategy to improve the quality of growth and body composition development in VLBW infants. Parat et al. (25) used HMA and individualized fortification to target a goal of 4 g/kg/day protein intake found that patients who received this targeted fortification for at least 30 days had increased fat-free mass at discharge (25). Morlacchi et al. (26) found that VLBW infants receiving targeted fortification HM had an increased fat-free mass compared to VLBW infants receiving preterm formula at term corrected age (26). While it has not been fully elucidated what early life nutrition interventions promote the best body composition outcomes in preterm infants, the goal of body composition development is becoming clearer. Increased lean body mass in preterm infants is increasingly associated with improved neurodevelopmental outcomes at 4 months (27), 1 year (28, 29), and 4 years of life (30). The potential to improve growth and body composition development in preterm infants is driving the incorporation of HMA into standard NICU care. However, HMA remains in the early phase of clinical implementation in US NICUs, as evidence that only one in 10 responding NICUs is performing HMA clinically.
Variation in the Method of Analysis
Several methods have been developed to analyze HM for use in individualized fortification and are described elsewhere (31). The two most commonly owned pieces of equipment in the surveyed population were the Creamatocrit PlusTM (n = 30) followed by the HM Analyzer by Miris (n = 12).
The Creamatocrit PlusTM (EKF Diagnostics) uses centrifugation to estimate the fat concentration and energy density of HM samples. Variations in measured fat content comparing the creamatocrit method to the gold standard gravimetric (or Mojonnier) method have been described in the literature; however, this method remains popular due to ease of use in clinical settings (32, 33). O'Neill et al. (34) found that the creamatocrit method reported mean fat content on average 46% higher and mean energy content 16% higher compared to gold standard analysis via the Mojonnier method (34). The same study found that the estimated mean fat content was 80% higher, and the mean energy content was 26% higher compared to milk analysis via mid-infrared spectroscopy (Calais HM Analyzer, Metron Instruments, Solon, OH) (34).
The differences in estimated compared to actual energy content could be related to variations in sample collection methods or due to limitations of the creamatocrit method. Concerns for the reliability of this method of HM analysis were noted by two of the NICUs that have access to this technology (as shown in Box 1).
The concerns for the reliability of analysis and the potential insufficiency of assessing only the fat and calorie composition of HM make mid-infrared spectroscopy analysis appealing for clinical use. The HM Analyzer by Miris measures macronutrient concentrations and estimates energy density within a 3 ml HM sample using mid-infrared spectroscopy; this technology was approved for clinical use by the FDA in December 2018. The Miris is the only multi-macronutrient analyzer that is currently approved for clinical use by the FDA and has been validated for macronutrient analysis in numerous studies (17, 35–37). This method of HMA does have significant barriers for many NICUs: it requires a representative sample for accurate analysis, it is an expensive piece of equipment to purchase and requires trained personnel to analyze the milk. Among the surveyed NICUs, the cost was a frequently mentioned barrier for the implementation of HMA.
An additional critical difference between the two most commonly owned HM analyzers is the type of information obtained from their analysis. The Creamatocrit PlusTM provides fat content and an estimated energy density of the milk sample. The Miris provides carbohydrate and protein content, in addition to the fat and energy density of measured milk samples. While there is no evidence that routine fat or carbohydrate supplementation alone improves growth (38, 39), ensuring adequate protein fortification has been associated with improved growth outcomes in preterm infants (38). Combining adequate protein and energy fortification in preterm infants is critical in optimizing growth outcomes. Relying solely on energy density to base individualized fortification decisions is likely not an effective method of improving growth outcomes in preterm infants (40, 41); again, as shown in Box 1.
Barriers to Clinical HMA Implementation
While the inadequacies of standard fortification methods have been identified, and the interest in individualized fortification is clear, there remain several barriers to the clinical implementation of HMA and individualized fortification in the typical NICU.
Barrier 1: Who Should Receive HMA and Individualized Fortification and How Often?
A systematic review and meta-analysis by Fabrizio et al. (42) determined that there was moderate- to low-certainty evidence supporting that individualized fortification (encompassing both targeted fortifications using HMA and adjustable fortification) for VLBW infants improved short-term growth compared to VLBW infants receiving standard fortification (42). Individual studies using HMA for targeted fortification have shown that this practice may improve growth and body composition outcomes (25, 43), but this finding is not universal (44). A lack of clarity in the literature on who individualized fortification would most benefit is reflected in the responses of the survey. From responding NICUs performing HMA clinically (n = 11), the majority (n = 6) perform HMA for infants that have complex medical or surgical conditions, including infants with poor growth of unclear etiology. Similarly, the frequency of HMA for an individual patient was typical as needed (n = 6); a total of two NICUs reported performing HMA at least weekly for an individual patient. Future research exploring both which NICU populations and what outcomes individualized fortification may most benefit from is required to help guide clinical implementation of this practice.
Barrier 2: Collecting a Representative Sample
Responses from the survey indicated a variety of sample collection practices for HMA, varying between single expression and 24-h sample collections. Similarly, procedures for sample collection intended for HMA vary in the published literature (45). The method of HM sample collection in a clinical setting must take into account the feasibility of sample collection for the lactating parent and NICU staff (46). However, the influence of HM sample collection on macronutrient composition must be considered when utilizing HMA for clinical nutrition decision-making. The fat content of HM is known to vary most significantly throughout the day and within a single feed (47–49). Protein content is known to decrease over multiple weeks and has also been reported to vary throughout 24 h (11, 15, 50). These macronutrient variations limit the efficacy of using a single feed milk sample for HM analysis in a clinical setting. Obtaining a representative sample from a pooled 24-h sample is essential to accurately capture the average macronutrient and caloric density of an HM sample to create clinical nutrition interventions (16, 51). Failure to obtain a representative sample and accurately perform HMA can drastically change results, fortification administered, and in turn, change the quality of the nutrition received. It is important to note that all samples used for milk analysis are discarded following testing, which may be a deterrent; however, the volume is minimized based on the technology used (e.g., Miris uses, 3 ml sample).
Barrier 3: Cost and Personnel
The personnel required for clinical implementation of HMA is a significantly greater cost than the technology alone. Personnel is needed to collect a representative sample (46), analyze the milk (16), create the fortification plan, and then actually prepare the individually fortified HM. Actual individualized fortification should be done in a designated feed preparation space away from the bedside and ideally in a milk lab (52).
Roles identified in this survey as individuals responsible for actually performing HMA included milk lab technicians (n = 5), registered dietitians (n = 4), physicians (n = 3), lactation consultants (n = 3), pharmacy technicians (n = 1), and research assistants (n = 1). Roles identified in this survey as being responsible for HMA quality were as follows: registered dietitians (n = 3), physicians (n = 3), lactation consultants (n = 3), and milk lab technicians (n = 2). The translation of a novel method of providing nutritional intervention in a clinical setting means that there is a limited framework in US NICUs on how HMA would best function and be afforded. Comparative effectiveness research to assess standard vs. individualized fortification in VLBW infants is needed to provide cost validation for this process in the typical clinical setting.
Solutions?
Human milk analysis and individualized fortification have been successfully implemented into clinical settings (43), but the barriers described above have contributed to this remaining an uncommon intervention in standard NICU practice. These barriers may help explain why only 11.8% of responding NICUs are performing HMA at the time of the survey. Research addressing the above translational gaps will improve the feasibility of the implementation of this practice in NICUs. While HMA remains accessible to only a fraction of NICUs, there appears to be an increasing practice of adjustable fortification, which is the practice of titrating protein fortification on top of a standard fortification based on BUN levels (20). A total of 41.3% of respondents to the survey reported utilizing adjusted fortification in their NICU. Adjusted fortification has been recommended by the European Milk Bank Association as a practical method to optimize HM fortification in the NICU (41). However, this method of fortification requires regular blood draws that may otherwise not be indicated. An adapted protein supplementation strategy proposed by Minarski et al. (23) utilizes a breast milk equation to suggest additional protein fortification based on days after delivery (23). These alternative methods of fortification may be more feasible for the typical NICU given current clinical practice in the US.
Strengths and Limitations
This study provides a general sense of the current practices of HMA and fortification in US NICUs, a practice that is currently transitioning from the research to the clinical stages of implementation. Formally, capturing typical clinical practices during such a transition period is useful in better identifying both implementation research gaps and practical barriers. Identifying the perspectives and practices of individuals utilizing HMA may help institutions considering HMA identify its usefulness and limitations.
One limitation to the study was the small number of responses from NICUs who are performing HMA in a clinical setting. While this could be due to sampling or a nonresponse error, this finding likely reflects how few NICUs are performing HMA in a clinical setting. An additional limitation to this study, as this was a self-administered survey by self-identified individuals, it is possible that the information collected was not entirely reflective of the practices of a single institution. We attempted to minimize measurement error by piloting the survey among a group of qualified NICU dietitians and utilizing internet groups and listservs that are known to be run by NICU dietitians. We selected NICU dietitians as the target population as we believed this group of professionals would be able to most accurately respond to the survey questions due to their unique professional scope. Some surveys were only partially completed, and it is possible that individuals with less standardized practice or content knowledge were more likely to terminate the survey early.
Conclusions
Human milk analysis and individualized fortification are emerging practices within NICUs in the US, with large variation in execution among respondents to this survey. This survey provides a general sense of current HM use and an assessment of HMA practices as it is gaining popularity and is becoming more accessible. Very few NICUs are using HMA and individualized fortification in the clinical setting. The survey has also identified many barriers and logistical concerns regarding the implementation of HMA and individualized fortification.
Though we received many responses, the results do not represent all US NICUs. Another limitation to the study was the small number of responses from NICUs who are performing HMA in a clinical setting. Future research may be beneficial to evaluate how practices change as HMA and individualized fortification gains popularity and are more commonly used in the clinical setting.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
SR, SM, and SG-W designed and distributed the survey. SR, SM, KM, and SG-W performed data analysis and interpreted the results. SR and SM drafted the manuscript. All authors read and approved the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2021.692600/full#supplementary-material
References
1. Patel AL, Johnson TJ, Engstrom JL, Fogg LF, Jegier BJ, Bigger HR, et al. Impact of early human milk on sepsis and health-care costs in very low birth weight infants. J Perinatol. (2013) 33:514–9. doi: 10.1038/jp.2013.2
2. Altobelli E, Angeletti PM, Verrotti A, Petrocelli R. The impact of human milk on necrotizing enterocolitis: a systematic review and meta-analysis. Nutrients. (2020) 12:1322. doi: 10.3390/nu12051322
3. Patel AL, Johnson TJ, Robin B, Bigger HR, Buchanan A, Christian E, et al. Influence of own mother's milk on bronchopulmonary dysplasia and costs. Arch Dis Child Fetal Neonatal Ed. (2017) 102:F256–61. doi: 10.1136/archdischild-2016-310898
4. Hair AB, Peluso AM, Hawthorne KM, Perez J, Smith DP, Khan JY, et al. Beyond necrotizing enterocolitis prevention: improving outcomes with an exclusive human milk-based diet. Breastfeed Med. (2016) 11:70–4. doi: 10.1089/bfm.2015.0134
5. Raghuveer TS, Zackula R. Strategies to prevent severe retinopathy of prematurity: a 2020 update and meta-analysis. Neoreviews. (2020) 21:e249–63. doi: 10.1542/neo.21-4-e249
6. McGee M, Unger S, Hamilton J, Birken CS, Pausova Z, Kiss A, et al. Adiposity and fat-free mass of children born with very low birth weight do not differ in children fed supplemental donor milk compared with those fed preterm formula. J Nutr. (2020) 150:331–9. doi: 10.1093/jn/nxz234
7. Eidelman AI, Schanler RJ. Breastfeeding and the use of human milk. Pediatrics. (2012) 129:e827–41. doi: 10.1542/peds.2011-3552
8. Schanler RJ. Evaluation of the evidence to support current recommendations to meet the needs of premature infants: the role of human milk. Am J Clin Nutr. (2007) 85:625s−8s. doi: 10.1093/ajcn/85.2.625S
9. Boyce C, Watson M, Lazidis G, Reeve S, Dods K, Simmer K, et al. Preterm human milk composition: a systematic literature review. Br J Nutr. (2016) 116:1033–45. doi: 10.1017/S0007114516003007
10. Bravi F, Wiens F, Decarli A, Dal Pont A, Agostoni C, Ferraroni M. Impact of maternal nutrition on breast-milk composition: a systematic review. Am J Clin Nutr. (2016) 104:646–62. doi: 10.3945/ajcn.115.120881
11. Groh-Wargo S, Valentic J, Khaira S, Super DM, Collin M. Considering human milk variability in the nutritional management of low-birth-weight infants. Infant Child Adol Nutr. (2014) 6:301–2. doi: 10.1177/1941406414536611
12. Maly J, Burianova I, Vitkova V, Ticha E, Navratilova M, Cermakova E. Preterm human milk macronutrient concentration is independent of gestational age at birth. Arch Dis Child Fetal Neonatal Ed. (2019) 104:F50–f6. doi: 10.1136/archdischild-2016-312572
13. R Development Core Team. R: A Language and Environment For Statistical Computing. Vienna: R Foundation for Statistical Computing (2020).
14. Newkirk M, Shakeel F, Parimi P, Rothpletz-Puglia P, Patusco R, Marcus AF, et al. Comparison of calorie and protein intake of very low birth weight infants receiving mother's own milk or donor milk when the nutrient composition of human milk is measured with a breast milk analyzer. Nutr Clin Pract. (2018) 33:679–86. doi: 10.1002/ncp.10060
15. Zachariassen G, Fenger-Gron J, Hviid MV, Halken S. The content of macronutrients in milk from mothers of very preterm infants is highly variable. Dan Med J. (2013) 60:A4631.
16. Kwan C, Fusch G, Rochow N, Fusch C. Milk analysis using milk analyzers in a standardized setting (MAMAS) study: a multicentre quality initiative. Clin Nutr. (2020) 39:2121–8. doi: 10.1016/j.clnu.2019.08.028
17. Fusch G, Rochow N, Choi A, Fusch S, Poeschl S, Ubah AO, et al. Rapid measurement of macronutrients in breast milk: how reliable are infrared milk analyzers? Clin Nutr. (2015) 34:465–76. doi: 10.1016/j.clnu.2014.05.005
18. Belfort M, Cherkerzian S, Bell K, Soldateli B, Cordova Ramos E, Palmer C, et al. Macronutrient intake from human milk, infant growth, and body composition at term equivalent age: a longitudinal study of hospitalized very preterm infants. Nutrients. (2020) 12:2249. doi: 10.3390/nu12082249
19. Kadioglu Simşek G, Alyamaç Dizdar E, Arayici S, Canpolat FE, Sari FN, Uraş N, et al. Comparison of the effect of three different fortification methods on growth of very low birth weight infants. Breastfeed Med. (2019) 14:63–8. doi: 10.1089/bfm.2018.0093
20. Arslanoglu S, Moro GE, Ziegler EE. Adjustable fortification of human milk fed to preterm infants: does it make a difference? J Perinatol. (2006) 26:614–21. doi: 10.1038/sj.jp.7211571
21. Rochow N, Fusch G, Ali A, Bhatia A, So HY, Iskander R, et al. Individualized target fortification of breast milk with protein, carbohydrates, and fat for preterm infants: a double-blind randomized controlled trial. Clin Nutr. (2021) 40:54–63. doi: 10.1016/j.clnu.2020.04.031
22. Morlacchi L, Mallardi D, Giannì ML, Roggero P, Amato O, Piemontese P, et al. Is targeted fortification of human breast milk an optimal nutrition strategy for preterm infants? An interventional study. J Transl Med. (2016) 14:195. doi: 10.1186/s12967-016-0957-y
23. Minarski M, Maas C, Engel C, Heinrich C, Böckmann K, Bernhard W, et al. Calculating protein content of expressed breast milk to optimize protein supplementation in very low birth weight infants with minimal effort-a secondary analysis. Nutrients. (2020) 12:1231. doi: 10.3390/nu12051231
24. Lingwood BE, Al-Theyab N, Eiby YA, Colditz PB, Donovan TJ. Body composition in very preterm infants before discharge is associated with macronutrient intake. Br J Nutr. (2020) 123:800–6. doi: 10.1017/S000711451900343X
25. Parat S, Raza P, Kamleh M, Super D, Groh-Wargo S. Targeted breast milk fortification for very low birth weight (VLBW) infants: nutritional intake, growth outcome and body composition. Nutrients. (2020) 12:1156. doi: 10.3390/nu12041156
26. Morlacchi L, Roggero P, Giannì ML, Bracco B, Porri D, Battiato E, et al. Protein use and weight-gain quality in very-low-birth-weight preterm infants fed human milk or formula. Am J Clin Nutr. (2018) 107:195–200. doi: 10.1093/ajcn/nqx001
27. Pfister KM, Gray HL, Miller NC, Demerath EW, Georgieff MK, Ramel SE. Exploratory study of the relationship of fat-free mass to speed of brain processing in preterm infants. Pediatr Res. (2013) 74:576–83. doi: 10.1038/pr.2013.138
28. Ramel SE, Gray HL, Christiansen E, Boys C, Georgieff MK, Demerath EW. Greater early gains in fat-free mass, but not fat mass, are associated with improved neurodevelopment at 1 year corrected age for prematurity in very low birth weight preterm infants. J Pediatr. (2016) 173:108–15. doi: 10.1016/j.jpeds.2016.03.003
29. Cooper DM, Girolami GL, Kepes B, Stehli A, Lucas CT, Haddad F, et al. Body composition and neuromotor development in the year after NICU discharge in premature infants. Pediatr Res. (2020) 88:459–65. doi: 10.1038/s41390-020-0756-2
30. Scheurer JM, Zhang L, Plummer EA, Hultgren SA, Demerath EW, Ramel SE. Body composition changes from infancy to 4 years and associations with early childhood cognition in preterm and full-term children. Neonatology. (2018) 114:169–76. doi: 10.1159/000487915
31. Fusch G, Kwan C, Kotrri G, Fusch C. Bed side human milk analysis in the neonatal intensive care unit: a systematic review. Clin Perinatol. (2017) 44:209–67. doi: 10.1016/j.clp.2016.11.001
32. Du J, Gay MCL, Lai CT, Trengove RD, Hartmann PE, Geddes DT. Comparison of gravimetric, creamatocrit and esterified fatty acid methods for determination of total fat content in human milk. Food Chem. (2017) 217:505–10. doi: 10.1016/j.foodchem.2016.08.114
33. Meier PP, Engstrom JL, Murtaugh MA, Vasan U, Meier WA, Schanler RJ. Mothers' milk feedings in the neonatal intensive care unit: accuracy of the creamatocrit technique. J Perinatol. (2002) 22:646–9. doi: 10.1038/sj.jp.7210825
34. O'Neill EF, Radmacher PG, Sparks B, Adamkin DH. Creamatocrit analysis of human milk overestimates fat and energy content when compared to a human milk analyzer using mid-infrared spectroscopy. J Pediatr Gastroenterol Nutr. (2013) 56:569–72. doi: 10.1097/MPG.0b013e31828390e4
35. Zhu M, Yang Z, Ren Y, Duan Y, Gao H, Liu B, et al. Comparison of macronutrient contents in human milk measured using mid-infrared human milk analyser in a field study vs. Chemical reference methods. Matern Child Nutr. (2017) 13:e12248. doi: 10.1111/mcn.12248
36. Billard H, Simon L, Desnots E, Sochard A, Boscher C, Riaublanc A, et al. Calibration adjustment of the mid-infrared analyzer for an accurate determination of the macronutrient composition of human milk. J Hum Lact. (2016) 32:Np19–27. doi: 10.1177/0890334415588513
37. Casadio YS, Williams TM, Lai CT, Olsson SE, Hepworth AR, Hartmann PE. Evaluation of a mid-infrared analyzer for the determination of the macronutrient composition of human milk. J Hum Lact. (2010) 26:376–83. doi: 10.1177/0890334410376948
38. Amissah EA, Brown J, Harding JE. Fat supplementation of human milk for promoting growth in preterm infants. Cochrane Database Syst Rev. (2020) 8:Cd000341. doi: 10.1002/14651858.CD000433.pub3
39. Amissah EA, Brown J, Harding JE. Carbohydrate supplementation of human milk to promote growth in preterm infants. Cochrane Database Syst Rev. (2020) 9:Cd000280. doi: 10.1002/14651858.CD000280.pub3
40. Bellagamba MP, Carmenati E, D'Ascenzo R, Malatesta M, Spagnoli C, Biagetti C, et al. One extra gram of protein to preterm infants from birth to 1800 g: a single-blinded randomized clinical trial. J Pediatr Gastroenterol Nutr. (2016) 62:879–84. doi: 10.1097/MPG.0000000000000989
41. Arslanoglu S, Boquien CY, King C, Lamireau D, Tonetto P, Barnett D, et al. Fortification of human milk for preterm infants: update and recommendations of the European milk bank association (EMBA) working group on human milk fortification. Front Pediatr. (2019) 7:76. doi: 10.3389/fped.2019.00076
42. Fabrizio V, Trzaski JM, Brownell EA, Esposito P, Lainwala S, Lussier MM, et al. Individualized versus standard diet fortification for growth and development in preterm infants receiving human milk. Cochrane Database Syst Rev. (2020) 11:CD013465. doi: 10.1002/14651858.CD013465
43. Rochow N, Fusch G, Choi A, Chessell L, Elliott L, McDonald K, et al. Target fortification of breast milk with fat, protein, and carbohydrates for preterm infants. J Pediatr. (2013) 163:1001–7. doi: 10.1016/j.jpeds.2013.04.052
44. McLeod G, Sherriff J, Hartmann PE, Nathan E, Geddes D, Simmer K. Comparing different methods of human breast milk fortification using measured v. assumed macronutrient composition to target reference growth: a randomised controlled trial. Br J Nutr. (2016) 115:431–9. doi: 10.1017/S0007114515004614
45. Leghi GE, Middleton PF, Netting MJ, Wlodek ME, Geddes DT, Muhlhausler BS. A systematic review of collection and analysis of human milk for macronutrient composition. J Nutr. (2020) 150:1652–70. doi: 10.1093/jn/nxaa059
46. Galante L, Vickers MH, Milan AM, Reynolds CM, Alexander T, Bloomfield FH, et al. Feasibility of standardized human milk collection in neonatal care units. Sci Rep. (2019) 9:14343. doi: 10.1038/s41598-019-50560-y
47. George AD, Gay MCL, Murray K, Muhlhausler BS, Wlodek ME, Geddes DT. Human milk sampling protocols affect estimation of infant lipid intake. J Nutr. (2020) 150:2924–30. doi: 10.1093/jn/nxaa246
48. Kent JC, Mitoulas LR, Cregan MD, Ramsay DT, Doherty DA, Hartmann PE. Volume and frequency of breastfeedings and fat content of breast milk throughout the day. Pediatrics. (2006) 117:e387–95. doi: 10.1542/peds.2005-1417
49. Moran-Lev H, Mimouni FB, Ovental A, Mangel L, Mandel D, Lubetzky R. Circadian macronutrients variations over the first 7 weeks of human milk feeding of preterm infants. Breastfeed Med. (2015) 10:366–70. doi: 10.1089/bfm.2015.0053
50. Khan S, Hepworth AR, Prime DK, Lai CT, Trengove NJ, Hartmann PE. Variation in fat, lactose, and protein composition in breast milk over 24 hours: associations with infant feeding patterns. J Hum Lact. (2013) 29:81–9. doi: 10.1177/0890334412448841
Keywords: human milk, human milk analysis, individualized fortification, nutrition, neonatal ICU
Citation: Ramey SR, Merlino Barr S, Moore KA and Groh-Wargo S (2021) Exploring Innovations in Human Milk Analysis in the Neonatal Intensive Care Unit: A Survey of the United States. Front. Nutr. 8:692600. doi: 10.3389/fnut.2021.692600
Received: 08 April 2021; Accepted: 30 June 2021;
Published: 03 September 2021.
Edited by:
Nadja Haiden, Medical University of Vienna, AustriaReviewed by:
Niels Rochow, Paracelsus Medical Private University, GermanyEkhard E. Ziegler, The University of Iowa, United States
Copyright © 2021 Ramey, Merlino Barr, Moore and Groh-Wargo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stacey R. Ramey, c3JyYW1leTE1QGdtYWlsLmNvbQ==
 Stacey R. Ramey
Stacey R. Ramey Stephanie Merlino Barr
Stephanie Merlino Barr Katie A. Moore2
Katie A. Moore2 Sharon Groh-Wargo
Sharon Groh-Wargo