- 1Colorectal Cancer Center and Department of Gastrointestinal Surgery, Shanghai Tenth People's Hospital, Tongji University School of Medicine, Shanghai, China
- 2Departments of Gastrointestinal Surgery, Beijing Shijitan Hospital, Capital Medical University, Beijing, China
- 3Department of Clinical Nutrition, Beijing Shijitan Hospital, Capital Medical University, Beijing, China
- 4The Radiology Imaging Center, The First Affiliated Hospital, Wenzhou Medical University, Wenzhou, China
- 5Department of Gastrointestinal Surgery, The First Affiliated Hospital, Wenzhou Medical University, Wenzhou, China
- 6Department of Gastrointestinal Surgery, The Second Affiliated Hospital, Wenzhou Medical University, Wenzhou, China
Background and Aims: Sarcopenia is negatively associated with clinical outcomes. However, the definitions of sarcopenia are inconsistent across international consensuses. Thus, the purpose of this study is to compare the impact of the basic definition components of sarcopenia and their combinations in post-operative complications and overall survival, aiming to find the best sarcopenia definition to stratify the prognosis in an Asian population.
Methods: A total of 1,307 patients who underwent curative surgery for gastric cancer from July 2014 to May 2019 were prospectively included. The basic sarcopenia components were measured pre-operatively, including low skeletal muscle mass index (LSMI), low skeletal muscle radiodensity (LSMD), low handgrip strength (LHGS), and low gait speed (LGS). Among them, LSMI and LSMD were measured using a CT post-processing software, LHGS was measured using an electronic hand dynamometer, and LGS was represented by a 6-m walk speed.
Results: For the single basic component, the muscle function parameters (LHGS or LGS) but not the muscle composition parameters (LSMI or LSMD) showed associations with post-operative complications and mortality. For the combination of the basic combinations, all statistically significant combinations included at least one muscle function parameter. The combination of muscle composition (LSMI or LSMD) and muscle function (LHGS or LGS) had a significantly higher area under the curve in the prediction of post-operative complications compared with the combinations of two muscle function parameters (LSMI plus LSMD) or two muscle composition parameters (LHGS plus LGS).
Conclusions: Compared with muscle composition parameters (LSMI and LSMD), muscle function parameters (LHGS and LGS) are better predictors of post-operative complications and overall survival, which should be considered as the principal determinant in the sarcopenia definition. The definition of sarcopenia consists of muscle function (LHGS or LGS) and muscle composition (LSMI or LSMD) separately, which is better than the combination of the two muscle function parameters (LHGS plus LGS) or two muscle composition parameters (LSMI plus LSMD).
Introduction
Gastric cancer is the sixth most common cancer and the third leading cause of cancer-related deaths worldwide. It is often diagnosed at an advanced stage and has a low survival rate (1). Despite significant improvements in treatment in recent years, the prognosis of gastric cancer remains poor. Patients with gastric cancer often experience appetite loss, diminished food intake, and a loss of muscle mass (2, 3). Sarcopenia severely influences patients with gastric cancer and is shown to be associated with disability, reduced therapy intolerance, decreased response to cancer therapy, increased post-operative complications, poor quality of life, and a shorter duration of survival (4–7).
Sarcopenia originally referred to the loss of muscle mass but is now considered a muscle disease characterized by several features, including altered muscle composition and the decline of muscle function. However, there is an ongoing debate about the best approach to define sarcopenia due to the different combinations of basic definition components. The European Working Group on Sarcopenia in Older People (EWGSOP) put forward the first practical diagnostic criteria for sarcopenia in 2010 (8), in which sarcopenia was determined by low muscle mass accompanied by low muscle strength or low physical performance. Subsequently published guidelines have proposed similar definitions, with low muscle mass as the prerequisite (9–12).
Although a loss of muscle mass and a loss of muscle function were frequently correlated, the loss of muscle function was often more predominant than that of muscle mass (13). An increasing number of studies in recent years have shown that handgrip strength and gait speed are strong predictors of adverse clinical outcomes (14–16). Moreover, muscle quality, such as muscle radiodensity, is emerging as a new indicator for muscle composition and shows a significant association with poor clinical outcomes (17, 18). Thus, there have been heated arguments regarding the principal determinant in defining sarcopenia. In 2019, EWGSOP updated its original definition (EWGSOP2) (19), with low muscle strength replacing the role of muscle mass as the principal determinant. According to EWGSOP2, patients were considered to have probable sarcopenia when low muscle strength was detected, and the diagnosis was further confirmed by the presence of low muscle quantity or quality.
Inconsistent with EWGSOP2, the Asian Working Group for Sarcopenia 2019 (AWGS2019) retained its previous definition of sarcopenia (12) and adopted wider ranges of cut-off values for low handgrip strength and low physical performance in the updated 2019 consensus (20), which added more confusion to the clinical application of sarcopenia diagnosis due to the inconsistency between EWGSOP2 and AWGS2019. Up until now, the basic components of sarcopenia definition generally consist of two groups and four sub-groups across different consensuses, namely, muscle composition (low muscle quantity and low muscle quality) and muscle function (low muscle strength and low physical performance). However, to date, there have been no studies investigating the various impacts of different combinations of these components on post-operative outcomes and mortality in patients with gastric cancer.
The purpose of this study is to investigate the impacts of the low skeletal muscle mass index (LSMI), low skeletal muscle radiodensity (LSMD), low handgrip strength (LHGS), low gait speed (LGS), and their combinations on clinical outcomes, to determine the best sarcopenia definition to stratify the risk of post-operative complications and mortality in patients with gastric cancer.
Materials and Methods
Patients
Patients who underwent surgical resection with curative intent for gastric cancer at the First Affiliated Hospital of Wenzhou Medical University were prospectively enrolled in this study. The inclusion criteria were: (1) at least 18 years of age; (2) had a histologically confirmed gastric adenocarcinoma; (3) planned to receive elective curative gastric surgery; (4) had abdominal CT scans within 1 month before surgery in our hospital. The exclusion criteria were as follows: (1) had a history of cancer; (2) had a local recurrence or distant metastasis of gastric cancer; (3) was unable to undergo functional assessments due to physical or mental causes; (4) data on muscle mass and muscle quality were unavailable due to unqualified CT images. Informed consent had been signed by all participants. All the patients signed informed consent after being informed that their clinical information will be used anonymously for research. This study was approved by the ethics committees of The First Affiliated Hospital of Wenzhou Medical University and all procedures followed were in accordance with the Helsinki Declaration of 1964 and later versions.
Assessments of Muscle Quantity and Quality
One of the gold standard methods in detecting body composition and abnormal body composition phenotypes is a CT assessment (19, 21). Both CT-derived total abdominal muscle areas and mean skeletal muscle radiodensity (SMD) were used to represent muscle quantity and quality according to EWGSOP2 and AWGS2019. The cross-sectional CT image at the third lumbar vertebra (L3) level was selected and a Hounsfield unit (HU) threshold of −29 to +150 was used to distinguish the muscle from other nearby tissues (22). To minimize measurement bias, a trained investigator (FMZ) identified the muscle, and the areas and mean SD were calculated automatically using a CT post-processing software (GE ADW 4.5). The muscle areas were divided by the square of the height to obtain the skeletal muscle mass index (SMI) (cm2/m2). Low muscle mass as represented by the LSMI was defined as <40.8 cm2/m2 for males and <34.9 cm2/m2 for females (23). Low muscle quality as represented by LSMD was defined as <38.5 HU for males and <8.6 HU for females (24).
Assessments of Muscle Strength and Physical Performance
Handgrip strength and gait speed (GS) were used to represent muscle strength and physical performance. Handgrip strength (HGS) was measured on the dominant hand with an electronic hand dynamometer (EH101; Camry, Guangdong Province, China). The patients were seated comfortably with their shoulder adducted and neutrally rotated, their elbow flexed at 90°, and the forearm and wrist in a neutral position, and then asked to squeeze the dynamometer in their dominant hand with full force (25). According to the AWGS2019, LHGS (A-LHGS) was defined as <28 kg for males and <18 kg for females; according to the EWGSOP2, LHGS (E-LHGS) was defined as <27 kg for males and <16 kg for females.
Physical performance was assessed by the usual GS on a 6-m course (26). The patients started to walk at a normal speed under the command of an examiner. The time was recorded between the first footfall and the first foot crossing the 6-m end line. According to the AWGS2019, LGS (A-LGS) was defined as <1 m/s; according to the EWGSOP2, LGS (E-LGS) was defined as ≤ 0.8 m/s.
The HGS and GS were assessed by trained investigators (SLW and ZLS) once the patients were hospitalized, and the maximal value of the HGS and GS were recorded in three consecutive tests.
Diagnosis of Sarcopenia
According to the AWGS2019, sarcopenia was defined as low muscle mass plus low muscle strength and/or low physical performance (20). According to the EWGSOP2, sarcopenia was defined as low muscle strength plus low muscle quantity and/or low muscle quality (19).
Data Collection
Clinical data were collected prospectively and maintained in a digital database. For each patient, the data were collected by trained surgeons (FMZ, SLW, and ZLS), and discrepancies were solved by referring to an adjudicator (CLZ). The following data were collected: age, gender, BMI, smoking history, alcohol drinking history, reduced food intake (<50% of energy requirements >1 week, or any reduction for >2 weeks), weight loss (>5% within the past 6 months or >10% beyond 6 months), nutritional risk screening (NRS) 2002, Charlson comorbidity index (CCI) score, American Society of Anesthesiologists (ASA) score, hemoglobin concentration (anemia was defined as hemoglobin concentration <120 g/L for males and <110 g/L for females), albumin concentration (hypoalbuminemia was defined as albumin concentration <35 g/L), tumor-nodule-metastasis (TNM) stage, laparoscopic surgery, post-operative hospital stay, cost, and 30-day post-operative complications classified as Grade II or above according to the Claviene–Dindo classification (27).
Follow-Up
All the patients received regular telephone interviews or outpatient reviews after surgery. A follow-up was conducted every 3 months for the first 2 years after surgery and once every 6 months thereafter. The content of the follow-up included post-operative life, physical examination, image logical examinations, endoscopy, and laboratory tests. Overall survival (OS) was calculated from the time of surgery to the time of death or the last follow-up. The latest follow-up date was February 2020.
Statistical Analysis
The categorical data were represented as counts with percentages and compared using a Pearson's chi-square test or Fisher's exact test. The continuous data were represented as mean with an SD or median with an interquartile range (IQR) and compared using Student's t-test or Mann–Whitney U-test. Sarcopenia definition has four basic components, namely, LSMI, LSMD, LHGS, and LGS; however, a sarcopenia definition that consists of three and more (up to four) basic components is not practical as the incidence of sarcopenia by this definition would be very low, which is inconsistent with reality. Logistic regression analyses were used to investigate the association of the single component of sarcopenia definition (LSMI, LSMD, LHGS, and LGS) and their pairwise combinations (LSMI plus LSMD, LSMI plus LHGS, LSMI plus LGS, LSMD plus LHGS, LSMD plus LGS, and LHGS plus LGS) with 30-day post-operative complications. The Cox proportional hazard regression model was used to investigate the association of those components and their combinations with mortality. To avoid multicollinearity, the basic sarcopenia components and their combinations were included separately in the multifactor analysis model. The proportional hazards assumption was checked for all variables using Kaplan–Meier curves or Schoenfeld residual plots. To further reduce the interference of confounding factors and to verify the stability of the results, a total of three incremental models with increasing numbers of varieties were created to investigate the impact of the incremental adjustment. Model 1 was unadjusted. Model 2 was adjusted for age and gender. Model 3 was adjusted for Model 2 plus smoking history, alcohol drinking history, BMI, reduced food intake, weight loss, NRS 2002, CCI score, ASA score, anemia, hypoalbuminemia, TNM stage, and laparoscopic surgery. All analyses were performed with SPSS statistics version 23 (IBM, Armonk, NY, USA).
Results
Characteristics of the Patients
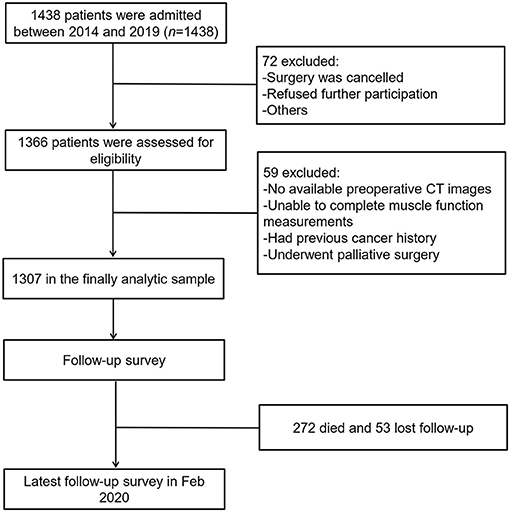
From July 2014 to May 2019, a total of 1,366 patients were enrolled in our study. Fifty-nine patients who did not meet the inclusion criteria were excluded, and 1,307 cases were analyzed. The process of patient selection is shown in Figure 1, and the different combinations of basic sarcopenia definitions are shown in Supplementary Figure 1. Of the 1,307 patients who underwent sarcopenia assessments, there were 409 with LSMI, 579 with LSMD, 480 with A-LHGS, 625 with A-LGS, 402 with E-LHGS, and 254 with E-LGS. The different combinations of the basic components of sarcopenia definition resulted in different population sizes, ranging from 127 to 334 cases (Supplementary Figure 1). Finally, 298 and 287 cases were diagnosed as AWGS2019-sarcopenia and EWGSOP2-sarcopenia, respectively.
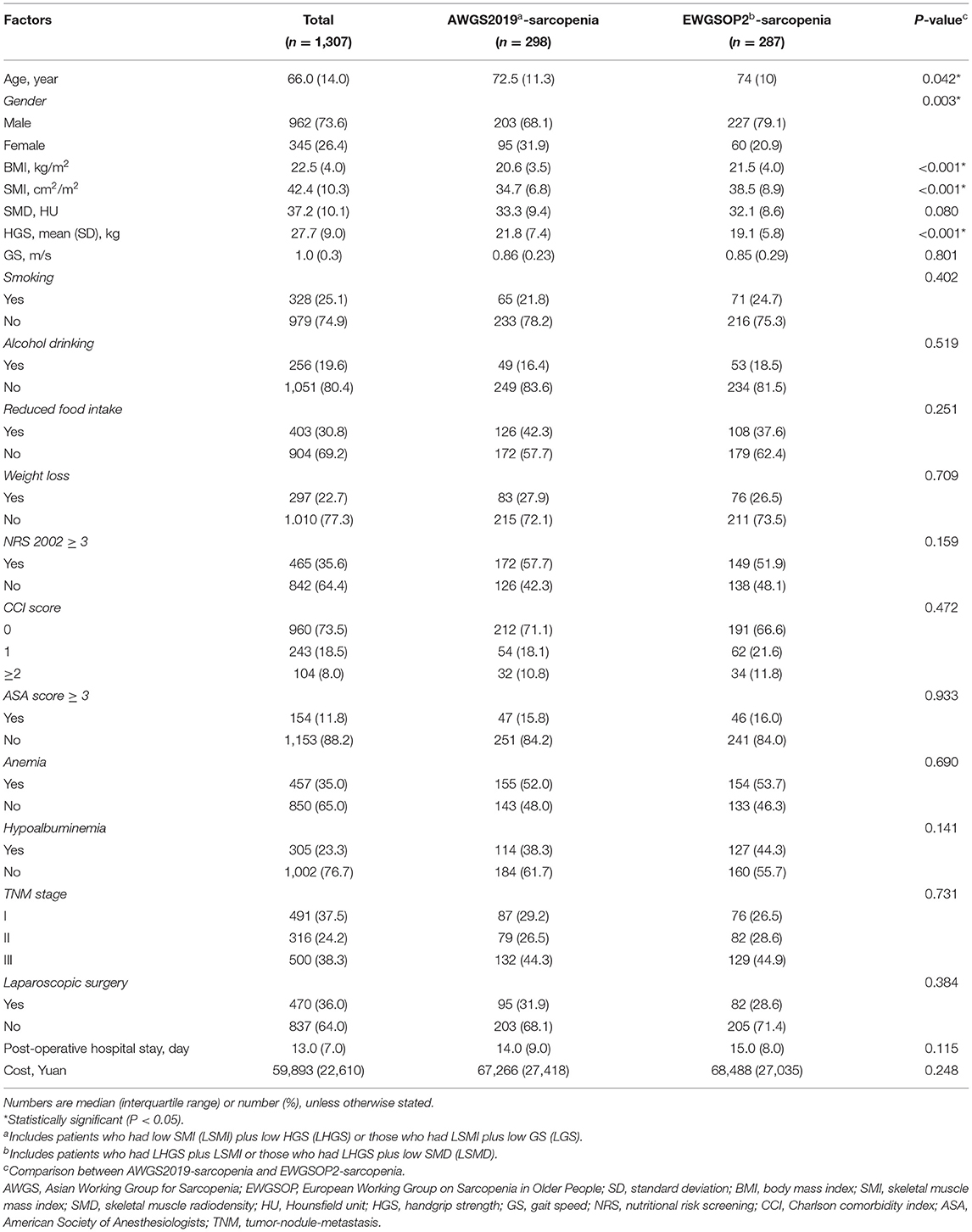
Baseline characteristics are shown in Table 1. The median age was 66 years, and 73.6% of patients were males. Compared with the total cohort, the patients with sarcopenia tended to be older, have lower BMI, SMI, SMD, HGS, and GS, and have increased hospital stay and cost. Compared with AWGS2019-sarcopenia, the patients with EWGSOP2-sarcopenia were older, more likely to be male, and had higher BMI and SMI but lower HGS. The other clinical characteristics were similar between AWGS2019 and EWGSOP2.
Impacts of the Basic Components of Sarcopenia Definition on the Post-operative Complication and Mortality
The odds ratio (OR) and hazard ratio (HR) of post-operative complications and mortality for the different basic components are shown in Figure 2, with the corresponding estimates presented in Supplementary Table 1. The incidence of post-operative complications was 21.7% (284/1,307) in the total cohort. In the final model, the muscle composition parameters including LSMI and LSMD were not associated with post-operative complications nor mortality. In contrast, A-LHGS (OR = 1.481, 95% CI = 1.092–2.007, P = 0.011) and E-LHGS (OR = 1.606, 95% CI = 1.177–2.191, P = 0.003) were significantly associated with post-operative complications. The E-LGS (HR = 1.582, 95% CI = 1.169–2.142, P = 0.003) was significantly associated with mortality.
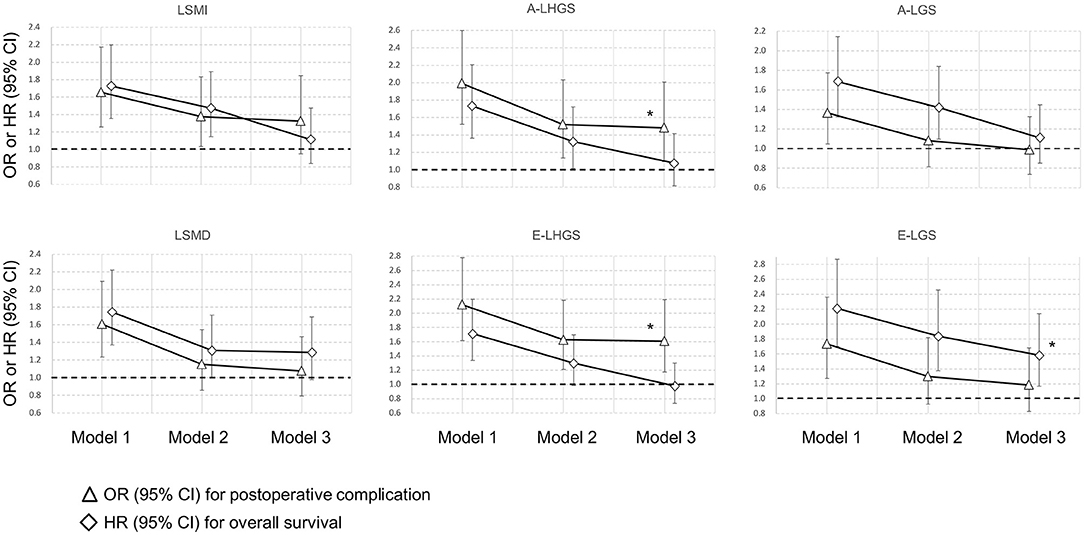
Figure 2. Association of basic sarcopenia components with clinical outcomes. *Statistically significant. A, Asian Working Group for Sarcopenia; E, European Working Group on Sarcopenia in Older People; LSMI, low skeletal muscle mass index; LSMD, low skeletal muscle radiodensity; LHGS, low handgrip strength; LGS, low gait speed.
Considering the significant and distinct impacts of E-LHGS and E-LGS on post-operative complications and mortality, we compared the SMI and SMD between E-LHGS and E-LGS (Supplementary Table 2). We found that the patients with E-LGS had significantly lower SMI (39 vs. 40.5 cm2/m2, P = 0.014) and SMD (32.5 vs. 35.0 HU, P < 0.001) compared with E-LHGS.
Impacts of Different Pairwise Combinations of Basic Components on the Post-operative Complication and Mortality
The OR and HR values with statistical significance are ranked in Figure 3, with the corresponding estimates presented in Supplementary Table 3. Whether for post-operative complications or mortality, all statistically significant combinations included at least one muscle function parameter, and the strongest combinations were those that consisted of both muscle function and muscle composition. For post-operative complications, the strongest combination was E-LHGS plus LSMI (OR = 1.659, 95% CI = 1.118–2.463, P = 0.012). For mortality, the strongest combination was E-LGS plus LSMD (HR = 1.6, 95% CI = 1.155–2.217, P = 0.005). The combination of LSMI plus LSMD was neither associated with post-operative complication nor mortality.
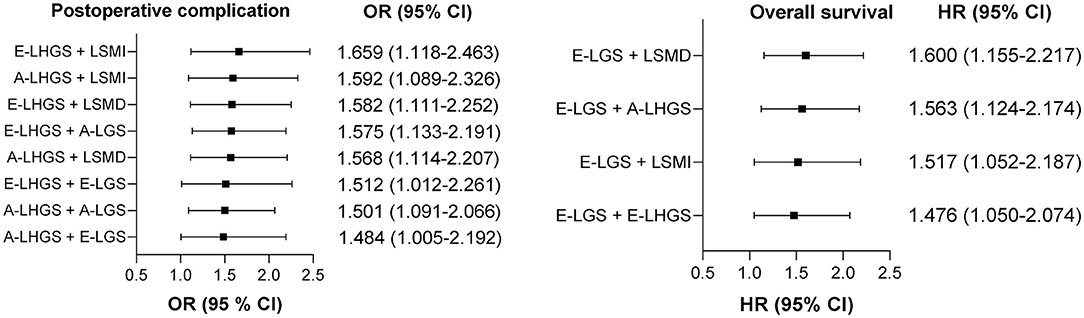
Figure 3. Ranking of the relationship strength of the associations between different pairwise combinations of basic components and clinical outcomes. A, Asian Working Group for Sarcopenia; E, European Working Group on Sarcopenia in Older People; LSMI, low skeletal muscle mass index; LSMD, low skeletal muscle radiodensity; LHGS, low handgrip strength; LGS, low gait speed.
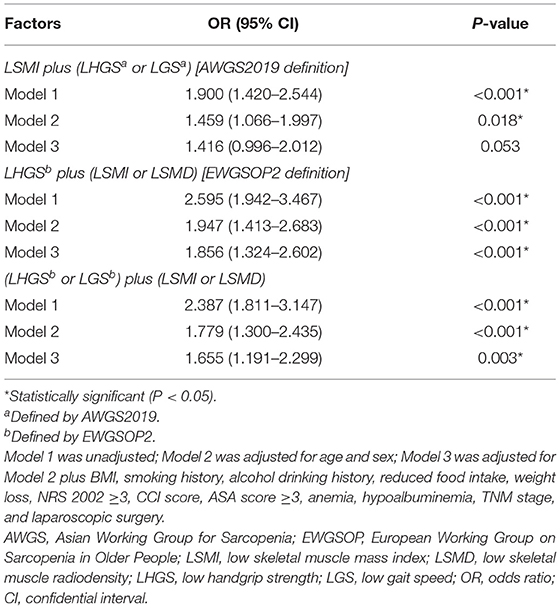
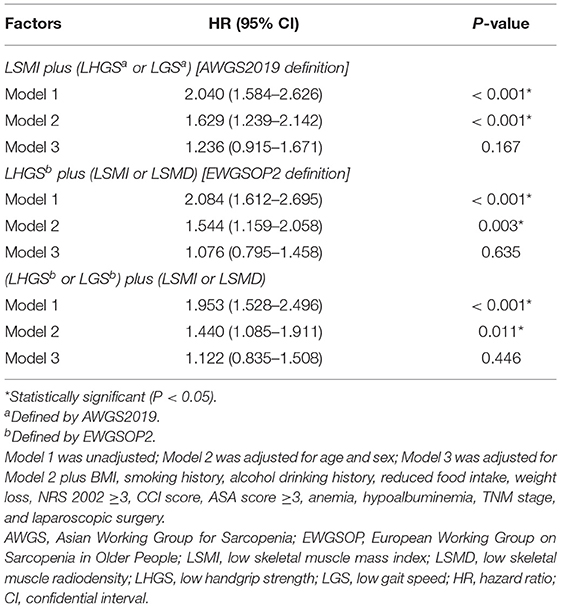
Given the important role of muscle function, we proposed an alternative sarcopenia definition, which was defined as the presence of E-LHGS or E-LGS plus LSMI or LSMD. For post-operative complications (Table 2), the EWGSOP2-sarcopenia (OR = 1.856, 95% CI = 1.324–2.602, P < 0.001) and the new-definition sarcopenia (OR = 1.655, 95% CI = 1.191–2.299, P = 0.003) showed statistical significance in the final model. However, none of the definitions were associated with mortality in the multivariate analysis (Table 3).
Additionally, to examine the impacts of these pairwise combinations on predicting post-operative complications and mortality, a receiver operating characteristic (ROC) curve was performed (Figure 4) with the corresponding estimates presented in Supplementary Tables 4, 5. The combination of muscle function (LSMI or LSMD) plus muscle composition (LHGS or LGS) had a significantly higher area under the curve [0.598, 95% CI = 0.57–0.626)] in the prediction of post-operative complications compared with the combinations of the two muscle function parameters (LSMI plus LSMD) [0.546, 95% CI = 0.517–0.575)] or two muscle composition parameters (LHGS plus LGS) [0.553, 95% CI = 0.524–0.581)]. These three types of combinations had no statistical difference in predicting mortality, but the combination of muscle composition plus muscle function showed a trend of a higher area under the ROC curve (AUC) in a longer follow-up period.
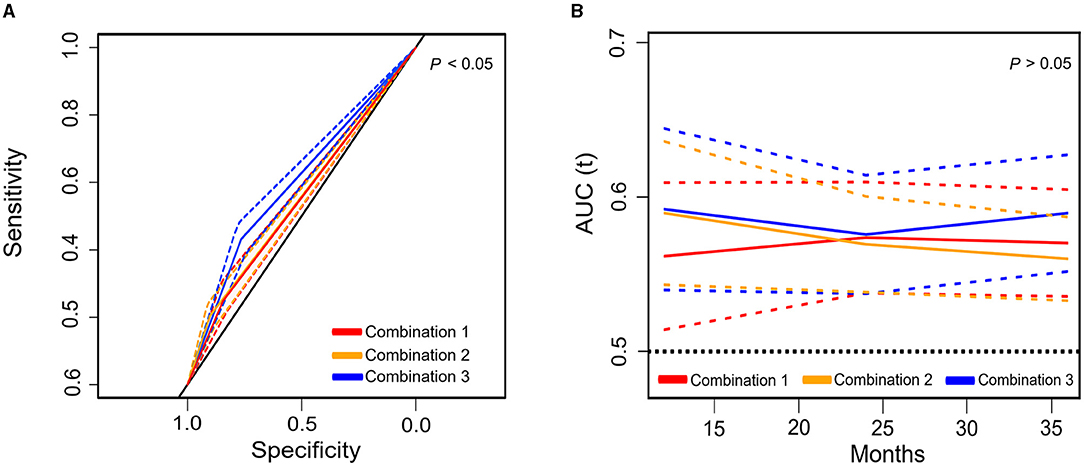
Figure 4. The area under the receiver operating characteristic (ROC) curve (solid line) and 95% CI (dotted line) of different pairwise combinations of basic components for post-operative complications (A) and mortality (B). Combination 1: LSMD plus LSMI; Combination 2: E-LHGS plus E-LGS; Combination 3: (E-LHGS or E-LGS) plus (LSMD or LSMI); E, European Working Group on Sarcopenia in Older People; ROC, receiver operating characteristic curve; LSMI, low skeletal muscle mass index; LSMD, low skeletal muscle radiodensity; LHGS, low handgrip strength; LGS, low gait speed; CI, confidential interval.
Discussion
In the current study, we demonstrated that the muscle function measured as LHGS and LGS was a better predictor of post-operative complications and mortality than the muscle composition measured as LSMI and LSMD. Additionally, we found that LHGS is a strong predictor of post-operative complications, while LGS is a strong predictor of mortality. Moreover, the sarcopenia definition consisting of both muscle function parameters (LHGS or LGS) and muscle composition parameters (LSMI or LSMD) had stronger impacts on the clinical outcomes when compared to the combinations of two muscle function parameters (LHGS plus LGS) or two muscle composition parameters (LSMI plus LSMD). These findings suggest that muscle function should be considered as the principal determinant in the diagnosis of sarcopenia, and muscle composition is also necessary for the diagnosis.
Previous studies have examined the association of muscle function and muscle composition with clinical outcomes. Sato et al. showed that pre-operative LHGS but not low lean body mass was significantly associated with grade 2 or higher morbidities in patients who underwent curative surgery for gastric cancer (28). A large, multiethnic, national study showed that LGS defined by <0.8m/s had a stronger association with death in the elderly compared with low lean mass (29). In our study, we obtained similar results, in which muscle function was found to be a better indicator as compared with muscle composition to predict post-operative complications and mortality. Notably, LHGS and LGS had different impacts on clinical outcomes; LHGS was strongly associated with post-operative complications, while LGS was strongly associated with mortality.
With respect to the different impacts of LHGS and LGS on clinical outcomes, Revenig et al. found that LHGS but not LGS showed a significant association with short-term morbidity and mortality in patients who underwent major abdominal operations (30). On the other hand, the HUNT II study reported no association of the different tertiles of HGS with mortality (31). An observational study conducted on more than 500,000 participants found that slow walking speed but not weak handgrip strength was associated with an increased risk of mortality in the low-BMI subset (15). A recent study also reported that it was LGS but not LHGS that showed significant association with mortality (32).
Although the exact reason for the different impacts of LHGS and LGS is unclear, this phenomenon may be partly explained in two ways. First, HGS as a form of explosive isometric force is significantly associated with the capacity of substrate reservation and utilization (33), systemic inflammation (34), and abnormal metabolism (35). Patients with LHGS are more likely to have increased complications due to their poor adaptability to surgical strikes. Second, LGS accounts for the 70% increase in the disability of cancer patients (32). The loss of mobility is likely to lead to the vicious cycle of decreased physical activity and contributes directly to a higher risk of mortality (26). Our analysis revealed that patients with LGS had worse muscle conditions than those with LHGS, which justified the hypothesis that the patients with LGS had worse survival due to their poor physical fitness. In the EWGSOP2, gait speed only serves as a severity indicator of sarcopenia diagnosis. In our study, LGS defined by the EWGSOP2 (<0.8 m/s), but not AWGS2019 (<1 m/s), and was found to be an independent risk factor for mortality. Our study indicated that LGS alone and its combination with muscle composition (LSMI or LSMD) were strong predictors of adverse clinical outcomes, which should also be considered as the prerequisite of sarcopenia definition like LHGS.
Few studies explored different combinations of the basic components of sarcopenia definition. Rodrigues et al. found that LSMI plus LSMD had the strongest association with 1-year mortality compared with LSMI alone or LSMD alone (36). However, their study did not adjust the nutrition-related variables in the analysis model, such as reduced food intake, weight loss, NRS 2002, anemia, and hypoalbuminemia. In our study, LSMI plus LSMD showed a higher risk of mortality than LSMI alone or LSMD alone in the unadjusted model, and the model adjusted for sex and age. However, the association attenuated to insignificance in the final model, which included more nutritional parameters.
Although LSMI or LSMD alone were not related to clinical outcomes, we found that their combinations with LHGS and LGS improved the predictive effect of LHGS alone or LGS alone in post-operative complications and mortality. In concordance with our study, Gan et al. found that the risk of non-alcoholic fatty liver disease increased when low muscle mass and LHGS were simultaneously detected compared with only one of them detected (37). Furthermore, Maurício et al. demonstrated that low muscle mass in combination with low muscle strength instead of other nutritional parameters had the strongest association with complications in patients with colorectal cancer (38).
However, the existing evidence is scattered. There is a paucity of previous studies to systematically compare the impacts of different combinations of the basic components of sarcopenia definition on clinical outcomes in one cohort. No study has yet discussed the impacts of LSMD plus LHGS, LSMD plus LGS, and LHGS plus LGS on clinical outcomes. Our findings extended the previous evidence by reporting that muscle function should be considered as the principal determinant in the diagnosis of sarcopenia. Sarcopenia defined by both muscle function and muscle composition had a stronger impact on the clinical outcomes when compared with that of muscle function alone or muscle composition alone. Our results indicated that the combination of muscle function plus muscle composition had the best ability to predict post-operative complications and a trend of higher mortality prediction ability in a longer follow-up period when compared with the combination of muscle composition plus muscle composition or muscle function plus muscle function.
Our results are supported by interventional research. With the development of perioperative management, pre-operative functional intervention gained increasing attention in the team-based approach and the enhanced recovery after surgery pathway. Pre-habilitation combining endurance and resistance training has been shown to improve physical capacity and muscle strength and decrease post-operative complications (39–41). Patients who do more exercise were observed to have a lower risk of mortality and recurrence (42). The associations of LHGS and LGS with clinical outcomes reported in our study emphasize the importance of pre-habilitation and rehabilitation in patients with gastric cancer.
The present study has some potential limitations. First, the observational design of our study does not allow us to draw firm conclusions on the causal role of muscle function and muscle composition in post-operative complications or mortality. However, data were prospectively collected to minimize the recall bias and many potential confounding factors were adjusted, including BMI, reduced food intake, and weight loss, which are strong predictors of clinical outcomes in patients who underwent abdominal operations (43). Second, the cut-off values for LSMI and LSMD were obtained from our previous large-scale studies (23, 24) due to the lack of a unified standard on the cut-off values for CT-assessed LSMI and LSMD. The existing cut-off values were mainly derived from populations that were not Chinese (44, 45). We believe that using cut-off values from Chinese-specific large sample studies can yield more accurate results under the consideration of the race differences between Chinese and other populations. Third, the analysis of this study was conducted in patients with gastric cancer, which may limit the generalization of the conclusion. Fourth, we were unable to calculate the sensitivity and specificity of our definitions in the present study because sarcopenia currently lacks a gold standard.
In conclusion, this study found that muscle function has stronger impacts on clinical outcomes compared with muscle composition. Low handgrip strength is a strong predictor of post-operative complications, and LGS is a strong predictor of mortality. The sarcopenia definition that consists of both muscle function and muscle composition showed the strongest impacts on clinical outcomes. These findings suggested that the definition of sarcopenia should be constructed using muscle function as the principal determinant and that this should be used together with muscle composition.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee for Clinical Research of the First Affiliated Hospital of Wenzhou Medical University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
C-LZ and ZY designed the study. ZZ provided technical support. F-MZ, S-LW, and Z-LS collected the data. X-ZZ, ZY, X-LC, and XS did the analysis and interpretation of data. F-MZ and H-PS wrote the article. X-ZZ and ZY revised the article and took the decision to submit the article for publication. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the National Natural Science Foundation of China (Nos. 81800795 and 81770884), the Shanghai Municipal Commission of Health and Family Planning (No. 20184Y0301), and the Key Technology of Palliative Care and Nursing for Cancer Patients (2017YFC1309200).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2021.709211/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality Worldwide for 36 cancers in 185 countries. Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Zhang Y, Wang J, Wang X, Gao T, Tian H, Zhou D, et al. The autophagic-lysosomal and ubiquitin proteasome systems are simultaneously activated in the skeletal muscle of gastric cancer patients with cachexia. Am J Clin Nutr. (2020) 111:570–9. doi: 10.1093/ajcn/nqz347
3. Chau I, Fuchs CS, Ohtsu A, Barzi A, Liepa AM, Cui ZL, et al. Association of quality of life with disease characteristics and treatment outcomes in patients with advanced gastric cancer: exploratory analysis of RAINBOW and REGARD phase III trials. Eur J Cancer. (2019) 107:115–23. doi: 10.1016/j.ejca.2018.11.013
4. Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. (2019) 393:2636–46. doi: 10.1016/S0140-6736(19)31138-9
5. Gourd E. Sarcopenia and adiposity linked to overall survival. Lancet Oncol. (2018) 19:e239. doi: 10.1016/S1470-2045(18)30284-5
6. Sandini M, Patino M, Ferrone CR, Alvarez-Pérez CA, Honselmann KC, Paiella S, et al. Association between changes in body composition and neoadjuvant treatment for pancreatic cancer. JAMA Surg. (2018) 153:809–15. doi: 10.1001/jamasurg.2018.0979
7. Caan BJ, Feliciano EMC, Prado CM, Alexeeff S, Kroenke CH, Bradshaw P, et al. Association of muscle and adiposity measured by computed tomography with survival in patients with nonmetastatic breast cancer. JAMA Oncol. (2018) 4:798–804. doi: 10.1001/jamaoncol.2018.0137
8. Cruzjentoft AJ, Baeyens J, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis Report of the European Working Group on Sarcopenia in Older People. Age Ageing. (2010) 39:412–23. doi: 10.1093/ageing/afq034
9. Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. (2011) 12:249–56. doi: 10.1016/j.jamda.2011.01.003
10. Morley JE, Abbatecola AM, Argiles JM, Baracos VE, Bauer JM, Bhasin S, et al. Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc. (2011) 12:403–9. doi: 10.1016/j.jamda.2011.04.014
11. Studenski SA, Peters KW, Alley DE, Cawthon PM, Mclean RR, Harris TB, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, final estimates. J Gerontol A Biol Sci Med Sci. (2014) 69:547–58. doi: 10.1093/gerona/glu010
12. Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. (2014) 15:95–101. doi: 10.1016/j.jamda.2013.11.025
13. Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquezmieyer P, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. (2009) 90:1579–85. doi: 10.3945/ajcn.2009.28047
14. Celis-Morales CA, Welsh P, Lyall DM, Steell L, Petermann F, Anderson J, et al. Associations of grip strength with cardiovascular, respiratory, and cancer outcomes and all cause mortality: prospective cohort study of half a million UK Biobank participants. BMJ. (2018) 361:k1651. doi: 10.1136/bmj.k1651
15. Yates T, Zaccardi F, Dhalwani NN, Davies MJ, Bakrania K, Celis-Morales CA, et al. Association of walking pace and handgrip strength with all-cause, cardiovascular, and cancer mortality: a UK Biobank observational study. Eur Heart J. (2017) 38:3232–40. doi: 10.1093/eurheartj/ehx449
16. Liu MA, DuMontier C, Murillo A, Hshieh TT, Bean JF, Soiffer RJ, et al. Gait speed, grip strength, and clinical outcomes in older patients with hematologic malignancies. Blood. (2019) 134:374–82. doi: 10.1182/blood.2019000758
17. Brown JC, Caan BJ, Prado CM, Weltzien E, Xiao J, Feliciano EMC, et al. Body composition and cardiovascular events in patients with colorectal cancer: a population-based retrospective cohort study. JAMA Oncol. (2019) 5:967–72. doi: 10.1001/jamaoncol.2019.0695
18. Martin L, Birdsell L, MacDonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. (2013) 31:1539–47. doi: 10.1200/JCO.2012.45.2722
19. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:16–31. doi: 10.1093/ageing/afy169
20. Chen L-K, Woo J, Assantachai P, Auyeung T-W, Chou M-Y, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. (2020) 21:300–7.e2. doi: 10.1016/j.jamda.2019.12.012
21. Cederholm T, Barazzoni R, Austin P, Ballmer P, Biolo G, Bischoff SC, et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr. (2017) 36:49–64. doi: 10.1016/j.clnu.2016.09.004
22. Mitsiopoulos N, Baumgartner R, Heymsfield S, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. (1998) 85:115–22. doi: 10.1152/jappl.1998.85.1.115
23. Zhuang C-L, Huang D-D, Pang W-Y, Zhou C-J, Wang S-L, Lou N, et al. Sarcopenia is an independent predictor of severe postoperative complications and long-term survival after radical gastrectomy for gastric cancer: analysis from a large-scale cohort. Medicine. (2016) 95:e22345. doi: 10.1097/MD.0000000000003164
24. Zhuang C-L, Shen X, Huang Y-Y, Zhang F-M, Chen X-Y, Ma L-L, et al. Myosteatosis predicts prognosis after radical gastrectomy for gastric cancer: a propensity score–matched analysis from a large-scale cohort. Surgery. (2019) 166:297–304. doi: 10.1016/j.surg.2019.03.020
25. Fess EE. Grip strength. In: Casanova JS, editor. Clinical Assessment Recommendations. 2nd edn. Chicago, IL: American Society of Hand Therapists (1992), p. 41–45.
26. Cesari M, Kritchevsky SB, Newman AB, Simonsick EM, Harris TB, Penninx BW, et al. Added value of physical performance measures in predicting adverse health-related events: results from the Health, Aging And Body Composition Study. J Am Geriatr Soc. (2009) 57:251–9. doi: 10.1111/j.1532-5415.2008.02126.x
27. Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. (2004) 240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae
28. Sato T, Aoyama T, Hayashi T, Segami K, Kawabe T, Fujikawa H, et al. Impact of preoperative hand grip strength on morbidity following gastric cancer surgery. Gastric Cancer. (2016) 19:1008–15. doi: 10.1007/s10120-015-0554-4
29. Cheung C-L, Lam KS, Cheung BM. Evaluation of cutpoints for low lean mass and slow gait speed in predicting death in the National Health and Nutrition Examination Survey 1999-2004. J Gerontol A Biol Sci Med. (2016) 71:90–5. doi: 10.1093/gerona/glv112
30. Revenig LM, Canter DJ, Kim S, Liu Y, Sweeney JF, Sarmiento JM, et al. Report of a simplified frailty score predictive of short-term postoperative morbidity and mortality. J Am Coll Surg. (2015) 220:904–11.e1. doi: 10.1016/j.jamcollsurg.2015.01.053
31. Karlsen T, Nauman J, Dalen H, Langhammer A, Wisløff U. The combined association of skeletal muscle strength and physical activity on mortality in older women: the HUNT2 study. Mayo Clin Proc. (2017) 92:710–8. doi: 10.1016/j.mayocp.2017.01.023
32. Williams GR, Chen Y, Kenzik KM, McDonald A, Shachar SS, Klepin HD, et al. Assessment of sarcopenia measures, survival, and disability in older adults before and after diagnosis with cancer. JAMA Netw Open. (2020) 3:e204783. doi: 10.1001/jamanetworkopen.2020.4783
33. Han Y-S, Proctor DN, Geiger PC, Sieck GC. Reserve capacity for ATP consumption during isometric contraction in human skeletal muscle fibers. J Appl Physiol. (2001) 90:657–64. doi: 10.1152/jappl.2001.90.2.657
34. Norman K, Stobäus N, Kulka K, Schulzke J. Effect of inflammation on handgrip strength in the non-critically ill is independent from age, gender and body composition. Eur J Clin Nutr. (2014) 68:155–8. doi: 10.1038/ejcn.2013.261
35. Wan J-j, Qin Z, Wang P-y, Sun Y, Liu X. Muscle fatigue: general understanding and treatment. Exp Mol Med. (2017) 49:e384. doi: 10.1038/emm.2017.194
36. Rodrigues CS, Chaves GV. Skeletal muscle quality beyond average muscle attenuation: a proposal of skeletal muscle phenotypes to predict short-term survival in patients with endometrial cancer. J Natl Compr Canc Netw. (2018) 16:153–60. doi: 10.6004/jnccn.2017.7028
37. Gan D, Wang L, Jia M, Ru Y, Ma Y, Zheng W, et al. Low muscle mass and low muscle strength associate with nonalcoholic fatty liver disease. Clin Nutr. (2019) 39:1124–30. doi: 10.1016/j.clnu.2019.04.023
38. Maurício SF, Xiao J, Prado CM, Gonzalez MC, Correia MITD. Different nutritional assessment tools as predictors of postoperative complications in patients undergoing colorectal cancer resection. Clin Nutr. (2018) 37:1505–11. doi: 10.1016/j.clnu.2017.08.026
39. Minnella EM, Awasthi R, Loiselle SE, Agnihotram RV, Ferri LE, Carli F. Effect of exercise and nutrition prehabilitation on functional capacity in esophagogastric cancer surgery: a randomized clinical trial. JAMA Surg. (2018) 153:1081–9. doi: 10.1001/jamasurg.2018.1645
40. Moran J, Guinan EM, Mccormick P, Larkin J, Mockler D, Hussey J, et al. The ability of prehabilitation to influence postoperative outcome after intra-abdominal operation: a systematic review and meta-analysis. Surgery. (2016) 160:1189–201. doi: 10.1016/j.surg.2016.05.014
41. Wynter-Blyth V, Moorthy K. Prehabilitation: preparing patients for surgery. BMJ. (2017) 358:j3702. doi: 10.1136/bmj.j3702
42. Cormie P, Zopf EM, Zhang X, Schmitz KH. The impact of exercise on cancer mortality, recurrence, and treatment-related adverse effects. Epidemiol Rev. (2017) 39:71–92. doi: 10.1093/epirev/mxx007
43. Skeie E, Tangvik RJ, Nymo LS, Harthug S, Lassen K, Viste A. Weight loss and BMI criteria in GLIM's definition of malnutrition is associated with postoperative complications following abdominal resections–Results from a National Quality Registry. Clin Nutr. (2020) 39:1593–9. doi: 10.1016/j.clnu.2019.07.003
44. Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. (2008) 9:629–35. doi: 10.1016/S1470-2045(08)70153-0
Keywords: sarcopenia, muscle mass, muscle radiodensity, handgrip strength, gait speed
Citation: Zhang F-M, Zhang X-Z, Shi H-P, Zhang Z, Wang S-L, Shen Z-L, Chen X-L, Shen X, Yu Z and Zhuang C-L (2021) Comparisons and Impacts of the Basic Components of Sarcopenia Definition and Their Pairwise Combinations in Gastric Cancer: A Large-Scale Study in a Chinese Population. Front. Nutr. 8:709211. doi: 10.3389/fnut.2021.709211
Received: 13 May 2021; Accepted: 17 September 2021;
Published: 20 October 2021.
Edited by:
Eloisa Colin-Ramirez, University of Alberta, CanadaReviewed by:
Sarah Peterson, Rush University Medical Center, United StatesLilia Castillo-Martinez, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (INCMNSZ), Mexico
Copyright © 2021 Zhang, Zhang, Shi, Zhang, Wang, Shen, Chen, Shen, Yu and Zhuang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cheng-Le Zhuang, emh1YW5nY2hlbmdsZUAxMjYuY29t; Zhen Yu, eXV6aGVuMDU3N0BnbWFpbC5jb20=
†These authors have contributed equally to this work
 Feng-Min Zhang1†
Feng-Min Zhang1† Zhen Yu
Zhen Yu Cheng-Le Zhuang
Cheng-Le Zhuang