- 1Department of Nutritional Sciences, School of Nutritional Sciences and Food Technology, Kermanshah University of Medical Sciences, Kermanshah, Iran
- 2Department of Community Medicine, School of Nutrition and Food Sciences, Tabriz University of Medical Sciences, Tabriz, Iran
- 3Department of Nutrition and Food Sciences, Maragheh University of Medical Sciences, Maragheh, Iran
- 4Poltekkes Kemenkes Palangka Raya, Palangka Raya, Indonesia
Background: The inflammatory potential of unhealthy diets can lead to the development of chronic diseases and also exacerbating their complications. Therefore, the present systematic review aimed to evaluate the association of dietary inflammatory index (DII) and quality of life (QOL) in human subjects.
Methods: A systematic search was conducted in PubMed, Web of Science, and Scopus databases, using the combination of all search terms related to DII and QOL until May 2022. All eligible human studies published in English were included.
Results: Three hundred twenty-seven studies were obtained from the first systematic search of the databases although, only eight studies were eligible for the evaluation. Seven studies reported that there was a significant reverse association between DII scores and overall QOL and/or its subscales in different populations including patients with asthma, osteoarthritis, hemodialysis patients, multiple sclerosis, obese women, and also in healthy subjects. While, one study on postmenopausal women found no evidence of this association.
Conclusion: This systematic review demonstrated that an anti-inflammatory diet might be associated with better QOL. However, future well-designed clinical trials can provide better conclusions especially regarding the quantifying of this relationship.
Introduction
The term Quality of Life (QOL) was first introduced in 1970s, as the multi-dimensional concept of well-being and health status regarding the physical, mental, emotional, and social aspects of life (1). QOL usually decreases during the aging and diseases (2, 3). It has been documented that low-grade inflammation, increasing pro-inflammatory cytokines in the body, is associated with different chronic disease (4–9), as well as impaired neurodevelopment (10) and adverse mental health outcomes (11), which can affect various aspects of patients’ QOL (12). Therefore, reversing the inflammatory pathways can increase QOL of patients.
Emerging evidence showed that healthy eating is associated with low inflammatory responses and can be a cost-effective intervention to improve the QOL. It was reported that a Western dietary pattern with high consumption of refined grains, processed meats, butter, and high-fat dairy products causes inflammation in the body. Whereas, a healthy diet like the Mediterranean diet which includes whole grains, vegetables, fish, and olive oil, can prevent inflammation or suppress inflammatory pathways (13–16). For this purpose, Shivappa et al. developed a tool to assess the inflammatory potential of the diet called the Dietary Inflammatory Index (DII) (17). Higher DII scores are associated with inflammatory cytokines such as interleukin (IL)-6, tumor necrosis factor (TNF-α), and high-sensitivity C-reactive protein (hs-CRP) (18, 19). Studies have shown that DII is associated with various diseases such as breast cancer (20), colorectal cancer (21), osteoarthritis (22), metabolic syndrome (23), and asthma (24).
Therefore, this study was conducted with the hypothesis that the inflammatory potential of the diet can lead to the development or progression of chronic diseases complications and thus decreases patients’ QOL. To the best of our knowledge, this is the first systematic review that has summarized and concluded the outcomes of related studies to assess the impact of DII on QOL.
Methods
The search strategy
This study was performed according to the PRISMA-P (Preferred Reporting Items for Systematic Reviews and Meta-
Analyses Protocols) 2015 statement. We searched through PubMed/Medline, Web of Science, and Scopus for relevant papers published in English until May 2022 using the following keywords: “dietary inflammatory score” OR “dietary inflammatory index” OR “DII” OR “inflammatory diet” OR “inflammatory potential of diet” OR “dietary inflammation potential” OR “potential inflammatory intake” OR “anti-inflammatory diet” OR “pro-inflammatory diet” in title/abstract AND “quality of life” OR “QOL” OR “health-related QOL” OR “HRQOL” OR “World Health Organization Quality of Life-Brief” OR “WHOQOL” OR “PedsQL” in the title/abstract.
The screening of studies
All detected articles were saved in an EndNote software file and duplicate articles were removed. Then, unrelated articles were identified and deleted by reviewing the titles and abstracts. The full text of remaining articles was then screened for eligibility and data extraction by two independent researchers. Discrepancies between the two authors were resolved by a third researcher.
Inclusion and exclusion criteria
Studies were included if they examined the association of a DII score and QOL. There was no restriction on study design and all English articles were eligible. Moreover, studies that assessed the association of DII with QOL in patients with knee osteoarthritis, multiple sclerosis (MS), asthma, and hemodialysis were included in this review.
Data extraction and quality assessment
The data were collected according to a standard extraction form to obtain the information about the first author’s name, geographical area, study design, population/sample size, mean ages of participants, interventional/control diet, duration of intervention, QOL/DII/food intake assessment tools, and the main outcomes.
For assessment of the articles quality, the adapted version of the Newcastle–Ottawa Scale (NOS) checklist was used for cross-sectional studies as it was shown in Supplementary Table 1 (25) and the Jadad checklist was used for experimental studies as it was shown in Supplementary Table 2 (26). In the NOS checklist, the score of ≥7 was interpreted as a low risk of bias, scores between 4 and 6 were interpreted as a high risk of bias, and the score of <4 was interpreted as a very high risk of bias (27). In the Jadad checklist, the score of ≥3 was considered to have superior quality (26).
Results
Selection of studies
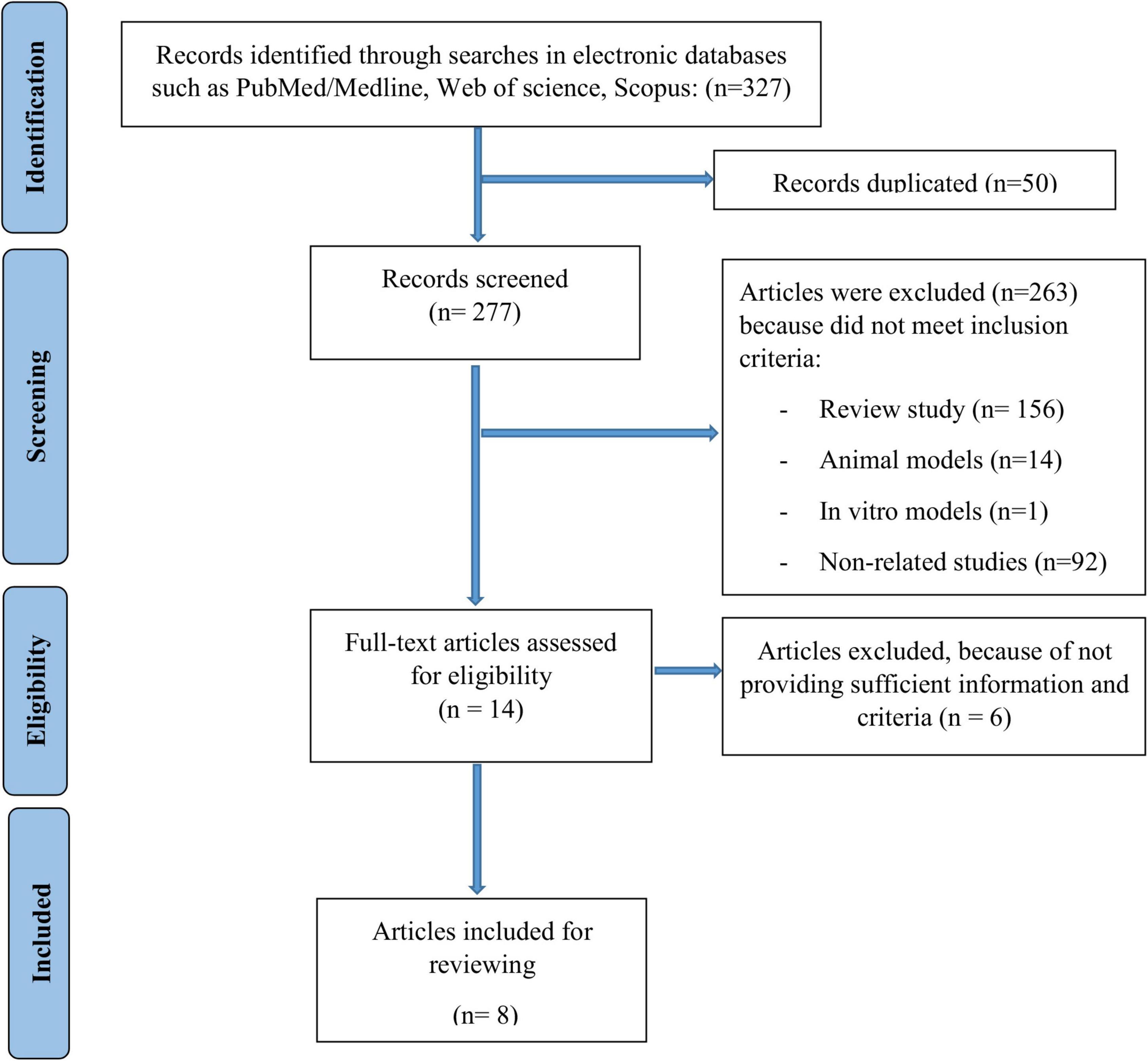
As it was shown in Figure 1, 327 potentially relevant articles were obtained by the search strategy. Of these records, 50 were excluded due to duplicate studies. Then, of 277 remained articles, 263 studies were excluded because they did not meet the inclusion criteria. Finally, 8 articles were included for analysis.
Characteristics of included studies
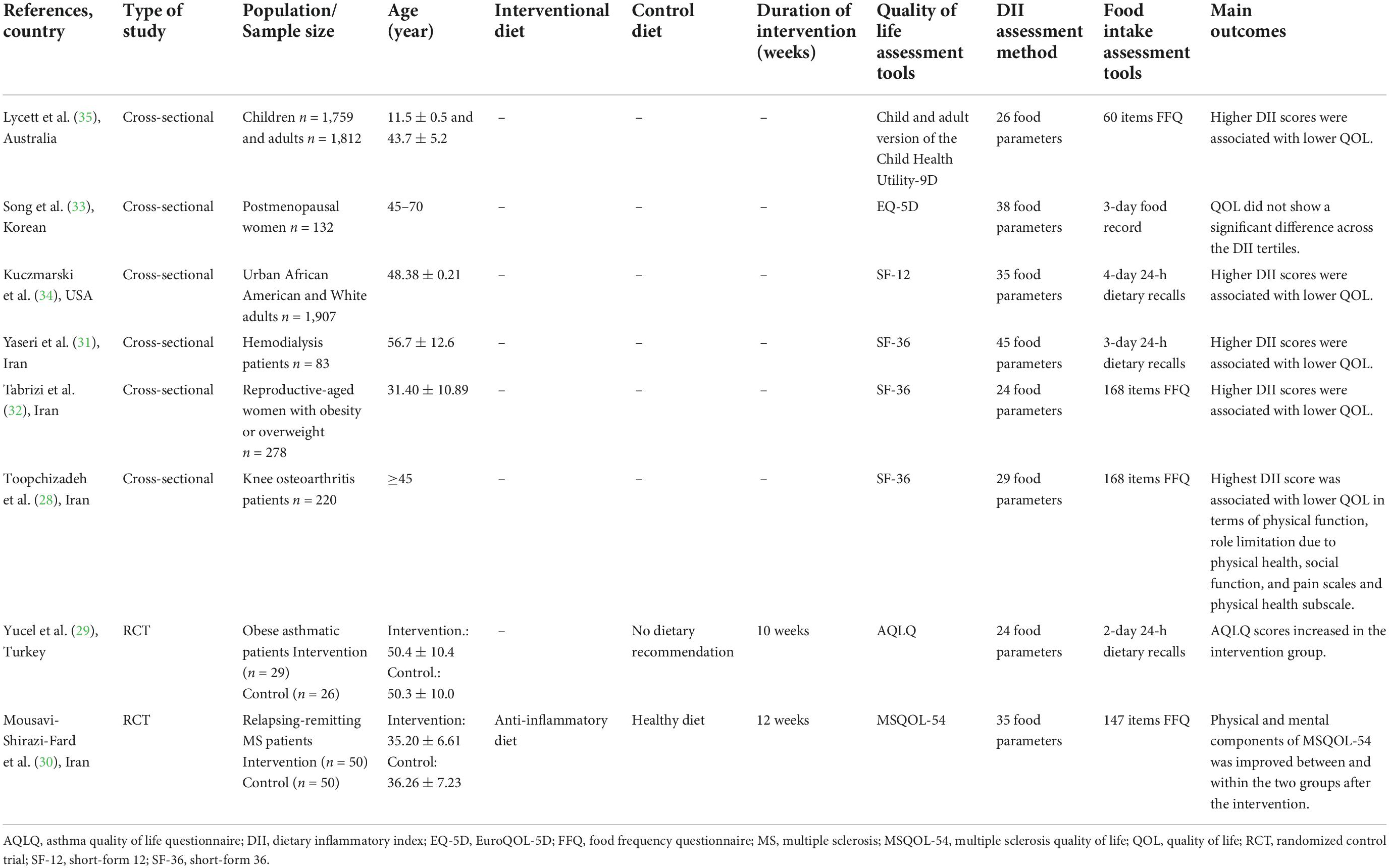
The study population of included studies were as follow: knee osteoarthritis (n = 1) (28), asthma (n = 1) (29), MS (n = 1) (30), hemodialysis patients (n = 1) (31), women with obesity or overweight (n = 1) (32), postmenopausal women (n = 1) (33), healthy people (n = 2) (34, 35). The details of each study are summarized in Table 1.
The population of studies were over 20 years of age, except for one research on children aged 11–12 (35). Of 8 included studies, 2 were randomized controlled trial (RCT) using the anti-inflammatory diets as the intervention for 10 (29) and 12 (30) weeks (see Table 1) and 6 articles were cross-sectional in design (28, 31–35).
Different questionnaires were used to assess QOL across the studies including Child and adult version of the Child Health Utility-9D (35), Short-Form 36 (SF-36) (28, 31, 32), Short-Form 12 (SF-12) (34), EuroQOL-5D (EQ-5D) (33), Asthma quality of life questionnaire (AQLQ) (29) and Multiple Sclerosis Quality of Life (MSQOL-54) (30).
There was also a heterogeneity in the assessment of dietary inflammatory index between studies. The food intake assessment tools were food frequency questionnaire (FFQ) (28, 30, 32, 35), 3-day food record (33), 3-day or 2-day 24-hour food recall (29, 31, 34).
Quality of the articles
Using the NOS checklist, it was determined that four cross-sectional studies had a high risk of bias (28, 31–33) and two of them had a low risk of bias (34, 35). Jadad’s checklist also showed that all interventional studies had superior quality (29, 30). The scores obtained from the NOS and Jadad checklists are shown in Supplementary Tables 1, 2.
Association between the dietary inflammatory index and quality of life
Five out of six cross-sectional studies found that higher DII scores were significantly associated with lower QOL (28, 31, 32, 34, 35). But the results of one cross-sectional study on post-menopausal women did not show any significant differences in QOL across the DII tertiles (33).
Moreover, the results of clinical trials showed that consumption of anti-inflammatory diet for 10 and 12 weeks significantly increased patients’ quality of life in terms of different physical and/or mental components (29, 30).
Discussion
To the best of our knowledge, the present study is the first systematic review of the association between DII and QOL. The majority of the studies included in this review showed the negative relationship between DII with QOL and/or its domains, with the exception of one study showing no association (33).
Healthy dietary patterns such as the Mediterranean diet and the Dietary Approaches to Stop Hypertension (DASH) promote eating healthy foods, which can reduce inflammation in the body (36–39), while Western dietary pattern has inflammatory properties (40). The effect of dietary patterns on QOL have also been previously studied and our results are consistent with these studies. Results of a systematic review by Govindaraju et al. showed that healthy dietary patterns like Mediterranean diet were associated with better QOL (41). Another review study found that in contrast to the Western and unhealthy diet, the Mediterranean diet was associated with better QOL in both physical and mental domains (42). Wu et al. reported that diet quality and dietary behavior were positively associated with various aspects of QOL, including physical, psychosocial, school, and emotional functioning in children and adolescents (43). Results of a most recent study showed that adherence to the Mediterranean diet was positively associated with quality of life in children and adolescents (44). Moreover, adhering to the DASH pattern led to the improvement of QOL in patients with heart failure (HF) during 3 months (45). It was also reported that healthy dietary patterns were associated with better sleep status, sexual function, and physical activity (46–50).
The most important mechanism for the health effects of the aforementioned healthy dietary patterns can be justified by reducing inflammation, suppressing pro-inflammatory responses, and the antioxidant effects. In this regard, focusing on the effect of diet in modulation of inflammation caused to the development of DII first in 2009 (51). DII is a validated dietary score that was introduced to assess the potential effects of people’s diet on their inflammatory status and health outcomes. Accordingly, a high DII score reflects the pro-inflammatory potential of diet, while the low scores of DII reflect the anti-inflammatory effect of diet (52).
It was reported that high DII scores were positively associated with systemic inflammation and also decreased lung function in people with asthma (24). Moreover, it was reported that consumption of a pro-inflammatory diet may have important role in knee osteoarthritis pathology (53). Studies showed a positive association between DII scores with postmenopausal complications such as osteoarthritis (33), lower bone density (54, 55), higher menopause-specific somatic score (56), hip fracture risk (54), increased risk of breast cancer (20), and proximal colorectal cancer (21).
Bohlouli et al. showed that adherence to an anti-inflammatory diet such as the Mediterranean diet improved fatigue severity in relapsing-remitting MS (57). Cross-sectional studies showed that the body composition and anthropometric measurements were directly associated with DII scores (58, 59). There are evidence that high DII scores have been positively associated with an increased risk of obesity in non-obese individuals and also the prevalence of overweight and obesity (60). Recently, Ferreira et al. showed that the comorbidities of obesity decreased after improving the DII scores of participants (61).
Dietary inflammatory index can trigger inflammatory responses in the body (24, 62) and the inflammatory cytokines are related to low QOL due to physical disability, psychosocial burdens, pain, mood, and sexual function (63–72) in different conditions and diseases like respiratory tract diseases (24, 73–75), osteoarthritis and synovitis (76, 77), MS (78), obesity (79, 80), postmenopausal women (81, 82), and hemodialysis patients (83).
However, Song et al. showed that there was no significant relationship between DII scores and QOL in post-menopausal women, which may be due to the low sample size of the study (33).
Figure 2 shows the association between pro-inflammatory diets and quality of life in different conditions.
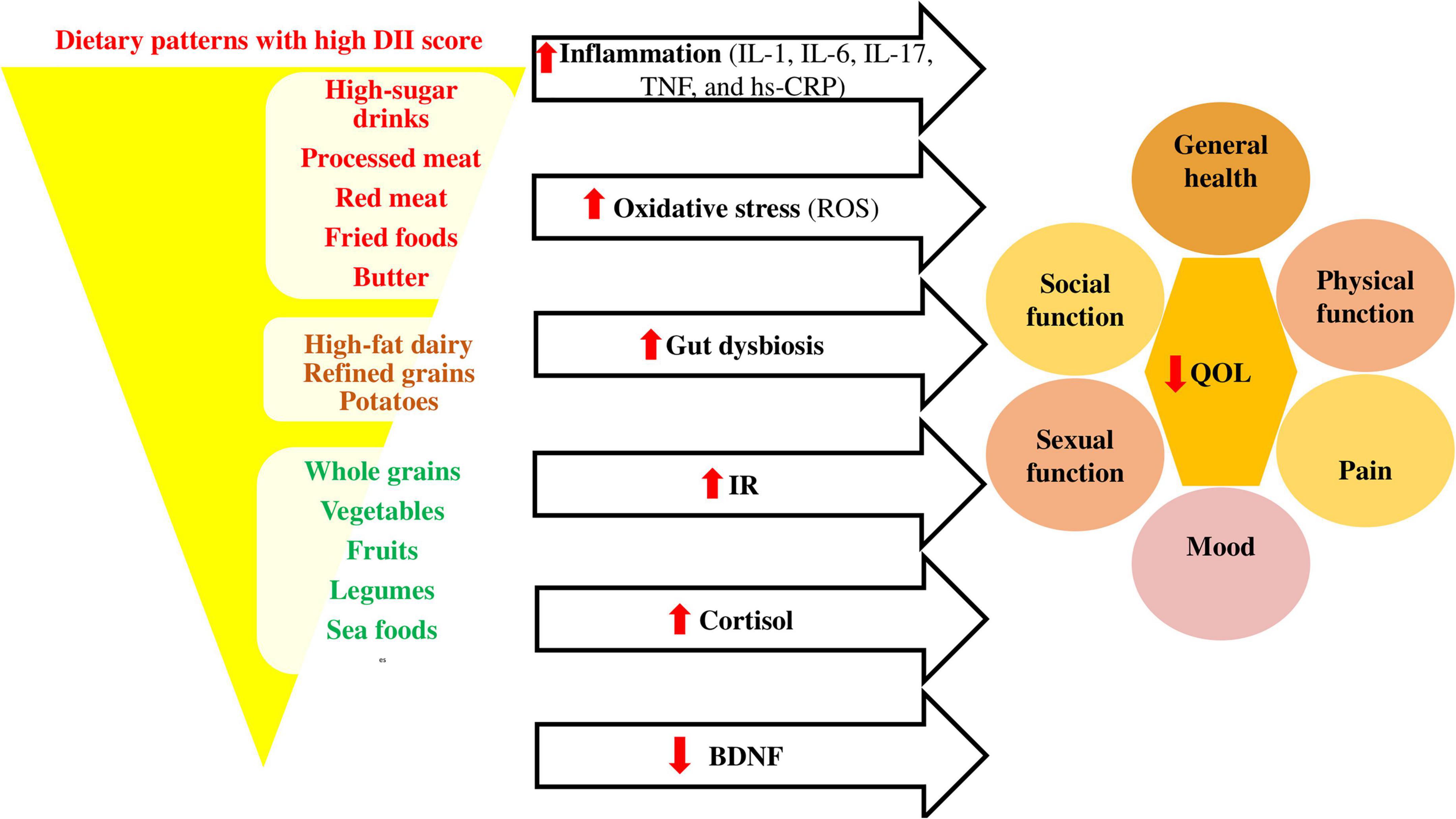
Figure 2. Association between pro-inflammatory diets and quality of life. Pro-inflammatory diets like Western diets (98) affect the inflammatory cytokines (99, 100), oxidative stress (101), gut microbiota composition (102), insulin resistance (IR) (103), cortisol (104), and brain derived neurotropic factor (BDNF) (105) levels, causing decrease in quality of life through its effects on different dimensions. BDNF, brain derived neurotropic factor; DII, dietary inflammatory index; hs-CRP, high-sensitivity C-reactive protein; IL, interleukin; IR, insulin resistance; QOL, quality of life; ROS, reactive oxygen species; TNF, tumor necrosis factor.
Healthy dietary patterns with low DII scores can also change the gut microbiota composition and correct the gut dysbiosis (84). These diets emphasize the consumption of vegetables, fruits, whole grain, beans, legumes, nuts, seeds, and olive oil (85) and improve the microbiome diversity by increasing growth of Bacteroides, Lactobacili, Bifidobacteria, Faecalibacterium, Oscillospira, Roseburia, Ruminococci, and their metabolic activities and decreasing growth of Firmicutes and Proteobacteria (86). Therefore, the production of short-chain fatty acids (SCFAs) will increase in the feces (84). SCFAs, especially butyrate bind to epithelial and immune cell G protein-coupled receptors (GPCRs) which leads to maintaining the integrity of the intestine and preventing inflammation, oxidative stress, and insulin resistance (87), while the western diets lead to metabolic endotoxemia by increasing intestinal permeability (86). Indeed, gut dysbiosis can affect various aspects of QOL, including physical and mental health (88–92).
The antidepressant effect of healthy diets with low DII score can also be explained through decreasing cortisol (93) and increasing brain derived neurotropic factor (BDNF) (94–97). Several limitations in the present study should be clarified when interpreting the results of this review including: (a) The number of studies on the association of DII and QOL was limited. (b) There was heterogeneity between studies’ populations (different diseases or conditions) and also questionnaires which assessed the QOL, DII, and food intake. (c) The majority of included studies in this systematic review were cross-sectional studies, which did not show causal and temporal associations. (d) The instruments used to examine diet and quality of life were both self-reported, which may be subject to recall and reporting biases.
Conclusion
This systematic review demonstrated that an anti-inflammatory diet might be associated with better QOL. However, future well-designed clinical trials on various disease can provide better conclusions especially regarding the quantifying of this relationship.
Data availability statement
The original contributions presented in this study are included in this article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
MG and SN contributed to designing the study, searching for resources, and writing the manuscript. MG, SK, VE, and JM cooperated in writing the manuscript. RS contributed to English revising of the manuscript. RM and MM cooperated in literature search. All authors contributed to the article and approved the submitted version.
Acknowledgments
We express our appreciation to the Research Vice-Chancellor of Tabriz and Maragheh University of Medical Sciences.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.1067468/full#supplementary-material
Abbreviations
AQLQ, asthma quality of life questionnaire; BDNF, brain derived neurotropic factor; DASH, dietary approaches to stop hypertension; DII, dietary inflammatory index; EQ-5D, EuroQOL-5D; FFQ, food frequency questionnaire; GPCRs, G protein-coupled receptors; HF, heart failure; 24HR, 24-hour food recall; hs-CRP, high-sensitivity C-reactive protein; IL, interleukin; IR, insulin resistance; MS, multiple sclerosis; MSQOL-54, multiple sclerosis quality of life; P, pain; PF, physical function; PH, physical health; PRISMA-P, preferred reporting items for systematic reviews and meta-analyses protocols; QOL, quality of life; RCT, randomized control trial; ROS, reactive oxygen species; RP, role limitation due to physical health; SCFAs, short-chain fatty acids; SF, social function; SF-12, short-form 12; SF-36, short-form 36; TNF-α, tumor necrosis factor.
References
1. Felce D, Perry J. Quality of life: its definition and measurement. Res Dev Disabil. (1995) 16:51–74. doi: 10.1016/0891-4222(94)00028-8
2. Megari K. Quality of life in chronic disease patients. Health Psychol Res. (2013) 1:e27. doi: 10.4081/hpr.2013.e27
3. Tourani S, Behzadifar M, Martini M, Aryankhesal A, Taheri Mirghaed M, Salemi M, et al. Health-related quality of life among healthy elderly Iranians: a systematic review and meta-analysis of the literature. Health Qual Life Outcomes. (2018) 16:1–9. doi: 10.1186/s12955-018-0845-7
4. Kay A. Asthma and inflammation. J Allergy Clin Immunol. (1991) 87:893–910. doi: 10.1016/0091-6749(91)90408-G
5. Cachofeiro V, Goicochea M, De Vinuesa SG, Oubiña P, Lahera V, Luño J. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease: new strategies to prevent cardiovascular risk in chronic kidney disease. Kidney Int. (2008) 74:S4–9. doi: 10.1038/ki.2008.516
6. Frischer JM, Bramow S, Dal-Bianco A, Lucchinetti CF, Rauschka H, Schmidbauer M, et al. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain. (2009) 132:1175–89. doi: 10.1093/brain/awp070
7. Goldring MB, Otero M. Inflammation in osteoarthritis. Curr Opin Rheumatol. (2011) 23:471. doi: 10.1097/BOR.0b013e328349c2b1
8. Ellulu MS, Patimah I, Khaza’ai H, Rahmat A, Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci. (2017) 13:851. doi: 10.5114/aoms.2016.58928
9. Guo Q, Wang Y, Xu D, Nossent J, Pavlos NJ, Xu J. Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res. (2018) 6:1–14. doi: 10.1038/s41413-018-0016-9
10. Jiang NM, Cowan M, Moonah SN, Petri WA Jr. The impact of systemic inflammation on neurodevelopment. Trends Mol Med. (2018) 24:794–804. doi: 10.1016/j.molmed.2018.06.008
11. Peirce JM, Alviña K. The role of inflammation and the gut microbiome in depression and anxiety. J Neurosci Res. (2019) 97:1223–41. doi: 10.1002/jnr.24476
12. Pengpid S, Peltzer K. The impact of chronic diseases on the quality of life of primary care patients in Cambodia, Myanmar and Vietnam. Iran J Public Health. (2018) 47:1308.
13. Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol. (2006) 48:677–85. doi: 10.1016/j.jacc.2006.03.052
14. Bulló M, Casas-Agustench P, Amigó-Correig P, Aranceta J, Salas-Salvadó J. Inflammation, obesity and comorbidities: the role of diet. Public Health Nutr. (2007) 10:1164–72. doi: 10.1017/S1368980007000663
15. Galland L. Diet and inflammation. Nutr Clin Pract. (2010) 25:634–40. doi: 10.1177/0884533610385703
16. Saghafi-Asl M, Mirmajidi S, Asghari Jafarabadi M, Vahid F, Shivappa N, Hébert JR, et al. The association of dietary patterns with dietary inflammatory index, systemic inflammation, and insulin resistance, in apparently healthy individuals with obesity. Sci Rep. (2021) 11:1–8. doi: 10.1038/s41598-021-86993-7
17. Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. (2014) 17:1689–96. doi: 10.1017/S1368980013002115
18. Tabung FK, Steck SE, Zhang J, Ma Y, Liese AD, Agalliu I, et al. Construct validation of the dietary inflammatory index among postmenopausal women. Ann Epidemiol. (2015) 25:398–405. doi: 10.1016/j.annepidem.2015.03.009
19. Na W, Kim M, Sohn C. Dietary inflammatory index and its relationship with high-sensitivity C-reactive protein in Korean: data from the health examinee cohort. J Clin Biochem Nutr. (2018) 62:83–8. doi: 10.3164/jcbn.17-22
20. Shivappa N, Blair CK, Prizment AE, Jacobs DR, Hébert JR. Prospective study of the dietary inflammatory index and risk of breast cancer in postmenopausal women. Mol Nutr Food Res. (2017) 61:1600592. doi: 10.1002/mnfr.201600592
21. Tabung FK, Steck SE, Ma Y, Liese AD, Zhang J, Caan B, et al. The association between dietary inflammatory index and risk of colorectal cancer among postmenopausal women: results from the women’s health initiative. Cancer Causes Control. (2015) 26:399–408. doi: 10.1007/s10552-014-0515-y
22. Veronese N, Shivappa N, Stubbs B, Smith T, Hébert JR, Cooper C, et al. The relationship between the dietary inflammatory index and prevalence of radiographic symptomatic osteoarthritis: data from the osteoarthritis initiative. Eur J Nutr. (2019) 58:253–60. doi: 10.1007/s00394-017-1589-6
23. Wirth M, Burch J, Shivappa N, Violanti JM, Burchfiel CM, Fekedulegn D, et al. Association of a dietary inflammatory index with inflammatory indices and the metabolic syndrome among police officers. J Occup Environ Med. (2014) 56:986. doi: 10.1097/JOM.0000000000000213
24. Wood LG, Shivappa N, Berthon BS, Gibson PG, Hebert JR. Dietary inflammatory index is related to asthma risk, lung function and systemic inflammation in asthma. Clin Exp Allergy. (2015) 45:177–83. doi: 10.1111/cea.12323
25. Herzog R, Álvarez-Pasquin M, Díaz C, Del Barrio JL, Estrada JM, Gil Á. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health. (2013) 13:154. doi: 10.1186/1471-2458-13-154
26. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. (1996) 17:1–12. doi: 10.1016/0197-2456(95)00134-4
27. Lo CKL, Mertz D, Loeb M. Newcastle-Ottawa scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. (2014) 14:45. doi: 10.1186/1471-2288-14-45
28. Toopchizadeh V, Dolatkhah N, Aghamohammadi D, Rasouli M, Hashemian M. Dietary inflammatory index is associated with pain intensity and some components of quality of life in patients with knee osteoarthritis. BMC Res Notes. (2020) 13:448. doi: 10.1186/s13104-020-05277-x
29. Yucel U, Ucar A, Soezener Z, Balaban S, Mungan D, Misirligil Z, et al. Effects of dietary intervention on diet inflammatory index and asthma characteristics in obese asthmatic individuals: randomized controlled trial. Eur J Pulmonol. (2021) 23:145–51.
30. Mousavi-Shirazi-Fard Z, Mazloom Z, Izadi S, Fararouei M. The effects of modified anti-inflammatory diet on fatigue, quality of life, and inflammatory biomarkers in relapsing-remitting multiple sclerosis patients: a randomized clinical trial. Int J Neurosci. (2021) 131:657–65. doi: 10.1080/00207454.2020.1750398
31. Yaseri M, Alipoor E, Hafizi N, Maghsoudi-Nasab S, Shivappa N, Hebert JR, et al. Dietary inflammatory index is a better determinant of quality of life compared to obesity status in patients with hemodialysis. J Renal Nutr. (2021) 31:313–9. doi: 10.1053/j.jrn.2020.07.006
32. Tabrizi FPF, Farhangi MA. Is there any mediatory association between health-related quality of life and eating behaviors to affect dietary inflammatory index (DII) among reproductive-aged women? A structural equation modeling approach. Nutr Clin Metab. (2021) 35:288–96. doi: 10.1016/j.nupar.2021.06.003
33. Song D, Kim J, Kang M, Park J, Lee H, Kim DY, et al. Association between the dietary inflammatory index and bone markers in postmenopausal women. PLoS One. (2022) 17:e0265630. doi: 10.1371/journal.pone.0265630
34. Kuczmarski MF, Orsega-Smith E, Mode NA, Rawal R, Evans MK, Zonderman AB. Healthy behaviors associated with changes in mental and physical strength in urban African American and white adults. Nutrients. (2021) 13:1824. doi: 10.3390/nu13061824
35. Lycett KM, Wijayawickrama DJ, Liu MJ, Grobler A, Burgner DP, Baur LA, et al. Does an inflammatory diet affect mental well-being in late childhood and mid-life? A cross-sectional study. Br J Nutr. (2022) 127:939–47. doi: 10.1017/S0007114521001616
36. Giugliano D, Esposito K. Mediterranean diet and metabolic diseases. Curr Opin Lipidol. (2008) 19:63–8. doi: 10.1097/MOL.0b013e3282f2fa4d
37. Asemi Z, Samimi M, Tabassi Z, Sabihi S-S, Esmaillzadeh A. A randomized controlled clinical trial investigating the effect of DASH diet on insulin resistance, inflammation, and oxidative stress in gestational diabetes. Nutrition. (2013) 29:619–24. doi: 10.1016/j.nut.2012.11.020
38. Saneei P, Hashemipour M, Kelishadi R, Esmaillzadeh A. The dietary approaches to stop hypertension (DASH) diet affects inflammation in childhood metabolic syndrome: a randomized cross-over clinical trial. Ann Nutr Metab. (2014) 64:20–7. doi: 10.1159/000358341
39. Nani A, Murtaza B, Sayed Khan A, Khan NA, Hichami A. Antioxidant and anti-inflammatory potential of polyphenols contained in mediterranean diet in obesity: molecular mechanisms. Molecules. (2021) 26:985. doi: 10.3390/molecules26040985
40. Christ A, Lauterbach M, Latz E. Western diet and the immune system: an inflammatory connection. Immunity. (2019) 51:794–811. doi: 10.1016/j.immuni.2019.09.020
41. Govindaraju T, Sahle BW, Mccaffrey TA, Mcneil JJ, Owen AJ. Dietary patterns and quality of life in older adults: a systematic review. Nutrients. (2018) 10:971. doi: 10.3390/nu10080971
42. Vajdi M, Farhangi MA. A systematic review of the association between dietary patterns and health-related quality of life. Health Qual Life Outcomes. (2020) 18:1–15. doi: 10.1186/s12955-020-01581-z
43. Wu XY, Zhuang LH, Li W, Guo HW, Zhang JH, Zhao YK, et al. The influence of diet quality and dietary behavior on health-related quality of life in the general population of children and adolescents: a systematic review and meta-analysis. Qual Life Res. (2019) 28:1989–2015. doi: 10.1007/s11136-019-02162-4
44. Romero-Robles MA, Ccami-Bernal F, Ortiz-Benique ZN, Pinto-Ruiz DF, Benites-Zapata VA, Casas Patiño D. Adherence to Mediterranean diet associated with health-related quality of life in children and adolescents: a systematic review. BMC Nutr. (2022) 8:57. doi: 10.1186/s40795-022-00549-0
45. Rifai L, Pisano C, Hayden J, Sulo S, Silver MA. Impact of the DASH diet on endothelial function, exercise capacity, and quality of life in patients with heart failure. Proc (Bayl Univ Med Cent). (2015) 28:151–6. doi: 10.1080/08998280.2015.11929216
46. Idelson PI, Scalfi L, Valerio G. Adherence to the mediterranean diet in children and adolescents: a systematic review. Nutr Metab Cardiovasc Dis. (2017) 27:283–99. doi: 10.1016/j.numecd.2017.01.002
47. La J, Roberts NH, Yafi FA. Diet and men’s sexual health. Sex Med Rev. (2018) 6:54–68. doi: 10.1016/j.sxmr.2017.07.004
48. Towe M, La J, El-Khatib F, Roberts N, Yafi FA, Rubin R. Diet and female sexual health. Sex Med Rev. (2020) 8:256–64. doi: 10.1016/j.sxmr.2019.08.004
49. Martinovic D, Tokic D, Martinovic L, Kumric M, Vilovic M, Rusic D, et al. Adherence to the mediterranean diet and its association with the level of physical activity in fitness center users: Croatian-based study. Nutrients. (2021) 13:4038. doi: 10.3390/nu13114038
50. Scoditti E, Tumolo MR, Garbarino S. Mediterranean diet on sleep: a health alliance. Nutrients. (2022) 14:2998. doi: 10.3390/nu14142998
51. Hébert JR, Shivappa N, Wirth MD, Hussey JR, Hurley TG. Perspective: the dietary inflammatory index (DII)—lessons learned, improvements made, and future directions. Adv Nutr. (2019) 10:185–95. doi: 10.1093/advances/nmy071
52. Kotemori A, Sawada N, Iwasaki M, Yamaji T, Shivappa N, Hebert JR, et al. Dietary inflammatory index is associated with inflammation in Japanese men. Front Nutr. (2021) 8:604296. doi: 10.3389/fnut.2021.604296
53. El-Ali Z, El-Kassas G, Ziade FM, Shivappa N, Hébert JR, Zmerly H, et al. Evaluation of circulating levels of interleukin-10 and interleukin-16 and dietary inflammatory index in lebanese knee osteoarthritis patients. Heliyon. (2021) 7:e07551. doi: 10.1016/j.heliyon.2021.e07551
54. Orchard T, Yildiz V, Steck SE, Hébert JR, Ma Y, Cauley JA, et al. Dietary inflammatory index, bone mineral density, and risk of fracture in postmenopausal women: results from the women’s health initiative. J Bone Miner Res. (2017) 32:1136–46. doi: 10.1002/jbmr.3070
55. Li R, Zhan W, Huang X, Wang J, Lv S, Liang L, et al. Associations between dietary inflammatory index (DII) and bone health among postmenopausal women in the United States. Int J Gynaecol Obstet. (2022) 158:663–70. doi: 10.1002/ijgo.14024
56. Aslani Z, Abshirini M, Heidari-Beni M, Siassi F, Qorbani M, Shivappa N, et al. Dietary inflammatory index and dietary energy density are associated with menopausal symptoms in postmenopausal women: a cross-sectional study. Menopause. (2020) 27:568–78. doi: 10.1097/GME.0000000000001502
57. Bohlouli J, Namjoo I, Borzoo-Isfahani M, Poorbaferani F, Moravejolahkami AR, Clark CC, et al. Modified Mediterranean diet v. traditional Iranian diet: efficacy of dietary interventions on dietary inflammatory index score, fatigue severity and disability in multiple sclerosis patients. Br J Nutr. (2022) 128:1274–84. doi: 10.1017/S000711452100307X
58. Ruiz-Canela M, Zazpe I, Shivappa N, Hébert JR, Sanchez-Tainta A, Corella D, et al. Dietary inflammatory index and anthropometric measures of obesity in a population sample at high cardiovascular risk from the PREDIMED (PREvencion con DIeta MEDiterranea) trial. Br J Nutr. (2015) 113:984–95. doi: 10.1017/S0007114514004401
59. Gholamalizadeh M, Ahmadzadeh M, Bourbour F, Vahid F, Ajami M, Majidi N, et al. Associations between the dietary inflammatory index with obesity and body fat in male adolescents. BMC Endocr Disord. (2022) 22:115. doi: 10.1186/s12902-022-01001-x
60. Wang YB, Shivappa N, Hébert JR, Page AJ, Gill TK, Melaku YA. Association between dietary inflammatory index, dietary patterns, plant-based dietary index and the risk of obesity. Nutrients. (2021) 13:1536. doi: 10.3390/nu13051536
61. Ferreira YAM, Kravchychyn ACP, Vicente SDCF, Campos RMDS, Tock L, Oyama LM, et al. An interdisciplinary weight loss program improves body composition and metabolic profile in adolescents with obesity: associations with the dietary inflammatory index. Front Nutr. (2019) 6:77. doi: 10.3389/fnut.2019.00077
62. Cavicchia PP, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, et al. A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J Nutr. (2009) 139:2365–72. doi: 10.3945/jn.109.114025
63. Amato M, Ponziani G, Rossi F, Liedl C, Stefanile C, Rossi L. Quality of life in multiple sclerosis: the impact of depression, fatigue and disability. Mult Scler J. (2001) 7:340–4. doi: 10.1177/135245850100700511
64. Janardhan V, Bakshi R. Quality of life in patients with multiple sclerosis: the impact of fatigue and depression. J Neurol Sci. (2002) 205:51–8. doi: 10.1016/S0022-510X(02)00312-X
65. Van Schoor N, Smit J, Twisk J, Lips P. Impact of vertebral deformities, osteoarthritis, and other chronic diseases on quality of life: a population-based study. Osteoporos Int. (2005) 16:749–56. doi: 10.1007/s00198-004-1744-9
66. Drayer RA, Piraino B, Reynolds Iii CF, Houck PR, Mazumdar S, Bernardini J, et al. Characteristics of depression in hemodialysis patients: symptoms, quality of life and mortality risk. Gen Hosp Psychiatry. (2006) 28:306–12. doi: 10.1016/j.genhosppsych.2006.03.008
67. Tepavcevic D, Kostic J, Basuroski I, Stojsavljevic N, Pekmezovic T, Drulovic J. The impact of sexual dysfunction on the quality of life measured by MSQoL-54 in patients with multiple sclerosis. Mult Scler J. (2008) 14:1131–6. doi: 10.1177/1352458508093619
68. Dixon JB. The effect of obesity on health outcomes. Mol Cell Endocrinol. (2010) 316:104–8. doi: 10.1016/j.mce.2009.07.008
69. Bayoumi M, Al Harbi A, Al Suwaida A, Al Ghonaim M, Al Wakeel J, Mishkiry A. Predictors of quality of life in hemodialysis patients. Saudi J Kidney Dis Transpl. (2013) 24:254. doi: 10.4103/1319-2442.109566
70. Booth S, Johnson MJ. Improving the quality of life of people with advanced respiratory disease and severe breathlessness. Breathe. (2019) 15:198–215. doi: 10.1183/20734735.0200-2019
71. Stephenson J, Smith C, Kearns B, Haywood A, Bissell P. The association between obesity and quality of life: a retrospective analysis of a large-scale population-based cohort study. BMC Public Health. (2021) 21:1990. doi: 10.1186/s12889-021-12009-8
72. Thapa P, Thebe P. Quality of life of postmenopausal women in rural area, Nepal. Post Reprod Health. (2021) 27:151–7. doi: 10.1177/20533691211014741
73. Dixon AE, Pratley RE, Forgione PM, Kaminsky DA, Whittaker-Leclair LA, Griffes LA, et al. Effects of obesity and bariatric surgery on airway hyperresponsiveness, asthma control, and inflammation. J Allergy Clin Immunol. (2011) 128:508–15.e1–5. doi: 10.1016/j.jaci.2011.06.009
74. Monadi M, Firouzjahi A, Hosseini A, Javadian Y, Sharbatdaran M, Heidari B. Serum C-reactive protein in asthma and its ability in predicting asthma control, a case-control study. Caspian J Intern Med. (2016) 7:37.
75. Zhang X, Zheng J, Zhang L, Liu Y, Chen GP, Wang L, et al. Systemic inflammation mediates the detrimental effects of obesity on asthma control. Allergy Asthma Proc. (2018) 39:43–50. doi: 10.2500/aap.2018.39.4096
76. Spector T, Hart D, Nandra D, Doyle D, Mackillop N, Gallimore J, et al. Low-level increases in serum C-reactive protein are present in early osteoarthritis of the knee and predict progressive disease. Arthritis Rheum. (1997) 40:723–7. doi: 10.1002/art.1780400419
77. Sokolove J, Lepus CM. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis. (2013) 5:77–94. doi: 10.1177/1759720X12467868
78. Lassmann H, Van Horssen J, Mahad D. Progressive multiple sclerosis: pathology and pathogenesis. Nat Rev Neurol. (2012) 8:647–56. doi: 10.1038/nrneurol.2012.168
79. Inui A, Meguid MM. Cachexia and obesity: two sides of one coin? Curr Opin Clin Nutr Metab Care. (2003) 6:395–9. doi: 10.1097/01.mco.0000078989.18774.74
80. Kirwan AM, Lenighan YM, O’reilly ME, Mcgillicuddy FC, Roche HM. Nutritional modulation of metabolic inflammation. Biochem Soc Trans. (2017) 45:979–85. doi: 10.1042/BST20160465
81. Barbour KE, Boudreau R, Danielson ME, Youk AO, Wactawski-Wende J, Greep NC, et al. Inflammatory markers and the risk of hip fracture: the women’s health initiative. J Bone Miner Res. (2012) 27:1167–76. doi: 10.1002/jbmr.1559
82. Tella SH, Gallagher JC. Prevention and treatment of postmenopausal osteoporosis. J Steroid Biochem Mol Biol. (2014) 142:155–70. doi: 10.1016/j.jsbmb.2013.09.008
83. Spittle M, Hoenich NA, Handelman G, Adhikarla R, Homel P, Levin NW. Oxidative stress and inflammation in hemodialysis patients. In: Avram MM editor. Improving Prognosis for Kidney Disorders. Dordrecht: Springer (2002). p. 45–52. doi: 10.1007/978-94-017-1848-6_5
84. De Filippis F, Pellegrini N, Vannini L, Jeffery IB, La Storia A, Laghi L, et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. (2016) 65:1812–21. doi: 10.1136/gutjnl-2015-309957
85. Bach-Faig A, Berry EM, Lairon D, Reguant J, Trichopoulou A, Dernini S, et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. (2011) 14:2274–84. doi: 10.1017/S1368980011002515
86. Merra G, Noce A, Marrone G, Cintoni M, Tarsitano MG, Capacci A, et al. Influence of mediterranean diet on human gut microbiota. Kompass Nutr Diet. (2022) 2:19–25. doi: 10.1159/000523727
87. Yoo JY, Groer M, Dutra SVO, Sarkar A, Mcskimming DI. Gut microbiota and immune system interactions. Microorganisms. (2020) 8:1587. doi: 10.3390/microorganisms8101587
88. Frati F, Salvatori C, Incorvaia C, Bellucci A, Di Cara G, Marcucci F, et al. The role of the microbiome in asthma: the gut–lung axis. Int J Mol Sci. (2018) 20:123. doi: 10.3390/ijms20010123
89. Kirby TO, Ochoa-Repáraz J. The gut microbiome in multiple sclerosis: a potential therapeutic avenue. Med Sci. (2018) 6:69. doi: 10.3390/medsci6030069
90. Tseng C-H, Wu C-Y. The gut microbiome in obesity. J Formos Med Assoc. (2019) 118:S3–9. doi: 10.1016/j.jfma.2018.07.009
91. Järbrink-Sehgal E, Andreasson A. The gut microbiota and mental health in adults. Curr Opin Neurobiol. (2020) 62:102–14. doi: 10.1016/j.conb.2020.01.016
92. Chisari E, Wouthuyzen-Bakker M, Friedrich AW, Parvizi J. The relation between the gut microbiome and osteoarthritis: a systematic review of literature. PLoS One. (2021) 16:e0261353. doi: 10.1371/journal.pone.0261353
93. Herbert J. Cortisol and depression: three questions for psychiatry. Psychol Med. (2013) 43:449–69. doi: 10.1017/S0033291712000955
94. Castrén E, Berninger B, Leingärtner A, Lindholm D. Regulation of brain-derived neurotrophic factor mRNA levels in hippocampus by neuronal activity. Prog Brain Res. (1998) 117:57–64. doi: 10.1016/S0079-6123(08)64007-8
95. Molteni R, Barnard R, Ying Z, Roberts C, Gomez-Pinilla F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience. (2002) 112:803–14. doi: 10.1016/S0306-4522(02)00123-9
96. Lopez-Garcia E, Schulze MB, Fung TT, Meigs JB, Rifai N, Manson JE, et al. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. (2004) 80:1029–35. doi: 10.1093/ajcn/80.4.1029
97. Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, Hu FB, Willett WC. Dietary patterns and markers of systemic inflammation among Iranian women. J Nutr. (2007) 137:992–8. doi: 10.1093/jn/137.4.992
98. Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. (2002) 13:3–9. doi: 10.1097/00041433-200202000-00002
99. Myles IA. Fast food fever: reviewing the impacts of the Western diet on immunity. Nutr J. (2014) 13:1–17. doi: 10.1186/1475-2891-13-61
100. Khayyatzadeh SS, Bagherniya M, Fazeli M, Khorasanchi Z, Bidokhti MS, Ahmadinejad M, et al. A Western dietary pattern is associated with elevated level of high sensitive C-reactive protein among adolescent girls. Eur J Clin Investig. (2018) 48:e12897. doi: 10.1111/eci.12897
101. Tomasello G, Mazzola M, Leone A, Sinagra E, Zummo G, Farina F, et al. Nutrition, oxidative stress and intestinal dysbiosis: influence of diet on gut microbiota in inflammatory bowel diseases. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. (2016) 160:461–6. doi: 10.5507/bp.2016.052
102. Zinöcker MK, Lindseth IA. The Western diet–microbiome-host interaction and its role in metabolic disease. Nutrients. (2018) 10:365. doi: 10.3390/nu10030365
103. Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, Hu FB, Willett WC. Dietary patterns, insulin resistance, and prevalence of the metabolic syndrome in women. Am J Clin Nutr. (2007) 85:910–8. doi: 10.1093/ajcn/85.3.910
104. Maurer M, Riesen W, Muser J, Hulter HN, Krapf R. Neutralization of Western diet inhibits bone resorption independently of K intake and reduces cortisol secretion in humans. Am J Physiol Renal Physiol. (2003) 284:F32–40. doi: 10.1152/ajprenal.00212.2002
Keywords: anti-inflammatory diet, chronic disease, dietary inflammatory index, inflammation, quality of life
Citation: Golmohammadi M, Kheirouri S, Ebrahimzadeh Attari V, Moludi J, Sulistyowati R, Nachvak SM, Mostafaei R and Mansordehghan M (2022) Is there any association between dietary inflammatory index and quality of life? A systematic review. Front. Nutr. 9:1067468. doi: 10.3389/fnut.2022.1067468
Received: 11 October 2022; Accepted: 23 November 2022;
Published: 22 December 2022.
Edited by:
Daniela Caetano Gonçalves, Federal University of São Paulo, BrazilReviewed by:
Everson Araujo Nunes, McMaster University, CanadaEvelyn Frias-Toral, Catholic University of Santiago de Guayaquil, Ecuador
Copyright © 2022 Golmohammadi, Kheirouri, Ebrahimzadeh Attari, Moludi, Sulistyowati, Nachvak, Mostafaei and Mansordehghan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seyed Mostafa Nachvak, c21uYWNodmFrQGhvdG1haWwuY29t
 Mona Golmohammadi
Mona Golmohammadi Sorayya Kheirouri
Sorayya Kheirouri Vahideh Ebrahimzadeh Attari
Vahideh Ebrahimzadeh Attari Jalal Moludi
Jalal Moludi Reny Sulistyowati4
Reny Sulistyowati4 Seyed Mostafa Nachvak
Seyed Mostafa Nachvak Roghayeh Mostafaei
Roghayeh Mostafaei Maryam Mansordehghan
Maryam Mansordehghan