- 1National Institute for Nutrition and Health, Chinese Center for Disease Control and Prevention, Beijing, China
- 2Ninghai Country Center for Disease Control and Prevention, Ningbo, China
- 3Hunan Provincial Center for Disease Control and Prevention, Changsha, China
- 4Shaanxi Provincial Center for Disease Control and Prevention, Xian, China
- 5School of Public Health, Hebei Medical University, Shijiazhuang, China
Background: The intake of certain food and nutrients may play a crucial role in cognitive health. However, research on the relationship between dietary patterns and cognitive function is limited. This study aims to investigate the associations between dietary patterns and multi-dimensional cognitive functions, such as global cognitive status and related domain profiles, mild cognitive impairment (MCI), and four major subtypes of Chinese adults.
Methods: Using the baseline data from the Community-based Cohort Study on Nervous System Diseases (2018–2019), we selected 4,309 Chinese adults aged 55 years and older as subjects with complete diet, cognition, and other related data. We collected food data for the past 12 months with a valid semi-quantitative food frequency questionnaire. Diving 49 food items into 13 subgroups, we used factor analysis to derive the main dietary patterns. We evaluated cognitive functions based on the scores of the Montreal Cognitive Assessment (MoCA) and used quantile regression and multivariable logistic regression to examine the relationship between dietary patterns and cognitive-related outcomes.
Results: We identified four dietary patterns, explaining 50.11% of the total variance: “meat-preferred” pattern, “plant-preferred” pattern, “eggs- and dairy-preferred” pattern, and “grain-preferred” pattern. After adjusting for all potential confounders, the “meat-preferred” pattern and the “plant-preferred” pattern were associated with higher scores of global cognition and several cognitive domains (p <0.05), while the “grain-preferred” pattern was associated with lower scores of global cognition (β = −0.36, p <0.05), execution (β = −0.19, p <0.05), visuospatial (β = −0.09, p <0.05), and language (β = −0.05, p <0.05). Adults adhering to the “meat-preferred” pattern and the “plant-preferred” pattern had decreased odds of MCI and some MCI subtypes (p-trend <0.05); in contrast, those in the top quartiles of the “grain-preferred” pattern had increased odds of MCI [adjusted odds ratio (AOR) = 1.34, 95% CI: 1.11–1.63, p-trend = 0.003].
Conclusions: Adhering to the “plant-preferred” pattern and the “meat-preferred” pattern may help improve the multi-dimensional cognitive functions; on the contrary, adhering to the “grain-preferred” pattern may worse cognitive health. More prospective studies in this field are needed to strengthen the evidence.
Introduction
There are 264.02 million people aged 60 years or older in China, accounting for 18.7% of the total population of 1.40 billion in 2020, an increase of about 5.4% over 2010. Due to the aging population, neurodegenerative disorders are expected to increase significantly. In 2020, it was reported that an estimated 9.83 million Chinese aged 60 years and older suffered from Alzheimer's disease (AD), whose cognition declines with age; and 38.77 million suffered from mild cognitive impairment (MCI), the most common outcome of pathological cognitive decline in the elderly (1). MCI, as an intermediate state, 8.1% of MCI may become AD (2) and 10–14% of MCI may return to normal cognition (3). To date, there are no effective measures to fight the progression of AD (4). Therefore, it is extremely important to fully investigate relevant risk factors associated with AD and provide sight into evidence-based intervention for early prevention among adults at early stages.
Diet may have an impact on the cognitive health. Studies in the United States, France, Brazil, and China found that regular consumption of fish, nuts, fruit, or vegetables is associated with improved cognitive function (5–7). Considering the complex interaction among various nutrients and food, research on the relationship between dietary patterns and cognition has received more and more attention. Observation studies showed that adherence to the Mediterranean diet can improve the global cognitive function (8) and decrease the risk of MCI in the elderly (9). Among the elderly in Japan, the “plant food and fish” pattern with high intake of vegetables, soy products, fruit, and fish determined by factor analysis is positively correlated with cognitive function score (10). Almost all of these studies have been carried out in Western countries, some of which have limitations in controlling cofounding factors. However, large-scale research in China is limited, and few studies have explored the associations between dietary patterns and cognitive outcomes in the subtypes of MCI. Accumulating more evidence in different regions of China is crucial to prevent the cognitive decline. Because of food items, nutrients in foods, and eating behaviors, especially the obvious nutrition transition in China. The beneficial dietary patterns identified in some regions may not be consistent with those in other regions (11).
This study aims to determine the main dietary patterns related to multi-dimensional cognitive functions, such as global cognitive status, six cognitive domain functions, prevalence of MCI, and four main MCI subtypes in Chinese residents aged 55 years and older, using the data from the Community-based Cohort Study on Nervous System Diseases (CCSNSD).
Materials and Methods
Study Population
Community-based Cohort Study on Nervous System Diseases, a longitudinal study established by the National Institute for Nutrition and Health, Chinese Center for Disease Control and Prevention in 2018–2019, focuses on potential factors associated with risks of epilepsy, AD and Parkinson's disease (PD). We used a multistage stratified random sampling approach to draw participants without such diseases in Hebei, Zhejiang, Shaanxi, and Hunan provinces. Detailed survey design has been reported elsewhere (12). The Institutional Review Board of the National Institute for Nutrition and Health approved the study (No. 2017020, November 6, 2017). All participants provided their written informed consent.
This study recruited 6,143 eligible subjects aged 55 years and older without clinical diagnosis of AD or comorbidities. In the analysis, we used a subsample of 4,309 subjects (1,956 men and 2,353 women) aged between 55 and 86 years, excluding 1,769 subjects with incomplete data on sociodemographic characteristic, disease history, cognitive examination, food frequency questionnaire (FFQ), psychological evaluation, or basic abilities of daily living, and further excluding 65 subjects unable to perform basic activities of daily living, such as eating, dressing, bathing, toileting, grooming, moving to bed or chair, walking through the room, or incontinence.
Identification of Dietary Patterns
We assessed dietary intake using a validated semi-quantitative FFQ covering 65 food items. Participants reported frequency categories (daily, weekly, monthly, annually, or never) and the average amount for each food item consumed in the last 12 months. We calculated their intake of each item based on the average intake frequency and quantity. If the subject reported the “never” intake frequency, we set his/her intake to 0 g/day or week. According to the participant's habitual intake and the similarity of types, we categorized 49 major food items into 13 subgroups, such as rice, wheat, tubers, legumes, fresh vegetables, fresh fruit, pork, beef and mutton, poultry, fish, eggs, dairy, and nuts. Supplementary Table S1 provides detailed information on the foods in each subgroup.
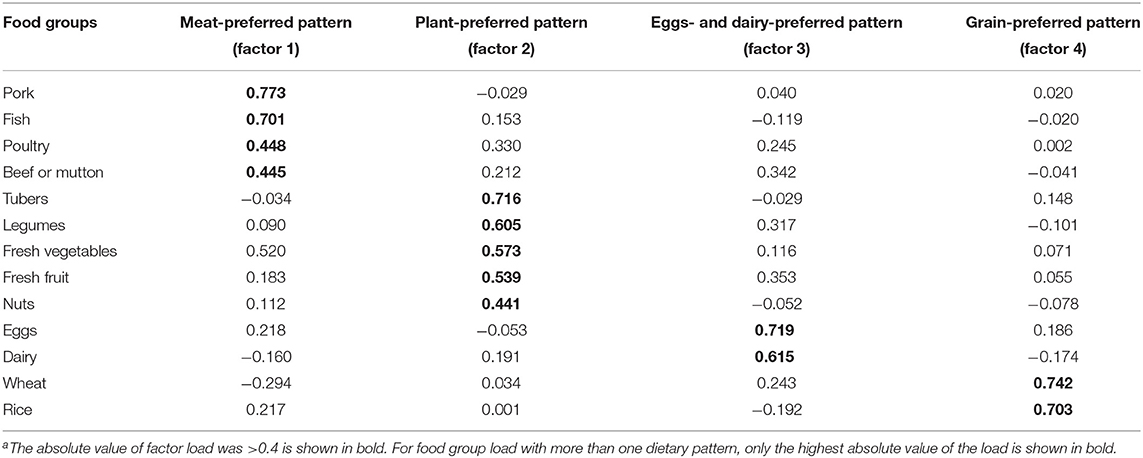
We used factor analysis with varimax rotation to analyze the 13 food subgroups and determined four factors, “meat-preferred” pattern, “plant-preferred” pattern, “eggs- and dairy-preferred” pattern, and “grain-preferred” pattern, based on eigenvalues >1.0, a scree plot, and the interpretability. Food items with an absolute factor load value >0.4 are the main contributors to the dietary pattern, representing the characteristics of each pattern. We calculated the factor scores of each participant for each pattern by summing the intake of food groups weighted by their factor loadings and grouped them into quartiles for further analysis. A higher quartile indicates more consistency with the pattern being calculated.
Assessment of Cognitive Function
We used the Montreal Cognitive Assessment (MoCA) to assess the cognitive function of participants. The sum of the scale items produces a total MoCA score ranging from 0 to 30, which is positively associated with the global cognitive function (13). The MCI criteria is based on the Chinese MoCA norms (14): total MoCA score ≤ 13 for illiterate individuals, ≤ 19 for individuals with 1–6 years of education, and ≤ 24 for individuals with 7 years of education or more.
We evaluated the cognitive domain functions of memory, execution, visuospatial, language, attention, and orientation by the memory index score (MIS), executive index score (EIS), visuospatial index score (VIS), language index score (LIS), attention index score (AIS), and orientation index score (OIS), respectively. We calculated these index scores based on the MoCA cognitive domain index score (13). We defined each impaired cognitive domain as participants who scored <1.5 SDs below the age- and education-adjusted mean value in that cognitive domain (15). We defined MCI subtypes as participants having MCI and characterized by different cognitive domain deficits (16, 17): amnestic MCI single domain (aMCI-SD): only memory impairment; non-amnestic MCI single domain (naMCI-SD): a deficit in one cognitive domain other than memory; amnestic MCI multiple domains (aMCI-MD): memory impairment plus one other impaired domain; and non-amnestic MCI multiple domains (naMCI-MD): deficits in at least 2 domains other than memory.
Assessment of Covariates
Interviewers with a degree in medicine or public health who have received two rounds of training by the national or provincial experts and passed the qualification examination collected all survey data, such as gender (male or female), age (years), education level (below primary school, primary school, or secondary school or above), residential area (rural or urban), current employment status (yes or no), and monthly household income per capita [ <1,000, 1,000–3,999, or ≥4,000 (Chinese yuan RMB)], smoking (never or ever/current), drinking (never or ever/current), physical activity (occupations, household chores, leisure time, and transportation activities), sleep duration (hours), and medication usage (yes or no), and other. We converted total physical activity hours into metabolic equivalent of tasks (METs) hours per week based on the Compendium of Physical Activities recommended by the American College of Sports Medicine Association (18), and divided them into tertiles (low, medium, and high). We categorized sleep hours based on the cutoff points recommended by the National Sleep Foundation, 7–9 h for participants aged between 55 and 64 years, and 7–8 h for participants 65 years and older (19). We calculated the total energy intake based on the China Food Composition Table.
We defined history of diet-related chronic diseases as participant having hypertension, diabetes, stroke, or myocardial infarction diagnosed or treated by physicians. We defined obesity as body mass index (BMI) ≥ 28 kg/m2, and central obesity as a waist circumference ≥90 cm for men and ≥85 cm for women. Detailed information on physical measurements has been reported elsewhere (12).
Statistical Analysis
For continuous variables, we used mean ± SD to describe the distribution and Wilcoxon signed rank test or Kruskal–Wallis H-test to examine the differences among groups as appropriate. For categorical variables, we used quantity (percentage) and the chi-square test. We used a series of quantile regression models to estimate the associations of each dietary pattern with the global cognitive score and cognitive domain subtypes. We built a set of modes, such as model without any adjustment (Model 1), model adjusted for age, gender, residential area, education level, current employment status, and household income level (Model 2), model further adjusted for physical activity, smoking, drinking, sleep duration, and total food energy (Model 3), and model further adjusted for diet-related chronic disease history, obesity, and central obesity (Model 4). We used multivariable logistic regression models to calculate the adjusted odds ratio (AOR) and 95% CI to estimate the relationship between each dietary pattern and the prevalence of MCI and its subtypes, controlling for covariates including age, gender, residential area, education level, current employment status, household income level, physical activity, smoking, drinking, sleep duration, total energy intake, diet-related chronic disease history, obesity, and central obesity. In addition, we tested the linear trends for the prevalence of MCI and its subtypes by assigning the median value to the quartiles of each dietary pattern score as a continuous variable. We used SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA) for all statistical analysis, and considered the statistical significance as a two-sided p < 0.05.
Results
Characteristics of Subjects
As shown in Table 1, the average age of the participants was 68.4 years. Among the 4,309 participants, 45.4% were men, 50.6% lived in rural areas, 85.8% achieved a primary education level or above, and 76.4% had a per capita monthly income more than 1,000 yuan. The proportion of participants with a history of diet-related chronic diseases, obesity, or central obesity were 36.3, 13.2, and 46.5%, respectively. Compared with participants with the normal cognitive function, MCI participants were older and poorer, and had lower energy intake (p < 0.05).
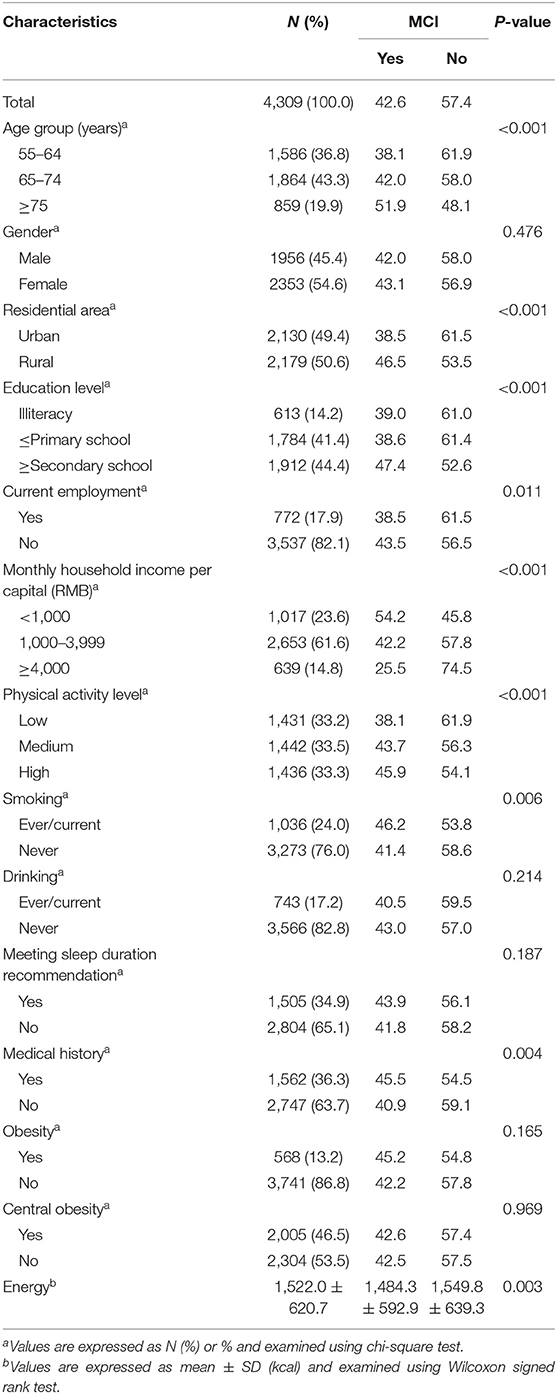
Table 1. Subject characteristics by cognitive status among Chinese adults aged 55 years and above in the Community-based Cohort Study on Nervous System Diseases (CCSNSD) 2018–2019.
Dietary Patterns
We identified four dietary patterns using factor analysis. Table 2 presents the factor load in each food group. These four patterns explained 50.1% of the variance in dietary intake (i.e., 23.0% by factor 1, 10.9% by factor 2, 8.9% by factor 3, and 7.3% by factor 4). The first factor “meat-preferred” pattern, was characterized by a large intake of pork, fish, poultry, and beef or lamb. The second pattern, “plant-preferred” pattern, included a large intake of tubers, legumes, fresh vegetables, fresh fruit, and nuts. The third pattern, “eggs- and dairy-preferred” pattern, referred to a large intake of eggs and dairy products. The fourth pattern, “grain-preferred” pattern, was characterized by a large intake of rice- and wheat-based foods. The intake of each food group was significantly different among the quartiles of each dietary pattern (p < 0.05, Supplementary Table S2).
Supplementary Table S3 presents sociodemographic status, lifestyle variables, and health-related factors for each quartile of dietary pattern scores. The distributions of residential area, education level, employment status, household income level, drinking, and energy intake were significantly different among the quartiles of the four dietary patterns (p < 0.05). Physical activity and smoking status were significantly different among the quartiles of the “meat-preferred” pattern, the “plant-preferred” pattern, and the “grain-preferred” pattern (p < 0.05). The history of diet-related chronic diseases, obesity, and central obesity were significantly different among the quartiles of the “meat-preferred” pattern and the “eggs- and dairy-preferred” pattern (p < 0.05).
Dietary Patterns and Global Cognition and Cognitive Domains
Table 3 presents the global cognitive score and cognitive domain subscores of the quartiles of each dietary pattern. Participants in the highest quartile of the “meat-preferred” pattern had higher global cognitive function scores and higher scores in most cognitive domains (p < 0.05) compared with those in other quartiles. The global cognitive function scores and cognitive domains were significantly increased as the “plant-preferred” pattern score increased from the first quartile to the fourth quartile (p < 0.05), while they were decreased as the “grain-preferred” pattern score from the lowest to the highest quartile (p < 0.05). The lowest and the highest scores of global cognitive function, execution, visuospatial, language, and attention were distributed in the third quartile and the fourth quartile of the “eggs- and dairy-preferred” pattern (p < 0.05).
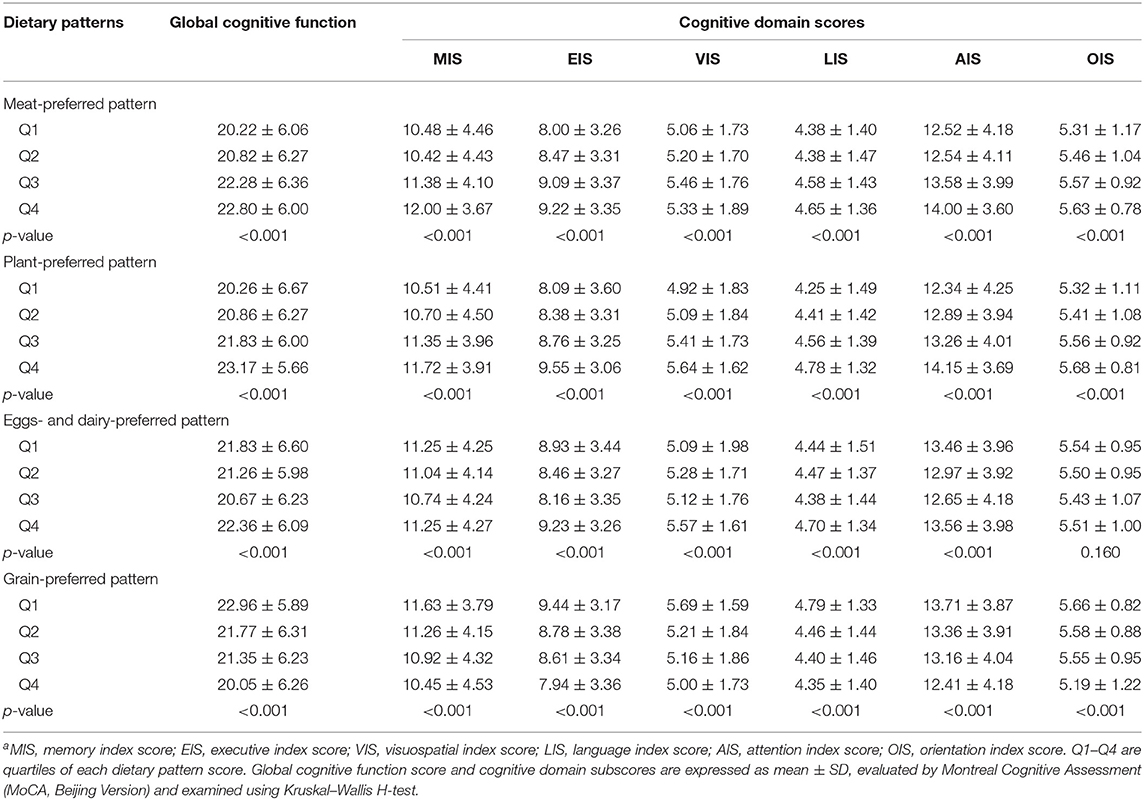
Table 3. Differences in global cognitive score and cognitive domain subscores by quartiles of each dietary pattern scorea.
Table 4 presents the association of the four dietary patterns with the global cognitive score and cognitive domain subscores. In the final multivariate models, the average scores of the global cognition, memory, execution, and attention were increased significantly as the quartiles of the “meat-preferred” pattern score increased (p < 0.05); similarly, participants with high intake of the “plant-preferred” pattern may have higher scores of 0.45, 0.25, 0.18, 0.13, 0.07, and 0.30 in the global cognition, memory, execution, visuospatial, language, and attention function (p < 0.05), respectively. In contrast, the average scores of global cognition, execution, visuospatial, and language function were decreased significantly as the quartiles of the “grain-preferred” pattern score increased (p < 0.05). After adjusting for all potential factors, only a positively significant relationship was observed between the visuospatial function score and the “eggs- and dairy-preferred” pattern (p < 0.05). No significant association was observed between the orientation function score and any of the four dietary patterns (p > 0.05).
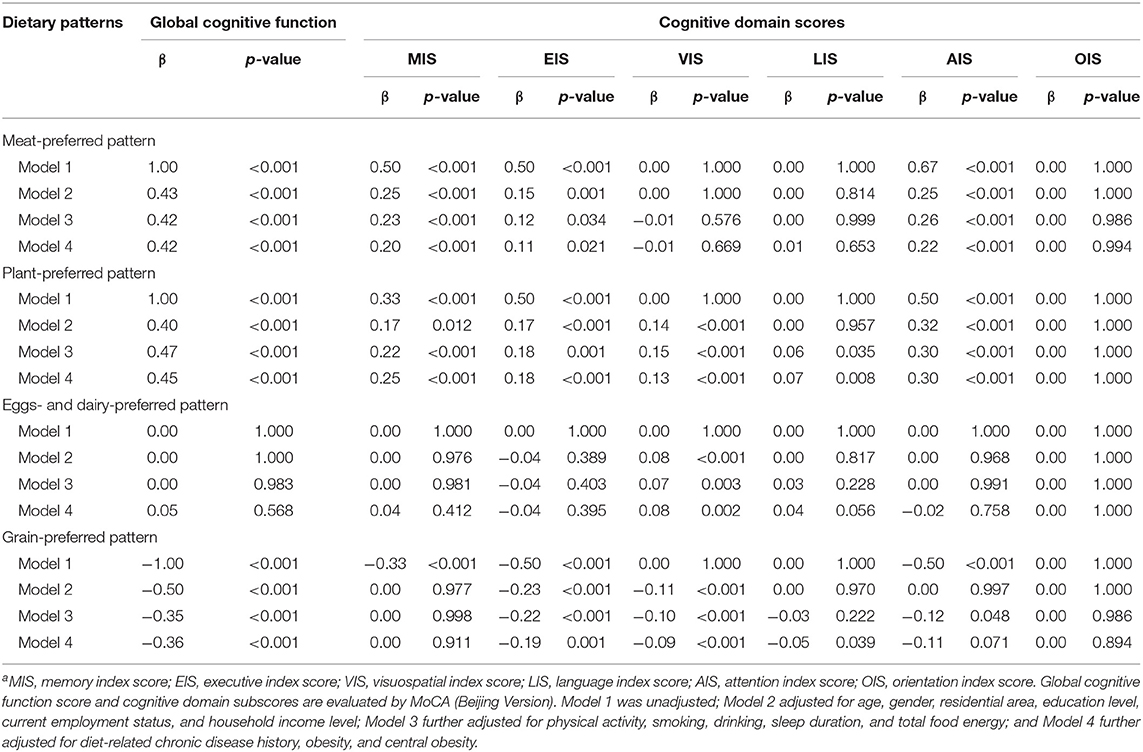
Table 4. Quantile regression analysis of associations of four dietary pattern scores with global cognitive score and cognitive domain subscoresa.
Dietary Patterns and MCI and Its Subtypes
As shown in Figure 1, the prevalence distributions of MCI and some of its subtypes differed among the quartiles of each dietary pattern. Participants in the highest quartile of the “meat-preferred” pattern had lower prevalence of MCI, aMCI-SD, naMCI-SD, and aMCI-MD (p < 0.05); and similarly, those in the highest quartile of the “plant-preferred” pattern had lower prevalence of MCI, aMCI-MD, and naMCI-MD (p < 0.05), compared with those in other quartiles of the corresponding pattern. In contrast, participants in the bottom quartile of the “eggs- and dairy-preferred” pattern had lower prevalence of MCI, aMCI-SD, and naMCI-SD (p < 0.05), and those in the bottom quartile of the “grain-preferred” pattern had lower prevalence of MCI, naMCI-SD, aMCI-MD, and naMCI-MD (p < 0.05).
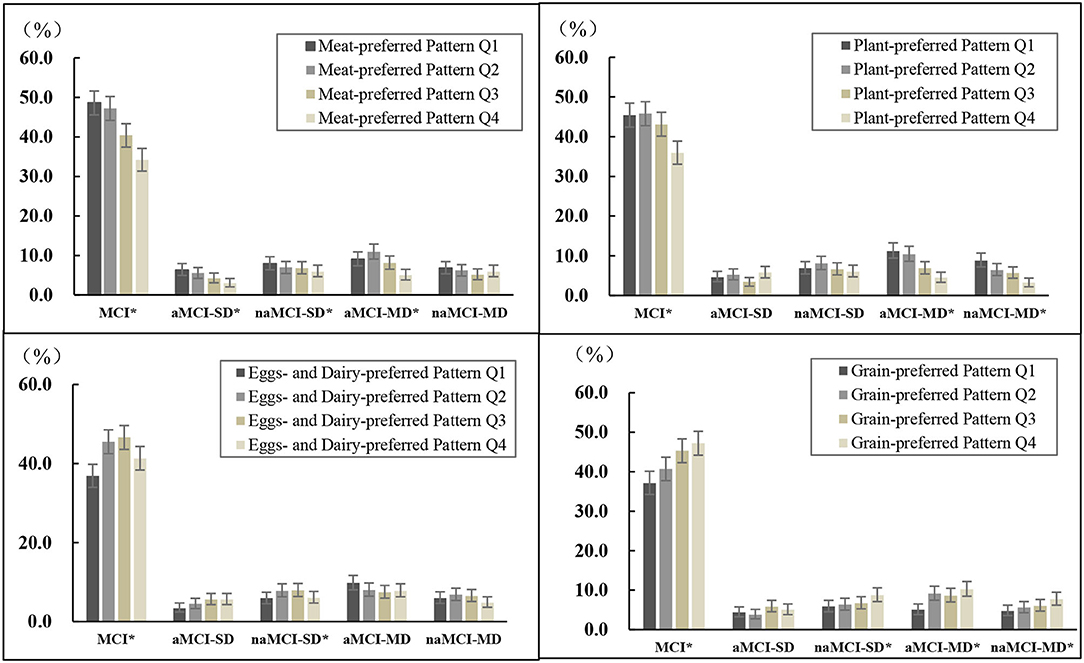
Figure 1. Prevalence of MCI and its subtypes by quartiles of each dietary pattern scorea. aQ1–Q4 are quartiles of each dietary pattern score. MCI, mild cognitive impairment; aMCI-SD, amnestic MCI single domain; naMCI-SD, non-amnestic MCI single domain; aMCI-MD, amnestic MCI multiple domains; naMCI-MD, non-amnestic MCI multiple domains. *p < 0.05 within dietary pattern score category, examined by chi-square test.
Figure 2 presents AOR and the 95% CI of MCI and its subtypes in the quartiles of each dietary pattern score. After controlling for multi-covariates, participants in the highest quartile of the “meat-preferred” pattern had 24 and 56% lower odds of MCI (AOR = 0.76, 95% CI 0.63–0.92) and aMCI-SD (AOR = 0.44, 95% CI 0.28–0.71), respectively, compared with those in the lowest quartile; similarly, those in the highest quartile of the “plant-preferred” pattern were less likely to have MCI, aMCI-MD, and naMCI-MD. The AOR was 0.72 (95% CI 0.60–0.80), 0.49 (95% CI 0.34–0.72), and 0.41 (95% CI 0.27–0.63), respectively. In addition, the increase in each quartile level of these pattern scores significantly reduced the odds of MCI and the corresponding subtypes mentioned above (p-trend < 0.05). On the contrary, participants in the highest quartile of the “grain-preferred” pattern score tended to have 34% (AOR = 1.34, 95% CI 1.11–1.63, p-trend = 0.003) and 65% (AOR = 1.65, 95% CI 1.13–2.40, p-trend = 0.007) higher odds of MCI and naMCI-SD compared with those in the lowest quartile, and those in the second quartile of the “eggs- and dairy-preferred” pattern score had an AOR of 1.22 (95% CI 1.02–1.47) for the prevalence of MCI.
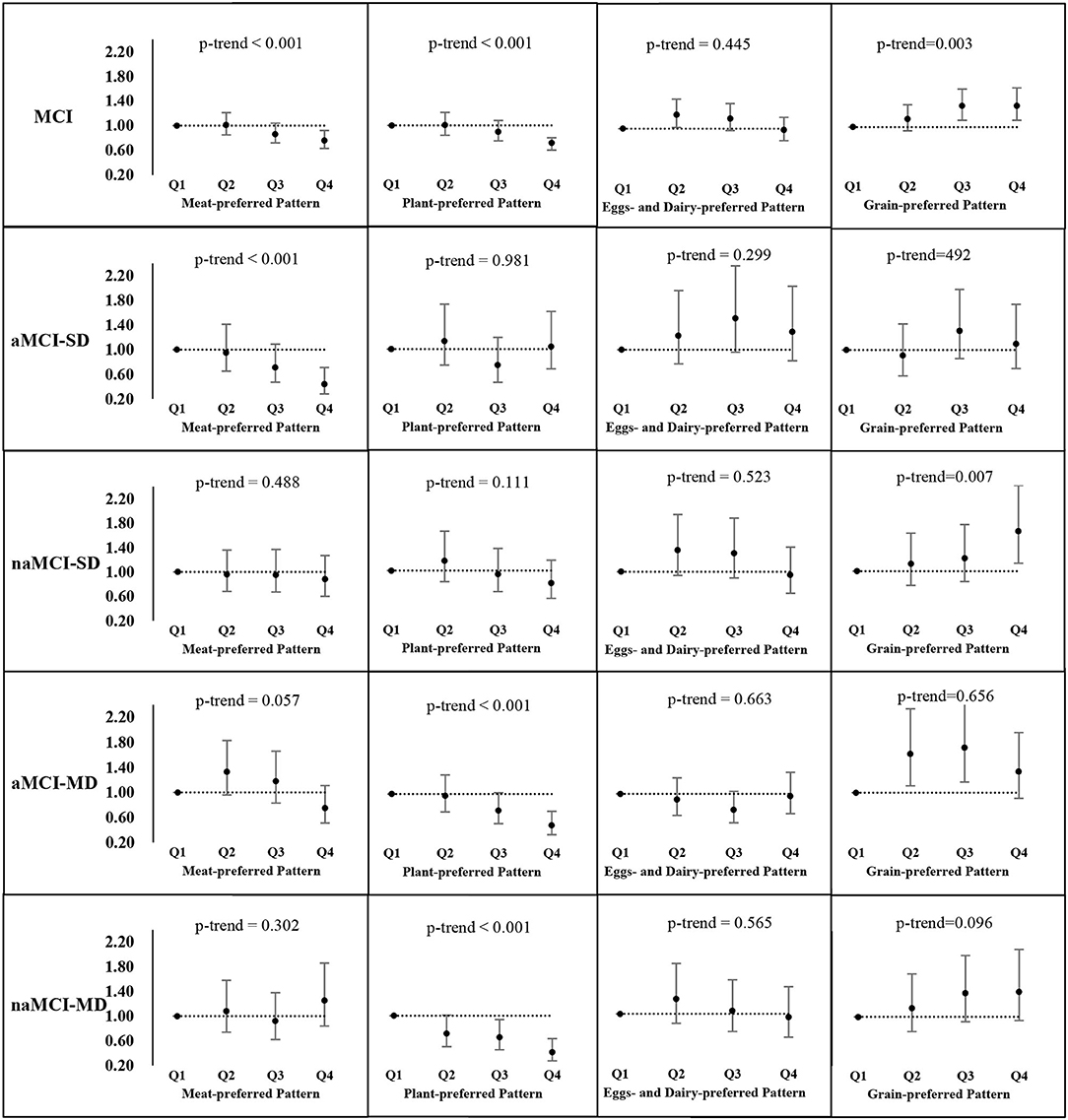
Figure 2. Adjusted odds ratios (AOR) and the corresponding 95% CIs for MCI and its subtypes across quartiles of each dietary pattern scorea. aMCI, mild cognitive impairment; aMCI-SD, amnestic MCI single domain; naMCI-SD, non-amnestic MCI single domain; aMCI-MD, amnestic MCI multiple domains; naMCI-MD, non-amnestic MCI multiple domains. Adjusted for age, gender, residential area, education level, current employment status, household income level, physical activity, smoking, drinking, sleep duration, total food energy, diet-related chronic disease history, obesity, and central obesity.
Discussion
In this cross-sectional study, we identified four different dietary patterns: “meat-preferred” pattern (pork, fish, poultry, and beef or mutton), “plant-preferred” pattern (tubers, legumes, fresh vegetables, fresh fruit, and nuts), “eggs- and dairy-preferred” pattern (eggs and dairy products), and “grain-preferred” pattern (rice- and wheat-based foods). We found that adherence to the “meat-preferred” pattern and the “plant-preferred” pattern increased the scores of the global cognitive function and several cognitive domain functions and decreased the odds of MCI and some subtypes after adjustments for all potential confounding variables. Conversely, better adherence to the “grain-preferred” pattern reduced cognitive function scores and increased the odds of MCI and naMCI-SD in adjusted models. In addition, we observed that the “eggs- and dairy-preferred” pattern was associated with higher cognitive domain scores of visuospatial function. Participants in the second quartile of this pattern score had a 22% (95% CI: 1.02–1.47) higher odds of MCI compared with those in the bottom quartile.
In this study, the “meat-preferred” pattern is conducive in improving the cognitive function and reducing the odds of MCI, aMCI-MD, and naMCI-MD, supported by its food intake profile that is characterized by high intake of pork, fish, poultry, beef, and lamb. Previous studies have showed that these foods play a potentially beneficial role in cognition (12, 20). Specifically, we found that participants in the third or top quartile intake of pork, beef or mutton, poultry, and fish had about 20–30% lower ORs of MCI and its some subtypes, respectively, compared with those in the bottom intake of the corresponding food group (12). Moreover, the results of a large longitudinal study of 5,934 French people 65 years and older with an average follow-up of 9.8 years showed that the low-meat intake is associated with an increased risk of cognitive impairment compared with regular intake frequency (7). In fact, some researchers thought that the high-meat intake with high saturated fat content increases inflammation and circulating cytokines, and have a negative impact on cognition (21), but this may be offset by the protein source itself which is beneficial to cognitive function by promoting the production and release of catecholamines, when maintaining a reasonable balance of this pattern. In addition, increasing the intake of fish rich in polyunsaturated fatty acids could significantly combat the pathologic cognitive impairment of the elderly shown by mechanism studies and epidemiologic analyses (22, 23), which supports that this pattern is related to superior cognitive function.
Our findings show that the “plant-preferred” pattern is positively associated with a variety of cognitive functions ranging from the global mental status to specific domains. Food groups that are good for to brain are fruit, vegetables, legumes, and nuts, which are rich in fiber, beta carotene, vitamins K and C, folate, and especially magnesium (24, 25). These antioxidants, which are considered to have anti-inflammatory properties, have shown to be significantly associated with the prevention of cognitive impairment (26), because oxidative stress and inflammation are the triggers of the cognitive decline process (27). Some epidemiological studies are consistent with the above findings, despite the dietary pattern analysis is different (28, 29). For example, a food pattern related to high intake of vegetables, fruit, legumes, and nuts, derived through a reduced rank regression analysis of 2,311 Chinese elderly adults aged 60–88 years, is associated with better memory and language function and a lower likelihood of MCI (29). Although the main criticism of plant-based diets is the risk of vitamin B12 deficiency (30, 31), which is correlated to cognitive dysfunction (e.g., fatigue, memory, and movement problems) (32). However, moderate intake of poultry, beef or lamb can provide efficient vitamin B12 intake. This is another important feature of the “plant-preferred” pattern in this study, providing a plausible explanation to our findings.
Eggs, milk, and dairy products are nutritious foods that contain a variety nutrients associated with improving cognitive function, such as folate, vitamin B12 (33), choline (34), and protein (35). In 2019, a systematic review of six studies summarized the effects of milk and dairy products intake on cognitive function, indicating that dairy products may help prevent cognitive decline (36). However, a study of 3,835 American adults 65 years conducted in the same year did not find a similar relationship between egg intake and cognitive health (37). Our study found that eggs and dairy foods intake is positively correlated with higher scores of the “eggs- and dairy-preferred” pattern, which may improve visuospatial ability. In addition, we observed that the intake of eggs in the third quartile of this pattern score reached the recommended amount (40–50 g/day) of the Chinese Dietary Guidelines, but even in the top quartile the dairy intake did not reach its recommended level (300 g/day). In this regard, the difference in the intake of eggs and dairy foods intakes in the first two quartiles of the pattern is not significant. Therefore, the observation that subjects in the second quartile tended to have higher odds of MCI than subjects in the bottom quartile may be caused by data variances.
In addition, in our study, adults who adhere to the “grain-preferred” pattern consume more rice- and wheat-based foods, and their cognitive functions are often incomplete, which is consisting with the findings in the literature. There is evidence that high glycemic index foods may cause cognitive impairment through adverse effects on neuronal integrity and glucose metabolism in the nervous system (38, 39). It is worth noting that there is evidence that moderate intake of rice is negatively associated with cognitive functions (12, 40). Given that wheat intake increased as the quartiles of this pattern score increased, but rice intake did not increase, although both intakes were dominant. Therefore, we speculate that the detrimental effects of this pattern on cognition may be mainly due to the large intake of wheat-based foods. Of course, large-scale prospective cohort studies are needed to provide more clues to the underlying mechanism.
This study has several limitations. First, the cross-sectional nature of our study cannot provide evidence of any causal conclusions between dietary patterns and multidimensional cognition. It must consider the possibility of reverse causality that changes in dietary patterns lead to cognitive decline that cannot be captured. Second, the intake of food groups comes from a valid, semi-quantitative FFQ covering 1 year. Ranking subjects based on the food intake and presenting the profiles information of dietary nutrients intake in patterns is not completely accurate and may also lead to recall bias. However, the census concluded that recall errors due to cognitive impairment are believed to bias the results toward the null hypothesis. Third, the factor analysis method is highly data-driven, and thus dominant food groups are still interacted with other second or minor food groups. Fourth, although we carefully adjusted some covariates during the data analysis process, we may not consider residual confounding factors (e.g., hyperlipidemia, social engagement, living conditions, etc.). Finally, because this study covers adults in four provinces of China only, any generalization of the results we obtained to other regions should be carried out with caution. However, the CCSNSD is specifically designed to investigate the risk factors of neurological diseases in China. The population-based design reduces the selection bias, and a comprehensive evaluation of participant's cognitive function by trained evaluators with a degree in medicine or public health increases the internal validity of the findings.
This study showed the associations of four distinct dietary patterns with cognitive functions among Chinese adults, and thus findings can contribute to the establishment of dietary guidelines targeting older Chinese adults to reduce the cognitive impairment. It is of great significance to provide upgraded scientific evidence and strategies for early nutrition intervention and have important theoretical and social significance for promoting healthy aging.
Conclusion
To conclude, the study is the first to examine the relationship between dietary patterns and multidimensional cognitive function, global cognitive status, six cognitive domain functions, and cognitive-related outcomes of MCI and its four subtypes in Chinese adults. The study showed that the “plant-preferred” pattern and the “meat-preferred” pattern are associated with higher cognitive function scores and lower odds of MCI and some of its subtypes, which further provide evidence that higher intake of diet rich in vegetables, fruit, legumes, nuts, and meat may help prevent cognitive decline, and people who prefer grain foods, especially wheat-based foods, are negatively associated with cognitive health. However, the exact role of the “eggs- and dairy-preferred” pattern in cognition remains to be determined. Future prospective cohort studies are needed to examine the effects of these patterns on brain health to prove the causal relationship between dietary patterns and cognitive function.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of the National Institute for Nutrition and Health (No. 2017020, November 6, 2017). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
QH, XJ, JZ, and ZW contributed to the conceptualization of the manuscript. QH and JZ performed the methodology. QH is responsible for the formal analysis and writing—original draft preparation. FH performed data curation. XJ, JZ, and ZW contributed to writing—review. ZW and BZ contributed to project administration. HW is responsible for supervision. FH, LW, HJ, MG, YH, WS, YM, and XZ contributed to the investigation. All authors read and approved the final manuscript.
Funding
This study was supported by grants from the Ministry of Finance of China, the National Key R&D Program of China, the Precision Medicine Project–Cohort Study on Nervous System Diseases (2017YFC0907700), and the Community-based Cohort Study on Nervous System Diseases (2017YFC0907701).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The present study uses data from the CCSNSD. We thank all of the participants and staff involved in the surveys.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.806871/full#supplementary-material
Abbreviations
AIS, Attention index score; EIS, Executive index score; LIS, Language index score; MCI, Mild cognitive impairment; MIS, Memory index score; OIS, Orientation index score; VIS, Visuospatial index score; aMCI-SD, Amnestic MCI single domain; aMCI-MD, Amnestic MCI multiple domains; naMCI-SD, Non-amnestic MCI single domain; naMCI-MD, Non-amnestic MCI multiple domains.
References
1. Jia L, Du Y, Chu L, Zhang Z, Li F, Lyu D, et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: a cross-sectional study. Lancet Public Health. (2020) 5:e661–71. doi: 10.1016/S2468-2667(20)30185-7
2. Davis M, O Connell T, Johnson S, Cline S, Merikle E, Martenyi F, et al. Estimating Alzheimer's disease progression rates from normal cognition through mild cognitive impairment and stages of dementia. Curr Alzheimer Res. (2018) 15:777–88. doi: 10.2174/1567205015666180119092427
3. Xue H, Hou P, Li Y, Mao X, Wu L, Liu Y. Factors for predicting reversion from mild cognitive impairment to normal cognition: a meta-analysis. Int J Geriatr Psychiatry. (2019) 34:1361–8. doi: 10.1002/gps.5159
4. Liu-Seifert H, Schumi J, Miao X, Tian Y, Rabbia M, Andersen SW, et al. Disease modification in Alzheimer's disease: current thinking. Ther Innov Regul Sci. (2020) 54:396–403. doi: 10.1007/s43441-019-00068-4
5. Solfrizzi V, Custodero C, Lozupone M, Imbimbo BP, Valiani V, Agosti P, et al. Shits with Alzheimer's disease and late-life cognitive disorders: a systematic review. J Alzheimers Dis. (2017) 59:815–49. doi: 10.3233/JAD-170248
6. Li M, Shi Z. A prospective association of nut consumption with cognitive function in chinese adults aged 55+ China health and nutrition survey. J Nutr Health Aging. (2019) 23:211–6. doi: 10.1007/s12603-018-1122-5
7. Ngabirano L, Samieri C, Feart C, Gabelle A, Artero S, Duflos C, et al. Intake of meat, fish, fruits, and vegetables and long-term risk of dementia and Alzheimer's disease. J Alzheimers Dis. (2019) 68:711–22. doi: 10.3233/JAD-180919
8. Mantzorou M, Vadikolias K, Pavlidou E, Tryfonos C, Vasios G, Serdari A, et al. Mediterranean diet adherence is associated with better cognitive status and less depressive symptoms in a Greek elderly population. Aging Clin Exp Res. (2021) 33:1033–40. doi: 10.1007/s40520-020-01608-x
9. Trichopoulou A, Kyrozis A, Rossi M, Katsoulis M, Trichopoulos D, La Vecchia C, et al. Mediterranean diet and cognitive decline over time in an elderly Mediterranean population. Eur J Nutr. (2015) 54:1311–21. doi: 10.1007/s00394-014-0811-z
10. Okubo H, Inagaki H, Gondo Y, Kamide K, Ikebe K, Masui Y, et al. Association between dietary patterns and cognitive function among 70-year-old Japanese elderly: a cross-sectional analysis of the SONIC study. Nutr J. (2017) 16:56. doi: 10.1186/s12937-017-0273-2
11. Zhang J, Wang Z, Du W, Huang F, Jiang H, Bai J, et al. Twenty-five-year trends in dietary patterns among Chinese adults from 1991 to 2015. Nutrients. (2021) 13:e1327. doi: 10.3390/nu13041327
12. Huang Q, Jia X, Zhang J, Huang F, Wang H, Zhang B, et al. Diet-cognition associations differ in mild cognitive impairment subtypes. Nutrients. (2021) 13:e1341. doi: 10.3390/nu13041341
13. Julayanont P, Brousseau M, Chertkow H, Phillips N, Nasreddine ZS. Montreal cognitive assessment memory index score (MoCA-MIS) as a predictor of conversion from mild cognitive impairment to Alzheimer's disease. J Am Geriatr Soc. (2014) 62:679–84. doi: 10.1111/jgs.12742
14. Lu J, Li D, Li F, Zhou A, Wang F, Zuo X, et al. Montreal cognitive assessment in detecting cognitive impairment in Chinese elderly individuals: a population-based study. J Geriatr Psychiatry Neurol. (2011) 24:184–90. doi: 10.1177/0891988711422528
15. Ding D, Zhao Q, Guo Q, Meng H, Wang B, Luo J, et al. Prevalence of mild cognitive impairment in an urban community in China: a cross-sectional analysis of the Shanghai Aging Study. Alzheimers Dement. (2015) 11:300–9.e302. doi: 10.1016/j.jalz.2013.11.002
16. Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, et al. Mild cognitive impairment–beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. (2004) 256:240–6. doi: 10.1111/j.1365-2796.2004.01380.x
17. Petersen RC. Mild cognitive impairment. Continuum (Minneap Minn). (2016) 22:404–18. doi: 10.1212/CON.0000000000000313
18. Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. (2000) 32:S498–504. doi: 10.1097/00005768-200009001-00009
19. Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, Doncarlos L, et al. National Sleep Foundation's sleep time duration recommendations: methodology and results summary. Sleep Health. (2015) 1:40–3. doi: 10.1016/j.sleh.2014.12.010
20. Zhu J, Xiang YB, Cai H, Li H, Gao YT, Zheng W, et al. A prospective investigation of dietary intake and functional impairments among the elderly. Am J Epidemiol. (2018) 187:2372–86. doi: 10.1093/aje/kwy156
21. Tan B, Norhaizan M. Effect of high-fat diets on oxidative stress, cellular inflammatory response and cognitive function. Nutrients. (2019) 11:2579. doi: 10.3390/nu11112579
22. Qin B, Plassman BL, Edwards LJ, Popkin BM, Adair LS, Mendez MA. Fish intake is associated with slower cognitive decline in Chinese older adults. J Nutr. (2014) 144:1579–85. doi: 10.3945/jn.114.193854
23. Zhang Y, Chen J, Qiu J, Li Y, Wang J, Jiao J. Intakes of fish and polyunsaturated fatty acids and mild-to-severe cognitive impairment risks: a dose-response meta-analysis of 21 cohort studies. Am J Clin Nutr. (2016) 103:330–40. doi: 10.3945/ajcn.115.124081
24. Chan R, Chan D, Woo J. A cross sectional study to examine the association between dietary patterns and cognitive impairment in older Chinese people in Hong Kong. J Nutr Health Aging. (2013) 17:757–65. doi: 10.1007/s12603-013-0348-5
25. Jiang X, Huang J, Song D, Deng R, Wei J, Zhang Z. Increased consumption of fruit and vegetables is related to a reduced risk of cognitive impairment and dementia: meta-analysis. Front Aging Neurosci. (2017) 9:18. doi: 10.3389/fnagi.2017.00018
26. Mcgrattan AM, Mcguinness B, Mckinley MC, Kee F, Passmore P, Woodside JV, et al. Diet and inflammation in cognitive ageing and Alzheimer's disease. Curr Nutr Rep. (2019) 8:53–65. doi: 10.1007/s13668-019-0271-4
27. Den H, Dong X, Chen M, Zou Z. Efficacy of probiotics on cognition, and biomarkers of inflammation and oxidative stress in adults with Alzheimer's disease or mild cognitive impairment - a meta-analysis of randomized controlled trials. Aging. (2020) 12:4010–39. doi: 10.18632/aging.102810
28. Rajaram S, Jones J, Lee GJ. Plant-based dietary patterns, plant foods, and age-related cognitive decline. Adv Nutr. (2019) 10:S422–s436. doi: 10.1093/advances/nmz081
29. Su X, Zhang J, Wang W, Ni C, Hu S, Shao P, et al. Dietary patterns and risk of mild cognitive impairment among Chinese elderly: a cross-sectional study. PLoS ONE. (2020) 15:e0235974. doi: 10.1371/journal.pone.0235974
30. Gilsing AM, Crowe FL, Lloyd-Wright Z, Sanders TA, Appleby PN, Allen NE, et al. Serum concentrations of vitamin B12 and folate in British male omnivores, vegetarians and vegans: results from a cross-sectional analysis of the EPIC-Oxford cohort study. Eur J Clin Nutr. (2010) 64:933–9. doi: 10.1038/ejcn.2010.142
31. Medawar E, Huhn S, Villringer A, Veronica Witte A. The effects of plant-based diets on the body and the brain: a systematic review. Transl Psychiatry. (2019) 9:226. doi: 10.1038/s41398-019-0552-0
32. Moore E, Mander A, Ames D, Carne R, Sanders K, Watters D. Cognitive impairment and vitamin B12: a review. Int Psychogeriatr. (2012) 24:541–56. doi: 10.1017/S1041610211002511
33. Morris MC. Nutritional determinants of cognitive aging and dementia. Proc Nutr Soc. (2012) 71:1–13. doi: 10.1017/S0029665111003296
34. Blusztajn JK, Slack BE, Mellott TJ. Neuroprotective actions of dietary choline. Nutrients. (2017) 9:e815. doi: 10.3390/nu9080815
35. La Rue A, Koehler KM, Wayne SJ, Chiulli SJ, Haaland KY, Garry PJ. Nutritional status and cognitive functioning in a normally aging sample: a 6-y reassessment. Am J Clin Nutr. (1997) 65:20–9. doi: 10.1093/ajcn/65.1.20
36. Cuesta-Triana F, Verdejo-Bravo C, Fernandez-Perez C, Martin-Sanchez FJ. Effect of milk and other dairy products on the risk of frailty, sarcopenia, and cognitive performance decline in the elderly: a systematic review. Adv Nutr. (2019) 10:S105–s119. doi: 10.1093/advances/nmy105
37. Bishop NJ, Zuniga KE. Egg consumption. Multi-domain cognitive performance, and short-term cognitive change in a representative sample of older US adults. J Am Coll Nutr. (2019) 38:537–46. doi: 10.1080/07315724.2019.1566035
38. Sunram-Lea SI, Owen L. The impact of diet-based glycaemic response and glucose regulation on cognition: evidence across the lifespan. Proc Nutr Soc. (2017) 76:466–77. doi: 10.1017/S0029665117000829
39. Taylor MK, Sullivan DK, Swerdlow RH, Vidoni ED, Morris JK, Mahnken JD, et al. A high-glycemic diet is associated with cerebral amyloid burden in cognitively normal older adults. Am J Clin Nutr. (2017) 106:1463–70. doi: 10.3945/ajcn.117.162263
Keywords: dietary patterns, cognition, cognitive domains, mild cognitive impairment, Chinese population
Citation: Huang Q, Jiang H, Zhang J, Jia X, Huang F, Wang H, Zhang B, Wang L, Gu M, Huang Y, Shi W, Ma Y, Zhang X and Wang Z (2022) Dietary Patterns Are Associated With Multi-Dimensional Cognitive Functions Among Adults Aged 55 and Older in China. Front. Nutr. 9:806871. doi: 10.3389/fnut.2022.806871
Received: 01 November 2021; Accepted: 17 January 2022;
Published: 17 February 2022.
Edited by:
Gang Wang, Shanghai Jiao Tong University, ChinaReviewed by:
Yongmei Shi, Shanghai Jiao Tong University, ChinaYong Zhao, Chongqing Medical University, China
Copyright © 2022 Huang, Jiang, Zhang, Jia, Huang, Wang, Zhang, Wang, Gu, Huang, Shi, Ma, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhihong Wang, d2FuZ3poQG5pbmguY2hpbmFjZGMuY24=
 Qiumin Huang
Qiumin Huang Hongru Jiang1
Hongru Jiang1 Jiguo Zhang
Jiguo Zhang Zhihong Wang
Zhihong Wang