Abstract
Background and Aims:
Vitamin D deficiency is a common disorder and has been linked with atrial fibrillation (AF) in several observational studies, although the causal relationships remain unclear. We conducted a Mendelian randomization (MR) analysis to determine the causal association between serum 25-hydroxyvitamin D [25(OH)D] concentrations and AF.
Methods and Results:
The analyses were performed using summary statistics obtained for single-nucleotide polymorphisms (SNPs) identified from large genome-wide association meta-analyses conducted on serum 25(OH)D (N = 79,366) and AF (N = 1,030,836). Six SNPs related to serum 25(OH)D were used as instrumental variables. The association between 25(OH)D and AF was estimated using both the fixed-effect and random-effects inverse variance weighted (IVW) method. The MR analyses found no evidence to support a causal association between circulating 25(OH)D level and risk of AF using random-effects IVW (odds ratio per unit increase in log 25(OH)D = 1.003, 95% CI, 0.841–1.196; P = 0.976) or fixed-effect IVW method (OR = 1.003, 95% CI, 0.876–1.148; P = 0.968). Sensitivity analyses yielded similar results. No heterogeneity and directional pleiotropy were detected.
Conclusion:
Using summary statistics, this MR study suggests that genetically predicted circulating vitamin D concentrations, especially for a non-deficient range, were not causally associated with AF in the general population. Future studies using non-linear design and focusing on the vitamin D deficiency population are needed to further evaluate the causal effect of vitamin D concentrations on AF.
Introduction
Atrial fibrillation (AF) is the most common persistent cardiac rhythm disorder encountered in clinical practice, and is associated with increased risks of stroke, dementia, heart failure (HF), and death (1). Currently, the estimated prevalence of AF in adults is between 2 and 4% and is strongly associated with advancing age, placing extensive economic and societal burden in terms of morbidity, mortality, increased healthcare resource utilization and loss of productivity (2, 3).
Vitamin D is an essential nutrient obtained from exposure to sunlight, diet, and dietary supplements (4). In the skin, solar ultraviolet B radiation converts 7-dehydrocholesterol to previtamin D3, which is immediately isomerized to vitamin D3. This precursor form of vitamin D is metabolized to 25-hydroxyvitamin D [25(OH)D] in the liver, which is used to determine vitamin D status in the routine clinical setting. 25-hydroxyvitamin D is biologically inactive and must be converted to its active form 1,25-dihydroxyvitamin D by 25-hydroxyvitamin D-1-α-hydroxylase mainly in the kidneys (4). Vitamin D deficiency is a global problem, as many as 37% of the general population worldwide having vitamin D deficiency under the definition of <20 ng/mL (<50 nmol/L). Using a definition of <12 ng/mL (<30 nmol/L), approximately 7% of the population may be at risk to manifest symptoms due to severe vitamin D deficiency (5).
In some observational studies, a low vitamin D level has been linked with a variety of cardiovascular diseases such as coronary artery disease (CAD), HF and AF (6, 7). However, these associations remain controversial. Moreover, given the nature of observational studies, these results may be confounded by multiple variables. Mendelian randomization (MR) uses genetic variants as instrumental variables to make causal inferences, avoiding potential biases caused from confounding and reverse causation in observational studies. Therefore, a MR study can be considered as analogous to a randomized controlled trial (RCT), inferring a causal association of the risk factor on the disease outcome (8). Whilst current evidence shows that there may be a relationship between vitamin D deficiency and AF, incomparable study designs and methodological limitations make existing evidence less reliable. In this study, we aimed to perform a two-sample MR analysis to determine the potential role of circulating vitamin D status on AF risk.
Methods
Study Design and Data Sources
This Mendelian randomization was reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization (STROBE-MR) guidelines (9). We performed a two-sample MR analysis to evaluate the causal effect of 25(OH)D concentrations on AF. Three assumptions need to be met to ensure a valid instrumental variable (Figure 1) (8). First, the genetic variants utilized as instrumental variables should be associated with the exposure of interest. Second, the genetic variants should not be associated with confounders. Third, the genetic variants should affect the outcome only through the exposure, but not by other pathways. Data on the association of genetic variants with 25(OH)D and AF were obtained from recently published genome-wide association studies (GWAS) (10, 11). The specific ethical reviews and informed consent had been obtained in the original studies.
Figure 1
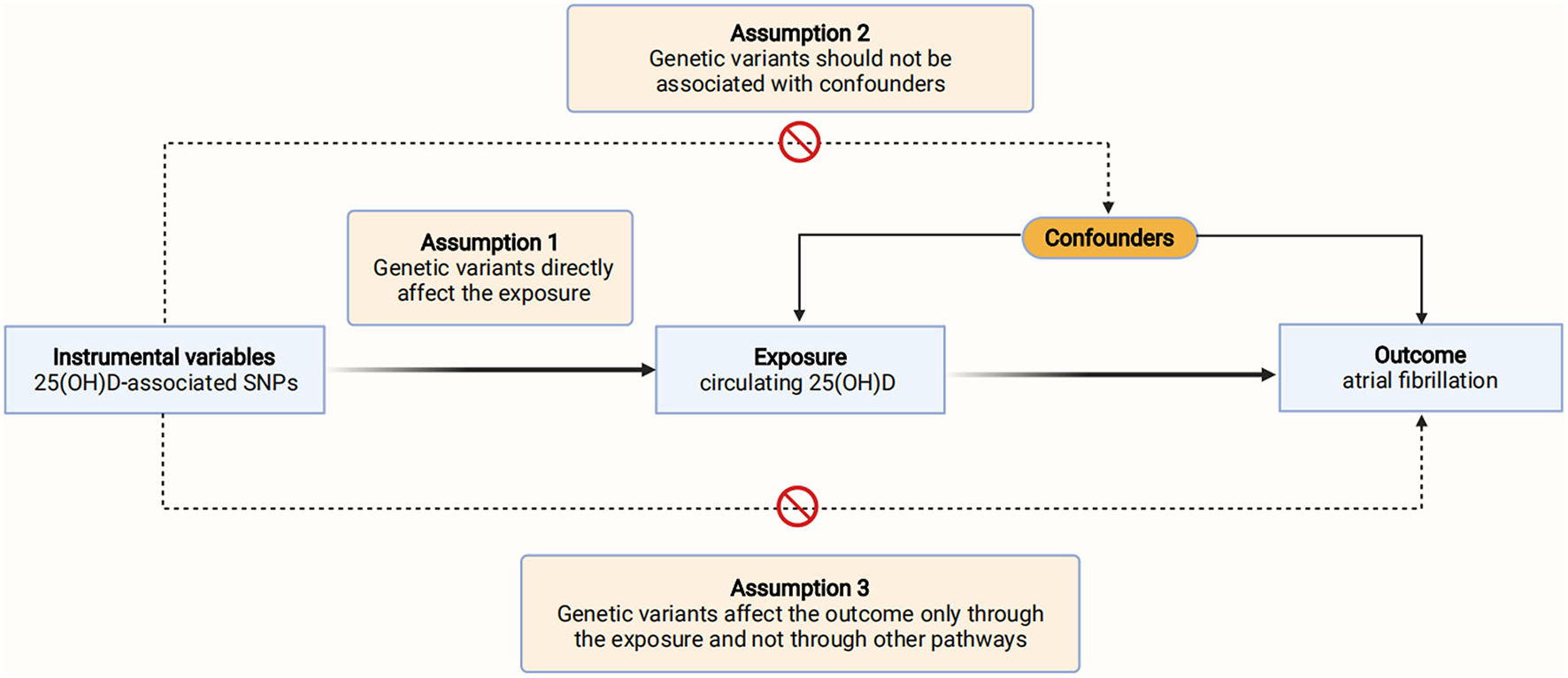
Schematic diagram of the Mendelian randomization assumptions. AF, atrial fibrillation; SNPs, single-nucleotide polymorphisms; 25(OH)D, 25-hydroxyvitamin D.
Selection of Genetic Variants
Summary statistics on the association between genetic variants and 25(OH)D concentrations from the recently published SUNLIGHT meta-GWAS were retrieved (10). This study is a large, multicenter, genome-wide association study involving 31 cohorts and case-control studies in Europe, Canada and USA, which was conducted through two stages. The first stage involved a discovery meta-analysis on a total of up to 79,366 European-ancestry individuals and the second stage replicated novel findings in 42,757 independent European-ancestry individuals (10). In the SUNLIGHT meta-GWAS, additive genetic models using linear regression on natural-log transformed 25(OH)D were fitted and a fixed-effect inverse variance weighted meta-analysis was performed across the contributing studies. Detailed descriptions of these 31 participating studies are presented in Supplementary Table 1, and the mean serum vitamin D concentrations of these studies were mostly in a non-deficient range. Information regarding the eligibility criteria, quality control, power calculation, genotyping platform and 25(OH)D measurements methods applied in each included study have been reported previously (10). Six SNPs were identified from the SUNLIGHT meta-GWAS at a genome-wide significance level (P < 5.0 × 10−8) (10). Linkage disequilibrium was not detected after preforming the linkage disequilibrium clumping process (r2 <0.001, distance >10,000 kb).
Utilizing the GWAS data, the pleiotropic associations between each of the six SNPs with several potential confounders were evaluated, including type 2 diabetes mellitus (T2DM) in 110,452 individuals (Diabetics Genetics Replication and Meta-analysis, DIAGRAM) (12), body mass index (BMI) in 339,224 individuals (Genetic Investigation of Anthropometric Traits, GIANT) (13), systolic and diastolic blood pressure in over 1 million individuals (International Consortium for Blood Pressure, ICBP) (14), alcohol consumption and smoking in up to 1.2 million individuals (GWAS and Sequencing Consortium of Alcohol and Nicotine use) (15). The Phenoscanner tool (http://www.phenoscanner.medschl.cam.ac.uk) was used to evaluate whether these SNPs were associated with other traits (16). The results showed that, at a genome-wide significance level (P < 5.0 × 10−8), none of these 6 SNPs had pleiotropic associations with T2DM, BMI, blood pressure, alcohol and tobacco use (Supplementary Table 2), or other potential confounders. Thus, six SNPs associated with circulating 25(OH)D concentrations were used as instrumental variables. An F statistic >10 indicated a low risk of weak instrument bias according to the formula: F = R2 × (N-2)/(1-R2), where R2 is the proportion of variance in vitamin D instruments using the formula: R2 = 2 × effect allele frequency × (1 – effect allele frequency) × (Beta/standard deviation (SD, equals 1))2 and N represents the sample size (17).
Outcome Data Source
Summary statistics for the associations of the six 25(OH)D-related SNPs with AF were extracted from the GWAS conducted by Nielsen et al. (11) which is currently the largest meta-GWAS focusing on the genetic architecture of AF, comparing a total of 60,620 cases and 970,216 controls of European ancestry from six contributing studies. Detailed descriptions of these 6 participating studies are presented in Supplementary Table 1. Cases were selected using International Classification of Diseases codes (ICD-9 or ICD-10), including AF and atrial flutter (11). Information regarding the eligibility criteria, power calculation, genotyping platform and quality control have been reported previously (11).
Statistical Analysis
Estimation of the overall causal effects between circulating 25(OH)D concentrations and AF was performed using both the fixed-effect and random-effects inverse variance weighted (IVW) method, which assume that all SNPs are valid instrumental variables based on the MR assumptions (18). In order to account for potential violations of the assumptions underlying the IVW method, we performed sensitivity analyses, including the weighted median, MR-Egger, simple median and penalized weighted median methods. The weighted median method provides a consistent effect estimate even when up to 50% of the genetic variants are invalid instruments using the inverse of the variance of the ratio estimates as weights (19). The MR-Egger approach provides a valid effect estimate even if all SNPs are invalid instruments and the intercept could be used to detect directional pleiotropy (20). A zero intercept for MR-Egger (P > 0.05) was considered to indicate no pleiotropic bias. The median-based estimator and MR-Egger can obtain consistent causal estimates under weaker assumptions. Whereas, these methods can be sensitive to genetic variants with heterogeneous causal estimates, therefore, the penalized weighted median method was also performed to provide a robust estimate by penalizing the weights of SNPs with heterogeneous causal estimates (21). Mendelian randomization pleiotropy residual sum and outlier (MR-PRESSO) test was performed to detect and correct for horizontal pleiotropic outliers (22). The Cochran Q test for heterogeneity was applied to test the presence of horizontal pleiotropy. The single SNP analysis using the Wald ratio method and leave-one-out sensitivity analysis were conducted to determine whether the association between 25(OH)D and AF was affected by single SNP. The odds ratios (ORs) are scaled per unit change in natural-log-transformed 25(OH)D level.
Power calculations were conducted using the mRnd power calculation tool (23). Given a sample size of 1,030,836 and 2.84% of variance in 25(OH)D explained by the genetic variants, this study had 80% power at an alpha rate of 5% to detect an OR of 0.931 for AF per unit increase in log 25(OH)D.
A two-side P < 0.05 was considered statistically significant in statistical tests for MR analyses. All statistical analyses were conducted using the “TwoSampleMR” package (24) and “MRPRESSO” package in the R software (version 4.0.4, R Development Core Team, Vienna, Austria).
Results
The characteristics of 6 SNPs and their association with 25(OH)D and AF are presented in Table 1. None of the individual 6 SNPs was associated with AF at Bonferroni corrected significance level P < 0.008 [(P < 0.05)/6 SNPs], and the allele frequencies between the exposure and outcome population were generally similar (Table 1). The F-statistic of each SNPs was more than 10, which indicated that they were all strong instrumental variables. There was no sample overlap between the exposure and outcome population (Table 1).
Table 1
| SNP | Position | Nearby gene | EA/NEA | 25(OH)D | Atrial fibrillation | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EAF | R2 (%) | F-statistic | Beta (SE) | P-value | EAF | Beta (SE) | P-value | ||||
| rs3755967 | 4:72828262 | GC | T/C | 0.28 | 0.319 | 254.28 | −0.089 (0.0023) | 4.74 × 10−343 | 0.28 | 0.0056 (0.0074) | 0.45 |
| rs12785878 | 11:70845097 | NADSYN1/ DHCR7 | T/G | 0.75 | 0.049 | 38.59 | 0.036 (0.0022) | 3.80 × 10−62 | 0.70 | 0.0011 (0.0075) | 0.89 |
| rs10741657 | 11:14871454 | CYP2R1 | A/G | 0.40 | 0.046 | 36.63 | 0.031 (0.0022) | 2.05 × 10−46 | 0.58 | −0.0117 (0.0067) | 0.08 |
| rs17216707 | 20:52165769 | CYP24A1 | T/C | 0.79 | 0.022 | 17.81 | 0.026 (0.0027) | 8.14 × 10−23 | 0.19 | −0.0156 (0.0087) | 0.07 |
| rs10745742 | 12:94882660 | AMDHD1 | T/C | 0.39 | 0.017 | 20.98 | 0.019 (0.0020) | 2.10 × 10−20 | 0.38 | −0.0074 (0.0068) | 0.28 |
| rs8018720 | 14:38625936 | SEC23A | C/G | 0.82 | 0.011 | 13.02 | −0.019 (0.0027) | 1.11 × 10−11 | 0.81 | 0.0058 (0.0088) | 0.51 |
Characteristics of the 25(OH)D-associated SNPs.
The beta coefficients for the association between SNPs and 25(OH)D are based on per effect allele per unit change in log 25(OH)D.
EA, effect allele; EAF, effect allele frequency; NEA, non-effect allele; SE, standard error; SNP, single nucleotide polymorphism.
In the overall analysis based on IVW method, the OR of AF per unit increase in log 25(OH)D was 1.003 (95% CI, 0.841–1.196; P = 0.976) for random-effects method and 1.003 (95% CI, 0.876–1.148; P = 0.968) for fixed-effect method, which was consistent with the results from weighted median [OR, 0.960, 95% CI (0.826–1.117); P = 0.600], MR-Egger [OR, 0.924, 95% CI (0.653–1.307); P = 0.678], simple median [OR, 0.984, 95% CI (0.776–1.247); P = 0.894] and penalized weighted median method [OR, 0.960, 95% CI (0.825–1.118); P = 0.602], indicating that the circulating 25(OH)D concentrations were not significantly associated with AF (Figure 2). Scatterplot depicting the relationship of the SNP effects on the 25(OH)D against the SNP effects on AF is shown in Supplementary Figure 1.
Figure 2
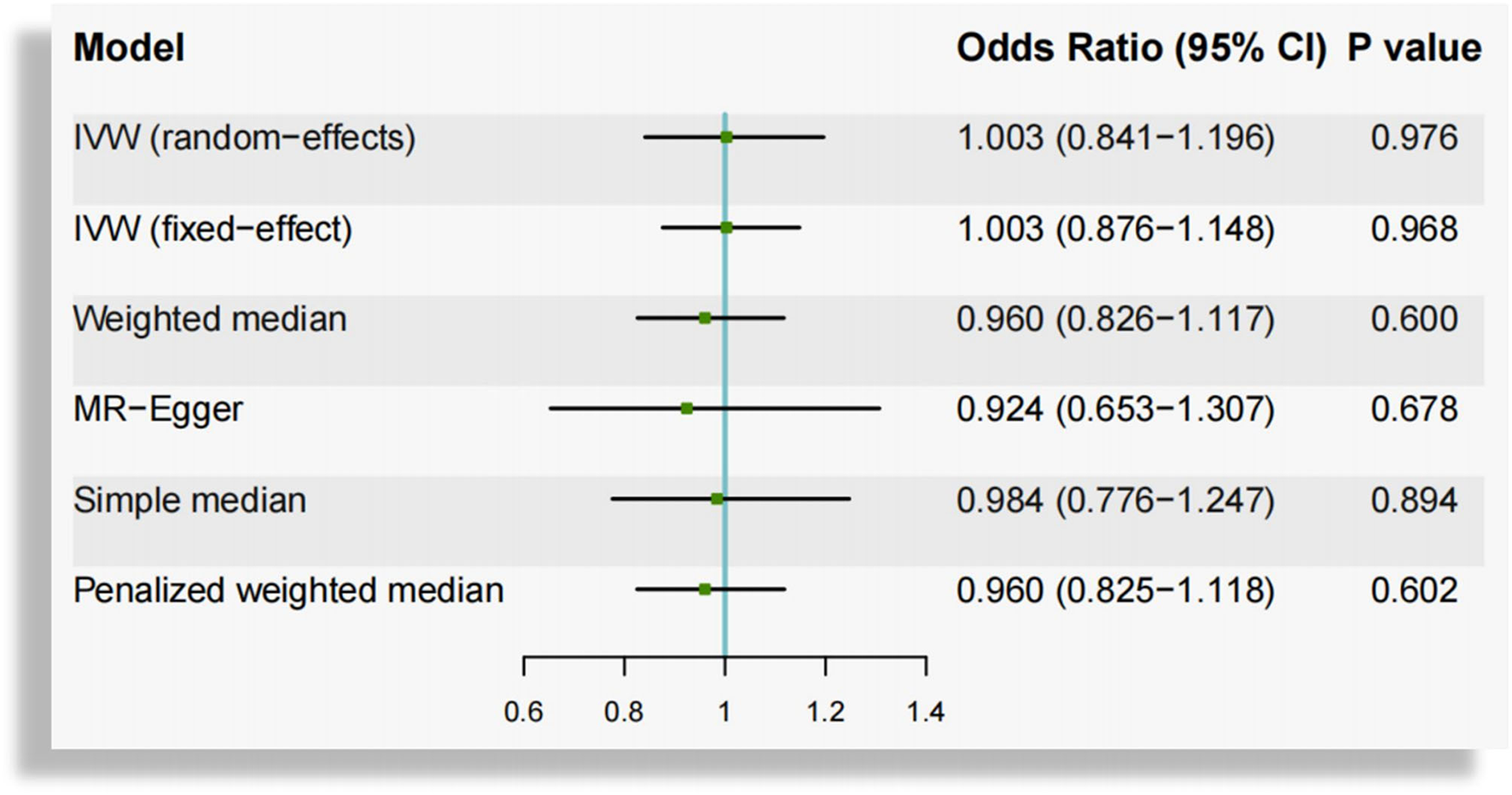
Mendelian randomization estimates of the association between genetically predicted circulating vitamin D and atrial fibrillation. CI, confidence interval; IVW, inverse-variance-weighted.
Heterogeneity among the causal estimates of the six SNPs was not observed (Q = 8.476, P = 0.132). There was no evidence of pleiotropy using MR-Egger (intercept = 0.004, se = 0.008, P = 0.610) or MR-PRESSO global test (P = 0.312). No outlier SNPs were identified in the MR-PRESSO analysis. According to leave-one-out analysis (Supplementary Figure 2) and single SNP analysis (Figure 3), the association between circulating 25(OH)D and AF was not driven by any individual SNP.
Figure 3
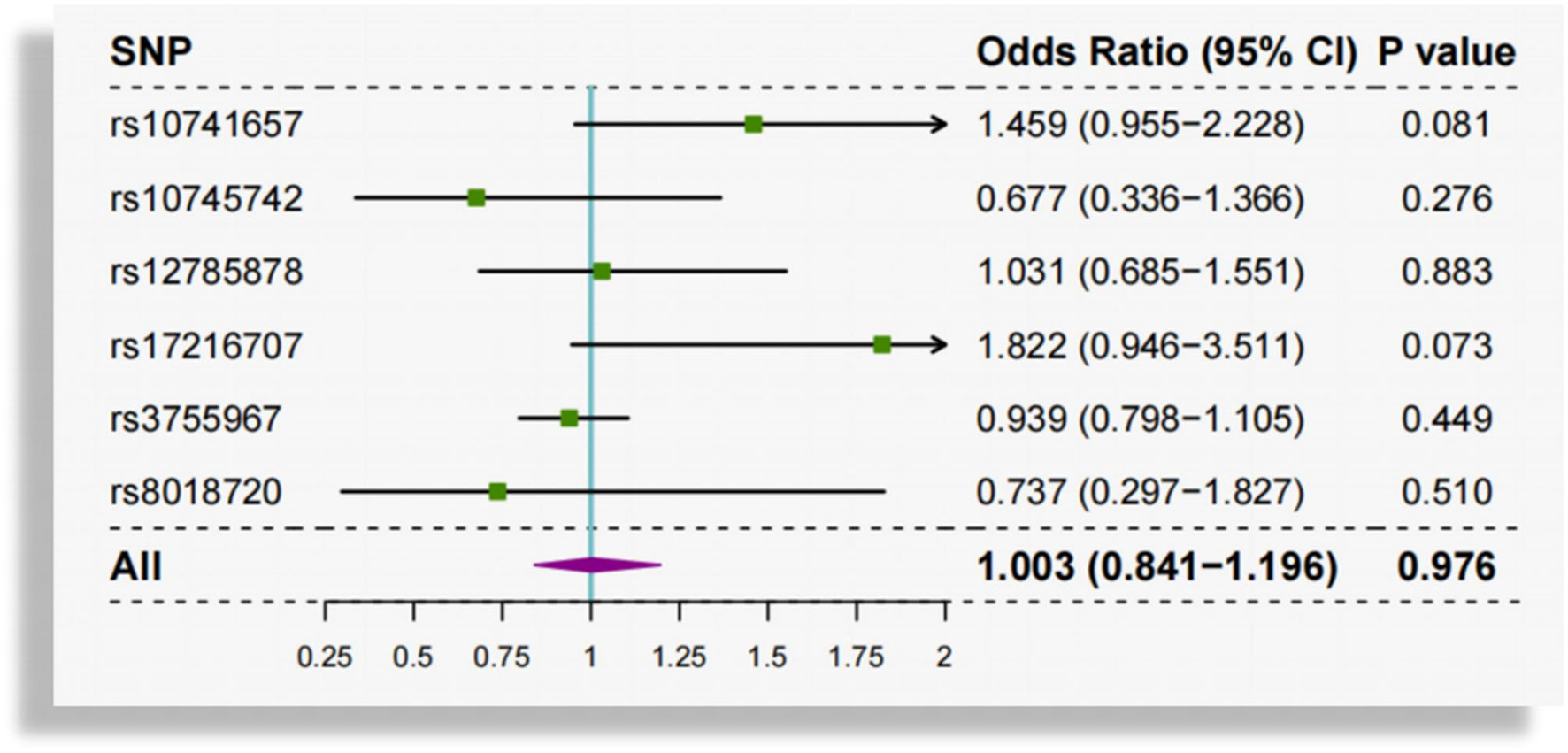
Single SNP analysis of genetically predicted circulating vitamin D and atrial fibrillation. SNP, single-nucleotide polymorphism.
Discussion
In the present MR analysis, we have capitalized on the summary statistics of two large meta-GWAS conducted for serum 25(OH)D and AF in European populations, without any sample overlap, and constructed a strong instrumental variable for 25(OH)D from six SNPs. We have employed a range of MR methods to investigate the association between 25(OH)D and AF. However, none of these analyses suggested a causal relationship between circulating 25(OH)D concentrations and AF in the general population.
As an essential nutrient for individuals, vitamin D performs an important role in the regulation of calcium, phosphorus, and bone metabolism. Besides, vitamin D receptors (VDR) are found in a variety of cells and tissues, including endothelial cells, vascular smooth muscle cells, cardiac myocytes and fibroblasts (25). Thus, it exerts many other important cellular regulatory functions include modulation of oxidative stress status, inflammatory response, mitochondrial function, insulin, and renin-angiotensin–aldosterone system (RAAS) (25).
In experimental study, Hanafy et al. (26) found that vitamin D increased the action potential duration and contractility in isolated rabbit left atrium tissue, revealed a direct electromechanical effect of vitamin D on preventing or terminating AF. Moreover, in our previous meta-analysis investigating the relationship between circulating vitamin D levels and AF risk which included observational studies mainly assessing for chronic AF, the results suggested that vitamin D deficiency modestly increases the risk of AF (7). More recently, another meta-analysis of observational studies also suggested that serum vitamin D deficiency (<20 ng/ml) was associated with an increased risks of AF in the general population and postoperative AF in patients underwent coronary artery bypass graft (CABG) (27). However, in the VITAL study (28), a large randomized clinical trial that recruited 25,119 adults for a median 5.3 years of daily doses (2,000 IU) of vitamin D supplementation and follow-up, the results did not support the use of vitamin D for the primary prevention of new-onset AF. Therefore, data concerning the causal association between vitamin D and AF are so far contradictory and controversial.
Currently, there are various studies looking at cardiovascular effects of vitamin D using MR, designed to minimize bias from confounding, however, most of these studies have found null effects (29). For example, a one-sample MR in 92,416 individuals of Danish descent failed to demonstrate any evidence of a causal association between plasma 25(OH)D levels and CAD or myocardial infarction (30). Similarly, a 2020 study in 417,580 Europeans from the UK Biobank found no evidence to suggest that vitamin D concentration is associated with CAD (31). In regarding to hypertension, the evidence from existing MR studies on the effects of predicted serum 25OHD levels on hypertension, systolic and diastolic blood pressure, consistently does not support any of these outcomes (29). In the current study, we also found no evidence that genetically predicted 25(OH)D is associated with increased risk of AF in the general population. Results of these MR studies suggests that the observed association of reduced 25(OH)D with CAD and AF may not represent a causal relationship, but more likely is due to confounding by unaccounted influences or due to reverse causation.
Notably, a recent non-linear MR study have suggested an L-shaped association of 25(OH)D with CVD risk, with increased CVD risk largely restricted to individuals with low vitamin D status (32). Moreover, in another similar study (33), the authors have demonstrated non-linear dose-response relationships of 25(OH)D with CAD, stroke and all-cause mortality in observational analysis, and identified an association of genetically-predicted 25(OH)D with all-cause mortality only for individuals with vitamin D deficiency (<25 ng/mL) but not for general population. Therefore, further MR studies using non-linear design and focusing on the vitamin D deficiency population are needed. Besides, categorical analysis using different cutpoints proposed for vitamin D deficiency is also warranted in order to identify the subsets of individuals who may benefit more from vitamin D supplementation. Additionally, in a MR study conducted in a small sized Chinese cohort of cardiac outpatients with stable CAD, in contrast to our study that evaluated individuals primarily form European ancestry, the result suggested genetically deprived vitamin D exposure is associated with increased risk of AF (34). Thus, the null effects observed in our study may also be due to the racial disparity. Future studies focusing the effects of vitamin D on risk of AF among different ethnic groups are needed.
Strengths and Limitations
This study has several strengths. First, using Mendelian randomization method, this study could provide a robust estimate of causal relationships between vitamin D and AF. Second, there was no sample overlap between studies included in the GWAS meta-analysis of vitamin D and AF, which could help reduce the severity of weak instrument bias. Third, the individuals included in these two GWAS meta-analysis were primarily of European ancestry, and the effect allele frequencies of the 6 vitamin D-associated SNPs were very similar between the two populations, which could mitigate the potential effect of population stratification. The results of this study also should be interpreted in conjunction with some limitations. First, though several approaches were conducted to assess and adjust for potential confounding or pleiotropic effects, we could not completely rule out the influence of unknown potential confounders. Second, access to only summarized data limits the range of analyses that can be performed, such as subgroup analyses, non-linear analysis and categorical analysis using different cutpoints for vitamin D deficiency.
Conclusion
Genetically predicted circulating vitamin D concentrations, especially for a non-deficient range, were not causally associated with AF in the general population. Future studies using non-linear design and focusing on the vitamin D deficiency population are needed to further evaluate the causal effect of 25(OH)D concentrations on AF.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Grant Number: 82100341 to ZZ, 81970270 and 82170327 to TL).
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The ethical reviews and informed consent had been obtained in the original studies, and were not required for the current analysis.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
GYHL has been a consultant and speaker for BMS/Pfizer, Boehringer Ingelheim and Daiichi-Sankyo. No fees are received personally. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.842392/full#supplementary-material
References
1.
MichaudGFStevensonWG. Atrial fibrillation. N Engl J Med. (2021) 384:353–61. 10.1056/NEJMcp2023658
2.
HindricksGPotparaTDagresNArbeloEBaxJJBlomstrom-LundqvistCet al. 2020 esc guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the european association for cardio-thoracic surgery (eacts): the task force for the diagnosis and management of atrial fibrillation of the european society of cardiology (esc) developed with the special contribution of the european heart rhythm association (ehra) of the esc. Eur Heart J. (2021) 42:373–498. 10.1093/eurheartj/ehab648
3.
BurdettPLipGYH. Atrial fibrillation in the united kingdom: Predicting costs of an emerging epidemic recognising and forecasting the cost drivers of atrial fibrillation-related costs. Eur Heart J Qual Care Clin Outcomes. (2020) 8:187–94. 10.1093/ehjqcco/qcaa093
4.
HolickMF. Vitamin d deficiency. N Engl J Med. (2007) 357:266–81. 10.1056/NEJMra070553
5.
GiustinaABouillonRBinkleyNSemposCAdlerRABollerslevJet al. Controversies in vitamin d: a statement from the third international conference. JBMR Plus. (2020) 4:e10417. 10.1002/jbm4.10417
6.
CosentinoNCampodonicoJMilazzoVDe MetrioMBrambillaMCameraMet al. Vitamin d and cardiovascular disease: current evidence and future perspectives. Nutrients. (2021) 13:3603. 10.3390/nu13103603
7.
ZhangZYangYNgCYWangDWangJLiGet al. Meta-analysis of vitamin d deficiency and risk of atrial fibrillation. Clin Cardiol. (2016) 39:537–43. 10.1002/clc.22563
8.
BurgessSButterworthAMalarstigAThompsonSG. Use of mendelian randomisation to assess potential benefit of clinical intervention. BMJ. (2012) 345:e7325. 10.1136/bmj.e7325
9.
SkrivankovaVWRichmondRCWoolfBARYarmolinskyJDaviesNMSwansonSAet al. Strengthening the reporting of observational studies in epidemiology using mendelian randomization: the strobe-mr statement. JAMA. (2021) 326:1614–21. 10.1001/jama.2021.18236
10.
JiangXO'ReillyPFAschardHHsuYHRichardsJBDupuisJet al. Genome-wide association study in 79,366 european-ancestry individuals informs the genetic architecture of 25-hydroxyvitamin d levels. Nat Commun. (2018) 9:260. 10.1038/s41467-017-02662-2
11.
NielsenJBThorolfsdottirRBFritscheLGZhouWSkovMWGrahamSEet al. Biobank-driven genomic discovery yields new insight into atrial fibrillation biology. Nat Genet. (2018) 50:1234–9. 10.1038/s41588-018-0171-3
12.
ReplicationDIGMeta-analysisCAsian Genetic Epidemiology Network Type 2 DiabetesCSouth Asian Type 2 DiabetesCMexican American Type 2 DiabetesCType 2 Diabetes Genetic Exploration by Nex-generation sequencing in muylti-Ethnic SamplesCet al. Genome-wide trans-ancestry meta-analysis provides insight into the genetic architecture of type 2 diabetes susceptibility. Nat Genet. (2014) 46:234–44. 10.1038/ng.2897
13.
LockeAEKahaliBBerndtSIJusticeAEPersTHDayFRet al. Genetic studies of body mass index yield new insights for obesity biology. Nature. (2015) 518:197–206. 10.1038/nature14177
14.
EvangelouEWarrenHRMosen-AnsorenaDMifsudBPazokiRGaoHet al. Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat Genet. (2018) 50:1412–25. 10.1038/s41588-018-0205-x
15.
LiuMJiangYWedowRLiYBrazelDMChenF.et al. Association studies of up to 12 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet. (2019) 51:237–44. 10.1038/s41588-018-0307-5
16.
StaleyJRBlackshawJKamatMAEllisSSurendranPSunBBet al. Phenoscanner: a database of human genotype-phenotype associations. Bioinformatics. (2016) 32:3207–9. 10.1093/bioinformatics/btw373
17.
PierceBLAhsanHVanderweeleTJ. Power and instrument strength requirements for mendelian randomization studies using multiple genetic variants. Int J Epidemiol. (2011) 40:740–52. 10.1093/ije/dyq151
18.
BurgessSScottRATimpsonNJDavey SmithGThompsonSGConsortiumE-I. Using published data in mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur J Epidemiol. (2015) 30:543–52. 10.1007/s10654-015-0011-z
19.
BowdenJDavey SmithGHaycockPCBurgessS. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. 10.1002/gepi.21965
20.
BowdenJDavey SmithGBurgessS. Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int J Epidemiol. (2015) 44:512–25. 10.1093/ije/dyv080
21.
ReesJMBWoodAMDudbridgeFBurgessS. Robust methods in mendelian randomization via penalization of heterogeneous causal estimates. PLoS ONE. (2019) 14:e0222362. 10.1371/journal.pone.0222362
22.
VerbanckMChenCYNealeBDoR. Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. 10.1038/s41588-018-0099-7
23.
BrionMJShakhbazovKVisscherPM. Calculating statistical power in mendelian randomization studies. Int J Epidemiol. (2013) 42:1497–501. 10.1093/ije/dyt179
24.
HemaniGZhengJElsworthBWadeKHHaberlandVBairdDet al. The mr-base platform supports systematic causal inference across the human phenome. Elife. (2018) 7:e34408. 10.7554/eLife.34408
25.
VassalleCParlantiAPingitoreABertiSIervasiGSabatinoL. Vitamin d, thyroid hormones and cardiovascular risk: exploring the components of this novel disease triangle. Front Physiol. (2021) 12:722912. 10.3389/fphys.2021.722912
26.
HanafyDAChangSLLuYYChenYCKaoYHHuangJHet al. Electromechanical effects of 1,25-dihydroxyvitamin d with antiatrial fibrillation activities. J Cardiovasc Electrophysiol. (2014) 25:317–23. 10.1111/jce.12309
27.
LiuXWangWTanZZhuXLiuMWanRet al. The relationship between vitamin d and risk of atrial fibrillation: a dose-response analysis of observational studies. Nutr J. (2019) 18:73. 10.1186/s12937-019-0485-8
28.
AlbertCMCookNRPesterJMoorthyMVRidgeCDanikJSet al. Effect of marine omega-3 fatty acid and vitamin d supplementation on incident atrial fibrillation: a randomized clinical trial. JAMA. (2021) 325:1061–73. 10.1001/jama.2021.1489
29.
BouillonRManousakiDRosenCTrajanoskaKRivadeneiraFRichardsJB. The health effects of vitamin d supplementation: evidence from human studies. Nat Rev Endocrinol. (2022) 18:96–110. 10.1038/s41574-021-00593-z
30.
Brondum-JacobsenPBennMAfzalSNordestgaardBG. No evidence that genetically reduced 25-hydroxyvitamin d is associated with increased risk of ischaemic heart disease or myocardial infarction: a mendelian randomization study. Int J Epidemiol. (2015) 44:651–61. 10.1093/ije/dyv078
31.
RevezJALinTQiaoZXueAHoltzYZhuZet al. Genome-wide association study identifies 143 loci associated with 25 hydroxyvitamin d concentration. Nat Commun. (2020) 11:1647. 10.1038/s41467-020-15421-7
32.
ZhouASelvanayagamJBHypponenE. Non-linear mendelian randomization analyses support a role for vitamin d deficiency in cardiovascular disease risk. Eur Heart J. (2021). 10.1093/eurheartj/ehab809. [Epub ahead of print].
33.
Emerging Risk Factors Collaboration E-CVDVDSC. Estimating dose-response relationships for vitamin d with coronary heart disease, stroke, and all-cause mortality: observational and mendelian randomisation analyses. Lancet Diabetes Endocrinol. (2021) 9:837–46. 10.1016/S2213-8587(21)00263-1
34.
ChanYHYiuKHHaiJJChanPHLamTHCowlingBJet al. Genetically deprived vitamin d exposure predisposes to atrial fibrillation. Europace. (2017) 19:iv25–31. 10.1093/europace/eux312
Summary
Keywords
vitamin D, Mendelian randomization, single-nucleotide polymorphisms, causal association, atrial fibrillation
Citation
Zhang N, Wang Y, Chen Z, Liu D, Tse G, Korantzopoulos P, Letsas KP, Goudis CA, Lip GYH, Li G, Zhang Z and Liu T (2022) Circulating Vitamin D Concentrations and Risk of Atrial Fibrillation: A Mendelian Randomization Study Using Non-deficient Range Summary Statistics. Front. Nutr. 9:842392. doi: 10.3389/fnut.2022.842392
Received
23 December 2021
Accepted
13 April 2022
Published
17 June 2022
Volume
9 - 2022
Edited by
Yap-Hang Chan, University of Hong Kong, Hong Kong SAR, China
Reviewed by
Jie Zhao, The University of Hong Kong, Hong Kong SAR, China; Tommaso Filippini, University of Modena and Reggio Emilia, Italy; Xia Jiang, Karolinska Institutet (KI), Sweden; Qiming Liu, Central South University, China
Updates
Copyright
© 2022 Zhang, Wang, Chen, Liu, Tse, Korantzopoulos, Letsas, Goudis, Lip, Li, Zhang and Liu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiwei Zhang zhangzhiweidoc@126.comTong Liu liutongdoc@126.com
This article was submitted to Clinical Nutrition, a section of the journal Frontiers in Nutrition
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.