- 1Changsha Social Work College, Changsha, China
- 2Department of Orthopaedics, Xiangya Hospital, Central South University, Changsha, China
- 3National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, China
Objective: The associations of dietary vitamin C and E intake with depression remains conflicting. This meta-analysis of observational study was therefore employed to clarify the issue further.
Methods: An extensive literature review (PubMed, Web of Science and Embase) was performed in January 2022 to identify the observational studies on the associations of dietary vitamin C and E intake with depression. The pooled relative risk (RR) of depression for the highest versus lowest dietary vitamin C and E intake category, and the weighted mean difference (WMD) of dietary vitamin C and E intake for depression versus control subjects, were calculated.
Results: A total of 25 observational studies (91966 participants) were included in this meta-analysis. The overall multi-variable adjusted RR demonstrated that dietary vitamin C intake was inversely associated with depression (RR = 0.72, 95% CI: 0.57 to 0.91; P = 0.005). In addition, the combined WMD showed that the dietary vitamin C intake in depression was lower than that in control subjects (WMD = −11.58, 95% CI: −14.88 to −8.29; P < 0.001). Similarly, the overall multi-variable adjusted RR demonstrated that dietary vitamin E intake was negatively associated with depression (RR = 0.84, 95% CI: 0.72 to 0.98; P = 0.02). Moreover, the combined WMD showed that the dietary vitamin E intake in depression was also lower than that in control subjects (WMD = −0.71, 95% CI: −1.07 to −0.34; P < 0.001).
Conclusion: The results of this meta-analysis suggest that both dietary vitamin C and E intake is inversely associated with depression. However, due to the limited evidence, more well-designed prospective cohort studies are still needed.
Introduction
As one of the most common mental disorders, depression affects females twice as much as males worldwide (1). The symptoms of depression are usually presented as exhaustion, sadness, lack of interest in daily activities, and even suicide (2). Affecting approximately 300 million people (3), depression is estimated to be the leading cause of disability worldwide by 2030 (4). Nevertheless, the treatment for depression is usually restricted to unsatisfactory curative effect, adverse side effects and costly pharmacotherapy (5). Recently, epidemiological evidence indicates that the dietary factors are associated with depression (6, 7). Thus, the identification of modifiable dietary factors for depression appears to be an important step in its clinical management.
Vitamin C (ascorbic acid), an essential water-soluble micronutrient, is traditionally employed to prevent and treat scurvy (8). However, vitamin E (also known as tocopherol) is a fat-soluble vitamin to modulate enzymes involved in signal transduction, gene expression and immunomodulatory capabilities (9, 10). Equipped with abundant vitamin C and E constituent, vegetable, fruit, legume and nut consumption are demonstrated to be inversely associated with depression (11–13). It is well known that vitamin C and E are served as common antioxidants that prevent other compounds from being oxidized (14–16), which donate electron and scavenge harmful free radicals. Since the oxidative stress is also considered to play a significant role in the pathophysiology of depression (17, 18), it seems naturally that dietary vitamin C and E intake is negatively associated with depression.
As far as we know, a number of observational studies have investigated the associations of dietary vitamin C and E intake with depression (19–43). However, their results are still conflicting. Therefore, this meta-analysis is employed to clarify the issue further. It is hypothesized that both dietary vitamin C and E intake is inversely associated with depression.
Materials and Methods
Search Strategy
We performed our meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (44). The PubMed, Web of Science and Embase electronic database were searched during January 2022 by using a combination of keywords and in-text words related to depression (‘depression,’ ‘depressive’), vitamin C (‘vitamin C,’ ‘ascorbic acid’) and vitamin E (‘vitamin E,’ ‘tocopherol’). No language restrictions were imposed. The titles and abstracts of all articles were first screened to identify eligible studies, and then the full articles were read to include the eligible studies. Moreover, the reference lists for the retrieved articles were also reviewed to identify additional studies.
Study Selection
Two researchers reviewed the titles, abstracts and full texts of all retrieved studies independently. Disagreements, if any, were resolved by discussions. The included studies were required to meet the following criteria: (1) observational studies; (2) the associations of dietary vitamin C and E intake with depression; and (3) relative risk (RR), odds ratio (OR) or weighted mean difference (WMD) with 95% confidence interval (CI) reported. The exclusion criteria were listed as follows: (1) duplicated or irrelevant articles; (2) reviews, letters or case reports; (3) randomized controlled trials; and (4) non-human studies.
Data Extraction
Two researchers extracted the data independently, and disagreements were resolved by discussion. The information about first author, year of publication, location, age, sex, sample size, study design, adjustments, dietary assessment, category of exposure, effect estimates and diagnostic criteria of depression, was collected respectively. The corresponding effect estimates with 95% CIs for the highest versus lowest dietary vitamin C and E intake category were extracted (adjusted for the maximum number of confounding variables). Moreover, the dietary vitamin C and E intake (mean ± SD) was also extracted for depression versus control subjects to calculate the WMD by Review Manager 5.3. The quality assessment was employed in accordance with the Newcastle-Ottawa Scale (NOS), which contains 8 items categorized into three dimensions: the selection of study groups, the comparability among different groups, and the ascertainment of either the exposure or outcome for case-control (Supplementary Table 1) and cohort studies (Supplementary Table 2), respectively.
Statistical Analyses
The RR for depression and WMD for dietary vitamin C and E intake were the outcome measures in our study. The I2 statistic, which measures the percentage of total variation across studies due to heterogeneity, was examined (I2 > 50% was considered heterogeneity). If significant heterogeneity was observed among the studies, the random-effects model was used; otherwise, the fixed effects model was utilized. Univariate meta-regression for publication year, sample size, location, age, sex, and dietary assessment was performed to explore the sources of heterogeneity. Begg’s test was employed to assess the publication bias (45, 46). Moreover, the subgroup analysis was employed for geographical region, dietary assessment, sex, population, study design, and diagnostic criteria of depression, respectively. In addition, a sensitivity analysis was also conducted to determine whether an individual study affected the pooled result.
Results
Study Identification and Selection
The detailed flow diagram of the study identification and selection was presented in Figure 1. A total of 1873 potentially relevant articles (548 for PubMed, 766 for Embase and 559 for Web of Science) were retrieved during the initial literature search. After eliminating 636 duplicated articles, 1237 articles were screened according to the titles and abstracts. 691 irrelevant studies were excluded. Then, 225 reviews, case reports or letters, 223 non-human studies, 76 randomized control trials studies were removed. Thereafter, 3 additional studies were acquired from the reference lists for the retrieved articles. Eventually, 25 studies were selected for this meta-analysis (19–43).
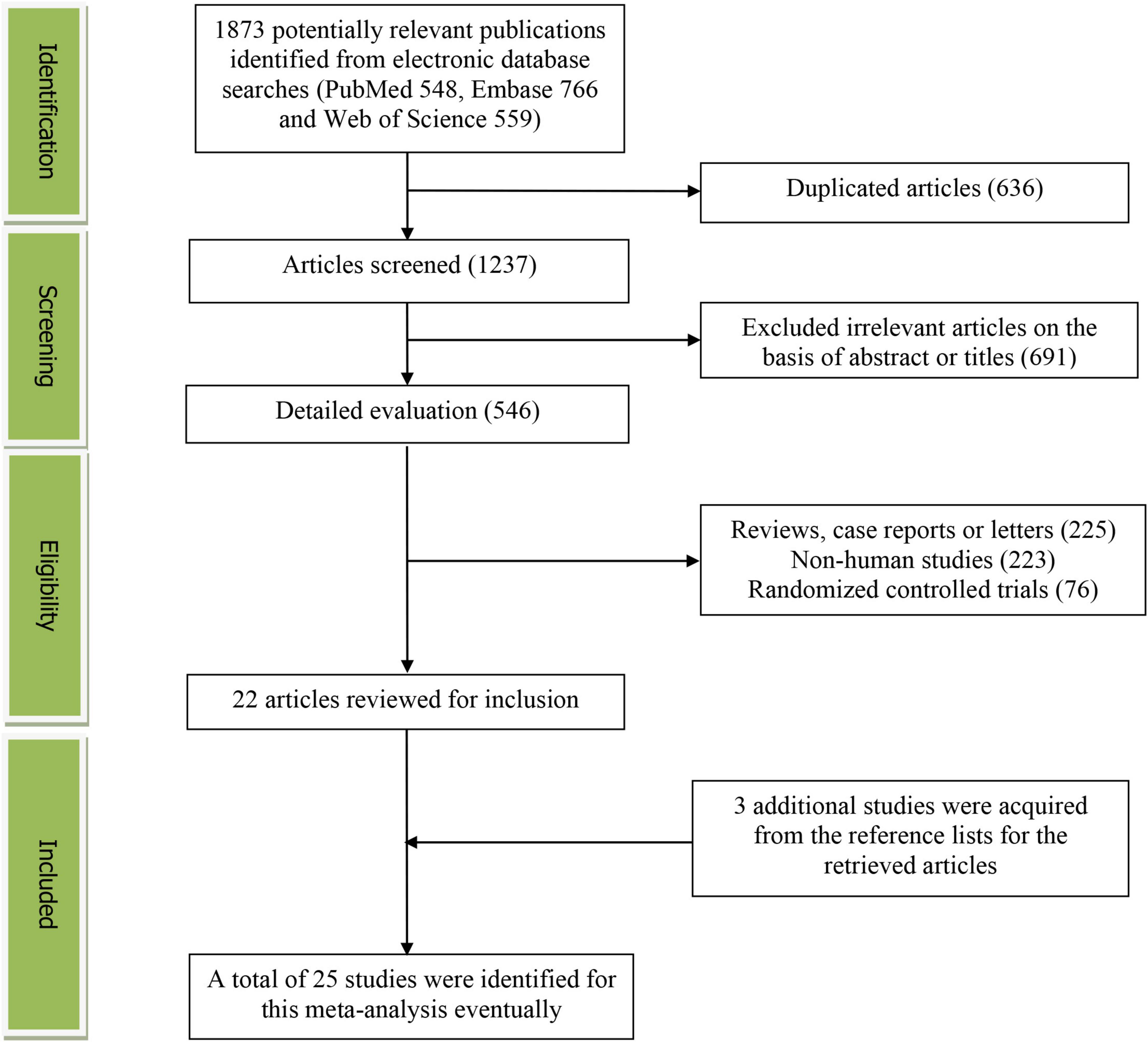
Figure 1. The detailed flow diagram of the study identification and selection in this meta-analysis.
Study Characteristics
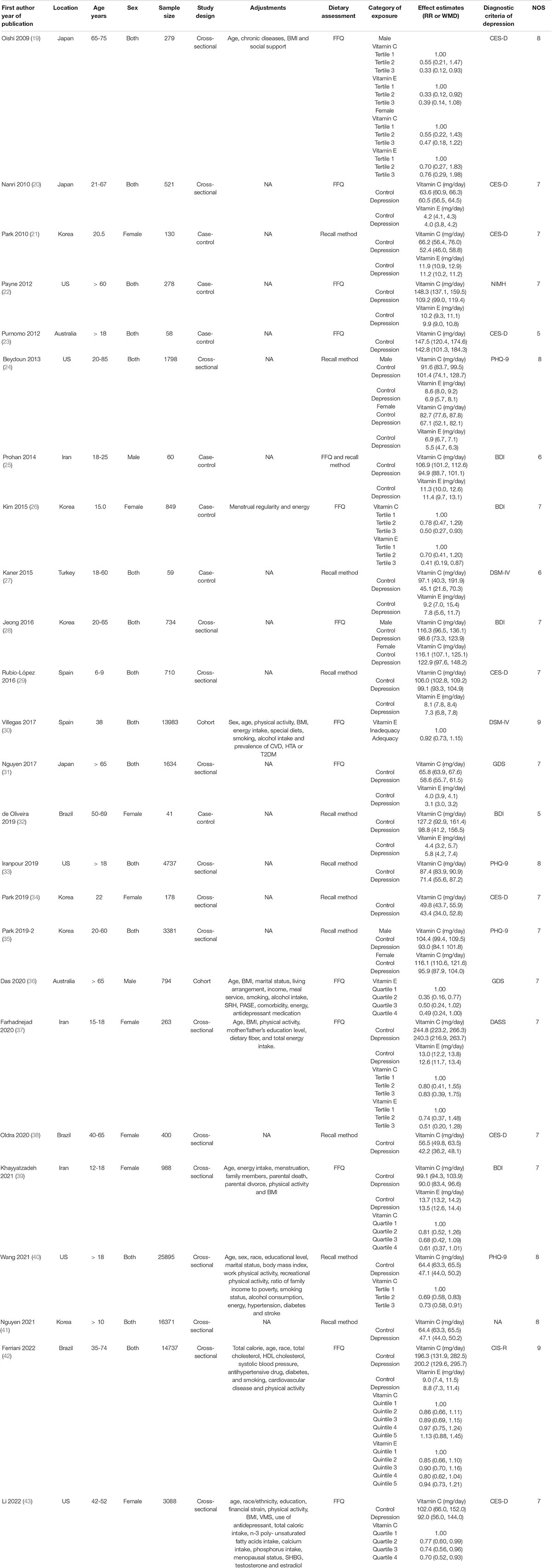
The main characteristics of the included studies were presented in Table 1. These studies were published between 2009 and 2022. 12 of the included studies were performed in Asian countries [Korea (21, 26, 28, 34, 35, 41), Iran (25, 37, 39) and Japan (19, 20, 31)], and the other ones were conducted in United States (22, 24, 33, 40, 43), Brazil (32, 38, 42), Australia (23, 36), Spain (29, 30), and Turkey (27). Male, female and both male and female participants were recruited in 2 (25, 36), 8 (21, 26, 32, 34, 37–39, 43), and 15 (19, 20, 22–24, 27–31, 33, 35, 40–42) studies, respectively. The sample size ranged from 41 to 25895 for a total number of 91966. The dietary vitamin C and E intake was assessed by food-frequency questionnaire (FFQ) in 14 studies (19, 20, 22, 23, 25, 26, 28, 30, 31, 36, 37, 39, 42, 43), and recall method in 12 studies (21, 24, 25, 27, 29, 32–35, 38, 40, 41). The diagnostic criteria of depression or depressive symptom were Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) (27, 30), Patient Health Questionnaire-9 (PHQ-9) (24, 33, 35, 40), Center for Epidemiological Studies Depression Scale (CES-D) (19–21, 23, 29, 34, 38, 43), Beck Depression Inventory (BDI) (25–27, 32, 39), Geriatric Depression Scale (GDS) (31, 36), Depression, Anxiety, Stress Scale (DASS) (37), National Institute of Mental Health (NIMH) (22), and Clinical Interview Schedule Revised (CIS-R) (42), respectively.
Relative Risk of Depression for the Highest Versus Lowest Dietary Vitamin C Intake Category
The overall multi-variable adjusted RR demonstrated that dietary vitamin C intake was negatively associated with depression (RR = 0.72, 95% CI: 0.57 to 0.91; P = 0.005) (Figure 2). A substantial level of heterogeneity was observed among various studies (P = 0.035, I2 = 53.5%). No evidence of publication bias existed according to the Begg’s rank-correlation test (P = 0.108), and the slope coefficient is 0.036. The results of meta-regression were showed as follow (Supplementary Table 3): publication year (P = 0.087), sample size (P = 0.296), location (P = 0.133), age (P = 0.169), sex (P = 0.307), dietary assessment (P = 0.911). The results of subgroup analysis were presented in Table 2. The negative relationship between dietary vitamin C intake and depression only existed in Asia (RR = 0.57, 95% CI: 0.42 to 0.78; P < 0.001), females (RR = 0.69, 95% CI: 0.59 to 0.80; P < 0.001), adolescent (RR = 0.61, 95% CI: 0.43 to 0.86; P = 0.005) and CES-D or BDI (RR = 0.62, 95% CI: 0.50 to 0.78; P < 0.001), but not in non-Asia (RR = 0.84, 95% CI: 0.62 to 1.13; P = 0.25), males (RR = 0.89, 95% CI: 0.54 to 1.47; P = 0.66), middle aged and elderly (RR = 0.76, 95% CI: 0.58 to 1.02; P = 0.07) and other diagnostic criteria of depression (RR = 0.89, 95% CI: 0.63 to 1.26; P = 0.52).
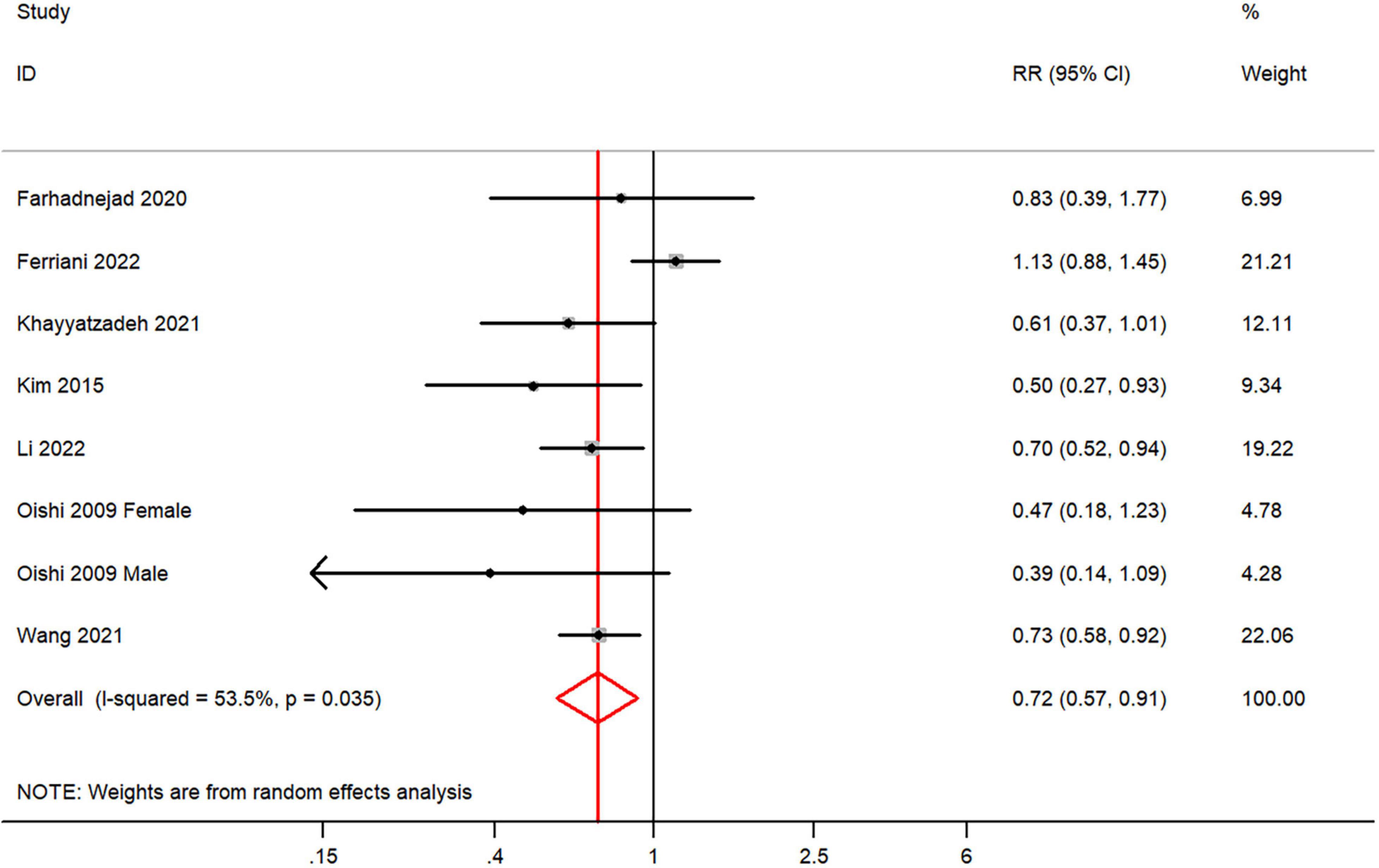
Figure 2. Forest plot of meta-analysis: overall multi-variable adjusted RR of depression for the highest versus lowest category of dietary vitamin C intake.
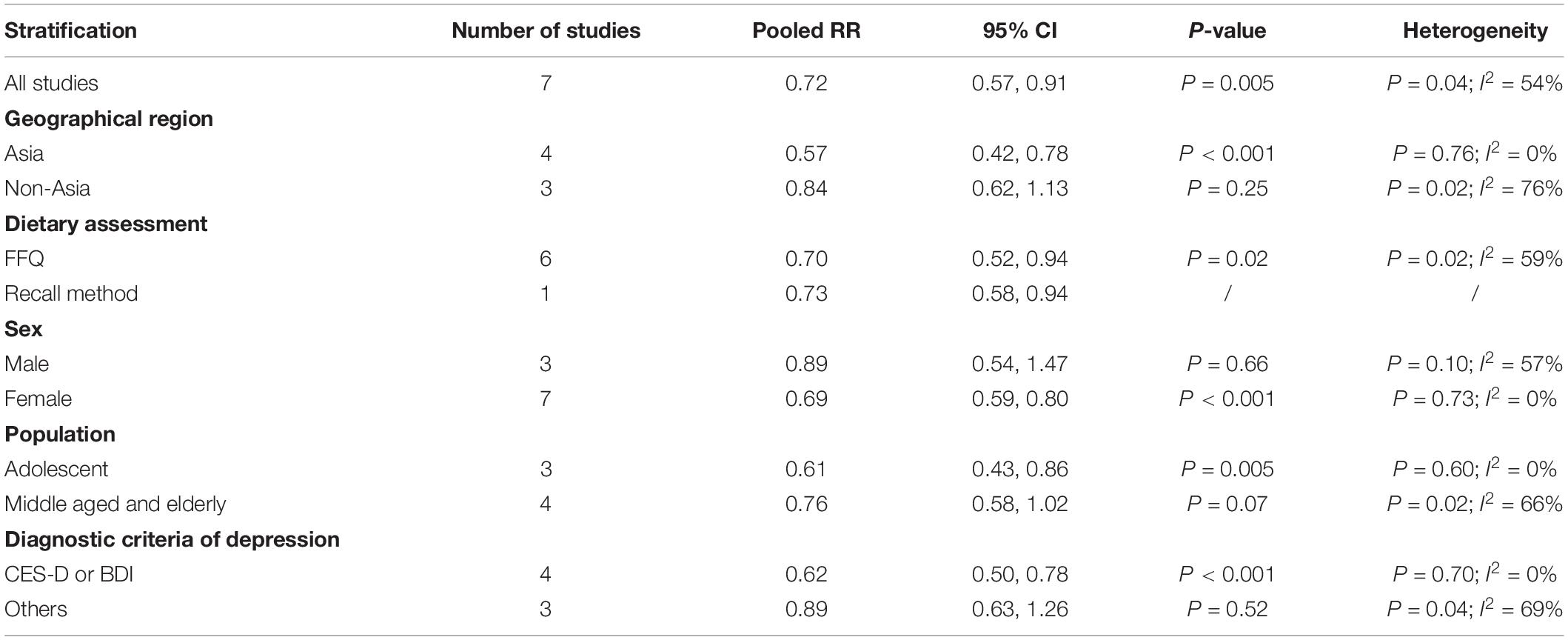
Table 2. Subgroup analysis of depression for the highest versus lowest dietary vitamin C intake category.
Weighted Mean Difference of Dietary Vitamin C Intake for Depression Versus Control Subjects
The overall combined WMD showed that dietary vitamin C intake in depression was lower than that in control subjects (WMD = −11.58, 95% CI: −14.88 to −8.29; P < 0.001) (Figure 3). A substantial level of heterogeneity was observed among the various studies (P < 0.001, I2 = 59.6%). No evidence of publication bias existed according to the Begg’s rank-correlation test (P = 0.503), and the slope coefficient is −10.377. The results of meta-regression were showed as follow (Supplementary Table 4): publication year (P = 0.661), sample size (P = 0.344), location (P = 0.068), age (P = 0.372), sex (P = 0.708), dietary assessment (P = 0.358). The results of subgroup analysis were presented in Table 3.
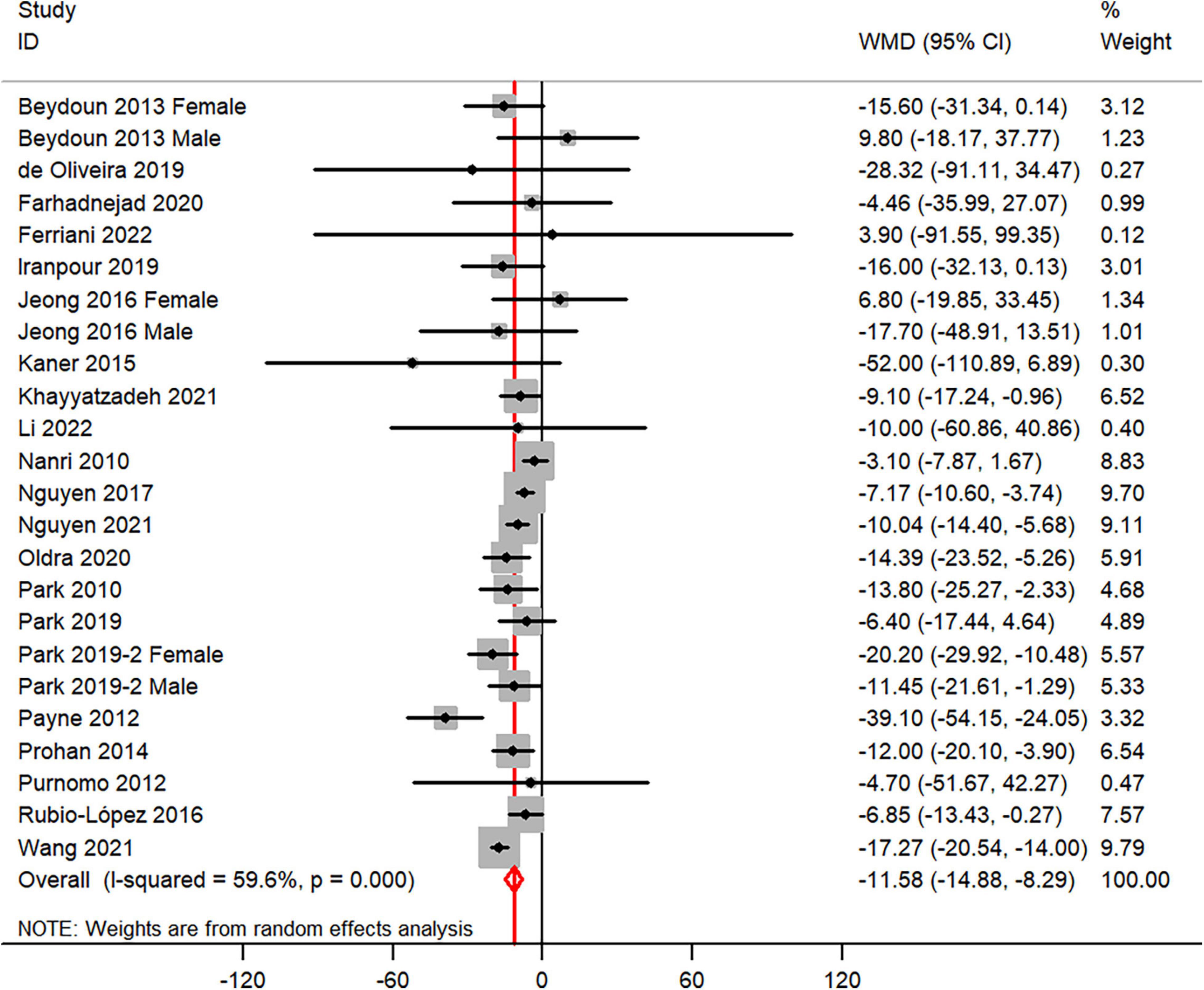
Figure 3. Forest plot of meta-analysis: WMD of dietary vitamin C intake for depression versus control subjects.
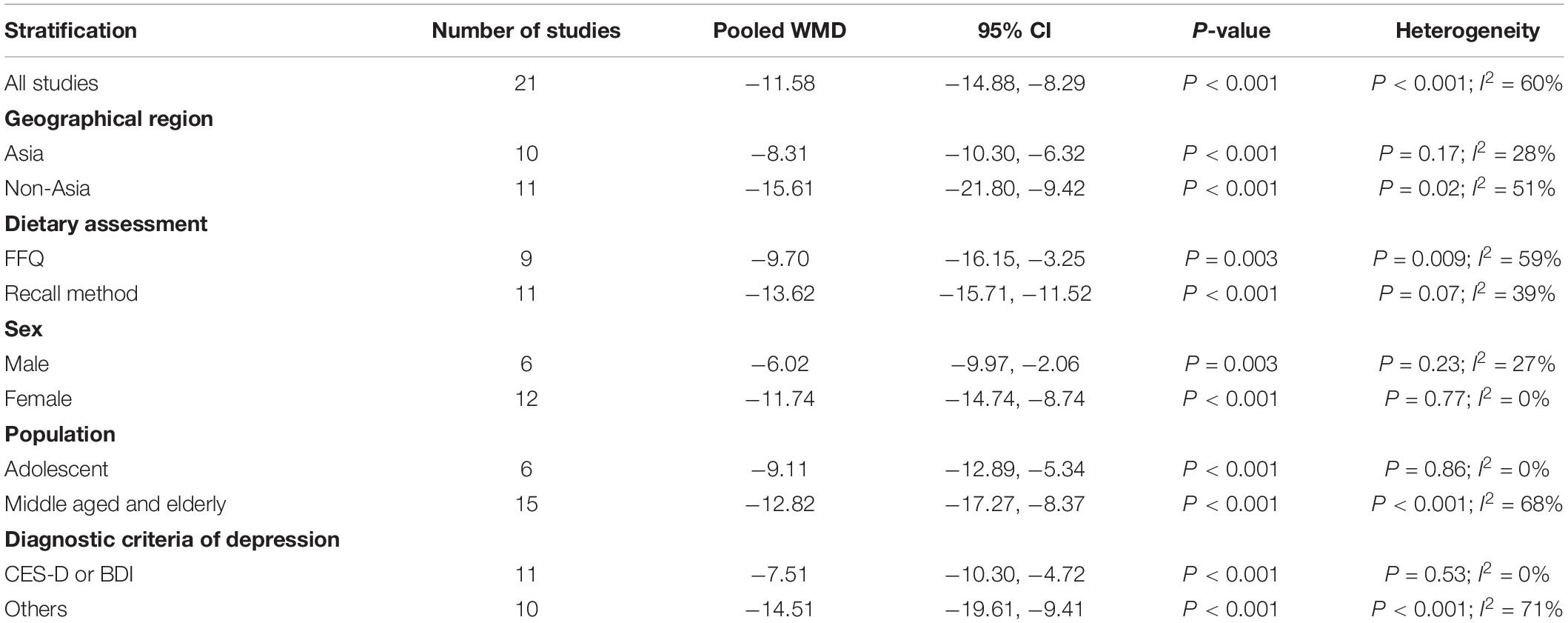
Table 3. Subgroup analysis for WMD of dietary vitamin C level in depression versus control subjects.
Relative Risk of Depression for the Highest Versus Lowest Dietary Vitamin E Intake Category
The overall multi-variable adjusted RR demonstrated that dietary vitamin E intake was negatively associated with depression (RR = 0.84, 95% CI: 0.72 to 0.98; P = 0.023) (Figure 4). No substantial level of heterogeneity was observed among various studies (P = 0.119, I2 = 40.9%). No evidence of publication bias existed according to the Begg’s rank-correlation test (P = 0.548), and the slope coefficient is 0.150. The results of meta-regression were showed as follow (Supplementary Table 5): publication year (P = 0.401), sample size (P = 0.031), location (P = 0.058), age (P = 0.100), sex (P = 0.105). The results of subgroup analysis were presented in Table 4. The negative relationship between dietary vitamin E intake and depression only existed in cross-sectional (RR = 0.81, 95% CI: 0.65 to 1.00; P = 0.05), Asia (RR = 0.49, 95% CI: 0.31 to 0.77; P = 0.002), adolescent (RR = 0.45, 95% CI: 0.25 to 0.81; P = 0.008), and CES-D or BDI (RR = 0.48, 95% CI: 0.29 to 0.81; P = 0.006), but not in prospective cohort (RR = 0.74, 95% CI: 0.41 to 1.32; P = 0.31), non-Asia (RR = 0.90, 95% CI: 0.76 to 1.06; P = 0.01), middle aged and elderly (RR = 0.87, 95% CI: 0.75 to 1.03; P = 0.10) and other diagnostic criteria of depression (RR = 0.88, 95% CI: 0.75 to 1.04; P = 0.13).
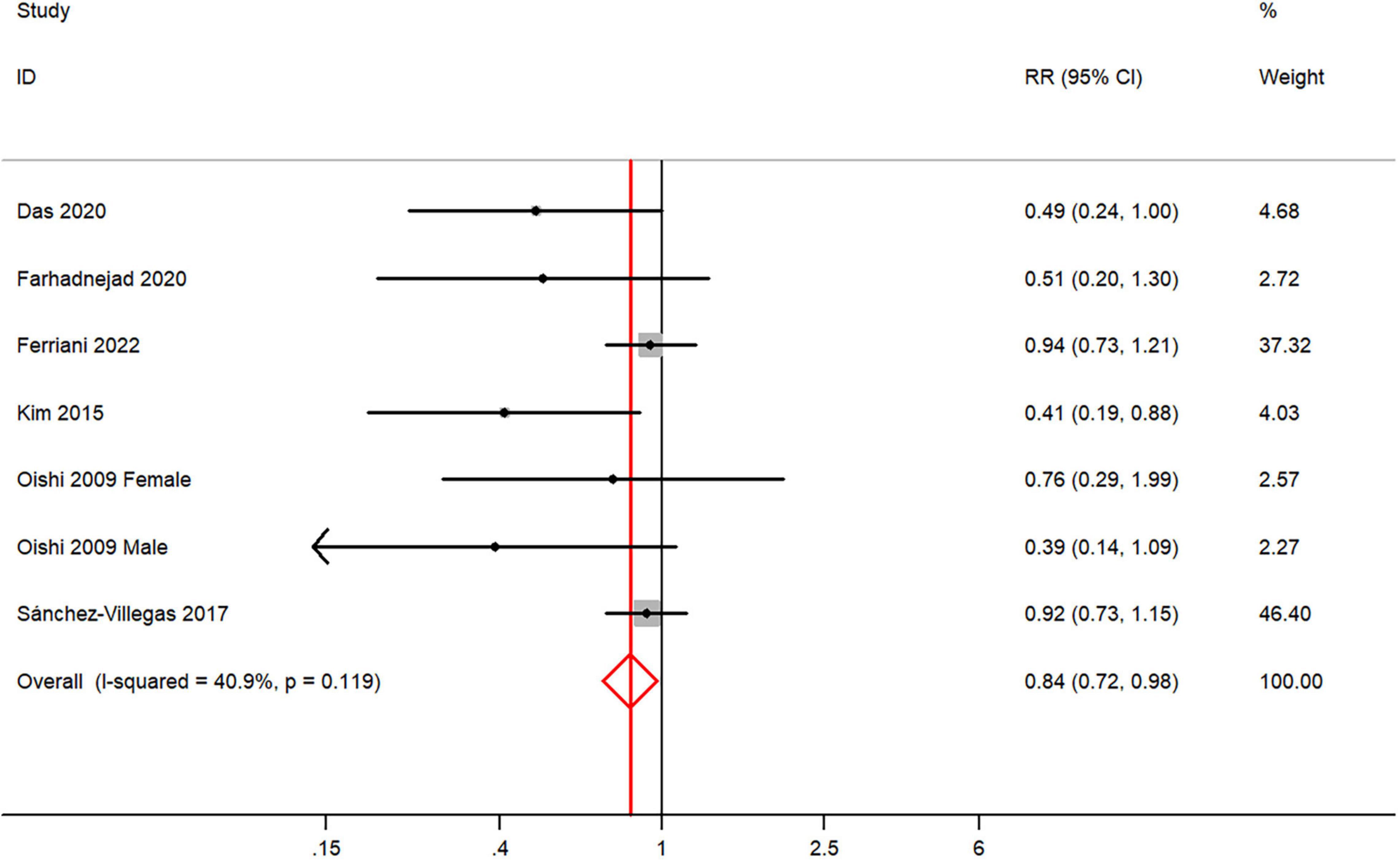
Figure 4. Forest plot of meta-analysis: overall multi-variable adjusted RR of depression for the highest versus lowest category of dietary vitamin E intake.
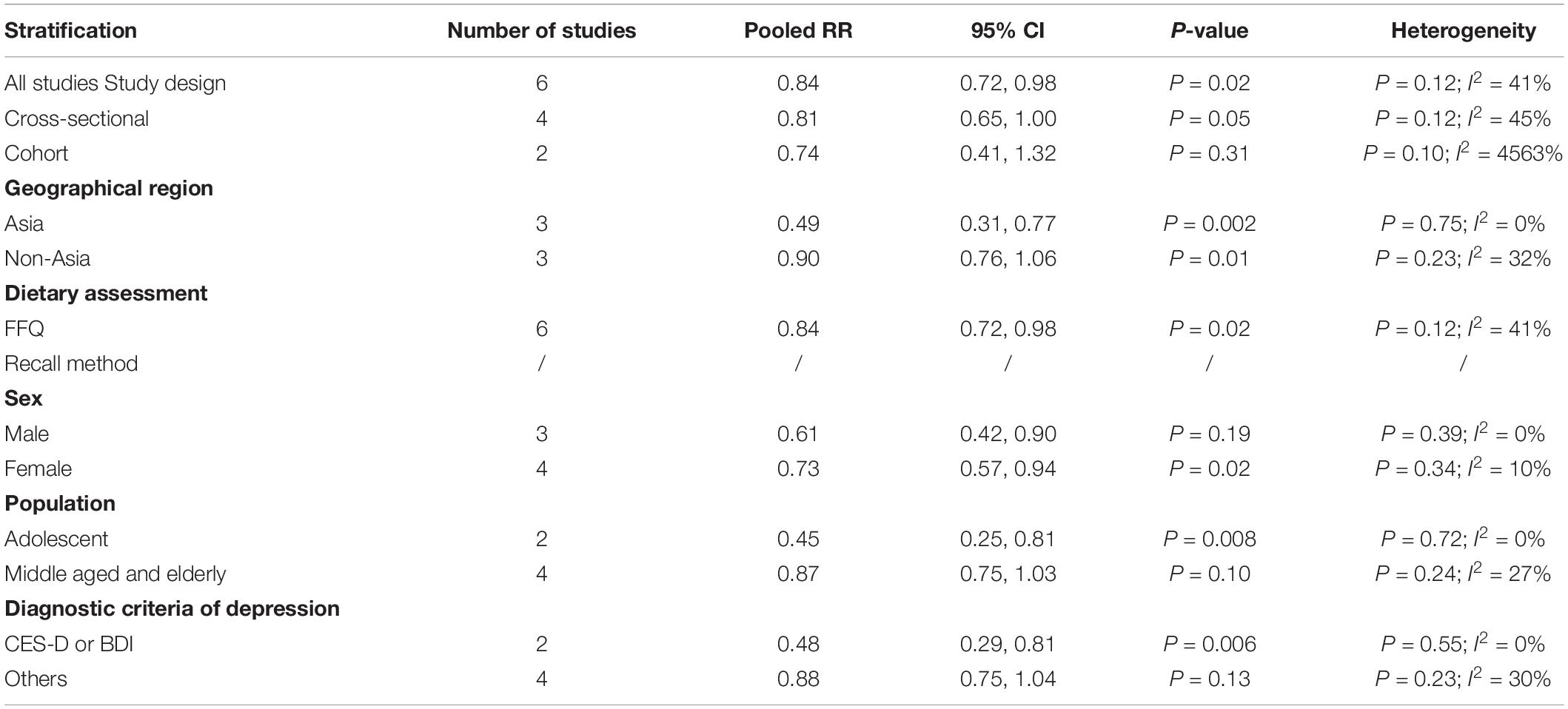
Table 4. Subgroup analysis of depression for the highest versus lowest dietary vitamin E intake category.
Weighted Mean Difference of Dietary Vitamin E Intake for Depression Versus Control Subjects
The overall combined WMD showed that dietary vitamin E intake in depression was lower than that in control subjects (WMD = −0.71, 95% CI: −1.07 to −0.34; P = 0.006) (Figure 5). A substantial level of heterogeneity was observed among the various studies (P < 0.001, I2 = 74.4%). No evidence of publication bias existed according to the Begg’s rank-correlation test (P = 0.951), and the slope coefficient is −0.760. The results of meta-regression were showed as follow (Supplementary Table 6): publication year (P = 0.737), sample size (P = 0.890), location (P = 0.164), age (P = 0.482), sex (P = 0.479), dietary assessment (P = 0.083). The results of subgroup analysis were presented in Table 5. The negative relationship between dietary vitamin E intake and depression only existed in recall method (WMD = −1.06, 95% CI: −1.46 to −0.65; P < 0.001), female (WMD = −0.40, 95% CI: −0.56 to −0.24; P < 0.001), but not in FFQ (WMD = −0.46, 95% CI: −0.98 to 0.06; P = 0.08) and males (WMD = −0.41, 95% CI: −1.23 to 0.40; P = 0.32).
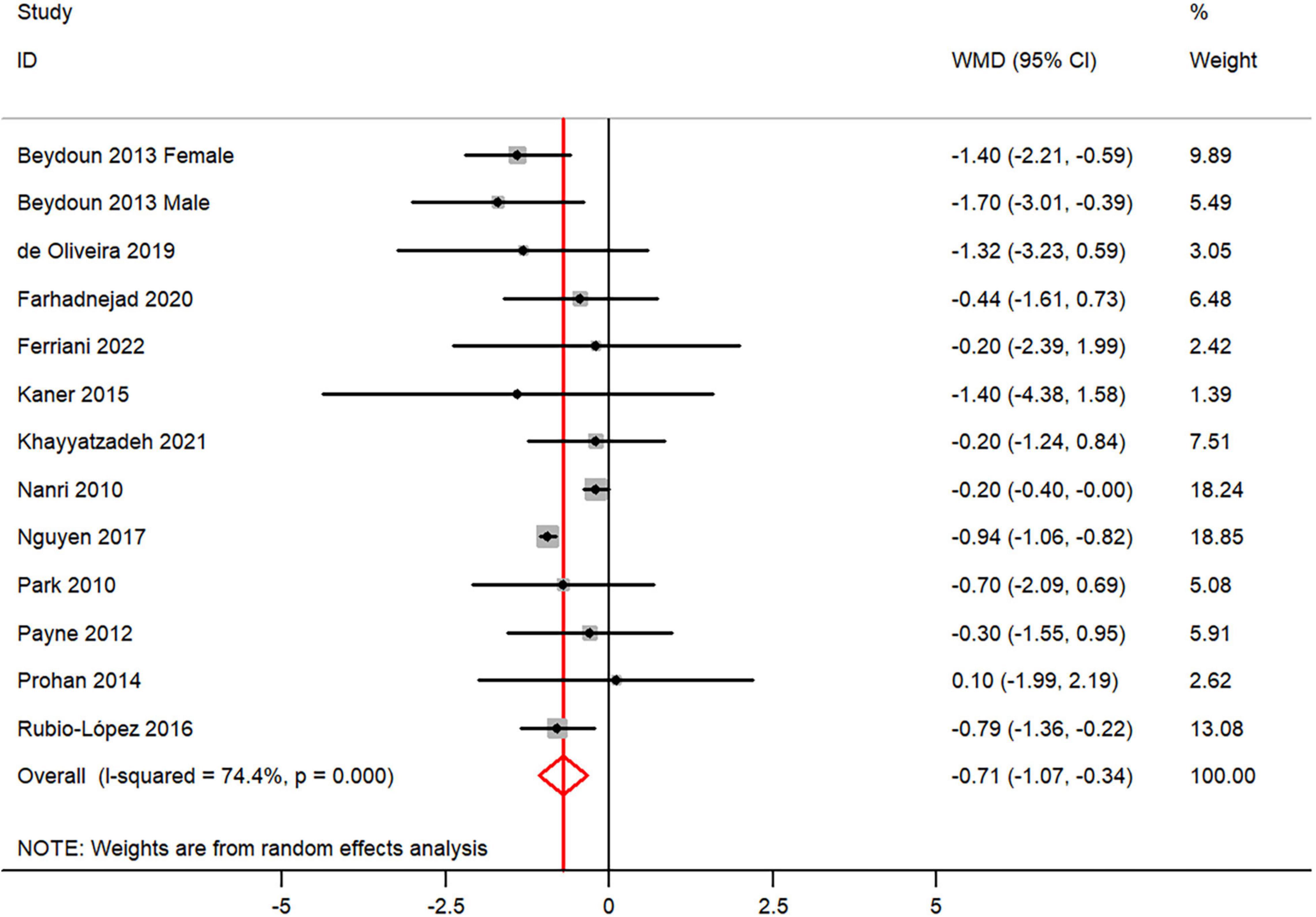
Figure 5. Forest plot of meta-analysis: WMD of dietary vitamin E intake for depression versus control subjects.
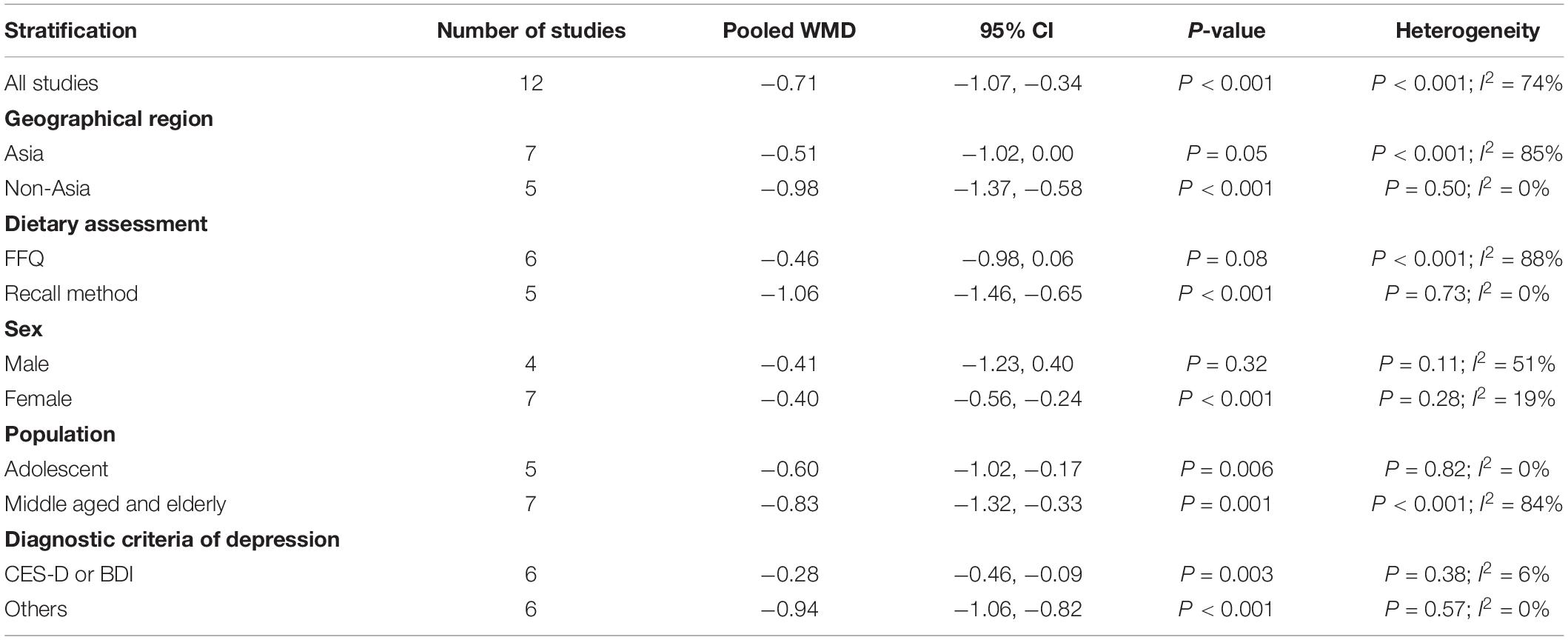
Table 5. Subgroup analysis for WMD of dietary vitamin E level in depression versus control subjects.
Sensitivity Analysis
The results of the sensitivity analysis showed only minimal changes in magnitude of the pooled effect estimate and heterogeneity when any one study was excluded from the meta-analysis, indicating that no individual study had excessive influence on these robust aggregated results (Supplementary Tables 7–10).
Discussion
A total of 25 observational studies were identified for examination in this meta-analysis, and the pooled analysis showed that both dietary vitamin C and E intake was inversely associated with depression.
The potential beneficial effect of dietary vitamin C and E intake on depression has been demonstrated by experimental evidence. Dulabi et al. finds that the chronic social isolation stress-induced weight gain and depressive-like behavior is protected by vitamin C (47). Moreover, Fraga et al. demonstrates that depressive-like behavior and hippocampal synaptic dysfunction induced by corticosterone is rapidly reversed by a single administration of vitamin C (48). In addition, Koizumi further indicates that vitamin C may impact social environment-related anxiety behavior and stress-induced anorexia in SMP30/GNL knockout mice (49). With regard to vitamin E, Manosso et al. reports that the depressive-like effect induced by TNF-α can be reduced by acute administration of α-tocopherol (30–100 mg/kg) (50). Parveen et al. further shows that the depression-like symptoms can be significantly improved by supplementation of 0.3 ml vitamin E/day for 4 weeks (51). Taken together, these above fundamental evidence strongly supports our results.
Interestingly, some of our findings are only confirmed in females (RR for vitamin C and WMD for vitamin E), which may be explained as follow: (1) the number of study for males is relative limited, which may influence the reliability of subgroup analysis; (2) females may be more precise and reliable in completing the dietary assessment (52); (3) some potential genetic sexual differences in diet-related pathology of depression may exist (53, 54). It should also be noted that some results of RR and WMD were not completely consistent (39). The reasons can be listed as follows: (1) the effect estimate for RR is adjusted by multi-variable, whereas WMD is not; (2) the classification of exposure vary among individuals (tertile or quartile). Therefore, it might be acceptable for the minor difference between RR and WMD result. Importantly, the inverse relationship between dietary vitamin E intake and depression is lost in prospective cohort study. Although the number of prospective cohort studies is rather limited (only 2), the factors that matter the dietary vitamin E intake may change after depression, which may reverse the causality directly (depressive subjects may consume less dietary vitamin E due to the reduced appetite). Moreover, subgroup analysis also suggests that geographic region, dietary assessment, diagnostic criteria of depression and population may influence the overall result. Therefore, these factors may be considered to contribute to the heterogeneity of our study. Taken together, more well-designed prospective cohort studies with sexual specification are still needed.
It should also be noted that vitamin C is a biomarker for vegetable and fruit, which contain a wide range of bioactive constituents for human health: nitrate (55), phytochemicals (56) and folate (57). Phytomedicine may be an alternative and effective treatment for depression when conventional drug is not applicable for its side effects, low effectiveness or inaccessibility (58). Moreover, the serum and dietary level of folate in depression is lower than that in controls (59), and folate supplementation improves the efficacy of traditional antidepressant medications (59). Therefore, folate is considered to be beneficial for the long-term management of depression (60). Taken together, the potential beneficial effect of these above bio-active constituents cannot be fully excluded, which should be considered in further study.
Our study has several strengthens. First, this is the first meta-analysis of observational studies on the associations of dietary vitamin C and E intake with depression. Second, our findings are consistence with the corresponding experimental fundamental evidence, which may provide helpful information to better consider the dietary effect on depression (e.g., vitamin C and E-rich food). The limitations of this study should also be acknowledged. First, the substantial level of heterogeneity might have distorted the reliability of our results. Second, due to the limitation in the relevant literature, only 2 prospective cohort studies are identified (preclude causal relationships). Third, the classification of exposure and diagnostic criteria of depression vary greatly among individuals. Fourth, the selection of adjusted factors is not uniform. Fifth, no included study has considered the severity of depression (e.g., major and minor depression) and dietary quality (the sources of vitamin C and E are partially similar), and some issues cannot be addressed. Last but not the least, the circulating level of vitamin C and E is not considered. These limitations may weaken the significance of this study.
Conclusion
Our results suggest that both dietary vitamin C and E intake is inversely associated with depression. However, due to the limited evidence, more well-designed prospective cohort studies with sexual specification are still needed.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
YZ and JD conceived the idea, drafted this manuscript, selected and retrieved relevant manuscript, and assessed each study. JD performed the statistical analysis. YZ was the guarantor of the overall content. Both authors revised and approved the final manuscript.
Funding
This study was supported by National Natural Science Foundation of China (82102581), National Postdoctoral Science Foundation of China (2021M693562), Provincial Outstanding Postdoctoral Innovative Talents Program of Hunan (2021RC2020), Young Investigator Grant of Xiangya Hospital, Central South University (2020Q14), and FuQing Postdoc Program of Xiangya Hospital, Central South University (176).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.857823/full#supplementary-material
Supplementary Figure 1 | Funnel plot with pseudo 95% confidence limits for the analysis of dietary vitamin C intake and depression.
Supplementary Figure 2 | Funnel plot with pseudo 95% confidence limits for the dietary vitamin C intake for depression versus control subjects.
Supplementary Figure 3 | Funnel plot with pseudo 95% confidence limits for the analysis of dietary vitamin E intake and depression.
Supplementary Figure 4 | Funnel plot with pseudo 95% confidence limits for the dietary vitamin E intake for depression versus control subjects.
References
1. Kessler RC. Epidemiology of women and depression. J Affect Disord. (2003) 74:5–13. doi: 10.1016/s0165-0327(02)00426-3
2. Yary T, Aazami S. Dietary intake of zinc was inversely associated with depression. Biol Trace Elem Res. (2012) 145:286–90. doi: 10.1007/s12011-011-9202-y
3. Jung A, Spira D, Steinhagen-Thiessen E, Ilja Demuth I, Norman K. Zinc deficiency is associated with depressive symptoms-results from the Berlin aging study II. J Gerontol A Biol Sci Med Sci. (2017) 72:1149–54. doi: 10.1093/gerona/glw218
4. Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. (2006) 3:e442. doi: 10.1371/journal.pmed.0030442
5. Mauskopf JA, Simon GE, Kalsekar A, Nimsch C, Dunayevich E, Cameron A. Nonresponse, partial response, and failure to achieve remission: humanistic and cost burden in major depressive disorder. Depress Anxiety. (2009) 26:83–97. doi: 10.1002/da.20505
6. Lai JS, Hiles S, Bisquera A, Hure AJ, McEvoy M, Attia J. A systematic review and meta-analysis of dietary patterns and depression in community-dwelling adults. Am J Clin Nutr. (2014) 99:181–97. doi: 10.3945/ajcn.113.069880
7. Zhang Y, Yang Y, Xie MS, Ding X, Li H, Liu ZC, et al. Is meat consumption associated with depression? A meta-analysis of observational studies. BMC Psychiatry. (2017) 17:409. doi: 10.1186/s12888-017-1540-7
8. Guo HB, Ding J, Liu Q, Li YS, Liang JY, Zhang Y. Vitamin C and Metabolic Syndrome: a meta-analysis of observational studies. Front Nutr. (2021) 8:728880. doi: 10.3389/fnut.2021.728880
9. Sherf-Dagan S, Buch A, Ben-Porat T, Sakran N, Sinai T. Vitamin E status among bariatric surgery patients: a systematic review. Surg Obes Relat Dis. (2021) 17:816–30. doi: 10.1016/j.soard.2020.10.029
10. Zhang Y, Ding J, Guo H, Liu Z, Liu Q, Li Y, et al. Associations of dietary and circulating vitamin E level with metabolic syndrome. A meta-analysis of observational studies. Front Nutr. (2021) 8:783990. doi: 10.3389/fnut.2021.783990
11. Saghafian F, Malmir H, Saneei P, Milajerdi A, Larijani B, Esmaillzadeh A. Fruit and vegetable consumption and risk of depression: accumulative evidence from an updated systematic review and meta-analysis of epidemiological studies. Br J Nutr. (2018) 119:1087–101. doi: 10.1017/S0007114518000697
12. Liu XQ, Yan Y, Li F, Zhang DF. Fruit and vegetable consumption and the risk of depression: a meta-analysis. Nutrition. (2016) 32:296–302. doi: 10.1016/j.nut.2015.09.009
13. Anjom-Shoae J, Sadeghi O, Keshteli AH, Afshar H, Esmaillzadeh A, Adibi P. Legume and nut consumption in relation to depression, anxiety and psychological distress in Iranian adults. Eur J Nutr. (2020) 59:3635–45. doi: 10.1007/s00394-020-02197-1
14. Padayatty SJ, Katz A, Wang Y, Eck P, Kwon O, Lee JH, et al. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr. (2003) 22:18–35. doi: 10.1080/07315724.2003.10719272
15. Pehlivan FE. Vitamin C: An antioxidant agent. In: Hamza AH editor. Vitamin C. London: IntechOpen (2017). p. 23–35.
16. Xiao JX, Khan MZ, Ma YL, Alugongo GM, Ma JY, Chen TY. The antioxidant properties of selenium and vitamin E; their role in periparturient dairy cattle health regulation. Antioxidants (Basel). (2021) 10:1555. doi: 10.3390/antiox10101555
17. Bajpai A, Verma AK, Srivastava M, Srivastava R. Oxidative stress and major depression. J Clin Diagn Res. (2014) 8:CC04–7.
18. Liu T, Zhong SM, Liao XX, Chen J, He TT, Lai SK. A meta-analysis of oxidative stress markers in depression. PLoS One. (2015) 10:e0138904. doi: 10.1371/journal.pone.0138904
19. Oishi J, Doi H, Kawakami N. Nutrition and depressive symptoms in community-dwelling elderly persons in Japan. Acta Med Okayama. (2009) 63:9–17. doi: 10.18926/AMO/31854
20. Nanri A, Kimura Y, Matsushita Y, Ohta M, Sato M, Mishima N, et al. Dietary patterns and depressive symptoms among Japanese men and women. Eur J Clin Nutr. (2010) 64:832–9. doi: 10.1038/ejcn.2010.86
21. Park JY, You JS, Chang KJ. Dietary taurine intake, nutrients intake, dietary habits and life stress by depression in Korean female college students: a case-control study. J Biomed Sci. (2010) 17(Suppl. 1):S40. doi: 10.1186/1423-0127-17-S1-S40
22. Payne ME, Steck SE, George RR, Steffens DC. Fruit, vegetable, and antioxidant intakes are lower in older adults with depression. J Acad Nutr Diet. (2012) 112:2022–7. doi: 10.1016/j.jand.2012.08.026
23. Purnomo J, Jeganathan S, Begley K, Houtzager L. Depression and dietary intake in a cohort of HIV-positive clients in Sydney. Int J STD AIDS. (2012) 23:882–6. doi: 10.1258/ijsa.2012.012017
24. Beydoun MA, Beydoun HA, Boueiz A, Shroff MR, Zonderman AB. Antioxidant status and its association with elevated depressive symptoms among US adults: national health and nutrition examination surveys 2005-6. Br J Nutr. (2013) 109:1714–29. doi: 10.1017/S0007114512003467
25. Prohan M, Amani R, Nematpour S, Jomehzadeh N, Haghighizadeh MH. Total antioxidant capacity of diet and serum, dietary antioxidant vitamins intake, and serum hs-CRP levels in relation to depression scales in university male students. Redox Rep. (2014) 19:133–9. doi: 10.1179/1351000214Y.0000000085
26. Kim TH, Choi JY, Lee HH, Park YS. Associations between dietary pattern and depression in Korean adolescent girls. J Pediatr Adolesc Gynecol. (2015) 28:533–7. doi: 10.1016/j.jpag.2015.04.005
27. Kaner G, Soylu M, Yüksel N, Inanç N, Ongan D, Başmısırlı E. Evaluation of nutritional status of patients with depression. Biomed Res Int. (2015) 2015:521481. doi: 10.1155/2015/521481
28. Jeong YJ, Han AL, Shin SR, Lee SY, Kim JH. Relationship between diet and prevalence of depression among Korean adults: Korea national health and nutrition examination survey 2010. J Agric Med Community Health. (2016) 41:75–84. doi: 10.5393/jamch.2016.41.2.075
29. Rubio-López N, Morales-Suárez-Varela M, Pico Y, Livianos-Aldana L, Llopis-González A. Nutrient intake and depression symptoms in Spanish children: the ANIVA study. Int J Environ Res Public Health. (2016) 13:352. doi: 10.3390/ijerph13030352
30. Sánchez-Villegas A, Pérez-Cornago A, Zazpe I, Santiago S, Lahortiga F, Martínez-González MA. Micronutrient intake adequacy and depression risk in the SUN cohort study. Eur J Nutr. (2018) 57:2409–19. doi: 10.1007/s00394-017-1514-z
31. Nguyen T, Tsujiguchi H, Kambayashi Y, Hara A, Miyagi S, Yamada Y. Relationship between vitamin intake and depressive symptoms in elderly Japanese individuals: differences with gender and body mass index. Nutrients. (2017) 9:1319. doi: 10.3390/nu9121319
32. de Oliveira NG, Teixeira IT, Theodoro H, Branco CS. Dietary total antioxidant capacity as a preventive factor against depression in climacteric women. Dement Neuropsychol. (2019) 13:305–11. doi: 10.1590/1980-57642018dn13-030007
33. Iranpour S, Sabour S. Inverse association between caffeine intake and depressive symptoms in US adults: data from national health and nutrition examination survey (NHANES) 2005-2006. Psychiatry Res. (2019) 271:732–9. doi: 10.1016/j.psychres.2018.11.004
34. Park SH, Oh EY, Kim SH, Chang KJ. Relationship among dietary taurine intake, dietary attitudes, dietary behaviors, and life stress by depression in Korean female college students. Adv Exp Med Biol. (2019) 1155:293–300. doi: 10.1007/978-981-13-8023-5_28
35. Park SY, Lum HA, Shin SR, Eo JE. Relationship between dietary intake and depression among Korean adults: Korea national health and nutrition examination survey 2014. Korean J Fam Pract. (2019) 9:139–46. doi: 10.21215/kjfp.2019.9.2.139
36. Das A, Cumming RG, Naganathan V, Ribeiro RV, Couteur D, Handelsman DJ. The association between antioxidant intake, dietary pattern and depressive symptoms in older Australian men: the Concord health and ageing in men project. Eur J Nutr. (2021) 60:443–54. doi: 10.1007/s00394-020-02255-8
37. Farhadnejad H, Tehrani AN, Salehpour A, Hekmatdoost A. Antioxidant vitamin intakes and risk of depression, anxiety and stress among female adolescents. Clin Nutr ESPEN. (2020) 40:257–62. doi: 10.1016/j.clnesp.2020.09.010
38. Oldra CM, Benvegnú DM, Silva D, Wendt G, Vieira AP. Relationships between depression and food intake in climacteric women. Climacteric. (2020) 23:474–81. doi: 10.1080/13697137.2020.1736025
39. Khayyatzadeh SS, Omranzadeh A, Miri-Moghaddam MM, Arekhi S, Naseri A, Ziaee A, et al. Dietary antioxidants and fibre intake and depressive symptoms in Iranian adolescent girls. Public Health Nutr. (2021) 24:5650–6. doi: 10.1017/S1368980020004838
40. Wang AN, Luo J, Zhang TH, Zhang DF. Dietary vitamin C and vitamin C derived from vegetables are inversely associated with the risk of depressive symptoms among the general population. Antioxidants (Basel). (2021) 10:1984. doi: 10.3390/antiox10121984
41. Nguyen HD, Oh HJ, Hoang N, Jo WH, Kim MS. Environmental science and pollution research role of heavy metal concentrations and vitamin intake from food in depression: a national cross-sectional study (2009-2017). Environ Sci Pollut Res Int. (2022) 29:4574–86. doi: 10.1007/s11356-021-15986-w
42. Ferriani LO, Silva DA, Molina M, Mill JG, Brunoni AR, da Fonseca M. Associations of depression and intake of antioxidants and vitamin B complex: results of the Brazilian longitudinal study of adult health (ELSA-Brasil). J Affect Disord. (2022) 297:259–68. doi: 10.1016/j.jad.2021.10.027
43. Li D, Xu WZ, Wu Q, Zheng HY, Li Y. Ascorbic acid intake is inversely associated with prevalence of depressive symptoms in US midlife women: a cross-sectional study. J Affect Disord. (2021) 299:498–503. doi: 10.1016/j.jad.2021.12.049
44. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. (2009) 339:b2700. doi: 10.1136/bmj.b2700
45. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
46. Lin LF, Shi LY, Chu HT, Murad MH. The magnitude of small-study effects in the Cochrane database of systematic reviews: an empirical study of nearly 30000 meta-analyses. BMJ Evid Based Med. (2020) 25:27–32. doi: 10.1136/bmjebm-2019-111191
47. Dulabi AN, Shakerin Z, Mehranfard N, Ghasemi M. Vitamin C protects against chronic social isolation stress-induced weight gain and depressive-like behavior in adult male rats. Endocr Regul. (2020) 54:266–74. doi: 10.2478/enr-2020-0030
48. Fraga DB, Camargo A, Olescowicz G, Padilha DA, Mina F, Budni J. A single administration of ascorbic acid rapidly reverses depressive-like behavior and hippocampal synaptic dysfunction induced by corticosterone in mice. Chem Biol Interact. (2021) 342:109476. doi: 10.1016/j.cbi.2021.109476
49. Koizumi M, Kondo Y, Isaka A, Ishigami A, Suzuki E. Vitamin C impacts anxiety-like behavior and stress-induced anorexia relative to social environment in SMP30/GNL knockout mice. Nutr Res. (2016) 36:1379–91. doi: 10.1016/j.nutres.2016.11.006
50. Manosso LM, Neis VB, Moretti M, Daufenbach JF, Freitas AE, Colla AR, et al. Antidepressant-like effect of α-tocopherol in a mouse model of depressive-like behavior induced by TNF-α. Prog Neuropsychopharmacol Biol Psychiatry. (2013) 46:48–57. doi: 10.1016/j.pnpbp.2013.06.012
51. Tahira Parveen BN, Amin N, Haleem DJ. Repeated administration of vitamin E decreases 5HIAA levels producing antidepressant effect and enhance behavioural effects. Pak J Biochem Mol Biol. (2010) 4:169–73.
52. Shin S, Lee HW, Kim CE, Lim JY, Lee JK, Lee SA, et al. Egg consumption and risk of metabolic syndrome in Korean adults: results from the health examinees study. Nutrients. (2017) 9:687. doi: 10.3390/nu9070687
53. Chang CC, Chang HA, Fang WH, Chang TC, Huang SY. Gender-specific association between serotonin transporter polymorphisms (5-HTTLPR and rs25531) and neuroticism, anxiety and depression in well-defined healthy Han Chinese. J Affect Disord. (2017) 207:422–8. doi: 10.1016/j.jad.2016.08.055
54. Wurtman JJ. Depression and weight gain: the serotonin connection. J Affect Disord. (1993) 29:183–92. doi: 10.1016/0165-0327(93)90032-f
55. LiuY Croft KD, Hodgson JM, Mori T, Ward NC. Mechanisms of the protective effects of nitrate and nitrite in cardiovascular and metabolic diseases. Nitric Oxide-Biol Chem. (2020) 96:35–43. doi: 10.1016/j.niox.2020.01.006
56. Francini-Pesenti F, Spinella P, Calo LA. Potential role of phytochemicals in metabolic syndrome prevention and therapy. Diab Metabol Syndr Obesity Targets Ther. (2019) 12:1987–2002. doi: 10.2147/dmso.s214550
57. Fu LW, Li YN, Luo DM, Deng SF, Hu YQ. Plausible relationship between homocysteine and obesity risk via MTHFR gene: a meta-analysis of 38,317 individuals implementing Mendelian randomization. Diab Metabol Syndr Obesity Targets Ther. (2019) 12:1201–12. doi: 10.2147/DMSO.S205379
58. Lee G, Bae H. Therapeutic effects of phytochemicals and medicinal herbs on depression. Biomed Res Int. (2017) 2017:6596241. doi: 10.1155/2017/6596241
59. Bender A, Hagan KE, Kingston N. The association of folate and depression: a meta-analysis. J Psychiatr Res. (2017) 95:9–18. doi: 10.1016/j.jpsychires.2017.07.019
Keywords: dietary vitamin C, dietary vitamin E, depression, meta-analysis, observational studies
Citation: Ding J and Zhang Y (2022) Associations of Dietary Vitamin C and E Intake With Depression. A Meta-Analysis of Observational Studies. Front. Nutr. 9:857823. doi: 10.3389/fnut.2022.857823
Received: 25 January 2022; Accepted: 04 March 2022;
Published: 07 April 2022.
Edited by:
Tuyen Van Duong, Taipei Medical University, TaiwanReviewed by:
Tommaso Filippini, University of Modena and Reggio Emilia, ItalyDongfeng Zhang, Qingdao University, China
Copyright © 2022 Ding and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Zhang, emhhbmd5aTAyMDVAY3N1LmVkdS5jbg==
 Jun Ding1
Jun Ding1 Yi Zhang
Yi Zhang