- 1College of Animal Science and Technology, Hunan Agricultural University, Changsha, China
- 2Hunan Co-Innovation Center of Animal Production Safety, Changsha, China
A trial was conducted to investigate the effects of different Se sources, including sodium selenite (S-Se) and selenium yeast (Y-Se) and different effective microorganism (EM) addition levels on growth performance, meat quality, and muscle fiber characteristics of three-yellow chickens and its potential mechanism. A total of 400 birds were randomly distributed into 4 groups (S-Se, S-Se + EM, Y-Se, and Y-Se + EM groups) consisting of a 2 × 2 factorial arrangement. The main factors were the source of Se (ISe = inorganic Se: 0.2 mg/kg S-Se; OSe = organic Se: 0.2 mg/kg Y-Se) and the level of EM (HEMB = high EM: 0.5% EM; ZEMB = low EM: 0% EM). Each treatment had 5 replicates and each replicate consisted of 20 broiler chickens. The trial lasted for 70 days. The results showed that, in breast muscle, the broiler chickens fed OSe source decreased the pH24h, drip loss, shear force, perimeter, cross-sectional area, and diameter, but increased the and density compared with the broiler chickens fed ISe source (p < 0.05); broiler chickens supplied with HEMB level decreased the cross-sectional area and diameter, but increased the pH24h, and density compared with the broiler chickens supplied with ZEMB level (p < 0.05). In thigh muscle, OSe source and HEMB level also could improve the meat quality and change muscle fiber characteristics of broiler chickens (p < 0.05). Meat quality was correlated with the muscle fiber characteristics (p < 0.05). OSe source and HEMB level could regulate the expression levels of muscle fiber-relative genes in the breast and thigh muscles (p < 0.05). In conclusion, OSe source and HEMB level could improve the meat quality of the breast and thigh muscles of three-yellow chickens by changing the muscle fiber characteristics, and they changed the muscle fiber characteristics by regulating the expression levels of muscle fiber-relative genes.
Introduction
Three-yellow chicken is one of the most famous indigenous breeds in China. They have a high content of linolenic acid and amino acid, and a low content of sinapic acid in the meat. As people's living standard has improved, the need for high-quality meat products has greatly increased (1). However, large-scale feeding of three-yellow chicken leads to a decrease in meat quality. Thus, more research has gravitated toward improving the meat quality of broiler chicken (2–4).
Sodium selenite (S-Se), selenium yeast (Y-Se), selenomethionine, and nano-selenium can be supplied in animal feed (5). Se is an essential trace element and a key part of glutathione peroxidase. Compared with S-Se, Y-Se has greater effects and is widely used (6). Dietary Y-Se supplementation can improve the carcass quality, antioxidant status, nutrition digestibility, Se deposition, and meat quality of Cobb 500 broilers, laying hen, and sheep (4, 7, 8). Probiotics such as Lactobacillus, yeast, and Bacillus subtilis as the possible substitute for antibiotics have attracted a lot of attention (2, 9). Previous studies had shown that, when supplied with Lactobacillus, it could improve the nutrient absorption, intestinal integrity, meat quality, and keep the balance of gut microbiota of weaning piglets, ducks, and Arbor Acres (AA) broilers (3, 10–12). Dietary yeast can improve the antioxidant status and meat quality of AA and Ross broilers (2, 10, 13). Effective microorganism (EM) mainly included Lactobacillus and yeast in this experiment. However, the effect of Y-Se and EM on the growth performance, meat quality, and muscle fiber characteristics of three-yellow chickens is still unknown.
Meat quality is reflected by many indexes such as pH value, color, flavor, drip loss, cooking loss, and shear force. The level of glycolysis and the content of lactic acid and oxidation level in the muscle can influence the meat quality (14–17). Previous studies had shown that, when supplied with Y-Se, Lactobacillus and yeast could increase the antioxidant capacity of ducks, aging mice, Ross broilers, and pigs by increasing the activities of glutathione peroxidase and superoxide dismutase and decreasing the contents of thiobarbituric acid reactive substances and malondialdehyde (2, 13, 18–20). The increase in antioxidant capacity prevents the oxidation of myoglobin, which can deepen the muscle color (21, 22), and it prevents the oxidation of muscle and keeps the cytomembrane integrity, thus decreasing the L*, drip loss, and cooking loss (14, 16, 23). Previous studies had found a correlation between meat quality and muscle fiber characteristics (24–26). However, there is less information about this correlation in broiler chickens. In this experiment, we investigated the effects of different Se sources and different EM addition levels on the growth performance, meat quality, and muscle fiber characteristics of three-yellow chickens, and studied the correlation between meat quality and muscle fiber characteristics, and then illustrated the underlying mechanism.
Materials and Methods
Animals and Diets
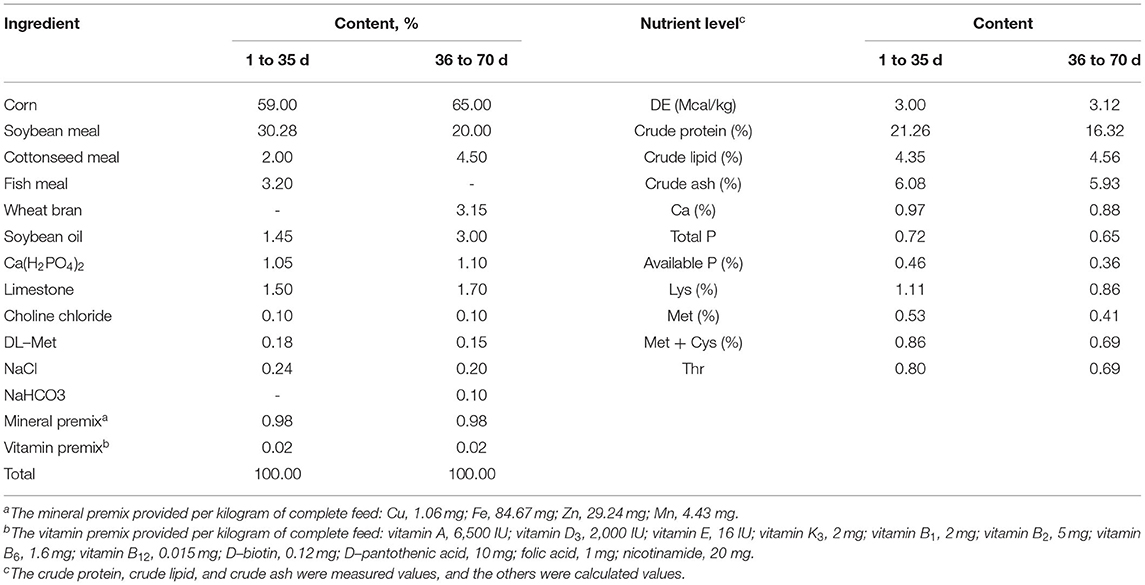
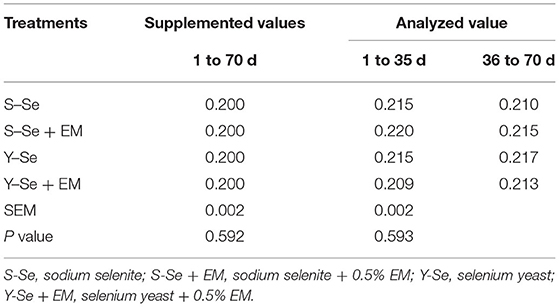
A total of 400 one-day-old healthy three-yellow chickens (male, average initial weight was 35.72 ± 0.05 g) were randomly distributed into 4 groups (S-Se, S-Se + EM, Y-Se, and Y-Se + EM groups) consisting of a 2 × 2 factorial arrangement. The main factors were the source of Se (ISe = inorganic Se: 0.2 mg/kg S-Se; OSe = organic Se: 0.2 mg/kg Y-Se) and the level of EM (HEMB = high EM: 0.5% EM; ZEMB = low EM: 0% EM). The basal diet met the nutrient requirements (except for microelement) of broiler chickens according to NRC 1994 and NY/T33 2004 (Table 1). The additional level of microelement was determined according to our previous study. The broiler chickens in the S-Se and S-Se + EM groups were fed the basal diet supplemented with 0.2 mg/kg S-Se, and in the Y-Se and Y-Se + EM groups were fed the basal diet supplemented with 0.2 mg/kg Y-Se. Meanwhile, the S-Se + EM and Y-Se + EM groups were fed the basal diet supplemented with 0.5% EM (Bokaxi, Changsha, China) which mainly included Lactobacillus and yeast at a dose of 2 × 108 cfu/kg and 3 × 107 cfu/kg, respectively. The analyzed value of Se in the diets is shown in Table 2. The trial lasted for 70 days. The birds had ad libitum access to food and water. Each treatment had 5 replicates, and each replicate consisted of 20 broiler chickens. Each replicate was raised in a single cage having the dimensions 160×160×100 cm (length × width × height). The room temperature was maintained constant at 22 ± 1°C. The relative humidity was maintained at 55 ± 5%. The lighting schedule was 23 h light:1 h dark. Birds were weighed in the morning at 1, 35, and 70 days, and feed intake was recorded each day. At the end of the trial, body weight (BW), average daily feed intake (ADFI), average daily gain (ADG), and feed conversion ratio (FCR) were calculated.
Sample Collection
At the end of the experiment, birds were fasted for 24 h before slaughter, and electrically stunned and euthanized by cervical dislocation. The right breast and thigh muscles were collected from one bird in each replicate. Some of the muscles were fixed in 4% formalin for morphometric analyses, some were used for meat quality examination, and some were immediately frozen at −80°C for quantitative real-time polymerase chain reaction (qRT-PCR) analysis.
Meat Quality
Meat quality of the right breast and thigh muscles was determined according to the method developed by Meng et al. with a slight modification (27). The pH values were measured at 45 min, 24 h, and 48 h postmortem using a portable pH meter (Mettler-Toledo, Shanghai, China). At the same time, meat colors, which included L*, a*, and b*, were determined by Minolta chroma meter (Konica Minolta, Tokyo, Japan). During 45 min after postmortem, the surface water of muscles (around 30 g) was removed, and the initial weight was recorded. After hanging vertically at 4°C for 24 h, the final weight was recorded. The drip loss was calculated as follows:
The muscles were trimmed to 2 cm × 2 cm × 1 cm, and the initial weight was recorded. The meats were packed by bag and immersed in 80°C water until the internal temperature of meats reached 74°C. The meats were cooled and the final weight was recorded after removing the surface water of meats. The cooking loss was calculated as follows:
After measuring the cooking loss, the shear force was measured using digital muscle shear apparatus (Stable Micro Systems, Surrey, UK).
Muscle Fiber Characteristics
The right breast and thigh muscles were embedded in paraffin and cut into 10 μm sections (horizontal axis). The samples were dehydrated via a series of incubations in xylene and ethanol solutions, and then stained with hematoxylin and eosin (HE staining), and observed through a microscope (400×) (Nikon, Tokyo, Japan). Three areas (each area was 0.15 mm2) in a slide (6 slides in each treatment) were randomly selected to examine the perimeter, cross-sectional area, diameter, and density of muscle fiber using CaseViewer.
qRT-PCR Analysis
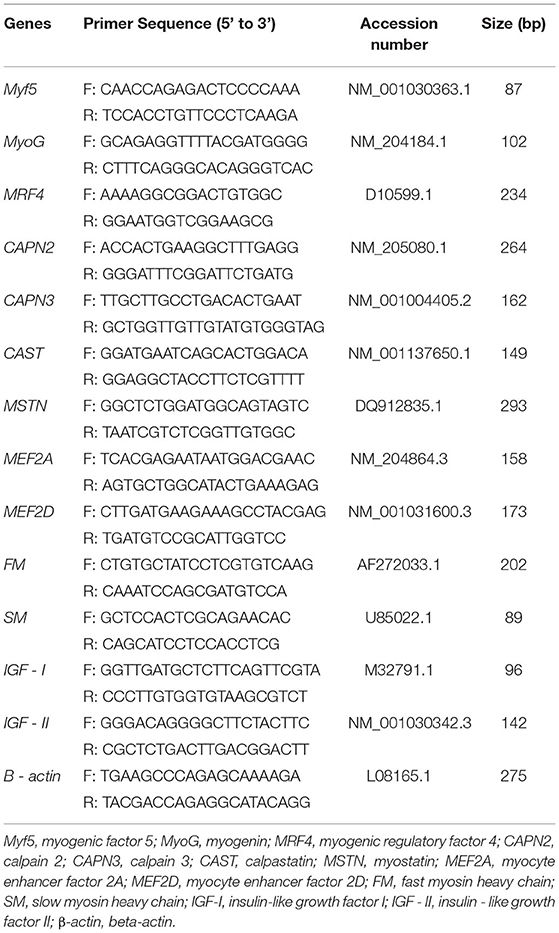
The relative expression levels of myogenic factor 5 (Myf5), myogenin (MyoG), myogenic regulatory factor 4 (MRF4), calpain 2 (CAPN2), calpain 3 (CAPN3), calpastatin (CAST), myostatin (MSTN), myocyte enhancer factor 2A (MEF2A), myocyte enhancer factor 2D (MEF2D), fast myosin heavy chain (FM), slow myosin heavy chain (SM), insulin-like growth factor I (IGF-I), and insulin-like growth factor II (IGF-II) in the right breast and thigh muscles were detected by qRT-PCR. The primers (Sangon Biotech, Shanghai, China) used are listed in Table 3. The β-actin gene was chosen as the reference gene for sample normalization. qRT-PCR reactions were carried out in a –Bio-Rad CFX96 touch qPCR system (Applied Biosystems, Foster City, USA) in 20 μl volumes that contained the following components: 10 μl of SYBR Green Mix (Takara, Changsha, China), 2 μl cDNA (1,000 ng/μl), 0.4 μl of each primer (10 mM), and 7.2 μl dH2O, followed by 40 cycles of 95°C for 30 s, 55°C−58°C for 30 s, and 72°C for 30 s. Finally, a melt curve analysis was used to detect the single product. The relative expression level was analyzed using the 2−ΔΔCT method. The amplification efficiencies of all primes ranged from 0.90 to 1.00. All samples were tested in triplicate.
Statistical Analysis
The experimental design was a 2 × 2 factorial arrangement, and the main factors were the source of Se and the level of EM. Data were analyzed by –two-way ANOVA using SPSS 22.0 (SPSS. Inc., Chicago, USA), which included the main effects of Se source, EM level, and their interaction (Se source × EM level). Tukey's multiple-range test was used to analyze the differences. All data were further subjected to one-way ANOVA. When overall differences were significant, the differences were tested by Duncan's multiple-range test (SPSS 22.0). The correlations between the meat quality and muscle fiber characteristics in the breast and thigh muscles were analyzed by SPSS 22.0. The level of significance was set at p < 0.05, and high level of significance was set at p < 0.01. The results are presented as the mean values and SEM.
Results
Growth Performance
The effect of Se source and EM level on growth performance is shown in Table 4. Se source influenced FCR of three-yellow chickens (p < 0.05). There was no interaction between Se source and EM level regarding growth performance (p > 0.05). Broiler chickens fed OSe source decreased the FCR during 1–35 days compared with the broiler chickens fed ISe source (p < 0.05). During 1–35 days, a greater FCR was observed in the S-Se + EM group compared with the Y-Se and Y-Se + EM groups (p < 0.05), whereas there were no significant differences among the S-Se, Y–Se, and Y-Se + EM groups, or between the S-Se and S-Se + EM groups (p > 0.05).
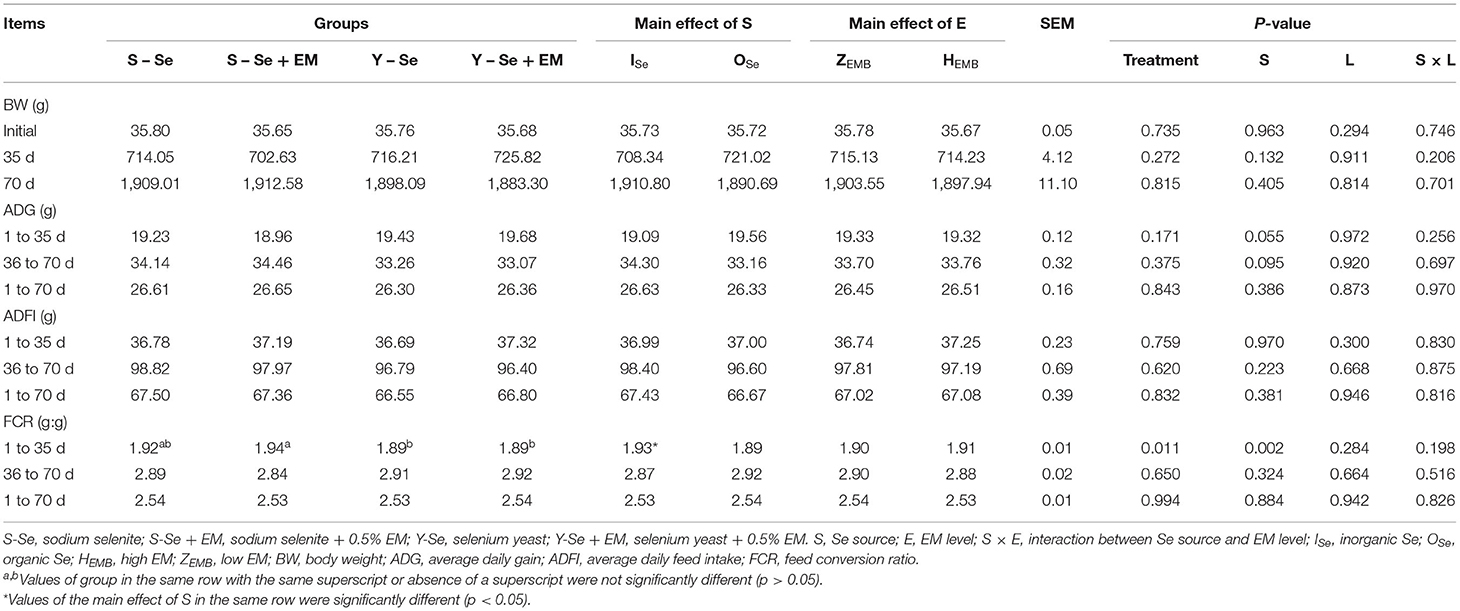
Table 4. Effects of different Se sources and different bacteria supplementation levels on the growth performance of three-yellow chickens.
Meat Quality in the Breast and Thigh Muscles
The effect of Se source and EM level on meat quality in the breast and thigh muscles is shown in Table 5. In breast muscle, the present experiment showed significant interactions between the Se source and EM level regarding pH24h and drip loss (p < 0.05). Compared with the broiler chickens fed ISe source, broiler chickens fed OSe source decreased the pH24h, drip loss, and shear force, and increased the (p < 0.05). Broiler chickens supplied with HEMB level increased pH24h and compared with the broiler chickens supplied with ZEMB level (p < 0.05). In detail, the S-Se + EM group had a greater pH24h compared with the other groups, and the S-Se group had a greater pH24h compared with the Y-Se + EM group (p < 0.05). The Y-Se + EM group had a greater compared with the other groups (p < 0.05). The drip loss in the Y-Se + EM group was lower than that in the S-Se + EM and Y-Se groups (p < 0.05). Shear force in the Y-Se + EM group was lower than that in the S-Se and S-Se + EM groups (p < 0.05).
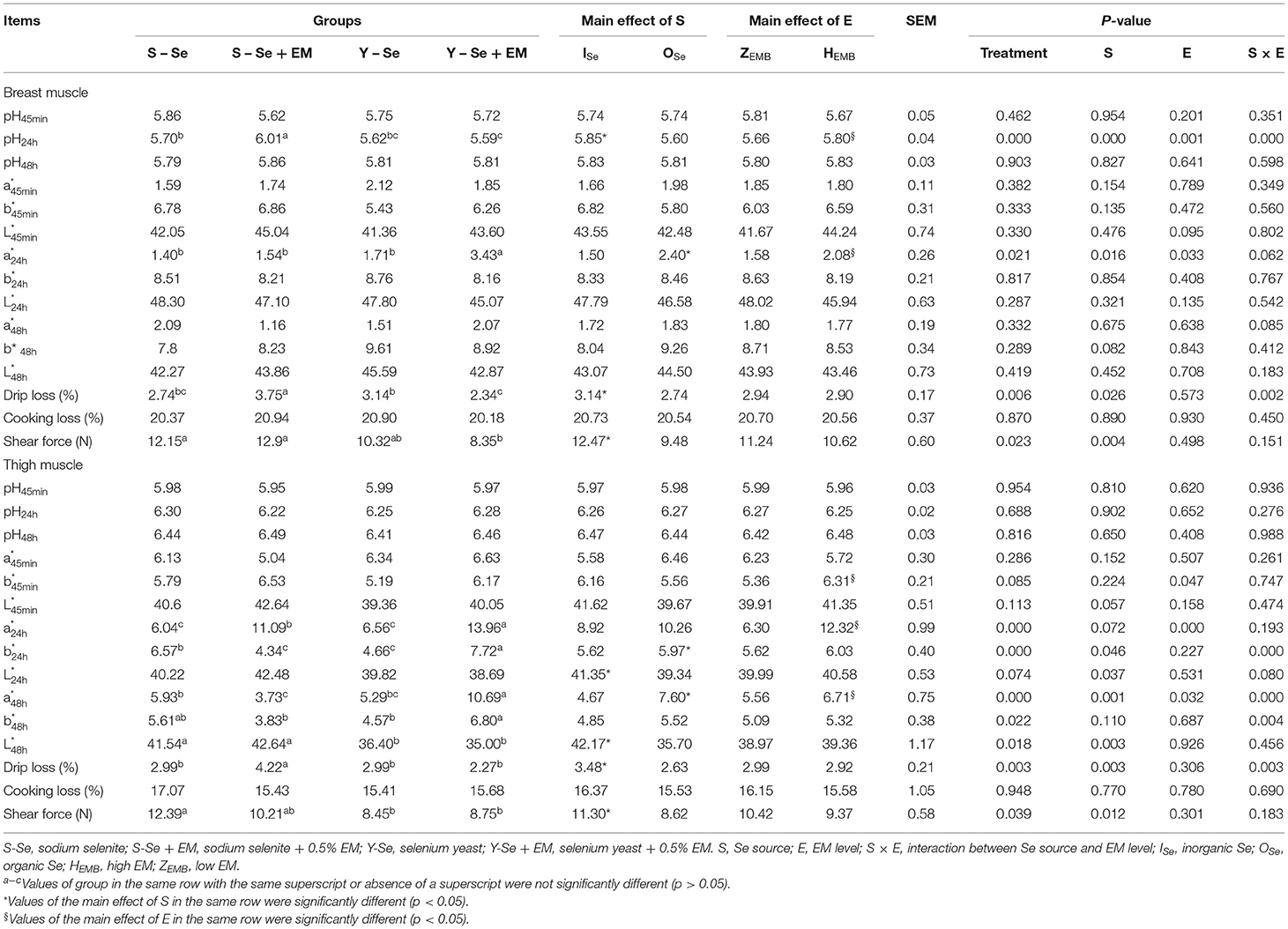
Table 5. Effects of different Se sources and different bacteria supplementation levels on the meat quality of three-yellow chickens.
In the thigh muscle, Se source and EM level showed significant interactions on , , , and drip loss (p < 0.05). Compared with broiler chickens supplied with ISe source, broiler chickens fed OSe source decreased the , , drip loss, and shear force, and increased the and (p < 0.05). Broiler chickens supplied with HEMB level increased the , , and compared with the broiler chickens supplied with ZEMB level (p < 0.05). In detail, the Y-Se + EM group had a greater compared with the other groups, and S-Se + EM group had a greater compared with the S-Se and Y-Se groups (p < 0.05). The Y-Se + EM group had a greater compared with the other groups, and the S-Se group had a greater compared with the S-Se + EM and Y-Se groups (p < 0.05). The Y-Se + EM group had a greater compared with the other groups, and the S-Se group had a greater compared with the S-Se + EM group (p < 0.05). The Y-Se + EM group had a greater compared with the S-Se + EM and Y-Se groups (p < 0.05). The S-Se and S-Se + EM groups had a higher compared with the Y-Se and Y-Se + EM groups (p < 0.05). The S-Se + EM group had a greater drip loss compared with the other groups (p < 0.05). The S-Se group had a greater shear force compared with the Y-Se and Y-Se + EM groups (p < 0.05).
Muscle Fiber Characteristics in the Breast and Thigh Muscles
The effect of Se source and EM level on muscle fiber characteristics in the breast and thigh muscles is shown in Figure 1 and Table 6. In the breast muscle, there were no interactions between Se source and EM level regarding the muscle fiber characteristics (p > 0.05). Compared with the broiler chickens fed ISe source, broiler chickens fed OSe source decreased the perimeter, cross-sectional area, and diameter, and improved the density (p < 0.05). Broiler chickens supplied with HEMB level decreased the cross-sectional area and diameter, and enhanced the density, compared with the broiler chickens supplied with ZEMB level (p < 0.05). In detail, the S-Se group had a greater cross-sectional area and diameter compared with the other groups (p < 0.05). The Y-Se + EM group had a greater density compared with the S-Se and S-Se + EM groups, and the Y-Se group had a greater density compared with the S-Se group (p < 0.05).
Figure 1. Hematoxylin and eosin staining of the right breast and thigh muscles (400 ×). S-Se, sodium selenite; S-Se + EM, sodium selenite + 0.5% EM; Y-Se, selenium yeast; Y-Se + EM, selenium yeast + 0.5% EM.
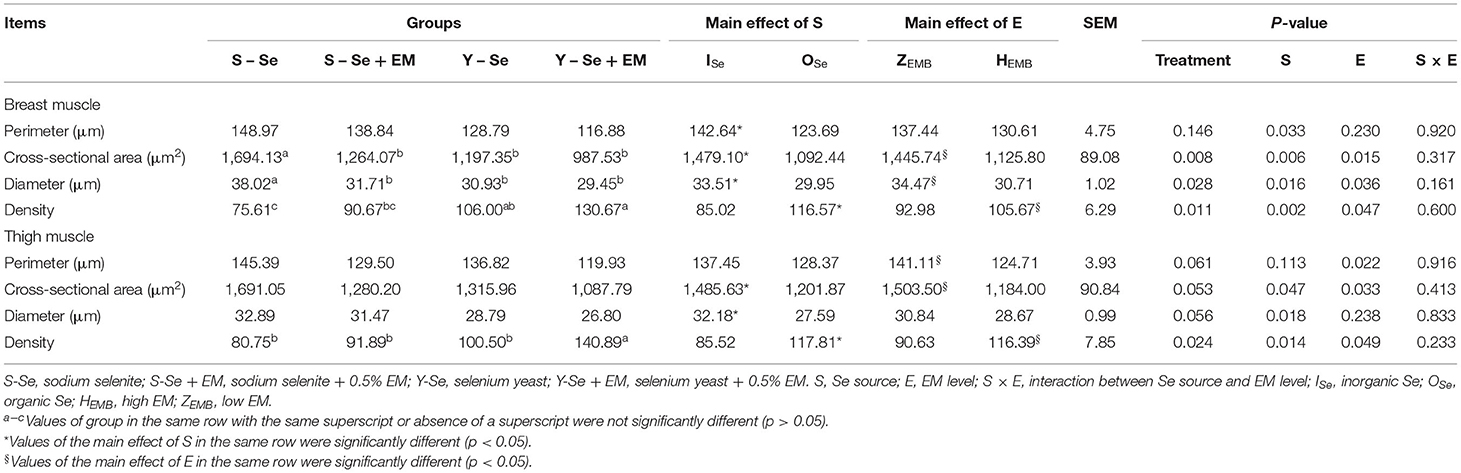
Table 6. Effects of different Se sources and different bacteria supplementation levels on the muscle fiber characteristic of three-yellow chickens.
In the thigh muscle, there were no interactions between Se source and EM level regarding the muscle fiber characteristics (p > 0.05). Compared with the broiler chickens fed ISe source, broiler chickens fed OSe source decreased the cross-sectional area and diameter, and increased the density (p < 0.05). Broiler chickens supplied with HEMB level decreased the perimeter and cross-sectional area, and increased the density, compared with the broiler chickens supplied with ZEMB level (p < 0.05). In detail, the Y-Se + EM group had a greater density compared with the other groups (p < 0.05).
The Correlations Between the Meat Quality and Muscle Fiber Characteristics
The correlations between the meat quality and muscle fiber characteristics in the breast and thigh muscles are shown in Figure 2. In the breast muscle, showed a negative correlation with perimeter, but shear force showed a positive correlation with perimeter (p < 0.05). The and were negatively related to cross-sectional area, and and shear force were positively related to cross-sectional area (p < 0.05). showed a negative correlation with diameter (p < 0.05). was negatively related to density (p < 0.05). In the thigh muscle, and showed negative correlations with perimeter, but shear force showed a positive correlation with perimeter (p < 0.05). and were negatively related to cross-sectional area; however, and shear force were positively related to cross-sectional area (p < 0.05). and showed negative correlations with diameter, but , , drip loss, and shear force showed positive correlations with diameter (p < 0.05). , , and drip loss were negatively related to density, but and were positively related to density (p < 0.05).
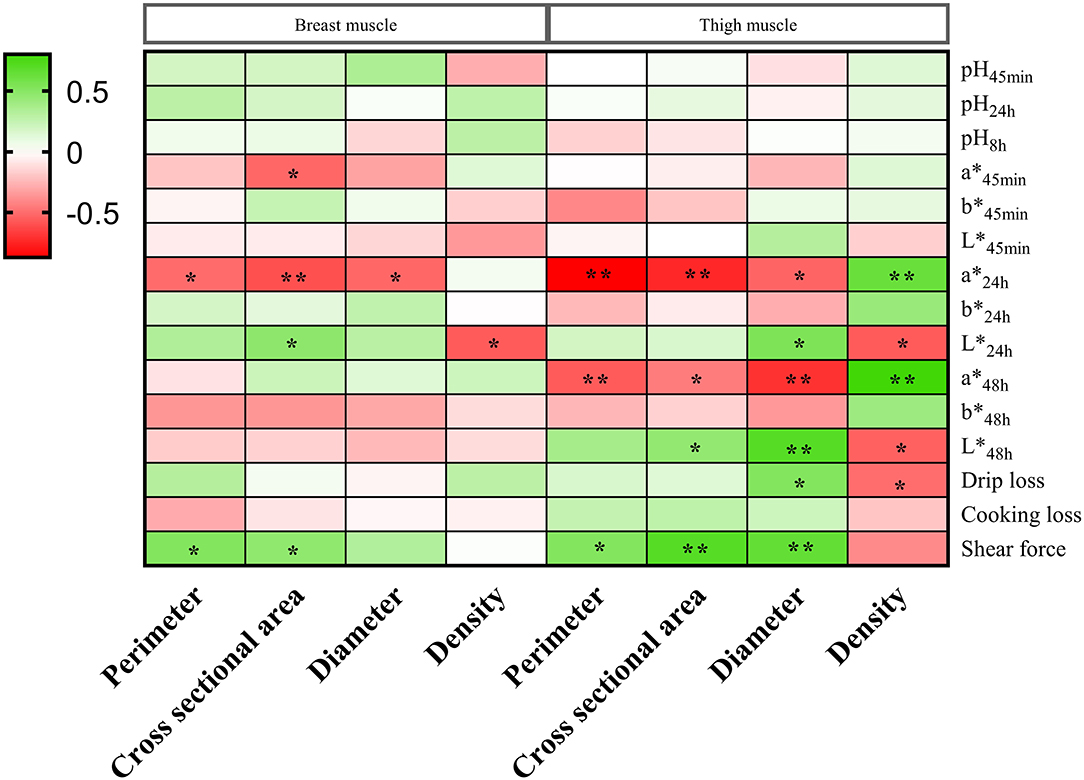
Figure 2. The correlations between the meat quality and muscle fiber characteristics in the breast and thigh muscles of three-yellow chickens. * means significant difference (p < 0.05); ** means highly significant difference (p < 0.01).
Expression Levels of Muscle Fiber-Relative Genes in the Breast and Thigh Muscles
The effect of Se source and EM level on the expression levels of muscle fiber-relative genes in the breast and thigh muscles is shown in Table 7. In the breast muscle, this study showed significant interactions between the Se source and EM level regarding the expression levels of CAST and SM (p < 0.05). Compared with the broiler chickens fed ISe source, broiler chickens fed OSe source decreased the expression level of CAST (p < 0.05) and increased the expression levels of MRF4 and CAPN2 (p < 0.05). Broiler chickens supplied with HEMB level increased the expression levels of MyoG and CAPN3 compared with the broiler chickens supplied with ZEMB level (p < 0.05). In detail, the S-Se + EM and Y-Se + EM groups increased the expression level of CAPN3 compared with the Y-Se group (p < 0.05). The S-Se group had a greater expression level of CAST compared with the other groups, and the S-Se + EM and Y-Se + EM groups had a greater expression level of CAST compared with the Y-Se group (p < 0.05).
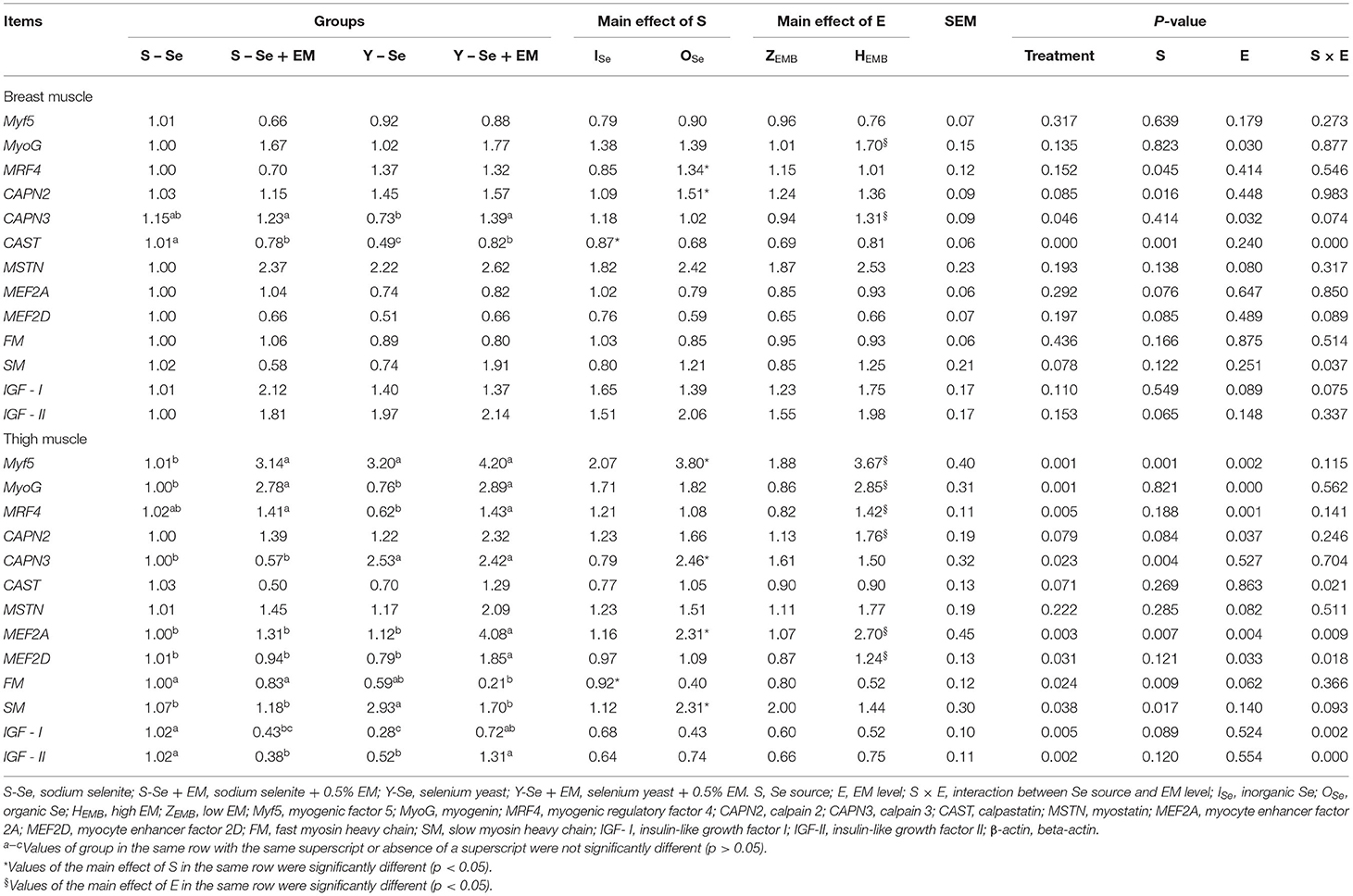
Table 7. Effects of different Se sources and different bacteria levels on the expression levels of muscle fiber-relative genes in the breast and thigh muscles.
In the thigh muscle, this study showed significant interactions between the Se source and EM level regarding the expression levels of CAST, MEF2A, MEF2D, IGF-I, and IGF-II (p < 0.05). Compared with the broiler chickens fed ISe source, broiler chickens fed OSe source decreased the expression level of FM and increased the expression levels of Myf5, CAPN3, MEF2A, and SM (p < 0.05). Broiler chickens supplied with HEMB level increased the expression levels of Myf5, MyoG, MRF4, CAPN2, MEF2A, and MEF2D compared with the broiler chickens supplied with ZEMB level (p < 0.05). In detail, the S-Se group had the lowest expression level of Myf5 compared with the other groups (p < 0.05). The S-Se + EM and Y-Se + EM groups increased the expression level of MyoG compared with the S-Se and Y-Se groups (p < 0.05). The S-Se + EM and Y-Se + EM groups increased the expression level of MRF4 compared with the Y-Se group (p < 0.05). The Y-Se and Y-Se + EM groups increased the expression level of CAPN3 compared with the S-Se and S-Se + EM groups (p < 0.05). The Y-Se + EM group had the highest expression levels of MEF2A and MEF2D compared with the other groups (p < 0.05). The S-Se and S-Se + EM groups increased the expression level of FM compared with the Y-Se + EM group (p < 0.05). The Y-Se group had the highest expression level of SM compared with the other groups (p < 0.05). The S-Se group increased the expression level of IGF-I compared with the S-Se + EM and Y-Se groups, and the Y-Se + EM group increased the expression level of IGF-I compared with the Y-Se group (p < 0.05). The S-Se and Y-Se + EM groups increased the expression level of IGF-II compared with the S-Se + EM and Y-Se groups (p < 0.05).
Discussion
Recently, the application of Y-Se and EM has received amounts of attention. The effect of dietary Y-Se supplementation on growth performance of chickens is controversial. Some studies show that dietary Y-Se supplementation increases the BW and weight gain, and decreases the FCR (5, 7). However, some studies show that dietary Y-Se supplementation has no influence on the BW, ADG, ADFI, and FCR of chicken during the overall period (28–30). Our results indicated that, compared with dietary ISe source, dietary OSe source only decreased the FCR during 1–35 days of three-yellow chickens. In this study, EM mainly included Lactobacillus and yeast. A previous study showed that dietary Lactobacillus increased the BW, ADG, and decreased the FCR (31). However, a previous study also showed that dietary Lactobacillus had no influence on the BW, ADG, ADFI, and increased the FCR (32). When supplied with 0.5% dried yeast, it decreases the ADFI of broiler chickens, but has no influence on the ADG and FCR (2). Our results indicated that compared with supplied with ZEMB level, those supplied with HEMB level had no influence on growth performance during the overall period. These discrepancies maybe related to the differences about the adding levels of bacteria and Se, bacteria species, and living environment.
Meat quality is an important aspect of the poultry industry and deeply influences the economic benefits. It can be reflected by the pH value, meat color, drip loss, cooking loss, and shear force. Meat colors include a*, b*, and L*, which is a major aspect to evaluate the products (33). Water-holding capacity, a key characteristic of meat quality, is reflected by drip loss and cooking loss (1). Shear force can reflect the tenderness of the meat (13), which is considered the most important index for eating quality (34). Previous studies had shown that dietary Y-Se supplementation decreased the drip loss and cooking loss of pigs and Ross 308 broilers (18, 22). Our results indicated that compared with dietary ISe source, the broiler chickens fed OSe source increased the meat quality by decreasing L*, drip loss, and shear force, and by increasing the a* and b* in the muscles of three-yellow chickens, which were in agreement with the previous studies. Dietary Lactobacillus supplement increases the b* in the thigh muscle of AA broilers (10), and dietary yeast supplement decreases the shear force in drumstick of Ross broilers (13). Our results indicated that compared with supplied with ZEMB level, broiler chickens supplied with HEMB level improved the meat quality by increasing the pH, a*, and b* in the muscles of three-yellow chickens. The mechanism of meat quality change may be related to the change in antioxidant levels. As an antioxidant mineral, Se is an essential co-factor in the antioxidant enzyme system (35). When supplied with Y-Se, Lactobacillus and yeast can increase the antioxidant capacity (2, 13, 18–20).
Our results showed that dietary OSe source and HEMB level decreased perimeter, cross-sectional area, and diameter, and increased density of muscle fiber on the breast and thigh muscles of three-yellow chickens. In the body of vertebrates, the most abundant tissue is skeletal muscle, and around 75–90% of skeletal muscle is composed of muscle fiber (36). Therefore, muscle fiber is a main factor to influence the meat quality. Muscle tenderness is negatively related to muscle fiber diameter and positively related to muscle fiber density (37). The glycogen concentration is low and the fat concentration is high in the slow muscle fiber (24), and it is positively related to pH and negatively related to drip loss and shear force (25, 38). However, the contents of myohemoglobin and chondriosome are low in the fast muscle fiber, which makes it easy to produce lactic acid (26), and it is positively related to drip loss, shear force, and L*, and negatively related to pH (24, 25). The present findings suggest that the muscle fiber characteristics were closely related to the meat quality, which were in agreement with the previous studies. Muscle fiber perimeter, cross-sectional area, and diameter were negatively related to a* and were positively related to the shear force. Muscle fiber density was positively related to a* and was negatively related to L* and drip loss. Our study illuminated that OSe source and HEMB level could improve the meat quality by regulating the muscle fiber characteristics.
Myf5, MyoG, and MRF4 are the members of myogenic regulatory factor family (39). Myf5, as a myogenic determination factor, takes part in the specification and proliferation of myoblasts (40). MyoG and MRF4 lead myoblast to form multinucleated myofiber (41, 42). Our results showed that in the breast and/or thigh muscles of three-yellow chickens, OSe source and/or HEMB level increased the expression levels of Myf5, MyoG, and MRF4 genes. These results explained how Y-Se and EM increased the density of muscle fiber. Calpain belongs to the papain superfamily, which includes CAPN1, CAPN2, and CAPN3, and is a Ca2+-regulated proteolytic enzyme (43). It degrades the myofibrillar protein (44), which affects the diameter of muscle fiber. Postmortem proteolysis of myofibrillar protein deeply influences the tenderness of the meat (45). CAST specifically inhibits the activity of calpain (46). Our results showed that OSe source and/or HEMB level increased the expression levels of CAPN2 and CAPN3 genes in the breast and/or thigh muscles of three-yellow chickens, and OSe source decreased the expression level of CAST gene in the breast muscle. This was a reason why Y-Se and EM decreased the perimeter, cross-sectional area, and diameter of muscle fiber, and it also explains how Y-Se decreased the shear force of muscles. MEF2A and MEF2D, as members of the MEF2 family, are DNA-binding transcription factors and play key roles in muscle development and differentiation (47). They are related to the formation of slow muscle fiber (48, 49). FM and SM are the fast myosin heavy chain and slow myosin heavy chain, respectively. Our results showed that in the thigh muscle OSe source and HEMB level increased the expression levels of MEF2A and MEF2D genes, and OSe source increased the expression level of SM gene and decreased the expression level of FM gene. These results indicated that Y-Se and EM increased the number of slow muscle fibers, and Y-Se decreased the number of fast muscle fibers. This was a reason why Y-Se and EM decreased the perimeter, cross-sectional area, and diameter of muscle fiber, and it also explains how Y-Se and EM increased the meat quality.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author.
Ethics Statement
The animal study was reviewed and approved by Committee of Laboratory Animal Management and Animal Welfare of Hunan Agricultural University.
Author Contributions
JX: conceptualization and writing–original draft preparation. CF: methodology and resources. RM: validation and supervision. RZ: software and data curation. YX: visualization. YQ: formal analysis. JT: investigation. RF: writing–review and editing, project administration, and funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the Double first-class construction project of Hunan Agricultural University and the National Key Research and Development Program of China, grant number 2018YFD0501403.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Oliveira TFB, Rivera DFR, Mesquita FR, Braga H, Ramos EM, Bertechini AG. Effect of different sources and levels of selenium on performance, meat quality, and tissue characteristics of broilers. J Appl Poultry Res. (2014) 23:15–22. doi: 10.3382/japr.2013-00761
2. Hussein E, Selim S. Efficacy of yeast and multi-strain probiotic alone or in combination on growth performance, carcass traits, blood biochemical constituents, and meat quality of broiler chickens. Livest Sci. (2018) 216:153–9. doi: 10.1016/j.livsci.2018.08.008
3. Li Y, Hou S, Chen J, Peng W, Wen W, Chen F, et al. Oral administration of Lactobacillus delbrueckii during the suckling period improves intestinal integrity after weaning in piglets. J Funct Foods. (2019) 63:103591. doi: 10.1016/j.jff.2019.103591
4. Lu J, Qu L, Shen MM, Wang XG, Guo J, Hu YP, et al. Effects of high-dose selenium-enriched yeast on laying performance, egg quality, clinical blood parameters, organ development, and selenium deposition in laying hens. Poultry Sci. (2019) 98:2522–30. doi: 10.3382/ps/pey597
5. Yang Y, Meng F, Wang P, Jiang Y, Yin Q, Chang J, et al. Effect of organic and inorganic selenium supplementation on growth performance, meat quality and antioxidant property of broilers. Afr J Biotechnol. (2014) 11:3031–6.
6. Yoon I, Werner TM, Butler JM. Effect of source and concentration of selenium on growth performance and selenium retention in broiler chickens. Poultry Sci. (2007) 86:727–30. doi: 10.1093/ps/86.4.727
7. Marković R, Cirić J, Drljačić A, Šefer D, Jovanović I, Jovanović D, et al. The effects of dietary Selenium-yeast level on glutathione peroxidase activity, tissue Selenium content, growth performance, and carcass and meat quality of broilers. Poultry Sci. (2018) 97:2861–70. doi: 10.3382/ps/pey117
8. Wang Z, Tan Y, Cui X, Chang S, Xiao X, Yan T, et al. Effect of different levels of selenium yeast on the antioxidant status, nutrient digestibility, selenium balances and nitrogen metabolism of Tibetan sheep in the Qinghai-Tibetan Plateau. Small Ruminant Res. (2019) 180:63–9. doi: 10.1016/j.smallrumres.2019.10.001
9. Zhang L, Jin P, Qin S, Liu J, Yang Z, Zhao H, et al. Effects of dietary supplementation with S. Platensis and probiotics on the growth performance, immune response and the fecal Lactobacillus spp And E Coli contents of weaned piglets. Livest Sci. (2019) 225:32–8. doi: 10.1016/j.livsci.2019.04.013
10. Zhu NH, Zhang RJ, Wu H, Zhang B. Effects of Lactobacillus cultures on growth performance, xanthophyll deposition, and color of the meat and skin of broilers. J Appl Poultry Res. (2009) 18:570–8. doi: 10.3382/japr.2009-00012
11. Vasaï F, Ricaud KB, Cauquil L, Daniel P, Peillod C, Gontier K, et al. Lactobacillus sakei modulates mule duck microbiota in ileum and ceca during overfeeding. Poultry Sci. (2014) 93:916–25. doi: 10.3382/ps.2013-03497
12. Tian Z, Cui Y, Lu H, Ma X. Effects of long-term feeding diets supplemented with Lactobacillus reuteri 1 on growth performance, digestive and absorptive function of the small intestine in pigs. J Funct Foods. (2020) 71:104010. doi: 10.1016/j.jff.2020.104010
13. Zhang AW, Lee BD, Lee SK, Lee KW, An GH, Song KB, et al. Effects of yeast (Saccharomyces cerevisiae) cell components on growth performance, meat quality, and ileal mucosa development of broiler chicks. Poultry Sci. (2005) 84:1015–21. doi: 10.1093/ps/84.7.1015
14. Cortinas L, Barroeta A, Villaverde C, Galobart J, Guardiola F, Baucells MD. Influence of the dietary polyunsaturation level on chicken meat quality: lipid oxidation. Poultry Sci. (2005) 84:48–55. doi: 10.1093/ps/84.1.48
15. Faustman C, Sun Q, Mancini R, Suman SP. Myoglobin and lipid oxidation interactions: Mechanistic bases and control. Meat Sci. (2010) 86:86–94. doi: 10.1016/j.meatsci.2010.04.025
16. Traore S, Aubry L, Gatellier P, Przybylski W, Jaworska D, Kajak-Siemaszko K, et al. Higher drip loss is associated with protein oxidation. Meat Sci. (2012) 90:917–24. doi: 10.1016/j.meatsci.2011.11.033
17. Zhang ZY, Jia GQ, Zuo JJ, Zhang Y, Lei J, Ren L, et al. Effects of constant and cyclic heat stress on muscle metabolism and meat quality of broiler breast fillet and thigh meat. Poultry Sci. (2012) 91:2931–7. doi: 10.3382/ps.2012-02255
18. Li J, Zhou J, Zhao H, Lei X, Xia X, Gao G, et al. Enhanced water-holding capacity of meat was associated with increased Sepw1 gene expression in pigs fed selenium-enriched yeast. Meat Sci. (2011) 87:95–100. doi: 10.1016/j.meatsci.2010.05.019
19. Sharma R, Kapila R, Kapasiya M, Saliganti V, Dass G, Kapila S. Dietary supplementation of milk fermented with probiotic Lactobacillus fermentum enhances systemic immune response and antioxidant capacity in aging mice. Nutr Res. (2014) 34:968–81. doi: 10.1016/j.nutres.2014.09.006
20. Baltić MŽ, Dokmanović Starčević M, Bašić M, Zenunović A, Ivanović J, Marković R, et al. Effects of selenium yeast level in diet on carcass and meat quality, tissue selenium distribution and glutathione peroxidase activity in ducks. Anim Feed Sci Tech. (2015) 210:225–33. doi: 10.1016/j.anifeedsci.2015.10.009
21. Sukumaran AT, Holtcamp AJ, Englishbey AK, Campbell YL, Kim T, Schilling MW, et al. Effect of deboning time on the growth of Salmonella, E. Coli, aerobic, and lactic acid bacteria during beef sausage processing and storage. Meat Sci. (2018) 139:49–55. doi: 10.1016/j.meatsci.2018.01.012
22. Bakhshalinejad R, Hassanabadi A, Swick RA. Dietary sources and levels of selenium supplements affect growth performance, carcass yield, meat quality and tissue selenium deposition in broilers. Animal Nutrition. (2019) 5:256–63. doi: 10.1016/j.aninu.2019.03.003
23. Meng Q, Velalar CN, Ruan R. Effects of epigallocatechin-3-gallate on mitochondrial integrity and antioxidative enzyme activity in the aging process of human fibroblast. Free Radical Bio Med. (2008) 44:1032–41. doi: 10.1016/j.freeradbiomed.2007.11.023
24. Choe JH, Choi YM, Lee SH, Shin HG Ryu YC, Hong KC, et al. The relation between glycogen, lactate content and muscle fiber type composition, and their influence on postmortem glycolytic rate and pork quality. Meat Sci. (2008) 80:355–62. doi: 10.1016/j.meatsci.2007.12.019
25. Hwang Y, Kim G, Jeong J, Hur S, Joo S. The relationship between muscle fiber characteristics and meat quality traits of highly marbled Hanwoo (Korean native cattle) steers. Meat Sci. (2010) 86:456–61. doi: 10.1016/j.meatsci.2010.05.034
26. Choi YM, Nam KW, Choe JH Ryu YC, Wick MP, Lee K, et al. Growth, carcass, fiber type, and meat quality characteristics in Large White pigs with different live weights. Livest Sci. (2013) 155:123–9. doi: 10.1016/j.livsci.2013.02.009
27. Meng Q, Sun S, Bai Y, Luo Z, Li Z, Shi B, et al. Effects of dietary resveratrol supplementation in sows on antioxidative status, myofiber characteristic and meat quality of offspring. Meat Sci. (2020) 167:108176. doi: 10.1016/j.meatsci.2020.108176
28. Wang Y, Xu B. Effect of different selenium source (sodium selenite and selenium yeast) on broiler chickens. Anim Feed Sci Tech. (2008) 144:306–14. doi: 10.1016/j.anifeedsci.2007.10.012
29. Han XJ, Qin P, Li WX, Ma QG Ji C, Zhang JY, et al. Effect of sodium selenite and selenium yeast on performance, egg quality, antioxidant capacity, and selenium deposition of laying hens. Poultry Sci. (2017) 96:3973–80. doi: 10.3382/ps/pex216
30. Khan MT, Mahmud A, Javed K, Zahoor I, Rehman MS. Organic and inorganic selenium in Aseel chicken diets: Effect on production performance. J Appl Poultry Res. (2018) 27:292–8. doi: 10.3382/japr/pfx070
31. Chen F, Gao SS, Zhu LQ, Qin SY, Qiu HL. Effects of dietary Lactobacillus rhamnosus CF supplementation on growth, meat quality, and microenvironment in specific pathogen-free chickens. Poultry Sci. (2018) 97:118–23. doi: 10.3382/ps/pex261
32. Mahrose KM, Alagawany M, Abd ElHack ME, Mahgoub SA, Attia FAM. Influences of stocking density and dietary probiotic supplementation on growing Japanese quail performance. An Acad Bras Ciênc. (2019) 91:e20180616. doi: 10.1590/0001-3765201920180616
33. Castaneda MP, Hirschler EM, Sams AR. Skin pigmentation evaluation in broilers fed natural and synthetic pigments. Poultry Sci. (2005) 84:143–7. doi: 10.1093/ps/84.1.143
34. Moeller SJ, Miller RK, Edwards KK, Zerby HN, Logan KE, Aldredge TL, et al. Consumer perceptions of pork eating quality as affected by pork quality attributes and endpoint cooked temperature. Meat Sci. (2010) 84:14–22. doi: 10.1016/j.meatsci.2009.06.023
35. Hassan F, Mobarez S, Mohamed M, Attia Y, Mekawy A, Mahrose K. Zinc and/or selenium enriched Spirulina as antioxidants in growing rabbit diets to alleviate the deleterious impacts of heat stress during summer season. Animals. (2021) 11:756. doi: 10.3390/ani11030756
36. Biressi S, Bjornson CRR, Carlig PMM, Nishijo K, Keller C, Rando TA. Myf5 expression during fetal myogenesis defines the developmental progenitors of adult satellite cells. Dev Biol. (2013) 379:195–207. doi: 10.1016/j.ydbio.2013.04.021
37. Zampiga M, Tavaniello S, Soglia F, Petracci M, Mazzoni M, Maiorano G, et al. Comparison of 2 commercial turkey hybrids: Productivity, occurrence of breast myopathies, and meat quality properties. Poultry Sci. (2019) 98:2305–15. doi: 10.3382/ps/pey607
38. Jeong JY, Hur SJ, Yang HS, Moon SH, Hwang YH, Park GB, et al. Discoloration characteristics of 3 major muscles from cattle during cold storage. J Food Sci. (2009) 74:C1–5. doi: 10.1111/j.1750-3841.2008.00983.x
39. Weintraub H, Da Vis R, Tapscott S, Thayer M, Krause M, Benezra R, et al. The myoD gene family: Nodal point during specification of the muscle cell lineage. Science. (1991) 251:761–6. doi: 10.1126/science.1846704
40. Steinbacher P, Haslett JR, Obermayer A, Marschallinger J, Bauer H, Sänger AM, et al. MyoD and Myogenin expression during myogenic phases in brown trout: A precocious onset of mosaic hyperplasia is a prerequisite for fast somatic growth. Dev Dynam. (2007) 236:1106–14. doi: 10.1002/dvdy.21103
41. Wright WE, Sassoon DA, Lin VK. Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell. (1989) 56:607–17. doi: 10.1016/0092-8674(89)90583-7
42. Hinits Y, Osborn DPS, Carvajal JJ, Rigby PWJ, Hughes SM. Mrf4 (myf6) is dynamically expressed in differentiated zebrafish skeletal muscle. Gene Expr Patterns. (2007) 7:738–45. doi: 10.1016/j.modgep.2007.06.003
43. Ono Y, Saido TC, Sorimachi H. Calpain research for drug discovery: challenges and potential. Nat Rev Drug Discov. (2016) 15:854–76. doi: 10.1038/nrd.2016.212
44. Koohmaraie M. The role of Ca2+-dependent proteases (calpains) in post mortem proteolysis and meat tenderness. Biochimie. (1992) 74:239–45. doi: 10.1016/0300-9084(92)90122-U
45. Gandolfi G, Pomponio L, Ertbjerg P, Karlsson AH, Nanni Costa L, Lametsch R, et al. Investigation on CAST, CAPN1 and CAPN3 porcine gene polymorphisms and expression in relation to post-mortem calpain activity in muscle and meat quality. Meat Sci. (2011) 88:694–700. doi: 10.1016/j.meatsci.2011.02.031
46. Zhou H, Hickford JGH. Allelic polymorphism of the caprine calpastatin (CAST) gene identified by PCR–SSCP. Meat Sci. (2008) 79:403–5. doi: 10.1016/j.meatsci.2007.10.015
47. Bhagavatula MRK, Fan C, Shen G, Cassano J, Plow EF, Topol EJ, et al. Transcription factor MEF2A mutations in patients with coronary artery disease. Hum Mol Genet. (2004) 13:3181–8. doi: 10.1093/hmg/ddh329
48. Potthoff MJ, Wu H, Arnold MA, Shelton JM, Olson EN. Histone deacetylase degradation and MEF2 activation promote the formation of slow-twitch myofibers. J Clin Invest. (2007) 117:2459–67. doi: 10.1172/JCI31960
Keywords: selenium yeast, effective microorganism, growth performance, meat quality, muscle fiber characteristic
Citation: Xue J, Fang C, Mu R, Zhuo R, Xiao Y, Qing Y, Tang J and Fang R (2022) Potential Mechanism and Effects of Different Selenium Sources and Different Effective Microorganism Supplementation Levels on Growth Performance, Meat Quality, and Muscle Fiber Characteristics of Three-Yellow Chickens. Front. Nutr. 9:869540. doi: 10.3389/fnut.2022.869540
Received: 04 February 2022; Accepted: 16 March 2022;
Published: 15 April 2022.
Edited by:
Zhaojun Wei, Hefei University of Technology, ChinaReviewed by:
Khalid M. Mahrose, Zagazig University, EgyptGuangtian Cao, China Jiliang University, China
Copyright © 2022 Xue, Fang, Mu, Zhuo, Xiao, Qing, Tang and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rejun Fang, ZmFuZ3JqNjNAMTI2LmNvbQ==
 Junjing Xue1,2
Junjing Xue1,2 Rejun Fang
Rejun Fang