Abstract
Background and aims:
Low-carbohydrate diets (LCD) and low-fat diets (LFD) have shown beneficial effects on the management of obesity. Epidemiological studies were conducted to compare the effects of the two diets. However, the results were not always consistent. This study aimed to conduct a meta-analysis to compare the long-term effects of LCD and LFD on metabolic risk factors and weight loss in overweight and obese adults.
Methods:
We performed a systematic literature search up to 30 March, 2022 in PubMed, EMBASE, and Cochrane Library. The meta-analysis compared the effects of LCD (carbohydrate intake ≤ 40%) with LFD (fat intake < 30%) on metabolic risk factors and weight loss for ≥6 months. Subgroup analyses were performed based on participant characteristics, dietary energy intake, and the proportions of carbohydrates.
Results:
33 studies involving a total of 3,939 participants were included. Compared with participants on LFD, participants on LCD had a greater reduction in triglycerides (–0.14 mmol/L; 95% CI, –0.18 to –0.10 mmol/L), diastolic blood pressure (–0.87 mmHg; 95% CI, –1.41 to –0.32 mmHg), weight loss (–1.33 kg; 95% CI, –1.79 to –0.87 kg), and a greater increase in high-density lipoprotein cholesterol (0.07 mmol/L; 95% CI, 0.06 to 0.09 mmol/L) in 6–23 months. However, the decrease of total cholesterol (0.14 mmol/L; 95% CI, 0.07 to 0.20 mmol/L) and low-density lipoprotein cholesterol (0.10 mmol/L; 95% CI, 0.06 to 0.14 mmol/L) was more conducive to LFD in 6–23 months. There was no difference in benefits between the two diets after 24 months. Subgroup analyses showed no significant difference in the reduction of total cholesterol, low-density lipoprotein cholesterol, and blood pressure between the two diets in participants with diabetes, hypertension, or hyperlipidemia.
Conclusion:
The results suggest that LCD and LFD may have specific effects on metabolic risk factors and weight loss in overweight and obese adults over 6 months. At 24 months, the effects on weight loss and improvement of metabolic risk factors were at least the same. These indicated that we might choose different diets to manage the overweight and obese subjects. However, the long-term clinical efficacy and effects of various sources of carbohydrates or fat in the two diets need to be studied in the future.
Introduction
Obesity is associated with an increased risk of hypertension, type 2 diabetes mellitus, dyslipidemia, metabolic syndrome, etc. The higher prevalence of obesity has become a significant global public health crisis issue. In 2016, more than 1.9 billion adults aged 18 years and older were overweight; of these, over 650 million were obese (1). The prevalence of obesity and its adverse consequences results in a heavy economic burden on the individual and on families and nations, including both developed and developing countries (2). Thus, improving the efficacy in preventing and controlling obesity worldwide is expected to have a great potential to reduce health costs and improve global health (3, 4).
Dietary factors play a vital role in the control of obesity. All methods of dietary intervention for obesity are based on reduced caloric diets (5). Among them, low-fat diets (LFD), especially reduced saturated fat intake, are the most widely used, which have been suggested in the dietary instruction for weight loss by the American Heart Association Nutrition Committee (6). However, in recent years, a number of studies have shown that other diets, such as low-carbohydrate diets (LCD), also have beneficial effects on significant weight loss, as well as increased energy expenditure, improved hyperinsulinemia and glycemic control, and decreased cardiometabolic risk (7–9). Thus, LCD has attracted more and more attention to the management of obesity.
Over the past 20 years, epidemiological studies have been conducted to compare the effects between LCD and LFD on metabolic risk factors and weight loss in overweight and obese adults (10–15); however, the results were not always consistent. These make people confused because both LFD and LCD have been suggested in the different dietary guidelines (6, 16, 17). Nadia et al. compared the effects of LCD and LFD on cardiovascular risk factors in healthy people (18). But this study only focused on persons without cardiometabolic diseases such as type 2 diabetes mellitus, myocardial infarction, stroke, etc., which are often accompanied by obesity and may benefit more from the two dietary patterns. Another earlier study by Hu et al. compared the effects of LCD versus LFD on metabolic risk factors in overweight and obese persons, indicating that LCD is at least as effective as LFD at decreasing weight and improving metabolic risk factors (19). After that, many new studies on this comparison are available (13–15, 20–24). The different effects on metabolic risk factors in overweight and obese persons between carbohydrate-restricted diets and fat-restricted diets still require further elucidation. Furthermore, the results of recent meta-analyses were usually conducted by medium- and short-term trials rather than separate analyses for longer-term studies, and they did not explore the effect on different populations such as patients with hyperlipidemia, diabetes, and hypertension. Therefore, we aimed to conduct the present study to compile the current evidence from all qualified randomized controlled trials to compare the long-term effects of the two diets on metabolic risk factors and weight loss in overweight and obese subjects.
Materials and methods
Literature search strategy
This meta-analysis was reported based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (PRISMA). To evaluate the effects of low carbohydrate diets (LCD) versus low fat diets (LFD) on metabolic risk factors and weight loss. Studies that were published on or before 30 March 2022 were selected. Keywords or medical subject-heading terms were used to screen as follows: LCD, carbohydrate-restricted diet, diet, or ketogenic combined with blood glucose or blood pressure or triglycerides or total cholesterol or high-density lipoprotein or low-density lipoprotein. First, titles and abstracts were filtered to exclude irrelevant studies. The full contents of the remaining literatures were next selected according to the pre-established criteria. Furthermore, references to the selected articles were also searched. The full search strategy is shown in the Supplementary materials.
Selection criteria
The inclusion criteria of this meta-analysis study were as follows: (1) the design of the study was a randomized controlled trial; (2) study participants were adults (at least 18 years old); (3) the subjects had a BMI ≥ 25 kg/m2, including overweight and obese, or BMI ≥ 30 kg/m2, including only obese (if the included study was from Asia, subjects with BMI ≥ 23 kg/m2, or BMI ≥ 30 kg/m2 were regarded as overweight or obese). (4) LCD and LFD were compared; (5) the intervention period was 6 months or longer; (6) both metabolic risk factors and body weight loss were included as the outcomes. Studies were excluded when other interventions such as drugs, surgery, and compulsory planned exercise were mentioned. The carbohydrate-restricted diets were defined as a prescribed intake of carbohydrates less than 40% of the total energy intake or a distinct reference to the Atkins diet, with an intake of only 20–40 g/d of carbohydrate in the first phase or carbohydrate intake of < 20% of total energy intake (25). The LFD was defined as a prescribed fat intake of less than 30% of total energy intake (25–27).
Data extraction and quality assessment
Two investigators (LL and JH) independently searched and screened all the potential related studies. The following information from each eligible study was extracted: (1) the basic characteristics of the included studies, including author’s name, year of publication, country of origin, duration of intervention, type of design, dietary composition, number, and rate of completion; (2) the characteristics of included persons, including sample size, gender, age, BMI, and health status such as basic diseases (diabetes, hypertension, cardiovascular diseases, and hyperlipemia); (3) the changes of metabolic risk factors compared with baseline, including triglycerides (TG), total cholesterol (TC), HDL-cholesterol (HDL-C), LDL-cholesterol (LDL-C), fasting blood glucose, systolic blood pressure (SBP), diastolic blood pressure (DBP), and body weight loss.
Two investigators independently assessed the risk of bias in the included studies using the Cochrane Collaboration’s tool (28), which contains the following criteria: (1) selection bias (random method); (2) performance bias; (3) detection bias (blind method for participants and results evaluation); (4) attrition bias (incomplete result data); (5) reporting bias (selective result reporting); and (6) other sources of bias. Studies were defined as having a high risk of bias: ≥1 item was a high risk of bias, and low risk of bias if all of the items were evaluated with a low risk of bias. The others were assessed as being at moderate risk of bias. Additionally, the quality of evidence for outcomes was evaluated using the Grading of Recommendations Assessment, Development and Evaluation (GRADE), which characterizes the evidence on the study limitations, imprecision, inconsistency, indirectness, and publication bias (29, 30).
Statistical analysis
Weighted mean differences (WMD) from baselines were calculated for the effects of LCD versus LFD on metabolic risk factors and weight loss and then a meta-analysis was performed. The baseline and outcomes values are shown in Supplementary Table 1. The heterogeneity was assessed by measuring the inconsistency (I2 statistic) of treatment effects among the trials. If there was significant heterogeneity across studies (I2 > 50%), the random effect model was used. Otherwise, the fixed effect model was used. If data were missing or incomplete, complete cases were analyzed. It should be noted that due to the different blood lipid and blood glucose units reported in the included studies, the data in mg/dL of blood lipid were converted to mmol/L by multiplying 0.0259 of TC, 0.0258 of HDL-C, 0.0259 of LDL-C, and 0.0113 of TG. The blood glucose value in mg/dL was converted to mmol/L by dividing 18. In addition, to determine whether different intervention time has different effects, the duration of intervention was stratified into 6–11 months, 12–23 months, and 24 months.
The publication bias was judged by the funnel plot and Egger’s regression test (31). Meta-regression and subgroup analyses were used to analyze the possible sources of heterogeneity, including hypertensive status, hyperlipidemia status, diabetic status, energy intake, and proportions of carbohydrates. Furthermore, sensitivity analyses were performed to explore the different potential influences by excluding each study in turn. All statistical analyses were performed using Stata statistical software (Version 14.0; Stata Corp.).
Results
Results of literature search
The flow diagram of the study screening procedure is shown in Figure 1. A total of 2,878 potentially relevant studies were retrieved. Based on the aforementioned criteria, 1921 articles were discharged after reviewing the titles and abstracts. After evaluating the full texts, 30 of the 63 studies did not meet the inclusion criteria and were removed. Finally, 33 studies met all inclusion criteria and were selected for further analysis in this study.
FIGURE 1
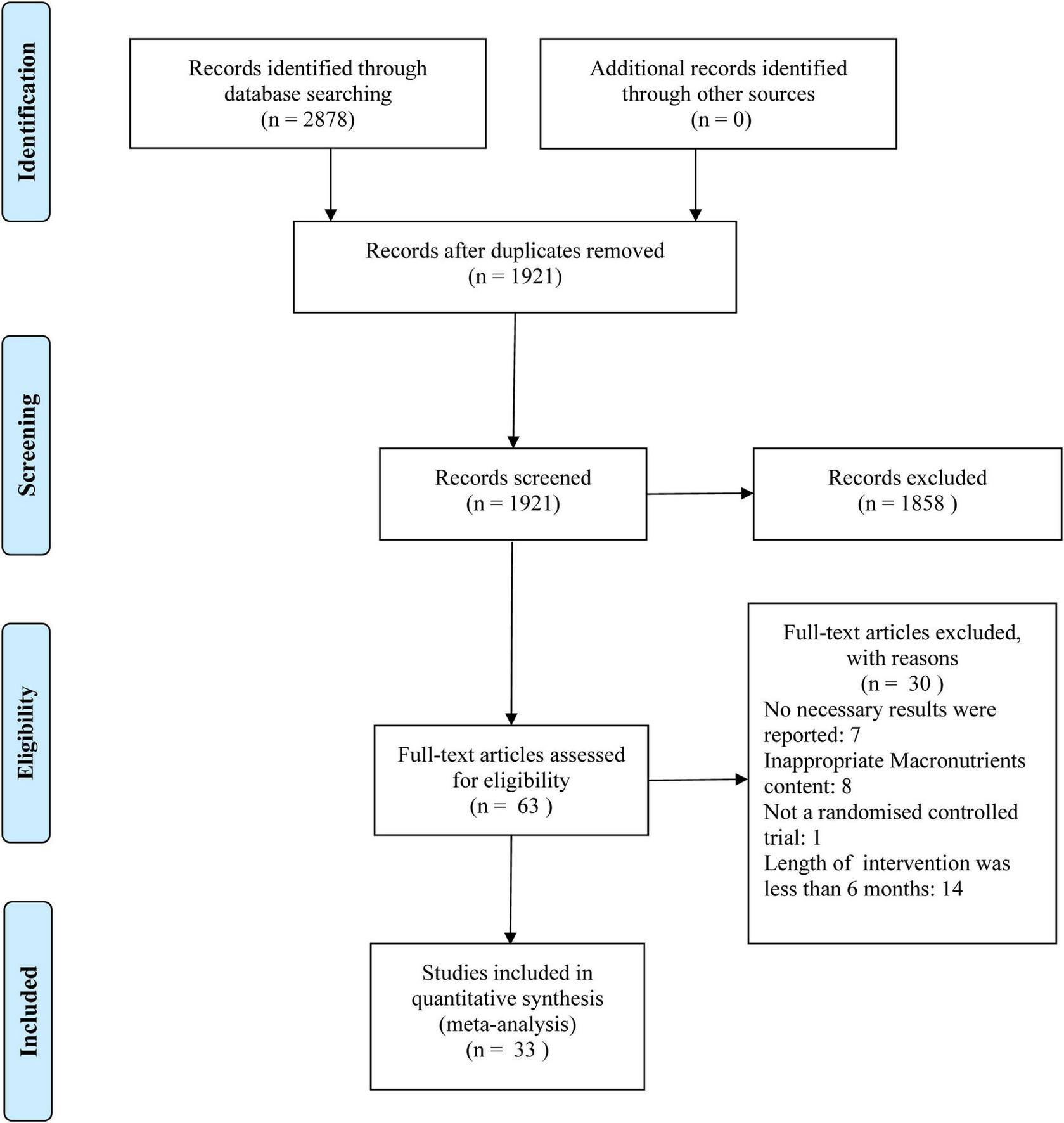
Flow diagram of study selection and meta-analysis.
Characteristics of the included studies
The basic characteristics of 33 randomized controlled trials included in this meta-analysis are shown in Table 1 (10–15, 20–24, 32–53). Nineteen of the included studies were conducted in North America, three in Asia, four in Oceania, and seven in Europe. A total of 3,939 participants, 1,978 on LCD and 1,961 on LFD, were included. The mean age of the participants at baseline ranged from 18 to 72 years. The follow-up period ranged from 6 to 24 months, eleven in 6 to 11 months, eighteen in 12 to 23 months, and four in 24 months. All studies were conducted among overweight or obese persons with or without basic diseases such as diabetes, hypertension, and hyperlipemia.
TABLE 1
| First author, (Reference no.) | Country | Duration of follow-up (months) | Design | No. of participants | Age (mean) | Male (%) | Population | Dietary composition | Completion % | Outcome measures | ||
| LCD | LFD | LCD | LFD | |||||||||
| Bazzano, (15) | American | 12 | Parallel | 148 | 46.8 | 12 | Overweight/obese; BMI: 30–45; no T2DM or CVD | Carbohydrate intake <40 g/d, no set energy goal | <30% of daily energy intake from total fat (with <7% from saturated fatty acids), 55% carbohydrate | 60 79% | 59 82% | WL, SBP, DBP, TC, TG, LDL-C, HDL, BG |
| Brinkworth, (35) | Australia | 12 | Parallel | 69 | 51.5 | 36 | Overweight/obese; BMI = 21; metabolic syndrome risk factor | 61% fat (20% saturated fatty acids), 4% carbohydrate, 35% protein | 30% fat (8% saturated fatty acids), 46% carbohydrate, 24% protein | 33 60% | 36 69% | TC, TG, HDL-C, LDL-C, WL, SBP, DBP, BG |
| Brehm, (10) | American | 6 | Parallel | 42 | 43.7 | 0 | Obese; BMI: 30–35; no DM or CVD | Carbohydrate intake =20 g/d | 30% fat, 55% carbohydrate, 15% protein | 22 85% | 20 74% | TC, TG, HDL-C, LDL-C, WL, SBP, DBP, BG |
| De Luis, (13) | Spain | 9 | Parallel | 331 | 50.1 | 25.7 | Obese; BMI: 35.4 ± 5.3; no DM or CVD | 40% fat, 33% carbohydrate (86.1 g/day), 20% protein | 27% fat, 53% carbohydrate, 20% protein | 168 100% | 163 100% | TG, TC, HDL-C, LDL-C, WL, SBP, DBP, BG |
| Davis, (37) | American | 12 | Parallel | 105 | 55 | 21.9 | Overweight/obese; BMI =25;T2DM | 49% fat, 24% carbohydrate, 27% protein | 25% fat, 53% carbohydrate, 22% protein | 55 100% | 50 100% | TC, TG, HDL-C, LDL-C, WL, SBP, DBP |
| Dansinger, (36) | American | 12 | Parallel | 41 | 48 | 52.5 | Overweight/obese; BMI: 27–42; at least one metabolic cardiac risk factors | Carbohydrate intake =20 g/d, and increasing up to 50 g/d | Vegetarian diet, 10% of calories from fat | 21 53% | 20 50% | TC, TG, HDL-C, LDL-C, WL, SBP, DBP, BG |
| Elhayany, (39) | Israel | 12 | Parallel | 124 | 56.4 | 53.2 | Obese; BMI: 27–34; T2DM | 45% fat (50% monounsaturated fatty acid), 35% carbohydrate, 20% protein | 30% fat, 50–55% carbohydrate, 15–20% protein | 61 72% | 63 71% | TC, TG, HDL-C, LDL-C, WL, BG |
| Ebbeling, (38) | American | 18 | Parallel | 73 | 27.5 | 20.5 | Obese; BMI: = 30; no DM | 35% fat, 40% carbohydrate, 25% protein | 20% fat,55% carbohydrate, 25% protein | 28 78% | 23 62% | TG, HDL-C, LDL-C, WL, SBP, DBP, BG |
| Foster, (50) | American | 24 | Parallel | 307 | 45.5 | 32.2 | Obese; BMI: 30–40; no DM | Carbohydrate intake 5 g/d per week | 30% fat, 55% carbohydrate, 15% protein | 153 58% | 154 68% | TG, HDL-C, LDL-C, WL, SBP, DBP |
| Frisch, (41) | Germany | 12 | Parallel | 200 | 47 | 31 | Overweight/obese; BMI: = 27; no DM | >35% fat, <40% carbohydrate, 25% protein | <30% fat, >55% carbohydrate, <15% protein | 100 85% | 100 80% | TC, TG, HDL-C, LDL-C, WL, SBP, DBP, BG |
| Foster, (40) | American | 6 | Parallel | 63 | 31.7 | 47 | Overweight/obese; BMI: =21; no DM | Carbohydrate intake <20 g/d | 25% fat, 60% carbohydrate, 15% protein | 33 61% | 30 57% | TC, TG, HDL-C, LDL-C, WL, SBP, DBP |
| Gardner, (23) | American | 12 | Parallel | 609 | 40 | 43 | Overweight/obese; BMI: 28–40; metabolic syndrome | The mean 12-month macronutrient distributions: 45% fat, 30% carbohydrate, 23% protein | The mean 12-month macronutrient distributions: 29% fat, 48% carbohydrate, 21% protein | 218 74% | 214 74% | TG, HDL-C, LDL-C, WL, SBP, DBP, BG |
| Guldbrand, (51) | Sweden | 24 | Parallel | 61 | 62 | 44.3 | Overweight/obese; BMI: 32; T2DM | 50% fat, 20% carbohydrate, 30% protein | 30% fat (<10 % saturated fatty acids), 55–60% carbohydrate, 10-15% protein | 30 100% | 31 100% | SBP, DBP, BG, LDL-C, HDL-C, TG |
| Gardner, (42) | American | 12 | Parallel | 153 | 42 | 0 | Overweight/obese; BMI: 32; no DM | Carbohydrate intake =50 g/d | <30% of total energy intake from fat | 68 88% | 59 78% | TG, HDL-C, LDL-C, WL, SBP, DBP, BG |
| Haufe, (20) | American | 6 | Parallel | 170 | 44 | 20.6 | Overweight/obese; BMI: 26.5–45.4, no T2DM | Carbohydrate intake =90 g/d | Fat intake of =20% of total energy | 55 66% | 56 64% | WL, TG, LDL-C, HDL-C, BG, TC |
| Hockaday, (43) | UK | 12 | Parallel | 93 | 51 | 55.9 | Weight: 76.4–82.2 kg | 40% fat, 20% carbohydrate, 20% protein | 26% fat, 54% carbohydrate, 20% protein | 54 NR | 39 NR | TG, BG |
| Jonasson, (14) | Sweden | 6 | Parallel | 61 | 62 | 44.2 | Overweight/obese; BMI: 33; DM | 43% fat, 20% carbohydrate, 31% protein | 30% fat, 55–60% carbohydrate | 30 100% | 31 100% | WL, TC, LDL-C, HDL-C, TG |
| Jenkins, (21) | Canada | 6 | Parallel | 39 | 55 | 38.5 | Overweight/obese; BMI: =27; hyperlipidemia | 43% fat, 26% carbohydrate,31% protein | 25% fat, 58% carbohydrate, 16% protein | 13 68% | 10 50% | LDL-C, HDL-C, TC, TG, BG, WL, SBP, DBP |
| Klemsdal, (44) | Norway | 12 | Parallel | 202 | 50 | 42 | Overweight/obese; BMI: 28–40; no DM or CVD | 35%-40% fat (20% saturated fatty acids), 30%-35% carbohydrate, 25-30% protein | 30% fat, 55%–60% carbohydrate, 15% protein | 78 78% | 86 84% | TC, TG, HDL-C, LDL-C, WL, SBP, DBP, BG |
| Lim, (45) | American | 15 | Parallel | 60 | 48.5 | 22 | Overweight/obese; BMI: 28–40; at least one CVD risk factor | 60% fat (20% saturated fatty acids), 4% carbohydrate, 35% protein | 10% fat (3% saturated fatty acids), 70% carbohydrate, 20% protein | 17 63% | 18 64% | TC, TG, HDL-C, LDL-C, WL, SBP, DBP, BG |
| Morgan, (11) | UK | 6 | Parallel | 115 | 40.7 | 27 | Overweight/obese; BMI: 27–40; no DM | Dr. Atkins’ New Diet Revolution | Rosemary Conley’s diet and fitness plan | 33 58% | 41 71% | TG, HDL-C, LDL-C, WL, BG |
| McAuley, (46) | New Zealand | 12 | Parallel | 62 | NR | 0 | Overweight; insulin resistance | Carbohydrate intake =20 g/d in the first 2 weeks, and increasing up to 50 g/day by 8 weeks | <30% fat (<10% saturated fatty acids), >55% carbohydrate, 15% protein | 24 75% | 24 75% | TC, TG, HDL-C, LDL-C, WL, SBP, DBP, BG |
| Saslow, (24) | American | 12 | Parallel | 34 | 59.7 | 26.5 | Overweight/obese; BMI: =25; T2DM | Carbohydrate intake <20–50 g/d | 45%–50% carbohydrate | 14 88% | 15 83% | TG, HDL-C, LDL-C, WL, SBP, DBP |
| Shai, (53) | Israel | 24 | Parallel | 214 | 52 | 94 | Obese; BMI: 31; T2DM | Carbohydrate intake <20 g and later 120 g | 30% fat (10 % saturated fatty acids), 55–60% carbohydrate, 10–15% protein | 85 78% | 84 90% | TG, HDL-C, LDL-C, WL, SBP, DBP, BG |
| Sacks, (52) | American | 24 | Factorial | 403 | 51 | 34.5 | Overweight/obese; BMI: 33; no DM or unstable CVD | 40% fat, 35% carbohydrate, 25% protein | 20% fat, 55% carbohydrate, 25% protein | 168 83% | 157 78% | TC, TG, HDL-C, LDL-C, WL, SBP, DBP, BG |
| Stern, (47) | American | 12 | Parallel | 132 | 53.5 | 82.6 | Obese; BMI =35; 83% DM or metabolic syndrome | Carbohydrate intake <30 g/d | To restrict caloric intake by 500 calories per day with <30% of calories from fat | 44 69% | 43 63% | TC, TG, HDL-C, LDL-C, WL, SBP, DBP, BG |
| Samaha, (32) | American | 6 | Parallel | 132 | 54 | 82.6 | Obese; BMI: =35; metabolic syndrome | Carbohydrate intake < 30 g/d | <30% of total energy intake from fat | 43 67% | 36 53% | TC, TG, HDL-C, LDL-C, WL, BG |
| Thomson, (33) | American | 6 | Parallel | 43 | 56.2 | 0 | Overweight/obese; BMI: 25–35; no DM or CVD. | 35% Carbohydrate, 25–30% protein, 35-40% fat | 55%–60% Carbohydrate,25% fat, 15%–20% protein | 19 90% | 21 95% | TC, TG, HDL-C, LDL-C, WL, SBP, DBP, BG |
| Tay, (12) | Australia | 6 | Parallel | 88 | 50.6 | 35.2 | Overweight/obese; BMI: 33.7; metabolic syndrome | 61% fat (20% saturated fat), 4% carbohydrate, 35% protein | 30% fat (< 8 % saturated fat), 46% carbohydrate, 24% protein | 45 82% | 43 80% | TC, TG, HDL-C, LDL-C, WL, SBP, DBP, BG |
| Wycherley, (49) | Australia | 13 | Parallel | 49 | 50.0 | 34.7 | Overweight/obese; BMI: 26–43; at least one metabolic syndrome risk factor | 61% fat (20% saturated fat), 4% carbohydrate, 35% protein | 30% fat (<8% saturated fat), 46% carbohydrate, 24% protein | 26 46% | 23 38% | TC, TG, HDL-C, LDL-C, WL, SBP, DBP, BG |
| Wolever, (48) | Canada | 12 | Parallel | 110 | 59.6 | 43.3 | Overweight/obese; BMI: 24–40; T2DM | 40.1% fat, 39.3% carbohydrate, 20.6% protein | Low-glycemic-index (low-fat) diet: 26.5% fat, 51.9% carbohydrates, 21.6% protein, | 53 98% | 55 98% | TC, TG, HDL-C, LDL-C, WL, SBP, BG |
| Yamada, (22) | Japan | 6 | Parallel | 24 | 63.3 | 50 | Obese; BMI: 25.8; T2DM | Carbohydrate intake <70-130 g/d | <25% fat, 50–60% carbohydrate, <20% protein | 12 100% | 12 100% | TG, HDL-C, LDL-C, WL, SBP, DBP, BG |
| Yancy, (34) | American | 6 | Parallel | 120 | 44.9 | 23.5 | Obese; BMI:30–60; hyperlipidemia | Carbohydrate(<20 g/d) decreased to <5 g/d | <30% fat (<10% saturated fatty acids) | 45 76% | 34 57% | TC, TG, HDL-C, LDL-C, WL, SBP, DBP |
Characteristics of randomized controlled trials included in the meta-analysis1.
1LCD, low carbohydrate diets; LFD, Low fat diets; DM, diabetes; T2DM, type 2 diabetes mellitus; CVD, cardiovascular disease; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TC, total cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure; BG, blood glucose; WL, weight loss; NR, not report.
Although the carbohydrate-restricted diets were prescribed as intake of carbohydrates less than 40% of the total energy intake (E%), actual carbohydrate intakes ranged from 4 to 41.4 E% in 6–11 months, from 4 to 43.5 E% in 12–23 months, and from 30 to 42.5 E% in 24 months. A total of 14 studies were very LCDs (VLCD) (carbohydrate intake ≤50 g/d) and 19 studies were moderate LCDs (MLCD) (carbohydrate intake >50 g/d). Similarly, although the LFD is prescribed as a fat intake of less than 30% of the total energy intake, actual fat intakes ranged from 2.8 to 33 E% in 6–11 months, from 20 to 30.8 E% in 12–23 months, and from 28.4 to 31 E% in 24 months. The completion rates of dietary interventions varied widely, ranging from 38 to 100%. It should be noted that though the intervention of the exercise program was discharged in our present study, a few studies also provided daily exercise volume.
Quality assessment
Two authors independently assessed the risk of bias in the included studies using the Cochrane Collaboration’s tool. The results of the quality evaluation of the included 33 RCT studies are shown in Supplementary Table 2, which shows that the study qualities of the selected trials were diverse. According to the possibility of bias, the study was assessed as being low risk, moderate risk, or high risk. A total of six studies were evaluated as high risk of bias, three studies were assessed as low risk of bias, and the other studies had a moderate risk of bias. The quality of evidence for outcomes was evaluated as low or very low, and details for the evaluation of the GRADE framework are presented in Supplementary Table 3.
Effects of low-carbohydrate diets versus low-fat diets on blood lipids
The individuals assigned to LCD showed a significantly greater decrease in TG (WMD, –0.14 mmol/L; 95% CI, –0.18 to –0.10 mmol/L; Figure 2) and a significantly greater increase in HDL-C (WMD, 0.07 mmol/L; 95% CI, 0.06–0.09 mmol/L) than the individuals assigned to LFD (Figure 3). However, the pooled effect comparing LCD versus LFD in TC (WMD, 0.14 mmol/L; 95% CI, 0.07 to 0.20 mmol/L; Figure 4) and LDL-C (WMD, 0.10 mmol/L; 95% CI, 0.06 to 0.14 mmol/L) indicates a significantly greater reduction in LFD (Figure 5). It is noteworthy that LCD significantly decreased TG (6–11 months: WMD, –0.17 mmol/L; 95% CI, –0.25 to –0.09 mmol/L; 12–23 months: –0.16 mmol/L; 95% CI, –0.21 to –0.10 mmol/L) and significantly increased HDL-C (6–11 months: WMD, 0.08 mmol/L; 95% CI, 0.05 to 0.11 mmol/L; 12–23 months: 0.08 mmol/L; 95% CI, 0.02 to 0.09 mmol/L) when compared to LFD in 6–23 months, but the reduction effect of LDL-L (6–11 months: WMD, 0.12 mmol/L; 95% CI, 0.04 to 0.21 mmol/L; 12–23 months: 0.11 mmol/L; 95% CI, 0.06 to 0.17 mmol/L) and TC (6–11 months: WMD, 0.12 mmol/L; 95% CI, 0.01 to 0.24 mmol/L; 12–23 months: 0.15 mmol/L; 95% CI, 0.06 to 0.23 mmol/L) was in favor of LFD in 6–23 months. However, these outcomes were not significant differences between the two diets at 24 months. The heterogeneity test showed that four outcomes were of low heterogeneity (TG: I2= 21,3%, P = 0.14; LDL-C: I2= 35%, P = 0.03; HDL-C: I2= 35%, P = 0.03; TC: I2= 29%, P = 0.09).
FIGURE 2
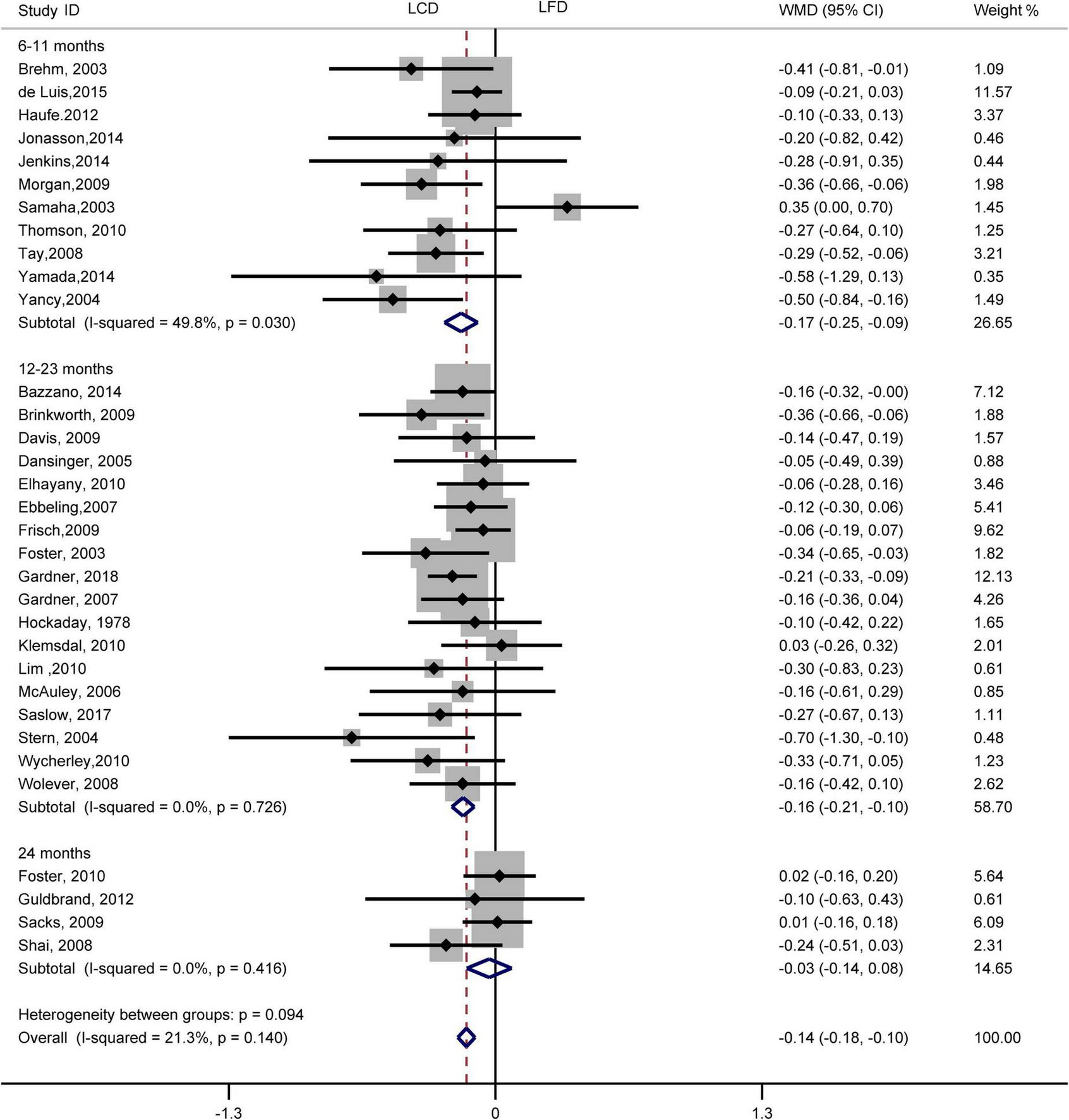
Forest plots showing weight mean differences (WMD) and 95% CI for triglyceride of the low-carbohydrate diets (LCD) in comparison with low-fat diets (LFD).
FIGURE 3
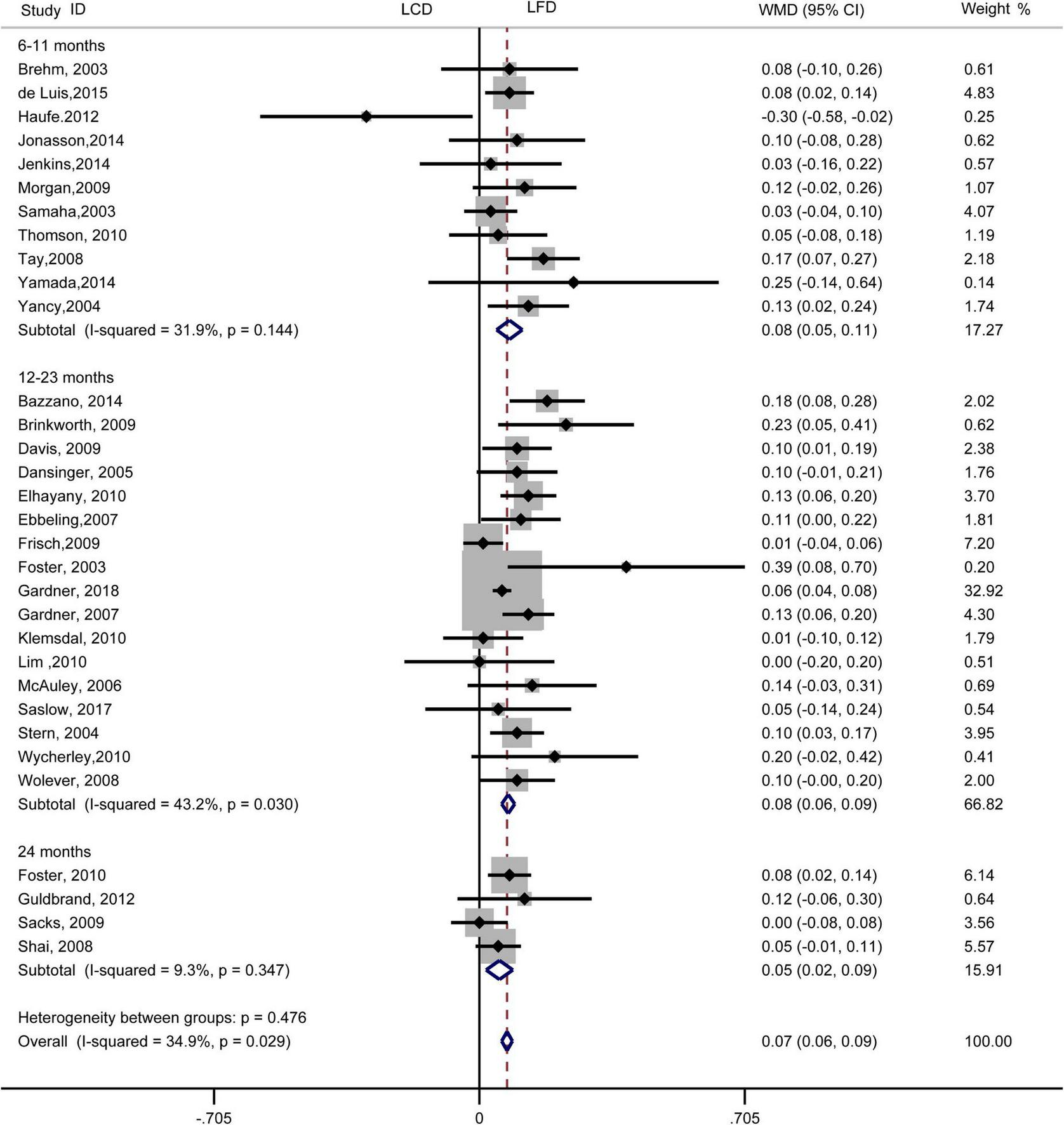
Forest plots showing weight mean differences (WMD) and 95% CI for HDL-cholesterol of the low-carbohydrate diets (LCD) in comparison with low-fat diets (LFD).
FIGURE 4
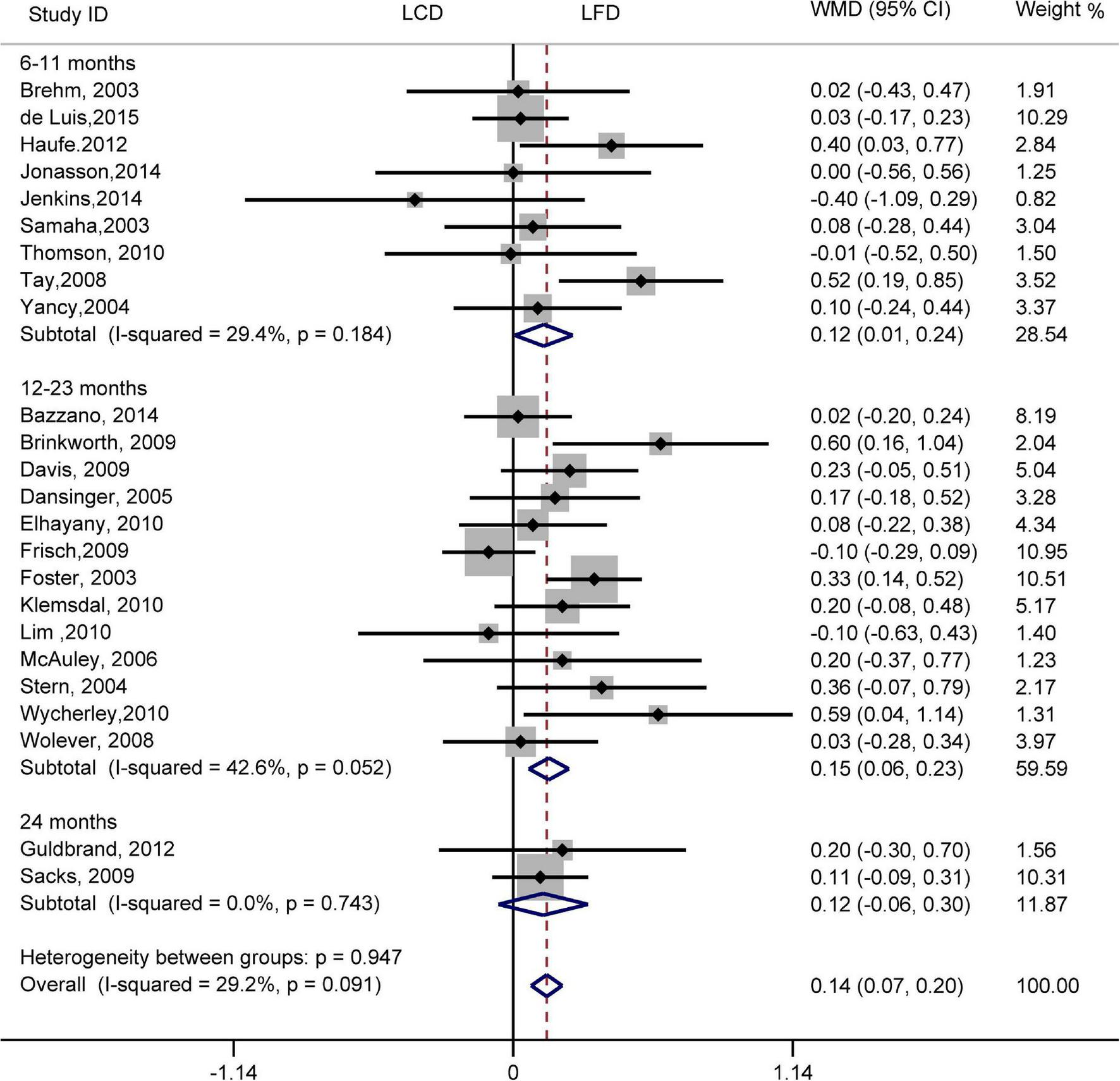
Forest plots showing weight mean differences (WMD) and 95% CI for total cholesterol of the low-carbohydrate diets (LCD) in comparison with low-fat diets (LFD).
FIGURE 5
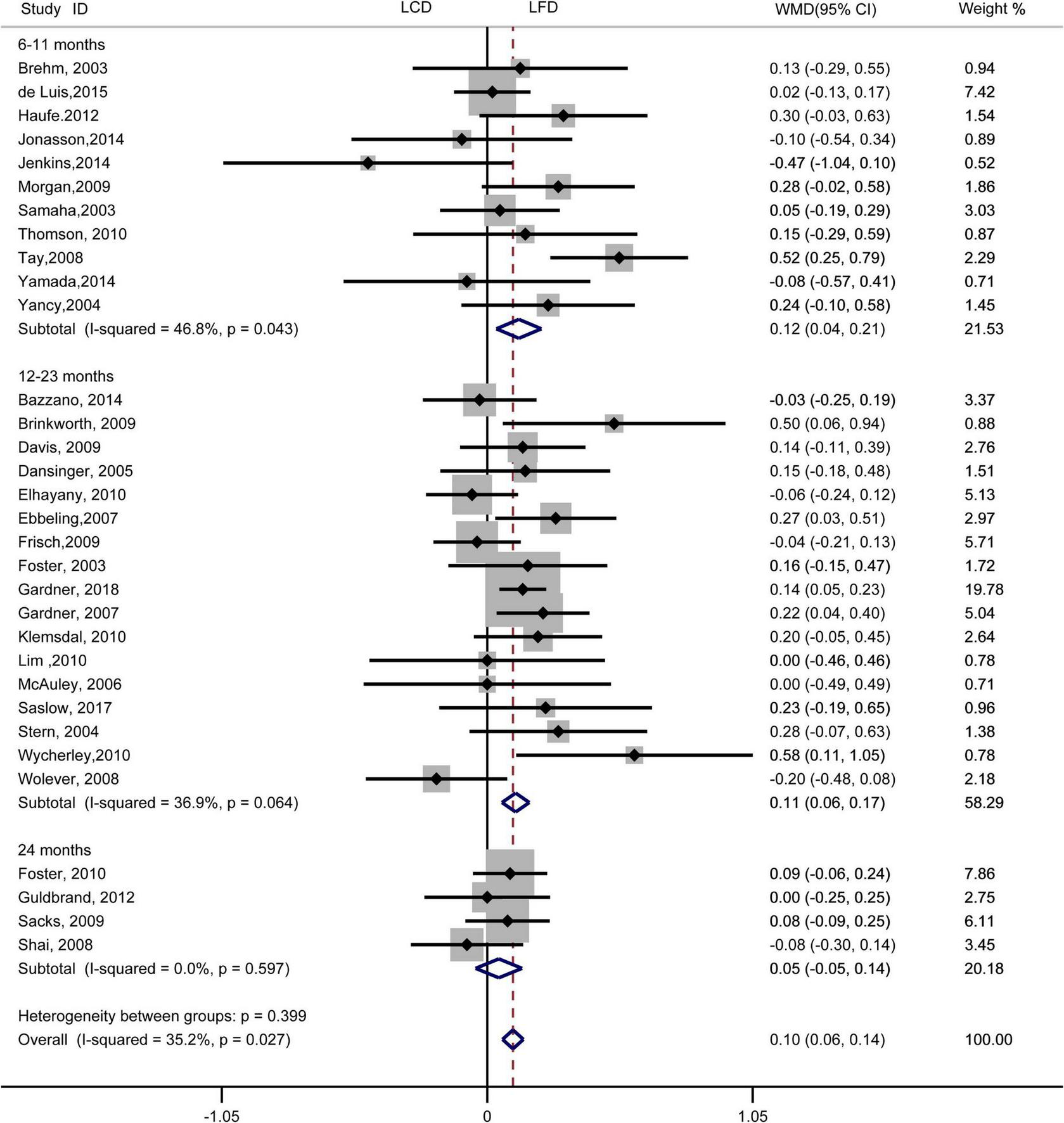
Forest plots showing weight mean differences (WMD) and 95% CI for LDL-cholesterol of the low-carbohydrate diets (LCD) in comparison with low-fat diets (LFD).
Effects of low-carbohydrate diets versus low-fat diets on blood pressure
There was no difference in the effect of two diets on SBP (WMD, –0.73 mmHg; 95% CI, –1.55 to 0.09 mmHg; I2 = 21%, P = 0.16; Supplementary Figure 1). However, compared with LFD, the decreased DBP was significantly greater in LCD (WMD, –0.87 mmHg; 95% CI, –1.41 to –0.32 mmHg; I2 = 0%, P = 0.62; Figure 6). The difference in the decrease of –1.03 mmHg (95% CI, –1.73 to –0.33 mmHg) also exists in 12–23 months. However, the trend was not significant at 6–11 months or 24 months. The heterogeneity test showed that the results of both SBP and DBP were low heterogeneity.
FIGURE 6
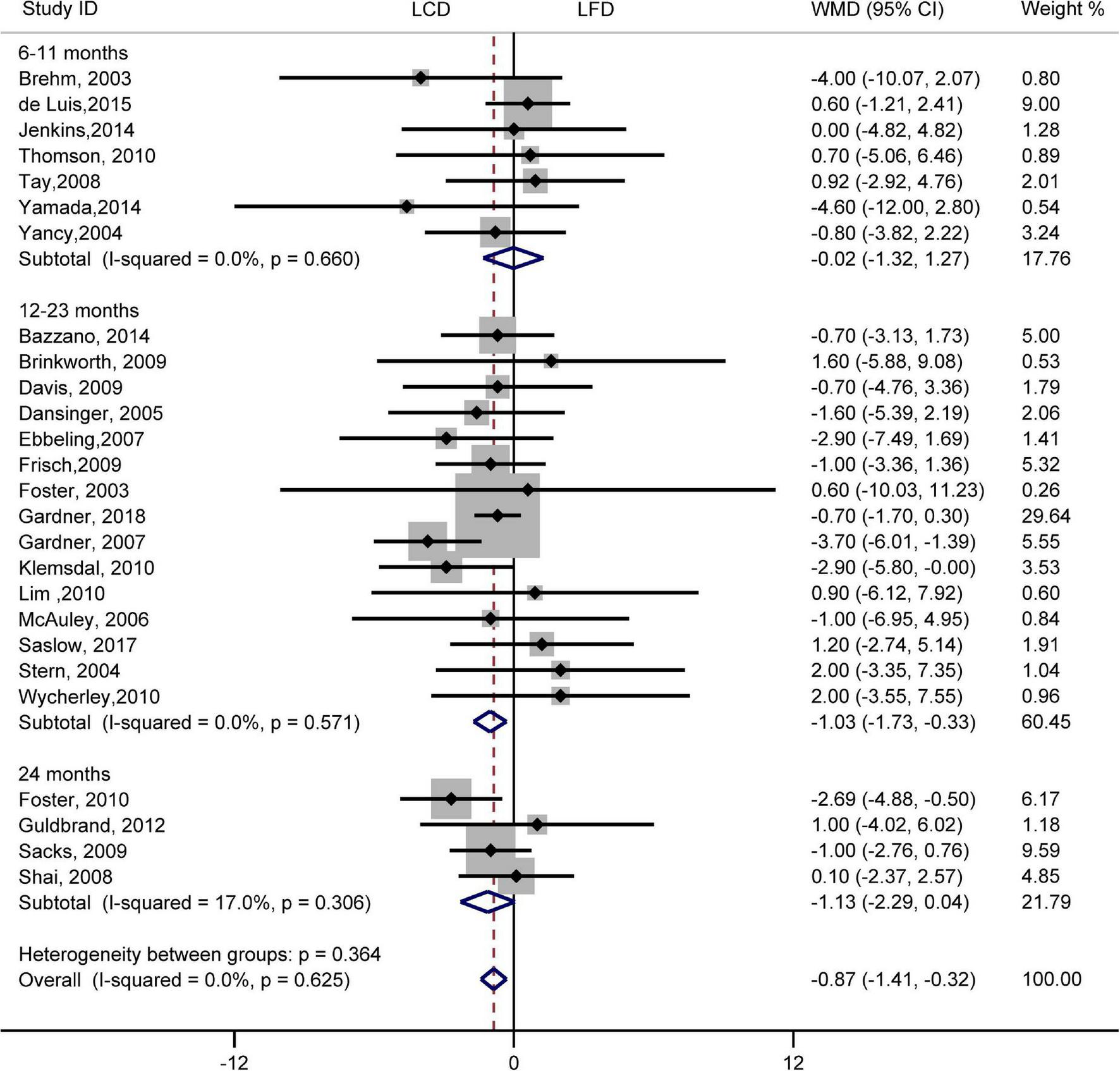
Forest plots showing weight mean differences (WMD) and 95% CI for diastolic blood pressure of the low-carbohydrate diets (LCD) in comparison with low-fat diets (LFD).
Effects of low-carbohydrate diets versus low-fat diets on blood glucose
There was no difference in blood glucose between LCD and LFD (WMD, –0.01 mmol/L; 95% CI, –0.05 to 0.03 mmol/L; Supplementary Figure 2). The heterogeneity test showed that blood glucose was low heterogeneity (I2= 40%, P = 0.02).
Effects of low-carbohydrate diets versus low-fat diets on weight loss
Results indicated that the individuals assigned to LCD showed a greater reduction in weight loss than the individuals assigned to LFD (WMD, –1.33 kg; 95% CI, –1.79 to –0.87 kg; Figure 7). Compared with LFD, the levels of weight loss in LCD decreased by –2.10 kg (95% CI, –3.07 to –1.14 kg) in 6–11 months and –1.21 kg (95% CI, –1.79 to –0.63 kg) in 12–23 months. However, there was no difference in weight loss between the two diets at 24 months. The heterogeneity test showed that weight loss was low heterogeneity (I2= 20%, P = 0.16).
FIGURE 7
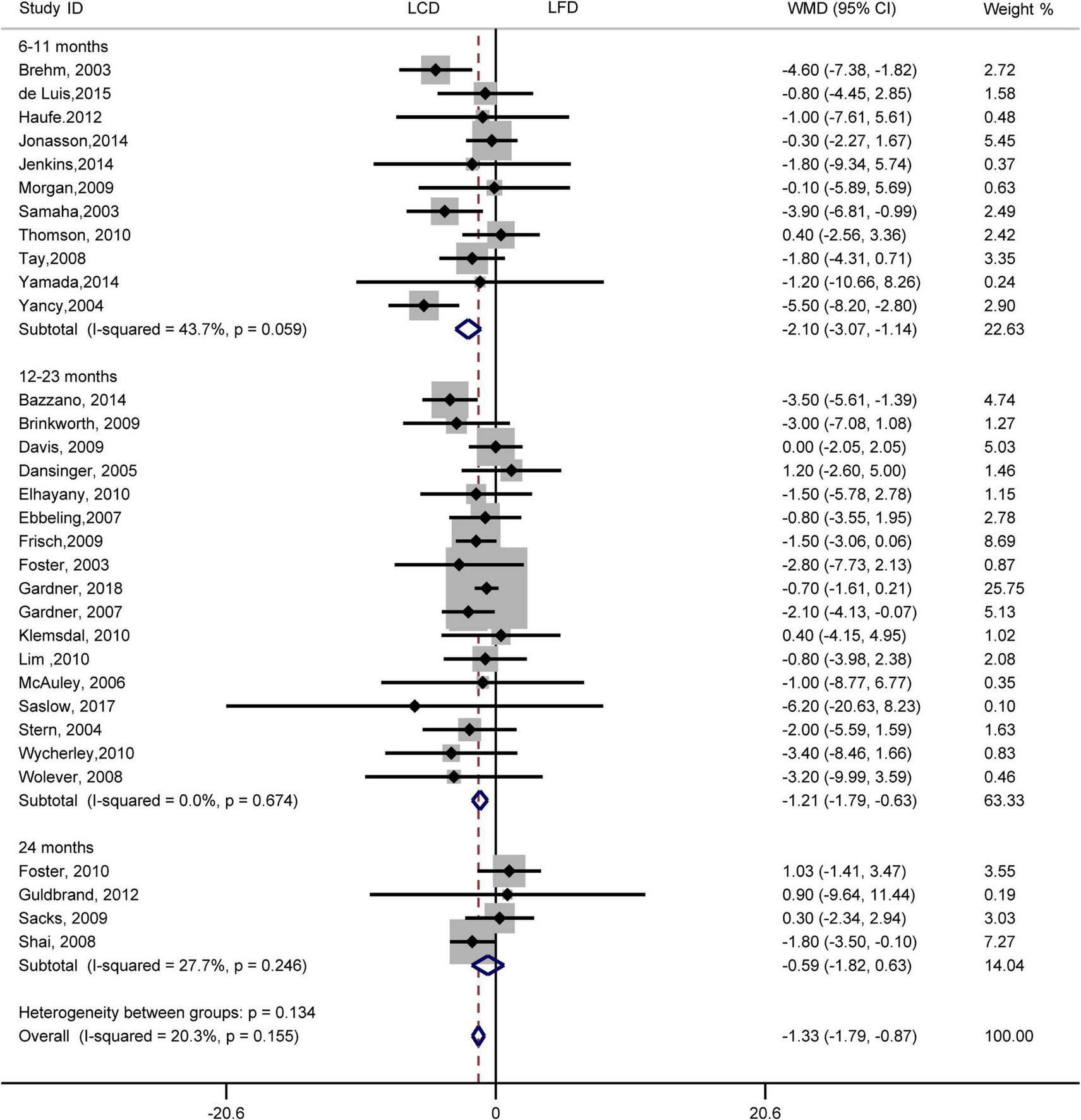
Forest plots showing weight mean differences (WMD) and 95% CI for weight loss of the low-carbohydrate diets (LCD) in comparison with low-fat diets (LFD).
Subgroup and sensitivity analyses
Subgroup analyses for metabolic risk factors were carried out according to study variables and participant characteristics, including hypertensive status, hyperlipidemia status, diabetic status, energy intake, and proportions of carbohydrates (Supplementary Tables 4–11). Overall, we found that the TC and LDL-C decreased more significantly in LFD. Still, SBP and DBP decreased more obviously in LCD in non-diabetic, non-hypertension, and non-hyperlipidemia participants. However, there were no significant differences between the two diets in participants with diabetes, hypertension, or hyperlipidemia. In identical caloric content and different caloric content subgroups, LCD had a stronger effect on SBP, DBP, and weight loss than LFD in identical caloric content subgroups, but no effect in different caloric content subgroups. When subgroup analyses were conducted based on proportions of carbohydrates, the reduction effect of DBP was favored in LCD in the moderate low carbohydrate subgroup, but not in the subgroup of very low carbohydrate. In the sensitivity analysis, the effects in the results remained unchanged after excluding one study at a time (Supplementary Figures 3–10). Meta-regression was used to explore heterogeneity, and we found that proportions of carbohydrates may be the source of heterogeneity (Supplementary Table 12).
Publication bias
Results of funnel plots showed that TC, TG, LDL-C, HDL-C, SBP, DBP, blood glucose, and weight loss were symmetric (Supplementary Figures 11–18). Results of the Egger’s tests also showed no significant publication bias (TC: P = 0.41; TG: P = 0.09; LDL-C: P = 0.57; HDL-C: P = 0.08; SBP: P = 0.61; DBP: P = 0.69; blood glucose: P = 0.66; weight loss: P = 0.38).
Discussion
Dietary intake, as a rule, follows a pattern of consumption and is one of the main factors that contribute directly to the impaired metabolic risk factors and obesogenic phenotype (54, 55). Numerous studies were performed to compare the effects on metabolic risk factors and weight loss in overweight and obese adults between LCD and LFD (10–15, 20–24, 32–53). However, the studies showed inconsistent results. In our present study, we performed a meta-analysis to the overall existing evidence from randomized controlled trials to compare LCD with LFD. Our results showed different effects on metabolic risk factors and weight loss in adults with overweight or obese between LCD and LFD. Compared with LFD, subjects on LCD had a greater reduction in TG, DBP, weight loss, and greater increases in HDL-C. However, participants on LFD had more decreases in LDL-C and TC. These indicated that we might choose different diets to manage the overweight and obese subjects according to their abnormal metabolic indicators and the need for weight loss.
Our results showed that LCD was more beneficial for improving TG and HDL-C, which was consistent with prior meta-analyses comparing the effect of the two diets in overweight and obese persons (56, 57). Even in healthy subjects in a meta-analysis including eleven randomized controlled trials with 1,369 participants, Nadia et al. also found that HDL-C and triglyceride levels had more favorable changes in LCD (18). The carbohydrate intake and macronutrient composition in LCD were related to the improvement of TG. The production of very low-density lipoprotein triglycerides in the liver is reduced in response to decreased carbohydrate substrate delivery (47). Moreover, the increase of HDL-C on the LCD may cause a greater decrease in TG via downregulation of hepatic scavenger receptor B1 levels because the receptor can bind HDL-C and promote the transportation of cholesterol to the liver (58). We also found that TC and LDL-C decreased significantly in LFD compared with LCD, which is different from previous reports (18, 19). The reasons may be that (1) we included more studies; (2) we included the overweight and obese subjects with or without basic diseases, but the other studies only included healthy persons. In this study, LCD did not cause a significant increase in TC and LDL-C, which may be related to our inclusion of more studies on MLCD. Our results were consistent with Hu et al. (19). However, Mansoor et al. and Lu et al. reported that LCD has adverse effects, which leads to an increase in TC and LDL-C (18, 59). Mansoor et al. only analyzed the effects of VLCD. Noteworthy, the VLCD caused higher levels of TC and LDL-C in many cases (60). The heterogeneity among the included studies was unavoidable. The source of heterogeneity was explored by meta-regression and subgroup analyses. The results found that the proportions of carbohydrates in LCD seem to be part of the source of heterogeneity. The research indicates that very low carbohydrate was related to better blood glucose control and greater weight loss (24), which means that the different content of carbohydrates in interventions may lead to heterogeneity. Moreover, subgroup analyses showed that LCD and LFD had different effects on blood pressure and blood lipids. Among participants with hypertension, hyperlipidemia, and diabetes, the reduction effect of blood pressure, TC, and LDL-C had no significant difference between the two diets. This would imply that the beneficial effects of LCD and LFD on blood pressure, TC, and LDL-C may be at least the same in participants with hypertension, hyperlipidemia, and diabetes. Subgroup analyses on energy intake indicated LCD had a significantly greater reduction of SBP, DBP, and weight loss in identical caloric content subgroups. Still, there was no significant difference in different caloric content subgroups. This difference between the subgroups may be due to the unequal dietary energy between the two diets in the studies because the energy intake of LCD was higher than that of LFD in three studies, and the energy intake of LFD was higher in three separate studies. It should be noted that LCD often increases the proportion of fat, which may cause a higher risk of some cardiovascular diseases or cancers (61). These causal relationships are long-term effects of high-fat diets, but studies included in our present and previous studies often persist from 6 to 24 months. Furthermore, it suggests that moderate replacement of carbohydrates with dietary fats may be a potential method to improve metabolic risk factors and simultaneously prevent increased risk for other diseases. On the other hand, some studies reduced dietary fats and replaced them with carbohydrates such as fruits and grains. However, this replacement did not affect atherogenic dyslipidemia among individuals with metabolic syndrome (62, 63).
Reducing dietary carbohydrates may produce clinical improvements in the management of blood pressure. We found that compared with LFD, individuals assigned to LCD showed a significantly greater reduction in diastolic blood pressure, not in systolic blood pressure, which is similar to a previous study (57). Studies have also shown that LCD with high monounsaturated fatty acids is beneficial for regulating blood pressure in some diseases such as type 2 diabetes (64). However, there was no difference in the improvement of blood pressure between the two diets in two earlier meta-analyses, including overweight and obese subjects (19, 56). The reason for the difference in our results may, at least in part, be more studies were included in the current research.
Both LCD and LFD are beneficial for weight loss. Previous studies have shown that compared with participants on LFD, those on LCD experienced a greater weight loss reduction (56, 65). Moreover, Mansoor et al. found that even in healthy subjects, LCD is more effective for weight loss than LFD (19). Our results are consistent with the effects reported in the above studies, suggesting that the individuals assigned to LCD showed a significantly stronger reduction in weight loss than the individuals assigned to LFD. High fat in LCD can stimulate more secretion of peptide YY, a peptide mainly produced by endocrine L cells, which can reduce appetite and increase satiety (66). Most of the LCDs increase protein intake, thereby increasing subjects’ satiety and reducing eating, which may be related to greater weight loss (33). A further study observed no difference in weight loss between the two diets lasting 24 months. It is similar to the results in an earlier study by Nordmann et al., which found that this different effect on weight loss between the two diets was no longer obvious after 12 months (56). However, the results were inconsistent. Some studies reported that the two diets are at least as effective in weight loss (19).
Reduction of carbohydrate intake has attracted more and more attention in recent years for its potential in health promotion and treatment of diseases, including decreasing body mass, improving fat and carbohydrate metabolism, producing clinical improvements in the management of type 2 diabetes mellitus, and reducing the predicted risk of atherosclerotic cardiovascular disease events (65, 67, 68). However, there are still controversial effects of LCD or the comparison between LCD and LFD (63, 65, 69). Some reasons that may be involved are as follows: (1) the criteria for included subjects are different. The criteria may include only overweight or obese persons or both, while in some studies, the criteria were the different BMI values. In addition, the included participants may be overweight and obese with or without basic diseases or healthy. (2) LCD often increases the percentage of energy from fat. Different fatty acids may have diverse effects. For instance, Abbasnezhad et al. found that LCD with high monounsaturated fatty acids benefits the regulation of blood pressure in some diseases such as type 2 diabetes (64). However, saturated fatty acids have been reported to increase both totals- and LDL-C (70). A study conducted by Sackner-Bernstein et al. showed that LCD is more significant in weight loss and in predicting ASCVD risk in overweight/obese subjects with health or dyslipidemia, but the outcomes were not stratified by follow-up time or different populations (68). Although Chawla et al. performed a stratified analysis of follow-up time, most of the studies were short-term trials (71). They found that LCD is more significant in improving weight loss, HDL-C, and TG within 12 months, but there is a lack of evidence to support the long-term effect of the two diets. This meta-analysis included more studies (over 12 months) and populations, and performed subgroup analyses of different populations and intervention durations to explore the short- and long-term effects of the two diets on metabolic risk factors.
It should be noted that LCD may have some adverse effects. First, LCD often increases the proportion of fat, which may cause a higher risk of some cardiovascular diseases or cancers (61). These causal relationships are long-term effects of high-fat diets, but studies included in our present and previous studies often persist from 6 to 24 months. Second, some meta-analyses based on observational studies have shown that long-term reduction of carbohydrate is related to a significantly increased risk of all-cause mortality (72, 73). Further, the study indicated that the source of food, especially the sources of protein and fat, notably modifies the association between carbohydrate intake and mortality (64). Third, some observational studies reported that in short-term interventional studies in humans, LCD has effects on mood and cognition, such as impaired cognitive function, attenuated performance on a memory-based task, and decreased cognitive processing speed (74–76). However, other studies have shown opposite effects, including having better sleep status, less involvement with mental disorders, and exerting a beneficial effect on depression (77, 78). A systematic review showed that reduction of carbohydrate intake has no stronger effect on psychosocial outcomes than diets of different macronutrient compositions, both in the short- and long-term (79). Thus, further studies are needed to investigate the effects of LCD on psychosocial outcomes.
Several potential limitations should be considered in our study. First, the definitions of LCDs are different. LCD is defined as a total carbohydrate intake of 20–60 g per day or less, or ≤45% of energy from carbohydrates. However, the definition of LFD is consistent, characterized as total fat intake ≤30% of energy from fat. Second, the duration is different, from 6 months to 2 years, and there is no trial lasting for more than 2 years. LCD may produce small short-term improvements in blood glucose control and weight loss, which are not sustained in the long term (80). Thus, the long-term effects of LCD on cardiovascular risk factors and weight loss require further research in the future. Third, only some studies reported the changed types and sources of carbohydrates or fat. Simple or complex carbohydrates have different effects on metabolic risk factors and weight loss (81, 82). Various fatty acids, including saturated, monounsaturated, or polyunsaturated fatty acids, also have diverse effects (83, 84). Therefore, further studies are needed to focus on the various types and sources of carbohydrates or fat in LCD and LFD in the future. Fourth, although we found significant differences in blood lipids, weight loss, and blood pressure between the two diets, most of the outcomes have weak differences, such as DBP, and lack of significant clinically significant. Large-scale clinical studies are needed to confirm the clinical effects of these metabolic risk makers in the future. Finally, the quality of evidence for outcomes ranges from low to very low in this study, not only because of study limitations and indirectness but also because of inconsistency. The dietary trials in participants who are not blinded may be one of the reasons for the low certainty evidence. The quality of evidence for study needs to be improved by well-designed randomized trials in the future.
Conclusion
In summary, our present meta-analysis found that individuals assigned to LCD showed a significantly greater reduction in TG, diastolic blood pressure, and weight loss, as well as a significant increase in HDL-C. However, LFD was associated with a significantly greater decrease in TC and LDL-C. Moderate restriction of carbohydrate intake in LCD did not cause adverse effects on LDL-C and TC. We also found that LCD was as effective as LFD on weight loss, and metabolic risk factors improvement lasted up to 2 years. However, few large-scale and high-quality studies have analyzed the long-term effects of LCD and LFD on metabolic risk factors. Hence, the long-term clinical efficacy and effects of various sources of carbohydrates or fat in the two diets are still worth further clarification.
Statements
Data availability statement
The original contributions presented in this study are included in the article/Supplementary materials, further inquiries can be directed to the corresponding author.
Author contributions
LL and JY designed the research. LL and JH were responsible for data acquisition, statistical analysis, and the interpretation of the results. LZ and YH were responsible for providing information and advice on the data synthesis and analysis. SH and JY contributed to the concept and design of the study, provided guidance during study selection, data analysis, draft development, and final submission. All authors read and approved the final manuscript.
Funding
This study was supported in part by the Key Program of The Third Affiliated Hospital of Chongqing Medical University (KY19024), Program of Chongqing Medical University for Youth Innovation in Future Medicine (W0085), and Li Jieshou Intestinal Barrier Research Foundation (Z-2017-24-2009).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.935234/full#supplementary-material
References
1.
World Health Organization. Obesity and Overweight. (2022). Available online at: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight(accessed March 30, 2022).
2.
TremmelMGerdthamUGNilssonPMSahaS. Economic burden of obesity: a systematic literature review.Int J Environ Res Public Health. (2017) 14:435. 10.3390/ijerph14040435
3.
HayesALungTWenLMBaurLRisselCHowardK. Economic evaluation of “healthy beginnings” an early childhood inter-vention to prevent obesity.Obesity (Silver Spring). (2014) 22:1709–15. 10.1002/oby.20747
4.
HollingworthWHawkinsJLawlorDABrownMMarshTKippingRR. Economic evaluation of lifestyle interventions to treat overweight or obesity in children.Int J Obes (Lond). (2012) 36:559–66. 10.1038/ijo.2011.272
5.
JensenMDRyanDHApovianCMArdJDComuzzieAGDonatoKAet al2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the american college of cardiology/american heart association task force on practice guidelines and the obesity society.Circulation. (2014) 129:S102–38. 10.1161/01.cir.0000437739.71477.ee
6.
American Heart Association Nutrition Committee, LichtensteinAHAppelLJBrandsMCarnethonMDanielsSet alDiet and lifestyle recommendations revision 2006: a scientific statement from the american heart association nutrition committee.Circulation. (2006) 114:82–96. 10.1161/CIRCULATIONAHA.106.176158
7.
O’NeillBJ. Effect of low-carbohydrate diets on cardiometabolic risk, insulin resistance, and metabolic syndrome.Curr Opin Endocrinol Diabetes Obes. (2020) 27:301–7. 10.1097/MED.0000000000000569
8.
HallKDChungST. Low-carbohydrate diets for the treatment of obesity and type 2 diabetes.Curr Opin Clin Nutr Metab Care. (2018) 21:308–12. 10.1097/MCO.0000000000000470
9.
KraussRMBlanchePJRawlingsRSFernstromHSWilliamsPT. Separate effects of reduced carbohydrate intake and weight loss on atherogenic dyslipidemia.Am J Clin Nutr. (2006) 83:1025–205. 10.1093/ajcn/83.5.1025
10.
BrehmBJSeeleyRJDanielsSRD’AlessioDA. A randomized trial comparing a very low carbohydrate diet and a calo-rie-restricted low fat diet on body weight and cardiovascular risk factors in healthy women.J Clin Endocrinol Metab. (2003) 88:1617–23. 10.1210/jc.2002-021480
11.
MorganLMGriffinBAMillwardDJDeLooyAFoxKRBaicSet alComparison of the effects of four commercially available weight-loss programmes on lipid-based cardiovascular risk factors.Public Health Nutr. (2009) 12:799–807. 10.1017/S1368980008003236
12.
TayJBrinkworthGDNoakesMKeoghJCliftonPM. Metabolic effects of weight loss on a very-low-carbohydrate diet com-pared with an isocaloric high-carbohydrate diet in abdominally obese subjects.J Am Coll Cardiol. (2008) 51:59–67. 10.1016/j.jacc.2007.08.050
13.
De LuisDAIzaolaOAllerRde la FuenteBBachillerRRomeroE. Effects of a high-protein/low carbohydrate versus a standard hypocaloric diet on adipocytokine levels and insulin resistance in obese patients along 9 months.J Diabetes Complicat. (2015) 29:950–4. 10.1016/j.jdiacomp.2015.06.002
14.
JonassonLGuldbrandHLundbergAKNystromFH. Advice to follow a low-carbohydrate diet has a favourable impact on low-grade inflammation in type 2 diabetes compared with advice to follow a low-fat diet.Ann Med. (2014) 46:182–7. 10.3109/07853890.2014.894286
15.
BazzanoLAHuTReynoldsKYaoLBunolCLiuYet alEffects of low-carbohydrate and low-fat diets: a randomized trial.Ann Intern Med. (2014) 161:309–18. 10.7326/M14-0180
16.
PerkJDe BackerGGohlkeHGrahamIReinerZVerschurenMet alEuropean guidelines on cardiovascular disease pre-vention in clinical practice (version 2012). The fifth joint task force of the european society of cardiology and other societies on cardiovascular disease rrevention in clinical practice (constituted by representatives of nine societies and by invited experts).Eur Heart J. (2012) 33:1635–701. 10.1093/eurheartj/ehs092
17.
No authors listed.Summary of revisions for the 2010 clinical practice recommendations.Diabetes Care. (2010) 33:S3. 10.2337/dc10-S003
18.
MansoorNVinknesKJVeierødMBRetterstølK. Effects of low-carbohydrate diets v. low-fat diets on body weight and cardiovascular risk factors: a meta-analysis of randomised controlled trials.Br J Nutr. (2016) 115:466–79. 10.1017/S0007114515004699
19.
HuTMillsKTYaoLDemanelisKEloustazMYancyWSJr.et alEffects of low-carbohydrate diets versus low-fat diets on metabolic risk factors: a meta-analysis of randomized controlled clinical trials.Am J Epidemiol. (2012) 176:S44–54. 10.1093/aje/kws264
20.
HaufeSUtzWEngeliSKastPBöhnkeJPofahlMet alLeft ventricular mass and function with reduced-fat or re-duced-carbohydrate hypocaloric diets in overweight and obese subjects.Hypertension. (2012) 59:70–5. 10.1161/HYPERTENSIONAHA.111.178616
21.
JenkinsDJWongJMKendallCWEsfahaniANgVWLeongTCet alEffect of a 6-month vegan low-carbohydrate (’Eco-Atkins’) diet on cardiovascular risk factors and body weight in hyperlipidaemic adults: a randomised controlled trial.BMJ Open. (2014) 4:e003505. 10.1136/bmjopen-2013-003505
22.
YamadaYUchidaJIzumiHTsukamotoYInoueGWatanabeYet alA non-calorie-restricted low-carbohydrate diet is effective as an alternative therapy for patients with type 2 diabetes.Intern Med. (2014) 53:13–9. 10.2169/internalmedicine.53.0861
23.
GardnerCDTrepanowskiJFDel GobboLCHauserMERigdonJIoannidisJPAet alEffect of low-fat vs low-carbohydrate diet on 12-month weight loss in overweight adults and the association with genotype pattern or insulin secretion: the DIETFITS randomized clinical trial.JAMA. (2018) 319:667–79. 10.1001/jama.2018.0245
24.
SaslowLRDaubenmierJJMoskowitzJTKimSMurphyEJPhinneySDet alTwelve-month outcomes of a randomized trial of a moderate-carbohydrate versus very low-carbohydrate diet in overweight adults with type 2 diabetes mellitus or prediabetes.Nutr Diabetes. (2017) 7:304. 10.1038/s41387-017-0006-9
25.
GeLSadeghiradBBallGDCda CostaBRHitchcockCLSvendrovskiAet alComparison of dietary macronutrient patterns of 14 popular named dietary programmes for weight and cardiovascular risk factor reduction in adults: systematic review and network meta-analysis of randomised trials.BMJ. (2020) 369:m696. 10.1136/bmj.m696
26.
TobiasDKChenMMansonJELudwigDSWillettWHuFB. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: a systematic review and meta-analysis.Lancet Diabetes Endocrinol. (2015) 3:968–79. 10.1016/S2213-8587(15)00367-8
27.
HooperLAbdelhamidASJimohOFBunnDSkeaffCM. Effects of total fat intake on body fatness in adults.Cochrane Database Syst Rev. (2020) 6:CD013636. 10.1002/14651858.CD013636
28.
HigginsJPAltmanDGGøtzschePCJüniPMoherDOxmanADet alThe Cochrane Collaboration’s tool for assessing risk of bias in randomised trials.BMJ. (2011) 343:d5928. 10.1136/bmj.d5928
29.
BalshemHHelfandMSchünemannHJOxmanADKunzRBrozekJet alGRADE guidelines: 3.rating the quality of evidence.J Clin Epidemiol. (2011) 64:401–6. 10.1016/j.jclinepi.2010.07.015
30.
MeaderNKingKLlewellynANormanGBrownJRodgersMet alA checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation.Syst Rev. (2014) 24:82. 10.1186/2046-4053-3-82
31.
EggerMDavey SmithGSchneiderMMinderC. Bias in meta-analysis detected by a simple, graphical test.BMJ. (1997) 315:629–34. 10.1136/bmj.315.7109.629
32.
SamahaFFIqbalNSeshadriPChicanoKLDailyDAMcGroryJet alA low-carbohydrate as compared with a low-fat diet in severe obesity.N Engl J Med. (2003) 348:2074–81. 10.1056/NEJMoa022637
33.
ThomsonCAStopeckATBeaJWCusslerENardiEFreyGet alChanges in body weight and metabolic indexes in overweight breast cancer survivors enrolled in a randomized trial of low-fat vs. reduced carbohydrate diets.Nutr Cancer. (2010) 62:1142–52. 10.1080/01635581.2010.513803
34.
YancyWSJr.OlsenMKGuytonJRBakstRPWestmanECA. low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: a randomized, controlled trial.Ann Intern Med. (2004) 140:769–77. 10.7326/0003-4819-140-10-200405180-00006
35.
BrinkworthGDNoakesMBuckleyJDKeoghJBCliftonPM. Long-term effects of a very-low-carbohydrate weight loss diet compared with an isocaloric low-fat diet after 12 mo.Am J Clin Nutr. (2009) 90:23–32. 10.3945/ajcn.2008.27326
36.
DansingerMLGleasonJAGriffithJLSelkerHPSchaeferEJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial.JAMA. (2005) 293:43–53.
37.
DavisNJTomutaNSchechterCIsasiCRSegal-IsaacsonCJSteinDet alComparative study of the effects of a 1-year dietary intervention of a low-carbohydrate diet versus a low-fat diet on weight and glycemic control in type 2 diabetes.Diabetes Care. (2009) 32:1147–52. 10.2337/dc08-2108
38.
EbbelingCBLeidigMMFeldmanHALoveskyMMLudwigDS. Effects of a low-glycemic load vs low-fat diet in obese young adults: a randomized trial.JAMA. (2007) 297:2092–102. 10.1001/jama.297.19.2092
39.
ElhayanyALustmanAAbelRAttal-SingerJVinkerS. A low carbohydrate Mediterranean diet improves cardiovascular risk factors and diabetes control among overweight patients with type 2 diabetes mellitus: a 1-year prospective randomized intervention study.Diabetes Obes Metab. (2010) 12:204–9. 10.1111/j.1463-1326.2009.01151.x
40.
FosterGDWyattHRHillJOMcGuckinBGBrillCMohammedBSet alA randomized trial of a low-carbohydrate diet for obesity.N Engl J Med. (2003) 348:2082–90. 10.1056/NEJMoa022207
41.
FrischSZittermannABertholdHKGöttingCKuhnJKleesiekKet alA randomized controlled trial on the efficacy of carbohydrate-reduced or fat-reduced diets in patients attending a telemedically guided weight loss program.Cardiovasc Diabetol. (2009) 8:36. 10.1186/1475-2840-8-36
42.
GardnerCDKiazandAAlhassanSKimSStaffordRSBaliseRRet alComparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial.JAMA. (2007) 297:969–77. 10.1001/jama.297.9.969
43.
HockadayTDHockadayJMMannJITurnerRC. Prospective comparison of modified fat-high-carbohydrate with standard low-carbohydrate dietary advice in the treatment of diabetes: one year follow-up study.Br J Nutr. (1978) 39:357–62. 10.1079/bjn19780045
44.
KlemsdalTOHolmeINerlandHPedersenTRTonstadS. Effects of a low glycemic load diet versus a low-fat diet in subjects with and without the metabolic syndrome.Nutr Metab Cardiovasc Dis. (2010) 20:195–201. 10.1016/j.numecd.2009.03.010
45.
LimSSNoakesMKeoghJBCliftonPM. Long-term effects of a low carbohydrate, low fat or high unsaturated fat diet compared to a no-intervention control.Nutr Metab Cardiovasc Dis. (2010) 20:599–607. 10.1016/j.numecd.2009.05.003
46.
McAuleyKASmithKJTaylorRWMcLayRTWilliamsSMMannJI. Long-term effects of popular dietary approaches on weight loss and features of insulin resistance.Int J Obes (Lond). (2006) 30:342–9. 10.1038/sj.ijo.0803075
47.
SternLIqbalNSeshadriPChicanoKLDailyDAMcGroryJet alThe effects of low-carbohydrate versus conventional weight loss diets in severely obese adults: one-year follow-up of a randomized trial.Ann Intern Med. (2004) 140:778–85. 10.7326/0003-4819-140-10-200405180-00007
48.
WoleverTMGibbsALMehlingCChiassonJLConnellyPWJosseRGet alThe canadian trial of carbohydrates in diabetes (CCD), a 1-y controlled trial of low-glycemic-index dietary carbohydrate in type 2 diabetes: no effect on glycated hemoglobin but reduction in C-reactive protein.Am J Clin Nutr. (2008) 87:114–25. 10.1093/ajcn/87.1.114
49.
WycherleyTPBrinkworthGDKeoghJBNoakesMBuckleyJDCliftonPM. Long-term effects of weight loss with a very low carbohydrate and low fat diet on vascular function in overweight and obese patients.J Intern Med. (2010) 267:452–61. 10.1111/j.1365-2796.2009.02174.x
50.
FosterGDWyattHRHillJOMakrisAPRosenbaumDLBrillCet alWeight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet: a randomized trial.Ann Intern Med. (2010) 153:147–57. 10.7326/0003-4819-153-3-201008030-00005
51.
GuldbrandHDizdarBBunjakuBLindströmTBachrach-LindströmMFredriksonMet alIn type 2 diabetes, randomisation to advice to follow a low-carbohydrate diet transiently improves glycaemic control compared with advice to follow a low-fat diet producing a similar weight loss.Diabetologia. (2012) 55:2118–27. 10.1007/s00125-012-2567-4
52.
SacksFMBrayGACareyVJSmithSRRyanDHAntonSDet alComparison of weight-loss diets with different compositions of fat, protein, and carbohydrates.N Engl J Med. (2009) 360:859–73. 10.1056/NEJMoa0804748
53.
ShaiISchwarzfuchsDHenkinYShaharDRWitkowSGreenbergIet alWeight loss with a low-carbohydrate, mediterranean, or low-fat diet.N Engl J Med. (2008) 359:229–41. 10.1056/NEJMoa0708681
54.
MazidiMShivappaNWirthMDHebertJRMikhailidisDPKengneAPet alDietary inflammatory index and cardiometabolic risk in US adults.Atherosclerosis. (2018) 276:23–7. 10.1016/j.atherosclerosis.2018.02.020
55.
NordmannAJNordmannABrielMKellerUYancyWSJr.BrehmBJet alEffects of low-carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors: a meta-analysis of randomized controlled trials.Arch Intern Med. (2006) 166:285–93. 10.1001/archinte.166.3.285
56.
MelaDJ. Eating behaviour, food preferences and dietary intake in relation to obesity and body-weight status.Proc Nutr Soc. (1996) 55:803–16. 10.1079/pns19960080
57.
BuenoNBde MeloISde OliveiraSLda Rocha AtaideT. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials.Br J Nutr (2013) 110:1178–87. 10.1017/S0007114513000548
58.
HatahetWColeLKudchodkarBJFungweTV. Dietary fats differentially modulate the expression of lecithin:cholesterol acyltransferase, apoprotein-A1 and scavenger receptor b1 in rats.J Nutr. (2003) 133:689–94. 10.1093/jn/133.3.689
59.
LuMWanYYangBHugginsCELiD. Effects of low-fat compared with high-fat diet on cardiometabolic indicators in people with overweight and obesity without overt metabolic disturbance: a systematic review and meta-analysis of randomised controlled trials.Br J Nutr. (2018) 119:96–108. 10.1017/S0007114517002902
60.
HarveyCJDCSchofieldGMZinnCThornleySJCroftsCMerienFLR. Low-carbohydrate diets differing in carbohydrate restriction improve cardiometabolic and anthropometric markers in healthy adults: a randomised clinical trial.PeerJ. (2019) 5:e6273. 10.7717/peerj.6273
61.
SasanfarBToorangFEsmaillzadehAZendehdelK. Adherence to the low carbohydrate diet and the risk of breast Cancer in Iran.Nutr J. (2019) 18:86. 10.1186/s12937-019-0511-x
62.
RajaieSAzadbakhtLKhazaeiMSherbafchiMEsmaillzadehA. Moderate replacement of carbohydrates by dietary fats affects features of metabolic syndrome: a randomized crossover clinical trial.Nutrition. (2014) 30:61–8. 10.1016/j.nut.2013.06.011
63.
SmithESSmithHABettsJAGonzalezJTAtkinsonGA. systematic review and meta-analysis comparing heterogeneity in body mass responses between low-carbohydrate and low-fat diets.Obesity (Silver Spring). (2020) 28:1833–42. 10.1002/oby.22968
64.
AbbasnezhadAFalahiEGonzalezMJKavehiPFouladvandFChoghakhoriR. Effect of different dietary approaches in comparison with high/low-carbohydrate diets on systolic and diastolic blood pressure in type 2 diabetic patients: a systematic review and meta-analysis.Prev Nutr Food Sci. (2020) 25:233–45. 10.3746/pnf.2020.25.3.233
65.
MacedoRCOSantosHOTinsleyGMReischak-OliveiraA. Low-carbohydrate diets: effects on metabolism and exercise - a comprehensive literature review.Clin Nutr ESPEN. (2020) 40:17–26. 10.1016/j.clnesp.2020.07.022
66.
EssahPALevyJRSistrunSNKellySMNestlerJE. Effect of macronutrient composition on postprandial peptide YY levels.J Clin Endocrinol Metab. (2007) 92:4052–5. 10.1210/jc.2006-2273
67.
HuntrissRCampbellMBedwellC. The interpretation and effect of a low-carbohydrate diet in the management of type 2 diabetes: a systematic review and meta-analysis of randomised controlled trials.Eur J Clin Nutr. (2018) 72:311–25. 10.1038/s41430-017-0019-4
68.
Sackner-BernsteinJKanterDKaulS. Dietary intervention for overweight and obese adults: comparison of low-carbohydrate and low-fat diets. A Meta-Analysis.PLoS One. (2015) 10:e0139817. 10.1371/journal.pone.0139817
69.
ChuruangsukCKheroufMCombetELeanM. Low-carbohydrate diets for overweight and obesity: a systematic review of the systematic reviews.Obes Rev. (2018) 19:1700–18. 10.1111/obr.12744
70.
MozaffarianDMichaRWallaceS. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials.PLoS Med. (2010) 7:e1000252. 10.1371/journal.pmed.1000252
71.
ChawlaSTessarolo SilvaFAmaral MedeirosSMekaryRARadenkovicD. The effect of low-fat and low-carbohydrate diets on weight loss and lipid levels: a systematic review and meta-analysis.Nutrients. (2020) 9:3774. 10.3390/nu12123774
72.
NotoHGotoATsujimotoTNodaM. Low-carbohydrate diets and all-cause mortality: a systematic review and meta-analysis of observational studies.PLoS One. (2013) 8:e55030. 10.1371/journal.pone.0055030
73.
SeidelmannSBClaggettBChengSHenglinMShahASteffenLMet alDietary carbohydrate intake and mortality: a pro-spective cohort study and meta-analysis.Lancet Public Health. (2018) 3:e419–28. 10.1016/S2468-2667(18)30135-X
74.
D’AnciKEWattsKLKanarekRBTaylorHA. Low-carbohydrate weight-loss diets. effects on cognition and mood.Appetite. (2009) 52:96–103. 10.1016/j.appet.2008.08.009
75.
HalyburtonAKBrinkworthGDWilsonCJNoakesMBuckleyJDKeoghJBet alLow- and high-carbohydrate weight-loss diets have similar effects on mood but not cognitive performance.Am J Clin Nutr. (2007) 86:580–7. 10.1093/ajcn/86.3.580
76.
WingRRVazquezJARyanCM. Cognitive effects of ketogenic weight-reducing diets.Int J Obes Relat Metab Disord. (1995) 19:811–6.
77.
DaneshzadEKeshavarzSAQorbaniMLarijaniBAzadbakhtL. Association between a low-carbohydrate diet and sleep status, depression, anxiety, and stress score.J Sci Food Agric. (2020) 100:2946–52. 10.1002/jsfa.10322
78.
RenMZhangHQiJHuAJiangQHouYet alAn almond-based low carbohydrate diet improves depression and glycometabolism in patients with type 2 diabetes through modulating gut microbiota and GLP-1: a randomized controlled trial.Nutrients. (2020) 12:3036. 10.3390/nu12103036
79.
HofmanZvan DrunenJDde LaterCKuipersH. The effect of different nutritional feeds on the postprandial glucose response in healthy volunteers and patients with type II diabetes.Eur J Clin Nutr. (2004) 58:1553–6. 10.1038/sj.ejcn.1602007
80.
SilveriiGABotarelliLDicembriniIGirolamoVSantagiulianaFMonamiMet alLow-carbohydrate diets and type 2 diabetes treatment: a meta-analysis of randomized controlled trials.Acta Diabetol. (2020) 57:1375–82. 10.1007/s00592-020-01568-8
81.
EspositoKPontilloADi PaloCGiuglianoGMasellaMMarfellaRet alEffect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial.JAMA. (2003) 289:1799–804. 10.1001/jama.289.14.1799
82.
QureshiAASamiSAKhanFA. Effects of stabilized rice bran, its soluble and fiber fractions on blood glucose levels and serum lipid parameters in humans with diabetes mellitus types I and II.J Nutr Biochem. (2002) 13:175–87. 10.1016/s0955-2863(01)00211-x
83.
RavautGLégiotABergeronKFMounierC. Monounsaturated fatty acids in obesity-related inflammation.Int J Mol Sci. (2020) 22:330. 10.3390/ijms22010330
84.
Silva FigueiredoPCarla InadaAMarcelinoGMaiara Lopes CardozoCde Cássia FreitasKde Cássia Avellaneda GuimarãesRet alFatty acids consumption: the role metabolic aspects involved in obesity and its associated disorders.Nutrients. (2017) 9:1158. 10.3390/nu9101158
Summary
Keywords
low-carbohydrate diets, low-fat diets, overweight, metabolic risk factors, obesity
Citation
Lei L, Huang J, Zhang L, Hong Y, Hui S and Yang J (2022) Effects of low-carbohydrate diets versus low-fat diets on metabolic risk factors in overweight and obese adults: A meta-analysis of randomized controlled trials. Front. Nutr. 9:935234. doi: 10.3389/fnut.2022.935234
Received
03 May 2022
Accepted
14 July 2022
Published
09 August 2022
Volume
9 - 2022
Edited by
Rafaela Rosário, University of Minho, Portugal
Reviewed by
Yoshitaka Hashimoto, Kyoto Prefectural University of Medicine, Japan; Shahab Alizadeh, Tehran University of Medical Sciences, Iran
Updates
Copyright
© 2022 Lei, Huang, Zhang, Hong, Hui and Yang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suocheng Hui, suochenghui@cqmu.edu.cnJian Yang, jianyang@hospital.cqmu.edu.cn
†These authors have contributed equally to this work
This article was submitted to Nutritional Epidemiology, a section of the journal Frontiers in Nutrition
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.