- 1Department of Cardiology, Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China
- 2Department of Guangdong Provincial Key Laboratory of Coronary Heart Disease Prevention, Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China
- 3The Second School of Clinical Medicine, Southern Medical University, Guangzhou, China
Background: Previous studies have shown that malnutrition is very common in patients with congestive heart failure (CHF) and is closely related to the occurrence of acute kidney injury. However, the relationship between malnutrition and contrast-associated acute kidney injury (CA-AKI) is unclear.
Method and results: We obtained data from 842 patients who were diagnosed with CHF following coronary angiography (CAG) or percutaneous coronary angiography (PCI) and had follow-up information from January 2013 to February 2016. The patients were divided into 3 groups according to the Controlling Nutritional Status Score before CAG or PCI procedure (Group 1: Normal; Group 2: Mild Malnutrition; Group 3: Moderate to Severe Malnutrition). The main endpoint was CA-AKI. Univariate and multivariable logistic regression analyses were performed. 556 (60.0%) patients suffered from malnutrition before CAG or PCI. During a median follow-up of 2.1 years, A total of 49 (5.82%) patients developed CA-AKI. Additionally, 5 (1.75%), 26 (6.27%) and 18 (12.77%) events were documented in patients with normal, mild and moderate or severe malnutrition, respectively (p < 0.01). In multivariable-adjusted models, patients with malnutrition showed a significantly higher incidence of CA-AKI than those in the normal group.
Conclusion: Malnutrition is an independent risk factor for CA-AKI in CHF patients following CAG.
Introduction
Contrast-associated acute kidney injury (CA-AKI), representing one third of all hospital-acquired acute kidney injuries, is associated with poor short-term and long-term outcomes (1–3). Adequate hydration is the most common way to prevent CA-AKI in patients following coronary angiography (CAG), while over hydration might induce acute heart failure (AHF) in patients with congestive heart failure (CHF) (4). Therefore, early identification of high-risk groups and active intervention are essential to preventing CA-AKI in chronic heart failure patients following CAG. Malnutrition, defined as any nutritional imbalance, is a major complication in hospitalized patients and is associated with increased healthcare costs and mortality, especially among CHF patients (5). The Controlling Nutritional Status Score (CONUT) was developed by Ulibarri et al. in 2005 as a screening tool for the nutritional status of hospitalized patients (6). Previous studies have shown that malnutrition is closely related to the occurrence of acute kidney injury (7–9). However, there has been a lack of research of the relationship between malnutrition and CA-AKI in congestive heart failure patients following CAG.
Materials and methods
Study population
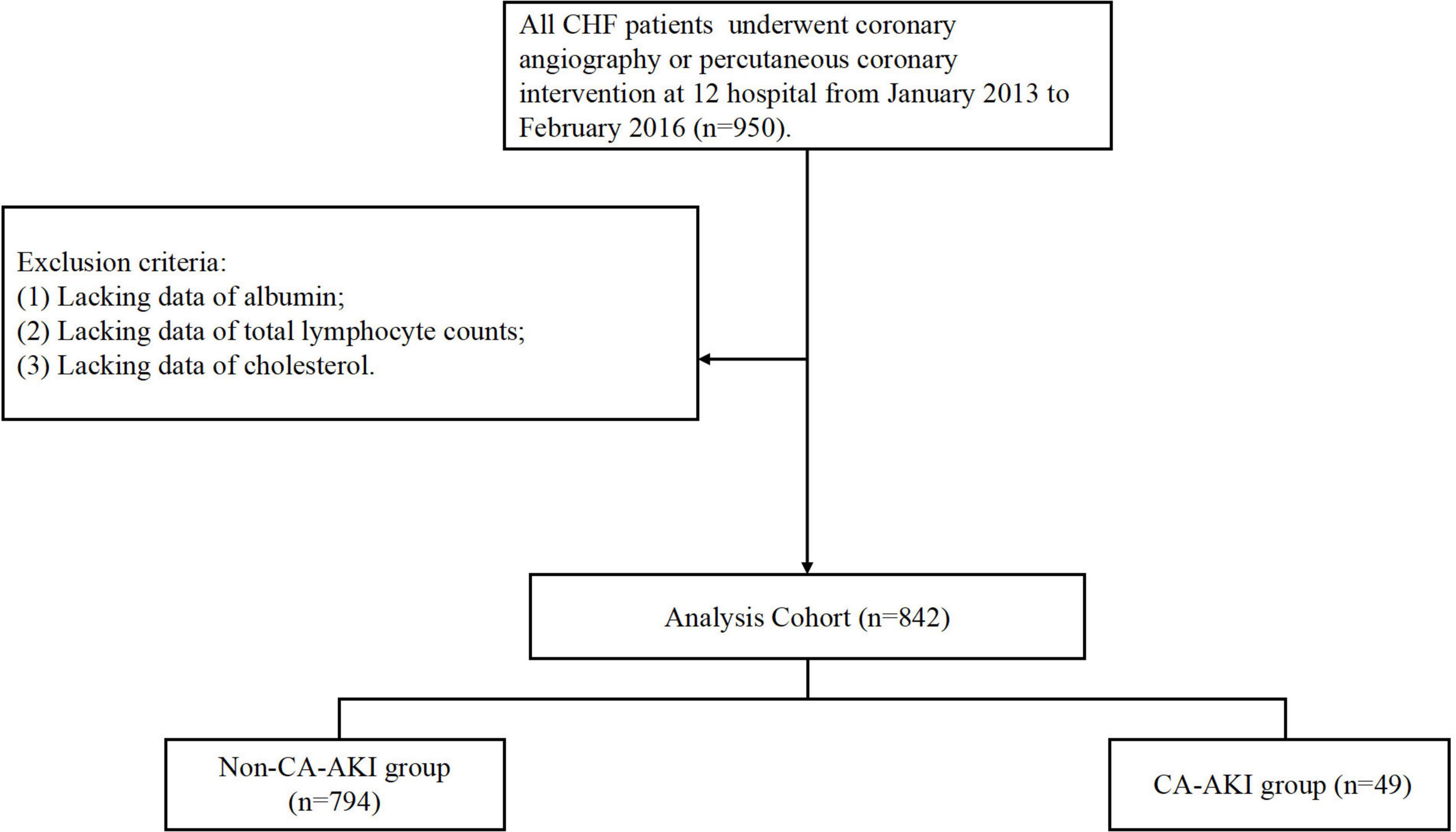
The present study was a prospective, multicenter, observational cohort study of patients who were admitted to one of twelve teaching hospitals in the Guangdong, Fujian and Xinjiang Provinces of China from January 2013 to February 2016 (NCT01402232; Supplementary Table 1). We included total of 950 patients who underwent coronary angiography (CAG) or percutaneous coronary angiography (PCI) and were diagnosed with CHF. A total of 842 patients were enrolled in the final analysis after excluding patients combined with conditions as follow: (a) lacking data of albumin (n = 67); (b) lacking data of total lymphocyte counts (n = 9); (c) lacking data of cholesterol (n = 32; Figure 1). The study and all of its protocols were approved by the institutional Ethics Research Committee of Guangdong Provincial People’s Hospital (No. GDREC2012141H). All patients gave written informed consent before undergoing CAG.
Malnutrition screening tools
The CONUT score that was developed by Ulibarri et al. in 2005 as a screening tool for the nutritional status of hospitalized patients, and has been validated for a wide range of populations, was applied to assess the nutritional state of patients with CHF (10, 11). It automatically assesses nutritional status, taking into account serum albumin, cholesterol, and total lymphocyte count (Supplementary Table 2). A score of 0–1 indicates a normal nutrition state, 2–4 reflects mild malnutrition, and 5–12 indicates moderate to severe malnutrition (12).
Data collection and definitions
The assessment of malnutrition was carried out before CAG or PCI according to the CONUT score. CAG procedures were performed according to standard clinical practice guidelines using standard guide catheters, guide wires, balloon catheters, and stents via the femoral or radial approach (4). The intravenous contrast agent administered was at the discretion of the interventional cardiologist. All patients received non-ionic, low-osmolarity contrast agents. The treatment and hydration protocols were based on current guidelines at the discretion of the cardiologists according to clinical conditions. High-risk patients were recommended to receive a continuous adequate intravenous infusion of isotonic saline or sodium bicarbonate solution at a rate of 1 ml/kg per hour (0.5 ml/kg per hour if the left ventricular ejection fraction was <35% or the New York Heart Association score was >2) for 12 h before and 12–24 h after the procedure (4). Preprocedural oral hydration volumes were recorded.
Baseline levels of blood indicators were measured. Serum creatinine was measured before CAG, on days 1, 2, and 3 postoperatively, and upon hospital discharge. The start time and end time of the procedure and the urine output during the 24 h after the procedure were recorded. Preprocedural interventions (i.e., normal saline injections and furosemide or statin administration), PCI technique, contrast agent details (non-ionic, low-osmolality, or isotonic), and contrast dose were selected at the discretion of the attending cardiologist based on their routine procedures and according to current practice guidelines. Patients were excluded if they received an intravenous contrast agent within 72 h after the procedure.
After discharge, adverse clinical events were assessed and registered by the attending physician or trained research assistant for a follow-up period of over 12 months. Data on patient and procedural characteristics was also obtained through original records and hospital electronic medical records. We calculated the estimated glomerular filtration rate (eGFR) by applying the Modification of Diet in Renal Disease (MDRD) equation (13). CHF was defined as a New York Heart Association (NYHA) class > 2 or Killip class > 1 (14).
Clinical outcomes
The primary endpoint was CA-AKI, defined as an increase in the level of Scr >0.3 mg/dl or >50% from the baseline level within the first 48 h after the procedure. The secondary endpoints were as follows: (1) 30-day mortality; (2) 1-year mortality; and (3) in-hospital dialysis.
Statistical analysis
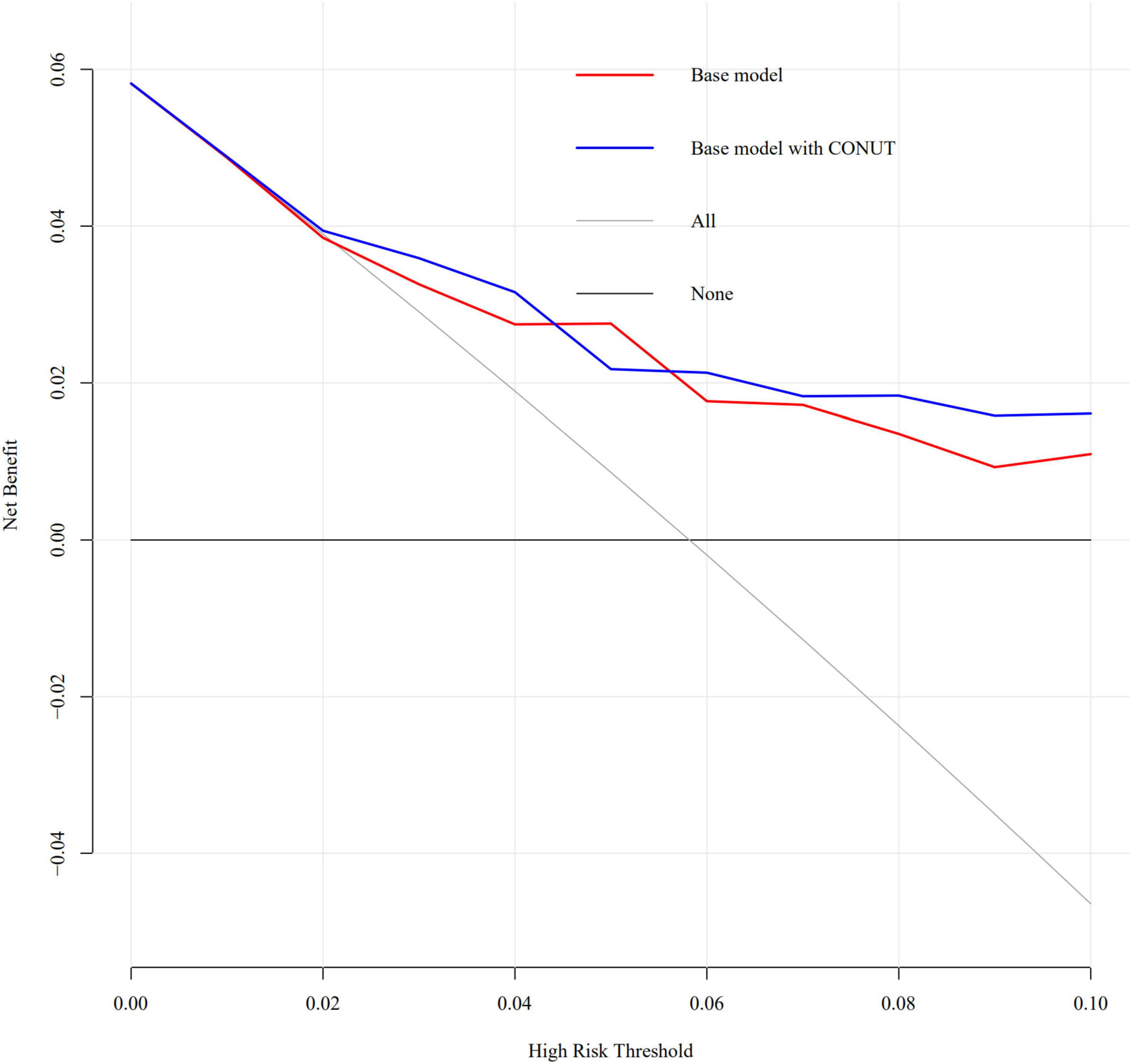
Descriptive statistics was applied, and the data were reported as the mean ± SD deviation in case of normal distribution and as the median and interquartile range when non-normally distributed. Categorical variables were expressed as numbers (percentages). The t-test and one-way ANOVA were used for comparisons of continuous data between the two groups, and comparisons of categorical variables between groups were performed by using a chi-square test. The associations between malnutrition and the study endpoints were assessed by univariate and multivariable logistic regression analysis, with model adjustments to include variables associated with known poor prognosis based on clinical plausibility. The results were presented as OR (95% CI). All P values <0.05 were considered significant. The decision curve analysis was adopted to quantify the predictive abilities of the multivariable logistic regression models (including multivariable logistic regression model and model added CONUT). The partial effect plot was used to express the value of CONUT in full model. The association between CA-AKI and the CONUT score level were evaluated on a continuous scale with restrictive cubic spline based on multivariable logistic regression model. The interaction between age and CONUT for prediction CA-AKI, also CRP and CONUT for prediction CA-AKI was evaluated with partial effect plot. The relative importance of item in CONUT were calculated, each item were included and removed, respectively, in full model and then Likelihood chi square, and Nagelkerke R2 value were calculated. All statistical analyses were performed using R (ver. 4.0.1).
Results
Baseline characteristics
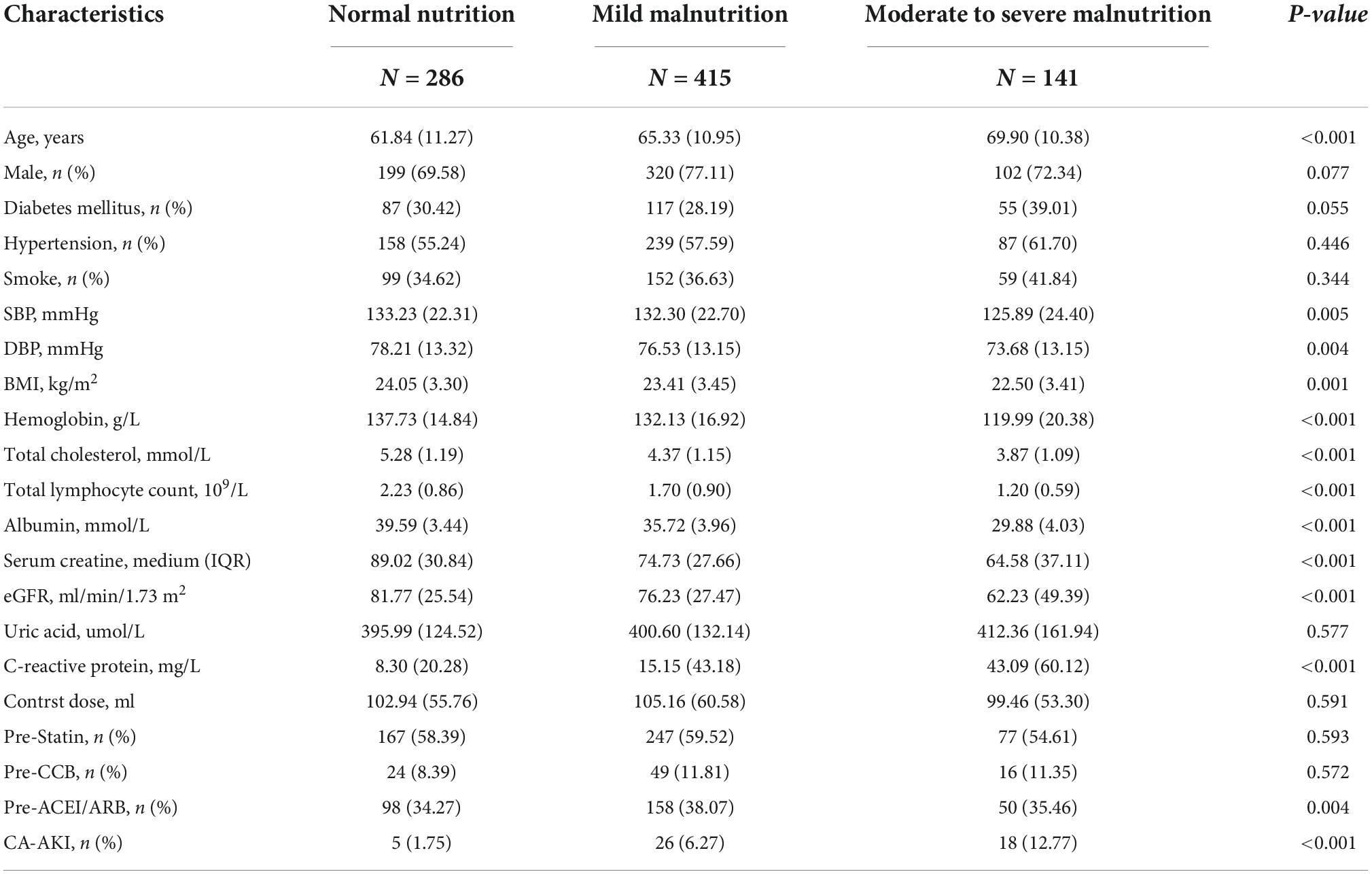
Among the 842 patients, included in the study, 556 (60.0%) suffered from malnutrition. The overall median age was 64.9 ± 11.3 years. Malnourished patients were older and more likely to be male. The baseline hemoglobin, total cholesterol, total lymphocyte count and albumin level were significantly lower in the group with malnutrition. We found that malnourished patients had worse underlying kidney function. More data on the baseline demographic and clinical characteristics of the patients are presented in Table 1.
Clinical outcomes
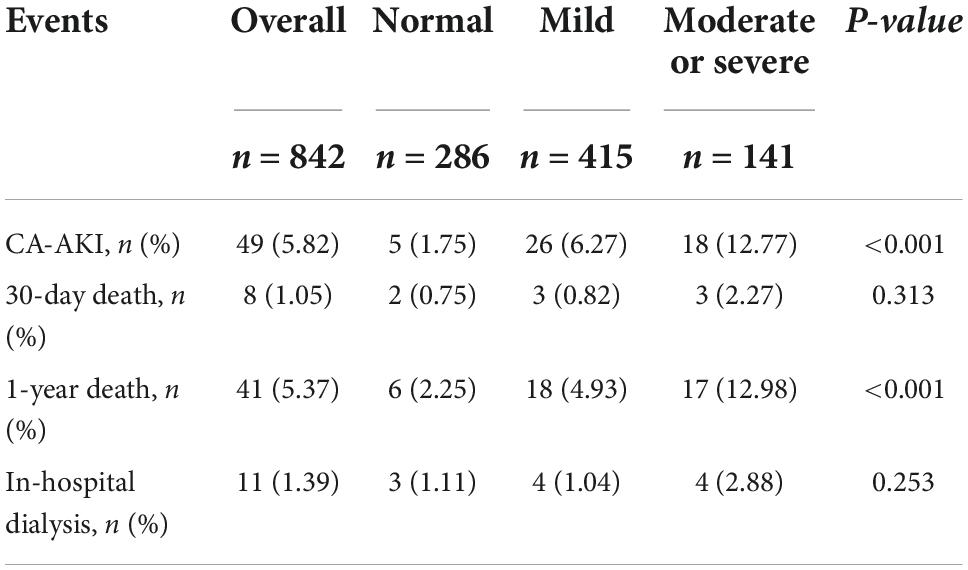
During hospitalization, 49 (5.82%) patients developed CA-AKI. In total, 5 (1.75%), 26 (6.27%) and 18 (12.77%) events were documented in patients with normal, mild or severe malnutrition states, respectively (p < 0.01). The incidence of CA-AKI and 1-year mortality were significantly increased in the malnutrition group. There were no significant differences in the incidence of dialysis and short-term mortality among the groups (Table 2).
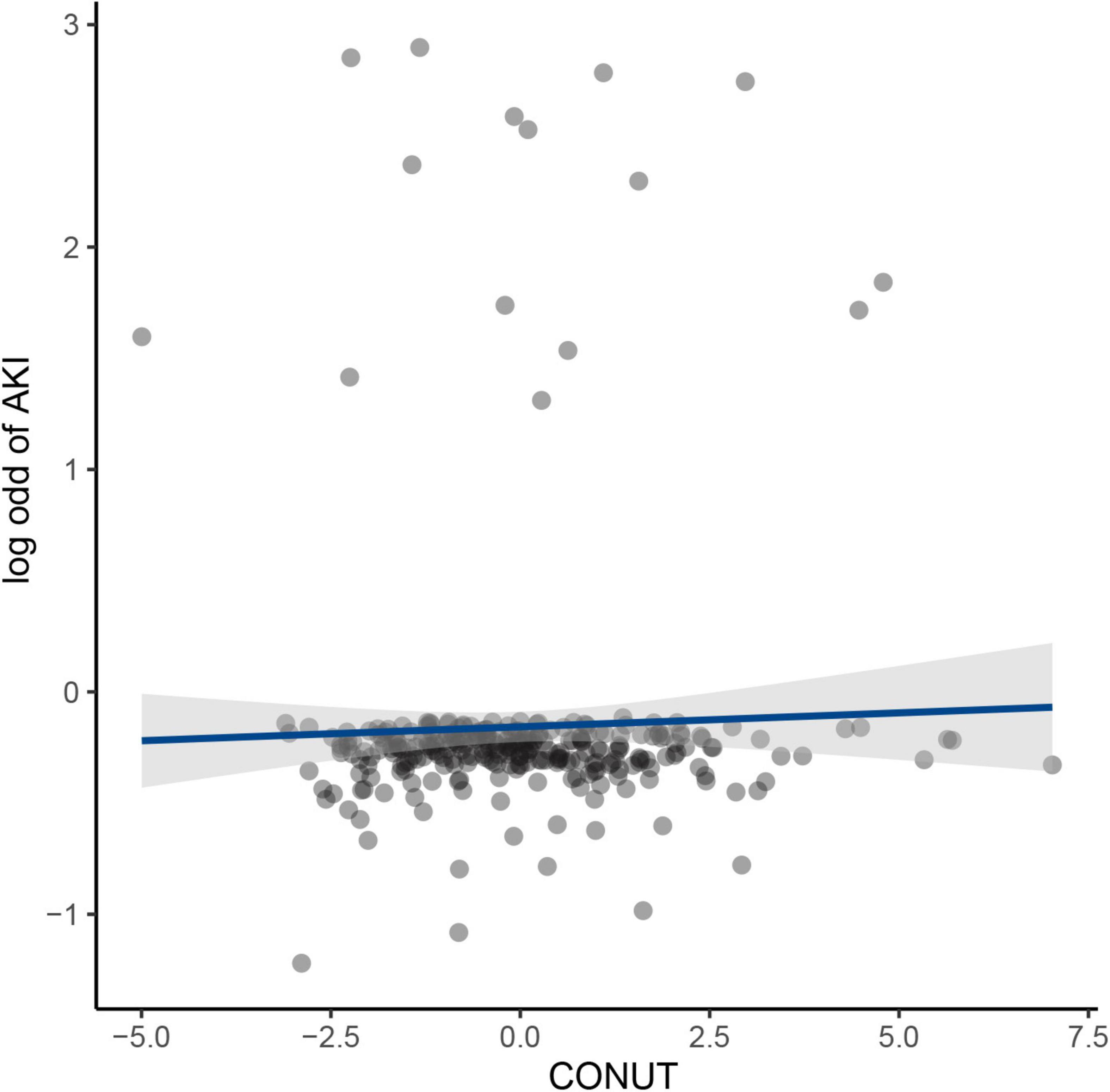
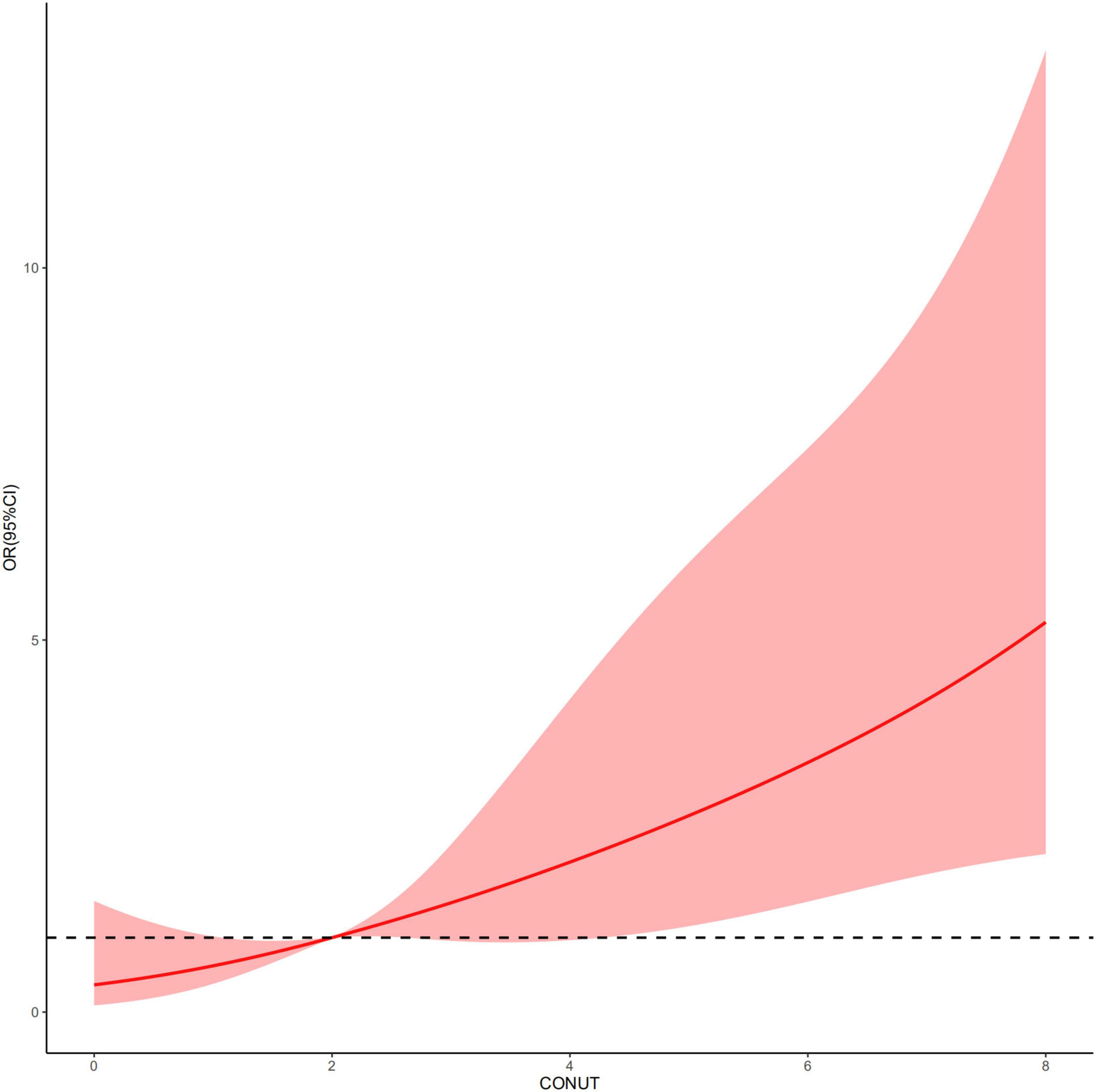
An increased CONUT value was associated with higher risk of CA-AKI. The univariate and multivariable logistic regression models showed that different levels of malnutrition were associated with the occurrence of CA-AKI in patients with CHF following CAG after adjusting for clinical variables (Table 3). In multivariable-adjusted model, patients with mild (2 ≤ CONUT score ≤ 4) [OR (95%CI) 3.15 (1.28-9.53)] and moderate (5 ≤ CONUT score ≤ 12) [OR (95%CI) 4.41 (1.57-14.45)] malnutrition showed a significantly higher incidence of CA-AKI than those in the normal group (p = 0.022 and 0.008, respectively). In decision curve analysis, model added CONUT showed higher net benefit when high risk threshold more than 5.5% (Figure 2). The partial effect plot showed the positive value of CONUT for higher predictive ability of CA-AKI (Figure 3). We observed the risk of CA-AKI increase with increase of the CONUT score level in the multivariable logistic regression model of restrictive cubic spline (Figure 4). We observed no significant evidence of interaction between CRP and CONUT (Supplementary Figure 1). We did not find any heterogeneity between age and CONUT for CA-AKI (Supplementary Figure 2). The importance of each item of the CONUT score were displayed (Supplementary Table 3).
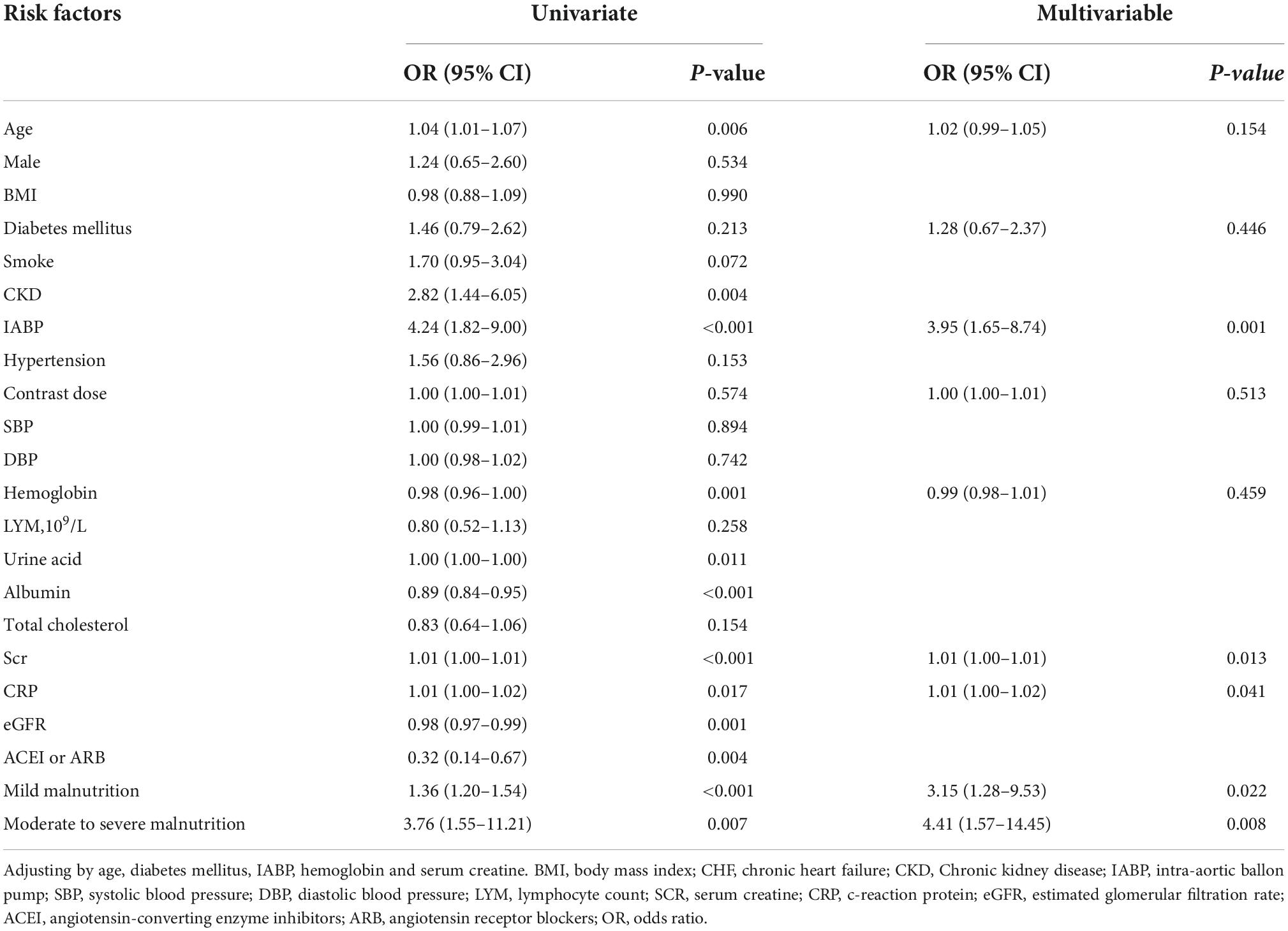
Table 3. Univariate and multivariate logistic regression analysis of the association between malnutrition and CA-AKI.
Discussion
To the best of our knowledge, this is the first study that attempts to investigate the relationship between malnutrition and CA-AKI in CHF patients. Our study shows that malnutrition levels were associated with an increased risk of developing CA-AKI in CHF patients. After adjusting for confounding factors, malnutrition levels were still a significant factor for CA-AKI in CHF patients.
A prospective observational study conducted by Li et al. included 46,549 in-hospital patients, and found that malnutrition is widespread among hospitalized patients and has a strong association with acute kidney injury (AKI) (7). Yu et al. enrolled 3,185 acute coronary syndrome patients and found that the risk of malnutrition was a helpful tool in identifying patients at high risk for AKI and mortality (8). Süleyman C et al. found a similar conclusion by enrolled in 360 consecutive patients with coronary angiography performed because of chronic coronary artery disease. They found that malnutrion were independently associated with the presence of CI-AKI in elderly patients (15). A prospective and observational study that included a total of 114 CHF patients reported that a poor nutritional status, as assessed using the CONUT score, is significantly associated with poor outcomes (16). In order to found a better tool to assess the influence of malnutrition to mortality, Shirley et al. simple tools (CONUT Score, Geriatric Nutritional Risk Index, and Prognostic Nutritional Index) and learned that CONUT Score have the best predictive power of mortality in patients with CHF (17). Our results are in agreement with these studies. Similarly to the previous reports, our study focused on malnutrition, and revealed that malnutrition is an independent risk factor for kidney dysfunction. However, our study included 842 CHF patients, and the sample size was significantly larger than that of the other studies on CHF patients. Moreover, we are the first prospective study to explore the relationship between malnutrition and CA-AKI.
Malnutrition is currently commonly defined as an acute, subacute or chronic nutritional state by The European Society for Clinical Nutrition and Metabolism (18). In this state, with or without inflammatory activity, different degrees of overnutrition or undernutrition will lead to changes in body composition and impaired bodily functions. Previous studies have revealed that the incidence of malnutrition among the elderly population is 5–12%, with the rate of 16–78% for elderly inpatients in developing countries and 29–61% in developed countries. The incidence of malnutrition in patients with cardiac insufficiency is 42–58% (10, 19–22).
A retrospective observational study revealed that malnutrition is common among patients with acute coronary syndrome and is strongly associated with increased rates of mortality and cardiovascular events. For mortality risk prediction, each of the 3 malnutrition scores had significant incremental prognostic value for the GRACE risk score. The highest incremental value was seen for the Control Nutritional Status (CONUT) score (12) that includes albumin (ALB), total cholesterol (TC), and whole blood lymphocyte (LYM) count, and is used to assess protein storage, calorie consumption and immune defense in the body (6).
A retrospective study found a strong independent association between increased neutrophil-to-lymphocyte ratios (NLRs) after cardiovascular surgery and AKI occurring in the first 7 days after surgery (23). A meta-analysis showed that low ALB could independently predict the occurrence of AKI, and correction of hypoproteinemia could prevent the occurrence of AKI (24).
The mechanisms underlying the association between malnutrition levels and CA-AKI are unclear. One potential mechanism may involve the body’s inflammatory response. LYM count is often considered an inflammatory response and immune status marker. Previous study suggested that inflammatory cells in general, and lymphocytes in particular are involved in the initiation, proliferation and recovery stages of AKI and infiltrate damaged kidney tissues and produce inflammatory mediators such as cytokines and chemokines (25). Another possible mechanism may involve a protein-calorie imbalance that could cause renal hemodynamic changes, reduce renal blood flow, decrease the glomerular filtration rate, and decrease the ability of renal tubules to excrete acid (26, 27).
Our study also has several limitations. First, we did not track changes in renal function among discharged patients. Second, whether improvements in malnutrition states could prevent CA-AKI was not assessed. More randomized clinical treatment studies on malnutrition and CA-AKI are needed.
Conclusion
In summary, malnutrition is an independent risk factor for CA-AKI in CHF patients following CAG. The higher the degree of malnutrition, the greater the risk of developing CA-AKI.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
MY and JY conceived and designed the study. ZH, YLn, BW, HH, QL, JL, YLu, and ZC collected the data and performed the analysis. MY, JY, and ZC were involved in the writing of the manuscript and is responsible for the integrity of the study. All authors have read and approved the final manuscript.
Funding
This research was funded and supported by the National Key Research and Development Program of China, Grant (2016YFC1301202); Multi-center study on key techniques for prevention, diagnosis and treatment of high risk coronary artery disease (DFJH2020026).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.937237/full#supplementary-material
Supplementary Figure 1 | Partial effect plot of interaction between CRP and Controlling Nutritional Status for CA-AKI.
Supplementary Figure 2 | Partial effect plot of interaction between age and Controlling Nutritional Status for CA-AKI.
References
1. Rear R, Bell RM, Hausenloy DJ. Contrast-induced nephropathy following angiography and cardiac interventions. Heart. (2016) 102:638–48. doi: 10.1136/heartjnl-2014-306962
2. James MT, Ghali WA, Knudtson ML, Ravani P, Tonelli M, Faris P, et al. Associations between acute kidney injury and cardiovascular and renal outcomes after coronary angiography. Circulation. (2011) 123:409–16.
3. Mehran R, Nikolsky E. Contrast-induced nephropathy: definition, epidemiology, and patients at risk. Kidney Int Suppl. (2006) 69:S11–5. doi: 10.1038/sj.ki.5000368
4. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. (2019) 40:87–165. doi: 10.1093/eurheartj/ehy394
5. Corkins MR, Guenter P, DiMaria-Ghalili RA, Jensen GL, Malone A, Miller S, et al. Malnutrition diagnoses in hospitalized patients: United States, 2010. JPEN J Parenter Enternal Nutr. (2014) 38:186–95. doi: 10.1177/0148607113512154
6. Ignacio de Ulíbarri J, González-Madroño A, de Villar NG, González P, González B, Mancha A, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. (2005) 20:38–45.
7. Li C, Xu L, Guan C, Zhao L, Luo C, Zhou B, et al. Malnutrition screening and acute kidney injury in hospitalised patients: a retrospective study over a 5-year period from China. Br J Nutr. (2020) 123:337–46. doi: 10.1017/s000711451900271x
8. Yu J, Li D, Jia Y, Li F, Jiang Y, Zhang Q, et al. Nutritional risk screening 2002 was associated with acute kidney injury and mortality in patients with acute coronary syndrome: insight from the REACP study. Nutr Metab Cardiovasc Dis. (2021) 31:1121–8. doi: 10.1016/j.numecd.2020.12.028
9. Berbel MN, Pinto MP, Ponce D, Balbi AL. Nutritional aspects in acute kidney injury. Rev Assoc Med Bras. (2011) 57:600–6. doi: 10.1590/s0104-42302011000500022
10. Sze S, Zhang J, Pellicori P, Morgan D, Hoye A, Clark AL. Prognostic value of simple frailty and malnutrition screening tools in patients with acute heart failure due to left ventricular systolic dysfunction. Clin Res Cardiol. (2017) 106:533–41. doi: 10.1007/s00392-017-1082-5
11. Sze S, Pellicori P, Kazmi S, Rigby A, Cleland JGF, Wong K, et al. Prevalence and prognostic significance of malnutrition using 3 scoring systems among outpatients with heart failure: a comparison with body mass index. JACC Heart Fail. (2018) 6:476–86. doi: 10.1016/j.jchf.2018.02.018
12. Raposeiras Roubín S, Abu Assi E, Cespón Fernandez M, Barreiro Pardal C, Lizancos Castro A, Parada JA, et al. Prevalence and prognostic significance of malnutrition in patients with acute coronary syndrome. J Am Coll Cardiol. (2020) 76:828–40. doi: 10.1016/j.jacc.2020.06.058
13. Aguiar-Souto P, Ferrante G, Del Furia F, Barlis P, Khurana R, Di Mario C. Frequency and predictors of contrast-induced nephropathy after angioplasty for chronic total occlusions. Int J Cardiol. (2010) 139:68–74. doi: 10.1016/j.ijcard.2008.10.006
14. Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. (2004) 44:1393–9. doi: 10.1016/j.jacc.2004.06.068
15. Efe SC, Karagöz A, Doðan C, Bayram Z, Cakmak EO, Kalkan S, et al. Prognostic significance of malnutrition scores in elderly patients for the prediction of contrast-induced acute kidney injury. Int J Clin Pract. (2021) 75:e14274. doi: 10.1111/ijcp.14274
16. Nakagomi A, Kohashi K, Morisawa T, Kosugi M, Endoh I, Kusama Y, et al. Nutritional status is associated with inflammation and predicts a poor outcome in patients with chronic heart failure. J Atheroscler Thromb. (2016) 23:713–27. doi: 10.5551/jat.31526
17. Sze S, Pellicori P, Zhang J, Weston J, Clark AL. The impact of malnutrition on short-term morbidity and mortality in ambulatory patients with heart failure. Am J Clin Nutr. (2021) 113:695–705. doi: 10.1093/ajcn/nqaa311
18. Cederholm T, Barazzoni R, Austin P, Ballmer P, Biolo G, Bischoff SC, et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr. (2017) 36:49–64. doi: 10.1016/j.clnu.2016.09.004
19. John BK, Bullock M, Brenner L, McGaw C, Scolapio JS. Nutrition in the elderly. Frequently asked questions. Am J Gastroenterol. (2013) 108:1252–66. doi: 10.1038/ajg.2013.125
20. Abd Aziz NAS, Teng N, Abdul Hamid MR, Ismail NH. Assessing the nutritional status of hospitalized elderly. Clin Interv Aging. (2017) 12:1615–25. doi: 10.2147/cia.S140859
21. La Rovere MT, Maestri R, Olmetti F, Paganini V, Riccardi G, Riccardi R, et al. Additional predictive value of nutritional status in the prognostic assessment of heart failure patients. Nutr Metab Cardiovasc Dis. (2017) 27:274–80. doi: 10.1016/j.numecd.2016.09.009
22. Cereda E, Klersy C, Pedrolli C, Cameletti B, Bonardi C, Quarleri L, et al. The geriatric nutritional risk index predicts hospital length of stay and in-hospital weight loss in elderly patients. Clin Nutr. (2015) 34:74–8. doi: 10.1016/j.clnu.2014.01.017
23. Kim WH, Park JY, Ok SH, Shin IW, Sohn JT. Association between the neutrophil/lymphocyte ratio and acute kidney injury after cardiovascular surgery: a retrospective observational study. Medicine. (2015) 94:e1867. doi: 10.1097/md.0000000000001867
24. Wiedermann CJ, Wiedermann W, Joannidis M. Hypoalbuminemia and acute kidney injury: a meta-analysis of observational clinical studies. Intensive Care Med. (2010) 36:1657–65. doi: 10.1007/s00134-010-1928-z
25. Weller S, Varrier M, Ostermann M. Lymphocyte function in human acute kidney injury. Nephron. (2017) 137:287–93. doi: 10.1159/000478538
26. Benabe JE, Martinez-Maldonado M. The impact of malnutrition on kidney function. Miner Electrolyte Metab. (1998) 24:20–6. doi: 10.1159/000057346
Keywords: malnutrition, congestive heart failure, contrast-associated acute kidney injury, mortality, coronary angiography (CAG)
Citation: Ying M, Yang J, Huang Z, Ling Y, Wang B, Huang H, Li Q, Liu J, Liu Y and Chen Z (2022) Association between malnutrition and contrast-associated acute kidney injury in congestive heart failure patients following coronary angiography. Front. Nutr. 9:937237. doi: 10.3389/fnut.2022.937237
Received: 06 May 2022; Accepted: 04 November 2022;
Published: 17 November 2022.
Edited by:
Scott W. Keith, Thomas Jefferson University, United StatesReviewed by:
Mohit Mathur, Visterra Inc., United StatesAli Karagoz, Istanbul Kartal Kocuyolu High Special Education and Research Hospital, Turkey
Copyright © 2022 Ying, Yang, Huang, Ling, Wang, Huang, Li, Liu, Liu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhujun Chen, Y2hlbnpodWp1bjExQHNpbmEuY29t; Yong Liu, bGl1eW9uZ0BnZHBoLm9yZy5jbg==
†These authors have contributed equally to this work
 Ming Ying
Ming Ying Junqing Yang1†
Junqing Yang1† Zhidong Huang
Zhidong Huang Yihang Ling
Yihang Ling Bo Wang
Bo Wang Haozhang Huang
Haozhang Huang Qiang Li
Qiang Li Jin Liu
Jin Liu Yong Liu
Yong Liu Zhujun Chen
Zhujun Chen