- 1Department of Clinical Epidemiology, Shengjing Hospital of China Medical University, Shenyang, China
- 2Clinical Research Center, Shengjing Hospital of China Medical University, Shenyang, China
- 3Liaoning Key Laboratory of Precision Medical Research on Major Chronic Disease, Shengjing Hospital of China Medical University, Shenyang, China
- 4Department of Obstetrics and Gynecology, Shengjing Hospital of China Medical University, Shenyang, China
- 5Center of Reproductive Medicine, Shengjing Hospital of China Medical University, Shenyang, China
Background: The intake of ultra-processed foods (UPFs) has increased rapidly in recent years. Evidence has suggested that UPFs has adverse effects on several health outcomes. This study aimed to first evaluate the association between the intake of UPFs and asthenozoospermia odds.
Methods: A hospital-based case-control study including 549 cases and 581 controls was performed in the infertility clinics of Shengjing Hospital of China Medical University from June 2020 to December 2020. Dietary intake was assessed using a validated food frequency questionnaire. Food items were categorized by the NOVA classification system based on the degree of processing. Semen parameters were analyzed according to the World Health Organization guidelines.
Results: The highest tertile of UPFs intake (% of total energy intake) was positively associated with the odds of asthenozoospermia (odds ratio [OR] = 1.53; 95% confidence interval [CI]: 1.12, 2.10; P for trend < 0.05), compared with the lowest tertile. Similar patterns were also found in subgroup analyses among participants with age ≥32 years (OR = 1.58; 95% CI: 1.04, 2.40), BMI ≥ 24 kg/m2 (OR = 1.52; 95% CI: 1.04, 2.22), ever cigarette smoking (OR = 1.78; 95% CI: 1.14, 2.79), and ever alcohol drinking (OR = 1.65; 95% CI: 1.01, 2.72), and in sensitivity analyses by using absolute amount (g/day) to calculate the intake of UPFs.
Conclusion: Higher consumption of UPFs was positively associated with the odds of asthenozoospermia. More studies are needed to confirm our findings.
Introduction
Infertility is a major issue of human reproductive health, affecting approximately 15% of couples worldwide (1). Half of all infertility cases are linked to male factors (1, 2). Asthenozoospermia, manifested as decreased sperm motility, is a major pathological indicator of male infertility (3, 4). An epidemiological study from China suggested that the prevalence of asthenozoospermia showed an upward trend during 2008–2016, and it could be more than 50% among 38,905 infertile male patients (5). Although the etiology of asthenozoospermia remains incompletely understood (6), several genetic and environmental factors have been reported to contribute to the risk of asthenozoospermia (7, 8). For example, a DNAH17 missense variant induces flagella destabilization and consequently causes asthenozoospermia (6), and tobacco smoking is associated with decreased sperm motility by impairing mitochondrial function in the sperm tail region (9).
As one of the most important environmental factors, the diet factor has also been proposed to play crucial roles in the etiology of asthenozoospermiais (10, 11). Ultra-processed foods (UPFs) are formulations of ingredients, mostly of exclusive industrial use, that result from a sequence of industrial processes (12), and they have been indicated to have many adverse effects on human health (13, 14). UPFs are often overconsumed due to their presentation, marketing, and the characteristics of highly palatable, attractive, and convenient. The contribution of UPFs can be more than 50% of total daily energy in some high-income countries, such as Britain and the United States (15, 16). Although the consumption of UPFs in China is relatively low, it is increasing rapidly in recent years (17). UPFs are commonly high in added sugar, total fat, saturated fat, and low in protein, dietary fiber, and micronutrients (18, 19), and evidence has suggested that UPFs can create suitable environment for microbes in the gut, then promote some inflammatory disease, such as obesity and metabolic syndrome (20). Notably, inflammation may be the major cause of the development of asthenozoospermia (21). In addition, a case-control study with 107 incident asthenozoospermic men and 235 age-matched controls suggested that the western diet pattern, characterized by high intake of UPFs, was positively associated with asthenozoospermia (odds ratio [OR] = 2.86; 95% confidence interval [CI]: 1.83, 2.97) (22). Thus, the above evidence hints that the intake of UPFs may have an adverse impact on asthenozoospermia.
To the best of our knowledge, there has been no efforts to explore the relationship between the intake of UPF and the odds of asthenozoospermia. Therefore, the purpose of this study was to investigate the potential association of aforementioned topic.
Materials and methods
Study design and participants
This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist (Supplemental Table 1). The details of the study design and participants have been described in previously published studies (3, 11). Briefly, a hospital-based case-control study was performed at Shengjing Hospital of China Medical University, in Liaoning, China. Participants were recruited in the infertility clinics from June, 2020 to December, 2020.
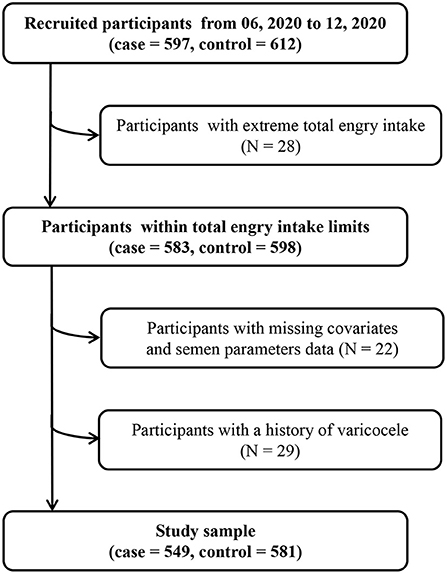
Asthenozoospermia cases (n = 597) were diagnosed according to the fifth edition of World Health Organization laboratory manual for the examination and processing of human semen, and defined as total motility (progressive + non-progressive) <40%, including both rapidly and slowly progressive motility, sluggish motility, and non-progressive motility (23). In addition, asthenozoospermia was also defined as progressive motility <32%, including rapidly and slowly progressive motility, and sluggish motility in the same class within 60 min of ejaculation over the past 3 months (23). The total number (or concentration) of spermatozoa and percentage of morphologically normal spermatozoa was equal to or above lower reference limits (24). Eligible controls (n = 612) were normozoospermic men from infertile couples, with ≥15 × 106 of sperms/mL, ≥40% total motility, ≥32% progressive motility, and ≥4% normal forms (11). According to the sample size calculation method of the unmatched case-control study design, we assumed that OR = 1.5, α = 0.05, β = 0.2, and the proportion of UPF intake in the control group was 67%, the PASS 11.0 software was used to calculate the sample size. The result showed that 469 participants were needed for both case and control groups. We included participants who consented to participate and returned the completed study questionnaire, and we excluded participants with total energy intake outside of predefined limits (total energy intake <800 or >4,200 kcal/d) (N = 28), missing covariates and semen parameters data (N = 22), and a history of varicocele (N = 29). Finally, 549 cases and 581 controls were included in our study and met the requirement of sample size. In addition, the participation rates of cases and controls were 91.96 and 94.93%, respectively (Figure 1). This study was approved by the ethics committee of Shengjing Hospital of China Medical University (No. 2017PS190K).
Semen collection and analysis
Semen samples were obtained by masturbation into a plastic tube in a dedicated semen collection room after 3–7 days abstinence periods. Condoms or lubricants were not allowed to be used. The samples were allowed to be liquefied for 45–60 min before analysis. Ejaculate volume was directly measured, and other semen parameters were measured using the WLJY9000 instrument, such as sperm concentration, total sperm count, motility, and morphology. Sperm DNA fragmentation and high sperm DNA staining were evaluated using BriCyte E6 flow cytometry. Pasteurization was applied for sperm smear observations and the sperm morphology was evaluated using optical microscope. All analyses were carried out by an experienced technician, and an external quality control was carried out throughout the study.
Data collection
Face-to-face interviews were performed by trained professional interviewers using a validated questionnaire. Demographic and lifestyle factors were collected, including age, annual family income (<50, 50 to <100, or ≥100 RMB thousand yuan), educational level (junior secondary or below, senior high school, technical secondary school, or junior college/university or above), dietary intake, dietary change status (yes or no), ever cigarette smoking (yes or no), ever alcohol drinking (yes or no), and physical activity. For dietary change status, participants were asked whether they had changed their diets since this year and before. Cigarette smoking and alcohol drinking were defined as at least one per day and once a week, respectively, for more than six consecutive months. In addition, physical activity was evaluated according to the previous study (25). Briefly, participants were asked the duration of activities spent on work, transportation, housework, and leisure-time exercise in the past 12 months (25), and the metabolic equivalent of tasks (MET) from the 2011 update of a major compendium of physical activities was used to calculate the amount of physical activity (26). Furthermore, physical examination data, such as height and weight, were also measured by trained health professionals. Body mass index (BMI) was used as a noninvasive proxy measure of body fatness and calculated as weight in kilograms divided by the square of the height in meters (kg/m2) (27). According to the cut-off point for Chinese, normal/underweight and overweight/obesity were defined as BMI ≤23.9 and ≥24 kg/m2, respectively (28).
Dietary assessment
A validated semi-quantitative food frequency questionnaire (FFQ) which included 110 food items with specified serving sizes was used to assess dietary intake for participants over the past year. The FFQ used in the present study was based on FFQ used in a large cohort study in the northeast of China (29, 30). The reproducibility and validity of the FFQ used in the current study were similar to FFQ used in previously published literature (11, 30–32). The FFQ included seven frequency categories (“never,” “two to three times per month,” “one time per week,” “two to three times per week,” “four to six times per week,” “one time per day,” and “more than two times per day”) for each food item. Daily consumption for each food item was calculated by multiplying the specified portion size by the frequency at which each food item was consumed per day. Daily intake of nutrients was calculated according to the Chinese Food Composition Table (33), and calculated by multiplying daily consumption for each food item by its nutrient content per gram and then adding the nutrient contributions of all food items.
To assess the overall nutritional quality, principal component analysis was used to generate major dietary patterns and factor loadings for all food items except UPF foods in grams (34). The suitability of data for factor analysis was examined using the Kaiser-Meyer-Olkin (KMO) and Bartlett's test of sphericity (35). The KMO value was 0.905 and Bartlett's test of sphericity was significant (P < 0.001), suggesting that data was suitable for factor analysis. Varimax rotation was used to increase data interpretability (30). After evaluating eigenvalues (>1) and conducting the screen test, five factors were identified. The factors were named descriptively according to the food groups showing high factor loadings (absolute value ≥0.3) in each dietary pattern (36), including vegetables pattern, fruit pattern, fish pattern, processed foods pattern, and tea pattern (Supplemental Table 2).
Exposure assessment
All food items were classified by the NOVA classification system, which categorizes foods into four groups according to the nature, extent, and purpose of food processing (37). Two investigators (J-LL and F-HL) independently classified food items according to previous studies (12, 19), and any discrepancies in this process were resolved by reaching a consensus with the third investigator (Q-JW). The definitions of the four food groups and the classification of food items of the FFQ were shown in Supplemental Table 3. Our study typically focused on the UPFs group, including instant noodles, bread, sausages, preserved egg, western-style pastry or cakes, Chinese pastries or cakes, cookies, sweet snacks or candies or jams, chocolate, ice cream, sesame/soybean paste, vegetable/fruit drinks, and soft drinks. The proportion of UPFs weighted by total energy intake (% total energy intake) for each participant was calculated.
Statistical analysis
The Kolmogorov-Smirnov test was used to assess the normality of continuous variables. To assess the characteristics of participants, continuous variables were all presented as median and interquartile range as they were not normally distributed, and categorical variables were presented as number and percentage. To compare differences between groups, the Kruskal-Wallis was used for continuous variables, and the Chi-square test was used for categorical variables. The proportion of UPFs weighted by total energy intake (% total energy intake) was categorized into three groups according to control distribution with the lowest tertile as the reference group. In the main analysis, Unconditional logistic regression analyses were used to assess associations between UPFs and the odds of asthenozoospermia. ORs and 95% CIs were calculated. The covariates included in the model were determined by univariate logistic regression and published literature for asthenozoospermia (3, 11, 24, 38). Model 1 was adjusted for age (continuous, years), BMI (continuous, kg/m2), and total energy intake (continuous, kcal). Model 2 was further adjusted for annual family income (< 50, 50 to <100, or ≥ 100 RMB thousand yuan), educational level (junior secondary or below, senior high school, technical secondary school, or junior college/university or above), ever cigarette smoking (yes or no), ever alcohol drinking (yes or no), abstinence time (continuous, days), physical activity (continuous, MET/h/week), and dietary change status (yes or no) based on Model 1. To consider the potential effect of nutritional quality, we further adjusted these five dietary patterns (continuous) in Model 3 based on Model 2. There was no collinearity among all variables in our analysis. Linear trends across tertiles were tested by modeling the median value of each tertile as a continuous variable.
Subgroup analyses were performed to examine potential effect modifications according to age (<32 or ≥32 years), BMI (<24 or ≥24 kg/m2), ever cigarette smoking (Yes or No), and ever alcohol drinking (Yes or No). And the likelihood test was used to evaluate potential interactions between UPFs intake and stratification variables.
We additionally evaluated the association between absolute amount of UPFs intake (g/day) and the odds of asthenozoospermia in sensitivity analyses to examine the robustness of the results. In the secondary analysis, we also assessed the effects of unprocessed or minimally processed foods and processed foods on asthenozoospermia. All statistical analyses were performed with SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA). All statistical tests were two sided and statistical significance was set at P < 0.05.
Results
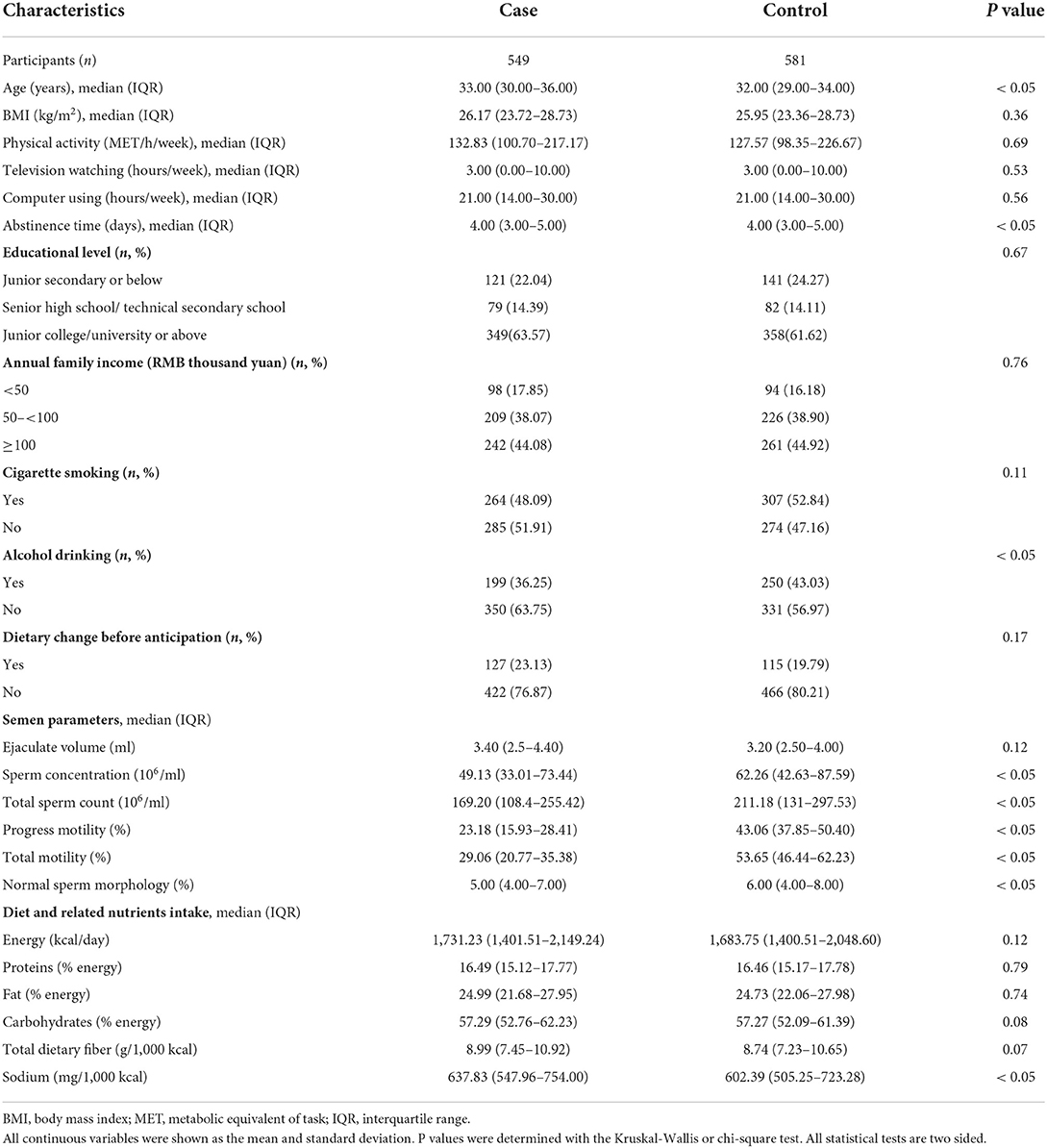
The distribution of general characteristics among cases and controls is shown in Table 1. Compared with controls, cases had significantly (P < 0.05) higher age and abstinence time, but lower proportion of ever alcohol drinking and semen parameters (sperm concentration, total sperm count, progress motility, total motility, and normal sperm morphology). In terms of diet and related nutrients intake, cases had significantly (P < 0.05) higher consumption of sodium than controls.
Table 2 displays the main associations between the intake of UPFs and the odds of asthenozoospermia. We found that the highest tertile of UPFs intake was positively associated with the odds of asthenozoospermia in model 1 (OR = 1.46; 95% CI: 1.08, 1.98; P for trend < 0.05) and model 2 (OR = 1.48; 95% CI: 1.09, 2.00; P for trend < 0.05), compared with the lowest tertile. And this positive association remained significant in the fully adjusted model 3 (OR = 1.53; 95% CI: 1.12, 2.10; P for trend < 0.05).
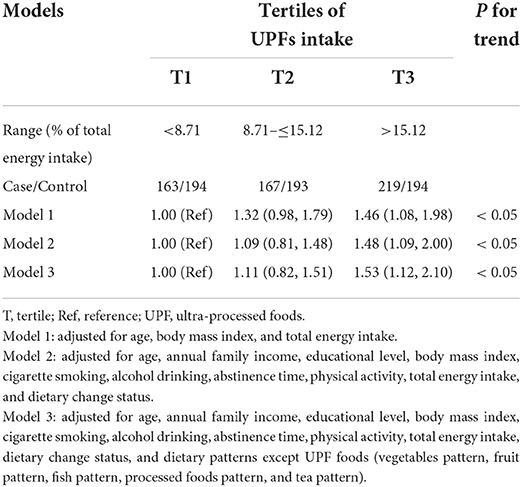
Table 2. Adjusted odds ratio (95% confidence interval) of ultra-processed foods intake and asthenozoospermia.
Table 3 presents the results of subgroup analyses. We observed that the intake of UPFs showed an association with a higher odds of asthenozoospermia among participants with age ≥ 32 years (OR = 1.58; 95% CI: 1.04, 2.40), BMI ≥ 24 kg/m2 (OR = 1.52; 95% CI: 1.04, 2.22), ever cigarette smoking (OR = 1.78; 95% CI: 1.14, 2.79), and ever alcohol drinking (OR = 1.65; 95% CI: 1.01, 2.72). However, no multiplicative interactions were found between UPFs intake and these stratifying variables (all P for interaction > 0.05).
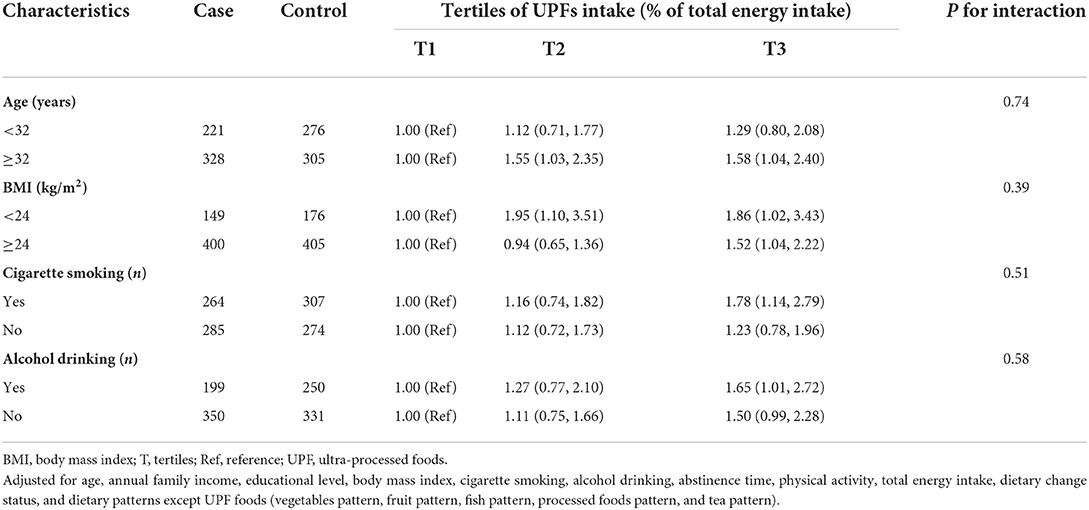
Table 3. Adjusted odds ratio (95% confidence interval) of ultra-processed foods intake and asthenozoospermia stratified by age, BMI, cigarette smoking, and alcohol drinking.
In the fully adjusted model (model 3), the positive association was also observed when evaluating the association between absolute amount of UPFs intake (g/day) and the odds of asthenozoospermia (Supplemental Table 4). In addition, we found that unprocessed or minimally processed foods intake was negatively associated with the odds of asthenozoospermia, and processed foods intake was positively associated with the odds of asthenozoospermia (Supplemental Table 5).
Discussion
In this hospital-based case-control study, to our knowledge, we first found that the highest tertile of UPFs intake was positively associated with the odds of asthenozoospermia, compared with the lowest tertile. Similar patterns were also observed among participants with age ≥ 32 yeas, BMI ≥ 24 kg/m2, ever cigarette smoking, and ever alcohol drinking in the subgroup analyses, and in sensitivity analyses by using absolute amount to calculate UPFs intake.
Although no epidemiological study assessed the association between UPFs intake and the odds of asthenozoospermia, some previous observational studies regarding diet factors and the odds of asthenozoospermia have been in line with our findings (22, 24, 39). Indeed, Eslamian et al. suggested that the higher intake of total saturated fatty acids (OR = 1.85; 95% CI: 1.83, 2.97) and total trans-fatty acids (OR = 2.53; 95% CI: 1.54, 3.92) was positively associated with the odds of asthenozoospermia (24), and the western dietary pattern could increase the odds of asthenozoospermia (OR = 2.86; 95% CI: 1.83, 2.97) (22). Of note, UPFs are characterized by being rich in total saturated fatty acids and trans-fatty acids, and are also representative foods of western dietary pattern. In addition, Eslamian et al. reported that the intakes of some specific UPFs were associated with a higher odds of asthenozoospermia. Indeed, the odds of asthenozoospermia significantly increased in the highest tertile of processed meat (OR = 2.03; 95% CI: 1.70, 2.44) and sweets intake (OR = 2.05, 95% CI: 1.09, 2.26) (39).
Although the underlying mechanism is not yet clear, there have been some possible explanations for the positive association between UPFs intake and the odds of asthenozoospermia. First, UPFs strongly affect the nutritional quality of diets (40), and are rich in energy, fat, and added sugar (40, 41). Evidence suggested that poorer nutritional quality of UPFs may be partly contribute to weight gain (42), and the consumption of energy, fat, and sugar might increase the risk of overweight and obesity (43). Meanwhile, evidence from a large cohort study with 10,665 patients consulting for a semen analysis demonstrated that increased BMI had an adverse effect on semen quality (44), and the result of a label-free quantitative LC-MS/MS proteomic analysis indicated that declines in endoplasmic reticulum protein 57 (ERp57) and actin-binding-related protein T2 (ACTRT2) expression may play critical roles in reducing sperm motility, thereby contributing to obesity-induced asthenozoospermia (45).
Second, UPFs usually contain food additives, and recent studies had indicated that food additives could cause microbiota-mediated adverse effects in the host (20). In fact, emulsifiers can alter microbiota composition and increase its proinflammatory potential, thereby promoting the development of chronic inflammatory diseases (46, 47). Interestingly, a prospective study demonstrated that the dysbiosis of gut microbiota was associated with male infertility (48), and an animal study found that the dysbiosis of gut microbiota was one of the main causes for the impaired spermatogenesis and sperm motility (49). Notably, inflammation seems to play an important role in the etiology of asthenozoospermia (50), and TNF-a as a central regulator of inflammation had been reported significantly higher in asthenozoospermia patients compared with health controls (51). Hence, food additives contained in UPFs may promote inflammatory disorders by causing the dysbiosis of gut microbiota, thereby increasing the risk of asthenozoospermia. At present, food additives are used widely; however, there is still a lack of data on the long-term effects of them on human health, and the potential effects are still largely unknown (42). Therefore, more research on the relationship between food additives and asthenozoospermia is needed in the future.
Third, some contaminants can be formed during the industrial process. Indeed phthalates can migrate from plastic packaging to foods, and act as endocrine disruptors (52). An epidemiological study suggested that there was a positive association between UPFs intake and urinary concentrations of phthalate metabolites (53). And an animal study suggested that mice prenatally exposed to Di-(2-ethylhexyl) phthalate (one of the most used phthalates) displayed a wide range of gonadal and epididymal abnormalities, including asthenozoospermia (54).
Strengths and limitations
Our study had some strengths. First, we provided a new sight for exploring the relationship between dietary factors and asthenozoospermia by considering the degree of food processing. Our findings supported the need for public health policies to limit the intake of ultra-processed foods and increase the intake of unprocessed or minimally processed foods instead. Second, our study had a relatively large sample size and high participation rates, which were conducive to reducing random errors. Third, our study adjusted for a variety of confounding factors to increase the credibility of our conclusions. However, our study also had some limitations. First, our FFQ was not designed by considering the NOVA classification, therefore the degree of processing of several food items was ambiguous. However, it is essential to reasonably recognize food items according to the NOVA classification. In order to reduce the bias caused by misclassification, the food items included in our FFQ were classified by two investigators independently, and any discrepancy was judged by the third investigator. Second, although FFQ was commonly used for dietary assessment, dietary intake of participants could not be calculated accurately based on the standard food portions and relatively limited food items number. Therefore, the measurement bias was unavoidable, and this might have led to the misestimation of the association between UPFs and asthenozoospermia. To reduce the bias, we excluded the participants who reported total energy intake values outside of predefined limits. Third, as a case-control study, selection biases and recall biases could not be avoided, and causation could not be confirmed either. To improve the comparability of case and control, the same clinic controls were applied, and highly trained and skilled researchers were selected to collect information for reducing the recall bias (55, 56). Fourth, although we had made the extensive adjustment, we still could not entirely exclude unmeasured or residual confounding factors, such as genetic factors and air pollution (57, 58). In addition, some important information, such as drug use history, was not considered because they were not cleaned. Last, our study was conducted in Chinese people, therefore the extrapolation of our findings to other populations was limited.
In conclusion, our study indicated that there was a positive association between the intake of UPFs and the odds of asthenozoospermia. Large prospective studies in different populations and settings are needed in the future to confirm our findings.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Shengjing Hospital of China Medical University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
J-LL, Q-JW, X-BW, B-CP, and Y-HZ conceived the study. Q-JW, B-CP, and Y-HZ contributed to the design. X-BW, QD, R-HG, XL, and B-CP collected the data. JL-L and F-HL cleaned the data, checked the discrepancy, and analyzed the data. JL-L, Q-JW, X-BW, and B-CP interpreted the data. All authors interpreted the data, read the manuscript, and approved the final vision.
Funding
This work was supported by the National Key R&D Program of China (No. 2017YFC0907403 to Y-HZ), Shengjing Hospital Clinical Research Project (No. M0071 to B-CP), 345 Talent Project of Shengjing Hospital of China Medical University (Q-JW), the Liaoning Revitalization Talents Program (No. XLYC1802095 to Y-HZ), and the unveiling and leading project of Liaoning province (No. 2021JH1/10400050 to Y-HZ).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.941745/full#supplementary-material
References
1. Jiao SY, Yang YH, Chen SR. Molecular genetics of infertility: loss-of-function mutations in humans and corresponding knockout/mutated mice. Hum Reprod Update. (2021) 27:154–89. doi: 10.1093/humupd/dmaa034
2. Castaneda JM, Hua R, Miyata H, Oji A, Guo Y, Cheng Y, et al. TCTE1 is a conserved component of the dynein regulatory complex and is required for motility and metabolism in mouse spermatozoa. Proc Natl Acad Sci U S A. (2017) 114:E5370–8. doi: 10.1073/pnas.1621279114
3. Liu FH, Wang XB, Wen ZY, Wang HY, Zhang M, Zhang S, et al. Dietary Inflammatory index and risk of asthenozoospermia: a hospital-based case-controlled study in China. Front Nutr. (2021) 8:706869. doi: 10.3389/fnut.2021.706869
4. Dong FN, Amiri-Yekta A, Martinez G, Saut A, Tek J, Stouvenel L, et al. Absence of CFAP69 causes male infertility due to multiple morphological abnormalities of the flagella in human and mouse. Am J Hum Genet. (2018) 102:636–48. doi: 10.1016/j.ajhg.2018.03.007
5. Wu ZG, Chen WK, Fei QJ, Liu YL, Liu XD, Huang H, et al. Analysis of semen quality of 38 905 infertile male patients during 2008-2016 in Wenzhou, China. Asian J Androl. (2021) 23:314–8. doi: 10.4103/aja.aja_83_20
6. Zhang B, Ma H, Khan T, Ma A, Li T, Zhang H, et al. A DNAH17 missense variant causes flagella destabilization and asthenozoospermia. J Exp Med. (2020) 217:e20182365. doi: 10.1084/jem.20182365
7. Jin ZR, Fang D, Liu BH, Cai J, Tang WH, Jiang H, et al. Roles of CatSper channels in the pathogenesis of asthenozoospermia and the therapeutic effects of acupuncture-like treatment on asthenozoospermia. Theranostics. (2021) 11:2822–44. doi: 10.7150/thno.51869
8. Li L, Chen S. Screening, identification and interaction analysis of key MicroRNAs and genes in Asthenozoospermia. Int J Med Sci. (2021) 18:1670–9. doi: 10.7150/ijms.54460
9. Asare-Anane H, Bannison SB, Ofori EK, Ateko RO, Bawah AT, Amanquah SD, et al. Tobacco smoking is associated with decreased semen quality. Reprod Health. (2016) 13:90. doi: 10.1186/s12978-016-0207-z
10. Eslamian G, Amirjannati N, Rashidkhani B, Sadeghi MR, Hekmatdoost A. Nutrient patterns and asthenozoospermia: a case-control study. Andrologia. (2017) 49:12624. doi: 10.1111/and.12624
11. Wang XB, Wu QJ, Liu FH, Zhang S, Wang HY, Guo RH, et al. The Association between dairy product consumption and asthenozoospermia risk: a hospital-based case-control study. Front Nutr. (2021) 8:714291. doi: 10.3389/fnut.2021.714291
12. Monteiro CA, Cannon G, Levy RB, Moubarac JC, Louzada ML, Rauber F, et al. Ultra-processed foods: what they are and how to identify them. Public Health Nutr. (2019) 22:936–41. doi: 10.1017/S1368980018003762
13. Fiolet T, Srour B, Sellem L, Kesse-Guyot E, Alles B, Mejean C, et al. Consumption of ultra-processed foods and cancer risk: results from NutriNet-Sante prospective cohort. BMJ. (2018) 360:k322. doi: 10.1136/bmj.k322
14. Silva Meneguelli T, Viana Hinkelmann J, Hermsdorff HHM, Zulet MA, Martinez JA, Bressan J. Food consumption by degree of processing and cardiometabolic risk: a systematic review. Int J Food Sci Nutr. (2020) 71:678–92. doi: 10.1080/09637486.2020.1725961
15. Monteiro CA, Moubarac JC, Levy RB, Canella DS, Louzada M, Cannon G. Household availability of ultra-processed foods and obesity in nineteen European countries. Public Health Nutr. (2018) 21:18–26. doi: 10.1017/S1368980017001379
16. Poti JM, Mendez MA, Ng SW, Popkin BM. Is the degree of food processing and convenience linked with the nutritional quality of foods purchased by US households? Am J Clin Nutr. (2015) 101:1251–62. doi: 10.3945/ajcn.114.100925
17. Baker P, Machado P, Santos T, Sievert K, Backholer K, Hadjikakou M, et al. Ultra-processed foods and the nutrition transition: Global, regional and national trends, food systems transformations and political economy drivers. Obes Rev. (2020) 21:e13126. doi: 10.1111/obr.13126
18. Monteiro CA, Cannon G, Moubarac JC, Levy RB, Louzada MLC, Jaime PC. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. (2018) 21:5–17. doi: 10.1017/S1368980017000234
19. Zhang S, Gan S, Zhang Q, Liu L, Meng G, Yao Z, et al. Ultra-processed food consumption and the risk of non-alcoholic fatty liver disease in the Tianjin Chronic Low-grade Systemic Inflammation and Health Cohort Study. Int J Epidemiol. (2021) 51:237–49. doi: 10.1093/ije/dyab174
20. Zinocker MK, Lindseth IA. The western diet-microbiome-host interaction and its role in metabolic disease. Nutrients. (2018) 10:365. doi: 10.3390/nu10030365
21. Tronchon V, Vialard F, El Sirkasi M, Dechaud H, Rollet J, Albert M, et al. Tumor necrosis factor-alpha−308 polymorphism in infertile men with altered sperm production or motility. Hum Reprod. (2008) 23:2858–66. doi: 10.1093/humrep/den277
22. Eslamian G, Amirjannati N, Rashidkhani B, Sadeghi MR, Baghestani AR, Hekmatdoost A. Adherence to the western pattern is potentially an unfavorable indicator of asthenozoospermia risk: a case-control study. J Am Coll Nutr. (2016) 35:50–8. doi: 10.1080/07315724.2014.936983
23. Organization WH. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Geneva: World Health Organization. (2010).
24. Eslamian G, Amirjannati N, Rashidkhani B, Sadeghi MR, Baghestani AR, Hekmatdoost A. Dietary fatty acid intakes and asthenozoospermia: a case-control study. Fertil Steril. (2015) 103:190–8. doi: 10.1016/j.fertnstert.2014.10.010
25. Du H, Bennett D, Li L, Whitlock G, Guo Y, Collins R, et al. Physical activity and sedentary leisure time and their associations with BMI, waist circumference, and percentage body fat in 05 million adults: the China Kadoorie Biobank study. Am J Clin Nutr. (2013) 97:487–96. doi: 10.3945/ajcn.112.046854
26. Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR Jr, Tudor-Locke C, et al. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc. (2011) 43:1575–81. doi: 10.1249/MSS.0b013e31821ece12
27. Stierman B, Ogden CL, Yanovski JA, Martin CB, Sarafrazi N, Hales CM. Changes in adiposity among children and adolescents in the United States, 1999-2006 to 2011-2018. Am J Clin Nutr. (2021) 114:1495–504. doi: 10.1093/ajcn/nqab237
28. Zhou BF. Cooperative Meta-Analysis Group of the Working Group on Obesity in China. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults–study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. (2002) 15:83–96.
29. Zhang HH, Zhao YH. Ambient air pollution exposure during pregnancy and gestational diabetes mellitus in Shenyang, China: a prospective cohort study. Environ Sci Pollut Res Int. (2021) 28:7806–14. doi: 10.1007/s11356-020-11143-x
30. Zhang HH, Xia Y, Chang Q, Gao S, Zhao YH. Dietary patterns and associations between air pollution and gestational diabetes mellitus. Environ Int. (2021) 147:106347. doi: 10.1016/j.envint.2020.106347
31. Wang XB, Wu QJ, Guo RH, Leng X, Du Q, Zhao YH, et al. Dairy product consumption and oligo-astheno-teratozoospermia risk: a hospital-based case-control study in china. Front Nutr. (2021) 8:742375. doi: 10.3389/fnut.2021.742375
32. Cui Q, Xia Y, Liu Y, Sun Y, Ye K, Li W, et al. Validity and reproducibility of a food frequency questionnaire for assessing dietary intake among residents of northeast china: northeast cohort study of China. Br J Nutr. (2022) 1−40. doi: 10.1017/S0007114522002318
33. Yang Y, Wang G, Pan X. China Food Composition. 2nd edn. Beijing: Peking University Medical Press. (2009).
34. Wen ZY, Liu C, Liu FH, Wei YF, Xu HL, Wang R, et al. Association between pre-diagnostic dietary pattern and survival of ovarian cancer: evidence from a prospective cohort study. Clin Nutr. (2022) 41:452–9. doi: 10.1016/j.clnu.2021.12.033
35. Bonetto C, Pace D, Bodini L, Colombi M, Van Bortel T, Lasalvia A. Development and psychometric validation of new questionnaires assessing experienced discrimination and internalised stigma among people with COVID-19. Epidemiol Psychiatr Sci. (2022) 31:e37. doi: 10.1017/S204579602200021X
36. Xia Y, Zhang Q, Liu L, Meng G, Wu H, Bao X, et al. Intermediary effect of inflammation on the association between dietary patterns and non-alcoholic fatty liver disease. Nutrition. (2020) 71:110562. doi: 10.1016/j.nut.2019.110562
37. Poti JM, Braga B, Qin B. Ultra-processed food intake and obesity: what really matters for health-processing or nutrient content? Curr Obes Rep. (2017) 6:420–31. doi: 10.1007/s13679-017-0285-4
38. Cui Q, Wang HH, Wu QJ, Wang XB, Guo RH, Leng X, et al. Diet quality scores and asthenoteratozoospermia risk: finding from a hospital-based case-control study in China. Front Nutr. (2022) 9:859143. doi: 10.3389/fnut.2022.859143
39. Eslamian G, Amirjannati N, Rashidkhani B, Sadeghi MR, Hekmatdoost A. Intake of food groups and idiopathic asthenozoospermia: a case-control study. Hum Reprod. (2012) 27:3328–36. doi: 10.1093/humrep/des311
40. Martinez Steele E, Popkin BM, Swinburn B, Monteiro CA. The share of ultra-processed foods and the overall nutritional quality of diets in the US: evidence from a nationally representative cross-sectional study. Popul Health Metr. (2017) 15:6. doi: 10.1186/s12963-017-0119-3
41. Louzada M, Ricardo CZ, Steele EM, Levy RB, Cannon G, Monteiro CA. The share of ultra-processed foods determines the overall nutritional quality of diets in Brazil. Public Health Nutr. (2018) 21:94–102. doi: 10.1017/S1368980017001434
42. Beslay M, Srour B, Méjean C, Allès B, Fiolet T, Debras C, et al. Ultra-processed food intake in association with BMI change and risk of overweight and obesity: a prospective analysis of the French NutriNet-Santé cohort. PLoS Med. (2020) 17:e1003256. doi: 10.1371/journal.pmed.1003256
43. Mendonca RD, Pimenta AM, Gea A, de la Fuente-Arrillaga C, Martinez-Gonzalez MA, Lopes AC, et al. Ultraprocessed food consumption and risk of overweight and obesity: the University of Navarra Follow-Up (SUN) cohort study. Am J Clin Nutr. (2016) 104:1433–40. doi: 10.3945/ajcn.116.135004
44. Belloc S, Cohen-Bacrie M, Amar E, Izard V, Benkhalifa M, Dalléac A, et al. High body mass index has a deleterious effect on semen parameters except morphology: results from a large cohort study. Fertil Steril. (2014) 102:1268–73. doi: 10.1016/j.fertnstert.2014.07.1212
45. Liu Y, Guo Y, Song N, Fan Y, Li K, Teng X, et al. Proteomic pattern changes associated with obesity-induced asthenozoospermia. Andrology. (2015) 3:247–59. doi: 10.1111/andr.289
46. Chassaing B, Van de Wiele T, De Bodt J, Marzorati M, Gewirtz AT. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut. (2017) 66:1414–27. doi: 10.1136/gutjnl-2016-313099
47. Chassaing B, Koren O, Goodrich JK, Poole AC, Srinivasan S, Ley RE, et al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. (2015) 519:92–6. doi: 10.1038/nature14232
48. Lundy SD, Sangwan N, Parekh NV, Selvam MKP, Gupta S, McCaffrey P, et al. Functional and taxonomic dysbiosis of the gut, urine, and semen microbiomes in male infertility. Eur Urol. (2021) 79:826–36. doi: 10.1016/j.eururo.2021.01.014
49. Ding N, Zhang X, Zhang XD, Jing J, Liu SS, Mu YP, et al. Impairment of spermatogenesis and sperm motility by the high-fat diet-induced dysbiosis of gut microbes. Gut. (2020) 69:1608–19. doi: 10.1136/gutjnl-2019-319127
50. Wang Y, Sun Y, Zhao X, Yuan R, Jiang H, Pu X. Downregulation of DJ-1 Fails to Protect Mitochondrial Complex I Subunit NDUFS3 in the Testes and Contributes to the Asthenozoospermia. Mediators Inflamm. (2018) 2018:6136075. doi: 10.1155/2018/6136075
51. Ghandehari-Alavijeh R, Zohrabi D, Tavalaee M, Nasr-Esfahani MH. Association between expression of TNF-α, P53 and HIF1α with asthenozoospermia. Hum Fertil (Camb). (2019) 22:145–51. doi: 10.1080/14647273.2018.1493750
52. Serrano SE, Braun J, Trasande L, Dills R, Sathyanarayana S. Phthalates and diet: a review of the food monitoring and epidemiology data. Environ Health. (2014) 13:43. doi: 10.1186/1476-069X-13-43
53. Buckley JP, Kim H, Wong E, Rebholz CM. Ultra-processed food consumption and exposure to phthalates and bisphenols in the US National Health and Nutrition Examination Survey, 2013-2014. Environ Int. (2019) 131:105057. doi: 10.1016/j.envint.2019.105057
54. Barakat R, Lin PP, Rattan S, Brehm E, Canisso IF, Abosalum ME, et al. Prenatal exposure to DEHP induces premature reproductive senescence in male mice. Toxicol Sci. (2017) 156:96–108. doi: 10.1093/toxsci/kfw248
55. D'Avanzo B, La Vecchia C, Katsouyanni K, Negri E, Trichopoulos D. An assessment, and reproducibility of food frequency data provided by hospital controls. Eur J Cancer Prev. (1997) 6:288–93. doi: 10.1097/00008469-199706000-00006
56. D' Avanzo B, La Vecchia C, Katsouyanni K, Negri E, Trichopoulos D. Reliability of information on cigarette smoking and beverage consumption provided by hospital controls. Epidemiology. (1996) 7:312–5. doi: 10.1097/00001648-199605000-00018
57. Wang W, Tu C, Nie H, Meng L, Li Y, Yuan S, et al. Biallelic mutations in CFAP65 lead to severe asthenoteratospermia due to acrosome hypoplasia and flagellum malformations. J Med Genet. (2019) 56:750–7. doi: 10.1136/jmedgenet-2019-106031
Keywords: asthenozoospermia, case-control study, diet, ultra-processed foods, China
Citation: Lv J-L, Wu Q-J, Wang X-B, Du Q, Liu F-H, Guo R-H, Leng X, Pan B-C and Zhao Y-H (2022) Intake of ultra-processed foods and asthenozoospermia odds: A hospital-based case-control study. Front. Nutr. 9:941745. doi: 10.3389/fnut.2022.941745
Received: 11 May 2022; Accepted: 28 September 2022;
Published: 20 October 2022.
Edited by:
Ghazaleh Eslamian, Shahid Beheshti University of Medical Sciences, IranReviewed by:
Morvarid Noormohammadi, Iran University of Medical Sciences, IranEmilia Ruggiero, Mediterranean Neurological Institute Neuromed (IRCCS), Italy
Copyright © 2022 Lv, Wu, Wang, Du, Liu, Guo, Leng, Pan and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo-Chen Pan, cGFuYmNAc2otaG9zcGl0YWwub3Jn; Yu-Hong Zhao, emhhb3l1aG9uZ0Bzai1ob3NwaXRhbC5vcmc=
†These authors have contributed equally to this work and share first authorship
 Jia-Le Lv1,2,3†
Jia-Le Lv1,2,3† Qi-Jun Wu
Qi-Jun Wu Xiao-Bin Wang
Xiao-Bin Wang Xu Leng
Xu Leng Bo-Chen Pan
Bo-Chen Pan Yu-Hong Zhao
Yu-Hong Zhao