Abstract
Honey has a long history of use for the treatment of digestive ailments. Certain honey types have well-established bioactive properties including antibacterial and anti-inflammatory activities. In addition, honey contains non-digestible carbohydrates in the form of oligosaccharides, and there is increasing evidence from in vitro, animal, and pilot human studies that some kinds of honey have prebiotic activity. Prebiotics are foods or compounds, such as non-digestible carbohydrates, that are used to promote specific, favorable changes in the composition and function of the gut microbiota. The gut microbiota plays a critical role in human health and well-being, with disturbances to the balance of these organisms linked to gut inflammation and the development and progression of numerous conditions, such as colon cancer, irritable bowel syndrome, obesity, and mental health issues. Consequently, there is increasing interest in manipulating the gut microbiota to a more favorable balance as a way of improving health by dietary means. Current research suggests that certain kinds of honey can reduce the presence of infection-causing bacteria in the gut including Salmonella, Escherichia coli, and Clostridiodes difficile, while simultaneously stimulating the growth of potentially beneficial species, such as Lactobacillus and Bifidobacteria. In this paper, we review the current and growing evidence that shows the prebiotic potential of honey to promote healthy gut function, regulate the microbial communities in the gut, and reduce infection and inflammation. We outline gaps in knowledge and explore the potential of honey as a viable option to promote or re-engineer a healthy gut microbiome.
Introduction
Gut microbiota plays a critical role in human health and well-being by aiding digestion, synthesizing vitamins, stimulating the immune system, and protecting against enteropathogenic infections (1–3). Disruptions to the symbiotic relationships within the gut microbiota and with its host, known as dysbiosis, can result in the development and progression of numerous diseases, ranging from inflammatory bowel disease and colon cancer to allergies, obesity, and mental health issues (4–8). As the composition and function of the gut microbiome are significantly influenced by diet (9–13), there is considerable interest in manipulating it to a more beneficial balance through dietary means (1, 14, 15). Prebiotics, which are typically non-digestible carbohydrates and other foodstuffs, have been used to promote specific, favorable changes in the gut that confer health benefits to the host (16). These benefits have been associated with increased numbers of potentially beneficial microbes including bifidobacteria and lactobacilli in the gut, and/or increased production of metabolites like short-chain fatty acids (SCFA) by gut microbes (14).
Honey has a long history of use as a therapeutic agent, including as a tonic to promote good digestive health (17, 18). It is now scientifically established that honey has many therapeutic properties, including antibacterial, anti-inflammatory, wound healing, and antioxidant activities (19, 20). Certain kinds of honey are especially “bioactive,” and this has been linked predominantly to their floral source (21, 22). Honey contains non-digestible oligosaccharides, and growing evidence from in vitro, animal, and pilot human studies suggests that some kinds of honey could have prebiotic capability to induce beneficial changes in the gut. In this paper, we summarize the history and composition of honey as a therapeutic for digestive health, the effect of the gut microbiome on human health and how it can be shaped by diet and prebiotics, and finally, explore the current evidence for, and future potential of, the honey as a prebiotic.
Honey as a Therapeutic Agent Throughout History
Honey in the Human Diet and Its Use for Digestive Health Throughout History
The importance of honey in the diets of human foragers throughout history has been well documented. Honey, as well as residual bee larvae in wild honey, may have been an important source of energy, fat, and protein for early humans (reviewed in (23)). It has been suggested that routine consumption of honey, an energy-dense and easily digestible food source, to supplement meat and plant foods, may have played an important role in shifting the diet from a low-calorie to an energy-rich, calorie-dense diet to support increasing brain activity during the evolution of larger hominin brains (23–25). The reduction of molar size, indicating the consumption of foods requiring less mechanical breakdown, along with the documented use of Oldowan tools (50,000–10,000 BCE) that may have been used for honey collecting as denoted in rock art also support this idea (23).
Honey has a long history as a treatment for gastrointestinal conditions. Circa 25 AD, Roman physicians prescribed different types of honey as a cure for both diarrhea and constipation, and Islamic holy scripts dating back to the 8th century show the prophet Muhammad recommending the use of honey for diarrhea (26, 27). In various books and records from eastern Europe and Arab countries, the use of honey in the prevention and treatment of peptic ulcers, gastritis, and gastroenteritis is often reported (28).
Many modern studies into the digestive health benefits of honey have shown that ingesting honey shortens the duration of bacterial diarrhea in children (29) and in critically ill tube-fed patients who were also reported to be less likely to suffer from organ failure on honey treatment (30). Honey also improved the recovery of patients with viral gastroenteritis (31). Other studies suggest that honey has a protective effect on the stomach (32). The consumption of relatively large amounts of honey (50–100 g) can also have a mild laxative effect, due to insufficient absorption of the fructose in honey (27).
The Composition and Therapeutic Properties of Honey
Honey is a naturally sweet substance produced by honey bees (Apis mellifera) from the nectar of flowers or from plant secretions. The composition of honey is complex with over 200 components, many of which are dependent on the floral source (28). The nectar collected by bees to make honey affects the flavor, color, and medicinal properties of different honeys (21). Honey is composed mostly of sugar (up to 80%) with the monosaccharides fructose and glucose making up the majority (∼70%), and di-, tri-, oligo-, and polysaccharides composing the remainder. Other components of honey include a water content of between 15 and 20%, proteins, organic acids (such as gluconic acid), minerals, plant phytochemicals, and vitamins (25, 33).
Honey has numerous nutritional and therapeutic benefits including antimicrobial, antioxidant, anti-inflammatory, and wound healing activities. Of these, the most extensively studied through in vitro and in vivo experiments and human trials has been antimicrobial activity (19, 22, 27, 34–37). The continued medicinal use of honey as a therapeutic agent can be attributed to its broad-spectrum antimicrobial properties, which have proven effective against many pathogenic organisms, including multi-drug resistant strains. The antimicrobial activity of honey is multi-factorial and is derived from osmolarity, acidity, the production of hydrogen peroxide, and the presence of non-peroxide factors (36). There have been no documented cases of microbial resistance to the inhibitory effects of honey and honey resistance cannot be induced (38–40). This is likely because honey has multiple mechanisms of antimicrobial action (41).
Relevant to the gut, honey inhibits undesirable microbes such as Listeria monocytogenes in milk, as well as Clostridium perfringens and Eubacterium aerofaciens (42). Additionally, honey also inhibits many enteropathogenic organisms, such as Salmonella species (multi-drug resistant strains); Shigella species; enteropathogenic E. coli (including multi-drug resistant strains), Enterobacter species, Yersinia enterocolitica, Campylobacter species, and Clostridium difficile (37, 43–49). Apart from its direct antibacterial activity, honey has been shown to prevent the attachment of Salmonella species to mucosal epithelial cells in vitro, thereby preventing the establishment of infection (50).
The antioxidant effect of honey is largely attributed to its phenolic compounds which, when ingested by an individual, can provide protection in the bloodstream and within cells (51). As with antimicrobial activity, the antioxidant capacity of honey is highly variable and dependent on floral sources. Generally, darker-colored honeys show higher levels of antioxidant activity than their lighter counterparts, as color is also determined by phenolic content. The phenolic content of honey has also been linked to its anti-inflammatory effects, and honey has been reported to downregulate pro-inflammatory cytokines, upregulate anti-inflammatory cytokines (52), and interrupt inflammation mediators (53, 54). Thus, the anti-inflammatory and antioxidant effects of honey are closely linked. The anti-inflammatory, antioxidant, antimicrobial, and wound healing properties of some honeys have been used extensively in the treatment of wounds, burns, and ulcers (20, 55–57); however less is known about their systemic effects when ingested.
Diet and the Gut Microbiome
The Gut Microbiome and Its Contribution to Human Health
The gut microbiome is recognized as playing a significant role in human health. Its composition varies significantly between individuals and within the same individual over time, influenced by factors such as age, sex, ethnicity, geographic location, medication usage, stress, gastrointestinal infections, smoking status, and diet (13, 58–61). Studies have implicated the gut microbiome in brain health and cognitive function, nervous system development and maturation, and the immune system and response, as well as asthma and allergies, cardiovascular health, and obesity (13, 14, 58, 59, 61–66). Consequently, there have been concentrated research efforts to identify a core ‘healthy’ human microbiome (58, 59, 67, 68).
Much of the earlier research was focused on profiling the microbiota of the gut to identify bacterial species and groups associated with beneficial outcomes—that is, probiotic species. Certain types of probiotic gut bacteria, such as bifidobacteria and lactobacilli, have been noted to lessen the severity of symptoms of rotavirus- and antibiotic-associated diarrhea in infants (69), aid in the breakdown of lactose in individuals with lactose intolerance, help with bile deconjugation, promote beneficial organic acid production, and compete with gastroenteritis-causing bacteria to prevent infection (70, 71). In contrast, an ‘unhealthy’ gut microbiome is linked to a reduction of beneficial bacteria, overgrowth of certain fungal species, increase in putrefactive bacteria, and increase in opportunistic pathogens (58). Although the association of specific commensal microbial types in health and disease is recognized, it is not always clear whether the microbes are the cause or effect (72, 73).
However, it is now more commonly accepted that a ‘healthy’ gut microbiome is one that performs desired metabolic functions and has a symbiotic relationship with its host, rather than only specific bacterial populations in greater or lesser numbers (58, 59, 74, 75). Molecular studies confirm that many genes encode for similar microbial functions across different bacterial species, including those associated with degradation and digestion of complex sugars, production of SCFA, energy production, and the synthesis of vitamins (59, 74, 76). A predominance of beneficial microbes, microbial activities, and resultant metabolites, acts to maintain a healthy gut barrier, facilitate immune homeostasis, and host metabolic health. Reductions in beneficial microbial activity in the gut, along with increased intestinal permeability, can increase interactions between microbial antigen and the immune system, triggering inflammatory processes both in the gut and systemically, and contribute to, or drive, poor host health (77). However, the ability to manipulate the gut microbiome using targeted nutritional approaches, which can reduce the severity of disease or improve health outcomes, is a key goal in translating an understanding of the gut microbiome into a therapeutic benefit (5, 73, 78).
The Impact of Diet and Prebiotics on Gut Microbiota
Diet plays a significant role in the functioning and composition of the gut microbiome (14, 79). The impact of diet on the gut microbiome has been shown as early as infancy, where the composition and diversity of the microbiota of breast-fed and formula-fed infants differed significantly (80). Studies have shown that the gut microbiome may co-evolve with diet. A study comparing the diet and gut microbiota of children from Europe and a rural African village showed that the African microbiome had a depletion of Firmicutes and was enriched with Acinetobacteria, Bacteroidetes, and a specific abundance of Xylanibacter and Prevotella that could improve the ability to extract calories from the indigestible plant polysaccharides that contributed to the diet of the African children (10). Long-term dietary patterns, particularly protein and animal fat as compared to carbohydrate/fiber intake, are linked to the assemblage of the gut microbial community and associated with population-wide patterns such as the relative abundance of Bacteroides and Prevotella (81). While the adult microbial community is relatively stable over time and linked to long-term diet (82, 83), it is possible to alter both the compositional makeup and function of the gut microbiota through short-term dietary alteration (84, 85).
Prebiotic foods, such as non-digestible carbohydrates, do not get absorbed in the upper gut and reach the colon intact where they are readily available for use as a selective substrate by gut microbiota. This results in selective stimulation of beneficial microbial populations and functions in the gut (16, 86). Dietary prebiotics have been linked to health-promoting effects including immunostimulation, improved digestion and absorption, vitamin synthesis, reduced cholesterol, reduced gas distension, regulation of opportunistic and invading pathogen growth, improved mineral (especially calcium) absorption, modulation of lipid metabolism via fermentation products, anti-inflammatory activity, and decreased risk of cancer and cardiovascular disease (11, 14, 87–95). The importance of bacterial functions related to carbohydrate metabolism in the colon is well established (4, 96). Indigestible complex carbohydrates, oligosaccharides, polysaccharides, and peptides are major drivers of gut microbial composition and activity (97). As such, there is a great interest in identifying sources of these carbohydrates for use as prebiotics.
The Prebiotic Potential of Honey
Evidence From Laboratory Studies
Although honey is predominantly made up of simple sugars (monosaccharides) that are rapidly absorbed in the small intestine, there are also di-, tri-, and oligosaccharides that are present in smaller quantities (98, 99). These oligosaccharides and low-weight polysaccharides in honey are likely to resist degradation by host enzymes and are capable of reaching the lower gut to exert prebiotic effects (100). Many studies suggest a prebiotic effect of various kinds of honeys of different floral varieties (Table 1). The proposed prebiotic effects of honey, and honey oligosaccharides, are summarized in Figure 1.
TABLE 1
| Honey type and source | Experimental approach | Prebiotic effect reported | References |
| in vitro studies | |||
| Honeydew (Spain) | Fecal bacteria fermentation | Increase in beneficial lactobacilli and bifidobacteria, reduction in enteric bacteria and Bacteroides. | (83) |
| Buckwheat (China) | 16S rDNA sequencing of V4 region | Increase in Bifidobacterium spp. | (84) |
| Juazeiro and Jurema-branca (Brazil) | Broth turbidity assay, with growth measured as turbidity | Increase in viable counts of Bifidobacterium lactis and Lactobacillus acidophilus | (85) |
| Manuka (New Zealand) | Microplate growth bioassay, with growth measured as optical density (turbidity) | Increase in Lactobacillus reuteri, L. rhamnosus and Bifidobacterium lactis. Inhibition of pathogenic bacteria: Escherichia coli, Salmonella typhimurium, and Staphylococcus aureus | (86) |
| Clover (United States) | Microbroth dilution, with growth measured as optical density (turbidity) | Increase in Bifidobacterium longum, B. adolescentis, B. breve, B. bifidum, and B. infantis Equally effective as commercial prebiotics: fructooligosaccharide, galactooligosaccharide, and inulin | (88) |
| Clover (United States) | Microbroth dilution, with growth measured as optical density (turbidity) | Increase in two commercial Bifidobacterium spp. strains (in skim milk supplemented with honey) | (93) |
| Sage, alfalfa and sourwood (United States) | Cultural enumeration (colony counts on agar plates) | Increase in Streptococcus, Lactobacillus, and Bifidobacterium strains | (92) |
| Acacia and chestnut (Saudi Arabia) | Agar disk diffusion assay, cultural enumeration (colony counts on agar plates) | Increase of bifidobacteria and lactobacilli, specifically by reducing doubling time Inhibition of pathogenic Listeria monocytogenes | (91) |
| Acacia and chestnut (Croatia) | Agar disk diffusion assay, cultural enumeration (colony counts on agar plates) | Increase in Bifidobacterium lactis | (94) |
| Unidentified floral source (India) | Viable colony counts on agar plates using bifidobacteria isolated from infant fecal samples, and identified via phenotypic and molecular (PCR) methods | Increase in all Bifidobacterium isolates | (124) |
| Sourwood, alfalfa, and sage (Unspecified) | Microbroth dilution, with growth measured as optical density (turbidity) | Increase in five Bifidobacterium species of human intestinal origin (B. longum, B. adolescentis, B. breve, B. bifidum, and B. infantis) Inhibition of C. perfringens and E. aerofaciens. | (96) |
| Unidentified floral source (Jordan) | Colony counts (CFU/ml) calculated from optical density (turbidity) readings | Significant increase in Bifidobacterium infantis and Lactobacillus acidophilus of intestinal origin | (97) |
| Tualang and multifloral (Malaysia) | Honey samples pre-treated to remove simple sugars, remaining fraction used to supplement skim milk; bacterial enumeration (colony counts on agar plates) | Increase in Bifidobacterium longum by all honey fractions with simple sugars removed | (112) |
| Clover (Unspecified) | Growth of probiotic pure cultures in skim milk supplemented with various sweeteners measured via cultural enumeration (colony counts on agar) | Honey best supports growth of probiotic strains, with significant increase in Bifidobacterium bifidum and Lactobacillus acidophilus numbers | (95) |
| in vivo and human studies | |||
| Generic, unknown floral source (India) | Wistar strain male albino rats (n = 36); small and large intestine collection, suspension and viable cell count | Increase in Lactobacillus acidophilus and Lactobacillus plantarum | (87) |
| Cotton (Egypt) | Swiss male albino mice (n = 42); cecum content collection, viable cell counts (bacterial enumeration on agar) of colonic bacteria | Increase in Bifidobacterium and Lactobacilli | (89) |
| Jarrah (Australian floral source, purchased in China) | BALB/c mice (n = 30); 16S rRNA sequencing of V3–V4 region Fecal water content measured via weighing fecal samples before and after drying | Gut microbiota equilibrium re-established, specifically by increasing abundance of key bacterial groups in the gut, and suppressing harmful bacteria Improvement in fecal water content, linked to alleviation of constipation | (90) |
| Prunella vulgaris, common name ‘self-heal’ (China) | Sprague Dawley male rats (n = 24) with induced colitis; histological analysis of colon samples, intestinal mRNA analysis, gut microbial community analysis (from caeca) via 16S rRNA sequencing of the V3–V4 region | Decrease in Bacteroidetes, and increase in Firmicutes; and at genus level increases in the beneficial Lactobacillus spp., and decrease in Lachnospiraceae, which is associated with the pathological features of colitis Overall reduction of symptoms associated with ulcerative colitis, mostly attributed to the abitlity of honey to modulate effects on gut microbiota | (113) |
| Unidentified floral source (Indonesia) | Pacific white shrimp fed honey (prebiotic), probiotic culture or synbiotic (combination of probiotic culture and honey); intestinal microbiota diversity analysis via DNA sequencing | Honey treatment most effective, showing increased intestinal microbiota diversity, and higher genus level abundance of beneficial (probiotic) bacteria Honey-fed shrimp showed highest survival rate post infection with Vibrio parahaemolyticus | (115) |
| Manuka (New Zealand) and multifloral (unspecified) | Pilot human clinical study where participants consumed daily dose (20 g) of honey; DNA from fecal sample sequenced for microbiota analysis | No significant changes (positive or negative) in gut microbiota populations, no antimicrobial effects of manuka honey on the beneficial populations of the gut | (119) |
Summary of the studies showing prebiotic effects of various honeys.
FIGURE 1
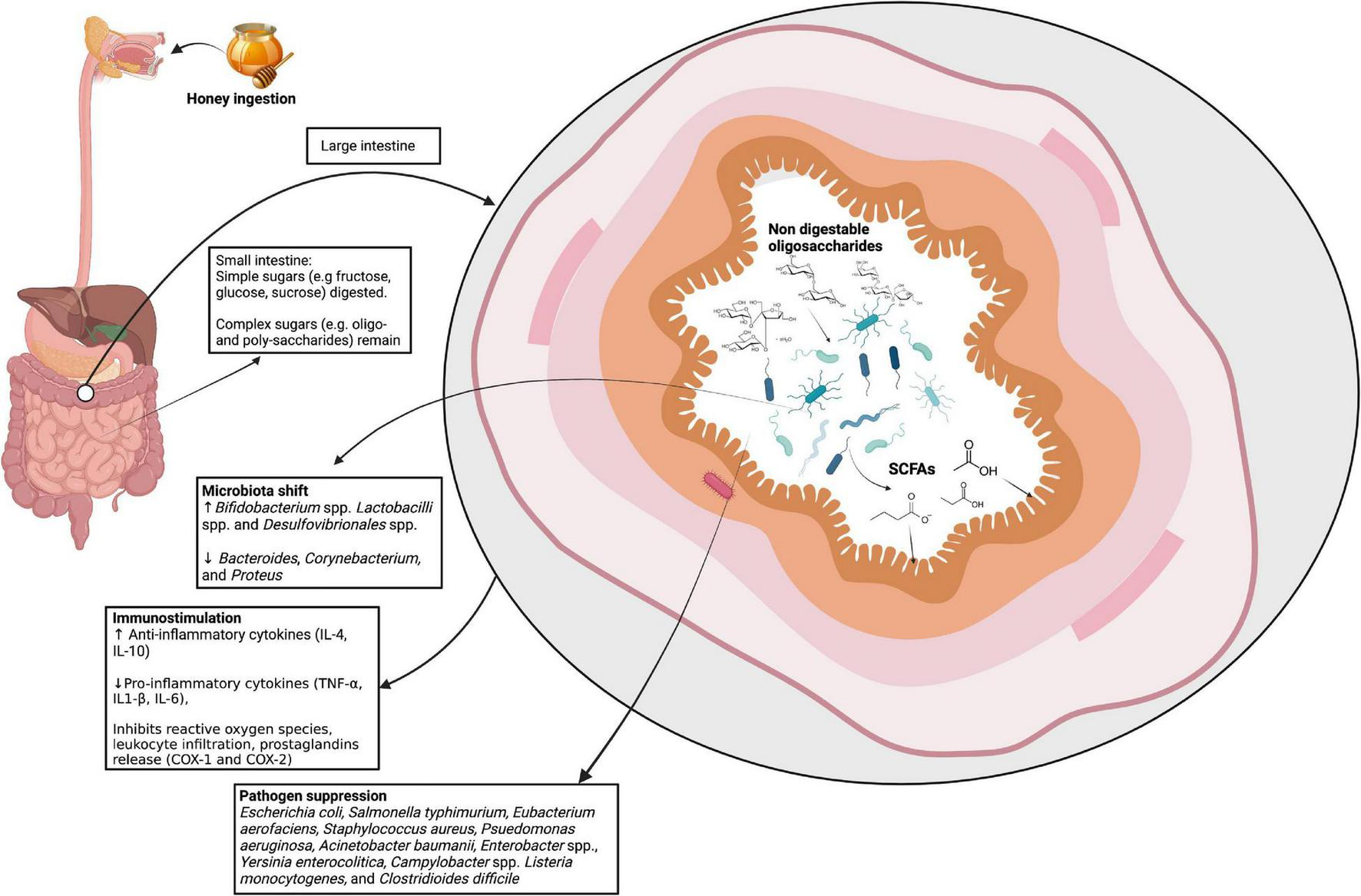
The proposed prebiotic effects of honey. Following ingestion, the simple sugars in honey are absorbed in the small intestine. The non-digestible components, including oligosaccharides, reach the lower intestines where they are proposed to be involved in immunostimulation, modulating the microbiota, and suppressing pathogens. SCFAs, short-chain fatty acids; IL, interleukin; TNF, tumor necrosis factor; COX, cyclooxegenase. Image created with BioRender.com.
There is significant evidence of the prebiotic potential of honey from in vitro studies that assess the effect of honey on the growth of probiotic bacteria (100–107) and in probiotic food products, such as milk or yogurt, supplemented with honey (108–111). Numerous studies show that honey supports and promotes the growth of probiotic Bifidobacterium and Lactobacillus species, including B. longum, B. adolescentis, B. breve, B. bifidum, and B. infantis, Lactobacillus. acidophilus, Lactobacillus plantarum, Lactobacillus reuteri, and Lactobacillus rhamnosus (103–107, 113). The growth-promoting effect of honey on bifidobacteria and lactobacilli is usually comparable to that of oligosaccharide prebiotics, including fructooligosaccharide (FOS), galactooligosaccharide (GOS), or inulin, where these prebiotics are included as controls (42, 104, 105, 110, 112, 113). Other studies have shown that honey not only promotes the growth of probiotic cultures but has a positive effect on the metabolism of bacterial strains from the human gut (95).
As oligosaccharide composition can affect prebiotic activity, it is not surprising that different honeys can have different prebiotic properties (114). Honey can contain source-specific oligosaccharides (99)for example, native New Zealand honeys showed high levels of isomaltose and melezitose (114, 115), while raffinose was reported in Italian honey (116); and also different concentrations of commonly occurring oligosaccharides (107) influencing their prebiotic potential.
Oligosaccharides isolated from honeydew had a positive impact on the growth of fecal bacteria, specifically by promoting the populations of the beneficial bifidobacteria and lactobacilli, and by reducing the numbers of the potentially harmful Bacteroides and clostridia (100), quantified by the prebiotic index that scores the ratio of potentially beneficial vs. harmful bacteria relative to the overall changes (117). The prebiotic index for the honey-derived oligosaccharides was similar to that of the commercial prebiotic, FOS. Similarly, three Malaysian Tualang honeys that had been pre-treated to remove simple sugars supported enhanced growth of the probiotic Bifidobacterium longum (118).
Evidence From Animal Studies and Pilot Human Trials
Numerous in vivo studies using animal models show that honey acts as a prebiotic, specifically by promoting the populations of probiotic bacteria, including Bifidobacterium spp. and Lactobacillus spp., (104, 106, 107, 119), and alleviating symptoms of constipation and ulcerative colitis (107, 119). The prebiotic effect of honey has also been reported in shrimp, where honey promoted the growth of known probiotics Microbavterium spp., Lactobacillus spp., and Neptumonas spp. (120). Shrimp receiving the honey prebiotic also had a higher abundance of gut microbes than the control or shrimp receiving either a probiotic or synbiotic. Another study investigating the prebiotic effect of honey on pacific white shrimp with Vibrio parahaemolyticus infection showed that those that were fed honey during the infection phase had a reduced pathogen load and higher survival rate compared to the control (no treatment) group (121).
The anti-inflammatory effect of honey can also contribute to its overall prebiotic potential, as many conditions in the gut (regardless of infection state) involve inflammation of the bowels. Various studies on the anti-inflammatory properties of honey, spanning both the gut and wound environment, suggest that honey promotes the upregulation of anti-inflammatory cytokines and downregulation of pro-inflammatory cytokines (38, 52, 53, 122, 123). In rats with acetic acid-induced gastric ulcers, a significant increase in the presence of pro-inflammatory cytokines tumor necrosis factor (TNF)-α, interleukin (IL)1-β, and IL-6 was noted. Following administration of manuka honey treatment, cytokine levels significantly decreased, the ulcers healed faster, and oxidative damage caused by acetic acid was reversed compared to the control group (122). Similarly, rats with dextran sodium sulfate-induced ulcerative colitis had a significant reduction in IL-1β and IL-6 in serum and TNF-α in colonic tissue samples after administration of Egyptian honey (124). The mechanisms suggested for inflammation reduction by honey include inhibition of reactive oxygen species, inhibition of leukocyte infiltration, inhibition of cyclooxygenase-1 and 2 (COX-1 and COX-2), and inducible nitric oxide synthase expression (53, 123). The main components in honey responsible for the anti-inflammatory and related antioxidant effects are the polyphenols, and polyphenols found in honey have been shown to alter the gut microbiome in rats with ulcerative colitis, showing both a reduction in inflammation and suppression of the populations of the potentially harmful organisms (54).
To date, there has been one human clinical study investigating the effect of daily honey consumption – specifically looking at the safety of eating manuka honey with high antibacterial activity compared to multi-floral honey (125). No significant changes in the numbers of five major bacterial groups in the gut were found, however, measuring prebiotic activity was not a primary aim of the study and the authors noted that any effects may have been masked due to interactions with other dietary components, the dose of honey used, as well as honey and storage conditions.
Gaps and Emerging Opportunities in the Study of Prebiotic Honey
Despite current marketing and increased consumer interest around “prebiotic honey,” there are limited published studies and human response data in this research area. The bioactive components in honey responsible for its prebiotic effect have not been fully identified. Additionally, whether honey can act as a prebiotic to remediate the gut microbiome in a state of dysbiosis, such as during infection or when the bowels are inflamed, is not well understood.
Although the variable composition and therapeutic properties of honey complicate mechanistic studies of its bioactivity, it provides the opportunity for a targeted approach for different health purposes, particularly given the antimicrobial, anti-inflammatory, and prebiotic potential of honey. These bioactivities can be aligned with the emerging area of personalized medicine, which focuses on enabling more targeted therapeutic treatment and preventative options for individuals (126).
Many chronic gut-related conditions, such as irritable bowel syndrome, colon cancer, Crohn’s disease, and C. difficile infection, are known to be exacerbated by inflammation of the bowels (127–129). Current therapies, in particular for irritable bowel syndrome and inflammatory bowel disease, include reducing foods that contribute to inflammation. The antibacterial and anti-inflammatory activity of honey is well documented throughout the literature (19, 33) and this combined with a prebiotic activity could place honey as a suitable treatment option to benefit the microbiota and reduce inflammation of the gut. As the health of gut microbiota is a key element in understanding whole-body health and is readily manipulated, targeted dietary interventions that alter the microbiome represent a strategy of significant benefit. Honey represents an attractive option in this space and with further validation could provide a means to benefit the gut microbiome in a healthy state and to remediate the microbiome from a dysbiotic state.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Author contributions
All authors listed have made a substantial, direct, and intellectual contributions to the work, and approved it for publication.
Funding
Funding for the current prebiotic honey research projects undertaken by our team was provided under the AgriFutures Australia Honey Bee & Pollination Program (Grant PRJ- 012227) and the NSW Bushfire Industry Recovery Package Sector Development Grants (BIP-SDG-135).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
GibsonGRBeattyERWangXCummingsJH. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin.Gastroenterology. (1995) 108:975–82. 10.1016/0016-5085(95)90192-2
2.
BäckhedFLeyRESonnenburgJLPetersonDAGordonJI. Host-bacterial mutualism in the human intestine.Science. (2005) 307:1915–20. 10.1126/science.1104816
3.
LeyREBäckhedFTurnbaughPLozuponeCAKnightRDGordonJI. Obesity alters gut microbial ecology.Proc Natl Acad Sci USA. (2005) 102:11070–5. 10.1073/pnas.0504978102
4.
KurokawaKItohTKuwaharaTOshimaKTohHToyodaAet alComparative metagenomics revealed commonly enriched gene sets in human gut microbiomes.DNA Res. (2007) 14:169–81. 10.1093/dnares/dsm018
5.
HaEM. The impact of gut microbiota in human health and diseases: implication for therapeutic potential.Biomol Ther. (2011) 19:155–73. 10.4062/biomolther.2011.19.2.155
6.
GentschewLFergusonLR. Role of nutrition and microbiota in susceptibility to inflammatory bowel diseases.Mol Nutr Food Res. (2012) 56:524–35. 10.1002/mnfr.201100630
7.
KnightRCallewaertCMarotzCHydeERDebeliusJWMcDonaldDet alThe microbiome and human biology.Annu Rev Genom Hum Genet. (2017) 18:65–86. 10.1146/annurev-genom-083115-022438
8.
Selber-HnativSRukundoBAhmadiMAkoubiHAl-BizriHAliuAFet alHuman gut microbiota: toward an ecology of disease.Front Microbiol. (2017) 8:1265. 10.3389/fmicb.2017.01265
9.
FlintHJDuncanSHScottKP. Louis :interactions and competition within the microbial community of the human colon: links between diet and health: minireview.Environ Microbiol. (2007) 9:1101–11. 10.1111/j.1462-2920.2007.01281.x
10.
de FilippoCCavalieriDdi PaolaMRamazzottiMPoulletJBMassartSet alImpact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa.Proc Natl Acad Sci USA. (2010) 107:14691–6. 10.1073/pnas.1005963107
11.
DewulfEMCaniPDClausSPFuentesSPuylaertPGBNeyrinckAMet alInsight into the prebiotic concept: lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women.Gut. (2013) 62:1112–21. 10.1136/gutjnl-2012-303304
12.
SommerFBäckhedF. The gut microbiota-masters of host development and physiology.Nat Rev Microbiol. (2013) 11:227–38. 10.1038/nrmicro2974
13.
CaniPD. Human gut microbiome: hopes, threats and promises.Gut. (2018) 67:1716–25. 10.1136/gutjnl-2018-316723
14.
GibsonGRScottKPRastallRATuohyKMHotchkissADubert-FerrandonAet alDietary prebiotics: current status and new definition.Food Sci Technol Bull. (2010) 7:1–19. 10.1616/1476-2137.15880
15.
RauchMLynchSV. The potential for probiotic manipulation of the gastrointestinal microbiome.Curr Opin Biotechnol. (2012) 23:192–201. 10.1016/j.copbio.2011.11.004
16.
GibsonGRHutkinsRSandersMEPrescottSLReimerRASalminenSJet alExpert consensus document: the international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics.Nat Rev Gastroenterol Hepatol. (2017) 14:491–502. 10.1038/nrgastro.2017.75
17.
El-soudNHA. Honey between traditional uses and recent medicine.Maced J Med Sci. (2012) 5:205–14.
18.
KuropatnickiAKKłósekMKucharzewskiM. Honey as medicine: historical perspectives.J Apic Res. (2018) 57:113–8. 10.1080/00218839.2017.1411182
19.
Alvarez-SuarezJMTulipaniSRomandiniSBertoliEBattinoM. Contribution of honey in nutrition and human health: a review.Med J Nutrition Metab. (2010) 3:15–23. 10.1007/s12349-009-0051-6
20.
MolanPC. Why honey is effective as a medicine: 2. The scientific explanation of its effects.Bee World. (2001) 82:22–40. 10.1080/0005772X.2001.11099498
21.
IrishJBlairSCarterDA. The antibacterial activity of honey derived from Australian flora.PLoS One. (2011) 6:e18229. 10.1371/journal.pone.0018229
22.
CarterDABlairSECokcetinNNBouzoDBrooksPSchothauerRet alTherapeutic manuka honey: no longer so alternative.Front Microbiol. (2016) 7:569. 10.3389/fmicb.2016.00569
23.
CrittendenAN. The importance of honey consumption in human evolution.Food Foodways. (2011) 19:257–73. 10.1080/07409710.2011.630618
24.
AllsopKAMillerJB. Honey revisited: a reappraisal of honey in pre-industrial diets.Br J Nutr. (1996) 75:513–20. 10.1079/BJN19960155
25.
SabaZSuzanaMAnumMY. Honey: food or medicine.Med Health. (2013) 2013:3–18.
26.
CraneE.The World History of Beekeeping and Honey Hunting.London: Duckworth (1999).
27.
BogdanovSJurendicTSieberRGallmannP. Honey for nutrition and health: a review.J Am Coll Nutr. (2008) 27:677–89. 10.1080/07315724.2008.10719745
28.
CraneE.A Book of Honey.Oxford: Oxford University Press (1980).
29.
HaffejeeIEMoosaA. Honey in the treatment of infantile gastroenteritis.Br Med J. (1985) 290:1866–7. 10.1136/bmj.290.6485.1866
30.
ShariatpanahiZVJamshidiFNasrollahzadehJAmiriZTeymourianH. Effect of Honey on diarrhea and fecal microbiotain in critically Ill tube-fed patients: a single center randomized controlled study.Anesth Pain Med. (2018) 8:62889.
31.
SalemSN. Honey regimen in gastrointestinal disorders.Bull Islam Med. (1981) 1:358–62.
32.
Al-SwayehOAMobarok AliATM. Effect of ablation of capsaicin-sensitive neurons on gastric protection by honey and sucralfate.Hepatogastroenterology. (1998) 45:297–302.
33.
MiguelMGAntunesMDFaleiroML. Honey as a complementary medicine.Integr Med Insights. (2017) 12:1–15. 10.1177/1178633717702869
34.
BogdanovS. Nature and origin of the antibacterial substances in honey.LWT Food Sci Technol. (1997) 30:748–53. 10.1006/fstl.1997.0259
35.
MolanPC. A brief review of the use of honey as a clinical dressing.Aust J Wound Manag. (1998) 6:148–58.
36.
MolanPC. Honey as an antimicrobial agent. In: MizrahiALenskyYeditors.Bee Products.Boston, MA: Springer (1997). p. 27–37. 10.1007/978-1-4757-9371-0_3
37.
WillixDJMolanPCHarfootCG. A comparison of the sensitivity of wound-infecting species of bacteria to the antibacterial activity of manuka honey and other honey.J Appl Bacteriol. (1992) 73:388–94. 10.1111/j.1365-2672.1992.tb04993.x
38.
BlairSECokcetinNNHarryEJCarterDA. The unusual antibacterial activity of medical-grade Leptospermum honey: antibacterial spectrum, resistance and transcriptome analysis.Eur J Clin Microbiol Infect Dis. (2009) 28:1199–208. 10.1007/s10096-009-0763-z
39.
CooperRAJenkinsLHenriquesAFMDugganRSBurtonNF. Absence of bacterial resistance to medical-grade manuka honey.Eur J Clin Microbiol Infect Dis. (2010) 29:1237–41. 10.1007/s10096-010-0992-1
40.
MaddocksSEJenkinsRE. Honey: a sweet solution to the growing problem of antimicrobial resistance?Future Microbiol. (2013) 8:1419–29. 10.2217/fmb.13.105
41.
BouzoDCokcetinNNLiLBallerinGBottomleyALLazenbyJet alCharacterizing the mechanism of action of an ancient antimicrobial, manuka honey, against Pseudomonas aeruginosa using modern transcriptomics.mSystems. (2020) 5:106–20. 10.1128/mSystems.00106-20
42.
ShinHSUstunolZ. Carbohydrate composition of honey from different floral sources and their influence on growth of selected intestinal bacteria: an in vitro comparison.Food Res Int. (2005) 38:721–8. 10.1016/j.foodres.2005.01.007
43.
MolanPC. Why honey is effective as a medicine. 2. The scientific explanation of its effects.Am J Clin Dermatol. (2001) 2:13–9.
44.
AdeboluTT. Effect of natural honey on local isolates of diarrhea-causing bacteria in Southwestern Nigeria.Afr J Biotechnol. (2005) 4:1172–4.
45.
BadawyOFShafiiSSATharwatEEKamalAM. Antibacterial activity of bee honey and its therapeutic usefulness against Escherichia coli O157: H7 and Salmonella typhimurium infection.Rev Sci Tech. (2004) 23:1011–22. 10.20506/rst.23.3.1543
46.
Al-WailiNSAkmalMSaloomKYAl-WailiFSAliA. The antimicrobial potential of honey from United Arab Emirates on some microbial isolates.Med Sci Monit. (2005) 11:433–8.
47.
LinSMMolanPCCursonsRT. The controlled in vitro susceptibility of gastrointestinal pathogens to the antibacterial effect of manuka honey.Eur J Clin Microbiol Infect Dis. (2011) 30:569–74. 10.1007/s10096-010-1121-x
48.
LinSMMolanPCCursonsRT. The in vitro susceptibility of Campylobacter sp:to the antibacterial effect of manuka honey.Eur J Clin Microbiol Infect Dis. (2009) 28:339–44. 10.1007/s10096-008-0630-3
49.
HammondENDonkorES. Antibacterial effect of manuka honey on Clostridium difficile.BMC Res Notes. (2013) 6:188. 10.1186/1756-0500-6-188
50.
AlnaqdyAAl-JabriAMahrooqiZNzeakoBNsanzeH. Inhibition effect of honey on the adherence of Salmonella to intestinal epithelial cells in vitro.Int J Food Microbiol. (2005) 103:347–51. 10.1016/j.ijfoodmicro.2004.11.042
51.
SchrammDDKarimMSchraderHRHoltRRCardettiMKeenCL. Honey with high levels of antioxidants can provide protection to healthy human subjects.J Agric Food Chem. (2003) 51:1732–5. 10.1021/jf025928k
52.
RannehYAkimAMHamidHAKhazaaiHFadelAZakariaZAet alHoney and its nutritional and anti-inflammatory value.BMC Complement Med Ther. (2021) 21:30. 10.1186/s12906-020-03170-5
53.
VallianouNG. Honey and its anti-inflammatory, anti-bacterial and anti-oxidant properties.Gen Med Open Access. (2014) 2:132. 10.4172/2327-5146.1000132
54.
ZhaoHChengNZhouWChenSWangQGaoHet alHoney polyphenols ameliorate DSS-induced ulcerative colitis via modulating gut microbiota in rats.Mol Nutr Food Res. (2019) 63:1900638. 10.1002/mnfr.201900638
55.
MolanPC. Selection of honey for use as a wound dressing.Primary Infection. (2000) 8:87–92.
56.
MolanPC. The role of honey in the management of wounds.J Wound Care. (1999) 8:415–8. 10.12968/jowc.1999.8.8.25904
57.
MolanPC. Re-introducing honey in the management of wounds and ulcers – theory and practice.Ostomy Wound Manage. (2002) 48:28–40.
58.
GagliardiATotinoVCacciottiFIebbaVNeroniBBonfiglioGet alRebuilding the gut microbiota ecosystem.Int J Environ Res Public Health. (2018) 15:1679. 10.3390/ijerph15081679
59.
DaveMHigginsPDMiddhaSRiouxK. The human gut microbiome: current knowledge, challenges, and future directions.Transl Res. (2012) 160:246–57. 10.1016/j.trsl.2012.05.003
60.
GaulkeCASharptonTJ. The influence of ethnicity and geography on human gut microbiome composition.Nat Med. (2018) 24:1495–6. 10.1038/s41591-018-0210-8
61.
CaniPDEverardA. Talking microbes: when gut bacteria interact with diet and host organs.Mol Nutr Food Res. (2016) 60:58–66. 10.1002/mnfr.201500406
62.
KeerthiTRNarayananRSreelekshmiKHoney ChandranC. Immunity and gut microbiome: role of probiotics and prebiotics. In: NaheedMMaryamDeditors.Probiotic Bacteria and Postbiotic Metabolites: Role in Animal and Human Health. (Berlin: Springer Nature) (2021). p. 61–83. 10.1007/978-981-16-0223-8_2
63.
ZahirFAlhewairiniSSMahamoodM. The gut–brain axis, cognition and honey. In: RehmanMUMajidSeditors.Therapeutic Applications of Honey and its Phytochemicals. (Singapore: Springer) (2020). 10.1007/978-981-15-6799-5_17
64.
CarabottiMSciroccoAMaselliMASeveriC. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems.Ann Gastroenterol. (2015) 28:203–9.
65.
ZhangDLiSWangNTanHYZhangZFengY. The cross-talk between gut microbiota and lungs in common lung diseases.Front Microbiol. (2020) 11:301. 10.3389/fmicb.2020.00301
66.
ChunxiLHaiyueLYanxiaLJianbingPJinS. The gut microbiota and respiratory diseases: new evidence.J Immunol Res. (2020) 2020:2340670. 10.1155/2020/2340670
67.
NeuATAllenEERoyK. Defining and quantifying the core microbiome: challenges and prospects.Proc Natl Acad Sci USA. (2021) 118:2104429118. 10.1073/pnas.2104429118
68.
RinninellaERaoulPCintoniMFranceschiFMiggianoGADGasbarriniAet alWhat is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases.Microorganisms. (2019) 7:1–14. 10.3390/microorganisms7010014
69.
O’CallaghanAvan SinderenD. Bifidobacteria and their role as members of the human gut microbiota.Front Microbiol. (2016) 7:925. 10.3389/fmicb.2016.00925
70.
SandineWE. Roles of Lactobacillus in the intestinal tract1.J Food Prot. (1979) 42:259–62. 10.4315/0362-028X-42.3.259
71.
ReidGBurtonJ. Use of Lactobacillus to prevent infection by pathogenic bacteria.Microbes Infect. (2002) 4:319–24. 10.1016/S1286-4579(02)01544-7
72.
ThomasLVOckhuizenT. New insights into the impact of the intestinal microbiota on health and disease: a symposium report.Br J Nutr. (2012) 107:S1–13. 10.1017/S0007114511006970
73.
CeapaCWopereisHRezaïkiLKleerebezemMKnolJOozeerR. Influence of fermented milk products, prebiotics and probiotics on microbiota composition and health.Best Pract Res Clin Gastroenterol. (2013) 27:139–55. 10.1016/j.bpg.2013.04.004
74.
Heintz-BuschartAWilmesP. Human gut microbiome: function matters.Trends Microbiol. (2018) 26:563–74. 10.1016/j.tim.2017.11.002
75.
ShanahanFGhoshTSO’ToolePW. The healthy microbiome—what is the definition of a healthy gut microbiome?Gastroenterology. (2021) 160:483–94. 10.1053/j.gastro.2020.09.057
76.
QinJLiRRaesJArumugamMBurgdorfSManichanhCet alA human gut microbial gene catalog established by metagenomic sequencing.Nature. (2010) 464:59–65. 10.1038/nature08821
77.
GasalyNde VosPHermosoMA. Impact of bacterial metabolites on gut barrier function and host immunity: a focus on bacterial metabolism and its relevance for intestinal inflammation.Front Immunol. (2021) 12:658354. 10.3389/fimmu.2021.658354
78.
WolterMGrantETBoudaudMSteimleAPereiraGVMartensECet alLeveraging diet to engineer the gut microbiome.Nat Rev Gastroenterol Hepatol. (2021) 18:885–902. 10.1038/s41575-021-00512-7
79.
SonnenburgJLBäckhedF. Diet-microbiota interactions as moderators of human metabolism.Nature. (2016) 535:56–64. 10.1038/nature18846
80.
SchwartzSFriedbergIIvanovIVDavidsonLAGoldsbyJSDahlDBet alA metagenomic study of diet-dependent interaction between gut microbiota and host in infants reveals differences in immune response.Genome Biol. (2012) 13:32. 10.1186/gb-2012-13-4-r32
81.
WuGDChenJHoffmannCBittingerKChenYYKeilbaughSAet alLinking long-term dietary patterns with gut microbial enterotypes.Science 2011. (1979) 334:105–8. 10.1126/science.1208344
82.
FragiadakisGKWastykHCRobinsonJLSonnenburgEDSonnenburgJLGardnerCD. Long-term dietary intervention reveals resilience of the gut microbiota despite changes in diet and weight.Am J Clin Nutr. (2020) 111:1127–36. 10.1093/ajcn/nqaa046
83.
LeemingERJohnsonAJSpectorTDRoyCIL. Effect of diet on the gut microbiota: rethinking intervention duration.Nutrients. (2019) 11:2862. 10.3390/nu11122862
84.
DavidLAMauriceCFCarmodyRNGootenbergDBButtonJEWolfeBEet alDiet rapidly and reproducibly alters the human gut microbiome.Nature. (2014) 505:559–63. 10.1038/nature12820
85.
TanesCBittingerKGaoYFriedmanESNesselLPaladhiURet alRole of dietary fiber in the recovery of the human gut microbiome and its metabolome.Cell Host Microbe. (2021) 29:392–407. 10.1016/j.chom.2020.12.012
86.
GibsonGRRoberfroidMB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics.J Nutr. (1995) 125:1401–12. 10.1093/jn/125.6.1401
87.
GrizardDBarthomeufC. Non-digestible oligosaccharides used as prebiotic agents: mode of production and beneficial effects on animal and human health.Reprod Nutr Dev. (1999) 39:563–88. 10.1051/rnd:19990505
88.
GibsonGRManningTS. Prebiotics.Best Pract Res Clin Gastroenterol. (2004) 18:287–98. 10.1016/j.bpg.2003.10.008
89.
RoberfroidMGibsonGRHoylesLMcCartneyALRastallRRowlandIet alPrebiotic effects: metabolic and health benefits.Br J Nutr. (2010) 104:S1–63. 10.1017/S0007114510003363
90.
ChauhanSVChorawalaMR. Probiotics, prebiotics, and synbiotics.Int J Pharm Sci Res. (2014) 3:711–26.
91.
RaschkaLDanielH. Mechanisms underlying the effects of inulin-type fructans on calcium absorption in the large intestine of rats.Bone. (2005) 37:728–35. 10.1016/j.bone.2005.05.015
92.
LoboVPatilAPhatakAChandraN. Free radicals, antioxidants and functional foods: impact on human health.Pharmacogn Rev. (2010) 4:118–26. 10.4103/0973-7847.70902
93.
Al-SherajiSHIsmailAManapMYMustafaSYusofRMHassanFA. Prebiotics as functional foods: a review.J Funct Foods. (2013) 5:1542–53. 10.1016/j.jff.2013.08.009
94.
NagpalRKaurA. Synbiotic effect of various prebiotics on in vitro activities of probiotic lactobacilli.Ecol Food Nutr. (2011) 50:63–8. 10.1080/03670244.2011.539161
95.
MohanAQuekSYGutierrez-MaddoxNGaoYShuQ. Effect of honey in improving the gut microbial balance.Food Qual Saf. (2017) 1:107–15. 10.1093/fqsafe/fyx015
96.
GillSRPopMDeBoyRTEckburgPBTurnbaughPJSamuelBSet alMetagenomic analysis of the human distal gut microbiome.Science 2006. (1979) 312:1355–9. 10.1126/science.1124234
97.
OttmanNSmidtHde VosWMBelzerC. The function of our microbiota: who is out there and what do they do?Front Cell Infect Microbiol. (2012) 2:104. 10.3389/fcimb.2012.00104
98.
BogdanovSRuoffKPersano OddoL. Physico-chemical methods for the characterisation of unifloral honeys: a review.Apidologie. (2004) 35:S1–17. 10.1051/apido:2004047
99.
SanzMLGonzálezMde LorenzoCSanzJMartínez-CastroI. Carbohydrate composition and physico chemical properties of artisanal honeys from madrid (Spain): occurence of Echium sp honey.J Sci Food Agric. (2004) 84:1577–84. 10.1002/jsfa.1823
100.
SanzMLPolemisNMoralesVCorzoNDrakoularakouAGibsonGRet alIn vitro investigation into the potential prebiotic activity of honey oligosaccharides.J Agric Food Chem. (2005) 53:2914–21. 10.1021/jf0500684
101.
JiangLXieMChenGQiaoJZhangHZengX. Phenolics and carbohydrates in buckwheat honey regulate the human intestinal microbiota.Evid Based Complement Alternat Med. (2020) 2020:6432942. 10.1155/2020/6432942
102.
de MeloFHCMenezesFNDDde SousaJMBdos Santos LimaMda Silva Campelo BorgesGde SouzaELet alPrebiotic activity of monofloral honeys produced by stingless bees in the semi-arid region of Brazilian Northeastern toward Lactobacillus acidophilus LA-05 and Bifidobacterium lactis BB-12.Food Res Int. (2020) 128:108809. 10.1016/j.foodres.2019.108809
103.
RosendaleDIMaddoxISMilesMCRodierMSkinnerMSutherlandJ. High-throughput microbial bioassays to screen potential New Zealand functional food ingredients intended to manage the growth of probiotic and pathogenic gut bacteria.Int J Food Sci Technol. (2008) 43:2257–67. 10.1111/j.1365-2621.2008.01863.x
104.
ShamalaTRShri JyothiYSaibabaP. Stimulatory effect of honey on multiplication of lactic acid bacteria under in vitro and in vivo conditions.Lett Appl Microbiol. (2000) 30:453–5. 10.1046/j.1472-765x.2000.00746.x
105.
KajiwaraSGandhiHUstunolZ. Effect of honey on the growth of and acid production by human intestinal Bifidobacterium spp.: an in vitro comparison with commercial oligosaccharides and inulin.J Food Prot. (2002) 65:214–8. 10.4315/0362-028X-65.1.214
106.
El-ArabAMEGirgisSMHegazyEMEl-KhalekABA. Effect of dietary honey on intestinal microflora and toxicity of mycotoxins in mice.BMC Complement Altern Med. (2006) 6:6. 10.1186/1472-6882-6-6
107.
LiYLongSLiuQMaHLiJXiaoqingWet alGut microbiota is involved in the alleviation of loperamide-induced constipation by honey supplementation in mice.Food Sci Nutr. (2020) 8:4388–98. 10.1002/fsn3.1736
108.
RayesAAH. Enhancement of probiotic bioactivity by some prebiotics to produce bio-fermented milk.Life Sci J. (2012) 9:2246–53.
109.
PopaDUstunolZ. Influence of sucrose, high fructose corn syrup and honey from different floral sources on growth and acid production by lactic acid bacteria and Bifidobacteria.Int J Dairy Technol. (2011) 64:247–53. 10.1111/j.1471-0307.2011.00666.x
110.
UstunolZGandhiH. Growth and viability of commercial Bifidobacterium sp:in honey-sweetened skim milk.J Food Prot. (2001) 64:1775–9. 10.4315/0362-028X-64.11.1775
111.
LucanMSlacanacVHardiJMastanjevicKBabicJKrstanovicVet alInhibitory effect of honey-sweetened goat and cow milk fermented with Bifidobacterium lactis Bb-12 on the growth of Listeria monocytogenes.Mljekarstvo. (2009) 59:96–106.
112.
ChickHShinHSUstunolZ. Growth and acid production by lactic acid bacteria and bifidobacteria grown in skim milk containing honey.J Food Sci. (2001) 66:478–81. 10.1111/j.1365-2621.2001.tb16134.x
113.
HaddadinMSYNazerIRaddadJARobinsonRK. Effect of honey on the growth and metabolism of two bacterial species of intestinal origin.Pak J Nutr. (2007) 6:693–7. 10.3923/pjn.2007.693.697
114.
KolayliSBoukraaLSahinHAbdellahF. Sugars in honey. In: PreedyVReditor.Dietary Sugars: Chemistry, Analysis, Function and Effects. (London: The Royal Society of Chemistry) (2012). p. 3–14. 10.1039/9781849734929-00003
115.
WestonRJBrocklebankLK. The oligosaccharide composition of some New Zealand honeys.Food Chem. (1999) 64:33–7. 10.1016/S0308-8146(98)00099-5
116.
OddoLPPiazzaMGSabatiniAGAccortiM. Characterization of unifloral honeys.Apidologie. (1995) 26:453–65. 10.1051/apido:19950602
117.
PalframanRGibsonGRRastallRA. Development of a quantitative tool for the comparison of the prebiotic effect of dietary oligosaccharides.Lett Appl Microbiol. (2003) 37:281–4. 10.1046/j.1472-765X.2003.01398.x
118.
Jan MeiSMohd NordinMSNorrakiahAS. Fructooligosaccharides in honey and effects of honey on growth of Bifidobacterium longum BB 536.Int Food Res J. (2010) 17:557–61.
119.
WangKWanZOuALiangXGuoXZhangZet alMonofloral honey from a medical plant, Prunella vulgaris, protected against dextran sulfate sodium-induced ulcerative colitis via modulating gut microbial populations in rats.Food Funct. (2019) 10:3828–38. 10.1039/C9FO00460B
120.
HasyimiWWidanarniWYuhanaM. Growth performance and intestinal microbiota diversity in pacific white shrimp Litopenaeus vannamei fed with a probiotic bacterium, honey prebiotic, and synbiotic.Curr Microbiol. (2020) 77:2982–90. 10.1007/s00284-020-02117-w
121.
FuandilaNNWidanarniWYuhanaM. Growth performance and immune response of prebiotic honey fed pacific white shrimp Litopenaeus vannamei to vibrio parahaemolyticus infection.J Appl Aquac. (2020) 32:221–35. 10.1080/10454438.2019.1615593
122.
AlmasaudiSBAbbasATAl-HindiRREl-ShitanyNAAbdel-DayemUAAliSSet alManuka honey exerts antioxidant and anti-inflammatory activities that promote healing of acetic acid-induced gastric ulcer in rats.Evid Based Complement Alternat Med. (2017) 2017:5413917. 10.1155/2017/5413917
123.
HadagaliMDChuaLS. The anti-inflammatory and wound healing properties of honey.Eur Food Res Technol. (2014) 239:1003–14. 10.1007/s00217-014-2297-6
124.
NoohHZNour-EldienNM. The dual anti-inflammatory and antioxidant activities of natural honey promote cell proliferation and neural regeneration in a rat model of colitis.Acta Histochem. (2016) 118:588–95. 10.1016/j.acthis.2016.06.006
125.
WallaceAEadySMilesMMartinHMcLachlanARodierMet alDemonstrating the safety of manuka honey UMF® 20+in a human clinical trial with healthy individuals.Br J Nutr. (2010) 103:1023–8. 10.1017/S0007114509992777
126.
McCarthyMI. Painting a new picture of personalised medicine for diabetes.Diabetologia. (2017) 60:793–9. 10.1007/s00125-017-4210-x
127.
RothfussKSStangeEFHerrlingerKR. Extraintestinal manifestations and complications in inflammatory bowel diseases.World J Gastroenterol. (2006) 12:4819–31. 10.3748/wjg.v12.i30.4819
128.
GeboesKRiddellRÖstAJensfeltBPerssonTLöfbergR. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis.Gut. (2000) 47:404–9. 10.1136/gut.47.3.404
129.
TackJFriedMHoughtonLASpicakJFisherG. Systematic review: the efficacy of treatments for irritable bowel syndrome – a European perspective.Aliment Pharmacol Ther. (2006) 24:183–205.
Summary
Keywords
honey, medicinal honey, prebiotic honey, prebiotics, gut microbiome, gut health, dietary remediation
Citation
Schell KR, Fernandes KE, Shanahan E, Wilson I, Blair SE, Carter DA and Cokcetin NN (2022) The Potential of Honey as a Prebiotic Food to Re-engineer the Gut Microbiome Toward a Healthy State. Front. Nutr. 9:957932. doi: 10.3389/fnut.2022.957932
Received
31 May 2022
Accepted
23 June 2022
Published
28 July 2022
Volume
9 - 2022
Edited by
Sudha Gupta, University of Kalyani, India
Reviewed by
Ahmad Ud Din, Sichuan University, China
Updates
Copyright
© 2022 Schell, Fernandes, Shanahan, Wilson, Blair, Carter and Cokcetin.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nural N. Cokcetin, nural.cokcetin@uts.edu.au
This article was submitted to Nutrition and Sustainable Diets, a section of the journal Frontiers in Nutrition
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.