Abstract
Background:
Obesity is a prevalent health problem in patients with schizophrenia, and calorie restriction diet (CRD) achieved effective weight loss and metabolic improvement; however, these have not been rigorously evaluated in obese patients with schizophrenia.
Objective:
To measure the effects of CRD on weight loss and metabolic status in hospitalized obese women with schizophrenia during a 4-week period.
Methods:
Participants were randomly assigned to two groups in a 1:1 ratio. The intervention group (n = 47) was asked to follow a CRD and the control group (n = 48) a normal diet for 4 weeks. Outcomes of body weight, body composition, as well as metabolic parameters were measured at baseline and following the intervention period.
Results:
Forty-five participants completed the 4-week research in both the intervention and control groups. Compared to the normal diet, adherence to the CRD significantly decreased body weight (2.38 ± 1.30 kg), body mass index (0.94 ± 0.52 kg/m2), waist circumference (4.34 ± 2.75 cm), hip circumference (3.37 ± 2.36 cm), mid-upper circumferences, triceps skin-fold thickness, fat mass and free fat mass with large effect sizes (p = <0.001, ηp2 range between 0.145 and 0.571), as well as total cholesterol (0.69 ± 0.70 mmol/L) with a medium effect size (p = 0.028, ηp2 = 0.054). There were no differences between the CRD and control groups in terms of pre-post changes in triglycerides, high- and low-density lipoprotein-cholesterols, as well as systolic and diastolic blood pressures (p > 0.05).
Conclusion:
CRD is preventative of weight gain, but not apparent in intervention for metabolic status in hospitalized obese women with schizophrenia.
Clinical trial registration: http://www.chictr.org.cn, ChiCTR-INR-16009185.
1. Introduction
Schizophrenia is a common severe mental illness with a global prevalence of about 1%, among the world’s top ten causes of long-term disability (1). A fair amount of patients suffer from psychosis, apathy, and withdrawal, and cognitive impairment (2). What’s worse, 15–72% of patients experience sustained weight gain after treatment, especially in the first 12 weeks (3). The prevalence of obesity is twice as common in people with schizophrenia as in the general population (4), which is linked to many adverse consequences such as cardiovascular diseases (5), higher mortality (6), reduced quality of life (7), and poor compliance (8).
Obesity is a chronic metabolic condition that develops with the excessive accumulation of adipose tissue in the body (9). The cause of the increase in obesity for patients with schizophrenia is multifactorial, including poor diet (10, 11) and physical inactivity due to mental symptoms (such as laziness, passivity, etc.) and antipsychotics (12, 13) and genetic predisposition (14, 15). Antipsychotics are drugs that affect the activity of many hormones and neuromodulators that are well expressed in the hypothalamus, pancreas, liver, adipose tissue and skeletal muscle and regulate glucose and lipid homeostasis throughout the body, making it highly susceptible to metabolic side effects (16). Moreover, many studies have found that schizophrenia and metabolic abnormalities share familial risk factors or a common genetic predisposition, which may lead to schizophrenia being more prone to metabolic abnormalities than the general population (14, 15, 17). Sex is also believed to play a role in obesity among patients with schizophrenia, with a higher obesity rate among female patients (18). Currently, lifestyle intervention such as physical exercise and dietary regimens based on calorie restriction is known as the preferred treatment options of obesity (19). A number of pieces of evidence suggested that diet intervention, such as dietary approaches to stop hypertension (DASH) (20), very-low-calorie ketogenic diets (VLCKD) (21), low-fat vegan diet (LFVD) (22), very-low-calorie diet (VLCD) (23), and calorie-restricted diet (CRD) (24–27), safely achieve substantial weight loss and improvement of metabolic parameters in common population with obesity. The efficacy of CRD intervention has been confirmed in a variety of population (25), including health obesity subjects (28), obese patients with and without type-2 diabetes (29), obese patients with psoriasis (30), obese postmenopausal women with and without the metabolic syndrome (31). However, studies related to calorie-restricted diets in obese patients with schizophrenia are scarce. From now on, only one study of descriptive correlational design, utilizing chart review and a convenience sample of 100 participants, was used to evaluate the effect of CRD on weight change in short-term acute care psychiatric patients receiving atypical antipsychotic medication (32). Apparently, the results were lacking in persuasiveness due to flaws in the study design.
This study intends to use a randomized controlled trial to evaluate the effect of CRD on the body weight, body composition, and metabolic status of obese women with schizophrenia. We hypothesized that CRD is beneficial for body weight control and improving metabolic status in obese women with schizophrenia, comparing with general diet, thus offering a successful dietary prescription for use in clinical practice.
2. Methods
2.1. Study design and eligibility
This randomized clinical trial using a single-center, open parallel design was conducted between September 2016 and October 2017 in the Second Affiliated Hospital of Xinxiang Medical University (also known as Henan Mental Hospital). Female inpatients with schizophrenia aged 18 to 65 years with a body mass index (BMI) over 28 kg/m2 were enrolled. The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) patient version was used for the diagnosis of schizophrenia by two experienced psychiatric physicians. Exclusion criteria included: clinically significant body weight change (≥5 %) or dieting attempts in the prior 30 days; eating disorders such as anorexia or binge eating; diabetes, hypertension, dyslipidemia; need special diets due to physical diseases. To eliminate the possible impact of changes of drug regimens, diets, and lifestyles on metabolic indices after hospitalization, a 2-week run-in period was set before carrying out the study. Participants who met inclusion criteria 2 weeks after admission were randomized assigned to consume the CRD or normal diet (ND) for 4 weeks of intervention period (average length of stay 50 days for patients in the hospital). This study was a randomized controlled trial, and participants were randomly assigned to either CRD group or ND group using computer-generated random number allocation. Blinding of patients and study personnel was not possible given the nature of the study.
The trial adhered to the ethical guidelines of the Declaration of Helsinki and the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline (33, 34). All subjects and their families in both groups voluntarily participated in this study with informed consent. This study was approved by the human ethics committees of the Second Affiliated Hospital of Xinxiang Medical University with the Approval number 2016023 and was registered at the Chinese Clinical Trials Registry1 with the registration number ChiCTR-INR-16009185 (data: September 10, 2016).
2.2. Intervention
After determining the group of intervention subjects, the clinical dietitian determines the energy intake of the subjects based on their past dietary history and anthropometric indicators. The energy distribution of breakfast, lunch, and dinner is roughly 30, 40, and 30%. Target calorie requirement of each patient was estimated based on resting energy expenditure (using Harris-Benedict equation) and physical activity level (35). The diet recipes are varied and do not repeat during the week, and the recipes are adjusted weekly according to the seasonal characteristics to choose fresh ingredients in season. Try to cook in healthy ways such as steaming, boiling, braising, boiling, and quick stir-frying.
The clinical dietitian conducted a nutritional check-up prior to the dietary intervention in order to know the patient’s eating habits, diet structure, and dietary contraindications (e.g., food allergies, ethnic habits, etc.). During the intervention period, the dietitian conducted weekly nutritional check-ups and kept an eye on the patients’ diet, asked them to avoid high-calorie snacks (such as nuts, instant noodle, alcohol, etc.) and allowed to consume fruits, tomato, cucumber, and plain milk in moderation. Since the food that patients obtained outside of their three meals is purchased through the commissary within the wards, the kinds and amounts of food they buy can be controlled within reasonable limits. Subjects’ charge nurses were responsible for monitoring their daily dietary compliance.
CRD: The dietary energy of CRD is reduced by about 500 kcal per day based on the target energy intake. The energy supply ratio of carbohydrates is 50–60%, fat is 20–30%, protein is 15–20%.
ND: Normal diet for control group followed a balanced diet with three macronutrients in appropriate proportions, with 55–65% carbohydrate, 20–30% fat, and 10–15% protein.
Health education: Individual nutrition counseling and dietary guidance for 30–60 min was offered to each participant of both groups within the first week of enrollment. In addition, weekly collective health lectures were held for 30–40 min each time, with topics including how to control weight, how to calculate and evaluate body mass index, types of food and their nutritional value, reasonable diet, scientific exercise, and the relationship between obesity and chronic diseases.
2.3. Outcomes measurements
Outcomes were measured at baseline and at week 4 from both groups. Body weight and composition were measured by body fat analyser (IOI 353, Danilsmc Co.,Ltd). Blood samples were drawn from the anterior elbow vein after one overnight fast and tested in 2 h by the hospital laboratory using standard techniques. Fasting blood glucose (FBG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL), and triglycerides were tested using Bechmann automatic biochemistry analyzer. The oxidation enzyme method was applied for FBG, TC and TG was measured using CHOD-PAP method and GPO-PAP method, respectively. HDL and LDL were tested using direct quantitation method (peroxidase scavenging and surfactant scavenging, respectively). Height (cm) measured using the Xiheng pointer height and weight scale (Wuxi, Jiangsu) to the nearest 0.1 cm without shoes, wearing lightweight clothes, barefoot and the head positioned in the Frankfurt horizontal plane. Blood pressure (BP) was measured by a standard digital sphygmomanometer (Omron HEM-7136, Omron Healthcare, Inc., Lake Forest, IL) with patients in a sitting position after a 5-min rest.
2.4. Sample size determination
The mean and standard deviation of BW changes in CRD group (−1.3, 2.0) and NC group (0.4, 2.4) obtained based on a pilot study (10 cases in each group), which were used for the calculation of the sample size of this study. Both group sample sizes of 37 achieve 90% power to reject the null hypothesis of equal BW change means and with a significance level of 0.05 using a two-sided two-sample unequal-variance t-test by PASS 15 software (NCSS LLC, Kaysville, UT, USA). Allowing for a dropout rate of 20%, we would need at least 47 patients in each group.
2.5. Statistics analysis
Mean along with SD and percentage were carried out for describing numeral variables and categorical variables. Distribution of data related to normality was evaluated using Shapiro Wilk test. The general characteristics between the two groups were compared using independent samples Student’s t-test or the Mann–Whitney U test (according to data normality) for numeral variables and Chi-square test or Fisher’s exact test for categorical variables. To identify intragroup differences (pre- and post- 4-week intervention), we applied paired samples t-tests. Additionally, a 95% confidence interval (CI) for the mean of the change from baseline (Δ = post-test - pre-test) was used to analyze significant changes in the variables. In addition, Cohen’s d was calculated as effect size (ES), which is a standardized measurement based on SD differences, used as a guide for substantive significance; while d = 0.2 was considered a small effect, d = 0.5 was a medium effect and d = 0.8 was a large effect. The effects of CRD on variables of body weight (BW), body component, FBG, blood lipid profile and BP were performed by analysis of covariance (ANCOVA) using pretest values as the covariate. Partial eta squared effect sizes (ηp2) were also reported on group as an indicator of effect size of ANCOVA. Suggested norms for ηp2: small = 0.01; medium = 0.06; large = 0.14. value of p lower than 0.05 was considered statistically significant. All statistical analyses were conducted using the SPSS version 18 (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Characteristics of the participants
A total of 95 female inpatients with schizophrenia and obesity were included in this study, including 47 in the intervention group and 48 in the control group. Two subjects in the intervention group were discharged on the 12th and 17th day of the study. Three subjects in the control group failed to complete the intervention according to the trial protocol. Among them, 1 patient was discharged on the 7th day after the intervention started, and the other 2 patients developed hyperglycemia, whose diet were adjusted to diabetic diet. No harms or unintended effects were observed in either group by diet intervention throughout the trial. Ninety subjects were included in the analysis, with the intervention group and control group 45 cases, respectively. The following medications were used by the patients either singly or in combination: risperidone (n = 53), olanzapine (n = 29), levomepromazine (n = 28), and aripiprazole (n = 24). Figure 1 presents a CONSORT diagram.
Figure 1
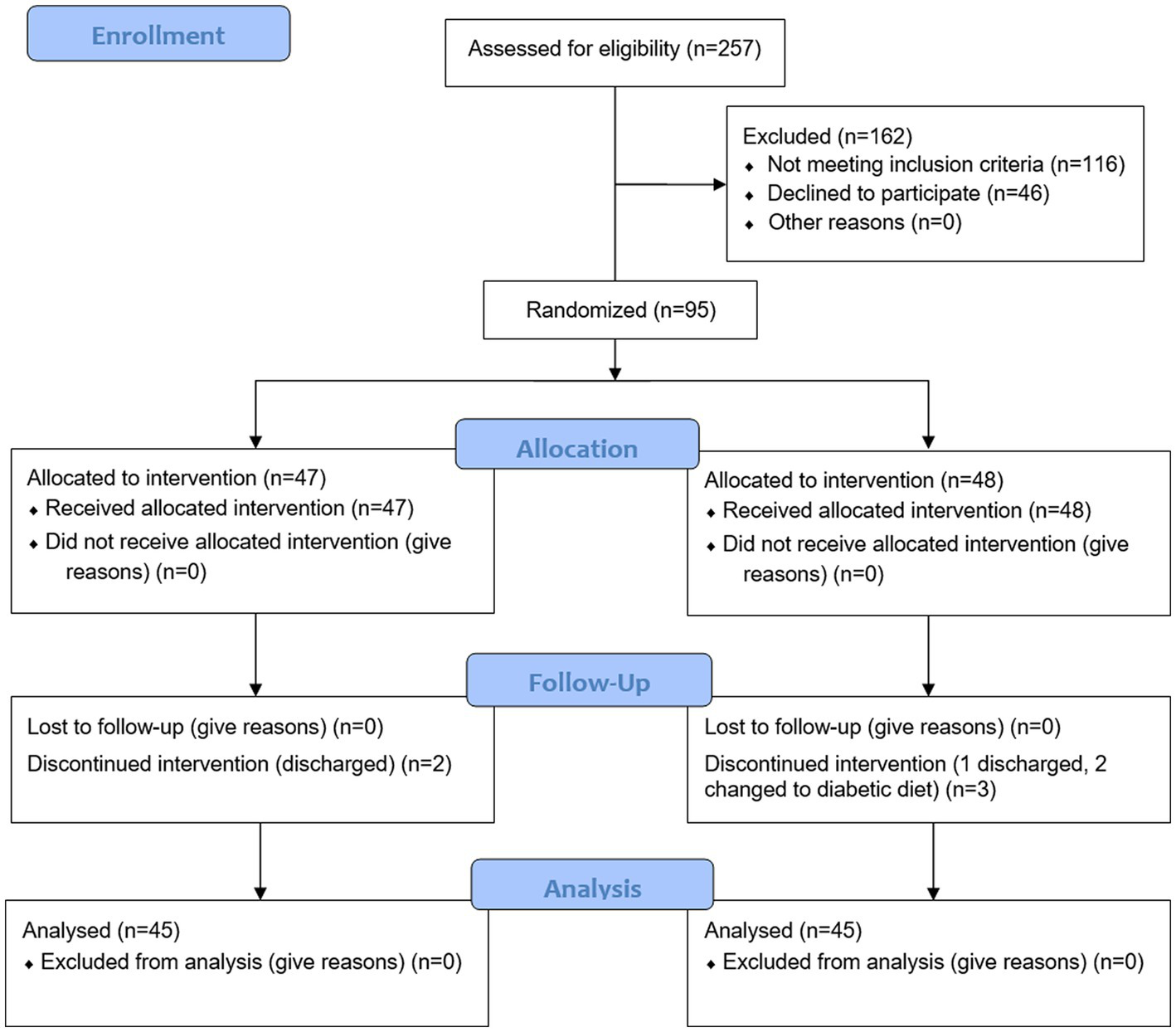
CONSORT flow diagram.
The average age of the study subjects included in the final data analysis was 35.63 ± 10.79 years old, and the average BMI was 31.40 ± 2.44 kg/m2. Descriptive statistics with baseline characteristics are summarized by groups in Table 1, and there were no statistical significances in demography (age, education, occupation, marriage), metabolic variables (FBG, lipid profile, BP), and mental illness status between two groups. The seven-day dietary schedule of a subject in CRD group and a seven-day dietary schedule of the ND group were sampled randomly to analysis for representing the intake of calories, macronutrients, and cholesterol between the two groups. The results are shown in Supplementary Table 1.
Table 1
| Intervention group (n = 45) | Control group (n = 45) | t/χ2 | Value of p | |
|---|---|---|---|---|
| Demography | ||||
| Age, years | 37.02 ± 10.56 | 34.24 ± 10.96 | −1.220 | 0.224 |
| Education | 2.464 | 0.482 | ||
| Elementary school or below | 13 | 10 | ||
| Middle School | 18 | 15 | ||
| Senior High School | 7 | 13 | ||
| University or above | 7 | 7 | ||
| Occupation | ||||
| Farmer | 34 | 34 | <0.001 | 1.000 |
| Others | 11 | 11 | ||
| Marriage | 0.811 | 0.906 | ||
| Unmarried | 10 | 11 | ||
| Married | 28 | 27 | ||
| Divorced | 4 | 4 | ||
| Remarried | 4 | 2 | ||
| Psychiatric history | ||||
| Family history | 0.090 | 0.764 | ||
| Yes | 6 | 7 | ||
| No | 39 | 38 | ||
| Disease duration, year | 10.14 ± 6.98 | 9.36 ± 7.05 | −0.530 | 0.595 |
| Anthropometric indicators | ||||
| Height, cm | 159.05 ± 5.58 | 159.91 ± 4.79 | 0.783 | 0.436 |
| BMI, kg/m2 | 31.84 ± 2.82 | 30.95 ± 1.90 | −1.754 | 0.083 |
| BW, kg | 80.63 ± 8.97 | 79.25 ± 7.24 | −0.802 | 0.425 |
| Metabolic indicators | ||||
| FBG, mmol/L | 5.34 ± 0.89 | 5.04 ± 0.66 | −1.827 | 0.071 |
| TC, mmol/L | 4.87 ± 0.69 | 4.64 ± 0.97 | −1.247 | 0.216 |
| TG, mmol/L | 2.08 ± 1.97 | 1.59 ± 0.65 | −1.576 | 0.119 |
| SBP, mmHg | 119.84 ± 12.94 | 117.16 ± 11.32 | −1.049 | 0.297 |
| DBP, mmHg | 78.20 ± 8.27 | 76.02 ± 7.39 | −1.317 | 0.191 |
Baseline characteristics of participants.
BMI, body mass index; BW, body weight, FBG, fasting blood-glucose; TC, total cholesterol; TG, triglycerides; SBP, systolic blood pressure; DBP, diastolic blood pressure.
3.2. Body weight and composition
After 4 weeks of calorie-restricted diets, compared to baseline, BW, body mass index (BMI), waist circumference (WC), hip circumference (HC), mid-upper arm circumference (MUAC), triceps skinfold thickness (TST), fat mass (FM) and free-fat mass (FFM) showed a significant reduction in CRD group, among which the decrease of BW (−2.38 ± 1.30), BMI (−0.94 ± 0.52) and FM (−1.59 ± 1.61) showed a moderate effect size (d > 0.2) and WC (−4.34 ± 2.75), HC (−3.37 ± 2.36), MUAC (−1.26 ± 1.71) and TST (−3.80 ± 3.17) showed a large effect size (d > 0.5), while, only FFM (−0.65 ± 1.17) showed small effect size (d = −0.141). Regarding control group, except for the MUAC (0.16 ± 1.01, p = 0.300) and FFM (0.36 ± 1.31, p = 0.073), all the above indicators demonstrate an increased change with statistic difference after 4 weeks compared with the beginning. In particular, the skinfold showed moderate effect size (d = 0.216), but BW, BMI, WC, HC, FM showed small effect size. According to the results by group, significant differences were observed over group in all the above indicators (p < 0.001) after 4 weeks test, as well as with a large effect size for all (ηp2 > 0.12). In addition, the same statistical analysis was implemented in indicators of FBG, lipid profiles, and BP (see Table 2 and Figure 2 for details).
Table 2
| Variables | Group | Baseline | End-of-trial | Change | pa | Cohen’d | pb | ηp2 |
|---|---|---|---|---|---|---|---|---|
| BW, kg | ND | 79.25 ± 7.24 | 80.06 ± 7.35 | 0.8 ± 2.14 | 0.014 | 0.111 | <0.001 | 0.452 |
| CRD | 80.63 ± 8.97 | 78.25 ± 8.57 | −2.38 ± 1.30 | <0.001 | −0.271 | |||
| BMI, kg/m2 | ND | 30.95 ± 1.90 | 31.28 ± 2.07 | 0.32 ± 0.87 | 0.017 | 0.161 | <0.001 | 0.424 |
| CRD | 31.84 ± 2.82 | 30.90 ± 2.64 | −0.94 ± 0.52 | <0.001 | −0.345 | |||
| WC, cm | ND | 106.10 ± 6.15 | 107.24 ± 5.84 | 1.14 ± 2.41 | 0.003 | 0.190 | <0.001 | 0.571 |
| CRD | 106.40 ± 6.51 | 102.06 ± 5.82 | −4.34 ± 2.75 | <0.001 | −0.703 | |||
| HC, cm | ND | 108.55 ± 5.99 | 109.56 ± 5.63 | 1.01 ± 2.22 | 0.004 | 0.174 | <0.001 | 0.485 |
| CRD | 110.06 ± 6.15 | 106.69 ± 5.89 | −3.37 ± 2.36 | <0.001 | −0.56 | |||
| MUAC, cm | ND | 31.81 ± 2.49 | 31.97 ± 2.65 | 0.16 ± 1.01 | 0.300 | 0.061 | <0.001 | 0.197 |
| CRD | 32.37 ± 2.29 | 31.12 ± 2.62 | −1.26 ± 1.71 | <0.001 | −0.510 | |||
| TST, cm | ND | 30.44 ± 5.75 | 31.64 ± 5.37 | 1.20 ± 2.28 | 0.001 | 0.216 | <0.001 | 0.469 |
| CRD | 32.10 ± 6.13 | 28.30 ± 5.30 | −3.80 ± 3.17 | <0.001 | −0.662 | |||
| FM, kg | ND | 29.83 ± 4.05 | 30.30 ± 3.92 | 0.47 ± 1.49 | 0.042 | 0.117 | <0.001 | 0.304 |
| CRD | 31.10 ± 4.61 | 29.51 ± 4.02 | −1.59 ± 1.61 | <0.001 | −0.368 | |||
| FFM, kg | ND | 44.87 ± 3.65 | 45.23 ± 3.80 | 0.36 ± 1.31 | 0.073 | 0.097 | <0.001 | 0.145 |
| CRD | 45.01 ± 4.63 | 44.36 ± 4.55 | −0.65 ± 1.17 | 0.001 | −0.141 | |||
Changes in body weight and composition during the study in the CRD intervention group vs. the ND group (mean ± SD).
ηp2, partial eta squared effect size; BW, body weight; BMI, body mass index; WC, waist circumference; HC, hip circumference; MUAC, mid-upper arm circumference; TST, triceps skinfold thickness; FM, fat mass; FFM, free-fat mass.
Figure 2
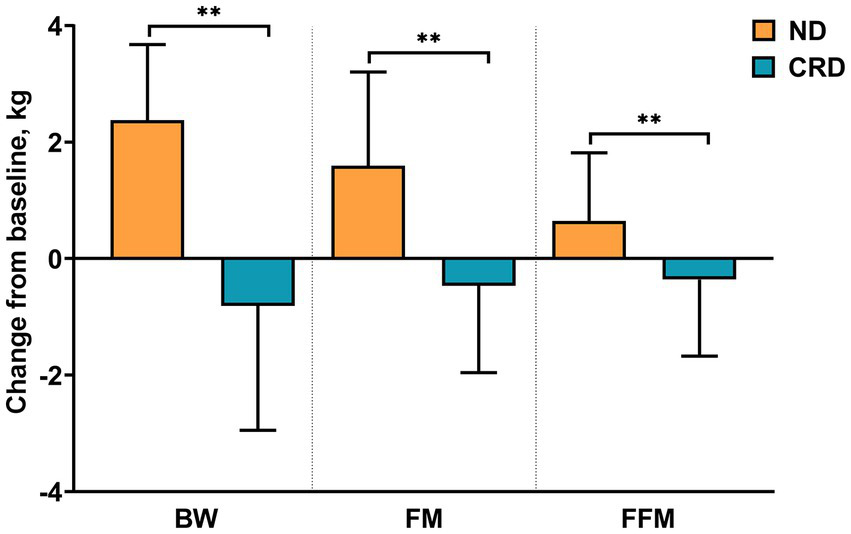
Changes from baseline in BW (body weight), FM (fat mass), and FFM (fat-free mass). ** presents the changes between calorie restriction diet (CRD) group and normal diet (ND) group considered statistical significance (p < 0.001).
3.3. FBG, blood lipid profiles, and BP
Regarding the FBG, there was a significant reduction in both groups compared to baseline, but the CRD show a large effect size (d = −0.730), while the CD group show a small effect (d = −0.382) (Table 3). With regard to blood lipid, the CRD group showed a significant reduction of both TC (−0.69 ± 0.70, p = <0.001) and low-density lipoprotein-cholesterol (LDL-C) (−0.40 ± 0.59, p = <0.001), along with moderate effect size (d > 0.5). Conversely, the ND group showed a significant increase of triglycerides (TG) (0.47 ± 0.93, p = 0.002), showing a moderate effect (d = 0.545). Adjustments for baseline values, compared with the control diet, the CRD dietary has resulted in significant reductions in TC (p = 0.028), but to a small size (ηp2 = 0.054).
Table 3
| Variables | Group | Baseline | End-of-trial | Change | pa | Cohen’d | pb | ηp2 |
|---|---|---|---|---|---|---|---|---|
| FBG, mmol/L | ND | 5.04 ± 0.66 | 4.77 ± 0.77 | −0.27 ± 0.77 | 0.022 | −0.382 | 0.361 | 0.010 |
| CRD | 5.34 ± 0.89 | 4.78 ± 0.65 | −0.57 ± 0.76 | <0.001 | −0.730 | |||
| TC, mmol/L | ND | 4.64 ± 0.97 | 4.44 ± 0.94 | −0.20 ± 1.06 | 0.202 | −0.214 | 0.028 | 0.054 |
| CRD | 4.87 ± 0.69 | 4.17 ± 0.69 | −0.69 ± 0.70 | <0.001 | −1.001 | |||
| TG, mmol/L | ND | 1.59 ± 0.65 | 2.06 ± 1.02 | 0.47 ± 0.93 | 0.002 | 0.545 | 0.076 | 0.036 |
| CRD | 2.08 ± 1.97 | 1.83 ± 0.77 | −0.25 ± 1.80 | 0.367 | −0.164 | |||
| HDL-C, mmol/L | ND | 1.19 ± 0.23 | 1.16 ± 0.29 | −0.03 ± 0.28 | 0.475 | −0.117 | 0.132 | 0.026 |
| CRD | 1.16 ± 0.27 | 1.06 ± 0.28 | −0.09 ± 0.32 | 0.056 | −0.341 | |||
| LDL-C, mmol/L | ND | 2.69 ± 0.61 | 2.61 ± 0.69 | −0.08 ± 0.67 | 0.437 | −0.121 | 0.100 | 0.031 |
| CRD | 2.95 ± 0.74 | 2.55 ± 0.59 | −0.40 ± 0.59 | <0.001 | −0.596 | |||
| SBP, mmHg | ND | 117.16 ± 11.32 | 117.20 ± 12.32 | 0.04 ± 14.00 | 0.983 | 0.004 | 0.079 | 0.035 |
| CRD | 119.84 ± 12.94 | 114.13 ± 9.90 | −5.71 ± 12.45 | 0.004 | −0.496 | |||
| DBP, mmHg | ND | 76.02 ± 7.39 | 76.11 ± 8.17 | 0.09 ± 10.14 | 0.953 | 0.011 | 0.219 | 0.017 |
| CRD | 78.20 ± 8.27 | 74.76 ± 7.48 | −3.44 ± 8.46 | 0.009 | −0.437 | |||
Changes in metabolic markers during the study in the CRD group vs. the ND group.
FBG, fasting blood-glucose; TC, total cholesterol; TG, triglycerides; HDL-C, high density lipoprotein-cholesterol; LDL-C, low density lipoprotein-cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure.
pa indicated the p-value of inra-group comparison between baseline and end-of-trial; pb indicated the p-value of inter-group comparison between changes of intervention group and control group.
3.4. Subgroups analysis
Subjects were split into three age subgroups, young (20 ≤ age < 30), middle-aged (30 ≤ age < 45) and elderly subgroup (45 ≤ age ≤ 60), and the foregoing analysis was conducted in each group (Supplementary Tables 2–7). Except for the middle-aged subgroup where the changes between the two groups in MUAC and FFM were not found, changes in all indicators of body weight and composition indicators were statistically different in all subgroups. Moreover, the statistical differences of TC changes, shown based on all subjects, were only found in the young subgroup, and both SBP (p = 0.011, ηp2 = 0.210) and DBP (p = 0.014, ηp2 = 0.196) in the middle-aged subgroup decreased significantly after the CRD intervention compared to ND.
4. Discussion
Our study aimed to evaluate the effect of CRD on body composition and metabolic indicators following a 4-week RCT program in hospitalized obese women with schizophrenia. To our knowledge, this is the first RCT study on obese patients with schizophrenia by CRD. The results demonstrate that CRD could prevent body weight gain, as well as waistline, hipline, MUAC, TST, FM, and FFM. In addition, TC was the only indicator that decreased after CRD intervention among all metabolic indicators compared with control group. The findings were largely consistent with our proposed research hypothesis.
For the primary research question, the most obvious finding to emerge from the analysis is that all the indicators of body mass and composition of the intervention group decreased. Weight loss of 5% with respect to baseline is generally accepted as a “clinically meaningful” amount (36). The 2013 Obesity Guidelines state that a 3% weight loss could lead to glucose and triglyceride improvements and a 5% weight loss could lead to HDL, LDL, and blood pressure improvements, which are clinically meaningful (37). In present study, the rate of weight loss in the intervention group was 2.95%, which did not meet the minimum recommended target. The short-term duration of the intervention was the primary explanation, which is the shortcoming of this study. CRD with relatively high protein contents may facilitate weight loss due to increased satiety and sustained energy expenditure via diet induced thermogenesis (38). We offered adequate protein for the CRD diet, but this did not prevent the loss of FFM. This may be due to the low energy intake of the CRD group, which resulted in gluconeogenesis by breaking down endogenous proteins. In accordance with this result, previous studies with general population also demonstrated the loss of FFM after CRD (31, 39). There is a general agreement that the loss of FFM should be avoided due to FFM’s protective effect against insulin resistance (28). A study in overweight participant showed that CRD encompassing endurance exercises could attenuate FFM loss at a similar degree of weight loss (40).
In addition, the present finding that BW, BMI, WC, HC, TST, and MUAC in the control group increased slightly at the end of the experiment compared with the baseline. This result indicates that there is excess energy intake in hospitalized patients with schizophrenia, which is mainly attributable to increased appetite or behavior inhibition caused by antipsychotics (41). It is commonly recognized that maintaining the weight loss is challenging for a person who returns to the same environment and behaviors that produced their weight gain (42). Undoubtedly, for obese persons with schizophrenia avoiding weight regain requires overcoming additional obstacles.
Besides changes of body weight and composition, CRD also contributed to commensurate changes in other metabolic indicators. For example, Hietaniemi et al. observed after 8-week CRD on women with obesity the triglyceride and fasting insulin concentrations decreased significantly (43). Rothberg et al. reported the improvements in blood lipid profile and the lowering of BP in those patients who had the WC decreasing after low-calorie diet (LCD) intervention. Rothberg et al. reported improved lipid profiles and decreased blood pressure in subjects who had declined WC after the LCD intervention (44). In current study, only TC observed a significant difference between intervention group and control group. Indicators other than TG and HDL-C, although there are statistical differences in the comparison before and after the experiment within the group, no difference in changes between the groups was found. Four weeks of intervention may not be sufficient to cause changes in metabolic outcomes. It has been shown that metabolic changes can only be produced when the rate of weight loss exceeds 5% or more (45), while the rate of weight loss in this study was only 2.95%. Furthermore, the blood glucose, lipid profiles, and blood pressure of the subjects were at the normal level when they were enrolled, resulting in their insensitivity to dietary intervention (46, 47). One unanticipated finding was that FBG decreased both in intervention group and control group, which were medium and small, respectively. The provision of health education to all participants could be the reason why both groups decreased; other reasons could be type 1 error of hypothesis testing.
To eliminate the possible influence of the wide age range of the study population on the results. We performed subgroup analyses for different age groups. We found that TC changes in the younger subgroup only differed more between the two intervention groups. There were also differences in blood pressure between two intervention methods in the middle-aged group. This suggests that age factors influence the effect of CRD intervention on metabolic status. Due to the limited sample size of each subgroup, the findings of the subgroup analysis need further validation.
This study has certain limitations and deficiencies, and the results of the study should be interpreted carefully. First, given the excess of female patients with schizophrenia over males and the stronger willingness of females to lose weight, we selected only female patients as the study population. Moreover, the study was conducted in a closed hospital setting, which can ensure the compliance of subjects, resulting in limited extrapolation of the findings. Secondly, this study did not carry out exercise intervention for the study subjects, obesity intervention should be carried out in many ways, especially diet and exercise. In addition, the duration of the intervention in this study was short, due to the average length of stay of the patients being 50 days, and the intervention subjects were not followed up, so the long-term effects of the intervention lacked evaluation. Weight regains after returning to normal or habitual diets is a common problem affecting the long-term effectiveness of dietary interventions.
5. Conclusion
In conclusion, we provide evidence that calorie-restricted diets improve weight and metabolic markers in obese patients hospitalized with schizophrenia in women. Future studies should include larger trials with long-term follow-up and a focus on weight maintenance following initial intervention.
Funding
This research was funded by the National Natural Science Foundation of China (81703216), the Open Project of Henan Key Lab of Biological Psychiatry (ZDSYS2021005), and the Joint Co-construction Project of Henan Medical Science and Technology Research Plan (LHGJ20220637).
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the human ethics committees of the Second Affiliated Hospital of Xinxiang Medical University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
LZ and YH: conceptualization. LZ and CY: methodology. LZ, MZ, ZZ, and XL: investigation. YH and LZ: writing—original draft preparation. CY and PH: validation. LL and PH: writing—review and editing. YH and CY: supervision. All authors read and agreed to the published version of the manuscript.
Acknowledgments
We are thankful to all the study participants for the substantial time and effort they spent in contributing to our study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1038070/full#supplementary-material
Footnotes
References
1.
HogerzeilSJvan HemertAMRosendaalFRSusserEHoekHW. Direct comparison of first-contact versus longitudinal register-based case finding in the same population: early evidence that the incidence of schizophrenia may be three times higher than commonly reported. Psychol Med. (2014) 44:3481–90. doi: 10.1017/S003329171400083X
2.
MueserKTMcGurkSR. Schizophrenia. Lancet. (2004) 363:2063–72. doi: 10.1016/S0140-6736(04)16458-1
3.
TekCKucukgoncuSGuloksuzSWoodsSWSrihariVHAnnamalaiA. Antipsychotic-induced weight gain in first-episode psychosis patients: a meta-analysis of differential effects of antipsychotic medications. Early Interv Psychiatry. (2016) 10:193–202. doi: 10.1111/eip.12251
4.
HoltRIHindDGossage-WorrallRBradburnMJSaxonDMcCronePet al. Structured lifestyle education to support weight loss for people with schizophrenia, schizoaffective disorder and first episode psychosis: the STEPWISE RCT. Health Technol Assess. (2018) 22:1–160. doi: 10.3310/hta22650
5.
LeuchtSBurkardTHendersonJMajMSartoriusN. Physical illness and schizophrenia: a review of the literature. Acta Psychiatr Scand. (2007) 116:317–33. doi: 10.1111/j.1600-0447.2007.01095.x
6.
HennekensCHHennekensARHollarDCaseyDE. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. (2005) 150:1115–21. doi: 10.1016/j.ahj.2005.02.007
7.
AllisonDBMackellJAMcDonnellDD. The impact of weight gain on quality of life among persons with schizophrenia. Psychiatr Serv. (2003) 54:565–7. doi: 10.1176/appi.ps.54.4.565
8.
WeidenPJMackellJAMcDonnellDD. Obesity as a risk factor for antipsychotic noncompliance. Schizophr Res. (2004) 66:51–7. doi: 10.1016/S0920-9964(02)00498-X
9.
WuLZhangLLiBJiangHDuanYXieZet al. AMP-activated protein kinase (AMPK) regulates energy metabolism through modulating thermogenesis in adipose tissue. Front Physiol. (2018) 9:122. doi: 10.3389/fphys.2018.00122
10.
McCreadieRMacdonaldEBlacklockCTilak-SinghDWilesDHallidayJet al. Dietary intake of schizophrenic patients in Nithsdale, Scotland: case-control study. BMJ. (1998) 317:784–5. doi: 10.1136/bmj.317.7161.784
11.
BrownSBirtwistleJRoeLThompsonC. The unhealthy lifestyle of people with schizophrenia. Psychol Med. (1999) 29:697–701. doi: 10.1017/S0033291798008186
12.
PillingerTMcCutcheonRAVanoLMizunoYArumuhamAHindleyGet al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. Lancet Psychiatry. (2020) 7:64–77. doi: 10.1016/S2215-0366(19)30416-X
13.
ChilizaBAsmalLOosthuizenPvan NiekerkEErasmusRKiddMet al. Changes in body mass and metabolic profiles in patients with first-episode schizophrenia treated for 12 months with a first-generation antipsychotic. Eur Psychiatry. (2015) 30:277–83. doi: 10.1016/j.eurpsy.2014.11.013
14.
van WinkelRRuttenBPPeerboomsOPeuskensJvan OsJDe HertM. MTHFR and risk of metabolic syndrome in patients with schizophrenia. Schizophr Res. (2010) 121:193–8. doi: 10.1016/j.schres.2010.05.030
15.
BoikoASPozhidaevIVPaderinaDZBocharovaAVMednovaIAFedorenkoOYet al. Search for possible associations of FTO gene polymorphic variants with metabolic syndrome, obesity and body mass index in schizophrenia patients. Pharmacogen Personal Med. (2021) 14:1123–31. doi: 10.2147/PGPM.S327353
16.
CarliMKolachalamSLongoniBPintaudiABaldiniMAringhieriSet al. Atypical antipsychotics and metabolic syndrome: from molecular mechanisms to clinical differences. Pharmaceuticals. (2021) 14:238. doi: 10.3390/ph14030238
17.
PapanastasiouE. The prevalence and mechanisms of metabolic syndrome in schizophrenia: a review. Ther Adv Psychopharmacol. (2013) 3:33–51. doi: 10.1177/2045125312464385
18.
AllisonDBFontaineKRHeoMMentoreJLCappelleriJCChandlerLPet al. The distribution of body mass index among individuals with and without schizophrenia. J Clin Psychiatry. (1999) 60:215–20. doi: 10.4088/JCP.v60n0402
19.
National Heart, LBI.Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary. Expert panel on the identification, evaluation, and treatment of overweight in adults. Am J Clin Nutr. (1998) 68:899–917.
20.
Razavi ZadeMTelkabadiMHBahmaniFSalehiBFarshbafSAsemiZ. The effects of DASH diet on weight loss and metabolic status in adults with non-alcoholic fatty liver disease: a randomized clinical trial. Liver Int. (2016) 36:563–71. doi: 10.1111/liv.12990
21.
BruciATuccinardiDTozziRBalenaASantucciSFrontaniRet al. Very low-calorie ketogenic diet: a safe and effective tool for weight loss in patients with obesity and mild kidney failure. Nutrients. (2020) 12:333. doi: 10.3390/nu12020333
22.
KahleovaHPetersenKFShulmanGIAlwarithJRembertETuraAet al. Effect of a low-fat vegan diet on body weight, insulin sensitivity, postprandial metabolism, and intramyocellular and hepatocellular lipid levels in overweight adults: a randomized clinical trial. JAMA Netw Open. (2020) 3:e2025454. doi: 10.1001/jamanetworkopen.2020.25454
23.
HaywoodCJPrendergastLAPurcellKLe FevreLLimWKGaleaMet al. Very low calorie diets for weight loss in obese older adults-a randomized trial. J Gerontol A Biol Sci Med Sci. (2017) 73:59–65. doi: 10.1093/gerona/glx012
24.
ZubrzyckiACierpka-KmiecKKmiecZWronskaA. The role of low-calorie diets and intermittent fasting in the treatment of obesity and type-2 diabetes. J. Physiol. Pharmacol. (2018) 69:663–683. doi: 10.26402/jpp.2018.5.02
25.
RuggenentiPAbbateMRuggieroBRotaSTrilliniMAparicioCet al. Renal and systemic effects of calorie restriction in patients with type 2 diabetes With abdominal obesity: a randomized controlled trial. Diabetes. (2017) 66:75–86. doi: 10.2337/db16-0607
26.
VinkRGRoumansNJMarimanECvan BaakMA. Dietary weight loss-induced changes in RBP4, FFA, and ACE predict weight regain in people with overweight and obesity. Physiol Rep. (2017) 5:e13450. doi: 10.14814/phy2.13450
27.
MorenoBBellidoDSajouxIGodayASaavedraDCrujeirasABet al. Comparison of a very low-calorie-ketogenic diet with a standard low-calorie diet in the treatment of obesity. Endocrine. (2014) 47:793–805. doi: 10.1007/s12020-014-0192-3
28.
ViljanenAPLautamäkiRJärvisaloMParkkolaRHuupponenRLehtimäkiTet al. Effects of weight loss on visceral and abdominal subcutaneous adipose tissue blood-flow and insulin-mediated glucose uptake in healthy obese subjects. Ann Med. (2009) 41:152–60. doi: 10.1080/07853890802446754
29.
LeslieWSTaylorRHarrisLLeanME. Weight losses with low-energy formula diets in obese patients with and without type 2 diabetes: systematic review and meta-analysis. Int J Obes (2005). 207;41(1):96–101. doi: 10.1038/ijo.2016.175
30.
JensenPZachariaeCChristensenRGeikerNRSchaadtBKStenderSet al. Effect of weight loss on the cardiovascular risk profile of obese patients with psoriasis. Acta Derm Venereol. (2014) 94:691–4. doi: 10.2340/00015555-1824
31.
GhachemAPrud'hommeDRabasa-LhoretRBrochuM. Effects of a 6-month caloric restriction induced-weight loss program in obese postmenopausal women with and without the metabolic syndrome: a MONET study. Menopause. (2017) 24:908–15. doi: 10.1097/GME.0000000000000862
32.
JacobowitzWDerbabianBSaundersA. The effect of a calorie-restricted diet on weight gain in short-term psychiatric inpatients receiving atypical antipsychotic medications. J Psychosoc Nurs Ment Health Serv. (2014) 52:30–7. doi: 10.3928/02793695-20140421-01
33.
SchulzKFAltmanDGMoherD. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. (2010) 340:c332. doi: 10.1136/bmj.c332
34.
StockhausenK. The declaration of Helsinki: revising ethical research guidelines for the 21st century. Med J Aust. (2000) 172:252–3. doi: 10.5694/j.1326-5377.2000.tb123936.x
35.
ZelloGA. Dietary reference intakes for the macronutrients and energy: considerations for physical activity. Appl Physiol Nutr Metab. (2006) 31:74–9. doi: 10.1139/h05-022
36.
WilliamsonDABrayGARyanDH. Is 5% weight loss a satisfactory criterion to define clinically significant weight loss?Obesity. (2015) 23:2319–20. doi: 10.1002/oby.21358
37.
RyanDHeanerM. Guidelines (2013) for managing overweight and obesity in adults. Preface to the full report. Obesity. (2014) 22:S1–3.
38.
JohanssonKNeoviusMHemmingssonE. Effects of anti-obesity drugs, diet, and exercise on weight-loss maintenance after a very-low-calorie diet or low-calorie diet: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. (2014) 99:14–23. doi: 10.3945/ajcn.113.070052
39.
CoutinhoSRWithERehfeldJFKulsengBTrubyHMartinsC. The impact of rate of weight loss on body composition and compensatory mechanisms during weight reduction: a randomized control trial. Clin Nutrit. (2018) 37:1154–62. doi: 10.1016/j.clnu.2017.04.008
40.
WeissEPJordanRCFreseEMAlbertSGVillarealDT. Effects of weight loss on Lean mass, strength, bone, and aerobic capacity. Med Sci Sports Exerc. (2017) 49:206–17. doi: 10.1249/MSS.0000000000001074
41.
CorrellCUNewcomerJWSilvermanBDiPetrilloLGrahamCJiangYet al. Effects of olanzapine combined with samidorphan on weight gain in schizophrenia: a 24-week phase 3 study. Am J Psychiatry. (2020) 177:1168–78. doi: 10.1176/appi.ajp.2020.19121279
42.
LeanMHankeyC. Keeping it off: the challenge of weight-loss maintenance. Lancet Diab Endocrinol. (2018) 6:681–3. doi: 10.1016/S2213-8587(17)30405-9
43.
HietaniemiMJokelaMRantalaMUkkolaOVuoristoJTIlvesMet al. The effect of a short-term hypocaloric diet on liver gene expression and metabolic risk factors in obese women. Nutr Metabol Cardiovasc Dis. (2009) 19:177–83. doi: 10.1016/j.numecd.2008.06.009
44.
RothbergAEMcEwenLNKraftsonATAjluniNFowlerCENayCKet al. Impact of weight loss on waist circumference and the components of the metabolic syndrome. BMJ Open Diabetes Res Care. (2017) 5:e000341. doi: 10.1136/bmjdrc-2016-000341
45.
RyanDHYockeySR. Weight loss and improvement in comorbidity: differences at 5, 10, 15%, and over. Curr Obes Rep. (2017) 6:187–94. doi: 10.1007/s13679-017-0262-y
46.
HoddyKKKroegerCMTrepanowskiJFBarnoskyABhutaniSVaradyKA. Meal timing during alternate day fasting: impact on body weight and cardiovascular disease risk in obese adults. Obesity. (2014) 22:2524–31. doi: 10.1002/oby.20909
47.
GabelKHoddyKKHaggertyNSongJKroegerCMTrepanowskiJFet al. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: a pilot study. Nutr Healthy Aging. (2018) 4:345–53. doi: 10.3233/NHA-170036
Summary
Keywords
calorie-restricted diet, obesity, women, schizophrenia, randomized controlled trial
Citation
Zhang L, Zhu M, Liu X, Zhao Z, Han P, Lv L, Yang C and Han Y (2023) Calorie-restricted diet mitigates weight gain and metabolic abnormalities in obese women with schizophrenia: a randomized controlled trial. Front. Nutr. 10:1038070. doi: 10.3389/fnut.2023.1038070
Received
05 October 2022
Accepted
10 April 2023
Published
05 May 2023
Volume
10 - 2023
Edited by
Lixin Na, Shanghai University of Medicine and Health Sciences, China
Reviewed by
Haiquan Xu, Chinese Academy of Agricultural Sciences, China; Achraf Ammar, Johannes Gutenberg University Mainz, Germany
Updates
Copyright
© 2023 Zhang, Zhu, Liu, Zhao, Han, Lv, Yang and Han.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chun Yang, linyingyangchun@163.comYong Han, hy_vip@126.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.