Abstract
Introduction:
strategy of periodic food restriction and fixed eating windows, could beneficially modify individuals by losing body weight, regulating glucose or lipid metabolism, reducing blood pressure, and modulating the immune system. Specific effects of IF and its mechanisms have not yet been assessed collectively. Thus, this systematic review aims to summarize and compare clinical trials that explored the immunomodulatory effects of IF.
Methods:
After screening, 28 studies were included in this systematic review.
Results:
In addition to weight loss, IF could benefit health subjects by strengthening their circadian rhythms, migrating immune cells, lower inflammatory factors, and enriching microbials. In addition of the anti-inflammatory effect by regulating macrophages, protection against oxidative stress with hormone secretion and oxidative-related gene expression plays a key beneficial role for the influence of IF on obese subjects.
Discussion:
Physiological stress by surgery and pathophysiological disorders by endocrine diseases may be partly eased with IF. Moreover, IF might be used to treat anxiety and cognitive disorders with its cellular, metabolic and circadian mechanisms. Finally, the specific effects of IF and the mechanisms pertaining to immune system in these conditions require additional studies.
1. Introduction
Fasting has recently received increasing attention for its advantages on body health (1). Dietary habits that involve fat-rich foods and snacks may lead to chronic diseases (2). Intermittent fasting (IF), as a dieting strategy, combines periodic energy restriction and fixed-duration eating windows (3). Different types of IF that incorporate varied combinations of fasting and eating windows have been proposed; examples include alternate-day fasting (36 h of fasting and 12 h of ad libitum eating) (4) and time-restricted fasting (16 h of fasting and 8 h of ad libitum eating) (5) (Figure 1).
Figure 1
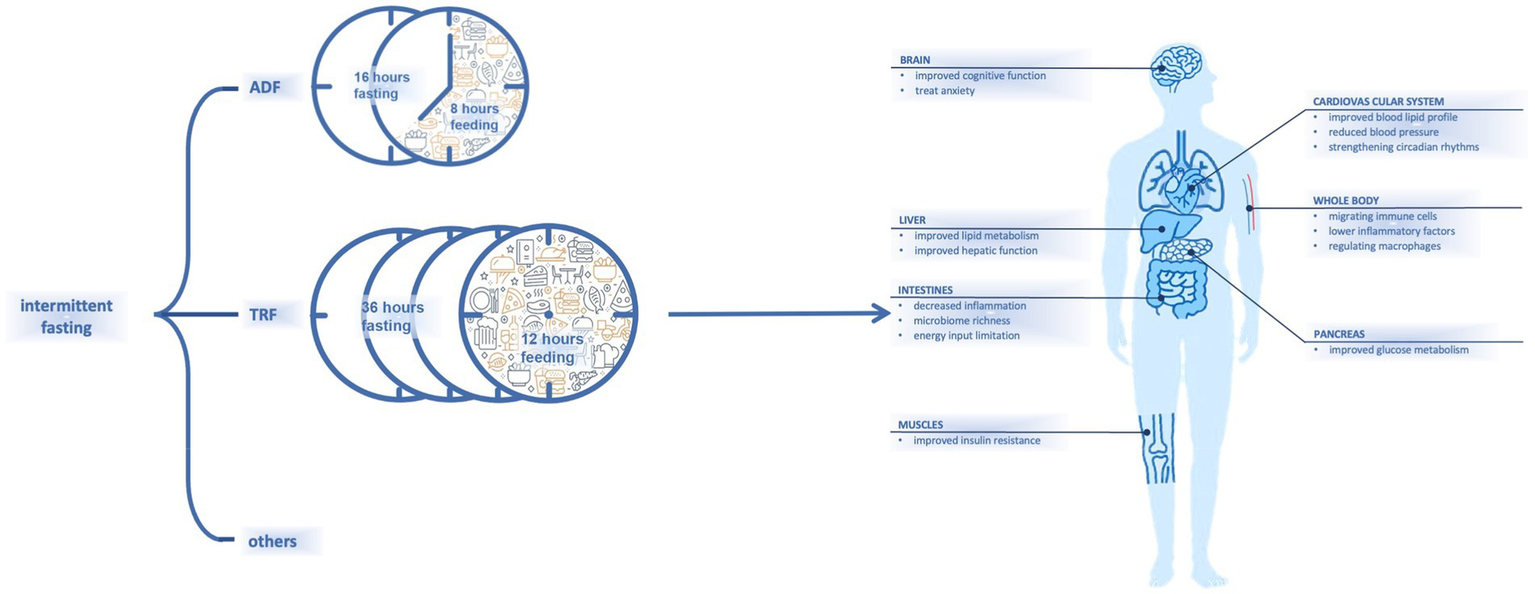
Content and presumed influences on body of intermittent fasting.
It has been shown that IF is effective for decreasing body weight (6), and it can help to regulate glucose or lipid metabolism and reduce blood pressure (7) (Figure 1). In one study, numerous subjects with metabolic syndrome experienced improvements in lipid and glucose metabolism after IF (8). Another study had also noted that healthy and lean people may experience metabolic improvements by resetting their dietary intake with a schedule of fasting and eating (9). As studies of additional parameters including pre-inflammatory markers have been conducted, other effects of fasting have been observed.
One area of great interest is the influence of fasting on the immune system, which responds to stressful and harmful events in the body (10). The immune system can be regulated by weight reduction; changes in lipid and glucose metabolism; and other processes, such as circadian rhythm changes (10–14). Whether the influence of fasting on the immune system would benefit different populations—including healthy people, people with metabolic syndromes, and those with other physiological or pathophysiological conditions—is subject to discussion.
In this systematic review, we summarize clinical trials that studied the immunomodulatory effects of IF. All types of subjects were included and divided into different groups including healthy subjects, obese subjects and others, to clarify the cross-effect between IF and subjects under different physiological and pathophysiological situations, including pregnancy, perioperative period,endocrine disease, cancer and autoimmune diseases. The purpose of this systematic review is to analyze and compare current trials on this topic and to provide insight into the possible influence of IF on the immune system.
2. Methods
This systematic review was conducted and presented according to the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA) guidelines (Supplementary Table S1) and Assessment of Multiple Systematic Reviews 2(AMSTAR 2) tools (Supplementary Table S2). Various databases were searched, including Cochrane, PubMed, and Embase, from January 2005 to August 2022. The terms used for the literature research were “time-restricted feeding,” “time-restricted eating,” “intermittent fasting,” “feeding schedule,” “food timing,” “meal frequency,” “compressed feeding,” and “restricted food intake.” These terms were then united with “OR.” In addition, the terms “normal human,” “adult,” “patient,” and “human” were linked with “OR.” The terms “immune,” “immunity,” “immunologic,” “lymphocyte,” “chemokine,” “interleukin,” “C-reactive protein,” “CRP,” “neutrophils,” “oxidative stress,” “oxidative burst,” “inflammatory,” “inflammation,” “immunoglobulin,” “autoimmune,” “lipid peroxidation,” “homocysteine,” “malondialdehyde,” “MDA,” “glutathione,” and “GSH” were united with “OR” and then added together with the aforementioned terms.
The inclusion criteria were as follows: randomized control trials and cohort studies; age > 18 years; one type of IF conducted; and at least one immunomodulatory marker analyzed. Exclusion criteria were as follows: intervention not strictly followed; no fasting procedure included in the intervention; IF combined with other eating interventions, such as liquid diet, protocol; and review articles.
A total of 3,558 potentially eligible articles were collected from the databases. After screening, 89 articles were selected for full-text review, of which 61 were excluded for unexpected interventions (Figure 2). Twenty-eight studies were later grouped into effects on healthy people, effects on obese subjects, and effects on other subjects according to the trial set. These grouping procedures were performed by two independent researchers before August 2022. The following parameters were extracted from the original articles for comparison: participants, trial length, intervention, control group, immunomodulatory effect, metabolic information, and body weight.
Figure 2
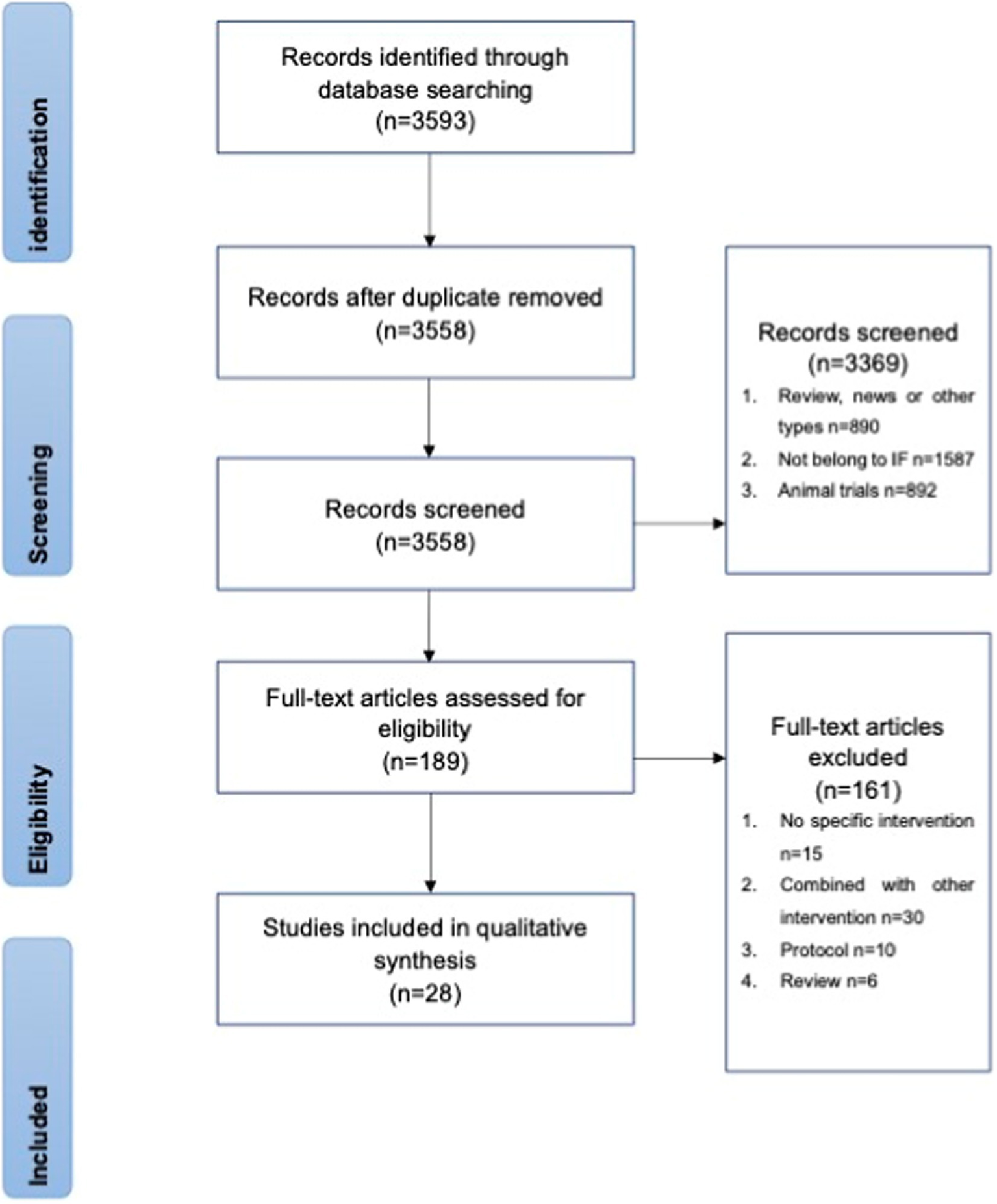
Search and study selection for systematic reviews (PRISMA) flow chart.
The Cochrane Collaboration tool (Supplementary Table S3) was applied to assess risk of bias in all included studies. The levels of evidence were as follows: randomized trials, nonrandomized controlled trials, historically controlled cohort studies, and single-arm noncontrolled trials. Because different trials had different levels of bias, a meta-analysis was not performed.
3. Results
3.1. Effects on non-obese healthy people
Eleven studies measured the immunomodulatory effect of IF on healthy people, and some included assessment of body weight changes or metabolic differences (Table 1).
Table 1
| Reference | Intervention | Control | Participants | Trial length | Immune immunomodulatory effect | Glucose metabolism | Lipid metabolism | Others | Body weight |
|---|---|---|---|---|---|---|---|---|---|
| Varady et al. (5) | ADF | RCT | BMI26 | 12w | CRP: ↓(p < 0.01) Leptin: ↓(p < 0.03) Adiponectin: ↑(p < 0.01) | TC: ⌀ LDL: ⌀ HDL: ⌀ TC*: ↓(p < 0.01) | DBP: ↓ (p < 0.05) SBP: ⌀ | ↓, −6.5 ± 1.0% (p < 0.001), on average 5.2 kg. | |
| Wegman et al. (15) | ADF | Crossover | BMI23 | 3w | Gene-upregulated*: SIRT1, SIRT3, SOD2, TFAM | Insulin: ↓(p = 0.0023) | |||
| Paoli et al. (16) | TRF | Before-after study | resistance-trained male | 8w | Adiponectin: ↑(p = 0.0000) Leptin: ↓(p = 0.0001) IL-1b: ↓(p = 0.0235) TT: ↓(p = 0.0476) IGF-1: ↓(p = 0.0397) IL-6*: ↓(p = 0.0035) TNF-α*: ↓(p = 0.0001) | Insulin: ↓(p = 0.0303) Glucose: ↓(p = 0.0011) | TG: ↓(p = 0.0201) HDL: ↑(p = 0.0142) LDL: ⌀ | ↓(p = 0.0448) | |
| Lauridsen et al. (3) | IF | Before-after study | lean | 4w | TNF-α: ⌀ IL-6: ⌀ IL-10: ⌀ Adiponectin: ⌀ Leptin: ⌀ Cortisol: ⌀ | Glucose: ⌀ Insulin: ⌀ HOMAIR: ⌀ HbA1c: ⌀ | HDL: ⌀ LDL: ⌀ TG: ⌀ TC: ⌀ | ALT: ⌀ SBP: ⌀ DBP: ⌀ | ↓(p = 0.05), on average 1.0 kg. |
| Gasmi et al. (17) | TRF | RCT | Young and aged | 12w | Red cells: ⌀ Monocytes: ⌀ Neutrophils: ↓ White blood cells: ↓ Lymphocytes: ↓ Natural killer cell: ↓ | ↓ young, (p < 0.05) | |||
| Madeo et al. (18) | ADF | Cross-sectional | healthy middle-aged | 4w | Monocytes: ⌀ Lymphocyte: ⌀ B cell: ⌀ CD4 T cell: ⌀ β-hydroxybutyrate*: ↓, (p = 0.003) | TC: ↓(p = 0.004) HDL: ⌀ LDL: ↓(p = 0.011) VLDL: ↓(p = 0.009) TG: ↓(p = 0.010) | SBP: ↓(p = 0.006) DBP: ↓(p = 0.0302) | ↓, (p < 0.0001), on average 3.5 kg. | |
| McAllister et al. (19) | TRF | RCT | BMI28 | 4w | Cortisol*: ↓ Adiponectin*: ↑ CRP: ↑ | Glucose: ⌀ Insulin*: ↑ | LDL: ↑ HDL: ⌀ TG: ↓ TC: ↓ | SBP: ↑ (P = 0.04) DBP: ⌀ | |
| Li et al. (20) | TRF | RCT | healthy man | 25d | IL-1β: ⌀ TNF-α: ⌀ Gene-upregulated: Bmal1(p = 0.0020), Clock(p = 0.0302), SIRT1(p = 00068) Microbial richness: ↑ (p < 0.005) | TC: ↓ (p < 0.0001) TG: ↓(p = 0.0052) LDL: ⌀ HDL: ↑(p < 0.0001) | AKP: ↓(p < 0.009) AST: ↓(p = 0.0268) ALT: ↓(p = 0.0174) Albumin: ↓, (p < 0.0001) | ||
| Moro et al. (9) | TRF | RCT | cyclist | 4w | TT: ↓(p = 0.0497) CRP: ⌀ ESR: ⌀ IL-6: ⌀ Adiponectin: ⌀ TNF: ⌀ TSH: ⌀ T3: ⌀ Cortisol*: ↓(p = 0.0005) IGF-1: ⌀ | Glucose: ⌀ Insulin: ⌀ | TC: ⌀ TG: ⌀ | Cr: ⌀ | ↓,2%(P = 0.04) |
| Paoli et al. (1) | TRF | RCT | healthy | 2 m/12 m | TT: ↓(p < 0.001) IGF-1: ↓(p = 0.039) Adiponectin: ↑(p = 0.001) Leptin: ↓(p < 0.001) Il-6: ↓(p = 0.038) IL-1β: ↓(p < 0.001) TNF-α: ↓(p = 0.042) | Glucose: ↓(p < 0.0001) Insulin: ↓(p < 0.0001) HOMA-IR: ↓(p < 0.0001) | TC: ⌀(p = 0.289) HDL: ↑(p < 0.001) LDL: ⌀(p = 0.129) TG: ↓(p < 0.0001) | ↓(p = 0.001), on average 2.89 kg. | |
| Mao et al. (21) | TRF | RCT | healthy | 5w | TNF-α: ↓(p = 0.024) IL-8: ↓(p = 0.045) CRP: ⌀ WBC: ⌀ Microbial-diversity: ↑ (p = 0.049) Resistin: ⌀ Leptin: ⌀ Ghrelin: ⌀ gene-upregulated: SIRT1, BMAL1, PER2, SIER1 | HOMA-IR: ↓, (p < 0.001, p = 0.002) Glucose: ↓(p = 0.005) | HDL: ⌀ LDL: ⌀ TC: ⌀ TG: ⌀ | SBP: ⌀ DBP: ⌀ AST: ↓(p = 0.046) ALT: ⌀ ALP: ⌀ GGT: ⌀ | ↓(P = 0.009), on average 1.6 kg. |
Effects on non-obese healthy objects.
WBC, White blood cells; NEUT, Neutrophile Granulocyte; PLT, Platelet; Hgb, hemoglobin; BCR/ABL, BCR/ABL gene; TT, testosterone; CRP, C-reactive protein; IGF-1, Insulin-Like Growth Factor 1; HOMA-IR, Homeostasis model assessment of insulin resistance; IF, intermittent fasting; RCT, randomized control study; CML, chronic myelogenous leukemia; PCOS, polycystic ovary syndrome; TC, total cholesterol; TG, triacylglycerol; LDL, low-density lipoprotein cholesterol; HDL, high-density lipoprotein cholesterol; AST, aspartate aminotransferase; ALT, glutamic-pyruvic transaminase; NK cell, natural killer cell; IL, interleukin; TNF, tumor necrotic factor; BP, blood pressure; ADF, alternative day fasting; TRF, time restricted feeding; TNF-a, tumor necrosis factor-alpha; SBP, systolic blood pressure; DBP, diastolic blood pressure; LDH, lactate dehydrogenase; GGT, gamma-glutamyl transpeptidase; ALP, alkaline phosphatase; HbA1c, glycosylated hemoglobin; VLDL, very low-density lipoprotein cholesterol; Cr, creatinine; ESR, erythrocyte sedimentation rate; TSH, Thyroid Stimulating Hormone; T3, triiodothyronine; ⌀, no significant results; ↑, significantly increasing; ↓, significantly decreasing. Some changes in values and p-values are missing as they were not presented in the original manuscript.*Indicates that the p-value was calculated based on the final and baseline values of participants in the TRF group because no comparison was made between changes in values in a TRF group and a normal diet control group in the original manuscript.
Various parameters were selected to investigate the immunomodulatory effects of IF in the eleven studies. Two studies measured the effects on immune cells but had different results. Madeo et al. found that almost all cell subsets remained the same (18), whereas Gasmi et al. observed that neutrophils, lymphocytes, and natural killer cells changed after a twelve-week trial of IF (17). Several studies have focused on classic inflammatory biomarkers. Lower levels of C-reactive protein (CRP), leptin, and adiponectin were observed in a study by Varady et al. (5). Similar results were reported by Paoli, both in an one-year (long-term) and an 8-week (short-term) trial (1)). However, Lauridsen et al. found that measurements of parameters such as tumor necrosis factor alpha (TNF-α), interleukin 6 (IL-6), and interleukin 10 (IL-10) were not significantly changed after a course of IF (3). Mao et al. reported that lower levels of TNF-α and IL-8 could be observed after 5 weeks of IF (21). A study by Mcallister et al. (19) measured cortisol levels and found no significant change and these results were replicated in a study by Moro et al. (9). Moro et al. also reported a significant decrease in testosterone levels (9). Two studies measured microbial diversity after IF and concluded that IF generated great richness (20). Li et al. attempted to explain this change and found that sirtuin1 (SIRT1) expression was higher after IF compared with baseline levels (20), which was regarded as a stimulator for circadian genes and correlated with microbial diversity. A study by Wegman et al. also measured Sirt-1–related genes and reported similar results (15).
In eight trials, the decrease of body weight was observed after several weeks; three additional studies did not assess this factor. With regard to glucose metabolism, seven studies measured levels of fasting insulin and fasting glucose and conducted the test of homeostatic model assessment of insulin resistance (HOMA-IR) (1). Two studies found no significant changes in these parameters (3, 9), whereas improvements in these parameters were observed in five other studies (1, 15, 16, 19, 21).
Nine studies measured parameters related to lipid metabolism, including total cholesterol (TC), triglycerides (TG), low-density lipoprotein (LDL), and high-density lipoprotein (HDL). Six of them found improvements in multiple parameters; IF was associated with higher HDL, lower TC, lower TG, and lower LDL (1, 5, 18, 20). The remaining three studies found no significant changes in these parameters (3, 9, 21). Effects on different parameters, such as systolic blood pressure, diastolic blood pressure, and alanine transaminase, have been reported in other studies (18, 20).
Sleep quality and appetite were evaluated in some studies (5, 19, 21), and there was no significance after IF (21). Another study showed that during fasting, satiety and fullness of subjects were lower than controlled group, but no differences were found in nausea scores between two groups (3). Alertness, focus perceiving and mood perceiving were measured insignificantly in one study (19).
3.2. Effects on obese subjects
The effects of IF on obese subjects have received much attention. Twelve studies that assessed this topic were identified (Table 2).
Table 2
| Reference | Intervention | Control | Participants | Trial length | Immune immunomodulatory effect | Glucose metabolism | Lipid metabolism | Others | Body weight |
|---|---|---|---|---|---|---|---|---|---|
| Varady et al. (22) | ADF | Before-after study | Obese | 8w | CRP: ⌀ Homocysteine: ⌀ Adiponectin: ↓, −30% (p < 0.05) Leptin: ↓, −21 ± 6% (p < 0.05) Resistin: ↓, −23 ± 6% | TC 4w: ↓, −20%(p < 0.05) LDL 4w: ↓, −31%(p < 0.05) HDL 4w: ⌀ TG: 4w ↓, −19% | ↓, −3.83%, on average 5.7 kg. | ||
| Varady et al. (2) | ADF | RCT | Obese | 12 m | CRP: ⌀ Homocysteine: ⌀ | Glucose: ⌀ Insulin: ⌀ | HDL: ↑ | BP: ⌀ | *↓, −6% |
| Peterson et al. (23) | TRF | RCT | Prediabetes | 5w | 8-isoprostane: ↓, −11 pg./ml (p = 0.05) TNF-α: ⌀ cortisol: ⌀ | Glucose: ⌀ Insulin: ↓(p = 0.13) | HDL: ⌀ LDL: ⌀ TC: ↑(p = 0.0007) | SBP: ↓, -11 mmHg (p = 0.03) DBP: ↓, -10 mmHg (p = 0.03) | *↓(p = 0.12) |
| Bowen et al. (24) | ADF | RCT | Obese | 24w (16w + 82) | CRP: ↓ | Insulin: ↓ Glucose: ↓ | HDL*: ↑ LDL*: ↓ TC*: ↓ TG*: ↓ | SBP*: ↓ DBP*: ↓ | *↓, on average 11.2 kg. |
| Haus et al. (5) | ADF | RCT | Obese | 24w | Adiponectin: ↓ Leptin: ↓ IL-6: ↑ TNF-α: ⌀ | Glucose: ↓, (p = 0.031) Insulin: ↓, (p = 0.115) HOMA-IR: ↓, (p = 0.031) | ↓, (p < 0.001) | ||
| Heilbronn et al. (25) | IF | RCT | Obese | 8w | TNF-α: ⌀ IL-6: ⌀ IL-10: ⌀ Macrophage: ↓ | HOMA-IR: ↓ | ↓ | ||
| Varady et al. (6) | TRF | RCT | Obese | 10w | 8-isoprostane: ↓(p = 0.02) TNF-α: ⌀ IL-6: ⌀ | Glucose: ⌀ Insulin: ↓, (p = 0.02, p = 0.04) Insulin resistance: ↓, (p = 0.03, p = 0.04) | LDL: ⌀ HDL: ⌀ TG: ⌀ | SBP: ⌀ DBP: ⌀ | ↓,3.2%(4 h) ↓,3.2%(6 h) |
| Zouhal et al. (26) | IF | RCT | Obese | 30d | IL-6*: ↓, (p = 0.02) TNF-α*: ↓, (p = 0.019) | AST: ⌀ ALT: ⌀ LDH: ⌀ Urea: ⌀ | ↓,2.7% (P = 0.002) | ||
| Mindikoglu et al. (10) | IF | Before-after study | Metabolic syndrome | 4w | leptin: ⌀ Adiponectin: ⌀ CRP: ⌀ Homocysteine: ↑ (p = 0.0004) IL-1: ⌀ IL-6: ⌀ IL-8: ⌀ TNF-α: ⌀ Gene-upregulated: AP5Z1, YPS8, INTS6, IGFBP5, POLRMT, KIT, CROCC, PIGR, CALU Gene-downregulated: POLK, CD109, SRGN, CAMP | HOMA-IR: ⌀ Glucose: ⌀ Insulin: ⌀ | TG: ⌀ HDL: ⌀ TC: ⌀ LDL: ⌀ | SBP: ↓(P = 0.023) DBP: ↓(p = 0.002) ALT: ⌀ AST: ⌀ GGT: ⌀ ALP: ⌀ Albumin: ⌀ | ↓(p < 0.0001), on average 2,5 kg. |
| Horne et al. (27) | IF | RCT | Metabolic syndrome | 4w/13w/26w | Galectin-3: ↑(p = 0.021) | ||||
| Heilbronn et al. (28) | IF | RCT | obese women | 8w | Gene-nonregulated: LIPE, ACACA, FASN, DGAT1 Gene-upregulated: PLIN5 Gene-downregulated: SOD1, SOD2 β-hydroxybutyrate:↑(p < 0.05) | ↓(p < 0.05) | |||
| Safavi et al. (8) | ADF | RCT | Metabolic syndrome | 4 m | CRP: ↓(p = 0.03) TNF-α: ↓(p = 0.60) IL-6: ↓(p = 0.49) PT: ↑(p < 0.001) APTT: ↑(p = 0.04) | Glucose: ↓(p = 0.03) | ↓(p = 0.02), on average 6.43 kg. |
Effects on obese subjects.
WBC, White blood cells; NEUT, Neutrophile Granulocyte; PLT, Platelet; Hgb, hemoglobin; BCR/ABL, BCR/ABL gene; TT, testosterone; CRP, C-reactive protein; IGF-1, Insulin-Like Growth Factor 1; HOMA-IR, Homeostasis model assessment of insulin resistance; IF, intermittent fasting; RCT, randomized control study; CML, chronic myelogenous leukemia; PCOS, polycystic ovary syndrome; TC, total cholesterol; TG, triacylglycerol; LDL, low-density lipoprotein cholesterol; HDL, high-density lipoprotein cholesterol; AST, aspartate aminotransferase;ALT, glutamic-pyruvic transaminase;NK cell, natural killer cell; IL, interleukin; TNF, tumor necrotic factor; BP, blood pressure; ADF, alternative day fasting; TRF, time restricted feeding; TNF-a, tumor necrosis factor-alpha; SBP, systolic blood pressure; DBP, diastolic blood pressure; LDH, lactate dehydrogenase; GGT, gamma-glutamyl transpeptidase; ALP, alkaline phosphatase; HbA1c, glycosylated hemoglobin; VLDL, very low-density lipoprotein cholesterol; Cr, creatinine; ESR, erythrocyte sedimentation rate; TSH, Thyroid Stimulating Hormone; T3, triiodothyronine; PT, prothrombin time; APTT, activated partial thromboplastin time; ⌀, no significant results; ↑, significantly increasing; ↓, significantly decreasing. Some changes in values and p-values are missing as they were not presented in the original manuscript.*Indicates that the p-value was calculated based on the final and baseline values of participants in the TRF group because no comparison was made between changes in values in a TRF group and a normal diet control group in the original manuscript.
Heilbronn et al. found that levels of TNF-α, IL-6, and IL-10 changed insignificantly during 8 weeks of IF, whereas macrophage counts increased significantly (25). Changes in CRP levels have been measured in several trials; however, almost no significant differences were observed (2, 10, 23, 24, 29). Conversely, Varady et al. found that 8-isoprostane decreased after 10 weeks of IF (6); these results were similar to those of a trial by Peterson et al. (23). Haus et al. reported that adiponectin and leptin levels decreased after a course of 24 weeks (29), and these results were confirmed by Varady et al. in a before–after study (22). Significant changes in IL-6 and TNF-α levels were observed in a study by Zouhal et al. (26). After a four-month trial conducted by Safavi et al., subjects had lower CRP levels (8). Mindikoglu et al. attempted to determine the immunomodulatory effects of gene expression like AP5Z1 after finding almost no significant change on inflammatory parameters (10). Heilbronn et al. also found that gene expression like PLIN5 may result in immune system changes (28).
Significant body weight reductions were observed in all studies except that of Horne et al., which only identified significant changes in galectin-3 levels (27). Because metabolic syndrome is often related to obesity, glucose and lipid metabolism have been extensively researched in obese subjects. Mindikoglu et al. compared fasting glucose and insulin levels before and after 4 weeks of IF and found no significant changes (10). Varady et al. also found no improvements in glucose metabolism in obese subjects who completed IF, but that study did identify higher level HDL (2). Six studies found that fasting insulin, fasting glucose, and HOMA-IR levels were improved after IF than before (6, 8, 23, 24, 26, 29). Augmentation of lipid metabolism was observed in a study by Varady et al. in obese subjects (22). However, other studies on lipid metabolism did not show such significant results. In addition to the collected metabolic findings, four studies found that IF could reduce blood pressure levels (10, 23, 24, 26).
3.3. Effects in other conditions
Five studies focused on the effects of IF on special populations, including individuals in special physiological states, such as during pregnancy or before or after an operation, and individuals with conditions such as polycystic ovary syndrome (PCOS), multiple sclerosis (MS), or chronic myelogenous leukemia (CML) (Table 3).
Table 3
| References | Intervention | Control | Participants | Trial length | Immune immunomodulatory effect | Glucose metabolism | Lipid metabolism | Others | Body weight |
|---|---|---|---|---|---|---|---|---|---|
| Ozturk et al. (30) | IF | RCT | Pregnant | 4w | Oxidative stress index (OSI): ⌀ Total oxidant status (TOS): ⌀ Total anti-oxidant status (TAS): ⌀ | ||||
| Nashwan et al. (31) | IF | Retrospective study | CML | WBC, NEUT, PLT, HGB*: ⌀ BCR/ABL*:⌀ | |||||
| Bing he et al. (32) | Eating on 8:00–16:00 | Before-after study | PCOS | 5w | TT: ↓(p = 0.048) CRP: ↓(p = 0.040) IGF-1: ↑(p = 0.006) | Glucose: ⌀ Insulin: ↓ (p = 0.017) HOMA-IR: ↓(p = 0.025) | TG: ⌀(p = 0.715) TC: ⌀(p = 0.328) LDL: ⌀(p = 0.984) | AST: ↓(p = 0.113) ALT: ↓(p = 0.027) | ↓(p < 0.001), on average 1.3 kg. |
| Fitzgerald et al. (33) | IF | RCT | Obese, multiple sclerosis | 8w | Leptin: ⌀ Adiponectin: ⌀ Memory T cell subsets: ↓ Naïve subset: ↑ Th1 cell: ↓ | ↓ | |||
| Ginhoven et al. (34) | IF | RCT | Kidney donation,BMI25 | CRP: ⌀ WBC, B cell, T cell: ⌀ NK cell: ↓after surgery (P < 0.001) IL-10, IL-6: ⌀ TNF-α: ⌀ before surgery, ↓after surgery Cytokine: ⌀ IL-8: ↑(p = 0.018) |
Effects in other conditions.
WBC, White blood cells; NEUT, Neutrophile Granulocyte; PLT, Platelet; Hgb, hemoglobin; BCR/ABL, BCR/ABL gene; TT, testosterone; CRP, C-reactive protein; IGF-1, Insulin-Like Growth Factor 1; HOMA-IR, Homeostasis model assessment of insulin resistance; IF, intermittent fasting; RCT, randomized control study; CML, chronic myelogenous leukemia; PCOS, polycystic ovary syndrome; TC, total cholesterol; TG, triacylglycerol; LDL, low-density lipoprotein cholesterol; HDL, high-density lipoprotein cholesterol; AST, aspartate aminotransferase;ALT, glutamic-pyruvic transaminase;NK cell, natural killer cell; IL, interleukin; TNF, tumor necrotic factor; BP, blood pressure; ADF, alternative day fasting; TRF, time restricted feeding; TNF-a, tumor necrosis factor-alpha; SBP, systolic blood pressure; DBP, diastolic blood pressure; LDH, lactate dehydrogenase; GGT, gamma-glutamyl transpeptidase; ALP, alkaline phosphatase; HbA1c, glycosylated hemoglobin; VLDL, very low-density lipoprotein cholesterol; Cr, creatinine; ESR, erythrocyte sedimentation rate; TSH, Thyroid Stimulating Hormone; T3, triiodothyronine; ⌀, no significant results; ↑, significantly increasing; ↓, significantly decreasing. Some changes in values and p-values are missing as they were not presented in the original manuscript. *Indicates that the p-value was calculated based on the final and baseline values of participants in the TRF group because no comparison was made between changes in values in a TRF group and a normal diet control group in the original manuscript.
Ozturk et al. conducted a study of Ramadan IF in pregnant women. Total antioxidant status, total oxidant status, and related indices were measured; however, none showed significant changes after 4 weeks of the intervention. Pregnancy complications and birth weights were measured but showed no significant results between the IF-treated group and the controlled group (30). A study by Ginhoven et al. focused on IF during the perioperative period; 30 subjects who underwent kidney donation surgery were randomly assigned into a 1-day fasting group and a four-day restriction group. Many indicators were examined including CRP, white blood cells (WBCs), B cells, T cells, natural killer cells, IL-10, IL-6, TNF-α, and lipopolysaccharide. No statistically significant preoperative differences between groups were observed, with the exception of IL-8, which peaked at 6 hours after surgery in both groups but was significantly higher in the restriction group (p = 0.018). After surgery, the restriction group showed lower natural killer cell counts, lower WBC counts, and lower TNF-α levels (34).
Yassin et al. conducted a retrospective study of the effects of IF in subjects with CML. Forty-nine subjects were enrolled and tested before, during, and after fasting. BCR-ABL expression levels were measured and showed no significant difference among the three time points. Various hematological parameters, including WBC, hemoglobin, and platelet levels, showed no significant changes (31).
An eight-hour IF was conducted in 15 women with PCOS for 5 weeks; participants reported significant decreases in body weight (32). Metabolic parameters were also assessed, and lipid metabolism had insignificant changes, whereas fasting insulin levels and HOMA-IR decreased significantly after IF compared with their baseline levels (32). Total testosterone decreased by approximately 10%, but changes in luteinizing hormone (LH) and follicle-stimulating hormone (FSH) levels were not significant (32). A reduction in high-sensitivity CRP (hsCRP) and alanine transaminase (ALT) levels was observed, and insulin-like growth factor 1 (IGF-1) was upregulated (32). Fitzgerald et al. found that IF could alter T-cell subsets and metabolic markers in subjects with multiple sclerosis. The subjects in that study lost an average of 3.0 kg after the eight-week trial and had no significant changes in leptin and adiponectin levels. Individuals in the IF group showed significant reductions in memory T cells and increased naïve cell subsets (33).
4. Discussion
For non-obese healthy people, it is believed that the body could maintain a steady state in which lipid and glucose metabolism are effective and the immune system works well (18). From some perspectives, IF could still benefit healthy people. After IF, WBC subsets in two trials changed in different ways (17, 18). The reduction in neutrophils may have resulted from the migration to extravascular lymphoid tissues (17). This process requires a long intervention, so another shorter trial conducted by Madeo et al. did not show such results (18). More studies showed that the elimination of old damaged cells would process during fasting, and more active immune cells would be generated when fasting ended (35). In this way, IF could protect various tissues against diseases with more active immune cells by hormesis mechanisms that increase cellular stress resistance (36). A decrease in natural killer cells is mainly linked to a decrease in IL-2 or IGF-1. Neither of which were measured in the trial by Madeo et al., but it could be observed in two studies by Paoli et al. (1, 16). Besides IGF-1, other measurements also show significant changes. Adiponectin may interact with adenosine 5′-monophosphate-activated protein kinase (AMPK) (19), which then helps to regulate insulin resistance (9). High level of adiponectin would stimulate fatty acid oxidation in skeletal muscle and inhibit glucose production in the liver, which benefit to energy homeostasis (37). Meanwhile, adiponectin is an anti-inflammatory agent, a reduction of inflammatory markers including CRP and TNF-α could be observed in some studies (1, 5). Changes in gene expression provide more information on immunomodulatory effects: Wegman et al. concluded that an increase in SIRT1 and sirtuin3 (SIRT3) expression could be detected after a 3-week trial (15). For SIRT1, other studies have also shown an increase level (21). SIRT1 is linked to circadian rhythms and cellular mechanisms, such as cell repair, division, metabolism, and growth (20). It could be concluded that IF could protect bodies from cardiovascular diseases. SIRT3 is a member of the sirtuin family of histone deacetylases, which are primary mitochondrial protein deacetylases. Moreover, it could regulate cell metabolism, thus maintaining myocardial energy steady. SIRT3 is also believed as a protection for cardiomyocytes from oxidative stress-mediated cell damage (38). Besides that, some animal studies showed more exciting results through SIRT3 regulation of IF. High expressions of SIRT3 in cerebral cortical and hippocampal cells could benefit for treating anxiety and cognitive disorders, which was found as considerable overlap mechanisms by which IF and exercise enhance brain function of Alzheimer’s Disease patients (39, 40). A study by Mao et al. investigated clock genes and showed that levels of genes such as BMAL1 and PER2 were elevated in a five-week trial (21), indicating that IF could partly modulate the immune system by improving the circadian rhythm. The reinforcement of circadian rhythm could benefit body immune through promoting system recovery and the clearance of harmful cellular element (41). Another potential immunomodulatory effect involves microbial diversity in two studies (20, 21): Low gut microbial diversity is associated with metabolic disease (42), and high diversity may be due to the high expression of SIRT1 and high levels of HDL (20) and improve body immune system, such as liver function mentioned in the study by Li et al. Emerging evidence showed that SIRT1 could promote gut microbial population shifts by influence inflammation and circadian rhythm (43). It has also been suggested that IF could benefit healthy people lose weight (9), even cyclists and men who practice resistance training (9, 16). After the trial, it was concluded that IF could lose almost fat and maintain muscle mass with the measurement of muscle area of the thigh and arm. Healthy individuals might already have high insulin sensitivity at baseline; thus, IF seems to have less influence on glucose metabolism in these non-obese and healthy individuals (18). Things were similar when focusing on lipid metabolism. A decrease in leptin was found in many studies (1, 16), which might suggest that IF could partly strengthen lipid metabolism in healthy individuals. To sum up, IF could benefit immune system of healthy people through migration of immune cells, regulation of oxidative-related and circadian-related genes, increasing gut microbial diversity and improvement of muscle-fat ratio. Trials with longer durations and more factors including anxiety degree, cognition state, microbial diversity (44–46), key gene expression, and inflammatory markers are needed to better clarify the immunomodulatory effects of IF on healthy people.
Most obese subjects would harbor inflamed adipose tissue, which could cause a persistent, low-grade, inflammatory response. Obesity is often associated with the metabolic syndrome, because fat accumulation would cause insulin resistance (47). And evidence accumulated that persistent inflammation of adipose tissue is a central mechanism through which obesity promotes cancer risk (48). From the perspective of immune cells, A decrease in macrophages was observed in a study by Heilbronn et al. (25). Most cytokines that are produced by adipose tissue originate from nonfat cells and macrophages (29), thus the result confirmed that IF could be beneficial for inflammation associated with obesity. Recent studies have suggested that IF inhibits the nuclear factor kappa-B signaling pathway, which is an important regulator of downstream parameters including TNF-α and IL-6 (25), which is consistent with the results that IF could partly eliminate the inflammation caused by adipose tissue, with lower CRP and TNF-α (26). There were insignificant changes of some inflammatory markers in some studies, which might be related with short trial duration and inadequate weight loss (6). The concentration of galectin-3, which plays various roles in humans, was measured increasingly by Horne et al. in 2021 (27). It has been shown that galectin-3 could stimulate the expression of some antiviral genes and protect against inflammation, which may result in improvements in glucose metabolism. Although changes in inflammatory factors were less significant in obese people compared with healthy subjects, the immunomodulatory effect of IF observed in obese people might reflect a suppression of oxidative stress (26). Heilbronn et al. found that the ketone bodies, especially β-hydroxybutyrate, which protects against lipotoxicity and stimulates lipid oxidation, was significantly elevated in obese subjects (28). As it was regarded as an epigenetic regulator in terms of histone methylation, acetylation, IF could help to delay various age-related diseases. A decrease in 8-isoprostane, a marker of oxidative stress in lipids, was observed in two studies (6, 23). Oxidative stress is a definition of the imbalance between the production and elimination of reactive oxygen species (49). Some other studies have suggested that, though IF might have little effect on inflammation, it may greatly influence oxidative stress, which is linked to insulin resistance (26). Interestingly, improvements in glucose metabolism were observed in two studies that reported decreased oxidative stress markers (6, 23). Significant changes in leptin, which is regarded as a special body weight regulating hormone, were also noted (29), meaning that the resistance to leptin is partly improved in obese subjects. Besides the ability to regulate metabolic syndrome, including lowering glucose and lipid synthesis (50), leptin is one of the mediators responsible for the inflammatory state (51). In addition to the findings about immune cells and inflammatory markers, other study conduct tests of gene expression. Heilbronn et al. found that genes related to oxidative stress were down-regulated such as SOD1 and SOD2 (28), and Mindikoglu et al. found that the expression of other genes including the tumor activators POLK, NIFK, SRGN, CAMP, and D109, were downregulated (10), which are consistent with remitting oxidative effect and lowering cancer risk by IF. To sum up, besides the advantages of IF on obese subjects including losing body weight, regulate lipid metabolism and improve insulin resistance, which was almost suggested in all studies, IF could reduce oxidative stress and remit inflammatory state through macrophage adjustment and hormone secretion. Moreover, although evidence is accumulating that gut microbial is involved in the etiology of obesity (52) and altered by modified IF (4), relevant researches were still rare. Another issue waiting for more studies was the influence between IF and nervous system on obese subjects. Neuroinflammation, which has emerged as a crucial cause of cognitive dysfunction, such as Alzheimer’s Disease, could be caused through inflamed adipose tissue of obesity (53). A study in obese rat showed that IF could prevent memory loss in comparison to ad libitum by regulating body metabolism (54), which offering a new sight for the advantages of IF to remit neuroinflammation.
Pregnancy is a state of high oxidative stress, which contributes to preeclampsia and restriction of fetal growth (30). Maternal IF resulted in detrimental influence on fetal development and maternal stress stage by changing the metabolite profiles in animal studies (55). However, IF has no significant influence on the high oxidative stress and fetal development in the human study (30). The reason could be the different circadian rhythms between rats and humans. A case related with gestational diabetes mellitus was reported that IF is a useful intervention to reduce maternal body weight, plasma glucose, and psychological distress without any adverse effects (56). Surgery is regarded as a shock or an acute stress, and IF is able to improve resistance to this stress. In a study by Ginhoyen et al. (34), a higher preoperative IL-8 level may counter the proinflammatory influence of subsequent surgery, thus TNF-α was lower in the food-restriction group after surgery. Compared with subjects in the non-fasting group, subjects in the restriction group showed a more moderate postoperative inflammatory response. For healthy people in special physiological states, such as those observed during the perioperative period, IF could reduce acute stress. More trials are needed to identify the influence on pregnant subjects, including the fetal and maternal safety, anti-stress effect and body metabolism regulation. It is worth nothing that study include in this review on pregnant subjects was a Ramadan IF trial, which might be less convincing as subjects in this study had experienced such interventions before.
PCOS is an endocrine condition closely linked to metabolic disorders. Because obesity is closely related to PCOS, it is not surprising that IF could provide benefits by reducing insulin resistance and easing hyperandrogenemia (32). Whether IF could be applied in subjects with cancer remains unclear (57), because it may also affect chemotherapy. In a study of subjects with CML, Yassin et al. reported that fasting did not result in significant immunological effects with measurements including BCR-ACL levels and hematological parameters (31). It was suggested that IF in some patients who have cancer could be capable of decreasing chemotherapy-related toxicity and tumor growth, however (58), more clinical trials were needed to clarify. MS is an autoimmune disease characterized by degeneration of the central nervous system (59). The epidemiology of this condition includes a history of childhood obesity. Although no significant changes in leptin or adiponectin levels have been observed in studies of IF in MS, an observed difference in T-cell subsets in intestines might explain the immunological effects of IF that have been reported in studies (33, 60), which was also a kind of possible therapy for MS (59). The components of the intestinal microbiome could also raise the propensity to develop MS strongly (59). Researches about gut microbial of the influence of IF on subjects who have MS were expected as a result of migration of intestine immune cell subsets. As mentioned before, IF is beneficial for nervous system by cellular, metabolic and circadian mechanisms and a promising therapy for brain disorders, future research should disentangle whether positive effects of IF could be applied in clinical situations (61). Besides IF, other types of diet, including energy-restricted fasting and ketogenic diet (62), were also evaluated as nutrition therapy for MS (63). Some advantages were concluded that ketone bodies produced in these diets could serve as an alternative energy source for the brain (62), and during 3-day cycles of a fasting mimicking diet, it was found that the clinical symptoms of experimental autoimmune encephalomyelitis mice. More results was put forward that the improvement of this diet was related with immune system, including reducing inflammatory cytokines and immune cell migration. However, these diets might cause deficiency of various nutrients in long term (63). To sum up, a special diet could serve as a unique nutrition therapy for MS with disadvantages of nutrition deficiency, which was nowadays a popular and promising topic.
The different evidence levels should be taken into consideration when analyzing the results of these studies. Of the 28 selected trials, 19 were randomized, controlled, parallel, or crossover studies. Some trials were cohort studies, and the trial focusing on CML was a retrospective study; the lack of a control group in that trial may lead to inaccurate conclusions. Trials differed in terms of baseline characteristics, study durations, meal types, and IF types. These differences may interfere with the final results. For example, Gasmi et al. studied whether young people and old people would act differently while undertaking IF (17), Paoli et al. compared all factors in a 2-month trial and in a 1-year trial (1), and Varady et al. focused on whether the influence of IF would vary with different durations of eating windows (6). In the future, more studies on this topic should be conducted to provide new data.
This systematic review finds substantial evidence that IF can modulate the immune system in non-obese healthy people, obese people, and subjects in other physiological or pathophysiological states and these effects were clinically relevant with cognitive improvement, lipid and metabolism regulation, and inflammatory state remission. The mechanisms influenced and regulated to drive changes in each population differ. For example, non-obese healthy people can metabolize lipids and glucose efficiently, so the immunomodulatory effect is reflected in immune cell subset migration, lower inflammatory factors, upregulation of circadian rhythm–related gene expression, and greater microbial diversity. Although weight reduction has also been observed in healthy people, changes in parameters of lipid and glucose metabolism remained insignificant in most cases. In obese people, IF contributes to body health by regulating macrophages, which is related to the inflammatory stage of adipose tissue. Although many inflammatory factors did not show significant changes in obese subjects, other important factors, including 9-isoprastane, leptin, and galectin-3, had significant changes. The gene expression of cancer activators and lipid oxidative activators provides insights into the mechanisms behind these immunomodulatory effects. In pregnant women, IF seems safe to be conducted and possibly useful to treat endocrine disorders during pregnancy. Moreover, IF is able to improve resistance to the stress of surgery. IF can be beneficial for the immune system of individuals with PCOS by improving endocrine function. Limited trials studying the effects of IF on cancer have been conducted. For nervous system, IF is believed to be applicable to treat anxiety and cognitive disorders by cellular, metabolic and circadian mechanisms. However, more trials are needed to better understand the effects and mechanisms by which IF modulates the immune system.
5. Conclusion
Our systematic review, analyzing data from IF studies in different populations, suggests that IF could have immunomodulatory effects in healthy people, obese people, and people with special physiological and pathophysiological conditions. Different mechanisms may contribute to these effects. IF can benefit non-obese healthy individuals by strengthening circadian rhythms, migrating immune cells, lower inflammatory factors, and enriching microbial diversity. In addition of the anti-inflammatory effect by regulating macrophages, protection against oxidative stress with hormone secretion and oxidative-related gene expression plays a key beneficial role for the influence of IF on obese subjects. Physiological stress by surgery and pathophysiological disorders by endocrine diseases may be partly eased with IF. Moreover, IF might be used to treat anxiety and cognitive disorders with its cellular, metabolic and circadian mechanisms. Finally, the specific effects of IF and the mechanisms pertaining to immune system in these conditions require additional studies.
Funding
This work was supported by grants from CAMS Innovation Fund for Medical Sciences (CIFMS) (No. 2021-I2M-1-058) and National High Level Hospital Clinical Research Funding (2022-PUMCH-B-034).
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
ZH, HX, HY, and YM contributed to conception and design of the study. ZH and HX organized the methodology, investigation, and data collection. ZH and CL performed the statistical analysis. ZH wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Acknowledgments
We would like to thank Editage (www.editage.cn) and Charlesworth (www.cwauthors.com.cn) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1048230/full#supplementary-material
References
1.
MoroTTinsleyGPacelliFQMarcolinGBiancoAPaoliA. Twelve months of time-restricted eating and resistance training improves inflammatory markers and Cardiometabolic risk factors. Med Sci Sports Exerc. (2021) 53:2577–85. doi: 10.1249/MSS.0000000000002738
2.
TrepanowskiJFKroegerCMBarnoskyAKlempelMCBhutaniSHoddyKKet al. Effect of alternate-day fasting on weight loss, weight maintenance, and Cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Intern Med. (2017) 177:930–8. doi: 10.1001/jamainternmed.2017.0936
3.
Harder-LauridsenNMRosenbergABenattiFBDammJAThomsenCMortensenELet al. Ramadan model of intermittent fasting for 28 d had no major effect on body composition, glucose metabolism, or cognitive functions in healthy lean men. Nutrition. (2017) 37:92–103. doi: 10.1016/j.nut.2016.12.015
4.
GuoYLuoSYeYYinSFanJXiaM. Intermittent fasting improves Cardiometabolic risk factors and alters gut microbiota in metabolic syndrome patients. J Clin Endocrinol Metab. (2021) 106:64–79. doi: 10.1210/clinem/dgaa644
5.
VaradyKABhutaniSKlempelMCKroegerCMTrepanowskiJFHausJMet al. Alternate day fasting for weight loss in normal weight and overweight subjects: a randomized controlled trial. Nutr J. (2013) 12:146. doi: 10.1186/1475-2891-12-146
6.
CienfuegosSGabelKKalamFEzpeletaMWisemanEPavlouVet al. Effects of 4-and 6-h time-restricted feeding on weight and Cardiometabolic health: a randomized controlled trial in adults with obesity. Cell Metab. (2020) 32:366–78.e3. doi: 10.1016/j.cmet.2020.06.018
7.
FangYGuYZhaoCLvYQianJZhuLet al. Impact of supervised beego, a traditional Chinese water-only fasting, on thrombosis and haemostasis. BMJ Nutr Prev Health. (2021) 4:4–17. doi: 10.1136/bmjnph-2020-000183
8.
RazaviRParvareshAAbbasiBYaghooblooKHassanzadehAMohammadifardNet al. The alternate-day fasting diet is a more effective approach than a calorie restriction diet on weight loss and hs-CRP levels. Int J Vitam Nutr Res. (2021) 91:242–50. doi: 10.1024/0300-9831/a000623
9.
MoroTTinsleyGLongoGGrigolettoDBiancoAFerrarisCet al. Time-restricted eating effects on performance, immune function, and body composition in elite cyclists: a randomized controlled trial. J Int Soc Sports Nutr. (2020) 17:65. doi: 10.1186/s12970-020-00396-z
10.
MindikogluALAbdulsadaMMJainAJalalPKDevarajSWilhelmZRet al. Intermittent fasting from dawn to sunset for four consecutive weeks induces anticancer serum proteome response and improves metabolic syndrome. Sci Rep. (2020) 10:18341. doi: 10.1038/s41598-020-73767-w
11.
LiuTXingYFanXChenZZhaoCLiuLet al. Fasting and overfeeding affect the expression of the immunity-or inflammation-related genes in the liver of poultry via endogenous retrovirus. Poult Sci. (2021) 100:973–81. doi: 10.1016/j.psj.2020.11.057
12.
AdawiM. The effects of the Ramadan fasting on metabolic and immunological disorders. Isr Med Assoc J. (2021) 23:251–2.
13.
WilhelmCSurendarJKaragiannisF. Enemy or ally? Fasting as an essential regulator of immune responses. Trends Immunol. (2021) 42:389–400. doi: 10.1016/j.it.2021.03.007
14.
OkawaTNagaiMHaseK. Dietary intervention impacts immune cell functions and dynamics by inducing metabolic rewiring. Front Immunol. (2021) 11:623989. doi: 10.3389/fimmu.2020.623989
15.
WegmanMPGuoMHBennionDMShankarMNChrzanowskiSMGoldbergLAet al. Practicality of intermittent fasting in humans and its effect on oxidative stress and genes related to aging and metabolism. Rejuvenation Res. (2015) 18:162–72. doi: 10.1089/rej.2014.1624
16.
MoroTTinsleyGBiancoAMarcolinGPacelliQFBattagliaGet al. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J Transl Med. (2016) 14:290. doi: 10.1186/s12967-016-1044-0
17.
GasmiMSellamiMDenhamJPaduloJKuvacicGSelmiWet al. Time-restricted feeding influences immune responses without compromising muscle performance in older men. Nutrition. (2018) 51-52:29–37. doi: 10.1016/j.nut.2017.12.014
18.
StekovicSHoferSJTripoltNAonMARoyerPPeinLet al. Alternate day fasting improves physiological and molecular markers of aging in healthy. Non-obese Humans Cell Metab. (2019) 30:462–76.e6. doi: 10.1016/j.cmet.2019.07.016
19.
McAllisterMJPiggBLRenteriaLIWaldmanHS. Time-restricted feeding improves markers of cardiometabolic health in physically active college-age men: a 4-week randomized pre-post pilot study. Nutr Res. (2020) 75:32–43. doi: 10.1016/j.nutres.2019.12.001
20.
ZebFWuXChenLFatimaSHaqIUChenAet al. Effect of time-restricted feeding on metabolic risk and circadian rhythm associated with gut microbiome in healthy males. Br J Nutr. (2020) 123:1216–26. doi: 10.1017/S0007114519003428
21.
XieZSunYYeYHuDZhangHHeZet al. Randomized controlled trial for time-restricted eating in healthy volunteers without obesity. Nat Commun. (2022) 13:1003. doi: 10.1038/s41467-022-28662-5
22.
BhutaniSKlempelMCBergerRAVaradyKA. Improvements in coronary heart disease risk indicators by alternate-day fasting involve adipose tissue modulations. Obesity (Silver Spring). (2010) 18:2152–9. doi: 10.1038/oby.2010.54
23.
SuttonEFBeylREarlyKSCefaluWTRavussinEPetersonCM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. (2018) 27:1212–21.e3. doi: 10.1016/j.cmet.2018.04.010
24.
BowenJBrindalEJames-MartinGNoakesM. Randomized trial of a high protein, partial meal replacement program with or without alternate day fasting: similar effects on weight loss, retention status, nutritional, metabolic, and behavioral outcomes. Nutrients. (2018) 10:1145. doi: 10.3390/nu10091145
25.
LiuBHutchisonATThompsonCHLangeKHeilbronnLK. Markers of adipose tissue inflammation are transiently elevated during intermittent fasting in women who are overweight or obese. Obes Res Clin Pract. (2019) 13:408–15. doi: 10.1016/j.orcp.2019.07.001
26.
ZouhalHBagheriRAshtary-LarkyDWongATrikiRHackneyACet al. Effects of Ramadan intermittent fasting on inflammatory and biochemical biomarkers in males with obesity. Physiol Behav. (2020) 225:113090. doi: 10.1016/j.physbeh.2020.113090
27.
HorneBDAndersonJLMayHTLeVTGalenkoODrakosSGet al. Intermittent fasting and changes in Galectin-3: a secondary analysis of a randomized controlled trial of disease-free subjects. Nutr Metab Cardiovasc Dis. (2022) 32:1538–48. doi: 10.1016/j.numecd.2022.03.001
28.
LiuBHutchisonATThompsonCHLangeKWittertGAHeilbronnLK. Effects of intermittent fasting or calorie restriction on markers of lipid metabolism in human skeletal muscle. J Clin Endocrinol Metab. (2021) 106:e1389–99. doi: 10.1210/clinem/dgaa707
29.
MirandaERFullerKNZPerkinsRKKroegerCMTrepanowskiJFVaradyKAet al. Endogenous secretory RAGE increases with improvements in body composition and is associated with markers of adipocyte health. Nutr Metab Cardiovasc Dis. (2018) 28:1155–65. doi: 10.1016/j.numecd.2018.07.009
30.
OzturkEBalatOUgurMGYazıcıogluCPenceSErelÖet al. Effect of Ramadan fasting on maternal oxidative stress during the second trimester: a preliminary study. J Obstet Gynaecol Res. (2011) 37:729–33. doi: 10.1111/j.1447-0756.2010.01419.x
31.
YassinMAGhasoubRSAldaptMBAbdullaMAChandraPShwayliaHMet al. Effects of intermittent fasting on response to tyrosine kinase inhibitors (TKIs) in patients with chronic myeloid leukemia: an outcome of European leukemia net project. Cancer Control. (2021) 28:107327482110092. doi: 10.1177/10732748211009256
32.
LiCXingCZhangJZhaoHShiWHeB. Eight-hour time-restricted feeding improves endocrine and metabolic profiles in women with anovulatory polycystic ovary syndrome. J Transl Med. (2021) 19:148. doi: 10.1186/s12967-021-02817-2
33.
FitzgeraldKCBhargavaPSmithMDVizthumDHenry-BarronBKornbergMDet al. Intermittent calorie restriction alters T cell subsets and metabolic markers in people with multiple sclerosis. EBioMedicine. (2022) 82:104124. doi: 10.1016/j.ebiom.2022.104124
34.
van GinhovenTMDikWAMitchellJRSmits-te NijenhuisMAvan Holten-NeelenCHooijkaasHet al. Dietary restriction modifies certain aspects of the postoperative acute phase response. J Surg Res. (2011) 171:582–9. doi: 10.1016/j.jss.2010.03.038
35.
BrandhorstSChoiIYWeiMChengCWSedrakyanSNavarreteGet al. A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and Healthspan. Cell Metab. (2015) 22:86–99. doi: 10.1016/j.cmet.2015.05.012
36.
MattsonMP. Dietary factors, hormesis and health. Ageing Res Rev. (2008) 7:43–8. doi: 10.1016/j.arr.2007.08.004
37.
FangHJuddRL. Adiponectin regulation and function. Compr Physiol. (2018) 8:1031–63. doi: 10.1002/cphy.c170046
38.
SunWLiuCChenQLiuNYanYLiuB. SIRT3: a new regulator of cardiovascular diseases. Oxidative Med Cell Longev. (2018) 2018:1–11. doi: 10.1155/2018/7293861
39.
LiuYChengALiYJYangYKishimotoYZhangSet al. SIRT3 mediates hippocampal synaptic adaptations to intermittent fasting and ameliorates deficits in APP mutant mice. Nat Commun. (2019) 10:1886. doi: 10.1038/s41467-019-09897-1
40.
CalabreseVCorneliusCDinkova-KostovaATCalabreseEJMattsonMP. Cellular stress responses, the hormesis paradigm, and vitagenes: novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid Redox Signal. (2010) 13:1763–811. doi: 10.1089/ars.2009.3074
41.
ScheiermannCKunisakiYFrenettePS. Circadian control of the immune system. Nat Rev Immunol. (2013) 13:190–8. doi: 10.1038/nri3386
42.
ÁlvarezJFernández RealJMGuarnerFGueimondeMRodríguezJMSaenz de PipaonM. Gut microbes and health. Gastroenterol Hepatol. (2021) 44:519–35. doi: 10.1016/j.gastrohep.2021.01.009
43.
HirahatakeKMSlavinJLMakiKCAdamsSH. Associations between dairy foods, diabetes, and metabolic health: potential mechanisms and future directions. Metabolism. (2014) 63:618–27. doi: 10.1016/j.metabol.2014.02.009
44.
MargalitOBoursiB. Tailoring bacterial taxa for immune cell modulation. Hepatobiliary Surg Nutr. (2021) 10:686–8. doi: 10.21037/hbsn-21-263
45.
ShengLJenaPKHuYWanYY. Age-specific microbiota in altering host inflammatory and metabolic signaling as well as metabolome based on the sex. Hepatobiliary Surg Nutr. (2021) 10:31–48. doi: 10.21037/hbsn-20-671
46.
GérardP. Beneficial effect of whole-grain wheat on liver fat: a role for the gut microbiota?Hepatobiliary Surg Nutr. (2021) 10:708–10. doi: 10.21037/hbsn-21-332
47.
EnginA. The definition and prevalence of obesity and metabolic syndrome. Adv Exp Med Biol. (2017) 960:1–17. doi: 10.1007/978-3-319-48382-5_1
48.
IyengarNMGucalpADannenbergAJHudisCA. Obesity and cancer mechanisms: tumor microenvironment and inflammation. J Clin Oncol. (2016) 34:4270–6. doi: 10.1200/JCO.2016.67.4283
49.
GrailleMWildPSauvainJJHemmendingerMGuseva CanuIHopfNB. Urinary 8-isoprostane as a biomarker for oxidative stress. A systematic review and meta-analysis. Toxicol Lett. (2020) 328:19–27. doi: 10.1016/j.toxlet.2020.04.006
50.
PereiraSClineDLGlavasMMCoveySDKiefferTJ. Tissue-specific effects of leptin on glucose and lipid metabolism. Endocr Rev. (2021) 42:1–28. doi: 10.1210/endrev/bnaa027
51.
Pérez-PérezAVilariño-GarcíaTFernández-RiejosPMartín-GonzálezJSegura-EgeaJJSánchez-MargaletV. Role of leptin as a link between metabolism and the immune system. Cytokine Growth Factor Rev. (2017) 35:71–84. doi: 10.1016/j.cytogfr.2017.03.001
52.
CanforaEEMeexRCRVenemaKBlaakEE. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat Rev Endocrinol. (2019) 15:261–73. doi: 10.1038/s41574-019-0156-z
53.
PugazhenthiSQinLReddyPH. Common neurodegenerative pathways in obesity, diabetes, and Alzheimer's disease. Biochim Biophys Acta Mol basis Dis. (2017) 1863:1037–45. doi: 10.1016/j.bbadis.2016.04.017
54.
ShinBKKangSKimDSParkS. Intermittent fasting protects against the deterioration of cognitive function, energy metabolism and dyslipidemia in Alzheimer's disease-induced estrogen deficient rats. Exp Biol Med (Maywood). (2018) 243:334–43. doi: 10.1177/1535370217751610
55.
AlkhalefahADunnWBAllwoodJWParryKLHoughtonFDAshtonNet al. Maternal intermittent fasting during pregnancy induces fetal growth restriction and down-regulated placental system a amino acid transport in the rat. Clin Sci (Lond). (2021) 135:1445–66. doi: 10.1042/CS20210137
56.
AliAMKunugiH. Intermittent fasting, dietary modifications, and exercise for the control of gestational diabetes and maternal mood dysregulation: a review and a case report. Int J Environ Res Public Health. (2020) 17:379. doi: 10.3390/ijerph17249379
57.
ZhaoXYangJHuangRGuoMZhouYXuL. The role and its mechanism of intermittent fasting in tumors: friend or foe?Cancer Biol Med. (2021) 18:63–73. doi: 10.20892/j.issn.2095-3941.2020.0250
58.
CliftonKKMaCXFontanaLPetersonLL. Intermittent fasting in the prevention and treatment of cancer. CA Cancer J Clin. (2021) 71:527–46. doi: 10.3322/caac.21694
59.
ReichDSLucchinettiCFCalabresiPA. Multiple Sclerosis. N Engl J Med. (2018) 378:169–80. doi: 10.1056/NEJMra1401483
60.
CignarellaFCantoniCGhezziLSalterADorsettYChenLet al. Intermittent fasting confers protection in CNS autoimmunity by altering the gut microbiota. Cell Metab. (2018) 27:1222–35.e6. doi: 10.1016/j.cmet.2018.05.006
61.
GuddenJArias VasquezABloemendaalM. The effects of intermittent fasting on brain and cognitive function. Nutrients. (2021) 13:3166. doi: 10.3390/nu13093166
62.
BahrLSBockMLiebscherDBellmann-StroblJFranzLPrüßAet al. Ketogenic diet and fasting diet as nutritional approaches in multiple sclerosis (NAMS): protocol of a randomized controlled study. Trials. (2020) 21:3. doi: 10.1186/s13063-019-3928-9
63.
AtabilenBAkdevelioğluY. Effects of different dietary interventions in multiple sclerosis: a systematic review of evidence from 2018 to 2022. Nutr Neurosci. (2022) 17:1–13. doi: 10.1080/1028415X.2022.2146843
Summary
Keywords
intermittent fasting, immune system, immunomodulatory effect, metabolic syndrome, obesity
Citation
He Z, Xu H, Li C, Yang H and Mao Y (2023) Intermittent fasting and immunomodulatory effects: A systematic review. Front. Nutr. 10:1048230. doi: 10.3389/fnut.2023.1048230
Received
19 September 2022
Accepted
09 February 2023
Published
28 February 2023
Volume
10 - 2023
Edited by
Reza Rastmanesh, The Nutrition Society, United Kingdom
Reviewed by
Eric Gumpricht, Isagenix International, LLC, United States; Friedemann Paul, Charité Universitätsmedizin Berlin, Germany; Cristoforo Scavone, University of São Paulo, Brazil
Updates
Copyright
© 2023 He, Xu, Li, Yang and Mao.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yilei Mao, pumch-liver@hotmail.com; Huayu Yang, dolphinyahy@hotmail.com
†These authors have contributed equally to this work
This article was submitted to Nutrition and Metabolism, a section of the journal Frontiers in Nutrition
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.