- 1Department of Gynecology and Obstetrics, The First Affiliated Hospital of Dalian Medical University, Dalian, Liaoning, China
- 2Department of Cardiology, The First Affiliated Hospital of Dalian Medical University, Dalian, Liaoning, China
Background: Previous studies have not established potential causal associations between coffee and caffeine consumption in endometrial cancer (EC) and its subgroups. Therefore, we used a two-sample MR method to assess the causal association between coffee and caffeine consumption and EC risk. We also evaluated the association between these genetically predicted exposures and EC prognosis.
Materials and methods: This study used 12 and two independent single-nucleotide polymorphisms (SNPs) associated with coffee and caffeine consumption as instrumental variables at a genome-wide significance level of p < 5 × 10–8. The EC Association Consortium (ECAC) performed a genome-wide association study (GWAS) analysis of 12,906 cases and 108,979 controls. FinnGen Consortium performed a GWAS analysis of 1,967 EC cases and 167,189 controls. The primary technique we employed was inverse-variance weighted, followed by the weighted median, MR-Egger regression, and MR robust adjusted profile score methods. We used the MR pleiotropy residual sum, Outlier test, and MR-Egger regression to assess Outlier and pleiotropic variants. We also conducted a sensitivity analysis through the leave-one-out method.
Results: Genetically predicted coffee consumption was not associated with EC and its subgroups in the ECAC, and the association was consistent in the FinnGen consortium. After excluding eight SNPs with confounding factors, the study performed sensitivity analyses, delivering consistent results. We also observed that caffeine consumption was not correlated with EC risk. As confirmed by MR analysis, selected SNPs determined that most do not significantly impact the likelihood of developing EC.
Conclusion: Our study indicated no convincing evidence supports coffee and caffeine consumption causing EC or impacting its prognosis. More studies are needed to validate the results.
1. Introduction
Endometrial cancer (EC) is one of the most common gynecologic malignancies. Its incidence was rising globally, with approximately 417,000 new cases in 2020 (1). If it continues its current trend, the number of women diagnosed with EC in the U.S. will reach 122,000 cases annually by 2030 (2).
It is recognized that Prolonged unopposed estrogen exposure is an established risk factor for EC. Metabolic factors such as obesity, insulin resistance, and dyslipidemia correlate with increased EC risk (3–6). Conversely, observational studies have shown that coffee and caffeine consumption negatively affect EC risk (7–9). Additionally, earlier research has linked higher coffee intake to lower levels of C-peptide and estrogen, two chemicals implicated in the development of endometrial cancer (10–12). However, the potential causal association of coffee and caffeine consumption with the risk of EC has yet to be established due to possible confounding factors and the lack of randomized controlled trials.
Mendelian randomization (MR) is a technique for assessing if an exposure factor has a causal effect on an outcome (13). MR strengthens causal inference by including genetic tools as exposure factors. It reduces reverse causation as alleles are randomly assigned during meiosis. The genetic tools are randomly assigned during conception and are usually not associated with confounding factors (14).
In this investigation, we evaluated the causal association of coffee and caffeine consumption with the risk of EC and its subgroups using a two-sample MR approach. We also assessed the correlation between the prognosis for EC and these genetically indicated exposures.
2. Materials and methods
2.1. Study design
Genetic variations serve as instrumental variables (IVs) in MR analyses to establish the causal link between exposure and outcome (15). MR analyses are focused on three essential hypotheses: (1) IVs are strongly associated with exposure factors; (2) IVs are not affected by any confounders; (3) IVs affect the outcome only through exposure factors, which are not related to the outcome (14). The flowchart of this MR study design is shown in Figure 1.
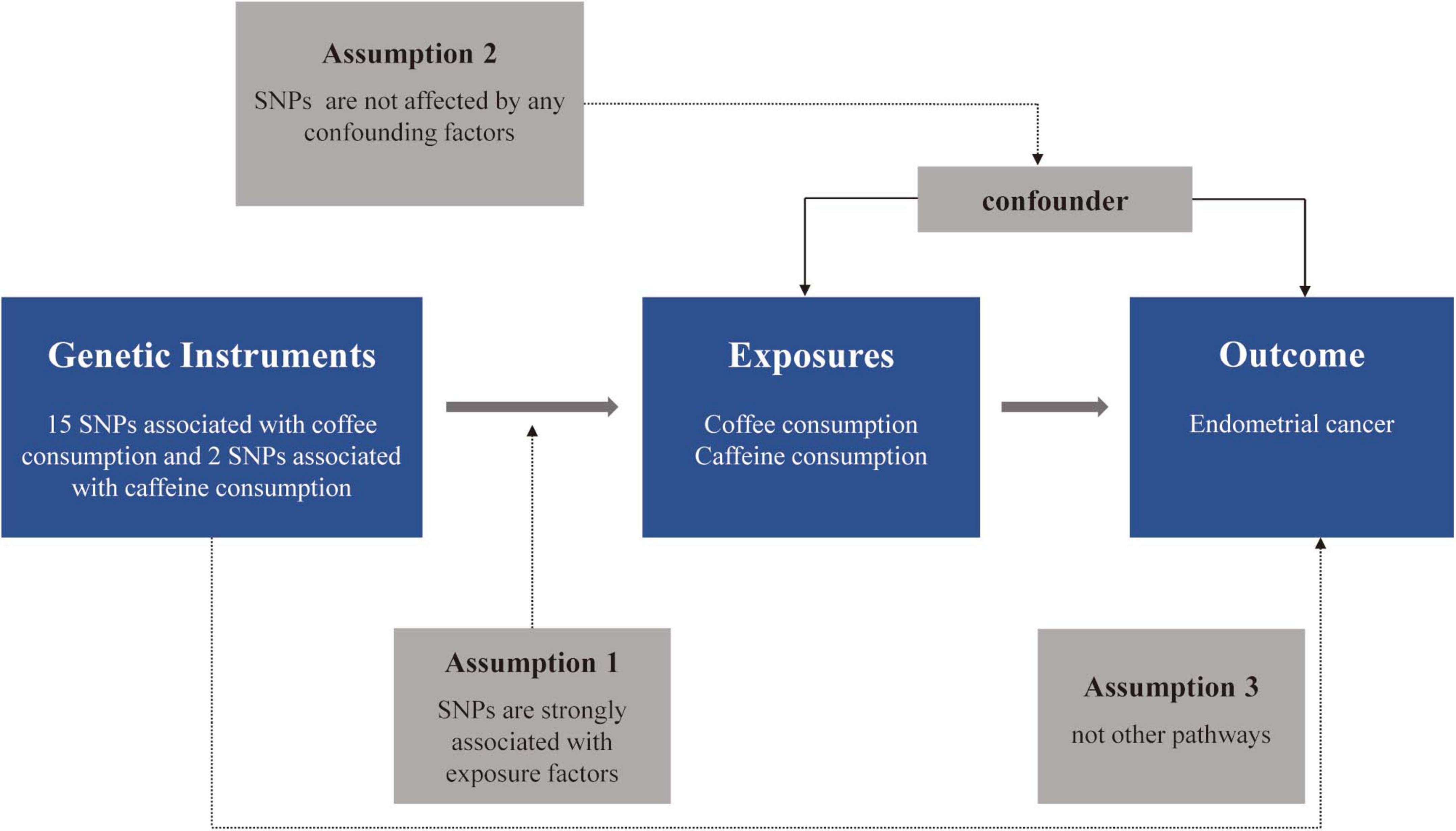
Figure 1. The flowchart of the Mendelian randomization (MR) study. SNP, single-nucleotide polymorphism.
2.2. Genetic instrument selection
The 15 single-nucleotide polymorphisms (SNPs) correlated with coffee consumption, derived from a meta-analysis of four large-scale genome-wide association studies (GWASs), involved 375,833 individuals (UK Biobank and three US cohorts) of European descent (16) (Supplementary Table 1). The GWASs adjusted for sex, age, total energy, body mass index, and top 20 principal components. In the United Kingdom Biobank (discovery phase), a touch screen questionnaire was applied to collect coffee consumption from all participants at baseline: “How many cups of coffee do you drink each day (including decaffeinated coffee)?” In the United States cohorts (replication phase), a semi-quantitative food frequency questionnaire was used to collect the regular and decaffeinated coffee consumption (16). The effect sizes of SNP-coffee associations increased by 50% (equivalently from 1 cup to 1.5 cups). To fulfill the first MR hypothesis, we selected SNPs that were reliably genetically variables (P < 5 × 10–8) and independently (linkage disequilibrium; LD r2 < 0.001 and cluster window > 10,000 kb) (17, 18) associated with exposures. Meanwhile, we calculated the F statistic (F > 10 indicates sufficient instrumental strength) (14). To fulfill the second MR hypothesis, we assessed the pleiotropic relationship between SNPs and potential confounders by searching the PhenoScanner V2 website (19, 20). Finally, to fulfill the third MR hypothesis, we excluded SNPs with P < 0.05 to ensure that IVs were not associated with the outcome (14). In the preliminary analysis, 11 SNPs were used as IVs for coffee consumption. Due to potential genome-wide confounders, we excluded eight SNPs and the remaining three as IVs in the sensitivity analysis (Supplementary Table 2).
The two variants associated with caffeine consumption came from a meta-analysis of 6 GWAS and included 9,876 individuals of European ancestry (21) (Supplementary Table 1). A self-reported questionnaire was used to find out how much caffeine people in coffee, tea, and cola drank. Pooled data on SNP-caffeine correlations were obtained from GWAS of 4,460 females and scaled to increase the caffeine measure by 80 mg, approximately equal to a cup of coffee (22). IVs were consistent with a P < 5 × 10–8, independent, and strongly correlated with the F-statistic, and were used to perform MR analyses. Detailed information on SNPs related to coffee and caffeine consumption is shown in Table 1.
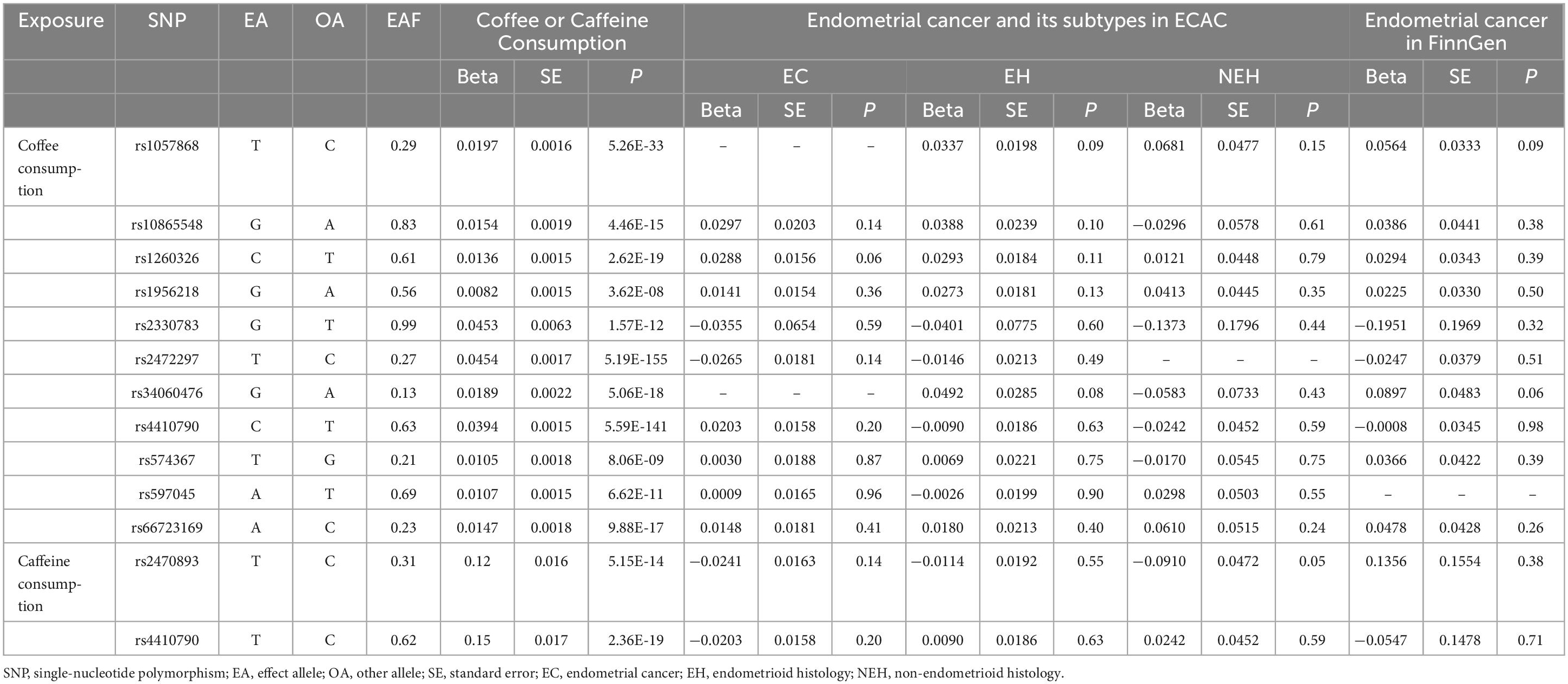
Table 1. Association of single nucleotide polymorphisms with coffee or caffeine consumption and endometrial cancer and its subtypes.
2.3. Data sources for endometrial cancer
Endometrial cancer-related data were obtained from the Endometrial Cancer Association Consortium (ECAC) and the FinnGen Consortium. ECAC performed a GWAS analysis of 12,906 cases and 108,979 controls (23). To avoid sample size overlap in the MR analysis, we removed the UK Biobank sample from the ECAC summary statistics, resulting in 12,270 EC cases and 46,126 controls (24). Furthermore, we analyzed the association of coffee and caffeine consumption with the risk of EC (8,758 patients with endometrioid histology and 1,230 cases with non-endometrioid histology) (25). We also performed Subgroup analyses in ECAC. GWAS analysis of 1,967 EC cases and 167,189 controls at the FinnGen Consortium (26). The ninth publication of the FinnGen Consortium database includes 377,277 individuals of Finnish ancestry, consisting of genes specific to the Finnish population, with high differential complementation accuracy and phenotypes from population-based registries. It includes cases from various disease domains, adjusted for age, sex, genetic principal components, and genotyping batches.
2.4. Data sources of BMI, smoking initiation, and alcohol consumption
Analyses were adjusted for differences in genetically predicted BMI, smoking initiation, and alcohol consumption using multivariable MR. The genetic variants linked to BMI and exposure variables were found through a GWAS meta-analysis in the Genetic Investigation of Anthropometric Traits1 consortium, which included 681,275 Europeans (27). GWAS data on 1,232,091 people showed summary-level information on how they started smoking (28). As was already said, GWAS data on 941,280 people showed that they drank alcohol, giving us summary-level statistics (28).
2.5. Statistical analysis
The inverse-variance weighted (IVW) was used as the primary statistical method. We used IVW with random effects to assess the relationships for genetically predicted coffee consumption. IVW fixed-effects models (analyses with several SNPs less than three) were used to estimate the interactions between genetic prediction and caffeine consumption. We also performed the weighted median (WM) and MR-Egger regression methods (29). The IVW method is applied to assume that all SNPs are valid and independent, and its meta-aggregation of multiple side effects in MR analyses of numerous SNPs (30). WM is the median obtained after weighting individual SNPs (31). The WM approach provides robust estimates, with at least 50% of the information coming from valid instrumental variables (32). The MR-Egger method allows for the inclusion of instrumental variables with a multivariate effect if the intercept P-value < 0.05 indicates the presence of horizontal multivariate validity (33). We applied the MR-pleiotropy residual sum and outlier (MR-PRESSO) methods to detect the presence of horizontal pleiotropy, and Cochran’s Q statistic was used to assess heterogeneity between SNPs in each analysis (34). We also conducted a sensitivity analysis through the leave-one-out method.
Moreover, we assessed the pathogenic impact of the selected SNPs on EC prognosis by MEndelian Randomization (SUMMER2), by the framework of MR analysis based on the Multi-Organomics Database of SUrvival-Related Cancers (35). All analyses were performed using the “TwoSampleMR” and “MR-PROESSO” packages in R software (version 4.3.1), and all statistical tests were two-sided.
3. Results
3.1. Causal relationship between coffee and caffeine consumption with risk of EC and its subtypes
The F-statistics for coffee (the 50% increase) and caffeine (the per 80-mg increase) in this study were 159 and 67, indicating that the IVs had sufficient instrumental strength. Furthermore, MR-PRESSO did not detect abnormal SNPs in preliminary and sensitivity analyses. We did not detect heterogeneity by IVW and MR-Egger regression.
In preliminary analyses, we found pleiotropy (p < 0.05) in the causal relationship between two data sets and coffee consumption by MR-Egger: endometrioid endometrial carcinoma (EEC) in the ECAC consortium and EC in the FinnGen consortium (Figure 2). To investigate the causality between coffee consumption and the risk of both EC and its subtypes, we processed the ECAC data by random-effects IVW. We found that coffee consumption was not associated with EC (OR = 1.217, 95% CI: 0.693–2.137). We also conducted a subgroup analysis of the ECAC data, which suggested that coffee consumption was not linked to non-endometrioid endometrial carcinoma (NEC) (OR = 1.187, 95% CI: 0.292–4.826). Furthermore, in the FinnGen consortium, coffee consumption did not affect EC (OR = 1.738, 95% CI: 0.587–5.142) (Figure 2). In sensitivity analyses, we did not find directed pleiotropy (p > 0.05). Additionally, we performed random-effects IVW analyses on the ECAC data and FinnGen consortium data, resulting in no significant differences from the preliminary analyses (Figure 3). Simultaneously, we adjusted pertinent variables such as body mass index, smoking initiation, and alcohol consumption and discovered that the outcomes did not deviate significantly from those of the primary analysis (Supplementary Table 3).

Figure 2. The association of genetically predicted coffee and caffeine consumption with endometrial cancer (EC) and its Subgroups. ECAC, the Endometrial Cancer Association Consortium; EH, Endometrioid histology; NEH, non-endometrioid histology; FinnGen, FinnGen Consortium; OR, odds ratio; CI, confidence interval.

Figure 3. Results of sensitivity analyses association of genetically predicted coffee consumption with EC. ECAC, the Endometrial Cancer Association Consortium; EH, Endometrioid histology; NEH, non-endometrioid histology; FinnGen, FinnGen Consortium; OR, odds ratio; CI, confidence interval.
To investigate the causal relationship between caffeine consumption and established outcomes, we analyzed the ECAC data by fixed-effects IVW, which showed that caffeine consumption was unrelated to the risk of EC (OR = 0.852, 95% CI: 0.852–1.003). We also performed subgroup analyses, which showed that caffeine consumption was not a factor in the pathogenesis of EEC (OR = 1.002, 95% CI: 0.827–1.214) and NEC (OR = 0.836, 95% CI: 0.523–1.337). In the FinnGen Consortium data analysis, we also observed that caffeine consumption was not correlated with EC risk (OR = 1.201, 95% CI: 0.258–5.587) (Figure 2).
The study also performed a leave-one-out analysis, which excluded the effect of individual SNPs on the overall causal estimate by removing each SNP stepwise and repeating the MR analysis. The leave-one-out analysis showed relatively stable results after removing each SNP (Supplementary Figures 1–4).
3.2. Effect of coffee and caffeine consumption on EC prognosis
Our analysis of selected SNPs has determined that most do not significantly impact the likelihood of developing EC, as confirmed by MR analysis. Shorter overall survival (OS) in EC was positively associated with the SNPs rs1956218 (HR: 1.275, P = 0.036) linked to coffee consumption, while the SNPs rs4410790 (HR: 0.702, P = 0.022) had the opposite effect. Additionally, coffee- and caffeine-consumption-associated SNPs rs4410790 (HR: 0.632, P = 0.012) and caffeine-consumption-associated SNPs rs73073176 (HR: 0.652, P = 0.048) were also identified to be associated with shorter cancer-specific survival (CSS) (Table 2; Figure 4).
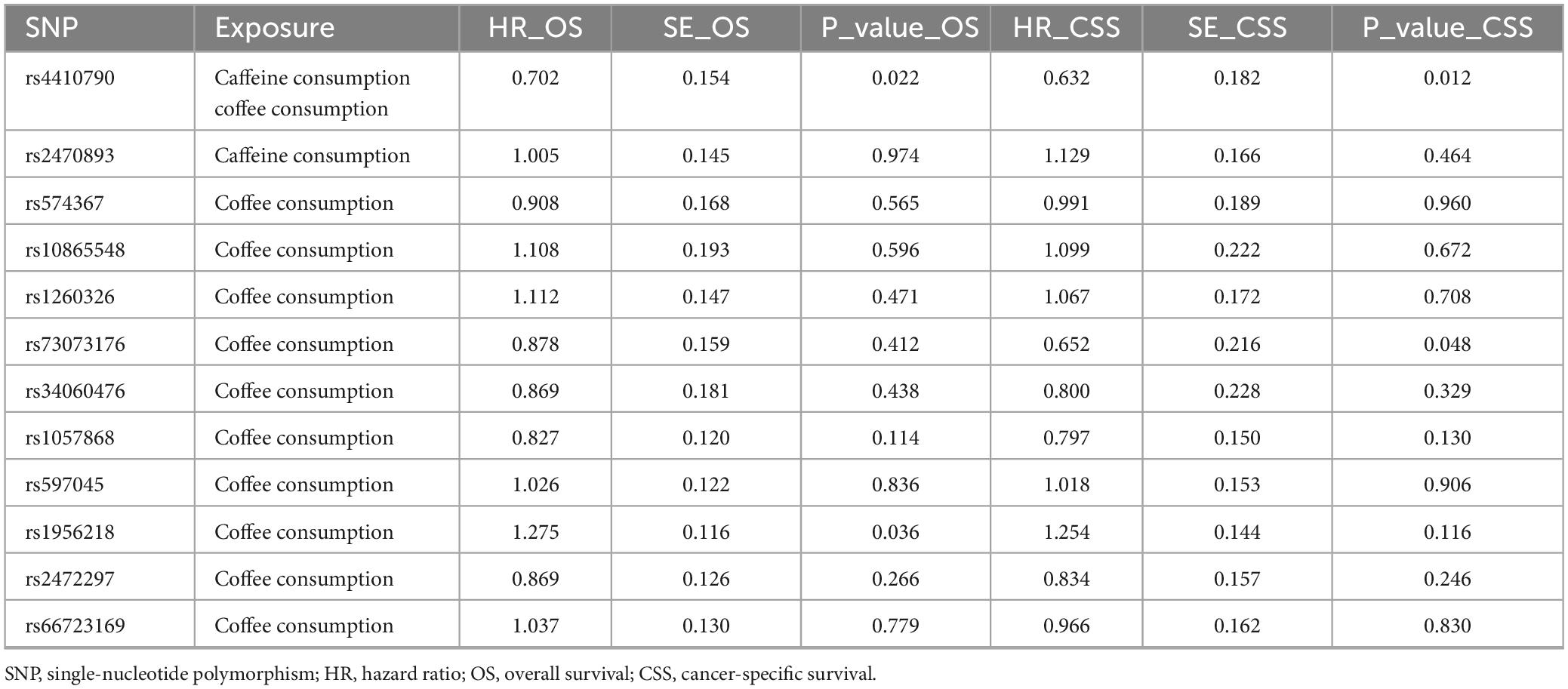
Table 2. Effect of coffee and caffeine consumption on overall survival and cancer-specific survival in all endometrial cancers.
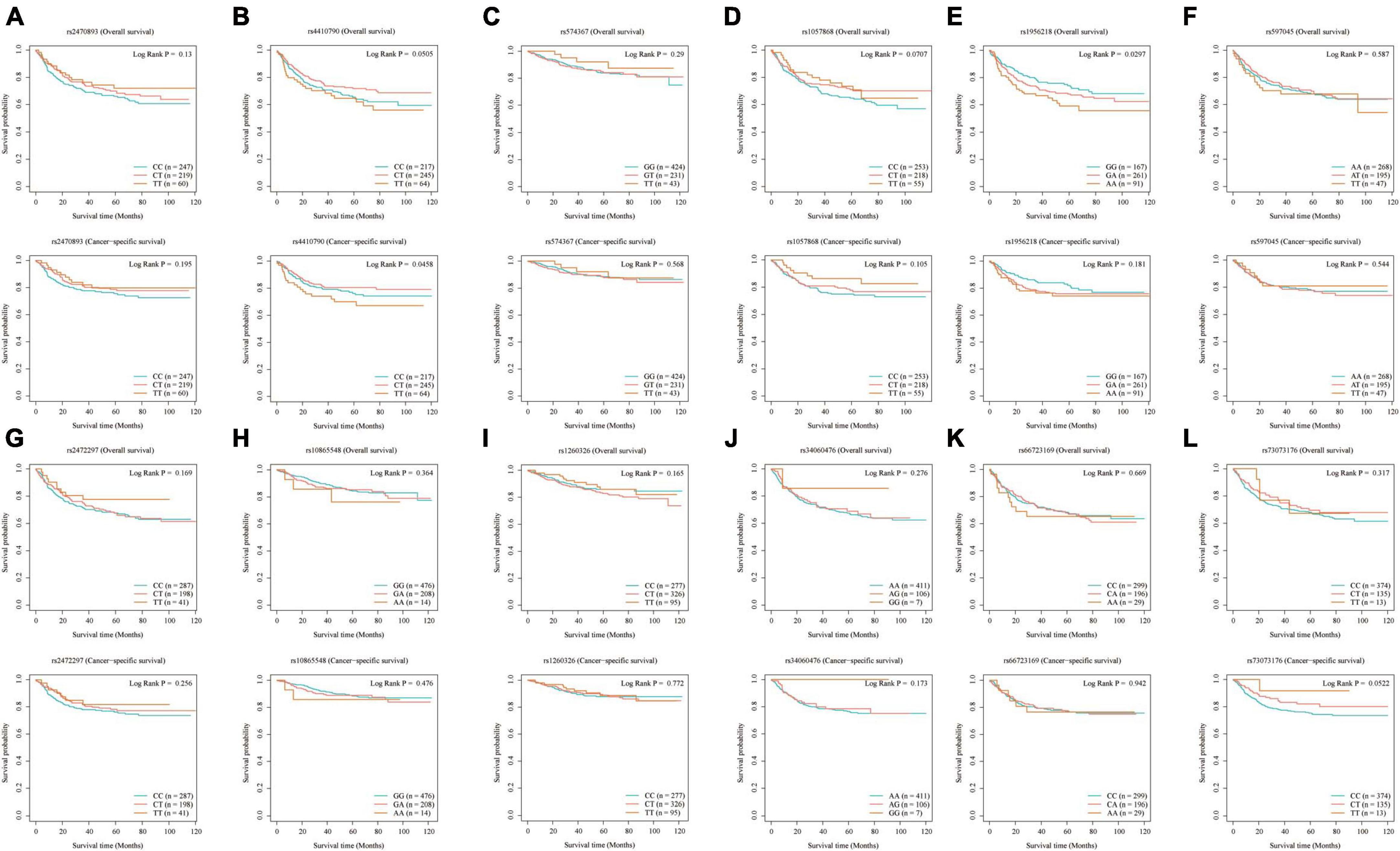
Figure 4. Kaplan–Meier plots of the effect of coffee and caffeine consumption on overall survival and cancer-specific survival in EC. (A) rs2470893; (B) rs4410790; (C) rs574367; (D) rs1057868; (E) rs1956218; (F) rs597045; (G) rs2472297; (H) rs10865548; (I) rs1260326; (J) rs34060476; (K) rs66723169; (L) rs73073176.
4. Discussion
Our study did not find genetically predicted associations between coffee and caffeine consumption regarding the risk of EC and its subgroups. No outlier SNPs were detected, although preliminary analyses detected pleiotropy in individual groups. Leave-one-out analyses also showed relatively stable results. After excluding SNPs with confounding factors, the study performed sensitivity analyses that did not detect pleiotropy or heterogeneity, delivering consistent results. Furthermore, we found that most SNPs were not associated with EC prognosis by MR analysis.
Previous research has been controversial regarding the association between coffee or caffeine consumption and the risk of EC. In recent times, a cross-sectional study (36) demonstrated that caffeine was not associated with the risk of EC [OR, 95% CI; 0.999 (0.996, 1.001), P = 0.297]. Moreover, a large prospective study (37) investigating the relationship between coffee and EC risk found that coffee intake was not significantly associated with EC risk. A published meta-analysis that resulted in this study also found a weak association between coffee consumption and EC risk. However, a meta-analysis including six cohort studies and 13 case-control studies supported coffee consumption’s potentially beneficial health effects on EC, especially in women with higher BMI (38). Meanwhile, in a meta-analysis of observational studies by Je et al. (39), an increase in coffee consumption of one cup/day was negatively associated with the risk ratio of EC and similar findings were reported by Yang et al. (37), Lafranconi et al. (40) and Lukic et al. (41). Despite this, most of the results supported that coffee and caffeine consumption were associated with a reduced risk of EC. However, these findings didn’t indicate that coffee and caffeine consumption was responsible for the reduced risk of EC. Due to methodological constraints and residual confounders, observational studies might only partially account for some factors influencing a result (such as the effects of a healthy lifestyle and diet).
Recently, there have been large-scale Mendelian randomization studies on coffee consumption and overall cancers, including EC, reported no causal relationship (42, 43). Nevertheless, our investigation not only examined the potential causal association of coffee and caffeine consumption with the risk of EC, but also assessed its impact on the relationship between EC progression. Confounders did not influence two-sample MR analyses, and we reduced reverse causality by using genetic variation as an instrumental variable. In this study, we assessed the association of selected SNPs with OS and CSS and produced Kaplan-Meier plots to illustrate. In terms of MR analysis, we applied two independent populations (ECAC and FinnGen consortium) separately, and the broadly consistent results ensured stability.
The study also has several disadvantages. Individual groups had horizontal pleiotropy in preliminary analyses, yet after excluding SNPs with potential confounders, we performed sensitivity analyses with generally consistent results. Second, most studies on coffee and caffeine used self-report methodologies, which were prone to bias. In addition, in this study, we did not stratify the menopausal status of EC patients, which might have led to the effect of coffee and caffeine intake on the risk of OC being influenced by menopausal status. Our studies were based on European populations and may need to be more generalizable to others.
5. Conclusion
Our MR investigation found no persuasive evidence to indicate a causal relationship between coffee and caffeine consumption and the risk of EC, and it was found to be largely irrelevant to the prognosis of EC. In the future, more clinical and basic studies are still needed to validate our results.
Data availability statement
The original contributions presented in this study are included in this article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
ZC: Conceptualization, Data curation, Investigation, Methodology, Software, Validation, Writing—original draft. CL: Investigation, Methodology, Software, Supervision, Validation, Writing—original draft. JW: Conceptualization, Investigation, Validation, Visualization, Writing—original draft. FK: Formal analysis, Methodology, Project administration, Resources, Writing—review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1291355/full#supplementary-material
Supplementary Figure 1 | Leave-one-out method of studies investigating the association between EC and coffee consumption in ECAC.
Supplementary Figure 2 | Leave-one-out method of studies investigating the association between EH and coffee consumption in ECAC.
Supplementary Figure 3 | Leave-one-out method of studies investigating the association between NEH and coffee consumption in ECAC.
Supplementary Figure 4 | Leave-one-out method of studies investigating the association between EC and coffee consumption in FinnGen Consortium.
Footnotes
- ^ https://portals.broadinstitute.org/collaboration/giant/index.php/GIANT_consortium_data_files (accessed April 28, 2021).
- ^ http://njmu-edu.cn:3838/SUMMER/
References
1. Sung H, Ferlay J, Siegel R, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Rahib L, Smith B, Aizenberg R, Rosenzweig A, Fleshman J, Matrisian L. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. (2014) 74:2913–21. doi: 10.1158/0008-5472.CAN-14-0155
3. Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial Cancer. Lancet. (2016) 387:1094–108. doi: 10.1016/S0140-6736(15)00130-0
4. Group S, Burke W, Orr J, Leitao M, Salom E, Gehrig P, et al. Endometrial Cancer: a review and current management strategies: Part I. Gynecol Oncol. (2014) 134:385–92. doi: 10.1016/j.ygyno.2014.05.018
5. Rosato V, Zucchetto A, Bosetti C, Dal Maso L, Montella M, Pelucchi C, et al. Metabolic syndrome and endometrial cancer risk. Ann Oncol. (2011) 22:884–9. doi: 10.1093/annonc/mdq464
6. Trabert B, Wentzensen N, Felix A, Yang H, Sherman M, Brinton L. Metabolic syndrome and risk of endometrial cancer in the United States: a study in the seer-medicare linked database. Cancer Epidemiol Biomark Prevent. (2015) 24:261–7. doi: 10.1158/1055-9965.EPI-14-0923
7. Loomis D, Guyton K, Grosse Y, Lauby-Secretan B, El Ghissassi F, Bouvard V, et al. Carcinogenicity of drinking coffee, mate, and very hot beverages. Lancet Oncol. (2016) 17:877–8. doi: 10.1016/S1470-2045(16)30239-X
8. Bohn S, Blomhoff R, Paur I. Coffee and cancer risk, epidemiological evidence, and molecular mechanisms. Mol Nutr Food Res. (2014) 58:915–30. doi: 10.1002/mnfr.201300526
9. Poole R, Kennedy O, Roderick P, Fallowfield J, Hayes P, Parkes J. Coffee consumption and health: umbrella review of meta-analyses of multiple health outcomes. BMJ. (2017) 359:j5024. doi: 10.1136/bmj.j5024
10. Lukanova A, Zeleniuch-Jacquotte A, Lundin E, Micheli A, Arslan A, Rinaldi S, et al. Prediagnostic Levels of C-Peptide, Igf-I, Igfbp -1, -2 and -3 and Risk of Endometrial Cancer. Int J Cancer. (2004) 108:262–8. doi: 10.1002/ijc.11544
11. Cust A, Allen N, Rinaldi S, Dossus L, Friedenreich C, Olsen A, et al. Serum Levels of C-Peptide, Igfbp-1 and Igfbp-2 and Endometrial Cancer Risk; Results from the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. (2007) 120:2656–64. doi: 10.1002/ijc.22578
12. Wu T, Willett W, Hankinson S, Giovannucci E. Caffeinated coffee, decaffeinated coffee, and caffeine in relation to plasma C-Peptide Levels, a Marker of Insulin Secretion, in U.S. Women. Diabetes Care. (2005) 28:1390–6. doi: 10.2337/diacare.28.6.1390
13. Smith G, Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. (2003) 32:1–22. doi: 10.1093/ije/dyg070
14. Burgess S, Thompson S. Mendelian Randomization: Methods for Using Genetic Variants in Causal Estimation. London: Chapman and Hall/CRC (2015). doi: 10.1201/b18084
15. Burgess S, Small D, Thompson SGA. Review of instrumental variable estimators for mendelian randomization. Stat Methods Med Res. (2017) 26:2333–55. doi: 10.1177/0962280215597579
16. Zhong V, Kuang A, Danning R, Kraft P, van Dam R, Chasman D, et al. A genome-wide association study of bitter and sweet beverage consumption. Hum Mol Genet. (2019) 28:2449–57. doi: 10.1093/hmg/ddz061
17. Li X, Cheng S, Cheng J, Wang M, Zhong Y, Yu A. Habitual coffee consumption increases risk of primary open-angle glaucoma: a mendelian randomization study. Ophthalmology. (2022) 129:1014–21. doi: 10.1016/j.ophtha.2022.04.027
18. Fang J, Song K, Zhang D, Liang Y, Zhao H, Jin J, et al. Coffee intake and risk of diabetic nephropathy: a mendelian randomization study. Front Endocrinol. (2023) 14:1169933. doi: 10.3389/fendo.2023.1169933
19. Kamat M, Blackshaw J, Young R, Surendran P, Burgess S, Danesh J, et al. Phenoscanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics. (2019) 35:4851–3. doi: 10.1093/bioinformatics/btz469
20. O’Neill C, Monteleone G, McLaughlin J, Paus R. The gut-skin axis in health and disease: a paradigm with therapeutic implications. Bioessays. (2016) 38:1167–76. doi: 10.1002/bies.201600008
21. Cornelis M, Kacprowski T, Menni C, Gustafsson S, Pivin E, Adamski J, et al. Genome-wide association study of caffeine metabolites provides new insights to caffeine metabolism and dietary caffeine-consumption behavior. Hum Mol Genet. (2016) 25:5472–82. doi: 10.1093/hmg/ddw334
22. McMahon G, Taylor A, Davey Smith G, Munafo M. Phenotype refinement strengthens the association of ahr and cyp1a1 genotype with caffeine consumption. PLoS One. (2014) 9:e103448. doi: 10.1371/journal.pone.0103448
23. O’Mara T, Glubb D, Amant F, Annibali D, Ashton K, Attia J, et al. Identification of nine new susceptibility loci for endometrial cancer. Nat Commun. (2018) 9:3166. doi: 10.1038/s41467-018-05427-7
24. Kho P, Mortlock S, Rogers PAW, Nyholt DR, Montgomery GW, Spurdle AB, et al. Genetic analyses of gynecological disease identify genetic relationships between uterine fibroids and endometrial cancer, and a novel endometrial cancer genetic risk region at the wnt4 1p36.12 locus. Hum Genet. (2021) 140:1353–65. doi: 10.1007/s00439-021-02312-0
25. Kurki M, Karjalainen J, Palta P, Sipila T, Kristiansson K, Donner K, et al. Author correction: finngen provides genetic insights from a well-phenotyped isolated population. Nature. (2023) 615:E19. doi: 10.1038/s41586-023-05837-8
26. Kurki M, Karjalainen J, Palta P, Sipila T, Kristiansson K, Donner K, et al. Finngen provides genetic insights from a well-phenotyped isolated population. Nature. (2023) 613:508–18. doi: 10.1038/s41586-022-05473-8
27. Yengo L, Sidorenko J, Kemper K, Zheng Z, Wood A, Weedon M, et al. Meta-analysis of genome-wide association studies for height and body mass index in approximately 700000 individuals of european ancestry. Hum Mol Genet. (2018) 27:3641–9. doi: 10.1093/hmg/ddy271
28. Liu M, Jiang Y, Wedow R, Li Y, Brazel D, Chen F, et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet. (2019) 51:237–44. doi: 10.1038/s41588-018-0307-5
29. Yuan S, Larsson S. Coffee and caffeine consumption and risk of kidney stones: a mendelian randomization study. Am J Kidney Dis. (2022) 79:9–14e1. doi: 10.1053/j.ajkd.2021.04.018
30. Burgess S, Butterworth A, Thompson S. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. (2013) 37:658–65. doi: 10.1002/gepi.21758
31. Bowden J, Davey Smith G, Haycock P, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965
32. Yavorska O, Burgess S. Mendelianrandomization: an R package for performing mendelian randomization analyses using summarized data. Int J Epidemiol. (2017) 46:1734–9. doi: 10.1093/ije/dyx034
33. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int J Epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080
34. Verbanck M, Chen C, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
35. Xin J, Gu D, Chen S, Ben S, Li H, Zhang Z, et al. Summer: a mendelian randomization interactive server to systematically evaluate the causal effects of risk factors and circulating biomarkers on pan-cancer survival. Nucleic Acids Res. (2023) 51:D1160–7. doi: 10.1093/nar/gkac677
36. Zhu G, Li Z, Tang L, Shen M, Zhou Z, Wei Y, et al. Associations of dietary intakes with gynecological cancers: findings from a cross-sectional study. Nutrients. (2022) 14:23. doi: 10.3390/nu14235026
37. Yang T, Crowe F, Cairns B, Reeves G, Beral V. Tea and coffee and risk of endometrial cancer: cohort study and meta-analysis. Am J Clin Nutr. (2015) 101:570–8. doi: 10.3945/ajcn.113.081836
38. Crous-Bou M, Du M, Gunter M, Setiawan V, Schouten L, Shu X, et al. Coffee consumption and risk of endometrial cancer: a pooled analysis of individual participant data in the epidemiology of endometrial cancer consortium (E2c2). Am J Clin Nutr. (2022) 116:1219–28. doi: 10.1093/ajcn/nqac229
39. Je Y, Giovannucci E. Coffee consumption and risk of endometrial cancer: findings from a large up-to-date meta-analysis. Int J Cancer. (2012) 131:1700–10. doi: 10.1002/ijc.27408
40. Lafranconi A, Micek A, Galvano F, Rossetti S, Del Pup L, Berretta M, et al. Coffee decreases the risk of endometrial cancer: a dose-response meta-analysis of prospective cohort studies. Nutrients. (2017) 9:11. doi: 10.3390/nu9111223
41. Lukic M, Guha N, Licaj I, van den Brandt P, Stayner L, Tavani A, et al. Coffee drinking and the risk of endometrial cancer: an updated meta-analysis of observational studies. Nutr Cancer. (2018) 70:513–28. doi: 10.1080/01635581.2018.1460681
42. Ong J, Law M, An J, Han X, Gharahkhani P, Whiteman D, et al. Association between coffee consumption and overall risk of being diagnosed with or dying from cancer among >300 000 uk biobank participants in a large-scale mendelian randomization study. Int J Epidemiol. (2019) 48:1447–56. doi: 10.1093/ije/dyz144
Keywords: endometrial cancer (EC), Mendelian randomization (MR), coffee consumption, caffeine consumption, endometrioid histology (EH)
Citation: Chen Z, Liu C, Wu J and Kong F (2023) Association of coffee and caffeine consumption with risk and prognosis of endometrial cancer and its subgroups: a Mendelian randomization. Front. Nutr. 10:1291355. doi: 10.3389/fnut.2023.1291355
Received: 14 September 2023; Accepted: 30 October 2023;
Published: 14 November 2023.
Edited by:
Yan Huang, University of Arkansas, United StatesReviewed by:
Weinan Zhou, University of Illinois Urbana-Champaign, United StatesXiaoming He, Mondelēz International, United Kingdom
Copyright © 2023 Chen, Liu, Wu and Kong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fandou Kong, a2ZkOTI2QG91dGxvb2suY29t
†These authors have contributed equally to this work and share first authorship
 Ziyu Chen1†
Ziyu Chen1† Chaosheng Liu
Chaosheng Liu Fandou Kong
Fandou Kong