- 1Department of Geography, The Pennsylvania State University, University Park, PA, United States
- 2Department of Health Behavior, Society and Policy, Newark, NJ, United States
- 3African Studies Program, The Pennsylvania State University, University Park, PA, United States
Food environments, or interfaces between consumers and their food systems, are a useful lens for assessing global dietary change. Growing inclusivity of nature-dependent societies in lower-and middle-income countries is driving recent developments in food environment frameworks. Downs et al. (2020) propose a food environment typology that includes: wild, cultivated, informal and formal market environments, where wild and cultivated are “natural food environments.” Drawing from transdisciplinary perspectives, this paper argues that wild and cultivated food environments are not dichotomous, but rather exist across diverse landscapes under varying levels of human management and alteration. The adapted typology is applied to a case study of Indigenous Pgaz K’Nyau food environments in San Din Daeng village, Thailand, using the Gallup Poll’s Thailand-adapted Diet Quality Questionnaire with additional food source questions. Wild-cultivated food environments, as classified by local participants, were the source of more food items than any other type of food environment (37% of reported food items). The case of Indigenous Pgaz K’Nyau food environments demonstrates the importance of understanding natural food environments along a continuum from wild to cultivated.
1 Introduction
Globalization is rapidly transforming diets and food choices (2, 3). An ongoing ‘nutrition transition’ toward calorie-dense and nutrient-poor ‘Western’ diets is exacerbating global burdens of disease (4), garnering much attention in the global public health literature. Changes to food environments are driving global dietary transitions (1). Often understood as the interface between the consumer and the food system (1, 5), food environments include physical environments where people acquire food (including built environments, such as homes, restaurants, schools, supermarkets; and natural environments, such as forests, home gardens and crop fields) with measurable characteristics that influence food decision-making (referred to as aspects or dimensions of the food environment, such as availability, access, affordability, convenience and desirability) (5–7).
Over the last few decades, food environment research has played a pivotal role in drawing attention to the structural factors shaping food access and choice (8, 9). Application of most food environment frameworks, however, remains limited to predominately high-income country and urban contexts with some notable exceptions (8, 9). The rest of the world (who still need to procure food and make dietary choices daily) are all-too-often overlooked. A recent systematic review of food environment research found no studies conducted in low-income countries (10). Since this systematic scoping review, a nascent food environment literature in low-income countries is emerging (11–15).
Geographic bias in the food environment literature toward high-income countries and urban contexts is responsible for an underrepresentation of some populations. The majority of the world’s Indigenous Peoples reside in low-and middle-income countries (LMICs). Indigenous communities are experiencing particularly stark agricultural and dietary transformations (16–18), associated with higher burdens of chronic disease (19). Indigenous food environments are shifting from wild and cultivated environments toward built food environments with reduced dependency on forest foods and increased market purchases (16, 20–22). Food environment frameworks designed for urban studies in high-income countries have not translated well to Indigenous and low-income country contexts, in which wild and cultivated landscapes and informal markets often provide important contributions to diet quality (1, 9, 15).
More inclusive frameworks for LMICs and Indigenous societies are beginning to garner attention (1, 12). Downs et al.’s typology of natural and built food environments is more inclusive of Indigenous and rural food environments, including those transitioning rapidly. Their framework fills a notable gap in the food environment literature for LMICs, where agricultural, pastoral, forested and aquatic natural environments provide affordable and healthy food sources in economically marginal contexts. Particularly commendable is the inclusion of wild foods. Though wild foods contribute substantially to the global food basket (23, 24), wild foods remain a key research gap in the food environment literature (5). Food environment research tends to underrepresent the contributions of wild foods and other non-market food sources in favor of the built market environment (6, 9).
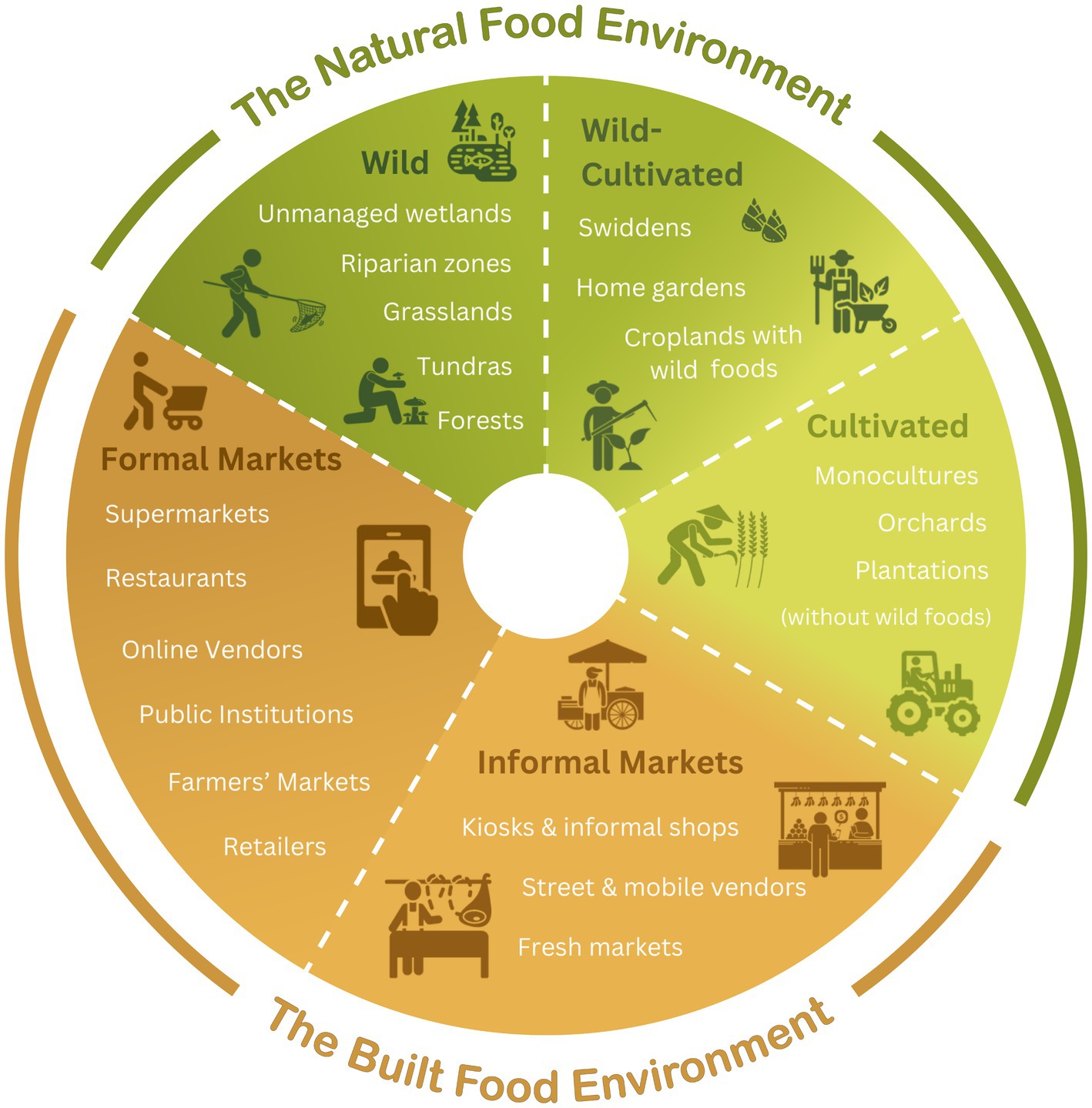
New frameworks effective at drawing attention to the dietary significance of wild and cultivated natural food environments could further benefit from transdisciplinary perspectives. By leveraging findings from diverse disciplines, this paper reevaluates the wild-cultivated and nature-built dichotomies that permeate current food environment frameworks. Proposed is an adapted food environment typology that dissolves wild-cultivated boundaries in favor of a continuum. The adapted typology is applied to a case study of Indigenous Pgaz K’Nyau food environments in San Din Daeng village, Thailand, using the Gallup Poll’s Thailand-adapted Diet Quality Questionnaire (DQ-Q) (25). The Pgaz K’Nyau case study showcases the dietary importance of the previously overlooked wild-cultivated type of food environment in a semi-subsistence Indigenous community.
2 What is wild?
Current conceptualizations of the natural food environment designate ‘wild’ and ‘cultivated’ as separate spheres (1). Growing consensus in wild foods literature, however, contends that wild and cultivated environments vary along a ‘wild-cultivated continuum’ (23), by domestication stage (23), adaptive niches (26) and management intensity (27).
Wild-cultivated boundary-bending is the norm in many of the world’s natural food environments. Natural food environments, such as swidden fallows and home gardens, act as ‘boundary elements’ that traverse a wild-cultivated divide. Wild-cultivated crossovers include orchards or forests with lightly managed wild fruit trees (i.e., pruning, mulching and watering). Other wild-cultivated food environments include cultivation zones with wild terrestrial or aquatic foods, such as: (i) home gardens with wild transplants, spontaneous edible plants and bushmeat, (ii) rice paddies with wild plants, shellfish and amphibians, and (iii) swidden fallows with spontaneous vegetables and forest species (see Figure 1). Home gardens, for instance, have been described as the “closest mimics of natural forests yet attained,” signaling a status that is not purely cultivated nor fully wild (28). Swidden fields provide another liminal space traversing the wild-cultivated divide. Swidden forest-farmers modify landscapes with fire to create successional patch mosaics of croplands and secondary forests that provide ecological niches for a spectrum of wild-cultivated foods (29–31).
Niche construction theory (applied in human-environment geography, archeology, anthropology, ethnobotany, human ecology, among others fields) provides a theoretical explanation for the range of edible species’ adaptive niches that span a wild-cultivated continuum (26). Niche construction theory posits that originally ‘wild’ organisms adapt to environmental niches formed through human management and landscape modification. Commonalities in organism and landscape modification strategies derived from global case studies include: (i) modifying plant communities, (ii) broadcasting wild annuals, (iii) transplanting edible tree and root crops, (iv) light management of perennials (e.g., mulching and pruning), and (v) landscape modification for enhanced food procurement (32).
At the landscape-scale, following niche construction theory, alterations through fire or other disturbances, create niches for edible wild species to adapt. Human-landscape interactions, according to anthropologist, Paul Roscoe, complicate: (33).
“…what constitutes “wild.” The very presence of consuming humans on a landscape affects food resources, blurring the lines between wild and domesticated and, hence, between hunting and pastoralism and between gathering and cultivation (e.g., 34, 35).”
‘Natural’ landscapes, as noted by Roscoe, tend to be products of human modification. Iconic ‘wildernesses’, such as Yosemite Valley (36), the Amazon rain forest (37, 38), African savannahs (39) and Australia’s arid deserts (40, 41) have long-standing histories of anthropogenic manipulation for enhanced food acquisition. Forest-dwelling peoples around the world have long modified surrounding landscapes for hunting and foraging (30, 32). Artificial forest islands have transformed Southwest Amazonia (42) and African savannahs (39). Even remote jungles of the Amazon Basin rain forest are shaped by over 13,000 years of human-environment interactions (43), including shifting horticulture and tree planting since 4,000 years ago (~2,050 BC) (43), soil fertility enhancement (44–46) and ‘large-scale forest transformations’ (47). Amazonia and its jungles have even been referred to as a ‘domesticated landscape’ (48). Paleoethnobotanical explorations of ancient landscape management and agroecosystems have unearthed the co-existence of both wild and cultivated species in overlapping spaces (49). Findings from around the world demonstrate millennia of human-environment co-evolution that overturn wild-cultivated dichotomies (38, 42).
At the scale of organisms, classifying plants and animals as either wild or cultivated is similarly difficult, given the expanse of semi-cultivated states (23). Sago (Metroxylon sagu Rottboell), for instance, is situated along a management gradient from remote sago forest stands (with no or minimal management) to cultivated sago patches in villages (50). Sago users of Nuaulu ethnicity do not differentiate between wild and cultivated sago (50). Ethnobiologist, Roy Ellen, while working with the Nuaulu concluded that “there is a continuum,” because “the distinction between cultivated and non-cultivated becomes a difficult one to make” (50).
Scholars from diverse disciplines continue to grapple with the perplexing question of what ‘wild’ or ‘wilderness’ is (23, 50, 51). Food environment literature is beginning to engage with wild foods and wild natural environments (1), but not yet with the subtleties of bounding ‘wild’, ‘wildness’ and ‘wilderness’ that is highly contested in other fields.
3 An adapted food environment typology integrating a wild-cultivated continuum
The proposed conceptual approach leverages transdisciplinary perspectives on complex wild-cultivated dynamics to build upon Downs et al.’s natural (wild and cultivated) and built (formal and informal market) food environment typology to integrate a wild-cultivated continuum (1) (see Figure 1).
The adapted typology recognizes the oftentimes porous boundaries and complex crossovers and migrations of foods between different types of food environments (depicted with a dashed line in Figure 1). Some food items, such as fish, may be sourced from multiple different food environments, regardless of their original source. An artisanal fisherperson consuming their own wild-caught fish would be interacting with a wild natural food environment. Consumers purchasing wild-caught or aquaculture farmed fish in a supermarket would be interacting with a formal market environment.
We apply the adapted food environment typology to quantify the dietary contributions from different types of food environments in the Pgaz K’Nyau community of San Din Daeng village, Thailand.
4 Case study of indigenous Pgaz K’Nyau food environments in Thailand
Pgaz K’Nyau Peoples (a Karen ethnic subgroup) traditionally practice rotational farming, a type of shifting cultivation with 6–12 year fallows that support agrobiodiversity and dietary diversity (52). Forest conservation policies and market integration pressures are driving conversions toward monoculture, agrochemicals and market reliance. Simultaneously, highland infrastructure projects (e.g., roads, electricity) are increasing market access and altering local diets, resulting in Pgaz K’Nyau food environment transitions (1).
Dietary diversity from different food environment types was assessed in San Din Daeng village, Chiang Mai province, Thailand. Emic local classifications of types of food environments were discussed in focus groups (n = 6 women). Focus group participants classified food sources under the following types of food environments: (i) Cultivated: monoculture animal feed corn fields (indirect dietary pathway via income generation reinvested in market food purchases), (ii) Wild: forests (though forests are sites of animal husbandry, participants considered forests mostly ‘wild’), (iii) Wild-Cultivated: home gardens, swiddens, agricultural streams, and rice paddies (rice paddies were included due to the presence of aquatic wild foods), (iv) Informal Market: fresh markets, kiosks, street vendors, informal shops and restaurants, and (v) Formal Market: supermarkets (e.g., Tesco Lotus, Big C and Macro) and convenience stores (e.g., 7-11) located in Chom Thong town.
The Gallup Poll’s Thailand-adapted Diet Quality Questionnaire (DQ-Q) (25) was administered to one adult woman (>18 years old) per household (n = 31; 94% of households) in late rainy season (late September – October, 2023). Sources of food items consumed the previous day were also recorded (e.g., Cultivated: monoculture non-swidden crop field; Wild-Cultivated: home garden, swidden, rice paddy, agricultural pond or stream; Wild: forest or forest stream; Informal Market: fresh market, village kiosk, informal shop, informal restaurant, street vendor; and Formal Market: convenience store or supermarket) (see Supplementary Information for survey questions).
The average Dietary Diversity Score was 5.4 (ranging from 3 to 9) with 68% of respondents exceeding the Women’s Minimum Dietary Diversity Score of 5 (21 out of 31 women). Wild-cultivated environments were the most frequented type of food environments with respondents reporting daily visits on average (compared to 4 times per week for informal markets, once a month for wild environments, and less than once a month for cultivated and formal market environments).
More food items were consumed from wild-cultivated environments than any other type of food environment (37% or 88 out of 240 food items reported in the DQ-Q; see Figure 2). Wild-cultivated food environments were the main source of micronutrient-rich food groups (vitamin A-rich fruits and vegetables, dark green leafy vegetables, other vegetables and other fruit) consumed the previous day. The majority of vitamin-A rich fruits and vegetables were obtained from home garden and swidden wild-cultivated environments (91%, or 8 out of 11 food items with an additional 2 shared food items). Wild-cultivated environments provided 65% of dark green leafy vegetables (10 out of 17 reported food items, and 1 shared food item), 68% of other fruits (15 out of 28 food items with an additional 4 shared items), and 39% of other vegetables (21 out of 67 food items with an additional 5 shared items) (see Supplementary Table S1 in Supplementary Information). Animal-sourced foods, such as meat, fish and eggs, were predominately obtained from informal markets. Carbohydrate staples, such as rice, were mostly acquired from wild-cultivated swiddens and rice paddies (31 out of 37 reported grain food items, or 84%).
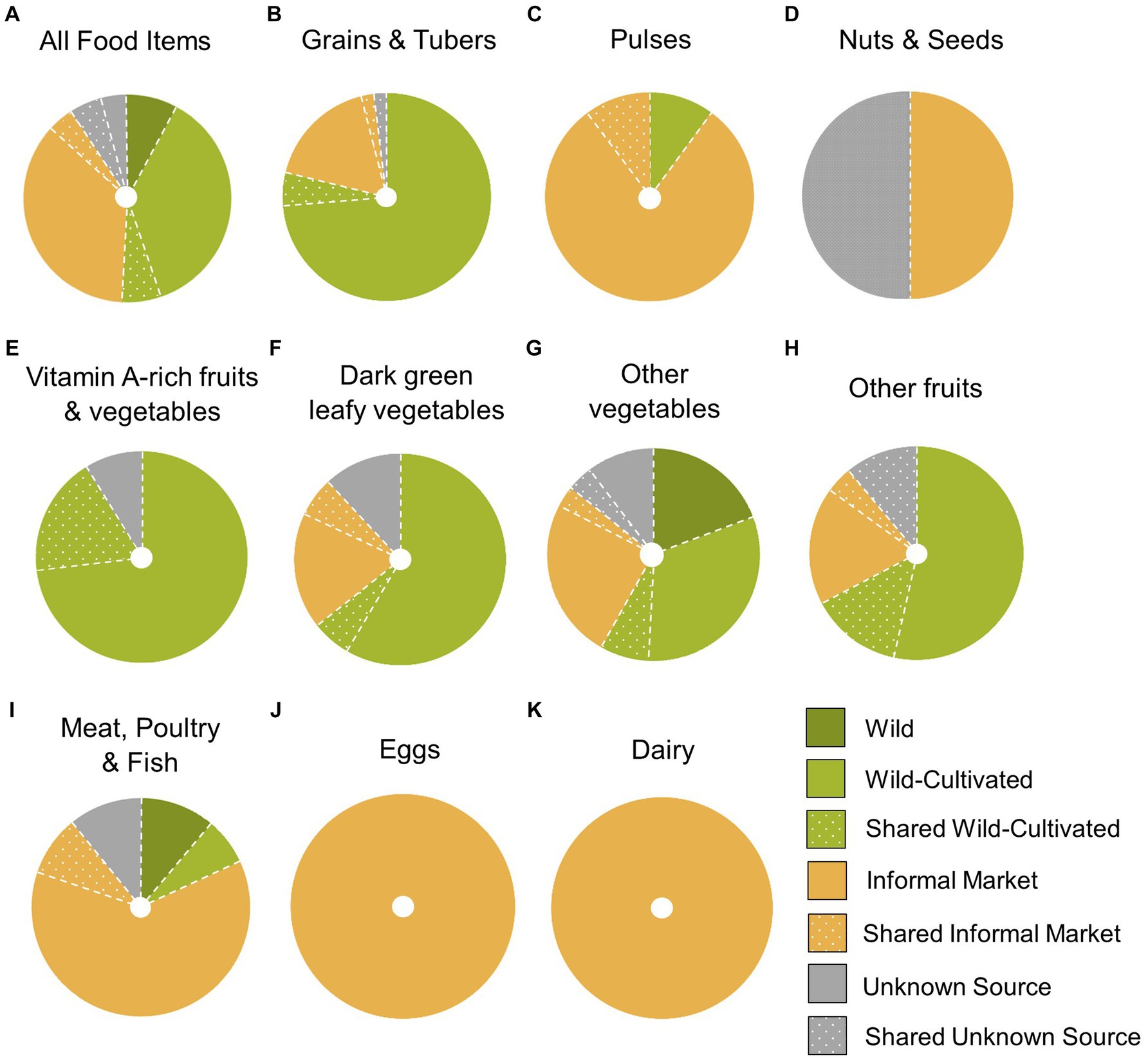
Figure 2. Proportions of food items acquired from each type of food environment per dietary diversity group (A-K), reported in a diet quality questionnaire in San Din Daeng village, Thailand (n = 31; 240 food items). ‘Shared’ refers to food items acquired from food sharing. E.g. ‘Shared wild-cultivated’ refers to food items acquired via food sharing from a wild-cultivated food source.
Despite rapid social-ecological change, San Din Daeng residents continue to rely heavily on natural food environments and particularly wild-cultivated environments. The formal market environment that has dominated food environment research is only marginal in this semi-subsistence setting (none of the food items reported in the DQ-Q were acquired from formal markets). The case of the Pgaz K’Nyau food environment of San Din Daeng village demonstrates that the previously overlooked wild-cultivated food environment can contribute substantially to local diets.
5 Discussion
Most food environment frameworks have underrepresented marginalized communities in LMICs, for whom the natural food environment presents a vital, affordable and healthy food source (1, 5, 53). With growing evidence on the nutritional importance of wild foods (54–57), the significance of natural food environments in LMICs is becoming more apparent (1, 5, 53). Though research on natural food environments is still embryonic, other disciplines have long engaged with forest-and nature-dependent peoples. Anthropologists, ethnobiologists, geographers, landscape ecologists, among others, have compiled an extensive body of knowledge on diverse food acquisition strategies, globally (41, 50, 55, 58). Greater emphasis on cross-disciplinary discussions is bringing decades of debates on wild-cultivated dynamics, plant-people interactions, traditional ecological and Indigenous knowledge, and nature-based ontologies into conversation with budding conceptual developments of natural food environments. Capitalizing on transdisciplinary theories, perspectives and bodies of knowledge can catalyze the development of effective food environment measurement tools that best capture the nuanced complexity of natural food environments.
Data availability statement
The datasets presented in this article are not readily available because data is only made available upon request at the authors’ discretion. Requests to access the datasets should be directed to bG16NTI4OEBwc3UuZWR1.
Ethics statement
The studies involving humans were approved by the Pennsylvania State University Institutional Review Board (STUDY00019694). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because some participants can not read or write. Participants signed or checked their names on a consent form. Formal written consent was obtained from the village leader.
Author contributions
LZ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Visualization, Writing – original draft, Writing – review & editing. SD: Methodology, Supervision, Visualization, Writing – review & editing. BP: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The lead author was funded by the Miller Fellowship from the Pennsylvania State University Geography Department, as well as by graduate student awards from the Pennsylvania State University (the Center for Landscape Diversity Graduate Student Travel Award, the Whiting Indigenous Knowledge Student Research Award and the EMS Centennial Graduate Research Travel Award).
Acknowledgments
Suwichan Phatthanaphraiwan assisted in coordinating the Pgaz K’Nyau case study. Claire Lorts and Salah Eddine Tabout assisted in graphic design. The residents of San Din Daeng village provided the empirical evidence of the dietary importance of wild-cultivated environments.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1343021/full#supplementary-material
References
1. Downs, SM, Ahmed, S, Fanzo, J, and Herforth, A. Food environment typology: advancing an expanded definition, framework, and methodological approach for improved characterization of wild, cultivated, and built food environments toward sustainable diets. Food Secur. (2020) 9:9. doi: 10.3390/foods9040532
2. Monterrosa, EC, Frongillo, EA, Drewnowski, A, de Pee, S, and Vandevijvere, S. Sociocultural influences on food choices and implications for sustainable healthy diets. Food Nutr Bull. (2020) 41:59S–73S. doi: 10.1177/0379572120975874
3. Hawkes, C . Uneven dietary development: linking the policies and processes of globalization with the nutrition transition, obesity and diet-related chronic diseases. Glob Health. (2006) 2:2. doi: 10.1186/1744-8603-2-4
4. Popkin, BM, and Ng, SW. The nutrition transition to a stage of high obesity and noncommunicable disease prevalence dominated by ultra-processed foods is not inevitable. Obes Rev. (2022) 23:e13366. doi: 10.1111/obr.13366
5. Turner, C, Aggarwal, A, Walls, H, Herforth, A, Drewnowski, A, Coates, J, et al. Concepts and critical perspectives for food environment research: a global framework with implications for action in low-and middle-income countries. Glob Food Sec. (2018) 18:93–101. doi: 10.1016/j.gfs.2018.08.003
6. Herforth, A, and Ahmed, S. The food environment, its effects on dietary consumption, and potential for measurement within agriculture-nutrition interventions. Food Secur. (2015) 7:505–20. doi: 10.1007/s12571-015-0455-8
7. Swinburn, B, Sacks, G, Vandevijvere, S, Kumanyika, S, Lobstein, T, Neal, B, et al. INFORMAS (international network for food and obesity/non-communicable diseases research, monitoring and action support): overview and key principles. Obes Rev. (2013) 14:1–12. doi: 10.1111/obr.12087
8. Davies, G, Frausin, G, and Parry, L. Are there food deserts in rainforest cities? Ann Am Assoc Geogr. (2017) 107:794–811. doi: 10.1080/24694452.2016.1271307
9. Battersby, J . Beyond the food desert: finding ways to speak about urban food security in South Africa. Geogr Ann Ser B. (2012) 94:141–59. doi: 10.1111/j.1468-0467.2012.00401.x
10. Turner, C, Kalamatianou, S, Drewnowski, A, Kulkarni, B, Kinra, S, and Kadiyala, S. Food environment research in low-and middle-income countries: a systematic scoping review. Adv Nutr. (2020) 11:387–97. doi: 10.1093/advances/nmz031
11. Turner, C, Bhogadi, S, Walls, H, Surendran, S, Kulkarni, B, Kinra, S, et al. Drivers of food acquisition practices in the food environment of peri-urban Hyderabad, India: a qualitative investigation. Health Place. (2022) 74:102763. doi: 10.1016/j.healthplace.2022.102763
12. Bogard, JR, Andrew, NL, Farrell, P, Herrero, M, Sharp, MK, and Tutuo, J. A typology of food environments in the Pacific region and their relationship to diet quality in Solomon Islands. Food Secur. (2021) 10:10. doi: 10.3390/foods10112592
13. Ganpule-Rao, AV, Roy, D, Karandikar, BA, Yajnik, CS, and Rush, EC. Food access and nutritional status of rural adolescents in India: Pune maternal nutrition study. Am J Prev Med. (2020) 58:728–35. doi: 10.1016/j.amepre.2019.10.017
14. Nguyen, T, Mai, HPT, Van Den Berg, M, Thanh, THT, and Béné, C. Interactions between food environment and (un)healthy consumption: evidence along a rural-urban transect in Viet Nam. Agriculture. (2021) 11:11. doi: 10.3390/agriculture11080789
15. Ghosh-Jerath, S, Kapoor, R, Barman, S, Singh, G, Singh, A, Downs, S, et al. Traditional food environment and factors affecting indigenous food consumption in Munda tribal Community of Jharkhand, India. Front Nutr. (2021) 7:70. doi: 10.3389/fnut.2020.600470
16. Kuhnlein, HV, and Receveur, O. Dietary change and traditional food Systems of Indigenous Peoples. Annu Rev Nutr. (1996) 16:417–42. doi: 10.1146/annurev.nutr.16.1.417
17. Kuhnlein, HV, Erasmus, B, and Spigelski, D. Indigenous peoples’ food systems: the many dimensions of culture, diversity and environment for nutrition and health. Rome: FAO (2009).
18. Kuhnlein, HV, Receveur, O, Soueida, R, and Egeland, GM. Arctic indigenous peoples experience the nutrition transition with changing dietary patterns and obesity. J Nutr. (2004) 134:1447–53. doi: 10.1093/jn/134.6.1447
19. Damman, S, Eide, WB, and Kuhnlein, HV. Indigenous peoples’ nutrition transition in a right to food perspective. Food Policy. (2008) 33:135–55. doi: 10.1016/j.foodpol.2007.08.002
20. D’Ambrosio, U, and Puri, RK. Foodways transitions in Ngäbe households of Costa Rica. Linking food plants, conservationist markets and worldviews in Conte-Burica. J Ethnobiol Ethnomed. (2011) 12:1–32. doi: 10.1186/s13002-015-0071-x
21. Chao, S . Gastrocolonialism: the intersections of race, food, and development in West Papua. Int J Hum Rights. (2022) 26:811–32. doi: 10.1080/13642987.2021.1968378
22. Reyes-García, V, Powell, B, Díaz-Reviriego, I, Fernández-Llamazares, Á, Gallois, S, and Gueze, M. Dietary transitions among three contemporary hunter-gatherers across the tropics. Food Secur. (2019) 11:109–22. doi: 10.1007/s12571-018-0882-4
23. Bharucha, Z, and Pretty, J. The roles and values of wild foods in agricultural systems. Philos Trans R Soc B. (2010) 365:2913–26. doi: 10.1098/rstb.2010.0123
25. Global diet quality project. (2023). Thailand. Available at:https://www.dietquality.org/countries/tha.
26. Smith, BD . A cultural niche construction theory of initial domestication. Biol Theory. (2011) 6:260–71. doi: 10.1007/s13752-012-0028-4
27. González-Insuasti, MS, and Caballero, J. Managing plant resources: how intensive can it be? Hum Ecol. (2007) 35:303–14. doi: 10.1007/s10745-006-9063-8
28. Kumar, BM, and Nair, PKR. The enigma of tropical homegardens. Agrofor Syst. (2004) 61:135–52. doi: 10.1007/978-94-017-2424-1_10
30. Hecht, SB, Morrison, KD, and Padoch, C. The social lives of forests: past, present, and future of woodland resurgence. Chicago: University of Chicago Press (2014).
31. Brookfield, H, and Padoch, C. Managing biodiversity in spatially and temporally complex agricultural landscapes In: DI Jarvis, C Padoch, and HD Cooper, editors. Managing biodiversity in agricultural ecosystems. New York: Columbia University Press (2007). 338–61.
32. Smith, BD . General patterns of niche construction and the management of “wild” plant and animal resources by small-scale pre-industrial societies. Philos Trans R Soc B. (2011) 366:836–48. doi: 10.1098/rstb.2010.0253
33. Roscoe, P . The hunters and gatherers of New Guinea. Curr Anthropol. (2002) 43:153–62. doi: 10.1086/338289
36. Cronon, W . The trouble with wilderness: or, getting back to the wrong nature. Environ Hist. (1996) 1:7–28. doi: 10.2307/3985059
37. Balée, W . Cultural forests of the Amazon: a historical ecology of people and their landscapes. Tuscaloosa: The University of Alabama Press (2013).
38. Piperno, DR, McMichael, C, and Bush, MB. Amazonia and the Anthropocene: what was the spatial extent and intensity of human landscape modification in the amazon basin at the end of prehistory? Holocene. (2015) 25:1588–97. doi: 10.1177/0959683615588374
39. Fairhead, J, and Leach, M. Misreading the African landscape: society and ecology in a forest-savanna mosaic. Cambridge: Cambridge University Press (1996).
40. Bliege Bird, R, Bird, DW, Codding, BF, Parker, CH, and Jones, JH. The “fire stick farming” hypothesis: Australian aboriginal foraging strategies, biodiversity, and anthropogenic fire mosaics. Proc Natl Acad Sci USA. (2008) 105:14796–801. doi: 10.1073/pnas.0804757105
41. Bliege Bird, R, and Bird, DW. Climate, landscape diversity, and food sovereignty in arid Australia: the firestick farming hypothesis. Am J Hum Biol. (2021) 33:e23527. doi: 10.1002/ajhb.23527
42. Lombardo, U, Iriarte, J, Hilbert, L, Ruiz-Pérez, J, Capriles, JM, and Veit, H. Early Holocene crop cultivation and landscape modification in Amazonia. Nature. (2020) 581:190–3. doi: 10.1038/s41586-020-2162-7
43. Roosevelt, AC . The Amazon and the Anthropocene: 13,000 years of human influence in a tropical rainforest. Anthropocene. (2013) 4:69–87. doi: 10.1016/j.ancene.2014.05.001
44. Woods, WI . Development of anthrosol research In: J Lehmann, DC Kern, B Glaser, and WI Wodos, editors. Amazonian dark earths. Dordrecht: Springer (2006)
45. Kern, DC, D’aquino, G, Rodrigues, TE, Frazao, FJL, Sombroek, W, and Myers, TP. Distribution of amazonian dark earths in the Brazilian amazon In: J Lehmann, DC Kern, B Glaser, and WI Wodos, editors. Amazonian dark earths. Dordrecht: Springer (2006)
46. Iriarte, J, Elliott, S, Maezumi, SY, Alves, D, Gonda, R, Robinson, M, et al. The origins of Amazonian landscapes: plant cultivation, domestication and the spread of food production in tropical South America. Quat Sci Rev. (2020) 248:106582. doi: 10.1016/j.quascirev.2020.106582
47. Heckenberger, MJ, Russell, JC, Toney, JR, and Schmidt, MJ. The legacy of cultural landscapes in the Brazilian Amazon: implications for biodiversity. Philos Trans R Soc B. (2007) 362:197–208. doi: 10.1098/rstb.2006.1979
48. Erickson, CL . Amazonia: the historical ecology of a domesticated landscape. New York: Springer; (2008), p. 157–183.
49. Slotten, V, Lentz, D, and Sheets, P. Landscape management and polyculture in the ancient gardens and fields at Joya de Cerén, El Salvador. J Anthropol Archaeol. (2020) 59:101191. doi: 10.1016/j.jaa.2020.101191
50. Ellen, R . Local knowledge and Management of Sago Palm (Metroxylon sagu ROTTBOELL) diversity in south central Seram, Maluku, eastern Indonesia. J Ethnobiol. (2006) 26:258–98. doi: 10.2993/0278
51. Harris, DR . An evolutionary continuum of people–plant interaction In: DR Harris and GC Hillman, editors. The emergence of agriculture. London: Routledge (2020). 26–44.
52. Schmidt-Vogt, D . Defining degradation: the impacts of swidden on forests in northern Thailand. Mt Res Dev. (1998) 18:135. doi: 10.2307/3673969
53. Marshall, Q, Fanzo, J, Barrett, CB, Jones, AD, Herforth, A, and McLaren, R. Building a global food systems typology: a new tool for reducing complexity in food systems analysis. Front Sustain Food Syst. (2021) 5:5. doi: 10.3389/fsufs.2021.746512
54. Cheek, JZ, Lambrecht, NJ, den Braber, B, Akanchha, N, Govindarajulu, D, Jones, AD, et al. Wild foods contribute to women’s higher dietary diversity in India. Nat Food. (2023) 4:476–82. doi: 10.1038/s43016-023-00766-1
55. Powell, B, Maundu, P, Kuhnlein, HV, and Johns, T. Wild foods from farm and Forest in the east Usambara Mountains, Tanzania. Ecol Food Nutr. (2013) 52:451–78. doi: 10.1080/03670244.2013.768122
56. Smith, E, Ahmed, S, Dupuis, V, Running Crane, M, Eggers, M, Pierre, M, et al. Contribution of wild foods to diet, food security, and cultural values amidst climate change. J Agric Food Syst Community Dev. (2019) 9:1–24. doi: 10.5304/jafscd.2019.09B.011
57. Rowland, D, Ickowitz, A, Powell, B, Nasi, R, and Sunderland, T. Forest foods and healthy diets: quantifying the contributions. Environ Conserv. (2017) 44:102–14. doi: 10.1017/S0376892916000151
Keywords: natural food environment, diet quality, dietary diversity, indigenous, wild foods, niche construction theory, diet, Swidden
Citation: Zeitler L, Downs S and Powell B (2024) Adapting food environment frameworks to recognize a wild-cultivated continuum. Front. Nutr. 11:1343021. doi: 10.3389/fnut.2024.1343021
Edited by:
Alessandra Durazzo, Council for Agricultural Research and Economics, ItalyReviewed by:
Dave Love, Johns Hopkins University, United StatesCopyright © 2024 Zeitler, Downs and Powell. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lilly Zeitler, bG16NTI4OEBwc3UuZWR1
 Lilly Zeitler
Lilly Zeitler Shauna Downs
Shauna Downs Bronwen Powell
Bronwen Powell