- 1Lishui City People's Hospital, Lishui, China
- 2Department of Critical Care Medicine, Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 3Department of General Surgery, Lishui People’s Hospital, Lishui, China
Background: Systemic inflammatory response represented by C-reactive protein to albumin ratio (CAR) was shown to be associated with long-term outcome in patients with hepatocellular carcinoma (HCC). We conducted a meta-analysis to investigate the prognostic value of preoperative CAR in patients undergoing hepatectomy for HCC.
Methods: We searched four databases (PubMed, Embase, Scopus and Cochrane Library) from inception to May 10th, 2024. Studies investigating the prognostic value of preoperative CAR in HCC patients after hepatectomy. The primary endpoints were overall survival (OS) and disease-free survival (DFS). Data from individual studies were aggregated to calculate the pooled hazard ratio (HR) using a random-effects model.
Results: A total of 11 studies included 4,066 patients were finally analyzed in the meta-analysis. Overall, the higher preoperative CAR was associated with poorer OS (HR 1.92, 95% CI 1.67 to 2.22, I2 = 0%) and DFS (HR 1.79, 95% CI 1.59 to 2.02, I2 = 0%) rate. Furthermore, subgroup analyses indicated that CAR could be a prognostic biomarker for patients with HCC regardless of regions and cut-off value.
Conclusion: Our meta-analysis indicates that higher preoperative CAR level is associated with poorer OS and DFS, it may be a good prognostic marker of survival outcomes after hepatectomy in patients with HCC. However, future prospective trials are necessary to validate the conclusion.
Systematic review registration: The study protocol was registered in the Open Science Framework (https://osf.io/uavt8).
Background
Despite advances in cancer treatment, liver cancer remains one of the most prevalent malignancies and constitutes a significant global public health challenge (1). According to the GLOBOCAN 2022, liver cancer ranks as the sixth most commonly diagnosed cancer and the third leading cause of cancer-related mortality, accounting for over 750,000 deaths worldwide in 2022 (2). Current treatment strategies available for liver cancer encompass surgery, chemotherapy, radiation, immunotherapies, or combinations thereof, with hepatectomy serves as the primary radical treatment for resectable liver cancer (3). However, a majority of patients experience high recurrence rates post-surgery, leading to suboptimal long-term survival outcomes (4). Consequently, the accurate identification of high-risk patients with poor prognoses through an efficient and convenient preoperative model is crucial. This identification facilitates the optimization of adjuvant therapy and potentially enhances long-term prognosis (5, 6).
Systemic inflammation within the tumor microenvironment is increasingly recognized as a crucial prognostic indicator in cancer patients (7). Serum C-reactive protein (CRP) and albumin (ALB), two significant acute phase-proteins, serve as markers reflecting the body’s nutritional status during a systemic inflammatory response (8). An increasing body of research has demonstrated their association with survival prognosis in cancer patients (9, 10). The CRP to ALB ratio (CAR) has recently emerged as a novel and potentially useful inflammation-based prognostic indicator for solid tumors. Recent studies have identified CAR as a reliable and effective prognostic marker for various cancers, including esophageal, gastric, pancreatic, colorectal, and nasopharyngeal cancers (11–15). Several recent studies have indicated that elevated CAR is associated with poorer survival rates and higher postoperative recurrence rates in patients with hepatocellular carcinoma (HCC) (16–19). Nevertheless, due to the heterogeneous biological behaviors of HCC, which can influence the clinical presentation of inflammation-related markers, the prognostic significance of CAR in assessing outcomes for HCC patients remains uncertain (20, 21). Therefore, we conducted this meta-analysis to investigate the prognostic value of CAR for overall survival (OS) and disease-free survival (DFS) in patients who have undergone hepatectomy for HCC.
Methods
Study selection
This meta-analysis was conducted in accordance with the updated PRISMA guidelines (22) (see Supplementary material 1). The study protocol was pre-registered in the Open Science Framework.1 Two authors independently conducted a comprehensive literature search in PubMed, Embase, Scopus, and Cochrane Library from their inception to May 10, 2024 for relevant articles published in English. The search strategy incorporated keywords such as “CRP,” “ALB,” “CAR,” and “liver cancer.” Detailed search strategies are provided in Supplementary material 2.
The inclusive criteria were as follows:
(1) Adult patients undergoing hepatectomy for HCC, with HCC confirmed by postoperative pathology;
(2) Evaluation of preoperative CRP and ALB levels in serum samples;
(3) Reported clinical outcomes including OS and DFS;
(4) Both prospective and retrospective studies, without design restrictions;
(5) Studies published in English only.
The exclusion criteria were as follows:
(1) Duplicate publications or studies involving heterogeneous patient types;
(2) Case reports, non-human studies, studies lacking sufficient information or relevant outcomes, and studies focusing on special populations (e.g., pediatric, pregnant);
(3) Studies including patients who did not undergo hepatectomy for HCC.
Data extraction and quality assessment
The retrieval of relevant studies were conducted independently by two authors (Shi Wang, and Shengqian Xu). All potentially eligible studies underwent full-text screening for inclusion. Discrepancies in this process were resolved through discussion and consensus. In cases where consensus could not be reached, a third co-author (Xiaopeng Fan) arbitrated to resolve the issue. A study-specific data abstraction form was adapted from the standardized Cochrane Data Collection template. Two authors (Jun Wang and Hailin Ye) independently extracted data, including the first author, publication year, country, sample size, population characteristics, cutoff value, and follow-up period. Predefined outcomes from the included studies were similarly extracted. When data were unavailable in the published articles, the corresponding authors were contacted to obtain essential missing information.
The quality of included studies was independently assessed by two reviewers (Shi Wang, and Shengqian Xu) using the Newcastle-Ottawa Scale (23). Publication bias was evaluated using the Egger’s regression test. When publication bias was identified, the trim-and-fill method was employed to further assess its potential impact on our meta-analysis (24). Any discrepancies throughout all phases were ultimately resolved through team consensus.
Statistical synthesis and analysis
We performed a pooled analysis to assess the relationship between CAR and OS and DFS in patients with HCC. A random-effects model was employed to estimate the hazard ratio (HR) along with a 95% confidence interval (CI). The I2 statistic was computed to gage heterogeneity between studies, where I2 values of <25%, 25 to 75, and > 75% signify low, moderate, and high heterogeneity, respectively (25). We stratified studies based on region and cutoff value (cutoff ≥0.02 versus ≥0.05) for subgroup analysis. Additionally, we conducted a sensitivity analysis to assess the impact of individual studies by omitting one at a time. All analyses were performed using the R software environment (version 4.3.1), with statistical significance set at p < 0.05.
Results
Study selection and study characteristics
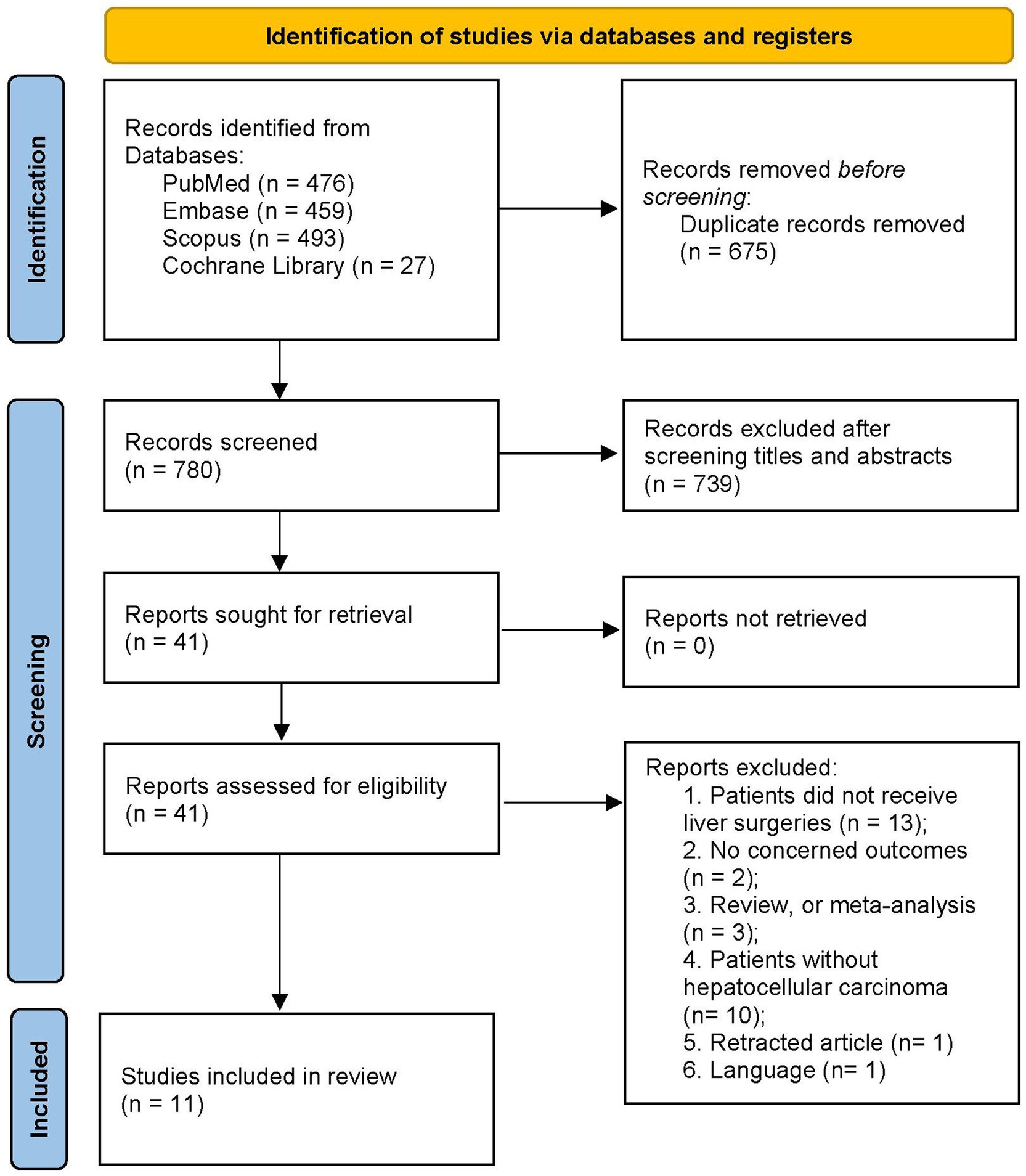
The initial search yielded a total of 1,455 articles from multiple sources: PubMed (n = 476), Embase (n = 459), Scopus (n = 493), and the Cochrane Library (n = 27). All records were imported into a document management software, from which 675 duplicated articles were automatically removed. Abstract screening resulted in the exclusion of 739 ineligible studies. Full-text evaluation led to the exclusion of 30 studies for various reasons (refer to Supplementary material 2). Finally, our meta-analysis included 11 studies (16, 17, 19, 26–33) encompassing 6,390 patients with HCC. Figure 1 illustrates the flow chart of the search and study selection processes.
Table 1 summarizes the basic characteristics of the included studies. The sample size ranged from 104 to 1,442 patients across the included studies. Most of included trials were conducted in Asian populations: five in Japan, four in China, one in Korea. One additional study was from Australia. Each study employed different CAR cutoff values, ranging from 0.027 up to 0.5. In addition, one study (28) reported the ALB to CRP ratio, which was converted to CAR for consistency.
All studies were assessed as high quality, with total scores exceeding 6 (Supplementary material 2). However, significant publication bias was detected for both OS and DFS outcomes (OS: p = 0.0039, DFS: p = 0.0115, Supplementary material 3). To address this bias, the trim-and-fill method was employed, resulting in adjusted pooled HRs of 1.76 (95%CI 1.55 to 2.01, I2 = 5%, Supplementary material 3) for OS and 1.74 (95%CI 1.55 to 1.95, I2 = 0%, Supplementary material 3) for DFS.
Meta-analysis results
All included studies reported OS rate. The random-effects model indicated that higher preoperative CAR was associated with poorer OS (HR 1.92, 95% CI 1.67 to 2.22, I2 = 0%, Figure 2A). DFS rate was evaluated in 9 included studies, with a pooled HR of 1.79 (95% CI 1.59 to 2.02, I2 = 0%, Figure 2B) for higher versus lower CAR groups.
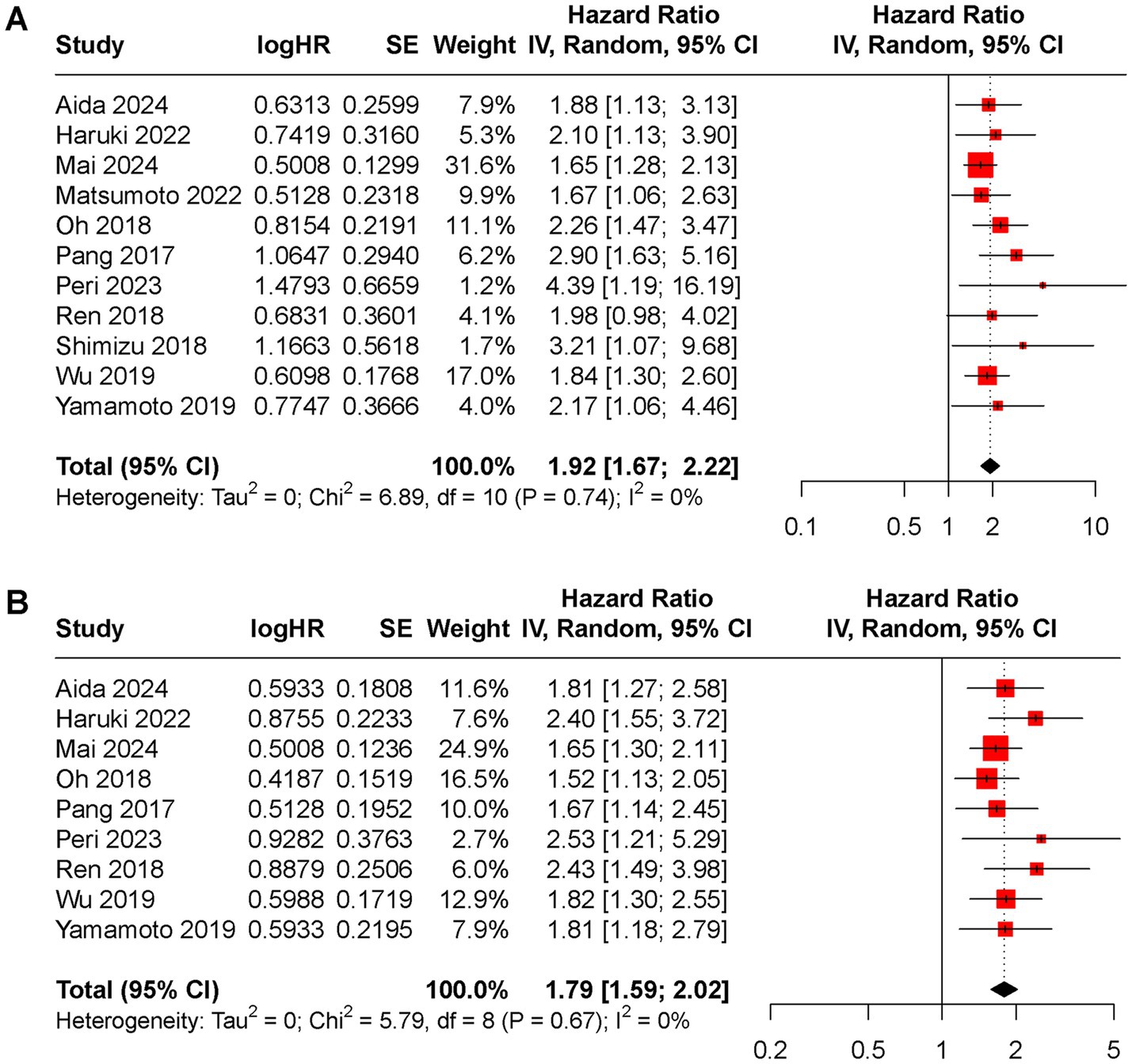
Figure 2. Forest plot of the pooled HR between low and high CAR groups for (A) OS rate, (B) DFS rate.
Subgroup analyses for OS and DFS stratified by regions and cut-off value were conducted (Figures 3, 4). Results indicated that preoperative CAR could serve as a prognostic biomarker for patients with HCC across different regions (Japan: HR for OS 1.94, 95% CI 1.49 to 2.17, I2 = 71%, HR for DFS 1.96, 95% CI 1.55 to 2.47, I2 = 0%; China: HR for OS 1.83, 95% CI 1.52 to 2.20, I2 = 4%, HR for DFS 1.77, 95% CI 1.50 to 2.08, I2 = 0%; Korea: HR for OS 2.26, 95% CI 1.47 to 3.47, HR for DFS 1.52, 95% CI 1.13 to 2.05; Australia: HR for OS 4.39, 95% CI 1.19 to 16.19, HR for DFS 2.53, 95% CI 1.21 to 5.29) and cut-off values (cutoff ≥0.02: HR for OS 2.00, 95% CI 1.57 to 2.56, I2 = 0%, HR for DFS 2.07, 95% CI 1.69 to 2.53, I2 = 0%; cutoff ≥0.05: HR for OS 1.92, 95% CI 1.57 to 2.36, I2 = 23%, HR for DFS 1.65, 95% CI 1.42 to 1.92, I2 = 0%).
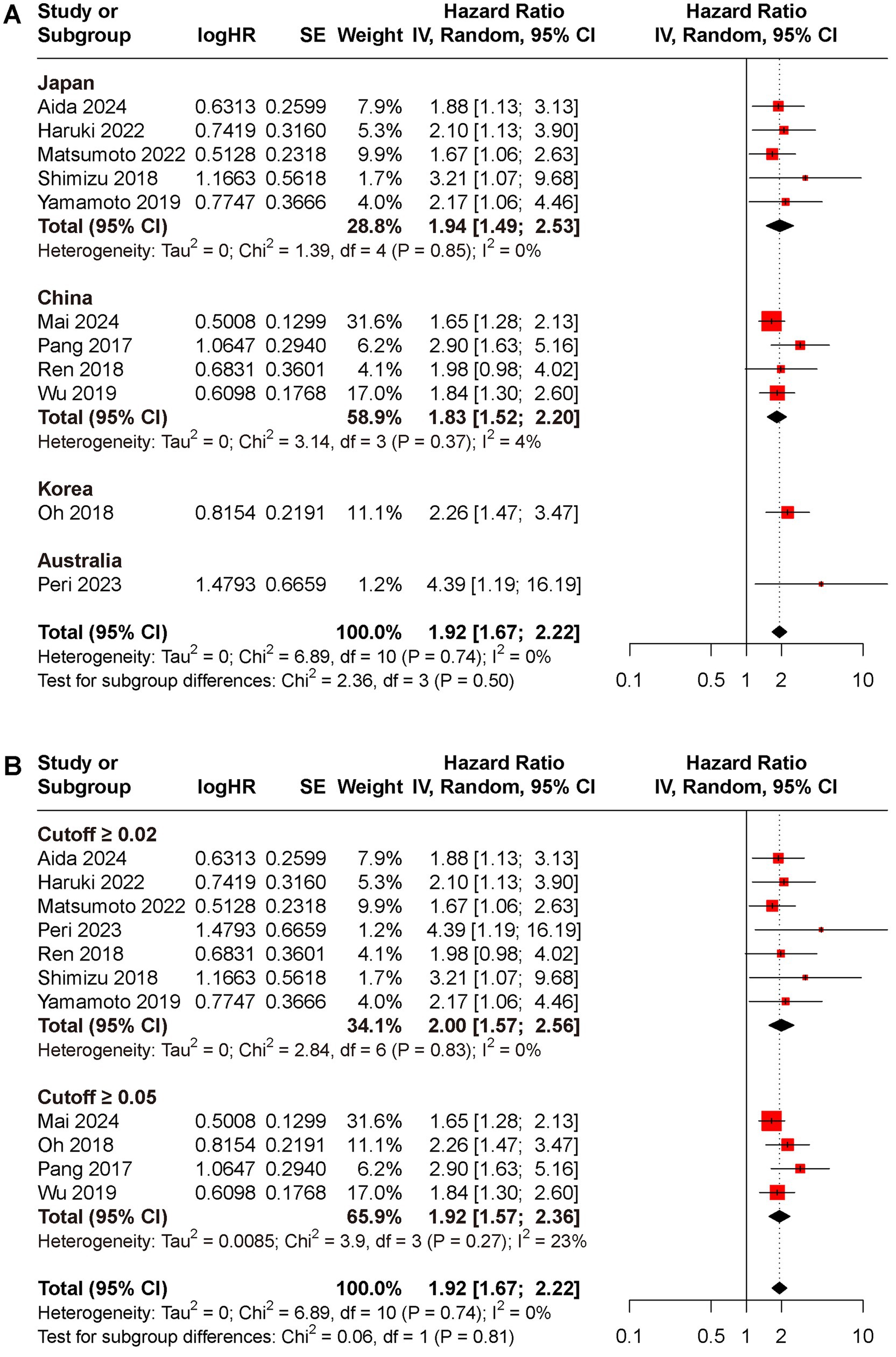
Figure 3. Subgroup analyses for OS rate between low and high CAR groups, (A) different reign; (B) cutoff ≥0.02 versus ≥0.05.
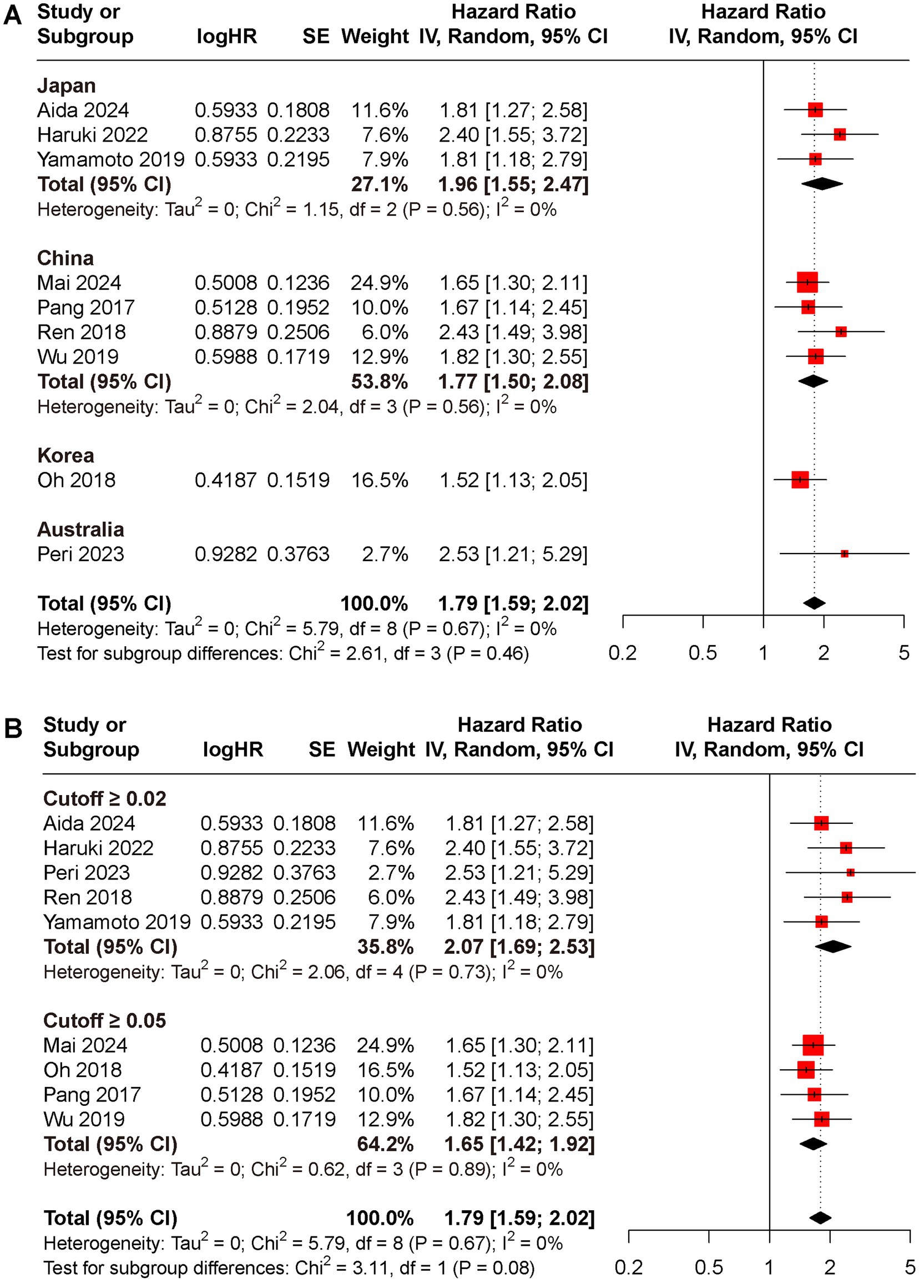
Figure 4. Subgroup analyses for DFS rate between low and high CAR groups, (A) different reign; (B) cutoff ≥0.02 versus ≥0.05.
Sensitivity analyses showed robust pooled effect estimates (Supplementary material 3), with HRs ranging from 1.87 (95%CI 1.62 to 2.17) to 2.07 (95%CI 1.74 to 2.46) for OS, and 1.75 (95%CI 1.54 to 1.98) to 1.85 (95%CI 1.62 to 2.11) for DFS.
Furthermore, the prognostic value of CAR was compared with alpha-fetoprotein (AFP) using data from nine studies that reported HRs for AFP in relation to OS and DFS. The pooled HRs for AFP were significant for both OS (HR 1.56, 95% CI 1.33 to 1.83, I2 = 5%, Figure 5A) and DFS (HR 1.59, 95% CI 1.35 to 1.88, I2 = 40%, Figure 5B).
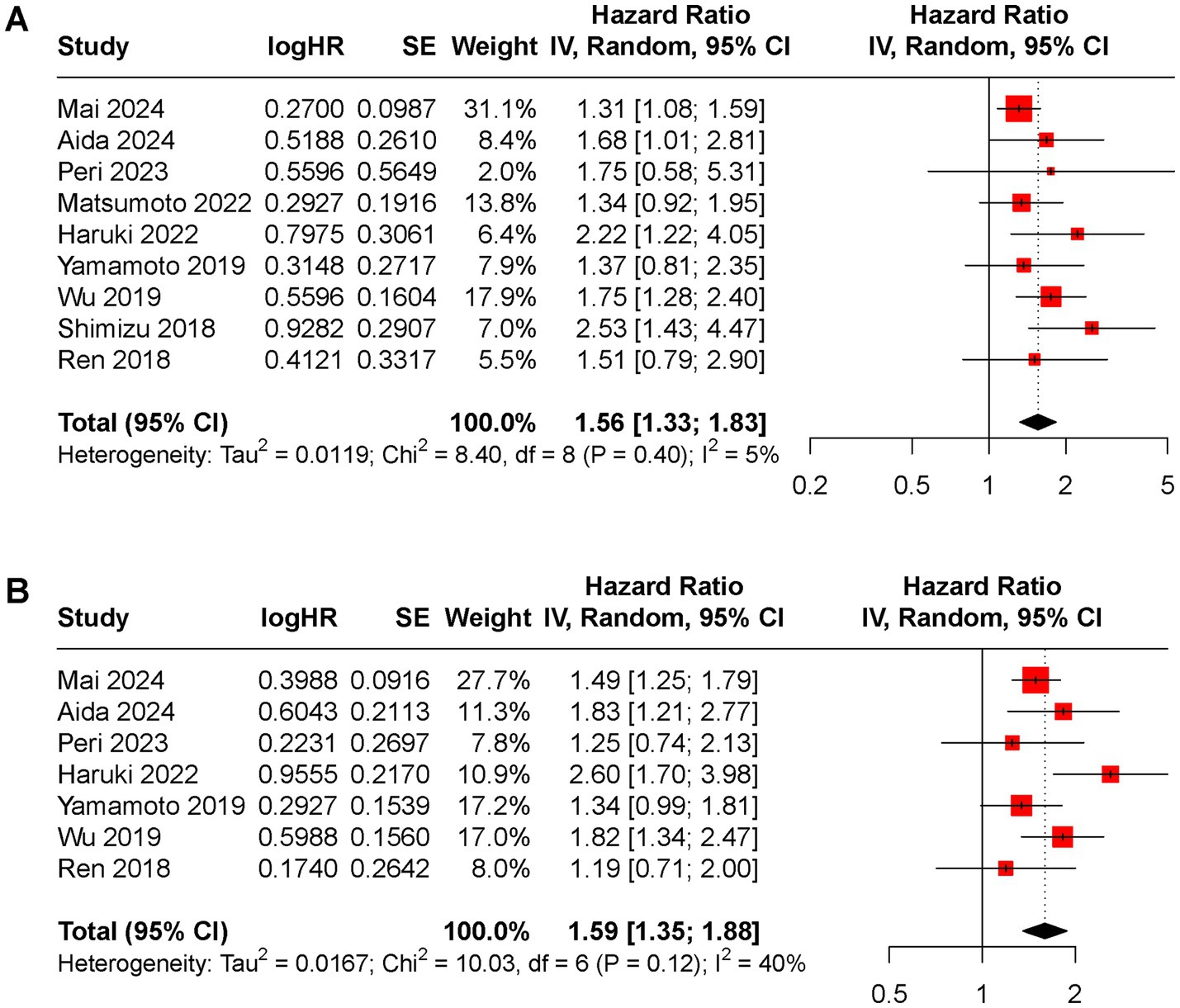
Figure 5. Forest plot of the pooled HR between low and high AFP groups for (A) OS rate, (B) DFS rate.
Discussion
In this updated meta-analysis investigating the prognostic value of preoperative CAR, we found that elevated preoperative CAR was significantly associated with poor OS and DFS rate among patients undergoing hepatectomy for HCC. Patients with high preoperative CAR exhibited a 1.78-fold reduction in OS and a 1.63-fold reduction in DFS compared to those with low CAR levels. This association remained consistent across different reigns, suggesting that preoperative CAR demonstrates promising prognostic value in various subgroups. Notably, preoperative CAR showed a stronger association with poor prognosis compared to AFP. However, the lack of a unified standard for establishing cutoff values presents a challenge that future studies need to address.
The inflammatory tumor microenvironment is closely implicated in all stages of cancer development, including initiation, promotion, and progression (34–36). Current evidence recognizes systemic inflammation response as a crucial factor in promoting tumorigenesis and cancer progression, with particular relevance to adverse outcomes in patients with HCC (37). Numerous studies have highlighted the significance of inflammation-based scoring systems in predicting the prognosis of HCC (38, 39). These scoring systems, such as the neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), platelet-to-lymphocyte ratio (PLR), and systemic immune-inflammation index (SII), typically comprise acute phase inflammatory cells from the circulatory system. Among these, the NLR, a predictive model based on circulating leukocytes, has demonstrated satisfactory prognostic value in patients with HCC (40, 41). Other studies have also found that the PLR, MLR, as well as SII, are useful predictors of outcomes in HCC patients (42, 43). However, a notable proportion of HCC patients undergo adjuvant therapy prior to hepatectomy, including hepatic artery infusion chemotherapy, targeted therapy, and radiofrequency ablation. These treatments can lead to a reduction in circulating inflammatory cells such as neutrophils, lymphocytes, monocytes, and platelet (37). Consequently, the leukocyte-based models mentioned earlier may not accurately reflect the true systemic inflammatory response status of HCC patients. As a result, they may be inadequate for evaluating the prognosis of HCC patients who have undergone hepatectomy.
Nutritional status is a crucial indicator significantly correlated with adverse outcomes in many cancer patients. Poor nutritional status heightens susceptibility to infection, vascular fragility, impaired wound healing, and dysfunctional coagulation, thus escalating the risk of serious postoperative complications, even mortality (44). CRP and ALB are vital acute-phase proteins reflecting the body’s nutritional state during systemic inflammatory responses and are pivotal in predicting cancer patient survival. The Glasgow prognostic score (GPS) and modified GPS (mGPS), comprising these acute-phase proteins, are recognized as independent prognostic markers HCC patients (45, 46). However, these markers are categorical. Conversely, the C-reactive protein to albumin ratio (CAR), also based on serum CRP and ALB, has been associated with a poor prognosis in HCC patients (20, 21). Despite using the same factors as GPS and mGPS, CAR can meticulously and rigorously stratify patient outcomes due to its continuous properties, potentially outperforming the sole use of serum CRP and albumin levels for scoring purposes.
To the best of our knowledge, this study represents the most comprehensive meta-analysis evaluating the prognostic value of preoperative CAR for survival outcomes after hepatectomy. A total of 11 studies comprised 4,066 patients with HCC undergoing hepatectomy were analyzed. The results indicated that high preoperative CAR was independently associated with poor OS and DFS among patients with HCC. Subgroup analyses demonstrated robust performance across different geographical locations. Collectively, our findings align with and substantiate the outcomes documented in previous meta-analyses (20, 21), thereby strengthening the existing body of evidence. Fan et al. (20) analyzed seven retrospective studies and found that HCC patients with high pre-treatment CAR had 2.48-fold reduced OS and 1.90-fold reduced DFS rate compared to those with low CAR. However, not all included patients received hepatectomy, potentially introducing selection bias due to differences in condition and treatment. Moreover, one of the included studies only reported the CRP to lymphocyte ratio instead of CRP, which could not be included in meta-analysis. Subsequently, Lin et al. (21) investigated the prognostic significance of CAR in Asian populations by including seven studies in China, Japan, and Korea. They discovered that the CAR was strongly correlated with the prognosis of patients with HCC and could serve as a noninvasive prognostic biomarker in clinical settings.
However, mechanism underlying the prognostic value of CAR in HCC remains unclear. Potential explanations may include the following: (1) Many solid tumors exhibit a strong association with inflammation, with CRP being one of the most common systemic inflammatory markers. (2) Nutritional status is a critical factor in the long-term prognosis of cancer patients, garnering increasing attention. ALB level serves as a simple marker to assess nutritional status. (3) ALB is exclusively synthesized in the liver, potentially enhancing the prognostic significance of CAR in HCC compared to other solid tumors.
Limitations
Nonetheless, this meta-analysis has several limitations that necessitate cautious interpretation of the findings. (1) The primary constraint of this meta-analysis pertains to the retrospective nature of all the included studies. This may introduce potential selection and recall biases, which could lead to an overestimation of the prognosis value of CAR. (2) Clinical heterogeneity is expected to contribute to statistical heterogeneity in the results. Despite efforts to account for this through subgroup analyses, confounding factors such as age, gender, liver function, and primary diseases may still affect the results. (3) Varying cut-off values for CAR across studies hindered the determination of an optimal threshold. This lack of standardization makes it challenging to establish a universally applicable prognostic value for CAR in clinical practice. (4) Despite comprehensive literature search efforts, the possibility of unpublished studies due to negative outcomes remains. This potential publication bias could skew the overall results toward a more positive association between CAR and prognosis. (5) Some incomplete data such as histopathological image after biopsy may have limited our ability to fully explore potential subgroup effects or moderating factors.
Conclusion
This meta-analysis provides evidence that elevated preoperative CAR is a significant predictor of poor OS and DFS in patients undergoing hepatectomy for HCC. Our findings suggest that CAR may serve as a valuable prognostic biomarker in this patient population. However, given the retrospective nature of the analyzed studies and the lack of a standardized cutoff value, further research is needed to verify the current findings. Furthermore, future multicenter, larger sample, and prospective randomized trials should investigate whether the application of CAR for patients with HCC can improve survival outcomes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
SW: Writing – review & editing, Writing – original draft, Methodology, Investigation, Data curation, Conceptualization. SX: Writing – review & editing, Writing – original draft, Supervision, Software, Project administration. JW: Writing – review & editing, Writing – original draft, Project administration, Formal analysis, Data curation. HY: Writing – review & editing, Writing – original draft, Project administration, Methodology, Formal analysis. XX: Writing – review & editing, Writing – original draft, Formal analysis, Data curation. KZ: Writing – review & editing, Software, Methodology, Investigation, Data curation. XF: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Resources, Methodology.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1444352/full#supplementary-material
Footnotes
References
1. Allemani, C, Matsuda, T, Di Carlo, V, Harewood, R, Matz, M, Nikšić, M, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. (2018) 391:1023–75. doi: 10.1016/s0140-6736(17)33326-3
2. Bray, F, Laversanne, M, Sung, H, Ferlay, J, Siegel, RL, Soerjomataram, I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
3. Sankar, K, Gong, J, Osipov, A, Miles, SA, Kosari, K, Nissen, NN, et al. Recent advances in the management of hepatocellular carcinoma. Clin Mol Hepatol. (2024) 30:1–15. doi: 10.3350/cmh.2023.0125
4. Hirokawa, F, Komeda, K, Taniguchi, K, Asakuma, M, Shimizu, T, Inoue, Y, et al. Is postoperative adjuvant Transcatheter arterial infusion therapy effective for patients with hepatocellular carcinoma who underwent hepatectomy? A prospective randomized controlled trial. Ann Surg Oncol. (2020) 27:4143–52. doi: 10.1245/s10434-020-08699-w
5. Beumer, BR, Buettner, S, Galjart, B, van Vugt, JLA, de Man, RA, JNM, IJ, et al. Systematic review and meta-analysis of validated prognostic models for resected hepatocellular carcinoma patients. Eur J Surg Oncol. (2022) 48:492–9. doi: 10.1016/j.ejso.2021.09.012
6. Innes, H, and Nahon, P. Statistical perspectives on using hepatocellular carcinoma risk models to inform surveillance decisions. J Hepatol. (2023) 79:1332–7. doi: 10.1016/j.jhep.2023.05.005
7. Jang, JH, Kim, DH, and Surh, YJ. Dynamic roles of inflammasomes in inflammatory tumor microenvironment. NPJ Precis Oncol. (2021) 5:18. doi: 10.1038/s41698-021-00154-7
8. Diakos, CI, Charles, KA, McMillan, DC, and Clarke, SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. (2014) 15:e493–503. doi: 10.1016/s1470-2045(14)70263-3
9. Cunha, GDC, Rosa, K, Wiegert, EVM, and de Oliveira, LC. Clinical relevance and prognostic value of inflammatory biomarkers: a prospective study in terminal Cancer patients receiving palliative care. J Pain Symptom Manag. (2021) 62:978–86. doi: 10.1016/j.jpainsymman.2021.04.009
10. Baitar, A, Kenis, C, Decoster, L, De Grève, J, Lobelle, JP, Flamaing, J, et al. The prognostic value of 3 commonly measured blood parameters and geriatric assessment to predict overall survival in addition to clinical information in older patients with cancer. Cancer. (2018) 124:3764–75. doi: 10.1002/cncr.31580
11. Tamagawa, H, Aoyama, T, Tamagawa, A, Komori, K, Maezawa, Y, Kano, K, et al. Influence of the preoperative C-reactive protein-to-albumin ratio on survival and recurrence in patients with esophageal Cancer. Anticancer Res. (2020) 40:2365–71. doi: 10.21873/anticanres.14205
12. Yue, L, Lu, Y, Li, Y, and Wang, Y. Prognostic value of C-reactive protein to albumin ratio in gastric Cancer: a Meta-analysis. Nutr Cancer. (2021) 73:1864–71. doi: 10.1080/01635581.2020.1817510
13. Kuboki, A, Kanaya, H, Nakayama, T, Konno, W, Goto, K, Nakajima, I, et al. Prognostic value of C-reactive protein/albumin ratio for patients with hypopharyngeal and laryngeal cancer undergoing invasive surgery involving laryngectomy. Head Neck. (2019) 41:1342–50. doi: 10.1002/hed.25565
14. Fan, Z, Fan, K, Gong, Y, Huang, Q, Yang, C, Cheng, H, et al. The CRP/albumin ratio predicts survival and monitors chemotherapeutic effectiveness in patients with advanced pancreatic Cancer. Cancer Manag Res. (2019) 11:8781–8. doi: 10.2147/cmar.S211363
15. Shibutani, M, Maeda, K, Nagahara, H, Iseki, Y, Ikeya, T, and Hirakawa, K. Prognostic significance of the preoperative ratio of C-reactive protein to albumin in patients with colorectal Cancer. Anticancer Res. (2016) 36:995–1001.
16. Mai, RY, Lu, TL, Lu, RJ, Zeng, C, Lian, F, Li, LQ, et al. C-reactive protein-albumin ratio (CAR): a more promising inflammation-based prognostic marker for patients undergoing curative hepatectomy for hepatocellular carcinoma. J Inflamm Res. (2024) 17:919–31. doi: 10.2147/jir.S441623
17. Aida, T, Haruki, K, Akaoka, M, Furukawa, K, Onda, S, Shirai, Y, et al. A novel combined C-reactive protein-albumin ratio and modified albumin-bilirubin score can predict long-term outcomes in patients with hepatocellular carcinoma after hepatic resection. Ann Gastroenterol Surg. (2024) 8:143–50. doi: 10.1002/ags3.12727
18. Sahakyan, MA, Brudvik, KW, Angelsen, JH, Dille-Amdam, RG, Sandvik, OM, Edwin, B, et al. Preoperative inflammatory markers in liver resection for colorectal liver metastases: a National Registry-Based Study. World J Surg. (2023) 47:2213–20. doi: 10.1007/s00268-023-07035-z
19. Peri, V, Lee, E, Fink, M, Starkey, G, Nikfarjam, M, Yoshino, O, et al. A single Centre experience with pre-operative markers in the prediction of outcomes after liver resection for hepatocellular carcinoma. J Gastrointest Surg. (2023) 27:1376–86. doi: 10.1007/s11605-023-05681-1
20. Fan, Y, Gu, X, and Gao, Z. Prognostic value of C-reactive protein to albumin ratio in patients with hepatocellular carcinoma: a meta-analysis. Clin Res Hepatol Gastroenterol. (2020) 44:241–3. doi: 10.1016/j.clinre.2019.07.014
21. Lin, N, Li, J, Ke, Q, Wang, L, Cao, Y, and Liu, J. Clinical significance of C-reactive protein to albumin ratio in patients with hepatocellular carcinoma: a Meta-analysis. Dis Markers. (2020) 2020:4867974–8. doi: 10.1155/2020/4867974
22. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
23. Wells, GA, Shea, B, O'Connell, D, Peterson, J, Welch, V, Losos, M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. (2020) [http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp]
24. Duval, S, and Tweedie, R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. (2000) 56:455–63. doi: 10.1111/j.0006-341x.2000.00455.x
25. Higgins, JP, Thompson, SG, Deeks, JJ, and Altman, DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
26. Haruki, K, Taniai, T, Yanagaki, M, Furukawa, K, Tsunematsu, M, Onda, S, et al. Sustained systemic inflammatory response predicts survival in patients with hepatocellular carcinoma after hepatic resection. Ann Surg Oncol. (2023) 30:604–13. doi: 10.1245/s10434-022-12464-6
27. Matsumoto, T, Kitano, Y, Imai, K, Kinoshita, S, Sato, H, Shiraishi, Y, et al. Clinical significance of preoperative inflammation-based score for the prognosis of patients with hepatocellular carcinoma who underwent hepatectomy. Surg Today. (2022) 52:1008–15. doi: 10.1007/s00595-021-02427-x
28. Wu, MT, He, SY, Chen, SL, Li, LF, He, ZQ, Zhu, YY, et al. Clinical and prognostic implications of pretreatment albumin to C-reactive protein ratio in patients with hepatocellular carcinoma. BMC Cancer. (2019) 19:538. doi: 10.1186/s12885-019-5747-5
29. Ren, Y, Fan, X, Chen, G, Zhou, D, Lin, H, and Cai, X. Preoperative C-reactive protein/albumin ratio to predict mortality and recurrence of patients with hepatocellular carcinoma after curative resection. Med Clin (Barc). (2019) 153:183–90. doi: 10.1016/j.medcli.2018.11.010
30. Shimizu, T, Ishizuka, M, Suzuki, T, Tanaka, G, Shiraki, T, Sakuraoka, Y, et al. The value of the C-reactive protein-to-albumin ratio is useful for predicting survival of patients with child-Pugh class A undergoing liver resection for hepatocellular carcinoma. World J Surg. (2018) 42:2218–26. doi: 10.1007/s00268-017-4446-0
31. Oh, TK, Choi, YR, Cho, JY, Yoon, YS, Han, HS, Park, IS, et al. The high-sensitivity C-reactive protein/albumin ratio predicts long-term oncologic outcomes after curative resection for hepatocellular carcinoma. J Clin Med. (2018) 7:139. doi: 10.3390/jcm7060139
32. Pang, S, Zhou, Z, Yu, X, Wei, S, Chen, Q, Nie, S, et al. The predictive value of integrated inflammation scores in the survival of patients with resected hepatocellular carcinoma: a retrospective cohort study. Int J Surg. (2017) 42:170–7. doi: 10.1016/j.ijsu.2017.04.018
33. Solaini, L, Atmaja, BT, Arumugam, P, Hutchins, RR, Abraham, AT, Bhattacharya, S, et al. The role of perioperative inflammatory-based prognostic systems in patients with colorectal liver metastases undergoing surgery. A cohort study. Int J Surg. (2016) 36:8–12. doi: 10.1016/j.ijsu.2016.10.010
34. Grivennikov, SI, Greten, FR, and Karin, M. Immunity, inflammation, and cancer. Cell. (2010) 140:883–99. doi: 10.1016/j.cell.2010.01.025
35. Hanahan, D, and Weinberg, RA. Hallmarks of cancer: the next generation. Cell. (2011) 144:646–74. doi: 10.1016/j.cell.2011.02.013
36. Greten, FR, and Grivennikov, SI. Inflammation and Cancer: triggers, mechanisms, and consequences. Immunity. (2019) 51:27–41. doi: 10.1016/j.immuni.2019.06.025
37. She, S, Shi, J, Zhu, J, Yang, F, Yu, J, and Dai, K. Impact of inflammation and the immune system on hepatocellular carcinoma recurrence after hepatectomy. Cancer Med. (2024) 13:e7018. doi: 10.1002/cam4.7018
38. Luo, QQ, Wang, T, and Zhang, KH. New indexes derived from routine blood tests and their clinical application in hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. (2022) 46:102043. doi: 10.1016/j.clinre.2022.102043
39. Roxburgh, CS, and McMillan, DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. (2010) 6:149–63. doi: 10.2217/fon.09.136
40. Xu, C, Wu, F, Du, L, Dong, Y, and Lin, S. Significant association between high neutrophil-lymphocyte ratio and poor prognosis in patients with hepatocellular carcinoma: a systematic review and meta-analysis. Front Immunol. (2023) 14:1211399. doi: 10.3389/fimmu.2023.1211399
41. Zhang, L, Feng, J, Kuang, T, Chai, D, Qiu, Z, Deng, W, et al. Blood biomarkers predict outcomes in patients with hepatocellular carcinoma treated with immune checkpoint inhibitors: a pooled analysis of 44 retrospective sudies. Int Immunopharmacol. (2023) 118:110019. doi: 10.1016/j.intimp.2023.110019
42. Lai, Q, Melandro, F, Larghi Laureiro, Z, Giovanardi, F, Ginanni Corradini, S, Ferri, F, et al. Platelet-to-lymphocyte ratio in the setting of liver transplantation for hepatocellular cancer: a systematic review and meta-analysis. World J Gastroenterol. (2018) 24:1658–65. doi: 10.3748/wjg.v24.i15.1658
43. Cui, S, Cao, S, Chen, Q, He, Q, and Lang, R. Preoperative systemic inflammatory response index predicts the prognosis of patients with hepatocellular carcinoma after liver transplantation. Front Immunol. (2023) 14:1118053. doi: 10.3389/fimmu.2023.1118053
44. Schwegler, I, von Holzen, A, Gutzwiller, JP, Schlumpf, R, Mühlebach, S, and Stanga, Z. Nutritional risk is a clinical predictor of postoperative mortality and morbidity in surgery for colorectal cancer. Br J Surg. (2010) 97:92–7. doi: 10.1002/bjs.6805
45. Li, MX, Bi, XY, Li, ZY, Huang, Z, Han, Y, Zhou, JG, et al. Prognostic role of Glasgow prognostic score in patients with hepatocellular carcinoma: a systematic review and Meta-analysis. Medicine (Baltimore). (2015) 94:e2133. doi: 10.1097/md.0000000000002133
Keywords: C-reactive protein to albumin ratio, prognostic marker, hepatocellular carcinoma, hepatectomy, meta-analysis
Citation: Wang S, Xu S, Wang J, Ye H, Zhang K, Fan X and Xu X (2024) Preoperative C-reactive protein to albumin ratio may be a good prognostic marker in patients undergoing hepatectomy for hepatocellular carcinoma: a meta-analysis. Front. Nutr. 11:1444352. doi: 10.3389/fnut.2024.1444352
Edited by:
Lucilla Crudele, University of Bari Aldo Moro, ItalyCopyright © 2024 Wang, Xu, Wang, Ye, Zhang, Fan and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoya Xu, eGlhb3lhX3h1QGFsaXl1bi5jb20=
 Shi Wang1
Shi Wang1 Hailin Ye
Hailin Ye Kai Zhang
Kai Zhang Xiaoya Xu
Xiaoya Xu