Abstract
Background:
The incidence of NAFLD is increasing. Preclinical evidences indicate that modulation of the gut microbiome could be a promising target in nonalcoholic fatty liver disease.
Method:
A systematic review and network meta-analysis was conducted to compare the effect of probiotics, synbiotics, prebiotics, fecal microbiota transplant, and antibiotics on the liver-enzyme, metabolic effects and liver-specific in patients with NAFLD. The randomized controlled trails (RCTs), limited to English language were searched from database such as Pubmed, Embase, Web of science and Cochrane Library from inception to November 2024. Review Manager 5.3 was used to to draw a Cochrane bias risk. Inconsistency test and publication-bias were assessed by Stata 14.0. Random effect model was used to assemble direct and indirect evidences. The effects of the intervention were presented as mean differences with 95% confidence interval.
Results:
A total of 1921 patients from 37 RCTs were eventually included in our study. 23 RCTs evaluated probiotics, 10 RCTs evaluated synbiotics, 4 RCTs evaluated prebiotics, 3 RCTs evaluated FMT and one RCT evaluated antibiotics. Probiotics and synbiotics were associated with a significantly reduction in alanine aminotransferase [ALT, (MD: −5.09; 95%CI: −9.79, −0.39), (MD: −7.38, 95CI%: −11.94, −2.82)] and liver stiffness measurement by elastograph [LSM, (MD: −0.37;95%CI: −0.49, −0.25), (MD: −1.00;95%CI: −1.59, −0.41)]. In addition to, synbiotics was superior to probiotics in reducing LSM. Synbiotics was associated with a significant reduction of Controlled Attenuation Parameter [CAP, (MD: −39.34; 95%CI: −74.73, −3.95)]. Both probiotics and synbiotics were associated with a significant reduction of aspartate transaminase [AST, (MD: −7.81; 95%CI: −15.49, −0.12), (MD: −13.32; 95%CI: −23, −3.64)]. Probiotics and Allogenic FMT was associated with a significant reduction of Homeostatic Model Assessment for Insulin Resistance [HOMA-IR, (MD: −0.7, 95%CI: −1.26, −0.15), (MD: −1.8, 95%CI: −3.53, − 0.07)]. Probiotics was associated with a significant reduction of body mass index [BMI, MD: −1.84, 95%CI: −3.35, −0.33].
Conclusion:
The supplement of synbiotics and probiotics maybe a promising way to improve liver-enzyme, LSM, and steatosis in patients with NAFLD. More randomized controlled trials are needed to determine the efficacy of FMT and antibiotics on NAFLD. And the incidence of adverse events of MTTs should be further explored.
Systematic review registration:
https://www.crd.york.ac.uk/prospero/, CRD42023450093.
1 Introduction
Nonalcoholic fatty liver disease (NAFLD) is a spectrum of liver tissue abnormalities that includes isolated hepatic steatosis, nonalcoholic steatohepatitis (NASH), liver fibrosis, cirrhosis, and liver cancer. With rapid economic development and lifestyle changes, NAFLD has become the leading cause of chronic liver disease, affecting 20–25% of adults worldwide, and estimated to affect 20% individuals with NASH (1). As a severe subtype of NAFLD, the incidence of NASH is increasing. It is projected to affect over 50% of the population by 2031, increasing incidence of liver cirrhosis, hepatocellular carcinoma, and mortality, imposing an economic burden on society (2, 3). There is no approved drug for NAFLD (4). Lifestyle modification, such as physical activity and weight loss, is a major intervention, that is difficult for overweight patients (5).
The gut contains a complex colony of trillions of microorganisms that live in harmony with the human body and help regulate metabolism, immunity, digestion, and nutrient absorption (6). Changes in the composition or function of the intestinal flora can participate in occurrence and development of various diseases by dysregulating host metabolism and immunity (7, 8). Preclinical studies have shown that regulating the gut microbiota can inhibit the development of obesity and hepatic steatosis, reduce liver inflammation, and delay the occurrence of NASH (9–11), indicating that targeting gut microbiota is a promising therapeutic measure.
Microbiome-targeted therapies (MTTs) have been proposed as a promising approach to regulate the gut microbiome, including several categories of antibiotics, probiotics, synbiotics, prebiotics and fecal microbiota transplantation (FMT). FMT is a process in which fecal flora collected from a healthy donor is transferred into a patient through a series of delivery routes, such as colonoscopy, nasogastric tube, and enema. Evidence shows that FMT could significantly reduce intracellular hepatic lipid and proinflammatory cytokine concentrations in high-fat diet-fed NAFLD mice and liver fibrosis and inflammatory infiltrates in NASH mice (12). In addition, Anecdotal. et al. also found a significant improvement in insulin sensitivity after the FMT from lean, healthy donors (13). Probiotics is live, non-pathogenic microorganisms that can improve gut health, as well as their critical metabolites. Acetate from probiotics could prevent NAFLD-HCC progression by binding with G-coupled protein receptor 43 (GPR43) and suppressing the IL-6/JAK1/STAT3 signaling pathway (14). Prebiotics can alleviate endotoxemia and inflammation, providing an alternative way to improve metabolic disorders. Beisner et al. found that prebiotics inulin and its fermentation product butyrate could attenuate weight gain and hepatic steatosis by promoting mucosal barrier integrity and strengthening paneth cell antimicrobial function (15). Synbiotics is a combination of probiotics and prebiotics. Alves et al. found that synbiotics supplementation could upregulate the expression of peroxisome proliferator-activated receptor α (PPAR-α) and downregulate sterol regulatory element-binding protein 1c (SREBP-1C) to reduce steatosis by increasing the β-oxidation process and modulating lipogenesis (16).
Recently, with increasing evidence from clinical trials demonstrating that MTTs play a significant role in improving NAFLD/NASH, the systematic review and meta-analysis by Sharpton et al. (17) was expanded to clearify the impact of probiotics, synbiotics, and prebiotics on NAFLD. However, due to the limited number of included literatures, only those on probiotics, synbiotics, and prebiotics were analyzed, and the efficacy and safety of MTTs in NAFLD treatment, including FMT and antibiotics, remains unevaluated. Moreover, few studies directly compare MTTs efficacy in NAFLD treatment (18). Therefore, given current literature limitations, our aim is to conduct a comprehensive systematic review and network meta-analysis, including as many pertinent literatures as possible, to evaluate the efficacy of MTTs in NAFLD/NASH treatment across hepatic inflammation, energy metabolism, and liver-specific outcomes.
2 Methods
This study follows the guidance of Preferred Reporting Items for meta-analysis and Systematic review of the network meta-analysis list. We established a protocol for the review, which was registered with PROSPERO prior to commencing the study1 (CRD42023450093).
3 Study selection
Included criteria: (1) study type: randomized controlled trails (RCTs); (2) Study object: NAFLD patients, which was defined by either Liver histology or noninvasive imagine modality (MRI, ultrasound, or elastography); (3) intervention measures: the experiment group was treated by MTTs, which was defined as interventions in any of the following 5 categories: prebiotics, synbiotics antibiotics, prebiotics and FMT. The control group was treat with placebo, usual care, and other MTTs different from the experiment group; (4) duration of therapy was≥4 week (excluding FMT trials); (5) Outcome indicators: one of the following outcomes was assessed: LSM, CAP, ALT, AST, TG, HDL-C, LDL-C, BMI, HOMA-IR. Excluded criteria: (1) hepatitis steatosis or fibrosis in patients were caused by hepatitis, liver cancer, autoimmune hepatitis, or other factors; (2) the study was not RCT; (3) the study did not acquire full text; and (4) the study was duplicated.
4 Search strategy
Pubmed Embase Web of science Cochrane Library were used as database for RCT research retrieval. The retrieval time was from the establishment of the database to November 2024. Keywords included were provided in Supplementary Table S1.
5 Data extraction and quality assessment
Two investigators independently read and screened the studies according to the inclusion criteria, and extracted data from the final included studies. The collected content included the authors of the included studies, the year of publication, the diagnostic criteria of the disease, the sample size, the age and gender of the participants, the intervention, follow-up duration, the outcomes, adverse reactions, and other relevant information. The mean and standard deviation (SD) values at the endpoint were directly extracted or calculated from the provided data. Cross-checks were conducted after screening, and a third party will be consulted to assist in judgment in case of disagreement.
The methodological quality of the included studies was assessed using the RCT risk of bias assessment tool in the Cochrane Handbook for Systematic Reviews. Two review authors independently evaluated each outcome in seven aspects: randomization sequence, allocation concealment, blinding of patients and staff, blinding of outcome assessors, completeness of outcome data, selective reporting of results, and other sources of bias. The risk of bias for each outcome was assessed as ‘low-risk’, ‘high-risk’, or ‘unclear’.
6 Statistical analysis
In this study, Review Manager 5.3 was used to draw a Cochrane bias risk. Stata 14.0 were used for network meta-analysis (NMA). We estimated summary mean difference (MD) for continuous outcomes using pairwise and network meta-analysis. The significance of an effect was expressed by 95% confidence interval (CI).
The results of included articles were described in the tables. Network evidence plots were used to show the relationship between interventions. In the Network evidence plots, the size of the dot represents the sample size of the treatment method. The larger dot is, the more the sample size is. The thickness of the line between two dots represents the number of studies. The thicker the line is, the more the number of studies is. The Surface Under the Cumulative Ranking (SUCRA) was used to reflect the probability order of different MTTs to be the best treatment option. A higher SUCRA score indicated a more effective or accepted treatment. Comparison adjusted funnel plots were used to assess the presence of publication bias. When there was a closed loop, we carried out inconsistency test. In the inconsistency test, if p < 0.05, it was considered that there existed inconsistency between the direction or indirection comparison. If there is an inconsistency, we chose random effect network meta-analysis model. We used the node splitting method to investigate the sources of inconsistency. Once node splitting method identifies the inconsistent node segments, we applied the stepwise exclusion method to pinpoint the specific studies or factors causing the inconsistency.
7 Results
7.1 Search results
According to the pre-determined retrieval strategy, 2,724 documents were retrieved from Pubmed, Web of science, Embase, and Cochrane library. 836 duplicated articles were removed, and the documents that did not meet the criteria were excluded by reading the abstract and full text of the documents. Finally, 37 articles were included. The specific retrieval process was shown in Figure 1.
Figure 1
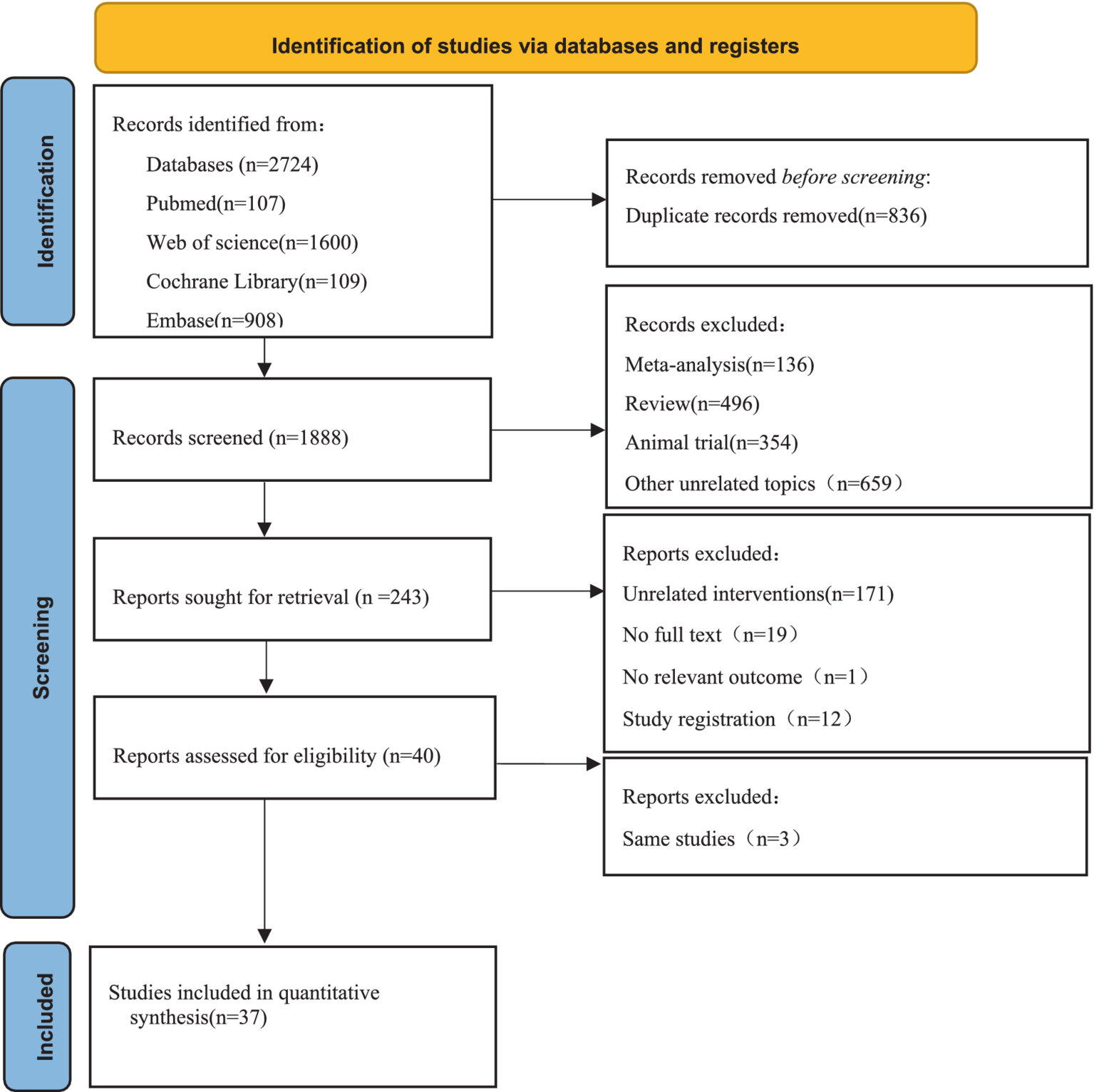
Flow diagram of the search strategy and study selection.
7.2 Characteristics of included studies
The 37 studies included 1921 patients, including 266 pediatrics and 1,655 adults. The articles were published from 2011 to 2024, and 35 of them are double-arm studies, one of them is three-arm study, and one of them is four-arm study. 5 MTTs were included: probiotics (23 RCTs), synbiotics (10 RCTs), prebiotics (4 RCTs), FMT (3 RCTs), antibiotics (1 RCT). More details of included studies were shown in Table 1.
Table 1
| Study ID | Diagnosis | Patient population | Diagnostic criteria | Sample size (M/F) | Age (year) | Intervention experimental group | Control group | Duration (week) | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Xue et al. (42) | NAFLD | Adult | (AASL) and Treatment of Non-Alcoholic Fatty Liver Disease (2018 revision) | 75(39/36) | E:(57.3 ± 13.4) C:(60.2 ± 8.5) |
Allogenic FMT | probiotics | 4 | BMI HOMA-IR UA AST ALT TBIL ALB TC TG LDL-C HDL-C LSM |
| Wong et al. (43) | NASH | Adult | Histology-proven | 20(13/7) | E:(42 ± 9) C:(55 ± 9) |
Probiotics and prebiotics | Usual care group | 24 | BMI ALT AST HDL-C LDL-C LSM Triglycerides |
| Witjes et al. (44) | NAFLD | Adult | Ultrasound | 21(19/2) | E:(51.2 ± 6.6) C:(48.5 ± 10.2) |
Allogenic FMT, | Autologous FMT1 | 24 | GGT AST ALT Cholesterol HDL-C LDL-C |
| Vajro et al. (19) | NAFLD | Pediatric | Ultrasound &ALT | 20(−/−) | / | Lactobacillus GG | Placebo | 8 | ALT BMI |
| Sepideh et al. (45) | NAFLD | Adult | Ultrasound | 42(28/14) | E:(42.10 ± 1.99) C:(47.33 ± 2.53) |
Probiotics | Placebo | 8 | HOMA-IR |
| Scorletti et al. (46) | NAFLD | Adult | / | 89(58/31) | E:50.2(12.4) C:51.6(13.1) |
Synbiotics | Placebo | 48 | BMI TC HDL-C LDL-C Triglycerides ALT AST GGT LSM CAP |
| Rodrigo et al. (47) | NAFLD/NASH | Pediatric | Ultrasound & AST/ALT ratio < 1 |
84(62/22) | E:(11.28 ± 1.87) C:(12.05 ± 1.45) |
Probiotics | Placebo | 24 | AST ALT CAP LSM BMI TC TG HDL LDL GGT |
| Nor et al. (48) | NAFLD | Adult | Ultrasound& CAP& ALT | 39(28/11) | E:54.70(10.19) C:52.47(16.73) |
Probiotics | Placebo | 24 | CAP TC TG ALT AST GGT LSM BMI |
| Mofidi et al. (49) | NAFLD | Adult | CAP &ALT | 42(23/19) | E:(40.09 ± 11.44) C:(44.61 ± 10.12) |
Synbiotics | Placebo | 28 | CAP LSM AST ALT HOMA-IR HDL-C LDL-C TC |
| Manzhalii et al. (50) | NASH | Adult | Ultrasound &ALT | 75(27/48) | E:(44.3 ± 1.5) C:(43.5 ± 1.3) |
Probiotics | Usual care | 12 | BMI TC TG ALT AST GGT LSM |
| Malaguarnera et al. (51) | NASH | Adult | Ultrasound &liver biopsy &ALT | 66(33/33) | E:(46.9 ± 5.4) C:(46.7 ± 55.7) |
Synbiotics | Placebo | 24 | BMI AST ALT TC TG HDL-C LDL-C HOMA-IR |
| Javadi et al. (20) | NAFLD | Adult | Ultrasound ALT | 75(60/15) | E:(43.9 ± 9.02); (38.7 ± 10); (43.2 ± 6.95); C:(42.2 ± 9.11) |
Probiotics\prebiotics\synbiotics | Placebo | 12 | BMI TC TG HDL-C LDL-C HOMA-IR |
| Goyal et al. (52) | NAFLD | Pediatrics | Ultrasound | 54(/) | E:(11.7 ± 2.21) C:(11.0 ± 1.20) | VSL#3 | Placebo | 16 | BMI AST ALT GGT LDL-C HDL-C Cholesterol |
| Ferolla et al. (53) | NASH | Adult | liver biopsy | 50(12/38) | / | Synbiotics | Usual care | 12 | BMI Cholesterol HDL-C LDL-C Triglycerides ALT AST |
| Famouri et al. (54) | NAFLD | Pediatric | Ultrasound | 64(32/32) | E:12.7(2.2) C:12.6(1.7) |
Probiotics | Placebo | 12 | AST ALT HDL-C LDL-C BMI Cholesterol Triglyceride |
| Eslamparast et al. (55) | NAFLD | Adult | Ultrasound | 52(25/27) | E:(46.35 ± 8.8) C:(45.69 ± 9.5) |
Synbiotics | Placebo | 28 | BMI ALT AST HOMA-IR LSM |
| Duseja et al. (56) | NAFLD | Adult | ALT& AST | 39(28/11) | E:38(10) C:33(6) |
Probiotics | Placebo | 48 | BMI AST BIL ALT |
| Craven et al. (57) | NAFLD | Adult | AASLD | 21(6/15) | E:47.6(14.9) C:57.5(13.0) |
Allogenic FMT | Autologous FMT | 24 | BMI |
| Chong et al. (58) | NAFLD | Adult | / | 35(28/7) | E:(57 ± 8) C:(58 ± 7) |
VSL#3 | Placebo | 10 | TC HDL LDL Triglycerides HOMA-IR ALT AST BMI |
| Cai et al. (59) | NAFLD | Adult | Ultrasound &liver biopsy &ALT |
140(85/55) | E:(46.13 ± 12.72) C:(49.62 ± 9.08) |
Probiotics | Usual care | 12 | ALT TBIL AST TG TC LDL-C HDL-C HOMA-IR |
| Bomhof et al. (60) | NASH | Adult | liver biopsy | 14(8/6) | E:(45.3 ± 5.6) C:(53.5 ± 4.8) |
Prebiotics | Placebo | 24 | BMI ALT HOMA-IR |
| Behrouz et al. (61) | NAFLD | Adult | ALT& Ultrasound | 89(63/26) | E1:(38.46 ± 7.11) E2:(38.41 ± 9.21) C:(38.43 ± 10.09) |
Probiotics/prebiotics | Placebo | 12 | BMI TG TC HDL-C LDL-C ALT AST Triglyceride |
| Asgharian et al. (62) | NAFLD | Adult | Ultrasound | 74(19/55) | E:(46.57 ± 1.7) C:(47.78 ± 1.7) |
Synbiotics | Placebo | 8 | ALT AST BMI |
| Aller et al. (63) | NAFLD | Adult | liver biopsy | 28(14/14) | E:(49.4 ± 10.9) C:(44.3 ± 15.1) |
Probiotics | Placebo | 12 | BMI TC LDL-C HDL-C TG HOMA-IR |
| Alisi et al. (64) | NAFLD | Pediatric | biopsy-proven | 44(24/20) | E:10(9,12) C:11(10,12) |
VSL3# | Placebo | 16 | Triglycerides HOMA-IR BMI ALT |
| Ahn et al. (65) | NAFLD | Adult | / | 65(33/32) | E:(41.7 ± 12.49) C:(44.71 ± 13.31) |
Probiotics | Placebo | 12 | BMI CAP LSM Cholesterol Triglyceride AST ALT |
| Abhari et al. (66) | NAFLD | Adult | CAP &ALT | 45(25/20) | E:(47.7 ± 11.4) C:(46.7 ± 12.4) |
Synbiotics | Placebo | 12 | CAP AST ALT BMI Cholesterol TG TC LDL-C HDL-C HOMA-IR |
| Sayari et al. (67) | NAFLD | Adult | ALT& Ultrasound | 140(85/55) | E:(42.48 ± 11.41) C:(43.42 ± 11.65) |
Synbiotics+ sitagliptin | Placebo (sitagliptin in both groups) | 16 | BMI TC ALT AST TG LDL HDL |
| Sadrkabir et al. (68) | NAFLD | Adult | Ultrasound | 61(40/21) | E:(43.26 ± 11.42) C:(43.72 ± 10.76) |
Gerilact | Placebo | 8 | BMI AST ALT TG LDL HDL CHOL |
| Kobyliak et al. (69) | NAFLD | Adult | AASLD | 58(−/−) | E:(53.4 ± 9.55) C:(57.29 ± 10.45) |
Probiotics | Placebo | 8 | BMI LSM ALT AST GGT TC TG HDL-C LDL-C |
| Ekhlasi et al. (70) | NAFLD | Adult | ALT &Ultrasound | 30(−/−) | / | Synbiotics | Placebo | 8 | BMI ALT AST |
| Abdel-Razik et al. (71) | NASH | Adult | Liver biopsy-proven | 50(16/34) | E:(40.2 ± 9.88) C:(38.4 ± 9.21) |
Rifaximin | Placebo | 24 | ALT AST HOMA-IR BMI |
| Ayob et al. (38) | NAFLD | Adult | ALT &Ultrasound | 40(29/11) | E:(55 ± 11.07) C:(49.95 ± 14.05) |
Probiotics | Placebo | 24 | BMI ALT AST TG TC |
| Derosa et al. (72) | NAFLD | Adult | ALT | 60(28/32) | E:(55.8 ± 7) C:(56.76 ± 7.7) |
VASL#3 | PLACEBO | 12 | BMI TC LDL-C HDL-C TG AST ALT |
| Escouto et al. (73) | NASH | Adult | Liver biopsy-proven | 48(10/38) | E:(58 ± 31.85) C:(57 ± 28.15) |
probiotics | Placebo | 24 | BMI ALT AST TC HDL-C LDL-C TG HOMA-IR |
| Reshef et al. (74) | NAFLD | Adult | Ultrasound | 50(15/35) | E:(45.72 ± 8.9) C:(46.48 ± 11.6) |
probiotics | UC | 12 | AST ALT |
| Naama et al. (75) | NAFLD | Adult | Ultrasound & ALT | 19(15/4) | C:(50 ± 14.52) E:(47.8 ± 10.37) |
Prebiotics | Placebo | 12 | BMI HOMA-IR TC HDL-C LDL-C TG ALT AST |
Characteristics of included studies.
“/” represents that it is not mentioned in the text; E: Experimental group; C: Control group.
7.3 Risk of bias
Review Manager 5.3 was used to draw a Cochrane bias risk. The risk assessment of 37 RCTs was shown in Figure 2. 20 studies used appropriate randomization methods, such as computer-generated random numbers or tables. 14 studies indicated allocation concealment, such as treatment allocation being packed in identical packages, sachets or same envelope. One non-blind study (19) was evaluated as “high risk.” 20 studies provided detailed descriptions of data integrity, including records of missing data and retention status at different stages. These were rated as “low risk.” None of the studies indicated selective reporting and were rated “unclear.” The result of risk assessment was shown in Figure 2.
Figure 2
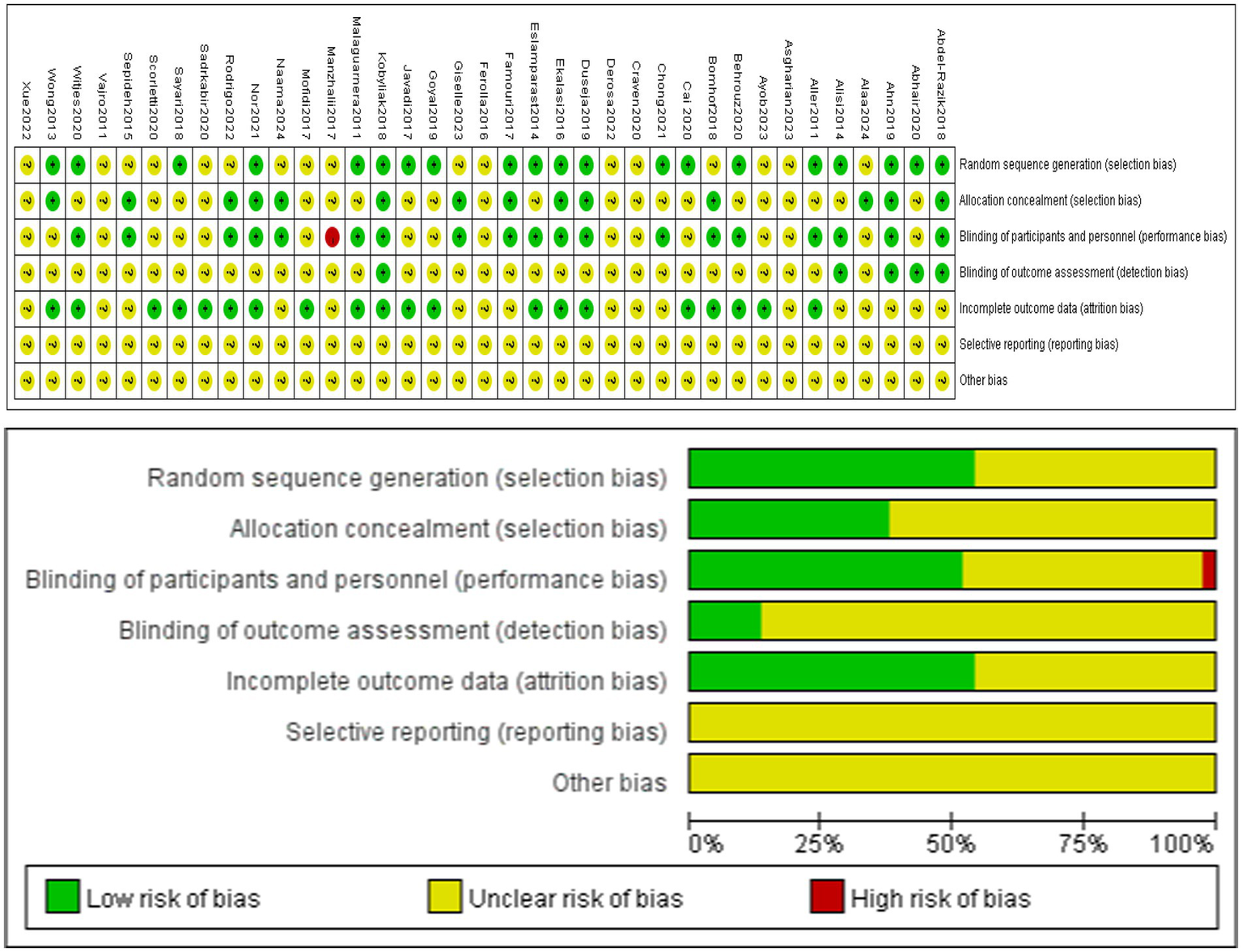
Risk of bias.
7.4 Network meta-analysis
7.4.1 Primary outcome
30 studies reported ALT, involving probiotics, prebiotics, synbiotics, FMT, of which the network relationship between the interventions is shown in Figure 3. In terms of ALT improvement, according to MD and 95%CI between all the pairwise interventions, probiotics (MD: −5.09; 95%CI: −9.79, −0.39), synbiotics (MD: −7.38; 95CI%: −11.94, −2.82) were superior to placebo. As shown in Table 2 and Figure 4. In addition, Autologous FMT, with the highest-ranking probability of SUCRA (76%), had the best effectiveness in reducing ALT, followed by prebiotics (62.8%) and synbiotics (71.8%). More details about the rank probability of SUCRA are shown in Figure 5.
Figure 3
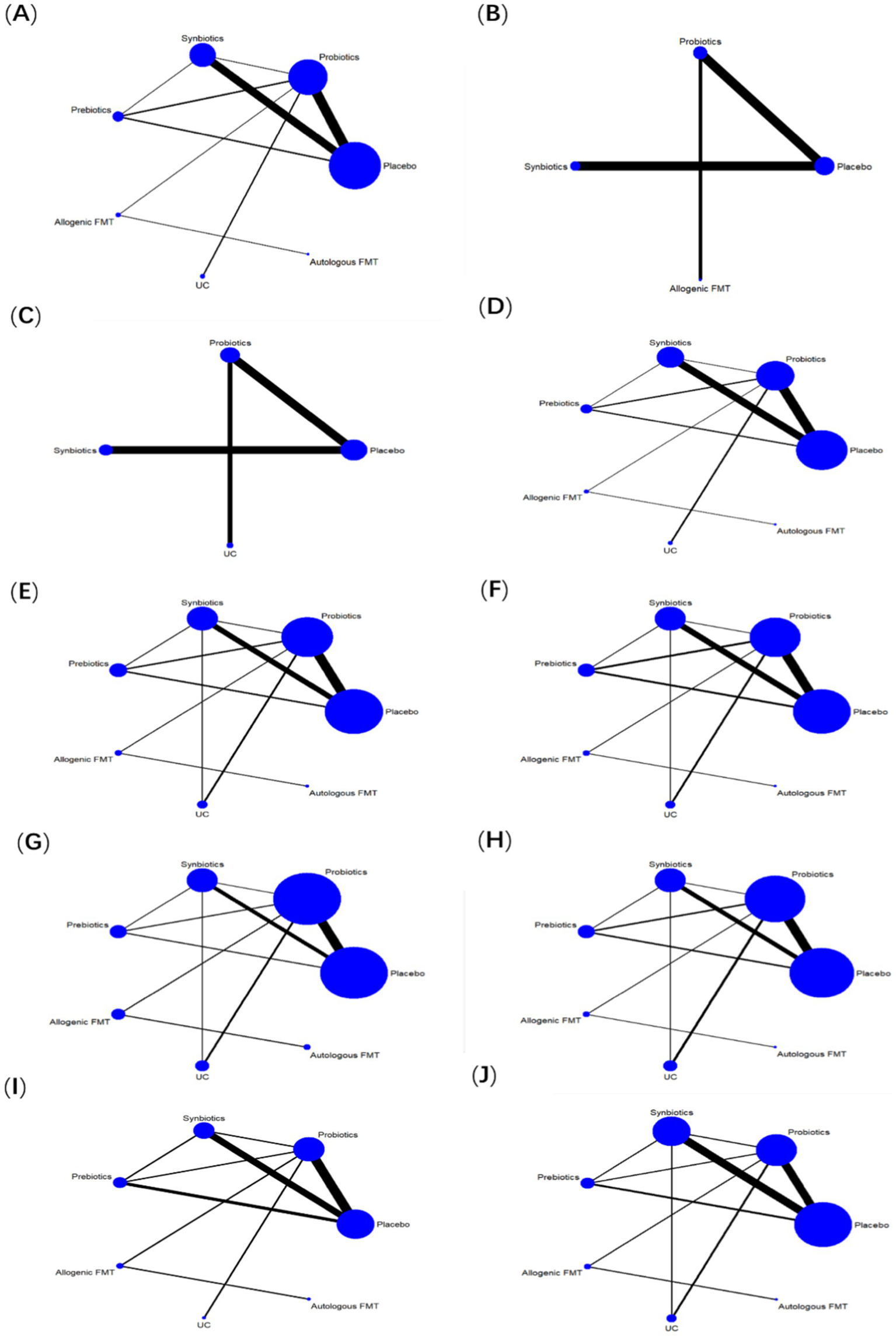
Network evidence plots. (A) ALT (B) CAP (C) LSM (D) AST (E) HDL-C (F) LDL-C (G) TG (H) TC (I) HOMA-IR (J) BMI.
Table 2
| Autologous FMT | - | - | - | - | - | - |
| −5.91 (−32.50, 20.68) | Synbiotics | - | −42.81 (−88.26, 2.64) | −24.21 (−88.24, 39.81) | - | −39.34 (−74.73, −3.95) |
| −6.78 (−33.91, 20.35) | −0.87 (−9.87, 8.12) | Prebiotics | - | - | - | - |
| −8.20 (−34.03, 17.63) | −2.29 (−8.59, 4.01) | −1.42 (−9.71, 6.88) | Probiotics | −18.60 (−63.70, 26.50) | - | −3.47 (−31.78, 24.84) |
| −9.90 (−31.92, 12.13) | −3.99 (−18.89, 10.91) | −3.12 (−18.96, 12.72) | −1.70 (−15.20, 11.80) | Allogenic FMT | - | −15.13 (−68.37, 38.12) |
| −12.98 (−39.97, 14.01) | −7.07 (−17.10, 2.96) | −6.20 (−17.59, 5.19) | −4.78 (−12.60, 3.04) | −3.08 (−18.68, 12.52) | UC | - |
| −13.29 (−39.54, 12.97) | −7.38 (−11.94, −2.82) | −6.51 (−14.79, 1.78) | −5.09 (−9.79, −0.39) | −3.39 (−17.68, 10.91) | −0.31 (−9.43, 8.81) | Placebo |
The league table of ALT and CAP.
Comparisons for ALT (bottom left) and CAP (upper right) of MTTs. Data of comparison for ALT and CAP, are MD(95% CI). The 95% CI confidence interval which does not range across 0 favors the column-defining treatment and is shown in bold.
Figure 4
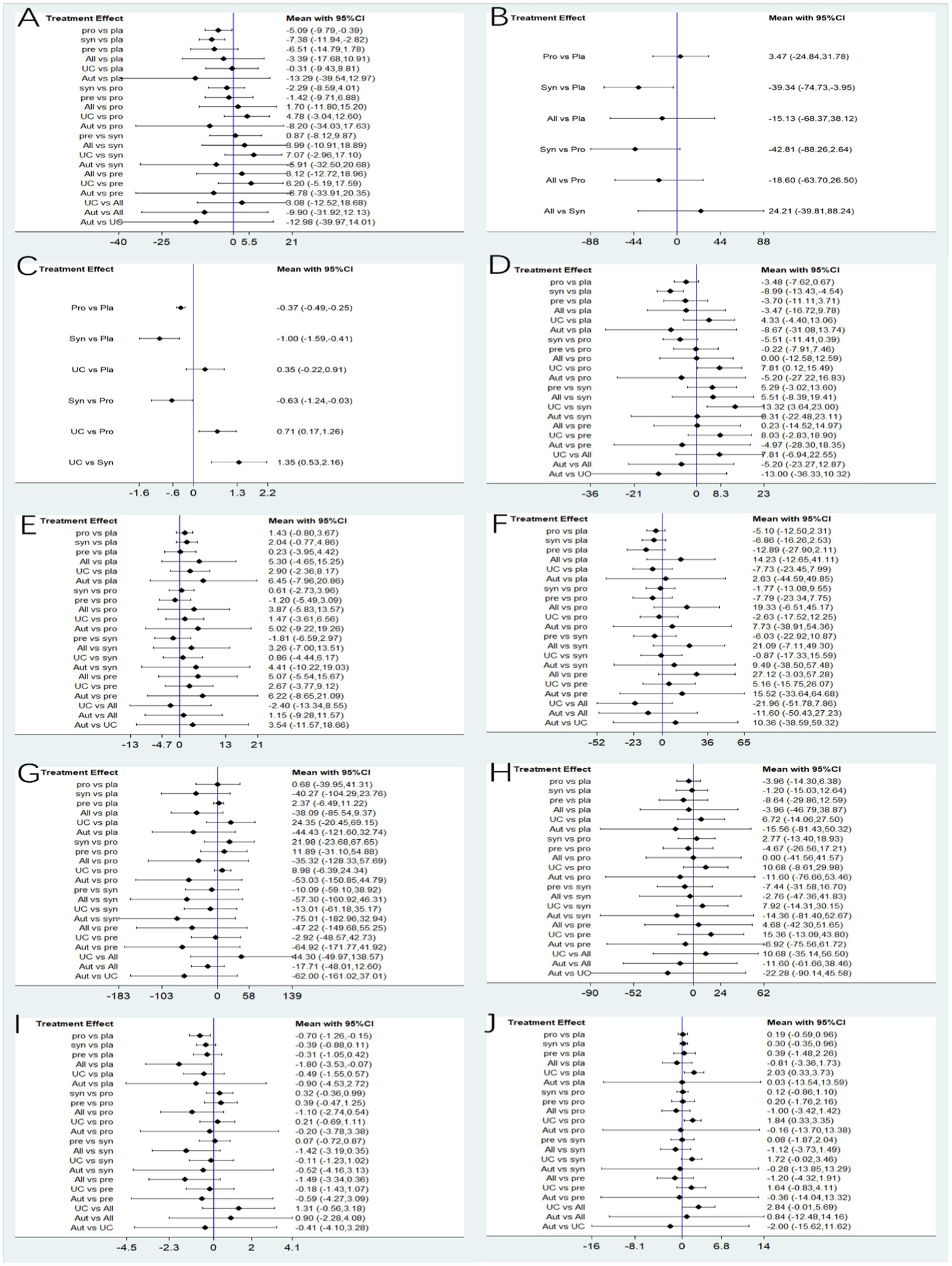
Forest plots. (A) ALT (B) CAP (C) LSM (D) AST (E) HDL-C (F) LDL-C (G) TG (H) TC (I) HOMA-IR (J) BMI.
Figure 5
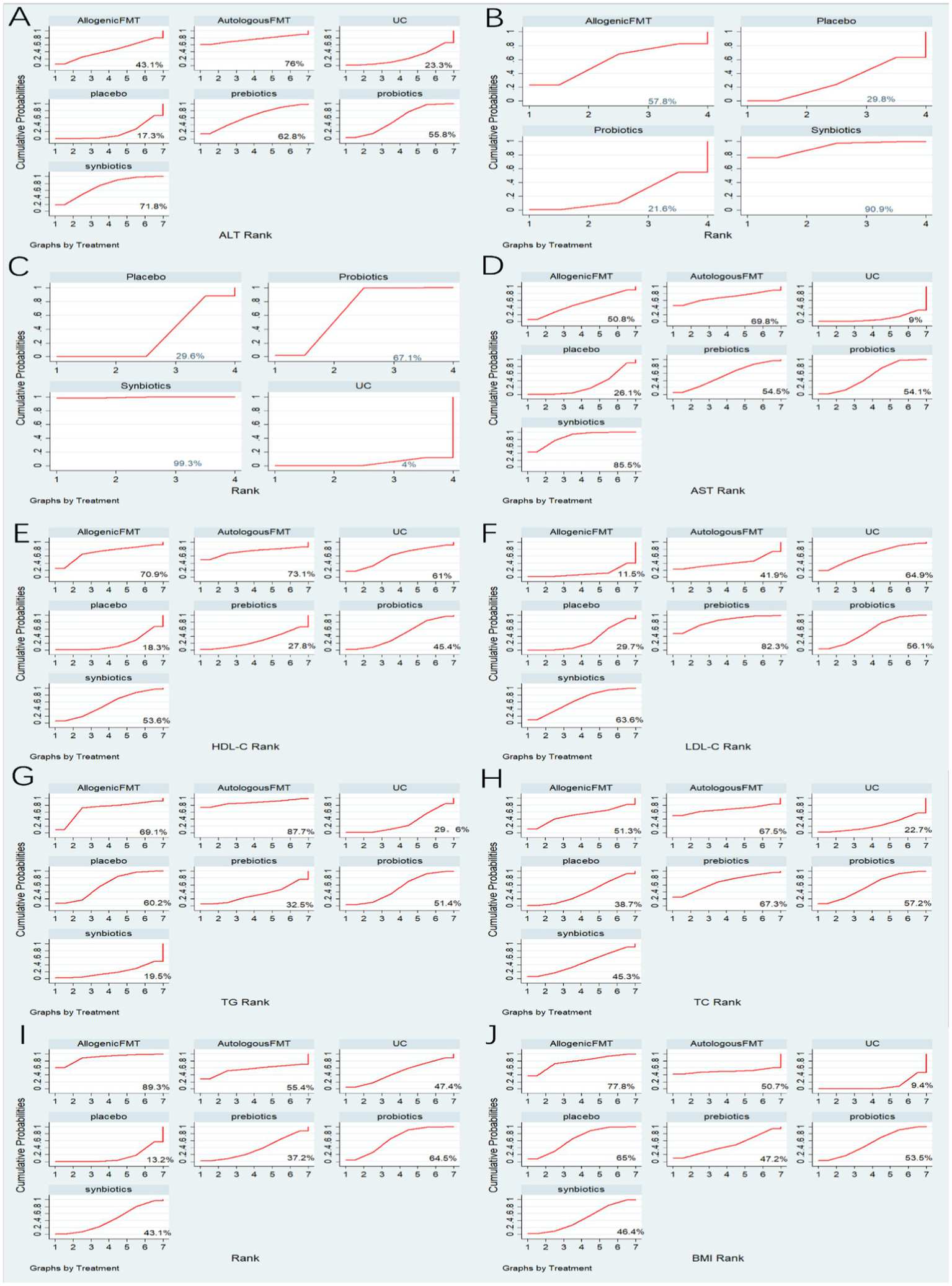
Summary of results from SUCRA. (A) ALT (B) CAP (C) LSM (D) AST (E) HDL-C (F) LDL-C (G) TG (H) TC (I) HOMA-IR (J) BMI.
7 studies reported CAP, involving probiotics, synbiotics, Allogenic FMT, of which the network relationship between the interventions is shown in Figure 3. In terms of CAP improvement, according to MD and 95%CI between all pairwise interventions, synbiotics (MD: −39.34; 95%CI: −74.73, −3.95) was superior to placebo. As shown in Table 2 and Figure 4. In addition, synbiotics (90.9%), had the best effectiveness in reducing CAP, followed by Alllogenic FMT (57.8%). More details about the rank probability of SUCRA are shown in Figure 5.
10 studies reported LSM, involving probiotics, synbiotics, of which the network relationship between the interventions is shown in Figure 3. In terms of LSM improvement, according to MD and 95%CI between all pairwise interventions, synbiotics (MD: −1.00; 95%CI: −1.59, −0.41), probiotics (MD: −0.37; 95%CI: −0.49, −0.25) was superior to placebo. Moreover, synbiotics (MD: −1.35; 95%CI: −12.16, −0.53), probiotics (MD: −0.71; 95%CI: −1.26, −0.17) was superior to UC. As shown in Table 3 and Figure 4. In addition, synbiotics (MD: −0.63; 95%CI: −1.24, −0.03) was better than probiotics. According to SUCRA, synbiotics (99.3%), had the best effectiveness in reducing LSM, followed by probiotics (67.1%). More details about the rank probability of SUCRA are shown in Figure 5.
Table 3
| Synbiotics | - | - | −0.63 (−1.24, −0.03) | −1.00 (−1.59, −0.41) | −1.35 (−2.16, −0.53) | |
| −0.31 (−23.11, 22.48) | Autologous FMT | - | - | - | - | - |
| −5.29 (−13.60, 3.02) | −4.97 (−28.30, 18.35) | Prebiotics | - | - | - | - |
| −5.51 (−11.41, 0.39) | −5.20 (−27.22, 16.83) | −0.22 (−7.91, 7.46) | Probiotics | - | −0.37 (−0.49, −0.25) | −0.71 (−1.26, −0.17) |
| −5.51 (−19.41, 8.39) | −5.20 (−23.27, 12.87) | −0.23 (−14.97, 14.52) | −0.00 (−12.59, 12.58) | Allogenic FMT | - | - |
| −8.99 (−13.43, −4.54) | −8.67 (−31.08, 13.74) | −3.70 (−11.11, 3.71) | −3.48 (−7.62, 0.67) | −3.47 (−16.72, 9.78) | Placebo | −0.35 (−0.91, 0.22) |
| −13.32 (−23.00, −3.64) | −13.00 (−36.33, 10.32) | −8.03 (−18.90, 2.83) | −7.81 (−15.49, −0.12) | −7.81 (−22.55, 6.94) | −4.33 (−13.06, 4.40) | UC |
The league table of AST and LSM.
Comparison for AST (bottom left) and LSM (upper right) of MTTs. Data of comparison for AST and LSM, are MD (95% CI). The 95%CI confidence interval which does not range across 0 favors the column-defining treatment and is shown in bold.
7.4.2 Secondary outcome
27 studies reported AST, involving probiotics, synbiotics, probiotics, Allogenic FMT, Autologous FMT. The network relationship between the interventions is shown in Figure 3. In terms of AST improvement, synbiotics (MD: −8.99; 95%CI: −13.43, −4.54) was superior to placebo. Moreover, synbiotics (MD: −13.32; 95%CI: −23, −3.64), and probiotics (MD: −7.81; 95%CI: −15.49, −0.12) were better than UC as shown in Table 3 and Figure 4. According to SUCRA, synbiotics (85.5%), had the best effectiveness in reducing AST, followed by Autologous FMT (69.8%), prebiotics (54.5%). More details about the rank probability of SUCRA are shown in Figure 5.
21 studies reported HDL-C, involving probiotics, synbiotics, probiotics, Allogenic FMT, Autologous FMT. The network relationship between the interventions is shown in Figure 3. In terms of HDL-C improvement, there was no significant difference between all interventions and placebo, and there was no difference between 5 interventions as shown in Table 4 and Figure 4. However, Autologous FMT (73.1%), Allogenic FMT (70.9%) with highest-ranking probability of SUCRA, had the best effectiveness in reducing HDL-C. More details about the rank probability of SUCRA are shown in Figure 5.
Table 4
| Allogenic FMT | −14.23 (−41.11, 12.65) | −19.33 (−45.17, 6.51) | −11.60 (−50.43, 27.23) | −8.71 (−57.91, 40.48) | −21.09 (−49.30, 7.11) | −21.96 (−51.78, 7.86) |
| 5.30 (−4.65, 15.25) | Placebo | −5.10 (−12.50, 2.31) | 2.63 (−44.59, 49.85) | −20.31 (−49.69, 9.06) | −6.86 (−16.26, 2.53) | −7.73 (−23.45, 7.99) |
| 3.87 (−5.83, 13.57) | −1.43 (−3.67, 0.80) | Probiotics | −7.73 (−54.36, 38.91) | −1.45 (−18.68, 15.79) | −1.77 (−13.08, 9.55) | −2.63 (−17.52, 12.25) |
| −1.15 (−11.57, 9.28) | −6.45 (−20.86, 7.96) | −5.02 (−19.26, 9.22) | AutologousFMT | −0.99 (−13.04, 11.06) | −9.49 (−57.48, 38.50) | −10.36 (−59.32, 38.59) |
| 5.07 (−5.54, 15.67) | −0.23 (−4.42, 3.95) | 1.20 (−3.09, 5.49) | 6.22 (−8.65, 21.09) | Prebiotics | −4.69 (−23.10, 13.72) | −6.72 (−16.53, 3.09) |
| 3.26 (−7.00, 13.51) | −2.04 (−4.86, 0.77) | −0.61 (−3.96, 2.73) | 4.41 (−10.22, 19.03) | −1.81 (−6.59, 2.97) | Synbiotics | −0.87 (−17.33, 15.59) |
| 2.40 (−8.55, 13.34) | −2.90 (−8.17, 2.36) | −1.47 (−6.56, 3.61) | 3.54 (−11.57, 18.66) | −2.67 (−9.12, 3.77) | −0.86 (−6.17, 4.44) | UC |
The league table of HDL and LDL.
Comparisons for HDL (bottom left) and LDL (upper right) of MTTs.
21 studies reported LDL-C, involving probiotics, synbiotics, probiotics, Allogenic FMT, Autologous FMT. The network relationship between the interventions is shown in Figure 3. In terms of LDL-C improvement, there was no significant difference between all interventions and placebo, and there was no difference between 5 interventions as shown in Table 4 and Figure 4. However, prebiotics (82.3%) with highest-ranking probability of SUCRA, had the best effectiveness in reducing LDL-C. More details about the rank probability of SUCRA are shown in Figure 5.
27 studies reported TG, involving probiotics, synbiotics, probiotics, Allogenic FMT, Autologous FMT. The network relationship between the interventions is shown in Figure 3. There was no significant difference between all interventions and placebo, and there was no difference between 5 interventions as shown in Table 5 and Figure 4. However, Autologous FMT (87.7%) with highest-ranking probability of SUCRA, had the best effectiveness in reducing TG, followed by Allogenic FMT (69.1%). More details about the rank probability of SUCRA are shown in Figure 5.
Table 5
| Autologous FMT | −11.60 (−61.66, 38.46) | −15.56 (−81.43, 50.32) | −11.60 (−76.66, 53.46) | −6.92 (−75.56, 61.72) | −22.28 (−90.14, 45.58) | −14.36 (−81.40, 52.67) |
| −17.71 (−48.01, 12.60) | Allogenic FMT | −3.96 (−46.79, 38.87) | −0.00 (−41.57, 41.56) | −4.68 (−51.65, 42.30) | −10.68 (−56.50, 35.14) | −2.76 (−47.36, 41.83) |
| −44.43 (−121.60, 32.74) | −38.09 (−85.54, 9.37) | Placebo | −3.96 (−14.30, 6.38) | −8.64 (−29.86, 12.59) | −6.72 (−27.50, 14.06) | −1.20 (−15.03, 12.64) |
| −53.03 (−150.85, 44.79) | −35.32 (−128.33, 57.69) | −0.68 (−41.31, 39.95) | Probiotics | −4.67 (−26.56, 17.21) | −10.68 (−29.98, 8.61) | −2.77 (−18.93, 13.40) |
| −64.92 (−171.77, 41.92) | −47.22 (−149.68, 55.25) | −2.37 (−11.22, 6.49) | −11.89 (−54.88, 31.10) | Prebiotics | −15.36 (−43.80, 13.09) | −7.44 (−31.58, 16.70) |
| −62.00 (−161.02, 37.01) | −44.30 (−138.57, 49.97) | −24.35 (−69.15, 20.45) | −8.98 (−24.34, 6.39) | 2.92 (−42.73, 48.57) | UC | −7.92 (−30.15, 14.31) |
| −75.01 (−182.96, 32.94) | −57.30 (−160.92, 46.31) | 40.27 (−23.76, 104.29) | −21.98 (−67.65, 23.68) | −10.09 (−59.10, 38.92) | −13.01 (−61.18, 35.17) | Synbiotics |
The league table of TG and TC.
Comparisons for TG (bottom left) and TC (upper right) of MTTs.
24 studies reported TC, involving probiotics, synbiotics, prebiotics, FMT. The network relationship between the interventions is shown in Figure 3. There was no significant difference between all interventions and placebo, and there was no difference between 5 interventions as shown in Table 5 and Figure 4. However, Autologous FMT (67.5%) with highest-ranking probability of SUCRA, had the best effectiveness in reducing TC, followed by prebiotics (67.3%) and probiotics (57.2%) More details about the rank probability of SUCRA are shown in Figure 5.
14 studies reported HOMA-IR, involving probiotics, synbiotics, probiotics, Allogenic FMT, Autologous FMT. The network relationship between the interventions is shown in Figure 3. Probiotics (MD: −0.7, 95%CI: −1.26, −0.15) and Allogenic FMT (MD: −1.8, 95%CI: −3.53, − 0.07) was better than placebo. Moreover, Allogenic FMT (89.3%) with highest-ranking probability of SUCRA, had the best effectiveness in reducing HOMA-IR, followed by probiotics (64.5%) and Autologous FMT (55.4%). More details about the rank probability of SUCRA are shown in Figure 5.
22 studies reported BMI, involving probiotics, synbiotics, probiotics, Allogenic FMT, Autologous FMT. The network relationship between the interventions is shown in Figure 3. Probiotics (MD: −1.84, 95%CI: −3.35, −0.33) was better than UC as shown in Table 6 and Figure 4. However, Allogenic FMT (77.8%) with highest-ranking probability of SUCRA, had the best effectiveness in reducing BMI, followed by placebo (65%). More details about the rank probability of SUCRA are shown in Figure 5.
Table 6
| Allogenic FMT | −1.00 (−3.42, 1.42) | −0.84 (−14.16, 12.48) | −2.84 (−5.69, 0.01) | −1.12 (−3.73, 1.49) | −1.20 (−4.32, 1.91) | −0.81 (−3.36, 1.73) |
| −1.10 (−2.74, 0.54) | Probiotics | 0.16 (−13.38, 13.70) | −1.84 (−3.35, −0.33) | −0.12 (−1.10, 0.86) | −0.20 (−2.16, 1.76) | −0.19 (−0.96, 0.59) |
| −0.90 (−4.08, 2.28) | 0.20 (−3.38, 3.78) | Autologous FMT | −2.00 (−15.62, 11.62) | −0.28 (−13.85, 13.29) | −0.36 (−14.04, 13.32) | −0.03 (−13.59, 13.54) |
| −1.31 (−3.18, 0.56) | −0.21 (−1.11, 0.69) | −0.41 (−4.10, 3.28) | UC | −1.72 (−3.46, 0.02) | −1.64 (−4.11, 0.83) | −2.03 (−3.73, −0.33) |
| −1.42 (−3.19, 0.35) | −0.32 (−0.99, 0.36) | −0.52 (−4.16, 3.13) | −0.11 (−1.23, 1.02) | Synbiotics | 0.08 (−1.87, 2.04) | −0.30 (−0.96, 0.35) |
| −1.49 (−3.34, 0.36) | −0.39 (−1.25, 0.47) | −0.59 (−4.27, 3.09) | −0.18 (−1.43, 1.07) | −0.07 (−0.87, 0.72) | Prebiotics | −0.39 (−2.26, 1.48) |
| −1.80 (−3.53, −0.07) | −0.70 (−1.26, −0.15) | −0.90 (−4.53, 2.72) | −0.49 (−1.55, 0.57) | −0.39 (−0.88, 0.11) | −0.31 (−1.05, 0.42) | Placebo |
The league table of HOMA-IR and BMI.
Comparisons for HOMA-IR (bottom left) and BMI (upper right) of MTTs. Data of comparison for HOMA-IR and BMI, are MD (95% CI). The 95% CI confidence interval which does not range across 0 favors the column-defining treatment and is shown in bold.
7.5 Adverse reaction
6 studies reported specific adverse reactions. Of these, two studies focused on the adverse effects of probiotics, mainly including symptoms of the digestive system such as diarrhea, flatulence, nausea, and other symptoms like mild headache, urinary tract infection, and adperianalrash. There was no difference in the incidence of adverse reactions in the digestive system between the probiotics group and placebo group (p = 0.857); two studies focused on the adverse effects of synbiotics, including moderate headaches, nausea, abdominal pain. There was no difference in the incidence of adverse reactions in the digestive system between the synbiotics group and placebo group (p = 0.62); one study reported the occurrence of flatulence in both the prebiotics group and placebo group. One study reported the adverse reactions of antibiotics, which were confined to the gastrointestinal adverse events, specifically comprising abdominal pain, nausea, vomiting, constipation and diarrhea. No significant difference was observed in the incidence rates between the intervention group and the control group. More details about adverse reactions are shown in Table 7 and Supplementary Table S2.
Table 7
| Study ID | Intervention of experiment group | Intervention of control group | Adverse reactions in experiment group | Adverse reactions in control group |
|---|---|---|---|---|
| Abhari et al. (66) | Synbiotics | Placebo | N | N |
| Ayob et al. (38) | Probiotics | Placebo | N | N |
| Ahn et al. (65) | Probiotics | Placebo | N | N |
| Alisi et al. (64) | VSL3# | Placebo | N | N |
| Aller et al. (63) | Probiotics | Placebo | N | N |
| Asgharian et al. (62) | Synbiotics | Placebo | N | N |
| Behrouz et al. (61) | Prebiotics/probrotic | Placebo | N | N |
| Bomhof et al. (60) | Oligofructose | Placebo | Flatulence (n = 1) | Flatulence (n = 1) |
| Cai et al. (59) | Probiotics | Usual Care | N | N |
| Chong et al. (58) | VSL#3 | Placebo | Urinary tract infection (n = 3); Bloating (n = 2); Nausea (n = 2); Genital thrush (n = 1); Adperianalrash (n = 1). |
Diarrhea (n = 1), Abdominal Cramps (n = 1); Back pain (n = 1); Traumatic toe infection (n = 1) |
| Craven et al. (57) | Allogenic FMT | Autologous FMT | N | N |
| Derosa et al. (72) | VSL#3 | Placebo | N | N |
| Duseja et al. (56) | Probiotics | Placebo | N | N |
| Ekalasi et al. (70) | Synbiotics | Placebo | N | N |
| Eslamparast et al. (55) | Synbiotics | Placebo | Moderate headaches (n = 1) | Abdominal pain (n = 1) |
| Famouri et al. (54) | Probiotics | Placebo | N | N |
| Ferolla et al. (53) | Synbiotics | Placebo | N | N |
| Goyal et al. (52) | VSL#3 | Placebo | N | N |
| Javadi et al. (20) | Probiotics/prebiotics/synbiotics | Placebo | N | N |
| Kobyliak et al. (69) | Probiotics | Placebo | Short-term Diarrhea (n = 1); Mild headaches (n = 1) |
Mild abdominal pain (n = 2); Nausea (n = 1). |
| Malaguarnera et al. (51) | Synbiotics | Placebo | Nausea (n = 1); Moderate headache (n = 1); Abdominal pain (n = 1). |
Nausea (n = 2); Fatigue (n = 1); Dizziness (n = 1) |
| Manzhalii et al. (50) | Probiotics | Placebo | N | N |
| Mofidi et al. (49) | Synbiotics | Placebo | N | N |
| Nor et al. (48) | Probiotics | Placebo | N | N |
| Rodrigo et al. (47) | Probiotics | Placebo | N | N |
| Sadrkabir et al. (68) | Synbiotics | Placebo | N | N |
| Sayari et al. (67) | Sitagliptin-synbiotics | Sitagliptin-placebo | N | N |
| Scorletti et al. (46) | Synbiotics | Placebo | N | N |
| Sepiden et al. (45) | Probiotics | Placebo | N | N |
| Vajro et al. (19) | Probiotics | Placebo | N | N |
| Witjes et al. (44) | Allogenic FMT | Autologous FMT | N | N |
| Wong et al. (43) | Probiotics and prebiotics | Usual Care | N | N |
| Xue et al. (42) | Allogenic FMT | Probiotics | N | N |
| Abdel-Razik et al. (71) | Rifaximin | Placebo | Abdominal pain (n = 1) Nausea (n = 2) Vomiting (n = 1) Constipation (n = 1) Diarrhea (n = 2) |
Abdominal pain (n = 2) Nausea (n = 2) Vomiting (n = 1) Constipation (n = 1) Diarrhea (n = 1) |
| Giselle et al. (73) | Probiotics | Placebo | N | N |
| Reshef et al. (74) | Probiotics | UC | N | N |
| Naama et al. (75) | Prebiotics | Placebo | N | N |
Adverse reaction.
7.6 Network inconsistency and publication bias
The network evidence diagrams of ALT, AST, TG, TC, HDL-C, LDL-C, BMI and HOMA-IR formed a closed loop, respectively. Network inconsistency was used to evaluate the inconsistency. The results of global-inconsistency assessment are shown in Supplementary Table S2. There were no evidences of inconsistency in the indicators ALT, AST, TG, TC, LDL-C, HDL-C and BMI. However, the p-value for test of global inconsistency of HOMA-IR is significant (p = 0.0006). The node-splitting method was performed to evaluate the inconsistency. The results of inconsistency showed that there was inconsistency in the comparison between prebiotics and placebo, as well as those between probiotics and prebiotics. Subsequently, leave-one-out method was used to remove the study (20) it was demonstrated that the inconsistency was not significant. It may be related to the differences in the measurement method and data processing of HOMA-IR compared to those in other studies. Comparison adjusted funnel plot for the ten outcomes is shown in Figure 6. The comparison adjusted funnel chart for the LSM outcome indicates poor symmetry, suggesting potential publication bias. The reason may be related to the small number of included studies and small sample size. In contrast, the comparison adjusted funnel chart for other nine outcome indicators ALT, CAP, AST, HDL-C, LDL-C, TG, TC, HOMA-IR and BMI showed symmetric distribution in the upper middle part and clustering towards the middle line.
Figure 6
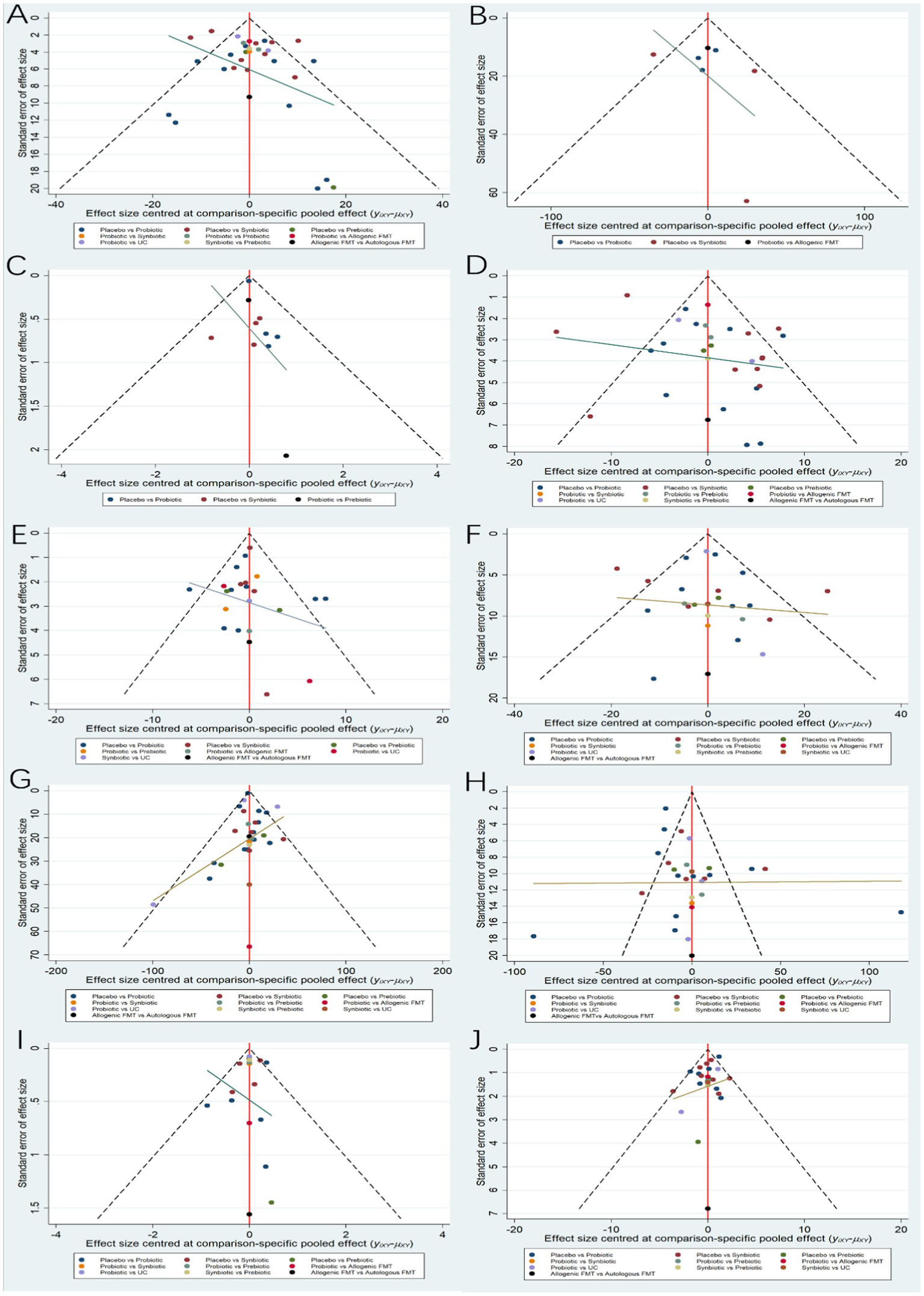
Funnel plots. (A) ALT (B) CAP (C) LSM (D) AST (E) HDL-C (F) LDL-C (G) TG (H) TC (I) HOMA-IR (J) BMI.
8 Discussion
With the changes in human lifestyle and the rapid development of society, the incidence of metabolic syndrome, including obesity and diabetes, is increasing annually. NAFLD, as a hepatic manifestation of metabolic syndrome, has become one of the leading causes of chronic liver disease (21). Furthermore, NAFLD is also a risk factor for cardiovascular disease, and atherosclerotic cardiovascular disease is also a leading cause of death in NAFLD patients (22, 23). Despite the dangers of NAFLD, few drugs are used to treat it. Healthy lifestyle and weight loss remain effective interventions to prevent and improve NAFLD. Evidence indicates that a 10% weight loss can reduce liver damage-levels and improve liver steatosis and fibrosis in NASH (24). Recently, studies have shown the potential mechanism of intestinal microbiota in NAFLD and the benefits of modulating the intestinal microbiome for NAFLD, which indicates the promising effect of MTTs on NAFLD/NASH treatment (25, 26).
This system review summarized the data from 37 randomized controlled trials, comparing synbiotics, probiotics, prebiotics, fecal transplantation, and antibiotics on liver enzymes (ALT, AST), glycolipid metabolism (TG, TC, LDL-C, HDL-C, HOMA-IR), and non-invasive steatosis and fibrosis in the liver (CAP, LSM) in 1921 patients with NAFLD. One study reported the effects of antibiotics on NAFLD, and network meta-analysis was not feasible, so the aim was to evaluate the quality and limitations of this study. The funnel plots showed that data points distributed relatively symmetrical and concentrated in the upper-middle area, indicating the reliablity of the data.
Our study found that probiotics and synbiotics were significantly superior to placebo in reducing liver enzyme markers AST and ALT, consistent with previous findings (27, 28). However, some studies have found that synbiotics cannot reduce ALT, and this may be related to the quantity of included articles (29). In addition, we found that synbiotics were also superior to conventional treatment in reducing AST, where conventional treatment includes diet management, exercise, weight loss, etc. Of the various interventions, there was no particular intervention that was superior to the others (30).
Our study found that probiotics and synbiotics were beneficial in improving liver steatosis and fibrosis. We evaluated the CAP score and liver stiffness measurement, which provide a quantitative, non-invasive evaluation of NAFLD by measuring hepatic steatosis and fibrosis. We found that probiotics and synbiotics could significantly reduce LSM. This may be related to the improvement of inflammatory response. Inflammatory factors such as IL-1β and TNF-α activate hepatic stellate cells in the liver to induce their differentiation into fibroblast-like cells, resulting in excessive deposition of a large amount of extracellular matrix in the liver, increasing liver stiffness. Previous studies have shown that probiotics can treat inflammatory diseases by regulating the release of intestinal inflammatory factors and increasing the secretion of anti-inflammatory factor IL-10 (31, 32). Furthermore, synbiotic supplementation has shown a better effect in reducing LSM compared to probiotics. Nevertheless, we cannot draw safely conclude that probiotics and synbiotics supplementation can improve fibrosis, considering the association between the reduced ALT levels and LSM (33). Synbiotics supplementation has shown superior function in reducing the CAP indicator, consistent with other meta-analyses (30). Synbiotics is the combination of probiotics and prebiotics in a formulation and, as such, has the advantage of producing increased levels of butyrate, which can upregulate GLP-1R expression to decrease hepatic steatosis (34). In addition, Alves et al. found that synbiotics can alter the expression of genes related to β-oxidation and lipogenesis (16).
Our study also found that probiotics was capable of reducing BMI, consistent with other studies (35). A study has shown that BMI reduction is dependent on NAFLD improvement, indicating that probiotics is a promising treatment for weight loss (36). HOMA-IR is a widely used model method for evaluating insulin resistance. Insulin resistance leads to increased insulin and blood glucose levels, reducing glucose uptake and increasing peripheral tissue decomposition. In this process, fatty acid accumulation in the liver and glucose metabolism disorders develop, contributing to NAFLD (37). We found that probiotics and Allogenic FMT could improve insulin resistance. We included 14 studies to evaluate the effect of MTTs on improving HOMA-IR. The inconsistency test (p < 0.05) indicated contradictions and irrationalities among different treatment measures. Subsequently, we employed the node-splitting method to explore the sources of inconsistency and found local inconsistencies in the comparisons between placebo and prebiotics, as well as between probiotics and prebiotics. Finally, using the stepwise exclusion method, we excluded the article by Javadi, after which the inconsistency test indicated no significant difference (p > 0.05). Javadi et al. (20) conducted a 12-week placebo-controlled trial comparing the efficacy of prebiotics, probiotics, and synbiotics in treating NAFLD. The measurement formula for HOMA-IR in this study differed from those in other studies, possibly due to the authors’ team using a formula adjusted for a specific human population. Additionally, the limited number of studies incorporating Allogenic FMT might affect the credibility of our conclusion regarding the improvement of HOMA-IR by MTTs.
Compared with other meta-analyses on MTTs, our study evaluated the effect of FMT and antibiotics on NAFLD. Interestingly, we did not find a positive effect of FMT on NAFLD, although there are a few studies indicating that FMT could alleviate high-fat-induced steatohepatitis and improve insulin sensitivity, which were correlated with an increase in the tight junction of small intestinal and butyrate-producing bacteria (12, 13). It may be related to the limitation of quantity of articles, and more RCT studies and long-term follow-ups are needed to verify their efficacy. Only one study evaluated the efficacy of 6-month rifaximin therapy in NAFLD patients. Rifaximin is an oral, non-absorbable antibiotics that reduces endotoxin absorption and intestinal bacterial overgrowth. Abdel-Razik et al. (38), conducted a randomized, double-blind experiment over a 6-month period to observe the effects of rifaximin on NASH. The group receiving the rifaximin for 6 months showed significant improvement (p < 0.05) in markers such as ALT, AST, and HOMA-IR compared to the placebo group. However, there were no changes in BMI, cholesterol, or TG. The number of cumulative adverse events between the placebo and rifaximin participants showed no significant difference. Some limitations of this study include the small sample size and the lack of a second liver biopsy to access the liver histopathology changes. Finally, we also focused on the adverse events of antibiotics, which included diarrhea, abdominal pain, nausea, and these may limit their widespread clinical application.
There were a few limitations to our study. First, the length of treatment varied. Most treatment cycles were 12 weeks and 24 weeks, and a few studies had treatment cycles of 8 weeks, which might cause clinical heterogeneity. Regarding the form of probiotics, synbiotics and prebiotics, only four studies explicitly stated that VSL#3 was used as an interventinal strategy, whereas others studies did not clearly specify the types, which may cause bias. Due to the limited number of included studies, we did not conduct a dose subgroup analysis, which may affect the accuracy of the results. Additionally, there were few studies on FMT and antibiotics for the NAFLD treatment, potentially influencing conclusions about their efficacy. Second, included studies were all small sample, reducing the satistical reliability. Third, there was a lack of long-term follow-up data, potentially impacting conclusion. The number of RCTs for FMT (3 RCTs) and antibiotics (1 RCT) was limited, and the results of the NMA merger may not be convincing enough. Given these limitations, it is recommended that future studies should note the following three points:1. It is suggested to carry out multi, large sample studies to clarify the exact effcacy of MTTs in the treatment of NAFLD. 2. It is recommended to conduct long-term follow-up RCT studies to obtain reliable data. 3. It is necessary to clarify the intervention measures to enhance the accuracy of the research conclusions.
In conclusion, we found that synbiotics and probiotics may significantly improve liver function, reduce enzyme levels, and ameliorate hepatic steatosis and fibrosis in patients with NAFLD. Thought-provokingly, sarcopenia, a condition shared by various diseases, was associated with higher risk of developing severe NAFLD (39). Growing evidence highlights the importance of the microbiota in the gut-brain-muscle axis, which is characterized by the involvement of gut flora that regulates skeletal muscle energy and muscle fiber conversion through its metabolites (40). A recent study showed that administration of prebiotics significantly improved muscle function, suggesting prospects for analyzing the MTTs efficacy on NAFLD with sarcopenia (41). And we found synbiotics provided the best effect on LSM reduction. However, no specific evidence was obtained from our study that antibiotics could improve patients with NAFLD due to the limited number of RCT. Finally, common adverse events such as diarrhea, abdominal pain, nausea, etc. should be noted, as they may limit the widespread application of MTTs.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
YS: Writing – original draft, Data curation, Formal analysis, Visualization, Writing – review & editing. SL: Writing – original draft, Conceptualization, Writing – review & editing. LZ: Writing – original draft, Supervision, Writing – review & editing. WZ: Writing – review & editing, Writing – original draft. YQ: Writing – review & editing, Supervision, Writing – original draft. ML: Writing – review & editing, Conceptualization, Funding acquisition, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was sponsored by National Natural Science Foundation of China (nos. 82205086 and 81904154), Young Elite Scientists Sponsorship Program by China Association for Science and Technology (nos. 2023QNRC001), Health Commission of Henan Province (nos. 2022JDZX114 and 2023ZXZX1162) and Henan Province Science and Technology Research Projects (nos. 232102310438 and 242102310500).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1470185/full#supplementary-material
Footnotes
References
1.
Mofidi F Poustchi H Yari Z Nourinayyer B Merat S Sharafkhah M et al . Synbiotic supplementation in lean patients with non-alcoholic fatty liver disease: a pilot, randomised, double-blind, placebo-controlled, clinical trial. British J Nut. (2017) 117:662–8. doi: 10.1017/S0007114517000204
2.
European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO . EASL–EASD–EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. (2016) 64:1388–402. doi: 10.1016/j.jhep.2015.11.004
3.
Wong VW Chan WK Chitturi S Chawla Y Dan Y Duseja A et al . Asia-Pacific working party on non-alcoholic fatty liver disease guidelines 2017—part 1: definition, risk factors and assessment. J Gastroenterol Hepatol. (2018) 33:70–85. doi: 10.1111/jgh.13857
4.
Long MT Noureddin M Lim JK . AGA clinical practice update: diagnosis and Management of Nonalcoholic Fatty Liver Disease in lean individuals: expert review. Gastroenterology. (2022) 163:764–774.e1. doi: 10.1053/j.gastro.2022.06.023
5.
Golabi P Sayiner M Fazel Y Koenig A Henry L Younossi ZM et al . Current complications and challenges in nonalcoholic steatohepatitis screening and diagnosis. Expert Rev Gastroenterol Hepatol. (2016) 10:63–71. doi: 10.1586/17474124.2016.1099433
6.
Lang S Schnabl B . Microbiota and fatty liver disease—the known, the unknown, and the future. Cell Host Microbe. (2020) 28:233–44. doi: 10.1016/j.chom.2020.07.007
7.
Albillos A de Gottardi A Rescigno M . The gut-liver axis in liver disease: pathophysiological basis for therapy. J Hepatol. (2020) 72:558–77. doi: 10.1016/j.jhep.2019.10.003
8.
Li D Li Y Yang S Lu J Jin X Wu M . Diet-gut microbiota-epigenetics in metabolic diseases: from mechanisms to therapeutics. Biomed Exp. (2022) 153:113290:113290. doi: 10.1016/j.biopha.2022.113290
9.
Kim DH Jeong D Kang IB Kim H Song KY Seo KH . Dual function ofLactobacillus kefiriDH5 in preventing high‐fat‐diet‐induced obesity: direct reduction of cholesterol and upregulation of PPAR‐α in adipose tissue. Mol Nutr Food Res. (2017) 61:252. doi: 10.1002/mnfr.201700252
10.
Wang W Xu AL Li ZC Li Y Xu SF Sang HC et al . Combination of probiotics and Salvia miltiorrhiza polysaccharide alleviates hepatic steatosis via gut microbiota modulation and insulin resistance improvement in high fat-induced NAFLD mice. Diabetes Metab J. (2020) 44:336–48. doi: 10.4093/dmj.2019.0042
11.
Lei Y Tang L Chen Q Wu L He W Tu D et al . Disulfiram ameliorates nonalcoholic steatohepatitis by modulating the gut microbiota and bile acid metabolism. Nat Commun. (2022) 13:6862. doi: 10.1038/s41467-022-34671-1
12.
Zhou D Pan Q Shen F Cao HX Ding WJ Chen YW et al . Total fecal microbiota transplantation alleviates high-fat diet-induced steatohepatitis in mice via beneficial regulation of gut microbiota. Sci Rep. (2017) 7:1529. doi: 10.1038/s41598-017-01751-y
13.
Vrieze A Van Nood E Holleman F Salojärvi J Kootte RS Bartelsman JF et al . Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. (2012) 143:913–916.e7. doi: 10.1053/j.gastro.2012.06.031
14.
Song Q Zhang X Liu W Wei H Liang W Zhou Y et al . Bifidobacterium pseudolongum-generated acetate suppresses non-alcoholic fatty liver disease-associated hepatocellular carcinoma. J Hepatol. (2023) 79:1352–65. doi: 10.1016/j.jhep.2023.07.005
15.
Beisner J Filipe Rosa L Kaden-Volynets V Stolzer I Günther C Bischoff SC . Prebiotic inulin and sodium butyrate attenuate obesity-induced intestinal barrier dysfunction by induction of antimicrobial peptides. Front Immunol. (2021) 12:678360. doi: 10.3389/fimmu.2021.678360
16.
Alves CC Waitzberg DL de L dos L Reis MB Guanabara CC et al . Prebiotic and synbiotic modifications of beta oxidation and lipogenic gene expression after experimental hypercholesterolemia in rat liver. Front Microbiol. (2017) 8:2010. doi: 10.3389/fmicb.2017.02010
17.
Sharpton SR Maraj B Harding-Theobald E Vittinghoff E Terrault NA . Gut microbiome-targeted therapies in nonalcoholic fatty liver disease: a systematic review, meta-analysis, and meta-regression. Am J Clin Nutr. (2019) 110:139–49. doi: 10.1093/ajcn/nqz042
18.
Carpi RZ Barbalho SM Sloan KP Laurindo LF Gonzaga HF Grippa PC et al . The effects of probiotics, prebiotics and synbiotics in non-alcoholic fat liver disease (NAFLD) and non-alcoholic Steatohepatitis (NASH): a systematic review. Int J Mol Sci. (2022) 23:8805. doi: 10.3390/ijms23158805
19.
Vajro P Mandato C Licenziati MR Franzese A Vitale DF Lenta S et al . Effects of Lactobacillus rhamnosus strain GG in Pediatric obesity-related liver disease. J Pediatr Gastroenterol Nutr. (2011) 52:740–3. doi: 10.1097/MPG.0b013e31821f9b85
20.
Javadi L Ghavami M Khoshbaten M Safaiyan A Barzegari A Pourghassem B . The effect of probiotic and/or prebiotic on liver function tests in patients with nonalcoholic fatty liver disease: a double blind randomized clinical trial. Iran Red Crescent Med J. (2017) 19:19. doi: 10.5812/ircmj.46017
21.
Guo X Yin X Liu Z Wang J . Non-alcoholic fatty liver disease (NAFLD) pathogenesis and natural products for prevention and treatment. Int J Mol Sci. (2022) 23:15489. doi: 10.3390/ijms232415489
22.
Duell PB Welty FK Miller M Chait A Hammond G Ahmad Z et al . American Heart Association Council on arteriosclerosis, thrombosis and vascular biology; council on hypertension; council on the kidney in cardiovascular disease; council on lifestyle and cardiometabolic health; and council on peripheral vascular disease. Nonalcoholic fatty liver disease and cardiovascular risk: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol. (2022) 42:e168–85. doi: 10.1161/ATV.0000000000000153
23.
Huang DQ Downes M Evans RM Witztum JL Glass CK Loomba R . Shared mechanisms between cardiovascular disease and NAFLD. Semin Liver Dis. (2022) 42:455–64. doi: 10.1055/a-1930-6658
24.
Vilar-Gomez E Martinez-Perez Y Calzadilla-Bertot L Torres-Gonzalez A Gra-Oramas B Gonzalez-Fabian L et al . Weight loss through lifestyle modification significantly reduces features of nonalcoholic Steatohepatitis. Gastroenterology. (2015) 149:367–378.e5. doi: 10.1053/j.gastro.2015.04.005
25.
Hu H Lin A Kong M Yao X Yin M Xia H et al . Intestinal microbiome and NAFLD: molecular insights and therapeutic perspectives. J Gastroenterol. (2020) 55:142–58. doi: 10.1007/s00535-019-01649-8
26.
Huang W Kong D . The intestinal microbiota as a therapeutic target in the treatment of NAFLD and ALD. Biomed Pharmacother. (2021) 135:111235. doi: 10.1016/j.biopha.2021.111235
27.
Wang LL Zhang PH Yan HH . Functional foods and dietary supplements in the management of non-alcoholic fatty liver disease: a systematic review and meta-analysis. Front Nutr. (2023) 10:1014010. doi: 10.3389/fnut.2023.1014010
28.
Rong L Ch'ng D Jia P Tsoi KKF Wong SH Sung JJY . Use of probiotics, prebiotics, and synbiotics in non‐alcoholic fatty liver disease: a systematic review and meta‐analysis. J Gastroenterol Hepatol. (2023) 38:1682–94. doi: 10.1111/jgh.16256
29.
Loman BR Hernández-Saavedra D An R Rector RS . Prebiotic and probiotic treatment of nonalcoholic fatty liver disease: a systematic review and meta-analysis. Nutr Rev. (2018) 76:822–39. doi: 10.1093/nutrit/nuy031
30.
Nian F Wu L Xia Q Tian P Ding C Lu X . Akkermansia muciniphila and Bifidobacterium bifidum prevent NAFLD by regulating FXR expression and gut microbiota. J Clin Transl Hepatol. (2023) 11:763–76. doi: 10.14218/JCTH.2022.00415
31.
Helwig U Lammers KM Rizzello F Brigidi P Rohleder V Caramelli E et al . Lactobacilli, bifidobacteria and E. coli nissle induce pro- and anti-inflammatory cytokines in peripheral blood mononuclear cells. World J Gastroenterol. (2006) 12:5978–86. doi: 10.3748/wjg.v12.i37.5978
32.
Siezen R.J. Kok J. Abee T. Schaafsma G. Lactic Acid Bacteria: Genetics, Metabolism and Applications. Springer Netherlands (1999):199–205. Available at: https://link.springer.com/book/10.1007/978-94-017-2027-4
33.
Liao Y Liu L Yang J Zhou X Teng X Li Y et al . Analysis of clinical features and identification of risk factors in patients with non-alcoholic fatty liver disease based on FibroTouch. Sci Rep. (2023) 13:14812. doi: 10.1038/s41598-023-41596-2
34.
Cai J Dong J Chen D Ye H . The effect of synbiotics in patients with NAFLD: a systematic review and meta-analysis. Ther Adv Gastroenterol. (2023) 16:17562848231174299. doi: 10.1177/17562848231174299
35.
Zhou D Chen YW Zhao ZH Yang RX Xin FZ Liu XL et al . Sodium butyrate reduces high-fat diet-induced non-alcoholic Steatohepatitis through upregulation of hepatic GLP-1R expression. Exp Mol Med. (2018) 50:1–12. doi: 10.1038/s12276-018-0183-1
36.
Finer N . Weight loss interventions and nonalcoholic fatty liver disease: optimizing liver outcomes. Diabetes Obes Metab. (2022) 24:44–54. doi: 10.1111/dom.14569
37.
Tarantino G Citro V Balsano C et al . Could SCGF-Beta levels be associated with inflammation markers and insulin resistance in male patients suffering from obesity-related NAFLD?Diagnostics (Basel). (2020) 10:395. doi: 10.3390/diagnostics10060395
38.
Ayob N Muhammad Nawawi KN Mohamad Nor MH Raja Ali RA Ahmad HF Oon SF et al . The effects of probiotics on small intestinal microbiota composition, inflammatory cytokines and intestinal permeability in patients with non-alcoholic fatty liver disease. Biomedicines. (2023) 11:640. doi: 10.3390/biomedicines11020640
39.
Tarantino G Sinatti G Citro V Santini SJ Balsano C . Sarcopenia, a condition shared by various diseases: can we alleviate or delay the progression?Intern Emerg Med. (2023) 18:1887–95. doi: 10.1007/s11739-023-03339-z. Epub 2023 Jul 25
40.
Petermann-Rocha F Gray SR Forrest E Welsh P Sattar N Celis-Morales C et al . Associations of muscle mass and grip strength with severe NAFLD: a prospective study of 333,295 UK biobank participants. J Hepatol. (2022) 76:1021–9. doi: 10.1016/j.jhep.2022.01.010
41.
Buigues C Fernández-Garrido J Pruimboom L Hoogland AJ Navarro-Martínez R Martínez-Martínez M et al . Effect of a prebiotic formulation on frailty syndrome: a randomized, double-blind clinical trial. Int J Mol Sci. (2016) 17:932. doi: 10.3390/ijms17060932
42.
Xue L Deng Z Luo W He X Chen Y . Effect of Fecal microbiota transplantation on non-alcoholic fatty liver disease: a randomized clinical trial. Front Cell Infect Microbiol. (2022) 12:759306. doi: 10.3389/fcimb.2022.759306
43.
Wong VW Wong G Chim AM Chu WC Yeung DK Li K et al . Treatment of nonalcoholic steatohepatitis with probiotics. A proof-of-concept study. Ann Hepatol. (2013) 12:256–62. doi: 10.1016/S1665-2681(19)31364-X
44.
Witjes JJ Smits LP Pekmez CT Prodan A Meijnikman AS Troelstra MA et al . Donor Fecal microbiota transplantation alters gut microbiota and metabolites in obese individuals with Steatohepatitis. Hepatol Commun. (2020) 4:1578–90. doi: 10.1002/hep4.1601
45.
Sepideh A Karim P Hossein A Leila R Hamdollah M Mohammad EG . et al., Effects of multistrain probiotic supplementation on glycemic and inflammatory indices in patients with nonalcoholic fatty liver disease: a double-blind randomized clinical trial. J Am Coll Nutr. (2016) 35:500–5. doi: 10.1080/07315724.2015.1031355
46.
Scorletti E Afolabi PR Miles EA Smith DE Almehmadi A Alshathry A et al . Synbiotics alter fecal microbiomes, but not liver fat or fibrosis, in a randomized trial of patients with nonalcoholic fatty liver disease. Gastroenterology. (2020) 158:1597–1610.e7. doi: 10.1053/j.gastro.2020.01.031
47.
Rodrigo T Dulani S Nimali S de A Fernando J de H et al . Effects of probiotics combined with dietary and lifestyle modification on clinical, biochemical, and radiological parameters in obese children with nonalcoholic fatty liver disease/nonalcoholic steatohepatitis: a randomized clinical trial. Clin Exp Pediatrics. (2022) 65:304–11. doi: 10.3345/cep.2021.00787
48.
Mohamad M Ayob N Mokhtar NM Raja R Tan GC Wong ZQ et al . The effect of probiotics (MCP® BCMC® strains) on hepatic steatosis, small intestinal mucosal immune function, and intestinal barrier in patients with non-alcoholic fatty liver disease. Nutrients. (2021) 13:93192. doi: 10.3390/nu13093192
49.
Mofidi F Yari Z Poustchi H Merat S Nourinayyer B Malekzadeh R et al . Effects of synbiotics supplementation in lean patients with nonalcoholic fatty liver disease: study protocol of a pilot randomized double-blind clinical trial. Arch Iran Med. (2016) 19:282–4. PMID:
50.
Manzhalii E Virchenko O Falalyeyeva T Beregova T Stremmel W . Treatment efficacy of a probiotic preparation for non‐alcoholic steatohepatitis: a pilot trial. J Dig Dis. (2017) 18:698–703. doi: 10.1111/1751-2980.12561
51.
Malaguarnera M Vacante M Antic T Giordano M Chisari G Acquaviva R et al . Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig Dis Sci. (2012) 57:545–53. doi: 10.1007/s10620-011-1887-4
52.
Goyal P Thapa BR Sharma NR Bhatia A . Probiotic and lifestyle modification in obese pediatrics with non-alcoholic fatty liver disease. Indian J Community Health. (2019) 31:50–6. doi: 10.47203/IJCH.2019.v31i01.009
53.
Ferolla SM Couto CA Costa-Silva L Armiliato GNA Pereira CAS Martins FS et al . Beneficial effect of Synbiotic supplementation on hepatic steatosis and anthropometric parameters, but not on gut permeability in a population with nonalcoholic Steatohepatitis. Nutrients. (2016) 8:397. doi: 10.3390/nu8070397
54.
Famouri F Shariat Z Hashemipour M Keikha M Kelishadi R . Effects of probiotics on nonalcoholic fatty liver disease in obese children and adolescents. J Pediatr Gastroenterol Nutr. (2017) 64:413–7. doi: 10.1097/MPG.0000000000001422
55.
Eslamparast T Poustchi H Zamani F Sharafkhah M Malekzadeh R Hekmatdoost A . Synbiotic supplementation in nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled pilot study. Am J Clin Nutr. (2014) 99:535–42. doi: 10.3945/ajcn.113.068890
56.
Duseja A Acharya SK Mehta M Chhabra S et al . High potency multistrain probiotic improves liver histology in non-alcoholic fatty liver disease (NAFLD): a randomised, double-blind, proof of concept study. Gastroenterology. (2019) 6:e000315. doi: 10.1136/bmjgast-2019-000315
57.
Craven L Rahman A Nair Parvathy S Beaton M Silverman J Qumosani K et al . Allogenic Fecal microbiota transplantation in patients with nonalcoholic fatty liver disease improves abnormal small intestinal permeability: a randomized control trial. Am J Gastroenterol. (2020) 115:1055–65. doi: 10.14309/ajg.0000000000000661
58.
Chong PL Laight D Aspinall RJ Higginson A Cummings MH . A randomised placebo controlled trial of VSL#3(®) probiotic on biomarkers of cardiovascular risk and liver injury in non-alcoholic fatty liver disease. BMC Gastroenterol. (2021) 21:144. doi: 10.1186/s12876-021-01660-5
59.
Cai GS Su H Zhang J . Protective effect of probiotics in patients with non-alcoholic fatty liver disease. Medicine. (2020) 99:E21464. doi: 10.1097/MD.0000000000021464
60.
Bomhof MR Parnell JA Ramay HR Crotty P Rioux KP Probert CS et al . Histological improvement of non-alcoholic steatohepatitis with a prebiotic: a pilot clinical trial. Eur J Nutr. (2019) 58:1735–45. doi: 10.1007/s00394-018-1721-2
61.
Behrouz V Aryaeian N Zahedi MJ Jazayeri S . Effects of probiotic and prebiotic supplementation on metabolic parameters, liver aminotransferases, and systemic inflammation in nonalcoholic fatty liver disease: a randomized clinical trial. J Food Sci. (2020) 85:3611–7. doi: 10.1111/1750-3841.15367
62.
Asgharian A Askari G Esmailzade A Feizi A Mohammadi V . The effect of symbiotic supplementation on liver enzymes, c-reactive protein and ultrasound findings in patients with non-alcoholic fatty liver disease: a clinical trial. Int J Prev Med. (2016) 7:59. doi: 10.4103/2008-7802.178533
63.
Aller R de DA Izaola O Conde R Gonzalez M Primo D et al . Effect of a probiotic on liver aminotransferases in nonalcoholic fatty liver disease patients: a double blind randomized clinical trial. Eur Rev Med Pharmacol Sci. (2011) 15:1090–5. PMID:
64.
Alisi A Bedogni G Baviera G Giorgio V Porro E Paris C et al . Randomised clinical trial: the beneficial effects ofVSL#3 in obese children with non‐alcoholic steatohepatitis. Aliment Pharmacol Ther. (2014) 39:1276–85. doi: 10.1111/apt.12758
65.
Ahn SB Jun DW Kang BK Lim JH Lim S Chung MJ . Randomized, double-blind, placebo-controlled study of a multispecies probiotic mixture in nonalcoholic fatty liver disease. Sci Rep. (2019) 9:5688. doi: 10.1038/s41598-019-42059-3
66.
Abhari K Saadati S Yari Z Hosseini H Hedayati M Abhari S et al . The effects of Bacillus coagulans supplementation in patients with non-alcoholic fatty liver disease: a randomized, placebo-controlled, clinical trial. Clin Nutr ESPEN. (2020) 39:53–60. doi: 10.1016/j.clnesp.2020.06.020
67.
Sayari S Neishaboori H Jameshorani M . Combined effects of synbiotic and sitagliptin versus sitagliptin alone in patients with nonalcoholic fatty liver disease. Clin Mol Hepatol. (2018) 24:331–8. doi: 10.3350/cmh.2018.0006
68.
Sadrkabir M Jahed S Sadeghi Z Isazadeh K . The effect of GeriLact on non-alcoholic fatty liver disease. J Kerman Univ Med Sci. (2020) 27:82–90. doi: 10.22062/jkmu.2020.89598
69.
Kobyliak N Abenavoli L Mykhalchyshyn G Kononenko L Boccuto L Kyriienko D et al . A multi-strain probiotic reduces the fatty liver index, cytokines and aminotransferase levels in NAFLD patients: evidence from a randomized clinical trial. J Gastrointestin Liver Dis. (2019) 27:41–9. doi: 10.15403/jgld.2014.1121.271.kby
70.
Ekhlasi G Zarrati M Agah S Hosseini AF Hosseini S Shidfar S et al . Effects of symbiotic and vitamin E supplementation on blood pressure, nitric oxide and inflammatory factors in non-alcoholic fatty liver disease. EXCLI J. (2017) 16:278–90. doi: 10.17179/excli2016-846
71.
Abdel-Razik A Mousa N Shabana W Refaey M Elzehery R Elhelaly R et al . Rifaximin in nonalcoholic fatty liver disease: hit multiple targets with a single shot. Eur J Gastroenterol Hepatol. (2018) 30:1237–46. doi: 10.1097/MEG.0000000000001232
72.
Derosa G Guasti L D’Angelo A Martinotti C Valentino MC di S et al . Probiotic therapy with VSL#3® in patients with NAFLD: a randomized clinical trial. Front Nutr. (2022) 9:846873. doi: 10.3389/fnut.2022.846873
73.
Escouto GS Port GZ Tovo CV Fernandes SA Peres A Dorneles GP et al . Probiotic supplementation, hepatic fibrosis, and the microbiota profile in patients with nonalcoholic Steatohepatitis: a randomized controlled trial. J Nutr. (2023) 153:1984–93. doi: 10.1016/j.tjnut.2023.05.019
74.
Reshef N Gophna U Reshef L Konikoff F Gabay G Zornitzki T et al . Prebiotic treatment in patients with nonalcoholic fatty liver disease (NAFLD)-a randomized pilot trial. Nutrients. (2024) 16:1571. doi: 10.3390/nu16111571
75.
Abd El Hamid AA Mohamed AE Mohamed MS Amin GEE Elessawy HAA Allam MF . The effect of probiotic supplementation on non-alcoholic fatty liver disease (NAFLD) fibrosis score in patients attending a tertiary hospital clinic in Cairo, Egypt. Gastroenterology. (2024) 24:354. doi: 10.1186/s12876-024-03424-3
Summary
Keywords
non-alcoholic fatty liver disease, microbiome-targeted therapies, network meta-analysis, fecal microbiota transplant, antibiotics, probiotics, synbiotics, prebiotics
Citation
Song Y, Liu S, Zhang L, Zhao W, Qin Y and Liu M (2025) The effect of gut microbiome-targeted therapies in nonalcoholic fatty liver disease: a systematic review and network meta-analysis. Front. Nutr. 11:1470185. doi: 10.3389/fnut.2024.1470185
Received
25 July 2024
Accepted
11 December 2024
Published
06 January 2025
Volume
11 - 2024
Edited by
Stavros Plessas, Democritus University of Thrace, Greece
Reviewed by
Giovanni Tarantino, University of Naples Federico II, Italy
Yasi Pan, The Chinese University of Hong Kong, China
Sudrishti Chaudhary, University of Pittsburgh, United States
Updates
Copyright
© 2025 Song, Liu, Zhang, Zhao, Qin and Liu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuanmei Qin, qinyuanmei69@163.comMinghao Liu, liumh015@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.