- 1Department of Metabolic & Bariatric Surgery, Shanghai Sixth People's Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Department of Gastrointestinal Surgery, Shanghai Fourth People’s Hospital, School of Medicine, Tongji University, Shanghai, China
- 3College of Fisheries and Life Science, Shanghai Ocean University, Shanghai, China
- 4Neuroimaging Research Branch, National Institute on Drug Abuse, National Institutes of Health, Baltimore, MD, United States
Background: Studies have shown that patients with obesity appear to be more susceptible to food addiction than the general population. Bariatric surgery stands as the most potent remedy for combating obesity, and it is believed to alleviate the manifestations of food addiction. However, the timing of bariatric surgery to improve food addiction has seldom been the focus of attention.
Methods: In this research, 78 individuals who underwent bariatric surgery were tracked over a period of 2 years. The Yale Food Addiction Scale 2.0 (YFAS 2.0) was employed to assess changes in food addiction tendencies post-surgery. Mixed linear modeling and cluster analysis were applied to investigate the timing of influence of bariatric surgery on the evolution of sub domains of food addiction.
Results: We found that: (1) Bariatric surgery significantly reduces food addiction scores; (2) Bariatric surgery rapidly reduces food addiction scores within first month of surgery and extends to 2 years after surgery; (3) Symptoms in the YFAS 2.0 could be divided into two domains (rapid decline / slow decline) based on their progression following surgery. Rapid decline domain experience rapid improvement shortly (usually 1st month) after the bariatric surgery and maintains a consistently low symptom level, while the slow decline domain improves slowly (usually 4th month) in the post-operative phase.
Discussion: Bariatric surgery induced rapid and sustained remission of food addiction, significantly reducing total food addiction scores within 1 month postoperatively. The effects maintained through 24 months, potentially through neurophysiological and gut microbiota alterations. Despite rapid remission of most food addiction symptoms, social/interpersonal problems, hazardous use, and large amount/longer showed delayed improvement, suggesting distinct behavioral persistence mechanisms.
Conclusion: Food addiction scores can rapidly decline postoperatively and remain consistently lower. However, bariatric surgery does not fully improve all addiction symptoms at the 1st month. This suggests the importance of establishing multidisciplinary clinics in bariatric metabolic surgery.
Introduction
Obesity is a pervasive and costly public health crisis (1), with higher prevalence of complications such as psychological disorders, such as anxiety (2), depression (3) and eating behavior disorders (4, 5). Bariatric surgery is the most effective form of treatment for patients with severe obesity and associated complications (6). However, not all those who have bariatric surgery can solve these complications, especially complex eating behavior disorders. Such as food addition, anorexia, bulimia, before and after surgery, can lead to weight regain and associated mental health challenge (7).
Food addiction as a complex multifactorial eating behaviors disorder has high prevalence in obesity and may be improved by bariatric surgery (8, 9). According to a recent meta-analysis, the prevalence of food addiction in individuals with obesity could be as high as 32%, while between 2 and 12% of healthy individuals are affected by food addiction (10). Even adjusting for health factors (such as smoking, substance use and physical activity), food addiction still showed a significant association with obesity (11–13). A prospective study has shown that food addiction can be reduced by bariatric surgery, with prevalence rates decreasing from 57.8% preoperatively to 7.2 and 13.7% at the 6 and 12-month postoperative time points, respectively (14). It has also been observed clinically that in the early post-operative period after bariatric surgery (within 6 months), there is acute improvements in eating behaviors (15, 16). However, the situation of how food addiction continues to change after surgery has not been explicitly explored due to the lack of high temporal frequency and long cohort studies.
Food addiction can be characterized by a lack of control over food intake, strong appetite, excessive food consumption despite negative health or social consequences, and repeated unsuccessful attempts to control intake (17) and divided into eleven different symptoms, each of which describes an addictive behavior with different positivity rate by the Yale Food Addiction Scale 2.0 (YFAS 2.0) (18). Symptoms of food addiction are different, some are better recognized, such as behavioral signs, and some are not so well recognized, such as social relationships, which are often difficult to intervene in. A recent study has shown that the most endorsed symptom is the symptom ‘large amount / longer’ (19) as a common phenomenon among people with obesity (20), which was defined as substance taken in larger amount and for longer period than intended by YFAS 2.0. Its discrimination parameter remains high, indicating its relatively good ability to delineate individuals who are higher on the latent trait (19). In contrast, Mohsen Saffari considered ‘social/interpersonal problems’ as the most adopted symptom (21), which represented continued consumption despite social or interpersonal problems. While there was another result that the symptom ‘hazardous use’, which represented food use in physically hazardous situation, had a scarcity of endorsement and the lowest discrimination parameter indicating a poor ability to delineate individuals who are higher vs. lower on the latent trait (19). Consequently, the symptom ‘hazardous use’ may not be improved or improved slowly after people with obesity lose weight through bariatric surgery. There are also previous researches which mentioned that the ‘persistent desire or repeated unsuccessful attempts to quit’ symptom is the most commonly recognized criterion (22, 23). These studies have collectively concluded that self-reported food addiction has different rates of positivity for different symptoms. Although the identification of symptoms of food addiction varies among subjects, there is a lack of nooses regarding whether different symptoms of food addiction are equally improved by bariatric surgery. Even when the total food addiction score decreases after bariatric surgery but these symptoms with more recognized and represented do not improve well, people with obesity may still be suffering from food addiction.
Because the available evidence suggests that bariatric surgery reduces the incidence of postoperative food addiction, the symptoms associated with eating behaviors are more readily identifiable and ameliorated. Therefore, we hypothesized that: (1) bariatric surgery could rapidly alleviate postoperative food addiction symptoms in obese patients; (2) bariatric surgery is ineffective in alleviating some of the symptoms of interpersonal problems symptoms of food addiction.
Methods
Subjects
This study was part of a larger prospective cohort study at Shanghai Sixth People’s Hospital. We obtained the following baseline information before patients underwent bariatric surgery: age, years of education, sex, body mass index (BMI), the YFAS 2.0 (food addiction score). Based on the Chinese criteria for overweight and obesity, the overweight or obesity (OW/OB) group comprised patients with overweight or obesity (BMI > 26 kg/m2) who were undergoing bariatric surgery at the Weight Loss Metabolism Clinic in Shanghai Sixth People’s Hospital. The healthy control (HC) group comprised individuals with healthy BMIs (18 kg/m2 < BMI < 24 kg/m2) who were randomly recruited. Postoperative follow-up examinations were performed at 1-month intervals until 18 months postoperatively and then at 6-month intervals until 24 months postoperatively. All participants had to meet the following inclusion criteria: (i) age between 18 and 65 years, (ii) the capacity to give informed consent, (iii) no untreated mental illness or unstable mental state, (iv) all participants with a history of bariatric surgery, and pregnant or breast-feeding females were excluded. The study and the survey obtained ethics approval by the independent IRB of the authors’ institution (Ethics Committee of Shanghai Sixth People’s Hospital, NO 2020–219-(1)).
Measurements
YFAS 2.0
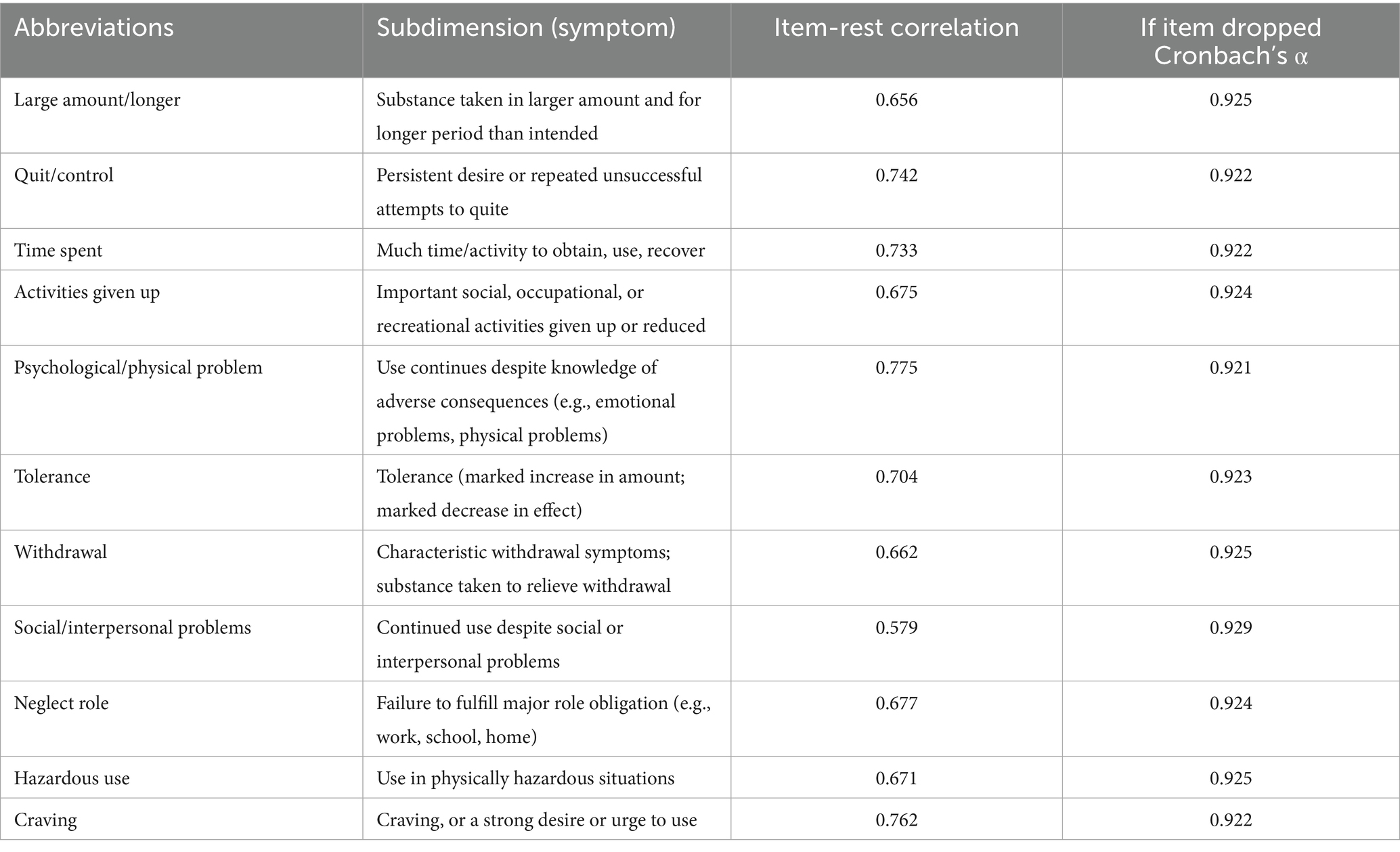
The YFAS 2.0, which contained a total of 11 judgment criteria and one clinical diagnostic criterion, was revised and published in 2016 by Gearhardt et al. on this basis (18). The method of analysis was a questionnaire containing personal information and the YFAS 2.0 carried out in an online platform (www.wjx.cn). The YFAS 2.0 consists of 35 questions scored on an 8-level Likert scale ranging from 0 to 7. It provides two scoring methods: symptom count or diagnostic threshold. The presence of no more than one symptom or the absence of a symptom 12 was classified as non-addiction, the presence of 2–3 symptoms and the occurrence of a symptom 12 was classified as mild addiction, the presence of 4–5 symptoms together with the symptom 12 addiction was classified as moderate and the occurrence of more symptoms with 12 was classified as severe addiction. The Chinese version of the YFAS 2.0 had good internal consistency in our study. Cronbach’s α for the YFAS 2.0 was 0.93. For convenience, we abbreviated each symptom using one or few words. Detailed description of the symptoms can be found in Table 1.
Statistical methods
All the statistical analyses were performed using Jamovi v2.3.16, GraphPad v 9.4.1 and R 4.2.3. The normally distributed data were analyzed by Student’s t-test and expressed as mean ± standard deviation. The non-normally distributed data were analyzed by the Mann–Whitney U test and expressed as median (quartiles [P25, P75]; the sex-related characteristics of the two groups were tested by the chi-square test).
For our primary endpoint, we conducted a mixed linear model (MLM) using food addiction score as the dependent variable. In this model, individual patients were included as a random effect, and the data timepoint for YFAS 2.0 collection as fixed effects. The MLM model was as follows:
Hierarchical clustering was performed using the Ward clustering algorithm and separated by Euclidean distance with factors arranged visually by time point and by groups. To assess the statistical significance of the clustering solution, we conducted an analysis of variance (ANOVA) to compare the means of variables across clusters. Our results indicated significant differences (p < 0.05) in the means of variables between clusters, supporting the validity of the clustering solution.
The model effectively controls for baseline heterogeneity between individuals by incorporating random intercept terms. It is suitable for longitudinal data analysis with repeated measurements. Model parameter estimation uses restricted maximum likelihood estimation (REML), and statistical significance is set at α = 0.05. Model fit was evaluated using the Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC). All statistical modeling procedures, including model fitting and validation, were implemented using dedicated open-source packages in R 4.4.3.
Results
Characteristics of the participants
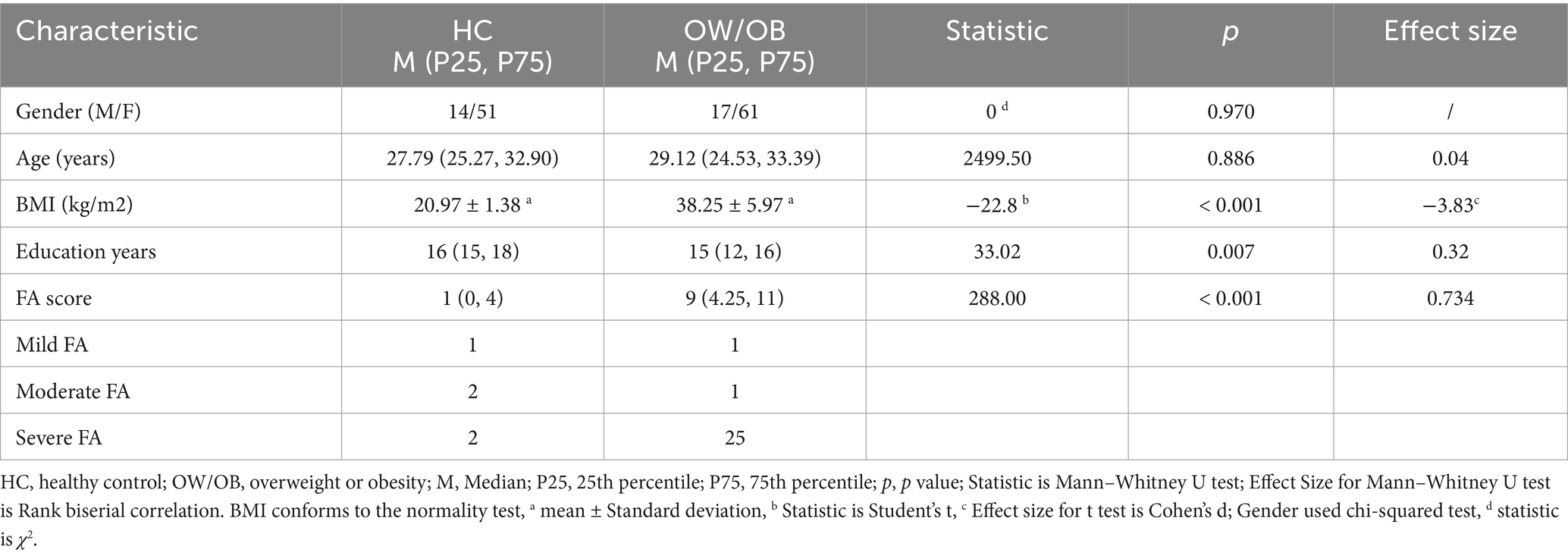
Group comparisons of the sociodemographic characteristics; the BMI; and the food addiction score are shown in Table 2. The OW/OB group consisted of 78 subjects (61 females and 17 males) with a median age of 29.12 years. The HC group consisted of 65 subjects (51 females and 14 males) with a median age of 27.79 years. There were no significant differences in gender (p = 0.0970) or age (27.79 [25.27, 32.90] vs. 29.12 [24.53, 33.39], respectively; U = 2499.50, p = 0.886) between the two groups. The BMI and food addiction score in the HC group significantly differed from those in the OW/OB group before surgery; specifically, the OW/OB group had higher food addiction score. The bar chart showed that before surgery, the median food addiction score was significantly higher in the HC than OW/OB group (1 [0, 4] vs. 9 [4.25, 11], respectively; U = 288.00, p < 0.001). 34.6% of individuals in the OW/OB group received a diagnosis of food addiction, while 7.7% of individuals in the HC group received a diagnosis of food addiction.
Prevalence of food addiction categorization after SG
To evaluate the assessment that SG could improve the situation of the food addiction, the MLM was used to describe the trend of the food addiction score, which took postoperative time as predictive variable, the food addiction score as predicted variable, subject as cluster. Compared with preoperative, the food addiction score of participants significantly decreased at 1 month postoperative (t = −4.64, p < 0.001), and it sustained until 24 months after bariatric surgery (t = −6.31, p < 0.001) (Figure 1; Supplementary Table S1). Finally, 25% of individuals in the OW/OB group received a diagnosis of food addiction at 24 months postoperative. In addition to the preoperative comparisons, repeated comparisons were made, i.e., changes were compared between two adjacent time points to determine at which postoperative time point the participants’ food addiction improvement occurred primarily. It was found that food addiction scores only decreased significantly in the first month postoperatively compared to the preoperative period, and otherwise did not change significantly compared to the previous month (Supplementary Table S2).
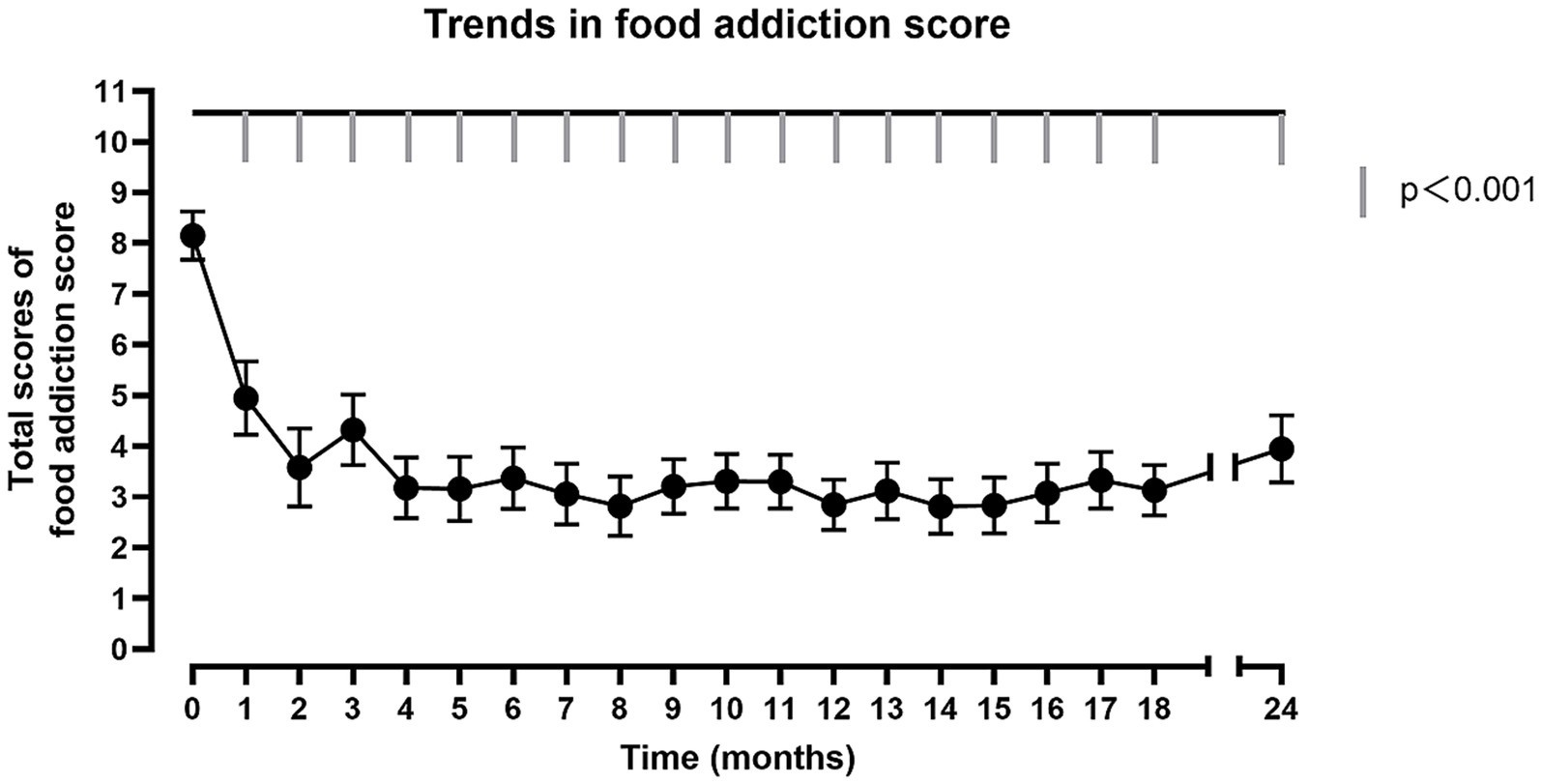
Figure 1. The trend of total food addiction score. The horizontal axis represents the post-operative follow-up time. The vertical axis represents the total score of food addiction score. The fold line reflects the trend in food addiction scores after surgery. Significant differences (p < 0.001) for each post-operative month compared to pre-operative.
Analysis of standardized residuals from the model indicated approximate normality in the overall error distribution. The residual median was −0.0729, demonstrating near-symmetry about zero. Interquartile ranges spanned from −0.454 (lower quartile) to 0.409 (upper quartile), satisfying the normality assumption for linear model errors. Extreme values ranged from −3.379 to 3.891, suggesting potential outliers. The model was estimated using restricted maximum likelihood (REML), with convergence achieved at an objective function value of 2,207. Model fit indices yielded an AIC of 2250.97 and a BIC of 2343.02.
Response of each symptom in YFAS 2.0 after SG
In order to observe how the proportion of positive individuals for each symptom in the YFAS 2.0 description varied, a cluster heat map was created based on each participant’s response to the questionnaire. In the heat map, each row corresponds to a symptom in the YFAS 2.0 description, whereas the columns represent the various pre- and post-operative time points. The values represented by each square are calculated according to a generalized MLM. Increasing brightness toward blue indicates a higher mean of possible rate, and white indicates a lower mean of possible rate. We found that out of a total of 11 symptoms, ‘psychological/ physical problem’, ‘craving’, ‘quit/control’, ‘time spent’, ‘activities given up’, ‘withdrawal’, ‘tolerance’, and ‘neglect role’ showed more similar trends, ‘social/interpersonal problems’, ‘large amount/longer’, and ‘hazardous use’ showed more similar trends (Figure 2).
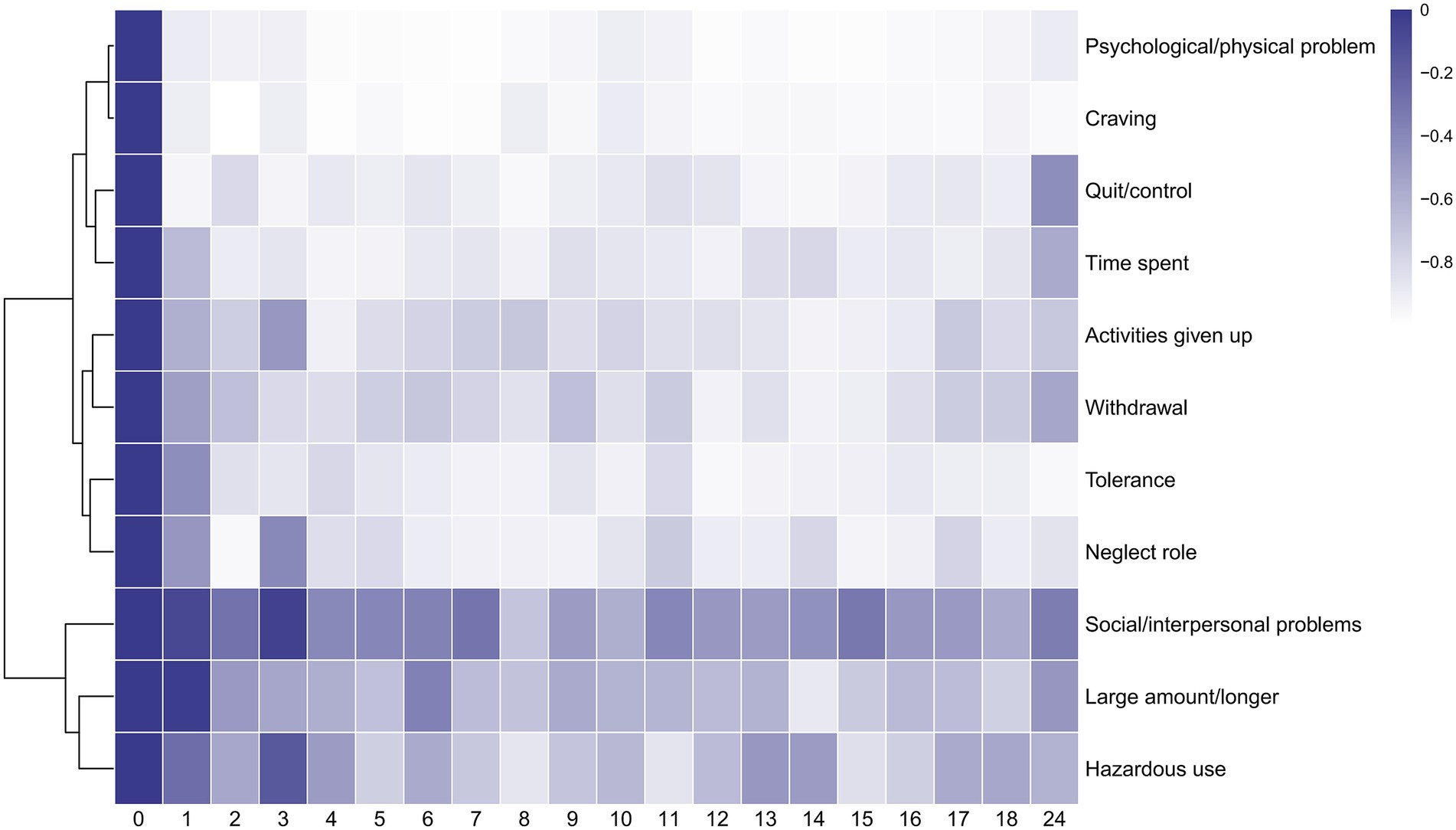
Figure 2. Heatmap of 11 symptoms of YFAS 2.0. The horizontal axis represents the post-operative follow-up time. The vertical axis represents 11 symptoms of YFAS 2.0(‘Large amount/longer’ for substance taken in larger amount and for longer period than intended. ‘Quit/control’ for persistent desire or repeated unsuccessful attempts to quit. ‘Time spent’ for much time/activity to obtain, use, recover. ‘Activities given up’ for important social, occupational, or recreational activities given up or reduced. ‘Psychological/physical problem’ for use continues despite knowledge of adverse consequences. ‘Tolerance’ for tolerance. ‘Withdrawal’ for characteristic withdrawal symptoms; substance taken to relieve withdrawal. ‘Social/interpersonal problems’ for continued use despite social or interpersonal problems. ‘Neglect role’ for failure to fulfill major role obligation. ‘Hazardous use’ for use in physically hazardous situations. ‘Craving’ for craving, or a strong desire or urge to use.) The algorithm for heatmap clustering was based on the Euclidean distance measure for similarity and Ward clustering algorithm.
In addition, the post-operative trends for each symptom were represented by equations calculated from a generalized MLM and presented as line graphs. For specific trends in each symptom, see Fig. S1 - Fig S11 in the Supporting Materials. We found that not all symptoms began to improve in the early postoperative period immediately. Among the symptoms ‘large amount/longer’ (z = −0.243, p > 0.05), ‘tolerance’ (z = −1.10, p > 0.05), ‘social/interpersonal problems’ (z = −1.27, p > 0.05), ‘hazardous use’ (z = −1.116, p > 0.05), there was no significant difference in the first month postoperatively compared to preoperatively. The symptom ‘large amount/longer’ began to improve significantly at the second month postoperatively (z = −2.571, p < 0.05). The symptom ‘hazardous use’ was improved significantly at the second month postoperatively (z = −2.039, p < 0.05) compared to the preoperative, while did not differ at the third month postoperatively (z = −0.719, p > 0.05) compared to the preoperative. The symptom ‘social/interpersonal problems’ was improved significantly at the second month postoperatively (z = −2.50, p < 0.05) compared to the preoperative, while did not differ at the third month postoperatively (z = −1.21, p > 0.05) compared to the preoperative. The symptom ‘hazardous use’ and ‘social/interpersonal problems’ were both improved from 4th month postoperatively until 24 months after bariatric surgery.
Discussion
This study sought to explore the interventional effects of bariatric surgery on food addiction by continuous measurements at intensive postoperative time points. Two main new findings were found: 1. Total food addiction scores were substantially reduced by bariatric surgery within 1 month postoperatively, and it could be maintained for at least 24 months postoperatively; 2. Bariatric surgery improved most of the symptoms of food addiction in the early postoperative period, however, it was slower to improve slow decline domain.
Bariatric surgery improves food addiction scores
Food addiction scores started to decline already early after bariatric surgery and leveled off afterwards. This study found that 34.6% of candidates for bariatric surgery have suffered from food addiction and exhibited elevated addiction scores, aligning with another observation reported by our research team (24). And it declined to 25% 2 years postoperatively. This is in line with the results of a recent meta-analysis shown that the absolute prevalence reduction of food addiction was decreased after bariatric surgery. Further, the effect was observed within 6 months of postoperative follow-up (10). We speculate that this may be due to neurophysiology, gut microbiota changes and eating behavior factors in the patients as a result of the bariatric surgery.
Food addiction is largely reduced due to a restriction of the gastric volume after bariatric surgical intervention, during which neuromodulation and hormone regulation are stimulated. The addiction is mediated by brain regions and neurotransmitters (25). Bariatric surgery reduces appetite and induces psychophysiological effects by influencing the expression of numerous brain neurotransmitters, including dopamine, serotonin and various neuropeptides such as neuropeptide Y, leptin, orexin, growth hormone-releasing peptide, growth hormone-releasing factor, leptin and glucagon-like peptide-1 levels (26). Bariatric surgery alters these neuropeptides’ secretion, which in turn reduces the patients’ food craving and lack of control over food intake (27, 28), important addiction symptoms in food addiction.
Bariatric surgery affects food addiction by altering a patient’s gut microbiota, which may in turn affect food addiction. Environmental factors and dietary patterns have a major influence on gut microbiota composition, and the overconsumption of highly palatable food may promote a gut microbiota dysbiosis that has been recently proposed to participate in the loss of eating control (29). Individuals with obesity, which may be promoted by food addiction, showed altered gut microbiota with a reduced diversity that facilitated energy absorption capacity and may affect host brain function (30). In the recent study, researchers demonstrated a translational link between mice and humans in gut microbiome composition associated with food addiction, supporting a link between gut microbiota and vulnerability to this behavioral disorder (31). While bariatric surgery affects the hosts’ metabolism by altering the gut microbiota (32) and modulates the inflammatory response through a variety of mechanisms that alter the patient’s physiology (33), which is a potential way to improve food addiction.
Food addiction is clinically associated with dysregulated eating patterns including grazing (34), emotional eating, and loss-of-control (LOC) eating (35). Grazing, operationally defined as the persistent, compulsive consumption of small-to-moderate food volumes without discrete meals or temporal structure (36), constitutes a distinct yet potentially mutually reinforcing behavioral phenotype relative to food addiction. Symptomatic overlap exists between grazing and food addiction (34), postoperative grazing may attenuate weight loss efficacy following bariatric surgery (37). Surgical gastric restriction necessitates structured nutritional protocols; consequently, postoperative guidance advocates planned, repetitive meal patterns with prescribed dietary composition. Some authorities contend that intention-regulated eating should be nosologically distinguished from maladaptive grazing (36). Some evidences suggests grazing incidence may increase postoperatively despite these interventions (37, 38). This investigation observed sustained reductions in food addiction scores across a 24-month postoperative follow-up. Crucially, this amelioration persisted even when accounting for putative risk factors associated with elevated postoperative grazing prevalence. A recent study proposed a cyclical reinforcement model wherein emotional eating drives dysregulated consumption, precipitating loss-of-control eating (35). Subsequent maladaptive attempts to regulate this behavior paradoxically perpetuate and intensify food addiction. This model emphasizes the mediating role of food-related cognitions (e.g., food craving) in facilitating LOC episodes, establishing cognitive processes as critical mechanisms in food addiction. Studies indicate bariatric surgery significantly attenuates emotional and LOC eating behaviors while enhancing cognitive restraint in candidates (39, 40). This suggests surgical intervention may ameliorate food addiction symptomatology through modulation of these core behavioral pathways. Notably, a meta-analysis revealed cognitive behavioral therapy (CBT) produces significant short-term reductions in emotional eating post-intervention (41). However, these gains demonstrate poor sustainability at long-term follow-up. Critically, CBT showed no significant effects on sustained LOC improvement at long-term follow-up. These findings remain constrained by limited sample sizes and methodological heterogeneity, precluding definitive conclusions regarding CBT’s long-term efficacy. This study documented postoperative food addiction score reduction absent concurrent CBT implementation.
Thus, the data from this study suggest that symptoms of food addiction in patients with obesity can be ameliorated by bariatric surgery. However, previous research on of postoperative outcomes in early food addiction has predominantly focused on follow-up assessments conducted beyond 3 months (42, 43). Although some studies have employed short-term follow-up at 1 month, sequential monthly assessments are notably lacking (44). More and longer longitudinal studies, preferably with experimental designs with control groups, are needed to assess the impact of bariatric surgery on the negative effects of food addiction. In addition, we should further investigate the mechanisms to provide a theoretical basis for finding new treatment pathways for food addiction in the future. As for the patients, we can better manage obesity in this population and reduce the symptoms of food addiction in patients.
Slow improvement in symptom ‘social/interpersonal problems’, ‘hazardous use’ and ‘large amount/longer’
All eleven symptoms can be classified into two domains based on postoperative trends, with the first domain improving rapidly postoperatively and the second domain improving slowly postoperatively. The rapid decline domain includes ‘psychological/ physical problem’, ‘craving’, ‘quit/control’, ‘time spent’, ‘activities given up’, ‘withdrawal’, ‘tolerance’, and ‘neglect role’; the slow decline domain includes ‘social/interpersonal problems’, ‘large amount/longer’, and ‘hazardous use’. The slowest improvement after surgery is seen in ‘social/interpersonal problems’.
Subjects are sensitive to the social and psychological problems caused by food addiction. The highest number of subjects (40/46) suffered from this problem before surgery, and the tendency to decline after surgery was moderate compared to other symptoms. It was obvious that the symptom for persistent desire or repeated unsuccessful attempts to quit was reduced by bariatric surgery rapidly in the early post-operative period. No previous study has followed up so intensively after surgery and analyzed serial changes in different symptoms. Patients involved in bariatric surgery are obese, a group of patients who face physical impairment, mobility difficulties, low self-esteem or self-control, and illnesses associated with negative body imagery (45, 46). Women may also be more concerned about aesthetic issues and may refuse and not want to disclose their weight. Whereas patients with obesity are often unable to perceive their weight correctly, even after bariatric surgery, patients seem unable to recognize changes in body image following significant weight loss (45). This can lead to patients refusing to participate in social activities for fear of overeating in front of their loved ones or friends, even though they have lost weight after bariatric surgery. This also explains why ‘social/interpersonal problems’ in the YFAS 2.0 is relatively slow to improve after surgery. A better understanding of this unique postoperative symptom may help to elucidate the underlying biological and psychological bases associated with these more severe symptoms, especially after bariatric surgery.
‘Hazardous use’ symptom is slow to improve and tend to rebound after bariatric surgery. ‘Hazardous use’ is the behavior of eating certain foods even when individuals know it was physically dangerous (such as eating sweets even with diabetes), which is one of the main symptoms of food addiction. Some studies have found that over time, cravings for sweets no longer differ from pre-operative (47). We advise patients to eat more foods rich in high protein postoperatively, and sweets such as sugar and beverages are strongly discouraged foods because eating sweets can cause patients to regain weight, as well as being detrimental to diabetes and insulin resistance controlling. This may lead patients to feel that they are hurting themselves by eating sweets, but when they still insist on eating sweets, they self-report as hazardous use. In addition, a recent finding suggests that some patients may substitute the concept of one reinforcer ‘food’ for another, such as ‘alcohol’, which is commonly referred to as ‘addiction transfer’ (48). And these patients with addiction shifts can have alcohol abuse, which may be contributing to the slow decline in symptom of ‘hazardous use’ after patients undergo bariatric surgery, despite a rapid decline in food addiction scores.
‘Large amount/longer’ begins to decline in the second month and remains at a lower level from the second month to the 24th month postoperatively. ‘Large amount/longer’ describes eating behaviors in which individuals eat more than planned or for more time than planned, persist in eating in the absence of hunger and eat to the point where they fell physically ill. Previous studies have shown that patients’ hunger, disinhibition and emotional eating decrease postoperatively, whereas restraint and postprandial fullness increase (49, 50). This may lead to an improvement in ‘large amount/longer’ postoperatively, which is in line with the trend we observed for postoperative changes. Besides, the new finding is that the improvement of ‘large amount/longer’ starts in the second postoperative month. We hypothesize that the unimprovement of the first month may be caused by the following reasons. Firstly, overall maladaptive eating behaviors (binge eating, night eating and uncontrolled eating) which are associated with ‘large amount/longer’ improve postoperatively and require an adaptation process (51). Secondly, the recovery of the postoperative diet starts with a liquid diet and gradually transitions to a semi-liquid, soft food and then to a normal diet. We commonly instruct the patients to eat slowly during the postoperative mission and to have an intermediate cut between ingesting solids and liquids at each meal, all of which may lead to an increase in the patient’s eating time. Thirdly, bariatric surgery alters the volume of the stomach, causing patients to feel fuller after surgery. In the early postoperative period, eating too quickly or eating too much at once may cause the patients to feel physically uncomfortable. These may explain why ‘large amount/longer’ did not improve significantly in the first month after surgery compared to the preoperative.
The following are a few limitations of this study. Prior research lacks studies utilizing MLM to characterize the continuous postoperative trajectory of food addiction scores. Consequently, no established reference exists to inform power calculations or sample size estimation for this specific analytical approach. Our sample size was therefore informed provisionally by effect sizes reported in studies examining short- and long-term food addiction improvement following bariatric surgery, with an anticipated attrition rate of 20% incorporated. During longitudinal assessment, attrition rates exceeded the projected 20% threshold at several follow-up intervals. The implementation of MLM for trajectory analysis— rather than reliance on mean food addiction score reporting— partially mitigates the impact of this missing data by accommodating subject-wise inclusion of all available observations. Consequently, despite acknowledged limitations in cohort retention, the reported model outputs remain methodologically robust and clinically interpretable. Future investigations should incorporate longitudinal assessments of dysregulated eating behaviors, trait-level food cravings, and dynamic psychological states. Integrating these multidimensional covariates would refine model specification accuracy and elucidate mechanistic pathways through which bariatric surgery ameliorates food addiction. This study was more of a questionnaire and a behavioral experiment to collect information about the patients, and more biochemical and neuroimaging indicators are needed to help us explain the mechanisms behind this phenomenon. Some patients may have had inadequate energy or time to complete the questionnaire on schedule, instead completing it a few days later than planned. All of the surgeries were performed using sleeve gastrectomy, and we can use the type of surgery as a controlled variable in the future study.
Conclusion
Bariatric surgery induced rapid and clinically meaningful improvement in food addiction symptomatology, with significant reductions observed as early as 1 month postoperatively. This therapeutic effect persisted throughout the 24-month follow-up period. However, the rates of improvement manifested significant heterogeneity across symptom domains. Notably, amelioration progressed more gradually for items assessing ‘Social/interpersonal problems’, ‘Hazardous use’, and ‘Large amount/longer’ compared to other symptoms.
Data availability statement
The data supporting the conclusions of this article will be made available by the authors upon request.
Ethics statement
The studies involving humans were approved by Ethics Committee of Shanghai Sixth People’s Hospital, NO 2020–219-(1). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
X-YB: Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. CW: Data curation, Methodology, Visualization, Writing – review & editing. TX: Writing – review & editing. LL: Data curation, Writing – review & editing. C-YY: Data curation, Writing – review & editing. H-LZ: Writing – review & editing. H-WZ: Writing – review & editing. X-DH: Writing – review & editing. HZ: Data curation, Methodology, Writing – review & editing. J-ZD: Funding acquisition, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Key R&D Program of China (Grant No. 2023YFC3043508), the National Natural Science Foundation of China (Grant No. 82370901), and the Shanghai Municipal Science and Technology Commission (Grant No. 24DZ2304800).
Acknowledgments
The authors would like to acknowledge Huiting Cai, Ziqi Liu for their help with data collection, data analysis and advice on writing the article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1535911/full#supplementary-material
SUPPLEMENTARY FIGURES S1-S11 | Trends in each symptom of the YFAS 2.0.
References
1. World Health Organization. (2021). Obesity and overweight. Available online at: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
2. Gariepy, G, Nitka, D, and Schmitz, N. The association between obesity and anxiety disorders in the population: a systematic review and meta-analysis. Int J Obes. (2010) 34:407–19. doi: 10.1038/ijo.2009.252
3. Alshehri, T, Mook-Kanamori, DO, de Mutsert, R, Penninx, BW, Rosendaal, FR, le Cessie, S, et al. The association between adiposity and atypical energy-related symptoms of depression: a role for metabolic dysregulations. Brain Behav Immun. (2023) 108:197–203. doi: 10.1016/j.bbi.2022.12.005
4. Heriseanu, AI, Hay, P, Corbit, L, and Touyz, S. Grazing in adults with obesity and eating disorders: a systematic review of associated clinical features and meta-analysis of prevalence. Clin Psychol Rev. (2017) 58:16–32. doi: 10.1016/j.cpr.2017.09.004
5. Macpherson-Sánchez, AE. Integrating fundamental concepts of obesity and eating disorders: implications for the obesity epidemic. Am J Public Health. (2015) 105:e71–85. doi: 10.2105/AJPH.2014.302507
6. Arterburn, DE, Telem, DA, Kushner, RF, and Courcoulas, AP. Benefits and risks of bariatric surgery in adults: a review. JAMA. (2020) 324:879–87. doi: 10.1001/jama.2020.12567
7. Allison, KC, Wu, J, Spitzer, JC, McCuen-Wurst, C, Ashare, RL, Tewksbury, C, et al. Changes in eating Behaviors and their relation to weight change 6 and 12 months after bariatric surgery. Obes Surg. (2023) 33:733–42. doi: 10.1007/s11695-022-06442-w
8. Cassin, SE, Sijercic, I, and Montemarano, V. Psychosocial interventions for food addiction: a systematic review. Curr Addict Rep. (2020) 7:9–19. doi: 10.1007/s40429-020-00295-y
9. Leary, M, Pursey, KM, Verdejo-Garcia, A, Burrows, TL, Cassin, SE, Sijercic, I, et al. Current intervention treatments for food addiction: a systematic review. Behav Sci. (2021) 11:6. doi: 10.3390/bs11060080
10. Praxedes, DRS, Silva-Júnior, AE, Macena, ML, Oliveira, AD, Cardoso, KS, Nunes, LO, et al. Prevalence of food addiction determined by the Yale food addiction scale and associated factors: a systematic review with meta-analysis. Eur Eat Disord Rev. (2022) 30:85–95. doi: 10.1002/erv.2878
11. Camacho-Barcia, L, Munguía, L, Lucas, I, De La Torre, R, Salas-Salvadó, J, Pintó, X, et al. Metabolic, affective and neurocognitive characterization of metabolic syndrome patients with and without food addiction. Implications for weight progression. Nutrients. (2021) 13:2779. doi: 10.3390/nu13082779
12. Fernandez-Aranda, F, Karwautz, A, and Treasure, J. Food addiction: a transdiagnostic construct of increasing interest. Eur Eat Disord Rev. (2018) 26:536–40. doi: 10.1002/erv.2645
13. Jiménez-Murcia, S, Agüera, Z, Paslakis, G, Munguia, L, Granero, R, Sánchez-González, J, et al. Food addiction in eating disorders and obesity: analysis of clusters and implications for treatment. Nutrients. (2019) 11:2633. doi: 10.3390/nu11112633
14. Sevinçer, GM, Konuk, N, Bozkurt, S, and Coşkun, H. Food addiction and the outcome of bariatric surgery at 1-year: prospective observational study. Psychiatry Res. (2016) 244:159–64. doi: 10.1016/j.psychres.2016.07.022
15. Nance, K, Eagon, JC, Klein, S, and Pepino, MY. Effects of sleeve gastrectomy vs. Roux-en-Y gastric bypass on eating behavior and sweet taste perception in subjects with obesity. Nutrients. (2017) 10:18. doi: 10.3390/nu10010018
16. Pepino, MY, Bradley, D, Eagon, JC, Sullivan, S, Abumrad, NA, and Klein, S. Changes in taste perception and eating behavior after bariatric surgery-induced weight loss in women. Obesity. (2014) 22:E13–20. doi: 10.1002/oby.20649
17. Gearhardt, AN, and Hebebrand, J. The concept of “food addiction” helps inform the understanding of overeating and obesity: debate consensus. Am J Clin Nutr. (2021) 113:274–6. doi: 10.1093/ajcn/nqaa345
18. Gearhardt, AN, Corbin, WR, and Brownell, KD. Development of the Yale food addiction scale version 2.0. Psychol Addict Behav. (2016) 30:113–21. doi: 10.1037/adb0000136
19. Chapron, S-A, Kervran, C, Da Rosa, M, Fournet, L, Shmulewitz, D, Hasin, D, et al. Does food use disorder exist? Item response theory analyses of a food use disorder adapted from the DSM-5 substance use disorder criteria in a treatment seeking clinical sample. Drug Alcohol Depend. (2023) 251:110937. doi: 10.1016/j.drugalcdep.2023.110937
20. Curtis, C, and Davis, C. A qualitative study of binge eating and obesity from an addiction perspective. Eat Disord. (2014) 22:19–32. doi: 10.1080/10640266.2014.857515
21. Saffari, M, Fan, C-W, Chang, Y-L, Huang, P-C, Tung, SEH, Poon, WC, et al. Yale food addiction scale 2.0 (YFAS 2.0) and modified YFAS 2.0 (mYFAS 2.0): Rasch analysis and differential item functioning. J Eat Disord. (2022) 10:185. doi: 10.1186/s40337-022-00708-5
22. Penzenstadler, L, Soares, C, Karila, L, and Khazaal, Y. Systematic review of food addiction as measured with the Yale food addiction scale: implications for the food addiction construct. Curr Neuropharmacol. (2019) 17:526–38. doi: 10.2174/1570159X16666181108093520
23. Pursey, KM, Stanwell, P, Gearhardt, AN, Collins, CE, and Burrows, TL. The prevalence of food addiction as assessed by the Yale food addiction scale: a systematic review. Nutrients. (2014) 6:4552–90. doi: 10.3390/nu6104552
24. Xu, T, Liu, JJ, Liu, ZQ, Qi, XG, Zhang, HW, Liu, L, et al. Altered asymmetry of amygdala volume mediates food addiction and weight gain. J Behav Addict. (2025) 14:522–33. doi: 10.1556/2006.2024.00073
25. Lüscher, C, and Malenka, R. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. (2011) 69:650–63. doi: 10.1016/j.neuron.2011.01.017
26. Huang, R, Ding, X, Fu, H, and Cai, Q. Potential mechanisms of sleeve gastrectomy for reducing weight and improving metabolism in patients with obesity. Surg Obes Relat Dis. (2019) 15:1861–71. doi: 10.1016/j.soard.2019.06.022
27. Wang, Q, Tang, W, Rao, W-S, Song, X, Shan, C-X, and Zhang, W. Changes of ghrelin/GOAT axis and mTOR pathway in the hypothalamus after sleeve gastrectomy in obese type-2 diabetes rats. World J Gastroenterol. (2017) 23:6231–41. doi: 10.3748/wjg.v23.i34.6231
28. Xu, T-R, Yang, Y, Ward, R, Gao, L, and Liu, Y. Orexin receptors: multi-functional therapeutic targets for sleeping disorders, eating disorders, drug addiction, cancers and other physiological disorders. Proc Natl Acad Sci. (2013) 25:2413–23. doi: 10.1016/j.cellsig.2013.07.025
29. Gupta, A, Osadchiy, V, and Mayer, EA. Brain–gut–microbiome interactions in obesity and food addiction. Nat Rev Gastroenterol Hepatol. (2020) 17:655–72. doi: 10.1038/s41575-020-0341-5
30. Barone, M, Garelli, S, Rampelli, S, Agostini, A, Matysik, S, D’Amico, F, et al. Multi-omics gut microbiome signatures in obese women: role of diet and uncontrolled eating behavior. BMC Med. (2022) 20:500. doi: 10.1186/s12916-022-02689-3
31. Samulėnaitė, S, García-Blanco, A, Mayneris-Perxachs, J, Domingo-Rodríguez, L, Cabana-Domínguez, J, Fernàndez-Castillo, N, et al. Gut microbiota signatures of vulnerability to food addiction in mice and humans. Gut. (2024) 73:1799–815. doi: 10.1136/gutjnl-2023-331445
32. Basso, N, Soricelli, E, Castagneto-Gissey, L, Casella, G, Albanese, D, Fava, F, et al. Insulin resistance, microbiota, and fat distribution changes by a new model of vertical sleeve gastrectomy in obese rats. Diabetes. (2016) 65:2990–3001. doi: 10.2337/db16-0039
33. Galanakis, CG, Daskalakis, M, Manios, A, Xyda, A, Karantanas, AH, and Melissas, J. Computed tomography-based assessment of abdominal adiposity changes and their impact on metabolic alterations following bariatric surgery. World J Surg. (2015) 39:417–23. doi: 10.1007/s00268-014-2826-2
34. Rossi, AA, Mannarini, S, Semonella, M, Castelnuovo, G, and Pietrabissa, G. The association between grazing and food addiction: the Italian version of the repetitive eating questionnaire (rep(eat)-Q) and its relationships with food addiction criteria. Nutrients. (2024) 16:949. doi: 10.3390/nu16070949
35. Rossi, AA. Tying food addiction to uncontrolled eating: the roles of eating-related thoughts and emotional eating. Nutrients. (2025) 17:369. doi: 10.3390/nu17030369
36. Conceição, EM, Mitchell, JE, Engel, SG, Machado, PP, Lancaster, K, and Wonderlich, SA. What is "grazing"? Reviewing its definition, frequency, clinical characteristics, and impact on bariatric surgery outcomes, and proposing a standardized definition. Surg Obes Relat Dis. (2014) 10:973–82. doi: 10.1016/j.soard.2014.05.002
37. Colles, SL, Dixon, JB, and O'Brien, PE. Grazing and loss of control related to eating: two high-risk factors following bariatric surgery. Obesity (Silver Spring). (2008) 16:615–22. doi: 10.1038/oby.2007.101 Erratum in: Obesity (Silver Spring). 2011 Nov;19(11):2287
38. Kofman, MD, Lent, MR, and Swencionis, C. Maladaptive eating patterns, quality of life, and weight outcomes following gastric bypass: results of an internet survey. Obesity (Silver Spring). (2010) 18:1938–43. doi: 10.1038/oby.2010.27
39. Barstad, LH, Johnson, LK, Borgeraas, H, Hofsø, D, Svanevik, M, Småstuen, MC, et al. Changes in dietary intake, food tolerance, hedonic hunger, binge eating problems, and gastrointestinal symptoms after sleeve gastrectomy compared with after gastric bypass; 1-year results from the Oseberg study-a randomized controlled trial. Am J Clin Nutr. (2023) 117:586–98. doi: 10.1016/j.ajcnut.2022.11.016
40. Nergård, BJ, Leifson, BG, Gislason, H, and Hedenbro, JL. Effect of different limb lengths on quality of life, eating patterns and gastrointestinal symptoms after Roux-en-Y gastric bypass in superobese patients: randomized study. BJS Open. (2020) 4:1109–16. doi: 10.1002/bjs5.50334
41. Gao, H, Wang, G, Zhang, X, Guo, H, Pang, L, and Gu, J. Meta-analysis of the effects of cognitive behavioral therapy on dietary habits and emotional outcomes in patients with obesity undergoing metabolic and bariatric surgery. J Nutr Educ Behav. (2025) 57:S1499-4046(25)00309-4. doi: 10.1016/j.jneb.2025.05.193
42. Halbeisen, G, Pahlenkemper, M, Sabel, L, Richardson, C, Agüera, Z, Fernandez-Aranda, F, et al. The prognostic role of food addiction for weight loss treatment outcomes in individuals with overweight and obesity: a systematic review and meta-analysis. Obes Rev. (2025) 26:e13851. doi: 10.1111/obr.13851
43. Reche-García, C, Piernas, C, García-Vizcaíno, EM, Lorente-Gallego, AM, Piuvezam, G, Frutos, MD, et al. Bariatric-metabolic surgery is the most effective intervention in reducing food addiction symptoms: a systematic review and meta-analysis. Obes Surg. (2024) 34:3475–92. doi: 10.1007/s11695-024-07107-6
44. Bach, P, Grosshans, M, Koopmann, A, Pfeifer, AM, Vollstädt-Klein, S, Otto, M, et al. Predictors of weight loss in participants with obesity following bariatric surgery - a prospective longitudinal fMRI study. Appetite. (2021) 163:105237. doi: 10.1016/j.appet.2021.105237
45. Meneguzzo, P, Behrens, SC, Favaro, A, Tenconi, E, Vindigni, V, Teufel, M, et al. Body image disturbances and weight Bias after obesity surgery: semantic and visual evaluation in a controlled study, findings from the BodyTalk project. Obes Surg. (2021) 31:1625–34. doi: 10.1007/s11695-020-05166-z
46. Westermann, S, Rief, W, Euteneuer, F, and Kohlmann, S. Social exclusion and shame in obesity. Eat Behav. (2015) 17:74–6. doi: 10.1016/j.eatbeh.2015.01.001
47. Tsouristakis, AI, Febres, G, McMahon, DJ, Tchang, B, Conwell, IM, Tsang, AJ, et al. Long-term modulation of appetitive hormones and sweet cravings after adjustable gastric banding and Roux-en-Y gastric bypass. Obes Surg. (2019) 29:3698. doi: 10.1007/s11695-019-04111-z
48. Wong, E, Fleishman, A, Brem, A, Jones, DB, and Wee, CC. High-risk alcohol use and disordered eating behavior before and 1 year after sleeve gastrectomy. BMC Med. (2022) 32:593–8. doi: 10.1007/s11695-021-05847-3
49. Al-Najim, W, Docherty, NG, and le Roux, CW. Food intake and eating behavior after bariatric surgery. Physiol Rev. (2018) 98:1113–41. doi: 10.1152/physrev.00021.2017
50. Wong, LY, Zafari, N, Churilov, L, Stammers, L, Price, S, Ekinci, EI, et al. Change in emotional eating after bariatric surgery: systematic review and meta-analysis. BMC Med. (2020) 4:995–1014. doi: 10.1002/bjs5.50318
Keywords: food addiction, bariatric surgery, YFAS 2.0, mixed linear model, cluster analysis
Citation: Ban X-Y, Wang C, Xu T, Liu L, Yin C-Y, Zhang H-L, Zhang H-W, Han X-D, Zheng H and Di J-Z (2025) When bariatric surgery reduces food addiction: a prospective study. Front. Nutr. 12:1535911. doi: 10.3389/fnut.2025.1535911
Edited by:
Mona Vintilă, West University of Timișoara, RomaniaReviewed by:
Marcela Gomes Reis, Faculty of Medical Sciences of Minas Gerais, BrazilAnwar Abouelnasr, Alexandria University, Egypt
Copyright © 2025 Ban, Wang, Xu, Liu, Yin, Zhang, Zhang, Han, Zheng and Di. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao-Dong Han, MTg5MzAxNzI5NDFAMTYzLmNvbQ==; Hui Zheng, emguZG10ckBnbWFpbC5jb20=; Jian-Zhong Di, ZGlqaWFuemhvbmdAc2p0dS5lZHUuY24=
†These authors have contributed equally to this work
‡ORCID: Hui Zheng, orcid.org/0000-0001-9735-5657
 Xu-Yan Ban
Xu-Yan Ban Chen Wang
Chen Wang Ting Xu
Ting Xu Lin Liu
Lin Liu Chuan-Yu Yin1,3
Chuan-Yu Yin1,3 Hui Zheng
Hui Zheng Jian-Zhong Di
Jian-Zhong Di