Abstract
Background:
The ketogenic diet, characterized by high fat, moderate protein, and extremely low carbohydrate intake, has been widely used as a medical treatment for various conditions and has gained increasing attention in recent years due to its health benefits.
Objectives:
This study aims to investigate the effectiveness of a ketogenic diet on outcomes in cancer patients compared to conventional non-ketogenic diets.
Materials and methods:
Studies that assigned cancer patients to either a ketogenic diet or a standard diet control group were included. Two reviewers independently extracted and analyzed the data.
Results:
This meta-analysis revealed that the ketogenic diet significantly reduced fat mass, visceral fat, insulin levels, blood glucose, fatigue, and insomnia compared to a non-ketogenic diet while improving low-density lipoprotein (LDL) cholesterol, total cholesterol, thyroid-stimulating hormone (TSH) levels, protein uptake, ketosis events, emotional function, and social function. Furthermore, the ketogenic diet induced ketosis by increasing β-hydroxybutyrate levels.
Conclusion:
The ketogenic diet was found to improve cancer patients’ outcomes more effectively than non-ketogenic diets. Notably, C-reactive protein levels showed greater improvement when the intervention lasted more than 12 weeks, with a diet composition of 2–4% carbohydrates, 16–18% protein, and 80–85% fat.
Systematic review registration:
(https://www.crd.york.ac.uk/PROSPERO/view/CRD42024553878) PROSPERO CRD4202455387.
Introduction
The World Health Organization (WHO) defines cancer as a group of diseases characterized by the uncontrolled growth of abnormal cells. According to global cancer data (1), there were 19.98 million new cancer cases in 2022, with a significant increase in less developed countries (2). Lung, breast, and colorectal cancers were the most common types (3). Cancer places a substantial burden on healthcare systems and is the second leading cause of death worldwide, making it one of the most pressing public health challenges (4).
Chemotherapy, radiotherapy, and surgery are the mainstays of conventional cancer treatments, but they often cause significant side effects (5). Recently, increasing attention has been directed toward the ketogenic diet (KD), a high-fat, low-carbohydrate diet, for its potential role in managing various types of cancer (6–9). Studies have suggested that KD may inhibit tumor growth by altering cellular metabolism and improve the tolerance of normal cells to radiotherapy and chemotherapy (10). Furthermore, KD may enhance the effectiveness of PD-1 blockade, a type of immunotherapy (11).
Several controlled clinical trials (CCTs) have investigated the effectiveness of KD in cancer treatment, with a focus on cancers such as glioma and breast cancer (12–18). These trials have demonstrated that KD may inhibit tumor progression by modulating metabolic pathways and enhancing the efficacy of standard treatments, such as chemotherapy and radiotherapy. Additionally, KD has been reported to improve patient tolerance to these therapies, reducing side effects. Based on these findings, researchers suggest that KD could be a safe and effective complementary therapy in cancer treatment, providing a potential new avenue for improving patient care. Despite ongoing debates about its overall effectiveness, the ketogenic diet continues to be explored as a potential adjuvant therapy in oncology.
The traditional KD, characterized by a 4:1 ketogenic ratio, is composed of approximately 90% fat, 8% protein, and 2% carbohydrates. However, several studies have suggested that a low-carbohydrate ketogenic diet (LCKD), with a composition of 70–80% fat, 5–10% carbohydrates, and 10–20% protein, may offer even greater benefits (19). More robust evidence is needed to identify the optimal KD composition for maximizing therapeutic outcomes in cancer treatment.
This meta-analysis aims to address this gap by collecting data from a limited number of studies examining KD as an adjuvant therapy in cancer patients. The analysis focuses on evaluating the significance of KD’s effects on body composition, lipid profile, immunologic factors, internal secretion, liver and kidney function, dietary intake, quality of life, and other factors such as ketosis events and adverse reactions. Additionally, we aim to explore how patients’ age, intervention duration, and dietary intervention ratios impact KD’s anti-tumor effects through subgroup analyses, providing further insights into its therapeutic potential.
Materials and methods
This systematic review was registered with PROSPERO (CRD 42024553878) and conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (20).
Search strategy and inclusion criteria
Medical Subject Headings (MeSH) and text words related to KD and cancer were used to identify the included studies. The search strategy was executed across six databases (PubMed, Web of Science, EMBASE, CINAHL, Medline, and the Cochrane Library) from inception through June 2024 (Supplementary Table). To ensure comprehensive coverage, a manual search of references was also conducted to identify potentially eligible studies. Screening and study selection were conducted independently by two authors (M Zhang and M Peng).
Studies were selected based on the PICOS principles (Table 1). Two authors (M. Zhang and M. Peng) independently reviewed the titles and abstracts of the selected articles, without being blinded to the authors or article titles. Full-text articles deemed potentially eligible were subsequently retrieved for further assessment. Any disagreements that arose during the selection process were resolved through consensus (Q. Zhang), with decisions made based on predefined inclusion and exclusion criteria.
Table 1
| Study participants (P) | Patients diagnosed with any kind of cancer or tumor. |
| Intervention (I) | Studies that used KD as an intervention. |
| Control (C) | A regular or standard diet in the control group. |
| Outcomes (O) | Body composition (fat mass, visceral fat mass, skeletal muscle mass, body cell mass), lipid profile (HDL cholesterol, LDL cholesterol, triglycerides, total cholesterol), immunologic factors (insulin, blood glucose, C-reactive protein, IGF-1, TNF-α, IL-10,serum β-hydroxybutyrate), internal secretion (TSH, FT3), liver and kidney function (GGT, creatinine, urea), diet amount(energy uptake, protein uptake, time to exhaustion), quality of life (quality of life score, emotional function, fatigue, insomnia, social function, future perspective, systemic therapy side effects) and other aspects (ketosis event, adverse event). |
| Study design (S) | Contrary to our original study protocol specification to only consider RCTs, we more generally decided to consider non-RCTs for meta-analysis, because the randomization is not always practical in certain cancer patients who might have their own diet preferences. |
Inclusion and exclusion criteria.
Data extraction and quality assessment
The two authors (M. Zhang and M. Peng) independently extracted data following the PICOS principle. Any discrepancies were resolved through discussion with the third author (Q Zhang), and decisions were made based on predefined inclusion/exclusion criteria and methodological quality considerations. The extracted data included the first author, publication year, study design, age of participants, cancer type, intervention and control diets, number of participants, duration of intervention, and outcome. Numerical data from the figures were extracted using Engauge Digitizer. When outcomes were reported in different formats, the results were standardized (mean ± standard deviation).
The risk of bias was assessed in accordance with the Cochrane Handbook guidelines, with a focus on key areas, including selection bias (21, 22). Discrepancies were resolved through discussion. Additionally, the quality of evidence was rated as high, moderate, low, or very low using the GRADE approach, which evaluates studies based on risk of bias, consistency, directness, precision, and publication bias (23).
Statistical analysis
Meta-analyses were conducted using RevMan software. The Cochran–Mantel–Haenszel method was applied for categorical data, and the inverse-variance method was used for continuous outcomes. To ensure consistency, data reported as medians, means, standard deviations (SD), or interquartile ranges (IQR) were converted to mean ± SD using established formulas (24–26). For studies reporting standard errors of the mean (SEM), SDs were calculated using the formula: SD = SEM × √N (27).
Effect sizes were expressed as standardized mean differences (SMDs) with 95% confidence intervals (CIs). Heterogeneity among studies was assessed using the I2 statistic. A random-effects model was used if I2 > 50%; otherwise, a fixed-effects model was applied. In cases of substantial heterogeneity, sensitivity analyses were performed by examining individual studies. Statistical significance was set at a p-value of < 0.05.
Result
Study selection
The initial database search yielded 1,820 citations. After removing duplicates, 1,496 articles remained for title and abstract screening. Of these, 122 full-text articles were selected for further evaluation. Ultimately, 108 articles were excluded: 48 were in vitro or animal studies, 55 had inappropriate designs, and five studies were excluded due to outcomes data not meeting requirements (n = 3) or being protocols (n = 2). A total of 14 clinical trials and 16 publications were included (12, 13, 15, 16, 18, 28–36). Figure 1 describes the literature search and study selection process.
Figure 1
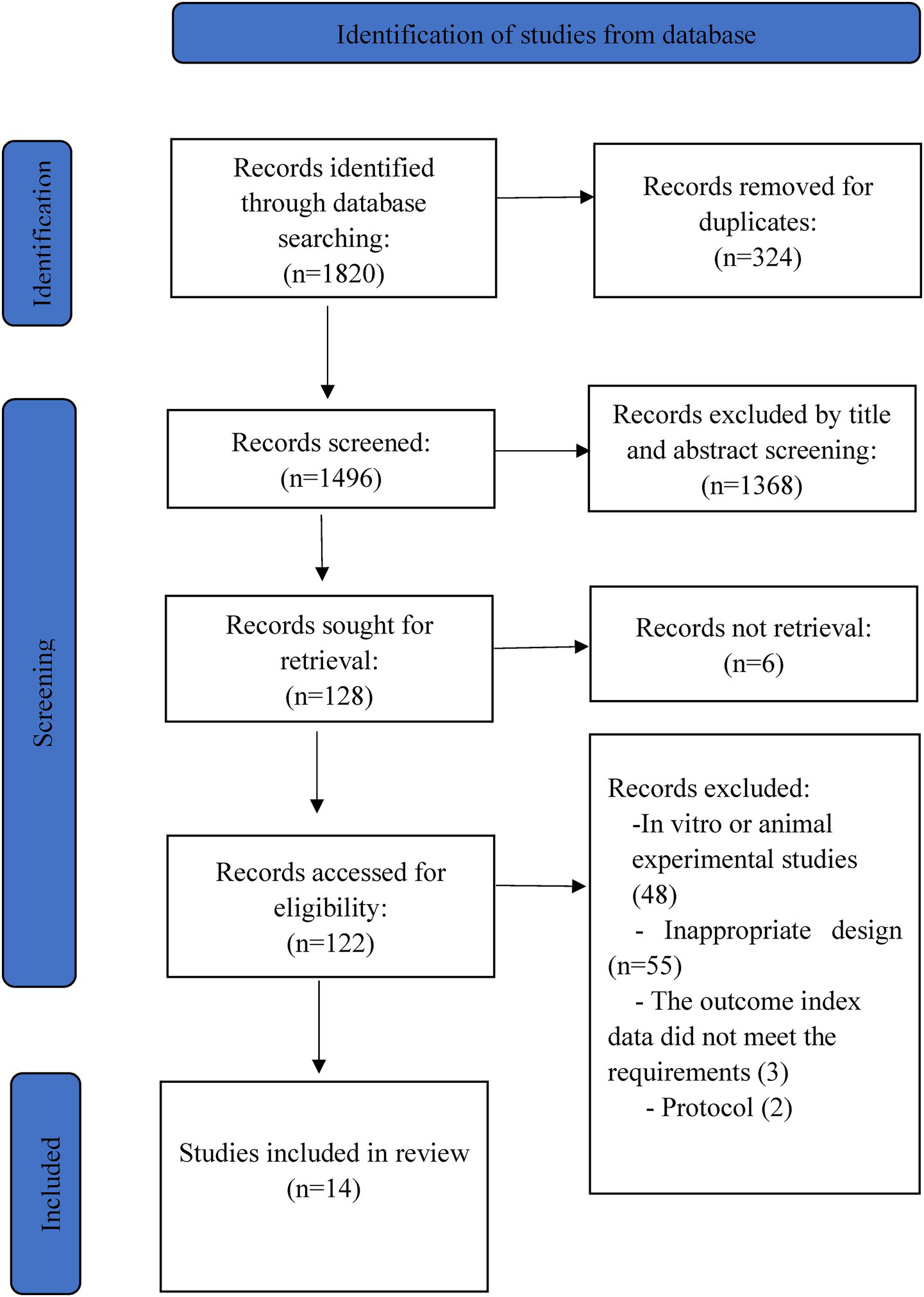
PRISMA flow chart.
Study characteristics
The included studies were conducted in Germany (n = 5) (13, 15, 16, 29, 30), the USA (n = 4) (18, 28, 32, 33), Iran (n = 3) (12), Korea (n = 2) (31, 34), Indonesia (n = 1) (35), and Brazil (n = 1) (36). The publication dates ranged from 2018 to 2020. A total of 11 trials were RCTs (12, 18, 28–33, 35), while five trials were non-RCTs (13, 15, 16, 34, 36), with the follow-up periods ranging from 6 days to 24 weeks. In this review, a KD was compared with either a standard or normal diet (12, 13, 15, 16, 29–31, 36) or other dietary approaches, including a low-carb diet (13), a general hospital diet (18, 33–35), or the American Cancer Society diet (28, 32).
In one study (13), the control group consisted of two separate groups: a standard group and a low-carbohydrate diet group. Participants across the included studies had various types of cancer, such as breast cancer (12, 13, 15), glioma (29, 30, 36), ovarian/endometrial cancer (29, 30), pancreatic biliary tract cancer (31, 34), carcinoma of the rectum (16), colorectal cancer (35). General characteristics are presented in Table 2.
Table 2
| General information | Data | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Country | Design | Type of cancer | Concurrent Treatment | Age (mean ± sd) years (I/C) | Sex, Participants Number of I/C | Intervening Measure | Duration(Week or day) | Outcome | Intervention | Control | ||||||
| T | C | T | C | Mean | SD | Number | Mean | SD | Number | ||||||||
| Cohen et al. (28) | USA | RCT | Ovarian or Endometrial cancer | Chemotherapy | 61.5 ± 8.5/58.6 ± 11.7 | F 25 | F 20 | KD:5% carbohydrate (≤20 g/d),25% protein (≤100 g/d),70%fat (≥125 g/d) | ACS: high-fiber, low-fat | 12 weeks | Fat mass | 32.7 | 14.9 | 25 | 41.2 | 19.6 | 20 |
| Visceral fat mass | 975 | 754.5 | 25 | 1,024 | 785.31 | 20 | |||||||||||
| Insulin | 6.7 | 4.1 | 23 | 12.1 | 6.7 | 20 | |||||||||||
| β-hydroxybutyrate | 0.91 | 0.73 | 23 | 0.25 | 0.18 | 20 | |||||||||||
| Blood glucose | 93 | 15.83 | 23 | 98.75 | 11.8 | 20 | |||||||||||
| CRP | 2 | 1.44 | 23 | 3 | 1.34 | 20 | |||||||||||
| IGF-1 | 100.7 | 44 | 23 | 111 | 59.9 | 20 | |||||||||||
| Voss et al. (29, 30) | Germany | RCT | Glioblastoma or Gliosarcoma | Chemotherapy | 57.74 ± 8.15/55.50 ± 11.45 | M/F 20 | M/F 20 | KD-IF: Carbohydrate limit is 50 g/day. The patient fasted on day 4 with 6 unlimited fluid intake. From day 10, patients no longer have dietary restrictions. | SD: 30 kcal/kg (about 60–80 g fat, 5 g /kg carbohydrate and 0.8 g /kg protein) | 6 days | Insulin | 6.9 | 3.7 | 22 | 17.25 | 19.17 | 18 |
| IGF-1 | 179.9 | 79.7 | 22 | 219.6 | 104.3 | 15 | |||||||||||
| Urea | 30.67 | 8.4 | 20 | 30.36 | 9.67 | 21 | |||||||||||
| Kang et al. (31) | Korea | RCT | Pancreatic biliary carcinoma | Operation | 58.3 ± 7.6/66.3 ± 9.8 | M 5\u00B0F 4 | M 6\u00B0F 3 | LCKD:80% kcal from fat, ketogenic ratio of 1.75:1 | GD | 4 weeks | TNF-α | 498.3 | 813.3 | 9 | 666.52 | 829.14 | 9 |
| Creatinine | 232 | 93.5 | 9 | 150.2 | 49.3 | 9 | |||||||||||
| β-hydroxybutyrate | 0.29 | 0.17 | 9 | 0.27 | 0.15 | 9 | |||||||||||
| Ketone body | 1.67 | 0.952 | 9 | 2.3 | 1.59 | 9 | |||||||||||
| Insulin | 11.58 | 10.52 | 9 | 6.14 | 2.63 | 9 | |||||||||||
| Blood glucose | 132.14 | 246.43 | 9 | 106.25 | 77.68 | 9 | |||||||||||
| Cohen et al. (32) | USA | RCT | Ovarian or Endometrial cancer | Chemotherapy | 61.5 ± 8.5/58.6 ± 11.7 | F 25 | F 20 | KD:70% fat, 25% protein, 5% carbohydrate | ACS: high-fiber, low-fat | 12 weeks | Total Cholesterol | 214 | 60 | 23 | 208 | 44 | 20 |
| HDL-Cholesterol | 72 | 13 | 23 | 65 | 15 | 20 | |||||||||||
| LDL-Cholesterol | 123 | 55 | 23 | 119 | 40 | 20 | |||||||||||
| Triglycericle(TG) | 97 | 33 | 23 | 111 | 46 | 20 | |||||||||||
| Energy uptake | 1,239 | 304 | 23 | 1,533 | 304 | 15 | |||||||||||
| Protein uptake | 29 | 0.7 | 23 | 18.5 | 1 | 15 | |||||||||||
| Khodabakhshi et al. (46) | Iran | RCT | Breast cancer | Chemotherapy | 44.8 ± 8.4/45.2 ± 15.0 | M/F 30 | M/F 30 | KD of medium chain triglyceride (MCT) (containing 6% caloric content from CHO, 19% protein, 20% MCT, 55% fat) | SD | 12 weeks | Fat mass | 29.1 | 7.1 | 30 | 30.8 | 7.5 | 30 |
| Blood glucose | 84.5 | 11.3 | 30 | 105.2 | 15.8 | 30 | |||||||||||
| Insulin | 5.7 | 4 | 30 | 6.9 | 4.5 | 30 | |||||||||||
| IGF-1 | 133 | 61 | 30 | 150 | 48 | 30 | |||||||||||
| Ketone body | 0.923 | 0.699 | 30 | 0.007 | 0.026 | 30 | |||||||||||
| Creatinine | 0.77 | 0.09 | 30 | 0.86 | 0.15 | 30 | |||||||||||
| TNF-α | 18 | 8.6 | 30 | 17.3 | 7.3 | 30 | |||||||||||
| Augustus et al. (33) | Trinidad | RCT | Breast cancer/Prostate/Colon/Rectum/Lung cancer/Cervix | Chemotherapy | 49.80 ± 6.72/51.80 ± 4.18 | M/F 17 | M/F 20 | KD = approximately 10% CHO, 15% Proteins, and 75% Fats | Usual diet | 16 weeks | Blood glucose | 74.8 | 6.204 | 17 | 84.4 | 7.358 | 20 |
| QoL score | 36.85 | 7.58 | 17 | 60.45 | 14.9 | 20 | |||||||||||
| Khodabakhshi et al. (12, 47) | Iran | RCT | Breast cancer | Chemotherapy | 44.8 ± 8.4/45.2 ± 15.0 | M/F 30 | M/F 30 | KD of medium chain triglyceride (MCT) (containing 6% caloric content from CHO, 19% protein, 20% MCT, 55% fat) | SD:55% CHO, 15% protein and 30% fat | 12 weeks | Tumor size | 27 | 25 | 30 | 34 | 26 | 30 |
| Energy uptake | 154.32 | 141.89 | 30 | 1603.8 | 65.68 | 30 | |||||||||||
| Protein uptake | 57.12 | 5.15 | 30 | 69.95 | 6.37 | 30 | |||||||||||
| CRP | 12 | 13 | 30 | 14.3 | 14 | 30 | |||||||||||
| IL-10 | 11.1 | 4.7 | 30 | 10.1 | 4.3 | 30 | |||||||||||
| 6 weeks | TNF-α | 19 | 9.1 | 30 | 16.4 | 6 | 30 | ||||||||||
| Insulin | 6.1 | 6.7 | 30 | 9.1 | 9.6 | 30 | |||||||||||
| CRP | 11 | 13 | 30 | 18.6 | 18 | 30 | |||||||||||
| IL-10 | 10.6 | 4.5 | 30 | 10 | 4.5 | 30 | |||||||||||
| QoL score | 75 | 20 | 30 | 62 | 20 | 30 | |||||||||||
| Emotional function | 62 | 23 | 30 | 60 | 21 | 30 | |||||||||||
| Social function | 91 | 17 | 30 | 87 | 17 | 30 | |||||||||||
| Fatigue | 33.95 | 8.82 | 30 | 35.62 | 5.39 | 30 | |||||||||||
| Insomnia | 4.88 | 8.88 | 30 | 31.1 | 12.25 | 30 | |||||||||||
| Future perspective | 62.08 | 8.09 | 30 | 40.97 | 16.42 | 30 | |||||||||||
| Systemic therapy side effects | 40.57 | 7.84 | 30 | 42.12 | 4.66 | 30 | |||||||||||
| IGF-1 | 140 | 63 | 30 | 136 | 34 | 30 | |||||||||||
| Ok et al. (34) | Korea | NRCT | Pancreatobiliary cancer after pancreatectomy | Pancreatectomy | 57.8 ± 7.3/66.3 ± 9.8 | M/F 10 | M/F 9 | KD: Carbohydrate: Protein: Fat = 3 ~ 6: 15 ~ 25:70 ~ 80 ketogenic ratio of 1.05 ~ 1.75:1 | Carbohydrate: Protein: Fat = 55–65:7–20:15–30 | 10 days | Fat mass | 14.2 | 6.4 | 8 | 17.1 | 4.9 | 9 |
| Triglycericle (TG) | 125.7 | 49.8 | 10 | 110.5 | 51.1 | 9 | |||||||||||
| HDL | 38.4 | 8.8 | 10 | 42.4 | 6.9 | 9 | |||||||||||
| LDL | 108.4 | 28.3 | 10 | 97.4 | 41.9 | 9 | |||||||||||
| Total cholesterol (TC) | 173.8 | 32.1 | 10 | 156 | 48.4 | 9 | |||||||||||
| CRP | 25.6 | 36.6 | 10 | 10.0 | 11.8 | 9 | |||||||||||
| LOS | 12 | 5.8 | 10 | 15.8 | 11.2 | 9 | |||||||||||
| Average meal compliance | 69.1 | 19.6 | 10 | 33.9 | 16.6 | 9 | |||||||||||
| Energy uptake | 61.31 | 19 | 10 | 38.5 | 21.9 | 9 | |||||||||||
| Dietary overall satisfaction | 6.2 | 1.8 | 10 | 3.8 | 1.1 | 9 | |||||||||||
| Urine acetone bodies | 50.8 | 35.1 | 10 | 22.2 | 23.7 | 9 | |||||||||||
| Kämmerer et al. (13) | Germany | NRCT | Breast cancer | Neoadjuvant chemotherapy | 52.5 ± 6.4/51.3 ± 8.1 | F 29 | F 92 | KD: Carbohydrate: Protein: Fat = 2–4%:16–18%:80–85% etogenic ratio of 1.6:1–2:1 | LCD: Carbohydrate: Protein: Fat = 20–30%:20–30%: 40–50% | 20 weeks | Fat mass | 19.5 | 6.91 | 19 | 24.31 | 7.00 | 70 |
| Visceral fat mass | 8.5 | 4.36 | 19 | 12.15 | 5.78 | 70 | |||||||||||
| Skeletal muscle mass | 40.86 | 3.68 | 19 | 41.97 | 5.38 | 70 | |||||||||||
| BCM: body cell mass | 22.02 | 2.19 | 19 | 22.57 | 3.21 | 70 | |||||||||||
| HDL | 75.83 | 22.21 | 19 | 67.64 | 13.71 | 70 | |||||||||||
| LDL | 150.59 | 37.38 | 19 | 142.13 | 33.33 | 70 | |||||||||||
| CRP | 2.05 | 2.74 | 19 | 1.60 | 2.17 | 70 | |||||||||||
| Insulin | 12.45 | 6.85 | 19 | 14.7 | 8.43 | 70 | |||||||||||
| IGF-1 | 13.99 | 12.27 | 19 | 6.94 | 3.54 | 70 | |||||||||||
| TSH | 1.83 | 1.3 | 19 | 1.61 | 0.94 | 70 | |||||||||||
| Quality of life score | 70.05 | 22.29 | 20 | 65.57 | 17.39 | 75 | |||||||||||
| Emotional function | 70.52 | 26.76 | 20 | 64.43 | 20.88 | 75 | |||||||||||
| Fatigue | 21.01 | 23.79 | 20 | 35.57 | 20.88 | 75 | |||||||||||
| Insomnia | 38.26 | 26.76 | 20 | 64.43 | 20.88 | 75 | |||||||||||
| Time to exhaution | 8.55 | 1.94 | 19 | 7.66 | 1.65 | 70 | |||||||||||
| Energy uptake | 32.5 | 1.5 | 19 | 24.30 | 0.7 | 70 | |||||||||||
| Protein uptake | 1.33 | 0.07 | 19 | 1.20 | 0.03 | 70 | |||||||||||
| 52.5 ± 6.4/51.3 ± 8.1 | F 29 | F 31 | KD: Carbohydrate: Protein: Fat = 2–4%:16–18%:80–85% etogenic ratio of 1.6:1–2:1 | SD: Carbohydrate: Protein: Fat = 52–62%:16–17%:28–31% | 20 weeks | Fat mass | 19.5 | 6.91 | 19 | 23.84 | 8.14 | 23 | |||||
| Visceral fat mass | 8.5 | 4.36 | 19 | 12.18 | 4.85 | 23 | |||||||||||
| Skeletal muscle mass | 40.86 | 3.68 | 19 | 39.71 | 6.07 | 23 | |||||||||||
| BCM: body cell mass | 22.02 | 2.19 | 19 | 22.14 | 3.86 | 23 | |||||||||||
| HDL | 75.83 | 22.21 | 19 | 65.38 | 12.96 | 23 | |||||||||||
| LDL | 150.59 | 37.28 | 19 | 124.48 | 22.29 | 23 | |||||||||||
| CRP | 2.05 | 2.74 | 19 | 1.96 | 1.94 | 23 | |||||||||||
| Insulin | 12.45 | 6.85 | 19 | 15.82 | 9.25 | 23 | |||||||||||
| IGF-1 | 13.99 | 12.27 | 19 | 7.83 | 5.08 | 23 | |||||||||||
| TSH | 1.83 | 1.3 | 19 | 1.20 | 0.88 | 23 | |||||||||||
| Quality of life score | 70.05 | 22.29 | 20 | 64.45 | 17.13 | 24 | |||||||||||
| Emotional function | 70.52 | 26.76 | 20 | 56.06 | 25.68 | 24 | |||||||||||
| Fatigue | 21.01 | 23.79 | 20 | 48.5 | 22.83 | 24 | |||||||||||
| Insomnia | 38.26 | 26.76 | 20 | 37.8 | 25.68 | 24 | |||||||||||
| Time to exhaution | 8.55 | 1.94 | 19 | 7.50 | 1.68 | 23 | |||||||||||
| Energy uptake | 32.5 | 1.5 | 19 | 26.4 | 1.2 | 23 | |||||||||||
| Protein uptake | 1.33 | 0.07 | 19 | 0.98 | 0.04 | 23 | |||||||||||
| Klement et al. (15) | Germany | NRCT | Breast cancer | Radiotherapy | 52.75 ± 14.14/49.73 ± 12.41 | F 29 | M 30 | KD = 75–80% calories from fat, and limiting carbohydrates to 50 g per day and 10 g per meal | Standard recommendations according to the German Nutrition Society | 12 weeks | GGT | 17.55 | 8.14 | 29 | 20.31 | 9.07 | 30 |
| Creatinine | 0.76 | 0.11 | 29 | 0.84 | 0.12 | 30 | |||||||||||
| Urea | 4.74 | 0.1 | 29 | 4.74 | 0.99 | 30 | |||||||||||
| Total cholesterol (TC) | 220.27 | 47.62 | 29 | 196.92 | 33.08 | 30 | |||||||||||
| HDL | 73.06 | 24.18 | 29 | 67.48 | 15.19 | 30 | |||||||||||
| LDL | 141.26 | 47.38 | 29 | 113.82 | 26.83 | 30 | |||||||||||
| Triglycericle (TG) | 80.24 | 24.43 | 29 | 108.53 | 55.63 | 30 | |||||||||||
| CRP | 3.98 | 5.95 | 29 | 3.34 | 5.27 | 30 | |||||||||||
| IGF-1 | 187.24 | 92.53 | 29 | 186.46 | 63.72 | 30 | |||||||||||
| Insulin | 9.29 | 7.6 | 29 | 8.35 | 4.97 | 30 | |||||||||||
| FT3 | 2.82 | 0.36 | 29 | 3.05 | 0.37 | 30 | |||||||||||
| TSH | 1.76 | 0.97 | 29 | 1.60 | 0.74 | 30 | |||||||||||
| Blood glucose | 103.15 | 16.29 | 29 | 96.76 | 9.31 | 30 | |||||||||||
| β-hydroxybutyrate | 0.67 | 0.55 | 29 | 0.36 | 0.63 | 30 | |||||||||||
| Quality of life score | 83.11 | 28.39 | 29 | 77.1 | 12.03 | 30 | |||||||||||
| Emotional functioning | 75 | 12.34 | 29 | 66.69 | 16.35 | 30 | |||||||||||
| Social functioning | 79.27 | 16.45 | 29 | 66.69 | 16.35 | 30 | |||||||||||
| Fatigue | 34.66 | 19.19 | 29 | 44.18 | 13.63 | 30 | |||||||||||
| Insomnia | 37.25 | 24.68 | 29 | 37.27 | 24.51 | 30 | |||||||||||
| Future perspective | 62.65 | 24.68 | 29 | 62.73 | 24.51 | 30 | |||||||||||
| Systemic therapy side effects | 19.01 | 9.4 | 29 | 24.97 | 15.17 | 30 | |||||||||||
| Klement et al. (16) | Germany | NRCT | Rectal cancer | Radiotherapy or Chemotherapy | 56.75 ± 10.7/62.25 ± 8.55 | M/F 18 | M/F 23 | KD = 75–80% calories from fat, and limiting carbohydrates to 50 g per day and 10 g per meal | Standard recommendations according to the German Nutrition Society | 12 weeks | Blood glucose | 108.73 | 22.51 | 29 | 131.27 | 61.96 | 23 |
| Insulin | 0.89 | 5.52 | 18 | 20.45 | 12.5 | 23 | |||||||||||
| IGF-1 | 135.77 | 47.21 | 18 | 175.72 | 79.33 | 23 | |||||||||||
| Total cholesterol(TC) | 184.26 | 28.54 | 18 | 182.89 | 40.7 | 23 | |||||||||||
| HDL | 64.69 | 14.27 | 18 | 62.79 | 17.89 | 23 | |||||||||||
| LDL | 109.47 | 26.62 | 18 | 103.03 | 35 | 23 | |||||||||||
| Triglycericle(TG) | 81.32 | 58.19 | 18 | 136.9 | 36.55 | 23 | |||||||||||
| β-hydroxybutyrate | 0.86 | 0.61 | 18 | 0.14 | 0.12 | 23 | |||||||||||
| Creatinine | 0.87 | 0.17 | 18 | 0.91 | 0.19 | 23 | |||||||||||
| GGT | 35.15 | 38.42 | 18 | 49.41 | 52.63 | 23 | |||||||||||
| Urea | 5.14 | 0.88 | 18 | 5.93 | 1.93 | 23 | |||||||||||
| CRP | 4.33 | 2.74 | 18 | 12.22 | 18.41 | 23 | |||||||||||
| FT3 | 2.26 | 0.43 | 18 | 3.00 | 0.47 | 23 | |||||||||||
| TSH | 6.21 | 9.17 | 18 | 2.79 | 3.00 | 32 | |||||||||||
| QoL score | 75.7 | 11.61 | 18 | 64.28 | 22.04 | 23 | |||||||||||
| Emotional function | 74.2 | 18.57 | 18 | 64.28 | 22.04 | 23 | |||||||||||
| Social function | 83.32 | 9.27 | 18 | 53.98 | 26.46 | 23 | |||||||||||
| Fatigue | 36.9 | 24.75 | 18 | 38.14 | 26.46 | 23 | |||||||||||
| Insomnia | 33.32 | 18.57 | 18 | 70.78 | 17.81 | 23 | |||||||||||
| Budipramana et al. (35) | Indonesia | RCT | Stage-IV colorectal adenocarcinoma | Operation | 40–65 range/17–65 range | M/F 12 | M/F 12 | Very low carbohydrate diet: Carbohydrate: Protein+Fat = 20%:80% | Uommon patients requirement | 3 weeks | Creatinine | 0.87 | 0.19 | 12 | 0.72 | 0.21 | 12 |
| CRP | 3.82 | 0.46 | 12 | 2.96 | 0.46 | 12 | |||||||||||
| Freedland et al. (18) | USA | RCT | Recurrent prostate cancer | Radiotherapy | 71.25 ± 1.47/70.76 ± 2.56 | M 27 | M 18 | The LCD = limit carbohydrate intake to ≤20 grams/day | Usual diet | 24 weeks | Triglycericle(TG) | 125.61 | 17.78 | 27 | 95.64 | 13.45 | 18 |
| HDL | 46.5 | 3 | 27 | 49.87 | 6.31 | 18 | |||||||||||
| LDL | 100 | 12.52 | 27 | 89.31 | 8.23 | 18 | |||||||||||
| Blood glucose | 98.63 | 3.76 | 27 | 106.53 | 5.21 | 18 | |||||||||||
| CRP | 2.18 | 0.58 | 27 | 2.41 | 0.78 | 18 | |||||||||||
| Santos et al. (36) | Brazil | NRCT | Recurrent glioblastoma | Radiotherapy or Chemotherapy | 49.5 ± 8.64/44.5 ± 8.06 | M/F 9 | M/F 8 | KD: Carbohydrate: Protein: Fat = 25%: 50%: 25% | SD | 12 weeks | Total cholesterol(TC) | 220.7 | 45.5 | 9 | 192.6 | 48 | 8 |
| LDL | 122.1 | 27.9 | 9 | 116.5 | 36 | 8 | |||||||||||
| Triglyceride (TG) | 154.4 | 93.3 | 9 | 99.1 | 42 | 8 | |||||||||||
Study characteristics and effect of ketogenic diet on outcomes of cancer patients.
RCT:randomized controlled trial; NRCT:non randomized controlled trial; ACS: American Cancer Society diet; KD: ketogenic diet; LCD: Low carb diet; SD: standard diet; GD: general hospital diet; CRP: C-reactive protein; FT3: free triiodothyronine; TSH: Thyroid Stimulating Hormone; TNF-α:tumor necrosis factor; GGT: gamma-glutamyl transpeptidase.
Assessment of the risk of bias
Eight trials included in the meta-analysis were rated as good quality, while four were considered poor quality and two were rated as fair. Seven trials showed a high risk of bias in random sequence generation, and five had significant bias in participant allocation concealment. Blinding was not possible due to the nature of the intervention, so it was excluded from the overall quality evaluation. Outcome assessment blinding was unclear in three studies. Additionally, three trials addressed incomplete outcome data, one had unclear selective reporting bias, and another one had an unspecified bias (Table 3).
Table 3
| General information | Data | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Country | Design | Type of cancer | Concurrent Treatment | Age (mean ± sd) years (I/C) | Sex, Participants Number of I/C | Intervening Measure | Duration (Week or day) | Outcome | Intervention | Control | ||||||
| T | C | T | C | Mean | SD | Number | Mean | SD | Number | ||||||||
| Cohen et al. (28) | USA | RCT | Ovarian or Endometrial cancer | Chemotherapy | 61.5 ± 8.5/58.6 ± 11.7 | F 25 | F 20 | KD:5% carbohydrate (≤20 g/d),25% protein (≤100 g/d),70%fat (≥125 g/d) | ACS: high-fiber, low-fat | 12 weeks | Ketosis Event | occurred | not occurred | 13/10 | occurred | not occurred | 2/18 |
| Cohen et al. (32) | USA | RCT | Ovarian or Endometrial Cancer | Chemotherapy | 61.5 ± 8.5/58.6 ± 11.7 | F 25 | F 20 | KD:5% carbohydrate (≤20 g/d),25% protein (≤100 g/d),70%fat (≥125 g/d) | ACS: high-fiber, low-fat | 12 weeks | Adverse Event | occurred | not occurred | 13/2 | occurred | not occurred | 4/96 |
| Voss et al. (29, 30) | Germany | RCT | Glioblastoma or Gliosarcoma | Radiotherapy | M/F 20 | M/F 20 | KD-IF: Carbohydrate limit is 50 g/day. The patient fasted on day 4 with 6 unlimited fluid intakes. From day 10, patients no longer have dietary restrictions. | SD: 30 kcal/kg (about 60–80 g fat, 5 g /kg carbohydrate and 0.8 g /kg protein) | 18 days | Adverse Event | occurred | not occurred | 5/20 | occurred | not occurred | 6/19 | |
| Ok et al. (34) | Korea | NRCT | Pancreatobiliary cancer after pancreatectomy | Pancreatectomy | 57.8 ± 7.3/66.3 ± 9.8 | M/F 10 | M/F 9 | KD: Carbohydrate: Protein: Fat = 3 ~ 6: 15 ~ 25:70 ~ 80 ketogenic ratio of 1.05 ~ 1.75:1 | Carbohydrate: Protein: Fat = 55–65:7–20:15–30 | 3 days | Ketosis Event | occurred | not occurred | 7/3 | occurred | not occurred | 2/7 |
| 10 days | Ketosis Event | occurred | not occurred | 5/5 | occurred | not occurred | 2/7 | ||||||||||
Study characteristics and effect of ketogenic diet on outcomes of cancer patients.
RCT: randomized controlled trial; NRCT: non randomized controlled trial; ACS: American Cancer Society diet; SD: standard diet.
Primary patient outcomes
When the included studies categorized participants into three groups—one KD group and two control groups—we treated this as data from two distinct groups. Furthermore, when a single study reported outcomes for the KD group and the control group at different intervention durations, we considered these as separate datasets (Table 4).
Table 4
| Study | Random sequence generation | Allocation concealment | Blinding | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias | Score | Overall quality |
|---|---|---|---|---|---|---|---|---|---|
| Augustus et al. (33) | + | − | − | − | ? | + | + | 3 | Fair |
| Budipramana et al. (35) | + | − | − | − | + | + | + | 4 | Good |
| Cohen et al. (28) | + | + | − | ? | + | + | + | 5 | Good |
| Cohen et al., (32) | + | + | − | ? | ? | + | + | 4 | Good |
| Freedland et al. (18) | + | + | − | − | + | + | + | 5 | Good |
| Kämmerer et al. (13) | − | − | − | − | ? | + | ? | 1 | Poor |
| Kang et al. (31) | + | ? | − | − | + | + | + | 4 | Good |
| Khodabakhshi et al. (12) | + | + | − | − | + | + | + | 5 | Good |
| Khodabakhshi et al. (12) | + | + | − | − | + | + | + | 5 | Good |
| Klement et al. (15) | − | − | − | − | + | + | + | 3 | Poor |
| Klement et al. (16) | − | − | − | − | + | + | + | 3 | Poor |
| Ok et al. (34) | + | ? | − | − | + | ? | − | 2 | Poor |
| Santos et al. (36) | ? | ? | − | ? | + | + | + | 3 | Fair |
| Voss et al. (29) | + | ? | − | − | + | + | + | 4 | Good |
Study quality and risk of bias assessment using the Cochrane collaboration’s tool.
Body composition
Fat mass
Of the 14 articles included, four reported post-intervention fat mass. A fixed-effects model showed a significant reduction (SMD = −0.48; 95% CI: −0.75 to −0.22; I2 = 0%), indicating extremely low heterogeneity. The overall effect was statistically significant, with a p-value of < 0.001 (Figure 2).
Figure 2
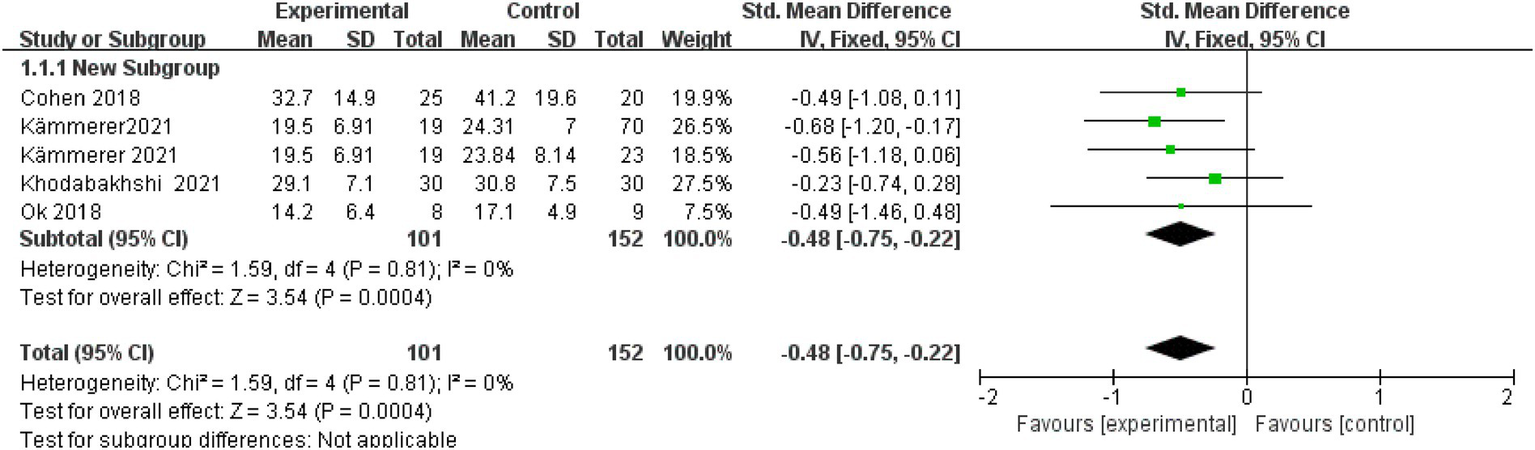
Fat mass.
Visceral fat mass
Two studies (with a total of 176 patients) reported that KD reduces visceral fat mass in cancer patients (SMD = −0.50, 95%CI: −0.83 to −0.17, p = 0.003) (Figure 3). Heterogeneity was mild (Q = 3.22, p = 0.200; I2 = 38%).
Figure 3
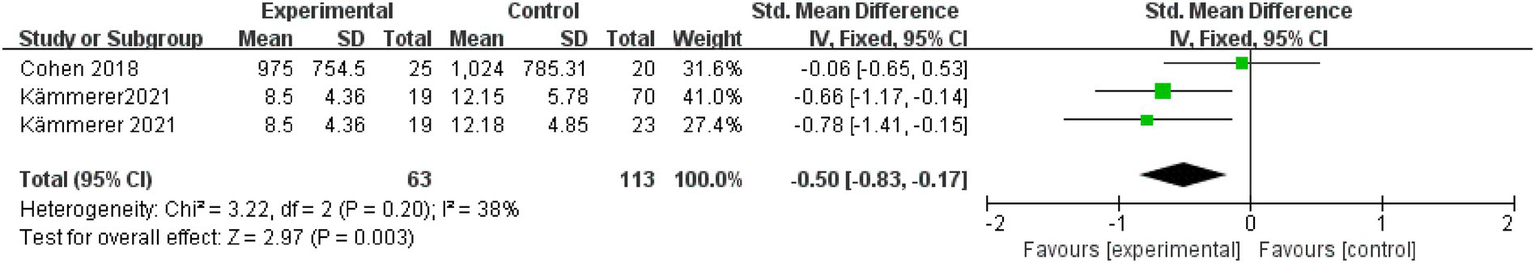
Visceral fat mass.
Blood constituent
LDL-cholesterol
Seven articles were assessed for LDL cholesterol. A fixed-effects model was applied after the intervention to examine the change in LDL cholesterol level, with an SMD of 0.46 (95% CI: 0.24 to 0.68), I2 = 13%, indicating low heterogeneity. The overall effect was statistically significant, with a p-value of < 0.001 (Figure 4).
Figure 4
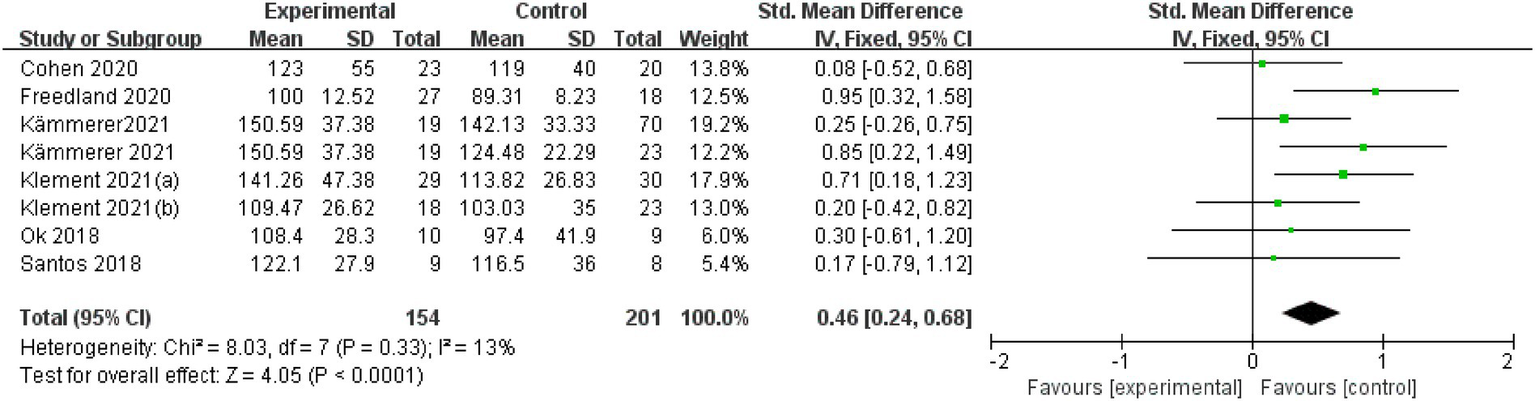
LDL-Cholesterol.
Total cholesterol (TC)
Four studies (patients’ number = 136) reported that KD reduces TC in cancer patients (SMD = 0.38, 95% CI: 0.04 to 0.72; p = 0.030) (Figure 5). Heterogeneity was low (Q = 1.82; p = 0.610; I2 = 0%).
Figure 5
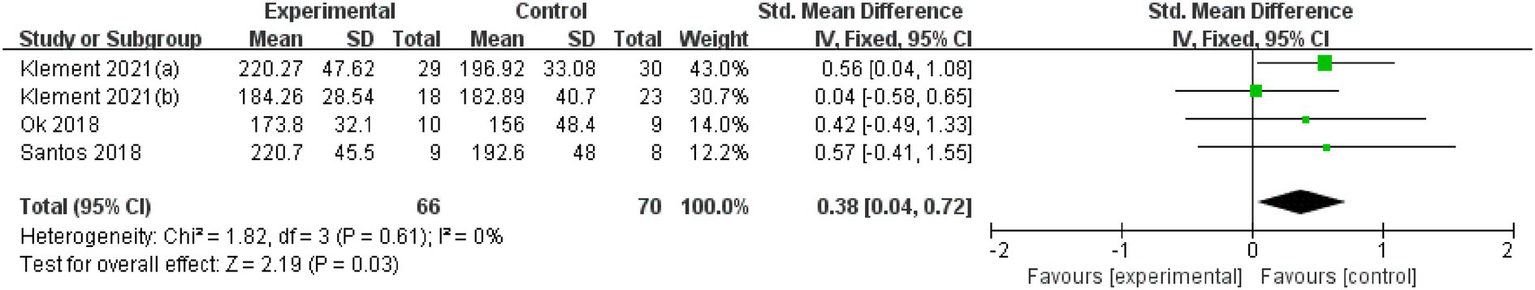
Total cholesterol (TC).
Secondary patient outcome
Immune-related index
Insulin
Eight articles assessed insulin levels. Post-intervention, a random-effects model yielded an SMD of −0.46 (95% CI: −0.85 to −0.08; I2 = 73%), indicating high heterogeneity. The overall effect was p = 0.020 (Supplementary Figure 1).
Blood glucose
Seven studies (involving 314 patients) reported that KD reduces blood glucose in cancer patients (SMD = −0.70, 95% CI: −1.35 to −0.05; p = 0.030) (Supplementary Figure 2). Heterogeneity was high (Q = 0.65; p < 0.001; I2 = 86%).
β-hydroxybutyrate
Four studies (with a total of 161 patients) reported that KD reduces β-hydroxybutyrate in cancer patients (SMD = 0.90, 95% CI: 0.26 to 1.54; p = 0.006) (Supplementary Figure 3). Heterogeneity was high (Q = 0.29; p = 0.020; I2 = 71%).
Internal secretion
Thyroid-stimulating hormone (TSH)
Three studies (patients’ number = 231) reported that KD reduces TSH in cancer patients (SMD = 0.34, 95% CI: 0.06 to 0.62; p = 0.020) (Supplementary Figure 4). Heterogeneity was low (Q = 1.43; p = 0.700; I2 = 0%).
Uptake
Three studies (with a total of 229 patients) reported that KD increases protein uptake in cancer patients (SMD = 4.67, 95% CI: 0.24 to 9.09; p < 0.001) (Supplementary Figure 5). Heterogeneity was low (Q = 19.64; p = 0.040; I2 = 0%).
Quality of life (QOL)
Emotional function
Four studies (patients’ number = 299) reported that KD enhances emotional function in cancer patients (SMD = 0.37, 95% CI: 0.12 to 0.61; p = 0.003) (Supplementary Figure 6). Heterogeneity was low (Q = 0.28, p = 0.680; I2 = 0%).
Fatigue
Four studies (patients’ number = 299) reported that KD reduces fatigue in cancer patients (SMD = −0.52, 95% CI: −0.72 to −0.27; p < 0.001) (Supplementary Figure 7). Heterogeneity was low (Q = 7.7, p = 0.100; I2 = 48%).
Insomnia
Four articles were assessed for insomnia. A random-effects model was used at post-intervention for insomnia, with an SMD of −1.10 (95% CI: −2.05 to −0.15), and I2 = 92%, suggesting high heterogeneity. The overall effect was p = 0.020 (Supplementary Figure 8).
Social function
Three studies (n = 160 patients) reported that KD increases social function in cancer patients (SMD = 0.76, 95% CI: 0.14 to 1.37; p = 0.020) (Supplementary Figure 9). Heterogeneity was high (Q = 0.21, p = 0.030; I2 = 72%).
Ketosis event and adverse event
Ketosis event
Two studies reported ketosis events, with urine testing being the most commonly used method for detecting ketone bodies. Results showed that KD had a significant effect on ketone bodies, with an odds ratio (OR) of 7.54 (95% CI, 2.57–22.13; p < 0.001) (Supplementary Figure 10).
CRP subgroup analysis
Dietary intervention cycle
The intervention periods for the four articles were less than 6 weeks, 6–12 weeks, and more than 12 weeks, respectively. There was a statistically significant overall effect (I2 = 0%, p < 0.001), suggesting that the dietary intervention cycle had an impact on the relationship between KD and CRP outcome measures. The greatest effect size for more than 12 weeks in improving CRP outcomes was SMD = −0.63 (95% CI: −1.03 to −0.24; p = 0.002). For less than 6 weeks, the effect size was SMD = −0.49 (95% CI: −1.46 to 0.48; p = 0.330). The 6–12 week group showed the smallest effect size: SMD = −0.34 (95% CI: −0.72 to 0.05; p = 0.090) (Supplementary Figure 11).
Ketogenic diet intervention ratio
CHO
The carbohydrate proportions reported in the four articles were 2–4%, 3–6%, 5, and 6%, respectively. The overall effect was statistically significant (I2 = 0%, p < 0.001), indicating that the CHO ratio had an effect on the relationship between KD and CRP outcomes. The greatest effect was observed in the 2–4% CHO group (SMD = −0.63; 95% CI: −1.03 to −0.24; p = 0.002), followed by the 3–6 and 5% CHO groups, with effect sizes of SMD = −0.49 (95% CI: −1.46 to 0.48; p = 0.330) and SMD = −0.49 (95% CI: −1.18 to 0.11; p = 0.110), respectively. The 6% CHO group showed the smallest effect size (SMD = −0.23; 95% CI: −0.74 to 0.28; p = 0.380) (Supplementary Figure 12).
Protein
The protein proportions in the four articles were 19, 15–20%, 16–18%, and 25%, respectively. The overall effect was statistically significant (I2 = 0%, p < 0.001), indicating that the protein ratio had a significant impact on the relationship between KD and CRP outcomes. The greatest effect size was observed in the 16–18% protein group (SMD = −0.63; 95% CI: −1.03 to −0.24; p = 0.0020), followed by the 15–20 and 25% protein groups, with effect sizes of SMD = −0.49 (95% CI: −1.46 to 0.48; p = 0.330) and SMD = −0.49 (95% CI: −1.08 to 0.11; p = 0.110), respectively. The 19% protein group showed the smallest effect size (SMD = −0.23; 95% CI: −0.74 to 0.28; p = 0.380) (Supplementary Figure 13).
Fat
The proportions of fat reported in the four articles were 55, 70%, 70–80, and 80–85%, respectively. The overall effect was statistically significant (I2 = 0%, p < 0.001), indicating that the fat ratio had a significant impact on the relationship between KD and CRP outcomes. The greatest effect size was observed in the 80–85% fat group (SMD = −0.63; 95% CI: −1.03 to −0.24; p = 0.002), followed by the 70–80 and 70% fat groups, with effect sizes of SMD = -0.49 (95% CI: −1.46 to 0.48; p = 0.330) and SMD = −0.49 (95% CI: −1.08 to 0.11; p = 0.110), respectively. The 55% fat group showed the smallest effect size (SMD = −0.23; 95% CI: −0.74 to 0.28; p = 0.380) (Supplementary Figure 14).
The implementation of a KD intervention in cancer patients did not result in significant changes in the following nine indicators: HDL cholesterol, triglycerides, CRP, IGF-1, TNF-α, creatinine, urea, energy intake, and age at the time of the dietary intervention (see Supplementary Figures 15–23).
Publication bias and sensitivity analysis
Funnel plot analysis revealed some asymmetry in the distribution of study sites, suggesting the possibility of publication bias. A sensitivity analysis was conducted by modifying the pooling model and comparing the results after sequentially removing each article. It was found that the combined results did not change significantly, indicating that our results were stable (Supplementary Figure 24).
Discussion
This systematic review found that the KD intervention has the potential to benefit cancer-related outcomes such as fat mass, visceral fat mass, LDL cholesterol, total cholesterol, thyroid-stimulating hormone, insulin, blood glucose, β-hydroxybutyrate, emotional function, fatigue, insomnia, social function, and ketosis events in cancer patients. It is a low-cost, easy-to-implement dietary intervention that should be recommended.
Evidence from this review suggests that KD can lead to a notable reduction in both fat mass and visceral fat mass, consistent with the findings of a previous study (37). Increased body fat mass can lead to chronic inflammation, which may further promote cancer development (38, 39). The KD, characterized by very low carbohydrate intake and high fat intake, encourages the body to convert fat into ketone bodies, which serve as the primary energy source (40). As body fat is utilized and consumed, body fat mass decreases. The KD, known for significantly reducing body fat mass, has been shown to exert anti-inflammatory, antiangiogenic, and pro-apoptotic effects on breast cancer cells (41). Evidence suggests that obesity and a surplus of adipose tissue support cancer growth through immune dysregulation, chronic inflammation, and increased insulin signaling, thereby reinforcing the causal link (41, 42). Adipose tissue, particularly around visceral organs, and its immune cells release pro-inflammatory cytokines such as interleukins and TNF-α, contributing to insulin resistance and tumor growth (43). Thus, the KD’s impact on reducing body weight and fat mass in cancer patients is beneficial. Additionally, this dietary pattern may alter the body’s metabolism to further reduce fat. However, future well-designed randomized controlled trials with broader populations are necessary to validate our findings and ultimately establish the KD as a routine adjunctive treatment for cancer patients.
Secondly, in terms of blood parameters, except for a significant decrease in low-density lipoprotein (LDL) and total cholesterol, no other outcome indicators showed significant changes. This finding is inconsistent with some previous studies and may be related to differences in intervention durations and disease types (44). In addition, the lack of significant effects for part indicators could also be attributed to the limited number of included studies and the presence of potential bias in methodological quality, as assessed by the risk of bias evaluation. Such biases may have weakened the statistical power and obscured potential associations. It is also worth noting that even in the absence of statistically significant differences, some observed trends may still hold potential clinical relevance. Future studies should consider both statistical and clinical significance when evaluating the impact of KD on metabolic and inflammatory outcomes.
Third, this review indicated that KD intervention was associated with significant improvements in emotional function, insomnia, social function, and fatigue among cancer patients; however, no significant effects were observed in other QoL domains. The improvements in emotional and social functioning align with findings from previous studies; however, the effects on fatigue and insomnia remain inconsistent across these studies (45). One possible explanation is that comprehensive improvements in QoL may require sustained adherence to the KD over a longer period. Most of the included studies only assessed short-term interventions, which may not have been sufficient to produce meaningful changes in overall QoL for patients. Therefore, further well-designed randomized controlled trials with longer follow-up durations are warranted to establish more definitive conclusions.
Furthermore, the results of this study indicate that changes in urea, creatinine, and adverse events were not significantly different, suggesting that a well-designed KD may reduce fat mass in cancer patients without causing serious adverse effects on liver and kidney function (12, 46). Therefore, we can preliminarily conclude that the studies included in this review do not show any detrimental effects of the KD on cancer patients, and it appears to have a certain level of safety.
Khodabakhshi et al. (47) suggest that the positive effects of ketogenic diet intervention on cancer patients are closely related to the duration of the intervention. The most significant finding of this study is that cancer patients experience the greatest improvement in C-reactive protein levels when following a KD for a duration of more than 12 weeks. In addition, it is pointed out that the proportion of KD gradients has a positive and significant effect on cancer patients (48). The results of this study indicate that 2–4% carbohydrates, 16–18% protein, and 80–85% fat are the maximum effective amounts for improving cancer patients’ outcomes. This provides precise recommendations for the design of future clinical trials. At present, there are significant differences in dietary recommendations, further highlighting the importance of evidence-based guidelines for ketogenic dietary intervention research (49). Currently, the implementation of KD interventions in clinical settings often lacks standardized guidelines and consistent food sources, which may affect the accuracy and consistency of the outcomes. The results of this study offer evidence that can help standardize KD interventions.
Finally, similar to earlier meta-analysis findings, our results demonstrate generally positive effects of KDs on weight loss or maintenance (50–52). Moreover, potential benefits on glycemic control and lipid regulation have also been consistently reported across studies (37, 45, 50). Importantly, this study addresses a critical gap in the current literature on precision nutritional interventions by stratifying outcomes based on cancer types, treatment stages, and dietary strategies. It further contributes novel insights into the application of synchronized intervention timing and dynamic monitoring of safety indicators. Nevertheless, future research with larger sample sizes and extended follow-up durations is necessary to confirm the long-term benefits of ketogenic dietary interventions.
Limitations
This study has several limitations. First, although the included studies were from different countries worldwide, there was limited evidence from regions such as Africa and Asia, which may have introduced regional bias. Second, the methodological quality of most included studies was low, resulting in a limited level of evidence. Third, the types, stages, and treatment methods of cancer included in this study were diverse, and the specific content of the KD interventions and outcome measurement standards varied, which supports the notion that obesity and a surplus of adipose tissue are not uniform, potentially increasing the heterogeneity of the results. Fourth, most studies only reported the short-term effects of KD interventions, lacking verification of long-term effects. Therefore, the conclusions drawn from this study require longer intervention periods to be substantiated. In conclusion, due to the small amount of evidence, the mixture of RCTs and non-RCTs, the inclusion of non-RCTs, and the small sample size, this may lead to high heterogeneity, which in turn may not lead to a definitive causality. The results derived from the present study represent only a preliminary exploration for the validation of the relevant results at a later stage. Finally, publication bias may have influenced the results of this review, as suggested by asymmetry in the funnel plot. This could be related to the limited number of studies, selective reporting of positive findings, and potential language or regional publication restrictions. Therefore, the findings should be interpreted with caution.
Implications for future studies
Despite the limitations of this meta-analysis, it may still offer valuable insights for future randomized controlled trials of KD interventions. Researchers should focus on methodological quality, employing rigorous and blinded study designs, and conducting large-sample, multicenter studies in specific cancer populations to enhance the level of evidence and verify the effectiveness and safety of KD interventions in cancer patients. Additionally, outcome measures should combine both subjective and objective indicators to establish the scientific validity of the conclusions from multiple perspectives. The KD intervention should be more standardized. For example, the KD intervention cycle, specific types of intervention diet, intervention frequency, single intervention time, intervention intensity, and other effects. Furthermore, researchers should conduct follow-up studies to observe the long-term effects of KD intervention, providing a more reliable basis for the treatment and care of cancer patients. This study suggests that future studies of the KD should enhance sample size, follow-up time, control group settings, and nutritional monitoring to further validate the reliability of the results derived in this study and to make a greater contribution to enriching the benefits of the KD for cancer patients.
Conclusion
This review found that KD results in improved cancer-related fat mass, visceral fat mass, LDL cholesterol, total cholesterol, β-hydroxybutyrate, thyroid-stimulating hormone, insulin, blood glucose, emotional function, fatigue, insomnia, social function, and ketosis events. Furthermore, the C-reactive protein outcome index had a greater impact when the intervention period exceeded 12 weeks, with a proportion of 2–4% CHO, 16–18% protein, and 80–85% fat. The potential benefits of a KD in cancer treatment highlight the necessity for well-designed clinical trials to better understand how this adjunctive approach impacts cancer patients’ nutritional status, prognosis, and QoL.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
MZ: Conceptualization, Methodology, Software, Writing – original draft, Data curation, Formal analysis. QZ: Conceptualization, Methodology, Software, Resources, Writing – review & editing. SH: Methodology, Investigation, Validation, Visualization, Writing – review & editing. YL: Investigation, Methodology, Data curation, Writing – review & editing. MP: Methodology, Conceptualization, Funding acquisition, Resources, Software, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Jiangsu Province Higher Education Institutions Basic Science (Natural Science) Research Top Project (24KJB320016).
Acknowledgments
We would like to express our heartfelt gratitude to all the researchers who conducted original studies. This study would not have been possible without their contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1535921/full#supplementary-material
References
1.
Worldwide cancer data . (2024). World Cancer Research Fund International. Available online at: https://www.wcrf.org/cancer-trends/worldwide-cancer-data/ [Accessed August 2024].
2.
Tyrovolas S Panagiotakos DB . The role of Mediterranean type of diet on the development of cancer and cardiovascular disease, in the elderly: a systematic review. Maturitas. (2010) 65:122–30. doi: 10.1016/j.maturitas.2009.07.003
3.
Warner WA Lee TY Badal K Williams TM Bajracharya S Sundaram V et al . Cancer incidence and mortality rates and trends in Trinidad and Tobago. BMC Cancer. (2018) 18:712. doi: 10.1186/s12885-018-4625-x
4.
Stewart BW Bray F Forman D Ohgaki H Straif K Ullrich A et al . Cancer prevention as part of precision medicine: 'plenty to be done'. Carcinogenesis. (2016) 37:2–9. doi: 10.1093/carcin/bgv166
5.
Sweeney CJ Chen YH Carducci M Liu G Jarrard DF Eisenberger M et al . Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. (2015) 373:737–46. doi: 10.1056/NEJMoa1503747
6.
Rieger J Bähr O Maurer GD Hattingen E Franz K Brucker D et al . ERGO: a pilot study of ketogenic diet in recurrent glioblastoma. Int J Oncol. (2014) 44:1843–52. doi: 10.3892/ijo.2014.2382
7.
Zahra A Fath MA Opat E Mapuskar KA Bhatia SK Ma DC et al . Consuming a ketogenic diet while receiving radiation and chemotherapy for locally advanced lung Cancer and pancreatic Cancer: the University of Iowa experience of two phase 1 clinical trials. Radiat Res. (2017) 187:743–54. doi: 10.1667/RR14668.1
8.
Iyikesici MS . Feasibility study of metabolically supported chemotherapy with weekly carboplatin/paclitaxel combined with ketogenic diet, hyperthermia and hyperbaric oxygen therapy in metastatic non-small cell lung cancer. Int J Hyperth. (2019) 36:446–55. doi: 10.1080/02656736.2019.1589584
9.
Branca JJ Pacini S Ruggiero M . Effects of pre-surgical vitamin D supplementation and ketogenic diet in a patient with recurrent breast cancer. Anticancer Res. (2015) 35:5525–32. PMID:
10.
Klement RJ . The influence of ketogenic therapy on the 5 r's of radiobiology. Int J Radiat Biol. (2019) 95:394–407. doi: 10.1080/09553002.2017.1380330
11.
Ferrere G Tidjani Alou M Liu P Goubet AG Fidelle M Kepp O et al . Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade. JCI Insight. (2021) 6:e145207. doi: 10.1172/jci.insight.145207
12.
Khodabakhshi A Akbari ME Mirzaei HR Seyfried TN Kalamian M Davoodi SH . Effects of ketogenic metabolic therapy on patients with breast cancer: a randomized controlled clinical trial. Clin Nutr (Edinb). (2021) 40:751–8. doi: 10.1016/j.clnu.2020.06.028
13.
Kämmerer U Klement RJ Joos FT Sütterlin M Reuss-Borst M . Low carb and ketogenic diets increase quality of life, physical performance, body composition, and metabolic health of women with breast cancer. Nutrients. (2021) 13:1029. doi: 10.3390/nu13031029
14.
Klement RJ Champ CE Kämmerer U Koebrunner PS Krage K Schäfer G et al . Impact of a ketogenic diet intervention during radiotherapy on body composition: III-final results of the KETOCOMP study for breast cancer patients. Breast Cancer Res. (2020) 22:94. doi: 10.1186/s13058-020-01331-5
15.
Klement RJ Weigel MM Sweeney RA . A ketogenic diet consumed during radiotherapy improves several aspects of quality of life and metabolic health in women with breast cancer. Clin Nutr (Edinb). (2021) 40:4267–74. doi: 10.1016/j.clnu.2021.01.023
16.
Klement RJ Meyer D Kanzler S Sweeney RA . Ketogenic diets consumed during radio-chemotherapy have beneficial effects on quality of life and metabolic health in patients with rectal cancer. Eur J Nutr. (2021) 61:69–84. doi: 10.1007/s00394-021-02615-y
17.
Klement RJ Koebrunner PS Meyer D Kanzler S Sweeney RA . Impact of a ketogenic diet intervention during radiotherapy on body composition: IV. Final results of the KETOCOMP study for rectal cancer patients. Clin Nutr (Edinb). (2021c) 40:4674–84. doi: 10.1016/j.clnu.2021.05.015
18.
Freedland SJ Allen J Jarman A Oyekunle T Armstrong AJ Moul JW et al . A randomized controlled trial of a 6-month low-carbohydrate intervention on disease progression in men with recurrent prostate Cancer: carbohydrate and prostate study 2 (CAPS2). Clin Cancer Res. (2020) 26:3035–43. doi: 10.1158/1078-0432.CCR-19-3873
19.
Westman EC Mavropoulos J Yancy WS Volek JS . A review of low-carbohydrate ketogenic diets. Curr Atheroscler Rep. (2003) 5:476–83. doi: 10.1007/s11883-003-0038-6
20.
Page MJ McKenzie JE Bossuyt PM Boutron I Hoffmann TC Mulrow CD et al . The PRISMA 2020 statement: an updated guideline for reporting systematic review. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
21.
Higgins JP Green S (2011). Cochrane handbook for systematic review of interventions version 5.1.0. Available online at: www.handbook.cochrane.org [Accessed August 2024].
22.
Higgins JP Altman DG Gøtzsche PC Jüni P Moher D Oxman AD et al . The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ Clin Res Ed. (2011) 343:d5928. doi: 10.1136/bmj.d5928
23.
Guyatt GH Oxman AD Vist GE Kunz R Falck-Ytter Y Alonso-Coello P et al . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ Clin Res Ed. (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
24.
Luo D Wan X Liu J Tong T . Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Med Res. (2018) 27:1785–805. doi: 10.1177/0962280216669183
25.
Hozo SP Djulbegovic B Hozo I . Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. (2005) 5:13. doi: 10.1186/1471-2288-5-13
26.
McGrath S Zhao X Steele R Thombs BD Benedetti A DEPRESsion Screening Data (DEPRESSD) Collaboration . Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat Methods Med Res. (2020) 29:2520–37. doi: 10.1177/0962280219889080
27.
Higgins J Green SR (2011). Cochrane handbook for systematic review of interventions. Version 5.1.0. Available online at: www.handbook.cochrane.org [Accessed August 2024].
28.
Cohen CW Fontaine KR Arend RC Alvarez RD Leath CA III Huh WK et al . A ketogenic diet reduces central obesity and serum insulin in women with ovarian or endometrial cancer. J Nutr. (2018) 148:1253–60. doi: 10.1093/jn/nxy119
29.
Voss M Wagner M von Mettenheim N Harter PN Wenger KJ Franz K et al . ERGO2: a prospective, randomized trial of calorie-restricted ketogenic diet and fasting in addition to reirradiation for malignant glioma. Int J Radiat Oncol Biol Phys. (2020) 108:987–95. doi: 10.1016/j.ijrobp.2020.06.021
30.
Voss M Wenger KJ von Mettenheim N Bojunga J Vetter M Diehl B et al . Short-term fasting in glioma patients: analysis of diet diaries and metabolic parameters of the ERGO2 trial. Eur J Nutr. (2022) 61:477–87. doi: 10.1007/s00394-021-02666-1
31.
Kang CM Yun B Kim M Song M Kim YH Lee SH et al . Postoperative serum metabolites of patients on a low carbohydrate ketogenic diet after pancreatectomy for pancreatobiliary cancer: a nontargeted metabolomics pilot study. Sci Rep. (2019) 9:16820. doi: 10.1038/s41598-019-53287-y
32.
Cohen CW Fontaine KR Arend RC Gower BA . A ketogenic diet is acceptable in women with ovarian and endometrial cancer and has no adverse effects on blood lipids: a randomized, controlled trial. Nutr Cancer. (2020) 72:584–94. doi: 10.1080/01635581.2019.1645864
33.
Augustus E Granderson I Rocke KD . The impact of a ketogenic dietary intervention on the quality of life of stage II and III cancer patients: a randomized controlled trial in the Caribbean. Nutr Cancer. (2021) 73:1590–600. doi: 10.1080/01635581.2020.1803930
34.
Ok JH Lee H Chung HY Lee SH Choi EJ Kang CM et al . The potential use of a ketogenic diet in pancreatobiliary cancer patients after pancreatectomy. Anticancer Res. (2018) 38:6519–27. doi: 10.21873/anticanres.13017
35.
Budipramana VS Arifin F . Very low carbohydrate diet effect on modified Glasgow prognostic score: a randomized controlled trial on stage-IV colorectal adenocarcinoma patients. Surg Gastroenterol Oncol. (2020) 25:280–5. doi: 10.21614/sgo-25-5-280
36.
Santos JG Da Cruz WMS Schönthal AH Salazar MD Fontes CAP Quirico-Santos T et al . Efficacy of a ketogenic diet with concomitant intranasal perillyl alcohol as a novel strategy for the therapy of recurrent glioblastoma. Oncol Lett. (2018) 15:1263–70. doi: 10.3892/ol.2017.7362
37.
Zhao H Jin H Xian J Zhang Z Shi J Bai X . Effect of ketogenic diets on body composition and metabolic parameters of cancer patients: a systematic review and meta-analysis. Nutrients. (2022) 14:4192. doi: 10.3390/nu14194192
38.
Danforth DN . The role of chronic inflammation in the development of breast cancer. Cancers. (2021) 13:3918. doi: 10.3390/cancers13153918
39.
Li CW Yu K Shyh-Chang N Jiang Z Liu T Ma S et al . Pathogenesis of sarcopenia and the relationship with fat mass: descriptive review. J Cachexia Sarcopenia Muscle. (2022) 13:781–94. doi: 10.1002/jcsm.12901
40.
Paoli A Rubini A Volek JS Grimaldi KA . Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr. (2013) 67:789–96. doi: 10.1038/ejcn.2013.116
41.
Wright C Simone NL . Obesity and tumor growth: inflammation, immunity, and the role of a ketogenic diet. Curr Opin Clin Nutr Metab Care. (2016) 19:294–9. doi: 10.1097/MCO.0000000000000286
42.
Lee YS Olefsky J . Chronic tissue inflammation and metabolic disease. Genes Dev. (2021) 35:307–28. doi: 10.1101/gad.346312.120
43.
Ahmed B Sultana R Greene MW . Adipose tissue and insulin resistance in obese. Biomed Pharmacother. (2021) 137:111315. doi: 10.1016/j.biopha.2021.111315
44.
Zhou C Wang M Liang J He G Chen N . Ketogenic diet benefits to weight loss, glycemic control, and lipid profiles in overweight patients with type 2 diabetes mellitus: a meta-analysis of randomized controlled trails. Int J Environ Res Public Health. (2022) 19:10429. doi: 10.3390/ijerph191610429
45.
Amanollahi A Khazdouz M Malekahmadi M Klement RJ Lee D Khodabakhshi A . Effect of ketogenic diets on cardio-metabolic outcomes in Cancer patients: a systematic review and Meta-analysis of controlled clinical trials. Nutr Cancer. (2023) 75:95–111. doi: 10.1080/01635581.2022.2117388
46.
Khodabakhshi A Seyfried TN Kalamian M Davoodi SH . Answer to the critical remarks to the article by Khodabakhshi and colleagues reporting results from a randomized study on ketogenic diet. Clin Nutr (Edinb). (2022) 41:2063–6. doi: 10.1016/j.clnu.2022.06.025
47.
Khodabakhshi A Seyfried TN Kalamian M Beheshti M Davoodi SH . Does a ketogenic diet have beneficial effects on quality of life, physical activity or biomarkers in patients with breast cancer: a randomized controlled clinical trial. Nutrients. (2022) 19:87. doi: 10.1186/s12937-020-00596-y
48.
Allen BG Bhatia SK Anderson CM Eichenberger-Gilmore JM Sibenaller ZA Mapuskar KA et al . Ketogenic diets as an adjuvant cancer therapy: history and potential mechanism. Redox Biol. (2014) 2:963–70. doi: 10.1016/j.redox.2014.08.002
49.
Champ CE Mishra MV Showalter TN Ohri N Dicker AP Simone NL . Dietary recommendations during and after cancer treatment: consistently inconsistent?Nutr Cancer. (2013) 65:430–9. doi: 10.1080/01635581.2013.757629
50.
López-Espinoza MÁ Chacón-Moscoso S Sanduvete-Chaves S Ortega-Maureira MJ Barrientos-Bravo T . Effect of a ketogenic diet on the nutritional parameters of obese patients: a systematic review and meta-analysis. Nutrients. (2021) 13:2946. doi: 10.3390/nu13092946
51.
Taftian M Beigrezaei S Arabi V Salehi-Abargouei A . The effect of ketogenic diet on weight loss in adult patients with Cancer: a systematic review and Meta-analysis of controlled clinical trials. Nutr Cancer. (2022) 74:1222–34. doi: 10.1080/01635581.2021.1942081
52.
Yang YF Mattamel PB Joseph T Huang J Chen Q Akinwunmi BO et al . Efficacy of low-carbohydrate ketogenic diet as an adjuvant cancer therapy: a systematic review and meta-analysis of randomized controlled trials. Nutrients. (2021) 13:1388. doi: 10.3390/nu13051388
Summary
Keywords
tumor, ketogenic diet, diets, ketogenic, systematic review and meta-analysis
Citation
Zhang M, Zhang Q, Huang S, Lu Y and Peng M (2025) Impact of ketogenic diets on cancer patient outcomes: a systematic review and meta-analysis. Front. Nutr. 12:1535921. doi: 10.3389/fnut.2025.1535921
Received
28 November 2024
Accepted
30 June 2025
Published
18 July 2025
Volume
12 - 2025
Edited by
Meghit Boumediene Khaled, University of Sidi-Bel-Abbès, Algeria
Reviewed by
Peter J. Voshol, Independent researcher, Culemborg, Netherlands
Dominic D’Agostino, University of South Florida, United States
Updates
Copyright
© 2025 Zhang, Zhang, Huang, Lu and Peng.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mengyun Peng, nine_779134944@yahoo.co.jp
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.