Abstract
Endometriosis is an estrogen-dependent chronic inflammatory disease which causes dysmenorrhea, chronic pelvic pain, and infertility in women of childbearing age, significantly impacting their quality of life and physical and mental health. The etiology of endometriosis remains unclear, with oxidative stress and inflammation currently thought to play pivotal roles in its pathophysiology. Epidemiological studies and clinical trials indicate that varying dietary patterns and specific nutrient supplementation can influence oxidative stress markers and levels of inflammatory factors and related pathways, potentially impacting the progression of endometriosis. In this review, we summarize the roles of oxidative stress and inflammation in endometriosis and thoroughly examine the current understanding of the effect of dietary patterns and nutrient supplementation in treating endometriosis. This study suggests that nutrients may prevent the occurrence of endometriosis by modulating levels of inflammatory factors, regulating angiogenesis, and influencing the metabolism of estrogen pathways. The findings might provide new insights into the treatment of endometriosis patients and the potential benefits of dietary patterns and nutrient supplementation in patients with endometriosis.
1 Introduction
Endometriosis is a common gynecological disorder characterized by the presence of endometrial tissue outside the uterus (1), typically presenting with severe pelvic pain and infertility, which affects 5–15% of reproductive-age women and as much as 3–5% of postmenopausal women (2). Endometriosis is a multifaceted, heterogeneous disease with no clear pathogenesis yet identified. Current research suggests that oxidative stress and inflammation play important roles in endometriosis. In the peritoneal fluid of patients with endometriosis, the concentration of numerous inflammatory factors, angiogenesis-related factors, and adhesion factors is elevated (3), and the activation of inflammation accelerates the progression of endometriosis (4). Endometriosis lesions stimulate the production of inflammatory factors and growth factors in the abdominal cavity. The persistent inflammatory response interacts with the central nervous system, resulting in chronic pain (5). Chronic pelvic pain significantly affects endometriosis patients’ quality of life and social functioning (6).
Dietary intervention and specific dietary patterns can influence the onset and outcomes of inflammation-related diseases, including cardiovascular disease, cancer, diabetes, and obesity (7). Different dietary patterns alter the levels of inflammatory factors and inflammation pathways in the body, affecting the occurrence of endometriosis. Ingestion of specific foods affects the levels of inflammatory factors, regulates angiogenesis, modulates the estrogen metabolism pathway in the body (8), and promotes or inhibits the development of endometriosis (9). The risk of endometriosis can be reduced by adjusting dietary intake, such as consuming nutrients with anti-inflammatory and antioxidant properties. Current research generally suggests that a high intake of green vegetables and fresh fruits (10), dairy products (11), legumes (12), polyphenols, and fish oil (13) significantly reduces the risk of endometriosis. Those with a high intake of red meat (14) and trans-fatty acids (15) have an increased risk of endometriosis. Women with endometriosis resort to non-medical methods to manage symptoms and enhance daily living (16). Self-care activities, complementary therapies, and positive doctor-patient relationships are critical components of endometriosis self-management.
However, a few studies have yielded inconsistent results, and there is still a lack of large-scale, high-quality studies to verify the relationship among dietary patterns, nutrients, and endometriosis. The role of different dietary patterns and nutrients in endometriosis is still ambiguous. This review aims to provide an overview of oxidative stress and inflammation in endometriosis, and comprehensively summarize the effects and potential mechanisms of various dietary patterns and nutrients in endometriosis (Figure 1).
Figure 1
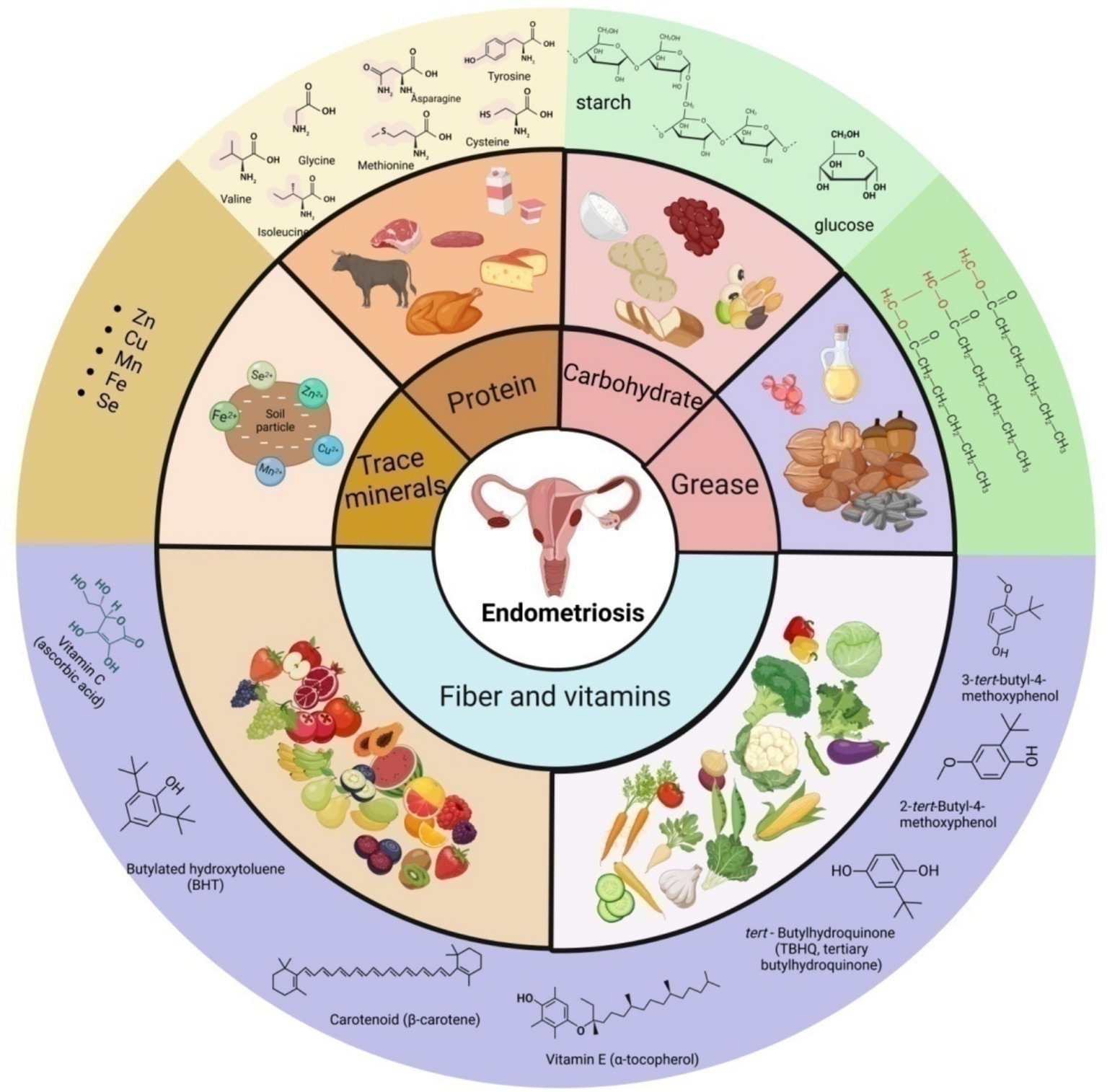
Schematic presentation of the main source, classification and some representative components of nutrition in endometriosis.
2 Oxidative stress and inflammation in endometriosis
There is evidence that endometriosis can be caused by retrograde menstruation (17), endometrial implantation, epithelial transformation (18), hormonal effects (19), and immune system dysfunction (20). Moreover, peritoneal fluid from people with endometriosis has unusual amounts of angiogenic and adhesion factors, as well as numerous inflammatory markers. This suggests that long-term inflammation plays a part in how endometriosis starts and gets worse (3). Endometriosis lesions stimulate the production of inflammatory cytokines and growth factors within the abdominal cavity, where the activation of inflammation further advances the progression of endometriosis (4).
Currently, it is believed that dietary ingredients with anti-proliferative, anti-inflammatory, antioxidant, analgesic, and estrogen-reducing properties can reduce the incidence of endometriosis. Nevertheless, the exact modus operandi remains obscure. Consequently, we have meticulously updated and comprehensively rearticulated these two mechanisms, with a particular focus on innovative and all-encompassing methodologies (Figure 2).
Figure 2

Schematic presentation of risk factors for endometriosis. (A) The exfoliated endometrial cells retrograde into the pelvic cavity and are implanted into the peritoneum and abdominal organs. (B) Epithelial-mesenchymal transformation is involved in the process of local inflammatory damage, repair, fibrosis, cell invasion and metastasis in endometriosis, as well as the formation of local lesions. (C) Adult stem cells are detected in endometrial suspension, which drive physiological endometrial regeneration and participate in the pathogenesis of endometriosis. (D) VEGF induces endothelial cell proliferation, migration and angiogenesis in endometriosis. (E) Elevated estrogen levels and the increased activity of estrogen receptors regulate immunity, inflammation and angiogenesis, and promote the continuous progression of endometrial lesions. (F) Abnormal activation of immune response and changes in peritoneal immune microenvironment are the basis of endometriosis.
2.1 The role of oxidative stress in endometriosis
Macrophages, red blood cells, and apoptotic endometrial tissue transplanted into the peritoneal cavity via retrograde menstruation serve as inducers of oxidative stress (21). The release of hemoglobin and iron from red blood cells activates macrophages, leading to the production of inflammatory factors including interleukin-1, interleukin-6, and tumor necrosis factor-alpha (22) and fostering the formation of damaging reactive oxygen species (23). The iron storage levels (ferritin load) in the peritoneal macrophages of patients with endometriosis are significantly elevated compared to the control group (24). The continuous increase in iron content triggers oxidative stress, resulting in local damage to the peritoneal mesothelium and creating adhesion sites for ectopic endometrial cells (25). Iron overload further exacerbates the condition by stimulating cell proliferation, thereby advancing endometriosis (26, 27).
ROS are intermediates generated during normal oxygen metabolism in the human body, with a delicate balance maintained between pro-oxidants and antioxidants in healthy individuals. Mitochondria generate ROS via the electron transport chain, a process termed oxidative phosphorylation (OXPHOS) (28). An excess of oxidants triggers oxidative stress (29), leading to a significant elevation in mitochondrial superoxide levels within ectopic endometrial tissue (30), which alters mitochondrial structure, particularly by expanding the surface area of the cristae. This influences OXPHOS function and leads to increased ROS production (31). Excess ROS regulates cell proliferation (32) and leads to the overactivation of nuclear factor kappa-B (NF-κB) by IL-1 and TNF-α. NF-κB enhances the invasive and adhesive capacities of endometriotic cells to the peritoneal surface by regulating the expression of matrix metalloproteinases, thereby stimulating angiogenesis and inflammation (33). Although mitochondria are the primary ROS producers, they are vulnerable to ROS attacks (30).
2.2 The role of inflammation in endometriosis
The coordinated action of macrophages, endometrial cells, endothelial cells, and activated lymphocytes, along with cytokines and chemokines, alters the abdominal immune microenvironment, facilitating pathological processes including cell proliferation, angiogenesis, lesion adhesion, growth, and invasion (34). Macrophage numbers significantly rise in peritoneal fluid and ectopic endometrium (35), and co-culturing macrophages with endometrial stromal cells (ESCs) enhances the proliferative and invasive capacities of ESCs (36). Co-culturing ESCs with monocyte-derived macrophages and NK cells from endometriosis patients showed that the interaction between ESCs and macrophages downregulates NK cell cytotoxicity by enhancing the release of IL-10 and TNF-β (37), potentially allowing endometrial fragments to evade immune clearance (38). Studies have reported that patients with endometriosis have an increased presence or activation of B lymphocytes (39). Anti-endometrial antibodies have been detected in the serum and peritoneal fluid of patients with endometriosis (40). These autoantibodies can stimulate the immune system, causing persistent inflammation, advancing endometriosis, and contributing to the formation of the inflammatory microenvironment.
The endometriosis microenvironment may enhance ERK activity in endometriotic cells. TNFα and IL-1β are capable of activating ERK and inducing the expression of IL-8 and IL-6 (41). The chemokine MCP1 also greatly increases the production of PGE2 (42), VEGF, IL-8, and MCP-1 in human endometriotic cells through a pathway specific to ERK (43).
The PI3K/AKT/mTOR pathway regulates cell growth, proliferation, differentiation, and apoptosis (44). Membrane-bound phosphoinositide 3-kinase (PI3K) is the most common mediator of mTOR activation, forming the core of the PI3K/AKT/mTOR pathway along with AKT (45). When PI3K is turned on, it causes PDK1 to be phosphorylated by AKT. This then turns on downstream mTOR receptors by interacting with TSC2 (46). The TSC complex, consisting of TSC1 and TSC2, interacts with GTPase-Ras, acting as a negative regulator of mTOR activity (47). When TSC2 is phosphorylated by kinases such as AKT, it dissociates from the complex, leading to the inactivation of the complex and the subsequent activation of mTORC1 by Rheb, which is bound to GTP (48). When activated, mTORC1 activates numerous downstream proteins, promoting protein synthesis and cell growth. Additionally, mTORC1 activates HIF-alpha through VEGF, serving as the primary angiogenic switch (49), thereby inducing new angiogenesis. LncRNA IGF2-AS facilitates the progression of endometriosis by targeting the miR-370-3p/IGF2 axis and activating the PI3K/AKT/mTOR signaling pathway (50). Interventions targeting HIF-1α and mTOR pathways may emerge as potential therapeutic targets for endometriosis (51).
Additionally, E2 regulates protein and DNA synthesis in uterine epithelial cells via the PKC/ERK/mTOR pathway, ultimately controlling cell proliferation (52). In endometrial stromal cells (ESC), TNFα-induced activation of the estrogen receptor ER leads to heightened ERK activation (53). Lipoprotein A4 suppresses inflammation and enhances autophagy via the AhR/mTOR/AKT pathway, thereby inhibiting endometriosis (54).
IL-37b, a unique member of the IL-1 family, may inhibit lesion growth by regulating proliferation, invasion, angiogenesis, and inflammation through the AKT and ERK1/2 signaling pathways (55). Recombinant human IL-37 enhances the Th1/Th2 ratio by inducing dendritic cell maturation, thus curbing the progression of endometriosis in mouse models (56). Exogenous IL-1β increases the production of nerve growth factor in primary endometrial stromal cells in endometriosis. This is associated with the onset of endometriosis and the concomitant pain (57). Interleukin-33 is a member of the IL-1β family. In mouse models that did not have IL-33, the size of endometriotic lesions was greatly reduced (58). When injected into a monkey model of endometriosis with its long-acting recovery antibody (AMY109), IL-8, a potent chemoattractant for angiogenic factors and immune cells, can inhibit neutrophil recruitment to endometrial lesions and suppress their production of monocyte chemotactic protein-1, thereby ameliorating endometriosis inflammation and fibrosis (59). IL-17 is upregulated in the serum, peritoneal fluid (PF), and endometrial lesions of patients with endometriosis (60), regulating the recruitment and M2 polarization of peritoneal macrophages in endometriosis (61).
HSP-70, a chaperone protein within the heat shock protein family, is produced by macrophages, smooth muscle cells, endometrial cells, dendritic cells, and vascular endothelial cells. It significantly stimulates the production of vascular endothelial growth factor, interleukin-6, and TNF-α in women with endometriosis. Furthermore, HSP-70 facilitates Toll-like receptor 4 (TLR4)-mediated growth of endometrial cells (62). The decrease in HSP-70 may facilitate unrestricted nuclear translocation of NF-κB, resulting in targeted transcription (Figure 3).
Figure 3
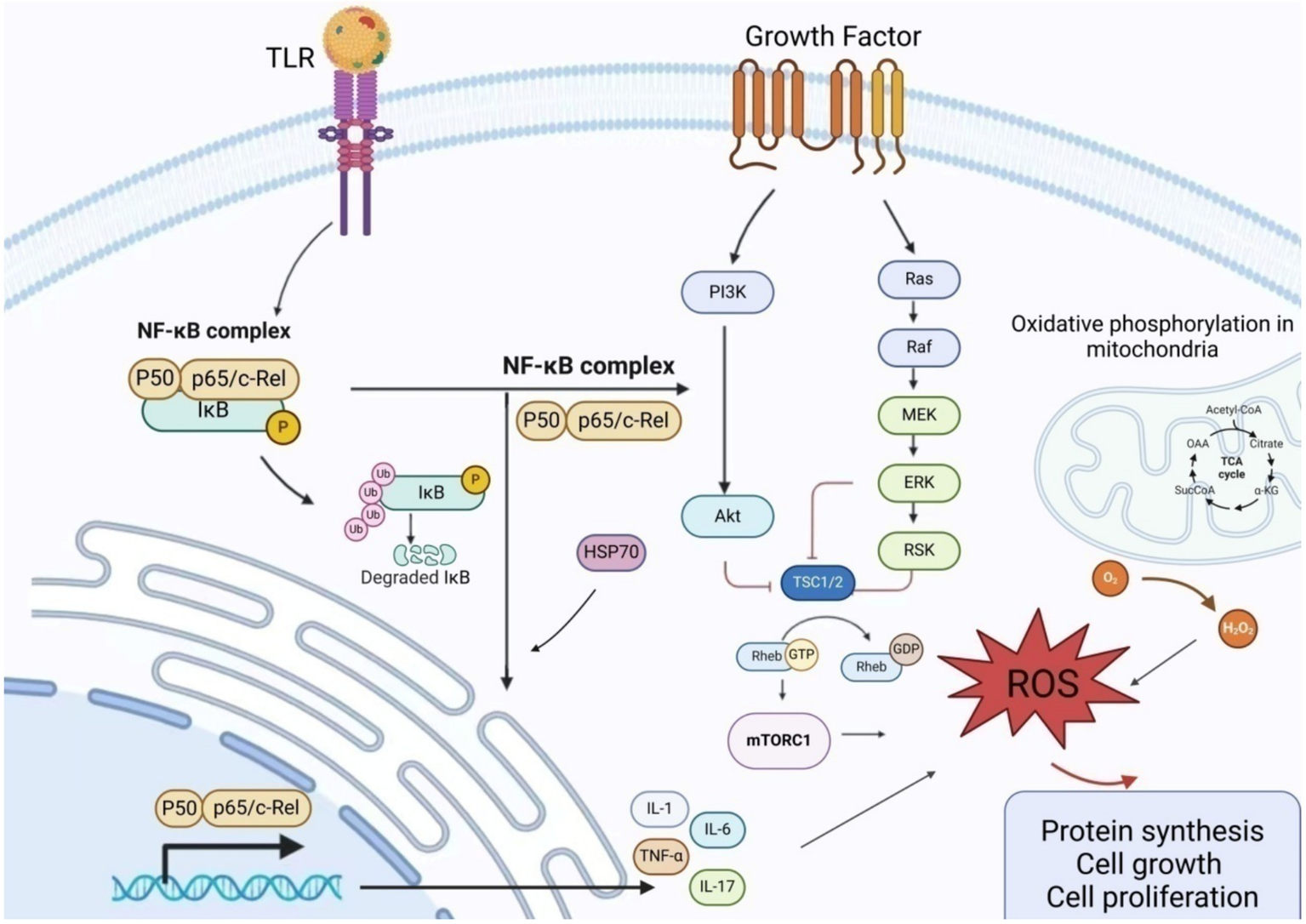
Schematic presentation of the mechanism of action of inflammatory factors in endometriosis NF-κB promotes the production and release of inflammatory factors by regulating the expression of matrix metalloproteinases. Excessive ROS regulates cell proliferation and induces excessive activation of NF-κB by IL-1 and TNF-α, thereby attacking mitochondria. The PI3K/AKT/mTOR pathway regulates cell growth, proliferation, differentiation, and apoptosis. Upon PI3K activation, AKT phosphorylation of PDK1 is stimulated, and the interaction between AKT and TSC2 activates downstream mTOR receptors. The TSC complex, consisting of TSC1 and TSC2, interacts with GTPase-Ras to serve as a negative regulator of mTOR activity. When TSC2 is phosphorylated by kinases (such as AKT), it dissociates from the complex, rendering it inactive. This leads to Rheb binding to GTP, activating mTORC1, and triggering an oxidative stress response. AKT, Protein Kinase B; ERK, Extracellular Signal-Regulated Kinase; GDP, Guanosine DiPhosphate; GTP, Guanosine TriPhosphate; MEK, Mitogen-Activated Protein Kinase; mTORC1, Mechanistic Target of Rapamycin Complex 1; NF-κB, Nuclear factor kappa-B; PI3K, Phosphatidylinositol 3-Kinase; RAF, RAF kinase; RAS, Rat Sarcoma Virus Oncogene; ROS, Reactive oxygen species; TRL, Toll-like receptor; TSC, Tuberous Sclerosis Complex.
3 Effect of dietary patterns in endometriosis
Endometriosis is characterized by hormone dependency and a high recurrence rate, necessitating lifelong management. Dietary intervention is currently one of the main methods for self-management for endometriosis patients, with numerous studies indicating that a higher intake of vitamin-rich foods reduces the risk of endometriosis (63–65). Excessive intake of red meat is associated with increased levels of estrogen sulfate, leading to higher levels of steroids and inflammatory factors that influence the progression of endometriosis (66, 67). Consuming dairy products, fruits, legumes, red meat, and potatoes is significantly associated with a reduced risk of endometriosis. Consuming fried potatoes, on the other hand, has a positive correlation with the risk of endometriosis. Intake of animal proteins, EPA, MUFA, and oleic acid is associated with a reduced risk of endometriosis (68). Those in the top fifth with the highest intake of trans-unsaturated fats have a 48% higher likelihood of being diagnosed with endometriosis (15). In the absence of ovarian dysfunction and insulin resistance in mice, high dietary fat intake increases the risk of endometriosis (69).
Most of the aforementioned studies focus on the association between specific foods and endometriosis, failing to establish a link between the participants’ overall dietary nutrient intake and the condition. When other factors are taken into account, the findings may be contradictory. Schwarz (70) found that the beneficial effects associated with fruit fiber vanished after adjusting for the healthy diet index. Furthermore, an elevated risk of endometriosis was noted when combining the total intake of vegetables and cruciferous fiber. Currently, there are no specific dietary recommendations for endometriosis patients. We have outlined the key features of prevalent dietary patterns and explored the connections between various dietary patterns and endometriosis.
3.1 Mediterranean diet
Mediterranean diet (MedDiet) includes eating a lot of fresh, seasonal, minimally processed plant-based foods, like vegetables, fruits, legumes, potatoes, bread, nuts, seeds, and other grains; a lot of olive oil (OO), especially virgin olive oil (VOO) and extra-virgin olive oil (EVOO), which is the main source of fat; some dairy products, like cheese and yogurt; and some poultry and fish, which are main sources of long-chain polyunsaturated fatty acids (PUFAs) (71), especially omega-3 fatty acids. Typically, the caloric intake from fat should not exceed 30%, with less than 8–10% from saturated fats.
The various nutrients in MedDiet, such as vitamins, minerals, polyphenols, fibers, nitrates, PUFAs, and monounsaturated fatty acids (MUFAs), are biologically active compounds that are beneficial to health (72, 73). They have synergistic and interactive effects in reducing inflammation (73) and affect different inflammatory markers in the body, such as IL-6 or TNF-α (74, 75). Flavonoids in grains, vegetables, fruits, and olive oil have potentially beneficial effects, including free radical scavenging, anti-inflammatory effects, and anti-Aβ neurotoxicity. Dietary polyphenols can also effectively combat ROS, reduce oxidative damage to genetic material, and enhance the antioxidant capacity of endothelial cells (76). The polyphenols in olive oil regulate the inflammation by inhibiting NF-κB (77). Resveratrol can improve the antioxidant status of Parkinson’s disease rats and reduce dopamine loss (78). When several polyphenols are used in combination, they exhibit stronger antioxidant capacity in terms of their activity (79). Hydroxytyrosol, which is found in extra virgin olive oil, can lower inflammation by stopping cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase from working (80). HT can also reduce superoxide ions and inhibit the excessive secretion of the important inflammatory mediator prostaglandin E2 in the human body (81).
Olive oil that is high in MUFA is crucial for keeping the lipid profile of mitochondrial membranes in check so that they can fight oxidative damage and age-related problems (82). People with a higher intake of olive oil have more MUFA in their mitochondrial membranes, while those who mainly consume sunflower oil have more omega-6 PUFA. These changes are consistent with the level of oxidative damage; namely, compared to those who consume sunflower oil, those who consume OO have fewer hydroperoxides in their body tissues. Therefore, a diet rich in olive oil produces membranes with less PUFA, which weakens the increase in lipid peroxidation (83). HT and oleuroside can reduce oxidative stress and optimize mitochondrial function (84). Fish oil is a beneficial source of omega-3 PUFAs. Giving older mice fish oil for 21 days increased the amount of omega-3 PUFA derivatives in their brains, which led to better mitochondrial function and ATP production (85). Fish oil omega-3 supplementation may reduce endometrial implant growth and inflammatory factor production, particularly in patients with stage III or IV endometriosis (13). Supplementing omega-3 polyunsaturated fatty acids alleviates pain symptoms related to endometriosis (86).
It is now believed that the long-term Mediterranean diet has a potential protective effect on cardiovascular diseases, stroke, obesity, diabetes, hypertension, malignant tumors, allergic diseases, as well as Alzheimer’s disease and Parkinson’s disease (87). Patients with endometriosis who follow the Mediterranean diet can reduce inflammatory factors in their bodies and alleviate pain symptoms (88).
3.2 Ketogenic diet
The characteristic of a low-carbon ketogenic diet is the consumption of extremely high-fat and low-carbohydrate diets, with carbohydrate intake ≤10% of energy consumption (89). This dietary pattern forces the system to shift from glucose metabolism to fatty acid metabolism, thereby producing ketones. A ketogenic diet increases low-density lipoprotein cholesterol in healthy, young, normal-weight women (90). Low carbohydrate intake causes physiological ketosis in patients with polycystic ovary syndrome, which reduces circulating insulin levels, lowers IGF-1 levels, and inhibits stimulation of androgen production, including in the ovaries and adrenal glands. Reducing circulating lipids, low-grade inflammation, and oxidative stress also helps prevent cardiovascular complications (91, 92). Low-fat and low-carbohydrate diets significantly reduce the concentrations of several serum inflammatory markers, such as TNF-α, IL-6, IL-8, MCP-1, etc. (93). After 21 days on a carefully formulated ketogenic diet (WFKD), women show improvements in body composition, blood pressure, and blood sugar; increased ketone bodies; and improvements in some, but not all, cholesterol markers (94). WFKD can improve fasting blood glucose, insulin resistance, weight, and body composition in women with stage IV metastatic breast cancer. WFKD serves as an adjunctive therapy, playing a positive role (95).
A low-carbohydrate ketogenic diet can alleviate chronic musculoskeletal pain, improve blood biomarkers, and enhance the quality of life of patients (96). The ketogenic diet regulates gut microbiota and short-chain fatty acids, which are associated with Alzheimer’s disease markers in subjects with mild cognitive impairment (97).
3.3 Low FODMAP diet
FODMAP stands for fermentable oligosaccharides, disaccharides, monosaccharides, and polyols. These osmotic carbohydrates are non-absorbable and undergo bacterial fermentation in the small intestine, leading to functional gastrointestinal symptoms such as cramps, bloating, and diarrhea (98). A short-term moderate-low-FODMAP diet can significantly alleviate gastrointestinal symptoms, with significant reductions in pain, bloating, diarrhea, and fullness scores (99).
The symptoms of endometriosis are closely related to other chronic diseases such as irritable bowel syndrome (IBS) (100), inflammatory bowel disease (IBD), and celiac disease (CD) (101). A low-FODMAP diet reduces irritable bowel symptoms in patients with endometriosis (102, 103). After 6 months on a low-FODMAP diet, women with endometriosis reported reduced pain and improved quality of life (104). After 3 months on a low-FODMAP diet, significant improvements were observed in all chronic pelvic pain, dysmenorrhea, and both intestinal and extraintestinal symptoms (105).
3.4 Gluten-free diet
GFD involves avoiding foods containing gluten (such as wheat, barley, spelled kham, rye, and triticale). The diet typically includes carbohydrates without gluten (GF) sources (such as rice, quinoa, corn, buckwheat, and legumes) and/or industrial GF products. Gluten-free products are typically high in saturated fats, sugars, and salts (106) and low in protein, fiber, and vitamins (107). Pain symptoms of endometriosis were alleviated after 12 months on a gluten-free diet (108). Celiac disease (CD) is an autoimmune disorder affecting 1% of the population, causing reversible inflammation of the small intestine mucosa and accompanied by acute symptoms such as diarrhea, constipation, bloating, nausea, and vomiting. A lifelong gluten-free diet (GFD) is a treatment for Crohn’s disease (109). However, some studies suggest that the increased gut side effects of a gluten-free diet may lead to adverse health outcomes (Table 1) (101).
Table 1
| Dietary patterns | Effect | Clinical research of endometriosis |
|---|---|---|
| Mediterranean diet (87) | 1. Reducing inflammation (73) 2. Oxidative stress (76) 3. Optimize mitochondrial function (84) | 1. Inflammatory factors ↓ (88) 2. Pain symptoms ↓ (88) |
| Ketogenic Diet | 1. Circulating lipids, low-grade inflammation, and oxidative stress ↓ (92) 2. TNF-α, IL-6, IL-8, MCP-1 ↓ (93) | 1. Increase LDL cholesterol ↑ (90) 2. Decreased triglycerides, total cholesterol, and low-density lipoprotein ↓ (91); High-density lipoprotein ↑ 3. Estradiol, progesterone, and SHBG were elevated ↑ (91) |
| Low FODMAP diet | 1. Pain, abdominal distension of IBS and IBD ↓ (101, 163) | 1. Abdominal distension and diarrhea ↓ (102, 103) 2. Pelvic pain ↓; Abdominal distension ↓ (104) |
| Gluten-free diet | 1. Irritable bowel symptoms ↓ (164) 2. Diarrhea, constipation, bloating ↓ (109) | 1. Dysmenorrhea ↓ (108) |
The effect of different dietary patterns on endometriosis.
IL-6, Interleukin-6; IL-8, Interleukin-8; LDL, Low density lipoprotein; MCP-1, Monocyte chemotactic protein-1; TNF-α, Tumor necrosis factor-α.
4 Effects of nutrient supplementation in endometriosis
The pathogenesis of endometriosis remains under investigation, prompting many affected women to adopt dietary interventions to manage symptoms and enhance daily living (16). A cross-sectional study revealed a dietary shift among endometriosis patients, with increased consumption of vegetables, fruits, grains, legumes, and fish, coupled with a decrease in dairy products, soy-based foods, and high saturated fat intake (110). An Italian study reported that 76% of women with endometriosis employed self-management strategies, with 44% opting for dietary changes, achieving a high score in dietary management effectiveness (111).
Various dietary patterns influence the onset of endometriosis by modifying the levels of inflammatory factors and the inflammation pathways within the body. Consuming specific foods influences the levels of inflammatory factors, regulates angiogenesis, affects the estrogen metabolism pathway (8), and can promote or inhibit the progression of endometriosis. Supplementation with antioxidant vitamins can effectively reduce dysmenorrhea severity, ameliorate chronic pelvic pain, and enhance the quality of life for these patients (112). The risk of endometriosis can be mitigated by dietary adjustments, particularly the consumption of nutrients with anti-inflammatory and antioxidant properties (113). We summarized the antioxidant capacity of various nutrients and their roles in animal models of endometriosis and endometriosis patients.
4.1 Vitamin C and vitamin E
VC can inhibit ROS and AKT/mTOR signaling, thereby preventing mesenchymal stem cell aging (114). Additionally, VC inhibits HIF1a transcription and increases HIF1 alpha-hydroxylase activity, leading to mitochondrial activation (115) and antioxidant effects. In mouse models, VC contributes to anti-ovarian aging by influencing collagen synthesis, angiogenesis, aging, cell proliferation, and differentiation (116). Intravenous vitamin C treatment prevents the induction of endometrial implants while inhibiting the regression of existing ones in mouse models (117). VE significantly lowers serum levels of CRP and IL-6 (118). Vitamin E enhances antioxidant status by increasing TAC levels in postmenopausal women, offering greater anxiety relief compared to the placebo group (119). Vitamin C and E supplementation alters the expression and production of vascular endothelial growth factor genes in patients with endometriosis (120), effectively reducing the severity and improving pelvic pain (121).
4.2 Vitamin D
VD can inhibit the activation of nuclear factor kappa B, reduce the expression of IL-1β, TNF-α, and IL-8, and decrease prostaglandin activity (122). In a mouse model, supplementation with VD induces an anti-inflammatory phenotype in macrophages and exhibits anti-proliferative properties (123). Serum levels of 25-hydroxyvitamin D3 negatively correlate with endometriosis (124). People with endometriosis who take vitamin D have much less pelvic pain and lower levels of hs-CRP, TAC, and total cholesterol/high-density lipoprotein cholesterol ratio (125). Supplementation with VD reduces pelvic pain in young patients with endometriosis (126) and ameliorates immune-inflammatory biomarkers in young postmenopausal women (127).
4.3 Epigallocatechin-3-gallate
The antioxidant and anti-inflammatory properties of tea polyphenols may have potential therapeutic effects on endometriosis (128). The amount of mRNA for nuclear factor kappa B and mitogen-activated protein kinase 1 goes up when epigallocatechin-3-gallate (EGCG) is present (129). Furthermore, in mice, EGCG selectively inhibits angiogenesis and blood perfusion in endometriotic lesions, leading to their regression (130). Pro-EGCG greatly slows down the development, growth, and formation of new blood vessels in experimental endometriosis, showing that it can both protect cells from damage and stop the growth of new blood vessels (131). Some genes, like vascular endothelial growth factor C (VEGFC) and the tyrosine kinase receptor VEGF receptor 2 (VEGFR2), are turned down by EGCG (132). EGCG downregulates VEGFC/VEGFR2 signaling through pathways involving c-JUN, interferon-γ, matrix metalloproteinase-9, and chemokine ligand 3, thereby inhibiting endothelial proliferation, inflammation, and cell migration. EGCG prevents the activation of MAPK and Smad signaling pathways stimulated by TGF-β1 in both endometrial and endometriotic stromal cells by a large amount. Animal experiments indicate that EGCG can prevent the progression of fibrosis in endometriosis (133).
4.4 Melatonin
In endometriosis, there is an increased expression of melatonin receptors (134). Melatonin mitigates oxidative stress, inflammation processes, and cell apoptosis by upregulating the Nrf2 signaling pathway and downregulating COX-2 protein levels (135), as well as SOD, GPx, CAT, and Bcl-2 activities (136). Melatonin inhibits the development of endometriosis by disrupting mitochondrial function and regulating siRNA expression in mouse models (137). Melatonin treatment reduces MMP-3 activity in mouse endometriosis models and facilitates endometriosis regression through caspase-3-mediated pathways, enhancing endometriotic cell apoptosis (138). Melatonin lowers the activity and expression of proMMP-9, which has anti-inflammatory effects and lessens the damage caused by peritoneal endometriosis in mice (139). A clinical study suggests that oral administration of 10 mg of melatonin before bedtime during menstruation provides better analgesic relief for dysmenorrheal (140).
4.5 Quercetin
Quercetin can reduce the expression of IL-6, IL-1β, and TNFα (141). Quercetin induces p21 CDK inhibitors that decrease pRb phosphorylation by capturing E2F1, thus inhibiting G1/S cell cycle progression. Low doses of quercetin induce mild DNA damage and activate Chk2, a key regulator of quercetin-induced p21 expression. Quercetin also lowers the levels of cyclin B1 and CDK1, which prevent transcription. This suggests that quercetin can stop the cell cycle from moving forward in physiologically relevant doses (142). Quercetin treatment effectively suppresses the growth of endometrial lesions in mice with endometriosis (143).
Quercetin induces the downregulation of ERK1/2, P38 MAPK, and AKT signaling molecules, leading to DNA fragmentation, mitochondrial membrane potential loss, and ROS production, thereby inducing cell apoptosis (144). Following intraperitoneal quercetin injection in mice, Ccnd1 mRNA expression was significantly reduced compared to the control group, leading to G0/G1 cell cycle arrest and reduced cell proliferation, accompanied by increased apoptosis of VK2/E6E7 and End1/E6E7 cells. In mouse models, the activity of oxidative stress markers is reduced in vivo through the Nrf2 signaling pathway (145). For example, it can prevent LPS from causing oxidative stress in the jejunum by activating the MAPK/Nrf2 signaling pathway. This can fix the damage that LPS does to the mitochondria in the jejunum and increase the expression of mitochondrial DNA copy number-related genes like COX1, ATP6, and ND1 (146).
4.6 Resveratrol
Resveratrol (RSV), a polyphenol found in red wine, regulates NF-κB activity (147). It inhibits Th17 cells, decreasing the production of the inflammatory factor IL-17 (148). When resveratrol is added to the stromal cells of women with endometriosis, the levels of IGF-1 and HGF go down (149).
Resveratrol decreases the concentrations of MCP1, VEGF (150), IL-6, IL-8, and TNF α in peritoneal fluid in vitro and in animal models (151), inhibiting angiogenesis and inflammation to regress endometriotic lesions (152). It reduces the invasiveness of endometrial stromal cells and curbs the progression of endometriosis in nude mouse models (153). Metastasis-associated protein 1 (MTA1) increases epithelial-mesenchymal transition (EMT) through its connection with ZEB2. Resveratrol suppresses ectopic lesions’ growth and MTA1 and ZEB2 expression. MTA1 may be a target for resveratrol (154). Taking 400 mg of resveratrol every day for 12–14 weeks lowers the amounts of matrix metalloproteinases MMP-2 and MMP-9 in endometriosis patients’ serum and peritoneal fluid, which lowers inflammation (155).
4.7 Curcumin
E2 levels in endometriosis lesions are significantly elevated; supplementing with curcumin can notably decrease estradiol levels, thereby inhibiting the growth rate of endometriosis (156). Curcumin can additionally suppress endometrial cells in endometriosis by downregulating the vascular endothelial growth factor (157). Curcumin contributes to the treatment of mouse models for endometriosis by modulating the HIF signaling pathway, ameliorating local hypoxia, and diminishing inflammation (158). Curcumin therapy reduces endometriosis in mice by preventing NFκB translocation and suppressing MMP-3 expression. It primarily enhances cell apoptosis in endometriosis via the cytochrome c-mediated mitochondrial pathway (159). Curcumin slows the progression of endometriosis in mice by inhibiting MMP-2 activity (160). Curcumin blocks endometriosis by reducing matrix metalloproteinase-9 activity (161). Curcumin supplementation can ameliorate oxidative stress levels (MDA and TAC) in postmenopausal women and lower inflammatory biomarkers (hs-CRP) (Table 2) (119).
Table 2
| Nutrient | Molecular mechanism | Experimental animal models | Clinical research |
|---|---|---|---|
| VC | 1. ROS and AKT/mTOR signal transduction ↓ (114) 2. Hif1-α hydroxylase activity ↑; HIF1-α transcription ↓; Mitochondrial activation ↑ (115) 3. Change VEGF gene expression and production (120) | 1. Rat: Anti-ovarian aging (116) 2. Rat: Inhibit the growth of endometriosis lesions (117) | Vitamin C and vitamin E supplements: 1. Dysmenorrhea ↓ (121) 2. Systemic oxidative stress index ↓ (121) |
| VE | CRP and IL-6 ↓ (118) | TAC ↑, antioxidant ↑, and anxiety ↓ in postmenopausal women (119) | |
| VD | 1. Activation of NF -κB ↓; IL-1β ↓, IL-8 ↓; prostaglandin activity ↓ (122) 2. IL-6 ↓, TNF-α1 ↓ (165) | Rat: Anti-proliferative properties and induces an anti-inflammatory phenotype in macrophages (123) | 1. Dysmenorrhea ↓ (126, 166) 2. Immuno-flammatory biomarkers ↓ (127) 3. Negatively associated with endometriosis in American women (124) 4. hs-CRP ↓, TAC ↑, and dysmenorrheal ↓ (125) |
| Viburnum opulus L. | Antioxidant capacity ↑ (167) | Rat: Induced lesion reduction (168) | NA |
| Melatonin | 1. Nrf2 signaling pathway ↑ COX-2 protein ↓ (135) 2. SOD, GPx, CAT and Bcl-2 activity ↑ (136) 3. The melatonin receptor ↑ (134) | 1. Rat: Disrupt mitochondrial function and regulate tiRNA to inhibit (137) 2. Rat: MMP-3 ↓, apoptosis ↑, Bcl-2 ↓, Bax ↑, caspase-9 activity ↑ (138) 3. Rat: proMMP-9 ↓, TIMP-1↑ (139) | 1. Dysmenorrhea ↓ (140) |
| EGCG (128) | 1. Inhibits estrogen-induced activation of endometrial cells (130) 2. NFκB ↑, mitogen-activated protein kinase1- mRNA ↑ (129) | 1. Rat: angiogenesis ↓, lesion ↓ (131) 2. Rat: VEGF-C/VEGFR2 expression ↓, lesion ↓, reduced VEGFR2 and ERK activation (132) 3. Rat: antifibrosis (133) 4. Rat: Selective inhibition VEGF-A (129) | NA |
| Trans-fatty acid | Tumor necrosis factor system hyperactive: TNFR, IL-6and CRP ↑ (169) | Rat: TNFα and redox status ↑, oxidative stress ↑, estrogen receptor 1 isoform and progesterone receptor ↓ (69) | The highest fifth of intakes were 48 percent more likely to be diagnosed with endometriosis (15) |
| Dairy | Rat: TNF-α ↓, IL-6 ↓ (170) | Dose-dependent reduction of endometriosis risk (11, 171) | |
| Quercetin | 1. IL-6, IL-1β, and TNF-α ↓ (141) 2. Induce p21 CDK inhibitor, inhibit the G1/S cell cycle; induced mild DNA damage and Chk2 activation; inhibits G2/M cell cycle, Transcriptional inhibition by inhibit NF-Y (142) 3. Inhibit VK2/E6E7, End1/ E6E7 and induce cell cycle arrest; ERK1/2 ↓, P38 MAPK ↓, and AKT ↓ (144) | 1. Rat: NF-κB ↓, oxidative stress ↓ (145) 2. Broiler chickens: oxidative stress ↓ by MAPK/Nrf2; Mitochondrial damage ↓; Nrf2 ↓; MAPK ↓, JNK ↓, ERK ↓, and p38MAPK ↓ (146) 3. mRNA of Ccnd1 ↓; VK2/E6E7↑ and End1 / E6E7 apoptosis ↑; miR-503-5p and miR-546↑ (144) | NA |
| Resveratrol (RSV) | 1. Th17 cells ↓, IL-17 ↓ (148) 2. IGF-1 ↓, HGF ↓ (149) | 1. Invasiveness ↓ (153) 2. Rat: Inflammatory cytokine ↓, COX enzyme ↓, prostaglandin ↓, inflammation ↓ (152) 3. Rat: MTA1 ↓, ZEB2 ↓ (154) | mRNA and protein levels of MMP-2 and MMP-9 ↓ (155), inflammation ↓ (155). |
| Curcumin | 1. Estradiol ↓ (156) 2. VEGF ↓ (157) | 1. Rat: HIF-1α mRNA and protein synthesis ↓; IL-1β, IL-6, and VEGFA ↓ (158) 2. NFκB ↓, MMP-3 ↓ (159) 3. MMP-2 ↓ (160) 4. MMP-9 ↓ (161) | MDA ↓, hs-CRP ↓ and TAC ↑ (119) in menopausal women |
The effect of nutrients in endometriosis.
Bcl-2, B-cell lymphoma-2; CAT, Catalase; COX2, Cyclo-oxygenase2; CRP, C-reactive protein; ERK, Extracellular regulated protein kinases; GPx, Glutathione Peroxidase; HGF, Hepatocyte growth factor; HIF1-α, Hypoxia inducible factor-1α; hs-CRP, High sensitivity C-reactive protein; IGF-1, Insulin-like growth factor-1; MAPK, Mitogen-activated protein kinases; MTA1, Metastasis associated protein 1; MMP, Matrix metalloproteinase; NF-κB, Nuclear factor kappa-B; Nrf2, Nuclear factor erythroid 2-related factor 2; p21 CDK inhibitor, P 21 cyclin dependent kinase inhibitor; ROS, Reactive oxygen species; SOD, Superoxide Dismutase; TAC, Total antioxidant capacity; TIMP, Tissue inhibitor of metalloproteinase; Th17, T helper 17 cell; VEGF, Vascular endothelial growth factor; VEGFR2, Vascular Endothelial Growth Factor Receptor 2; ZEB2, Zinc Finger E-Box Binding Homeobox 2.
5 Conclusion
Oxidative stress and inflammatory factors play crucial roles in endometriosis. This article summarizes the roles of oxidative stress and inflammatory factors in endometriosis and reviews the risk factors of endometriosis. Additionally, we have summarized the current mainstream dietary patterns and the impact of common nutrients on endometriosis. Supplementing nutrients can inhibit the progression of endometriosis in various ways, such as reducing levels of inflammatory factors, decreasing oxidative stress responses, inhibiting cell proliferation, and reducing angiogenesis. The Mediterranean dietary paradigm, renowned as a quintessential anti-inflammatory dietary regimen, is capable of curtailing the levels of inflammatory mediators within the body and mitigating the symptoms experienced by patients afflicted with endometriosis. Nutrients endowed with anti-inflammatory properties, including vitamins (such as vitamin C and vitamin E), quercetin, resveratrol, curcumin, and epigallocatechin-3-gallate (EGCG), hold the potential to diminish the lesions associated with endometriosis and thus exhibit latent therapeutic implications. Analyzing endometriosis patients’ dietary traits and formulating new preventive dietary strategies (162) promise to mitigate disease incidence and lessen the patients’ burden. The anti-inflammatory and antioxidant properties of nutrients may be key areas of focus. Currently, there are no dietary guidelines for endometriosis patients, and previous studies were limited in sample size and primarily descriptive. Existing research on dietary patterns and the role of nutrients in endometriosis is insufficiently comprehensive. There is a pressing need to delve deeper into the pathogenesis of endometriosis and the impact of varied dietary patterns, aiming to identify optimal dietary regimes for these patients. This approach holds significant promise for alleviating symptoms and halting disease progression.
Statements
Author contributions
LZ: Data curation, Writing – original draft, Writing – review & editing. BL: Investigation, Writing – original draft. XJ: Formal analysis, Methodology, Writing – original draft. LJ: Conceptualization, Visualization, Writing – review & editing. KL: Project administration, Resources, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Scientific research funding project of Liaoning Provincial Department of Science and Technology (No. 2020JH2/10300050).
Acknowledgments
The authors thank the support of the funding “Scientific research funding project of Liaoning Provincial Department of Science and Technology (No. 2020JH2/10300050)” to this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
- COX2
Cyclo-oxygenase2
- ESCs
Endometrial stromal cells
- ERK
Extracellular regulated protein kinases
- HIF-α
Hypoxia inducible factor-1 α
- HT
Hydroxytyrosol
- MAPK
Mitogen-activated protein kinases
- MCP-1
Monocyte chemotactic protein-1
- MUFA
Monounsaturated fatty acids
- mtDNA
Mitochondria DNA
- NGF
Nerve growth factor
- NF-κB
Nuclear factor kappa-B
- OO
Olive oil
- OS
Oxidative stress
- OXPHOS
Oxidative Phosphorylation
- PKC
Phosphokinase C
- PI3K
Phosphate inositol 3 kinase
- PUFA
Polyunsaturated fatty acids
- RSV
Resveratrol
- ROS
Reactive oxygen species
- TSC
Tuberculous protein sclerosis complex
- TLR4
Toll like receptor 4
- VEGF
Vascular endothelial growth factor
- WFKD
Well-formulated ketogenic diet
Glossary
References
1.
UpsonK. Environmental risk factors for endometriosis: a critical evaluation of studies and recommendations from the epidemiologic perspective. Curr Epidemiol Rep. (2020) 7:149–70. doi: 10.1007/s40471-020-00236-3
2.
ViganoPParazziniFSomiglianaEVercelliniP. Endometriosis: epidemiology and aetiological factors. Best Pract Res Clin Obstet Gynaecol. (2004) 18:177–200. doi: 10.1016/j.bpobgyn.2004.01.007
3.
AgarwalAAponte-MelladoAPremkumarBJShamanAGuptaS. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol. (2012) 10:49. doi: 10.1186/1477-7827-10-49
4.
CapobiancoAMonnoACottoneLVenneriMABiziatoDdi PuppoFet al. Proangiogenic Tie2(+) macrophages infiltrate human and murine endometriotic lesions and dictate their growth in a mouse model of the disease. Am J Pathol. (2011) 179:2651–9. doi: 10.1016/j.ajpath.2011.07.029
5.
McKinnonBDBertschiDBersingerNAMuellerMD. Inflammation and nerve fiber interaction in endometriotic pain. Trends Endocrinol Metab. (2015) 26:1–10. doi: 10.1016/j.tem.2014.10.003
6.
KalfasMChisariCWindgassenS. Psychosocial factors associated with pain and health-related quality of life in endometriosis: a systematic review. Eur J Pain. (2022) 26:1827–48. doi: 10.1002/ejp.2006
7.
KoeneRJPrizmentAEBlaesAKonetySH. Shared risk factors in cardiovascular disease and cancer. Circulation. (2016) 133:1104–14. doi: 10.1161/CIRCULATIONAHA.115.020406
8.
Vennberg KarlssonJPatelHPrembergA. Experiences of health after dietary changes in endometriosis: a qualitative interview study. BMJ Open. (2020) 10:e032321. doi: 10.1136/bmjopen-2019-032321
9.
NirgianakisKEggerKKalaitzopoulosDRLanzSBallyLMuellerMD. Effectiveness of dietary interventions in the treatment of endometriosis: a systematic review. Reprod Sci. (2022) 29:26–42. doi: 10.1007/s43032-020-00418-w
10.
ParazziniFChiaffarinoFSuraceMChatenoudLCiprianiSChianteraVet al. Selected food intake and risk of endometriosis. Hum Reprod. (2004) 19:1755–9. doi: 10.1093/humrep/deh395
11.
QiXZhangWGeMSunQPengLChengWet al. Relationship between dairy products intake and risk of endometriosis: a systematic review and dose-response meta-analysis. Front Nutr. (2021) 8:701860. doi: 10.3389/fnut.2021.701860
12.
SamanehYShahidehJahanianSAzadehMAnoshirvanK. The association of food consumption and nutrient intake with endometriosis risk in Iranian women: a case-control study. Int J Reprod Biomed. (2019) 17:661–70. doi: 10.18502/ijrm.v17i9.5102
13.
KhanakiKNouriMArdekaniAMGhassemzadehAShahnaziVSadeghiMRet al. Evaluation of the relationship between endometriosis and omega-3 and omega-6 polyunsaturated fatty acids. Iran Biomed J. (2012) 16:38–43. doi: 10.6091/ibj.1025.2012
14.
YamamotoAHarrisHRVitonisAFChavarroJEMissmerSA. A prospective cohort study of meat and fish consumption and endometriosis risk. Am J Obstet Gynecol. (2018) 219:178.e1–178.e10. doi: 10.1016/j.ajog.2018.05.034
15.
MissmerSAChavarroJEMalspeisSBertone-JohnsonERHornsteinMDSpiegelmanDet al. A prospective study of dietary fat consumption and endometriosis risk. Hum Reprod. (2010) 25:1528–35. doi: 10.1093/humrep/deq044
16.
O'HaraRRoweHFisherJ. Self-management in condition-specific health: a systematic review of the evidence among women diagnosed with endometriosis. BMC Womens Health. (2019) 19:80. doi: 10.1186/s12905-019-0774-6
17.
SampsonJA. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am J Pathol. (1927) 3:93–110.43. PMID:
18.
FergusonBRBenningtonJLHaberSL. Histochemistry of mucosubstances and histology of mixed mullerian pelvic lymph node glandular inclusions. Evidence for histogenesis by mullerian metaplasia of coelomic epithelium. Obstet Gynecol. (1969) 33:617–25. PMID:
19.
Parente BarbosaCBentes de SouzaAMBiancoBChristofoliniDM. The effect of hormones on endometriosis development. Minerva Ginecol. (2011) 63:375–86. PMID:
20.
UlukusMAriciA. Immunology of endometriosis. Minerva Ginecol. (2005) 57:237–48. PMID:
21.
DonnezJBindaMMDonnezODolmansMM. Oxidative stress in the pelvic cavity and its role in the pathogenesis of endometriosis. Fertil Steril. (2016) 106:1011–7. doi: 10.1016/j.fertnstert.2016.07.1075
22.
SimoniJSimoniOLoxCDMcGunegleDEFeolaM. Cytokines and PAF release from human monocytes and macrophages: effect of hemoglobin and contaminants. Artif Cells Blood Substit Immobil Biotechnol. (1994) 22:525–34. doi: 10.3109/10731199409117880
23.
Van LangendoncktACasanas-RouxFDonnezJ. Oxidative stress and peritoneal endometriosis. Fertil Steril. (2002) 77:861–70. doi: 10.1016/S0015-0282(02)02959-X
24.
LousseJCDefrèreSvan LangendoncktAGrasJGonzález-RamosRColetteSet al. Iron storage is significantly increased in peritoneal macrophages of endometriosis patients and correlates with iron overload in peritoneal fluid. Fertil Steril. (2009) 91:1668–75. doi: 10.1016/j.fertnstert.2008.02.103
25.
ArumugamKYipYC. De novo formation of adhesions in endometriosis: the role of iron and free radical reactions. Fertil Steril. (1995) 64:62–4. doi: 10.1016/S0015-0282(16)57655-9
26.
DefrèreSLangendoncktAVVaesenSJouretMGonzálezRRet al. Iron overload enhances epithelial cell proliferation in endometriotic lesions induced in a murine model. Hum Reprod. (2006) 21:2810–6. doi: 10.1093/humrep/del261
27.
WyattJFernandoSMPowellSGHillCJArshadIProbertCet al. The role of iron in the pathogenesis of endometriosis: a systematic review. Hum Reprod Open. (2023) 2023:hoad033. doi: 10.1093/hropen/hoad033
28.
StaniekKGilleLKozlovAVNohlH. Mitochondrial superoxide radical formation is controlled by electron bifurcation to the high and low potential pathways. Free Radic Res. (2002) 36:381–7. doi: 10.1080/10715760290021225
29.
LuJWangZCaoJChenYDongY. A novel and compact review on the role of oxidative stress in female reproduction. Reprod Biol Endocrinol. (2018) 16:80. doi: 10.1186/s12958-018-0391-5
30.
NgôCChéreauCNiccoCWeillBChapronCBatteuxFet al. Reactive oxygen species controls endometriosis progression. Am J Pathol. (2009) 175:225–34. doi: 10.2353/ajpath.2009.080804
31.
AssafLEidAANassifJ. Role of AMPK/mTOR, mitochondria, and ROS in the pathogenesis of endometriosis. Life Sci. (2022) 306:120805. doi: 10.1016/j.lfs.2022.120805
32.
ScutieroGIannonePBernardiGBonaccorsiGSpadaroSVoltaCAet al. Oxidative stress and endometriosis: a systematic review of the literature. Oxidative Med Cell Longev. (2017) 2017:7265238. doi: 10.1155/2017/7265238
33.
KaponisAIwabeTTaniguchiFItoMDeuraIDecavalasGet al. The role of NF-kappaB in endometriosis. Front Biosci (Schol Ed). (2012) 4:1213–34. doi: 10.2741/s327
34.
SymonsLKMillerJEKayVRMarksRMLiblikKKotiMet al. The immunopathophysiology of endometriosis. Trends Mol Med. (2018) 24:748–62. doi: 10.1016/j.molmed.2018.07.004
35.
BerbicMSchulkeLMarkhamRTokushigeNRussellPFraserIS. Macrophage expression in endometrium of women with and without endometriosis. Hum Reprod. (2009) 24:325–32. doi: 10.1093/humrep/den393
36.
ChanRWSLeeCLNgEHYYeungWSB. Co-culture with macrophages enhances the clonogenic and invasion activity of endometriotic stromal cells. Cell Prolif. (2017) 50:e12330. doi: 10.1111/cpr.12330
37.
YangHLZhouWJChangKKMeiJHuangLQWangMYet al. The crosstalk between endometrial stromal cells and macrophages impairs cytotoxicity of NK cells in endometriosis by secreting IL-10 and TGF-beta. Reproduction. (2017) 154:815–25. doi: 10.1530/REP-17-0342
38.
MacerMLTaylorHS. Endometriosis and infertility: a review of the pathogenesis and treatment of endometriosis-associated infertility. Obstet Gynecol Clin N Am. (2012) 39:535–49. doi: 10.1016/j.ogc.2012.10.002
39.
RiccioLGCBaracatECChapronCBatteuxFAbrãoMS. The role of the B lymphocytes in endometriosis: a systematic review. J Reprod Immunol. (2017) 123:29–34. doi: 10.1016/j.jri.2017.09.001
40.
RandallGWGanttPAPoe-ZeiglerRLBergmannCANoelMEStrawbridgeWRet al. Serum antiendometrial antibodies and diagnosis of endometriosis. Am J Reprod Immunol. (2007) 58:374–82. doi: 10.1111/j.1600-0897.2007.00523.x
41.
YoshinoOOsugaYHirotaYKogaKHirataTHaradaMet al. Possible pathophysiological roles of mitogen-activated protein kinases (MAPKs) in endometriosis. Am J Reprod Immunol. (2004) 52:306–11. doi: 10.1111/j.1600-0897.2004.00231.x
42.
CarliCMetzCNAl-AbedYNaccachePHAkoumA. Up-regulation of cyclooxygenase-2 expression and prostaglandin E2 production in human endometriotic cells by macrophage migration inhibitory factor: involvement of novel kinase signaling pathways. Endocrinology. (2009) 150:3128–37. doi: 10.1210/en.2008-1088
43.
VeillatVCarliCMetzCNAl-AbedYNaccachePHAkoumAet al. Macrophage migration inhibitory factor elicits an angiogenic phenotype in human ectopic endometrial cells and triggers the production of major angiogenic factors via CD44, CD74, and MAPK signaling pathways. J Clin Endocrinol Metab. (2010) 95:E403–12. doi: 10.1210/jc.2010-0417
44.
HennessyBTSmithDLRamPTLuYMillsGB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. (2005) 4:988–1004. doi: 10.1038/nrd1902
45.
McKinnonBDKocbekVNirgianakisKBersingerNAMuellerMD. Kinase signalling pathways in endometriosis: potential targets for non-hormonal therapeutics. Hum Reprod Update. (2016) 22:382–403. doi: 10.1093/humupd/dmv060
46.
ManningBD. Balancing Akt with S6K: implications for both metabolic diseases and tumorigenesis. J Cell Biol. (2004) 167:399–403. doi: 10.1083/jcb.200408161
47.
LiYCorradettiMNInokiKGuanKL. TSC2: filling the GAP in the mTOR signaling pathway. Trends Biochem Sci. (2004) 29:32–8. doi: 10.1016/j.tibs.2003.11.007
48.
LaplanteMSabatiniDM. mTOR signaling at a glance. J Cell Sci. (2009) 122:3589–94. doi: 10.1242/jcs.051011
49.
KararJMaityA. PI3K/AKT/mTOR pathway in angiogenesis. Front Mol Neurosci. (2011) 4:51. doi: 10.3389/fnmol.2011.00051
50.
JinXFengJChengX. LncRNA IGF2-AS promotes endometriosis progression through targeting miR-370-3p/IGF2 axis and activating PI3K/AKT/mTOR signaling pathway. J Assist Reprod Genet. (2022) 39:2699–710. doi: 10.1007/s10815-022-02638-2
51.
BadaryDMAbou-TalebHAIbrahimM. Hypoxia-inducible factor-1alpha and mTOR as a potential therapeutic target in endometriosis: an immunohistochemical study. Appl Immunohistochem Mol Morphol. (2023) 31:629–34. doi: 10.1097/PAI.0000000000001148
52.
WangYZhuLKuokkanenSPollardJW. Activation of protein synthesis in mouse uterine epithelial cells by estradiol-17beta is mediated by a PKC-ERK1/2-mTOR signaling pathway. Proc Natl Acad Sci USA. (2015) 112:E1382–91. doi: 10.1073/pnas.1418973112
53.
GoriIPellegriniCStaedlerDRussellRJanCCannyGO. Tumor necrosis factor-alpha activates estrogen signaling pathways in endometrial epithelial cells via estrogen receptor alpha. Mol Cell Endocrinol. (2011) 345:27–37. doi: 10.1016/j.mce.2011.06.043
54.
HuangZXHeXRDingXYChenJHLeiYHBaiJBet al. Lipoxin A4 depresses inflammation and promotes autophagy via AhR/mTOR/AKT pathway to suppress endometriosis. Am J Reprod Immunol. (2023) 89:e13659. doi: 10.1111/aji.13659
55.
HeYXiongTGuoFduZFanYSunHet al. Interleukin-37b inhibits the growth of murine endometriosis-like lesions by regulating proliferation, invasion, angiogenesis and inflammation. Mol Hum Reprod. (2020) 26:240–55. doi: 10.1093/molehr/gaaa014
56.
LiLLiaoZYeMJiangJ. Recombinant human IL-37 inhibited endometriosis development in a mouse model through increasing Th1/Th2 ratio by inducing the maturation of dendritic cells. Reprod Biol Endocrinol. (2021) 19:128. doi: 10.1186/s12958-021-00811-3
57.
PengBAlotaibiFTSediqiSBedaiwyMAYongPJ. Role of interleukin-1beta in nerve growth factor expression, neurogenesis and deep dyspareunia in endometriosis. Hum Reprod. (2020) 35:901–12. doi: 10.1093/humrep/deaa017
58.
KatoTYasudaKMatsushitaKIshiiKJHirotaSYoshimotoTet al. Interleukin-1/−33 signaling pathways as therapeutic targets for endometriosis. Front Immunol. (2019) 10:2021. doi: 10.3389/fimmu.2019.02021
59.
Nishimoto-KakiuchiASatoINakanoKOhmoriHKayukawaYTanimuraHet al. A long-acting anti-IL-8 antibody improves inflammation and fibrosis in endometriosis. Sci Transl Med. (2023) 15:eabq5858. doi: 10.1126/scitranslmed.abq5858
60.
ShiJLZhengZMChenMShenHHLiMQShaoJ. IL-17: an important pathogenic factor in endometriosis. Int J Med Sci. (2022) 19:769–78. doi: 10.7150/ijms.71972
61.
MillerJEAhnSHMarksRMMonsantoSPFazleabasATKotiMet al. IL-17A modulates peritoneal macrophage recruitment and M2 polarization in endometriosis. Front Immunol. (2020) 11:108. doi: 10.3389/fimmu.2020.00108
62.
KhanKNKitajimaMImamuraTHirakiKFujishitaASekineIet al. Toll-like receptor 4-mediated growth of endometriosis by human heat-shock protein 70. Hum Reprod. (2008) 23:2210–9. doi: 10.1093/humrep/den195
63.
HarrisHREkeACChavarroJEMissmerSA. Fruit and vegetable consumption and risk of endometriosis. Hum Reprod. (2018) 33:715–27. doi: 10.1093/humrep/dey014
64.
DarlingAMChavarroJEMalspeisSHarrisHR. A prospective cohort study of vitamins B, C, E, and multivitamin intake and endometriosis. J Endometr. (2013) 5:17–26. doi: 10.5301/je.5000151
65.
Mier-CabreraJAburto-SotoTBurrola-MéndezSJiménez-ZamudioLTolentinoMCCasanuevaEet al. Women with endometriosis improved their peripheral antioxidant markers after the application of a high antioxidant diet. Reprod Biol Endocrinol. (2009) 7:54. doi: 10.1186/1477-7827-7-54
66.
ArisAParisK. Hypothetical link between endometriosis and xenobiotics-associated genetically modified food. Gynecol Obstet Fertil. (2010) 38:747–53. doi: 10.1016/j.gyobfe.2010.08.030
67.
AfrinSAlAshqarAel SabehMMiyashita-IshiwataMReschkeLBrennanJTet al. Diet and nutrition in gynecological disorders: a focus on clinical studies. Nutrients. (2021) 13:1747. doi: 10.3390/nu13061747
68.
ArabAKarimiEVingrysKKelishadiMRMehrabaniSAskariG. Food groups and nutrients consumption and risk of endometriosis: a systematic review and meta-analysis of observational studies. Nutr J. (2022) 21:58. doi: 10.1186/s12937-022-00812-x
69.
HeardMEMelnykSBSimmenFAYangYPabonaJMPSimmenRCM. High-fat diet promotion of endometriosis in an immunocompetent mouse model is associated with altered peripheral and ectopic lesion redox and inflammatory status. Endocrinology. (2016) 157:2870–82. doi: 10.1210/en.2016-1092
70.
SchwartzNRMAfeicheMCTerryKLFarlandLVChavarroJEMissmerSAet al. Glycemic index, glycemic load, fiber, and gluten intake and risk of laparoscopically confirmed endometriosis in premenopausal women. J Nutr. (2022) 152:2088–96. doi: 10.1093/jn/nxac107
71.
RománGCJacksonREGadhiaRRománANReisJ. Mediterranean diet: the role of long-chain omega-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev Neurol (Paris). (2019) 175:724–41. doi: 10.1016/j.neurol.2019.08.005
72.
ShannonOMStephanBCMMinihaneAMMathersJCSiervoM. Nitric oxide boosting effects of the Mediterranean diet: a potential mechanism of action. J Gerontol A Biol Sci Med Sci. (2018) 73:902–4. doi: 10.1093/gerona/gly087
73.
TostiVBertozziBFontanaL. Health benefits of the Mediterranean diet: metabolic and molecular mechanisms. J Gerontol A Biol Sci Med Sci. (2018) 73:318–26. doi: 10.1093/gerona/glx227
74.
EstruchRMartínez-GonzálezMÁCorellaDSalas-SalvadóJRuiz-GutiérrezVCovasMIet al. Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial. Ann Intern Med. (2006) 145:1–11. doi: 10.7326/0003-4819-145-1-200607040-00004
75.
Salas-SalvadóJGarcia-ArellanoAEstruchRMarquez-SandovalFCorellaDFiolMet al. Components of the Mediterranean-type food pattern and serum inflammatory markers among patients at high risk for cardiovascular disease. Eur J Clin Nutr. (2008) 62:651–9. doi: 10.1038/sj.ejcn.1602762
76.
ZamboninLCalicetiCVieceli Dalla SegaFFiorentiniDHreliaLLandiSet al. Dietary phenolic acids act as effective antioxidants in membrane models and in cultured cells, exhibiting proapoptotic effects in leukaemia cells. Oxidative Med Cell Longev. (2012) 2012:839298. doi: 10.1155/2012/839298
77.
Andreo-LópezMCContreras-BolívarVMuñoz-TorresMGarcía-FontanaB. Influence of the Mediterranean diet on healthy aging. Int J Mol Sci. (2023) 24:4491. doi: 10.3390/ijms24054491
78.
KhanMMAhmadAIshratTKhanMBHodaMNKhuwajaGet al. Resveratrol attenuates 6-hydroxydopamine-induced oxidative damage and dopamine depletion in rat model of Parkinson's disease. Brain Res. (2010) 1328:139–51. doi: 10.1016/j.brainres.2010.02.031
79.
KurinEMucajiPNagyM. In vitro antioxidant activities of three red wine polyphenols and their mixtures: an interaction study. Molecules. (2012) 17:14336–48. doi: 10.3390/molecules171214336
80.
ZhangXCaoJZhongL. Hydroxytyrosol inhibits pro-inflammatory cytokines, iNOS, and COX-2 expression in human monocytic cells. Naunyn Schmiedeberg's Arch Pharmacol. (2009) 379:581–6. doi: 10.1007/s00210-009-0399-7
81.
RosignoliPFuccelliRFabianiRServiliMMorozziG. Effect of olive oil phenols on the production of inflammatory mediators in freshly isolated human monocytes. J Nutr Biochem. (2013) 24:1513–9. doi: 10.1016/j.jnutbio.2012.12.011
82.
Fernández del RíoLGutiérrez-CasadoEVarela-LópezAVillalbaJM. Olive oil and the hallmarks of aging. Molecules. (2016) 21:163. doi: 10.3390/molecules21020163
83.
JilgeBMeyerH. Coprophagy-dependant changes of the anaerobic bacterial flora in stomach and small intestine of the rabbit. Z Versuchstierkd. (1975) 17:308–14. PMID:
84.
SchafferSPodstawaMVisioliFBoganiPMüllerWEEckertGP. Hydroxytyrosol-rich olive mill wastewater extract protects brain cells in vitro and ex vivo. J Agric Food Chem. (2007) 55:5043–9. doi: 10.1021/jf0703710
85.
AfshordelSHaglSWernerDRöhnerNKögelDBazanNGet al. Omega-3 polyunsaturated fatty acids improve mitochondrial dysfunction in brain aging – impact of Bcl-2 and NPD-1 like metabolites. Prostaglandins Leukot Essent Fatty Acids. (2015) 92:23–31. doi: 10.1016/j.plefa.2014.05.008
86.
AbokhraisIMDenisonFCWhitakerLHRSaundersPTKDoustAWilliamsLJet al. A two-arm parallel double-blind randomised controlled pilot trial of the efficacy of Omega-3 polyunsaturated fatty acids for the treatment of women with endometriosis-associated pain (PurFECT1). PLoS One. (2020) 15:e0227695. doi: 10.1371/journal.pone.0227695
87.
CiebieraMEsfandyariSSibliniHPrinceLElkafasHWojtyłaCet al. Nutrition in gynecological diseases: current perspectives. Nutrients. (2021) 13:1178. doi: 10.3390/nu13041178
88.
CirilloMArgentoFRBecattiMFiorilloCCocciaMEFatiniC. Mediterranean diet and oxidative stress: a relationship with pain perception in endometriosis. Int J Mol Sci. (2023) 24:14601. doi: 10.3390/ijms241914601
89.
VinciguerraFGrazianoMHagnäsMFrittittaLTumminiaA. Influence of the Mediterranean and ketogenic diets on cognitive status and decline: a narrative review. Nutrients. (2020) 12:1019. doi: 10.3390/nu12041019
90.
BurénJEricssonMDamascenoNRTSjödinA. A ketogenic low-carbohydrate high-fat diet increases LDL cholesterol in healthy, young, Normal-weight women: a randomized controlled feeding trial. Nutrients. (2021) 13:814. doi: 10.3390/nu13030814
91.
PaoliAMancinLGiaconaMCBiancoACaprioM. Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. J Transl Med. (2020) 18:104. doi: 10.1186/s12967-020-02277-0
92.
BuenoNBde MeloISVde OliveiraSLda Rocha AtaideT. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. (2013) 110:1178–87. doi: 10.1017/S0007114513000548
93.
ForsytheCEPhinneySDFernandezMLQuannEEWoodRJBibusDMet al. Comparison of low fat and low carbohydrate diets on circulating fatty acid composition and markers of inflammation. Lipids. (2008) 43:65–77. doi: 10.1007/s11745-007-3132-7
94.
SaenzCHooperSOrangeTKnightABarraganMLynchTet al. Effect of a free-living ketogenic diet on feasibility, satiety, body composition, and metabolic health in women: the grading level of optimal carbohydrate for women (GLOW) study. J Am Coll Nutr. (2021) 40:295–306. doi: 10.1080/07315724.2021.1875338
95.
BugaAHarperDGSapperTNHydePNFellBDickersonRet al. Feasibility and metabolic outcomes of a well-formulated ketogenic diet as an adjuvant therapeutic intervention for women with stage IV metastatic breast cancer: the keto-CARE trial. PLoS One. (2024) 19:e0296523. doi: 10.1371/journal.pone.0296523
96.
FieldRPourkazemiFRooneyK. Effects of a low-carbohydrate ketogenic diet on reported pain, blood biomarkers and quality of life in patients with chronic pain: a pilot randomized clinical trial. Pain Med. (2022) 23:326–38. doi: 10.1093/pm/pnab278
97.
NagpalRNethBJWangSCraftSYadavH. Modified Mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer's disease markers in subjects with mild cognitive impairment. EBioMedicine. (2019) 47:529–42. doi: 10.1016/j.ebiom.2019.08.032
98.
SyedKIswaraK. Low-FODMAP diet In: StatPearls. Treasure Island (FL): StatPearls Publishing (2024)
99.
van MegenFSkodjeGILergenmullerSZühlkeSAabakkenLVeierødMBet al. A low FODMAP diet reduces symptoms in treated celiac patients with ongoing symptoms-a randomized controlled trial. Clin Gastroenterol Hepatol. (2022) 20:2258–2266.e3. doi: 10.1016/j.cgh.2022.01.011
100.
AlgeraJPDemirDTörnblomHNybackaSSimrénMStörsrudS. Low FODMAP diet reduces gastrointestinal symptoms in irritable bowel syndrome and clinical response could be predicted by symptom severity: a randomized crossover trial. Clin Nutr. (2022) 41:2792–800. doi: 10.1016/j.clnu.2022.11.001
101.
BrounsFvan HaapsAKeszthelyiDVenemaKBongersMMaasJet al. Diet associations in endometriosis: a critical narrative assessment with special reference to gluten. Front Nutr. (2023) 10:1166929. doi: 10.3389/fnut.2023.1166929
102.
VarjúPFarkasNHegyiPGaramiASzabóIIllésAet al. Low fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAP) diet improves symptoms in adults suffering from irritable bowel syndrome (IBS) compared to standard IBS diet: a meta-analysis of clinical studies. PLoS One. (2017) 12:e0182942. doi: 10.1371/journal.pone.0182942
103.
MooreJSGibsonPRPerryREBurgellRE. Endometriosis in patients with irritable bowel syndrome: specific symptomatic and demographic profile, and response to the low FODMAP diet. Aust N Z J Obstet Gynaecol. (2017) 57:201–5. doi: 10.1111/ajo.12594
104.
van HaapsAPWijbersJVSchreursAMFVlekSTuynmanJde BieBet al. The effect of dietary interventions on pain and quality of life in women diagnosed with endometriosis: a prospective study with control group. Hum Reprod. (2023) 38:2433–46. doi: 10.1093/humrep/dead214
105.
BorghiniRPorporaMGCasaleRMarinoMPalmieriEGrecoNet al. Irritable bowel syndrome-like disorders in endometriosis: prevalence of nickel sensitivity and effects of a low-nickel diet. An open-label pilot study. Nutrients. (2020) 12:341. doi: 10.3390/nu12020341
106.
PellegriniNAgostoniC. Nutritional aspects of gluten-free products. J Sci Food Agric. (2015) 95:2380–5. doi: 10.1002/jsfa.7101
107.
ViciGBelliLBiondiMPolzonettiV. Gluten free diet and nutrient deficiencies: a review. Clin Nutr. (2016) 35:1236–41. doi: 10.1016/j.clnu.2016.05.002
108.
MarzialiMVenzaMLazzaroSLazzaroAMicossiCStolfiVM. Gluten-free diet: a new strategy for management of painful endometriosis related symptoms?Minerva Chir. (2012) 67:499–504. PMID:
109.
ItzlingerABranchiFElliLSchumannM. Gluten-free diet in celiac disease-forever and for all?Nutrients. (2018) 10:1796. doi: 10.3390/nu10111796
110.
MazzaETroianoEMazzaSFerroYAbbinanteAAgnetaMTet al. The impact of endometriosis on dietary choices and activities of everyday life: a cross-sectional study. Front Nutr. (2023) 10:1273976. doi: 10.3389/fnut.2023.1273976
111.
ArmourMSinclairJChalmersKJSmithCA. Self-management strategies amongst Australian women with endometriosis: a national online survey. BMC Complement Altern Med. (2019) 19:17. doi: 10.1186/s12906-019-2431-x
112.
ZhengSHChenXXChenYWuZCChenXQLiXL. Antioxidant vitamins supplementation reduce endometriosis related pelvic pain in humans: a systematic review and meta-analysis. Reprod Biol Endocrinol. (2023) 21:79. doi: 10.1186/s12958-023-01126-1
113.
AbramiukMMertowskaPFrankowskaKŚwiechowska-StarekPSatoraMPolakGet al. How can selected dietary ingredients influence the development and progression of endometriosis?Nutrients. (2024) 16:154. doi: 10.3390/nu16010154
114.
YangMTengSMaCYuYWangPYiC. Ascorbic acid inhibits senescence in mesenchymal stem cells through ROS and AKT/mTOR signaling. Cytotechnology. (2018) 70:1301–13. doi: 10.1007/s10616-018-0220-x
115.
FujisawaKHaraKTakamiTOkadaSMatsumotoTYamamotoNet al. Evaluation of the effects of ascorbic acid on metabolism of human mesenchymal stem cells. Stem Cell Res Ther. (2018) 9:93. doi: 10.1186/s13287-018-0825-1
116.
AbdollahifarMAAzadNSajadiEShams MofaraheZZareFMoradiAet al. Vitamin C restores ovarian follicular reservation in a mouse model of aging. Anat Cell Biol. (2019) 52:196–203. doi: 10.5115/acb.2019.52.2.196
117.
ErtenOUEnsariTADilbazBCakirogluHAltinbasSKÇaydereMet al. Vitamin C is effective for the prevention and regression of endometriotic implants in an experimentally induced rat model of endometriosis. Taiwan J Obstet Gynecol. (2016) 55:251–7. doi: 10.1016/j.tjog.2015.07.004
118.
AsbaghiOSadeghianMNazarianBSarreshtedariMMozaffari-KhosraviHMalekiVet al. The effect of vitamin E supplementation on selected inflammatory biomarkers in adults: a systematic review and meta-analysis of randomized clinical trials. Sci Rep. (2020) 10:17234. doi: 10.1038/s41598-020-73741-6
119.
Farshbaf-KhaliliAOstadrahimiAMirghafourvandMAtaei-AlmanghadimKDoustiSIranshahiAMet al. Clinical efficacy of curcumin and vitamin E on inflammatory-oxidative stress biomarkers and primary symptoms of menopause in healthy postmenopausal women: a triple-blind randomized controlled trial. J Nutr Metab. (2022) 2022:6339715. doi: 10.1155/2022/6339715
120.
AnsariniyaHHadinedoushanHJavaheriAZareF. Vitamin C and E supplementation effects on secretory and molecular aspects of vascular endothelial growth factor derived from peritoneal fluids of patients with endometriosis. J Obstet Gynaecol. (2019) 39:1137–42. doi: 10.1080/01443615.2019.1601167
121.
AminiLChekiniRNateghiMRHaghaniHJamialahmadiTSathyapalanTet al. The effect of combined vitamin C and vitamin E supplementation on oxidative stress markers in women with endometriosis: a randomized, triple-blind placebo-controlled clinical trial. Pain Res Manag. (2021) 2021:5529741. doi: 10.1155/2021/5529741
122.
MiyashitaMKogaKIzumiGSueFMakabeTTaguchiAet al. Effects of 1,25-dihydroxy vitamin D3 on endometriosis. J Clin Endocrinol Metab. (2016) 101:2371–9. doi: 10.1210/jc.2016-1515
123.
LopezACruzMLChompreGHernándezSIsidroRAFloresIet al. Influence of stress on the vitamin D-vitamin D receptor system, macrophages, and the local inflammatory milieu in endometriosis. Reprod Sci. (2020) 27:2175–86. doi: 10.1007/s43032-020-00235-1
124.
XieBLiaoMHuangYHangFMaNHuQet al. Association between vitamin D and endometriosis among American women: national health and nutrition examination survey. PLoS One. (2024) 19:e0296190. doi: 10.1371/journal.pone.0296190
125.
MehdizadehkashiARokhgirehSTahermaneshKEslahiNMinaeianSSamimiM. The effect of vitamin D supplementation on clinical symptoms and metabolic profiles in patients with endometriosis. Gynecol Endocrinol. (2021) 37:640–5. doi: 10.1080/09513590.2021.1878138
126.
NodlerJLDiVastaADVitonisAFKareviciusSMalschMSardaVet al. Supplementation with vitamin D or omega-3 fatty acids in adolescent girls and young women with endometriosis (SAGE): a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. (2020) 112:229–36. doi: 10.1093/ajcn/nqaa096
127.
Bueloni-DiasFNOrsattiCLCangussuLMPoloniPFSpadoto-DiasDNahas-NetoJet al. Isolated vitamin D supplementation improves the immune-inflammatory biomarkers in younger postmenopausal women: a randomized, double-blind, placebo-controlled trial. Menopause. (2018) 25:897–903. doi: 10.1097/GME.0000000000001106
128.
ChenXManGCWHungSWZhangTFungLWYCheungCWet al. Therapeutic effects of green tea on endometriosis. Crit Rev Food Sci Nutr. (2023) 63:3222–35. doi: 10.1080/10408398.2021.1986465
129.
XuHLuiWTChuCYNgPSWangCCRogersMS. Anti-angiogenic effects of green tea catechin on an experimental endometriosis mouse model. Hum Reprod. (2009) 24:608–18. doi: 10.1093/humrep/den417
130.
LaschkeMWSchwenderCScheuerCVollmarBMengerMD. Epigallocatechin-3-gallate inhibits estrogen-induced activation of endometrial cells in vitro and causes regression of endometriotic lesions in vivo. Hum Reprod. (2008) 23:2308–18. doi: 10.1093/humrep/den245
131.
WangCCXuHManGCWZhangTChuKOChuCYet al. Prodrug of green tea epigallocatechin-3-gallate (pro-EGCG) as a potent anti-angiogenesis agent for endometriosis in mice. Angiogenesis. (2013) 16:59–69. doi: 10.1007/s10456-012-9299-4
132.
XuHBeckerCMLuiWTChuCYDavisTNKungALet al. Green tea epigallocatechin-3-gallate inhibits angiogenesis and suppresses vascular endothelial growth factor C/vascular endothelial growth factor receptor 2 expression and signaling in experimental endometriosis in vivo. Fertil Steril. (2011) 96:1021–1028.e1. doi: 10.1016/j.fertnstert.2011.07.008
133.
MatsuzakiSDarchaC. Antifibrotic properties of epigallocatechin-3-gallate in endometriosis. Hum Reprod. (2014) 29:1677–87. doi: 10.1093/humrep/deu123
134.
MosherAATsoulisMWLimJTanCAgarwalSKLeylandNAet al. Melatonin activity and receptor expression in endometrial tissue and endometriosis. Hum Reprod. (2019) 34:1215–24. doi: 10.1093/humrep/dez082
135.
GüneyMOralBKarahanN. Regression of endometrial explants in a rat model of endometriosis treated with melatonin. Fertil Steril. (2008) 89:934–42. doi: 10.1016/j.fertnstert.2007.04.023
136.
KocOGunduzBTopcuogluABugdayciGYilmazFDuranB. Effects of pinealectomy and melatonin supplementation on endometrial explants in a rat model. Eur J Obstet Gynecol Reprod Biol. (2010) 153:72–6. doi: 10.1016/j.ejogrb.2010.06.012
137.
ParkSHamJYangCParkWParkHAnGet al. Melatonin inhibits endometriosis development by disrupting mitochondrial function and regulating tiRNAs. J Pineal Res. (2023) 74:e12842. doi: 10.1111/jpi.12842
138.
PaulSBhattacharyaPdas MahapatraPSwarnakarS. Melatonin protects against endometriosis via regulation of matrix metalloproteinase-3 and an apoptotic pathway. J Pineal Res. (2010) 49:156–68. doi: 10.1111/j.1600-079X.2010.00780.x
139.
PaulSSharmaAVMahapatraPDBhattacharyaPReiterRJSwarnakarS. Role of melatonin in regulating matrix metalloproteinase-9 via tissue inhibitors of metalloproteinase-1 during protection against endometriosis. J Pineal Res. (2008) 44:439–49. doi: 10.1111/j.1600-079X.2007.00547.x
140.
SödermanLEdlundMBöttigerY. Adjuvant use of melatonin for pain management in dysmenorrhea - a randomized double-blinded, placebo-controlled trial. Eur J Clin Pharmacol. (2022) 78:191–6. doi: 10.1007/s00228-021-03234-6
141.
BournivalJPlouffeMRenaudJProvencherC. Quercetin and sesamin protect dopaminergic cells from MPP+-induced neuroinflammation in a microglial (N9)-neuronal (PC12) coculture system. Oxidative Med Cell Longev. (2012) 2012:921941. doi: 10.1155/2012/921941
142.
JeongJHAnJYKwonYTRheeJGLeeYJ. Effects of low dose quercetin: cancer cell-specific inhibition of cell cycle progression. J Cell Biochem. (2009) 106:73–82. doi: 10.1002/jcb.21977
143.
ZhangLMohankumarKMartinGMariyamFParkYHanSJet al. Flavonoids quercetin and Kaempferol are NR4A1 antagonists and suppress endometriosis in female mice. Endocrinology. (2023) 164:bqad133. doi: 10.1210/endocr/bqad133
144.
ParkSLimWBazerFWWhangKYSongG. Quercetin inhibits proliferation of endometriosis regulating cyclin D1 and its target microRNAs in vitro and in vivo. J Nutr Biochem. (2019) 63:87–100. doi: 10.1016/j.jnutbio.2018.09.024
145.
ZhaoPHuZMaWZangLTianZHouQ. Quercetin alleviates hyperthyroidism-induced liver damage via Nrf2 signaling pathway. Biofactors. (2020) 46:608–19. doi: 10.1002/biof.1626
146.
SunLXuGDongYLiMYangLLuW. Quercetin protects against lipopolysaccharide-induced intestinal oxidative stress in broiler chickens through activation of Nrf2 pathway. Molecules. (2020) 25:1053. doi: 10.3390/molecules25051053
147.
LeiroJArranzJAFraizNSanmartínMLQuezadaEOralloF. Effect of cis-resveratrol on genes involved in nuclear factor kappa B signaling. Int Immunopharmacol. (2005) 5:393–406. doi: 10.1016/j.intimp.2004.10.006
148.
LanzilliGCottarelliANicoteraGGuidaSRavagnanGFuggettaMP. Anti-inflammatory effect of resveratrol and polydatin by in vitro IL-17 modulation. Inflammation. (2012) 35:240–8. doi: 10.1007/s10753-011-9310-z
149.
ArablouTDelbandiAAKhodaverdiSArefiSKolahdouz-MohammadiRHeidariSet al. Resveratrol reduces the expression of insulin-like growth factor-1 and hepatocyte growth factor in stromal cells of women with endometriosis compared with nonendometriotic women. Phytother Res. (2019) 33:1044–54. doi: 10.1002/ptr.6298
150.
ErgenoğluAMYenielAÖErbaşOAktuğHYildirimNUlukuşMet al. Regression of endometrial implants by resveratrol in an experimentally induced endometriosis model in rats. Reprod Sci. (2013) 20:1230–6. doi: 10.1177/1933719113483014
151.
Bayoglu TekinYGuvenSKirbasAKalkanYTumkayaLGuvendag GuvenES. Is resveratrol a potential substitute for leuprolide acetate in experimental endometriosis?Eur J Obstet Gynecol Reprod Biol. (2015) 184:1–6. doi: 10.1016/j.ejogrb.2014.10.041
152.
Ozcan CenksoyPOktemMErdemOKarakayaCCenksoyCErdemAet al. A potential novel treatment strategy: inhibition of angiogenesis and inflammation by resveratrol for regression of endometriosis in an experimental rat model. Gynecol Endocrinol. (2015) 31:219–24. doi: 10.3109/09513590.2014.976197
153.
Bruner-TranKLOsteenKGTaylorHSSokalskaAHainesKDulebaAJ. Resveratrol inhibits development of experimental endometriosis in vivo and reduces endometrial stromal cell invasiveness in vitro. Biol Reprod. (2011) 84:106–12. doi: 10.1095/biolreprod.110.086744
154.
KongXXuXZhouLZhuMYaoSDingYet al. MTA1, a target of resveratrol, promotes epithelial-mesenchymal transition of endometriosis via ZEB2. Mol Ther Methods Clin Dev. (2020) 19:295–306. doi: 10.1016/j.omtm.2020.09.013
155.
KodarahmianMAmidiFMoiniAKashaniLShabani NashtaeiMPazhohanAet al. The modulating effects of resveratrol on the expression of MMP-2 and MMP-9 in endometriosis women: a randomized exploratory trial. Gynecol Endocrinol. (2019) 35:719–26. doi: 10.1080/09513590.2019.1576612
156.
ZhangYCaoHYuZPengHYZhangCJ. Curcumin inhibits endometriosis endometrial cells by reducing estradiol production. Iran J Reprod Med. (2013) 11:415–22. PMID:
157.
CaoHWeiYXZhouQZhangYGuoXPZhangJ. Inhibitory effect of curcumin in human endometriosis endometrial cells via downregulation of vascular endothelial growth factor. Mol Med Rep. (2017) 16:5611–7. doi: 10.3892/mmr.2017.7250
158.
DingJMeiSChengWNiZYuC. Curcumin treats endometriosis in mice by the HIF signaling pathway. Am J Transl Res. (2022) 14:2184–98. PMID:
159.
JanaSPaulSSwarnakarS. Curcumin as anti-endometriotic agent: implication of MMP-3 and intrinsic apoptotic pathway. Biochem Pharmacol. (2012) 83:797–804. doi: 10.1016/j.bcp.2011.12.030
160.
JanaSRudraDSPaulSSnehasiktaS. Curcumin delays endometriosis development by inhibiting MMP-2 activity. Indian J Biochem Biophys. (2012) 49:342–8. PMID:
161.
SwarnakarSPaulS. Curcumin arrests endometriosis by downregulation of matrix metalloproteinase-9 activity. Indian J Biochem Biophys. (2009) 46:59–65. PMID:
162.
PiecuchMGarbiczJWaliczekMMalinowska-BorowskaJRozentrytP. I am the 1 in 10-what should I eat? A research review of nutrition in endometriosis. Nutrients. (2022) 14:5283. doi: 10.3390/nu14245283
163.
BlackCJStaudacherHMFordAC. Efficacy of a low FODMAP diet in irritable bowel syndrome: systematic review and network meta-analysis. Gut. (2022) 71:1117–26. doi: 10.1136/gutjnl-2021-325214
164.
MeliniVMeliniF. Gluten-free diet: gaps and needs for a healthier diet. Nutrients. (2019) 11:170. doi: 10.3390/nu11010170
165.
NonnLPengLFeldmanDPeehlDM. Inhibition of p38 by vitamin D reduces interleukin-6 production in normal prostate cells via mitogen-activated protein kinase phosphatase 5: implications for prostate cancer prevention by vitamin D. Cancer Res. (2006) 66:4516–24. doi: 10.1158/0008-5472.CAN-05-3796
166.
AmzajerdiAKeshavarzMGhorbaliEPezaroSSarviF. The effect of vitamin D on the severity of dysmenorrhea and menstrual blood loss: a randomized clinical trial. BMC Womens Health. (2023) 23:138. doi: 10.1186/s12905-023-02284-5
167.
KraujalytėVVenskutonisPRPukalskasAČesonienėLDaubarasR. Antioxidant properties and polyphenolic compositions of fruits from different European cranberrybush (Viburnum opulus L.) genotypes. Food Chem. (2013) 141:3695–702. doi: 10.1016/j.foodchem.2013.06.054
168.
SaltanGSüntarIOzbilginSIlhanMDemirelMAOzBEet al. Viburnum opulus L.: a remedy for the treatment of endometriosis demonstrated by rat model of surgically-induced endometriosis. J Ethnopharmacol. (2016) 193:450–5. doi: 10.1016/j.jep.2016.09.029
169.
MozaffarianD. Trans fatty acids - effects on systemic inflammation and endothelial function. Atheroscler Suppl. (2006) 7:29–32. doi: 10.1016/j.atherosclerosissup.2006.04.007
170.
ZemelMBSunX. Dietary calcium and dairy products modulate oxidative and inflammatory stress in mice and humans. J Nutr. (2008) 138:1047–52. doi: 10.1093/jn/138.6.1047
171.
TrabertBPetersUde RoosAJScholesDHoltVL. Diet and risk of endometriosis in a population-based case-control study. Br J Nutr. (2011) 105:459–67. doi: 10.1017/S0007114510003661
Summary
Keywords
endometriosis, inflammation, oxidative stress, dietary patterns, nutrition
Citation
Zhou L, Liu B, Jian X, Jiang L and Liu K (2025) Effect of dietary patterns and nutritional supplementation in the management of endometriosis: a review. Front. Nutr. 12:1539665. doi: 10.3389/fnut.2025.1539665
Received
04 December 2024
Accepted
21 February 2025
Published
12 March 2025
Volume
12 - 2025
Edited by
Maria Luisa Mizgier, University of the Andes, Chile
Reviewed by
Małgorzata Mizgier, Poznan University of Physical Education, Poland
Marija Stojanovic, University of Belgrade, Serbia
Updates
Copyright
© 2025 Zhou, Liu, Jian, Jiang and Liu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lili Jiang, jiangll311@163.com; Kuiran Liu, liukr0412@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.