- 1Transplant Research Center, Clinical Research Institute, Mashhad University of Medical Sciences, Mashhad, Iran
- 2Student Research Committee, Mashhad University of Medical Sciences, Mashhad, Iran
- 3Department of Nutrition, Food Sciences and Clinical Biochemistry, School of Medicine, Social Determinants of Health Research Center, Gonabad University of Medical Science, Gonabad, Iran
- 4Rajaei Cardiovascular Medical and Research Center, Iran University of Medical Sciences, Tehran, Iran
- 5Clinical Research Development Unit, Imam Reza Hospital, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
- 6Department of Infectious Diseases and Tropical Medicine, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
- 7Imam Khomeini Hospital Complex, Tehran University of Medical Sciences, Tehran, Iran
Background: Although a large number of trials have observed the anti-inflammatory properties of propolis, the currently available research remains controversial regarding its beneficial health effects. Hence, the purpose of this study was to examine the effect of propolis on inflammatory and oxidative stress markers in adults.
Methods: A comprehensive search was performed in Scopus, Web of Science, and PubMed/Medline to find relevant randomized controlled trials (RCTs) until January 2024. The overall effect sizes were calculated using the random-effects model and expressed as weighted mean differences (WMD) with a 95% confidence interval (CI). The possible heterogeneity between included trials was assessed by performing Cochran’s Q test.
Results: In total, 27 trials with 29 treatment arms were eligible for inclusion in this review. This meta-analysis revealed that propolis consumption led to a significant decrease in C-reactive protein (CRP) (WMD: –1.23; 95%CI: –1.76, –0.69; p < 0.001), Interleukin-6 (IL-6) (WMD: –1.52; 95%CI: –2.10, –0.93; p < 0.001), Tumor necrosis factor-α (WMD: –1.15; 95%CI: –1.75, –0.55; p < 0.001), and Monocyte chemoattractant protein-1 (MCP-1) (WMD: –35.33; 95%CI: –50.28, –20.37; p < 0.001), and a significant increase in total antioxidant capacity (TAC) (WMD: 0.32; 95%CI: 0.12, 0.51; p = 0.001), Glutathione (GSH) (WMD: 4.71; 95%CI: 3.17, 6.25; p < 0.001), and Glutathione peroxidase (GPx) (WMD: 44.75; 95%CI: 5.10, 84.40; p = 0.02). However, there were no significant effects on IL-10, IL-2, IL-8, pro-oxidant-antioxidant balance (PAB), malondialdehyde (MDA), and superoxide dismutase (SOD) in comparison to the control group.
Conclusion: Propolis supplementation appears effective in reducing inflammation and oxidative stress by enhancing antioxidant capacity and reducing specific inflammatory markers. However, variations in study designs, dosages, and participant characteristics contribute to the heterogeneity of results. Further well-designed RCTs are needed to confirm these findings and determine the optimal dosage and long-term effects. Given its potential anti-inflammatory and antioxidant properties, propolis may serve as a complementary approach in managing inflammation-related conditions, though its clinical application requires further validation.
Systematic review registration: https://clinicaltrials.gov/, identifier CRD42023474033.
1 Introduction
Honeybees (mostly Apis mellifera) create propolis, a natural resinous mixture, by combining exudate collected from various plant sources with salivary enzymes and wax (1). Bees use propolis to patch up damaged areas of their honeycombs, keeping the interior at a constant temperature and humidity while also creating a sterile space and guarding the entrance from potential predators. Traditional medicine has used propolis for a very long time because it has many health benefits (2). The chemical composition of propolis varies greatly depending on a number of factors, including the time of year, the type of vegetation at the collection site, and the species of bees involved. The active components of propolis have been identified in more than 300 samples from multiple regions of the world. These include phenolic acids and related esters, flavonoids, terpenes, aromatic aldehydes and alcohols, stilbenes, b-steroids, and fatty acids (1, 3, 4). Numerous chronic diseases have been found to be helped by propolis because of its antimicrobial, antiviral, antifungal, antiprotozoal, antioxidant, anti-inflammatory, immunomodulatory, antihyperglycemic, antihypertensive, antiproliferative, and hepatoprotective characteristics (1). The propolis used in previous studies has antioxidant and anti-inflammatory properties (5–7). In addition, a recent systematic review suggested that propolis could alleviate oxidative stress, renal damage, and inflammation status (8).
Propolis modulates the immune system by targeting both the innate and adaptive immune responses (9). This natural product can raise the levels of anti-inflammatory agents such as Interleukin-10 (IL-10) (10) and lower the levels of pro-inflammatory factors like Interferon (IFN-γ), IL-1β (11), Tumor necrosis factor-α (TNF-α), IL-6 (12), ICAM-1 (intercellular adhesion molecule), leukotrienes D4, and prostaglandins E2 and F2α (10). Recent scientific research has indicated that propolis may play a significant role in the treatment of inflammatory diseases (13) and immunological disorders (9). Earlier meta-analyses showed that propolis supplementation significantly decreased C-reactive protein (CRP), TNF-α, and IL-6 (12). Another key antioxidant component of propolis is caffeic acid phenethyl ester (CAPE), which works by blocking the production of reactive oxygen species (ROS) (14). Propolis may reduce oxidative stress and inflammation, according to several studies (15–17). Today, we know that propolis has a lot of flavonoids, which are plant-based chemicals that stop the production of nitric oxide (NO), IL-1, and IL-6 (18). Phenolic acids, which have been found in abundance in propolis, are an additional immunomodulatory substance. Their molecular activity decreases the levels of NO, cytokines, and neutrophils by scavenging free radicals and inhibiting the production of nitric oxide and inflammatory cytokines by macrophages and/or neutrophils (19).
With a focus on propolis and inflammation, some studies found evidence of a possible connection between propolis and inflammation and oxidative stress. Two systematic reviews have been completed on the effects of propolis on inflammation and oxidative stress, respectively (12, 20). Because of the inconsistent evidence, availability of new data, and limitations of previous reviews, we aimed to conduct a new systematic review and meta-analysis of available randomized controlled trials (RCT) to investigate the effects of propolis supplementation on inflammatory and oxidative stress markers in adults.
2 Materials and methods
The Preferred Reporting Items of Systematic Reviews and Meta-Analysis (PRISMA) framework was considered the foundation for every step of the planning and execution of this systematic review and meta-analysis (21). Also, in the PROSPERO database, this systematic review’s protocol is available with the registration ID: CRD42023474033.
2.1 Search strategy
To find relevant RCTs that examined the impact of propolis on oxidative stress and inflammatory markers, the ISI Web of Science, PubMed, and Scopus databases were comprehensively searched until January 2024. There were no time or language constraints on this search. The search strategy that was used in each database contains main keywords such as: (intervention OR “randomized clinical trial” OR RCT OR “randomized controlled trial” OR “clinical trial” OR “trial” OR blinded OR parallel OR “Cross-Over”) AND (“propolis”). Lastly, in order to prevent missing any eligible trials, the Google Scholar search engine was manually searched and the reference lists of relevant papers were carefully examined.
2.2 Eligibility criteria
Two authors (M.R. and H.B.) independently screened the trials that were found through primary searches using the inclusion criteria of the current study. The eligibility criteria were designed by applying the PICOS framework as follows: Participant: adults, Intervention: propolis consumption, Comparison: control group, Outcomes: oxidative stress, and inflammation markers, Study: randomized controlled trials (22). All included studies had to meet the following criteria: (a) human interventional studies, (b) RCTs design, (c) propolis consumption as an intervention, (d) reporting the changes in the levels of inflammatory and oxidative markers, and (e) Intervention on the adult population (≥ 18 years).
2.3 Exclusion criteria
Studies met the following criteria excluded from the present review: non-RCT studies, and observational research such as case-control, cohort, cross-sectional, etc. Furthermore, short communication, review articles, letters to the editor, studies conducted on people younger than 18 years, comminution therapy, duration of intervention less than 1 week, and lack of an appropriate control group were other exclusion criteria.
2.4 Data extraction
Relevant required data was independently extracted from included trials by two investigators (M.A. and H.G.). The extracted items include the name of the first author, publication year, region or country, sample size for each group, characteristics of participants [health status, mean age, gender, and mean body mass index (BMI)]. Type of control group, features of intervention with propolis (type, dosage, and duration), and mean changes and SD of each marker level changes (or the level of each marker in the first and the end of intervention).
2.5 Quality assessment
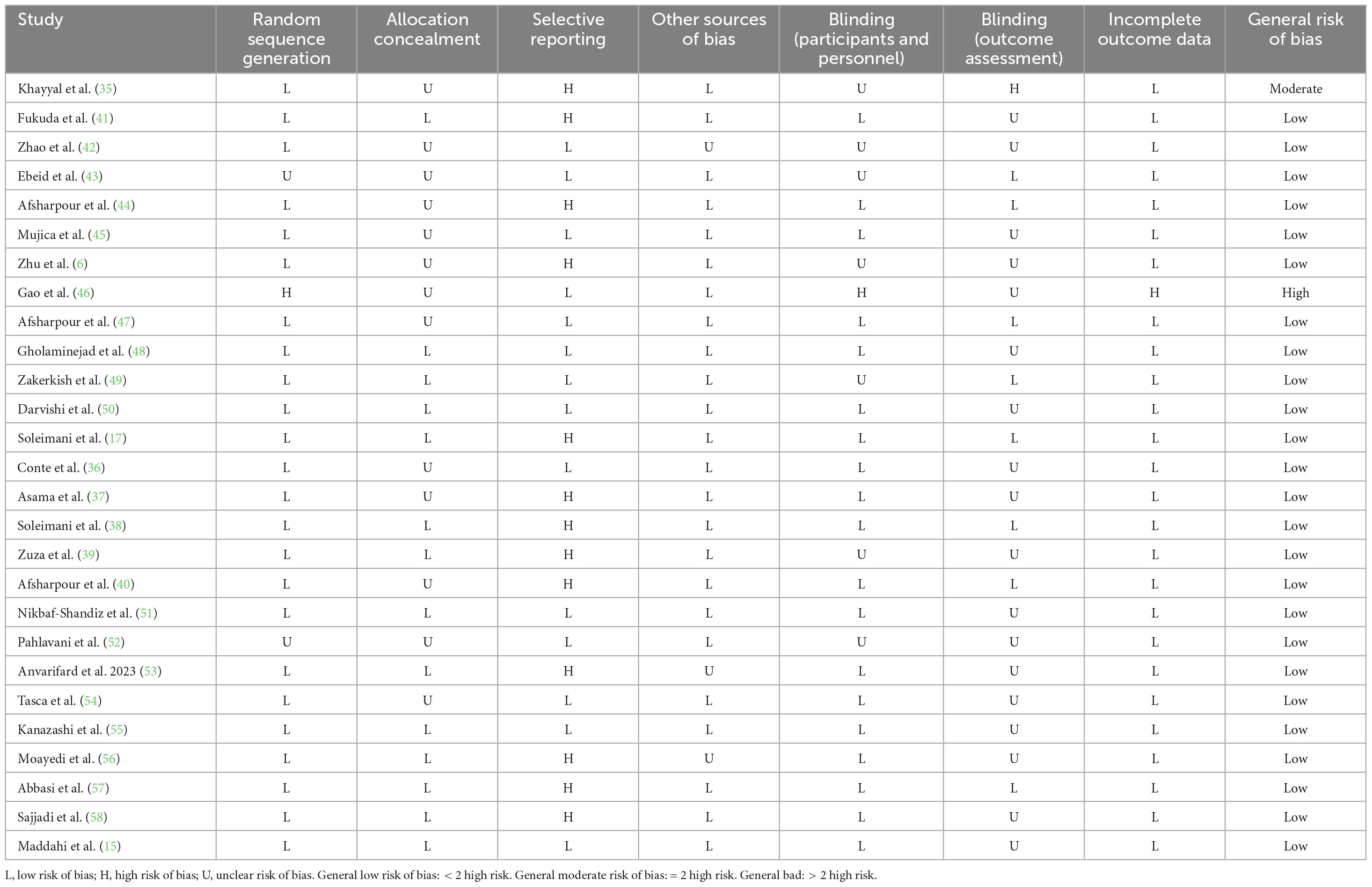
By applying the approach proposed by the Cochrane Collaboration, the general risk of bias for each included study was evaluated (23). Based on this tool’s framework, the risk of bias was assessed in the following seven domains: incomplete outcome data, blinding of outcome assessment, random sequence generation, selective reporting, allocation concealment, other biases, and blinding of participants and personnel. Each subclass’s risk of bias was categorized into three levels: high, unclear, and low. If the number of high-risk bias subclasses is more than two, the general risk of bias is considered high. If there are two subclasses, the general risk of bias is deemed moderate, and if there are fewer than two, the general risk of bias is regarded as low (24, 25).
2.6 Data synthesis and statistical analysis
All conducted analyses were executed by using version 17 of STATA software (Stata Corp., College Station, TX). Furthermore, p-values less than 0.05 were identified as statistically significant (two-tailed). In order to assess the influence of propolis intake on identified outcomes, the pooled effect sizes were calculated according to the random effect model based on the mean changes and SDs in both intervention and control groups. Also, The overall effect size was expressed as weighted mean differences (WMD) and 95% confidence interval (95% CI) (26). The mean changes in the case of non-reporting were directly estimated by subtracting the level of the markers at the beginning of the intervention from the end. SD was also determined by using the following formula: Change SD = square root [(SDbaseline)2 + (SDfinal)2-(2 × R × SDbaseline × SDfinal)] (27). Interquartile range (IQR), standard Error (SEs), and 95% confidence interval by applying the method of Hozo et al. convert to SDs (28). Cochran’s Q-test and the I-squared statistic (I2) were used to assess the heterogeneity among the included studies (29). P-value < 0.05 was considered as the significant between-studies heterogeneity. In addition, if a significant heterogeneity was detected, then based on the I2 statistics measure the interpretation of levels of heterogeneity among the pooled effect size was done as follows: 40% < I2 < 75% identified as moderate, and 75% < I2 as high heterogeneity among combined effect sizes.
Subgroup analysis was conducted to find the source of heterogeneity among included studies based on the following pre-defined criteria (30): gender (males, females, and both sexes), duration of propolis intake (< 12 and ≥ 12 weeks), propolis dosage (<1,000 and ≥ 1,000 mg/day), age of subjects (< 50 and > 50 years), participants’ health status (non-alcoholic fatty liver disease (NAFLD), type 2 diabetes mellitus (T2DM), other conditions, and healthy), and baseline BMI (normal, overweight, and obesity). Egger’s regression test and visual examination of funnel plots were used to assess publication bias for each outcome. (31). The impact of each of the effect sizes on the overall effect size was investigated for each outcome by conducting a sensitivity test with the leave-one-out approach (32). Polynomial modeling analyses and Meta-regression were performed to investigate the non-linear and linear relationship between the characteristics of propolis intervention (dose and duration) and changes in the levels of each of the oxidative and inflammatory stress markers, respectively (33).
2.7 GRADE analysis
In this meta-analysis, the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) protocol was applied to assess the level of the evidence’s certainty (34). Based on this framework, the limitations of the evidence were evaluated in 5 sections: inconsistency, indirectness, publication bias, risk of bias, and imprecision.
Limitations in each domain were categorized into the following three classes: very serious limitations, serious limitations, and no serious limitations. Lastly, four degrees of evidence quality were identified: very high, high, moderate, and low.
3 Results
3.1 Study selection
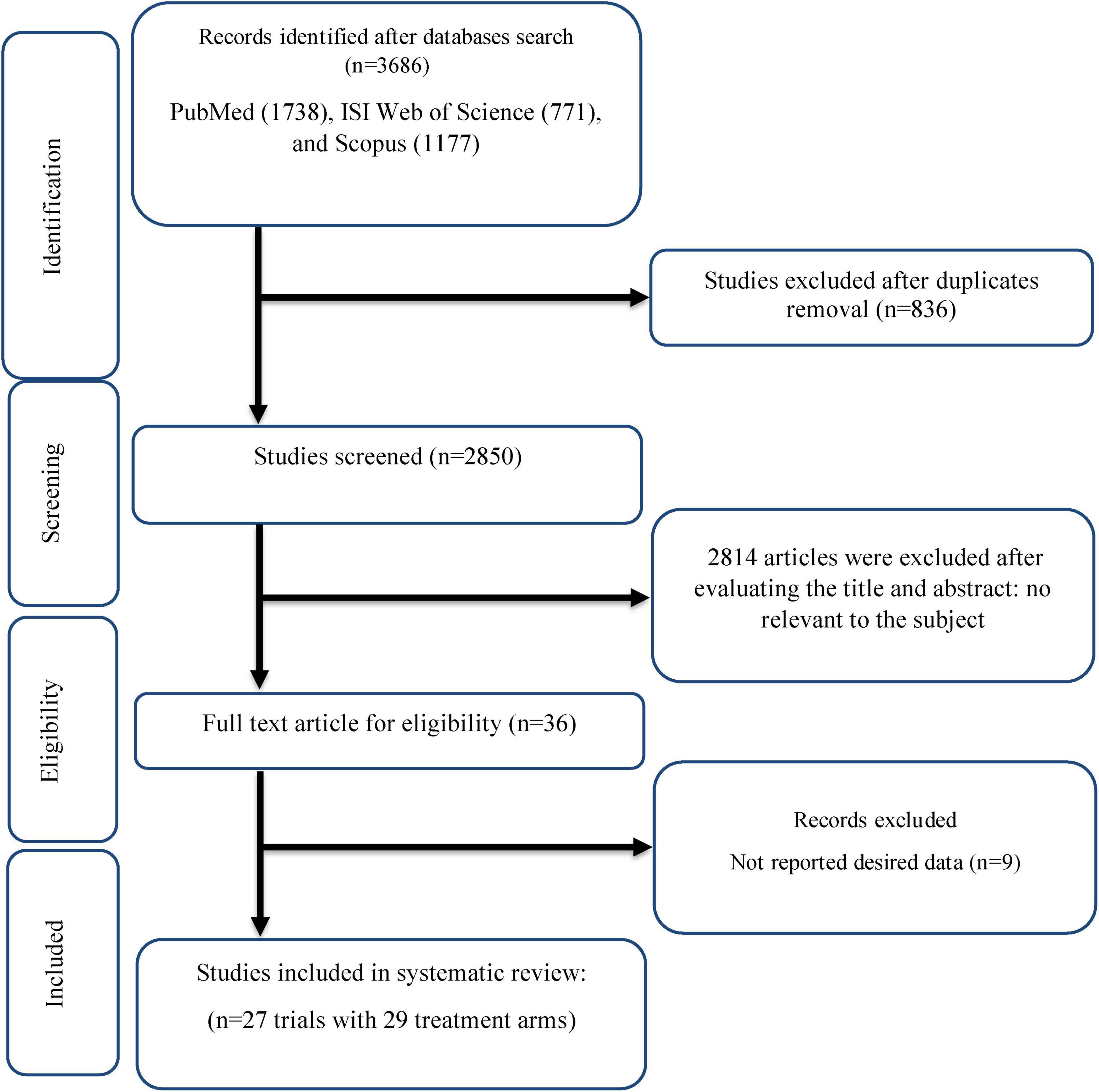
As shown in Figure 1, After 3,686 studies were obtained by initial search, 836 duplicate papers were removed. Among the remaining 2,850 studies that were screened, 2,814 did not meet the eligibility criteria for this review. The full text of 36 studies was evaluated, of which nine trials were excluded because they did not provide the necessary data. Finally, 27 studies (with 29 arm treatments) with 1,539 participants were included in this review (Figure 1) (6, 15, 17, 35–58).
3.2 Study characteristics
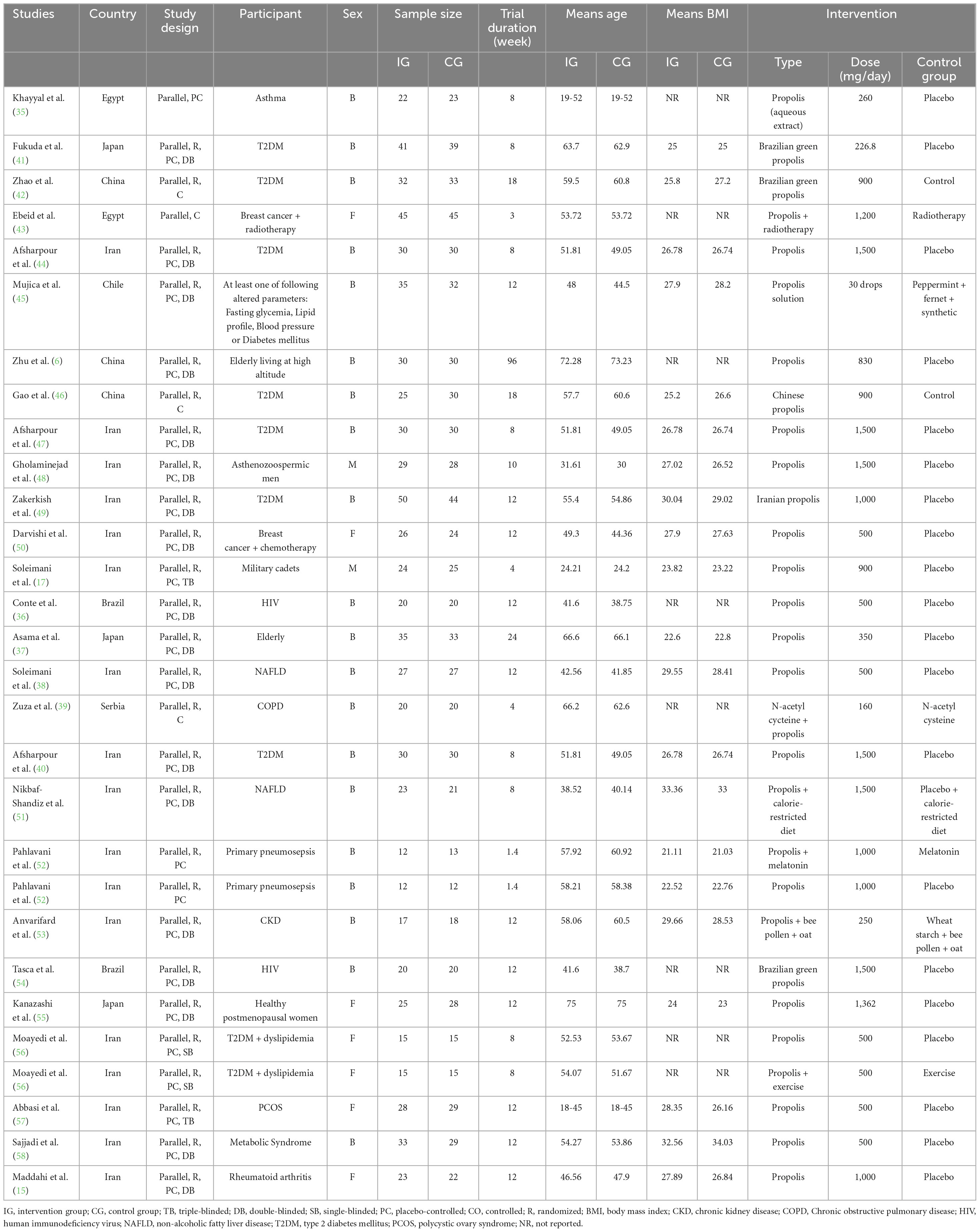
The included trials were published between 2003 and 2023. The study countries included Egypt (35, 43), Japan (37, 41, 55), China (6, 42, 46, 55), Iran (15, 17, 38, 40, 44, 47–53, 56–58) Chile (45), Brazil (36, 54), and Serbia (39). Among the included trials, 6 were conducted on females (15, 43, 50, 55–57) 2 on males (17, 48), and 20 on both sexes (6, 35–42, 44–47, 49–54, 58). The sample size of the treatment arms ranged from 24 (52) to 99 participants (49). The mean age of the participants varied from 24.2 (17) to 75 years (55). Also, the mean BMI varied from 21.07 (52), and 33.29 (58) kg/m2. The participants in the four included studies were healthy (6, 37, 45, 55). Also, in one included study, participants were patients with cardiovascular disease, diabetes mellitus, or overweight, or at least one altered parameter in the following markers: lipid profile, fasting glycemia, and blood pressure (45). Furthermore, the participants of the rest of the trials were conducted on individuals with Asthma (35), T2DM (40–42, 44, 46, 47, 49), Breast cancer (43, 50), Asthenozoospermia (48), HIV (36, 54), COPD (39), Primary pneumosepsis (52), chronic kidney disease (CKD) (53), NAFLD (38, 51), T2DM and dyslipidemia (56), polycystic ovary syndrome (PCOS) (57), Metabolic Syndrome (58), or Rheumatoid arthritis (15). The propolis received in the included studies was in the form of drops (45), syrup (52), sachets (35), and pills (tablets and capsules) (6, 15, 17, 36–44, 46–51, 53–58). The daily dosage of propolis ranged from 160 mg (lowest dosage) (39) to 1,500 mg (highest dosage) (40, 44, 47, 48, 51, 54). Also, the intervention duration in the eligible trials was between 1.4 (shortest duration) (52) to 96 weeks (longest duration) (6). The features of the eligible trials are provided in Table 1.
3.3 Quality assessment
Based on the risk of bias assessment performed using the approach proposed by Cochrane, the general risk of bias was identified as high for 1 (46), and moderate for 1 (35), eligible trial. At the same time, the rest of the included trials had a low general risk of bias. Table 2 provides the results of the risk of bias assessment in each domain.
3.4 Meta-analysis
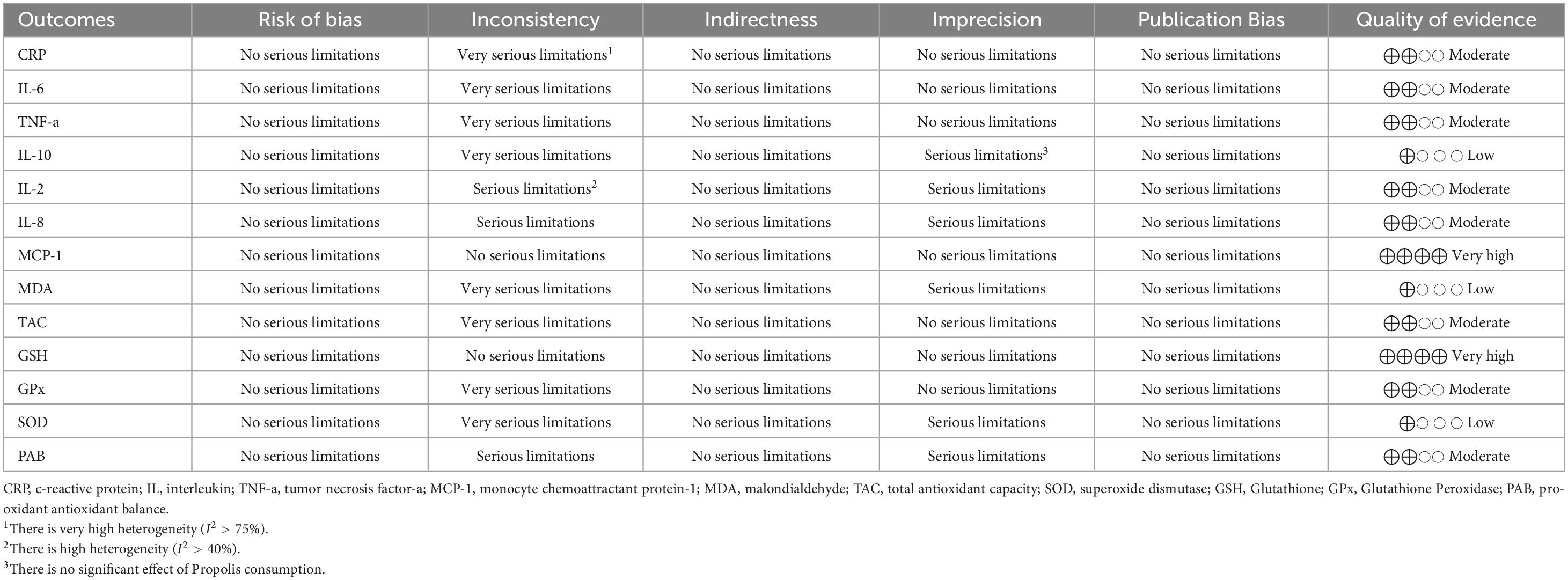
3.4.1 Impact of supplementing with propolis on CRP levels
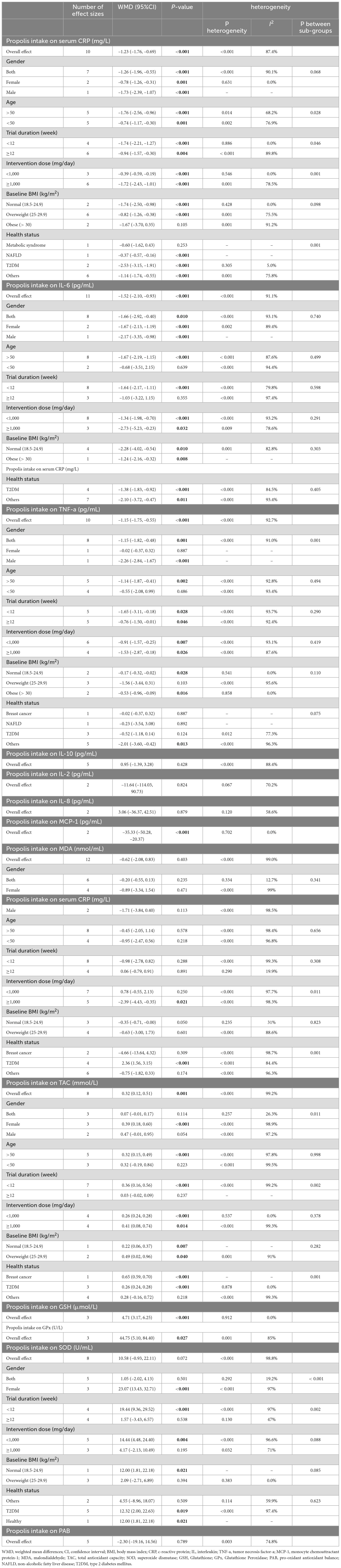
Pooling 10 effect sizes demonstrated that propolis supplementation led to a significant decrease in the serum level of CRP (WMD: –1.23 mg/l; 95% CI –1.76 to –0.69; p < 0.001) (Figure 2A). However, significant heterogeneity was observed between the pooled trials (p < 0.001). Based on the I2 statistics measures (I2 = 87.4%) the heterogeneity among the included studies was identified as high (I2 > 75%). Subgroup analysis revealed that consumption of propolis in individuals with metabolic syndrome or obesity did not have a significant impact on the serum CRP levels (Table 3). Meta-regression demonstrated that the dose (coefficients = –316.08, Plinearity = 0.08; Supplementary Figure 1A) and duration (coefficients = 2.26, Plinearity = 0.23; Supplementary Figure 2A) of propolis supplementation were not sources of heterogeneity. Also, no significant linear relationship was detected between them and serum CRP changes.
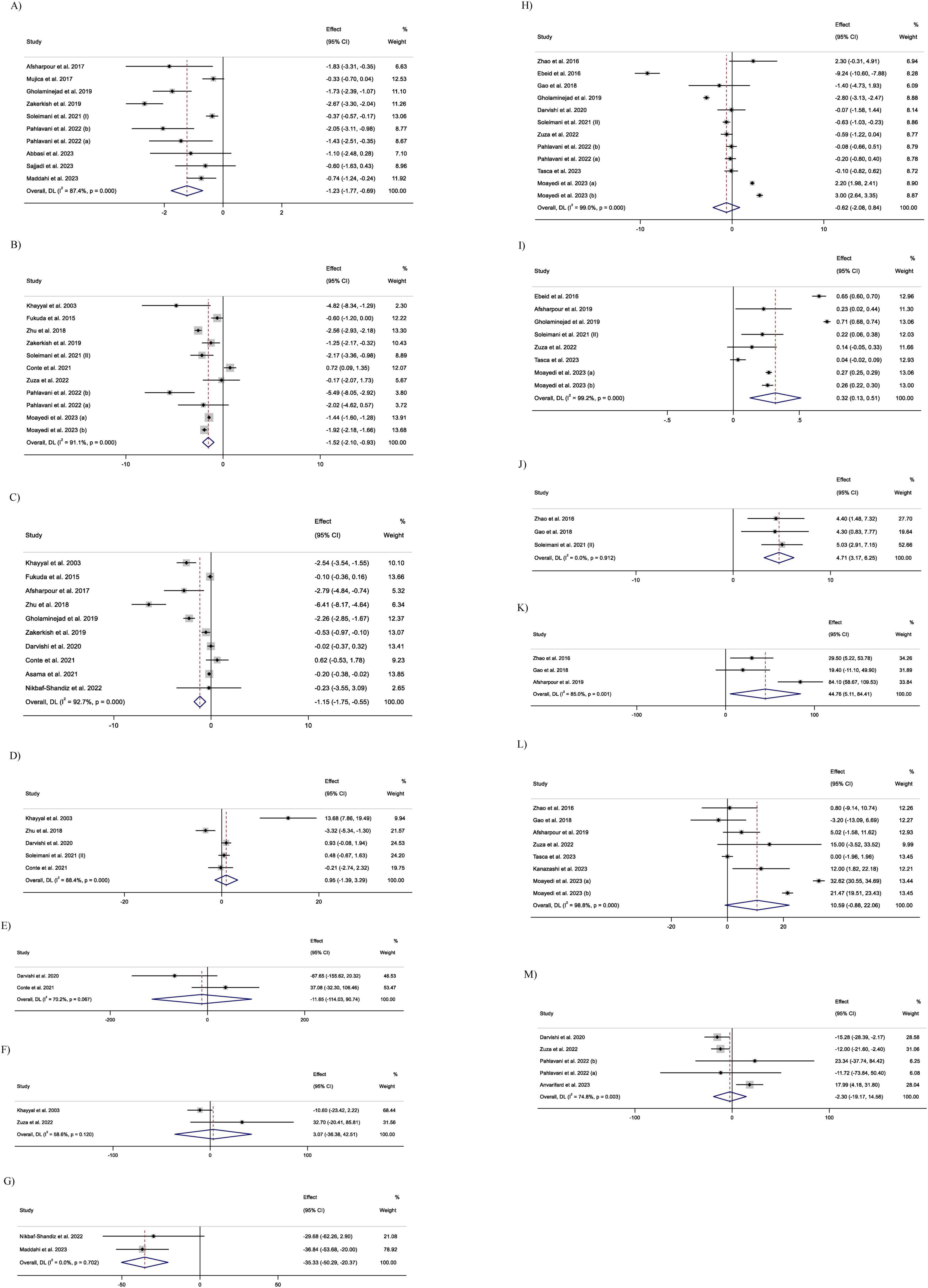
Figure 2. Forest plot detailing weighted mean difference and 95% confidence intervals (CIs) for the effect of Propolis intake on: (A) CRP (C-reactive protein, mg/L); (B) IL-6 (Interleukin-6, pg/mL); (C) TNF-α (Tumor necrosis factor-alpha, pg/mL); (D) IL-10 (Interleukin-10, pg/mL); (E) IL-2 (Interleukin-2, pg/mL); (F) IL-8 (Interleukin-8, pg/mL); (G) MCP-1 (Monocyte chemoattractant protein-1, pg/mL); (H) MDA (Malondialdehyde, nmol/mL); (I) TAC (Total Antioxidant Capacity, mmol/L); (J) GSH (Glutathione, μmol/L); (K) GPx (Glutathione Peroxidase, U/L); (L) SOD (Superoxide Dismutase, U/mL); and (M) PAB (Pro-oxidant-antioxidant balance).
3.4.2 Impact of supplementing with propolis on IL-6 levels
After combining 11 effect sizes, a significant reduction in IL-6 serum levels was detected in the groups that received propolis (WMD: –1.52 pg/mL; 95% CI –2.10 to –0.93; p < 0.001) (Figure 2B). Furthermore, heterogeneity between the pooled trials was significant (; p < 0.001). I2 level (I2 = 91.1%), demonstrated a high heterogeneity among the combined effect sizes (I2 > 75%). Subgroup analysis demonstrated that propolis intake in trials conducted on participants aged less than 50 years, and in the studies with a duration of receiving propolis ≥ 12 weeks did not have a significant influence on the IL-6 serum levels (Table 3). Meta-regression revealed that the intervention features, including dosage and duration of propolis intake, were not sources of heterogeneity. Also, no significant linear relationship was identified between the characteristics of propolis intake and changes in IL-6 levels (dose: coefficients = -58.64, P linearity = 0.32; Supplementary Figure 1B), and duration (coefficients = -0.61, P linearity = 0.90; Supplementary Figure 2B).
3.4.3 Impact of supplementing with propolis on TNF-α levels
Meta-analyzing 10 effect sizes demonstrated a significant decrease in serum TNF-α levels followed by propolis supplementation (WMD: –1.15 pg/mL; 95% CI –1.75 to –0.55; P < 0.001) (Figure 2C). However, a significant heterogeneity was observed between the included trials (P < 0.001). In addition, I2 statistics measures (I2 = 92.7%) indicated a high level of heterogeneity among pooled effect sizes (I2 > 75%). Subgroup analysis demonstrated a non-significant influence of propolis consumption on the TNF-a levels in the trials conducted on only females, subjects aged < 50 years, and participants with breast cancer, NAFLD, and type 2 diabetes (Table 3).
Meta-regression indicated that the dosage of propolis supplementation was not a source of heterogeneity, and there was no significant linear relationship between propolis dosage and changes in serum TNF-a levels (coefficients = -59.92, P linearity = 0.50; Supplementary Figure 1C). However, the duration of intervention with propolis was identified as a source of heterogeneity. Also, a significant linear relationship was observed between the changes in the TNF-a level and duration of propolis supplementation (coefficients = –9.94, P linearity = 0.009; Supplementary Figure 2C).
3.4.4 Impact of supplementing with propolis on IL-10 levels
Meta-analyzing five effect sizes revealed that propolis supplementation had no significant impact on serum IL-10 levels (WMD: 0.95 pg/mL; 95% CI –1.39 to 3.28; P = 0.42) (Figure 2D). Also, a significant heterogeneity was identified between the pooled effect sizes (P < 0.001). I2 statistics (I2 = 85.9%) indicated a high level of heterogeneity among the included effect sizes (I2 > 75%).
3.4.5 Impact of supplementing with propolis on IL-2 levels
Pooling of two effect sizes showed the non-significant influence of the propolis intake on IL-2 serum levels (WMD: –11.64 pg/mL; 95% CI (–114.03 to 90.73; P = 0.82) (Figure 2E). However, no significant heterogeneity was observed between the pooled effect sizes (I2 = 70.2%; P = 0.06).
3.4.6 Impact of supplementing with propolis on IL-8 levels
Combining two effect sizes mentioned that propolis consumption had no significant impact on IL-8 serum levels [WMD: 3.06 pg/mL; 95% CI (–36.37 to 42.51; P = 0.87)] (Figure 2F). Furthermore, no significant heterogeneity was detected between the included trials (I2 = 58.6%; P = 0.12).
3.4.7 Impact of supplementing with propolis on Monocyte chemoattractant protein-1 levels
Meta-analyzing two effect sizes revealed that propolis intake had a significant lowering effect on MCP-1 serum levels (WMD: –35.33 pg/mL; 95% CI (–50.28 to –20.37; P < 0.001) (Figure 2G). At the same time, no significant heterogeneity was detected between the pooled trials (I2 = 0.0%; P = 0.70).
3.4.8 Impact of supplementing with propolis on Malondialdehyde levels
Meta-analyzing of 12 effect sizes demonstrated that propolis intake had no significant influence on MDA levels (WMD: –0.62 nmol/mL; 95% CI –2.08 to 0.83; P = 0.40) (Figure 2H). Furthermore, a significant heterogeneity was detected between the included effect sizes (P < 0.001). I2-values (I2 = 99.0%), indicated a high level of heterogeneity among the pooled effect sizes (I2 > 75%). Subgroup analysis indicated that consuming propolis with a dose of ≥ 1,000 mg/day and in the trials conducted on participants with type 2 diabetes led to a significant decrease in MDA levels (Table 3). Meta-regression showed that the dose (coefficients = –57.17, Plinearity = 0.15; Supplementary Figure 1E) and duration (coefficients = 0.52, P linearity = 0.37; Supplementary Figure 2E) of propolis supplementation were not sources of heterogeneity. Also, no significant linear relationship was observed between them and changes in MDA levels.
3.4.9 Impact of supplementing with propolis on total antioxidant capacity (TAC) levels
Combining eight effect sizes revealed that propolis consumption significantly increased the TAC levels (WMD: 0.32 nmol/mL; 95% CI 0.12–0.51; P = 0.001) (Figure 2I). However, a significant heterogeneity was detected between included trials (P < 0.001). Based on the I2 measures (I2 = 99.2%), levels of heterogeneity among the pooled studies identified as high (I2 > 75%). Subgroup analysis demonstrated that propolis intake did not significantly change TAC levels in studies conducted only on males or on individuals aged < 50 years (Table 3). Meta-regression reported the absence of a significant linear relationship between the features (dose and duration) of the propolis intake and changes in TAC. It also showed that the dose (coefficients = 724.26, P linearity = 0.43; Supplementary Figure 1F) and duration (coefficients = -2.33, P linearity = 0.67; Supplementary Figure 2F) of propolis supplementation were not sources of heterogeneity.
3.4.10 Impact of supplementing with propolis on Glutathione (GSH) levels
Meta-analysis of three effect sizes demonstrated that propolis intake significantly increased the GSH levels (WMD: 4.71 μmol/L; 95% CI 3.17 to 6.25; P < 0.001) (Figure 2J). Also, there was no significant heterogeneity between the pooled effect sizes (I2 = 0.0%; p = 0.91).
3.4.11 Impact of supplementing with propolis on Glutathione peroxidase (GPx) levels
Combining three effect sizes revealed that propolis consumption significantly increased GPx levels (WMD: 44.75 U/L; 95% CI 5.10–84.40; P = 0.02) (Figures 2K, 3). Also, a significant heterogeneity was observed between the pooled effect sizes (P = 0.001). Also, I2 levels (I2 = 85%) identified the levels of heterogeneity among the combined trials as high (I2 > 75%).
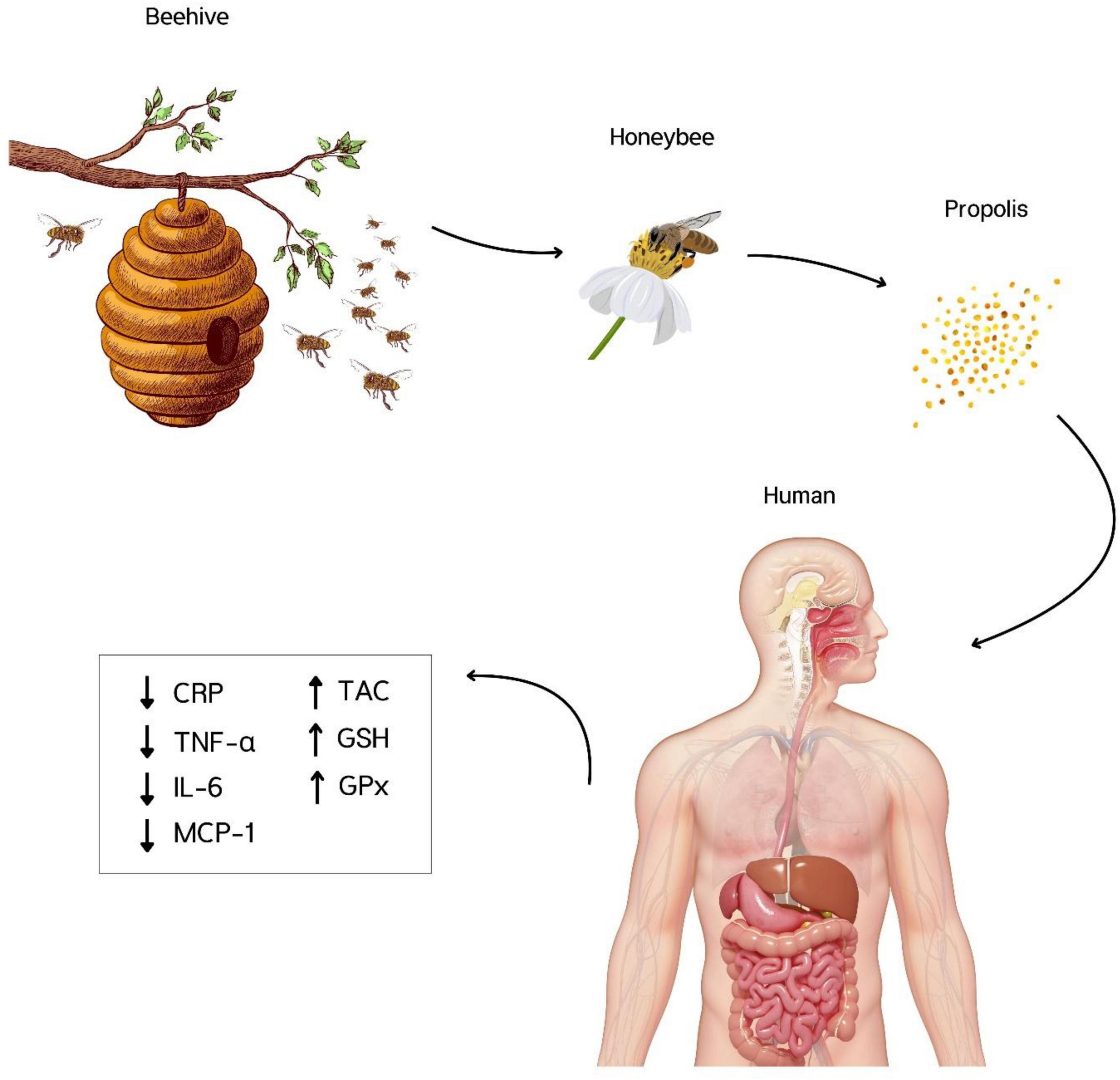
Figure 3. Propolis consumption significantly reduced C-reactive protein (CRP), Interleukin-6 (IL-6), Tumor necrosis factor-α (TNF-α), and Monocyte chemoattractant protein-1 (MCP-1), and increased total antioxidant capacity (TAC), Glutathione (GSH), and Glutathione peroxidase (GPx). There were no significant effects on IL-10, IL-2, IL-8, pro-oxidant-antioxidant balance, malondialdehyde, and superoxide dismutase.
3.4.12 Impact of supplementing with propolis on Superoxide dismutase (SOD) levels
Pooling eight effect sizes demonstrated that propolis intake did not significantly change SOD levels (WMD: 10.58 U/mL; 95% CI –0.93 to 22.11; P = 0.07) (Figure 2L). Also, a significant heterogeneity was mentioned between the included trials (P < 0.001). In addition, I2 measures (I2 = 98.8%) indicated a high heterogeneity among the pooled effect sizes (I2 > 75%). The subgroup analysis reported the significant enhancing effect of propolis consumption in studies conducted on only females, trials with an intervention duration of < 12 weeks or propolis supplemental dosage of <1,000 mg/day (Table 3). In addition, propolis intake in healthy individuals or patients with type 2 diabetes led to a significant increase in SOD levels.
Meta-regression reported a significant linear relationship between the duration of propolis intake and changes in SOD levels. Also, the duration of supplementation was identified as a source of heterogeneity (coefficients = -0.14, P linearity = 0.01; Supplementary Figure 2G). However, the dosage of propolis supplementation was not the source of heterogeneity (coefficients = –24.14, Plinearity = 0.12; Supplementary Figure 1G). Furthermore, no significant linear relationship was detected between supplementation dosage and SOD level changes.
3.4.13 Impact of supplementing with propolis on Pro-oxidant-antioxidant balance (PAB) levels
Combining five effect sizes demonstrated that propolis consumption had no significant impacts on PAB levels (WMD: –2.30 U/mL; 95% CI –19.16 to 14.56; P = 0.78) (Figure 2M). However, a significant heterogeneity was observed between the included effect sizes (P = 0.003). Based on the I2 levels (I2 = 74.8%), the heterogeneity among pooled trials was identified as moderate (40% < I2 < 75%).
3.5 Non-linear dose-response analysis
Fractional polynomial modeling demonstrated a significant non-linear relationship between propolis supplementation dosage and changes in SOD levels (coefficients = 14.77, P non–linearity = 0.02). This analysis suggests that a daily supplement of 500 mg of propolis may induce a more pronounced increase in SOD levels compared to other dosages reported in the trials. Furthermore, it showed a significant non-linear relationship between the duration of propolis supplementation and changes in TNF-a (coefficients = –29.39, P non–linearity = 0.01), MDA (coefficients = 10.05, P non–linearity = 0.01), and SOD levels (coefficients = 39.18, P non–linearity = 0.02).
It seemed that the duration of 8 weeks is an optimum duration for propolis intake to increase SOD compared to other duration of included trial duration. However, no significant non-linear relationship between the features (dose and duration) of propolis intake and changes in the levels of other outcomes was found (Supplementary Figures 3A-P).
3.6 Sensitivity analysis
The sensitivity analysis, which was performed to investigate the effect of the quality of each of the included studies on the overall effect size of each of the outcomes, reported that the impact of propolis supplementation on MCP-1 levels after removing the study conducted by Maddahi et al. (WMD: –29.68 pg/mL 95%CI: –62.25, 2.89), and for GPx after excluding Zhao et al. (WMD: 52.32 U/L 95%CI: –11.07, 115.71) changed significantly. Furthermore, omitting the Gao et al. (WMD: 12.51 U/mL 95%CI: 0.22, 24.81), or Tasca et al. (WMD: 12.55 U/mL 95%CI: 3.63, 21.47) led to a significant change in finding regarding the impact of propolis consumption on SOD levels. However, the pooled effect sizes of CRP, IL-6, TNF-a, IL-10, IL-2, IL-8, MDA, TAC, GSH, and PAB were not significantly affected by the presence of an effect size among pooled items.
3.7 Publication bias
The visual interpretation of funnel plots and the implementation of Egger regression and Begg rank correlation analyses showed that there was no significant publication bias among the evidence investigating the impact of propolis supplementation on any of the outcomes, including CRP (pBegg = 0.28), IL-6 (pBegg = 0.64), TNF-a (pBegg = 0.07), IL-10 (pEgger = 0.65), MDA (pBegg = 0.73), TAC (pEgger = 0.71), GSH (pEgger = 0.29), GPx (pEgger = 0.75), SOD (pEgger = 0.64), and PAB (pEgger = 0.66) (Supplementary Figures 4A-M).
3.8 GRADE analysis
The quality of evidence investigating the impact of propolis intake on MCP-1 or GSH was upgraded to very high due to a lack of serious limitations in none of the GRADE domains. The quality of evidence was considered moderate for CRP, IL-6, TNF-a, TAC, and GPx due to very serious inconsistency. Also, due to serious imprecision and serious inconsistency the quality of evidence for IL-2, IL-8, and PAB was identified as moderate, too. However, the certainty of evidence was downgraded to low quality for IL-10, SOD, and MDA due to serious imprecision and very serious inconsistency. The GRADE profile is shown in Table 4.
4 Discussion
Oxidative stress is a key factor in promoting inflammation and contributing to the onset of chronic conditions like cardiovascular diseases, diabetes, neurodegenerative disorders, and cancer (59). The accumulation of ROS resulting from an imbalance between their production and neutralization via DNA damage, lipid peroxidation, and protein modifications leads to tissue damage, activation of pro-inflammatory signaling pathways, and direct cell damage (60). Therefore, strategies to reduce oxidative stress and maintain a balance between ROS and antioxidants may help prevent or manage chronic diseases.
This current systematic review and meta-analysis study reveals that propolis reduces inflammation through the reduction of inflammatory markers including, CRP, TNF-α, and IL-6 in the intervention of more than 12 weeks and at the age of more than 50 years. It also lowers oxidative stress by decreasing MDA levels in doses of more than 1,000 mg/day and increasing TAC, GSH, GPX, and SOD in healthy people, women, and diabetes, and in the intervention of less than 12 weeks and the dose of less than 1,000 mg/day. In addition, the dose-response analysis in the present study showed that the optimum dose and duration for increasing SOD is 500 mg/day and 8 weeks, respectively.
Propolis supplementation shows a dose-dependent effect, with higher doses generally providing more pronounced benefits, particularly in reducing oxidative stress and improving glycemic control (47, 61, 62). The optimal duration for supplementation varies, with effective outcomes observed from as short as 1 week to as long as 6 months, depending on the health condition and desired outcomes (47, 55, 63, 64). These findings suggest that both the dose and duration of propolis supplementation should be tailored to the specific health goals of the individual.
The findings of this study are consistent with the meta-analysis of Hallajzadeh et al. (20). The difference is that in the present study, more studies on inflammatory factors and oxidative stress have been meta-analyzed, and the subgroup analyses performed show significant changes in oxidative stress markers with propolis intervention. The most recent meta-analysis also shows that intervention with propolis increases TAC, GSH, GPX, and MDA decreases with a dose ≥ 1,000 mg/day (61).
Propolis contains a variety of bioactive components, such as flavonoids, phenolic acids, and terpenoids, which contribute to its antioxidant activity (59). These compounds scavenge free radicals and inhibit ROS production, thereby reducing oxidative stress. Propolis flavonoids, specifically quercetin, and kaempferol derivatives, can directly neutralize ROS and suppress oxidative stress-induced damage (16).
Propolis has been demonstrated to influence signaling pathways related to inflammation. For example, it can suppress the activation of nuclear factor-kappa B (NF-κB), a crucial regulator of inflammation which demonstrated reduced NF-κB activation and subsequent downregulation of pro-inflammatory cytokines (65).
Furthermore, propolis has been discovered to regulate inflammatory mediators like cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) (16). These enzymes are involved in the production of inflammatory molecules. A type of flavonoid in propolis, caffeic acid phenethyl ester (CAPE) inhibits the release of arachidonic acid from the cell membrane and prevents gene expression of LOX and COX enzymes (66). The compound CAPE disrupts the interaction between the ligand, LPS, and the receptor complex, TLR4/MD2, leading to the inhibition of Toll-like receptor 4 (TLR4) activation. The binding of LPS to a hydrophobic pocket in MD2 initiates the assembly of a receptor multimer consisting of two TLR4/MD2/LPS complexes. This, in turn, recruits adaptor proteins and activates intracellular signaling pathways. Dysregulation of the TLR4 receptor has been implicated in chronic inflammatory diseases (67).
Furthermore, propolis may modulate intracellular signaling pathways related to oxidative stress and inflammation. Experimental evidence demonstrated that propolis activates the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway (68). Nrf2 is a master regulator of antioxidant defence and can promote the expression of antioxidant enzymes and phase II detoxifying enzymes, counteracting oxidative stress and reducing inflammation (69).
Although propolis is generally considered safe for consumption, it’s important to note that individuals may have varying sensitivities or allergies to bee products. While specific clinical trial studies on propolis supplements’ side effects are scarce, a few potential side effects such as allergic reactions, contact dermatitis, and gastrointestinal upset have been mentioned in general research and anecdotal reports. It’s worth emphasizing that these side effects are generally infrequent and mild (70).
The ability of propolis to modulate inflammatory and oxidative stress markers positions it as a potentially valuable treatment option for a range of diseases beyond its traditional uses. However, further research and standardization are necessary to fully realize its therapeutic potential. As scientific understanding of propolis grows, it could become an integral part of treatment strategies for neurodegenerative diseases, cardiovascular conditions, metabolic disorders, autoimmune diseases, and even cancer.
Propolis has shown promise in managing metabolic syndrome (MetS) and its associated chronic diseases, which are significant contributors to global mortality. Its antioxidant and anti-inflammatory properties help ameliorate symptoms by inhibiting advanced glycation end products (AGEs) and their receptors (RAGEs), as well as pro-inflammatory signaling cascades (71). In patients with type 2 diabetes mellitus (T2DM) and chronic periodontitis, propolis supplementation has been found to improve glycemic control and periodontal health. A clinical trial demonstrated significant reductions in hemoglobin A1c, fasting plasma glucose, and serum Nε-(carboxymethyl) lysine levels, alongside improved periodontal parameters (63). Propolis has been evaluated for its effects on non-alcoholic fatty liver disease (NAFLD), showing protective effects against hepatic steatosis and fibrosis. It significantly reduced liver stiffness and high-sensitivity C-reactive protein levels, indicating its potential as a therapeutic agent for NAFLD (38). Propolis may also benefit patients with rheumatoid arthritis (RA) by reducing inflammation and oxidative stress. It inhibits inflammatory pathways and reduces reactive oxygen species, potentially alleviating pain and improving disease control (72). Propolis has been studied for its anticancer and neuroprotective properties. Brazilian green propolis, in particular, has shown a potential to improve cognitive functions and protect against neurodegenerative damage due to its antioxidant properties (73). Propolis has been explored for its potential against SARS-CoV-2 infection mechanisms. It inhibits key pathways involved in viral entry and inflammation, suggesting its utility in managing COVID-19 and related respiratory conditions (74).
Propolis demonstrates complementary effects across different demographics and metabolic conditions. Its benefits vary by gender, with males potentially experiencing more pronounced effects (75). In older adults, propolis aids in reducing body fat and oxidative stress (55, 76), while in metabolic disorders like PCOS and diabetes, it improves insulin sensitivity and lipid profiles. Overall, propolis supports metabolic regulation through its impact on gut microbiota, adipogenesis, and inflammation, making it a versatile nutraceutical for metabolic health (49, 77).
The current study is a comprehensive review of the effect of propolis on inflammatory factors and oxidative stress, which has been examined by a larger number of RCTs than in previous reviews. Also, dose-response analysis has determined the optimal dose and duration of propolis consumption to reduce inflammation and oxidative stress.
However, the present meta-analysis has some limitations that should be mentioned: based on the GRADE of most of the obtained results, they are weak to moderate, so it is still not possible to draw a definite conclusion about the effectiveness of propolis in reducing oxidative stress and inflammation. Another limitation of this study is the result of sensitivity analysis for MCP-1, GPx, and SOD variables, which is associated with uncertainty in the conclusions. On the other hand, different types of propolis have been used in RCTs, which have different flavonoid compounds depending on their geographical location and other factors, and as a result, their effectiveness will be different. Also, the heterogeneity of the intervention population in different RCTs is another limitation of this study. The standardization of propolis formulations is challenged by chemical variability, diverse extraction methods, and complex correlations between chemical composition and biological activity. These limitations impact the reproducibility and comparability of study outcomes, highlighting the need for standardized criteria and methodologies to enhance the reliability of propolis-based research and applications. Future research on propolis should prioritize standardization, clinical efficacy, and understanding its mechanisms of action. Additionally, exploring its potential in aging and neurological health, alongside improving production methods, will significantly advance our knowledge and application of propolis in medicine.
5 Conclusion
In conclusion, propolis exerts its effects on reducing oxidative stress and inflammation by reducing CRP, IL-6, TNF-α, and MCP-1 and enhancing TAC, GSH, and GPx. These findings support the traditional use of propolis in treating various diseases related to oxidative stress and inflammation. More RCTs are needed to draw definitive conclusions about the best dose and duration of intervention.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
HB: Conceptualization, Formal Analysis, Writing – review & editing. MS: Writing – original draft. MA: Resources, Validation, Writing – review & editing. MR: Writing – original draft. HG: Data curation, Investigation, Writing – original draft. IR: Writing – review & editing. RK: Supervision, Writing – review & editing. MM: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1542184/full#supplementary-material
Abbreviations
BMI, Body mass index; CAPE, Caffeic acid phenethyl ester; CKD, Chronic kidney disease; CRP, C-reactive protein; COX-2, cyclooxygenase-2; GSH, Glutathione; GPx, Glutathione peroxidase; IL, Interleukin; iNOS, inducible nitric oxide synthase; MCP-1, Monocyte chemoattractant protein-1; MDA, Malondialdehyde; NO, Nitric oxide; NF-κ B, Nuclear factor-kappa B; Nrf2, Nuclear factor erythroid 2-related factor 2; PAB, Pro-oxidant-antioxidant balance; ROS, Reactive oxygen species; SOD, Superoxide dismutase; TAC, Total antioxidant capacity; TLR4, Toll-like receptor 4; TNF-α, Tumor necrosis factor-alpha.
References
1. Rivera-Yañez N, Rivera-Yañez C, Pozo-Molina G, Méndez-Catalá C, Méndez-Cruz A, Nieto-Yañez O. Biomedical properties of propolis on diverse chronic diseases and its potential applications and health benefits. Nutrients. (2020) 13:78. doi: 10.3390/nu13010078
2. Balica G, Vostinaru O, Stefanescu C, Mogosan C, Iaru I, Cristina A, et al. Potential role of propolis in the prevention and treatment of metabolic diseases. Plants (Basel). (2021) 10:883. doi: 10.3390/plants10050883
3. Braakhuis A. Evidence on the health benefits of supplemental propolis. Nutrients. (2019) 11:2705. doi: 10.3390/nu11112705
4. Kocot J, Kiełczykowska M, Luchowska-Kocot D, Kurzepa J, Musik I. Antioxidant potential of propolis, bee pollen, and royal jelly: Possible medical application. Oxid Med Cell Longev. (2018) 2018:7074209. doi: 10.1155/2018/7074209
5. Imai H, Era S, Hayashi T, Negawa T, Matsuyama Y, Okihara K, et al. Effect of propolis supplementation on the redox state of human serum albumin during high-intensity kendo training. Adv Exerc Sports Physiol. (2005) 11:109–13.
6. Zhu A, Wu Z, Zhong X, Ni J, Li Y, Meng J, et al. Brazilian green propolis prevents cognitive decline into mild cognitive impairment in elderly people living at high altitude. J Alzheimers Dis. (2018) 63:551–60. doi: 10.3233/JAD-170630
7. Necip A, Demirtas I, Tayhan S, Işık M, Bilgin S, Turan ÝF, et al. Isolation of phenolic compounds from eco-friendly white bee propolis: Antioxidant, wound-healing, and anti-Alzheimer effects. Food Sci Nutr. (2024) 12:1928–39. doi: 10.1002/fsn3.3888
8. Anvarifard P, Anbari M, Ostadrahimi A, Ardalan M, Ghoreishi Z. A comprehensive insight into the molecular and cellular mechanisms of the effects of Propolis on preserving renal function: A systematic review. Nutr Metab (Lond). (2022) 19:1–23. doi: 10.1186/s12986-021-00639-z
9. Al-Hariri M. Immune’s-boosting agent: Immunomodulation potentials of propolis. J Family Community Med. (2019) 26:57–60. doi: 10.4103/jfcm.JFCM_46_18
10. Kurek-Górecka A, Kłósek M, Pietsz G, Balwierz R, Olczyk P, Czuba Z. Ethanolic extract of propolis and CAPE as cardioprotective agents against LPS and IFN-α stressed cardiovascular injury. Nutrients. (2024) 16:627. doi: 10.3390/nu16050627
11. Bueno-Silva B, Kawamoto D, Ando-Suguimoto E, Alencar S, Rosalen P, Mayer M. Brazilian red propolis attenuates inflammatory signaling cascade in LPS-activated macrophages. PLoS One. (2015) 10:e0144954. doi: 10.1371/journal.pone.0144954
12. Shang H, Bhagavathula A, Aldhaleei W, Rahmani J, Karam G, Rinaldi G, et al. Effect of propolis supplementation on C-reactive protein levels and other inflammatory factors: A systematic review and meta-analysis of randomized controlled trials. J King Saud Univer Sci. (2020) 32:1694–701. doi: 10.1016/j.jksus.2020.01.003
13. Hori J, Zamboni D, Carrão D, Goldman G, Berretta A. The inhibition of inflammasome by Brazilian Propolis (EPP-AF). Evid Based Complement Alternat Med. (2013) 2013:418508. doi: 10.1155/2013/418508
14. Vecchi C, dos Santos R, da Silva J, Rissi C, Machado R, de Castro-Hoshino L, et al. Development of microneedles for propolis delivery: The effect on the production of reactive oxygen species and in vitro immunostimulating activity. J Drug Delivery Sci Technol. (2023) 89:105065. doi: 10.1016/j.jddst.2023.105065
15. Maddahi M, Nattagh-Eshtivani E, Jokar M, Barati M, Tabesh H, Safarian M, et al. The effect of propolis supplementation on cardiovascular risk factors in women with rheumatoid arthritis: A double-blind, placebo, controlled randomized clinical trial. Phytother Res. (2023) 37:5424–34. doi: 10.1002/ptr.7996
16. Pahlavani N, Malekahmadi M, Firouzi S, Rostami D, Sedaghat A, Moghaddam A, et al. Molecular and cellular mechanisms of the effects of Propolis in inflammation, oxidative stress and glycemic control in chronic diseases. Nutr Metab (Lond). (2020) 17:65. doi: 10.1186/s12986-020-00485-5
17. Soleimani D, Miryan M, Hadi V, Gholizadeh Navashenaq J, Moludi J, Sayedi S, et al. Effect of propolis supplementation on athletic performance, body composition, inflammation, and oxidative stress following intense exercise: A triple-blind randomized clinical trial. Food Sci Nutr. (2021) 9:3631–40. doi: 10.1002/fsn3.2319
18. Wang K, Ping S, Huang S, Hu L, Xuan H, Zhang C, et al. Molecular mechanisms underlying the in vitro anti-inflammatory effects of a flavonoid-rich ethanol extract from chinese propolis (poplar type). Evid Based Complement Alternat Med. (2013) 2013:127672. doi: 10.1155/2013/127672
19. Szliszka E, Kucharska A, Sokół-Łêtowska A, Mertas A, Czuba Z, Król W. Chemical composition and anti-inflammatory effect of ethanolic extract of brazilian green propolis on activated J774A.1 macrophages. Evid Based Complement Alternat Med. (2013) 2013:976415. doi: 10.1155/2013/976415
20. Hallajzadeh J, Milajerdi A, Amirani E, Attari V, Maghsoudi H, Mirhashemi S. Effects of propolis supplementation on glycemic status, lipid profiles, inflammation and oxidative stress, liver enzymes, and body weight: A systematic review and meta-analysis of randomized controlled clinical trials. J Diabetes Metab Disord. (2021) 20:831–43. doi: 10.1007/s40200-020-00696-w
21. Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med. (2009) 151:264–9.W64. doi: 10.7326/0003-4819-151-4-200908180-00135
22. Methley A, Campbell S, Chew-Graham C, McNally R, Cheraghi-Sohi S. PICO, PICOS and SPIDER: A comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res. (2014) 14:579. doi: 10.1186/s12913-014-0579-0
23. Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.0. 2 [updated Sept 2009], The Cochrane Collaboration, 2009. (2010).
24. Golalipour E, Hosseininasab D, Nikbaf-Shandiz M, Rasaei N, Bahari H, Hajmir M, et al. The effect of acarbose treatment on anthropometric indices in adults: A systematic review and meta-analysis of randomized clinical trials. Clin Nutr Open Sci. (2024) 56:166–91. doi: 10.1016/j.nutos.2024.06.004
25. Ghodoosi N, Rasaei N, Goudarzi K, Hashemzadeh M, Dolatshahi S, Omran H, et al. The effects of conjugated linoleic acid supplementation on glycemic control, adipokines, cytokines, malondialdehyde and liver function enzymes in patients at risk of cardiovascular disease: A GRADE-assessed systematic review and dose-response meta-analysis. Nutr J. (2023) 22:47. doi: 10.1186/s12937-023-00876-3
26. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
27. Borenstein M, Hedges L, Higgins J, Rothstein H. Introduction to Meta-Analysis. Hoboken, NJ: John Wiley & Sons (2021).
28. Hozo S, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. (2005) 5:13. doi: 10.1186/1471-2288-5-13
29. Higgins J, Thompson S, Deeks J, Altman D. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
30. Higgins J, Thompson S. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
31. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
32. Tobias A. Assessing the influence of a single study in the meta-analysis estimate. Stata Tech. Bull. (1999) 47:15–7.
33. Xu C, Doi S. The robust error meta-regression method for dose-response meta-analysis. Int J Evid Based Healthc. (2018) 16:138–44. doi: 10.1097/XEB.0000000000000132
34. Guyatt G, Oxman A, Vist G, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
35. Khayyal M, El-Ghazaly M, El-Khatib AS, Hatem AM, de Vries PJ, El-Shafei S, et al. A clinical pharmacological study of the potential beneficial effects of a propolis food product as an adjuvant in asthmatic patients. Fundam Clin Pharmacol. (2003) 17:93–102. doi: 10.1046/j.1472-8206.2003.00117.x
36. Conte F, Tasca K, Santiago K, de Oliveira Cardoso E, Romagnoli G, de Assis Golim M, et al. Propolis increases Foxp3 expression and lymphocyte proliferation in HIV-infected people: A randomized, double blind, parallel-group and placebo-controlled study. Biomed Pharmacother. (2021) 142:111984. doi: 10.1016/j.biopha.2021.111984
37. Asama T, Hiraoka T, Ohkuma A, Okumura N, Yamaki A, Urakami K. Cognitive improvement and safety assessment of a dietary supplement containing propolis extract in elderly Japanese: A placebo-controlled, randomized, parallel-group, double-blind human clinical study. Evid Based Complement Alternat Med. (2021) 2021:6664217. doi: 10.1155/2021/6664217
38. Soleimani D, Rezaie M, Rajabzadeh F, Gholizadeh Navashenaq J, Abbaspour M, Miryan M, et al. Protective effects of propolis on hepatic steatosis and fibrosis among patients with nonalcoholic fatty liver disease (NAFLD) evaluated by real-time two-dimensional shear wave elastography: A randomized clinical trial. Phytother Res. (2021) 35:1669–79. doi: 10.1002/ptr.6937
39. Žuža O, Minić R, Kotur-Stevuljević J, Žujović D, Ðorąević B, Ilić A. A combination of N-acetyl cysteine and propolis attenuates oxidative-inflammatory parameters during COPD exacerbation. Eur Rev Med Pharmacol Sci. (2022) 26:2467–77. doi: 10.26355/eurrev_202204_28481
40. Afsharpour F, Javadi M, Hashemipour S, Koushan Y, Haghighian H. Changes in lipid profile, liver enzymes and inflammatory factors following oral supplementation with propolis in patients with type 2 diabetes. Clin Diabetol. (2022) 11:224–31. doi: 10.5603/DK.a2022.0033
41. Fukuda T, Fukui M, Tanaka M, Senmaru T, Iwase H, Yamazaki M, et al. Effect of Brazilian green propolis in patients with type 2 diabetes: A double-blind randomized placebo-controlled study. Biomed Rep. (2015) 3:355–60. doi: 10.3892/br.2015.436
42. Zhao L, Pu L, Wei J, Li J, Wu J, Xin Z, et al. Brazilian green propolis improves antioxidant function in patients with type 2 diabetes mellitus. Int J Environ Res Public Health. (2016) 13:498. doi: 10.3390/ijerph13050498
43. Ebeid S, Abd El Moneim NA, El-Benhawy SA, Hussain NG, Hussain MI. Assessment of the radioprotective effect of propolis in breast cancer patients undergoing radiotherapy. New perspective for an old honey bee product. J Radiation Res Appl Sci. (2016) 9:431–40. doi: 10.1016/j.jrras.2016.06.001
44. Afsharpour F, Hashemipour S, Khadem-Haghighian H, Koushan Y. Effects of Iranian propolis on glycemic status, inflammatory factors, and liver enzyme levels in type 2 diabetic patients: A randomized, double-blind, placebo-controlled, clinical trial. J Nutr Sci Dietetics. (2017):9–14.
45. Mujica V, Orrego R, Pérez J, Romero P, Ovalle P, Zúñiga-Hernández J, et al. The role of propolis in oxidative stress and lipid metabolism: A randomized controlled trial. Evid Based Complement Alternat Med. (2017) 2017:4272940. doi: 10.1155/2017/4272940
46. Gao W, Pu L, Wei J, Yao Z, Wang Y, Shi T, et al. Serum antioxidant parameters are significantly increased in patients with type 2 diabetes mellitus after consumption of chinese propolis: A randomized controlled trial based on fasting serum glucose Level. Diabetes Ther. (2018) 9:101–11. doi: 10.1007/s13300-017-0341-9
47. Afsharpour F, Javadi M, Hashemipour S, Koushan Y, Haghighian H. Propolis supplementation improves glycemic and antioxidant status in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled study. Complement Ther Med. (2019) 43:283–8. doi: 10.1016/j.ctim.2019.03.001
48. Gholaminejad F, Javadi M, Karami A, Alizadeh F, Haghighian H. Propolis supplementation effects on semen parameters, oxidative stress, Inflammatory biomarkers and reproductive hormones in infertile men with asthenozoospermia; A randomized clinical trial. Int J Med Lab. (2019) 6:21–32. doi: 10.18502/ijml.v6i1.504
49. Zakerkish M, Jenabi M, Zaeemzadeh N, Hemmati A, Neisi N. The effect of Iranian propolis on glucose metabolism, lipid profile, insulin resistance, renal function and inflammatory biomarkers in patients with type 2 diabetes mellitus: A randomized double-blind clinical trial. Sci Rep. (2019) 9:7289. doi: 10.1038/s41598-019-43838-8
50. Darvishi N, Yousefinejad V, Akbari M, Abdi M, Moradi N, Darvishi S, et al. Antioxidant and anti-inflammatory effects of oral propolis in patients with breast cancer treated with chemotherapy: A Randomized controlled trial. J Herbal Med. (2020) 23:100385. doi: 10.1016/j.hermed.2020.100385
51. Nikbaf-Shandiz M, Tutunchi H, Khoshbaten M, Nazari Bonab H, Ebrahimi-Mameghani M. Propolis supplementation in obese patients with non-alcoholic fatty liver disease: Effects on glucose homeostasis, lipid profile, liver function, anthropometric indices and meta-inflammation. Food Funct. (2022) 13:11568–78. doi: 10.1039/d2fo01280d
52. Pahlavani N, Malekahmadi M, Sedaghat A, Rostami A, Alkadir O, Taifi A, et al. Effects of melatonin and propolis supplementation on inflammation, oxidative stress, and clinical outcomes in patients with primary pneumosepsis: A randomized controlled clinical trial. Complement Med Res. (2022) 29:275–85. doi: 10.1159/000523766
53. Anvarifard P, Ostadrahimi A, Ardalan M, Anbari M, Ghoreishi Z. The effects of propolis on pro-oxidant-antioxidant balance, glycemic control, and quality of life in chronic kidney disease: A randomized, double-blind, placebo-controlled trial. Sci Rep. (2023) 13:9884. doi: 10.1038/s41598-023-37033-z
54. Tasca K, Conte F, Correa C, Santiago K, Cardoso E, Manfio V, et al. Propolis consumption by asymptomatic HIV-individuals: Better redox state? A prospective, randomized, double-blind, placebo-controlled trial. Biomed Pharmacother. (2023) 162:114626. doi: 10.1016/j.biopha.2023.114626
55. Kanazashi M, Iida T, Nakanishi R, Tanaka M, Ikeda H, Takamiya N, et al. Brazilian propolis intake decreases body fat mass and oxidative stress in community-dwelling elderly females: A randomized placebo-controlled trial. Nutrients. (2023) 15:364. doi: 10.3390/nu15020364
56. Moayedi F, Taghian F, Jalali Dehkordi K, Hosseini S. Cumulative effects of exercise training and consumption of propolis on managing diabetic dyslipidemia in adult women: A single-blind, randomized, controlled trial with pre-post-intervention assessments. J Physiol Sci. (2023) 73:1–18. doi: 10.1186/s12576-023-00872-6
57. Abbasi E, Bagherniya M, Soleimani D, Ghasemi-Tehrani H, Abbaspour M, Clark C, et al. The effects of propolis supplementation on high-sensitivity C-reactive protein, testosterone hormone, and metabolic profile in women with polycystic ovary syndrome: A randomized, triple-blinded, placebo-controlled clinical trial. Phytother Res. (2023) 37:5366–77. doi: 10.1002/ptr.7977
58. Sajjadi S, Bagherniya M, Soleimani D, Siavash M, Askari G. Effect of propolis on mood, quality of life, and metabolic profiles in subjects with metabolic syndrome: A randomized clinical trial. Sci Rep. (2023) 13:4452. doi: 10.1038/s41598-023-31254-y
59. Malekahmadi M, Pahlavani N, Heshmati J, Clayton Z, Beigmohammadi M, Navashenaq J, et al. Effect of propolis supplementation on oxidative stress markers: A systematic review of randomized controlled trials. J Herbal Med. (2023) 40:100679. doi: 10.1016/j.hermed.2023.100679
60. Prata C, Maraldi T, Angeloni C. Strategies to counteract oxidative stress and inflammation in chronic-degenerative diseases. Int J Mol Sci. (2022) 23:6439. doi: 10.3390/ijms23126439
61. Nazari-Bonab H, Jamilian P, Radkhah N, Zarezadeh M, Ebrahimi-Mameghani M. The effect of propolis supplementation in improving antioxidant status: A systematic review and meta-analysis of controlled clinical trials. Phytother Res. (2023) 37:3712–23. doi: 10.1002/ptr.7899
62. Silveira M, De Jong D, Berretta A, Galvão E, Ribeiro J, Cerqueira-Silva T, et al. Efficacy of Brazilian green propolis (EPP-AF®) as an adjunct treatment for hospitalized COVID-19 patients: A randomized, controlled clinical trial. Biomed Pharmacother. (2021) 138:111526. doi: 10.1016/j.biopha.2021.111526
63. El-Sharkawy H, Anees M, Van Dyke T. Propolis improves periodontal status and glycemic control in patients with type 2 diabetes mellitus and chronic periodontitis: A randomized clinical trial. J Periodontol. (2016) 87:1418–26. doi: 10.1902/jop.2016.150694
64. Tsuchiya Y, Hirata N, Asama T, Osakabe N, Hirata K, Akagi R. Can a short-term daily oral administration of propolis improve muscle fatigue and recovery? Int J Sports Med. (2022) 43:859–64. doi: 10.1055/a-1808-6319
65. Rius-Pérez S, Pérez S, Martí-Andrés P, Monsalve M, Sastre J. Nuclear factor kappa B signaling complexes in acute inflammation. Antioxid Redox Signal. (2020) 33:145–65. doi: 10.1089/ars.2019.7975
66. Molehin O, Adefegha S, Ajiboye I, Ogunleye T. Modulation of Phospholipases by Phenolic Compounds: Novel Targets in the Management of Inflammatory Diseases. Phospholipases in Physiology and Pathology. Amsterdam: Elsevier (2023). p. 91–110.
67. Zhao W, Wang L, Yang J, Li L, Xu W, Li T. Caffeic acid phenethyl ester attenuates pro-inflammatory and fibrogenic phenotypes of LPS-stimulated hepatic stellate cells through the inhibition of NF-κB signaling. Int J Mol Med. (2014) 33:687–94. doi: 10.3892/ijmm.2013.1613
68. Xu W, Lu H, Yuan Y, Deng Z, Zheng L, Li H. The antioxidant and anti-inflammatory effects of flavonoids from propolis via Nrf2 and NF-κB pathways. Foods. (2022) 11:2439. doi: 10.3390/foods11162439
69. Saito Y, Tsuruma K, Ichihara K, Shimazawa M, Hara H. Brazilian green propolis water extract up-regulates the early expression level of HO-1 and accelerates Nrf2 after UVA irradiation. BMC Complement Altern Med. (2015) 15:421. doi: 10.1186/s12906-015-0945-4
70. de Groot A. Propolis: A review of properties, applications, chemical composition, contact allergy, and other adverse effects. Dermatitis. (2013) 24:263–82. doi: 10.1097/DER.0000000000000011
71. Zulhendri F, Ravalia M, Kripal K, Chandrasekaran K, Fearnley J, Perera C. Propolis in metabolic syndrome and its associated chronic diseases: A narrative review. Antioxidants (Basel). (2021) 10:348. doi: 10.3390/antiox10030348
72. Nattagh-Eshtivani E, Pahlavani N, Ranjbar G, Gholizadeh Navashenaq J, Salehi-Sahlabadi A, Mahmudiono T, et al. Does propolis have any effect on rheumatoid arthritis? A review study. Food Sci Nutr. (2022) 10:1003–20. doi: 10.1002/fsn3.2684
73. Bhargava P, Mahanta D, Kaul A, Ishida Y, Terao K, Wadhwa R, et al. Experimental evidence for therapeutic potentials of propolis. Nutrients. (2021) 13:2528. doi: 10.3390/nu13082528
74. Berretta A, Silveira M, Cóndor Capcha J, De Jong D. Propolis and its potential against SARS-CoV-2 infection mechanisms and COVID-19 disease: Running title: Propolis against SARS-CoV-2 infection and COVID-19. Biomed Pharmacother. (2020) 131:110622. doi: 10.1016/j.biopha.2020.110622
75. Zheng Y, Wu Y, Tao L, Chen X, Jones T, Wang K, et al. Chinese propolis prevents obesity and metabolism syndromes induced by a high fat diet and accompanied by an altered gut microbiota structure in mice. Nutrients. (2020) 12:959. doi: 10.3390/nu12040959
76. Scorza C, Goncalves V, Finsterer J, Scorza F, Fonseca F. Exploring the prospective role of propolis in modifying aging hallmarks. Cells. (2024) 13:390. doi: 10.3390/cells13050390
Keywords: propolis, oxidative stress, inflammation, systematic review, meta-analysis
Citation: Bahari H, Shahraki Jazinaki M, Aliakbarian M, Rashidmayvan M, Golafrouz H, Rahnama I, Khodashahi R and Malekahmadi M (2025) Propolis supplementation on inflammatory and oxidative stress biomarkers in adults: a systematic review and meta-analysis of randomized controlled trials. Front. Nutr. 12:1542184. doi: 10.3389/fnut.2025.1542184
Received: 09 December 2024; Accepted: 25 April 2025;
Published: 12 May 2025.
Edited by:
Ana Sanches Silva, National Institute for Agricultural and Veterinary Research (INIAV), PortugalReviewed by:
Muthukumar Serva Peddha, Central Food Technological Research Institute (CSIR), IndiaEndre Mathe, University of Debrecen, Hungary
Copyright © 2025 Bahari, Shahraki Jazinaki, Aliakbarian, Rashidmayvan, Golafrouz, Rahnama, Khodashahi and Malekahmadi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rozita Khodashahi, cmtob2Rhc2hhaGlAeWFob28uY29t; S2hvZGFzaGFoaVJAbXVtcy5hYy5pcg==; Mahsa Malekahmadi, bWFsZWthaG1hZGltYWhzYUBnbWFpbC5jb20=
 Hossein Bahari
Hossein Bahari Mostafa Shahraki Jazinaki
Mostafa Shahraki Jazinaki Mohsen Aliakbarian1
Mohsen Aliakbarian1 Mohammad Rashidmayvan
Mohammad Rashidmayvan Haniyeh Golafrouz
Haniyeh Golafrouz Rozita Khodashahi
Rozita Khodashahi Mahsa Malekahmadi
Mahsa Malekahmadi