Abstract
Background and aims:
The relationship between oxidative stress (OS) and preserved ratio impaired spirometry (PRISm) remains unclear. We aimed to utilize the oxidation balance score (OBS), a validated instrument for assessing the overall OS status, to investigate the association between OBS and PRISm.
Methods:
We included data from 7,180 participants in the National Health and Nutrition Examination Survey (NHANES). The OBS was calculated using 20 components of diet and lifestyle. Binary logistic regression analyses were conducted to investigate the association between OBS and PRISm. Subsequent analyses were performed for non-smokers and smokers across different OBS levels.
Results:
OBS was inversely associated with PRISm in all models (all p-values < 0.001). In subsequent analyses, the odds ratio (OR) for PRISm increased sequentially from non-smokers with high OBS, to non-smokers with low OBS, to smokers with high OBS, and to smokers with low OBS (dose–response p-values in all models ≤ 0.003); smokers with low OBS exhibited the highest PRISm incidence in the fully adjusted model (OR = 1.83, 95% CI = 1.27–2.64, p = 0.001).
Conclusion:
A lower OBS was associated with an increased incidence of PRISm, particularly among smokers, suggesting that OS may play a pivotal role in this relationship.
1 Introduction
Unlike chronic obstructive pulmonary disease (COPD), preserved ratio impaired spirometry (PRISm) is characterized by the absence of airflow obstruction with reduced lung function (1). PRISm is defined as a normal or preserved ratio of forced expiratory volume in 1 s (FEV1) to forced vital capacity (FVC; FEV1: FVC ≥ 0.7), coupled with an FEV1 below 80% of the predicted value (1). With a global prevalence ranging from 5 to 20%, PRISm is associated with an increase in respiratory symptoms (1, 2) and a higher risk of adverse clinical outcomes (3–5). A prospective cohort study with a 4.5-year follow-up reported that 50% of PRISm patients progressed to COPD, while 15% reverted to normal lung function (6). Given its reversible nature, PRISm represents a promising target for preventive strategies that can avert a poor prognosis. While previous studies have identified several risk factors for PRISm, including smoking status, abnormal body mass index (BMI), and age (1, 2, 7), the pathogenesis remains unclear, and interventions to reverse PRISm and restore normal lung function are limited.
Oxidative stress (OS), a term first coined by Helmut Sies in 1985, refers to an imbalance in which prooxidants outnumber antioxidants, leading to tissue damage and subsequent organ dysfunction (8). Previous research has confirmed that OS induced by smoking contributes to the decline in lung function among smokers (9). Numerous exogenous factors influence OS levels in the body; for instance, physical activity and certain nutrients, such as vitamin C, calcium, and selenium, act as antioxidants, whereas obesity, smoking status, and excessive alcohol consumption are prooxidant factors (10). The oxidation balance score (OBS), which incorporates 20 dietary and lifestyle-related pro-oxidant and antioxidant components, is a validated tool for assessing the overall impact of exogenous factors on OS status, with higher OBS values indicating a greater capacity to resist oxidative damage (10). A recent study found that a higher OBS score was associated with a lower incidence of COPD (11). However, few studies have investigated the association of OBS with PRISm. We therefore aimed to investigate the relationship between OBS and PRISm. Furthermore, considering that smoking is a risk factor for PRISm and that smoking-induced OS impairs lung function, we hypothesize that a healthy diet and lifestyle, by acting as antioxidant strategies, may mitigate the impact of smoking on PRISm risk. Thus, to test this hypothesis, we compared the risk of PRISm in non-smokers and smokers with different OBS levels.
2 Methods
This study was exempt from additional ethical review, as it involved secondary analysis of a public database.
2.1 Study population
This study utilized data from the National Health and Nutrition Examination Survey (NHANES), conducted by the National Center for Health Statistics (NCHS), to assess the health and nutritional status of the US population. NHANES procedures include interviews, physical examinations conducted in mobile examination centers, and laboratory tests. Further details regarding the research protocol and written informed consent are available on the NHANES website.1
Data from three consecutive NHANES cycles (2007–2008, 2009–2010, and 2011–2012), encompassing 30,442 participants with available spirometry data, were used. Participants were excluded based on the following criteria: age < 20 years old, pregnancy, lack of spirometry data, or data quality below grade B (B refers to meeting American Thoracic Society data collection standards: three acceptable curves and two reproducible curves, with two observed values within 150 mL), missing data on diet and lifestyle factors necessary for OBS calculation, and evidence of airflow obstruction (FEV1: FVC < 0.70; Figure 1).
Figure 1
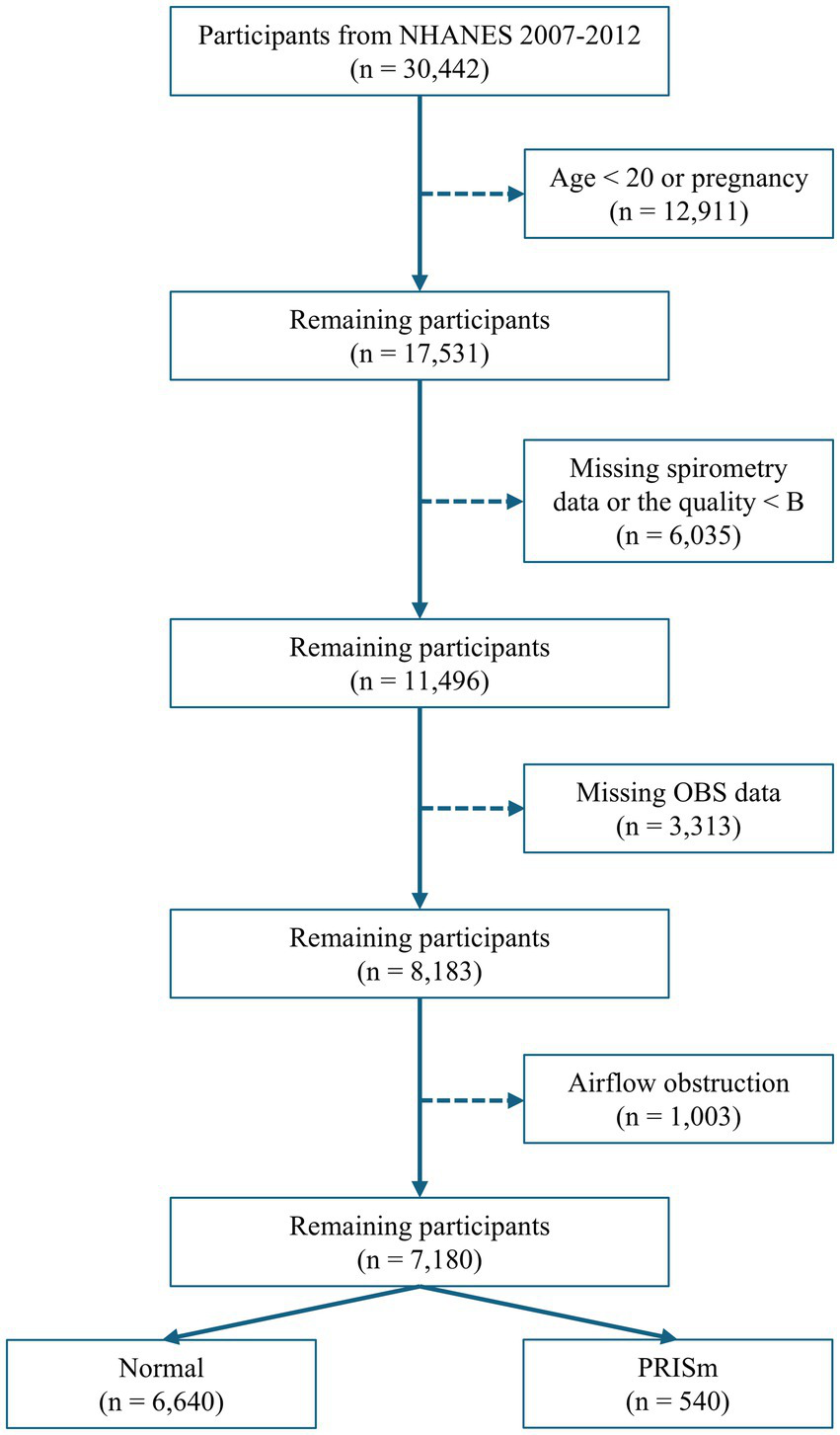
Flowchart for selecting participants.
2.2 Definition of PRISm
PRISm was defined as FEV1: FVC ≥ 0.7 and FEV1 < 80% of the predicted value. FEV1 and FVC data were obtained from the SPX dataset. Predicted FEV1 values were calculated using the new “race-neutral” Global Lung Function Initiative (GLI) equation (12).
2.3 OBS calculation
Based on data from the interview questionnaire—which includes computer-assisted personal interviewing and audio computer-assisted self-interviewing systems, both programmed with built-in consistency checks to reduce data entry errors, and with the computer-assisted personal interviewing system using online help screens to assist interviewers in defining key terms used in the questionnaire—physical examination, and laboratory tests, 20 components—comprising 16 dietary nutrients and 4 lifestyle factors—were categorized into 5 prooxidants and 15 antioxidants for the OBS calculation, following gender stratification (Supplementary Table 1). Alcohol consumption was categorized into non-drinkers, light drinkers (female < 15 g/day versus male < 30 g/day), and heavy drinkers (female ≥ 15 g/day versus male ≥ 30 g/day), with points assigned as 2, 1, and 0, respectively. The remaining 4 prooxidants were assigned 2, 1, and 0 points for the low, medium, and high tertile groups, respectively; conversely, all 15 antioxidants were assigned 0, 1, and 2 points (10).
2.4 Covariates
Age (categorized as young adult < 25 years, adult < 45 years, middle-aged < 65 years, and aged ≥ 65 years), gender, ethnicity, poverty–income ratio (categorized as low ≤ 1.3, middle ≤ 3.5, and high > 3.5), education level (classified into less than 9th grade, 9–11th grade, high school grade, some college or AA degree, and college graduate or above), marital status (defined as “not single” including “living with a partner” and “married,” or “single”), BMI (categorized as underweight < 18.5, normal < 25, overweight < 30, and obese ≥ 30), smoking status (defined as non-smoker): “not smoked 100 cigarettes in life,” former smoker (smoked at least 100 cigarettes in life but not smoking now,” and current smoker), and drinking frequency (defined as non-drinker, < 5 days per month, 5–10 days per month, and ≥10 days per month) were analyzed as covariates.
2.5 Statistical analysis
2.5.1 Baseline characteristics
The baseline characteristics of PRISm cases and normal controls were presented, with continuous variables presented as medians and categorical variables presented as percentages. Pearson’s chi-squared and Wilcoxon rank-sum tests were used for categorical and continuous variables, respectively.
2.5.2 Relationship between OBS and PRISm
Binary logistic regression analyses were conducted to assess the relationship between OBS or quartile OBS and PRISm in model 1. These results were then adjusted for age, gender, and ethnicity in model 2 and further adjusted for economic conditions, education, marital status, BMI, smoking status, and alcohol consumption in model 3. The results are expressed as odds ratio (OR) and 95% confidence interval (CI).
2.5.3 Association of smoking in addition to OBS with PRISm
Smoking status was redefined as non-smokers (smoking−) and current smokers (smoking+)—former smokers were classified as non-smokers. A new OBS was recalculated using 19 components, excluding the cotinine component, and categorized as either higher OBS (OBS+) or lower OBS (OBS−) based on the 50th percentile. Participants were then reclassified into four mutually exclusive groups based on the combination of OBS and smoking status: smoking−/OBS+, smoking−/OBS−, smoking+/OBS+, and smoking+/OBS−. Finally, ORs for PRISm were calculated for the four groups.
2.5.4 Sensitivity analysis
To address concerns about the instability of the PRISm definition caused by the use of different predicted-FEV1 equations, we replaced the GLI equation with the NHANES III equation and reran all analyses (13).
Continuous variables are represented as medians with interquartile ranges, while categorical variables are represented by counts and percentages. Pearson’s chi-squared and Wilcoxon rank-sum tests were used for categorical and continuous variables, respectively. A binary logistic regression model was used for the primary analysis, and various models adjusting for different covariates were used. Trend tests were conducted to evaluate the dose–response effect when the exposure factor was treated as an ordinal variable. All statistical analyses were performed using R version 4.4.1. A two-tailed p-value of < 0.05 was considered statistically significant.
3 Results
3.1 Baseline characteristics by PRISm status
Among the 7,180 participants, 540 (7.5%) were classified as PRISm cases, while 6,640 (92.5%) served as controls. Significant differences were observed between the PRISm and control groups in terms of age, ethnicity, household income, education level, marital status, BMI, smoking status, and alcohol consumption. The median OBS was 18 in the PRISm group versus 21 in the control group (p-value of < 0.001; Table 1).
Table 1
| Characteristic | Normal N = 6,6401 | PRISm N = 5401 | p-value2 |
|---|---|---|---|
| OBS | 21 [15, 26] | 18 [12, 23] | <0.001 |
| Age | <0.001 | ||
| Young adult | 842 (12.68%) | 42 (7.78%) | |
| Adult | 2,947 (44.38%) | 186 (34.44%) | |
| Middle aged | 2,183 (32.88%) | 241 (44.63%) | |
| Elderly | 668 (10.06%) | 71 (13.15%) | |
| Gender | 0.2 | ||
| Female | 3,258 (49.07%) | 281 (52.04%) | |
| Male | 3,382 (50.93%) | 259 (47.96%) | |
| Ethnicity | <0.001 | ||
| Non-Hispanic White | 3,123 (47.03%) | 111 (20.56%) | |
| Non-Hispanic Black | 1,057 (15.92%) | 327 (60.56%) | |
| Mexican American | 1,156 (17.41%) | 18 (3.33%) | |
| Other Hispanic | 730 (10.99%) | 21 (3.89%) | |
| Other ethnicities | 574 (8.64%) | 63 (11.67%) | |
| Household Income | 0.005 | ||
| Low | 1,774 (28.81%) | 152 (30.40%) | |
| Middle | 2,140 (34.75%) | 201 (40.20%) | |
| High | 2,244 (36.44%) | 147 (29.40%) | |
| Not recorded | 482 | 40 | |
| Education | <0.001 | ||
| Less than 9th grade | 512 (7.72%) | 25 (4.63%) | |
| 9–11th grade | 875 (13.19%) | 78 (14.44%) | |
| High school grade | 1,394 (21.01%) | 146 (27.04%) | |
| Some college or AA degree | 2,055 (30.97%) | 192 (35.56%) | |
| College graduate or above | 1,800 (27.12%) | 99 (18.33%) | |
| Not recorded | 4 | 0 | |
| Marital Status | <0.001 | ||
| Not single | 4,031 (60.74%) | 277 (51.39%) | |
| Single | 2,606 (39.26%) | 262 (48.61%) | |
| Not recorded | 3 | 1 | |
| BMI | <0.001 | ||
| Normal | 1,940 (29.22%) | 111 (20.56%) | |
| Underweight | 64 (0.96%) | 13 (2.41%) | |
| Overweight | 2,318 (34.91%) | 136 (25.19%) | |
| Obesity | 2,318 (34.91%) | 280 (51.85%) | |
| Smoking | 0.013 | ||
| Non-smoker | 3,899 (58.76%) | 290 (53.70%) | |
| Former smoker | 1,408 (21.22%) | 114 (21.11%) | |
| Current smoker | 1,329 (20.03%) | 136 (25.19%) | |
| Not recorded | 4 | 0 | |
| Drinking | <0.001 | ||
| Non-drinker | 683 (12.12%) | 89 (20.18%) | |
| 1–5 drinks/month | 3,342 (59.29%) | 260 (58.96%) | |
| 5–10 drinks/month | 616 (10.93%) | 37 (8.39%) | |
| 10+ drinks/month | 996 (17.67%) | 55 (12.47%) | |
| Not recorded | 1,003 | 99 |
Participants’ characteristics at baseline.
Data are presented as the median [25th–75th percentile] or n (%).
The p-value was calculated using the Wilcoxon rank-sum test or Pearson’s chi-squared test.
PRISm, preserved ratio impaired spirometry; OBS, oxidation balance score; BMI, body mass index.
3.2 Association between OBS and PRISm
In model 1, OBS was inversely associated with PRISm (OR = 0.95, 95% CI = 0.93–0.96, p < 0.001). This inverse association remained significant after adjusting for age, gender, and ethnicity in model 2 (OR = 0.96, 95% CI = 0.95–0.98, p < 0.001) and remained stable in the fully adjusted model 3 (OR = 0.97, 95% CI = 0.95–0.98, p < 0.001). Compared to the lowest quartile (Q1), the highest quartile (Q4) of OBS was associated with a significantly lower incidence of PRISm across all models (all p- and p-trend values < 0.001). In the fully adjusted model 3, the OR was 0.53 (95% CI = 0.37–0.75; Table 2).
Table 2
| Exposure | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| OR (95% CI) p-value | OR (95% CI) p-value | OR (95% CI) p-value | |
| OBS | 0.95 (0.93–0.96) | 0.96 (0.95–0.98) | 0.97 (0.95–0.98) |
| < 0.001 | < 0.001 | < 0.001 | |
| OBS (Quartile) | |||
| Q1 (4–15) | Reference | Reference | Reference |
| Q2 (16–21) | 0.73 (0.58–0.90) | 0.81 (0.64–1.02) | 0.82 (0.62–1.07) |
| 0.004 | 0.075 | 0.15 | |
| Q3 (22–26) | 0.57 (0.44–0.72) | 0.68 (0.52–0.88) | 0.76 (0.56–1.02) |
| < 0.001 | 0.003 | 0.073 | |
| Q4 (27–36) | 0.37 (0.28–0.48) | 0.51 (0.38–0.67) | 0.53 (0.37–0.75) |
| < 0.001 | < 0.001 | < 0.001 | |
| P for Trend | < 0.001 | < 0.001 | < 0.001 |
Binary logistic regression analysis of the relationship between OBS and PRISm.
Model 1, unadjusted; Model 2, adjusted for age, gender, and race; Model 3, adjusted for age, gender, ethnicity, economic conditions, education, marital status, BMI, smoking, and drinking.
3.3 Relationship between smoking status with OBS and PRISm
The smoking−/OBS+ group served as the reference and was compared to the other three mutually exclusive groups: the smoking−/OBS− group, the smoking+/OBS+ group, and the smoking+/OBS− group. Logistic regression analyses demonstrated that the smoking−/OBS− group was a significant risk factor for PRISm in all models (all p-values < 0.009); in model 3, the OR was 1.43 (95% CI = 1.10–1.88). Similarly, the smoking+/OBS− group exhibited a positive association with PRISm across the three models (all p-values ≤ 0.001); in model 3, the OR was 1.83 (95% CI = 1.27–2.64). Interestingly, smoking+/OBS+ was positively correlated with PRISm in models 1 and 2 (both p-values < 0.05), but this statistical significance was not observed in the fully adjusted model 3. Most notably, a significant dose–response relationship was observed across the groups, with OR values progressively increasing from the smoking−/OBS+ group (reference group, OR = 1.00), followed by the smoking−/OBS− and smoking+/OBS+ groups, and finally reaching the highest in the smoking+/OBS− group (in all three models, for the dose–response trend, all p-values ≤ 0.003; Table 3).
Table 3
| OR | 95% CI | p-value | p for trend | |
|---|---|---|---|---|
| Model 1 | < 0.001 | |||
| Smoking− OBS+ | Reference | |||
| Smoking− OBS− | 1.94 | 1.57–2.40 | < 0.001 | |
| Smoking+ OBS+ | 1.57 | 1.09–2.22 | 0.013 | |
| Smoking+ OBS− | 2.26 | 1.71–2.97 | < 0.001 | |
| Model 2 | < 0.001 | |||
| Smoking− OBS+ | Reference | |||
| Smoking− OBS− | 1.57 | 1.26–1.97 | < 0.001 | |
| Smoking+ OBS+ | 1.51 | 1.02–2.19 | 0.034 | |
| Smoking+ OBS− | 1.96 | 1.46–2.64 | < 0.001 | |
| Model 3 | 0.003 | |||
| Smoking− OBS+ | Reference | |||
| Smoking− OBS− | 1.43 | 1.10–1.88 | 0.009 | |
| Smoking+ OBS+ | 1.50 | 0.96–2.31 | 0.070 | |
| Smoking+ OBS− | 1.83 | 1.27–2.64 | 0.001 |
PRISm odds ratio for the four mutually exclusive groups based on OBS and smoking status.
Model 1, unadjusted; Model 2, adjusted for age, gender, and ethnicity; Model 3, adjusted for age, gender, ethnicity, economic conditions, education, marital status, BMI, smoking, and drinking.
3.4 Sensitivity analysis
The sensitivity analysis aligned with the primary analysis, suggesting that the results are robust to the instability of the PRISm definition when using different equations for predicted FEV1 (Supplementary Tables 2, 3).
4 Discussion
We used OBS as an index of overall OS status induced by exogenous factors and investigated its relationship with PRISm, along with the consideration of smoking status. Our findings indicate that co-exposure to OS and smoking is associated with a higher risk of PRISm. If these relationships were causal, a healthy diet and lifestyle could potentially aid in the recovery of smokers with PRISm, serving as a form of antioxidant therapy.
4.1 Therapy for PRISm
Although PRISm, with its high prevalence, is associated with clinically significant respiratory symptoms and poor prognosis (1–5), therapeutic options for PRISm are limited. Consequently, respiratory medications are often used improperly (14, 15). In the SPIROMICS study, 42% of PRISm patients used bronchodilators, and 23% used inhaled glucocorticoids (15). In contrast, in the COPDGene study, 20% used respiratory medication, and this high rate was related to worse symptoms (14). However, the RETHINC study, a randomized controlled trial (RCT), demonstrated that inhaled dual bronchodilator therapy is ineffective in relieving symptoms (16). Since smoking is the primary cause of PRISm, smoking cessation remains a primary goal; nevertheless, interventions to alleviate symptoms and reverse PRISm to normal lung function remain necessary. The lack of effective treatments for PRISm is partly due to its unclear pathogenesis.
4.2 OS in PRISm
OS is implicated in a myriad of diseases, including atherosclerosis (17), Alzheimer’s disease (18), cancer (19), and COPD (20). It can be categorized into two types based on its etiological role: primary versus secondary, or direct versus indirect (8). Smoking-induced OS in COPD is a classic example of a secondary cause contributing to disease progression. Specifically, cigarette smoking, a rich source of oxidants, induces OS, which, in turn, inhibits α1-antitrypsin and neutrophil elastase in the lungs—the pathological hallmark of COPD (21). Furthermore, OS triggers inflammation and cell death-and fibrosis-related pathological cascades, thereby accelerating the pathological progression of COPD (22). Moreover, OS-related gene polymorphisms serve as “susceptibility factors” in the progression to COPD (9). Given its role in COPD, OS is likely also involved in PRISm. Our study found an association between OBS and PRISm, thereby supporting this assumption and illuminating potential mechanisms. Further experimental studies on the pathogenesis are necessary to obtain conclusive evidence.
4.3 Antioxidant strategies against OS in PRISm
With COPD, potential antioxidant strategies to reduce OS include thiol-based antioxidants, antioxidant mimetics, peroxidase inhibitors, mitochondria-targeted antioxidants, and dietary antioxidants. However, the majority of therapeutic targets remain either unsatisfactory or have not been sufficiently studied, with the exception of nutritional antioxidants (8, 22). Diets rich in prooxidants are linked to poor lung function and contribute to the development of COPD (23), whereas a Mediterranean diet rich in antioxidants is associated with a lower risk of COPD (24). L-ascorbic acid (vitamin C) and α-tocopherol (vitamin E) are well-known dietary antioxidants, and their role in OS has been extensively studied (25–27). Given that a single nutrient cannot represent the combined effect of all OS-related nutrients, the OBS provides a comprehensive assessment of OS-related nutrients and lifestyle, reflecting the levels of OS induced by exogenous factors (10). In other words, the OBS can estimate how well a certain diet and lifestyle can withstand OS. We found that, compared with smoking−/OBS+, smoking+/OBS+ had a higher PRISm risk, and the OR increased further in smoking+/OBS−. This trend suggests that a healthy diet and lifestyle may offer antioxidant therapy for PRISm. However, more RCTs focusing on the impact of a healthy diet and lifestyle on PRISm are essential.
4.4 Strengths
This study is the first to demonstrate an association between PRISm and OS, offering novel insights into the pathogenesis of PRISm and guiding future research. Moreover, the variation in PRISm incidence across the four mutually exclusive groups combining smoking status and OBS suggests potential intervention strategies, underscoring the role of a healthy diet and lifestyle in the management of PRISm. NHANES data, with their large sample size and rigorous survey design, ensured the reliability of our findings. Sensitivity analysis using different predicted FEV1 equations further corroborated our findings.
4.5 Limitations
This study has several limitations. First, the incidence of PRISm may have been overestimated due to the use of prebronchodilator spirometry data, although the impact of bronchodilator administration on PRISm incidence has not been fully established. Second, as an index, the OBS can only indirectly reflect exogenous OS status, while the endogenous part was not evaluated in our study. This may limit our interpretation of OS in PRISm. Third, as this is a cross-sectional study, logistic regression cannot establish causality. Fourth, although multiple models were used to adjust for several common confounding factors, some unmeasurable confounders remain inevitable and may bias our conclusions.
5 Conclusion
A higher OBS was associated with a lower incidence of PRISm, suggesting that OS may play a key role in this relationship. A healthy diet and lifestyle may function as an antioxidant therapy for PRISm, particularly among smokers.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Review Board of the National Center for Health Statistics (NCHS). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SG: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft. XD: Conceptualization, Data curation, Formal analysis, Methodology, Resources, Software, Writing – review & editing. XG: Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1551237/full#supplementary-material
Abbreviations
BMI, Body mass index; COPD, Chronic obstructive pulmonary disease; FEV1, Forced expiratory volume in the first second; FVC, Forced vital capacity; GLI, Global Lung Function Initiative; NHANES, National Health and Nutrition Examination Survey; NCHS, National Center for Health Statistics; OBS, Oxidation balance score; OS, Oxidative stress; PRISm, Preserved ratio impaired spirometry.
Footnotes
References
1.
WanESCastaldiPJChoMHHokansonJEReganEAMakeBJet al. Epidemiology, genetics, and subtyping of preserved ratio impaired spirometry (PRISm) in COPDGene. Respir Res. (2014) 15:89. doi: 10.1186/s12931-014-0089-y
2.
GuerraSCarsinAEKeidelDSunyerJLeynaertBJansonCet al. Health-related quality of life and risk factors associated with spirometric restriction. Eur Respir J. (2017) 49:1602096. doi: 10.1183/13993003.02096-2016
3.
WanESBaltePSchwartzJEBhattSPCassanoPACouperDet al. Association between preserved ratio impaired spirometry and clinical outcomes in US adults. JAMA. (2021) 326:2287–98. doi: 10.1001/jama.2021.20939
4.
WanESFortisSReganEAHokansonJHanMKCasaburiRet al. Longitudinal phenotypes and mortality in preserved ratio impaired spirometry in the COPDGene study. Am J Respir Crit Care Med. (2018) 198:1397–405. doi: 10.1164/rccm.201804-0663OC
5.
ZhengJZhouRZhangYSuKChenHLiFet al. Preserved ratio impaired spirometry in relationship to cardiovascular outcomes: a large prospective cohort study. Chest. (2023) 163:610–23. doi: 10.1016/j.chest.2022.11.003
6.
WijnantSRADe RoosEKavousiMStrickerBHTerzikhanNLahousseLet al. Trajectory and mortality of preserved ratio impaired spirometry: the Rotterdam study. Eur Respir J. (2020) 55:1901217. doi: 10.1183/13993003.01217-2019
7.
KurthLHnizdoE. Change in prevalence of restrictive lung impairment in the U.S. population and associated risk factors: the National Health and nutrition examination survey (NHANES) 1988-1994 and 2007-2010. Multidiscip Respir Med. (2015) 10:7. doi: 10.1186/s40248-015-0003-6
8.
FormanHJZhangH. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat Rev Drug Discov. (2021) 20:689–709. doi: 10.1038/s41573-021-00233-1
9.
HeJQRuanJConnettJEAnthonisenNRParéPDSandfordAJ. Antioxidant gene polymorphisms and susceptibility to a rapid decline in lung function in smokers. Am J Respir Crit Care Med. (2002) 166:323–8. doi: 10.1164/rccm.2111059
10.
ZhangWPengSFChenLChenHMChengXETangYH. Association between the oxidative balance score and telomere length from the National Health and nutrition examination survey 1999-2002. Oxidative Med Cell Longev. (2022) 2022:1345071. doi: 10.1155/2022/1345071
11.
LiuZZengHZhangH. Association of the oxidation balance score with the prevalence of chronic obstructive pulmonary disease from the NHANES 2007-2012: a large-scale cross-sectional study. Heart Lung. (2024) 65:84–92. doi: 10.1016/j.hrtlng.2024.02.005
12.
BowermanCBhaktaNRBrazzaleDCooperBRCooperJGochicoa-RangelLet al. A race-neutral approach to the interpretation of lung function measurements. Am J Respir Crit Care Med. (2023) 207:768–74. doi: 10.1164/rccm.202205-0963OC
13.
HankinsonJLOdencrantzJRFedanKB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. (1999) 159:179–87.
14.
ReganEALynchDACurran-EverettDCurtisJLAustinJHGrenierPAet al. Clinical and radiologic disease in smokers with Normal spirometry. JAMA Intern Med. (2015) 175:1539–49. doi: 10.1001/jamainternmed.2015.2735
15.
WoodruffPGBarrRGBleeckerEChristensonSACouperDCurtisJLet al. Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med. (2016) 374:1811–21. doi: 10.1056/NEJMoa1505971
16.
HanMKYeWWangDWhiteEArjomandiMBarjaktarevicIZet al. Bronchodilators in tobacco-exposed persons with symptoms and preserved lung function. N Engl J Med. (2022) 387:1173–84. doi: 10.1056/NEJMoa2204752
17.
SabaLCauRVergalloRKooiMEStaubDFaaGet al. Carotid artery atherosclerosis: mechanisms of instability and clinical implications. Eur Heart J. (2025) 46:904–21. doi: 10.1093/eurheartj/ehae933
18.
ButterfieldDAHalliwellB. Oxidative stress, dysfunctional glucose metabolism and Alzheimer's disease. Nat Rev Neurosci. (2019) 20:148–60. doi: 10.1038/s41583-019-0132-6
19.
SiesHBerndtCJonesDP. Oxidative stress. Annu Rev Biochem. (2017) 86:715–48. doi: 10.1146/annurev-biochem-061516-045037
20.
ZhuYDuttaSHanYChoiDPolverinoFOwenCAet al. Oxidative stress promotes lipid-laden macrophage formation via CYP1B1. Redox Biol. (2025) 79:103481. doi: 10.1016/j.redox.2024.103481
21.
SunSWangCHuJZhaoPWangXBalchWE. Spatial covariance reveals isothiocyanate natural products adjust redox stress to restore function in alpha-1-antitrypsin deficiency. Cell Rep Med. (2025) 6:101917. doi: 10.1016/j.xcrm.2024.101917
22.
BarnesPJ. Oxidative stress-based therapeutics in COPD. Redox Biol. (2020) 33:101544. doi: 10.1016/j.redox.2020.101544
23.
ScodittiEMassaroMGarbarinoSToraldoDM. Role of diet in chronic obstructive pulmonary disease prevention and treatment. Nutrients. (2019) 11:1357. doi: 10.3390/nu11061357
24.
FischerAJohanssonIBlombergASundströmB. Adherence to a Mediterranean-like diet as a protective factor against COPD: a nested case-control study. COPD. (2019) 16:272–7. doi: 10.1080/15412555.2019.1634039
25.
MłynarskaEBiskupLMożdżanMGrygorcewiczOMożdżanZSemeradtJet al. The role of oxidative stress in hypertension: the insight into antihypertensive properties of vitamins a, C and E. Antioxidants (Basel). (2024) 13:848. doi: 10.3390/antiox13070848
26.
MoniruzzamanMLeeSParkYMinTBaiSC. Evaluation of dietary selenium, vitamin C and E as the multi-antioxidants on the methylmercury intoxicated mice based on mercury bioaccumulation, antioxidant enzyme activity, lipid peroxidation and mitochondrial oxidative stress. Chemosphere. (2021) 273:129673. doi: 10.1016/j.chemosphere.2021.129673
27.
NohWBaikI. Associations of serum vitamin a and E concentrations with pulmonary function parameters and chronic obstructive pulmonary disease. Nutrients. (2024) 16:3197. doi: 10.3390/nu16183197
Summary
Keywords
preserved ratio impaired spirometry, oxidative balance score, oxidative stress, dietary quality and lifestyle, antioxidant strategy
Citation
Gu S, Du X and Gu X (2025) Association between the oxidative balance score and preserved ratio impaired spirometry in US adults: NHANES 2007–2012. Front. Nutr. 12:1551237. doi: 10.3389/fnut.2025.1551237
Received
25 December 2024
Accepted
15 July 2025
Published
06 August 2025
Volume
12 - 2025
Edited by
Paula Ravasco, Catholic University of Portugal, Portugal
Reviewed by
Margaret Sarolta McElrea, Queensland Children’s Hospital, Children's Health Queensland, Australia
Siew Tin Tan, International Medical University, Malaysia
Updates
Copyright
© 2025 Gu, Du and Gu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shengyuan Gu, gootionyuan@126.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.