Abstract
Objectives:
The purpose was to assess the levels of iron (Fe), magnesium (Mg), copper (Cu), calcium (Ca), zinc (Zn) in the blood of pregnant women during early pregnancy, and to evaluate their potential association with gestational diabetes mellitus (GDM).
Methods:
We enrolled 9,112 pregnant women who underwent testing for essential metal elements at Guangdong Women and Children Hospital during the first trimester in 2015–2022. The basic information of pregnant women and peripheral blood samples were collected, and five essential metal elements in whole blood were detected by atomic absorption spectrometry. The relationship between these essential metal elements and GDM was analyzed using the generalized linear regression model (GLM), weighted quantile sum regression (WQS), quantile g-computation regression (QGC), and Bayesian kernel machine regression (BKMR).
Results:
Analysis of the correlation between essential metal elements and GDM revealed significant associations. Compared with the first quantile concentration level, the fourth quantile level of Fe (OR = 1.347, 95%CI: 1.158 ~ 1.568), Zn (OR = 1.379, 95%CI: 1.185 ~ 1.606) and Mg (OR = 1.192, 95%CI: 1.022 ~ 1.392) exhibited significant associations with GDM. Restricted cubic spline (RCS) analysis showed a positive linear relationship between Fe, Zn, and Mg and GDM risk (Poverall < 0.05 and Pnon-linear > 0.05). WQS analysis showed that the WQS index had a significant positive correlation with GDM (OR = 1.129, 95%CI: 1.023 ~ 1.247), with Fe (0.446) having greater weight. QGC analysis revealed a positive correlation between the combined action of five essential metal elements and GDM risk (OR = 1.161, 95%CI: 1.075 ~ 1.248), with Zn (0.454) and Fe (0.417) showing greater influence. In BKMR analysis, the combined effect of all essential metal elements on GDM showed an overall upward trend, with Fe (PIP = 0.772) having the most significant influence. No interaction between essential metal elements and GDM was found in this study.
Conclusion:
Higher levels of Fe, Zn and Mg were positively correlated with GDM risk. The combined action of five essential metal elements was positively correlated with GDM, with Fe identified as playing the most significant role.
Highlights
The aim of this study was to evaluate the levels of iron (Fe), magnesium (Mg), copper (Cu), calcium (Ca), and zinc (Zn) in the blood of pregnant women during early pregnancy and to explore their individual, combined, and interactive effects with gestational diabetes mellitus (GDM).
This prospective cohort study enrolled 9,112 pregnant women who underwent testing for essential trace elements at Guangdong Women and Children Hospital during the first trimester between 2015 and 2022.
The study found that higher levels of Fe, Zn, and Mg were positively correlated with the risk of GDM, highlighting the importance of maintaining appropriate levels of essential trace elements in pregnant women during early pregnancy. Furthermore, the research revealed a positive correlation between the combined exposure to essential trace elements and GDM risk, with Fe playing a predominant role.
1 Introduction
Gestational diabetes mellitus (GDM) was defined as diabetes mellitus that develops during pregnancy in women who previously had normal glucose metabolism, representing one of the most prevalent complications during gestation (1). In recent years, the global prevalence of GDM has been rising due to increasing rates of overweight and obesity among women of childbearing age, as well as an aging population (2, 3). Numerous studies have highlighted the short-term and long-term adverse health effects of GDM on both pregnant women and their offspring (4). GDM not only contributes to adverse pregnancy outcomes such as premature delivery, dystocia, and macrosomia, but also elevates the risk of future cardiovascular and metabolic diseases (5–7). Moreover, maternal hyperglycemia during early pregnancy epigenetically programmed offspring’s metabolic health, as highlighted in the DOHaD paradigm (8, 9). At present, the etiology of GDM was not clear, which brought difficulties to clinical diagnosis and treatment.
The common risk factors for GDM included genetic predisposition, unhealthy lifestyle, and social factors (10–12). Some researchers also found that intestinal flora imbalance caused diabetes through inflammation, insulin resistance, and nutritional signals (13, 14). In addition, increasing animal and metabolic studies demonstrated that the steady-state imbalance of essential metal elements including iron (Fe), calcium (Ca), zinc (Zn), copper (Cu) and magnesium (Mg) was also an important risk factor leading to GDM (15, 16). Among these biological samples, blood levels of essential metal elements were relatively stable (17, 18). Therefore, whole blood samples were often used to monitor the levels of these five essential metals to assess the health status of pregnant women (19). They were also used to intervene in the dietary supplementation, so as to maintain the normal physiological and metabolic functions of pregnant women (20).
The deficiency or excess of essential metal elements in pregnant women may be associated with inflammation and oxidative stress, influencing glucose metabolism and insulin sensitivity and thereby increasing the risk of GDM (19). Studies have found that oxidative stress mediated by Fe overload may be related to the occurrence of gestational diabetes (21, 22). In a prospective study of pregnant women, the high level of plasma Cu in early pregnancy was positively correlated with the glucose level in the middle and late pregnancy (23). Zhao et al. (24) also found that Impairment of β-cells by Cu homeostasis is related to the occurrence of GDM. In addition, more and more studies showed that other essential metal elements such as Zn and Mg were also related to GDM (25). A retrospective cohort study discovered that lower Ca and Mg concentrations within a certain range before 24 weeks’ gestation might prospectively impair fasting plasma glucose levels during pregnancy (26). However, the research on the relationship between essential metal elements and GDM was limited, and the research results were still inconsistent (27, 28). Variations in sample sizes and research methodologies may compromise the consistency of conclusions drawn from these studies (27–29).
Furthermore, most current research focused on the impact of individual essential metal elements on GDM (30–33), overlooking the simultaneous consumption and potential interactions among multiple essential metals that pregnant women typically experience (34). Few studies explored the combined effects of various essential metal elements on GDM (35). Previous studies suggested that it was necessary to carry out related research in pregnant women to verify the association between essential metal elements and GDM, so as to identify and intervene GDM-related risk factors early.
Therefore, we hypothesize that there was a correlation between the levels of essential metal elements during early pregnancy and GDM. This study aimed to assess the levels of five essential metal elements in the blood of pregnant women in the first trimester and explore their individual associations with GDM. Given that pregnant women are exposed to multiple essential metal elements simultaneously in the first trimester, using a combined exposure model, the relationship between the joint action and interaction of essential metal elements and GDM was analyzed.
2 Materials and methods
2.1 Study design and participants
This prospective cohort study enrolled 9,801 pregnant women who met inclusion criteria between January 2015 and December 2022. Participants were included after completing baseline data collection during their initial pregnancy examination, with follow-up conducted through the second and third trimesters for oral glucose tolerance tests (OGTT) in outpatient settings. Following application of exclusion criteria, 9,112 participants remained for analysis, based on their baseline and follow-up survey data. The study received approval from the Ethics Committee of Guangdong Women and Children Hospital (ID: 202401082).
Inclusion criteria: (1) Age 18 ~ 45 years; (2) Singleton pregnancy; (3) Being a Guangdong resident planning to deliver at the study hospital; (4) Detection of blood essential metal elements in early pregnancy (<14 weeks). Exclusion criteria: (1) Pre-pregnancy patients complicated with metabolic diseases such as diabetes and severe organ diseases; (2) Abortion and induced labor during pregnancy; (3) Suffering from other serious medical diseases during pregnancy; (4) Incomplete clinical information.
This study performed a sample size estimation based on a cohort study design. With α = 0.05 and β = 0.10, the calculated required sample size was 3,870. Considering potential issues such as loss to follow-up, the sample size was increased by 20%. The final sample size included in this study meets the required criteria (Figure 1).
Figure 1
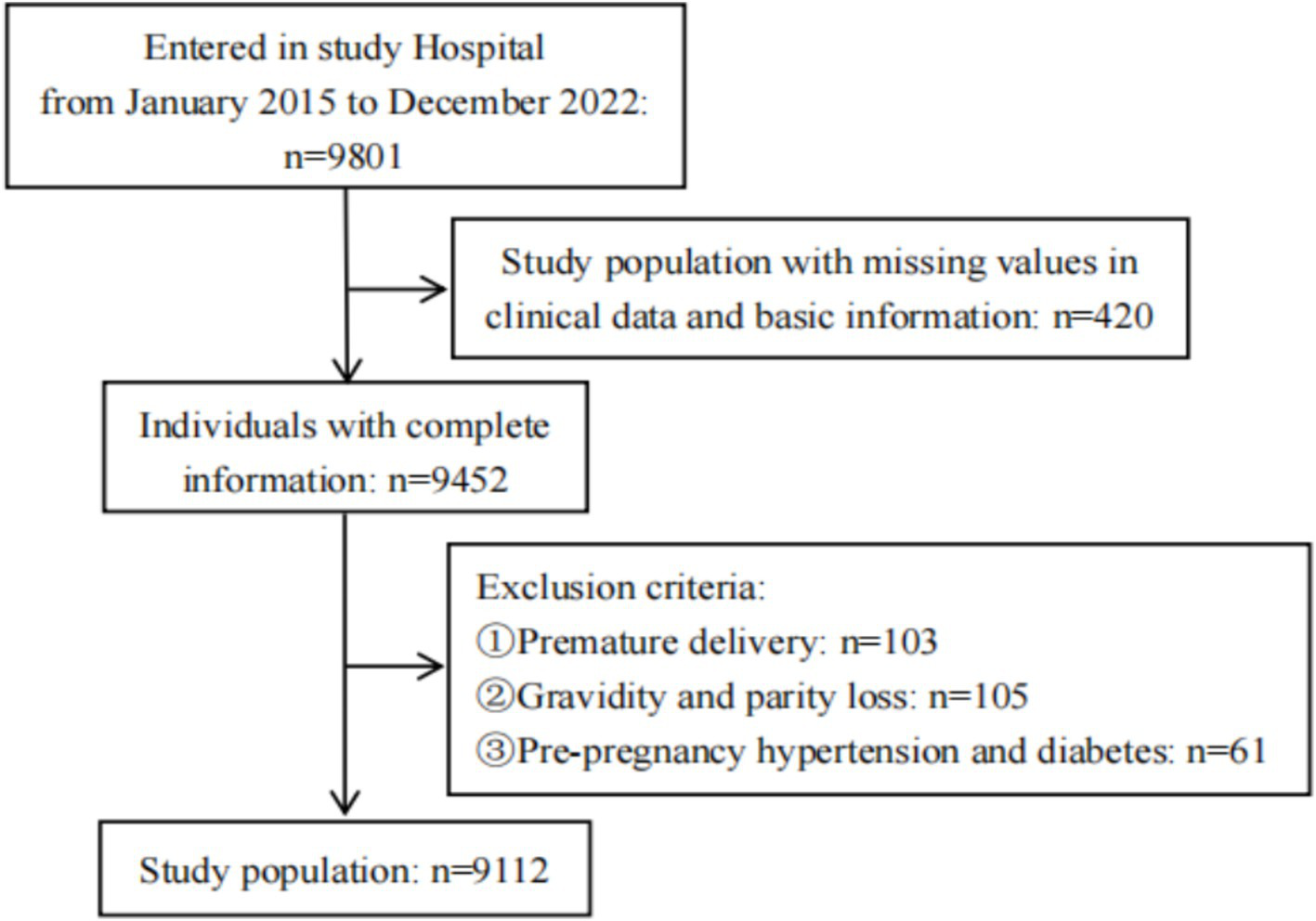
Flowchart of study population.
2.2 Definitions
In this study, GDM was taken as the outcome index, and the diagnosis information was obtained from the electronic medical record system. Diagnostic criteria of GDM: All pregnant women who have not been diagnosed with pre-pregnancy diabetes or gestational diabetes were given 75 g OGTT at the first visit after 24–28 weeks of pregnancy. The diagnostic thresholds for the 75 g OGTT were as follows: Fasting plasma glucose (FPG) < 5.1 mmol/L, or 1-h plasma glucose (1hPG) < 10.0 mmol/L, or 2-h plasma glucose (2hPG) < 8.5 mmol/L (36).
2.3 Basic data collection
During the initial pregnancy examination, basic information including the age, pre-pregnancy height and weight, last menstrual period, maternity history, and medical history of the pregnant women was collected. This information was self-reported and recorded in the electronic medical record system, which was queried for data retrieval. Pre-pregnancy BMI was calculated by dividing self-reported pre-pregnancy weight by the square of measured height (37).
2.4 Measurement of trace elements
Whole blood samples were collected from pregnant women in the first trimester (12.27 ± 0.9 weeks) by trained nurses. Two mL venous blood was collected with EDTA-K2 vacuum anticoagulant tubes from the antecubital region. Samples were transported at room temperature and refrigerated at 4°C, and analyzed within 3 days of collection.
The flame atomic absorption spectrometer (BH7100S), calibration solution, and quality control materials were sourced from Beijing Bohui Innovation Biotechnology Co., Ltd. The instrument utilized gas flow control technology in the tungsten boat metal furnace. Prior to testing, whole blood samples were thoroughly mixed using a vortex for at least 1 min. Subsequently, 40 μL of the whole blood sample was diluted with 1.96 mL of diluent. After mixing, the elemental concentrations in all blood samples were measured via flame atomic absorption spectrometry within 2 h. The instrument and element lamp were preheated for 30 min to ensure baseline stability without ignition during this period.
2.5 Quality control
Participants were selected in strict accordance with the inclusion and exclusion criteria. Data integrity was maintained by carefully cleaning and verifying participant information and laboratory results. Covariates were included in all statistical analysis models, and sensitivity analysis was conducted to evaluate the reliability of the results.
Biological samples were collected following a standardized protocol, and essential metal elements were measured using a unified method. To ensure accuracy and precision, regular calibration and quality control were performed. Before testing each batch of samples, a standard curve along with low and high-level quality control samples were conducted. The quality control results for each batch met the requirement of a standard deviation within twice the target value. For the five essential metal elements, the baseline stability was maintained within 0.005 Abs. Instrument sensitivity was confirmed by spraying the sensitivity standard detection solution, with the characteristic concentrations for each metal element as follows: Cu 0.035 mg/L/1%, Zn 0.015 mg/L/1%, Ca 0.080 mg/L/1%, Mg 0.040 mg/L/1%, and Fe 1.05 mg/L/1%.
To address potential batch effects due to the extended collection period, only data from pregnant women’s first visits were used. Consistency in experimental equipment, measurement protocols, and procedures was maintained through regular calibration and maintenance to ensure instrument stability. The standard deviation for each batch was set to 2 times the target value, keeping batch-to-batch variability within an acceptable range. During the experimental design stage, potential sources of variation were strictly controlled, and standardized preprocessing steps were applied. Additionally, principal component analysis (PCA) was used to detect batch effects, and methods were applied, when necessary, to correct residual batch-related variability, ensuring result reliability.
2.6 Statistical analysis
In this study, all data analyses were performed using R version 4.3.1 software. Assessment of statistical significance was based on two-tailed p-values, with a p < 0.05 threshold considered to be statistically significant. No missing data were included in the analysis, as cases with missing values were excluded during participant selection. In descriptive analysis, classified variables were characterized by frequency and composition ratio, while continuous variables were presented as mean ± standard deviation (SD). Independent sample t-tests were used to compare two groups of continuous variables assumed to follow a normal distribution, and χ2 tests were utilized for comparing constituent ratios. Normality tests confirmed that concentrations of five essential metal elements in pregnant women’s blood approximated a normal distribution. Pearson correlation analysis was conducted to examine associations between these essential metal elements.
The study utilized generalized linear regression (GLM) evaluate the relationship between a single essential metal element and GDM. According to the quartile of five essential metal elements, the subjects were divided into four groups, and the risk of GDM in other quantile levels before and after adjusting covariate was estimated with the first quantile level as reference. The median level of the quartile array of each essential metal element was included in the model for trend test (P for trend), and then the trend test is corrected by using the false discovery rate (FDR) to control the false positive rate (38). To explore the dose–response relationship, restricted cubic splines (RCS) were employed for visual analysis, setting nodes at the 10th, 50th, and 90th percentiles with the 50th percentile serving as the reference value. Both overall correlation (Poverall) and non-linear correlation (Pnon-linear) were found to be less than 0.05, indicating a non-linear dose–response relationship. A Poverall < 0.05 with Pnon-linear > 0.05 suggested a linear dose–response relationship (39).
Bayesian kernel machine regression can analyze the complexity of the combined action of chemicals. This study primarily aimed to evaluate the combined effect of five essential metal elements in blood on GDM, identify the essential metal elements that significantly impact the outcome, fit the dose–response relationship of this combined effect, and explore potential interactions between these essential metal elements (40). The relative importance of each essential metal element was determined by calculating the posterior inclusion probability (PIP). The closer the PIP value is to 1, the greater the role of the essential metal element in the joint effect. In this study, BKMR model was run for 25,000 iterations. The r package used was “bkmr.”
In order to verify the robustness of our main results, two sensitivity analyses were performed. Firstly, weighted quantile sum regression (WQS) and quantile g-computation regression (QGC) were used to verify the results of mixed exposure analysis. Both models are classical mixed exposure models (41). The overall effect of the combined action of essential metal elements on GDM was estimated. Additionally, the contribution of each individual essential metal element was analyzed and displayed. The R-package used were “gWQS” and “qgcomp.” Secondly, in order to verify the interaction between essential metal elements in the results of BKMR model, the essential metal elements are divided into two levels according to the median. Evaluation of multiplication terms in Logistic regression analysis of multiplication interaction. The additive interaction was evaluated by using R-package “epiR” and three indicators: relative excess risk due to interaction (RERI), attributable promotion due to interaction (AP) and synergy index (S). If 95%CI of RERI and AP index does not contain 0 and 95%CI of S index does not contain 1, it is considered that there is additive interaction between essential metal elements, in which RERI value and AP value > 0 are synergistic, RERI < 0 and AP < 0 are antagonistic, S value > 1 is synergistic and S < 1 is antagonistic (42).
To improve clarity, we have specified the covariates included in each model (GLM, BKMR, WQS, QGC). All models included the following covariates: maternal age in years at enrollment (continuous) (43), pre-pregnancy BMI (kg/m2, continuous) (44), parity (45), season (categorized as spring, summer, autumn, winter) (46–48) and gestational week (weeks, continuous) (49) at blood specimen collection. Because factors such as gestational week and season may affect the content of essential metal elements, attention should be paid to these variables (50).
3 Results
3.1 Characteristics of the study population
The population characteristics of the pregnant women recruited in our study were summarized in Table 1. The average age of 9,112 pregnant women was 30.47 ± 4.37 years old, and the average BMI before pregnancy was 20.79 ± 2.68 kg/m2. Of these women, 18.62% (1,697/9112) pregnant women were subsequently diagnosed with GDM. The age, parity, gravidity and pre-pregnancy BMI of pregnant women with GDM were higher than those of pregnant women without GDM (p < 0.001).
Table 1
| Variables | GDM (n = 1,697) | Non-GDM (n = 7,415) | t/χ2 | P |
|---|---|---|---|---|
| Maternal age (years) | 32.04 ± 4.43 | 30.14 ± 4.29 | 16.041 | <0.001 |
| 18 ~ 25 | 43 (2.53) | 575 (7.75) | 215.610 | <0.001 |
| 26 ~ 30 | 606 (35.71) | 3,657 (49.32) | ||
| 31 ~ 45 | 1,048 (61.76) | 3,183 (42.93) | ||
| Pre-pregnancy BMI (kg/m2) | 21.56 ± 2.92 | 20.63 ± 2.60 | 12.046 | <0.001 |
| <18.5 | 233 (13.73) | 1,515 (20.43) | 117.100 | <0.001 |
| 18.5 ~ 23.9 | 1,127 (66.41) | 5,079 (68.50) | ||
| ≥24.0 | 337 (19.86) | 821 (11.07) | ||
| Parity | ||||
| Primiparity | 821 (48.38) | 4,013 (54.12) | 18.041 | <0.001 |
| Multiparity | 876 (51.62) | 3,402 (45.88) | ||
| Gravidity (times) | ||||
| 1 | 526 (31.00) | 2,844 (38.35) | 31.772 | <0.001 |
| ≥2 | 1,171 (69.00) | 4,571 (61.65) | ||
Demographic characteristics of research objects (N = 9,112).
Continuous variables in the table are represented by −x ± s, and classified variables are represented by n (%). Bold black font indicates statistically significant differences between groups (P < 0.05).
3.2 Element concentrations in whole blood
The characteristics of essential metal elements in blood of pregnant women in the first trimester were shown in Table 2. Compared with the non-GDM group, the concentration levels of essential metal elements such as Fe (p < 0.001), Zn (p < 0.001), Cu (p = 0.008) and Mg (p < 0.001) in GDM group were higher.
Table 2
| Variables | GDM (n = 1,697) | Non-GDM (n = 7,415) | t | P |
|---|---|---|---|---|
| Fe (mmol/L) | 7.83 ± 0.68 | 7.74 ± 0.67 | 4.639 | < 0.001 |
| Ca (mmol/L) | 1.55 ± 0.10 | 1.55 ± 0.10 | 1.318 | 0.187 |
| Zn (μmol/L) | 85.82 ± 12.37 | 84.29 ± 12.02 | 4.633 | < 0.001 |
| Cu (μmol/L) | 23.06 ± 4.65 | 22.73 ± 4.5 | 2.651 | 0.008 |
| Mg (mmol/L) | 1.40 ± 0.13 | 1.38 ± 0.13 | 4.409 | < 0.001 |
Characteristics of essential metal elements in pregnant women’s blood (N = 9,112).
Continuous variables in the table are represented by −x ± s. Bold black font indicates statistically significant differences between groups (P < 0.05).
3.3 Correlation analysis between essential metal elements
The results of Pearson correlation analysis between five essential metal elements were shown in Figure 2, and there was a positive correlation between most essential metal elements. Among them, Fe and Mg had the strongest correlation (r = 0.605, p < 0.001), and there was a negative correlation between Fe and Ca (r = −0.208, p < 0.001).
Figure 2
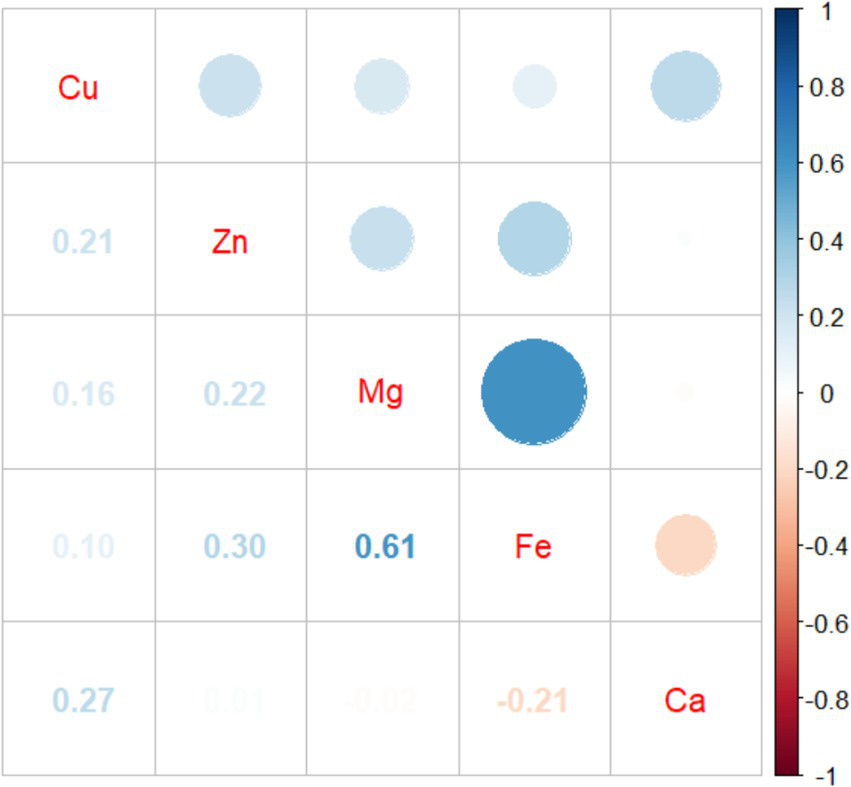
Pearson correlation analysis between five essential metal elements.
3.4 Regression models of GDM risk
3.4.1 Correlation between individual essential metal element and GDM
The results of logistics regression were shown in Table 3. After adjusting the age, pre-pregnancy BMI, parity, detection season and gestational week of essential metal elements, Fe (OR = 1.171, 95%CI: 1.080 ~ 1.272), Zn (OR = 1.008, 95%CI: 1.004 ~ 1.013) and Mg (OR = 1.906, 95%CI: 1.237 ~ 2.937) levels showed positive correlations with GDM risk. However, no significant correlations were found between Ca or Cu levels and GDM (p > 0.05). Analysis by quartiles of essential metal elements revealed that higher quartile levels of Fe, Zn, and Mg were associated with increased GDM risk (PFDR < 0.05). Compared with the first quantile level, the fourth quantile level of Fe (OR = 1.347, 95%CI: 1.158 ~ 1.568), Zn (OR = 1.379, 95%CI: 1.185 ~ 1.606) and Mg (OR = 1.192, 95%CI: 1.022 ~ 1.392) were positively correlated with GDM. Upon further adjustment for other essential metal elements, only Zn maintained a positive correlation with GDM risk (OR = 1.006, 95%CI: 1.001 ~ 1.011). Notably, fourth quartile levels of Fe (OR = 1.221, 95%CI: 1.009 ~ 1.478) and Zn (OR = 1.272, 95%CI: 1.081 ~ 1.496) remained positively associated with GDM risk.
Table 3
| Variables | Total(n) | GDM (n, %) | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |||
| Fe (mmol/L) | 9,112 | 1,697 (18.62) | 1.210 (1.118, 1.311) | <0.001 | 1.171 (1.080, 1.272) | <0.001 | 1.104 (0.992, 1.230) | 0.070 |
| Quartile 1 (5.42 ~ 7.32) | 2,270 | 380 (4.17) | Ref | Ref | Ref | |||
| Quartile 2 (7.33 ~ 7.78) | 2,276 | 401 (4.40) | 1.064 (0.912, 1.241) | 0.432 | 1.027 (0.877, 1.202) | 0.742 | 1.008 (0.856, 1.186) | 0.927 |
| Quartile 3 (7.79 ~ 8.21) | 2,280 | 405 (4.44) | 1.074 (0.921, 1.253) | 0.361 | 1.058 (0.904, 1.239) | 0.480 | 1.008 (0.848, 1.199) | 0.924 |
| Quartile 4 (8.22 ~ 9.65) | 2,286 | 511 (5.61) | 1.432 (1.236, 1.660) | <0.001 | 1.347 (1.158, 1.568) | <0.001 | 1.221 (1.009, 1.478) | 0.041 |
| P for trend | <0.001 | <0.001 | 0.022 | |||||
| Ca (mmol/L) | 9,112 | 1,697 (18.62) | 0.706 (0.420, 1.185) | 0.189 | 0.878 (0.515, 1.496) | 0.634 | 0.891 (0.502, 1.579) | 0.693 |
| Quartile 1 (1.25 ~ 1.48) | 2066 | 398 (4.37) | Ref | Ref | Ref | |||
| Quartile 2 (1.49 ~ 1.55) | 2,402 | 444 (4.87) | 0.950 (0.818, 1.105) | 0.506 | 0.989 (0.848, 1.154) | 0.889 | 1.001 (0.856, 1.170) | 0.993 |
| Quartile 3 (1.56 ~ 1.62) | 2,308 | 425 (4.66) | 0.946 (0.813, 1.101) | 0.473 | 0.989 (0.847, 1.156) | 0.891 | 1.004 (0.855, 1.179) | 0.964 |
| Quartile 4 (1.63 ~ 1.88) | 2,336 | 430 (4.72) | 0.945 (0.813, 1.100) | 0.468 | 1.008 (0.864, 1.178) | 0.915 | 1.024 (0.867, 1.208) | 0.783 |
| P for trend | 0.496 | 0.904 | 0.759 | |||||
| Zn (μmol/L) | 9,112 | 1,697 (18.62) | 1.010 (1.006, 1.015) | <0.001 | 1.008 (1.004, 1.013) | <0.001 | 1.006 (1.001, 1.011) | 0.014 |
| Quartile 1 (46.30 ~ 76.60) | 2,249 | 380 (4.06) | Ref | Ref | Ref | |||
| Quartile 2 (76.61 ~ 84.10) | 2,293 | 401 (4.43) | 1.086 (0.930, 1.268) | 0.296 | 1.100 (0.939, 1.288) | 0.237 | 1.071 (0.913, 1.255) | 0.399 |
| Quartile 3 (84.11 ~ 92.10) | 2,268 | 405 (4.47) | 1.111 (0.952, 1.297) | 0.184 | 1.091 (0.932, 1.278) | 0.277 | 1.028 (0.874, 1.210) | 0.737 |
| Quartile 4 (92.11 ~ 121.90) | 2,302 | 511 (5.66) | 1.467 (1.265, 1.703) | <0.001 | 1.379 (1.185, 1.606) | <0.001 | 1.272 (1.081, 1.496) | 0.004 |
| P for trend | <0.001 | <0.001 | 0.005 | |||||
| Cu (μmol/L) | 9,112 | 1,697 (18.62) | 1.016 (1.004, 1.028) | 0.007 | 1.012 (0.999, 1.024) | 0.051 | 1.007 (0.994, 1.020) | 0.305 |
| Quartile 1 (10.10 ~ 19.70) | 2,266 | 406 (4.45) | Ref | Ref | Ref | |||
| Quartile 2 (19.71 ~ 22.70) | 2,175 | 370 (4.06) | 0.939 (0.804, 1.097) | 0.427 | 0.913 (0.779, 1.070) | 0.264 | 0.901 (0.768, 1.057) | 0.202 |
| Quartile 3 (22.71 ~ 26.00) | 2,362 | 454 (4.98) | 1.090 (0.940, 1.265) | 0.254 | 1.048 (0.900, 1.221) | 0.543 | 1.013 (0.867, 1.184) | 0.869 |
| Quartile 4 (26.01 ~ 38.40) | 2,309 | 467 (5.13) | 1.161 (1.002, 1.3477) | 0.047 | 1.107 (0.951, 1.290) | 0.190 | 1.044 (0.887, 1.229) | 0.603 |
| P for trend | 0.013 | 0.073 | 0.438 | |||||
| Mg (mmol/L) | 9,112 | 1,697 (18.62) | 2.575 (1.693, 3.916) | <0.001 | 1.906 (1.237, 2.937) | 0.003 | 1.169 (0.676, 2.020) | 0.577 |
| Quartile 1 (0.98 ~ 1.30) | 2,167 | 360 (3.95) | Ref | Ref | Ref | |||
| Quartile 2 (1.31 ~ 1.38) | 2,243 | 403 (4.42) | 1.099 (0.940, 1.286) | 0.235 | 1.035 (0.883, 1.215) | 0.670 | 0.972 (0.823, 1.147) | 0.735 |
| Quartile 3 (1.39 ~ 1.47) | 2,329 | 439 (4.82) | 1.165 (0.999, 1.360) | 0.051 | 1.092 (0.934, 1.278) | 0.270 | 0.986 (0.831, 1.171) | 0.873 |
| Quartile 4 (1.48 ~ 1.73) | 2,373 | 495 (5.43) | 1.323 (1.139, 1.538) | <0.001 | 1.192 (1.022, 1.392) | 0.026 | 0.998 (0.827, 1.205) | 0.982 |
| P for trend | <0.001 | 0.017 | 0.995 | |||||
Logistic regression analysis of the relationship between individual essential metal elements and GDM (N = 9,112).
Ref, reference group; OR, odds ratio; CI, confidence interval; GDM, gestational diabetes mellitus.
Model 1: not adjusted any variables.
Model 2: adjusted the age of pregnant women, pre-pregnancy BMI, parity, season and gestational week at blood specimen collection.
Model 3: adjusted for all variables in Model 2 and other four trace metals (continuous variables).
P for trend was obtained from the conditional logistic regression models by using the median of each quartile of essential metal element concentrations as continuous variables.
The bold black font indicates that the association is statistically significant (P < 0.05).
3.4.2 Exposure-response relationship between individual essential metal element and GDM
Furthermore, Figure 3 illustrated the results of RCS analysis. After adjusting for covariates, a positive linear relationship was observed between Fe, Zn, and Mg levels and the risk of GDM (Pnon-linear > 0.05 and Pnon-linear > 0.05). Among them, the inflection point of Fe was 7.68 mmol/L, Zn was 87.75 μmol/L and Mg was 1.42 mmol/L. There was no significant correlation between Ca and Cu levels and the risk of GDM (Poverall > 0.05 and Pnon-linear > 0.05).
Figure 3
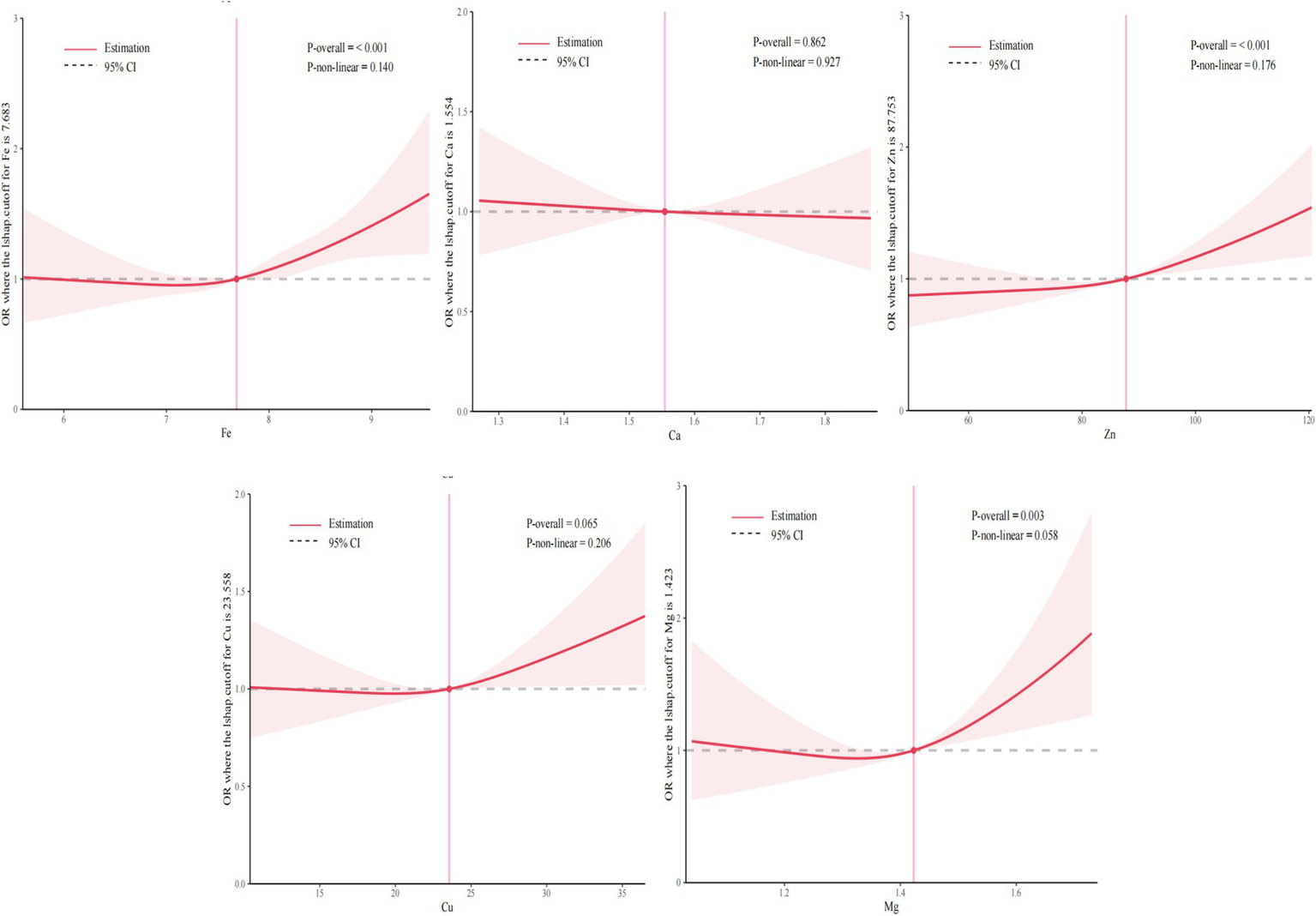
Exposure-reaction relationship between five essential metal elements and GDM. The model adjusted the age of pregnant women, pre-pregnancy BMI, parity, season and gestational week at blood specimen collection.
3.4.3 Correlation between multiple essential metal elements and GDM
In BKMR, the PIP values of the essential metal elements were shown in Table 4, and Fe (PIP = 0.772) had the greatest influence on the combined action. The combined effects of five essential metal elements on GDM were shown in Figure 4, and the combined effects of all essential metal elements on GDM generally showed an upward trend. The individual effect of a single essential metal element on GDM risk was shown in Figure 5, in which Fe played a key role in the combined effect of essential metal elements and GDM. In addition, the exposure-response relationship between each essential metal element and GDM was shown in Figure 6, and the effects of Fe and Zn on GDM showed a trend of first decreasing and then increasing, which was more in line with the non-linear correlation. No obvious interaction between essential metal elements was found in this study, and the results were shown in Figure 7.
Table 4
| Variables | PIP |
|---|---|
| Fe | 0.772 |
| Ca | <0.001 |
| Zn | 0.166 |
| Cu | 0.007 |
| Mg | 0.005 |
PIP of each essential metal elements in BKMR (N = 9,112).
The model adjusted the age of pregnant women, pre-pregnancy BMI, parity, season and gestational week at blood specimen collection.
Figure 4
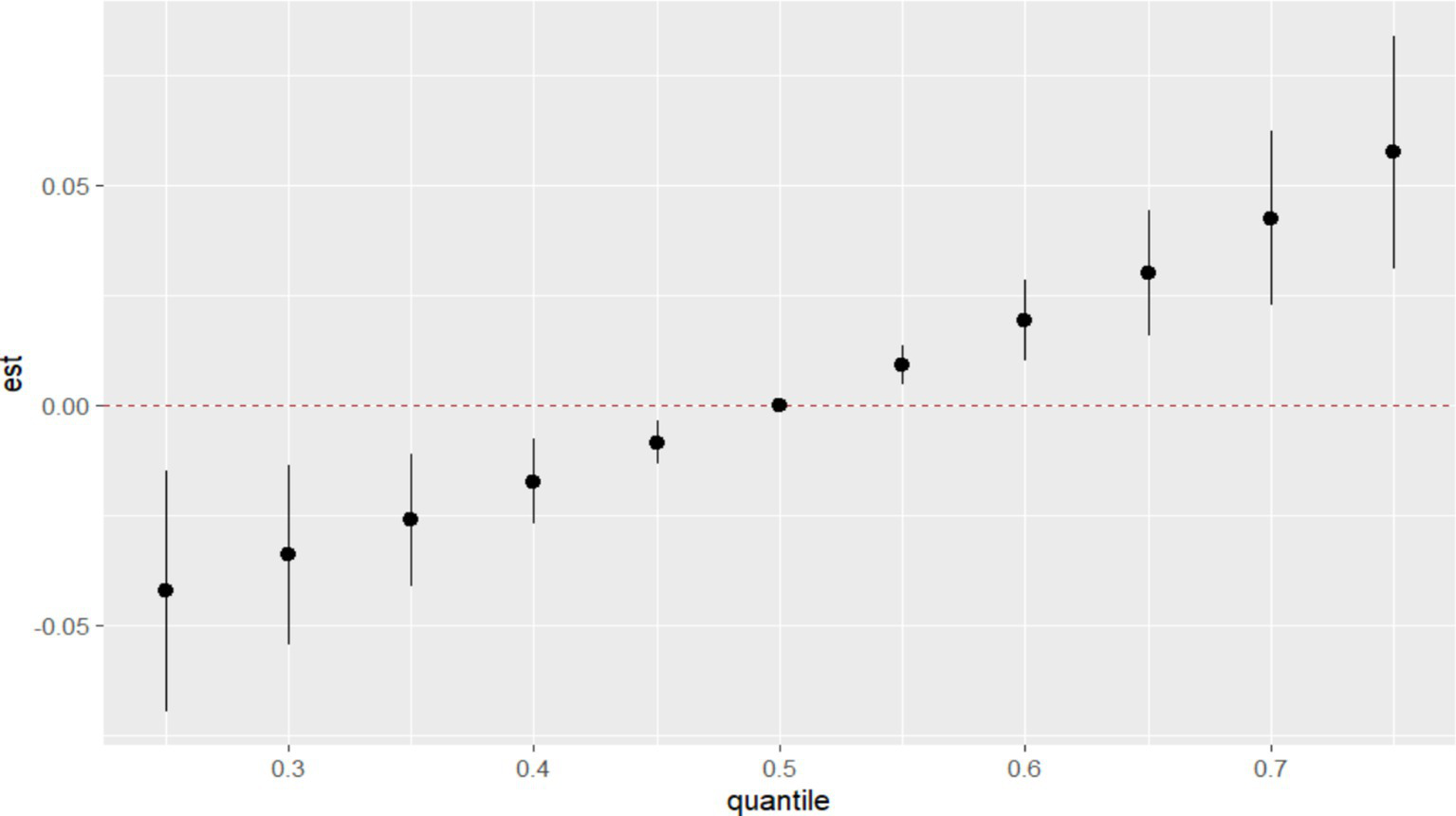
Combined effect of essential metal elements on GDM.
Figure 5
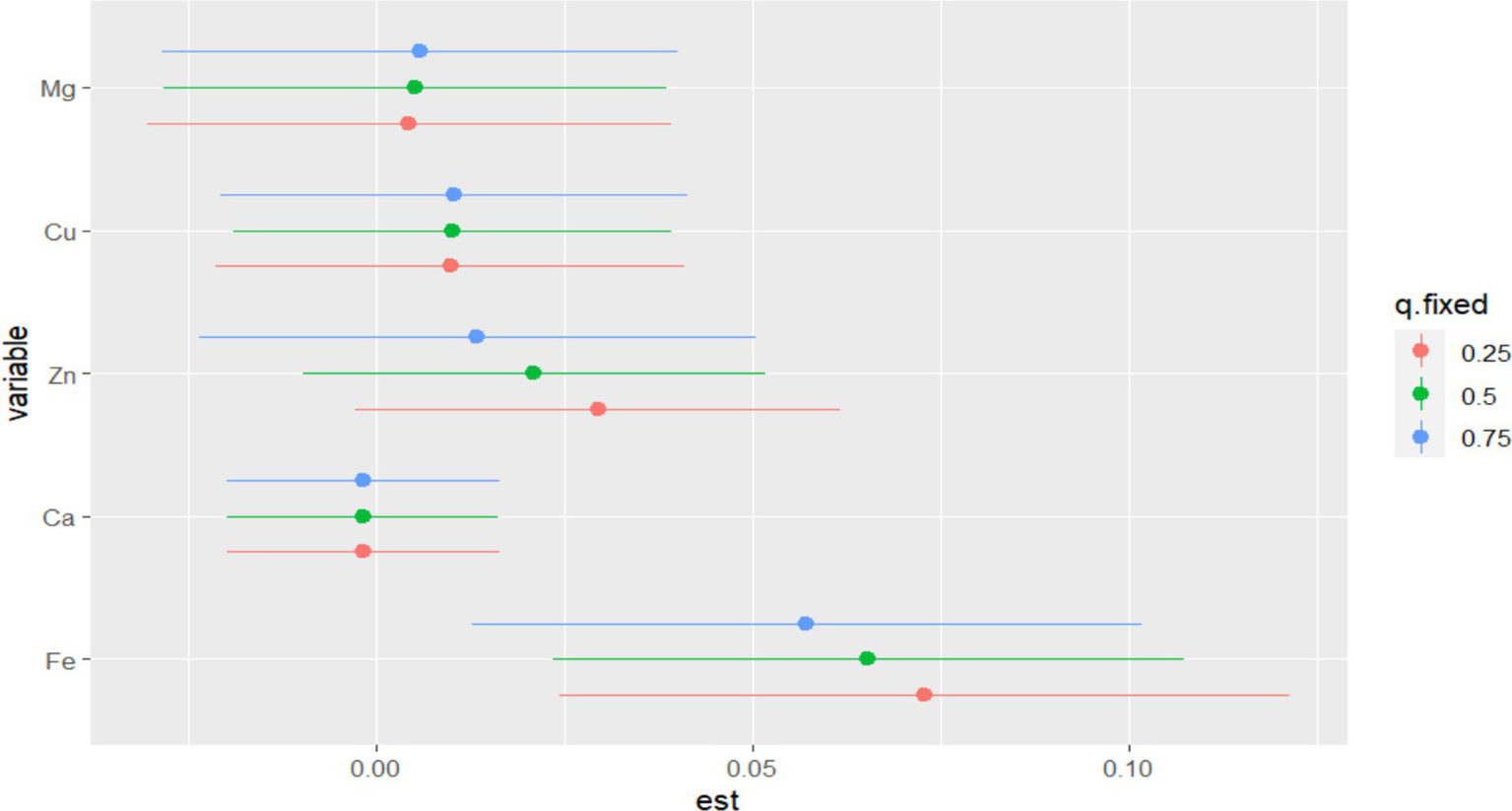
Individual effects of essential metal elements on GDM correlation.
Figure 6
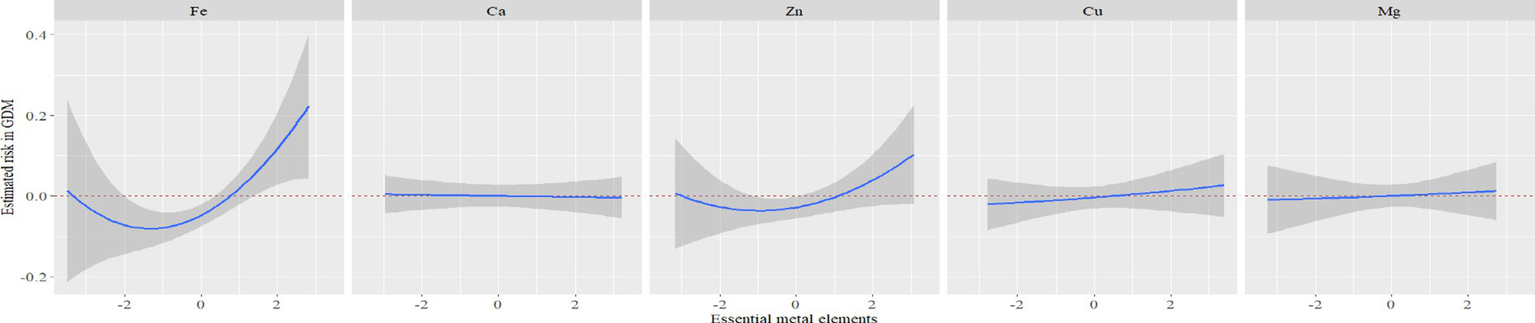
Exposure-response relationship between individual essential metal elements and GDM.
Figure 7
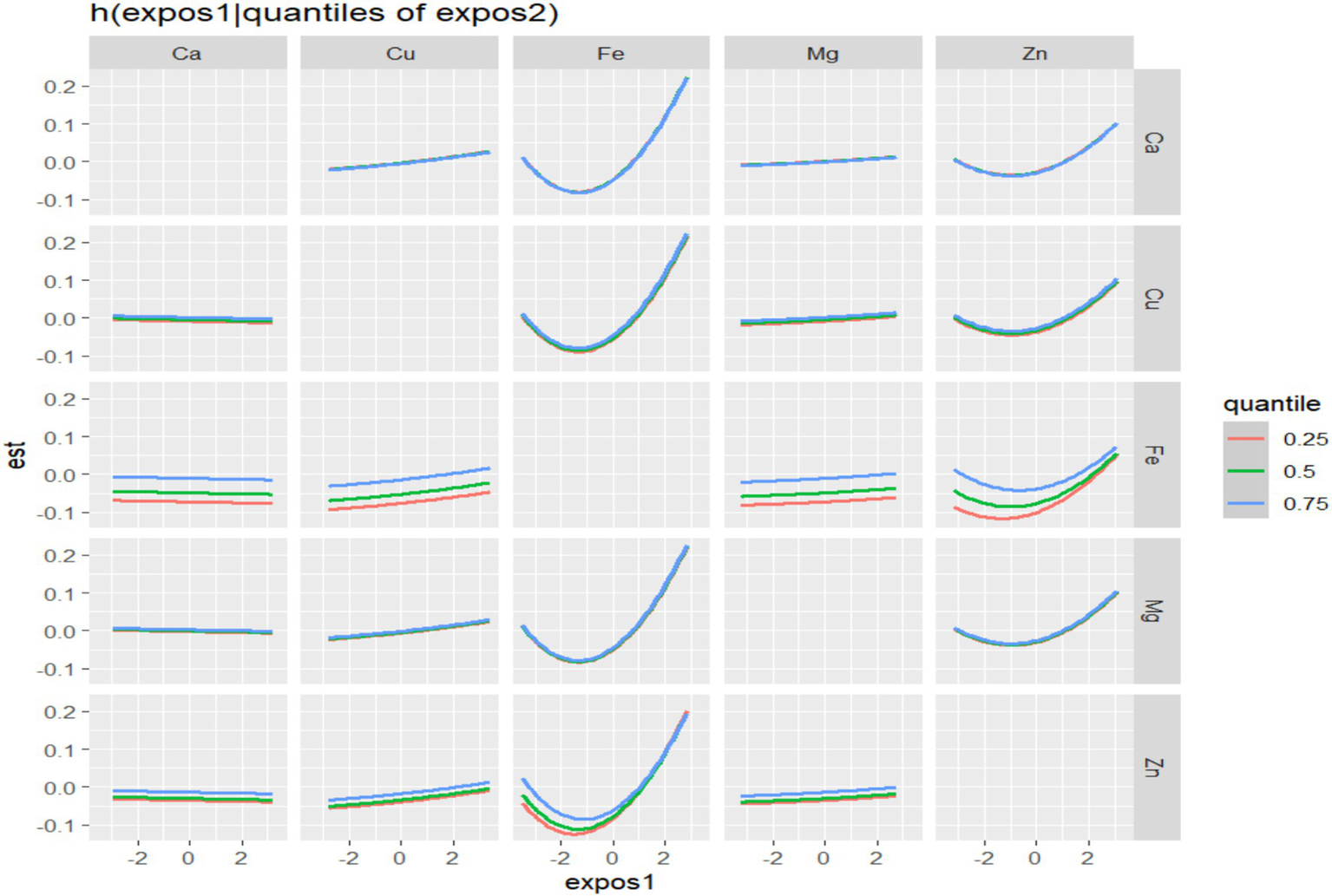
Bivariate exposure-effect curve of essential metal elements associated with GDM.
3.5 Sensitivity analyses
Supplementary Tables S1, S2 presents the results of WQS and QGC. After covariate correction, the combined action amount of five essential metal elements and the risk of GDM showed a monotonically increasing positive relationship, in which Fe had a larger positive weight in the relationship between the mixture and GDM (Supplementary Figures S1, S2).
All the above analysis results showed that Fe played an important role in GDM, and BKMR model had not found any interaction between essential metal elements on GDM, so this study further used multiplication interaction and addition interaction to verify whether Fe and other essential metal elements interact with GDM. The multiplication interaction results were shown in Supplementary Table S3 and the addition interaction results were shown in Supplementary Table S4. After adjusting the covariate, there was still no multiplication or addition interaction between the essential metal elements and GDM.
4 Discussion
The occurrence of GDM was a complex process, and its pathogenesis was still not fully clarified. Previous studies have shown heterogeneity in the relationship between different levels of essential metals. This study provided reliable results to explore the influence of essential metal elements in early pregnancy on GDM risk.
Fe was one of the important trace essential metal elements in human body (51). The results from the BKMR and GLM model indicated Fe were associated with an increased risk of GDM. The negative correlation found between Ca and GDM in the BKMR model might offset the positive correlations observed between other essential metal elements and GDM to some extent (52). This underscored the importance of combining classical single exposure effect models with mixed exposure effect models (53). On the other hand, it was emphasized that Fe was associated with an increased risk of GDM in the comprehensive effect. A study using data from the Ma’anshan birth cohort (MABC) recruited 3,289 pregnant women. Their results showed that the risk of GDM in pregnant women with the highest level of serum Fe was 1.63 times higher than that in the middle level (54). The Wuhan twin birth cohort (WTBC) study from 2013 to 2016 also found that the risk of GDM in pregnant women with serum Fe concentration in the fourth quartile was 5.13 times higher than that in the first quartile (55). Elevated Fe levels may contribute to increased oxidative stress, potentially leading to damage to pancreatic β cells, reduced insulin secretion, and impaired glucose regulation (56). High levels of Fe increased the burden on the liver, reduced the sensitivity of the liver to insulin, induced insulin resistance, reduced the synthesis of liver glycogen and weakened the sensitivity to insulin signals (57). High levels of Fe can also reduce the supply of glucose oxidation energy and enhance fatty acid metabolism in skeletal muscle cells, reduce insulin-induced glucose transport in adipocytes, and ultimately lead to GDM (58).
As an auxiliary factor of many enzymes, Zn was second only to Fe in the whole-body content (59). Clinical and animal studies have shown that Zn can directly regulate insulin activity and glucose homeostasis (60). More and more evidence was consistent with the results of this study, supporting the relationship between Zn and GDM (61, 62). The results of this study showed that the risk of GDM in pregnant women with Zn at the fourth quantile level (92.10 ~ 121.90 μ mol/L) was 1.379 times that at the lowest quantile level. The mechanism between high levels of Zn and GDM includes the direct effects of Zn on oxidative stress, immune regulation and insulin activity (63). In addition, Zn has estrogen activity (64), which can simulate some characteristics of insulin secreted by pancreatic β cells and interfere with insulin metabolism and glucose homeostasis, thus increasing the risk of GDM (65). However, the correlation between Zn and GDM in some previous studies was not consistent with the results of this study. A previous case–control study in Wuhan, China showed that compared with the lowest quartile level, the risk of GDM in pregnant women with Zn at the highest quartile level in the first trimester was 0.30 (95%CI: 0.18 ~ 0.50) (55). Zhang and Liang (66) recruited pregnant women who received obstetric examination in the obstetric clinic of Shanxi Provincial People’s Hospital as the research objects, and found that there was no correlation between Zn level and pregnant women’s blood sugar level. A prospective cohort study of pregnant women in Australia by Wilson et al. (67) also showed that Zn level in early pregnancy was not related to GDM.
Mg, involved in various enzymatic reactions in the body (68), affecting glucose metabolism stability and insulin sensitivity (69). Impairment of Mg homeostasis at insulin receptor will lead to insulin resistance and decrease of insulin secretion in β cells, leading to an increase in GDM risk (70). The results of this study showed that the higher level of Mg was positively correlated with the risk of GDM (OR = 1.192, 95%CI: 1.022 ~ 1.392), and the risk of GDM increased significantly when the blood Mg level was greater than 1.42 mmol/L. The results were similar to a prospective cohort study with 65 pregnant women, indicating that the risk of GDM increased 3.923 times per unit level of Mg (66). However, a previous case–control study included 610 pregnant women as the research object, and the study showed that there was no significant correlation between Mg and GDM (55). Differences in sample size might explain the discrepancies in the findings.
In addition to the main findings, our research found no correlation between Cu, calcium and GDM. However, studies have shown that there was a negative correlation between Ca and GDM in early pregnancy (RR = 0.84, 95%CI: 0.73, 0.97), and a positive correlation between Cu and GDM (RR = 1.23, 95%CI: 1.02, 1.49) (55). Although the essential metal elements examined in their study are consistent with our research, differences detection methods for essential metal elements, and covariates may contribute to the discrepancies in the findings. Ca homeostasis affected insulin secretion and the survival of β cells, while calcium channel blockers had shown efficacy in preventing GDM (71). Excessive Cu would increase oxidative stress, leading to β cell death and glucose metabolism disorder (72). Future studies with larger sample sizes, more refined measurement techniques, and careful control of confounding factors may provide a clearer understanding of these trace elements’ role in GDM.
This study suggested that while essential for normal metabolic function, the combined action of these five elements may also elevate the risk of GDM. Some studies supported the results of this study (73–75). Li et al. (76) used BKMR model to evaluate the correlation between FPG and the combined effects of five elements, including urinary arsenic (As), nickel (Ni), cadmium (Cd) and plasma selenium (Se) and Zn. The results showed that these five elements were also positively associated with FPG (76). However, in this study, besides Se and Zn, three harmful elements, AS, Ni and Cd, were also included in the overall effect evaluation. The biological samples used in the above study were also different from this study, which used urine and plasma samples to detect elements, while this study used whole blood samples. A retrospective cohort study included 8,169 pregnant women in China, which proved that the WQS index of essential metal elements such as manganese (Mn), Cu, lead (Pb), Ca, Zn and Mg was significantly positively correlated with FBG (26). Among the six essential metal elements included in the above study, Mn was the most important in the combined effect. The difference of results may be related to the types of metal elements and the mechanism between essential metals and GDM was not clear.
Previous studies have shown that different essential metal elements may have synergistic or antagonistic interactions on health effects, particularly in relation to pregnancy and maternal health (77). Zheng et al. (78) evaluated the relationship between Zn, Se, Cu and molybdenum as a mixture and the glucose level in pregnancy in a multi-ethnic pregnancy cohort, and found that the synergistic effect of Cu and Zn would increase the glucose level during pregnancy (78). It may be related to the synergistic activation of Cu/Zn superoxide dismutase by Cu and Zn (79). Cu/Zn superoxide dismutase promotes the production of ROS by acting as superoxide reductase or superoxide oxidase, which leads to metabolic disorders and affects glucose levels (80). Nested case–control study in pregnant women in China also showed that Ca and Fe had significant synergistic effect on GDM (55). In addition, Mg and Ca had a strong synergistic effect, which affected FPG and increases the risk of GDM (81). The synergistic effect of Mg and Ca may be related to the secretion of parathyroid hormone. Thyroid function can mediate the relationship between essential metal elements and GDM (66). The interaction mode of essential metals was influenced by dose level, dose ratio or mixture composition (82). Therefore, it was still necessary to explain the interaction between essential metal elements (83).
It was worth noting that with the development of economy, pregnant women pay more attention to the supplement of nutrients during pregnancy, and whether healthy pregnant women need regular supplement of essential metal elements needs further evaluation. Previous studies have shown that Fe supplementation for pregnant women without Fe deficiency anemia in the first trimester is positively correlated with the increase of hemoglobin level and blood sugar level, and the risk of GDM also increases (84). High levels of Fe can also produce reactive oxygen species (ROS) and cause tissue damage, leading to premature delivery, low birth weight infants and neurodevelopmental defects in children and other adverse pregnancy outcomes (85, 86). Zinc played a crucial and direct role in the development of the fetal nervous system and the prevention of pregnancy complications (87). Additionally, studies suggested that magnesium reduced oxidative stress and helped prevent complications like pregnancy-induced hypertension and preeclampsia (88). The WHO suggested that prenatal health care should promote positive pregnancy experience, and the population size, distribution and Fe deficiency of anemia should be fully considered under strict research background before Fe supplements can be recommended (89). Zinc and Mg supplements were not recommended as part of routine pregnancy care, but only in the context of rigorous research (90). Essential metal element detection should be included in routine prenatal exams, as early detection is crucial to prevent imbalances that could harm both mother and fetus. Additionally, mobile health tools (mHealth) were used to monitor various health indicators in pregnant women with GDM and played a key role in improving their dietary compliance and behavior changes (91). In the future, greater attention should be given to this area.
5 Limitations
Single center design was one of the main limitations of this study. This study was observational and only identified an association between essential metal elements and GDM, without determining a causal relationship. At the same time, this study did not collect the information of pregnant women’s dietary intake, dietary supplements intake, atmospheric environment during pregnancy and household fuel use. These factors were closely related to the level of essential metal elements, and related research should be further included in the above factors in the future. Only five essential metal elements, such as Fe, Cu, Zn, Ca and Mg, were included in the assessment of combined effect. Pregnant women may be exposed to a wider range of environment in the real world, and indicators of other essential metal elements need to be collected.
6 Conclusion
The research findings unveiled a notable association between essential metal elements and GDM. Particularly, higher concentrations of Fe, Zn, and Mg manifested a significant positive correlation with GDM, emphasizing the criticality of adequate intake of these elements during pregnancy. The combined impact of these elements manifested a monotonically increasing relationship with the risk of GDM, with Fe exerting the most substantial influence, suggesting Fe as a potential biomarker for GDM risk. However, no significant interactions between the essential metal elements and GDM were observed. Given the complexity of trace element balance during pregnancy, further research is needed to investigate their collective impact on GDM development.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Guangdong Women and Children Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
GC: Writing – original draft, Writing – review & editing. LW: Conceptualization, Data curation, Writing – review & editing. CJ: Methodology, Software, Supervision, Writing – review & editing. JX: Formal analysis, Project administration, Validation, Writing – review & editing. GL: Resources, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1554840/full#supplementary-material
References
1.
ShangJDolikunNTaoXZhangPWoodwardMHackettMLet al. The effectiveness of postpartum interventions aimed at improving women’s mental health after medical complications of pregnancy: a systematic review and meta-analysis. BMC Pregnancy Childbirth. (2022) 22:809. doi: 10.1186/s12884-022-05084-1
2.
JuanJYangH. Prevalence, prevention, and lifestyle intervention of gestational diabetes mellitus in China. Int J Environ Res Public Health. (2020) 17:9517. doi: 10.3390/ijerph17249517
3.
ZhuHZhaoZXuJChenYZhuQZhouLet al. The prevalence of gestational diabetes mellitus before and after the implementation of the universal two-child policy in China. Front Endocrinol. (2022) 13:960877. doi: 10.3389/fendo.2022.960877
4.
SzmuilowiczEDJosefsonJLMetzgerBE. Gestational diabetes mellitus. Endocrinol Metab Clin N Am. (2019) 48:479–93. doi: 10.1016/j.ecl.2019.05.001
5.
Täufer CederlöfELundgrenMLindahlBChristerssonC. Pregnancy complications and risk of cardiovascular disease later in life: a Nationwide cohort study. J Am Heart Assoc. (2022) 11:e023079. doi: 10.1161/JAHA.121.023079
6.
BrenerALewnardIMackinnonJJonesCLohrNKondaSet al. Missed opportunities to prevent cardiovascular disease in women with prior preeclampsia. BMC Womens Health. (2020) 20:217. doi: 10.1186/s12905-020-01074-7
7.
GrandiSMFilionKBYoonSAyeleHTDoyleCMHutcheonJAet al. Cardiovascular disease-related morbidity and mortality in women with a history of pregnancy complications. Circulation. (2019) 139:1069–79. doi: 10.1161/CIRCULATIONAHA.118.036748
8.
GluckmanPDHansonMA. Developmental origins of disease paradigm: a mechanistic and evolutionary perspective. Pediatr Res. (2004) 56:311–7. doi: 10.1203/01.PDR.0000135998.08025.FB
9.
ElliottHRSharpGCReltonCLLawlorDA. Epigenetics and gestational diabetes: a review of epigenetic epidemiology studies and their use to explore epigenetic mediation and improve prediction. Diabetologia. (2019) 62:2171–8. doi: 10.1007/s00125-019-05011-8
10.
LoweWL. Genetics and epigenetics: implications for the life course of gestational diabetes. Int J Mol Sci. (2023) 24:6047. doi: 10.3390/ijms24076047
11.
MeazawMWChojentaCMulunehMDLoxtonD. Systematic and meta-analysis of factors associated with preeclampsia and eclampsia in sub-Saharan Africa. PLoS One. (2020) 15:e0237600. doi: 10.1371/journal.pone.0237600
12.
Aguilar-CorderoMJLasserrot-CuadradoAMur-VillarNLeón-RíosXARivero-BlancoTPérez-CastilloIM. Vitamin D, preeclampsia and prematurity: a systematic review and meta-analysis of observational and interventional studies. Midwifery. (2020) 87:102707. doi: 10.1016/j.midw.2020.102707
13.
Balleza-AlejandriLRPeña-DuránEBeltrán-RamírezAReynoso-RoaASSánchez-AbundisLDGarcía-GalindoJJet al. Decoding the gut microbiota-gestational diabetes link: insights from the last seven years. Microorganisms. (2024) 12:1070. doi: 10.3390/microorganisms12061070
14.
PonzoVFedeleDGoitreILeoneFLezoAMonzeglioCet al. Diet-gut microbiota interactions and gestational diabetes mellitus (GDM). Nutrients. (2019) 11:330. doi: 10.3390/nu11020330
15.
XuRMengXPangYAnHWangBZhangLet al. Associations of maternal exposure to 41 metals/metalloids during early pregnancy with the risk of spontaneous preterm birth: does oxidative stress or DNA methylation play a crucial role?Environ Int. (2022) 158:106966. doi: 10.1016/j.envint.2021.106966
16.
MoraisJBSSeveroJSBeserraJBde OiveiraARSCruzKJCde Sousa MeloSRet al. Association between cortisol, insulin resistance and zinc in obesity: a Mini-review. Biol Trace Elem Res. (2019) 191:323–30. doi: 10.1007/s12011-018-1629-y
17.
AshrapPWatkinsDJMukherjeeBRosario-PabónZVélez-VegaCMAlshawabkehAet al. Performance of urine, blood, and integrated metal biomarkers in relation to birth outcomes in a mixture setting. Environ Res. (2021) 200:111435. doi: 10.1016/j.envres.2021.111435
18.
ChenYOuQXChenYZhuQLTanMHZhangMMet al. Association between trace elements and preeclampsia: a retrospective cohort study. J Trace Elem Med Biol. (2022) 72:126971. doi: 10.1016/j.jtemb.2022.126971
19.
XieSZhangEGaoSSuSLiuJZhangYet al. Associations of systemic immune-inflammation index and systemic inflammation response index with maternal gestational diabetes mellitus: evidence from a prospective birth cohort study. Chin Med J. (2025) 138:729–37. doi: 10.1097/CM9.0000000000003236
20.
KomarovaTMcKeatingDPerkinsAVTinggiU. Trace element analysis in whole blood and plasma for reference levels in a selected Queensland population, Australia. Int J Environ Res Public Health. (2021) 18:2652. doi: 10.3390/ijerph18052652
21.
Shaji GeethaNBobbyZDorairajanGJacobSE. Increased hepcidin levels in preeclampsia: a protective mechanism against iron overload mediated oxidative stress?J Matern Fetal Neonatal Med. (2022) 35:636–41. doi: 10.1080/14767058.2020.1730322
22.
ZhangYXuSZhongCLiQWuMZhangGet al. Periconceptional iron supplementation and risk of gestational diabetes mellitus: a prospective cohort study. Diabetes Res Clin Pract. (2021) 176:108853. doi: 10.1016/j.diabres.2021.108853
23.
ZhengYZhangCWeisskopfMWilliamsPLParsonsPJPalmerCDet al. A prospective study of early pregnancy essential metal(loid)s and glucose levels late in the second trimester. J Clin Endocrinol Metab. (2019) 104:4295–303. doi: 10.1210/jc.2019-00109
24.
ZhaoDChenJLiXHuangYZhangYZhaoFet al. A prospective study of early pregnancy metal concentrations and gestational diabetes mellitus based on a birth cohort in Northwest China. BMC Pregnancy Childbirth. (2025) 25:387. doi: 10.1186/s12884-025-07336-2
25.
KotKŁanocha-ArendarczykNKupnickaPSzymańskiSMalinowskiWKalisińskaEet al. Selected metal concentration in maternal and cord blood. Int J Environ Res Public Health. (2021) 18:12407. doi: 10.3390/ijerph182312407
26.
ZhouZChenGLiPRaoJWangLYuDet al. Prospective association of metal levels with gestational diabetes mellitus and glucose: a retrospective cohort study from South China. Ecotoxicol Environ Saf. (2021) 210:111854. doi: 10.1016/j.ecoenv.2020.111854
27.
XiangHTaoYZhangBLiangCLiZFengLet al. Protective effect of high zinc levels on preterm birth induced by mercury exposure during pregnancy: a birth cohort study in China. J Trace Elem Med Biol. (2019) 55:71–7. doi: 10.1016/j.jtemb.2019.06.004
28.
YuYGaoMWangXGuoYPangYYanHet al. Recommended acceptable levels of maternal serum typical toxic metals from the perspective of spontaneous preterm birth in Shanxi Province, China. Sci Total Environ. (2019) 686:599–605. doi: 10.1016/j.scitotenv.2019.05.413
29.
FreireCAmayaEGilFMurciaMLLopSCasasMet al. Placental metal concentrations and birth outcomes: the environment and childhood (INMA) project. Int J Hyg Environ Health. (2019) 222:468–78. doi: 10.1016/j.ijheh.2018.12.014
30.
XingYXiaWZhangBZhouAHuangZZhangHet al. Relation between cadmium exposure and gestational diabetes mellitus. Environ Int. (2018) 113:300–5. doi: 10.1016/j.envint.2018.01.001
31.
XiaXLiangCShengJYanSHuangKLiZet al. Association between serum arsenic levels and gestational diabetes mellitus: a population-based birth cohort study. Environ Pollut. (2018) 235:850–6. doi: 10.1016/j.envpol.2018.01.016
32.
MuñozMPValdésMMuñoz-QuezadaMTLuceroBRubilarPPinoPet al. Urinary inorganic arsenic concentration and gestational diabetes mellitus in pregnant women from Arica, Chile. Int J Environ Res Public Health. (2018) 15:1418. doi: 10.3390/ijerph15071418
33.
Ashley-MartinJDoddsLArbuckleTEBouchardMFShapiroGDFisherMet al. Association between maternal urinary speciated arsenic concentrations and gestational diabetes in a cohort of Canadian women. Environ Int. (2018) 121:714–20. doi: 10.1016/j.envint.2018.10.008
34.
FangMHuLChenDGuoYLiuJLanCet al. Exposome in human health: utopia or wonderland?Innovation. (2021) 2:100172. doi: 10.1016/j.xinn.2021.100172
35.
WangYZhangPChenXWuWFengYYangHet al. Multiple metal concentrations and gestational diabetes mellitus in Taiyuan, China. Chemosphere. (2019) 237:124412. doi: 10.1016/j.chemosphere.2019.124412
36.
Association AD. 2. Classification and diagnosis of diabetes: standards of medical Care in Diabetes-2018. Diabetes Care. (2018) 41:S13–s27. doi: 10.2337/dc18-S002
37.
ZhouBForceFebCM-AGOCOT. Predictive values of body mass index and waist circumference to risk factors of related diseases in Chinese adult population. Zhonghua Liu Xing Bing Xue Za Zhi. (2002) 23:5–10. doi: 10.3760/cma.j.issn.0254-6450.2002.01.103
38.
FengWCuiXLiuBLiuCXiaoYLuWet al. Association of urinary metal profiles with altered glucose levels and diabetes risk: a population-based study in China. PLoS One. (2015) 10:e0123742. doi: 10.1371/journal.pone.0123742
39.
LusaLAhlinČ. Restricted cubic splines for modelling periodic data. PLoS One. (2020) 15:e0241364. doi: 10.1371/journal.pone.0241364
40.
BobbJFValeriLClaus HennBChristianiDCWrightROMazumdarMet al. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics. (2015) 16:493–508. doi: 10.1093/biostatistics/kxu058
41.
KeilAPBuckleyJPO'BrienKMFergusonKKZhaoSWhiteAJ. A quantile-based g-computation approach to addressing the effects of exposure mixtures. Environ Health Perspect. (2020) 128:47004. doi: 10.1289/EHP5838
42.
PerzJFArmstrongGLFarringtonLAHutinYJFBellBP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. (2006) 45:529–38. doi: 10.1016/j.jhep.2006.05.013
43.
LozanoMMurciaMSoler-BlascoRCasasMZuberoBRiutort-MayolGet al. Exposure to metals and metalloids among pregnant women from Spain: levels and associated factors. Chemosphere. (2022) 286:131809. doi: 10.1016/j.chemosphere.2021.131809
44.
ZhengQXWangHWJiangXMLinYLiuGHPanMet al. Prepregnancy body mass index and gestational weight gain are associated with maternal and infant adverse outcomes in Chinese women with gestational diabetes. Sci Rep. (2022) 12:2749. doi: 10.1038/s41598-022-06733-3
45.
LewandowskaMWięckowskaBSajdakSLubińskiJ. First trimester microelements and their relationships with pregnancy outcomes and complications. Nutrients. (2020) 12:1108. doi: 10.3390/nu12041108
46.
ModzelewskiRStefanowicz-RutkowskaMMMatuszewskiWBandurska-StankiewiczEM. Gestational diabetes mellitus-recent literature review. J Clin Med. (2022) 11:5736. doi: 10.3390/jcm11195736
47.
LiangCMWuXYHuangKYanSQLiZJXiaXet al. Trace element profiles in pregnant women's sera and umbilical cord sera and influencing factors: repeated measurements. Chemosphere. (2019) 218:869–78. doi: 10.1016/j.chemosphere.2018.11.115
48.
LiaoLWeiXLiuMGaoYYinYZhouR. The association between season and hypertensive disorders in pregnancy: a systematic review and meta-analysis. Reprod Sci. (2023) 30:787–801. doi: 10.1007/s43032-022-01010-0
49.
LinQYangJDengL. Analysis of calcium, copper, iron, magnesium and zinc in 6 698 pregnant women. Henan J Prev Med. (2020) 31:514–7. doi: 10.13515/j.cnki.hnjpm.1006-8414.2020.07.012
50.
LeineweberCGeislerGPeesMÖfnerSMarschangRE. Trace elements in Hermann's tortoises (Testudo hermanni) according to sex, season, and sampling region in Central Europe. Animals. (2024) 14:2178. doi: 10.3390/ani14152178
51.
SharashenidzeAPanchulidzeLSanikidzeT. Alterations in placenta redox-status during experimental model of hypoxia-induced preeclampsia. Georgian Med News. (2017) 268–269:86–90.
52.
ZhangYDongTHuWWangXXuBLinZet al. Association between exposure to a mixture of phenols, pesticides, and phthalates and obesity: comparison of three statistical models. Environ Int. (2019) 123:325–36. doi: 10.1016/j.envint.2018.11.076
53.
RenMZhaoJWangBAnHLiYJiaXet al. Associations between hair levels of trace elements and the risk of preterm birth among pregnant women: a prospective nested case-control study in Beijing birth cohort (BBC), China. Environ Int. (2022) 158:106965. doi: 10.1016/j.envint.2021.106965
54.
ZhuBLiangCXiaXHuangKYanSHaoJet al. Iron-related factors in early pregnancy and subsequent risk of gestational diabetes mellitus: the Ma'anshan birth cohort (MABC) study. Biol Trace Elem Res. (2019) 191:45–53. doi: 10.1007/s12011-018-1595-4
55.
ZhuGZhengTXiaCQiLPapandonatosGDMingYet al. Plasma levels of trace element status in early pregnancy and the risk of gestational diabetes mellitus: a nested case-control study. J Trace Elem Med Biol. (2021) 68:126829. doi: 10.1016/j.jtemb.2021.126829
56.
PlowsJFStanleyJLBakerPNReynoldsCMVickersMH. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. (2018) 19:3342. doi: 10.3390/ijms19113342
57.
HuXCaiXMaRFuWZhangCduX. Iron-load exacerbates the severity of atherosclerosis via inducing inflammation and enhancing the glycolysis in macrophages. J Cell Physiol. (2019) 234:18792–800. doi: 10.1002/jcp.28518
58.
NakamuraTNaguroIIchijoH. Iron homeostasis and iron-regulated ROS in cell death, senescence and human diseases. Biochim Biophys Acta, Gen Subj. (2019) 1863:1398–409. doi: 10.1016/j.bbagen.2019.06.010
59.
OlechnowiczJTinkovASkalnyASuliburskaJ. Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J Physiol Sci. (2018) 68:19–31. doi: 10.1007/s12576-017-0571-7
60.
LiXTYuPFGaoYGuoWHWangJLiuXet al. Association between plasma metal levels and diabetes risk: a case-control study in China. Biomed Environ Sci. (2017) 30:482–91. doi: 10.3967/bes2017.064
61.
LiuBFengWWangJLiYHanXHuHet al. Association of urinary metals levels with type 2 diabetes risk in coke oven workers. Environ Pollut. (2016) 210:1–8. doi: 10.1016/j.envpol.2015.11.046
62.
RoversoMDi MarcoVBadoccoDPastorePCalanducciMCosmiEet al. Maternal, placental and cordonal metallomic profiles in gestational diabetes mellitus. Metallomics. (2019) 11:676–85. doi: 10.1039/c8mt00331a
63.
BonaventuraPBenedettiGAlbarèdeFMiossecP. Zinc and its role in immunity and inflammation. Autoimmun Rev. (2015) 14:277–85. doi: 10.1016/j.autrev.2014.11.008
64.
Del PupLMantovaniALuceACavaliereCFacchiniGDi FranciaRet al. Endocrine disruptors and female cancer: informing the patients (review). Oncol Rep. (2015) 34:3–11. doi: 10.3892/or.2015.3997
65.
Cooper-CapetiniVde VasconcelosDAAMartinsARHirabaraSDonato JrJCarpinelliAet al. Zinc supplementation improves glucose homeostasis in high fat-fed mice by enhancing pancreatic β-cell function. Nutrients. (2017) 9:1150. doi: 10.3390/nu9101150
66.
ZhangWLiangH. Relationships between maternal selected metals (cu, mg, Zn and Fe), thyroid function and blood glucose levels during pregnancy. Biol Trace Elem Res. (2023) 201:3603–12. doi: 10.1007/s12011-022-03455-5
67.
WilsonRLBianco-MiottoTLeemaqzSYGrzeskowiakLEDekkerGARobertsCT. Early pregnancy maternal trace mineral status and the association with adverse pregnancy outcome in a cohort of Australian women. J Trace Elem Med Biol. (2018) 46:103–9. doi: 10.1016/j.jtemb.2017.11.016
68.
TakayaJYamatoFKuroyanagiYHigashinoHKanekoK. Intracellular magnesium of obese and type 2 diabetes mellitus children. Diabetes Ther. (2010) 1:25–31. doi: 10.1007/s13300-010-0003-7
69.
DrenthenLCAde BaaijJHFRodwellLvan HerwaardenAETackCJde GalanBE. Oral magnesium supplementation does not affect insulin sensitivity in people with insulin-treated type 2 diabetes and a low serum magnesium: a randomised controlled trial. Diabetologia. (2024) 67:52–61. doi: 10.1007/s00125-023-06029-9
70.
RamadassSBasuSSrinivasanAR. Serum magnesium levels as an indicator of status of diabetes mellitus type 2. Diabetes Metab Syndr. (2015) 9:42–5. doi: 10.1016/j.dsx.2014.04.024
71.
ArdissinoMSlobEAWRajasundaramSReddyRKWoolfBGirlingJet al. Safety of beta-blocker and calcium channel blocker antihypertensive drugs in pregnancy: a Mendelian randomization study. BMC Med. (2022) 20:288. doi: 10.1186/s12916-022-02483-1
72.
RoyDMaityNCKumarSMaityARathaBNBiswasRet al. Modulatory role of copper on hIAPP aggregation and toxicity in presence of insulin. Int J Biol Macromol. (2023) 241:124470. doi: 10.1016/j.ijbiomac.2023.124470
73.
YuanYXiaoYYuYLiuYFengWQiuGet al. Associations of multiple plasma metals with incident type 2 diabetes in Chinese adults: the Dongfeng-Tongji cohort. Environ Pollut. (2018) 237:917–25. doi: 10.1016/j.envpol.2018.01.046
74.
YangAChengNPuHLiuSDaiMZhengTet al. Occupational metal exposures, smoking and risk of diabetes and prediabetes. Occup Med. (2017) 67:217–23. doi: 10.1093/occmed/kqw078
75.
MenkeAGuallarECowieCC. Metals in urine and diabetes in U.S. adults. Diabetes. (2016) 65:164–71. doi: 10.2337/db15-0316
76.
LiZXuYHuangZWeiYHouJLongTet al. Association between exposure to arsenic, nickel, cadmium, selenium, and zinc and fasting blood glucose levels. Environ Pollut. (2019) 255:113325. doi: 10.1016/j.envpol.2019.113325
77.
MansouriBRezaeiASharafiKAzadiNPirsahebMRezaeiMet al. Mixture effects of trace element levels on cardiovascular diseases and type 2 diabetes risk in adults using G-computation analysis. Sci Rep. (2024) 14:5743. doi: 10.1038/s41598-024-56468-6
78.
ZhengYZhangCWeisskopfMGWilliamsPLClaus HennBParsonsPJet al. Evaluating associations between early pregnancy trace elements mixture and 2nd trimester gestational glucose levels: a comparison of three statistical approaches. Int J Hyg Environ Health. (2020) 224:113446. doi: 10.1016/j.ijheh.2019.113446
79.
WdowiakABrzozowskiIBojarI. Superoxide dismutase and glutathione peroxidase activity in pregnancy complicated by diabetes. Ann Agric Environ Med. (2015) 22:297–300. doi: 10.5604/12321966.1152083
80.
LiuTZhangMRahmanMLWangXHinkleSNZhangCet al. Exposure to heavy metals and trace minerals in first trimester and maternal blood pressure change over gestation. Environ Int. (2021) 153:106508. doi: 10.1016/j.envint.2021.106508
81.
XiaoLZanGFengXBaoYHuangSLuoXet al. The associations of multiple metals mixture with accelerated DNA methylation aging. Environ Pollut. (2021) 269:116230. doi: 10.1016/j.envpol.2020.116230
82.
TianYLuanMZhangJYangHWangYChenH. Associations of single and multiple perfluoroalkyl substances exposure with folate among adolescents in NHANES 2007-2010. Chemosphere. (2022) 307:135995. doi: 10.1016/j.chemosphere.2022.135995
83.
KristensenTNKetolaTKronholmI. Adaptation to environmental stress at different timescales. Ann N Y Acad Sci. (2020) 1476:5–22. doi: 10.1111/nyas.13974
84.
SiSShenYXinXMoMShaoBWangSet al. Hemoglobin concentration and iron supplement during pregnancy were associated with an increased risk of gestational diabetes mellitus. J Diabetes. (2021) 13:211–21. doi: 10.1111/1753-0407.13101
85.
SangkhaeVFisherALGanzTNemethE. Iron homeostasis during pregnancy: maternal, placental, and fetal regulatory mechanisms. Annu Rev Nutr. (2023) 43:279–300. doi: 10.1146/annurev-nutr-061021-030404
86.
GuoYZhangNZhangDRenQGanzTLiuSet al. Iron homeostasis in pregnancy and spontaneous abortion. Am J Hematol. (2019) 94:184–8. doi: 10.1002/ajh.25341
87.
BracchiIGuimarãesJRodriguesCAzevedoRCoelhoCMPinheiroCet al. Essential trace elements status in Portuguese pregnant women and their association with maternal and neonatal outcomes: a prospective study from the IoMum cohort. Biology (Basel). (2023) 12:1351. doi: 10.3390/biology12101351
88.
GunabalasingamSDe Almeida Lima SlizysDQuotahOMageeLWhiteSLRigutto-FarebrotherJet al. Micronutrient supplementation interventions in preconception and pregnant women at increased risk of developing pre-eclampsia: a systematic review and meta-analysis. Eur J Clin Nutr. (2023) 77:710–30. doi: 10.1038/s41430-022-01232-0
89.
WHO Guidelines Approved by the Guidelines Review Committee. WHO antenatal care recommendations for a positive pregnancy experience: Nutritional interventions update: Multiple micronutrient supplements during pregnancy. Geneva: World Health Organization (2020).
90.
WHO Guidelines Approved by the Guidelines Review Committee. WHO antenatal care recommendations for a positive pregnancy experience: Nutritional interventions update: Zinc supplements during pregnancy. Geneva: World Health Organization (2021).
91.
EberleCLoehnertMStichlingS. Effectivness of specific mobile health applications (mHealth-apps) in gestational diabtetes mellitus: a systematic review. BMC Pregnancy Childbirth. (2021) 21:808. doi: 10.1186/s12884-021-04274-7
Summary
Keywords
essential metal elements, gestational diabetes mellitus, inflammatory indicators, mixed exposure, mediation
Citation
Chen G, Wu L, Ji C, Xia J and Liu G (2025) Association between blood essential metal elements in early pregnancy and gestational diabetes mellitus. Front. Nutr. 12:1554840. doi: 10.3389/fnut.2025.1554840
Received
03 January 2025
Accepted
26 May 2025
Published
19 June 2025
Volume
12 - 2025
Edited by
Javier Diaz-Castro, University of Granada, Spain
Reviewed by
Hajo Haase, Technical University of Berlin, Germany
Phyllis Ohene-Agyei, The University of Auckland, New Zealand
Daniel Osmar Suárez Rico, University of Guadalajara, Mexico
Updates
Copyright
© 2025 Chen, Wu, Ji, Xia and Liu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guocheng Liu, hnliuguocheng@126.com; Jianhong Xia, xixixia58@126.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.